Attached files
| file | filename |
|---|---|
| EX-5.1 - EXHIBIT 5.1 - Health-Right Discoveries, Inc. | s102086_ex5-1.htm |
| EX-10.1 - EXHIBIT 10.1 - Health-Right Discoveries, Inc. | s102086_ex10-1.htm |
| EX-23.1 - EXHIBIT 23.1 - Health-Right Discoveries, Inc. | s102086_ex23-1.htm |
As filed with the Securities and Exchange Commission on October 30, 2015
Registration No. 333-206839
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1 TO FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
HEALTH-RIGHT DISCOVERIES, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 2834 | 45-3588650 | ||
|
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
18851 NE 29th Avenue, Suite 700
Aventura, Florida 33180
(305) 705-3247
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive officer)
David Hopkins
President
18851 NE 29th Avenue, Suite 700
Aventura, Florida 33180
(305) 705-3247
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies to:
Dale S. Bergman, Esq.
Gutierrez Bergman Boulris, P.L.L.C.
100 Almeria Avenue, Suite 340
Coral Gables, Florida 33134
(305) 358-5100
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☐ | Smaller reporting company ☒ |
|
(Do not check if a smaller reporting company) |
CALCULATION OF REGISTRATION FEE
Title of each class of Securities to be Registered(1) |
Amount to be Registered |
Proposed Maximum Offering Price Per Share(2)(3) |
Proposed Maximum Aggregate Offering Price |
Amount of Registration Fee |
||||||||||||
| Common stock, par value $0.001 per share | 5,309,152 | $ | 0.25 | $ | 1,327,288 | $ | 133.71 | (4) | ||||||||
(1) This registration statement covers the resale by our selling shareholders of up to 5,309,152 shares of common stock previously issued to such selling shareholders.
(2) The offering price has been estimated solely for the purpose of computing the amount of the registration fee in accordance with Rule 457. The proposed maximum offering price is based on the estimated high end of the range at which the common stock will initially be sold.
(3) The selling shareholders will offer their shares at $0.25 per share until the Company’s shares are quoted on the OTCQX or the OTCQB operated by OTC Markets Group and, assuming we secure this qualification, thereafter at prevailing market prices or privately negotiated prices.
(4) Previously paid.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities nor may offers to buy these securities be accepted until the registration statement filed with the Securities and Exchange Commission becomes effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED OCTOBER 30, 2015
PROSPECTUS
HEALTH-RIGHT DISCOVERIES, INC.
5,309,152 Shares of Common Stock
The selling shareholders named in this prospectus are offering up to 5,309,152 shares of common stock through this prospectus. We will not receive any proceeds from this offering and have not made any arrangements for the sale of these securities.
Our common stock is presently not traded on any market or securities exchange. Given the foregoing, the selling shareholders will offer their shares at $0.25 per share until the shares are quoted on the OTCQX or the OTCQB operated by OTC Markets Group. Although we intend to apply for quotation of our common stock on the OTCQX or the OTCQB operated by OTC Markets Group, we may not secure this qualification and even if we do an active public market for our common stock may never materialize. If we secure this qualification, the sale price to the public of the shares registered hereunder will be at prevailing market prices or privately negotiated prices.
The Company is an emerging growth company under the Jumpstart Our Business Startups Act of 2012 (the “Jobs Act”) and as such, may elect to comply with certain reduced public company reporting requirements for future filings.
The purchase of the shares of common stock offered through this prospectus involves a high degree of risk. See the section of this prospectus entitled “Risk Factors” beginning at page 5.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
The date of this prospectus is ____________, 2015
TABLE OF CONTENTS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission (the “SEC”) using the SEC’s registration rules for a delayed or continuous offering and sale of securities. Under the registration rules, using this prospectus and, if required, one or more prospectus supplements, the selling shareholders named herein may distribute the shares of common stock covered by this prospectus. This prospectus also covers any shares of common stock that may become issuable as a result of stock splits, stock dividends or similar transactions. A prospectus supplement may add, update or change information contained in this prospectus.
You should rely only on the information contained in this prospectus. We have not authorized any dealer, salesperson or other person to provide you with information concerning us, except for the information contained in this prospectus. The information contained in this prospectus is complete and accurate only as of the date on the front cover page of this prospectus, regardless when the time of delivery of this prospectus or the sale of any common stock. This prospectus is not an offer to sell, nor is it a solicitation of an offer to buy, our common stock in any jurisdiction in which the offer or sale is not permitted.
| 1 |
This summary provides an overview of all material information contained in this prospectus. It does not contain all the information you should consider before making a decision to purchase the shares our selling shareholders are offering. You should very carefully and thoroughly read the more detailed information in this prospectus and review our financial statements and all other information that is incorporated by reference in this prospectus.
Unless the context otherwise requires, references in this prospectus to “HRD,” “Health-Right,” “the Company,” “we,” “our” and “us” refers to Health-Right Discoveries, Inc. All share and per share information in this prospectus has been adjusted to give retroactive effect to a two-for-one stock split implemented in November 2014.
Overview
Health-Right is a natural biotech company that combines science and nutrition to develop branded ingredients, formulations and products that seek to provide a better quality of life for consumers who primarily suffer from stress-induced viruses and diseases. These formulations and products are developed naturally, by utilizing and scientifically combining various ingredients to help positively influence the interrelationship between stress and the immune system. The Company is planning to explore the application of its formulation platform to include cannabidiol (“CBD”) derived from industrial hemp. CBD, which is a cannabis-derived compound that may offer some of the benefits of medical marijuana without the side effects (i.e. the feeling of being “high” that comes from other cannabis compounds such as THC). The Company’s efforts in this regard will be dependent upon the U.S. Food and Drug Administration (“FDA”) clarifying its position with respect to the classification of hemp-derived CBD as a dietary supplement and an ingredient deemed to be Generally Described as Safe (“GRAS’), as opposed to cannabis-derived CBD, which is not so classified (the FDA has not as yet differentiated hemp-derived CBD from cannabis-derived CBD).
The Company believes the formulation platform it has developed in order to help address the negative nutritional interrelationship between stress, a weakened immune system and certain conditions and diseases is now in place. At the end of 2014, Health-Right completed initial test-marketing of Advanced H-Plex Defense Formula 11, its first product (“H-Plex Defense”), an all-natural dietary supplement whose formulation seeks to address less than optimal nutrition and nutritional deficiencies to aid persons afflicted with Herpes Simplex Virus 1 (“HSV-1”). Based on customer feedback, including a reorder rate exceeding industry norms, HRD believes that it has developed an alternative approach to help counteract and improve the causes/triggers of HSV-1, a virus that affects over an estimated 100 million people in the U.S. alone with symptoms including tingling, itching, blisters, sores and rashes with an estimated $5 billion marketplace for treatments by 2017, according to Global Industry Analysts, Inc.
Notwithstanding the foregoing, the Company has not conducted formal clinical trials of H-Plex Defense. Subject to receipt of necessary funding, the Company intends to do so, utilizing the services of Miami Research Associates (“MRA”), one of the largest, multi-special clinical research centers in the United States. Management has had multiple meetings and discussion with MRA and has negotiated Protocol Development Agreement for clinical trials, the finalization and execution of which is subject to receipt of necessary funding.
We believe that Health-Right is uniquely positioned because its approach of not just responding to stress induced conditions and diseases, but proactively addressing stress-induced problems before they wreak havoc on multiple body systems. The Company’s research, formulation and nutritional theories offer wide ranging potential because we believe that our formulation platform has the potential to be successfully applied In the prescription nutritional/medical foods, OTC monograph drug and all-natural dietary supplement/nutraceutical arenas. HRD anticipates that it will be able to leverage the results of its planned clinical trials in order to market ingredients, branded ingredients, private label ingredients and finished products. We also believe that we will be positioned to license our formulations to strategic partners in order to expedite and achieve product commercialization and cross-promotion.
In addition to its initial product, the Company plans to focus its efforts on areas where it believes that HRD can commercialize and market products on an expedited basis. We believe that are other potential applications for our formulations that we have in the pipeline, including all-natural products designed to relieve symptoms associated with:
| · | compromised immune systems and related muscle and joint pain; |
| · | the common cold and flu viruses; and |
| · | constipation resulting from prescription medications. |
By adapting its formulation platform to applications such as these, the Company believes that it will be able to generate revenues in the short term in the following markets:
| · | the Over-the-Counter (“OTC”) monograph drug industry; |
| · | the prescription nutritional marketplace; and |
| · | the natural products space. |
| 2 |
The Company intends to use its contacts in the healthcare, medical foods and natural products industries to market and sell its proposed products either directly or through strategic partners and licensees.
To date, the Company has generated limited revenues and has operated with limited capital. The Company will require significant capital to implement its business plan. There can be no assurance that the Company can raise the necessary funds, on favorable terms or otherwise. Failure to obtain sufficient capital will substantially harm the Company’s prospects.
Corporate Information
The Company was incorporated in the state of Florida on October 11, 2011 under the name “Four Plex Partners, Inc.” and changed its name to “Health-Right Discoveries, Inc.” on March 22, 2012.
Our executive offices are located at 18851 NE 29th Avenue, Suite 700, Aventura, Florida 33180 and our telephone number is (305) 705-3247. Our corporate website is www.health-right.com. Information appearing on our corporate website is not part of this prospectus.
Selling Shareholders
The Company previously sold or issued an aggregate of 17,133,332 shares of common stock to its founders, investors in private transactions (the “Private Placements”) and service providers.
The Company issued an aggregate of 14,133,332 shares of common stock to its founders from inception to June 30, 2015 for capital contributions of $25,000 and for services rendered to the Company, including 6,766,666 shares to David Hopkins, 6,366,666 shares to James Pande and 1,000,000 shares to Michael Fabiano. Mr. Hopkins subsequently gifted 950,000 of the shares of common stock held by him to five non-affiliated parties in October and December 2013 and transferred 153,156 shares to one party in March 2015 a private transaction in satisfaction of obligations owed to it by Mr. Hopkins. The resale of 3,257,574 shares of common stock currently held by our founders and 551,578 of the 1,103,156 shares of common stock transferred by Mr. Hopkins to third parties is covered by this prospectus.
In the Private Placements which were conducted from December 2011 to September 2012, the Company sold an aggregate of 1,350,000 shares of common stock to seven investors from inception to June 30, 2015, which sales generated gross proceeds of $165,000. The resale of 675,000 of these shares is covered by this prospectus.
We have also issued an additional 1,650,000 shares of common stock to third party service providers for legal and other business services. The resale of 825,000 of these shares is covered by this prospectus.
The Offering
This prospectus relates to the resale from time to time by the selling shareholders identified in this prospectus of 5,309,152 shares of our common stock, par value $0.001 per share. No shares are being offered for sale by the Company.
| Common stock offered by selling shareholders: | 5,309,172 shares of common stock. |
| Common stock outstanding on October 30, 2015: | 17,133,332 shares of common stock (1). |
| Terms of the Offering: | The selling shareholders will determine when and how they will sell the shares of common stock offered in this prospectus. |
| Use of proceeds: | We will not receive any proceeds from the sale of the shares of common stock offered by the selling shareholders under this prospectus. |
| Risk factors: | The common stock offered hereby involves a high degree of risk and should not be purchase by investors who cannot afford the loss of their entire investment. |
(1) Does not include 3,000,000 shares of our common stock reserved for issuance under our 2015 Stock Incentive Plan.
| 3 |
The following summary financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the Financial Statements and Notes thereto, included elsewhere in this prospectus.
| For the Years Ended December 31, | For the Six Months Ended June 30, | |||||||||||||||
| Statement Of Operations | 2014 | 2013 | 2015 | 2014 | ||||||||||||
| (Unaudited) | (Unaudited) | |||||||||||||||
| Revenues | $ | 59,811 | $ | 12,724 | $ | 13,581 | $ | 26,199 | ||||||||
| Cost of Sales | $ | 10,276 | $ | 11,424 | $ | 2,664 | $ | 4,380 | ||||||||
| Other Selling, General and Interest Expenses | $ | 241,114 | $ | 340,977 | $ | 38,740 | $ | 75,490 | ||||||||
| Net Loss | $ | (191,579 | ) | $ | (339,677 | ) | $ | (27,823 | ) | $ | (53,671 | ) | ||||
| As of | ||||||||
| December 31, 2014 | June 30, 2015 | |||||||
| Balance Sheet Data | (Unaudited) | |||||||
| Cash | $ | 609 | $ | 748 | ||||
| Total Assets | $ | 1,822 | $ | 5,413 | ||||
| Total Liabilities | $ | 201,133 | $ | 231,147 | ||||
| Total Stockholders’ Deficiency | $ | (199,311 | ) | $ | (226,134 | ) | ||
| 4 |
The shares of our common stock being offered for resale by the selling shareholders are highly speculative in nature, involve a high degree of risk and should be purchased only by persons who can afford to lose the entire amount invested in the common stock. Before purchasing any of the shares of common stock, you should carefully consider the following factors relating to our business and prospects. If any of the following risks actually occurs, our business, financial condition or operating results could be materially adversely affected. In such case, you may lose all or part of your investment. You should carefully consider the risks described below and the other information in this process before investing in our common stock.
We are an early stage company with a limited operating history.
The Company was incorporated in 2011, is an early stage company and has generated only limited revenues to date, with its primary focus being applying its limited capital resources in large part to product development activities. It is subject to all the problems, expenses, difficulties, complications and delays encountered in establishing a new business. The Company does not know if it will become commercially viable and ever generate significant revenues or operate at a profit.
The Company will require additional financing to become commercially viable.
To date, the Company has funded its development activities primarily through the Private Placements, which have generated approximately $165,000 and from inception in 2011 through June 30, 2015, respectively, $25,000 in capital contributions from principal shareholders and additional loans from principal shareholders. The Company will require additional financing to complete development of, commercially launch and market its planned products. The Company incurred net losses of $191,579 and $339,677 for the years ended December 31, 2014 and 2013, respectively and $27,823 and $53,671 for the six months ended June 30, 2015 and 2014, respectively. Moreover, as of June 30, 2015, we had a total shareholders’ deficiency of $226,134. Our independent auditors report for the year ended December 31, 2014 includes an explanatory paragraph stating that our lack of revenues and working capital raise substantial doubt about our ability to continue as a growing concern. While we are seeking to raise additional financing through additional securities offerings, there can be no assurance that additional financing will be available to the Company when needed, on favorable terms or otherwise. Moreover, any such additional financing may dilute the interests of purchasers of the shares offered hereby. The absence of additional financing, when needed, could cause the Company to delay implementation of its business plan in whole or in part, curtail its business activities and seriously harm the Company and its prospects.
Our success will be dependent in part on our ability to successfully develop, commercialize and market a portfolio of products utilizing our formulation platform and market acceptance of our planned products.
