Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Revance Therapeutics, Inc. | d47972d8k.htm |
| EX-99.2 - EX-99.2 - Revance Therapeutics, Inc. | d47972dex992.htm |

BELMONT Phase 2 Active Comparator Trial 24-Week Topline Interim Results DAN BROWNE President & CEO OCTOBER 29, 2015 Exhibit 99.1

Forward-Looking Statements/Safe Harbor This presentation contains forward-looking statements, including statements relating to: our RT002 investigational drug product candidate, including but not limited to statements about the current and potential features and benefits, the process and timing of anticipated future clinical development, our business strategy, goal and plan and prospects, timing and outcome of our clinical trials, our ability to obtain regulatory approval, the potential benefits and of our product candidates and our technologies, demand for our product candidates, and drivers of demand. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from our expectations. These risks and uncertainties include, but are not limited to: the outcome, cost and timing of our product development activities and clinical trials; the uncertain clinical development process, including the risk that clinical trials may not have an effective design; our ability to obtain and maintain regulatory approval of our product candidates; our ability to obtain funding for our operations; our plans to research, develop and commercialize our product candidates; our ability to achieve market acceptance of our product candidates; unanticipated costs or delays in research, development and commercialization efforts; the applicability of clinical study results to actual outcomes; the size and growth potential of the markets for our product candidates; our ability to successfully commercialize our product candidates and the timing of commercialization activities; the rate and degree of market acceptance of our product candidates; our ability to develop sales and marketing capabilities; the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for financing; and our ability to continue obtaining and maintaining intellectual property protection for our product candidates. These and other risks are described in the “Risk Factors” section of our Form 10-Q filed with the Securities and Exchange Commission on August 7, 2015. The “Risk Factors” section of 10-Q speaks only as of the date thereof. These forward-looking statements speak only as of the date hereof or the date specified. Revance disclaims any obligation to update these forward-looking statements. “Revance Therapeutics”, TransMTS® and the Revance logo are registered trademarks of Revance Therapeutics, Inc. All other trademarks or registered trademarks are the property of their respective owners. Confidential and Proprietary
BELMONT 24-Week Results Positive Planning to Accelerate RT002 Injectable into Phase 3 in 2H 2016 Confidential and Proprietary *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc. Efficacy & Safety Highlights: As measured by investigators, all three RT002 doses achieved 100% one-point or greater response rates at Week 4 which was maintained through Week 8 Excellent safety profile, appears well-tolerated with no serious adverse events based on interim analysis ZERO eyelid ptosis for 20U and 40U RT002 Excellent risk-to-benefit ratio at all doses Dose response observed - dose selected Plan to engage FDA and accelerate 40U dose into Phase 3 All three doses of RT002 (20U, 40U, 60U) achieved highly statistically significant investigator-reported primary efficacy (p < 0.001) at Week 4 As measured by patients, RT002 achieved at or near 100% response rates for no or mild wrinkles in all dose groups at Week 4
BELMONT 24-Week Results Positive Planning to Accelerate RT002 Injectable into Phase 3 in 2H 2016 Confidential and Proprietary Duration Highlights: Achieved 6-month duration of effect with high responder rates Statistically superior duration to VISTABEL/BOTOX Cosmetic 6-month median duration of at least 1-point improvement as measured by investigators in the 40U dose, with 23.6 weeks vs.18.8 weeks for BOTOX Cosmetic (p=0.020) At 24 Weeks, RT002 at 40U and 60U doses continued to deliver clinically meaningful higher response rates vs. BOTOX Cosmetic 40U dose is statistically superior in duration to BOTOX on all key responder definitions measured by investigators, including at least 1- and 2-point improvement and none or mild wrinkles Consistent Phase 3 results should support labeling of 6 months *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc.

BELMONT Study Design A Phase 2, Randomized, Double-Blind, Dose Ranging, Active and Placebo Controlled, Multi-Center Study to Evaluate the Safety and Efficacy and Duration of Effect of RT002, a Botulinum Toxin Type A for Injection, to Treat Glabellar Lines Confidential and Proprietary Protocol Title Objectives To determine the safety and efficacy of a single treatment of RT002 at three dosage levels for the treatment of glabellar lines versus VISTABEL®/BOTOX® Cosmetic RT002: 20U, 40U, 60U VISTABEL®/BOTOX® Cosmetic: 20U Placebo To assess the duration of effect of a single treatment of RT002 at three dosage levels versus VISTABEL®/BOTOX® Cosmetic *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc.

