Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PUMA BIOTECHNOLOGY, INC. | d104127d8k.htm |
| Exhibit 99.1
|
An Open-Label Study to Characterize the Incidence and Severity of Diarrhea in Patients with Early-Stage HER2+ Breast Cancer Treated with Neratinib and Intensive Loperamide Prophylaxis
Copyright 2015 Puma Biotechnology
1
|
|
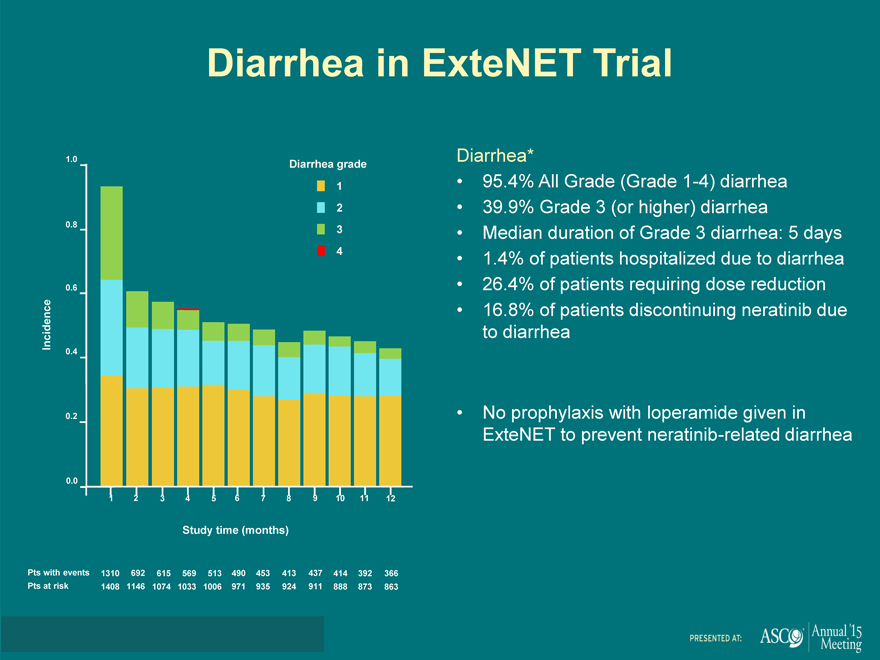
Diarrhea in ExteNET Trial
1.0 Diarrhea grade
1
2
0.8 3
4
0.6
Incidence 0.4
0.2
0.0
1 2 3 4 5 6 7 8 9 10 11 12
Study time (months)
Pts with events 1310 692 615 569 513 490 453 413 437 414 392 366
Pts at risk 1408 1146 1074 1033 1006 971 935 924 911 888 873 863
Diarrhea*
95.4% All Grade (Grade 1-4) diarrhea
39.9% Grade 3 (or higher) diarrhea
Median duration of Grade 3 diarrhea: 5 days
