Attached files
| file | filename |
|---|---|
| EX-99.2 - EXHIBIT 99.2 - WINDTREE THERAPEUTICS INC /DE/ | ex99_2.htm |
| 8-K - DISCOVERY LABORATORIES, INC 8-K 11-12-2015 - WINDTREE THERAPEUTICS INC /DE/ | form8k.htm |
Exhibit 99.1

AEROSURF® Phase 2 Program UpdateInvestor Conference CallNovember 12, 2015

Forward Looking Statement To the extent that statements in this presentation are not strictly historical, including statements about the Company’s business strategy, outlook, objectives, plans, intentions, goals, future financial conditions, future collaboration agreements, the success of the Company’s product development, or otherwise as to future events, such statements are forward-looking, and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. The forward-looking statements contained in this presentation are subject to certain risks and uncertainties that could cause actual results to differ materially from the statements made. These risks are further described in the Company's periodic filings with the Securities and Exchange Commission (SEC), including the most recent reports on Forms 10-K, 8-K and 10-Q, and any amendments thereto (“Company Filings”).

Primary characteristic is surfactant deficiency in underdeveloped lungs of premature infants (born with a lack of natural lung surfactant required for open airways and proper gas exchange – O2 in and CO2 out)American Academy of Pediatrics guidelines recommend providing surfactant replacement within the first hours of life1Neonatologists believe the highest unmet need in RDS is the ability to deliver surfactant non-invasively to patients2 Respiratory Distress Syndrome (RDS) 1. AAP guidelines, 20132. Discovery Labs’ primary market research (2014)

Aerosolized Surfactant for RDS Proprietary Synthetic KL4 Surfactant Designed to be structurally similar to human lung surfactantSURFAXIN® - Liquid KL4 surfactant (intratracheal instillate) for RDS approved by the FDA Innovative Aerosol Delivery Technology Designed specifically for the capability to aerosolize and deliver surfactant Potential to transform the treatment of premature infants with RDS by making surfactant therapy available through non-invasive delivery technology. ®

Current Treatment of RDS: Intubate or Not? Surfactant replacement therapy (SRT) - requires intubation and mechanical ventilation (MV); available surfactants are animal-derivedInvasive intubation and MV can result in serious respiratory conditions and other complications, such as higher risk of infection and bronchopulmonary dysplasia (BPD) Considered less invasive but does not address underlying condition – surfactant deficiencyMany infants respond poorly and require delayed rescue SRT via intubation and MV (“nCPAP failure”) Earlier SRT produces better outcomes compared to late SRT1 Invasive Intubation Nasal continuous positive airway pressure (nCPAP) 1. AAP guidelines, 2013
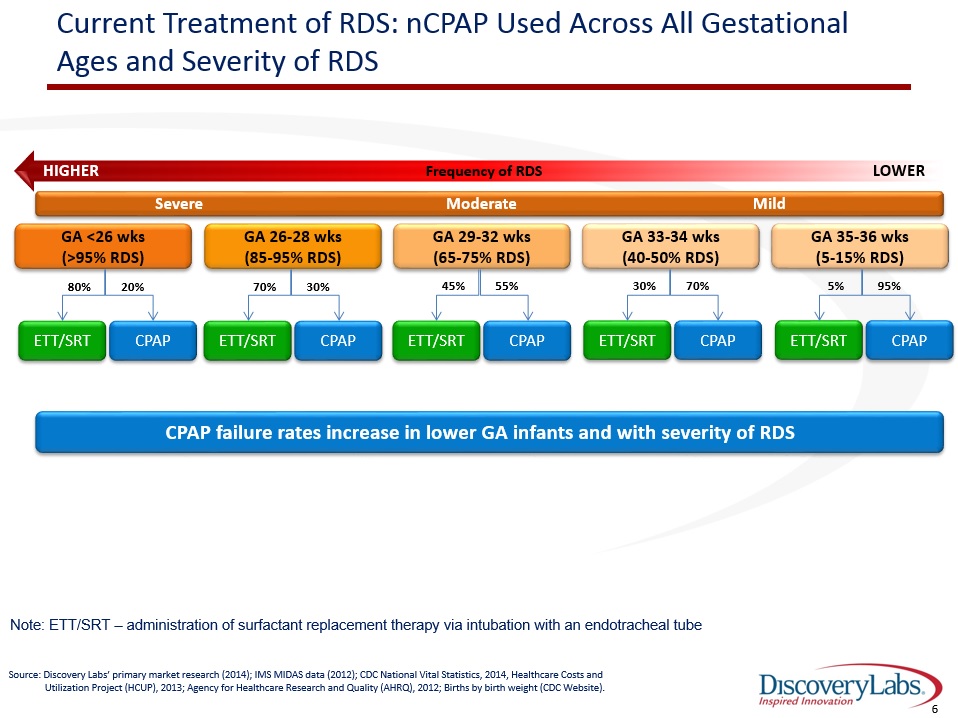
Current Treatment of RDS: nCPAP Used Across All Gestational Ages and Severity of RDS Frequency of RDS HIGHER LOWER Severe Moderate Mild GA <26 wks(>95% RDS) GA 26-28 wks(85-95% RDS) GA 29-32 wks(65-75% RDS) GA 33-34 wks(40-50% RDS) GA 35-36 wks(5-15% RDS) 20% ETT/SRT CPAP 80% 30% ETT/SRT CPAP 70% 55% ETT/SRT CPAP 45% 70% ETT/SRT CPAP 30% 95% ETT/SRT CPAP 5% Source: Discovery Labs’ primary market research (2014); IMS MIDAS data (2012); CDC National Vital Statistics, 2014, Healthcare Costs and Utilization Project (HCUP), 2013; Agency for Healthcare Research and Quality (AHRQ), 2012; Births by birth weight (CDC Website). Note: ETT/SRT – administration of surfactant replacement therapy via intubation with an endotracheal tube CPAP failure rates increase in lower GA infants and with severity of RDS

