Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zyla Life Sciences | a16-1463_28k.htm |
Exhibit 99.1
January 2016 Corporate Update NASDAQ: EGLT 1

Forward-Looking Statements 2 Statements included that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management's current expectations, and are subject to known and unknown uncertainties and risks. Actual results could differ materially from those discussed due to a number of factors, including, but not limited to: the success of integrating recent acquisitions; our ability to obtain regulatory approval of our product candidates; our ability to successfully commercialize SPRIX® Nasal Spray and OXAYDO®; competitive factors; general market conditions; and other risk factors described in Egalet's filings with the United States Securities and Exchange Commission. Egalet assumes no obligation to update or revise any forward-looking statements contained in this presentation whether as a result of new information or future events, except as may be required by law. Please refer to oxaydo.com and sprix.com for full prescribing information (PI) including the Boxed Warning for SPRIX.

What Makes Egalet Different 3 Established commercial infrastructure Product & pipeline target large markets & designed to address top FDA priority Proprietary Guardian™ Technology platform
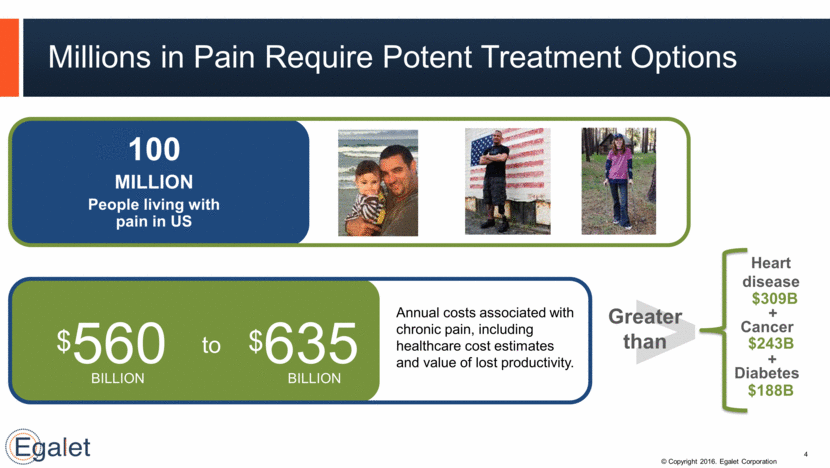
Millions in Pain Require Potent Treatment Options 100 MILLION People living with pain in US 4 People abuse opioids worldwide3 $560 to $635 Annual costs associated with chronic pain, including healthcare cost estimates and value of lost productivity. $ BILLION BILLION > Heart disease Greater than Cancer Diabetes $309B $243B $188B + +
