Attached files
| file | filename |
|---|---|
| EX-31.2 - EXHIBIT 31.2 - SIRONA DENTAL SYSTEMS, INC. | t1502747_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - SIRONA DENTAL SYSTEMS, INC. | t1502747_ex31-1.htm |
| EX-32.1 - EXHIBIT 32.1 - SIRONA DENTAL SYSTEMS, INC. | t1502747_ex32-1.htm |
| EX-32.2 - EXHIBIT 32.2 - SIRONA DENTAL SYSTEMS, INC. | t1502747_ex32-2.htm |
| EX-21.1 - EXHIBIT 21.1 - SIRONA DENTAL SYSTEMS, INC. | t1502747_ex21-1.htm |
| EX-23.1 - EXHIBIT 23.1 - SIRONA DENTAL SYSTEMS, INC. | t1502747_ex23-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
| FORM 10-K |
| (Mark One) | þ | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
| For the fiscal year ended September 30, 2015 | ||
| or | ||
| ¨ | Transition Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 | |
| For the transition period from ___________to ___________ |
Commission file number 000-22673

Sirona Dental Systems, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 11-3374812 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
30-30 47th Avenue, Suite 500, Long Island City, New York |
11101 | |
| (Address of principal executive offices) | (Zip Code) |
(718) 482-2011
(Registrant’s telephone number, including area code)
| Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. |
Yes þ No ¨
|
| Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. |
Yes ¨ No þ
|
| Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. |
Yes þ No ¨
|
| Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). | Yes þ No ¨ |
| Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. | ¨ |
| Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one): |
Large accelerated filer þ Accelerated filer ¨ Non-accelerated filer ¨ Smaller reporting company ¨ |
| Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). | Yes ¨ No þ |
The aggregate market value of common stock held by non-affiliates of the registrant as of March 31, 2015, the last business day of the registrant’s most recently completed second fiscal quarter, was approximately $2,834,488,012. Such aggregate market value is computed by reference to the closing sale price of the Common Stock on such date.
As of November 17, 2015, the number of shares outstanding of the Registrant’s Common Stock, par value $.01 per share, was 55,896,887.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement for its 2015 annual meeting of stockholders to be filed pursuant to Regulation 14A not later than 120 days after the end of the fiscal year (September 30, 2015) are incorporated by reference into Part III of this report on Form 10-K.
FORWARD-LOOKING STATEMENTS
This Form 10-K Annual Report contains forward-looking statements that involve risks and uncertainties. All statements, other than statements of historical facts, included in this Annual Report regarding the Company, its financial position, products, business strategy, and plans and objectives of management of the Company for future operations, are forward-looking statements. When used in this Annual Report, words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “objectives,” “plans”, and similar expressions, or the negatives thereof or variations thereon or comparable terminology as they relate to the Company, its products or its management, identify forward-looking statements. Such forward-looking statements are based on the beliefs of the Company’s management, as well as assumptions made by and information currently available to the Company’s management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of various factors, including, but not limited to, those contained in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in Item 7 of this Annual Report and the “Risk Factors” set forth in Item 1A of this Annual Report. All forward looking statements speak only as of the date of this Annual Report and are expressly qualified in their entirely by the cautionary statements included in this report. The Company undertakes no obligation to update or revise forward-looking statements which may be made to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated events other than required by law.
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
i
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
OVERVIEW
Sirona Dental Systems, Inc. ( “Sirona,” the “Company,” “we,” “us,” and “our” refer to Sirona Dental Systems, Inc. and its consolidated subsidiaries and their predecessors, except as otherwise indicated or unless context otherwise requires) is the leading global manufacturer of high-quality, technologically-advanced dental equipment, and is focused on developing, manufacturing, and marketing innovative solutions for dentists around the world. The Company is uniquely positioned to benefit from several trends in the global dental industry, such as technological innovation, the shift to digital imaging, favorable demographic trends, and growing patient focus on dental health and cosmetic appearance. The Company’s headquarters is in Long Island City, New York, and its largest facility is located in Bensheim, Germany.
Sirona has a long tradition of innovation in the dental industry. The Company introduced the first dental electric drill over 130 years ago, the first dental X-ray unit approximately 100 years ago, the first dental computer-aided design/computer-aided manufacturing (CAD/CAM) system 30 years ago, and numerous other significant innovations in dentistry. Sirona continues to make significant investments in research and development (“R&D”), and its track record of innovative and profitable new products continues today. Sirona has the broadest product portfolio in the industry and is capable of fully outfitting and integrating a dental practice.
The majority of our revenues derive from the manufacture and sale of dental equipment. In addition, we also provide sales and after-sales service support to dentists and distributors through our growing sales and service infrastructure.
Sirona manages its commercial operations on both a product and geographic basis and maintains four reporting segments: 1) Dental CAD/CAM Systems, 2) Imaging Systems, 3) Treatment Centers, and 4) Instruments. Products from each category are marketed in all geographical sales regions.
The Company’s business has grown substantially in the past several years, driven by numerous high-tech product introductions, a continued expansion of its global sales and service infrastructure, strong relationships with key distribution partners, namely Patterson and Henry Schein, and an international dealer network.
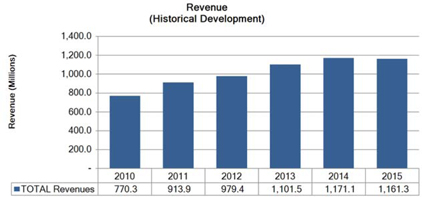
| 1 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
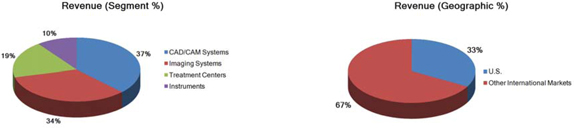
Segment and geographic breakouts of Sirona’s revenues for the fiscal year ended September 30, 2015 were as follows:
HISTORY
Sirona dates back to the establishment of Reiniger, Gebbert & Schall, which introduced the first dental electrical drill in 1882. In 1925, the Company became part of Siemens & Halske Group and in 1934 launched the smallest x-ray in the world, enabling dental x-rays for the first time. In 1956, Siemens introduced Sirona as a brand for treatment centers, and in 1958 the group developed the first ball-bearing turbine for dental drills.
In 1997, funds advised by the financial sponsor, Permira, acquired the Sirona dental business from Siemens in a leveraged buy-out transaction. Following the transaction, Sirona substantially increased its international sales and intensified its focus on product innovation. In November 2003, Permira sold Sirona to the Scandinavian financial sponsor, EQT, and management in a leveraged buy-out transaction that closed in February 2004. In April 2005, funds managed by Madison Dearborn Partners, a private equity firm, and Sirona’s management entered into an agreement to acquire Sirona in a leveraged buy-out transaction that closed in June 2005.
In September 2005, Schick Technologies, Inc. (“Schick”) entered into an Exchange Agreement with Sirona Holdings Luxco S.C.A. (“Luxco”) and Sirona Holding GmbH (“Sirona Holding”) providing for the issuance of 36,972,480 shares of Schick common stock to Luxco in exchange for Luxco’s entire economic interest in Sirona Holding, which consisted of all of the issued and outstanding share capital of Sirona Holding and the existing indebtedness of Sirona Holding owed to Luxco in the principal amount of Euro 151.0 million ($182 million) plus accrued interest (the “Exchange”). In June 2006, the Exchange closed and Schick, a Delaware corporation formed in 1997, was renamed Sirona Dental Systems, Inc. Although Sirona Holding became a subsidiary of Schick upon the completion of the Exchange, Sirona Holding was deemed the acquiring corporation for accounting purposes because Luxco received a controlling ownership interest in the Company, Sirona Holding's designees constituted a majority of the members of the Company’s board of directors and Sirona Holding's senior management represented a majority of the senior management of the Company. In May 2011, Luxco sold all of its remaining shares of Sirona common stock pursuant to an underwritten follow-on public offering.
On September 15, 2015, the Company and DENTSPLY International, Inc. (“DENTSPLY”) announced that the Board of Directors of both companies had unanimously approved a definitive Agreement and Plan of Merger (the “Merger Agreement”) under which the companies will combine in an all-stock merger (the “Merger”). DENTSPLY is a leading manufacturer and distributor of dental and other consumable medical device products. The Merger Agreement provides that, upon the terms and subject to the conditions set forth in the Merger Agreement, Sirona will merge with and into a wholly-owned subsidiary of DENTSPLY, with Sirona surviving as a wholly-owned subsidiary of DENTSPLY. Upon completion of the Merger, the combined company's name will be changed to DENTSPLY SIRONA Inc. Subject to the terms and conditions of the Merger Agreement, if the Merger is completed, each outstanding share of Sirona common stock will be converted into the right to receive 1.8142 shares of common stock of DENTSPLY, with cash paid in lieu of any fractional shares of common stock of DENTSPLY that a Sirona stockholder would otherwise have been entitled to receive.
| 2 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The transaction, which is expected to be completed in the second quarter of Fiscal 2016, is subject to the receipt of regulatory approvals and other customary closing conditions, including the approval of stockholders of both Sirona and DENTSPLY. For additional information related to the Merger refer to DENTSPLY’S Registration Statement on Form S-4 which was filed with the SEC on October 29, 2015.
Our common stock is currently traded publicly on the NASDAQ Global Select Market under the trading symbol “SIRO”.
INDUSTRY/PRODUCTS
Overview
The global dental market encompasses the diagnosis, treatment and prevention of disease and ailments of the teeth, gums and supporting bone. This market has enjoyed steady growth, driven by a number of factors, including an increased desire for aesthetics, a demographic shift towards an aging population coupled with a desire to retain tooth structure later in life, growth in disposable income, a desire for more convenience on the part of both dentists and patients, a shift towards private pay, a greater need for dental preventative care.
The global dental market has benefited from technological innovation, which increase productivity for the dentist. This is particularly important in markets facing increased demand for dental services with little or no increase in the number of dentists servicing those markets. In addition, technological developments allow dentists to offer higher quality treatment to patients. We believe that the high-tech end of the dental market is growing at a faster pace than the overall dental market and that this trend will continue over time.
Recent technological advancements in the dental equipment industry include 3D radiography, digital radiography, CAD/CAM technology, and intra oral cameras.
Sirona serves the high-tech dental equipment and technology market for dental practitioners and laboratories. We are the only manufacturing company that can fully outfit a dental practitioner’s office with dental equipment, including treatment centers, imaging systems, dental CAD/CAM systems, and instruments. Our products represent important investments by dental practitioners, and some of these products can have a life span of 10-20 years (shorter for instruments and software), depending on the nature and quality of the product.
Products
Our principal products are generally classified into the following segments: Dental CAD/CAM Systems, Imaging Systems, Treatment Centers, and Instruments.
A brief description of each of our segments follows. See Note 11 to our consolidated financial statements for revenues and gross profit by segment for each of the last three fiscal years, and assets by segment, at September 30, 2015 and 2014.
| Segment Revenue Contribution | Year ended | |||||
| September 30, | ||||||
| (In percent) | 2015 | 2014 | 2013 | |||
| Dental CAD/CAM Systems | 37% | 37% | 37% | |||
| Imaging Systems | 34% | 34% | 35% | |||
| Treatment Centers | 19% | 19% | 19% | |||
| Instruments | 10% | 10% | 9% | |||
| Total | 100% | 100% | 100% | |||
| 3 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Dental CAD / CAM Systems
Dental CAD/CAM Systems address the worldwide market for dental restorations, which includes several types of restorations, such as inlays, onlays, veneers, crowns, bridges, copings and bridge frameworks made from ceramic, metal or composite blocks. The global market for dental restorations can be divided into two sub-segments: in-mouth fillings and out-of-mouth pre-shaped restorations. CAD/CAM-produced ceramic restorations represent a growing portion of the out-of-mouth restoration market and the number of out-of-mouth restorations prepared with CAD/CAM systems has increased substantially over the past few years. At the same time, the number of dental practitioners and dental laboratories using CAD/CAM technology has increased. Sirona estimates that as of the end of fiscal year 2015, the market penetration for in-office CAD/CAM systems had grown to approximately 16% in the United States and Germany.
Sirona pioneered the application of high-tech CAD/CAM techniques to the traditional lab-based restoration process with the commercialization of the CEramic REConstruction, or CEREC, method. Sirona’s CEREC system is an in-office application that enables dentists to produce high quality restorations from ceramic material and insert them into the patient’s mouth during a single appointment. CEREC has a number of advantages compared to the traditional out-of-mouth pre-shaped restoration method, as CEREC does not require a physical model, restorations can be created in the dentist’s office and the procedure can be completed in a single visit. The CEREC system consists of an imaging unit and a milling unit. The imaging unit scans the damaged area, captures the image of the tooth or teeth requiring restoration and proposes the specifications for the restoration. The milling unit then mills the ceramic restoration to the required specifications based upon the captured image and the dentist’s design specifications. The result is a biocompatible, non-metallic, natural-looking restoration made of durable, high-quality ceramic materials completed in a single treatment session. Independent studies indicate that CEREC ceramic restorations are as durable as gold and can replace conventional restoration materials for most procedures. In addition, CEREC restorations are aesthetically pleasing and have the benefit of a natural-looking appearance.
Sirona offers a service contract on its CEREC product, which includes software updates and upgrades and maintenance on software-related hardware.
In addition to CEREC, Sirona also offers CAD/CAM products for dental laboratories, including the inLab restoration fabrication system and the extra-oral inEos scanner. These products are designed to improve efficiency and reduce costs for the dental laboratory. The inLab system scans the models received from the dentists and then mills ceramic or composite block restorations, such as crown copings and bridge frameworks to the specifications of the captured image.
Summary Innovation Timeline (last 10 years) | |
| Dental CAD/CAM Systems | |
| Year | Product / Event |
| 2005 | Launch of the inEos scanner, which is a high speed extra-oral scanner which produces 3D digital images from a single tooth up to a jaw, directly from the plaster model. |
| 2007 | Launch of the MC XL next generation milling unit, which produces high quality, precisely fitted restorations in about half the time that the older CEREC milling units required. |
| Introduction of Sirona’s Biogeneric software, which virtually automated the design portion of the CAD/CAM process for inlays and onlays. | |
| Launch of the next generation inLab milling unit, the inLab MC XL. The inLab MC XL milling and grinding unit opens up a broad range of production options for the dental laboratory. Milling performance and precision have been greatly enhanced, and a switch from grinding to milling can be accomplished in just a few, simple steps. | |
| 2008 | Expansion of the central restoration service business to the United States. |
| Expanded our CEREC offering with the introduction of Sirona Connect. Sirona Connect is a web-based service that facilitates the electronic transmission of digital impressions acquired with a CEREC acquisition unit to inLab laboratories. Laboratories can use the digital impression to create final restorations. This process eliminates the need to take physical impressions, resulting in increased accuracy, less reworking of restorations and productivity savings. | |
| 4 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 2009 | Launch of a new CEREC camera, based on the Company’s proprietary Bluecam technology, which was faster, more accurate, and improved the workflow for practitioners. |
| 2010 | Further enhancement Sirona’s Biogeneric software with the introduction of Version 3.8, which has the ability to create crowns and bridges. |
| Introduction of the CEREC AC Connect stand-alone digital impression unit. CEREC AC Connect allows dental professionals to scan digital impressions and then send them to the inLab® dental laboratory of their choice. | |
| Introduction of the successor inEos scanner, the inEos Blue. inEos Blue is based on the Bluecam technology, is easy to use, fast, precise, flexible, and its auto capture function allows for substantial time savings. | |
| 2011 | Introduction of CEREC 4.0 software, an entirely redesigned software that gives CEREC users enhanced capabilities and speeds up the restoration process. In addition, CEREC 4.0 enables dentists to design and manufacture multiple restorations simultaneously, further enhancing productivity and profitability. |
| 2012 | Launch of new CEREC Omnicam camera, which allows dentists to generate precise whole-arch scans in the shortest possible time. Three features of the CEREC Omnicam are particularly notable: video streaming, digitization of jaw structures in their natural color, and powderless scanning of tooth surfaces. This introduction further strengthens Sirona’s leadership position in the dental CAD/CAM market. |
| Introduction of inLab 4.0 software, which offers an extended spectrum of clinical applications. New design tools facilitate a customized and direct workflow. The completely revised platform provides a secure basis for integrating future applications. | |
| 2013 | Launch of updated and expanded CEREC 4.2 software, further differentiated its CAD/CAM milling product line with the units MC X and MC XL Premium, and introduced the Apollo DI digital imaging system, all in March. These introductions expanded our portfolio and enable Sirona to offer “CAD/CAM for Everyone”, an approach which seeks to address the various needs of the widest possible range of dentists. |
| Launch of enhanced inLab MC XL. | |
| Introduction of the successor inEos scanner, the inEos X5. The 5-axis in Eos X5 is unrivaled in precision and has flexible handling, quick scanning times, and a comprehensive application spectrum for all digitization tasks. | |
| Launch of inLab4.2 software, which introduced further applications, such as smart design with virtual articulation, smile design, and other features. | |
| 2014 | Launch of CEREC 4.3 software, which enables carbide milling for optimal fabrication of zirconium oxide and polymer materials. Improved and simplified workflow for Omnicam. Virtual articulation is now calculated automatically. Integrated button to automatically upload the current case in Sirona Connect. CEREC 4.3 also allows a screw-retained implant workflow. Together with the introduction of additional TiBases and Scanpost, this leads to strong growth in implantology consumables. |
| Launch of precolored inCoris TZI C blocks that remove the need to use coloring liquids, thus simplifying the operating process with an improved result. | |
| 2015 | Introduction of inLab MC X5, new milling and grinding unit for dental labs. First Sirona disc processing unit with 5 axis designed specifically for the commercial dental lab. |
| Launch of CEREC Ortho 1.1 software: CEREC can now also be used for digital impressions for orthodontic indications, e.g., for treatment with transparent aligners. The new guided scanning procedure enables precise, full-arch digital impressions. The CEREC Ortho software creates a digital model of the entire arch. The data obtained can then be sent for planning the orthodontic treatment and manufacturing the required appliances, thus eliminating the necessity to create and send a physical model. A cooperation agreement with Align Technology allows dentists to also use digital impressions for aligner therapy with Invisalign. | |
| Launch of CEREC Guide 2: the surgical guide can now be produced chairside and cost-effectively in the practice. The guide is designed based upon the ideal prosthetic and surgical positioning via combination of CEREC intra-oral digital impressions and Sirona’s 3D X-ray volume data. Once designed, the guide is then milled from PMMA on one of the CEREC MC X or CEREC MC XL Premium Package milling units. The guide can be manufactured within an hour and requires no models or a radiographic guide with reference bodies. |
| 5 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| Launch of CEREC 4.4 software: CEREC 4.4 provides simple and user-friendly operation. The software also includes several new functions that further improve the workflow and produce more accurate results throughout the design process. The new Biojaw algorithm uses the entire scanned area as a reference for the restoration to be created and generates initial restoration proposals. The latest software uses improved algorithms that allow smoother surfaces and deeper fissures when grinding feldspathic, glass and silicate ceramics. With the new, extra-fine grinding tools of the 4-motor CEREC MC XL Premium Package in particular, designs are milled with more detail and greater precision. | |
| Introduction of CEREC AF/AI: In addition to the CEREC AC cart version, two new acquisition versions are available: the CEREC AF flexible tabletop version and the CEREC AI integrated in the treatment center TENEO. Both new models use the CEREC Omnicam powder-free color camera. |
Imaging Systems
Imaging Systems comprise a broad range of systems for diagnostic imaging in the dental practice. Sirona has developed a comprehensive range of imaging systems for 2D or 3D, panoramic, and intra-oral applications that allow the dentist to provide a full range of diagnostics and treat the patient in a more efficient manner, resulting in safer, faster, and better dentistry.
Intra-oral x-ray systems use image-capture sensor devices, which are inserted into the mouth behind the diagnostic area, and typically take images of one or two teeth. Panoramic x-ray systems produce images of the entire jaw structure by means of an x-ray tube and an image capture device, which rotates around the head.
Summary Innovation Timeline (last 10 years) | |
| Imaging Systems | |
| Year | Product / Event |
| 2006 | Expansion of our imaging system product line to include Schick's intraoral sensor portfolio based on CMOS technology, as a result of the Exchange. |
| 2007 | Introduction of the GALILEOS Comfort 3-D imaging unit. Today, three-dimensional imaging is offering dentists advanced diagnostic and therapeutic options in the fields of surgery, implantology, prosthetics, orthodontics, and restorative dentistry. GALILEOS integrates these capabilities efficiently into dental practices. |
| 2008 | Launch of GALILEOS Compact, which is specifically tailored to meet the needs of the general practitioner. |
| 2009 | Introduction of software that facilitates the integration of 3D X-ray volume (bone level data) with a CEREC AC CAD/CAM scan (surface level information). This software allows the practitioner to plan both the implant surgery and the prosthetic at the start of the implant treatment session. This integrated process reduces the number of treatment sessions, resulting in greater accuracy and superior implant alignment. With this new software, the dental practitioner can now place more focus on the desired aesthetic outcome throughout the entire treatment process. |
| 2011 | Introduction of the flagship Orthophos XG 3DReady, which provides dental practitioners with a wide variety of diagnostic possibilities and is upgradeable to a 3D unit. Other models of the family include the Orthophos XG 5 and the basic model Orthophos XG 3. |
| Launch of the Orthophos XG 3D imaging unit. This system gives the practitioner traditional 2D panoramic imaging capability and the ability to scan and view a large, 8x8 centimeter 3D field of view (a scan big enough to capture the entire jaw). Orthophos XG 3D is also available with cephalometric options, orthodontic, implant, and other specialty programs. | |
| 2012 | Launch of the next generation of intraoral digital radiography – the Schick 33 sensor and image management system. Schick 33 is the most advanced sensor in dentistry, delivering an unparalleled combination of high-resolution images and dynamic image management. |
| 2013 | Launch of the GALILEOS CompactPLUS , which is specifically tailored to meet the needs of the general practitioner, orthodontist, and oral surgeon. |
| 2014 | Launch of the SICAT function - the first software which visualizes real, patient-specific movement of the lower jaw within the 3D module of the GALILEOS. |
| 6 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 2015 | Introduction of the Orthophos SL imaging unit. This system ensures image quality in 2D and 3D x-rays via Sharp Layer, a capture technology that automatically adapts the panoramic curve of the sensor to the patient's individual jaw anatomy, thus producing a sharper image. The digital conversion sensor (DCS) technology generates electrical signals directly from the x-ray beams without intermediate conversion to light, thus producing highly precise x-ray images with reduced radiation doses. |
| Launch of the SIDEXIS 4 software, which based on workflows, provides access to all pertinent patient image data and acts as a central hub for the integration of diagnostic image data of any kind - not only prepared during the course of the actual treatment, but also image data received from other dentists for long-term patients. With SIDEXIS 4, the dentist is able to display a full overview of the patient's treatment history in a timeline. |
Treatment Centers
Treatment Centers comprise a broad range of products from basic dentist chairs to sophisticated chair-based units with integrated diagnostic, hygiene, and ergonomic functionalities, as well as specialist centers used in preventative treatment and for training purposes. Sirona offers specifically configured products to meet the preferences of dentists within each region in which it operates. Sirona’s treatment center configurations and system integration are designed to enhance productivity by creating a seamless workflow within the dental practice. Sirona’s centers therefore allow the dentist to both improve productivity and increase patient satisfaction, significant factors in adding value to his or her practice.
Summary Innovation Timeline (last 10 years) | |
| Treatment Centers | |
| Year | Product / Event |
| 2008 | Launch of the TENEO treatment center, which combines industry-leading technology with a timeless design that provides both patient and dentist with the ultimate in convenience and comfort. |
| 2011 | Introduction of the SINIUS treatment center, a comfort class treatment center, which enables the dentist to maximize time and flexibility of their practice. SINIUS is fully networked and is easily integrated into any dental practice. |
| 2014 | Launch of the INTEGO treatment center. INTEGO offers the design of the new generation of Sirona treatment centers with top quality and flexible configuration options at an attractive price. The fully-networked treatment center comes in two versions: INTEGO and INTEGO pro with extended functionality. |
Instruments
Sirona offers a wide range of instruments, including handheld and power-operated handpieces for cavity preparation, endodontics, periodontology and prophylaxis, which are regularly updated and improved. The instruments are supplemented by multi-function tips, supply and suction hoses, as well as care and hygiene systems for instrument preparation. Sirona’s instruments are often sold as packages in combination with treatment centers. Sirona’s unique handpiece cleaning and sterilizing machine, the DAC Universal, is the only fully automatic system for instruments hygiene – with its unique features it has defined the standard of care for infection prevention in the dental office.
Sirona intends to continue to strengthen the position of its Instruments segment as a diversified supplier of high-quality, reliable, user-friendly and cost-efficient dental instruments.
| 7 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Summary Innovation Timeline (last 10 years) | |
| Instruments | |
| Year | Product / Event |
| 2005 | Launch of first generation SIROLaser - the smallest diode laser worldwide, redefining ergonomics in laser dentistry. |
| Launch of SIROEndo, a device for endodontic treatment with built-in APEX Location, seamlessly adaptable to the treatment center, and optimal integration into daily workflow. | |
| 2007 | Launch of SIROPure - the first handpiece not requiring any lubrication over its entire product lifetime, keeps the cavity oil-free, eliminates maintenance errors, and lowers total cost of ownership. |
| 2009 | Launch of new diode laser, SIROLaser Advance / SIROLaser Xtend. |
| Launch of 3rd generation of DAC Universal, allowing the processing of wrapped instruments. | |
| 2011 | Launch of SIROBoost high performance power turbine line with a high torque level, allowing faster, more efficient, and comfortable operation. |
| 2012 | Launch of the new handpiece program: T2 Line / T3 Line. |
| 2013 | Launch of new turbine generation (T1/T2/T3) and the T4 Line. |
| Launch of SIROInspect - fluorescence-based cavity detection device, whose safe and simple operation significantly reduces the risk of secondary cavities. | |
| 2014 | Launch of new T3 Racer, with 30 watts the most powerful turbine on the market. |
| 2015 | Launch of new SIROLaser Blue, beeing the first dental diode laser to have a blue, an infrared, and a red diode. This enables it to cover a range of 21 indications. |
Manufacturing and Suppliers
Our main manufacturing and assembly activities are located in Bensheim, approximately 60 kilometers south of Frankfurt am Main, Germany. We also operate smaller manufacturing sites in New York, Italy, Denmark and China. All of our facilities are in good condition.
All of our manufacturing facilities have established and maintain a Quality Management System that is registered to ISO 9001:2000 and ISO 13485:2003. Our New York and Bensheim facilities also maintain a Device Establishment Registration with the United States Food and Drug Administration.
Manufacturing consists primarily of assembly, systems integration and testing. We generally outsource manufacturing of parts and components used in the assembly of our products but own the design and tools used by our key component suppliers. We do, however, manufacture most of the precision parts used for our instruments.
We purchase various components for our products from a number of outside suppliers. We currently have established relationships with approximately 1,400 suppliers, of which we view approximately 160 as “key suppliers.” Each supplier is selected according to stringent quality criteria, which are reviewed regularly. We do not believe we are dependent on one or a small group of suppliers and believe we could locate alternative suppliers if needed. For reasons of quality assurance or cost effectiveness, the Company relies on single sources for certain purchased components, e.g. sensors, which we use in our imaging segment. We work closely with our suppliers to help ensure continuity of supply while maintaining high quality and reliability. We have agreements in place and use a number of techniques, including security or consignment stock commitments, to address potential disruptions of the supply chain. We also own any custom tooling used in manufacturing these components. The Company has not experienced any significant difficulty in the past in obtaining the materials necessary to meet its production schedule. However, the need to replace one of our single source suppliers could cause a disruption in our ability to timely deliver certain of our products or increase costs. See Item 1A Risk Factors — “We are dependent upon a limited number of suppliers for critical components. If these suppliers delay or discontinue the manufacture of these components, we may experience delays in shipments, increased costs and cancellation of orders for our products.”
| 8 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Sales and Marketing
Our sales and marketing efforts are directed through regional managers who oversee our sales professionals. These professionals work closely with our distribution partners to maximize the efficiency and productivity of their sales efforts. Our marketing initiatives are focused on highlighting Sirona’s leading role as a high-tech systems provider and industry innovator. In order to promote our brand and increase client loyalty, our distribution partners are supported through wide ranging advertising activities. In addition, we have been a key presenter at all major dental exhibitions, which are critical forums for raising brand awareness and new product introductions. Lastly, our product information is actively made available to business publications, dentists, journals, professional organizations and dental schools, our website (www.sirona.com), and social media offerings (Facebook, Twitter, Sirona Blog, etc.) are important interactive platforms for end-users as well as for distributors.
Distribution
Sirona distributes its products globally to dental practices, clinics and laboratories through an international network of more than 490 distributors and increasingly through our own sales and service infrastructure. See Note 11 to our consolidated financial statements for a description of our net sales and long-lived assets by geographic region for the last three fiscal years. Because distributors typically cover both dental equipment and consumables, they have regular contact with the dentist and are therefore optimally positioned to identify new equipment sale opportunities. Sirona’s primary distributors are Patterson Companies and Henry Schein, two of the world‘s largest dental distributors. In the United States, Patterson is Sirona’s primary distributor. Outside of the United States, Henry Schein is the company‘s largest distributor. Patterson Companies and Henry Schein accounted for 33% and 14%, respectively, of Sirona’s worldwide revenue for the fiscal year ended September 30, 2015. Sirona distributes elsewhere through a well-developed network of independent regional distributors. Sirona works closely with its distributors by training their technicians and sales representatives with respect to its products. With over 10,000 sales and service professionals trained each year, Sirona seeks to ensure high standards of quality in after-sale service and the best marketing of its products. The success of Sirona’s products is evidenced by their importance to its distribution partners, and in many cases are among their best-selling offerings. The Company continues to expand its sales and service infrastructure in selected countries around the world. These investments allow us to support our distributors’ selling efforts and strengthen the Sirona brand in these key markets. These investments, and the subsequent expansion of our infrastructure, have enabled Sirona to grow revenues and profitability at a faster rate.
On April 27, 1998, Sirona and Patterson Companies entered into an exclusive distribution agreement (the ‘‘CAD/CAM Distribution Agreement’’) pursuant to which Patterson was appointed as the exclusive distributor of Sirona’s CEREC CAD/CAM products within the United States and Canada. Under the terms of the CAD/CAM Distribution Agreement, Patterson’s exclusivity was to terminate on September 30, 2007. On June 30, 2005, Sirona and Patterson entered into an amendment of the CAD/CAM Distribution Agreement which extended Patterson’s exclusivity from October 1, 2007 through September 30, 2017. As consideration for the extension of its exclusivity, Patterson agreed to make a one-time payment to Sirona in the amount of $100 million (the ‘‘Exclusivity Fee’’). In July 2005, Patterson paid the Exclusivity Fee, in its entirety, to Sirona. The full amount of the Exclusivity Fee was recorded as deferred income and has been recognized on a straight-line basis since October 1, 2007. The extension did not modify or alter the underlying provisions of the companies’ agreement through 2007, including the performance criteria necessary to maintain the exclusivity. The performance criteria are benchmark thresholds which afford Sirona the opportunity to abandon the exclusivity or to terminate the agreement with Patterson, but do not create minimum purchase obligations under a take-or-pay arrangement. The CAD/CAM Distribution Agreement was amended in May 2011 to revise the parameters for inLab sales in the United States and Canada.
In April 2000, Schick and Patterson entered into an exclusive distribution agreement (the “Schick Distribution Agreement”) covering the United States and Canada; and as of May 1, 2000, Schick began marketing and selling its CDR dental products in the United States and Canada through Patterson. This contract was amended in July 2005, March 2007, and May 2010 and expired on December 31, 2012.
In May 2012, the Company and Patterson amended and restated the terms of their business relationship set forth in CAD/CAM Distribution Agreement and the Schick Distribution Agreement with respect to distribution of certain products throughout the United States and in October 2013 entered into new distribution agreements covering Canada. The amendment and restatement of both the CAD/CAM Distribution Agreement and Schick Distribution Agreement addressed issues related to pricing, termination and annual minimum purchase quotas, and provided growth targets which, if achieved, extend the companies’ exclusivity period.
| 9 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Sirona executes separate contracts with Henry Schein for each product group in each of the various jurisdictions in which Henry Schein distributes its products. The contracts governing most of the products distributed through Henry Schein are non-exclusive. Each of the contracts provides for minimum annual purchases, which are set annually. The contracts have terms of up to five years. Either party is entitled to terminate any of the contracts upon six months’ notice but generally not before the third anniversary of the contract. Sirona may terminate a contract upon 30 days’ notice in case of Henry Schein’s default under the terms of the contract.
Competition
Competition in the global dental market is fragmented by both geography and products. We compete with a variety of companies, including large international companies as well as smaller companies that compete regionally or on a narrower product line. Sirona competes on the basis of its comprehensive and innovative product line and its global distribution network.
Research and Development
Sirona commits significant resources to research and development, with a particular focus on developing products that offer new diagnostic and treatment options, while increasing comfort for both users and patients and streamlining process efficiency. Research and development statistics for the last three fiscal years are as follows:
| Research and Development | Year ended | |||||
| September 30, | ||||||
| (In millions, except otherwise noted) | 2015 | 2014 | 2013 | |||
| Research and development expenses | $ | 54.8 | $ | 64.6 | $ | 60.2 |
| Research and development expenses (% of revenue) | 4.7% | 5.5% | 5.5% | |||
| Number of R&D professionals employed globally | 343 | 320 | 286 | |||
Sirona also cooperates in its research efforts with partners in research facilities and dental practices around the world. In fiscal year 2011, Sirona opened the Center of Innovation in Bensheim, Germany. The Center underscores Sirona’s ongoing commitment to innovation in dentistry. Housing the majority of research and development professionals under one roof will ensure the Company maximum collaboration, creativity, technological advancement, and idea sharing.
Patents, Trade Secrets and Proprietary Rights
We seek to protect our intellectual property through a combination of patent, trademark and trade secret protection. We believe that our future success will depend in part on our ability to obtain and enforce patents for our products and processes, preserve our trade secrets and operate without infringing the proprietary rights of others.
Patents
We have an active corporate patent program, the goal of which is to secure patent protection for our technology. Sirona owns and/or maintains approximately 1,047 patents and patent applications throughout the world. The patents expire at various dates through 2031. We also license or sublicense some of the technology used in our products from third parties.
| 10 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Trademarks
We generally attempt to build brand awareness of our products through the use of trademark registrations. “Sirona,” “CEREC,” “Orthophos,” “Heliodent,” “inLab,” “CDR,” and “Schick” are some of our key registered trademarks. In addition, we have common law trademark rights in several other names we use commercially in connection with our products.
Trade Secrets
In addition to patent protection, we own trade secrets and proprietary know-how, which we seek to protect through agreements with employees and other appropriate individuals. These agreements generally allow assignment of confidential information developed by or made known to the individual by the Company during the course of the individual's relationship with the Company as confidential and not to be disclosed to third parties, except in specific limited circumstances. The agreements also generally assign to the Company all inventions conceived by the individual in the course of rendering services to the Company. However, there can be no assurance that the Company will be successful in enforcing this policy in each case, that the Company would have adequate remedies available for any breach or that the Company's trade secrets will not otherwise become known to, or independently developed by, its competitors.
Regulation
Medical Devices
Most of our products require certain forms of regulatory clearance, including, but not limited to, marketing clearance by the United States Food and Drug Administration (the “FDA”) in accordance with the Federal Food, Drug and Cosmetic Act, as amended (the "FD&C Act") and by our Notified Body in accordance with the European Union's Medical Device Directive 93/42/EEC ("MDD").
The FDA and MDD review process typically requires extended proceedings pertaining to product safety and efficacy. We believe that our future success will depend to a large degree upon commercial sales of improved versions of our current products and sales of new products; we will not be able to market such products in the U.S. or in the European Union without FDA or MDD clearance, respectively. There can be no assurance that any products developed by us in the future will be granted clearance by applicable regulatory authorities or that additional regulations will not be adopted or current regulations amended in such a manner as to adversely affect us.
Pursuant to the FD&C Act, the FDA regulates the introduction, manufacture, advertising, labeling, packaging, marketing and distribution of, and record-keeping for, dental devices. The FDA classifies medical devices intended for human use into three classes: Class I, Class II, and Class III. The Company’s products are classified by the FDA into Class I or II that renders them subject only to general controls that apply to all medical devices, in particular regulations regarding alteration, misbranding, notification, record-keeping and good manufacturing practices.