Current Treatment of RDS: Trends in Non-Invasive Care of Neonates – Increasing Use of nCPAP to Avoid Intubation However, still experience high nCPAP failure rates First-line nCPAP use has been trending up across all gestational ages up to 32 weeks GA Est. GA: < 24 wks ~24-27 wks ~28-29 wks ~30-32 wks Source: Soll, Obstetric and Neonatal Care Practices for Infants 501 to 1500 g From 2000 to 2009; Pediatrics; July 2013 Source: Soll, Obstetric and Neonatal Care Practices for Infants 501 to 1500 g From 2000 to 2009; Pediatrics; July 2013

RDS: Clinicians seeking a non-invasive way to deliver SRT What is wanted1:An approach that effectively delivers surfactant without intubation or mechanical ventilationPossibility of repeat dosesAvoids clinical instability associated with bolus administrationAdministration by non-specialist staffReduce cost of treating premature infants 1. Pillow & Minocchieri: Neonatology, 2012 “…optimization of less invasive method of surfactant administration will be one of the most important subjects for research in the field of surfactant therapy of RDS in coming years”. Kribs A. How best to administer surfactant to VLBW infants. Arch Dis Child Fetal Neonatal Ed 2011;doi:10.1136.

Potential to Transform Management of RDS Capillary Drug pumped through capillary Energy Input Aerosolized KL4 Surfactant via nCPAP Goal is to administer surfactant without invasive intubation and early in the management of RDS in premature infants ® We are conducting the AEROSURF® development program with the goal of establishing AEROSURF as the first aerosolized surfactant therapy to address RDS

Bridge the Surfactant / RDS Gap in the First 72 Hours 48 to 72 Hours Surfactant Deficient Endogenous Surfactant Production Birth ® GoalProvide surfactant therapy to premature infants until they can produce their own endogenous surfactantAllow for single or repeat non-invasive doses of aerosolized surfactant with nCPAP Initial AEROSURF dose Potential Repeat dose Additional dose, if needed Phase 2 development program is primarily to assess safety and understand the proper dosing regimen to support premature infants to surfactant self-sufficiency

Comprehensive Phase 2 Program Phase 2a Phase 2a Expansion Phase 2a Phase 2b Gestational Age (wks) 29 – 34 26 - 28 26 – 32(Begin with 29 – 32) Dose Groups 15 min; 30 min; 45 min( 25, 50, 75 TPL mg/kg)(8 active, 8 control per group)Single dose 60 min; 90 min (100 and 150 TPL mg/kg)(8 active, 8 control per group)Primarily single dose 30 min; 45 min (50 and 75 TPL mg/kg)(8 active, 8 control per group)Up to two doses 25 min; 50 min; Control(40 and 80 TPL mg/kg)Up to 3 doses # of patients 48 32 32 Up to 250 Objective(s) Safety and tolerabilityPhysiological data suggesting delivery of KL4 surfactant to the lungsPerformance of aerosol delivery system Safety and tolerability of higher doses and determine therapeutic index (safety window)Continue physiological assessment Safety and tolerabilityPhysiological assessment Provide evidence of efficacy on an acceptable endpointIdentify dose regimens for phase 3 studyProvide estimate of effect size # of sites Initiated with 3; increased to 8 (US) 12 (US) Up to 20 (US) 50+ (US, EU, Canada, LATAM) Timeline / Milestones Completed May 2015; key objectives achieved Completed Oct 2015 Initiated; results expected Q1 2016 Expect to initiate Q4 2015; target enrollment completion – mid - 2016 ®