To date, we have only developed and test marketed our initial product H-Plex Defense. Our ultimate success will be dependent in part on our ability to successfully develop, commercialize and market a portfolio of products utilizing our formulation platform. In addition, market acceptance by and demand for our planned products from consumers will also be key factors in our ability to succeed. If we are unable to develop, commercialize and market our portfolio of planned products or if they are not accepted by consumers, our business and financial condition could be seriously harmed.
We will be dependent on third parties to help formulate and manufacture our planned products.
We intend to enter into agreements with third parties to assist in the formulation of and manufacture our planned products, including furnishing the necessary ingredients. While Health-Right believes that there are multiple firms who can provide such services, we have not entered into formal agreements with any such third parties. Should we not be able to do so on commercially reasonable terms or if subsequent to doing so, a good relationship with our formulation and manufacturing partners is not maintained or the supply of ingredients is interrupted, our business may be significantly harmed.
We have not undertaken any significant marketing efforts and we have only limited marketing experience.
As we are in the development stage, we have not undertaken any significant marketing efforts for our planned products beyond our initial trial marketing of H-Plex Defense. Moreover, we have only limited marketing experience and will likely rely on third parties to assist with product marketing. Accordingly, there is no assurance that any marketing strategy we develop can be successfully implemented or if implemented, that it will result in significant sales of our planned portfolio of products.
| 5 |
As we develop and commercialize our planned product portfolio, we may be increasingly subject to government regulation.
Although we do not anticipate that our planned products will be subject to the lengthy and costly FDA application processes for new drug approvals, we will be subject to some degree of regulation depending on the intended applications and markets our planned products. For example, our ability to manufacture and market products containing hemp-derived CBD will be dependent upon the FDA clarifying its classification of CBD, so that hemp-derived CBD (as opposed to cannabis-derived CBD), is classified as a dietary supplement and deemed to be an ingredient which is GRAS by the FDA (the FDA has not as yet differentiated hemp-derived CBD from cannabis-derived CBD, which is not classified as GRAS). All of our products will need to be manufactured in facilities that comply with current Good Manufacturing Processes (“cGMP”) as promulgated by the FDA from time to time. In addition, even though are planned products are expected to contain ingredients which are deemed to be GRAS by the FDA; we may need to conduct clinical trials to support our claims of efficacy. Moreover, we will be subject to various labeling requirements and registration requirements, such as securing a National Drug Code (“NDC”) for products we develop which are intended to be eligible for insurance reimbursement, such as those for the prescription nutritional marketplace. There can also be no assurance that future regulatory changes will be implemented and if implemented that we will be able to comply with such future regulations, at commercially reasonable cost, if at all.
As we develop and commercialize our planned products, we may become subject to risks entailed in securing reimbursement from private and public insurance plans.
To the extent that we formulate and commercialize products that may be eligible for insurance reimbursement, such as products for the prescription nutritional marketplace, we will be subject to various requirements in order to obtain reimbursement from private insurance or public insurance, such as Medicare and Medicaid. There is no certainty that we will be able to secure and maintain these requirements for insurance reimbursement. If we do not do so, we may not be able to successfully market these products and generate revenues therefrom. Even if we do so, revenues generated from these products will be subject to potential delays in receiving reimbursement payments from insurers.
Our business plan depends on our intellectual property rights and if we are unable to protect them, our competitive position may suffer.
Our business plan is predicated on part on our intellectual property rights, including our proprietary formulation platform and individual product formulations. Accordingly, protecting our intellectual property rights is critical to our continued success and our ability to maintain our competitive position. We protect our proprietary rights through a combination of trade secret and copyright law, confidentiality agreements and technical measures. We generally enter into non-disclosure agreements with our consultants and limit access to our trade secrets. We cannot assure you that the steps we have taken will prevent misappropriation of our proprietary property. Misappropriation of our intellectual property would have an adverse effect on our competitive position. In addition, we may have to engage in litigation in the future to enforce or protect our intellectual property rights or to defend against claims of invalidity, and we may incur substantial costs and the diversion of management’s time and attention as a result.
If we are deemed to infringe on the proprietary rights of third parties, we could incur unanticipated expense and be prevented from providing our planned products.
We could be subject to intellectual property infringement claims as the number of our competitors grows and our products overlap with competitive products. While we do not believe that we have infringed or are infringing on any proprietary rights of third parties, we cannot assure you that infringement claims will not be asserted against us or that those claims will be unsuccessful. We could incur substantial costs and diversion of management resources defending any infringement claims whether or not such claims are ultimately successful. Furthermore, a party making a claim against us could secure a judgment awarding substantial damages, as well as injunctive or other equitable relief that could effectively block our ability to provide products or services. In addition, we cannot assure you that licenses for any intellectual property of third parties that might be required for our products or services will be available on commercially reasonable terms, or at all.
The Company will face significant competition.
The markets for natural biotechnology products are and will continue to be highly competitive. The Company will face significant competition from other companies who are developing, commercializing and marketing competitive products, as well as those who may elect to do so in the future. Some of these competitors or potential competitors have greater experience, more extensive industry contacts and greater financial resources than the Company. There can be no assurance that the Company can effectively compete.
We currently rely on our President and his loss could have an adverse effect on the Company.
Until we build up our management infrastructure, our success depends upon our President, who is our sole executive officer. The loss of his services would currently have a material adverse effect on HRD. We are not party to an employment agreement with our President and do not anticipate having key man insurance in place on him in the foreseeable future.
| 6 |
The Company’s success will be dependent in part upon its ability to attract qualified personnel and consultants.
The Company’s success will be dependent in part upon its ability to attract qualified management, administrative, product development and marketing and sales personnel and consultants. The inability to do so on favorable terms may harm the Company’s proposed business.
We intend to become subject to the periodic reporting requirements of the Securities Exchange Act of 1934 that will require us to incur audit fees and legal fees in connection with the preparation of such reports. These additional costs could reduce or eliminate our ability to earn a profit.
Following the effective date of our registration statement of which this prospectus is a part, we will be required to file periodic reports with the SEC pursuant to the Securities Exchange Act of 1934 (the “Exchange Act”) and the rules and regulations promulgated thereunder. In order to comply with these requirements, our independent registered public accounting firm will have to review our financial statements on a quarterly basis and audit our financial statements on an annual basis. Moreover, our legal counsel will have to review and assist in the preparation of such reports. The costs charged by these professionals for such services cannot be accurately predicted at this time because factors such as the number and type of transactions that we engage in and the complexity of our reports cannot be determined at this time and will have a major effect on the amount of time to be spent by our auditors and attorneys. However, the incurrence of such costs will obviously be an expense to our operations and thus have a negative effect on our ability to meet our overhead requirements and earn a profit. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
Our internal controls may be inadequate, which could cause our financial reporting to be unreliable and lead to misinformation being disseminated to the public.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Rule 13a-15(f) under the Exchange Act, internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| · | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company; |
| · | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and/or directors of the Company; and |
| · | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company’s assets that could have a material effect on the financial statements. |
We will be required to include a report of management on the effectiveness of our internal control over financial reporting. We expect to incur additional expenses and diversion of management’s time as a result of performing the system and process evaluation, testing and remediation required in order to comply with the management certification requirements.
We do not have a sufficient number of employees to segregate responsibilities and may be unable to afford increasing our staff or engaging outside consultants or professionals to overcome our lack of employees. During the course of our testing, we may identify other deficiencies that we may not be able to timely remediate. In addition, if we fail to achieve and maintain the adequacy of our internal controls, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act (“Sarbanes-Oxley”). Moreover, effective internal controls, particularly those related to revenue recognition, are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock, if a market ever develops, could drop significantly.
| 7 |
The Jobs Act has reduced the information that the Company is required to disclose, which could adversely affect the price of the common stock.
Under the Jobs Act, the information that the Company will be required to disclose has been reduced in a number of ways.
Before the adoption of the Jobs Act, the Company was required to register the common stock under the Exchange Act within 120 days after the last day of the first fiscal year in which the Company had total assets exceeding $1,000,000 and 500 record holders of the common stock; the Jobs Act has changed this requirement such that the Company must register the common stock under the Exchange Act within 120 days after the last day of the first fiscal year in which the Company has total assets exceeding $10,000,000 and 2,000 record holders or 500 record holders who are not “accredited investors.” As a result, the Company is now required to register the Common Stock under the Exchange Act substantially later than previously.
As a company that had gross revenues of less than $1 billion during the Company’s last fiscal year, the Company is an “emerging growth company,” as defined in the Jobs Act (an “EGC”). The Company will retain that status until the earliest of (a) the last day of the fiscal year which the Company has total annual gross revenues of $1,000,000,000 (as indexed for inflation in the manner set forth in the Jobs Act) or more; (b) the last day of the fiscal year of following the fifth anniversary of the date of the first sale of the common stock pursuant to an effective registration statement under the Securities Act; (c) the date on which the Company has, during the previous three year period, issued more than $1,000,000,000 in non-convertible debt; or (d) the date on which the Company is deemed to be a “large accelerated filer,” as defined in Rule 12b-2 under the Exchange Act or any successor thereto. As an EGC, the Company is relieved from the following:
| • | The Company is excluded from Section 404(b) of Sarbanes-Oxley, which otherwise would have required the Company’s auditors to attest to and report on the Company’s internal control over financial reporting. The JOBS Act also amended Section 103(a)(3) of Sarbanes-Oxley to provide that (i) any new rules that may be adopted by the PCAOB requiring mandatory audit firm rotation or changes to the auditor’s report to include auditor discussion and analysis (each of which is currently under consideration by the PCAOB) shall not apply to an audit of an EGC; and (ii) any other future rules adopted by the PCAOB will not apply to the Company’s audits unless the SEC determines otherwise. |
| • | The Jobs Act amended Section 7(a) of the Securities Act to provide that the Company need not present more than two years of audited financial statements in an initial public offering registration statement and in any other registration statement, need not present selected financial data pursuant to Item 301 of Regulation S-K for any period prior to the earliest audited period presented in connection with such initial public offering. In addition, the Company is not required to comply with any new or revised financial accounting standard until such date as a private company (i.e., a company that is not an “issuer” as defined by Section 2(a) of Sarbanes-Oxley) is required to comply with such new or revised accounting standard. Corresponding changes have been made to the Exchange Act, which relates to periodic reporting requirements, which would be applicable if the Company were required to comply with them. |
| • | As long as the Company is an EGC, the Company may comply with Item 402 of Regulation S-K, which requires extensive quantitative and qualitative disclosure regarding executive compensation, by disclosing the more limited information required of a “smaller reporting company.” |
| • | In the event that the Company registers the common stock under the Exchange Act as it intends to do, the Jobs Act will also exempt the Company from the following additional compensation-related disclosure provisions that were imposed on U.S. public companies pursuant to the Dodd-Frank Act: (i) the advisory vote on executive compensation required by Section 14A(a) of the Exchange Act; (ii) the requirements of Section 14A(b) of the Exchange Act relating to shareholder advisory votes on “golden parachute” compensation; (iii) the requirements of Section 14(i) of the Exchange Act as to disclosure relating to the relationship between executive compensation and our financial performance; and (iv) the requirement of Section 953(b)(1)of the Dodd-Frank Act, which requires disclosure as to the relationship between the compensation of the Company’s chief executive officer and median employee pay. |
Since the Company is not required, among other things, to file reports under Section 13 of the Exchange Act or to comply with the proxy requirements of Section 14 of the Exchange Act until such registration occurs or to comply with certain provisions of Sarbanes-Oxley and the Dodd-Frank Act and certain provisions and reporting requirements of or under the Securities Act and the Exchange Act or to comply with new or revised financial accounting standards as long as the Company is an EGC, and the Company’s officers, directors and 10% shareholders are not required to file reports under Section 16(a) of the Exchange Act until such registration occurs, the Jobs Act has had the effect of reducing the amount of information that the Company and its officers, directors and 10% shareholders are required to provide for the foreseeable future.
As a result of such reduced disclosure, the price for the Common Stock may be adversely affected.
| 8 |
The costs of being a public company could result in us being unable to continue as a going concern.
As a public company, we will have to comply with numerous financial reporting and legal requirements, including those pertaining to audits and internal control. The costs of this compliance could be significant. If our revenues do not increase and/or we cannot satisfy many of these costs through the issuance of our shares, we may be unable to satisfy these costs in the normal course of business which would result in our being unable to continue as a going concern.
Our Articles of Incorporation and By Laws provide for indemnification of officers and directors at our expense and limit their liability that may result in a major cost to us and hurt the interests of our shareholders because corporate resources may be expended for the benefit of officers and/or directors.
Our Articles of Incorporation and Bylaws provide for the indemnification of our officers and directors. We have been advised that, in the opinion of the SEC, indemnification for liabilities arising under federal securities laws is against public policy as expressed in the Securities Act of 1933 (the “Securities Act”) and is, therefore, unenforceable.
The offering price of the shares has been arbitrarily determined by the Company.
The offering price of the shares has been arbitrarily determined by the Company and bears no relationship to the Company’s assets, book value, potential earnings or any other recognized criterion of value.
Currently, there is no public market for our securities, and we cannot assure you that any public market will ever develop and it is likely to be subject to significant price fluctuations.
Currently, there is no public market for our common stock and our common stock may never be traded on any exchange, or, if traded, a public market may not materialize. Even if we are successful in developing a public market, there may not be enough liquidity in such market to enable shareholders to sell their stock.
Our common stock is unlikely to be followed by any market analysts, and there may be few or no institutions acting as market makers for the common stock. Either of these factors could adversely affect the liquidity and trading price of our common stock. Until our common stock is fully distributed and an orderly market develops in our common stock, if ever, the price at which it trades is likely to fluctuate significantly. Prices for our common stock will be determined in the marketplace and may be influenced by many factors, including the depth and liquidity of the market for shares of our common stock, developments affecting our business, including the impact of the factors referred to elsewhere in these Risk Factors, investor perception of the Company, and general economic and market conditions. No assurances can be given that an orderly or liquid market will ever develop for the shares of our common stock.
| 9 |
If a trading market should develop for our common stock, it is likely that it will initially be deemed to be a “penny stock.” Therefore, trading of our common stock may be restricted by the SEC’s penny stock regulations and FINRA’s sales practice requirements, which may limit a shareholder’s ability to buy and sell our common stock.
In addition to the “penny stock” rules promulgated by the SEC, the Financial Industry Regulatory Authority (“FINRA”) has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, the FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. FINRA’s requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock.
The market for penny stocks has experienced numerous frauds and abuses that could adversely impact investors in our stock.
Company management believes that the market for penny stocks has suffered from patterns of fraud and abuse. Such patterns include:
| · | Control of the market for the security by one or a few broker-dealers that are often related to a promoter or issuer; |
| · | Manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; |
| · | “Boiler room” practices involving high pressure sales tactics and unrealistic price projections by sales persons; |
| · | Excessive and undisclosed bid-ask differentials and markups by selling broker-dealers; and |
| · | Wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the inevitable collapse of those prices with consequent investor losses. |
Any trading market that may develop for our common stock may be restricted by virtue of state securities “Blue Sky” laws that prohibit trading absent compliance with individual state laws. These restrictions may make it difficult or impossible to sell shares in those states.
