Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - CATALYST BIOSCIENCES, INC. | d106627d8k.htm |
Exhibit 99.1
|
|
Exhibit 99.1
Catalyst Biosciences
Exceptional Science. Essential Medicines.
Company Overview December 2015
| 1 |
|
|
|
Forward Looking Statements
This presentation includes forward-looking statements relating to the Catalyst Biosciences, Inc. (the “Company”). Forward-looking statements include statements about the potential markets for the Company’s product candidates, the potential advantages of the Company’s product candidates, product development plans and timelines, potential safety and efficacy of the Company’s product candidates, potential sales of product candidates, if approved, the Company’s intellectual property and any statement of belief or assumptions underlying any of the foregoing. These statements reflect the current views of the Company’s senior management with respect to future events. Forward-looking statements address matters that involve risks and uncertainties, such as the timing of, costs associated with and outcomes of development, clinical and regulatory activities, risks associated with third-party arrangements, including the risk that Catalyst must negotiate with Pfizer about obtaining manufacturing technology and know-how related to CB 813d, potential adverse effects arising from the testing or use of the Company’s drug candidates, risks related to the Company’s ability to develop, manufacture and commercialize product candidates, to obtain regulatory approval of product candidates and to obtain marketplace acceptance of product candidates, to avoid infringing patents held by other parties and to secure and defend patents of the Company, and to manage and obtain capital, including through any future financing or the conversion of outstanding convertible promissory notes. Further information regarding these and other risks is included in the Company’s Form 10-Q for the quarter ending September 30, 2015 filed with the Securities and Exchange Commission on November 5, 2015, under the heading “Risk Factors.
| 2 |
|
|
|
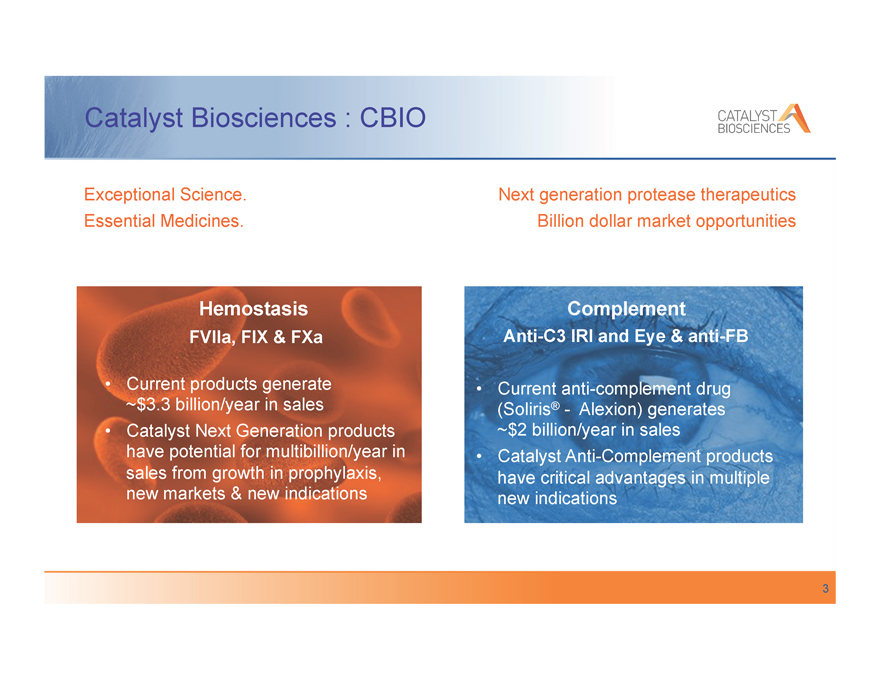
Catalyst Biosciences : CBIO
Exceptional Science. Essential Medicines.
