Attached files
| file | filename |
|---|---|
| 8-K - 8-K - HTG MOLECULAR DIAGNOSTICS, INC | d121064d8k.htm |
Exhibit 99.1

HTG
HTG

HTG
This presentation
contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our timeline strategy, future operations,
future product development, prospects and plans and objectives are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,”
“predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our
forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in forward-looking statements, including due to risks involved in the development and commercialization of products,
replacement of existing technologies and outcome of relationships with third parties. The forward-looking statements contained in this presentation reflect our current views with respect to future events, and we assume no obligation to update any
forward-looking statements except as required by applicable law.
8 January 2016 © HTG Molecular Diagnostics, Inc. 2
HTG investment highlights
HTG
Disruptive technology positioned to replace & consolidate
existing tests in $2B+ markets. ( FISH, Flo Cytometry, IHC)
Growing pipeline of late stage BioPharma collaborations with potential to lead to high valued companion
diagnostic tests
Well positioned for continued execution of strategic milestones
Enabling precision medicine at the local level
1/8/2016 3
Clinical molecular testing transformed
HTG’s solution for the future
HTG
Today
Centralized, remote testing labs
Slow, 2-4 week turnaround time for results
Samples are limited And often insufficient for
multiple tests and platforms
Poor integration of data Creates confusion among doctors and frustrates patients
HTG’s vision
Full molecular profiling in every lab
With 1-2 day turnaround and improved workflow for results
Sample use is optimized Profile
thousands of genes with a few square millimeters of tissue
Personalized therapies Optimized drug selection delivered at the local level
HTG
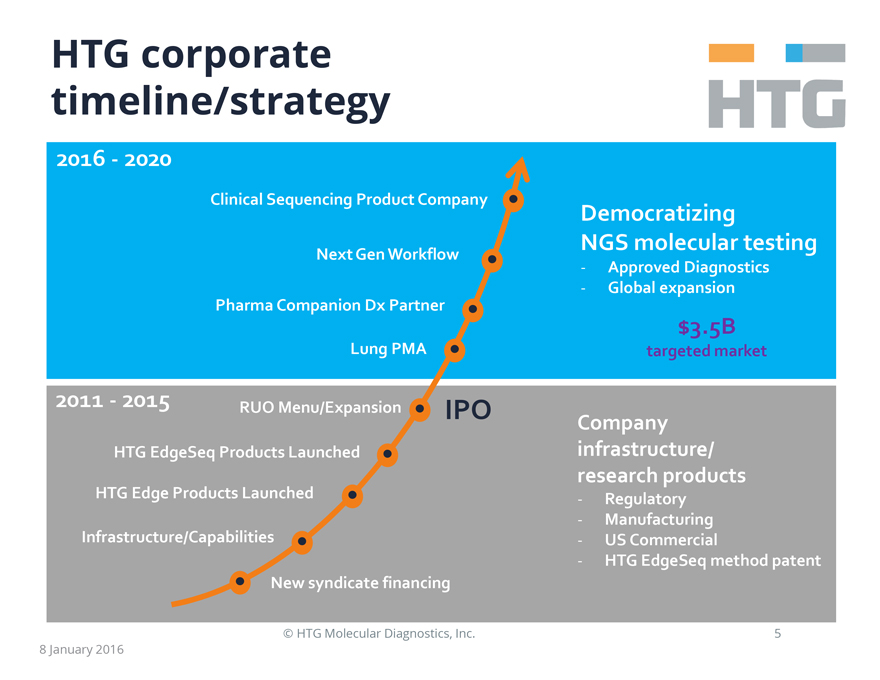
HTG corporate
timeline/strategy
2016 - 2020
Clinical Sequencing Product Company
Next Gen Workflow
Pharma Companion Dx Partner
Lung PMA
Democratizing NGS molecular testing
- Approved Diagnostics
- Global expansion
$3.5B
targeted market
2011 - 2015
RUO Menu/Expansion IPO
HTG EdgeSeq Products Launched
HTG Edge Products Launched
Infrastructure/Capabilities
New syndicate financing
Company infrastructure/research products
- Regulatory
- Manufacturing
- US Commercial
- HTG EdgeSeq method patent
© HTG Molecular Diagnostics, Inc.
