Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2015
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File No. 0-20190
AUTHENTIDATE HOLDING CORP.
(Exact Name of Issuer as Specified in Its Charter)
| Delaware | 14-1673067 | |
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
Connell Corporate Center, 300 Connell Drive, 5th Floor, Berkeley Heights, NJ 07922
(Address of principal executive offices) (Zip Code)
Issuer’s telephone number, including area code (908) 787-1700
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of Each Class |
Name of Each Exchange on Which Registered | |
| Common Stock, par value $.001 per share | The Nasdaq Stock Market, LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15 (d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (ss.229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer. See definitions of “accelerated filer and large accelerated filer” and in Rule 12b-2 of the Exchange Act. (check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | |||
| Non-accelerated filer ¨ | (Do not check if a smaller reporting company) | Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter (December 31, 2014): $31,900,000
APPLICABLE ONLY TO CORPORATE REGISTRANTS
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date: 42,194,841 as of September 25, 2015.
DOCUMENTS INCORPORATED BY REFERENCE
List hereunder the following documents if incorporated by reference and the Part of the Form 10-K (e.g., Part I, Part II, etc.) into which the document is incorporated: (1) Any annual report to security holders; (2) Any proxy or information statement; and (3) Any prospectus filed pursuant to Rule 424(b) or (e) under the Securities Act of 1933.
None
Table of Contents
Table of Contents
THIS ANNUAL REPORT ON FORM 10-K, INCLUDING ITEM 1 (“BUSINESS”) AND ITEM 7 (“MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS”), CONTAINS CERTAIN FORWARD-LOOKING STATEMENTS WITHIN THE MEANING OF SECTION 27A OF THE SECURITIES ACT OF 1933 AND SECTION 21E OF THE SECURITIES EXCHANGE ACT OF 1934. STATEMENTS THAT ARE NOT HISTORICAL FACT ARE FORWARD-LOOKING STATEMENTS. WHEN USED IN THIS REPORT, THE WORDS “BELIEVE,” “ANTICIPATE,” “THINK,” “INTEND,” “PLAN,” “WILL BE,” “EXPECT”, AND SIMILAR EXPRESSIONS IDENTIFY SUCH FORWARD-LOOKING STATEMENTS. SUCH STATEMENTS REGARDING FUTURE EVENTS AND/OR OUR FUTURE FINANCIAL PERFORMANCE ARE SUBJECT TO CERTAIN RISKS AND UNCERTAINTIES, WHICH COULD CAUSE ACTUAL EVENTS OR OUR ACTUAL FUTURE RESULTS TO DIFFER MATERIALLY FROM ANY FORWARD-LOOKING STATEMENT. SUCH RISKS AND UNCERTAINTIES INCLUDE, AMONG OTHER THINGS, RISKS ASSOCIATED WITH MARKET ACCEPTANCE OF OUR SOFTWARE, PRODUCTS AND SERVICES, OUR ABILITY TO IMPLEMENT OUR BUSINESS PLAN, COMPETITION, MANAGEMENT OF GROWTH, PRICING, TECHNOLOGICAL CHANGES, THE AVAILABILITY OF ANY NEEDED FINANCING AND OTHER RISKS AND UNCERTAINTIES THAT MAY BE DETAILED FROM TIME TO TIME IN OUR REPORTS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. THE COMPANY CAUTIONS INVESTORS THAT ANY FORWARD-LOOKING STATEMENTS MADE BY THE COMPANY ARE NOT GUARANTEES OR INDICATIVE OF FUTURE PERFORMANCE. IMPORTANT ASSUMPTIONS AND OTHER IMPORTANT FACTORS THAT COULD CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE FORWARD-LOOKING STATEMENTS WITH RESPECT TO THE COMPANY INCLUDE, BUT ARE NOT LIMITED TO, THE RISKS AND UNCERTAINTIES AFFECTING IT’S BUSINESS DESCRIBED IN ITEM 1A OF THIS ANNUAL REPORT ON FORM 10-K AND IN OTHER SECURITIES FILINGS BY THE COMPANY. ALTHOUGH THE COMPANY BELIEVES THAT ITS PLANS, INTENTIONS AND EXPECTATIONS REFLECTED IN OR SUGGESTED BY SUCH FORWARD-LOOKING STATEMENTS ARE REASONABLE, ACTUAL RESULTS COULD DIFFER MATERIALLY FROM A PROJECTION OR ASSUMPTION IN ANY OF ITS FORWARD-LOOKING STATEMENTS. THE COMPANY’S FUTURE FINANCIAL CONDITION AND RESULTS OF OPERATIONS, AS WELL AS ANY FORWARD-LOOKING STATEMENTS, ARE SUBJECT TO CHANGE AND INHERENT RISKS AND UNCERTAINTIES. THE FORWARD-LOOKING STATEMENTS CONTAINED IN THIS ANNUAL REPORT ON FORM 10-K ARE MADE ONLY AS OF THE DATE HEREOF AND THE COMPANY DOES NOT HAVE OR UNDERTAKE ANY OBLIGATION TO UPDATE OR REVISE ANY FORWARD-LOOKING STATEMENTS WHETHER AS A RESULT OF NEW INFORMATION, SUBSEQUENT EVENTS OR OTHERWISE, UNLESS OTHERWISE REQUIRED BY LAW.
| ITEM 1. | BUSINESS |
General
Authentidate Holding Corp. (Authentidate or the company) and its subsidiaries provide secure web-based revenue cycle management applications and telehealth products and services that enable healthcare organizations to increase revenues, improve productivity, reduce costs, coordinate care for patients and enhance related administrative and clinical workflows and compliance with regulatory requirements. Our web-based services are delivered as Software as a Service (SaaS) to our customers interfacing seamlessly with billing, information and document management systems. These solutions incorporate multiple features and security technologies such as business-rules based electronic forms, intelligent routing, transaction management, electronic signatures, identity credentialing, content authentication, automated audit trails and remote patient management capabilities. Both web and fax-based communications are integrated into automated, secure and trusted workflow solutions.
Our telehealth solutions provide in-home patient vital signs monitoring systems and services to improve care for patients and reduce the cost of care by delivering results to their healthcare providers via the Internet.
2
Table of Contents
Our telehealth solutions combine our tablet or Electronic House Call™ patient vital signs monitoring appliances or our Interactive Voice Response patient vital signs monitoring solution with a web-based management and monitoring software module. Both solutions enable unattended measurements of patients’ vital signs and related health information and are designed to aid wellness and preventative care and deliver better care to specific patient segments that require regular monitoring of medical and behavioral health conditions. Healthcare providers can easily view each specific patient’s vital statistics and make adjustments to the patient’s care plans securely via the Internet. The service provides a combination of care plan schedule reminders and comprehensive disease management education as well as intelligent routing to alert on-duty caregivers whenever a patient’s vital signs are outside of the practitioner’s pre-set ranges. Healthcare providers and health insurers are also expected to benefit by having additional tools to improve patient care and reduce in-person and emergency room patient visits and hospital readmissions.
We operate our business in the United States with technology and service offerings that address emerging growth opportunities based on the regulatory and legal requirements specific to each market. Our business is engaged in the development and sale of web-based services largely based on our Inscrybe® platform and related capabilities and our telehealth products and services. In recent years we have focused our efforts on developing and introducing solutions for use in the healthcare information technology industry.
As described in greater detail below, more recently we have focused on providing our solutions to commercial marketplace end-users in the healthcare information technology industry.
We believe there are a number of on-going factors that will be favorable for the healthcare information technology industry in the near future. These factors include regulatory reforms in the U.S. focused on controlling costs, automating medical records and processes and expanding the availability of healthcare coverage, and healthcare industry trends to significantly reduce costs, shorten the length of hospital stays, reduce hospital readmissions, shift patient care towards wellness and preventative care programs and automate healthcare records and processes. Furthermore, we believe our business will benefit as the recent U.S. Supreme Court decisions upholding the healthcare law are better recognized in the marketplace. Because healthcare information technology solutions play an important role in healthcare by improving safety, efficiency and reducing cost, they are often viewed as more strategic than other capital purchases. In addition, government agencies, as well as politicians and policymakers appear to agree that the growing cost of our healthcare system is unsustainable and the intelligent use of information systems will improve health outcomes and, correspondingly, drive down costs. The broad recognition that healthcare information technology is essential to help control healthcare costs and improve quality contributed to the inclusion of healthcare information technology incentives in the American Recovery and Reinvestment Act (ARRA) and accompanying Health Information Technology for Economic and Clinical Health (HITECH) provisions which include more than $35 billion in incentives for healthcare organizations to modernize operations through “meaningful use” of healthcare information technology. Further, as more consumers are provided with insurance coverage, healthcare providers may face increased volumes that could create capacity constraints, and they may find it challenging to profitably provide care at the planned reimbursement rates under the expanded coverage models. Another aspect of the market for healthcare information technology is the shift away from fee-for-service or volume-based reimbursement towards value-based or outcomes-based reimbursement. Payers, including health insurance companies and federal and state governments, are implementing programs to link reimbursement to quality measurements and outcomes, and this alignment creates significant financial motivation for adoption of healthcare information technology products and services. We believe that there are substantial sums of reimbursement funds that are tied to incentive programs such as value based purchasing, 30-day readmission rules and quality reporting requirements. There are also a growing number of third-party studies that document how telehealth can positively impact the way healthcare is delivered. From chronic care to behavioral health and wellness programs, a wide range of patient populations can benefit from telehealth. We believe that telehealth products are helping physicians and patients to accomplish a number of goals, including, shifting visits away from high-cost settings; reducing the cost of managing patients with chronic diseases; reducing unnecessary hospital readmissions; reducing the duration of hospital stays; improving access to care for patients located in
3
Table of Contents
remote areas; and improving outcomes. We believe the factors discussed above will create strong incentives for providers to maximize efficiency and create the need for additional investments in healthcare information technology solutions and services. We also believe that the healthcare information technology industry will likely benefit as healthcare providers and governments continue to recognize that these solutions and services contribute to safer, more efficient healthcare.
General Business Developments during and subsequent to Fiscal Year 2015
During fiscal 2015 we have focused primarily on marketing our telehealth products and services and our referral and order management and hospital discharge solutions, and in recent months have further narrowed our area of focus to the commercial market. We have also continued to take steps to refine our core product and service offerings, significantly expand our addressable markets, manage operating costs and position the company for long-term growth. We believe the company is well positioned for growth as the healthcare markets it serves are growing rapidly and expected to continue to grow over the next several years according to third party analyst market assessments. Additionally, our products and services target the “12 month episode of care” market, which is the largest and fastest growing market segment for remote patient management services. As discussed above, we also believe our business will benefit as government healthcare reforms are implemented and as healthcare industry trends take hold. Although we have taken steps to focus our business in these areas, our progress will be impacted by the timing of customer contracts and implementations and the market acceptance of our products and services.
On March 6, 2015 we announced that the Department of Veterans Affairs (VA) informed the company that it did not intend to exercise the fourth and final option year under our contract for telehealth products and services. The company’s contract with the VA was originally awarded in April 2011 and consisted of a base year and four one-year option years which were exercisable at the VA’s sole discretion. The current option year under the contract expired on May 15, 2015 and the transition process with the VA was completed by that date. Our VA revenue included both recurring service revenues as well as hardware sales. As a result of the non-renewal of the VA contract we have reported significantly reduced revenues and expect that trend to continue over the next several quarters. Accordingly, due to this development, we have taken steps to reduce our operating costs and better align our resources with the growth opportunities we intend to pursue. The VA had been our largest customer, accounting for approximately 45% and 58% of our total revenue for the years ended June 30, 2015 and 2014, respectively. As a result, we have implemented a number of changes to our business plan with the ultimate goal to increase revenues and positive cash flow from operations, including a recalibration of marketing and sales efforts that have already resulted in growth from existing customers and sales to new customers. These changes include cost reductions from reducing our workforce and use of consultants that we made in the third quarter of fiscal 2015 and additional workforce reductions through August 31, 2015. During the third quarter the company recorded charges of approximately $70,000 related to workforce severance during the period and did not incur any additional severance costs for the additional workforce reductions made through June 30, 2015 or the more recent workforce reductions made in August 2015. These reductions are expected to reduce operating expenses by approximately $4,151,000 on an annualized basis. We have also taken actions to realign our data center operations for an expected annualized cost reduction of $203,000 and have executed an agreement with our landlord to relocate our current offices, which is anticipated to result in annualized savings of $372,000.
We believe that our experience with the VA telehealth project has enabled us to refine our telehealth products and services and position the company for success in the commercial market which we believe provides a significantly larger opportunity for the company as this market develops. We have continued to develop lower cost solutions for the commercial market that we were not able to deploy at the VA and we have already reduced our operating costs in excess of the monthly recurring revenues and equipment margins that we were generating from the VA project. Moving forward, we plan to repurpose our volume-tested, web-based management and monitoring solutions and investments to focus on offering a broader array of cost effective solutions to the healthcare market and increase our sales efforts on commercial market opportunities which we believe offer greater potential for growth.
4
Table of Contents
We intend to continue our efforts to market our web-based services and related products in our target markets. We also intend to focus on identifying additional applications and markets where our technology can address customer needs. However, the company has incurred significant recurring losses and our operations and product development activities have required substantial capital investment to date. These factors, among others, raise substantial doubt about our ability to continue as a going concern.
Our current revenues consist principally of transaction fees for web-based hosted software services and revenues from hardware sales, monthly monitoring services and maintenance fees from our telehealth business. For the fiscal year ended June 30, 2015 one customer accounted for approximately 45% of our consolidated revenues. Growth in our business is affected by a number of factors, including general economic and business conditions, and is characterized by long sales cycles. The timing of customer contracts, implementations and ramp-up to full utilization can have a significant impact on results and we believe our results over a longer period of time provide better visibility into our performance.
Non-binding Letter of Intent with Peachstate Health Management, LLC
On August 25, 2015, we announced that we entered into a non-binding letter of intent with Peachstate Health Management, LLC d/b/a AEON Clinical Laboratories, an expanding clinical laboratory based in Gainesville, GA (“AEON”) for the acquisition of all of the outstanding membership interests of AEON in exchange for shares of a newly created class of our Series E preferred stock (the “Series E Shares”). The letter of intent contemplates that the AEON members will be issued Series E Shares convertible into 19.9% of the outstanding shares of our common stock on the date of the closing of the merger transaction, and an additional 5% of the outstanding shares of the company’s common stock upon approval of the merger transaction by our shareholders. Additional Series E Shares will be issued to AEON members in 2016 and 2020 if AEON achieves certain financial results. The additional 2016 Series E Shares will be convertible into 24% of the outstanding shares of our common stock on the date of the closing and will be issued to the AEON members provided AEON achieves $16 million of EBITDA in calendar year 2015. The AEON members will be issued another tranche of Series E Shares in 2020 which, including the previously issued Series E Shares, will be convertible into 85% of the outstanding shares of our common stock (on a partially diluted basis as defined) provided AEON achieves $65.9 million in EBITDA, in the aggregate, in calendar years 2017 and 2018, or $99 million in EBITDA, in the aggregate, for calendar years 2016, 2017 and 2018. The letter of intent also provides for the issuance of Series E Shares as bonus shares for the achievement of $117 million in net income for the four fiscal years ending December 31, 2019, convertible into 5% of the outstanding shares of our common stock (on a partially diluted basis as defined). The holders of the Series E Shares will have certain preferential rights, including the right to vote separately as a class to nominate and elect one director for each 10% of the outstanding shares of the company’s common stock into which the outstanding Series E Shares shall be convertible.
The letter of intent is non-binding and any agreement is subject to the negotiation and execution of a definitive transaction agreement which may vary from the terms set forth in the letter of intent. The final transaction is also subject to material conditions, including, but not limited to, the approval of: (i) the respective boards of directors of the companies, (ii) the shareholders of the company and the members of AEON, (iii) the Nasdaq Stock Market, and (iv) other customary conditions for a transaction of this nature. Accordingly, there can be no assurance that a definitive agreement will be reached by the parties, or that any agreement will result in the completion of the merger transaction.
AEON Clinical Laboratories is a growing comprehensive and efficient clinical laboratory using state of the art testing equipment. AEON has developed proprietary methodologies that are designed to provide fast and reliable urine and oral fluid (saliva) test results. AEON provides health care professionals with four primary tests: Medical Toxicology, Pharmacogenomics, Cancer Genetic Testing, and Molecular Biology.
5
Table of Contents
Products and Services
We provide web-based revenue cycle management applications and telehealth products and services that (i) accelerate patient placement at care facilities or care providers; (ii) speed care order (referral) completion and approval and (iii) connect patients to their care providers through remote monitoring and communications. Our solutions incorporate workflow automation, electronic signature, and transaction management capabilities based on our Inscrybe® platform and remote patient management solutions using our tablet or Electronic House Call™ patient vital signs monitoring appliances or our Interactive Voice Response products and services. Our services are designed for ease of use and flexibility, and can be easily customized to meet the needs of specific industries or companies. Our products and services are scalable, facilitating the migration from existing paper processes and patient care practices and are designed to be seamlessly interfaced with billing, electronic medical records and hospital information and patient record systems. Our customers only require an Internet connection and web browser to access our web-based applications thereby utilizing previous investments in systems and technology. The ability of Inscrybe® to permit customers to choose the modules they want to implement, as well as the platform’s ability to support mixed-modal forms of communication contribute to the platform’s functionality and versatility.
Inscrybe® Healthcare—Inscrybe® Healthcare is a secure web-based revenue cycle management workflow automation solution that enables healthcare industry participants to securely exchange and track a variety of documents, certificates, authorizations, and other information over different modes of communication, including electronic and fax delivery. Inscrybe Healthcare incorporates electronic signatures, business- rules based electronic forms, content authentication using AuthentiProof, workflow intelligence for routing and transaction management, and identity credentialing and verification. Inscrybe Healthcare allows users to simplify complex clinical and administrative processes required for patient care, and facilitates order processing, online review and electronic signature of healthcare documentation, while validating the identity of the parties involved. Further, it is designed to comply with Health Insurance Portability and Accountability Act (HIPAA) guidelines. We designed the system in a modular fashion so it is easily configurable to meet customer needs and allows for the migration from current paper-based processes to an efficient paperless automated work environment. It is used to track and manage all kinds of structured and unstructured data and can be interfaced with existing in-house and external systems, including Health Information Exchange infrastructures. Inscrybe Healthcare includes the following workflow automation solutions:
| • | Inscrybe Referral and Order Management—provides an automated process for the exchange, update, completion and management of healthcare orders including certificates of medical necessity, plan-of-care forums, written orders, interim orders, prior authorizations, claim attachments and other supporting documentation required by healthcare payors for reimbursement of medical equipment and service claims from care providers. The physician module available with this solution provides physicians and their staff members with the ability to automate the referral order entry and tracking process and enables physicians to electronically sign order documents securely on the web. Physicians can also use this solution to refer patients to other physicians, communicate with patients using secure e-mail and track billable signatures and time spent managing patient care plans to support reimbursements. The solution’s community portal and workflow features enable home care and post-acute care providers, hospitals, health insurers and physicians to streamline important workflow processes which facilitate timely patient care, accelerate referral completion and reimbursement, maximize productivity, enhance compliance and reduce costs. |
| • | Inscrybe Hospital Discharge—automates the hospital discharge planning process and enables hospital case managers, social workers, and discharge planners to optimize the patient discharge process. The Hospital Discharge solution uses defined workflows for patient discharge referrals, eligibility verification and acceptance, and automatic notifications to suitable care facilities or home care providers. The solution improves hospital facility utilization by optimizing patient length-of-stay and bed turnover and can incorporate input from family members into the discharge process resulting in a more efficient, cost-effective discharge planning process and enhanced compliance with patient care |
6
Table of Contents
| plans. Hospitals can also use this solution to monitor post-discharge patient care to help reduce hospital readmissions and related costs imposed by recent regulatory reforms. |
Telehealth Solutions—provide an advanced in-home patient vital signs monitoring system and a web-based management and monitoring software module for use by healthcare providers. Our remote patient monitoring tablet and Electronic House Call devices work with peripheral devices, such as blood pressure monitors, weight scales, thermometers, glucometers, sleep apnea devices and wound care cameras, for unassisted patient vital signs measurement and monitoring. The system allows for manual entry or automatically takes vital signs from peripherals (both wired and wireless devices) and communicates with the practitioner over the Internet for analysis and intervention. Patients can access the information on the monitoring software interface to review their own vital statistics history, as well as to obtain reminders of their scheduled medications, practitioner instructions, and therapy regimen. The system also includes onscreen patient treatment information, disease management education, and intelligent routing to alert on-duty caregivers if any vital statistics fall outside of the range of parameters pre-set by the practitioner. The Interactive Voice Response (IVR) patient vital signs monitoring solution offers patients an alternative to using a dedicated vital signs in-home monitoring device. Using any touch-tone phone, patients can answer their session questions and enter their vital signs test results verbally or by entering their answers on their phone keypad. The IVR solution uses the same peripheral devices as the device based solution and answers to session questions and measurements are viewable by caregivers on the same web-based service. The IVR solution is designed for patients who require a flexible, mobile solution for care plan reminders and vital signs monitoring. We also provide a telehealth tablet and a telehealth software application to complement our current products and services. Additionally, physicians and their staff can order supplies and services for patients using the Inscrybe Healthcare feature provided by the system.
AuthentiProof™—a content integrity and time-and-date stamp application, enables a user to have a digital record of a transaction created and stored by a trusted third party that can be used to verify the content, date, time and parties related to the transaction in the future. AuthentiProof can be used to verify the authenticity of a document or file sent electronically as of a specific point in time and allows users to detect whether or not documents or files with an AuthentiProof seal have been altered or modified. AuthentiProof incorporates our proprietary content authentication technology.
Sales and Marketing
We sell our web-based services and telehealth products and services through a direct sales effort, reseller arrangements and group purchasing organizations (GPOs). Our resellers and GPOs typically receive a commission based on a percentage of the value of customer agreements we enter into due to their efforts. In cases where our contracts have a term exceeding one year, we generally defer service revenue derived from these contracts and recognize it over the life of the contract. We have also retained professional consultants to support our marketing and sales efforts by providing us with expertise in specific markets. Consultants may receive fixed fees, commissions or equity-based compensation. The markets for healthcare devices and solutions include integrated delivery networks, physician groups and networks, managed and accountable care organizations, hospitals, medical centers, home health agencies, pharmacies, governments and public health organizations.
Supply Relationships
We use AT&T Inc., to provide and maintain a secure hosting center at a facility in New York to host our web-based services. We believe that there are sufficient alternative suppliers of these services. We augment our own staff from time-to-time by using third party consultants and our software and services incorporate products and services which we license from unaffiliated third parties. We also use a contract manufacturer to assemble our Electronic House Call telehealth devices and several suppliers for our tablets, peripherals and various component parts and services. We believe that adequate alternative suppliers of these products and services exist on commercially reasonable terms so as to mitigate any adverse impacts caused by the termination of any of our existing relationships.
7
Table of Contents
Competition
We compete in markets for our web-based services and our telehealth products and services that are highly competitive and rapidly changing. Although we believe there is no single company that directly competes with all of our services and solutions, we do face intense competition from other companies with respect to our various offerings. Further, we are aware of efforts by other companies to develop products or services to either compete directly with our services, solutions, and products or that could be used as alternatives to our offerings. We believe that the principal competitive factors affecting the market for our services and solutions include features such as ease of use, quality/reliability of our offerings, scalability, features and functionality, customer service and support and price. Although we believe that our services, solutions and products compete favorably in respect of all these factors, there can be no assurance that we can maintain our competitive position against current or potential competitors.
These companies offer fax products, web-based processing of medical forms, signature solutions and patient monitoring products and services that could compete with our services, solutions and products. Almost all of these competitors are substantially larger or have more experience and market share than we do in their respective markets. In addition, companies with which we do not presently directly compete may become competitors in the future through their product development in the area of secure online services and telehealth services and such companies may have greater financial, technological, and marketing resources than we do. Therefore, these competitors may be able to respond more quickly than we can to new or changing opportunities, technologies, standards and customer requirements. Many of these competitors also have broader and more established distribution channels that may be used to deliver competing products or services directly to customers through bundling or other means. If such competitors were to bundle competing products or services for their customers, the demand for our products and services might be substantially reduced and the ability to distribute our products successfully and the utilization of our services would be substantially diminished.
New technologies and the expansion of existing technologies may increase competitive pressure. We cannot assure you that competing technologies developed by others or the emergence of new industry standards will not adversely affect our competitive position or render our services or technologies noncompetitive or obsolete. In addition, our markets are characterized by announcements of collaborative relationships involving our competitors. The existence or announcement of any such relationships could adversely affect our ability to attract and retain customers. As a result of the foregoing and other factors, we may not be able to compete effectively with current or future competitors, and competitive pressures that we face could materially harm our business.
Patents and Trademarks
Presently, we have one issued U.S. patent and one pending patent application. We also have been granted a license to one issued U.S. patent by Authentidate International AG, two issued U.S. patents by our former joint venture partner and their affiliate and one issued U.S. patent by a third party. We also entered into a license and settlement agreement with Robert Bosch Healthcare Systems, Inc. providing for the resolution and dismissal, with prejudice, of the purported patent infringement lawsuit filed by Bosch against Express MD in January 2012 and a license to the various asserted patents. Some of the technology embodied in some of our current products cannot be patented. We have registered the trademarks “Authentidate”, “Inscrybe”, “InscrybeMD”, “AuthentiProof” and “Inscrybe Office” in the U.S., the trademark “Authentidate” in the European Community and Canada, “AuthentiProof” in Canada, Mexico and the European Community, “Inscrybe” in the European Community and Canada “Inscrybe Office,” and a number of other trademarks as Madrid Protocol international registrations. We continue to take steps to protect our intellectual property rights including filing additional trademark and patent applications where appropriate. There can be no assurance that any patents or registrations will be issued or that any such patents or registrations that do issue will be effective to protect our products and services or trademarks from duplication by other manufacturers or developers or to prevent our competitors from offering similar products and services.
8
Table of Contents
Research and Development
The market for our web-based services and telehealth products is characterized by rapid technological change involving the application of a number of advanced technologies, including those relating to computer hardware and software, communication technologies, mass storage devices, electronic signatures, content authentication and other related technologies. Our ability to be competitive depends upon our ability to anticipate and effectively react to technological changes, changing market conditions and the requirements of our customers. Product development expenses for the fiscal years ended June 30, 2015, 2014 and 2013, were $1,180,000, $1,108,000 and $1,085,000 respectively.
Our product development activities are focused primarily on enhancing our products and services to address customer and healthcare market needs. During the past months, our research and development activities have been reduced in light of the non-renewal of our agreement with the VA and the associated reduction in force we implemented to realign our operating costs. We capitalize software development costs and amortize those costs in accordance with our policy disclosed in Note 1 of Notes to Consolidated Financial Statements included in this Annual Report on Form 10-K.
Intellectual Property
Other companies operating in our market may independently develop substantially equivalent proprietary information or otherwise obtain access to our know-how. In addition, there can be no assurance that we will be able to afford the expense of any litigation which may be necessary to enforce or defend our rights under any patent. Although we believe that the web-based services and telehealth products we sell do not and will not infringe upon the patents or violate the proprietary rights of others, it is possible that such infringement or violation has occurred or may occur. In the event that the web-based services and telehealth products we sell are deemed to infringe upon the patents or proprietary rights of others, we could be required to modify our offerings or obtain a license for the use and/or sale of such products and services. There can be no assurance that, in such an event, we would be able to do so in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do any of the foregoing could have a material adverse effect upon our business. In addition, if our current or proposed offerings are deemed to infringe upon the patents or proprietary rights of others, we could, under certain circumstances, become liable for damages or subject to an injunction, which could also have a material adverse effect on our business. It is our policy to investigate allegations of third party intellectual property rights to the extent that they are brought to our attention or to the extent that we become independently aware of such third party intellectual property rights to ensure that our current and proposed products and services do not infringe on any such rights. We cannot provide any assurances that our products or services do not infringe upon any other patents, including the patents that we have investigated.
In addition, with respect to our telehealth offerings, in connection with the termination of the joint venture our former joint venture partner and an affiliate licensed to us certain intellectual property assets to enable us to continue to commercialize and develop the Electronic House Call™ remote patient monitoring products and services. Accordingly, our right to utilize any such intellectual property is subject to the terms of this agreement. Further, and similar to the intellectual property owned by us, there can be no assurance that the intellectual property licensed to us will be effective to protect our products and services from duplication by other manufacturers or developers or to prevent our competitors from offering similar products and services.
As described in greater detail in Item 3—Legal Proceedings, on May 22, 2015, the company, together with its subsidiary Express MD Solutions, LLC entered into a license and settlement agreement with Robert Bosch Healthcare Systems, Inc. providing for the resolution and dismissal, with prejudice, of the purported patent infringement lawsuit filed by Bosch against Express MD in January 2012. While the Company does not believe that it was or is infringing any of the asserted patents, in order to mitigate its risk and avoid further costs and distractions of litigation, it entered into the license and settlement agreement. The agreement provides for the company to be granted by Bosch a worldwide, non-exclusive, non-assignable, royalty bearing license under the patents and a covenant not to be sued under certain other patents owned by Bosch.
9
Table of Contents
Employees
At June 30, 2015, we employed 25 full-time employees throughout our operations, including our senior management and have reduced our staff to approximately 17 during the first quarter of fiscal 2016. None of our employees are represented by a collective bargaining agreement and we believe that our employee relations are satisfactory. In the normal course of business, we have also used part-time employees and contracted with third parties to provide support for various projects.
Government Regulation
Government Matters
Compliance with federal, state, local, and foreign laws, including laws enacted for the protection of the environment has to date had no material effect upon our capital expenditures, results of operations, or competitive position. Although we do not anticipate any material adverse effects in the future based on the nature of our operations, no assurance can be given such laws, or any future laws enacted, will not have a material adverse effect on our business.
Government Regulation of Medical Devices
Government authorities in the United States at the federal, state and local levels and foreign countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, promotion, advertising, distribution, sampling, marketing, and import and export of medical devices. Various federal, state, local and foreign statutes and regulations also govern testing, manufacturing, safety, labeling, storage, distribution and record-keeping related to such products and their marketing. The process of obtaining these approvals and clearances, and the subsequent process of maintaining substantial compliance with appropriate federal, state, local, and foreign statutes and regulations, can require the expenditure of substantial time and financial resources. In addition, statutes, rules, regulations and policies may change and new legislation or regulations may be issued that could delay such approvals.
Under the Federal Food Drug and Cosmetic Act, medical devices are classified into one of three classes: Class I, Class II or Class III. The classification of a device into one of these three classes generally depends on the degree of risk associated with the medical device and the extent of control needed to ensure safety and effectiveness. Class I and II devices must be able to demonstrate safety and efficacy by adhering to a set of general controls, including compliance with the applicable portions of the FDA’s Quality System Regulation, which sets forth good manufacturing practice requirements; facility registration, device listing and product reporting of adverse medical events; truthful and non-misleading labeling; and promotion of the device only for its cleared or approved intended uses. Class II devices are also subject to these general controls, and any other special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. Review and clearance by the FDA for these devices is typically accomplished through the so-called 510(k) pre-market notification procedure. When 510(k) clearance is sought, a sponsor must submit a pre-market notification demonstrating that the proposed device is substantially equivalent to a legally marketed Class II device (for example, a device previously cleared through the 510(k) pre-market notification process). If the FDA agrees that the proposed device is substantially equivalent to the predicate device, then 510(k) clearance to market will be granted. After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, requires a new 510(k) clearance or could require pre-market approval, or PMA. The FDA has categorized our telehealth product as a Class II device.
Both before and after a medical device is commercially distributed, manufacturers and marketers of the device have ongoing responsibilities under FDA regulations. The FDA reviews design and manufacturing practices, labeling and record keeping, and manufacturers’ required reports of adverse experiences and other information to identify potential problems with marketed medical devices. Device manufacturers are subject to periodic and unannounced inspection by the FDA for compliance with the Quality System Regulation, which sets
10
Table of Contents
forth the current good manufacturing practice requirements that govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, servicing, labeling, storage, installation and distribution of all finished medical devices intended for human use.
FDA regulations prohibit the advertising and promotion of a medical device for any use outside the scope of a 510(k) clearance or PMA approval or for unsupported safety or effectiveness claims. Although the FDA does not regulate physicians’ practice of medicine, the FDA does regulate manufacturer communications with respect to off-label use. If the FDA finds that a manufacturer has failed to comply with FDA laws and regulations or that a medical device is ineffective or poses an unreasonable health risk, it can institute or seek a wide variety of enforcement actions and remedies, ranging from a public warning letter to more severe actions such as:
| • | fines, injunctions and civil penalties; |
| • | recall or seizure of products; |
| • | operating restrictions, partial suspension or total shutdown of production; |
| • | refusing requests for 510(k) clearance or PMA approval of new products; |
| • | withdrawing 510(k) clearance or PMA approvals already granted; and |
| • | criminal prosecution. |
The FDA also has the authority to require repair, replacement or refund of the cost of any medical device.
Third-Party Reimbursement
Our telehealth products are used for medical purposes generally covered by government or private health plans. In general, a third-party payor only covers a medical product or procedure when the plan administrator is satisfied that the product or procedure improves health outcomes, including quality of life or functional ability, in a safe and cost-effective manner. Even if a device has received clearance or approval for marketing by the FDA, there is no assurance that third-party payors will cover the cost of the device and related procedures. In many instances, third-party payors use price schedules that do not vary to reflect the cost of the products and equipment used in performing those procedures. In other instances, payment or reimbursement is separately available for the products and equipment used, in addition to payment or reimbursement for the procedure itself. Even if coverage is available, third-party payors may place restrictions on the circumstances where they provide coverage or may offer reimbursement that is not sufficient to cover the cost of our products.
Third-party payors who cover the cost of medical products or equipment, in addition to allowing a general charge for the procedure, often maintain lists of exclusive suppliers or approved lists of products deemed to be cost-effective. Authorization from those third-party payors is required prior to using products that are not on these lists as a condition of reimbursement. If our products are not on the approved lists, healthcare providers must determine if the additional cost and effort required in order to obtain prior authorization, and the uncertainty of actually obtaining coverage, is justified by any perceived clinical benefits from using our products. If hospitals and physicians cannot obtain adequate reimbursement for our products or the procedures in which they are used, our business, financial condition, results of operations, and cash flows could suffer a material adverse impact.
Health Care Reform
On March 23, 2010, the Patient Protection and Affordable Care Act was signed into law and on March 30, 2010, the Health Care and Education Reconciliation Act of 2010 was signed into law. Together, the two measures make the most sweeping and fundamental changes to the U.S. healthcare system since the creation of Medicare and Medicaid. The Health Care Reform laws include a large number of health-related provisions to take effect over the next four years, including expanding Medicaid eligibility, requiring most individuals to have health insurance, establishing new regulations on health plans, establishing health insurance exchanges, requiring
11
Table of Contents
manufacturers to report payments or other transfers of value made to physicians and teaching hospitals, and modifying certain payment systems to encourage more cost-effective care and a reduction of inefficiencies and waste, including through new tools to address fraud and abuse. In 2013, a 2.3% excise tax on the sale by manufacturers, producers and importers of certain medical devices that are not exempted from such tax was imposed under these health care reform laws. Further, as administrative rules implementing healthcare reform under the legislation are not yet finalized or have been modified, the impact of the healthcare reform legislation on our business is unknown, and there can be no assurances that healthcare reform legislation will not adversely impact either our operational results or the manner in which we operate our business. Healthcare industry participants may respond by reducing their investments or postponing investment decisions, including investments in our solutions and services.
In addition, various healthcare reform proposals have also emerged at the state level. We cannot predict the exact effect newly enacted laws or any future legislation or regulation will have on us. However, the implementation of new legislation and regulation may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business, possibly materially. In addition, the enacted excise tax may materially and adversely affect our operating expenses and results of operations.
Fraud and Abuse Laws
We are subject to various federal and state laws pertaining to healthcare fraud and abuse, which, among other things, prohibit the offer or acceptance of remuneration intended to induce or in exchange for the purchase of products or services reimbursed under a federal healthcare program and the submission of false or fraudulent claims with the government. These laws include the federal Anti-Kickback Statute, the False Claim Act and comparable state laws. These laws regulate the activities of entities involved in the healthcare industry, such as us, by limiting the kinds of financial arrangements such entities may have with healthcare providers who use or recommend the use of medical products (including for example, sales and marketing programs, advisory boards and research and educational grants). In addition, in order to ensure that healthcare entities comply with healthcare laws, the Office of Inspector General, or OIG, of the U.S. Department of Health and Human Services recommends that healthcare entities institute effective compliance programs. To assist in the development of effective compliance programs, the OIG has issued model Compliance Program Guidance, or CPG, materials for a variety of healthcare entities which, among other things, identify practices that may implicate the federal Anti-Kickback Statute and other relevant laws and describes elements of an effective compliance program. Violations of these laws can lead to civil and criminal penalties, damages, imprisonment, fines, exclusion from participation in Medicare, Medicaid and other federal healthcare programs, and the curtailment or restructuring of our operations. Any such violations could have a material adverse effect on our business, financial condition, results of operations or cash flows.
HIPAA
Two federal crimes were created under the Health Insurance Portability and Accountability Act of 1996, or HIPAA: healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private payors. The false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Individually identifiable health information is subject to an array of federal and state regulation. Federal rules promulgated pursuant to HIPAA regulate the use and disclosure of health information by “covered entities.” Covered entities include individual and institutional healthcare providers from which we may receive individually identifiable health information. These regulations govern, among other things, the use and disclosure of health information for research purposes, and require the covered entity to obtain the written authorization of the individual before using or disclosing health information for research. Failure of the covered entity to obtain such authorization could subject the covered entity to civil and criminal penalties. We may experience delays and complex negotiations as we deal with each entity’s differing interpretation of the regulations and what is required for compliance. Also, where our customers or
12
Table of Contents
contractors are covered entities, including hospitals, universities, physicians or clinics, we may be required by the HIPAA regulations to enter into “business associate” agreements that subject us to certain privacy and security requirements. In addition, many states have laws that apply to the use and disclosure of health information, and these laws could also affect the manner in which we conduct our research and other aspects of our business. Such state laws are not preempted by the federal privacy law where they afford greater privacy protection to the individual. While activities to assure compliance with health information privacy laws are a routine business practice, we are unable to predict the extent to which our resources may be diverted in the event of an investigation or enforcement action with respect to such laws. HIPAA regulations also require national standards for some types of electronic health information transactions and the data elements used in those transactions, security standards to ensure the integrity and confidentiality of health information and standards to protect the privacy of individually identifiable health information. Moreover, the Health Information Technology for Economic and Clinical Health Act (HITECH) provisions of the American Recovery and Reinvestment Act of 2009 (ARRA,) and associated regulatory requirements, extend many of the HIPAA obligations, formerly imposed only upon covered entities, to business associates as well. The extension of these HIPAA obligations to business associates by law has created additional liability risks related to the privacy and security of individually identifiable health information.
In addition, in accordance with requirements under HIPAA, the U.S. Department of Health and Human Services (“HHS”) is implementing a new version of the standards for HIPAA-covered electronic transactions, including claims, remittance advices, and requests and responses for eligibility. These standards are called ANSI-5010. Additionally, HIPAA requires all entities that are covered by HIPAA to upgrade to the tenth revision of the International Statistical Classification of Diseases and Related Health Problems promulgated by the World Health Organization, also known as ICD-10, for use in reporting medical diagnoses and inpatient procedures.
Available Information
We file registration statements, periodic and current reports, proxy statements, and other materials with the Securities and Exchange Commission (SEC). You may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Room 1580, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a web site at www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC, including our filings. We make our public filings with the SEC, including our Annual Report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all exhibits and amendments to these reports available free of charge on our web site, http://www.authentidate.com, as soon as reasonably practicable after we file such material with the SEC. We also make available on our web site reports filed by our executive officers and directors on Forms 3, 4 and 5 regarding their ownership of our securities. These materials are available in the “Investor Relations” portion of our web site, under the link “SEC Filings.” We also use our web site to make generally available important information about our company. Important information, including press releases, presentation and financial information regarding our company, is routinely posted on and accessible on the Investor Relations subpage of our web site, which is accessible by clicking on the tab labeled “Investors” on our web site home page. Therefore, investors should look to the “Investor Relations” subpage of our web site for important information. Information contained on our web site is not part of this Annual Report on Form 10-K.
Corporate Information
Authentidate Holding Corp. was organized in August 1985 as Bitwise Designs, Inc. and reincorporated under the laws of the state of Delaware in May 1992. We changed our name to Authentidate Holding Corp. in March 2001. Our executive office is presently located at the Connell Corporate Center, 300 Connell Drive, 5th Floor, Berkeley Heights, New Jersey 07922, and our telephone number is (908) 787-1700. Authentidate, Inc. was organized as a majority-owned subsidiary during our 2000 fiscal year and we presently own 100% of the outstanding capital stock of this company. ExpressMD Solutions LLC was formed in June, 2008 and we presently own 100% of the membership interests of this company.
13
Table of Contents
As provided for under the Private Securities Litigation Reform Act of 1995, we wish to caution shareholders and investors that the following important factors, among others discussed throughout this Annual Report on Form 10-K for the fiscal year ended June 30, 2015, have affected, and in some cases could affect, our actual results of operation and cause our results to differ materially from those anticipated in forward looking statements made herein. Our business, results of operations and financial condition may be materially and adversely affected due to any of the following risks. The risks described below are not the only ones we face. Additional risks we are not presently aware of or that we currently believe are immaterial may also impair our business operations. The trading price of our common stock could decline due to any of these risks. In assessing these risks, you should also refer to the other information contained or incorporated by reference in this Annual Report on Form 10-K, including our financial statements and related notes.
Risks Related to Our Financial Condition
Failure to increase our revenue and keep our expenses consistent with revenues could prevent us from achieving and maintaining profitability in the near term as we seek to generate new business revenues to offset the loss of our largest customer.