1.4% of patients hospitalized due to diarrhea
26.4% of patients requiring dose reduction
16.8% of patients discontinuing neratinib due
to diarrhea
No prophylaxis with loperamide given in
ExteNET to prevent neratinib-related diarrhea
|
|
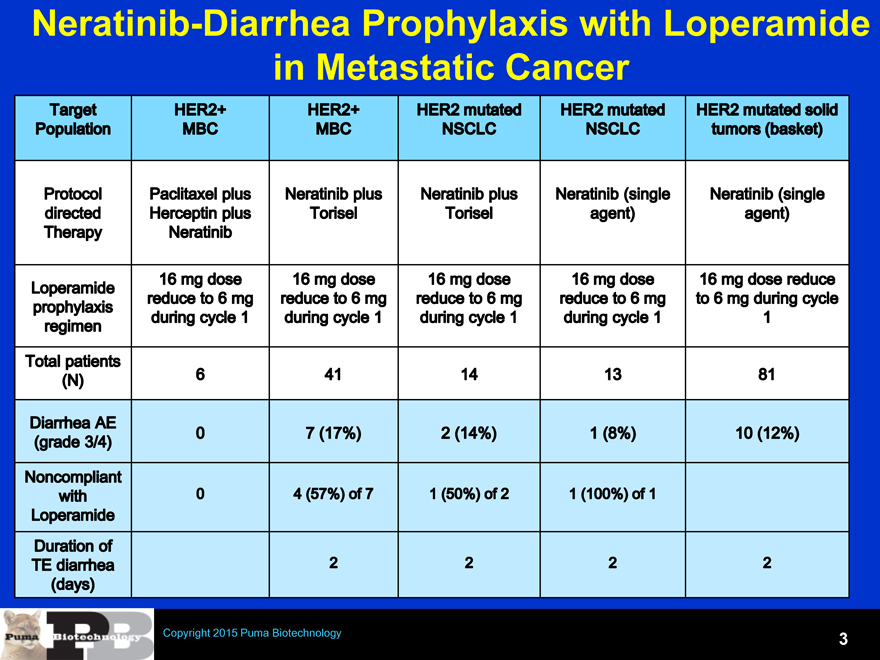
Neratinib-Diarrhea Prophylaxis with Loperamide in Metastatic Cancer
Copyright 2015 Puma Biotechnology
3
|
|
Phase II Open-Label Study to Characterize the Incidence and Severity of Diarrhea in Patients with Early-Stage HER2+ Breast Cancer Treated with Neratinib and Intensive Loperamide Prophylaxis
Goal: Investigate the use of loperamide given prophylactically to reduce neratinib related diarrhea in patients treated for extended adjuvant HER2 positive breast cancer
Copyright 2015 Puma Biotechnology
4