There is currently no established public market for our common stock, and there can be no assurance that any established public market will develop in the foreseeable future. Transfer of our common stock may also be restricted under the securities laws and regulations promulgated by various states and foreign jurisdictions, commonly referred to as “blue sky” laws. Absent compliance with such individual state laws, our common stock may not be traded in such jurisdictions. Because the securities being registered hereunder have not been registered for resale under the blue sky laws of any state, the holders of such shares, and persons who desire to purchase them in any trading market that might develop in the future, should be aware that there may be significant state blue sky law restrictions upon the ability of investors to sell the securities and of purchasers to purchase the securities. These restrictions prohibit the secondary trading of our common stock. We currently do not intend to and may not be able to qualify securities for resale in a number of states that do not offer manual exemptions and require shares to be qualified before they can be resold by our shareholders. Accordingly, investors should consider the secondary market for our securities to be a limited one.
Our board of directors has the authority, without shareholder approval, to issue preferred stock with terms that may not be beneficial to common shareholders and with the ability to affect adversely shareholder voting power and perpetuate their control over us.
Our Amended and Restated Articles of Incorporation allows us to issue shares of preferred stock without any vote or further action by our shareholders. Our board of directors has the authority to fix and determine the relative rights and preferences of preferred stock. As a result, our board of directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock.
The ability of our executive officers and directors, who are our principal shareholders, to control our business may limit or eliminate minority shareholders’ ability to influence corporate affairs.
Our executive officers and directors, who are our principal shareholders, own and assuming the sale of the shares registered by them hereunder as selling shareholders, will continue to own a majority of our issued and outstanding common stock. Accordingly, they will be able to effectively control the election of directors, as well as all other matters requiring shareholder approval. The interests of our principal shareholders may differ from the interests of other shareholders with respect to the issuance of shares, business transactions with or sales to other companies, selection of other directors and other business decisions. The minority shareholders have no way of overriding decisions made by our principal shareholders. This level of control may also have an adverse impact on the market value of our shares because our principal shareholders may institute or undertake transactions, policies or programs that result in losses may not take any steps to increase our visibility in the financial community and / or may sell sufficient numbers of shares to significantly decrease our price per share.
| 10 |
We do not expect to pay cash dividends in the foreseeable future.
We have never paid cash dividends on our common stock. We do not expect to pay cash dividends on our common stock at any time in the foreseeable future. The future payment of dividends directly depends upon our future earnings, capital requirements, financial requirements and other factors that our board of directors will consider. Since we do not anticipate paying cash dividends on our common stock, return on your investment, if any, will depend solely on an increase, if any, in the market value of our common stock.
Because we are not subject to compliance with rules requiring the adoption of certain corporate governance measures, our shareholders have limited protection against interested director transactions, conflicts of interest and similar matters.
Sarbanes-Oxley, as well as rule changes proposed and enacted by the SEC, the New York Stock Exchange/NYSE/AMEX and the Nasdaq Stock Market, as a result of Sarbanes-Oxley, require the implementation of various measures relating to corporate governance. These measures are designed to enhance the integrity of corporate management and the securities markets and apply to securities that are listed on those exchanges or the Nasdaq Stock Market. Because we are not presently required to comply with many of the corporate governance provisions and because we chose to avoid incurring the substantial additional costs associated with voluntary compliance, we have not yet adopted these measures.
We do not currently have independent audit or compensation committees. As a result, directors have the ability, among other things, to determine their own level of compensation. Until we comply with such corporate governance measures, regardless of whether such compliance is required, the absence of such standards of corporate governance may leave our shareholders without protections against interested director transactions, conflicts of interest, if any, and similar matters and investors may be reluctant to provide us with funds necessary to expand our operations as a result thereof.
We intend to comply with all corporate governance measures relating to director independence as and when required. However, we may find it very difficult or be unable to attract and retain qualified officers, directors and members of board committees required to provide for our effective management as a result of Sarbanes-Oxley enactment of Sarbanes-Oxley has resulted in a series of rules and regulations by the SEC that increase responsibilities and liabilities of directors and executive officers. The perceived increased personal risk associated with these recent changes may make it more costly or deter qualified individuals from accepting these roles.
We will not receive any proceeds from the sale of the common stock offered through this prospectus by the selling shareholders. We have agreed to bear the expenses (other than any underwriting discounts or commissions or broker’s commissions) in connection with the registration of the common stock being offered hereby by the selling shareholders.
| 11 |
DETERMINATION OF OFFERING PRICE
All shares being offered will be sold by selling shareholders without our involvement, consequently the actual price of the stock will be determined by prevailing market prices at the time of sale or by private transactions negotiated by the selling shareholders. The offering price will thus be determined by market factors and the independent decisions of the selling shareholders.
The common stock to be sold by the selling shareholders is common stock that is currently issued and outstanding. Accordingly, there will be no dilution to our existing shareholders.
This prospectus covers the resale from time to time by the selling shareholders identified in the table below of up to 5,309,152 shares of our common stock, which were issued in various transactions exempt from registration under the Securities Act. We are registering the shares to permit the selling shareholders and any of their pledgees, donees, transferees, assignees and successors-in-interest to, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions when and as they deem appropriate in the manner described in “Plan of Distribution.” As of the date of this prospectus there are 17,133,332 shares of our common stock issued and outstanding.
The following table sets forth, as of the date of this prospectus, the name of each selling shareholder, the number and percentage of shares of our common stock beneficially owned by each selling shareholder prior to the offering for resale of the shares under this prospectus, the number of shares of our common stock beneficially owned by each selling shareholder that may be offered from time to time under this prospectus, and the number and percentage of shares of our common stock beneficially owned by the selling shareholder after the offering of the shares (assuming all of the offered shares are sold by the selling shareholder.
There are no agreements between the Company and any selling shareholder pursuant to which the shares subject to this registration statement were issued. Other than James Pande, a founder and director, David Hopkins, a founder, officer and director and Michael Fabiano, a founder, none of the selling shareholders ever been an officer or director of the Company and other than rendering services in the ordinary course of business, has had a material relationship with us at any time within the past three years.
Beneficial ownership is determined in accordance with the rules of the SEC, and includes any shares of common stock as to which a person has sole or shared voting power or investment power and any shares of common stock which the person has the right to acquire within sixty (60) days through the exercise of any option, warrant or right, through conversion of any security or pursuant to the automatic termination of a power of attorney or revocation of a trust, discretionary account or similar arrangement.
| 12 |
| Name of Selling Shareholder | Total Shares Owned by Selling Shareholder |
Total Shares to be Registered Pursuant to this Offering |
Percentage of Common Stock Before Offering |
Number of Shares Owned by Selling Shareholder After Offering |
||||||||||||
| James Pande | 6,366,666 | 1,591,696 | 37.2% | 4,774,970 | ||||||||||||
| David Hopkins | 5,663,510 | 1,415,878 | 33.1% | 4,247,632 | ||||||||||||
| Michael Fabiano | 1,000,000 | 250,000 | 5.8% | 750,000 | ||||||||||||
| Zach Hollman(1) | 500,000 | 250,000 | 2.9% | 250,000 | ||||||||||||
| Dale S. Bergman(2) | 400,000 | 200,000 | 2.3% | 200,000 | ||||||||||||
| John W. Koelle | 400,000 | 200,000 | 2.3% | 200,000 | ||||||||||||
| Charlie Bird(3) | 290,000 | 145,000 | 1.7% | 145,000 | ||||||||||||
| Dominic Joseph Lewis(4) | 500,000 | 250,000 | 2.9% | 250,000 | ||||||||||||
| Douglas B. Porter (5) | 250,000 | 125,000 | 1.4% | 125,000 | ||||||||||||
| Trenton Pande | 200,000 | 100,000 | 1.2% | 100,000 | ||||||||||||
| Timothy and Kyle Crotty | 200,000 | 100,000 | 1.2% | 100,000 | ||||||||||||
| David Petoskey | 200,000 | 100,000 | 1.2% | 100,000 | ||||||||||||
| Julian Cameron(6) | 200,000 | 100,000 | 1.2% | 100,000 | ||||||||||||
| Viviana Hammons | 150,000 | 75,000 | * | 75,000 | ||||||||||||
| Robert Brenner | 150,000 | 75,000 | * | 75,000 | ||||||||||||
| Jay Carr | 150,000 | 75,000 | * | 75,000 | ||||||||||||
| Preston J. Fields | 100,000 | 50,000 | * | 50,000 | ||||||||||||
| Brian K. Linstrand | 100,000 | 50,000 | * | 50,000 | ||||||||||||
| Kevin Henderson | 100,000 | 50,000 | * | 50,000 | ||||||||||||
| Joel Mayersohn | 50,000 | 25,000 | * | 25,000 | ||||||||||||
| Mark Purvis | 10,000 | 5,000 | * | 5,000 | ||||||||||||
| Blaine Heckaman(7) | 153,156 | 76,578 | * | 76,578 | ||||||||||||
*Less than 1%.
| (1) | Represents shares held of record by NE1 Design Studios, LLC, of which firm Mr. Holman is a managing member. |
| (2) | Represents shares held of record by Almeria Group Holdings, LLC, an affiliate of the Company’s legal counsel, of which firm Mr. Bergman is a managing member. |
| (3) | Represents shares held of record by The Better Image Company, of which firm Mr. Bird is President. |
| (4) | Includes 50,000 shares purchased by Dr. Lewis for the benefit of his daughter. |
| (5) | Includes 200,000 shares held of record by William B. Porter Group, of which firm Mr. Porter is President. |
| (6) | Represents shares held of record by the JA Cameron Revocable Trust, of which trust Mr. Cameron is Trustee. |
| (7) | Represents shares held of record by Kaufman, Rossin & Company, P.A., of which firm Mr. Heckaman is Managing Principal. |
The selling shareholders and any of their pledgees, donees, transferees, assignees and successors-in-interest may, from time to time, sell any or all of their shares of common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. As there is currently no trading market for our shares, the selling shareholders will offer their shares at $0.25 per share until the Company’s shares are quoted on the OTCQX or the OTCQB operated by the OTC Markets Group. Assuming we secure this qualification, thereafter the shares may be sold these at fixed or negotiated prices. The selling shareholders may use any one or more of the following methods when selling shares:
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits investors; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| 13 |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | to cover short sales made after the date that this registration statement is declared effective by the SEC; |
| · | broker-dealers may agree with the selling shareholders to sell a specified number of such shares at a stipulated price per share; |
| · | through the distribution of common stock by any selling shareholder to its partners, members or shareholders; |
| · | any other method permitted pursuant to applicable law; and |
| · | a combination of any such methods of sale. |
Broker-dealers engaged by the selling shareholders may arrange for broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts the selling shareholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling shareholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved.
The selling shareholders may from time to time pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell shares of common stock from time to time under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling shareholders to include the pledgee, transferee or other successors in interest as selling shareholders under this prospectus.
Upon a selling shareholder’s notification to us that any material arrangement has been entered into with a broker-dealer for the sale of such shareholder’s common stock through a block trade, special offering, exchange distribution or secondary distribution or a purchase by a broker or dealer, a supplement to this prospectus will be filed, if required, pursuant to Rule 424(b) under the Securities Act disclosing (i) the name of each such selling shareholder and of the participating broker-dealer(s), (ii) the number of shares involved, (iii) the price at which such shares of common stock were sold, (iv) the commissions paid or discounts or concessions allowed to such broker-dealer(s), where applicable, (v) that such broker-dealer(s) did not conduct any investigation to verify the information set out or incorporated by reference in this prospectus, and (vi) other facts material to the transaction. In addition, upon our being notified in writing by a selling shareholder that a donee or pledgee intends to sell more than 500 shares of common stock, a supplement to this prospectus will be filed if then required in accordance with applicable securities law.
The selling shareholders also may transfer the shares of common stock in other circumstances, in which case the donees, assignees, transferees, pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus and may sell the shares of common stock from time to time under this prospectus after we have filed any necessary supplements to this prospectus under Rule 424(b), or other applicable provisions of the Securities Act supplementing or amending the list of selling shareholders to include such donee, assignee, transferee, pledgee, or other successor-in-interest as a selling shareholder under this prospectus.
In the event that the selling shareholders are deemed to be “underwriters,” any broker-dealers or agents that are involved in selling the shares will be deemed to be “underwriters” within the meaning of the Securities Act, in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Discounts, concessions, commissions and similar selling expenses, if any, that can be attributed to the sale of the shares of common stock will be paid by the selling shareholder and/or the purchasers. Each selling shareholder has represented and warranted to us that it acquired the securities subject to this registration statement for his/her own account for investment and not for the benefit of any other person and not with a view to distribute or sell in violation of the Securities Act or any state securities laws or rules and regulations promulgated thereunder.
| 14 |
If a selling shareholder uses this prospectus for any sale of the common stock, it will be subject to the prospectus delivery requirements of the Securities Act. The selling shareholders will be responsible to comply with the applicable provisions of the Securities Act and the Exchange Act and the rules and regulations thereunder promulgated, including, without limitation, Regulation M, as applicable to such selling shareholders in connection with resales of their respective shares under this registration statement.
We are required to pay all fees and expenses incident to the registration of the shares, but we will not receive any proceeds from the sale of the common stock.
| 15 |
Overview
Health-Right is a natural biotech company that combines science and nutrition to develop branded ingredients, formulations and products that seek to provide a better quality of life for consumers who primarily suffer from stress-induced viruses and diseases. These formulations and products are developed naturally, by utilizing and scientifically combining various ingredients to help positively influence the interrelationship between stress and the immune system. The Company is planning to explore the application of its formulation platform to include CBD derived from industrial hemp. CBD, which is a cannabis-derived compound that may offer some of the benefits of medical marijuana without the side effects (i.e. the feeling of being “high” that comes from other cannabis compounds such as THC). The Company’s efforts in this regard will be dependent upon the FDA clarifying its position with respect to the classification of hemp-derived CBD as a dietary supplement and an ingredient deemed to be GRAS, which is not so classified (the FDA has not as yet differentiated hemp-derived CBD from cannabis-derived CBD).
The Company believes the formulation platform it has developed in order to help address the negative nutritional inter-relationship between stress, a weakened immune system and certain conditions and diseases is now in place. At the end of 2014, Health-Right completed initial test marketing of H-Plex Defense, its first product, an all-natural dietary supplement whose formulation seeks to address less than optimal nutrition and nutritional deficiencies to aid persons afflicted with HSV-1. Based on customer feedback, including a reorder rate exceeding industry norms, HRD believes that it has developed an alternative approach to help counteract and improve the causes/triggers of HSV-1, a virus that affects over an estimated 100 million people in the U.S. alone with symptoms including tingling, itching, blisters, sores and rashes with an estimated $5 billion marketplace for treatments by 2017, according to Global Industry Analysts, Inc.