BELMONT Study Assessments Efficacy evaluations versus baseline Every 4 weeks for up to 36 weeks using Investigator Global Assessment-Facial Wrinkle Severity (IGA-FWS) All subjects were followed for at least 24 weeks Primary Efficacy Assessments ≥ 1-point improvement IGA-FWS Duration of response Risk-to-benefit ratio Secondary Efficacy Assessments Investigator (IGA-FWS) Subject Global Aesthetic Improvement Scale (GAIS) Patient Facial Wrinkle Scale (PFWS) Confidential and Proprietary

Key Efficacy, Safety and Duration Data BELMONT 24-Week Interim Results
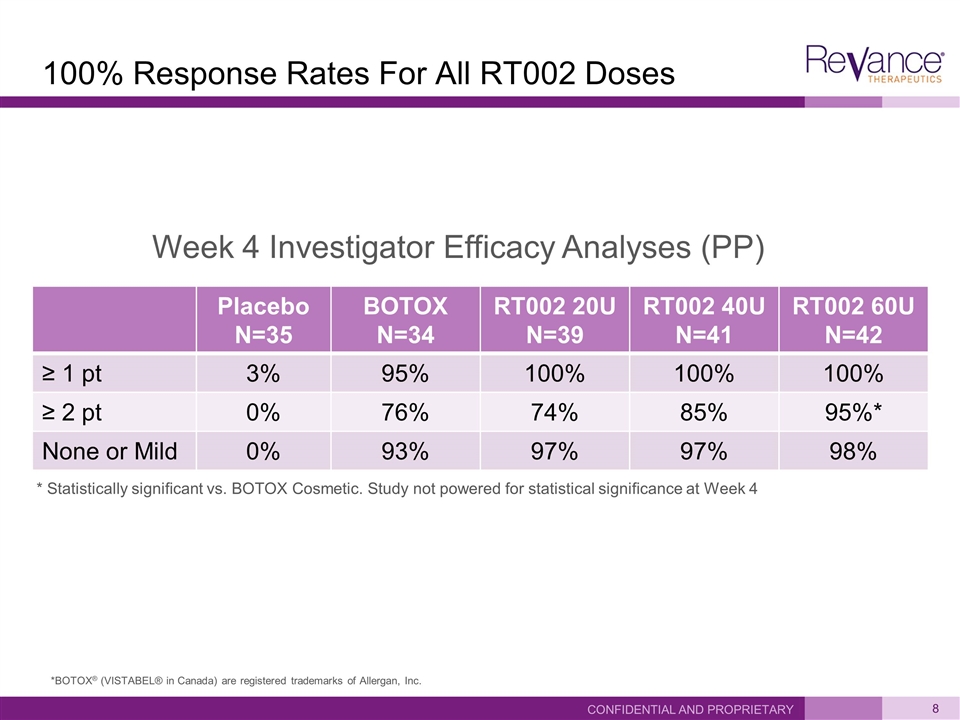
100% Response Rates For All RT002 Doses Placebo N=35 BOTOX N=34 RT002 20U N=39 RT002 40U N=41 RT002 60U N=42 ≥ 1 pt 3% 95% 100% 100% 100% ≥ 2 pt 0% 76% 74% 85% 95%* None or Mild 0% 93% 97% 97% 98% Confidential and Proprietary * Statistically significant vs. BOTOX Cosmetic. Study not powered for statistical significance at Week 4 Week 4 Investigator Efficacy Analyses (PP) *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc.
