Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Inspyr Therapeutics, Inc. | v426465_8k.htm |
| EX-99.01 - EXHIBIT 99.01 - Inspyr Therapeutics, Inc. | v426465_ex99-01.htm |
Exhibit 99.02

1 ICV Conference December 8 - 9, 2015 OTC/QB : GNSZ www.GenSpera.com Craig A. Dionne, PhD President & CEO

2 Forward - Looking Statements Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, the outcome of its clinical trials, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the Company's filings and future filings made with the U.S. Securities and Exchange Commission. Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera directly. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock. This presentation is based upon information available to the public, as well as other information from sources management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of the presentation and are subject to change without notice.

3 • Developing a naturally occurring toxin in a peptide delivery system to target solid tumors without the side - effect profile of chemotherapeutic agents • Large market opportunities in multiple cancers including liver, brain and prostate with potential for combination with immunotherapy • Efficacy being demonstrated in mid - stage clinical trials in 2 orphan indications • Positive proof - of - concept Phase 2 liver cancer trial • Highly encouraging data from ongoing Phase 2 brain cancer trial • Mechanism of action protected by robust , global patent estate including exclusive license to technology developed at Johns Hopkins University • Exclusive rights with world - class partners for commercial - scale production and raw material supply • Management has a history of success in oncology drug discovery and development Investment Highlights
