Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REVA Medical, Inc. | a15-21258_18k.htm |
Exhibit 99.1

Positive Initial Results for Fantom Released at TCT
San Diego, California and Sydney, Australia (Friday, 16 October 2015, AEST) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”) is pleased to announce that Dr. J. Ribamar Costa from the Institute Dante Pazzanese of Cardiology, in Sao Paulo, Brazil, presented results from REVA’s clinical trial program for its Fantom® sirolimus-eluting bioresorbable scaffold at the Transcatheter Cardiovascular Therapeutics (“TCT”) Conference, which is being held October 11th through 15th in San Francisco, California.
Dr. Costa presented data from both the FANTOM I pilot study and the FANTOM II CE Mark clinical trial during the Bioresorbable Vascular Scaffolds, Part 2 session, which was held on Thursday, October 15th.
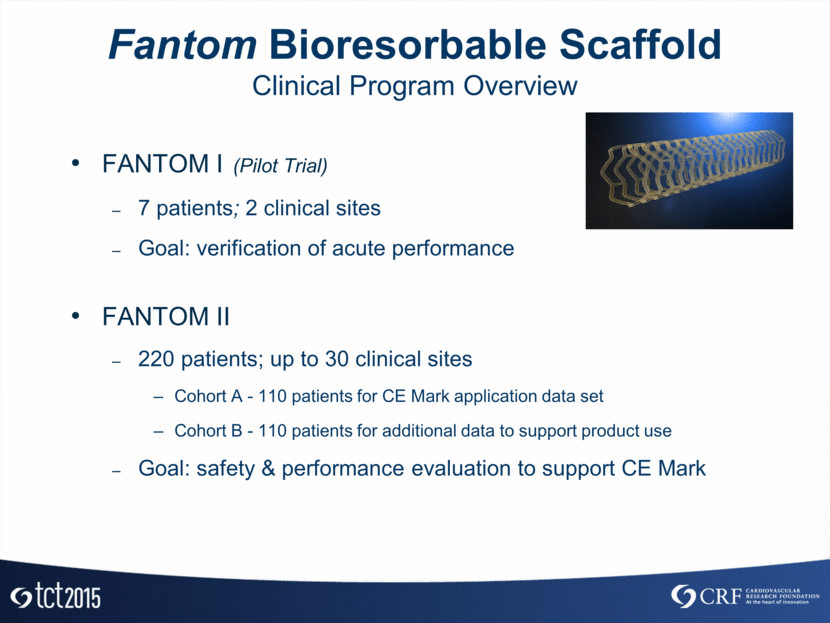
The FANTOM I pilot study enrolled seven patients, all of whom have now completed their follow-up evaluations through the six-month time point, including the primary imaging assessment at four months. The FANTOM II CE Mark clinical trial has completed enrollment of 110 patients who will have their primary follow-up evaluations during the next six months. Results released on both sets of patients showed excellent acute procedural outcomes and safety results for Fantom. Additionally, the initial results confirm sustained restoration of blood flow and very low reported Major Adverse Cardiac Events (“MACE”) to date.
Data from the FANTOM II six-month primary endpoint is scheduled to be presented at EuroPCR, which will be held next May in Paris, France. This data will be the basis of REVA’s CE Mark application, which is planned for mid-2016.
The presentation materials delivered at the Bioresorbable Vascular Scaffolds session are attached hereto. The presentation materials are also available in the Investor Relations section of REVA’s website at www.revamedical.com and are being filed with the U.S. Securities and Exchange Commission.
About REVA
REVA is a clinical stage medical device company located in San Diego, California, USA, that is working to commercialize its proprietary bioresorbable stents, which are called “scaffolds” because of their temporary nature. The Company’s scaffolds have been developed as an alternative to metal stents, which are small tube-like devices permanently implanted into an artery to treat coronary artery disease. Scaffolds provide restoration of blood flow, support the artery through the healing process, then disappear (or “resorb”) from the body over a period of time. This resorption allows the return of natural movement and function of the artery, a result not attainable with permanent metal stents. The Company’s initial product, the Fantom® scaffold, has been designed to offer an ideal balance of thinness and strength and distinct ease-of-use features including complete scaffold visibility under x-ray, expansion with one continuous inflation, and no procedural time limitations. REVA will require successful clinical trial results and regulatory approval before it can commercialize Fantom or any other products.