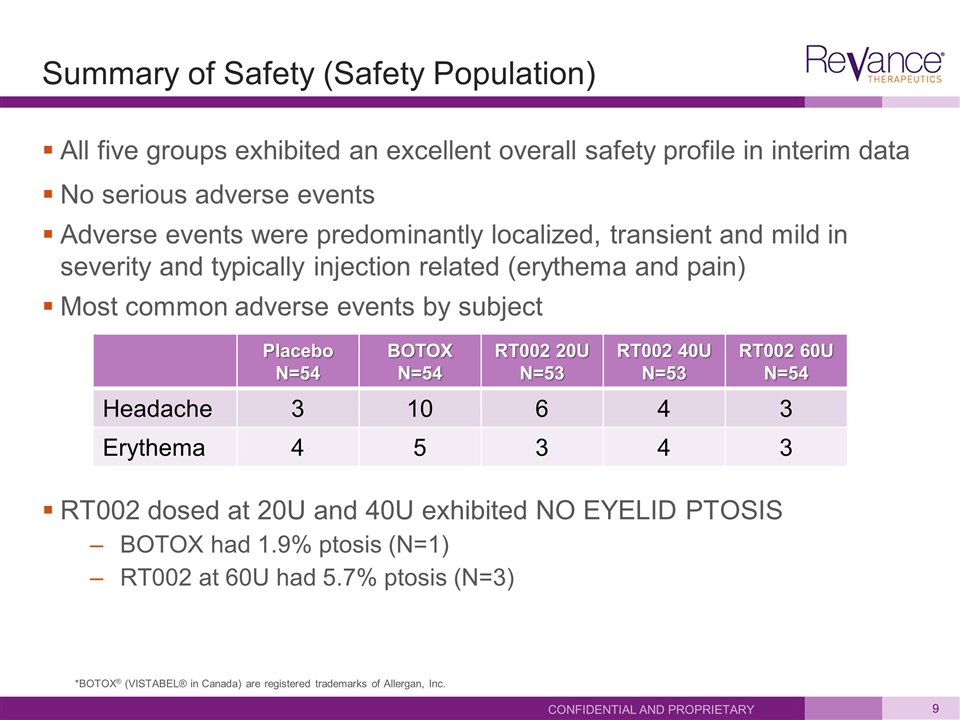
Summary of Safety (Safety Population) All five groups exhibited an excellent overall safety profile in interim data No serious adverse events Adverse events were predominantly localized, transient and mild in severity and typically injection related (erythema and pain) Most common adverse events by subject RT002 dosed at 20U and 40U exhibited NO EYELID PTOSIS BOTOX had 1.9% ptosis (N=1) RT002 at 60U had 5.7% ptosis (N=3) Confidential and Proprietary Placebo N=54 BOTOX N=54 RT002 20U N=53 RT002 40U N=53 RT002 60U N=54 Headache 3 10 6 4 3 Erythema 4 5 3 4 3 *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc.
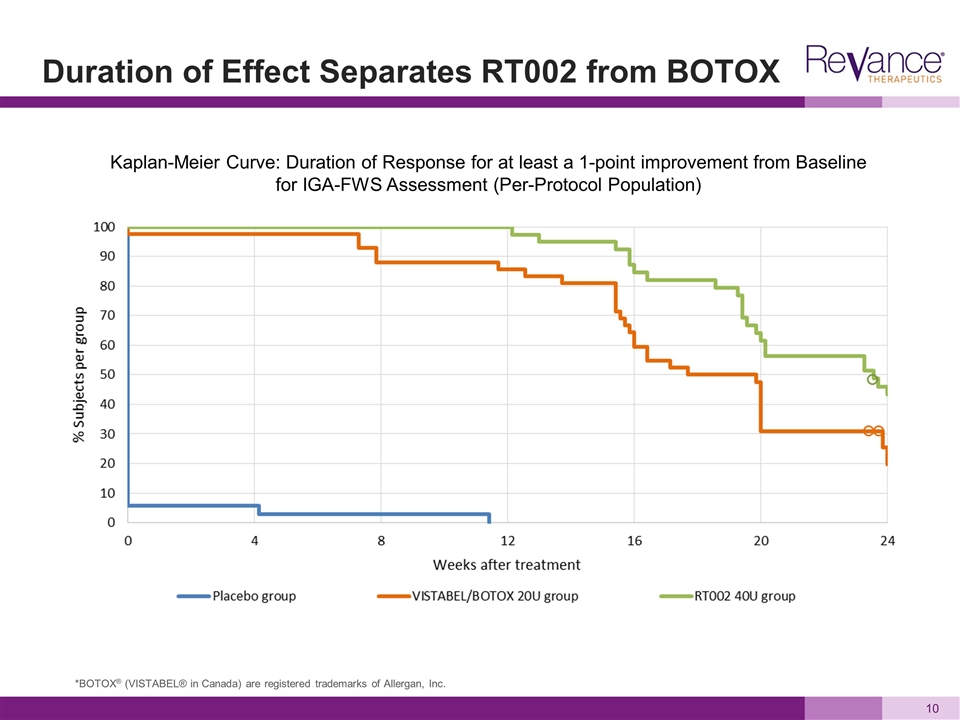
Duration of Effect Separates RT002 from BOTOX Kaplan-Meier Curve: Duration of Response for at least a 1-point improvement from Baseline for IGA-FWS Assessment (Per-Protocol Population) *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc.