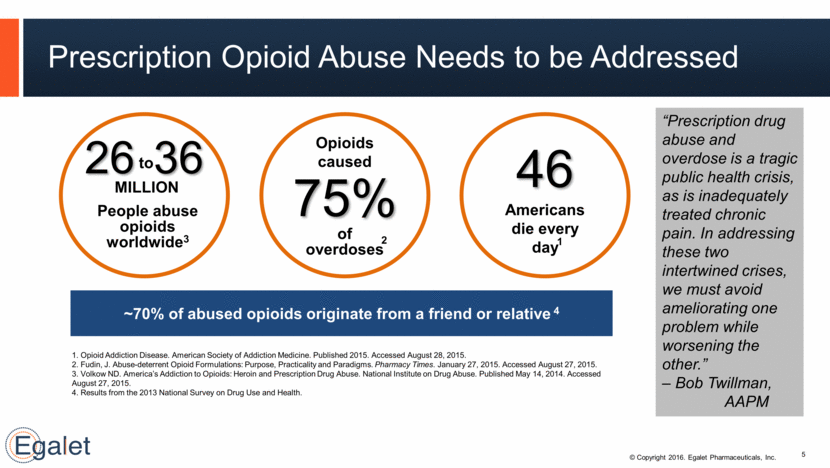
Prescription Opioid Abuse Needs to be Addressed 5 $ BILLION 1. Opioid Addiction Disease. American Society of Addiction Medicine. Published 2015. Accessed August 28, 2015. 2. Fudin, J. Abuse-deterrent Opioid Formulations: Purpose, Practicality and Paradigms. Pharmacy Times. January 27, 2015. Accessed August 27, 2015. 3. Volkow ND. America’s Addiction to Opioids: Heroin and Prescription Drug Abuse. National Institute on Drug Abuse. Published May 14, 2014. Accessed August 27, 2015. 4. Results from the 2013 National Survey on Drug Use and Health. 75% Opioids caused of overdoses 2 46 Americans die every day 1 to 26 36 MILLION People abuse opioids worldwide3 ~70% of abused opioids originate from a friend or relative 4 “Prescription drug abuse and overdose is a tragic public health crisis, as is inadequately treated chronic pain. In addressing these two intertwined crises, we must avoid ameliorating one problem while worsening the other.” – Bob Twillman, AAPM
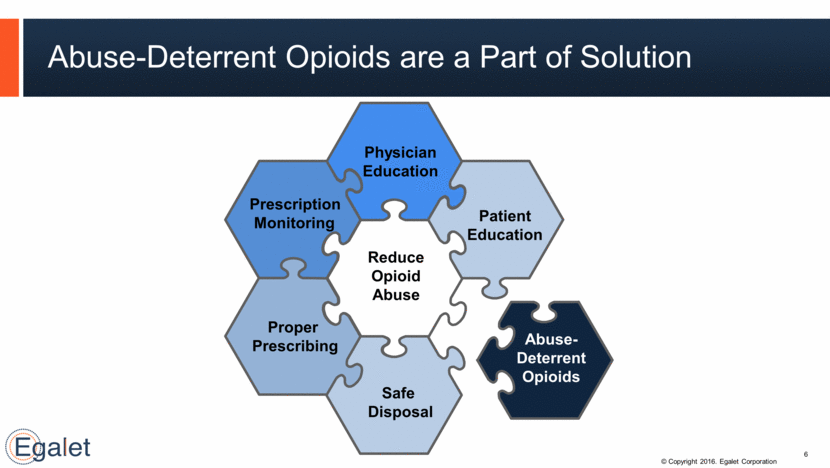
Abuse-Deterrent Opioids are a Part of Solution 6 Physician Education Prescription Monitoring Abuse-Deterrent Opioids Patient Education Proper Prescribing Safe Disposal Reduce Opioid Abuse
FDA Shows Strong Commitment to AD Opioids 7 Issued final guidance April 2015 Have six AD opioids: OxyContin, OXAYDO*, Targiniq, Embeda, Hysingla, MorphaBond Throckmorton presented at NASCSA meeting Oct 2015: No more non-AD opioid approvals Generics need to be abuse deterrent Future should be all AD opioids *OXAYDO was approved prior to FDA guidance for AD opioids.