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 · +1 (858) 966-3000 · +1 (858) 966-3099 (FAX) · www.revamedical.com
AUSTRALIAN OFFICE: Suite 4, Level 14, 6 O’Connell Street, Sydney NSW 2000 · +61 2 9237 2800
ARBN 146 505 777 · REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders have limited liability
Forward-Looking Statements
This announcement contains or may contain forward-looking statements that are based on management’s beliefs, assumptions and expectations and on information currently available to management. All statements that are not statements of historical fact, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements, such as those statements regarding our ability to obtain regulatory approvals, timely and successfully complete our clinical trials, protect our intellectual property position, commercialize our products if and when approved, develop and commercialize new products, recruit and retain our key personnel, and estimates regarding our capital requirements and financial performance. You should not place undue reliance on forward-looking statements. Although management believes forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause our actual results to vary materially from those expressed in forward-looking statements, including the risks and uncertainties that are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the US Securities and Exchange Commission (the “SEC”) on March 30, 2015, and as may be updated in our periodic reports thereafter. Any forward-looking statements in this announcement speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
|
United States |
|
Australia |
|
Australia |
|
Investor & Media Enquiries: |
|
Investor Enquiries: |
|
Media Enquiries: |
|
REVA Medical, Inc. |
|
Inteq Limited |
|
Buchan Consulting |
|
Cheryl Liberatore |
|
Kim Jacobs |
|
Rebecca Wilson |
|
Director, Communications |
|
+61 2 9229 2700 |
|
+61 3 9866 4722 |
|
+1 858-966-3045 |
|
|
|
Annabel Murphy |
|
|
|
|
|
+61 2 9237 2800 |
FantomTM Differentiating Features and Clinical Trial Insights Jose de Ribamar Costa Jr, MD, PhD, FACC Instituto Dante Pazzanese de Cardiologia HCOR São Paulo - Brazil

Disclosure Statement of Financial Interest I, J Ribamar Costa Jr, DO NOT have a financial interest/arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context of the subject of this presentation.

Fantom Bioresorbable Scaffold Fantom™ Sirolimus-Eluting Bioresorbable Scaffold Desaminotyrosine Polycarbonate Key Scaffold Features Complete scaffold visibility under x-ray Single-step continuous inflation Clinically significant expansion range Radial strength at 125 µm strut thickness Vasomotion restoration < 1 year No special storage or handling Deliverability Vessel Patency Visibility
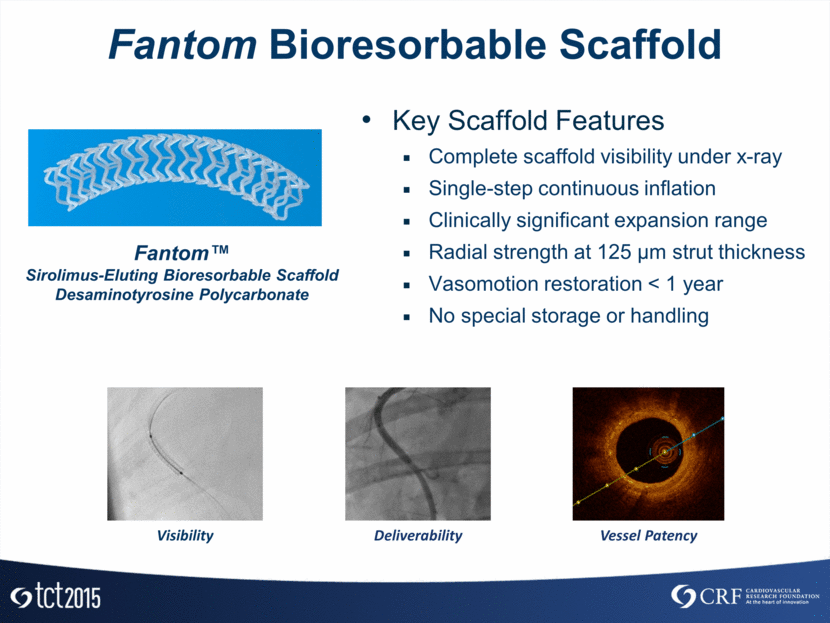
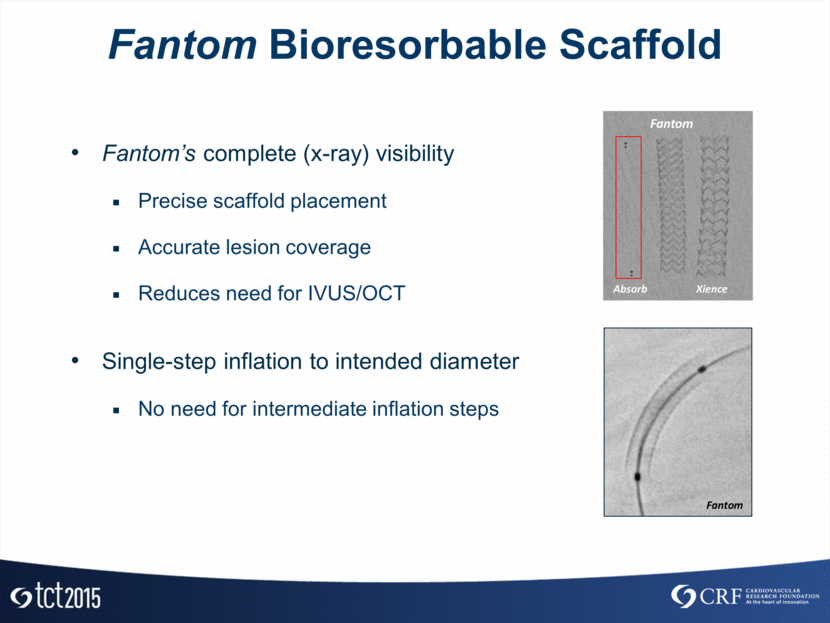
Fantom Bioresorbable Scaffold Fantom’s complete (x-ray) visibility Precise scaffold placement Accurate lesion coverage Reduces need for IVUS/OCT Single-step inflation to intended diameter No need for intermediate inflation steps Absorb Fantom Xience Fantom