We incurred net losses of approximately $9,700,000, $7,143,000 and $11,349,000 for the fiscal years ended June 30, 2015, 2014 and 2013, respectively and had an accumulated deficit of approximately $206,485,000 at June 30, 2015. We have expended, and will continue to be required to expend, substantial funds to pursue product development projects, enhance our marketing and sales efforts and to otherwise operate our business. Therefore, we will need to generate higher revenues to achieve and maintain profitability and cannot assure you that we will be profitable in any future period. Further, our ability to generate the level of revenues we require to achieve profitability was adversely impacted by the decision of the Department of Veterans Affairs not to exercise the remaining renewal period of our contract. The loss of this customer has increased and accelerated our need to diversify our business by adding new customers. Our prospects should be considered in light of the difficulties frequently encountered in connection with the establishment of a new business line, which characterizes our business, such as the difficulty in creating a viable market, the significant related development and marketing costs and the overall competitive environment in which we operate. Accordingly, there can be no assurance that we will be able to achieve profitable operations in future operating periods. Our business results are likely to remain uncertain as we are unable to reliably predict revenues from our current customers. Revenue levels achieved from our customers, the mix of products and solutions that we offer, our ability to introduce new products as planned and our ability to reduce and manage our operating expenses will affect our financial results. Consequently, we may not be profitable in any future period. Unless we can generate additional revenues from sales or rentals of products and services to existing customers or obtain new customers, we will continue to generate substantial operating losses and may need to curtail our business and incur additional restructuring or exit costs, which may be material. These factors, among others, raise substantial doubt about our ability to continue as a going concern. Our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Our capital requirements have been significant and until our revenues can sufficiently support our operating costs, we expect to raise additional capital to finance our operations and repay outstanding debt obligations.
Our capital requirements have been and will continue to be significant. We have been substantially dependent upon private placements and registered offerings of our securities and on short-term and long-term debt transactions to fund such requirements. We are expending significant amounts of capital to develop, promote and market our software, services and products. Due to these expenditures, we have incurred significant losses to date. We used approximately $4,951,000, $4,726,000 and $5,214,000 in cash for operating activities for the fiscal years ended June 30, 2015, 2014 and 2013, respectively. Our available cash and cash equivalents as of June 30, 2015 totaled approximately $247,000. Our current estimated monthly operational requirements after
14
Table of Contents
giving effect to our cost cutting measures, but not to any special or one-time events is approximately $350,000. Since February 2015, we have completed a number of debt financing transactions resulting in total proceeds of approximately $3.50 million, and have approximately $1.45 million in notes that must be repaid in October 2015. We have an immediate need for additional capital and are continuing to explore additional potential transactions to improve our capital position. We expect our existing resources, revenues generated from operations, net proceeds from our debt financing transactions, other transactions we are considering and proceeds received from the exercise of outstanding warrants or restructuring of debt (of which there can be no assurance) to satisfy our working capital requirements for at least the next twelve months; however, no assurances can be given, that we will be able to attain sales levels and support our costs through revenues derived from operations or generate sufficient cash flow to satisfy our other obligations. These factors, among others, raise substantial doubt about our ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments to the recoverability and classification of assets carrying amounts or the amounts and classifications of liabilities that might result from the outcome of these uncertainties. Unless we are able to increase revenues substantially or generate additional capital from other transactions, our current cash resources will only satisfy our working capital needs for a limited period of time. In addition to our recently announced letter of intent for a business combination transaction, we are continuing to explore additional potential transactions to improve our capital position to ensure we are able to meet our financing and working capital requirements. We would expect to raise additional funds through public or private equity offerings, debt financings or strategic alliances. Raising additional funds by issuing equity or convertible debt securities may cause our stockholders to experience substantial dilution in their ownership interests and new investors may have rights superior to the rights of our other stockholders. Raising additional funds through debt financing or preferred stock, if available, may involve covenants that restrict our business activities and options and such additional securities may have powers, designations, preferences or rights senior to our currently outstanding securities. We may also enter into financing transactions which involve the granting of liens on our assets or which grant preferences of payment from our revenue streams, all of which could adversely impact our ability to rely on our revenue from operations to support our ongoing operating costs. Alternatively, we may seek to obtain new financing from existing security holders, which may include reducing the exercise or conversion prices of outstanding securities, or the issuance of additional equity securities. Currently, the company does not have any definitive agreements with any third-parties for such transactions and there can be no assurance, however, that we will be successful in completing the transaction contemplated by the letter of intent, raising additional capital or securing financing when needed or on terms satisfactory to the company. If we are unable to raise additional capital when required, or on acceptable terms, we will need to reduce costs and operations substantially or potentially suspend operations, any of which would have a material adverse effect on our business, financial condition and results of operations. Our future capital requirements will depend on, and could increase substantially as a result of many factors, including:
| • | our need to utilize cash to support research and development activities and to make incremental investments in our organization; |
| • | our ability to achieve targeted revenue, gross profit margins and cost management objectives; |
| • | our ability to reach break-even or profitability; |
| • | the success of our sales and marketing efforts; |
| • | our need to repay indebtedness; |
| • | the extent and terms of any development, marketing or other arrangements; and |
| • | changes in economic, regulatory or competitive conditions, including the continuing economic weakness and federal budgetary uncertainty. |
15
Table of Contents
We depend on a limited number of customers for a substantial portion of our revenues, and the loss of the VA as a customer and the loss, or a significant reduction in purchases by, one or more of our other important customers will adversely affect our operating results.
We receive a significant amount of our revenues from a limited number of customers. Over recent years, the Department of Veterans Affairs (VA) had been our largest customer, generating approximately 45% of our total consolidated revenues for the fiscal year ended June 30, 2015. On March 6, 2015, the company announced that the VA informed the company that it was not exercising the fourth and final option year under the contract for telehealth services. Our VA revenue included both recurring services revenue as well as hardware sales. Due to this, the company expects to report significantly reduced revenues over the next several quarters and the loss of this business will materially adversely affect our business, results of operations and financial condition until we are able to replace the revenues that had been generated through our VA contract. We will need to attract new clients and attempt to diversify our customer base from a limited number of potential customers. In general, most of our customer orders for our telehealth business have been and are expected to continue to be made on a purchase order basis, which does not generally require any long-term commitments nor any minimum purchase requirements. Therefore, these customers may alter their past purchasing behavior with little or no notice to us for various reasons. If our customers alter their past (or expected) purchasing behavior, or if we encounter any problems collecting amounts due from them, our financial condition and results of operations could be negatively impacted.
Our revenues may be affected by changes in technology spending levels.
In the past, unfavorable or uncertain macroeconomic conditions and reduced global technology spending rates have adversely affected the markets in which we operate. Current economic conditions and ongoing uncertainty about the economic recovery could reduce the demand for our products and services and negatively impact revenues and operating profit. We are unable to predict changes in general macroeconomic conditions and when global spending rates will be affected. Furthermore, even if spending rates increase, we cannot be certain that the market for our products and services will be positively impacted. If there are future reductions in spending rates, or if spending rates do not increase, our revenues, operating results and financial condition may be adversely affected.
Economic volatility and uncertainty may negatively impact our business, results of operations, financial condition or liquidity.
Our markets have been and will continue to be affected by general macroeconomic and market conditions. Developments, such as the lingering economic instability in the U.S., Europe and China, the imposition of government spending restrictions in the U.S. (such as through sequestration), and the inflationary risks associated with higher commodity prices, among other developments, have generated significant instability in the credit and financial markets and may have an adverse effect on our results of operations and financial condition. Volatile, negative or uncertain economic conditions in our significant markets have undermined and could in the future undermine business confidence in our markets and cause our clients to reduce or defer their spending on new technologies or initiatives or terminate existing contracts, which would negatively affect our business. While the U.S. economy appears to have stabilized somewhat relative to its performance in recent years, the ultimate impact of these developments cannot be predicted, and they may have a material adverse effect on our liquidity and financial condition if our ability to obtain financing for operations or obtain credit from trade creditors were to be impaired. If general economic conditions further deteriorate or economic uncertainty continues, we or our customers might experience future deterioration of their businesses, cash flow shortages and financing difficulties.
Healthcare policy changes, including recent laws to reform the U.S. healthcare system, may have a material adverse effect on us.
Healthcare costs have risen significantly over the past decade. There have been, and continue to be, proposals by legislators, regulators, and third-party payors to keep these costs down. Certain proposals, if passed,
16
Table of Contents
could impose limitations on the prices we will be able to charge for our products, or the amounts of reimbursement available for our products from governmental agencies or third-party payors. These limitations could have a material adverse effect on our financial position and results of operations.
On March 23, 2010, the Patient Protection and Affordable Care Act was signed into law and on March 30, 2010, the Health Care and Education Reconciliation Act of 2010 was signed into law. Together, the two measures make the most sweeping and fundamental changes to the U.S. healthcare system since the creation of Medicare and Medicaid. The Health Care Reform laws include a large number of health-related provisions to take effect over the next four years, including expanding Medicaid eligibility, requiring most individuals to have health insurance, establishing new regulations on health plans, establishing health insurance exchanges, requiring manufacturers to report payments or other transfers of value made to physicians and teaching hospitals, and modifying certain payment systems to encourage more cost-effective care and a reduction of inefficiencies and waste, including through new tools to address fraud and abuse. In 2013, a 2.3% excise tax on the sale by manufacturers, producers and importers of certain medical devices that are not exempted from such tax was imposed under these health care reform laws. Further, as administrative rules implementing healthcare reform under the legislation are not yet finalized or have been modified, the impact of the healthcare reform legislation on our business is unknown, and there can be no assurances that healthcare reform legislation will not adversely impact either our operational results or the manner in which we operate our business. Healthcare industry participants may respond by reducing their investments or postponing investment decisions, including investments in our solutions and services.
In addition, various healthcare reform proposals have also emerged at the state level. We cannot predict the exact effect newly enacted laws or any future legislation or regulation will have on us. However, the implementation of new legislation and regulation may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business, possibly materially. In addition, the enacted excise tax may materially and adversely affect our operating expenses and results of operations.
We depend on growth in the software as a service market, and lack of growth or contraction in this market could materially adversely affect our sales and financial condition.
Our hosted software and web-based solutions compete with other “software as a service” solutions. Demand for our solutions and software offerings is driven by several factors, including an increased focus on protecting business-critical applications, government and industry regulations requiring data protection and integrity, and the growth in the market for software as a service. Segments of the computer and software industry have in the past experienced significant economic downturns and decreases in demand as a result of changing market factors. A change in the market factors that are driving demand for offerings of software as a service could adversely affect our sales, profitability and financial condition.
We depend on third parties for the supply and manufacture of our telehealth products, which may result in delays and quality-control issues and new regulations related to conflict minerals could adversely impact our business.
We do not own or lease any manufacturing facilities. Accordingly, in order to market our telehealth solution we purchase finished products and components from unaffiliated suppliers and use a contract manufacturer to produce our Electronic House Call device. In addition, we may use unaffiliated third parties to provide distribution services for this solution. If the agreements with these third parties are terminated or if they are unable to perform their obligations under such agreements, it could take several months to establish and qualify alternative suppliers and manufacturing and distribution partners for our products and we may not be able to fulfill our customers’ orders in a timely manner. At the present time we believe that if existing third party relationships terminate, alternative providers are available on commercially reasonable terms. However, there can be no assurance that the future production capacity of our current manufacturer will be sufficient to satisfy our requirements or that alternative providers of components or manufacturing or distribution services will be
17
Table of Contents
available on commercially reasonable terms, or at all. The failure to identify suitable alternative suppliers, manufacturers or distributors could adversely impact our customer relationships and our financial condition. In addition, due to our use of third-party manufacturers and distributors, we do not have control over the timing of product shipments. Delays in shipment could result in the deferral or cancellation of purchases of our products, which would harm our results of operations in any particular quarter. Revenue for a period may be lower than predicted if large orders forecasted for that period are delayed or are not realized, which could impact cash flow or result in a decline in our stock price.
Moreover, pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act, the SEC promulgated new rules applicable to public companies concerning the use of certain minerals and metals, known as conflict minerals, in their products. The rules require us to undertake measures to understand the origin and, as need be, source of any conflict minerals within our supply chain and commencing in May 2014, to disclose, among other things, those measures and whether or not any such conflict minerals in our products originated from the Democratic Republic of the Congo and adjoining countries. We expect that we will incur additional costs in complying with this rule, including for diligence measures undertaken to understand the origin and, as need be, source of conflict minerals used in certain of our products, in addition to the potential cost of implementing changes to products, processes, or sources of supply as a consequence of such verification activities. In addition, the implementation of these rules could adversely affect the sourcing, supply, and pricing of materials used in certain of our products by reducing the supply of “conflict free” components and parts. We cannot be sure that we will be able to obtain the necessary information on conflict minerals from our suppliers in any future periods, or that we will be able to determine that all of our products are conflict free. As a result, we may face reputational challenges with our customers, stockholders and other stakeholders if we determine that our products contain minerals not determined to be conflict free or if we are unable to sufficiently verify the origins for all conflict minerals used in our products through the procedures we implement.
Our business may be adversely affected by legal proceedings.
We have been in the past, and may become in the future, involved in legal proceedings. You should carefully review and consider the various disclosures we make in our reports filed with the SEC regarding legal matters that may affect our business. As described in greater detail below, on May 22, 2015, the company, together with its subsidiary Express MD Solutions, LLC entered into a license and settlement agreement with Robert Bosch Healthcare Systems, Inc. providing for the resolution and dismissal, with prejudice, of the purported patent infringement lawsuit filed by Bosch against Express MD in January 2012 in the U.S. District Court for the Northern District of California, Case No. 5:12-cv-00068-JW. While the company does not believe that it is infringing any of the asserted patents, in order to mitigate its risk and avoid further costs and distractions of litigation, it entered into the license and settlement agreement. As previously reported by the company, the complaint alleged that the company’s “Electronic House Call” product infringes one or more claims of certain patents allegedly owned by Bosch. The agreement provides for the company to be granted by Bosch a worldwide, non-exclusive, non-assignable, royalty bearing license under the patents and a covenant not to be sued under certain other patents owned by Bosch. In consideration thereof, the company agreed to pay Bosch for the license and related matters and to pay certain royalties to Bosch for a three year period thereafter.
The expense of defending such litigation may be substantial and the time required to defend the actions could divert management’s attention from the day-to-day operations of our business, which could adversely affect our business, results of operations and cash flows. We cannot predict with certainty the outcome of any legal proceedings in which we become involved and it is difficult to estimate the possible costs to us stemming from any such matters. In addition, an unfavorable outcome in such litigation could have a material adverse effect on our business, results of operations, financial position and cash flows.
18
Table of Contents
Our success is dependent on the performance of our management and the cooperation, performance and retention of our executive officers and key employees.
Our business and operations are substantially dependent on the performance of our senior management team and executive officers. If our management team is unable to perform it may adversely impact our results of operations and financial condition. We do not maintain “key person” life insurance on any of our executive officers. The loss of one or several key employees could seriously harm our business. Any reorganization or reduction in the size of our employee base could harm our ability to attract and retain other valuable employees critical to the success of our business.
If we lose key personnel or fail to integrate replacement personnel successfully, our ability to manage our business could be impaired.
Our future success depends upon the continued service of our key management, technical, sales, finance, and other critical personnel. Other than with respect to employment agreements that we entered into with our executive officers, our key personnel do not have employment agreements and we cannot assure you that we will be able to retain them. Key personnel have left our company in the past and there likely will be additional departures of key personnel from time to time in the future. Further, following the non-renewal of our telehealth contract with the VA, in order to reduce our operating costs, we have implemented several reductions in staff and the use of external resources and to date have eliminated a number of positions throughout the company, or approximately 50% of our workforce. The loss of any key employee could result in significant disruptions to our operations, including adversely affecting the timeliness of product releases, the successful implementation and completion of company initiatives, the effectiveness of our disclosure controls and procedures and our internal control over financial reporting, and the results of our operations. In addition, hiring, training, and successfully integrating replacement sales and other personnel could be time consuming, may cause additional disruptions to our operations, and may be unsuccessful, which could negatively impact future revenues.
Developing and implementing new or updated software and services and other product offerings may take longer and cost more than expected.
We rely on a combination of internal development, strategic relationships, licensing and acquisitions to develop our products and services. The cost of developing new software, services and other product offerings, such as Inscrybe Healthcare and related modules, and our telehealth offerings is inherently difficult to estimate. Our development and implementation of proposed software, services or other product offerings may take longer than originally expected, require greater investment of cash resources than initially expected, require more testing than originally anticipated and require the acquisition of additional personnel and other resources. Accordingly, we expect to face substantial uncertainties with respect to the performance and market acceptance of new software and services and other product offerings. If we are unable to develop new or updated software, services or other product offerings on a timely basis and implement them without significant disruptions to the existing systems and processes of our customers, we may lose potential revenues and harm our relationships with current or potential customers.
The success of any of our product acquisition and licensing activities is subject to uncertainty and any completed acquisitions or licenses may reduce our earnings, be difficult to integrate, not perform as expected or require us to obtain additional financing.
We regularly evaluate selective acquisitions and look to continue to enhance our product line by acquiring rights to additional products and services. Such acquisitions may be carried out through the purchase of assets, joint ventures and licenses or by acquiring other companies. However, we cannot assure you that we will be able to complete acquisitions or in-licensing arrangements that meet our target criteria on satisfactory terms, if at all. Successfully integrating a product or service acquisition or in-licensing arrangement can be a lengthy and complex process. The diversion of our management’s attention and any delays or difficulties encountered in
19
Table of Contents
connection with any of our acquisitions or arrangements could result in the disruption of our ongoing business or inconsistencies in standards, controls, procedures and policies that could negatively affect our ability to maintain relationships with customers, suppliers, employees and others with whom we have business dealings. In addition, other companies, including those with substantially greater resources than ours, may compete with us for the acquisition of product or in-licensing candidates and approved products, resulting in the possibility that we devote resources to potential acquisitions or arrangements that are never completed. If we do engage in any such acquisition or arrangement, we will incur a variety of costs, and we may never realize the anticipated benefits of the acquisition or arrangement in light of those costs. If we fail to realize the expected benefits from acquisitions or arrangements we may consummate in the future, whether as a result of unidentified risks, integration difficulties, regulatory setbacks or other events, our business, results of operations and financial condition could be adversely affected.
In addition, our product acquisition and licensing activities may require us to obtain additional debt or equity financing, resulting in increased debt obligations or dilution of ownership to our existing stockholders, as applicable. Therefore, we may not be able to finance acquisitions on terms satisfactory to us, if at all.
New or updated software, services and product offerings will not become profitable unless they achieve sufficient levels of market acceptance, which may require significant efforts and costs.
There can be no assurance that customers and potential customers will accept from us new or updated software, services and other products. The future results of our business will depend, in significant part, on the success of our software, services or other product offerings. Current and potential customers may choose to use similar products and services offered by our competitors or may not purchase new or updated software, services or products, especially when they are initially offered and if they require changes in equipment or workflow. For software, services and products we are developing or may develop in the future, there can be no assurance that we will attract sufficient customers or that such offerings will generate sufficient revenues to cover their associated development, marketing and maintenance costs. Furthermore, there can be no assurance that any pricing strategy that we implement for any new software and services or other product offerings will be economically viable or acceptable to the target markets. Failure to achieve broad penetration in target markets with respect to new or updated software, services and product offerings could have a material adverse effect on our business prospects. Further, achieving market acceptance for new or updated software, services and product offerings is likely to require substantial marketing efforts and expenditure of significant funds to create awareness and demand by potential customers.
We do not have patents on all the technology we use, which could harm our competitive position.
Presently, we have one issued U.S. patent and one pending patent application. We also have been granted a license to one issued U.S. patent by Authentidate International AG, two issued U.S. patents by our former joint venture partner and their affiliate and one issued U.S. patent by a third party. We also entered into a license and settlement agreement with Robert Bosch Healthcare Systems, Inc. providing for the resolution and dismissal, with prejudice, of the purported patent infringement lawsuit filed by Bosch against Express MD in January 2012 and a license to the various asserted patents. Some of the technology embodied in some of our current products cannot be patented. We have registered the trademarks “Authentidate”, “Inscrybe”, “InscrybeMD”, “AuthentiProof” and “Inscrybe Office” in the U.S., the trademark “Authentidate” in the European Community and Canada, “AuthentiProof” in Canada, Mexico and the European Community, “Inscrybe” in the European Community and Canada, “Inscrybe Office,” and a number of other trademarks as Madrid Protocol international registrations. We continue to take steps to protect our intellectual property rights including filing additional trademark and patent applications where appropriate. We rely on confidentiality agreements with our key employees to the extent we deem it to be necessary. We further intend to file patent applications for any new products we may develop, to the extent that we believe that any technology included in such products is patentable. There can be no assurance that any patents in fact, will be issued or that any such patents that do issue will be effective to protect our products and services from duplication by other manufacturers or developers or to
20
Table of Contents
prevent our competitors from offering similar products and services. Other companies operating within our business segments may independently develop substantially equivalent proprietary information or otherwise obtain access to our know-how, much of which is maintained as trade secrets and there can be no assurance that we will be able to afford the expense of any litigation which may be necessary to enforce our rights under any patent.
In addition, with respect to our telehealth offerings, in connection with the termination of the joint venture, our former joint venture partner and an affiliate licensed to us certain intellectual property assets to enable us to continue to commercialize and develop the ExpressMD Solutions remote patient monitoring products and services. Accordingly, our right to utilize any such intellectual property is subject to the terms of this agreement. Further, and similar to the intellectual property owned by us, there can be no assurance that the intellectual property licensed to us will be effective to protect our products and services from duplication by other manufacturers or developers or to prevent our competitors from offering similar products and services.
We have investigated patents held by third parties of which we are aware and we believe that our products and services, including our telehealth offerings, do not infringe on the claims of these patents. However, we cannot provide any assurances that our products and services do not infringe upon any third party patents or violate the proprietary rights of others, including the patents we have investigated, and it is possible that such infringement or violation has occurred or may occur. As described in greater detail below, on May 22, 2015, the company, together with its subsidiary Express MD Solutions, LLC entered into a license and settlement agreement with Robert Bosch Healthcare Systems, Inc. providing for the resolution and dismissal, with prejudice, of the purported patent infringement lawsuit filed by Bosch against Express MD in January 2012 in the U.S. District Court for the Northern District of California, Case No. 5:12-cv-00068-JW. While the company does not believe that it was or is infringing any of the asserted patents, in order to mitigate its risk and avoid further costs and distractions of litigation, it entered into the license and settlement agreement. As previously reported by the company, the complaint alleged that the company’s “Electronic House Call” product infringes one or more claims of certain patents allegedly owned by Bosch. The agreement provides for the company to be granted by Bosch a worldwide, non-exclusive, non-assignable, royalty bearing license under the patents and a covenant not to be sued under certain other patents owned by Bosch. In consideration thereof, the company agreed to pay Bosch for the license and related matters and to pay certain royalties to Bosch for a three year period thereafter.
In the event that products we sell or services we provide are deemed to infringe upon the patents or proprietary rights of others, we could be required to modify our products and/or services or obtain a license for the manufacture, use and/or sale of such products and services. There can be no assurance that, in such an event, we would be able to do so in a timely manner, upon acceptable terms and conditions, or at all, and the failure to do any of the foregoing could have a material adverse effect upon our business. Moreover, there can be no assurance that we will have the financial or other resources necessary to defend against a patent infringement or proprietary rights violation action. In addition, if our products, services or proposed products or services are deemed to infringe upon the patents or proprietary rights of others, we could, under certain circumstances, become liable for damages or subject to an injunction, which could also have a material adverse effect on our business.
Because we currently derive a majority of our revenues from a few telehealth products and services, hosted software and web-based service offerings, any decline in demand for these offerings could severely harm our ability to generate revenues.
We currently derive a majority of our revenues from a limited number of telehealth products and services, hosted software and web-based service offerings. In addition, our focus on building our business is concentrated on markets for telehealth solutions, hosted software and web-based services where content integrity, workflow automation, electronic signatures, time and date stamping and web-based services are important to customers. As a result, we are particularly vulnerable to fluctuations in demand for these offerings, whether as a result of competition, product obsolescence, technological change, customer spending, or other factors. If our revenues
21
Table of Contents
derived from our offerings were to decline significantly, our business and operating results would be adversely affected. As a result, if our relationships with significant customers were disrupted we could lose a significant percentage of our anticipated revenues which could have material adverse effect on our business.
Some of our hosted software and web-based service offerings have long and unpredictable sales cycles, which may impact our quarterly operating results.
Transactions for some of our hosted software and web-based service offerings may require customers to undertake customized installations to integrate the solutions into their legacy systems and require them to modify existing business practices. The period from our initial contact with a potential customer until the execution of an agreement is difficult to predict and can be in excess of six to twelve months. The sales cycles for these transactions can be long and unpredictable due to a number of uncertainties such as:
| • | customers’ budgetary constraints; |
| • | the need to educate potential customers about our software and service offerings; |
| • | the timing of customers’ budget cycles; |
| • | delays caused by customers’ internal review processes; |
| • | customers’ willingness to invest resources and modify their network infrastructures to make use of our offerings; and |
| • | for sales to government customers, governmental regulatory approval and purchasing requirements. |
We are unable to control or influence many of these factors. Further, we have experienced delays in the pace of adoption and use by our customers of our transaction-based offerings, such as Inscrybe Healthcare, which has adversely affected our earnings. We may experience similar delays with our other products and services and products and services currently under development. During the sales cycle and the implementation period, we may expend substantial time, effort and money preparing contract proposals, negotiating contracts and implementing solutions without receiving any related revenue. In addition, many of our expenses are relatively fixed in the short term, including personnel costs and technology and infrastructure costs. Accordingly, our inability to generate sufficient revenues from these offerings has a direct impact on our results of operations.
The failure to properly manage our growth could cause our business to lose money.
We are using our sales and marketing efforts in order to develop and pursue existing and potential market opportunities. This growth is expected to place a significant demand on management and operational resources. In order to manage growth effectively, we must implement and improve our operational systems and controls on a timely basis. If we fail to implement these systems and controls, our business, financial condition, results of operations and cash flows may be materially and adversely affected.
Healthcare industry consolidation could impose pressure on our price, reduce our potential client base and reduce demand for our offerings.
Many hospitals and health care centers have consolidated to create larger healthcare enterprises with greater market power. If this consolidation trend continues, it could reduce the size of our potential customer base and give the resulting enterprises greater bargaining power, which may lead to erosion of the prices for our products and services. In addition, this consolidation could also erode our revenue base.
Our hosted software and web-based services and web site may be subject to intentional disruption.
Although we believe we have sufficient controls in place to prevent intentional disruptions, such as software viruses specifically designed to impede the performance of our software and web-based services, we may be
22
Table of Contents
affected by such efforts in the future. Further, despite the implementation of security measures, this infrastructure or other systems that we interface with, including the Internet and related systems, may be vulnerable to physical break-ins, hackers, improper employee or contractor access, programming errors, attacks by third parties or similar disruptive problems, resulting in the potential misappropriation of our proprietary information or interruptions of our services. Any compromise of our security, whether as a result of our own systems or systems that they interface with, could substantially disrupt our operations, harm our reputation and reduce demand for our services.
Performance problems with our systems, security breaches and other disruptions could cause us to lose business or incur liabilities.
Our customer satisfaction and our business could be harmed if we experience transmission delays or failures or loss of data in the systems we use to provide services to our customers, including transaction-related services. Further, in the ordinary course of our business, we collect and store sensitive data, including our proprietary information and that of our customers in our data center and on our networks. These systems are complex and, despite testing and quality control and security measures, we cannot be certain that problems will not occur or that they will be detected and corrected promptly if they do occur, and our information technology systems may be vulnerable to attacks by hackers or breached due to error or malfeasance. In providing these services, we rely on internal systems as well as communications and hosting services provided by third parties, such as the Internet. To operate without interruption, both we and the service providers we use must guard against:
| • | damage from fire, power loss and other natural disasters; |
| • | communications failures; |
| • | software and hardware errors, failures or crashes; |
| • | security breaches, computer viruses and similar disruptive problems; and |
| • | other potential interruptions. |
We have experienced periodic system interruptions in the past, and we cannot guarantee that they will not occur again. In the event of a catastrophic event at our data center or any third party facility we use, we may experience an extended period of system unavailability, which could negatively impact our business. Further, any compromise of our electronic systems, including the unauthorized access, use or disclosure of sensitive information, the disruption or breach of our networks or security measures, the loss of stored data, could have a material adverse impact on our business, expose us to reputational damage, result in legal claims and cause us to incur material liabilities. Such events would also be likely to require us to incur significant costs to improve cyber security, including through organizational changes, deploying additional personnel and protection technologies, further training of employees, and engaging third party experts and consultants. In addition, any real or perceived compromise of our security or disclosure of sensitive information may result in lost revenues by, deterring clients from using or purchasing our products and services in the future or by clients electing to use competing suppliers. Although we maintain insurance for our business, we cannot guarantee that our insurance will be adequate to compensate us for all losses that may occur or that this coverage will continue to be available on acceptable terms or in sufficient amounts.
In addition, some of our web-based services may, at times, be required to accommodate higher than expected volumes of traffic. At those times, we may experience slower response times or system failures. Any sustained or repeated interruptions or disruptions in these systems or slowdown in their response times could damage our relationships with customers. Further, the Internet has experienced, and is likely to continue to experience, significant growth in the number of users and the amount of traffic. If the Internet continues to experience increased usage, the Internet infrastructure may be unable to support the demands placed on it which could harm its reliability and performance. Any significant interruptions in our services or increases in response time could result in a loss of potential or existing users of services and, if sustained or repeated, could reduce the attractiveness of our services.
23
Table of Contents
We are subject to product liability risks associated with the production, marketing and sale of products used in the healthcare industry.
The production, marketing and sale of devices used in the healthcare industry have inherent risks of liability in the event of product failure or claim of harm caused by product operation. Furthermore, even meritless claims of product liability may be costly to defend against. The commercialization of the telehealth device exposes us to such claims. These types of product liability claims may result in decreased demand for this product, injury to our reputation, related litigation costs, and substantial monetary awards to plaintiffs. We attempt to limit by contract our liability, however, the limitations of liability set forth in the contracts may not be enforceable in certain jurisdictions or may not otherwise protect us from liability for damages. We may also be subject to claims that are not covered by contract, such as a claim directly by a patient. Although we currently maintain product liability insurance, we may not have sufficient insurance coverage, and we may not be able to obtain sufficient coverage at a reasonable cost. Our inability to obtain product liability insurance at an acceptable cost or to otherwise protect against potential product liability claims could inhibit the commercialization of any products that we develop. If we are sued for any injury caused by our products or processes, then our liability could exceed our product liability insurance coverage and our total assets.
We need to comply with ongoing regulatory requirements applicable to our telehealth product and our results of operations may be adversely impacted by any failure to comply with these requirements.
Our telehealth product is a medical device that is subject to extensive regulation in the United States. Unless an exemption applies, each medical device that we wish to market in the United States must receive either 510(k) clearance or premarket approval from the U.S. Food and Drug Administration, or the FDA, before the product can be sold. Either process can be lengthy and expensive. The FDA’s 510(k) clearance procedure, also known as “premarket notification,” is the process we have used for our current telehealth product. The regulatory clearance for our telehealth product provides for its use for its intended purposes. In addition, we are subject to inspection and marketing surveillance by the FDA to determine our compliance with all regulatory requirements and the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, distribution and record-keeping for approved products are subject to extensive regulation. If the FDA determines that our promotional materials or activities constitute promotion of an unapproved use or we otherwise fail to comply with other FDA regulations, we may be subject to regulatory enforcement actions, including a public warning letter, injunction, civil fines, suspensions, loss of regulatory clearance, product recalls or product seizures. In the more egregious cases, criminal prosecution, civil penalties, or disgorgement of profits are possible. The subsequent discovery of previously unknown problems may also result in restrictions on the marketing of our products, and could include voluntary or mandatory recall or withdrawal of products from the market. Further, we cannot predict the likelihood, nature or extent of adverse government regulation that may arise from future legislation or administrative action. In addition, the FDA has increased its focus on regulating computer software intended for use in a healthcare setting, including applications meant to run on a mobile platform or on a browser tailored for use on a mobile platform. If our software solutions or applications are deemed to be actively regulated medical devices by the FDA, we could be subject to more extensive requirements governing pre- and post-marketing activities. As described above, complying with these regulations could be time consuming and expensive, and may require FDA clearance or pre-market approval. If we are not able to maintain regulatory compliance with any of our products, we may be subject to regulatory enforcement actions as described above and may not be permitted to market our products, which would have a material adverse impact on our results of operations, cash flows and financial condition.
Further, any modification to an FDA-cleared medical device that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, requires a new FDA 510(k) clearance or, possibly, a premarket approval. The FDA requires every manufacturer to make its own determination as to whether a modification requires a new 510(k) clearance or premarket approval, but the FDA may review and disagree with any decision reached by the manufacturer. In the future, we may make modifications to our telehealth products and, in appropriate circumstances, determine that new clearance or
24
Table of Contents
approval is unnecessary. Regulatory authorities may disagree with our decisions not to seek new clearance or approval and may require us to obtain clearance or approval for modifications to our products. If that were to occur for a previously cleared or approved product, we may be required to cease marketing or recall the modified device until we obtain the necessary clearance or approval. Under these circumstances, we may also be subject to significant regulatory fines or other penalties.
In February 2014, the FDA conducted a routine inspection of our business premises related to our telehealth products and upon the completion of their inspection issued us inspectional observations on FDA Form 483. We have provided the FDA with written responses to the Form 483 that describes the actions we have taken to address their observations and we believe that we have responded to the observations noted by the FDA. In February 2015, the FDA issued an Establishment Inspection Report (EIR) and considered the inspection “closed”. We would expect another inspection in 2016 based on the typical biennial inspections cycle from the FDA. Beyond incurring compliance-related expenses, we are presently unable to predict what impact, if any, these matters or ensuing proceedings, if any, will have on our financial condition, results of operations or cash flows.
Our ability to generate revenues from our telehealth products is subject to our ability to obtain acceptable prices or an adequate level of reimbursement from payors of healthcare costs.
Our ability to commercialize our telehealth product successfully will depend in part on the extent to which appropriate coverage and reimbursement levels for the cost of this product are obtained by us or by our direct customers from governmental authorities, private health insurers and other organizations. The ability of customers to obtain appropriate reimbursement for their products and services from private and governmental payors is critical to the success of medical technology device companies as the availability of reimbursement affects which products customers purchase and the prices they are willing to pay. The cost containment measures that healthcare payors and providers are instituting and the effect of any healthcare reform could materially and adversely affect our ability to generate revenues from this product and our profitability. In addition, given ongoing federal and state government initiatives directed at lowering the total cost of healthcare, the United States Congress and state legislatures will likely continue to focus on healthcare reform and the reform of the Medicare and Medicaid payment systems. While we cannot predict whether any proposed cost-containment measures will be adopted, the announcement or adoption of these proposals could reduce the price that we receive for our telehealth product in the future. We cannot predict the outcomes of any of legislative or regulatory efforts at reducing costs of providing healthcare and regulatory changes in this regard may have a material adverse effect on our business.
The healthcare industry is highly regulated at the local, state and federal level.
In addition to regulatory requirements concerning the commercialization of medical devices, we are subject to a significant and wide-ranging number of regulations both within the United States and elsewhere, such as regulations in the areas of healthcare fraud and the security and privacy of patient data. Existing and new laws and regulations affecting the health care industry could create unexpected liabilities for us, cause us to incur additional costs, and restrict our operations. Many health care laws are complex, and their application to specific services and relationships may not be clear and these laws and regulations may be applied to us in ways that we do not anticipate, particularly as we develop and release new and more sophisticated products and services. Our failure to accurately anticipate the application of these laws and regulations, or our other failure to comply with them, could create liability for us, result in adverse publicity, and negatively affect our business. Some of the risks we face from health care regulation are described below:
Healthcare Fraud. Federal and state governments continue to enhance regulation of and increase their scrutiny over practices involving healthcare fraud affecting healthcare providers whose services are reimbursed by Medicare, Medicaid and other government healthcare programs. Our healthcare provider clients are subject to laws and regulations on fraud and abuse which, among other things, prohibit the direct or indirect payment or
25
Table of Contents
receipt of any remuneration for patient referrals, or arranging for or recommending referrals or other business paid for in whole or in part by these federal or state healthcare programs. Federal enforcement personnel have substantial funding, powers and remedies to pursue suspected or perceived fraud and abuse. The effect of this government regulation on our clients is difficult to predict. Many of the regulations applicable to our clients and that may be applicable to us, including those relating to marketing incentives offered in connection with medical device sales, are vague or indefinite and have not been interpreted by the courts. They may be interpreted or applied by a prosecutorial, regulatory or judicial authority in a manner that could broaden their applicability to us or require our clients to make changes in their operations or the way in which they deal with us. If such laws and regulations are determined to be applicable to us and if we fail to comply with any applicable laws and regulations, we could be subject to civil and criminal penalties, sanctions or other liability, including exclusion from government health programs, which could have a material adverse effect on our business, results of operations and financial condition.
Security and Privacy of Patient Information. Federal, state and local laws regulate the confidentiality of patient records and the circumstances under which those records may be released. These regulations govern both the disclosure and use of confidential patient medical record information and require the users of such information to implement specified security measures. United States regulations currently in place governing electronic health data transmissions continue to evolve and are often unclear and difficult to apply. Similarly, laws in non-U.S. jurisdictions may have similar or even stricter requirements related to the treatment of patient information. In the United States, HIPAA regulations require national standards for some types of electronic health information transactions and the data elements used in those transactions, security standards to ensure the integrity and confidentiality of health information and standards to protect the privacy of individually identifiable health information. Covered entities under the Health Insurance Portability and Accountability Act of 1996 (HIPAA), which include healthcare organizations such as our clients, and our claims transmission services, are required to comply with the privacy standards, the transaction regulations and the security regulations. Moreover, the Health Information Technology for Economic and Clinical Health Act (HITECH) provisions of the American Recovery and Reinvestment Act of 2009 (ARRA) and associated regulatory requirements, extend many of the HIPAA obligations, formerly imposed only upon covered entities, to business associates as well. As a business associate of our clients who are covered entities, we were in most instances already contractually required to ensure compliance with the HIPAA regulations as they pertain to handling of covered client data. However, the extension of these HIPAA obligations to business associates by law has created additional liability risks related to the privacy and security of individually identifiable health information. Evolving HIPAA and HITECH related laws or regulations and regulations in non-U.S. jurisdictions could restrict the ability of our clients to obtain, use or disseminate patient information. This could adversely affect demand for our solutions if they are not re-designed in a timely manner in order to meet the requirements of any new interpretations or regulations that seek to protect the privacy and security of patient data or enable our clients to execute new or modified healthcare transactions. We may need to expend additional capital, software development and other resources to modify our solutions and devices to address these evolving data security and privacy issues. Furthermore, our failure to maintain confidentiality of sensitive personal information in accordance with the applicable regulatory requirements could damage our reputation and expose us to breach of contract claims (although we contractually limit liability, when possible and where permitted), fines and penalties.
Electronic Health Records Laws and Associated Interoperability Standards. A number of federal and state laws govern the use and content of electronic health record systems. For example, ARRA requires “meaningful use of certified electronic health record technology” by health care providers in order to receive incentive payments. Regulations have been issued that identify standards and implementation specifications and establish the certification standards for qualifying electronic health record technology. Nevertheless, these standards and specifications are subject to interpretation by the entities designated to certify such technology. While we have not sought to obtain certification of our software solutions for “meaningful use” under the criteria adopted by the U.S. Department of Health and Human Services regarding electronic health records, our software solutions operate in the framework of facilitating the electronic exchange of health care information by our customers and our solutions must be designed in a manner that facilitates our customers’ compliance with these laws. Further,
26
Table of Contents
there is increasing demand among customers, industry groups and government authorities that healthcare software and systems provided by various vendors be compatible with each other. Market forces or governmental/regulatory authorities could create software interoperability standards that would apply to our solutions, health care devices or solutions, and if our software solutions, health care devices or services are not consistent with those standards, we could be forced to incur substantial additional development costs to conform. We may incur increased development costs and delays in delivering solutions if we need to upgrade our software solutions to either obtain compliance with these varying and evolving standards or to facilitate such compliance by our customers. In addition, any standards applicable to our products and solutions may lengthen our sales and implementation cycle. To the extent that any such standards are narrowly construed or delayed in publication, or that we are delayed in achieving any certification that is necessary or desirable, our sales could be impaired and we may have to invest significantly in changes to our software solutions or devices. Because this is a topic of increasing state and federal regulation, we expect additional and continuing modification of the current legal and regulatory environment. We cannot predict the content or effect of possible future regulation on our business activities.
In addition, in accordance with requirements under HIPAA, the U.S. Department of Health and Human Services (“HHS”) is implementing a new version of the standards for HIPAA-covered electronic transactions, including claims, remittance advices, and requests and responses for eligibility. These standards are called ANSI-5010. Additionally, HIPAA required all entities that are covered by HIPAA to upgrade to the tenth revision of the International Statistical Classification of Diseases and Related Health Problems promulgated by the World Health Organization, also known as ICD-10, for use in reporting medical diagnoses and inpatient procedures.
If we are unable to generate sufficient demand for our current telehealth products and services, we may not be able to recover our inventory and other investments. Further, modifications to our current telehealth products may require new marketing clearances or approvals or require us to cease marketing or recall the modified products until such clearances or approvals are obtained.
In connection with our manufacturing and sales plans for our current telehealth products, we have purchased certain components and contract manufacturing services for the production of our Electronic House Call monitoring appliance. Our ability to recover our investment in building inventories of our current telehealth products is subject to risks. If we are unable to generate sufficient demand for our products, or incur regulatory penalties relating to our telehealth products, we may not be able to recover the cost of our investments in our telehealth business and our financial condition and results of operations could be negatively impacted.
If our manufacturer and suppliers for our telehealth products fail to comply with the FDA’s Quality System Regulation, or QSR, and other applicable post market requirements, our operations could be disrupted, our product sales and profitability could suffer, and we may be subject to a wide variety of FDA enforcement actions.