Notwithstanding the foregoing, the Company has not conducted formal clinical trials of H-Plex Defense. Subject to receipt of necessary funding, the Company intends to do so, utilizing the services of MRA, one of the largest, multi-special clinical research centers in the United States. Management has had multiple meetings and discussion with MRA and has negotiated Protocol Development Agreement for clinical trials, the finalization and execution of which is subject to receipt of necessary funding.
We believe that Health-Right is uniquely positioned because its approach of not just responding to stress induced conditions and diseases, but proactively addressing stress-induced problems before they wreak havoc on multiple body systems. The Company’s research, formulation and nutritional theories offer wide ranging potential because we believe that our formulation platform has the potential to be successfully applied In the prescription nutritional/medical foods, OTC monograph drug and all-natural dietary supplement/nutraceutical arenas. HRD anticipates that it will be able to leverage the results of its planned clinical trials in order to market ingredients, branded ingredients, private label ingredients and finished products. We also believe that we will be positioned to license our formulations to strategic partners in order to expedite and achieve product commercialization and cross-promotion.
In addition to its initial product, the Company plans to focus its efforts on areas where it believes that HRD can commercialize and market products on an expedited basis. We believe that are other potential applications for our formulations that we have in the pipeline, including all-natural products designed to relieve symptoms associated with:
| · | compromised immune systems and related muscle and joint pain; |
| · | the common cold and flu viruses; and |
| · | constipation resulting from prescription medications. |
By adapting its formulation platform to applications such as these, the Company believes that it will be able to generate revenues in the short term in the following markets:
| · | the OTC monograph drug industry; |
| · | the prescription nutritional marketplace; and |
| · | the natural products space. |
The Company intends to use its contacts in the healthcare, medical foods and natural products industries to market and sell its proposed products either directly or through strategic partners and licensees.
To date, the Company has generated limited revenues and has operated with limited capital. The Company will require significant capital to implement its business plan. There can be no assurance that the Company can raise the necessary funds, on favorable terms or otherwise. Failure to obtain sufficient capital will substantially harm the Company’s prospects.
| 16 |
The Problem – Stress Contributing to Disease
Introduction
Stress may come to be seen as the primary contributing cause of most disease in the future. Today’s research continues to link stress to more and more symptoms and diseases, both acute and chronic.
So what exactly is stress? Stress is our reaction to our external environment as well as our inner thoughts and feelings. Simply put, stress is our body’s natural response to dangers, the “fight or flight” mechanisms—the body’s preparedness to do battle with or flee from danger. This response involves a complex biochemical-hormonal process that can offer different results for each individual.
Stress can generate a number of symptoms and diseases caused by changes in the immune system function, hormonal response and biochemical reactions. When this happens, it then can influence body functions in our digestive tract as well as our cardiovascular, neurological or musculoskeletal systems.
Types of Stress
Physical Stress—exercise, hard labor or even giving birth.
Mental Stress—high responsibility, long hours or fatigue, anxiety and worrying
Emotional Stress—anger, fear, sadness, frustration, betrayal or bereavement
Traumatic Stress—infection, injury, surgery or burns and extreme temperatures
Nutritional Stress—vitamin and mineral deficiencies, protein or fat excesses or deficiencies, even food allergies
Chemical Stress—environmental pollution such as exposure to pesticides and cleaning solvents, and the personal use of chemicals such as drugs, alcohol, caffeine and nicotine
Psycho-spiritual Stress—financial or career pressures, relationships, life goals, spiritual alignment and the general state of happiness.
The Compromised Immune System
Weaker immune systems can leave people more vulnerable to stress-related conditions, infections and diseases. Some common causes that may weaken the immune system are:
| Causes of Physical and Emotional Stress | |
| Pain and inflammation | Lack of sleep |
| Viral infections | Internal toxins |
| Poor dietary choices | Chemicals |
| Worrying | Excessive alcohol consumption |
| Sunburn | Surgery |
| Allergic Reactions | Fatigue |
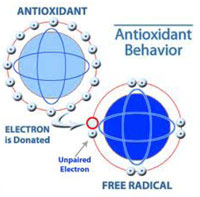
Our immune system becomes weaker when a free radical is attacking our body. This free radical is an atom or group of atoms that contains at least one unpaired electron. Free radicals have the ability to join easily with other compounds, which can create dramatic, negative changes in the body. Each free radical may only exist for a tiny fraction of a second, but the damage it leaves behind can be irreversible; particularly damage to heart muscle cells, nerve cells and certain immune system sensor cells. The formation of a large number of free radicals stimulates the formation of more free radicals, leading to even greater cellular damage and immune system impairment.
 |
 |
People are routinely advised to “minimize negative stress” in an effort to improve their state of physical and emotional wellness. As most of us know, not all stress is negative. However, for the majority of people, the constant daily stress we endure damages our bodies and weakens immunity, thereby dramatically increasing our susceptibility to illness and disease. It is generally accepted in the medical community that stress contributes or exacerbates a wide spectrum of illnesses. Simply not getting enough quality, daily sleep can dramatically increase a person’s stress.
| 17 |
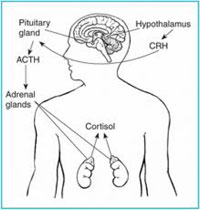
Almost all body functions and organs react to stress. During stressful events, which can be mental/emotional and/or physical, the pituitary gland increases its production of adrenocorticotropic hormone (ACTH), which in turn stimulates the release of the hormones Cortisone and Cortisol. These two hormones have the effect of inhibiting the functioning of disease-fighting white blood cells and suppressing the immune response. A stressful event actually triggers a state of physical changes in the body, and this is called the “fight or flight” response, which is primarily designed to prepare a person to face or flee from immediate/eminent danger. The increased production of adrenal hormones like Cortisol is what is believed to be responsible for most of the symptoms associated with stress. This “fight or flight” response causes the body to step up its metabolism of proteins, fats and carbohydrates to quickly produce energy for the body. The response also causes the body to excrete amino acids, potassium and phosphorus, deplete magnesium stored in the muscle tissue and decreases calcium. Stress also promotes the formation of free radicals that can become oxidized and damage body tissues, especially cell membranes.
During a stress response, our bodies begin excreting potassium, magnesium and calcium. Although these essential minerals are essential to good health and wellness, poor dietary and lifestyle choices rob our bodies of these vital minerals. In terms of micro-nutrients, minerals can be even more important than vitamins. Potassium and magnesium are some of the more helpful minerals for rebalancing the electrical properties of our cells, in addition to helping balance calcium.
The Health-Right Solution
Introduction
Health-Right believes that through solid research, it has developed a formulation platform to address the less than optimal nutrition and the nutritional deficiencies resulting from a stress-induced state. Pending clinical research, the Company believes that there are a number of applications for our products that can be rolled out in three different areas:
| · | the OTC monograph drug business; |
| · | the niche prescription nutritionals/medical foods arena; and |
| · | the natural markets for consumers who insist on natural alternatives. |
OTC Monograph Drug Business
HRD has identified two monographs, which, subject to receipt of required funding, HRD believes should allow it to access the OTC monograph drug business mass marketplace in 2016 with oral and topical formulations of H-Plex Defense. Once funding is in place, Health-Right will complete any formulation adjustments and, if necessary, file a New Drug Application (“NDA”) with the FDA. As it is basing its products on monograph drugs for the OTC marketplace, the Company anticipates that the FDA approval process will be fairly rapid. Once approved, we believe that by utilizing existing relationships, we can gain access to the food and drug mass merchant distribution channel. If we are able to successfully market our initial product in this manner, we intend to apply its H-Plex Defense formulation platform to other monograph drugs in order to develop additional products for distribution in the OTC marketplace.
Prescription Nutritional/Medical Foods Market
As defined by the Orphan Drug Act (1988 Amendment), a prescription nutritional or a medical food is “a food which is formulated to be consumed or administered enterally (orally) under the supervision of a physician, and which is intended for specific dietary management of a disease or condition for which distinctive nutritional requirements, based on recognized scientific principles, are established by medical evaluation.”
Prescription nutritionals or medical foods must be shown by medical evaluation to meet the distinctive nutritional needs of a specific, diseased patient population being targeted, prior to marketing. In contrast, dietary supplements are intended for normal, healthy adults and require no pre-market efficacy tests. Furthermore, as indicated by their nomenclature, prescription nutritionals or medical foods require physician supervision and a prescription. Simply put, prescription nutritionals or medical foods are medical products for a specific nutritional purpose while dietary supplements are a consumer product to supplement the diet and maintain good health and regular function.
Subject to receipt of necessary funding, HRD intends to conduct clinical trials to demonstrate the benefits of its formulation platform. HRD believes that its formulation platform could be applicable to the development of various products designed specifically for the dietary management of disease. HRD has identified at least three potential applications for its formulation platform in the prescription nutritional/medical foods marketplace.
We would anticipate that our prescription nutritional products, when and if successfully developed, would be marketed through one or more strategic partners with experience and relationships in this marketplace, whom we intend to ally with.
| 18 |
Natural Market
Today’s life is faster than ever, filled with constant change, uncertainty and stress. It often results in a sleep-deprived, stressed and depressed population. Consumers spend literally billions of dollars annually to fight fatigue and stress through mood enhancers, sleep aids, energy drinks, coffee, etc. However, these “solutions” are not combating the problem, they’re compounding the problem. A large and rapidly growing number of consumers are desperately seeking healthier, alternative solutions to neutralize the negative impact their fast-paced and stressful lives take on their bodies.
Consumers also have more reinforcement of their interest in dietary supplements. CRN’s (Council for Responsible Nutrition) 2007 Healthcare Professionals Impact Study found that “more than three-quarters of U.S. physicians (79%) and nurses (82%) recommend dietary supplements to their patients.”
Pipeline/Products/Methodology
Viral Marketplace
The first product we formulated and test marketed is H-Plex Defense, an oral natural product designed to help manage triggers that cause recurrent cold sore outbreaks in persons afflicted with HSV-1. A complementary topical version is currently in the development stage.
Our market test took place from July 2013 through December 2014. We marketed H-Plex Defense nationwide online through a website. During the market trial, 1,215 distinct customers purchased the product; 369 (or 30%) of these customers reordered the product or subscribed to a monthly purchase program and 846 customers (or 70%) did not reorder. The Company believes that the 30% reorder rate exceeds industry norms.
Of the reorder customers:
| · | 78% reordered at least one time; |
| · | 41% reordered for 4-6 months; and |
| · | 30% ordered for 6 months or more. |
During the market test, feedback from reordering customers demonstrated that the product not only improved the quality of life for sufferers of HSV-1 (the cold sore virus) but also demonstrated that it afforded relief to those also discovered it helped consumers with Herpes Simplex Virus 2 (“HSV-2”). More than 3.7 billion people under the age of 50 worldwide (including over 300 million people in the United States),, or 67% of the population, are infected with HSV-1 according to an October 2015 study from the World Health Organization (“WHO”), WHO’s first global estimates of the HSV-1 infection.
The new estimates highlight, however, that HSV-1 is also an important cause of genital herpes. Some 140 million people aged 15-49 years are infected with genital HSV-1 infection, primarily in the Americas, Europe and Western Pacific. Fewer people in high-income countries are becoming infected with HSV-1 as children, likely due to better hygiene and living conditions, and are instead at risk of contracting it genitally through oral sex after they become sexually active.
WHO also estimated that 417 million people aged 15-49 years have HSV-2 infection, which causes genital herpes. Taken together, the estimates reveal that over half a billion people between the ages of 15-49 years have genital infection caused by either HSV-1 or HSV-2. Given the lack of a permanent and curative treatment for both HSV-1 and HSV-2, WHO and partners are working to accelerate development of vaccines and topical microbicides, which will have a crucial role in preventing these infections in the future.
The WHO study highlights the significant potential for the Company’s H-Plex Defense, HSV-1 product.
Joint Pain/Arthritis Marketplace
With the help of Dr. Dominic Lewis, a prominent orthopedic surgeon who is also a shareholder of the Company, HRD was able to utilize its formulation platform for an application designed to help stabilize the immune system while simultaneously addressing arthritis and/or joint pain. Individuals who suffer from constant joint pain and arthritis are under a lot of stress, which in turn can cause their immune system to be severely compromised. Dr. Lewis consulted with the Company on the components of the formulation intended to address the joint pain/arthritis marketplace, appropriate dosage amounts and other aspects of product development and formulation.
Scientific research is pointing more and more ever year indicating that stress is becoming a significant contributing factor to major illness. A great majority of physicians believe that the reduction of stress leads to a healthier, stronger, more stable immune system. It sounds elementary but our Company knows of no pharmaceutical or drug companies addressing these serious issues with prescription medication.
HRD’s formulation can be adjusted in order to be marketable in the natural, OTC and prescription nutritional marketplaces. Management is not aware of any other product on the market that is taking this approach or has developed a research platform to address more than just consumer pain.
Much like the viral marketplace, HRD is developing a complimentary topical for specific pain management to provide direct relief to arthritis and joint sufferers. This topical could be used as a combination treatment in the prescription nutritional markets, as well as the OTC markets.
Gastrointestinal Marketplace
Using our formulation platform, the Company has developed and did some anecdotal testing of an application for people with digestive tract irregularities resulting from stress and prescription medication—they seem to go hand in hand. According to various sources, such as www.WEBMD.com, people who are under tremendous stress or take certain prescription medication, which can cause severe constipation problems. The anecdotal testing consisted of use of the product by approximately ten “friends and family” individuals who were taking prescription medication for post-surgery pain or anxiety. The anecdotal testing was not a clinical trial and the results thereof are in no manner a substitute for a formal clinical trial.
Stress is a known factor in constipation and many prescription medication side effects cause constipation. The stress is only increased when a person cannot have healthy bowel movements. We believe that by helping patients regain regularity, their stress levels will be reduced.
| 19 |
Management believes this application can also be modified to help patients address post-surgery prescription constipation.
CBD, Stress/Anxiety and Immune System Health
The Company has commenced preliminary research to explore the development of a product portfolio that utilizes CBD from industrial hemp in powder form, in combination with certain other natural ingredients, to help combat the negative effects that stress and anxiety have on the immune system. This innovation could possibly play a part in changing the landscape regarding natural products assisting with quality of life issues for consumers who solely relied on prescription medication. We believe that the focus of this CBD-related product platform will involve improving anxiety and the interrelationship between stress, a compromised immune system and viruses.
What is the Nutritional Rationale Behind our Methodology?
HRD developed what it believes to be a synergistic and targeted formulation platform for stress-induced viruses and diseases that addresses multiple body systems in an effort to help neutralize certain negative effects of symptomatic stress. Based on a compendium of research we assembled with respect to the key components of H-Plex Defense, we believe that our formulation provides positive immune system benefits by supporting the elimination of harmful free radicals, boosting immune system defenses through the use of specific natural herbs which are shown to have anti-viral effects, providing a stress management component to help minimize the negative effects of Cortisol, and offering supplement minerals and Vitamin D to aid in the restoration of depleted minerals and maintenance of calcium balance.