Phase 2a Clinical Program (29 to 34 weeks GA)Top-Line Data Review ®

Phase 2a Study (29 to 34 wks GA)Study Design Evaluate the safety and tolerability of aerosolized KL4 surfactant (lucinactant 30 mg TPL/ml) for inhalation, administered to preterm neonates 29 to 34 weeks post-menstrual age (PMA) receiving nCPAP for RDS, compared to neonates receiving nCPAP alone.DesignMulticenter, randomized, controlled, dose-escalation studyPreterm neonates within the first 21 hours after birth and who have had implementation of controlled nCPAP within 1 hour of birth.8 control and 8 active per dose group (80 total: 40 active; 40 pooled control)Treatment GroupsDose Group 1 – 15 min (25 mg TPL/kg) single dose Dose Group 2 – 30 min (50 mg TPL/kg) single doseDose Group 3 – 45 min (75 mg TPL/kg) single doseDose Group 4 – 60 min (100 mg TPL/kg) single dose* Dose Group 5 – 90 min (150 mg TPL/kg) single dose** Dose Groups 4 & 5 could have repeat doses if oxygen requirement was > 0.35 at least 2 hours after dosingMethod of AdministrationReconstituted lyophilized KL4 surfactant aerosolized by the investigational device (capillary aerosol generator), and introduced into the nCPAP circuit. 13 ®

Inclusion CriteriaGestational age 29 to 34 weeks PMAImplementation of controlled nCPAP within 60 minutes after birthSpontaneous breathingChest radiograph consistent with RDSWithin the first 21 hours after birth, requires the following to maintain SpO2 of 88% - 95%nCPAP of 5 to 6 cm H2OFiO2 of 0.30 to 0.50 for at least 60 minutes FiO2 of 0.25 to 0.50 for at least 120 minutes (amendment)FiO2 of 0.25 to 0.50 for at least 30 minutes (amendment)Exclusion CriteriaHemodynamically unstableMajor congenital / chromosomal abnormalities Recurrent apnea 14 Phase 2a Study (29 to 34 wks GA)Study Design (cont’d) ®

Phase 2a Study (29 to 34 wks GA)Study Objectives Primary ObjectiveSafety and tolerability of AEROSURF® compared to nCPAP aloneOther Key ObjectivesAssess physiological data indicating that aerosolized KL4 surfactant is being delivered into the lungs of premature infantsGas exchange: FiO2 requirements and changes in CO2Need for rescue therapy and requirement for invasive respiratory supportAcceptable performance of the AEROSURF Delivery System in the NICU 15 ®
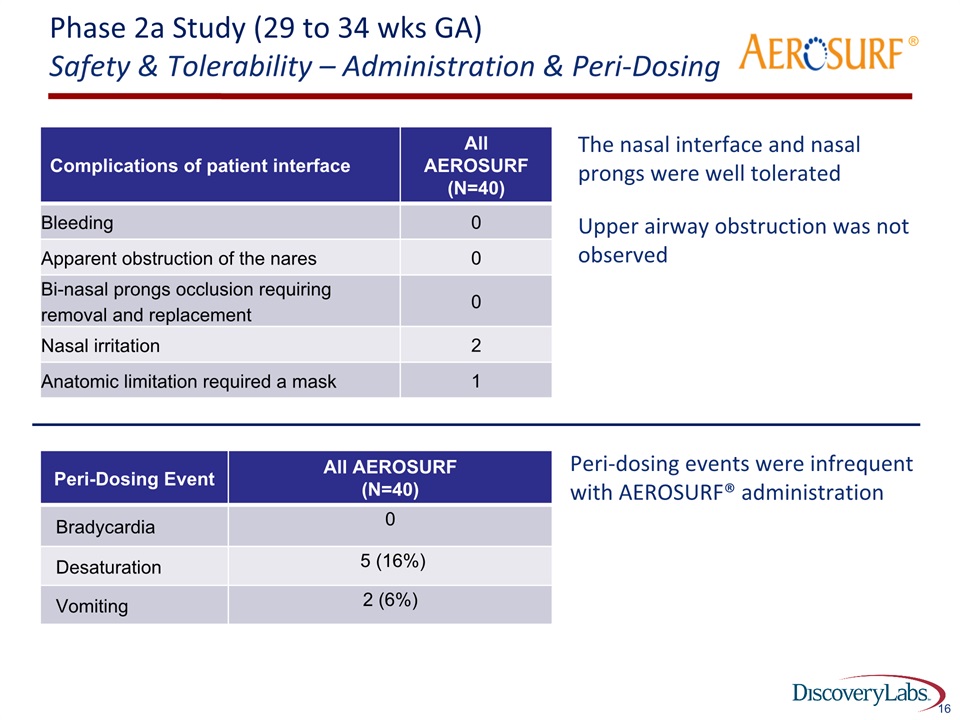
Phase 2a Study (29 to 34 wks GA)Safety & Tolerability – Administration & Peri-Dosing The nasal interface and nasal prongs were well tolerated Upper airway obstruction was not observed 16 Complications of patient interface All AEROSURF (N=40) Bleeding 0 Apparent obstruction of the nares 0 Bi-nasal prongs occlusion requiring removal and replacement 0 Nasal irritation 2 Anatomic limitation required a mask 1 ® Peri-Dosing Event All AEROSURF(N=40) Bradycardia 0 Desaturation 5 (16%) Vomiting 2 (6%) Peri-dosing events were infrequent with AEROSURF® administration

