Attached files
| file | filename |
|---|---|
| EX-23.1 - EX-23.1 - ViewRay, Inc. | d73715dex231.htm |
Table of Contents
Index to Financial Statements
As filed with the Securities and Exchange Commission on December 30, 2015.
Registration No. 333-207347
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 3
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
ViewRay, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware | 3845 | 42-1777485 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
2 Thermo Fisher Way
Oakwood Village, OH 44146
(440) 703-3210
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Chris A. Raanes
President & Chief Executive Officer
ViewRay, Inc.
2 Thermo Fisher Way
Oakwood Village, OH 44146
(440) 703-3210
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Mark V. Roeder, Esq. Brian D. Paulson, Esq. |
D. David Chandler Chief Financial Officer ViewRay, Inc. 2 Thermo Fisher Way Oakwood Village, OH 44146 Telephone: (440) 703-3210 Facsimile: (800) 417-3459 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | x | |||
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of Each Class of Securities to be Registered |
Amount to be Registered |
Proposed Maximum Offering Price Per Share |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee | ||||
| Shares of common stock, par value $0.01 per share |
38,128,672(1) | $5.00(2) | $190,643,360 | $19,254(3) | ||||
|
| ||||||||
| (1) | Consists of (a) 37,929,912 outstanding shares of the registrant’s common stock and (b) 198,760 shares of the registrant’s common stock issuable upon exercise of common stock purchase warrants. Pursuant to Rule 416 under the Securities Act of 1933, as amended, there is also being registered hereby such indeterminate number of additional shares of common stock of the registrant as may be issued or issuable because of stock splits, stock dividends, stock distributions, and similar transactions. |
| (2) | Estimated solely for purposes of calculating the registration fee according to Rule 457(c) under the Securities Act based on the average of the bid and asked price of our common stock quoted on the OTC Markets, OTCQB tier of OTC Markets Group, Inc. on December 15, 2015. |
| (3) | Previously paid. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
Index to Financial Statements
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PROSPECTUS (Subject to Completion) | Dated December 30, 2015 |
38,128,672 Shares

Common Stock
This prospectus relates to the offering and resale by the selling stockholders identified herein of up to 38,128,672 shares of common stock, par value $0.01 per share, of ViewRay, Inc. Of the shares being offered, 37,929,912 are presently issued and outstanding and 198,760 are issuable upon exercise of common stock purchase warrants. These shares include an aggregate of (i) 5,884,504 shares of common stock issued and sold to accredited investors in a private placement offering in a series of closings on July 23, 2015, August 13, 2015 and August 17, 2015, or the Private Placement, (ii) 770,000 shares of our common stock that were held by one of our stockholders immediately prior to the closing of our merger transaction on July 23, 2015, or the Merger, (iii) 31,275,408 shares of our common stock issued in the Merger to the former stockholders of ViewRay Technologies, Inc. in connection with the closing of the Merger and (iv) 198,760 shares of common stock issuable upon exercise of common stock purchase warrants, or the Placement Agent Warrants, issued as compensation to Northland Securities, Inc., Katalyst Securities LLC, Trout Capital LLC and MLV & Co. LLC, as co-exclusive placement agents and their designees in connection with the Private Placement, or the Placement Agents. All shares of common stock issued in the Private Placement were sold at a purchase price of $5.00 per share.
The selling stockholders may sell the shares of common stock on any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale, in the over-the-counter market, in one or more transactions otherwise than on these exchanges or systems, such as privately negotiated transactions, or using a combination of these methods, and at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. See the disclosure under the heading “Plan of Distribution” elsewhere in this prospectus for more information about how the selling stockholders may sell or otherwise dispose of their shares of common stock hereunder.
The selling stockholders may sell any, all or none of the securities offered by this prospectus and we do not know when or in what amount the selling stockholders may sell their shares of common stock hereunder following the effective date of this registration statement.
We will not receive any proceeds from the sale of our common stock by the selling stockholders in the offering described in this prospectus.
Our common stock is eligible for quotation for trading on the OTCQB tier of OTC Markets Group, Inc. under the symbol “VRAY.” On December 29, 2015, the last quoted sale price for our common stock as reported on the OTCQB was $5.10 per share.
Investing in our common stock involves a high degree of risk. Before making any investment in our common stock, you should read and carefully consider the risks described in this prospectus under “Risk Factors” beginning on page 8 of this prospectus.
You should rely only on the information contained in this prospectus or any prospectus supplement or amendment hereto. We have not authorized anyone to provide you with different information.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
, 2015
Table of Contents
Index to Financial Statements
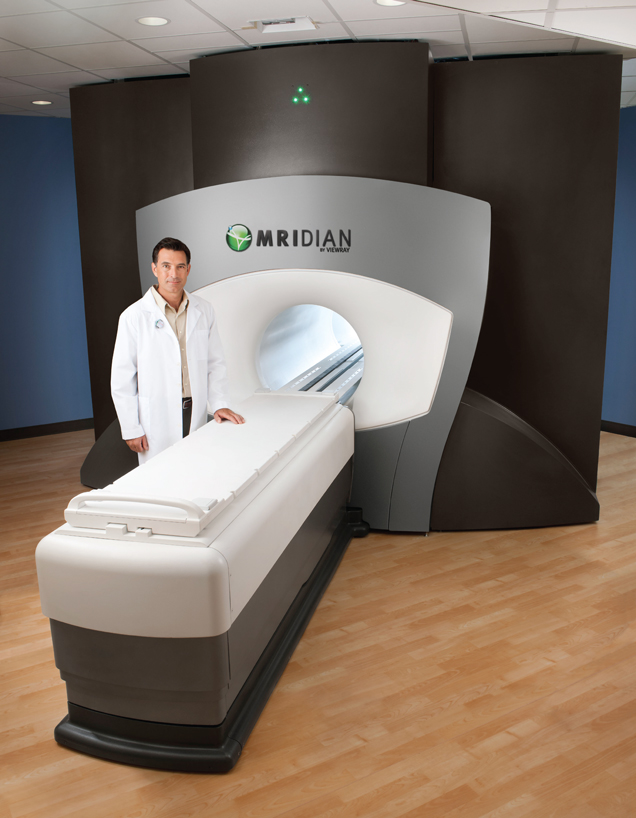
Table of Contents
Index to Financial Statements
You should rely only on the information contained in this prospectus or in any free writing prospectus prepared by us or on our behalf. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
Information contained on our website is not part of this prospectus.
ViewRay®, MRIdian® and our logo are some of our trademarks used in this prospectus. This prospectus also includes trademarks, tradenames, and service marks that are the property of other organizations. Solely for convenience, our trademarks and tradenames referred to in this prospectus may appear without the ® and ™ symbols, but those references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights, or the right of the applicable licensor to these trademarks and tradenames.
i
Table of Contents
Index to Financial Statements
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes before deciding to buy shares of our common stock. Unless the context requires otherwise, references in this prospectus to “the company,” “we,” “us” and “our” refer to ViewRay, Inc.
Overview
We design, manufacture and market MRIdian, the first and only MRI-guided radiation therapy system that images and treats cancer patients simultaneously. MRI is a broadly used imaging tool which has the ability to differentiate between types of soft tissue clearly, unlike X-ray or computed tomography, or CT, the most commonly used imaging technologies in radiation therapy today. MRIdian integrates MRI technology, radiation delivery and our proprietary software to locate, target and track the position and shape of soft-tissue tumors while radiation is delivered. These capabilities allow MRIdian to deliver radiation to the tumor accurately while delivering less radiation to healthy tissue than existing radiation therapy treatments. We believe this innovation leads to improved patient outcomes and reduced side effects from off-target radiation delivery. We received 510(k) marketing clearance from the U.S. Food and Drug Administration, or FDA, for MRIdian in May 2012 and received permission to affix the CE mark in November 2014. Patients are actively receiving treatment on MRIdian systems at three cancer centers.
Cancer is a leading cause of death globally and the second leading cause of death in the United States. Radiation therapy is a common method used to treat cancer that uses lethal doses of ionizing energy to damage genetic material in cells. Nearly two-thirds of all treated cancer patients in the United States will receive some form of radiation therapy during the course of their illness, according to estimates by the American Society for Radiation Oncology, or ASTRO. In 2013, IMV Medical Information Division, Inc., or IMV, reported that 93% of patients receiving radiation therapy in the United States were treated by a linear accelerator, or linac. The global linac market was $2.8 billion in 2011 and was expected to grow to $3.7 billion by 2016 according to a 2012 Markets and Markets report. IAEA Human Health Campus reported that there are over 11,000 linacs installed at over 7,500 centers worldwide. We believe the addressable market for MRIdian is the annual market for linacs due to MRIdian’s ability to treat a broad spectrum of disease sites. However, we believe that MRIdian may be used more frequently for complex cancer cases that may be difficult to treat on a standard linac due to the location of the tumor in relation to the surrounding soft tissues. We currently estimate the annual market for linacs to be 1,100 units per year globally, the majority of which are replacement units.
Despite the prevalence of MRI for diagnostic purposes and its ability to image soft tissue clearly, the radiation therapy industry has been unable to integrate MRI into external-beam radiation therapy systems. Existing radiation therapy systems use X-ray-based imaging technologies, such as CT, which cannot differentiate between types of soft tissue or provide an accurate visualization of a tumor and its position in relation to critical organs. In addition, existing systems that offer imaging during the course of a treatment are limited by the rate at which they can image due to the level of radiation to which they expose the patient. These constraints make it difficult for a clinician to locate a tumor accurately, track its motion in real-time or adapt treatment as anatomy changes. It is very difficult to irradiate a tumor while minimizing the amount of radiation hitting critical organs without the ability to see the tumor’s exact location and shape. If a tumor is insufficiently irradiated, it may not respond to treatment, resulting in a lower probability of survival for the patient. If organs and other healthy soft tissues are irradiated, side effects can be severe, including organ failure and secondary cancers.
1
Table of Contents
Index to Financial Statements
MRIdian is a next-generation, radiation therapy solution that enables treatment and real-time imaging of a patient’s anatomy simultaneously. The high-quality images that it generates clearly differentiate the targeted tumor, surrounding soft tissue and nearby critical organs. MRIdian also records the level of radiation dose that the treatment area has received, enabling physicians to adapt the prescription between treatments as needed. We believe this improved visualization and accurate dose recording will enable better treatment, improve patient outcomes and reduce side effects. Key benefits to users and patients include improved imaging and patient alignment, on-table adaptive treatment planning, motion management and an accurate recording of the delivered radiation dose. Physicians have already used MRIdian to treat a broad spectrum of radiation therapy patients with more than 20 different types of cancer, as well as patients for whom radiation therapy was previously not an option.
We currently market MRIdian through a direct sales force in the United States and distributors in the rest of the world. At September 30, 2015, we had four MRIdian systems installed and had 14 signed orders for new systems for a backlog value of $78.0 million. We generated revenue of $5.8 million and $5.9 million during the nine months ended September 30, 2015 and 2014 and had net losses of $30.9 million and $24.2 million during the nine months ended September 30, 2015 and 2014. We generated revenue of $3.2 million in 2013 and $6.4 million in 2014 and had net losses of $27.2 million in 2013 and $33.8 million in 2014.
Current Radiation Therapy Process and Limitations
We believe the key limitations of existing radiation therapy technologies are the following:
| • | Inability to accurately locate a tumor for treatment alignment. To locate a tumor, current radiation therapy systems rely on on-table CT scans that are unable to differentiate between types of soft tissue. Therefore, surrogate registration markers, including existing bone structures, external marks and surgically implanted fiducials, are frequently used to align a patient to the treatment beams prior to commencing treatment. By relying on a proxy for tumor location rather than the tumor itself, clinicians risk missing the tumor and risk hitting healthy tissue when they deliver treatment beams into a patient’s body because the spatial relationship between the tumor, healthy tissues and these markers frequently changes. |
| • | Inability to adapt treatment on table. A physician designs a treatment plan based on images that are captured at the beginning of therapy. Creating a treatment plan can take one to two weeks, and treatment itself can take up to seven weeks. However, during the course of therapy, tumors often change size, orientation or shape and patient anatomy can change for reasons including weight loss or gain. Adjusting for these changes would require replanning, which may take several days and is resource intensive. As a result of these limitations, replanning is infrequently performed. |
| • | Inability to track tumor and organ motion accurately. In addition to difficulty locating a tumor accurately in a patient’s body, a further challenge is accounting for ongoing tumor movement during treatment. Tumors have been shown to move multiple centimeters relative to surrogate registration markers over the course of only a few seconds. Although physicians use internal markers and external cameras and blocks to track respiratory and other motion, they are unable to track the tumor itself and its location relative to other soft tissues. This limitation increases the probability of missing the targeted treatment area. As a result, physicians usually enlarge the total region to be radiated, causing an additional risk of side effects. |
| • | Inability to record cumulative radiation delivered. Currently, there are no methods to record the actual dose of radiation delivered to a tumor or surrounding healthy tissue during the course of treatment. Therefore, physicians must assume that the radiation is delivered according to plan, rather than making decisions based on actual dose delivered. |
2
Table of Contents
Index to Financial Statements
Each of these limitations increases the risk of missing a tumor and hitting healthy tissue during treatment. If a tumor is insufficiently irradiated, it may not respond to treatment, resulting in a lower probability of survival for the patient. If organs and other healthy soft tissues are irradiated, side effects can be severe, including organ failure and secondary cancers.
Our Solution
We have developed MRIdian to address the key limitations of existing radiation therapy technologies. We believe that MRIdian provides the following clinical and commercial benefits to physicians, hospitals and patients:
| • | Improved tumor visibility and patient alignment. The soft-tissue contrast of MRIdian’s on-board MRI enables clinicians to locate, target and track the tumor and healthy tissues and accurately align a patient to the treatment beams without the use of surrogate registration markers, X-rays or CT. |
| • | On-table adaptive planning. Using an MR image captured at the beginning of each therapy session, MRIdian automatically maps the patient’s soft tissue anatomy in 3D and calculates the dose that would be delivered using the current treatment plan. If the initial prescribed treatment is not clinically acceptable to the physician, MRIdian has the ability to automatically recalculate and adapt the plan to changing anatomy while the patient is on the table at the time of treatment, a capability unique to MRIdian. We believe hospitals will be able to bill incrementally for this replanning. |
| • | Ability to track tumors and manage patient motion. MRIdian can capture multiple soft-tissue imaging planes concurrently during treatment and refresh the image multiple times per second. This real-time imaging enables the physician to track the movement of the tumor and the surrounding healthy tissue as treatment is delivered. If a tumor or critical organ moves beyond a physician-defined boundary, the treatment beam automatically pauses. This beam control becomes especially important in the situations where a tumor may be in close proximity to a critical organ. |
| • | Record and evaluate the delivered dose. After each treatment MRIdian, calculates the dose delivered using a proprietary algorithm and advanced MR imaging, enabling the physician to review and re-optimize the patient’s treatment sessions if needed. |
| • | Fits into existing treatment paradigms and workflow. MRIdian can be used with standard planning methodologies and is used to treat a broad spectrum of disease sites. In addition, we believe MRIdian’s increased target accuracy will allow physicians to treat with higher doses over fewer treatment fractions and potentially improve patient throughput and efficiency. MRIdian fits inside most standard radiation therapy vaults without significant modifications and is supported by existing codes that are available for linac reimbursement. |
We believe the ability to image with MRI and treat cancer patients simultaneously will lead to improved patient outcomes and reduced side effects from off-target radiation delivery.
Our Strategy
Our objective is to make MRI-guided radiation delivery the standard of care for radiation therapy. To achieve this goal, we intend to do the following:
| • | target top-tier hospitals in initial global sales efforts to influence and increase market adoption; |
| • | commercialize MRIdian with a targeted sales force in the United States and through distributors in international markets; |
3
Table of Contents
Index to Financial Statements
| • | increase broader awareness of MRIdian’s capabilities to expand our share of the radiation therapy market; |
| • | maintain our competitive lead in MRI-guided radiation therapy through continued innovation; |
| • | continue to work with leading hospitals to optimize efficiency and patient throughput; and |
| • | drive cost reductions in the design and manufacture of MRIdian and improve our margins. |
Risks Associated with Our Business
Our business is subject to numerous risks and uncertainties, including those highlighted in the section titled “Risk Factors” immediately following this prospectus summary. These risks include, but are not limited to, the following:
| • | We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. |
| • | If clinicians do not widely adopt MRI-guided radiation therapy or MRIdian fails to achieve and sustain sufficient market acceptance, we will not generate sufficient revenue and our growth prospects, financial condition and results of operations could be harmed. |
| • | We may not be able to generate sufficient revenue from the commercialization of MRIdian to achieve and maintain profitability. |
| • | We are an early, commercial-stage company and have a limited history commercializing MRIdian, which may make it difficult to evaluate our current business and predict our future performance. |
| • | If third-party payors do not provide coverage and adequate reimbursement to our customers, it could negatively impact sales of MRIdian. |
| • | The long sales cycle and low unit volume sales of MRIdian, as well as other factors, may contribute to substantial fluctuations in our operating results and stock price and make it difficult to compare our results of operations across periods. |
| • | Our ability to achieve and maintain profitability depends substantially on increasing our gross margins by reducing product costs and improving our economies of scale, which we may not be able to achieve or sustain. |
| • | We face competition from numerous companies, many of whom have greater resources than we do or offer alternative technologies at lower prices than our MRIdian systems, which may make it more difficult for us to achieve significant market penetration and profitability. |
| • | We rely on a limited number of third-party suppliers or, in some cases, sole suppliers, for the majority of our components, subassemblies and materials and may not be able to find replacements or immediately transition to alternative suppliers. |
| • | We depend on third-party distributors to market and distribute MRIdian in international markets. If our distributors fail to successfully market and distribute MRIdian, our business will be harmed. |
| • | We may need to raise additional capital to fund our existing commercial operations, develop and commercialize new features for MRIdian and new products and expand our operations. |
| • | If we are unable to adequately protect our proprietary technology or maintain issued patents that are sufficient to protect MRIdian, others could compete against us more directly, which could harm our business, financial condition and results of operations. |
4
Table of Contents
Index to Financial Statements
| • | There is not now, nor has there been since our inception, any significant trading activity in our common stock, and an active trading market for our shares may never develop or be sustained, which may make it difficult for you to sell your shares of our common stock. |
| • | Our share price may be volatile and may be influenced by numerous factors, some of which are beyond our control. |
| • | We may be exposed to additional risks as a result of “going public” by means of a reverse acquisition transaction. |
Corporate Information
We were incorporated in Nevada as Mirax Corp. on September 6, 2013, and reincorporated in Delaware as ViewRay, Inc. on July 21, 2015.
On July 23, 2015, our wholly-owned subsidiary, Vesuvius Acquisition Corp., a corporation formed in the State of Delaware on July 16, 2015, or the Acquisition Sub, merged with and into ViewRay Technologies, Inc., a corporation incorporated in 2004 in the State of Florida originally under the name of ViewRay Incorporated, subsequently reincorporated in the State of Delaware in 2007 as ViewRay Incorporated, and changed its name to ViewRay Technologies Inc. in July 2015, referred to herein as ViewRay. On July 23, 2015, ViewRay, Inc., Acquisition Sub and ViewRay entered into an Agreement and Plan of Merger and Reorganization, or the Merger Agreement, which closed on the same date. Pursuant to the terms of the Merger Agreement, Acquisition Sub merged with and into ViewRay, which was the surviving corporation and thus became our wholly-owned subsidiary. All of the outstanding capital stock of ViewRay was converted into shares of our common stock.
In connection with the Merger and pursuant to the Split-Off Agreement, we transferred our pre-Merger assets and liabilities to our pre-Merger majority stockholder, in exchange for the surrender by her and cancellation of 4,150,171 shares of our common stock, or the Split-Off. Upon the closing of the Merger, we discontinued our pre-Merger business and acquired the business of ViewRay and will continue the existing business operations of ViewRay as a publicly-traded company under the name ViewRay, Inc.
Upon the closing of the Merger, we ceased to be a “shell company” under applicable rules of the Securities and Exchange Commission, or the SEC. On July 23, 2015, in connection with the Merger, our Board of Directors changed our fiscal year from a fiscal year ending on November 30 to one ending on December 31 of each year, which was the fiscal year of ViewRay.
On July 23, 2015, we entered into a securities purchase agreement, or the Securities Purchase Agreement, with certain accredited investors, providing for the issuance and sale to such investors of an aggregate of 5,884,504 shares of common stock issued and sold to accredited investors in a private placement offering in a series of closings on July 23, 2015, August 13, 2015 and August 17, 2015, at a purchase price per share of $5.00 and for aggregate gross proceeds to us of $29.4 million, or the Private Placement. After deducting for placement agent and other fees and expenses, the aggregate net proceeds from the Private Placement were $26.9 million. Northland Securities, Inc., Katalyst Securities LLC, Trout Capital LLC and MLV & Co. LLC, served as co-exclusive placement agents, or, along with their sub-agents, the Placement Agents, for the Private Placement.
The Securities Purchase Agreement also contains certain anti-dilution provisions. Those anti-dilution provisions provide that, subject to certain exceptions, if we issue and sell Common Stock or Common Stock equivalents at a purchase price per share of lower than $5.00 within the six month period following July 23, 2015, each investor in the Private Placement shall be entitled to receive such number of additional shares of our common stock as they would have received had such lower purchase price per share been applicable in the Private Placement.
5
Table of Contents
Index to Financial Statements
At the closings of the Private Placement we issued to the Placement Agents and their designees, warrants, or the Placement Agent Warrants, to acquire up to 198,760 shares of our common stock at an exercise price of $5.00 per share. Each of the Placement Agent Warrants is exercisable at any time at the option of the holder until the five-year anniversary of its date of issuance.Our authorized capital stock currently consists of 300,000,000 shares of common stock, and 10,000,000 shares of the preferred stock. Our common stock is quoted on the OTC Markets (OTCQB) under the symbol “VRAY,” which changed from “MRXC” on July 20, 2015.
Our principal executive offices are located at 2 Thermo Fisher Way, Oakwood Village, Ohio 44146. Our telephone number is (440) 703-3210. Our website address is www.viewray.com. (The information contained on, or that can be accessed through, our website is not a part of this prospectus.)
We are an emerging growth company as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. We will remain an emerging growth company until the earlier of December 31, 2019, the end of the fiscal year following the fifth anniversary of the date of the first sale of our common stock pursuant to an effective registration statement filed under the Securities Act, the last day of the year in which we have total annual gross revenue of at least $1.0 billion, the date on which we are deemed to be a large accelerated filer (this means the market value of our common stock that is held by non-affiliates exceeds $700.0 million at the end of the second quarter of that year), or the date on which we have issued more than $1.0 billion in nonconvertible debt securities during the prior three-year period. An emerging growth company may take advantage of specified reduced reporting requirements and is relieved of certain other significant requirements that are otherwise generally applicable to public companies. As an emerging growth company, we will:
| • | avail ourselves of the exemption from the requirement to obtain an attestation and report from our auditors on the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; |
| • | provide less extensive disclosure about our executive compensation arrangements; and |
| • | not be required to hold stockholder non-binding advisory votes on executive compensation or golden parachute arrangements. |
However, we are choosing to “opt out” of the extended transition periods available under the JOBS Act for complying with new or revised accounting standards.
6
Table of Contents
Index to Financial Statements
THE OFFERING
This prospectus relates to the resale from time to time by the selling stockholders identified herein of up to 38,128,672 shares of our common stock. We are not offering any shares for sale under the registration statement of which this prospectus is a part.
| Common stock outstanding prior to this offering |
38,200,088 shares(1) |
| Common stock offered by the selling stockholders hereunder |
38,128,672 shares(2) |
| Common stock outstanding after this offering |
38,200,088 shares(3) |
| Use of proceeds |
We will not receive any proceeds from the sale of our common stock offered by the selling stockholders under this prospectus. We may, however, receive proceeds from warrants exercised by selling stockholders in the event that such warrants are exercised for cash. See “Use of Proceeds” beginning on page 70 of this prospectus. |
| Lock-Up Agreements |
Selling stockholders who hold an aggregate of 32,552,131 shares of the common stock included in this offering are subject to lock-up agreements, which restrict the sale of such shares for a period of six (6) months following the consummation of the Merger. See “Market Price of and Dividends on Our Common Stock and Related Stockholder Matters—Lock-Up Agreements.” |
| Risk factors |
See “Risk Factors” beginning on page 8 and other information included in this prospectus for a discussion of factors that you should consider carefully before deciding to invest in our common stock. |
| OTC symbol |
VRAY |
| (1) | As of October 1, 2015. |
| (2) | Includes (a) 37,929,912 shares of common stock outstanding and (b) 198,760 shares of common stock issuable upon exercise of the Placement Agent Warrants. |
| (3) | Excludes (a) 9,238,026 shares of our common stock reserved for issuance under our 2008 Stock Option and Incentive Plan, or 2008 Plan, and our 2015 Equity Incentive Award Plan, or 2015 Plan, and (b) 123,231 shares of our common stock issuable upon the exercise of an outstanding warrant with an exercise price of $5.85 per share, which is not being registered pursuant to the registration statement of which this prospectus is a part. As of November 19, 2015, there were outstanding options to purchase 4,313,183 shares of our common stock under our 2008 Plan, with a weighted average exercise price of $0.91 per share, and 1,672,416 shares of our common stock under our 2015 Plan, with a weighted average exercise price of $5.15 per share. |
7
Table of Contents
Index to Financial Statements
Investing in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus. While we believe that the risks and uncertainties described below are the material risks currently facing us, additional risks that we do not yet know of or that we currently think are immaterial may also arise and materially affect our business. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that case, the trading price of our common stock could decline.
Risks Related to Our Business and Strategy
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. These factors raise substantial doubt about our ability to continue as a going concern.
We have historically incurred substantial net losses, including net losses of $10.3 million and $7.6 million during the three months ended September 30, 2015 and 2014, $30.9 million and $24.2 million during the nine months ended September 30, 2015 and 2014, respectively. We expect our net losses to continue as a result of ongoing expansion of our commercial operations, including increased manufacturing, sales and marketing costs. These net losses have had, and will continue to have, a negative impact on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability could harm our business, financial condition, results of operations and cash flows.
Further, the net losses we incur may fluctuate significantly from quarter-to-quarter and year-to-year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance quarter-to-quarter and year-to-year, due to factors including the timing of clinical trials, any litigation that we may file or that may be filed against us, the execution of collaboration, licensing or other agreements and the timing of any payments we make or receive thereunder. These factors raise substantial doubt about our ability to continue as a going concern.
Our independent registered public accounting firm has issued an opinion on our 2014 financial statements that included an explanatory paragraph referring to our ability to continue as a going concern. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty. We believe the closing of the Private Placement enables us to continue as a going concern.
If clinicians do not widely adopt MRI-guided radiation therapy or MRIdian fails to achieve and sustain sufficient market acceptance, we will not generate sufficient revenue and our growth prospects, financial condition and results of operations could be harmed.
Our MRI-guided radiation therapy system, MRIdian, may never gain significant acceptance in the marketplace and, therefore, may never generate substantial revenue or allow us to achieve or maintain profitability. Widespread adoption of MRI-guided radiation therapy depends on many factors, including acceptance by clinicians that MRI-guided radiation therapy is clinically-effective and cost-effective in treating a wide range of cancers, demand by patients for such treatment, successful education of clinicians on the various aspects of this therapeutic approach and coverage and adequate reimbursement for procedures performed using MRI-guided radiation therapy. If we are not successful in conveying to hospitals that MRI-guided radiation therapy provides equivalent or superior radiation therapy compared to existing technologies, we may experience reluctance or refusal on the part of hospitals to order, and third-party payors to pay for performing, a treatment in which MRIdian is utilized. Our ability to achieve commercial market acceptance for MRIdian or any other future
8
Table of Contents
Index to Financial Statements
products also depends on the strength of our sales, marketing and distribution organizations. In addition, our expectations regarding cost savings from using MRIdian may not be accurate. These hurdles may make it difficult to demonstrate to physicians, hospitals and other healthcare providers that MRIdian is an appropriate option for radiation therapy, may be superior to available radiation therapy systems and may be more cost-effective than alternative technologies.
Furthermore, we may encounter difficulty in gaining inclusion in cancer treatment guidelines and gaining broad market acceptance by healthcare providers, third-party payors and patients. Healthcare providers may have difficulty in obtaining adequate reimbursement from government and/or third-party payors for cancer treatment, which may negatively impact adoption of MRIdian.
We may not be able to generate sufficient revenue from the commercialization of MRIdian to achieve and maintain profitability.
We rely solely on the commercialization of MRIdian to generate revenue, and we expect to generate substantially all of our revenue in the future from sales of MRIdian. We have installed only four systems that are currently treating patients. During the three months and nine months ended September 30, 2015, we recognized revenue of $5.1 million and $5.5 million, respectively, from installed MRIdian systems at Washington University and Siteman Cancer Center at Barnes Jewish Hospital, or Washington University in St. Louis, University of California, Los Angeles and Health System and Jonsson Comprehensive Cancer Center, or UCLA, The University of Wisconsin Carbone Cancer Center, or the University of Wisconsin—Madison, and Seoul National University Hospital, South Korea. In order to successfully commercialize MRIdian, we will need to continue to expand our marketing efforts to develop new relationships and expand existing relationships with customers, to receive clearance or approval for MRIdian in additional countries, to achieve and maintain compliance with all applicable regulatory requirements and to develop and commercialize new features for MRIdian. We cannot assure you that we will be able to achieve or maintain profitability. If we fail to successfully commercialize MRIdian, we may never receive a return on the substantial investments in product development, sales and marketing, regulatory compliance, manufacturing and quality assurance we have made, as well as further investments we intend to make, which may cause us to fail to generate revenue and gain economies of scale from such investments.
In addition, potential customers may decide not to purchase MRIdian, or our customers may decide to cancel orders due to changes in treatment offerings, research and product development plans, difficulties in obtaining coverage or reimbursement for MRI-guided radiation therapy treatment, complications with facility build-outs, utilization of MRI-guided radiation therapy or other cancer treatment methods developed by other parties, lack of financing or the inability to obtain or delay in obtaining a certificate of need from state regulatory agencies or zoning restrictions, all of which are circumstances outside of our control.
In addition, demand for MRIdian systems may not increase as quickly as we predict, and we may be unable to increase our revenue levels as we expect. Even if we succeed in increasing adoption of MRIdian systems by hospitals and other healthcare providers, maintaining and creating relationships with our existing and new customers and developing and commercializing new features for MRIdian, we may not be able to generate sufficient revenue to achieve or maintain profitability.
We are an early, commercial-stage company and have a limited history commercializing MRIdian, which may make it difficult to evaluate our current business and predict our future performance.
We are an early, commercial-stage company and have a limited operating history. We commenced operations as a Florida corporation in 2004 and subsequently reincorporated in Delaware in 2007. However, we did not begin commercial operations until 2013. Our limited history commercializing MRIdian may make it difficult to evaluate our current business and predict our future performance. Any assessment of our profitability or prediction about our future success or viability is subject to significant uncertainty. We have encountered and
9
Table of Contents
Index to Financial Statements
will continue to encounter risks and difficulties frequently experienced by early, commercial-stage companies in rapidly evolving industries. If we do not address these risks successfully, our business could be harmed.
If MRIdian does not perform as expected, or if we are unable to satisfy customers’ demands for additional product features, our reputation, business and results of operations will suffer.
Our success depends on the market’s confidence that MRIdian can provide reliable, high-quality MRI-guided radiation therapy. There are only four MRIdian systems being used in commercial practice, and therefore we have very few statistics regarding the efficacy or reliability of MRIdian. We believe that our customers are likely to be particularly sensitive to product defects and errors, including functional downtime that limits the number of patients that can be treated using the system or a failure that is costly to repair. For example, in January 2014, we initiated a correction of the system at Washington University in St. Louis due to a defect we identified in an advanced software feature in the treatment planning system of MRIdian. We promptly updated our software to resolve this defect and notified the U.S. Food and Drug Administration, or FDA, of this correction. We cannot assure that similar product defects or other errors will not occur in the future. This could also include the mistreatment of a patient with MRIdian caused by human error on the part of MRIdian’s operators or prescribing physicians or as a result of a machine malfunction. We may be subject to regulatory enforcement action or legal claims arising from any defects or errors that may occur. Any failure of MRIdian to perform as expected could harm our reputation, business and results of operations.
Furthermore, the Cobalt-60 radioactive materials used in MRIdian systems decay over time, which eventually leads to longer treatment times and may have a negative impact on the number of patients a hospital can treat during a day. U.S. regulations require inspection of Cobalt-60 every five years, at which time customers may consider replacing the Cobalt-60 source. This natural decay or a customer’s failure to replace the Cobalt-60 may have a negative impact on MRIdian performance.
In addition, our customers are technologically well informed and at times have specific demands or requests for additional functionality. If we are unable to meet those demands through the development of new features for MRIdian or future products, those new features or products do not function at the level that our customers expect, we are unable to increase throughput as expected or we are unable to obtain regulatory clearance or approval of those new features or products, where applicable, our reputation, business and results of operations could be harmed.
The safety and efficacy of MRIdian for certain uses is not currently supported by long-term clinical data, and MRIdian may therefore be less safe and effective than initially anticipated.
MRIdian has received premarket clearance by the FDA under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or FDCA. In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. This process is typically shorter and generally requires the submission of less supporting documentation than the FDA’s premarket approval process and does not always require long-term clinical studies. Additionally, to date, we have not been required to complete long-term clinical studies in connection with the sale of MRIdian outside the United States. As a result, we currently lack the breadth of published long-term clinical data supporting the efficacy of MRIdian and the benefits it offers that might have been generated in connection with other approval processes. In addition, because MRIdian has only been on the market since 2013, we have limited complication or patient survival rate data with respect to treatment using the system. If future patient studies or clinical testing do not support our belief that MRIdian offers a more advantageous treatment for a wide variety of cancer types, market acceptance of the system could fail to increase or could decrease and our business could be harmed.
If we choose to, or are required to, conduct additional studies, such studies or experience could reduce the rate of coverage and reimbursement by both public and private third-party payors for procedures that are performed with
10
Table of Contents
Index to Financial Statements
MRIdian, slow the market adoption of our product by physicians, significantly reduce our ability to achieve expected revenues and prevent us from becoming profitable. In addition, if future studies and experience indicate that MRIdian causes unexpected or serious complications or other unforeseen negative effects, we could be subject to mandatory product recalls or suspension or withdrawal of FDA clearance, and our reputation with physicians, patients and healthcare providers may suffer.
There have been instances of patients’ severe injury or death due to either operator misuse or system malfunction with other radiation therapy systems. If our redundant safety systems do not operate as we expect, or were misused by operators, MRIdian could severely injure or kill a patient. This could result in lawsuits, fines or damage to our reputation.
We may be delayed or prevented from implementing our long-term sales strategy if we fail to educate clinicians and patients about the benefits of MRIdian.
In order to increase revenue, we must increase awareness of the range of benefits that we believe MRIdian offers to both existing and potential customers, primarily cancer clinicians. An important part of our sales strategy involves educating and training clinicians to utilize the entire functionality of MRIdian. In addition, we must further educate clinicians about the ability of MRIdian to treat a wide range of cancer types effectively and efficiently. If clinicians are not properly educated about the use of MRIdian for radiation therapy, they may be unwilling or unable to take advantage of the full range of functionality that we believe MRIdian offers, which could have a negative impact on MRIdian sales. Clinicians may decide that certain tumors can be adequately treated using traditional radiation therapy systems, notwithstanding the benefits of MRIdian. Cobalt-60 systems have historically had certain limitations which have resulted in an increased use of linacs and a decreased use of Cobalt-60 systems. These historical limitations included imprecise radiation dose applications and an unsharp, wide-beam edge. If we do not adequately educate physicians about the functionality of our Cobalt-60 system to address some of the limitations that have affected Cobalt-60 systems, we may be delayed or prevented from implementing our long-term sales strategy. We must also succeed in educating clinicians about the potential for reimbursement for procedures performed using MRIdian. In addition, we need to increase awareness of MRIdian among potential patients, who are increasingly educated about cancer treatment options and therefore impact adoption of new technologies by clinicians. If our efforts to expand sales of MRIdian in the long-term are not successful, our business and results of operations will be harmed.
We may not be able to gain the support of leading hospitals and key opinion leaders, or to publish the results of our clinical trials in peer-reviewed journals, which may make it difficult to establish MRIdian as a standard of care and achieve market acceptance.
Our strategy includes developing relationships with leading hospitals and key opinion leaders in our industry. If these hospitals and key industry thought leaders determine that MRIdian is not clinically effective or that alternative technologies are more effective, or if we encounter difficulty promoting adoption or establishing MRIdian as a standard of care, our ability to achieve market acceptance of MRIdian could be significantly limited.
We believe that publication of scientific and medical results in peer-reviewed journals and presentation of data at leading conferences are critical to the broad adoption of MRIdian. Publication in leading medical journals is subject to a peer-review process, and peer reviewers may not consider the results of studies involving MRIdian sufficiently novel or worthy of publication.
We have a limited history of manufacturing, assembling and installing MRIdian in commercial quantities and may encounter related problems or delays that could result in lost revenue.
The pre-installation manufacturing processes for MRIdian include sourcing components from various third-party suppliers, subassembly, assembly, system integration and testing. We must manufacture and assemble MRIdian in compliance with regulatory requirements and at an acceptable cost in order to achieve and maintain
11
Table of Contents
Index to Financial Statements
profitability. We have only a limited history of manufacturing, assembling and installing MRIdian and, as a result, we may have difficulty manufacturing, assembling and installing MRIdian in sufficient quantities in a timely manner. To manage our manufacturing and operations with our suppliers, we forecast anticipated product orders and material requirements to predict our inventory needs up to a year in advance and enter into purchase orders on the basis of these requirements. Our limited manufacturing history may not provide us with sufficient data to accurately predict future component demand and to anticipate our costs effectively.
Further, we have experienced and may in the future experience delays in obtaining components from suppliers and installing our systems at customer sites associated with contractor timing delays, which could impede our ability to manufacture, assemble and install MRIdian on our expected timeline. Alternatively, delays or postponements of scheduled customer installations could lead to excess inventory due to our limited flexibility to postpone or delay component shipments from suppliers. Accordingly, we may encounter difficulties in production of MRIdian, including problems with quality control and assurance, component supply shortages or surpluses, increased costs, shortages of qualified personnel and difficulties associated with compliance with local, state, federal and foreign regulatory requirements. In addition, if we are unable to maintain larger-scale manufacturing capabilities, our ability to generate revenue will also be limited and our reputation could be harmed. If we cannot achieve the required level and quality of production, we may need to make changes in our supply chain or enter into licensing and other arrangements with third parties who possess sufficient manufacturing facilities and capabilities in compliance with regulatory requirements. Even if we outsource necessary production or enter into licensing or other third-party arrangements, the associated cost could reduce our gross margin and harm our financial condition and results of operations.
We have limited experience in marketing and selling MRIdian, and if we are unable to adequately address our customers’ needs, it could negatively impact sales and market acceptance of MRIdian and we may never generate sufficient revenue to achieve or sustain profitability.
We have limited experience in marketing and selling MRIdian. We have only been selling MRIdian since 2013 and our four MRIdian systems installed have only been used for treating patients since early 2014. In addition, MRIdian is a new technology in the radiation therapy systems sector and our future sales will largely depend on our ability to increase our marketing efforts and adequately address our customers’ needs. We believe it is necessary to maintain a sales force that includes sales representatives with specific technical backgrounds that can support our customers’ needs. We will also need to attract and develop sales and marketing personnel with industry expertise. Competition for such employees is intense and we may not be able to attract and retain sufficient personnel to maintain an effective sales and marketing force. If we are unable to adequately address our customers’ needs, it could negatively impact sales and market acceptance of MRIdian and we may never generate sufficient revenue to achieve or sustain profitability.
The long sales cycle and low unit volume sales of MRIdian, as well as other factors, may contribute to substantial fluctuations in our operating results and stock price and make it difficult to compare our results of operations to prior periods and predict future financial results.
Because of the relatively small number of systems we expect to install in any period, each installation of a MRIdian will represent a significant percentage of our revenue for a particular period. Additionally, customer site construction, certificate of need and additional zoning and licensing permits are often required in connection with the sale of a MRIdian, any of which may further delay the installation process. When we are responsible for installing a system, we only recognize revenue from the sale of a MRIdian after the system has been installed and accepted by the customer. When a qualified third party is responsible for the installation, we only recognize revenue when title is transferred. Therefore, if we do not install a MRIdian or transfer title when anticipated, our operating results will vary significantly from our expectations. We have had experiences with customers postponing installation of MRIdian systems due to delays in facility build-outs, which are often lengthy and costly processes for our existing and potential customers. In addition, if our customers delay or cancel purchases, we may be required to modify or terminate contractual arrangements with our suppliers, which may result in the
12
Table of Contents
Index to Financial Statements
loss of deposits. Due to future fluctuations in revenue and costs, as well as other potential fluctuations, you should not rely upon our operating results in any particular period as an indication of future performance. In addition to the other risks described herein, the following factors may also contribute to these fluctuations:
| • | timing of when we are able to recognize revenue associated with sales of MRIdian; |
| • | actions relating to regulatory matters, including regulatory requirements in some states for a certificate of need prior to the installation of a MRIdian; |
| • | delays in shipment due to, for example, unanticipated construction delays at customer locations where MRIdian is to be installed, labor disturbances or natural disasters; |
| • | delays in our manufacturing processes or unexpected manufacturing difficulties; |
| • | timing of the announcements of contract executions or other customer and commercial developments; |
| • | timing of the announcement, introduction and delivery of new products or product features by us and by our competitors; |
| • | timing and level of expenditures associated with expansion of sales and marketing activities and our overall operations; |
| • | fluctuations in our gross margins and the factors that contribute to such fluctuations, as described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” elsewhere in this prospectus; |
| • | our ability to effectively execute on our strategic and operating plans; |
| • | the extent to which MRIdian gains market acceptance and the timing of customer demand for MRIdian; |
| • | our ability to protect our proprietary rights and defend against third-party challenges; |
| • | disruptions in the supply or changes in the costs of raw materials, labor, product components or transportation services; and |
| • | changes in third-party coverage and reimbursement, government regulation or in a customer’s ability to obtain financing. |
These factors are difficult to forecast and may contribute to fluctuations in our reported revenue and results of operations and variation from our expectations, particularly during the periods in which our sales volume is low. Any such fluctuations in our financial results may cause volatility in our stock price.
Each MRIdian is a major capital equipment item and is subject to a lengthy sales cycle. The time from initial customer contact to execution of a contract can take 18 to 24 months or more. Following execution of a contract, it generally takes nine to 12 months for a customer to customize an existing facility or construct a new vault, which is inclusive of the time from when a customer places the order to when the system is delivered. During this time, facilities support and transitioning, as well as permitting, are typically required, which can take several months. The time required to customize an existing facility, including modifications of a standard vault to accommodate an MRI, is currently three months. If a customer does not have an existing vault available, it may take longer to construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures. If a small number of customers defer installation of a MRIdian for even a short period, recognition of a significant amount of revenue may be deferred to a subsequent period. Because our operating costs are relatively fixed, our inability to recognize revenue in a particular period may impact our profitability in that period. As a result, the inability to recognize revenue in a particular period may make it difficult to compare our operating results with prior periods. The price of a MRIdian requires a portion of our target customers to obtain outside financing before committing to purchase a MRIdian system. Such financing may be difficult for our customers to obtain in any given period, if at all. The requirement of site-specific
13
Table of Contents
Index to Financial Statements
modifications or construction may also delay adoption or overall demand. In addition, while we believe that our backlog of orders provides a better measure at any particular point in time of the long-term performance prospects of our business than our operating results for a particular period, investors may attribute significant weight to our operating results for a particular period, which may be volatile and as a result cause fluctuations in our stock price.
A large portion of our revenue in any given reporting period will be derived from a small number of contracts.
Given a significant portion of the purchase price for MRIdian will generally be recognized as revenue in a single reporting period, we expect a small number of contracts in any given reporting period to account for a substantial part of our revenue in any such period, and we expect this trend to continue. Any decrease in revenue from these contracts could harm our operating results. Accordingly, our revenue and results of operations may vary from period to period. We are also subject to credit risk associated with the concentration of our accounts receivable from our customers. If one or more of our customers at any given time were either to terminate their contracts with us, cease doing business with us or to fail to pay us on a timely basis, our business, financial condition and results of operations could be harmed.
The payment structure we use in our customer arrangements may lead to fluctuations in operating cash flows in a given period.
While our customers typically provide a deposit upon entering into a sales contract with us, the substantial majority of the payment owed for a MRIdian is not due until the time of shipment of a MRIdian or following final acceptance by the customer upon installation. If we miss targeted shipments or our customers do not actively work towards completing installation, our receipt of payments and our operating cash flows could be impacted. In addition, if customers do not adhere to our payments terms, our operating cash flows could be impacted in any given period. Due to these fluctuations in operating cash flows and other potential fluctuations, you should not rely upon our operating results in any particular period as an indication of future performance.
Amounts included in backlog may not result in actual revenue and are an uncertain indicator of our future earnings.
We define backlog as the accumulation of all orders for which revenue has not been recognized and we consider valid. The determination of backlog includes, among other factors, our subjective judgment about the likelihood of an order becoming revenue and the regulatory approval required in the customer’s jurisdiction, if any. Our judgments in this area have been, and in the future may be, incorrect and we cannot assure you that, for any order included in backlog, we will recognize revenue with respect to such order. In addition, orders can be delayed for a number of reasons, many of which are beyond our control, including supplier delays which may cause delays in our manufacturing process, customer delays in commencing or completing construction of its facility, delays in obtaining zoning or other approvals and delays in obtaining financing. We may not be aware of these delays affecting our suppliers and customers and as a result may not consider them when evaluating the contemporaneous effect on backlog. Moreover, orders generally do not have firm dates by when a customer must take delivery, which could allow a customer to delay the order without cancelling the contract. Further, our backlog could be reduced due to cancellation of orders by customers. Should a cancellation occur, our backlog and anticipated revenue would be reduced unless we were able to replace such order. Reported reductions in our backlog could negatively impact our future results of operations or the price of our common stock.
We evaluate our backlog at least quarterly to determine if the orders continue to meet our criteria for inclusion in backlog. Our criteria include an outstanding and effective written agreement for the delivery of a MRIdian signed by customers, receipt of a minimum customer deposit or a letter of credit, any changes in customer or distributor plans or financial conditions, the customer’s or distributor’s continued intent and ability to fulfill the order contract, changes to regulatory requirements, the status of regulatory approval required in the customer’s
14
Table of Contents
Index to Financial Statements
jurisdiction, if any, or reasons for cancellation of order contracts. We may adjust our reported backlog as a result of these factors and due to changes in our judgment about the timing of shipment of a system for particular projects or the status of our regulatory approval in a particular jurisdiction, where applicable. Projects we once categorized as included within our backlog may be removed if we determine, based on the aforementioned criteria, that a particular order or orders no longer constitute valid backlog. In addition, one or more of our contracts have in the past and may in the future contribute to a material portion of our backlog in any one year. Because revenue will not be recognized until we have fulfilled our obligations to a customer, there may be a significant amount of time from signing a contract with a customer or shipping a system and revenue recognition. We cannot assure you that our backlog will result in revenue on a timely basis or at all, or that any cancelled contracts will be replaced.
Our ability to achieve profitability depends substantially on increasing our gross margins by reducing costs of MRIdian and improving our economies of scale, which we may not be able to achieve.
We are not, and never have been, profitable. The MRIdian purchase contracts we have entered into to date have been at a range of selling prices. Generally, earlier contracts have been at lower prices and more recent contracts have been at higher prices. Our earlier contracts resulted in negative gross margins. In order to become profitable we will need to be able to enter into contracts at increased prices. Our intention is to enter into purchase contracts for MRIdian systems with selling prices that are increasingly closer to our list price of a MRIdian. Our ability to enter into contracts at higher selling prices depends on a number of factors including:
| • | our ability to achieve commercial market acceptance for our system; |
| • | the pricing of competitors’ cancer therapy systems; |
| • | availability of coverage and adequate reimbursement by commercial and government payors; and |
| • | our ability to manufacture and install our systems in a timely and cost-effective manner. |
We bear the risk of warranty claims on all products we supply, including equipment and component parts manufactured by third parties. We cannot assure you that we will be successful in claiming recovery under any warranty or indemnity provided to us by our suppliers or vendors in the event of a successful warranty claim against us by a customer or that any recovery from such vendor or supplier would be adequate. In addition, warranty claims brought by our customers related to third-party components may arise after our ability to bring corresponding warranty claims against such suppliers expires, which could result in additional costs to us. There is a risk that warranty claims made against us will exceed our warranty reserve and our business, financial condition and results of operations could be harmed.
Our customer contracts provide that our customers commit to purchase a MRIdian for a fixed price, and a MRIdian will generally not be delivered for 11 to 15 months. In some circumstances, delivery can be postponed several months due to customer delays related to construction, vault preparation or concurrent facility expansion, and the cost of product supplies may increase significantly in the intervening time period. In addition, inflation may generally reduce the real value of the purchase price payable upon the achievement of future progress payment milestones. Either of these occurrences could cause our gross margins to decline or cause us to lose money on the sale of a MRIdian.
Moreover, our gross margins may decline in a given period due in part to significant replacement rates for components, resulting in increased warranty expense, negative profit margins on service contracts and customer dissatisfaction. If we are unable to reduce our expenses and improve or maintain quality and reliability, our profitability may be negatively impacted. Additionally, we may face increased demands for compensation from customers who are not satisfied with the quality and reliability of MRIdian, which could increase our service costs or require us to issue credits against future service payments and negatively impact future product sales. For example, we may have to extend a warranty period due to our failure to meet up-time requirements. Even if we are able to implement cost reduction and quality improvement efforts successfully, our service operations may
15
Table of Contents
Index to Financial Statements
remain unprofitable given the relatively small size and geographic dispersion of our installed base, which prevents us from achieving significant economies of scale for the provision of services. If we are unable to continue to sell MRIdian at increasingly higher prices that result in higher gross margins, we may never become profitable.
We may not be able to develop new products or enhance the capabilities of MRIdian to keep pace with our industry’s rapidly changing technology and customer requirements.
Our industry is characterized by rapid technological changes, new product introductions and enhancements and evolving industry standards. Our business prospects depend on our ability to develop new products and applications for our technology in new markets that develop as a result of technological and scientific advances, while improving the performance and cost-effectiveness of MRIdian. New technologies, techniques or products could emerge that might offer better combinations of price and performance than MRIdian systems. The market for radiation therapy treatment products is characterized by rapid innovation and advancement in technology. It is important that we anticipate changes in technology and market demand, as well as physician, hospital and healthcare provider practices to successfully develop, obtain clearance or approval, if required, and successfully introduce new, enhanced and competitive technologies to meet our prospective customers’ needs on a timely and cost-effective basis. Nevertheless, we must carefully manage our introduction of new products. If potential customers believe that such products will offer enhanced features or be sold for a more attractive price, they may delay purchases until such products are available. We may also have excess or obsolete inventory as we transition to new products, and we have no experience in managing product transitions. If we do not successfully innovate and introduce new technology into our anticipated product lines or effectively manage the transitions of our technology to new product offerings, our business, financial condition and results of operations could be harmed.
We face competition from numerous companies, many of whom have greater resources than we do or offer alternative technologies at lower prices than our MRIdian systems, which may make it more difficult for us to achieve significant market penetration and profitability.
The market for radiation therapy equipment is characterized by intense competition and pricing pressure. In particular, we compete with a number of existing therapy equipment companies, including Elekta AB, Varian Medical Systems, Inc. and Accuray Incorporated. Many of these competitors are large, well-capitalized companies with significantly greater market share and resources than we have. As a result, these companies may be better positioned than we are to spend more aggressively on marketing, sales, intellectual property and other product initiatives and research and development activities. In addition, we may compete with certain MRI-linear accelerator research projects that are currently in development and may be commercialized, including projects by the University of Alberta’s Cross Cancer Institute and a partnership of the University of Sydney, Ingham Institute and the University of Queensland.
Existing technologies may offer certain advantages compared to the MRI technology used by our MRidian system. For example, computed tomography, or CT, is known to hold certain potential advantages over MRI technology for use in radiation therapy. Diagnostic CT is currently the most widely adopted imaging modality for treatment planning, and can be used to directly measure the electron density of patient tissues, which enables more accurate dose computation. In addition, CT imaging provides superior imaging of bones and boney anatomy than MRI, which is advantageous when imaging those structures for planning and alignment for treatment. Finally, CT is a less expensive technology than MRI and might be preferred by customers seeking a lower cost solution.
Our current competitors or other potential competitors may develop new products for the treatment of cancer at any time. In addition, competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards or customer requirements. If we are unable to develop products that compete effectively against the products of existing or future competitors, our future revenue could be
16
Table of Contents
Index to Financial Statements
negatively impacted. Some of our competitors may compete by changing their pricing model or by lowering the price of their therapy systems. If these competitors’ pricing techniques are effective, it could result in downward pressure on the price of all therapy systems. If we are unable to maintain or increase our selling prices in the face of competition, we may not improve our gross margins.
In addition to the competition that we face from technologies performing similar functions to MRIdian, competition also exists for the limited capital expenditure budgets of our customers. A potential purchaser may be forced to choose between two items of capital equipment. Our ability to compete may also be negatively impacted when purchase decisions are based largely upon price, because MRIdian is a premium-priced system relative to other capital expenditures and alternative radiation therapy technologies. In certain circumstances, a purchaser may decide that an alternative radiation therapy system priced below MRIdian may be sufficient for its patient population given the relative upfront cost savings. In addition to the cost of the MRIdian system, U.S. customers are required to inspect the Cobalt-60 every five years, and our customers may incur significant costs associated with the inspection, replacement and disposal of Cobalt-60.
Negative press regarding MRI-guided radiation therapy for the treatment of cancer could harm our business.
The comparative efficacy and overall benefits of MRI-guided radiation therapy are not yet well understood, particularly with respect to certain types of cancer. These types of reports could negatively impact the market’s acceptance of MRI-guided radiation therapy, and therefore our ability to generate revenue could be negatively impacted.
We may acquire other businesses, form joint ventures or make investments in other companies or technologies that could negatively affect our operating results, dilute our stockholders’ ownership, increase our debt or cause us to incur significant expense.
We may pursue acquisitions of businesses and assets. We also may pursue strategic alliances and joint ventures that leverage our proprietary technology and industry experience to expand our offerings or distribution. We have no experience with acquiring other companies and limited experience with forming strategic partnerships. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in the incurrence of debt, contingent liabilities or future write-offs of intangible assets or goodwill, any of which could have a negative impact on our cash flows, financial condition and results of operations. Integration of an acquired company also may disrupt ongoing operations and require management resources that we would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could harm our financial condition and results of operations. We may not realize the anticipated benefits of any acquisition, strategic alliance or joint venture.
Foreign acquisitions involve unique risks in addition to those mentioned above, including those related to integration of operations across different cultures and languages, currency risks and the particular economic, political and regulatory risks associated with specific countries.
To finance any acquisitions or joint ventures, we may choose to issue shares of common stock as consideration, which could dilute the ownership of our stockholders. Additional funds may not be available on terms that are favorable to us, or at all. If the price of our common stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration.
17
Table of Contents
Index to Financial Statements
Risks Related to Our Reliance on Third Parties
We rely on a limited number of third-party suppliers and, in some cases, sole suppliers, for the majority of our components, subassemblies and materials and may not be able to find replacements or immediately transition to alternative suppliers.
We rely on several sole suppliers, including Japan Superconductor Technology, Inc., Siemens AG, Best Theratronics Ltd., Manufacturing Sciences Corporation, Tesla Engineering Limited, PEKO Precision Products, Inc., Euromechanics GmbH and Quality Electrodynamics, LLC, for certain components of MRIdian. These sole suppliers, and any of our other suppliers, may be unwilling or unable to supply components of MRIdian to us reliably and at the levels we anticipate or are required by the market. For us to be successful, our suppliers must be able to provide us with products and components in substantial quantities, in compliance with regulatory requirements, in accordance with agreed upon specifications, at acceptable costs and on a timely basis. An interruption in our commercial operations could occur if we encounter delays or difficulties in securing these components, and if we cannot then obtain an acceptable substitute. Any such interruption could harm our reputation, business, financial condition and results of operations.
If we are required to transition to new third-party suppliers for certain components of MRIdian, we believe that there are only a few other manufacturers that are currently capable of supplying the necessary components. In addition, the use of components or materials furnished by these alternative suppliers could require us to alter our operations. Furthermore, if we are required to change the manufacturer of a critical component of MRIdian, we will be required to verify that the new manufacturer maintains facilities, procedures and operations that comply with our quality and applicable regulatory requirements, which could further impede our ability to manufacture MRIdian in a timely manner. We currently do not carry inventory for components for more than two or three systems or have open purchase orders for components for more than four or six systems at any given time. Transitioning to a new supplier could be time-consuming and expensive, may result in interruptions in our operations and product delivery, could affect the performance specifications of MRIdian or could require that we modify the design of MRIdian. If the change in manufacturer results in a significant change to MRIdian, a new 510(k) clearance from the FDA or similar international regulatory authorization may be necessary, which could cause substantial delays. The occurrence of any of these events could harm our ability to meet the demand for MRIdian in a timely manner or cost-effectively.
We cannot assure you that we will be able to secure alternative equipment and materials and utilize such equipment and materials without experiencing interruptions in our workflow. If we should encounter delays or difficulties in securing, reconfiguring or revalidating the equipment and components we require for MRIdian, our reputation, business, financial condition and results of operations could be negatively impacted.
We depend on third-party distributors to market and distribute MRIdian in international markets.
A significant portion of our backlog is composed of international sales, and we expect a significant amount of our revenue to come from international sales. We depend on a number of distributors for sales in these international markets, and we have not to date completed the installation of any MRIdian systems internationally. We cannot control the efforts and resources our third-party distributors will devote to marketing MRIdian. Our distributors may not be able to successfully market and sell MRIdian and may not devote sufficient time and resources to support the marketing and selling efforts that enable the product to develop, achieve or sustain market acceptance. In some jurisdictions, we rely on our distributors to manage the regulatory process, and we are dependent on their ability to do so effectively. In addition, if a dispute arises with a distributor or if a distributor is terminated by us or goes out of business, it may take time to locate an alternative distributor, to seek appropriate regulatory approvals and to train such distributor’s personnel to market MRIdian, and our ability to sell and service MRIdian in the region formerly serviced by such terminated distributor could be harmed. Any of our distributors could become insolvent or otherwise become unable to pay amounts owed to us when due. Any of these factors could reduce our revenue from affected international markets, increase our costs in those markets or damage our reputation. In addition, if we are unable to attract additional international distributors, our international revenue may not grow.
18
Table of Contents
Index to Financial Statements
Failures by our third-party distributors to timely deliver or properly install MRIdian could harm our reputation.
We rely on arrangements with third-party distributors for sales and installation of MRIdian in international markets. To date, our third-party distributors have not completed the installation of any MRIdian systems internationally. As a result of our reliance on third-party distributors, we may be subject to disruptions and increased costs due to factors beyond our control, including labor strikes, third-party error and other issues. If the services of any of these distributors become unsatisfactory, including the failure of such distributors to properly install MRIdian, we may experience delays in meeting our customers’ product demands and we may not be able to find a suitable replacement on a timely basis or on commercially reasonable terms. Any failure to deliver, install or service products in a timely manner may damage our reputation and could cause us to lose current or potential customers.
We rely on third parties to store our inventory and to perform spare parts shipping and other logistics functions on our behalf. A failure or disruption with our logistics providers could harm our business.
Customer service is a critical element of our sales strategy. Third-party logistics providers store most of our spare parts inventory in depots around the world and perform a significant portion of our spare parts logistics and shipping activities. If any of our logistics providers suffers an interruption in its business or experiences delays, disruptions or quality control problems in its operations or we have to change and qualify alternative logistics providers for our spare parts, shipments of spare parts to our customers may be delayed and our reputation, business, financial condition and results of operations could be negatively harmed.
If third-party payors do not provide coverage and adequate reimbursement to our customers, it could negatively impact sales of MRIdian.
In the United States, hospitals and other healthcare providers who purchase MRIdian generally rely on third-party payors to reimburse all or part of the costs and fees associated with the treatments performed with our system, including the cost of MRIdian. Accordingly, sales of MRIdian depend, in part, on whether coverage and adequate reimbursement for standard planning methodologies are available to our customers from third-party payors, such as government healthcare insurance programs, including the Medicare and Medicaid programs, private insurance plans, health maintenance organizations and preferred provider organizations. In general, third-party payors in the United States have become increasingly cost-conscious, which has limited coverage for, and reimbursement of, certain procedures such as MRI-guided radiation therapy. Third-party payors have also increased utilization controls related to the use of products such as ours by healthcare providers.
Furthermore, there is no uniform policy on coverage and reimbursement for MRI-guided radiation therapy among third-party payors. Payors continue to review their coverage policies carefully for existing and new therapies and can, without notice, deny coverage for treatments that include the use of MRIdian.
The Medicare program is increasingly used as a model for how private payors and other governmental payors develop their coverage and reimbursement policies for medical services and procedures. Medicare coverage of advanced and conventional radiation therapies using MRIdian currently varies depending upon the geographic location in which the services are provided. The Centers for Medicare & Medicaid Services, or CMS, has not adopted national coverage determination for such therapies that would determine coverage nationally. In the absence of such a national coverage determination, Medicare Administrative Contractors, or MACs, with jurisdiction over specific geographic regions have the discretion to determine whether and when the use of MRI-guided radiation therapy will be considered medically necessary and covered in their respective regions. A number of MACs have adopted or proposed local coverage determinations covering MRI-guided radiation therapy. However, these local coverage determinations do not ensure that coverage will be available for MRI-guided radiation therapy for all types of cancer as the coverage policies may limit coverage to only certain types of cancer.
19
Table of Contents
Index to Financial Statements
Even if MRI-guided radiation therapy is covered and reimbursed by third-party payors, adverse changes in payors’ coverage and reimbursement policies that affect MRIdian could harm our ability to market and sell MRIdian. We cannot be sure that third-party payors will reimburse our customers for procedures using MRIdian at a level that will enable us to achieve or maintain adequate sales and price levels for MRIdian. Without coverage and adequate reimbursement from third-party payors, the market for MRIdian may be limited.
Third-party payors regularly update reimbursement amounts and also, from time to time, revise the methodologies used to determine reimbursement amounts. This includes annual updates to payments to physicians, hospitals and ambulatory surgery centers for the radiation treatments performed with MRIdian. Because the cost of MRIdian generally is recovered by the healthcare provider as part of the payment for performing the treatment and not separately reimbursed, these updates could directly impact the demand for MRIdian. An example of payment updates is the Medicare program’s updates to hospital and physician payments, which are done on an annual basis using a prescribed statutory formula.
Under the Medicare Physician Fee Schedule, or MPFS, rule for 2015, in the past, when the application of the formula resulted in lower payment, Congress has passed interim legislation to prevent the reductions. In April 2015, however, the Medicare Access and CHIP Reauthorization Act of 2015, or MACRA, was signed into law, which repealed and replaced the statutory formula for Medicare payment adjustments to physicians. MACRA provides a permanent end to the annual interim legislative updates that had previously been necessary to delay or prevent significant reductions to payments under the Medicare Physician Fee Schedule. MACRA extended existing payment rates through June 30, 2015, with a 0.5% update for July 1, 2015 through December 31, 2015, and for each calendar year through 2019, after which there will be a 0% annual update each year through 2025. In addition, MACRA requires the establishment of the Merit-Based Incentive Payment System, beginning in 2019, under which physicians may receive performance-based payment incentives or payment reductions based on their performance with respect to clinical quality, resource use, clinical improvement activities and meaningful use of electronic health records. MACRA also requires the Centers for Medicare & Medicaid Services, or CMS, beginning in 2019, to provide incentive payments for physicians and other eligible professionals that participate in alternative payment models, such as accountable care organizations, that emphasize quality and value over the traditional volume-based fee-for-service model. It is unclear what impact, if any, MACRA will have on our business and operating results, but any resulting decrease in payment may result in reduced demand for our services.
With respect to hospital payments, in its final rule for the Hospital Outpatient Prospective Payment System, or HOPPS, effective January 1, 2015, CMS will implement changes in the coding system to simplify the billing of conventional radiation therapy performed in the hospital outpatient setting into three levels—simple, intermediate or complex—and to simplify the billing of intensity modulated radiation therapy, or IMRT, into two levels, simple or complex. Simple IMRT treatments (e.g., breast and prostate) may be paid at a lower rate than complex IMRT, which includes all other disease sites. We believe that MRIdian will tend to be used for a higher proportion of complex treatments than other radiation therapy systems, and accordingly, we believe that the new HOPPS rules will have a minimal impact on our customers.
On October 30, 2015, the Centers for Medicare and Medicaid Services (CMS) issued the final rule for 2016 Medicare payment rates for hospital outpatient services, physicians and services performed in the freestanding center setting. The final rule included certain proposals that impact reimbursement rates for radiation therapy services, which have resulted in small changes in reimbursement in the freestanding center setting. These coding changes will be implemented in 2016 and do not appear to be significant for services delivered with our products.
Any significant cuts in reimbursement rates or changes in reimbursement methodology or administration for MRI-guided radiation therapy, or concerns or proposals regarding further cuts or changes in methodology or administration, could further increase uncertainty, influence our customers’ decisions, reduce demand for MRIdian, cause customers to cancel orders and impact our revenue and harm our business.
20
Table of Contents
Index to Financial Statements
Foreign governments also have their own healthcare reimbursement systems, which vary significantly by country and region, and we cannot be sure that adequate reimbursement will be made available with respect to MRIdian under any foreign reimbursement system.
Our employees, consultants and commercial partners may engage in misconduct or other improper activities, including insider trading and non-compliance with regulatory standards and requirements.
We are exposed to the risk that our employees, consultants and commercial partners may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless or negligent conduct or disclosure of unauthorized activities to us that violates the regulations of the FDA and non-U.S. regulators, including those laws requiring the reporting of true, complete and accurate information to such regulators, manufacturing standards, healthcare fraud and abuse laws and regulations in the United States and abroad or laws that require the true, complete and accurate reporting of financial information or data. In particular, sales, marketing and business arrangements in the healthcare industry, including the sale of medical devices, are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations.
Risks Related to Our Financial Condition and Capital Requirements
We may need to raise additional capital to fund our existing commercial operations, develop and commercialize new features for MRIdian and new products and expand our operations.
Based on our current business plan, we believe net proceeds from the Private Placement, together with our current cash and cash equivalents, cash receipts from sales of MRIdian systems and additional available draw down from the CRG Term Loan, will enable us to conduct our planned operations for at least the next 12 months. The additional available draw down from the CRG Term Loan is available at our option on or before June 26, 2016, upon the occurrence of either (i) an initial public offering of our common stock on a nationally recognized securities exchange that raises a minimum of $40.0 million in net cash proceeds with a minimum of $120.0 million post-money valuation, or Qualifying IPO, or (ii) the achievement of a minimum of $25.0 million gross revenue from the sales of the MRIdian system during any consecutive 12 months before March 31, 2016. If our available cash balances, net proceeds from the Private Placement and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements including because of lower demand for MRIdian as a result of lower than currently expected rates of reimbursement from commercial third-party payors and government payors or due to other risks described herein, we may seek to sell common or preferred equity or convertible debt securities, enter into an additional credit facility or another form of third-party funding or seek other debt financing.
We may consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities or for other reasons, including to:
| • | increase our sales and marketing efforts to increase market adoption of MRIdian and address competitive developments; |
21
Table of Contents
Index to Financial Statements
| • | provide for supply and inventory costs associated with plans to accommodate potential increases in demand for MRIdian systems; |
| • | fund development and marketing efforts of any future products or additional features to then-current products; |
| • | acquire, license or invest in new technologies; |
| • | acquire or invest in complementary businesses or assets; and |
| • | finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| • | our ability to achieve revenue growth and improve gross margins; |
| • | our rate of progress in establishing coverage and reimbursement arrangements with domestic and international commercial third-party payors and government payors; |
| • | the cost of expanding our operations and offerings, including our sales and marketing efforts; |
| • | our rate of progress in, and cost of the sales and marketing activities associated with, establishing adoption of MRIdian; |
| • | the cost of research and development activities; |
| • | the effect of competing technological and market developments; |
| • | costs related to international expansion; and |
| • | the potential cost of and delays in product development as a result of any regulatory oversight applicable to MRIdian. |
The various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, dilution to our stockholders could result. Any equity securities issued also could provide for rights, preferences or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences and privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to certain components contained within MRIdian, or grant licenses on terms that are not favorable to us.
We will incur significant costs as a result of operating as a public company and our management expects to devote substantial time to public company compliance programs.
As a public company, we will incur significant legal, accounting and other expenses due to our compliance with regulations and disclosure obligations applicable to us, including compliance with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as rules implemented by the Securities and Exchange Commission, or SEC, and the OTC Markets. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact, in ways we cannot currently anticipate, the manner in which we operate our business. Our management and other personnel will devote a substantial amount of time to these compliance programs and monitoring of public company reporting obligations and as a result of the new corporate governance and executive compensation related rules, regulations and guidelines prompted by the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, and further regulations and disclosure obligations expected in the future, we will likely need to devote additional time and costs to comply with such compliance programs and rules. These rules and regulations will cause us to incur significant legal and financial compliance costs and will make some activities more time-consuming and costly.
22
Table of Contents
Index to Financial Statements
To comply with the requirements of being a public company, we may need to undertake various actions, including implementing new internal controls and procedures and hiring new accounting or internal audit staff. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that information required to be disclosed in reports under the Securities Exchange Act of 1934, or the Exchange Act, is accumulated and communicated to our principal executive and financial officers. Our current controls and any new controls that we develop may become inadequate and weaknesses in our internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls could negatively impact the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we may be required to include in our periodic reports we will file with the SEC under Section 404 of the Sarbanes-Oxley Act, harm our operating results, cause us to fail to meet our reporting obligations or result in a restatement of our prior period financial statements. In the event that we are not able to demonstrate compliance with the Sarbanes-Oxley Act, that our internal control over financial reporting is perceived as inadequate or that we are unable to produce timely or accurate financial statements, investors may lose confidence in our operating results and the price of our common stock could decline. In addition, if we are unable to continue to meet these requirements, our common stock may not be able to remain eligible for quotation on the OTC Markets or meet the eligibility requirements for the NASDAQ Stock Market, or NASDAQ.
We are not currently required to comply with the SEC rules that implement Section 404 of the Sarbanes-Oxley Act and are therefore not yet required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. As a public company, we will be required to comply with certain of these rules, which require management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second annual report. We are just beginning the costly and challenging process of compiling the system and processing documentation needed to comply with such requirements. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective.
Our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting until the later of our second annual report or the first annual report required to be filed with the SEC following the date we are no longer an “emerging growth company” as defined in the JOBS Act depending on whether we choose to rely on certain exemptions set forth in the JOBS Act. If we are unable to assert that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, which could harm our business.
Compliance with recently adopted rules of the SEC relating to “conflict minerals” may require us and our suppliers to incur substantial expense and may result in disclosure by us that certain minerals used in products we manufacture or contract to manufacture are not “DRC conflict free.”