The FD&C Act further provides that, unless exempted by regulation, medical devices may not be commercially distributed in the U.S. unless they have been cleared by the FDA. There are two review procedures by which medical devices can receive such clearance. Some products may qualify for clearance under a Section 510(k) procedure, in which the manufacturer submits to the FDA a pre-market notification that it intends to begin marketing the product, and shows that the product is substantially equivalent to another legally marketed product (i.e., that it has the same intended use and that it is as safe and effective as a legally marketed device, and does not raise different questions of safety and effectiveness than does a legally marketed device). Certain Class I devices are exempt from the 510(k) pre-market notification requirement and manufacturers of such products may proceed to market without any submission to the FDA. In some cases, the 510(k) notification must include data from human clinical studies.
Marketing in the U.S. may commence once the FDA issues a clearance letter finding such substantial equivalence. According to FDA regulations, the agency has 90 days in which to respond to a Class I or II 510(k) notification. There can be no assurance, however, that the FDA will provide a timely response, or that it will reach a finding of substantial equivalence.
| 11 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
If a product does not qualify for the 510(k) procedure (either because it is not substantially equivalent to a legally marketed device or because it is a Class III device), the FDA must approve a Pre-Market Approval ("PMA") application before marketing can begin. PMA applications must demonstrate, among other things, that the medical device is safe and effective. A PMA application is typically a complex submission that includes the results of clinical studies. Preparation of such an application is a detailed and time-consuming process. Once a PMA application has been submitted, the FDA's review process may be lengthy and include requests for additional data. By statute and regulation, the FDA may take 180 days to review a PMA application, although such time may be extended. Furthermore, there can be no assurance that the FDA will approve a PMA application.
The products that we distribute in the European Union bear the "CE Mark," a European Union symbol of compliance with the MDD. In order to market our products in the member countries of the European Union, it is necessary that those products conform to the requirements of the MDD. Our Bensheim facility which is engaged in the manufacturing of Class IIa and Class IIb medical devices as defined by the MDD is ISO 13485 certified. It is also necessary that our products comply with any revisions which may be made to these standards or the MDD.
Medical devices are subject to ongoing regulatory oversight by the FDA and a Notified Body. The FD&C Act requires that all medical device manufacturers and distributors register annually with the FDA and submit a list of those medical devices which they distribute commercially. The MDD requires that Class IIa devices or higher bear a CE mark with a Notified Body Number. The FD&C Act and the MDD also requires that all manufacturers of medical devices comply with labeling requirements and manufacture their products and maintain their documents in a prescribed manner with respect to manufacturing, testing, and quality control activities. The FDA's Medical Device Reporting regulation and the MDD subject medical devices to post-market reporting requirements for death or serious injury, and for certain malfunctions that would be likely to cause or contribute to a death or serious injury if malfunction were to recur. In addition, the FDA and the MDD prohibit a device which has received marketing clearance from being marketed for applications for which marketing clearance has not been obtained. Furthermore, the FDA generally requires that medical devices not cleared for marketing in the U.S. receive FDA marketing clearance before they are exported, unless an export certification has been granted. The FDA and the ISO Notified Bodies regularly inspect our registered and/or certified facilities.
Failure to comply with applicable regulatory requirements can, among other consequences, result in fines, injunctions, civil penalties, suspensions or loss of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution. In addition, governmental regulations may be established that could prevent or delay regulatory clearance of our products. Delays in receipt of clearance, failure to receive clearance or the loss of previously received clearance would have a material adverse effect on our business, financial condition and results of operations.
Environmental, Health and Safety Matters
In addition to the laws and regulations discussed above, we are subject to government regulations applicable to all businesses, including, among others, regulations related to occupational health and safety, workers' benefits and environmental protection. The extent of government regulation that might result from any future legislation or administrative action cannot be accurately predicted. Failure to comply with regulatory requirements could have a material adverse effect on our business, financial condition and results of operations.
Employees
As of September 30, 2015, the Company had 3,458 employees. The Company believes that its relations with its employees are good. No Company employees are represented by labor unions or are subject to a collective bargaining agreement in the United States. Approximately 25% of our German employees are members of the IG Metall union. We have not experienced any work stoppages due to labor disputes.
Executive Officers
See Part III, Item 10 of this 10-K Report for information about Executive Officers of the Company.
| 12 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Available Information
Information about the Company’s products and services, stockholder information, press releases, and filings with the Securities and Exchange Commission (“SEC”) can be found on the Company’s website at www.sirona.com. The information contained on our website is for informational purposes only and is not incorporated by reference into this report. The Company’s Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and other SEC filings, and any amendments to such reports and filings, are available free of charge in the Investor Relations section of the Company’s website as soon as reasonably practical after the Company’s material is filed with, or furnished to, the SEC.
| ITEM 1A. | RISK FACTORS |
INTRODUCTION
These risk factors may be important to understanding any statement in this Annual Report on Form 10-K or elsewhere. The following information should be read in conjunction with Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A), and the consolidated financial statements and related notes incorporated by reference in this report.
Our businesses routinely encounter and address risks, some of which will cause our future results to be different - sometimes materially different - than we anticipate. Discussion about the material operational risks that our businesses encounter can be found in our MD&A, in the business descriptions in Item 1 of this report and in previous SEC filings. Below, we have described our present view of the material risks facing our business.
RISKS RELATED TO OUR BUSINESS
We must continue to develop new products and adapt to rapid technological change and evolving industry standards to remain competitive.
We are currently developing new products and enhancements to existing products. We cannot assure you that we will initiate, continue with and/or succeed in our efforts to develop or enhance such products. There can be no assurance that any new products will be developed by us, or if developed, will be approved by, or receive marketing clearance from, applicable domestic and/or international governmental or regulatory authorities.
The market for our products is characterized by rapid and significant technological change, evolving industry standards and new product introductions. Our products require significant planning, design, development and testing which requires significant capital commitments and investment by us. There can be no assurance that our products or proprietary technologies will not become noncompetitive or obsolete as a result of technological change, evolving industry standards or new product introductions or that we will be able to generate any economic return on our investment in product development. If our products or technologies become noncompetitive or obsolete, our business could be negatively affected.
If we cannot obtain or maintain approval from government agencies, we will not be able to sell our products.
We must obtain certain approvals by, and marketing clearances from, governmental authorities, including the FDA and similar health authorities in foreign countries to market and sell our products in those countries. These regulatory agencies regulate the marketing, manufacturing, labeling, packaging, advertising, sale and distribution of medical devices. The FDA enforces additional regulations regarding the safety of X-ray emitting devices. Our products are currently regulated by such authorities and certain of our new products will require approval by, or marketing clearance from, various governmental authorities, including the FDA. Various states also impose similar regulations.
| 13 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The FDA review process typically requires extended proceedings pertaining to the safety and efficacy of new products. A 510(k) application is required in order to market a new or modified medical device. If specifically required by the FDA, a pre-market approval, or PMA, may be necessary. Such proceedings, which must be completed prior to marketing a new medical device, are potentially expensive and time consuming. They may delay or hinder a product’s timely entry into the marketplace. Moreover, there can be no assurance that the review or approval process for these products by the FDA or any other applicable governmental authority will occur in a timely fashion, if at all, or that additional regulations will not be adopted or current regulations amended in such a manner as will adversely affect us. The FDA also oversees the content of advertising and marketing materials relating to medical devices which have received FDA clearance. Failure to comply with the FDA’s advertising guidelines may result in the imposition of penalties.
We are also subject to other federal, state and local laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices. The extent of government regulation that might result from any future legislation or administrative action cannot be accurately predicted. Failure to comply with regulatory requirements could have a material adverse effect on our business.
Similar to the FDA review process, the EU review process typically requires extended proceedings pertaining to the safety and efficacy of new products. Such proceedings, which must be completed prior to marketing a new medical device, are potentially expensive and time consuming and may delay or prevent a product’s entry into the marketplace.
Our profitability may be negatively impacted by adverse general macroeconomic conditions in the geographic markets in which we sell our products.
Our profitability depends in part on the varying economic and other conditions of the global dental market, which in turn is impacted by general macroeconomic conditions in the geographic markets in which we sell our products. Growth in the global dental market over the past few years has been driven by a number of factors, including a growth in disposable income, a shift towards private pay, a greater need for dental preventative care and an increased emphasis on aesthetics. Demand for our products would be negatively impacted by a decline in the economy in general, including interest rate and tax changes, that impact the financial strength of our customers, as well as by changes in the economy in general that reduce disposable income among dental consumers in the markets we sell our products, which would in turn reduce the demand for preventative and aesthetic dental services.
The ongoing disruptions in the overall world economy and financial markets could reduce disposable income among dental consumers and negatively affect the demand for dental services, which could be harmful to our financial position and results of operations. Furthermore, there can be no assurances that government responses to the disruptions in the financial markets will stabilize the markets or increase liquidity and the availability of credit for our customers. Difficult economic conditions may also result in a higher rate of losses on our accounts receivable. As a result, our business, results of operations or financial condition could be materially adversely affected.
We are dependent upon a limited number of distributors for a significant portion of our revenue, and loss of these key distributors could result in a loss of a significant amount of our revenue.
Historically, a substantial portion of our revenue has come from a limited number of distributors. For example, Patterson Dental Company, Inc. accounted for 33% of revenue for the fiscal year ended September 30, 2015. In addition, 14% of our revenue for the fiscal year ended September 30, 2015, was attributable to sales to Henry Schein, Inc. It is anticipated that Patterson and Henry Schein will continue to be the largest contributors to our revenue for the foreseeable future. There can be no assurance that Patterson and Henry Schein will purchase any specified minimum quantity of products from us or that they will continue to purchase any products at all. If Patterson or Henry Schein ceases to purchase a significant volume of products from us, it could have a material adverse effect on our results of operations and financial condition.
| 14 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Competition in the markets for our products is intense, and we may not be able to compete effectively.
Competition relating to our current products is intense and includes various companies, both within and outside of the United States. We anticipate that competition for our future products will also be intense and include various companies, both within and outside of the United States, Asia and Europe. Our competitors and potential competitors include large companies with substantially greater financial, sales and marketing, and technical resources, larger and more experienced research and development staffs, more extensive physical facilities and substantially greater experience in obtaining regulatory approvals and in marketing products than we have. In addition, we cannot assure you that our competitors are not currently developing, or will not attempt to develop, technologies and products that are more effective than those being developed by us or that would otherwise render our existing and new technology and products obsolete or noncompetitive. We may not be able to compete successfully and may lose market share to our competitors.
Our failure to obtain issued patents and, consequently, to protect our proprietary technology could hurt our competitive position.
Our success will depend in part on our ability to obtain and enforce claims in our patents directed to our products, technologies and processes, both in the United States and in other countries. Risks and uncertainties that we face with respect to our patents and patent applications include the following:
| · | the pending patent applications that we have filed, or to which we have exclusive rights, may not result in issued patents or may take longer than we expect to result in issued patents; |
| · | the allowed claims of any patents that issue may not provide meaningful protection; |
| · | we may be unable to develop additional proprietary technologies that are patentable; |
| · | the patents licensed or issued to us may not provide a competitive advantage; |
| · | other companies may challenge patents licensed or issued to us; |
| · | disputes
may arise regarding inventions and corresponding ownership rights in inventions and know-how resulting from the joint creation or use of intellectual property by us and our respective licensors; and |
| · | other companies may design around the technologies patented by us. |
Our revenue and operating results are likely to fluctuate.
Our quarterly and annual operating results have varied in the past, and our operating results are likely to continue to fluctuate in the future. These variations result from a number of factors, many of which are substantially outside of our control, including:
| · | the timing of new product introductions by us and our competitors; |
| · | timing of industry tradeshows, particularly the International Dental Show; |
| · | changes in relationships with distributors; |
| · | the timing of operational decisions by distributors and end users; |
| · | developments in government reimbursement policies; |
| · | changes in product mix; |
| · | our ability to supply products to meet customer demand; |
| · | fluctuations in manufacturing costs; |
| · | tax incentives; |
| · | currency fluctuations; and |
| · | general economic conditions, as well as those specific to the healthcare industry and related industries. |
| 15 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Our financial results may be adversely affected by fluctuations in foreign currency exchange rates.
We are exposed to currency exchange risk with respect to the U.S. Dollar in relation to the Euro, because a large portion of our revenue and expenses are denominated in Euros. In addition, we have an increasing portion of revenue and expenses denominated in other foreign currencies, e.g. Yen, Australian Dollar, Brazilian Real, and Yuan Renminbi. We monitor changes in our exposure to exchange rate risk. While we enter into hedging arrangements to protect our business against certain currency fluctuations, these hedging arrangements do not provide comprehensive protection, and our results of operations and prospects could be materially and adversely affected by foreign exchange fluctuations.
Our hedging and cash management transactions may expose us to loss or limit our potential gains.
As part of our risk management program, we use foreign currency exchange forward contracts. While intended to reduce the effects of exchange rate fluctuations, these transactions may limit our potential gains or expose us to loss. Should our counterparties to such transactions or the sponsors of the exchanges through which these transactions are offered fail to honor their obligations due to financial distress or otherwise, we would be exposed to potential losses or the inability to recover anticipated gains from these transactions.
We enter into foreign currency exchange forward contracts as economic hedges of trade commitments or anticipated commitments denominated in currencies other than the functional currency to mitigate the effects of changes in currency rates. Although we do not enter into these instruments for trading purposes or speculation, and although our management believes all of these instruments are economically effective as hedges of underlying physical transactions, these foreign exchange commitments are dependent on timely performance by our counterparties. Their failure to perform could result in our having to close these hedges without the anticipated underlying transaction and could result in losses if foreign currency exchange rates have changed.
We enter into interest rate swap agreements from time to time to manage some of our exposure to interest rate volatility. These swap agreements involve risks, such as the risk that counterparties may fail to honor their obligations under these arrangements. In addition, these arrangements may not be effective in reducing our exposure to changes in interest rates. If such events occur, our results of operations may be adversely affected.
Most of our cash deposited with banks is not insured and would be subject to the risk of bank failure. Our total liquidity also depends in part on the availability of funds under our Senior Facility Agreement. The failure of any bank in which we deposit our funds or that is part of our Senior Facility Agreement could reduce the amount of cash we have available for operations and additional investments in our business.
If we lose our key management personnel or are unable to attract and retain qualified personnel, it could adversely affect our results of operations or delay or hurt our research and product development efforts.
Our success is dependent, in part, upon our ability to hire and retain management, sales, technical, research and other personnel who are in high demand and are often subject to competing employment opportunities. It is possible that the loss of the services of one or a combination of our senior executives or key managers could have an adverse effect on our operations.
Work stoppages and other labor relations matters may make it substantially more difficult or expensive for us to produce our products, which could result in decreased sales or increased costs, either of which would negatively impact our financial condition and results of operations.
A significant part of our foreign employees are subject to collective bargaining agreements, and some of our employees are unionized; therefore, we are subject to the risk of work stoppages and other labor relations matters. While we have not experienced prolonged work stoppages in recent years and believe our relations with employees are satisfactory, any prolonged work stoppage or strike at any one of our principal facilities could have a negative impact on our business, financial condition, or results of operations.
| 16 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
We may experience difficulties managing our growth, which could adversely affect our results of operations.
It is expected that we will grow in certain areas of our operations as we develop and, assuming receipt of the necessary regulatory approvals, market our products. We will therefore need to recruit personnel, particularly sales and marketing personnel, and expand our capabilities, which may strain our managerial, operational, financial and other resources. To compete effectively and manage our growth, we must:
| · | train, manage, motivate and retain a growing employee base; |
| · | accurately forecast demand for, and revenue from, our product candidates; and |
| · | expand existing operational, financial and management information systems to support our development and planned commercialization activities and the multiple locations of our offices. |
Our failure to manage these challenges effectively could materially harm our business.
Since we operate in markets outside of the United States and Europe, we are subject to additional risks.
We anticipate that sales outside of the United States and Europe will continue to account for a significant percentage of our revenue. Such revenue is subject to a number of uncertainties, including, but not limited to, the following:
| · | economic and political instability; |
| · | import or export licensing requirements; |
| · | trade restrictions; |
| · | longer payment cycles; |
| · | unexpected changes in regulatory requirements and tariffs; |
| · | fluctuations in currency exchange rates; |
| · | potentially adverse tax consequences; and |
| · | potentially weak protection of intellectual property rights. |
These risks may impair our ability to generate revenue from our sales efforts. In addition, many countries outside of the United States and Europe have their own regulatory approval requirements for the sale of products. As a result, the introduction of new products into, and our continued sale of existing products in, these markets could be prevented, and/or costly and/or time-consuming, and we cannot assure you that we will be able to obtain the required regulatory approvals on a timely basis, if at all.
Our business is subject to extensive, complex, and changing laws, regulations, and orders that failure to comply with could subject us to civil or criminal penalties or other liabilities.
We are subject to extensive laws, regulations, and orders which are administered by various international, federal, and state governmental authorities, including, among others, the FDA, the Office of Foreign Assets Control of the United States Department of the Treasury ("OFAC"), the United States Federal Trade Commission, the United States Department of Justice, and other similar domestic and foreign authorities. These regulations include, but are not limited to, the U.S. Foreign Corrupt Practices Act and similar international anti-bribery laws, regulations concerning the supply of conflict minerals, various environmental regulations and regulations relating to trade, import and export controls and economic sanctions. Such laws, regulations, and orders may be complex and are subject to change.
Compliance with the numerous applicable existing and new laws, regulations and orders could require us to incur substantial regulatory compliance costs. Although the Company has implemented policies and procedures to comply with applicable laws, regulations and orders, there can be no assurance that governmental authorities will not raise compliance concerns or perform audits to confirm compliance with such laws, regulations, and orders. Failure to comply with applicable laws, regulations, or orders could result in a range of governmental enforcement actions, including fines or penalties, injunctions, and/or criminal or other civil proceedings. Any such actions could result in higher than anticipated costs or lower than anticipated revenue and could have a material adverse effect on the Company's reputation, business, financial condition, and results of operations.
| 17 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
We may be exposed to liabilities under the Foreign Corrupt Practices Act, and any determination that we violated the Foreign Corrupt Practices Act could have a material adverse effect on our business.
To the extent that we operate outside the United States, we are subject to the Foreign Corrupt Practices Act (the “FCPA”) which generally prohibits U.S. companies and their intermediaries from bribing foreign officials for the purpose of obtaining or keeping business or otherwise obtaining favorable treatment. In particular, we may be held liable for actions taken by our strategic or local partners even though such partners are foreign companies that are not subject to the FCPA. Any determination that we violated the FCPA could result in sanctions that could have a material adverse effect on our business.
Regulations related to conflict minerals could adversely impact our business.
The Dodd-Frank Wall Street Reform and Consumer Protection Act contains provisions designed to improve transparency and accountability concerning the supply of certain minerals, known as conflict minerals, originating from the Democratic Republic of Congo (DRC) and adjoining countries. As a result, in August 2012 the SEC adopted annual disclosure and reporting requirements for those companies who use conflict minerals mined from the DRC and adjoining countries in their products. There are additional costs associated with complying with these disclosure requirements, including for diligence to determine the sources of conflict minerals used in our products and other potential changes to products, processes or sources of supply as a consequence of such verification activities. The implementation of these rules could adversely affect the sourcing, supply, and pricing of materials used in our products. As there may be only a limited number of suppliers offering conflict-free minerals, we cannot be sure that we will be able to obtain necessary conflict minerals from such suppliers in sufficient quantities or at competitive prices. Also, we may face reputational challenges if we determine that certain of our products contain minerals not determined to be conflict free or if we are unable to sufficiently verify the origins for all conflict minerals used in our products through the procedures we may implement.
We may be a party to legal actions that are not covered by insurance.
We may be a party to a variety of legal actions, such as employment and employment discrimination-related suits, employee benefit claims, breach of contract actions, tort claims, stockholder suits, including securities fraud, governmental investigations and intellectual property related litigation. In addition, because of the nature of our business, we are subject to a variety of legal actions relating to our business operations. Although we have maintained insurance coverage for some of these potential liabilities, we cannot assure you that such insurance coverage will continue to be available or, if available, that it can be obtained in sufficient amounts or at reasonable cost or that it will be sufficient to cover any claims that may arise. Other potential liabilities may not be covered by insurance, insurers may dispute coverage, or the amount of insurance may not be sufficient to cover the damages awarded. In addition, certain types of damages, such as punitive damages, may not be covered by insurance and/or insurance coverage for all or certain forms of liability may become unavailable or prohibitively expensive in the future.
We are dependent upon a limited number of suppliers for critical components. If these suppliers delay or discontinue the manufacture of these components, we may experience delays in shipments, increased costs and cancellation of orders for our products.
We rely on key suppliers for various critical components and procure certain components from outside sources which are sole suppliers. The availability and prices of these components may be subject to change due to interruptions in production, changing market conditions and other events. Any delays in delivery of or shortages in these components could interrupt and delay manufacturing of our products and result in the cancellation of orders for our products. In addition, these suppliers could discontinue the manufacture or supply of these components at any time. We may not be able to identify and integrate alternative sources of supply in a timely fashion or at all. Any transition to alternate suppliers may result in delays in shipment and increased expenses and may limit our ability to deliver products to our customers. If we are unable to develop reasonably-priced alternative sources in a timely manner, or if we encounter delays or other difficulties in the supply of such products and other materials from third parties, our business and results of operations may be harmed. In past years, semiconductors have been subject to significant price fluctuations.
| 18 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
While we have, in the past, attempted to mitigate the effects of such potential fluctuations, we cannot assure you that we will continue to do so or that we will be able to successfully mitigate the effect of future price increases on our results of operations and financial condition. See Item 1 Business – Manufacturing and Suppliers.
Our profitability could suffer if third parties infringe upon our proprietary technology.
Our profitability could suffer if third parties infringe upon our intellectual property rights or misappropriate our technologies and trademarks for their own businesses. To protect our rights to our intellectual property, we rely on a combination of patent and trademark law, trade secret protection, confidentiality agreements and contractual arrangements with our employees, strategic partners and others. We cannot assure you that any of our patents, any of the patents of which we are a licensee or any patents which may be issued to us or which we may license in the future, will provide us with a competitive advantage or afford us protection against infringement by others, or that the patents will not be successfully challenged or circumvented by third parties, including our competitors. The protective steps we have taken may be inadequate to deter misappropriation of our proprietary information. We may be unable to detect the unauthorized use of, or take appropriate steps to enforce, our intellectual property rights. Effective patent, trademark and trade secret protection may not be available in every country in which we will offer, or intend to offer, our products. Any failure to adequately protect our intellectual property could devalue our proprietary content and impair our ability to compete effectively. Further, defending our intellectual property rights could result in the expenditure of significant financial and managerial resources.
Our profitability may suffer if our products are found to infringe the intellectual property rights of others.
Litigation may be necessary to enforce our patents or to defend against any claims of infringement of patents owned by third parties that are asserted against us. In addition, we may have to participate in one or more interference proceedings declared by the United States Patent and Trademark Office, the European Patent Office or other foreign patent governing authorities, to determine the priority of inventions, which could result in substantial costs.
If we become involved in litigation or interference proceedings, we may incur substantial expense, and the proceedings may divert the attention of our technical and management personnel, even if we ultimately prevail. An adverse determination in proceedings of this type could subject us to significant liabilities, allow our competitors to market competitive products without obtaining a license from us, prohibit us from marketing our products or require us to seek licenses from third parties that may not be available on commercially reasonable terms, if at all. If we cannot obtain such licenses, we may be restricted or prevented from commercializing our products.
The enforcement, defense and prosecution of intellectual property rights, including the United States Patent and Trademark Office’s, the European Patent Office’s and other foreign patent offices’ interference proceedings, and related legal and administrative proceedings in the United States and elsewhere, involve complex legal and factual questions. As a result, these proceedings are costly and time-consuming, and their outcome is uncertain. Litigation may be necessary to:
| · | assert against others or defend us against claims of infringement; |
| · | enforce patents owned by, or licensed to us from, another party; |
| · | protect our trade secrets or know-how; or |
| · | determine
the enforceability, scope and validity of our proprietary rights or the proprietary rights of others. |
Changes in the healthcare industry could adversely affect our business.
The healthcare industry has undergone, and is in the process of undergoing, significant changes driven by efforts to reduce costs. These changes include legislative healthcare reform, the reduction of spending budgets by government and private insurance programs, such as Medicare, Medicaid and corporate health insurance plans; trends toward managed care; consolidation of healthcare distribution companies; consolidation of healthcare manufacturers; collective purchasing arrangements and consolidation among office-based healthcare practitioners; and changes in reimbursements to customers. Some of these potential changes may cause a decrease in demand for and/or reduce the prices of our products. These changes could adversely affect our revenues and profitability. In addition, similar legislative efforts in the future could adversely impact our business.
| 19 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The implementation of the Health Care Reform Law could adversely affect our business.
The Patient Protection and Affordable Care Act as amended by the Health Care and Education Reconciliation Act, each enacted in March 2010, generally known as the Health Care Reform Law, significantly expand health insurance coverage to uninsured Americans and changes the way health care is financed by both governmental and private payers. We expect expansion of access to health insurance to increase the demand for our products and services, but other provisions of the Health Care Reform Law could affect us adversely. Additionally, further federal and state proposals for health care reform are likely. We cannot predict what further reform proposals, if any, will be adopted, when they may be adopted, or what impact they may have on us.
The Health Care Reform Law contains many provisions designed to generate the revenues necessary to fund the coverage expansions and to reduce costs of Medicare and Medicaid, including imposing a 2.3% excise tax on domestic sales of many medical devices by manufacturers and importers that began in 2013, which may adversely affect sales and cost of goods sold.
The implementation of the reporting and disclosure obligations of the Physician Payment Sunshine Act provisions of the Health Care Reform Law could adversely affect our business.
A Health Care Reform Law provision, generally referred to as the Physician Payment Sunshine Act or Open Payments Program, has imposed new reporting and disclosure requirements for drug and device manufacturers with regard to payments or other transfers of value made to certain practitioners (including physicians, dentists and teaching hospitals), and for such manufacturers and for group purchasing organizations, with regard to certain ownership interests held by physicians in the reporting entity. On February 1, 2013, the Centers for Medicare and Medicaid Services (“CMS”) released the final rule to implement the Physician Payment Sunshine Act. We published our first disclosure report in March 2015. As required under the Physician Payment Sunshine Act, CMS will publish information from these reports on a publicly available website, including amounts transferred and physician, dentist and teaching hospital identities.
The final rule implementing the Physician Payment Sunshine Act is complex, ambiguous, and broad in scope. CMS commentary on the final rule and more recent CMS communications indicate that wholesale drug and device distributors which take title to such products are to be treated as “applicable manufacturers” subject to full reporting requirements. In addition, certain of our subsidiaries manufacture devices. Accordingly, we are required to collect and report detailed information regarding certain financial relationships we have with dentists and teaching hospitals. The Physician Payment Sunshine Act preempts similar state reporting laws, although we or our subsidiaries may be required to continue to report under certain of such state laws. While we have substantially compliant programs and controls in place to comply with the Physician Payment Sunshine Act requirements, our compliance with the final rule imposes additional costs on us.
If we fail to comply with laws and regulations relating to health care fraud, we could suffer penalties or be required to make significant changes to our operations, which could adversely affect our business.
We are subject to federal and state (and similar foreign) laws and regulations relating to health care fraud. Some of these laws, referred to as “false claims laws,” prohibit the submission or causing the submission of false or fraudulent claims for reimbursement to federal, state and other health care payers and programs. Other laws, referred to as “anti-kickback laws,” prohibit soliciting, offering, receiving or paying remuneration in order to induce the referral of a patient or ordering, purchasing, leasing or arranging for or recommending ordering, purchasing or leasing, of items or services that are paid for by federal, state and other health care payers and programs.
| 20 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The government has expressed concerns about financial relationships between suppliers on the one hand and physicians and dentists on the other. As a result, we regularly review and revise our marketing practices as necessary to facilitate compliance. In addition, under the reporting and disclosure obligations of the Physician Payment Sunshine Act provisions of the Health Care Reform Law, the general public and government officials will be provided with new access to detailed information with regard to payments or other transfers of value to certain practitioners (including physicians, dentists and teaching hospitals) by applicable drug and device manufacturers subject to such reporting and disclosure obligations, which includes us. This information may lead to greater scrutiny, which may result in modifications to established practices and additional costs.
Failure to comply with health care fraud laws and regulations could result in significant civil and criminal penalties and costs, including the loss of licenses and the ability to participate in federal and state health care programs, and could have a material adverse impact on our business. Also, these laws may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could require us to make changes in our operations or incur substantial defense and settlement expenses. Even unsuccessful challenges by regulatory authorities or private relators could result in reputational harm and the incurring of substantial costs. In addition, many of these laws are vague or indefinite and have not been interpreted by the courts, and have been subject to frequent modification and varied interpretation by prosecutorial, regulatory authorities, increasing compliance risks.
While we believe that we are substantially compliant with the foregoing laws and regulations promulgated thereunder, and have adequate compliance programs and controls in place to ensure substantial compliance, we cannot predict whether changes in applicable law, or interpretation of laws, or changes in our services or marketing practices in response, could adversely affect our business.
If we fail to comply with laws and regulations relating to the confidentiality of sensitive personal information or standards in electronic health data transmissions, we could be required to make significant changes to our products, or incur penalties or other liabilities.
Certain of our businesses involve access to personal health, medical, financial and other information of individuals, and are accordingly directly or indirectly subject to numerous federal, state, local and foreign laws and regulations that protect the privacy and security of such information, and require, among other things, the implementation of various recordkeeping, operational, notice and other practices intended to safeguard that information, limit its use to allowed purposes, and notify individuals in the event of privacy and security breaches. Failure to comply with these laws can result in substantial penalties and other liabilities. As a result of the federal Health Information Technology for Economic and Clinical Health Act (“HITECH Act”), which was passed in 2009, some of our businesses that were previously only indirectly subject to federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) privacy and security rules became directly subject to such rules because such businesses serve as “business associates” of HIPAA covered entities, such as health care providers. On January 17, 2013 the Office for Civil Rights of the Department of Health and Human Services released a final rule implementing the HITECH Act and making certain other changes to HIPAA privacy and security requirements. Compliance with the rule was required by September 23, 2013, and increases the requirements applicable to some of our businesses.
In addition, our affiliates handle personally identifiable information pertaining to our members and paying subscribers. Both we and our affiliates are subject to laws and regulations related to Internet communications (including the CAN-SPAM Act of 2003), consumer protection (including the Telephone Consumer Protection Act and similar state laws), advertising, privacy, security and data protection. If we or our affiliates are found to be in violation of these laws and regulations, we may become subject to administrative fines or litigation, which could materially increase our expenses and cause the value of our securities to decline.
Product liability claims exposure could be significant.
We may face exposure to product liability claims and recalls for unforeseen reasons from consumers, distributors or others. We may experience material product liability losses in the future, and we may incur significant costs to defend these claims. In addition, if any of our products are or are alleged to be defective; we may be required to participate in a recall involving those products. End-users of our products may look to us for contribution when faced with product recalls or product liability claims. Although we have maintained insurance coverage related to product liability claims, we cannot assure you that product liability insurance coverage will continue to be available or, if available, that it can be obtained in sufficient amounts or at reasonable cost or that it will be sufficient to cover any claims that may arise. We may not maintain any insurance relating to potential recalls of our products. A successful product liability claim brought against us in excess of available insurance coverage or a requirement to participate in any product recall could reduce our profits and/or impair our financial condition, and damage our reputation.
| 21 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Product warranty claims exposure could be significant.
We generally warrant each of our products against defects in materials and workmanship for a period of one year from the date of shipment plus any extended warranty period purchased by the customer. The future costs associated with providing product warranties could be material. A successful warranty claim brought against us could reduce our profits and/or impair our financial condition, and damage our reputation.
Adverse publicity regarding the safety of our technology or products could negatively impact us.
Despite any favorable safety tests that may be completed with respect to our products, adverse publicity regarding application of X-ray products or other products being developed or marketed by others could negatively affect us. If other researchers’ studies raise or substantiate concerns over the safety of our technology approach or product development efforts generally, our reputation could be harmed, which would adversely impact our business.
Inadequate levels of reimbursement from governmental or other third-party payers for procedures using our products may cause our revenue to decline.
Third-party payers, including government health administration authorities, private health care insurers and other organizations regulate the reimbursement of fees related to certain diagnostic procedures or medical treatments. Third-party payers are increasingly challenging the price and cost-effectiveness of medical products and services. While we cannot predict what effect the policies of government entities and other third-party payers will have on future sales of our products, there can be no assurance that such policies would not cause our revenue to decline.
We have developed and must continue to maintain internal controls.
Effective internal controls are necessary for us to provide assurance with respect to our financial reports and to effectively prevent fraud. If we cannot provide reasonable assurance with respect to our financial reports and effectively prevent fraud, our operating results could be harmed. The Sarbanes-Oxley Act of 2002 requires us to furnish a report by management on internal control over financial reporting, including managements’ assessment of the effectiveness of such control. Internal control over financial reporting may not prevent or detect misstatements because of its certain limitations, including the possibility of human error, the circumvention or overriding of controls, or fraud. As a result, even effective internal controls may not provide reasonable assurances with respect to the preparation and presentation of financial statements. In addition, projections of any evaluation of effectiveness of internal control over financial reporting to future periods are subject to the risk that the control may become either obsolete or inadequate as a result of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. If we fail to maintain adequate internal controls, including any failure to implement required new or improved controls, or if we experience difficulties in implementing new or revised controls, our business and operating results could be harmed and we could fail to meet our reporting obligations.
We may be required to record a significant charge to earnings if our goodwill or other intangible assets become impaired.
Our balance sheet includes goodwill and other identifiable intangible assets. If impairment of our goodwill or other identifiable intangible assets is determined, we may be required to record a significant charge to earnings in the period of such determination under U.S. generally accepted accounting principles (GAAP).
Compliance with changing regulation of corporate governance and public disclosure will result in additional expenses and pose challenges for our management
Changing laws, regulations and standards relating to corporate governance and public disclosure, including the Dodd-Frank Wall Street Reform and Consumer Protection Act and the rules and regulations promulgated thereunder, the Sarbanes-Oxley Act and SEC regulations, have created uncertainty for public companies and significantly increased the costs and risks associated with accessing the U.S. public markets. Our management team will need to devote significant time and financial resources to comply with both existing and evolving standards for public companies, which will lead to increased general and administrative expenses and a diversion of management time and attention from revenue generating activities to compliance activities.
We Are Subject to Payments-Related Risks
We accept payments using a variety of methods, including credit card, debit card, credit accounts, direct debit from a customer’s bank account, consumer invoicing, physical bank check, and payment upon delivery. For existing and future payment options we offer to our customers, we may become subject to additional regulations and compliance requirements (including obligations to implement enhanced authentication processes that could result in significant costs and reduce the ease of use of our payments products), as well as fraud. For certain payment methods, including credit and debit cards, we pay interchange and other fees, which may increase over time and raise our operating costs and lower profitability. We rely on third parties to provide certain payment methods and payment processing services, including the processing of credit cards, debit cards, electronic checks, and promotional financing. In each case, it could disrupt our business if these companies become unwilling or unable to provide these services to us. We are also subject to payment card association operating rules, including data security rules, certification requirements, and rules governing electronic funds transfers, which could change or be reinterpreted to make it difficult or impossible for us to comply. If we fail to comply with these rules or requirements, or if our data security systems are breached or compromised, we may be liable for card issuing banks’ costs, subject to fines and higher transaction fees, and lose our ability to accept credit and debit card payments from our customers, process electronic funds transfers, or facilitate other types of online payments, and our business and operating results could be adversely affected.
| 22 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
RISKS RELATED TO OUR COMMON STOCK
Certain provisions of our certificate of incorporation and bylaws and Delaware law could discourage, delay, or prevent a merger or acquisition at a premium price.
The provisions of our certificate of incorporation and bylaws may also deter, delay or prevent a third-party from acquiring us. These provisions include:
| · | limitations on the ability of stockholders to amend our charter documents, including stockholder supermajority voting requirements; |
| · | the authority of the board of directors to adopt amendments to our bylaws without shareholder approval; |
| · | the inability of stockholders to act by written consent or to call special meetings; |
| · | a classified board of directors with staggered three-year terms; |
| · | advance notice requirements for nominations for election to the board of directors and for stockholder proposals; and |
| · | the authority of our board of directors to issue, without stockholder approval, up to 5,000,000 shares of preferred stock with such terms as the board of directors may determine and to issue additional shares of our common stock. |
We are also subject to the protections of Section 203 of the Delaware General Corporation Law, which prevents us from engaging in a business combination with a person who acquires at least 15% of our common stock for a period of three years from the date such person acquired such common stock, unless board or stockholder approval were obtained.
In addition, in the event of a “change of control” as defined in our senior facilities agreement, we may be required to, among other things, repay all of our obligations outstanding under the senior facilities agreement, with interest thereon, which could materially adversely impact the value of our common stock.
These provisions could have the effect of delaying, deferring or preventing a change in control of our company, discourage others from making tender offers for our shares, lower the market price of our stock or impede the ability of our stockholders to change our management, even if such changes would be beneficial to our stockholders.