After a device is placed on the market, numerous regulatory requirements also apply to our manufacturer and suppliers. The manufacturing processes of some of our vendors must comply with the FDA’s Quality System Regulation, or QSR, which governs the methods used in, and the facilities and controls used for, the design, testing, manufacture, control, quality assurance, installation, servicing, labeling, packaging, storage and shipping of medical devices. The FDA enforces the QSR through unannounced inspections. If one of our suppliers fails a QSR inspection, or if a corrective action plan adopted by a supplier is not sufficient, the FDA may bring an enforcement action, and our operations could be disrupted and the manufacturing of our products delayed. We are also subject to the FDA’s general prohibition against promoting our products for unapproved or “off-label” uses, the FDA’s adverse event reporting requirements and the FDA’s reporting requirements for field correction or product removals. The FDA has recently placed increased emphasis on its scrutiny of compliance with the QSR and these other post market requirements. If we or our manufacturer or suppliers violate the FDA’s requirements or fail to take adequate corrective action in response to any significant compliance issue raised by the FDA, the FDA can take various enforcement actions which could cause our product sales and profitability to suffer.
27
Table of Contents
Our hosted software and web-based services and other product offerings may not be accepted by the market, which would seriously harm our business.
Demand and market acceptance for our currently available hosted software and web-based services and other product offerings remain subject to a high level of uncertainty. Achieving widespread acceptance of these or future offerings will continue to require substantial marketing efforts and the expenditure of significant funds to create and maintain brand recognition and customer demand for such offerings. Demand for our software, services and other product offerings depends on, among other things:
| • | the perceived ability of our offerings to address real customer problems; |
| • | the perceived quality, price, ease-of-use and interoperability of our offerings as compared to our competitors’ offerings; |
| • | the market’s perception of the ease or difficulty in deploying our software or services, especially in complex network environments; |
| • | the continued evolution of electronic commerce as a viable means of conducting business; |
| • | market acceptance and use of new technologies and standards; |
| • | the ability of network infrastructures to support an increasing number of users and services; |
| • | the pace of technological change and our ability to keep up with these changes; and |
| • | general economic conditions, which influence how much money our customers and potential customers are willing to allocate to their information technology budgets. |
There can be no assurance that adequate marketing arrangements will be made and continued for our products and services and there can be no assurance that any of these offerings will ever achieve or maintain widespread market acceptance or that such offerings will be profitable.
If we cannot continuously enhance our hosted software and web-based service offerings in response to rapid changes in the market, our business will be harmed.
The software-based services industry and computer industry are characterized by extensive research and development efforts which result in the frequent introduction of new products and services which render existing products and services obsolete. Our ability to compete successfully in the future will depend in large part on our ability to maintain a technically competent research and development staff and our ability to adapt to technological changes in the industry and enhance and improve our hosted software and web-based service offerings and successfully develop and market new offerings that meet the changing needs of our customers. Although we are dedicated to continued improvement of our offerings with a view towards satisfying market needs with the most advanced capabilities, there can be no assurance that we will be able to continue to do so on a regular basis and remain competitive with products offered by other manufacturers. At the present time, we do not have a targeted level of expenditures for research and development. We will evaluate all opportunities but believe the majority of our research and development will be devoted to enhancements of our existing offerings.
If our hosted software and web-based service offerings and telehealth solutions are not competitive, our business will suffer.
We are engaged in the highly competitive businesses of developing hosted software and web-based workflow management services and telehealth solutions. These markets are continually evolving and, in some cases, subject to rapid technological change. Many of our competitors have greater financial, technical, product development, marketing and other resources than we do. These organizations may be better known than we are and have more customers than we do. We cannot provide assurance that we will be able to compete successfully against these organizations. We believe that the principal competitive factors affecting our markets include
28
Table of Contents
performance, ease of use, quality/reliability of our offerings, scalability, features and functionality, price and customer service and support. There can be no assurance that we will be able to successfully incorporate these factors into our software and web-based services or telehealth solutions and compete against current or future competitors or that competitive pressure we face will not harm our business. If we are unable to develop and market products to compete with the products of competitors, our business will be materially and adversely affected.
Our business, including Inscrybe Healthcare and our telehealth products and services are relatively new business lines and although the level of competition for these offerings is uncertain at this point in time, the field of software-based solutions in which we compete is highly competitive. There can be no assurances, however, that any of our offerings will achieve market acceptance.
We also expect that competition will increase as a result of industry consolidations and the formation of new companies with new, innovative offerings. Current and potential competitors have established or may establish cooperative relationships among themselves or with third parties to increase the ability of their software and service offerings to address the needs of our prospective customers. Accordingly, it is possible that new competitors or alliances among competitors may emerge and rapidly acquire significant market share. Increased competition is likely to result in price reductions, reduced operating margins and loss of market share, any of which could harm our business.
Our hosted software and web-based services are complex and are operated in a wide variety of computer configurations, which could result in errors or product failures.
Our hosted software and web-based services are complex and may contain undetected errors, failures or bugs that may arise when they are first introduced or when new versions are released. These offerings may be used in large-scale computing environments with different operating systems, system management software and equipment and networking configurations, which may cause errors or failures in our offerings or may expose undetected errors, failures or bugs in such offerings. Our customers’ computer environments are often characterized by a wide variety of configurations that make pre-release testing for programming or compatibility errors difficult and time-consuming. Despite testing by us and by others, errors, failures or bugs may not be found in new products or releases after commencement of commercial use. Errors, failures or bugs in our offerings could result in negative publicity, returns, loss of or delay in market acceptance of our hosted software or web-based services or claims by customers or others. Alleviating these problems could require significant expenditures of our capital and resources and could cause interruptions, delays or cessation of our licenses which could cause us to lose existing or potential customers and would adversely affect our financial conditions, results of operations and cash flows. Most of our license agreements with customers contain provisions designed to limit our exposure to potential product liability claims. It is possible, however, that these provisions may not prove effective in limiting our liability.
We have a significant amount of net operating loss carry forwards which we may not be able to utilize in certain circumstances.
At June 30, 2015, we had net operating loss, or NOL, carry forwards for federal income tax purposes of approximately $162,000,000 available to offset future taxable income. Under Section 382 of the Internal Revenue Code, following an “ownership change,” special limitations apply to the use by a “loss corporation” of its (i) NOL carry forwards arising before the ownership change and (ii) net unrealized built-in losses (if such losses existed immediately before the ownership change and exceed a statutory threshold amount) recognized during the five years following the ownership change ((i) and (ii) are referred to collectively as the “Applicable Tax Attributes”). After an ownership change, the amount of the loss corporation’s taxable income for each post-change taxable year that may be offset by the Applicable Tax Attributes is limited to the product of the “long-term tax-exempt rate” (published by the IRS for the month of the ownership change) multiplied by the value of the loss corporation’s stock (the “Section 382 Limitation”). To the extent that the loss corporation’s Section 382 Limitation in a given taxable year exceeds its taxable income for the year, that excess increases the Section 382 Limitation in future taxable years.
29
Table of Contents
Risks Related to Our Common Stock and Other Securities
Our stock price is volatile and could decline.
The price of our common stock has been, and is likely to continue to be, volatile. Our stock price during the fiscal year ended June 30, 2015 traded as low as $0.16 per share and as high as $1.20 per share. We cannot assure you that your initial investment in our common stock will not fluctuate significantly. The market price of our common stock may fluctuate significantly in response to a number of factors, some of which are beyond our control, including:
| • | quarterly variations in our operating results; |
| • | announcements we make regarding significant contracts, acquisitions, dispositions, strategic partnerships, or joint ventures; |
| • | additions or departures of key personnel; |
| • | the introduction of competitive offerings by existing or new competitors; |
| • | uncertainty about and customer confidence in the current economic conditions and outlook; |
| • | reduced demand for any given product on web-based service offering; and |
| • | sales of our common stock. |
In addition, the stock market in general, including companies whose stock is listed on The NASDAQ Capital Market, have experienced extreme price and volume fluctuations that have often been disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance.
The failure to maintain compliance with The Nasdaq Capital Market listing standards could result in delisting and adversely affect the market price and liquidity of our common stock.
Our common stock is currently traded on The Nasdaq Capital Market under the symbol “ADAT”. If we fail to meet any of the continued listing standards of The Nasdaq Capital Market, our common stock will be delisted from The Nasdaq Capital Market. These continued listing standards include specifically enumerated criteria, such as a $1.00 minimum closing bid price and a minimum stockholders’ equity requirement, which requires listed companies to maintain stockholders’ equity of at least $2.5 million.
On January 28, 2015, we received a staff deficiency letter from The Nasdaq Stock Market notifying us that for the prior 30 consecutive business days, the closing bid price per share of our common stock was below the $1.00 minimum bid price requirement for continued listing on The Nasdaq Capital Market, as required by Listing Rule 5550(a)(2) (the “Bid Price Rule”). Nasdaq provided us with 180 calendar days, or until July 27, 2015, to regain compliance with the Bid Price Rule. To regain compliance with the Bid Price Rule, the closing bid price of our common stock must meet or exceed $1.00 per share for a minimum of ten consecutive business days during the 180 day grace period.
Subsequently, on May 28, 2015, we received a second staff deficiency letter from The Nasdaq Stock Market notifying us that we did not comply with Nasdaq Listing Rule 5550(b)(1), the minimum stockholders’ equity requirement for continued listing on the Nasdaq Capital Market, which requires listed companies to maintain stockholders’ equity of at least $2.5 million. Nasdaq provided us with 45 calendar days, to submit a plan to regain compliance with the minimum stockholders’ equity standard. Pursuant to an extension granted by the staff, we submitted our compliance plan on July 21, 2015.
On July 29, 2015, we received a determination letter from the staff of The Nasdaq Stock Market stating that the company has not regained compliance with The Nasdaq Capital Market minimum bid price of $1.00
30
Table of Contents
requirement for continued listing set forth in Nasdaq Listing Rule 5550(a)(2). The Nasdaq determination letter also stated that the company is not eligible for an additional 180-day extension to regain compliance with the minimum bid price rule because the company does not meet the minimum stockholders’ equity initial listing requirement for the Nasdaq Capital Market. The determination letter also stated that the Company did not maintain a minimum $2.5 million in stockholders equity for continued listing and did not meet the alternatives of market value of listed securities or net income as required under Listing Rule 5550(b) and that such deficiency serves as an additional basis for delisting. Pursuant to the determination letter, we requested a hearing to appeal this determination on August 5, 2015, and were granted a hearing on September 10, 2015. At the hearing we presented our plan to regain compliance with both the minimum bid price requirement of Listing Rule 5550(a)(2) and the minimum shareholders’ equity requirement of Listing Rule 5550(b)(1).
On September 16, 2015, we received written notice that the Nasdaq Hearings Panel (the “Panel”) granted our request to remain listed on The NASDAQ Stock Market, LLC, subject to the condition that, on or before January 25, 2016, we shall announce and inform the Panel that the company’s proposed business combination has closed and that NASDAQ’s Listing Qualifications Staff (the “Staff”) has approved the combined entity’s application for initial listing on NASDAQ. In its written notice, the Panel stated that during the granted exception period the company must promptly notify the Panel of any significant developments, particularly any event, condition or circumstance that may impact its ability to meet the terms of the exception granted by the Panel and that the Panel reserves the right to reconsider the granted exception in such an instance. The company is diligently working to timely satisfy the terms of the Panel’s decision; however, there can be no assurance that the company will be able to do so. In the event that the company is unable to meet the exception requirement, the Panel will issue a final determination to delist the company’s shares and suspend trading of the company’s shares on The Nasdaq Capital Market.
If our common stock were to be delisted from The Nasdaq Capital Market, trading of our common stock most likely will be conducted in the over-the-counter market on an electronic bulletin board established for unlisted securities such as the OTC Bulletin Board. Such trading will reduce the market liquidity of our common stock. As a result, an investor would find it more difficult to dispose of, or obtain accurate quotations for the price of, our common stock and the price of our common stock could suffer a significant decline. Delisting may also impair our ability to raise capital. If our common stock is delisted from The Nasdaq Capital Market and the trading price remains below $5.00 per share, trading in our common stock might also become subject to the requirements of certain rules promulgated under the Exchange Act, which require additional disclosure by broker-dealers in connection with any trade involving a stock defined as a “penny stock” (generally, any equity security not listed on a national securities exchange or quoted on Nasdaq that has a market price of less than $5.00 per share, subject to certain exceptions). Many brokerage firms are reluctant to recommend low-priced stocks to their clients. Moreover, various regulations and policies restrict the ability of shareholders to borrow against or “margin” low-priced stocks, and declines in the stock price below certain levels may trigger unexpected margin calls. Additionally, because brokers’ commissions on low-priced stocks generally represent a higher percentage of the stock price than commissions on higher priced stocks, the current price of the common stock can result in an individual shareholder paying transaction costs that represent a higher percentage of total share value than would be the case if our share price were higher. This factor may also limit the willingness of institutions to purchase our common stock. Finally, the additional burdens imposed upon broker-dealers by these requirements could discourage broker-dealers from facilitating trades in our common stock, which could severely limit the market liquidity of the stock and the ability of investors to trade our common stock.
Since we have not paid dividends on our common stock, you may not receive income from this investment.
We have not paid any dividends on our common stock since our inception and do not contemplate or anticipate paying any dividends on our common stock in the foreseeable future. Earnings, if any, will be used to finance the development and expansion of our business. Accordingly, you may have to sell some or all of your common stock in order to generate cash from your investment. You may not receive a gain on your investment when you sell our common stock and may lose the entire amount of your investment.
31
Table of Contents
Trading in our stock over the last twelve months has been limited, so investors may not be able to sell as much stock as they want at prevailing prices.
The average daily trading volume in our common stock for the year ended June 30, 2015 was approximately 97,000 shares. If limited trading in our stock continues, it may be difficult for investors to sell their shares in the public market at any given time at prevailing prices. Moreover, the market price for shares of our common stock may be made more volatile because of the relatively low volume of trading in our common stock. When trading volume is low, significant price movement can be caused by the trading in a relatively small number of shares. Volatility in our common stock could cause stockholders to incur substantial losses.
Additional financings could result in dilution to existing stockholders and otherwise adversely impact the rights of our common stockholders.
As stated above, we require additional financings in order to obtain additional capital with which to operate our business. Any such financings will dilute the percentage ownership interests of our stockholders and may adversely affect our earnings and net book value per share. In addition, we may not be able to secure any such additional financing on terms acceptable to us, if at all. We have the authority to issue additional shares of common stock and preferred stock, as well as additional classes or series of warrants or debt obligations which may be convertible into any one or more classes or series of ownership interests. We are authorized to issue 190 million shares of common stock and 5 million shares of preferred stock. Subject to compliance with the requirements of the NASDAQ Stock Market, such securities may be issued without the approval or other consent of our stockholders.
We filed a “shelf” registration statement on Form S-3 with the Securities and Exchange Commission in August 2012, which was declared effective by the Commission in December 2012. There is approximately $29 million available for future issuances under this registration statement, subject to SEC limitations. This disclosure shall not constitute an offer to sell or a solicitation of an offer to buy the securities, nor shall there by any sale of these securities in any jurisdiction in which an offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such jurisdiction. Any offer of the securities will be solely by means of the prospectus included in the registration statement and one or more prospectus supplements that will be issued at the time of the offering.
In the event that any future financing should be in the form of, be convertible into or exchangeable for, equity securities, and upon the conversion or exercise of such securities, investors may experience additional dilution. Moreover, we may issue undesignated shares of preferred stock, the terms of which may be fixed by our board of directors and which terms may be preferential to the interests of our common stockholders. We have issued preferred stock in the past, and our board of directors has the authority, without stockholder approval, to create and issue one or more additional series of such preferred stock and to determine the voting, dividend and other rights of holders of such preferred stock. Any debt financing, if available, may involve restrictive covenants that impact our ability to conduct our business. The issuance of any of such series of preferred stock or debt securities may have an adverse effect on the holders of common stock.
The number of shares of our common stock outstanding has increased substantially as a result of our recent financings, and the exercise or conversion of the warrants and shares of preferred stock issued in these transactions could result in further dilution to holders of our common stock and adversely impact the market price of our common stock.
During our fiscal year ended June 30, 2015, we completed several transactions that have resulted in our issuance of a substantial number of shares of common stock and securities convertible into, or exercisable for, additional shares of common stock. The issuance of these securities has resulted in substantial dilution to stockholders who held our common stock prior to such transactions and will result in additional dilution in the future if shares of convertible preferred stock are converted into common stock and common stock purchase
32
Table of Contents
warrants are exercised. The conversion of preferred shares and the exercise of warrants could also adversely affect the market price of our common stock if the holders of these securities immediately sell some or all of the shares of common stock issued upon conversion or exercise or there is a perception in the market that the holders of a large number of shares intend to sell their shares.
In February 2015, the company entered into a securities purchase agreement with an accredited investor pursuant to which we issued a promissory note in the aggregate principal amount of $100,000 and common stock purchase warrants to purchase up to 80,000 shares of common stock for gross proceeds of $100,000. The warrants vest in equal monthly installments over twelve months if the notes are outstanding and, subject to vesting requirements, are exercisable for a period of 54 months commencing on the six month anniversary of the issuance date at an initial exercise price of $1.01 per share. Although the securities purchase agreement we entered into with this investor contemplated a second closing for $900,000, the second closing did not occur and we did not receive the additional funds. The vested warrants related to this transaction were exchanged for warrants issued in connection with the June 8, 2015 transaction discussed below.
On February 17, 2015, in a separate transaction, the company issued a short-term promissory note in the aggregate principal amount of $950,000 and warrants to purchase 99,500 shares of common stock to an accredited investor for gross proceeds of $950,000. The warrants are exercisable for a period of 54 months commencing on the six month anniversary of the issuance date and have an initial exercise price of $1.01 per share. On April 3, 2015, the company entered into an amendment agreement with this investor to extend the maturity date of this note from March 19, 2015 to July 2, 2015 and to grant the holder the right to exchange the principal amount of the short-term note (and unpaid interest thereon) into the securities of the company sold in the next financing, as defined in the amendment agreement. This investor subsequently agreed not to participate in the convertible debt financing for which a closing was held June 8, 2015 and in consideration of such election, the participation right was modified to allow him to exchange such note for comparable securities in an alternative transaction. In consideration of waivers previously granted by the holder of potential events of default under the short-term note and the amendment to extend the maturity date, the company agreed to issue the holder warrants to purchase 3,166,667 shares of common stock of the company. The warrants are exercisable for a period of 54 months commencing six months following the date of issuance and have an exercise price equal to $0.31 per share. The holder of the short-term note is an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of the board of directors of the company. Through a series of amendments to the agreement, the note has been extended through October 16, 2015.
On April 24, 2015, the company issued a short-term promissory note in the aggregate principal amount of $500,000 to Lazarus Investment Partners LLLP, the beneficial owner of approximately 29.4% of our common stock immediately prior to the note transaction, for gross proceeds of $500,000. In consideration for this loan, the company agreed to reduce the exercise price on approximately 6,233,600 warrants held by Lazarus to $0.25, based on the most recent closing bid price of the common stock prior to the note transaction, and to extend the expiration date of the warrants to October 25, 2019. Through a series of amendments to the agreement, the note has been extended through October 16, 2015.
On June 8, 2015, the company issued an aggregate principal amount of $900,000 of senior secured convertible debentures and common stock purchase warrants to purchase 3,626,667 shares of common stock. Subject to certain limitations, the debentures are convertible at any time at the option of the holder into shares of our common stock at an initial conversion price of $0.25 per share. Each debenture matures on the one-year anniversary of the issuance date. The warrants are exercisable for a period of 54 months commencing on the six month anniversary of the issuance date at an initial exercise price of $0.30 per share. Although the securities purchase agreement we entered into with this investor contemplated a second closing for up to $2.1 million, the second closing has not occurred and we have not received any of the additional funds to date.
On August 7, 2015, the company issued a senior secured note in the aggregate principal amount of $320,000 to MKA 79, LLC, an entity affiliated with J. David Luce, a member of the company’s board of directors. In
33
Table of Contents
consideration of the loan, the company agreed to reduce the exercise price on approximately 5,474,829 warrants held by MKA 79 and an affiliate to $0.17, based on the most recent closing bid price of the common stock prior to the note transaction, and to extend the expiration date of the warrants to December 13, 2019.
In September 2015, the company has issued promissory notes in the aggregate principal amount of $525,000 to accredited investors in a private transaction. The notes are unsecured and are not convertible into equity securities of the company. The notes bear interest at 20% per annum, payable in arrears, and are due upon the earlier of (i) September 18, 2016, or (ii) within 30 days of the closing of a sale of equity or debt securities of the company, or series of closings, as part of the same transaction, of equity or debt securities within a period of 90 days, in the gross amount of at least $5,000,000 in cash proceeds. The company also issued the investors warrants to purchase an aggregate of 1,050,000 shares of common stock. The warrants are exercisable commencing twelve months following their issuance for a period of 54 months at an exercise price of $0.30 per share. These closings are part of an offering of up to $1 million of notes and 2 million warrants.
Due to our need for additional capital, we may be required to issue additional shares of common stock or securities convertible into or exercisable for common stock at prices reflecting a dilution to existing stockholders. We may seek to obtain capital from the holders of existing warrants or convertible securities by reducing the exercise or conversion prices and may include additional issuance of equity securities. All of these potential transactions may result in dilution to existing stockholders.
Our executive officers, directors and significant stockholders will be able to influence matters requiring stockholder approval
As of the date of this report, our executive officers, directors and largest shareholder (Lazarus Investment Partners, LLLP) possess beneficial ownership (without, however, giving effect to any limitations on the ability of such persons to convert shares of Series D preferred stock or exercise warrants) of approximately 44.5% of our common stock and within this amount, Lazarus Investment Partners beneficially owns approximately 29.3% of our outstanding common stock. Due to such ownership position, these persons have increased influence over the outcome of future stockholder votes, including the election of directors and other significant business matters that require stockholder approval, and their interests may differ from the interests of other stockholders. This concentration of ownership may also have the effect of delaying, preventing or deterring a change in control of our company, could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale or merger of our company and may negatively affect the market price of our common stock. These transactions might include proxy contests, tender offers, mergers or other business combinations or purchases of common stock that could give our stockholders the opportunity to realize a premium over the then-prevailing market price for shares of our common stock. In addition, the sale of these shares of common stock may adversely affect the market price of our common stock and our stock price may decline substantially.
The exercise of our outstanding options and warrants, or conversion of our outstanding shares of convertible preferred stock, may depress our stock price and dilute your ownership of the company.
As of June 30, 2015, the following options, restricted stock units and warrants were outstanding:
| • | Stock options to purchase 4,316,000 shares of common stock at exercise prices ranging from $0.18 to $9.00 per share, not all of which are immediately exercisable. The weighted average exercise price of the outstanding stock options is $1.44 per share. These stock options are employee and non-executive director options. |
| • | Warrants to purchase 33,669,000 shares of common stock with a weighted average exercise price of $.84 per share. |
| • | An aggregate of 888,000 unvested restricted stock units. |
In addition, there are currently outstanding 28,000 shares of our Series B convertible preferred stock which the holder may convert into shares of our common stock at a conversion price equal to $2.80 per share.
34
Table of Contents
Accordingly, the outstanding 28,000 shares of Series B convertible preferred stock are presently convertible into an aggregate of 250,000 shares of our common stock, which will be available for immediate resale in accordance with the provisions of Rule 144 under the Securities Act. Further, there are currently outstanding 665,000 shares of Series D preferred stock, which are initially convertible into an aggregate of 6,125,024 shares of common stock at the initial conversion rate of $1.08571 per share commencing six months after the original issue date of the Series D preferred stock (exclusive of any additional shares of common stock that we may elect to issue in lieu of paying cash dividends on the Series D preferred stock). Shares of common stock issued upon conversion of Series D preferred stock may be resold from time to time by a holder in accordance with Rule 144 under the Securities Act.
Further, on June 8, 2015, we issued a principal amount of 900,000 of senior secured convertible debentures and common stock purchase warrants. Subject to certain limitations, the debentures are convertible at any time at the option of the holder into an initial amount of 3.6 million shares of our common stock at an initial conversion price of $0.25 per share. The conversion rate of these debentures is subject to adjustment, including if we issue or sell shares of its common stock or other equity securities for a price per share that is less than the conversion price then in effect, in which event the conversion price will be decreased to equal 85% of such lower price.
To the extent that these securities are exercised or converted, or we issue additional common shares, dilution to our stockholders will occur. Moreover, the terms upon which we will be able to obtain additional equity capital may be adversely affected, since the holders of these securities can be expected to exercise or convert them at a time when we would, in all likelihood, be able to obtain any needed capital on terms more favorable to us than the exercise and conversion terms provided by those securities. Further, in the event the conversion price of our outstanding convertible debentures or shares of convertible preferred stock is lower than the actual trading price on the day of conversion, the holders could immediately sell their converted common shares, which would have a dilutive effect on the value of the outstanding common shares. Furthermore, the significant downward pressure on the trading price of our common stock as preferred stock or debentures holders converted these securities and sell the common shares received on conversion could encourage short sales by the holders of preferred stock or other security holders. This would place further downward pressure on the trading price of our common stock. Even the mere perception of eventual sales of common shares issued on the conversion of the shares of preferred stock or debentures could lead to a decline in the trading price of our common stock.
Our currently outstanding shares of convertible preferred stock or the issuance of additional shares of preferred stock could adversely affect the rights of the holders of shares of our common stock.
We have issued a total of 28,000 shares of Series B preferred stock and 665,000 shares of Series D preferred stock and our board is authorized to issue up to an additional 4,307,000 shares of preferred stock without any further action on the part of our stockholders. Pursuant to our certificate of incorporation, our board has the authority to fix and determine the voting rights, rights of redemption and other rights and preferences of preferred stock. Our board may, at any time, authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock, and the right to the redemption of the shares, before the redemption of our common stock, which may have a material adverse effect on the rights of the holders of our common stock. In addition, our board, without further stockholder approval (but subject to the rules of the Nasdaq Stock Market) may, at any time, issue large blocks of preferred stock. Pursuant to the certificates of designations governing the rights and preferences of our outstanding shares of Series B preferred stock and Series D preferred stock, each share of preferred stock has certain rights and preferences, including the right to receive dividends in preference to our common stockholders. In addition, we must obtain the approval of the holders of a majority of the shares of outstanding convertible preferred stock in order to: (i) amend, alter or repeal any provisions of our Certificate of Incorporation which would materially adversely affect any of the preferences, rights, powers or privileges of such preferred stock (ii) create, authorize or issue any other class or series of preferred stock on a parity with, or having greater or preferential rights than, the outstanding convertible preferred stock, (iii) redeem, repurchase or otherwise acquire for value, or set aside for payment or make
35
Table of Contents
available for a sinking fund for the purchase or redemption of, any stock ranking junior to on a parity with the outstanding convertible preferred stock, or (iv) enter into any agreement which would prohibit or restrict our right to pay dividends on the outstanding convertible preferred stock. The need to obtain the approval of holders of our convertible preferred stock before taking these actions could impede our ability to take certain actions that management or our board may consider to be in the best interests of our stockholders. Any failure to obtain such approval could limit our business flexibility, harm our business and result in a decrease in the value of our common stock or convertible preferred stock.
We have granted liens on all of our assets to the holders of the certain debt instruments and the senior secured convertible debentures we have issued include additional provisions that could adversely affect the interests of the holders of our common stock. If we are required to repay our outstanding debt securities on their scheduled due date, our financial condition may be adversely affected.
On June 8, 2015, we entered into definitive agreements relating to a private placement of up to a principal amount of $3.0 of senior secured convertible debentures (the “Convertible Debentures”) and common stock purchase warrants. An initial closing for an aggregate principal amount of $900,000 of these debentures and warrants to purchase 3,626,667 shares of common stock was held on June 8, 2015. A final closing of up to $2.1 million of these debentures and warrants to purchase 8,400,000 shares of common stock was expected to occur prior to June 24, 2015, but did not transpire.
Subject to certain exceptions, the Convertible Debentures rank senior to our existing and future indebtedness and these debentures mature on the one-year anniversary of the issuance date. Subject to certain limitations, the Convertible Debentures are convertible at any time at the option of the holder into shares of our common stock at an initial conversion price of $0.25 per share. If we issue or sell shares of our common stock, rights to purchase shares of its common stock, or securities convertible into shares of its common stock for a price per share that is less than the conversion price then in effect, the conversion price will be decreased to equal 85% of such lower price. Similarly, the warrants issued with the Convertible Debentures have an initial exercise price of $0.30, but such exercise price is subject to adjustment if we issue or sell shares of our common stock or other securities convertible into or exercisable for shares of common stock for a price per share that is less than the conversion price of the Convertible Debentures. The Convertible Debentures bear interest at 9% per annum with interest payable upon maturity or on any earlier redemption date. If we are unable to consummate an additional financing prior to the maturity date of the Convertible Debentures, we will be required to repay these securities, which may have an adverse effect on our cash position. In addition, the Convertible Debentures are secured by a first priority lien on our assets related to our “Inscrybe Referral and Order Management” and “Inscrybe Hospital Discharge” solutions (the “Inscrybe Solutions Business”) in accordance with, and subject to, a security agreement between us and the investors.
The Convertible Debentures contain covenants and events of default customary for similar transactions. Accordingly, without the consent of the holders of the Convertible Debentures we must comply with certain restrictions against incurring additional indebtedness and granting additional security interests on our assets. Among the defined events of default are defaults of our payment obligations, breach of any material covenant or representation of the Convertible Debentures or the related transaction agreements, and the commencement of proceedings under applicable U.S. federal or state bankruptcy, insolvency, reorganization or other similar laws either against us or by us. Upon the occurrence of an event of default under the Convertible Debentures, a holder may require us to repay all or a portion of the Convertible Debentures in cash, at a price equal to 110% of the principal and accrued and unpaid interest. If we are unable to repay the Convertible Debentures when due, or upon an event of default, the holders could foreclose on our encumbered assets.
In addition, in August 2015, we issued a senior secured promissory note (the “August Note”) in the aggregate principal amount of $320,000 to MKA 79, LLC, in a private transaction. The August Note is due and payable on December 31, 2015 and interest shall accrue on the August Note at the rate of 10.0% per annum. The August Note is not convertible into any of our equity securities and it contains terms and events of default
36
Table of Contents
customary for similar transactions. Accordingly, without the consent of the holder of the August Note we must comply with certain restrictions against incurring additional indebtedness and granting additional security interests on our assets. The events of default defined in the August Note include customary terms, including a default of our payment obligation, a breach of material terms of the August Note and the commencement of bankruptcy proceedings. The August Note is secured by a first priority lien on certain of our assets, as described in a security agreement entered into between the company and the purchaser, which collateral consists of those of our assets other than those covered by the security agreement we entered into with the holders of the Convertible Debentures. In consideration of the loan, we agreed to amend certain of the terms of the existing 5,474,829 Common Stock Purchase Warrants currently held by the lender and an affiliate. In amending these warrants, we agreed to reduce the exercise price of such warrants to $0.17 and to extend the expiration date of the warrants to December 13, 2019. The initial exercise prices of these warrants were between $0.95 and $1.34. The Purchaser is an entity affiliated with J. David Luce, a member of the Company’s board of directors.
Provisions in our charter documents and Delaware law could discourage or prevent a takeover, even if an acquisition would be beneficial to our stockholders.
Provisions of our certificate of incorporation and bylaws, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us, even if doing so would be beneficial to our stockholders. These provisions include:
| • | authorizing the issuance of “blank check” preferred that could be issued by our board of directors to increase the number of outstanding shares and thwart a takeover attempt; |
| • | prohibiting cumulative voting in the election of directors, which would otherwise allow less than a majority of stockholders to elect director candidates; and |
| • | advance notice provisions in connection with stockholder proposals that may prevent or hinder any attempt by our stockholders to bring business to be considered by our stockholders at a meeting or replace our board of directors. |
Together these provisions may delay, deter or prevent a change in control of us, adversely affecting the market price of our common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS
There are no unresolved staff comments.
Our executive offices and certain operations are located at the Connell Corporate Center, 300 Connell Drive, 5th Floor, Berkeley Heights, NJ 07922.
We entered into the lease agreement for our executive offices on July 11, 2005. The lease was for a term of ten years and four months, with a commencement date of October 1, 2005 and covers approximately 19,700 total rentable square feet. The annual rent in the first year was $324,000 increasing to $512,000 in year 2 and increasing at regular intervals until year 10 when the annual rent was approximately $561,000. Effective February 1, 2010, we amended our lease to reduce the annual rent to approximately $512,000 for the remaining term and extended the lease term for one year through January 2017. As part of the lease agreement, we posted a letter of credit securing our lease payments which was reduced to approximately $256,000. On September 23, 2015, we amended our lease to relocate our executive offices to approximately 5,200 total rentable square feet in the same building. The lease amendment is dated as of September 15, 2015 and will be effective upon the completion of renovations to the premises or, if earlier, the date that the company occupies the space. The amended lease has a term of six years following the occupancy date and annual rentals ranging from
37
Table of Contents
approximately $135,000 in the first year to $148,000 in the final year. The lease also provides us with a one-time option to renew the lease for a term of five years at the then-current market rate and, provided we pay an early termination fee, allows us an early termination option on each of the 18-month, 27-month and 36-month anniversary dates of the effective date of the amendment. As part of the lease agreement, we will reduce our letter of credit securing our lease payments to approximately $135,000.
On May 22, 2015, the company, together with its subsidiary Express MD Solutions, LLC entered into a license and settlement agreement with Robert Bosch Healthcare Systems, Inc. providing for the resolution and dismissal, with prejudice, of the purported patent infringement lawsuit filed by Bosch against Express MD in January 2012 in the U.S. District Court for the Northern District of California, Case No. 5:12-cv-00068-JW. While the company does not believe that it is infringing any of the asserted patents, in order to mitigate its risk and avoid further costs and distractions of litigation, it entered into the license and settlement agreement. As previously reported by the company, the complaint alleged that the company’s “Electronic House Call” product infringes one or more claims of certain patents allegedly owned by Bosch. The agreement provides for the company to be granted by Bosch a worldwide, non-exclusive, non-assignable, royalty bearing license under the patents and a covenant not to be sued under certain other patents owned by Bosch. In consideration thereof, the company agreed to pay Bosch for the license and related matters and to pay certain royalties to Bosch for a three year period thereafter.
We are also subject to claims and litigation arising in the ordinary course of business. Our management considers that any liability from any reasonably foreseeable disposition of such claims and litigation, individually or in the aggregate, would not have a material adverse effect on our consolidated financial position, results of operations or cash flows.
ITEM 4. MINE SAFETY DISCLOSURE.
Not applicable.
38
Table of Contents
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Our common stock currently trades on The NASDAQ Capital Market under the symbol “ADAT.” After giving effect to our reverse stock split, the following is the range of high and low sales prices for our common stock on The NASDAQ Capital Market for the periods indicated below:
| High | Low | |||||||
| Fiscal Year 2015 |
||||||||
| 1st Quarter |
$ | 0.88 | $ | 0.44 | ||||
| 2nd Quarter |
$ | 1.20 | $ | 0.60 | ||||
| 3rd Quarter |
$ | 0.94 | $ | 0.20 | ||||
| 4th Quarter |
$ | 0.32 | $ | 0.16 | ||||
| Fiscal Year 2014 |
||||||||
| 1st Quarter |
$ | 0.97 | $ | 0.82 | ||||
| 2nd Quarter |
$ | 1.88 | $ | 0.88 | ||||
| 3rd Quarter |
$ | 1.59 | $ | 0.97 | ||||
| 4th Quarter |
$ | 1.14 | $ | 0.61 | ||||
As of September 18, 2015 there were approximately 400 holders of record of our common stock. The number of record holders may not be representative of the number of beneficial owners because many of the shares of our common stock are held by depositories, brokers or other nominees. We believe that there are approximately 3,500 holders of our common stock.
On January 28, 2015, we received a staff deficiency letter from The Nasdaq Stock Market notifying us that for the prior 30 consecutive business days, the closing bid price per share of our common stock was below the $1.00 minimum bid price requirement for continued listing on The Nasdaq Capital Market, as required by Listing Rule 5550(a)(2) (the “Bid Price Rule”). Nasdaq provided us with 180 calendar days, or until July 27, 2015, to regain compliance with the Bid Price Rule. To regain compliance with the Bid Price Rule, the closing bid price of our common stock must meet or exceed $1.00 per share for a minimum of ten consecutive business days during the 180 day grace period.
Subsequently, on May 28, 2015, we received a second staff deficiency letter from The Nasdaq Stock Market notifying us that we did not comply with the minimum stockholders’ equity requirement for continued listing on the Nasdaq Capital Market, which requires listed companies to maintain stockholders’ equity of at least $2.5 million. Nasdaq provided us with 45 calendar days, or until July 13, 2015, to submit a plan to regain compliance with the minimum stockholders’ equity standard. Pursuant to an extension granted by the staff, we submitted our compliance plan on July 21, 2015.
On July 29, 2015, we received a determination letter from the staff of The Nasdaq Stock Market stating that the company has not regained compliance with The Nasdaq Capital Market minimum bid price of $1.00 requirement for continued listing set forth in Nasdaq Listing Rule 5550(a)(2). The Nasdaq determination letter also stated that the company is not eligible for an additional 180-day extension to regain compliance with the minimum bid price rule because the company does not meet the minimum stockholders’ equity initial listing requirement for the Nasdaq Capital Market. The determination letter also stated that the Company did not maintain a minimum $2.5 million in stockholders equity for continued listing and did not meet the alternatives of market value of listed securities or net income as required under Listing Rule 5550(b) and that such deficiency serves as an additional basis for delisting. Pursuant to the determination letter, we requested a hearing to appeal this determination on August 5, 2015, and were granted a hearing on September 10, 2015. At the hearing we presented our plan to regain compliance with both the minimum bid price requirement of Listing Rule 5550(a)(2) and the minimum shareholders’ equity requirement of Listing Rule 5550(b)(1). On September 16, 2015, we
39
Table of Contents
received written notice that the Panel granted our request to remain listed on The NASDAQ Stock Market, LLC, subject to the condition that, on or before January 25, 2016, we shall announce and inform the Panel that the company’s proposed business combination has closed and that NASDAQ’s Listing Qualifications Staff (the “Staff”) has approved the combined entity’s application for initial listing on NASDAQ. In its written notice, the Panel stated that during the granted exception period the company must promptly notify the Panel of any significant developments, particularly any event, condition or circumstance that may impact its ability to meet the terms of the exception granted by the Panel and that the Panel reserves the right to reconsider the granted exception in such an instance. The company is diligently working to timely satisfy the terms of the Panel’s decision; however, there can be no assurance that the company will be able to do so. In the event that the company is unable to meet the exception requirement, the Panel will issue a final determination to delist the company’s shares and suspend trading of the company’s shares on The Nasdaq Capital Market.
Dividend Policy
We have not paid any dividends on our common stock since our inception. We do not expect to pay any dividends on our common stock in the foreseeable future and plan to retain earnings, if any, to finance the development and expansion of our business. Further, our Certificate of Incorporation authorizes our board of directors to issue Preferred Stock with a preferential right to dividends. We currently have 28,000 shares of Series B preferred stock outstanding which have the right to receive dividends equal to an annual rate of 10% of the issue price payable on a semi-annual basis and 665,000 shares of Series D preferred stock outstanding which have the right to receive dividends equal to an annual rate of 5% of the issue price payable on a semi-annual basis in cash or shares of common stock, at our option.
Sales of Unregistered Securities
Except as previously reported and as described elsewhere in this Annual Report on Form 10-K, we did not sell unregistered securities during the quarter ended June 30, 2015.
For the quarter ended June 30, 2015, we issued options to purchase an aggregate of 739,915 shares of common stock to our non-executive directors that elected to receive options and in July 2015, we issued an aggregate of 78,854 restricted shares of our common stock to our non-executive directors who served on our board during the fiscal quarter that elected to receive shares of common stock in lieu of the cash fees earned for their service as members of our board of directors pursuant to our 2011 Omnibus Equity Incentive Plan. The options are exercisable for a period of ten years at an exercise price of $0.19 per share. Such securities were issued for service on our board during the quarter ended June 30, 2014. These securities were issued pursuant to the exemption from registration provided by Section 4(a)(2) of the Securities Act of 1933, as amended.
Effective as of June 30, 2015, the company issued an aggregate of 151,869 shares of common stock in lieu of the cash payment of accrued dividends of $164,884 on its outstanding shares of Series D convertible preferred stock, in accordance with the applicable terms of the Series D convertible preferred stock. Certain officers, directors and significant stockholders of the company own shares of Series D convertible preferred stock and received shares of common stock, as follows: an entity affiliated with one of our directors, J. David Luce, and his spouse, were issued a total of 60,518 shares; an entity affiliated with Mr. Luce’s sibling received 3,997 shares; Todd A. Borus, a director, was issued 571 shares; William A. Marshall, our chief financial officer was issued 2,284 shares; and Lazarus Investment Partners, which owns in excess of 20% of our common stock, was issued 45,674 shares. The foregoing shares of common stock were issued pursuant to Section 4(a)(2) of the Securities Act of 1993, as amended.
Repurchase of Equity Securities
There were no stock repurchases during the year ended June 30, 2015.
Securities Authorized for Issuance under Equity Compensation Plans
Disclosure pursuant to this item is provided below in Item 12 of this Annual Report.
40
Table of Contents
Stock Performance Graph
The following performance graph shall not be deemed “filed” for purposes of Section 18 of the Exchange Act or otherwise subject to the liabilities under that Section and shall not be deemed to be incorporated by reference into any filing of Authentidate under the Securities Act or the Exchange Act.
Comparison of Cumulative Total Return—June 30, 2010 to June 30, 2015
Set forth below is a line graph comparing the total cumulative return on Authentidate’s common stock and the Nasdaq Composite Index and the Nasdaq Computer Index. Authentidate’s common stock is listed for trading in the Nasdaq Capital Market under the trading symbol ADAT. The comparisons in the graph below are based on historical data and are not intended to forecast the possible future performance of Authentidate common stock.