Section 1502 of the Dodd-Frank Act required the SEC to promulgate rules requiring disclosure by a public company of any “conflict minerals” (tin, tungsten, tantalum and gold) necessary to the functionality or production of a product manufactured or contracted to be manufactured by the public company. The SEC adopted final rules in 2012 that took effect at the end of January 2013. Because we manufacture or contract to manufacture a product that contains tin, tungsten, tantalum or gold, we will be required under these rules to determine whether those minerals are necessary to the functionality or production of MRIdian and, if so, conduct a country of origin inquiry with respect to all such minerals. If any such minerals may have originated in the Democratic Republic of the Congo, or DRC, or any of its adjoining countries, or covered countries, then we must
23
Table of Contents
Index to Financial Statements
conduct diligence on the source and chain of custody of those conflict minerals to determine if they originated in one of the covered countries and, if so, whether they financed or benefited armed groups in the covered countries. Disclosures relating to the products that may contain conflict minerals, the country of origin of those minerals and whether they are “DRC conflict free” must be provided in a Form SD (and accompanying conflict minerals report, if required, to disclose the diligence undertaken by us in sourcing the minerals and our conclusions relating to such diligence). If we are required to submit a conflict minerals report, after 2015 that report must be audited by an independent auditor pursuant to existing government auditing standards. Compliance with this new disclosure rule may be very time-consuming for management and our supply chain personnel (as well as time-consuming for our suppliers) and could involve the expenditure of significant amounts of money by us and them. Disclosures, mandated by this new rule, which can be perceived by the market to be “negative,” may cause customers to refuse to purchase MRIdian. We cannot assure you that the cost of compliance with the rule will not harm our business, financial condition or results of operations.
Our loan and security agreement with Capital Royalty Partners II L.P., Capital Royalty Partners II - Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Parallel Investment Opportunities Partners II L.P., or together, CRG, contains operating and financial covenants that may restrict our business and financing activities.
At September 30, 2015, we had $30 million in outstanding debt to CRG. Borrowings under our loan and security agreement with CRG are secured by substantially all of our personal property, including our intellectual property. Our loan and security agreement restricts our ability to, among other things:
| • | dispose of or sell our assets; |
| • | make material changes in our business; |
| • | merge with or acquire other entities or assets; |
| • | incur additional indebtedness; |
| • | create liens on our assets; |
| • | pay dividends; |
| • | make investments; and |
| • | pay off subordinated indebtedness. |
Our loan and security agreement also requires that we comply with certain financial covenants. We are required to:
| • | hold cash and certain cash equivalents, in accounts subject to control agreements in favor of CRG, in an amount greater than or equal to the greater of (i) $2 million, increasing to $5 million upon the occurrence of a qualifying IPO, and (ii) the minimum cash balance required by any lender or lenders providing us with accounts receivable or inventory financing, as permitted by the loan and security agreement; and |
| • | have revenue from the sales, marketing and distribution of MRIdian systems and related services in an amount of at least $45 million for the twenty-four month period beginning January 1, 2015, with gradual step-ups to at least $120 million for the twenty-four month period beginning January 1, 2019. |
If we fail to meet the minimum required revenue for any twenty-four month period, within 90 days of the end of such period we are permitted to (i) issue additional capital stock of the company in exchange for cash or (ii) borrow additional subordinated debt, in each case, in amount equal to (x) two multiplied by (y) the minimum required revenue for the applicable twenty-four month period less the company’s actual revenue over the relevant testing period for such minimum required revenue in order to make up the shortfall.
24
Table of Contents
Index to Financial Statements
The operating and financial restrictions and covenants in our loan and security agreement, as well as any future financing agreements into which we may enter, may restrict our ability to finance our operations and engage in, expand or otherwise pursue our business activities and strategies. Our ability to comply with these covenants may be affected by events beyond our control, and future breaches of any of these covenants could result in a default under our loan and security agreement. If not waived, future defaults could cause all of the outstanding indebtedness under our loan and security agreement to become immediately due and payable and terminate all commitments to extend further credit.
If we do not have or are unable to generate sufficient cash available to repay our debt obligations when they become due and payable, either upon maturity or in the event of a default, we may not be able to obtain additional debt or equity financing on favorable terms, if at all, which may negatively impact our ability to operate and continue our business as a going concern.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
At December 31, 2014, we had federal net operating loss carryforwards, or NOLs, of $125.3 million, which begin to expire in the year ended December 31, 2024, and state NOLs of approximately $1.5 million, which begin to expire in the year ended December 31, 2019. We also had federal research and development tax credit carryforwards of $2.4 million for the year ended December 31, 2014. These credits expire at various dates through the year ending December 31, 2024. Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its NOLs to offset future taxable income. We believe we have experienced at least one ownership change in the past. We are currently analyzing the tax impacts of such ownership change on our federal NOLs and credit carryforwards. Future changes in our stock ownership, including this or future offerings, as well as other changes that may be outside of our control, could result in additional ownership changes under Section 382 of the Code. Our NOLs may also be limited under similar provisions of state law. We have recorded a full valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future tax benefits of such assets.
We face risks related to the current global economic environment, which could delay or prevent our customers from obtaining financing to purchase MRIdian and implement the required facilities, which could harm our business, financial condition and results of operations.
The state of the global economy continues to be uncertain. The current global economic conditions and uncertain credit markets and concerns regarding the availability of credit pose a risk that could impact customer demand for MRIdian, as well as our ability to manage normal commercial relationships with our customers, suppliers and creditors, including financial institutions. If the current global economic environment deteriorates, our business could be negatively affected.
Risks Related to Administrative, Organizational and Commercial Operations and Growth
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
We anticipate growth in our business operations. This future growth could create a strain on our organizational, administrative and operational infrastructure, including manufacturing operations, quality control, technical support and customer service, sales force management and general and financial administration. We may not be able to maintain the quality of or installation timelines of MRIdian or satisfy customer demand as it grows. Our ability to manage our growth properly will require us to continue to improve our operational, financial and management controls, as well as our reporting systems and procedures. We may implement new enterprise software systems in a number of areas affecting a broad range of business processes and functional areas. The time and resources required to implement these new systems is uncertain and failure to complete this in a timely and efficient manner could harm our business.
25
Table of Contents
Index to Financial Statements
If we are unable to support demand for MRIdian and our future products, including ensuring that we have adequate resources to meet increased demand, or we are unable to successfully manage the evolution of our MRI-guided radiation technology, our business could be harmed.
As our commercial operations and sales volume grow, we will need to continue to increase our workflow capacity for manufacturing, customer service, billing and general process improvements and expand our internal quality assurance program, among other things. We will also need to purchase additional equipment, some of which can take several months or more to procure, set up and validate, and increase our manufacturing, maintenance, software and computing capacity to meet increased demand. We cannot assure you that any of these increases in scale, expansion of personnel, purchase of equipment or process enhancements will be successfully implemented.
The loss of our President and Chief Executive Officer or Chief Scientific Officer or our inability to attract and retain highly skilled scientists and salespeople could negatively impact our business.
Our success depends on the skills, experience and performance of our President and Chief Executive Officer, Chris A. Raanes, and our Chief Scientific Officer and founder, James F. Dempsey, Ph.D. The individual and collective efforts of these employees will be important as we continue to develop MRIdian and as we expand our commercial activities. The loss or incapacity of existing members of our executive management team could negatively impact our operations if we experience difficulties in hiring qualified successors. Our executive officers have employment agreements; however, the existence of an employment agreement does not guarantee the retention of the executive officer for any period of time.
Our commercial, manufacturing and research and development programs and operations depend on our ability to attract and retain highly skilled engineers, scientists and technicians. We may not be able to attract or retain qualified managers, engineers, scientists and technicians in the future due to the competition for qualified personnel among medical device businesses, particularly in California and Ohio. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. Recruiting and retention difficulties can limit our ability to support our commercial, manufacturing and research and development programs. All of our employees are at-will, which means that either we or the employee may terminate his or her employment at any time.
If we were sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale and use of MRIdian could lead to the filing of product liability claims were someone to allege that MRIdian did not effectively treat the conditions its users were intending to target, caused other serious medical conditions or otherwise failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of or inappropriate reliance upon the information we provide in the ordinary course of our business activities, such as customer support or operating instructions. A product liability claim could result in substantial damages and be costly and time-consuming for us to defend.
We maintain product liability insurance in the amount of $9.0 million per occurrence and $9.0 million in the aggregate, but this insurance may not fully protect us from the financial impact of defending against product liability claims. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could lead to regulatory investigations, product recalls or withdrawals, damage our reputation or cause current vendors, suppliers and customers to terminate existing agreements and potential customers and partners to seek other suppliers of radiation therapy systems, any of which could negatively impact our results of operations.
26
Table of Contents
Index to Financial Statements
Sanctions against Russia, and Russia’s response to those sanctions, could harm our business, financial condition and results of operations.
Due to Russia’s recent military intervention in Ukraine and annexation of Crimea, the United States and the European Union, or EU, have imposed sanctions on certain individuals and one financial institution in Russia and have proposed the use of broader economic sanctions. In response, Russia has imposed entry bans on certain U.S. lawmakers and officials. We have engaged a third-party distributor and are currently in discussions with potential customers in Russia. If the United States or the EU were to impose sanctions on Russian businesses, or if Russia were to take retaliatory action against U.S. companies operating in Russia, our sales and marketing efforts in Russia could be harmed.
We face risks associated with our international business.
In addition to our marketing and sales of MRIdian in the United States, we also market MRIdian in North America, Europe and the Pacific Rim, with contracts signed with customers and distributors in Taiwan, Turkey, Korea, China, the United Arab Emirates, Hong Kong, Japan, Italy and Russia. Our international business operations are subject to a variety of risks, including:
| • | difficulties in staffing and managing foreign and geographically dispersed operations; |
| • | having to comply with various U.S. and international laws, including export control laws and the U.S. Foreign Corrupt Practices Act of 1977, or the FCPA, and anti-money laundering laws; |
| • | differing regulatory requirements for obtaining clearances or approvals to market MRIdian; |
| • | changes in uncertainties relating to foreign rules and regulations that may impact our ability to sell MRIdian, perform services or repatriate profits to the United States; |
| • | tariffs, export or import restrictions, restrictions on remittances abroad, imposition of duties or taxes that limit our ability to move MRIdian out of these countries or interfere with the import of essential materials into these countries; |
| • | limitations on our ability to enter into cost-effective arrangements with distributors of MRIdian, or at all; |
| • | fluctuations in foreign currency exchange rates; |
| • | imposition of limitations on production, sale or export of MRI-guided radiation therapy systems in foreign countries; |
| • | imposition of limitations on or increase of withholding and other taxes on remittances and other payments by foreign subsidiaries or joint ventures; |
| • | differing multiple payor reimbursement regimes, government payors or patient self-pay systems; |
| • | imposition of differing labor laws and standards; |
| • | economic, political or social instability in foreign countries and regions; |
| • | an inability, or reduced ability, to protect our intellectual property, including any effect of compulsory licensing imposed by government action; and |
| • | availability of government subsidies or other incentives that benefit competitors in their local markets that are not available to us. |
We expect that we will begin expanding into other target markets; however, we cannot assure you that our expansion plans will be realized, or if realized, be successful. We expect each market to have particular regulatory and funding hurdles to overcome and future developments in these markets, including the uncertainty relating to governmental policies and regulations, could harm our business. If we expend significant time and resources on expansion plans that fail or are delayed, our reputation, business and financial condition may be harmed.
27
Table of Contents
Index to Financial Statements
Our results may be impacted by changes in foreign currency exchange rates.
Currently, the majority of our international sales contracts are denominated in U.S. dollars. We pay certain of our suppliers in a foreign currency under the terms of their supply agreements, and we may pay other suppliers in the future in foreign currency. As a result, an increase in the value of the U.S. dollar relative to foreign currencies could require us to reduce our selling price or risk making MRIdian less competitive in international markets or our costs could increase. Also, if our international sales increase, we may enter into a greater number of transactions denominated in non-U.S. dollars, which could expose us to foreign currency risks, including changes in currency exchange rates. We do not currently engage in any hedging transactions. If we are unable to address these risks and challenges effectively, our international operations may not be successful and our business could be harmed.
We could be negatively impacted by violations of applicable anti-corruption laws or violations of our internal policies designed to ensure ethical business practices.
We operate in a number of countries throughout the world, including in countries that do not have as strong a commitment to anti-corruption and ethical behavior that is required by U.S. laws or by corporate policies. We are subject to the risk that we, our U.S. employees or our employees located in other jurisdictions or any third parties such as our sales agents and distributors that we engage to do work on our behalf in foreign countries may take action determined to be in violation of anti-corruption laws in any jurisdiction in which we conduct business, including the FCPA and the Bribery Act of 2010, or the U.K. Anti-Bribery Act. In addition, we operate in certain countries in which the government may take an ownership stake in an enterprise and such government ownership may not be readily apparent, thereby increasing potential anti-corruption law violations. Any violation of the FCPA and U.K. Anti-Bribery Act or any similar anti-corruption law or regulation could result in substantial fines, sanctions, civil and/or criminal penalties and curtailment of operations in certain jurisdictions and might harm our business, financial condition or results of operations. In addition, we have internal ethics policies with which we require our employees to comply in order to ensure that our business is conducted in a manner that our management deems appropriate. If these anti-corruption laws or internal policies were to be violated, our reputation and operations could also be substantially harmed. Further, detecting, investigating and resolving actual or alleged violations is expensive and can consume significant time and attention of our senior management.
We are subject to export restrictions and laws affecting trade and investments, and the future sale of our MRIdian system may be further limited or prohibited in the future by a government agency or authority.
As a global company headquartered in the United States, our MRIdian system is subject to U.S. laws and regulations that may limit, restrict or require a license to export (and re-export from other countries) our MRIdian system and related product and technical information due to its use of hazardous materials, including Cobalt-60, lead and depleted uranium. We are also subject to the export and import laws of those foreign jurisdictions to which we sell or from which we re-export our MRIdian system. Compliance with these laws and regulations could significantly limit our operations and our sales in the future and failure to comply, even indirectly, could result in a range of penalties, including restrictions on exports of our MRIdian system for a specified time period, or forever, and severe monetary penalties. In certain circumstances, these restrictions may affect our ability to interact with any of our future foreign subsidiaries and otherwise limit our trade with third parties, including suppliers and customers, operating inside and outside the United States. In addition, if we introduce new products, we may need to obtain licenses or approvals from the United States and other governments to ship them into foreign countries. Failure to receive the appropriate approvals may mean that our commercial efforts and expenses related to such efforts may not result in any revenue, which could harm our business.
We depend on our information technology systems, and any failure of these systems could harm our business.
We depend on information technology and telecommunications systems for significant elements of our operations. We have developed propriety software for the management and operation of MRIdian by our
28
Table of Contents
Index to Financial Statements
customers. We have installed, and expect to expand, a number of enterprise software systems that affect a broad range of business processes and functional areas, including for example, systems handling human resources, financial controls and reporting, contract management, regulatory compliance and other infrastructure operations. In addition to the aforementioned business systems, we intend to extend the capabilities of both our preventative and detective security controls by augmenting the monitoring and alerting functions, the network design and the automatic countermeasure operations of our technical systems. These information technology and telecommunications systems support a variety of functions, including sales and marketing, manufacturing operations, customer service support, billing and reimbursement, research and development activities and general administrative activities.
Information technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our information technology and telecommunications systems, failures or significant downtime of our information technology or telecommunications systems or those used by our third-party service providers could prevent us from providing maintenance and support services to our customers, conducting research and development activities and managing the administrative aspects of our business. Any disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could harm our business.
Our operations are vulnerable to interruption or loss due to natural or other disasters, power loss, strikes and other events beyond our control.
We conduct a significant portion of our activities, including administration and data processing, at facilities located in California, Ohio and other areas that have experienced major earthquakes, tornadoes and other natural disasters. A major earthquake, tornado or other disaster (such as a major fire, hurricane, flood, tsunami, volcanic eruption or terrorist attack) affecting our facilities, or those of our suppliers, could significantly disrupt our operations, and delay or prevent product shipment or installation during the time required to repair, rebuild or replace our suppliers’ damaged manufacturing facilities; these delays could be lengthy and costly. If any of our customers’ facilities are negatively impacted by a disaster, shipments of MRIdian could be delayed. Additionally, customers may delay purchases of MRIdian until operations return to normal. Even if we are able to quickly respond to a disaster, the ongoing effects of the disaster could create some uncertainty in the operations of our business. In addition, our facilities may be subject to a shortage of available electrical power and other energy supplies. Any shortages may increase our costs for power and energy supplies or could result in blackouts, which could disrupt the operations of our affected facilities and harm our business. Further, MRIdian is typically shipped from a limited number of ports, and any disaster, strike or other event blocking shipment from these ports could delay or prevent shipments and harm our business. In addition, concerns about terrorism, the effects of a terrorist attack, political turmoil or an outbreak of epidemic diseases, such as ebola or influenza, could have a negative effect on our operations, those of our suppliers and customers and the ability to travel, which could harm our business, financial condition and results of operations.
Risks Related to Intellectual Property
Litigation or other proceedings or third-party claims of intellectual property infringement could require us to spend significant time and money and could prevent us from selling MRIdian or impact our stock price.
There is considerable intellectual property litigation and contested patent disputes in the medical device area. Third parties may, in the future, assert claims that we are employing their proprietary technology without authorization, including claims from competitors or from non-practicing entities that have no relevant product revenue and against whom our own patent portfolio may have no deterrent effect. As we continue to commercialize MRIdian in its current or an updated form, launch new products and enter new markets, we expect that competitors may claim that MRIdian infringes their intellectual property rights as part of business strategies
29
Table of Contents
Index to Financial Statements
designed to impede our successful commercialization and entry into new markets. Although we are presently unaware of any basis by which a third party would be justified in making such claims, in the future, we may receive letters or other threats or claims from third parties inviting us to take licenses under, or alleging that we infringe, their patents. Third parties may have obtained, and may in the future obtain, patents under which such third parties may claim that the use of our technologies constitutes patent infringement.
Moreover, we may become party to future adversarial proceedings regarding our patent portfolio or the patents of third parties. Such proceedings could include contested post-grant proceedings such as oppositions, inter partes review, reexamination, interference or derivation proceedings before the U.S. Patent and Trademark Office or foreign patent offices. The legal threshold for initiating litigation or contested proceedings is low, so that even lawsuits or proceedings with a low probability of success might be initiated. Litigation and contested proceedings can also be expensive and time-consuming, and our adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we can.
We could incur substantial costs and divert the attention of our management and technical personnel in defending ourselves against any of these claims or in any of such proceedings. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a negative impact on our cash position and stock price. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement or misappropriation against us, we may be required to pay damages, obtain one or more licenses from third parties or be prohibited from selling certain products, all of which could have a negative impact on our cash position, business and financial condition.
In addition, we may be unable to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. Moreover, we could encounter delays in product introductions while we attempt to develop alternative methods or products. Defense of any lawsuit or adversarial proceeding or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of MRIdian or future products could impact our ability to grow and maintain profitability and harm our business.
If we are unable to adequately protect our proprietary technology or maintain issued patents that are sufficient to protect MRIdian, others could compete against us more directly, which could harm our business, financial condition and results of operations.
Our commercial success will depend in part on our success in obtaining and maintaining issued patents and other intellectual property rights in the United States and elsewhere and protecting our proprietary technology. If we do not adequately protect our intellectual property and proprietary technology, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
We hold the exclusive worldwide license for certain patents and applications covering our combination of MRI and radiation therapy technologies. Specifically, we hold a license to three issued U.S. patents, 14 issued foreign patents (eight of which were issued in Great Britain, Germany, France and the Netherlands as a result of two patent applications filed and allowed through the European Patent Office), one pending U.S. application and four pending foreign patent applications at September 30, 2015. We own an additional eight issued U.S. patents, eight issued foreign patents, 17 pending U.S. patent applications and 68 pending foreign patent applications, and, at September 30, 2015, two of our foreign patent applications are allowed. Assuming all required fees are paid, individual patents or patent applications owned or licensed by us will expire between 2025 and 2035. We also have a joint ownership interest with Case Western Reserve University in one issued patent and one U.S. patent application.
30
Table of Contents
Index to Financial Statements
We cannot provide any assurances that any of our patents have, or that any of our pending patent applications that mature into issued patents will include, claims with a scope sufficient to protect MRIdian, any additional features we develop for MRIdian or any new products. Other parties may have developed technologies that may be related or competitive to our platform, may have filed or may file patent applications and may have received or may receive patents that overlap or conflict with our patent applications, either by claiming the same methods or devices or by claiming subject matter that could dominate our patent position. The patent positions of medical device companies, including our patent position, involve complex legal and factual questions, and, therefore, the issuance, scope, validity and enforceability of any patent claims that we may obtain cannot be predicted with certainty. Patents, if issued, may be challenged, deemed unenforceable, invalidated or circumvented. U.S. patents and patent applications may also be subject to supplemental examination or contested post-grant proceedings such as inter partes review, reexamination, interference or derivation proceedings before the U.S. Patent and Trademark Office and challenges in district court. Patents may be subjected to opposition, post-grant review or comparable proceedings lodged in various foreign, both national and regional, patent offices. These proceedings could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such proceedings may be costly. Thus, any patents that we may own or exclusively license may not provide any protection against competitors. Furthermore, an adverse decision in an interference proceeding can result in a third party receiving the patent right sought by us, which in turn could affect our ability to commercialize MRIdian.
Furthermore, though an issued patent is presumed valid and enforceable, its issuance is not conclusive as to its validity or its enforceability and it may not provide us with adequate proprietary protection or competitive advantages against competitors with similar products. Competitors may also be able to design around our patents. Other parties may develop and obtain patent protection for more effective technologies, designs or methods. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or trade secrets by consultants, suppliers, vendors, former employees and current employees. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries. If any of these developments were to occur, they each could have a negative impact on our sales.
Our ability to enforce our patent rights depends on our ability to detect infringement. It is difficult to detect infringers who do not advertise the components that are used in their products. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product. Any litigation to enforce or defend our patent rights, even if we were to prevail, could be costly and time-consuming and would divert the attention of our management and key personnel from our business operations. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded if we were to prevail may not be commercially meaningful.
In addition, proceedings to enforce or defend our patents could put our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Such proceedings could also provoke third parties to assert claims against us, including that some or all of the claims in one or more of our patents are invalid or otherwise unenforceable. If any of our patents covering MRIdian are invalidated or found unenforceable, our financial position and results of operations could be negatively impacted. In addition, if a court found that valid, enforceable patents held by third parties covered MRIdian, our financial position and results of operations could be harmed.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| • | any of our patents, or any of our pending patent applications, if issued, will include claims having a scope sufficient to protect MRIdian or any other products; |
| • | any of our pending patent applications will issue as patents; |
| • | we will be able to successfully commercialize MRIdian on a substantial scale before our relevant patents expire; |
31
Table of Contents
Index to Financial Statements
| • | we were the first to make the inventions covered by each of our patents and pending patent applications; |
| • | we were the first to file patent applications for these inventions; |
| • | others will not develop similar or alternative technologies that do not infringe our patents; |
| • | any of our patents will be found to ultimately be valid and enforceable; |
| • | any patents issued to us will provide a basis for an exclusive market for our commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
| • | we will develop additional proprietary technologies or products that are separately patentable; or |
| • | our commercial activities or products will not infringe upon the patents of others. |
We rely upon unpatented trade secrets, unpatented know-how and continuing technological innovation to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our employees and our collaborators and consultants. We also have agreements with our employees and selected consultants that obligate them to assign their inventions to us and have non-compete agreements with some, but not all, of our consultants. It is possible that technology relevant to our business will be independently developed by a person that is not a party to such an agreement. Furthermore, if the employees and consultants who are parties to these agreements breach or violate the terms of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could otherwise become known or be independently discovered by our competitors.
If we are not able to meet the requirements of our license agreement with the University of Florida Research Foundation, Inc., we could lose access to the technologies licensed thereunder and be unable to manufacture, market or sell MRIdian.
We license patents and patent applications from the University of Florida Research Foundation, Inc., or UFRF, covering our combination of MRI and radiation therapy, and other key technologies, incorporated into MRIdian under a license agreement that requires us to pay royalties to UFRF. In addition, the license agreement obligates us to pursue an agreed development plan and to submit periodic reports and restricts our ability to take actions to defend the licensed patents. The license agreement terminates when the underlying patents expire in 2025, although UFRF has the right to unilaterally terminate the agreement if we do not meet our royalty payment obligations, including minimum royalty payments of $50,000 per quarter, or if we fail to satisfy other development and commercialization obligations related to our utilization of the technology. If UFRF were to terminate the agreement or if we were to otherwise lose the ability to exploit the licensed patents, our competitive advantage could be reduced, we may not be able to find a source to replace the licensed technology and we may be prevented from selling MRIdian. The license agreement reserves to UFRF the initial right to defend or prosecute any claim arising with respect to the licensed technology. If UFRF does not vigorously defend the patents, we may be required to engage in expensive patent litigation to enforce our rights and any competitive advantage we have based on the licensed technology may be hampered. Any of these events could harm our business, financial condition and results of operations.
Recent changes in U.S. patent laws may limit our ability to obtain, defend or enforce our patents.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. The Leahy-Smith America Invents Act, or the Leahy-Smith Act, includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and also affect patent litigation. The United States Patent Office recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to
32
Table of Contents
Index to Financial Statements
file provisions, only became effective on March 16, 2013. The first to file provisions limit the rights of an inventor to patent an invention if not the first to file an application for patenting that invention, even if such invention was the first invention. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business.
However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the enforcement and defense of our issued patents. For example, the Leahy-Smith Act provides that an administrative tribunal known as the Patent Trial and Appeals Board, or PTAB, provides a venue for companies to challenge the validity of competitor patents at a cost that is much lower than district court litigation and on timelines that are much faster. Although it is not clear what, if any, long-term impact the PTAB proceedings will have on the operation of our business, the initial results of patent challenge proceedings before the PTAB since its inception in 2013 have resulted in the invalidation of many U.S. patent claims. The availability of the PTAB as a lower-cost, faster and potentially more potent tribunal for challenging patents could therefore increase the likelihood that our own patents will be challenged, thereby increasing the uncertainties and costs of maintaining and enforcing them. Moreover, if such challenges occur with regard to our UFRF-licensed patents, as indicated above, we have only limited rights to control the defense.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed.
In addition to patent protection, we also rely upon copyright and trade secret protection, as well as non-disclosure agreements and invention assignment agreements with our employees, consultants and third parties, to protect our confidential and proprietary information. For example, significant elements of MRIdian are based on unpatented trade secrets and know-how that are not publicly disclosed. In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
33
Table of Contents
Index to Financial Statements
Third parties may assert ownership or commercial rights to inventions we develop.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. We have written agreements with collaborators that provide for the ownership of intellectual property arising from our collaborations. These agreements provide that we must negotiate certain commercial rights with collaborators with respect to joint inventions or inventions made by our collaborators that arise from the results of the collaboration. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party collaborator’s materials where required, or if disputes otherwise arise with respect to the intellectual property developed with the use of a collaborator’s technology, we may be limited in our ability to capitalize on the market potential of these intellectual property rights. In addition, we may face claims by third parties that our agreements with employees, contractors or consultants obligating them to assign intellectual property to us are ineffective or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such intellectual property. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property or may lose our exclusive rights in that intellectual property. Either outcome could harm our business.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities or other medical device companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Risks Related to Regulatory Matters
MRIdian and our operations are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business.
MRIdian is a medical device that is subject to extensive regulation in the United States and elsewhere, including by the FDA and its foreign counterparts. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices:
| • | design, development and manufacturing; |
| • | testing, labeling, content and language of instructions for use and storage; |
| • | clinical trials; |
| • | product safety; |
| • | marketing, sales and distribution; |
| • | premarket clearance and approval; |
| • | record keeping procedures; |
| • | advertising and promotion; |
| • | recalls and field safety corrective actions; |
34
Table of Contents
Index to Financial Statements
| • | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
| • | post-market approval studies; and |
| • | product import and export. |
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive either clearance under Section 510(k) of the FDCA or approval of a premarket approval, or PMA, application from the FDA, unless an exemption applies. In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, in order to clear the proposed device for marketing. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence. In the PMA process, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices.
Modifications to products that are approved through a PMA application generally require FDA approval. Similarly, certain modifications made to products cleared through a 510(k) may require a new 510(k) clearance. Both the PMA approval and the 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to 12 months, but can last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is filed with the FDA. In addition, PMA generally requires the performance of one or more clinical trials. Despite the time, effort and cost, we cannot assure you that any particular device will be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory approvals could harm our business.
In the United States, we have obtained 510(k) premarket clearance from the FDA to market MRIdian for the provision of stereotactic radiosurgery and precision radiotherapy for lesions, tumors and conditions anywhere in the body where radiation treatment is indicated. An element of our strategy is to continue to upgrade MRIdian to incorporate new software and hardware enhancements. We expect that such upgrades, as well as other future modifications to MRIdian, may require new 510(k) clearance; however, future upgrades may be subject to the substantially more costly, time-consuming and uncertain PMA process. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, product introductions or modifications could be delayed or canceled, which could cause our sales to decline.
| • | The FDA can delay, limit or deny clearance or approval of a device for many reasons, including: |
| • | we may not be able to demonstrate to the FDA’s satisfaction that MRIdian is substantially equivalent to the proposed predicate device or safe and effective for its intended use; |
| • | the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and |
| • | the manufacturing process or facilities we use may not meet applicable requirements. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared product on a timely basis. For example, in response
35
Table of Contents
Index to Financial Statements
to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) clearance process, the FDA initiated an evaluation, and in January 2011, announced several proposed actions intended to reform the clearance process. The FDA intends these reform actions to improve the efficiency and transparency of the clearance process, as well as bolster patient safety. In addition, as part of the Food and Drug Administration Safety and Innovation Act, or FDASIA, enacted in 2012, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms, which are further intended to clarify and improve medical device regulation both pre- and post-clearance and approval. Some of these proposals and reforms could impose additional regulatory requirements upon us that could delay our ability to obtain new 510(k) clearances, increase the costs of compliance or restrict our ability to maintain our current clearances.
Even after we have obtained the proper regulatory clearance or approval to market a product, we have ongoing responsibilities under FDA regulations. The failure to comply with applicable regulations could jeopardize our ability to sell MRIdian and result in enforcement actions such as:
| • | warning letters; |
| • | fines; |
| • | injunctions; |
| • | civil penalties; |
| • | termination of distribution; |
| • | recalls or seizures of products; |
| • | delays in the introduction of products into the market; |
| • | total or partial suspension of production; |
| • | refusal to grant future clearances or approvals; |
| • | withdrawals or suspensions of current clearances or approvals, resulting in prohibitions on sales of MRIdian; and |
| • | in the most serious cases, criminal penalties. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and harm our reputation, business, financial condition and results of operations.
In order to sell MRIdian in member countries of the European Economic Area, or EEA, MRIdian must comply with the essential requirements of the EU Medical Devices Directive (Council Directive 93/42/EEC). Compliance with these requirements is a prerequisite to be able to affix the Conformité Européene, or CE, mark to MRIdian, without which they cannot be sold or marketed in the EEA. To demonstrate compliance with the essential requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the EU Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a Member State of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
36
Table of Contents
Index to Financial Statements
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device (e.g., product labeling and instructions for use) are supported by suitable evidence. We received the CE mark for MRIdian in November 2014. If we fail to remain in compliance with applicable European laws and directives, we would not be able to continue to affix the CE mark to MRIdian, which would prevent us from selling MRIdian within the EEA. We will also need to obtain regulatory approval in other foreign jurisdictions in which we plan to market and sell MRIdian.
Modifications to MRIdian and our future products may require new 510(k) clearances or PMA approvals, or may require us to cease marketing or recall the modified products until clearances are obtained.
In the United States, we have obtained 510(k) premarket clearance from the FDA to market MRIdian for the provision of stereotactic radiosurgery and precision radiotherapy for lesions, tumors and conditions anywhere in the body where radiation treatment is indicated. Any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance or, possibly, approval of a PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. We have made modifications to MRIdian in the past and have determined based on our review of the applicable FDA regulations and guidance that in certain instances new 510(k) clearances or PMA approvals were not required. We may make similar modifications or add additional features in the future that we believe do not require a new 510(k) clearance or approval of a PMA. If the FDA disagrees with our determination and requires us to submit new 510(k) notifications or PMA applications for modifications to our previously cleared products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties.
Furthermore, the FDA’s ongoing review of the 510(k) clearance process may make it more difficult for us to make modifications to our previously cleared products, either by imposing more strict requirements on when a new 510(k) notification for a modification to a previously cleared product must be submitted, or applying more onerous review criteria to such submissions. For example, the FDA is currently reviewing its guidance describing when it believes a manufacturer is obligated to submit a new 510(k) for modifications or changes to a previously cleared device. The FDA is expected to issue revised guidance to assist device manufacturers in making this determination. It is unclear whether the FDA’s approach in this new guidance will result in substantive changes to existing policy and practice regarding the assessment of whether a new 510(k) is required for changes or modifications to existing devices. The FDA continues to review its 510(k) clearance process, which could result in additional changes to regulatory requirements or guidance documents, which could increase the costs of compliance or restrict our ability to maintain current clearances.
If treatment guidelines for cancer radiation therapies change or the standard of care evolves, we may need to redesign and seek new marketing authorization from the FDA for MRIdian.
If treatment guidelines for cancer radiation therapies or the standard of care evolves, we may need to redesign MRIdian and seek new clearances or approvals from the FDA for MRIdian. Our 510(k) clearance from the FDA is based on current treatment guidelines. If treatment guidelines change so that different treatments become desirable, the clinical utility of MRIdian could be diminished and our business could suffer. For example, competition by other forms of cancer treatment, in particular personalized medicine approaches in targeting drugs and biologics, could reduce the use of radiation therapy as a standard of care in certain indications.
37
Table of Contents
Index to Financial Statements
The misuse or off-label use of MRIdian may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
Clinicians or physicians may misuse MRIdian or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If MRIdian is misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizeable damage awards against us that may not be covered by insurance. In addition, any of the events described above could harm our business.
In addition, MRIdian has been cleared by the FDA for specific treatments. We train our marketing and direct sales force to not promote MRIdian for uses outside of the FDA-cleared indications for use, known as “off-label uses.” For example, MRIdian has not been indicated for diagnostic use. We cannot, however, prevent a physician from using MRIdian off-label, when in the physician’s independent professional medical judgment he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use MRIdian off-label. Furthermore, the use of MRIdian for indications other than those cleared by the FDA or approved by any foreign regulatory body may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
If the FDA or any foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance or imposition of an untitled letter, which is used for violators that do not necessitate a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action under other regulatory authority, such as false claims laws, if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment of our operations.
Our MRIdian systems may cause or contribute to adverse medical events that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our MRIdian systems, or a recall of our MRIdian systems either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.
We are subject to the FDA’s medical device reporting regulations and similar foreign regulations, which require us to report to the FDA when we receive or become aware of information that reasonably suggests that MRIdian may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, it could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of MRIdian. If we fail to comply with our reporting obligations, the FDA could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device clearance, seizure of MRIdian or delay in clearance of future products.
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could
38
Table of Contents
Index to Financial Statements
occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. For example, in January 2014, we initiated a correction of the system at Washington University in St. Louis due to a defect we identified in an advanced software feature in the treatment planning system of MRIdian. We promptly updated our software to resolve this defect and notified the FDA of this correction, but the FDA has not formally classified this correction as a recall. We cannot assure you that similar product defects or other errors will not occur in the future. Recalls involving MRIdian could be particularly harmful to our business, financial condition and results of operations because it is currently our only product.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for MRIdian in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales.
If we or our distributors do not obtain and maintain international regulatory registrations or approvals for MRIdian, we will not be able to market and sell MRIdian outside of the United States.
Sales of our devices outside the United States are subject to foreign regulatory requirements that vary widely from country to country. In addition, the FDA regulates exports of medical devices from the United States. While the regulations of some countries may not impose barriers to marketing and selling MRIdian or only require notification, others require that we or our distributors obtain the approval of a specified regulatory body. We have applied for and received regulatory approval in Europe, the United Arab Emirates, Taiwan, Korea and Italy, where regulatory approval is required in addition to the CE mark, and are seeking regulatory approval to market MRIdian in China, Japan, Canada, Russia, Hong Kong and Turkey. We currently have orders to deliver MRIdian to customers in the United States, Taiwan, Korea, Italy and the United Arab Emirates, which we include in our backlog due to the status of each sales order and our regulatory approval processes in these countries. Complying with foreign regulatory requirements, including obtaining registrations or approvals, can be expensive and time-consuming, and we cannot be certain that we or our distributors will receive regulatory approvals in each country in which we plan to market MRIdian or that we will be able to do so on a timely basis. The time required to obtain registrations or approvals, if required by other countries, may be longer than that required for FDA clearance, and requirements for such registrations or approvals may significantly differ from FDA requirements. If we modify MRIdian, we or our distributors may need to apply for additional regulatory approvals before we are permitted to sell the modified product. In addition, we may not continue to meet the quality and safety standards required to maintain the authorizations that we or our distributors have received. If we or our distributors are unable to maintain our authorizations in a particular country, we will no longer be able to sell MRIdian in that country, which could harm our business.
Regulatory approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others.
We must manufacture MRIdian in accordance with federal and state regulations, and we could be forced to recall our installed systems or terminate production if we fail to comply with these regulations.
The methods used in, and the facilities used for, the manufacture of MRIdian must comply with the FDA’s Quality System Regulation, or QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, servicing and shipping of MRIdian. Furthermore, we are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical
39
Table of Contents
Index to Financial Statements
device manufacturing facilities, which may include the facilities of subcontractors. MRIdian is also subject to similar state regulations and various laws and regulations of foreign countries governing manufacturing.
We cannot guarantee that we or any subcontractors will take the necessary steps to comply with applicable regulations, which could cause delays in the delivery of MRIdian. In addition, failure to comply with applicable FDA requirements or later discovery of previously unknown problems with MRIdian or manufacturing processes could result in, among other things:
| • | warning letters or untitled letters; |
| • | fines, injunctions or civil penalties; |
| • | suspension or withdrawal of approvals or clearances; |
| • | seizures or recalls of MRIdian; |
| • | total or partial suspension of production or distribution; |
| • | administrative or judicially imposed sanctions; |
| • | FDA’s refusal to grant pending or future clearances or approvals for MRIdian; |
| • | clinical holds; |
| • | refusal to permit the import or export of MRIdian; and |
| • | criminal prosecution of us or our employees. |
Any of these actions could significantly and negatively impact supply of MRIdian. If any of these events occurs, our reputation could be harmed, we could be exposed to product liability claims and we could lose customers and suffer reduced revenue and increased costs.
Legislative or regulatory reforms in the United States or the EU may make it more difficult and costly for us to obtain regulatory clearances or approvals for MRIdian or to produce, market or distribute MRIdian after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulation of medical devices or the reimbursement thereof. In addition, the FDA or Nuclear Regulatory Commission, or NRC, regulations and guidance are often revised or reinterpreted by the FDA or NRC in ways that may significantly affect our business and our MRIdian systems. For example, in response to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) clearance process, the FDA initiated an evaluation, and in January 2011, announced several proposed actions intended to reform the clearance process. In addition, as part of FDASIA, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms, which are further intended to clarify and improve medical device regulation both pre- and post-clearance or approval. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to manufacture, market or distribute MRIdian or future products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| • | additional testing prior to obtaining clearance or approval; |
| • | changes to manufacturing methods; |
| • | recall, replacement or discontinuance of MRIdian or future products; or |
| • | additional record keeping. |
40
Table of Contents
Index to Financial Statements
Any of these changes could require substantial time and cost and could harm our business and our financial results.
In September 2012, the European Commission published proposals for the revision of the EU regulatory framework for medical devices. The proposal would replace the EU Medical Devices Directive and the Active Implantable Medical Devices Directive with a new regulation (the Medical Devices Regulation). Unlike the Directives that must be implemented into national laws, the Regulation would be directly applicable in all EEA Member States and so is intended to eliminate current national differences in regulation of medical devices.
In October 2013, the European Parliament approved a package of reforms to the European Commission’s proposals. Under the revised proposals, only designated “special notified bodies” would be entitled to conduct conformity assessments of high-risk devices. These special notified bodies will need to notify the European Commission when they receive an application for a conformity assessment for a new high-risk device. The European Commission will then forward the notification and the accompanying documents on the device to the Medical Devices Coordination Group, or MDCG (a new, yet to be created, body chaired by the European Commission, and representatives of EEA Member States), for an opinion. These new procedures may result in a longer or more burdensome assessment of our new products.
If finally adopted, the Medical Devices Regulation is expected to enter into force sometime in 2016 and become applicable three years thereafter. In its current form it would, among other things, also impose additional reporting requirements on manufacturers of high-risk medical devices, impose an obligation on manufacturers to appoint a “qualified person” responsible for regulatory compliance and provide for more strict clinical evidence requirements.
Our business involves the use of hazardous materials and we and our third-party manufacturers must comply with environmental laws and regulations, which may be expensive and restrict how we do business.
Our third-party manufacturers’ activities and our own activities involve the controlled storage, use and disposal of hazardous materials, including Cobalt-60, lead and depleted uranium. We and our manufacturers are subject to federal, state, local and foreign laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these hazardous materials. We currently carry no insurance specifically covering environmental claims relating to the use of hazardous materials, but we do reserve funds to address these claims at both the federal and state levels. Although we believe that our safety procedures for handling and disposing of these materials and waste products comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental injury or contamination from the use, storage, handling or disposal of hazardous materials. In the event of an accident, state or federal or other applicable authorities may curtail our use of these materials and interrupt our business operations. In addition, if an accident or environmental discharge occurs, or if we discover contamination caused by prior operations, including by prior owners and operators of properties we acquire, we could be liable for cleanup obligations, damages and fines. If such unexpected costs are substantial, this could significantly harm our financial condition and results of operations.
If we are found to have violated laws protecting the confidentiality of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
There are a number of federal and state laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information.
In particular, the U.S. Department of Health and Human Services has promulgated rules governing the privacy and security of individually identifiable health information under the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, or HIPAA. These privacy and security rules protect medical records and other personal health
41
Table of Contents
Index to Financial Statements
information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information, limiting most uses and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose, and requiring administrative, technical and physical safeguards. Although we are not a covered entity under HIPAA, we have entered into agreements with certain covered entity customers, such as health care providers, under which we are considered to be a “business associate” under HIPAA. As a business associate, we are contractually bound and may also be directly responsible under HIPAA, as amended by HITECH, to implement policies, procedures and reasonable and appropriate security measures to protect any individually identifiable health information we may create, receive, maintain or transmit on behalf of covered entities. We may also be subject to state laws protecting the confidentiality of medical records where those state laws have stricter provisions than HIPAA. Our failure to protect or secure any individually identifiable health information received on behalf of customers could subject us to civil and criminal liability, including the imposition of monetary fines, and adverse publicity, and could harm our business and impair our ability to attract new customers.
We are subject to federal and state fraud and abuse laws and health information privacy and security laws, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
There are numerous U.S. federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback, false claims and physician transparency laws. Our relationships with providers and hospitals are subject to scrutiny under these laws. We may also be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual or furnishing or arranging for a good or service, for which payment may be made, in whole or in part, under federal healthcare programs, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent; |
| • | HIPAA, which created federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
| • | the federal physician sunshine requirements under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the Affordable Care Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, which is defined broadly to include other healthcare providers and teaching hospitals and ownership and investment interests held by physicians and their immediate family members; |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; |
| • | state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; and |
42
Table of Contents
Index to Financial Statements
| • | state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. |
These laws, among other things, constrain our business, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers of medical devices. We have a variety of arrangements with our customers that could implicate these laws. Due to the breadth of these laws, the narrowness of statutory exceptions and safe harbors available, and the range of interpretations to which they are subject to, it is possible that some of our current or future practices might be challenged under one or more of these laws. Even an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to respond to, and thus could harm our business, financial condition and results of operations.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment and the curtailment or restructuring of our operations, any of which could negatively impact our ability to operate our business and our results of operations.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, could harm our cash flows, financial condition and results of operations.
In March 2010, the Affordable Care Act was enacted in the United States, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other things, the Affordable Care Act:
| • | requires each medical device manufacturer to pay a sales tax equal to 2.3% of the price for which such manufacturer sells its medical devices; |
| • | establishes a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; |
| • | implements payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and |
| • | establishes an Independent Payment Advisory Board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. |
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2024 unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for MRIdian or additional pricing pressure.
43
Table of Contents
Index to Financial Statements
Risks Related to Ownership of Our Common Stock
The price of our common stock may be volatile and may be influenced by numerous factors, some of which are beyond our control.
Factors that could cause volatility in the market price of our common stock include, but are not limited to:
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | commercial success and market acceptance of MRIdian; |
| • | success of our competitors in discovering, developing or commercializing products; |
| • | ability to commercialize or obtain regulatory approval for MRIdian, or delays in commercializing or obtaining regulatory approval; |
| • | strategic transactions undertaken by us; |
| • | additions or departures of key personnel; |
| • | product liability claims; |
| • | prevailing economic conditions; |
| • | disputes concerning our intellectual property or other proprietary rights; |
| • | FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry; |
| • | healthcare reform measures in the United States; |
| • | sales of our common stock by our officers, directors or significant stockholders; |
| • | future sales or issuances of equity or debt securities by us; |
| • | business disruptions caused by earthquakes, tornadoes or other natural disasters; and |
| • | issuance of new or changed securities analysts’ reports or recommendations regarding us. |
In addition, the stock markets in general, and the markets for medical device companies in particular, have experienced extreme volatility that have been often unrelated to the operating performance of the issuer. These broad market fluctuations may negatively impact the price or liquidity of our common stock. In the past, when the price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business.
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
44
Table of Contents
Index to Financial Statements
In addition, Section 102 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. An “emerging growth company” can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
Future sales of our common stock or securities convertible or exchangeable for our common stock may cause our stock price to decline.
If our existing stockholders or optionholders sell, or indicate an intention to sell, substantial amounts of our common stock in the public market after the lock-up and legal restrictions on resale lapse, the price of our common stock could decline. The perception in the market that these sales may occur could also cause the price of our common stock to decline. Based on shares of ViewRay common stock outstanding at September 30, 2015, we have outstanding a total of 38,200,288 shares of common stock. Of those shares, only 260,000 are currently freely tradable, without restriction, in the public market. We have entered into registration agreements to register for resale under the Securities Act 5,884,504 shares of common stock, which we issued and sold in the Private Placement, 770,000 shares of our common stock that were held by one of our stockholders immediately prior to the closing of the Merger, 31,275,408 shares of our common stock, which we issued to former stockholders of ViewRay Technologies, Inc. in connection with the closing of the Merger, and 198,760 shares of common stock issuable to holders of the warrants issued to placement agents in connection with the Private Placement. Such shares represent approximately 99% of the outstanding shares of common stock as of September 30, 2015. Upon the effectiveness of any such registration statement, or other registration statement we could elect to file with respect to any other outstanding shares of common stock, any sales of those shares or any perception in the market that such sales may occur could cause the trading price of our common stock to decline. As of the date of effectiveness of such registration statement, such shares registered for resale will be freely tradable without restriction, except for 32,552,131 shares of our common stock, which will become freely tradable upon the expiration of certain lock-up restrictions applicable to those shares, which prohibit their sale, disposition or other transfer for a period of six months following July 23, 2015; however, in the case of certain of our former shareholders, the lock-up restrictions prohibit the sale, disposition or other transfer of approximately 80% of such shareholder’s shares.
In addition, based on the number of shares subject to outstanding awards under our 2008 Stock Option and Incentive Plan, or 2008 Plan, or available for issuance thereunder, at September 30, 2015, and including the initial reserves under our 2015 Equity Incentive Award Plan, or 2015 Plan, 9,238,026 shares of common stock that are either subject to outstanding options, outstanding but subject to vesting or reserved for future issuance under the 2008 Plan or 2015 Plan will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. As further described elsewhere in our Current Report on Form 8-K filed on July 29, 2015, as amended on August 13, 2015, we also plan to file a registration statement permitting shares of common stock issued in the future pursuant to the 2008 Plan and 2015 Plan to be freely resold by plan participants in the public market, subject to the lock-up agreements, applicable vesting schedules and, for shares held by directors, executive officers and other affiliates, volume limitations under Rule 144 under the Securities Act. The 2015 Plan contains provisions for the annual increase of the number of shares reserved for issuance under such plan, which shares we also intend to register. If the shares we may issue from time to time under the 2008 Plan or 2015 Plan are sold, or if it is perceived that they will be sold, by the award recipients in the public market, the price of our common stock could decline.
Holders of our common stock, including shares issuable upon exercise of our common stock warrants, are entitled to rights with respect to the registration of their shares under the Securities Act. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the
45
Table of Contents
Index to Financial Statements
Securities Act, except for shares purchased by affiliates, subject to the lock-up agreements described above in the section entitled “The Merger and Related Transactions—Lock-up Agreements and Other Restrictions.” Sales of such shares could also cause the price of our common stock to decline.
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders and the purchasers of our common stock offered hereby. We are authorized to issue an aggregate of 300,000,000 shares of common stock and 10,000,000 shares of “blank check” preferred stock. We may issue additional shares of our common stock or other securities that are convertible into or exercisable for our common stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our common stock may create downward pressure on the trading price of the common stock. We may need to raise additional capital in the near future to meet our working capital needs, and there can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with these capital raising efforts, including at a price (or exercise prices) below the price you paid for your stock.
Furthermore, in connection with the Private Placement, we entered into agreements containing certain anti-dilution provisions. Those anti-dilution provisions provide that, subject to certain exceptions, if we issue and sell common stock and common stock equivalents at a purchase price per share of lower than $5.00 within the six month period following July 2015, each investor in the Private Placement shall be entitled to receive such number of additional shares of our common stock as they would have received had such lower purchase price per share been applicable in the Private Placement, which could result in additional dilution and cause the market price of our securities to decline.
There may never be an active, liquid and orderly trading market for our common stock, which may make it difficult for you to sell your shares of our common stock.
Our common stock is quoted on OTC Markets, OTCQB tier of OTC Markets Group Inc., an over-the-counter quotation system, and there is not now, nor has there been since our inception, any significant trading activity in our common stock, and an active trading market for our shares may never develop or be sustained. In that venue, our stockholders may find it difficult to obtain accurate quotations as to the market value of their shares of our common stock, and may find few buyers to purchase their stock and few market makers to support its price. As a result of these and other factors, you may be unable to resell your shares of our common stock at or above the price for which you purchased them, at or near quoted bid prices, or at all. Further, an inactive market may also impair our ability to raise capital by selling additional equity in the future, and may impair our ability to enter into strategic partnerships or acquire companies or products by using shares of our common stock as consideration.
We intend to list shares of our common stock on a national securities exchange in the future, but we do not now, and may not in the future, meet the initial listing standards of any national securities exchange, which is often a more widely-traded and liquid market. Some, but not all, of the factors which may delay or prevent the listing of our common stock on a more widely-traded and liquid market include the following: our stockholders’ equity may be insufficient; the market value of our outstanding securities may be too low; our net income from operations may be too low; our common stock may not be sufficiently widely held; we may not be able to secure market makers for our common stock; and we may fail to meet the rules and requirements mandated by the several exchanges and markets to have our common stock listed. Should we fail to satisfy the initial listing standards of the national exchanges, or our common stock is otherwise rejected for listing, and remains listed on the OTC Markets or is suspended from the OTC Markets, the trading price of our common stock could suffer and
46
Table of Contents
Index to Financial Statements
the trading market for our common stock may be less liquid and our common stock price may be subject to increased volatility.
Our common stock is and may continue to be subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in the stock.
Rule 15g-9 under the Exchange Act establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require: (a) that a broker or dealer approve a person’s account for transactions in penny stocks; and (b) the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must: (a) obtain financial information and investment experience objectives of the person and (b) make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form: (a) sets forth the basis on which the broker or dealer made the suitability determination; and (b) confirms that the broker or dealer received a signed, written agreement from the investor prior to the transaction. Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our common stock and cause a decline in the market value of our common stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker or dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Our operating results for a particular period may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to fluctuations. Our operating results will be affected by numerous factors, including:
| • | variations in the level of expenses related to MRIdian or future development programs; |
| • | level of underlying demand for MRIdian and any other products we develop; |
| • | addition or termination of clinical trials or funding support; |
| • | receipt, modification or termination of government contracts or grants, and the timing of payments we receive under these arrangements; |
| • | our execution of any collaborative, licensing or similar arrangements, and the timing of payments we may make or receive under these arrangements; |
| • | any intellectual property infringement lawsuit or opposition, interference or cancellation proceeding in which we may become involved; and |
| • | regulatory developments affecting MRIdian or our competitors. |
47
Table of Contents
Index to Financial Statements
If our operating results for a particular period fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any fluctuations in our operating results may, in turn, cause the price of our common stock to fluctuate substantially. We believe that comparisons of our financial results from various reporting periods are not necessarily meaningful and should not be relied upon as an indication of our future performance.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Based on the beneficial ownership of our common stock at October 1, 2015, our officers and directors, together with holders of 5% or more of our outstanding common stock and their respective affiliates, will beneficially own approximately 83.0% of our common stock. Accordingly, these stockholders will continue to have significant influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transaction. The interests of these stockholders may not be the same as or may even conflict with your interests. For example, these stockholders could delay or prevent a change in control of the company, even if such a change in control would benefit our other stockholders, which could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of the company or our assets and might affect the prevailing price of our common stock. The significant concentration of stock ownership may negatively impact the price of our common stock due to investors’ perception that conflicts of interest may exist or arise.
Shares of our common stock that have not been registered under federal securities laws are subject to resale restrictions imposed by Rule 144, including those set forth in Rule 144(i) which apply to a former “shell company.”
Prior to the closing of the Merger, we were deemed a “shell company” under applicable SEC rules and regulations because we had no or nominal operations and either no or nominal assets, assets consisting solely of cash and cash equivalents, or assets consisting of any amount of cash and cash equivalents and nominal other assets. Pursuant to Rule 144 promulgated under the Securities Act of 1933, as amended, or the Securities Act, sales of the securities of a former shell company, such as us, under that rule are not permitted (i) until at least 12 months have elapsed from July 29, 2015, the date on which our Current Report on Form 8-K, reflecting our status as a non-shell company, was filed with the SEC and (ii) unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than Form 8-K reports. We intend to register such shares for sale under the Securities Act, but are currently a “voluntary filer” and are not subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act. As a result, unless we register such shares for sale under the Securities Act, most of our stockholders will be forced to hold their shares of our common stock for at least that 12-month period before they are eligible to sell those shares, and even after that 12-month period, sales may not be made under Rule 144 unless we and the selling stockholders are in compliance with other requirements of Rule 144. Further, it will be more difficult for us to raise funding to support our operations through the sale of debt or equity securities unless we agree to register such securities under the Securities Act, which could cause us to expend significant time and cash resources. Additionally, our previous status as a shell company could also limit our use of our securities to pay for any acquisitions we may seek to pursue in the future (although none are currently planned). The lack of liquidity of our securities as a result of the inability to sell under Rule 144 for a longer period of time than a non-former shell company could cause the market price of our securities to decline.
Provisions of our charter documents or Delaware law could delay or prevent an acquisition of the company, even if such an acquisition would be beneficial to our stockholders, which could make it more difficult for you to change management.
Provisions in our certificate of incorporation and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which
48
Table of Contents
Index to Financial Statements
stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors. These provisions include:
| • | a classified board of directors so that not all directors are elected at one time; |
| • | a prohibition on stockholder action through written consent; |
| • | no cumulative voting in the election of directors; |
| • | the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director; |
| • | a requirement that special meetings of stockholders be called only by the board of directors, the chairman of the board of directors, the chief executive officer or, in the absence of a chief executive officer, the president; |
| • | an advance notice requirement for stockholder proposals and nominations; |
| • | the authority of our board of directors to issue preferred stock with such terms as our board of directors may determine; and |
| • | a requirement of approval of not less than 66 2/3% of all outstanding shares of our capital stock entitled to vote to amend any bylaws by stockholder action, or to amend specific provisions of our certificate of incorporation. |
In addition, Delaware law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person who, together with its affiliates, owns, or within the last three years has owned, 15% or more of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Delaware law may discourage, delay or prevent a change in control of the company. Furthermore, our certificate of incorporation will specify that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for most legal actions involving actions brought against us by stockholders. We believe this provision benefits us by providing increased consistency in the application of Delaware law by chancellors particularly experienced in resolving corporate disputes, efficient administration of cases on a more expedited schedule relative to other forums and protection against the burdens of multi-forum litigation. However, the provision may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any applicable action brought against us, a court could find the choice of forum provisions contained in our certificate of incorporation to be inapplicable or unenforceable in such action.
Provisions in our charter documents and other provisions of Delaware law could limit the price that investors are willing to pay in the future for shares of our common stock.
We do not anticipate paying any cash dividends on our common stock in the foreseeable future; therefore, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
We have never declared or paid cash dividends on our common stock. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. In addition, our current loan and security agreement with CRG contains, and our future loan arrangements may contain, terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
49
Table of Contents
Index to Financial Statements
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend, in part, on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on the company. If no securities or industry analysts commence coverage of the company, the price for our common stock could be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our common stock or publish inaccurate or unfavorable research about our business, our stock price could decline. In addition, if our operating results fail to meet the forecast of analysts, our stock price could decline. If one or more of these analysts cease coverage of the company or fail to publish reports on us regularly, demand for our common stock could decrease, which might cause our stock price and trading volume to decline.
* * *
The risks above do not necessarily comprise all of those associated with an investment in the company. This prospectus contains forward looking statements that involve unknown risks, uncertainties and other factors that may cause the actual results, financial condition, performance or achievements of the company to be materially different from any future results, performance or achievements expressed or implied by such forward looking statements. Factors that might cause such a difference include, but are not limited to, those set out above.
50
Table of Contents
Index to Financial Statements
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements, including, without limitation, in the sections captioned “Description of Business,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Plan of Operations,” and elsewhere. Any and all statements contained in this prospectus that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this prospectus may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the development of commercially viable pharmaceuticals, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the SEC and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above.
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation:
| • | market acceptance of MRI-guided radiation therapy; |
| • | the benefits of MRI-guided radiation therapy; |
| • | our ability to successfully sell and market MRIdian in our existing and expanded geographies; |
| • | the performance of MRIdian in clinical settings; |
| • | competition from existing technologies or products or new technologies and products that may emerge; |
| • | the pricing and reimbursement of MRI-guided radiation therapy; |
| • | the implementation of our business model and strategic plans for our business and MRIdian; |
| • | the scope of protection we are able to establish and maintain for intellectual property rights covering MRIdian; |
| • | our ability to obtain regulatory approval in targeted markets for MRIdian; |
| • | estimates of our future revenue, expenses, capital requirements and our need for additional financing; |
| • | our financial performance; |
| • | our expectations related to the use of proceeds from the Private Placement; |
| • | developments relating to our competitors and the healthcare industry; and |
| • | other risks and uncertainties, including those listed under the section titled “Risk Factors.” |
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking statements contained in this prospectus to reflect any new information or future events or circumstances or otherwise, except as required by law.
51
Table of Contents
Index to Financial Statements
Readers should read this prospectus in conjunction with the discussion under the caption “Risk Factors,” our financial statements and the related notes thereto in this prospectus, and other documents which we may file from time to time with the SEC.
52
Table of Contents
Index to Financial Statements
This prospectus covers the resale from time to time by the selling stockholders identified in the table below of up to an aggregate of 38,128,672 shares of our common stock, which includes (i) 5,884,504 shares of our common stock issued and sold to investors in the Private Placement, (ii) 770,000 shares of our common stock that were held by one of our stockholders immediately prior to the closing of the Merger, (iii) 31,275,408 shares of our common stock, issued in the Merger to the former stockholders of ViewRay Technologies, Inc. in connection with the closing of the Merger and (iv) 198,760 shares of our common stock issuable upon exercise of common stock warrants by the holders of the Placement Agent Warrants issued as compensation in connection with the Private Placement.
Pursuant to the Registration Rights Agreement entered into with each of the investors in the Private Placement, we have filed with the SEC the registration statement of which this prospectus forms a part in order to register such resales of our common stock under the Securities Act. We have also agreed to cause this registration statement to become effective and to keep such registration statement effective within and for the time periods set forth in the Registration Rights Agreement. Our failure to satisfy the filing or effectiveness deadlines set forth in the Registration Rights Agreement may subject us to payment of certain monetary penalties pursuant to the terms of the Registration Rights Agreement.
The selling stockholders identified in the table below may from time to time offer and sell under this prospectus any or all of the shares of common stock described under the column “Shares of Common Stock Being Offered in this offering” in the table below. The table below has been prepared based upon information furnished to us by the selling stockholders as of the dates represented in the footnotes accompanying the table. The selling stockholders identified below may have sold, transferred or otherwise disposed of some or all of their shares since the date on which the information in the following table is presented in transactions exempt from or not subject to the registration requirements of the Securities Act. Information concerning the selling stockholders may change from time to time and, if necessary, we will amend or supplement this prospectus accordingly and as required.
We have been advised, as noted in the footnotes in the table below, that certain of the selling stockholders are broker-dealers and/or affiliates of a broker-dealer. Those selling stockholders have informed us that they bought our securities in the ordinary course of business, and that none of these selling stockholders had, at the time of their purchase of our securities, any agreements or understandings, directly or indirectly, with any person to distribute such securities.
The following table and footnote disclosure following the table sets forth the name of each selling stockholder, the nature of any position, office or other material relationship, if any, that the selling stockholder has had within the past three years with us or with any of our predecessors or affiliates, and the number of shares of our common stock beneficially owned by the selling stockholder before this offering. The number of shares reflected are those beneficially owned, as determined under applicable rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under applicable SEC rules, beneficial ownership includes any shares of common stock as to which a person has sole or shared voting power or investment power and any shares of common stock which the person has the right to acquire within 60 days after October 1, 2015 through the exercise of any option, warrant or right or through the conversion of any convertible security. Unless otherwise indicated in the footnotes to the table below and subject to community property laws where applicable, we believe, based on information furnished to us, that each of the selling stockholders named in this table has sole voting and investment power with respect to the shares indicated as beneficially owned.
We have assumed that all shares of common stock reflected in the table as being offered in the offering covered by this prospectus will be sold from time to time in this offering. We cannot provide an estimate as to the number of shares of common stock that will be held by the selling stockholders upon termination of the offering covered by this prospectus because the selling stockholders may offer some, all or none of their shares of common stock being offered in the offering.