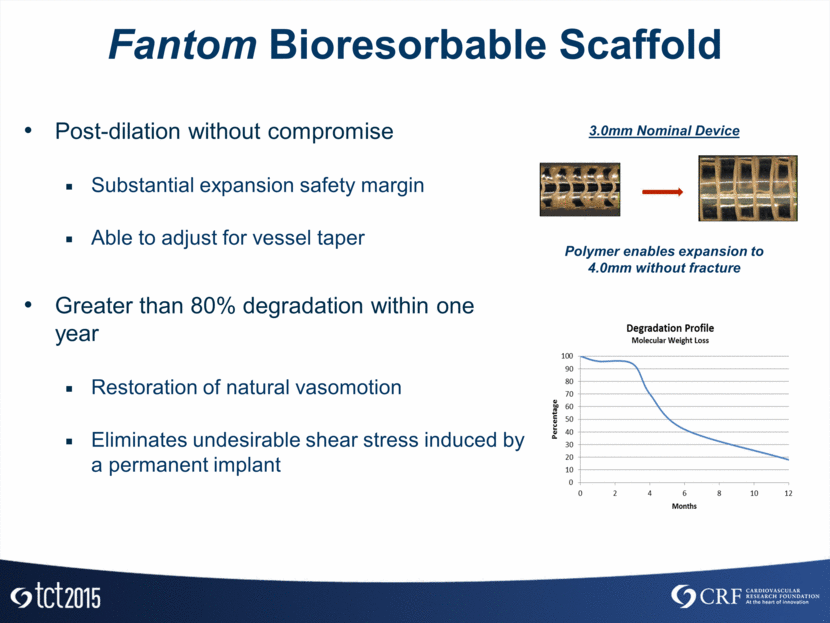
Fantom Bioresorbable Scaffold Post-dilation without compromise Substantial expansion safety margin Able to adjust for vessel taper Greater than 80% degradation within one year Restoration of natural vasomotion Eliminates undesirable shear stress induced by a permanent implant 3.0mm Nominal Device Polymer enables expansion to > 4mm without fracture Polymer enables expansion to 4.0mm without fracture 3.0mm Nominal Device
Fantom Bioresorbable Scaffold Clinical Program Overview FANTOM I (Pilot Trial) 7 patients; 2 clinical sites Goal: verification of acute performance FANTOM II 220 patients; up to 30 clinical sites Cohort A - 110 patients for CE Mark application data set Cohort B - 110 patients for additional data to support product use Goal: safety & performance evaluation to support CE Mark
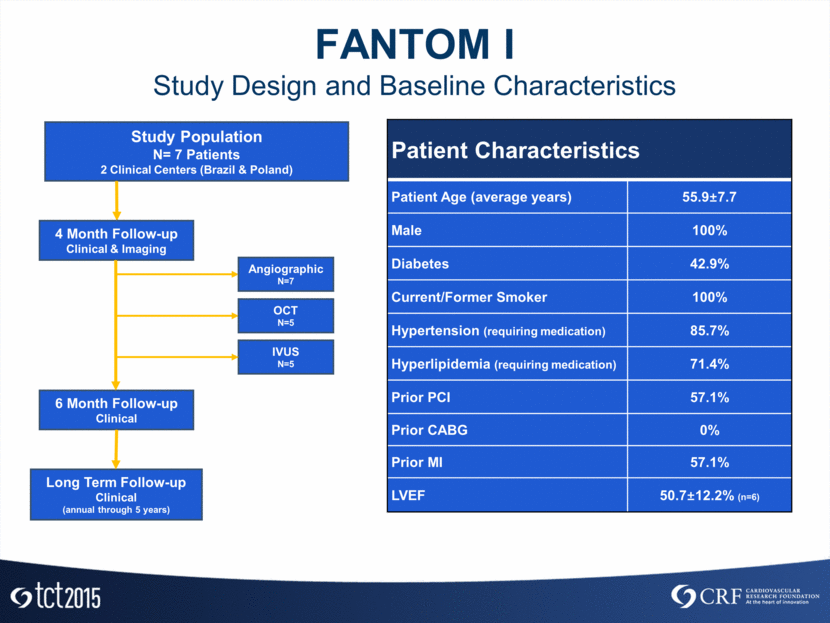
FANTOM I Study Design and Baseline Characteristics Patient Characteristics Patient Age (average years) 55.9±7.7 Male 100% Diabetes 42.9% Current/Former Smoker 100% Hypertension (requiring medication) 85.7% Hyperlipidemia (requiring medication) 71.4% Prior PCI 57.1% Prior CABG 0% Prior MI 57.1% LVEF 50.7±12.2% (n=6) Study Population N= 7 Patients 2 Clinical Centers (Brazil & Poland) 4 Month Follow-up Clinical & Imaging 6 Month Follow-up Clinical Angiographic N=7 OCT N=5 IVUS N=5 Long Term Follow-up Clinical (annual through 5 years)