Hemostasis
FVIIa, FIX & FXa
, Current products generate
~$3.3 billion/year in sales
, Catalyst Next Generation products have potential for multibillion/year in sales from growth in prophylaxis, new markets & new indications
Next generation protease therapeutics Billion dollar market opportunities
Complement
Anti-C3 IRI and Eye & anti-FB
, Current anti-complement drug (Soliris®— Alexion) generates
~$2 billion/year in sales
, Catalyst Anti-Complement products have critical advantages in multiple new indications
| 3 |
|
|
|
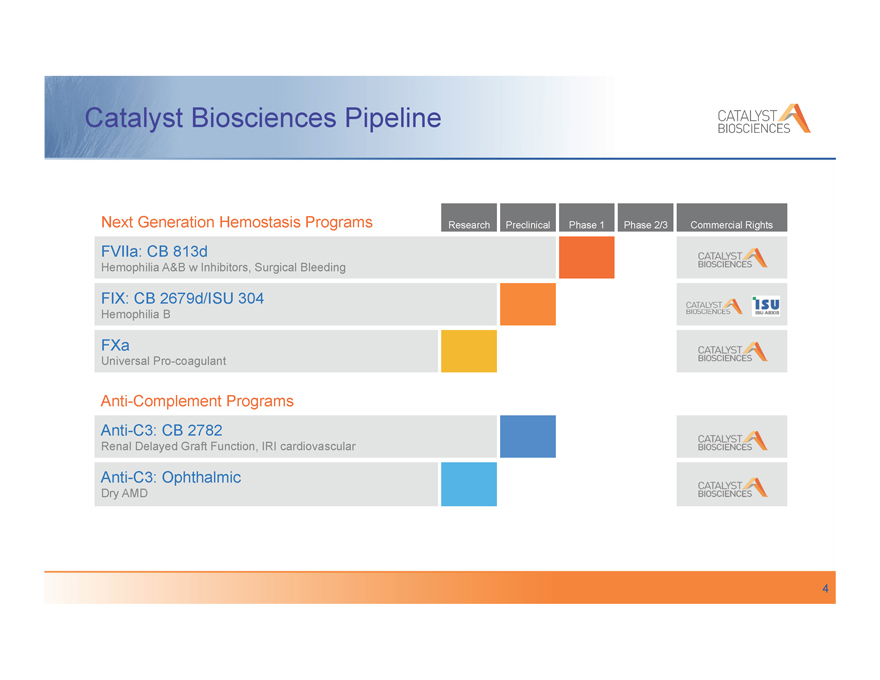
Catalyst Biosciences Pipeline
Next Generation Hemostasis Programs Research Preclinical Phase 1 Phase 2/3 Commercial Rights
FVIIa: CB 813d
Hemophilia A&B w Inhibitors, Surgical Bleeding
FIX: CB 2679d/ISU 304
Hemophilia B
FXa
Universal Pro-coagulant
Anti-Complement Programs
Anti-C3: CB 2782
Renal Delayed Graft Function, IRI cardiovascular
Anti-C3: Ophthalmic
Dry AMD
| 4 |
|
|
|
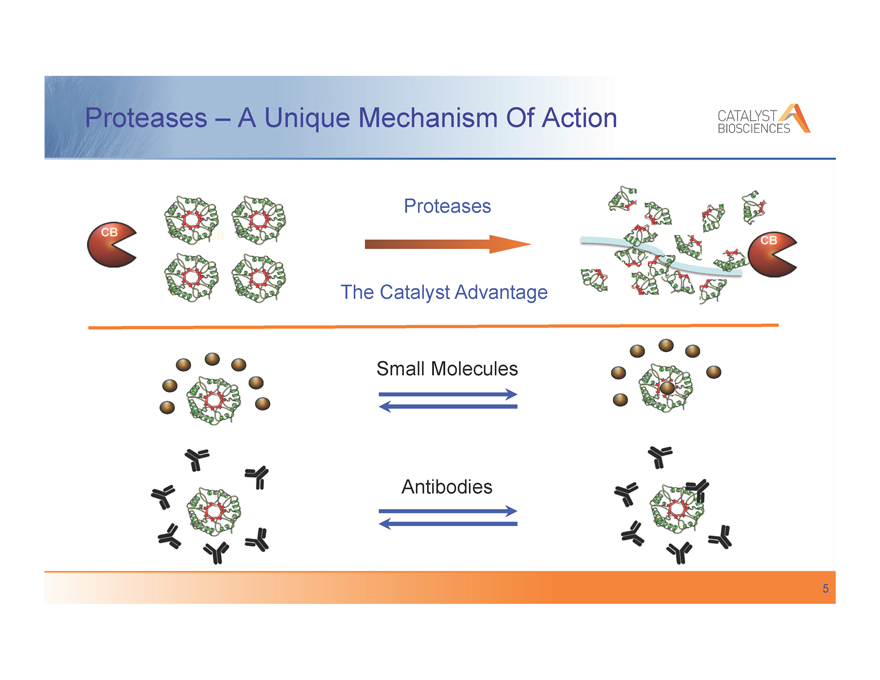
Proteases – A Unique Mechanism Of Action
Proteases
The Catalyst Advantage
Small Molecules
Antibodies
| 5 |
|
|
|
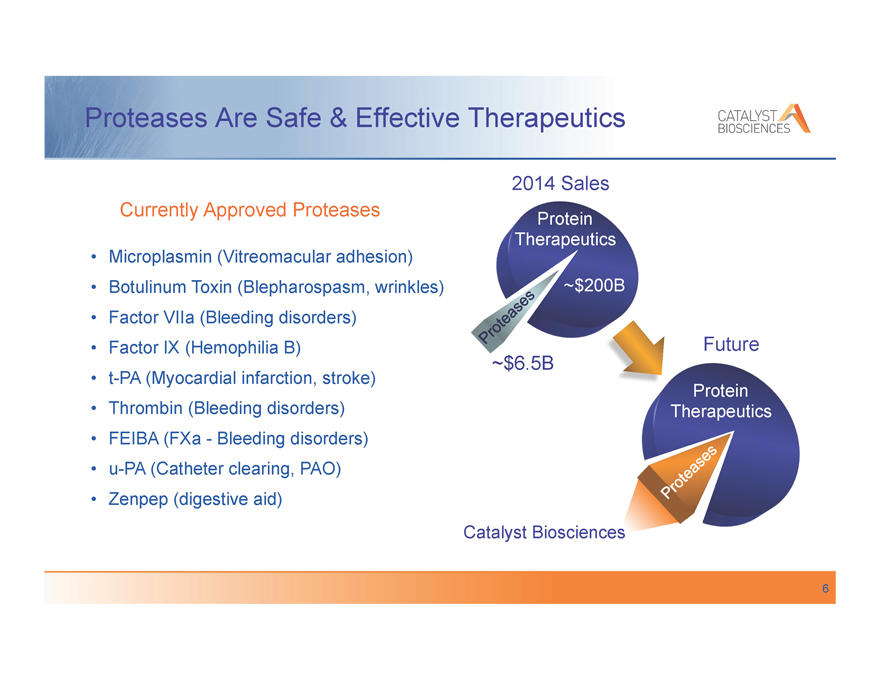
Proteases Are Safe & Effective Therapeutics
Currently Approved Proteases
Microplasmin (Vitreomacular adhesion) Botulinum Toxin (Blepharospasm, wrinkles) Factor VIIa (Bleeding disorders) Factor IX (Hemophilia B) t-PA (Myocardial infarction, stroke) Thrombin (Bleeding disorders) FEIBA (FXa—Bleeding disorders) u-PA (Catheter clearing, PAO) Zenpep (digestive aid)
2014 Sales
Protein
Therapeutics
