Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Kite Pharma, Inc. | d18180d8k.htm |
Exhibit 99.1
As used in this Current Report on Form 8-K, all references to “we,” “us,” “our,” “Kite Pharma,” the “Company” and similar designations refer to Kite Pharma, Inc. and its subsidiaries on a consolidated basis. This Current Report on Form 8-K contains common law, unregistered trademarks for Kite Pharma and eACT based on use of the trademarks in the United States. Other trademarks referred to in this Current Report on 8-K are the property of their respective owners.
FORWARD-LOOKING STATEMENTS
FORWARD-LOOKING STATEMENTS
This Current Report on Form 8-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, or the Exchange Act, which are subject to the “safe harbor” created by those sections. We may, in some cases, use words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes to identify these forward-looking statements. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. Forward-looking statements may include, but are not limited to, statements about:
| • | the success, cost and timing of our product development activities and clinical trials; |
| • | the ability and willingness of the NCI to continue research and development activities relating to eACT pursuant to the CRADA; |
| • | our ability to obtain and maintain regulatory approval of KTE-C19 and any other product candidates, and any related restrictions, limitations and/or warnings in the label of an approved product candidate; |
| • | the ability to license additional intellectual property relating to a product candidate targeting the EGFRvIII antigen from a third party and relating to additional product candidates from the National Institutes of Health and to comply with our existing license agreements; |
| • | our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; |
| • | the commercialization of our product candidates, if approved; |
| • | our plans and ability to research, develop and commercialize our product candidates, including under our research collaboration with Amgen Inc.; |
| • | our ability to attract collaborators with development, regulatory and commercialization expertise; |
| • | our ability to integrate T-Cell Factory B.V., or TCF, a Dutch company we acquired in March 2015, and our ability to potentially significantly expand our pipeline of TCR-based product candidates using TCF’s proprietary TCR-GENErator technology platform; |
| • | future agreements with third parties in connection with the commercialization of our product candidates and any other approved product; |
| • | the size and growth potential of the markets for our product candidates, and our ability to serve those markets; |
| • | the rate and degree of market acceptance of our product candidates; |
1
| • | regulatory developments in the United States and foreign countries; |
| • | our ability to develop, validate and utilize our own clinical manufacturing facility and commercial manufacturing facility; |
| • | our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; |
| • | the success of competing therapies that are or may become available; |
| • | our ability to attract and retain key scientific or management personnel; |
| • | the accuracy of our estimates regarding expenses, future revenue, capital requirements and needs for additional financing; |
| • | our ability to in-license, acquire, or invest in complementary businesses, technologies, products or assets to further expand our portfolio of eACT-based product candidates or to complement our eACT-based product candidates; and |
| • | our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates. |
These forward-looking statements reflect our management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the date of Current Report on Form 8-K and are subject to risks and uncertainties. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these forward-looking statements. We discuss in greater detail many of these risks and uncertainties under the heading “Risk Factors” in this Current Report on Form 8-K. We qualify all of the forward-looking statements in this Current Report on Form-8-K by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
2
Our Business
We are a clinical-stage biopharmaceutical company focused on the development and commercialization of novel cancer immunotherapy products designed to harness the power of a patient’s own immune system to target and kill cancer cells. We do this using our engineered autologous cell therapy, or eACT, which we believe is a market-redefining approach to the treatment of cancer. eACT involves the genetic engineering of T cells to express either chimeric antigen receptors, or CARs, or T cell receptors, or TCRs. These modified T-cells are designed to recognize and destroy cancer cells.
We are currently conducting four company-sponsored pivotal studies of our lead product candidate, KTE-C19, a CAR-based therapy. We are conducting a Phase 2 clinical trial (ZUMA-1) of KTE-C19 in patients with refractory diffuse large B cell lymphoma, or DLBCL, including primary mediastinal B cell lymphoma, or PMBCL, and transformed follicular lymphoma, or TFL. DLBCL, PMBCL and TFL are types of aggressive non-Hodgkin lymphoma, or NHL. We plan to report interim data from the Phase 2 trial in 2016. If we believe the data are compelling, we plan to discuss with the U.S. Food and Drug Administration, or FDA, the filing of a Biologics License Application for accelerated approval of KTE-C19 as a treatment for patients with refractory DLBCL, PMBCL and TFL. If approved, we plan to commercially launch KTE-C19 in 2017.
We are also conducting a Phase 2 clinical trial (ZUMA-2) of KTE-C19 in patients with relapsed/refractory mantle cell lymphoma, a Phase 1-2 clinical trial (ZUMA-3) of KTE-C19 in adult patients with relapsed/refractory acute lymphoblastic leukemia, or ALL, and a Phase 1-2 clinical trial (ZUMA-4) of KTE-C19 in pediatric patients with relapsed/refractory ALL. We plan to report data from ZUMA-2 and the Phase 2 portions of ZUMA-3 and ZUMA-4 in 2017. If we believe the data are compelling, we plan to pursue FDA approval for these additional indications.
We also have a Cooperative Research and Development Agreement, or CRADA, with the U.S. Department of Health and Human Services, as represented by the National Cancer Institute, or the NCI, through which we are funding the research and development of eACT-based product candidates utilizing CARs and TCRs for the treatment of advanced solid and hematological malignancies. We currently fund multiple early single phase clinical trials of CAR- and TCR-based therapies that are each being conducted by our collaborator, the NCI.
3
Our clinical trials of KTE-C19, and those being conducted in collaboration with the NCI, are summarized below.
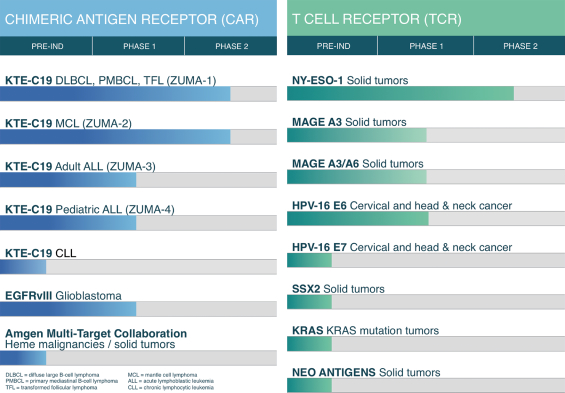
Recent Developments
Phase 1 Results from ZUMA-1
In December 2015, at the annual meeting of the American Society of Hematology (ASH), we announced data from the Phase 1 portion of ZUMA-1. Seven patients were treated with KTE-C19, of which four achieved complete remissions and one achieved a partial remission, representing an overall objective response rate of 71%. All complete remissions were first observed at one month. Three patients had ongoing complete remissions at three months. The patient who achieved a complete remission but relapsed was retreated and achieved a partial remission. The one patient who initially achieved a partial remission relapsed and died off protocol.
One patient experienced dose-limiting toxicities of cytokine release syndrome and neurotoxicity that were life-threatening toxicities. This patient died within 30 days of KTE-C19 infusion. The death was due to intracranial hemorrhage deemed unrelated to KTE-C19 per the investigator. Overall, KTE-C19 related adverse events included severe and life threatening toxicities, consisting predominantly of cytokine release syndrome and neurotoxicity, which were reversible except in the patient with the dose-limiting toxicities. Supportive care, tocilizumab, and corticosteroids were used to manage these adverse events.
The ZUMA-1 results were generally consistent with the results from the NCI Phase 1-2a clinical trial of anti-CD19 CAR T cell therapy in patients with relapsed/refractory lymphomas and leukemias. As of August 31, 2015, the objective response rate of the 36 evaluable patients in the NCI study was 78%, with 47% having complete remissions. As of August 31, 2015, 15 of the patients were in remission for more than one year. Twenty-two of the 36 evaluable patients in the NCI study had relapsed DLBCL, PMBCL or TFL. The objective response rate of these 22 patients was 68% and the complete remission rate was 41%. Severe and life threatening toxicities relating to cytokine release syndrome and neurotoxicity occurred mostly in the first two weeks after cell infusion and generally resolved within three weeks.
Breakthrough Therapy Designation
In December 2015, the FDA granted breakthrough therapy designation status to our lead product candidate, KTE-C19, for the treatment of patients with refractory DLBCL, PMBCL and TFL.
Breakthrough therapy designation is granted by the FDA to expedite the development and review of new therapies to treat serious or life-threatening conditions. The criteria for breakthrough therapy designation require preliminary clinical evidence that demonstrates the therapy may have substantial improvement on at least one clinically significant endpoint over available therapy. This designation conveys all fast track program features, as well as more intensive FDA guidance on an efficient drug development program and eligibility for rolling review and priority review.
Initiation of ZUMA-3 and ZUMA-4
In December 2015, we initiated a Phase 1-2 clinical trial (ZUMA-3) of KTE-C19 in adult patients with relapsed/refractory ALL, and a Phase 1-2 clinical trial (ZUMA-4) of KTE-C19 in pediatric patients with relapsed/refractory ALL. Both studies will proceed as a single-arm, open-label, multi-center study in patients with ALL whose disease is refractory to or has relapsed following standard chemotherapy or hematopoietic stem cell transplantation. The Phase 1 portion of each study will assess the safety of KTE-C19, and the Phase 2 portion will assess efficacy and safety. The target enrollment in each study is a total of 75 patients.
GE Global Research Strategic Collaboration
In November 2015, we entered into a strategic research collaboration with GE Global Research to develop a next generation, functionally integrated and automated manufacturing system for engineered T cell therapy. We believe this collaboration will accelerate the development of automation technologies for engineered T cell therapy that have the potential to reduce cost, improve speed and minimize variability. Under the terms of the agreement, Kite and GE Global Research will each contribute resources and relevant expertise to the partnership.
4
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below and all other information in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference, and in any free writing prospectus that we have authorized for use in connection with this offering. The occurrence of any of these risks could harm our business, financial condition, results of operations and growth prospects. In such an event, the market price of our common stock could decline and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
Risks Related to Our Business and Industry
We have incurred net losses in every year since our inception and anticipate that we will continue to incur net losses in the future.
