Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Tracon Pharmaceuticals, Inc. | a15-21358_18k.htm |
Exhibit 99.1
TRACON PHARMACEUTICALS October 2015 NASDAQ: TCON

This presentation contains statements that are, or may be deemed to be, "forward-looking statements." In some cases these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” "expects,” “plans,” "intends,” “may,” “could,” “might,” “will,” “should,” “approximately,” “potential,” or, in each case, their negatives or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. These statements relate to future events or our future financial performance or condition, business strategy, current and prospective product candidates, planned clinical trials and preclinical activities, product approvals, research and development costs, current and prospective collaborations, timing and likelihood of success, plans and objectives of management for future operations, and future results of anticipated product candidates. These statements involve substantial known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, but are not limited to, risks associated with conducting clinical trials, whether any of our product candidates will be shown to be safe and effective, our ability to finance continued operations, our reliance on third parties for various aspects of our business, competition in our target markets, our ability to protect our intellectual property, and other risks and uncertainties described in our filings with the Securities and Exchange Commission, including under the heading “Risk Factors”. In light of the significant uncertainties in our forward-looking statements, you should not place undue reliance on these statements or regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward-looking statements contained in this presentation represent our estimates and assumptions only as of the date of this presentation and, except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise after the date of this presentation. This presentation also contains estimates, projections and other information concerning our industry, our business, and the markets for our drug candidates, as well as data regarding market research, estimates and forecasts prepared by our management. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances reflected in this information. Forward-Looking Statements

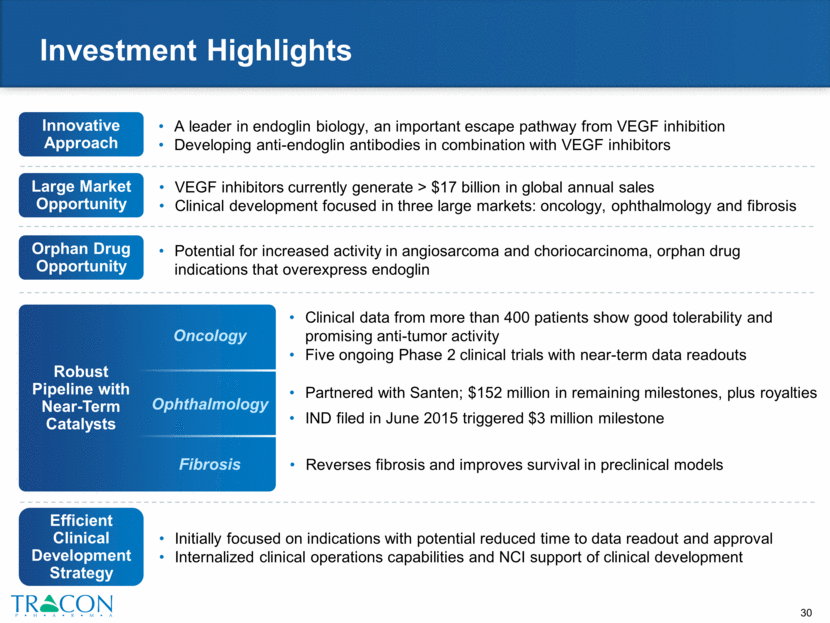
Robust Pipeline with Near-Term Catalysts Innovative Approach Investment Highlights Initially focused on indications with potential reduced time to data readout and approval Internalized clinical operations capabilities and NCI support of clinical development Clinical data from more than 400 patients show good tolerability and promising anti-tumor activity Five ongoing Phase 2 clinical trials with near-term data readouts A leader in endoglin biology, an important escape pathway from VEGF inhibition Developing anti-endoglin antibodies in combination with VEGF inhibitors Oncology Experienced Team VEGF inhibitors currently generate > $17 billion in global annual sales Clinical development focused in three large markets: oncology, ophthalmology and fibrosis Ophthalmology Fibrosis Partnered with Santen; $152 million in remaining milestones, plus royalties IND filed in June 2015 triggered $3 million milestone Reverses fibrosis and improves survival in preclinical models Potential for increased activity in angiosarcoma and choriocarcinoma, orphan drug indications that overexpress endoglin Large Market Opportunity Orphan Drug Opportunity Efficient Clinical Development Strategy