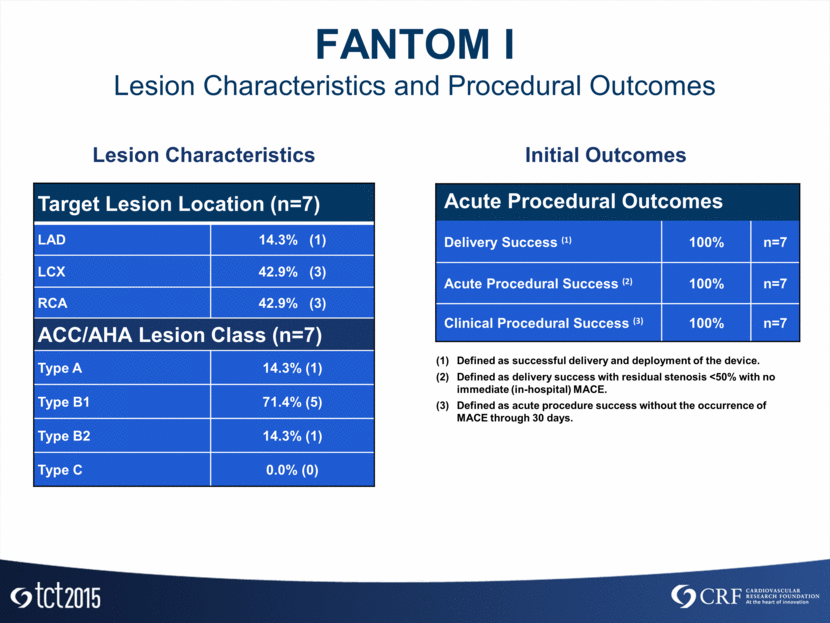
FANTOM I Lesion Characteristics and Procedural Outcomes Target Lesion Location (n=7) LAD 14.3% (1) LCX 42.9% (3) RCA 42.9% (3) ACC/AHA Lesion Class (n=7) Type A 14.3% (1) Type B1 71.4% (5) Type B2 14.3% (1) Type C 0.0% (0) Lesion Characteristics Initial Outcomes Acute Procedural Outcomes Delivery Success (1) 100% n=7 Acute Procedural Success (2) 100% n=7 Clinical Procedural Success (3) 100% n=7 Defined as successful delivery and deployment of the device. Defined as delivery success with residual stenosis <50% with no immediate (in-hospital) MACE. Defined as acute procedure success without the occurrence of MACE through 30 days.
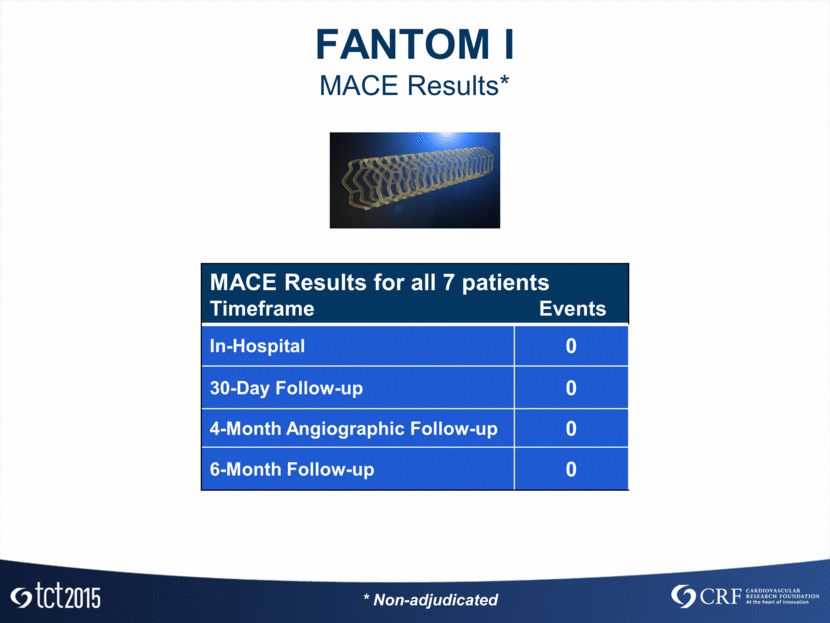
FANTOM I MACE Results* MACE Results for all 7 patients Timeframe Events In-Hospital 0 30-Day Follow-up 0 4-Month Angiographic Follow-up 0 6-Month Follow-up 0 * Non-adjudicated
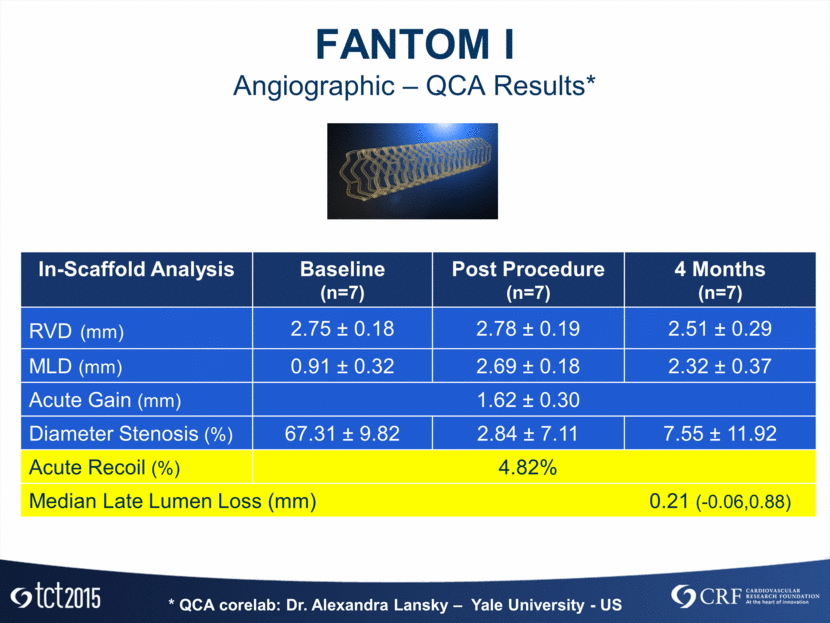
FANTOM I Angiographic – QCA Results* In-Scaffold Analysis Baseline (n=7) Post Procedure (n=7) 4 Months (n=7) RVD (mm) 2.75 ± 0.18 2.78 ± 0.19 2.51 ± 0.29 MLD (mm) 0.91 ± 0.32 2.69 ± 0.18 2.32 ± 0.37 Acute Gain (mm) 1.62 ± 0.30 Diameter Stenosis (%) 67.31 ± 9.82 2.84 ± 7.11 7.55 ± 11.92 Acute Recoil (%) 4.82% Median Late Lumen Loss (mm) 0.21 (-0.06,0.88) * QCA corelab: Dr. Alexandra Lansky – Yale University - US