8 January 2016 5
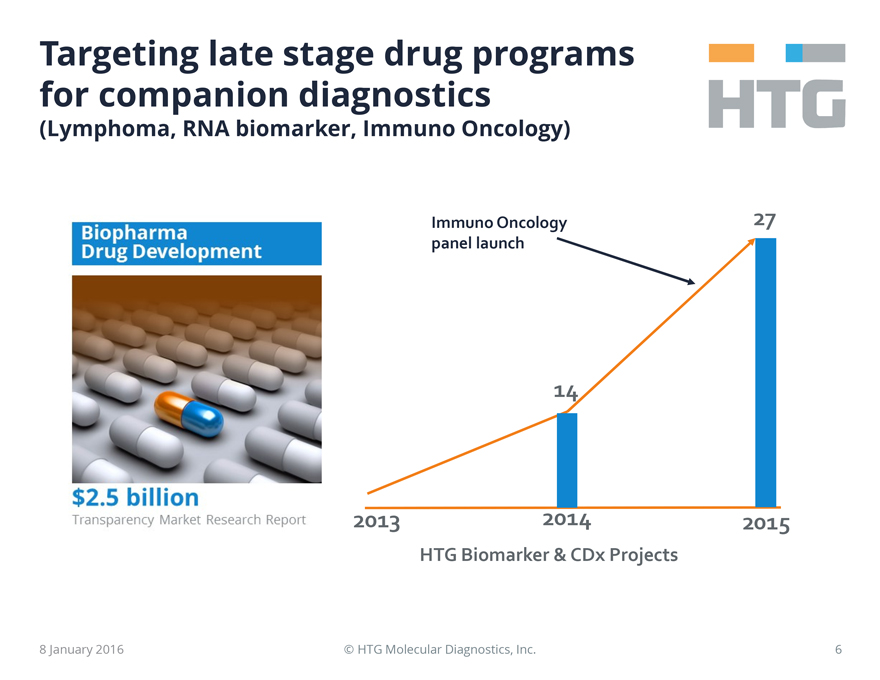
Targeting late stage drug programs for companion diagnostics
(Lymphoma, RNA biomarker, Immuno Oncology)
HTG
Biopharma Drug Development
$2.5 billion
Transparency Market Research Report
Immuno Oncology panel launch
27
14
2013 2014 2015
HTG Biomarker & CDx Projects
8 January 2016 © HTG Molecular Diagnostics, Inc. 6
BioPharma A
The Case of
the Assay Failure
HTG
Problem Statement – in a 300 case phase II study,
over 50% of the samples failed to yield a diagnostic result by RNASeq because of lack of sample or poor sample quality causing clinical development of the compound to be stopped
HTG Solution – A subset of these samples from this same cohort tested using HTG EdgeSeq Oncology Biomarker Panel Assay (OBP) yielded diagnostic results in over 90% of the
cases because HTG assay requires far less sample than RNASeq
Resulting Status – the BioPharma has now contracted with HTG to test the rest of the 300 cases
from the phase II study and reassess clinical development for the compound.
8 January 2016 © HTG Molecular Diagnostics, Inc. 7

Targeting translational research centers for diagnostic development
HTG
Translational Medicine
Science Translational Medicine
$7.7 billion
Wells Fargo Genomic Tools Report
Stanford MEDICINE
Freidenrich Center for Translational Research
THE UNIVERSITY OF TEXAS
MD Anderson Cancer Center
Making Cancer History*
Worldwide innovative networking in personalized cancer medicine
NIH NATIONAL CANCER INSTITUTE
Inova Translational Medicine INSTITUTE
PROOF
Centre of | Centre d’ EXCELLENCE Biomarker solutions for health care. Biomarqueurs - Solutions en soins de sante.
UNC
SCHOOL OF MEDICINE
School of Medicine
UNIVERSITY OF COLORADO ANSCHUTZ MEDICAL CAMPUS
8 January 2016 © HTG Molecular Diagnostics, Inc. 8
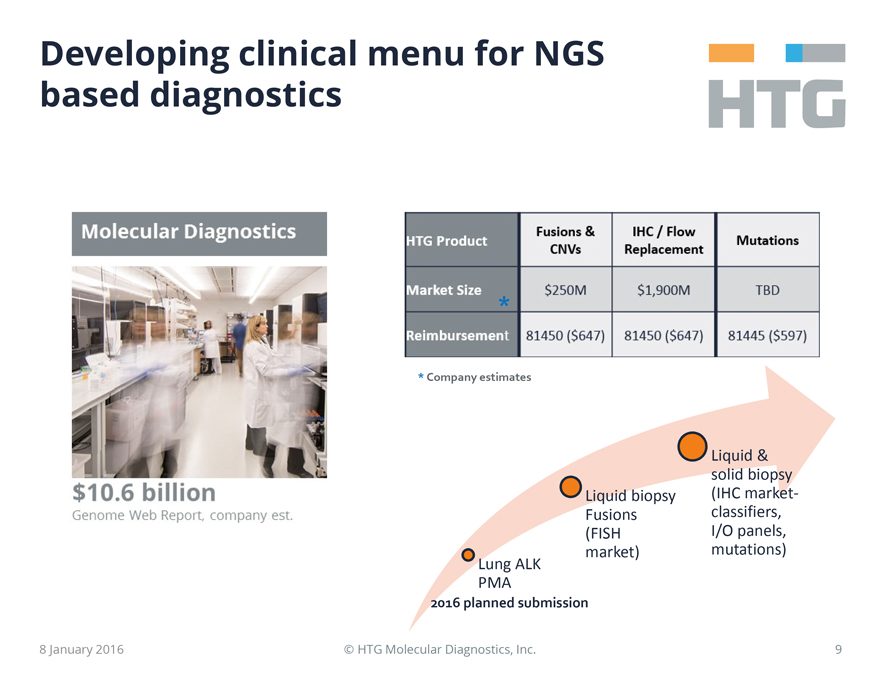
HTG
Developing clinical
menu for NGS based diagnostics
Molecular Diagnostics
$10.6 billion
Genome Web Report, company est.