~$200B
Future
~$6.5B
Protein
cs
Catalyst Biosciences
| 6 |
|
|
|
Hemophilia Overview
Disease
, Hereditary, chronic condition—orphan disease with a growing population
, Two primary forms: hemophilia A (FVIII) and hemophilia B (FIX), combined ~400,000 patients WW*
, Patients have complete or severe deficiency of a clotting factor (receive FVIII or FIX) needed to form stable blood clots or have antibodies (inhibitors) against their replacement factor (receive FVIIa or FEIBA)
, Internal bleeding in joints causes substantial pain, inflammation, joint damage, and loss of mobility
*Bolton-Maggs & Pasi, The Lancet 2003, v361 p1831
Market Characteristics
, Recombinant “replacement” factors, FVIII or FIX, or FVIIa/FEIBA are the dominant modes of treatment
, Drugs administered intravenously by patients or caregivers
, P1 trials are in hemophilia patients with PD efficacy endpoints
, Recent registration trials have been single P2/3s
, Small sales force requirement
Key Unmet Needs
, Products that enable prophylactic treatment, prevent internal joint damage
, Faster-acting and more efficacious products for bleeds
, One product that does both
7
|
|
FVIIa Program: CB 813d
, Current FVIIa market ~$1.5B (NovoSeven®)
, Leading next-generation FVIIa in the clinic
, Significant improvements (6-9 fold) in potency, duration of effect and an improved therapeutic index vs NovoSeven in multiple pre-clinical animal models
, Phase I in severe hemophilia patients
(± inhibitors) demonstrated Proof-of-Mechanism (PoM) with excellent safety and tolerability