We are a clinical-stage biopharmaceutical company with a limited operating history. Investment in biopharmaceutical product development is highly speculative because it entails substantial upfront capital expenditures and significant risk that any potential product candidate will fail to demonstrate adequate effect or an acceptable safety profile, gain regulatory approval and become commercially viable. We have no products approved for commercial sale and have not generated any revenue from product sales to date, and we continue to incur significant research and development and other expenses related to our ongoing operations. As a result, we are not profitable and have incurred losses in each period since our inception in June 2009. For the years ended December 31, 2014 and 2013, we reported a net loss of $42.6 million and $6.4 million, respectively. For the three months ended September 30, 2015 and 2014, we reported a net loss of $27.4 million and $9.1 million, respectively. For the nine months ended September 30, 2015 and 2014, we reported a net loss of $63.4 million and $29.6 million, respectively. As of September 30, 2015, we had an accumulated deficit of $121.4 million. We expect to continue to incur significant expenditures for the foreseeable future, and we expect these expenditures to increase as we continue our research and development of, and seek regulatory approvals for, product candidates based on our engineered autologous cell therapy, or eACT. Even if we succeed in commercializing one or more of our product candidates, we will continue to incur substantial research and development and other expenditures to develop and market additional product candidates. We may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital.
eACT represents a novel approach to cancer treatment that creates significant challenges for us.
eACT involves (1) harvesting T cells from the patient’s blood, (2) genetically engineering T cells to express cancer-specific receptors, (3) increasing the number of engineered T cells and (4) infusing the functional cancer-specific T cells back into the patient. Advancing this novel and personalized therapy creates significant challenges for us, including:
| • | Educating medical personnel regarding the potential side effect profile of eACT, such as the potential adverse side effects related to cytokine release and neurotoxicity; |
| • | Using medicines to manage adverse side effects of eACT, such as tocilizumab and corticosteroids, which may not adequately control the side effects and/or may have a detrimental impact on the efficacy of the treatment; |
| • | Sourcing clinical and, if approved, commercial supplies for the materials used to manufacture and process our eACT-based product candidates; |
| • | Developing a robust and reliable process, while limiting contamination risks, for engineering a patient’s T cells ex vivo and infusing the engineered T cells back into the patient; |
| • | Conditioning patients with chemotherapy in conjunction with delivering eACT, which may increase the risk of adverse side effects; |
| • | Obtaining regulatory approval, as the U.S. Food and Drug Administration, or FDA, and other regulatory authorities have limited experience with commercial development of T cell therapies for cancer; and |
5
| • | Establishing sales and marketing capabilities upon obtaining any regulatory approval to gain market acceptance of a novel therapy. |
In addition, we expect to use manufacturing and processing approaches to produce engineered T cells that are based on the original approach used by our collaborator, the National Cancer Institute, or NCI. While the NCI is using CAR- and TCR-based therapies in a Phase 2 clinical trial and in Phase 1-2a clinical trials that we are funding under a Cooperative Research and Development Agreement, or CRADA, we cannot be sure that our engineered T cell therapy will obtain the same safety and efficacy results as those obtained by the NCI using its own original production methods.
Our business is highly dependent on the success of KTE-C19, our lead product candidate. KTE-C19 and our other product candidates will require significant additional clinical testing before we can seek regulatory approval and potentially launch commercial sales.
Our business and future success depends on our ability to obtain regulatory approval of and then successfully commercialize our lead product candidate, KTE-C19. Even though the NCI is studying an anti-CD19 CAR T cell therapy that uses the identical construct and viral vector as KTE-C19, the differences in manufacturing may render the product incomparable, particularly with respect to clinical results. While the NCI has begun using the same manufacturing process that is being used for KTE-C19, the NCI has only treated a limited number of patients using this manufacturing process. All of our product candidates, including KTE-C19, will require additional clinical and non-clinical development, regulatory review and approval in multiple jurisdictions, substantial investment, access to sufficient commercial manufacturing capacity and significant marketing efforts before we can generate any revenue from product sales. In addition, because KTE-C19 is our most advanced product candidate, and because our other product candidates are based on similar technology, if KTE-C19 or the NCI’s study of anti-CD19 CAR T cell therapy with the identical construct and viral vector encounters safety or efficacy problems, developmental delays or regulatory issues or other problems, our development plans and business would be significantly harmed.
We are highly dependent on the National Cancer Institute for research and development and early clinical testing of our product candidates and on the National Institutes of Health for licensing intellectual property rights to future product candidates.
A substantial portion of our research and development has been conducted by the NCI under the CRADA. Pursuant to the CRADA, we provide $3.0 million annually to largely fund a Phase 2 and Phase 1-2a clinical trials of various eACT-based product candidates.
The NCI, with Dr. Steven A. Rosenberg as the principal investigator, is conducting a Phase 2 and multiple Phase 1-2a clinical trials of engineered T cell therapy targeting various antigens, including CD19, in small numbers of patients. We have limited control over the nature or timing of the NCI’s clinical trials and limited visibility into their day-to-day activities, including with respect to how they are providing and administering T cell therapy. For example, the research we are funding constitutes only a small portion of the NCI’s overall research. Other research being conducted by Dr. Rosenberg may at times receive higher priority than research on our programs. While we have used the results of the NCI’s clinical trial of anti-CD19 CAR T cell therapy to support our investigational new drug application, or IND, for our Phase 1-2 clinical trial of KTE-C19, these factors could adversely affect the timing of our future IND filings and our ability to conduct future planned clinical trials.
Under the CRADA, we have an exclusive option to negotiate commercialization licenses from the National Institutes of Health, or the NIH, to intellectual property relating to CAR- and TCR-based product candidates developed in the course of the CRADA research plan. However, we would have to negotiate with the NIH for such a license. There can be no assurance that we would be able to successfully complete such negotiations and ultimately acquire the rights to the intellectual property surrounding the additional product candidates that we may seek to acquire. Further, to the extent we would like to negotiate a license to a patent filed before the CRADA was entered into, another party may object to the NIH granting us a license during a 30-day public notification period, and the NIH may decide not to grant us the license.
Though the CRADA has a five-year term expiring on August 30, 2017, the NCI may unilaterally terminate the CRADA at any time for any reason or for no reason upon at least 60 days prior written notice. If the NCI unilaterally terminates the CRADA, the research and development of eACT would be suspended, and we may be unable to research, develop and license future product candidates.
6
We may not be able to file INDs to commence additional clinical trials on the timelines we expect, and even if we are able to, the FDA may not permit us to proceed.
We expect that with the early clinical work performed by the NCI pursuant to the CRADA and the additional research we are performing in-house, including pursuant to the Amgen Agreement, we may pursue the filing with the FDA of a number of INDs over the next several years. We expect to submit an IND for a second product candidate, a TCR-based product candidate, and an IND to be filed for a CAR product candidate researched under the Amgen Agreement, by the end of 2016. However, our timing of filing on the TCR product candidate will be primarily dependent on receiving human proof-of-concept data from the NCI’s clinical trials and our timing of filing on all product candidates is subject to further research. We cannot be sure that submission of an IND will result in the FDA allowing further clinical trials to begin, or that, once begun, issues will not arise that suspend or terminate such clinical trials. For instance, the FDA may not allow us to use the NCI clinical trial data to support our INDs. Additionally, even if such regulatory authorities agree with the design and implementation of the clinical trials set forth in an IND or clinical trial application, we cannot guarantee that such regulatory authorities will not change their requirements in the future.
Our clinical trials may fail to demonstrate adequately the safety and efficacy of any of our product candidates, which would prevent or delay regulatory approval and commercialization.
Before obtaining regulatory approvals for the commercial sale of our product candidates, including KTE-C19, we must demonstrate through lengthy, complex and expensive preclinical testing and clinical trials that our product candidates are both safe and effective for use in each target indication. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of our product candidates may not be predictive of the results of later-stage clinical trials. We expect there may be greater variability in results for products processed and administered on a patient-by-patient basis, like eACT, than for “off-the-shelf” products, like many drugs. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy profile despite having progressed through preclinical studies and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising results in earlier trials. Most product candidates that commence clinical trials are never approved as products.
Data from the NCI preclinical studies and Phase 1 and Phase 1-2a clinical trials of anti-CD19 CAR T cell therapy should not be relied upon as evidence that later or larger-scale clinical trials will succeed. We have designed our ongoing Phase 1-2 single-arm multicenter clinical trial of KTE-C19 primarily to assess safety and efficacy in patients with refractory diffuse large B cell lymphoma, or DLBCL, primary mediastinal B cell lymphoma, or PMBCL, and transformed follicular lymphoma, or TFL. The NCI clinical trials of anti-CD19 CAR T cell therapy involves a limited number of patients, and only a subset of these patients have been diagnosed with DLBCL, PMBCL and TFL. Our clinical trials may also evaluate different CAR T cell doses and chemotherapy conditioning regimens based on emerging safety and efficacy data. The results of the NCI trials to date may not predict results for our ongoing trial or any future studies. In addition, our proposed manufacturing process for KTE-C19 includes what we believe are process improvements that are not part of the anti-CD19 CAR T cell production process that the NCI originally used in its clinical trials. Accordingly, our safety and efficacy results with KTE-C19 may not be in line with the NCI’s results.
In addition, even if the trials are successfully completed, we cannot guarantee that the FDA or foreign regulatory authorities will interpret the results as we do, and more trials could be required before we submit our product candidates for approval. To the extent that the results of the trials are not satisfactory to the FDA or foreign regulatory authorities for support of a marketing application, approval of our product candidates may be significantly delayed, or we may be required to expend significant additional resources, which may not be available to us, to conduct additional trials in support of potential approval of our product candidates.
7
We have limited experience as a company conducting clinical trials.
While we are currently conducting clinical trials of KTE-C19, all of the other preclinical and clinical trials relating to our product candidates have been and are being conducted by the NCI. Although we have recruited a team that has significant experience with clinical trials, we have limited experience as a company in conducting clinical trials. In part because of this lack of experience, we cannot be certain that our ongoing clinical trials will be completed on time or if the planned clinical trials will begin or be completed on time, if at all. Large-scale trials would require significant additional financial and management resources, and reliance on third-party clinical investigators, contract research organizations, or CROs, or consultants. Relying on third-party clinical investigators or CROs may force us to encounter delays that are outside of our control.
Monitoring of patient safety in our ongoing and planned sponsored multicenter clinical trials will be more challenging than the monitoring currently being done by the NCI, which could adversely affect our ability to obtain regulatory approval.
The NCI is a center of excellence for clinical trials in cancer patients, with a large group of experienced staff and a clinical trial support system. Patients enrolled in the NCI clinical trials are generally hospitalized for extended periods of time for dedicated observation, and there is a ready availability of specialized health care professionals and intensive care unit beds if necessary. For our ongoing clinical trials of KTE-C19 and in our planned sponsored multicenter clinical trials of KTE-C19 and other product candidates, we have and expect to contract with universities and academic centers experienced in the assessment and management of toxicities arising during clinical trials. Nonetheless, the extent of patient observation and the appropriate treatment of toxicities may not be as optimal as is currently being done at the NCI, whether due to personnel changes, inexperience, changes of shift, house staff coverage or related issues. This could lead to more severe or prolonged toxicities or even patient deaths, which could result in us or the FDA delaying, suspending or terminating one or more of our clinical trials, and which could jeopardize regulatory approval. Medicines used at sites to help manage adverse side effects of KTE-C19, such as tocilizumab and corticosteroids, may not adequately control the side effects and/or may have a detrimental impact on the efficacy of the treatment. Use of these medicines may increase as we initiate new trial sites. All patients in the Phase 1 portion of ZUMA-1 received tocilizumab and most received corticosteroids. These medicines were infrequently used in the NCI Phase 1-2a clinical trial of anti-CD19 CAR T cell therapy.
Our product candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential or result in significant negative consequences.
Undesirable side effects caused by our product candidates could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities. Results of our trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics.