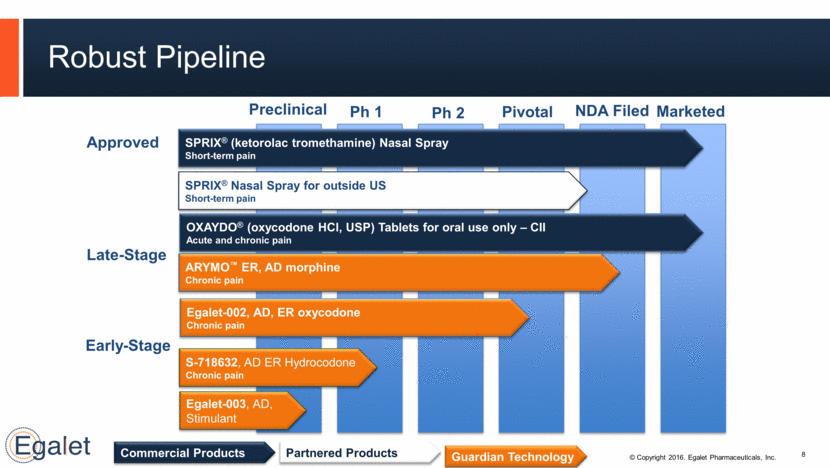
Robust Pipeline 8 Approved Late-Stage Early-Stage SPRIX® (ketorolac tromethamine) Nasal Spray Short-term pain ARYMO™ ER, AD morphine Chronic pain Egalet-002, AD, ER oxycodone Chronic pain S-718632, AD ER Hydrocodone Chronic pain OXAYDO® (oxycodone HCl, USP) Tablets for oral use only – CII Acute and chronic pain Egalet-003, AD, Stimulant Ph 1 Ph 2 Pivotal NDA Filed Marketed Guardian Technology Commercial Products SPRIX® Nasal Spray for outside US Short-term pain Preclinical Partnered Products
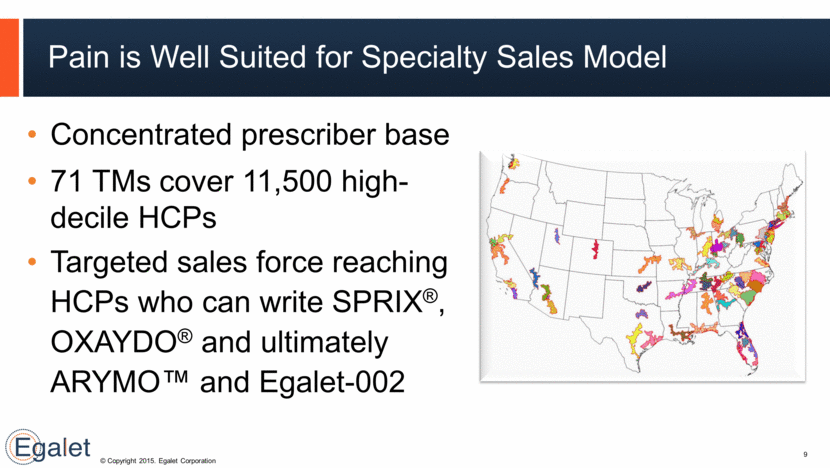
Concentrated prescriber base 71 TMs cover 11,500 high-decile HCPs Targeted sales force reaching HCPs who can write SPRIX®, OXAYDO® and ultimately ARYMO™ and Egalet-002 Pain is Well Suited for Specialty Sales Model 9
SPRIX® Nasal Spray, a Potent NSAID w/ Rapid Absorption 10

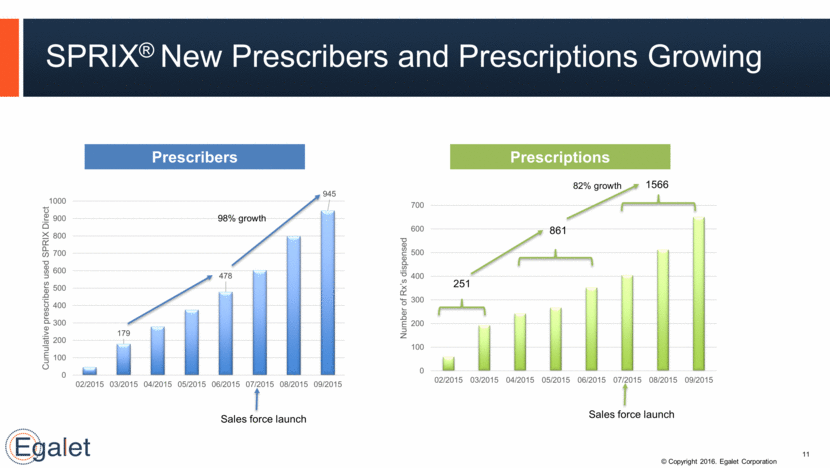
SPRIX® New Prescribers and Prescriptions Growing 98% growth 251 861 1566 Sales force launch Sales force launch Prescribers Prescriptions 11 179 478 945 0 100 200 300 400 500 600 700 800 900 1000 02/2015 03/2015 04/2015 05/2015 06/2015 07/2015 08/2015 09/2015 Cumulative prescribers used SPRIX Direct 0 100 200 300 400 500 600 700 02/2015 03/2015 04/2015 05/2015 06/2015 07/2015 08/2015 09/2015 Number of Rx’s dispensed 82% growth
OXAYDO®, Only IR Oxy Designed to Discourage Abuse 12

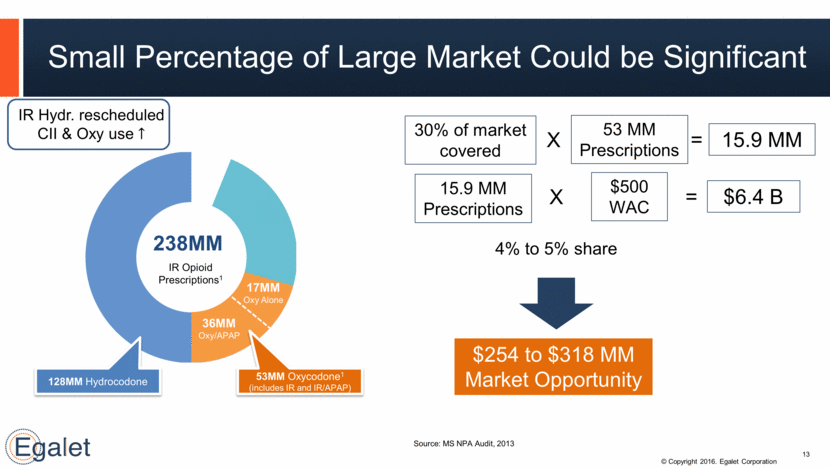