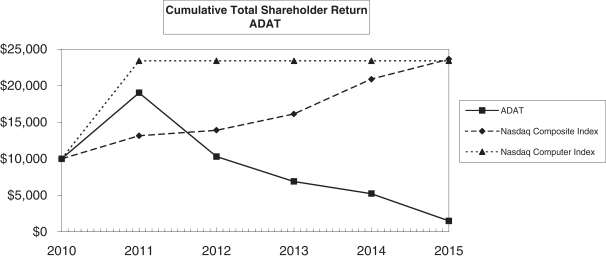
Listed below is the value of a $10,000 investment at each of the fiscal year ends presented:
Cumulative Total Shareholder Return at June 30,
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | |||||||||||||||||||
| ADAT |
$ | 10,000 | $ | 19,048 | $ | 10,317 | $ | 6,905 | $ | 5,238 | $ | 1,508 | ||||||||||||
| Nasdaq Composite Index |
$ | 10,000 | $ | 13,149 | $ | 13,915 | $ | 16,135 | $ | 20,899 | $ | 23,643 | ||||||||||||
| Nasdaq Computer Index |
$ | 10,000 | $ | 23,403 | $ | 23,403 | $ | 23,403 | $ | 23,403 | $ | 23,403 | ||||||||||||
| (1) | Assumes $10,000 was invested at June 30, 2010 in Authentidate and each Index presented. |
| (2) | The comparison indices were chosen in good faith by management. Most of our peers are divisions of large multi-national companies, therefore a comparison may not be meaningful. |
41
Table of Contents
ITEM 6. SELECTED FINANCIAL DATA
The following selected financial data are derived from our consolidated financial statements, which have been audited. The data set forth below should be read in conjunction with the consolidated financial statements, including the related notes, “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and the other financial information included elsewhere in this Report.
| Year Ended June 30, | ||||||||||||||||||||
| (in thousands, except per share amounts) |
2015 | 2014 | 2013 | 2012 | 2011 | |||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||
| Continuing operations |
||||||||||||||||||||
| Revenues |
$ | 3,689 | $ | 5,556 | $ | 4,827 | $ | 3,188 | $ | 2,936 | ||||||||||
| Operating expenses |
12,395 | 12,673 | 12,198 | 10,895 | 10,087 | |||||||||||||||
| Loss |
(9,700 | ) | (7,143 | ) | (11,349 | ) | (8,352 | ) | (6,786 | ) | ||||||||||
| Basic and diluted net loss per common share |
(0.25 | ) | (0.26 | ) | (0.45 | ) | (0.35 | ) | (0.32 | ) | ||||||||||
| Net loss |
(9,700 | ) | (7,143 | ) | (11,349 | ) | (8,352 | ) | (12,555 | ) | ||||||||||
| Other Financial Data: |
||||||||||||||||||||
| Continuing operations: |
||||||||||||||||||||
| Net cash used by operating activities |
$ | (4,951 | ) | $ | (4,726 | ) | $ | (5,214 | ) | $ | (6,513 | ) | $ | (6,465 | ) | |||||
| Net cash (used) provided in investing activities (1) |
(546 | ) | 370 | (471 | ) | (1,277 | ) | 1,680 | ||||||||||||
| Net cash provided by financing activities |
4,450 | 1,935 | 7,154 | 7,612 | 4,557 | |||||||||||||||
| Net (decrease) increase in cash, cash equivalents and marketable securities |
(1,047 | ) | (2,421 | ) | 1,469 | (178 | ) | 1,069 | ||||||||||||
| Net cash provided by discontinued operations |
— | — | — | — | 1,260 | |||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Current assets |
$ | 1,602 | $ | 5,254 | $ | 9,184 | $ | 8,449 | $ | 8,229 | ||||||||||
| Current liabilities |
4,540 | 2,884 | 4,333 | 3,647 | 3,290 | |||||||||||||||
| Working capital |
(2,938 | ) | 2,370 | 4,851 | 4,802 | 4,939 | ||||||||||||||
| Total assets |
4,163 | 8,228 | 13,193 | 12,861 | 10,835 | |||||||||||||||
| Total long term liabilities |
88 | 126 | 184 | 3,147 | 140 | |||||||||||||||
| Redeemable preferred stock |
— | — | — | 3,254 | 2,931 | |||||||||||||||
| Shareholders’ (deficit) equity |
(465 | ) | 5,218 | 8,676 | 2,813 | 4,474 | ||||||||||||||
| (1) | Excludes purchases and sales of marketble securities |
42
Table of Contents
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
Overview
Authentidate Holding Corp. (Authentidate or the company) and its subsidiaries provide secure web-based revenue cycle management applications and telehealth products and services that enable healthcare organizations to increase revenues, improve productivity, reduce costs, coordinate care for patients and enhance related administrative and clinical workflows and compliance with regulatory requirements. Our web-based services are delivered as Software as a Service (SaaS) to our customers interfacing seamlessly with billing and document management systems. These solutions incorporate multiple features and security technologies such as business- rules based electronic forms, intelligent routing, transaction management, electronic signatures, identity credentialing, content authentication, automated audit trails and remote patient management capabilities. Both web and fax-based communications are integrated into automated, secure and trusted workflow solutions.
Our telehealth solutions provide in-home patient vital signs monitoring systems and services to improve care for patients and reduce the cost of care by delivering results to their healthcare providers via the Internet. Our telehealth solutions combine our tablet or Electronic House Call™ patient vital signs monitoring appliances or our Interactive Voice Response patient vital signs monitoring solution with a web-based management and monitoring software module. Both solutions enable unattended measurements of patients’ vital signs and related health information and are designed to aid wellness and preventative care, and deliver better care to specific patient segments that require regular monitoring of medical and behavioral health conditions. Healthcare providers can easily view each specific patient’s vital statistics and make adjustments to the patient’s care plans securely via the Internet. This service provides a combination of care plan schedule reminders and comprehensive disease management education as well as intelligent routing to alert on-duty caregivers whenever a patient’s vital signs are outside of the practitioner’s pre-set ranges. Healthcare providers and health insurers are also expected to benefit by having additional tools to improve patient care and reduce in-person and emergency room patient visits and hospital readmissions.
We operate our business in the US with technology and service offerings that address emerging growth opportunities based on the regulatory and legal requirements specific to each market. Our business is engaged in the development and sale of web-based services largely based on our Inscrybe® platform and related capabilities and our telehealth products and services. In recent years we have focused our efforts on developing and introducing solutions for use in the healthcare information technology industry.
We believe there are a number of on-going factors that will be favorable for the healthcare information technology industry in the near future. These factors include regulatory reforms in the U.S. focused on controlling costs, automating medical records and processes and expanding the availability of healthcare coverage, and healthcare industry trends to significantly reduce costs, shorten the length of hospital stays, reduce hospital readmissions, shift patient care towards wellness and preventative care programs and automate healthcare records and processes. Furthermore, we believe our business will benefit as the recent U.S. Supreme Court decisions upholding the healthcare law are better recognized in the marketplace. Because healthcare information technology solutions play an important role in healthcare by improving safety, efficiency and reducing cost, they are often viewed as more strategic than other capital purchases. In addition, government agencies, as well as politicians and policymakers appear to agree that the growing cost of our healthcare system is unsustainable and the intelligent use of information systems will improve health outcomes and, correspondingly, drive down costs. The broad recognition that healthcare information technology is essential to help control healthcare costs and improve quality contributed to the inclusion of healthcare information technology incentives in the American Recovery and Reinvestment Act (ARRA) and accompanying Health Information Technology for Economic and Clinical Health (HITECH) provisions which include more than $35 billion in incentives for healthcare organizations to modernize operations through “meaningful use” of healthcare information technology. Further, as more consumers are provided with insurance coverage, healthcare providers may face increased volumes that could create capacity constraints, and they may find it challenging to profitably
43
Table of Contents
provide care at the planned reimbursement rates under the expanded coverage models. Another aspect of the market for healthcare information technology is the shift away from fee-for-service or volume-based reimbursement towards value-based or outcomes-based reimbursement. Payers, including health insurance companies and federal and state governments, are implementing programs to link reimbursement to quality measurements and outcomes, and this alignment creates significant financial motivation for adoption of healthcare information technology products and services. We believe that there are substantial sums of reimbursement funds that are tied to incentive programs such as value based purchasing, 30-day readmission rules and quality reporting requirements. There are also a growing number of third-party studies that document how telehealth can positively impact the way healthcare is delivered. From chronic care to behavioral health and wellness programs, a wide range of patient populations can benefit from telehealth. We believe that telehealth products are helping physicians and patients to accomplish a number of goals, including, shifting visits away from high-cost settings; reducing the cost of managing patients; reducing unnecessary hospital readmissions; reducing the duration of hospital stays; improving access to care for patients located in remote areas; and improving outcomes. We believe the factors discussed above will create strong incentives for providers to maximize efficiency and create the need for additional investments in healthcare information technology solutions and services. We also believe that the healthcare information technology industry will likely benefit as healthcare providers and governments continue to recognize that these solutions and services contribute to safer, more efficient healthcare.
We have experienced net losses and negative cash flow from operating activities while we have been focused on developing our products and services, refining our business strategies and repositioning our businesses for growth. Although we believe we are well positioned for such growth, we expect to continue to generate net losses and negative cash flow for the foreseeable future as we seek to expand our potential markets and generate increased revenues. As discussed in more detail below, we have completed several financing transactions, and sold non-core assets to fund our working capital needs. See “Liquidity and Capital Resources”.
During fiscal 2015 we have focused primarily on marketing our telehealth products and services and our referral and order management and hospital discharge solutions. We have also continued to take steps to refine our core product and service offerings, significantly expand our addressable markets, manage operating costs and position the company for long-term growth. We believe the company is well positioned for growth as the healthcare markets it serves are growing rapidly and expected to continue to grow over the next several years according to third party analyst market assessments. Additionally, our products and services target the “12 month episode of care” market, which is the largest and fastest growing market segment for remote patient management services. As discussed above, we also believe our business will benefit as federal government healthcare reforms are implemented and healthcare industry trends take hold. Although we have taken steps to focus our business in these areas, our progress will be impacted by the timing of customer contracts and implementations and the market acceptance of our products and services.
On March 6, 2015 we announced that the Department of Veterans Affairs (VA) informed the company that it did not intend to exercise the fourth and final option year under our contract for telehealth products and services. The company’s contract with the VA was originally awarded in April 2011 and consisted of a base year and four one-year option years which were exercisable at the VA’s sole discretion. The current option year under the contract expired on May 15, 2015 and the transition process with the VA was completed by that date. Our VA revenue included both recurring service revenues as well as hardware sales. As a result of the non-renewal of the VA contract we expect to report significantly reduced revenues over the next several quarters and we have taken steps to reduce our operating costs and better align our resources with the growth opportunities we intend to pursue. The VA had been our largest customer, accounting for approximately 45% and 58% of our total revenue for the years ended June 30, 2015 and 2014, respectively. As a result, we have implemented a number of changes to our business plan with the ultimate goal to increase revenues and positive cash flow from operations, including a recalibration of marketing and sales efforts that have already resulted in growth from existing customers and sales to new customers. These changes include cost reductions from reducing our workforce and use of consultants that we made in the third quarter of fiscal 2015 and additional workforce reductions through
44
Table of Contents
August 31, 2015. During the third quarter the company recorded charges of approximately $70,000 related to workforce severance during the period and did not incur any additional severance costs for the additional workforce reductions made through June 30, 2015 or the more recent workforce reductions made in August 2015. These reductions are expected to reduce operating expenses by approximately $4,151,000 on an annualized basis. We have also taken actions to realign our data center operations for an expected annualized cost reduction of $203,000 and have executed an agreement with our landlord to relocate our corporate offices, which is expected to result in annualized savings of $372,000.
We believe that our experience with the VA telehealth project has enabled us to refine our telehealth products and services and position the company for success in the commercial market which we believe provides a significantly larger opportunity for the company as this market develops. We have continued to develop lower cost solutions for the commercial market that we were not able to deploy at the VA and we have already reduced our operating costs in excess of the monthly recurring revenues and equipment margins that we were generating from the VA project. Moving forward, we plan to repurpose our volume-tested, web-based management and monitoring solutions and investments to focus on offering a broader array of cost effective solutions to the healthcare market and increase our sales efforts on commercial market opportunities which we believe offer greater potential for growth.
Our current revenues consist principally of transaction fees for web-based hosted software services and revenues from hardware sales, monthly monitoring services and maintenance fees from our telehealth business. Growth in our business is affected by a number of factors, including general economic and business conditions, and is characterized by long sales cycles. The timing of customer contracts, implementations and ramp-up to full utilization can have a significant impact on results and we believe our results over a longer period of time provide better visibility into our performance.
We intend to continue our efforts to market our web-based services and related products in our target markets. We also intend to focus on identifying additional applications and markets where our technology can address customer needs.
Critical Accounting Policies
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States and the rules of the Securities and Exchange Commission. The preparation of our consolidated financial statements and related notes in accordance with generally accepted accounting principles requires us to make estimates, which include judgments and assumptions that affect the reported amounts of assets, liabilities, revenue, and expenses, and related disclosure of contingent assets and liabilities. We have based our estimates on historical experience and on various assumptions that we believe to be reasonable under the circumstances. We evaluate our estimates on a regular basis and make changes accordingly. Actual results may differ from these estimates under different assumptions or conditions. To the extent that there are material differences between these estimates and actual results, our financial condition or results of operations will be affected.
A critical accounting estimate is based on judgments and assumptions about matters that are uncertain at the time the estimate is made. Different estimates that reasonably could have been used or changes in accounting estimates could materially impact our financial statements. We believe that the policies described below represent our critical accounting policies, as they have the greatest potential impact on our consolidated financial statements. However, you should also review our Summary of Significant Accounting Policies beginning on page F-7 of Notes to Consolidated Financial Statements contained elsewhere in this Annual Report on Form 10-K.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases.
45
Table of Contents
Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amounts expected to be realized.
Long-Lived Assets
Long-lived assets, including property and equipment, software development costs, patent costs, trademarks and licenses are reviewed for impairment using an undiscounted cash flow approach whenever events or changes in circumstances such as significant changes in the business climate, changes in product offerings, or other circumstances indicate that the carrying amount may not be recoverable.
Revenue Recognition
Revenue is derived from web-based hosted software services, telehealth products and post contract customer support services. Revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed and collectability is reasonably assured. Multiple-element arrangements are assessed to determine whether they can be separated into more than one unit of accounting. A multiple-element arrangement is separated into more than one unit of accounting if all of the following criteria are met: the delivered item has value to the customer on a standalone basis; there is objective and reliable evidence of the fair value of the undelivered items in the arrangement; if the arrangement includes a general right of return relative to the delivered items, and delivery or performance of the undelivered item is considered probable and substantially in our control. If these criteria are not met, then revenue is deferred until such criteria are met or until the period over which the last undelivered element is delivered, which is typically the life of the contract agreement. If these criteria are met, we allocate total revenue among the elements based on the sales price of each element when sold separately which is referred to as vendor specific objective evidence or VSOE.
Revenue from web-based hosted software and related services and post contract customer support services is recognized when the related service is provided and, when required, accepted by the customer. Revenue from telehealth products is recognized when such products are delivered. Revenue from multiple element arrangements that cannot be allocated to identifiable items is recognized ratably over the contract term which is generally one year.
Management Estimates
Preparing financial statements requires management to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses. Examples include estimates of loss contingencies and product life cycles, assumptions such as elements comprising a software arrangement, including the distinction between upgrades/enhancements and new products; when technological feasibility is achieved for our products; the potential outcome of future tax consequences; and determining when investment or other impairments exist. We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We make estimates on the future recoverability of capitalized amounts, we record a valuation allowance against deferred tax assets when we believe it is more likely than not that such deferred tax assets will not be realized and we make assumptions in connection with the calculations of share-based compensation expense. Actual results and outcomes may differ from management’s estimates, judgments and assumptions. We have based our estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances and we evaluate our estimates on a regular basis and make changes accordingly. Historically, our estimates relative to our critical accounting estimates have not differed materially from actual results; however, actual results may differ from these estimates under different conditions. If actual results differ from these estimates and other considerations used in estimating amounts reflected in our consolidated financial statements, the resulting changes could have a material
46
Table of Contents
adverse effect on our consolidated statement of operations, and in certain situations, could have a material adverse effect on liquidity and our financial condition.
Share-Based Compensation
Option-based employee compensation expense is determined using the Black-Scholes option pricing model which values options based on the stock price at the grant date, the exercise price of the option, the expected life of the option, the estimated volatility, expected dividend payments and the risk-free interest rate over the expected life of the options.
The company computed the estimated fair values of all option based compensation using the Black-Scholes option pricing model and the assumptions set forth in the following table. The company based its estimate of the life of these options on historical averages over the past five years and estimates of expected future behavior. The expected volatility was based on the company’s historical stock volatility. The assumptions used in the company’s Black-Scholes calculations for fiscal 2015, 2014 and 2013 are as follows:
| Risk Free Interest Rate |
Dividend Yield |
Volatility Factor |
Weighted Average Option Life (Months) |
|||||||||||||
| Fiscal year 2015 |
0.6 | % | 0 | % | 84 | % | 48 | |||||||||
| Fiscal year 2014 |
1.4 | % | 0 | % | 89 | % | 48 | |||||||||
| Fiscal year 2013 |
0.6 | % | 0 | % | 107 | % | 48 | |||||||||
The Black-Scholes option-pricing model requires the input of highly subjective assumptions. Because the company’s stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models may not provide a reliable single measure of the fair value of share-based compensation for employee and director stock options. Management will continue to assess the assumptions and methodologies used to calculate estimated fair value of share-based compensation as circumstances change and additional data becomes available over time, which may result in changes to these assumptions and methodologies. Such changes could materially impact the company’s fair value determination.
Concentrations of Credit Risk
Financial instruments which subject us to concentrations of credit risk consist of cash and cash equivalents, marketable securities and trade accounts receivable. To reduce credit risk, we place our cash, cash equivalents and investments with high credit quality financial institutions and typically invest in AA or better rated investments. We monitor our credit customers and we establish an allowance for doubtful accounts based upon factors surrounding the credit risk of specific customers, historical trends and other information.
The following analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and notes thereto contained elsewhere in this Annual Report on Form 10-K.
Results of Operations
Fiscal Year 2015 Compared to Fiscal Year 2014
Revenues were $3,689,000 for the year ended June 30, 2015 compared to $5,556,000 for the prior year period. These results reflect a decrease in revenues from both our hosted software services and our telehealth products and services due primarily to lower transaction volumes and equipment sales, respectively.
47
Table of Contents
Cost of revenues decreased to $1,877,000 for the year ended June 30, 2015 compared to $3,759,000 for the same period in the prior year, due primarily to the lower costs related to telehealth revenues, lower telecommunications expenses for our hosted software services and lower data center personnel, hosting and maintenance expenses.
Selling general and administrative (SG&A) expenses increased to $8,379,000 for the year ended June 30, 2015 compared to $7,040,000 for the prior year period. The increase is due primarily to higher personnel, severance, consulting, legal and inventory adjustment expenses which offset lower selling expenses and other savings. These results do not give full effect to the impact of our recent work force reductions and other cost-savings measures implemented beginning in March 2015. The prior year period also includes a state payroll tax credit of approximately $175,000 which reduced SG&A expenses for the prior year period.
Product development expenses were $1,180,000 for the year ended June 30, 2015 compared to $1,108,000 for the prior year period. The increase is due primarily to higher personnel expenses and does not give full effect to our recent work force reductions.
Depreciation and amortization expense was $959,000 for the year ended June 30, 2015 compared to $766,000 for the prior year period. This change is due primarily to higher expenses for the amortization of other assets and acquired licenses.
Other expense was $994,000 for the year ended June 30, 2015 compared to $26,000 for the prior year period. The increase in other expense consists primarily of the non-cash loss on the extinguishment of certain notes recorded in connection with the modification and extension of such notes and the amortization of the debt discount and deferred financing costs on the company’s notes payable. The loss on extinguishment consists of the remaining unamortized debt discount and deferred financing costs related to the modified notes and the Black-Scholes value of any new warrants issued in connection with the note modifications. The prior year amount reflects the non-cash amortization of debt discount and deferred financing costs on senior secured notes repaid on October 31, 2013 offset in part by a gain of approximately $101,000 on the sale of certain non-core assets.
Net loss for the year ended June 30, 2015 was $9,700,000, or $0.25 per share, compared to $7,143,000, or $0.26 per share, for the prior year period. The increase in net loss for the year is due primarily to the increases in SG&A expenses, non-cash expenses for debt extinguishment and debt discount amortization and the other factors discussed above.
Fiscal Year 2014 Compared to Fiscal Year 2013
Revenues were $5,556,000 for the year ended June 30, 2014 compared to $4,827,000 for the prior year period. These results reflect an increase in revenues from our telehealth products and services which offset lower revenues from our hosted software services due primarily to lower transaction volumes.
Cost of revenues increased to $3,759,000 for the year ended June 30, 2014 compared to $3,454,000 for the same period in the prior year, due primarily to the higher telehealth revenues which offset lower data center maintenance expenses.
Selling general and administrative (SG&A) expenses increased to $7,040,000 for the year ended June 30, 2014 compared to $6,816,000 for the prior year period. The increase is due primarily to higher stock compensation, selling and consulting expenses which were offset in part by lower legal and investor relation expenses and state payroll tax credits.
Product development expenses were $1,108,000 for the year ended June 30, 2014 compared to $1,085,000 for the prior year period. The increase is due primarily to higher personnel expenses.
48
Table of Contents
Depreciation and amortization expense was $766,000 for the year ended June 30, 2014 compared to $843,000 for the prior year period. This change is due primarily to lower expenses for amortization of capitalized software offset in part by the amortization of acquired licenses.
Other expense was $26,000 for the year ended June 30, 2014 compared to $3,978,000 for the prior year period. Other expense consists primarily of the non-cash amortization of the debt discount on the company’s senior secured notes payable. The decrease in other expense for the current period reflects the early extinguishment of debt in June 2013 and a gain of approximately $101,000 on the sale of certain non-core assets in fiscal 2014. In fiscal 2013, other expense also includes a non-cash loss of approximately $1,060,000 on the early extinguishment of certain senior secured notes in exchange for Series D preferred stock and warrants to purchase shares of common stock. The amount of the loss is equal to the remaining unamortized debt discount and deferred financing costs related to such notes as of the extinguishment date.
Net loss for the year ended June 30, 2014 was $7,143,000, or $0.26 per share, compared to $11,349,000, or $0.45 per share, for the prior year period. The decrease in net loss for the year is due primarily to the decrease in non-cash debt discount amortization and the other factors discussed above.
Liquidity and Capital Resources
Overview
Our operations and product development activities have required substantial capital investment to date. Our primary sources of funds have been the issuance of equity and the incurrence of third party debt. As described in greater detail in Notes 8, 17 and 18 of Notes to Consolidated Financial Statements, we completed an equity-financing transaction in September 2014 for net proceeds of approximately $2.1 million and since then, we have completed several debt financings for proceeds of approximately $3.5 million during and subsequent to the end of our 2015 fiscal year to fund our operations. We are using the proceeds from these transactions for working capital and general corporate purposes, including supporting the rollout of our telehealth products and services. As discussed in more detail below, our recurring operating losses and capital needs, among other factors, raise substantial doubt about our ability to continue as a going concern. Our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
For the year ended June 30, 2015, expenditures for data center equipment and other assets totaled approximately $185,000 and expenditures for software licenses and other intangible assets totaled approximately $361,000. We have developed and intend to continue to develop new applications to grow our business and address new markets.
In August 2012, we filed with the SEC a registration statement on Form S-3 and a pre-effective amendment to such registration statement under the Securities Act in December 2012. The shelf registration, was declared effective by the SEC on December 2012 and allows us to sell, from time to time in one or more public offerings, shares of our common stock, shares of our preferred stock, debt securities or warrants to purchase common stock, preferred stock or debt securities, or any combination of such securities, for proceeds in the aggregate amount of up to $40 million, subject to SEC limitations. Following our recent offering in August 2014, there is approximately $29 million available under this registration statement for future transactions, subject to SEC limitations. The terms of any such future offerings, if any, and the type of equity or debt securities would be established at the time of the offering. This disclosure shall not constitute an offer to sell or a solicitation of an offer to buy the securities, nor shall there be any sale of these securities in any jurisdiction in which an offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such jurisdiction. Any offer of the securities will be solely by means of the prospectus included in the registration statement and one or more prospectus supplements that will be issued at the time of the offering.
At the company’s adjourned annual meeting of stockholders held on June 30, 2015, our stockholders approved an amendment to the company’s certificate of incorporation to increase the number of authorized shares of the company’s common stock from 100 million shares to 190 million shares of common stock.
49
Table of Contents
Cash Flows
At June 30, 2015, cash, cash equivalents amounted to approximately $247,000 and total assets at that date were $4,163,000. Since June 30, 2014 cash, cash equivalents and marketable securities decreased by approximately $1,047,000 reflecting the issuance of company securities offset by cash used principally to fund operating losses, product development activities, changes in working capital and capital expenditures during the year ended June 30, 2015. Our current estimated monthly operational requirements after giving effect to our cost cutting measures, but not to any special or one-time events, is approximately $350,000. Cash used for the period includes investments in data center and related infrastructure equipment; investments in manufacturing, licenses and other assets; and the prepayment of certain insurance premiums and maintenance contracts. We expect to continue to use cash to fund operating losses, changes in working capital, product development activities and capital expenditures for the foreseeable future.
Net cash used by operating activities for the year ended June 30, 2015 was approximately $4,951,000 compared to $4,726,000 for the prior year period reflecting an increase in cash used for operations for the current period. Net cash used by investing activities, excluding purchases and sales of marketable securities, was $546,000 for the year ended June 30, 2015 compared to net cash provided of $370,000 for the prior year period. This change is due primarily to proceeds of approximately $851,000 from the sale of certain non-core assets during the prior year period. Net cash provided by financing activities for the year ended June 30, 2015 was approximately $4,450,000 compared to $1,935,000 for the prior year period. The amount for the current period reflects the net proceeds from the August 2014 private placement transaction and the issuance of short-term promissory notes less the payment of certain preferred stock dividends and the repayment of a short-term promissory note. The amount for prior year reflects the net proceeds from the private placement transaction in fiscal 2014 less the repayment of senior secured notes and the payment of certain preferred stock dividends.
Recent Financing Activities
To date we have been largely dependent on our ability to sell additional shares of our common stock or other equity and debt securities to obtain financing to fund our operating deficits, product development activities, business acquisitions, capital expenditures and telehealth activities. In September 2014, we completed an equity-based financing and received approximately $2.1 million of net proceeds in a registered direct offering to certain accredited investors. Since February 2015, we have completed debt financing transactions resulting in total proceeds of approximately $3.5 million. The material terms of these debt transactions are summarized below.
In February 2015, we issued a promissory note in the aggregate principal amount of $100,000 and common stock purchase warrants to purchase up to 80,000 shares of common stock for gross proceeds of $100,000. The note was an unsecured obligation of the company, was not convertible into equity securities of the company and accrued interest at a rate of 8% per annum. The warrants vested in equal monthly installments over twelve months if the notes were outstanding and, subject to vesting requirements, were exercisable for a period of 54 months commencing on the six month anniversary of the issuance date at an initial exercise price of $1.01 per share. Although the securities purchase agreement that the company entered into with this investor contemplated a second closing for $900,000 of additional proceeds, the second closing did not occur and the company did not receive the additional funds. This note and the vested portion of the warrants were exchanged for notes and warrants issued in the June 8, 2015 transaction discussed below and the warrants issued for this transaction were cancelled.
Further, in February 2015, in a separate transaction, we issued an additional promissory note in the aggregate principal amount of $950,000 and warrants to purchase 99,500 shares of common stock to an accredited investor for gross proceeds of $950,000. This note is an unsecured obligation of the company, is not convertible into equity securities of the company and accrues interest at a rate of 5.76% per annum. This note was due and payable on the first to occur of the one month anniversary of the issue date or the date on which the company received at least $950,000 in proceeds from equity or debt financing. The short-term note contains
50
Table of Contents
covenants and events of default customary for similar transactions. The warrants are exercisable for a period of 54 months commencing on the six month anniversary of the issuance date and have an initial exercise price of $1.01 per share. On April 3, 2015, we entered into an amendment agreement with the holder of this note to extend its maturity date from March 19, 2015 to July 2, 2015. In addition, pursuant to the amendment agreement, we also agreed to grant the holder the right to exchange the principal amount of the note into the securities of the company sold in the next financing, as defined in the amendment agreement. This investor subsequently agreed not to participate in the convertible debt financing for which a closing was held June 8, 2015 and in consideration of such election, the participation right was modified to allow him to exchange such note for comparable securities in an alternative transaction. In consideration of waivers previously granted by the holder of potential events of default under the note and the amendment to extend the maturity date, we issued the holder warrants to purchase 3,166,667 shares of common stock. The warrants are exercisable for a period of 54 months commencing six months following the date of issuance and have an exercise price equal to $0.31 per share. Subsequently, the note has been extended several times through October 16, 2015.
On April 24, 2015, we issued a promissory note in the aggregate principal amount of $500,000 to Lazarus Investment Partners LLLP, the beneficial owner of approximately 29.3% of the company’s common stock, in a private transaction. The note is an unsecured obligation of the company and is not convertible into equity securities of the company. The note was originally due and payable on the first to occur of July 2, 2015 or the date on which the company received at least $900,000 in proceeds from equity or debt financing transactions and has been extended several times through October 16, 2015. Interest on the note was originally 5.76% per annum payable at maturity and has been increased to 12% in connection with the most recent extension. The note contains terms and events of default customary for similar transactions. In consideration of the loan, the company and Lazarus entered into a warrant amendment agreement pursuant to which the company agreed to amend certain of the terms of the existing 6,233,636 common stock purchase warrants held by Lazarus to reduce the exercise price of such warrants to $0.25, which was $0.01 above the most recently reported closing consolidated bid price of the company’s common stock prior to the execution of the transaction documents, and to extend the expiration date of the warrants to October 25, 2019. The warrants amended were issued in various transactions from 2010 through 2013 at exercise prices ranging between $0.88 and $2.00.
On June 8, 2015, we entered into definitive agreements relating to a private placement of up to $3.0 million in principal amount of senior secured convertible debentures and common stock purchase warrants. An initial closing for an aggregate principal amount of $900,000 of convertible debentures and warrants to purchase 3,626,667 shares of common stock was held on June 8, 2015. The warrants are exercisable on or after the six month anniversary of the date of issuance at an initial exercise price of $0.30 per share and will expire 54 months from the initial exercise date. In addition, subject to certain limitations, the warrants provide that, beginning six months from issuance, the company shall have the right to cause the holder to exercise the warrants provided that the following conditions are satisfied: (i) the closing bid price of the company’s common stock is at least $0.45 for the 20 consecutive trading days prior to the date of the mandatory exercise notice and (ii) all of the warrants issued in the transaction are called by the company for a mandatory exercise. A final closing for up to $2.1 million of convertible debentures and additional warrants to purchase 8,400,000 shares of common stock did not occur. As a condition of the initial closing, the holder of an aggregate principal amount of $100,000 of previously issued promissory notes was required to exchange such notes and certain warrants held by it for the convertible debentures and warrants issued in this transaction. These convertible debentures, subject to certain exceptions, rank senior to existing and future indebtedness of the company and are secured to the extent and as provided in the security agreement entered into between the company and the purchasers. The convertible debentures mature on the one-year anniversary of the issuance date thereof and, subject to certain limitations, are convertible at any time at the option of the holder into shares of the company’s common stock at an initial conversion price of $0.25 per share. Subject to certain exemptions, if we issue or sell shares of our common stock, rights to purchase shares of our common stock, or securities convertible into shares of common stock for a price per share that is less than the conversion price then in effect, the conversion price will be decreased to equal 85% of such lower price and the exercise price of the warrants will be decreased to a lower price based on the amount by which the conversion price of the convertible debentures was reduced. The convertible debentures bear interest at 9% per annum with
51
Table of Contents
interest payable upon maturity or on any earlier redemption date. The convertible debentures are secured by a first priority lien on the company’s assets related to its “Inscrybe Referral and Order Management” and “Inscrybe Hospital Discharge” solutions.
On August 7, 2015, we issued a senior secured promissory note in the aggregate principal amount of $320,000 to an accredited investor in a private transaction dated August 7, 2015. The note is due and payable on December 31, 2015 and interest shall accrue on the note at the rate of 10.0% per annum. The note is not convertible into equity securities of the company and it contains terms and events of default customary for similar transactions. The note is secured by a first priority lien on certain of our assets, as described in a security agreement entered into between the company and the purchaser dated as of August 7, 2015. In consideration of the loan, we agreed to amend certain of the terms of the existing 5,474,829 common stock purchase warrants currently held by the purchaser and an affiliate to reduce the exercise price of such warrants to $0.17 and to extend the expiration date of the warrants to December 13, 2019. The warrants amended were issued in various transactions from 2012 through 2013 at exercise prices ranging between $0.95 and $1.34. The purchaser is an entity affiliated with J. David Luce, a member of our board of directors.
On August 26, 2015, we issued promissory notes in the aggregate principal amount of $400,000 to Lazarus Investment Partners LLLP, and an entity affiliated with J. David Luce, a member of our board of directors, in a private transaction. The notes are unsecured obligations of the company and are not convertible into equity securities of the company. The notes bear interest at 20% per annum, payable in arrears, and are due upon the earlier of (i) August 26, 2016, or (ii) within 30 days of the closing of the contemplated acquisition, merger or similar transaction with Peachstate Health Management, LLC (d/b/a AEON Clinical Laboratories) as described above, or a similar alternative acquisition, merger or similar transaction with an unaffiliated third party, or (iii) the closing of a sale of equity or debt securities of the company, or series of closings, as part of the same transaction, of equity or debt securities within a period of 90 days, in the gross amount of at least $5,000,000 in cash proceeds. The holders have the right to convert interest and principal due on the note into any alternative financing that may be undertaken by the company while the notes are outstanding.
In September 2015, the company issued promissory notes in the aggregate principal amount of $525,000 to accredited investors in a private transaction. The notes are unsecured and are not convertible into equity securities of the company. The notes bear interest at 20% per annum, payable in arrears, and are due upon the earlier of (i) September 18, 2016, or (ii) within 30 days of the closing of a sale of equity or debt securities of the company, or series of closings, as part of the same transaction, of equity or debt securities within a period of 90 days, in the gross amount of at least $5,000,000 in cash proceeds. The company also issued the investors warrants to purchase an aggregate of 1,050,000 shares of common stock. The warrants are exercisable commencing twelve months following their issuance for a period of 54 months at an exercise price of $0.30 per share. These closings are part of an offering of up to $1 million of notes and 2 million warrants.
However, we have an immediate need for additional capital and are exploring additional potential transactions to improve our capital position, ensure we are able to meet our working capital requirements and provide funds to pay these debt obligations which are due in the next twelve months. The company has incurred significant losses and our operations and product development activities have required substantial capital investment to date.
Under our current operating plan to grow our business, our ability to improve operating cash flow has been highly dependent on the market acceptance of our offerings. As discussed more fully in the Overview section above, the VA, our largest customer, did not renew our contract beyond May 15, 2015 and we expect to report significantly reduced revenues over the next several quarters. We have taken steps to reduce our operating costs and better align our resources with our growth opportunities; however, and based on our business plan, we expect our existing resources, revenues generated from operations, net proceeds from our debt financing transactions, other transactions we are considering and proceeds received from the exercise of outstanding warrants (of which there can be no assurance) or a restructuring of outstanding debt obligations (of which there can be no assurance) to satisfy our working capital requirements for at least the next twelve months. If necessary, management of the
52
Table of Contents
company believes that it can raise additional equity or debt financing to satisfy its working capital requirements. However, no assurances can be given that we will be able to support our costs or pay debt obligations through revenues derived from operations or generate sufficient cash flow to satisfy our other obligations. These factors, among others, raise substantial doubt about our ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments to the recoverability and classification of assets carrying amounts or the amounts and classifications of liabilities that might result from the outcome of these uncertainties. Unless we are able to increase revenues substantially or generate additional capital from other transactions, our current cash resources will only satisfy our working capital needs for a limited period of time. As discussed above, we have an immediate need for additional capital and in addition to our recently announced letter of intent for a business combination transaction, as discussed above, we are continuing to explore additional potential transactions to improve our capital position to ensure we are able to meet our financing and working capital requirements. We would expect to raise additional funds through public or private equity offerings, debt financings or strategic alliances or a restructuring of outstanding debt obligations. Raising additional funds by issuing equity or convertible debt securities may cause our stockholders to experience substantial dilution in their ownership interests and new investors may have rights superior to the rights of our other stockholders. Raising additional funds through debt financing or preferred stock, if available, may involve covenants that restrict our business activities and options and such additional securities may have powers, designations, preferences or rights senior to our currently outstanding securities or may require the granting of liens on our assets or preferences on revenue sources. We may also enter into financing transactions which involve the granting of liens on our assets or which grant preferences of payment from our revenue streams, all of which could adversely impact our ability to rely on our revenue from operations to support our ongoing operating costs. Alternatively, we may seek to obtain new financing from existing security holders, which may include reducing the exercise or conversion prices of outstanding securities, or the issuance of additional equity securities. Currently, the company does not have any definitive agreements with any third parties for such transactions and there can be no assurances that the company will be successful in completing the transaction contemplated by the letter of intent, raising additional capital or securing financing when needed or on terms satisfactory to the company. If we are unsuccessful in raising additional capital we will need to reduce costs and operations substantially or potentially suspend operations. Accordingly, any inability to obtain required financing on sufficiently favorable terms could have a material adverse effect on our business, results of operations and financial condition.
Our future capital requirements may vary materially from those now planned. The amount of capital that we will need in the future will depend on many factors, including:
| • | our relationships with suppliers and customers; |
| • | the market acceptance of our products and services; |
| • | the levels of promotion and advertising that will be required to launch our new offerings and achieve and maintain a competitive position in the marketplace; |
| • | price discounts on our products and services to our customers; |
| • | our pursuit of strategic transactions; including our ability to complete the transaction contemplated by our recently announced letter of intent for a business combination; |
| • | our need to repay existing indebtedness; |
| • | our business, product, capital expenditure and research and development plans and product and technology roadmaps; |
| • | the level of accounts receivable and inventories that we maintain; |
| • | technological advances; |
| • | our decision to redeem our outstanding shares of preferred stock; and |
| • | our competitors’ response to our offerings. |
53
Table of Contents
Financing Activities
Except as discussed above, we have not engaged in any external financing activities in fiscal 2015 and 2014.
Other Matters
The events and contingencies described below have impacted or may impact our liquidity and capital resources.
In August 2015, we announced that our board was focused on exploring strategic alternatives in order to enhance shareholder value. Subsequently, on August 25, 2015, we announced that we had entered into a non-binding letter of intent with Peachstate Health Management, LLC d/b/a AEON Clinical Laboratories, an expanding clinical laboratory based in Gainesville, GA (“AEON”) for the acquisition of all of the outstanding membership interests of AEON in exchange for shares of a newly created class of Series E preferred stock of Authentidate. As described in greater detail above, the letter of intent provides that if certain financial results are achieved during the next four years, the AEON members would be issued Series E Shares convertible into 85% of the outstanding shares of the company’s common stock on a partially diluted basis.
Presently, 28,000 shares of our Series B preferred stock, originally issued in a private financing in October 1999, remain outstanding. As of October 1, 2004, our right to redeem these shares of Series B preferred stock is vested. Accordingly, we have the right to repurchase such shares at a redemption price equal to $25.00 per share, plus accrued and unpaid dividends. The holder, however, has the right to convert these shares of preferred stock into an aggregate of 250,000 shares of our common stock at a conversion rate of $2.80. In the event we elect to redeem these securities, the holder will be able to exercise its conversion right subsequent to the date that we issue a notice of redemption but prior to the deemed redemption date as would be set forth in such notice. As of June 30, 2015, no shares of the Series B preferred stock have been redeemed.
In connection with our private placement of Series D preferred stock in June 2013, we issued 665,000 shares of Series D 5% convertible preferred stock. The Series D preferred stock is convertible into 6,125,024 shares of our common stock at an initial conversion rate of $1.08571 per share. Each share of Series D preferred stock has a stated value of $10.00 per share. The company has the right to repurchase these shares at the stated value per share, plus accrued and unpaid dividends, starting in June 2015 and to require the holders to convert such securities into common stock starting in June 2016. Each holder of our Series D preferred stock has the right to convert such shares into common stock at any time commencing on the six month anniversary date of the issue date. The Series D preferred stock will pay dividends at the rate of 5% per annum, payable in cash or shares of common stock, at the company’s option subject, however, to limitations required by the Nasdaq stock market.
Commitments
Office Lease Commitments
We entered into the lease agreement for our executive offices on July 11, 2005. The lease was for a term of ten years and four months, with a commencement date of October 1, 2005 and covers approximately 19,700 total rentable square feet. The annual rent in the first year was $324,000 increasing to $512,000 in year 2 and increasing at regular intervals until year 10 when the annual rent would be approximately $561,000. Effective February 1, 2010, we amended our lease to reduce the annual rent to approximately $512,000 for the remaining lease term and extended the lease term for one year through January 2017. As part of the lease agreement, we posted a letter of credit securing our lease payments which was reduced to approximately $256,000. On September 23, 2015, we amended our lease to relocate our executive offices to approximately 5,200 total rentable square feet in the same building. The lease amendment is dated as of September 15, 2015 and will be effective upon the completion of renovations to the premises or, if earlier, the date that the company occupies the space. The amended lease has a term of six years following the occupancy date and annual rentals ranging from approximately $135,000 in the first year to $148,000 in the final year. The lease also provides us with a one-time
option to renew the lease for a term of five years at the then-current market rate and, provided we pay an early termination fee, allows us an early termination option on each of the 18-month, 27-month and 36-month anniversary dates of the effective date of the amendment. As part of the lease agreement, we will reduce our letter of credit securing our lease payments to approximately $135,000.