In the NCI Phase 1-2a clinical trial of anti-CD19 CAR T cell therapy, when the patients received the CAR T cells, all of them had low blood counts due to the chemotherapy conditioning regimen. While the trial is ongoing, most prominent acute toxicities have included symptoms thought to be associated with the release of cytokines, such as fever, low blood pressure and kidney dysfunction. Most patients also experienced neurotoxicity, such as ataxia, confusion, somnolence and speech impairment. There have been life threatening events related to cytokine release syndrome and neurotoxicity. Some of these events required intense medical intervention such as intubation or pressor support. Several patients have died, but the deaths were not attributed to the CAR T cells. In addition, in the NCI Phase 1 clinical trial of pediatric or young adult patients with relapsed/refractory acute lymphoblastic leukemia, or ALL, infusion of anti-CD19 CAR T cells was associated with significant, acute toxicities, including febrile neutropenia, cytokine release syndrome, chemical laboratory abnormalities, low blood counts and neurotoxicity.
As an anti-CD19 CAR T cell therapy, KTE-C19 has caused similar toxicities as the therapy in the NCI clinical trials as seen in the Phase 1 portion of the ZUMA-1 trial. As presented at ASH, the KTE-C19 related adverse events included severe and life threatening toxicities, consisting predominantly of cytokine release syndrome and neurotoxicity, which were reversible except in the patient that experienced dose-limiting toxicities. This patient died within thirty days of treatment, but the death was deemed unrelated to KTE-C19.
8
Patients in the NCI clinical trials of the CAR-based product candidate targeting the EGFRvIII antigen and the TCR-based product candidates are expected to receive high dose Interleukin-2, which is associated with toxicities such as capillary leak syndrome, hypotension, impaired kidney and liver function, and mental status changes.
If unacceptable toxicities arise in the development of our product candidates, we or the NCI could suspend or terminate our trials or the FDA or comparable foreign regulatory authorities could order us to cease clinical trials or deny approval of our product candidates for any or all targeted indications. Treatment-related side effects could also affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff, particularly outside of the NCI as toxicities resulting from personalized T cell therapy are not normally encountered in the general patient population and by medical personnel. We have trained and expect to have to train medical personnel using eACT to understand the side effect profile of eACT for both our clinical trials and upon any commercialization of any eACT-based product candidates. Inadequate training in recognizing or managing the potential side effects of eACT could result in patient deaths. Any of these occurrences may harm our business, financial condition and prospects significantly.
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons. The timely completion of clinical trials in accordance with their protocols depends, among other things, on our ability to enroll a sufficient number of patients who remain in the study until its conclusion. The enrollment of patients depends on many factors, including:
| • | the patient eligibility criteria defined in the protocol; |
| • | the size of the patient population required for analysis of the trial’s primary endpoints; |
| • | the proximity of patients to study sites; |
| • | the design of the trial; |
| • | our ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| • | our ability to obtain and maintain patient consents; and |
| • | the risk that patients enrolled in clinical trials will drop out of the trials before the manufacturing of KTE-C19 or trial completion. |
In addition, our clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as our product candidates, and this competition will reduce the number and types of patients available to us, because some patients who might have opted to enroll in our trials may instead opt to enroll in a trial being conducted by one of our competitors. Since the number of qualified clinical investigators is limited, we expect to conduct some of our clinical trials at the same clinical trial sites that some of our competitors use, which will reduce the number of patients who are available for our clinical trials in such clinical trial site. Moreover, because our product candidates represent a departure from more commonly used methods for cancer treatment, potential patients and their doctors may be inclined to use conventional therapies, such as chemotherapy and hematopoietic cell transplantation, rather than enroll patients in any future clinical trial.
Delays in patient enrollment may result in increased costs or may affect the timing or outcome of our ongoing clinical trial and planned clinical trials, which could prevent completion of these trials and adversely affect our ability to advance the development of our product candidates.
Clinical trials are expensive, time-consuming and difficult to design and implement.
Human clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Because our product candidates are based on new technology and engineered on a patient-by-patient basis, we expect that they will require extensive research and development and have substantial manufacturing and processing costs. In addition, costs to treat patients with relapsed/refractory cancer and to treat potential side effects that may result from eACT can be significant. Accordingly, our clinical trial costs are likely to be significantly higher than for more conventional therapeutic technologies or drug products. In addition, our proposed personalized product candidates involve several complex and costly manufacturing and processing steps, the costs of which will be borne by us.
9
The market opportunities for our product candidates may be limited to those patients who are ineligible for or have failed prior treatments and may be small.
The FDA often approves new therapies initially only for use in patients with relapsed or refractory metastatic disease. We expect to initially seek approval of our product candidates in this setting. Subsequently, for those products that prove to be sufficiently beneficial, if any, we would expect to seek approval in earlier lines of treatment and potentially as a first line therapy, but there is no guarantee that our product candidates, even if approved, would be approved for earlier lines of therapy, and, prior to any such approvals, we may have to conduct additional clinical trials.
Our projections of both the number of people who have the cancers we are targeting, as well as the subset of people with these cancers in a position to receive second or third line therapy, and who have the potential to benefit from treatment with our product candidates, are based on our beliefs and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these cancers. The number of patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for our product candidates may be limited or may not be amenable to treatment with our product candidates. For instance, we expect our lead product candidate, KTE-C19, to initially target a small patient population that suffers from aggressive non-Hodgkin lymphomas, or NHL, and ALL. Even if we obtain significant market share for our product candidates, because the potential target populations are small, we may never achieve profitability without obtaining regulatory approval for additional indications.
KTE-C19 has received orphan drug status, and breakthrough therapy designation, but we may be unable to obtain breakthrough therapy designation for other product candidates or maintain the benefits associated with orphan drug status, including market exclusivity.
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biologic intended to treat a rare disease or condition or for which there is no reasonable expectation that the cost of developing and making available in the United States a drug or biologic for a disease or condition will be recovered from sales in the United States for that drug or biologic. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full Biologics License Application, or BLA, to market the same biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity. In 2012, the FDA established a breakthrough therapy designation which is intended to expedite the development and review of products that treat serious or life-threatening conditions.
We have received orphan drug status for KTE-C19 for the treatment of DLBCL, but exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan designated indication and may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. The European Commission has also granted KTE-C19 orphan drug designation for the treatment of DLBCL, PMBCL, ALL, mantle cell lymphoma, or MCL, chronic lymphocytic leukemia/small lymphocytic lymphoma, and follicular lymphoma. The designation may provide ten years of market exclusivity in Europe, but is subject to certain limited exceptions.
In addition, we have received breakthrough therapy designation for KTE-C19 for the treatment of refractory DLBCL, PMBCL and TFL, but there can be no assurance that such designation will result in expedited review or approval. The FDA may also rescind the breakthrough therapy designation for KTE-C19 if subsequent data no longer support the designation. Breakthrough therapy designation does not change the standards for product approval. While we intend to seek orphan drug designation and breakthrough therapy designation for other product candidates, we may never receive such designations.
10
If we fail to develop additional product candidates, our commercial opportunity will be limited.
We expect to initially develop our lead product candidate, KTE-C19. However, one of our strategies is to pursue clinical development of additional product candidates. Developing, obtaining regulatory approval for and commercializing additional product candidates, including additional TCR- or CAR-based product candidates, will require substantial additional funding and is prone to the risks of failure inherent in medical product development. We cannot provide you any assurance that we will be able to successfully advance any of these additional product candidates through the development process.
Even if we receive FDA approval to market additional product candidates for the treatment of cancer, we cannot assure you that any such product candidates will be successfully commercialized, widely accepted in the marketplace or more effective than other commercially available alternatives. If we are unable to successfully develop and commercialize additional product candidates, our commercial opportunity will be limited. Moreover, a failure in obtaining regulatory approval of additional product candidates may have a negative effect on the approval process of any other, or result in losing approval of any approved, product candidate.
We intend to develop our own clinical manufacturing facility and commercial manufacturing facility, which will require significant resources and we may fail to successfully develop either or both facilities, which could adversely affect our clinical trials and the commercial viability of our product candidates.
We currently rely on outside vendors to manufacture clinical supplies and process our product candidates, which is currently and will continue to be done on a patient-by-patient basis. We have not yet caused our product candidates to be manufactured or processed on a commercial scale, and may not be able to achieve manufacturing and processing on our own, including on a patient-by-patient basis, to satisfy demands for any of our product candidates. While we believe the manufacturing and processing approach we intend to employ at our manufacturing facilities is comparable to the current approach undertaken by the NCI, we have limited experience in managing the T cell engineering process, and our process may be more difficult or expensive than the NCI’s current approach. We cannot be sure that even minor changes in the production process will not result in significantly different T cells or that such cells will be as safe and effective as those used in any NCI-based T cell therapy.
We have leased approximately 18,000 square feet near our headquarters in Santa Monica, California, which we expect to use as our clinical manufacturing facility and have also leased approximately 43,500 square feet in El Segundo, California to develop our commercial manufacturing facility. While we have completed construction of our clinical manufacturing facility, we have to provide certain information to the FDA prior to using the facility for any of our clinical trials. The FDA could object to our use of our clinical manufacturing facility. Also, the commercial facility requires substantial improvements and we do not yet have sufficient information to reliably estimate the cost of the clinical and commercial manufacturing and processing of our product candidates, and the actual cost to manufacture and process our product candidates could materially and adversely affect the commercial viability of our product candidates. As a result, we may never be able to develop a commercially viable product. In addition, the commercial manufacturing facility we develop will require FDA approval, which we may never obtain. Even if approved, we would be subject to ongoing periodic unannounced inspection by the FDA, the Drug Enforcement Administration and corresponding state agencies to ensure strict compliance with current good manufacturing practices, or cGMPs, and other government regulations.
The manufacture of medical products is complex and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. Manufacturers of cell therapy products often encounter difficulties in production, particularly in scaling out and validating initial production and ensuring the absence of contamination. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if contaminants are discovered in our supply of product candidates or in the manufacturing facilities, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination. We cannot assure you that any stability or other issues relating to the manufacture of our product candidates will not occur in the future.
We may also fail to manage the logistics of collecting and shipping patient material to the manufacturing site and shipping the product candidate back to the patient. Logistical and shipment delays and problems, whether or not caused by us or our vendors, could prevent or delay the delivery of product candidates to patients.
11
We may also experience manufacturing difficulties due to resource constraints or as a result of labor disputes. If we were to encounter any of these difficulties, our ability to provide our product candidates to patients would be jeopardized.
We currently have no marketing and sales organization and have no experience in marketing products. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to generate product revenue.
We currently have no sales, marketing or distribution capabilities and have no experience in marketing products. We intend to develop an in-house marketing organization and sales force, which will require significant capital expenditures, management resources and time. We will have to compete with other pharmaceutical and biotechnology companies to recruit, hire, train and retain marketing and sales personnel. If we are unable or decide not to establish internal sales, marketing and distribution capabilities, we will pursue collaborative arrangements regarding the sales and marketing of our products, however, there can be no assurance that we will be able to establish or maintain such collaborative arrangements, or if we are able to do so, that they will have effective sales forces. Any revenue we receive will depend upon the efforts of such third parties, which may not be successful. We may have little or no control over the marketing and sales efforts of such third parties and our revenue from product sales may be lower than if we had commercialized our product candidates ourselves. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our product candidates.
There can be no assurance that we will be able to develop in-house sales and distribution capabilities or establish or maintain relationships with third-party collaborators to commercialize any product in the United States or overseas.
A variety of risks associated with conducting research and clinical trials abroad and marketing our product candidates internationally could materially adversely affect our business.