HTG Product Fusions & CNVs IHC / Flow
Replacement Mutations
Market Size * $250M $1,900M TBD
Reimbursement 81450
($647) 81450 ($647) 81445 ($597)
* Company estimates
Lung ALK PMA
2016 planned submission
Liquid biopsy Fusions (FISH market)
Liquid & solid biopsy (IHC market-classifiers, I/O panels, mutations)
8 January 2016
© HTG Molecular Diagnostics, Inc. 9
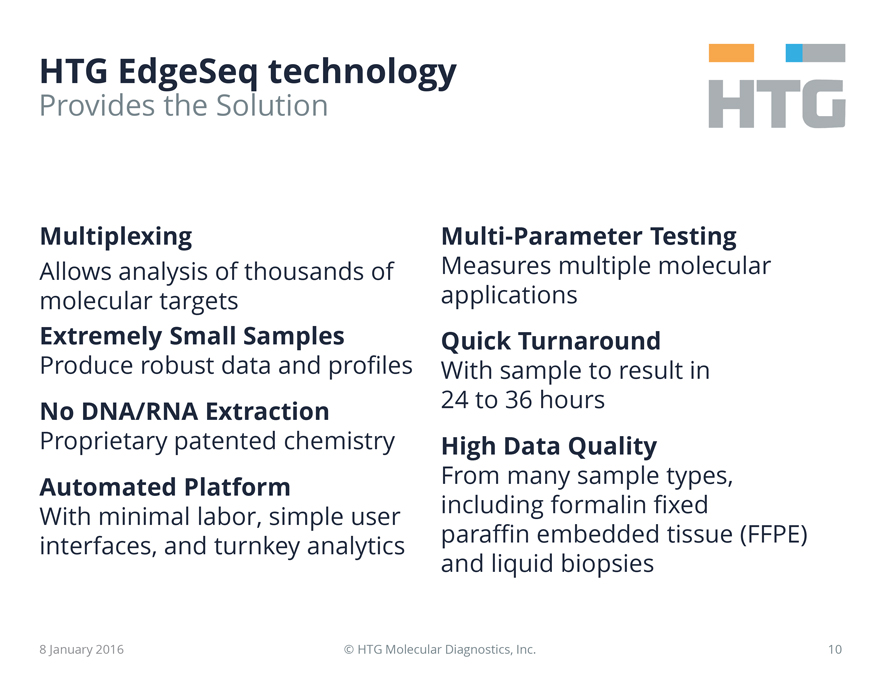
HTG EdgeSeq technology
Provides the Solution
HTG
Multiplexing
Allows analysis of thousands of molecular targets
Extremely Small Samples
Produce robust data and profiles
No DNA/RNA Extraction
Proprietary patented chemistry
Automated Platform
With minimal labor, simple user interfaces, and turnkey analytics
Multi-Parameter Testing
Measures multiple molecular applications
Quick Turnaround
With sample to result in 24 to 36 hours
High Data Quality
From many sample types, including formalin fixed paraffin embedded tissue
(FFPE) and liquid biopsies
8 January 2016 © HTG Molecular Diagnostics, Inc. 10
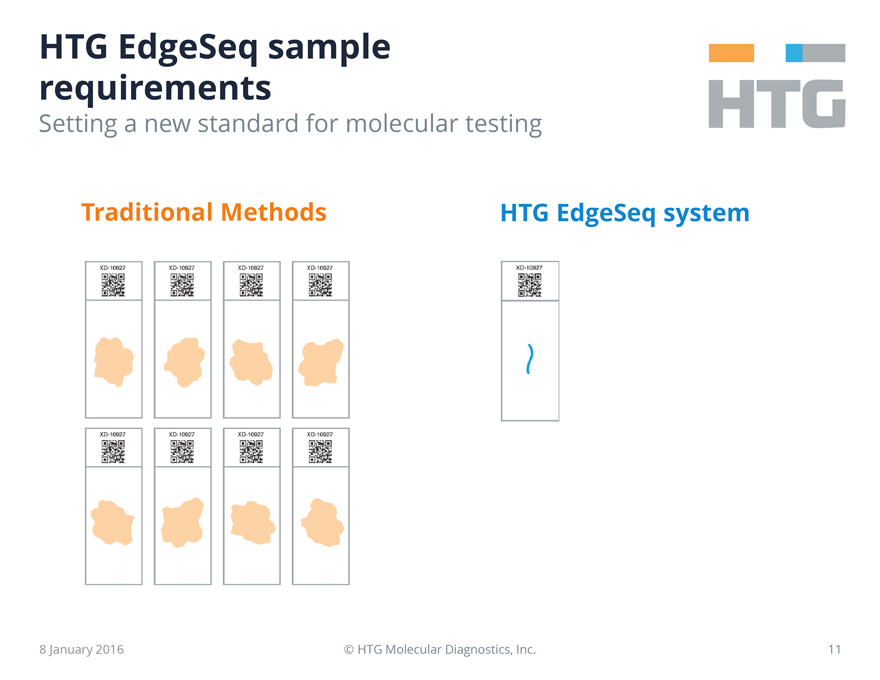
HTG EdgeSeq sample requirements
Setting a new standard for molecular testing
HTG
Traditional Methods
XD-10927 XD-10927 XD-10927 XD-10927
XD-10927 XD-10927 XD-10927 XD-10927
HTG EdgeSeq system
XD-10927