The market price of our common stock may fluctuate significantly, and this may make it difficult for holders to resell our common stock when they want or at prices that they find attractive.
The price of our common stock on the NASDAQ Global Select Market constantly changes. We expect that the market price of our common stock will continue to fluctuate. The market price of our common stock may fluctuate as a result of a variety of factors, many of which are beyond our control. These factors include:
| · | changes in the price of DENTSPLY common stock; |
| · | changes in market conditions; |
| · | quarterly variations in our operating results; |
| · | operating results that vary from the expectations of management, securities analysts and investors; |
| · | changes in expectations as to our future financial performance; |
| 23 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| · | announcements of strategic developments, significant contracts, acquisitions and other material events by us, our competitors, or our distribution partners; |
| · | the operating and securities price performance of other companies that investors believe are comparable to us; |
| · | future sales of our equity or equity-related securities; |
| · | changes in the economy and the financial markets; |
| · | departures of key personnel; |
| · | changes in governmental regulations; and |
| · | geopolitical conditions, such as acts or threats of terrorism or military conflicts. |
In addition, in recent years, the stock market in general has experienced extreme price and volume fluctuations. This volatility has had a significant effect on the market price of securities issued by many companies for reasons often unrelated to their operating performance. These broad market fluctuations may adversely affect the market price of our common stock, regardless of our operating results.
RISKS RELATED TO THE MERGER
The exchange ratio is fixed and will not be adjusted in the event of any change in either our or DENTSPLY’s stock price.
Upon closing of the Merger, each share of our common stock will be converted into the right to receive 1.8142 shares of DENTSPLY common stock. This exchange ratio will not be adjusted for changes in the market price of either DENTSPLY common stock or our common stock between the date of signing the Merger agreement and completion of the Merger. Changes in the price of DENTSPLY common stock prior to the Merger will affect the value of DENTSPLY common stock that our common stockholders will receive on the date of the Merger. The exchange ratio will be adjusted appropriately to fully reflect the effect of any stock dividend or distribution, reclassification, stock split (including a reverse stock split), recapitalization, split-up, combination, exchange of shares, readjustment or other similar transaction with respect to the shares of either DENTSPLY common stock or our common stock prior to the closing of the Merger.
The prices of DENTSPLY common stock and our common stock at the closing of the Merger may vary from their prices on the date the Merger agreement was executed. As a result, the value represented by the exchange ratio will also vary.
These variations could result from changes in the business, operations or prospects of DENTSPLY or us prior to or following the Merger, regulatory considerations, general market and economic conditions and other factors both within and beyond our or DENTSPLY’s control. We may complete the Merger with DENTSPLY a considerable period after the dates of both the DENTSPLY special meeting and our special meeting. Therefore, at the time of our special stockholders meeting, our stockholders will not know with certainty the value of the shares of DENTSPLY common stock that they will receive upon completion of the Merger.
The Merger is subject to the receipt of consents and clearances from domestic and foreign regulatory authorities that may impose conditions that could have an adverse effect on us, DENTSPLY, or the combined company or, if not obtained, could prevent completion of the Merger.
Before the Merger may be completed, applicable waiting periods must expire or terminate under antitrust and competition laws. In deciding whether to grant regulatory clearances, the relevant governmental entities will consider the effect of the Merger on competition within their relevant jurisdiction. The terms and conditions of the approvals that are granted may impose requirements, limitations or costs or place restrictions on the conduct of the combined company’s business. The Merger agreement may require us and/or DENTSPLY to comply with conditions imposed by regulatory entities and, in certain circumstances, either company may refuse to close the Merger on the basis of those regulatory conditions. There can be no assurance that regulators will not impose conditions, terms, obligations or restrictions and that such conditions, terms, obligations or restrictions will not have the effect of delaying completion of the Merger or imposing additional material costs on or materially limiting the revenues of the combined company following the Merger. In addition, neither we nor DENTSPLY can provide assurance that any such conditions, terms, obligations or restrictions will not result in the delay or abandonment of the Merger.
| 24 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Any delay in completing the Merger may reduce or eliminate the benefits expected to be achieved thereunder.
In addition to the required regulatory clearances, the Merger is subject to a number of other conditions beyond our and DENTSPLY’s control that may prevent, delay or otherwise materially adversely affect its completion. Either party cannot predict whether and when these other conditions will be satisfied. Furthermore, the requirements for obtaining the required clearances and approvals could delay the completion of the Merger for a significant period of time or prevent it from occurring. Any delay in completing the Merger could cause the combined company not to realize, or to be delayed in realizing, some or all of the synergies that we expect to achieve if the Merger is successfully completed within its expected time frame.
Uncertainties associated with the Merger may cause a loss of management personnel and other key employees which could adversely affect the future business and operations of the combined company.
We and DENTSPLY are dependent on the experience and industry knowledge of their respective officers and other key employees to execute their business plans. The combined company’s success after the Merger will depend in part upon the ability of us and DENTSPLY to retain key management personnel and other key employees. Our and DENTSPLY’s current and prospective employees may experience uncertainty about their roles within the combined company following the Merger, which may have an adverse effect on the ability of each of us and DENTSPLY to attract or retain key management and other key personnel. Accordingly, no assurance can be given that the combined company will be able to attract or retain key management personnel and other key employees of ours and DENTSPLY to the same extent that we and DENTSPLY have previously been able to attract or retain each of our own employees. A failure by us, DENTSPLY, or, following the completion of the Merger, the combined company to attract, retain and motivate executives and other key employees during the period prior to or after the completion of the Merger could have a negative impact on their respective businesses.
Lawsuits have been filed against each of our and DENTSPLY’s board of directors challenging the Merger and an adverse ruling may prevent the Merger from being completed.
DENTSPLY, Merger Sub and the members of our board of directors were named as defendants in lawsuits brought by our stockholders challenging the Merger and seeking, among other things, injunctive relief to enjoin the defendants from completing the Merger on the agreed-upon terms. Additional lawsuits may be filed against DENTSPLY, Merger Sub, us and/or each of our respective directors or officers in connection with the Merger.
One of the conditions to the closing of the Merger is the absence of any order, injunction, decree, statute, rule or regulation by a court or other governmental entity that makes illegal or prohibits the consummation of the Merger or the other transactions contemplated by the Merger agreement. Consequently, if a settlement or other resolution is not reached in the lawsuits referenced above and the plaintiffs secure injunctive or other relief prohibiting, delaying or otherwise adversely affecting the parties’ ability to complete the Merger, then such injunctive or other relief may prevent the Merger from becoming effective within the expected time frame or at all.
Failure to complete the Merger could negatively impact the stock prices and the future business and financial results of ours and DENTSPLY’s.
Completion of the Merger is not assured and is subject to risks, including the risks that approval of the transactions by our and DENTSPLY’s stockholders or by governmental entities will not be obtained or that certain other closing conditions will not be satisfied. If the Merger is not completed, our and/or DENTSPLY’s ongoing businesses and financial results may be adversely affected and we and/or DENTSPLY will be subject to several risks, including the following:
| 25 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| · | having to pay certain significant costs relating to the Merger without receiving the benefits of the Merger, including, in certain circumstances, a termination fee of $280 million, in the case of DENTSPLY, and a termination fee of $205 million, in our case; |
| · | the potential loss of key personnel during the pendency of the Merger as employees may experience uncertainty about their future roles with the combined company; |
| · | We and DENTSPLY will have been subject to certain restrictions on the conduct of their businesses which may have prevented them from making certain acquisitions or dispositions or pursuing certain business opportunities while the Merger was pending; and |
| · | having had the focus of each companies’ management on the Merger instead of on pursuing other opportunities that could have been beneficial to the companies. |
If the Merger is not completed, we and DENTSPLY cannot assure each of our respective stockholders that these risks will not materialize and will not materially adversely affect our or DENTSPLY’s businesses, financial results and stock prices.
The Merger agreement contains provisions that could discourage a potential competing acquirer of either us or DENTSPLY.
The Merger agreement contains “no shop” provisions that, subject to limited exceptions, restrict each of our and DENTSPLY’s ability to solicit, initiate or knowingly encourage and induce, or take any other action designed to facilitate competing third-party proposals relating to a merger, reorganization or consolidation of the company or an acquisition of the company’s stock or assets. Further, even if the DENTSPLY board of directors or our board of directors withdraws or qualifies its recommendation with respect to the Merger, we or DENTSPLY, as the case may be, will still be required to submit each of their merger-related proposals to a vote at each of our respective special meetings. In addition, the other party generally has an opportunity to offer to modify the terms of the Merger in response to any competing acquisition proposals before the board of directors of the company that has received a third-party proposal may withdraw or qualify its recommendation with respect to the Merger.
These provisions could discourage a potential third-party acquirer that might have an interest in acquiring all or a significant portion of us or DENTSPLY from considering or proposing that acquisition, even if it were prepared to pay consideration with a higher per share cash or market value than the market value proposed to be received or realized in the Merger or might result in a potential third-party acquirer proposing to pay a lower price to the stockholders than it might otherwise have proposed to pay because of the added expense of the $280 million or $205 million termination fee, as applicable, that may become payable in certain circumstances.
If the Merger agreement is terminated and either we or DENTSPLY determine to seek another business combination, it may not be able to negotiate a transaction with another party on terms comparable to, or better than, the terms of the Merger.
Our and DENTSPLY’s executive officers and directors have certain interests in the Merger that may be different from, or in addition to, the interests of DENTSPLY and our stockholders generally.
Our and DENTSPLY’s executive officers and directors have certain interests in the Merger that may be different from, or in addition to, the interests of DENTSPLY stockholders and our stockholders generally. Our and DENTSPLY’s executive officers negotiated the terms of the Merger agreement. Our and DENTSPLY’s executive officers have arrangements with us or DENTSPLY, as applicable, that provide for severance benefits if their employment is terminated under certain circumstances following the completion of the Merger. In addition, certain of our compensation and benefit plans and arrangements provide for payment or accelerated vesting or distribution of certain rights or benefits upon completion of the Merger. In the case of DENTSPLY, under the Merger agreement, DENTSPLY may act before completion of the Merger to accelerate the vesting of equity awards (stock options and RSUs denominated in DENTSPLY common stock) held by some or all of its non-employee directors who will not continue as directors of the combined company after the Merger. Executive officers and directors also have rights to indemnification and directors’ and officers’ liability insurance that will survive completion of the Merger.
| 26 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Upon completion of the Merger, the board of directors of the combined company will be comprised of eleven members, consisting of six of DENTSPLY’s current directors and five of our current directors. Mr. Slovin, currently a director and our president and chief executive officer, will serve as a director and as chief executive officer of the combined company, and Mr. Wise, the current chairman and chief executive officer of DENTSPLY, will serve as executive chairman of the board of directors of the combined company. Additionally, the combined company’s management team will include executives from each of us and DENTSPLY. From DENTSPLY, Christopher T. Clark (the current president and chief financial officer of DENTSPLY) will serve as president and chief operating officer, technologies of the combined company, and James G. Mosch (the current executive vice president and chief operating officer of DENTSPLY) will serve as president and chief operating officer, dental and healthcare consumables of the combined company. From us, Ulrich Michel (our current executive vice president and chief financial officer) will serve as executive vice president and chief financial officer of the combined company.
Each of our and DENTSPLY’s boards of directors were aware of these interests at the time each approved the Merger and the transactions contemplated by the Merger agreement. These interests, including the continued employment of certain of our and DENTSPLY’s executive officers by the combined company, the continued positions of certain of our and DENTPLY’s directors as directors of the combined company and the indemnification of former directors and officers by the combined company, may cause our and DENTSPLY’s directors and executive officers to view the Merger proposal differently and more favorably than you may view it.
Current holders of our and DENTSPLY’s common stock will have a reduced ownership and voting interest after the Merger and will exercise less influence over management.
Current holders of our and DENTSPLY common stock have the right to vote in the election of the board of directors and on other matters affecting us and DENTSPLY, respectively. Upon the completion of the Merger, each of our stockholders who receives shares of DENTSPLY common stock will become a stockholder of the combined company with a percentage ownership of the combined company that is smaller than such stockholder’s percentage ownership of us. Similarly, after completion of the Merger, the shares of combined company common stock retained by each DENTSPLY stockholder will represent a smaller percentage ownership of the combined company. It is currently expected that our stockholders immediately prior to the effective time of the Merger as a group will receive shares in the Merger constituting approximately 42% of the shares of combined company common stock on a fully diluted basis immediately after the Merger. As a result, stockholders of DENTSPLY immediately prior to the effective time of the Merger as a group will own approximately 58% of the shares of combined company common stock on a fully diluted basis immediately after the Merger. Because of this, DENTSPLY and our stockholders will have less influence on the management and policies of the combined company than they now have on the management and policies of DENTSPLY and us, respectively.
RISKS RELATED TO THE COMBINED COMPANY FOLLOWING THE MERGER
The combined company may be unable to integrate successfully our and DENTSPLY’s businesses and realize the anticipated benefits of the Merger.
The success of the Merger will depend, in large part, on the ability of the combined company to realize the anticipated benefits, including cost savings, from combining our and DENTSPLY’s businesses. To realize these anticipated benefits, our and DENTSPLY’s businesses must be successfully integrated. This integration will be complex and time consuming. The failure to integrate successfully and to manage successfully the challenges presented by the integration process may result in the combined company not fully achieving the anticipated benefits of the Merger. Potential difficulties the combined company may encounter as part of the integration process include the following:
| · | the inability to successfully combine our and DENTSPLY’s businesses in a manner that permits the combined company to achieve the full revenue and cost synergies anticipated to result from the Merger; |
| 27 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| · | complexities associated with managing the combined businesses, including the challenge of integrating complex systems, technology, networks and other assets of each of the companies in a seamless manner that minimizes any adverse impact on customers, suppliers, employees and other constituencies; |
| · | coordinating geographically separated organizations, systems and facilities; |
| · | addressing possible differences in business backgrounds, corporate cultures and management philosophies; |
| · | integrating the workforces of the two companies while maintaining focus on providing consistent, high quality customer service; and |
| · | potential unknown liabilities and unforeseen increased or new expenses, delays or regulatory conditions associated with the Merger. |
In addition, we and DENTSPLY have operated and, until the completion of the Merger, will continue to operate independently. It is possible that the integration process could result in:
| · | diversion of the attention of each company’s management; |
| · | disruption of existing relationships with distributors and other manufacturers in the industry that drive a substantial amount of revenues to each company; and |
| · | the disruption of, or the loss of momentum in, each company’s ongoing businesses or inconsistencies in standards, controls, procedures and policies, any of which could adversely affect each company’s ability to maintain relationships with customers, suppliers, employees and other constituencies our or DENTSPLY’s ability to achieve the anticipated benefits of the Merger, or which could reduce each company’s earnings or otherwise adversely affect the business and financial results of the combined company. |
The Merger may not be accretive and may cause dilution to the combined company’s adjusted earnings per share, which may negatively affect the market price of the combined company’s common stock.
We and DENTSPLY currently anticipate that the Merger will be accretive to stockholders on an adjusted earnings per share basis within the first full year following the completion of the Merger. This expectation is based on preliminary estimates, which may materially change. The combined company could also encounter additional transaction and integration-related costs or other factors such as the failure to realize all of the benefits anticipated in the Merger. All of these factors could cause dilution to the combined company’s adjusted earnings per share or decrease or delay the expected accretive effect of the Merger and cause a decrease in the market value of the combined company’s common stock.
The future results of the combined company will suffer if the combined company does not effectively manage its expanded operations following the Merger.
Following the Merger, the size of the business of the combined company will increase significantly beyond the current size of either our or DENTSPLY’s business. The combined company’s future success depends, in part, upon its ability to manage this expanded business, which will pose substantial challenges for management, including challenges related to the management and monitoring of new operations and associated increased costs and complexity. There can be no assurances that the combined company will be successful or that it will realize the expected operating efficiencies, cost savings, revenue enhancements and other benefits currently anticipated from the Merger.
The combined company is expected to incur substantial expenses related to the Merger and the integration of us and DENTSPLY.
The combined company is expected to incur substantial expenses in connection with the Merger and the integration of us and DENTSPLY. There are a large number of processes, policies, procedures, operations, technologies and systems that must be integrated, including purchasing, accounting and finance, sales, payroll, pricing, revenue management, manufacturing, research and development, marketing and benefits. While we and DENTSPLY have assumed that a certain level of expenses would be incurred, there are many factors beyond each of our controls that could affect the total amount or the timing of the integration expenses. Moreover, many of the expenses that will be incurred are, by their nature, difficult to estimate accurately. These expenses could, particularly in the near term, exceed the savings that the combined company expects to achieve from the elimination of duplicative expenses and the realization of economies of scale and cost savings. These integration expenses likely will result in the combined company taking significant charges against earnings following the completion of the Merger, and the amount and timing of such charges are uncertain at present.
| 28 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
The Company leases its headquarters in Long Island City, New York. The lease expires in November 2017. The leased space houses executive offices and group functions including legal affairs and investor relations, sales and marketing, research and development laboratories and production and shipping facilities.
The Company has its largest facility in Bensheim, Germany. It is composed of a number of buildings housing the Company’s primary manufacturing and assembly facility. It also houses executive offices, finance, sales, customer service and marketing, research and development laboratories, and shipping facilities. In fiscal year 2011, the Company expanded these facilities with inauguration of the Center of Innovation, which houses the research and development professionals in Germany under one roof. In fiscal year 2013, the Company once again invested in these facilities by significantly expanding and enhancing its Instruments manufacturing capacity. In fiscal year 2014, the Company purchased the main administrative building on the Bensheim campus.
In addition, since September 2007, the Company leases space in Salzburg, Austria. The leased space houses executive offices and group functions including strategy, sales, finance, accounting, human resources, marketing, and legal affairs.
The Company also maintains manufacturing facilities in China, Italy and Denmark and certain sales and service offices worldwide.
The Company believes that its properties and facilities will be adequate for its needs for the foreseeable future and that, if such space proves to be inadequate, it will be able to procure additional or replacement space that will be adequate for its needs.
| ITEM 3. | LEGAL PROCEEDINGS |
The Company is involved in various legal proceedings that are incidental to the conduct of the Company’s business. The Company is not involved in any pending or threatened legal proceedings that the Company believes could reasonably be expected to have a material adverse effect on the financial condition or results of operations.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
| 29 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock is currently traded publicly on the NASDAQ Global Select Market. Our trading symbol is “SIRO”.
QUARTERLY INFORMATION ON THE PRICE RANGE OF COMMON STOCK
The following table presents quarterly information on the price range of our common stock. This information indicates the high and low sale prices, as quoted on NASDAQ commencing October 1, 2013. These prices do not include retail markups, markdowns or commissions.
| Quarterly Price Range of Common Stock | ||||
| Range | ||||
| (In Dollars) | High | Low | ||
| Fiscal Year Ended September 30, 2015 | ||||
| First Quarter | $ | 90.20 | $ | 74.38 |
| Second Quarter | 93.51 | 86.29 | ||
| Third Quarter | 102.89 | 87.90 | ||
| Fourth Quarter | 105.37 | 90.92 | ||
| Fiscal Year Ended September 30, 2014 | ||||
| First Quarter | $ | 73.94 | $ | 66.04 |
| Second Quarter | 77.31 | 67.20 | ||
| Third Quarter | 82.84 | 72.11 | ||
| Fourth Quarter | 84.95 | 75.16 | ||
On November 17, 2015, there were approximately 67 holders of record of the Company’s common stock. However, the Company believes that the number of beneficial owners of its common stock is substantially higher.
Historically, Sirona has not paid any dividends to holders of its common stock. The Company may consider paying dividends in the future, but currently has no plans to do so. The payment of dividends is within the discretion of the Board of Directors and will depend upon the Company’s earnings, its capital requirements, financial condition and other relevant factors.
For information relating to securities authorized for issuance under equity compensation plans, see Part III, Item 12.
ISSUER PURCHASES OF EQUITY SECURITIES
In May 2013, the Company’s Board of Directors announced a stock repurchase program (the “2013 Program”) to purchase up to an aggregate of $100 million of its common stock in open market or privately-negotiated transactions effective through June 2016. The Company is not obligated to acquire any particular amount of common stock and may suspend the program at any time at its discretion without prior notice.
| 30 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
During the fourth quarter of the fiscal year ended September 30, 2015, there were no share purchases made pursuant to this program. The approximate dollar value of shares that may yet be purchased under this program was $68.3 million as of September 30, 2015.
PERFORMANCE MEASUREMENT COMPARISON
The following graph compares the Company’s cumulative stockholder return on its common stock with the return on the Russell 2000 Index and the Dow Jones US Medical Equipment Index from September 30, 2010 through September 30, 2015, the end of the Company’s fiscal year. The graph assumes investments of $100 on September 30, 2010, the last trading day of that fiscal year, in the Company’s common stock, the Russell 2000 Index, and the US Medical Equipment Index and assumes the reinvestment of all dividends.
COMPARISON OF 6 YEAR CUMULATIVE TOTAL RETURN* Among Sirona Dental Systems, Inc, The Russell 2000 Index And The Dow Jones US Medical Equipment Index |
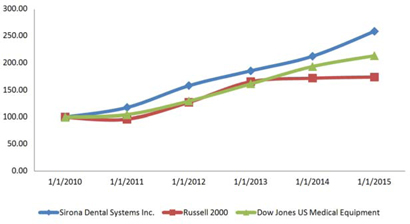 |
| *$100 invested on 9/30/2010 in stock or index-including reinvestment of dividends. |
| Comparison of 6-Year Cumulative Total Return | ||||||||||||
| (In Dollars) | 9/30/2010 | 9/30/2011 | 9/30/2012 | 9/30/2013 | 9/30/2014 | 9/30/2015 | ||||||
| Sirona Dental Systems Inc. | $ | 100.00 | $ | 117.67 | $ | 158.05 | $ | 185.71 | $ | 212.76 | $ | 258.99 |
| Russell 2000 | 100.00 | 96.47 | 127.25 | 165.50 | 172.01 | 174.15 | ||||||
| Dow Jones US Medical Equipment | 100.00 | 104.60 | 129.50 | 161.37 | 193.80 | 213.71 | ||||||
| 31 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| ITEM 6. | SELECTED FINANCIAL DATA |
The selected historical consolidated financial data of Sirona included below and elsewhere in this document are not necessarily indicative of future performance. This information is only a summary and should be read in conjunction with the sections entitled ‘‘Management’s Discussion and Analysis of Financial Condition and Results of Operations’’ and the consolidated financial statements contained elsewhere in this document.
| Selected Income Statement Data | Year Ended | |||||||||
| September 30, | ||||||||||
| (In millions, except for per share amounts) | 2015 | 2014 | 2013 | 2012 | 2011 | |||||
| REVENUE | $ | 1,161.3 | $ | 1,171.1 | $ | 1,101.5 | $ | 979.4 | $ | 913.9 |
| GROSS PROFIT | 648.2 | 641.7 | 591.4 | 524.0 | 483.7 | |||||
| OPERATING INCOME | 258.3 | 238.1 | 212.8 | 185.8 | 160.9 | |||||
| INCOME BEFORE TAXES | 242.9 | 230.4 | 197.5 | 178.3 | 159.5 | |||||
| NET INCOME | 188.1 | 177.4 | 148.5 | 135.6 | 123.8 | |||||
| NET INCOME ATTRIBUTABLE TO SIRONA DENTAL SYSTEMS, INC. | $ | 186.2 | $ | 175.7 | $ | 146.7 | $ | 133.8 | $ | 121.8 |
INCOME PER SHARE (attributable to Sirona Dental Systems, Inc. common shareholders): |
||||||||||
| Basic | $ | 3.35 | $ | 3.18 | $ | 2.67 | $ | 2.41 | $ | 2.19 |
| Diluted | $ | 3.30 | $ | 3.13 | $ | 2.61 | $ | 2.36 | $ | 2.13 |
| Selected Balance Sheet Data | As of | |||||||||
| September 30, | ||||||||||
| (In millions) | 2015 | 2014 | 2013 | 2012 | 2011 | |||||
| CASH AND CASH EQUIVALENTS | $ | 517.8 | $ | 382.8 | $ | 241.7 | $ | 151.1 | $ | 345.9 |
| WORKING CAPITAL (1), (2) | 581.5 | 449.8 | 317.0 | 222.9 | 46.2 | |||||
| TOTAL ASSETS | 1,902.3 | 1,811.0 | 1,738.4 | 1,494.7 | 1,726.1 | |||||
| NON-CURRENT LIABILITIES (2) | 266.3 | 312.5 | 334.3 | 315.9 | 255.0 | |||||
| TOTAL LIABILITIES | 561.4 | 549.8 | 580.4 | 502.3 | 790.2 | |||||
| RETAINED EARNINGS | 946.1 | 759.9 | 584.2 | 437.5 | 303.6 | |||||
| SIRONA DENTAL SYSTEMS, INC. SHAREHOLDERS' EQUITY | 1,338.2 | 1,258.8 | 1,155.6 | 989.4 | 932.3 | |||||
| TOTAL SHAREHOLDERS' EQUITY | 1,340.9 | 1,261.2 | 1,158.0 | 992.4 | 935.9 | |||||
| NET CASH (DEBT) (3) | $ | 437.6 | $ | 303.3 | $ | 166.3 | $ | 75.6 | $ | (22.5) |
| (1) Working capital is defined as current assets less current liabilities. | ||||||||||
| (2) The significant decrease in working capital and non-current liabilities in fiscal year 2011 is due to the reclassification of the final tranche of the senior term loan due in November 2011 as current. The balance of these senior term loans was $364.8 as of September 30, 2011. | ||||||||||
| (3)Net cash (debt) is defined as cash and cash equivalents less short and long-term financial liabilities. | ||||||||||
| 32 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
INTRODUCTION
Except as otherwise indicated or unless context otherwise requires, the terms “Sirona”, the “Company”, “we”, “us”, and “our” refer to Sirona Dental Systems, Inc. and its consolidated subsidiaries.
Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) is intended to provide the reader of our financial statements with a narrative, from the perspective of our management, on our business, financial condition, results of operations, liquidity, and certain other factors that may affect our future results. Unless expressly stated otherwise, the comparisons presented in this MD&A refer to the same period in the prior year.
Our MD&A should be read in conjunction with the Consolidated Financial Statements included elsewhere in this report. Actual results and the timing of certain events may differ significantly from those projected in forward-looking statements due to a number of factors, including those set forth in “Operations Review” in this Item and elsewhere in this Report.
All amounts in this section are reported in millions of U.S. Dollars ($), except as otherwise disclosed.
Non-GAAP Financial Measures
Certain income statement information is presented on an adjusted basis. Such information represents non-GAAP financial measures. Sirona supplementally presents this information because it believes doing so facilitates a better comparison of its operating results from period to period without regard to certain significant items, which management believes do not reflect Sirona’s operating performance in the ordinary, ongoing and customary course of its operations. For a listing and definitions of our current non-GAAP financial measures as well as a reconciliation of these measures to the most comparable GAAP measure, please refer to “Non-GAAP Financial Measures” within “Operations Review” in this MD&A.
EXECUTIVE OVERVIEW OF SIRONA’S BUSINESS AND PERFORMANCE
Business
Sirona is the leading global manufacturer of high-quality, technologically-advanced dental equipment, and is focused on developing, manufacturing, and marketing innovative systems and solutions for dentists around the world. The Company is uniquely positioned to benefit from several trends in the global dental industry, such as technological innovation, increased use of CAD/CAM systems in restorative dentistry, the shift to digital imaging, favorable demographic trends, and growing patient focus on dental health and cosmetic appearance. The Company’s headquarters is in Long Island City, New York, and its largest facility is located in Bensheim, Germany.
Sirona has a long tradition of innovation in the dental industry. The Company introduced the first dental electric drill over 130 years ago, the first dental X-ray unit approximately 100 years ago, the first dental computer-aided design/computer-aided manufacturing (CAD/CAM) system 30 years ago, and numerous other significant innovations in dentistry. Sirona continues to make significant investments in R&D, and its track record of innovative and profitable new products continues today. Sirona has the broadest product portfolio in the industry and is capable of fully outfitting and integrating a dental practice.
The majority of our revenues derive from the manufacture and sale of dental equipment. In addition, we also provide sales and after-sales service support to dentists and distributors through our growing sales and service infrastructure.
| 33 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Sirona manages its commercial operations on both a product and geographic basis and maintains four reporting segments: 1) Dental CAD/CAM Systems, 2) Imaging Systems, 3) Treatment Centers, and 4) Instruments. Products from each category are marketed in all geographical sales regions.
Our business has grown substantially in the past several fiscal years, driven by numerous high-tech product introductions, a continued expansion of our global sales and service infrastructure, and strong relationships with key distribution partners, namely Patterson in the U.S. and Henry Schein in Europe.
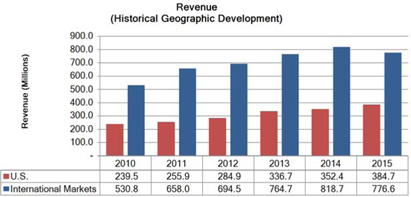
U.S.: Revenues have been driven by innovative products, particularly in the CAD/CAM and Imaging segments.
International Markets: Sirona has been able to grow revenues in international markets by gaining market share with its innovative, best-in-class product solutions as well as through expansion of its local presence and distribution channels by establishing sales and service locations in countries such as Australia, Brazil, China, Italy, Japan, Russia, Slovakia, South Africa, South Korea, and Turkey. The expansion helped to increase market share but also contributed to higher SG&A expenses.
Sirona has been able to grow by often being first-to-market and establishing a strong distribution network as these countries’ dental markets expanded. Additionally, increasing demand for best-in-class dental technology and growing middle class populations demanding better dental care in these markets have increased demand for Sirona’s products.
Current Performance at a Glance
The following is a synopsis of our performance for the fiscal year ended September 30, 2015:
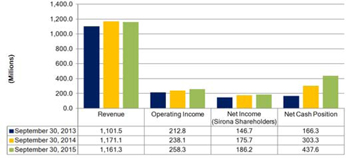
| · | Revenue: For the fiscal year ended September 30, 2015, reported revenue decreased 0.8%. On a Local Currency basis, total revenues were up 9.8% over the prior-year period (prior year period: up 6.4% Local Currency). We continued to see growing demand for products across all segments. |
| 34 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CAD/CAM grew in all regions, especially in International Markets. Instruments continued to benefit from our expanded state-of-the-art manufacturing facility in Bensheim, Germany, while Treatment Centers saw steady increase in demand across all product lines. Geographically, Local Currency growth was driven by all regions.
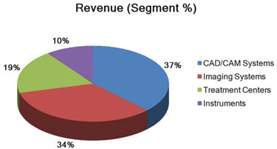 |
 |
| · | Operating Income: Operating income increased $20.2 million, or 8.5%, compared to the prior year. |
Highlights: Gross profit increased by $6.5 million despite the decrease in reported revenues. Gross profit margin benefited from foreign exchange developments and improved to 55.8% (prior year period: 54.8%). We continue to invest in the expansion of our sales and service infrastructure in key markets and in research and development for new products and services; however, the benefits from foreign exchange translation more than offset these investments.
As a result, SG&A expenses decreased $6.8 million, while R&D expenses were below the prior-year period by $9.8 million.
| · | Net Income: Net income attributable to Sirona shareholders was $186.2 million, an increase of $10.5 million, or 6.0%, over the prior year. The effective tax rate for the fiscal year ended September 30, 2015, was 22.6% (prior year period: 23.0%). |
| · | Cash Position: At September 30, 2015, the Company had cash and cash equivalents of $517.8 million and total debt of $80.2 million, resulting in net cash of $437.6 million, or an increase of $134.3 million compared to September 30, 2014. |
Significant Factors Affecting Our Operating Performance
Foreign Currency Fluctuations
Although the U.S. Dollar is Sirona’s reporting currency, it conducts its business in many currencies, and its functional currencies vary depending on the country of operation. Fluctuations in exchange rates impact Sirona’s financial results. The single largest influencing factor is the U.S. Dollar/Euro exchange rate.
Although Sirona does not apply hedge accounting for foreign currency derivatives, it has entered into foreign exchange forward contracts to help mitigate foreign currency exposure. As these agreements are short-term (generally not exceeding six months) and do not cover all underlying exposures, continued fluctuation in exchange rates could materially affect Sirona’s results of operations.
Loans made to Sirona under the Senior Facilities Agreement entered into on November 14, 2011, are denominated in the functional currency of the respective borrowers. See “Liquidity and Capital Resources” for a discussion of our Senior Facilities Agreement. However, intra-group loans and other intra-group monetary assets and liabilities are denominated in the functional currency of only one of the parties to the agreements. Where intra-group loans are of a long-term investment nature, the potential fluctuations in exchange rates are reflected within other comprehensive income, whereas exchange rate fluctuations for short-term intra-group loans and other short-term intra-group transactions are recorded in the consolidated statements of income. These fluctuations may be significant in any period due to changes in exchange rates, especially between the Euro and the U.S. Dollar.
| 35 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The MDP Transaction and the Exchange
On June 30, 2005, Sirona Holdings Luxco S.C.A. (‘‘Luxco’’), a Luxembourg-based holding entity owned by funds managed by Madison Dearborn Partners, Beecken Petty O’Keefe, management and employees of Sirona, obtained control over the Sirona business. The transaction was effected by using new legal entities, Sirona Holding GmbH and its wholly-owned subsidiary Sirona Dental Services GmbH, to acquire 100% of the interest in Sirona Dental Systems Beteiligungs- und Verwaltungs GmbH, the former parent of the Sirona business through a leveraged buy-out transaction (the ‘‘MDP Transaction’’).
The assets and liabilities acquired in the MDP Transaction and the Exchange were partially stepped up to fair value, and a related deferred tax liability was recorded. The excess of the total purchase price over the fair value of the net assets acquired, including IPR&D, which were expensed at the date of closing of the MDP Transaction and the Exchange, was allocated to goodwill and is subject to periodic impairment testing.
Sirona’s cost of goods sold, R&D, SG&A expense, and operating results have been and will continue to be materially affected by depreciation and amortization costs resulting from the step-up to fair value of Sirona’s assets and liabilities.
Fluctuations in Operating Results
Sirona’s operating results have varied in the past and are likely to vary in the future. These variations result from a number of factors, many of which are substantially outside its control, including:
| · | the timing of new product introductions by us and our competitors; |
| · | timing of industry tradeshows, particularly the International Dental Show (“IDS”); |
| · | changes in relationships with distributors; |
| · | the timing of operational decisions by distributors and end users; |
| · | developments in government reimbursement policies; |
| · | changes in product mix; |
| · | our ability to supply products to meet customer demand; |
| · | fluctuations in manufacturing costs; |
| · | tax incentives; |
| · | currency fluctuations; and |
| · | general economic conditions, as well as those specific to the healthcare industry and related industries. |
Due to the variations which Sirona has experienced in its operating results, it does not believe that period-to-period comparisons of results of operations of Sirona are necessarily meaningful or reliable as indicators of future performance.
Effective Tax Rate
Sirona’s effective tax rate may vary significantly from period to period and, as a global enterprise, can be influenced by many factors. These factors include, but are not limited to, changes in the mix of earnings in countries with differing statutory tax rates (including the result of business acquisitions and dispositions), changes in the valuation of deferred tax assets and liabilities, the results of audits and examinations of previously filed tax returns, tax planning initiatives, tax characteristics of income, changes in exchange rates, as well as the timing and deductibility of expenses for tax purposes. The Company’s effective tax rate differs from the U.S. federal statutory rate of 35% primarily as a result of lower effective tax rates on certain earnings outside of the United States.
