Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Intra-Cellular Therapies, Inc. | d13740d8k.htm |
| EX-99.3 - EX-99.3 - Intra-Cellular Therapies, Inc. | d13740dex993.htm |
| EX-99.1 - EX-99.1 - Intra-Cellular Therapies, Inc. | d13740dex991.htm |
Exhibit 99.2
|
|
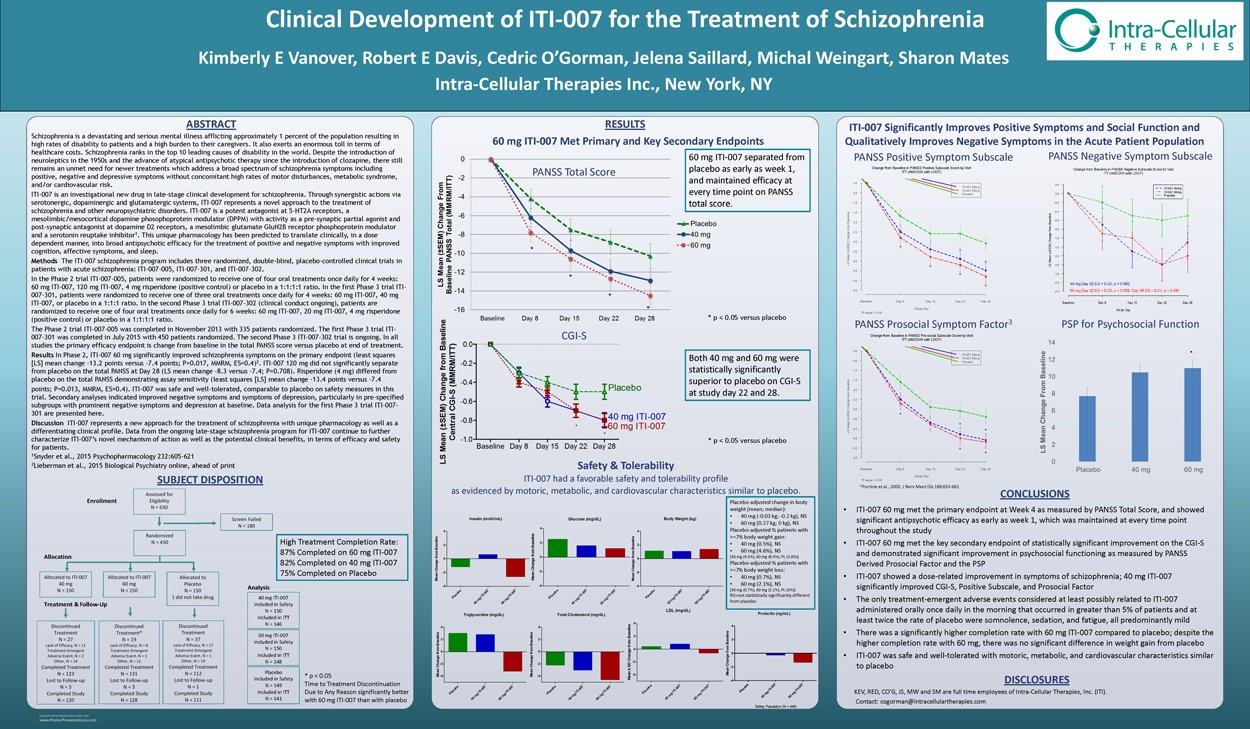
Further Characterizing Brain Receptor Occupancy with ITI-007:
Results from a Positron Emission Tomography (PET) Study in Patients with Schizophrenia
Kimberly E Vanover1, Robert E Davis1, Yun Zhou2, Weiguo Ye2, Cedric O’Gorman1, Jelena Saillard1, Michal Weingart1, Robert Litman3, Sharon Mates1, Dean Wong2 1Intra-Cellular Therapies Inc., New York, NY; 2Johns Hopkins University School of Medicine, Baltimore, MD; 3CBH Health, Rockville, MD
ABSTRACT RESULTS
[11C]-Raclopride 6
All medications approved in the USA to treat schizophrenia, to date, have to a varying extent, dopamine D2 receptor 60 mg ITI-007 occupancy (D2RO) as a feature of their pharmacology. Nonetheless, different antipsychotics exhibit different threshold levels of striatal D2RO. Most antipsychotics are D2 receptor antagonists, both pre- and post- synaptically, and have ~40% Mean Peak Striatal D2 Receptor Occupancy demonstrated antipsychotic efficacy in association with about a 65 – 80% striatal D2RO, while only slightly higher striatal At an effective antipsychotic dose, ITI-007 demonstrated relatively low striatal D2RO D2RO (>80%) has been associated with the development of extrapyramidal side effects and hyperprolactinemia. Dopamine receptor partial agonists differ in this regard. For example, aripiprazole, which is both a pre- and post- synaptic partial agonist, demonstrates higher (>80%) D2RO in association with efficacy. At therapeutic doses in patients with schizophrenia, Baseline BP %D RO clozapine is an exception, with efficacy associated with relatively lower D2RO occupancy (<50%). ND 2
Group Baseline
ITI-007 is a first-in-class dopamine receptor phosphoprotein modulator (DPPM), acting as a presynaptic partial agonist and postsynaptic antagonist1. This allows for reduced release of dopamine presynaptically with ITI-007 compared to pre- Start Time Mean for Mean synaptic antagonists along with blockade of dopamine postsynaptically for more efficient reduction of dopaminergic Subjecta of Scan Caudate Putamen Caudate Putamen Striatum ± SD signaling than most other antipsychotic drugs. ITI-007 also benefits from potent serotonin 5-HT2A receptor antagonism,
N001b 3.04 3.149 17.9 18 18