, Safe and well tolerated, no serious TEAEs
, Improved correction of PT and aPTT (vs NovoSeven) for up to 48 h
http://clinicaltrials.gov/ct2/show/NCT01439971,term=FVIIa&rank=2
Activated blood coagulation Factor VII
| 8 |
|
|
|
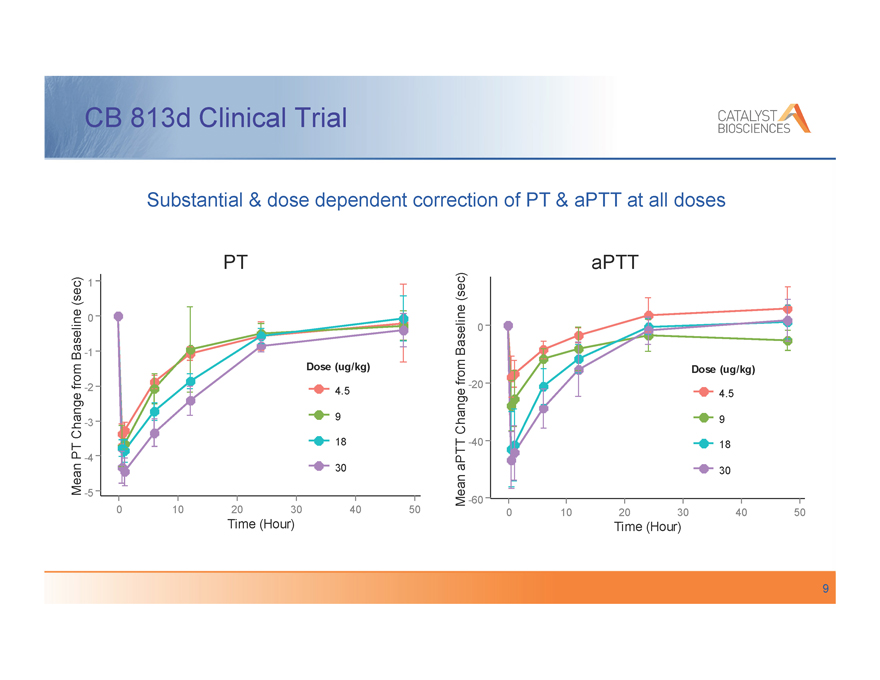
CB 813d Clinical Trial
Substantial & dose dependent correction of PT & aPTT at all doses
PT
(sec) 1
0
Baseline -1
Dose (ug/kg)
from -2 4.5
Change -3 9
18
PT -4
Mean 30
-5
0 10 20 30 40 50
Time (Hour)
aPTT
(sec)
Baseline 0
from Dose (ug/kg)
-20
4.5
Change 9
aPTT -40 18
30
Mean -60
0 10 20 30 40 50
Time (Hour)
9
|
|
CB 813d Clinical Trial
, Single doses up to 30 µg/kg were very well tolerated when administered to 25 hemophilia A and B patients in the non-bleeding state
, There were no instances of thrombosis or bleeding
, Evidence of pharmacologic activity was observed with dose-dependent changes of PT, aPTT, PF1+2, and TGA for up to 48 hours
, The terminal half-life of CB 813d was approximately 3.5 hours and was similar across all dose groups
, The results for safety and pharmacologic activity support further clinical development of CB 813d for treatment of individuals with hemophilia and inhibitors to FVIII or FIX
, Phase 2/3 trial anticipated to begin in Q1 2017
10
|
|
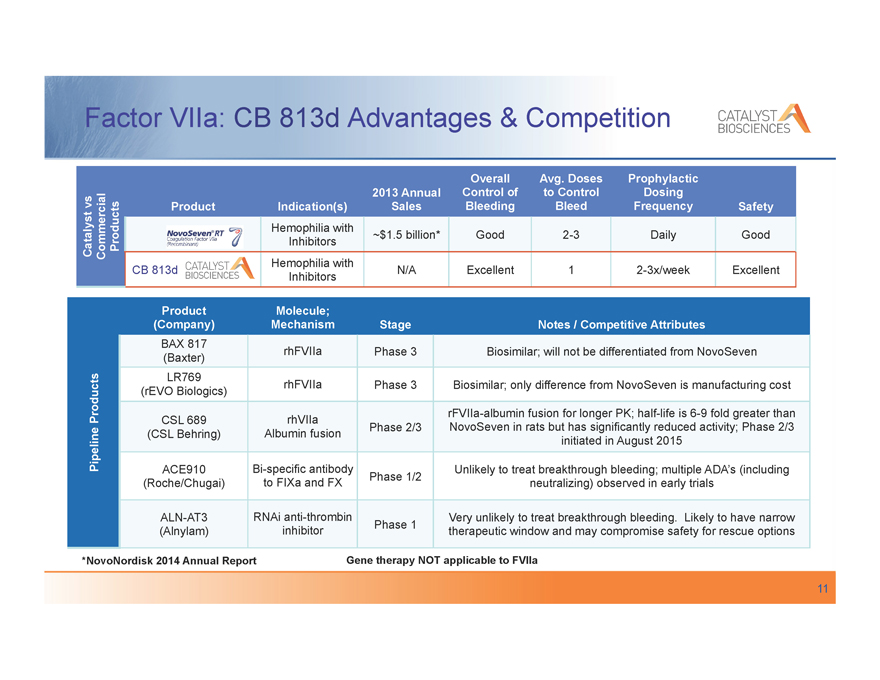
Factor VIIa: CB 813d Advantages & Competition
Overall Avg. Doses Prophylactic
2013 Annual Control of to Control Dosing
vs Product Indication(s) Sales Bleeding Bleed Frequency Safety
Hemophilia with