54
Table of Contents
Contractual Commitments
A summary of the contractual commitments associated with our lease obligations as of June 30, 2015 is as follows (in thousands):
| Total | Less than 1 year |
1-3 years | 4-5 years | More than 5 years |
||||||||||||||||
| Leases |
||||||||||||||||||||
| Operating |
$ | 811 | $ | 512 | $ | 299 | $ | — | ||||||||||||
| Capital |
44 | 16 | 28 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total lease obligations |
$ | 855 | $ | 528 | $ | 327 | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Effective as of January 15, 2014, we entered into agreements with each of our former chief executive officer and chief financial officer in order to continue the compensation modification program implemented in February 2010. Pursuant to these agreements, both officers agreed to continue the reduction in their base salary to 70% of their original base salary commencing January 16, 2014 and continuing until the earlier of (i) such time as the company achieves “cash flow breakeven” or (ii) January 15, 2015. These agreements have not been renewed. Pursuant to these continuation agreements, the term “cash flow breakeven” is defined to mean that the company has achieved positive cash flow from operations for two consecutive fiscal quarters determined by reference to the revenues and other amounts received by the company from its operations. The term “cash flow from operations”, however, shall not include (a) amounts received from the sale, lease or disposition of (i) fixed or capital assets, except for amounts received in the ordinary course of business; or (ii) any subsidiary company; (b) capital expenditures; (c) interest income and expense; and (d) other non-operating items as determined in accordance with generally accepted accounting principles in the United States as consistently applied during the periods involved. In consideration for these agreements, we granted these executives restricted stock units under our 2011 Omnibus Equity Incentive Plan based on 30% of their base salary for the period commencing January 16, 2014 through January 15, 2015. The number of restricted stock units granted was determined by dividing the total amount of base salary that is reduced pursuant to the new modification agreements by the closing price of our common stock on the date of grant. In connection with the program we granted our former chief executive officer 66,412 restricted stock units and our chief financial officer 59,542 restricted stock units. The options and restricted stock units granted in connection with the compensation modification program for prior years shall only vest upon either the date determined that the company achieves cash flow breakeven, as defined above, or in the event of a termination of employment either without “cause” or for “good reason”, as such terms were defined in the employment agreements we previously entered with each such persons.
In connection with the termination of the company’s employment relationship with its former chief executive officer and president in February 2015, the company is presently reviewing its severance obligations to him and the vesting and other post-termination provisions of certain of the unexercised stock options and other unvested stock options and unvested restricted stock units held by him as of the effective date of his separation from the company.
Off-Balance Sheet Arrangements
We have not created, and are not party to, any special-purpose or off-balance sheet entities for the purpose of raising capital, incurring debt or operating parts of our business that are not consolidated into our financial statements. We do not have any arrangements or relationships with entities that are not consolidated into our financial statements that are reasonably likely to materially affect our liquidity or the availability of our capital resources. We have entered into various agreements by which we may be obligated to indemnify the other party with respect to certain matters. Generally, these indemnification provisions are included in contracts arising in the normal course of business under which we customarily agree to hold the indemnified party harmless against losses arising from a breach of representations related to such matters as intellectual property rights. Payments by us under such indemnification clauses are generally conditioned on the other party making a claim. Such claims
55
Table of Contents
are generally subject to challenge by us and to dispute resolution procedures specified in the particular contract. Further, our obligations under these arrangements may be limited in terms of time and/or amount and, in some instances, we may have recourse against third parties for certain payments made by us. It is not possible to predict the maximum potential amount of future payments under these indemnification agreements due to the conditional nature of our obligations and the unique facts of each particular agreement. Historically, we have not made any payments under these agreements that have been material individually or in the aggregate. As of June 30, 2015, we were not aware of any obligations under such indemnification agreements that would require material payments.
Effects of Inflation and Changing Prices
The impact of general inflation on our operations has not been significant to date and we believe inflation will continue to have an insignificant impact on us.
Present Accounting Standards Not Yet Adopted
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers” (Topic 606). This ASU is intended to clarify the principles for recognizing revenue by removing inconsistencies and weaknesses in revenue requirements; providing a more robust framework for addressing revenue issues; improving comparability of revenue recognition practices across entities, industries, jurisdictions and capital markets; and providing more useful information to users of financial statements through improved revenue disclosure requirements. The new standard is required to be applied retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying it recognized at the date of initial application. The provisions of this ASU were effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2016. Early application is not permitted. In August 2015, the FASB issued ASU 2015-14, “Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date”, which defers the effective date to fiscal periods beginning after December 15, 2017. Early adoption is permitted for fiscal periods beginning after December 15, 2016. The standard permits the use of either the retrospective or cumulative effect transition method. We are currently evaluating the impact of this standard on our consolidated financial statements.
In June 2014, the FASB issued ASU No. 2014-12, “Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period.” This ASU requires a reporting entity to treat a performance target that affects vesting and that could be achieved after the requisite service period as a performance condition, and apply existing guidance under the Stock Compensation Topic of the ASC as it relates to awards with performance conditions that affect vesting to account for such awards. The provisions of this ASU are effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2015. We are currently evaluating the impact of this standard on our consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern.” This ASU is intended to define management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. Specifically, ASU 2014-15 provides a definition of the term substantial doubt and requires an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). It also requires certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans and requires an express statement and other disclosures when substantial doubt is not alleviated. The new standard will be effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2016, with early adoption permitted. We are currently evaluating the impact of this standard on our consolidated financial statements.
56
Table of Contents
In April 2015, the FASB issued ASU 2015-03, “Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs.” This ASU is intended to simplify the presentation of debt issuance costs and conform to the guidance in International Financial Reporting Standards, which requires that transaction costs be deducted from the carrying value of the financial liability and not recorded as separate assets. Additionally, the requirement to recognize debt issuance costs as deferred charges conflicts with the guidance in FASB Concepts Statement No. 6, “Elements of Financial Statements,” which states that debt issuance costs are similar to debt discounts and in effect reduce the proceeds of borrowing, thereby increasing the effective interest rate. FASB Concepts Statement No. 6 further states that debt issuance costs cannot be an asset because they provide no future economic benefit. The amendments in this ASU require that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the amendments in this ASU. For public business entities, the amendments in this ASU are effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2015. In August 2015, the FASB issued ASU No. 2015-15, “Interest—Imputation of Interest (Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements—Amendments to SEC Paragraphs Pursuant to Staff Announcement at June 18, 2015 EITF Meeting.” This ASU adds SEC paragraphs pursuant to the SEC Staff Announcement at the June 18, 2015, Emerging Issues Task Force meeting about the presentation and subsequent measurement of debt issuance costs associated with line-of-credit arrangements to this topic. Given the absence of authoritative guidance within ASU 2015-03 for debt issuance costs related to line-of-credit arrangements, the SEC staff would not object to an entity deferring and presenting debt issuance costs as an asset and subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. We are currently evaluating the impact of this standard on our consolidated financial statements.
In April 2015, the FASB issued ASU No. 2015-05, “Customer’s Accounting for Fees Paid in a Cloud Computing Arrangement”, which provides explicit guidance to help companies evaluate the accounting for fees paid by a customer in a cloud computing arrangement. The new guidance clarifies that if a cloud computing arrangement includes a software license, the customer should account for the license consistent with its accounting for other software licenses. If the arrangement does not include a software license, the customer should account for the arrangement as a service contract. ASU 2015-05 is effective for public business entities for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2015. An entity can elect to adopt the amendments either prospectively for all arrangements entered into or materially modified after the effective date, or retrospectively. Early adoption is permitted for all entities. We are currently evaluating the impact of this standard on our consolidated financial statements.
In May 2015, the FASB issued ASU 2015-08, “Business Combinations—Pushdown Accounting—Amendment to SEC Paragraphs Pursuant to Staff Accounting Bulletin No. 115.” This ASU was issued to amend various SEC paragraphs pursuant to the issuance of Staff Accounting Bulletin No. 115. This update is not expected to have a significant impact on our consolidated financial statements.
In June 2015, the FASB issued ASU 2015-10, “Technical Corrections and Improvements.” The amendments in this update represent changes to clarify the Codification, correct unintended application of guidance, or make minor improvements to the Codification that are not expected to have a significant effect on current accounting practice or create a significant administrative cost to most entities. Transition guidance varies based on the amendments in this Update. The amendments that require transition guidance are effective for all entities for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2015. Early adoption is permitted, including adoption in an interim period. All other amendments will be effective upon the issuance of this Update. We are currently evaluating the impact of this standard on our consolidated financial statements.
In July 2015, the FASB issued ASU 2015-11, “Inventory (Topic 330): Simplifying the Measurement of Inventory.” The amendments in this ASU require an entity to measure in scope inventory at the lower of cost and
57
Table of Contents
net realizable value. Net realizable value is the estimated selling prices in the ordinary course of business, less reasonably predictable costs of completion, disposal, and transportation. Subsequent measurement is unchanged for inventory measured using LIFO or the retail inventory method. The amendments do not apply to inventory that is measured using last-in, first-out (LIFO) or the retail inventory method. The amendments apply to all other inventory, which includes inventory that is measured using first-in, first-out (FIFO) or average cost. The amendments in this ASU are effective for public business entities for fiscal years beginning after December 15, 2016, including interim periods within those fiscal years. A reporting entity should apply the amendments prospectively with earlier application permitted as of the beginning of an interim or annual reporting period. We are currently evaluating the impact of this standard on our consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
As of June 30, 2015, we are not exposed to significant financial market risks from changes in foreign currency exchange rates and are only minimally impacted by changes in interest rates. However, in the future, we may enter into transactions denominated in non-U.S. currencies or increase the level of our borrowings, which could increase our exposure to these market risks. We have not used, and currently do not contemplate using, any derivative financial instruments.
Interest Rate Risk
At any time, fluctuations in interest rates could affect interest earnings on our cash and marketable securities. We believe that the effect, if any, of reasonably possible near term changes in interest rates on our financial position, results of operations, and cash flows would not be material. Currently, we do not hedge these interest rate exposures. The primary objective of our investment activities is to preserve capital. We have not used derivative financial instruments in our investment portfolio.
At June 30, 2015, our unrestricted cash totaled approximately $247,000 and was in non-interest bearing checking accounts used to pay operating expenses.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The Financial Statements are annexed hereto at Part IV, Item 15 of this Annual Report on Form 10-K.
| ITEM 9. CHANGES | IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
We have had no disagreements with our accountants on any accounting or financial disclosures.
ITEM 9A CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our chief executive officer and chief financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) or 15d-15(e) under the Exchange Act) as of the end of the period covered by this report, have concluded that, based on the evaluation of these controls and procedures, our disclosure controls and procedures were effective at the reasonable assurance level to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, to allow timely decisions regarding required disclosure.
Our management, including our chief executive officer and chief financial officer, do not expect that our disclosure controls and procedures or our internal controls will prevent all errors and all fraud. A control system,
58
Table of Contents
no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. Our management, however, believes our disclosure controls and procedures are in fact effective to provide reasonable assurance that the objectives of the control system are met.
Management’s Report on Internal Control over Financial Reporting
Our management, under the supervision of our chief executive officer and chief financial officer, is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended). Our internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The company’s internal control over financial reporting includes those policies and procedures that:
(i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company;
(ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and
(iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Management, including our chief executive officer and chief financial officer, conducted an evaluation of the effectiveness of our internal control over financial reporting as of June 30, 2015. In making this evaluation, management used the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission in 2013 (COSO). Based on our evaluation under the framework in Internal Control—Integrated Framework, our management has concluded that our internal control over financial reporting was effective as of June 30, 2015.
This annual report does not include an attestation report of our independent registered public accounting firm regarding our internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to rules of the Securities and Exchange Commission that permit us to provide only management’s report in this annual report.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Changes in Internal Control over Financial Reporting
There was no change in our system of internal control over financial reporting (as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934) during our quarter ended June 30, 2015 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
The information set forth under the caption “Sales of Unregistered Securities” in Item 5 of Part II of this Annual Report on Form 10-K regarding issuances of equity securities is incorporated herein by reference.
The information set forth in Item 2 of Part I of this Annual Report on Form 10-K regarding the amendment to our office lease is incorporated herein by reference.
59
Table of Contents
In July 2015, the company granted 100,000 common stock purchase warrants to a consultant in consideration of its provision of consulting services. The warrants are exercisable beginning six months following the grant date at a price of $0.19 per share, expire five years from the grant date and contain “piggy-back” registration rights. Under this consulting arrangement, the company may grant up to an additional 800,000 warrants to this consultant based on the achievement of performance targets. Unless such performance targets are achieved, no additional warrants would be issued under this arrangement.
On October 9, 2015, the company entered into agreements with the holders of outstanding promissory notes in the aggregate principal amount of $1,450,000 that were due on October 9, 2015 (the “Prior Notes”) in order to further extend the maturity date of the Prior Notes to October 16, 2015. No other terms of the Prior Notes were modified. The holders of the Prior Notes are Lazarus Investment Partners LLLP, which is the beneficial owner of approximately 29.3% of the Company’s common stock and which holds a Prior Note in the principal amount of $500,000, and an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of the Board of Directors of the Company, which holds a Prior Note in the principal amount of $950,000. The Company intends to continue holding further discussions with the holders of the Prior Notes regarding a further extension of the maturity of these obligations.
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Management
Our executive officers and directors are as follows:
| Name |
Age | Office | ||||
| Charles C. Lucas III |
53 | Chairman of the Board of Directors | ||||
| Ian C. Bonnet |
45 | Chief Executive Officer, President and Director | ||||
| William P. Henry |
48 | Interim Chief Strategy Officer and Director | ||||
| Roy E. Beauchamp |
70 | Director | ||||
| Todd A. Borus, M.D. |
42 | Director | ||||
| Marc A. Horowitz |
55 | Director | ||||
| J. David Luce |
54 | Director | ||||
| William A. Marshall |
63 | Chief Financial Officer, Treasurer and Principal Accounting Officer | ||||
In November 2014, the board expanded its size to seven directors and elected Roy E. Beauchamp to serve as a director for an initial term expiring at our annual meeting of stockholders to be held in 2015 and until his successor shall have been elected and qualified. As described in greater detail below, on December 10, 2014, we entered into an agreement with Lazarus Investment Partners pursuant to which we granted them the right to nominate a second individual for election to our board. In accordance with the terms of this second agreement, Marc A. Horowitz was elected to our board of directors as of December 10, 2014. See “Summary of Lazarus Board Agreements”.
On December 9, 2014, J. Edward Sheridan notified the board of directors that he had decided to retire from the board, effective immediately. Mr. Sheridan, a director, had also served as Chairman of the Audit Committee and the Nominating and Corporate Governance Committee and as a member of the Management Resources and Compensation Committee of the Board.
On February 18, 2015, Ian C. Bonnet was appointed as our president and chief executive officer, and elected to our board, following the termination of Mr. O’Connell Benjamin as our president and chief executive officer and his resignation from our board of directors.
On June 18, 2015, the board expanded its size to eight directors and elected William P. Henry to serve as a director for an initial term of one year and until his successor shall have been elected and qualified.
On July 1, 2015, Jeffrey Beunier, a member of the board of directors notified the board that he decided to resign from the board, effective immediately. Mr. Beunier had also served as Chairman of the Audit Committee of the board and was designated as the Audit Committee Financial Expert. Mr. Beunier stated that his resignation was due to his pursuit of other interests.
60
Table of Contents
Our by-laws provide that the number of persons on the board of directors shall be between three and fifteen persons, as determined by the board of directors. Our board of directors currently consists of seven members. All directors hold office until the next annual meeting of shareholders or until their successors are elected and qualify. Officers are elected annually by, and serve at the discretion of, the board of directors. There are no familial relationships between or among any of our officers or directors.
Biographical Information
The principal occupations and brief summary of the background of each of our current directors and executive officer is as follows:
Directors and Executive Officers
Charles C. Lucas III joined our board of directors in December 2012 and was appointed as Chairman of the Board on May 1, 2014. He is currently the general counsel of Elevation LLC, a position he has held since January 2011. Elevation LLC is an institutional broker-dealer focused on macro-based research and agency execution. Prior to joining Elevation LLC, Mr. Lucas was a partner with The McAulay Firm, an executive search firm, from 1996 to December 2010. Prior to joining The McAulay Firm, Mr. Lucas engaged in the private practice of law with the firm of Robinson, Bradshaw & Hinson, P.A. Mr. Lucas received a Bachelor of Arts degree from the University of North Carolina and a Juris Doctor from the Duke University School of Law. Mr. Lucas is active in numerous civic and philanthropic organizations and since October 2004 has been a Trustee of The Duke Endowment and since 2008 has served on the Board of Visitors of Duke University School of Law. In addition, Mr. Lucas is a past and current member of the Board of Trustees of the University of North Carolina School of the Arts and has served as the Chairman of this Board since 2008.
Ian C. Bonnet was appointed as our president and chief executive officer, and elected to our board, on February 18, 2015. Mr. Bonnet has twenty years of leadership and management experience in the healthcare industry spanning work with hospitals, health plans, technology companies, and government agencies. Mr. Bonnet was Vice President Exchange Execution and ICD-10 Execution at Anthem (formerly WellPoint) from April 2010 to October 2014. Prior to Anthem, he held leadership positions with Deloitte Consulting for over six years serving numerous clients while developing Deloitte’s ICD-10 practice and federal health practice. Prior to Deloitte, he served in key positions with Ernst & Young. Mr. Bonnet received his MHSA from Arizona State University and his BA in Biology from the College of Charleston. Mr. Bonnet also attended The Citadel.
William P. Henry was elected to our board of directors in July 2015 and on July 23, 2015 was appointed as our interim chief strategy officer. Mr. Henry is a technology executive with over twenty-five years of experience in the telecommunications and software industries. From July 2012 through April 2015, Mr. Henry served as the Chief Executive Officer and a board member of Omnico Group, a multi-national point of sale technology provider. Prior to that, from April 2010 to April 2012, Mr. Henry was the Chief Executive Officer of Masternaut Group, a privately held telematics and mobile workforce management technology company. Earlier, Mr. Henry served as the Chief Executive Officer of Tele Atlas, a global provider of digital maps and dynamic content used in navigation and location-based services and the Chief Operating Officer of iSOFT, plc, an international supplier of healthcare software applications. Mr. Henry earned an MBA from the Wharton School of Business and an undergraduate degree from the University of California at San Diego.
Roy E. Beauchamp was elected to our board of directors in November 2014 and is the President of Beauchamp and Associates, LLC, which was established in October 2007 and which provides consulting and advisory services on logistics, technology introduction, construction business opportunity development and organizational and leadership development. Gen. Beauchamp served in the United States Army for thirty-seven years, retiring as a Lieutenant General in 2002. In the course of his military career he served in a wide variety of senior positions involving technology management, production management, industrial base management, project management, contract management and logistics management. These positions included Commanding General—Defense Industrial Supply Center; Deputy Chief of Staff for Research, Development and Acquisition, Army Materiel Command; Commanding General, Tank-Automotive and Armaments Command; and Director of
61
Table of Contents
Logistics and Security Assistance, U.S. Central Command. His last assignment prior to retirement was Deputy Commanding General of the Army Materiel Command. Following his retirement, Gen. Beauchamp joined the Washington Group International, where he served as Senior Vice President for Domestic Operations in the Defense Business Unit from October 2002 until September 2005, as Program Director for the Katrina Program Management Office until September 2006, and as a Special Assistant to the Chief Operating Officer until June 2007. Gen. Beauchamp was responsible for developing and managing a portfolio that included Department of Defense infrastructure projects, Homeland Security projects and support to the Department of State and other government agencies. Presently, Gen. Beauchamp is the Chairman of BDD, LLC, a service disabled veteran owned business and an executive of a number of privately-held companies. Gen. Beauchamp received a Bachelor’s Degree from the University of Nebraska, a Master of Business Administration from the University of Dayton and an M.A. in Public Administration from Central Michigan University.
Todd A. Borus, M.D., was elected to our board of directors in May 2011 and is a board certified orthopedic surgeon in Vancouver, WA and Portland, OR. Since August 2006, Dr. Borus has been a partner with Northwest Surgical Specialists, a private orthopedic surgery group, based in Vancouver, WA. Dr. Borus is affiliated with the Southwest Washington Medical Center and Legacy Salmon Creek Hospital and since January 2010, Dr. Borus has held the position of Section Chief, Department of Orthopedic Surgery, at Legacy Salmon Creek Hospital. Dr. Borus graduated with a degree in economics from Williams College and attended medical school at the Mount Sinai school of medicine in New York City. His orthopedic surgery training was completed at the University of Michigan. From August 2005 to July 2006, Dr. Borus completed a fellowship training in adult reconstruction and joint replacement surgery at the Brigham and Women’s Hospital, Harvard University, in Boston, MA. In addition, since January 2009, Dr. Borus has served on the Physician Advisory Council of the Southwest Washington Medical Center and currently serves as a consultant to Mako Surgical Corp., a medical device company. Dr. Borus is the brother of Justin Borus, the manager of the general partner of Lazarus Investment Partners, LLP, which is the beneficial owner of approximately 29.3% of our common stock.
Marc A. Horowitz was elected to our board of directors in December 2014. Mr. Horowitz is an entrepreneur in the healthcare information technology market with over twenty-five years of leadership and experience. In January 2015, Mr. Horowitz was appointed as Executive Vice President of Invidasys, a cloud based provider of technology to the health insurance sector. Most recently, from February 2009 until January 2015, Mr. Horowitz served as Senior Vice President of Health Language, Inc., now part of Wolters Kluwer, which provides users with the platform to manage standard and enhanced clinical terminologies on an enterprise scale. In 1997, Mr. Horowitz was a co-founder of Health Language and acted in various executive leadership roles through 2004 and again upon rejoining Health Language in February 2009. From 2004 to 2009, Mr. Horowitz was the Group Business Development Director of iSOFT, plc, now part of CSC, an international supplier of healthcare software applications. Mr. Horowitz has also held a number of other leadership, business development and marketing positions in the healthcare information technology sector. In December 2014, he completed serving two consecutive terms as the Chairman of the Affiliate Forum of the International Health Terminology Standards Development Organization, a multi-national not-for-profit organization that works to develop standards for health systems. The Affiliate Forum brings together users of SNOMED CT (Systematized Nomenclature of Medicine—Clinical Terms) to advise and support the IHTSDO.
J. David Luce joined our board of directors in February 2003. Following our annual meeting of stockholders on May 1, 2014, Mr. Luce was named Vice Chairman of the Board and served as Co-Chairman from June 25, 2013 until May 1, 2014. From 1990 to August 2009, Mr. Luce was a Senior Vice President of Fixed Income Sales with Barclays PLC (formerly Lehman Brothers). Prior to joining Lehman Brothers, Mr. Luce served as a Vice President, Fixed Income Sales, at Kidder Peabody. Subsequent to his departure from Barclays PLC, Mr. Luce acts as a fixed income portfolio manager at ICC Capital Management and during such time has provided advisory services to the fixed income departments at Elevation LLC and MIP Global, Inc. Mr. Luce graduated from Duke University in 1983 with a B.A. in Economics.
William A. Marshall joined Authentidate Holding Corp. as Chief Financial Officer and Treasurer in February 2006. Mr. Marshall has more than 30 years of experience as a Chief Financial Officer, audit partner and senior
62
Table of Contents
management advisor. Most recently, he served as Chief Financial Officer and Treasurer for NEON Communications, Inc., a former publicly traded provider of optical networking solutions, from 2001 to January 2005, when the company was acquired. From September 1999 to September 2001, he was Chief Financial Officer and Treasurer for Vitts Networks, Inc., a provider of high-speed Internet communications. From 1995 to September 1999, he served as Chief Financial Officer and Treasurer for Viisage Technology, Inc., a software technology company where he led the company’s initial public offering in 1996. From 1987 to 1994, Mr. Marshall was a Partner at KPMG LLP where he provided audit, accounting, financial and SEC reporting, business advisory, public offering and merger and acquisition services for a variety of growing middle market companies. Prior to 1987, Mr. Marshall held various positions in audit and business advisory services at KPMG LLP. Mr. Marshall has a B.S. in Accounting from Elizabethtown College in Pennsylvania and is a Certified Public Accountant.
Qualifications of Directors
The following table summarizes the specific experience, qualifications, attributes or skills of our directors that led our Nominating and Corporate Governance Committee to conclude that such person should serve as a director of Authentidate:
| Directors |
Relevant Experience and Qualifications | |
| Charles C. Lucas |
Significant business and legal experience, including his executive positions with Elevation LLC and The McAulay Firm. Significant experience in governance and leadership derived from his positions as a trustee of The Duke Endowment and service on the Board of Visitors of Duke University School of Law and the Board of Trustees of the University of North Carolina School of the Arts and current service as Chairman. | |
| Ian C. Bonnet |
Possesses significant leadership and business experience in the healthcare industry, particularly with respect to the development and commercialization of healthcare information technology solutions in both the commercial and government sectors. Mr. Bonnet was appointed as our president and chief executive officer in February 2015 and has a unique perspective on our company’s business and prospects due to such role. Mr. Bonnet also possesses significant experience in the managerial, sales and marketing functions and offers significant industry knowledge and insight in the board’s consideration of corporate strategy, opportunities and industry trends. | |
| William P. Henry |
Significant business, leadership and operations experience serving as a technology executive in the healthcare, telecommunications and software industries. Mr. Henry has extensive experience as the Chief Operating Officer of an international supplier of healthcare software applications and the Chief Executive Officer of a multi-national point of sale technology provider and a privately held telematics and mobile workforce management technology company. | |
| Roy E. Beauchamp |
Extensive leadership experience in the government and private business sectors having achieved the rank of Lieutenant General in the U.S. Army, which included his service in a number of leadership positions involving technology management, production management, industrial base management, project management, contract management and logistics management. These positions included Commanding General—Defense Industrial Supply Center and Director of Logistics and Security Assistance, U.S. Central Command. Mr. Beauchamp also possesses significant business leadership experience gained from his service as an executive with the Washington Group International and through his consulting and advisory business. | |
| Todd A. Borus, M.D. |
Significant knowledge of the healthcare industry and possessing healthcare leadership and management experience through his medical practice, particularly his role as Section Chief, Department of Orthopedic Surgery, at Legacy Salmon Creek Hospital and service on the Physician Advisory Council of the Southwest Washington Medical Center. Experience in advising a medical device company based on his consultancy with Mako Surgical Corp. | |
63
Table of Contents
| Directors |
Relevant Experience and Qualifications | |
| Marc A. Horowitz |
Significant leadership and business experience in the healthcare information technology industry. Director executive business experience in the healthcare technology field was gained through role as a founder and executive of Health Language, Inc. and an executive with iSOFT, plc. His role as the Chairman of the Affiliate Forum of the International Health Terminology Standards Development Organization, a multi-national not-for-profit organization that works to develop standards for health systems, provides him with significant knowledge and experience concerning the healthcare information technology industry. | |
| J. David Luce |
Significant business and financial experience, including serving as Senior Vice President with Barclays Capital (formerly Lehman Brothers) and background in private investment banking. Breadth of knowledge about Authentidate’s business as a result of service on our board since 2003. | |
Meetings of the Board of Directors; Independence
During the fiscal year ended June 30, 2015, our board of directors met on eighteen occasions and acted on written consent on two occasions. No member of the board of directors other than Dr. Borus attended less than 75% of the aggregate number of (i) the total number of meetings of the board of directors or (ii) the total number of meetings held by all committees of the board of directors during the fiscal year ended June 30, 2015. Our independent directors meet in executive sessions periodically during the course of the year.
Our board of directors currently consists of seven individuals, four of whom are currently independent directors as defined in the Marketplace Rules of The NASDAQ Stock Market. Our independent directors are Charles C. Lucas, Todd A. Borus, M.D. Roy E. Beauchamp, and Marc A. Horowitz. Mr. Bonnet is not an independent director due to the fact that he serves as our chief executive officer and president and Mr. Henry is not independent due to the fact that he is serving as the interim chief strategy officer. Our board determined on August 6, 2009, in connection with its approval of an award of 250,000 options to Mr. Luce that Mr. Luce no longer satisfied the independence criteria of The NASDAQ Stock Market following such grant as such grant was in consideration for services rendered in connection with our ExpressMD™ Solutions subsidiary and former joint venture.
Committees of the Board of Directors
The board of directors presently has four committees:
| • | Audit Committee; |
| • | Management Resources and Compensation Committee; |
| • | Nominating and Corporate Governance Committee; and |
| • | Strategy and Risk Analysis Committee |
Audit Committee. The members of the Audit Committee are presently Marc A. Horowitz, Charles C. Lucas and Roy E. Beauchamp. Mr. Horowitz currently serves as chairman of this committee. Mr. Sheridan and Mr. Beunier served on the Audit Committee during our 2015 fiscal year. Mr. Sheridan served as the chairman of the committee until his retirement in December 2014 and Mr. Beunier served as chairman of the committee from December 2014 through his retirement on July 1, 2015. Dr. Borus also served on this committee until January 14, 2014. Each of the individuals that currently serve on the Audit Committee is, and that served on the Audit Committee during our fiscal year ended June 30, 2015 was, an independent member of our board of directors. In addition, the board of directors has determined that the members of the Audit Committee that served during fiscal 2015 and at present meet the additional independence criteria required for audit committee membership set forth in Rule 10 A-3 promulgated by the SEC under the Securities Exchange Act of 1934, as amended.
64
Table of Contents
The Audit Committee acts to: (i) acquire a complete understanding of our audit functions; (ii) review with management the finances, financial condition and our interim financial statements; (iii) review with our independent auditors the year-end financial statements; and (iv) review implementation with the independent auditors and management any action recommended by the independent auditors. Our board of directors adopted a Restated and Amended Charter governing the activities of the Audit Committee, which is available on our corporate website at www.authentidate.com under the following tabs: “about us—corporate governance—Board Committees—Audit”. During the fiscal year ended June 30, 2015, the Audit Committee met on four occasions.
Audit Committee Financial Expert. Our board of directors has determined that Audit Committee member Charles C. Lucas is our audit committee financial expert, as defined under applicable SEC regulations, and is an independent member of our board. Until his retirement in December 2014 Mr. Sheridan was our Audit Committee financial expert and Mr. Beunier was our financial expert from December 2014 through his retirement on July 1, 2015.
Management Resources and Compensation Committee. The members of the Management Resources and Compensation Committee are currently Charles C. Lucas, Roy E. Beauchamp and Todd A. Borus, each of whom is an independent member of our board of directors. Mr. Lucas currently serves as the chairman of this committee. The functions of this committee include administration of our stock option programs and the negotiation and review of all employment agreements of our executive officers. During the fiscal year ended June 30, 2015, this committee held six meetings and acted on written consent on one occasion. The Management Resources and Compensation Committee has authority to select, engage, compensate and terminate independent compensation consultants, legal counsel and such other advisors as it deems necessary and advisable to assist it in carrying out its responsibilities and functions. The Management Resources and Compensation Committee is governed by a written charter approved by our board of directors which is available on our corporate web site at www.authentidate.com under the following tabs: “about us—corporate governance—Board Committees—Management Resources and Compensation”.
Nominating and Corporate Governance Committee. The members of this committee are currently Charles C. Lucas, Roy E. Beauchamp and Todd A. Borus, each of whom is an independent member of our board of directors. Mr. Lucas currently serves as the chairman of this committee. Mr. Sheridan was the chairman of this committee until his retirement in December 2014. Our board of directors has adopted a charter governing the activities of the Nominating and Corporate Governance Committee, which may be viewed online on our web site at www.authentidate.com under the following tabs: “about us—corporate governance—Board Committees—Nominating and Corporate Governance”. Pursuant to its charter, this committee’s tasks include reviewing and recommending to the board issues relating to the board’s composition and structure; establishing criteria for membership and evaluating corporate policies relating to the recruitment of board members; implementing and monitoring policies regarding principles of corporate governance in order to ensure the board’s compliance with its fiduciary duties to the company and its stockholders; and making recommendations regarding proposals submitted by stockholders. During the fiscal year ended June 30, 2015, this committee held four meetings.
Strategy and Risk Analysis Committee. The members of this committee are currently William P. Henry, Roy E. Beauchamp, Marc Horowitz and Charles C. Lucas. Mr. Henry currently serves as the chairman of this committee. On August 6, 2015, the board reconstituted the membership of this committee which was originally established in November 2014. Previously, the members of this committee were Jeffrey Beunier (chairman), J. David Luce and Roy E. Beauchamp. The purpose of this committee is to assist the board in monitoring the financial management of the company, assessing risks facing the company and reviewing strategic transactions that it may consider. The charter governing the activities of this committee may be viewed online on our web site at www.authentidate.com under the following tabs: “about us—corporate governance—Board Committees—Strategy-and-Risk-Analysis-Committee”. This committee has met on one occasion since its establishment.
65
Table of Contents
Corporate Governance
We maintain a corporate governance page on our corporate web site which includes important information about our corporate governance practices, including our Corporate Governance Principles, our Code of Ethics and Business Conduct, and charters for the committees of the board of directors. The corporate governance page can be found at www.authentidate.com, by clicking on “about us-corporate governance.”
Board Leadership Structure
Pursuant to our corporate governance policies, in the event the board elects to appoint the chief executive officer as the Chairman of the Board, the board shall also appoint an independent member of the board to serve as “Lead Director,” who shall be responsible for coordinating the activities of the other independent directors and to perform various other duties. Our Chairman, Mr. Lucas, is an independent member of the board.
Board’s Role in Oversight of Risk
As described above, in November 2014, the company’s board of directors established the Strategy and Risk Analysis Committee to assist the board in assessing risks facing the company. With respect to risk management, this committee focuses on identifying and assessing the risks facing the company and reviewing with management the company’s funding requirements and capital planning. In addition, in managing the company’s overall risk exposure, the board of directors mitigates risks through discussing with management the appropriate level of risk for the company and evaluating the risk information received from management. These risks include financial, technological, competitive, and operational risks. Further, the Audit Committee receives updates from senior management and assesses risk in satisfaction of their risk management role in accordance with the Audit Committee charter. Our Audit Committee charter provides that the Audit Committee is responsible for monitoring material financial and operating risks of the company. On a quarterly basis, management reports to the Audit Committee regarding our various risk areas. In addition, each of the other committees of the board of directors considers risks within its area of responsibility.
Lazarus Board Agreement
Under the terms of the Board Nomination and Observer Agreement dated September 25, 2012 (the “Board Agreement”) between us and Lazarus Investment Partners, LLLP (“Lazarus Partners”), we granted Lazarus Partners the right to appoint either an observer to our board of directors or to nominate an individual for election to our board of directors. The agreement was amendment on several occasions to extend the initial exercise period to December 18, 2013. Under the Board Agreement, so long as Lazarus Partners owns at least 10% of our outstanding common stock, it has the right to designate one person to be nominated for election to the board, or in the alternative, if it owns at least 5% of our outstanding common stock, the right to designate an observer to attend all meetings of our board in a non-voting capacity for a period of two years. We agreed that if Lazarus Partners nominates an individual for election to our board, we shall promptly increase the size of the board, appoint such nominee as a member of the board and, subject to the terms of the Board Agreement, use our best efforts to include such nominee in the slate of nominees recommended for election as a director for three years. In October 2013, Lazarus Investment Partners exercised its appointment right and nominated Mr. Jeffrey A. Beunier to our board. Our board subsequently increased its size to six members and elected Jeffrey A. Beunier to serve as a director on October 31, 2013. Mr. Beunier subsequently resigned from the board on July 1, 2015.
Subsequently, on December 10, 2014, we entered into a new Board Nomination Agreement (the “2014 Board Agreement”) with Lazarus Partners and certain of its affiliates (the “Lazarus Group”), which presently beneficially owns approximately 29.3% of our common stock. Pursuant to the 2014 Board Agreement, we granted the Lazarus Group the right to nominate a second individual for election to our board and agreed to promptly appoint such nominee as a member of the board. Pursuant to this agreement, the Lazarus Group designated Mr. Horowitz and we elected him to our board of directors, effective immediately. Further, under the
66
Table of Contents
2014 Board Agreement, we agreed to use our best efforts to include Mr. Horowitz in our slate of nominees recommended for election as a director during a three year designation period, as defined under the 2014 Board Agreement. The 2014 Board Agreement further provides that if a board vacancy occurs during the designation period solely because of the death, disability, disqualification, resignation or removal of their designee, the Lazarus Group shall be entitled to designate such person’s successor.
Procedures for Determining Executive and Director Compensation
The Management Resources and Compensation Committee was formed to, among other things, assist our board of directors in the discharge of its responsibilities with respect to compensation of our executive officers and non-employee directors. In accordance with its charter, the compensation committee has authority to determine the amount, form and terms of compensation of our chief executive officer and other officers, and to take such action, and to direct us to take such action, as it deems necessary or advisable to compensate our chief executive officer and other officers in a manner consistent with its determinations, and shall deliberate and vote on all such actions outside the presence of our chief executive officer and other officers. The committee is responsible for reviewing, at least annually, the performance of our chief executive officer and other officers, including in light of any goals and objectives established for such performance, and, in light of such review, determining each officer’s compensation. In accordance with its charter, the committee also has authority to establish our general compensation policies and practices and to administer plans and arrangements established pursuant to such policies and practices. In addition, the committee has authority to administer our equity compensation programs, including without limitation to recommend the adoption of such plans, to recommend the reservation of shares of our common stock for issuance thereunder, to amend and interpret such plans and the awards and agreements issued pursuant thereto, and to make awards to eligible persons under the plans and determine the terms of such awards, including any such awards to our chief executive officer and other officers. With respect to non-employee director compensation, the committee reviews such compensation practices and policies and makes recommendations to our board of directors as to the amount, form and terms of non-employee director compensation.
The Management Resources and Compensation Committee did not retain outside consultants during the 2015 fiscal year to assist it in implementing these policies or making specific decisions relating to executive compensation. However, the committee does, from time to time, review general information regarding the compensation practices of other companies, including some that may compete with Authentidate for the services of its executives and employees and that information is a factor used by the committee in its decisions and in its general oversight of compensation practices. However, the committee does not use that information to generate specific compensation amounts or targets. Instead, in each compensation decision, the committee exercises its business judgment regarding the appropriateness of types and amounts of compensation in light of the value to the company of specific individuals.
With respect to fiscal 2015 compensation for our executives, the Management Resources and Compensation Committee took into account recommendations made by the Chairman of the Board and chief executive officer of Authentidate with respect to determinations of the types and amounts of compensation to be paid to the other executive officers and also discussed with the Chairman of the Board and the chief executive officer the types and amounts such individuals believed would be appropriate to pay each of them in light of the amounts being recommended for the other executives. Authentidate’s senior management generally applies a similar philosophy and similar policies to determine the compensation of officers and managers who are not executive officers and reports to the Management Resources and Compensation Committee regarding these matters.
Within the context of the overall objectives of our compensation program, we determined the specific amounts of compensation to be paid to each of our executives during fiscal 2015 based on a number of factors including: our understanding of the amount of compensation generally paid by similarly situated companies to their executives with similar roles and responsibilities; our executives’ performance during the fiscal year in general and as measured against predetermined company and individual performance goals; the roles and
67
Table of Contents
responsibilities of our executives; the individual experience and skills of, and expected contributions from, our executives; the amounts of compensation being paid to our other executives; our executives’ historical compensation and performance at our company; and any contractual commitments we have made to our executives regarding compensation. Such information is reflected in the Summary Compensation Table under “Executive Compensation” below. With respect to the compensation payable to its new chief executive officer, the committee utilized generally available data in determining the components of the compensation package it agreed to in entering into its employment agreement with Mr. Bonnet. The company’s employment agreement included a cash base salary and equity incentive award designed to achieve the committee’s goals of retaining a new chief executive and tying additional benefits to performance goals.
Regarding the compensation of the company’s non-employee directors, in June 2011, the Management Resources and Compensation Committee, engaged Frederic W. Cook & Co., Inc., a nationally-recognized compensation consulting firm, to provide advice regarding the company’s non-employee director compensation policies and practices and present the committee with its assessment and recommendations. Following their assessment and taking their recommendations into account, the committee made a recommendation to our board of directors regarding a new equity incentive plan as well as modifications to the company’s then-current director compensation policy. In July 2011, our board of directors adopted the compensation policies recommended by the committee. No changes to these policies were made during the fiscal year ended June 30, 2015 and the committee did not engage any compensation consultant in fiscal 2015.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our directors and executive officers, and persons who own, directly or indirectly, more than 10% of a registered class of our equity securities, to file with the Securities and Exchange Commission (“SEC”) initial reports of ownership and reports of changes in ownership of common stock and other equity securities we issue. Officers, directors and greater than 10% shareholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms that they file. Based solely on a review of the copies of such reports furnished to us and representations that no other reports were required during the fiscal year ended June 30, 2015, we believe that all Section 16(a) filing requirements applicable to our officers, directors and 10% shareholders were complied with during the 2015 fiscal year.
Code of Ethics
On July 31, 2003, our board of directors approved the Code of Ethics and Business Conduct for our company. Our Code of Ethics and Business Conduct covers all our employees and Directors, including our chief executive officer and chief financial officer. During the fiscal year ended June 30, 2015, we did not waive any provisions of the Code of Ethics and Business Conduct. Our Code of Ethics and Business Conduct was filed as Exhibit 14 to our Annual Report on Form 10-K for the fiscal year ended June 30, 2003. We have also posted our Code of Ethics and Business Conduct on our web site at http://www.authentidate.com, and may be found as follows:
| • | From our main web page, first click on “About Us” |
| • | Then click on “Corporate Governance” |
| • | Next, under “Corporate Governance,” click on “Code of Ethics” |
We will post any amendments to or waivers from our Code of Ethics and Business Conduct at that location.