Research and Development
We have developed our formulation platform and H-Plex Defense, our initial product, in conjunction with various consultants and third party research and development firms experienced in providing those services. We anticipate continuing to do so as well as performing any needed clinical trials through such third parties for the foreseeable future.
While there is no pre-approval mechanism at the FDA for medical food products, all such products must have validation of their effectiveness prior to being marketed. Because all medical food products are required to contain ingredients that are GRAS, there are no safety testing requirements. We plan to validate the efficacy of our products by clinical testing, including double blind, randomized clinical trials.
Manufacturing and Sources of Supply
We intend to outsource the manufacturing of our planned products to third parties whose facilities are cGMP compliant. While we have no formal contractual relationship with any such firm, we believe that there are numerous cGMP compliant third party manufacturers who can be secured on a short or long term basis at commercially reasonable cost. We utilized one such manufacturer located in Miami, Florida, for product in connection with our test marketing of H-Plex Defense. We expect to provide each contract manufacturer we use with a formula and manufacturing specifications. The manufacturer will then source and purchase raw ingredients and manufactures the products to our specifications. We believe that the raw materials used in our formulations are readily available from various sources.
Sales and Marketing
We intend to distribute our products through a network of independent distributors, who market to the various industry segments who we intend to target, such as mass merchants, healthcare networks, chain drug stores and similar facilities. We do not as yet have agreements with any distributor and there can be no assurance that we will be able to enter into any such agreement on commercially reasonable terms. Subject to the availability of capital, we may supplement such efforts with an internal sales force. We also intend to market our products on line. We plan to support these sales efforts through marketing campaigns in both traditional broadcast and online media, including radio and television commercials, infomercials, print advertisements and social media and other online marketing campaigns. Our ability to undertake and the extent of marketing of our products we can undertake will be dependent in large part on our capital resources.
Government Regulation
Introduction
Although our planned products will not be subject to the lengthy and costly FDA application processes for new drug approvals, we will be subject to some degree of regulation depending on the intended applications and markets our planned products. There can be no assurance that future regulatory changes will be implemented and if implemented that we will be able to comply with such future regulations, at commercially reasonable cost, if at all.
| 20 |
Dietary Supplements
Congress defined the term "dietary supplement" in the Dietary Supplement Health and Education Act (“DSHEA”) of 1994. A dietary supplement is a product taken by mouth that contains a "dietary ingredient" intended to supplement the diet. The "dietary ingredients" in these products may include: vitamins, minerals, herbs or other botanicals, amino acids, and substances such as enzymes, organ tissues, glandulars, and metabolites. Dietary supplements can also be extracts or concentrates, and may be found in many forms such as tablets, capsules, soft gels, gel caps, liquids, or powders. They can also be in other forms, such as a bar, but if they are, information on their label must not represent the product as a conventional food or a sole item of a meal or diet. Whatever their form may be, DSHEA places dietary supplements in a special category under the general umbrella of "foods," not drugs, and requires that every supplement be labeled a dietary supplement.
The DSHEA, which amended the Federal Food, Drug, and Cosmetic Act, created a new regulatory framework for the safety and labeling of dietary supplements. Under DSHEA, a firm is responsible for determining that the dietary supplements it manufactures or distributes are safe and that any representations or claims made about them are substantiated by adequate evidence to show that they are not false or misleading. This means that dietary supplements do not need approval from FDA before they are marketed. Except in the case of a new dietary ingredient, where pre-market review for safety data and other information is required by law, a firm does not have to provide FDA with the evidence it relies on to substantiate safety or effectiveness before or after it markets its products. We believe that H-Plex Defense meets the definition of a dietary supplement under DSHEA as its formulation consists of existing (and no new dietary ingredients).
H-Plex Defense will be required to comply with FDA labelling regulations for dietary supplements. FDA regulations require that certain information appear on dietary supplement labels. Information that must be on a dietary supplement label includes: a descriptive name of the product stating that it is a "supplement;" the name and place of business of the manufacturer, packer, or distributor; a complete list of ingredients; and the net contents of the product. In addition, each dietary supplement (except for some small volume products or those produced by eligible small businesses) must have nutrition labeling in the form of a "Supplement Facts" panel. This label must identify each dietary ingredient contained in the product. Failure to comply with these regulations could result in an adverse impact on the Company’s business.
Prescription Nutritionals/Medical Foods
Prescription nutritionals or medical foods are deemed to be foods by the FDA and not drugs and, therefore, are not subject to any regulatory requirements that specifically apply to drugs. For example, any of our planned prescription nutritional/medical foods products will not have to undergo premarket review or approval by the FDA. Prescription nutritionals or medical foods are exempt from the labeling requirements for health claims and nutrient content claims under the Nutrition Labeling and Education Act of 1990. Rather, their labeling must comply with all food labeling requirements except for those specific requirements from which medical foods are exempt.
OTC Monograph Drugs
OTC drugs are defined as drugs that are safe and effective for use by the general public without seeking treatment by a health professional. Because there are over 300,000 marketed OTC drug products, the FDA reviews the active ingredients and the labeling of over 80 therapeutic classes of drugs, for example analgesics or antacids, instead of individual drug products. For each category, an OTC drug monograph is developed and published in the Federal Register. OTC drug monographs are a kind of "recipe book" covering acceptable ingredients (those that are deemed to be GRAS), doses, formulations, and labeling. Once a final monograph is implemented, companies can make and market an OTC product without the need for FDA pre-approval. These monographs define the safety, effectiveness, and labeling of all marketing OTC active ingredients. New products that conform to a final monograph may be marketed without further FDA review. Those that do not conform must be reviewed by the New Drug Application (NDA) process. A drug company may also petition to change a final monograph to include additional ingredients or to modify labeling. As we intend to initially market OTC drugs that conform to existing monographs and use ingredients which are deemed to be GRAS by the FDA, we do not anticipate having to go through a lengthy approval process, if at all.
Clinical Trials
While we do not anticipate requiring clinical trials of our planned products in order to bring them to market, we do plan to conduct such trials to support our product efficacy claims.
cGMP Compliance
All of our products will need to be manufactured in facilities that comply with cGMP. The contract manufacturer we used for our initial test market, as well as those we are exploring in connection with commercial production of our planned products have facilities which are cGMP compliant.
| 21 |
Insurance Reimbursement
To the extent that we formulate and commercialize products that may be eligible for insurance reimbursement, such as products for the prescription nutritional marketplace, we will be subject to various requirements in order to obtain reimbursement from private insurance or public insurance, such as Medicare and Medicaid. There is no certainty that we will be able to secure and maintain these requirements for insurance reimbursement. If we do not do so, we may not be able to successfully market these products and generate revenues therefrom. Even if we do so, revenues generated from these products will be subject to potential delays in receiving reimbursement payments from insurers.
Intellectual Property
Our business plan is predicated on part on our intellectual property rights, including our proprietary formulation platform and individual product formulations. Accordingly, protecting our intellectual property rights is critical to our continued success and our ability to maintain our competitive position. We currently protect our proprietary rights through a combination of trade secret and copyright law, confidentiality agreements and technical measures. We generally enter into non-disclosure agreements with our consultants and limit access to our trade secrets. To the extent our future development efforts result in patentable inventions or discoveries, we may seek patent and/or trademark protection. We cannot assure you that the steps we have taken will prevent misappropriation of our proprietary property. Misappropriation of our intellectual property would have an adverse effect on our competitive position. In addition, we may have to engage in litigation in the future to enforce or protect our intellectual property rights or to defend against claims of invalidity, and we may incur substantial costs and the diversion of management’s time and attention as a result.
Employees
As of the date of this prospectus the Company’s sole employee is its President and the Company relies on independent third party consultants in large part to perform additional services as needed. As we implement our business plan and subject to the availability of capital, additional employees will be hired in the future as our business expands.
Properties
We do not own any real property. We maintain an office mailing address at 18829 NE 29th Avenue, Suite 700, Aventura, Florida 33180 at nominal cost and pay for the utilization of office and conference space at such location on an as needed basis. The Company anticipates renting commercial office and warehouse space in the South Florida area once it has raised sufficient capital. We believe that there is a significant amount of suitable space available at commercially reasonable cost.
Legal Proceedings
Currently there are no legal proceedings pending or threatened against us. However, from time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in any such matter may harm our business.
MARKET FOR COMMON EQUITY AND RELATED SHAREHOLDER MATTERS
There is presently no public market for our common stock and there has never been a market for our common stock. We anticipate applying for quotation of our common stock on the OTCQX or the OTCQB operated by OTC Markets Group following the effectiveness of the registration statement of which this prospectus forms a part. However, we cannot assure you that our shares will be quoted on any tier of OTC Markets Group or, if quoted, that a public market will develop and if developed, be liquid and be sustained.
A market maker sponsoring a company’s securities is required to obtain a quotation of the securities on any of the public trading markets, including the OTCQX or OTCQB. If we are unable to obtain a market maker for our common stock, we will be unable to develop a trading market for our common stock. We may be unable to locate a market maker that will agree to sponsor our securities. Even if we do locate a market maker, there is no assurance that our securities will be able to meet the requirements for a quotation or that the securities will be accepted for quotation by OTC Markets Group on the OTCQX or OTCQB.
We intend to apply for quotation of the securities on the OTCQX or OTCQB operated by OTC Markets Group, but there can be no assurance that we will be able to obtain either listing. OTCQX and OTCQB securities are not quoted and traded on the floor of an organized national or regional stock exchange. Instead, securities transactions are conducted through a telephone and computer network connecting dealers in stocks. OTCQX and OTCQB stocks are traditionally smaller companies that do not meet the financial and other listing requirements of a regional or national stock exchange.
| 22 |
Holders of our Common Stock
As of the date of this prospectus, we had 17,133,332 shares of common stock issued and outstanding and 24 shareholders of record of our common stock.
Transfer Agent
Following effectiveness of this registration statement of which this prospectus forms a part, we intend to appoint VStock Transfer, LLC, Woodmere, New York, as transfer agent for our common stock.
Dividend Policy
The payment by us of dividends, if any, in the future rests within the discretion of our Board of Directors and will depend, among other things, upon our earnings, capital requirements and financial condition, as well as other relevant factors. We have not paid any dividends since our inception and we do not intend to pay any cash dividends in the foreseeable future, but intend to retain all earnings, if any, for use in our business.
Rule 144 Shares
Rule 144 under the Securities Act provides that a person who is not an affiliate and has held restricted securities for a prescribed period of at least six months (if the issuer is a reporting company) or 12 months (if the issuer is a non-reporting company, as is the case herein), may, under certain conditions, sell all or any of his shares without volume limitation. Affiliates, however, may not sell shares in excess of 1% of the Company’s outstanding common stock in any three month period. There is no limit on the amount of restricted securities that may be sold by a non-affiliate (i.e., a shareholder who has not been an officer, director or control person for the three months prior to sale) after the restricted securities have been held by the owner for the aforementioned prescribed period of time. All of our remaining 11,824,180 shares of common stock not covered by this prospectus will be eligible for public sale pursuant to Rule 144, commencing 90 days after the date the registration statement of which this prospectus forms a part in declared effective by the SEC.
| 23 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Results of Operations
Six Months Ended June 30, 2015 Compared to Six Months Ended June 30, 2014
We had revenues of $13,581 for the six months ended June 30, 2015, as compared to revenues of $26,199 for the six months ended June 30, 2014, reflecting the completion of our trial-marketing of our initial planned product, H-Plex Defense in the 2015 period. Cost of sales similarly declined to $2,664 in the 2015 period from $4,380 in the 2014 period, resulting in a corresponding decline in gross profit to $10,917 in the 2015 period from $21,819 in the 2014 period.
General and administrative costs decreased to $36,355 in the 2015 period from $74,488 for the six months ended June 30, 2014, while interest expense increased to $2,385 for the six months ended June 30, 2015, from $1,002 for the six months ended June 30, 2014, reflecting the increased principal balance of shareholder loans.
Net losses for six months ended June 30, 2015 and June 30, 2014 were $27,823 and $53,671, respectively.
Year Ended December 31, 2014 Compared to Year Ended December 31, 2013
We had revenues of $59,811 for the year ended December 31, 2014, as compared to revenues of $12,724 for the year ended December 31, 2015, reflecting the increased trial-marketing of H-Plex Defense in 2014. Cost of sales declined to $10,276 in 2014 from $11,424 in 2013, offset by write-offs of obsolete inventory of $5,840 and $10,830 in 2014 and 2013, respectively, which resulted in gross profit of $49,535 in 2014, as compared to $1,300 in 2013.
General and administrative costs were $236,314 in 2014, as compared to $339,331 in 2013, while interest expense increased to $4,800 in 2014 from $1,626 in 2013, reflecting the increased principal balance of shareholder loans.
Net losses for the years ended December 31, 2014 and December 31, 2013, were $191,579 and $339,677, respectively.
Liquidity and Capital Resources
As of June 30, 2015, total current assets were $5,413 as compared to $1,822 on December 31, 2014. Total current liabilities as of June 30, 2015 were $231,547, as compared to $201,133 as of December 31, 2014.
For the six month period ended June 30 2015, we raised $7,500 through a shareholder loan, which was offset by repayment of a $4,678 shareholder loan.
Net cash used in operating activities was $2,683 in the first six months of 2015 compared to $22,959 for the same period in 2014.
Net cash provided by financing activities in the first six months of 2015 was $2,822 compared to $3,720 in the first six months of 2014.
Our primary source of capital to develop and implement our business plan has been from private placements of our securities and shareholder loans.
The Company will require additional financing to complete development to commercially launch and market its planned products. Our independent auditors report for the year ended December 31, 2010 includes an explanatory paragraph strategy that our lack of revenues and working capital raise substantial doubt about our ability to continue a growing concern. While we are seeking to raise additional financing, either through government grants and loans or additional securities or offerings, there can be no assurance that additional financing will be available to the Company when needed, on favorable terms or otherwise. Moreover, any such additional financing may dilute the interests of existing shareholders. The absence of additional financing, when needed, could cause the Company to delay implementation of its business plan in whole or in part, curtail its business activities and seriously harm the Company and its prospects.
| 24 |
The Company will require additional financing to complete development of, commercially launch and market its planned products. Our independent auditors report for the year ended December 31, 2014 includes an explanatory paragraph stating that our lack of revenues and working capital raise substantial doubt about our ability to continue as a growing concern. While we are seeking to raise additional financing through additional securities offerings, there can be no assurance that additional financing will be available to the Company when needed, on favorable terms or otherwise. Moreover, any such additional financing may dilute the interests of purchasers of the shares offered hereby. The absence of additional financing, when needed, could cause the Company to delay implementation of its business plan in whole or in part, curtail its business activities and seriously harm the Company and its prospects.