Catalyst Commercial Products Inhibitors ~$1.5 billion* Good 2-3 Daily Good
Hemophilia with
CB 813d N/A Excellent 1 2-3x/week Excellent
Inhibitors
Product Molecule;
(Company) Mechanism Stage Notes / Competitive Attributes
BAX 817
(Baxter) rhFVIIa Phase 3 Biosimilar; will not be differentiated from NovoSeven
LR769
(rEVO Biologics) rhFVIIa Phase 3 Biosimilar; only difference from NovoSeven is manufacturing cost
Products rFVIIa-albumin fusion for longer PK; half-life is 6-9 fold greater than
CSL 689 rhVIIa
(CSL Behring) Albumin fusion Phase 2/3 NovoSeven in rats but has significantly reduced activity; Phase 2/3
Pipeline initiated in August 2015
ACE910 Bi-specific antibody Unlikely to treat breakthrough bleeding; multiple ADA’s (including
Phase 1/2
(Roche/Chugai) to FIXa and FX neutralizing) observed in early trials
ALN-AT3 RNAi anti-thrombin Very unlikely to treat breakthrough bleeding. Likely to have narrow
Phase 1
(Alnylam) inhibitor therapeutic window and may compromise safety for rescue options
*NovoNordisk 2014 Annual Report Gene therapy NOT applicable to FVIIa
11
|
|
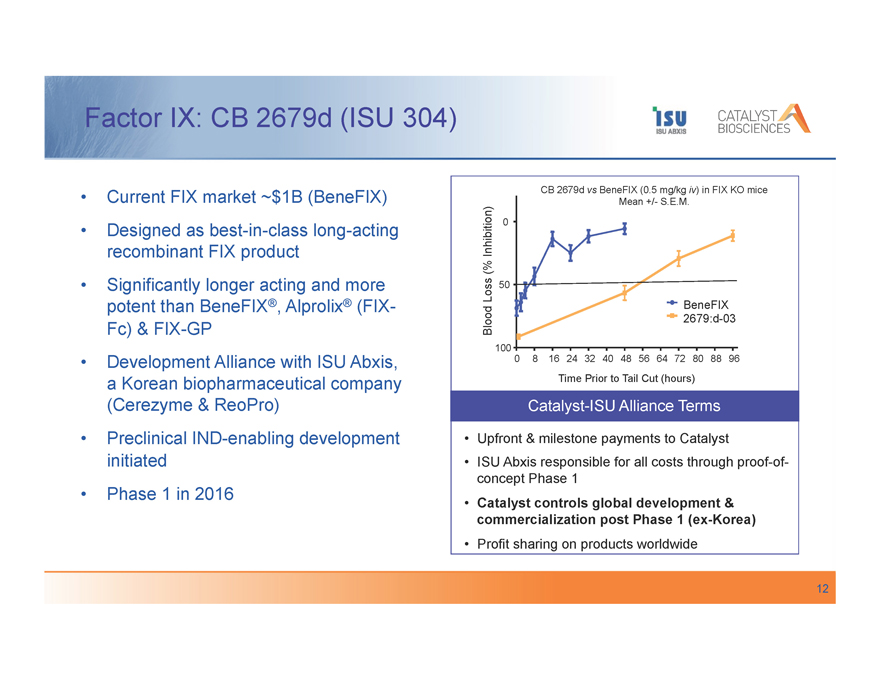
Factor IX: CB 2679d (ISU 304)
Current FIX market ~$1B (BeneFIX) Designed as best-in-class long-acting recombinant FIX product Significantly longer acting and more potent than BeneFIX®, Alprolix® (FIX-Fc) & FIX-GP
Development Alliance with ISU Abxis, a Korean biopharmaceutical company (Cerezyme & ReoPro) Preclinical IND-enabling development initiated Phase 1 in 2016
CB 2679d vs BeneFIX (0.5 mg/kg iv) in FIX KO mice
Mean +/- S.E.M.