68
Table of Contents
ITEM 11. EXECUTIVE COMPENSATION
The following table sets forth certain information concerning all cash and non-cash compensation awarded to, earned by or paid to our chief executive officer and our chief financial officer (the “Named Executive Officers”), during the fiscal years ended June 30, 2015 and 2014:
Summary of Executive Compensation
| Name and Principal Position |
Fiscal Year |
Salary ($) |
Bonus ($) (2) |
Stock Awards ($) |
Option Awards ($) (3) |
Non-Equity Incentive Plan Compensation ($) |
Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||||||||||||||
| Ian Bonnet, |
2015 | $ | 100,128 | $ | — | $ | 195,000 | $ | — | $ | — | $ | — | $ | — | $ | 295,128 | |||||||||||||||||||
| Chief Executive Officer and President (1) |
||||||||||||||||||||||||||||||||||||
| O’Connell Benjamin, |
2015 | $ | 129,200 | $ | — | $ | — | $ | 105,000 | $ | — | $ | — | $ | — | $ | 234,200 | |||||||||||||||||||
| Former Chief Executive Officer and President (4) (5) |
2014 | $ | 203,000 | $ | — | $ | 87,000 | $ | 169,500 | $ | — | $ | — | $ | — | $ | 459,500 | |||||||||||||||||||
| William A. Marshall, |
2015 | $ | 217,750 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 217,750 | |||||||||||||||||||
| Chief Financial Officer and Treasurer (4) |
2014 | $ | 182,000 | $ | — | $ | 78,000 | $ | — | $ | — | $ | — | $ | — | $ | 260,000 | |||||||||||||||||||
| (1) | On February 18, 2015, we entered into an employment arrangement with Mr. Bonnet. The agreement was for an initial term of six months and has been extended through September 18, 2015. Mr. Bonnet’s base salary is $275,000 per annum and he may receive a bonus in the discretion of the Management Resources and Compensation Committee. Mr. Bonnet was awarded 250,000 restricted stock units that vest as described below. |
| (2) | Our Named Executive Officers may receive a performance-based bonus of up to 50% of their base salary if certain performance targets are met, pursuant to their respective employment agreements with us. As of date hereof, no bonus amounts for fiscal 2015 have been determined or awarded. No bonus amounts were paid for fiscal 2014. |
| (3) | Reflects the grant date fair value of the options granted during the period that are expected to vest. Estimated value of stock options represents the expense as calculated in accordance with FASB Accounting Standards Codification Topic 718: Compensation—Stock Compensation. A discussion of the methods used to calculate these values may be found in Note 2 to our Consolidated Financial Statements contained elsewhere in this Annual Report on Form 10-K for the fiscal year ended June 30, 2015. |
| (4) | On February 18, 2010 a compensation modification program was implemented. Pursuant to this program Mr. Benjamin and Mr. Marshall accepted a reduction in their current base salary to 85% of their base salary until such time as the company achieves cash flow breakeven, as defined. This continued through January 14, 2013. On January 15, 2013, a modification to the compensation modification program was implemented. Pursuant to this program, Mr. Benjamin and Mr. Marshall accepted a further reduction in their current base salary to 70% of their base salary until the earlier of (i) such time as the company achieves cash flow breakeven or (ii) January 14, 2014. In January 2014 these agreements were extended through January 15, 2015. In consideration of such agreements, Mr. Benjamin and Mr. Marshall received restricted stock units of the company’s common stock of 62,431 and 55,972 units, respectively, during fiscal 2013 and 66,412 units and 59,542 units, respectively, during fiscal 2014 which will vest as the company achieves cash flow breakeven. This program was not extended after January 15, 2015. |
| (5) | Mr. Benjamin is our former chief executive officer and president. His employment with the company ended February 18, 2015. On November 25, 2014, we had entered into an employment agreement with Mr. Benjamin. The agreement was effective as of such date and was for a term expiring on September 30, 2015. Mr. Benjamin’s base salary was $290,000 per annum. The employment agreement provided that Mr. Benjamin may receive a bonus of up to $75,000 in the event the company achieved cash flow breakeven, as defined in the employment agreement. Moreover, Mr. Benjamin was eligible to receive a one-time bonus of $150,000 if our common stock had a closing price at or above $3.00 for 15 consecutive trading days during our fiscal year ended June 30, 2015. In addition, he was eligible for an additional bonus in the discretion of the Management Resources and Compensation Committee. Such events did not transpire. Under this agreement, the company granted Mr. Benjamin 300,000 stock options, with 200,000 options subject to performance-based vesting conditions, which did not occur. In connection with the termination of the company’s employment relationship with Mr. Benjamin in February 2015, the company has considered its severance obligations to him and the vesting and other post-termination provisions of certain of the unexercised stock options and other unvested stock options and unvested restricted stock units held by him as of the effective date of his separation from the company. As of the date hereof, the company has not reached a final resolution of this matter. |
69
Table of Contents
Discussion of Summary Compensation Table
A summary of certain material terms of our compensation plans and arrangements is set forth below. Each of the primary elements of our executive compensation is discussed in detail below. In the descriptions below, we highlight particular compensation objectives that we have designed our executive compensation program to address. However, it should be noted that we have designed the various elements of our compensation program to complement each other and thereby collectively serve all of our executive compensation objectives. Accordingly, whether or not specifically mentioned below, we believe that each element of our executive compensation program, to a greater or lesser extent, serves each of our compensation objectives.
Base Salary
The base salaries payable to our named executive officers reflect the initial base salaries that we negotiated with them at the time of their initial employment or promotion and our subsequent adjustments to these amounts to reflect market increases, the growth and stage of development of our company, our executives’ performance and increased experience, any changes in our executives’ roles and responsibilities and other factors. Employment agreements which we have entered into with each our named executive officers of are summarized below.
As described in greater detail below under the caption “Executive Compensation—Employment Agreements with Named Executive Officers” on February 18, 2010, we entered into agreements with each of O’Connell Benjamin, our former chief executive officer and president, and William A. Marshall, our chief financial officer, to implement a compensation modification program approved by the Management Resources and Compensation Committee of the board of directors. Pursuant to these agreements, both our chief executive officer and chief financial officer agreed to accept a reduction in their base salary to 85% of their current base salary until such time as we achieve “Cash Flow Breakeven”, as defined in such agreements. In consideration for their agreement to accept a reduction in their base salary, we granted such officers options to purchase such number of shares of common stock as is equal to 15% of their base salary. Accordingly, we granted our former chief executive officer 21,750 options and granted our chief financial officer 19,500 options. The options were granted under the our 2000 Employee Stock Option Plan, are exercisable for a period of ten (10) years at a per share exercise price of $2.02 and shall only vest and become exercisable upon either the date determined that we achieve “cash flow breakeven” or in the event of a termination of employment either without “cause” or for “good reason”, as such terms are defined in the employment agreements previously entered into between us and our former chief executive officer and chief financial officer. This arrangement was continued in 2011 and 2012. In consideration of their agreement to extend the modification program, on February 4, 2011, we granted Messrs. Benjamin and Marshall new options under our 2010 Employee Stock Option Plan, which are exercisable at $0.88 per share and on June 21, 2012, we granted our former chief executive officer 36,250 options and granted our chief financial officer 32,500 options exercisable at $1.30 per share. In addition, we also amended the vesting for the options granted in February 2010 and 2011 to provided that the measurement period to determine whether the vesting criteria of achieving “Cash Flow Breakeven” has been satisfied shall expire at the end of the fiscal quarter ending September 30, 2013.
On January 15, 2013, we entered into agreements with each of our former chief executive officer and chief financial officer to continue this compensation modification program and both officers agreed to a further reduction in their base salary to 70% of their original base salary commencing January 16, 2013 and continuing until the earlier of (i) such time as the company achieves “cash flow breakeven” or (ii) January 15, 2014. In consideration for these agreements, we granted Messrs. Benjamin and Marshall restricted stock units under our 2011 Omnibus Equity Incentive Plan based on (i) 15% of their base salary for the period commencing January 16, 2013 through September 30, 2013 attributable to the incremental 15% reduction in base salary through the expiration date of the prior salary reduction program; plus (ii) 30% of their base salary for the period commencing October 1, 2013 through January 15, 2014. The number of restricted stock units granted was determined by dividing the total amount of base salary that is reduced pursuant to the new modification agreements by the closing price of our common stock on the date of grant. Based on the foregoing, we granted
70
Table of Contents
our former chief executive officer 62,431 restricted stock units and our chief financial officer 55,972 restricted stock units. Effective January 15, 2014 we extended this program until the earlier of (i) such time as the company achieves “cash flow breakeven” or (ii) January 15, 2015. At this time, we granted our former chief executive officer 66,412 restricted stock units and our chief financial officer 59,542 restricted stock units. The restricted stock units shall only vest upon either the date determined that the company achieves cash flow breakeven, as defined above, or in the event of a termination of employment either without “cause” or for “good reason”, as such terms were defined in the employment agreements we previously entered with each such officer. In addition, in January 2013, we also amended the vesting for the options granted in February 2010 and 2011 and June 2012 to our employees, including executive officers, to eliminate the specific measurement period to determine whether the vesting criteria of achieving “cash flow breakeven performance” has been satisfied. This program was not extended after January 15, 2015.
In February 2015, Mr. Benjamin’s employment with the company terminated and we hired Ian C. Bonnet as our new chief executive officer and president. Pursuant to our employment agreement with Mr. Bonnet, we agreed to pay him a base salary of $275,000 per annum, based on our determination as to the salary level necessary to retain Mr. Bonnet as our new chief executive.
Equity Compensation.
Stock option awards provide our executive officers with the right to purchase shares of our common stock at a fixed exercise price typically for a period of up to ten years, subject to continued employment with our company. Stock options are earned on the basis of continued service to us and generally vest over three years, beginning with one-third vesting one year after the date of grant with the balance then vesting in equal monthly installments over the following two year period. Such vesting is intended as an incentive to such executive officers to remain with us and to provide a long-term incentive. However, we have also sought to base vesting of options on overall corporate performance. For example, as discussed above, the options granted to our named executive officers in connection with the compensation modification agreements they entered into with us in February 2010, February 2011 and June 21, 2012 and the restricted stock units awarded in January 2013 and January 2014, will vest either on the date determined that we achieve Cash Flow Breakeven or in the event of a termination of employment either without “cause” or for “good reason”, as such terms are defined in their employment agreements with us.
Options are generally exercisable for a limited period of time after termination of employment (other than termination for cause) if vested, subject to certain rights that were negotiated in connection with the employment agreements we entered into with our named executive officers. We do not require that any portion of the shares acquired be held until retirement, we do not have a policy prohibiting a director or executive officer from hedging the economic risks of his or her stock ownership and we do not have any minimum stock ownership requirements for executive officers. Stock options have been granted pursuant to our 2000 Employees Stock Option Plan (the “2000 Plan”), our 2010 Employee Stock Option Plan (the “2010 Plan”), and our 2011 Omnibus Equity Incentive Plan (the “2011 Plan”). See “Payments Upon Termination or Change-in-Control” for a discussion of the change-in-control provisions related to stock awards. The exercise price of each stock option granted under our equity compensation plans is based on the fair market value of our common stock on the grant date and the Management Resources and Compensation Committee may set the exercise price of the options granted to our named executive officers at a price equal to or greater than the fair market value in order to reinforce the incentive nature of the award. Options granted in fiscal 2015 have an exercise price equal to the market price on the grant date, which was considered appropriate by the Management Resources and Compensation Committee based on the market price of our common stock.
As described in greater detail below, during our 2015 fiscal year, we granted 250,000 restricted stock units to our new chief executive officer in connection with his employment agreement. Further, we also granted 300,000 stock options to Mr. Benjamin in connection with the employment agreement we entered into with him in November 2014, the details of which are also described below.
71
Table of Contents
Employment Agreements with Named Executive Officers
The following are summaries of the employment agreements with our named executive officers. The agreements provide the general framework and some of the specific terms for the compensation of the named executive officers. See “Payments Upon Termination or Change-in-Control” below for a discussion of payments due to our named executive officers upon the termination of his employment or a change-in-control of our company.
Ian C. Bonnet
We entered into an employment letter dated February 18, 2015 (the “Employment Letter”) with Ian C. Bonnet, our new president and chief executive officer, which discusses the terms of his employment. The Employment Letter provided that Mr. Bonnet’s employment commences on February 18, 2015 (the “Start Date”) and expired on the six month anniversary of the Start Date. As described below under the caption “Material Board and Compensation Committee Actions After Fiscal 2015”, on September 30, 2015 we entered into a new employment agreement with Mr. Bonnet. The initial Employment Letter with Mr. Bonnet provided for the following compensation terms:
| • | Base Salary and Bonus. Mr. Bonnet received an annual base salary payable at the rate of $275,000 per year with a bonus potential as determined by the Management Resources and Compensation Committee of the board in its sole discretion and based upon achievement of performance conditions. |
| • | Initial Equity Grant. Mr. Bonnet was granted an initial equity award under our 2011 Omnibus Equity Incentive Plan (the “2011 Plan”) consisting of 250,000 restricted stock units (the “Initial RSUs”) which vested as follows: (i) 150,000 shares covered by the Initial RSUs vested on the start date and (ii) 100,000 shares covered by the Initial RSUs shall vest on the six month anniversary of the start date, provided that the following conditions have occurred: (A) Mr. Bonnet shall continuously remain in our employment during the initial term, (B) he shall have entered into a new employment agreement (or an amendment to the original agreement) no later than the six month anniversary of the Start Date which shall provide for an employment term of at least twelve (12) months, and (C) he shall have submitted a restructuring plan which has been approved by the board for implementation. The Employment Letter also provided that if within 90 days of a change in control, as such term is defined in the 2011 Plan, Mr. Bonnet’s employment is terminated or his title, position or responsibilities is materially reduced and he terminates his employment, then all of the shares subject to the Initial RSUs shall be deemed fully vested on the termination date. |
| • | Severance Terms. If we terminate Mr. Bonnet’s employment without cause or if Mr. Bonnet resigns for “good reason” (each as defined in the Employment Letter), we will pay him severance benefits including, among other things, (i) the accrued compensation and (ii) subject to (A) his ongoing compliance with the employee assignment and confidentiality agreement we entered into with him, (B) his execution of a general release in favor of the company, and (C) resignation from the board (and any committees thereof), a severance payment of an amount equal to 33.3% of the base salary payable to him during the initial six month term of the Employment Letter. |
| • | Other Benefits and Terms. We will reimburse Mr. Bonnet for reasonable temporary living expenses incurred during the initial six month term of the Employment Letter in addition to other reasonable business-related expenditures. Mr. Bonnet has entered into the company’s standard form of Employee Invention Assignment and Confidentiality Agreement. |
72
Table of Contents
William A. Marshall
Mr. Marshall, our chief financial officer and treasurer entered into an at-will employment agreement with us effective as of February 15, 2006. The following is a summary of Mr. Marshall’s employment agreement:
| • | Annual base salary of $260,000. |
| • | Annual bonus targeted at 50% of base salary, in the discretion of the board, or if the board so designates, the Management Resources and Compensation Committee of the board, based on the annual performance of the company, except that the bonus for the fiscal year ending June 30, 2006 was guaranteed, pro rata, from the date his employment commenced. Mr. Marshall was also provided with an allowance of $75,000 for reimbursement of temporary living and relocation expenses and will be covered by health and similar benefits made available to the company’s senior management. |
| • | Grant of options to purchase 150,000 shares of the our common stock at an exercise price of $9.00, which options vest as follows: 50,000 shares vest on the one-year anniversary of the date of grant and the balance of 100,000 options shall vest monthly, as long as Mr. Marshall continues to be an employee of the company, in equal amounts over the subsequent 24 months. |
| • | Mr. Marshall will be entitled to a severance payment of 12 months in accordance with the terms of his employment agreement. |
| • | With respect to the options granted to Mr. Marshall, in the event his employment is terminated by the company without “cause” or by him for “good reason,” then all options granted to him shall become immediately fully vested and the exercise period in which he may exercise such options shall be extended to the duration of the original term of the option. In the event Mr. Marshall’s employment is terminated by Authentidate for “cause,” then all options granted and not exercised as of the termination date shall terminate immediately and be null and void. In the event that Mr. Marshall terminates his employment other than for “good reason,” then the options, to the extent vested as of the termination date, shall remain exercisable in accordance with their terms for a period of three months following the termination date, but in no event after the expiration of the exercise period. |
| • | The employment agreement contains confidentiality obligations that survive indefinitely and non-solicitation and non-competition obligations that end on the first anniversary of the date of cessation of Mr. Marshall’s employment. |
O’Connell Benjamin
On February 18, 2015, Mr. Benjamin’s employment as our president and chief executive officer terminated. On November 25, 2014, we had entered into a new employment agreement with Mr. Benjamin pursuant to which we continued his employment until September 30, 2015, unless sooner terminated as provided in the agreement. The following is a summary description of the terms of the 2014 employment agreement (the “2014 Employment Agreement”). Under the 2014 Employment Agreement, Mr. Benjamin’s base salary was $290,000 per annum, subject to the impact of the compensation modification agreement dated January 15, 2014, as described above. The 2014 Employment Agreement provided for cash-based bonus awards if we achieve certain performance goals. Mr. Benjamin was eligible to receive a bonus of up to $75,000 in cash if we achieved cashflow breakeven and a one-time bonus of $150,000 if our common stock has a closing price at or above $3.00 per share for 15 consecutive trading days during our fiscal year ending June 30, 2015. In addition, Mr. Benjamin was eligible for additional bonuses in the discretion of the Management Resources and Compensation Committee. No bonuses were earned prior to the termination of his employment. Mr. Benjamin was also granted options to purchase a maximum of 300,000 shares of the company’s common stock at an exercise price equal to the fair market value of the common stock on the date of execution of the employment agreement. Of the options awarded, options to purchase 100,000 shares were immediately vested and 200,000 options, which were subject to performance-based vesting requirements, did not vest prior to the termination of his employment. The 2014 Employment Agreement also provided for benefits and payments in the event Mr. Benjamin’s employment was terminated by
73
Table of Contents
us without “cause”, by Mr. Benjamin for “good reasons” or due to disability. See the discussion under the caption “Payments Upon Termination or Change in Control—O’Connell Benjamin” for additional information concerning the termination of Mr. Benjamin’s employment. The 2014 Employment Agreement contains confidentiality obligations that survive termination and non-solicitation and non-competition obligations that end on the first anniversary of the date of cessation of Mr. Benjamin’s employment.
Material Board and Compensation Committee Actions After Fiscal 2015
William P. Henry
On July 23, 2015, we appointed William P. Henry, a member of the board of directors, to serve as our interim chief strategy officer. On August 24, 2015, we entered into an employment agreement with Mr. Henry, the effectiveness of which is retroactive to July 23, 2015. The employment agreement provides that Mr. Henry shall serve as our interim chief strategy officer on an at-will basis.
| • | Salary and Bonus. Mr. Henry will be entitled to receive a base salary of payable at the rate of $250,000 per year, which will be payable upon the expiration of the term of the Employment Letter. In addition, Mr. Henry will be entitled to a bonus of $200,000 in the event we complete a transaction resulting in a “change in control” during the term of the employment agreement or within 150 days thereafter. |
| • | Equity Grants. Pursuant to the employment agreement, Mr. Henry was granted an initial equity award under our 2011 Omnibus Equity Incentive Plan (the “Plan”) of 475,000 stock options (the “Initial Options”). The Initial Options vest and are exercisable immediately and are exercisable for a period of ten years, subject to the terms of the Plan and the stock option agreement evidencing such award. The exercise price of the Initial Options is $0.25 per share, and is at a premium above the closing price of the company’s common stock on August 24, 2015, the date of execution of the employment agreement, which was $0.12 per share. In addition, the employment agreement provides that commencing on the date of execution of the agreement (the “Measurement Date”), Mr. Henry will be eligible to receive grants of additional stock options under the Plan based on the duration of the term of the employment agreement. Under this arrangement, commencing on the Measurement Date, Mr. Henry shall be granted one or more additional awards of 125,000 stock options under the Plan for each thirty (30) day period thereafter while the agreement remains in effect (the “Additional Options”). Awards of Additional Options shall be granted on (i) the Measurement Date and (ii) each monthly anniversary thereafter during the term of the agreement. Each grant of Additional Options shall vest on the thirty (30) day anniversary date following the date on which such award was granted. Further, each award of Additional Options is exercisable immediately upon the approval of the Company’s shareholders of an amendment to the Plan to increase the number of shares of Common Stock available for awards to be issued thereunder. The exercise price of each grant of Additional Options shall be equal to the greater of $0.25 per share or “Fair Market Value” as determined under the Plan, and, to the extent exercisable, such Additional Options shall be exercisable for a term of ten years. |
| • | Other Benefits and Terms. We will reimburse Mr. Henry for reasonable business-related expenditures. Mr. Henry has also entered into the company’s standard form of Employee Invention Assignment and Confidentiality Agreement. If Mr. Henry’s employment is terminated for any reason, we shall pay him all accrued compensation due and owing to him and he is not entitled to any severance or other benefits following any termination of his employment with the company except that if his employment is terminated for any reason other than cause (as defined in the employment agreement), any unvested option awards granted under the agreement shall automatically vest and the exercise period of the options granted under the agreement shall be extended to the duration of their original term. Further, Mr. Henry, who also serves as a member of the company’s board of directors, will not receive remuneration for serving as a director while he is also serving as interim chief strategy officer. |
74
Table of Contents
New Employment Letter with Ian C. Bonnet
On September 28, 2015, we entered into a new employment agreement (the “New Employment Letter”) with Mr. Bonnet which sets forth the terms of his continued employment as our president and chief executive officer. The New Employment Letter provides that Mr. Bonnet shall serve in such capacities on an at-will basis on the following terms and conditions.
Base Salary and Bonus. Mr. Bonnet will continue to receive an annual base salary of payable at the rate of $275,000 per year. Mr. Bonnet’s bonus potential will be determined by the Management Resources and Compensation Committee of the board in its sole discretion and based upon its assessment of the company’s achievement of performance conditions.
Other Benefits and Terms. We will continue to reimburse Mr. Bonnet for reasonable temporary living expenses in additional to other reasonable business-related expenditures. Further, we will provide him with standard group health and other insurance benefits generally available to senior management and agreed to evaluate additional lines of insurance which may be appropriate, including key man life insurance and accidental death and dismemberment insurance. Mr. Bonnet also agreed that he remains subject to the terms and condition of the standard form of Employee Invention Assignment and Confidentiality Agreement, which was executed in connection with his original employment letter.
Equity Grants. We granted Mr. Bonnet an award of 300,000 stock options under our 2011 Omnibus Equity Incentive Plan (the “2011 Plan”) pursuant to the New Employment Letter. These options are subject to time-based vesting requirements with 50% of the options vesting on the six month anniversary of the grant date and the remainder vesting on the twelve month anniversary of the grant date. The options are exercisable for a period of ten years at an exercise price of $0.30 per share and are subject to the terms of the 2011 Plan and the stock option agreement evidencing such award. The Management Resources and Compensation Committee also determined that the vesting conditions applicable to the 100,000 unvested restricted stock units granted to Mr. Bonnet pursuant to his original employment letter were satisfied.
Severance Terms. The New Employment Letter provides that we will make certain payments and provide benefits to Mr. Bonnet upon the termination of his employment. These terms and conditions are discussed below under the caption “Payments upon Termination or Change-in-Control—Ian C. Bonnet”.
75
Table of Contents
Outstanding Equity Awards
The following table sets forth certain information with respect to outstanding equity awards at June 30, 2015 with respect to the Named Executive Officers.
Outstanding Equity Awards At Fiscal Year-End
| Options Awards (1) | Stock Awards | |||||||||||||||||||||||||||||||
| Name |
Number of Securities Underlying Unexercised Options— Exercisable (#) |
Number
of Securities Underlying Unexercised Options— Unexercisable (#) |
Option Exercise Price ($) |
Option Expiration Date (2) |
Number of Shares or Units of Stock That Have Not Vested (#) |
Market Value of Shares or Units of Stock That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested ($) |
||||||||||||||||||||||||
| Ian Bonnet |
— | — | 100,000 | (3) | 19,000 | — | — | |||||||||||||||||||||||||
| O’Connell Benjamin (4) |
25,000 | — | 9.00 | 08/23/15 | 62,431 | (11) | 41,204 | — | — | |||||||||||||||||||||||
| 25,000 | — | 2.72 | 08/08/17 | 66,412 | (11) | 43,832 | — | — | ||||||||||||||||||||||||
| 50,000 | — | 2.50 | 12/05/17 | — | — | — | — | |||||||||||||||||||||||||
| 66,667 | — | (5) | 0.78 | 05/06/19 | — | — | — | — | ||||||||||||||||||||||||
| — | 21,750 | (6) | 2.02 | 02/18/20 | — | — | — | — | ||||||||||||||||||||||||
| — | 21,750 | (7) | 0.88 | 02/04/21 | — | — | — | — | ||||||||||||||||||||||||
| 75,000 | — | 2.28 | 04/14/21 | — | — | — | — | |||||||||||||||||||||||||
| 45,833 | 4,167 | (8) | 1.60 | 09/06/21 | — | — | — | — | ||||||||||||||||||||||||
| — | 36,250 | (9) | 1.30 | 06/21/22 | — | — | — | — | ||||||||||||||||||||||||
| 29,167 | 20,833 | (10) | 1.42 | 09/10/22 | — | — | — | — | ||||||||||||||||||||||||
| 100,000 | 200,000 | (12) | 0.89 | 09/30/23 | — | — | — | — | ||||||||||||||||||||||||
| 100,000 | — | (13) | 1.05 | 11/25/24 | — | — | — | — | ||||||||||||||||||||||||
| — | — | — | — | |||||||||||||||||||||||||||||
| William A. Marshall |
150,000 | — | 9.00 | 02/15/16 | 55,972 | (11) | 10,635 | — | — | |||||||||||||||||||||||
| 25,000 | — | 2.72 | 08/08/17 | 59,542 | (11) | 11,313 | — | — | ||||||||||||||||||||||||
| — | 19,500 | (6) | 2.02 | 02/18/20 | — | — | — | — | ||||||||||||||||||||||||
| — | 19,500 | (7) | 0.88 | 02/04/21 | — | — | — | — | ||||||||||||||||||||||||
| — | 32,500 | (9) | 1.30 | 06/21/22 | — | — | — | — | ||||||||||||||||||||||||
| (1) | Stock option grants reported in the table above were granted under, and are subject to, our 2000, 2010 and 2011 Plans. The option expiration date shown above is the normal expiration date, and the last date that the options may be exercised. For each Named Executive Officer, the unexercisable options shown above are also unvested. Unvested shares are generally forfeited if the Named Executive Officer’s employment terminates, except to the extent otherwise provided in an employment agreement. For information regarding the effect on vesting of options on the death, disability or termination of employment of a Named Executive Officer or a change in control of our company, see “Payments Upon Termination or Change in Control” below. If a Named Executive Officer’s employment is terminated by us for cause, options (including the vested portion) are generally forfeited. The exercisable options shown above, and any unexercisable options shown above that subsequently become exercisable, will generally expire earlier than the normal expiration date if the Named Executive Officer’s employment terminates, except as otherwise specifically provided in the Named Executive Officer’s employment agreement. For a description of the material terms of the Named Executive Officer’s employment agreements, see “Employment Agreements with Named Executive Officers” above. |
| (2) | Effective January 17, 2007, we amended all of the outstanding options held by our employees solely to modify the expiration date to be ten years from the original grant date. |
| (3) | On February 18, 2015, Mr. Bonnet was granted 250,000 restricted stock units that vest as follows: (i) 150,000 restricted stock units vested on the start date and (ii) 100,000 restricted stock units shall vest on the six month anniversary of the start date, provided that the following conditions have occurred: (a) Mr. Bonnet shall continuously remain in our employment during the initial term, (b) he shall have entered into a new employment agreement (or an amendment to the original agreement) no later than the six month anniversary of the start date which shall provide for an employment term of at least twelve (12) months, and (c) he shall have submitted a restructuring plan for the company to the board which has been approved by the board for implementation. This table excludes the options granted to Mr. Bonnet pursuant to his September 2015 employment agreement. |
| (4) | Mr. Benjamin was our former chief executive officer and president. His employment with the company ended February 18, 2015. Although certain option awards in the table are shown as vested, in connection with the termination of the company’s employment relationship with Mr. Benjamin in February 2015, the company is presently considering its severance obligations to him including the vesting and other |
76
Table of Contents
| post-termination provisions of certain of the unexercised stock options and other unvested stock options and unvested restricted stock units held by him as of the effective date of his separation from the company. In connection with this review process, the company may seek to limit Mr. Benjamin’s entitlement to these benefits. |
| (5) | On May 6. 2009, Mr. Benjamin was granted 200,000 options. This option grant vests as follows: 66,667 options are subject to the time-based vesting requirements with 33.3% of such amount vesting on the one year anniversary of the grant date and the balance vesting in equal installments of 33.3% on each of the next two anniversaries of the grant date. The remaining 133,333 shares covered by the option award were subject to performance-based vesting conditions and were forfeited since these vesting conditions were not met. |
| (6) | The options granted on February 18, 2010 were granted in conjunction with the implementation of a compensation modification program. Pursuant to this program Mr. Benjamin and Mr. Marshall accepted a reduction in their current base salary to 85% of their base salary until such time as the company achieves cash flow breakeven. The number of granted options was equal to 15% of their base salary (before the August 2012 one-for-two reverse stock split) and shall only vest and become exercisable upon either the date determined that the company achieves cash flow breakeven or in the event of a termination of employment either for “cause” or “good reason”. |
| (7) | The options granted on February 4, 2011 were granted in conjunction with the continuation of a compensation modification program. Pursuant to this program Mr. Benjamin and Mr. Marshall continued the reduction in their current base salary to 85% of their base salary until such time as the company achieves cash flow breakeven. The number of granted options was equal to 15% of their base salary (before the one-for-two reverse stock split) and shall only vest and become exercisable upon either the date determined that the company achieves cash flow breakeven or in the event of a termination of employment either for “cause” or “good reason”. |
| (8) | On September 9, 2011, Mr. Benjamin was granted 150,000 options. This option grant vests as follows: 50,000 options are subject to the time-based vesting requirements with 33,3% of such amount vesting on the one year anniversary of the grant date and the balance vesting in equal monthly installments thereafter. The remaining 100,000 shares covered by the option award were subject to performance-based vesting conditions and were forfeited since these vesting conditions were not met. |
| (9) | The options granted on June 21, 2012, were granted in conjunction with the continuation of a compensation modification program. Pursuant to this program Mr. Benjamin and Mr. Marshall continued the reduction in their current base salary to 85% of their base salary until such time as the company achieves cash flow breakeven. The number of granted options was equal to 15% of their base salary for the period February 1, 2012 through September 30, 2013 (before the one-for-two reverse stock split) and shall only vest and become exercisable upon either the date determined that the company achieves cash flow breakeven or in the event of a termination of employment either for “cause” or “good reason”. |
| (10) | On September 10, 2012, Mr. Benjamin was granted 200,000 options. This option grant vests as follows: 50,000 options are subject to the time-based vesting requirements with 33.3% of such amount vesting on the one year anniversary of the grant date and the balance vesting in equal monthly installments thereafter. The remaining 150,000 shares covered by the award were subject to performance-based vesting conditions and were forfeited since the conditions were not met. |
| (11) | Restricted stock units granted on January 15, 2013 and January 28, 2014, were granted in conjunction with the continuation of a compensation modification program. Pursuant to this program Mr. Benjamin and Mr. Marshall agreed to a further reduction in their current base salary to 70% of their base salary until the earlier of (i) such time as the company achieves cash flow breakeven or (ii) January 15, 2015 under the current agreements. The number of restricted stock units granted was based on the amount of the reduction in base salary and these units shall only vest and become exercisable upon either the date determined that the company achieves cash flow breakeven or in the event of a termination of employment either for “cause” or “good reason”. |
| (12) | On September 30, 2013, Mr. Benjamin was granted 600,000 options. This option grant vests as follows: 300,00 options are subject to the time based vesting requirements with 33.3% of such amounts vesting on the grant date and the balance vesting in equal installments of 100,000 shares on each of the one year and two year anniversaries of the grant date. The remaining 300,000 shares covered by the award were subject to performance-based vesting conditions and were forfeited since these conditions were not met. |
| (13) | On November 25, 2014, Mr. Benjamin was granted 300,000 options. This option grant vests as follows: 100,000 options vested immediately and the remaining 200,000 shares covered by the award were subject to performance-based vesting conditions and were forfeited since these conditions were not met. |
Payments upon Termination or Change-in-Control
The discussion below reflects the estimated benefits that would be paid or accrue to each of the Named Executive Officers in the event of the following hypothetical scenarios:
| • | termination without cause, or constructive (“good reason”) termination (including upon the occurrence of a change in control of a company); |
| • | termination for cause; |
| • | upon an executive’s disability; or |
| • | in the event of the executive’s death. |
77
Table of Contents
Ian C. Bonnet
Death or Disability. Pursuant to the Employment Letter and New Employment Letter, if Mr. Bonnet’s employment is terminated as a result of his death, Mr. Bonnet or his estate, as applicable, would receive any accrued but unpaid, base salary, bonus and expense reimbursement amounts through the date of his death. Under the New Employment Letter, if Mr. Bonnet’s employment is terminated as a result of disability, Mr. Bonnet would be entitled to the same payments and benefits as if his employment was terminated without cause.
Cause. The Employment Letter and New Employment Letter provide that if Mr. Bonnet’s employment is terminated for cause or he terminates his employment without good reason, he would be entitled to his base salary and expense reimbursement through the date of termination, and he shall have no further entitlement to any other compensation or benefits. In the event of termination for cause, all stock options that have not been exercised as of the date of termination for cause shall be deemed to have expired as of such date. In the event of termination without good reason, options vested as of the date of termination may be exercised for a limited period in accordance with the terms of the equity compensation plan under which such option was granted.
Without Cause or for Good Reason. Under the Employment Letter, if Mr. Bonnet’s employment is terminated without cause, or by Mr. Bonnet for good reason, we would be obligated to: (a) pay any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date of termination; and (b) pay a severance payment equal to 33.3% of his base salary in effect on the termination date. Under the New Employment Letter, if Mr. Bonnet’s employment was terminated by us without cause or by him for good reason, we shall pay him (i) the accrued compensation, (ii) a severance payment of an amount equal to three (3) months of base salary; and (iii) continued participation in the health and welfare plans (or comparable plans) provided by us for a period equal to the shorter of six months from the date of termination or, until he is eligible for comparable coverage with a subsequent employer. The severance and continuation of benefits are subject to his ongoing compliance with the Employee Assignment and Confidentiality Agreement, execution of a general release and resignation from the board. Further, under the New Employment Letter, if we terminate his employment without cause, he resigns for good reason, or termination is due to his death or disability, then any unvested options shall immediately vest and the exercise period in which he may exercise these options shall be extended to the duration of their original term.
Change of Control. The New Employment Letter also provides that if (1) during the period commencing on the date the company enters into a definitive agreement with respect to a transaction that would constitute a change in control (including a definitive merger or acquisition agreement contemplated by that certain Letter of Intent dated August 19, 2015 between the Company and Peachstate Health Management, LLC) and ending on the date the definitive agreement therefor is terminated or the change in control is consummated, the company terminates his employment without cause or (2) during the period commencing upon the consummation of the change in control and ending six (6) months thereafter, either (i) the company or, if applicable, the surviving or successor entity, terminates his employment without cause or (ii) he resigns for good reason, then we will pay and provide to him: (A) the accrued compensation, and (B) subject to his ongoing compliance with the Employee Assignment and Confidentiality Agreement, execution of a general release in favor of the company, and resignation from the board, (1) a severance payment of an amount equal to three months of base salary and (2) continued participation in the health and welfare plans (or comparable plans) for a period equal to the shorter of six months from the date of termination or, until he is eligible for comparable coverage with a subsequent employer. The New Employment Letter also provides that unvested options shall immediately vest and the exercise period in which he may exercise such options shall be extended to the duration of their original term if his employment is terminated by the company without cause or by him for good reason in anticipation of, or within 180 days following, a change in control.
Employee Covenants. Mr. Bonnet agreed to keep confidential and not disclose any confidential or proprietary information owned by, or received by or on behalf of, us or any of our affiliates, during the term of the agreement or at any time thereafter. He also agreed to return such confidential and proprietary information to us immediately in the event of any termination of employment. Mr. Bonnet also agreed, during his employment
78
Table of Contents
with the company and for a period of one year thereafter, to not in any manner enter into or engage in any business that is engaged in any business directly competitive with our business anywhere in the world, with limited exceptions. Moreover, Mr. Bonnet agreed, during his employment with the company and for a period of 12 months thereafter, to not, directly or indirectly, without our prior written consent: (i) solicit or induce any employee of us or any of our affiliates to leave such employ; or (ii) solicit the business of any customer with respect to products or services that compete directly with the products or services provided or supplied by us.
William A. Marshall
Death or Disability. Pursuant to the terms of his employment agreement, if Mr. Marshall’s employment is terminated as a result of his death, Mr. Marshall or his estate, as applicable, would receive any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date of his death. If Mr. Marshall’s employment is terminated as a result of disability, Mr. Marshall or his estate, as applicable, would receive (a) any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date on which the disability occurs; (b) a severance payment equal to 12 months of his base salary in effect on the termination date and (c) continued participation in our benefit plans (or comparable plans) for the longer of the natural expiration of the agreement or the end of the month of the one-year anniversary of the termination of his employment. Further, in the event of a termination due to his death or disability, Mr. Marshall’s (or his estate’s or legal representative’s) right to purchase shares of common stock pursuant to any stock option or stock option plan to the extent vested as of the termination date shall remain exercisable for a period of twelve months following such date, but in no event after the expiration of the exercise period.
Cause. If Mr. Marshall’s employment is terminated for cause or he terminates his employment without good reason, he would be entitled to his base salary, other accrued compensation and expense reimbursement through the date of termination, and he shall have no further entitlement to any other compensation or benefits. All stock options that have not been exercised as of the date of termination for cause shall be deemed to have expired as of such date otherwise, options vested as of the date of termination may be exercised for a period of three months thereafter.
Without Cause or for Good Reason. If Mr. Marshall’s employment is terminated without cause, or by Mr. Marshall for good reason, we would be obligated to: (a) pay any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date of termination; (b) pay a severance payment of 12 months of his base salary in effect on the termination date, but in no event less than $260,000; and (c) provide for his continued participation in our benefit plans (or comparable plans) for the longer of the natural expiration of the agreement or the end of the month of the one-year anniversary of the termination of his employment. Further, in the event of such a termination event, his right to purchase shares of common stock pursuant to any stock option shall immediately fully vest and become exercisable, and the exercise period in which he may exercise his options shall be extended to the duration of their original term. In addition, pursuant to the compensation modification agreements we entered into with Mr. Marshall, each of the option awards and restricted stock units will vest upon either the company achieving cash flow breakeven or in the event of a termination of employment without “cause” or for “good reason” as such terms are defined in his employment agreement, subject to limitation in the compensation modification agreements applicable to restricted stock units.
Change of Control. The benefits Mr. Marshall would receive upon termination without cause or for good reason shall not be adversely affected in the event of a change of control.
Employee Covenants. In his employment agreement, Mr. Marshall agreed to keep confidential and not disclose any confidential or proprietary information owned by, or received by or on behalf of, us or any of our affiliates, during the term of the agreement or at any time thereafter. He also agreed to return such confidential and proprietary information to us immediately in the event of any termination of employment. Mr. Marshall also agreed, during his employment with the company and for a period of one year thereafter, to not in any manner enter into or engage in any business that is engaged in any business directly competitive with our business anywhere in the world, with limited exceptions. Moreover, Mr. Marshall agreed, during his employment with the
79
Table of Contents
company and for a period of 12 months thereafter, to not, directly or indirectly, without our prior written consent: (i) solicit or induce any employee of us or any of our affiliates to leave such employ; or (ii) solicit the business of any customer with respect to products or services that compete directly with the products or services provided or supplied by us.
William P. Henry
Death or Disability. Pursuant to our employment agreement with him, if Mr. Henry’s employment is terminated as a result of his death, he or his estate, as applicable, would receive any accrued but unpaid, base salary, bonus and expense reimbursement amounts through the date of his death. If Mr. Henry’s employment is terminated as a result of disability, he or his estate, as applicable, would receive any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date on which the disability occurs.
Cause. If Mr. Henry’s employment is terminated for cause, he would be entitled to his base salary and expense reimbursement through the date of termination, and he shall have no further entitlement to any other compensation or benefits. In the event of termination for cause, all stock options that have not been exercised as of the date of termination for cause shall be deemed to have expired as of such date.
Without Cause. If Mr. Henry’s employment is terminated without cause, we would be obligated to: (a) pay any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date of termination and (b) options granted to him will be deemed vested and shall be exercisable for the duration of their original term.
Change of Control. The benefits Mr. Henry would receive upon termination without cause or for good reason shall not be adversely affected in the event of a change of control.
Employee Covenants. Mr. Henry agreed to keep confidential and not disclose any confidential or proprietary information owned by, or received by or on behalf of, us or any of our affiliates, during the term of the agreement or at any time thereafter. He also agreed to return such confidential and proprietary information to us immediately in the event of any termination of employment. Mr. Henry also agreed, during his employment with the company and for a period of one year thereafter, to not in any manner enter into or engage in any business that is engaged in any business directly competitive with our business anywhere in the world, with limited exceptions. Moreover, Mr. Henry agreed, during his employment with the company and for a period of 12 months thereafter, to not, directly or indirectly, without our prior written consent: (i) solicit or induce any employee of us or any of our affiliates to leave such employ; or (ii) solicit the business of any customer with respect to products or services that compete directly with the products or services provided or supplied by us.
O’Connell Benjamin
The following discussion summarizes the company’s post-termination obligations to Mr. Benjamin arising under its prior employment agreement with him. As stated above, in connection with Mr. Benjamin’s departure from the company in February 2015, the company is presently considering its severance obligations to him and the vesting and other post-termination provisions of certain of the unexercised stock options and other unvested stock options and unvested restricted stock units held by Mr. Benjamin as of the effective date of his separation from the company. In connection with this review process, the company may seek to limit Mr. Benjamin’s entitlement to these benefits.
Death or Disability. Pursuant to the terms of his 2014 Employment Agreement, if Mr. Benjamin’s employment is terminated as a result of his death, Mr. Benjamin or his estate, as applicable, would receive any accrued but unpaid, base salary, bonus and expense reimbursement amounts through the date of his death. If Mr. Benjamin’s employment is terminated as a result of disability, under the 2014 Employment Agreement Mr. Benjamin or his estate, as applicable, would receive (a) any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date on which the disability occurs; (b) a severance payment of 12 months of his base salary in effect on the termination date and (c) continued participation in our benefit plans
80
Table of Contents
(or comparable plans) for the longer of the natural expiration of the agreement or the end of the month of the one-year anniversary of the termination of his employment. Further, in the event of a termination due to his death or disability, under the 2014 Employment Agreement Mr. Benjamin’s (or his estate’s or legal representative’s) right to purchase shares of common stock pursuant to any stock option or stock option plan to the extent vested as of the termination date shall remain exercisable in accordance with the terms of the equity compensation plan under which such option was granted.