|
|
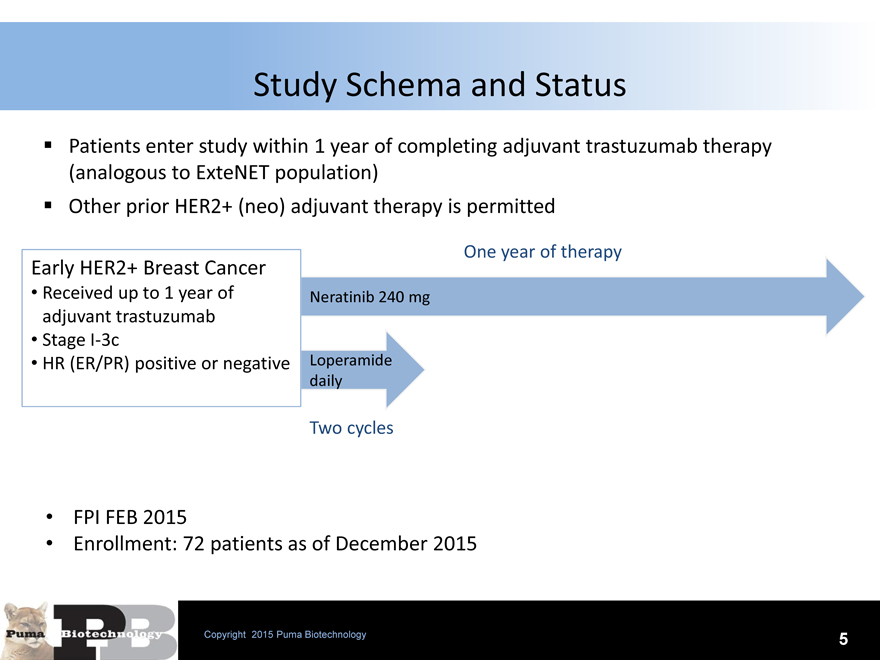
Study Schema and Status
Patients enter study within 1 year of completing adjuvant trastuzumab therapy (analogous to ExteNET population) Other prior HER2+ (neo) adjuvant therapy is permitted
Early HER2+ Breast Cancer
Received up to 1 year of adjuvant trastuzumab
Stage I-3c
HR (ER/PR) positive or negative
One year of therapy
Neratinib 240 mg
Loperamide
daily
Two cycles
FPI FEB 2015
Enrollment: 72 patients as of December 2015
Copyright 2015 Puma Biotechnology
5
|
|
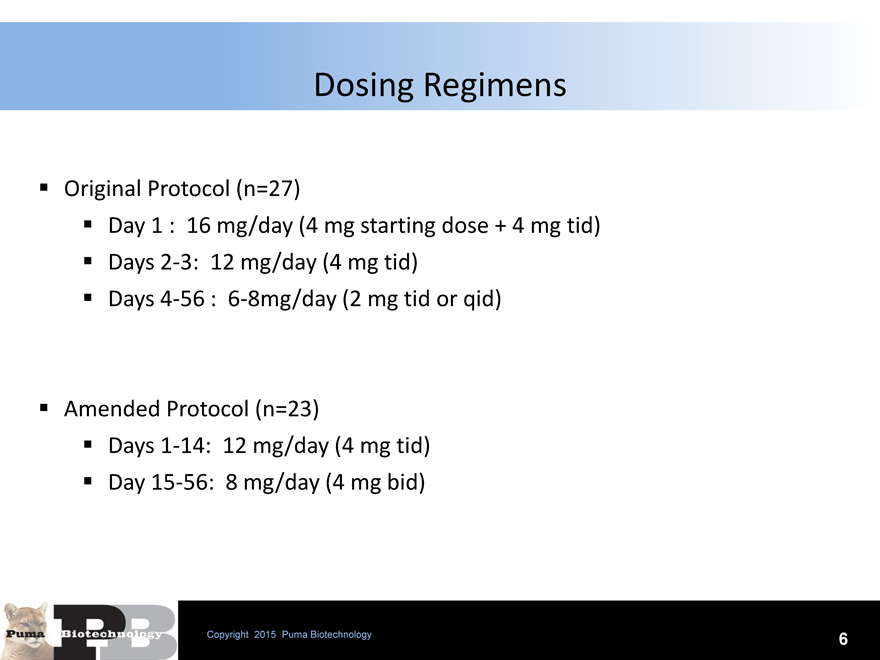
Dosing Regimens
Original Protocol (n=27)
Day 1 : 16 mg/day (4 mg starting dose + 4 mg tid) Days 2-3: 12 mg/day (4 mg tid) Days 4-56 : 6-8mg/day (2 mg tid or qid)
Amended Protocol (n=23)
Days 1-14: 12 mg/day (4 mg tid) Day 15-56: 8 mg/day (4 mg bid)