We plan to initiate a clinical program for KTE-C19 in Europe in 2016 and ultimately seek regulatory approval of our product candidates outside of the United States. Accordingly, we expect that we will be subject to additional risks related to operating in foreign countries, including:
| • | differing regulatory requirements in foreign countries; |
| • | unexpected changes in tariffs, trade barriers, price and exchange controls and other regulatory requirements; |
| • | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| • | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| • | foreign taxes, including withholding of payroll taxes; |
| • | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| • | difficulties staffing and managing foreign operations; |
| • | workforce uncertainty in countries where labor unrest is more common than in the United States; |
| • | differing payor reimbursement regimes, governmental payors or patient self-pay systems, and price controls; |
| • | potential liability under the Foreign Corrupt Practices Act of 1977 or comparable foreign regulations; |
| • | challenges enforcing our contractual and intellectual property rights, especially in those foreign countries that do not respect and protect intellectual property rights to the same extent as the United States; |
| • | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| • | business interruptions resulting from geo-political actions, including war and terrorism. |
These and other risks associated with our international operations, including the operations of our European subsidiary, Kite Pharma EU B.V., may materially adversely affect our ability to attain or maintain profitable operations.
12
We face significant competition from other biotechnology and pharmaceutical companies, and our operating results will suffer if we fail to compete effectively.
The biopharmaceutical industry is characterized by intense competition and rapid innovation. Our competitors may be able to develop other compounds or drugs that are able to achieve similar or better results. Our potential competitors include major multinational pharmaceutical companies, established biotechnology companies, specialty pharmaceutical companies and universities and other research institutions. Many of our competitors have substantially greater financial, technical and other resources, such as larger research and development staff and experienced marketing and manufacturing organizations and well-established sales forces. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in our competitors. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors, either alone or with collaborative partners, may succeed in developing, acquiring or licensing on an exclusive basis drug or biologic products that are more effective, safer, more easily commercialized or less costly than our product candidates or may develop proprietary technologies or secure patent protection that we may need for the development of our technologies and products. We believe the key competitive factors that will affect the development and commercial success of our product candidates are efficacy, safety, tolerability, reliability, convenience of use, price and reimbursement.
Specifically, genetically engineering T cells faces significant competition in both the CAR and TCR technology space from multiple companies. Even if we obtain regulatory approval of our product candidates, the availability and price of our competitors’ products could limit the demand and the price we are able to charge for our product candidates. We may not be able to implement our business plan if the acceptance of our product candidates is inhibited by price competition or the reluctance of physicians to switch from existing methods of treatment to our product candidates, or if physicians switch to other new drug or biologic products or choose to reserve our product candidates for use in limited circumstances. For additional information regarding our competition, see “Item 1. Business—Competition” in our Annual Report on Form 10-K for the year ended December 31, 2014, or Annual Report, which has been filed with the SEC.
We are highly dependent on our key personnel, and if we are not successful in attracting and retaining highly qualified personnel, we may not be able to successfully implement our business strategy.
Our ability to compete in the highly competitive biotechnology and pharmaceutical industries depends upon our ability to attract and retain highly qualified managerial, scientific and medical personnel. We are highly dependent on our management, scientific and medical personnel, including our President and Chief Executive Officer, our Executive Vice President of Research & Development and Chief Medical Officer, our Chief Scientific Officer and our Chief Financial Officer & Chief Operating Officer. The loss of the services of any of our executive officers, other key employees, and other scientific and medical advisors, and our inability to find suitable replacements could result in delays in product development and harm our business. Our strong relationship with the NCI is bolstered by our President and Chief Executive Officer’s relationship with Dr. Rosenberg of the NCI. If we lose our President and Chief Executive Officer or if Dr. Rosenberg leaves the NCI, our relationship with the NCI may deteriorate and our business could be harmed. We conduct substantially all of our operations at our facilities in Southern California. This region is headquarters to many other biopharmaceutical companies and many academic and research institutions. Competition for skilled personnel in our market is intense and may limit our ability to hire and retain highly qualified personnel on acceptable terms or at all.
To induce valuable employees to remain at our company, in addition to salary and cash incentives, we have provided stock options that vest over time. The value to employees of stock options that vest over time may be significantly affected by movements in our stock price that are beyond our control, and may at any time be insufficient to counteract more lucrative offers from other companies. Despite our efforts to retain valuable employees, members of our management, scientific and development teams may terminate their employment with us on short notice. Although we have employment agreements with our key employees, these employment agreements provide for at-will employment, which means that any of our employees could leave our employment at any time, with or without notice. We do not maintain “key man” insurance policies on the lives of these individuals or the lives of any of our other employees. Our success also depends on our ability to continue to attract, retain and motivate highly skilled junior, mid-level and senior managers as well as junior, mid-level and senior scientific and medical personnel.
13
We have grown rapidly and will need to continue to grow the size of our organization, and we may experience difficulties in managing this growth.
As our development and commercialization plans and strategies develop, and as we continue to transition into operating as a public company, we have rapidly expanded our employee base and expect to continue to add managerial, operational, sales, marketing, financial and other personnel. Current and future growth imposes significant added responsibilities on members of management, including:
| • | identifying, recruiting, integrating, maintaining and motivating additional employees; |
| • | managing our internal development efforts effectively, including the clinical and FDA review process for our product candidates, while complying with our contractual obligations to contractors and other third parties; and |
| • | improving our operational, financial and management controls, reporting systems and procedures. |
Our future financial performance and our ability to commercialize our product candidates will depend, in part, on our ability to effectively manage our growth, and our management may also have to divert a disproportionate amount of its attention away from day-to-day activities in order to devote a substantial amount of time to managing these growth activities.
We currently rely, and for the foreseeable future will continue to rely, in substantial part on certain independent organizations, advisors and consultants to provide certain services, including substantially all aspects of regulatory approval, clinical management and clinical manufacturing. There can be no assurance that the services of independent organizations, advisors and consultants will continue to be available to us on a timely basis when needed, or that we can find qualified replacements. In addition, if we are unable to effectively manage our outsourced activities or if the quality or accuracy of the services provided by consultants is compromised for any reason, our clinical trials may be extended, delayed or terminated, and we may not be able to obtain regulatory approval of our product candidates or otherwise advance our business. There can be no assurance that we will be able to manage our existing consultants or find other competent outside contractors and consultants on economically reasonable terms, or at all.
If we are not able to effectively expand our organization by hiring new employees and expanding our groups of consultants and contractors, we may not be able to successfully implement the tasks necessary to further develop and commercialize our product candidates and, accordingly, may not achieve our research, development and commercialization goals.
We may form or seek strategic alliances or enter into additional licensing arrangements in the future, and we may not realize the benefits of such alliances or licensing arrangements.
We may form or seek strategic alliances, create joint ventures or collaborations or enter into additional licensing arrangements with third parties that we believe will complement or augment our development and commercialization efforts with respect to our product candidates and any future product candidates that we may develop. Any of these relationships may require us to incur non-recurring and other charges, increase our near and long-term expenditures, issue securities that dilute our existing stockholders or disrupt our management and business. In addition, we face significant competition in seeking appropriate strategic partners and the negotiation process is time-consuming and complex. Moreover, we may not be successful in our efforts to establish a strategic partnership or other alternative arrangements for our product candidates because they may be deemed to be at too early of a stage of development for collaborative effort and third parties may not view our product candidates as having the requisite potential to demonstrate safety and efficacy. Any delays in entering into new strategic partnership agreements related to our product candidates could delay the development and commercialization of our product candidates in certain geographies for certain indications, which would harm our business prospects, financial condition and results of operations.
If we license products or businesses, we may not be able to realize the benefit of such transactions if we are unable to successfully integrate them with our existing operations and company culture. For instance, our research collaboration with Amgen Inc. and our collaboration with bluebird bio, Inc. require significant research and development commitments that may not result in the development and commercialization of additional product candidates. In addition, our collaboration with GE Global Research may not result in automation technologies that
14
improve engineered T cell manufacturing. We cannot be certain that, following a strategic transaction or license, we will achieve the results, revenue or specific net income that justifies such transaction.
We may not realize the benefits of acquisitions, including our acquisition of T-Cell Factory, B.V., or other strategic transactions.
We acquired T-Cell Factory, B.V., or TCF, on March 17, 2015 and renamed the acquired company Kite Pharma EU B.V. We actively evaluate various strategic transactions on an ongoing basis. We may acquire other businesses, products or technologies as well as pursue joint ventures or investments in complementary businesses. The success of acquisitions, including our acquisition of TCF, and any future strategic transactions depends on the risks and uncertainties involved including:
| • | unanticipated liabilities related to acquired companies; |
| • | difficulties integrating acquired personnel, technologies and operations into our existing business; |
| • | retention of key employees; |
| • | diversion of management time and focus from operating our business to management of strategic alliances or joint ventures or acquisition integration challenges; |
| • | increases in our expenses and reductions in our cash available for operations and other uses; |
| • | disruption in our relationships with collaborators or suppliers as a result of such a transaction; and |
| • | possible write-offs or impairment charges relating to acquired businesses. |
If any of these risks or uncertainties occur, we may not realize the anticipated benefit of any acquisition or strategic transaction. For example, TCF’s TCR-GENErator technology platform may fail to identify TCR-based product candidates that are safe and effective, or at all. Additionally, foreign acquisitions, including our acquisition of TCF, a Dutch company, are subject to additional risks, including those related to integration of operations across different cultures and languages, currency risks, potentially adverse tax consequences of overseas operations and the particular economic, political and regulatory risks associated with specific countries. For instance, we owe significant milestone payments to the sellers of TCF in euros, rather than dollars, and we have not hedged these payments.
Future acquisitions or dispositions could result in potentially dilutive issuances of our equity securities, the incurrence of debt, contingent liabilities or amortization expenses or write-offs of goodwill, any of which could harm our financial condition.
If we fail to obtain additional financing, we may be unable to complete the development and commercialization of our product candidates.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial amounts to continue the clinical development of our product candidates, including KTE-C19. If approved, we will require significant additional amounts in order to launch and commercialize our product candidates.
Changing circumstances may cause us to consume capital significantly faster than we currently anticipate, and we may need to spend more money than currently expected because of circumstances beyond our control. We may require additional capital for the further development and commercialization of our product candidates, including funding our internal manufacturing capabilities and our subsidiary, and may need to raise additional funds sooner if we choose to expand more rapidly than we presently anticipate.
We cannot be certain that additional funding will be available on acceptable terms, or at all. We have no committed source of additional capital and if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of our product candidates or other research and development initiatives. Our license agreements and CRADA may also be terminated if we are unable to meet the payment obligations under the agreements. We could be required to seek collaborators for our product candidates at an earlier stage than otherwise would be desirable or on terms that are less favorable than might otherwise be available or relinquish or license on unfavorable terms our rights to our product candidates in markets where we otherwise would seek to pursue development or commercialization ourselves.
Any of the above events could significantly harm our business, prospects, financial condition and results of operations and cause the price of our common stock to decline.
15
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of public and private equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a stockholder. The incurrence of indebtedness would result in increased fixed payment obligations and could involve certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms unfavorable to us.
Our internal computer systems, or those used by our CROs or other contractors or consultants, may fail or suffer security breaches.