53
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| Adriana Murillo & Carlos Enrique Vargas Moncaleano |
6,835 | * | 6,835 | — | * | |||||||||||||||
| Aaron Lehmann |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Aaron Segal |
§ | 4 | 3,108 | * | 3,108 | — | * | |||||||||||||
| Abbot Thayer |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Aiden Thomas Leith |
42,000 | * | 42,000 | — | * | |||||||||||||||
| Aisling Capital II, LP |
5 | 7,461,923 | 19.53% | 7,461,923 | — | * | ||||||||||||||
| Albert & Heidi Gentile JTWROS |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Allan Lipkowitz Revocable Living Trust 8/26/2005 |
6 | 5,000 | * | 5,000 | — | * | ||||||||||||||
| Allen Chase Foundation—Special Investment Account |
7 | 4,000 | * | 4,000 | — | * | ||||||||||||||
| American Portfolios Financial Services Inc. |
§§ | 8 | 5,498 | * | 5,498 | — | * | |||||||||||||
| Anders Lindholm |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Andrew Brenner |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Andrew Rosen |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Andrew Wahl |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Anthony Apicella |
9 | 12,653 | * | 1,127 | 11,526 | * | ||||||||||||||
| Anthony Naer |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Anthony Prisco |
2,200 | * | 2,200 | — | * | |||||||||||||||
| Anton Bogner and Barbara D. Bogner JTWROS |
40,000 | * | 40,000 | — | * | |||||||||||||||
| Arielle E. Crothers |
3,500 | * | 3,500 | — | * | |||||||||||||||
| Armin Langenegger |
4,370 | * | 4,370 | — | * | |||||||||||||||
| Aton Select Fund Limited |
10 | 100,001 | * | 100,001 | — | * | ||||||||||||||
| Bacon LLC |
11 | 5,334 | * | 5,334 | — | * | ||||||||||||||
| Barclay Armitage |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Barry Cohn |
§ | 12 | 2,670 | * | 2,670 | — | * | |||||||||||||
| Barry J. Shemaria |
6,000 | * | 6,000 | — | * | |||||||||||||||
| Beacon Bioventures Fund II Limited Partnership |
13 | 7,989,923 | 20.92% | 7,989,923 | — | * | ||||||||||||||
| Benjamin Bowen |
§ | 14 | 3,040 | * | 3,040 | — | * | |||||||||||||
| Bianka Yankov |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Biodex Partners, LLC |
15 | 2,665 | * | 2,665 | — | * | ||||||||||||||
| Boris Eligulashvili |
14,125 | * | 14,125 | — | * | |||||||||||||||
| Byron Hughey |
3,000 | * | 3,000 | — | * | |||||||||||||||
| C. James Prieur and Karen Prieur JTWROS |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Carl Domino |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Carol Cody |
20,000 | * | 20,000 | — | * | |||||||||||||||
54
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| Charles Medici and Diane Medici JTWROS |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Choi Family Trust Dated March 15, 2001 |
16 | 20,000 | * | 20,000 | — | * | ||||||||||||||
| Christopher Cozzolino |
§ | 17 | 264 | * | 264 | — | * | |||||||||||||
| Christopher Innace |
50,000 | * | 50,000 | — | * | |||||||||||||||
| Christopher P. Figoni |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Christopher Pisacano |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Claryton Struve |
40,000 | * | 40,000 | — | * | |||||||||||||||
| Claude M. Penchina Revocable Family Trust |
18 | 16,000 | * | 16,000 | — | * | ||||||||||||||
| Clem LLC |
19 | 5,000 | * | 5,000 | — | * | ||||||||||||||
| Craig R. Whited |
50,000 | * | 50,000 | — | * | |||||||||||||||
| Currie Family Trust |
20 | 20,000 | * | 20,000 | — | * | ||||||||||||||
| Dale A. & Pamela J. Dettmer, as Trustees |
8,936 | * | 8,936 | — | * | |||||||||||||||
| Dana Michael Inman |
1,365 | * | 1,365 | — | * | |||||||||||||||
| Daniel B. Salvas |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Daryl R. Schaller |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Daryl Squicciarini or Jennifer M. Squicciarini |
30,000 | * | 30,000 | — | * | |||||||||||||||
| Daugherty David |
511 | * | 511 | — | * | |||||||||||||||
| David A. Diehl |
2,000 | * | 2,000 | — | * | |||||||||||||||
| David Frydrych |
20,000 | * | 20,000 | — | * | |||||||||||||||
| David Landskowsky |
§ | 21 | 8,115 | * | 8,115 | — | * | |||||||||||||
| David M. Rickey Trust Dtd 5/8/2002 |
22 | 5,000 | * | 5,000 | — | * | ||||||||||||||
| David Stollwerk and Susan Stollwerk JTWROS |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Deborah Carnall |
39,159 | * | 39,159 | — | * | |||||||||||||||
| Dennis R. DeLoach, Jr. |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Dennis Reilly |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Donald Reilly |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Douglas Baker |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Douglas Kohrs |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Douglas M. Fambrough, III |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Dyke Rogers |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Edward J. King |
30,000 | * | 30,000 | — | * | |||||||||||||||
| Edward O’Connell |
5,000 | * | 5,000 | — | * | |||||||||||||||
| EFD Capital, Inc. |
§ | 23 | 6,657 | * | 6,657 | — | * | |||||||||||||
| Elena Laguta |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Eleven II Partners Ltd. |
24 | 2,000 | * | 2,000 | — | * | ||||||||||||||
| Elizabeth Crothers |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Eric Rubenstein |
§ | 25 | 8,115 | * | 8,115 | — | * | |||||||||||||
55
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| Ernest C. and Tina L. Euler, as Trustees of the Ernest C. Euler Trust |
5,964 | * | 5,964 | — | * | |||||||||||||||
| F&M Star Alliance Inc. |
26 | 10,000 | * | 10,000 | — | * | ||||||||||||||
| F. Henry Beguelin |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Fern P. O’Brian |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Frank Lombardo |
10,515 | * | 10,515 | — | * | |||||||||||||||
| Garretson B. Trudeau |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Gasper Pisacano |
1,000 | * | 1,000 | — | * | |||||||||||||||
| George Kingsley and Nadine J. Kingsley JTWROS |
9,000 | * | 9,000 | — | * | |||||||||||||||
| George Reynolds |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Gerald Fought |
4,036 | * | 4,036 | — | * | |||||||||||||||
| Gerald Lowrey |
150,215 | * | 150,215 | — | * | |||||||||||||||
| Gibralt Capital Corporation |
27 | 100,000 | * | 100,000 | — | * | ||||||||||||||
| Giles Sandvik and Jeanne Sandvik JTWROS |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Glenn Raudins |
28 | 6,595 | * | 407 | 6,188 | * | ||||||||||||||
| Gordon DeMeester |
5,133 | * | 5,133 | — | * | |||||||||||||||
| Gurmit Lotey |
4,591 | * | 4,591 | — | * | |||||||||||||||
| H Investment Company LLC |
29 | 20,000 | * | 20,000 | — | * | ||||||||||||||
| Harbour Tycoon Limited |
30 | 3,403,942 | 8.91% | 3,403,942 | — | * | ||||||||||||||
| Harold O. LaFlash and Greta G. LaFlash Revocable Trust |
31 | 4,000 | * | 4,000 | — | * | ||||||||||||||
| Igor Belousov |
60,000 | * | 60,000 | — | * | |||||||||||||||
| Imagiance LLC |
32 | 2,000 | * | 2,000 | — | * | ||||||||||||||
| Inna Mamuta |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Irwin Blitt Revocable Trust DTD 1-28-79 |
33 | 10,000 | * | 10,000 | — | * | ||||||||||||||
| Itochu Corporation |
34 | 880,546 | 2.31% | 880,546 | — | * | ||||||||||||||
| James Carnall |
39,160 | * | 39,160 | — | * | |||||||||||||||
| James F. Dempsey, Ph.D |
35 | 813,415 | 2.09% | 182,602 | 630,813 | 1.53% | ||||||||||||||
| James L. Johnson |
23,400 | * | 23,400 | — | * | |||||||||||||||
| James Saylor and Dorothy Saylor JTWROS |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Jay Bradbury |
18,000 | * | 18,000 | — | * | |||||||||||||||
| Jay Haft |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Jeff Rennell and Christine Kellye Rennell JTWROS |
40,000 | * | 40,000 | — | * | |||||||||||||||
| Jeffrey Adair |
1,463 | * | 1,463 | — | * | |||||||||||||||
| Jeffrey Fitzsimmons |
9,614 | * | 9,614 | — | * | |||||||||||||||
| Jeffrey Kuhr |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Jeffrey Paul Peterson |
§ | 36 | 2,280 | * | 2,280 | — | * | |||||||||||||
| Jeffrey Simon |
3,205 | * | 3,205 | — | * | |||||||||||||||
56
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| JMSK Ltd. |
37 | 44,837 | * | 44,837 | — | * | ||||||||||||||
| John Buddenbaum |
2,391 | * | 2,391 | — | * | |||||||||||||||
| John N. Randall |
2,000 | * | 2,000 | — | * | |||||||||||||||
| John O. Gallant |
5,000 | * | 5,000 | — | * | |||||||||||||||
| John Patrick |
56,992 | * | 56,992 | — | * | |||||||||||||||
| John V. Wagner |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Jonathan & Gina Blatt Children’s Trust UA 02/20/2002 |
38 | 4,000 | * | 4,000 | — | * | ||||||||||||||
| Jonathan Blatt and Gina Blatt, JTWROS |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Jonathan Li |
5,334 | * | 5,334 | — | * | |||||||||||||||
| Joseph Biancaniello |
4,200 | * | 4,200 | — | * | |||||||||||||||
| Joseph C. Garone and Jennifer J. Garone JTWROS |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Joseph F. Grassi |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Joseph H. McCall |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Joseph Rubino |
6,000 | * | 6,000 | — | * | |||||||||||||||
| Joseph Schump |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Julie A. Wineman |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Kaushal Pandya |
2,500 | * | 2,500 | — | * | |||||||||||||||
| Kearny Venture Partners Enrepreneurs’ Fund, L.P. |
39 | 50,563 | * | 50,563 | — | * | ||||||||||||||
| Kearny Venture Partners, L.P. |
40 | 2,479,359 | 6.49% | 2,479,359 | — | * | ||||||||||||||
| Kelli A. Lanphere |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Kenneth Richard Olivier |
2,707 | * | 2,707 | — | * | |||||||||||||||
| Ketal Patel |
17,139 | * | 17,139 | — | * | |||||||||||||||
| Kevin Filosa |
§ | 41 | 3,060 | * | 3,060 | — | * | |||||||||||||
| Kevin C. McDonough |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Kevin Gollon |
5,613 | * | 5,613 | — | * | |||||||||||||||
| Kimberly Hanley |
§ | 42 | 31 | * | 31 | — | * | |||||||||||||
| Larry Caillouet |
6,000 | * | 6,000 | — | * | |||||||||||||||
| Laura Renfro |
1,065 | * | 1,065 | — | * | |||||||||||||||
| Lee Harrison Corbin |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Leland Zurich and Valerie Zurich JTWROS |
30,000 | * | 30,000 | — | * | |||||||||||||||
| Leon Radomsky |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Lindsay B. Jacobson |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Lindsey S. McGrandy |
§ | 43 | 70 | * | 70 | — | * | |||||||||||||
| Lisa A. Piper |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Lisa Torsiello |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Livingston Securities, LLC |
§§ | 44 | 5,250 | * | 5,250 | — | * | |||||||||||||
| Louis Tagliaferro |
§ | 45 | 80 | * | 80 | — | * | |||||||||||||
| LRFA, LLC |
46 | 50,000 | * | 50,000 | — | * | ||||||||||||||
| Lynn Zheng |
16,000 | * | 16,000 | — | * | |||||||||||||||
57
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| Mackie Klingbeil |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Magna Equities II LLC |
47 | 40,000 | * | 40,000 | — | * | ||||||||||||||
| Mahipal Ravipati |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Mark Gold, M.D. |
48 | 28,215 | * | 3,206 | 25,009 | * | ||||||||||||||
| Mark Patterson |
2,222 | * | 2,222 | — | * | |||||||||||||||
| Mark Richard |
5,272 | * | 5,272 | — | * | |||||||||||||||
| Mark Tompkins |
49 | 1,070,000 | 2.80% | 1,070,000 | — | * | ||||||||||||||
| Martillo Finance Limited |
50 | 30,000 | * | 30,000 | — | * | ||||||||||||||
| Martin Junge |
23,000 | * | 23,000 | — | * | |||||||||||||||
| Mary Renfro |
1,065 | * | 1,065 | — | * | |||||||||||||||
| Mat 9 LLC |
51 | 45,200 | * | 45,200 | — | * | ||||||||||||||
| Matthew Longo |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Mauricio Martinez |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Maximilian Scheder—Bieschin, IRA |
10,000 | * | 10,000 | — | * | |||||||||||||||
| MCK Corporation |
52 | 2,000 | * | 2,000 | — | * | ||||||||||||||
| Meredith Stone |
7,623 | * | 7,623 | — | * | |||||||||||||||
| Michael Bird |
§ | 53 | 1,360 | * | 1,360 | — | * | |||||||||||||
| Michael Golub and Barbara Golub JTWROS |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Michael J. Colandrea |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Michael J. Pierce |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Michael L. Willis and Sharon D. Willis JTWROS |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Michael Levine |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Michael Silverman |
§ | 54 | 28,216 | * | 28,215 | 50,001 | * | |||||||||||||
| Michael T. Smith |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Michael Zimmerman |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Mikhail Dank |
5,000 | * | 5,000 | — | * | |||||||||||||||
| MJSK, LTD. |
55 | 54,129 | * | 54,129 | — | * | ||||||||||||||
| MLV & Co. LLC |
§§ | 56 | 2,350 | * | 2,350 | — | * | |||||||||||||
| Mohammed S. Rais |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Morgan Janssen |
§ | 57 | 4,320 | * | 4,320 | — | * | |||||||||||||
| Murray W. Grigg |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Nancy & William Price Mendenhall |
2,665 | * | 2,665 | — | * | |||||||||||||||
| Nicholas S. Cucinelli |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Nitin Bhatnagar |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Noel P. McGee |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Norma Simione |
139 | * | 139 | — | * | |||||||||||||||
| Northland Securities, Inc. |
§§ | 58 | 7,600 | * | 7,600 | — | * | |||||||||||||
| Northlea Partners LLLP |
59 | 5,000 | * | 5,000 | — | * | ||||||||||||||
| O. Stuart Chase |
2,000 | * | 2,000 | — | * | |||||||||||||||
| OrbiMed Associates III, LP |
60 | 75,371 | * | 75,371 | — | * | ||||||||||||||
58
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| OrbiMed Private Investments III LP |
61 | 7,914,545 | 20.72% | 7,914,545 | — | * | ||||||||||||||
| OSPREY I LLC |
62 | 10,000 | * | 10,000 | — | * | ||||||||||||||
| Park City Capital Offshore Master Ltd. |
63 | 200,000 | * | 200,000 | — | * | ||||||||||||||
| Patricia E. King |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Paul Ehrenstein |
§ | 64 | 4,873 | * | 4,873 | — | * | |||||||||||||
| Paul C. Sarahan |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Paul Tompkins |
50,000 | * | 40,000 | 10,000 | * | |||||||||||||||
| Peter D’Amico |
1,365 | * | 1,365 | — | * | |||||||||||||||
| Peter E. Bennett |
§ | 65 | 2,280 | * | 2,280 | — | * | |||||||||||||
| Peter Rentrop |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Peter K. Janssen |
§ | 66 | 1,824 | * | 1,824 | — | * | |||||||||||||
| Qingguo Zeng |
8,311 | * | 8,311 | — | * | |||||||||||||||
| R. Lea Bone |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Randal S. Milch and Amelia S. Salzman JTWROS |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Randall A. Sebring and Alice M. Sebring JTWROS |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Ravi Gutta |
14,900 | * | 14,900 | — | * | |||||||||||||||
| Raymond Warenik |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Ren Le |
565 | * | 565 | — | * | |||||||||||||||
| Richard E. Renfro and Tenessa L. Renfro |
1,261 | * | 1,261 | — | * | |||||||||||||||
| Ricardo Yanez |
339 | * | 339 | — | * | |||||||||||||||
| Richard Kern and Ellen Kern JTWROS |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Richard Madison |
15,000 | * | 15,000 | — | * | |||||||||||||||
| Richard Pisacano |
§ | 67 | 5,100 | * | 5,100 | — | * | |||||||||||||
| Richard Salzar & Anca M. Cretan Salzar, JTWROS |
7,000 | * | 7,000 | — | * | |||||||||||||||
| Richard Stark |
41,034 | * | 41,034 | — | * | |||||||||||||||
| Richard W. Baskerville |
2,000 | * | 2,000 | — | * | |||||||||||||||
| RMR Wealth Management LLC |
§ | 68 | 7,680 | * | 7,680 | — | * | |||||||||||||
| Robert A. and Mary R. Renfro |
7,582 | * | 7,582 | — | * | |||||||||||||||
| Robert A. Zlotecki, MD |
1,332 | * | 1,332 | — | * | |||||||||||||||
| Robert Amdur |
13,333 | * | 13,333 | — | * | |||||||||||||||
| Robert Burkhardt |
12,000 | * | 12,000 | — | * | |||||||||||||||
| Robert Crothers |
§ | 69 | 33,141 | * | 33,141 | — | * | |||||||||||||
| Robert D. Frankel |
6,000 | * | 6,000 | — | * | |||||||||||||||
| Robert G. Curtin |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Robert Jacob and Christine Jacob JTWROS |
3,000 | * | 3,000 | — | * | |||||||||||||||
59
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| Robert McGrath |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Robert Schoenbacher |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Robinson Crothers |
§ | 70 | 5,675 | * | 5,675 | — | * | |||||||||||||
| Rod Manning |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Roman Livson |
§ | 71 | 7,736 | * | 7,736 | — | * | |||||||||||||
| Ronald Bartlett & Valerie Bartlett JTWROS |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Rory McGee |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Royal Seal Holding Co., Limited |
72 | 854,884 | 2.24% | 854,884 | — | * | ||||||||||||||
| Russell S. Donda |
158,618 | * | 158,618 | — | * | |||||||||||||||
| Ryan Konik |
§ | 73 | 1,800 | * | 1,800 | — | * | |||||||||||||
| S. Tucker S. Johnson |
26,352 | * | 26,352 | — | * | |||||||||||||||
| Sabin Tucker Snow, Jr. Trust u/d/t 12/23/76 |
74 | 2,707 | * | 2,707 | — | * | ||||||||||||||
| Sachin S. Kamath |
40,111 | * | 40,111 | — | * | |||||||||||||||
| Sack Family Investment Fund, LLC |
75 | 20,000 | * | 20,000 | — | * | ||||||||||||||
| Sameer Keole |
2,665 | * | 2,665 | — | * | |||||||||||||||
| Scott Cardone |
§ | 76 | 498 | * | 498 | — | * | |||||||||||||
| Scott Rapfogel |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Seena Bernhard and Steven Bernhard JTWROS |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Sergey Sergeev |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Siemens Venture Capital Healthcare GmbH |
77 | 146,947 | * | 146,947 | — | * | ||||||||||||||
| Simpson Family Trust |
78 | 5,000 | * | 5,000 | — | * | ||||||||||||||
| Souheil F. Haddad |
10,000 | * | 10,000 | — | * | |||||||||||||||
| SP Capital Partners, LLC |
79 | 20,000 | * | 20,000 | — | * | ||||||||||||||
| Stan Rabinovich |
25,000 | * | 25,000 | — | * | |||||||||||||||
| Stanton J. Rowe |
20,000 | * | 20,000 | — | * | |||||||||||||||
| Stephanie H. Warrington |
931 | * | 931 | — | * | |||||||||||||||
| Stephen C. Rush |
7,999 | * | 7,999 | — | * | |||||||||||||||
| Stephen DiChiara |
5,000 | * | 5,000 | — | * | |||||||||||||||
| Stephen Pisacano |
3,400 | * | 3,400 | — | * | |||||||||||||||
| Stephen Renaud |
§ | 80 | 83,216 | * | 33,215 | 50,001 | * | |||||||||||||
| Steven K. and Deborah S. Nelson JTWROS |
3,000 | * | 3,000 | — | * | |||||||||||||||
| Susan & Joseph Nehmen |
5,518 | * | 5,518 | — | * | |||||||||||||||
| Synogen Investment Trust, LLC |
81 | 5,215 | * | 5,215 | — | * | ||||||||||||||
| Technology Ventures II Venture Capital Investment Limited Partnership |
82 | 145,310 | * | 145,310 | — | * | ||||||||||||||
60
Table of Contents
Index to Financial Statements
| Selling Stockholder(1) |
Broker- Dealer or Broker- Dealer Affiliate |
Footnote, if any |
Shares of Common Stock Beneficially Owned Before this Offering |
Percentage of Common Stock Beneficially Owned Before this Offering(2) |
Shares of Common Stock Being Offered in this Offering |
Shares of Common Stock Beneficially Owned Upon Completion of this Offering(3) |
Percentage of Outstanding Common Stock Beneficially Owned Upon Completion of this Offering(2)(3) | |||||||||||||
| The Mastin-Allen Stock Trust |
83 | 13,038 | * | 13,038 | — | * | ||||||||||||||
| Thomas F. LaMarca |
6,000 | * | 6,000 | — | * | |||||||||||||||
| Thomas Norgiel |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Thomas Weisel Healthcare Venture Partners, L.P. |
84 | 2,035,731 | 5.33% | 2,035,731 | — | * | ||||||||||||||
| Thomas Zahavi |
10,000 | * | 10,000 | — | * | |||||||||||||||
| Timothy Hermann |
§ | 85 | 642 | * | 642 | — | * | |||||||||||||
| Tina L. and Ernest C. Euler |
987 | * | 987 | — | * | |||||||||||||||
| Tina L. and Ernest C. Euler, as Trustees of the Tina L. Euler Trust |
630 | * | 630 | — | * | |||||||||||||||
| Todd Harrigan |
§ | 86 | 1,613 | * | 1,613 | — | * | |||||||||||||
| Tom McGurk, Jr. |
4,000 | * | 4,000 | — | * | |||||||||||||||
| Trout Capital LLC |
§§ | 87 | 7,200 | * | 7,200 | — | * | |||||||||||||
| University of Florida Research Foundation, Inc. |
88 | 33,652 | * | 33,652 | — | * | ||||||||||||||
| Walter G. Gans |
2,000 | * | 2,000 | — | * | |||||||||||||||
| Whiting Holdings, LP |
89 | 20,000 | * | 20,000 | — | * | ||||||||||||||
| William A. Crothers |
3,000 | * | 3,000 | — | * | |||||||||||||||
| William A. Crothers, Jr. |
6,000 | * | 6,000 | — | * | |||||||||||||||
| William C. Sykes |
34,000 | * | 34,000 | — | * | |||||||||||||||
| William E. & Catherine P. Simon Trust dated April 23, 2012 & Catherine P. Simon Trust dated April 23, 2001 |
90 | 24,168 | * | 24,168 | — | * | ||||||||||||||
| William E. & Catherine P. Simon |
4,486 | * | 4,486 | — | * | |||||||||||||||
| William Jeffrey Kadi |
20,000 | * | 20,000 | — | * | |||||||||||||||
| William Strawbridge |
5,000 | * | 5,000 | — | * | |||||||||||||||
| William Wells |
88,886 | * | 88,886 | — | * | |||||||||||||||
| Xiaodong Lu |
1,023 | * | 1,023 | — | * | |||||||||||||||
| * | Less than 1%. |
| § | The selling stockholder is an affiliate of a broker-dealer. |
| §§ | The selling stockholder is a broker-dealer. |
| 1 | All information regarding investors in the Private Placement is provided as of August 17, 2015. |
| 2 | Percentage ownership is based on a denominator equal to the sum of (i) 38,200,088 shares of our common stock outstanding as of August 17, 2015, and (ii) the number of shares of common stock issuable upon exercise or conversion of convertible securities beneficially owned by the applicable selling stockholder. |
| 3 | Assumes that all shares of common stock being registered under the registration statement of which this prospectus forms a part are sold in this offering, and that none of the selling stockholders acquire additional shares of our common stock after the date of this prospectus and prior to completion of this offering. |
| 4 | Includes 3,108 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
61
Table of Contents
Index to Financial Statements
| 5 | Aisling Capital Partners, LP, or Aisling GP, is the general partner of the selling stockholder. Aisling Capital Partners LLC, or Aisling Partners, is the general partner of Aisling GP. The individual managing members, or the Aisling Managers, of Aisling Partners are Dennis Purcell, Andrew Schiff, M.D. and Steve Elms. By virtue of these relationships, Aisling GP, Aisling Partners and the Aisling Managers may be deemed to have voting and investment power over the shares held by the selling stockholder. Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, an affiliate of the selling stockholder. Voting and dispositive decisions with respect to shares held by the selling stockholder are not made by Dr. Bilenker; he disclaims beneficial ownership of the shares held by the selling stockholder, except to the extent of any pecuniary interest therein, if any. |
| 6 | Allan Lipkovitz, the trustee of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 7 | O. Stuart Chase is the Headmaster Emeritus of the selling stockholder has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 8 | Includes 5,498 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 9 | Includes 11,526 shares of our common stock underlying an option held by the selling stockholder and exercisable on or within 60 days following October 1, 2015 that are not being offered in this offering. |
| 10 | David Dawes has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 11 | Richard E. Rush, Manager of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 12 | Includes 2,670 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 13 | Beacon Bioventures Advisors Fund II Limited Partnership, or BBA II, is the general partner of the selling stockholder. Impresa Management LLC, or Impresa, is the general partner of BBA II. By virtue of these relationships, BBA II and Impresa may be deemed to have voting and investment power over the shares held by the selling stockholder. Impresa is managed on a day-to-day basis by its President, Paul L. Mucci, and as such, Mr. Mucci may be deemed to have voting and dispositive power with respect to all shares held by the selling stockholder. Each of the individuals and entities listed above expressly disclaims beneficial ownership of the shares held by the selling stockholder, except to the extent of any pecuniary interest therein, if any. |
| 14 | Includes 3,040 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 15 | John R. Bennett, the Managing Partner of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 16 | Jong Tae Choi, the trustee of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 17 | Includes 264 shares the selling stockholder has the right to acquire through the exercise of common stock warrants. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 18 | Claude M. Penchina, the trustee of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 19 | Mark Bildner, member of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 20 | Malcolm R. Currie and Barbara L. Currie, trustees of the selling stockholder, have the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
62
Table of Contents
Index to Financial Statements
| 21 | Includes 8,115 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 22 | David M. Rickey, the trustee of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 23 | Includes 6,657 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. Barbara J. Glenns, President of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 24 | Eleven II Management LLC is the General Partner of the selling stockholder. Klaus Fosmark, Member of the Eleven II Management LLC, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 25 | Includes 8,115 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 26 | Roman Ryzhkov, President of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 27 | Ryan Chan, the Chief Financial Officer of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 28 | Includes 6,188 shares of our common stock underlying an option held by the selling stockholder and exercisable on or within 60 days following October 1, 2015 that are not being offered in this offering. |
| 29 | Pamela Baker, the Manager of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 30 | Aditya Puri, a member of our board of directors, is an Investments Director at Xeraya Capital, an affiliate of Harbour Tycoon Limited. |
| 31 | Harold LaFlash and Greta LaFlash, trustees of the selling stockholder, have the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 32 | Olivier Jarry, Member of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 33 | Irwin Blitt, the trustee of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 34 | Masahiro Okafuji has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 35 | Includes 588,912 shares of our common stock underlying an option held by the selling stockholder and exercisable on or within 60 days following October 1, 2015 that are not being offered in this offering. The selling stockholder is our Secretary and Chief Scientific Officer and is currently a member of our board of directors. |
| 36 | Includes 2,280 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 37 | Janice Gold, the wife of Dr. Gold, a member of our board of directors, is the President of MJSK, Ltd., and Steven Gold, the son of Dr. Gold, is the General Partner of JMSK, Ltd. Voting and dispositive decisions with respect to shares held by MJSK, Ltd. and JMSK, Ltd. are not made by Dr. Gold; he disclaims beneficial ownership of the shares held by MJSK, Ltd. and JMSK, Ltd. except to the extent of any pecuniary interest therein, if any. |
| 38 | H. Joshua Blatt, the trustee of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 39 | Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., or KVP, Kearny Venture Associates, L.L.C., or KVA, and Thomas Weisel Capital Management LLC, or TWCM. KVA is the general partner of each of KVP and Kearny Venture Partners |
63
Table of Contents
Index to Financial Statements
| Entrepreneurs’ Fund, L.P., or KVPE. TWCM is the managing member of Thomas Weisel Healthcare Venture Partners, LLC, the general partner of Thomas Weisel Healthcare Venture Partners, L.P., or TWHVP. Voting and dispositive decisions with respect to shares held by KVP, KVPE and TWHVP are made by Dr. Castelein; however, he disclaims beneficial ownership of the shares held by KVP, KVPE and TWHVP, except to the extent of any pecuniary interest therein, if any. |
| 40 | Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., or KVP, Kearny Venture Associates, L.L.C., or KVA, and Thomas Weisel Capital Management LLC, or TWCM. KVA is the general partner of each of KVP and Kearny Venture Partners Entrepreneurs’ Fund, L.P., or KVPE. TWCM is the managing member of Thomas Weisel Healthcare Venture Partners, LLC, the general partner of Thomas Weisel Healthcare Venture Partners, L.P., or TWHVP. Voting and dispositive decisions with respect to shares held by KVP, KVPE and TWHVP are made by Dr. Castelein; however, he disclaims beneficial ownership of the shares held by KVP, KVPE and TWHVP, except to the extent of any pecuniary interest therein, if any. |
| 41 | Includes 3,060 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 42 | Includes 31 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 43 | Includes 70 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 44 | Includes 5,250 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is a broker-dealer that acted as a sub-placement agent in the Private Placement. Scott B. Livingston, Chief Executive Officer of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 45 | Includes 80 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 46 | David Welch, President of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 47 | Joshua Sason, Managing Member of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 48 | Includes 19,630 shares of our common stock underlying an option held by the selling stockholder and exercisable on or within 60 days following October 1, 2015 that are not being offered in this offering. The selling stockholder is currently a member of our board of directors. |
| 49 | Includes 300,000 shares owned by the selling stockholder that are not being offered in this offering. |
| 50 | WT Director Limited is the controlling entity of the selling stockholder. Each of Alison Blackwood and Du Preez Vermeulen, authorized signatories of WT Director Limited, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. Ms. Blackwood and Mr. Vermeulen disclaim beneficial ownership of such securities, except to the extent of her or his pecuniary interest therein. |
| 51 | Ralph Pastore, Managing Member of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 52 | John M. Petras, President of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 53 | Includes 1,360 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
64
Table of Contents
Index to Financial Statements
| 54 | Includes 28,215 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 55 | Janice Gold, the wife of Dr. Gold, a member of our board of directors, is the President of MJSK, Ltd., and Steven Gold, the son of Dr. Gold, is the General Partner of JMSK, Ltd. Voting and dispositive decisions with respect to shares held by MJSK, Ltd. and JMSK, Ltd. are not made by Dr. Gold; he disclaims beneficial ownership of the shares held by MJSK, Ltd. and JMSK, Ltd. except to the extent of any pecuniary interest therein, if any. |
| 56 | Includes 2,350 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is a broker-dealer who served as a placement agent in the Private Placement. The selling stockholder is a wholly owned subsidiary of FBR Capital Markets Holdings, Inc. FBR Capital Markets Holdings, Inc. is a wholly owned subsidiary of FBR & Co., a public company. |
| 57 | Includes 4,320 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 58 | Includes 7,600 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is a broker-dealer who served as a placement agent in the Private Placement. |
| 59 | Dr. John Abeles, Manager of the General Partner of the the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 60 | OrbiMed Capital GP III LLC, or GP III, is the general partner of OrbiMed Private Investments III, LP, or OPI III. OrbiMed Advisors LLC, or OrbiMed, is the managing member of GP III and the general partner of OrbiMed Associates III, LP, or OA III. Samuel D. Isaly is the managing member of and owner of a controlling interest in OrbiMed. By virtue of such relationships, GP III, OrbiMed and Mr. Isaly may be deemed to have voting and investment power over the shares held by OPI III and OA III. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner of OrbiMed. Each of GP III, OrbiMed, Mr. Isaly and Dr. Bonita disclaims beneficial ownership of the shares held by OPI III and OA III, except to the extent of its or his pecuniary interest therein, if any. |
| 61 | OrbiMed Capital GP III LLC, or GP III, is the general partner of OrbiMed Private Investments III, LP, or OPI III. OrbiMed Advisors LLC, or OrbiMed, is the managing member of GP III and the general partner of OrbiMed Associates III, LP, or OA III. Samuel D. Isaly is the managing member of and owner of a controlling interest in OrbiMed. By virtue of such relationships, GP III, OrbiMed and Mr. Isaly may be deemed to have voting and investment power over the shares held by OPI III and OA III. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner of OrbiMed. Each of GP III, OrbiMed, Mr. Isaly and Dr. Bonita disclaims beneficial ownership of the shares held by OPI III and OA III, except to the extent of its or his pecuniary interest therein, if any. |
| 62 | Dale Burns, Manager of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 63 | Michael J. Fox, Director of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 64 | Includes 4,873 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 65 | Includes 2,280 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 66 | Includes 1,824 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
65
Table of Contents
Index to Financial Statements
| 67 | Includes 3,500 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 68 | Includes 7,680 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. Philip Rabinovich, Managing Member of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 69 | Includes 33,141 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 70 | Includes 5,675 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 71 | Includes 7,736 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 72 | Cowealth Medical Holding Co., Ltd, a Cayman Company publicly held and listed in Gretai Market, Taiwan (Taipei Stock Exchange), has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 73 | Includes 1,800 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 74 | S. Tucker S. Johnson and Gretchen W. Johnson, trustees of the selling stockholder, each have the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 75 | Burton M. Sack, Managing Member of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 76 | Includes 498 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 77 | Dr. Ralf Schnell and Klaus Grünfelder, the Chief Executive Officer and Chief Financial Officer of the selling stockholder, respectively, each have the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 78 | Paul V. Simpson and Maria N. Simpson, trustees of the selling stockholder, each have the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 79 | Stan Rabinovich, Managing Member of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 80 | Includes 28,215 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 81 | Richard R. Allen, Managing Director of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 82 | Sinzo Nakano has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 83 | Richard R. Allen, trustee of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 84 | Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., or KVP, Kearny Venture Associates, L.L.C., or KVA, and Thomas Weisel Capital Management LLC, or TWCM. KVA is the general partner of each of KVP and Kearny Venture Partners Entrepreneurs’ Fund, L.P., or KVPE. TWCM is the managing member of Thomas Weisel Healthcare |
66
Table of Contents
Index to Financial Statements
| Venture Partners, LLC, the general partner of Thomas Weisel Healthcare Venture Partners, L.P., or TWHVP. Voting and dispositive decisions with respect to shares held by KVP, KVPE and TWHVP are made by Dr. Castelein; however, he disclaims beneficial ownership of the shares held by KVP, KVPE and TWHVP, except to the extent of any pecuniary interest therein, if any. |
| 85 | Includes 642 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 86 | Includes 1,613 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is an affiliate of a broker-dealer that acted as a sub-placement agent in the Private Placement. |
| 87 | Includes 7,200 shares the selling stockholder has the right to acquire through the exercise of a common stock warrant. The selling stockholder is a broker-dealer who served as a placement agent in the Private Placement. The selling stockholder is a limited liability company whose managing member and CEO is Jonathan Fassberg. Accordingly, Mr. Fassberg may be deemed to have voting and investment power over the shares held by the selling stockholder. Mr. Fassberg disclaims beneficial ownership with respect to such shares, except to the extent of their pecuniary interest therein, if any. |
| 88 | David P. Norton, Michael V. McKee and George C. Kolb, Jr., the President, Treasurer and Secretary, respectively, of the selling stockholder, each have the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 89 | Mark S. Whiting, Partner of the selling stockholder, has the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
| 90 | William E. Simon and Catherine P. Simon, the trustees of the selling stockholder, have the power to vote or dispose of the securities held of record by the selling stockholder and may be deemed to beneficially own those securities. |
67
Table of Contents
Index to Financial Statements
The selling stockholders and any of their pledgees, donees, transferees, assignees or other successors-in-interest may, from time to time, sell, transfer or otherwise dispose of any or all of their shares of common stock or interests in shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices. The selling stockholders may use one or more of the following methods when disposing of the shares or interests therein:
| • | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| • | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| • | through brokers, dealers or underwriters that may act solely as agents; |
| • | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| • | an exchange distribution in accordance with the rules of the applicable exchange; |
| • | privately negotiated transactions; |
| • | through the writing or settlement of options or other hedging transactions entered into after the effective date of the registration statement of which this prospectus is a part, whether through an options exchange or otherwise; |
| • | broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share; |
| • | a combination of any such methods of disposition; and |
| • | any other method permitted pursuant to applicable law. |
The selling stockholders may also sell shares under Rule 144 under the Securities Act of 1933, as amended, or Securities Act, if available, rather than under this prospectus, provided that they meet the criteria and conform to the requirements of these provisions, including the requirements of Rule 144(i) applicable to former “shell companies.”
Broker-dealers engaged by the selling stockholders may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
The selling stockholders may from time to time pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell shares of common stock from time to time under this prospectus, or under a supplement or amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
Upon being notified in writing by a selling stockholder that any material arrangement has been entered into with a broker-dealer for the sale of common stock through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, we will file a supplement to this prospectus, if required, pursuant to Rule 424(b) under the Securities Act, disclosing (i) the name of each such selling stockholder and of the participating broker-dealer(s), (ii) the number of shares involved, (iii) the price at which such shares of common stock were sold, (iv) the commissions paid or discounts or concessions allowed to such
68
Table of Contents
Index to Financial Statements
broker-dealer(s), where applicable, (v) that such broker-dealer(s) did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus, and (vi) other facts material to the transaction. In addition, upon being notified in writing by a selling stockholder that a donee or pledge intends to sell more than 500 shares of common stock, we will file a supplement to this prospectus if then required in accordance with applicable securities law.
The selling stockholders also may transfer the shares of common stock in other circumstances, in which case the transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus.
In connection with the sale of the shares of common stock or interests in shares of common stock, the selling stockholders may enter into hedging transactions after the effective date of the registration statement of which this prospectus is a part with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The selling stockholders may also sell shares of common stock short after the effective date of the registration statement of which this prospectus is a part and deliver these securities to close out their short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into option or other transactions after the effective date of the registration statement of which this prospectus is a part with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The selling stockholders and any broker-dealers or agents that are involved in selling the shares may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act and the rules of the Financial Industry Regulatory Authority (FINRA).
We have advised the selling stockholders that they are required to comply with Regulation M promulgated under the Securities and Exchange Act during such time as they may be engaged in a distribution of the shares. The foregoing may affect the marketability of the common stock.
The aggregate proceeds to the selling stockholders from the sale of the common stock offered by them will be the purchase price of the common stock less discounts or commissions, if any. Each of the selling stockholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents. We will not receive any of the proceeds from the sale of common stock offered by the selling stockholders.
We are required to pay all fees and expenses incident to the registration of the shares. We have agreed to indemnify the selling stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act or otherwise.
We have agreed with the selling stockholders to keep the registration statement of which this prospectus constitutes a part effective until the earlier of (a) the date that is two years from the date it is declared effective by the SEC and (b) the date on which all the securities registered hereunder have been sold under this prospectus or pursuant to Rule 144.
69
Table of Contents
Index to Financial Statements
DETERMINATION OF OFFERING PRICE
There currently is a limited public market for our common stock. The selling stockholders will determine at what price they may sell the offered shares, and such sales may be made at prevailing market prices or at privately negotiated prices. See “Plan of Distribution” above for more information.
70
Table of Contents
Index to Financial Statements
We are registering the shares of common stock issued or issuable to the selling stockholders to permit the resale of these shares of common stock by the selling stockholders from time to time after the date of this prospectus. We will not receive any proceeds from the sale of our common stock offered by the selling stockholders under this prospectus. We may, however, receive proceeds from warrants exercised by selling stockholders in the event that such warrants are exercised for cash.
We will bear all fees and expenses incident to our obligation to register the shares of our common stock being offered for resale hereunder by the selling stockholders.
71
Table of Contents
Index to Financial Statements
We have authorized capital stock consisting of 300,000,000 shares of common stock and 10,000,000 shares of preferred stock. As of the date of this prospectus, we had 38,200,088 shares of common stock issued and outstanding, and no shares of preferred stock issued and outstanding.
Common Stock
The holders of outstanding shares of common stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and in such amounts as the board from time to time may determine. Holders of common stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting of the election of directors then standing for election. The common stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of our company, the assets legally available for distribution to stockholders are distributable ratably among the holders of the common stock after payment of liquidation preferences, if any, on any outstanding payment of other claims of creditors. Each outstanding share of common stock is duly and validly issued, fully paid and non-assessable.
Holders of common stock purchased in the Private Placement have anti-dilution protection with respect to such shares such that, if within 180 days after the initial closing date of July 23, 2015, we issue additional shares of common stock or common stock equivalents (subject to customary exceptions, including but not limited to shares of common stock issued or issuable pursuant to an acquisition, joint venture or technology license agreement, securities issued to financial institutions or lessors in connection with credit arrangements, equipment financings, lease arrangements, etc., in the aggregate not exceeding 5% of the common stock outstanding, issuances of awards under the 2015 Plan) for a consideration per share less than the Private Placement offering price of $5.00 per share, each such investor will be entitled to receive from us additional shares of common stock in an amount such that, when added to the number of shares of common stock initially purchased by such investor and still held of record and beneficially owned by such investor at the time of the dilutive issuance, will equal the number of shares of common stock that such investor’s Private Placement subscription amount for the previously held shares would have purchased at the lower price. Holders of a majority of the then previously held shares may waive the anti-dilution right of all Private Placement investors with respect to a particular issuance.
Preferred Stock
Shares of preferred stock may be issued from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by our board of directors prior to the issuance of any shares thereof. Preferred stock will have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated in such resolution or resolutions providing for the issue of such class or series of preferred stock as may be adopted from time to time by the board of directors prior to the issuance of any shares thereof. The number of authorized shares of preferred stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of our capital stock entitled to vote generally in the election of the directors, voting together as a single class, without a separate vote of the holders of the preferred stock, or any series thereof, unless a vote of any such holders is required pursuant to any preferred stock designation.
While we do not currently have any plans for the issuance of additional preferred stock, the issuance of such preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of the common stock until the Board of Directors determines the specific rights of the holders of the preferred stock; however, these effects may include:
| • | Restricting dividends on the common stock; |
72
Table of Contents
Index to Financial Statements
| • | Diluting the voting power of the common stock; |
| • | Impairing the liquidation rights of the common stock; or |
| • | Delaying or preventing a change in control of the company without further action by the stockholders. |
Other than in connection with shares of preferred stock (as explained above), which preferred stock is not currently designated nor contemplated by us, we do not believe that any provision of our amended and restated charter or bylaws would delay, defer or prevent a change in control.
Warrants
As of the date hereof:
| • | the Placement Agent Warrants entitle their holders to purchase 198,760 shares of common stock, with a term of five years and an exercise price of $5.00 per share; and |
| • | other warrants entitle their holder to purchase 128,231 shares of common stock, expiring on December 16, 2023 and with an exercise price of $5.85 per share. |
The Placement Agent Warrants contain “weighted average” anti-dilution protection in the event that we issue common stock or securities convertible into or exercisable for shares of common stock at a price lower than the subject warrant’s exercise price, subject to certain customary exceptions, as well as customary provisions for adjustment in the event of stock splits, subdivision or combination, mergers, etc.
This summary descriptions of the warrants described above is qualified in their entirety by reference to the forms of such warrants filed as an exhibit to this prospectus.
Options
Options to purchase 4,357,180 shares of our common stock that were originally granted under our 2008 Plan to certain of our employees, officers and directors with a weighted average exercise price of $0.80 per share were assumed by us in connection with the Merger. Options to purchase 1,507,147 shares of our common stock have been granted under our 2015 Plan to certain of our employees and officers, with an exercise price of $5.00 per share.
Other Convertible Securities
As of the date hereof, other than the securities described above, the Company does not have any outstanding convertible securities.
Anti-Takeover Effects of Provisions of our Certificate of Incorporation, our Bylaws and Delaware Law
Some provisions of Delaware law, our certificate of incorporation and our bylaws that will be in effect immediately prior to the consummation of the Merger contain provisions that could make the following transactions more difficult: acquisition of us by means of a tender offer; acquisition of us by means of a proxy contest or otherwise; or removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions that might result in a premium over the price of our common stock.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability
73
Table of Contents
Index to Financial Statements
to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a person deemed an “interested stockholder” from engaging in a “business combination” with a publicly held Delaware corporation for three years following the date such person becomes an interested stockholder unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors, such as discouraging takeover attempts that might result in a premium over the price of our common stock.
Undesignated Preferred Stock
The ability to authorize undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of the company. These and other provisions may have the effect of deterring hostile takeovers or delaying changes in control or management of the company.
Special Stockholder Meetings
Our bylaws provide that a special meeting of stockholders may be called only by our board of directors, our chairman of the board of directors, chief executive officer, or in the absence of a chief executive officer, the president.
Requirements for Advance Notification of Stockholder Nominations and Proposals
Our bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors.
Elimination of Stockholder Action by Written Consent
Our certificate of incorporation eliminates the right of stockholders to act by written consent without a meeting.
Classified Board; Election and Removal of Directors
Our board of directors is divided into three classes. The directors in each class will serve for a three-year term, one class being elected each year by our stockholders, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, our stockholders holding a majority of the shares of our common stock outstanding will be able to elect all of our directors. In addition, our directors may not be removed without cause, and removal of our directors for cause will require a majority stockholder vote. For more information on the classified board of directors, see the section titled “Management—Board Composition.” This system of electing and removing directors may tend to discourage a third party from making a tender offer or otherwise attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of the directors.
74
Table of Contents
Index to Financial Statements
Choice of Forum
Our certificate of incorporation will provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our certificate of incorporation or our bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine.
Amendment of Charter Provisions
The amendment of any of the above provisions, except for the provision making it possible for our board of directors to issue convertible preferred stock, would require approval by holders of at least 66 2⁄3% of the voting power of our then outstanding voting stock.
The provisions of the Delaware General Corporation Law, our certificate of incorporation and our bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in our management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Limitations of Liability and Indemnification Matters
For a discussion of liability and indemnification, please see the section titled “Directors, Executive Officers, Promoters and Control Persons—Limitation on Liability and Indemnification Matters.”
Transfer Agent
The transfer agent and registrar for our common stock is Globex Transfer, LLC. The transfer agent and registrar’s address is 780 Deltona Blvd., Suite 202, Deltona, FL 32725 and its telephone number is 813-344-4490.
75
Table of Contents
Index to Financial Statements
MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY AND
RELATED STOCKHOLDER MATTERS
Our common stock is quoted on the OTC Markets (OTCQB) under the symbol “VRAY,” which changed from “MRXC” on July 20, 2015.
Our common stock is currently eligible for quotation for trading on OTC Markets, OTCQB tier of OTC Markets Group, Inc. under the ticker symbol “VRAY.” To date, there has been a limited public trading market for our common stock on the OTC Markets. During the year ended December 31, 2014 and during the quarters ended March 31 and June 30, 2015, there was no market for our common stock. For the quarter ended September 30, 2015, the high and low closing bid quotations for our common stock, as reported by the OTC Markets, was $7.50 and $0.15. On December 15, 2015, the last quoted sale price for our common stock as reported on the OTCQB tier was $5.00 per share. As of the date of this prospectus, there are: (i) outstanding options to purchase 5,763,450 shares of our common stock; (ii) outstanding warrants to purchase 326,991 shares of our common stock; and (iii) 38,200,088 outstanding shares of our common stock, 260,000 of which have been registered under the Securities Act and are freely tradable, 31,275,408 were issued to former stockholders of ViewRay Technologies, Inc. in connection with the Merger, and 5,884,504 of which were issued and sold in the Private Placement.
As of the date of this prospectus, we have 38,200,088 shares of common stock outstanding held by 265 stockholders of record.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our common stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our board of directors and will be dependent upon financial condition, results of operations, capital requirements and such other factors as the board of directors deems relevant.
Shares Eligible for Future Sale
Future sales of our common stock, including shares issued upon the exercise of outstanding options or warrants, in the public market, or the perception that those sales may occur, could cause the prevailing price for our common stock to fall or impair our ability to raise equity capital in the future. As described below, only a limited number of shares of our common stock will be available for sale in the public market for a period of several months after consummation of the Merger due to contractual and legal restrictions on resale described below. Future sales of our common stock in the public market either before (to the extent permitted) or after restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing price of our common stock at such time and our ability to raise equity capital at a time and price we deem appropriate.
Upon the completion of the Private Placement, we had 38,200,088 shares of common stock outstanding, of which our directors and executive officers beneficially own an aggregate of 24,571,046 shares. Of those outstanding shares, 260,000 shares of our common stock are freely tradeable, without restriction, as of the date of this prospectus. All shares issued in connection with the Merger and the Private Placement were issued as restricted securities, and as such none of those shares can be publicly sold unless and until they become eligible for sale under Rule 144 promulgated under the Securities Act or they are registered for resale under an effective registration statement under the Securities Act. We are registering under the registration statement for which this prospectus forms a part the shares issued in connection with the Merger and the Private Placement.
76
Table of Contents
Index to Financial Statements
Lock-up Agreements
In connection with the Merger, holders of 32,552,131 shares of our common stock have agreed, subject to certain exceptions, not to dispose of or hedge any shares of common stock or securities convertible into or exchangeable for shares of common stock during the period from the date of the lock-up agreement continuing through the date six months after the date of the Merger, except with our prior written consent.
The foregoing description of the lock-up agreement does not purport to be complete, and is qualified in its entirety by the complete form of lock-up agreement included as an exhibit to the Securities Purchase Agreement entered in connection with the Private Placement attached as an exhibit to this prospectus, the text of which is incorporated herein by reference.
Rule 144
Pursuant to Rule 144(i) promulgated under the Securities Act, sales of the securities of a former shell company, such as us, under that rule are not permitted (i) until at least 12 months have elapsed from the date on which we filed our “Form 10 information” on our Current Report on Form 8-K, filed on July 29, 2015, reflecting our status as a non-shell company and (ii) unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than Form 8-K reports. We intend to register such shares for sale under the Securities Act, but are currently a “voluntary filer” and are not subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act. As a result, unless we register such shares for sale under the Securities Act, most of our stockholders will be forced to hold their shares of our common stock for at least that 12-month period before they are eligible to sell those shares, and even after that 12-month period, sales may not be made under Rule 144 unless we and the selling stockholders are in compliance with other requirements of Rule 144.
In general, subject to Rule 144(i), Rule 144 provides that (i) any of our non-affiliates that has held restricted common stock for at least 6 months is thereafter entitled to sell its restricted stock freely and without restriction, provided that we remain compliant and current with our SEC reporting obligations, and (ii) any of our affiliates, which includes our directors, executive officers and other person in control of us, that has held restricted common stock for at least 12 months is thereafter entitled to sell its restricted stock subject to the following restrictions: (a) we are compliant and current with our SEC reporting obligations, (b) certain manner of sale provisions are satisfied, (c) a Form 144 is filed with the SEC, and (d) certain volume limitations are satisfied, which limit the sale of shares within any three-month period to a number of shares that does not exceed the greater of 1% of the total number of outstanding shares. A person who has ceased to be an affiliate at least three months immediately preceding the sale and who has owned such shares of common stock for at least one year is entitled to sell the shares under Rule 144 without regard to any of the limitations described above.
Regulation S
Regulation S under the Securities Act provides that shares owned by any person may be sold without registration in the U.S., provided that the sale is effected in an offshore transaction and no directed selling efforts are made in the U.S. (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that our shares of common stock may be sold in some other manner outside the U.S. without requiring registration in the U.S.
Rule 701
In general, under Rule 701 as currently in effect, any of our employees, directors, officers, consultants or advisors who acquired common stock from us in connection with a written compensatory stock or option plan or other written agreement, in compliance with Rule 701 under the Securities Act, before the effective date of the
77
Table of Contents
Index to Financial Statements
Merger (to the extent such common stock is not subject to a lock-up agreement) is entitled to rely on Rule 701 to resell such shares beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act in reliance on Rule 144, but without compliance with the holding period requirements contained in Rule 144. Accordingly, subject to any applicable lock-up agreements, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, under Rule 701 persons who are not our “affiliates,” as defined in Rule 144, may resell those shares without complying with the minimum holding period or public information requirements of Rule 144, and persons who are our “affiliates” may resell those shares without compliance with Rule 144’s minimum holding period requirements (subject to the terms of the lock-up agreement referred to above, if applicable).
Registration Rights
In connection with the Private Placement, we entered into a Registration Rights Agreement, pursuant to which we have agreed that promptly, but no later than 90 calendar days from the final closing of the Private Placement, the Company will file a registration statement with the SEC, or the Registration Statement, covering (a) the shares of common stock issued in the Private Placement, (b) the shares of common stock issuable upon exercise of the Placement Agent Warrants, (c) the shares of common stock issued in exchange for the equity securities of ViewRay outstanding prior to the Merger, and (d) shares of common stock held by certain pre-Merger security holders of the Company, or collectively, the Registrable Shares. The Company will use its commercially reasonable efforts to ensure that such Registration Statement is declared effective within 180 calendar days after the final closing of the Private Placement. If the Company is late in filing the Registration Statement, if the Registration Statement is not declared effective within 180 days after the final closing of the Private Placement, the Company fails to maintain the Registration Statement continuously effective as to all Registrable Shares included in such Registration Statement or the Company fails to satisfy the current public information as required under Rule 144(c), the Company will make payments to each holder of Registrable Shares as monetary penalties at a rate equal to 12% of the Private Placement Price per annum for each share affected during the period; provided, however, that in no event will the aggregate of any such penalties exceed 5% of the Private Placement Price per share. No monetary penalties will accrue with respect to any Registrable Shares removed from the Registration Statement in response to a comment from the staff of the SEC limiting the number of shares of common stock which may be included in the Registration Statement, or Cutback Comment.
The Company must keep the Registration Statement effective for two years from the date it is declared effective by the SEC or until (i) the Registrable Shares have been sold in accordance with such effective Registration Statement, or (ii) the Registrable Shares have been previously sold in accordance with Rule 144.
The holders of Registrable Shares (including any shares of common stock removed from the Registration Statement as a result of a Cutback Comment) and the stockholders of the Company prior to the Merger (but not holders of the shares issued to the stockholders of ViewRay in consideration for the Merger) will have “piggyback” registration rights for such Registrable Shares with respect to any registration statement filed by the Company following the effectiveness of the Registration Statement that would permit the inclusion of such shares, subject to customary cutback in an underwritten offering, which would be pro rata.
We will pay all expenses in connection with any registration obligation provided in the Registration Rights Agreement, including, without limitation, all registration, filing, stock exchange fees, printing expenses, all fees and expenses of complying with applicable securities laws, and the fees and disbursements of our counsel and of our independent accountants and reasonable fees and disbursements of counsel to the investors, in an amount not to exceed $35,000. Each investor will be responsible for its own sales commissions, if any, transfer taxes and the expenses of any attorney or other advisor such investor decides to employ.
All descriptions of the Registration Rights Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
78
Table of Contents
Index to Financial Statements
Stock Plans
As soon as practicable after the effective date of this Registration Statement, we intend to file with the SEC a registration statement under the Securities Act covering the shares of common stock that we may issue (i) upon exercise of outstanding options under the assumed 2008 Plan, and (ii) that are outstanding or reserved for issuance under the 2015 Plan and the ESPP. Such registration statement is expected to be filed and become effective as soon as practicable after the registration statement of which this prospectus forms a part becomes effective. Accordingly, shares registered under such registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the lock-up agreements described above, if applicable.
79
Table of Contents
Index to Financial Statements
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
The following management’s discussion and analysis should be read in conjunction with the historical financial statements and the related notes thereto contained in this prospectus. The management’s discussion and analysis contains forward-looking statements, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect” and the like, and/or future tense or conditional constructions (“will,” “may,” “could,” “should,” etc.), or similar expressions, identify certain of these forward-looking statements. These forward-looking statements are subject to risks and uncertainties, including those under “Risk Factors” in this prospectus, that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. The company’s actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of several factors. The company does not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this prospectus.
As previously reported, on July 23, 2015, our wholly-owned subsidiary, Vesuvius Acquisition Corp., a corporation formed in the State of Delaware on July 16, 2015, or the Acquisition Sub, merged with and into ViewRay Technologies, Inc., a corporation incorporated in 2004 in the State of Florida originally under the name of ViewRay Incorporated, subsequently reincorporated in the State of Delaware in 2007. Pursuant to this transaction, or the Merger, ViewRay Technologies, Inc. was the surviving corporation and became our wholly-owned subsidiary. All of the outstanding capital stock of ViewRay Technologies, Inc. was converted into shares of our common stock, as described in more detail below.
Also as previously reported, immediately prior to the closing of the Merger, under the terms of a split-off agreement, or the Split-Off Agreement, and a general release agreement, we transferred all of its pre-Merger operating assets and liabilities to its wholly-owned special-purpose subsidiary, Vesuvius Acquisition Corp., a Nevada corporation, or the Split-Off Subsidiary, formed on July 16, 2015.
In connection with the Merger and pursuant to the Split-Off Agreement, we transferred our pre-Merger assets and liabilities to our pre-Merger majority stockholder, in exchange for the surrender and cancellation of 4,150,171 shares of our common stock.
As a result of the Merger and Split-Off, we discontinued our pre-Merger business and acquired the business of ViewRay and will continue the existing business operations of ViewRay as a publicly-traded company under the name ViewRay, Inc.
As the result of the Merger and the change in business and operations of the Company, a discussion of the past financial results of the Company is not pertinent, and under applicable accounting principles the historical financial results of ViewRay, the accounting acquirer, prior to the Merger are considered the historical financial results of the Company.
The following discussion highlights ViewRay’s results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described, and provides information that management believes is relevant for an assessment and understanding of the statements of financial condition and results of operations presented herein. The following discussion and analysis are based on ViewRay’s unaudited condensed consolidated financial statements contained in this prospectus which we have prepared in accordance with United States generally accepted accounting principles. You should read the discussion and analysis together with such condensed consolidated financial statements and the related notes thereto.
80
Table of Contents
Index to Financial Statements
Basis of Presentation
The unaudited condensed consolidated financial statements of ViewRay for the three months and nine months ended September 30, 2015 and 2014, contained herein include a summary of our significant accounting policies and should be read in conjunction with the discussion below. In the opinion of management, all material adjustments necessary to present fairly the results of operations for such periods have been included in these condensed consolidated financial statements. All such adjustments are of a normal recurring nature.
Company Overview
We design, manufacture and market MRIdian, the first and only MRI-guided radiation therapy system to image and treat cancer patients simultaneously. MRI is a broadly used imaging tool that has the ability to differentiate between types of soft tissue clearly, unlike X-ray or computed tomography, or CT, which are the most commonly used imaging technologies in radiation therapy today. MRIdian integrates MRI technology, radiation delivery and our proprietary software to locate, target and track the location and shape of soft-tissue tumors while radiation is delivered. These capabilities allow MRIdian to accurately deliver radiation to the tumor while reducing the amount delivered to healthy tissue, as compared to other radiation therapy treatments today. We believe this leads to improved patient outcomes and reduced side effects from off-target radiation delivery.
We received initial 510(k) marketing clearance from the FDA for our treatment planning and delivery software in January 2011 and for MRIdian in May 2012. We also received permission to affix the CE mark in November 2014, allowing MRIdian to be sold within the European Economic Area. At September 30, 2015, patients had received radiation treatment on MRIdian systems at three cancer centers located at Washington University in St. Louis, University of California, Los Angeles and the University of Wisconsin—Madison. In September 2015, Seoul National University Hospital in South Korea became the fourth cancer center and the first international location to operate a MRIdian system. Seoul National University Hospital began treating patients in October 2015.
We currently market MRIdian through a direct sales force in the United States and distributors in the rest of the world. We market MRIdian to a broad range of worldwide customers, including university research and teaching hospitals, community hospitals, private practices, government institutions and freestanding cancer centers. Our sales and revenue cycle varies based on the customer and can be lengthy, sometimes lasting up to 18 to 24 months or more from initial customer contact to sales contract execution. Following execution of a sales contract, it generally takes nine to 12 months for a customer to customize an existing facility or construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures.
We generated product, service and grant revenue of $5.3 million and $2.6 million, and had net losses of $10.3 million and $7.6 million during the three months ended September 30, 2015 and 2014, respectively. We generated product, service and grant revenue of $5.8 million and $5.9 million, and had net losses of $30.9 million and $24.2 million during the nine months ended September 30, 2015 and 2014, respectively. At September 30, 2015, we had 14 signed orders representing backlog value of $78.0 million.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We expect our expenses will increase substantially in connection with our ongoing activities, as we:
| • | add personnel to support our product development and commercialization efforts; |
| • | continue our research and development efforts; |
| • | seek regulatory approval for MRIdian in foreign countries; and |
| • | operate as a public company. |
81
Table of Contents
Index to Financial Statements
Accordingly, we may seek to fund our operations through public or private equity or debt financings or other sources. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and our ability to develop enhancements to and integrate new technologies into MRI-guided radiation therapy systems.
Merger
On July 23, 2015, ViewRay, Inc. (f/k/a Mirax Corp.), and ViewRay Technologies, Inc. (f/k/a ViewRay Incorporated), consummated an Agreement and Plan of Merger and Reorganization, or Merger Agreement. Pursuant to the Merger Agreement, the stockholders of ViewRay Technologies, Inc. contributed all of their equity interests to ViewRay, Inc. for shares of the ViewRay, Inc.’s common stock and merged with the Company’s subsidiary, which resulted in ViewRay Technologies, Inc. becoming a wholly-owned subsidiary of ViewRay, Inc. or the Merger. Effective as of July 23, 2015, ViewRay Inc. amended and restated its Certificate of Incorporation to increase its authorized common stock to 300,000,000 shares and 10,000,000 shares of “blank check” preferred stock, par value of $0.01 per share.
Upon closing of the Merger, under the terms of the Split-Off Agreement, dated July 23, 2015 among ViewRay, Inc., ViewRay Technologies, Inc. and Vesuvius Acquisition Sub, Inc., the acquisition subsidiary of Mirax, and a general release agreement dated July 23, 2015, or the General Release Agreement, ViewRay Inc. transferred all of its pre-Merger operating assets and liabilities to wholly- owned special-purpose subsidiary incorporated in Nevada, Vesuvius Acquisition Sub, Inc., or the Split-Off Subsidiary. Thereafter, Mirax transferred all of the outstanding shares of capital stock of the Split-Off Subsidiary to certain pre-Merger insiders of Mirax in exchange for the surrender and cancellation of shares of Mirax common stock held by such persons (the Split-Off).
Together with the Merger, on July 23, 2015, ViewRay Technologies, Inc. effected a 2.975-for-1 stock split of its then outstanding common stock and convertible preferred stock (collectively referred to as “Capital Stock”) and convertible preferred stock warrants, in which (i) each share of outstanding Capital Stock was increased into 2.975 shares of Capital Stock; (ii) the number of outstanding options to purchase each Capital Stock was proportionately increased on a 2.975-for-1 basis; (iii) number of shares reserved for future option grants under the 2008 Plan were proportionately increased on a 2.975-for-1 basis; (iv) the exercise price of each such outstanding option was proportionately decreased on a 2.975-for-1 basis; and (v) each share of outstanding convertible preferred stock warrant was increased into 2.975 shares of convertible preferred stock warrant. All of the share and per share amounts have been adjusted, on a retroactive basis, to reflect this 2.975-for-1 stock split.
Private Placement
At the closing of the Merger, ViewRay, Inc. conducted a private placement offering, or the Private Placement, of its securities for $26.2 million through the sale of 5,884,504 shares of the common stock of the surviving corporation, at an offering price of $5.00 per share, net of offering cost. Existing ViewRay investors purchased $17.0 million of shares in the Private Placement. Certain shareholders of Mirax retained, after giving effect to the Split-Off, 1,000,005 shares of the common stock of the surviving corporation upon the Private Placement.
The Merger is being accounted for as a reverse-merger and recapitalization. ViewRay Technologies, Inc. is the acquirer for financial reporting purposes, and ViewRay, Inc. is the acquired company under the acquisition method of accounting in accordance with FASB ASC Topic 805, Business Combination. Consequently, the assets, liabilities and operations that will be reflected in the historical financial statements prior to the Merger will be those of ViewRay Technologies, Inc. and will be recorded at the historical cost basis, and the condensed consolidated financial statements after completion of the Merger will include the assets, liabilities and results of operations of ViewRay Technologies, Inc. up to the day prior to the closing of the Merger and the assets, liabilities and results of operations of the combined company from and after the closing date of the Merger.
82
Table of Contents
Index to Financial Statements
New Orders
New orders are defined as the sum of gross product orders recorded during the period.
Backlog
We define backlog as the accumulation of all orders for which revenue has not been recognized and we consider valid. Backlog includes customer deposits received which are recorded as a liability on the balance sheet. Orders may be revised or cancelled according to their terms or upon mutual agreement between the parties. Therefore, it is difficult to predict with certainty the amount of backlog that will ultimately result in revenue. The determination of backlog includes objective and subjective judgment about the likelihood of an order contract becoming revenue. We perform a quarterly review of backlog to verify that outstanding orders in backlog remain valid, and based upon this review, orders that are no longer expected to result in revenue are removed from backlog. Our criteria include an outstanding and effective written agreement for the delivery of a MRIdian signed by customers, receipt of a minimum customer deposit or a letter of credit, any changes in customer or distributor plans or financial conditions, the customer’s or distributor’s continued intent and ability to fulfill the order contract, changes to regulatory requirements, the status of regulatory approval required in the customer’s jurisdiction, if any, or reasons for cancellation of order contracts.
During the nine months ended September 30, 2015 and the year ended December 31, 2014, our new orders were $28.8 million and $37.6 million, respectively. At September 30, 2015, we had 14 signed sales contracts for MRIdian systems in backlog with a total value of $78.0 million. At December 31, 2014, we had 10 signed sales contracts for MRIdian systems in backlog with a total value of $54.7 million.
Components of Statements of Operations
Revenue
Product Revenue. Product revenue consists of sales of MRIdian systems, as well as optional components, such as additional planning workstations and body coils.
Following execution of a sales contract, it generally takes nine to 12 months for a customer to customize an existing facility or construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures. On-site training takes approximately one week and can be conducted concurrent with installation and acceptance testing. Sales contracts generally include customer deposits upon execution of the agreement, and in certain cases, additional amounts due at shipment or commencement of installation, and final payment due generally upon customer acceptance.
Service Revenue. We generally offer maintenance service at no cost to customers to cover parts, labor and maintenance for one to two years. In addition, we offer multi-year, post-installation maintenance and support contracts that provide various levels of service support, which enables our customers to select the level of on-going support services, including parts and labor, which they require. These post-installation contracts are for a period of one to five years and provide services ranging from 24/7 on-site parts, labor and preventative maintenance to labor only with a longer response time. We also offer technology upgrades to our MRIdian systems, when and if available, for an additional fee. Service revenue is recognized on a straight-line basis over the term during which the contracted services are provided.
Grant Revenue. On December 15, 2008, the Company entered into an agreement with the county redevelopment fund in the State of Ohio for a loan of up to $800 thousand to fund the renovation of the Company’s Ohio headquarters. The loan, which bore interest at 6% annually through the maturity date of December 31, 2009, is secured by the Company’s leasehold improvements. Under the terms of the loan agreement, the lender may forgive $240 thousand if the Company meets certain permanent job creation
83
Table of Contents
Index to Financial Statements
requirements within the State of Ohio. In July 2010, $560 thousand of principal and accrued interest through the loan maturity date were repaid. In September 2015, the $240 thousand was forgiven and was recognized as grant revenue for the nine months ended September 30, 2015.
Cost of Revenue
Product Cost of Revenue. Product cost of revenue primarily consists of the cost of materials, installation and services associated with the manufacture and installation of MRIdian systems, as well as medical device excise tax and royalty payments to the University of Florida Research Foundation. Product cost of revenue also includes lower of cost or market inventory, or LCM, adjustments if the carrying value of the inventory is greater than its net realizable value. For strategic reasons, we sold our initial MRIdian systems prior to the receipt of FDA marketing clearance at prices lower than our projected costs to manufacture and install. As we accumulated materials, installation and other costs for these systems, we regularly assessed the carrying value of the related inventory value and recorded charges, or LCM adjustments, to reduce inventory to the lower of cost and net realizable value. The remaining realizable value of inventory was charged to product cost of revenue as those initial sites were completed and accepted. This resulted in LCM charges of $1.0 million and $0.6 million for the nine months ended September 30, 2015 and 2014, respectively. LCM charges were higher in 2014 since the majority of the LCM charges related to the two MRIdian systems installed in the nine months ended September 30, 2014 were already recorded in 2013 when the systems were built.
We expect our materials, installation and service costs to decrease as we continue to scale our operations, improve product designs and work with our third-party suppliers to lower costs.
Service Cost of Revenue. Service cost of revenue is comprised primarily of personnel costs, training and travel expenses to service and maintenance of installed MRIdian systems. Service cost of revenue also includes the costs of replacement parts under maintenance and support contracts.
Operating Expenses
Research and Development. Research and development expenses consist primarily of compensation and related costs for personnel, including stock-based compensation, employee benefits and travel. Other significant research and development costs arise from development, manufacturing and commercialization of MRIdian. These costs consist of third-party consulting services, laboratory supplies, research materials, medical equipment, computer equipment and licensed technology, and related depreciation and amortization. We expense research and development expenses as incurred. As we continue to invest in improving MRIdian and developing new technologies, we expect research and development expenses to increase in absolute dollars.
Selling and Marketing. Selling and marketing expenses consist primarily of compensation and related costs for personnel, including stock-based compensation, employee benefits and travel associated with our selling and marketing organization, including our direct sales force and sales management and our marketing and customer support personnel. Selling and marketing expenses also include costs related to trade shows and marketing programs. We expense selling and marketing costs as incurred. We expect selling and marketing expenses to increase in future periods as we expand our sales force and our marketing and customer support organization and increase our participation in trade shows and marketing programs.
General and Administrative. Our general and administrative expenses consist primarily of compensation and related costs for personnel, including stock-based compensation, employee benefits and travel, for our finance, human resources, regulatory and other administrative personnel. In addition, general and administrative expenses include third-party consulting, legal, audit, accounting services, quality and regulatory functions and facilities costs, and gain or loss on the disposal of property and equipment. We expect general and administrative expenses to increase in absolute dollars following the consummation of the Merger due to additional legal, accounting, insurance, investor relations and other costs associated with being a public company, as well as other costs associated with growing our business.
84
Table of Contents
Index to Financial Statements
Interest Income
Interest income consists primarily of interest income received on our cash and cash equivalents.
Interest Expense
Interest expense consists primarily of interest and amortization of the debt discount related to our long-term debt entered in 2013 with Hercules Technology III, L.P. and Hercules Technology Growth Capital, Inc., or together, Hercules, convertible promissory notes issued in 2014 and, long-term debt entered in 2015 with Capital Royalty II L.P and Parallel Investment Opportunities Partners II L.P., or together, CRG.
Other Income (Expense), Net
Other income (expense), net consists primarily of foreign currency exchange gains and losses and changes in the fair value of a convertible preferred stock warrant.
The outstanding convertible stock warrant is re-measured to fair value at each balance sheet date with the corresponding gain or loss from the adjustment recorded as a component of other income (expense), net. In July 2015, upon the closing of the Merger, the convertible preferred stock warrants were converted into warrants to purchase common stock. The aggregate fair value of these warrants upon the closing of the Merger was reclassified from liabilities to common stock additional paid-in-capital, a component of stockholder’s equity (deficit), and we ceased recording further related periodic fair value.
Results of Operations
The following tables set forth our results of operations for the periods presented (in thousands):
| Nine Months Ended September 30, | Years Ended December 31, | |||||||||||||||
| 2015 | 2014 | 2014 | 2013 | |||||||||||||
| Revenue: |
||||||||||||||||
| Product |
$ | 5,119 | $ | 5,702 | $ | 5,988 | $ | 2,253 | ||||||||
| Service |
419 | 229 | 411 | 12 | ||||||||||||
| Grant |
240 | — | — | 894 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
5,778 | 5,931 | 6,399 | 3,159 | ||||||||||||
| Cost of revenue: |
||||||||||||||||
| Product |
6,311 | 7,858 | 8,176 | 8,173 | ||||||||||||
| Service |
1,390 | 413 | 975 | 14 | ||||||||||||
| Total cost of revenue |
7,701 | 8,271 | 9,151 | 8,187 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross margin |
(1,923 | ) | (2,340 | ) | (2,752 | ) | (5,028 | ) | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
7,408 | 7,451 | 9,404 | 8,780 | ||||||||||||
| Selling and marketing |
3,315 | 3,572 | 4,681 | 3,781 | ||||||||||||
| General and administrative |
15,779 | 9,395 | 14,742 | 9,508 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses: |
26,502 | 20,418 | 28,827 | 22,069 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(28,425 | ) | (22,758 | ) | (31,579 | ) | (27,097 | ) | ||||||||
| Interest income |
1 | — | 1 | 4 | ||||||||||||
| Interest expense |
(2,376 | ) | (1,505 | ) | (2,243 | ) | (97 | ) | ||||||||
| Other income (expense), net |
(89 | ) | 82 | 21 | (32 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before provision for income taxes |
(30,889 | ) | (24,180 | ) | (33,800 | ) | (27,222 | ) | ||||||||
| Provision for income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
(30,889 | ) | (24,180 | ) | (33,800 | ) | (27,222 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
85
Table of Contents
Index to Financial Statements
Comparison of the Nine Months Ended September 30, 2015 and 2014
Revenue
| Nine Months Ended September 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| (unaudited) | ||||||||||||
| Product |
$ | 5,119 | $ | 5,702 | $ | (583 | ) | |||||
| Service |
419 | 229 | 190 | |||||||||
| Grant |
240 | — | 240 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
$ | 5,778 | $ | 5,931 | $ | (153 | ) | |||||
|
|
|
|
|
|
|
|||||||
Total revenue during the nine months ended September 30, 2015 decreased $0.2 million compared to the nine months ended September 30, 2014, primarily due to one system installed during the nine months ended September 30, 2015 at full price, compared to two systems installed during the nine months ended September 30, 2014 at discounted prices.
Product Revenue. Product revenue during the nine months ended September 30, 2015 decreased $0.6 million compared to the nine months ended September 30, 2014. This decrease was due to one MRIdian system sold to Seoul National University Hospital, South Korea, during the nine months ended September 30, 2015 compared to the sale of two MRIdian systems to University of California, Los Angeles and to University of Wisconsin-Madison during the nine months ended September 30, 2014, which were both at discounted prices.
Service Revenue. Service revenue during the nine months ended September 30, 2015 increased $0.2 million compared to the nine months ended September 30, 2014. This increase was due to a higher number of months of amortization of maintenance service revenue recognized for additional MRIdian system installed at each of University of California, Los Angeles and the University of Wisconsin-Madison during the nine months ended September 30, 2015 compared to the nine months ended September 30, 2014.
Grant Revenue. Grant revenue during the nine months ended September 30, 2015 increased $0.2 million compared to the nine months ended September 30, 2014. This increase was due to the Company’s note payable to the county redevelopment fund in the State of Ohio being forgiven based on meeting certain employment requirements in July 2015 and was recognized as grant revenue during the nine months ended September 30, 2015.
Cost of Revenue
| Nine Months Ended September 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| (unaudited) | ||||||||||||
| Product |
$ | 6,311 | $ | 7,858 | $ | (1,547 | ) | |||||
| Service |
1,390 | 413 | 977 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cost of revenue |
$ | 7,701 | $ | 8,271 | $ | (570 | ) | |||||
|
|
|
|
|
|
|
|||||||
Product Cost of Revenue. Product cost of revenue decreased $1.5 million during the nine months ended September 30, 2015, compared to the nine months ended September 30, 2014. This decrease was due to one MRIdian system sold to Seoul National University Hospital, South Korea, during the nine months ended September 30, 2015 compared to the sale of MRIdian to University of California, Los Angeles and to University of Wisconsin-Madison during the nine months ended September 30, 2014. Portion of the LCM charges as product cost of revenue for University of California, Los Angeles and University of Wisconsin-Madison were also recorded in 2013.
86
Table of Contents
Index to Financial Statements
Service Cost of Revenue. Service cost of revenue during the nine months ended September 30, 2015 increased $1.0 million, compared to the nine months ended September 30, 2014. The increase in service cost of revenue was due to the provision of services for the MRIdian system installed at Seoul National University Hospital, South Korea.
Operating Expenses
| Nine Months Ended September 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| (unaudited) | ||||||||||||
| Research and development |
$ | 7,408 | $ | 7,451 | $ | (43 | ) | |||||
| Selling and marketing |
3,315 | 3,572 | (257 | ) | ||||||||
| General and administrative |
15,779 | 9,395 | 6,384 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
$ | 26,502 | $ | 20,418 | $ | 6,084 | ||||||
|
|
|
|
|
|
|
|||||||
Research and Development. Research and development expenses during the nine months ended September 30, 2015 remained comparable to the nine months ended September 30, 2014.
Selling and Marketing. Selling and marketing expenses during the nine months ended September 30, 2015 decreased $0.3 million compared to the nine months ended September 30, 2014. This decrease was primarily due to a $0.5 million decrease in trade show and public relationship expenses during the nine months ended September 30, 2015 as majority of the trade shows in 2015 will take place in the last quarter as compared to in the third quarter in 2014, partially offset by a $0.2 million increase in consulting activities as a result of engaging more consulting services for roadshow preparation and other marketing activities during the nine months ended September 30, 2015.
General and Administrative. General and administrative expenses during the nine months ended September 30, 2015 increased $6.4 million, compared to the nine months ended September 30, 2014. This increase was primarily attributable to a $2.9 million write-off of deferred IPO financing costs in June 2015, a $2.0 million increase in personnel and related costs as a result of higher employee headcount, a $0.7 million increase in facility costs as we leased new office space in September 2014, a $0.4 million increase in accounting and legal fees related to our originally planned IPO and reverse merger activities, and a $0.3 million increase in consulting expenses as a result of engaging more consulting services during the nine months ended September 30, 2015.
Interest Expense
| Nine Months Ended September 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| (unaudited) | ||||||||||||
| Interest expense |
$ | (2,376 | ) | $ | (1,505 | ) | $ | (871 | ) | |||
Interest expense increased $0.9 million during the nine months ended September 30, 2015, which was primarily due a higher loan balance related to the CRG Term Loan obtained in June 2015.
Other Income (Expense), Net
| Nine Months Ended September 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| (unaudited) | ||||||||||||
| Other income (expense), net |
$ | (89 | ) | $ | 82 | $ | (171 | ) | ||||
87
Table of Contents
Index to Financial Statements
Other income (expense), net during the nine months ended September 30, 2015 consisted primarily of the change in fair value of our convertible preferred stock warrant liability prior to the Merger and foreign exchange loss related to customer’s deposits denominated in the Euro.
Comparison of the Years Ended December 31, 2014 and 2013
Revenue
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Product |
$ | 5,988 | $ | 2,253 | $ | 3,735 | ||||||
| Service |
411 | 12 | 399 | |||||||||
| Grant |
— | 894 | (894 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
$ | 6,399 | $ | 3,159 | $ | 3,240 | ||||||
|
|
|
|
|
|
|
|||||||
Total revenue in 2014 increased $3.2 million compared to 2013, primarily due to an increase in product revenue of $3.7 million and an increase in service revenue of $0.4 million, partially offset by a $0.9 million decrease in grant revenue due to expiration of the Grant Agreement with the State of Ohio in 2013.
Product Revenue. Product revenue in 2014 increased $3.7 million compared to 2013. This increase was due to the acceptance of a MRIdian system at each of University of California, Los Angeles and the University of Wisconsin—Madison in 2014, partially offset by the acceptance of a MRIdian system at Washington University in St. Louis in 2013.
Service Revenue. Service revenue in 2014 increased $0.4 million compared to 2013. This increase was due to maintenance service revenue recognized for the MRIdian system installed at Washington University in St. Louis in 2013 and the MRIdian system installed at each of University of California, Los Angeles and the University of Wisconsin—Madison in 2014.
Cost of Revenue
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Product cost of revenue |
$ | 8,176 | $ | 8,173 | $ | 3 | ||||||
| Service cost of revenue |
975 | 14 | 961 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cost of revenue |
$ | 9,151 | $ | 8,187 | $ | 964 | ||||||
|
|
|
|
|
|
|
|||||||
Product Cost of Revenue. The change in product cost of revenue in 2014 was insignificant compared to 2013. Product cost of revenue in 2013 consisted of $3.6 million in inventory costs related to customer acceptance of a MRIdian system at Washington University in St. Louis and LCM adjustments of $4.6 million. In 2014, product cost of revenue consisted primarily of the release of inventory costs of $5.6 million for accepted MRIdian systems at University of California, Los Angeles and University of Wisconsin—Madison, additional site preparation and installation costs incurred in 2014 of $2.0 million and LCM adjustments of $0.6 million. The LCM adjustments in 2014 decreased $4.0 million compared to 2013 as a result of increase in the selling prices of our MRIdian system and decrease in our product costs through a continued effort to improve product design and supply chain management.
88
Table of Contents
Index to Financial Statements
We currently expect that margins on current orders will continue this trend and show improvements from historic margins. We expect to achieve cost savings of approximately $1.0 million per current system from the initial MRIdian systems installed in 2013 and 2014. We believe that the combination of higher system prices and lower projected inventory costs as we increase our sales volume and leverage our supplier relationships will enable us to continue to improve our margins.
Service Cost of Revenue. Service cost of revenue in 2014 increased $0.9 million, compared to 2013. The increase in service cost of revenue was due to the provisioning of services for the MRIdian system installed at each of Washington University in St. Louis, University of California, Los Angeles and University of Wisconsin–Madison.