Copyright 2015 Puma Biotechnology
6
|
|
Study Preliminary Results
Copyright 2015 Puma Biotechnology
7
|
|
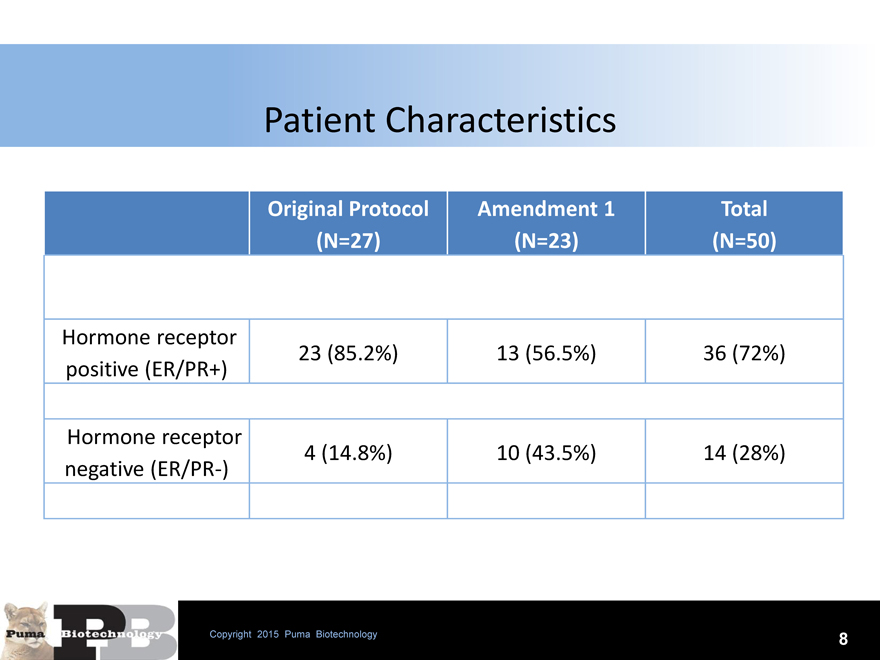
Patient Characteristics
Original Protocol Amendment 1 Total
(N=27)(N=23)(N=50)
Hormone receptor
23 (85.2%) 13 (56.5%) 36 (72%)
positive (ER/PR+)
Hormone receptor
4 (14.8%) 10 (43.5%) 14 (28%)
negative (ER/PR-)
Copyright 2015 Puma Biotechnology
8
|
|
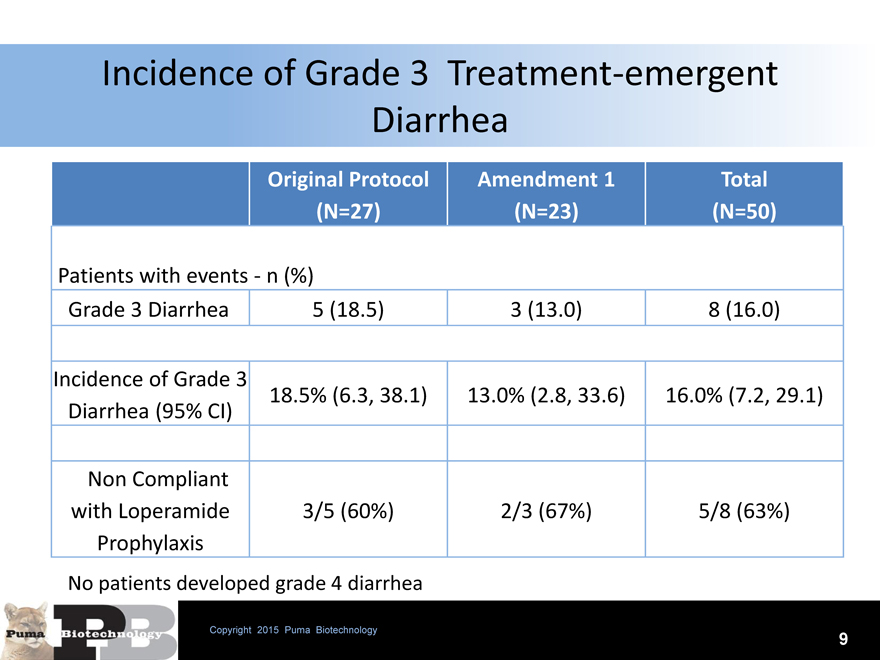
Incidence of Grade 3 Treatment-emergent Diarrhea
Original Protocol Amendment 1 Total
(N=27)(N=23)(N=50)
Patients with events—n (%)
Grade 3 Diarrhea 5 (18.5) 3 (13.0) 8 (16.0)
Incidence of Grade 3
18.5% (6.3, 38.1) 13.0% (2.8, 33.6) 16.0% (7.2, 29.1)
Diarrhea (95% CI)
Non Compliant
with Loperamide 3/5 (60%) 2/3 (67%) 5/8 (63%)