serotonin transporter (SERT) inhibition, and increased phosphorylation of glutamatergic N-methyl-D-aspartate (NMDA)
GluN2B receptors likely downstream of dopamine D1 receptor activation in mesolimbic brain regions1. Together, this unique N002 4.033 5.234 43.1 39.2 41 pharmacology predicts more efficient dopamine modulation with antipsychotic efficacy at relatively low levels of D2RO. N003 4.216 5.519 35.5 34 35 39% Positron emission tomography (PET) data in healthy volunteers (Clinical trial ITI-007-003) was previously presented2. The 1 h
N004 3.399 4.556 49.8 47.5 49 ± 12%
results from this PET study indicated ITI-007 (10–40 mg) was safe and well tolerated and rapidly entered the brain with long-lasting and dose-related occupancy. ITI-007 (10 mg) demonstrated high occupancy (>80 %) of cortical 5-HT2A receptors N005 3.641 4.619 52.3 50 51 and low occupancy of striatal D2 receptors (~12%). D2RO increased with dose and significantly correlated with plasma N006 3.684 4.074 41 34.1 38 concentration. ITI-007 (40 mg) resulted in mean occupancy of 29% and peak occupancy up to 39% of striatal D2R and peak N011 3.872 4.476 36.8 36.1 36 60 mg ITI-007 occupancy up to 33% of striatal serotonin transporters. ITI-007 (60 mg) was projected to have ~50% striatal D2RO.
N012 2.939 3.419 24.1 21.5 23 34%
The primary objective of the present study (ITI-007-008) was to determine the striatal D2RO of ITI-007 in patients with 3 h
3 N013 3.965 4.6 35.7 36 36 ± 8%
schizophrenia at a dose of 60 mg that has previously demonstrated antipsychotic efficacy .
N002 0
Methods Patients with stable schizophrenia (N=14) volunteered and were washed off their antipsychotic medications for N014 3.079 3.653 41.8 40.3 41
participation in this inpatient open-label study. After a drug-free period of at least two weeks, patients received a N011 3.872 4.476 16 16.1 16 A simplified reference tissue model with a spatially-constraint linear regression-based parametric imaging
baseline scan followed by administration of 60 mg ITI-007 once daily for two weeks and up to three subsequent post-
N012 2.939 3.419 12.8 10.8 12 19%
treatment scan(s). Carbon-11-Raclopride was used as the radiopharmaceutical for imaging striatal D2 receptors. Brain 7.5 h algorithm was used to generate BP image from 90-min dynamic [11C]raclopride PET (Zhou et al., regions of interest were outlined using magnetic resonance tomography (MRT) with cerebellum as the reference region. N013 3.965 4.6 13.8 16 15 ± 10%
NeuroImage, 2003, pp. 975-989).