FANTOM I IVUS Results* In-Scaffold Analysis Post Procedure (n=5) 4 Months (n=7) Mean vessel area, mm² 12.82 ± 1.85 14.19 ± 2.90 Mean scaffold area, mm² 6.15 ± 0.69 6.00 ± 1.10 Mean scaffold diameter, mm² 2.81 ± 0.16 2.77 ± 0.25 Mean lumen area, mm² 6.15 ± 0.68 5.60 ± 0.67 Mean lumen diameter, mm 2.69 ± 0.18 2.32 ± 0.37 Malapposed Volume, mm³ 0.15 ± 0.37 0.06 ± 0.05 NIH area, mm² 0.41 ± 0.64 % of NIH obstruction 3.14 ± 2.04 * IVUS corelab: Dr. J. Ribamar Costa Jr – CRC (São Paulo)
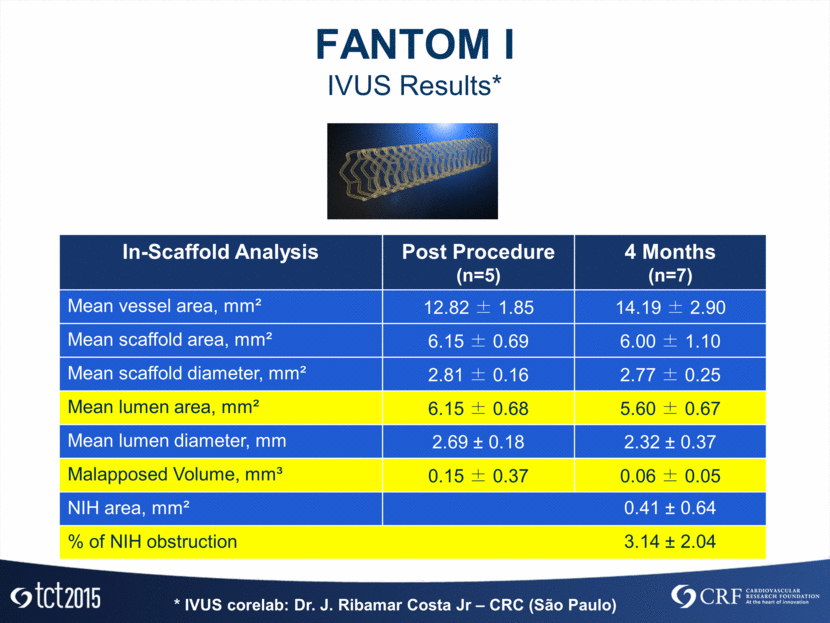
Absorb ABBOTT Fantom REVA MEDICAL DESolve NX ELIXIR Differences and Similarities Poly-L-lactic acid Poly-L-lactic acid poly(I2DAT-co-lactic acid) 157µm 125 µm 150µm Crossing profile ~1.5mm Crossing profile ~1.5mm Crossing profile ~1.3mm Fantom Optical Properties Comparison* * Courtesy Dr. D. Chamie – CRC OCT corelab – São Paulo
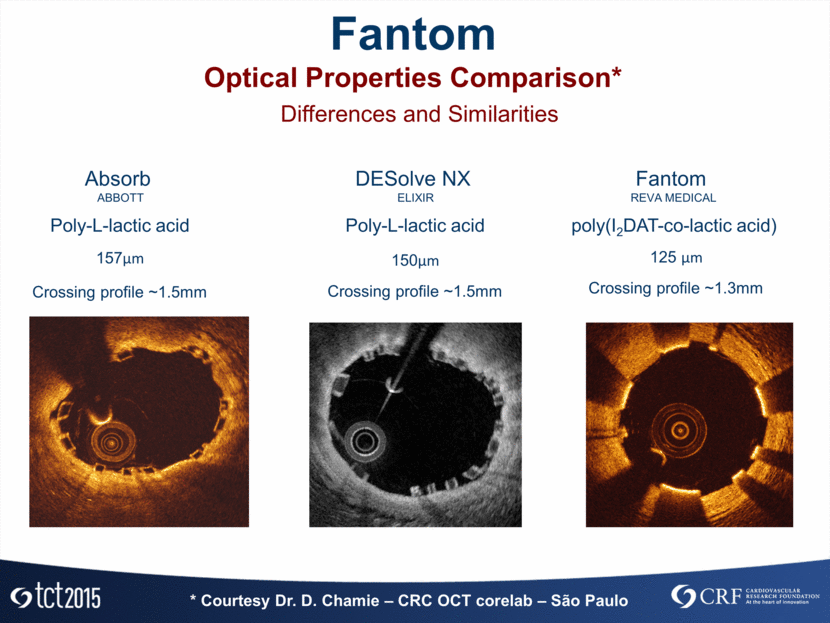
* OCT corelab: Dr. D. Chamie – CRC – São Paulo NIH Quantification FANTOM I Main OCT Results * Serruys PW, et .a Circulation 2010;122;2301-2312 N I H T o ta l 1.56 ± 0.28 1.25 ± 0.36 * * Se rru ys PW , e t . a C i rcu l a t i o n 2 0 1 0 ; 1 2 2 ; 2 3 0 1 -2 3 1 2 67.7% 32.3% I n t e rst ru t O b st ru ct i ve 1.56 ± 0.28 1.04 ± 0.07 0.52 ± 0.22 NIH Area N I H A r e a : % fr o m T o ta l 67.7% 32.3% I n t e rst ru t O b st ru ct i ve 1.56 ± 0.28 1.04 ± 0.07 0.52 ± 0.22 NIH Area N I H A r e a : % fr o m T o ta l
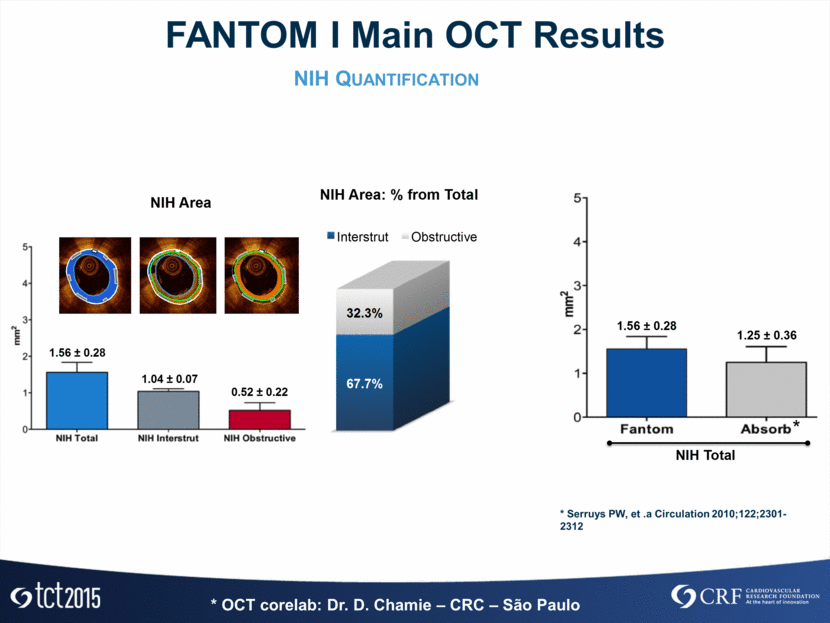