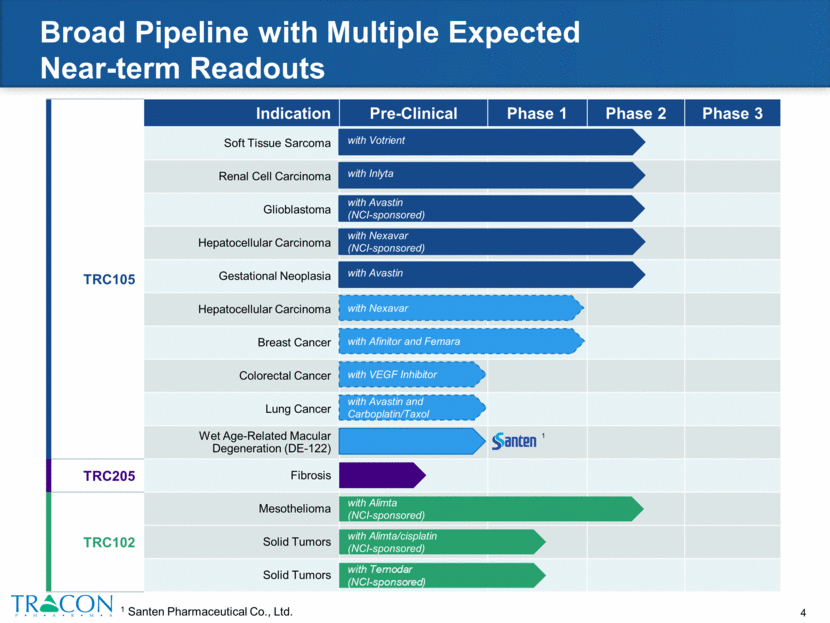
TRC105 Indication Pre-Clinical Phase 1 Phase 2 Phase 3 Soft Tissue Sarcoma Renal Cell Carcinoma Glioblastoma Hepatocellular Carcinoma Gestational Neoplasia Hepatocellular Carcinoma Breast Cancer Colorectal Cancer Lung Cancer Wet Age-Related Macular Degeneration (DE-122) TRC205 Fibrosis TRC102 Mesothelioma Solid Tumors Solid Tumors Broad Pipeline with Multiple Expected Near-term Readouts 1 Santen Pharmaceutical Co., Ltd. with Alimta (NCI-sponsored) with Alimta/cisplatin (NCI-sponsored) with Inlyta with Votrient with Avastin (NCI-sponsored) with Nexavar (NCI-sponsored) with Nexavar with VEGF Inhibitor with Afinitor and Femara with Avastin and Carboplatin/Taxol 1 with Avastin 1
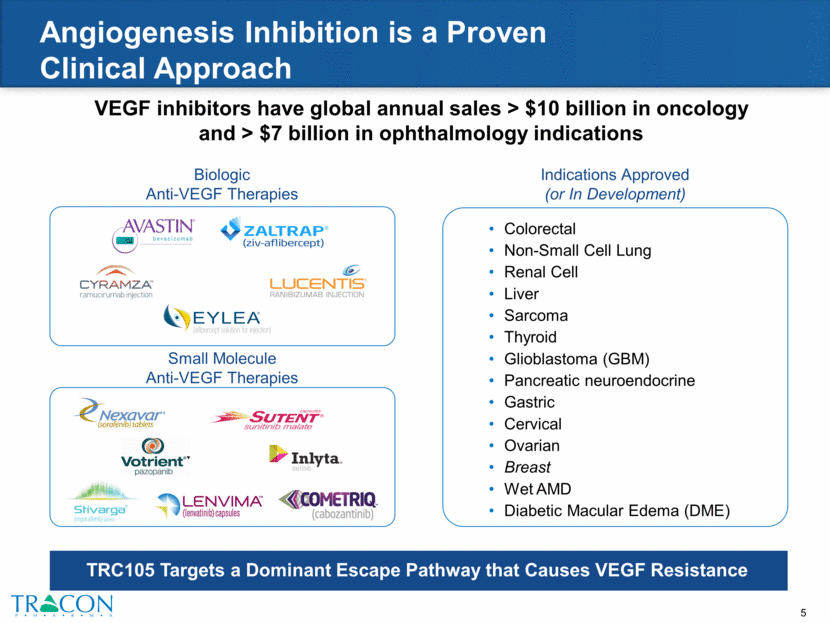
VEGF inhibitors have global annual sales > $10 billion in oncology and > $7 billion in ophthalmology indications Angiogenesis Inhibition is a Proven Clinical Approach Colorectal Non-Small Cell Lung Renal Cell Liver Sarcoma Thyroid Glioblastoma (GBM) Pancreatic neuroendocrine Gastric Cervical Ovarian Breast Wet AMD Diabetic Macular Edema (DME) TRC105 Targets a Dominant Escape Pathway that Causes VEGF Resistance Biologic Anti-VEGF Therapies Small Molecule Anti-VEGF Therapies Indications Approved (or In Development)
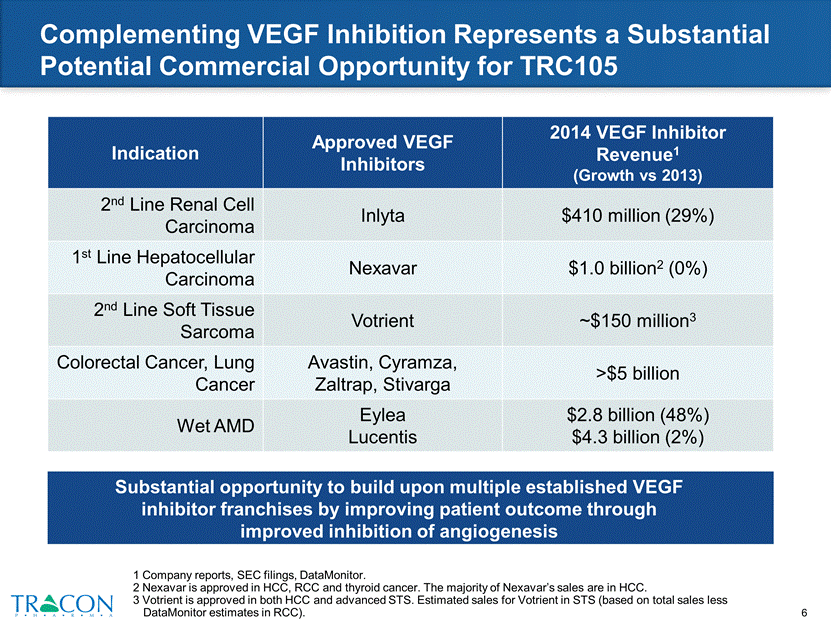
Complementing VEGF Inhibition Represents a Substantial Potential Commercial Opportunity for TRC105 1 Company reports, SEC filings, DataMonitor. 2 Nexavar is approved in HCC, RCC and thyroid cancer. The majority of Nexavar’s sales are in HCC. 3 Votrient is approved in both HCC and advanced STS. Estimated sales for Votrient in STS (based on total sales less DataMonitor estimates in RCC). Indication Approved VEGF Inhibitors 2014 VEGF Inhibitor Revenue1 (Growth vs 2013) 2nd Line Renal Cell Carcinoma Inlyta $410 million (29%) 1st Line Hepatocellular Carcinoma Nexavar $1.0 billion2 (0%) 2nd Line Soft Tissue Sarcoma Votrient ~$150 million3 Colorectal Cancer, Lung Cancer Avastin, Cyramza, Zaltrap, Stivarga >$5 billion Wet AMD Eylea Lucentis $2.8 billion (48%) $4.3 billion (2%) Substantial opportunity to build upon multiple established VEGF inhibitor franchises by improving patient outcome through improved inhibition of angiogenesis
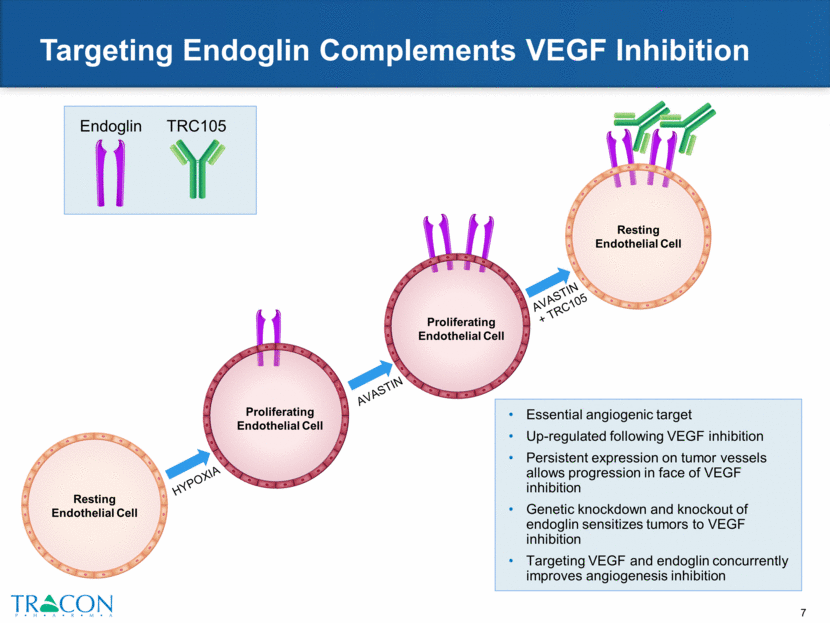
Targeting Endoglin Complements VEGF Inhibition Resting Endothelial Cell Essential angiogenic target Up-regulated following VEGF inhibition Persistent expression on tumor vessels allows progression in face of VEGF inhibition Genetic knockdown and knockout of endoglin sensitizes tumors to VEGF inhibition Targeting VEGF and endoglin concurrently improves angiogenesis inhibition Resting Endothelial Cell TRC105 Endoglin Proliferating Endothelial Cell HYPOXIA AVASTIN AVASTIN + TRC105 Proliferating Endothelial Cell
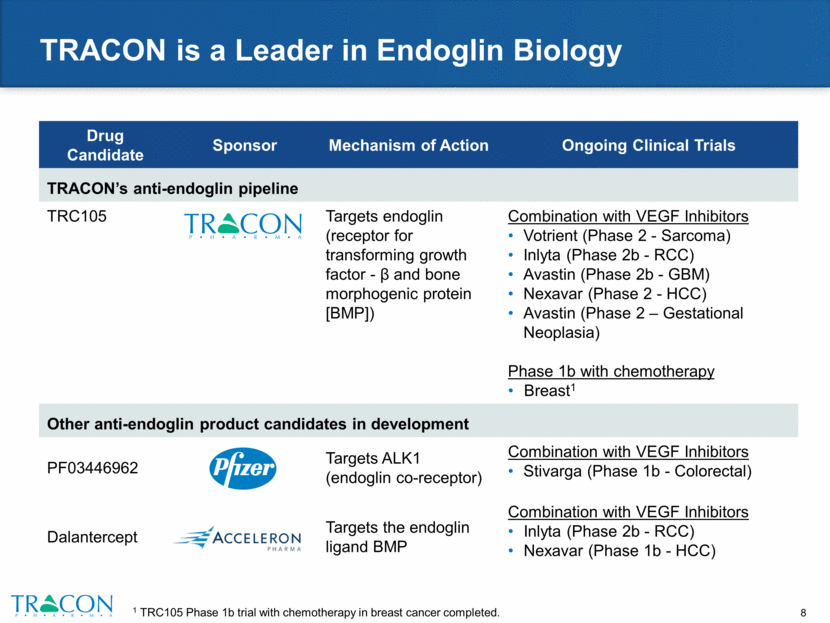
TRACON is a Leader in Endoglin Biology 1 TRC105 Phase 1b trial with chemotherapy in breast cancer completed. Drug Candidate Sponsor Mechanism of Action Ongoing Clinical Trials TRACON’s anti-endoglin pipeline TRC105 Targets endoglin (receptor for transforming growth factor - and bone morphogenic protein [BMP]) Combination with VEGF Inhibitors Votrient (Phase 2 - Sarcoma) Inlyta (Phase 2b - RCC) Avastin (Phase 2b - GBM) Nexavar (Phase 2 - HCC) Avastin (Phase 2 – Gestational Neoplasia) Phase 1b with chemotherapy Breast1 Other anti-endoglin product candidates in development PF03446962 Targets ALK1 (endoglin co-receptor) Combination with VEGF Inhibitors Stivarga (Phase 1b - Colorectal) Dalantercept Targets the endoglin ligand BMP Combination with VEGF Inhibitors Inlyta (Phase 2b - RCC) Nexavar (Phase 1b - HCC)