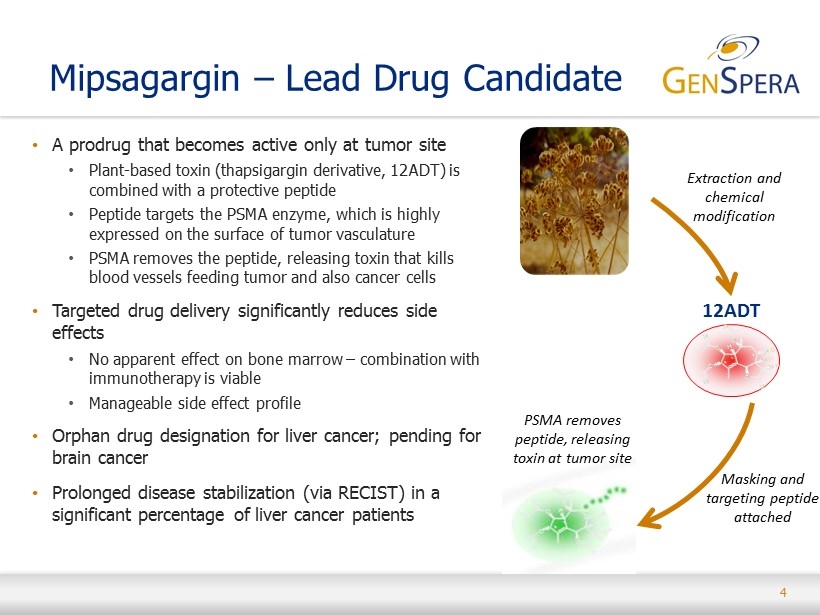
4 • A prodrug that becomes active only at tumor site • Plant - based toxin (thapsigargin derivative, 12ADT) is combined with a protective peptide • Peptide targets the PSMA enzyme, which is highly expressed on the surface of tumor vasculature • PSMA removes the peptide, releasing toxin that kills blood vessels feeding tumor and also cancer cells • Targeted drug delivery significantly reduces side effects • No apparent effect on bone marrow – combination with immunotherapy is viable • Manageable side effect profile • Orphan drug designation for liver cancer; pending for brain cancer • Prolonged disease stabilization (via RECIST) in a significant percentage of liver cancer patients Mipsagargin – Lead Drug Candidate 12ADT Extraction and chemical modification Masking and targeting peptide attached PSMA removes peptide, releasing toxin at tumor site
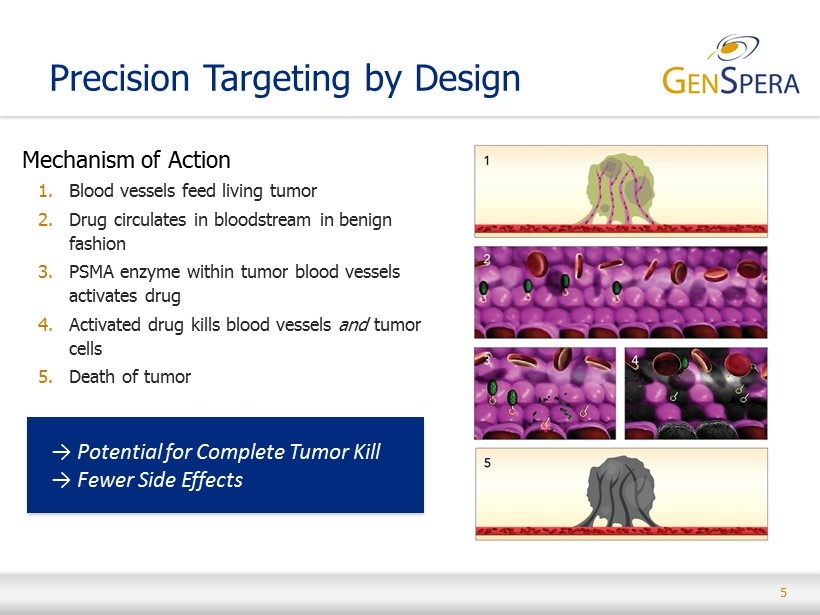
5 Mechanism of Action 1. Blood vessels feed living tumor 2. Drug circulates in bloodstream in benign fashion 3. PSMA enzyme within tumor blood vessels activates drug 4. Activated drug kills blood vessels and tumor cells 5. Death of t umor Precision Targeting by Design → Potential for Complete Tumor Kill → Fewer Side Effects
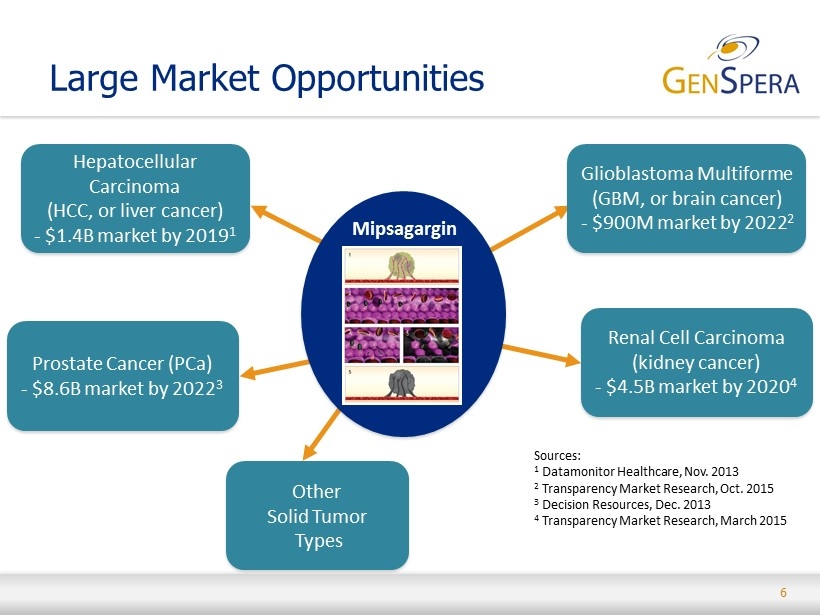
6 Large Market Opportunities Mipsagargin Hepatocellular Carcinoma (HCC, or liver cancer ) - $1.4B market by 2019 1 Glioblastoma Multiforme (GBM, or brain cancer ) - $900M market by 2022 2 Prostate Cancer (PCa) - $8.6B market by 2022 3 Renal Cell Carcinoma (kidney cancer) - $4.5B market by 2020 4 Other Solid Tumor Types Sources: 1 Datamonitor Healthcare, Nov. 2013 2 Transparency Market Research, Oct. 2015 3 Decision Resources, Dec. 2013 4 Transparency Market Research, March 2015