Cause. If Mr. Benjamin’s employment is terminated for cause or he terminates his employment without good reason, he would be entitled to his base salary and expense reimbursement through the date of termination, and he shall have no further entitlement to any other compensation or benefits. In the event of termination for cause, all stock options that have not been exercised as of the date of termination for cause shall be deemed to have expired as of such date. In the event of termination without good reason, options vested as of the date of termination may be exercised for a limited period in accordance with the terms of the equity compensation plan under which such option was granted.
Without Cause or for Good Reason. Under the 2014 Employment Agreement, if Mr. Benjamin’s employment is terminated without cause, by Mr. Benjamin for good reason, or either (1) we fail to timely notify him or our intent to renew his agreement or (2) after providing such notice, we fail to reach an agreement on a new employment agreement with him prior to the expiration date, then we would be obligated to: (a) pay any accrued but unpaid base salary, bonus and expense reimbursement amounts through the date of termination; (b) pay a severance payment equal to 12 months of his base salary in effect on the termination date, but in no event less than $290,000; (c) provide for his continued participation in our benefit plans (or comparable plans) for the longer of the natural expiration of the agreement or the end of the month of the one-year anniversary of the termination of his employment. Further, in the event of such a termination event, certain of the options and other equity incentive awards granted to Mr. Benjamin will be deemed vested and shall be exercisable for the duration of their original term, subject to the conditions and limitations of the 2014 Employment Agreement. In addition, pursuant to the compensation modification agreements we entered into with Mr. Benjamin, as described above, each of the option awards and restricted stock units will vest upon either the company achieving cash flow breakeven or in the event of a termination of employment without “cause” or for “good reason”, as such terms are defined in his employment agreement, subject to the limitations described above applicable to the restricted stock units.
Change of Control. In the event of a change of control, under the 2014 Employment Agreement, Mr. Benjamin shall have the right to terminate his employment with us for any reason within a limited period of time of such change of control and such termination shall be deemed for good reason. In such an event, we would be required to pay Mr. Benjamin the amounts described above.
Employee Covenants. In the 2014 Employment Agreement, Mr. Benjamin agreed to keep confidential and not disclose any confidential or proprietary information owned by, or received by or on behalf of, us or any of our affiliates, during the term of the agreement or at any time thereafter. He also agreed to return such confidential and proprietary information to us immediately in the event of any termination of employment. Mr. Benjamin also agreed, during his employment with the company and for a period of one year thereafter, to not in any manner enter into or engage in any business that is engaged in any business directly competitive with our business anywhere in the world, with limited exceptions. Moreover, Mr. Benjamin agreed, during his employment with the company and for a period of 12 months thereafter, to not, directly or indirectly, without our prior written consent: (i) solicit or induce any employee of us or any of our affiliates to leave such employ; or (ii) solicit the business of any customer with respect to products or services that compete directly with the products or services provided or supplied by us.
2011 Omnibus Equity Incentive Plan
Adjustments upon Merger or Change in Control. The 2011 Plan provides that in the event of a merger with or into another corporation or “change in control,” including the sale of all or substantially all of our assets,
81
Table of Contents
unless otherwise provided in an award agreement, in the event of a change in control in which the successor company assumes or substitutes for an option, stock appreciation right, restricted stock award, restricted stock unit award or other share-based award, if a participant’s employment or service as a director with such successor company terminates within 24 months following such change in control (or such other period set forth in the award agreement): (i) options and stock appreciation rights outstanding as of the date of such termination of employment will immediately vest, become fully exercisable, and may thereafter be exercised for 24 months (or such other period of time set forth in the award agreement), (ii) the restrictions, limitations and other conditions applicable to restricted stock and restricted stock units outstanding as of the date of such termination of employment shall lapse and such awards shall become free of all restrictions, and (iii) the restrictions, limitations and other conditions applicable to any other share-based awards or any other awards shall lapse, and such awards shall become free of all restrictions. However, unless otherwise provided in an award agreement, in the event of a change in control, if the successor company does not assume or substitute for an option, stock appreciation right, restricted stock award, restricted stock unit award or other share-based award, then immediately prior to the change in control: (i) those options and stock appreciation rights outstanding as of the date of the change in control that are not assumed or substituted for shall immediately vest and become fully exercisable, (ii) restrictions, limitations and other conditions applicable to restricted stock and restricted stock units that are not assumed or substituted for shall lapse and the restricted stock and restricted stock units shall become free of all restrictions, and (iii) the restrictions, other limitations and other conditions applicable to any other share-based awards or any other awards that are not assumed or substituted for shall lapse, and such other share-based awards or such other awards shall become free of all restrictions.
Termination of Employment. Under the 2011 Plan, if a grantee’s employment or service is terminated for cause, any unexercised option shall terminate effective immediately upon such termination of employment or service. Except as otherwise provided by in an award agreement, if a grantee’s employment or service terminates on account of death or disability, then any unexercised option, to the extent exercisable on the date of such termination of employment or service, may be exercised, in whole or in part, within the first twelve (12) months after such termination of employment or service (but only during the option term) by his or her personal representative or by the person to whom the option is transferred by will or the applicable laws of descent and distribution.
The 2011 Plan provides that except as otherwise provided by the Committee in the award agreement, if a grantee’s employment or service terminates for any reason other than for cause, death, disability or pursuant to a change of control, then any unexercised option, to the extent exercisable immediately before the grantee’s termination of employment or service, may be exercised in whole or in part, not later than three (3) months after such termination of employment or service (but only during the option term); and, to the extent that any such option was not exercisable on the date of such termination of employment or service, it will immediately terminate.
Equity Compensation Plans
2011 Omnibus Equity Incentive Plan
At the company’s special meeting of stockholders held on August 23, 2011, our stockholders approved the 2011 Omnibus Equity Incentive Plan (the “2011 Plan”). Our board of directors adopted the 2011 Plan on July 19, 2011, subject to stockholder approval at the special meeting. In May 2014, our stockholders approved an amended to the 2011 Plan to increase the maximum number of shares available for awards under the 2011 Plan. The purpose of the 2011 Plan is to assist us and our subsidiaries in attracting and retaining selected individuals who, serving as our employees, directors, consultants and/or advisors, are expected to contribute to our success and to achieve long-term objectives which will benefit our stockholders through the additional incentives inherent in the awards under the 2011 Plan. The maximum number of shares of our common stock that are available for awards under the 2011 Plan, as amended, (subject to the adjustment provisions of the 2011 Plan) is 6,750,000 shares. Under the 2011 Plan, options, stock appreciation rights, restricted stock awards, restricted stock
82
Table of Contents
unit awards, other share-based awards and performance awards may be granted to eligible participants. Subject to the reservation of authority by our board of directors to administer the 2011 Plan and act as the committee thereunder, the 2011 Plan will be administered by the Management Resources and Compensation Committee (the “Committee”), which has the authority to determine the terms and conditions of awards, and to interpret and administer the 2011 Plan. As of September 1, 2015, there were outstanding (i) 3,772,761 options to purchase shares under the 2011 Plan, with exercise prices ranging from $0.18 to $1.72 and (ii) a total of 888,437 shares granted pursuant to restricted stock awards and restricted stock units under the 2011 Plan.
The 2011 Plan replaced both the 2001 Director Plan and the 2010 Employee Plan as the company’s vehicle for granting equity awards to its employees, directors and consultants. Accordingly, as of August 23, 2011, all equity awards granted to our employees, directors and eligible consultants will be made pursuant to the 2011 Plan and shares remaining under our existing plans will no longer be available for grants under such plans. Holders of unexercised options granted under the 2001 Director Plan and the 2010 Employee Plan will be able to exercise those options in accordance with the terms of such grants, until the expiration date set forth in their option certificates.
Summary of the 2011 Plan
Shares Available. The maximum number of shares of our common stock that are available for awards under the 2011 Plan (subject to the adjustment provisions described under “Adjustments upon Changes in Capitalization” below), as amended on May 1, 2014, is 6,750,000 shares. If any shares of common stock subject to an award under the 2011 Plan, or an award under the 2010 Employee Plan, are forfeited, expire or are settled for cash (in whole or in part), the shares subject to the award may be used again for awards under the 2011 Plan to the extent of the forfeiture, expiration or cash settlement.
Eligibility. Options, stock appreciation rights (“SARs”), restricted stock awards, restricted stock unit awards, other share-based awards and performance awards may be granted under the 2011 Plan. Options may be either “incentive stock options,” as defined in Section 422 of the Code, or nonstatutory stock options. Awards may be granted under the 2011 Plan to any employee, non-employee member of our board of directors, consultant or advisor who is a natural person and provides services to us or a subsidiary, except for incentive stock options which may be granted only to employees.
Administration. Subject to the reservation of authority by our board of directors to administer the 2011 Plan and act as the committee thereunder, the 2011 Plan will be administered by the Committee. The Committee has the authority to determine the terms and conditions of awards, and to interpret and administer the 2011 Plan.
Stock Options. The Committee may grant either nonstatutory stock options or incentive stock options. A stock option entitles the recipient to purchase a specified number of shares of our common stock at a fixed price subject to terms and conditions set by the Committee. The purchase price of shares of common stock covered by a stock option cannot be less than 100% of the fair market value of the common stock on the date the option is granted. Fair market value of the common stock is generally equal to the closing price for the common stock on the Principal Exchange on the date the option is granted (or if there was no closing price on that date, on the last preceding date on which a closing price was reported). Options are subject to terms and conditions set by the Committee. Options granted under the 2011 Plan expire no later than 10 years from the date of grant.
Stock Appreciation Rights. The Committee is authorized to grant SARs in conjunction with a stock option or other award granted under the 2011 Plan, and to grant SARs separately. The grant price of a SAR may not be less than 100% of the fair market value of a share of our common stock on the date the SAR is granted. The term of an SAR may be no more than 10 years from the date of grant. SARs are subject to terms and conditions set by the Committee. Upon exercise of an SAR, the participant will have the right to receive the excess of the fair market value of the shares covered by the SAR on the date of exercise over the grant price.
83
Table of Contents
Restricted Stock Awards. Restricted stock awards may be issued either alone or in addition to other awards granted under the 2011 Plan, and are also available as a form of payment of performance awards and other earned cash-based incentive compensation. The Committee determines the terms and conditions of restricted stock awards, including the number of shares of common stock granted, and conditions for vesting that must be satisfied, which may be based principally or solely on continued provision of services, and also may include a performance-based component. Unless otherwise provided in the award agreement, the holder of a restricted stock award will have the rights of a stockholder from the date of grant of the award, including the right to vote the shares of common stock and the right to receive distributions on the shares. Except as otherwise provided in the award agreement, any shares or other property (other than cash) distributed with respect to the award will be subject to the same restrictions as the award.
Restricted Stock Unit Awards. Awards of restricted stock units having a value equal to an identical number of shares of common stock may be granted either alone or in addition to other awards granted under the 2011 Plan, and are also available as a form of payment of performance awards granted under the 2011 Plan and other earned cash-based incentive compensation. The Committee determines the terms and conditions of restricted stock units, including conditions for vesting that must be satisfied, which may be based principally or solely on continued provision of services, and also may include a performance-based component. The holder of a restricted stock unit award will not have voting rights with respect to the award. Except as otherwise provided in the award agreement, any shares or other property (other than cash) distributed with respect to the award will be subject to the same restrictions as the award.
Other Share-Based Awards. The 2011 Plan also provides for the award of shares of our common stock and other awards that are valued by reference to our common stock or other property (“Other Share-Based Awards”). Other Share-Based Awards may be paid in cash, shares of our common stock or other property, or a combination thereof, as determined by the Committee. The Committee determines the terms and conditions of Other Share-Based Awards, including any conditions for vesting that must be satisfied.
Performance Awards. Performance awards provide participants with the opportunity to receive shares of our common stock, cash or other property based on performance and other vesting conditions. Performance awards may be granted from time to time as determined at the discretion of the Committee. Subject to the share limit and maximum dollar value set forth above under “Limits on Awards to Participants,” the Committee has the discretion to determine (i) the number of shares of common stock under, or the dollar value of, a performance award and (ii) the conditions that must be satisfied for grant or for vesting, which typically will be based principally or solely on achievement of performance goals.
No Repricing. The 2011 Plan prohibits option and SAR repricings (other than to reflect stock splits, spin-offs or other corporate events described under “Adjustments upon Changes in Capitalization” below, or in connection with a change in control of the company) unless stockholder approval is obtained.
Nontransferability of Awards. No award under the 2011 Plan, and no shares subject to awards that have not been issued or as to which any applicable restriction, performance or deferral period has not lapsed, is transferable other than by will or the laws of descent and distribution, and an award may be exercised during the participant’s lifetime only by the participant or the participant’s estate, guardian or legal representative, except that the Committee may provide in an award agreement that a participant may transfer an award without consideration to certain family members, family trusts, or other family-owned entities, or for charitable donations under such terms and conditions determined by the Committee.
Amendment and Termination. The 2011 Plan may be amended or terminated by our board of directors except that stockholder approval is required for any amendment to the 2011 Plan which increases the number of shares of common stock available for awards under the 2011 Plan, expands the types of awards available under the 2011 Plan, materially expands the class of persons eligible to participate in the 2011 Plan, permits the grant of options or SARs with an exercise or grant price of less than 100% of fair market value on the date of grant,
84
Table of Contents
amends the provisions of the 2011 Plan prohibiting the repricing of options and SARs as described above, increases the limits on shares subject to awards, or otherwise materially increases the benefits to participants under the 2011 Plan. The 2011 Plan will expire on the 10th anniversary of the Effective Date, except with respect to awards then outstanding, and no further awards may be granted thereafter.
Other Option Plans
2010 and 2000 Employee Stock Option Plans
In May 2010, our stockholders approved the 2010 Employee Stock Option Plan (the “2010 Plan”) which provided for the grant of options to purchase up to 5,000,000 shares of our common stock. The 2010 Plan served as our primary equity incentive plan for our employees and other eligible participants until the approval by our shareholders of the 2011 Plan. The board of directors unanimously approved the 2010 Plan on January 20, 2010 and our stockholders approved the 2010 Plan on May 19, 2010. The 2010 Plan and 2000 Plan were administered by the Management Resources and Compensation Committee designated by our board of directors. The board or committee had full authority to interpret the 2010 Plan and 2000 Plan and to establish and amend rules and regulations relating thereto. Under the terms of the 2010 Plan, options granted there under were designated as options which qualify for incentive stock option treatment (“ISOs”) under Section 422 of the Code, or options which do not so qualify (“Non-ISOs”). As of June 30, 2015, there were 237,225 options outstanding under the 2010 Plan, with exercise prices ranging from $0.88 to $2.40.
In March 2001, our stockholders approved the 2000 Employees Stock Option Plan (the “2000 Plan”) which, as amended, provided for the grant of options to purchase up to 5,000,000 shares of our common stock. to our employees, until its expiration in 2010. Under the terms of the 2000 Plan, options granted there under were designated as ISOs or Non-ISOs. As of June 30, 2015, there were 771,967 options outstanding under the 2000 Plan, with exercise prices ranging from $0.78 to $9.00
2001 Non-Executive Director Stock Option Plan
In January 2002, our stockholders approved the 2001 Non-Executive Director Stock Option Plan. Awards were granted under the 2001 Director Plan until the approval by our shareholders of the 2011 Plan to (i) non-executive directors as defined and (ii) members of any advisory board we may establish who are not full-time employees of us or any of our subsidiaries. Under the 2001 Director Plan, each non-executive director was automatically be granted an option to purchase 20,000 shares upon joining the board and an option to purchase 5,000 shares each September 1st thereafter, pro rata, based on the time the director has served during the prior year. The term non-executive director refers to those of our directors who are not otherwise a full-time employee of Authentidate or any subsidiary. In addition, each eligible member of an advisory board will receive, upon joining the advisory board, and on each anniversary of the effective date of his appointment, an option to purchase 2,500 shares of our common stock. The 2001 Director Plan expired ten years following its adoption. As of June 30, 2015 there were 80,000 outstanding options granted under the 2001 Director Plan. The options outstanding have exercise prices ranging from $1.16 to $6.00. As stated above, following the approval of the 2011 Plan by our shareholders, no further awards were granted under the 2001 Plan.
Director Compensation
On July 19, 2011, our board approved the following new compensation policy for our non-employee directors:
| • | The annual director fee for our non-executive directors is $30,000; |
| • | Committee chairmen are paid an additional annual fee as follows: (a) Chairman of the Board—$25,000 per annum; (b) Chairman of the Audit Committee—$15,000 per annum; (c) Chairman of the Management Resources and Compensation Committee—$7,500 per annum; and (d) other Committee Chairmen—$5,000 per annum; and |
85
Table of Contents
| • | Meeting fees for our independent directors are $1,500 for each meeting of the board of directors, and $1,500 for each meeting of a committee of the board of directors. For meetings held by conference call, fees are $750 per meeting. Reasonable and customary expenses incurred in attending the board and committee meetings are reimbursable. |
In addition to the foregoing cash compensation, effective with the approval by the company’s stockholders of the 2011 Plan, each non-employee director will receive (i) upon initial election to the board of directors, a nonstatutory stock option for the purchase of 20,000 shares of the company’s common stock which vests immediately upon election and (ii) an annual stock option grant, to be granted on September 1, for the purchase of 10,000 shares of the company’s common stock which also vests immediately; which award amount was increased to 15,000 options in June 2012; provided, that any non-employee director, who has not served as a director for an entire year prior to September 1st of the reference year shall receive a pro rata number of options determined as follows:
| Date of Membership |
Options Granted | |||
| September 1 through November 30 |
15,000 | |||
| December 1 through February 28 |
11,250 | |||
| March 1 through May 30 |
7,500 | |||
| June 1 through August 31 |
3,500 | |||
On September 1, 2015, we granted an aggregate of 71,250 options to our non-employee directors pursuant to the 2011 Plan. These options have an exercise price of $0.28 and are exercisable for a period of ten years from the grant date. The exercise price of such options is equal to the fair market value of our common stock on the grant date, as determined under the 2011 Plan. With respect to such options, upon the termination of service of a director, options shall terminate on the second anniversary of the date of termination of service, except that if termination of service is due to optionee’s death or permanent disability (as determined by the board), the option shall terminate on the earlier of the expiration date of such option or 12 months following the date of death or termination for permanent disability and if an optionee is removed from the board for cause, as determined by the board, the option awards held by such optionee would terminate immediately upon removal.
Through December 2012, non-employee directors continued to have the option to elect to receive up to 100% of their cash director compensation, including amounts payable for committee service, service as a committee chair and per meeting fees, in restricted shares of our common stock issued under the 2011 Plan. Under this program, prior to the commencement of each fiscal year, each non-employee director had the right to elect to receive a percentage (up to 100%) of all cash compensation payable to such non-employee director for the fiscal year ending the following June 30 in restricted shares of our common stock. Notwithstanding the foregoing, however, on one occasion during each fiscal quarter, a non-employee director, prior to the first day of the last month of each fiscal quarter, may notify the company of his decision to modify a prior election, with any such revised allocation to be effective for any subsequent fiscal quarter during the remainder of the fiscal year. If a non-employee director elects to receive a percentage of his or her cash compensation in restricted shares, the number of restricted shares that will be issued to such director will be calculated by dividing the cash amount to be converted into restricted shares by the fair market value of the company’s common stock as of the date the fees are earned, which date shall be deemed to be the last trading day of each fiscal quarter. Fair market value will be determined in accordance with the provisions of the 2011 Plan.
On December 18, 2012, the board approved a modification to our non-employee director compensation policy to require that all director fees be paid in the form of either non-qualified stock options or restricted shares of common stock to be issued under the 2011 Plan, effective January 1, 2013. Each director elected the form of payment to be received. As the company pays director fees on a quarterly basis in arrears, the securities to be issued to our non-employee directors for each fiscal quarter while this policy modification is in effect will be issued following the close of each such fiscal quarter. Under this new policy, if a non-employee director elects to receive payment of director fees in the form of non-qualified stock options, the number of options issued will be
86
Table of Contents
calculated by dividing the cash amount to be converted into options by the fair value of an option as determined by the Black-Scholes option pricing model as of the last trading day of each fiscal quarter. If a non-employee director elects to receive payment in restricted shares, the number of shares to be issued to such director will be determined as described above. Restricted shares will be restricted from public resale in accordance with the provisions of Rule 144, as adopted by the Securities and Exchange Commission under the Securities Act of 1933, as amended. The options to be granted to non-employee directors under the 2011 Plan are exercisable for a period of ten years from the grant date. The exercise price of such options shall be equal to the fair market value of our common stock on the grant date, as determined under the 2011 Plan. Upon the termination of service of a director, these options shall remain exercisable to the same extent as pertains to the annual option awards granted to non-employee directors, as described above.
Further, in July 2011, the board also adopted stock ownership guidelines applicable to our non-employee directors. The Non-Employee Director Stock Ownership Guidelines require all non-employee directors to hold shares of our common stock with a value equal to four times the amount of the base annual retainer fee paid to non-employee directors for service on the board, excluding additional committee retainer fees, if any. This ownership guideline is initially calculated using the base annual retainer fee for service as a non-employee director as of the date we adopted these guidelines for current directors or for any new members of our board, such person first became subject to the guidelines. These ownership guidelines will be re-calculated following any adjustment to the applicable annual non-employee director retainer fees. These guidelines will be based on the applicable annual board retainer fee in effect on such calculation date.
Non-employee directors are required to achieve the applicable level of ownership within five years of the later of the date the guidelines were adopted and the date the person first became a non-employee member of the board. Shares that count toward satisfaction of the guidelines include shares owned outright by the director or his or her immediate family members residing in the same household and shares held in trust for the benefit of the director or his or her family. Unexercised and/or unvested equity awards do not count toward satisfaction of the guidelines. The value of a share will be measured on the date of the company’s annual meeting each year as the greater of (i) the average closing price over the 12 months preceding the date of calculation or (ii) the purchase price actually paid by the person for such share of the company’s stock. The purchase price for shares acquired pursuant to restricted stock units, performance shares and other similar full value awards is zero. Our Non-Employee Director Stock Ownership Guidelines may be waived, at the discretion of the board’s Management Resources and Compensation Committee if compliance would create undue hardship or prevent a director from complying with a court order, as in the case of a divorce settlement.
On September 10, 2012, we entered into indemnification agreements with each of the non-employee members of our board of directors. The indemnification agreements provide, subject to the procedures, limitations and exclusions set forth in the agreements: (i) that we will indemnify the indemnitee to the fullest extent permitted by applicable law in the event the indemnitee is, or is threatened to be made, a party to or a participant in an action, suit or other proceeding by reason of the fact that the indemnitee is or was one of our directors or is or was serving at our request as a director, officer, employee, agent or fiduciary of another enterprise; (ii) that we will advance, to the fullest extent not prohibited by applicable law, the expenses incurred by the indemnitee in connection with any such proceeding; (iii) that the rights of the indemnitee under the agreement are in addition to any other rights the indemnitee may have otherwise; and (iv) that the agreement shall continue until and terminate upon 10 years after the latest date that the indemnitee shall have ceased to serve as one of our directors or as a director, officer, employee, agent or fiduciary of any other enterprise at our request. We are required to advance such person’s expenses in connection with his or her defense, provided that the indemnitee undertakes to repay all amounts advanced if it is ultimately determined that such person is not entitled to be indemnified by us. We have entered into materially similar indemnification agreements with each of our non-employee directors that were elected to board subsequent to September 10, 2012.
87
Table of Contents
A summary of non-executive director compensation for the fiscal year ended June 30, 2015 is as follows:
Summary of Non-Executive Director Compensation
| Name (1) |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) (2) |
Option Awards ($) (3) |
Non-Equity Incentive Plan Compensation ($) |
Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||||||||
| Charles C Lucas III |
$ | — | $ | — | $ | 100,058 | $ | — | $ | — | $ | — | $ | 100,058 | ||||||||||||||
| William P. Henry (4) |
$ | 1,071 | $ | — | $ | 2,660 | $ | — | $ | — | $ | — | $ | 3,731 | ||||||||||||||
| Roy E. Beauchamp (5) |
$ | 5,495 | $ | — | $ | 37,650 | $ | — | $ | — | $ | — | $ | 43,145 | ||||||||||||||
| Todd A. Borus |
$ | — | $ | — | $ | 55,800 | $ | — | $ | — | $ | — | $ | 55,800 | ||||||||||||||
| Marc A. Horowitz (6) |
$ | 2,543 | $ | — | $ | 39,080 | $ | — | $ | — | $ | — | $ | 41,623 | ||||||||||||||
| J. David Luce |
$ | — | $ | 51,637 | $ | 10,800 | $ | — | $ | — | $ | — | $ | 62,437 | ||||||||||||||
| J. Edward Sheridan (7) |
$ | — | $ | — | $ | 39,561 | $ | — | $ | — | $ | — | $ | 39,561 | ||||||||||||||
| Jeffrey Beunier (8) |
$ | — | $ | — | $ | 68,053 | $ | — | $ | — | $ | — | $ | 68,053 | ||||||||||||||
| (1) | As of June 30, 2015, each of our current directors had the following number of options outstanding: Mr. Lucas—603,989 options; Mr. Henry 20,000 options; Mr. Beauchamp—210,824 options; Mr. Borus—396,918 options; Mr. Horowitz—207,054 options; and Mr. Luce—85,000 options (not including 250,000 vested options he was granted in August 2009 as a fee for services rendered in connection with our former ExpressMD Solutions joint venture). Includes options issued to certain directors in lieu of cash director fees during fiscal 2015 as follows: Mr. Lucas—424,415 options; Mr. Beauchamp 190,824; Mr. Borus 216,493; and Mr. Horowitz 187,054. |
| (2) | For the year ended June 30, 2015, the following directors elected to receive a portion of their cash director fees in shares of restricted common stock. The number of shares earned for service during fiscal 2015 is as follows: Mr. Luce—151,213 shares. As of the fiscal year ended June 30, 2015, the aggregate number of shares of restricted stock issued in lieu of cash director fees to our current non-executive directors was as follows: Mr. Lucas 0 shares; Mr. Henry 0 shares; Mr. Beauchamp 0 shares; Mr. Borus 47,495 shares, Mr. Horowitz 0 shares and Mr. Luce—344,163 shares. |
| (3) | Reflects the dollar amount recognized for financial statement reporting purposes for the fiscal year ended June 30, 2015 computed in accordance with FASB Accounting Standards Codification Topic 718: Compensation—Stock Compensation, and thus may include amounts from awards granted in and prior to 2014. A discussion of the methods used to calculate these values may be found in Note 2 to our Consolidated Financial Statements contained elsewhere in this Annual Report on Form 10-K for the fiscal year ended June 30, 2015. |
| (4) | Mr. Henry was elected to the board on June 18, 2015. |
| (5) | Mr. Beauchamp was elected to the board on November 13, 2014. |
| (6) | Mr. Horowitz was elected to the board on December 10, 2014. |
| (7) | Mr. Sheridan retired from the board effective as of December 9, 2014. As of June 30, 2015, Mr. Sheridan had a total of 342,754 options outstanding and had been issued a total of 36,352 shares in lieu of cash director fees. |
| (8) | Mr. Beunier resigned from the board effective July 1, 2015. As of June 30, 2015, Mr. Beunier had a total of 318,095 options outstanding and had been issued a total of 26,515 shares in lieu of cash director fees. |
88
Table of Contents
Compensation Committee Interlocks and Insider Participation
There are no compensation committee interlocks between the members of our Management Resources and Compensation Committee and any other entity. Currently, Messrs. Lucas, Beauchamp and Borus are the members of this committee and during fiscal 2015 Mr. Sheridan also served on this committee until his retirement. None of the members of the Management Resources and Compensation Committee (a) was an officer or employee of the company during the last fiscal year; (b) was formerly an officer of the company or any of its subsidiaries; or (c) had any relationship with the company requiring disclosure under Item 404 of Regulation S-K.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table sets forth certain information as of September 5, 2015 with respect to (i) each director and and person listed as a named executive officer, (ii) and all directors and executive officers in the Summary Compensation Table appearing below in this Annual Report on Form 10-K, and (iii) persons (including any “group” as that term is used in Section 13(d)(3) of the Securities Exchange Act of 1934, as amended), known by us to be the beneficial owner of more than five percent of our common stock. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed outstanding shares of common stock that are (a) subject to stock options or warrants held by that person that are currently exercisable or exercisable within 60 days of the date of this table, (b) shares of common stock issuable upon conversion of shares of convertible preferred stock held by that person that are currently convertible or convertible within 60 days of the date of this table, and (c) shares of common stock issuable upon the vesting of restricted stock units (RSUs) within 60 days of such date. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
| Common Stock | ||||||||
| Name and Address of Beneficial Holder |
Amount and Nature of Beneficial Ownership (++) |
Percent of Class (#) |
||||||
| 5% Stockholders |
||||||||
| Lazarus Investment Partners LLLP 3200 Cherry Creek South Drive, Suite 670 Denver, Colorado 80209 |
14,763,709 | (1) | 29.3 | % | ||||
| Directors and Executive Officers |
||||||||
| Charles C. Lucas III c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
626,989 | (2) | 1.5 | % | ||||
| Ian C. Bonnet c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
250,000 | (3) | * | |||||
| William P. Henry c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
620,000 | (4) | 1.4 | % | ||||
89
Table of Contents
| Common Stock | ||||||||
| Name and Address of Beneficial Holder |
Amount and Nature of Beneficial Ownership (++) |
Percent of Class (#) |
||||||
| Roy E. Beauchamp c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
225,824 | (5) | * | |||||
| Todd A. Borus, M.D. c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
553,885 | (6) | 1.3 | % | ||||
| Marc A. Horowitz c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
218,304 | (7) | * | |||||
| J. David Luce c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
1,334,755 | (8) | 3.1 | % | ||||
| William A. Marshall c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
685,074 | (9) | 1.6 | % | ||||
| O’Connell Benjamin c/o Authentidate Holding Corp. Connell Corporate Center 300 Connell Drive, 5th Floor Berkeley Heights, NJ 07922 |
1,554,910 | (10) | 3.6 | % | ||||
| Directors and Executive Officers as a group (2)(3)(4)(5)(6)(7)(8)(9) |
4,511,831 | 10.0 | % | |||||
| ++ | Unless otherwise indicated below, each director, officer and 5% stockholder has sole voting and sole investment power with respect to all shares that it beneficially owns. |
| # | Based on 42,194,841 shares of common stock outstanding as of September 5, 2015. |
| * | Represents less than 1% of the issued and outstanding shares of common stock. |
| (1) | Based on Schedule 13D/A filed by the listed stockholder on September 14, 2015. The securities reported on this table as beneficially owned by Lazarus Management Company, LLC (“Lazarus Management”) are held by or for the benefit of Lazarus Investment Partners LLLP (“Lazarus Partners”). Includes warrants to purchase an aggregate of 6,233,636 shares of common stock and 1,842,113 shares of common stock which may be issued upon conversion of 200,000 shares of Series D preferred stock. Also includes 7,500 shares of common stock beneficially owned by Lazarus Macro Micro Partners LLLP; Lazarus Investment Partners LLLP holds no interest in these securities and Lazarus Management Company LLC and Justin B. Borus disclaims beneficial ownership except to the extent of their pecuniary interest therein. Lazarus Management, as the investment |
90
Table of Contents
| adviser and general partner of Lazarus Partners, and Justin B. Borus, as the managing member of Lazarus Management, may be deemed to beneficially own the securities held by Lazarus Partners for the purposes of Rule 13d-3 of the Securities Exchange Act of 1934, insofar as they may be deemed to have the power to direct the voting or disposition of those securities. Neither the filing of this report nor any of its contents shall be deemed to constitute an admission that Lazarus Management or Mr. Borus is, for any other purpose, the beneficial owner of any of the securities, and each of Lazarus Management and Mr. Borus disclaims beneficial ownership as to the securities, except to the extent of his or its pecuniary interests therein. |
| (2) | Includes vested options to purchase 618,989 shares of common stock. Excludes options granted September 30, 2015 pursuant to the company’s non-employee director compensation policy. |
| (3) | Includes 250,000 shares of common stock and excludes unvested options to purchase 300,000 shares of common stock. |
| (4) | Includes vested options to purchase 495,000 shares of common stock and unvested options to purchase 125,000 shares of common stock. Excludes unvested options to purchase 125,000 shares of common stock granted on September 24, 2015. Excludes options granted September 30, 2015 pursuant to the company’s non-employee director compensation policy. |
| (5) | Includes vested options to purchase 225,824 shares of common stock. Excludes options granted September 30, 2015 pursuant to the company’s non-employee director compensation policy. |
| (6) | Includes vested options to purchase 411,918 shares of common stock, warrants to purchase 35,982 shares of common stock and 23,026 shares of common stock issuable upon conversion of 2,500 shares of Series D preferred stock. Dr. Borus is the brother of the manager of the general partner of Lazarus Investment Partners, LLLP; however, Dr. Borus expressly disclaims beneficial ownership interest in our securities which are beneficially owned by Lazarus Investment Partners. Excludes options granted September 30, 2015 pursuant to the company’s non-employee director compensation policy. |
| (7) | Includes vested options to purchase 218,304 shares of common stock. Excludes options granted September 30, 2015 pursuant to the company’s non-employee director compensation policy. |
| (8) | Includes vested options to purchase 220,000 shares of common stock. Includes 753,105 shares of common stock owned by affiliated entities and 17,487 shares of common stock owned by his spouse. Excludes unvested options to purchase 125,000 shares of common stock. Excludes warrants to purchase an aggregate of 5,474,829 shares of common stock issued in connection with our issuances of secured notes during 2012 and our sale of Series D preferred stock, which are held by an entity affiliated with the reporting person and his spouse, which warrants contain a blocker provision under which the holder thereof does not have the right to exercise such warrants to the extent that such exercise would result in beneficial ownership by the holder thereof or any of its affiliates, of more than 4.99% of our common stock. Also excludes 2,440,799 shares of common stock issuable upon conversion of an aggregate of 265,000 shares of Series D preferred stock which were issued June 20, 2013 in our Series D preferred stock financing to an entity affiliated with the reporting person and his spouse, which securities contain a blocker provision under which the holder thereof does not have the right to convert such securities to the extent that such event would result in the holder or any of its affiliates acquiring more than 4.99% of our common stock. Excludes common shares granted as of September 30, 2015 pursuant to the company’s non-employee director compensation policy. |
| (9) | Includes vested options to purchase 175,000 shares of common stock, warrants to purchase 225,167 shares of common stock and 92,106 shares of common stock issuable upon conversion of 10,000 shares of Series D preferred stock. Excludes unvested options to purchase 71,500 shares of common stock and 115,514 restricted stock units which are subject to vesting requirements. |
| (10) | Mr. Benjamin’s employment as our president and chief executive officer terminated, and he resigned as a member of the board, effective February 18, 2015. The information reported for Mr. Benjamin is based on information available to the company as of his termination date and may not reflect current beneficial ownership. Includes (i) vested options to purchase 636,110 shares of common stock, (ii) warrants to purchase an aggregate of 422,131 shares of common stock and (iii) 253,291 shares of common stock issuable upon conversion of 27,500 shares of Series D preferred stock. Excludes unvested options to purchase 185,307 shares of common stock and 128,843 restricted stock units, which are subject to vesting requirements. See the discussion under the caption “Payments Upon Termination or Change in Control—O’Connell Benjamin” for further information about the terms and conditions applicable to Mr. Benjamin’s |
91
Table of Contents
| unexercised stock options and unvested options and restricted stock units following the termination of his employment. Certain stock options and restricted stock units held by him are shown as unvested in the above table as the company is presently determining Mr. Benjamin’s rights with respect to such securities under the employment agreement we had entered into with him, dated November 25, 2014. In connection with this review process, the company may seek to limit Mr. Benjamin’s entitlement to these benefits. |
Equity Compensation Plan Information
The following table provides information about our common stock that may be issued upon the exercise of options under all of our equity compensation plans as of June 30, 2015 which consisted of the 2011 Plan, the 2010 Employee Stock Option Plan, 2000 Employee Stock Option Plan, as amended, and the 2001 Non-Employee Director Stock Option Plan, as amended. Information concerning each of the aforementioned plans is set forth above.
| Plan Category |
Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights |
Weighted Average Exercise Price of Outstanding Options and Warrants |
Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans Excluding Securities Reflected in Column (A) |
|||||||||
| Equity Compensation Plans Approved by Stockholders |
4,747,000 | (1) | $ | 1.47 | (4) | 2,402,000 | (2) | |||||
| Equity Compensation Plans Not |
||||||||||||
| Approved by Stockholders |
925,000 | (3) | 1.36 | N/A | ||||||||
|
|
|
|
|
|
|
|||||||
| Total |
5,672,000 | $ | 1.45 | 2,402,000 | ||||||||
|
|
|
|
|
|
|
|||||||
| (1) | Includes 80,000 options issued pursuant to our 2001 Director Plan, as amended, 1,009,000 options issued to employees pursuant to our 2000 Plan and 2010 Plan and 3,227,000 options and 1,038,000 restricted stock units issued pursuant to our 2011 Plan, but does not include 71,250 options granted under our 2011 Plan on September 1, 2015. |
| (2) | Reflects the remaining shares available for issuance as of June 30, 2015 pursuant to our 2011 Plan. |
| (3) | See Note 12 to Consolidated Financial Statements included in this Annual Report on Form 10-K for information related to common stock purchase warrants issued to certain consultants. |
| (4) | The calculation of the weighted average exercise price of the outstanding options excludes shares of common stock included in column (a) that are issuable upon the vesting of then outstanding RSUs. |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
Except as disclosed herein, we have not entered into any material transactions or series of similar transactions with any director, executive officer or any security holder owning 5% or more of our common stock since the beginning of our last fiscal year. For information concerning employment and indemnification agreements with, and compensation of, our executive officers and directors, see the disclosure in the section of this Annual Report on Form 10-K captioned “Executive Compensation” and “Summary of Non-Executive Director Compensation”.
As of June 11, 2013, we entered into a securities purchase agreement with certain accredited investors pursuant to which we agreed to issue a total of 665,000 shares of Series D preferred stock and warrants to purchase 6,650,000 shares of common stock. The shares of Series D preferred stock and warrants were sold as units, with each unit consisting of one share of Series D preferred stock and ten warrants. Investors that held an aggregate principal amount of $6,500,000 of senior notes agreed to surrender their notes in consideration of the issuance of the shares of Series D preferred stock and warrants and other investors purchased $150,000 of such securities. At closing, which occurred on June 20, 2013, we received an aggregate of $6,650,000 in cancellation of indebtedness and the $150,000 of additional funds. An aggregate principal amount of $4,850,000 of the senior
92
Table of Contents
notes surrendered in this transaction were held by certain of our officers, directors and our largest stockholder, as follows: an entity affiliated with J. David Luce, a member of our board of directors, and his spouse, held an aggregate principal amount of $2,650,000 of senior notes, and O’Connell Benjamin, our former chief executive officer and a member of our board of directors, and our chief financial officer, William Marshall, each held an aggregate principal amount of $100,000 of senior notes. The parties affiliated with Mr. Luce purchased 265,000 shares of Series D preferred stock and 2,650,000 warrants and each of Mr. Benjamin and Mr. Marshall purchased 10,000 shares of Series D preferred stock and 100,000 warrants. Further, Lazarus Investment Partners LLLP, which was the beneficial owner of approximately 24.9% of our outstanding shares of common stock immediately prior to this transaction, held an aggregate principal amount of $2,000,000 of senior notes. We issued to Lazarus Investment Partners a total of 200,000 shares Series D preferred stock and 2,000,000 warrants. The manager of the general partner of Lazarus Investment Partners, LLLP, is the brother of Dr. Todd A. Borus, a member of our board of directors. In addition, Dr. Todd A. Borus participated in this transaction as an investor and purchased 2,500 shares of Series D preferred stock and 25,000 warrants. As previously reported by the company and as described elsewhere herein, the company has issued the holders of its Series D preferred stock including the related persons noted above, shares of common stock in lieu of the cash payment of dividends on the outstanding shares of Series D preferred stock.
As described in greater detail in Note 25 of Notes to Consolidated Financial Statements, on August 28, 2014, the company entered into a securities purchase agreement with certain accredited and/or institutional investors relating to a registered direct offering by the company for an aggregate of 3,041,454 shares of common stock and warrants to purchase up to an aggregate of 1,003,678 shares of common stock. One of the investors in the offering, Lazarus Investment Partners LLLP, was the beneficial owner of approximately 29.6% of our outstanding shares of common stock immediately prior to the offering, agreed to purchase $500,000 worth of shares of common stock and warrants (704,225 shares and 232,394 warrants). The manager of the general partner of Lazarus Investment Partners, LLLP, is the brother of Dr. Todd A. Borus, a member of our board of directors. Dr. Borus agreed to purchase 33,278 shares of common stock and 10,982 warrants (subscription proceeds of $25,000). Further, Sarah Trent Harris, a family member of Charles C. Lucas, the Chairman of our Board, agreed to purchase 211,268 shares of common stock and 69,718 warrants (subscription proceeds of $150,000). In addition, an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of our board of directors, agreed to purchase 352,113 shares of common stock and 116,197 warrants (subscription proceeds of $250,000). O’Connell Benjamin, our former chief executive officer agreed to purchase 133,111 shares of common stock and 43,927 warrants (subscription proceeds of $100,000) and William A. Marshall, our chief financial officer, agreed to purchase 66,556 shares of common stock and 21,963 warrants (subscription proceeds of $50,000).
On December 10, 2014, we entered into a second Board Nomination Agreement (the “Board Agreement”) with Lazarus Investment Partners, LLLP and certain of its affiliates (the “Lazarus Group”), the beneficial owner of approximately 29.3% of the company’s common stock. Pursuant to the Board Agreement, we granted the Lazarus Group the right to nominate a second individual for election to our Board and agreed to promptly appoint such nominee as a member of the Board. As discussed above in greater detail, the Lazarus Group designated Mr. Marc A. Horowitz and the company agreed to appoint him to its Board of Directors, effective immediately. Further, under the Board Agreement, we agreed to use our best efforts to include Mr. Horowitz in the company’s slate of nominees recommended for election as a director during a three year designation period, as defined under the Board Agreement. The Board Agreement further provides that if a Board vacancy occurs during the designation period solely because of the death, disability, disqualification, resignation or removal of their designee, the Lazarus Group shall be entitled to designate such person’s successor. Mr. Horowitz was subsequently elected to serve as a director by our stockholders at our May 2015 annual meeting.