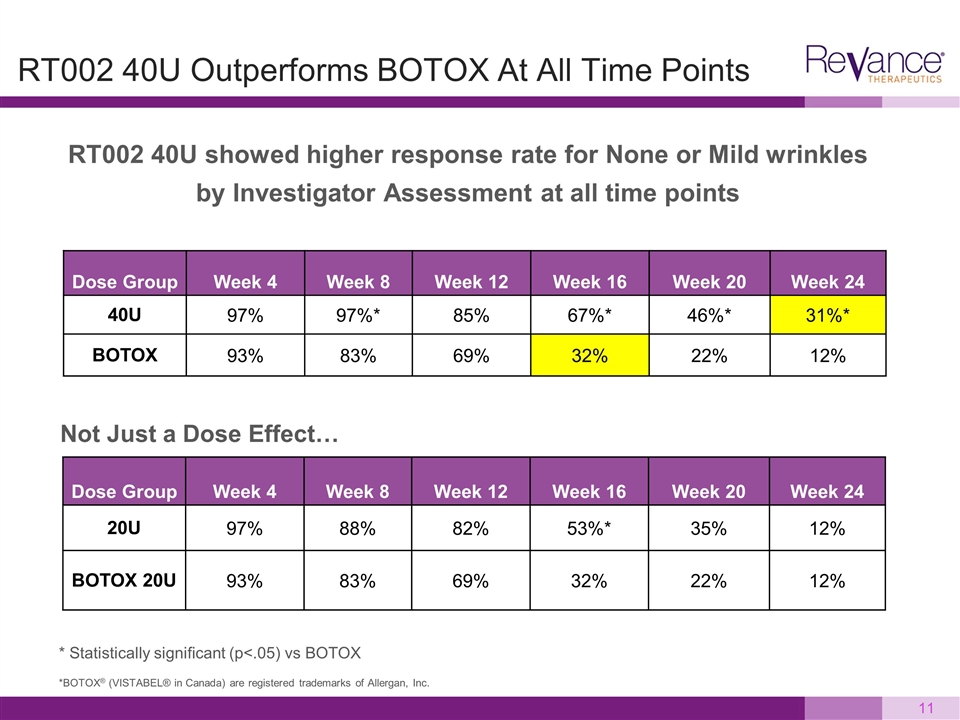
RT002 40U showed higher response rate for None or Mild wrinkles by Investigator Assessment at all time points RT002 40U Outperforms BOTOX At All Time Points Dose Group Week 4 Week 8 Week 12 Week 16 Week 20 Week 24 40U 97% 97%* 85% 67%* 46%* 31%* BOTOX 93% 83% 69% 32% 22% 12% * Statistically significant (p<.05) vs BOTOX *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc. Dose Group Week 4 Week 8 Week 12 Week 16 Week 20 Week 24 20U 97% 88% 82% 53%* 35% 12% BOTOX 20U 93% 83% 69% 32% 22% 12% Not Just a Dose Effect…
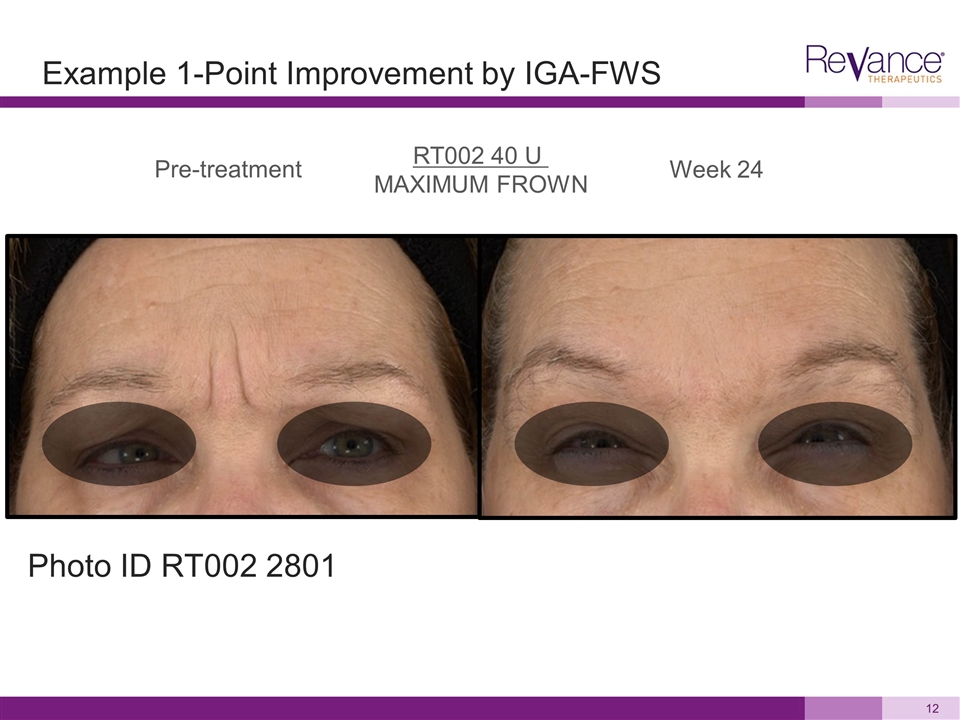
Pre-treatment Week 24 RT002 40 U MAXIMUM FROWN Example 1-Point Improvement by IGA-FWS Photo ID RT002 2801
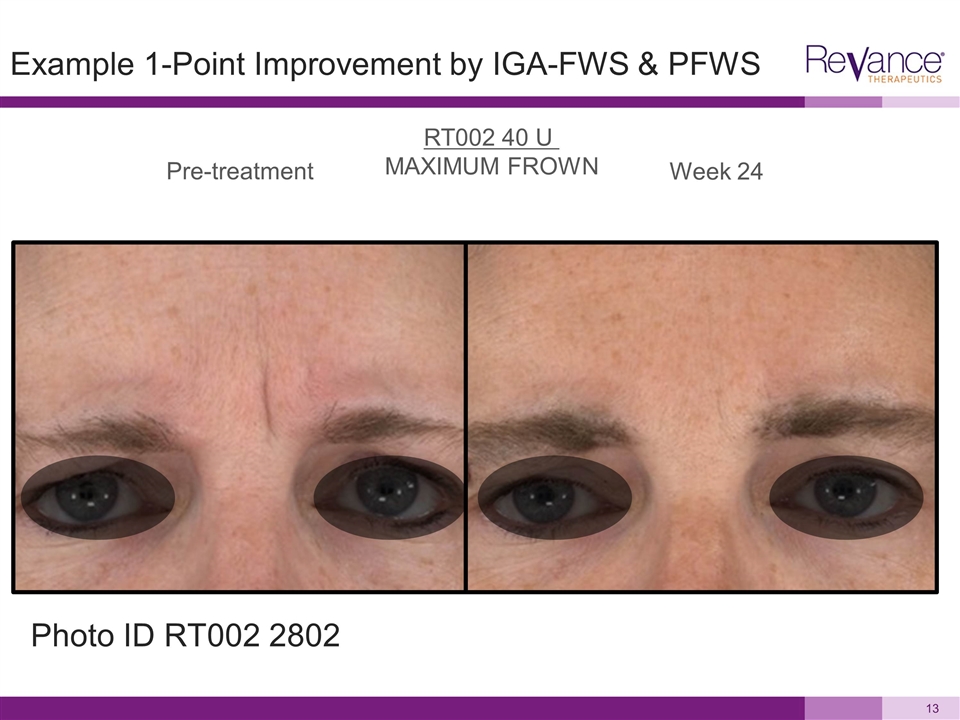