Critical Accounting Policies
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates included deferred revenue, costs incurred related to deferred revenue, the useful lives of property and equipment, the useful lives of intangible assets and accounting for the business combination.
Income Taxes
The Company accounts for income taxes in accordance with ASC 740, Accounting for Income Taxes, as clarified by ASC 740-10, Accounting for Uncertainty in Income Taxes. Under this method, deferred income taxes are determined based on the estimated future tax effects of differences between the financial statement and tax basis of assets and liabilities given the provisions of enacted tax laws. Deferred income tax provisions and benefits are based on changes to the assets or liabilities from year to year. In providing for deferred taxes, the Company considers tax regulations of the jurisdictions in which the Company operates, estimates of future taxable income, and available tax planning strategies. If tax regulations, operating results or the ability to implement tax-planning strategies vary, adjustments to the carrying value of deferred tax assets and liabilities may be required. Valuation allowances are recorded related to deferred tax assets based on the “more likely than not” criteria of ASC 740.
ASC 740-10 requires that the Company recognize the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the “more-likely-than-not” threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority.
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
| 25 |
Directors and Executive Officers
Our directors and executive officers and their respective ages and titles are as follows:
| Name | Age | Position(s) and Office(s) Held | ||
| David Hopkins | 47 | President and Director | ||
| James Pande | 56 | Director | ||
Set forth below is a brief description of the background and business experience of our directors and executive officers.
David Hopkins founded Health-Right in 2011 and has served as President and a director since that time. From November 2009 until he founded the Company, Mr. Hopkins was a founder and managing member of Envirocare Solutions, LLC, a privately-held technology-driven manufacturer and wholesale distributor of products designed to deliver cold air micro-mist diffusion safely into the environment. In addition, since 2001, Mr. Hopkins has been a principal of Hopkins & Associates, a consulting firm providing turnaround and business development services to various private and public held companies engaged in a variety of industries, ranging from the production and distribution of soft drinks to biotechnology and environmental products. Mr. Hopkins holds a B.S. degree from Carroll University in Wisconsin.
James Pande has served as a director of the Company since its inception in 2011. Since 2010, he has served as Vice President of Marketing and Sales Aldora Aluminum and Glass Products, based in Miramar, Florida. Prior thereto, he owned Smith Mountain Impact Systems in Miami, Florida, which he built into a respected manufacturer of hurricane impact entrance doors. Mr. Pande holds a bachelor’s degree from the Cornell School of Hotel and Restaurant Management.
Following effectiveness of the registration statement of which this prospectus forms a part, we intend to seek to expand our board of directors to include additional members, including “independent” directors.
Terms of Office
Our directors are appointed for a one-year term to hold office until the next annual meeting of our shareholders and until a successor is appointed and qualified, or until their removal, resignation, or death. Executive officers serve at the pleasure of the board of directors.
Board Committees
Our board of directors does not currently have an audit committee, a compensation committee, or a corporate governance committee. We plan to establish such committees in the near future.
Code of Ethics
We do not currently have a Code of Ethics that applies to employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. We plan to adopt a Code of Ethics in near future.
Advisory Board
The Company plans to establish an advisory board, whose members will meet periodically in person or by telephone with management and/or the board of directors to advise on product development and marketing matters. Members of the advisory board will serve at the pleasure of the board of directors. It is anticipated that members of the advisory board will be compensated through the grant of stock option awards under our 2015 Incentive Stock Plan.
| 26 |
Summary Compensation Table
The table below summarizes all compensation awarded to, earned by, or paid to our President, who was our sole executive officer for 2014 and 2013, including amounts accrued but not paid.
SUMMARY COMPENSATION TABLE
| Name and principal position | Year | Salary ($) | Bonus ($) | Stock Awards (#) | Option Awards (#) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
| David Hopkins, President | 2014 | 52,000 | (1) | 0 | 0 | 0 | 0 | 0 | 0 | 52,000 | (1) | |||||||||||||||||||
| 2013 | 52,000 | (1) | 0 | 0 | 0 | 0 | 0 | 0 | 52,000 | (1) |
| (1) | Represents $52,000 in salary accrued but not paid to Mr. Hopkins in each of 2014 and 2013. |
Employment Agreements
The Company is presently not party to an employment agreement with its executive officer.
Outstanding Equity Awards at Fiscal Year-End Table
The table below summarizes all unexercised options, stock that has not vested, and equity incentive plan awards for each named executive officer outstanding as of the end of our last completed fiscal year.
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||||||||||||||||||||||||||||
| OPTION AWARDS | STOCK AWARDS | |||||||||||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Shares of Stock That Have Not Vested (#) |
Market Value of Shares or Shares of Stock That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Shares or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Shares or Other Rights That Have Not Vested (#) |
|||||||||||||||||||||||||||
| David Hopkins | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
Compensation of Directors Table
The table below summarizes all compensation paid to our directors for our last completed fiscal year.
DIRECTOR COMPENSATION |
||||||||||||||||||||||||||||
| Name | Fees Earned or Paid in Cash ($) |
Stock Awards ($) |
Option Awards ($) |
Non-Equity Incentive Plan Compensation ($) |
Non-Qualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||||||||
| David Hopkins | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
| James Pande | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||
| 27 |
Narrative Disclosure to the Director Compensation Table
We currently do not compensate our non-employee directors. When we expand our board we will intend to implement a plan and compensate them with a combination of cash and stock option awards, depending on our financial resources at that time.
2015 Incentive Stock Plan
Our 2015 Incentive Stock Plan provides for equity incentives to be granted to our employees, executive officers or directors or to key advisers or consultants. Equity incentives may be in the form of stock options with an exercise price not less than the fair market value of the underlying shares as determined pursuant to the 2015 Incentive Stock Plan, restricted stock awards, other stock based awards, or any combination of the foregoing. The 2015 Incentive Stock Plan is administered by the board of directors. 3,000,000 shares of our common stock are reserved for issuance pursuant to the exercise of awards under the 2015 Incentive Stock Plan. The number of shares so reserved automatically adjusts upward on January 1 of each year, so that the number of shares covered by the 2015 Incentive Stock Plan is equal to 15% of our issued and outstanding common stock. No awards are outstanding as of the date of this prospectus.
| 28 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of the date of this prospectus, the beneficial ownership of our common stock by each director and executive officer, by each person known by us to beneficially own 5% or more of the our common stock and by directors and executive officers as a group. Unless otherwise stated, the address of the persons set forth in the table is c/o the Company, 18851 NE 29th Avenue, Suite 700, Aventura, Florida 33180.
|
Names and addresses of beneficial owners |
Number of shares
|
Percentage of class (%) | ||||||
| Directors and executive officers: | ||||||||
| David Hopkins | 5,663,510 | 33.1% | ||||||
| James Pande | 6,366,666 | 37.1% | ||||||
| All directors and executive officers as a group (two (2) persons) | 12,030,176 | 70.2% | ||||||
| Other 5% or greater shareholders: | ||||||||
|
Michael Fabiano |
1,000,000 | 5.8% | ||||||
The persons named above have full voting and investment power with respect to the shares indicated. Under
the rules of the SEC, a person (or group of persons) is deemed to be a “beneficial owner” of a security if he or she,
directly or indirectly, has or shares the power to vote or to direct the voting of such security, or the power to dispose of or
to direct the disposition of such security. Accordingly, more than one person may be deemed to be a beneficial owner
of the same security.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Related Party Transactions
Since inception, the Company has relied in large part on loans from James Pande and David Hopkins, its principal shareholders, to fund its operations.
From inception through December 31, 2014, the Company borrowed an aggregate of $76,385 from two of its shareholders. The amounts were recorded as a loan payable to related parties on the accompanying balance sheet. These loans are non-interest bearing and due on demand. The balance on these notes at December 31, 2014 and 2013 were $35,084 and $25,505, respectively.
One of the Company’s founders has made loan the Company through direct charges on the founders’ credit card to pay for Company expenses. The balance of this loan at December 31, 2014 and 2013 was $9,827 and $14,794, respectively. The founder is charging the Company 15.5% interest on the unpaid monthly balances associated with this loan.
The above loans are classified on the accompanying balance sheet as loans payable – related party.
In August, 2013 the Company converted certain loans payable referred to above and accrued salary and expenses to the shareholders in the aggregate amount of $82,499 into secured notes payable to the shareholders. The notes bear interest at 3% per annum, are secured by substantially all assets of the Company, and are due on demand. The balance on the notes at December 31, 2014 and 2013 were $49,200 and $67,499, respectively.
Accrued interest on the above debts to related parties aggregated $4,877 and $1,378 at December 31, 2014 and 2013, respectively.
The Company’s board of directors approved a salary to the Company’s president in the amount of $52,000 per annum plus a car allowance of $600 per month. As of December 31, 2014 and 2013 the amount unpaid and accrued amounted to$78,000 and $25,000, respectively, which is reflected on the accompanying Balance sheet at that date. Effective in August 2013, the president waived his car allowance which amount has not been accrued since that date.
Mr. Hopkins has also made a loan to the Company through direct charges on his credit card to pay for certain Company expenses. The balance of this loan at June 30, 2015 was $5,499. Mr. Hopkins is charging the Company 15.5% interest on the unpaid monthly balances associated with this loan, which is equal to the interest rate he pays on the credit card.
In August, 2013 the Company converted of the certain loans payable referred to above and accrued salary and expenses to Mr. Hopkins in the aggregate amount of $82,499 into secured notes payable to the shareholders. The notes bear interest at 3% per annum, are secured by substantially all assets of the Company, and are due on demand. The balance on the notes at June 30, 2015 was $48,850.
In July 2015, Mr. Pande extended a $75.000 one-year credit facility to the Company, pursuant to which the Company may, subject to Mr. Pande’s consent, borrow (but not reborrow) up to such amount in one or more installments during its term, which expires on July 30, 2016. Interest at the rate of 7-1/2% on the principal balance outstanding from time to time accrues and is payable monthly in arrears. The entire principal balance together with accrued but unpaid interest is due and payable at maturity and is mandatorily prepayable if the Company consummates a financing prior to maturity which generates gross proceeds of at least $500,000. The parties also entered into a security agreement pursuant to which the outstanding principal balance under the credit facility and the outstanding principal balance under the demand notes made in favor of Mr. Pande are secured by a first priority security interest in substantially all of the Company’s assets.
| 29 |
Review, Approval and Ratification of Related Party Transactions
Given our small size and limited financial resources, we had not adopted formal policies and procedures for the review, approval or ratification of transactions with our executive officers, directors and significant shareholders. However, we intend that such transactions will, on a going-forward basis, be subject to the review, approval or ratification of our board of directors, or an appropriate committee thereof.
Capital Stock
Our authorized capital stock consists of 100,000,000 shares of common stock, par value $0.001 and 5,000,000 shares of preferred stock, par value $0.001.
Common Stock
As of the date of this prospectus, 17,133,332 shares of common stock are issued as outstanding. The shares of common stock presently outstanding are fully paid and non-assessable. Each holder of common stock is entitled to one vote for each share owned on all matters voted upon by shareholders, and a majority vote is required for all actions to be taken by shareholders. In the event we liquidate, dissolve or wind-up our operations, the holders of the common stock are entitled to share equally and ratably in our assets, if any, remaining after the payment of all our debts and liabilities and the liquidation preference of any shares of preferred stock that may then be outstanding. The common stock has no preemptive rights, no cumulative voting rights, and no redemption, sinking fund, or conversion provisions.
Holders of common stock are entitled to receive dividends, if and when declared by the board of directors, out of funds legally available for such purpose, subject to the dividend and liquidation rights of any preferred stock that may then be outstanding.
Preferred Stock
Our board of directors has the authority, without further action by the shareholders, to issue shares of preferred stock in one or more series and to fix the rights, preferences and the number of shares constituting any series or the designation of such series. While our Amended and Restated Articles of Incorporation and bylaws do not contain any provisions that may delay, defer or prevent a change in control, the issuance of preferred stock may have the effect of delaying or preventing a change in control or make removal of our management more difficult. No shares of preferred stock are outstanding as of the date of this prospectus.
The validity of the common stock being offered hereby has been passed upon by Gutierrez Bergman Boulris, P.L.L.C., Coral Gables, Florida. A limited liability company affiliated with such law firm beneficially owns 400,000 shares of our common stock, whose resale is covered by the prospectus forming a part of this registration statement.
The audited financial statements included in this prospectus and elsewhere in the registration have so been included in reliance upon the report of Paritz and Company, P. A., independent registered public accountants, upon the authority of said firm as experts in accounting and auditing in giving said report.
We have filed a registration statement on Form S-1 under the Securities Act with the SEC with respect to the shares of our common stock offered through this prospectus. This prospectus is filed as a part of that registration statement, but does not contain all of the information contained in the registration statement and exhibits. Statements made in the registration statement are summaries of the material terms of the referenced contracts, agreements or documents of the company. We refer you to our registration statement and each exhibit attached to it for a more detailed description of matters involving the company. You may inspect the registration statement, exhibits and schedules filed with the SEC at the SEC’s principal office in Washington, D.C. Copies of all or any part of the registration statement may be obtained from the Public Reference Section of the SEC, 100 F Street, N.E. Washington, D.C. 20549. Please Call the Commission at 1-800-SEC-0330 for further information on the operation of the public reference rooms. The SEC also maintains a web site at http://www.sec.gov that contains reports, proxy Statements and information regarding registrants that files electronically with the SEC. Our registration statement and the referenced exhibits can also be found on this site.
| 30 |
DISCLOSURE OF SEC POSITION ON INDEMNIFICATION
FOR SECURITIES ACT LIABILITIES
In accordance with the provisions in our Amended and Restated Articles of Incorporation, we will indemnify an officer, director, or former officer or director, to the full extent permitted by law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
| 31 |
HEALTH-RIGHT DISCOVERIES, INC.
INDEX TO FINANCIAL STATEMENTS
| F-1 |
| Paritz |
& Company, P.A |
15 Warren Street, Suite 25 Hackensack, New Jersey 07601 (201) 342-7753 Fax: (201) 342-7598 E-Mail: PARITZ@paritz.com | |
| Certified Public Accountants | |||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors
Health-Right Discoveries, Inc.
We have audited the accompanying balance sheet of Health-Right Discoveries, Inc. as of December 31, 2014 and 2013 and the related statements of operations, changes in stockholders’ equity (deficiency) and cash flows for the years ended December 31, 2014 and 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Health-Right Discoveries, Inc.as of December 31, 2014 and 2013, and the results of its operations and cash flows for the years ended December 31, 2014 and 2013 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As disclosed in Note 3the Company has generated minimal revenue since inception, has sustained losses since inception, and has a stockholders’ deficiency of $199,311 at December 31, 2014. These factors, among others, raise substantial doubt about the ability of the Company to continue as a going concern. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/S/Paritz & Company, P.A.