8 January 2016 © HTG Molecular Diagnostics, Inc. 11
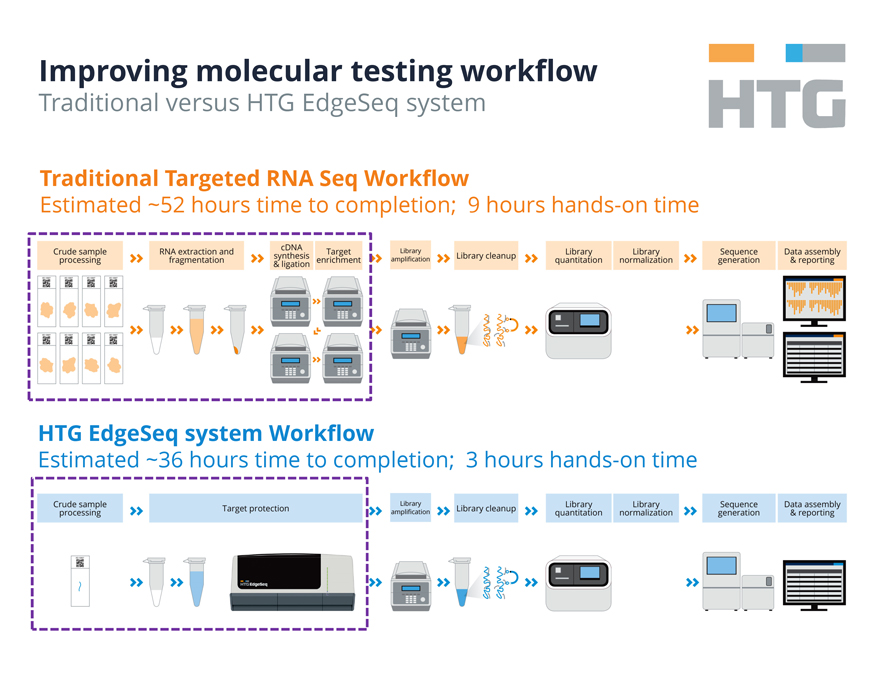
Improving molecular testing workflow
Traditional versus HTG EdgeSeq system
HTG
Traditional Targeted RNA Seq Workflow
Estimated ~52 hours time to completion; 9 hours hands-on
time
Crude sample processing RNA extraction and fragmentation cDNA synthesis & ligation Target enrichment
Library amplification Library cleanup Library quantitation Library normalization Sequence generation Data assembly & reporting
HTG EdgeSeq system Workflow
Estimated ~36 hours time to completion; 3 hours hands-on time
Crude sample processing Target protection Library amplification Library cleanup Library quantitation Library normalization Sequence generation Data assembly &
reporting
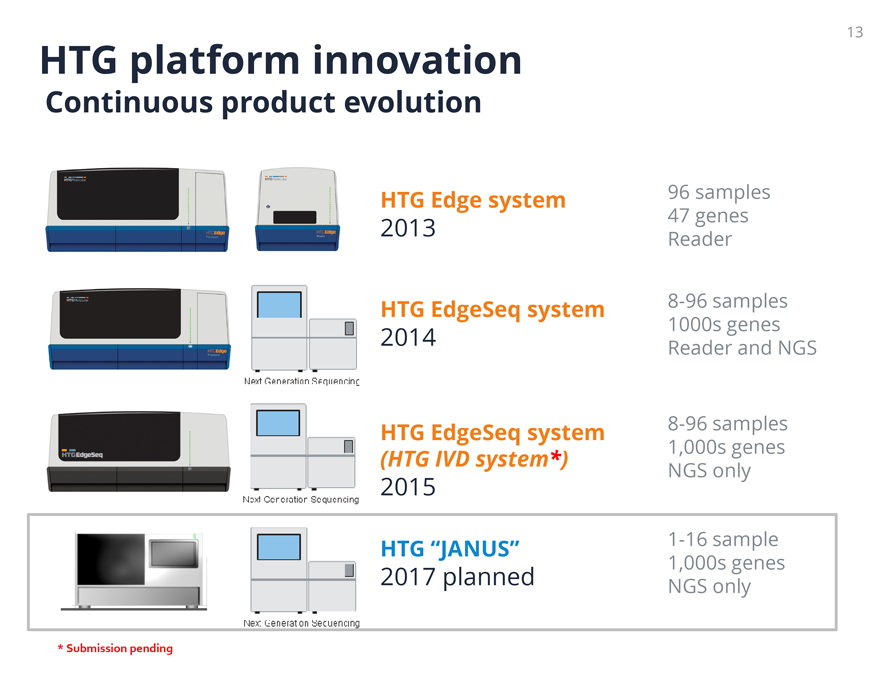
HTG platform innovation
Continuous product evolution
HTG Edge system 2013 96 samples 47 genes Reader
Next Generation Sequencing HTG EdgeSeq system 2014 8-96 samples 1000s genes Reader and NGS
Next Generation Sequencing HTG EdgeSeq system (HTG IVD system*) 2015 8-96 samples 1,000s genes NGS only
HTGEdgeSeq
Next Generation Sequencing HTG “JANUS” 2017 planned 1-16 sample 1,000s
genes NGS only
* Submission pending
13
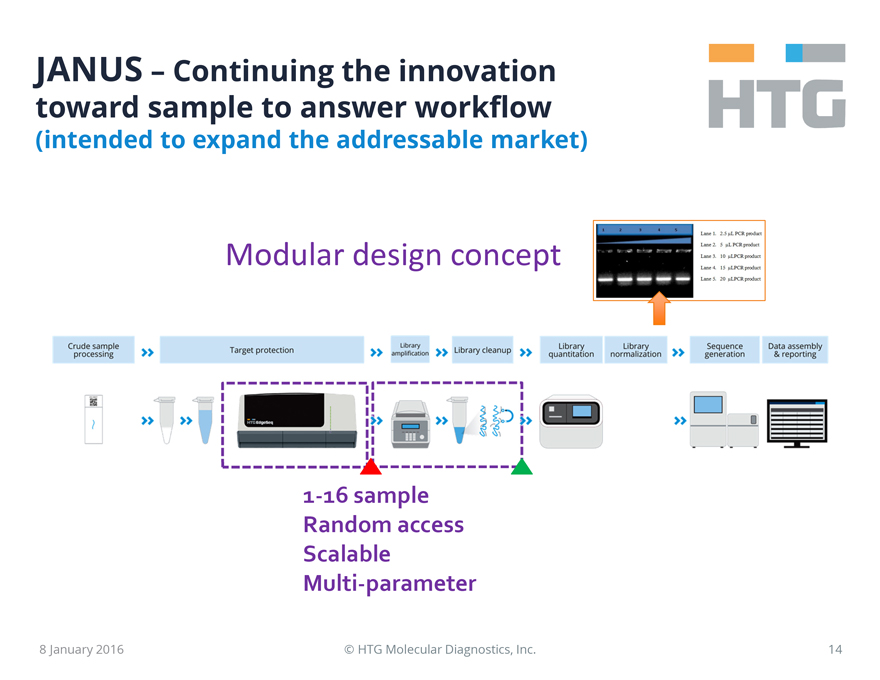