Pre-treatment Week 24 RT002 40 U MAXIMUM FROWN Example 1-Point Improvement by IGA-FWS & PFWS Photo ID RT002 2802
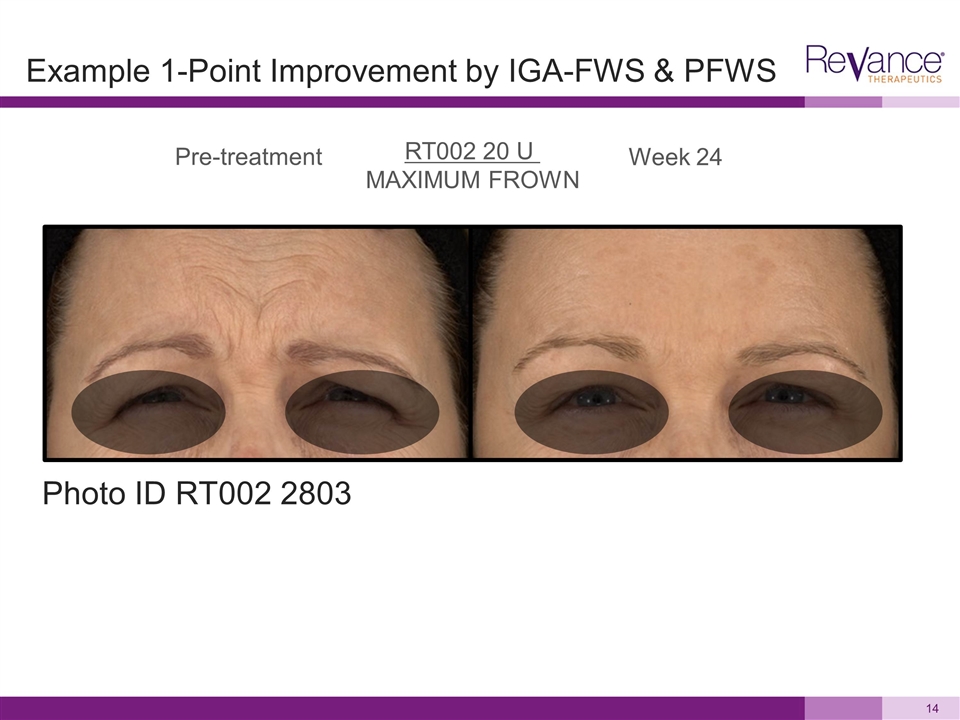
Pre-treatment Week 24 RT002 20 U MAXIMUM FROWN Photo ID RT002 2803 Example 1-Point Improvement by IGA-FWS & PFWS
Conclusions From Topline Interim Analysis Confidential and Proprietary RT002 achieves statistically significant 6-month duration of effect with higher responder rates compared to BOTOX RT002 40U appears well-tolerated with no ptosis. Efficacy was statistically superior at the majority of time points compared to BOTOX. RT002 40U results indicate 31% of subjects maintain none or mild wrinkles compared to BOTOX at 12% at 6 months BELMONT results support selection of the RT002 40U dose to move forward into Phase 3 RT002 achieves 100% investigator and at or near 100% patient response in all doses at the 28-day primary efficacy assessment *BOTOX® (VISTABEL® in Canada) are registered trademarks of Allergan, Inc.
BELMONT Subject Rating of Duration Importance Study included subject questionnaire to determine attitudes around duration of effect: 87% said duration was Somewhat Important, Important or Very Important 79% said duration was Important or Very Important More than half (51%) rated duration the Very Important Confidential and Proprietary
Planned Next Steps for RT002 in Glabellar Lines Plan to complete study and report final results 1H 2016 Expect to complete End-of-Phase 2 meeting with FDA 1H 2016 Anticipate initiating Phase 3 pivotal studies 2H 2016

Transforming the Neurotoxin Market