Hackensack, New Jersey
September 1, 2015
| F-2 |
The accompanying notes are an integral part of these financial statements
| F-3 |
The accompanying notes are an integral part of these financial statements
| F-4 |
| HEALTH-RIGHT DISCOVERIES, INC. |
| STATEMENTS OF CASH FLOWS |
| Year ended December 31, | ||||||||
| 2014 | 2013 | |||||||
| OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (191,579 | ) | $ | (339,677 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock based compensation | 89,583 | 225,417 | ||||||
| Accrued salary to related party | 53,000 | 59,200 | ||||||
| Inventory write-off | 5,840 | 10,830 | ||||||
| Accrued interest | 3,949 | |||||||
| Changes in operating assets and liabilities | ||||||||
| Inventories | 1,289 | (811 | ) | |||||
| Prepaid expenses | — | 3,507 | ||||||
| Accrued expenses | ||||||||
| Credit card payable | 422 | 8,232 | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | (37,496 | ) | (33,302 | ) | ||||
| FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance common stock | 15,000 | — | ||||||
| Proceeds of loan from related parties | 11,663 | 53,726 | ||||||
| Proceeds from note payable | 15,000 | |||||||
| Repayment of related party loan | (25,350 | ) | — | |||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 16,313 | 53,726 | ||||||
| INCREASE (DECREASE) IN CASH | (21,183 | ) | 20,424 | |||||
| CASH - BEGINNING OF YEAR | 21,792 | 1,368 | ||||||
| CASH - END OF YEAR | $ | 609 | $ | 21,792 | ||||
| $ | — | — | ||||||
| Supplemental disclosures of cash flow information: | ||||||||
| Non-cash financing activities | ||||||||
| Issuance of common stock for services classified as prepaid expense | $ | — | $ | 70,000 | ||||
The accompanying notes are an integral part of these financial statements
| F-5 |
| HEALTH-RIGHT DISCOVERIES, INC. |
| STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIENCY) |
| Additional | ||||||||||||||||||||
| ------COMMON STOCK------ | Paid-In | Accumulated | ||||||||||||||||||
| Shares | Amount | Capital | Deficit | Total | ||||||||||||||||
| BALANCE – January 1, 2013 | 13,823,332 | $ | 13,823 | $ | 222,427 | $ | (234,305 | ) | $ | 1,945 | ||||||||||
| Common stock issued for services | 2,750,000 | 2,750 | 272,250 | 275,000 | ||||||||||||||||
| Net Loss | (339,677 | ) | (339,677 | ) | ||||||||||||||||
| BALANCE – December 31, 2013 | 16,573,332 | $ | 16,573 | $ | 494,677 | $ | (573,982 | ) | $ | (62,732 | ) | |||||||||
| Common stock issued in private placement | 150,000 | 150 | 14,850 | 15,000 | ||||||||||||||||
| Common stock issued for services | 400,000 | 400 | 39,600 | 40,000 | ||||||||||||||||
| Net loss | (191,579 | ) | (191,579 | ) | ||||||||||||||||
| BALANCE – December 31, 2014 | 17,123,332 | $ | 17,123 | $ | 549,127 | $ | (765,561 | ) | $ | (199,311 | ) | |||||||||
The accompanying notes are an integral part of these financial statements
| F-6 |
HEALTH-RIGHT DISCOVERIES, INC.
NOTE 1 – Business
Health-Right Discoveries, Inc. (the “Company”) was formed under the laws of the State of Florida on October 12, 2011 under the name Four Plex Partners, Inc. The company changed its name to Health-Right Discoveries, Inc. on March 22, 2012. The Company’s business is to develop and market an innovative portfolio of both prescription nutritional, OTC monograph and natural products that primarily focus on factors relating to stress-induced conditions and diseases. The Company believes it has developed a formulation platform to vastly improve the negative interrelationship between stress, a weakened immune system and certain stress-related conditions and diseases.
NOTE 2 - Summary of Significant Accounting Policies
Use of Estimates
The preparation of the financial statements in conformity with Generally Accepted Accounting Principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenue and expenses during the reporting periods. Actual results could differ from those estimates. Certain of the Company’s estimates could be affected by external conditions, including those unique to its industry, and general economic conditions. It is possible that these external factors could have an effect on the Company’s estimates that could cause actual results to differ from its estimates. The Company re-evaluates all of its accounting estimates at least quarterly based on these conditions and record adjustments when necessary.
Cash
The Company considers all short-term highly liquid investments with an original maturity at the date of purchase of three months or less to be cash equivalents.
Revenue Recognition
The Company follows the guidance of the Accounting Standards Codification (“ASC”) Topic 605, “Revenue Recognition”. We record revenue when persuasive evidence of an arrangement exists, product delivery has occurred, the selling price to the customer is fixed or determinable and collectability of the revenue is reasonably assured. The Company has not experienced any significant returns from customers and accordingly, in management’s opinion, no reserve for returns has been provided.
Inventories
Inventories, which consist of the Company’s product held for resale, are stated at the lower of cost, determined using the first-in, first-out, and net realizable value. Net realizable value is the estimated selling price, in the ordinary course of business, less estimated costs to complete and dispose of the product.
| F-7 |
If the Company identifies excess, obsolete or unsalable items, its inventories are written down to their realizable value in the period in which the impairment is first identified. Shipping and handling costs incurred for inventory purchases and product shipments are recorded in cost of sales in the Company’s statements of operations. During the year ended December 31, 2014 and 2013, the Company recorded a loss of $5,840 and $10,830, respectively due to management’s estimation of obsolete inventory.
Fair Value Measurements
The Company adopted the provisions of ASC Topic 820, “Fair Value Measurements and Disclosures”, which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments. The carrying amounts of our short and long term credit obligations approximate fair value because the effective yields on these obligations, which include contractual interest rates, are comparable to rates of returns for instruments of similar credit risk.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 — inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
The Company does not have any assets or liabilities measured at fair value on a recurring basis.
Stock-based compensation
The Company recognizes compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee stock-based awards, the fair value of the award is calculated on the date of grant using the Black-Scholes method for stock options and the quoted price of our common stock for common shares; the expense is recognized over the service period for awards expected to vest. For non-employee stock-based awards, the fair value of the award on the date of grant is calculated in the same manner as employee awards. However, the awards are revalued at the end of each reporting period and the pro rata compensation expense is adjusted accordingly until such time the nonemployee award is fully vested, at which time the total compensation recognized to date equals the fair value of the stock-based award as calculated on the measurement date, which is the date at which the award recipient’s performance is complete. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from original estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised.
| F-8 |
Advertising
Advertising and marketing expenses are charged to operations as incurred.
Income Taxes
The company uses the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company has no material uncertain tax positions for any of the reporting periods presented.
NOTE 3 – Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has generated minimal revenues since inception. The Company has sustained losses since inception, and has a stockholders’ deficiency of $199,311 at December 31, 2014. These factors among others raise substantial doubt about the ability of the Company to continue as a going concern for a reasonable period of time.
The continuing operations of the Company are dependent upon its ability to continue to raise adequate financing and to commence profitable operations in the future and repay its liabilities arising from normal business operations as they become due. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 4 – Stockholders’ Equity
The Company has authorized 100,000,000 shares of common stock $.001 par value and 5,000,000 shares of preferred stock $.001 par value.
On October 31, 2014, the board of directors approved an amendment to the Company’s Certificate of Incorporation, as amended, to effect a 2-for-1 stock split on the issued and outstanding common shares. All relevant information relating to numbers of shares and per share information have been retrospectively adjusted to reflect the stock split for all periods presented.
| F-9 |
During the year ended December 31, 2013, the Company issued 2,750,000 shares of common stock for services rendered which were valued at $275,000. The Company valued these shares based on the per share price in which unaffiliated investors purchased shares of common stock in a private placement. Of the amount referred to above, $70,000 was recorded as a prepaid expense and amortized over the life of the specific agreement. During the year ended December 31, 2014 and 2013, $49,583 and $20,417, respectively of this amount was amortized and recorded in the statement of operations.
During the year ended December 31, 2014, the Company issued 150,000 shares of common stock in a private placement for proceeds of $15,000.
During the year ended December 31, 2014, the Company issued 400,000 shares of common stock for services rendered which were valued at $40,000. The Company valued these shares based on the per share price in which unaffiliated investors purchased shares of common stock in the private placement referred to above.
NOTE 5 – Related Party
From inception through December 31, 2014, the Company borrowed an aggregate of $76,385 from two of its shareholders. The amounts were recorded as a loan payable to related parties on the accompanying balance sheet. These loans are non-interest bearing and due on demand. The balance on these notes at December 31, 2014 and 2013 were $35,084 and $25,505, respectively.
One of the Company’s founders has made loan the Company through direct charges on the founders’ credit card to pay for Company expenses. The balance of this loan at December 31, 2014 and 2013 was $9,827 and $14,794, respectively. The founder is charging the Company 15.5% interest on the unpaid monthly balances associated with this loan.
The above loans are classified on the accompanying balance sheet as loans payable – related party.
In August, 2013 the Company converted certain loans payable referred to above and accrued salary and expenses to the shareholders in the aggregate amount of $82,499 into secured notes payable to the shareholders. The notes bear interest at 3% per annum, are secured by substantially all assets of the Company, and are due on demand. The balance on the notes at December 31, 2014 and 2013 were $49,200 and $67,499, respectively.
Accrued interest on the above debts to related parties aggregated $4,877 and $1,378 at December 31, 2014 and 2013, respectively.
The Company’s board of directors approved a salary to the Company’s president in the amount of $52,000 per annum plus a car allowance of $600 per month. As of December 31, 2014 and 2013 the amount unpaid and accrued amounted to$78,000 and $25,000, respectively, which is reflected on the accompanying Balance sheet at that date. Effective in August 2013, the president waived his car allowance which amount has not been accrued since that date.
NOTE 6 – Note payable
On January 10, 2014, the Company entered into a note payable to an unrelated party that bears interest at 3% per annum and is payable upon a funding event of at least $50,000 to the Company or 9 months after the date of issuance, whichever comes first. The maturity date has passed and the lender has verbally agreed to extend the note, which is now considered due on demand.
| F-10 |
NOTE 6 – Credit card payable
The Company utilizes a credit card for working capital purposes. The card has a credit limit of $9,000, $8,695 and $8,273 outstanding at December 31, 2014 and 2013, respectively and bears interest at 21.24%.
NOTE 7 – Income Taxes
The reconciliation of income tax benefit at the U.S. statutory rate of 34% for the years ended December 31, 2013 and 2012 to the Company’s effective tax rate is as follows:
| Years Ended | ||||||||
| December
31, 2014 | December
31, 2013 | |||||||
| U.S. federal statutory rate | (34 | )% | (34 | )% | ||||
| State income tax, net of federal benefit | (6 | )% | (6 | )% | ||||
| Change in valuation allowance | (40 | )% | (40 | )% | ||||
| Income Tax provision (benefit) | 0 | % | 0 | % | ||||
The tax effects of temporary differences that give rise to the Company’s net deferred tax liability as of December 31, 2014 and 2013 are as follows:
| Years Ended | ||||||||
| December 31, 2014 | December 31, 2013 | |||||||
| Deferred Tax Assets | ||||||||
| Net operating losses | $ | 306,000 | $ | 230,000 | ||||
| Less: Valuation allowance | (306,000 | ) | (230,000 | ) | ||||
| $ | — | $ | — | |||||
As of December 31, 2014 and 2013, the Company had approximately $765,000 and $574,000 of federal and state net operating loss carryovers (“NOLs”) which begin to expire in 2031. Utilization of the NOLs may be subject to limitation under the Internal Revenue Code Section 382 should there be a greater than 50% ownership change as determined under regulations.
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. Based on the assessment, management has established a full valuation allowance against the entire deferred tax asset relating to NOLs for every period because it is more likely than not that all of the deferred tax asset will not be realized.
| F-11 |
The Company files U.S. federal and state of Florida tax returns that are subject to audit by tax authorities beginning with the year ended December 31, 2011. The Company’s policy is to classify assessments, if any, for tax and related interest and penalties as tax expense.
NOTE 8 – Subsequent events
Management has evaluated subsequent events through September 1, 2015, the date which the financial statements were available to be issued.
| F-12 |
| HEALTH-RIGHT DISCOVERIES, INC. | |||||
| BALANCE SHEETS | |||||
| (Unaudited) |
| June 30, 2015 | December 31, 2014 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash | $ | 748 | $ | 609 | ||||
| Inventories | 4,665 | 1,213 | ||||||
| Total Current Assets | 5,413 | 1,822 | ||||||
| TOTAL ASSETS | $ | 5,413 | $ | 1,822 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Credit card payable | $ | 8,817 | $ | 8,695 | ||||
| Loans payable - related parties | 48,083 | 44,911 | ||||||
| Notes payable - related parties | 48,850 | 49,200 | ||||||
| Accrued interest - related parties | 6,122 | 4,877 | ||||||
| Accrued interest - other | 675 | 450 | ||||||
| Salaries payable - related party | 104,000 | 78,000 | ||||||
| Note payable | 15,000 | 15,000 | ||||||
| Total Current Liabilities | 231,547 | 201,133 | ||||||
| STOCKHOLDERS’ DEFICIENCY | ||||||||
| Preferred Stock, .001 par value, 5,000,000 shares authorized No shares issued and outstanding June 30, 2015 and December 31, 2014 | ||||||||
| Common Stock, .001 par value, 100,000,000 shares authorized 17,133,332 and 16,573,332 shares issued and outstanding December 31, 2014 and 2013, respectively | 17,133 | 17,123 | ||||||
| Additional Paid in Capital | 550,117 | 549,127 | ||||||
| Accumulated Deficit | (793,384 | ) | (765,561 | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIENCY | (226,134 | ) | (199,311 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIENCY | $ | 5,413 | $ | 1,822 | ||||
| The accompanying notes are an integral part of these financial statements |
| F-13 |
HEALTH-RIGHT DISCOVERIES, INC.