Operating Expenses
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Research and development |
$ | 9,404 | $ | 8,780 | $ | 624 | ||||||
| Selling and marketing |
4,681 | 3,781 | 900 | |||||||||
| General and administrative |
14,742 | 9,508 | 5,234 | |||||||||
| Total operating expenses |
$ | 28,827 | $ | 22,069 | $ | 6,758 | ||||||
Research and Development. Research and development expenses in 2014 increased $0.6 million, or 7%, compared to 2013. This increase was primarily attributable to a $0.7 million increase in project material costs and a $0.2 million increase in travel expenses, offset by a $0.2 million decrease in grant project contractor expense due to the expiration of the State of Ohio grant in April 2013.
Selling and Marketing. Selling and marketing expenses in 2014 increased $0.9 million, or 24%, compared to 2013. This increase was primarily attributable to a $0.3 million increase in travel-related expenses to promote international sales, a $0.2 million increase in trade show expenses, a $0.2 million increase in public relations and website redesign expenses and a $0.1 million increase in marketing consultant expenses.
General and Administrative. General and administrative expenses in 2014 increased $5.2 million, or 55%, compared to 2013. This increase was primarily attributable to a $1.3 million increase in accounting and legal fees, a $1.2 million increase in travel expenses, a $1.2 million increase in personnel and related costs as a result of higher employee headcount, and a $1.0 million increase in regulatory consulting expenses. The change was also attributable to a $0.5 million increase in rent and facility expenses due to our new office lease in Mountain View, California, which commenced during the third quarter of 2014.
Interest Expense
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Interest expense |
$ | (2,243 | ) | $ | (97 | ) | $ | (2,146 | ) | |||
Interest expense increased $2.1 million in 2014, which was primarily due to the long-term debt we incurred in December 2013.
89
Table of Contents
Index to Financial Statements
Other Income (Expense), Net
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Other income (expense), net |
$ | 21 | $ | (32 | ) | $ | 53 | |||||
Other income (expense), net in 2014 consisted primarily of the change in fair value of our convertible preferred stock warrant liability.
Liquidity and Capital Resources
Since ViewRay’s inception in 2004 as a Florida corporation, we have incurred significant net losses and negative cash flows from operations. During 2014 and the nine months ended September 30, 2015, we had net losses of $33.8 million and $30.9 million, respectively. At December 31, 2014 and September 30, 2015, we had an accumulated deficit of $152.0 million and $182.9 million, respectively.
At December 31, 2014 and September 30, 2015, we had cash and cash equivalents of $11.1 million and $32.1 million, respectively. To date, we have financed our operations principally through private placements of ViewRay’s common stock, private placements of ViewRay’s convertible preferred stock, issuances of convertible promissory notes, issuances of term loans and receipts of customer deposits for new orders and payments from customers for systems installed. We expect that our existing cash and cash equivalents, together with cash receipts from sales of MRIdian systems, the net proceeds from the Private Placement and the additional available draw down from the CRG Term Loan, will enable us to conduct our planned operations for at least the next 12 months.
We could potentially use our available financial resources sooner than we currently expect, and we may incur additional indebtedness to meet future financing needs. Adequate additional funding may not be available to us on acceptable terms or at all. In addition, although we anticipate being able to obtain additional financing through non-dilutive means, we may be unable to do so. Our failure to raise capital as and when needed could have significant negative consequences for our business, financial condition and results of operations. Our future capital requirements and the adequacy of available funds will depend on many factors, including those set forth in the section titled “Risk Factors.”
The following table summarizes our cash flows for the periods presented:
| Nine Months Ended September 30, | Year Ended December 31, | |||||||||||||||
| 2015 | 2014 | 2014 | 2013 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Cash used in operating activities |
$ | (31,305 | ) | $ | (27,207 | ) | $ | (27,469 | ) | $ | (25,371 | ) | ||||
| Cash used in investing activities |
(2,406 | ) | (1,497 | ) | (2,603 | ) | (1,593 | ) | ||||||||
| Cash provided by (used in) financing activities |
54,646 | 3,888 | 14,672 | 49,395 | ||||||||||||
Operating Activities
We have historically experienced negative cash outflows as we developed MRIdian and continued to expand our business. Our net cash used in operating activities primarily results from our net loss adjusted for non-cash expenses and changes in working capital components as we have grown our business, and is influenced by the timing of cash payments for inventory purchase and cash receipts from our customers. Our primary source of cash flow from operating activities is cash receipts from customers including sales of MRIdian systems and, to a lesser extent, by up-front payments from customers. Our primary uses of cash from operating activities are amounts due to vendors for purchased components and employee-related expenditures. Our cash flows from operating activities will continue to be affected principally by our working capital requirements and the extent to which we collect cash receipts from our customers, build up our inventory balances, increase spending on personnel and other operating activities as our business grows.
90
Table of Contents
Index to Financial Statements
During the nine months ended September 30, 2015, operating activities used $31.3 million in cash, primarily as a result of our net loss of $30.9 million and $6.3 million net change in our operating assets and liabilities, partially offset by aggregate non-cash charges of $5.9 million. The net change in our operating assets and liabilities was primarily the result of decrease in accounts payable, purchase of inventory and making prepaid payments on inventory components, offset by increase in customer deposits and deferred revenue, and accounts receivable. The $6.0 million decrease in accounts payable was the result of timing of payments as a result of the growth in our business. The $2.4 million increase in inventory and $2.2 million increase in deposits on purchased inventory were due to installations of MRIdian systems. The decrease in our operating assets and liabilities was partially offset by $3.6 million increase in customer deposits and deferred revenue primarily due to new sales contracts entered, and $0.9 million cash receipts from accounts receivable during the nine months ended September 30, 2015. Non-cash charges primarily included $2.9 million write-off of deferred offering cost, $1.0 million inventory lower of cost or market charges related to expected MRIdian system installation in Korea, $0.9 million of depreciation and amortization charges, $0.6 million for amortization of debt discount and accrued interest related to our debt incurred in December 2013 and June 2015, and $0.5 million for stock-based compensation.
During the nine months ended September 30, 2014, operating activities used $27.2 million in cash, primarily as a result of our net loss of $24.2 million and a $4.7 million net change in our operating assets and liabilities, which was partially offset by aggregate non-cash charges of $1.7 million. The net change in our operating assets and liabilities was primarily the result of higher accounts receivables balances and increased purchases of inventory, offset by higher customer deposits and increases in accrued expenses and other long-term liabilities. The increase in accounts receivable of $2.0 million and customer deposits of $2.1 million was primarily due to revenue and new sales order growth in the nine months ended September 30, 2014. The increase in inventory of $3.0 million and deposits on purchased components of $1.4 million was primarily due to installations of MRIdian systems. The decrease of $0.6 million in accrued expenses and other long-term liabilities was mainly due to $1.5 million payment of our accrued purchase commitments, partially offset by a $0.9 million increase in accrued expenses and other long-term liabilities attributable to higher accrued personnel costs due to growth in headcount, higher accrued sales tax and medical device exercise tax liabilities, and increase in accrued interest as a result of debt incurred in December 2013. Non-cash charges primarily included $0.7 million for depreciation and amortization, $0.3 million in stock-based compensation and $0.6 million of LCM adjustments related to the reduction of the carrying value of inventory to its net realizable value.
During 2014, operating activities used $27.5 million in cash, primarily as a result of our net loss of $33.8 million offset by $4.0 million net change in our operating assets and liabilities, and aggregate non-cash charges of $2.3 million. The net change in our operating assets and liabilities was primarily the result of higher accounts payable, customer deposits and deferred revenue balances, offset by higher deferred costs and accounts receivable balances, increased purchases of inventory, and decreases in accrued expenses and other long-term liabilities. The increase in accounts payable of $3.2 million was due to timing of payments as a result of the growth in our business. The increase in accounts receivable of $0.7 million and the increase in customer deposits and deferred revenue of $10.1 million was primarily due to revenue and new sales order growth in 2014. These changes were offset by a $4.7 million increase in deferred costs and a $3.8 million increase in inventory and deposits on purchased inventory due to installations of MRIdian systems. The decrease of $0.2 million in accrued expenses and other long-term liabilities was mainly due to $1.5 million payment of our accrued purchase commitments, offset by a $1.3 million increase in accrued expenses and other long-term liabilities attributable to higher accrued personnel costs due to growth in headcount, higher accrued sales tax and medical device excise tax liabilities, and increase in accrued interest as a result of debt incurred in December 2013. Non-cash charges primarily included $1.0 million for depreciation and amortization, $0.3 million for stock-based compensation, $0.6 million of LCM adjustments related to the reduction of the carrying value of inventory to its net realizable value and $0.4 million for amortization of debt discount and accrued interest related to our 2014 convertible promissory notes and debt incurred in December 2013.
91
Table of Contents
Index to Financial Statements
During 2013, operating activities used $25.4 million in cash, primarily as a result of our net loss of $27.2 million and a $4.3 million net change in our operating assets and liabilities, which was partially offset by aggregate non-cash charges of $6.1 million. The net change in our operating assets and liabilities was primarily the result of increased purchases of inventory and lower accounts payable, offset by increase in accrued expenses and other long-term liabilities. The increase in inventory of $5.2 million and deposits on purchased components of $0.9 million was mainly due to the installation of MRIdian systems. The decrease in accounts payable of $0.7 million was due to timing differences in making payments when compared to December 31, 2012. These changes were offset by a $2.1 million increase in accrued expenses and other long-term liabilities attributable to higher accrued personnel costs due to growth in headcount. Non-cash charges primarily included $1.1 million for depreciation and amortization and $4.6 million of LCM adjustments related to the reduction of the carrying value of inventory to its net realizable value.
Investing Activities
Cash used in investing activities during the nine months ended September 30, 2015 of $2.4 million primarily resulted from capital expenditures to purchase property and equipment.
Cash used in investing activities during 2014 of $2.6 million primarily resulted from capital expenditures to purchase property and equipment of $2.0 million and an increase in restricted cash of $0.6 million.
Cash used in investing activities during 2013 of $1.6 million primarily resulted from capital expenditures to purchase property and equipment of $1.2 million and to purchase an intellectual property license of $0.5 million.
Financing Activities
On June 26, 2015, we entered into a Term Loan Agreement, or the Term Loan, with Capital Royalty Partners II L.P and Parallel Investment Opportunities Partners II L.P., or together, CRG, for up to $50.0 million of which $30.0 million was made available to us upon closing with the remaining $20.0 million to be available on or before June 26, 2016 at our option the occurrence of either (i) an initial public offering of our common stock on a nationally recognized securities exchange that raises a minimum of $40.0 million in net cash proceeds with a minimum of $120.0 million post-money valuation, or Qualifying IPO, or (ii) the achievement of a minimum of $25.0 million gross revenue from the sales of the MRIdian system during any consecutive 12 months before March 31, 2016. We drew down the first $30.0 million on closing date. The Term Loan has a maturity date of June 26, 2020 (i.e., 5 years) and bears cash interest at a rate of 12.50% per annum to be paid quarterly during the first 3 years, or the interest-payment-only period. The interest-payment-only period can be extended for another year till June 26, 2019 if we complete a Qualifying IPO on or before June 26, 2018. During the interest-payment-only period, we have the option to elect to pay only 8% of the 12.5% per annum interest in cash, and the remaining 4.5% of the 12.5% per annum interest as compounded interest, or deferred payment in-kind interest, added to the aggregate principal amount of the Term Loan. Principal payment and any deferred payment in-kind interest will be paid quarterly in equal installments following the end of the interest-payment-only period through maturity date.
The Term Loan is subject to a prepayment penalty of 3% on the outstanding balance during the first 12 months following the funding of the Term Loan, 2% on the outstanding balance after year 1 but on or before year 2, 1% on the outstanding balance after year 2 but on or before year 3, and 0% on the outstanding loan if prepaid after year 3 thereafter until maturity. The Term Loan is also subject to a facility fee of 5% based on the sum of the Term Loan drawn and any outstanding payment in-kind payable on maturity date or the date such Term Loan becomes due for whatever reason. All direct financing costs were accounted for as a discount on the Term Loan and will be amortized to interest expense during the life of the Term Loan using the effective interest method. The Term Loan is subject to financial covenants and is collateralized by essentially all our assets and limits the Company’s ability with respect to additional indebtedness, investments or dividends, among other things, subject to customary exceptions.
92
Table of Contents
Index to Financial Statements
On June 26, 2015, the Company paid off in full the $15.0 million outstanding term debt with Hercules using part of the proceeds received from the CRG Term Loan.
During the nine months ended September 30, 2015, financing activities provided $54.6 million in cash primarily from the net proceeds of $27.4 million related to the draw-down of long-term debt, net of debt discount, $27.1 million from the net proceeds from the Private Placement and $15.7 million from proceeds from issuance of Series C convertible preferred stock, which was partially offset by $15.0 million payments of term loan and $0.6 million payment of costs related to the initial public offering.
During 2014, financing activities provided $14.7 million in cash primarily from the net proceeds related to the issuance of convertible promissory notes of $9.9 million and net proceeds from the issuance of convertible preferred stock of $5.0 million.
During 2013, financing activities provided $49.4 million in cash from the net proceeds related to the issuance of convertible preferred stock of $34.9 million, net of issuance costs, and net proceeds of $14.5 million related to the term loan we entered into with Hercules in December 2013.
Off-Balance Sheet Arrangements
During the nine months ended September 30, 2015 and December 31, 2014, we did not have any off-balance sheet arrangements as defined by applicable SEC regulations.
Critical Accounting Policies and Estimates
Our condensed consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these condensed consolidated financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, expenses and related disclosures. We evaluate our estimates and assumptions on an ongoing basis. Our estimates are based on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Our actual results could differ from these estimates.
We believe that the assumptions and estimates have the greatest potential impact on our condensed consolidated financial statements. Therefore, we consider these to be our critical accounting policies and estimates. For further information on all of our significant accounting policies, see the notes to our condensed consolidated financial statements.
Revenue Recognition
Revenue recognition for systems that we install generally occurs when the customer acknowledges that the system operates in accordance with our standard product specifications, the customer accepts the installed unit and we transfer title and risk of loss to the customer. Service revenue is recognized on a straight-line basis over the term during which contracted services are provided. We use judgment to estimate revenue allocations from sales arrangements with multiple deliverables between the product and service revenue. In situations where a deliverable in a multi-element arrangement has a value to the customer on a stand-alone basis, we are required to allocate the fair value of the various elements based on the selling price of each element. The principal deliverables consist of (i) sale of MRIdian systems, which generally includes installation, site preparation and software, and (ii) product support, which includes extended service and maintenance. We determine selling prices using vendor specific objective evidence, or VSOE, if it exists, or third-party evidence, or TPE. If neither VSOE or TPE exists for a deliverable, we use best estimated selling price, or BESP. We allocate revenue to multiple elements generally using the relative fair values as determined by BESP. We regularly review VSOE, TPE and BESP for all of our products and services.
93
Table of Contents
Index to Financial Statements
We also receive payments for cost reimbursement of allowable expenditures and payments for the achievement of certain milestones under government grants in return for qualifying property and equity purchases and research and development activities over a contractually defined period. These payments are nonrefundable. Government grants generally provide us with fixed payments and a contractually defined period of research. Grant revenues are recognized as associated expenses incurred and are billed to grantors in conjunction with the terms of the grants.
Stock-Based Compensation
Stock-based compensation expense is measured and recognized in the condensed consolidated financial statements based on the fair value of the awards granted. The fair value of each option award is estimated on the grant date using the Black-Scholes option-pricing model. Stock-based compensation expense is recognized, net of forfeitures, over the requisite service periods of the awards, which is generally four years. At September 30, 2015, total unrecognized compensation cost related to stock-based awards granted to employees, net of estimated forfeitures, was $5.6 million which is expected to be recognized over a weighted-average period of 3.5 years.
Our use of the Black-Scholes option-pricing model requires the input of highly subjective assumptions, including expected term of the option, expected volatility of the price of our common stock, risk-free interest rates and the expected dividend yield of our common stock. The assumptions used in our option-pricing model represent management’s best estimates. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, our stock-based compensation expense could be materially different in the future.
Common Stock Warrant
In December 2013 in connection with the Term Loan, we issued a warrant to Hercules to purchase 128,231 shares of our preferred stock with an exercise price of $5.85 per share, subject to certain adjustments, which were converted to warrant to purchase our common stock upon the closing of the Merger in July 2015. This warrant is exercisable in whole or in part at any time prior to the expiration date of the warrant, which is the later of (i) December 16, 2023 and (ii) the date that is five years following the effective date of the registration statement of an initial underwritten public offering of our common stock.
Prior to the Merger, the preferred stock warrant is recorded as preferred stock warrant liability and adjusted to fair value at each balance sheet date, with the change in fair value being recorded as a component of other income (expense), net in the condensed consolidated statements of operations.
Upon the closing of the Merger on July 23, 2015, all shares of Series C convertible preferred stock were converted into common stock, and the warrant to purchase Series C convertible preferred stock was converted into the warrant to purchase 128,231 shares of our common stock. Fair value of these warrants at the closing date were transferred into common stock additional paid-in capital, and we ceased to adjust the fair value of the converted common stock warrants.
Related to the Private Placement, the Company issued 198,760 shares of common stock warrants at an exercise price of $5.00 per share to private placement agents as payment for services provided in July and August 2015. These placement agent warrants are exercisable at any time at the option of the holder until the five year anniversary of its date of issuance. The number of shares of common stock issuable upon the exercise of each placement warrant is adjustable in the event of certain stock dividends, stock splits, combinations of shares and similar transactions. Upon exercise, the aggregate exercise price of the warrants issued are payable by the holders in cash.
94
Table of Contents
Index to Financial Statements
Inventory Valuation
Inventory consists primarily of purchased components for assembling MRIdian systems and other direct costs associated with MRIdian system installation. Inventory is stated at the lower of cost (on a first-in, first-out basis) or market value. When the net realizable value of the inventory is lower than related costs, we reduce the carrying value of the inventory for the difference while recording a corresponding charge to cost of product revenues. The assumptions we used in estimating the net realizable value of the inventory primarily include the total cost to complete the applicable MRIdian system. We recorded an inventory lower of cost and market adjustment of $1.0 million and $0.6 million during the nine months ended September 30, 2015 and 2014, respectively.
Prior to January 1, 2015, our inventory cost is measured on a first-in, first-out basis through specific identification. To support the increasing MRIdian system installations and inventory purchase activities, starting January 1, 2015, we elected to change inventory cost measurement to weighted average basis. The accounting principle change does not have an impact on prior periods’ financial statements, therefore no retrospective adjustment is required. The accounting principle change does not have an impact on product cost of revenue or net loss for the three months and nine months ended September 30, 2015.
Income Taxes
We are subject to income taxes in the United States, and we use estimates in determining our provision for income taxes. We use the asset and liability method of accounting for income taxes. Under this method, we calculate deferred tax asset or liability account balances at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect our taxable income.
We estimate actual current tax exposure together with assessing temporary differences resulting from differences in accounting for reporting purposes and tax purposes for certain items, such as accruals and allowances not currently deductible for tax purposes. These temporary differences result in deferred tax assets and liabilities, which are included in our condensed consolidated balance sheets. In general, deferred tax assets represent future tax benefits to be received when certain expenses previously recognized in our condensed consolidated statements of operations and comprehensive loss become deductible expenses under applicable income tax laws or when net operating loss or credit carryforwards are utilized. Accordingly, realization of our deferred tax assets is dependent on future taxable income against which these deductions, losses and credit carryforwards can be utilized.
We assess the likelihood that our deferred tax assets will be recovered from future taxable income, and to the extent we believe that recovery is not likely, establish a valuation allowance. At September 30, 2015 and December 31, 2014, we have a full valuation allowance set up for our net deferred tax assets.
Under federal and similar state tax statutes, changes in our ownership, including ownership changes resulting from the Merger, may limit our ability to use our available net operating loss and tax credit carryforwards. The annual limitation, as a result of a change of ownership, may result in the expiration of net operating losses and credits before utilization. We believe we have experienced at least one ownership change in the past. We are currently analyzing the tax impact of such ownership change on our federal NOLs and credit carryforwards. Our ability to use our remaining net operating loss carryforwards may be further limited if we experience an ownership change or as a result of future changes in our stock ownership.
JOBS Act Accounting Election
We are an “emerging growth company” within the meaning of the JOBS Act. Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would
95
Table of Contents
Index to Financial Statements
otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies that are not emerging growth companies.
Recently Issued and Adopted Accounting Pronouncements
We review new accounting standards to determine the expected financial impact, if any, that the adoption of each such standard will have. For the recently issued accounting standards that we believe may have an impact on our condensed consolidated financial statements, see the section entitled “Notes to Condensed Consolidated Financial Statements—Note 2—Summary of Significant Accounting Policies” in the condensed consolidated financial statement.
96
Table of Contents
Index to Financial Statements
Corporate Information
We were incorporated in Nevada as Mirax Corp. on September 6, 2013, and reincorporated in Delaware as ViewRay, Inc. on July 21, 2015. Upon the closing of the Merger, we discontinued our pre-Merger business and acquired the business of ViewRay and will continue the existing business operations of ViewRay. ViewRay commenced operations as a Florida corporation in 2004, subsequently reincorporated in Delaware in in 2007, and changed its name to ViewRay Technologies, Inc. in July 2015.
Our authorized capital stock currently consists of 300,000,000 shares of common stock, and 10,000,000 shares of the preferred stock. Our common stock is quoted on the OTC Markets (OTCQB) under the symbol “VRAY,” which changed from “MRXC” on July 20, 2015.
Our principal executive offices are located at 2 Thermo Fisher Way, Oakwood Village, Ohio 44146. Our telephone number is (440) 703-3210. Our website address is www.viewray.com. (The information contained on, or that can be accessed through, our website is not a part of this prospectus.)
Company Overview
We design, manufacture and market MRIdian, the first and only MRI-guided radiation therapy system that images and treats cancer patients simultaneously. MRI is a broadly used imaging tool which has the ability to differentiate between types of soft tissue clearly, unlike X-ray or computed tomography, or CT, the most commonly used imaging technologies in radiation therapy today. MRIdian integrates MRI technology, radiation delivery and our proprietary software to locate, target and track the position and shape of soft-tissue tumors while radiation is delivered. These capabilities allow MRIdian to deliver radiation to the tumor accurately while delivering less radiation to healthy tissue than existing radiation therapy treatments. We believe this innovation leads to improved patient outcomes and reduced side effects from off-target radiation delivery. We received 510(k) marketing clearance from the U.S. Food and Drug Administration, or FDA, for MRIdian in May 2012 and received permission to affix the CE mark in November 2014. Patients are actively receiving treatment on MRIdian systems at three cancer centers.
Cancer is a leading cause of death globally and the second leading cause of death in the United States. Radiation therapy is a common method used to treat cancer that uses lethal doses of ionizing energy to damage genetic material in cells. Nearly two-thirds of all treated cancer patients in the United States will receive some form of radiation therapy during the course of their illness, according to estimates by the American Society for Radiation Oncology, or ASTRO. In 2013, IMV Medical Information Division, Inc., or IMV, reported that 93% of patients receiving radiation therapy in the United States were treated by a linear accelerator, or linac. The global linac market was $2.8 billion in 2011 and was expected to grow to $3.7 billion by 2016 according to a 2012 Markets and Markets report. IAEA Human Health Campus reported that there are over 11,000 linacs installed at over 7,500 centers worldwide. We believe the addressable market for MRIdian is the annual market for linacs due to MRIdian’s ability to treat a broad spectrum of disease sites. However, we believe that MRIdian may be used more frequently for complex cancer cases that may be difficult to treat on a standard linac due to the location of the tumor in relation to the surrounding soft tissues. We currently estimate the annual market for linacs to be 1,100 units per year globally, the majority of which are replacement units.
Despite the prevalence of MRI for diagnostic purposes and its ability to image soft tissue clearly, the radiation therapy industry has been unable to integrate MRI into external-beam radiation therapy systems. Existing radiation therapy systems use X-ray-based imaging technologies, such as CT, which cannot differentiate between types of soft tissue or provide an accurate visualization of a tumor and its position in relation to critical organs. In addition, existing systems that offer imaging during the course of a treatment are limited by the rate at which they can image due to the level of radiation to which they expose the patient. These constraints make it difficult for a
97
Table of Contents
Index to Financial Statements
clinician to locate a tumor accurately, track its motion in real-time or adapt treatment as anatomy changes. It is very difficult to irradiate a tumor while minimizing the amount of radiation hitting critical organs without the ability to see the tumor’s exact location and shape. If a tumor is insufficiently irradiated, it may not respond to treatment, resulting in a lower probability of survival for the patient. If organs and other healthy soft tissues are irradiated, side effects can be severe, including organ failure and secondary cancers.
MRIdian is a next-generation, radiation therapy solution that enables treatment and real-time imaging of a patient’s anatomy simultaneously. The high-quality images that it generates clearly differentiate the targeted tumor, surrounding soft tissue and nearby critical organs. MRIdian also records the level of radiation dose that the treatment area has received, enabling physicians to adapt the prescription between treatments as needed. We believe this improved visualization and accurate dose recording will enable better treatment, improve patient outcomes and reduce side effects. Key benefits to users and patients include improved imaging and patient alignment, on-table adaptive treatment planning, motion management and an accurate recording of the delivered radiation dose. Physicians have already used MRIdian to treat a broad spectrum of radiation therapy patients with more than 20 different types of cancer, as well as patients for whom radiation therapy was previously not an option.
We currently market MRIdian through a direct sales force in the United States and distributors in the rest of the world. At September 30, 2015, we had four MRIdian systems installed and had 14 signed orders for new systems for a backlog value of $78.0 million. We generated revenue of $5.8 million and $5.9 million during the nine months ended September 30, 2015 and 2014 and had net losses of $30.9 million and $24.2 million during the nine months ended September 30, 2015 and 2014. We generated revenue of $3.2 million in 2013 and $6.4 million in 2014 and had net losses of $27.2 million in 2013 and $33.8 million in 2014.
Cancer and Radiation Therapy Market
Incidence of Cancer
Cancer is a leading cause of death globally and the second leading cause of death in the United States behind cardiovascular disease. According to the American Cancer Society, approximately 1.6 million people were expected to be diagnosed with cancer in the United States during 2014 and approximately 0.6 million were expected to die from cancer, accounting for nearly one of every four deaths. As a result of a growing and aging population, The World Health Organization’s (the “WHO”), Global Initiative for Cancer Registry Development estimates that the number of new cancer cases worldwide will grow from 14.1 million in 2008 to 19.3 million in 2025.
Cancer Therapy
The primary goal of cancer therapy is to kill cancerous tissues while minimizing damage to healthy tissues. There are three main ways to treat cancer: surgery, chemotherapy and radiation. Surgery attempts to physically remove the tumor from the body, while minimizing trauma to healthy tissue and preventing the spread or translocation of the disease to other parts of the body. Surgery is particularly effective because the surgeon can directly observe the tumor and surrounding healthy tissue throughout the course of the procedure and adapt his or her plan mid-procedure accordingly. Chemotherapy uses drugs to kill cancer cells. Unlike surgery, most forms of chemotherapy circulate systemically to reach cancer cells almost anywhere in the body. Chemotherapy is most effective at destroying microscopic levels of disease. Radiation is used to damage genetic material in cells with a lethal dose of ionizing energy. Effective radiation therapy balances destroying cancer cells with minimizing damage to normal cells. It can be used at high doses to ablate a tumor, an effect similar to surgery, or at moderate doses to target local microscopic disease, as is done with chemotherapy. Other, more recently developed ways of treating cancer include hormone therapy and targeted therapy, such as immunotherapy.
98
Table of Contents
Index to Financial Statements
Radiation Therapy
Radiation therapy has become widespread, with nearly two-thirds of all treated cancer patients in the United States receiving some form of radiation therapy during the course of their treatment, according to estimates by ASTRO. For most cancer types treated with radiation therapy, at least 75% of the patients are treated with the intent to cure the cancer. For lung and brain cancers, that number is somewhat lower, with 59% of lung cancer patients and 50% of brain cancer patients being treated with the goal of curing cancer. The remainder of cases are treated with palliative intent to relieve pain. Radiation therapy is a non-invasive outpatient procedure with little or no recovery time and can be used on patients who are inoperable. According to IMV, 93% of patients receiving radiation therapy in the United States are treated using a linac.
Radiation is used to kill cancer cells primarily by damaging their DNA, but can also kill healthy cells in the same way or cause them to become cancerous themselves. As a result, the goal of curative radiation therapy is to balance delivery of a sufficiently high dose of radiation to a tumor to kill the cancer cells while, at the same time, minimizing damage to healthy cells, particularly those in critical organs. Normal cells are better able to repair themselves after radiation than tumor cells, so doses of radiation are often fractionated, or delivered in separate sessions with rest periods in between. As a result, standard radiation therapy is often given once a day, five times a week, for one to seven weeks. In 2012, patients made an estimated 20.9 million radiation therapy treatment visits in the United States.
Radiation Therapy Equipment Market
The global linac market was $2.8 billion in 2011 and was expected to grow to $3.7 billion by 2016 according to a 2012 Markets and Markets report. According to IAEA Human Health Campus, there are 11,000 linacs installed at over 7,500 centers worldwide. In the United States, there are 3,800 linacs installed at over 2,500 centers. The annual market for linacs is estimated to be 1,100 units per year globally, the majority of which are replacement units.
In the radiation therapy market, new technologies have historically been adopted at a rapid rate. According to IMV, the percentage of centers performing intensity modulated radiation therapy, or IMRT, grew from 30% in 2002 to 96% in 2012. The percentage of sites utilizing image guided radiation therapy, or IGRT, grew more quickly: from 15% in 2004 to 83% in 2012. The majority of IGRT uses on-board X-ray systems. As leading cancer centers adopt and study MRI-guided radiation therapy, we believe that our next-generation system will also follow a rapid adoption curve among the broader linac replacement market.
Radiation Therapy Treatment Process
Following diagnosis of the disease state, radiation treatment generally consists of the following steps.
| • | Imaging and tumor contouring. To design the treatment plan, physicians obtain initial images of the tumor. This is done most commonly using a CT scan, often supplemented by an MRI, a positron emission tomography, or PET, scan, or both. These images, also known as simulation scans, are then imported into a treatment planning software system and aligned to each other. Based on clinical experience, a physician will manually draw, or contour, specific areas on the aligned images to characterize the location and extent of the tumor highlighting the following: |
| • | Gross tumor volume, or GTV, a volumetric region encompassing the visible tumor. |
| • | Clinical target volume, or CTV, a larger area encompassing the GTV, where the cancer may have already or may be likely to spread. |
| • | Planning target volume, or PTV, a further enlarged area to allow for inexact imaging, patient movement during treatment or tumor movement between planning and treatment. The PTV may be sized multiple times larger than the CTV, risking radiation damage to healthy tissue, including in many cases critical organs. |
99
Table of Contents
Index to Financial Statements
| • | Treatment planning and dose prescription. Once the clinician has a three-dimensional map of the tumor, surrounding healthy tissues and nearby critical organs, a physician determines a treatment plan using one of the methods below. Creation of these plans typically takes one to two weeks. A typical curative radiation therapy treatment dose will be delivered over the course of several weeks with 10 to 35 radiation therapy sessions, referred to as fractions, lasting from a few minutes to an hour or more depending on the treatment plan. |
| • | 3D-CRT planning. Using a method called three-dimensional conformal radiation therapy, or 3D-CRT, a clinician will decide what beam angles and shapes to use to target a tumor and how long each beam will irradiate it. A computer will then calculate the potential dose delivered, and a clinician will manually adjust the plan to arrive at an acceptable dose. |
| • | IMRT planning. Using a method called intensity modulated radiation therapy, or IMRT, a physician will use computer software to optimize a treatment plan to achieve a more precise dose distribution than 3D-CRT by using thousands of beamlets, IMRT has been shown to result in better patient outcomes than 3D-CRT. |
| • | SRS and SBRT planning. Stereotactic radiosurgery, or SRS, and stereotactic body radiation therapy, or SBRT, are methods of delivery using 3D-CRT or IMRT designed to deliver high doses of precisely targeted radiation in a reduced number of sessions, usually one to five fractions. SRS is used in brain and spine applications, and has been shown to be particularly effective in those areas, while SBRT is used in the rest of the body, and has been shown to be particularly effective in early-stage lung cancer. |
| • | Alignment. Prior to radiation delivery, clinicians typically take images to assist with patient alignment. Most systems use a form of on-board CT called cone-beam CT to image, which delivers inferior contrast and a higher radiation dose than diagnostic CT. A less commonly used imaging technology is fluoroscopy, a real-time 2D X-ray system that can expose a patient to even higher doses of radiation than cone-beam CT. Because of the limited soft tissue contrast of X-ray-based imaging, clinicians often use registration markers such as nearby bone structures or surgically implanted fiducial markers to align patients with the treatment beams. Patients may also be immobilized by restraining devices, or techniques such as respiratory control or abdominal compression, which are employed to minimize motion due to breathing. To track breathing and other body motions during treatment, specific trackers may be used, also known as 4D radiation therapy. Use of any image or registration marker to help with alignment is called image-guided radiation therapy, or IGRT. |
| • | Delivery. Based on alignment with these images, markers or other radiation therapy trackers, treatment begins and radiation is delivered to the patient. In some cases, additional 2D X-ray images are taken intermittently or registration makers are monitored during treatment to try to account for tumor movement. |
| • | Review. After a treatment session, a physician will review the delivered treatment to ensure that it is proceeding according to plan. Currently, there are no methods to record the actual dose that was delivered to the tumor and nearby critical structures. In those rare occasions when a physician is able to observe changes in the size or shape of a tumor, he or she may decide to adjust the treatment plan. However, revising a treatment plan may take several days and delays treatment. |
Limitations of Radiation Therapy
Limitations with radiation therapy arise as a result of imaging technologies that make accurate visualization of a tumor and its relation to critical organs difficult or impossible during treatment. As a result, we believe treatments are not as effective or safe as they could be.
| • | Inability to accurately locate a tumor for treatment alignment. To locate a tumor, current radiation therapy systems rely on on-table CT scans which are unable to differentiate between types of soft |
100
Table of Contents
Index to Financial Statements
| tissue. Therefore, surrogate registration markers, including existing bone structures, external marks and surgically implanted fiducials, are frequently used to align a patient to the treatment beams prior to commencing treatment. |
Comparison of On-Table CT Images to On-Table MRIdian Images
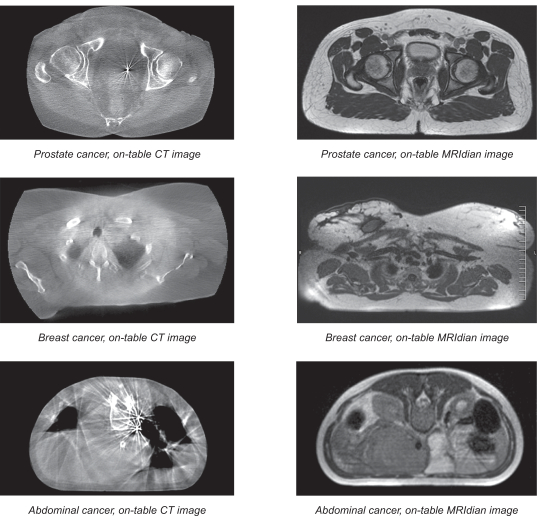
The spatial relationship between the tumors, particularly those in soft tissue, and registration markers is likely to change between initial imaging and the first treatment session. By relying on a proxy for tumor location rather than the tumor itself, clinicians risk missing the tumor when they deliver treatment beams into a patient’s body. Furthermore, fiducial markers can migrate inside the body, are unable to track changes in the tumor shape, may interfere with imaging, are invasive, require time to heal and have a high incidence of side effects and complications.
| • | Inability to adapt treatment on table. A physician designs a treatment plan and dose prescription based on images that are captured at the beginning of therapy. Creating a treatment plan can take one to two weeks, and treatment itself can take up to seven weeks. However, during the course of therapy, tumors often change size, orientation or shape and patient anatomy can change for reasons such as weight loss or gain. Adjusting for these changes would require replanning which may take several days and is resource intensive. In addition, due to limitations in imaging technologies, physicians may be unaware |
101
Table of Contents
Index to Financial Statements
| of changes in the tumor and surrounding anatomy and continue to dose according to the original treatment plan. As a result of these limitations, replanning is infrequently performed. |
| • | Inability to track tumor and organ motion accurately. In addition to difficulty locating a tumor accurately in a patient’s body, a further challenge is accounting for ongoing tumor movement during treatment. Tumors have been shown to move multiple centimeters relative to surrogate registration markers over the course of only a few seconds. Although physicians use internal markers and external cameras and blocks to track respiratory and other motion, they are unable to track the tumor itself and its location relative to other soft tissues. This limitation increases the probability of missing the targeted treatment area. As a result, physicians usually enlarge the total region to be irradiated, causing an additional risk of side effects. |
| • | Inability to record cumulative radiation delivered. In order to determine treatment effectiveness, it is important to track how much radiation has been delivered to a tumor or surrounding healthy tissue. Currently, there are no methods to record the actual dose of radiation that was delivered to the tumor and nearby critical structures. Therefore, physicians must assume that the radiation is delivered according to plan, rather than making decisions based on actual dose delivered. |
Each of these limitations increases the risk of missing a tumor and hitting healthy tissue during treatment. If a tumor is insufficiently irradiated, it may not respond to treatment, resulting in a lower probability of survival for the patient. The ability to avoid irradiating healthy tissue has been shown to reduce side effects. If healthy tissues, particularly critical organs, are irradiated, the side effects can be severe, including scarring of lung tissue, fibrosis and cardiotoxicity in lung and breast cancers, incontinence and sexual dysfunction in pelvic and prostate cancers, infertility in pediatric cancers, memory loss, seizures and necrosis in brain cancer and secondary cancers.
Although MR technology is an imaging tool broadly used to differentiate between types of soft tissue in diagnostic settings, to date such technology has not been used with radiation therapy because the magnetic field generated by an MRI interferes with the linac’s ability to accelerate electrons and the linac produces radio frequencies that distort the MR images. Current forms of CT have improved over time, but issues with radiation dose and image quality limit the utility of these technologies. Fluoroscopy and cone-beam CT involve the use of X-rays, a form of ionizing radiation, and pose an increased risk of radiation-induced cancer to the patient.
Our Solution
We have developed MRIdian to address the key limitations of existing external-beam radiation therapy technologies. MRIdian employs MRI-based technology to provide real-time imaging that clearly defines the targeted tumor from the surrounding soft tissue and other critical organs during radiation treatment. MRIdian allows physicians to record the level of radiation exposure that the tumor has received and adapt the prescription between fractions as needed. We believe this combination of enhanced visualization and accurate dose recording will significantly improve the safety and efficacy of radiation therapy, leading to better outcomes for patients.
We believe that MRIdian provides the following clinical and commercial benefits to physicians, hospitals and patients:
| • | Improved tumor visibility and patient alignment. The soft-tissue contrast of MRidian’s on-board MRI enables clinicians to locate, target and track the tumor and healthy tissues and accurately align a patient to the treatment beams without the use of X-ray, CT or surrogate registration markers. If the clinician prefers, the software has the ability to automatically map the patient’s soft tissue anatomy each treatment session in less than one minute, and MRIdian can use that information to automatically align the patient. |
| • | On-table adaptive planning. Due to changing anatomy the clinician may be unable to obtain an optimal match between the patient on the table and the treatment plan. Using an MR image captured at |
102
Table of Contents
Index to Financial Statements
| the beginning of each therapy session, MRIdian automatically maps the patients’ soft tissue anatomy in 3D and calculates the dose that would be delivered using the current treatment plan. If the prescribed treatment is not clinically acceptable to the physician, MRIdian has the ability to automatically recalculate and adapt the plan to changing anatomy at the time of treatment. Utilizing our proprietary Monte Carlo algorithm and software, replanning can be done in less than two minutes while the patient is on the table. We believe hospitals will be able to bill incrementally for this replanning. |
| • | Ability to track tumors and manage patient motion. MRIdian can capture multiple soft-tissue imaging planes concurrently during treatment, refreshing the image multiple times per second. This real-time imaging enables the physician to track the movement of the tumor and the surrounding healthy tissue directly, rather than relying on registration markers such as existing bones or implanted fiducials. If a tumor or critical organ moves beyond a physician-defined boundary, the treatment beam automatically pauses. This beam control becomes especially important in the situations where a tumor may be in close proximity to a critical organ, such as the heart during lung and breast cancer treatments or the rectum during prostate cancer treatments. This knowledge of the tumor location has enabled physicians to treat patients who would not previously have been considered radiation therapy candidates. |
| • | Record and evaluate the delivered dose. Using our proprietary algorithm and advanced MR imaging, MRIdian calculates the dose delivered after each treatment, enabling the physician to review and re-optimize the patient’s treatment session if needed. In addition, MRIdian can utilize diagnostic CT images that are fused with the MR images at each treatment in order to more accurately calculate dose. MRIdian also captures and records a video, known as a MRIdian Movie™, of the delivered treatments which can be evaluated by the physician or shared with patients. |
| • | Fits into existing treatment paradigms and workflow. MRIdian can be used for 3D-CRT, IMRT, IGRT, SBRT and SRS and can also be used to treat a broad spectrum of disease sites. In addition, we believe MRIdian’s increased target accuracy will allow physicians to treat patients with higher doses over fewer treatment fractions and potentially improve patient throughput and efficiency. MRIdian fits inside most standard radiation therapy vaults without significant modifications and is supported by existing codes that are available for linac reimbursement. |
We believe the ability to image with MRI and treat cancer patients simultaneously will lead to improved patient outcomes and reduced side effects from off-target radiation delivery.
Our Strategy
Our objective is to make MRI-guided radiation delivery the standard of care for radiation therapy. To achieve this goal, we intend to do the following:
| • | Target top-tier hospitals in initial global sales efforts to influence and increase market adoption. We intend to market MRIdian to a broad range of customers worldwide, including university research and teaching hospitals, private practices, community hospitals, government institutions and freestanding cancer centers. We are initially focusing on the leading hospitals worldwide which are typically early adopters of best-in-class technology and are able to influence and promote adoption by other centers both locally and globally. We plan to continue to work with these institutions to position MRIdian as a marketing tool they can use to differentiate their offerings from their peers and promote broader market awareness on the benefits of MRI-guided radiation therapy. |
| • | Commercialize MRIdian with a targeted sales force in the United States and through distributors in international markets. We intend to market MRIdian through a combination of direct sales and distributors. We are building a small, specialty sales force for the United States and Canada and are using distributors in international markets. At September 30, 2015, we had six signed orders with U.S. customers and eight signed orders with customers outside the United States for new MRIdian systems, and we intend to continue to expand our presence in key markets to capitalize on the growing |
103
Table of Contents
Index to Financial Statements
| international opportunity for MRIdian. We are engaging distributors and seeking government approval where needed to market MRIdian in China, Japan, Canada, Russia, Hong Kong and Turkey, and we intend to work with distributors and regulators in other countries in the future. |
| • | Increase broader awareness of MRIdian’s capabilities to expand our share of the radiation therapy market. We intend to educate radiation oncologists about the capabilities and resulting benefits of MRIdian over traditional radiation therapy systems. In order to drive awareness and adoption, we also intend to support the publication of clinical and scientific data and analysis, work with key opinion leaders, present at leading academic conferences and engage in outreach at leading hospitals worldwide. We also plan to leverage our existing customer network as a reference for new potential users to experience our technology in use in the clinical setting. |
| • | Maintain our competitive lead in MRI-guided radiation therapy through continued innovation. We plan to continue to invest in our technology to maintain our leadership position in the emerging MRI-guided radiation therapy market. We intend to develop and introduce enhancements to the system and software to provide improved capabilities for MRIdian users and patients. In addition, we plan to explore potential benefits of integrating our MRI technology with alternative beam technologies. We believe we have a strong intellectual property portfolio that covers the MRIdian system as well as critical design elements and key aspects of its subsystem and components. We will continue to enhance this portfolio as we develop new features and technologies. |
| • | Continue to work with leading hospitals to optimize efficiency and patient throughput. We strive to maximize the efficiency and effectiveness of the MRIdian system for our customers. We plan to continue to work closely with key opinion leaders, clinicians and hospitals in a proactive manner to determine how best to refine and improve MRIdian’s features, optimize workflow and maximize patient throughput. We utilize this customer feedback to guide product development and increase MRIdian’s reliability and efficiency, which we believe will result in positive experiences for our customers and ensure their continued usage and recommendation of MRIdian. |
| • | Drive cost reductions in the design and manufacture of MRIdian and improve our margins. We plan to continue to explore ways to bring down our cost of goods to improve margins for MRIdian. We believe we can reduce costs in the design and manufacture of MRIdian. |
104
Table of Contents
Index to Financial Statements
MRIdian
MRIdian is a next-generation, MRI-guided radiation therapy system that is comprised of four major components, (i) the MRI system, (ii) the radiation delivery system, (iii) integrated treatment planning and delivery software and (iv) a safety and control system.
MRIdian
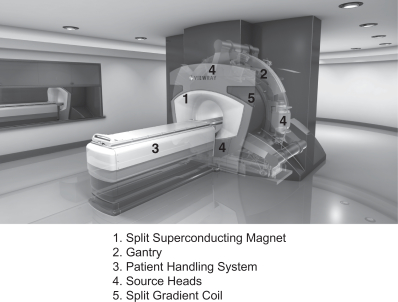
MRI System
The MRI system is the component of MRIdian that captures soft tissue images of the patient’s body. To address the technical complications that arise from combining an MRI with a linac, we have designed a proprietary split superconducting magnet that allows treatment through a central gap, eliminating MRI components in the path of the beam. Our MRI system captures and displays live, high-quality images in three planes at two frames per second or in one plane at four frames per second. The images are used to track tissues and control radiation treatment beam delivery.
While other MRI systems in development utilize a high field strength magnet to provide a clearer image, use of higher field strength magnets results in distortions of soft tissue images by up to approximately six millimeters at 1.5 tesla and unfavorable dose distribution distortions, which are unacceptable for delivering accurate radiation therapy.
We have engineered our MRI system to be able to produce clear images using a low field strength 0.35 tesla magnet which enables us to avoid the image and dose distortions that are a result of using a higher field strength magnet. In addition, MRIdian’s 0.35 tesla field strength prevents heating of the patient during uninterrupted imaging, which could occur in a higher field strength magnet requiring the imaging to be discontinued or interrupted.
105
Table of Contents
Index to Financial Statements
MRI System
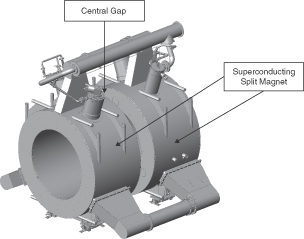
Radiation Delivery System
Radiation is delivered from three Cobalt-60 radiation therapy heads symmetrically mounted on a rotating ring gantry, providing full 360 degree coverage and simultaneous dose delivery, as opposed to prior Cobalt-60 systems that have historically been limited by imprecise radiation dose applications. Each head is equipped with a double focused multi-leaf collimator, designed to overcome the wide-beam edge of previous-generation Cobalt-60 systems and shape the beam for precision radiation therapy treatments. It allows the delivery of treatment plans for 3D-CRT, IMRT and SBRT that are clinically equivalent to those produced on the most advanced linear accelerators available today. Stereotactic procedures are possible with a positioning accuracy of less than one millimeter. Cobalt-60 is used because it does not create any radio frequency which interferes with the MRI.
Comparison of Previous-Generation Cobalt Beam to ViewRay Cobalt Beam
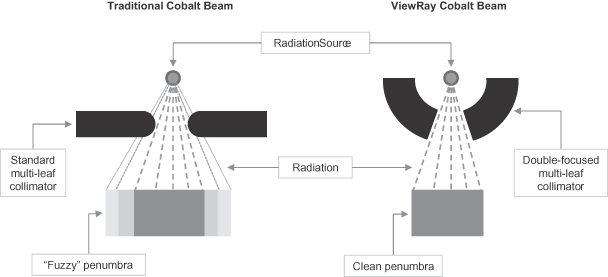
Integrated Treatment Planning and Delivery Software
Our treatment planning and delivery software can create treatment plans and manage the treatment delivery process. It is designed to create optimized 3D-CRT, IMRT and SBRT plans for delivery on MRIdian. Using this
106
Table of Contents
Index to Financial Statements
software, the on-table adaptive planning process typically takes less than two minutes, and includes: auto-contouring, dose prediction and treatment plan optimization. For contouring, the software will automatically draw the outline of the tumor and nearby organs. The clinician can then make refinements before treatment, if necessary. Dose prediction can be calculated immediately before treatment, allowing the current state of the patient’s anatomy to be taken into account. The software can generate an optimal treatment plan solution in less than one minute, allowing it to re-plan while the patient is on the treatment couch.
MRIdian has soft-tissue tracking beam control capability. While the radiation dose is being delivered, the software analyzes the acquired images and can determine tumor or organ location relative to set tolerances. If the targeted tumor or a critical organ moves beyond a physician-defined boundary, the treatment beams will automatically pause. When the tumor moves back into the target zone, the treatment will automatically resume. Physicians can set both spatial and time thresholds for pausing treatment delivery. This enables the system to account for tumor and patient motion during treatment.
The software archives all the information generated during treatment and builds a database of patient-specific planning, delivery and imaging data. It also includes a review tool which provides clinicians with a visual comparison of the delivered versus planned treatment. At the end of each treatment, the software determines the delivered dose by combining the recorded actions of the radiation delivery system with the daily image and auto-contouring of the patient. With this information, clinicians can fine-tune prescriptions based on the actual dose delivered. In addition, it provides a MRIdian Movie™ of each delivered treatment which can be evaluated by the physician or exported and shared with the patient.
Safety and Control System
In addition to complying with the applicable FDA and Nuclear Regulatory Commission, or NRC, requirements, the radiation delivery subsystem also meets a double fault tolerant design standard and has redundant safety systems. If any two components in the radiation delivery subsystem fail simultaneously, such as power and pneumatics, the system reverts to a safe state. MRIdian also contains redundant computer control for safety and system logging and double encoders on all axes of motion for safety. The control system continuously monitors performance to ensure all systems are performing and communicating appropriately.
Installed Base and Clinical Use
We received initial 510(k) marketing clearance from the FDA for our treatment planning and delivery software in January 2011 and for MRIdian in May 2012. We also received permission to affix the CE mark in November 2014, allowing MRIdian to be sold within the EEA. We received a license and permission to import MRIdian into the United Arab Emirates in December 2014. We received regulatory approval in Italy in January 2015 and Korea in September 2015, which is required in addition to the CE mark. We are currently seeking government approval to market MRIdian in China, Japan, Canada, Russia, Hong Kong and Turkey. Other countries where we will be seeking approval in the near term are Canada, Egypt and Australia. We may also seek required approvals in other countries in the future.
We have three units installed at three leading cancer centers in the United States including Washington University and Siteman Cancer Center at Barnes-Jewish Hospital, or Washington University in St. Louis; University of California, Los Angeles Health System and Jonsson Comprehensive Cancer Center, or University of California, Los Angeles; and The University of Wisconsin Carbone Cancer Center, or the University of Wisconsin–Madison. In January 2014, Washington University in St. Louis, a National Cancer Institute Designated Comprehensive Cancer Center, became the first center to treat patients with MRIdian. Washington University in St. Louis is scaling up its use of MRIdian in its clinical practice, and is now treating as many as 15 patients per day. In September 2014, Washington University in St. Louis used MRIdian to perform the first on-table adaptive treatments as part of an ongoing clinical service. Also in September 2014, the University of Wisconsin–Madison treated its first patients with MRIdian and became the first center to employ the soft-tissue
107
Table of Contents
Index to Financial Statements
tracking beam control capability unique to MRIdian. In October 2014, University of California, Los Angeles, became the third center to use MRIdian in clinical practice. We are working with each of these centers to determine how best to refine and improve MRIdian’s features, optimize workflow and maximize patient throughput.
At September 30, 2015, patients have received treatment sessions at these centers using MRIdian systems. These included cancers of the prostate, breast, lung, colorectal and bladder, which are among the most prevalent types of cancer in the United States according to the Centers for Disease Control and Prevention, or CDC, as well as the liver, stomach, esophagus and pancreas, which are among the most prevalent types of cancer outside of the United States according to the WHO.
Backlog
In 2013, we executed new sales contracts with a total value of $17.4 million, and in 2014 we executed new sales contracts with a total value of $37.6 million. At September 30, 2015, we had four MRIdian systems installed and had 14 signed orders for new systems for a backlog value of $78.0 million.
We define backlog as the accumulation of all orders for which revenue has not been recognized and we consider valid. Backlog includes customer deposits received which are recorded as a liability on the balance sheet. Orders may be revised or cancelled according to their terms or upon mutual agreement between the parties. Therefore, it is difficult to predict with certainty the amount of backlog that will ultimately result in revenue. The determination of backlog includes objective and subjective judgment about the likelihood of an order contract becoming revenue. We perform a quarterly review of backlog to verify that outstanding orders in backlog remain valid, and based upon this review, orders that are no longer expected to result in revenue are removed from backlog.
Among other criteria, to consider a sale to be in backlog we must possess an outstanding and effective written agreement for the delivery of a MRIdian signed by the customer, as well as receipt of a minimum customer deposit or letter of credit. For removal of an order from our backlog, the following criteria are considered: changes in customer or distributor plans or financial conditions; the customer’s or distributor’s continued intent and ability to fulfil the order contract; changes to regulatory requirements; the status of regulatory approval required in the customer’s jurisdiction, if any; and other reasons for potential cancellation of order contracts.
Installation Process
Following execution of a contract, it generally takes nine to 12 months for a customer to prepare an existing facility or construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures. MRIdian is designed to fit into a typical radiation therapy vault, similar to other replacement linear accelerators. MRIdian’s components all fit through standard hospital vault entrances for assembly. On-site training takes approximately one week and can be conducted concurrent with installation and acceptance testing.
Our customers are responsible for removing any outgoing linear accelerator and preparing the mounting pad, power and support system connections. Additional room modifications required are consistent with those generally required for MRI systems such as radio frequency shielding of the room and additional power.
Clinical Development
To date, we have primarily relied on clinical symposia and case studies presented at ASTRO and ESTRO to raise awareness of MRI-guided radiation therapy and to market MRIdian to leading cancer centers. In order to promote
108
Table of Contents
Index to Financial Statements
broader adoption rates at other cancer centers and hospitals, we plan to work with our customers to collect and publish data on clinical efficacy, treatment times and clinical results for patients who have been treated on a MRIdian. While we do not currently have statistically significant, objective evidence that MRIdian improves patient outcomes or decreases healthcare costs, we are currently sponsoring two studies at Washington University in St. Louis to compare MRIdian to other IGRT systems. We plan to continue to support further studies to demonstrate the benefits of MRI-guided radiation therapy and adaptive treatment planning. As data accumulate from the use of MRIdian, we plan to work with professional healthcare organizations to further support global marketing efforts, additional product clearances, approvals and/or registrations and potential improvements in reimbursement.
Selling and Marketing
We currently market MRIdian through a direct sales force in the United States and distributors in the rest of the world. We market MRIdian to a broad range of worldwide customers, including university research and teaching hospitals, community hospitals, private practices, government institutions and freestanding cancer centers. As with the traditional linac market, our sales and revenue cycle varies based on the customer and can be lengthy, sometimes lasting up to 18 to 24 months or more from initial customer contact to contract execution.
To sell MRIdian globally, we use a combination of sales executives, sales directors and a network of international third-party distributors with internal support from sales operations, product management and application specialists. A targeted group of three sales directors and one vice president are responsible for selling MRIdian within the United States. Our product management function helps market MRIdian and works with our engineering group to identify and develop upgrades and enhancements. We also have a team of application specialists who provide post-sales support.
Although we do not currently engage in advertising efforts, our selling and marketing practices include participating in trade shows and symposia.
Competition
We compete directly with companies marketing IGRT devices for the treatment of cancer using CT, ultrasound, optical tracking and X-ray imaging. We also compete with companies developing next-generation IGRT devices, specifically those developing MRI-guided devices, amongst others. We expect technological advances, including the ability to provide real-time imaging, clinical outcomes, size, price, operational complexity and operational efficiency to drive competitive market dynamics.
Our major competitors with devices approved for distribution in the United States or globally include Varian Medical Systems, Inc., or Varian, Elekta AB, or Elekta, and Accuray Incorporated. Many of our direct competitors have greater financial, sales and marketing, service infrastructure and research and development capabilities than we do, as well as more established reputations and current market share. The main limitations of currently approved devices are the lack of real-time, clear images before and during the treatment, as well as the ability to perform on-table adaptive planning.
We are also aware of one commercial and two academic ongoing research efforts to develop radiation therapy systems incorporating MRI. Elekta and Royal Philips have formed a consortium to develop a commercial Elekta-Philips MRI-linac. The University of Sydney, Ingham Institute and the University of Queensland have formed a partnership to develop an MRI-linac and the University of Alberta’s Cross Cancer Institute is working on an MRI-linac as well. Although these academic research efforts may not compete directly with us commercially, if one of our competitors were to form a partnership with one of these institutions to commercialize their system, it could impact our sales negatively. Of these three, we believe the Elekta-Philips MRI-linac is the most advanced in development, although we believe this system is still years away from approval. MRIdian is the first and only commercially available MRI-guided radiation therapy device to image and treat cancer patients simultaneously.
109
Table of Contents
Index to Financial Statements
The limited capital expenditure budgets of our customers results in all suppliers to these entities competing for a limited pool of funds. Our customers may be required to select between two items of capital equipment. For example, some of our potential customers are considering expensive proton therapy systems which could consume a significant portion of their capital expenditure budgets.
Manufacturing
We have adopted a model in which we rely on subsystem manufacturing, assembly and testing by our key suppliers. The MRIdian system is integrated at the customer site. Through this approach, we avoid the majority of the fixed cost structure of manufacturing facilities. We purchase major components and subsystems for MRIdian from national and international third-party OEM suppliers and contract manufacturers. These major components include the magnet, MRI electronics, ring gantry, radiation therapy heads, Cobalt-60 sources, multi-leaf collimators, patient-treatment table and computers. We also directly purchase minor components and parts. At the customer site, we assemble and integrate these components with our proprietary software and perform multiple levels of testing and qualification. The system undergoes a final acceptance test, which is performed in conjunction with the customer.
Many of the major subsystems and components of MRIdian are currently procured through single and sole source suppliers. Among these are the magnet, MRI electronics, MRI coils, ring gantry, radiation-therapy heads, Cobalt-60 sources, multi-leaf collimators and the patient-treatment table. We have entered into multi-year supply agreements for all major components and subsystems. Our restated joint development and supply agreement with Euromechanics Medical GmbH, as amended, has expired by its terms. We do not believe that that the expiration will have a negative impact on our manufacturing operations or business because we are currently negotiating an extension of the agreement and we are continuing to operate under the agreement as if the agreement had not expired during these negotiations. Except for the MRI power, control and image reconstruction subsystem, we own the design of all other major subsystems and components.
We manage our supplier relationships with scheduled business reviews and periodic program updates. We closely monitor supplier quality and delivery performance to ensure compliance with all MRIdian system specifications. We believe our supply chain has adequate capacity to meet our projected sales over the next several years.
Intellectual Property
The proprietary nature of, and protection for, MRIdian components, new technologies, processes and know-how are important to our business. Our policy is to seek patent protection in the United States and in certain foreign jurisdictions for our MRIdian systems and other technology where available and when appropriate. We also in-license technology, inventions and improvements we consider important to the development of our business.
We hold the exclusive worldwide license for certain patents and patent applications covering our combination of MRI and radiation therapy technologies. Specifically, we hold a license to two issued U.S. patents, one allowed U.S. patent application, 14 issued foreign patents (eight of which were issued in Great Britain, Germany, France and the Netherlands as a result of two patent applications filed and allowed through the European Patent Office) and four pending foreign patent applications at July 13, 2015. We own an additional eight issued U.S. patents, seven issued foreign patents, 16 pending U.S. patent applications and 48 pending foreign patent applications, and, at July 13, 2015, one of our foreign patent applications is allowed. Assuming all required fees are paid, individual patents or patent applications owned or licensed by us will expire between 2025 and 2034. We also have a joint ownership interest with Case Western Reserve University in one issued patent and one U.S. patent application.
Our portfolio includes patents and patent applications directed to system-wide aspects of MRIdian and to key aspects of its subsystems and components. The initial licensed patents for our core technology broadly cover the
110
Table of Contents
Index to Financial Statements
simultaneous use of MR imaging and isotopic external-beam radiation therapy. Such patents have been granted in the United States, Europe, Hong Kong, Australia and Japan, and additional related patent applications remain pending in China, Canada, the United States, Australia and Japan. In the United States we have pending continuation applications of the licensed patents that extend this core technology to alternate beam technologies. Additionally, we have patents and patent applications that cover critical design elements including, among others, our approach to Cobalt IMRT, our methods for integrating MRI and the radiation delivery system, and the design of our disassemblable (“pop apart”) magnet which enables the MRI sub-system to fit into most standard radiation therapy vaults. The U.S. patent application on our approach to Cobalt IMRT has been issued, the patent application on our split gradient coil has been issued in the United States, Japan, Australia and China and numerous applications on other design elements are pending in the United States and foreign jurisdictions. In addition, we have a U.S. patent and U.S. and foreign patent applications that cover the use of MRI imaging at a frequency sufficient to account for real-time organ motion to provide video rate tissue tracking in disciplines outside of radiation therapy.
We continue to review new technological developments in our system and in the field as a whole in order to make decisions about what filings would be most appropriate for us. An additional key component of our intellectual property is our proprietary software used in planning and delivering MRIdian’s therapeutic radiation dose.
In December 2004, we entered into a licensing agreement with the University of Florida Research Foundation, Inc., or UFRF, whereby UFRF granted us a worldwide exclusive license to certain of UFRF’s patents in exchange for 33.653 shares of common stock and a royalty from sales of products developed and sold by us utilizing the licensed patents. We were obligated to meet certain product development and commercialization milestones by various dates through December 31, 2014. The significant milestones met prior to December 31, 2013 included: (i) completion of a business plan and Small Business Technology Transfer grant application; (ii) securing a minimum of $20.0 million venture financing; (iii) successful relocation and build out of the company’s headquarters; (iv) receipt of the first magnet from an OEM partner; (v) hiring of a chief executive officer with industry experience in developing and commercializing similar products; and (vi) filing for FDA approval. The final milestone, which required us to recognize the first commercial sale of the MRIdian system to retail customers by December 31, 2014, was met during the year ended December 31, 2013. If these milestones had not been accomplished, UFRF would have had the right to terminate the licensing agreement. Royalty payments are based on 1% of net sales, defined as the amount collected on sales of licensed products and/or licensed processes after deducting trade and/or quantity discounts, credits on returns and allowances, outbound transportation costs paid and sales tax. Minimum quarterly royalty payments of $50,000 commenced with the quarter ended March 31, 2014 and are payable in advance. Minimum royalties paid in any calendar year will be credited against earned royalties for such calendar year. The royalty payments continue until the earlier of (i) the date that no licensed patents remain enforceable or (ii) the payment of earned royalties, once begun in 2014, cease for more than four consecutive calendars quarters.
In addition to our patents, we also rely upon trade secrets, know-how, trademarks, copyright protection and continuing technological and licensing opportunities to develop and maintain our competitive position. We have periodically monitored and continue to monitor the activities of our competitors and other third parties with respect to their use of intellectual property. We require our employees, consultants and outside scientific collaborators to execute confidentiality and invention assignment agreements upon commencing employment or consulting relationships with us. Despite these safeguards, any of our know-how or trade secrets not protected by a patent could be disclosed to, or independently developed by, a competitor.
Coverage and Reimbursement
Reimbursement rates in the United States have generally supported a favorable return on investment for the purchase of new radiotherapy equipment, including MRIdian. Payments for standard radiation therapy treatments using MRIdian, including 3D-CRT, IMRT and SBRT, are generally covered and reimbursed under existing
111
Table of Contents
Index to Financial Statements
Current Procedural Terminology, or CPT, codes and policies currently in place. User experience to date indicates that our initial customers have treated a wide spectrum of different patients and treatment modalities using MRIdian. Physicians use the MRIdian system’s on-board MRI to perform a complex simulation weekly for IMRT or daily for SBRT, special physics consult and adaptive re-planning. Each of these are distinct procedures which can be billed by physicians using existing CPT codes, so long as such procedures meet medical necessity and other coverage criteria as established by government and other third-party payors.
Third-party payors, including governmental healthcare programs such as Medicare and Medicaid, establish coverage policies and reimbursement rates for diagnostic examinations and therapeutic procedures performed by physicians in hospitals and free-standing clinics. Private insurers often model their payment rates and coverage policies based on those established by the government. The U.S. Congress from time to time considers various Medicare and other healthcare reform proposals that could affect both private and public third-party payor coverage and reimbursement for healthcare services provided in hospitals and clinics. In addition, third-party payors regularly update reimbursement amounts, including annual updates to payments to physicians, hospitals and clinics for medical procedures, including radiation treatments using MRIdian.
The changes to simplify the billing for conventional radiation therapy and IMRT by CMS in its final rule for the Hospital Outpatient Prospective Payment System, or HOPPS, effective January 1, 2015, are further indicative of an overall U.S. health policy trend towards cost containment. Whether this trend results in capitated payments per patient or case payment per procedure, we believe MRIdian’s capability of enabling physicians to visualize the treatment area and adapt therapy in real-time to maximize the clinical benefit to the patient will result in cost savings to both providers and third-party payors.
We plan to work with our customers to collect and publish data on clinical results for patients who have undergone procedures on MRIdian. We are currently sponsoring a study that is estimated to be completed in 2017 at Washington University in St. Louis to compare MRIdian to other IGRT systems, and we plan to continue to support further studies to demonstrate the benefits of MRI-guided radiation therapy and adaptive treatment planning. As data accumulate from the use of our system, we plan to work with professional healthcare organizations to further support global marketing efforts, additional product clearances, approvals and/or registrations and potential improvements in reimbursement. Additionally, we currently provide reimbursement support to our customers through a third-party vendor.
Foreign Reimbursement Regulations
Internationally, reimbursement and healthcare payment systems vary from country to country and include single-payor, government managed systems as well as systems in which private payors and government-managed systems exist side-by-side. In general, the process of obtaining coverage approvals is slower outside of the United States. Our ability to achieve adoption of MRIdian as well as significant sales volume in international markets we enter will depend in part on the availability of reimbursement for procedures performed using MRIdian.
Research and Development
Continued innovation and development of advanced technologies is critical to our goal of making MRI-guided radiation therapy the standard of care for cancer treatment. Our current development activities include improvements in and expansion of product capabilities, continued clinical workflow refinements, design improvements to reduce system costs and improvements in reliability.
The modular design of MRIdian enables the development of new capabilities and performance enhancements by generally allowing each subsystem to evolve within the overall platform design. Access to regular MRIdian upgrades protects customer investment in MRIdian, and facilitates the adoption of new features and capabilities among existing installed base customers. We expect these upgrades will generally consist of new software
112
Table of Contents
Index to Financial Statements
features and MRI imaging enhancements designed to leverage the capabilities of MRIdian’s on-table adaptive planning, real-time tumor tracking, and the other inherent capabilities of MRI imaging technologies, as well as hardware enhancements as they become available. Ultimately we have a vision of expanding the system’s on-table adaptive feature into a continuously-adaptive capability, which adjusts and corrects for any changes as they happen, on the table, in real-time.
In addition, we believe our existing and expanding IP portfolio will enable us to continuously develop innovative technologies to further strengthen the differentiation of MRIdian in the marketplace. Magnetic resonance imaging is a powerful and versatile measurement technique and is widely used throughout radiology and medicine because of its ability to generate information about tissues and disease states.
At September 30, 2015, we had a total of 36 employees in our research and development departments. Research and development expenses were $7.4 million and $7.5 million during the nine months ended September 30, 2015 and 2014, and $9.4 million and $8.8 million for the years ended December 31, 2014 and 2013. We plan to continue to increase our investment in research and development in future periods.
Government Regulation
U.S. Medical Device Regulation and Nuclear Materials Regulation
As a manufacturer and seller of medical devices and devices that deliver radiation, we and some of our suppliers and distributors are subject to extensive and rigorous regulation by the FDA, the NRC, other federal, state and local authorities in the United States and foreign regulatory authorities. Regulations promulgated by the FDA relating to medical devices and radiation-producing devices govern, among other things, the following activities that we perform or that are performed on our behalf, and that we will continue to perform or have performed on our behalf:
| • | product design, development and testing; |
| • | manufacturing; |
| • | approval or clearance; |
| • | packaging, labeling and storage; |
| • | marketing, advertising and promotion; |
| • | distribution, including importing and exporting; |
| • | installation; |
| • | possession and disposal; |
| • | record keeping; |
| • | service and surveillance, including post-approval monitoring and reporting; |
| • | complaint handling; and |
| • | repair or recall of products and issuance of field safety corrective actions. |
FDA Clearance and Approval of Medical Devices
The FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, promotion, distribution, and production of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. Unless an exemption applies, the FDA requires that all new medical devices and all marketed medical devices that have been significantly changed, or that will be marketed with a new indication for use, obtain either clearance via a 510(k) pre-market notification or approval
113
Table of Contents
Index to Financial Statements
via a Premarket Approval, or PMA, application before the manufacturer may commercially distribute the product in the United States. The type of marketing authorization necessary is generally linked to the classification of the device. The FDA classifies medical devices into one of three classes. Devices deemed to pose the lowest risk are placed in Class I, and most Class I devices are exempt from premarket notification requirements. Class I devices are those for which safety and effectiveness can be reasonably assured by adherence to a set of regulations referred to as General Controls, which require compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, and regulations regarding facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. However, some Class I devices, called Class I reserved devices, also require premarket clearance by the FDA through the 510(k) premarket notification process described below.
Moderate risk devices are placed in Class II and are subject to General Controls as well as Special Controls, which can include performance standards, guidelines and post-market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA pursuant to Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or FDCA.
Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device are placed in Class III. Class III devices require FDA approval of a PMA prior to marketing.
MRIdian has been classified as a Class II medical device subject to the 510(k) clearance process.
510(k) clearance process. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process. Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent” to either:
| • | a device that was legally marketed prior to May 28, 1976, the date upon which the Medical Device Amendments of 1976 were enacted; or |
| • | another commercially available, similar device that was cleared through the 510(k) process. |
To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence.
The process of obtaining 510(k) clearance usually takes from three to 12 months from the date the application is filed and generally requires submitting supporting design and test data, which can be extensive and can prolong the process for a considerable period of time. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device. We received our 510(k) clearances for the treatment planning and delivery software system in January 2011 and for MRIdian in May 2012.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in the intended use of the device, may require a new 510(k) clearance or, depending on the modification, could require approval of a PMA. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with the manufacturer’s decision, it may retroactively require the manufacturer to submit a request for 510(k) clearance or PMA approval and can require the manufacturer to cease marketing and/or recall the product until 510(k) clearance or PMA approval is obtained. Since obtaining 510(k) clearances in 2011 and 2012, we have made changes to MRIdian that we believe do not require new 510(k) clearance.
Premarket application approval process. Submission and approval of a PMA is required before marketing of a Class III product may proceed. Under the PMA application process, the applicant must generally conduct at
114
Table of Contents
Index to Financial Statements
least one clinical investigation and submit extensive data and clinical information demonstrating reasonable assurance of the safety and effectiveness of the device for its intended use to the FDA’s satisfaction. Accordingly, a PMA application typically includes, but is not limited to, extensive technical information regarding device design and development, pre-clinical and clinical trial data, manufacturing information, labeling and financial disclosure information for the clinical investigators in device studies. The PMA process is much more demanding than the 510(k) premarket notification process.