7 Compelling Preclinical Data Efficacy Pharmacology • Complete tumor regression with monotherapy in mouse tumor models • No need to combine with other drugs • Fewer side effects • Safety margin of 7 - fold to 10 - fold in mouse • No acquired resistance to mipsagargin Bioanalytical • No released active 12ADT in blood (only intact, inactive mipsagargin) • 12ADT found at very high levels in tumors, less in liver and kidneys, not found elsewhere Toxicology • No effect on liver or cardiovascular system • No effect on bone marrow – no immunosuppression or anemia • Kidney is affected at high doses – dose - dependent, non - cumulative, easily monitored and 100% rapidly reversible
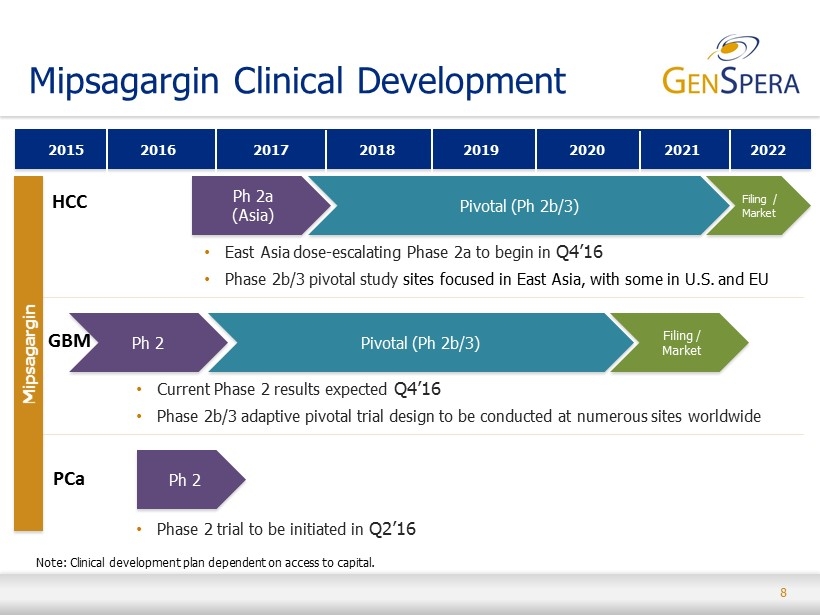
8 Mipsagargin Clinical Development • Phase 2 trial to be initiated in Q2’16 2020 2015 2017 2019 2016 2018 Ph 2 Pivotal (Ph 2b/3) Filing / Market • East Asia dose - escalating Phase 2a to begin in Q4’16 • Phase 2b/3 pivotal study sites focused in East Asia, with some in U.S. and EU • Current Phase 2 results expected Q4’16 • Phase 2b/3 adaptive pivotal trial design to be conducted at numerous sites worldwide HCC Ph 2a (Asia) Ph 2 Note: Clinical development plan dependent on access to capital . GBM PCa Mipsagargin 2021 Pivotal (Ph 2b/3) 2022 Filing / Market
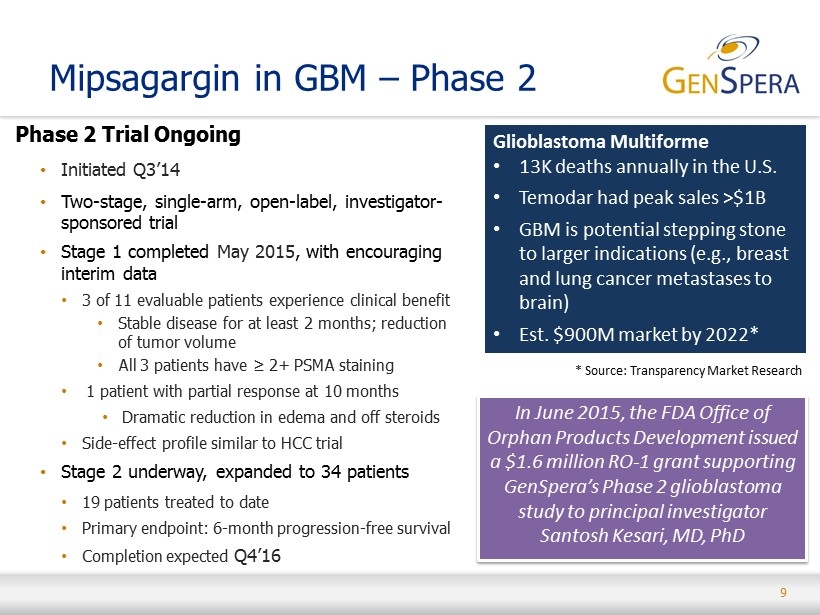
9 Mipsagargin in GBM – Phase 2 Phase 2 Trial Ongoing • Initiated Q3’14 • Two - stage, single - arm, open - label, investigator - sponsored trial • Stage 1 completed May 2015 , with encouraging interim data • 3 of 11 evaluable patients experience clinical benefit • Stable disease for at least 2 months; reduction of tumor volume • All 3 patients have ≥ 2+ PSMA staining • 1 patient with partial response at 10 months • Dramatic reduction in edema and off steroids • Side - effect profile similar to HCC trial • Stage 2 underway, expanded to 34 patients • 19 patients treated to date • Primary endpoint: 6 - month progression - free survival • Completion expected Q4’16 Glioblastoma Multiforme • 13K deaths annually in the U.S. • Temodar had peak sales >$1B • GBM is potential stepping stone to larger indications (e.g., breast and lung cancer metastases to brain ) • Est. $900M market by 2022* In June 2015, the FDA Office of Orphan Products Development issued a $1.6 million RO - 1 grant supporting GenSpera’s Phase 2 glioblastoma study to principal investigator Santosh Kesari, MD, PhD * Source: Transparency Market Research
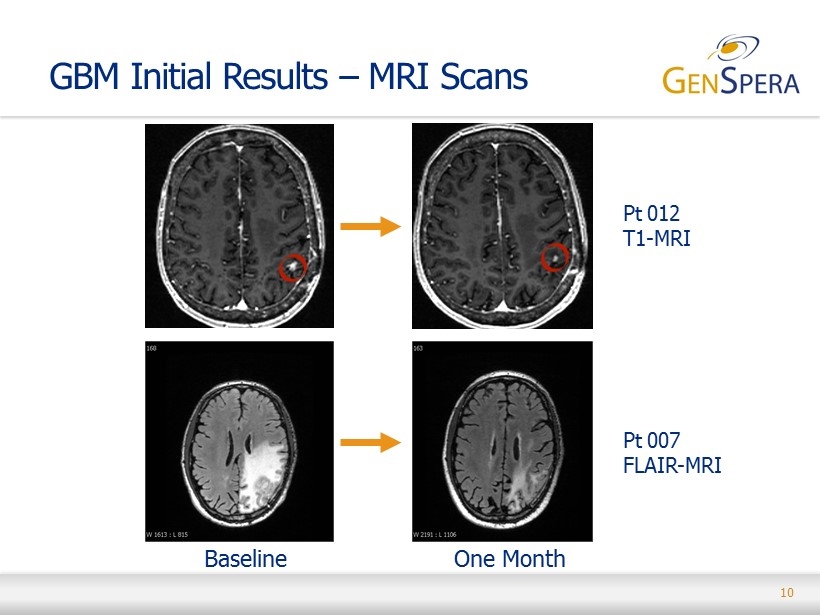
10 Pt 012 T1 - MRI Pt 007 FLAIR - MRI GBM Initial Results – MRI Scans Baseline One Month
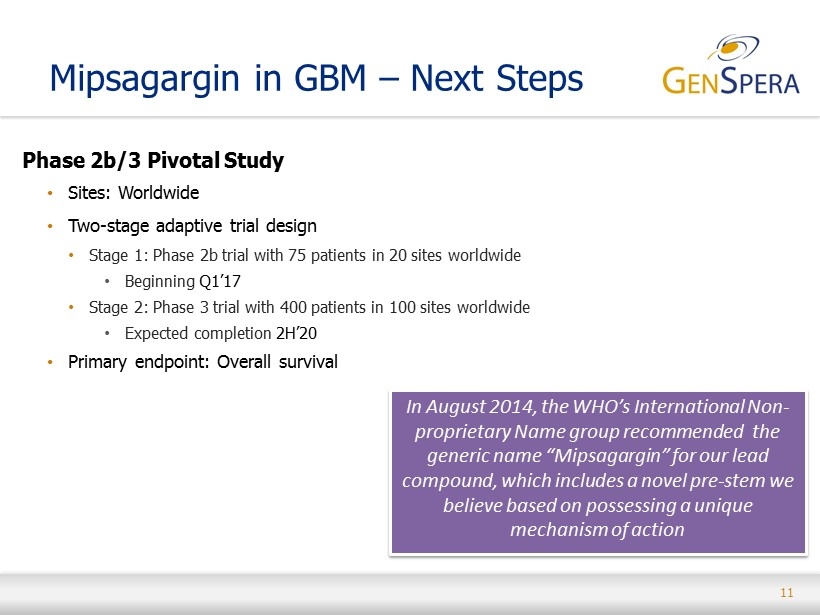
11 Mipsagargin in GBM – Next Steps Phase 2b/3 Pivotal Study • Sites: Worldwide • Two - stage adaptive trial design • Stage 1: Phase 2b trial with 75 patients in 20 sites worldwide • Beginning Q1’17 • Stage 2: Phase 3 trial with 400 patients in 100 sites worldwide • Expected completion 2H’20 • Primary endpoint: Overall survival In August 2014, the WHO’s International Non - proprietary Name group recommended the generic name “Mipsagargin” for our lead compound, which includes a novel pre - stem we believe based on possessing a unique mechanism of action