Prophylaxis
No patients developed grade 4 diarrhea
Copyright 2015 Puma Biotechnology
9
|
|
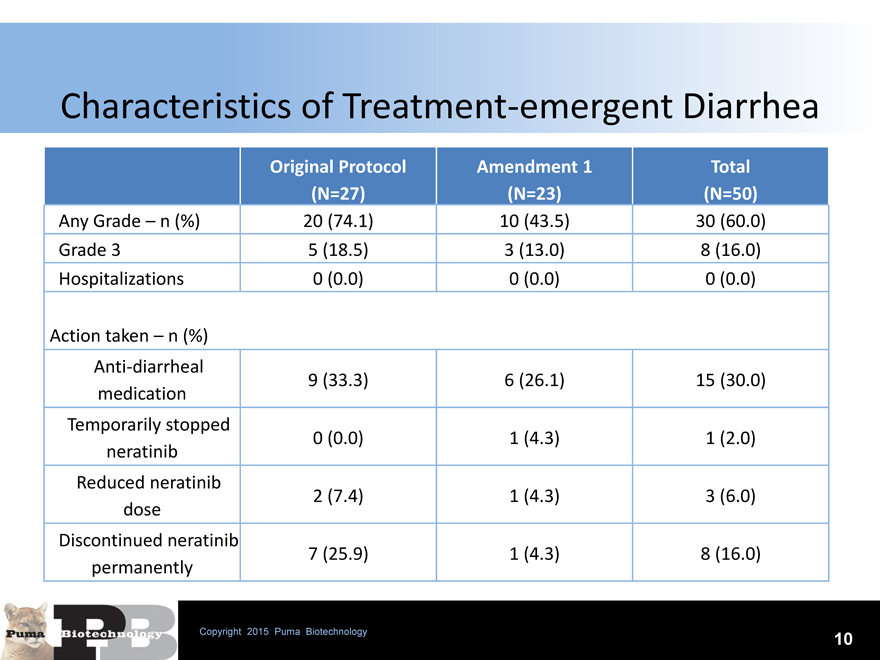
Characteristics of Treatment-emergent Diarrhea
Original Protocol Amendment 1 Total
(N=27)(N=23)(N=50)
Any Grade – n (%) 20 (74.1) 10 (43.5) 30 (60.0)
Grade 3 5 (18.5) 3 (13.0) 8 (16.0)
Hospitalizations 0 (0.0) 0 (0.0) 0 (0.0)
Action taken – n (%)
Anti-diarrheal
9 (33.3) 6 (26.1) 15 (30.0)
medication
Temporarily stopped
0 (0.0) 1 (4.3) 1 (2.0)
neratinib
Reduced neratinib
2 (7.4) 1 (4.3) 3 (6.0)
dose
Discontinued neratinib
7 (25.9) 1 (4.3) 8 (16.0)
permanently
Copyright 2015 Puma Biotechnology
10
|
|
Copyright 2015 Puma Biotechnology
11
|
|
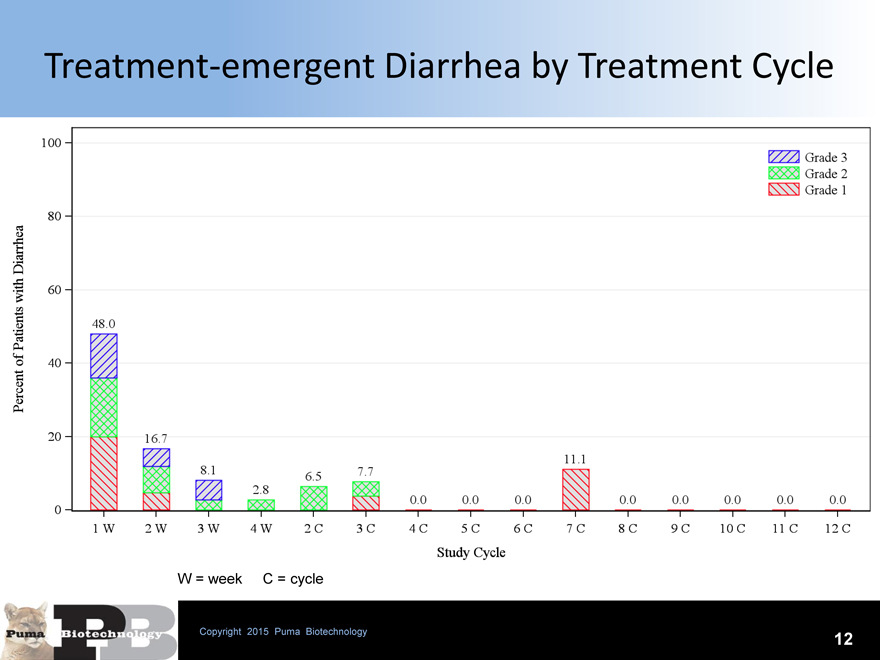
Treatment-emergent Diarrhea by Treatment Cycle