Despite the implementation of security measures, our internal computer systems and those of our future CROs and other contractors and consultants are vulnerable to damage from computer viruses and unauthorized access. While we have not experienced any such material system failure or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from completed or future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on NCI for research and development of our product candidates and other third parties for the manufacture of our product candidates and to conduct clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed.
Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses.
Our operations, and those of our CROs and other contractors and consultants, could be subject to earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions, for which we are predominantly self-insured. In addition, we are reliant on the NCI for conducting research and development of our product candidates, and the NCI may be affected by government shutdowns or withdrawn funding. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses. We rely on third-party manufacturers to produce and process our product candidates on a patient by patient basis. Our ability to obtain clinical supplies of our product candidates could be disrupted if the operations of these suppliers are affected by a man-made or natural disaster or other business interruption. Our corporate headquarters, the location of our manufacturer of the CAR gene, and processing location are in California near major earthquake faults and fire zones. The ultimate impact on us, our significant suppliers and our general infrastructure of being located near major earthquake faults and fire zones and being consolidated in certain geographical areas is unknown, but our operations and financial condition could suffer in the event of a major earthquake, fire or other natural disaster.
Our employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk of employee fraud or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with the laws of the FDA and other similar foreign regulatory bodies, provide true, complete and accurate information to the FDA and other similar foreign regulatory bodies, comply with manufacturing standards we have established, comply with healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws, or report financial information or data accurately or to disclose unauthorized activities to us. If we obtain FDA approval of any of our product candidates and begin commercializing those products in the United States, our potential exposure under such laws will increase significantly, and our costs associated with compliance with such laws are also likely to increase. These laws may impact, among other things,
16
our current activities with principal investigators and research patients, as well as proposed and future sales, marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials. The laws that may affect our ability to operate include, but are not limited to:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe, or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, either the referral of an individual, or the purchase, lease, order or recommendation of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment or approval from Medicare, Medicaid, or other third-party payors that are false or fraudulent or knowingly making a false statement to improperly avoid, decrease or conceal an obligation to pay money to the federal government; |
| • | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations, or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; |
| • | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans, and healthcare clearinghouses as well as their respective business associates that perform services for them that involve the use, or disclosure of, individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization; |
| • | the federal Physician Payment Sunshine Act, created under the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, which we refer to collectively as the Healthcare Reform Act, and its implementing regulations, which require certain manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the United States Department of Health and Human Services, or HHS, information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members; and |
| • | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers. |
Additionally, we are subject to state and foreign equivalents of each of the healthcare laws described above, among others, some of which may be broader in scope and may apply regardless of the payor.
We have adopted a code of business conduct and ethics, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent inappropriate conduct may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. Efforts to ensure that our business arrangements will comply with applicable healthcare laws may involve substantial costs. It is possible
17
that governmental and enforcement authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations. In addition, the approval and commercialization of any of our product candidates outside the United States will also likely subject us to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability as a result of the clinical testing of our product candidates and will face an even greater risk if we commercialize any products. For example, we may be sued if our product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | decreased demand for our product candidates; |
| • | injury to our reputation; |
| • | withdrawal of clinical trial participants; |
| • | initiation of investigations by regulators; |
| • | costs to defend the related litigation; |
| • | a diversion of management’s time and our resources; |
| • | substantial monetary awards to trial participants or patients; |
| • | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| • | loss of revenue; |
| • | exhaustion of any available insurance and our capital resources; |
| • | the inability to commercialize any product candidate; and |
| • | a decline in our share price. |
Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of products we develop, alone or with corporate collaborators. Our insurance policies may also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. While we have obtained clinical trial insurance for our clinical trials of KTE-C19, we may have to pay amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Our ability to utilize our net operating loss carryforwards and certain other tax attributes will be limited.
Under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended, if a corporation undergoes an “ownership change” (generally defined as a greater than 50% change (by value) in its equity ownership over a three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change income may be limited. As of December 31, 2014 we have U.S. net operating loss carryforwards of approximately $32.2 million. As a result of our private placements and our initial public offering, we have triggered two “ownership changes.” We may also experience ownership changes in the future as a result of subsequent shifts in our stock ownership. Accordingly, if we earn net taxable income, our ability to use our
18
pre-change net operating loss carryforwards to offset U.S. federal taxable income will be subject to limitations, which will result in increased future tax liability to us.
Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price.
As widely reported, global credit and financial markets have experienced extreme volatility and disruptions in the past several years, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates and uncertainty about economic stability. There can be no assurance that further deterioration in credit and financial markets and confidence in economic conditions will not occur. Our general business strategy may be adversely affected by any such economic downturn, volatile business environment or continued unpredictable and unstable market conditions. If the current equity and credit markets deteriorate, or do not improve, it may make any necessary debt or equity financing more difficult, more costly, and more dilutive. Failure to secure any necessary financing in a timely manner and on favorable terms could have a material adverse effect on our growth strategy, financial performance and stock price and could require us to delay or abandon clinical development plans. In addition, there is a risk that one or more of our current service providers, manufacturers and other partners may not survive these difficult economic times, which could directly affect our ability to attain our operating goals on schedule and on budget.
At September 30, 2015, we had approximately $173.0 million of cash and cash equivalents and $195.6 million of marketable securities. While we are not aware of any downgrades, material losses or other significant deterioration in the fair value of our cash equivalents and marketable securities since September 30, 2015, no assurance can be given that further deterioration of the global credit and financial markets would not negatively impact our current portfolio of cash equivalents and marketable securities or our ability to meet our financing objectives. Furthermore, our stock price may decline due in part to the volatility of the stock market and the general economic downturn.
Risks Related to Our Reliance on Third Parties
We rely and will rely on third parties to conduct our clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval of or commercialize our product candidates.
We depend and will depend upon independent investigators and collaborators, such as universities, medical institutions, CROs and strategic partners to conduct our preclinical and clinical trials under agreements with us, including without limitation the NCI. We negotiate budgets and contracts with CROs and study sites, which may result in delays to our development timelines and increased costs. We will rely heavily on these third parties over the course of our clinical trials, and we control only certain aspects of their activities. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with applicable protocol, legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our regulatory responsibilities. We and these third parties are required to comply with current good clinical practices, or cGCPs, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for product candidates in clinical development. Regulatory authorities enforce these cGCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of these third parties fail to comply with applicable cGCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that, upon inspection, such regulatory authorities will determine that any of our clinical trials comply with the cGCP regulations. In addition, our clinical trials must be conducted with biologic product produced under cGMPs and will require a large number of test patients. Our failure or any failure by these third parties to comply with these regulations or to recruit a sufficient number of patients may require us to repeat clinical trials, which would delay the regulatory approval process. Moreover, our business may be implicated if any of these third parties violates federal or state fraud and abuse or false claims laws and regulations or healthcare privacy and security laws.
Any third parties conducting our clinical trials are and will not be our employees and, except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our ongoing preclinical, clinical and nonclinical programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other drug development activities, which could affect their performance on our behalf. If these third parties do not
19
successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to complete development of, obtain regulatory approval of or successfully commercialize our product candidates. As a result, our financial results and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenue could be delayed.
Switching or adding third parties to conduct our clinical trials involves substantial cost and requires extensive management time and focus. In addition, there is a natural transition period when a new third party commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines.
We expect to rely on third parties to manufacture our clinical product supplies, and we may have to rely on third parties to produce and process our product candidates, if approved.
We currently rely on outside vendors to manufacture supplies and process our product candidates. Our anticipated reliance on a limited number of third-party manufacturers for clinical product supplies, and if we are unable to develop our own commercial manufacturing facility, for any commercial product supplies, exposes us to the following risks:
| • | We may be unable to identify manufacturers on acceptable terms or at all because the number of potential manufacturers is limited and the FDA may have questions regarding any replacement contractor. This may require new testing and compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our products after receipt of FDA questions, if any. |
| • | Our third-party manufacturers might be unable to timely formulate and manufacture our product or produce the quantity and quality required to meet our clinical and commercial needs, if any. |
| • | Contract manufacturers may not be able to execute our manufacturing procedures appropriately. |
| • | Our future contract manufacturers may not perform as agreed or may not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our products. |
| • | Manufacturers are subject to ongoing periodic unannounced inspection by the FDA, the Drug Enforcement Administration and corresponding state agencies to ensure strict compliance with cGMP and other government regulations and corresponding foreign standards. We do not have control over third-party manufacturers’ compliance with these regulations and standards. |
| • | We may not own, or may have to share, the intellectual property rights to any improvements made by our third-party manufacturers in the manufacturing process for our products. |
| • | Our third-party manufacturers could breach or terminate their agreement with us. |
Our contract manufacturers are also subject to the same risks we face in developing our own manufacturing capabilities, as described above. Each of these risks could delay our clinical trials, the approval, if any of our product candidates by the FDA or the commercialization of our product candidates or result in higher costs or deprive us of potential product revenue. In addition, we will rely on third parties to perform release tests on our product candidates prior to delivery to patients. If these tests are not appropriately done and test data are not reliable, patients could be put at risk of serious harm.
Cell-based therapies rely on the availability of specialty raw materials, which may not be available to us on acceptable terms or at all.
eACT requires many specialty raw materials, some of which are manufactured by small companies with limited resources and experience to support a commercial product. In addition, those suppliers normally support blood-based hospital businesses and generally do not have the capacity to support commercial products manufactured under cGMP by biopharmaceutical firms. The suppliers may be ill-equipped to support our needs, especially in non-routine circumstances like an FDA inspection or medical crisis, such as widespread contamination. We also do not have contracts with many of these suppliers, and may not be able to contract with them on acceptable terms or at all. Accordingly, we may experience delays in receiving key raw materials to support clinical or commercial manufacturing.
20
In addition, some raw materials are currently available from a single supplier, or a small number of suppliers. We cannot be sure that these suppliers will remain in business, or that they will not be purchased by one of our competitors or another company that is not interested in continuing to produce these materials for our intended purpose.
If we or our third-party manufacturers use hazardous and biological materials in a manner that causes injury or violates applicable law, we may be liable for damages.
Our research and development activities involve the controlled use of potentially hazardous substances, including chemical and biological materials, by our third-party manufacturers. Our manufacturers are subject to federal, state and local laws and regulations in the United States governing the use, manufacture, storage, handling and disposal of medical and hazardous materials. Although we believe that our manufacturers’ procedures for using, handling, storing and disposing of these materials comply with legally prescribed standards, we cannot completely eliminate the risk of contamination or injury resulting from medical or hazardous materials. We intend to develop our own manufacturing facilities, which would make us subject to these same laws, regulations and risks. As a result of any such contamination or injury, we may incur liability or local, city, state or federal authorities may curtail the use of these materials and interrupt our business operations. In the event of an accident, we could be held liable for damages or penalized with fines, and the liability could exceed our resources. We do not have any insurance for liabilities arising from medical or hazardous materials. Compliance with applicable environmental laws and regulations is expensive, and current or future environmental regulations may impair our research, development and production efforts, which could harm our business, prospects, financial condition or results of operations.
Risks Related to Government Regulation
The FDA regulatory approval process is lengthy and time-consuming, and we may experience significant delays in the clinical development and regulatory approval of our product candidates.