12 Mipsagargin in HCC – Phase 1 Phase 1: First In - Human Study • Completed August 2013 • Multicenter, dose - escalation, 3+3 design • 44 patients with advanced solid tumors treated, including 5 with HCC at 40/66.8/66.8 mg/m 2 • Observation: 2 of 5 HCC patients with prolonged disease stabilization at 12 and 25 months Hepatocellular Carcinoma • No. 3 cancer - killer worldwide (over 600K deaths annually) • Over 700K new cases diagnosed annually • Estimated $1.4B market by 2019* * Source: Datamonitor Healthcare
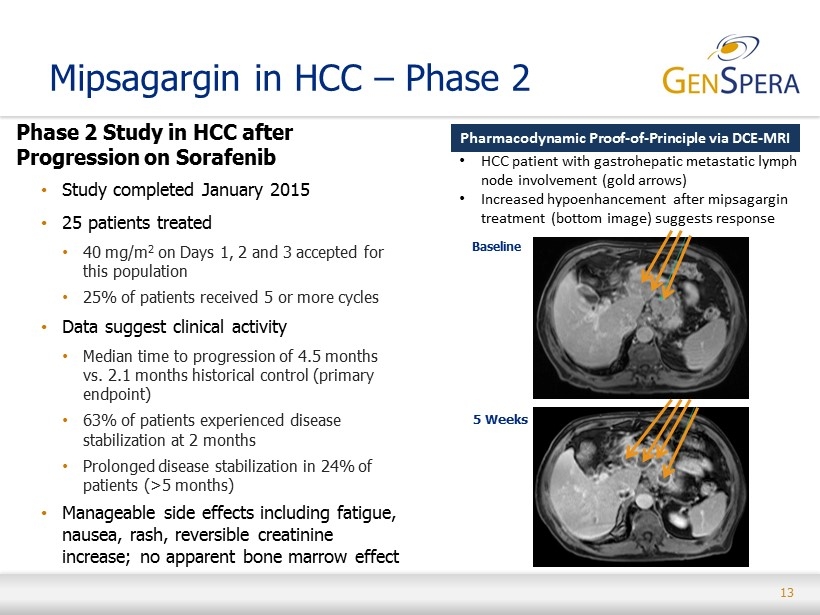
13 Phase 2 Study in HCC after Progression on Sorafenib • Study completed January 2015 • 25 patients treated • 40 mg/m 2 on Days 1, 2 and 3 accepted for this population • 2 5% of patients received 5 or more cycles • Data suggest clinical activity • Median time to progression of 4.5 months vs. 2.1 months historical control (primary endpoint) • 63% of patients experienced disease stabilization at 2 months • Prolonged disease stabilization in 24% of patients (> 5 months) • Manageable side effects including fatigue, nausea, rash, reversible creatinine increase; no apparent bone marrow effect Mipsagargin in HCC – Phase 2 Pharmacodynamic Proof - of - Principle via DCE - MRI Baseline 5 Weeks • HCC patient with gastrohepatic metastatic lymph node involvement ( gold arrows) • Increased hypoenhancement after mipsagargin treatment (bottom image) suggests response
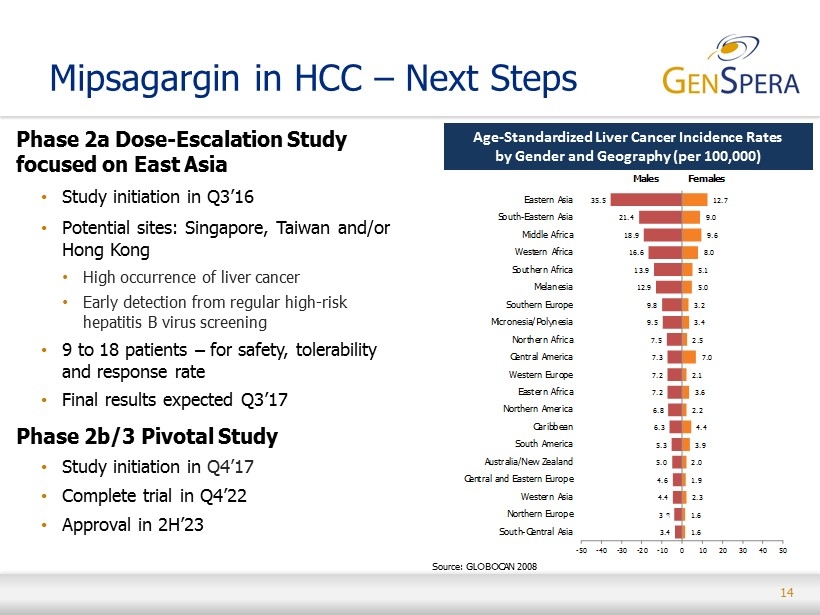
14 Mipsagargin in HCC – Next Steps Phase 2a Dose - Escalation Study focused on East Asia • Study initiation in Q3’16 • Potential sites: Singapore, Taiwan and/or Hong Kong • High occurrence of liver cancer • Early detection from regular high - risk hepatitis B virus screening • 9 to 18 patients – for safety, tolerability and response rate • Final results expected Q3’17 Phase 2b/3 Pivotal