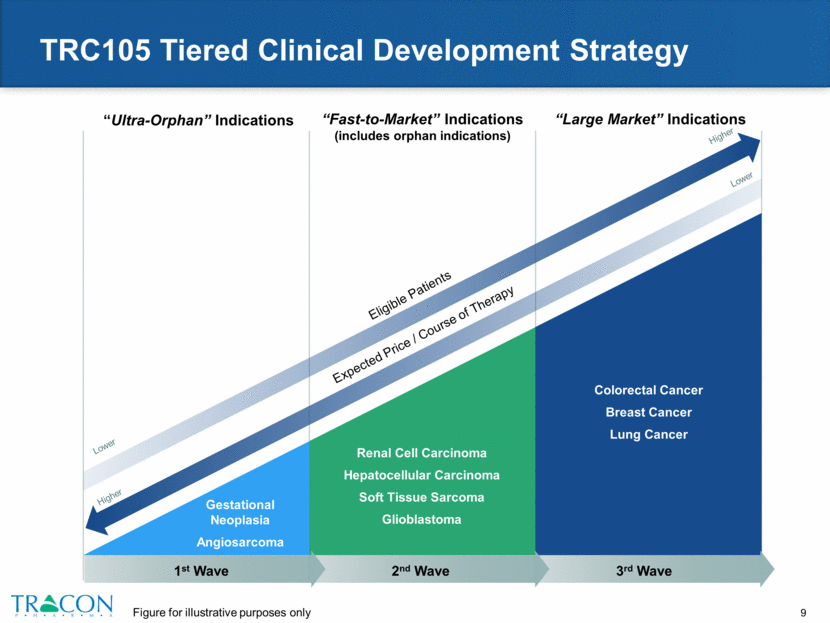
TRC105 Tiered Clinical Development Strategy “Ultra-Orphan” Indications “Fast-to-Market” Indications (includes orphan indications) “Large Market” Indications Expected Price / Course of Therapy Higher Lower Lower Higher Eligible Patients Gestational Neoplasia Angiosarcoma Renal Cell Carcinoma Hepatocellular Carcinoma Soft Tissue Sarcoma Glioblastoma Colorectal Cancer Breast Cancer Lung Cancer 3rd Wave 1st Wave 2nd Wave Figure for illustrative purposes only
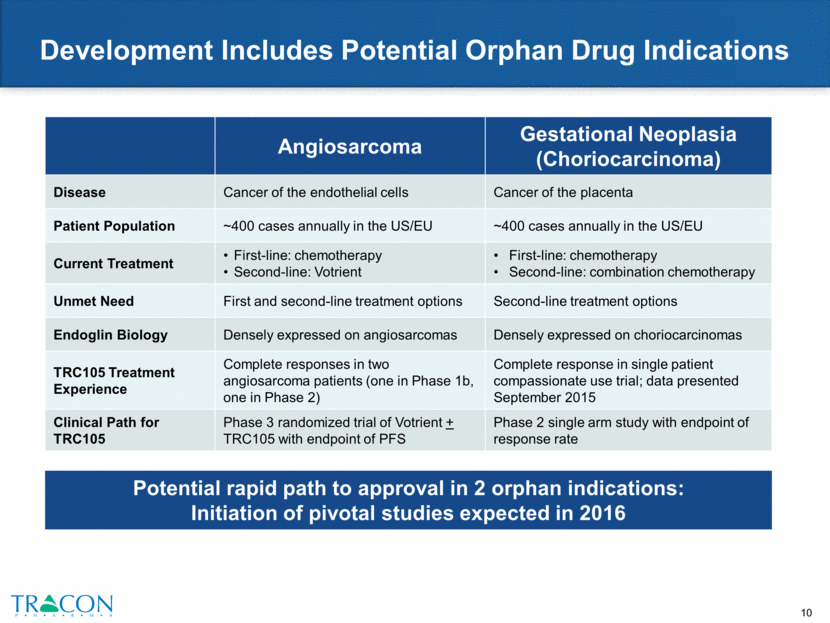
Development Includes Potential Orphan Drug Indications Angiosarcoma Gestational Neoplasia (Choriocarcinoma) Disease Cancer of the endothelial cells Cancer of the placenta Patient Population ~400 cases annually in the US/EU ~400 cases annually in the US/EU Current Treatment First-line: chemotherapy Second-line: Votrient First-line: chemotherapy Second-line: combination chemotherapy Unmet Need First and second-line treatment options Second-line treatment options Endoglin Biology Densely expressed on angiosarcomas Densely expressed on choriocarcinomas TRC105 Treatment Experience Complete responses in two angiosarcoma patients (one in Phase 1b, one in Phase 2) Complete response in single patient compassionate use trial; data presented September 2015 Clinical Path for TRC105 Phase 3 randomized trial of Votrient + TRC105 with endpoint of PFS Phase 2 single arm study with endpoint of response rate Potential rapid path to approval in 2 orphan indications: Initiation of pivotal studies expected in 2016
TRC105 Phase 1 Clinical Trial Summary Safety: The Maximum Tolerated Dose was 10 mg/kg weekly or 15 mg/kg every other week On target effects included signs seen in Osler-Weber-Rendu syndrome, a syndrome associated with superior cancer survival, and anemia No hypertension, heart attack, stroke, fistula, pulmonary bleeding, impaired wound-healing, redness or soreness of the palms, or kidney dysfunction No anti-drug antibodies detected to TRC105 Pharmacokinetics: At 10 mg/kg weekly and 15 mg/kg every two weeks, concentrations of TRC105 needed to saturate target receptors were maintained continuously Efficacy: Reduced tumor burden, tumor markers, and symptoms were achieved in patients with advanced refractory cancer (e.g., sarcoma and prostate cancer) Effects on angiogenic biomarkers
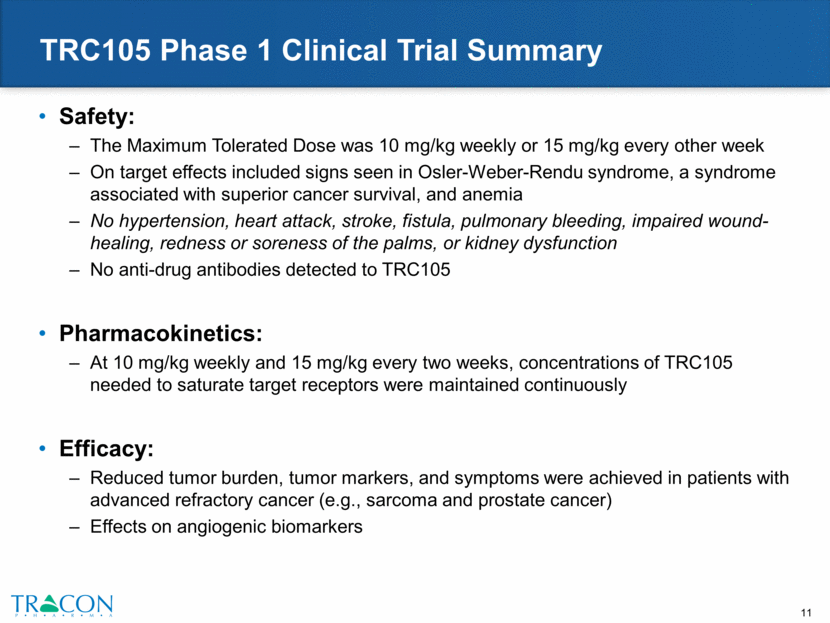
Well Tolerated with Multiple VEGF inhibitors with Evidence of Clinical Activity Combination Well Tolerated Signs of Activity in Phase 1b/2 Ongoing Development TRC105 + Avastin Tumor reductions in Avastin-refractory patients; complete durable response in choriocarcinoma patient Randomized Phase 2 trial in GBM; global Phase 2 trial planned in gestational neoplasia TRC105 + Inlyta PFS of 11.3 mos. and ORR of 29% in clear cell RCC exceeded reported Inlyta1 PFS of 4.8 mos. and ORR of 11% Randomized Phase 2 trial in clear cell RCC TRC105 + Nexavar ORR of 40% at top dose levels of TRC105 in HCC exceeded reported Nexavar2 ORR of 2% Phase 2 trial of TRC105 + Nexavar in HCC TRC105 + Votrient Complete durable responses in angiosarcoma Randomized global Phase 3 trial in angiosarcoma planned for 2016 1 Inlyta results from separate Inlyta Phase 3 AXIS trial following VEGFR treatment. Inlyta results from head-to-head comparison in same clinical trial may differ. 2 Nexavar results from separate Phase 3 SHARP trial. Nexavar results from head-to-head comparison in same clinical trial may differ.