W = week C = cycle
Copyright 2015 Puma Biotechnology
12
|
|
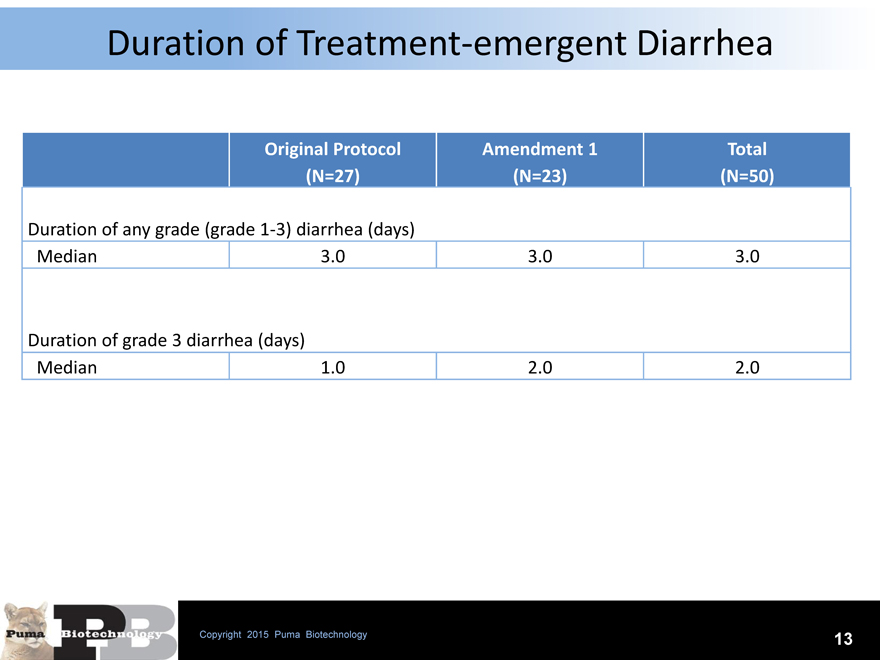
Duration of Treatment-emergent Diarrhea
Original Protocol Amendment 1 Total
(N=27)(N=23)(N=50)
Duration of any grade (grade 1-3) diarrhea (days)
Median 3.0 3.0 3.0
Duration of grade 3 diarrhea (days)
Median 1.0 2.0 2.0
Copyright 2015 Puma Biotechnology
13
|
|
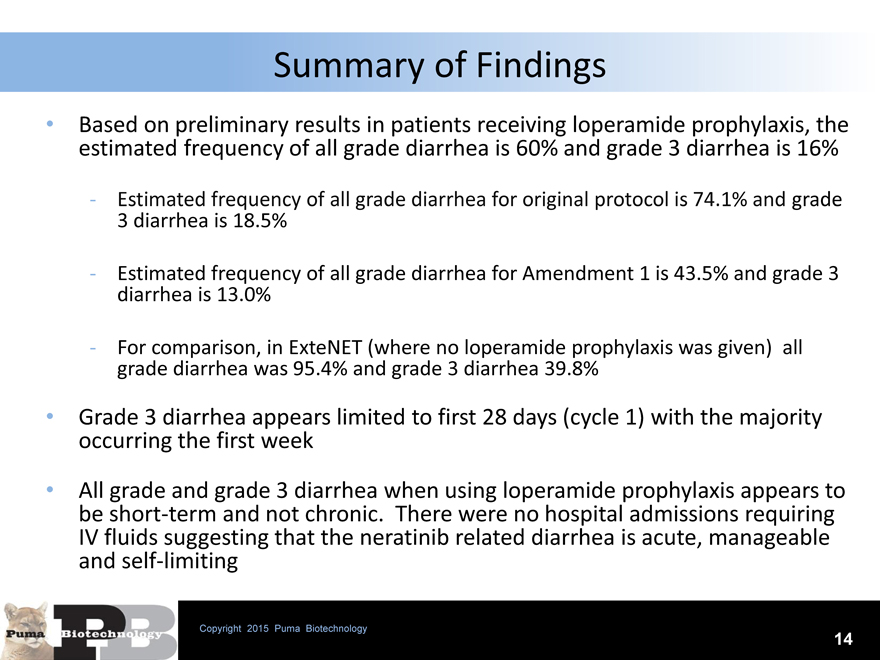
Summary of Findings
Based on preliminary results in patients receiving loperamide prophylaxis, the estimated frequency of all grade diarrhea is 60% and grade 3 diarrhea is 16%
- Estimated frequency of all grade diarrhea for original protocol is 74.1% and grade 3 diarrhea is 18.5%
- Estimated frequency of all grade diarrhea for Amendment 1 is 43.5% and grade 3 diarrhea is 13.0%