Phase 2a Study (29 to 34 wks GA)Safety & Tolerability - Adverse Events Co-morbidities and adverse events occur frequently in this patient population Adverse Event Observations:The most common adverse events in the AEROSURF® and control groups were:Neonatal jaundice ConstipationApnea AnemiaAll observed adverse events were as expected for this patient population Incidence of adverse events was generally comparable between AEROSURF and control groupsThere was no pattern of increased adverse events with increasing AEROSURF dose 17 ®
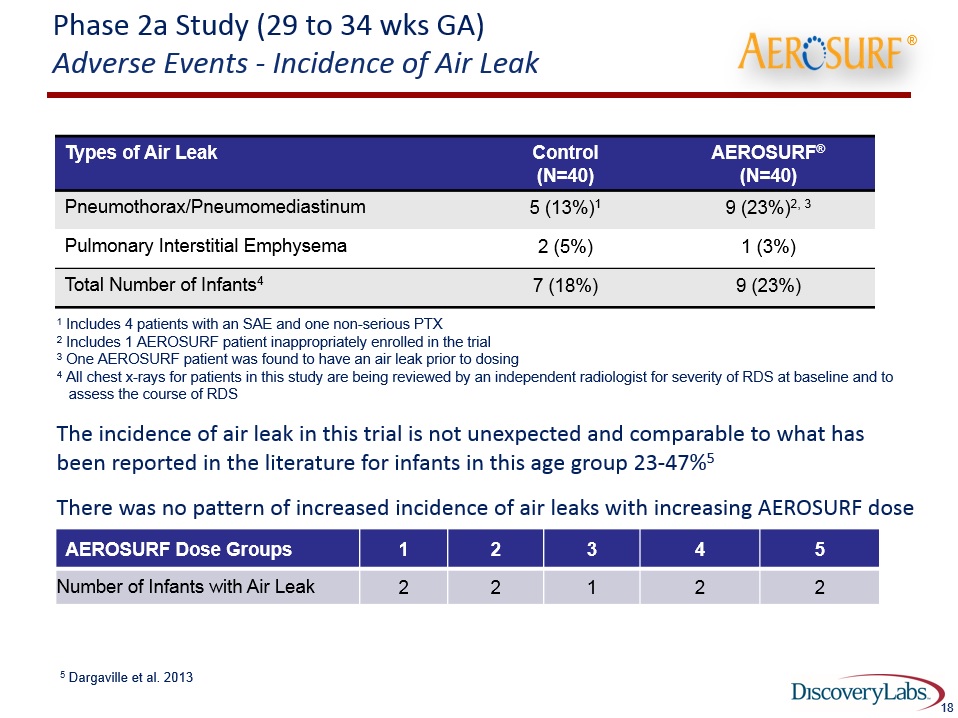
Phase 2a Study (29 to 34 wks GA) Adverse Events - Incidence of Air Leak 18 Types of Air Leak Control(N=40) AEROSURF®(N=40) Pneumothorax/Pneumomediastinum 5 (13%)1 9 (23%)2, 3 Pulmonary Interstitial Emphysema 2 (5%) 1 (3%) Total Number of Infants4 7 (18%) 9 (23%) 1 Includes 4 patients with an SAE and one non-serious PTX2 Includes 1 AEROSURF patient inappropriately enrolled in the trial3 One AEROSURF patient was found to have an air leak prior to dosing4 All chest x-rays for patients in this study are being reviewed by an independent radiologist for severity of RDS at baseline and to assess the course of RDSThe incidence of air leak in this trial is not unexpected and comparable to what has been reported in the literature for infants in this age group 23-47%5 There was no pattern of increased incidence of air leaks with increasing AEROSURF dose 5 Dargaville et al. 2013 ®