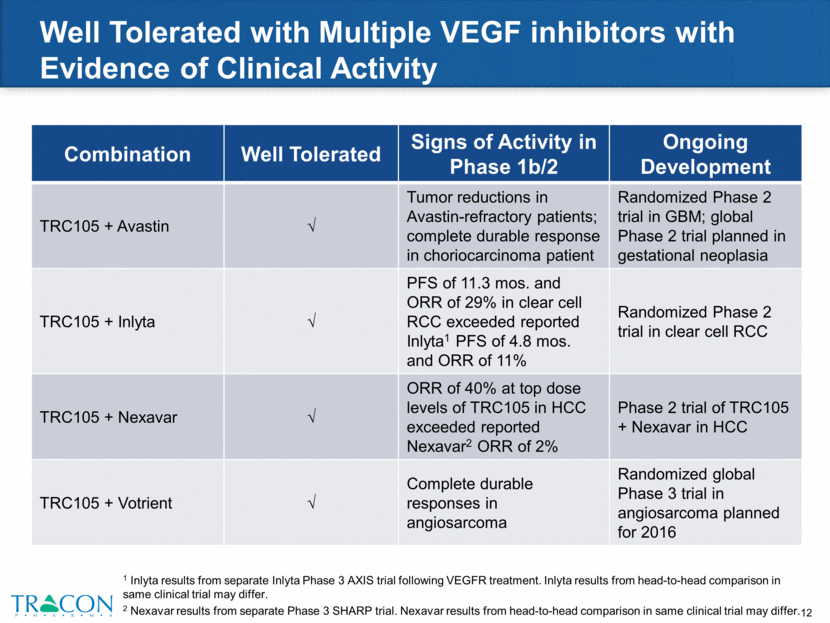
TRC105 + Avastin Demonstrated Impressive Results in a VEGF-Refractory Population 1 Patients with reductions in measurable disease on TRC105 and Avastin with longer duration of treatment than the most recent prior VEGF inhibitor treatment. 2 Patient achieved stable disease. Maximum percentage change in target lesion size in cancer patients treated with TRC105 and Avastin Best Overall Response Progressive Disease Durable Partial Responders by Choi Criteria1 Stable Disease Prior VEGF inhibitor therapy Partial Response by Choi Criteria by either investigator assessment or per independent review 2 (60%) (50%) (40%) (30%) (20%) (10%) 0% 10% 20% 30% 40% 50% 60% Maximum % Change in Target Lesions
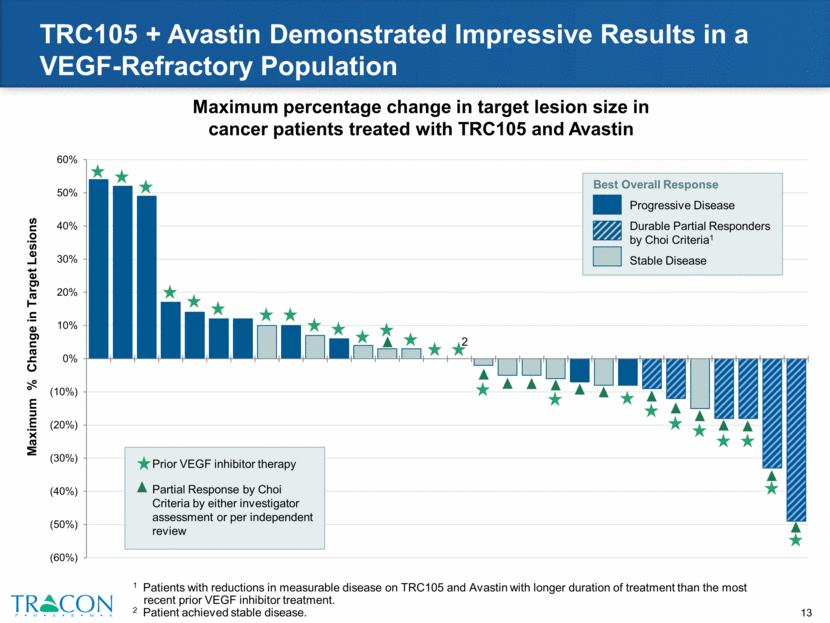
Summary of TRC105 + Avastin Response Duration Improvement in response duration in patients with significant tumor reductions on TRC105 + Avastin and who remained without cancer progression for longer than during prior VEGF therapy 6 prior cancer regimens (1 w/Avastin) 5 prior cancer regimens (1 w/VEGFR TKI) 3 prior cancer regimens (2 w/Avastin) 7 prior cancer regimens (4 w/Avastin) 8 prior cancer regimens (1 w/Avastin) 2 prior cancer regimens (1 w/Avastin) Weeks on Treatment 18 4 5 21 20 10 23 23 123 23 31 32 Ovarian (56, F) Colorectal (66, F) Colorectal (53, M) Colorectal (55, M) Ovarian (71, F) Ovarian (81, F) Duration on TRC105 + Avastin Tx Duration on Last Prior VEGF Tx
TRC105 + Avastin in Gestational Neoplasia A 37 year old woman with widely metastatic choriocarcinoma who progressed following five chemotherapeutic regimens and stem cell transplant developed a complete response to treatment with TRC105 + Avastin, following four months of treatment, that is ongoing at month seven Global Phase 2 study in gestational neoplasia, including choriocarcinoma, is planned with response rate as the primary endpoint

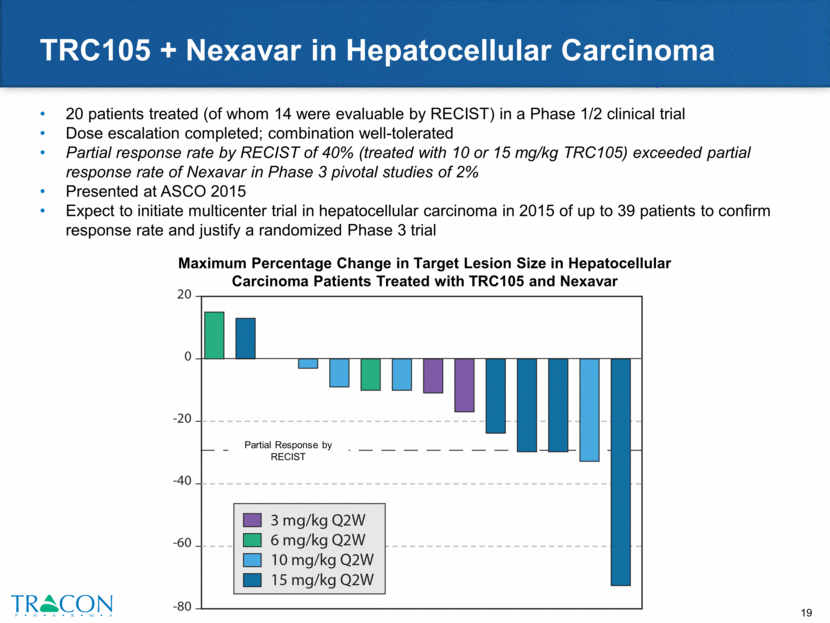
TRC105 + Inlyta in Renal Cell Carcinoma 18 patients treated in a Phase 1b clinical trial who failed at least one VEGF inhibitor Dose escalation completed; combination well-tolerated Partial response rate by RECIST of 29% (4 of which were in the fourth line setting) exceeded partial response rate of Inlyta following VEGFR TKI treatment in the Inlyta Phase 3 AXIS trial of 11% Improved activity in clear cell (8 of 12 patients with Choi responses, including 4 RECIST PRs) Median PFS in clear cell RCC of 11.3 months by Kaplan-Meier exceeded PFS of Inlyta following VEGFR TKI treatment in the Inlyta Phase 3 AXIS trial of 4.8 months Presented at GU ASCO 2015 Maximum Percentage Change in Target Lesion Size in Renal Cell Carcinoma Patients Treated with TRC105 and Inlyta Best Response (% From Baseline) Stable/Progressive Disease Partial Responders by Choi Criteria Patient who progressed on Inlyta immediately prior to study entry Ongoing treatment Progressive Disease by RECIST Partial Response by RECIST PFS = 8.4 months (all RCC histologies) PFS = 11.3 months (clear cell RCC) Best Response (n=17) Partial Response (PR) 5 Stable Disease (SD) 10 Progressive Disease (PD) 2