Study • Study initiation in Q4’17 • Complete trial in Q4’22 • Approval in 2H’23 3.4 3.8 4.4 4.6 5.0 5.3 6.3 6.8 7.2 7.2 7.3 7.5 9.5 9.8 12.9 13.9 16.6 18.9 21.4 35.5 1.6 1.6 2.3 1.9 2.0 3.9 4.4 2.2 3.6 2.1 7.0 2.5 3.4 3.2 5.0 5.1 8.0 9.6 9.0 12.7 -50 -40 -30 -20 -10 0 10 20 30 40 50 South-Central Asia Northern Europe Western Asia Central and Eastern Europe Australia/New Zealand South America Caribbean Northern America Eastern Africa Western Europe Central America Northern Africa Micronesia/Polynesia Southern Europe Melanesia Southern Africa Western Africa Middle Africa South-Eastern Asia Eastern Asia Males Females Age - Standardized Liver Cancer Incidence Rates by Gender and Geography (per 100,000) Source : GLOBOCAN 2008
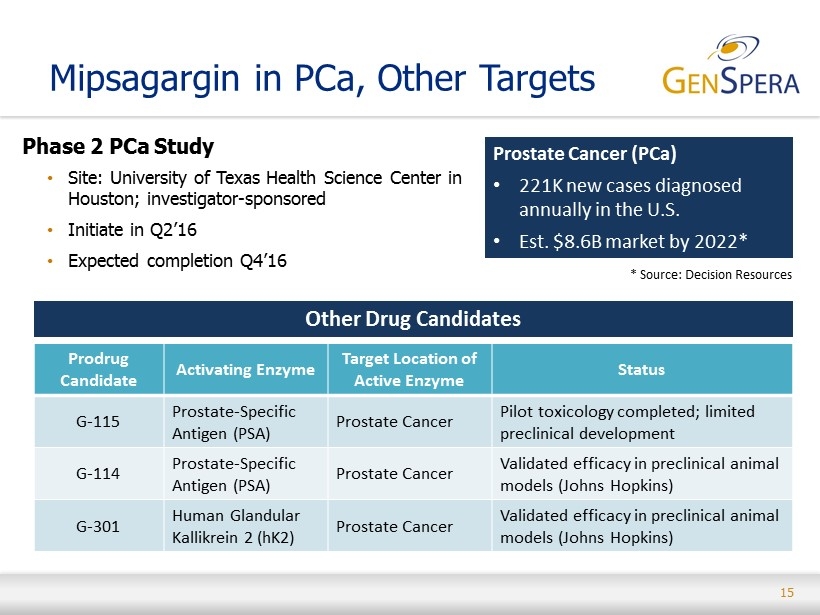
15 Mipsagargin in PCa, Other Targets Phase 2 PCa Study • Site: University of Texas Health Science Center in Houston ; investigator - sponsored • Initiate in Q2’16 • Expected completion Q4’16 Prostate Cancer (PCa) • 221K new cases diagnosed annually in the U.S. • Est. $8.6B market by 2022* * Source: Decision Resources Prodrug Candidate Activating Enzyme Target Location of Active Enzyme Status G - 115 Prostate - Specific Antigen (PSA) Prostate Cancer Pilot toxicology completed; limited preclinical development G - 114 Prostate - Specific Antigen (PSA) Prostate Cancer Validated efficacy in preclinical animal models (Johns Hopkins) G - 301 Human Glandular Kallikrein 2 (hK2) Prostate Cancer Validated efficacy in preclinical animal models (Johns Hopkins) Other Drug Candidates