The company makes no provision for deferred U.S. income taxes on undistributed foreign earnings because as of September 30, 2015, it remained management’s intention to continue to indefinitely reinvest such earnings in foreign operations. The distribution of lower-taxed foreign earnings to the U.S. would generally increase the Company’s effective tax rate.
| 36 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
OPERATIONS REVIEW
CURRENT FISCAL YEAR
| Operations Review | Year ended | |||||||||||
| September 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| ($ in millions) | ||||||||||||
| REVENUE | $ | 1,161.3 | 100.0% | $ | 1,171.1 | 100.0% | $ | (9.8) | (0.8%) | |||
| Dental CAD/CAM Systems | 434.9 | 37.4% | 425.8 | 36.4% | 9.1 | 2.1% | ||||||
| Imaging Systems | 391.5 | 33.7% | 399.8 | 34.1% | (8.3) | (2.1%) | ||||||
| Treatment Centers | 216.6 | 18.7% | 225.3 | 19.2% | (8.7) | (3.9%) | ||||||
| Instruments | 118.3 | 10.2% | 120.2 | 10.3% | (1.9) | (1.6%) | ||||||
| COST OF GOODS SOLD | (513.1) | (44.2%) | (529.4) | (45.2%) | 16.3 | 3.1% | ||||||
| GROSS PROFIT (1) | 648.2 | 55.8% | 641.7 | 54.8% | 6.5 | 1.0% | ||||||
| Dental CAD/CAM Systems | 304.5 | 70.0% | 291.1 | 68.4% | 13.4 | 4.6% | ||||||
| Imaging Systems | 224.3 | 57.3% | 232.6 | 58.2% | (8.3) | (3.6%) | ||||||
| Treatment Centers | 88.6 | 40.9% | 91.5 | 40.6% | (2.9) | (3.2%) | ||||||
| Instruments | 48.5 | 41.0% | 51.0 | 42.4% | (2.5) | (4.9%) | ||||||
| Corporate (unallocated) | (17.7) | (24.5) | 6.8 | 27.8% | ||||||||
| Selling, general and administrative expense | (344.1) | (29.6%) | (350.9) | (30.0%) | 6.8 | 1.9% | ||||||
| Research and development | (54.8) | (4.7%) | (64.6) | (5.5%) | 9.8 | 15.2% | ||||||
| Net other operating income (expense) | 9.0 | 0.8% | 11.9 | 1.0% | (2.9) | (24.4%) | ||||||
| OPERATING INCOME | 258.3 | 22.2% | 238.1 | 20.3% | 20.2 | 8.5% | ||||||
| Gain (loss) on foreign currency transactions, net | (17.9) | (1.5%) | (0.3) | (0.0%) | (17.6) | (5,866.7%) | ||||||
| Gain (loss) on derivative instruments | 1.7 | 0.1% | (2.5) | (0.2%) | 4.2 | 168.0% | ||||||
| Interest expense, net | (4.2) | (0.4%) | (2.9) | (0.2%) | (1.3) | (44.8%) | ||||||
| Other income (expense) | 5.0 | 0.4% | (2.0) | (0.2%) | 7.0 | 350.0% | ||||||
| INCOME BEFORE TAXES | 242.9 | 20.9% | 230.4 | 19.7% | 12.5 | 5.4% | ||||||
| Income tax provision | (54.8) | (4.7%) | (53.0) | (4.5%) | (1.8) | (3.4%) | ||||||
| NET INCOME | 188.1 | 16.2% | 177.4 | 15.1% | 10.7 | 6.0% | ||||||
| Less: Net income attributable to noncontrolling interests | (1.9) | (0.2%) | (1.7) | (0.1%) | (0.2) | (11.8%) | ||||||
| NET INCOME ATTRIBUTABLE TO SIRONA DENTAL SYSTEMS, INC. | $ | 186.2 | 16.0% | $ | 175.7 | 15.0% | $ | 10.5 | 6.0% | |||
| (1) Percentages refer to the percent of total revenues except for segment gross profit information, where percentages refer to segment gross profit margin. | ||||||||||||
| 37 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Revenue
For the fiscal year ended September 30, 2015, revenue was $1,161.3 million, a decrease of $9.8 million, or 0.8% (increased 9.8% Local Currency). Local Currency growth of 9.8% (including $11.3 million of growth via business acquisitions) was offset by a 10.6% unfavorable foreign exchange impact on our reported revenue. This was driven by the weakness of the Euro and other major currencies (such as Australian Dollar, Brazilian Real, Japanese Yen, etc.) throughout the fiscal year and impacted all segments. Revenue developed by segment and geographically as follows:
By segment:
| · | CAD/CAM Systems (increased 2.1% - up 12.1% Local Currency): Segment revenues were $434.9 million (prior year period: $425.8 million), an increase of $9.1 million reported compared to an increase of $51.4 million in Local Currency ($40.1 million organic growth and $11.3 million via business acquisitions). All regions experienced growth, with especially strong demand in international markets. |
| · | Imaging Systems (decreased 2.1% - up 4.9% Local Currency): Segment revenues were $391.5 million (prior year period: $399.8 million), a decrease of $8.3 million reported compared to an increase of $19.6 million in Local Currency. The U.S., where we continue to experience increasing demand for our 3D product lines, and Asia-Pacific regions drove segment growth. |
| · | Treatment Centers (decreased 3.8% - up 11.8% Local Currency): Segment revenues were $216.6 million (prior year period: $225.3 million), a decrease of $8.7 million reported compared to an increase of $26.6 million in Local Currency. Our Treatment Center segment continued to witness steady increase in demand across all product lines. Segment growth was especially strong in Europe. Sales of our treatment centers in the U.S gained traction in the latter part of the fiscal year with market launches via our expanded distribution agreement with Patterson. |
| · | Instruments (decreased 1.6% - up 14.1% Local Currency): Segment revenues were $118.3 million (prior year period: $120.2 million), a decrease of $1.9 million reported compared to an increase of $16.9 million in Local Currency. Instruments continued to experience strong growth. Germany and other international markets drove Local Currency revenue growth due to strong demand for our laser and hygiene products and handpieces. |
Geographically:
| · | U.S. (increased 9.2% - increased 6% excluding the impact of business acquisitions): Revenues benefited primarily from strong demand for our CAD/CAM Systems and Imaging products. Sales in the latter part of the fiscal year showed signs of a promising future growth in the U.S. for our Treatment Center segment. |
| · | International Markets (decreased 5.1% - increased 10.0% Local Currency): Local Currency revenues grew significantly across all regions, especially Asia-Pacific, while reported revenues were negatively impacted by the weakening of the Euro and other major currencies by 15.2%. Treatment Centers and CAD/CAM Systems drove our 10.0% Local Currency growth in International Markets. |
Cost of Goods Sold and Gross Profit
Cost of Goods Sold
For the fiscal year ended September 30, 2015, cost of goods sold was $513.1 million, a decrease of $16.3 million, or 3.1%. Cost of goods sold as a percentage of sales decreased to 44.2% (prior year period: 45.2%). Cost of goods sold benefitted mainly from the weakening of the Euro during the period. Amortization and depreciation expense resulting from the step-up to fair values of tangible and intangible assets, included in cost of goods sold, declined by $8.4 million compared to the prior-year period.
| 38 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Gross Profit
For the fiscal year ended September 30, 2015, gross profit was $648.2 million, an increase of $6.5 million, or 1.0%, compared to a decrease in revenue of $9.8 million, or 0.8%. Gross profit margin was 55.8% (prior year period: 54.8%). The 1.0% increase in the gross profit margin was mainly due to the abovementioned favorable foreign exchange effects of 2.4%, which more than offset the 1.4% negative operational impact.
By segment, gross profit developed in the fiscal year ended September 30, 2015, compared to the fiscal year ended September 30, 2014 as follows:
| · | CAD/CAM Systems: Segment gross profit was $304.5 million (prior year period: $291.1 million), an increase of $13.4 million compared to an increase of $9.1 million in reported segment revenue. Segment gross profit margin was 70.0% (prior year period: 68.4%). The 1.6% improvement was primarily due to foreign exchange fluctuations. |
| · | Imaging Systems: Segment gross profit was $224.3 million (prior year period: $232.6 million), a decrease of $8.3 million compared to a decrease of $8.3 million in reported segment revenue. Segment gross profit margin was 57.3% (prior year period: 58.2%). The 0.9% decline was primarily due to unfavorable product mix/price concessions. |
| · | Treatment Centers: Segment gross profit was $88.6 million (prior year period: $91.5 million), a decrease of $2.9 million compared to a decrease of $8.7 million in reported segment revenue. Segment gross profit margin was 40.9% (prior year period: 40.6%). The 0.3% improvement was primarily driven by benefits from the weakness of the Euro, with strong sales of our standard and comfort lines – especially in Europe. |
| · | Instruments: Segment gross profit was $48.5 million (prior year period: $51.0 million), a decrease of $2.5 million compared to a decrease of $1.9 million in reported segment revenue. Segment gross profit margin was 41.0% (prior year period: 42.4%). The 1.4% decline was primarily the result of unfavorable product/regional mix. |
Selling, General, and Administrative
For the fiscal year ended September 30, 2015, SG&A expense was $344.1 million, a decrease of $6.8 million, or 1.9%.
The decrease in SG&A expense was primarily driven by the weakening of the Euro and other major currencies. SG&A expense for the period included $3.1 million of marketing expenditures for the International Dental Show (“IDS”) that was held in March in Germany as well as $4.6 million of merger and acquisition-related expenses. We continue to investment in the expansion of our sales and service infrastructure in growth markets.
Research and Development
R&D expense for the fiscal year ended September 30, 2015, was $54.8 million, a decrease of $9.8 million, or 15.2%.
The decrease was mainly driven by the weakening of the Euro. As a percentage of revenue, R&D expense was 4.7% (prior year period: 5.5%).
| 39 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Net Other Operating Income (Loss)
Net other operating income (loss) for the fiscal year ended September 30, 2015, compared to the fiscal year ended September 30, 2014 was as follows:
| Net Other Operating Income (Loss) | Year ended | |||
| September 30, | ||||
| (In millions) | 2015 | 2014 | ||
| Income resulting from the amortization of the deferred income related to the Patterson exclusivity payment | $ | 10.0 | $ | 10.0 |
| Gain (loss) from patent infringement settlement (1) | (0.7) | - | ||
| Other miscellaneous gain (loss) (2) | (0.3) | 1.9 | ||
| Net other operating income (loss) | $ | 9.0 | $ | 11.9 |
| (1) The gain (loss) from patent infringement settlement for the periods under report represents net amounts received (paid) for past lost profits in out-of-court settlements of patent defense suits in the normal course of business. | ||||
| (2) The other miscellaneous gain for the year ended September 30, 2014, represents a gain on disposal of certain business assets. | ||||
Gain (Loss) on Foreign Currency Transactions and Derivative Instruments
Foreign Currency Transactions
The loss on foreign currency transactions for the fiscal year ended September 30, 2015 amounted to $17.9 million (prior year period: loss of $0.3 million). The components of these results are as follows:
| Gain (Loss) on Foreign Currency Transactions | Year ended | |||
| September 30, | ||||
| (In millions) | 2015 | 2014 | ||
| Unrealized non-cash foreign exchange gain (loss) from translation adjustment of deferred income related to the Patterson exclusivity payment | $ | (3.2) | $ | (2.0) |
| Unrealized non-cash foreign exchange gain (loss) on short-term intra-group loans | (2.8) | (0.1) | ||
| Gain (loss) on other foreign currency transactions (1) | (11.9) | 1.8 | ||
| Gain (Loss) on Foreign Currency Transactions | $ | (17.9) | $ | (0.3) |
| (1) For the periods under report, the gain (loss) on other foreign currency transactions related to the revaluation of short-term assets and liabilities and realized transactions, both of which were primarily impacted by the fluctuations between the Euro/U.S. Dollar, Real/Euro, and Yen/Euro exchange rates. | ||||
Derivative Instruments
For the fiscal year ended September 30, 2015, the gain on derivative instruments was $1.7 million (prior year period: loss of $2.5 million). In both periods, the results related to foreign currency hedges.
Other Income (Expense)
Other income (expense) for the fiscal year ended September 30, 2015 was a net income of $5.0 million (prior-year period: net expense of $2.0 million). In the current period under report, other income (expense) included the positive effect of $6.0 million from a net reduction in earn-out liabilities from business acquisitions (prior-year period: $1.5 million expense).
| 40 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Income Tax Provision
For the fiscal year ended September 30, 2015, Sirona recorded a profit before income taxes of $242.9 million (prior year period: $230.4 million), an income tax provision of $54.8 million (prior year period: $53.0 million), and an effective tax rate of 22.6% (prior year period: 23.0%). The effective tax rate is primarily driven by the mix of earnings across different jurisdictions.
The 22.6% effective tax rate for the fiscal year ended September 30, 2015 includes the effect from a tax audit in Germany for fiscal years 2010 through 2013 that was completed in the fourth quarter of the current fiscal year. Without consideration for the effect from the German tax audit, the effective tax rate for fiscal year ended September 30, 2015 was 22.2%.
| 41 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
PRIOR FISCAL YEAR
| Operations Review | Year ended | |||||||||||
| September 30, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| ($ in millions) | ||||||||||||
| REVENUE | $ | 1,171.1 | 100.0% | $ | 1,101.5 | 100.0% | $ | 69.6 | 6.3% | |||
| Dental CAD/CAM Systems | 425.8 | 36.4% | 409.3 | 37.2% | 16.5 | 4.0% | ||||||
| Imaging Systems | 399.8 | 34.1% | 378.0 | 34.3% | 21.8 | 5.8% | ||||||
| Treatment Centers | 225.3 | 19.2% | 210.7 | 19.1% | 14.6 | 6.9% | ||||||
| Instruments | 120.2 | 10.3% | 103.5 | 9.4% | 16.7 | 16.1% | ||||||
| COST OF GOODS SOLD | (529.4) | (45.2%) | (510.1) | (46.3%) | (19.3) | (3.8%) | ||||||
| GROSS PROFIT (1) | 641.7 | 54.8% | 591.4 | 53.7% | 50.3 | 8.5% | ||||||
| Dental CAD/CAM Systems | 291.1 | 68.4% | 276.7 | 67.6% | 14.4 | 5.2% | ||||||
| Imaging Systems | 232.6 | 58.2% | 221.9 | 58.7% | 10.7 | 4.8% | ||||||
| Treatment Centers | 91.5 | 40.6% | 81.4 | 38.6% | 10.1 | 12.4% | ||||||
| Instruments | 51.0 | 42.4% | 43.2 | 41.7% | 7.8 | 18.1% | ||||||
| Corporate (unallocated) | (24.5) | (31.8) | 7.3 | 23.0% | ||||||||
| Selling, general and administrative expense | (350.9) | (30.0%) | (332.8) | (30.2%) | (18.1) | (5.4%) | ||||||
| Research and development | (64.6) | (5.5%) | (60.2) | (5.5%) | (4.4) | (7.3%) | ||||||
| Net other operating income (expense) | 11.9 | 1.0% | 14.4 | 1.3% | (2.5) | (17.4%) | ||||||
| OPERATING INCOME | 238.1 | 20.3% | 212.8 | 19.3% | 25.3 | 11.9% | ||||||
| Gain (loss) on foreign currency transactions, net | (0.3) | (0.0%) | (12.4) | (1.1%) | 12.1 | 97.6% | ||||||
| Gain (loss) on derivative instruments | (2.5) | (0.2%) | 0.4 | 0.0% | (2.9) | (725.0%) | ||||||
| Interest expense, net | (2.9) | (0.2%) | (3.4) | (0.3%) | 0.5 | 14.7% | ||||||
| Other income (expense) | (2.0) | (0.2%) | 0.2 | 0.0% | (2.2) | (1,100.0%) | ||||||
| INCOME BEFORE TAXES | 230.4 | 19.7% | 197.6 | 17.9% | 32.8 | 16.6% | ||||||
| Income tax provision | (53.0) | (4.5%) | (49.0) | (4.4%) | (4.0) | (8.2%) | ||||||
| NET INCOME | 177.4 | 15.1% | 148.6 | 13.5% | 28.8 | 19.4% | ||||||
| Less: Net income attributable to noncontrolling interests | (1.7) | (0.1%) | (1.8) | (0.2%) | 0.1 | 5.6% | ||||||
| NET INCOME ATTRIBUTABLE TO SIRONA DENTAL SYSTEMS, INC. | $ | 175.7 | 15.0% | $ | 146.8 | 13.3% | $ | 28.9 | 19.7% | |||
| (1) Percentages refer to the percent of total revenues except for segment gross profit information, where percentages refer to segment gross profit margin. | ||||||||||||
| 42 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Revenue
For the fiscal year ended September 30, 2014, revenue was $1,171.1 million, an increase of $69.6 million, or 6.3% (increased 6.4% Local Currency). Reported revenue across all segments was favorably impacted by the strengthening of the Euro throughout most of the fiscal year, entirely offset by the weakening of other major currencies (such as Australian Dollar, Brazilian Real, Japanese Yen, etc.). Revenue developed by segment and geographically as follows:
By segment:
| · | CAD/CAM Systems (increased 4.0% - up 5.0% Local Currency): Revenue growth was driven by international markets, benefiting from new-user demand for our best-in-class products. CAD/CAM generally faced a difficult year-over-year comparison, with strong prior-year growth. The U.S. also faced a difficult year-over-year comparison due to the successful Omnicam trade-up program in fiscal 2013. |
| · | Imaging Systems (increased 5.8% - up 5.7% Local Currency): Revenue growth was driven by increasing demand for our intraoral and Orthophos product lines, particularly in the U.S. |
| · | Treatment Centers (increased 6.9% - up 5.9% Local Currency): Treatment Center revenues were boosted by the launch of our new Intego unit in the fourth quarter; however, segment revenues faced an overall challenging year-over-year comparison due to strong revenues in the prior year in connection with the IDS in Cologne, Germany, as well as the last-edition program for our well-renowned M1+ unit. |
| · | Instruments (increased 16.1% - up 15.4% Local Currency): Revenue growth was driven by international markets, especially in the hygiene and traditional business. Our increased manufacturing capacity contributed to market expansion. |
Geographically:
| · | U.S. (increased 4.7%): U.S. revenues continued to benefit from strong, new-user demand for both our CAD/CAM and Imaging products, but faced a difficult overall year-over-year comparison, with prior-year growth of 18.2% benefiting from trade-up programs. |
| · | International Markets (increased 7.1% - increased 7.1% Local Currency): Germany, our second-largest individual market, faced a challenging year-over-year comparison due to very strong sales in connection with the IDS in March 2013, with prior-year growth of 23.7% Local Currency. Strong local sales growth in other international markets (led by Japan, China, and Brazil) was offset by the weakening of major currencies in these markets. |
Cost of Goods Sold and Gross Profit
Cost of Goods Sold
For the fiscal year ended September 30, 2014, cost of goods sold was $529.4 million, an increase of $19.3 million, or 3.8%. Cost of goods sold as a percentage of sales decreased to 45.2% (prior year period: 46.3%). Cost of goods sold benefited primarily from improvements in product/regional mix. Amortization and depreciation expense resulting from the step-up to fair values of tangible and intangible assets, included in cost of goods sold, declined by $4.5 million compared to the prior-year period.
Gross Profit
For the fiscal year ended September 30, 2014, gross profit was $641.7 million, an increase of $50.3 million, or 8.5%, compared to an increase in revenue of $69.6 million, or 6.3%. Gross profit margin was 54.8% (prior year period: 53.7%). The 1.1% increase in the gross profit margin was mainly due to improvements in product/regional mix.
| 43 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
By segment, gross profit developed in the fiscal year ended September 30, 2014, compared to the fiscal year ended September 30, 2013 as follows:
| · | CAD/CAM Systems: Segment gross profit was $291.1 million (prior year period: $276.7 million), an increase of $14.4 million compared to an increase of $16.5 million in reported segment revenue. Segment gross profit margin was 68.4% (prior year period: 67.6%). The 0.8% improvement was driven by product and regional mix with a higher share of new-user sales versus trade ups. |
| · | Imaging Systems: Segment gross profit was $232.6 million (prior year period: $221.9 million), an increase of $10.7 million compared to an increase of $21.8 million in reported segment revenue. Segment gross profit margin was 58.2% (prior year period: 58.7%). The 0.5% decline was mainly due to negative foreign currency effects. |
| · | Treatment Centers: Segment gross profit was $91.5 million (prior year period: $81.4 million), an increase of $10.1 million compared to an increase of $14.6 million in reported segment revenue. Segment gross profit margin was 40.6% (prior year period: 38.6%). The 2.0% improvement was primarily driven by gains in productivity. |
| · | Instruments: Segment gross profit was $51.0 million (prior year period: $43.2 million), an increase of $7.8 million compared to an increase of $16.7 million in reported segment revenue. Segment gross profit margin was 42.4% (prior year period: 41.7%). The 0.7% improvement was the result of favorable product/regional mix. |
Selling, General, and Administrative
For the fiscal year ended September 30, 2014, SG&A expense was $350.9 million, an increase of $18.1 million, or 5.4%.
The increase in SG&A expense was primarily driven by the general increase in sales volume and continuing investment in the expansion of our sales and service infrastructure to capitalize on opportunities to gain market share and build up our presence in key growth markets.
Research and Development
R&D expense for the fiscal year ended September 30, 2014, was $64.6 million, an increase of $4.4 million, or 7.3%.
The increase was mostly driven by additional investment in R&D projects. As a percentage of revenue, R&D expense was 5.5% (prior year period: 5.5%).
| 44 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Net Other Operating Income (Loss)
Net other operating income (loss) for the fiscal year ended September 30, 2014, compared to the fiscal year ended September 30, 2013 was as follows:
| Net Other Operating Income (Loss) | Year ended | |||
| September 30, | ||||
| (In millions) | 2014 | 2013 | ||
| Income resulting from the amortization of the deferred income related to the Patterson exclusivity payment | $ | 10.0 | $ | 10.0 |
| Gain (loss) from patent infringement settlement (1) | - | 4.4 | ||
| Other miscellaneous gain (loss) (2) | 1.9 | - | ||
| Net other operating income (loss) | $ | 11.9 | $ | 14.4 |
| (1) The gain from patent settlement for the fiscal year ended September 30, 2013, represents amounts received for past lost profits in an out-of-court settlement of a patent defense suit in the normal course of business. | ||||
| (2) The other miscellaneous gain for the year ended September 30, 2014, represents a gain on disposal of certain business assets. | ||||
Gain (Loss) on Foreign Currency Transactions and Derivative Instruments
Foreign Currency Transactions
The loss on foreign currency transactions for the fiscal year ended September 30, 2014 amounted to $0.3 million (prior year period: loss of $12.4 million). The components of these results are as follows:
| Gain (Loss) on Foreign Currency Transactions | Year ended | |||
| September 30, | ||||
| (In millions) | 2014 | 2013 | ||
| Unrealized non-cash foreign exchange gain (loss) from translation adjustment of deferred income related to the Patterson exclusivity payment | $ | (2.0) | $ | 1.9 |
| Unrealized non-cash foreign exchange gain (loss) on short-term intra-group loans | (0.1) | (0.9) | ||
| Gain (loss) on other foreign currency transactions (1) | 1.8 | (13.4) | ||
| Gain (Loss) on Foreign Currency Transactions | $ | (0.3) | $ | (12.4) |
| (1) For the periods under report, the gain (loss) on other foreign currency transactions related to the revaluation of short-term assets and liabilities and realized transactions, both of which were primarly impacted by the fluctuations between the Yen/Euro, Euro/U.S. Dollar, and Real/Euro exchange rates. | ||||
Derivative Instruments
For the fiscal year ended September 30, 2014, the loss on derivative instruments was $2.5 million (prior year period: gain of $0.4 million). In both periods, the results related to foreign currency hedges.
| 45 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Income Tax Provision
For the fiscal year ended September 30, 2014, Sirona recorded a profit before income taxes of $230.4 million (prior year period: $197.5 million), an income tax provision of $53.0 million (prior year period: $49.0 million), and an effective tax rate of 23.0% (prior year period: 24.8%). The effective tax rate is primarily driven by the mix of earnings across different jurisdictions.
The income tax provision as of September 30, 2013, included the effect from a local trade tax increase at our principal German operations, which was enacted in and effective beginning in the first quarter of fiscal year 2013. This tax rate change resulted in a tax expense of $2.2 million from a non-cash remeasurement of deferred tax assets and liabilities. Excluding this amount, the effective tax rate in fiscal year 2013 was 23.7%.
NON-GAAP FINANCIAL MEASURES (unaudited)
To supplement our consolidated financial statements, operations review, and our business outlook, we currently use the following non-GAAP financial measures (unaudited):
| · | Local Currency, |
| · | Non-GAAP Adjusted Net Income, and |
| · | Non-GAAP Adjusted Earnings Per Diluted Share, |
Management recognizes that the use of these non-GAAP measures has limitations, including the fact that they might not be comparable with similar non-GAAP measures used by other companies and that management must exercise judgment in determining which types of charges and other items should be excluded from its non-GAAP financial measures. Management currently compensates for these limitations by providing full disclosure of each non-GAAP financial measure and a reconciliation to the most directly comparable GAAP measure. The presentation of this financial information is not intended to be considered in isolation or as a substitute for, or superior to, the financial information prepared and presented in accordance with GAAP.
We use these non-GAAP financial measures for financial and operational decision making and as a means to evaluate period-to-period comparisons. Our management believes that these non-GAAP financial measures provide meaningful supplemental information regarding Sirona’s operating performance in the ordinary, ongoing and customary course of its operations. Accordingly, management excludes the impact of acquisition-related intangible depreciation and amortization in order to compare our underlying financial performance to prior periods, certain charges and income related to currency revaluation of assets and liabilities that do not reflect our period-to-period core operating performance, and to the extent relevant in a particular period, any other significant cash or non-cash items that management does not view as indicative of its ongoing operating performance. Each item is evaluated on an individual basis, taking into consideration both quantitative and qualitative aspects of their nature. We believe that both management and investors benefit from referring to these non-GAAP financial measures in assessing our performance and when planning, forecasting and analyzing future periods. These non-GAAP financial measures also facilitate management's internal evaluation of period-to-period comparisons. We believe these non-GAAP financial measures are useful to investors both because (1) they allow for greater transparency with respect to key metrics used by management in its financial and operational decision making, and (2) they are provided to and used by our institutional investors and the analyst community to facilitate comparisons with prior and subsequent reporting periods.
Local Currency
Certain revenue information is presented on a local currency basis (“Local Currency”). Sirona supplementally presents this revenue information because it believes doing so facilitates a comparison of its operating results from period to period without regard to changes resulting solely from fluctuations in currency rates. Sirona calculates Local Currency revenue growth by comparing current-period revenues to prior-period revenues with both periods converted at the U.S. Dollar/local currency average foreign exchange rate for each month of the prior period for the currencies in which we do business.
| 46 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Adjusted Net Income and Adjusted Earnings per Diluted Share
These non-GAAP measures (both attributable to Sirona’s shareholders) exclude, as applicable, the following:
| · | amortization and depreciation expense resulting from the step-up to fair values of intangible and tangible assets related to past business combinations, |
| · | gains and losses on foreign currency transactions and derivative instruments, |
| · | any tax effects related to the above, and |
| · | to the extent relevant in a particular period, any other significant cash or non-cash items that management does not view as indicative of its ongoing operating performance. |
The following tables reconcile the above non-GAAP financial measures to their most directly comparable GAAP financial measures for the period(s) under report:
| Non-GAAP Financial Measures (GAAP reconciliation) | Year ended | ||||||||||
| September 30, | |||||||||||
| (In millions, except for per share and percent amounts) | 2015 | 2014 | |||||||||
| GAAP Net Income attributable to Sirona shareholders | $ | 186.2 | $ | 3.30 | 1) | $ | 175.7 | $ | 3.13 | 1) | |
| Adjustments (after tax 2)) | |||||||||||
| Amortization and depreciation expense resulting from the step-up to fair values of intangible assets related to past business combinations | $ | 20.4 | 27.5 | ||||||||
| (Gain)/loss on foreign currency transactions, net | 13.9 | 0.2 | |||||||||
| (Gain)/loss on derivative instruments | (1.3) | 1.9 | |||||||||
| Other items: | |||||||||||
| Merger and acquisition-related expenses | 3.6 | - | |||||||||
| Management Transition | 0.6 | 2.5 | |||||||||
| Discrete tax items | 1.2 | - | |||||||||
| One-time gain on sale of certain operating assets | - | (1.5) | |||||||||
| Adjusted Net Income attributable to Sirona shareholders | $ | 224.5 | $ | 3.98 | 1) | $ | 206.4 | $ | 3.67 | 1) | |
| 1) per diluted share | |||||||||||
| 2) tax impact calculated using estimated effective tax rate of | 22.6% | 23.0% | |||||||||
| 47 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
LIQUIDITY AND CAPITAL RESOURCES
Summary
Historically, Sirona’s principal uses of cash, apart from operating requirements (including research and development efforts), have been for interest payments, debt repayment, and acquisitions. Operating capital expenditures typically are approximately equal to operating depreciation (excluding any effects from the increased amortization and depreciation expense resulting from the step-up to fair values of Sirona’s and Schick’s assets and liabilities required under purchase accounting). These expenditures may temporarily exceed operating depreciation for larger-scale infrastructure and other investment activities that the Company may undertake from time to time. The Company also uses cash for occasional purchases of treasury shares pursuant to stock repurchase programs.
At September 30, 2015, the Company had cash and cash equivalents of $517.8 million and total debt of $80.2 million, resulting in net cash of $437.6 million. We believe our ability to generate cash from operating activities is one of our fundamental strengths. The near-term outlook for our business remains strong, and we expect to continue generating significant cash flows from operations in the future. The Company typically does not raise capital through issuance of stock; instead, we use debt financing to lower our overall cost of capital and increase our return on shareholders’ equity. We believe that our operating cash flows, available cash, and available but unused revolving credit facilities, in combination, provide us with the necessary financial flexibility to fund our working capital needs, research and development efforts, and anticipated capital expenditures for the foreseeable future.
We have significant operations outside of the U.S. and earn a significant portion of our consolidated operating income and income before taxes through our foreign subsidiaries. Cash and cash equivalents of $428.6 million held by our foreign subsidiaries generally are not subject to restrictions prohibiting such amounts from being available in the United States. The distribution of lower-taxed foreign earnings to the United States, however, would generally increase our effective tax rate. It is management’s intention to continue to indefinitely reinvest such earnings in foreign operations.
Debt Financing
On November 14, 2011, the Company entered into a senior facilities agreement (the “Senior Facilities Agreement’) with Sirona Dental Systems, Inc. and all significant subsidiaries of Sirona as original borrowers and original guarantors, and as of November 16, 2011, Sirona fully repaid its obligations under the Prior Senior Facilities Agreement. Initial borrowings under the Senior Facilities Agreement were used to retire the outstanding borrowings under the Company's previous credit facilities. Please see “Capital Resources - Senior Facilities Agreement” within this section and Note 19 to our consolidated financial statements for a complete description of this Senior Facilities Agreement.
The Senior Facilities Agreement contains restrictive covenants that limit Sirona’s ability to make loans, to incur additional indebtedness, and to make disposals, subject to agreed exceptions. The Company has agreed to certain financial debt covenants in relation to the financing. The covenants stipulate that the Company must maintain certain ratios in respect of consolidated total net debt to consolidated adjusted EBITDA. If the Company breaches any of the covenants, the loans will become repayable on demand.
The financial covenants require that the Company maintain a debt coverage ratio (“Debt Cover Ratio”) of consolidated total net debt to consolidated adjusted EBITDA (“Consolidated Adjusted EBITDA”), determined on the basis of the last twelve months, of no more than 3.00 to 1. The Company is required to determine its compliance with the covenants as of September 30 and March 31. As of September 30, 2015, the most recent period for which this ratio was calculated, the Company was in compliance:
| 48 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| Covenants Ratios | September 30, | March 31, | September 30, | |||
| (In millions, except for ratio amounts) | 2015 | 2015 | 2014 | |||
| Consolidated Total Net Debt | $ | (437.6) | $ | (321.8) | $ | (303.3) |
| Consolidated Adjusted EBITDA | $ | 349.8 | $ | 326.9 | $ | 321.6 |
| Debt Cover Ratio (1) | N/A | N/A | N/A | |||
| as set by covenants (less than or equal to) | 3.00 | 3.00 | 3.00 | |||
| (1) Not meaningful in the absence of net debt. | ||||||
Cash Flows
The following table summarizes our cash flows from operating, investing, and financing activities for each of the periods under report:
| Cash Flows | Year ended | |||||||||||
| September 30, | Change | |||||||||||
| (In millions, except for percent amounts) | 2015 | 2014 | 2013 | 2015 vs. 2014 | 2014 vs. 2013 | |||||||
| Net cash provided by (used in): | ||||||||||||
| OPERATING activities | $ | 239.2 | $ | 248.4 | $ | 232.0 | $ | (9.2) | (3.7%) | $ | 16.4 | 7.1% |
| INVESTING activities | (70.9) | (81.6) | (106.8) | 10.7 | 13.1% | 25.2 | 23.6% | |||||
| FINANCING activities | (12.6) | (10.0) | (38.1) | (2.6) | (26.0%) | 28.1 | 73.8% | |||||
| Increase (decrease) in cash during the period | $ | 155.7 | $ | 156.8 | $ | 87.1 | $ | (1.1) | (0.7%) | $ | 69.7 | 80.0% |
Net Cash Provided by (Used in) Operating Activities
Net cash provided by operating activities represents net cash from operations, returns on investments, and payments for interest and taxation.
Fiscal Year 2015 vs. Fiscal Year 2014
Net cash provided by operating activities was $239.2 million for the fiscal year ended September 30, 2015 (prior year period: $248.4 million), or a decrease of $9.2 million compared to an increase of $10.7 million in net income.
Influencing factors on the year-over-year change in operating cash flows were:
| · | The favorable adjustments to reconcile net income to net operating cash flow increased by $25.9 million over the prior-year period. Driving factors for this increase were: |
| o | $15.7 million more favorable adjustments from net deferred taxes related to normal, ongoing business, |
| o | $13.4 million more favorable adjustment from derivative instruments and foreign currency transactions, and |
| 49 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| o | $6.5 million less favorable adjustment from depreciation and amortization due to decreasing amortization resulting from the step-up to fair value of tangible and intangible assets. |
| o | The remaining changes only had a minor impact on the change in net cash provided by operating activities. |
| · | The changes in assets and liabilities were $45.8 million more unfavorable than in the prior year. The year-over-year factors were: |
| o | The change in receivables and inventories was $69.3 million less favorable than in the prior year. This negative development was primarily driven by the $70.4 million increase in receivables. Accounts receivable increased significantly over the prior year due to a higher concentration of revenues in the latter part of the fourth quarter in the current year compared to the prior year. |
| o | The change in trade accounts payable and other assets and liabilities was $12.5 million more favorable than in the prior year. This positive development reflects the timing of purchases. |
| o | The change in current income tax liabilities was $11.0 million more favorable than in the prior year. This positive development was due to the timing of income tax payments. |
Fiscal Year 2014 vs. Fiscal Year 2013
Net cash provided by operating activities was $248.4 million for the fiscal year ended September 30, 2014 (prior year period: $232.0 million), an increase of $16.4 million, or 7.1%.
Influencing factors on the year-over-year change in operating cash flows were:
| · | The favorable adjustments to reconcile net income to net operating cash flow decreased by $22.1 million over the prior-year period. Driving factors for this decrease were: |
| o | $11.1 million less favorable adjustments from net deferred taxes related to normal, ongoing business. In the prior year, the acquisition of a technology company and the trade tax increase at our primary facility in Bensheim, Germany, both resulted in favorable adjustments, |
| o | $9.2 million less favorable adjustment from derivative instruments and foreign currency transactions, |
| o | $0.7 million more favorable adjustment from depreciation and amortization due to decreasing amortization resulting from the step-up to fair value of tangible and intangible assets, |
| o | In fiscal 2014, the gain on the sale of certain business assets resulted in an unfavorable impact of $1.9 million in the other adjustments, and |
| o | The remaining changes only had a minor impact on the change in net cash provided by operating activities. |
| · | The unfavorable effect of changes in assets and liabilities resulted in a decrease over the prior-year period of $9.6 million. The year-over-year factors were: |
| o | The change in receivables and inventories was $35.6 million more favorable than in the prior year. This positive difference was primarily driven by the $33.7 million favorable development in receivables. Accounts receivable decreased significantly over the prior year due in part to a lower concentration of revenues in the latter part of the fourth quarter in the current year compared to the prior year as well as a shift in customer structure, with increased sales in the fourth quarter to customers with shorter payment terms. |
| o | The change in trade accounts payable and other assets and liabilities was $23.9 million less favorable than in the prior year. This negative development reflects the timing of supplier payments in the current period as well as the favorable impacts in the prior year resulting from the acquisition of a technology company. |
| 50 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| o | The change in current income tax liabilities was $2.1 million less favorable than in the prior year. This negative development was due to the timing of income tax payments. |
Net Cash Provided by (Used in) Investing Activities
Net cash used in investing activities represents cash used for capital expenditures in the normal course of operating activities, financial investments, acquisitions, asset disposals, and divestitures.
Net cash used in investing activities was $70.9 million for the fiscal year ended September 30, 2015 (prior year period: $81.6 million), or a decrease of $10.7 million.
For the fiscal year ended September 30, 2015, net cash used in investing activities represented:
| · | The acquisition of a dental company for $18.5 million, and |
| · | capital expenditures in the course of normal operating activities. |
For the fiscal year ended September 30, 2014, net cash used in investing activities represented:
| · | the acquisition of the main administrative building in Bensheim, Germany for $26.7 million, |
| · | the sale of certain business assets for $11.5 million, |
| · | investment in additional machinery for the instruments manufacturing facility in Bensheim, Germany, and |
| · | capital expenditures in the course of normal operating activities. |
For the fiscal year ended September 30, 2013, net cash used in investing activities represented:
| · | the acquisition of a dental technology company for $35.0 million, |
| · | the completion of the expansion of the new instruments manufacturing facility in Bensheim, Germany, and |
| · | capital expenditures in the course of normal operating activities. |
Net Cash Provided by (Used in) Financing Activities
Net cash used in financing activities represents net cash from debt financing activities, dividends, treasury share transactions, and share-based compensation activities.