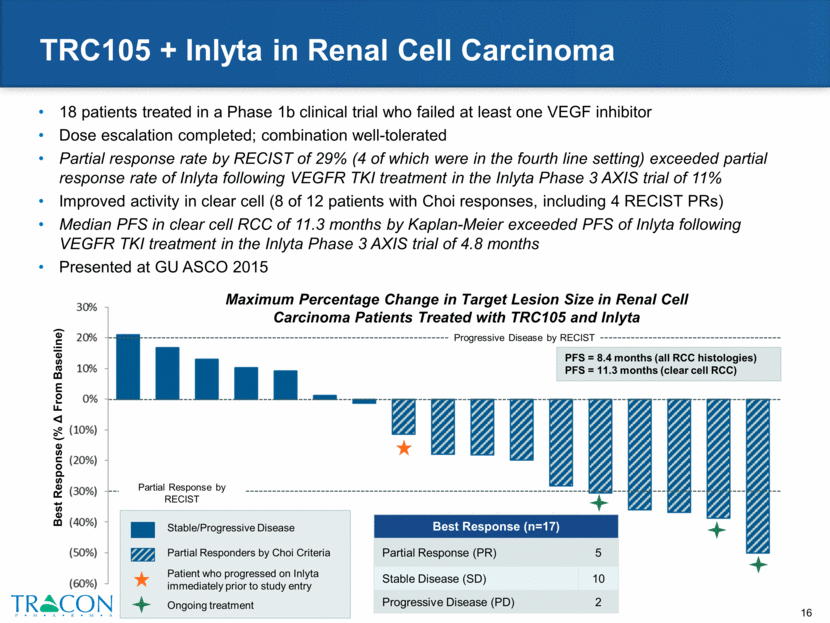
Ongoing Phase 2 Multicenter Randomized Trials PHASE 2 in RCC Advanced or metastatic clear cell RCC Progression following 1 prior VEGF inhibitor 1 prior mTOR inhibitor allowed 1 prior immunotherapy allowed Randomized (n=150) Primary Endpoint: PFS Inlyta + TRC105 Inlyta 1:1 Randomization PHASE 2 in GBM Progression following chemoradiation (no prior Avastin) Randomized (n=86) Primary Endpoint: PFS Avastin + TRC105 Avastin 1:1 Randomization
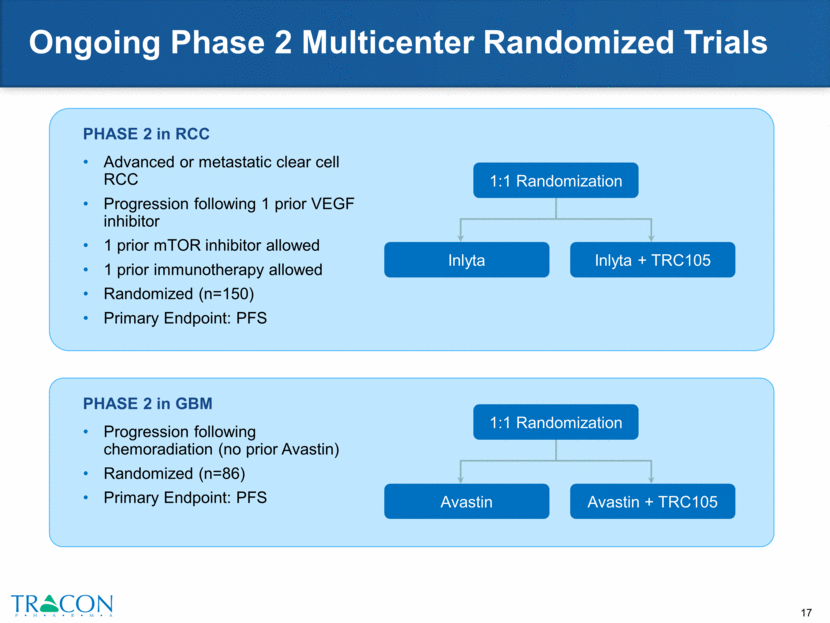
TRC105 + Votrient in Sarcoma Two-part Phase 2 clinical trial is expected to enroll 94 patients (18 in Phase 1b and 76 in Phase 2a, including a 13 patient angiosarcoma-specific cohort) In Phase 1b portion, 6/18 patients had >10% tumor reductions Phase 2 trial employs a biomarker strategy based on endoglin expression on sarcoma tissue to identify responsive subtypes Could serve as basis for enrollment into a biomarker-directed Phase 3 trial in soft tissue sarcoma Two ongoing complete responses in angiosarcoma, a sarcoma subtype that consistently overexpresses endoglin, and may therefore serve as its own biomarker of responsiveness to TRC105 treatment Expect to initiate global Phase 3 study in angiosarcoma in 2016 Phase 1b Complete Response Day 48 Day 0 Day 0 Day 37 Phase 2 Complete Response
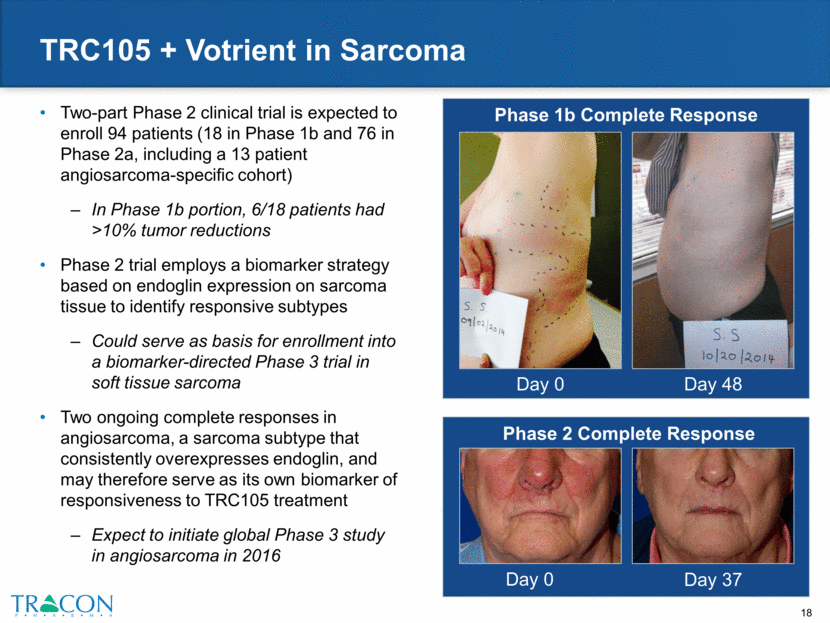
TRC105 + Nexavar in Hepatocellular Carcinoma Maximum Percentage Change in Target Lesion Size in Hepatocellular Carcinoma Patients Treated with TRC105 and Nexavar 20 patients treated (of whom 14 were evaluable by RECIST) in a Phase 1/2 clinical trial Dose escalation completed; combination well-tolerated Partial response rate by RECIST of 40% (treated with 10 or 15 mg/kg TRC105) exceeded partial response rate of Nexavar in Phase 3 pivotal studies of 2% Presented at ASCO 2015 Expect to initiate multicenter trial in hepatocellular carcinoma in 2015 of up to 39 patients to confirm response rate and justify a randomized Phase 3 trial Partial Response by RECIST
TRC105 Tiered Product Development Strategy 1Progression free survival. 2Overall response rate. 3Overall survival. 4TRACON internal targets based on marketed drugs for similar indications. Subject to regulatory and healthcare payor requirements. Companion Therapy Indications Commercial Rationale Target Efficacy Threshold for Approval/Reimbursement4 Ultra-Orphan Votrient Angiosarcoma Endoglin expressed on angiosarcoma; Votrient approved as single agent; short time to endpoint (PFS1) 50% improvement in PFS Avastin Gestational Neoplasia Endoglin expressed on choriocarcinoma; short time to expected endpoint (ORR2) 15% response rate Fast-to-Market Votrient Sarcoma: 2nd Line Votrient approved as single agent; short time to endpoint (PFS) 50% improvement in PFS in an enriched population Inlyta Renal cell: 2nd Line Inlyta approved as single agent; short time to endpoint (PFS) in a vascular tumor 30% improvement in PFS Avastin GBM: 2nd Line Avastin