Small Percentage of Large Market Could be Significant Source: MS NPA Audit, 2013 238M IR Opioid Prescriptions1 128MM Hydrocodone 53MM Oxycodone1 (includes IR and IR/APAP) 36MM Oxy/APAP 17MM Oxy Alone 238MM 13 53 MM Prescriptions 30% of market covered 15.9 MM 4% to 5% share $254 to $318 MM Market Opportunity X = 15.9 MM Prescriptions $500 WAC $6.4 B X = IR Hydr. rescheduled CII & Oxy use
Manufacturing Process Drives Differentiation with Guardian™ Technology 14

ARYMO™ ER: AD Morphine Could be on Market in ‘16* *for the management of pain severe enough to require daily, around-the-clock, opioid treatment and for which alternative treatments are inadequate 15 Hard matrix Resistant to particle size reduction & chemical extraction Forms viscous hydrogel on contact with liquid can’t draw up into syringe Reduces risk of accidental misuse via chewing Reduces risk of intential abuse via IV injection or snorting NDA submitted 12/14/2105 PDUFA 4Q2016 7.1 MM morphine prescriptions Most prescribed ER opioid
ARYMO™ ER Designed to Deter Abuse by Injection 16 Karsten Lindhardt, Ph.D., SVP, R&D

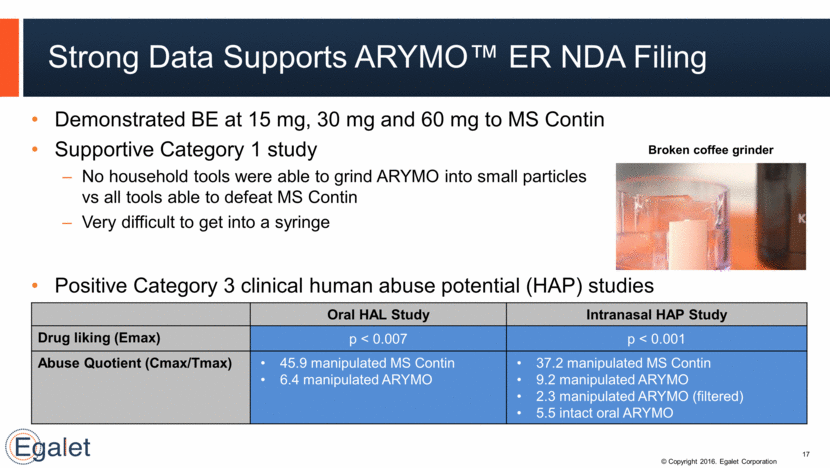
Strong Data Supports ARYMO™ ER NDA Filing Demonstrated BE at 15 mg, 30 mg and 60 mg to MS Contin Supportive Category 1 study No household tools were able to grind ARYMO into small particles vs all tools able to defeat MS Contin Very difficult to get into a syringe 17 Oral HAL Study Intranasal HAP Study Drug liking (Emax) p < 0.007 p < 0.001 Abuse Quotient (Cmax/Tmax) 45.9 manipulated MS Contin 6.4 manipulated ARYMO 37.2 manipulated MS Contin 9.2 manipulated ARYMO 2.3 manipulated ARYMO (filtered) 5.5 intact oral ARYMO Broken coffee grinder Positive Category 3 clinical human abuse potential (HAP) studies
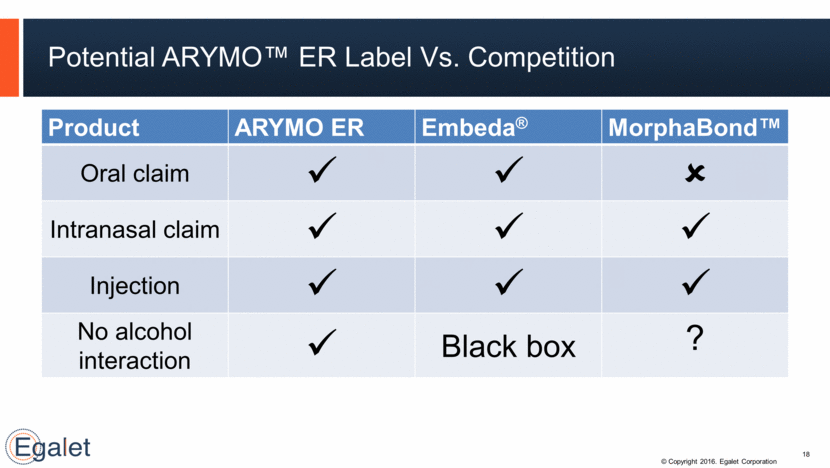