Following receipt of a PMA application, the FDA conducts an administrative review to determine whether the application is sufficiently complete to permit a substantive review. If it is not, the agency will refuse to file the PMA. If it is, the FDA will accept the application for filing and begin the review. The FDA, by statute and by regulation, has 180 days to review a filed PMA application, although the review of an application more often occurs over a significantly longer period of time. During this review period, the FDA may request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA. Before approving or denying a PMA, an FDA advisory committee may review the PMA at a public meeting and provide the FDA with the committee’s recommendation on whether the FDA should approve the submission, approve it with specific conditions, or not approve it. Overall, the PMA application process typically takes between one to three years, but may take significantly longer. The FDA may approve a PMA application with post-approval conditions intended to ensure the safety and effectiveness of the device including, among other things, restrictions on labeling, user training requirements, restrictions on promotion, sale and distribution, and requirements for the collection of long-term follow-up data.
MRIdian is not currently approved under a PMA approval, and we have no plans for any indication or system improvements or extensions that we believe would require a PMA.
Clinical trials. Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) clearance. Such trials require submission of an investigational device exemption, or IDE, application to the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. If an IDE is required, the FDA and the appropriate institutional review boards, or IRBs, at the clinical sites must approve the study before clinical trials may begin. If the device is considered a non-significant risk device, IDE submission to FDA is not required. Instead, only approval from the IRB overseeing the clinical trial is required. Clinical trials are subject to extensive monitoring, record keeping and reporting requirements. Clinical trials must be conducted under the oversight of an IRB for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, the patient’s informed consent must be obtained in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. The clinical trial sponsor, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and effectiveness of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product.
Continuing FDA regulation. Any devices we manufacture or distribute pursuant to 510(k) clearance or PMA approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions.
In addition, our manufacturing operations for medical devices and those of our suppliers must comply with the FDA’s QSR. The QSR requires that each manufacturer, including third party manufacturers, establish and implement a quality system by which the manufacturer monitors the manufacturing process and maintains records that show compliance with FDA regulations and the manufacturer’s written specifications and procedures. Among other things, the QSR requires that manufacturers establish performance requirements before
115
Table of Contents
Index to Financial Statements
production and follow stringent requirements applicable to the device design, testing, production, control, record keeping, documentation, labeling and installation, as well as supplier/contractor selection, complaint handling and other quality assurance procedures during all aspects of the manufacturing process. Compliance with the QSR is necessary to be able to continue to market medical devices that have received FDA approval or clearance, and to receive FDA clearance or approval to market new or significantly modified medical devices. The FDA makes announced and unannounced inspections of medical device manufacturers, and these inspections may include the manufacturing facilities of subcontractors. Following an inspection, the FDA may issue reports, known as FDA Form 483 reports, listing the investigator’s observations of conditions or practices which indicate the possibility that an FDA-regulated product may be in violation of FDA’s requirements. FDA may also issue warning letters documenting regulatory violations observed during an inspection. The manufacturer’s failure to adequately respond to such reports or warning letters may result in FDA enforcement action against the manufacturer and related consequences, including, among other things, fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, FDA refusal to grant 510(k) clearance or PMA approval to new devices, withdrawal of existing clearances or approvals, and criminal prosecution.
Manufacturers must also comply with post-market surveillance regulations, including medical device reporting regulations, which require that manufacturers review and report to the FDA any incident in which their device may have caused or contributed to a death or serious injury, or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. In addition, corrections and removal reporting regulations require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act that may present a risk to health. The FDA may also order a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death.
The FDA and the Federal Trade Commission, or FTC, also regulate the promotion and advertising of MRIdian. In general, we may not promote or advertise MRIdian for uses not within the scope of our clearances or approvals or make unsupported safety and effectiveness claims.
Failure to comply with applicable FDA requirements, including delays in or failures to report incidents to the FDA or off-label promotion, can result in enforcement action by the FDA, which can include any of the following sanctions:
| • | warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties; |
| • | customer notifications or repair, replacement, refunds, recall, administrative detention or seizure of our MRIdian systems; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying requests for 510(k) clearance or PMA approval of new or modified products; |
| • | withdrawing 510(k) clearances or PMA approvals that have already been granted; |
| • | refusal to grant export approval for products; or |
| • | criminal prosecution. |
Radiological health. We are also regulated by the FDA under the Electronic Product Radiation Control provisions of the FDCA because MRIdian contains gamma radiation producing components, and because we assemble these components during manufacturing and service activities. The Electronic Product Radiation Control provisions require gamma radiation producing products to comply with certain regulations and applicable performance standards. Manufacturers are required to certify in product labeling and reports to the FDA that their products comply with all necessary standards as well as maintain manufacturing, testing and sales records for their products. The Electronic Product Radiation Control provisions also require manufacturers to report product defects and affix appropriate labeling to covered products. Failure to comply with these requirements could result in enforcement action by the FDA, which can include any of the sanctions described above.
116
Table of Contents
Index to Financial Statements
Nuclear Regulatory Commission and U.S. State Agencies
In the United States, as a manufacturer of medical devices and devices utilizing radioactive byproduct material (i.e. depleted uranium shielding and Cobalt-60 sources) we are subject to extensive regulation by not only federal governmental authorities, such as the NRC, but also by state and local governmental authorities, such as the Ohio Department of Health, to ensure such devices are safe and effective. In Ohio, the Department of Health, by agreement with the NRC, regulates the possession, use, and disposal of radioactive byproduct material as well as the manufacture of devices containing radioactive sealed sources to ensure compliance with state and federal laws and regulations. We have received sealed source device approval from the Ohio Department of Health for MRIdian and have entered into a standby letter of credit with PNC for $103,000 to provide certification of financial assurance for decommissioning Cobalt-60 radioactive materials in accordance with Ohio Department of Health regulations. The company and/or its supplier of radiation sources must also comply with NRC and U.S. Department of Transportation regulations on the labeling and packaging requirements for shipment of radiation sources to hospitals or other users of MRIdian. Compliance with NRC, state and local requirements is required for distribution, installation, use and service within each state that we intend to install MRIdian systems.
Existing radiation therapy facilities practicing nuclear medicine, brachytherapy or Gamma Knife therapy are already required to have necessary NRC and/or state licenses and a radiation safety program requiring compliance to various provisions under 10 CFR 35 “Medical uses of byproduct material.” Use of MRIdian is regulated under 10 CFR 35.1000 “Other medical uses of byproduct material or radiation from byproduct material.” In 2013, the NRC released a licensing guidance under 10 CFR 35.1000 to guide our customers in the NRC requirements applicable for the use of MRIdian. We believe that this guidance is favorable in that it is consistent with clinical use of existing image-guided radiation therapy devices.
Moreover, our use, management, and disposal of certain radioactive substances and wastes are subject to regulation by several federal and state agencies depending on the nature of the substance or waste material. We believe that we are in compliance with all federal and state regulations for this purpose.
Outside the United States, various laws apply to the import, distribution, installation and use of MRIdian, in consideration of the nuclear materials within MRIdian.
U.S. Privacy and Security Laws
We may also be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and their respective implementing regulations, including the final omnibus rule published on January 25, 2013, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. Further, “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity are also subject to certain HIPAA privacy and security standards. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
U.S. Fraud and Abuse Laws and Regulations
The healthcare industry is also subject to a number of fraud and abuse laws and regulations, including physician anti-kickback, false claims and physician payment transparency laws. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in federal healthcare programs and significant
117
Table of Contents
Index to Financial Statements
monetary penalties, among others. These laws, among other things, constrain the sales, marketing and other promotional activities of manufacturers of medical products, such as us, by limiting the kinds of financial arrangements we may have with hospitals, physicians and other potential purchasers of medical products who may seek reimbursement from a federal or state health care program such as Medicare or Medicaid.
Anti-kickback laws. The federal Anti-Kickback Statute makes it a criminal offense to knowingly and willfully solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase, order, lease of any good, facility, item or service, that are reimbursable by a state or federal health care program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value. The Anti-Kickback Statute has been interpreted to apply to the purchase of medical devices from a particular manufacturer or the referral of patients to a particular supplier of diagnostic services utilizing such devices. Although, there are established statutory exceptions and regulatory safe harbors that define certain financial transactions and practices that are not subject to the Anti-Kickback Statute, the exceptions and safe harbors are drawn narrowly. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances.
Generally, courts have taken a broad interpretation of the scope of the Anti-Kickback Statute, holding that the statute may be violated if merely one purpose of a payment arrangement is to induce referrals or purchases. Further, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Violations of this law are punishable by up to five years in prison, and can also result in criminal fines, administrative civil money penalties and exclusion from participation in federal healthcare programs. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. Many states have also adopted statutes similar to the federal Anti-Kickback Statute, some of which apply to payments in connection with the referral of patients for healthcare items or services reimbursed by any source, not only governmental payor programs.
False Claims Act. The federal civil False Claims Act prohibits anyone from knowingly and willfully presenting, or causing to be presented, claims for payment, that are false or fraudulent, for services not provided as claimed. In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud. Because the complaint is initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. If the government is ultimately successful in obtaining redress in the matter or if the plaintiff succeeds in obtaining redress without the government’s involvement, then the plaintiff will receive a percentage of the recovery. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties ranging from $5,500 to $11,000 for each separate false claim, and may be excluded from participation in federal health care programs, and, although the federal False Claims Act is a civil statute, violations may also implicate various federal criminal statutes. Several states have also adopted comparable state false claims act, some of which apply to all payors.
Civil monetary penalties laws. The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Other fraud and abuse laws. HIPAA also created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare
118
Table of Contents
Index to Financial Statements
benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, the intent standard for certain healthcare fraud statutes under HIPAA was amended by the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Affordable Care Act, such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Physician payment transparency laws. There has been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers and entities. The Affordable Care Act, among other things, imposed new reporting requirements on certain manufacturers, including certain device manufacturers, for payments provided to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately, and completely the required information may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for “knowing failures.” Device manufacturers were required to begin collecting data on August 1, 2013 and submit reports on aggregate payment data to the government for the first reporting period (August 1, 2013—December 31, 2013) by March 31, 2014, and to report detailed payment data for the first reporting period and submit legal attestation to the accuracy of such data by June 30, 2014. Thereafter, device manufacturers must submit reports by the 90th day of each subsequent calendar year. CMS made all reported data publicly available on September 30, 2014.
Certain states also mandate implementation of compliance programs, impose restrictions on device manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to healthcare providers and entities.
The laws and regulations and their enforcement are constantly undergoing change, and we cannot predict what effect, if any, changes may have on our business. In addition, new laws and regulations may be adopted which adversely affect our business. There has been a trend in recent years, both in the United States and internationally, toward more stringent regulation and enforcement of requirements applicable to medical device manufacturers and requirements regarding protection and confidentiality of personal data.
State Certificate of Need Laws
In some states, a certificate of need, or CON, or similar regulatory approval is required by hospitals and other healthcare providers prior to the acquisition of high-cost capital items, including MRIdian, or the provision of new services. These laws generally require appropriate state agency determination of public need and approval prior to the acquisition of such capital items or addition of new services. CON requirements may preclude our customers from acquiring, or significantly delay acquisition of, MRIdian and/or from performing treatments using MRIdian. CON laws are the subject of ongoing legislative activity, and a significant increase in the number of states regulating the offering and use of MRIdian through CON or similar requirements could adversely affect us.
Healthcare Reform
In the United States and foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system seeking, among other things, to reduce healthcare costs that could affect our results of operations.
119
Table of Contents
Index to Financial Statements
By way of example, in the United States, the Affordable Care Act was signed into law in March 2010, which is expected to substantially change the way healthcare is delivered and financed by both governmental and private insurers. Among other things, the Affordable Care Act:
| • | imposed an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States; |
| • | established a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; |
| • | implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and |
| • | created an independent payment advisory board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. |
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This included reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and will stay in effect through 2024 unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals and imaging centers.
We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for MRIdian or additional pricing pressure.
Foreign Regulation of Medical Devices
Our activities outside the United States are subject to regulatory requirements that vary from country to country and frequently differ significantly from those in the United States. Failure to obtain and maintain regulatory approval or clearance in any foreign country in which we market or plan to market MRIdian may have a negative effect on our ability to generate revenue and harm our business.
In general, MRIdian is regulated outside the United States as medical devices by foreign governmental agencies similar to the FDA and the FTC. In addition, in foreign countries where we have operations or sell MRIdian, we are subject to laws and regulations applicable to manufacturers of medical devices, radiation producing devices and to the healthcare industry, and laws and regulation of general applicability relating to environmental protection, safe working conditions, manufacturing practices and other matters. These laws and regulations are often comparable to, or more stringent than U.S. laws and regulations. Our sales of MRIdian in foreign countries are also subject to regulation of matters such as product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. We rely in some countries on our foreign distributors to assist us in complying with applicable regulatory requirements.
Regulation in the EU
In the European Union, or EU, we are required under the European Medical Device Directive (Council Directive 93/42/EEC) to affix the CE mark to our MRIdian systems in order to sell the MRIdian systems in member countries of the EU. The CE mark is an international symbol that represents adherence to certain essential principles of safety and effectiveness mandated in the European Medical Device Directive (the so-called
120
Table of Contents
Index to Financial Statements
“essential requirements”). Once affixed, the CE mark enables a product to be sold within the European Economic Area, which is composed of the 28 Member States of the EU plus Norway, Iceland and Liechtenstein.
To demonstrate compliance with the essential requirements, we must undergo a conformity assessment procedure which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I with no measuring function and which are not sterile) where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a Member State of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This Certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
We received the CE Certificate of Conformity from our Notified Body in November 2014, allowing us to affix the CE mark to MRIdian in order to sell it throughout the EEA.
If we modify MRIdian we may need to apply for permission to affix the CE mark to the modified product. Additionally, we will need to apply for a CE mark for any new products that we may develop in the future. We cannot be certain that we will be able to obtain permission to affix the CE mark for modified or new products or that we will continue to meet the quality and safety standards required to maintain the permissions that we have already received or may receive in the future. In addition, if we are unable to obtain permission to affix the CE mark to our future products, we would be unable to sell them in EU member countries.
Regulation in Other Countries
We will be subject to additional regulations in foreign countries in which we intend to market, sell and import MRIdian. We or our distributors must receive all necessary approvals or clearance prior to marketing and importing MRIdian in those international markets. We received a license and permission to import MRIdian into the United Arab Emirates in December 2014. We received regulatory approval in Italy in January 2015 and in Korea in September 2015, which is required in addition to the CE mark. We are currently seeking government approval to market MRIdian in China, Japan, Canada, Russia, Hong Kong and Turkey. Other countries where we will be seeking approval in the near term are Canada, Egypt, and Australia. We will seek approvals in other countries as may be required in the future.
The International Standards Organization promulgates internationally recognized standards, including those for the requirements of quality systems. We are certified to the ISO 13485:2003 standard, which specify the quality system requirements for medical device manufacturers. To support our ISO certifications, we are subject to surveillance audits by a Notified Body yearly and recertification audits every three years that assess our continued compliance with the relevant ISO standards. Our most recent recertification audit occurred in March 2015.
Employees
At September 30, 2015, we had 93 full-time employees. Within our workforce at September 30, 2015, 36 employees are engaged in research and development and 57 employees in business development, finance, human resources, facilities, and general management and administration. We have no collective bargaining agreements with our employees, and we have not experienced any work stoppages. We consider our relations with our employees to be good.
121
Table of Contents
Index to Financial Statements
Facilities
Our corporate headquarters are located in Oakwood Village, Ohio, where we lease and occupy approximately 41,000 square feet of office space. The current term of our Oakwood Village lease expires on October 31, 2017, with an option to extend the term through October 31, 2027. We also maintain an office in Mountain View, California, where we lease and occupy approximately 25,500 square feet of office space. The current term of our Mountain View lease expires on November 30, 2019. In connection with our Mountain View, California lease, we entered into a standby letter of credit with PNC Bank, National Association for $0.5 million, which is still outstanding at September 30, 2015.
We believe that our existing facilities are adequate for our current needs. When our leases expire, we may exercise our renewal option or look for additional or alternate space for our operations and we believe that suitable additional or alternative space will be available in the future on commercially reasonable terms.
122
Table of Contents
Index to Financial Statements
Directors and Executive Officers
Below are the names of and certain information regarding the Company’s current executive officers and directors who were appointed effective as of September 30, 2015:
| Name |
Age | Position(s) | ||||
| Executive Officers |
||||||
| Chris A. Raanes |
50 | President, Chief Executive Officer and Director | ||||
| James F. Dempsey, Ph.D. |
45 | Chief Scientific Officer and Director | ||||
| Michael Brandt |
52 | Senior Vice President of Sales | ||||
| D. David Chandler |
58 | Chief Financial Officer | ||||
| Douglas H. Keare |
51 | Chief Operating Officer | ||||
| Non-Employee Directors |
||||||
| Joshua Bilenker, M.D.(2) |
43 | Director | ||||
| David Bonita, M.D.(1)(3) |
40 | Director | ||||
| Caley Castelein, M.D.(2)(3) |
44 | Director | ||||
| Mark S. Gold, M.D.(3) |
66 | Director | ||||
| Aditya Puri(1)(2) |
44 | Director | ||||
| Brian K. Roberts(1) |
44 | Director | ||||
| Robert Weisskoff, Ph.D. |
53 | Director | ||||
| (1) | Member of audit committee. |
| (2) | Member of compensation committee. |
| (3) | Member of nominating and corporate governance committee. |
Executive Officers
Chris A. Raanes has served as our President and Chief Executive Officer and as a member of the board of directors since February 2013. Mr. Raanes brings over 15 years of experience in the private and public medical device field. As our President and Chief Executive Officer, Mr. Raanes has supported our growth and strategic initiatives, including our worldwide commercial expansion of MRIdian. Previously, Mr. Raanes was Executive Vice President from July 2011 to November 2012 and Chief Operating Officer and Senior Vice President from September 2002 to July 2011 at Accuray Incorporated, a medical device company. He also served as Vice President and General Manager, Digital Imaging at PerkinElmer Inc., a healthcare company, from December 1999 to March 2002. Mr. Raanes holds a B.S. and an M.S. in Electrical Engineering from the Massachusetts Institute of Technology. We believe Mr. Raanes is qualified to serve on our board of directors because of his extensive management experience and his expertise in radiation therapy device commercialization and operations.
James F. Dempsey, Ph.D. has served as our Chief Scientific Officer since founding ViewRay in March 2004. Dr. Dempsey has been a member of the board of directors since January 2008. Dr. Dempsey brings more than 17 years of experience in the field of radiotherapy medical physics to ViewRay. He previously served as a faculty member in the University of Florida Department of Radiation Oncology, as Assistant Professor from July 2001 to July 2007 and Associate Professor from July 2007 to January 2008. Dr. Dempsey holds a B.S. in Radiochemistry from San Jose State University and a Ph.D. in Nuclear Chemistry from Washington University in St. Louis. We believe Dr. Dempsey is qualified to serve on our board of directors based on his in-depth knowledge of our product, business and industry, as well as his expertise in nuclear chemistry and physics and medical physics.
Michael Brandt has served as our Senior Vice President of Sales since January 2012. He previously served as a general manager of Accuray Incorporated’s Americas division from October 2009 to December 2012, and as
123
Table of Contents
Index to Financial Statements
general manager of Accuray Incorporated’s Europe, Middle East, India and Africa division from September 2008 to October 2009. Prior to Accuray Incorporated, Mr. Brandt worked at Philips Medical Systems, a healthcare company, serving as Senior Director MRI International from September 2007 to September 2008, as Senior Director MRI, North America from 2005 to September 2007 and as Marketing Director MRI, North America from 2001 to 2005. Mr. Brandt holds an N.D. in Electrical Engineering from Vaal University of Technology in South Africa.
D. David Chandler has served as our Chief Financial Officer since November 2010. Mr. Chandler has over 30 years of experience in finance, strategic planning, investor relations and accounting. Before joining ViewRay, Mr. Chandler served as a Practice Manager and consulting Chief Financial Officer with vcfo Holdings, Inc., a financial consulting firm, from October 2007 to November 2010. Prior to that, he served as Chief Operating Officer and Chief Financial Officer of Straitshot Communications, Inc., a network solutions company, from June 2003 to July 2007 and Chief Financial Officer of Stellar One Corporation, a technology company, from July 1998 to April 2003. Prior to Stellar One Corporation, Mr. Chandler held a variety of financing and accounting management roles, including Business Assurance Manager with PricewaterhouseCoopers LLP. Mr. Chandler received a B.A. in Business from the University of Washington, Michael G. Foster School of Business and a B.A. in German from the University of Washington.
Douglas H. Keare has served as our Chief Operating Officer since April 2015. Mr. Keare has over 20 years of technology and medical device executive experience. Before joining ViewRay, Mr. Keare was doing consulting work with and/or advising a number of startup companies from January 2014 to April 2015. He founded and served as CEO of RallyOn, Inc., a software company focused on corporate health and wellness, from October 2008 to December 2013. Prior to that, Mr. Keare served as Vice President of Customer and Technical Support at Accuray Inc. from December 2002 to January 2007. Mr. Keare also served as the President and Chief Operating Officer for Pricing Dynamics from July 2000 to July 2002. He held several positions at ADAC Laboratories in Customer Support, Operations and Quality from October 1992 to March 1999. As Vice President of Quality, he led ADAC’s successful effort to win the Malcolm Baldridge National Quality Award in 1996. Mr. Keare received a B.A. in Economics from Dartmouth College and an M.B.A. from Stanford University’s Graduate School of Business.
Board Composition
Non-Employee Directors
Joshua Bilenker, M.D. has served as a member of our board of directors since January 2008. Dr. Bilenker joined Aisling Capital LLC in April 2006 and has served as an Operating Partner since November 2013. He has served as Chief Executive Officer of Loxo Oncology, Inc., an Aisling Capital LLC portfolio company, since July 2013. Previously, Dr. Bilenker served as a Medical Officer in the Office of Oncology Drug Products at the FDA from August 2004 to April 2006. Dr. Bilenker has served on the boards of directors of Loxo Oncology, Inc. since July 2008, T2 Biosystems, Inc. since August 2011 and Roka Bioscience, Inc. since January 2012, as well as on the boards of directors of several private companies. He is also a board member of the NCCN Foundation and BioEnterprise. Dr. Bilenker holds an A.B. in English from Princeton University and an M.D. from the Johns Hopkins School of Medicine. We believe Dr. Bilenker is qualified to serve on our board of directors based on his oncology background, experience at the FDA and his extensive service as a director or officer of, and as an investor in, other healthcare companies.
David Bonita, M.D. has served as a member of our board of directors since January 2008. Dr. Bonita has served as a Private Equity Partner at OrbiMed Advisors LLC, or OrbiMed, since June 2013. Dr. Bonita joined OrbiMed in June 2004 as a Private Equity Senior Associate, and was promoted to Private Equity Principal in December 2007. Prior to OrbiMed, he was a corporate finance analyst in the healthcare investment banking group of Morgan Stanley & Co. from February 1998 to July 1999, and a corporate finance analyst in the healthcare investment banking group of UBS AG from August 1997 to February 1998. Dr. Bonita has also served on the board of directors of Loxo Oncology, Inc. since October 2013, as well as on the boards of directors of
124
Table of Contents
Index to Financial Statements
several private companies. Dr. Bonita holds an A.B. in Biological Sciences from Harvard College and an M.D. and M.B.A. from Columbia University in the City of New York. We believe Dr. Bonita is qualified to serve on our board of directors due to his extensive investment experience in the healthcare industry.
Caley Castelein, M.D. has served as a member of our board of directors since January 2008. Dr. Castelein has served as a Managing Director of Kearny Venture Partners, L.P. since September 2006. Prior to that, Dr. Castelein served as a Managing Director at Thomas Weisel Partners, which was acquired by Stifel, Nicolaus & Company, Incorporated in July 2010, from March 2003 to September 2006. Dr. Castelein has served on the boards of directors of several private companies. Dr. Castelein holds an A.B. in Biological Sciences from Harvard College and an M.D. from the University of California, San Francisco. We believe Dr. Castelein is qualified to serve on our board of directors based on his extensive investment experience in the healthcare industry.
Mark S. Gold, M.D. has served as a member of our board of directors since our founding in March 2004. Dr. Gold was a Professor, Distinguished Professor and Eminent Scholar of Neuroscience, Psychiatry, Community Health & Family Medicine at the University of Florida College of Medicine from 1990 until his retirement in 2014. Dr. Gold was a Founding Member and Executive Committee Member of the McKnight Brain Institute, and 17th Distinguished Alumni Professor of the University of Florida. Dr. Gold has worked for over 40 years in basic science and clinical research, translating neuroscientific research into clinical practice. He has been a consultant and senior advisor to banks and private equity and venture capital firms on medical devices, pharmaceuticals and health care services throughout his career. He was a Founding Director of the Somerset Valley Bank and Somerset Valley Financial from 1991 to 1999. Dr. Gold has served on the board of directors of Axogen, Inc. (formerly LecTec Corporation) since September 2011. Dr. Gold is also a member of the boards of directors of Magstim Ltd, Wales, UK and RiverMend Health, Atlanta, Georgia. We believe Dr. Gold is qualified to serve on our board of directors because of his academic expertise and his extensive research experience across medical specialties and institutions.
Aditya Puri has served as a member of our board of directors since February 2015. Mr. Puri has served as an Investments Director at Xeraya Capital, which is responsible for life sciences investments for Khazanah Nasional Berhad, since October 2012. Previously, he was a Director in Khazanah Nasional’s Life Sciences unit since November 2011, which was responsible for Khazanah’s life sciences investments. Prior to that, Mr. Puri consulted part time in the greater Boston area for various healthcare and cleantech startups affiliated with Harvard University and Massachusetts Institute of Technology, or MIT, from 2009 to 2011. Mr. Puri also served as Managing Director of global development at Salary.com from July 2007 to April 2008. Mr. Puri was at the Yankee Group, a global technology research and consulting company, from September 2000 to March 2007, finishing his tenure as a Vice-President and member of the leadership team. Between March 1997 and April 2000, he was at Boston Scientific, a Fortune 500 medical device manufacturer. Mr. Puri serves on several boards of directors of private companies in the investment and healthcare fields. Mr. Puri has a B.S. from the University of Southern Maine and received an M.B.A. from the MIT Sloan School of Management. We believe Mr. Puri is qualified to serve on our board of directors because of his extensive experience in life sciences investment and growth.
Brian K. Roberts has served as a member of our board of directors since December 2015. Mr. Roberts also served as the Chief Financial Officer at Avedro, Inc., a privately held pharmaceutical and medical device company, since January 2015, and currently serves as the Chief Operating and Financial Officer since December 2015. Prior to Avedro, Mr. Roberts was the Chief Financial Officer at Insulet Corporation, also a medical device company, since March 2009. Mr. Roberts also previously served as the Chief Financial Officer at Jingle Networks from August 2007 to January 2009 and as Chief Financial Officer of Digitas from June 2001 to July 2007. Mr. Roberts has also held finance positions at Idiom Technologies, Inc., the Monitor Group and has served as an auditor with Ernst & Young LLP. He holds a B.S. in Accounting and Finance from Boston College. We believe Mr. Roberts is qualified to serve on the Board because of his over 20 years of financial, operational and strategic experience in private and public companies.
125
Table of Contents
Index to Financial Statements
Robert Weisskoff, Ph.D. has served as a member of our board of directors since September 2015. Dr. Weisskoff has served as a Partner of Fidelity Biosciences, a division of FMR LLC, since November 2004. Prior to joining FMR LLC, he was Vice President of Business Development at EPIX Pharmaceuticals, Inc. from 1998 to October 2004. He previously served on the staff of Massachusetts General Hospital/Harvard Medical School as a Director and Associate Professor of Radiology from 1990 to 1998. From July 2007 to March 2013, Dr. Weisskoff served on the board of directors of Tetraphase Pharmaceuticals, Inc., and currently serves on the boards of directors of several private companies. Dr. Weisskoff holds an A.B. in Physics from Harvard University, a Ph.D. in Physics from the Massachusetts Institute of Technology and an M.B.A. from Columbia University in the City of New York. We believe Dr. Weisskoff is qualified to serve on our board of directors because of his investment experience in the healthcare industry and his extensive research and development and business development experience at medical device companies.
Director Independence
Our board of directors currently consists of eight members. We are not currently subject to listing requirements of any national securities exchange that has requirements that a majority of the board of directors be “independent.” Nevertheless, our board of directors has determined that all of our directors, other than Mr. Raanes and Dr. Dempsey, qualify as “independent” directors in accordance with listing requirements of The NASDAQ Stock Market, or NASDAQ. Mr. Raanes and Dr. Dempsey are not considered independent because each is an employee of ViewRay. The NASDAQ independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by NASDAQ rules, our board of directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
Classified Board of Directors
In accordance with our certificate of incorporation, our board of directors will be divided into three classes with staggered, three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. Our directors are divided among the three classes as follows:
| • | the Class I directors will be Chris A. Raanes, Aditya Puri and Robert Weisskoff, Ph.D., and their terms will expire at the annual meeting of stockholders to be held in 2016; |
| • | the Class II directors will be Josh Bilenker, M.D., James F. Dempsey, Ph.D. and Mark S. Gold, M.D., and their terms will expire at the annual meeting of stockholders to be held in 2017; and |
| • | the Class III directors will be David Bonita, M.D., Caley Castelein, M.D. and Brian K. Roberts, and their terms will expire at the annual meeting of stockholders to be held in 2018. |
Our certificate of incorporation will provide that the authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control of the company.
126
Table of Contents
Index to Financial Statements
Role of Board in Risk Oversight Process
Risk assessment and oversight are an integral part of our governance and management processes. Our board of directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the board of directors at regular board meetings as part of management presentations that focus on particular business functions, operations or strategies, and presents the steps taken by management to mitigate or eliminate such risks.
Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure, and our audit committee is responsible for overseeing our major financial risk exposures and the steps our management has taken to monitor and control these exposures. The audit committee also monitors compliance with legal and regulatory requirements. Our nominating and governance committee monitors the effectiveness of our corporate governance guidelines and considers and approves or disapproves any related-person transactions. Our compensation committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Board Committees
Audit Committee
Our audit committee oversees our corporate accounting and financial reporting process. Among other matters, the audit committee:
| • | appoints our independent registered public accounting firm; |
| • | evaluates our independent registered public accounting firm’s qualifications, independence and performance; |
| • | determines the engagement of our independent registered public accounting firm; |
| • | reviews and approves the scope of the annual audit and the audit fee; |
| • | discusses with management and our independent registered public accounting firm the results of the annual audit and the review of our quarterly financial statements; |
| • | approves the retention of our independent registered public accounting firm to perform any proposed permissible non-audit services; |
| • | monitors the rotation of partners of our independent registered public accounting firm on our engagement team as required by law; |
| • | is responsible for reviewing our financial statements and our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; |
| • | reviews our critical accounting policies and estimates; and |
| • | annually reviews the audit committee charter and the committee’s performance. |
The current members of our audit committee are Dr. Bonita, Mr. Puri and Mr. Roberts. Mr. Roberts serves as the chairperson of the audit committee. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and NASDAQ. Our board of directors has
127
Table of Contents
Index to Financial Statements
determined that Mr. Roberts is an audit committee financial expert as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of NASDAQ. Under the rules of the SEC, members of the audit committee must also meet heightened independence standards. However, a minority of the members of the audit committee may be exempt from the heightened audit committee independence standards for one year from the date of the Merger. Our board of directors has determined that each of Dr. Bonita, Mr. Puri and Mr. Roberts are independent under the applicable rules of NASDAQ. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and NASDAQ.
Compensation Committee
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The compensation committee reviews and recommends corporate goals and objectives relevant to compensation of our chief executive officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives and recommends to our board of directors the compensation of these officers based on such evaluations. The compensation committee also recommends to our board of directors the issuance of stock options and other awards under our stock plans. The compensation committee will review and evaluate, at least annually, the performance of the compensation committee and its members, including compliance by the compensation committee with its charter. The current members of our compensation committee are Drs. Bilenker and Castelein and Mr. Puri. Dr. Castelein serves as the chairperson of the compensation committee. Each of the members of our compensation committee is independent under the applicable rules and regulations of NASDAQ, is a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act and is an “outside director” as that term is defined in Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended, or the Code. The compensation committee operates under a written charter.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for making recommendations to our board of directors regarding candidates for directorships and the size and composition of our board of directors. In addition, the nominating and corporate governance committee is responsible for overseeing our corporate governance policies and reporting and making recommendations to our board of directors concerning governance matters. The current members of our nominating and corporate governance committee are Drs. Bonita, Castelein and Gold. Dr. Castelein serves as the chairperson of the nominating and corporate governance committee. Each of the members of our nominating and corporate governance committee is an independent director under the applicable rules and regulations of NASDAQ relating to nominating and corporate governance committee independence. The nominating and corporate governance committee operates under a written charter.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has at any time been one of our officers or employees. None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our board of directors or compensation committee.
128
Table of Contents
Index to Financial Statements
Board Diversity
Upon consummation of the Merger, our nominating and corporate governance committee will be responsible for reviewing with the board of directors, on an annual basis, the appropriate characteristics, skills and experience required for the board of directors as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the nominating and corporate governance committee, in recommending candidates for election, and the board of directors, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including the following:
| • | personal and professional integrity; |
| • | ethics and values; |
| • | experience in corporate management, such as serving as an officer or former officer of a publicly held company; |
| • | experience in the industries in which we compete; |
| • | experience as a director or executive officer of another publicly held company; |
| • | diversity of expertise and experience in substantive matters pertaining to our business relative to other board members; |
| • | conflicts of interest; and |
| • | practical and mature business judgment. |
Currently, our board of directors evaluates, and following the consummation of the Merger will evaluate, each individual in the context of the board of directors as a whole, with the objective of assembling a group that can best maximize the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics is available on our website at www.viewray.com. We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website. The reference to our web address does not constitute incorporation by reference of the information contained at or available through our website.
Limitation on Liability and Indemnification Matters
Our certificate of incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
| • | any breach of the director’s duty of loyalty to us or our stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | any transaction from which the director derived an improper personal benefit. |
Our certificate of incorporation and bylaws provide that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding,
129
Table of Contents
Index to Financial Statements
and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his, her or its actions in that capacity regardless of whether we would otherwise be permitted to indemnify him, her or it under Delaware law.
In addition to the indemnification required in our certificate of incorporation and bylaws, we have entered or intend to enter into indemnification agreements with each of our directors, officers and certain other employees prior to the consummation of the Merger. These agreements will provide for the indemnification of our directors, officers and certain other employees for all reasonable expenses and liabilities incurred in connection with any action or proceeding brought against them by reason of the fact that they are or were our agents. We believe that these provisions in our certificate of incorporation, bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. This description of the limitation of liability and indemnification provisions of our certificate of incorporation, of our bylaws and of our indemnification agreements is qualified in its entirety by reference to these documents, each of which is attached as an exhibit to this prospectus.
The limitation of liability and indemnification provisions in our certificate of incorporation and may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. There is no pending litigation or proceeding naming any of our directors, officers or employees as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director, officer or employee.
Director Compensation
From our inception to the date of this prospectus, no compensation was earned by or paid to Dinara Akzhigitova, who was our sole director. Ms. Akzhigitova resigned as our sole officer and director effective as of July 23, 2015 in connection with the Merger.
ViewRay became our wholly owned subsidiary upon the closing of the Merger on July 23, 2015. The following summarizes the compensation earned by ViewRay’s non-employee directors in ViewRay’s fiscal year ending December 31, 2014.
In 2014, ViewRay paid Dr. Gold, who is not affiliated with any of ViewRay’s major investors, an aggregate in cash of $14,000, which represents a quarterly retainer of $2,000 plus $2,000 per board meeting attended. Otherwise, ViewRay did not pay any cash compensation to any of the non-employee members of ViewRay’s board of directors, and ViewRay did not pay director fees to our directors who are ViewRay’s employees. However, ViewRay reimbursed ViewRay’s non-employee directors for travel and other necessary business expenses incurred in the performance of their services for ViewRay.
In addition, in April 2014, ViewRay granted each of Drs. Gold and Roemer options to purchase ViewRay common stock, which were converted into options to purchase 4,736 shares of our common stock, each having an exercise prices of $0.75 per share. These options vest and become exercisable in 48 monthly installments on each monthly anniversary of May 13, 2013 for Dr. Gold and November 13, 2013 for Dr. Roemer, subject to the individual continuing to provide services through each such vesting date.
130
Table of Contents
Index to Financial Statements
In connection with the Merger, we approved a compensation policy for our non-employee directors, or the Director Compensation Program. Pursuant to the Director Compensation Program, our non-employee directors will receive cash compensation, paid quarterly in arrears, as follows:
| • | Each non-employee director will receive an annual cash retainer in the amount of $40,000 per year. |
| • | Any non-employee Chairman will receive an additional annual cash retainer in the amount of $35,000 per year, and a lead independent director, if appointed, will receive an additional annual cash retainer in the amount of $7,500 per year. |
| • | The chairperson of the audit committee will receive additional annual cash compensation in the amount of $20,000 per year for such chairperson’s service on the audit committee. Each non-chairperson member of the audit committee will receive additional annual cash compensation in the amount of $10,000 per year for such member’s service on the audit committee. |
| • | The chairperson of the compensation committee will receive additional annual cash compensation in the amount of $15,000 per year for such chairperson’s service on the compensation committee. Each non-chairperson member of the compensation committee will receive additional annual cash compensation in the amount of $7,000 per year for such member’s service on the compensation committee. |
| • | The chairperson of the nominating and corporate governance committee will receive additional annual cash compensation in the amount of $10,000 per year for such chairperson’s service on the nominating and corporate governance committee. Each non-chairperson member of the nominating and corporate governance committee will receive additional annual cash compensation in the amount of $5,000 per year for such member’s service on the nominating and corporate governance committee. |
Under the Director Compensation Program, upon the director’s initial appointment or election to our board of directors, each non-employee director will receive an option (the Initial Grant) to purchase that number of shares of our common stock such that the award has an aggregate grant date fair value (as defined below) equal to $176,400, rounded down to the nearest whole share (subject to adjustment as provided in the applicable equity plan). In addition, each non-employee director who has been serving as a director and will continue to serve as a director immediately following each annual stockholder meeting, will be automatically granted, on the date of such annual stockholder meeting, an option (the Annual Grant) to purchase that number of shares of our common stock such that the award has an aggregate grant date fair value equal to $70,200, rounded down to the nearest whole share (subject to adjustment as provided in the applicable equity plan). For purposes of the Initial Grant and the Annual Grant, “grant date fair value” will mean the fair value of an award as of the date of grant as determined in accordance with ASC Topic 718, “Share-Based Payment”, using the Black-Scholes pricing model and the valuation assumptions used by the company in accounting for options as of such date of grant. The Initial Grant will vest as to 1/36th of the shares subject to Initial Grant on each monthly anniversary of the applicable grant date, subject to continued service through each applicable vesting date, and the Annual Grant will vest as to 1/12th of the shares subject to the Annual Grant on each month anniversary of the applicable grant date, subject to continued service through such vesting date. In addition, pursuant to the terms of the Director Compensation Program, all equity awards outstanding and held by a non-employee director will vest in full immediately prior to the occurrence of a change in control (as defined in the applicable equity plan such awards were granted under).
Upon the consummation of the Merger, we granted each of Drs. Bilenker, Bonita, Castelein, Gold and Mr. Puri an option to purchase 19,556 shares of our common stock at an exercise price per share equal to $5. The options vest and become exercisable in substantially equal monthly installments over the 12 months following the grant date, subject to the individual continuing to provide services to us through the applicable vesting date.
131
Table of Contents
Index to Financial Statements
2014 Director Compensation Table
The following table sets forth information for the year ended December 31, 2014 regarding the compensation awarded to, earned by or paid to ViewRay’s non-employee directors as if ViewRay been a reporting company on December 31, 2014. We did not pay any compensation to our one director, Dinara Akzhigitova, in fiscal year 2014:
| Name |
Fees Earned or Paid in Cash($) |
Option Awards ($)(1) |
Total($) | |||||||||
| Joshua Bilenker, M.D. |
$ | — | $ | — | $ | — | ||||||
| David Bonita, M.D. |
— | — | — | |||||||||
| Caley Castelein, M.D. |
— | — | — | |||||||||
| Mark S. Gold, M.D. |
14,000 | 2,079 | 16,079 | |||||||||
| Peter Roemer, Ph.D. |
1,225 | 2,079 | 3,304 | |||||||||
| Robert Weisskoff, Ph.D. |
— | — | — | |||||||||
| Philip Yang |
— | — | — | |||||||||
| (1) | The amounts reported in the Option Awards column represent the grant date fair values of stock options granted during the year ended December 31, 2014 as calculated in accordance with ASC Topic 718, excluding the impact of estimated forfeitures related to service-based vesting provisions. See Note 13 to our audited financial statements included elsewhere in this prospectus for the assumptions used in calculating these amounts. As of December 31, 2014, each of Drs. Gold and Roemer held options to purchase an aggregate of 17,404 shares of common stock (as converted pursuant to the Merger such that the numbers reflect the shares subject to the options as if they had been granted by us and not ViewRay). None of ViewRay’s other non-employee directors held any options or stock awards. |
Involvement in Certain Legal Proceedings
None of our directors or executive officers has been involved in any of the following events during the past 10 years:
| • | any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| • | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
| • | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; or |
| • | being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
132
Table of Contents
Index to Financial Statements
From our inception to the date of this prospectus, no compensation was earned by or paid to our sole named executive officer, Dinara Akzhigitova, who served as our principal executive. Ms. Akzhigitova resigned as our sole officer and director effective as of July 23, 2015 in connection with the Merger.
ViewRay became our wholly-owned subsidiary upon the closing of the Merger on July 23, 2015. The following summarizes the compensation earned in each of ViewRay’s fiscal years ended December 31, 2014 and includes a discussion of compensation arrangements by the individuals who would have been deemed its named executive officers, or NEOs, had ViewRay been a reporting company on December 31, 2014, which would have been as follows:
| • | Chris A. Raanes, President and Chief Executive Officer; |
| • | James F. Dempsey, Ph.D., Chief Scientific Officer; and |
| • | Michael Brandt, Senior Vice President of Sales. |
This discussion contains forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from currently planned programs as summarized in this discussion.
2014 Summary Compensation Table
The following table shows information regarding the compensation of the NEOs for services performed in the years ended December 31, 2014. All amounts reflect compensation received from ViewRay.
| Name and Principal Position | Year | Salary($) | Bonus($) | Option Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($)(2) |
All Other Compensation ($)(3) |
Total($) | |||||||||||||||||||||
| Chris A. Raanes |
2014 | 415,000 | — | 236,368 | 155,625 | 826,724 | ||||||||||||||||||||||
| President and Chief Executive Officer |
||||||||||||||||||||||||||||
| James F. Dempsey, Ph.D. |
2014 | 260,000 | — | 44,999 | 68,250 | — | 373,249 | |||||||||||||||||||||
| Chief Scientific Officer |
||||||||||||||||||||||||||||
| Michael Brandt |
2014 | 250,000 | — | 14,222 | 106,625 | — | 370,847 | |||||||||||||||||||||
| Senior Vice President of Sales |
||||||||||||||||||||||||||||
| (1) | The amounts reported in the Option Awards columns represent the grant date fair values of stock options granted during 2014 as calculated in accordance with ASC Topic 718, excluding the impact of estimated forfeitures related to service-based vesting provisions. See Note 13 to our audited financial statements included elsewhere in this prospectus for the assumptions used in calculating these amounts. |
| (2) | The amounts reported in the Non-Equity Incentive Plan Compensation column represent the annual cash performance-based bonuses earned by the NEOs pursuant to the achievement of certain company performance objectives. These amounts for 2014 were paid to the NEOs in early 2015. For Mr. Brandt, the amount also includes $41,000 for 2014 and $34,107 for 2013 that was payable pursuant to achievement of certain sales commissions under ViewRay’s sales compensation plan. For more information on the 2014 bonuses paid to the NEOs, please see the descriptions of the annual performance bonuses paid to the NEOs in “Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End—Terms and Conditions of Annual Bonuses” below and the description of the sales commissions paid to Mr. Brandt in “Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End—Terms and Conditions of Sales Compensation Plan” below. |
| (3) | The amounts reported in the All Other Compensation column in 2014 for Mr. Raanes represents commercial air travel of $11,367, and transportation and housing in the greater Cleveland area of $8,364 reimbursed by |
133
Table of Contents
Index to Financial Statements
| ViewRay pursuant to the terms and conditions of the Raanes Agreement (as defined below), whereby ViewRay reimburses the costs incurred by Mr. Raanes for commuting from his residence to ViewRay’s offices in Oakwood Village, Ohio. For more information, please see the description of the Raanes Agreement in “Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End—Terms and Conditions of Employment Agreement with Chris A. Raanes” below. |
Outstanding Equity Awards at 2014 Year-End
The following table sets forth all outstanding equity awards held by each of the NEOs at December 31, 2014: These options were converted into options to purchase our common stock in connection with the Merger, and the table below reflects all outstanding options as of December 31, 2014 as if they had been granted by us.
| Option Awards | ||||||||||||||||||||
| Name |
Vesting Commencement Date(1) |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||||||
| Chris A. Raanes |
2/4/2013 | (2) | 400,101 | 584,760 | 0.71 | 2/7/2023 | ||||||||||||||
| 5/13/2013 | 154,494 | 235,807 | 0.76 | 4/11/2024 | ||||||||||||||||
| 11/13/2013 | 40,230 | 108,313 | 0.76 | 4/11/2024 | ||||||||||||||||
| James F. Dempsey, Ph.D. |
1/8/2008 | (2) | 75,243 | — | 0.81 | 6/17/2018 | ||||||||||||||
| 9/30/2014 | (3) | — | 51,291 | 0.81 | 9/30/2018 | |||||||||||||||
| 6/17/2010 | (4) | 61,752 | — | 0.69 | 6/29/2020 | |||||||||||||||
| 7/14/2010 | 197,635 | — | 0.69 | 6/29/2020 | ||||||||||||||||
| 3/1/2012 | 43,494 | 19,762 | 0.71 | 8/8/2022 | ||||||||||||||||
| 5/13/2013 | 38,062 | 58,086 | 0.76 | 4/11/2024 | ||||||||||||||||
| 11/13/2013 | 64,503 | 173,653 | 0.76 | 4/11/2024 | ||||||||||||||||
| Michael Brandt |
1/9/2012 | (2) | 64,328 | 23,895 | 0.69 | 12/1/2021 | ||||||||||||||
| 3/1/2012 | 11,468 | 5,209 | 0.71 | 8/8/2022 | ||||||||||||||||
| 5/13/2013 | 10,031 | 15,315 | 0.76 | 4/11/2024 | ||||||||||||||||
| 11/13/2013 | 1,915 | 5,155 | 0.76 | 2/7/2023 | ||||||||||||||||
| (1) | Except as otherwise noted, options vest and become exercisable in 48 installments on each monthly anniversary of the vesting commencement date, such that all awards will be vested on the fourth anniversary of the vesting commencement date, subject to the holder continuing to provide services to the company through such vesting date. |
| (2) | The option vests and becomes exercisable as to 25% of the shares on the first anniversary of the vesting commencement date, and in 36 installments thereafter on each monthly anniversary of the vesting commencement date, such that all awards will be vested on the fourth anniversary of the vesting commencement date, subject to the holder continuing to provide services to the company through such vesting date. For Dr. Dempsey’s option, in the event Dr. Dempsey is terminated without “cause” within 12 months following a “change of control” (each as defined below in the section titled “—Offer Letter to James F. Dempsey, Ph.D.”), the vesting and exercisability of the option shall accelerate in full as of the date of such termination. For Mr. Raanes’ option, (i) if Mr. Raanes is terminated without “cause” or resigns for “good reason” (each as defined below in the section titled “—Employment Agreement with Chris A. Raanes”), the vesting and exercisability of the option shall accelerate with respect to the number of shares that would have vested in the 12 months following Mr. Raanes’ termination; and (ii) in the event Mr. Raanes is terminated without cause or resigns for good reason within the period of time commencing three months prior to a “change of control” (as defined below in the section titled “—Employment Agreement with Chris A. Raanes”) and ending 18 months following such change of control, then the vesting and exercisability of the option shall accelerate in full as of the date of such termination. |
134
Table of Contents
Index to Financial Statements
| (3) | The option vests and becomes exercisable in full upon the occurrence of one of the following events on or before December 31, 2014: (a) immediately prior to the closing of a “corporate reorganization” (as defined in ViewRay’s contingent equity agreement, effective January 8, 2008) in which the company and/or our stockholders receive at least $500 million; or (b) immediately prior to the closing of a firm commitment underwritten public offering of shares of common” stock to the public at a pre-money valuation of at least $500 million. The option did not vest on or before December 31, 2014. |
| (4) | The option vests and becomes exercisable in 36 installments on each monthly anniversary of the vesting commencement date, such that all awards will be vested on the third anniversary of the vesting commencement date, subject to Dr. Dempsey continuing to provide services to the company through such vesting date. |
Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End
In connection with the Merger, each of the NEOs continues to be employed with us under the terms of their employment agreement or offer letter, as applicable, with ViewRay.
Employment Agreement with Chris A. Raanes
In January 2013, ViewRay entered into an employment agreement with Mr. Raanes, or the Raanes Agreement, to serve as our President and Chief Executive Officer and as a member of our board of directors, providing for base salary, target annual bonus opportunity and standard employee benefit plan participation. Mr. Raanes’ base salary is subject to annual increases in the sole discretion of the board of directors. Mr. Raanes’ base salary for 2014 was $415,000, and he had an annual bonus target of 50% of base salary that is earned based on the achievement of certain milestones. Please see the section below titled “Terms and Conditions of Annual Bonuses” for a further description of the annual bonuses awarded to Mr. Raanes. In 2013, Mr. Raanes was also paid a $50,000 signing bonus, 50% of such signing bonus was paid to Mr. Raanes on the date he commenced employment with ViewRay and the remaining 50% of such signing bonus was paid on the six-month anniversary of such date. In addition, ViewRay reimbursed or directly paid for the costs incurred by Mr. Raanes for housing and transportation in the greater Cleveland area and commercial air travel from his residence in California to its offices in Ohio. In 2014, ViewRay reimbursed $8,364 in the aggregate for Mr. Raanes’ housing and transportation in the greater Cleveland area and $11,367 for commercial air travel from his residence to its offices in Ohio. Mr. Raanes is now based in our headquarters in Mountain View, California. Pursuant to the Raanes Agreement, ViewRay granted Mr. Raanes a stock option to purchase 984,861 shares of common stock, which equaled 5% of our issued and outstanding shares as of January 18, 2013. Under the Raanes Agreement, Mr. Raanes’ employment is terminable at-will. Mr. Raanes has also executed ViewRay’s standard confidential information and invention assignment agreement, which contains certain non-competition covenants.
The Raanes Agreement also provides Mr. Raanes with certain severance and change in control benefits. Mr. Raanes’ was eligible to participate in any carveout plan that ViewRay adopted, with minimum levels of compensation at various transaction price levels as set forth in the Raanes Agreement. ViewRay never adopted any such carveout plan (and as a result Mr. Raanes was not eligible for any such carveout payments).
In addition, pursuant to the Raanes Agreement, if Mr. Raanes’ employment is terminated without cause or Mr. Raanes resigns for “good reason” (as defined below) at any time three months prior to or 18 months following a change of control, then the vesting and exercisability of Mr. Raanes’ initial option granted under the Raanes Agreement will accelerate in full.
Additionally, in the event that Mr. Raanes is terminated without cause or resigns for good reason, subject to his executing and not revoking a general release of all claims, then Mr. Raanes will become entitled to receive (i) a severance payment equal to 12 months of his annual base salary, payable in substantially equal installments, (ii) a lump sum cash payment equal to a pro-rated portion of his annual performance bonus payable on the later of (a) the annual date bonuses are made to current employees and (b) the first installment payment for the base
135
Table of Contents
Index to Financial Statements
salary severance, (iii) payment or reimbursement by us of COBRA premiums for up to 12 months, and (iv) accelerated vesting of Mr. Raanes’ option granted under the Raanes Agreement with respect to that number of shares that would have vested had he remained employed with the company for an additional 12 months.
Under the Raanes Agreement, “change of control” means (i) a sale of all or substantially all of the assets of the company and its subsidiaries taken as a whole or (ii) a merger, consolidation or other similar business combination involving the company, if, upon completion of such transaction, the beneficial owners of voting equity securities of the company immediately prior to the transaction beneficially own less than 50% of the successor entity’s voting equity securities; provided that “change of control” will not include a transaction where the consideration received or retained by the holders of the then-outstanding capital stock of the company does not consist primarily of (a) cash or cash equivalent consideration, (b) securities which are registered under the Securities Act or any successor statute thereto, or (c) securities for which the company or any other issuer thereof has agreed, including pursuant to a demand, to file a registration statement within 90 days of completion of the transaction for resale to the public pursuant to the Securities Act.
Under the Raanes Agreement, “cause” means Mr. Raanes’ (i) dishonesty of a material nature; (ii) theft or embezzlement of our funds or assets; (iii) conviction of, or guilty or no contest plea to, a felony charge or misdemeanor involving moral turpitude, or the entry of a consent decree with any governmental body; (iv) noncompliance in any material respect with any laws or regulations, foreign or domestic; (v) violation of any express direction or any rule, regulation or policy established by the board of directors that is consistent with the terms of the Raanes Agreement; (vi) material breach of the Raanes Agreement or material breach of Mr. Raanes’ fiduciary duties to the company; (vii) gross incompetence, gross neglect or gross misconduct in the performance of his duties; or (viii) repeated and consistent failure to perform the duties under the Raanes Agreement during normal business hours except during vacation periods or absences due to temporary illness. If the board of directors determines in good faith that cause exists, Mr. Raanes will be given written notice by the board of directors that provides the factual basis for the determination prior to that determination being final and Mr. Raanes will have 10 business days to respond and to attempt to cure the condition, although no cure period need be offered if the board of directors reasonably determines that the conditions are not subject to cure.
Under the Raanes Agreement, “good reason” means a resignation that occurs within 30 days following Mr. Raanes’ first having knowledge of any (i) material reduction in his base salary, (ii) material breach of the Raanes Agreement by the company, or (iii) material diminution of Mr. Raanes’ title as Chief Executive Officer or responsibility as Chief Executive Officer imposed by the board of directors (other than in response to an event constituting cause). With respect to subsection (i), any reduction consistent with general reductions in the base salaries of other executives as part of a plan to avoid insolvency or manage any financial distress or hardship of the company will not be deemed to constitute a material reduction in his base salary; and with respect to subsection (ii), good reason will only exist where Mr. Raanes’ has provided the company with written notice of the breach and the company has failed to cure such breach within 10 business days of such written notice.
Offer Letter to James F. Dempsey, Ph.D.
In October 2010, ViewRay entered into an offer letter with Dr. Dempsey that provides for employment at-will and annual base salary, annual target bonus, option awards and certain other benefits. Dr. Dempsey’s base salary for 2014 was $260,000. In addition, for 2014, Dr. Dempsey has an annual target bonus of 35% of base salary awarded based on the achievement of certain milestones. Please see the section titled “Terms and Conditions of Annual Bonuses” below for a further description of the annual bonuses awarded to Dr. Dempsey. His offer letters also contain certain non-disparagement and non-competition restrictive covenants (during Dr. Dempsey’s employment and for 12 months following termination).
The offer letter also provides Dr. Dempsey with certain severance and change of control benefits.
In the event that Dr. Dempsey is terminated without cause, subject to executing and not revoking a general release of all claims, then Dr. Dempsey is entitled to receive a severance payment equal to 12 months of his base
136
Table of Contents
Index to Financial Statements
salary plus his annual bonus for the year preceding the termination date, payable in substantially equal installments over the six-month period following his termination.
“Change of control” has the same meaning as under the Raanes Agreement. “Cause” means Dr. Dempsey’s (i) willful failure to perform his material duties, other than a failure resulting from his complete or partial incapacity due to long-term physical or mental illness or impairment, (ii) willful act that constitutes gross misconduct and that is injurious to the company, (iii) willful breach of a provision of the offer letter, (iv) material or willful violation of a federal or state law or regulation applicable to the business of the company, or (v) conviction or plea of guilty or no contest to a felony.
Offer Letter to Michael Brandt
In December 2011, ViewRay entered into an offer letter with Mr. Brandt that provides for employment at-will and other terms and conditions similar to Dr. Dempsey, except as provided below. Mr. Brandt’s base salary for 2014 was $250,000. Mr. Brandt is also eligible for additional compensation under the company’s sales compensation plan. Please see the section below titled “Terms and Conditions of Sales Compensation Plan” for a further description of Mr. Brandt’s sales commissions.
The offer letter also provides Mr. Brandt with certain severance and change of control benefits. In the event Mr. Brandt is terminated without cause or resigns for “good reason” (as defined below) at any time within 12 months following a change of control, then in addition to the severance payments described below, the vesting and exercisability of Mr. Brandt’s option granted under his offer letter will accelerate in full.
In addition, in the event that Mr. Brandt is terminated without cause or resigns for good reason, subject to executing and not revoking a general release of all claims, then Mr. Brandt is entitled to receive a severance payment equal to four months of his base salary plus one-third of his annual bonus for the year preceding the termination date payable in substantially equal installments over the four-month period following his termination. “Change of control” has the same meaning as under the Raanes Agreement. “Cause” has the same meaning as under Dr. Dempsey’s offer letter, “Good reason” means the occurrence of one or more of the following conditions, without Mr. Brandt’s consent and without remedy by the company: (i) a material reduction in his compensation, including but not limited to his level of base salary and annual bonus opportunity, other than reductions approved by the board of directors that are applicable to all employees; (ii) a material, non-voluntary, reduction in his authority, duties, position, title or responsibilities or a material, adverse change in his reporting structure; or (iii) a reduction in the kind or level of his benefits to which he was entitled immediately prior to such reduction, other than reductions approved by the board of directors that are applicable to all employees of the company.
Terms and Conditions of Annual Bonuses
For 2014, all of the NEOs were eligible for cash performance-based bonuses pursuant to the achievement of certain performance objectives. The performance targets are approved annually by ViewRay’s board of directors. When determining the 2014 performance bonus program for the NEOs, in late 2013, the board of directors set certain performance goals, using a mixture of performance objectives after receiving input from ViewRay’s Chief Executive Officer. These performance objectives included installing MRIdian systems and commencing patient treatment, obtaining new sales orders, building manufacturing capabilities, implementing cost-reduction programs and building a world class management and employee team. There was no specific weighting for each performance goal when determining the overall bonus amount, and instead the board of directors evaluated the overall achievement of all performance goals based on the importance to the success of the company. For each of these performance goals under the annual bonus program, the board of directors set general performance goals, but there was no minimum or maximum achievement for each performance target; instead, the board of directors weighed the achievement, partial achievement or non-achievement for each performance target when deciding the overall achievement level. These performance goals were not expected to be attained based on average or below-average performance. The board of directors intended for the performance targets to require significant
137
Table of Contents
Index to Financial Statements
effort on the part of the NEOs and, therefore, set these targets at levels they believed would be difficult to achieve, such that average or below-average performance would not satisfy these targets.
Each NEO’s target bonus opportunity is expressed as a percentage of base salary which can be achieved by meeting corporate goals. For each of the NEOs, his target bonus opportunity is originally set in his employment agreement or offer letter, as applicable, with the company as described above. The board of directors reviews these target percentages to ensure they are adequate, and, while reviewing these target percentages the board of directors does not follow a formula but rather uses the factors as general background information prior to determining the target bonus opportunity rates for the participating NEOs. The board of directors sets these rates based on each participating executive’s experience in his role with the company and the level of responsibility held by each executive, which the board of directors believes directly correlates to his ability to influence corporate results. For 2014, the board of directors used a guideline target bonus opportunity of 50% for Mr. Raanes and 35% for Dr. Dempsey and Mr. Brandt.
Corporate goals and performance targets are reviewed and approved by the board of directors prior to any allocation of the annual bonuses. In early 2015, the board of directors reviewed ViewRay’s 2014 company-wide performance with respect to determining bonuses for executive officers and determined achievement of the performance goals at 75%. Following its review and determinations, the board of directors awarded cash bonuses to the NEOs at 75% of their target bonus opportunity ($155,625 for Mr. Raanes, $68,250 for Dr. Dempsey and $65,625 for Mr. Brandt). The NEOs’ 2014 annual bonuses are set forth in the “2014 Summary Compensation Table” above.
Terms and Conditions of Sales Compensation Plan
For 2014, in addition to the target performance bonus amount described above, Mr. Brandt was also eligible for performance-based cash incentives under a sales compensation plan. For 2014, under his sales compensation plan, Mr. Brandt was eligible to earn commissions based upon the achievement of certain sales targets. For 2014, each of these sales targets were set at levels we determined would require significant effort to achieve and would not be met by average or below-average performance. Commissions for the sales targets are payable monthly or semi-monthly, at our sole discretion. For 2014, Mr. Brandt was awarded $41,000 for achievement of the sales commissions goals.
Terms and Conditions of Equity Awards
In 2014, ViewRay granted two options to each of our NEOs. In April 2014, the board of directors granted the following options to purchase ViewRay’s common stock to each of the NEOs: 390,302 shares and 148,544 shares with vesting commencement dates of May 11, 2013 and November 11, 2013, respectively, for Mr. Raanes; 96,149 shares and 238,156 shares with vesting commencement dates of May 11, 2013 and November 11, 2013, respectively, for Dr. Dempsey; and 25,347 shares and 7,071 shares with vesting commencement dates of May 11, 2013 and November 11, 2013, respectively, for Mr. Brandt. These options were converted into options to purchase our common stock in connection with the Merger, so the foregoing numbers reflect the number of shares of our common stock as if they had been granted by us in 2014. Each of these options had an exercise price of $0.76 per share, which the board determined was the fair market value on the date of grant (as converted to reflect our common stock). The options vest and become exercisable in 48 monthly installments on each monthly anniversary of the applicable vesting commencement date, such that all awards will be vested on the four-year anniversary of the vesting commencement date, subject to the individual continuing to provide services to us through each such vesting date.
Upon the consummation of the Merger, Mr. Raanes, Dr. Dempsey and Mr. Brandt received an option to purchase 454,776, 273,039 and 40,987 shares of our common stock, respectively, at an exercise price per share equal to $5. The options will vest and become exercisable in substantially equal monthly installments over the four years following the grant date, subject to the individual continuing to provide services to us through the applicable vesting date.
138
Table of Contents
Index to Financial Statements
Terms and Conditions of 401(k) Plan
In June 2008, ViewRay adopted our 401(k) Retirement Savings Plan for employees. The 401(k) plan is intended to qualify under Section 401(k) of the Code so that contributions to the 401(k) plan by employees or by ViewRay, and the investment earnings thereon, are not taxable to the employees until withdrawn from the 401(k) plan, and so that contributions by ViewRay, if any, will be deductible by us when made. Under the 401(k) plan, employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit and to have the amount of such reduction contributed to the 401(k) plan. ViewRay does not currently make any matching contributions under our 401(k) plan. We have assumed the ViewRay 401(k) plan in connection with the Merger.
Equity Compensation Plans
The principal features of our equity incentive plans and agreements are summarized below. These summaries are qualified in their entirety by reference to the text of the plans or agreements, which are filed as exhibits to this prospectus.
2015 Equity Incentive Award Plan
In connection with the Merger, we have adopted the 2015 Plan, which was effective immediately prior to the consummation of the Merger. The principal purpose of the 2015 Plan is to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards and cash-based performance bonus awards. The material terms of the 2015 Plan are summarized below.
Share Reserve. Under the 2015 Plan, 4,708,343 shares of common stock will be initially reserved for issuance pursuant to a variety of stock-based compensation awards, including stock options, stock appreciation rights, or SARs, restricted stock awards, restricted stock unit awards, deferred stock awards, dividend equivalent awards, stock payment awards, performance awards and other stock-based awards. As of the date of this prospectus, and in connection with the consummation of the Merger, options to purchase 1,375,786 shares of our common stock have been granted under the 2015 Plan to our executive officers and directors, and options to purchase 131,361 shares have been granted under the 2015 Plan to other employees and consultants. The number of shares initially reserved for issuance or transfer pursuant to awards under the 2015 Plan will be increased by an annual increase on the first day of each year beginning in 2017 and ending in 2026, equal to the least of (a) four percent (4%) of the shares of stock outstanding (on an as-converted basis) on the last day of the immediately preceding year and (b) such smaller number of shares of stock as determined by our board of directors; provided, however, that no more than 15,000,000 shares of common stock may be issued upon the exercise of incentive stock options, or ISOs. The following counting provisions will be in effect for the share reserve under the 2015 Plan:
| • | to the extent that an award terminates, expires or lapses for any reason or an award is settled in cash without the delivery of shares, any shares subject to the award at such time will be available for future grants under the 2015 Plan; |
| • | to the extent shares are tendered or withheld to satisfy the grant, exercise price or tax withholding obligation with respect to any award under the 2015 Plan, such tendered or withheld shares will be available for future grants under the 2015 Plan; |
| • | to the extent that shares of common stock are repurchased by us prior to vesting so that shares are returned to us, such shares will be available for future grants under the 2015 Plan; |
| • | the payment of dividend equivalents in cash in conjunction with any outstanding awards will not be counted against the shares available for issuance under the 2015 Plan; and |
| • | to the extent permitted by applicable law or any exchange rule, shares issued in assumption of, or in substitution for, any outstanding awards of any entity acquired in any form of combination by us or any of our subsidiaries will not be counted against the shares available for issuance under the 2015 Plan. |
139
Table of Contents
Index to Financial Statements
Administration. The compensation committee is expected to administer the 2015 Plan unless our board of directors assumes authority for administration. The compensation committee must consist of at least three members of our board of directors, each of whom is intended to qualify as an “outside director,” within the meaning of Section 162(m) of the Code, a “non-employee director” for purposes of Rule 16b-3 under the Exchange Act and an “independent director” within the meaning of the NASDAQ rules. The 2015 Plan provides that the board of directors or compensation committee may delegate its authority to grant awards to employees other than executive officers to a committee consisting of one or more members of our board of directors or one or more of our officers, other than awards made to our non-employee directors, which must be approved by our full board of directors.
Subject to the terms and conditions of the 2015 Plan, the administrator has the authority to select the persons to whom awards are to be made, to determine the number of shares to be subject to awards and the terms and conditions of awards, and to make all other determinations and to take all other actions necessary or advisable for the administration of the 2015 Plan. The administrator is also authorized to adopt, amend or rescind rules relating to administration of the 2015 Plan. Our board of directors may at any time remove the compensation committee as the administrator and revest in itself the authority to administer the 2015 Plan. The full board of directors will administer the 2015 Plan with respect to awards to non-employee directors.
Eligibility. Options, SARs, restricted stock and all other stock-based and cash-based awards under the 2015 Plan may be granted to individuals who are then our officers, employees or consultants or are the officers, employees or consultants of certain of our subsidiaries. Such awards also may be granted to our directors. Only employees of the company or certain of our subsidiaries may be granted ISOs.
Awards. The 2015 Plan provides that the administrator may grant or issue stock options, SARs, restricted stock awards, restricted stock unit awards, deferred stock awards, deferred stock unit awards, dividend equivalent awards, performance awards, stock payment awards and other stock-based and cash-based awards, or any combination thereof. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
| • | Nonstatutory Stock Options, or NSOs, will provide for the right to purchase shares of common stock at a specified price that may not be less than the fair market value of a share of common stock on the date of grant, and usually will become exercisable (at the discretion of the administrator) in one or more installments after the grant date, subject to the participant’s continued employment or service with us and/or subject to the satisfaction of corporate performance targets and individual performance targets established by the administrator. NSOs may be granted for any term specified by the administrator that does not exceed 10 years. |
| • | Incentive Stock Options will be designed in a manner intended to comply with the provisions of Section 422 of the Code and will be subject to specified restrictions contained in the Code. Among such restrictions, ISOs must have an exercise price of not less than the fair market value of a share of our common stock on the date of grant, may only be granted to employees, and must not be exercisable after a period of 10 years measured from the date of grant. In the case of an ISO granted to an individual who owns (or is deemed to own) at least 10% of the total combined voting power of all classes of our capital stock, the 2015 Plan provides that the exercise price must be at least 110% of the fair market value of a share of our common stock on the date of grant and the ISO must not be exercisable after a period of five years measured from the date of grant. |
| • | Restricted Stock Awards may be granted to any eligible individual and made subject to such restrictions as may be determined by the administrator. Restricted stock, typically, may be forfeited for no consideration or repurchased by us at the original purchase price if the conditions or restrictions on vesting are not met. In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Purchasers of restricted stock, unlike recipients of options, will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions |
140
Table of Contents
Index to Financial Statements
| lapse; however, extraordinary dividends will generally be placed in escrow, and will not be released until restrictions are removed or expire. |
| • | Restricted Stock Unit Awards may be awarded to any eligible individual, typically without payment of consideration, but subject to vesting conditions based on continued employment or service or on performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| • | Deferred Stock Awards represent the right to receive shares of common stock on a future date. Deferred stock may not be sold or otherwise hypothecated or transferred until issued. Deferred stock will not be issued until the deferred stock award has vested, and recipients of deferred stock generally will have no voting or dividend rights prior to the time when the vesting conditions are satisfied and the shares are issued. Deferred stock awards generally will be forfeited, and the underlying shares of deferred stock will not be issued, if the applicable vesting conditions and other restrictions are not met. |
| • | Deferred Stock Units are denominated in unit equivalent of shares of common stock, and vest pursuant to a vesting schedule or performance criteria set by the administrator. The common stock underlying deferred stock units will not be issued until the deferred stock units have vested, and recipients of deferred stock units generally will have no voting rights prior to the time when vesting conditions are satisfied. |
| • | Stock Appreciation Rights may be granted in connection with stock options or other awards, or separately. SARs granted in connection with stock options or other awards typically will provide for payments to the holder based upon increases in the price of our common stock over a set exercise price. The exercise price of any SAR granted under the 2015 Plan must be at least 100% of the fair market value of a share of our common stock on the date of grant. Except as required by Section 162(m) of the Code with respect to a SAR intended to qualify as performance-based compensation as described in Section 162(m) of the Code, there are no restrictions specified in the 2015 Plan on the exercise of SARs or the amount of gain realizable therefrom, although restrictions may be imposed by the administrator in the SAR agreements. SARs under the 2015 Plan will be settled in cash or shares of common stock, or in a combination of both, at the election of the administrator. |
| • | Dividend Equivalent Awards represent the value of the dividends, if any, per share paid by us, calculated with reference to the number of shares covered by the award. Dividend equivalents may be settled in cash or shares and at such times as determined by our compensation committee or board of directors, as applicable. |
| • | Performance Awards may be granted by the administrator on an individual or group basis. Generally, these awards will be based upon specific performance targets and may be paid in cash or in common stock or in a combination of both. Performance awards may include “phantom” stock awards that provide for payments based upon the value of our common stock. Performance awards may also include bonuses that may be granted by the administrator on an individual or group basis and that may be payable in cash or in common stock or in a combination of both. |
| • | Stock Payment Awards may be authorized by the administrator in the form of common stock or an option or other right to purchase common stock as part of a deferred compensation or other arrangement in lieu of all or any part of compensation, including bonuses, that would otherwise be payable in cash to the employee, consultant or non-employee director. |
Change in Control. In the event of a change in control where the acquirer does not assume or replace awards granted prior to the consummation of such transaction, awards issued under the 2015 Plan will be subject to accelerated vesting such that 100% of such awards will become vested and exercisable or payable, as
141
Table of Contents
Index to Financial Statements
applicable. Performance awards will vest in accordance with the terms and conditions of the applicable award agreement. In the event that, within the 12 month period immediately following a change in control, a participant’s services with us are terminated by us other than for cause (as defined in the 2015 Plan) or by such participant for good reason (as defined in the 2015 Plan), then the vesting and, if applicable, exercisability of 100% of the then-unvested shares subject to the outstanding equity awards held by such participant under the 2015 Plan will accelerate effective as of the date of such termination. The administrator may also make appropriate adjustments to awards under the 2015 Plan and is authorized to provide for the acceleration, cash-out, termination, assumption, substitution or conversion of such awards in the event of a change in control or certain other unusual or nonrecurring events or transactions. Under the 2015 Plan, a change in control is generally defined as:
| • | the transfer or exchange in a single transaction or series of related transactions by our stockholders of more than 50% of our voting stock to a person or group; |
| • | a change in the composition of our board of directors over a two-year period such that the members of the board of directors who were approved by at least two-thirds of the directors who were directors at the beginning of the two-year period or whose election or nomination was so approved cease to constitute a majority of the board of directors; |
| • | a merger, consolidation, reorganization or business combination in which we are involved, directly or indirectly, other than a merger, consolidation, reorganization or business combination that results in our outstanding voting securities immediately before the transaction continuing to represent a majority of the voting power of the acquiring company’s outstanding voting securities and after which no person or group beneficially owns 50% or more of the outstanding voting securities of the surviving entity immediately after the transaction; or |
| • | stockholder approval of our liquidation or dissolution. |
Adjustments of Awards. In the event of any stock dividend, stock split, spin-off, recapitalization, distribution of our assets to stockholders (other than normal cash dividends) or any other corporate event affecting the number of outstanding shares of our common stock or the share price of our common stock other than an “equity restructuring” (as defined below), the administrator may make appropriate, proportionate adjustments to reflect the event giving rise to the need for such adjustments, with respect to:
| • | the aggregate number and type of shares subject to the 2015 Plan; |
| • | the number and kind of shares subject to outstanding awards and terms and conditions of outstanding awards (including, without limitation, any applicable performance targets or criteria with respect to such awards); and |
| • | the grant or exercise price per share of any outstanding awards under the 2015 Plan. |
In the event of one of the adjustments described above or other corporate transactions, in order to prevent dilution or enlargement of the potential benefits intended to be made available under the 2015 Plan, the administrator has the discretion to make such equitable adjustments and may also:
| • | provide for the termination or replacement of an award in exchange for cash or other property; |
| • | provide that any outstanding award cannot vest, be exercised or become payable after such event; |
| • | provide that awards may be exercisable, payable or fully vested as to shares of common stock covered thereby; or |
| • | provide that an award under the 2015 Plan cannot vest, be exercised or become payable after such event. |
In the event of an equity restructuring, the administrator will make appropriate, proportionate adjustments to the number and type of securities subject to each outstanding award and the exercise price or grant price thereof, if
142
Table of Contents
Index to Financial Statements
applicable. In addition, the administrator will make equitable adjustments, as the administrator in its discretion may deem appropriate to reflect such equity restructuring, with respect to the aggregate number and type of shares subject to the 2015 Plan. The adjustments upon an equity restructuring are nondiscretionary and will be final and binding on the affected holders and the Company.