approved as single agent; short time to endpoint (OS3) in a vascular tumor 30% improvement in OS Nexavar Hepatocellular: 1st Line Nexavar approved as single agent in first line; short time to endpoint (OS) 30% improvement in OS Large Market VEGF inhibitor Colon cancer: Last Line Stivarga approved as single agent; short time to endpoint (OS) 30% improvement in OS Afinitor + Femara Breast cancer: Neoadjuvant Neoadjuvant setting allows approval based on pathologic complete response rate (pCR) 30% improvement in pCR Avastin + chemo Lung cancer: 1st Line Significant Avastin commercial franchise 30% improvement in OS
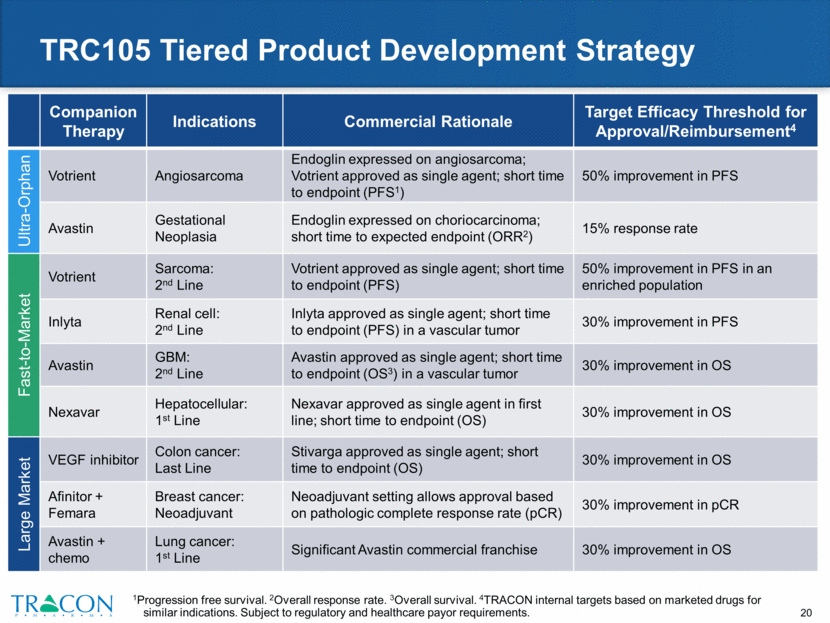
TRC105 – Multiple Expected Near-Term Readouts Companion Therapy 2015 2016 2017 Indication Votrient Sarcoma Angiosarcoma Inlyta RCC Avastin GBM Nexavar Hepatocellular Avastin Gestational Neoplasia Avastin + Carbo/Taxol Lung Afinitor + Femara Breast VEGF Inhibitor CRC Phase 2 data expected Data presentation Phase 1B Phase 2B Phase 1B/ 2 Phase 1B/2A Phase 2A Phase 2B Phase 1B Combination Trial: TRC105+VEGF Planned Combination Trial Phase 1B/2 Single Patient Phase 2 Phase 2 Phase 3
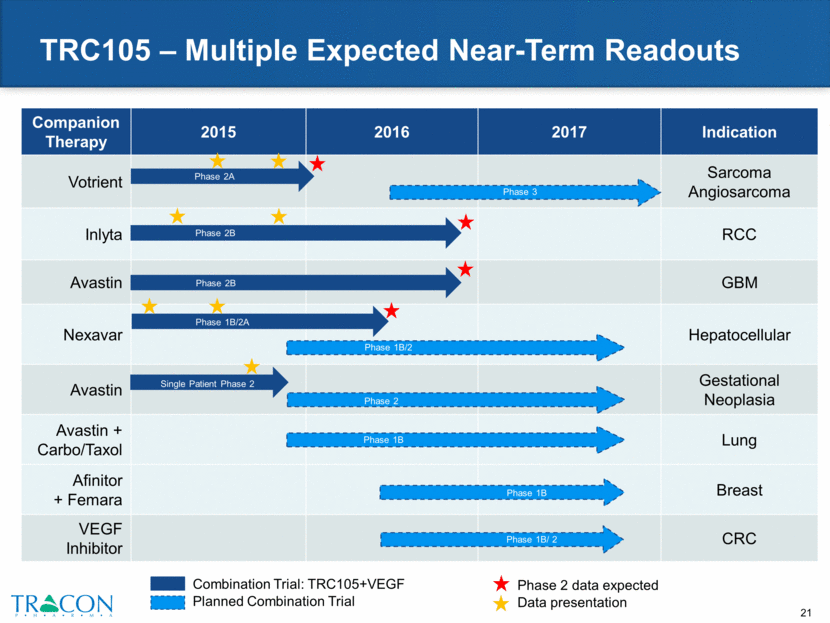
Development in AMD Partnered with Santen Santen, a global ophthalmology company with $1.4 billion in annual revenue, will lead global development and commercialization efforts for TRC105 in wet AMD and other eye diseases Deal terms $10 million upfront Santen pays for all development costs Up to $155 million in total milestone payments ($152 million remaining) Royalties in the high single digits to low teens Data from Ophthotech indicate that vision in AMD can be improved by targeting complementary pathways in combination with VEGF inhibitors TRC105 preclinical proof of concept established in a model of AMD IND filed in June 2015, triggering $3 million milestone payment
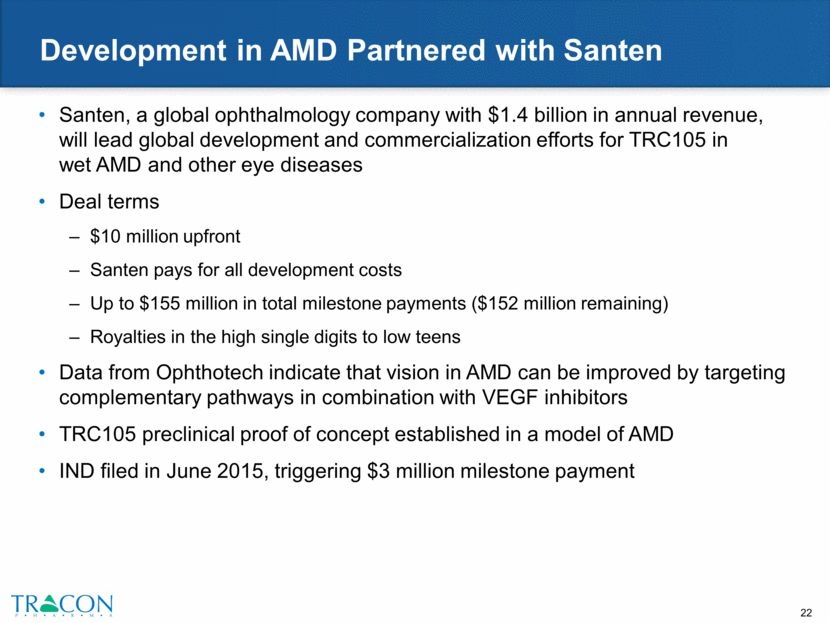
TRC205: Anti-Endoglin Antibody for Fibrosis Nearly 50% of all deaths in developed world are associated with some type of chronic fibroproliferative disease Fibrosis alters architecture of organs and tissues, thereby disrupting normal function and is associated with a wide variety of diseases and injuries Two recent FDA-approved treatments for idiopathic pulmonary fibrosis (IPF) – Esbriet (Roche) and Ofev (Boehringer Ingelheim) No FDA-approved treatments for non-alcoholic steatohepatitis (NASH) SYSTEMIC FIBROTIC DISEASES Scleroderma Nephrogenic Systemic Fibrosis Cystic Fibrosis Chronic Graft vs. Host Disease Atherosclerosis INJURY ASSOCIATED FIBROTIC DISEASE Surgical-induced Fibrosis Burn-induced Scarring & Contraction Radiation-Induced Fibrosis HEART Heart Attack (MI) Congestive Heart Failure (CHF) PULMONARY Idiopathic Pulmonary Fibrosis Scleroderma Lung Disease COPD ARDS Areas of Fibrotic Disease OPHTHALMIC Glaucoma Age-Related Macular Degeneration (AMD) Diabetic Macular Edema (DME) Diabetic Retinopathy Dry Eye Disease LIVER Alcoholic Cirrhosis Hepatitis C Cirrhosis Non-Alcoholic Steato Hepatitis (NASH) RENAL Transplant Nephropathy Diabetic Nephropathy Lupus Nephritis BONE MARROW Idiopathic & Drug Induced Myelofibrosis SKIN Hypertrophic Scarring Keloids