Fiscal Year 2015 vs. Fiscal Year 2014
Net cash used in financing activities was $12.6 million for the fiscal year ended September 30, 2015 (prior year period: $10.0 million), or an increase of $2.6 million.
Influencing factors on the year-over-year change in financing cash flows were:
| · | $9.6 million lower volume of treasury stock purchases compared to the prior period, and |
| · | $2.6 million lower proceeds and $9.7 million higher tax effects from shares issued under share-based compensation plans. |
Fiscal Year 2014 vs. Fiscal Year 2013
Net cash used in financing activities was $10.0 million for the fiscal year ended September 30, 2014 (prior year period: $38.1 million), or a decrease of $28.1 million.
Influencing factors on the year-over-year change in financing cash flows were:
| · | $28.1 million lower volume of treasury stock purchases compared to the prior period, |
| 51 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| · | $1.1 million lower proceeds and $0.3 million higher tax effects from shares issued under share-based compensation plans, and |
| · | $1.3 million lower amounts for the purchase of shares from and dividends paid to noncontrolling interests. |
Capital Resources
Senior Facilities Agreement
On November 14, 2011, the Company entered into the Senior Facilities Agreement with Sirona Dental Systems, Inc. and all significant subsidiaries of Sirona as original borrowers and original guarantors. As of November 16, 2011, Sirona fully repaid its obligations under the Prior Senior Facilities Agreement. Initial borrowings under the Senior Facilities Agreement were used to retire the outstanding borrowings under the Company's previous credit facilities.
The Senior Facilities Agreement includes:
| (1) | a term loan in an aggregate principal amount of $75 million ("Facility A Term Loan") available to Sirona or Sirona Dental, as borrower; |
| (2) | a 120 million Euro revolving credit facility (“Revolving Facility B”) available to Sirona Dental Systems GmbH and Sirona Dental Services GmbH, as initial borrowers; and |
| (3) | a $100 million revolving credit facility (“Revolving Facility C”) available to Sirona or Sirona Dental, as initial borrowers. |
The Revolving Facility B is available for borrowing in Euro or any other freely available currency agreed to by the facility agent. The facilities are made available on an unsecured basis. Subject to certain limitations, each European guarantor guarantees the performance of each European borrower, except itself, and each U.S. guarantor guarantees the performance of each U.S. borrower, except itself.
Of the amount borrowed under the Facility A Term Loan, 30% is due on November 16, 2015, and the balance is due on November 16, 2016. The loans under the Senior Facilities Agreement bear interest of EURIBOR, for Euro-denominated loans, and LIBOR for the other loans, plus an initial margin of 160, 85 and 110 basis points for the Facility A Term Loan, Revolving Facility B and Revolving Facility C, respectively.
The Senior Facilities Agreement contains a margin ratchet. Pursuant to this provision, which applies from March 31, 2012 onwards, the applicable margin varies depending on the Company’s leverage multiple (i.e. the ratio of consolidated total net debt to consolidated adjusted EBITDA as defined in the Senior Facilities Agreement) between 160 basis points and 215 basis points for the Facility A Term Loan, 85 basis points and 140 basis points for the Revolving Facility B, and 110 basis points and 165 basis points for the Revolving Facility C.
The Senior Facilities Agreement contains restrictive covenants that limit Sirona's ability to make loans, to incur additional indebtedness, and to make disposals, subject to agreed-upon exceptions. The Company has agreed to certain financial debt covenants in relation to the financing. The covenants stipulate that the Company must maintain certain ratios in respect of consolidated total net debt to consolidated adjusted EBITDA. If the Company breaches these covenants, the loans will become repayable on demand.
On November 16, 2011, Sirona entered into 5-year payer interest rate swaps to fully hedge its 3-month LIBOR exposure for the Facility A Term Loan. The terms of the swap reflect the term structure of the underlying loan. The effective nominal interest rate is 1.2775% plus the applicable margin. Settlement of the swaps is required on a quarterly basis.
Debt issuance costs of $2.8 million were incurred in relation to the financing in November 2011 and have been capitalized as deferred charges and are amortized using the effective interest method over the term of the loans.
As of September 30, 2015 and September 30, 2014, the Facility A Term Loan was fully drawn to the amount of $75.0 million. The Revolving Facilities B and C remained undrawn as of September 30, 2015 and September 30, 2014.
| 52 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONTRACTUAL OBLIGATIONS AND COMMERCIAL COMMITMENTS
The following table summarizes contractual obligations and commercial commitments as of September 30, 2015:
| Contractual Obligations and Commercial Commitments | Payments due by period | |||||||||
| (In millions) | Total | < 1 year | 1-3 years | 3-5 years | > 5 years | |||||
| Long-term debt (1) | $ | 78.1 | $ | 25.3 | $ | 52.8 | $ | - | $ | - |
| Capital lease obligations | 4.6 | 0.2 | 0.4 | - | 4.0 | |||||
| Operating lease obligations | 34.5 | 10.6 | 14.5 | 6.8 | 2.6 | |||||
| Pension | 28.5 | 3.3 | 5.1 | 5.8 | 14.3 | |||||
| Purchase commitments (2) | 35.2 | - | 28.8 | 6.4 | - | |||||
| Total | $ | 180.9 | $ | 39.4 | $ | 101.6 | $ | 19.0 | $ | 20.9 |
| (1) includes expected interest payments and agency/commitment fees | ||||||||||
| (2) represents unconditional purchase commitments with remaining terms in excess of one year | ||||||||||
OFF-BALANCE SHEET ARRANGEMENTS
Sirona does not have any off-balance sheet financing arrangements other than its derivatives.
SIGNIFICANT ACCOUNTING POLICIES AND ESTIMATES
Introduction
The preparation of financial statements in conformity with U.S. GAAP requires Sirona to make estimates and assumptions that affect amounts reported in its consolidated financial statements and accompanying notes. These estimates and assumptions are evaluated on an ongoing basis based on historical developments, market conditions, industry trends and other information Sirona believes to be reasonable under the circumstances. There can be no assurance that actual results will conform to Sirona’s estimates and assumptions, and that reported results of operations will not be materially adversely affected by the need to make accounting adjustments to reflect changes in its estimates and assumptions from time to time. The following is a discussion of significant accounting policies and estimates important for the understanding of certain events and disclosures for the period under report. For a comprehensive listing and discussion of our significant accounting policies and estimates, please refer to Note 2 “Basis of Presentation and Summary of Significant Accounting Policies” in this report.
| 53 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Significant Policies and Estimates
Revenue Recognition
The Company’s main revenue stream results from the delivery of dental equipment. The Company also enters into revenue arrangements that consist of multiple deliverables of its product and service offerings. Additionally, certain products, primarily in our CAD/CAM and Imaging segments, may contain embedded software that functions together with the product to deliver the product’s essential functionality.
Revenue, net of related discounts and allowances, is recognized when products or equipment have been shipped, when persuasive evidence of the arrangement exists, the price is fixed or determinable, collectability is reasonably assured, title and risk of loss has passed to customers based on the shipping terms, no significant obligations remain, and allowances for discounts, returns, and customer incentives can be reliably estimated. The Company offers discounts to its distributors if certain conditions are met. Discounts and allowances are primarily based on the volume of products purchased or targeted to be purchased by the individual customer or distributor. Discounts are deducted from revenue at the time of sale or when the discount is offered, whichever is later. The Company estimates volume discounts based on the individual customer’s historical and estimated future product purchases. Returns of products, excluding warranty related returns, are infrequent and insignificant. Amounts received from customers in advance of product shipment are classified as deferred income until the revenue can be recognized in accordance with the Company’s revenue recognition policy.
| · | Services: Service revenue is generally recognized ratably over the contract term as the specified services are performed. Amounts received from customers in advance of rendering of services are classified as deferred income until the revenue can be recognized upon rendering of those services. |
| · | Extended Warranties: The Company offers its customers an option to purchase extended warranties on certain products. The Company recognizes revenue on these extended warranty contracts ratably over the life of the contract. The costs associated with these extended warranty contracts are recognized when incurred. |
| · | Multiple-Element Arrangements (“MEAs”): Arrangements with customers may include multiple deliverables, including any combination of equipment, services, and extended warranties. The deliverables included in the Company’s MEAs are separated into more than one unit of accounting when (i) the delivered equipment has value to the customer on a stand-alone basis, and (ii) delivery of the undelivered service element(s) is probable and substantially in the control of the Company. Arrangement consideration is then allocated to each unit, delivered or undelivered, based on the relative selling price (“RSP”) of each unit of accounting based first on vendor-specific objective evidence (“VSOE”) if it exists and then based on estimated selling price (“ESP”). |
VSOE: In most instances, products are sold separately in stand-alone arrangements. Services are also sold separately through renewals of contracts with varying periods. The Company determines VSOE based on its pricing and discounting practices for the specific product or service when sold separately, considering geographical, customer, and other economic or marketing variables, as well as renewal rates or stand-alone prices for the service element(s).
ESP: The estimated selling price represents the price at which the Company would sell a product or service if it were sold on a stand-alone basis. When VSOE does not exist for all elements, the Company determines ESP for the arrangement element based on sales, cost and margin analysis, as well as other inputs based on its pricing practices. Adjustments for other market and Company-specific factors are made as deemed necessary in determining ESP.
After separating the elements into their specific units of accounting, total arrangement consideration is allocated to each unit of accounting according to the nature of the revenue as described above and application of the RSP method. Total recognized revenue is limited to the amount not contingent upon future transactions.
Pensions and 401(k) Plan
The Company has defined benefit and defined contribution pension plans and an early retirement plan. Sirona recognizes changes in the funded status of its benefit plans, not yet recognized in the income statement, in other comprehensive income until they are amortized as a component of net periodic benefit cost.
| 54 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Pension expense is recognized on an accrual basis over the employee’s approximate service periods. Defined benefit pension costs are determined by using an actuarial method, which provides for the deferral of actuarial gains and losses (in excess of a specified corridor) that result from changes in assumptions or actual experience differing from that assumed. Costs relating to changes in the benefit plan as well as the transition obligation are amortized. Disclosure of the components of periodic pension cost is also required. When purchase accounting is applied, pension liabilities are recognized for the projected benefit obligation in excess of plan assets.
The key assumption used in the actuarial calculations for the defined benefit pension plans is the selection of the appropriate discount rate. The discount rate has been selected by reference to market interest rates. The discount rate used reflects the rates available on high quality fixed income investment of appropriate duration at the measurement dates of each year. Fluctuations in market interest rates could impact the amount of pension expense recorded for these plans. The discount rate assumed at September 30, 2015 was 2.15% (prior year period: 2.30%). Accordingly, this change in discount rate affected the amount of pension obligation recorded at September 30, 2015.
Plan assets consist of insurance policies with a guaranteed minimum return by the insurance company and an excess profit participation feature for a portion of the benefits. Sirona pays the premiums on the insurance policies but does not manage the investment of the funds; the insurance company makes all decisions on investment of funds, including the allocation to asset groups. The fair value of the plan assets such as equity securities, fixed-income investments, and others is based on the cash surrender values reported by the insurance company.
Contributions made to the defined contribution pension plans and the 401(k) savings plan for U.S. employees are accrued based on the contributions required by the plan.
The Company also has an early retirement plan, Altersteilzeit (‘‘ATZ’’), which allows certain German employees who have been accepted into the plan to retire at 60 rather than at the legal retirement age of 67. Eligible employees are those who have attained the age of 59, have completed 12 years of service, and have been accepted to participate in the ATZ plan. Accepted employees join for a period of 2-4 years, during which they work in full active service for 50% of the agreed ATZ plan period, the remaining 50% of the plan period being the passive phase during which the employee does not work. Alternatively, the employee may work for 50% of the time for the entire agreed ATZ plan period. The alternative actually executed is decided via mutual agreement between Sirona and the employee. During the active service period, the employees receive 50% of their salary plus a bonus payment equal to 35% of their salary, and the remaining 50% of their salary, plus a bonus payment equal to 35% of their salary, is paid during the inactive service period. The Company recognizes the salary component of the ATZ plan over the period from the beginning of the ATZ period to the end of the active service period.
Income Taxes
Sirona recognizes deferred tax assets and liabilities based on the differences between the financial statement carrying amounts and the tax basis of assets and liabilities. Sirona regularly reviews its deferred tax assets for recoverability and establishes a valuation allowance, as necessary, based on historical taxable income, projected future taxable income, the expected timing of the reversals of existing temporary differences and the implementation of tax-planning strategies. If Sirona is unable to generate sufficient future taxable income in certain tax jurisdictions, or if there is a material change in the actual effective tax rates or time period within which the underlying temporary differences become taxable or deductible, it could be required to increase its valuation allowance against its deferred tax assets resulting in an increase in its effective tax rate and an adverse impact on operating results. As of September 30, 2015, Sirona had recorded valuation allowances against its deferred tax assets in the amount of $3.8 million (prior year period: $3.1 million). Further information on income taxes is provided in Note 9 to the consolidated financial statements appearing elsewhere in this report.
Management believes it is more likely than not that forecasted income, including income that may be generated as a result of certain tax planning strategies, together with the tax effects of the deferred tax liabilities, will be sufficient to fully recover the remaining deferred tax assets. In the event that the Company determines all or part of the net deferred tax assets are not realizable in the future, the Company will make an adjustment to the valuation allowance that would be charged to earnings in the period such determination is made. In addition, the calculation of tax liabilities involves significant judgment in estimating the impact of uncertainties in the application of ASC 740 and other complex tax laws. Resolution of these uncertainties in a manner inconsistent with management’s expectations could have a material impact on the Company’s financial condition and operating results.
| 55 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Impairment of Long-Lived and Finite-Lived Assets
Sirona assesses all its long-lived assets for impairment whenever events or circumstances indicate their carrying value may not be recoverable. Sirona’s management assesses whether there has been an impairment by comparing anticipated undiscounted future cash flows from operating activities with the carrying value of the asset. The factors considered by Sirona’s management in this assessment include operating results, trends and prospects, as well as the effects of obsolescence, demand, competition and other economic factors. If an impairment is deemed to exist, management records an impairment charge equal to the excess of the carrying value over the fair value of the impaired assets. This could result in a material charge to earnings.
Impairment of Indefinite-Lived Assets
Goodwill is allocated to each of our reporting units, which we regard to be our operating segments (Dental CAD/CAM Systems, Imaging Systems, Treatment Centers, and Instruments). Sirona assesses goodwill for impairment annually on September 30 unless an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying value at an earlier date. This evaluation begins with a qualitative assessment to determine if the fair value of its reporting units is more likely than not less than their carrying values. The Company evaluates such qualitative factors as
| (i) | the results of the last quantitative impairment assessment, |
| (ii) | macro- and industry economic conditions such as significant changes in the business and legal climate and competition, and |
| (iii) | Company-specific assumptions including historical data and experience, operating performance indicators, projections of revenues and expenses and related cash flows, expected long-term growth rates, sale or disposition of a significant portion of the business, the development of its stock price, and other factors. |
If we determine that the fair value is more likely than not less than the carrying value, or we decide to bypass the qualitative assessment for a reporting unit, goodwill is tested for impairment under the two-step valuation test. The first step is to estimate the fair value of each reporting unit and compare this estimated fair value with each reporting unit’s carrying value. If the fair value is less than the carrying value, additional steps, including an allocation of the estimated fair value to the assets and liabilities of the reporting unit, would be necessary to determine the amount, if any, of goodwill impairment. In this second step, a fair value exercise similar to a business combination would be performed where the individual identifiable assets and liabilities of the reporting unit are valued at fair value with the difference between the fair value of the reporting unit being the implied fair value of goodwill. As of September 30, 2015, based on the qualitative assessment, the Company determined that step one of the impairment test is not required. If we would determine the fair value of a reporting unit, we would use a discounted future cash flow model to estimate reporting unit fair value. Significant assumptions in a discounted cash flow model would include discount rate, revenue and gross profit margin growth and terminal growth rates based on our judgments, estimates and assumptions.
Sirona evaluates trademarks and in-process research and development (“IPR&D”), which are considered indefinite-lived intangible assets until the associated projects are completed, for impairment at least annually or whenever events or circumstances indicate their carrying value might be impaired. In performing this assessment, Sirona’s management employs a systematic methodology that considers qualitative and quantitative evidence in evaluating whether an impairment is likely to have occurred. These factors include operating results, trends and prospects, as well as the effects of obsolescence, demand, competition and other economic factors. If an impairment is likely to have occurred, as estimate of the fair value of the indefinite-lived intangible assets is performed. The carrying value is considered impaired when it exceeds the fair market value. In such an event, an impairment loss is recognized equal to the amount of that excess. Key assumptions in determining fair value include using the expected discounted cash flows. Once an impairment is determined, an impairment charge is recorded in the consolidated statement of income.
| 56 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
ACCOUNTING STANDARDS ISSUED BUT NOT YET ADOPTED
Please see Note 3 to the consolidated financial statements for any discussions of recently issued accounting standards that have not yet been adopted.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Sirona is exposed to economic risk from foreign currency exchange rates as well as interest rates. Portions of these risks are hedged; however, they may impact our operating performance.
The following discussion should be read in conjunction with Notes 2, 17, and 19 to Sirona’s audited consolidated financial statements appearing elsewhere in this report, which provide further information on Sirona’s derivative instruments.
Foreign Currency
Sirona’s primary market risk exposure is foreign currency risk. Certain transactions, assets, and liabilities are exposed to foreign currency risk, which can adversely affect our revenues and operating profits. To help mitigate this risk and maximize the economic effectiveness of our foreign currency positions, Sirona enters into forward exchange contracts where practicable.
The Euro is the functional currency for the majority of Sirona’s subsidiaries, including its German operations, which are the primary sales and manufacturing operations of Sirona. Sales from other Sirona operations are denominated in various foreign currencies. As a percent of total revenues, sales and operating expenses in Euro, U.S. Dollar, and all other currencies (most importantly: Japanese Yen, Australian Dollar, Chinese Yuan Renminbi, and Brazilian Real) for fiscal year 2015 were approximately as follows:
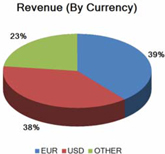 |
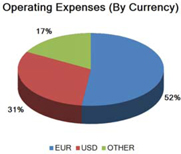 |
| 57 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The most significant influencing factor is the U.S. Dollar/Euro exchange rate. During the periods under review, the U.S. Dollar/Euro exchange rate has fluctuated significantly. The following table presents the relevant U.S. Dollar/Euro exchange rate information for the period(s) under report:
| Fluctuations in Exchange Rates (USD/EUR) | Year ended | |||||
| September 30, | ||||||
| (In U.S. Dollars) | 2015 | 2014 | 2013 | |||
| Exchange rate used to calculate items in Sirona's financial statements: | ||||||
| Period-end(1) | $ | 1.1215 | $ | 1.2592 | $ | 1.3499 |
| Average(2) | 1.1492 | 1.3573 | 1.3121 | |||
| Fluctuations during the period: | ||||||
| Low(3) | $ | 1.0526 | $ | 1.2592 | $ | 1.2710 |
| High(4) | 1.2814 | 1.3956 | 1.3639 | |||
| (1) Closing rate as of the balance sheet date. | ||||||
| (2) Year-to-date average exchange rate for the fiscal year. | ||||||
| (3) Lowest daily exchange rate for the fiscal year. | ||||||
| (4) Highest daily exchange rate for the fiscal year. | ||||||
In order to hedge portions of the transactional exposure to fluctuations in exchange rates, based on forecasted and firmly committed cash flows, Sirona enters into forward foreign currency contracts. Currently, the principal currencies hedged against the Euro are the U.S. Dollar, Japanese Yen, and Australian Dollar. These forward foreign currency contracts are intended to reduce short-term effects of changes in exchange rates. Sirona does not apply hedge accounting to these forward foreign currency contracts.
As of September 30, 2014, we changed our disclosure method of estimating and quantifying our foreign currency risks from a tabular presentation of the assets subject to foreign currency risk without an estimate of the possible effects on future earnings to a value-at-risk (“VAR”) model. We made this change because we believe that use of a VAR method provides our shareholders with a better understanding and estimate of the potential impacts on our earnings due to foreign currency risk.
VAR is defined as the expected gain or loss, for a given confidence level, in the fair value of our portfolio due to positive or adverse market movements over a defined time horizon. The VAR model is not intended to represent actual gains or losses in fair value, including determinations of other-than-temporary losses in fair value in accordance with U.S. GAAP, but is used as a risk estimation and management tool. The distribution of the potential changes in total market value of all transactional asset, and liability positions is computed based on the historical volatilities and correlations among foreign currency exchange rates, assuming normal market conditions.
The VAR is calculated as the total gain or loss that will not be exceeded at the 95.0 percentile confidence level. Several risk factors are not captured in the model, including liquidity, operational, and legal risks. The following table sets forth the one-day VAR for substantially all of our positions for the fiscal years ended September 30, 2015 and 2014:
| Foreign Currency Risk | Value at Risk | |||||||
| Average | Maximum | |||||||
| (In millions) | Net Exposure (1) | Gain (Loss)(2) |
Gain (2) | (Loss) (2) | ||||
| September 30, 2015 | $ | 365.3 | $ | 0.2 | $ | 4.7 | $ | (4.6) |
| September 30, 2014 | 215.5 | (0.1) | 1.6 | (1.7) | ||||
| (1) as of the balance sheet date. Represents the net asset (liability) position exposed to foreign currency risk. | ||||||||
| (2) for the fiscal years ended | ||||||||
| 58 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Interest Rate
Sirona is also exposed to interest rate risk associated with short and long-term bank loans bearing variable interest rates. To help mitigate this interest rate risk exposure, Sirona enters into interest rate swap agreements.
On November 16, 2011, Sirona entered into interest rate swaps to fully hedge its interest exposure in connection with the Senior Facilities Agreement dated November 14, 2011. See “Management’s Discussion and Analysis of Financial Conditions and Results of Operations - Long-Term Debt” for further details. Since Sirona’s interest exposure is fully hedged and nearly 100% effective as of September 30, 2015, any sensitivity analysis (such as a hypothetical, instantaneous increase of one percentage point in the interest rates applicable to the variable interest rate debt) would not have had a material impact on interest expense for the periods under report.
Please refer to Note 19 for information on interest rates and scheduled maturities of principal by fiscal year for our outstanding variable-rate indebtedness as of September 30, 2015.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The response to this item is included as a separate section of this Annual Report on Form 10-K, beginning on page F-1.
ITEM
9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
None.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our chief executive officer (principal executive officer) and chief financial officer (principal financial officer), evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934), as of September 30, 2015. Based upon this evaluation, our chief executive officer and chief financial officer concluded that, as of September 30, 2015, the Company’s disclosure controls and procedures are effective. Our disclosure controls and procedures are designed to ensure that information relating to the Company, including our consolidated subsidiaries, that is required to be disclosed in the reports we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in Commission’s rules and forms, and is accumulated and communicated to our management, including our principal executive officer and our principal financial officer, as appropriate to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over the Company’s financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act). Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
| 59 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Our management assessed the effectiveness of the Company’s internal control over financial reporting as of September 30, 2015. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control Integrated Framework (2013). Based on our assessment, management believes that, as of September 30, 2015, our internal control over financial reporting is effective based on those criteria.
The independent registered public accounting firm, which audited the Company’s financial statements included in this Form 10-K, has issued an attestation report on the Company’s internal control over financial reporting. Please see the attestation report on page 71.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting (as defined in rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the quarter ended September 30, 2015, that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
None.
| 60 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The information required by this item not set forth herein is incorporated by reference to the proxy statement for our 2015 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission no later than 120 days after the end of the fiscal year (September 30, 2015).
ITEM 11. EXECUTIVE COMPENSATION
The information required by this item not set forth herein is incorporated by reference to the proxy statement for our 2015 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission no later than 120 days after the end of the fiscal year (September 30, 2015).
ITEM
12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND
RELATED STOCKHOLDER MATTERS
The information required by this item not set forth herein is incorporated by reference to the proxy statement for our 2015 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission no later than 120 days after the end of the fiscal year (September 30, 2015).
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
The information required by this item not set forth herein is incorporated by reference to the proxy statement for our 2015 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission no later than 120 days after the end of the fiscal year (September 30, 2015).
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
The information required by this item not set forth herein is incorporated by reference to the proxy statement for our 2015 Annual Meeting of Stockholders to be filed with the Securities and Exchange Commission no later than 120 days after the end of the fiscal year (September 30, 2015).
| 61 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a) (1) Financial Statements, See Financial Information Table of Contents on Page 69.
(b) The following Exhibits are included in this report:
| 62 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
|
Exhibit No. |
Item Title |
| 2.1 | Exchange Agreement, by and among Sirona Holdings Luxco S.C.A, Blitz 05-118 GmbH and Schick Technologies, Inc., dated September 25, 2005 (incorporated by reference to Exhibit 99.1 to Form 8-K, filed on September 26, 2005) |
| 2.2 | Amendment No. 1 to Exchange Agreement, dated May 11, 2006 (incorporated by reference to Exhibit 99.1 to Form 8-K, filed on May 16, 2006) |
| 2.3 | Agreement and Plan of Merger, dated September 15, 2015, by and among DENTSPLY International Inc., the Company and Dawkins Merger Sub Inc. (incorporated by reference to Exhibit 2.1 to Form 8-K, filed on September 16, 2015) |
| 3.1 | Amended and Restated Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form S-1, File No. 333-33731, filed on June 30, 1997) |
| 3.2 | Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.2 to Form 8-K filed on June 20, 2006) |
| 3.3 | Certificate of Amendment to Amended and Restated Certificate of Incorporation of the Company (incorporated by reference to Exhibit 3.1 to Form 8-K filed on February 24, 2014) |
| 3.4 | Bylaws of the Company effective as of September 20, 2010 (incorporated by reference to Exhibit 3.2 to Form 8-K, filed on September 23, 2010) |
| 3.5 | First Amendment to the By-Laws of the Company, dated September 15, 2015 (incorporated by reference to Exhibit 3.1 to Form 8-K, filed on September 16, 2015) |
| 4.1 | Form of Common Stock certificate of the Company (incorporated by reference to Exhibit 4.4 to the Company’s Registration Statement on Form S-3, File No. 333-153092, filed on August 20, 2008) |
| 10.1 | 1996 Employee Stock Option Plan, as amended (incorporated by reference to Exhibit 10.1 to Form 10-K, filed on July 13, 2001)† |
| 10.2 | Amendment to 1996 Employee Stock Option Plan (incorporated by reference to the Company’s definitive proxy statement on Schedule 14A, filed on May 16, 2006)† |
| 10.3 | 1997 Stock Option Plan for Non-Employee Directors, as amended (incorporated by reference to Exhibit 10.2 to Form 10-K, filed on June 18, 2003)† |
| 10.4 | Sirona Dental Systems, Inc. Equity Incentive Plan (incorporated by reference to the Company’s definitive proxy statement on Schedule 14A, filed on January 26, 2007)† |
| 10.5 | Form of Stock Option Notice under Sirona Dental Systems, Inc. Equity Incentive Plan (incorporated by reference Exhibit 10.2 to Form 8-K filed on February 28, 2007)† |
| 10.6 | Distributorship Agreement, dated April 6, 2000, by and between Schick Technologies, Inc. and Patterson Dental Company (incorporated by reference to Exhibit 10.34 to Form 10-K, filed on June 29, 2000)** |
| 10.7 | Amendment No. 1 to Distributorship Agreement, dated July 1, 2005 by and between Schick Technologies, Inc. and Patterson Dental Company (incorporated by reference to Exhibit 10.1 to Form 10-Q/A, filed on March 24, 2006)** |
| 10.8 | Consulting and Non-Competition Agreement between Schick Technologies, Inc. and David B. Schick, dated May 7, 2004 (incorporated by reference to Exhibit 10.33 to Form 10-K, filed on June 25, 2004) |
| 10.9 | Transaction Services Agreement by and between Blitz F04-506 GmbH, Sirona Dental Services GmbH & Co KG, Sirona Dental Systems GmbH, MDP IV Offshore GP, LP and Harry M. Jansen Kraemer, Jr., dated July 6, 2005 (incorporated by reference to Exhibit 10.7 to Form 10-K, filed on December 11, 2006) |
| 63 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 10.10 | Registration Agreement between the Company and Luxco, dated as of June 20, 2006 (incorporated by reference to Exhibit 2.1 to Form 8-K filed on June 20, 2006) |
| 10.11 | Employment Agreement between the Company and Jeffrey T. Slovin, dated as of June 14, 2006 (incorporated by reference to Exhibit 2.2 to Form 8-K filed on June 20, 2006)† |
| 10.12 | Employment Agreement between the Company and Michael Stone, dated as of June 14, 2006 (incorporated by reference to Exhibit 2.3 to Form 8-K filed on June 20, 2006)† |
| 10.13 | Transition and Severance Agreement between the Company and Zvi Raskin, dated as of June 14, 2006 (incorporated by reference to Exhibit 2.4 to Form 8-K filed on June 20, 2006)† |
| 10.14 | Employment Agreement between Sirona Beteiligungs- und Verwaltungsgesellschaft mbH (represented by its shareholder Sirona Dental Systems SARL) and Jost Fischer, dated as of January 25, 2002 (incorporated by reference to Exhibit 10.5 to Form 10-Q, filed on August 9, 2006)† |
| 10.15 | Employment Agreement between Sirona Beteiligungs- und Verwaltungsgesellschaft mbH (represented by its shareholder Sirona Dental Systems SARL) and Simone Blank, dated as of June 27, 2001 (incorporated by reference to Exhibit 10.6 to Form 10-Q, filed on August 9, 2006)† |
| 10.16 | Consolidated and Restated Amendment to Distributorship Agreement between Sirona Dental Systems GmbH and Patterson Companies, Inc. (incorporated by reference to Exhibit 10.8 to Form 10-Q, filed on August 9, 2006)** |
| 10.17 | Senior Facilities Agreement (incorporating amendments made on December 5, 2006 and January 19, 2007) among Sirona Dental Systems, Inc., Schick Technologies, Inc., Sirona Dental Systems GmbH, Sirona Dental Services GmbH, Sirona Dental Systems LLC, Sirona Holding GmbH, Sirona Immobilien GmbH, J.P. Morgan PLC, UBS Limited, JPMorgan Chase Bank, N.A., and J.P. Morgan Europe Limited, dated November 22, 2006 (incorporated by reference to Exhibit 10.1 to Form 10-Q, filed on May 10, 2007) |
| 10.18 | Description of the Sirona Dental Systems, Inc. EVA Plan (incorporated by reference to Exhibit 10.18 to Form 10-K filed on December 7, 2007)† |
| 10.19 | Employment Agreement between Schick Technologies, Inc. and Jeffrey T. Slovin, dated June 9, 2004 (superseded by the employment agreement dated June 20, 2006 (the “2006 employment agreement”) incorporated by reference as Exhibit 10.11 to this Form 10-K, except for the bonus information contained in Section IV referenced in the 2006 employment agreement)† |
| 10.20 | Company’s 2008 Executive Bonus Plan (incorporated by reference to Exhibit 10.1 to Form 10-Q, filed on May 8, 2008)† |
| 10.21 | Company’s 2009 Executive Bonus Plan (incorporated by reference to Exhibit 10.21 to Form 10-K, filed on December 4, 2008)† |
| 10.22 | Amended and Restated Service Agreement between Sirona Dental GmbH, the Company and Jost Fischer, dated as of December 2, 2008 (superseding an Executive Service Agreement between Sirona Dental GmbH and Jost Fischer, dated as of October 10, 2007, which superseded the Employment Agreement between Sirona Beteiligungs- und Verwaltungsgesellschaft mbH (represented by its shareholder Sirona Dental Systems SARL) and Jost Fischer, dated as of January 25, 2002) (incorporated by reference to Exhibit 10.22 to Form 10-K, filed on December 4, 2008)† |
| 64 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 10.23 | Amended and Restated Service Agreement between Sirona Dental GmbH, the Company and Simone Blank, dated as of December 2, 2008 (superseding an Executive Service Agreement between Sirona Dental GmbH and Simone Blank, dated as of October 1, 2007, which superseded the Employment Agreement between Sirona Beteiligungs- und Verwaltungsgesellschaft mbH (represented by its shareholder Sirona Dental Systems SARL) and Simone Blank, dated as of June 27, 2001) (incorporated by reference to Exhibit 10.23 to Form 10-K, filed on December 4, 2008)† |
| 10.24 | Amendment to Employment Agreement, dated as of December 2, 2008, between the Company and Jeffrey T. Slovin (amending the Employment Agreement between the Company and Jeffrey T. Slovin, dated as of June 14, 2006 and superseding the Employment Agreement between the Company and Jeffrey T. Slovin dated as of June 9, 2004) (incorporated by reference to Exhibit 10.24 to Form 10-K, filed on December 4, 2008)† |
| 10.25 | Sirona Dental Systems, Inc. Equity Incentive Plan, as amended (incorporated by reference to Exhibit 10.1 to Form 8-K, filed on March 3, 2009)† |
| 10.26 | Schick Technologies, Inc. 1996 Stock Option Plan, as amended (incorporated by reference to Exhibit 10.2 to Form 8-K, filed on March 3, 2009)† |
| 10.27 | Renewal Letter Agreement, dated as of May 4, 2009, between Sirona Dental Services GmbH, a corporation organized under the laws of Germany (“Sirona GmbH”) and Sirona Holdings Luxco S.C.A., a société en commandite par actions organized under the laws of the Grand Duchy of Luxembourg (“Luxco”), to the Advisory Services Agreement dated October 1, 2005 between Sirona GmbH and Luxco, together with the Assignment and Assumption Agreement dated May 4, 2009 among Sirona GmbH, Sirona Dental Systems, Inc. and Luxco (incorporated by reference to Exhibit 10.1 to Form 10-Q, filed on May 5, 2009) |
| 10.28 | Form of Restricted Stock Unit Agreement for December 8, 2009 restricted stock unit grants (incorporated by reference to Exhibit 10.1 to Form 8-K, filed on December 11, 2009)† |
| 10.29 | Amendment to Distributorship Agreement, dated May 5, 2010, by and between Schick Technologies, Inc. and Patterson Companies, Inc. (incorporated by reference to Exhibit 10.1 to Form 10-Q, filed on May 5, 2010)** |
| 10.30 | Amendment No. 2 to Amended and Restated Employment Agreement, dated as of September 20, 2010, between the Company and Jeffrey T. Slovin (amending the Employment Agreement between the Company and Jeffrey T. Slovin, dated as of June 14, 2006) (incorporated by reference to Exhibit 10.1 to Form 8-K, filed on September 23, 2010)† |
| 10.31 | Employment Agreement, dated as of September 13, 2007, as amended on October 15, 2008, by and between Sirona Dental GmbH and Walter Petersohn (incorporated by reference to Exhibit 10.1 to Form 8-K, filed on September 23, 2010)† |
| 10.32 | Supplement Agreement to Service Agreement between Sirona Dental GmbH, the Company and Jost Fischer, dated as of November 15, 2010, as amended by the Amended and Restated Service Agreement between Sirona Dental GmbH, the Company and Jost Fischer, dated as of December 2, 2008 (incorporated by reference to Exhibit 10.32 to Form 10-K, filed on November 18, 2010)†** |
| 10.33 | Supplement Agreement to Service Agreement between Sirona Dental GmbH, the Company and Simone Blank, dated as of November 15, 2010, as amended by the Amended and Restated Service Agreement between Sirona Dental GmbH, the Company and Simone Blank, dated as of December 2, 2008 (incorporated by reference to Exhibit 10.33 to Form 10-K, filed on November 18, 2010)†** |
| 10.34 | Amendment to Consolidated and Restated Amendment to Distributorship Agreement, dated May 3, 2011, between Patterson Companies. Inc. and Sirona Dental Systems GMBH (incorporated by reference to Exhibit 10.1 to Form 10-Q, filed on May 6, 2011)** |
| 65 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 10.35 | Term and Revolving Facilities Agreement between, among others, Sirona Dental Systems, Inc., Schick Technologies, Inc., Sirona Dental Systems, LLC, Sirona Dental Services GmbH, Sirona Dental Systems GmbH, Sirona Immobilien GmbH, Sirona Technologie GmbH & Co. KG, JPMorgan Limited, UniCredit Bank AG and J.P. Morgan Europe Limited, dated November 14, 2011. (incorporated by reference to Exhibit 10.35 to Form 8-K, filed on November 18, 2011) |
| 10.36 | Senior Facilities Agreement, dated November 4, 2011, by and among Sirona Dental Systems, Inc., J.P. Morgan Limited, Unicredit Bank AG and J.P. Morgan Europe Limited. (incorporated by reference to Exhibit 10.1 to Form 8-K, filed on November 18, 2011) |
| 10.37 | Amended and Restated U.S. Distributorship Agreement, dated May 31, 2012, by and between Patterson Companies, Inc. and Sirona Dental Systems, Inc. (incorporated by reference to Exhibit 10.1 to Form 8-K/A, filed on July 12, 2012) |
| 10.38 | Amended and Restated U.S. CAD-CAM Distributorship Agreement, dated May 31, 2012, by and between Patterson Companies, Inc. and Sirona Dental Systems GmbH. (incorporated by reference to Exhibit 10.2 to Form 8-K/A, filed on July 12, 2012) |
| 10.39 | Letter Amendment to Amended and Restated Employment Agreement, dated as of October 1, 2012, between Sirona Dental Systems, Inc. and Jeffrey T. Slovin (incorporated by reference to Exhibit 10.1 to Form 8-K, filed on October 5, 2012)† |
| 10.40 | Transition Agreement by and between Sirona Dental GmbH, Sirona Dental Systems, Inc. and Jost Fischer, dated November 16, 2012 (incorporated by reference to Exhibit 10.39 to Form 10-K, filed on November 16, 2012)† |
| 10.41 | Employment Contract between Sirona Dental Services GmbH and Rainer Berthan, dated February 20, 2012 (incorporated by reference to Exhibit 10.40 to Form 10-K, filed on November 16, 2012)† |
| 10.42 | Amendment to Amended and Restated Employment Agreement, dated as of May 7, 2013, between Sirona Dental Systems, Inc. and Jeffrey T. Slovin (incorporated by reference to Exhibit 10.1 to Form 10-Q, filed on May 10, 2013)† |
| 10.43 | Executive Employment Agreement, dated July 29, 2013, by and between Sirona Dental Systems, Inc. and Ulrich Michel (incorporated by reference to Exhibit 10.2 to Form 10-Q, filed on August 2, 2013)† |
| 10.44 | Separation Agreement, dated August 1, 2013, by and between Sirona Dental GmbH, Sirona Dental Systems, Inc. and Simone Blank (incorporated by reference to Exhibit 10.1 to Form 10-Q, filed on August 2, 2013)† |
| 10.45 | Sirona Dental Systems, Inc. 2015 Long-Term Incentive Plan (incorporated by reference to the Company’s definitive proxy statement on Schedule 14A, filed on January 28, 2015)† |
| 10.46 | Letter Amendment to Amended and Restated Employment Agreement of Jeffrey T. Slovin, dated September 15, 2015, between Sirona Dental Systems, Inc. and Jeffrey T. Slovin (incorporated by reference to Exhibit 10.1 to Form 8-K, filed on September 16, 2015)† |
| 14.1 | Code of Ethics (incorporated by reference to Exhibit 14.1 to the Company’s Annual Report on Form 10-K, filed on June 25, 2004) |
| 16.1 | Letter from Grant Thornton LLP to the Securities and Exchange Commission confirming statements made about it by Company in connection with changes to the Company's certifying accountant (incorporated by reference to Exhibit 16.1 to Form 8-K, filed on June 26, 2006) |
| 21.1 | List of Subsidiaries of Company* |
| 23.1 | Consent of Independent Registered Public Accounting Firm* |
| 31.1 | Certification of Principal Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* |
| 31.2 | Certification of Principal Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002* |
| 32.1 | Section 1350 Certification of Chief Executive Officer* |
| 66 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 32.2 | Section 1350 Certification of Chief Financial Officer* |
| 101.INS | XBRL Instance Document*** |
| 101.SCH | XBRL Taxonomy Extension Schema Document*** |
| 101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document*** |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document*** |
| 101.LAB | XBRL Taxonomy Labels Linkbase Document*** |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document*** |
| † | Compensatory plan or arrangement |
| * | Filed herewith |
| ** | Certain information in this exhibit has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request under Rule 24b-2 promulgated under the Securities Exchange Act of 1934, as amended. |
| *** | Attached as Exhibit 101 to this report are the following documents formatted in XBRL (eXtensible Business Reporting Language): (i) Consolidated Statements of Income for the years ended September 30, 2015, 2014 and 2013, (ii) Consolidated Statements of Comprehensive Income for the years ended September 30, 2015, 2014 and 2013, (iii) Consolidated Balance Sheets as of September 30, 2015 and 2014, (iv) Consolidated Statements of Shareholders' Equity for the years ended September 30, 2015, 2014 and 2013, (v) Consolidated Statements of Cash Flows for the years ended September 30, 2015, 2014 and 2013, and (vi) Notes to Consolidated Condensed Financial Statements. |
| 67 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the registrant has duly caused this Annual Report on Form 10-K to be signed on its behalf by the undersigned, thereunto duly authorized.