16 • Exclusive license to use technology developed at Johns Hopkins University and the University of Copenhagen • Current i nfusion composition • Patent coverage in U.S. to 2023 • Data exclusivity outside of U.S. − up to 10 years after drug approval in EU • New composition - of - matter Patent Application • Worldwide exclusivity expected through 2033 • Other applications filed for methods of use, formulations, process improvements • Exclusive partnership with Phyton Biotech for commercial - scale thapsigargin production • The name mipsagargin has novel stem – new drug class differentiation value Intellectual Property

17 Business Strategy • O ptimize value across multiple indications, territories, and technologies • Continue developing drug candidates independently • Expand management and staffing • Continue business development discussions and partnership opportunities • Develop pipeline through internal efforts, licensing, or acquisition

18 Leadership and Scientific Teams Management and Board (Prior Experience / Affiliations) Craig A. Dionne, PhD Chairman and Chief Executive Officer Russell Richerson, PhD COO and Corporate Secretary Peter E. Grebow, PhD Director Bo Jesper Hansen, MD, PhD Director Scott V. Ogilvie Director Scientific Advisory Board John T. Isaacs, PhD Chief Scientific Advisor, Co - Founder Professor at Johns Hopkins School of Medicine, co - inventor of GenSpera’s technologies Samuel R. Denmeade, MD Chief Clinical Advisor and Co - Founder Professor at Johns Hopkins School of Medicine and board - certified medical oncologist, co - inventor of GenSpera’s technologies Søren Brøgger Christensen, PhD Scientific Advisory Board Member Professor at University of Copenhagen; expert in chemistry of thapsigargin, exploring further derivatives and manufacturing efficiencies for thapsigargin AFIN International, Inc. Gulf Enterprises International, Ltd.