Inhibition) 0
(%
Loss 50
BeneFIX
Blood 2679:d-03
100
0 8 16 24 32 40 48 56 64 72 80 88 96
Time Prior to Tail Cut (hours)
Catalyst-ISU Alliance Terms
, Upfront & milestone payments to Catalyst
, ISU Abxis responsible for all costs through proof-of-concept Phase 1
, Catalyst controls global development & commercialization post Phase 1 (ex-Korea)
, Profit sharing on products worldwide
12
|
|
Anti-Complement Opportunity
, Complement targets are biologically & clinically validated
–, KO mice studies
–, Human genetics
–, Approved drug (Soliris®) for PNH & aHUS;
–, Positive P2 data for Dry Age-Related Macular Degeneration (AMD) – Geographic Atrophy (GA)
, Multiple acute indications mediated by complement driven ischemia reperfusion injury
–, Transplant rejection: Initial indication – anti-C3 to prevent Renal Delayed Graft Function (DGF)
–, Cardiovascular: CABG, MI & Stroke (label expansion potential for a DGF drug)
, Chronic indications
–, Ocular: Initial indication – anti-C3 (ocular) to slow the progression of GA in Dry AMD
–, Asthma
–, Autoimmune
13
|
|
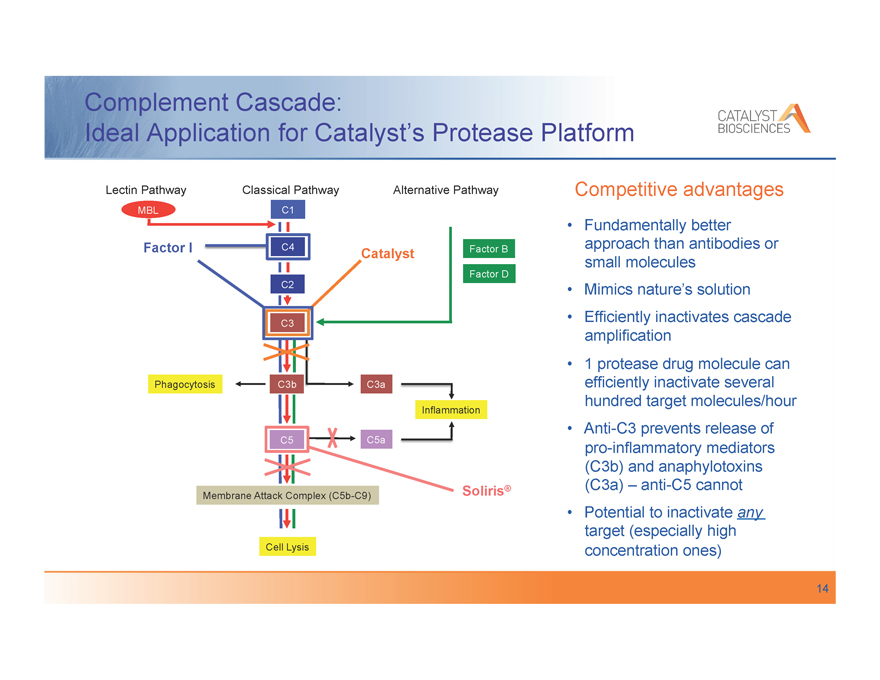
Complement Cascade:
Ideal Application for Catalyst’s Protease Platform
Lectin Pathway Classical Pathway Alternative Pathway
MBL C1
Factor I C4 Catalyst Factor B
Factor D
C2 C2
C3
Phagocytosis C3b C3a
Inflammation
C5 C5a
Membrane Attack Complex (C5b-C9) Soliris®
Cell Lysis
Competitive advantages
Fundamentally better approach than antibodies or small molecules Mimics nature’s solution Efficiently inactivates cascade amplification 1 protease drug molecule can efficiently inactivate several hundred target molecules/hour Anti-C3 prevents release of pro-inflammatory mediators (C3b) and anaphylotoxins (C3a) – anti-C5 cannot Potential to inactivate any target (especially high concentration ones)
14
|
|
Anti-C3 for Dry AMD
, Advanced dry AMD, or geographic atrophy
(GA), leads to loss of RPE photoreceptors,
blindness
, No approved drugs
, Global wet AMD market is >$4 billion
annually
, GA prevalence is equivalent to wet AMD
, Strong genetic evidence for complement in
pathogenesis of dry AMD*
, Complement is the only validated anti-GA
target
, Roche anti-Factor D antibody @ 10 mg/eye
intravitreal injection showed 20-44% inhibition
of GA progression with monthly dosing;
dosing every 2 months failed
C3 is the “best target” in the complement cascade