Potential ARYMO™ ER Label Vs. Competition 18 Product ARYMO ER Embeda® MorphaBond™ Oral claim Intranasal claim Injection No alcohol interaction Black box ?
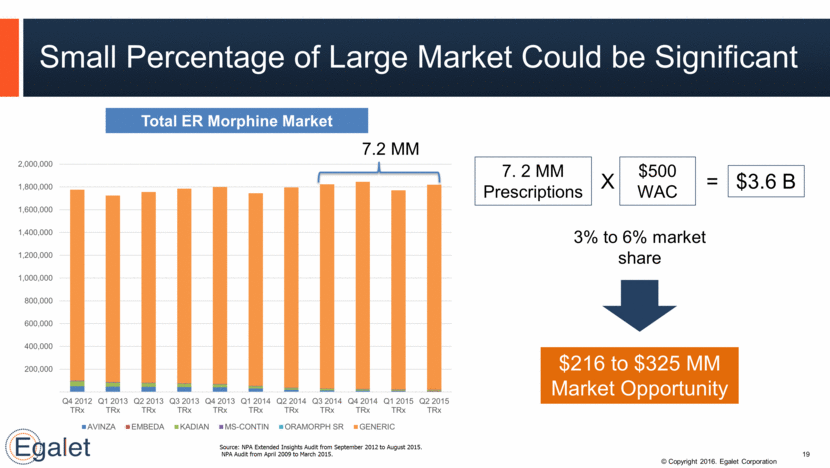
Small Percentage of Large Market Could be Significant Source: NPA Extended Insights Audit from September 2012 to August 2015. NPA Audit from April 2009 to March 2015. 7.2 MM Total ER Morphine Market 19 7. 2 MM Prescriptions $500 WAC $3.6 B 3% to 6% market share $216 to $325 MM Market Opportunity X = 200,000 400,000 600,000 800,000 1,000,000 1,200,000 1,400,000 1,600,000 1,800,000 2,000,000 Q4 2012 TRx Q1 2013 TRx Q2 2013 TRx Q3 2013 TRx Q4 2013 TRx Q1 2014 TRx Q2 2014 TRx Q3 2014 TRx Q4 2014 TRx Q1 2015 TRx Q2 2015 TRx AVINZA EMBEDA KADIAN MS-CONTIN ORAMORPH SR GENERIC
Egalet-002, an AD, ER Oxycodone, in Late-Stage Development* *for the management of pain severe enough to require daily, around-the-clock, opioid treatment and for which alternative treatments are inadequate 20 Pivotal Phase 3 program started NDA submission mid-17 Hard matrix surrounded by hard shell Resistant to particle size reduction & chemical extraction Forms viscous hydrogel on contact with liquid can’t draw up into a syringe Reduces risk of accidental misuse via chewing Reduces risk of intential abuse via snorting or IV injection Oxycodone is highest selling ER opioid; $2.5B in sales in 2013
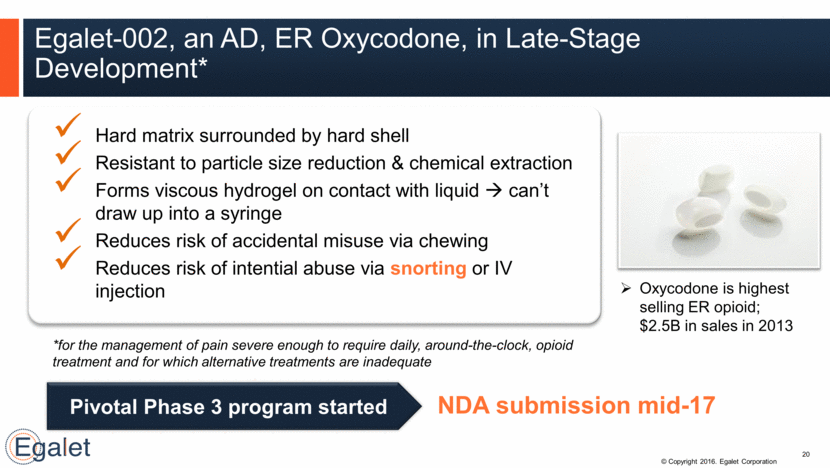
Extended Drug Release by Tablet Erosion 21 Well-defined erosion area allows constant extended drug release Shell is excreted & degrades to its monomer lactic acid w/ environmental impact Designed w/ improved PK profile compared to OxyContin