For purposes of the 2015 Plan, “equity restructuring” means a nonreciprocal transaction between us and our stockholders, such as a stock dividend, stock split, spin-off, rights offering or recapitalization through a large, nonrecurring cash dividend, that affects the number or kind of shares (or other securities) or the share price of our common stock (or other securities) and causes a change in the per share value of the common stock underlying outstanding stock-based awards granted under the 2015 Plan. In the event of a stock split in connection with an offering, the administrator will proportionately adjust (i) the number of shares subject to any outstanding award under the 2015 Plan, (ii) the exercise or grant price of any such awards, if applicable, and (iii) the aggregate number of shares subject to the 2015 Plan.
Amendment and Termination. Our board of directors or the compensation committee (with board approval) may terminate, amend or modify the 2015 Plan at any time and from time to time. However, we must generally obtain stockholder approval:
| • | to increase the number of shares available under the 2015 Plan (other than in connection with certain corporate events, as described above); |
| • | reduce the price per share of any outstanding option or SAR granted under the 2015 Plan; |
| • | cancel any option or SAR in exchange for cash or another award when the option or SAR price per share exceeds the fair market value of the underlying shares; or |
| • | to the extent required by applicable law, rule or regulation (including any NASDAQ rule). |
Termination. Our board of directors may terminate the 2015 Plan at any time. No ISOs may be granted pursuant to the 2015 Plan after the 10th anniversary of the effective date of the 2015 Plan, and no additional annual share increases to the 2015 Plan’s aggregate share limit will occur from and after such anniversary. Any award that is outstanding on the termination date of the 2015 Plan will remain in force according to the terms of the 2015 Plan and the applicable award agreement.
We intend to file with the SEC a registration statement on Form S-8 covering the shares of common stock issuable under the 2015 Plan.
2008 Stock Option and Incentive Plan
We assumed the 2008 Plan in connection with the Merger, and it continues to govern the ViewRay stock options assumed and converted by us in connection with the Merger. No further awards will be granted under the 2008 Plan. The 2008 Plan was amended to increase the share reserve on June 17, 2010, July 14, 2010, September 16, 2011, August 8, 2012, February 7, 2013, May 8, 2013 and November 18, 2013. The 2008 Plan provided for the grant of ISOs, NSOs, restricted stock awards, restricted stock units and SARs. At September 30, 2015, options to purchase 6,011,160 shares of common stock at a weighted-average exercise price per share of $2.11 remained outstanding under the 2008 Plan. No other equity awards have been granted under the 2008 Plan.
Administration. Our board of directors, or a committee thereof appointed by our board of directors, has the authority to administer the 2008 Plan and the awards granted under it. The administrator has the authority to select the employees to whom awards will be granted under the 2008 Plan, the size and type of awards to be subject to those awards under the 2008 Plan, and the terms and conditions of the awards granted. In addition, the administrator has the authority to construe and interpret the 2008 Plan, to establish, amend or waive rules and resolutions for the 2008 Plan’s administration and to amend the terms and conditions of any outstanding award as allowed under the 2008 Plan and such awards. The administrator may make all other determinations that may be necessary or advisable for the administration of the 2008 Plan.
143
Table of Contents
Index to Financial Statements
Awards. The 2008 Plan provides that the administrator may grant or issue stock incentive awards, including ISOs and NSOs. In addition, no more than 206,896 shares subject to equity awards can be granted to any one participant in any calendar year. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
| • | Stock Option Awards. The 2008 Plan provides for the grant of ISOs under the federal tax laws or NSOs. ISOs may be granted only to employees. NSOs may be granted to employees, directors or consultants. The exercise price of ISOs granted to employees who at the time of grant own stock representing more than 10% of the voting power of all classes of our common stock may not be less than 110% of the fair market value per share of our common stock on the date of grant, and the exercise price of ISOs granted to any other employees may not be less than 100% of the fair market value per share of our common stock on the date of grant. The exercise price of NSOs granted to employees, directors or consultants may not be less than (i) the minimum price required by applicable state law, (ii) the minimum price required by the company’s governing instrument or (iii) $0.01, whichever is greater. However, any option that is intended to avoid taxation under Section 409A of the Code must be granted with an exercise price equal to or greater than 100% of the fair market value per share of our common stock on the date of grant. Shares subject to options under the 2008 Plan generally vest in a series of installments over an optionee’s period of service. In general, the maximum term of options granted is 10 years. The maximum term of ISOs granted to an optionee who owns stock representing more than 10% of the voting power of all classes of our common stock is five years. Payment of the exercise price is generally made in cash, through a net share exercise election, or a promissory note. In general, options granted under the 2008 Plan will vest and become exercisable at such time as determined by the administrator. |
| • | Stock Appreciation Rights. The 2008 Plan provides that we may issue SARs. Each SAR will be governed by a SAR agreement and may be granted in connection with stock options or other awards, or separately. SARs granted in connection with stock options or other awards typically will provide for payments to the holder based upon increases in the price of our common stock over a set exercise price. The exercise price of any SAR granted under the 2008 Plan will not be less than the exercise price for the option if the SAR is granted in connection with an option; otherwise the exercise price must be at least 85% of the fair market value of a share of our common stock on the date of grant; provided that any SAR that is intended to avoid taxation under Section 409A of the Code must be granted with an exercise price equal to or greater than 100% of the fair market value per share of our common stock on the date of grant. There are no restrictions specified in the 2008 Plan on the exercise of SARs or the amount of gain realizable therefrom, although restrictions may be imposed by the administrator in the SAR agreements; provided that a SAR will not be exercisable for six months if the recipient of the SAR received a hardship distribution under our 401(k) plan. SARs under the 2008 Plan will be settled in cash or shares of our common stock, or in a combination of both, at the election of the administrator. In general, SARs may not be sold or otherwise transferred and the maximum term of SARs granted is 10 years. |
| • | Restricted Stock Awards. The 2008 Plan provides that we may issue restricted stock awards. Each restricted stock award will be governed by a restricted stock award agreement. Certain restrictions will be placed on the restricted shares of common stock that will vest as determined by the administrator. The administrator has the discretion to require a cash payment in exchange for a grant of restricted stock, but such payment is not required. In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Holders of restricted stock will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse. |
| • | Restricted Stock Unit Awards. The 2008 Plan provides that we may issue restricted stock unit awards. Each restricted stock unit award will be governed by a restricted stock unit award agreement and may be awarded to any eligible individual, typically without payment of consideration although the administrator may require payment in consideration of the restricted stock units, but subject to vesting conditions based on continued employment or service or on performance criteria established by the |
144
Table of Contents
Index to Financial Statements
| administrator. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| • | Performance-Based Stock Awards. The 2008 Plan provides that we may issue performance-based stock awards. Each performance-based stock award will be governed by a performance-based stock award agreement and may be granted by the administrator as any of the above described equity awards. Generally, these awards will be based upon specific performance targets, in order to satisfy the requirements under Section 162(m) of the Code. Section 162(m) of the Code did not apply to us while we were a private company, so we did not issue any awards subject to this provision prior to termination of the 2008 Plan. |
Adjustments. In the event of certain corporate transactions or capitalization changes, the administrator of the 2008 Plan may adjust the maximum number of shares of common stock that may be delivered under the 2008 Plan, the limit on the number of shares that may be granted during a calendar year, the number and type of shares subject to outstanding wards and the exercise price of any options or SARs; provided that the administrator will be required to make such adjustments if such corporate transactions or capitalization changes constitute an “equity restructuring” as defined in ASC Topic 718.
Change of Control. If a change of control occurs, and if the agreements effectuating the change of control do not provide for the assumption or substitution of all options, SARs and restricted stock units granted under the 2008 Plan, then the administrator, may, with respect to any or all of such non-assumed options, SARs and restricted stock units, take any or all of the following actions to be effective as of the date of the change of control (or as of any other date fixed by the administrator occurring within the 30-day period ending on the date of the change of control, but only if such action remains contingent upon the effectuation of the change of control): (i) accelerate the vesting and/or exercisability of any non-assumed options, SARs and restricted stock units; (ii) cancel any such options, SARs and restricted stock units which have not vested and/or which have not become exercisable, if applicable; (iii) cancel any such options, SARs and restricted stock units in exchange for shares or a cash payment or such other property; (iv) cancel any such options and SARs after a specified date after providing the holders of such options or SARs with an opportunity to exercise such options or SARs to the extent vested and/or exercisable and reasonable notice of such opportunity to exercise; (v) require and cause the exercise of any such options and SARs by a cashless or net share exercise; and/or (vi) cancel any such options, SARs and restricted stock units and notify the holders of such options, SARs or restricted stock units of such action, but only if the fair market value of the shares subject to the awards that are options or SARs does not exceed the exercise price of the options or SARs, or in the case of restricted stock units, the fair market value of the underlying shares is zero. If a change of control occurs, then, each other award besides an option, SAR or restricted stock unit will be governed by applicable law and the documents effectuating the change of control.
Amendment; Termination. Our board of directors may amend, suspend or terminate the 2008 Plan or any portion thereof at any time, but no action will diminish the rights of a holder of an outstanding award without the holder’s consent, unless there is a dissolution or liquidation of the company or as required by applicable law. Our board of directors can reprice all or any portion of outstanding options and SARs as it deems appropriate without the need for any stockholder approval. In connection with the Merger, the 2008 Plan has been terminated and no further awards will be granted under the 2008 Plan. However, all outstanding ViewRay stock options assumed in connection with the Merger will continue to be governed by their existing terms.
We intend to file with the SEC a registration statement on Form S-8 covering our shares of our common stock issuable under the 2008 Plan to cover the ViewRay stock options assumed in the Merger.
145
Table of Contents
Index to Financial Statements
2015 Employee Stock Purchase Plan
In connection with the Merger, we have adopted the ESPP, which became effective immediately prior to the closing of the Merger. The ESPP is designed to allow our eligible employees to purchase shares of our common stock, at semi-annual intervals, with their accumulated payroll deductions. The ESPP is intended to qualify under Section 423 of the Code.
Plan Administration. Subject to the terms and conditions of the ESPP, our compensation committee will administer the ESPP. Our compensation committee can delegate administrative tasks under the ESPP to the services of an agent and/or employees to assist in the administration of the ESPP. The administrator will have the discretionary authority to administer and interpret the ESPP. Interpretations and constructions of the administrator of any provision of the ESPP or of any rights thereunder will be conclusive and binding on all persons. We will bear all expenses and liabilities incurred by the ESPP administrator.
Shares Available Under ESPP. The maximum number of our shares of our common stock which will be authorized for sale under the ESPP is equal to the sum of (a) 285,621 shares of common stock and (b) an annual increase on the first day of each year beginning in 2016 and ending in 2025, equal to the lesser of (i) 1% of the shares of common stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year and (ii) such number of shares of common stock as determined by our board of directors; provided, however, no more than 1,675,000 shares of our common stock may be issued under the ESPP. The shares made available for sale under the ESPP may be authorized but unissued shares or reacquired shares reserved for issuance under the ESPP.
Eligible Employees. Employees eligible to participate in the ESPP for a given offering period generally include employees who are employed by us or one of our designated subsidiaries on the first day of the offering period, or the enrollment date. Our employees and any employees of our subsidiaries who customarily work less than five months in a calendar year or are customarily scheduled to work less than 20 hours per week will not be eligible to participate in the ESPP. Finally, an employee who owns (or is deemed to own through attribution) 5% or more of the combined voting power or value of all our classes of stock or of one of our subsidiaries will not be allowed to participate in the ESPP.
Participation. Employees will enroll under the ESPP by completing a payroll deduction form permitting the deduction from their compensation of at least 1% of their compensation but not more than the lesser of 15% of their compensation and $30,000 per offering period. Such payroll deductions are expressed as a whole number percentage and the accumulated deductions will be applied to the purchase of shares on each semi-annual purchase date. However, a participant may not purchase more than 3,000 shares in each offering period, and may not subscribe for more than $25,000 in fair market value of shares our common stock (determined at the time the option is granted) per calendar year falling in the offering period. The ESPP administrator has the authority to change these limitations for any subsequent offering period.
Offering. Under the ESPP, participants are offered the option to purchase shares of our common stock at a discount during a series of successive offering periods. The offering periods will commence and end on dates as determined by the ESPP administrator. However, in no event may an offering period be longer than 27 months in length.
The option purchase price will be the lower of 85% of the closing trading price per share of our common stock on the first trading date of an offering period in which a participant is enrolled or 85% of the closing trading price per share on the semi-annual purchase date, which will occur on the last trading day of each offering period.
Unless a participant has previously canceled his or her participation in the ESPP before the purchase date, the participant will be deemed to have exercised his or her option in full as of each purchase date. Upon exercise, the participant will purchase the number of whole shares that his or her accumulated payroll deductions will buy at the option purchase price, subject to the participation limitations listed above.
146
Table of Contents
Index to Financial Statements
A participant may cancel his or her payroll deduction authorization at any time prior to the end of the offering period. Upon cancellation, the participant will have the option to either (a) receive a refund of the participant’s account balance in cash without interest or (b) exercise the participant’s option for the current offering period for the maximum number of shares of common stock on the applicable purchase date, with the remaining account balance refunded in cash without interest. Following at least one payroll deduction, a participant may also decrease (but not increase) his or her payroll deduction authorization once during any offering period. If a participant wants to increase or decrease the rate of payroll withholding, he or she may do so effective for the next offering period by submitting a new form before the offering period for which such change is to be effective.
A participant may not assign, transfer, pledge or otherwise dispose of (other than by will or the laws of descent and distribution) payroll deductions credited to a participant’s account or any rights to exercise an option or to receive shares of our common stock under the ESPP, and during a participant’s lifetime, options in the ESPP shall be exercisable only by such participant. Any such attempt at assignment, transfer, pledge or other disposition will not be given effect.
Adjustments upon Changes in Recapitalization, Dissolution, Liquidation, Merger or Asset Sale. In the event of any increase or decrease in the number of issued shares of our common stock resulting from a stock split, reverse stock split, stock dividend, combination or reclassification of the common stock, or any other increase or decrease in the number of shares of common stock effected without receipt of consideration by us, we will proportionately adjust the aggregate number of shares of our common stock offered under the ESPP, the number and price of shares which any participant has elected to purchase pursuant under the ESPP and the maximum number of shares which a participant may elect to purchase in any single offering period.
If there is a proposal to dissolve or liquidate us, then the ESPP will terminate immediately prior to the consummation of such proposed dissolution or liquidation, and any offering period then in progress will be shortened by setting a new purchase date to take place before the date of our dissolution or liquidation. We will notify each participant of such change in writing at least ten business days prior to the new exercise date. If we undergo a merger with or into another corporation or sale of all or substantially all of our assets, each outstanding option will be assumed or an equivalent option substituted by the successor corporation or the parent or subsidiary of the successor corporation. If the successor corporation refuses to assume the outstanding options or substitute equivalent options, then any offering period then in progress will be shortened by setting a new purchase date to take place before the date of our proposed sale or merger. We will notify each participant of such change in writing at least ten business days prior to the new exercise date.
Amendment and Termination. Our board of directors may amend, suspend or terminate the ESPP at any time. However, the board of directors may not amend the ESPP without obtaining stockholder approval within 12 months before or after such amendment to the extent required by applicable laws. The ESPP will terminate on the 10th anniversary of the date of its initial approval of our stockholders, unless earlier terminated.
We intend to file with the SEC a registration statement on Form S-8 covering our shares issuable under the ESPP.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from the director or executive officer. The director or executive officer may amend or terminate the plan in limited circumstances. Our directors and executive officers may also buy or sell additional shares of common stock outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
147
Table of Contents
Index to Financial Statements
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
SEC rules require us to disclose any transaction or currently proposed transaction in which the Company is a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000 or 1% of the average of the Company’s total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of the Company’s common stock, or an immediate family member of any of those persons.
The following is a description of transactions since January 1, 2012 to which we have been a party, in which the amount involved exceeded or will exceed $120,000, and in which any of our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest, other than compensation and other arrangements that are described in the section titled “Executive Compensation.” The following description is historical and has not been adjusted to give effect to the Merger or the share conversion ratio pursuant to the Merger Agreement.
Sales and Purchases of Securities
Original Series C Convertible Preferred Stock Financing, Second Tranche
In March 2012, ViewRay issued an aggregate of 1,546,472 shares of Series C convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $26.3 million to 18 accredited investors, which included 646,114 shares of Series C convertible preferred stock that were issued pursuant to a conversion of $10.3 million aggregate principal amount of convertible promissory notes issued in our 2011 bridge financing. The shares of Series C convertible preferred stock issued in March 2012 were exchanged for shares of ViewRay Series B convertible preferred stock or ViewRay common stock, depending upon whether the holders of such shares participated in ViewRay’s Series D-2 convertible preferred stock financing, in our recapitalization effected in connection with the issuance of Series D-2 convertible preferred stock in June 2013, or the Recapitalization. Participating holders of ViewRay Series C convertible preferred stock received shares of Series B convertible preferred stock on a 1:1 basis in the Recapitalization, and non-participating holders of Series C convertible preferred stock received shares of ViewRay common stock on a 10:1 basis in the Recapitalization. The table below sets forth the number of shares of Series C convertible preferred stock sold or issued to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our common stock in connection with the Merger.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Principal Amount Under Convertible Promissory Notes |
Accrued Interest Under Convertible Promissory Notes |
Additional Cash |
Aggregate Purchase Price($)(1) |
|||||||||||||||
| OrbiMed Private Investments III, LP(2) |
$ | 427,950 | $ | 2,529,634 | $ | 148,036 | $ | 3,794,451 | $ | 6,472,120 | ||||||||||
| Beacon Bioventures Fund II Limited Partnership(3) |
427,951 | 2,529,634 | 148,036 | 3,794,451 | 6,472,120 | |||||||||||||||
| Aisling Capital II, LP(4) |
427,951 | 2,529,634 | 148,036 | 3,794,451 | 6,472,120 | |||||||||||||||
| Kearny Venture Partners, L.P.(5) |
244,542 | 14,447 | 845 | 21,671 | 3,698,300 | |||||||||||||||
| Affiliates of Mark Gold, M.D.(6) |
18,078 | 200,000 | 8,416 | 65,000 | 273,416 | |||||||||||||||
| (1) | Includes the principal amount owed and unpaid accrued interest at a rate of 8% per annum with respect to the Series C convertible preferred stock issued pursuant to the conversion of convertible promissory notes issued in our 2011 bridge financing. |
| (2) | Includes 175,383 shares issued due to conversion of convertible promissory note principal and accrued interest and 248,530 shares purchased with additional cash issued to OrbiMed Private Investments III, LP and 1,670 shares issued due to conversion of convertible promissory note principal and accrued interest and |
148
Table of Contents
Index to Financial Statements
| 2,367 shares purchased with additional cash by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (3) | Includes 177,053 shares issued due to conversion of convertible promissory note principal and accrued interest and 250,897 shares purchased with additional cash. Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited Partnership. |
| (4) | Includes 177,053 shares issued due to conversion of convertible promissory note principal and accrued interest and 250,897 shares purchased with additional cash. Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (5) | Includes 49,575 shares issued due to conversion of convertible promissory note principal and accrued interest and 70,252 shares purchased with additional cash by Kearny Venture Partners, L.P., 1,011 shares issued due to conversion of convertible promissory note principal and accrued interest and 1,432 shares purchased with additional cash by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 50,586 shares issued due to conversion of convertible promissory note principal and accrued interest and 71,684 shares purchased with additional cash by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
| (6) | Includes 10,335 shares issued due to conversion of convertible promissory note principal and accrued interest and 2,148 shares purchased with additional cash by MJSK, Ltd. and 3,445 shares issued due to conversion of convertible promissory note principal and accrued interest and 2,148 shares purchased with additional cash by Translational Research Family LP. Translational Research Family LP subsequently sold its shares to JMSK, Ltd. Janice Gold, the wife of Mark Gold, M.D., is the President of MJSK, Ltd. and a Partner of Translational Research Family LP. Steven Gold, the son of Mark Gold, M.D., is the General Partner of JMSK, Ltd. Mark Gold, M.D. is a member of our board of directors. |
Series D-1 Convertible Preferred Stock Financing, First Tranche
In November 2012, ViewRay issued an aggregate of 388,290 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.9 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in November 2012 were exchanged for shares of ViewRay Series B convertible preferred stock in the Recapitalization on a 1:1 basis. The table below sets forth the number of shares of Series D-1 convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our common stock in connection with the Merger.
| Name |
Number of Shares of Series D-1 Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| OrbiMed Private Investments III, LP(1) |
108,721 | $ | 1,644,260 | |||||
| Beacon Bioventures Fund II Limited Partnership(2) |
108,722 | 1,644,260 | ||||||
| Aisling Capital II, LP(3) |
108,722 | 1,644,260 | ||||||
| Kearny Venture Partners, L.P.(4) |
62,125 | 939,574 | ||||||
| (1) | Includes 107,696 shares purchased by OrbiMed Private Investments III, LP and 1,025 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity |
149
Table of Contents
Index to Financial Statements
| Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited Partnership. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes 30,442 shares purchased by Kearny Venture Partners, L.P., 620 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 31,063 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
Series D-1 Convertible Preferred Stock Financing, Second Tranche
In February 2013, ViewRay amended and restated its Series D-1 convertible preferred stock purchase agreement to issue an additional 330,608 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.0 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in February 2013 were exchanged for shares of ViewRay Series B convertible preferred stock in the Recapitalization on a 1:1 basis. The table below sets forth the number of shares of Series D-1 convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our common stock in connection with the Merger.
| Name |
Number of Shares of Series D-1 Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| OrbiMed Private Investments III, LP(1) |
92,570 | $ | 1,400,000 | |||||
| Beacon Bioventures Fund II Limited Partnership(2) |
92,571 | 1,400,000 | ||||||
| Aisling Capital II, LP(3) |
92,571 | 1,400,000 | ||||||
| Kearny Venture Partners, L.P.(4) |
52,896 | 799,998 | ||||||
| (1) | Includes 91,697 shares purchased by OrbiMed Private Investments III, LP and 873 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited Partnership. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes 25,920 shares purchased by Kearny Venture Partners, L.P., 528 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 26,448 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
Series D-2 Convertible Preferred Stock Financing
In May and June 2013, ViewRay issued an aggregate of 996,021 shares of Series D-2 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $15.3 million to 27 accredited investors. The
150
Table of Contents
Index to Financial Statements
shares of Series D-2 convertible preferred stock issued in May and June 2013 were immediately exchanged for shares of ViewRay Series B convertible preferred stock in the Recapitalization on a 1:1 basis. The table below sets forth the number of shares of Series D-2 convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our common stock in connection with the Merger.
| Name |
Number of Shares of Series D-2 Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| OrbiMed Private Investments III, LP(1) |
277,713 | $ | 4,200,000 | |||||
| Beacon Bioventures Fund II Limited Partnership(2) |
277,713 | 4,200,000 | ||||||
| Aisling Capital II, LP(3) |
277,713 | 4,200,000 | ||||||
| Kearny Venture Partners, L.P.(4) |
158,692 | 2,399,997 | ||||||
| Mark Gold, M.D. and affiliates (5) |
4,190 | 63,404 | ||||||
| (1) | Includes 275,093 shares purchased by OrbiMed Private Investments III, LP and 2,620 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited Partnership. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes 77,760 shares purchased by Kearny Venture Partners, L.P., 1,586 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 79,346 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
| (5) | Includes 168 shares purchased by Mark Gold, M.D., 2,651 shares purchased by MJSK, Ltd. and 1,371 shares purchased by JMSK, Ltd. Janice Gold, the wife of Mark Gold, M.D., is the President of MJSK, Ltd. Steven Gold, the son of Mark Gold, M.D., is the General Partner of JMSK, Ltd. Mark Gold, M.D. is a member of our board of directors. |
151
Table of Contents
Index to Financial Statements
Series C Convertible Preferred Stock Financing
In November 2013, ViewRay issued an aggregate of 862,064 shares of Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $15.0 million to eight accredited investors. Shares of ViewRay Series C convertible preferred stock converted into our common stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the number of shares of ViewRay Series C convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| OrbiMed Private Investments III, LP(1) |
160,919 | 2,800,001 | ||||||
| Beacon Bioventures Fund II Limited Partnership(2) |
160,919 | 2,800,001 | ||||||
| Aisling Capital II, LP(3) |
160,919 | 2,800,001 | ||||||
| Kearny Venture Partners, L.P.(4) |
91,951 | 1,599,991 | ||||||
| (1) | Includes 159,401 shares purchased by OrbiMed Private Investments III, LP and 1,518 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited Partnership. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes 45,057 shares purchased by Kearny Venture Partners, L.P., 918 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 45,976 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
Convertible Promissory Note Purchase Agreement
In August and November 2014, ViewRay issued convertible promissory notes for an aggregate principal amount of $10.0 million to seven accredited investors. In December 2014, all outstanding principal and interest under the 2014 Notes were converted into 584,675 shares of ViewRay Series C convertible preferred stock at a price of $17.40 per share. See the section below titled “—Series C Convertible Preferred Stock Financing Extension” for further information. Shares of ViewRay Series C convertible preferred stock converted into our common stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the principal amount of the convertible promissory notes sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof.
| Name |
Aggregate Purchase Price($) |
|||
| Royal Seal Holding Co., Limited(1) |
$ | 4,999,999 | ||
| OrbiMed Private Investments III, LP(2) |
2,800,001 | |||
| Beacon Bioventures Fund II Limited Partnership(3) |
2,800,001 | |||
| Aisling Capital II, LP(4) |
2,800,001 | |||
| Kearny Venture Partners, L.P.(5) |
1,599,997 | |||
152
Table of Contents
Index to Financial Statements
| (1) | Philip Yang, who was a member of our board of directors, is Vice President of Cowealth Medical Holding Co. Ltd., which is the sole shareholder of Royal Seal Holding Co., Limited. |
| (2) | Includes convertible promissory notes with an aggregate principal amount of $2,773,586 purchased by OrbiMed Private Investments III, LP and convertible promissory notes with an aggregate principal amount of $26,415 purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (3) | Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited Partnership. |
| (4) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (5) | Includes convertible promissory notes with an aggregate principal amount of $784,007 purchased by Kearny Venture Partners, L.P., convertible promissory notes with an aggregate principal amount of $15,990 purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and convertible promissory notes with an aggregate principal amount of $799,999 purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
Series C Convertible Preferred Stock Financing Extension
In December 2014 and January 2015, ViewRay issued an aggregate of 935,248 shares of its Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $16.3 million to 10 accredited investors, including the conversion of all outstanding principal and interest under the 2014 Notes into shares of ViewRay Series C convertible preferred stock at a price of $17.40 per share. The aggregate gross consideration received from the sale of Series C convertible preferred stock was $6.1 million, and the aggregate gross consideration received from the conversion of all outstanding principal and interest of the 2014 Notes was $10.2 million. Shares of ViewRay Series C convertible preferred stock converted into our common stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the number of shares of ViewRay Series C convertible preferred stock issued to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Principal Amount Under 2014 Notes |
Accrued Interest Under 2014 Notes |
Additional Cash |
Aggregate Purchase Price($)(1) |
|||||||||||||||
| OrbiMed Private Investments III, LP(2) |
163,709 | $ | 2,800,001 | $ | 48,548 | $ | — | $ | 2,848,549 | |||||||||||
| Beacon Bioventures Fund II Limited Partnership(3) |
163,709 | 2,800,001 | 48,548 | — | 2,848,549 | |||||||||||||||
| Aisling Capital II, LP(4) |
163,709 | 2,800,001 | 48,548 | — | 2,848,549 | |||||||||||||||
| Kearny Venture Partners, L.P.(5) |
93,548 | 1,599,997 | 27,742 | — | 1,627,739 | |||||||||||||||
| Mark Gold, M.D. and affiliates(6) |
5,747 | — | — | 100,000 | 100,000 | |||||||||||||||
| (1) | Includes the outstanding principal amount owed and unpaid accrued interest at a rate of 8% per annum with respect to the Series C convertible preferred stock issued pursuant to the conversion of the 2014 Notes. |
| (2) | Includes 162,175 shares issued due to conversion of convertible promissory note principal and accrued interest to OrbiMed Private Investments III, LP and 1,544 shares issued due to conversion of convertible promissory note principal and accrued interest to OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
153
Table of Contents
Index to Financial Statements
| (3) | Includes 163,709 shares issued due to conversion of convertible promissory note principal and accrued interest. Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited Partnership. |
| (4) | Includes 163,709 shares issued due to conversion of convertible promissory note principal and accrued interest. Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (5) | Includes 45,839 shares issued due to conversion of convertible promissory note principal and accrued interest to Kearny Venture Partners, L.P., 935 shares issued due to conversion of convertible promissory note principal and accrued interest to Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 46,774 shares issued due to conversion of convertible promissory note principal and accrued interest to Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
| (6) | Includes 5,747 shares purchased with cash by JMSK, Ltd. Steven Gold, the son of Mark Gold, M.D., is the General Partner of JMSK, Ltd. Mark Gold, M.D. is a member of our board of directors. |
In February 2015, ViewRay issued an aggregate of 862,068 shares of its Series C convertible preferred stock at a price of $17.40 per share for aggregate gross consideration of $15.0 million to one accredited investor. Shares of ViewRay Series C convertible preferred stock converted into our common stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the number of shares of ViewRay Series C convertible preferred stock issued to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or an immediate family member thereof.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| Harbour Tycoon Limited(1) |
862,068 | $ | 15,000,000 | |||||
| (1) | Aditya Puri, a member of our board of directors, is an Investments Director at Xeraya Capital, an affiliate of Harbour Tycoon Limited. |
Participation in the Private Placement
Certain of our existing institutional investors, including investors affiliated with certain of our directors, have purchased an aggregate of 3,400,003 of shares of our common stock in the Private Placement, for an aggregate purchase price of $17,000,015 based on the offering price of $5.00 per share. Such purchases were made on the same terms as the shares that were sold to other investors in the Private Placement and not pursuant to any pre-existing contractual rights or obligations. See the footnotes to the beneficial ownership table in “Security Ownership of Certain Beneficial Owners and Management” for more details.
License Agreement with University of Florida Research Foundation, Inc.
In December 2004, we entered into a Standard Exclusive License Agreement with Sublicensing Terms with University of Florida Research Foundation, Inc., or UFRF, under which we licensed certain patents from UFRF in exchange for royalty payments and an equity issuance, or the UFRF License Agreement. We entered into an amendment of the UFRF License Agreement in December 2007. Since December 2004, we have paid UFRF $226,000 in royalties and $63,000 in patent and legal fees pursuant to the terms of the UFRF License Agreement. In addition, we have issued 11,312 shares of common stock to UFRF pursuant to the terms of the UFRF License Agreement, which required us to issue UFRF a certain number of shares of common stock upon execution of the UFRF License Agreement, as well as issue UFRF additional shares of common stock to maintain UFRF’s ownership of 5% of our outstanding equity until certain financing conditions were satisfied. We have satisfied these financing conditions and have no further obligations to issue UFRF shares of our common stock pursuant to
154
Table of Contents
Index to Financial Statements
the terms UFRF License Agreement. Prior to the consummation of the Private Placement, UFRF was a beneficial owner of approximately 0.10% of our capital stock on an as-converted basis. In connection with his former employment at the University of Florida and his role in the development of the licensed patents under the UFRF License Agreement, as amended, James F. Dempsey, Ph.D., our Chief Scientific Officer and a member of our board of directors, receives a percentage of the royalty payments we pay to UFRF and is entitled to a percentage of any proceeds from the sale of common stock by UFRF. Specifically, under the University of Florida’s intellectual property policy, Dr. Dempsey is entitled to (i) 40% of any royalty payments we pay to UFRF or proceeds from the sale of common stock by UFRF up to $500 thousand and then (ii) 25% of any royalty payments we pay to UFRF or proceeds from the sale of common stock by UFRF over $500 thousand. Mark Gold, M.D., a member of our board of directors since our founding in March 2004, was a Professor, Distinguished Professor and Chairman of Psychiatry at the University of Florida from 1990 until his retirement in June 2014.
Purchase Order with Cowealth Medical Holding Co. Ltd.
In 2014, we received two purchase orders from Cowealth Medical Holding Co. Ltd. (“Cowealth”), each for the purchase of a MRIdian system at $5.4 million per system. Also in 2014, we received a total of $1.5 million deposit in connection with these purchase orders. At December 31, 2014, these purchase orders were included in our backlog at a value of $10.8 million. Cowealth is the sole shareholder of Royal Seal Holding Co., Limited, which, prior to the consummation of this offering, beneficially owns approximately 3% of our outstanding capital stock on an as-converted basis. Philip Yang, who was a member of the our board of directors in 2014, is Vice President of International Procurement of Cowealth, where his responsibilities involve discovering, purchasing and sourcing the technology that Cowealth distributes as part of its international business.
Indemnification Agreements and Directors’ and Officers’ Liability Insurance
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer.
Employment Agreements and Offer Letters
In connection with the Merger, each of our new executive officers became employed with us under the terms of their employment agreement or offer letter, as applicable, with ViewRay. For more information regarding these employment agreements for Messrs. Raanes and Brandt and Dr. Dempsey, see the section titled “Executive Compensation—Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End.”
Offer Letter to Doug Keare, Chief Operating Officer
In April 2015, ViewRay entered an offer letter with Mr. Keare that provides for employment at-will and annual base salary, annual target bonus (which is pro-rated for 2015), option awards and certain other benefits. Mr. Keare is also eligible for a sign-on bonus of $15,000, which is paid in 12 equal monthly installments beginning on April 30, 2015 (in connection with his commencement of employment with ViewRay). Mr. Keare will forfeit any unpaid portion of this bonus if he terminates his employment prior to April 30, 2016. His offer letter also contains certain non-disparagement and cooperation restrictive covenants (during Mr. Keare’s employment). Mr. Keare has also executed ViewRay’s standard confidential information and invention assignment agreement. Mr. Keare’s employment is terminable at-will.
The offer letter also provides Mr. Keare with certain severance and change of control benefits. In the event Mr. Keare is terminated without “cause” or resigns for “good reason” (each, as defined his employment agreement) at any time within 12 months following a change of control, then in addition to the severance payments described below, the vesting of Mr. Keare’s option granted under his offer letter will accelerate in full.
155
Table of Contents
Index to Financial Statements
In addition, in the event that Mr. Keare is terminated without cause or resigns for good reason, subject to executing and not revoking a general release of all claims, then Mr. Keare is entitled to receive a severance payment equal to six months of his base salary plus one-half of his annual bonus for the year preceding the termination date payable in substantially equal installments over the six-month period following his termination.
Offer Letter to David Chandler, Chief Financial Officer
In November 2010, ViewRay entered into an offer letter with Mr. Chandler that provides for employment at-will and annual base salary, annual target bonus, option awards and certain other benefits. His offer letter also contains certain non-disparagement and non-competition restrictive covenants (during Mr. Chandler’s employment and for 12 months following termination of employment). Mr. Chandler has also executed ViewRay’s standard confidential information and invention assignment agreement, which contains certain non-competition covenants. In addition, ViewRay reimbursed or directly paid for the costs incurred by Mr. Chandler for housing and transportation in the greater Cleveland and San Francisco areas and commercial air travel from his residence to its offices in Ohio and California. Mr. Chandler’s employment is terminable at-will.
The offer letter also provides Mr. Chandler with certain severance and change of control benefits.
In addition, in the event that Mr. Chandler is terminated without cause or resigns for good reason (each, as defined in his offer letter), subject to executing and not revoking a general release of all claims, then Mr. Chandler is entitled to receive a severance payment equal to six months of his base salary plus his annual bonus for the year preceding the termination date payable in substantially equal installments over the six-month period following his termination.
Other Transactions
We have granted stock options to our executive officers. For a description of these stock options granted to Messrs. Raanes and Brandt and Dr. Dempsey, see the section titled “Executive Compensation.” Upon the consummation of the Merger, each of Messrs. Keare and Chandler was granted an option to purchase 269,684 and 92,661 shares of our common stock, respectively, at an exercise price per share equal to $5. Mr. Keare’s options have a vesting commencement date of April 30, 2015 and will vest and become exercisable as to 25% of the shares on the first anniversary of the vesting commencement date, and in 36 installments thereafter on each monthly anniversary of that date, such that all shares will be vested on the fourth anniversary of the vesting commencement date, subject to his continuing to provide services to us through the applicable vesting date. Mr. Chandler’s options will vest and become exercisable in substantially equal monthly installments over the 4 years following the grant date, subject to his continuing to provide services to us through the applicable vesting date. We have also granted stock options to certain members of the board of directors. For a description of these stock options, see the section titled “Management—2013 Director Compensation Table.”
Policies and Procedures for Related-Person Transactions
Our board of directors has adopted a written related-person transaction policy, to be effective upon the consummation of the Merger, setting forth the policies and procedures for the review and approval or ratification of related-person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s-length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
156
Table of Contents
Index to Financial Statements
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information relating to the beneficial ownership of our common stock at December 14, 2015, by:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of ViewRay’s pre-Merger outstanding shares of common stock; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all current directors and executive officers as a group. |
The number of shares beneficially owned by each entity, person, director or executive officer is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days of December 14, 2015 through the exercise of any stock option, warrants or other rights. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of common stock held by such person.
The percentage of shares beneficially owned is computed on the basis of 38,200,088 shares of common stock outstanding at December 14, 2015. Shares of common stock that a person has the right to acquire within 60 days of December 14, 2015 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated below, the address for each beneficial owner listed in the table is c/o ViewRay, Inc., 2 Thermo Fisher Way, Oakwood Village, Ohio 44146.
| Name and Address of Beneficial Owner |
Number of Shares Beneficially Owned |
Number of Shares Exercisable Within 60 Days |
Number of Shares Beneficially Owned |
Percentage of Beneficial Ownership |
||||||||||||
| 5% and Greater Stockholders |
||||||||||||||||
| Aisling Capital II, LP(1) |
7,461,923 | — | 7,461,923 | 19.53 | % | |||||||||||
| Beacon Bioventures Fund II Limited Partnership Partnership(2) |
7,989,923 | — | 7,989,923 | 20.92 | ||||||||||||
| Entities affiliated with OrbiMed Private Investments III, LP(3) |
7,989,916 | — | 7,989,916 | 20.92 | ||||||||||||
| Entities affiliated with Kearny Venture Partners, L.P.(4) |
4,565,653 | — | 4,565,653 | 11.95 | ||||||||||||
| Harbour Tycoon Limited(5) |
3,403,942 | — | 3,403,942 | 8.91 | ||||||||||||
| Named Executive Officers and Directors |
||||||||||||||||
| Chris A. Raanes(6) |
952,164 | 72,180 | 1,024,344 | 2.61 | ||||||||||||
| James F. Dempsey, Ph.D.(7) |
785,481 | 27,934 | 813,415 | 2.09 | ||||||||||||
| Michael Brandt(8) |
125,572 | 5,580 | 131,152 | * | ||||||||||||
| Joshua Bilenker, M.D.(9) |
7,468,443 | 3,260 | 7,471,703 | 19.55 | ||||||||||||
| David Bonita, M.D.(10) |
7,996,463 | 3,260 | 7,999,696 | 20.94 | ||||||||||||
| Caley Castelein, M.D.(11) |
4,572,173 | 3,260 | 4,575,433 | 11.97 | ||||||||||||
| Mark S. Gold, M.D.(12) |
126,837 | 3,550 | 130,387 | * | ||||||||||||
| Aditya Puri(13) |
3,410,462 | 3,260 | 3,413,722 | 8.93 | ||||||||||||
| Brian K. Roberts(14) |
0 | 1,630 | 1,630 | * | ||||||||||||
| Robert Weisskoff, Ph.D.(15) |
7,994,813 | 3,260 | 7,998,073 | 20.93 | ||||||||||||
| All current directors and executive officers as a group (11 persons)(16) |
33,621,255 | 134,014 | 33,755,269 | 83.84 | ||||||||||||
| * | Indicates beneficial ownership of less than 1% of the total outstanding common stock. |
157
Table of Contents
Index to Financial Statements
| (1) | Includes 7,461,923 shares held by Aisling Capital II, LP, or Aisling. Aisling Capital Partners, LP, or Aisling GP, is the general partner of Aisling. Aisling Capital Partners LLC, or Aisling Partners, is the general partner of Aisling GP. The individual managing members, or the Aisling Managers, of Aisling Partners are Dennis Purcell, Andrew Schiff, M.D. and Steve Elms. By virtue of these relationships, Aisling GP, Aisling Partners and the Aisling Managers may be deemed to have voting and investment power over the shares held by Aisling. Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, an affiliate of Aisling. Voting and dispositive decisions with respect to shares held by Aisling are not made by Dr. Bilenker; he disclaims beneficial ownership of the shares held by Aisling, except to the extent of any pecuniary interest therein, if any. The address of Aisling is c/o Aisling Capital, LLC, 888 Seventh Avenue, 30th Floor, New York, New York 10106. |
| (2) | Includes 7,989,923 shares held by Beacon Bioventures Fund II Limited Partnership Partnership, or Beacon. Beacon Bioventures Advisors Fund II Limited Partnership, or BBA II, is the general partner of Beacon. Impresa Management LLC, or Impresa, is the general partner of BBA II. By virtue of these relationships, BBA II and Impresa may be deemed to have voting and investment power over the shares held by Beacon. Impresa is managed on a day-to-day basis by its President, Paul L. Mucci, and as such, Mr. Mucci may be deemed to have voting and dispositive power with respect to all shares held by Beacon. Each of the individuals and entities listed above expressly disclaims beneficial ownership of the shares held by Beacon, except to the extent of any pecuniary interest therein, if any. Robert Weisskoff, Ph.D., a member of our board of directors, is a Partner of Fidelity Biosciences, an affiliate of Beacon. Voting and dispositive decisions with respect to shares held by Beacon are not made by Dr. Weisskoff; he disclaims beneficial ownership of the shares held by Beacon, except to the extent of any pecuniary interest therein, if any. The address of Beacon is c/o Fidelity Biosciences, One Main Street, 13th Floor, Cambridge, Massachusetts 02142. |
| (3) | Includes (i) 7,914,545 shares held by OrbiMed Private Investments III, LP, or OPI III, and (ii) 75,371 shares held by OrbiMed Associates III, LP, or OA III. OrbiMed Capital GP III LLC, or GP III, is the general partner of OPI III. OrbiMed Advisors LLC, OrbiMed, is the managing member of GP III and the general partner of OA III. Samuel D. Isaly is the managing member of and owner of a controlling interest in OrbiMed. By virtue of such relationships, GP III, OrbiMed and Mr. Isaly may be deemed to have voting and investment power over the shares held by OPI III and OA III. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner of OrbiMed. Each of GP III, OrbiMed, Mr. Isaly and Dr. Bonita disclaims beneficial ownership of the shares held by OPI III and OA III, except to the extent of its or his pecuniary interest therein, if any. The address of OrbiMed Investments and OrbiMed Associates is c/o OrbiMed Advisors LLC, 601 Lexington Avenue, 54th Floor, New York, New York 10022. |
| (4) | Includes (i) 2,479,359 shares held by Kearny Venture Partners, L.P., or KVP, (ii) 50,563 shares held by Kearny Venture Partners Entrepreneurs’ Fund, L.P., or KVPE, and (iii) 2,035,731 shares held by Thomas Weisel Healthcare Venture Partners, L.P., or TWHVP. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of KVP, Kearny Venture Associates, L.L.C., or KVA, and Thomas Weisel Capital Management LLC, or TWCM. KVA is the general partner of each of KVP and KVPE. TWCM is the managing member of Thomas Weisel Healthcare Venture Partners, LLC, the general partner of TWHVP. Voting and dispositive decisions with respect to shares held by KVP, KVPE and TWHVP are made by Dr. Castelein; however, he disclaims beneficial ownership of the shares held by KVP, KVPE and TWHVP, except to the extent of any pecuniary interest therein, if any. The address of KVP, KVPE and TWHVP is c/o Kearny Venture Partners, 88 Kearny Street, Suite 1800, San Francisco, California 94108. |
| (5) | Includes 3,403,942 shares held by Harbour Tycoon Limited. Aditya Puri, a member of our board of directors, is an Investments Director at Xeraya Capital, an affiliate of Harbour Tycoon Limited. The address of Harbour Tycoon Limited is c/o 2nd Floor, The Grand Pavilion Commercial Centre, 802 West Bay Road, P.O. Box 1338, Grand Cayman KY1-1003, Cayman Islands. |
| (6) | Consists of 1,024,344 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015 by Mr. Raanes. |
| (7) | Consists of (i) 182,602 shares held and (ii) 630,813 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015 by Dr. Dempsey. |
158
Table of Contents
Index to Financial Statements
| (8) | Consists of 131,152 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015 by Mr. Brandt. |
| (9) | Consists of (i) the shares held by Aisling and (ii) 9,780 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015. See footnote 1. Dr. Bilenker disclaims beneficial ownership of the shares held by Aisling, except to the extent of any pecuniary interest therein, if any. |
| (10) | Consists of (i) the shares held by OPI III and OA III and (ii) 9,780 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015. See footnote 3. |
| (11) | Consists of (i) the shares held by KVP, KVPE and TWHVP and (ii) 9,780 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015. See footnote 4. Dr. Castelein disclaims beneficial ownership of the shares held by KVP, KVPE and TWHVP, except to the extent of any pecuniary interest therein, if any. |
| (12) | Consists of (i) 3,206 shares held by Dr. Gold, (ii) 54,129 shares held by MJSK, Ltd., (iii) 44,837 shares held by JMSK, Ltd. and (iv) 28,215 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015 by Dr. Gold. Janice Gold, the wife of Dr. Gold, is the President of MJSK, Ltd., and Steven Gold, the son of Dr. Gold, is the General Partner of JMSK, Ltd. Voting and dispositive decisions with respect to shares held by MJSK, Ltd. and JMSK, Ltd. are not made by Dr. Gold; he disclaims beneficial ownership of the shares held by MJSK, Ltd. and JMSK, Ltd. except to the extent of any pecuniary interest therein, if any. |
| (13) | Consists of (i) the shares held by Harbour Tycoon Limited and (ii) 9,780 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015. See footnote 5. |
| (14) | Consists of 1,630 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015 by Mr. Roberts. |
| (15) | Consists of (i) the shares held by Beacon and (ii) 8,150 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015. See footnote 2. Voting and dispositive decisions with respect to shares held by Beacon are not made by Dr. Weisskoff; he disclaims beneficial ownership of the shares held by Beacon, except to the extent of any pecuniary interest therein, if any. |
| (16) | Includes (i) 31,411,357 shares held by entities affiliated with certain of our directors and (ii) 33,755,269 shares beneficially owned by our executive officers and directors, which includes the 31,411,357 shares held by such entities and 134,014 shares that may be acquired pursuant to the exercise of stock options within 60 days of December 14, 2015. |
159
Table of Contents
Index to Financial Statements
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following discussion is a summary of the material U.S. federal income tax consequences to Non-U.S. Holders (as defined below) of the purchase, ownership and disposition of our common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or non-U.S. tax laws are not discussed. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service (the “IRS”), in each case in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a Non-U.S. Holder. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position to that discussed below regarding the tax consequences of the purchase, ownership and disposition of our common stock.
This discussion is limited to Non-U.S. Holders that hold our common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a Non-U.S. Holder’s particular circumstances, including the impact of the Medicare contribution tax on net investment income. In addition, it does not address consequences relevant to Non-U.S. Holders subject to special rules, including, without limitation:
| • | U.S. expatriates and former citizens or long-term residents of the United States; |
| • | persons subject to the alternative minimum tax; |
| • | persons holding our common stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment; |
| • | banks, insurance companies, and other financial institutions; |
| • | brokers, dealers or traders in securities; |
| • | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors therein); |
| • | tax-exempt organizations or governmental organizations; |
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| • | persons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
| • | tax-qualified retirement plans. |
If an entity treated as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
160
Table of Contents
Index to Financial Statements
Definition of a Non-U.S. Holder
For purposes of this discussion, a “Non-U.S. Holder” is any beneficial owner of our common stock that is neither a “U.S. person” nor an entity treated as a partnership for U.S. federal income tax purposes. A U.S. person is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| • | an individual who is a citizen or resident of the United States; |
| • | a corporation created or organized under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate, the income of which is subject to U.S. federal income tax regardless of its source; or |
| • | a trust that (1) is subject to the primary supervision of a U.S. court and all substantial decisions of which are subject to the control of one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code), or (2) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
Distributions
As described in the section entitled “Dividend Policy,” we do not anticipate paying any cash dividends on our common stock in the foreseeable future. However, if we do make distributions of cash or property on our common stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a Non-U.S. Holder’s adjusted tax basis in its common stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below under “—Sale or Other Taxable Disposition.”
Subject to the discussion below on effectively connected income, dividends paid to a Non-U.S. Holder will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty, provided the Non-U.S. Holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate). A Non-U.S. Holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. Holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
If dividends paid to a Non-U.S. Holder are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such dividends are attributable), the Non-U.S. Holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the Non-U.S. Holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying that the dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States.
Any such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the regular graduated rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected dividends, as adjusted for certain items. Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
161
Table of Contents
Index to Financial Statements
Sale or Other Taxable Disposition
A Non-U.S. Holder will not be subject to U.S. federal income tax on any gain realized upon the sale or other taxable disposition of our common stock unless:
| • | the gain is effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the Non-U.S. Holder maintains a permanent establishment in the United States to which such gain is attributable); |
| • | the Non-U.S. Holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the disposition and certain other requirements are met; or |
| • | our common stock constitutes a U.S. real property interest (“USRPI”) by reason of our status as a U.S. real property holding corporation (“USRPHC”) for U.S. federal income tax purposes. |
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the regular graduated rates. A Non-U.S. Holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items.
Gain described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty), which may be offset by certain U.S. source capital losses of the Non-U.S. Holder (even though the individual is not considered a resident of the United States), provided the Non-U.S. Holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we currently are not, and do not anticipate becoming, a USRPHC. Because the determination of whether we are a USRPHC depends, however, on the fair market value of our USRPIs relative to the fair market value of our non-U.S. real property interests and our other business assets, there can be no assurance we currently are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition by a Non-U.S. Holder of our common stock will not be subject to U.S. federal income tax if our common stock is “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, and such Non-U.S. Holder owned, actually and constructively, 5% or less of our common stock throughout the shorter of the five-year period ending on the date of the sale or other taxable disposition or the Non-U.S. Holder’s holding period.
Non-U.S. Holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
Payments of dividends on our common stock will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know the holder is a United States person and the holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any dividends on our common stock paid to the Non-U.S. Holder, regardless of whether any tax was actually withheld. In addition, proceeds of the sale or other taxable disposition of our common stock within the United States or conducted through certain U.S.-related brokers generally will not be subject to backup withholding or information reporting, if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such holder is a United States person, or the holder otherwise establishes an exemption. Proceeds of a disposition of our common stock conducted through a non-U.S. office of a non-U.S. broker that does not have certain enumerated relationships with the United States generally will not be subject to backup withholding or information reporting.
162
Table of Contents
Index to Financial Statements
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the Non-U.S. Holder resides or is established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a Non-U.S. Holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts
Withholding taxes may be imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the Foreign Account Tax Compliance Act, or “FATCA”) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends on, or gross proceeds from the sale or other disposition of, our common stock paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States-owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Under the applicable Treasury Regulations and administrative guidance, withholding under FATCA generally applies to payments of dividends on our common stock, and will apply to payments of gross proceeds from the sale or other disposition of such stock on or after January 1, 2019.
Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our common stock.
163
Table of Contents
Index to Financial Statements
The validity of the common stock being offered in this prospectus has been passed upon by Latham & Watkins LLP.
The financial statements included in this Prospectus have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein (which report expresses an unqualified opinion on the financial statements and includes an explanatory paragraph referring to the company’s ability to continue as a going concern). Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
Upon the effectiveness of registration statement of which this prospectus forms a part or our earlier registration of a class of our securities under Section 12 of the Exchange Act, we will be required to file annual, quarterly and current reports and proxy statements and other information with the SEC. Prior to that time, we are not required to file such reports, but expect to do so as a “voluntary filer” under the Exchange Act. You may read and copy any document that we file at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549, on official business days during the hours of 10:00 am and 3:00 pm. Please call the SEC at 1-800-SEC-0330 for further information on the Public Reference Room. All filings we make with the SEC are also available on the SEC’s web site at http://www.sec.gov. Our website address is http://www.viewray.com. We have not incorporated by reference into this prospectus the information on our website, and you should not consider it to be a part of this document.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock being offered by this prospectus. This prospectus is part of that registration statement. This prospectus does not contain all of the information set forth in the registration statement or the exhibits to the registration statement. For further information with respect to us and the shares we are offering pursuant to this prospectus, you should refer to the complete registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract, agreement or other document referred to are not necessarily complete, and you should refer to the copy of that contract or other documents filed as an exhibit to the registration statement. You may read or obtain a copy of the registration statement at the SEC’s public reference room and website referred to above.
164
Table of Contents
Index to Financial Statements
VIEWRAY INCORPORATED
| Page | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| Statements of Convertible Preferred Stock and Stockholders’ Deficit |
F-5 | |||
| F-6 | ||||
| F-7 | ||||
F-1
Table of Contents
Index to Financial Statements
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of
ViewRay Incorporated
We have audited the accompanying balance sheets of ViewRay Incorporated (the “Company”) as of December 31, 2014 and 2013, and the related statements of operations, convertible preferred stock and stockholders’ deficit and cash flows for each of the two years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements for the years ended December 31, 2014 and 2013 have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, the Company’s recurring losses and negative cash flows from operations, stockholders’ capital deficiency and limited liquidity raise substantial doubt about its ability to continue as a going concern. Management’s plans concerning these matters are also discussed in Note 1 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ Deloitte & Touche LLP |
| Cleveland, Ohio |
| February 20, 2015 |
| (March 25, 2015 as to the effects of the reverse stock split described in Note 18 and July 23, 2015 as to the effects of the stock conversion described in Note 18) |
F-2
Table of Contents
Index to Financial Statements
VIEWRAY INCORPORATED
(in thousands, except share and per share data)
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| ASSETS |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 26,529 | $ | 11,129 | ||||
| Accounts receivable |
254 | 904 | ||||||
| Inventory |
5,557 | 8,238 | ||||||
| Deposits on purchased inventory |
2,319 | 2,798 | ||||||
| Deferred cost of revenue |
— | 4,712 | ||||||
| Prepaid expenses and other current assets |
277 | 626 | ||||||
|
|
|
|
|
|||||
| Total current assets |
34,936 | 28,407 | ||||||
| Property and equipment, net |
1,786 | 2,931 | ||||||
| Restricted cash |
453 | 1,053 | ||||||
| Intangible assets, net |
431 | 264 | ||||||
| Deferred offering costs |
— | 1,419 | ||||||
| Other assets |
59 | 31 | ||||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
$ | 37,665 | $ | 34,105 | ||||
|
|
|
|
|
|||||
| LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT |
||||||||
| Current liabilities: |
||||||||
| Notes payable |
$ | 240 | $ | 240 | ||||
| Accounts payable |
2,346 | 6,134 | ||||||
| Accrued liabilities |
4,048 | 4,436 | ||||||
| Customer deposits |
2,853 | 6,100 | ||||||
| Deferred revenue, current portion |
425 | 7,361 | ||||||
| Long-term debt, current portion |
— | 5,493 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
9,912 | 29,764 | ||||||
| Deferred revenue, net of current portion |
128 | — | ||||||
| Long-term debt, net of current portion |
14,384 | 9,149 | ||||||
| Convertible preferred stock warrant liability |
158 | 138 | ||||||
| Other long-term liabilities |
196 | 567 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES |
24,778 | 39,618 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 6) |
||||||||
| Convertible preferred stock, par value $0.01 per share; 67,460,997 and 74,460,997 shares authorized at December 31, 2013 and 2014; 25,036,330 and 27,654,928 shares issued and outstanding at December 31, 2013 and 2014; aggregate liquidation preference of $131,434 and $146,732 at December 31, 2013 and 2014 |
130,037 | 145,110 | ||||||
| Stockholders’ deficit: |
||||||||
| Common stock, par value $0.01 per share; 81,000,000 and 88,000,000 shares authorized at December 31, 2013 and 2014; 878,717 and 907,037 shares issued and outstanding at December 31, 2013 and 2014 |
9 | 9 | ||||||
| Additional paid-in capital |
1,096 | 1,414 | ||||||
| Accumulated deficit |
(118,255 | ) | (152,046 | ) | ||||
|
|
|
|
|
|||||
| TOTAL STOCKHOLDERS’ DEFICIT |
(117,150 | ) | (150,623 | ) | ||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ DEFICIT |
$ | 37,665 | $ | 34,105 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these financial statements.
F-3
Table of Contents
Index to Financial Statements
VIEWRAY INCORPORATED
(in thousands, except share and per share data)
| Year Ended December 31, |
||||||||
| 2013 | 2014 | |||||||
| Revenue: |
||||||||
| Product |
$ | 2,253 | $ | 5,988 | ||||
| Service |
12 | 411 | ||||||
| Grant |
894 | — | ||||||
|
|
|
|
|
|||||
| Total revenue |
3,159 | 6,399 | ||||||
| Cost of revenue: |
||||||||
| Product |
8,173 | 8,176 | ||||||
| Service |
14 | 975 | ||||||
|
|
|
|
|
|||||
| Total cost of revenue |
8,187 | 9,151 | ||||||
| Gross margin |
(5,028 | ) | (2,752 | ) | ||||
| Operating expenses: |
||||||||
| Research and development |
8,780 | 9,404 | ||||||
| Selling and marketing |
3,781 | 4,681 | ||||||
| General and administrative |
9,508 | 14,742 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
22,069 | 28,827 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(27,097 | ) | (31,579 | ) | ||||
| Interest income |
4 | 1 | ||||||
| Interest expense |
(97 | ) | (2,243 | ) | ||||
| Other income (expense), net |
(32 | ) | 21 | |||||
|
|
|
|
|
|||||
| Loss before provision for income taxes |
(27,222 | ) | (33,800 | ) | ||||
| Provision for income taxes |
— | — | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (27,222 | ) | $ | (33,800 | ) | ||
|
|
|
|
|
|||||
| Cumulative dividends on convertible preferred stock |
(2,898 | ) | — | |||||
| Deemed capital contribution on conversion of Series C convertible preferred stock into common stock |
8,783 | — | ||||||
| Deemed dividend on convertible preferred stock extinguishment |
(6,863 | ) | — | |||||
| Deemed capital contribution on repurchase of Series A convertible preferred stock |
— | 9 | ||||||
|
|
|
|
|
|||||
| Net loss attributable to common stockholders |
$ | (28,200 | ) | $ | (33,791 | ) | ||
|
|
|
|
|
|||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (34.59 | ) | $ | (37.87 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares used in computing net loss per share attributable to common stockholders, basic and diluted |
815,340 | 892,315 | ||||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
Index to Financial Statements
VIEWRAY INCORPORATED
Statements of Convertible Preferred Stock and Stockholders’ Deficit
(in thousands, except share data)
| Convertible Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Deficit |
Total Stockholders’ Deficit |
||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
| Balance at January 1, 2013 |
16,748,405 | $ | 79,540 | 730,203 | $ | 8 | $ | 773 | $ | (90,055 | ) | $ | (89,274 | ) | ||||||||||||||
| Issuance of common stock from option exercises |
— | — | 1,463 | — | 1 | — | 1 | |||||||||||||||||||||
| Issuance of Series D-1 convertible preferred stock (net of issuance cost of $99) |
983,558 | 4,901 | — | — | — | — | — | |||||||||||||||||||||
| Issuance of Series D-2 convertible preferred stock (net of issuance cost of $291) |
3,013,797 | 15,030 | — | — | — | — | — | |||||||||||||||||||||
| Accrued dividends |
— | — | — | — | — | (2,898 | ) | (2,898 | ) | |||||||||||||||||||
| Conversion of Series C convertible preferred stock and related dividends into common stock and deemed capital contribution |
(1,470,485 | ) | (7,475 | ) | 147,051 | 1 | 103 | 8,783 | 8,887 | |||||||||||||||||||
| Conversion of accrued dividends into Series D-2 convertible preferred stock |
3,196,417 | 16,249 | — | — | — | — | — | |||||||||||||||||||||
| Extinguishment of Series B-1, Series C, Series D-1 and Series D-2 convertible preferred stock |
(22,308,230 | ) | (105,217 | ) | — | — | — | — | — | |||||||||||||||||||
| Exchange of new Series B convertible preferred stock and deemed dividend |
22,308,230 | 112,080 | — | — | — | (6,863 | ) | (6,863 | ) | |||||||||||||||||||
| Issuance of new Series C convertible preferred stock (net of issuance cost of $71) |
2,564,638 | 14,929 | — | — | — | — | — | |||||||||||||||||||||
| Stock-based compensation |
— | — | — | — | 219 | — | 219 | |||||||||||||||||||||
| Net loss |
— | — | — | — | — | (27,222 | ) | (27,222 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2013 |
25,036,330 | 130,037 | 878,717 | 9 | 1,096 | (118,255 | ) | (117,150 | ) | |||||||||||||||||||
| Issuance of common stock from option exercises |
— | — | 28,320 | — | 21 | — | 21 | |||||||||||||||||||||
| Repurchase of Series A convertible preferred stock and deemed capital contribution |
(1,353 | ) | (25 | ) | — | — | — | 9 | 9 | |||||||||||||||||||
| Issuance of Series C convertible preferred stock (net of issuance cost of $181) |
880,546 | 4,969 | — | — | — | — | — | |||||||||||||||||||||
| Conversion of convertible promissory notes into Series C convertible preferred stock, including accrued interest of $173 (net of unamortized debt discount of $44) |
1,739,405 | 10,129 | — | — | — | — | — | |||||||||||||||||||||
| Stock-based compensation |
— | — | — | — | 297 | — | 297 | |||||||||||||||||||||
| Net loss |
— | — | — | — | — | (33,800 | ) | (33,800 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at December 31, 2014 |
27,654,928 | $ | 145,110 | 907,037 | $ | 9 | $ | 1,414 | $ | (152,046 | ) | $ | (150,623 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
Table of Contents
Index to Financial Statements
VIEWRAY INCORPORATED
(in thousands)
| Year Ended December 31, |
||||||||
| 2013 | 2014 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: |
||||||||
| Net loss |
$ | (27,222 | ) | $ | (33,800 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
1,149 | 1,020 | ||||||
| Stock-based compensation |
219 | 318 | ||||||
| Change in fair value of convertible preferred stock warrant liability |
— | (20 | ) | |||||
| Inventory lower of cost and market adjustment |
4,582 | 598 | ||||||
| Loss on disposal of property and equipment |
176 | 8 | ||||||
| Amortization of debt discount and interest accrual |
8 | 446 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable |
(254 | ) | (650 | ) | ||||
| Inventory |
(5,175 | ) | (3,279 | ) | ||||
| Deposits on purchased inventory |
(879 | ) | (479 | ) | ||||
| Deferred costs |
— | (4,712 | ) | |||||
| Prepaid expenses and other current assets |
(167 | ) | 13 | |||||
| Other assets |
(37 | ) | 28 | |||||
| Accounts payable |
(666 | ) | 3,230 | |||||
| Accrued expenses and other long-term liabilities |
2,074 | (245 | ) | |||||
| Customer deposits and deferred revenue |
821 | 10,055 | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(25,371 | ) | (27,469 | ) | ||||
|
|
|
|
|
|||||
| CASH FLOWS FROM INVESTING ACTIVITIES: |
||||||||
| Purchase of property and equipment |
(1,198 | ) | (2,003 | ) | ||||
| Purchase of intellectual property license |
(500 | ) | — | |||||
| Change in restricted cash |
105 | (600 | ) | |||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(1,593 | ) | (2,603 | ) | ||||
|
|
|
|
|
|||||
| CASH FLOWS FROM FINANCING ACTIVITIES: |
||||||||
| Proceeds from issuance of convertible preferred stock, net |
34,860 | 4,969 | ||||||
| Proceeds from the exercise of stock options |
1 | 21 | ||||||
| Proceeds from issuance of long-term debt, net |
14,534 | — | ||||||
| Proceeds from issuance of convertible promissory notes, net |
— | 9,941 | ||||||
| Repurchase of Series A convertible preferred stock |
— | (37 | ) | |||||
| Payments of costs related to the initial public offering |
— | (222 | ) | |||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
49,395 | 14,672 | ||||||
|
|
|
|
|
|||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS |
22,431 | (15,400 | ||||||
| CASH AND CASH EQUIVALENTS—Beginning of period |
4,098 | 26,529 | ||||||
|
|
|
|
|
|||||
| CASH AND CASH EQUIVALENTS—End of period |
$ | 26,529 | $ | 11,129 | ||||
|
|
|
|
|
|||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: |
||||||||
| Cash paid for interest |
$ | — | $ | 1,504 | ||||
|
|
|
|
|
|||||
| Cash paid for income taxes |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
| NON-CASH INVESTING AND FINANCING ACTIVITIES: |
||||||||
| Conversion of convertible notes into new Series C convertible preferred stock |
$ | — | $ | 10,129 | ||||
|
|
|
|
|
|||||
| Cumulative dividends on convertible preferred stock |
$ | 2,898 | $ | — | ||||
|
|
|
|
|
|||||
| Conversion of Series C convertible preferred stock and related dividends into common stock |
$ | 8,887 | $ | — | ||||
|
|
|
|
|
|||||
| Conversion of accrued dividends into Series D-2 convertible preferred stock |
$ | 16,249 | $ | — | ||||
|
|
|
|
|
|||||
| Exchange of new Series B convertible preferred stock upon extinguishment of prior convertible |
$ | 6,863 | $ | — | ||||
|
|
|
|
|
|||||
| Issuance of convertible preferred stock warrant |
$ | 158 | $ | — | ||||
|
|
|
|
|
|||||
| Purchase of equipment in accounts payable |
$ | 59 | $ | 62 | ||||
|
|
|
|
|
|||||
| Costs related to the initial public offering included in accounts payable and accrued liabilities |
$ | — | $ | 1,197 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these financial statements.
F-6
Table of Contents
Index to Financial Statements
Notes to Financial Statements
| 1. | BACKGROUND AND ORGANIZATION |
ViewRay Incorporated, or the Company, designs, manufactures and markets MRIdian, the first and only MRI-guided radiation therapy system to image and treat cancer patients simultaneously.
Since inception, the Company has devoted substantially all of its efforts towards research and development, initial selling and marketing activities, raising capital and preparing for the manufacturing and shipment of MRIdian systems. In May 2012, the Company was granted clearance from the U.S. Food and Drug Administration, or FDA, to sell MRIdian. In November 2013, the Company received its first clinical acceptance of a MRIdian at a customer site, and the first patient was treated with that system in January 2014. The Company received permission to affix the CE mark in November 2014.
The Company has incurred losses and negative cash flows from operations since inception and has an accumulated deficit of $152.0 million at December 31, 2014. The Company anticipates incurring additional losses until such time that it can generate significant revenues from MRIdian systems. The Company’s primary source of liquidity to date has been through sales of convertible preferred stock, proceeds from various debt arrangements, initial sales of MRIdian systems and customer deposits received on future orders. In 2014, the Company raised a total of $14.9 million net proceeds through issuance of convertible promissory notes and Series C convertible preferred stock. In January and February 2015, the Company raised a total of $15.6 million net proceeds through issuance of Series C convertible preferred stock. The Company is involved in active financing negotiations; however, if a financing event does not occur, the Company is expected to exhaust its cash and cash equivalents during 2015. Substantial additional financing will be needed by the Company to fund its operations and research and development efforts. There is no assurance that such financing will be available when needed or on acceptable terms. The Company is also subject to those risks associated with any early stage operating company that has working capital requirements and substantial expenditures for research and development. There can be no assurance that MRIdian will be commercially viable. In addition, the Company operates in an environment of rapid technological change, and is largely dependent on the services of its employees. These factors raise substantial doubt about the Company’s ability to continue as a going concern.
Management is currently evaluating different strategies to obtain the required funding of future operations. These strategies may include, but are not limited to: additional funding from current or new investors, refinancing existing debt obligations, and/or obtaining additional debt financing, and/or an initial public offering of the Company’s common stock. There can be no assurance that these future funding efforts will be successful.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and satisfaction of liabilities in the normal course of business. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might result from the outcome of this uncertainty.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
The accompanying financial statements reflect the application of certain significant accounting policies, as described below and elsewhere in the accompanying notes to the financial statements.
Basis of Presentation
The financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, or GAAP, and pursuant to the rules and regulation of the Securities and Exchanges Commission, or SEC.
F-7
Table of Contents
Index to Financial Statements
Use of Estimates
The preparation of the accompanying financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amount of expenses during the reporting period. Such management estimates include those relating to the allocation of revenue to its multiple deliverable elements, inventory write-downs to reflect net realizable value, assumptions used in the valuation of stock-based awards and a convertible preferred stock warrant, accrued losses from purchase commitments and valuation allowances against deferred tax assets. Actual results could differ from those estimates.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with an original maturity of three months or less to be cash equivalents. The Company deposits its cash primarily in checking and money market accounts.
Restricted Cash
At December 31, 2013, the Company had irrevocable standby letters of credit of $453 thousand to a governmental agency in connection with certain regulatory requirements for the Company’s radiation therapy product and to a customer in connection with the Company’s contractual obligations with such customer.