JANUS - Continuing the innovation toward sample to answer workflow
(intended to expand the addressable market)
HTG
Modular design concept
1 2 3 4 5
Lane 1. 2.5 uL PCR product
Lane 2. 5 uL PCR product
Lane 3. 10 uLPCR product
Lane 4. 15 uLPCR product
Lane 5. 20 uLPCR product
Crude sample processing Target protection Library amplification
Library cleanup Library quantitation Library normalization Sequence generation Data assembly & reporting
1-16 sample Random access Scalable Multi-parameter
8 January 2016 © HTG Molecular Diagnostics, Inc. 14

HTG forms IVD test development agreement with Illumina
October 30, 2014
HTG
HTG EdgeSeq
HTG Molecular
Sample Plate
Lot 0000
Part 00000000
Store at 15-30°C
Expires 12.31.25
illumina®
8 January 2016 © HTG Molecular Diagnostics, Inc. 15
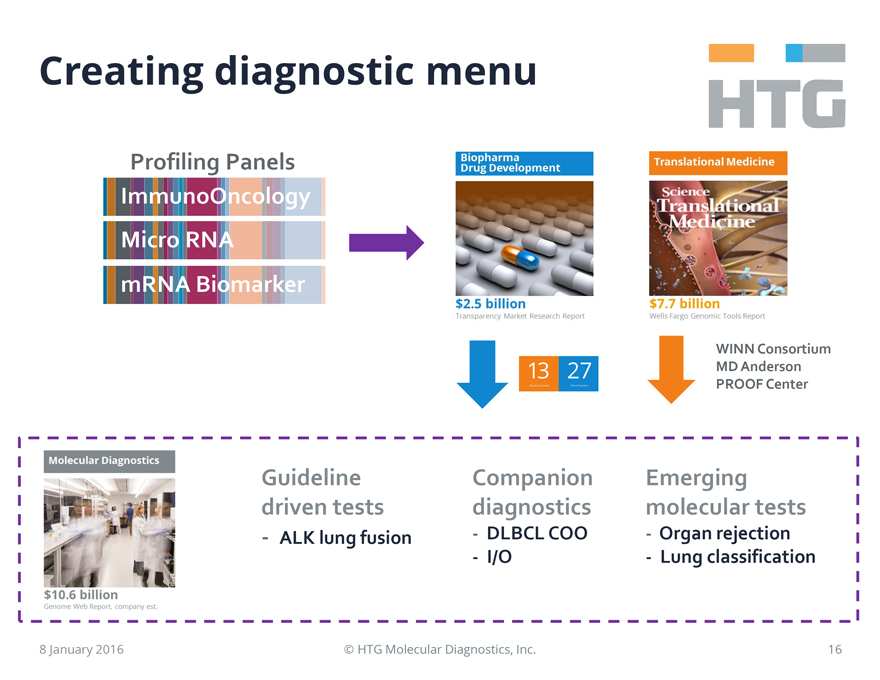
Creating diagnostic menu
HTG
Profiling Panels
ImmunoOncology
Micro RNA
mRNA Biomarker
Biopharma Drug Development
$2.5 billion
Transparency Market Research Report
13 Biopharma Clients
27 Active Programs
Translational Medicine
Science Translational Medicine
$7.7 billion Wells Fargo Genomic Tools Report
WINN Consortium MD Anderson PROOF Center
Molecular Diagnostics
$10.6 billion
Genome Web Report, company est.