FANTOM I OCT Results* Cross-Section Level Analysis Post-Procedure N=6 4-Month FU N=6 Difference (95% CI) P Analyzed Scaffold Length, mm 17.63 ± 2.09 18.45 ± 1.47 -0.81 (-3.68 to 2.05) 0.916 Analyzed cross-sections per lesion 30.0 ± 3.57 31.16 ± 2.31 -1.16 (-5.63 to 3.30) 0.684 Mean Scaffold Area, mm2 7.05 ± 0.78 6.55 ± 0.76 0.49 (-0.14 to 1.12) 0.116 Min. Scaffold Area, mm2 6.03 ± 0.86 5.49 ± 0.67 0.53 (-0.09 to 1.16) 0.080 Strut Core Area, mm2 0.04 ± 0.00 0.03 ± 0.00 0.00 (-0.002 to 0.005) 0.317 Mean Scaffold Expansion, % 125.66 ± 27.96 126.02 ± 19.36 -0.35 (-23.48 to 22.76) 0.893 Mean Lumen Area, mm2 6.89 ± 0.68 5.08 ± 0.83 1.80 (1.18 to 2.43) 0.028 Min. Lumen Area, mm2 5.63 ± 0.91 3.87 ± 0.82 1.76 (0.62 to 2.90) 0.028 Flow Area, mm2 6.51 ± 0.62 5.08 ± 0.83 1.42 (0.75 to 2.10) 0.028 Analyzed struts per scaffolds 251.83 ± 32.65 255.50 ± 19.39 -3.66 (-34.21 to 26.88) 0.917 Freq. of covered struts per lesion, % N/A 99.14 ± 1.01 Freq. malapposed struts per lesion, % 2.07 ± 3.69 0.00 Mean NIH thickness over cov. struts, mm N/A 0.09 ± 0.03 * OCT corelab: Dr. D. Chamie – CRC – São Paulo
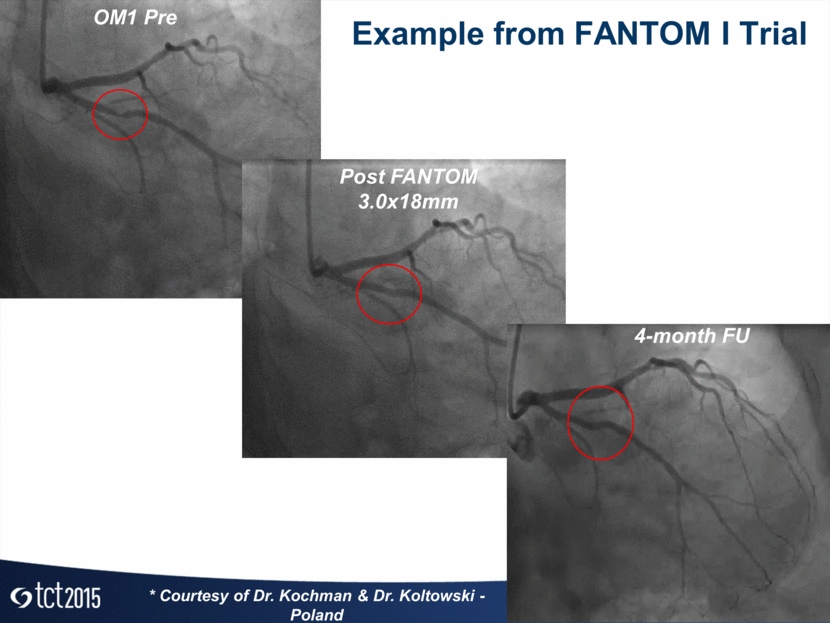
OM1 Pre Post FANTOM 3.0x18mm 4-month FU Example from FANTOM I Trial * Courtesy of Dr. Kochman & Dr. Koltowski - Poland
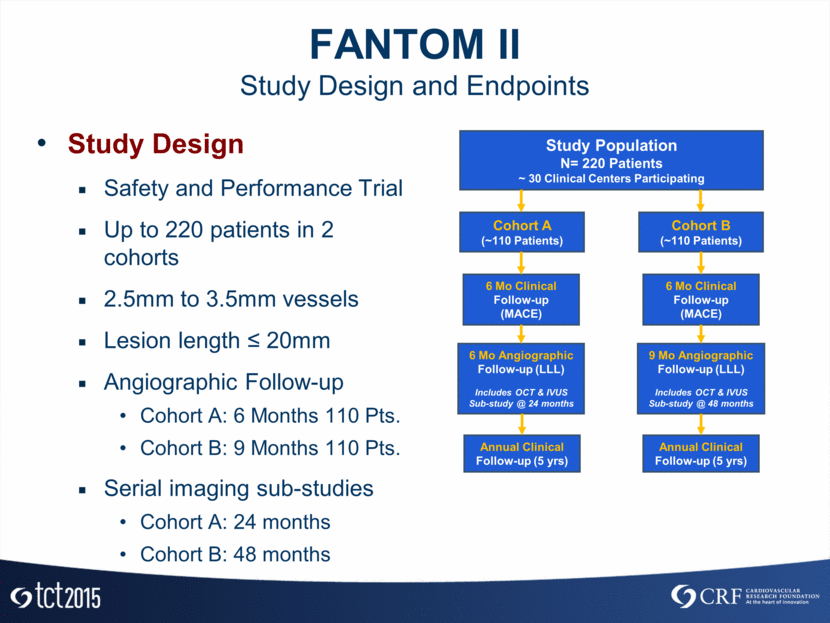
FANTOM II Study Design and Endpoints Study Design Safety and Performance Trial Up to 220 patients in 2 cohorts 2.5mm to 3.5mm vessels Lesion length < 20mm Angiographic Follow-up Cohort A: 6 Months 110 Pts. Cohort B: 9 Months 110 Pts. Serial imaging sub-studies Cohort A: 24 months Cohort B: 48 months Study Population N= 220 Patients ~ 30 Clinical Centers Participating Cohort A (~110 Patients) 