Binding potentials were estimated using a simplified reference tissue model. D2RO was expressed as percent change in the N014 3.079 3.653 36 31.2 34 binding potentials before and after ITI-007 administration. CONCLUSIONS
N011 3.872 4.476 4.7 5.9 5
Results Mean striatal D2RO levels observed with the dose of 60 mg ITI-007 was ~40%, with peak occupancy up to 51%. ITI-
N012 2.939 3.419 0.5 -3.6 negligible 7% • At an effective antipsychotic dose of 60 mg, ITI-007 demonstrated
007 was safe and well tolerated in this study. 24-27 h
Discussion This PET study in patients with stable schizophrenia further adds to the information gleaned regarding brain N013 3.965 4.6 9.6 12.5 11 ± 7% relatively low (~40%) mean peak striatal D2RO in patients with receptor occupancy levels from a prior PET study in healthy volunteers. ITI-007 demonstrates relatively low striatal D2RO N014 3.079 3.653 12.9 14.7 14 at the antipsychotic efficacious dose of 60 mg. In this regard, ITI-007 is more clozapine-like with efficacy at relatively low schizophrenia at plasma steady state.
D2RO. Yet, ITI-007 demonstrates a more favorable safety profile. Consistent with low striatal D2RO, ITI-007 has a lesser liability for D2 mediated side effects, such as extrapyramidal side effects, including akathisia, and hyperprolactinemia than a An additional 2 subjects were evaluated at 20 mg ITI-007 and an additional 2 subjects were evaluated at 120 mg ITI-007, but • Time course data revealed peak brain occupancy as early as 1 h post-many antipsychotic drugs. Together, ITI-007 represents an exciting new potential treatment for schizophrenia. baseline binding potentials were low with no measureable D2RO post-dose in one subject at each dose (N008 and N010); therefore
dose that was sustained through the 3 h post-dose measure and
BACKGROUND these data (N=1/dose) are considered exploratory: in subject N009, a mean of 27% striatal D2RO was measured 1 h after a dose of 20 mg ITI-007; in subject N007, a mean of 47% striatal D2RO was measured 1 h after a dose of 120 mg ITI-007. gradually tapered off over the course of 24 h.
ITI-007 has a first-in-class pharmacological profile b
N001 showed low plasma levels at the start of the scan, indicating the dose may have been missed; a reanalysis without this low • Consistent with low striatal D2RO, ITI-007 has a relatively low liability for 1 outlier confirmed the initial analysis result with mean striatal D RO approximately 40% with 60 mg ITI-007, but, as would be
via serotonergic, dopaminergic and glutamatergic pathways 2 expected, with less variability (43% ± 7%; range 35% to 51%; 41% median). D2 mediated side effects, such as extrapyramidal side effects, including
5-HT2A receptor antagonist akathisia, and hyperprolactinemia; in efficacy studies, 60 mg ITI-007 was
Dopamine phosphoprotein modulator (DPPM)
SAFETY & TOLERABILITY not different from placebo on such side effects.
Glutamatergic phosphoprotein modulator
ITI-007 represents a first-in-class new potential treatment for
Serotonin reuptake inhibitor
ITI-007 was safe and well tolerated in this study. to Binding Affinities schizophrenia and other psychiatric and neurological disorders.
There were no clinically significant changes in vital signs, ECGs,
ITI-007 is currently in Phase 3 clinical development for the treatment
ITI 007 IC200131 or clinical chemistry laboratory values.
Ki nM) Ki (nM) schizophrenia and bipolar depression.
The most frequent adverse events (occurring in more than 2
5-HT2A 5 61 A low dose strategy for the treatment of behavioral disturbances patients) that were reported to be at least possibly related to
D2 574 associated with dementia is being pursued, for which even lower (5 –
ITI-007 were mild headache and mild sedation.
10%) striatal D2RO may be beneficial without the accompanying adverse
>1000 Note: IC200131 is the
~70 major circulating active • There were no adverse event reports of akathisia or other effects associated with many antipsychotic drugs. metabolite of ITI-007 extrapyramidal side effects.
Sn 2015 ology 232:605-621 DISCLOSURES
Mean values of motor function as measured by BARS and SAS
Da Psychopharmac logy 232:2863-2872 KEV, RED, CO’G, JS, MW and SM are full time employees of Intra-Cellular Therapies, Inc. (ITI).
indicated no motor disturbances with ITI-007 treatment.
Lie ., 2015 Biological Psychiatry online, ahead of print Contact: kvanover@intracellulartherapies.com
RESEARCH POSTER PRESENTATION DESIGN © 2012
www.PosterPresentations.com