Phase 2a Study (29 to 34 wks GA)Safety & Tolerability - Serious Adverse Events (SAE) 19 SAE Control(N=40) AEROSURF®(N=40) Apnea 0 1 (3%) Oxygen saturation decreased 0 1 (3%) Necrotising enterocolitis 1 (3%) 2 (5%) Cardio-respiratory arrest 1 (3%) 1 (3%)1 Hydrocephalus 1 (3%) 0 Pneumothorax/Pneumomediastinum 4 (10%) 9 (23%)2, 3 Pulmonary Hemorrhage 1 (3%)1 0 Death 1 (3%) 1 (3%) 1 SAEs associated with mortality - both considered unrelated to study drug or trial procedures2 Includes 1 AEROSURF patient inappropriately enrolled in the trial3 One AEROSURF patient was found to have an air leak prior to dosing and the SAE ® These are SAEs that you would expect to see in this populationThere was no pattern of increased incidence of SAEs with increasing AEROSURF doseAll air leaks resolved without complication.

Phase 2a Study (29 to 34 wks GA)Safety and Tolerability - Summary The adverse events seen in this trial were expected for this patient population The adverse events and complications of prematurity were generally comparable between AEROSURF® and control groupsThere was no pattern of increased adverse events or serious adverse events with increasing AEROSURF doseThe AEROSURF Delivery System delivered KL4 surfactant to the infants in a way that was generally safe and well toleratedThe Independent Safety Review Committee supports proceeding to the next studies in our program 20 ®
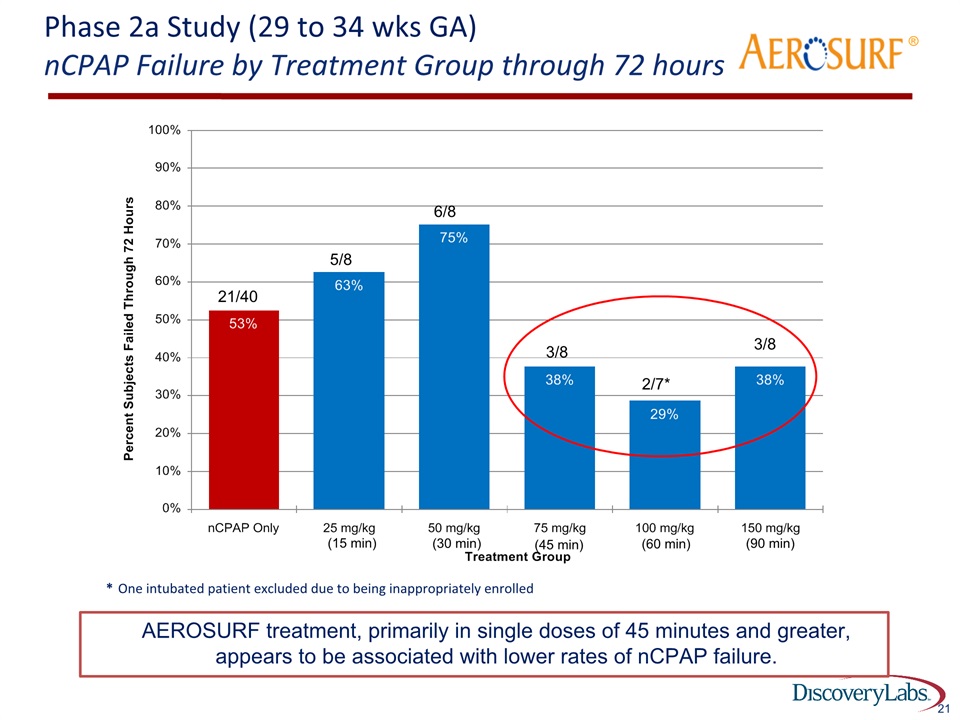
Phase 2a Study (29 to 34 wks GA)nCPAP Failure by Treatment Group through 72 hours 21 * One intubated patient excluded due to being inappropriately enrolled 2/7* 3/8 3/8 21/40 5/8 6/8 ® AEROSURF treatment, primarily in single doses of 45 minutes and greater, appears to be associated with lower rates of nCPAP failure. (15 min) (30 min) (45 min) (60 min) (90 min)