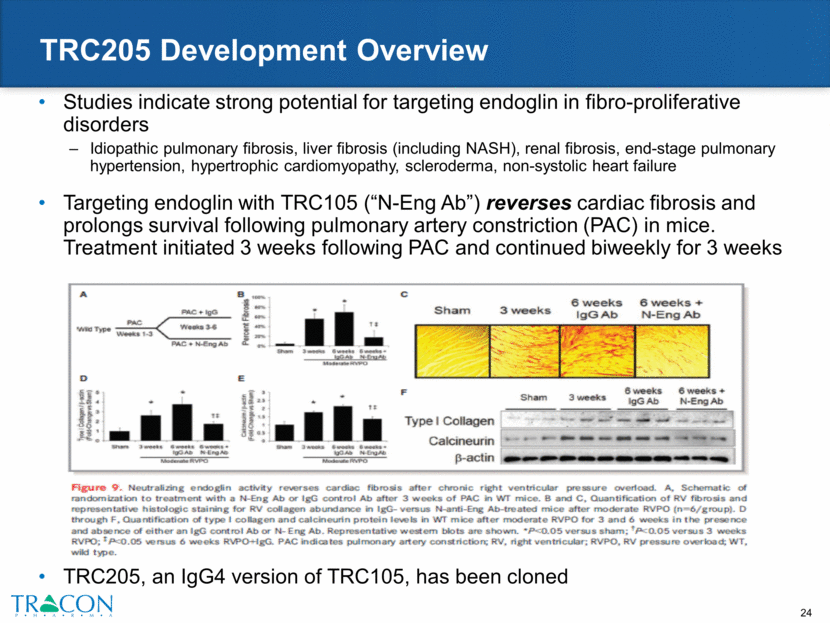
TRC205 Development Overview Studies indicate strong potential for targeting endoglin in fibro-proliferative disorders Idiopathic pulmonary fibrosis, liver fibrosis (including NASH), renal fibrosis, end-stage pulmonary hypertension, hypertrophic cardiomyopathy, scleroderma, non-systolic heart failure Targeting endoglin with TRC105 (“N-Eng Ab”) reverses cardiac fibrosis and prolongs survival following pulmonary artery constriction (PAC) in mice. Treatment initiated 3 weeks following PAC and continued biweekly for 3 weeks TRC205, an IgG4 version of TRC105, has been cloned
TRC102: Reversing Resistance to Chemotherapy Small molecule designed to reverse resistance to chemotherapy and complement poly ADP-ribose polymerase (PARP) inhibitors Inhibits base excision repair, a dominant pathway of DNA repair that allows for resistance to alkylating chemotherapy (e.g., Temodar) and antimetabolite chemotherapy (e.g., Alimta) Completed three Phase 1 clinical trials: with Alimta published in Investigational New Drugs in 2012, with Fludara presented at ASH 2014, and with Temodar presented at ASCO 2015 Well-tolerated with daily oral dosing at doses that achieved target plasma levels and pharmacodynamic endpoints; partial responses and stable disease observed in refractory disease Selected for development by NCI in 2013, which is expected to fund multiple Phase 2 trials In combination with Alimta/Cisplatin in solid tumors (Phase 1 initiated) In combination with Alimta in mesothelioma (Phase 2 initiated) In combination with Temodar in GBM (Phase 2) In combination with chemoradiation in lung cancer (Phase 1) Recently expanded IP estate with Case Western Reserve; sponsoring research into potential predictive biomarkers

Capital Efficient Clinical Development Strategy Beneficial relationship with NCI Multiple TRC105 and TRC102 clinical trials conducted in collaboration with NCI Mechanism used by Genentech to fund the majority of Avastin Phase 3 clinical trials Clinical Operations Internal system of clinical trial execution, including data management, allowing the company to conduct clinical trials without a CRO Validated in-house clinical operations and data management More efficient access to clinical data at lower cost Initial focus on indications with potential for reduced time to approval Significant Cost Savings to TRACON
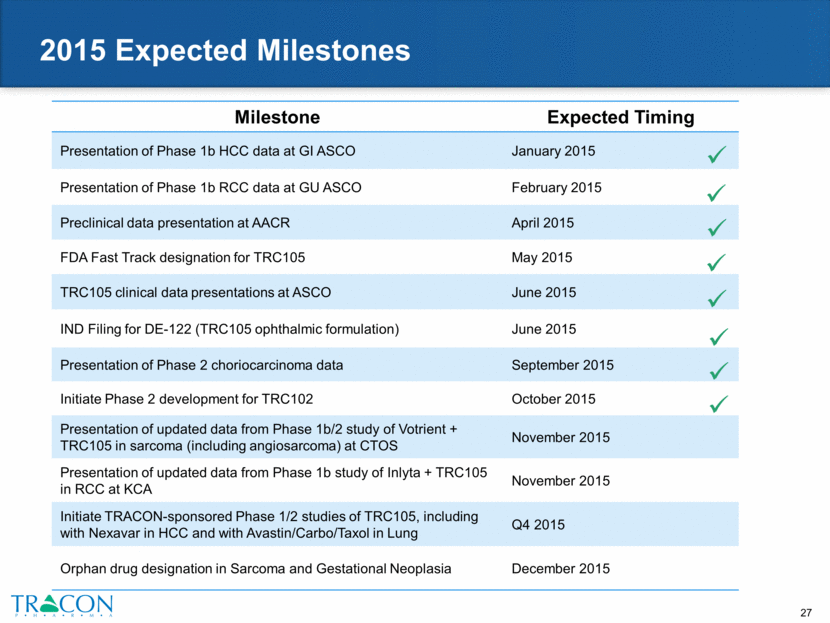
2015 Expected Milestones Milestone Expected Timing Presentation of Phase 1b HCC data at GI ASCO January 2015 Presentation of Phase 1b RCC data at GU ASCO February 2015 Preclinical data presentation at AACR April 2015 FDA Fast Track designation for TRC105 May 2015 TRC105 clinical data presentations at ASCO June 2015 IND Filing for DE-122 (TRC105 ophthalmic formulation) June 2015 Presentation of Phase 2 choriocarcinoma data September 2015 Initiate Phase 2 development for TRC102 October 2015 Presentation of updated data from Phase 1b/2 study of Votrient + TRC105 in sarcoma (including angiosarcoma) at CTOS November 2015 Presentation of updated data from Phase 1b study of Inlyta + TRC105 in RCC at KCA November 2015 Initiate TRACON-sponsored Phase 1/2 studies of TRC105, including with Nexavar in HCC and with Avastin/Carbo/Taxol in Lung Q4 2015 Orphan drug designation in Sarcoma and Gestational Neoplasia December 2015