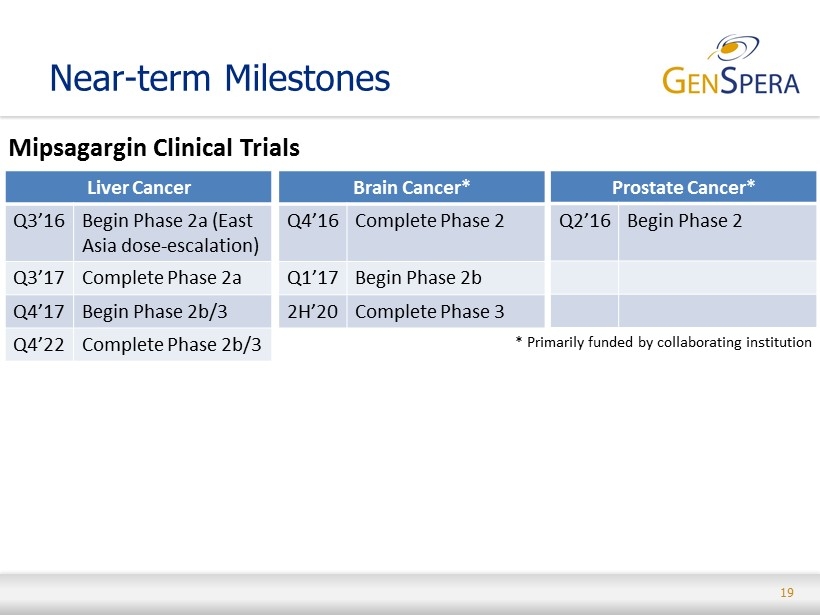
19 Near - term Milestones Mipsagargin Clinical Trials Liver Cancer Q3’16 Begin Phase 2a (East Asia dose - escalation) Q3’17 Complete Phase 2a Q4’17 Begin Phase 2b/3 Q4’22 Complete Phase 2b/3 Brain Cancer* Q4’16 Complete Phase 2 Q1’17 Begin Phase 2b 2H’20 Complete Phase 3 Prostate Cancer* Q2’16 Begin Phase 2 * Primarily funded by collaborating institution

20 • Developing a naturally occurring toxin in a peptide delivery system to target solid tumors without the side - effect profile of chemotherapeutic agents • Large market opportunities in multiple cancers including liver, brain and prostate with potential for combination with immunotherapy • Efficacy being demonstrated in mid - stage clinical trials in 2 orphan indications • Positive proof - of - concept Phase 2 liver cancer trial • Highly encouraging data from ongoing Phase 2 brain cancer trial • Mechanism of action protected by robust , global patent estate including exclusive license to technology developed at Johns Hopkins University • Exclusive rights with world - class partners for commercial - scale production and raw material supply • Management has a history of success in oncology drug discovery and development Investment Highlights