| Dated: November 20, 2015 | SIRONA DENTAL SYSTEMS, INC. |
| By: | /s/ JEFFREY T. SLOVIN |
| Jeffrey T. Slovin | |
| President, Chief Executive Officer, and Director |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| SIGNATURE | TITLE | DATE | |
| /s/ JEFFREY T. SLOVIN | President, Chief Executive Officer, and Director | November 20, 2015 | |
| Jeffrey T. Slovin | (Principal Executive Officer) | ||
| /s/ ULRICH MICHEL | Executive Vice President and Chief Financial Officer | November 20, 2015 | |
| Ulrich Michel | (Principal Financial Officer) | ||
| /s/ STEPHAN MITSDOERFFER | Chief Accounting Officer | November 20, 2015 | |
| Stephan Mitsdoerffer | (Principal Accounting Officer) | ||
| /s/ THOMAS JETTER | Chairman of the Board and Director | November 20, 2015 | |
| Thomas Jetter | |||
| /s/ DAVID BEECKEN | Director | November 20, 2015 | |
| David Beecken | |||
| /s/ WILLIAM K. HOOD | Director | November 20, 2015 | |
| William K. Hood | |||
| /s/ ARTHUR D. KOWALOFF | Director | November 20, 2015 | |
| Arthur D. Kowaloff | |||
| /s/ HARRY M. JANSEN KRAEMER, JR. | Director | November 20, 2015 | |
| Harry M. Jansen Kraemer, Jr. | |||
| /s/ TIMOTHY P. SULLIVAN | Director | November 20, 2015 | |
| Timothy P. Sullivan | |||
| 68 |
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
FINANCIAL INFORMATION
TABLE OF CONTENTS
| 69 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors
Sirona Dental Systems, Inc.:
We have audited the accompanying consolidated balance sheets of Sirona Dental Systems, Inc. and subsidiaries as of September 30, 2015 and 2014, and the related consolidated statements of income, comprehensive income, shareholders’ equity, and cash flows for each of the years in the three-year period ended September 30, 2015. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Sirona Dental Systems, Inc. and subsidiaries as of September 30, 2015 and 2014, and the results of their operations and their cash flows for each of the years in the three-year period ended September 30, 2015 in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Sirona Dental Systems, Inc.’s internal control over financial reporting as of September 30, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO), and our report dated November 20, 2015 expressed an unqualified opinion on the effectiveness of Sirona Dental Systems, Inc.’s internal control over financial reporting.
/s/ KPMG AG Wirtschaftsprüfungsgesellschaft
Frankfurt, Germany
November 20, 2015
| 70 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors
Sirona Dental Systems, Inc.:
We have audited Sirona Dental Systems, Inc.’s internal control over financial reporting as of September 30, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). Sirona Dental Systems, Inc.’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting under Item 9A. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Sirona Dental Systems, Inc. maintained, in all material respects, effective internal control over financial reporting as of September 30, 2015, based on criteria established in Internal Control—Integrated Framework (2013) issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Sirona Dental Systems, Inc. and subsidiaries as of September 30, 2015 and 2014, and the related consolidated statements of income, comprehensive income, shareholders’ equity, and cash flows for each of the years in the three-year period ended September 30, 2015, and our report dated November 20, 2015 expressed an unqualified opinion on those consolidated financial statements.
/s/ KPMG AG Wirtschaftsprüfungsgesellschaft
Frankfurt, Germany
November 20, 2015
| 71 |
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONSOLIDATED STATEMENTS OF INCOME
| Year ended September 30, | ||||||||
| (In millions, except for share and per share amounts) | Notes | 2015 | 2014 | 2013 | ||||
| REVENUE | $ | 1,161.3 | $ | 1,171.1 | $ | 1,101.5 | ||
| Cost of goods sold | (513.1) | (529.4) | (510.1) | |||||
| GROSS PROFIT | 648.2 | 641.7 | 591.4 | |||||
| Selling, general and administrative expense | (344.1) | (350.9) | (332.8) | |||||
| Research and development expense | (54.8) | (64.6) | (60.2) | |||||
| Net other operating income (loss) | 6 | 9.0 | 11.9 | 14.4 | ||||
| OPERATING INCOME | 258.3 | 238.1 | 212.8 | |||||
| Gain (loss) on foreign currency transactions | (17.9) | (0.3) | (12.4) | |||||
| Gain (loss) on derivative instruments | 7 | 1.7 | (2.5) | 0.4 | ||||
| Interest income (expense) | (4.2) | (2.9) | (3.4) | |||||
| Other income (expense) | 5.0 | (2.0) | 0.1 | |||||
| INCOME BEFORE TAXES | 242.9 | 230.4 | 197.5 | |||||
| Income tax benefit (expense) | 9 | (54.8) | (53.0) | (49.0) | ||||
| NET INCOME | 188.1 | 177.4 | 148.5 | |||||
| Net (income) loss attributable to noncontrolling interests | (1.9) | (1.7) | (1.8) | |||||
| NET INCOME ATTRIBUTABLE TO SIRONA DENTAL SYSTEMS, INC. | $ | 186.2 | $ | 175.7 | $ | 146.7 | ||
|
INCOME PER SHARE (attributable to Sirona Dental Systems, Inc. common shareholders) |
10 | |||||||
| Basic | $ | 3.35 | $ | 3.18 | $ | 2.67 | ||
| Diluted | $ | 3.30 | $ | 3.13 | $ | 2.61 | ||
| Weighted average shares - basic | 55,640,225 | 55,269,606 | 54,979,044 | |||||
| Weighted average shares - diluted | 56,377,863 | 56,203,970 | 56,213,992 | |||||
The accompanying Notes are an integral part of these financial statements.
| 72 |
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| Year ended | ||||||||
| September 30, | ||||||||
| (In millions) | Notes | 2015 | 2014 | 2013 | ||||
| NET INCOME | $ | 188.1 | $ | 177.4 | $ | 148.5 | ||
| OTHER COMPREHENSIVE INCOME (LOSS), NET OF TAX | 12 | |||||||
| Cumulative translation adjustment | (103.7) | (88.7) | 36.7 | |||||
| Derivative financial hedging instruments | 0.2 | 0.3 | 0.6 | |||||
| Unrecognized elements of pension cost | (3.0) | 6.8 | 0.9 | |||||
| TOTAL OTHER COMPREHENSIVE INCOME (LOSS) | (106.5) | (81.6) | 38.2 | |||||
| TOTAL COMPREHENSIVE INCOME (LOSS) | 81.6 | 95.8 | 186.7 | |||||
| Comprehensive (income) loss attributable to noncontrolling interests | (1.7) | (1.5) | (1.8) | |||||
| COMPREHENSIVE INCOME (LOSS) ATTRIBUTABLE TO SIRONA DENTAL SYSTEMS, INC. SHAREHOLDERS | $ | 79.9 | $ | 94.3 | $ | 184.9 | ||
The accompanying Notes are an integral part of these financial statements.
| 73 |
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONSOLIDATED BALANCE SHEETS
| September 30, | ||||||
| (In millions, except for share and par value amounts) | Notes | 2015 | 2014 | |||
| ASSETS | ||||||
| CURRENT ASSETS | ||||||
| Cash and cash equivalents | $ | 517.8 | $ | 382.8 | ||
| Restricted cash | 0.6 | 0.8 | ||||
| Accounts receivable, net of allowance for doubtful accounts of $1.4 and $2.4, respectively | 154.9 | 115.6 | ||||
| Inventories, net | 14 | 129.4 | 123.4 | |||
| Deferred tax assets | 9 | 26.0 | 29.7 | |||
| Prepaid expenses and other current assets | 33.7 | 26.8 | ||||
| Income tax receivable | 9 | 14.2 | 8.0 | |||
| TOTAL CURRENT ASSETS | 876.6 | 687.1 | ||||
| Property, plant and equipment, net of accumulated depreciation of $191.4 and $171.3, respectively | 208.3 | 221.0 | ||||
| Goodwill | 585.9 | 629.3 | ||||
| Restricted cash | 0.5 | - | ||||
| Intangible assets, net of accumulated amortizationof $495.7 and $511.3, respectively | 216.8 | 252.8 | ||||
| Other non-current assets | 3.3 | 5.3 | ||||
| Deferred tax assets | 9 | 10.9 | 15.5 | |||
| TOTAL ASSETS | $ | 1,902.3 | $ | 1,811.0 | ||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||
| CURRENT LIABILITIES | ||||||
| Trade accounts payable | $ | 65.3 | $ | 59.9 | ||
| Short-term financial liabilities | 23.1 | 1.5 | ||||
| Income taxes payable | 9 | 14.8 | 6.1 | |||
| Deferred tax liabilities | 9 | 0.7 | 2.2 | |||
| Accrued liabilities and deferred income | 191.2 | 167.6 | ||||
| TOTAL CURRENT LIABILITIES | 295.1 | 237.3 | ||||
| Long-term financial liabilities | 57.1 | 78.0 | ||||
| Deferred tax liabilities | 9 | 100.5 | 111.8 | |||
| Other non-current liabilities | 21.5 | 25.1 | ||||
| Pension related provisions | 62.4 | 71.7 | ||||
| Deferred income | 24.8 | 25.9 | ||||
| TOTAL LIABILITIES | 561.4 | 549.8 | ||||
| COMMITMENTS & CONTINGENCIES | ||||||
| SHAREHOLDERS' EQUITY | ||||||
| Preferred stock ($0.01 par value; 5,000,000 shares authorized; | 0 | 0 | ||||
| none issued and outstanding) | ||||||
| Common stock ($0.01 par value; 95,000,000 shares authorized; | 0.6 | 0.6 | ||||
|
58,367,468 shares issued; 55,895,969 shares outstanding at Sep. 30, 2015; 57,776,336 shares issued; 55,364,617 shares outstanding at Sept. 30, 2014 |
||||||
| Additional paid-in capital | 702.6 | 697.9 | ||||
| Treasury stock, at cost | (132.0) | (126.8) | ||||
|
2,471,499 shares held at cost at Sep. 30, 2015; 2,411,719 shares held at cost at Sept. 30, 2014 |
||||||
| Retained earnings | 946.1 | 759.9 | ||||
| Accumulated other comprehensive income (loss) | 12 | (179.1) | (72.8) | |||
| TOTAL SIRONA DENTAL SYSTEMS, INC. SHAREHOLDERS' EQUITY | 1,338.2 | 1,258.8 | ||||
| NONCONTROLLING INTERESTS | 2.7 | 2.4 | ||||
| TOTAL SHAREHOLDERS' EQUITY | 1,340.9 | 1,261.2 | ||||
| TOTAL LIABILITIES & SHAREHOLDERS' EQUITY | $ | 1,902.3 | $ | 1,811.0 | ||
The accompanying Notes are an integral part of these financial statements.
| 74 |
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
| September 30, | |||||||
| (In millions, except for share amounts) | Notes | 2015 | 2014 | 2013 | |||
| SIRONA DENTAL SYSTEMS, INC. SHAREHOLDERS' EQUITY | |||||||
| NUMBER OF COMMON SHARES ISSUED AND OUTSTANDING | |||||||
| Balance at beginning of period | 55,364,617 | 54,999,436 | 55,051,673 | ||||
| Issuance of common stock upon exercise of options | 591,132 | 562,721 | 615,570 | ||||
| Purchase of treasury stock | (59,780) | (197,540) | (667,807) | ||||
| Balance at end of period | 55,895,969 | 55,364,617 | 54,999,436 | ||||
| COMMON SHARE CAPITAL | $ | 0.6 | $ | 0.6 | $ | 0.6 | |
| ADDITIONAL PAID-IN CAPITAL | |||||||
| Balance at beginning of period | 697.9 | 674.2 | 650.2 | ||||
| Issuance of common stock upon exercise of options and net effect of vesting of RSUs/PSUs | 4.2 | 6.8 | 7.9 | ||||
| Stock compensation | 13.7 | 12.2 | 12.8 | ||||
| Tax effect of stock options exercised and net effect of vesting of RSUs/PSUs | (13.2) | 4.7 | 3.9 | ||||
| Purchase of shares from noncontrolling interests | - | - | (0.6) | ||||
| Balance at end of period | 702.6 | 697.9 | 674.2 | ||||
| TREASURY STOCK | |||||||
| Balance at beginning of period | (126.8) | (112.0) | (69.1) | ||||
| Purchase of treasury stock (at cost) | (5.2) | (14.8) | (42.9) | ||||
| Balance at end of period | (132.0) | (126.8) | (112.0) | ||||
| RETAINED EARNINGS | |||||||
| Balance at beginning of period | 759.9 | 584.2 | 437.5 | ||||
| Net income (loss) | 186.2 | 175.7 | 146.7 | ||||
| Balance at end of period | 946.1 | 759.9 | 584.2 | ||||
| ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS) | 12 | ||||||
| Balance at beginning of period | (72.8) | 8.6 | (29.8) | ||||
| Other comprehensive income (loss) | (106.3) | (81.4) | 38.4 | ||||
| Balance at end of period | (179.1) | (72.8) | 8.6 | ||||
| TOTAL SIRONA DENTAL SYSTEMS, INC. SHAREHOLDERS' EQUITY | $ | 1,338.2 | $ | 1,258.8 | $ | 1,155.6 | |
| EQUITY ATTRIBUTABLE TO NONCONTROLLING INTERESTS | |||||||
| Balance at beginning of period | $ | 2.4 | $ | 2.4 | $ | 3.0 | |
| Purchase of shares from noncontrolling interests | - | - | (0.8) | ||||
| Dividend distribution to noncontrolling interests | (1.3) | (1.5) | (1.4) | ||||
| Net income (loss) attributable to noncontrolling interests | 1.9 | 1.7 | 1.8 | ||||
| Cumulative translation adjustment | (0.3) | (0.2) | (0.2) | ||||
| TOTAL EQUITY ATTRIBUTABLE TO NONCONTROLLING INTERESTS | $ | 2.7 | $ | 2.4 | $ | 2.4 | |
| TOTAL SHAREHOLDERS' EQUITY | $ | 1,340.9 | $ | 1,261.2 | $ | 1,158.0 | |
The accompanying Notes are an integral part of these financial statements.
| 75 |
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year ended September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| OPERATING ACTIVITIES | ||||||
| NET INCOME | $ | 188.1 | $ | 177.4 | $ | 148.5 |
| ADJUSTMENTS TO RECONCILE NET INCOME TO NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | ||||||
| Depreciation and amortization | 69.8 | 76.3 | 75.6 | |||
| (Gain) loss on derivative instruments and foreign currency transactions | 16.2 | 2.8 | 12.0 | |||
| Deferred income taxes | 1.9 | (13.8) | (2.7) | |||
| Share-based compensation expense | 13.7 | 12.2 | 12.8 | |||
| Other adjustments | 0.5 | (1.3) | 0.6 | |||
| TOTAL ADJUSTMENTS TO RECONCILE NET INCOME TO OPERATING CASH FLOWS | 102.1 | 76.2 | 98.3 | |||
| CHANGES IN ASSETS AND LIABILITIES | ||||||
| Accounts receivable | (49.9) | 20.5 | (13.2) | |||
| Inventories | (23.8) | (24.9) | (26.8) | |||
| Trade accounts payable | 11.2 | (9.5) | 16.4 | |||
| Other current and non-current assets | (11.4) | 2.9 | (15.3) | |||
| Other current and non-current liabilities | 20.4 | 14.3 | 30.5 | |||
| Current income taxes | 2.5 | (8.5) | (6.4) | |||
| EFFECT OF CHANGES IN ASSETS AND LIABILITIES ON OPERATING CASH FLOWS | (51.0) | (5.2) | (14.8) | |||
| NET CASH PROVIDED BY (USED IN) OPERATING ACTIVITIES | 239.2 | 248.4 | 232.0 | |||
| INVESTING ACTIVITIES | ||||||
| Investment in property, plant and equipment | (52.8) | (93.7) | (70.7) | |||
| Proceeds from sale of property, plant and equipment | 0.9 | 1.1 | 0.1 | |||
| Purchase of intangible assets | (0.5) | (0.5) | (1.2) | |||
| Acquisition of business, net of cash acquired | (18.5) | - | (35.0) | |||
| Sale of business, net of cash sold | - | 11.5 | - | |||
| NET CASH PROVIDED BY (USED IN) INVESTING ACTIVITIES | $ | (70.9) | $ | (81.6) | $ | (106.8) |
The accompanying Notes are an integral part of these financial statements.
| 76 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year ended September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| FINANCING ACTIVITIES | ||||||
| Repayments of short-term and long-term debt | $ | (0.1) | $ | - | $ | (0.1) |
| Purchase of treasury stock | (5.2) | (14.8) | (42.9) | |||
| Purchase of shares from noncontrolling interest | - | - | (1.4) | |||
| Dividend distributions to noncontrolling interest | (1.3) | (1.5) | (1.4) | |||
| Common shares issued under share based compensation plans | 4.2 | 6.8 | 7.9 | |||
| Tax effect of common shares issued under share based compensation plans | (10.2) | (0.5) | (0.2) | |||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | (12.6) | (10.0) | (38.1) | |||
| CHANGE IN CASH AND CASH EQUIVALENTS | 155.7 | 156.8 | 87.1 | |||
| Effect of exchange rate change on cash and cash equivalents | (20.7) | (15.7) | 3.5 | |||
| Cash and cash equivalents at beginning of period | 382.8 | 241.7 | 151.1 | |||
| CASH AND CASH EQUIVALENTS AT END OF PERIOD | $ | 517.8 | $ | 382.8 | $ | 241.7 |
| SUPPLEMENTAL INFORMATION | ||||||
| GENERAL | ||||||
| Interest paid | $ | 3.7 | $ | 2.7 | $ | 2.8 |
| Interest capitalized | 0.3 | 0.4 | 0.2 | |||
| Income taxes paid | 40.3 | 60.3 | 65.7 | |||
| ACQUISITION OF BUSINESS | ||||||
| Current assets | $ | 3.7 | $ | - | $ | 5.2 |
| Non-current assets | 25.1 | - | 61.2 | |||
| Current liabilities | (4.8) | - | (7.8) | |||
| Non-current liabilities | (5.4) | - | (12.0) | |||
| 18.6 | - | 46.6 | ||||
| Cash paid | (18.6) | - | (36.7) | |||
| Settlement of balances | - | - | (4.5) | |||
| Fair value of liabilities incurred | $ | - | $ | - | $ | 5.4 |
The accompanying Notes are an integral part of these financial statements.
| 77 |
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 1 | GENERAL |
THE COMPANY AND ITS OPERATIONS
Sirona Dental Systems, Inc. (“Sirona,” the “Company,” “we,” “us,” and “our” refer to Sirona Dental Systems, Inc. and its consolidated subsidiaries) is the leading global manufacturer of high-quality, technologically-advanced dental equipment, and is focused on developing, manufacturing, and marketing innovative systems and solutions for dentists around the world. We offer a broad range of products across all major segments of the dental technology market including CEREC and our other CAD/CAM systems, digital intra oral and 2D and 3D panoramic imaging systems, treatment centers, and instruments. The Company acquired Schick Technologies, Inc. (“Schick”) in 2006, in a transaction accounted for as a reverse acquisition (the “Exchange”), further expanding our global presence and product offerings and strengthening our research and development capabilities. Sirona has served equipment dealers and dentists worldwide for more than 130 years. The Company’s headquarters is located in Long Island City, New York with its primary facility located in Bensheim, Germany, as well as other support, manufacturing, assembling, and sales and service facilities located around the globe.
| 2 | BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
BASIS OF PRESENTATION
The accompanying financial statements have been prepared in accordance with accounting principles generally accepted in the United States (‘‘U.S. GAAP’’). All amounts are reported in millions of U.S. Dollars ($), except per share amounts or as otherwise disclosed.
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Fiscal Year
The Company’s fiscal year is October 1 to September 30.
Principles of Consolidation
The consolidated financial statements include, after eliminating inter-company transactions and balances, the accounts of Sirona Dental Systems, Inc. and its subsidiaries.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the reported amount of revenues and expenses during the reporting periods. Actual results could differ from estimates. Some of the more significant estimates include allowances for doubtful accounts, inventory valuation reserves, purchase accounting assumptions, depreciable lives of assets, amortization periods, impairment of long-lived assets, deferred tax asset valuation allowance, discounts to customers, pension reserves, provisions and warranty reserves.
| 78 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Foreign Currency
The functional currency for foreign operations has been determined in all cases to be the local currency. Adjustments resulting from translating foreign functional-currency assets and liabilities are recorded in shareholders’ equity as a component of accumulated other comprehensive income. Gains or losses resulting from transactions in other than the functional currency are reflected in the consolidated statements of income, except for intra-group transactions of a long-term nature, which are recorded in shareholders’ equity as a component of accumulated other comprehensive income.
Comprehensive Income
In addition to net income, comprehensive income includes other charges or credits to equity other than those resulting from transactions with shareholders. Accumulated other comprehensive income relates to foreign currency translation adjustments related to the Company’s foreign subsidiaries, changes in the fair value of cash flow hedges, as well as to the pension adjustment resulting from the application of ASC 715-30, Compensation-Retirement Benefits – Defined Benefit Plans-Pension.
The Company presents all non-owner changes in shareholders’ equity in two separate but consecutive statements: Consolidated Statements of Income and Consolidated Statement of Comprehensive Income. The details for these items (category and type of change, current period movements, and related tax effects) are reported separately in Note 12 to the consolidated financial statements.
Revenue Recognition
The Company’s main revenue stream results from the delivery of dental equipment. The Company also enters into revenue arrangements that consist of multiple deliverables of its product and service offerings. Additionally, certain products, primarily in our CAD/CAM and Imaging segments, may contain embedded software that functions together with the product to deliver the product’s essential functionality.
Revenue, net of related discounts and allowances, is recognized when products or equipment have been shipped, when persuasive evidence of the arrangement exists, the price is fixed or determinable, collectability is reasonably assured, title and risk of loss has passed to customers based on the shipping terms, no significant obligations remain, and allowances for discounts, returns, and customer incentives can be reliably estimated. The Company offers discounts to its distributors if certain conditions are met. Discounts and allowances are primarily based on the volume of products purchased or targeted to be purchased by the individual customer or distributor. Discounts are deducted from revenue at the time of sale or when the discount is offered, whichever is later. The Company estimates volume discounts based on the individual customer’s historical and estimated future product purchases. Returns of products, excluding warranty related returns, are infrequent and insignificant. Amounts received from customers in advance of product shipment are classified as deferred income until the revenue can be recognized in accordance with the Company’s revenue recognition policy.
| · | Services: Service revenue is generally recognized ratably over the contract term as the specified services are performed. Amounts received from customers in advance of rendering of services are classified as deferred income until the revenue can be recognized upon rendering of those services. |
| · | Extended Warranties: The Company offers its customers an option to purchase extended warranties on certain products. The Company recognizes revenue on these extended warranty contracts ratably over the life of the contract. The costs associated with these extended warranty contracts are recognized when incurred. |
| · | Multiple-Element Arrangements (“MEAs”): Arrangements with customers may include multiple deliverables, including any combination of equipment, services, and extended warranties. The deliverables included in the Company’s MEAs are separated into more than one unit of accounting when (i) the delivered equipment has value to the customer on a stand-alone basis, and (ii) delivery of the undelivered service element(s) is probable and substantially in the control of the Company. Arrangement consideration is then allocated to each unit, delivered or undelivered, based on the relative selling price (“RSP”) of each unit of accounting based first on vendor-specific objective evidence (“VSOE”) if it exists and then based on estimated selling price (“ESP”). |
| 79 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
VSOE: In most instances, products are sold separately in stand-alone arrangements. Services are also sold separately through renewals of contracts with varying periods. The Company determines VSOE based on its pricing and discounting practices for the specific product or service when sold separately, considering geographical, customer, and other economic or marketing variables, as well as renewal rates or stand-alone prices for the service element(s).
ESP: The estimated selling price represents the price at which the Company would sell a product or service if it were sold on a stand-alone basis. When VSOE does not exist for all elements, the Company determines ESP for the arrangement element based on sales, cost and margin analysis, as well as other inputs based on its pricing practices. Adjustments for other market and Company-specific factors are made as deemed necessary in determining ESP.
After separating the elements into their specific units of accounting, total arrangement consideration is allocated to each unit of accounting according to the nature of the revenue as described above and application of the RSP method. Total recognized revenue is limited to the amount not contingent upon future transactions.
Research and Development
Amounts spent by the Company for research and development (R&D) efforts are recorded as R&D expenses when incurred. R&D costs relate primarily to internal costs for salaries, direct overhead costs and outside vendors. The Company capitalizes costs of equipment used for general R&D if it has alternative future use. The depreciation related to this capitalized equipment is included in the Company’s R&D costs. Software development costs incurred prior to the attainment of technological feasibility are considered R&D and are expensed as incurred. Once technological feasibility is established, software development costs are capitalized until the product is available for general release to customers. Amortization of these costs is included in cost of goods sold over the estimated life of the products.
Warranty Expense
The Company offers warranties on its products for periods between one and three years. Estimated future warranty obligations related to product sales are charged to operations in the period in which the related revenue is recognized. These estimates are based on historical warranty experience and other relevant information of which the Company is aware. Estimated warranty expenses are recorded as an accrued liability and selling, general and administrative expense.
| Warranty Expense | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Warranty expense | $ | 23.9 | $ | 23.5 | $ | 18.3 |
Shipping and Handling Costs
Shipping and handling costs charged to customers are included in revenues, and the associated expense is recorded in cost of sales for all periods presented.
Advertising Costs
Advertising costs are expensed as incurred and recorded within selling, general and administrative expense. During the periods under report, advertising expense was as follows:
| 80 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| Advertising Costs | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Advertising costs | $ | 40.3 | $ | 41.4 | $ | 39.3 |
Pension Benefits
The Company has defined benefit and defined contribution pension plans and an early retirement plan. Sirona recognizes changes in the funded status of its benefit plans, not yet recognized in the income statement, in other comprehensive income until they are amortized as a component of net periodic benefit cost in accordance with the provisions of ASC 715-30, Compensation-Retirement Benefits – Defined Benefit Plans-Pension.
Pension expense is recognized on an accrual basis over the employee’s approximate service periods. Defined benefit pension costs are determined by using an actuarial method, which provides for the deferral of actuarial gains and losses (within a specified corridor) that result from changes in assumptions or actual experience differing from that assumed. Costs relating to changes in the benefit plan are amortized. Disclosure of the components of periodic pension cost is also required. When purchase accounting is applied, pension liabilities are recognized for the projected benefit obligation in excess of plan assets.
For the defined contribution pension plans, the net pension cost is equal to the contributions required by the plan.
The Company also has an early retirement plan, Altersteilzeit (‘‘ATZ’’), which allows certain German employees who have been accepted into the plan to retire at 60 rather than at the legal retirement age of 67. Eligible employees are those who have attained the age of 59, have completed 12 years of service, and have been accepted to participate in the ATZ plan. Accepted employees join for a period of 2-4 years, during which they work in full active service for 50% of the agreed ATZ plan period, the remaining 50% of the plan period being the passive phase during which the employee does not work. Alternatively, the employee may work for 50% of the time for the entire agreed ATZ plan period. The alternative actually executed is decided via mutual agreement between Sirona and the employee. During the active service period, the employees receive 50% of their salary plus a bonus payment equal to 35% of their salary, and the remaining 50% of their salary, plus a bonus payment equal to 35% of their salary, is paid during the inactive service period. The Company recognizes the salary component of the ATZ plan over the period from the beginning of the ATZ period to the end of the active service period.
Income Taxes
Differences between the basis of assets and liabilities for financial statement purposes and for tax return purposes are recorded as deferred tax assets or deferred tax liabilities in the accompanying consolidated financial statements. Deferred taxes represent the tax consequences in future years of these differences at each balance sheet date, based on the enacted tax laws and statutory rates applicable to the periods in which the differences are expected to affect taxable income. The provision (benefit) for income taxes represents the tax payable for the period and the change during the period in deferred tax assets and liabilities. A valuation allowance is established when it is more likely than not that the deferred tax assets are not realizable. The effect on deferred tax assets and liabilities of a change in the tax rates is recognized in income as an adjustment to income tax expense in the period that includes the enactment date. See Note 9, “Income Taxes” for additional information.
Cash and Cash Equivalents
All highly liquid investments with an original maturity of three months or less are considered to be cash equivalents. Investments in money market funds are carried at fair value. All other cash equivalents are stated at cost, which approximates fair value.
| 81 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Restricted Cash
Restricted cash represents cash balances that are i) pledged as collateral to financial institutions that provide security for prepayments from customers and other bonds or ii) held as security deposits for leased office space.
Accounts Receivable
Accounts receivable are stated at the invoiced amount, less allowances for doubtful accounts, which approximates fair value given their short-term due dates. Collectability of accounts receivable is regularly reviewed and is based upon management’s knowledge of customers and compliance with credit terms. The allowance for doubtful accounts is adjusted based on such evaluation, with the corresponding expense included in selling, general, and administrative expense in the Consolidated Statements of Income. Accounts receivable balances are written off when management deems the balances uncollectible.
Inventory
Inventory is carried at the lower of cost or market value. Cost is determined using standard costing, which approximates the weighted average cost method. In addition to direct material and direct labor costs, certain costs related to the overhead and production expenses are included in inventory. Inventory reserves are provided for risks relating to slow moving, unmarketable, and obsolete items.
Business Acquisitions
The Company acquires businesses as well as partial interests in businesses. Acquired businesses are accounted for using the acquisition method of accounting, which requires that all assets and liabilities are recorded at their respective fair values. Any excess of the purchase price over estimated fair values of net assets is recorded as goodwill. The assumptions made in determining fair value assigned to assets and liabilities acquired as well as asset lives can materially impact the results of operations.
The Company obtains information during due diligence and through other sources to arrive at respective fair values. Examples of factors and information that the Company uses to determine the fair values include: tangible and intangible asset evaluations and appraisals; evaluations of existing contingencies and liabilities; product line integration information; and information systems compatibilities. If the initial accounting for an acquisition is incomplete by the end of the quarter in which the acquisition occurred, the Company will record a provisional estimate in the financial statements. The provisional estimate will be finalized as soon as information becomes available but no later than one year from the acquisition date.
Investments in Companies
Investments in associated companies over which the Company can exercise significant influence but not control are accounted for using the equity method. Investments in associated companies over which the Company cannot exercise significant influence or control are accounted for at cost.
Property, Plant and Equipment
Property, plant, and equipment are recorded at historical cost plus the fair value of asset retirement costs, if any and if reasonably estimable, less accumulated depreciation. Additions, improvements and major renewals, which extend the useful life of the asset, are capitalized; maintenance and repairs are expensed as incurred. When assets are retired or disposed of, the assets and related accumulated depreciation and amortization are removed from the balance sheet and the resulting gain or loss is reflected in current operating income. Development costs for software to be sold, leased, or otherwise marketed incurred after the establishment of technological feasibility are capitalized and amortized to cost of goods sold on a straight-line basis over the expected useful life of the software. Costs of software developed for internal use incurred during the development of the application are capitalized and amortized to operating expense on a straight-line basis over the expected useful life of the software. Prepayments for property, plant, and equipment are classified as property, plant, and equipment and are not depreciated until the assets are received and placed into service.
| 82 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The cost of property, plant and equipment is depreciated using the straight-line method over the following estimated useful lives of the respective assets:
|
Property, Plant, and Equipment (Useful Lives) |
Useful Life | |||
| (In years) | Minimum | Maximum | ||
| Buildings | 25 | 50 | ||
| Building improvements and leasehold improvements | 5 | 10 | ||
| Machinery and technical equipment | 3 | 10 | ||
| Software and software licenses | 3 | 5 | ||
Finite-Lived Intangible Assets
Finite-lived intangible assets are amortized according to the pattern in which the economic benefit of the asset is used up over their estimated useful lives, as shown below:
|
Finite-Lived Intangible Assets (Useful Lives) |
Useful Life | |||
| (In years) | Minimum | Maximum | ||
| Patents and licenses | 10 | 13 | ||
| Technologies and Dealer Relationships | 1 | 15 | ||
Impairment of Long-Lived and Finite-Lived Assets
Long lived assets held for use by the Company are reviewed for impairment whenever events or circumstances provide evidence that suggests the carrying amount of the asset may not be recoverable. The Company performs ongoing impairment analysis on technology-related intangible assets. Determination of whether an impairment exists is based upon a comparison of the identifiable undiscounted cash flows of the assets or groups of assets to the carrying amount of the assets or groups of assets. If impaired, the resulting charge reflects the excess of the asset’s carrying amount over its fair value.