Guideline driven tests
- ALK lung fusion
Companion
diagnostics
- DLBCL COO
- I/O
Emerging molecular tests
- Organ rejection
- Lung classification
8 January 2016
© HTG Molecular Diagnostics, Inc.
16
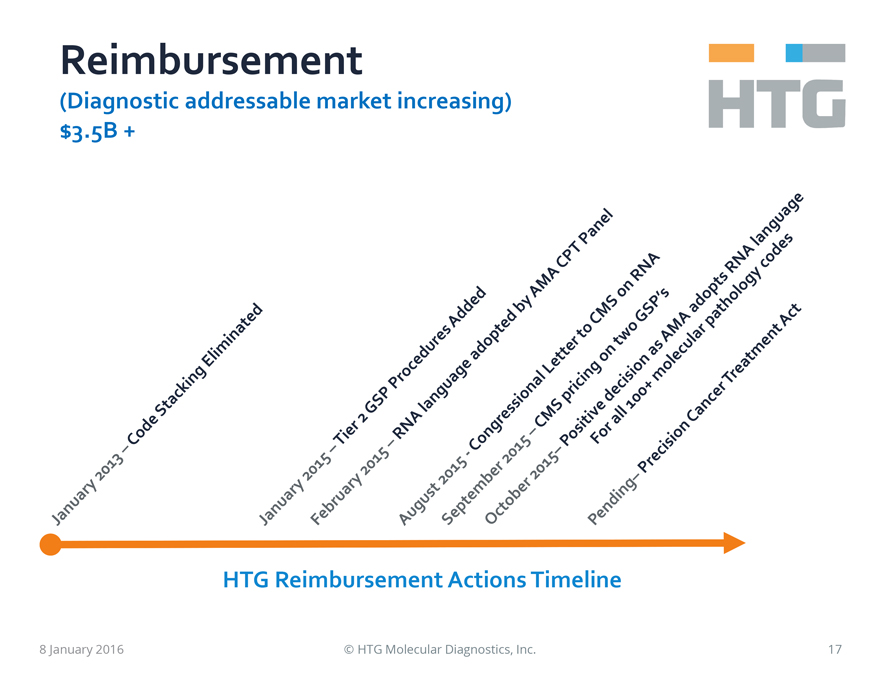
Reimbursement
(Diagnostic
addressable market increasing) $3.5B +
HTG
January 2013 – Code Stacking
Eliminated
January 2015 – Tier 2 GSP Procedures Added
February 2015
– RNA language adopted by AMA CPT Panel
August 2015 – Congressional Letter to CMS on RNA
September 2015 – CMS pricing on two GSP’s
October 2015 – Positive decision as
AMA adopts RNA language
For all 100+ molecular pathology codes
Pending –
Precision Cancer Treatment Act
HTG Reimbursement Actions Timeline
8 January
2016 © HTG Molecular Diagnostics, Inc. 17
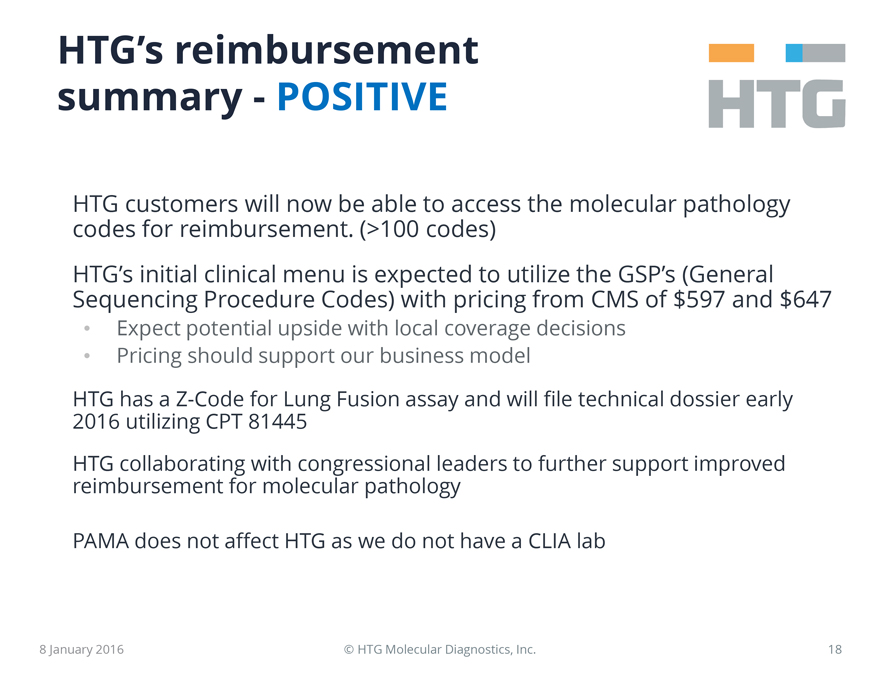
HTG’s reimbursement summary – POSITIVE
HTG
HTG customers will now be able to access the molecular pathology codes for reimbursement.