In February 2014, $350 thousand in outstanding irrevocable letters of credit were cancelled upon the satisfaction of the Company’s contractual obligations with such customer. In July 2014, the Company issued an irrevocable standby letter of credit in the amount of $450 thousand as a guarantee to a new lease agreement signed in 2014. In December 2014, as a performance guarantee in connection with the Company’s contractual obligations with a distributor, the Company issued another irrevocable standby letter of credit for $500 thousand.
At December 31, 2014, the Company had an aggregate of $1.1 million of outstanding letters of credit. The letters of credit are collateralized by a restricted cash deposit account, which is presented as part of noncurrent assets on the balance sheets because the Company is not certain when the restriction will be lifted on the collateralized letters of credit. As of December 31, 2013 and 2014, no amounts were drawn on the letters of credit.
Concentration of Credit Risk, Other Risks and Uncertainties
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash and cash equivalents and accounts receivable. Cash and cash equivalents are deposited in checking and money market accounts at one financial institution. At times, cash balances may be in excess of the amounts insured by the Federal Deposit Insurance Corporation. Management believes the financial risk associated with these balances is minimal and has not experienced any losses to date. The Company performs periodic credit evaluations of its customers’ financial condition and generally requires deposits from its customers. The Company’s accounts receivable was derived from grant revenue earned from the State of Ohio at December 31, 2013 (see Note 8), and from billing to a customer at December 31, 2014. The Company’s customers representing greater than 10% of accounts receivable and revenue for the periods presented were as follows:
| Revenue | Accounts Receivable | |||||||||||||||
| Year Ended December 31, |
December 31, | |||||||||||||||
| Customers |
2013 | 2014 | 2013 | 2014 | ||||||||||||
| State of Ohio |
28 | % | * | 100 | % | * | ||||||||||
| Customer A |
72 | % | * | * | * | |||||||||||
| Customer B |
* | 52 | % | * | * | |||||||||||
| Customer C |
* | 40 | % | * | * | |||||||||||
| Customer D |
* | * | * | 100 | % | |||||||||||
F-8
Table of Contents
Index to Financial Statements
The Company’s future results of operations involve a number of risks and uncertainties. Factors that could affect the Company’s future operating results and cause actual results to vary materially from expectations include, but are not limited to, rapid technological change, continued acceptance of MRIdian, competition from substitute products and larger companies, protection of proprietary technology, ability to maintain distributor relationships and dependence on key individuals. Furthermore, new products to be developed by the Company require approval from the FDA or other international regulatory agencies prior to commercial sales. There can be no assurance that the Company’s future products will receive the necessary clearances.
The Company relies on a concentrated number of suppliers to manufacture essentially all of the components used in MRIdian. The Company’s suppliers may encounter problems during manufacturing due to a variety of reasons, including failure to comply with applicable regulations, including the FDA’s Quality System Regulation, equipment malfunction and environmental factors, any of which could delay or impede their ability to meet demand.
Accounts Receivables and Allowance for Doubtful Accounts
Accounts receivable are recorded at the invoiced amount, net of any allowance for doubtful accounts, and do not bear interest. The allowance for doubtful accounts, if any, is based on the assessment of the collectability of customer accounts.
Based on the specific customer and the current economic conditions, there was no allowance for doubtful accounts recorded at December 31, 2013 and 2014.
Fair Value of Financial Instruments
Financial instruments consist of cash and cash equivalents, accounts receivable, restricted cash, prepaid expenses and other current assets, accounts payable, accrued liabilities, notes payable, convertible preferred stock warrant liability and long-term debt. Cash equivalents are stated at amortized cost, which approximates fair value at the balance sheet dates, due to the short period of time to maturity. Accounts receivable, prepaid expenses and other current assets, accounts payable, accrued liabilities and notes payable are stated at their carrying value, which approximates fair value due to the short time to the expected receipt or payment date. The convertible preferred stock warrant liability is carried at fair value. The carrying amount of the Company’s long-term debt approximates fair value as the stated interest rate approximates market rates currently available to the Company.
Inventory and Deposits on Purchased Inventory
Inventory consists of purchased components for assembling MRIdian systems and other direct and indirect costs associated with MRIdian system installation. Inventory is stated at the lower of cost (on a first-in, first-out basis) or market value. All inventories expected to be placed in service during the Company’s normal operating cycle for the delivery and assembly of MRIdian systems, including items expected to be on hand for more than one year, are classified as current assets. The Company reduces the carrying value of its inventory for the difference between cost and net realizable value and records a charge to cost of product revenues for the amount required to reduce the carrying value of inventory to net realizable value. The Company recorded an inventory lower of cost and market adjustment of $4.6 million and $598 thousand during the years ended December 31, 2013 and 2014, respectively.
The Company records inventory items which have been paid for but not yet received and titles have not yet transferred to the Company as deposits on purchased inventory. Deposits on purchased inventory are included within current assets as the related inventory items are expected to be received and used in MRIdian systems within the Company’s normal operating cycle. The Company assesses the recoverability of deposits on purchased inventory based on credit assessments of the vendors and their history supplying these assets. At December 31, 2014, the Company did not have any instances whereby deposits for purchased inventory were written off or the purchased inventory was not delivered.
F-9
Table of Contents
Index to Financial Statements
Shipping and Handling Costs
Shipping and handling costs for product shipments to customers are included in cost of product revenue. Shipping and handling costs incurred for inventory purchases are capitalized in inventory and expensed in cost of product revenue. These costs are not passed on to customers.
Property and Equipment
Property and equipment are recorded at cost. Depreciation is computed over estimated useful lives, ranging from two to 15 years of the related assets using the straight-line method. Acquired software is recorded at cost. Amortization of acquired software generally occurs over three years using the straight-line method. Leasehold improvements are amortized on a straight-line basis over the shorter of the useful life or term of the lease. Upon retirement or sale, the cost and related accumulated depreciation are removed from the balance sheet and the resulting gain or loss is recorded to general and administrative expense in the accompanying statements of operations. Routine expenditures for maintenance and repairs are expensed as incurred.
Depreciation and amortization periods for property and equipment are as follows:
| Property and Equipment |
Estimated Useful Life | |
| Prototype | 2 years | |
| Machinery and equipment | 5 – 15 years | |
| Furniture and fixture | 5 – 10 years | |
| Software | 3 years | |
| Leasehold improvements | Lesser of estimated useful life or remaining lease term |
Intangible Assets
The Company capitalizes the costs incurred in obtaining certain licenses. During the year ended December 31, 2013, the Company acquired a license to intellectual property associated with certain technology components incorporated into the Company’s systems for $500 thousand. The license cost is being amortized on a straight-line basis over its estimated useful life of three years.
Impairment of Long-Lived Assets
The Company reviews the recoverability of long-lived assets, including equipment, leasehold improvements, software and intangible assets when events or changes in circumstances occur that indicate that the carrying value of the asset may not be recoverable. The assessment of possible impairment is based on the ability to recover the carrying value of the assets from the expected future cash flows (undiscounted and without interest charge) of the related operations. If these cash flows are less than the carrying value of such assets, an impairment loss for the difference between the estimated fair value and carrying value is recorded. There was no impairment loss recognized during the years ended December 31, 2013 and 2014.
Deferred Offering Costs
The Company capitalizes qualified legal, accounting and other direct costs related to its efforts to raise capital through a public sale of its common stock in its planned IPO. These costs are recorded in deferred offering costs in the accompanying balance sheets and will be deferred until the completion of the IPO, at which time they will be reclassified to additional paid-in capital as a reduction of the IPO proceeds. If the Company terminates its plan for an IPO or significantly delays such plan, any deferred costs will be expensed at that time. At December 31, 2014, the Company capitalized $1.4 million of deferred IPO costs. No amounts were deferred as of December 31, 2013.
F-10
Table of Contents
Index to Financial Statements
Comprehensive Loss
Comprehensive loss is the change in equity of a company during a period from transactions and other events and circumstances, excluding transactions resulting from investment owners and distribution to owners. For the periods presented, comprehensive loss did not differ from net loss.
Revenue Recognition
The Company derives revenue primarily from the sale of the systems and related services, which are predominantly sales of MRIdian, as well as support and maintenance services on purchased systems. In all sales arrangements, the Company recognizes revenues when there is persuasive evidence of an arrangement, the fee is fixed or determinable, collection of the fee is reasonably assured and delivery has occurred. For sales of MRIdian systems, the Company currently recognizes product revenue upon receipt of customer acceptance. For sales of the related support and maintenance services, the Company recognizes service revenue on a straight-line basis over the service contract term, which is typically 12 months.
Multiple Elements
Based on the nature of the Company’s business, it frequently enters into sales arrangements with customers that contain multiple elements or deliverables. In situations where a deliverable in a multi-element arrangement has value to the customer on a stand-alone basis, the Company is required to allocate the fair value of the various elements based on the selling price of each element. The principal deliverables consist of (i) sale of MRIdian systems, which generally includes installation, site preparation and software, and (ii) product support, which includes extended service and maintenance.
The Company determines selling prices using vendor specific objective evidence, or VSOE, if it exists, or third party evidence, or TPE. If neither VSOE nor TPE exists for a deliverable, the Company uses best estimated selling price, or BESP. The Company allocates revenue to its multiple elements generally using the relative fair values as determined by BESP. The Company regularly reviews VSOE, TPE and BESP for all of its MRIdian systems and services.
Product Revenue
Product revenue is derived primarily from the sales of MRIdian. The system contains both software and non-software components that together deliver essential functionality. However, because MRIdian includes hardware products as well as software components that function together with the hardware components to deliver MRIdian’s essential functionality, the revenue from the sale of MRIdian systems does not fall within the scope of the software revenue recognition rules.
The related customer contracts currently call for on-site assembly of the system components and system integration. Once the system installation is completed, the Company performs a detailed demonstration with the customer showing that MRIdian meets the standard product specifications. After successful demonstration, the customer signs a document indicating customer acceptance. All contracts include customer deposits upon signing of the agreement with final payment generally due upon customer acceptance.
Revenue recognition for MRIdian systems generally occurs when the customer acknowledges that the system operates in accordance with standard product specifications, the customer accepts the installed unit and title and risk of loss are transferred to the customer.
Service Revenue
Service revenue is derived primarily from maintenance services. Service revenue is deferred and recognized ratably over the service period.
F-11
Table of Contents
Index to Financial Statements
Grant Revenue
The Company receives payments for the achievement of certain milestones under government grants over a contractually defined period. These payments are nonrefundable. Government grants generally provide the Company with fixed payments and a contractually defined period. Grant revenues are recognized as milestones under the grant program are achieved and is earned through reimbursements for the qualifying expenses incurred by the Company.
The Company retains ownership and exclusive rights to all inventions made under these arrangements. Upon the completion of the Company’s government grants, no further obligations exist under these arrangements. The Company retains the rights to commercialize the technology it developed under government grants without any royalty obligations.
Customer Deposits
Customer deposits represent payments received in advance of system installation. For domestic sales, advance payments received prior to customer acceptance are recorded as customer deposits. For international sales, advance payments are initially recorded as customer deposits and are subsequently reclassified to deferred revenue upon inventory shipment when the title and risk of loss of inventory items transferred to customers. All customer deposits, including those that are expected to be a deposit for more than one year, are classified as current liabilities based on consideration of the Company’s normal operating cycle (the time between acquisition of the inventory components and the final cash collection from customers on these inventory components) which is in excess of one year.
Deferred Revenue and Deferred Cost of Revenue
Deferred revenue consists of deferred product revenue and deferred service revenue. Deferred product revenue arises from timing differences between the fulfillment of other contract deliverables and satisfaction of all revenue recognition criteria consistent with the Company’s revenue recognition policy. Deferred service revenue results from the advance billing for services to be delivered over a period of time. Deferred revenues expected to be realized within one year are classified as current liabilities.
Deferred cost of revenue consists of cost for inventory items that have been shipped with title and risk of loss transferred to customer but the customer acceptance has not been received. Deferred cost of revenue is included as part of current assets as the corresponding deferred product revenue are expected to be realized within one year. The inventories recorded in deferred cost of revenue are also included in the inventory lower of cost or market analysis. At December 31, 2014, no reserve was required for deferred cost of revenue.
Research and Development Costs
Expenditures, including payroll, contractor expenses and supplies, for research and development of products and manufacturing processes are expensed as incurred.
Software development costs incurred subsequent to establishing technological feasibility are capitalized through the general release of MRIdian systems that contain the embedded software elements. Technological feasibility is demonstrated by the completion of a working model. The Company has not capitalized any software development costs at December 31, 2013 or 2014, since the costs incurred subsequent to achieving technological feasibility and completing the research and development for the software components were immaterial.
Stock-Based Compensation
The Company uses the Black-Scholes option-pricing model as the method for estimating the fair value of stock options. The Black-Scholes option-pricing model requires the use of highly subjective and complex assumptions
F-12
Table of Contents
Index to Financial Statements
that determine the fair value of share-based awards, including the options’ expected term and the price volatility of the underlying stock. The fair value of the portion of the award that is ultimately expected to vest is recognized as compensation expense over the awards’ requisite service periods in the statements of operations. The Company attributes the value of share-based compensation to expense using the straight-line method.
Medical Device Excise Tax
Medical Device Excise Tax, or MDET, Section 4191 of the Internal Revenue Code enacted by the Health Care and Education Reconciliation Act of 2010, in conjunction with the Patient Protection and Affordable Care Act, established a 2.3% excise tax on medical devices sold domestically beginning on January 1, 2013. The Company included the cost of MDET in cost of product revenue during the year ended December 31, 2014, net of amounts directly billed to the customer for this tax, if any.
Deferred Commissions
Deferred commissions are the direct and incremental costs directly associated with the MRIdian system contracts with customers, which primarily consist of sales commissions to our direct sales force. The commissions are deferred and expensed in proportion to the revenue recognized upon the acceptance of the MRIdian system. At December 31, 2014, the Company had $221 thousand deferred commissions recorded as part of prepaid expenses and other current assets on the balance sheet.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes. Deferred tax assets and liabilities are recognized for the estimated future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. Deferred tax expense or benefit is the result of changes in the deferred tax assets and liabilities. Valuation allowances are established when necessary to reduce deferred tax assets where, based upon the available evidence, management concludes that it is more-likely-than not that the deferred tax assets will not be realized. Because of the uncertainty of the realization of the deferred tax assets, the Company has recorded a full valuation allowance against its net deferred tax assets.
In evaluating the ability to recover its deferred income tax assets, the Company considers all available positive and negative evidence, including its operating results, ongoing tax planning and forecasts of future taxable income on a jurisdiction-by-jurisdiction basis. In the event the Company was to determine that it would be able to realize its deferred income tax assets in the future in excess of their net recorded amount, it would make an adjustment to the valuation allowance which would reduce the provision for income taxes.
Reserves are provided for tax benefits for which realization is uncertain. Such benefits are only recognized when the underlying tax position is considered more likely than not to be sustained on examination by a taxing authority, assuming they possess full knowledge of the position and facts. It is the Company’s policy to include any penalties and interest related to income taxes in its income tax provision; however, the Company currently has no penalties or interest related to income taxes. The earliest year that the Company is subject to examination is the year ended December 31, 2004.
Convertible Preferred Stock Warrant Liability
The Company’s warrant to purchase convertible preferred stock is classified as a liability on the balance sheets at fair value upon issuance because the warrant is exercisable for contingently redeemable preferred stock which is classified outside of stockholders’ deficit. The warrant is subject to re-measurement to fair value at each balance sheet date, and any change in fair value is recognized in the statements of operations as other income (expense),
F-13
Table of Contents
Index to Financial Statements
net. The Company will continue to adjust the liability for changes in fair value until the earlier of the exercise or expiration of the warrant, the conversion of the underlying shares of convertible preferred stock or the completion of a liquidation event or the completion of an IPO. Upon the exercise, expiration or the completion of the liquidation event, the related warrant liability will be reclassified to additional paid-in capital.
Accrued Purchase Commitments
The Company has certain non-cancellable purchase commitments from outstanding purchase orders related to the manufacture of MRIdian systems. As part of the inventory lower of cost and market adjustment charged to cost of product revenue, the Company accrued the total purchase commitments of $1.5 million at December 31, 2013 as it relates to the determination of the total cost to complete a MRIdian system. The accrued purchase commitments are recorded as part of accrued liabilities in the accompanying balance sheets as the Company expects to receive the inventory within its normal operating cycle.
The Company did not have any outstanding non-cancellable purchase commitments at December 31, 2014.
Segment and Geographic Information
The Company has one business activity, which is radiation therapy technology combined with magnetic resonance imaging, and operates in one reportable segment. The Company’s chief operating decision-maker, its chief executive officer, reviews its operating results on an aggregate basis for purposes of allocating resources and evaluating financial performance. Also, the Company does not have segment managers as the Company manages its operations as a single operating segment, and all of the Company’s revenues have been derived from customers located in the United States for the years ended December 31, 2013 and 2014. At December 31, 2013 and 2014, all long-lived assets are located in the United States.
Net Loss per Share
The Company’s basic net loss per share attributable to common stockholders is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of shares of common stock outstanding for the period. The net loss attributable to common stockholders was not allocated to the convertible preferred stock under the two-class method as the convertible preferred stock do not have a contractual obligation to share in the net loss attributable to common stockholders. The diluted net loss per share attributable to common stockholders is computed by giving effect to all potential common stock equivalents outstanding for the period determined using the treasury stock method. For purposes of this calculation, convertible preferred stock, stock options and a warrant to purchase convertible preferred stock are considered to be common stock equivalents but have been excluded from the calculation of diluted net loss per share attributable to common stockholders as their effect is anti-dilutive.
Recent Accounting Pronouncements
In July 2013, the Financial Accounting Standards Board, or FASB, issued authoritative guidance that addresses the presentation of an unrecognized tax benefit when a net operating loss carryforward, a similar tax loss or a tax credit carryforward exists. This new standard requires the netting of unrecognized tax benefits, or UTBs, against a deferred tax asset for a loss or other carryforward that would apply in settlement of the uncertain tax positions. UTBs will be netted against all available same-jurisdiction loss or other tax carryforwards that would be utilized, rather than only against carryforwards that are created by the UTBs. This new guidance is effective for the Company beginning January 1, 2014, with early adoption permitted. The Company adopted this guidance in 2014 and it did not have a material impact on the Company’s financial statements.
In May 2014, the FASB issued an update to supersede nearly all existing revenue recognition guidance under GAAP. This new standard requires an entity to recognize the amount of revenue to which it expects to be entitled
F-14
Table of Contents
Index to Financial Statements
for the transfer of promised goods or services to customers. The new guidance is effective for the Company on January 1, 2017. Early adoption is not permitted. The new standard permits the use of either the retrospective or cumulative effect transition method. The Company is evaluating the effect that the new guidance will have on its financial statements and related disclosures. The Company has not yet selected a transition method nor has it determined the effect of the standard on its ongoing financial reporting.
In June 2014, the FASB issued an amendment to eliminate the accounting and reporting differences in GAAP between development stage entities and other operating entities, including the presentation of inception-to-date financial statement information and the development stage entity financial statement label. The amendment also clarified that the guidance related to Risks and Uncertainties is applicable to entities that have not commenced planned principal operations. These changes will provide more consistent consolidation analysis and decisions among reporting entities. While these amendments are retrospectively effective for annual reporting periods beginning after December 15, 2014, early adoption is permitted for any annual reporting period or interim period for which the entity’s financial statements have not yet been issued. The Company elected early adoption in the year ended December 31, 2013. The Company’s adoption of this standard did not have a significant impact on its financial position, results of operations or cash flows.
In August 2014, the FASB issued an explicit requirement for management to assess if there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures in certain circumstances. In connection with each annual and interim period, management must assess if there is substantial doubt about an entity’s ability to continue as a going concern within one year after the financial statement issuance date. Disclosures are required if conditions give rise to substantial doubt. The new standard is effective for all entities in the first annual period ending after December 15, 2016. The Company has not elected to early adopt this accounting pronouncement.
| 3. | BALANCE SHEET COMPONENTS |
Property and Equipment
Property and equipment consist of the following (in thousands):
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Prototype |
$ | 6,053 | $ | 6,342 | ||||
| Machine and equipment |
2,714 | 4,214 | ||||||
| Leasehold improvements |
1,154 | 1,270 | ||||||
| Furniture and fixtures |
232 | 263 | ||||||
| Software |
595 | 647 | ||||||
|
|
|
|
|
|||||
| Property and equipment, gross |
10,748 | 12,736 | ||||||
| Less: accumulated depreciation and amortization |
(8,962 | ) | (9,805 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 1,786 | $ | 2,931 | ||||
|
|
|
|
|
|||||
Depreciation and amortization expense related to property and equipment was $1.1 million and $853 thousand during the years ended December 31, 2013 and 2014, respectively.
F-15
Table of Contents
Index to Financial Statements
Intangible Assets
Intangible assets consist of the following (in thousands):
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Intangible assets—license cost |
$ | 500 | $ | 500 | ||||
| Accumulated amortization |
(69 | ) | (236 | ) | ||||
|
|
|
|
|
|||||
| Intangible assets, net |
$ | 431 | $ | 264 | ||||
|
|
|
|
|
|||||
Intangible asset amortization was $69 thousand and $167 thousand during the years ended December 31, 2013 and 2014, respectively.
The estimated future amortization expense of purchased intangible assets at December 31, 2014 was as follows (in thousands):
| Year Ending December 31, | Estimated Future Amortization Expense |
|||
| 2015 |
$ | 167 | ||
| 2016 |
97 | |||
|
|
|
|||
| Total |
$ | 264 | ||
|
|
|
|||
Accrued Liabilities
Accrued liabilities consist of the following (in thousands):
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Accrued payroll and related benefits |
$ | 1,233 | $ | 1,652 | ||||
| Accrued non-cancellable purchase commitments |
1,502 | — | ||||||
| Accrued accounts payable |
517 | 946 | ||||||
| Sales tax and medical device excise tax payable |
98 | 499 | ||||||
| Accrued legal and accounting |
84 | 901 | ||||||
| Accrued interest |
78 | 142 | ||||||
| Other |
536 | 296 | ||||||
|
|
|
|
|
|||||
| Total accrued liabilities |
$ | 4,048 | $ | 4,436 | ||||
|
|
|
|
|
|||||
Deferred Revenue
Deferred revenue consists of the following (in thousands):
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Deferred revenue: |
||||||||
| Products |
$ | 286 | $ | 6,919 | ||||
| Service |
267 | 442 | ||||||
|
|
|
|
|
|||||
| Total deferred revenue |
553 | 7,361 | ||||||
| Less: current portion of deferred revenue |
(425 | ) | (7,361 | ) | ||||
|
|
|
|
|
|||||
| Noncurrent portion of deferred revenue |
$ | 128 | $ | — | ||||
|
|
|
|
|
|||||
F-16
Table of Contents
Index to Financial Statements
| 4. | FAIR VALUE FINANCIAL MEASUREMENTS |
Assets and liabilities recorded at fair value on a recurring basis in the balance sheets are categorized based upon the level of judgment associated with the inputs used to measure their fair values. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The standard describes a fair value hierarchy based on three levels of inputs, of which the first two are considered observable and the last unobservable, that may be used to measure fair value which are the following:
Level 1—Quoted prices in active markets for identical assets or liabilities.
Level 2—Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The assets’ or liabilities’ fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
The Company’s financial instruments that are carried at fair value consist of Level 1 assets and Level 3 liabilities. Level 1 assets include highly liquid bank deposits and money market funds, which were not material at December 31, 2013 or 2014. Level 3 liabilities consist of the convertible preferred stock warrant liability. The convertible preferred stock warrant liability was valued using the Black-Scholes option-pricing model. Generally, increases (decreases) in the fair value of the underlying stock and estimated term would result in a directionally similar impact to the fair value of the warrant (see Note 13).
The convertible preferred stock warrant was issued in December 2013 and, therefore, was outstanding at December 31, 2013 and 2014. The following table sets forth the fair value of the Company’s financial liabilities by level within the fair value hierarchy (in thousands):
| December 31, 2013 | ||||||||||||||||
| Fair Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Convertible preferred stock warrant liability |
$ | 158 | $ | — | $ | — | $ | 158 | ||||||||
| December 31, 2013 | ||||||||||||||||
| Fair Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Convertible preferred stock warrant liability |
$ | 138 | $ | — | $ | — | $ | 138 | ||||||||
The following table sets forth a summary of the changes in the fair value of the Company’s Level 3 financial liabilities (in thousands):
| Year ended December 31, |
||||||||
| 2013 | 2014 | |||||||
| Fair value, beginning of period |
$ | — | $ | 158 | ||||
| Issuance of convertible preferred stock warrant |
158 | — | ||||||
| Change in fair value of Level 3 financial liabilities |
— | (20 | ) | |||||
|
|
|
|
|
|||||
| Fair value, end of period |
158 | 138 | ||||||
|
|
|
|
|
|||||
The gains and losses from re-measurement of Level 3 financial liabilities are recorded as part of other income (expense), net in the statements of operations.
F-17
Table of Contents
Index to Financial Statements
| 5. | DEBT |
Notes Payable
On December 15, 2008, the Company entered into an agreement with the county redevelopment fund in the State of Ohio for a loan of up to $800 thousand to fund the renovation of the Company’s Ohio headquarters. The loan, which bore interest at 6% annually through the maturity date of December 31, 2009, is secured by the Company’s leasehold improvements. Under the terms of the loan agreement, the lender may forgive $240 thousand if the Company meets certain permanent job creation requirements within the State of Ohio. In July 2010, $560 thousand of principal and accrued interest through the loan maturity date were repaid. At December 31, 2013 and 2014, the Company had not met the permanent job creation requirements and as such the $240 thousand was not forgiven and remains a current liability. The notes payable of $240 thousand are due and demandable at any time and do not bear interest.
Term Loan
In December 2013, the Company entered into a Loan and Security Agreement, or the Term Loan, with Hercules Technology Growth Capital, Inc. and Hercules Technology III, L.P., or together, Hercules, for $15.0 million that was outstanding at December 31, 2013 and 2014. Borrowings under the Term Loan bear cash interest at the greater of the annual prime rate plus 7.0% or 10.25%, which was 10.25% at December 31, 2013 and 2014. In addition, borrowings under the Term Loan bear deferred payment in-kind interest at 1.5% per annum. Interest only payments began in January 2014, with monthly principal and interest payments beginning on January 1, 2015 and the entire balance of the Term Loan are to be paid in full by the June 1, 2017 maturity date. The Term Loan is subject to a prepayment penalty of 5% on the outstanding balance during the first 12 months following the funding of the Term Loan and 1% on the outstanding balance thereafter until maturity. The Term Loan was issued at a discount of $466 thousand, which will be amortized to interest expense during the life of the Term Loan using the effective interest method. The discount included the fair value of a convertible preferred stock warrant that was issued with the Term Loan, as discussed in the following paragraph, and the related transaction costs. The Term Loan is collateralized by essentially all the Company’s assets and limits the Company’s ability with respect to additional indebtedness, investments or dividends, among other things, subject to customary exceptions.
In connection with the issuance of the Term Loan, the Company entered into a Warrant Agreement with Hercules to issue a fully vested and exercisable warrant to purchase128,231 shares of Series C convertible preferred stock with an exercise price of $5.84 per share. The warrant is exercisable any time before the later of 10 years from issuance or five years after an IPO. The warrant will be automatically exercised immediately prior to expiration if the fair market value of one share of Series C convertible preferred stock is greater than the exercise price at the time of expiration. The warrant provides for anti-dilution rights on the Series C convertible preferred stock, which includes one-time down-round protection. The fair value of the warrant upon issuance of $158 thousand was recorded as convertible preferred stock warrant liability and a discount to the carrying value of the Term Loan. The fair value of the warrant at the time of issuance was estimated using the Black-Scholes option-pricing model with the following assumptions: expected term of two years, expected volatility of 30%, risk-free interest rate of 0.4% and expected dividend yield of 0%. See Note 12 for assumptions used to estimate the fair value of convertible preferred stock warrant liability at December 31, 2013 and 2014.
F-18
Table of Contents
Index to Financial Statements
The Company’s scheduled future payments on the Term Loan at December 31, 2013 are as follows (in thousands):
| Year Ending December 31, | ||||
| 2015 |
$ | 6,827 | ||
| 2016 |
6,827 | |||
| 2017 |
4,118 | |||
|
|
|
|||
| Total future payments |
17,772 | |||
| Less: amount representing interest |
(2,772 | ) | ||
|
|
|
|||
| Total principal amount |
15,000 | |||
| Less: unamortized debt discount |
(358 | ) | ||
|
|
|
|||
| Carrying value of long-term debt |
14,642 | |||
| Less: current portion |
(5,493 | ) | ||
|
|
|
|||
| Long-term portion |
$ | 9,149 | ||
|
|
|
|||
2014 Convertible Promissory Notes
In August 2014, the Company entered into a Convertible Promissory Note Agreement, or the Convertible Note Agreement, with a majority of its current investors to sell convertible promissory notes in an aggregate principal amount of $10.0 million with the option to raise an additional $1.5 million, or 2014 Notes. The Company received gross proceeds of $3.9 million in August 2014 and $6.1 million in November 2014 under the Convertible Note Agreement. The 2014 Notes carried a simple interest rate of 8% per annum and were subordinated in right of payment to all of the Company’s other indebtedness. The 2014 Notes were to mature in November 2015 unless previously converted. The Convertible Note Agreement provided for the conversion of the 2014 Notes at the option of the majority investors, and at any time, into shares of Series C convertible preferred stock at the then applicable conversion price. In December 2014, the holders of the 2014 Notes opted to convert the outstanding principal and accrued interest of $10.2 million into 1,739,405 shares of Series C convertible preferred stock at a price of $5.84 per share in accordance with the terms of the Convertible Note Agreement. As of December 31, 2014, the 2014 Notes are no longer outstanding. In addition, the option to raise an additional $1.5 million under the Convertible Note Agreement expired unexercised in December 2014 and no more 2014 Notes will be issued under this agreement.
| 6. | COMMITMENTS AND CONTINGENCIES |
Operating Leases
The Company leases office space in Oakwood Village, Ohio and Mountain View, California under non-cancellable operating leases. At December 31, 2014, the future minimum payments for the operating leases are as follows (in thousands):
| At December 31, 2014 |
Operating Leases | |||
| 2015 |
$ | 1,086 | ||
| 2016 |
1,113 | |||
| 2017 |
1,106 | |||
| 2018 |
963 | |||
| 2019 and thereafter |
823 | |||
|
|
|
|||
| Total future minimum payments |
$ | 5,091 | ||
|
|
|
|||
Rent expense incurred under operating leases was $308 thousand and $683 thousand during the years ended December 31, 2013 and 2014, respectively.
F-19
Table of Contents
Index to Financial Statements
Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company records a provision for a liability when it believes that it is both probable that a liability has been incurred and the amount can be reasonably estimated. Significant judgment is required to determine both probability and the estimated amount.
In the normal course of business, the Company may become involved in legal proceedings. The Company will accrue a liability for such matters when it is probable that a liability has been incurred and the amount can be reasonably estimated. When only a range of possible loss can be established, the most probable amount in the range is accrued. If no amount within this range is a better estimate than any other amount within the range, the minimum amount in the range is accrued. The accrual for a litigation loss contingency might include, for example, estimates of potential damages, outside legal fees and other directly related costs expected to be incurred. At December 31, 2014, the Company was not involved in any material legal proceedings.
Purchase Commitments
At December 31, 2014, the Company had no outstanding firm purchase commitments.
| 7. | LICENSING AGREEMENT |
In December 2004, the Company entered into a licensing agreement with the University of Florida Research Foundation, Inc., or UFRF, whereby UFRF granted the Company a worldwide exclusive license to certain of UFRF’s patents in exchange for 33,652 shares of common stock and a royalty from sales of products developed and sold by the Company utilizing the licensed patents. The Company was obligated to meet certain product development and commercialization milestones by various dates through December 31, 2014. The significant milestones met prior to December 31, 2013 included: (i) completion of a business plan and Small Business Technology Transfer grant application; (ii) securing a minimum of $20.0 million venture financing; (iii) successful relocation and build out of Company headquarters; (iv) receipt of the first magnet from an OEM partner; (v) hiring of a chief executive officer with industry experience in developing and commercializing similar products; and (vi) filing for FDA approval. The final milestone, which required the Company to recognize the first commercial sale of the MRIdian system to retail customers by December 31, 2014, was met during the year ended December 31, 2013. If these milestones had not been accomplished, UFRF would have had the right to terminate the licensing agreement. Royalty payments are based on 1% of net sales, defined as the amount collected on sales of licensed products and/or licensed processes after deducting trade and/or quantity discounts, credits on returns and allowances, outbound transportation costs paid and sales tax. Minimum quarterly royalty payments of $50 thousand commenced with the quarter ended March 31, 2014 and are payable in advance. Minimum royalties paid in any calendar year will be credited against earned royalties for such calendar year. The royalty payments continue until the earlier of (i) the date that no licensed patents remain enforceable or (ii) the payment of earned royalties, once begun in 2014, cease for more than four consecutive calendars quarters. Royalty expenses based on 1% of net sales were $26 thousand and $137 thousand during the years ended December 31, 2013 and 2014, respectively, and were recorded as product cost of revenue in the accompanying statements of operations. The minimum royalty payments in excess of 1% of net sales were nil and $63 thousand during the years ended December 31, 2013 and 2014, respectively, and were recorded as general and administrative expenses in the accompanying statements of operations.
| 8. | GRANT REVENUE |
In April 2009, the Company and other collaborators were awarded a grant from the State of Ohio of up to $5.0 million in total support pursuant to the Third Frontier Biomedical Research Commercialization Program. The Company’s portion of this grant is $2.8 million. Consistent with the grant agreement, the funds become due to the Company upon written request to the grantor subsequent to the achievement of milestone and qualifying
F-20
Table of Contents
Index to Financial Statements
expenditures being incurred. The terms of the grant obligate the Company to develop and commercialize MRIdian primarily at its headquarters in the State of Ohio, to raise certain amounts of new equity investment and to incur certain levels of expenditures to develop and market MRIdian. The grant revenue from this arrangement was recognized as these milestones were achieved during the years ended December 31, 2012 and 2013, before the arrangement expired in April 2013.
| 9. | DISTRIBUTION AGREEMENT |
In December 2014, the Company entered into a distribution agreement with ltochu Corporation, or ltochu, a Japanese entity, pursuant to which the Company appointed ltochu as its exclusive distributor for the sale and delivery of the Company’s MRIdian products within Japan. In consideration of the exclusive distribution rights granted, ltochu agreed to pay a distribution fee of $4.0 million in three installments: (i) the first installment of $1.0 million was due upon execution of the distribution agreement; (ii) the second installment of $1.0 million is due within 10 business days following submission of the application for regulatory approval of the Company’s product to the Japan regulatory authority; and (iii) the final installment of $2.0 million is due within 10 business days following receipt of approval for the Company’s product from the Japan regulatory authority. The distribution fee paid by ltochu is refundable if the Company fails to obtain the approval from the Japan regulatory authority before December 31, 2017. The first installment of $1.0 million was received in December 2014 and was recorded as customer deposits in the accompanying balance sheets at December 31, 2014.
The exclusive distribution agreement has an initial term of 10 years, and contains features customary in such distribution agreements. Under this distribution agreement, the Company will supply its products and services to ltochu based upon the Company’s then-current pricing. In conjunction with the distribution agreement, Itochu also purchased $5.2 million of Series C convertible preferred stock in December 2014 at a price of $5.84 per share and became a stockholder of the Company (see Note 11).
| 10. | COMMON STOCK RESERVED FOR ISSUANCE |
The common stock reserved for future issuance at December 31, 2013 and 2014 was as follows:
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Conversion of outstanding convertible preferred stock |
25,036,330 | 27,654,928 | ||||||
| Shares underlying outstanding stock options |
2,723,406 | 4,248,504 | ||||||
| Shares available for future stock option grants |
1,848,367 | 295,101 | ||||||
| Warrant to purchase convertible preferred stock |
128,231 | 128,231 | ||||||
|
|
|
|
|
|||||
| Total shares of common stock reserved |
29,736,334 | 32,326,764 | ||||||
|
|
|
|
|
|||||
| 11. | CONVERTIBLE PREFERRED STOCK |
In February 2013, the Company raised $5.0 million through the sale and issuance of 983,558 shares of Series D-1 convertible preferred stock for $5.08 per share.
Recapitalization
In May and June 2013, the Company effected a recapitalization in connection with a Series D-2 convertible preferred stock financing, or the Series D-2 Offering. The Series D-2 Offering consisted of (i) the sale and issuance of 3,013,797 shares of Series D-2 convertible preferred stock for $15.3 million, or $5.08 per share, and (ii) the issuance of 3,196,417 shares of Series D-2 convertible preferred stock to participating investors in exchange for all then-outstanding dividends accrued on shares of Series B-1, Series C and Series D-1 convertible
F-21
Table of Contents
Index to Financial Statements
preferred stock at an exchange rate equal to the sales price of the Series D-2 Offering of $5.08 per share. Non-participating holders of 1,470,485 shares of Series C convertible preferred stock received 147,051 shares of common stock, on a 10:1 basis, in exchange for these shares and accrued dividends of $1.4 million, or the Non-participating Exchange. At the closing of the Series D-2 Offering, all then-outstanding shares of Senior Convertible Preferred Stock (defined as all outstanding shares of Series B-1, Series C, Series D-1 and Series D-2 convertible preferred stock) were exchanged for an equal number of newly-issued shares of Series B convertible preferred stock, or the Participating Exchange, containing substantially similar rights to those contained in the Senior Convertible Preferred Stock except that (i) rights to dividends are no longer cumulative and therefore dividends accrue to holders of Series B convertible preferred stock only when declared and (ii) the liquidation preference for the prior shares of Series B-1 convertible preferred stock was increased. All shares of Senior Convertible Preferred Stock were surrendered and cancelled after the recapitalization.
The recapitalization was accounted for as an extinguishment of the Senior Convertible Preferred Stock which resulted in the following:
| • | For the Non-participating Exchange, the Company recognized a gain on extinguishment of Series C convertible preferred stock in the amount $8.8 million as the difference between the carrying value of the securities surrendered (i.e., carrying value of non-participating Series C convertible preferred stock and the related accrued dividends) and the fair value of the common stock issued in exchange. The $8.8 million gain was a deemed capital contribution to the holders of the common stock that was recognized as a decrease to net loss attributable to common stockholders and a decrease to accumulated deficit. |
| • | For the Participating Exchange, the Company recognized a charge on extinguishment of participating Senior Convertible Preferred Stock as the difference between the carrying value of the securities surrendered (i.e., carrying value of the participating Senior Convertible Preferred Stock) and the fair value of the Series B convertible preferred stock issued in exchange. The $6.9 million charge was a deemed dividend that was recognized as an increase to net loss attributable to common stockholders and an increase to accumulated deficit. |
In November 2013, the Company raised $15.0 million through the sale and issuance of 2,564,638 shares of Series C convertible preferred stock for $5.84 per share.
In December 2014, the Company issued 2,619,951 shares of Series C convertible preferred stock, consisting of (i) the issuance of 880,546 shares for $5.2 million, or $5.84 per share, to Itochu in conjunction with the distribution agreement, and (ii) the issuance of 1,739,405 shares upon conversion of the outstanding principal and accrued interest of the 2014 Notes (see Note 5).
F-22
Table of Contents
Index to Financial Statements
The changes in the shares of convertible preferred stock and common stock during the years ended December 31, 2013 and 2014 were as follows:
| Series A Convertible Preferred Stock |
Extinguished Series B-1 Convertible Preferred Stock |
Extinguished Series C Convertible Preferred Stock |
Extinguished Series D-1 Convertible Preferred Stock |
Extinguished Series D-2 Convertible Preferred Stock |
New Series B Convertible Preferred Stock |
New Series C Convertible Preferred Stock |
Total Convertible Preferred Stock |
Common Stock |
||||||||||||||||||||||||||||
| Balance at December 31, 2012 |
163,462 | 6,321,238 | 9,108,533 | 1,155,172 | — | — | — | 16,748,405 | 730,203 | |||||||||||||||||||||||||||
| Issuance of Series D-1 convertible preferred stock |
— | — | — | 983,558 | — | — | — | 983,558 | — | |||||||||||||||||||||||||||
| Issuance of Series D-2 convertible preferred stock |
— | — | — | — | 3,013,797 | — | — | 3,013,797 | — | |||||||||||||||||||||||||||
| Conversion of Series C convertible preferred stock and related dividends into common stock and deemed capital contribution |
— | — | (1,470,485 | ) | — | — | — | — | (1,470,485 | ) | 147,051 | |||||||||||||||||||||||||
| Conversion of accrued dividends into Series D-2 convertible preferred stock |
— | — | — | — | 3,196,417 | — | — | 3,196,417 | — | |||||||||||||||||||||||||||
| Extinguishment of Series B-1, Series C, Series D-1 and Series D-2 convertible preferred stock |
— | (6,321,238 | ) | (7,638,048 | ) | (2,138,730 | ) | (6,210,214 | ) | — | — | (22,308,230 | ) | — | ||||||||||||||||||||||
| Exchange of new Series B convertible preferred stock and deemed dividend |
— | — | — | — | — | 22,308,230 | — | 22,308,230 | — | |||||||||||||||||||||||||||
| Issuance of new Series C convertible preferred stock |
— | — | — | — | — | — | 2,564,638 | 2,564,638 | — | |||||||||||||||||||||||||||
| Issuance of common stock from option exercises |
— | — | — | — | — | — | — | — | 1,463 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance at December 31, 2013 |
163,462 | — | — | — | — | 22,308,230 | 2,564,638 | 25,036,330 | 878,717 | |||||||||||||||||||||||||||
| Repurchase of Series A convertible preferred stock and deemed capital contribution |
(1,353 | ) | — | — | — | — | — | — | (1,353 | ) | — | |||||||||||||||||||||||||
| Issuance of Series C convertible preferred stock |
— | — | — | — | — | — | 880,546 | 880,546 | — | |||||||||||||||||||||||||||
| Conversion of convertible promissory notes into Series C convertible preferred stock |
— | — | — | — | — | — | 1,739,405 | 1,739,405 | — | |||||||||||||||||||||||||||
| Issuance of common stock from option exercises |
— | — | — | — | — | — | — | — | 28,320 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Balance at December 31, 2014 |
162,109 | — | — | — | — | 22,308,230 | 5,184,589 | 27,654,928 | 907,037 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
F-23
Table of Contents
Index to Financial Statements
Prior to the completion of the recapitalization in June 2013, the rights, privileges and preferences of Series A, Series B-1, Series C and Series D-1 convertible preferred stock were as follows:
Voting Rights
Each share of Series A, Series B-1, Series C and Series D-1 convertible preferred stock was entitled to voting rights equal to the number of shares of common stock into which each share could be converted. The shares possessed contain certain customary rights and protective provisions including anti-dilution protection, pre-emptive and protective rights in the event of future issuances of equity securities by the Company, rights to limit the ability of the Company to incur indebtedness without prior approval of a majority of the Series D-1 convertible preferred stock holders, registration rights in the event of a public offering of the Company’s common stock, right of first refusal and co-sale rights in the event of certain transfers of shares by or among shareholders, the ability of a majority of holders of convertible preferred stock to require the tender of all shares in the event of a sale of the Company and information rights.
The Company’s board of directors should consist of 11 members. The holders of Series A, voting as a separate class, are entitled to elect one member of the board of directors. The holders of Series B-1, voting as a separate class, are entitled to elect seven members of the board of directors. The holders of Series C, voting as a separate class, are entitled to elect one member of the board of directors. The holders of common stock, voting as a separate class, are entitled to elect two members of the board of directors.
Conversion Rights
Each share of Series A, Series B-1, Series C and Series D-1 convertible preferred stock was convertible by any holder at any time into common stock. The conversion rate was determined by dividing the purchase price applicable to such shares of convertible preferred stock ($3.99 for Series A and Series B-1 and $5.08 for Series C and Series D-1 convertible preferred stock) by the conversion price ($3.99 for Series A and Series B-1 and $5.08 for Series C and Series D-1 convertible preferred stock). Conversion of such shares was automatic upon the closing of an underwritten public offering with proceeds equal to or exceeding $15.25 per share, and in which the net proceeds received by the Company equal or exceed $50.0 million or the affirmative vote of holders of shares of convertible preferred stock representing at least a majority of the voting power of the then outstanding shares of Series A, Series B-1, Series C and Series D-1 convertible preferred stock, voting together as a single class.
Adjustment of Conversion Price for Qualifying Dilutive Issuances
In the event the Company issued additional shares of common stock after the Series D-1 convertible preferred stock original issue date without consideration or for a consideration per share less than the conversion price in effect immediately prior to such issuance, then and in each such event the conversion price would have been reduced to a price equal to such conversion price multiplied by the following fraction:
| • | the numerator of which is equal to the sum of (i) the product of the number of shares of common stock outstanding or deemed to be outstanding immediately prior to such issuance and the conversion price in effect immediately prior to such issuance) and (ii) the product of the number of additional shares of common stock so issued and the average price per share received by the Company for the additional shares of common stock so issued); and |
| • | the denominator of which is equal to the number of shares of common stock outstanding or deemed to be outstanding immediately prior to such issuance plus the number of additional shares of common stock so issued. |
Dividends
Holders of Series B-1, Series C and Series D-1 convertible preferred stock, in preference to the holders of Series A convertible preferred stock and common stock, were entitled to receive cash dividends at the per annum rate of
F-24
Table of Contents
Index to Financial Statements
8% of the convertible preferred stock purchase price. Such payments were to be paid in order of preference (Series D-1, Series C, Series B-1 convertible preferred stock). Dividends were cumulative from the date of issuance of the respective convertible preferred stock until paid. Holders of Series B-1 convertible preferred stock were entitled to receive, when, as and if declared by the board of directors, cash dividends at the per annum rate of 8% of the Series B-1 convertible preferred stock original issue price of $3.99 per share and such dividend was cumulative. Holders of Series C and Series D-1 convertible preferred stock were entitled to receive, when, as and if declared by the board of directors, cash dividends at the per annum rate of 8% of the Series C and Series D-1 convertible preferred stock original issue price of $5.08 per share and such dividend was cumulative.
So long as 20% of the Series D-1 convertible preferred stock remained outstanding, the Company would not pay or declare any dividend on shares other than Series D-1 convertible preferred stock without the consent of the Series D-1 convertible preferred stockholders. In the event dividends were paid on any share of common stock, the Company would have paid an additional dividend on all outstanding shares of Series A, Series B-1, Series C and Series D-1 convertible preferred stock in a per share amount equal (on an as-if-converted-to-common-stock-basis) to the amount paid or set aside for each share of common stock.
Liquidation Preferences
Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, or a corporate organization, the holders of Series B-1, Series C and Series D-1 convertible preferred stock were entitled to be paid out of Company assets before any payment was to be made to the holders of Series A convertible preferred stock and common stock. Holders of Series A convertible preferred stock were entitled to be paid out of any remaining assets in the event of a liquidation event before any payment was to be made to the holders of common stock. Upon the completion of distributions required to satisfy the convertible preferred stock liquidation preferences, any remaining assets were to be distributed pro rata among the holders of convertible preferred stock and common stock, treating all convertible preferred stock as if it were converted to common stock.
The Series C and D-1 convertible preferred stock liquidation preference was equal to the sum of $5.08 per share plus any accrued but unpaid dividends, whether or not declared and any declared but unpaid dividends on such shares. The Series B-1 convertible preferred stock liquidation preference was equal to the sum of $3.99 per share plus any declared but unpaid dividends on such shares. The Series A convertible preferred stock liquidation preference was $18.52 per share plus any declared but unpaid dividends on such shares. The liquidation preference of the convertible preferred stock was subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other recapitalization affecting such shares.
Redemption
The Series A, Series B-1, Series C and Series D-1 convertible preferred stock was not redeemable at the option of the holder. The convertible preferred stock was classified outside of stockholders’ deficit because, in the event of certain “liquidation events” that are not solely within the Company’s control (including a dissolution, change of control, acquisition, asset sale or winding up of the Company), the shares would become redeemable at the option of the holders. The Company did not adjust the carrying values of the convertible preferred stock to the deemed liquidation values of such shares since a liquidation event was not probable at any of the balance sheet dates. Subsequent adjustments to increase or decrease the carrying values to the ultimate liquidation values will be made only if and when it becomes probable that such a liquidation event will occur.
F-25
Table of Contents
Index to Financial Statements
Convertible preferred stock at December 31, 2013 and 2014 consisted of the following (in thousands, except share data):
| December 31, 2013 | ||||||||||||||||
| Shares Authorized |
Shares Issued and Outstanding |
Aggregate Liquidation Preference |
Net Carrying Value |
|||||||||||||
| Series A |
398,500 | 163,462 | $ | 3,029 | $ | 3,028 | ||||||||||
| Series B |
60,500,000 | 22,308,230 | 113,405 | 112,080 | ||||||||||||
| Series C |
6,562,497 | 2,564,638 | 15,000 | 14,929 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| 67,460,997 | 25,036,330 | $ | 131,434 | $ | 130,037 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 31, 2014 | ||||||||||||||||
| Shares Authorized |
Shares Issued and Outstanding |
Aggregate Liquidation Preference |
Net Carrying Value |
|||||||||||||
| Series A |
398,500 | 162,109 | $ | 3,004 | $ | 3,003 | ||||||||||
| Series B |
60,500,000 | 22,308,230 | 113,405 | 112,080 | ||||||||||||
| Series C |
13,562,497 | 5,184,589 | 30,323 | 30,027 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| 74,460,997 | 27,654,928 | $ | 146,732 | $ | 145,110 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
At December 31, 2013 and 2014, the rights, privileges and preferences of Series A, Series B and Series C convertible preferred stock (collectively, convertible preferred stock) are as follows:
Voting Rights
Each share of convertible preferred stock is entitled to voting rights equal to the number of shares of common stock into which each share can be converted.
The convertible preferred stock terms contain certain customary rights and protective provisions including anti-dilution protection, pre-emptive and protective rights in the event of future issuances of equity securities by the Company, rights to limit the ability of the Company to incur indebtedness without prior approval of a majority of the convertible preferred stock holders, registration rights in the event of a public offering of the Company’s common stock, right of first refusal and co-sale rights in the event of certain transfers of shares by or among shareholders, the ability of a majority of holders of convertible preferred stock to require the tender of all shares in the event of a sale of the Company and information rights.
The Company’s board of directors consists of 10 members. The holders of Series A convertible preferred stock, voting as a separate class, are entitled to elect one member of the board of directors. The holders of Series B convertible preferred stock, voting as a separate class, are entitled to elect eight members of the board of directors. The holders of Series C convertible preferred stock, voting as a separate class, are entitled to elect one member of the board of directors. The holders of common stock, voting as a separate class, are entitled to elect two member of the board of directors.
Conversion Rights
Each share of convertible preferred stock is convertible by any holder at any time into common stock. The conversion rate is determined by dividing the purchase price applicable to such shares of convertible preferred stock ($3.99 for Series A, $5.08 for Series B and $5.84 for Series C convertible preferred stock) by the conversion price ($3.99 for Series A, $5.08 for Series B and $5.84 for Series C convertible preferred stock). Conversion of convertible preferred stock is automatic upon the closing of an underwritten public offering with proceeds equal to or exceeding $18.55 per share, and in which the net proceeds received by the Company equal
F-26
Table of Contents
Index to Financial Statements
or exceed $50.0 million or the affirmative vote of holders of shares of convertible preferred stock representing at least a majority of the voting power of the then outstanding shares of Series A, Series B and Series C convertible preferred stock, voting together as a single class.
Adjustment of Conversion Price for Qualifying Dilutive Issuances
In the event the Company issues additional shares of common stock after the Series C convertible preferred stock original issue date without consideration or for a consideration per share less than the conversion price in effect immediately prior to such issuance, then and in each such event the conversion price will be reduced to a price equal to such conversion price multiplied by the following fraction:
| • | the numerator of which is equal to the sum of (i) the product of the number of shares of common stock outstanding or deemed to be outstanding immediately prior to such issuance and the conversion price in effect immediately prior to such issuance) and (ii) the product of the number of additional shares of common stock so issued and the average price per share received by the Company for the additional shares of common stock so issued); and |
| • | the denominator of which is equal to the number of shares of common stock outstanding or deemed to be outstanding immediately prior to such issuance plus the number of additional shares of common stock so issued. |
Dividends
Holders of Series B and Series C convertible preferred stock, in preference to the holders of Series A convertible preferred stock and common stock, are entitled to receive, when, as and if declared by the board of directors, cash dividends at the per annum rate of 8% of the convertible preferred stock purchase price and such dividend is noncumulative. Holders of Series B convertible preferred stock are entitled to receive, when, as and if declared by the board of directors, cash dividends at the per annum rate of 8% of the Series B convertible preferred stock original issue price of $5.08 per share. Holders of Series C convertible preferred stock are entitled to receive, when, as and if declared by the board of directors, cash dividends at the per annum rate of 8% of the Series C convertible preferred stock original issue price of $5.84 per share and such dividend is noncumulative.
So long as any senior shares of convertible preferred stock are outstanding, the Company will not pay or declare any dividend on Series A convertible preferred stock or common stock until all dividends on the Series B and Series C convertible preferred stock have been declared and paid. In the event dividends are paid on any share of Series A convertible preferred stock or common stock, the Company will pay an additional dividend on all outstanding shares of convertible preferred stock in a per share amount equal (on an as-if-converted-to-common-stock-basis) to the amount paid or set aside for each share of common stock.
Liquidation Preferences
Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary, or corporate reorganization, holders of Series B and Series C convertible preferred stock are entitled to be paid out of Company assets before any payment will be made to the holders of Series A convertible preferred stock and common stock. Holders of Series A convertible preferred stock are entitled to be paid out of any remaining assets in the event of a liquidation event before any payment will be made to the holders of common stock. Upon the completion of distributions required to satisfy the convertible preferred stock liquidation preferences, any remaining assets will be distributed pro rata among the holders of convertible preferred stock and common stock, treating all convertible preferred stock as if it were converted to common stock.
The Series C convertible preferred stock liquidation preference is equal to the sum of $5.84 per share plus any declared but unpaid dividends on such shares. The Series B convertible preferred stock liquidation preference is equal to the sum of $5.08 per share plus any declared but unpaid dividends on such shares. The Series A
F-27
Table of Contents
Index to Financial Statements
convertible preferred stock liquidation preference is $18.52 per share plus any declared but unpaid dividends on such shares. The liquidation preference of the convertible preferred stock is subject to appropriate adjustment in the event of any stock dividend, stock split, combination or other recapitalization affecting such shares.
Redemption
The convertible preferred stock is not redeemable at the option of the holder. The convertible preferred stock was classified outside of stockholders’ deficit because, in the event of certain “liquidation events” that are not solely within the Company’s control (including a dissolution, change of control, acquisition, asset sale or winding up of the Company), the shares would become redeemable at the option of the holders. The Company did not adjust the carrying values of the convertible preferred stock to the deemed liquidation values of such shares since a liquidation event was not probable at any of the balance sheet dates. Subsequent adjustments to increase or decrease the carrying values to the ultimate liquidation values will be made only if and when it becomes probable that such a liquidation event will occur.
| 12. | CONVERTIBLE PREFERRED STOCK WARRANT |
The Company has an outstanding convertible preferred stock warrant related to a 2013 debt financing (see Note 5) whereby the Company issued a warrant to purchase 128,231 shares of Series C convertible preferred stock. The convertible preferred stock warrant was recorded as a liability and is adjusted to fair value at each balance sheet date, with the change in fair value being recorded as a component of other income (expense), net in the statements of operations. Upon issuance, the fair value of the warrant was estimated to be $158 thousand. Due to the close proximity of the issuance date in December 2013 to the year end, no mark to market adjustment was recognized during the year ended December 31, 2013. The Company recorded a gain of $20 thousand related to the change in fair value of preferred stock warrant liability as part of other income (expense), net in the accompanying statements of operations for the year ended December 31, 2014.
The key terms of the outstanding convertible preferred stock warrant and the convertible preferred stock warrant liability at December 31, 2013 and 2014 were as follows (in thousands):
| Issuance Date |
Expiration Date | Exercise Price per Share |
Shares | Fair Value of Warrant | ||||||||||||||||
| December 31, 2013 |
December 31, 2014 |
|||||||||||||||||||
| Series C Warrant |
December 2013 |
The later of December 2023 or five years after an IPO |
$ | 5.84 | 128,231 | $ | 158 | $ | 138 | |||||||||||
The Company used the Black-Scholes option-pricing model to estimate the fair value of the convertible preferred stock warrant with the following assumptions:
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Series C Warrant: |
||||||||
| Expected term (in years) |
2.0 | 5.3 | ||||||
| Expected volatility |
30.0 | % | 30.0 | % | ||||
| Risk-free interest rate |
0.4 | % | 1.7 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
F-28
Table of Contents
Index to Financial Statements
| 13. | STOCK-BASED COMPENSATION |
2008 Stock Option and Incentive Plan
The Company’s 2008 Stock Option and Incentive Plan, or the 2008 Plan, provides for the grant of stock and stock-based awards to employees, officers, directors, advisors and consultants, including stock options, nonqualified stock options, restricted stock awards, restricted stock units and stock appreciation rights. The purpose of the 2008 Plan is to promote the interests of the Company by providing the opportunity to purchase or receive shares or to receive compensation that is based upon appreciation in the value of the shares to eligible recipients, as defined, in order to attract and retain employees and provide additional incentive to work to increase the value of shares and a stake in the future of the Company. During 2008, the Company issued to an officer a stock option for 38,059 shares outside of the 2008 Plan with an exercise price of $0.80 per share which vested over three years. These options were unexercised and expired in September 2014. The compensation expense related to stock options outside of the 2008 Plan were insignificant during the years ended December 31, 2013 and 2014.
Options granted may be either incentive stock options or nonstatutory stock options. Incentive stock options may be granted to employees with exercise prices of no less than the fair value of the common stock on the grant date and nonstatutory options may be granted to employees or consultants at exercise prices of no less than 85% of the fair value of the common stock on the grant date, as determined by the board of directors. If, at the time of grant, the optionee owns stock representing more than 10% of the voting power of all classes of stock of the Company, a 10% shareholder, the exercise price must be at least 110% of the fair value of the common stock on the grant date as determined by the board of directors. Options become exercisable generally ratably over four years. Options granted under the 2008 Plan expire in 10 years from the date of grant, or five years from the date of grant for 10% shareholders.
A summary of the Company’s stock option activity and related information is as follows:
| Options Outstanding | ||||||||||||||||||||
| Shares Available for Grant |
Number of Stock Options Outstanding |
Weighted-Average Exercise Price |
Weighted-Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Balance at January 1, 2013 |
306,097 | 1,907,644 | $ | 0.71 | 6.3 | $ | 28 | |||||||||||||
| Additional shares authorized |
2,359,662 | |||||||||||||||||||
| Granted |
(1,190,349 | ) | 1,190,349 | 0.70 | ||||||||||||||||
| Exercised |
— | (1,463 | ) | 0.68 | ||||||||||||||||
| Cancelled |
372,957 | (372,957 | ) | 0.69 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2013 |
1,848,367 | 2,723,573 | 0.71 | 7.7 | 138 | |||||||||||||||
| Granted |
(1,642,799 | ) | 1,642,799 | 0.84 | ||||||||||||||||
| Exercised |
— | (28,320 | ) | 0.73 | ||||||||||||||||
| Cancelled |
89,533 | (89,533 | ) | 0.74 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2014 |
295,101 | 4,248,519 | $ | 0.76 | 7.7 | $ | 8,343 | |||||||||||||
|
|
|
|
|
|||||||||||||||||
| Vested and exercisable at December 31, 2013 |
1,360,757 | $ | 0.71 | 6.4 | $ | 67 | ||||||||||||||
| Vested and expected to vest at December 31, 2013 |
2,333,655 | $ | 0.71 | 7.4 | $ | 118 | ||||||||||||||
| Vested and exercisable at December 31, 2014 |
2,379,076 | $ | 0.72 | 6.8 | $ | 4,764 | ||||||||||||||
| Vested and expected to vest at December 31, 2014 |
3,777,913 | $ | 0.78 | 7.5 | $ | 7,452 | ||||||||||||||
F-29
Table of Contents
Index to Financial Statements
The weighted-average grant date fair value of options granted to employees for the years ended December 31, 2013 and 2014 was $0.35 and $0.42 per share, respectively. The grant date fair value of options vested during the years ended December 31, 2013 and 2014 was $72 thousand and $339 thousand, respectively.
Aggregate intrinsic value represents the difference between the estimated fair value of the underlying common stock and the exercise price of outstanding, in-the-money options. The aggregate intrinsic value of options exercised was insignificant during the years ended December 31, 2013 and 2014.
At December 31, 2014, total unrecognized compensation cost related to stock-based awards granted to employees, net of estimated forfeitures, was $682 thousand, which is expected to be recognized over a weighted-average period of 2.9 years.
Determination of Fair Value
The determination of the fair value of stock options on the date of grant using an option-pricing model is affected by the estimated fair value of the Company’s common stock, as well as assumptions regarding a number of complex and subjective variables. The variables used to calculate the fair value of stock options using the Black-Scholes option-pricing model include actual and projected employee stock option exercise behaviors, expected price volatility of the Company’s common stock, the risk-free interest rate and expected dividends. Each of these inputs is subjective and generally requires significant judgment to determine.
Fair Value of Common Stock
The fair value of the common stock underlying the stock-based awards was determined by the Company’s board of directors, with input from management and third-party valuations.
Expected Term
The expected term represents the period that the Company’s option awards are expected to be outstanding. The Company considers several factors in estimating the expected term of options granted, including the expected lives used by a peer group of companies within the Company’s industry that the Company considers to be comparable to its business and the historical option exercise behavior of its employees, which the Company believes is representative of future behavior.
Expected Volatility
As the Company does not have a trading history for its common stock, the expected stock price volatility for the Company’s common stock was estimated by taking the average historic price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the stock option grants. Industry peers consist of several public companies in the Company’s industry which were the same as the comparable companies used in the common stock valuation analysis. The Company intends to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of its own share price becomes available, or unless circumstances change such that the identified companies are no longer similar to the Company, in which case, more suitable companies whose share prices are publicly available would be used in the calculation.
Risk-Free Interest Rate
The risk-free interest rate is based on the zero coupon U.S. Treasury notes, with maturities similar to the expected term of the options.
Expected Dividend Yield
The Company does not anticipate paying any dividends in the foreseeable future and, therefore, uses an expected dividend yield of zero in the Black-Scholes option-valuation model.
F-30
Table of Contents
Index to Financial Statements
In addition to the Black-Scholes assumptions discussed immediately above, the estimated forfeiture rate also has a significant impact on the related stock-based compensation. The forfeiture rate of stock options is estimated at the time of grant and revised in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data to estimate pre-vesting option forfeitures and records stock-based compensation expense only for those awards that are expected to vest.
The fair value of employee stock options was estimated at the date of grant using a Black-Scholes option-pricing model with the following weighted-average assumptions:
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Expected term (in years) |
6.2 | 5.7 | ||||||
| Expected volatility |
52.4 | % | 50.7 | % | ||||
| Risk-free interest rate |
1.2 | % | 1.8 | % | ||||
| Expected dividend yield |
0 | % | 0 | % | ||||
Stock-Based Compensation Expense
Total stock-based compensation expense recognized in the Company’s statements of operations is classified as follows (in thousands):
| Year Ended December 31, | ||||||||
| 2013 | 2014 | |||||||
| Research and development |
$ | 29 | $ | 85 | ||||
| Selling and marketing |
9 | 15 | ||||||
| General and administrative |
181 | 218 | ||||||
|
|
|
|
|
|||||
| Total stock-based compensation expense |
$ | 219 | $ | 318 | ||||
|
|
|
|
|
|||||
During the years ended December 31, 2013 and 2014, there were no stock-based compensation expenses capitalized as a component of inventory or recognized in cost of revenue. Stock-based compensation relating to stock-based awards granted to consultants was insignificant for the years ended December 31, 2013 and 2014.
| 14. | INCOME TAXES |
The following reconciles the differences between income taxes computed at the federal income tax rate and the provision for income taxes:
| Year Ended December 31, | ||||||||
| 2013 | 2014 | |||||||
| Expected income tax benefit at the federal statutory rate |
34.0 | % | 34.0 | % | ||||
| State taxes, net of federal benefit |
1.5 | 3.7 | ||||||
| Change in effective tax rate |
0.0 | 1.3 | ||||||
| Non-deductible items and other |
(2.1 | ) | 0.4 | |||||
| Federal and state credits |
2.0 | 1.2 | ||||||
| Change in valuation allowance |
(35.4 | ) | (40.6 | ) | ||||
|
|
|
|
|
|||||
| Total |
0.0 | % | 0.0 | % | ||||
|
|
|
|
|
|||||
F-31
Table of Contents
Index to Financial Statements
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. The principal components of the Company’s net deferred tax assets consisted of the following at December 31, 2013 and 2014 (in thousands):
| December 31, | ||||||||
| 2013 | 2014 | |||||||
| Net operating loss carryforwards |
$ | 33,248 | $ | 46,024 | ||||
| Research and development tax credits |
2,041 | 2,441 | ||||||
| Reserves and accruals |
2,728 | 724 | ||||||
| Other |
376 | 2,948 | ||||||
|
|
|
|
|
|||||
| 38,393 | 52,137 | |||||||
| Total deferred tax assets |
(38,393 | ) | (52,137 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
The Company maintains a valuation allowance related to its deferred tax asset position when management believes it is more likely than not that the net deferred tax assets will not be realized in the future. The Company’s valuation allowance increased by $13.7 million during the year ended December 31, 2014.
At December 31, 2014, the Company had federal net operating loss carryforwards of $125.3 million, which begin to expire in the year ended December 31, 2024, and a tax benefit of $1.5 million related to state net operating loss carryforwards, which begin to expire in the year ending December 31, 2019. The Company had federal research and development tax credit carryforwards of $2.4 million at the year ended December 31, 2014. These credits expire at various dates through the year ending December 31, 2024.
Under the provisions of the Internal Revenue Code, or IRC, net operating loss and credit carryforwards and other tax attributes may be subject to limitation if there has been a significant change in ownership of the Company, as defined by the IRC. The Company believes it has experienced at least one ownership change in the past. The Company is currently analyzing the tax impact of such ownership change on its federal net operating loss and credit carryforwards. Future owner or equity shifts, including an IPO, could result in limitations on net operating loss and credit carryforwards.
Because of the net operating loss and credit carryforwards, all of the Company’s federal tax returns and state returns since the year ended December 31, 2004 remain subject to federal and California examination.
The Company accounts for uncertain tax positions using a “more-likely-than-not” threshold. The evaluation of uncertain tax positions is based on factors including, but not limited to, changes in tax law, the measurement of tax positions taken or expected to be taken in tax returns, the effective settlement of matters subject to audit, new audit activity and changes in facts or circumstances related to a tax position. The Company evaluates these tax positions on an annual basis. In addition, the Company also accrues for potential interest and penalties related to unrecognized tax benefits in income tax expense. At December 31, 2013 and 2014, the Company had no unrecognized tax benefits.
F-32
Table of Contents
Index to Financial Statements
| 15. | NET LOSS PER SHARE |
The following table sets forth the computation of the Company’s basic and diluted net loss per share for the periods presented (in thousands, except share and per share data):
| Year Ended December 31, | ||||||||
| 2013 | 2014 | |||||||
| Net loss attributable to common stockholders |
$ | (28,200 | ) | $ | (33,791 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares used in computing net loss per share attributable to common stockholders, basic and diluted |
815,340 | 892,315 | ||||||
|
|
|
|
|
|||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (34.59 | ) | $ | (37.87 | ) | ||
|
|
|
|
|
|||||
The following weighted-average common stock equivalents were excluded from the calculation of diluted net loss per share for the periods presented because including them would have had an anti-dilutive effect:
| Year Ended December 31, | ||||||||
| 2013 | 2014 | |||||||
| Convertible preferred stock (if converted) |
20,901,591 | 25,078,396 | ||||||
| Options to purchase common stock |
2,799,118 | 3,766,704 | ||||||
| Convertible preferred stock warrant (if converted) |
5,619 | 128,231 | ||||||
| 16. | EMPLOYEE BENEFITS |
The Company has a 401(k) Plan, 401(k) Plan, which covers its eligible employees. The 401(k) Plan permits the participants to defer a portion of their compensation in accordance with the provisions of Section 401(k) of the IRC. At its discretion, the Company can match a portion of the participants’ contributions or make profit-sharing contributions. There was no matching or profit-sharing contributions during the years ended December 31, 2013 or 2014.
| 17. | RELATED PARTY TRANSACTIONS |
As discussed in Note 7, the Company pays a royalty to UFRF, a common stockholder, related to a licensing agreement.
| 18. | SUBSEQUENT EVENTS |
The Company has evaluated subsequent events through the date the financial statements were issued.
In January 2015, the Company issued an aggregate of 162,407 shares of Series C convertible preferred stock to Itochu and another new investor at a price of $5.84 per share for a total gross consideration of $950 thousand (see Note 9).
In February 2015, the Company issued 2,564,652 shares of Series C convertible preferred stock to a new investor at a price of 5.84 per share for total gross consideration of $15.0 million (see Note 11). The new investor will have the right to appoint one director to serve on our board.
At March 25, 2015, the Company effected a 1-for-7.25 reverse stock split of the Company’s then outstanding common stock and convertible preferred stock (collectively referred to as “Capital Stock”) and convertible preferred stock warrants, in which (i) each 7.25 shares of outstanding Capital Stock were combined into 1 share of Capital Stock; (ii) the number of outstanding options to purchase each Capital Stock was proportionately reduced on a 1-for-7.25 basis; (iii) number of shares reserved for future option grants under the 2008 Plan were proportionately reduced on a 1-for-7.25 basis; (iv) the exercise price of each such outstanding option was
F-33
Table of Contents
Index to Financial Statements
proportionately increased on a 1-for-7.25 basis; and (v) each 7.25 shares of outstanding convertible preferred stock warrant were combined into 1 share of convertible preferred stock warrant. All of the share and per share amounts have been adjusted, on a retroactive basis, to reflect this 1-for-7.25 reverse stock split (Notes 2, 5, 9, 10, 11, 12, 13 and 15).
At July 23, 2015, the Company effected a 2.975-for-1 stock split of the Company’s then outstanding common stock and convertible preferred stock (collectively referred to as “Capital Stock”) and convertible preferred stock warrants, in which (i) each share of outstanding Capital Stock was increased into 2.975 shares of Capital Stock; (ii) the number of outstanding options to purchase each Capital Stock was proportionately increased on a 2.975-for-1 basis; (iii) number of shares reserved for future option grants under the 2008 Plan were proportionately increased on a 2.975-for-1 basis; (iv) the exercise price of each such outstanding option was proportionately decreased on a 2.975-for-1 basis; and (v) each share of outstanding convertible preferred stock warrant was increased into 2.975 shares of convertible preferred stock warrant. All of the share and per share amounts have been adjusted, on a retroactive basis, to reflect this 2.975-for-1 stock split (Notes 2, 5, 9, 10, 11, 12, 13 and 15).
F-34
Table of Contents
Index to Financial Statements
VIEWRAY, INC.
| Page | ||||
| F-36 | ||||
| F-37 | ||||
| Condensed Consolidated Statements of Convertible Preferred Stock and Stockholders’ Deficit |
F-38 | |||
| F-39 | ||||
| F-40 | ||||
F-35
Table of Contents
Index to Financial Statements
VIEWRAY, INC.