AEROSURF® Phase 2a Study (29 to 34 wks GA) Patients on Room Air at 1 Hour Post Start of Treatment1 % of Subjects on Room Air at 1 Hour After Dosing 22 1 For control patients time is 1 hour after randomization At 1 hour after start of treatment, 28% of all AEROSURF® patients were at 21% O2 (room air) compared to 8% of control patients. (n = 40) ® (n = 40)
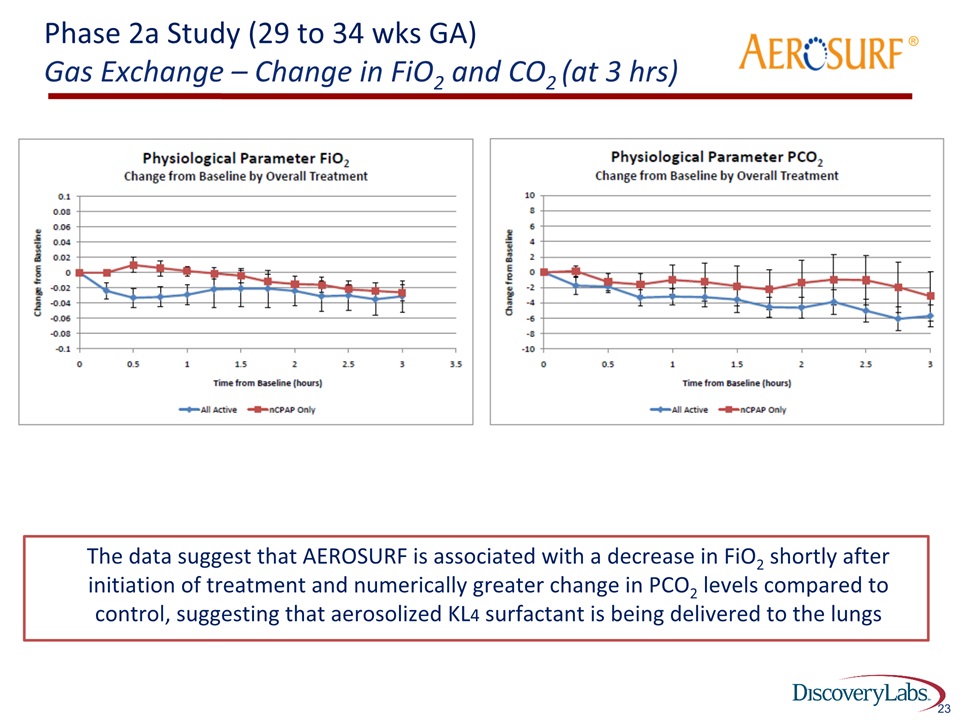
23 Phase 2a Study (29 to 34 wks GA)Gas Exchange – Change in FiO2 and CO2 (at 3 hrs) ® The data suggest that AEROSURF is associated with a decrease in FiO2 shortly after initiation of treatment and numerically greater change in PCO2 levels compared to control, suggesting that aerosolized KL4 surfactant is being delivered to the lungs

Bridge the Surfactant / RDS Gap in the First 72 Hours 48 to 72 Hours Surfactant Deficient Endogenous Surfactant Production Birth ® GoalProvide surfactant therapy to premature infants until they can produce their own endogenous surfactantAllow for single or repeat non-invasive doses of aerosolized surfactant with nCPAP Initial AEROSURF dose Potential Repeat dose Additional dose, if needed Phase 2 development program is primarily to assess safety and understand the proper dosing regimen to support premature infants to surfactant self-sufficiency

Phase 2a Study (29 to 34 wks GA) Time to Intubation for nCPAP Failure for Control Group ® 25 nCPAP failures due to RDS normally occur within 72 hours; in the control group (n=40) majority of nCPAP failures occurred within 48 hours of life