(Unaudited)
| Six Months Ended June 30 | Three Months Ended June 30 | |||||||||||||||
| 2015 | 2014 | 2015 | 2014 | |||||||||||||
| Sales | 13,581 | 26,199 | 5,887 | 11,849 | ||||||||||||
| Cost of Sales | 2,664 | 4,380 | 1,047 | 2,120 | ||||||||||||
| Gross Profit | 10,917 | 21,819 | 4,840 | 9,729 | ||||||||||||
| COSTS AND EXPENSES: | ||||||||||||||||
| General and administrative | 36,355 | 74,488 | 18,156 | 32,753 | ||||||||||||
| Interest expense - related parties | 2,385 | 1,002 | 685 | 490 | ||||||||||||
| Total Cost and expenses | 38,740 | 75,490 | 18,841 | 33,243 | ||||||||||||
| Loss before income tax provision | (27,823 | ) | (53,671 | ) | (14,001 | ) | (23,514 | ) | ||||||||
| Income tax provision | — | — | — | — | ||||||||||||
| NET LOSS | (27,823 | ) | (53,671 | ) | (14,001 | ) | (23,514 | ) | ||||||||
| Loss per common share | $ | (0.00 | ) | $ | (0.00 | ) | $ | (0.00 | ) | $ | (0.00 | ) | ||||
| Weighted average common shares outstanding | 17,128,832 | 16,573,332 | 17,133,332 | 16,573,332 | ||||||||||||
| The accompanying notes are an integral part of these financial statements |
| F-14 |
| HEALTH-RIGHT DISCOVERIES, INC. | |||||
| STATEMENTS OF CASH FLOWS | |||||
| (Unaudited) |
| Six months ended June 30 | ||||||||
| 2015 | 2014 | |||||||
| OPERATING ACTIVITIES: | ||||||||
| Net loss | $ | (27,823 | ) | $ | (53,671 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock based compensation | 1,000 | — | ||||||
| Accrued salary to related party | 26,000 | 26,000 | ||||||
| Accrued interest | 1,470 | 939 | ||||||
| Changes in operating assets and liabilities | ||||||||
| Inventories | (3,452 | ) | 1,354 | |||||
| Credit card payable | 122 | 2,419 | ||||||
| NET CASH USED IN OPERATING ACTIVITIES | (2,683 | ) | (22,959 | ) | ||||
| FINANCING ACTIVITIES: | ||||||||
| Proceeds of loan from related parties | 7,500 | 12,720 | ||||||
| Proceeds from note payable | — | 15,000 | ||||||
| Repayment of related party loan | (4,678 | ) | (24,000 | ) | ||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 2,822 | 3,720 | ||||||
| INCREASE (DECREASE) IN CASH | 139 | (19,239 | ) | |||||
| CASH - BEGINNING OF YEAR | 609 | 21,792 | ||||||
| CASH - END OF YEAR | $ | 748 | $ | 2,553 | ||||
| $ | — | — | ||||||
The accompanying notes are an integral part of these financial statements
| F-15 |
HEALTH-RIGHT DISCOVERIES, INC.
June 30, 2015
(Unaudited)
NOTE 1 – Business
Health-Right Discoveries, Inc. (“the Company”) was formed under the laws of the State of Florida on October 12, 2011 under the name Four Plex Partners, Inc. The company changed its name to Health-Right Discoveries, Inc. on March 22, 2012. The Company’s business is to develop and market an innovative portfolio of both prescription nutritional, OTC monograph and natural products that primarily focus on factors relating to stress-induced conditions and diseases. The Company believes it has developed a formulation platform to vastly improve the negative interrelationship between stress, a weakened immune system and certain stress-related conditions and diseases.
NOTE 2 - Summary of Significant Accounting Policies
Basis of Presentation
The accompanying unaudited financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial statements and Article 10 of Regulation S-X of the United States Securities and Exchange Commission (“SEC”). Accordingly, they do not contain all information and footnotes required by accounting principles generally accepted in the United States of America for annual financial statements. In the opinion of the Company’s management, the accompanying unaudited financial statements contain all the adjustments necessary (consisting only of normal recurring accruals) to present the financial position of the Company as of June 30, 2015 and the results of operations and cash flows for the periods presented. The results of operations for the six and three months ended June 30, 2015 are not necessarily indicative of the operating results for the full fiscal year or any future period. These unaudited financial statements should be read in conjunction with the financial statements for the year ended December 31, 2014, and related notes thereto included in the elsewhere in this filing.
Use of Estimates
The preparation of the financial statements in conformity with Generally Accepted Accounting Principles (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements and the reported amounts of revenue and expenses during the reporting periods. Actual results could differ from those estimates. Certain of the Company’s estimates could be affected by external conditions, including those unique to its industry, and general economic conditions. It is possible that these external factors could have an effect on the Company’s estimates that could cause actual results to differ from its estimates. The Company re-evaluates all of its accounting estimates at least quarterly based on these conditions and record adjustments when necessary.
| F-16 |
Cash
The Company considers all short-term highly liquid investments with an original maturity at the date of purchase of three months or less to be cash equivalents.
Revenue Recognition
The Company follows the guidance of the Accounting Standards Codification (“ASC”) Topic 605, “Revenue Recognition”. We record revenue when persuasive evidence of an arrangement exists, product delivery has occurred, the selling price to the customer is fixed or determinable and collectability of the revenue is reasonably assured. The Company has not experienced any significant returns from customers and accordingly, in management’s opinion, no reserve for returns has been provided.
Inventories
Inventories, which consist of the Company’s product held for resale, are stated at the lower of cost, determined using the first-in, first-out, and net realizable value. Net realizable value is the estimated selling price, in the ordinary course of business, less estimated costs to complete and dispose of the product.
If the Company identifies excess, obsolete or unsalable items, its inventories are written down to their realizable value in the period in which the impairment is first identified. Shipping and handling costs incurred for inventory purchases and product shipments are recorded in cost of sales in the Company’s statements of operations.
Fair Value Measurements
The Company adopted the provisions of ASC Topic 820, “Fair Value Measurements and Disclosures”, which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents, accounts receivable, accounts payable and accrued expenses are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments. The carrying amounts of our short and long term credit obligations approximate fair value because the effective yields on these obligations, which include contractual interest rates, are comparable to rates of returns for instruments of similar credit risk.
ASC 820 defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
| F-17 |
Level 3 — inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
The Company does not have any assets or liabilities measured at fair value on a recurring basis.
Stock-based compensation
The Company recognizes compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee stock-based awards, the fair value of the award is calculated on the date of grant using the Black-Scholes method for stock options and the quoted price of our common stock for common shares; the expense is recognized over the service period for awards expected to vest. For non-employee stock-based awards, the fair value of the award on the date of grant is calculated in the same manner as employee awards. However, the awards are revalued at the end of each reporting period and the pro rata compensation expense is adjusted accordingly until such time the nonemployee award is fully vested, at which time the total compensation recognized to date equals the fair value of the stock-based award as calculated on the measurement date, which is the date at which the award recipient’s performance is complete. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from original estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised.
Advertising
Advertising and marketing expenses are charged to operations as incurred.
Income Taxes
The company uses the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company has no material uncertain tax positions for any of the reporting periods presented.
NOTE 3 – Going Concern
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has generated minimal revenues since inception. The Company has sustained losses since inception, and has a stockholders’ deficiency of $226,134 at June 30, 2015. These factors among others raise substantial doubt about the ability of the Company to continue as a going concern for a reasonable period of time.
| F-18 |
The continuing operations of the Company are dependent upon its ability to continue to raise adequate financing and to commence profitable operations in the future and repay its liabilities arising from normal business operations as they become due. The accompanying financial statements do not include any adjustments that might result from the outcome of this uncertainty.
NOTE 4 – Stockholders’ Equity
The Company has authorized 100,000,000 shares of common stock $.001 par value and 5,000,000 shares of preferred stock $.001 par value.
On October 31, 2014, the board of directors approved an amendment to the Company’s Certificate of Incorporation, as amended, to effect a 2-for-1 stock split on the issued and outstanding common shares. All relevant information relating to numbers of shares and per share information have been retrospectively adjusted to reflect the stock split for all periods presented.
In March 2015, the Company issued 10,000 shares of common stock for services rendered which were valued at $1,000. The Company valued these shares based on the per share price in which unaffiliated investors purchased shares of common stock in ae private placement.
NOTE 5 – Related Party
From inception through June 30, 2015, the Company borrowed an aggregate of $83,885 from two of its shareholders. The amounts were recorded as a loan payable to related parties on the accompanying balance sheet. These loans are non-interest bearing and due on demand. The balance on these notes at June 30, 2015 was 42,584
One of the Company’s founders has made loan the Company through direct charges on the founders’ credit card to pay for Company expenses. The balance of this loan at June 30, 2015 was 5,499. The founder is charging the Company 15.5% interest on the unpaid monthly balances associated with this loan.
The above loans a classified on the accompanying balance sheet as loans payable – related party.
In August, 2013 the Company converted certain loans payable referred to above and accrued salary and expenses to the shareholders in the aggregate amount of $82,499 into secured notes payable to the shareholders. The notes bear interest at 3% per annum, are secured by substantially all assets of the Company, and are due on demand. The balance on the notes at June 30, 2015 was 48,850.
Accrued interest on the above debts to related parties aggregated $6,122 and $4,877 at June 30, 2015 and December 31, 2014 respectively.
The Company’s board of directors approved a salary to the Company’s president in the amount of $52,000 per annum plus a car allowance of $600 per month. As June 30, 2015 the amount unpaid and accrued amounted to$104,000, which is reflected on the accompanying Balance sheet at that date. Effective in August 2013, the president waived his car allowance which has not been accrued since that date.
| F-19 |
NOTE 6 – Note payable
On January 10, 2014, the Company entered into a note payable to an unrelated party that bears interest at 3% per annum and is payable upon a funding event of at least $50,000 to the Company or 9 months after issuance, whichever comes first. The maturity date has passed and the lender has verbally agreed to extend the note, which is now considered due on demand.
NOTE 6 – Credit card payable
The Company utilizes a credit card for working capital purposes. The card has a credit limit of $9,000, $8,817 outstanding at June 30, 2015 and bears interest at 21.24%.
NOTE 7 – Income Taxes
As of June 30, 2015, the Company had approximately $793,000 of federal and state net operating loss carryovers (“NOLs”)
which begin to expire in 2031. Utilization of the NOLs may be subject to limitation under the Internal Revenue Code
Section 382 should there be a greater than 50% ownership change as determined under regulations.
In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. Based on the assessment, management has established a full valuation allowance against the entire deferred tax asset relating to NOLs for every period because it is more likely than not that all of the deferred tax asset will not be realized.
The Company files U.S. federal and state of Florida tax returns that are subject to audit by tax authorities beginning with the year ended December 31, 2011. The Company’s policy is to classify assessments, if any, for tax and related interest and penalties as tax expense.
NOTE 8 – Subsequent events
Management has evaluated subsequent events through September 1, 2015, the date which the financial statements were available to be issued.
| F-20 |
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
| Registration Fees | $ | 133.71 | ||
| Transfer Agent Fees | $ | 1,500.00 | ||
| Accounting Fees and Expenses | $ | 6,500.00 | ||
| Legal Fees and Expenses | $ | 25,000.00 | ||
| Miscellaneous Fees and Expenses | $ | 1,500.00 | ||
| Total | $ | 34,633.71 |
All amounts are estimates other than the SEC’s registration fee. We are paying all expenses of the offering listed above. No portion of these expenses will be borne by the selling shareholders. The selling shareholders, however, will pay any other expenses incurred in selling their common stock, including any brokerage commissions or costs of sale.
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS
Our Amended and Restated Articles of Incorporation and bylaws provide for indemnification of our officers and directors to the fullest extent permitted by the Florida Business Corporation Act.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
During the past three years, we effected the following transactions in reliance upon exemptions from registration under the Securities Act, as amended (all share numbers have been adjusted to give retroactive effect to a two-for-one stock split in the form of a stock dividend implemented by the Company on October 31, 2014 pursuant to the exemption from registration afforded by Section 3(a) (9) under the Securities Act):
On June 5, 2013, the Company issued 200,000 shares of common stock to one individual for advisory services.
On October 10, 2013, the Company issued 500,000 shares of common stock to one entity for technology/marketing services.
On October 23, 2013, the Company issued 800,000 and 1,200,000 shares of common stock to James Pande and David Hopkins, respectively, for services rendered to the Company.
On October 23, 2013, the Company issued 50,000 shares of common stock to one individual for legal services.
On July 12, 2014, the Company issued 100,000 shares of common stock to one individual for $10,000.
On September 2, 2014, the Company issued 50,000 shares of common stock to one individual for $5,000.
On October 22, 2014, the Company issued 400,000 shares of common stock to one entity for legal services.
On March 23, 2015, the Company issued 10,000 shares of common stock to one individual for consulting services.
All of the foregoing securities were issued in accordance with the exemption from registration pursuant to Section 4(a) (2) promulgated under the Securities Act, as amended, as the persons receiving such shares having provided the Company with appropriate investment representations.
| II-1 |
ITEM 16. EXHIBITS
Exhibit Number |
Description | |
| 3.1(i) | Amended and Restated Articles of Incorporation* | |
| 3.2 | By-Laws* | |
| 5.1 | Opinion of Gutierrez Bergman Boulris, P.L.L.C.** | |
| 10.1 | 2015 Stock Incentive Plan** | |
| 23.1 | Consent of Paritz and Company, P.A.** | |
| 23.2 | Consent of Gutierrez Bergman Boulris, P.L.L.C. (Included in Exhibit 5.1)** | |
| 24 | Power of Attorney (included in signature page to this registration statement) |
*Previously filed.
**Filed herewith.
ITEM 17. UNDERTAKINGS
The undersigned registrant hereby undertakes:
1. To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement;
(a) to include any prospectus required by Section 10(a) (3) of the Securities Act of 1933;
(b) to reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement; and Notwithstanding the forgoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation From the low or high end of the estimated maximum offering range may be reflected in the form of prospects filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in the volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.; and
(c) to include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in the registration statement.
2. That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered herein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
3. To remove from registration by means of a post-effective amendment any of the securities being registered hereby which remain unsold at the termination of the offering.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to our directors, officers and controlling persons pursuant to the provisions above, or otherwise, we been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by us of expenses incurred or paid by one of our directors, officers, or controlling persons in the successful defense of any action, suit or proceeding, is asserted by one of our directors, officers, or controlling persons in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification is against public policy as expressed in the Securities Act of 1933, and we will be governed by the final adjudication of such issue.
| II-2 |
Each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B of the Securities Act or other than prospectuses filed in reliance on Rule 430A of the Securities Act, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
| II-3 |
SIGNATURES
In accordance with the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and authorized this registration statement to be signed on its behalf by the undersigned, in Aventura, Florida, on October 30, 2015.
| HEALTH-RIGHT DISCOVERIES, INC. | ||
| By: | /s/ David Hopkins | |
| David Hopkins, President, Chief Executive Officer and Chief Financial Officer | ||
| (Principal Executive, Financial and Accounting Officer) | ||
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints David Hopkins as a true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, for each of them and in each name, place and stead, in any and all capacities, to sign any and all pre- or post-effective amendments to this registration statement, and to file the same with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as fully to all intents and purposes as each might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent or his substitute, may lawfully do or cause to be done by virtue hereof. In accordance with the requirements of the Securities Act of 1933, as amended, this registration statement was signed by the following person in the capacities and on the dates stated.
| Signatures | Title(s) | Date | |||
| By: | /s/ David Hopkins |
President and Director |
October 30, 2015 | ||
| David Hopkins | (Principal Executive, Financial and Accounting Officer) | ||||
| By: | /s/ James Pande | Director | October 30, 2015 | ||
| James Pande | |||||
| II-4 |