-, Targeting either individual pathways, e.g., alternative (Factor D) or C5 appears to be “leaky”, consequently limiting efficacy
Efficient inactivation of C3 expected to provide greater efficacy compared with competing anti-Factor D or anti-C5 strategies Catalytic turn-over of target expected to support efficacious dosing every 2 months or less frequently Less than 40% inhibition of GA progression over 18 months would be acceptable if dosing was less frequent than monthly
*Science, April 2005; JAMA, July 2006; NEJM, July, 2007; and others
15
|
|
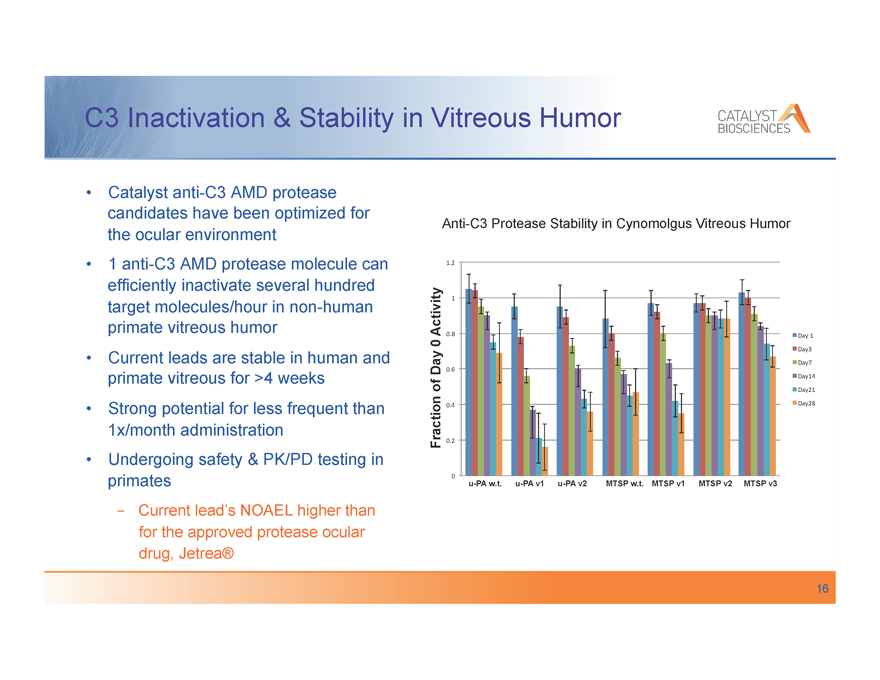
C3 Inactivation & Stability in Vitreous Humor
Catalyst anti-C3 AMD protease candidates have been optimized for the ocular environment 1 anti-C3 AMD protease molecule can efficiently inactivate several hundred target molecules/hour in non-human primate vitreous humor Current leads are stable in human and primate vitreous for >4 weeks Strong potential for less frequent than 1x/month administration Undergoing safety & PK/PD testing in primates
-, Current lead’s NOAEL higher than for the approved protease ocular drug, Jetrea®
Anti-C3 Protease Stability in Cynomolgus Vitreous Humor
1.2”
Activity 1”
0.8” Day“1”
0 Day3”
Day7”
Day 0.6”
Day14”
of Day21”
0.4” Day28”
Fraction 0.2”
0”
u-PA w.t. u-PA v1 u-PA v2 MTSP w.t. MTSP v1 MTSP v2 MTSP v3
16
|
|
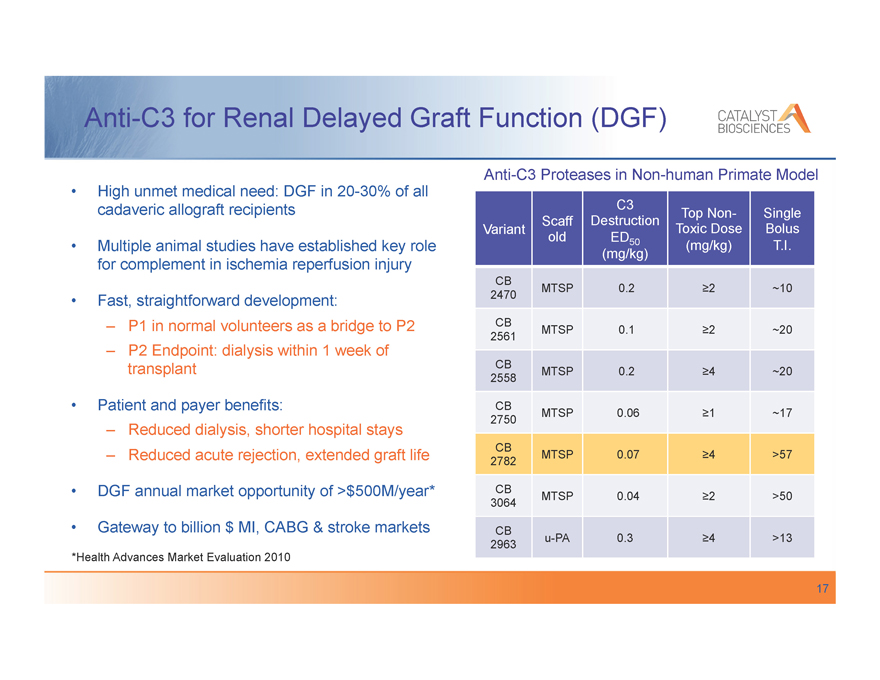
Anti-C3 for Renal Delayed Graft Function (DGF)
High unmet medical need: DGF in 20-30% of all cadaveric allograft recipients
Multiple animal studies have established key role for complement in ischemia reperfusion injury
Fast, straightforward development:
–, P1 in normal volunteers as a bridge to P2
–, P2 Endpoint: dialysis within 1 week of transplant
Patient and payer benefits:
–, Reduced dialysis, shorter hospital stays
–, Reduced acute rejection, extended graft life DGF annual market opportunity of >$500M/year* Gateway to billion $ MI, CABG & stroke markets
*Health Advances Market Evaluation 2010
Anti-C3 Proteases in Non-human Primate Model
C3
Scaff Destruction Top Non- Single
Variant Toxic Dose Bolus
old ED50 (mg/kg) T.I.
(mg/kg)
CB
2470 MTSP 0.2 ,2 ~10
CB
2561 MTSP 0.1 ,2 ~20
CB
2558 MTSP 0.2 ,4 ~20
CB
2750 MTSP 0.06 ,1 ~17
CB
2782 MTSP 0.07 ,4 >57
CB
3064 MTSP 0.04 ,2 >50
CB
2963 u-PA 0.3 ,4 >13
17
|
|
Intellectual Property
Comprehensive protection of all programs & protease engineering technologies