Phase 2a Study (29 to 34 wks GA) Time to Intubation for nCPAP Failure by Treatment Group ® 26 AEROSURF may prolong the time to intubation; repeat dosing may be important to extend this effect to surfactant self-sufficiency Control Dose Groups 3,4 & 5 Dose Groups 1 & 2 (15 & 30 min) (45, 60 & 90 min)

Phase 2a Study (29 to 34 wks GA)nCPAP Failure by Treatment Group through 72 hours 27 * One intubated patient excluded due to being inappropriately enrolled 2/7* 3/8 3/8 21/40 5/8 6/8 ® AEROSURF treatment, primarily in single doses of 45 minutes and greater, appears to be associated with lower rates of nCPAP failure Focus for dose selection going forward (15 min) (30 min) (45 min) (60 min) (90 min)
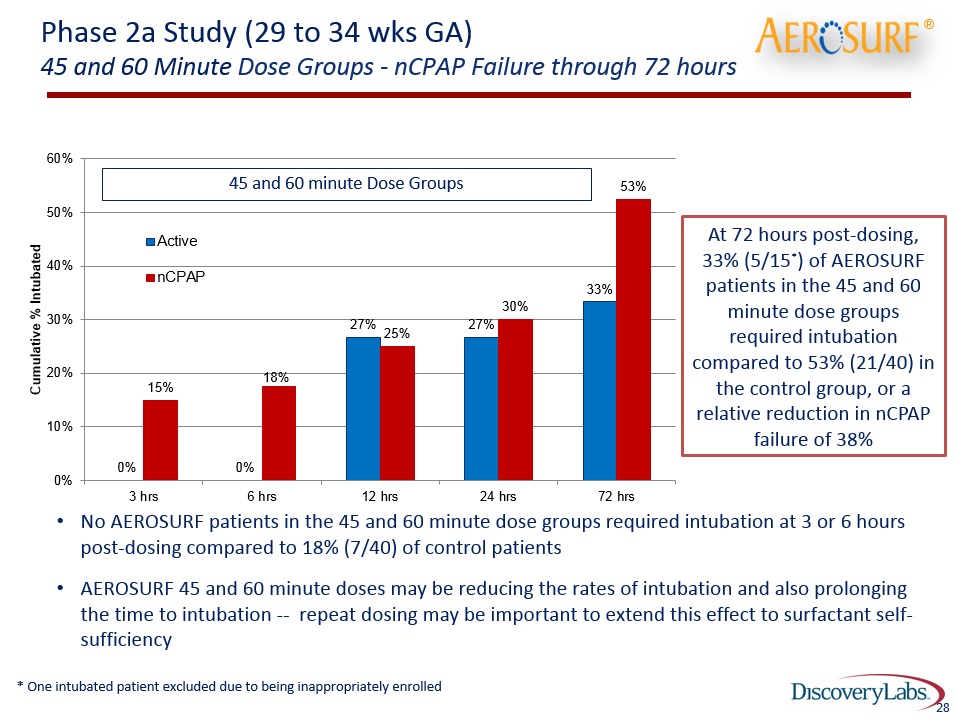
Phase 2a Study (29 to 34 wks GA)45 and 60 Minute Dose Groups - nCPAP Failure through 72 hours No AEROSURF patients in the 45 and 60 minute dose groups required intubation at 3 or 6 hours post-dosing compared to 18% (7/40) of control patientsAEROSURF 45 and 60 minute doses may be reducing the rates of intubation and also prolonging the time to intubation -- repeat dosing may be important to extend this effect to surfactant self-sufficiency 45 and 60 minute Dose Groups ® 28 At 72 hours post-dosing, 33% (5/15*) of AEROSURF patients in the 45 and 60 minute dose groups required intubation compared to 53% (21/40) in the control group, or a relative reduction in nCPAP failure of 38% * One intubated patient excluded due to being inappropriately enrolled