We have not previously submitted a BLA to the FDA, or similar approval filings to comparable foreign authorities. A BLA must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety and effectiveness for each desired indication. The BLA must also include significant information regarding the chemistry, manufacturing and controls for the product. We expect the novel nature of our product candidates to create further challenges in obtaining regulatory approval. For example, the FDA has limited experience with commercial development of T cell therapies for cancer. We also intend to obtain regulatory approval of future TCR-based product candidates regardless of cancer type or origin, which the FDA may have difficulty accepting if our clinical trials only involved cancers of certain origins. Accordingly, the regulatory approval pathway for our product candidates may be uncertain, complex, expensive and lengthy, and approval may not be obtained.
We may also experience delays in completing planned clinical trials for a variety of reasons, including delays related to:
| • | the availability of financial resources to commence and complete the planned trials; |
| • | reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | obtaining approval at each clinical trial site by an independent institutional review board, or IRB; |
| • | recruiting suitable patients to participate in a trial; |
| • | having patients complete a trial or return for post-treatment follow-up; |
| • | clinical trial sites deviating from trial protocol or dropping out of a trial; |
| • | adding new clinical trial sites; or |
| • | manufacturing sufficient quantities of qualified materials under cGMPs and applying them on a subject by subject basis for use in clinical trials. |
We could also encounter delays if physicians encounter unresolved ethical issues associated with enrolling patients in clinical trials of our product candidates in lieu of prescribing existing treatments that have established safety and efficacy profiles. Further, a clinical trial may be suspended or terminated by us, the IRBs for the institutions in which such trials are being conducted, the Data Monitoring Committee for such trial, or by the FDA or other regulatory authorities due to a number of factors, including failure to conduct the clinical trial in accordance with
21
regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. The FDA’s review of our data of our ongoing clinical trials of KTE-C19 may, depending on the data, also result in the delay, suspension or termination of one or more clinical trials of KTE-C19, which would also delay or prevent the initiation of our other planned clinical trials. If we experience termination of, or delays in the completion of, any clinical trial of our product candidates, the commercial prospects for our product candidates will be harmed, and our ability to generate product revenue will be delayed. In addition, any delays in completing our clinical trials will increase our costs, slow down our product development and approval process and jeopardize our ability to commence product sales and generate revenue.
The NCI may also experience similar difficulties in completing ongoing clinical trials and conducting future clinical trials of product candidates. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may ultimately lead to the denial of regulatory approval of our product candidates.
The FDA may disagree with our regulatory plan and we may fail to obtain regulatory approval of our product candidates.
We are currently conducting a Phase 1-2 clinical trial of KTE-C19 in patients with refractory DLBCL, PMBCL and TFL. We are also conducting a Phase 2 clinical trial of KTE-C19 in patients with relapsed/refractory MCL and Phase 1-2 clinical trials of KTE-C19 in adult patients with relapsed/refractory ALL and pediatric patients with relapsed/refractory ALL. If the results are sufficiently compelling, we intend to discuss with the FDA filing a BLA for approval of KTE-C19 for the treatment of refractory DLBCL, PMBCL and TFL, and subsequently seek approval for relapsed/refractory MCL and relapsed/refractory ALL. However, the general approach for FDA approval of a new biologic or drug is dispositive data from two well-controlled, Phase 3 clinical studies of the relevant biologic or drug in the relevant patient population. Phase 3 clinical studies typically involve hundreds of patients, have significant costs and take years to complete. We believe our accelerated approval strategy is warranted given the limited alternatives for patients with refractory DLBCL, PMBCL and TFL, but the FDA may ultimately require a Phase 3 clinical trial prior to approval, particularly since eACT represents a novel and personalized treatment.
Our Phase 1-2 clinical trial results may also not support approval. In addition, our product candidates could fail to receive regulatory approval for many reasons, including the following:
| • | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of our clinical trials; |
| • | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that our product candidates are safe and effective for any of their proposed indications; |
| • | the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval, including due to the heterogeneity of patient populations; |
| • | we may be unable to demonstrate that our product candidates’ clinical and other benefits outweigh their safety risks; |
| • | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| • | the data collected from clinical trials of our product candidates may not be sufficient to the satisfaction of the FDA or comparable foreign regulatory authorities to support the submission of a BLA or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere; |
| • | the FDA or comparable foreign regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which we contract for clinical supplies or of any manufacturing facility we develop; and |
| • | the approval policies or regulations of the FDA or comparable foreign regulatory authorities may significantly change in a manner rendering our clinical data insufficient for approval. |
22
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not mean that we will be successful in obtaining regulatory approval of our product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of our product candidates in one jurisdiction does not guarantee that we will be able to obtain or maintain regulatory approval in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that we intend to charge for our products is also subject to approval.
We may also submit marketing applications in other countries. Regulatory authorities in jurisdictions outside of the United States have requirements for approval of product candidates with which we must comply prior to marketing in those jurisdictions. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our products in certain countries. If we fail to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be harmed.
Even if we receive regulatory approval of our product candidates, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense and we may be subject to penalties if we fail to comply with regulatory requirements or experience unanticipated problems with our product candidates.
Any regulatory approvals that we receive for our product candidates will require surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a risk evaluation and mitigation strategy in order to approve our product candidates, which could entail requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. In addition, if the FDA or a comparable foreign regulatory authority approves our product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping for our product candidates will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and cGCPs for any clinical trials that we conduct post-approval. Later discovery of previously unknown problems with our product candidates, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of our product candidates, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, warning letters or holds on clinical trials; |
| • | refusal by the FDA to approve pending applications or supplements to approved applications filed by us or suspension or revocation of license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of our product candidates; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of our product candidates. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained and we may not achieve or sustain profitability.
23
In addition, if we were able to obtain accelerated approval of KTE-C19, the FDA would require us to conduct a confirmatory study to verify the predicted clinical benefit and additional safety studies. The results from the confirmatory study may not support the clinical benefit, which would result in the approval being withdrawn.
Even if we obtain regulatory approval of our product candidates, the products may not gain market acceptance among physicians, patients, hospitals, cancer treatment centers and others in the medical community.
The use of engineered T cells as a potential cancer treatment is a recent development and may not become broadly accepted by physicians, patients, hospitals, cancer treatment centers and others in the medical community. We expect physicians in the large bone marrow transplant centers to be particularly influential and we may not be able to convince them to use eACT for many reasons. For example, certain of the product candidates that we will be developing target a cell surface marker that may be present on cancer cells as well as non-cancerous cells. It is possible that our product candidates may kill these non-cancerous cells, which may result in unacceptable side effects, including death. Additional factors will influence whether our product candidates are accepted in the market, including:
| • | the clinical indications for which our product candidates are approved; |
| • | physicians, hospitals, cancer treatment centers and patients considering our product candidates as a safe and effective treatment; |
| • | the potential and perceived advantages of our product candidates over alternative treatments; |
| • | the prevalence and severity of any side effects; |
| • | product labeling or product insert requirements of the FDA or other regulatory authorities; |
| • | limitations or warnings contained in the labeling approved by the FDA; |
| • | the timing of market introduction of our product candidates as well as competitive products; |
| • | the cost of treatment in relation to alternative treatments; |
| • | the availability of coverage, adequate reimbursement and pricing by third-party payors and government authorities; |
| • | the willingness of patients to pay out-of-pocket in the absence of coverage by third-party payors and government authorities; |
| • | relative convenience and ease of administration, including as compared to alternative treatments and competitive therapies; and |
| • | the effectiveness of our sales and marketing efforts. |
In addition, although we are not utilizing embryonic stem cells or replication competent vectors, adverse publicity due to the ethical and social controversies surrounding the therapeutic use of such technologies, and reported side effects from any clinical trials using these technologies or the failure of such trials to demonstrate that these therapies are safe and effective may limit market acceptance of our product candidates. If our product candidates are approved but fail to achieve market acceptance among physicians, patients, hospitals, cancer treatment centers or others in the medical community, we will not be able to generate significant revenue.
Even if our products achieve market acceptance, we may not be able to maintain that market acceptance over time if new products or technologies are introduced that are more favorably received than our products, are more cost effective or render our products obsolete.
Coverage and reimbursement may be limited or unavailable in certain market segments for our product candidates, which could make it difficult for us to sell our product candidates profitably.
Successful sales of our product candidates, if approved, depend on the availability of coverage and adequate reimbursement from third-party payors. In addition, because our product candidates represent new approaches to the treatment of cancer, we cannot accurately estimate the potential revenue from our product candidates.
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Obtaining coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance.
24
Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs and treatments they will cover and the amount of reimbursement. Reimbursement by a third-party payor may depend upon a number of factors, including, but not limited to, the third-party payor’s determination that use of a product is:
| • | a covered benefit under its health plan; |
| • | safe, effective and medically necessary; |
| • | appropriate for the specific patient; |
| • | cost-effective; and |
| • | neither experimental nor investigational. |
Obtaining coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process that could require us to provide to the payor supporting scientific, clinical and cost-effectiveness data for the use of our products. Even if we obtain coverage for a given product, the resulting reimbursement payment rates might not be adequate for us to achieve or sustain profitability or may require co-payments that patients find unacceptably high. Patients are unlikely to use our product candidates unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our product candidates.
In the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. Therefore, coverage and reimbursement for products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our product candidates to each payor separately, with no assurance that coverage and adequate reimbursement will be obtained.
We intend to seek approval to market our product candidates in both the United States and in selected foreign jurisdictions. If we obtain approval in one or more foreign jurisdictions for our product candidates, we will be subject to rules and regulations in those jurisdictions. In some foreign countries, particularly those in the EU, the pricing of biologics is subject to governmental control. In these countries, pricing negotiations with governmental authorities can take considerable time after obtaining marketing approval of a product candidate. In addition, market acceptance and sales of our product candidates will depend significantly on the availability of coverage and adequate reimbursement from third-party payors for our product candidates and may be affected by existing and future health care reform measures.
Third-party payors, whether domestic or foreign, or governmental or commercial, are developing increasingly sophisticated methods of controlling healthcare costs. In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory changes to the health care system that could impact our ability to sell our products profitably. In particular, in 2010, the Healthcare Reform Act was enacted. The Healthcare Reform Act and its implementing regulations, among other things, revised the methodology by which rebates owed by manufacturers to the state and federal government for covered outpatient drugs and certain biologics, including our product candidates, under the Medicaid Drug Rebate Program are calculated, increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program, extended the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations, subjected manufacturers to new annual fees and taxes for certain branded prescription drugs, and provided incentives to programs that increase the federal government’s comparative effectiveness research.
Other legislative changes have been proposed and adopted in the United States since the Healthcare Reform Act was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect on April 1, 2013 and will remain in effect until 2024, unless additional congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, or the ATRA, which, among other things, reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
25
There have been, and likely will continue to be, legislative and regulatory proposals at the foreign, federal and state levels directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. We cannot predict the initiatives that may be adopted in the future. The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare and/or impose price controls may adversely affect:
| • | the demand for our product candidates, if we obtain regulatory approval; |
| • | our ability to set a price that we believe is fair for our products; |
| • | our ability to generate revenue and achieve or maintain profitability; |
| • | the level of taxes that we are required to pay; and |
| • | the availability of capital. |
Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors, which may adversely affect our future profitability.