Goodwill and Indefinite-Lived Intangible Assets
Goodwill and indefinite lived intangible assets, consisting of certain trademarks and in-process research and development (IPR&D), are not amortized, but are reviewed for potential impairment on an annual basis as of September 30, or whenever events or circumstances indicate that the carrying amount may not be recoverable. First, a qualitative assessment is performed on reporting units to determine if further quantitative impairment testing is necessary. If this qualitative assessment indicates that a possible impairment exists, a quantitative impairment test is performed. Goodwill impairment tests are based upon a comparison of the fair value of the reporting units to their respective carrying amount. If the carrying amount of the reporting unit exceeds its fair value, the goodwill impairment loss is measured as the excess of the carrying amount of goodwill over its implied fair value. If impairment is identified on indefinite-lived intangibles, the resulting charge reflects the excess of the asset’s carrying amount over its fair value.
| 83 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Other non-current assets
Other non-current assets and prepaid expenses include capitalized debt issuance costs. The costs are amortized using the effective interest method. The non-current unamortized balance of such debt issuance costs was $0.1 million as of September 30, 2015 (prior year period: $0.6 million).
Derivative Financial Instruments
The Company enters into forward foreign currency contracts in order to manage currency risks arising from its forecasted and firmly committed foreign currency denominated cash flows. The Company enters into these contracts to limit the foreign exchange rate risk for periods generally not to exceed six months. The Company also enters into interest rate swaps to manage its interest rates on its long term debt.
The Company does not utilize financial instruments for speculative purposes. The Company accounts for derivative financial instruments in accordance with ASC 815, Derivatives and Hedging. This Topic prescribes requirements for designation and documentation of hedging relationships and ongoing assessments of effectiveness in order to qualify for hedge accounting. The Company has designated its interest rate swaps as qualifying hedge instruments and therefore applies hedge accounting. The Company has not designated any of its foreign currency derivatives as qualifying for hedge accounting under ASC 815. All derivative instruments are recognized as either assets or liabilities in the consolidated balance sheet at fair value. The fair value of the forward foreign currency contracts and interest rate swaps are included within prepaid and other current assets or current accrued liabilities, depending on whether they are an asset or a liability. The change in fair value is recognized within ‘‘Gains (losses) on derivative instruments’’ in the consolidated statement of income for the forward foreign currency contracts and the ineffective portion of the interest rate swaps. The effective portion of interest rate swaps is recognized within “Accumulated other comprehensive income/(loss)” in the consolidated balance sheet.
Fair Value of Financial Instruments
Financial instruments consist of cash, cash equivalents, accounts receivable, accounts payable, bank loans, foreign currency forward contracts, interest rate swaps, and certain liabilities related to fixed and intangible asset purchases and liabilities for business acquisitions primarily from earn-out features. The carrying values of cash, cash equivalents, accounts receivable, and accounts payable approximate their respective fair values because of the short-term nature of these items. The fair value of the foreign currency forward contracts and interest rate swaps are estimated based on information such as quotes from financial institutions. The fair values of the acquisition-related liabilities are based on discounted valuations of commercial assumptions made by Company management of stipulations governed in the underlying purchase agreements.
| 84 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 3 | ACCOUNTING STANDARDS ISSUED |
Changes to accounting principles generally accepted in the United States (“U.S. GAAP”) are established by the Financial Accounting Standards Board (“FASB”) in the form of Accounting Standards Updates (“ASUs”). The Company considers the applicability and impact of all ASUs. ASUs not listed below were assessed and determined to be either (i) not applicable, (ii) are expected to have a minimal impact on our consolidated financial statements, or (iii) their adoption had an immaterial impact on our consolidated financial statements.
Adopted
There were no ASUs adopted during the current period, which had a material impact on our consolidated financial statements.
Not Yet Adopted
In May 2015, the FASB issued ASU 2015-05, Customer’s Accounting for Fees Paid in a Cloud Computing Arrangement, which provides guidance to customers about whether a cloud computing arrangement includes a software license. If such an arrangement contains a software license, the software license element of that arrangement should be accounted for consistent with the acquisition of other software licenses. If such an arrangement does not include a software license, the arrangement should be accounted for as a service contract. ASU 2015-05 is effective for public entities for fiscal years, and interim periods within those years, beginning after December 15, 2015, which corresponds to the Company’s fiscal year beginning October 1, 2016, with early adoption permitted. The new guidance permits either prospective or retrospective adoption. We are currently evaluating the potential impact of adoption on our consolidated financial statements and have not yet selected an adoption method.
In April 2015, the FASB issued ASU 2015-03, Simplifying the Presentation of Debt Issuance Costs, which requires an entity to present such costs related to a recognized debt liability as a direct deduction from the carrying amount of that debt liability on the balance sheet rather than as an asset. ASU 2015-03 is effective for public entities for fiscal years, and interim periods within those years, beginning after December 15, 2015, which corresponds to the Company’s fiscal year beginning October 1, 2016, with early adoption permitted. Currently, we do not expect a material impact of adoption on our consolidated financial statements.
In May 2014, the FASB issued ASU 2014-09, Revenue from Contracts with Customers, which provides updated guidance on revenue recognition principles that will supersede most current conceptual and industry-specific revenue recognition guidance and thus enhance comparability of revenue recognition practices across entities, industries, jurisdictions, and capital markets. The key principle of this updated guidance is that entities should recognize revenue to depict the transfer of goods or services to customers at an amount reflecting the consideration expected in exchange for those goods or services. The new guidance prescribes a five-step analysis of transactions to determine how and when to recognize revenue. In addition, the new guidance provides for capitalization of certain costs of obtaining or fulfilling a contract with a customer as well as enhanced disclosure requirements to enable a better understanding of the nature, amount, timing, and uncertainty of revenue and cash flows arising from contracts with customers. ASU 2014-09 is effective for public entities for fiscal years, and interim periods within those years, beginning after December 15, 2016. The new guidance permits the use of either a retrospective or cumulative effect transition method. In July 2015, the FASB approved a one-year deferral of the effective date to apply to fiscal years, and interim periods within those years, beginning after December 15, 2017, which corresponds to the Company’s fiscal year beginning October 1, 2018, with early adoption as of the original effective date permitted. We have not yet selected a transition method or adoption date and are evaluating the expected impact of adoption on our consolidated financial statements.
| 85 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 4 | BUSINESS COMBINATIONS |
ACQUISITIONS
On June 30, 2015, the Company acquired 100% of the outstanding shares of capital stock of a dental marketing services and software company. The results of its operations have been included in the consolidated financial statements since this date. The fair value of total consideration transferred for this acquisition totaled $18.6 million, consisting entirely of cash. The purchase price allocation resulted in goodwill acquired of $12.9 million. The results of operations for this acquisition included in the current period under report was as follows: revenues of $11.3 million and $0.2 million of net income.
MERGERS
On September 15, 2015, the Company and DENTSPLY International, Inc. (“DENTSPLY”) announced that the Board of Directors of both companies had unanimously approved a definitive Agreement and Plan of Merger (the “Merger Agreement”) under which the companies will combine in an all-stock merger. DENTSPLY is a leading manufacturer and distributor of dental and other consumable medical device products. The Merger Agreement provides that, upon the terms and subject to the conditions set forth in the Merger Agreement, Sirona will merge with and into a wholly-owned subsidiary of DENTSPLY, with Sirona surviving as a wholly-owned subsidiary of DENTSPLY. Upon completion of the merger, the combined company's name will be changed to DENTSPLY SIRONA Inc. Subject to the terms and conditions of the Merger Agreement, if the merger is completed, each outstanding share of Sirona common stock will be converted into the right to receive 1.8142 shares of common stock of DENTSPLY, with cash paid in lieu of any fractional shares of common stock of DENTSPLY that a Sirona stockholder would otherwise have been entitled to receive.
The Merger Agreement contains certain termination rights for both the Company and DENTSPLY, including if the merger is not consummated on or before March 15, 2016 (which is subject to extension under certain circumstances but generally not beyond December 15, 2016) and if the approval of the stockholders of either the Company or DENTSPLY is not obtained. The Merger Agreement further provides that, upon termination of the Merger Agreement under specified circumstances, including termination of the Merger Agreement by the Company or DENTSPLY as a result of an adverse change in the recommendation of the other party’s board of directors, (i) the Company may be required to pay a termination fee of $205.0 million to DENTSPLY and DENTSPLY may be required to pay a termination fee of $280.0 million to the Company and (ii) either company may be required to reimburse the other company for merger-related expenses of up to $15.0 million.
The transaction, which is expected to be completed in the second quarter of Fiscal 2016, is subject to the receipt of regulatory approvals and other customary closing conditions, including the approval of stockholders of both Sirona and DENTSPLY. For additional information related to the merger refer to DENTSPLY’S Registration Statement on Form S-4 which was filed with the SEC on October 29, 2015.
| 5 | EMPLOYEE SHARE-BASED COMPENSATION |
ASC 718, Compensation – Stock Compensation, requires that all share based compensation arrangements, including grants of stock option awards to employees, be recognized based on the estimated fair value of the share-based payment award.
EQUITY INCENTIVE PLAN
Stock options, restricted stock shares, restricted stock units (“RSU”), and performance-based stock units (“PSU”) have been issued to employees, directors, and consultants under the Company’s 2006 Equity Incentive Plan (“2006 Plan”). The 2006 Plan provided for granting in total up to 4,550,000 stock options, incentive stock, restricted stock shares, RSU’s, and PSU’s. The 2006 Plan received stockholder approval at the Company’s Annual Meeting of Stockholders held on February 27, 2007, and was amended on February 25, 2009. At the Company’s Annual Meeting of Stockholders held on February 25, 2015, stockholders approved the Company’s new 2015 Long-Term Incentive Plan (“2015 Plan”) to replace the 2006 Plan. The 2015 Plan provides for granting in total up to 6,825,000 stock options, incentive shares, restricted stock shares, RSU’s, PSU’s, and other forms of equity-based awards to employees, directors, consultants, and advisers. Shares under the new 2015 Plan were first registered pursuant to a registration statement dated April 2, 2015, at which time these shares became available for grant and any remaining shares available under the 2006 Plan were nullified. To cover the exercise of options and vesting of RSU’s and PSU’s, the Company generally issues new shares from its authorized but unissued share pool. As of September 30, 2015, 6,804,157 shares were available for future grant under the 2015 Plan.
| 86 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Restricted and Performance-Based Stock Units
In the fiscal year ended September 30, 2015, the Company granted 139,387 RSU’s and 37,004 PSU’s with an average value of $87.29, representing the average of closing prices of the Company’s common stock at the grant dates.
RSU’s generally vest in annual tranches over a period of four to five years, while PSU’s generally vest after three years. The PSU’s were granted to certain Company executives and vest three years from the date of the grant provided the Company achieves performance targets specified in the grant. RSU’s and PSU’s do not have voting rights or rights to dividends prior to vesting. The value of each RSU and PSU grant is determined by the closing price at the date of grant. Share-based compensation expense for the entire award is recognized straight-line over the service period of the last separately vesting tranche of the award.
Stock Options
In the fiscal year ended September 30, 2015, the Company granted 160,262 stock options with a weighted average exercise price of $86.00 and weighted average fair value of $22.90 at the grant date. Grants generally vest in annual tranches over a period of four to five years. All grants expire ten years after the date of the grant.
The fair value of options granted under the 2006 Plan were estimated using the Black-Scholes option pricing model using assumptions in the following table. The exercise price is equal to the fair market value of Sirona’s stock at the grant date. Expected volatility is based on the Company’s historical stock price volatility. The risk-free rate is based on the U.S. Treasury yield curve in effect at the day of grant and has a term equal to the expected life of the option. The expected life represents the period of time the options are expected to be outstanding based on anticipated grantee behavior. The expected dividend yield is based on the Company’s history of not paying regular dividends in the past and the Company’s current intention not to pay dividends in the foreseeable future.
| Black-Scholes Assumptions |
Year ended | |||
| September 30, | ||||
| 2015 | 2014 | |||
| Expected Volatility | 26.77% | 33.27% | ||
| Risk-free rate | 1.58% | 1.35% | ||
| Expected term (in years) | 5 | 5 | ||
| Expected dividends | - | - | ||
| 87 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Compensation Costs
The following tables summarize compensation expense charged to income for stock-based compensation and additional information for the fiscal years ended September 30, 2015, 2014, and 2013, respectively:
| Compensation Expense | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Compensation expense (1), (2), (3) | $ | 13.7 | $ | 12.2 | $ | 12.8 |
| (1) For the fiscal year 2015, this included a compensation charge of $0.6 for share-based awards in connection with the CFO Transition. | ||||||
| (2) For the fiscal year 2014, this included a compensation charge of $3.3 for share-based awards in connection with the CFO Transition. | ||||||
| (3) For the fiscal year 2013, this includes a compensation charge of $3.8 for the modification of share based awards in connection with the Transition Agreement for the former CEO and Chairman. | ||||||
| Additional Information | ||||||
| Year ended | ||||||
| September 30, | ||||||
| (In millions, except where noted) | 2015 | 2014 | 2013 | |||
| Tax Information | ||||||
| Income tax benefit recognized for share-based compensation | $ | (3.9) | $ | (3.4) | $ | (3.7) |
| Tax benefit realized from share-based compensation | $ | (26.3) | $ | (9.2) | $ | (9.8) |
| Future Costs | ||||||
| Total compensation cost to be recognized in future periods related to outstanding non-vested share-based compensation awards | $ | 28.5 | $ | 23.6 | $ | 19.6 |
| Weighted-average period expected for recognition of cost (in years) | 2.9 | 2.4 | 2.6 | |||
| 88 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Restricted and Performance-Based Stock Unit Activity
The following is a summary of Sirona’s RSU and PSU activity for the last three fiscal years:
| Restricted Stock Units Activity | Year ended | |||||||||||
| September 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Number of shares |
Weighted average market price at grant |
Number of shares |
Weighted average market price at grant |
Number of shares |
Weighted average market price at grant | |||||||
| Outstanding at beginning of period | 491,781 | $ | 55.81 | 570,928 | $ | 46.37 | 585,187 | $ | 38.47 | |||
| Granted | 139,387 | 87.63 | 161,835 | 68.13 | 180,036 | 63.19 | ||||||
| Vested | (189,841) | 47.30 | (205,599) | 39.93 | (157,039) | 37.37 | ||||||
| Forfeited | (6,593) | 62.83 | (35,383) | 52.17 | (37,256) | 41.49 | ||||||
| Outstanding at end of period | 434,734 | 69.62 | 491,781 | $ | 55.81 | 570,928 | $ | 46.37 | ||||
| Performance-based Stock Units Activity | Year ended | |||||||||||
| September 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Number of shares |
Weighted average market price at grant |
Number of shares |
Weighted average market price at grant |
Number of shares |
Weighted average market price at grant | |||||||
| Outstanding at beginning of period | 42,566 | $ | 66.63 | 13,000 | $ | 36.78 | 13,000 | $ | 36.78 | |||
| Granted | 37,004 | 86.00 | 43,466 | 66.63 | - | - | ||||||
| Vested | - | - | (13,000) | 36.78 | - | - | ||||||
| Forfeited | - | - | (900) | 66.64 | - | - | ||||||
| Outstanding at end of period | 79,570 | 75.64 | 42,566 | $ | 66.63 | 13,000 | $ | 36.78 | ||||
| 89 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Stock Option Activity
The following is a summary of Sirona’s stock option activity for the last three fiscal years:
| Stock Option Activity | Year ended | ||||||||||
| September 30, | |||||||||||
| 2015 | 2014 | 2013 | |||||||||
| Number of options |
Weighted average exercise price |
Number of options |
Weighted average exercise price |
Number of options |
Weighted average exercise price | ||||||
| Outstanding at beginning of period | 1,560,592 | $ | 29.13 | 1,778,102 | $ | 21.93 | 2,157,113 | $ | 17.63 | ||
| Granted | 160,262 | 86.00 | 224,565 | 67.77 | 154,500 | 63.45 | |||||
| Exercised | (751,227) | 16.28 | (406,300) | 16.75 | (512,221) | 15.49 | |||||
| Forfeited | (3,163) | 51.59 | (35,775) | 54.34 | (21,290) | 42.17 | |||||
| Outstanding at end of period | 966,464 | $ | 48.48 | 1,560,592 | $ | 29.13 | 1,778,102 | $ | 21.93 | ||
| thereof vested and exercisable | 561,756 | 1,189,950 | 1,469,883 | ||||||||
| Additional Information (In millions, except where noted) | |||||||||||
| Intrinsic value of options exercised | $ | 58.6 | $ | 22.5 | $ | 27.3 | |||||
| Total fair value of options vested | $ | 2.3 | $ | 2.4 | $ | 2.0 | |||||
| Aggregate intrinsic value of exercisable stock options | $ | 34.4 | $ | 68.0 | $ | 75.2 | |||||
| Weighted average remaining contractual life (in years) | 4.0 | 2.6 | 3.3 | ||||||||
| 90 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 6 | NET OTHER OPERATING INCOME (LOSS) |
The components of net other operating income (loss) for the periods under report are as follows:
| Net Other Operating Income (Loss) | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Income resulting from the amortization of the deferred income related to the Patterson exclusivity payment | $ | 10.0 | $ | 10.0 | $ | 10.0 |
| Gain (loss) from patent infringement settlement (1) | (0.7) | - | 4.4 | |||
| Other miscellaneous gain (loss) (2) | (0.3) | 1.9 | - | |||
| Net other operating income (loss) | $ | 9.0 | $ | 11.9 | $ | 14.4 |
| (1) The gain (loss) from patent infringement settlement for the periods under report represents net amounts received (paid) for past lost profits in out-of-court settlements of patent defense suits in the normal course of business. | ||||||
| (2) The other miscellaneous gain for the fiscal year ended September 30, 2014, represents a gain on disposal of certain business assets. | ||||||
DEFERRED INCOME
On June 30, 2005, Sirona and its largest distributor, Patterson, amended the terms of an existing distribution agreement to extend Patterson’s rights as exclusive distributor of certain Sirona products within the U.S. and Canada from October 1, 2007 through September 30, 2017. As consideration for the extension of its exclusivity rights, Patterson made a one-time payment of $100 million to Sirona in July 2005. Sirona recorded the full amount of the payment as deferred income and started amortizing the amount on a straight-line basis over ten years on October 1, 2007. Sirona accounts for the deferred income related to the Patterson payment as a monetary liability. The deferred income is amortized and recognized in net other operating income on a straight-line basis over the term of the contract ($10 million per year). The current portion of deferred income is reported within accrued liabilities and deferred income in the consolidated balance sheets. Effects of remeasurement of the amount from U.S. Dollar to Euro are reflected in the statement of income. For the periods under report, these effects were as follows:
|
Deferred Income (Patterson Exclusivity Payment) |
Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Foreign currency transaction gain (loss) resulting from the remeasurement of the deferred income related to the Patterson exclusivity payment | $ | (3.2) | $ | (2.0) | $ | 1.9 |
| 91 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 7 | DERIVATIVE INSTRUMENTS AND HEDGING STRATEGIES |
RISKS AND EXPOSURE
Our operations are exposed to market risks from changes in foreign currency exchange rates and interest rates. In the normal course of business, these risks are managed through a variety of strategies, including the use of derivatives.
Interest Rate Risk
The Company is exposed to interest rate risk associated with fluctuations in the interest rates on its variable interest rate debt. In order to manage this risk, the Company enters into interest rate swap agreements, when appropriate, based upon market conditions.
Foreign Currency Exposure
Although the U.S. Dollar is Sirona’s reporting currency, it conducts its business in many currencies, and its local functional currency varies depending on the country of operation, which exposes the Company to market risk associated with foreign currency exchange rate movements. The Company hedges certain foreign currency transaction exposure through foreign exchange forward contracts.
CASH FLOW HEDGES
Interest Rate
The Company uses interest rate swaps to convert a portion of its debt's variable interest rate to a fixed interest rate. Interest rate swaps have been established for 100% of the interest for the Facility A Term Loan under the Senior Facilities Agreement until November 2016. The interest rate swaps fix the LIBOR element of interest payable on 100% of the principal amount of the Facility A Term Loan for defined three month interest periods over the entire term of the loan. The defined interest rates fixed for each three month interest period range from 1.270% to 1.285%. Settlement of the swaps is required on a quarterly basis. These swaps are designated as hedging instruments under ASC 815. These instruments were immaterial for all periods under report. The Company enters into interest rate swap contracts infrequently as they are only used to manage interest rate risk on long-term debt instruments.
Foreign Currency
The Euro is the functional currency for many of Sirona’s subsidiaries, including its primary sales and manufacturing operations in Germany. During the periods under review, exchange rates fluctuated significantly, thereby impacting Sirona’s financial results. In order to hedge portions of the transactional exposure to fluctuations in exchange rates, based on forecasted and firmly-committed cash flows, Sirona enters into foreign exchange forward contracts (currently: USD, AUD, and JPY). These forward foreign currency contracts are intended to reduce short-term effects of changes in exchange rates. The Company enters into forward contracts that are considered to be economic hedges but which are not considered hedging instruments under ASC 815. As of September 30, 2015, these contracts had notional amounts totaling $35.3 million (prior year period: $31.1 million). These agreements are relatively short-term (generally not exceeding six months).
The fair value carrying amount of the Company's derivative instruments at September 30, 2015 is described in Note 21 Fair Value Measurements.
| 92 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The following tables summarize the impact of gains and losses from the fair value changes of the Company’s derivative instruments reported in our consolidated statements of income for the periods under report:
| Derivatives Designated as Cash Flow Hedging Instruments | ||||||
| Amount of Gain (Loss) Recognized in Accumulated Other Comprehensive Income | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Interest rate swap contracts | $ | 0.4 | $ | 0.5 | $ | 1.0 |
| Derivatives Not Designated as Hedging Instruments | |||||||
| Amount of Gain (Loss) Recognized in Income on Derivative Instruments | Year ended | ||||||
| September 30, | |||||||
| (In millions) | 2015 | 2014 | 2013 | Affected Line Item in the Statement of Income | |||
| Foreign exchange contracts | $ | 1.7 | $ | (2.5) | $ | 0.4 | Gain (loss) on derivative instruments, net |
| 8 | INTEREST INCOME (EXPENSE) |
| Interest Income (Expense) | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Interest income | $ | 0.7 | $ | 1.0 | $ | 0.6 |
| Interest expense | (4.9) | (3.9) | (4.0) | |||
| Interest income (expense) | $ | (4.2) | $ | (2.9) | $ | (3.4) |
| 9 | INCOME TAXES |
The following discussion and tables present the Company’s relevant income tax positions for the periods under report.
| 93 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
INCOME TAX (PROVISION) BENEFIT
The income tax (provision) benefit is comprised of the following:
| (Provision) Benefit for Income Tax | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Current | ||||||
| Domestic (U.S) | $ | (1.6) | $ | (8.9) | $ | (9.1) |
| Foreign | (55.2) | $ | (53.5) | $ | (55.5) | |
| Total current | (56.8) | (62.4) | (64.6) | |||
| Deferred | ||||||
| Domestic (U.S) | $ | (3.9) | $ | 5.4 | $ | 4.8 |
| Foreign | 5.9 | $ | 4.0 | $ | 10.8 | |
| Total deferred | 2.0 | 9.4 | 15.6 | |||
| (Provision) benefit for income tax | $ | (54.8) | $ | (53.0) | $ | (49.0) |
DEFERRED TAX ASSETS (LIABILITIES)
Components of Net Asset (Liability)
At September 30, 2015 the Company had total deferred tax assets of $46.1 million (prior year period $56.9 million), and total deferred tax liabilities of $110.4 million (prior year period $125.6 million).
The significant components of our net asset (liability) representing deferred income tax balances are as follows:
| Components of Net Deferred Tax Asset (Liability) | September 30, | |||
| (In millions) | 2015 | 2014 | ||
| Employee share-based compensation | $ | 6.1 | $ | 15.7 |
| Receivables | 0.7 | 0.7 | ||
| Inventory reserve | 2.4 | 2.1 | ||
| Property, plant, and equipment | (14.3) | (14.9) | ||
| Intangible assets and goodwill | (92.7) | (105.0) | ||
| Debt issuance costs | (1.7) | (1.9) | ||
| Employee benefit accruals | 11.2 | 11.1 | ||
| Deferred income | 6.2 | 5.1 | ||
| Valuation allowances | (3.8) | (3.1) | ||
| Tax loss carryforward | 8.8 | 11.0 | ||
| IC profit elimination | 9.4 | 8.1 | ||
| Other | 3.4 | 2.4 | ||
| Net deferred income tax asset (liability) | $ | (64.3) | $ | (68.7) |
In assessing the recoverability of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon sufficient taxable income within the carry-back years and the generation of future taxable income during the periods in which those temporary differences and tax loss carry-forwards become deductible. Management considers taxable income in the carry-back years, if carry back is permitted in the tax law, the projected future taxable income (including the realization of future taxable temporary differences), and tax planning strategies in making this assessment.
| 94 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Tax Loss Carry-forwards
For the periods under report, the Company had gross tax loss carry-forwards subject to expiration as follows:
| Tax Loss Carry-forwards | As of | |||
| September 30, | ||||
| (In millions) | 2015 | 2014 | ||
| Year of expiration: | ||||
| 2015 | $ | $ | 1.0 | |
| 2016 | - | - | ||
| 2017 | 1.3 | 1.4 | ||
| 2018 | 1.1 | 1.2 | ||
| 2019 | 4.2 | 4.3 | ||
| 2020 | 0.9 | - | ||
| 15 years thereafter | 4.8 | 7.7 | ||
| Subtotal | 12.3 | 15.6 | ||
| Indefinite | 16.6 | 20.1 | ||
| Total tax loss carry-forwards | $ | 28.9 | $ | 35.7 |
|
Deferred Tax Assets (Valuation Allowance Recognized(1)) |
Additions | |||||||||
| Charged to | ||||||||||
| (In millions) | Beginning balance |
Costs / expenses |
Other accounts |
Deductions | Ending balance | |||||
| For the year ended September 30, | ||||||||||
| 2015 | $ | 3.1 | $ | - | $ | 1.0 | $ | 0.3 | $ | 3.8 |
| 2014 | 3.2 | - | - | 0.1 | 3.1 | |||||
| 2013 | $ | 0.8 | $ | - | $ | 2.4 | $ | - | $ | 3.2 |
| (1) predominantly relating to tax loss carry-forwards. Management believes that it is more likely than not that the benefits of those existing tax loss carry-forwards will not be realized within the period those tax losses are deductible. | ||||||||||
| 95 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
RECONCILIATION OF (PROVISION) BENEFIT FOR INCOME TAXES
The difference between the U.S. federal income tax rate and the Company’s income tax provision included in the consolidated statements of income consisted of the following:
| Reconciliation of (Provision) Benefit for Income Taxes | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Income before income taxes | $ | 242.9 | $ | 230.4 | $ | 197.5 |
| Reconciliation of (provision) benefit for income taxes: | ||||||
| Computed tax provision | (85.1) | (80.7) | (69.8) | |||
| Foreign tax differential | 34.2 | 30.2 | 26.5 | |||
| Nondeductible expenses | 0.6 | (1.4) | (0.2) | |||
| Permanent differences relating to German trade taxes | (1.0) | (1.1) | (1.4) | |||
| Share-based compensation | (0.3) | 1.5 | 0.7 | |||
| Tax income (expense) from prior periods | (0.2) | (0.2) | 0.2 | |||
| Tax free income and tax credits | 0.6 | 0.5 | 0.6 | |||
| Additional state taxes | (0.4) | (0.8) | (1.1) | |||
| Change in tax rate (1) | - | - | (2.2) | |||
| Change in valuation allowance | (3.0) | (1.4) | (2.4) | |||
| Other | (0.2) | 0.4 | 0.1 | |||
| (Provision) benefit for income taxes (2) | $ | (54.8) | $ | (53.0) | $ | (49.0) |
| (1) The change in tax rate from prior periods of $2.2 million at September 30, 2013, predominantly relates to a non-cash remeasurement of deferred tax assets and liabilities resulting from an increase in the local trade tax rate at our principal German operations. | ||||||
| (2) The income tax provision at September 30, 2015, includes expenses of $1.0 million related to a tax audit in Germany covering fiscal years 2010 until 2013. | ||||||
In August 2007, a tax law was enacted that may limit the Company’s deductibility of interest in Germany (“Zinsschranke”). For the periods under report, the Company’s deductibility of interest was not limited as a result of this German tax law.
COMPONENTS OF INCOME BEFORE TAXES
| Components of Income before Taxes | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Germany | $ | 116.0 | $ | 133.7 | $ | 125.3 |
| United States | 31.5 | 25.1 | 23.2 | |||
| Other foreign | 95.4 | 71.6 | 49.0 | |||
| Income before income taxes | $ | 242.9 | $ | 230.4 | $ | 197.5 |
| 96 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
ADDITIONAL INFORMATION
None of the goodwill recognized in the Exchange or in the business combinations completed in any of the periods presented is tax deductible.
The company makes no provision for deferred U.S. income taxes on undistributed foreign earnings and profits because as of September 30, 2015, it remained management’s intention to continue to indefinitely reinvest these amounts in foreign operations. These earnings relate to ongoing operations and, as of September 30, 2015, the approximate amount of undistributed foreign earnings amounted to $710 million (prior year period: $552 million). Because of the availability of U.S. foreign tax credits as well as other factors, it is not practicable to determine the income tax liability that would be payable if such earnings were not reinvested indefinitely.
For the periods under report, the Company had no unrecognized tax benefits.
With limited exception, the Company and its subsidiaries are no longer subject to U.S. federal, state and local or non-U.S. income tax audits (including Germany) by taxing authorities for tax returns filed with respect to periods prior to fiscal year 2012. During the fourth quarter of fiscal year ending September 30, 2015, the German tax authority closed an income tax audit of the Company’s principal German operations for the fiscal years 2010 through 2013.
The Company classifies interest and penalties associated with income taxes as interest and other operating expense, respectively. Amounts of interest or penalties have not been material in any period.
| 10 | INCOME PER SHARE |
The computation of basic and diluted income per share is as follows:
| Income per Share | Year ended | |||||
| September 30, | ||||||
| (In millions, except share and per share amounts) | 2015 | 2014 | 2013 | |||
| Net income attributable to Sirona Dental Systems, Inc. common shareholders | $ | 186.2 | $ | 175.7 | $ | 146.7 |
| Weighted average shares outstanding - basic | 55,640,225 | 55,269,606 | 54,979,044 | |||
| Dilutive effect of stock-based compensation (1) | 737,638 | 934,364 | 1,234,948 | |||
| Weighted average shares outstanding - diluted | 56,377,863 | 56,203,970 | 56,213,992 | |||
| Net income per share | ||||||
| Basic | $ | 3.35 | $ | 3.18 | $ | 2.67 |
| Diluted | 3.30 | 3.13 | 2.61 | |||
| (1) There were no stock options excluded from the computation of diluted earnings per share for the periods under report. | ||||||
| 11 | SEGMENT REPORTING |
Sirona manages its business on both a product and geographic basis and has four reporting segments: (1) Dental CAD/CAM Systems, (2) Imaging Systems, (3) Treatment Centers, and (4) Instruments. Sirona’s products are distributed globally through a network of independent distributors or our own sales and service infrastructure to dental practices, clinics, and laboratories. The Electronic Center is a shared facility that manufactured electronic components and provides services for all Sirona segments, and to a very limited extent, external parties. The Electronic Center was outsourced beginning with fiscal year 2013. Further shared functions are operated centrally: including customer service, logistics, site management, IT, and administration.
| 97 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
DESCRIPTION OF THE COMPANY’S SEGMENTS
Dental CAD/CAM Systems
Dental CAD/CAM Systems address the worldwide market for dental restorations: inlays, onlays, veneers, crowns, bridges, copings, bridge frameworks, etc. made from ceramic, metal, or composite blocks. Dental CAD/CAM Systems products comprise in-office systems for dentists (CEREC) and laboratories (inLab, inEOS, etc.), as well as a central manufacturing service for copings and bridge-frameworks. The CEREC system allows dentists to prepare restorations in an ‘‘out-of-mouth pre-shaped’ process and insert them into the patient’s mouth during a single visit.
Imaging Systems
Imaging systems products comprise a broad range of digital systems for diagnostic imaging in the dental practice. Sirona has developed a broad range of imaging systems for 2D and 3D panoramic and intra-oral applications that allow the dentist to accommodate the patient in a more efficient manner.
Treatment Centers
Sirona’s treatment centers comprise a broad range of products from basic dentist chairs to sophisticated chair-based centers with integrated diagnostic, hygiene, and ergonomic functionalities (such as Teneo, Sinius, C8+, etc.) as well as specialist centers used in preventative treatment and for training purposes.
Instruments
Sirona offers a wide range of instruments, including handheld and power-operated handpieces for cavity preparation, endodontics, periodontology, and prophylaxis, which are regularly updated and improved. The instruments are supplemented by multi-function tips, supply and suction hoses, as well as care and hygiene systems for instrument preparation. Sirona’s instruments are often sold as complete packages in combination with treatment centers. The segment also supplies parts for other divisions, especially Treatment Centers (OEM turbines and tubes) and Dental CAD/CAM Systems.
SEGMENT RESULTS
The following tables reflect the results of the Company’s reportable segments under the Company’s management reporting system. The segment performance measure used to monitor segment performance is gross profit (‘‘Segment Performance Measure’’) excluding the impact of the acquisition of control of the Sirona business by Sirona Holdings Luxco S.C.A. (“Luxco”) – the former controlling shareholder, a Luxembourg-based holding entity owned by funds managed by Madison Dearborn Partners (“MDP”), Beecken Petty O’Keefe, and management of Sirona through a leveraged buyout transaction on June 30, 2005 (the “MDP Transaction”) – and the Exchange. This measure is considered by management to better reflect the performance of each segment as it eliminates the need to allocate centrally-incurred costs and significant purchase accounting impacts that the Company does not believe are representative of the performance of the segments. Furthermore, the Company monitors performance geographically by region. As the Company manages its business on both a product and a geographical basis, U.S. GAAP requires segmental disclosure based on product information.
| 98 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| Segment Performance | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Revenue External | ||||||
| Dental CAD/CAM Systems | $ | 434.9 | $ | 425.8 | $ | 409.3 |
| Imaging Systems | 391.5 | 399.8 | 378.0 | |||
| Treatment Centers | 216.6 | 225.3 | 210.7 | |||
| Instruments | 118.3 | 120.2 | 103.5 | |||
| Total Segments | 1,161.3 | 1,171.1 | 1,101.5 | |||
| Total | $ | 1,161.3 | $ | 1,171.1 | $ | 1,101.5 |
| Revenue Internal | ||||||
| Instruments | 12.1 | 13.2 | 12.8 | |||
| Intercompany elimination | (12.1) | (13.2) | (12.8) | |||
| Revenue Total | ||||||
| Dental CAD/CAM Systems | $ | 434.9 | $ | 425.8 | $ | 409.3 |
| Imaging Systems | 391.5 | 399.8 | 378.0 | |||
| Treatment Centers | 216.6 | 225.3 | 210.7 | |||
| Instruments | 130.4 | 133.4 | 116.3 | |||
| Total Segments | 1,173.4 | 1,184.3 | 1,114.3 | |||
| Total | $ | 1,173.4 | $ | 1,184.3 | $ | 1,114.3 |
|
Segment performance measure (gross profit) |
||||||
| Dental CAD/CAM Systems | $ | 304.5 | $ | 291.1 | $ | 276.7 |
| Imaging Systems | 224.3 | 232.6 | 221.9 | |||
| Treatment Centers | 88.6 | 91.5 | 81.4 | |||
| Instruments | 48.5 | 51.0 | 43.2 | |||
| Total Segments | 665.9 | 666.2 | 623.2 | |||
| Corporate | 7.1 | 8.8 | 6.0 | |||
| Total | $ | 673.0 | $ | 675.0 | $ | 629.2 |
| Depreciation and amortization | ||||||
| Dental CAD/CAM Systems | $ | 16.4 | $ | 15.6 | $ | 13.5 |
| Imaging Systems | 9.0 | 7.5 | 6.6 | |||
| Treatment Centers | 7.6 | 6.6 | 7.4 | |||
| Instruments | 6.0 | 6.6 | 5.0 | |||
| Total Segments | 39.0 | 36.3 | 32.5 | |||
| Corporate | 3.9 | 3.8 | 2.5 | |||
| Total | $ | 42.9 | $ | 40.1 | $ | 35.0 |
| 99 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
RECONCILIATION OF THE RESULTS OF THE SEGMENT PERFORMANCE MEASURE TO THE CONSOLIDATED STATEMENTS OF INCOME
The following table and discussion provide a reconciliation of the total results of operations of the Company’s business segments under management reporting to the consolidated financial statements. The differences shown between management reporting and U.S. GAAP for the periods under report are mainly due to the impact of purchase accounting and certain other corporate items. These effects are not included in the segment performance measure as the Company does not believe these to be representative of the performance of each segment. These adjustments relate primarily to the exclusion of amortization and depreciation related to the step-up to fair value of the intangible and tangible assets as a result of the MDP Transaction (refer to Note 16 for more information).