Egalet-002 in Late-Stage Development 22 NDA submission mid-17 Study Status Category 1 abuse-deterrent studies Completed Clinical alcohol interaction study Completed Category 3 oral HAP (will compare to OxyContin) To start in H1:16 Category 3 intranasal HAP (will compare to OxyContin) To start in H1:16 Phase 3 open label, long-term safety study Underway Randomized withdrawal efficacy/safety study Underway
Egalet Leadership in AD Opioid Development Organized a Category 1 Focus Group Industry meeting with several FDA attendees 23 Speaker at ADF meetings ALERRT™ visual analog scale Working with Pinney Associates on new tool to assess level of effort and resources required for tampering

Next Horizon: Stimulant Abuse is a Big Problem ~ 2/3 of college seniors will be offered prescription stimulants for nonmedical use during their college career2 ~900,000 Americans abuse prescription stimulants every month 10.8 MILLION Americans abuse prescription stimulants annually 20% of students report abusing prescription stimulants at least once in their lifetime3 1 Stimulant Addiction and Abuse. Addiction Center Web Site. https://www.addictioncenter.com/stimulants/ Published October 1 2015. Accessed January 4 2016. 2 Prescription Drug Abuse Statistics. CLAAD Web Site. http://claad.org/rx-drug-abuse-stats/ Accessed January 4 2016. 3 New Survey: Misuse and Abuse of Prescription Stimulants Becoming Normalized Behavior Among College Students, Young Adults. Partnership for Drug-Free Kids. http://www.drugfree.org/newsroom/adhd-survey-2014 Published November 13 2015. Accessed January 4 2016.