Condensed Consolidated Balance Sheets
(In thousands, except share and per share data)
| September 30, 2015 |
December 31, 2014 |
|||||||
| (Unaudited) | ||||||||
| ASSETS |
||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 32,064 | $ | 11,129 | ||||
| Accounts receivable |
1 | 904 | ||||||
| Inventory |
9,649 | 8,238 | ||||||
| Deposits on purchased inventory |
5,021 | 2,798 | ||||||
| Deferred cost of revenue |
4,303 | 4,712 | ||||||
| Prepaid expenses and other current assets |
1,290 | 626 | ||||||
|
|
|
|
|
|||||
| Total current assets |
52,328 | 28,407 | ||||||
| Property and equipment, net |
5,296 | 2,931 | ||||||
| Restricted cash |
553 | 1,053 | ||||||
| Intangible assets, net |
139 | 264 | ||||||
| Deferred offering costs |
— | 1,419 | ||||||
| Other assets |
61 | 31 | ||||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
$ | 58,377 | $ | 34,105 | ||||
|
|
|
|
|
|||||
| LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT) |
||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,625 | $ | 6,134 | ||||
| Accrued liabilities |
5,014 | 4,436 | ||||||
| Customer deposits |
11,412 | 6,100 | ||||||
| Deferred revenue, current portion |
5,249 | 7,361 | ||||||
| Long-term debt, current portion |
— | 5,493 | ||||||
| Notes payable |
— | 240 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
23,300 | 29,764 | ||||||
| Long-term debt, net of current portion |
27,543 | 9,149 | ||||||
| Convertible preferred stock warrant liability |
— | 138 | ||||||
| Deferred revenue, net of current portion |
374 | — | ||||||
| Other long-term liabilities |
650 | 567 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES |
51,867 | 39,618 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 8) |
||||||||
| Convertible preferred stock, par value $0.01 per share; 80,710,997 and 74,460,997 shares authorized at September 30, 2015 (unaudited) and December 31, 2014; nil and 27,654,928 shares issued and outstanding at September 30, 2015 (unaudited) and December 31, 2014; aggregate liquidation preference of nil and $146,732 at September 30, 2015 (unaudited) and December 31, 2014 |
— | 145,110 | ||||||
| Stockholders’ equity (deficit): |
||||||||
| Common stock, par value of $0.01 per share; 90,000,000 and 88,000,000 shares authorized at September 30, 2015 (unaudited) and December 31, 2014; 38,200,088 and 907,037 shares issued and outstanding at September 30, 2015 (unaudited) and December 31, 2014 |
372 | 9 | ||||||
| Additional paid-in capital |
189,073 | 1,414 | ||||||
| Accumulated deficit |
(182,935 | ) | (152,046 | ) | ||||
|
|
|
|
|
|||||
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) |
6,510 | (150,623 | ) | |||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES, CONVERTIBLE PREFERRED STOCK AND STOCKHOLDERS’ EQUITY (DEFICIT) |
$ | 58,377 | $ | 34,105 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these financial statements.
F-36
Table of Contents
Index to Financial Statements
VIEWRAY, INC.
Condensed Consolidated Statements of Operations
(In thousands, except share and per share data)
| Nine Months Ended September 30, |
||||||||
| 2015 | 2014 | |||||||
| (Unaudited) | ||||||||
| Revenue: |
||||||||
| Product |
$ | 5,119 | $ | 5,702 | ||||
| Service |
419 | 229 | ||||||
| Grant |
240 | — | ||||||
|
|
|
|
|
|||||
| Total revenue |
5,778 | 5,931 | ||||||
| Cost of revenue: |
||||||||
| Product |
6,311 | 7,858 | ||||||
| Service |
1,390 | 413 | ||||||
|
|
|
|
|
|||||
| Total cost of revenue |
7,701 | 8,271 | ||||||
|
|
|
|
|
|||||
| Gross margin |
(1,923 | ) | (2,340 | ) | ||||
| Operating expenses: |
||||||||
| Research and development |
7,408 | 7,451 | ||||||
| Selling and marketing |
3,315 | 3,572 | ||||||
| General and administrative |
15,779 | 9,395 | ||||||
|
|
|
|
|
|||||
| Total operating expenses |
26,502 | 20,418 | ||||||
|
|
|
|
|
|||||
| Loss from operations |
(28,475 | ) | (22,758 | ) | ||||
| Interest income |
1 | 1 | ||||||
| Interest expense |
(2,376 | ) | (1,505 | ) | ||||
| Other income (expense), net |
(89 | ) | 82 | |||||
|
|
|
|
|
|||||
| Loss before provision for income taxes |
$ | (30,889 | ) | $ | (24,180 | ) | ||
| Provision for income taxes |
— | — | ||||||
|
|
|
|
|
|||||
| Net loss |
$ | (30,889 | ) | $ | (24,180 | ) | ||
|
|
|
|
|
|||||
| Deemed capital contribution on repurchase of Series A preferred stock |
$ | — | $ | 9 | ||||
|
|
|
|
|
|||||
| Net loss attributable to common stockholders |
$ | (30,889 | ) | $ | (24,171 | ) | ||
|
|
|
|
|
|||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (2.96 | ) | $ | (27.25 | ) | ||
|
|
|
|
|
|||||
| Weighted-average common shares used to compute net loss per share attributable to common stockholders, basic and diluted |
10,433,051 | 887,189 | ||||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these financial statements.
F-37
Table of Contents
Index to Financial Statements
Condensed Consolidated Statements of Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(In thousands, except share data)
| Convertible Preferred Stock |
Common Stock | Additional Paid-in Capital |
Accumulated Deficit |
Total Stockholders’ Equity (Deficit) |
||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||
| Balance at December 31, 2014 |
27,654,928 | $ | 145,110 | 907,037 | $ | 9 | $ | 1,414 | $ | (152,046 | ) | $ | (150,623 | ) | ||||||||||||||
| Issuance of common stock from option exercises (unaudited) |
— | — | 26,555 | — | 19 | — | 19 | |||||||||||||||||||||
| Stock-based compensation (unaudited) |
— | — | — | — | 531 | — | 531 | |||||||||||||||||||||
| Issuance of Series C convertible preferred stock, net of offering costs of $221 (unaudited) |
2,727,059 | 15,729 | — | — | — | — | — | |||||||||||||||||||||
| Conversion of convertible preferred stock to common stock in connection with the Merger (unaudited) |
(30,381,987 | ) | (160,839 | ) | 30,381,987 | 304 | 160,535 | — | 160,839 | |||||||||||||||||||
| Issuance of common stock upon private placement, net of offering costs of $3,223 (unaudited) |
— | — | 5,884,504 | 59 | 26,165 | — | 26,224 | |||||||||||||||||||||
| Issuance of common stock to Mirax (unaudited) |
— | — | 1,000,005 | — | — | — | — | |||||||||||||||||||||
| Conversion of convertible preferred stock warrants to common stock warrants (unaudited) |
— | — | — | — | 93 | — | 93 | |||||||||||||||||||||
| Issuance of common stock warrants (unaudited) |
— | — | — | — | 316 | — | 316 | |||||||||||||||||||||
| Net loss (unaudited) |
— | — | — | — | (30,889 | ) | (30,889 | ) | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at September 30, 2015 (unaudited) |
— | $ | — | 38,200,088 | $ | 372 | $ | 189,073 | $ | (182,935 | ) | $ | 6,510 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
F-38
Table of Contents
Index to Financial Statements
VIEWRAY, INC.
Condensed Consolidated Statements of Cash Flows
(In thousands)
(Unaudited)
| Nine Months Ended September 30, |
||||||||
| 2015 | 2014 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: |
||||||||
| Net loss |
$ | (30,889 | ) | $ | (24,180 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||
| Depreciation and amortization |
913 | 737 | ||||||
| Stock-based compensation |
531 | 262 | ||||||
| Change in fair value of convertible preferred stock warrant liability |
(45 | ) | (74 | ) | ||||
| Inventory lower of cost and market adjustment |
995 | 598 | ||||||
| Write-off of deferred offering cost |
2,920 | — | ||||||
| Amortization of debt discount and interest accrual |
573 | 138 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable |
903 | (2,004 | ) | |||||
| Inventory |
(2,406 | ) | (3,037 | ) | ||||
| Deposits on purchased inventory |
(2,223 | ) | (1,387 | ) | ||||
| Deferred costs |
409 | — | ||||||
| Prepaid expenses and other current assets |
(664 | ) | (214 | ) | ||||
| Accounts payable |
(6,048 | ) | 8 | |||||
| Notes payable |
(240 | ) | — | |||||
| Accrued expenses and other long-term liabilities |
392 | (576 | ) | |||||
| Customer deposits and deferred revenue |
3,574 | 2,522 | ||||||
|
|
|
|
|
|||||
| Net cash used in operating activities |
(31,305 | ) | (27,207 | ) | ||||
|
|
|
|
|
|||||
| CASH FLOWS FROM INVESTING ACTIVITIES: |
||||||||
| Purchase of property and equipment |
(2,906 | ) | (1,397 | ) | ||||
| Change in restricted cash balance |
500 | (100 | ) | |||||
|
|
|
|
|
|||||
| Net cash used in investing activities |
(2,406 | ) | (1,497 | ) | ||||
|
|
|
|
|
|||||
| CASH FLOWS FROM FINANCING ACTIVITIES: |
||||||||
| Proceeds from issuance of convertible notes, net |
— | 3,904 | ||||||
| Repurchase of Series A convertible preferred stock |
— | (37 | ) | |||||
| Proceeds from issuance of convertible preferred stock, net |
15,729 | — | ||||||
| Proceeds from draw down of long-term debt, net |
27,381 | — | ||||||
| Payments of long-term debt |
(15,000 | ) | — | |||||
| Proceeds from common stock private placement, gross |
29,447 | — | ||||||
| Payment of offering costs related to common stock private placement |
(2,302 | ) | — | |||||
| Payments of costs related to the initial public offering |
(628 | ) | — | |||||
| Proceeds from the exercise of stock options |
19 | 21 | ||||||
|
|
|
|
|
|||||
| Net cash provided by financing activities |
54,646 | 3,888 | ||||||
|
|
|
|
|
|||||
| NET INCREASE (DECREASE) IN CASH |
20,935 | (24,816 | ) | |||||
| CASH—BEGINNING OF PERIOD |
11,129 | 26,529 | ||||||
|
|
|
|
|
|||||
| CASH—END OF PERIOD |
$ | 32,064 | $ | 1,713 | ||||
|
|
|
|
|
|||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
||||||||
| Cash paid for interest |
$ | 1,711 | $ | 1,111 | ||||
|
|
|
|
|
|||||
| Cash paid for taxes |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
| SUPPLEMENTAL NON-CASH INVESTING AND FINANCING ACTIVITIES: |
||||||||
| Purchase of fixed assets in accounts payable and accrued expenses |
$ | (309 | ) | $ | 150 | |||
|
|
|
|
|
|||||
| Fair value of common stock warrants issued to placement agents as service payment |
$ | 316 | $ | — | ||||
|
|
|
|
|
|||||
| Offering cost in accounts payable and accrued expenses |
$ | 605 | $ | — | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of these condensed consolidated financial statements.
F-39
Table of Contents
Index to Financial Statements
VIEWRAY, INC.
Notes to Condensed Consolidated Financial Statements
(Unaudited)
| 1. | Background and Organization |
On July 23, 2015, ViewRay, Inc. (f/k/a Mirax Corp.), or the Company, and ViewRay Technologies, Inc. (f/k/a ViewRay Incorporated), consummated an Agreement and Plan of Merger and Reorganization, or Merger Agreement. Pursuant to the Merger Agreement, the stockholders of ViewRay Technologies, Inc. contributed all of their equity interests to the Company for shares of the Company’s common stock and merged with the Company’s subsidiary, which resulted in ViewRay Technologies, Inc. becoming a wholly-owned subsidiary of the Company (the Merger). Refer to Note 4 for further information on the Merger.
ViewRay, Inc. and its wholly owned subsidiary ViewRay Technologies, Inc., designs, manufactures and markets MRIdian, the first and only MRI-guided radiation therapy system to image and treat cancer patients simultaneously.
Since inception, ViewRay Technologies, Inc. has devoted substantially all of its efforts towards research and development, initial selling and marketing activities, raising capital and preparing for the manufacturing and shipment of MRIdian systems. In May 2012, ViewRay Technologies, Inc. was granted clearance from the U.S. Food and Drug Administration, or FDA, to sell MRIdian. In November 2013, ViewRay Technologies, Inc. received its first clinical acceptance of a MRIdian at a customer site, and the first patient was treated with that system in January 2014. ViewRay Technologies, Inc. received permission to affix the CE mark in November 2014.
| 2. | Summary of Significant Accounting Policies |
The accompanying condensed consolidated financial statements reflect the application of certain significant accounting policies, as described below and elsewhere in the accompanying notes to the condensed consolidated financial statements.
Basis of Presentation
The condensed consolidated financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, or GAAP, and pursuant to the rules and regulation of the Securities and Exchanges Commission, or SEC. In the opinion of management, all adjustments, including normal recurring adjustments, considered necessary for a fair presentation of the Company’s unaudited condensed consolidated financial statements have been included. The results of operations for the three months and nine months ended September 30, 2015 are not necessarily indicative of the results that may be expected for the year ending December 31, 2015 or any future period. These unaudited condensed consolidated financial statements and notes thereto should be read in conjunction with the audited consolidated financial statements and notes thereto for the year ended December 31, 2014.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported and disclosed in the condensed consolidated financial statements and accompanying notes. Such estimates include, but are not limited to, allocation of revenue to its multiple deliverable elements, inventory write-downs to reflect net realizable value, assumptions used in the valuation of stock-based awards, accrued losses from purchase commitments, and valuation allowances against deferred tax assets. Actual results could differ from those estimates.
F-40
Table of Contents
Index to Financial Statements
Inventory
Inventory consists of purchased components for assembling MRIdian systems and other direct and indirect costs associated with MRIdian system installation. Inventory is stated at the lower of cost or market value. All inventories expected to be placed in service during ViewRay Technologies, Inc. normal operating cycle for the delivery and assembly of MRIdian systems, including items expected to be on hand for more than one year, are classified as current assets.
Effective January 1, 2015, ViewRay Technologies, Inc. made a voluntary change to its accounting policy for inventory cost basis. Under the previous accounting policy, the Company recorded inventory items on a first-in, first-out basis through specific identification. Purchased components were assigned to each MRIdian system at original cost. Under the new accounting policy, ViewRay Technologies, Inc. recorded inventory at weighted average cost basis.
The Company believes that this change is preferable because it will be more efficient for the Company to keep track of its inventory cost. The first-in, first-out cost basis through specific identification accounting policy was manageable at the time since ViewRay Technologies, Inc. had limited MRIdian system installations (one MRIdian system installation during the year ended December 31, 2013, and another two MRIdian system installation during the year ended December 31, 2014). However, due to the Company’s growing business and sales, the number of planned MRIdian system installation has been increasing. Purchased components are no longer assigned to specific MRIdian system installation. Along with the Company’s increased components purchasing activities, the new accounting policy will significantly reduce the Company’s burden and cost of inventory management.
In accordance with applicable accounting literature, a change in inventory cost basis is treated as a change in accounting principle and requires retrospective application. The accounting policy change has no cumulative effect on ViewRay Technologies, Inc. annual statements of operations prior to January 1, 2015, an immaterial effect on ViewRay Technologies, Inc. interim condensed consolidated statements of operations for the year ended December 31, 2014. Therefore, no retrospective adjustment of the Company’s annual consolidated financial statements are required, and the Company’s condensed consolidated statements of operations for the three and nine months ended September 30, 2014 is not revised for the immaterial impact from the accounting policy change.
Recent Accounting Pronouncements
In July 2015 the FASB issued ASU 2015-11, Simplifying the Measurement of Inventory, which requires entities to measure most inventory at the lower of cost and net realizable value, thereby simplifying the current guidance under which an entity must measure inventory at the lower of cost or market. ASU 2015-11 is effective prospectively for annual periods beginning after December 15, 2016 and interim periods therein. Early application is permitted. The Company is reviewing the provisions of ASU 2015-11 and expects that the new guidance will not have a material impact on the Company’s financial reporting.
In April 2015, the Financial Accounting Standards Board (FASB) issued ASU 2015-03, Simplifying the Presentation of Debt Issuance Costs, which requires all debt issuance costs be presented in the balance sheet as a direct deduction from the carrying value of the associated debt. Under ASU 2015-03, the presentation of debt issuance costs is consistent with the presentation for a debt discount, which is a direct adjustment to the carrying value of the debt. Accordingly, the amortization of such costs should continue to be calculated using the interest method and be reported as interest expense. ASU 2015-03 is effective for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company has recorded the debt issuance cost related to the Term Loan issued in June 2015 as a debt discount, and amortized to interest expense during the life of the Term Loan using the effective interest method. See Note 7, Term Loan.
F-41
Table of Contents
Index to Financial Statements
| 3. | Going Concern |
The Company’s unaudited condensed consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company currently has limited working capital, and has not completed its efforts to establish a stabilized source of revenues sufficient to cover operating costs over an extended period of time.
Management anticipates that the Company will be dependent, for the near future, on additional investment capital to fund operating expenses. The Company intends to position itself so that it may be able to raise additional funds through the capital markets. There are no assurances that the Company will be successful in this or any of its endeavors or become financially viable and continue as a going concern.
| 4. | Merger |
On July 23, 2015, ViewRay, Inc. (f/k/a Mirax Corp.), or the Company, and ViewRay Technologies, Inc. (f/k/a ViewRay Incorporated), consummated an Agreement and Plan of Merger and Reorganization, or Merger Agreement. Pursuant to the Merger Agreement, the stockholders of ViewRay Technologies, Inc. contributed all of their equity interests to the Company for shares of the Company’s common stock and merged with the Company’s subsidiary, which resulted in ViewRay Technologies, Inc. becoming a wholly-owned subsidiary of the Company (the Merger). Effective as of July 23, 2015, the Company amended and restated its Certificate of Incorporation to increase its authorized common stock to 300,000,000 shares and 10,000,000 shares of “blank check” preferred stock, par value of $0.01 per share.
Upon the closing of the Merger, under the terms of the Split-Off Agreement, dated July 23, 2015 among the Company, ViewRay Technologies, Inc. and Vesuvius Acquisition Sub, Inc., the acquisition subsidiary of the Company (the Split-Off Agreement), and a general release agreement dated July 23, 2015 (the General Release Agreement), the Company transferred all of its pre-Merger operating assets and liabilities to wholly- owned special-purpose subsidiary incorporated in Nevada, Vesuvius Acquisition Sub, Inc. (the Split-Off Subsidiary). Thereafter, the Company transferred all of the outstanding shares of capital stock of the Split-Off Subsidiary to certain pre-Merger insiders of the Company in exchange for the surrender and cancellation of shares of the Company’s common stock held by such persons.
Together with the Merger, on July 23, 2015, ViewRay Technologies, Inc. effected a 2.975-for-1 stock split of its then outstanding common stock and convertible preferred stock (collectively referred to as “Capital Stock”) and convertible preferred stock warrants, in which (i) each share of outstanding Capital Stock was increased into 2.975 shares of Capital Stock; (ii) the number of outstanding options to purchase each Capital Stock was proportionately increased on a 2.975-for-1 basis; (iii) number of shares reserved for future option grants under the 2008 Plan were proportionately increased on a 2.975-for-1 basis; (iv) the exercise price of each such outstanding option was proportionately decreased on a 2.975-for-1 basis; and (v) each share of outstanding convertible preferred stock warrant was increased into 2.975 shares of convertible preferred stock warrant. All of the share and per share amounts have been adjusted, on a retroactive basis, to reflect this 2.975-for-1 stock split.
At the closing of the Merger, the Company conducted a private placement offering, or the Private Placement, of its securities for $26.2 million, net of offering cost, through the sale of 5,884,504 shares of the common stock of the surviving corporation, at an offering price of $5.00 per share. Investors in ViewRay Technologies, Inc. purchased $17.0 million of shares in the Private Placement. Certain shareholders of the Company retained, after giving effect to the Split-Off, 1,000,005 shares of the common stock of the surviving corporation upon the Private Placement. The former stockholders of ViewRay Technologies Inc. collectively own approximately 90.9% of the outstanding shares of the Company’s common stock.
F-42
Table of Contents
Index to Financial Statements
Immediately following the closing of the Merger, the Company’s outstanding shares of common stock (on a fully diluted basis) are owned as follows:
| • | Former holders of the ViewRay Technologies, Inc.’s capital stock hold an aggregate of 34,715,582 shares of the Company’s common stock, or approximately 72.7% on a fully diluted basis; |
| • | The Private Placement, resulted in an aggregate of 5,884,504 shares of the Company’s common stock, consisting of 3,400,003 shares held by ViewRay Technologies, Inc. shareholders and 2,484,501 shares issued to new shareholders, or together approximately 12.3% on a fully diluted basis; |
| • | 128,231 shares of ViewRay Technologies, Inc.’s preferred stock warrant were converted to the Company’s common stock warrant, or approximately 0.3% on a fully diluted basis; |
| • | 198,760 shares of common stock issued as warrants to placement agents as payment for services provided, or approximately 0.4% on a fully diluted basis; |
| • | Holders of the Company’s common stock prior to the closing of the Merger hold an aggregate of 1,000,005 shares of the Company’s common stock, or approximately 2.1% on a fully diluted basis; and |
| • | 9,225,397 shares of common stock are reserved for issuance under the 2008 Stock Incentive Plan, or the 2008 Plan, and the 2015 Equity Incentive Plan of ViewRay, or the 2015 Plan, collectively representing approximately 19.3% on a fully diluted basis. Upon closing, 1,507,147 options to purchase shares of the Company’s common stock are granted to employees under the 2015 Plan. In addition, the Board of Directors of the Company has adopted a 285,621-share Employee Stock Purchase Plan (ESPP). |
The Merger is being accounted for as a reverse-merger and recapitalization. ViewRay Technologies, Inc. is the acquirer for financial reporting purposes, and ViewRay, Inc. is the acquired company under the acquisition method of accounting in accordance with FASB ASC Topic 805, Business Combination. Consequently, the assets, liabilities and operations that will be reflected in the historical consolidated financial statements prior to the Merger will be those of ViewRay Technologies, Inc. and will be recorded at the historical cost basis, and the condensed consolidated financial statements after completion of the Merger will include the assets, liabilities and results of operations of ViewRay Technologies, Inc. up to the day prior to the closing of the Merger and the assets, liabilities and results of operations of the combined company from and after the closing date of the Merger.
| 5. | Balance Sheet Components |
Property and Equipment
Property and equipment consisted of the following (in thousands):
| September 30, 2015 |
December 31, 2014 |
|||||||
| (Unaudited) | ||||||||
| Prototype |
$ | 6,471 | $ | 6,342 | ||||
| Machine and equipment |
5,605 | 4,214 | ||||||
| Leasehold improvements |
1,274 | 1,270 | ||||||
| Furniture and fixtures |
347 | 263 | ||||||
| Software |
790 | 647 | ||||||
| Construction in progress |
1,402 | — | ||||||
|
|
|
|
|
|||||
| Property and equipment, gross |
15,889 | 12,736 | ||||||
| Less: accumulated depreciation and amortization |
(10,593 | ) | (9,805 | ) | ||||
|
|
|
|
|
|||||
| Property and equipment, net |
$ | 5,296 | $ | 2,931 | ||||
|
|
|
|
|
|||||
F-43
Table of Contents
Index to Financial Statements
Depreciation and amortization expense related to property and equipment was $280 thousand, $207 thousand, $788 thousand and $612 thousand during the three months ended September 30, 2015 and 2014 and the nine months ended September 30, 2015 and 2014, respectively.
Intangible Assets
Intangible assets consisted of the following (in thousands):
| September 30, 2015 |
December 31, 2014 |
|||||||
| (Unaudited) | ||||||||
| Intangible assets—license cost |
$ | 500 | $ | 500 | ||||
| Accumulated amortization |
(361 | ) | (236 | ) | ||||
|
|
|
|
|
|||||
| Intangible assets, net |
$ | 139 | $ | 264 | ||||
|
|
|
|
|
|||||
Intangible amortization expense was $42 thousand, $42 thousand, $125 thousand and $125 thousand during the three months ended September 30, 2015 and 2014 and the nine months ended September 30, 2015 and 2014, respectively, which were recorded in general and administrative expenses in the condensed consolidated statements of operations.
At September 30, 2015, the estimated future amortization expense of purchased intangible assets was as follows (in thousands):
| Year Ending December 31, |
Estimated Future Amortization Expense |
|||
| (Unaudited) | ||||
| The remainder of 2015 |
$ | 42 | ||
| 2016 |
97 | |||
|
|
|
|||
| Total amortization expense |
$ | 139 | ||
|
|
|
|||
Accrued Liabilities
Accrued liabilities consisted of the following (in thousands):
| September 30, 2015 |
December 31, 2014 |
|||||||
| (Unaudited) | ||||||||
| Accrued payroll and related benefits |
$ | 1,779 | $ | 1,652 | ||||
| Accrued accounts payable |
478 | 946 | ||||||
| Sales tax and medical device excise tax payable |
33 | 499 | ||||||
| Accrued legal and accounting |
410 | 901 | ||||||
| Accrued interest |
— | 142 | ||||||
| Accrued debt facility fee |
1,500 | — | ||||||
| Other |
814 | 296 | ||||||
|
|
|
|
|
|||||
| Total accrued liabilities |
$ | 5,014 | $ | 4,436 | ||||
|
|
|
|
|
|||||
F-44
Table of Contents
Index to Financial Statements
Deferred Revenue
Deferred revenue consisted of the following (in thousands):
| September 30, 2015 |
December 31, 2014 |
|||||||
| (Unaudited) | ||||||||
| Deferred revenue: |
||||||||
| Product |
$ | 5,050 | $ | 6,919 | ||||
| Services |
573 | 442 | ||||||
|
|
|
|
|
|||||
| Total deferred revenue |
5,623 | 7,361 | ||||||
| Less: current portion of deferred revenue |
(5,249 | ) | (7,361 | ) | ||||
|
|
|
|
|
|||||
| Noncurrent portion of deferred revenue |
$ | 374 | $ | — | ||||
|
|
|
|
|
|||||
| 6. | Fair Value of Financial Instruments |
The Company’s financial instruments that are carried at fair value mainly consist of Level 1 assets and Level 3 liabilities. Level 1 assets include highly liquid bank deposits and money market funds, which were not material at September 30, 2015 and December 31, 2014. Level 3 liabilities consist of the convertible preferred stock warrant liability. The convertible preferred stock warrant liability was valued using the Black-Scholes option-pricing model. Generally, increases (decreases) in the fair value of the underlying stock and estimated term would result in a directionally similar impact to the fair value of the warrant (see Note 8).
The convertible preferred stock warrants were issued in December 2013 and were still outstanding at December 31, 2014. In July 2015, upon the Merger of the Company and ViewRay Technologies, Inc., and the Private Placement, the convertible preferred stock warrants were converted into warrants to purchase the Company’s common stock. The aggregate fair value of these warrants upon the closing of the Merger is $93 thousand which was reclassified from liabilities to common stock additional paid-in-capital, a component of condensed consolidated stockholder’s equity (deficit), and the Company ceased recording further related periodic fair value change adjustments.
The following table sets forth the fair value of the Company’s financial liabilities by level within the fair value hierarchy (in thousands):
| At September 30, 2015 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| (Unaudited) | ||||||||||||||||
| Convertible preferred stock warrant liability |
$ | — | $ | — | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| At December 31, 2014 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Convertible preferred stock warrant liability |
$ | — | $ | — | $ | 138 | $ | 138 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
F-45
Table of Contents
Index to Financial Statements
The following table sets forth a summary of the changes in the fair value of the Company’s Level 3 financial liabilities (in thousands):
| Nine Months Ended September 30, 2015 |
||||
| (Unaudited) | ||||
| Fair value, beginning of period |
$ | 138 | ||
| Change in fair value of Level 3 financial liabilities |
(45 | ) | ||
| Reclassification of preferred stock warrant liabilities to additional paid-in-capital in conjunction with the conversion of the convertible preferred stock into common stock upon closing of the Merger |
(93 | ) | ||
|
|
|
|||
| Fair value, end of period |
$ | — | ||
|
|
|
|||
The gains and losses from re-measurement of Level 3 financial liabilities are recorded as part of other income (expense), net in the condensed consolidated statements of operations.
| 7. | Term Loan |
2015 Term Loan
On June 26, 2015, ViewRay Technologies, Inc. entered into a Term Loan Agreement, or the Term Loan, with Capital Royalty Partners II L.P and Parallel Investment Opportunities Partners II L.P or together, CRG, for up to $50.0 million of which $30.0 million was made available to us upon closing with the remaining $20.0 million to be available on or before June 26, 2016 at our option upon the occurrence of either (i) an initial public offering of our common stock on a nationally recognized securities exchange that raises a minimum of $40.0 million in net cash proceeds with a minimum of $120.0 million post-money valuation, or Qualifying IPO, or (ii) achievement of a minimum of $25.0 million gross revenue from the sales of the MRIdian system during any consecutive 12 months before March 31, 2016. We drew down the first $30.0 million on closing date. The Term Loan has a maturity date of June 26, 2020 and bears cash interest at a rate of 12.5% per annum to be paid quarterly during the first 3 years, or the interest-payment-only period. The interest-payment-only period can be extended for another year until June 26, 2019 if the Company completes a Qualifying IPO on or before June 26, 2018. During the interest-payment-only period, the Company has the option to elect to pay only 8% of the 12.5% per annum interest in cash, and the remaining 4.5% of the 12.5% per annum interest as compounded interest, or deferred payment in-kind interest, added to the aggregate principal amount of the Term Loan. Principal payment and any deferred payment in-kind interest will be paid quarterly in equal installments following the end of the interest-payment-only period through maturity date.
The Term Loan is subject to a prepayment penalty of 3% on the outstanding balance during the first 12 months following the funding of the Term Loan, 2% on the outstanding balance after year 1 but on or before year 2, 1% on the outstanding balance after year 2 but on or before year 3, and 0% on the outstanding loan if prepaid after year 3 thereafter until maturity. The Term Loan is also subject to a facility fee of 5% based on the sum of the Term Loan drawn and any outstanding payment in-kind payable on maturity date or the date such Term Loan becomes due for whatever reason. All direct financing costs were accounted for as a discount on the Term Loan and will be amortized to interest expense during the life of the Term Loan using the effective interest method. The Term Loan is subject to financial covenants and is collateralized by essentially all our assets and limits the Company’s ability with respect to additional indebtedness, investments or dividends, among other things, subject to customary exceptions.
On June 26, 2015, ViewRay Technologies, Inc. paid off in full the outstanding term loan, the related interest and other penalty fee with Hercules Technology III, L.P. and Hercules Technology Growth Capital, Inc., or together, Hercules, using part of the proceeds received from the CRG Term Loan. Hercules Term Loan was entered in December 2013.
F-46
Table of Contents
Index to Financial Statements
| 8. | Commitments and Contingencies |
Operating Leases
The Company leases office space in Oakwood Village, Ohio and Mountain View, California under non-cancellable operating leases. At December 31, 2014, the future minimum payments for the operating leases are as follows (in thousands):
| Year Ending December 31, |
Future Minimum Payments |
|||
| 2015 |
$ | 1,086 | ||
| 2016 |
1,113 | |||
| 2017 |
1,106 | |||
| 2018 |
963 | |||
| 2019 and thereafter |
823 | |||
|
|
|
|||
| Total future minimum payments |
$ | 5,091 | ||
|
|
|
|||
Contingencies
The Company is subject to claims and assessments from time to time in the ordinary course of business. The Company records a provision for a liability when it believes that it is both probable that a liability has been incurred and the amount can be reasonably estimated. Significant judgment is required to determine both probability and the estimated amount.
In the normal course of business, the Company may become involved in legal proceedings. The Company will accrue a liability for such matters when it is probable that a liability has been incurred and the amount can be reasonably estimated. When only a range of possible loss can be established, the most probable amount in the range is accrued. If no amount within this range is a better estimate than any other amount within the range, the minimum amount in the range is accrued. The accrual for a litigation loss contingency might include, for example, estimates of potential damages, outside legal fees and other directly related costs expected to be incurred. At September 30, 2015 and December 31, 2014, the Company was not involved in any material legal proceedings.
Purchase Commitments
At September 30, 2015, the Company had no outstanding firm purchase commitments.
| 9. | Convertible Preferred Stock |
In January 2015, the Company issued an aggregate of 162,407 shares of Series C convertible preferred stock to a new investor at a price of $5.84 per share for a total gross consideration of $950 thousand.
In February 2015, the Company issued 2,564,652 shares of Series C convertible preferred stock to another investor at a price of $5.84 per share for total gross consideration of $15.0 million.
The rights, privileges and preferences of the issued Series C convertible preferred stock in January and February 2015 are the same as the Series C convertible preferred stock outstanding as of December 31, 2014.
On July 23, 2015, upon the closing of the Merger, all of ViewRay Technologies, Inc.’s 30,381,987 shares of outstanding convertible preferred stock were converted into the Company’s common stock at a 1:1 conversion rate.
F-47
Table of Contents
Index to Financial Statements
Convertible preferred stock as of September 30, 2015 and December 31, 2014 consisted of the following (in thousands, except share data):
| September 30, 2015 | ||||||||||||||||
| Shares Authorized |
Shares Issued and Outstanding |
Aggregate Liquidation Preference |
Net Carrying Value |
|||||||||||||
| (Unaudited) | ||||||||||||||||
| Series A |
398,500 | — | $ | — | $ | — | ||||||||||
| Series B |
60,500,000 | — | — | — | ||||||||||||
| Series C |
19,812,497 | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
80,710,997 | — | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| December 31, 2014 | ||||||||||||||||
| Shares Authorized |
Shares Issued and Outstanding |
Aggregate Liquidation Preference |
Net Carrying Value |
|||||||||||||
| Series A |
398,500 | 162,109 | $ | 3,004 | $ | 3,003 | ||||||||||
| Series B |
60,500,000 | 22,308,230 | 113,405 | 112,080 | ||||||||||||
| Series C |
13,562,497 | 5,184,589 | 30,323 | 30,027 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
74,460,997 | 27,654,928 | $ | 146,732 | $ | 145,110 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| 10. | Stock Warrant |
Related to a 2013 debt financing, the Company issued a warrant to purchase 128,231 shares of Series C convertible preferred stock. The convertible preferred stock warrant was recorded as a liability and is adjusted to fair value at each balance sheet date, with the change in fair value being recorded as a component of other income (expense), net in the condensed consolidated statements of operations. Upon issuance, the fair value of the warrant was estimated to be $158 thousand. As of December 31, 2014, the warrant had not been exercised and was still outstanding. Change in the fair value of the warrant was ($6) thousand and $19 thousand for the three months ended September 30, 2015 and 2014, and $45 thousand and $74 thousand for the nine months ended September 30, 2015 and 2014, were recognized in the condensed consolidated statements of operations.
All shares of Series C convertible preferred stock were converted into common stock, and the warrant to purchase Series C convertible preferred stock was converted into the warrant to purchase 128,231 shares of the Company’s common stock, upon the closing of the Merger on July 23, 2015. The fair value of the preferred stock warrant liability of $93 thousand was reclassed into common stock additional paid-in capital in the accompanying balance sheets for the nine months ended September 30, 2015.
Related to the Merger and the Private Placement, in July and August 2015, the Company issued 198,760 shares of common stock warrants at an exercise price of $5.00 per share to private placement agents as payment for services provided. These placement warrants are exercisable at any time at the option of the holder until the five year anniversary of its date of issuance. The number of shares of common stock issuable upon the exercise of each placement warrant is adjustable in the event of certain stock dividends, stock splits, combinations of shares and similar transactions. Upon exercise, the aggregate exercise price of the warrants issued are payable by the holders in cash.
The Company estimated the aggregate fair value of the warrants issued to placement agents as of the grant date to be $316 thousand and recorded as an offering cost against the total proceeds from the Private Placement recorded in common stock additional paid-in capital in the accompanying condensed consolidated balance sheets and condensed consolidated statements of convertible preferred stock and stockholders’ equity (deficit) as of September 30, 2015.
F-48
Table of Contents
Index to Financial Statements
Pursuant to ASC 815-15 and ASC 815-40, the fair value of the placement warrants was recorded as equity awards on the grant date.
The placement warrants were valued at their grant dates using the Black-Scholes pricing model and the following weighted average assumptions:
| September 30, 2015 |
||||
| Common Stock Warrant: |
||||
| Expected term (in years) |
5.0 | |||
| Expected volatility |
31.8 | % | ||
| Risk-free interest rate |
1.6 | % | ||
| Expected dividend yield |
0 | % | ||
| 11. | Stock-Based Compensation |
A summary of the Company’s stock option activity and related information is as follows:
| Options Outstanding | ||||||||||||||||||||
| Shares Available for Grant |
Number of Stock Options Outstanding |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Balance at December 31, 2014 |
295,101 | 4,248,519 | $ | 0.76 | 7.7 | $ | 8,343 | |||||||||||||
| Additional authorized (unaudited) |
4,708,447 | |||||||||||||||||||
| Granted (unaudited) |
(1,911,137 | ) | 1,911,137 | 5.04 | — | — | ||||||||||||||
| Exercised (unaudited) |
— | (26,555 | ) | 0.73 | — | — | ||||||||||||||
| Cancelled (unaudited) |
121,941 | (121,941 | ) | 1.30 | — | — | ||||||||||||||
|
|
|
|
|
|||||||||||||||||
| Balance at September 30, 2015 (unaudited) |
3,214,352 | 6,011,160 | $ | 2.11 | 7.8 | $ | 18,755 | |||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Vested and exercisable at September 30, 2015 (unaudited) |
2,911,734 | $ | 0.82 | 6.5 | $ | 12,702 | ||||||||||||||
| Vested and expected to vest at September 30, 2015 (unaudited) |
4,576,826 | $ | 1.88 | 7.6 | $ | 15,329 | ||||||||||||||
The weighted-average grant date fair value of options granted to employees was $3.13 per share during the nine months ended September 30, 2015. The grant date fair value of options vested was $390 thousand during nine months ended September 30, 2015.
Aggregate intrinsic value represents the difference between the estimated fair value of the underlying common stock and the exercise price of outstanding, in-the-money options.
At September 30, 2015, total unrecognized compensation cost related to stock-based awards granted to employees, net of estimated forfeitures, was $5.6 million which is expected to be recognized over a weighted-average period of 3.5 years.
Determination of Fair Value
The determination of the fair value of stock options on the date of grant using an option-pricing model is affected by the estimated fair value of the Company’s common stock, as well as assumptions regarding a number of complex and subjective variables. The variables used to calculate the fair value of stock options using the
F-49
Table of Contents
Index to Financial Statements
Black-Scholes option-pricing model include actual and projected employee stock option exercise behaviors, expected price volatility of the Company’s common stock, the risk-free interest rate and expected dividends. Each of these inputs is subjective and generally requires significant judgment to determine.
Fair Value of Common Stock
Prior to the Merger, the fair value of the common stock underlying the stock-based awards was determined by ViewRay Technologies, Inc.’s board of directors, with input from management and third-party valuations. Post-Merger, our common stock shares are listed on the OTC Bulletin Board. Fair value of the common stock is the adjusted closing price of the Company’s common stock on the trading date.
Expected Term
The expected term represents the period that the Company’s option awards are expected to be outstanding. The Company considers several factors in estimating the expected term of options granted, including the expected lives used by a peer group of companies within the Company’s industry that the Company considers to be comparable to its business and the historical option exercise behavior of its employees, which the Company believes is representative of future behavior.
Expected Volatility
As the Company does not have a sufficient trading history for its common stock, the expected stock price volatility for the Company’s common stock was estimated by taking the average historic price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the stock option grants. Industry peers consist of several public companies in the Company’s industry which were the same as the comparable companies used in the common stock valuation analysis. The Company intends to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of its own share price becomes available, or unless circumstances change such that the identified companies are no longer similar to the Company, in which case, more suitable companies whose share prices are publicly available would be used in the calculation.
Risk-Free Interest Rate
The risk-free interest rate is based on the zero coupon U.S. Treasury notes, with maturities similar to the expected term of the options.
Expected Dividend Yield
The Company does not anticipate paying any dividends in the foreseeable future and, therefore, uses an expected dividend yield of zero in the Black-Scholes option-valuation model.
In addition to the Black-Scholes assumptions discussed immediately above, the estimated forfeiture rate also has a significant impact on the related stock-based compensation. The forfeiture rate of stock options is estimated at the time of grant and revised in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data to estimate pre-vesting option forfeitures and records stock-based compensation expense only for those awards that are expected to vest.
For the nine months ended September 30, 2015, the weighted average fair value of employee stock options was estimated at the date of grant using a Black-Scholes option-pricing model with the following assumptions. 1,911,137 shares were granted during the nine months ended September 30, 2015.
F-50
Table of Contents
Index to Financial Statements
| Nine Months Ended September 30, 2015 |
||||
| (Unaudited) | ||||
| Expected term (in years) |
6.0 | |||
| Expected volatility% |
68.7 | % | ||
| Risk-free interest rate% |
1.8 | % | ||
| Expected dividend yield% |
0.0 | % | ||
Stock-Based Compensation Expense
Total stock-based compensation expense recognized in the Company’s condensed consolidated statements of operations is classified as follows (in thousands):
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2015 | 2014 | 2015 | 2014 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| Research and development |
$ | 95 | $ | 14 | $ | 143 | $ | 70 | ||||||||
| Selling and marketing |
16 | 3 | 26 | 11 | ||||||||||||
| General and administrative |
272 | 41 | 362 | 181 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total stock-based compensation expense |
$ | 383 | $ | 58 | $ | 531 | $ | 262 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
During the three months and nine months ended September 30, 2015 and 2014, there were no stock-based compensation expenses capitalized as a component of inventory or recognized in cost of revenue. Stock-based compensation relating to stock-based awards granted to consultants was insignificant for the three months and nine months ended September 30, 2015 and 2014.
| 12. | Net Loss per Share |
The following table sets forth the computation of the Company’s basic and diluted net loss per share for the periods presented (in thousands, except share and per share data):
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2015 | 2014 | 2015 | 2014 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| Net loss |
$ | (10,260 | ) | $ | (7,569 | ) | $ | (30,889 | ) | $ | (24,180 | ) | ||||
| Gain due to repurchase of Series A preferred stock |
— | — | — | 9 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss attributable to common stockholders |
$ | (10,260 | ) | $ | (7,569 | ) | $ | (30,889 | ) | $ | (24,171 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Weighted-average common shares used in computing net loss per share, basic and diluted |
29,157,069 | 900,062 | 10,433,051 | 887,189 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per share, basic and diluted |
$ | (0.35 | ) | $ | (8.41 | ) | $ | (2.96 | ) | $ | (27.25 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
F-51
Table of Contents
Index to Financial Statements
The following weighted-average common stock equivalents were excluded from the calculation of diluted net loss per share for the periods presented because including them would have had an anti-dilutive effect:
| Three Months Ended September 30, |
Nine Months Ended September 30, |
|||||||||||||||
| 2015 | 2014 | 2015 | 2014 | |||||||||||||
| (Unaudited) | ||||||||||||||||
| Convertible preferred stock (if converted) |
7,265,258 | 25,034,977 | 22,138,427 | 25,035,220 | ||||||||||||
| Options to purchase common stock |
5,535,394 | 4,164,151 | 4,701,033 | 3,627,700 | ||||||||||||
| Stock warrants (if converted) |
269,079 | 128,231 | 175,696 | 128,231 | ||||||||||||
| 13. | Subsequent Events |
The Company has evaluated subsequent events through December 30, 2015, the date on which these condensed consolidated financial statements were issued. No significant subsequent events to this date would have had material impact on the Company’s condensed consolidated financial statements as of and for the nine months ended September 30, 2015.
F-52
Table of Contents
Index to Financial Statements
VIEWRAY INCORPORATED AND MIRAX CORP.
| Page | ||||
| F-54 | ||||
F-53
Table of Contents
Index to Financial Statements
VIEWRAY INCORPORATED AND MIRAX CORP.
UNAUDITED PRO FORMA COMBINED FINANCIAL INFORMATION
On July 23, 2015, ViewRay Technologies, Inc. (f/k/a ViewRay Incorporated), or the Company, and ViewRay, Inc. (f/k/a Mirax Corp.), or Mirax, consummated an Agreement and Plan of Merger and Reorganization, or Merger Agreement. Pursuant to the Merger Agreement, the stockholders of ViewRay contributed all of their equity interests in the Company to Mirax for shares of Mirax common stock, which resulted in the Company becoming a wholly-owned subsidiary of Mirax (the Merger).
Effective as of July 23, 2015, Mirax amended and restated its Certificate of Incorporation to increase its authorized common stock to 300,000,000 shares and 10,000,000 shares of “blank check” preferred stock, par value of $0.01 per share.
Upon closing of the Merger, under the terms of the Split-Off Agreement, dated July 23, 2015 among the Company, Mirax and Vesuvius Acquisition Sub, Inc., the acquisition subsidiary of Mirax (the Split-Off Agreement), and a general release agreement dated July 23, 2015 (the General Release Agreement), Mirax transferred all of its pre-Merger operating assets and liabilities to wholly- owned special-purpose subsidiary incorporated in Nevada, Mirax Enterprise Corp. (the Split-Off Subsidiary). Thereafter, Mirax transferred all of the outstanding shares of capital stock of the Split-Off Subsidiary to certain pre-Merger insiders of Mirax in exchange for the surrender and cancellation of shares of Mirax common stock held by such persons.
At the closing of the merger, the surviving corporation conducted and completed a private placement offering of its securities for $29.4 million through the sale of 5,884,504 shares of the common stock of the surviving corporation, at an offering price of $5.00 per share. Existing ViewRay investors purchased $17.0 million of shares in the private placement offering. Certain shareholders of Mirax retained, after giving effect to the Split-Off, 1,000,005 shares of the common stock of the surviving corporation upon the private placement offering. The former stockholders of the Company collectively own approximately 90.88% of the outstanding shares of the surviving corporation’s common stock.
Immediately following the closing of the Merger, the surviving corporation’s outstanding shares of common stock (on a fully diluted basis) are owned as follows:
| • | Former holders of the Company’s capital stock hold an aggregate of 31,315,579 shares of the surviving corporation’s common stock, or approximately 66.58% on a fully diluted basis; |
| • | The Private Placement Offering, or the PPO, resulted in an aggregate of 5,884,504 shares of the surviving corporation’s common stock, consisting of 3,400,003 shares held by existing Company shareholders and 2,484,502 shares issued to new shareholders, or together approximately 12.32% on a fully diluted basis; |
| • | 198,760 shares of common stock issued as warrants to placement agents as payment for services provided, or approximately 0.4% on a fully diluted basis; |
| • | Holders of Mirax common stock prior to the closing of the Merger hold an aggregate of 1,000,005 shares of the surviving corporation’s common stock, or approximately 2.09% on a fully diluted basis; and |
| • | 9,225,397 shares of common stock are reserved for issuance under the 2008 Stock Incentive Plan, or the 2008 Plan, and the 2015 Equity Incentive Plan of ViewRay, or the 2015 Plan, collectively representing approximately 19.32% on a fully diluted basis. Upon closing, 1,507,147 options to purchase shares of the surviving corporation’s common stock are granted to employees under the 2015 Plan. In addition, the Board of Directors of the surviving corporation will adopt a 285,621-share Employee Stock Purchase Plan (ESPP). |
The Merger is being accounted for as a reverse-merger and recapitalization. The Company is the acquirer for financial reporting purposes, and Mirax is the acquired company under the acquisition method of accounting in
F-54
Table of Contents
Index to Financial Statements
accordance with FASB ASC Topic 805, Business Combination. Consequently, the assets, liabilities and operations that will be reflected in the historical financial statements prior to the Merger will be those of the Company and will be recorded at the historical cost basis of the Company, and the consolidated financial statements after completion of the Merger will include the assets, liabilities and results of operations of the Company up to the day prior to the closing of the Merger and the assets, liabilities and results of operations of the combined company from and after the closing date of the Merger. The unaudited pro forma combined financial information is based on individual historical financial statements of the Company and Mirax prepared under U.S. GAAP and is adjusted to give effect to the Merger Agreement.
The historical financial statements have been adjusted in the pro forma combined financial statements to give effects to events that are (1) directly attributable to the Merger, (2) factually supportable, and (3) with respect to the statement of operations, expected to have a continuing impact on the combined entities. The unaudited pro forma combined statements of operations eliminate any non-recurring charges directly related to the Merger that the combined entities incur upon completion of the Merger.
Since the Merger took place in the third quarter of the Company’s fiscal year 2015, the unaudited pro forma combined statements of operations combine the Company’s historical statements of operations for the nine months ended September 30, 2015 with Mirax historical statements of operations for the six months ended May 31, 2015, giving effect to the events that are directly attributable to the Merger, at the Merger closing date of July 23, 2015, and that are expected to have a continuing impact on the combined company. Between the six months ended period of May 31, 2015 and the Merger date of July 23, 2015, Mirax did not have any material transactions, therefore there was no material charge for Mirax statement of operations for the period ended July 23, 2015 compared to the six months ended May 31, 2015 as presented in the unaudited pro forma combined statements of operations. The difference in fiscal periods between the Company and Mirax does not result to material misstatement in the combined pro-forma financial statements.
The unaudited pro forma combined financial information does not purport to represent what the combined company’s results of operations or financial position would actually have been had the Merger occurred on the dates described above or to project the combined company’s results of operations or financial position for any future date or period.
The unaudited pro forma combined financial information should be read together with Viewray, Inc.’s unaudited balance sheet as of September 30, 2015 and statements of operations, statements of convertible preferred stock and stockholders’ deficit and statements of cash flows for the nine months ended September 30, 2015 and the accompanying notes, and (2) Mirax Corp. unaudited balance sheet as of May 31, 2015 and the related statements of operations and statements of cash flows for the six months ended May 31, 2015 and the accompanying notes.
F-55
Table of Contents
Index to Financial Statements
| ViewRay Incorporated and Mirax Corp. Unaudited Pro Forma Combined Statements of Operations Year Ended December 31, 2014 |
||||||||||||||||||||
| ViewRay Incorporated |
Mirax Corp. | Merger Pro Forma Adjustments |
Combined Pro Forma |
|||||||||||||||||
| Revenue: |
||||||||||||||||||||
| Product |
$ | 5,988 | $ | — | $ | — | $ | 5,988 | ||||||||||||
| Service |
411 | — | — | 411 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total Revenues |
6,399 | — | — | 6,399 | ||||||||||||||||
| Cost of revenues: |
||||||||||||||||||||
| Product |
8,176 | — | — | 8,176 | ||||||||||||||||
| Service |
975 | — | — | 975 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total cost of revenues |
9,151 | — | — | 9,151 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Gross profit |
(2,752 | ) | — | — | (2,752 | ) | ||||||||||||||
| Operating expenses: |
||||||||||||||||||||
| Research and development expenses |
9,404 | — | — | 9,404 | ||||||||||||||||
| Selling and marketing |
4,681 | — | — | 4,681 | ||||||||||||||||
| General and administrative expenses |
14,742 | 24 | — | 14,766 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total operating expenses |
28,827 | 24 | — | 28,851 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss from operations |
(31,579 | ) | (24 | ) | — | (31,603 | ) | |||||||||||||
| Other income (expense): |
||||||||||||||||||||
| Interest income |
1 | — | — | 1 | ||||||||||||||||
| Interest expense |
(2,243 | ) | — | — | (2,243 | ) | ||||||||||||||
| Other income (expense) |
21 | — | (20 | ) | A | 1 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Loss before provision for income taxes |
(33,800 | ) | (24 | ) | (20 | ) | (33,844 | ) | ||||||||||||
| Provision (credit) for income taxes |
— | — | — | — | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss |
$ | (33,800 | ) | $ | (24 | ) | $ | (20 | ) | $ | (33,844 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Deemed capital contribution on repurchase of Series A convertible preferred stock |
9 | — | — | 9 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss attributable to common stock holders |
(33,791 | ) | (24 | ) | (20 | ) | (33,835 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (37.87 | ) | $ | (0.01 | ) | — | $ | (1.30 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
| Weighted-average common shares used to compute net loss per share attributable to common stockholders, basic and diluted |
892,316 | 3,778,906 | — | 25,970,712 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||
F-56
Table of Contents
Index to Financial Statements
| ViewRay Incorporated and Mirax Corp. Unaudited Pro Forma Combined Statements of Operations Nine Months Ended September 30, 2015 |
||||||||||||
| ViewRay Technologies, Inc. |
Mirax Corp. | Combined Pro Forma |
||||||||||
| Revenue: |
||||||||||||
| Product |
$ | 5,119 | $ | — | $ | 5,119 | ||||||
| Service |
419 | — | 419 | |||||||||
| Grant |
240 | — | 240 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Revenue |
5,778 | — | 5,778 | |||||||||
| Cost of revenue: |
||||||||||||
| Product |
6,311 | — | 6,311 | |||||||||
| Service |
1,390 | — | 1,390 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cost of revenue |
7,701 | — | 7,701 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross margin |
(1,923 | ) | — | (1,923 | ) | |||||||
| Operating expenses: |
||||||||||||
| Research and development |
7,408 | — | 7,408 | |||||||||
| Selling and marketing |
3,315 | — | 3,315 | |||||||||
| General and administrative |
15,779 | 2 | 15,781 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
26,502 | 2 | 26,504 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(28,425 | ) | (2 | ) | (28,427 | ) | ||||||
| Interest income |
1 | — | 1 | |||||||||
| Interest expense |
(2,376 | ) | — | (2,376 | ) | |||||||
| Other income (expense), net |
(89 | ) | — | (89 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss before provision for income taxes |
(30,889 | ) | (2 | ) | (30,891 | ) | ||||||
| Provision for income taxes |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (30,889 | ) | $ | (2 | ) | $ | (30,891 | ) | |||
|
|
|
|
|
|
|
|||||||
| Deemed capital contribution on repurchase of Series A convertible preferred stock |
$ | 9 | $ | — | $ | 9 | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss attributable to common stock holders |
$ | (33,791 | ) | $ | (24 | ) | $ | (33,835 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per share attributable to common stockholders, basic and diluted |
$ | (2.96 | ) | $ | (0.00 | ) | $ | (2.96 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted-average common shares used to compute net loss per share attributable to common stockholders, basic and diluted |
10,433,051 | 4,343,339 | 10,433,051 | |||||||||
|
|
|
|
|
|
|
|||||||
Merger Pro Forma Adjustments
A – The adjustment reflects the conversion of 43,103 shares of the Company’s Series C preferred stock warrants into 128,231 shares of warrants to purchase common stock upon the Merger, and the elimination of the change in fair value of convertible preferred stock warrant liability.
F-57
Table of Contents
Index to Financial Statements
PART II
Information Not Required in Prospectus
Item 13. Other Expenses of Issuance and Distribution.
Set forth below is an estimate (except for registration fees, which are actual) of the approximate amount of the types of fees and expenses listed below that were paid or are payable by us in connection with the issuance and distribution of the shares of common stock to be registered by this registration statement. None of the expenses listed below are to be borne by any of the selling stockholders named in the prospectus that forms a part of this registration statement.
| Item |
Amount to be paid |
|||
| SEC registration fee |
$ | 19,254 | ||
| Printing and engraving expenses |
25,000 | |||
| Legal fees and expenses |
225,000 | |||
| Accounting fees and expenses |
84,000 | |||
| Transfer agent fees and expenses |
3,000 | |||
| Miscellaneous expenses |
746 | |||
|
|
|
|||
| Total |
$ | 357,000 | ||
|
|
|
|||
Item 14. Indemnification of Directors and Officers.
As permitted by Section 102 of the Delaware General Corporation Law, we have adopted provisions in our amended and restated certificate of incorporation and amended and restated bylaws that limit or eliminate the personal liability of our directors for a breach of their fiduciary duty of care as a director. The duty of care generally requires that, when acting on behalf of the corporation, directors exercise an informed business judgment based on all material information reasonably available to them. Consequently, a director will not be personally liable to us or our stockholders for monetary damages for breach of fiduciary duty as a director, except for liability for:
| • | any breach of the director’s duty of loyalty to us or our stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | any act related to unlawful stock repurchases, redemptions or other distributions or payment of dividends; or |
| • | any transaction from which the director derived an improper personal benefit. |
These limitations of liability do not affect the availability of equitable remedies such as injunctive relief or rescission. Our amended and restated certificate of incorporation also authorizes us to indemnify our officers, directors and other agents to the fullest extent permitted under Delaware law.
As permitted by Section 145 of the Delaware General Corporation Law, our amended and restated bylaws provide that:
| • | we may indemnify our directors, officers and employees to the fullest extent permitted by the Delaware General Corporation Law, subject to limited exceptions; |
| • | we may advance expenses to our directors, officers and employees in connection with a legal proceeding to the fullest extent permitted by the Delaware General Corporation Law, subject to limited exceptions; and |
II-1
Table of Contents
Index to Financial Statements
| • | the rights provided in our amended and restated bylaws are not exclusive. |
Our amended and restated certificate of incorporation and our amended and restated bylaws provide for the indemnification provisions described above and elsewhere herein. We have entered into separate indemnification agreements with our directors and officers that may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. These indemnification agreements generally require us, among other things, to indemnify our directors and officers against liabilities that may arise by reason of their status or service as directors or officers, other than liabilities arising from willful misconduct. These indemnification agreements also generally require us to advance any expenses incurred by the directors or officers as a result of any proceeding against them as to which they could be indemnified. In addition, we have purchased a policy of directors’ and officers’ liability insurance that insures our directors and officers against the cost of defense, settlement or payment of a judgment in some circumstances. These indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of directors and officers for liabilities, including reimbursement of expenses incurred, arising under the Securities Act of 1933, as amended, or the Securities Act.
Item 15. Recent Sales of Unregistered Securities.
The following list sets forth information as to all ViewRay and our securities sold since January 1, 2015 through October 1, 2015, which were not registered under the Securities Act. The descriptions of ViewRay issuances are historical and have not been adjusted to give effect to the Merger or the share conversion ratio pursuant to the Merger Agreement.
| 1. | In March 2012, ViewRay issued an aggregate of 1,546,472 shares of Series C convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $26.3 million to 18 accredited investors, which included 646,114 shares of Series C convertible preferred stock which were issued pursuant to the conversion of $10.3 million aggregate principal amount of convertible promissory notes. |
| 2. | In November 2012, ViewRay issued an aggregate of 388,290 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.9 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in November 2012 were exchanged for shares of Series B convertible preferred stock in our recapitalization. |
| 3. | In February 2013, ViewRay amended and restated the Series D-1 convertible preferred stock purchase agreement to issue an additional 330,608 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.0 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in February 2013 were exchanged for shares of Series B convertible preferred stock in our recapitalization. |
| 4. | In May and June 2013, ViewRay issued an aggregate of 996,021 shares of Series D-2 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $15.3 million to 27 accredited investors. The shares of Series D-2 convertible preferred stock issued in May and June 2013 were immediately exchanged for shares of Series B convertible preferred stock in our recapitalization. |
| 5. | In November 2013, ViewRay issued an aggregate of 862,064 shares of Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $15.0 million to eight accredited investors. |
| 6. | In August and November 2014, ViewRay issued convertible promissory notes for an aggregate principal amount of $10.0 million to seven accredited investors. |
| 7. | In December 2014 and January 2015, ViewRay issued an aggregate of 935,248 shares of Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $16.3 million to 10 accredited investors, including the conversion of all outstanding principal and interest under the 2014 Notes into shares of Series C convertible preferred stock. |
II-2
Table of Contents
Index to Financial Statements
| 8. | In February 2015, ViewRay issued 862,068 shares of Series C convertible preferred stock at a price per share of $17.40 for gross consideration of $15.0 million to one accredited investor. |
| 9. | In July and August 2015, we issued an aggregate of (i) 5,884,504 shares of common stock to accredited investors in the Private Placement, (ii) 1,000,005 shares of our common stock issued to former stockholders of Mirax Corp. in connection with the Merger, (iii) 31,315,579 shares of our common stock issued to former stockholders of ViewRay Technologies, Inc. in connection with the closing of the Merger and (iv) 198,760 shares of common stock issuable upon exercise of the Placement Agent Warrants. |
| 10. | We granted stock options and stock awards to employees, directors and consultants under the 2008 Plan covering an aggregate of 1,863,031 shares of common stock, at a weighted-average exercise price of $2.57 per share. Additionally, we granted stock options and stock awards to employees, directors and consultants under the 2015 Plan covering an aggregate of 1,507,147 shares of common stock, at a weighted-average exercise price of $5.00 per share. Of these, options covering an aggregate of 329,198 shares were canceled without being exercised. |
| 11. | We sold an aggregate of 69,203 shares of common stock to employees, directors and consultants for cash consideration in the aggregate amount of $153,223 upon the exercise of stock options and stock awards. |
We claimed exemption from registration under the Securities Act for the sale and issuance of securities in the transactions described in paragraphs (1)-(9) by virtue of Section 4(a)(2) of the Securities Act and/or Regulation D promulgated under the Securities Act as transactions not involving any public offering. All of the purchasers of unregistered securities for which we relied on Section 4(a)(2) and/or Regulation D represented that they were accredited investors as defined under the Securities Act. We claimed such exemption on the basis that (a) the purchasers in each case represented that they intended to acquire the securities for investment only and not with a view to the distribution thereof and that they either received adequate information about the Registrant or had access, through employment or other relationships, to such information and (b) appropriate legends were affixed to the stock certificates issued in such transactions.
We claimed exemption from registration under the Securities Act for the sales and issuances of securities in the transactions described in paragraphs (10)-(11) above under Section 4(a)(2) of the Securities Act, in that such sales and issuances did not involve a public offering, or under Rule 701 promulgated under the Securities Act, in that they were offered and sold either pursuant to written compensatory plans or pursuant to a written contract relating to compensation, as provided by Rule 701.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits. See the Exhibit Index attached to this Registration Statement, which is incorporated by reference herein.
(b) Financial Statement Schedules. Schedules not listed above have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| 1. | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| a. | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; |
| b. | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration |
II-3
Table of Contents
Index to Financial Statements
| statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| c. | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| 2. | That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of the securities at that time shall be deemed to be the initial bona fide offering thereof. |
| 3. | To remove from registration by means of a post-effective amendment any of the securities being registered that remain unsold at the termination of the offering. |
| 4. | That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§ 230.430A of Title 17 of the Code of Federal Regulations), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
| 5. | That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
| 6. | The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| a. | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§230.424 of Title 17 of the Code of Federal Regulations); |
| b. | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| c. | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| d. | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant
II-4
Table of Contents
Index to Financial Statements
has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-5
Table of Contents
Index to Financial Statements
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Amendment No. 3 to Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in Oakwood Village, Ohio, on December 30, 2015.
| VIEWRAY, INC. | ||
| By: | /s/ Chris A. Raanes | |
| Chris A. Raanes President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act, this Amendment No. 3 to Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ Chris A. Raanes Chris A. Raanes |
Director, President and Chief Executive Officer (Principal Executive Officer) |
December 30, 2015 | ||
| /s/ D. David Chandler D. David Chandler |
Chief Financial Officer (Principal Financial and Accounting Officer) |
December 30, 2015 | ||
| * Joshua Bilenker, M.D. |
Director | |||
| * David Bonita, M.D. |
Director | |||
| * Caley Castelein, M.D. |
Director | |||
| * James F. Dempsey, Ph.D. |
Director and Chief Scientific Officer | |||
| * Mark S. Gold, M.D. |
Director | |||
| * Aditya Puri |
Director | |||
| * Robert Weisskoff, Ph.D. |
Director | |||
| *By: | /s/ D. David Chandler | |
| D. David Chandler | ||
| Attorney-in-fact | ||
II-6
Table of Contents
Index to Financial Statements
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below hereby constitutes and appoints Chris A. Raanes and D. David Chandler, and each of them acting individually, as his or her true and lawful attorneys-in-fact and agents, each with full power of substitution, for him or her in any and all capacities, to sign any and all amendments to this Registration Statement, including post-effective amendments or any abbreviated registration statement and any amendments thereto filed pursuant to Rule 462(b) increasing the number of securities for which registration is sought, and to file the same, with all exhibits thereto and other documents in connection therewith, with the SEC, granting unto said attorneys-in-fact and agents, with full power of each to act alone, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully for all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or his, her or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| /s/ Brian K. Roberts Brian K. Roberts |
Director | December 30, 2015 | ||
II-7
Table of Contents
Index to Financial Statements
EXHIBIT INDEX
| Exhibit |
Description | |
| 2.1* | Agreement and Plan of Merger and Reorganization, dated as of July 23, 2015, by and among ViewRay Inc., Acquisition Sub and ViewRay Technologies, Inc. | |
| 3.1* | Amended and Restated Certificate of Incorporation of ViewRay, Inc., filed July 23, 2015. | |
| 3.2* | Amended and Restated Bylaws of ViewRay, Inc., effective as of July 23, 2015. | |
| 3.3* | Certificate of Merger of Acquisition Sub with and into ViewRay Technologies, Inc. filed July 23, 2015. | |
| 4.1* | Form of Common Stock Certificate. | |
| 4.2* | Form of Registration Rights Agreement, by and among ViewRay, Inc. and certain investors named therein. | |
| 5.1* | Opinion of Latham & Watkins LLP. | |
| 10.1* | Split-Off Agreement, dated as of July 23, 2015, by and among ViewRay, Inc., Mirax Enterprise Corp. and Dinara Akzhigitova. | |
| 10.2* | General Release Agreement, dated as of July 23, 2015, by and among ViewRay, Inc., Mirax Enterprise Corp. and Dinara Akzhigitova. | |
| 10.3* | Form of Lock-Up and No Short Selling Agreement between ViewRay, Inc., and the officers, directors and shareholders party thereto. | |
| 10.4* | Form of Securities Purchase Agreement between ViewRay, Inc., and the investors party thereto. | |
| 10.5* | Engagement Letter, dated June 9, 2015, between ViewRay, Inc. and the Placement Agents. | |
| 10.6* | Form of Placement Agent Warrant for Common Stock of ViewRay, Inc. | |
| 10.7(a)* | Office Lease, effective April 17, 2008, by and between Cleveland Industrial Portfolio, LLC and ViewRay Incorporated. | |
| 10.7(b)* | First Amendment to the Office Lease, effective April 16, 2013 by and between Cleveland Industrial Portfolio, LLC and ViewRay Incorporated. | |
| 10.7(c)* | Second Amendment to the Office Lease, effective August 15, 2014 by and between Cleveland Industrial Portfolio, LLC and ViewRay Incorporated. | |
| 10.8* | Office Lease, effective June 19, 2014, by and between BXP Research Park LP and ViewRay Incorporated. | |
| 10.9†* | Employment Agreement, effective January 18, 2013, by and between ViewRay Incorporated and Chris A. Raanes. | |
| 10.10†* | Offer Letter, effective November 11, 2010, by and between ViewRay Incorporated and D. David Chandler. | |
| 10.11†* | First Amended and Restated Offer Letter, dated October 6, 2010, by and between ViewRay Incorporated and James F. Dempsey, Ph.D. | |
| 10.12†* | Offer Letter, dated December 9, 2011, by and between ViewRay Incorporated and Michael Brandt. | |
| 10.13#* | Manufacturing and Supply Agreement, effective September 18, 2013, by and between ViewRay Incorporated and Japan Superconductor Technology, Inc. | |
| 10.14(a)# | Development and Supply Agreement, effective May 29, 2008, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
Table of Contents
Index to Financial Statements
| Exhibit |
Description | |
| 10.14(b)#* | Amendment No. 1 to the Development and Supply Agreement, effective December 1, 2009, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(c)#* | Amendment No. 2 to the Development and Supply Agreement, effective May 4, 2010, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(d)#* | Amendment No. 3 to the Development and Supply Agreement, effective February 9, 2011, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(e)#* | Amendment No. 4 to the Development and Supply Agreement, effective May 11, 2012, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(f)#* | Amendment No. 5 to the Development and Supply Agreement, effective May 30, 2012, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(g)#* | Amendment No. 6 to the Development and Supply Agreement, effective February 21, 2014, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.15#* | Cobalt-60 Source Supply and Removal Agreement, effective December 19, 2013, by and between ViewRay Incorporated and Best Theratronics, Ltd. | |
| 10.16#* | Development and Supply Agreement, effective June 24, 2009, by and between ViewRay Incorporated and Manufacturing Sciences Corporation. | |
| 10.17(a)#* | Development and Supply Agreement, effective July 9, 2009, by and between ViewRay Incorporated and Tesla Engineering Limited. | |
| 10.17(b)#* | Amendment No. 1 to the Development and Supply Agreement, effective January 20, 2015, by and between ViewRay Incorporated and Tesla Engineering Limited. | |
| 10.18#* | Development and Supply Agreement, effective July 2, 2010, by and between ViewRay Incorporated and PEKO Precision Products, Inc. | |
| 10.19(a)#* | Amended and Restated Joint Development and Supply Agreement, effective May 15, 2008, by and between ViewRay Incorporated and 3D Line GmbH. | |
| 10.19(b)#* | Amendment No. 1 to the Amended and Restated Joint Development and Supply Agreement, effective August 13, 2008, by and between ViewRay Incorporated and Euromechanics Medical GmbH. | |
| 10.19(c)#* | Amendment No. 2 to the Amended and Restated Joint Development and Supply Agreement, effective November 27, 2009, by and between ViewRay Incorporated and Euromechanics Medical GmbH. | |
| 10.20#* | Development and Supply Agreement, effective June 1, 2010, by and between ViewRay Incorporated and Quality Electrodynamics, LLC. | |
| 10.21(a)#* | Standard Exclusive License Agreement with Sublicensing Terms, effective December 15, 2004, by and between ViewRay Incorporated and the University of Florida Research Foundation, Inc. | |
| 10.21(b)#* | Amendment No. 1 to the Standard Exclusive License Agreement with Sublicensing Terms, effective December 6, 2007, by and between ViewRay Incorporated and the University of Florida Research Foundation, Inc. | |
| 10.22#* | Term Loan Agreement, effective June 26, 2015, by and among ViewRay Incorporated, the Subsidiary Guarantors (as defined therein), Capital Royalty Partners II L.P., Capital Royalty Partners II—Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Parallel Investment Opportunities Partners II L.P. | |
| 10.23* | Warrant Agreement, effective December 16, 2013, by and between ViewRay Incorporated and Hercules Technology III, L.P. | |
Table of Contents
Index to Financial Statements
| Exhibit |
Description | |
| 10.24(a)†* | ViewRay Incorporated 2008 Stock Incentive Plan. | |
| 10.24(b)†* | Form of Incentive Stock Option and Reverse Vesting Agreement (Change of Control) under the 2008 Plan. | |
| 10.24(c)†* | Form of Incentive Stock Option and Reverse Vesting Agreement under the 2008 Plan. | |
| 10.24(d)†* | Form of Nonstatutory Stock Option and Reverse Vesting Agreement under the 2008 Plan. | |
| 10.25†* | Contingent Equity Agreement, effective January 8, 2008, by and among ViewRay Incorporated, James F. Dempsey, Ph.D., Russell S. Donda, Jim Carnall and William Wells. | |
| 10.26(a)†* | ViewRay, Inc. 2015 Equity Incentive Award Plan. | |
| 10.26(b)†* | Form of Option Agreement under the 2015 Plan. | |
| 10.26(c)†* | Form of Restricted Stock Agreement under the 2015 Plan. | |
| 10.26(d)†* | Form of Restricted Stock Unit Agreement under the 2015 Plan. | |
| 10.27†* | Form of Indemnification Agreement for directors and executive officers. | |
| 10.28†* | Agreement, effective June 11, 2008, by and among ViewRay Incorporated, James F. Dempsey, Ph.D., William W. Wells, James D. Carnall and Russell S. Donda. | |
| 10.29†* | ViewRay, Inc. 2015 Employee Stock Purchase Plan. | |
| 10.30†* | Offer Letter, dated April 30, 2015, by and between ViewRay, Inc. and Doug Keare. | |
| 23.1 | Consent of Deloitte & Touche LLP. | |
| 23.2* | Consent of Latham & Watkins LLP (contained in Exhibit 5.1). | |
| 24.1 | Power of Attorney (previously included on the signature page of Registrant’s Registration Statement on Form S-1, filed on October 9, 2015). | |
| 101.INS | XBRL Instance Document. | |
| 101.SCH | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document. | |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document. | |
| * | Filed previously. |
| † | Management contract or compensatory plan or arrangement. |
| # | Confidential treatment granted. Portions of this exhibit (indicated by asterisks) have been omitted and this exhibit has been filed separately with the SEC. |