Risks Related to Our Intellectual Property
We depend on intellectual property licensed from third parties and termination of any of these licenses could result in the loss of significant rights, which would harm our business.
We are dependent on patents, know-how and proprietary technology, both our own and licensed from others.
We have several license agreements, including with Cabaret Biotech Ltd., or Cabaret, and Dr. Zelig Eshhar, the NIH, Amgen Inc. and Alpine Immune Sciences, Inc. These licenses may be terminated upon certain conditions, as further described under “Item 1. Business—Our Research and Development and License Agreements” in our Annual Report. Any termination of these licenses could result in the loss of significant rights and could harm our ability to commercialize our product candidates. In addition, Cabaret in-licenses some of the intellectual property rights it is licensing to us. To the extent Cabaret fails to meet its obligations under its license agreement, which we are not in control of, we may lose the benefits of our license agreement with Cabaret.
Disputes may also arise between us and our licensors regarding intellectual property subject to a license agreement, including:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | whether and the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | our right to sublicense patent and other rights to third parties under collaborative development relationships; |
| • | our diligence obligations with respect to the use of the licensed technology in relation to our development and commercialization of our product candidates, and what activities satisfy those diligence obligations; and |
| • | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners. |
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
In addition, our subsidiary, Kite Pharma EU B.V., has licenses to certain intellectual property rights relating to its TCR-GENErator platform, and we are subject to the same risks of termination and disputes with respect to our subsidiary’s licenses. See “Item 1. Business—T-Cell Factory Acquisition” in our Annual Report. We are generally also subject to all of the same risks with respect to protection of intellectual property that we license, as we are for intellectual property that we own, which are described below. If we or our licensors fail to adequately protect this intellectual property, our ability to commercialize products could suffer.
26
If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively in our market.
We rely upon a combination of patents, trade secret protection and license agreements to protect the intellectual property related to our technologies. Any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market.
We primarily rely on our license agreement with Cabaret with respect to CAR-based product candidates generally and KTE-C19 specifically, and rely and expect to rely on license agreements with the NIH for other product candidates. Certain intellectual property which is covered by these agreements has been developed with funding from the U.S. government. As such, our rights in this intellectual property are subject to certain research and other rights of the government.
We anticipate additional patent applications will be filed both in the United States and in other countries, as appropriate. However, we cannot predict:
| • | if and when patents will issue; |
| • | the degree and range of protection any issued patents will afford us against competitors including whether third parties will find ways to invalidate or otherwise circumvent our patents; |
| • | whether or not others will obtain patents claiming aspects similar to those covered by our patents and patent applications; or |
| • | whether we will need to initiate litigation or administrative proceedings which may be costly whether we win or lose. |
Composition of matter patents for biological and pharmaceutical products such as CAR- or TCR-based product candidates are generally considered to be the strongest form of intellectual property protection for those types of products, as such patents provide protection without regard to any method of use. We cannot be certain that the claims in our pending patent applications covering composition of matter of our product candidates will be considered patentable by the United States Patent and Trademark Office, or the USPTO, or by patent offices in foreign countries, or that the claims in any of our issued patents will be considered patentable by courts in the United States or foreign countries. Method of use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method. Moreover, even if competitors do not actively promote their product for our targeted indications, physicians may prescribe these products “off-label.” Although off-label prescriptions may infringe or contribute to the infringement of method of use patents, the practice is common and such infringement is difficult to prevent or prosecute.
The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates or uses thereof in the United States or in other foreign countries. Even if the patents do successfully issue, third parties may challenge the validity, enforceability or scope thereof, for example through inter partes review, or IPR, post-grant review or ex parte reexamination before the USPTO or oppositions and other comparable proceedings in foreign jurisdictions, which may result in such patents being cancelled, narrowed, invalidated or held unenforceable. For example, on November 16, 2015, an anonymous party filed for an ex parte reexamination of a patent that we licensed pursuant to our agreement with Cabaret. If, as a result, one or more of the claims in this patent are determined to be invalid or unenforceable, our ability to block certain third party CAR products with this patent could be seriously impaired. Even if our patents and applications are unchallenged, they may not adequately protect our intellectual property or prevent others from designing around our claims. If the breadth or strength of protection provided by the patents and patent applications we hold with respect to our product candidates is threatened, it could dissuade companies from collaborating with us to develop, and threaten our ability to commercialize, our product candidates. Further, if we encounter delays in our clinical trials, the period of time during which we could market our product candidates under patent protection would be reduced. United States patent applications containing or at any time contained a claim not entitled to a priority date before March 16, 2013 are subject to the “first to file” system implemented by the America Invents Act (2011).
27
This first to file system will require us to be cognizant going forward of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain that we were the first to file any patent application related to our product candidates. Furthermore, for United States applications in which all claims are entitled to a priority date before March 16, 2013, an interference proceeding can be provoked by a third-party or instituted by the USPTO, to determine who was the first to invent any of the subject matter covered by the patent claims of our applications.
In addition to the protection afforded by patents, we seek to rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our product discovery and development processes that involve proprietary know-how, information, or technology that is not covered by patents. Although we require all of our employees to assign their inventions to us, and require all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information, or technology to enter into confidentiality agreements, we cannot be certain that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Furthermore, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent unauthorized material disclosure of our intellectual property to third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially adversely affect our business, operating results and financial condition.
Third-party claims of intellectual property infringement may prevent or delay our product discovery and development efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation involving patents and other intellectual property rights in the biotechnology and pharmaceutical industries. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are developing our product candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may give rise to claims of infringement of the patent rights of others.
Third parties may assert that we are employing their proprietary technology without authorization. We are aware of a U.S. patent held by one of our competitors relating to certain CAR compositions of matter. Generally, conducting clinical trials and other development activities in the United States to develop data supporting an FDA new drug application is not considered “infringement.” If and when KTE-C19 or another of our CAR-based product candidates is approved by the FDA, that competitor may then seek to enforce its patent by filing a patent infringement lawsuit against us. On August 13, 2015, we filed a petition with the USPTO to institute an IPR proceeding of this competitor’s patent, requesting a determination that the claims in the patent are invalid. By law, the USPTO has until February 2016 to determine whether to grant or deny our petition to institute the IPR. If the USPTO grants our petition, it is required to reach a determination within approximately 12 months from when it institutes the IPR proceeding. If the USPTO grants our petition and finds any particular claim of the patent invalid, and that determination is sustained (if appealed), that particular claim would not impede commercialization of KTE-C19 or any of our other CAR-based product candidates. If the USPTO grants our petition and upholds validity, we as petitioner would be estopped from asserting in court any ground of invalidity that we raised or reasonably could have raised in the IPR proceeding. Any claim of this patent that has not been held to be invalid could potentially be used as a basis to claim infringement and, consequently, to try and block our ability to commercialize our CAR-based product candidates, unless we obtain a license under the applicable patent, or until the patent expires. If the USPTO denies our petition and does not institute an IPR proceeding on the patent, this would have no binding effect on subsequent claims or defenses of the parties.
Additionally, there may be third-party patents of which we are currently unaware with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any
28
third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of our product candidates, constructs or molecules used in or formed during the manufacturing process, or any final product itself, the holders of any such patents may be able to block our ability to commercialize the product candidate unless we obtained a license under the applicable patents, or until such patents expire or they are finally determined to be held invalid or unenforceable. In the U.S., issued patents enjoy a presumption of validity that can be overcome in litigation only with evidence that is clear and convincing,” a heightened standard. Similarly, if any third-party patent were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, including combination therapy or patient selection methods, the holders of any such patent may be able to block our ability to develop and commercialize the product candidate unless we obtained a license or until such patent expires or is finally determined to be held invalid or unenforceable. In either case, such a license may not be available on commercially reasonable terms or at all. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, or at all, our ability to commercialize our product candidates may be impaired or delayed, which could in turn significantly harm our business.
Parties making claims against us may seek and obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure. We cannot predict whether any such license would be available at all or whether it would be available on commercially reasonable terms. Furthermore, even in the absence of litigation, we may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates. We may fail to obtain any of these licenses at a reasonable cost or on reasonable terms, if at all. In that event, we would be unable to further develop and commercialize our product candidates, which could harm our business significantly.
We may not be successful in obtaining or maintaining necessary rights to product components and processes for our development pipeline through acquisitions and in-licenses.
Presently we have rights to the intellectual property, through licenses from third parties and under patent applications that we own or will own, to develop KTE-C19 and certain other product candidates. Because our programs may involve additional product candidates that may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license or use these proprietary rights. For instance, while we have certain intellectual property directed to a CAR-based product candidate that targets the EGFRvIII antigen, we may require an additional license relating to the EGFRvIII scFv target binding site in order to commercialize a CAR-based product candidate that targets the EGFRvIII antigen. In addition, while we have patent rights directed to certain CAR constructs, we do not have, and do not expect to obtain, any intellectual property to broad TCR constructs. Rather, any intellectual property directed to TCR-based product candidates that we may obtain would be product specific.
Our product candidates may also require specific formulations to work effectively and efficiently and these rights may be held by others. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology.
The licensing and acquisition of third-party intellectual property rights is a competitive area, and companies, which may be more established, or have greater resources than we do, may also be pursuing strategies to license or acquire third-party intellectual property rights that we may consider necessary or attractive in order to commercialize our product candidates. More established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, for licenses to additional product candidates, we would have to negotiate a license with the NIH or other third parties for the rights to certain patents and patent applications relating to such product candidates. There
29
can be no assurance that we will be able to successfully complete such negotiations and ultimately acquire the rights to the intellectual property surrounding the additional eACT product candidates that we may seek to acquire.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that one or more of our patents is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign our infringing products, which may be impossible or require substantial time and monetary expenditure.
Interference proceedings provoked by third parties or brought by the USPTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could result in a loss of our current patent rights and could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or interference proceedings may result in a decision adverse to our interests and, even if we are successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our trade secrets or confidential information, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
Issued patents covering our product candidates could be found unpatentable, invalid or unenforceable if challenged in court or the USPTO.
If we or one of our licensing partners initiate legal proceedings against a third party to enforce a patent covering one of our product candidates, the defendant could counterclaim that the patent covering our product candidate, as applicable, is invalid and/or unenforceable. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms
30
include IPR, ex parte re-examination and post grant review in the United States, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings).For example, on November 16, 2015, an anonymous party filed for an ex parte reexamination of a patent that we licensed pursuant to our agreement with Cabaret. Such proceedings could result in revocation or amendment to our patents in such a way that they no longer cover our product candidates. The outcome following legal assertions of unpatentability, invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we, our patent counsel and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on our product candidates. Such a loss of patent protection could have a material adverse impact on our business.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. For example, in the recent case, Assoc. for Molecular Pathology v. Myriad Genetics, Inc., the U.S. Supreme Court held that certain claims to DNA molecules are not patentable. While we do not believe that any of the patents owned or licensed by us will be found invalid based on this decision, we cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patents.
We have limited foreign intellectual property rights and may not be able to protect our intellectual property rights throughout the world.