(>100 codes)
HTG’s initial clinical menu is expected to utilize the GSP’s (General Sequencing Procedure Codes) with pricing from CMS of $597 and $647
Expect potential upside with local coverage decisions
Pricing should support
our business model
HTG has a Z-Code for Lung Fusion assay and will file technical dossier early 2016 utilizing CPT 81445
HTG collaborating with congressional leaders to further support improved reimbursement for molecular pathology
PAMA does not affect HTG as we do not have a CLIA lab
8 January 2016 © HTG Molecular
Diagnostics, Inc. 18
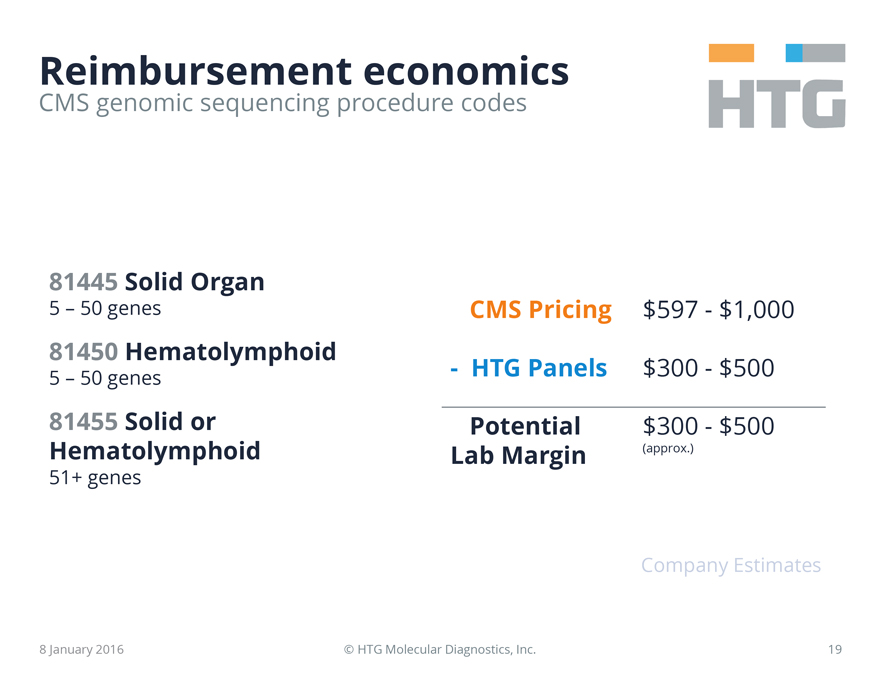
Reimbursement economics
CMS genomic sequencing procedure codes
HTG
81445 Solid Organ
5 – 50 genes
81450 Hematolymphoid
5 – 50 genes
81455 Solid or Hematolymphoid
51+ genes
CMS Pricing $597 - $1,000
- HTG Panels $300 - $500
Potential Lab Margin $300 - $500 (approx.)