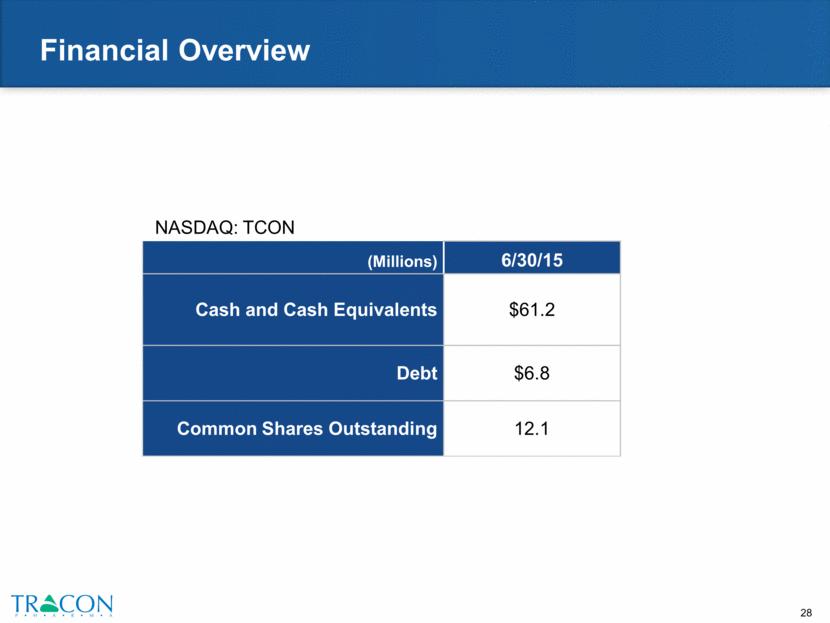
Financial Overview NASDAQ: TCON (Millions) 6/30/15 Cash and Cash Equivalents $61.2 Debt $6.8 Common Shares Outstanding 12.1
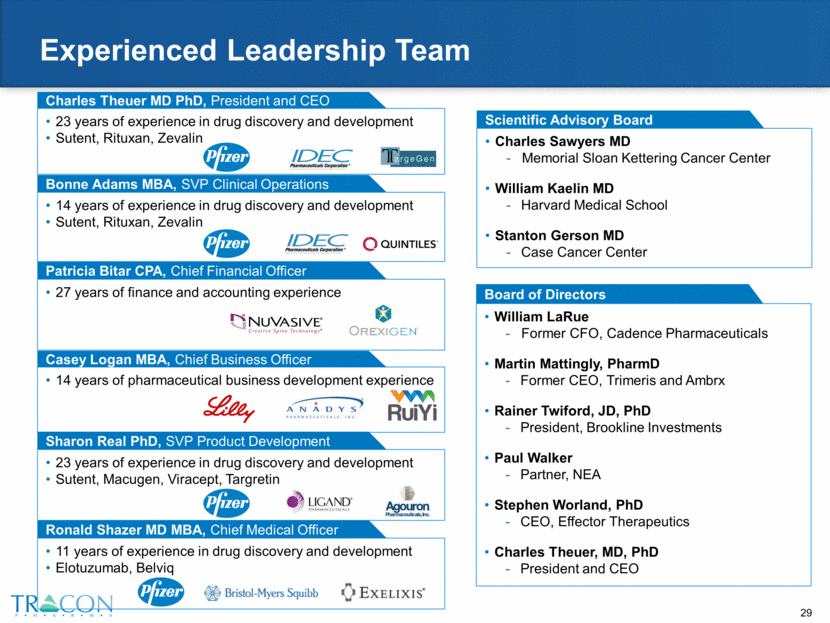
Experienced Leadership Team Scientific Advisory Board Charles Sawyers MD Memorial Sloan Kettering Cancer Center William Kaelin MD Harvard Medical School Stanton Gerson MD Case Cancer Center Casey Logan MBA, Chief Business Officer 14 years of pharmaceutical business development experience Charles Theuer MD PhD, President and CEO 23 years of experience in drug discovery and development Sutent, Rituxan, Zevalin Sharon Real PhD, SVP Product Development 23 years of experience in drug discovery and development Sutent, Macugen, Viracept, Targretin Patricia Bitar CPA, Chief Financial Officer 27 years of finance and accounting experience Board of Directors William LaRue Former CFO, Cadence Pharmaceuticals Martin Mattingly, PharmD Former CEO, Trimeris and Ambrx Rainer Twiford, JD, PhD President, Brookline Investments Paul Walker Partner, NEA Stephen Worland, PhD CEO, Effector Therapeutics Charles Theuer, MD, PhD President and CEO Bonne Adams MBA, SVP Clinical Operations 14 years of experience in drug discovery and development Sutent, Rituxan, Zevalin Ronald Shazer MD MBA, Chief Medical Officer 11 years of experience in drug discovery and development Elotuzumab, Belviq
Robust Pipeline with Near-Term Catalysts Innovative Approach Investment Highlights Initially focused on indications with potential reduced time to data readout and approval Internalized clinical operations capabilities and NCI support of clinical development Clinical data from more than 400 patients show good tolerability and promising anti-tumor activity Five ongoing Phase 2 clinical trials with near-term data readouts A leader in endoglin biology, an important escape pathway from VEGF inhibition Developing anti-endoglin antibodies in combination with VEGF inhibitors Oncology Experienced Team VEGF inhibitors currently generate > $17 billion in global annual sales Clinical development focused in three large markets: oncology, ophthalmology and fibrosis Ophthalmology Fibrosis Partnered with Santen; $152 million in remaining milestones, plus royalties IND filed in June 2015 triggered $3 million milestone Reverses fibrosis and improves survival in preclinical models Potential for increased activity in angiosarcoma and choriocarcinoma, orphan drug indications that overexpress endoglin Large Market Opportunity Orphan Drug Opportunity Efficient Clinical Development Strategy
TRACON PHARMACEUTICALS October 2015 NASDAQ: TCON