We have limited intellectual property rights outside the United States, and, in particular, our patents directed to CAR constructs do not extend outside of the United States. Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our products and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biopharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
31
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
We have received confidential and proprietary information from third parties. In addition, we employ individuals who were previously employed at other biotechnology or pharmaceutical companies. We may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of these third parties or our employees’ former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial cost and be a distraction to our management and employees.
Risks Related to Our Common Stock
The price of our stock has been and may continue to be highly volatile, and you could lose all or part of your investment.
Prior to our initial public offering in 2014, there was no public market for our common stock. We cannot assure you that an active, liquid trading market for our shares will develop or persist. You may not be able to sell your shares quickly or at a recently reported market price if trading in our common stock is not active. The trading price of our common stock following our initial public offering has been and is likely to continue to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control, including limited trading volume. In addition to the factors discussed in this “Risk Factors” section, these factors include:
| • | the commencement, enrollment or results of our ongoing and planned clinical trials of our product candidates or any future clinical trials we may conduct, or changes in the development status of our product candidates; |
| • | any delay in our regulatory filings for our product candidates and any adverse development or perceived adverse development with respect to the applicable regulatory authority’s review of such filings, including without limitation the FDA’s issuance of a “refusal to file” letter or a request for additional information; |
| • | adverse results or delays in clinical trials; |
| • | our or NCI’s decision to initiate a clinical trial, not to initiate a clinical trial or to terminate an existing clinical trial; |
| • | adverse regulatory decisions, including failure to receive regulatory approval of our product candidates; |
| • | changes in laws or regulations applicable to our products, including but not limited to clinical trial requirements for approvals; |
| • | adverse developments concerning our manufacturers; |
| • | our inability to obtain adequate product supply for any approved product or inability to do so at acceptable prices; |
| • | our inability to establish collaborations if needed; |
| • | our failure to commercialize our product candidates; |
| • | additions or departures of key scientific or management personnel; |
| • | unanticipated serious safety concerns related to the use of our product candidates; |
| • | introduction of new products or services offered by us or our competitors; |
| • | announcements of significant acquisitions, strategic partnerships, joint ventures or capital commitments by us or our competitors; |
| • | our ability to effectively manage our growth; |
| • | the size and growth of our initial cancer target markets; |
| • | our ability to successfully treat additional types of cancers or at different stages, including the ability of Kite Pharma EU B.V. to discover new TCR-based product candidates; |
| • | actual or anticipated variations in quarterly operating results; |
| • | our cash position; |
| • | our failure to meet the estimates and projections of the investment community or that we may otherwise provide to the public; |
32
| • | publication of research reports about us or our industry, or immunotherapy in particular, or positive or negative recommendations or withdrawal of research coverage by securities analysts; |
| • | changes in the market valuations of similar companies; |
| • | overall performance of the equity markets; |
| • | sales of our common stock by us or our stockholders in the future; |
| • | trading volume of our common stock; |
| • | changes in accounting practices; |
| • | ineffectiveness of our internal controls; |
| • | disputes or other developments relating to proprietary rights, including patents, litigation matters and our ability to obtain patent protection for our technologies; |
| • | significant lawsuits, including patent or stockholder litigation; |
| • | general political and economic conditions; and |
| • | other events or factors, many of which are beyond our control. |
In addition, the stock market in general, and The NASDAQ Global Select Market and biopharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. In the past, securities class action litigation has often been instituted against companies following periods of volatility in the market price of a company’s securities. This type of litigation, if instituted, could result in substantial costs and a diversion of management’s attention and resources, which would harm our business, operating results or financial condition.
We do not intend to pay dividends on our common stock so any returns will be limited to the value of our stock.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Any return to stockholders will therefore be limited to the appreciation of their stock.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of September 30, 2015, our executive officers, directors, and 10% stockholders beneficially owned approximately 37.6% of our voting stock, a significant portion of which is beneficially owned by Arie Belldegrun, our President, Chief Executive Officer and Chairman. These stockholders may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents, or approval of any merger, sale of assets, or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders.
We are an emerging growth company, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act, or the JOBS Act, enacted in April 2012. While we no longer expect to be an emerging growth company as of December 31, 2015, we have been able to take advantage of exemptions from various reporting requirements as an emerging growth company, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding nonbinding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our common stock less attractive because we relied on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. As a result, changes in
33
rules of U.S. generally accepted accounting principles or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations.
Failure to establish and maintain adequate finance infrastructure and accounting systems and controls could impair our ability to comply with the financial reporting and internal controls requirements for publicly traded companies.
As a public company, we operate in an increasingly demanding regulatory environment, including with respect to more complex accounting rules. Company responsibilities required by the Sarbanes-Oxley Act include establishing and maintaining corporate oversight and adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud.
Our compliance with Section 404 of the Sarbanes-Oxley Act will require that we incur substantial accounting expense and expend significant management efforts. We expect to have to comply with Section 404 at December 31, 2015 and our testing, and the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls that we would be required to remediate in a timely manner so as to be able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act each year. If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner each year, we could be subject to sanctions or investigations by the SEC, NASDAQ or other regulatory authorities which would require additional financial and management resources and could adversely affect the market price of our common stock. Furthermore, if we cannot provide reliable financial reports or prevent fraud, our business and results of operations could be harmed and investors could lose confidence in our reported financial information.
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we have incurred and will continue to incur significant legal, accounting and other expenses that we did not incur as a private company. We are subject to the reporting requirements of the Exchange Act, which requires, among other things, that we file with the SEC annual, quarterly and current reports with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act, as well as rules subsequently adopted by the SEC and The NASDAQ Global Select Market to implement provisions of the Sarbanes-Oxley Act, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, in July 2010, the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, was enacted. There are significant corporate governance and executive compensation related provisions in the Dodd-Frank Act that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Recent legislation permits emerging growth companies to implement many of these requirements over a longer period and up to five years from the pricing of our initial public offering. We intend to take advantage of this new legislation but cannot guarantee that we will not be required to implement these requirements sooner than budgeted or planned and thereby incur unexpected expenses. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
We expect the rules and regulations applicable to public companies to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. If these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition and results of operations. The increased costs will decrease our net income or increase our net loss, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
34
Sales of a substantial number of shares of our common stock by our existing stockholders in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market or the perception that these sales might occur, could depress the market price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
Sales of our common stock by current stockholders may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate, and make it more difficult for you to sell shares of our common stock.
Certain holders of our securities are entitled to rights with respect to the registration of their shares under the Securities Act. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock.
We have registered on Form S-8 all shares of common stock that are issuable under our 2014 Equity Incentive Plan, as amended, or the EIP. As a consequence, these shares can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates.
In connection with our recently announced public offering of common stock, we and our directors and executive officers have entered into lock-up agreements for a period of 90 days, respectively, following the offering, subject to specified exceptions. We and our directors and executive officers may be released from lock-up prior to the expiration of the lock-up period at the sole discretion of Jefferies LLC and Citigroup Global Markets Inc. Upon expiration or earlier release of the lock-up, we and our directors and executive officers may sell shares into the market, which could adversely affect the market price of shares of our common stock.
Actual or potential sales of our common stock by our employees, including our directors and executive officers, pursuant to pre-arranged stock trading plans could cause our stock price to fall or prevent it from increasing for numerous reasons, and actual or potential sales by such persons could be viewed negatively by other investors.
In accordance with the guidelines specified under Rule 10b5-1 of the Exchange Act, and our policies regarding stock transactions, a number of our employees, including certain executive officers have adopted and may continue to adopt stock trading plans pursuant to which they arrange to sell shares of our common stock from time to time in the future. Generally, sales under such plans by our executive officers require public filings. Actual or potential sales of our common stock by such persons could cause the price of our common stock to fall or prevent it from increasing for numerous reasons. For example, a substantial number of shares of our common stock becoming available (or being perceived to become available) for sale in the public market could cause the market price of our common stock to fall or prevent it from increasing. Also, actual or potential sales by such persons could be viewed negatively by other investors.
Future sales and issuances of our common stock or rights to purchase common stock, including pursuant to the EIP, could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
We expect that significant additional capital may be needed in the future to continue our planned operations, including conducting clinical trials, commercialization efforts, expanded research and development activities and costs associated with operating a public company. To raise capital, we may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing stockholders, and new investors could gain rights, preferences and privileges senior to the holders of our common stock.
Pursuant to the EIP, our management is authorized to grant stock options to our employees, directors and consultants.
The number of shares of our common stock reserved for issuance under our EIP will automatically increase on January 1 of each year continuing through and including January 1, 2024, by 5% of the total number of shares of our capital stock outstanding on December 31 of the preceding calendar year, or such lesser number of shares
35
determined by our board of directors. In addition, the number of shares of our common stock reserved for issuance under our 2014 Employee Stock Purchase Plan, or ESPP, will automatically increase on January 1 of each year continuing through and including January 1, 2024, by the lessor of (1) 1% of the total number of shares of our common stock outstanding on December 31 of the preceding calendar year, (2) 720,000 shares, or (3) a number determined by our board of directors that is less than (1) and (2). Unless our board of directors elects not to increase the number of shares underlying our EIP and ESPP each year, our stockholders may experience additional dilution, which could cause our stock price to fall.
Anti-takeover provisions under our charter documents and Delaware law could delay or prevent a change of control which could limit the market price of our common stock and may prevent or frustrate attempts by our stockholders to replace or remove our current management.
Our amended and restated certificate of incorporation and amended and restated bylaws contain provisions that could delay or prevent a change of control of our company or changes in our board of directors that our stockholders might consider favorable. Some of these provisions include:
| • | a board of directors divided into three classes serving staggered three-year terms, such that not all members of the board will be elected at one time; |
| • | a prohibition on stockholder action through written consent, which requires that all stockholder actions be taken at a meeting of our stockholders; |
| • | a requirement that special meetings of stockholders be called only by the chairman of the board of directors, the chief executive officer, or by a majority of the total number of authorized directors; |
| • | advance notice requirements for stockholder proposals and nominations for election to our board of directors; |
| • | a requirement that no member of our board of directors may be removed from office by our stockholders except for cause and, in addition to any other vote required by law, upon the approval of not less than two-thirds of all outstanding shares of our voting stock then entitled to vote in the election of directors; |
| • | a requirement of approval of not less than two-thirds of all outstanding shares of our voting stock to amend any bylaws by stockholder action or to amend specific provisions of our certificate of incorporation; and |
| • | the authority of the board of directors to issue preferred stock on terms determined by the board of directors without stockholder approval and which preferred stock may include rights superior to the rights of the holders of common stock. |
In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporate Law, which may prohibit certain business combinations with stockholders owning 15% or more of our outstanding voting stock. These anti-takeover provisions and other provisions in our amended and restated certificate of incorporation and amended and restated bylaws could make it more difficult for stockholders or potential acquirors to obtain control of our board of directors or initiate actions that are opposed by the then-current board of directors and could also delay or impede a merger, tender offer or proxy contest involving our company. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing or cause us to take other corporate actions you desire. Any delay or prevention of a change of control transaction or changes in our board of directors could cause the market price of our common stock to decline.
If securities or industry analysts issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if the clinical trials and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more analysts do not initiate coverage of us, cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
36