Phase 2a Study (29 to 34 wks GA) 45 and 60 Minute Dose Groups - nCPAP Failure through 72 hours 21/40 5/15* ® Time to nCPAP Failure 29 nCPAP Failure AEROSURF treated patients experienced a 20% absolute reduction or a 38% relative reduction in nCPAP failure compared to control (45 & 60 min) (45 & 60 min) * One intubated patient excluded due to being inappropriately enrolled *
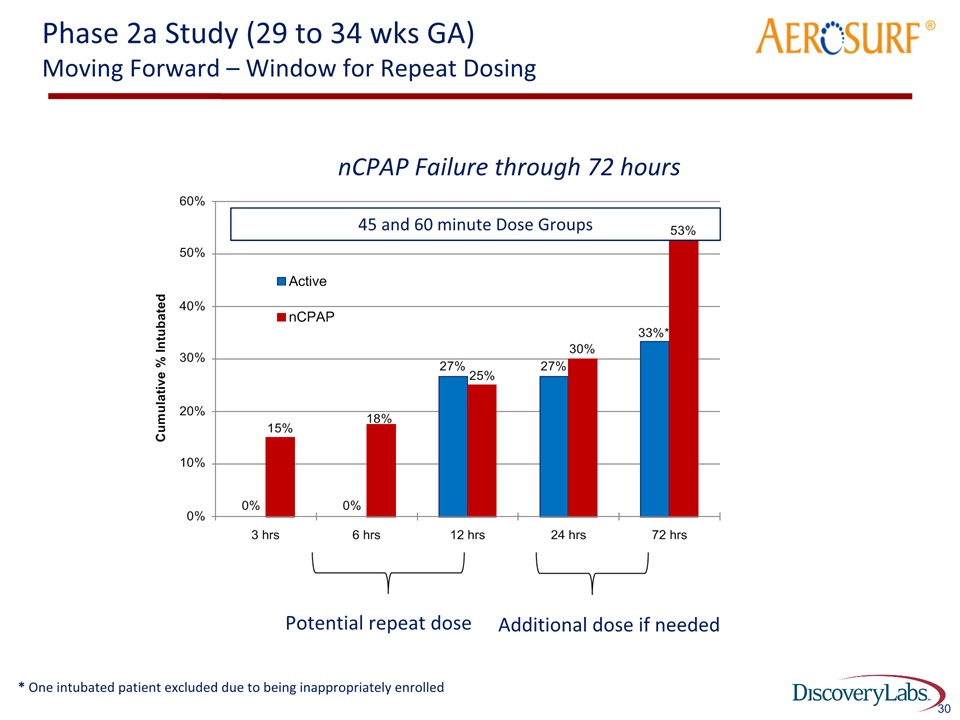
Phase 2a Study (29 to 34 wks GA)Moving Forward – Window for Repeat Dosing ® 30 Potential repeat dose nCPAP Failure through 72 hours Additional dose if needed 45 and 60 minute Dose Groups * One intubated patient excluded due to being inappropriately enrolled

Phase 2a Study (29 to 34 wks GA) Summary to Date Overall, the safety and tolerability profile of the AEROSURF group in the trial was generally comparable to the control group There was acceptable performance by the novel aerosol delivery technology in the NICUAerosolized surfactant produces physiological changes that are expected with surfactant replacement therapyThe goal of decreasing nCPAP failure and intubations appears achievable - data to date suggest that AEROSURF may be decreasing nCPAP failure and the need for intubationRepeat dosing may be important to enhance the reduction in nCPAP failuresResults from the phase 2a program in 29-34 week GA premature infants has informed the phase 2b study in premature infants 29-32 week GA and the phase 2a study in 26-28 week GA premature infants ® 31

Comprehensive Phase 2 Program Phase 2a Phase 2a Expansion Phase 2a Phase 2b Gestational Age (wks) 29 – 34 26 - 28 26 – 32(Begin with 29 – 32) Dose Groups 15 min; 30 min; 45 min( 25, 50, 75 TPL mg/kg)(8 active, 8 control per group)Single dose 60 min; 90 min (100 and 150 TPL mg/kg)(8 active, 8 control per group)Primarily single dose 30 min; 45 min (50 and 75 TPL mg/kg)(8 active, 8 control per group)Up to two doses 25 min; 50 min; Control(40 and 80 TPL mg/kg)Up to 3 doses # of patients 48 32 32 Up to 250 Objective(s) Safety and tolerabilityPhysiological data suggesting delivery of KL4 surfactant to the lungsPerformance of aerosol delivery system Safety and tolerability of higher doses and determine therapeutic index (safety window)Continue physiological assessment Safety and tolerabilityPhysiological assessment Provide evidence of efficacy on an acceptable endpointIdentify dose regimens for phase 3 studyProvide estimate of effect size # of sites Initiated with 3; increased to 8 (US) 12 (US) Up to 20 (US) 50+ (US, EU, Canada, LATAM) Timeline / Milestones Completed May 2015; key objectives achieved Completed Oct 2015 Initiated; results expected Q1 2016 Expect to initiate Q4 2015; target enrollment completion – mid - 2016 ®

AEROSURF® Phase 2 Program UpdateInvestor Conference CallNovember 12, 2015