Egalet-003 Development Path to Registration We have formulation experience with stimulants Regulatory strategy is a 505(b)(2) path based on bioequivalence (BE) Development plan similar to ARYMO™ Initial in vitro formulation design to achieve desired release profile Initial clinical PK study to identify optimal formulation in vivo Establish IVIVC model Pivotal PK studies to demonstrate BE across the dosage range Food effect study at highest dosage strength Full complement of Category 1, 2 & 3 AD studies consistent with FDA Guidance 25 Goal: File Egalet-003 IND second half of 2016
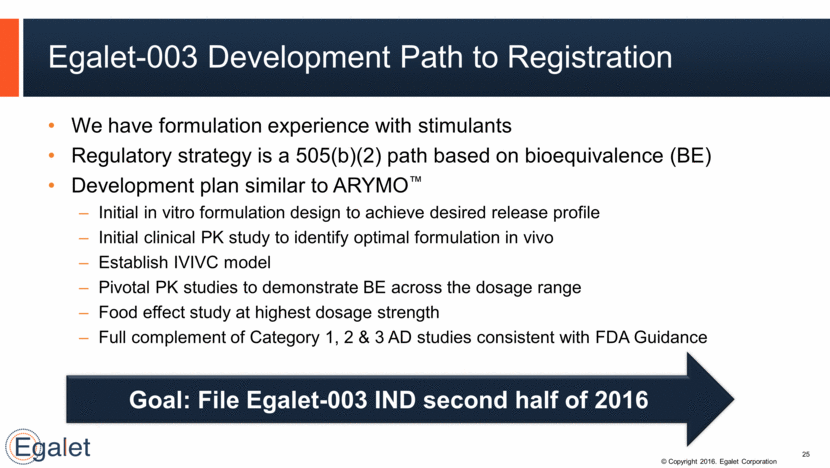
Strong Cash Position 26 (in $ millions) Mar 31, 2015 Jun 30, 2015 Sep 30, 2015 Cash & marketable securities $ 53.9 $ 108.4 $169.1 Current assets less current liabilities 41.6 84.9 146.8 Total debt outstanding 15.0 76.0 76.0 Acquired SPRIX & licensed OXAYDO $15MM in venture debt from Hercules Technology Closed $61MM offering of convertible senior notes Launched SPRIX Closed $86.3 MM equity follow-on offering Launched OXAYDO
Financial Expectations for 2016 2015 3Q2015 reported cash used in operating activities of $18.7 million 4Q2015 expect cash used in operating activities to increase due to: ARYMO NDA filing fee of $2.3 million SPRIX sNDA fee of $1.2 million 2016 Growth of SPRIX and OXAYDO revenue Expenses expected to be $22 to $25 million a quarter Full year impact of 2015 deployment of 50-person field sales force and the addition of 21 territories in 2016 Commercial expenses for SPRIX and OXAYDO R&D spend will increase given Egalet-002 Phase 3 studies Interest expense will increase due to full year impact of 2015 convertible debt Investment in commercial manufacturing expansion of $6-$8 million between 4Q14-3Q16 27