Company Estimates
8 January 2016 © HTG Molecular Diagnostics, Inc. 19
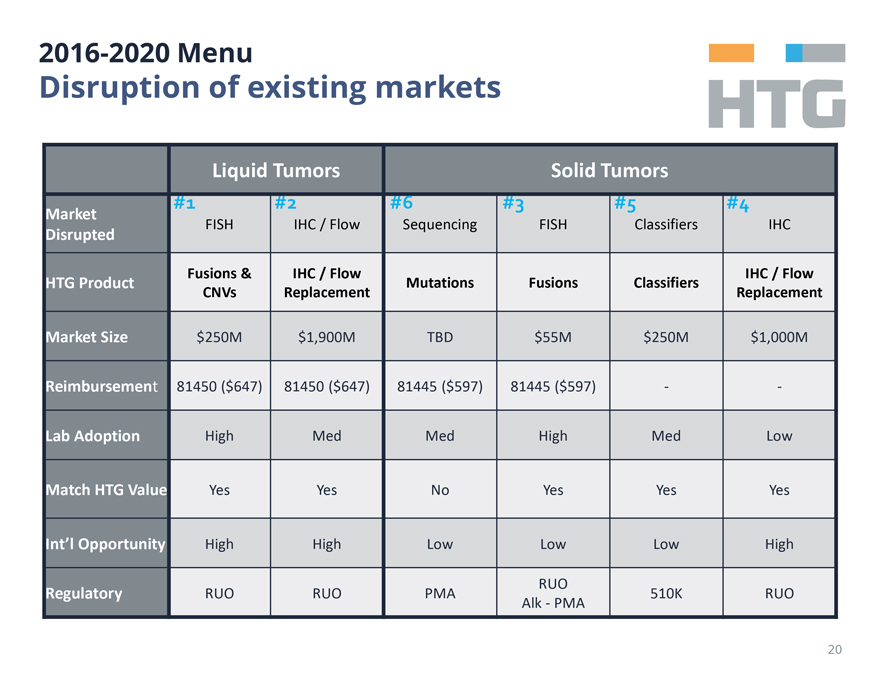
2016-2020 Menu
Disruption
of existing markets
HTG
Liquid Tumors Solid Tumors
#1 FISH #2 IHC / Flow #6 Sequencing #3 FISH #5 Classifiers #4 IHC
Market Disrupted
HTG Product Fusions & CNVs IHC / Flow Replacement Mutations Fusions Classifiers IHC / Flow Replacement
Market Size $250M $1,900M TBD $55M $250M $1,000M
Reimbursement 81450 ($647) 81450 ($647) 81445
($597) 81445 ($597) - -
Lab Adoption High Med Med High Med Low
Match HTG
Value Yes Yes No Yes Yes Yes
Int’l Opportunity High High Low Low Low High
Regulatory RUO RUO PMA RUO Alk-PMA 510K RUO
20
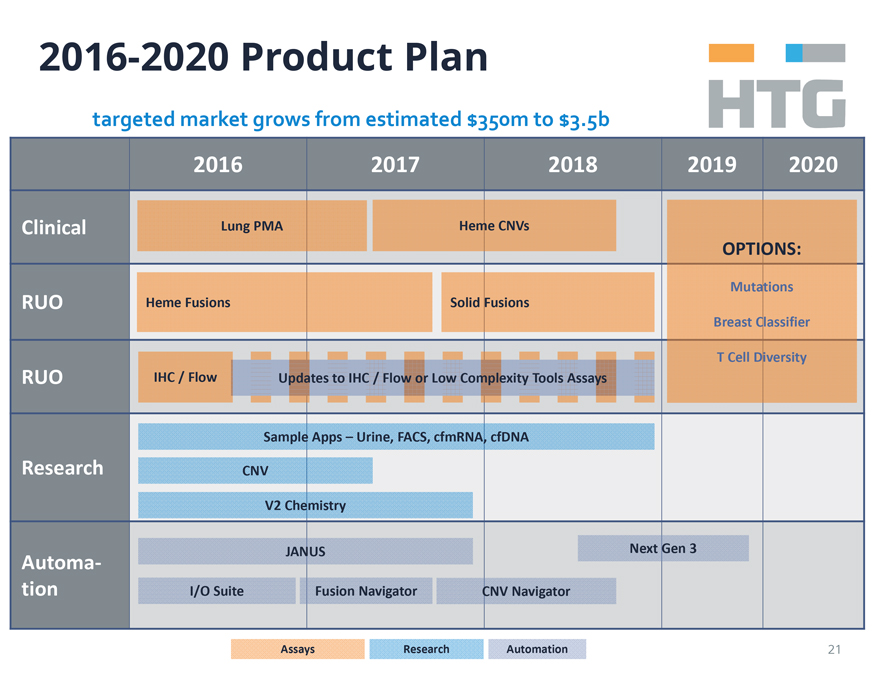
2016-2020 Product Plan
targeted market grows from estimated $350m to $3.5b
HTG
2016 2017 2018
Clinical Lung PMA Heme CNVs OPTIONS:
RUO Heme Fusions Solid Fusions Mutations Breast Classifier
RUO IHC / Flow Updates to IHC /
Flow or Low Complexity Tools Assays T Cell Diversity
Sample Apps – Urine, FACS, cfmRNA, cfDNA
Research CNV V2 Chemistry
Automa-
tion JANUS Next Gen 3 I/O Suite Fusion Navigator CNV Navigator
Assays Research Automation 21

Empowering precision medicine at the local level
HTG
HTGEdgeSeq
8 January 2016 © HTG Molecular Diagnostics, Inc. 22