Inter-segment transactions are based on amounts which management believes are approximate to the amounts of transactions with unrelated third parties.
Reconciliation of Segment Performance Measure to Consolidated Statement of Income |
Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Revenue | ||||||
| Total segments (external) | $ | 1,161.3 | $ | 1,171.1 | $ | 1,101.5 |
| Consolidated revenue | $ | 1,161.3 | $ | 1,171.1 | $ | 1,101.5 |
| Depreciation and amortization | ||||||
| Total segments | $ | 39.0 | $ | 36.3 | $ | 32.5 |
| Differences management reporting vs. U.S. GAAP (including corporate items and step-up depreciation and amortization) | 30.8 | 40.0 | 43.1 | |||
| Consolidated depreciation and amortization | 69.8 | 76.3 | 75.6 | |||
| Segment performance measure (gross profit) | ||||||
| Total segments | 665.9 | 666.2 | 623.2 | |||
| Differences management reporting vs. U.S. GAAP (including step-up depreciation and amortization and certain other corporate items) | (17.7) | (24.5) | (31.8) | |||
| Consolidated gross profit | 648.2 | 641.7 | 591.4 | |||
| Selling, general and administrative expense | (344.1) | (350.9) | (332.8) | |||
| Research and development expense | (54.8) | (64.6) | (60.2) | |||
| Net other operating income (loss) | 9.0 | 11.9 | 14.4 | |||
| Gain (loss) on foreign currency transactions | (17.9) | (0.3) | (12.4) | |||
| Gain (loss) on derivative instruments | 1.7 | (2.5) | 0.4 | |||
| Interest income (expense) | (4.2) | (2.9) | (3.4) | |||
| Other income (expense) | 5.0 | (2.0) | 0.1 | |||
| Income before taxes | $ | 242.9 | $ | 230.4 | $ | 197.5 |
| 100 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONCENTRATION OF REVENUE
A substantial portion of our revenue comes from two distributors accounting for more than 10% of revenues. Patterson Companies, Inc. (“Patterson”) and Henry Schein, Inc. (“Henry Schein”) accounted for revenues and accounts receivable for the periods under report as shown in the table below. These revenues were earned across all segments, with a significant portion of revenues with Patterson being earned in the CAD/CAM and Imaging segments. No other customer accounted for more than 10% of revenues.
| Concentration of Revenue | Year ended | |||||
| September 30, | ||||||
| (In millions, except for percent amounts) | 2015 | 2014 | 2013 | |||
| Top Customers | ||||||
| Revenues | ||||||
| Patterson Dental Company, Inc. | 33% | 31% | 33% | |||
| Henry Schein, Inc. | 14% | 14% | 14% | |||
| Total of customers > 10% revenues | 47% | 45% | 47% | |||
| Accounts Receivable | ||||||
| Patterson Dental Company, Inc. | $ | 50.9 | $ | 38.9 | $ | 61.3 |
| Henry Schein, Inc. | 15.0 | 14.6 | 12.2 | |||
| Total Accounts Receivable of customers > 10% revenues | $ | 65.9 | $ | 53.5 | $ | 73.5 |
ADDITIONAL SEGMENT ALLOCATIONS
The tables that follow present additional segment allocations in accordance with U.S. GAAP.
Asset Allocations
| Segment Asset Allocation | Year ended | |||||||
| September 30, | ||||||||
| 2015 | 2014 | |||||||
| (In millions) | Goodwill | Total assets |
Goodwill | Total assets | ||||
| Dental CAD/CAM Systems | $ | 275.4 | $ | 913.1 | $ | 295.8 | $ | 869.2 |
| Imaging Systems | 175.8 | 513.6 | 188.8 | 489.0 | ||||
| Treatment Centers | 82.0 | 304.4 | 88.1 | 289.8 | ||||
| Instruments | 52.7 | 171.2 | 56.6 | 163.0 | ||||
| Total | $ | 585.9 | $ | 1,902.3 | $ | 629.3 | $ | 1,811.0 |
| 101 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Geographic Allocations
| Geographic Information | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Revenue (1) | ||||||
| Unites States | $ | 384.7 | $ | 352.4 | $ | 336.7 |
| Germany | 174.9 | 200.3 | 198.6 | |||
| Rest of world | 601.7 | 618.4 | 566.2 | |||
| Total revenue | $ | 1,161.3 | $ | 1,171.1 | $ | 1,101.5 |
| (1) Revenue is allocated to the country in which the customer is located. | ||||||
| Long-Lived Assets (2) | ||||||
| United States | $ | 26.3 | $ | 6.7 | ||
| Germany | 157.0 | 192.6 | ||||
| Rest of world | 28.2 | 27.0 | ||||
| Total long-lived assets | $ | 211.5 | $ | 226.3 | ||
| (2) Long-lived assets exclude all intangible assets and deferred tax assets. | ||||||
| 12 | ACCUMULATED OTHER COMPREHENSIVE INCOME (LOSS) |
The components and development of accumulated other comprehensive income (loss) for the periods under report are as follows:
Accumulated Other Comprehensive Income (Loss) |
Year ended | |||||||
| September 30, | ||||||||
| 2015 | ||||||||
| (In millions) | Cumulative translation adjustments |
Unrecognized elements of pension cost |
Net
gain |
Total | ||||
| Balance at beginning of period | $ | (85.6) | $ | 13.2 | $ | (0.4) | $ | (72.8) |
| Current increase (decrease) | (103.7) | (4.1) | 0.4 | (107.4) | ||||
| Income tax (expense) benefit | - | 1.1 | (0.2) | 0.9 | ||||
| Balance at end of period | (189.3) | 10.2 | (0.2) | (179.3) | ||||
| Other comprehensive income (loss) attributable to noncontrolling interests, net of tax | (0.2) | - | - | (0.2) | ||||
| Balance at end of period attributable to Sirona Dental Systems, Inc. shareholders | $ | (189.1) | $ | 10.2 | $ | (0.2) | $ | (179.1) |
| 102 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Accumulated Other Comprehensive Income (Loss) |
Year ended | |||||||
| September 30, | ||||||||
| 2014 | ||||||||
| (In millions) | Cumulative translation adjustments |
Unrecognized elements of pension cost |
Net gain hedging instruments |
Total | ||||
| Balance at beginning of period | $ | 2.9 | $ | 6.4 | $ | (0.7) | $ | 8.6 |
| Current increase (decrease) | (88.7) | 9.4 | 0.5 | (78.8) | ||||
| Income tax (expense) benefit | - | (2.6) | (0.2) | (2.8) | ||||
| Balance at end of period | (85.8) | 13.2 | (0.4) | (73.0) | ||||
| Other comprehensive income (loss) attributable to noncontrolling interests, net of tax | (0.2) | - | - | (0.2) | ||||
| Balance at end of period attributable to Sirona Dental Systems, Inc. shareholders | $ | (85.6) | $ | 13.2 | $ | (0.4) | $ | (72.8) |
| 13 | ACCOUNTS RECEIVABLE |
The allowance for doubtful accounts developed as follows:
Accounts Receivable (Allowance for Doubtful Accounts) |
Additions | |||||||||
| Charged to | ||||||||||
| (In millions) | Beginning balance |
Costs / expenses |
Other accounts |
Deductions | Ending balance | |||||
| For the year ended September 30, | ||||||||||
| 2015 | $ | 2.4 | $ | 0.2 | $ | - | $ | 1.2 | $ | 1.4 |
| 2014 | 1.6 | 1.0 | - | 0.2 | 2.4 | |||||
| 2013 | $ | 1.4 | $ | 0.8 | $ | - | $ | 0.6 | $ | 1.6 |
| 14 | INVENTORIES |
The components of net inventories for the periods under report are as follows:
| 103 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| Inventories | September 30, | |||
| (In millions) | 2015 | 2014 | ||
| Finished goods | $ | 92.9 | $ | 86.8 |
| Work in progress | 14.1 | 14.3 | ||
| Raw materials | 42.0 | 39.7 | ||
| Inventories, gross | 149.0 | 140.8 | ||
| Inventory reserve | (19.6) | (17.4) | ||
| Inventories, net | $ | 129.4 | $ | 123.4 |
| 15 | PROPERTY, PLANT AND EQUIPMENT |
Property, Plant and Equipment (Details) |
September 30, | |||||||||||
| 2015 | 2014 | |||||||||||
| (In millions) | Gross | Accum. depr. / amort. |
Net | Gross | Accum. depr. / amort. |
Net | ||||||
| Land | $ | 10.5 | $ | - | $ | 10.5 | $ | 11.9 | $ | - | $ | 11.9 |
| Buildings, building improvements, and leasehold improvements (1) | 83.6 | (21.9) | 61.7 | 79.0 | (19.5) | 59.5 | ||||||
| Machinery and technical equipment | 198.1 | (125.6) | 72.5 | 200.3 | (118.3) | 82.0 | ||||||
| Software and software licenses | 85.2 | (43.9) | 41.3 | 79.3 | (33.5) | 45.8 | ||||||
| Prepayments for property, plant, and equipment | 22.3 | - | 22.3 | 21.8 | - | 21.8 | ||||||
| Total property, plant, and equipment | $ | 399.7 | $ | (191.4) | $ | 208.3 | $ | 392.3 | $ | (171.3) | $ | 221.0 |
| (1) thereof office space leased under operating leases to third parties | $ | 1.0 | $ | 0.2 | $ | 1.2 | $ | 0.3 | ||||
| Additional Information | Year ended | |||||||||||
| September 30, | ||||||||||||
| (In millions) | 2015 | 2014 | 2013 | |||||||||
| Depreciation and amortization expense | $ | 42.9 | $ | 40.1 | $ | 35.0 | ||||||
| thereof Amortization of Capitalized Software Development Costs | $ | 11.2 | $ | 9.2 | $ | 8.8 | ||||||
| 104 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 16 | INTANGIBLE ASSETS AND GOODWILL |
COMPONENTS AND DEVELOPMENT
The components and development of intangible assets and goodwill for the periods under report are as follows:
Intangible Assets (Details) |
September 30, | |||||||||||
| 2015 | 2014 | |||||||||||
| Accum. | Accum. | |||||||||||
| (In millions) | Gross | amort. | Net | Gross | amort. | Net | ||||||
| Patents & Licenses | $ | 126.5 | $ | (102.1) | $ | 24.4 | $ | 141.9 | $ | (103.9) | $ | 38.0 |
| Trademarks | 117.7 | (1.1) | 116.6 | 123.7 | (0.9) | 122.8 | ||||||
| Technologies and dealer relationships | 442.9 | (392.5) | 50.4 | 470.0 | (406.5) | 63.5 | ||||||
| In-process research & development (IPR&D) | 25.4 | - | 25.4 | 28.5 | - | 28.5 | ||||||
| Identifiable intangible assets | 712.5 | (495.7) | 216.8 | 764.1 | (511.3) | 252.8 | ||||||
| Goodwill | 585.9 | - | 585.9 | 629.3 | - | 629.3 | ||||||
| Total intangible assets | $ | 1,298.4 | $ | (495.7) | $ | 802.7 | $ | 1,393.4 | $ | (511.3) | $ | 882.1 |
Additional Information (finite-lived identifiable intangible assets) |
Year ended | |||||||||||
| September 30, | ||||||||||||
| (In millions) | 2015 | 2014 | 2013 | |||||||||
| Amortization expense | $ | 26.9 | $ | 36.1 | $ | 40.6 | ||||||
| Annual Estimated Amortization Expense (5-Year Future) | ||||||||||||
| Year ending September 30, | ||||||||||||
| 2016 | $ | 21.1 | ||||||||||
| 2017 | 12.7 | |||||||||||
| 2018 | 9.3 | |||||||||||
| 2019 | 5.0 | |||||||||||
| 2020 | 5.0 | |||||||||||
| 105 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
RECONCILIATIONS
The following table provides information reconciling the changes in the value of intangible assets for the current fiscal year:
Intangible Assets (Reconciliations of Changes in Value and Additional Information) |
September 30, | |||||||||||||||
| 2015 | 2014 | |||||||||||||||
| (In millions, except where noted) | Goodwill | IPR&D | All other assets |
Total | Goodwill | IPR&D | All other assets |
Total | ||||||||
| Beginning balance, net | $ | 629.3 | $ | 28.5 | $ | 224.3 | $ | 882.1 | $ | 672.1 | $ | 30.6 | $ | 271.1 | $ | 973.8 |
| Amortization | - | - | (26.9) | (26.9) | - | - | (36.1) | (36.1) | ||||||||
| Purchases (disposals) in the normal course of operations | - | - | (0.2) | (0.2) | (5.5) | - | 0.5 | (5.0) | ||||||||
| Effect of business combinations (1) | 12.9 | - | 10.6 | 23.5 | - | - | - | - | ||||||||
| Foreign currency fluctuations | (55.5) | (3.1) | (18.8) | (77.4) | (36.8) | (2.1) | (10.8) | (49.7) | ||||||||
| Reclassifications within intangible assets | (0.8) | - | 2.4 | 1.6 | (0.5) | - | (0.4) | (0.9) | ||||||||
| Other | - | - | - | - | - | - | ||||||||||
| Ending balance, net | $ | 585.9 | $ | 25.4 | $ | 191.4 | $ | 802.7 | $ | 629.3 | $ | 28.5 | $ | 224.3 | $ | 882.1 |
| (1) The effect of business combinations for the year ended September 30, 2015 resulted from the acquisition of a dental company in the third quarter. | ||||||||||||||||
| The total carrying value of IPR&D as of September 30, 2015, represented a single project: | ||||||||||||||||
| Estimated completion of development phase (in %)… | 55% | |||||||||||||||
| Estimated remaining cost to complete… | $ | 11.0 | ||||||||||||||
| Estimated percentage of completion for the full project (in %)… | 40% | |||||||||||||||
| Estimated timing of project completion… | 2018 | |||||||||||||||
| The remaining steps prior to product release… | further development, prototype finalization and testing, integration and field testing, and regulatory approvals. | |||||||||||||||
| 106 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
ADDITIONAL INFORMATION
On June 30, 2005, Sirona Holdings Luxco S.C.A. (‘‘Luxco’’), a Luxembourg-based holding entity owned by funds managed by Madison Dearborn Partners, Beecken Petty O’Keefe, management and employees of Sirona, obtained control over the Sirona business. The transaction was effected by using new legal entities, Sirona Holding GmbH and its wholly owned subsidiary Sirona Dental Services GmbH, to acquire 100% of the interest in Sirona Dental Systems Beteiligungs- und Verwaltungs GmbH, the former parent of the Sirona business through a leveraged buy-out transaction (the ‘‘MDP Transaction’’). The MDP Transaction was accounted for as a leveraged buyout transaction, in a manner similar to a business combination. Certain members of Sirona management who were deemed to be in the control group held equity interests in Sirona Group prior to and subsequent to the MDP Transaction (‘‘Continuing Shareholders’’). The interests of the Continuing Shareholders have been reflected at the predecessor basis, resulting in 9.15% of each asset and liability acquired being valued at historical cost at June 30, 2005. The remaining 90.85% interest in each asset and liability was recognized at fair value at June 30, 2005, and the excess of purchase price over predecessor basis is included in additional paid-in capital in shareholders’ equity. Intangible assets and goodwill were primarily recorded in the MDP Transaction and the reverse acquisition of Schick on June 30, 2006.
| 17 | SHORT-TERM FINANCIAL LIABILITIES |
The components of short-term financial liabilities for the periods under report are as follows:
| Short-Term Financial Liabilities | September 30, | |||
| (In millions) | 2015 | 2014 | ||
| Bank loans | ||||
| Senior Term Loan ("Facility A" Term Loan, variable rate) | ||||
| Installment repayable in November 2015 | $ | 22.5 | $ | - |
| Accrued interest on long-term bank loans | 0.3 | 0.3 | ||
| Other short-term bank loans | 0.1 | 0.1 | ||
| Bank loans | 22.9 | 0.4 | ||
| Other short-term financial liabilities | 0.2 | 1.1 | ||
| Short-Term Financial Liabilities | $ | 23.1 | $ | 1.5 |
| 107 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 18 | ACCRUED LIABILITIES AND DEFERRED INCOME |
The components of accrued liabilities and deferred income for the periods under report are as follows:
| Accrued Liabilities and Deferred Income | September 30, | |||
| (In millions) | 2015 | 2014 | ||
Employee benefits (e. g. bonuses, vacation, overtime, holiday payment) |
$ | 36.1 | $ | 41.0 |
| Product warranty | 7.8 | 7.6 | ||
| Customer rebates and bonuses | 33.5 | 29.2 | ||
| Deferred income | 21.6 | 27.0 | ||
| Other provision and liabilities | 92.2 | 62.8 | ||
| Accrued liabilities and deferred income | $ | 191.2 | $ | 167.6 |
PRODUCT WARRANTY
The following table provides the components of and changes in the product warranty accrual for the periods under report:
| Product Warranty | Year ended | |||
| September 30, | ||||
| (In millions) | 2015 | 2014 | ||
| Balance at beginning of the period | $ | 7.6 | $ | 9.7 |
| Accruals for warranties issued | 24.8 | 21.9 | ||
| Warranty settlements made | (23.9) | (23.5) | ||
| Translation adjustment | (0.7) | (0.5) | ||
| Balance at end of the period | $ | 7.8 | $ | 7.6 |
| 108 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 19 | LONG-TERM FINANCIAL LIABILITIES |
The components of long-term financial liabilities for the periods under report are as follows:
| Long-Term Financial Liabilities | September 30, | |||
| (In millions, except percentages) | 2015 | 2014 | ||
| Bank loans | ||||
| Senior Term Loan ("Facility A" Term Loan, variable rate) (1) | ||||
| Installment repayable in November 2016 | $ | 52.8 | $ | 75.3 |
| Bank loans | 52.8 | 75.3 | ||
| Other long-term financial liabilities | 4.6 | 3.0 | ||
| 57.4 | 78.3 | |||
| Less current portion | (0.3) | (0.3) | ||
| Long-Term Financial Liabilities | $ | 57.1 | $ | 78.0 |
| Additional Information | ||||
| (1) Actual interest rate | 2.88% | 2.88% | ||
| (1) Average interest rate for the fiscal year ended | 2.88% | 2.88% | ||
SENIOR TERM LOANS
Senior Facilities Agreement
On November 14, 2011, the Company entered into a senior facilities agreement (the “Senior Facilities Agreement”) with Sirona Dental Systems, Inc. and all significant subsidiaries of Sirona as original borrowers and original guarantors. As of November 16, 2011, Sirona fully repaid its obligations under the Prior Senior Facilities Agreement. Initial borrowings under the Senior Facilities Agreement were used to retire the outstanding borrowings under the Company's previous credit facilities.
The Senior Facilities Agreement includes:
| (1) | a term loan in an aggregate principal amount of $75 million ("Facility A Term Loan") available to Sirona or Sirona Dental, as borrower; |
| (2) | a 120 million Euro revolving credit facility (“Revolving Facility B”) available to Sirona Dental Systems GmbH and Sirona Dental Services GmbH, as initial borrowers; and |
| (3) | a $100 million revolving credit facility (“Revolving Facility C”) available to Sirona or Sirona Dental, as initial borrowers. |
The Revolving Facility B is available for borrowing in Euro or any other freely available currency agreed to by the facility agent. The facilities are made available on an unsecured basis. Subject to certain limitations, each European guarantor guarantees the performance of each European borrower, except itself, and each U.S. guarantor guarantees the performance of each U.S. borrower, except itself. There are no cross-border guarantees.
Of the amount borrowed under the Facility A Term Loan, 30% is due on November 16, 2015, and the balance is due on November 16, 2016. The loans under the New Senior Facilities Agreement bear interest of EURIBOR, for Euro-denominated loans, and LIBOR for the other loans, plus an initial margin of 160, 85 and 110 basis points for the Facility A Term Loan, Revolving Facility B and Revolving Facility C, respectively.
The Senior Facilities Agreement contains a margin ratchet. Pursuant to this provision, which applies from March 31, 2012 onwards, the applicable margin varies depending on the Company’s leverage multiple (i.e. the ratio of consolidated total net debt to consolidated adjusted EBITDA as defined in the Senior Facilities Agreement) between 160 basis points and 215 basis points for the Facility A Term Loan, 85 basis points and 140 basis points for the Revolving Facility B, and 110 basis points and 165 basis points for the Revolving Facility C.
| 109 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The Senior Facilities Agreement contains restrictive covenants that limit Sirona's ability to make loans, to incur additional indebtedness, and to make disposals, subject to agreed-upon exceptions. The Company has agreed to certain financial debt covenants in relation to the financing. The covenants stipulate that the Company must maintain certain ratios in respect of consolidated total net debt to consolidated adjusted EBITDA. If the Company breaches these covenants, the loans will become repayable on demand.
On November 16, 2011, Sirona entered into 5-year payer interest rate swaps to fully hedge its 3-month LIBOR exposure for the Facility A Term Loan. The terms of the swap reflect the term structure of the underlying loan. The effective nominal interest rate is 1.2775% plus the applicable margin. Settlement of the swaps is required on a quarterly basis.
Debt issuance costs of $2.8 million were incurred in relation to the financing in November 2011 and have been capitalized as deferred charges and are amortized using the effective interest method over the term of the loans.
As of September 30, 2015 and September 30, 2014, the Facility A Term Loan was fully drawn to the amount of $75.0 million. The Revolving Facilities B and C remained undrawn as of September 30, 2015 and September 30, 2014.
The table below reflects the contractual maturity dates of the various borrowings as of September 30, 2015:
| Contractual Maturity Dates | As of | |||
| September 30, | ||||
| (In millions) | 2015 | 2014 | ||
| Year Ending September 30, | ||||
| 2015 | $ | - | $ | 0.3 |
| 2016 | 22.8 | 22.5 | ||
| 2017 | 52.5 | 52.5 | ||
| 2018 | - | - | ||
| 2019 | - | - | ||
| 2020 | - | - | ||
| Total | $ | 75.3 | $ | 75.3 |
| 20 | PENSION PLANS |
DEFINED BENEFIT PLANS
In Germany, the Company traditionally had an unfunded defined benefit pension plan whose benefits are based primarily on years of service and wage and salary group. As of January 1, 2001, the Company replaced its unfunded defined benefit pension plan with a new defined contribution plan. All new hires after that date only receive defined contributions to a pension plan based on a percentage of the employee’s eligible compensation. However, due to grandfathering provisions for certain existing employees hired before that date, the Company continues to be obligated to provide pension benefits which are at a minimum equal to benefits that would have been available under the terms of the traditional defined benefit plans (Grandfathered Benefit). The Grandfathered Benefit and contributions to the Company’s pension plan made for those employees after January 1, 2001 are included in the disclosures for defined benefit plans. The Company accounts for the Grandfathered Benefit by recognizing the higher of the defined contribution obligation or the defined benefit obligation for the minimum benefit. As of September 30, 2015 and 2014, contributions made through the defined contribution plan for those employees are adequate to cover the Grandfathered Benefit obligation. Therefore, the Company accounts for that portion of its pension obligation as a fully funded plan with a funded status of zero.
| 110 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The Company uses an actuarial measurement date of September 30.
| Defined Benefit Plans (Details) | September 30, | |||||
| (In millions, except where noted) | 2015 | 2014 | ||||
| Change in Funded Status of Defined Benefit Plans | ||||||
| Projected benefit obligation | ||||||
| Balance at beginning of period | $ | (85.9) | $ | (80.5) | ||
| Service cost | (0.9) | (1.1) | ||||
| Interest cost | (1.5) | (2.2) | ||||
| Actuarial gain (loss) | 1.1 | (11.0) | ||||
| Investment earnings | (0.5) | (0.5) | ||||
| Benefits paid | 2.7 | 3.1 | ||||
| Currency translation | 9.4 | 6.3 | ||||
| Balance at end of period | (75.6) | (85.9) | ||||
| Fair value of plan assets | ||||||
| Fair value at beginning of period | 14.2 | 14.5 | ||||
| Actual return on plan assets | 0.5 | 0.5 | ||||
| Employer's contribution | 0.7 | 0.9 | ||||
| Benefits paid | (0.7) | (0.7) | ||||
| Currency Translation | (1.5) | (1.0) | ||||
| Fair value at end of period | 13.2 | 14.2 | ||||
| Funded status: net asset (liability) | $ | (62.4) | $ | (71.7) | ||
| Accumulated Benefit Obligation | $ | (61.6) | $ | (70.8) | ||
| Long-Term Estimated Rate of Return on Plan Assets (1) | 3.05% | 3.56% | ||||
| (1) per annum. This rate was based on an appropriate long-term rate for the plan assets held and is applied to the extent the defined benefit obligation is recognized for the Grandfathered Benefit. | ||||||
| Defined Benefit Plans (Additional Information) | Year Ended | |||||
| September 30, | ||||||
| (In millions, except where noted) | 2015 | 2014 | 2013 | |||
| Components of Net Periodic Benefit Costs | ||||||
| Service cost, net | $ | 0.2 | $ | 0.2 | $ | 0.2 |
| Interest cost | 1.5 | 2.2 | 2.1 | |||
| Amortization of actuarial (gains) losses | 1.0 | 0.2 | 0.2 | |||
| Net periodic benefit cost | $ | 2.7 | $ | 2.6 | $ | 2.5 |
| Weighted Average Assumptions | ||||||
| Discount rate used for determining benefit obligations (current-year rate) and net periodic benefit costs (prior-year rate). | 2.15% | 2.30% | 3.40% | |||
| 111 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Plan assets consist of insurance policies with a guaranteed minimum return by the insurance company and an excess profit participation feature for a portion of the benefits. Sirona pays the premiums on the insurance policies but does not manage the investment of the funds; the insurance company makes all decisions on investment of funds, including the allocation to asset groups. The fair value of the plan assets such as equity securities, fixed-income investments, and others is based on the cash surrender values reported by the insurance company.
The benefits and contributions expected to be paid in cash in each of the following five years, and in aggregate for the fiscal years thereafter, are as follows:
Defined Benefit Pension Plans (Expected Cash Payments) |
As of | |||||||
| September 30, | ||||||||
| 2015 | 2014 | |||||||
| (In millions) | For benefits | For contributions |
For benefits | For contributions | ||||
| Year ending September 30, | ||||||||
| 2015 | $ | $ | $ | 3.6 | $ | 1.2 | ||
| 2016 | 3.3 | 1.0 | 2.9 | 1.2 | ||||
| 2017 | 2.4 | 1.0 | 2.7 | 1.2 | ||||
| 2018 | 2.7 | 1.0 | 3.0 | 1.1 | ||||
| 2019 | 2.8 | 1.0 | 3.2 | 1.1 | ||||
| 2020 | 3.0 | 1.0 | ||||||
| 5 Years thereafter | 14.3 | 7.8 | 16.3 | 9.8 | ||||
| Total expected cash payments | $ | 28.5 | $ | 12.8 | $ | 31.7 | $ | 15.6 |
DEFINED CONTRIBUTION PLANS
The Company also offers defined contribution benefits under the terms of a Section 401(k) plan in the U.S. as well as a number of other foreign plans. For the periods under report, the Company made payments to these plans as follows:
| Defined Contribution Plans | Year ended | |||
| September 30, | ||||
| (In millions) | 2015 | 2014 | ||
| Contributions made to: | ||||
| U.S. plans (1) | $ | 0.9 | $ | 0.8 |
| Foreign plans | 1.0 | 1.1 | ||
| Total contributions to defined contribution plans | $ | 1.9 | $ | 1.9 |
| (1) The Company is obligated to match employee contributions as defined in the plans. | ||||
| 112 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
| 21 | FAIR VALUE MEASUREMENTS |
The Company applies the provisions of ASC 820, Fair Value Measurements and Disclosures, for assets and liabilities that are recognized or disclosed at fair value in the financial statements on a recurring basis. ASC 820 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities that are required to be recorded or disclosed at fair value, the Company considers the principal or most advantageous market in which it would transact and the market-based risk measurements or assumptions that market participants would use in pricing the asset or liability, such as inherent risk, transfer restrictions, and the credit risk of the Company and counterparties to the arrangement.
ASC 820 establishes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three levels. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is available and significant to the fair value measurement. ASC 820 establishes and prioritizes the following three levels of inputs that may be used to measure fair value:
| Level 1 | Quoted prices in active markets for identical assets or liabilities. |
| Level 2 | Observable inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. |
| Level 3 | Inputs that are generally unobservable and typically reflect management’s estimates of assumptions that market participants would use in pricing the asset or liability. |
FAIR VALUE OF FINANCIAL INSTRUMENTS
Financial instruments consist of cash, cash equivalents, accounts receivable, accounts payable, foreign currency forward contracts, interest rate swaps, and certain liabilities related to business acquisitions primarily resulting from earn-out features. The carrying amounts of cash, cash equivalents, accounts receivable and accounts payable approximate their respective fair values because of the short maturity and nature of these items. The fair value of the foreign currency forward contracts and interest rate swaps is determined by the estimated cash flows of those contracts and swaps. The fair values of the acquisition-related liabilities are based on discounted valuations of commercial assumptions made by Company management of stipulations governed in the underlying purchase agreements.
| 113 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
ASSETS/LIABILITIES MEASURED AT FAIR VALUE ON A RECURRING BASIS
The following table presents the Company’s assets and liabilities measured at fair value on a recurring basis for the periods under report:
Assets and Liabilities Measured at Fair Value (Recurring Basis) |
September 30, | |||||||
| 2015 | ||||||||
Quoted prices in active markets for identical instruments (Level 1) |
Significant other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
Total | |||||
| (In millions) | Foreign exchange | |||||||
| Assets | ||||||||
Cash equivalents (money market funds) |
$ | 213.9 | $ | - | $ | - | $ | 213.9 |
| Derivative assets | - | 0.6 | - | 0.6 | ||||
| Liabilities | ||||||||
| Derivative liabilities | - | (0.1) | - | (0.1) | ||||
| Business combination-related liabilities | - | - | (7.1) | (7.1) | ||||
| Total | $ | 213.9 | $ | 0.5 | $ | (7.1) | $ | 207.3 |
Assets and Liabilities Measured at Fair Value (Recurring Basis) |
September 30, | |||||||
| 2014 | ||||||||
Quoted prices in active markets for identical instruments (Level 1) |
Significant other observable inputs (Level 2) |
Significant unobservable inputs (Level 3) |
Total | |||||
| (In millions) | Foreign exchange | |||||||
| Assets | ||||||||
Cash equivalents (money market funds) |
$ | 211.2 | $ | - | $ | - | $ | 211.2 |
| Liabilities | ||||||||
| Derivative liabilities | - | (1.5) | - | (1.5) | ||||
| Business combination-related liabilities | - | - | (14.2) | (14.2) | ||||
| Total | $ | 211.2 | $ | (1.5) | $ | (14.2) | $ | 195.5 |
| 114 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
The change in the fair value of the business acquisition-related liabilities was $7.1 million for the period under report, and a total for all acquisitions of $6.0 million income was recorded in other income (expense) in the income statement for the fiscal year ended September 30, 2015.
In the Company’s September 30, 2015 and 2014 Consolidated Balance Sheet, derivative assets and derivative liabilities are classified as prepaid expenses and other current assets and accrued liabilities and deferred income, respectively.
The Company did not elect the fair value option for any other eligible financial instruments.
| 22 | COMMITMENTS AND CONTINGENCIES |
OPERATING LEASE COMMITMENTS
The Company leases certain buildings (including in New York and Charlotte (USA), Salzburg (Austria), and various other locations across the globe), vehicles, and IT equipment from unrelated third parties. The leases are non-cancellable and have terms of more than one year. Lease expense for the periods under report was as follows:
| Lease Expense | Year ended | |||||
| September 30, | ||||||
| (In millions) | 2015 | 2014 | 2013 | |||
| Lease expense | $ | 16.1 | $ | 17.5 | $ | 16.4 |
Future minimum lease payments under non-cancelable operating lease agreements as of September 30, 2015 are as follows:
Future Minimum Lease Payments Under Non-cancelable Operating Leases |
As of | |||
| September 30, | ||||
| (In millions) | 2015 | 2014 | ||
| Year ending September 30, | ||||
| 2015 | $ | $ | 8.9 | |
| 2016 | 10.6 | 6.7 | ||
| 2017 | 8.3 | 3.8 | ||
| 2018 | 6.2 | 2.5 | ||
| 2019 | 3.8 | 3.3 | ||
| 2020 | 3.0 | 0.0 | ||
| thereafter | 2.6 | 2.1 | ||
| Total future minimum lease payments | $ | 34.5 | $ | 27.3 |
UNCONDITIONAL PURCHASE COMMITMENTS
As of September 30, 2015, the Company had unconditional purchase commitments of $35.2 million (prior year period $20.0 million), mainly for purchases of raw material and components, which are due over a period of from one to three years.
| 115 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
CONTINGENCIES
The Company may be involved in lawsuits, claims, investigations and proceedings, including patent and commercial matters that arise in the ordinary course of business. At September 30, 2015, there are no such matters pending that the Company expects to be material in relation to its business, consolidated financial position, results of operations or cash flows.
| 23 | SELECTED QUARTERLY INFORMATION (UNAUDITED) |
The following is a summary of the Company’s unaudited quarterly operating results for the periods under report:
Selected Quarterly Information (Unaudited) |
Quarter ended | Year ended | ||||||||
| (In millions, except for share and per share amounts) | December |
March 31, 2015 |
June 30, 2015 |
September |
September | |||||
| REVENUE | $ | 293.0 | $ | 257.3 | $ | 306.1 | $ | 304.9 | $ | 1,161.3 |
| GROSS PROFIT | 162.9 | 143.8 | 174.6 | 166.9 | 648.2 | |||||
| OPERATING INCOME | 62.8 | 50.0 | 80.4 | 65.1 | 258.3 | |||||
| INCOME BEFORE TAXES | 60.8 | 47.2 | 77.2 | 57.7 | 242.9 | |||||
| NET INCOME | 46.8 | 36.4 | 59.4 | 45.5 | 188.1 | |||||
| NET INCOME ATTRIBUTABLE TO SIRONA DENTAL SYSTEMS, INC. | $ | 46.0 | $ | 36.0 | $ | 58.8 | $ | 45.4 | $ | 186.2 |
INCOME PER SHARE (attributable to Sirona Dental Systems, Inc. common shareholders) |
||||||||||
| Basic | $ | 0.83 | $ | 0.65 | $ | 1.06 | $ | 0.81 | $ | 3.35 |
| Diluted | 0.82 | 0.64 | 1.04 | 0.80 | 3.30 | |||||
| 116 |
SIRONA DENTAL SYSTEMS, INC.
AND SUBSIDIARIES
FORM 10-K
FOR THE FISCAL YEAR ENDED SEPTEMBER 30, 2015
Selected Quarterly Information (Unaudited) |
Quarter ended | Year ended | ||||||||
December |
March 31, | June 30, | September |
September | ||||||
| (In millions, except for share and per share amounts) | 2013 | 2014 | 2014 | 2014 | 2014 | |||||
| REVENUE | $ | 298.7 | $ | 282.7 | $ | 299.7 | $ | 290.0 | $ | 1,171.1 |
| GROSS PROFIT | 162.7 | 151.5 | 167.6 | 159.9 | 641.7 | |||||
| OPERATING INCOME | 62.1 | 50.4 | 67.3 | 58.3 | 238.1 | |||||
| INCOME BEFORE TAXES | 58.8 | 49.6 | 67.7 | 54.3 | 230.4 | |||||
| NET INCOME | 45.0 | 37.9 | 51.8 | 42.7 | 177.4 | |||||
| NET INCOME ATTRIBUTABLE TO SIRONA DENTAL SYSTEMS, INC. | $ | 44.2 | $ | 37.3 | $ | 51.5 | $ | 42.7 | $ | 175.7 |
INCOME PER SHARE (attributable to Sirona Dental Systems, Inc. common shareholders) |
||||||||||
| Basic | $ | 0.80 | $ | 0.67 | $ | 0.93 | $ | 0.78 | $ | 3.18 |
| Diluted | 0.79 | 0.66 | 0.92 | 0.76 | 3.13 | |||||
| 117 |