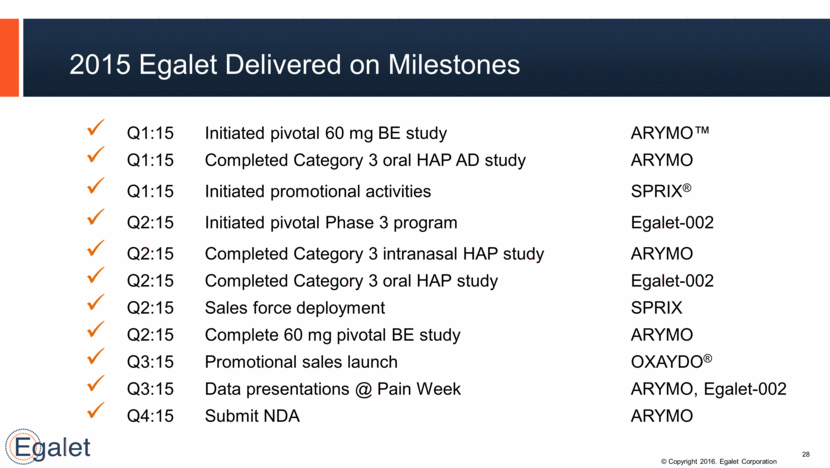
Q1:15 Initiated pivotal 60 mg BE study ARYMO™ Q1:15 Completed Category 3 oral HAP AD study ARYMO Q1:15 Initiated promotional activities SPRIX® Q2:15 Initiated pivotal Phase 3 program Egalet-002 Q2:15 Completed Category 3 intranasal HAP study ARYMO Q2:15 Completed Category 3 oral HAP study Egalet-002 Q2:15 Sales force deployment SPRIX Q2:15 Complete 60 mg pivotal BE study ARYMO Q3:15 Promotional sales launch OXAYDO® Q3:15 Data presentations @ Pain Week ARYMO, Egalet-002 Q4:15 Submit NDA ARYMO 2015 Egalet Delivered on Milestones 28
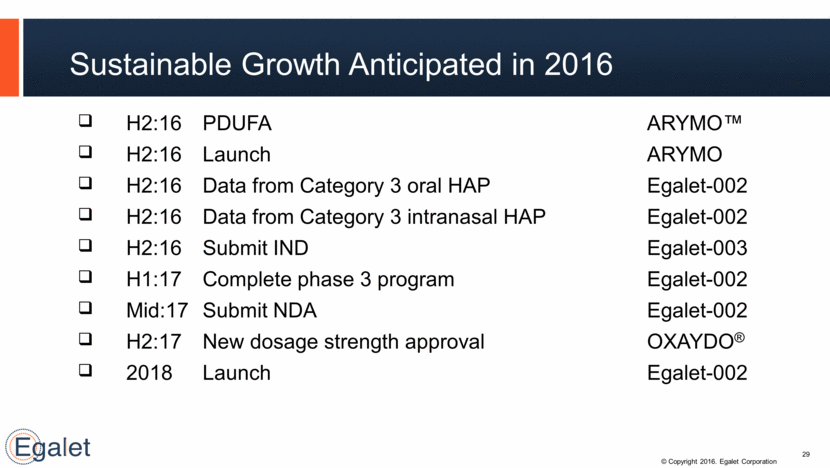
H2:16 PDUFA ARYMO™ H2:16 Launch ARYMO H2:16 Data from Category 3 oral HAP Egalet-002 H2:16 Data from Category 3 intranasal HAP Egalet-002 H2:16 Submit IND Egalet-003 H1:17 Complete phase 3 program Egalet-002 Mid:17 Submit NDA Egalet-002 H2:17 New dosage strength approval OXAYDO® 2018 Launch Egalet-002 Sustainable Growth Anticipated in 2016 29
What Makes Egalet Different 30 Established commercial infrastructure Product & pipeline target large markets & designed to address top FDA priority Proprietary Guardian™ Technology platform
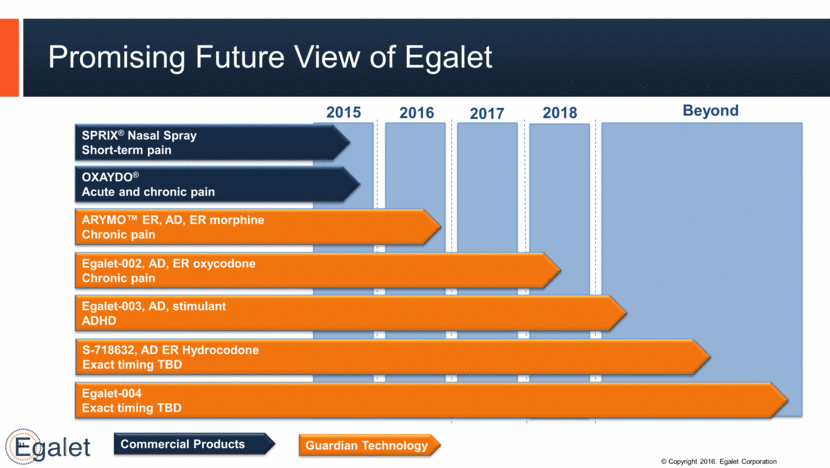
31 OXAYDO® Acute and chronic pain ARYMO™ ER, AD, ER morphine Chronic pain Egalet-002, AD, ER oxycodone Chronic pain 2016 2017 2018 Promising Future View of Egalet 2015 Egalet-003, AD, stimulant ADHD S-718632, AD ER Hydrocodone Exact timing TBD Egalet-004 Exact timing TBD Beyond SPRIX® Nasal Spray Short-term pain Guardian Technology Commercial Products
Thank You 32