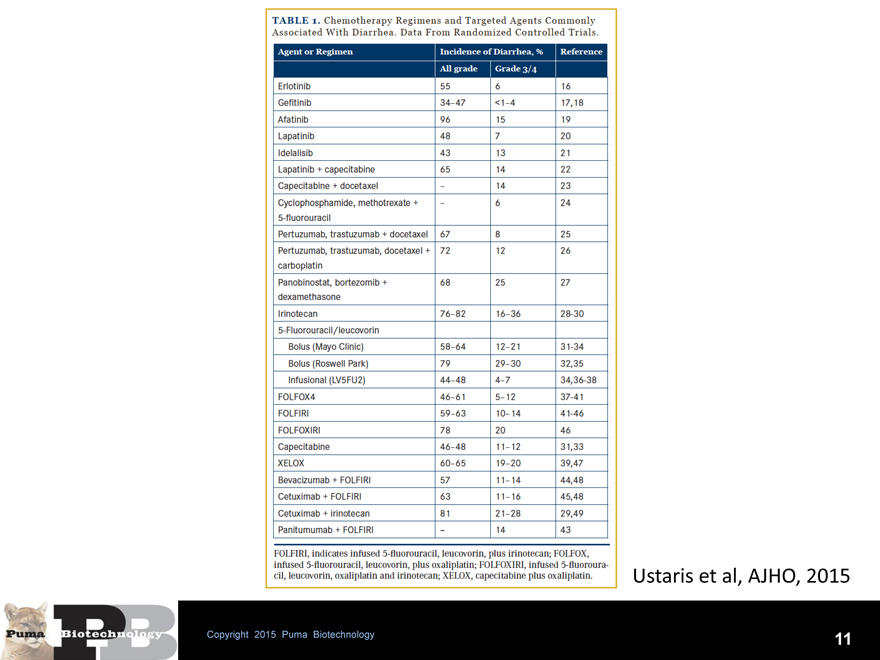
- For comparison, in ExteNET (where no loperamide prophylaxis was given) all grade diarrhea was 95.4% and grade 3 diarrhea 39.8%
Grade 3 diarrhea appears limited to first 28 days (cycle 1) with the majority occurring the first week
All grade and grade 3 diarrhea when using loperamide prophylaxis appears to be short-term and not chronic. There were no hospital admissions requiring IV fluids suggesting that the neratinib related diarrhea is acute, manageable and self-limiting
Copyright 2015 Puma Biotechnology
14
|
|
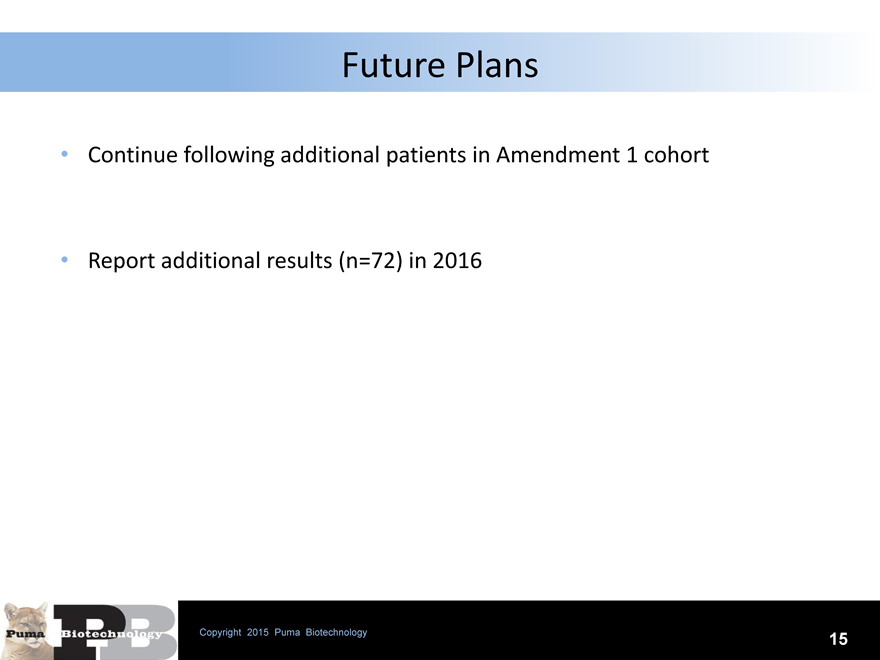
Future Plans
Continue following additional patients in Amendment 1 cohort
Report additional results (n=72) in 2016
Copyright 2015 Puma Biotechnology
15