, Factor VIIa: Worldwide patents encompassing clinical candidate(s) and uses thereof, granted and pending, providing coverage through 2029-2031
, Factor IX: Worldwide patents (including composition of matter) granted and pending providing coverage through 2031
, Factor Xa: Worldwide patents (including composition of matter) filed and pending providing coverage through 2033
, Anti-complement programs: Current granted and pending worldwide, patents (including composition of matter) providing coverage through 2025, with new material >2035
, Technology platform: Worldwide issued and pending patents covering multiple novel protease screening and discovery technologies with through 2026
18
|
|
Catalyst Milestones
, 2016
–, Receive ISU FIX milestones
–, Publish data from the FVIIa, FIX, and anti-complement programs
–, Initiate CB 2679d FIX Phase 1 in hemophilia B patients
–, Demonstrate bi-monthly dosing feasibility in the Dry AMD program
–, Manufacture CB 813d FVIIa for P2/3 trial
–, Report initial Phase 1 proof-of-mechanism efficacy and safety data for CB 2679d FIX in hemophilia B patients
, 2017
–, Initiate CB 813d FVIIa P2/3 trial in hemophilia A + B inhibitor patients
–, Complete CB 2679d FIX Phase 1 in hemophilia B patients
–, Report on-demand efficacy and multi-dose safety in CB 813d FVIIa trial
19
|
|
Catalyst Biosciences Investor Highlights—CBIO
, Clinical Stage Public Protease-Based Hemostasis and Anti-Complement Company
–, Design of improved, second generation proteases: FVIIa & FIX
–, Proprietary platform that creates novel proteases: Anti-complement
, Leading next generation, long-acting Factor VIIa for hemophilia A/B inhibitor patients in ~$1.5B market with significant growth potential
–, Proof of mechanism, safety & tolerability demonstrated in hemophilia patients
–, Phase 1 Clinical Data presented at ISTH in June 2015
–, Phase 2/3 trial to initiate in Q1 2017
, Best-in-class Factor IX in hemophilia B; fully-funded to clinical Proof of Mechanism in late 2016
, Three additional programs
–, Highly differentiated, clinically-validated anti-complement approach to multibillion dollar Dry AMD market
–, Novel, anti-complement orphan program (renal delayed graft function) ready for IND-enabling studies
–, Best-in-class Factor Xa for hemophilia and surgical bleeding with strong pre-clinical efficacy
20
|
|
Catalyst Biosciences
Exceptional Science. Essential Medicines.
www.catalystbiosciences.com
21