6 Mo Clinical Follow-up (MACE) Cohort B (~110 Patients) 6 Mo Clinical Follow-up (MACE) 6 Mo Angiographic Follow-up (LLL) Includes OCT & IVUS Sub-study @ 24 months 9 Mo Angiographic Follow-up (LLL) Includes OCT & IVUS Sub-study @ 48 months Annual Clinical Follow-up (5 yrs) Annual Clinical Follow-up (5 yrs)
FANTOM II Current Status Cohort A Enrollment complete September 2015 Follow-up ongoing Presentation of primary endpoint targeted for EuroPCR Cohort B Enrollment ongoing
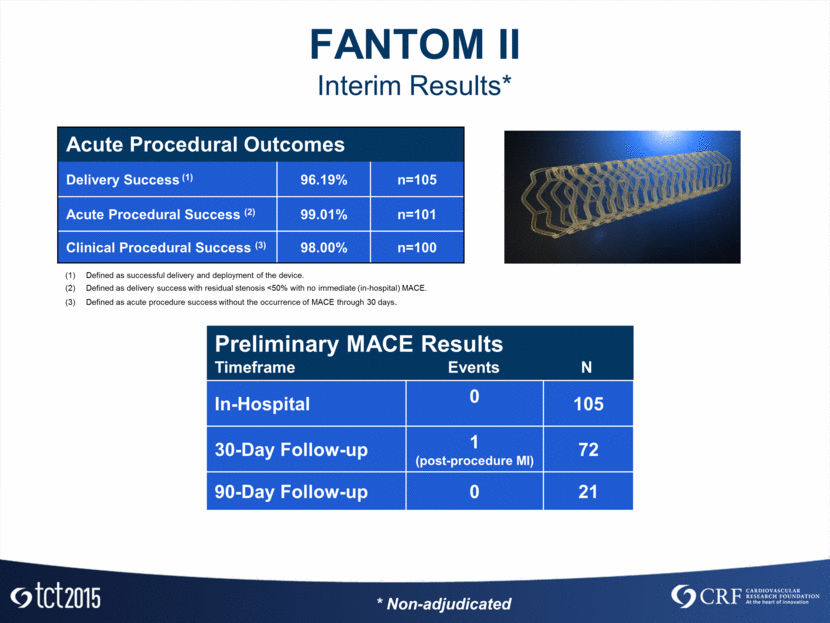
FANTOM II Interim Results* Preliminary MACE Results Timeframe Events N In-Hospital 0 105 30-Day Follow-up 1 (post-procedure MI) 72 90-Day Follow-up 0 21 Acute Procedural Outcomes Delivery Success (1) 96.19% n=105 Acute Procedural Success (2) 99.01% n=101 Clinical Procedural Success (3) 98.00% n=100 Defined as successful delivery and deployment of the device. Defined as delivery success with residual stenosis <50% with no immediate (in-hospital) MACE. Defined as acute procedure success without the occurrence of MACE through 30 days. * Non-adjudicated
FANTOM Program Summary New and interesting features: Radiopacity Lower crossing profile Faster resorption Direct inflation to intended diameter Initial clinical data suggest: Good acute performance High rate of device deliverability Minimal residual stenosis and acute recoil Encouraging imaging performance at 4 months (minimal NIH formation, all struts covered and apposed) Low MACE rate More clinical data: EuroPCR 2016!