On February 17, 2015, we issued a short-term promissory note (the “Short Term Note”) in the aggregate principal amount of $950,000 to an accredited investor and also issued this investor warrants to purchase an additional 99,500 shares of common stock. The company received funds in the amount of $950,000 from this investor on the same date. The Short Term Note is an unsecured obligation of the company and is not convertible into equity securities of the company. The Short Term Note was originally due and payable on the first to occur
93
Table of Contents
of the one month anniversary of the issue date or the date on which the company receives at least $950,000 in proceeds from equity or debt financing transactions. Interest shall accrue on the Short Term Note at the rate of 0.48% per month and the Short Term Note contains terms and events of default customary for similar transactions. The warrants issued to the holder of the Short Term Note are exercisable for a period of 54 months commencing on the six month anniversary of the date on which they are issued and will have an initial exercise price of $1.01 per share. The exercise price of these warrants is subject to adjustment in the case of stock splits, stock dividends, combinations of shares and similar recapitalization transactions. On April 3, 2015, we entered into an agreement with the holder of the Short Term Note to extend the maturity date of the Short Term Note from March 19, 2015 to July 2, 2015. In addition, pursuant to the amendment agreement, we granted the holder the right to exchange the principal amount of the Short Term Note (and unpaid interest thereon) into the securities that we issue in the next financing, as defined in the amendment agreement. This investor subsequently agreed not to participate in the convertible debt financing for which a closing was held June 8, 2015 and in consideration of such election, the participation right was modified to allow him to exchange such note for comparable securities in an alternative transaction. In consideration of waivers previously granted by the holder of potential events of default under the Short Term Note and the extension of its maturity date, we agreed to issue the holder warrants to purchase an aggregate of 3,166,667 shares of our common stock exercisable for a period of 54 months commencing six months following the date of issuance, at an exercise price equal to $0.31 per share. The exercise price of these warrants is subject to adjustment in the case of stock splits, stock dividends, combinations of shares and similar recapitalization transactions. We have subsequently further extended the maturity date of the Short Term Note on multiple occasions, and presently, the Short Term Note has a maturity date of October 16, 2015. The holder of the Short Term Note is an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of our board of directors.
On April 24, 2015, we issued a short-term promissory note in the aggregate principal amount of $500,000 to Lazarus Investment Partners LLLP, the beneficial owner of approximately 29.4% of our common stock immediately prior to the note transaction, for gross proceeds of $500,000. In consideration for the short-term note transaction, we agreed to reduce the exercise price on approximately 6,233,600 warrants held by Lazarus to $0.25 based on the most recent closing bid price of the common stock prior to the note transaction, and to extend the expiration date of the warrants to October 25, 2019. The short-term note is an unsecured obligation of the company, is not convertible into equity securities of the company and accrues interest at a rate of 5.76% per annum. The short-term note was originally due and payable on the first to occur of July 2, 2015, or the date on which the company receives at least $900,000 in cash proceeds from an equity or debt financing. However, we have subsequently further extended the maturity date of the short term note on multiple occasions, and presently, the it has a maturity date of October 16, 2015. The short-term note contains covenants and events of default customary for similar transactions. The net proceeds from this transaction were approximately $500,000. The warrants, as amended, are exercisable for a period of approximately 54 months and have an initial exercise price of $0.25 per share.
On August 7, 2015, we issued a senior secured promissory note (the “Senior Note”) in the aggregate principal amount of $320,000 to MKA 79, LLC (the “Purchaser”), in a private transaction. The Senior Note is due and payable on December 31, 2015 and interest shall accrue on the Senior Note at the rate of 10.0% per annum. The Senior Note is not convertible into equity securities of the company and it contains terms and events of default customary for similar transactions. The Senior Note is secured by a first priority lien on certain of our assets, as described in a security agreement entered into between the company and the Purchaser dated as of August 7, 2015. In consideration of the loan, the company and the Purchaser entered into a Warrant Amendment Agreement pursuant to which the company agreed to amend certain of the terms of the existing 5,474,829 Common Stock Purchase Warrants (the “Warrants”) currently held by the Purchaser and an affiliate of the Purchaser. In amending the Warrants, the company agreed to reduce the exercise price of such Warrants to $0.17 and to extend the expiration date of the Warrants to December 13, 2019. The Warrants, as amended by the Warrant Amendment Agreement were issued in various transactions on or about March 14, 2012, September 28, 2012, and June 20, 2013 at exercise prices ranging between $0.95 and $1.34. The Purchaser is an entity affiliated with J. David Luce, a member of the company’s board of directors. The company is using the net proceeds from the transaction for general business and working capital purposes.
94
Table of Contents
Effective August 26, 2015, we received loan proceeds in the aggregate amount of $400,000 from two separate lenders, MKA 79 LLC and Lazarus Investment Partners LLLP. As evidence of the loans, we issued a promissory note in the principal amount of $200,000 to each of the lenders. The loans bear interest at 20% per annum, payable in arrears, and are due upon the earlier of (i) August 26, 2016 or (ii) within 30 days of the closing of the contemplated acquisition, merger or similar transaction with (A) Peachstate Health Management, LLC (d/b/a AEON Clinical Laboratories) as described in the company’s Current Report on Form 8-K, as filed with the Securities and Exchange Commission on August 25, 2015, or a similar alternative acquisition, merger or similar transaction with an unaffiliated third party or (iii) the closing of a sale of equity or debt securities of the company, or series of closings, as part of the same transaction, of equity or debt securities within a period of 90 days, in the gross amount of at least $5,000,000 in cash proceeds. The notes are neither secured by any assets nor convertible into equity securities of the company. Further, the lenders have the right, at their option, to convert interest and principal due on the note into any alternative financing that may be undertaken by the company while the notes are outstanding. MKA 79 LLC is an entity affiliated with J. David Luce, who is a member of the board of directors of the company. Lazarus Investment Partners LLP is the beneficial owner of approximately 29.3% of the company’s common stock.
Approval for Related Party Transactions
Although we have not adopted a formal policy relating to the approval of proposed transactions that we may enter into with any of our executive officers, directors and principal stockholders, including their immediate family members and affiliates, our Audit Committee, all of the members of which, are independent, reviews the terms of proposed material related party transactions. The results of this review are then communicated to the entire board of directors, which has the ultimate authority as to whether or not we enter into such transactions. In approving or rejecting the proposed related party transaction, our Audit Committee and our board of directors shall consider the relevant facts and circumstances available and deemed relevant to them, including, but not limited to the risks, costs and benefits to us, the terms of the transaction, the availability of other sources for comparable services or products, and, if applicable, the impact on a director’s independence. We shall approve only those agreements that, in light of known circumstances, are in, or are not inconsistent with, our best interests, as our Audit Committee and our board of directors determine in the good faith exercise of their discretion.
Independence of our Board of Directors and its Committees
The listing rules established by the Nasdaq Stock Market, LLC require that a majority of the members of a listed company’s board of directors qualify as “independent” as affirmatively determined by the board, meaning that each independent director has no direct or indirect material relationship with a company other than as a director and/or a stockholder. Our board of directors consults with legal counsel to ensure that our board’s determination with respect to the definition of “independent” is consistent with current Nasdaq listing rules. Our board of directors reviewed all relevant transactions or relationships between each director, or any of his family members, and our company and has affirmatively determined that each of our directors, other than J. David Luce, Ian C. Bonnet and William P. Henry, are independent directors under the applicable guidelines noted above. However, as Mr. Henry is serving currently as our interim chief strategy officer, it is our intention that following the expiration of his service in such capacity, he will be considered as an independent member of our board of directors. In considering their independence, our board considered each of the relationships and transactions involving our directors described above under the caption “Certain Relationships and Related Transactions and Director Independence”. Our board of directors currently has four active committees: the Audit Committee, the Management Resources and Compensation Committee, the Nominating and Corporate Governance Committee and the Strategy and Risk Analysis Committee. All of the members of our Audit Committee meet the standards for independence required under current Nasdaq Stock Market listing rules, SEC rules, and applicable securities laws and regulations. All of the members of our Management Resources and Compensation Committee and our Nominating and Corporate Governance Committee satisfy the applicable independence standards.
95
Table of Contents
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
The board of directors of Authentidate has selected EisnerAmper LLP as its independent registered public accounting firm for the current fiscal year. During the 2015 fiscal year, the audit services provided by EisnerAmper LLP consisted of an audit of the financial statements and services related to filings with the SEC. The following table presents the total fees billed for professional audit and non-audit services rendered by our independent registered public accounting firm for the years ended June 30, 2015 and 2014, and fees billed for other services rendered by our independent registered public accounting firm during those periods.
| Year Ended June 30, | ||||||||
| 2015 | 2014 | |||||||
| Audit Fees (1) |
$ | 178,000 | $ | 176,000 | ||||
|
|
|
|
|
|||||
| Total |
$ | 178,000 | $ | 176,000 | ||||
|
|
|
|
|
|||||
| (1) | Audit services consist of audit work performed on financial statements, audit work performed on internal control over financial reporting, reviews of Annual Reports on Form 10-K, reviews of financial statements and related Quarterly Reports on Form 10-Q during the fiscal year, as well as work that generally only the independent registered public accounting firm can reasonably be expected to provide, including consents for registration statement filings and responding to SEC comment letters on annual and quarterly filings. During the fiscal years ended June 30, 2015 and 2014, all reported amounts were for services provided by EisnerAmper LLP. |
Our Audit Committee has determined that the services provided by our independent registered public accounting firms and the fees we expensed for such services has not compromised the independence of our independent auditors.
Consistent with SEC policies regarding auditor independence, the Audit Committee has responsibility for appointing, setting compensation and overseeing the work of the independent auditor. In recognition of this responsibility, the Audit Committee has established a policy to pre-approve all audit and permissible non-audit services provided by the independent registered public accounting firm. Prior to engagement of the independent auditor for the next year’s audit, management will submit a detailed description of the audit and permissible non-audit services expected to be rendered during that year for each of four categories of services described above to the Audit Committee for approval. In addition, management will also provide to the Audit Committee for its approval a fee proposal for the services proposed to be rendered by the independent auditor. Prior to the engagement of the independent auditor, the Audit Committee will approve both the description of audit and permissible non-audit services proposed to be rendered by the independent auditor and the budget for all such services. The fees are budgeted and the Audit Committee requires the independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service.
During the year, circumstances may arise when it may become necessary to engage the independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, the Audit Committee requires separate pre-approval before engaging the independent registered public accounting firm. To ensure prompt handling of unexpected matters, the Audit Committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report, for informational purposes only, any pre-approval decisions to the Audit Committee at its next scheduled meeting. The four categories of services provided by the independent registered public accounting firm are as defined in the footnotes to the fee table set forth above.
Each of the permitted non-audit services has been pre-approved by the Audit Committee or the Audit Committee’s Chairman pursuant to delegated authority by the Audit Committee. The Audit Committee has not authorized our independent registered public accounting firm to provide any additional non-audit services.
96
Table of Contents
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements
The following Financial Statements of AHC are set forth below:
| • | Report of Independent Registered Public Accounting Firm; |
| • | Consolidated Balance Sheets as of June 30, 2015 and 2014; |
| • | Consolidated Statements of Operations and Comprehensive Operations for the years ended June 30, 2015, 2014 and 2013; |
| • | Consolidated Statements of Shareholders’ (Deficit) Equity for the years ended June 30, 2015, 2014, and 2013; |
| • | Consolidated Statements of Cash Flows for the years ended June 30, 2015, 2014, and 2013; and |
| • | Notes to Consolidated Financial Statements |
(a)(2) Financial Statement Schedules
There are no schedules required for any of the years in the three year period ended June 30, 2015 pursuant to item 15 (d)
(a)(3) Exhibits
The exhibits required by Item 15 (a) (3) are set forth in the Exhibit Index
97
Table of Contents
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| AUTHENTIDATE HOLDING CORP. | ||
| By: | /s/ IAN C. BONNET | |
| Ian C. Bonnet | ||
| Chief Executive Officer and President | ||
Dated: October 13, 2015
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| Signature |
Capacity |
Date | ||
| /s/ CHARLES C. LUCAS III Charles C. Lucas III |
Chairman of the Board |
October 13, 2015 | ||
| /s/ IAN C. BONNET Ian C. Bonnet |
Chief Executive Officer, President |
October 13, 2015 | ||
| /s/ WILLIAM P. HENRY William P. Henry |
Interim Chief Strategy Officer and Director |
October 13, 2015 | ||
| /s/ ROY E. BEAUCHAMP Roy E. Beauchamp |
Director |
October 13, 2015 | ||
| /s/ TODD A. BORUS, M.D. Todd A. Borus, M.D. |
Director |
October 13, 2015 | ||
| /s/ MARC A. HOROWITZ Marc A. Horowitz |
Director |
October 13, 2015 | ||
| /s/ J. DAVID LUCE J. David Luce |
Director |
October 13, 2015 | ||
| /s/ WILLIAM A. MARSHALL William A. Marshall |
Chief Financial Officer and Principal Accounting Officer |
October 13, 2015 | ||
98
Table of Contents
EXHIBIT INDEX
The exhibits designated with an asterisk (*) are filed herewith. All other exhibits have been previously filed with the Commission and, pursuant to 17 C.F.R. ss. 230.411, are incorporated by reference to the document referenced in brackets following the descriptions of such exhibits. A management contract or compensation plan or arrangement is indicated with (§§). Certain portions of exhibits marked with the symbol (++) have been granted confidential treatment by the Securities and Exchange Commission. Such portions were omitted and filed separately with the Securities and Exchange Commission.
| Item No. |
Description | |
| 2.1 | Share Purchase Agreement by and among Authentidate Holding Corp. and Exceet Group, dated March 9, 2011 (filed as exhibit 2.1 to Current Report on Form 8-K filed on April 7, 2011). | |
| 2.2 | Joint Venture Termination Agreement dated November 21, 2011 (filed as Exhibit 2.1 to Current Report on Form 8-K filed on November 28, 2011). | |
| 3.1 | Certificate of Incorporation (Exhibit 3.3.1 to Registration Statement on Form S-18, File No. 33-46246-NY). | |
| 3.1.1 | Certificate of Amendment to Certificate of Incorporation (filed as Exhibit 3 to Definitive Proxy Statement dated February 16, 2001 as filed with the Securities and Exchange Commission). | |
| 3.1.2 | Certificate of Amendment to Certificate of Incorporation (filed as Exhibit C to Definitive Proxy Statement dated December 31, 2003 as filed with the Securities and Exchange Commission). | |
| 3.1.3 | Certificate of Amendment to Certificate of Incorporation (filed as Exhibit 3.1 to Current Report on Form 8-K as filed with the Securities and Exchange Commission on December 23, 2011). | |
| 3.1.4 | Certificate of Amendment to Certificate of Incorporation (filed as Exhibit 3.1 to Current Report on Form 8-K as filed with the Securities and Exchange Commission on August 30, 2012). | |
| 3.1.5 | Certificate of Amendment to Certificate of Incorporation (filed as Exhibit 3.1 to Current Report on Form 8-K as filed with the Securities and Exchange Commission on July 7, 2015). | |
| 3.2 | Certificate of Designation of Series B Preferred Stock (Exhibit 3.2.1 to Form 10-KSB dated October 4, 1999). | |
| 3.2.1 | Certificate of Amendment of Certificate of Designations, Preferences and Rights and Number of Shares of Series B Convertible Preferred Stock (filed as Exhibit 3.1 to Form 10-Q for the quarter ended December 31, 2002). | |
| 3.3 | Certificate of Designations, Preferences and Rights and Number of Shares of Series D Convertible Preferred Stock (filed as Exhibit 3.1 to Current Report filed on June 12, 2013). | |
| 3.4 | By-Laws, as amended (filed as Exhibit 3.2.1 to Form 10-Q for the quarter ended March 31, 2004). | |
| 3.4.1 | Amendment to By-laws (filed as Exhibit 3.1 to Current Report on Form 8-K, dated November 15, 2007). | |
| 4.1 | Form of Common Stock Certificate (filed as Exhibit 4.1 to Registration Statement on Form S-18, File No. 33-46246-NY). | |
| 4.2 | Form of Series B Preferred Stock Certificates (Exhibit 4.5 to the Registration Statement on form SB-2, File No. 33-76494). | |
| 4.3 | Form of Warrant granted to consultant (filed as Exhibit 4.6 to Annual Report on Form 10-K for the fiscal year ended June 30, 2010). | |
| 4.4 | Form of Warrant issued in private placement from October 2010 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on October 14, 2010). | |
99
Table of Contents
| Item No. |
Description | |
| 4.5 | Form of Warrant (filed as Exhibit 4.1 to Current Report on Form 8-K dated October 12, 2011). | |
| 4.6 | Form of Warrants issued March 14, 2012 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on March 14, 2012). | |
| 4.7 | Form of Warrants issued April 10, 2012 (filed as Annex C to Definitive Proxy Statement dated March 13, 2012). | |
| 4.8 | Form of Warrants issued September 28, 2012 (filed as Exhibit 4.2 to Current Report on Form 8-K filed on September 28, 2012). | |
| 4.9 | Form of Extension Warrants issued September 28, 2012 (filed as Exhibit 4.3 to Current Report on Form 8-K filed on September 28, 2012). | |
| 4.10 | Warrant issued as of December 1, 2012 (filed as Exhibit 4.1 to Quarterly Report on Form 10-Q for the quarter ended December 31, 2012). | |
| 4.11 | Form of Warrant Agreement between Authentidate Holding Corp. and Continental Stock Transfer Company, including form of certificate of warrants issuable to investors pursuant to Underwriting Agreement (filed as Exhibit 4.1 to Current Report on Form 8-K filed on June 12, 2013). | |
| 4.12 | Specimen of Series D Convertible Preferred Stock Certificate (filed as Exhibit 4.2 to Current Report on Form 8-K filed on June 12, 2013). | |
| 4.13 | Form of Warrant issuable pursuant to Securities Purchase Agreement dated June 11, 2013 (filed as Exhibit 4.3 to Current Report on Form 8-K filed on June 12, 2013). | |
| 4.14 | Form of Warrant issued to consultant dated as of September 19, 2013 (filed as Exhibit 4.2 to Quarterly Report on Form 10-Q filed on February 14, 2014). | |
| 4.15 | Form of Warrant issued to consultant dated as of September 19, 2013 (filed as Exhibit 4.3 to Quarterly Report on Form 10-Q filed on February 14, 2014). | |
| 4.16 | Form of Warrant issuable to Investors dated as of November 11, 2013 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on November 13, 2013). | |
| 4.17 | Form of Warrant issued to consultant dated as of December 5, 2013 (filed as Exhibit 4.3 to Quarterly Report on Form 10-Q filed on February 14, 2014). | |
| 4.18 | Form of Warrant issued pursuant to Securities Purchase Agreement dated as of August 28, 2014 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on September 2, 2014). | |
| 4.19 | Form of Warrant issued to consultant dated as of September 16, 2014 (filed as Exhibit 4.22 to Annual Report on Form 10-K for the fiscal year ended June 30, 2014). | |
| 4.20 | Note issued to VER 83, LLC dated February 17, 2015 (filed as Exhibit 4.3 to Current Report on Form 8-K filed on February 23, 2015). | |
| 4.21 | Warrant issued to VER 83, LLC dated February 17, 2015 (filed as Exhibit 4.4 to Current Report on Form 8-K filed on February 23, 2015). | |
| 4.22 | Note Extension Agreement dated April 3, 2015 between Authentidate Holding Corp. and VER 83, LLC (filed as Exhibit 10.1 to Current Report on Form 8-K filed on April 9, 2015). | |
| 4.23 | Form of Warrant Issued April 3, 2015 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on April 9, 2015). | |
| 4.24 | Form of Note issued to Lazarus Investment Partners, dated April 24, 2015 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on April 28, 2015). | |
100
Table of Contents
| Item No. |
Description | |
| 4.25 | Warrant Amendment Agreement dated April 24, 2015 between Authentidate Holding Corp. and Lazarus Investment Partners, LLLP (filed as Exhibit 10.1 to Current Report on Form 8-K filed on April 28, 2015). | |
| 4.26§§ | Form of Restricted Stock Unit Grant to Ian C. Bonnet dated February 18, 2015 (filed as Exhibit 10.2 to Quarterly Report on Form 10-Q for the fiscal quarter ended March 31, 2015). | |
| 4.27* | Form of Convertible Senior Secured Debenture issued June 8, 2015 | |
| 4.28* | Form of Warrant issued June 8, 2015. | |
| 4.29 | Form of Promissory Note issued to MKA 79, LLC dated August 7, 2015 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on August 12, 2015). | |
| 4.30 | Warrant Amendment Agreement dated August 7, 2015 between Authentidate Holding Corp. and MKA 79, LLC (filed as Exhibit 10.2 to Current Report on Form 8-K filed on August 12, 2015). | |
| 4.31 | Form of Note issued August 26, 2015 (filed as Exhibit 10.1 to Current Report on Form 8-K filed on August 28, 2015). | |
| 4.32* | Form of Warrant issued to Consultant as of July 1, 2015 | |
| 4.33 | Form of Note issued September 18, 2015 (filed as Exhibit 4.1 to Current Report on Form 8-K filed on September 24, 2015). | |
| 4.34 | Form of Warrant issued September 18, 2015 (filed as Exhibit 4.2 to Current Report on Form 8-K filed on September 24, 2015). | |
| 10.1§§ | 2000 Employee Stock Option Plan, as amended (filed as Exhibit B to Definitive Proxy Statement dated December 31, 2003 as filed with the Securities and Exchange Commission). | |
| 10.2§§ | Form of Stock Option Award Pursuant to 2000 Employee Stock Option Plan, as amended (filed as Exhibit 10.30.1 to Annual Report on Form 10-K for the fiscal year ended June 30, 2004). | |
| 10.3§§ | Form of Stock Option Award Pursuant to 2001 Non-Executive Director Stock Option Plan, as amended (filed as Exhibit 10.31.1 to Annual Report on Form 10-K for the fiscal year ended June 30, 2004). | |
| 10.4 | Lease Agreement dated as of July 5, 2005 between Authentidate Holding Corp. and The Connell Company (filed as Exhibit 10.1 to Current Report on Form 8-K dated July 11, 2005). | |
| 10.5§§ | Employment Agreement between William A. Marshall and Authentidate Holding Corp. (filed as Exhibit 10.1 to Current Report on Form 8-K dated February 15, 2006). | |
| 10.6§§ | Compensation Modification Agreement with O’Connell Benjamin (filed as Exhibit 10.1 to Current Report on Form 8-K dated February 22, 2010). | |
| 10.7§§ | Compensation Modification Agreement with William Marshall (filed as Exhibit 10.2 to Current Report on Form 8-K dated February 22, 2010). | |
| 10.8§§ | 2010 Employee Stock Option Plan (filed as Exhibit A to definitive Proxy Statement dated April 14, 2010). | |
| 10.9§§ | Form of Stock Option Award Pursuant to 2010 Employee Stock Option Plan (filed as Exhibit 10.22 to Annual Report on Form 10-K for the fiscal year ended June 30, 2010). | |
| 10.10§§ | 2001 Non-Executive Director Stock Option Plan, as amended (filed as Exhibit 10.2 to Current Report on Form 8-K dated May 25, 2010). | |
| 10.11 | Form of Securities Purchase Agreement dated October 12, 2010 by and among Authentidate Holding Corp. and the Investors named therein (filed as Exhibit 10.1 to Current Report on Form 8-K filed on October 14, 2010). | |
101
Table of Contents
| Item No. |
Description | |
| 10.12 | Form of Registration Rights Agreement dated October 12, 2010 by and among Authentidate Holding Corp. and the Investors named therein (filed as Exhibit 10.2 to Current Report on Form 8-K filed on October 14, 2010). | |
| 10.13§§ | Compensation Modification Agreement with O’Connell Benjamin (filed as Exhibit 10.5 to Quarterly Report on Form 10-Q for the quarter ended December 31, 2010). | |
| 10.14§§ | Compensation Modification Agreement with William Marshall (filed as Exhibit 10.6 to Quarterly Report on Form 10-Q for the quarter ended December 31, 2010). | |
| 10.15§§ | 2011 Omnibus Equity Incentive Plan (filed as Appendix A to the definitive proxy statement dated July 27, 2011). | |
| 10.16§§ | Form of Incentive Stock Option Grant Agreement under the 2011 Omnibus Equity Incentive Plan (filed as Exhibit 10.32 to the Annual Report on Form 10-K for the year ended June 30, 2011). | |
| 10.17§§ | Form of Non-Statutory Stock Option Grant Agreement under the 2011 Omnibus Equity Incentive Plan (filed as Exhibit 10.33 to the Annual Report on Form 10-K for the year ended June 30, 2011). | |
| 10.18++ | Intellectual Property License and Supply Agreement dated November 21, 2011 (filed as Exhibit 10.1 to Current Report on Form 8-K filed on November 28, 2011). | |
| 10.19 | Registration Rights Agreement dated November 21, 2011 (filed as Exhibit 10.2 to Current Report on Form 8- filed on November 28, 2011). | |
| 10.20§§ | Compensation Modification Agreement dated June 21, 2012 with O’Connell Benjamin (filed as Exhibit 10.1 to Current Report on Form 8-K filed on June 27, 2012). | |
| 10.21§§ | Compensation Modification Agreement dated June 21, 2012 with William A. Marshall (filed as Exhibit 10.2 to Current Report on Form 8-K filed on June 27, 2012. | |
| 10.22 | Form of Indemnification Agreement (filed as Exhibit 10.1 to Current Report on Form 8-K filed on September 12, 2012). | |
| 10.23 | Board Nominating and Observer Agreement between the Company and Lazarus Investment Partners, LLLP (filed as Exhibit 10.4 to Current Report on Form 8-K filed on September 28, 2012). | |
| 10.24§§ | Compensation Modification Agreement with O’Connell Benjamin (filed as Exhibit 10.1 to Current Report on Form 8-K filed on January 17, 2013). | |
| 10.25§§ | Compensation Modification Agreement with William A. Marshall (filed as Exhibit 10.2 to Current Report on Form 8-K filed on January 17, 2013). | |
| 10.26§§ | Amendment to Employment Agreement with William A. Marshall (filed as Exhibit 10.4 to Current Report on Form 8-K filed on January 17, 2013). | |
| 10.27§§ | Form of Restricted Stock Unit Agreement (filed as Exhibit 10.5 to Current Report on Form 8-K filed on January 17, 2013). | |
| 10.28 | Form of Amendment Agreement dated March 22, 2013 (filed as Exhibit 10.1 to Current Report on Form 8-K filed on March 27, 2013). | |
| 10.29 | Amendment to Board Nomination and Observer Agreement dated March 22, 2013 (filed as Exhibit 10.2 to Current Report on Form 8-K filed on March 27, 2013). | |
| 10.30 | Form of Registration Rights Agreement dated as of June 11, 2013(filed as Exhibit 10.3 to Current Report on Form 8-K filed on June 12, 2013). | |
| 10.31 | Amendment No. 2 to Board Nomination and Observer Agreement dated as of June 11, 2013 (filed as Exhibit 10.1 to Current Report on Form 8-K filed on June 12, 2013). | |
102
Table of Contents
| Item No. |
Description | |
| 10.32 | Amendment No. 3 to Board Nomination and Observer Agreement (filed as Exhibit 10.45 to Annual Report on Form 10-K filed on September 26, 2013). | |
| 10.33§§ | Form of Stock Option Agreement between Authentidate Holding Corp. and O’Connell Benjamin dated September 30, 2013 (filed as Exhibit 10.3 to Quarterly Report on Form 10-Q filed November 14, 2013). | |
| 10.34§§ | Form of Performance-based Stock Option Agreement between Authentidate Holding Corp. and O’Connell Benjamin dated September 30, 2013 (filed as Exhibit 10.4 to Quarterly Report on Form 10-Q filed November 14, 2013). | |
| 10.35 | Form of Registration Rights Agreement dated as of November 11, 2013 (filed as Exhibit 10.2 to Current Report on Form 8-K filed November 11, 2013). | |
| 10.36§§ | Compensation Modification Agreement with O’Connell Benjamin dated January 28, 2014 (filed as Exhibit 10.1 to Current Report on Form 8-K filed January 30, 2014). | |
| 10.37§§ | Compensation Modification Agreement with William A. Marshall dated January 28, 2014 (filed as Exhibit 10.2 to Current Report on Form 8-K filed January 30, 2014). | |
| 10.38§§ | Form of Restricted Stock Unit Agreement granted January 28, 2014 (filed as Exhibit 10.3 to Current Report on Form 8-K filed January 30, 2014). | |
| 10.39§§ | 2011 Omnibus Equity Incentive Plan, as amended (filed as Annex A to the definitive Proxy Statement dated March 21, 2014). | |
| 10.40 | Form of Securities Purchase Agreement dated as of August 28, 2014 (filed as Exhibit 10.1 to Current Report on Form 8-K filed September 2, 2014). | |
| 10.41 | Board Nomination Agreement with Lazarus Investment Partners, LLLP dated December 10, 2014 (filed as Exhibit 10.1 to Current Report on Form 8-K filed December 15, 2014). | |
| 10.42§§ | Employment Agreement with Ian C. Bonnet dated February 18, 2015 (filed as Exhibit 10.1 to Current Report on Form 8-K filed February 18, 2015). | |
| 10.43* | Securities Purchase Agreement dated May 29, 2015. | |
| 10.44* | Registration Rights Agreement dated May 29, 2015. | |
| 10.45* | Security Agreement dated May 29, 2015. | |
| 10.46* | Amendment to Securities Purchase Agreement and Registration Agreement dated May 29, 2015, entered into on July 30, 2015. | |
| 10.47 | Note Extension Agreement with Lazarus Investment Partners, LLLP dated July 2, 2015 (filed as Exhibit 10.1 to Current Report on Form 8-K filed July 7, 2015). | |
| 10.48 | Note Extension Agreement with VER 83, LLC dated July 2, 2015 (filed as Exhibit 10.2 to Current Report on Form 8-K filed July 7, 2015). | |
| 10.49§§* | Employment Agreement with William P. Henry. | |
| 10.50 | Security Agreement between the Company and MKA79, LLC dated August 7, 2015 (filed as Exhibit 10.1 to Current Report on Form 8-K filed August 12, 2015). | |
| 10.51§§* | Amendment to Employment Agreement with Ian C. Bonnet dated as of August 18, 2015. | |
| 10.52* | Note Extension Agreement with VER 83, LLC dated September 25, 2015. | |
| 10.53* | Note Extension Agreement with Lazarus Investment Partners, LLLP dated September 25, 2015. | |
103
Table of Contents
| Item No. |
Description | |
| 10.54 | Form of Purchase Agreement dated September 15, 2015 (filed as Exhibit 10.1 to Current Report on Form 8-K filed September 24, 2015). | |
| 10.55§§ | Second Amendment to Employment Agreement with Ian C. Bonnet (filed as Exhibit 10.2 to Current Report on Form 8-K filed September 24, 2015). | |
| 10.56* | Amendment to Lease dated as of September 15, 2015. | |
| 10.57§§ | Employment Letter with Ian C. Bonnet dated September 28, 2015 (filed as Exhibit 10.1 to Current Report on Form 8-K filed September 30, 2015). | |
| 14 | Code of Ethics (Exhibit 14 to Annual Report on Form 10-K for the fiscal year ended June 30, 2003). | |
| 21* | Subsidiaries of Registrant | |
| 23.1* | Consent of EisnerAmper LLP | |
| 31.1* | Certification of Chief Executive Officer | |
| 31.2* | Certification of Chief Financial Officer | |
| 32* | Section 1350 Certification of Chief Executive Officer and Chief Financial Officer | |
| 101.1* | The following financial information from the Authentidate Holding Corp.’s Annual Report on Form 10-K for the fiscal year ended June 30, 2015, formatted in XBRL (eXtensible Business Reporting Language) and furnished electronically herewith: (i) the Consolidated Balance Sheets; (ii) the Consolidated Statements of Operations and Comprehensive Operations; (iii) the Consolidated Statements of Shareholders’ Equity; (iv) the Consolidated Statements of Cash Flows; and, (v) the Notes to Consolidated Financial Statements. | |
104
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Index to Consolidated Financial Statements
June 30, 2015, 2014 and 2013
| Page | ||||
| F-2 | ||||
| Consolidated Financial Statements |
||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Shareholders
Authentidate Holding Corp.
We have audited the accompanying consolidated balance sheets of Authentidate Holding Corp. and subsidiaries (the “Company”) as of June 30, 2015 and 2014, and the related consolidated statements of operations and comprehensive operations, shareholders’ (deficit) equity and cash flows for each of the years in the three-year period ended June 30, 2015. The financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Authentidate Holding Corp. and subsidiaries as of June 30, 2015 and 2014, and the consolidated results of their operations and their cash flows for each of the years in the three-year period ended June 30, 2015, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company’s recurring losses from operations and negative cash flows from operations raise substantial doubt about its ability to continue as a going concern. Management’s plans considering these matters are described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ EisnerAmper LLP
New York, New York
October 13, 2015
F-2
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Consolidated Balance Sheets
| June 30, | ||||||||
| (in thousands, except per share data) |
2015 | 2014 | ||||||
| Assets |
||||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 247 | $ | 1,084 | ||||
| Restricted cash |
256 | 256 | ||||||
| Marketable securities |
— | 210 | ||||||
| Accounts receivable, net |
274 | 508 | ||||||
| Inventory, net |
603 | 2,937 | ||||||
| Prepaid expenses and other current assets |
222 | 259 | ||||||
|
|
|
|
|
|||||
| Total current assets |
1,602 | 5,254 | ||||||
| Property and equipment, net |
301 | 448 | ||||||
| Other assets |
||||||||
| Licenses, net |
1,904 | 1,933 | ||||||
| Other assets, net |
356 | 593 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 4,163 | $ | 8,228 | ||||
|
|
|
|
|
|||||
| Liabilities and Shareholders’ (Deficit) Equity |
||||||||
| Current liabilities |
||||||||
| Accounts payable, accrued expenses and other liabilities |
$ | 2,109 | $ | 2,806 | ||||
| Notes payable, net of unamortized discount |
2,150 | — | ||||||
| Warrant liability |
214 | — | ||||||
| Deferred revenue |
67 | 78 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
4,540 | 2,884 | ||||||
| Long-term deferred revenue |
88 | 126 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
4,628 | 3,010 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies (Note 13) |
||||||||
| Shareholders’ (deficit) equity |
||||||||
| Preferred stock, $.10 par value; 5,000 shares authorized, Series B, 28 shares and Series D, 665 shares issued and outstanding on June 30, 2015 and 2014, respectively |
69 | 69 | ||||||
| Common stock, $.001 par value; 190,000 shares authorized, 42,116 and 38,511 shares issued and outstanding on June 30, 2015 and 2014, respectively |
42 | 39 | ||||||
| Additional paid-in capital |
205,909 | 201,492 | ||||||
| Accumulated deficit |
(206,485 | ) | (196,382 | ) | ||||
|
|
|
|
|
|||||
| Total shareholders’ (deficit) equity |
(465 | ) | 5,218 | |||||
|
|
|
|
|
|||||
| Total liabilities and shareholders’ (deficit) equity |
$ | 4,163 | $ | 8,228 | ||||
|
|
|
|
|
|||||
The accompanying notes are an integral part of the consolidated financial statements.
F-3
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Consolidated Statements of Operations and Comprehensive Operations
| Year Ended June 30, | ||||||||||||
| (in thousands, except per share data) |
2015 | 2014 | 2013 | |||||||||
| Revenues |
||||||||||||
| Hosted software services |
$ | 1,928 | $ | 2,239 | $ | 2,706 | ||||||
| Telehealth products and services |
1,761 | 3,317 | 2,121 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenues |
3,689 | 5,556 | 4,827 | |||||||||
| Operating expenses |
||||||||||||
| Cost of revenues |
1,877 | 3,759 | 3,454 | |||||||||
| Selling, general and administrative |
8,379 | 7,040 | 6,816 | |||||||||
| Product development |
1,180 | 1,108 | 1,085 | |||||||||
| Depreciation and amortization |
959 | 766 | 843 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
12,395 | 12,673 | 12,198 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(8,706 | ) | (7,117 | ) | (7,371 | ) | ||||||
| Other (expense) income, net |
(994 | ) | (26 | ) | (3,978 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (9,700 | ) | $ | (7,143 | ) | $ | (11,349 | ) | |||
|
|
|
|
|
|
|
|||||||
| Basic and diluted loss per common share |
$ | (0.25 | ) | $ | (0.26 | ) | $ | (0.45 | ) | |||
|
|
|
|
|
|
|
|||||||
| Comprehensive operations |
||||||||||||
| Net loss |
$ | (9,700 | ) | $ | (7,143 | ) | $ | (11,349 | ) | |||
|
|
|
|
|
|
|
|||||||
| Comprehensive loss |
$ | (9,700 | ) | $ | (7,143 | ) | $ | (11,349 | ) | |||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-4
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Consolidated Statements of Shareholders’ (Deficit) Equity
| (in thousands) |
Number of Shares |
Preferred Stock |
Number of Shares |
Common Stock |
Additional Paid-in Capital |
Accumulated Deficit |
Total Shareholders’ (Deficit)/Equity |
|||||||||||||||||||||
| Balance, June 30, 2012 |
— | $ | — | 26,999 | $ | 27 | $ | 179,890 | $ | (177,104 | ) | $ | 2,813 | |||||||||||||||
| Preferred stock dividends |
(384 | ) | (384 | ) | ||||||||||||||||||||||||
| Share-based compensation expense |
305 | 305 | ||||||||||||||||||||||||||
| Restricted shares and stock options issued for services |
104 | 217 | 217 | |||||||||||||||||||||||||
| Warrants issued with secured debt |
2,895 | 2,895 | ||||||||||||||||||||||||||
| Warrants issued for services |
180 | 180 | ||||||||||||||||||||||||||
| Cost for shares issued for business acquisition |
(6 | ) | (6 | ) | ||||||||||||||||||||||||
| Reclass Series B preferred stock |
28 | 3 | 697 | 700 | ||||||||||||||||||||||||
| Conversion of Series C preferred stock |
3,552 | 4 | 2,838 | 2,842 | ||||||||||||||||||||||||
| Issuance of common stock, net |
4,684 | 4 | 3,930 | 3,934 | ||||||||||||||||||||||||
| Issuance of Series D preferred stock, net |
665 | 66 | 6,463 | 6,529 | ||||||||||||||||||||||||
| Net loss |
(11,349 | ) | (11,349 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, June 30, 2013 |
693 | $ | 69 | 35,339 | $ | 35 | $ | 197,409 | $ | (188,837 | ) | $ | 8,676 | |||||||||||||||
| Preferred stock dividends |
(402 | ) | (402 | ) | ||||||||||||||||||||||||
| Restricted stock issued for preferred stock dividends |
275 | 1 | 342 | 343 | ||||||||||||||||||||||||
| Share-based compensation expense |
587 | 587 | ||||||||||||||||||||||||||
| Issuance of common stock, net |
2,348 | 3 | 2,393 | 2,396 | ||||||||||||||||||||||||
| Exercise of warrants |
483 | 459 | 459 | |||||||||||||||||||||||||
| Restricted shares and stock options issued for services |
66 | 216 | 216 | |||||||||||||||||||||||||
| Warrants issued for services |
86 | 86 | ||||||||||||||||||||||||||
| Net loss |
(7,143 | ) | (7,143 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, June 30, 2014 |
693 | $ | 69 | 38,511 | $ | 39 | $ | 201,492 | $ | (196,382 | ) | $ | 5,218 | |||||||||||||||
| Preferred stock dividends |
(403 | ) | (403 | ) | ||||||||||||||||||||||||
| Restricted stock issued for preferred stock dividends |
306 | 333 | 333 | |||||||||||||||||||||||||
| Share-based compensation expense |
150 | 475 | 475 | |||||||||||||||||||||||||
| Share based severance |
153 | 153 | ||||||||||||||||||||||||||
| Issuance of common stock, net |
3,041 | 3 | 2,115 | 2,118 | ||||||||||||||||||||||||
| Restricted shares and stock options issued for services |
108 | 358 | 358 | |||||||||||||||||||||||||
| Warrants issued with debt |
902 | 902 | ||||||||||||||||||||||||||
| Warrants issued for services |
81 | 81 | ||||||||||||||||||||||||||
| Net loss |
(9,700 | ) | (9,700 | ) | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance, June 30, 2015 |
693 | $ | 69 | 42,116 | $ | 42 | $ | 205,909 | $ | (206,485 | ) | $ | (465 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Consolidated Statements of Cash Flows
| Year Ended June 30, | ||||||||||||
| (in thousands) |
2015 | 2014 | 2013 | |||||||||
| Cash flows from operating activities |
||||||||||||
| Net Loss |
$ | (9,700 | ) | $ | (7,143 | ) | $ | (11,349 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities |
||||||||||||
| Amortization of debt discount and deferred financing costs |
386 | 127 | 2,931 | |||||||||
| Loss on extinguishment of notes |
557 | — | 1,060 | |||||||||
| Inventory reserve |
200 | — | — | |||||||||
| Depreciation and amortization |
959 | 766 | 843 | |||||||||
| Share-based compensation |
475 | 587 | 305 | |||||||||
| Share-based severance |
153 | — | — | |||||||||
| Warrants issued for services |
81 | 176 | 105 | |||||||||
| Restricted shares and stock options issued for services |
358 | 216 | 217 | |||||||||
| Net gain on sale of non-core assets |
— | (101 | ) | — | ||||||||
| Changes in assets and liabilities |
||||||||||||
| Accounts receivable |
234 | 58 | 79 | |||||||||
| Inventory |
538 | 1,022 | 57 | |||||||||
| Prepaid expenses and other current assets |
37 | 339 | 674 | |||||||||
| Accounts payable, accrued expenses and other liabilities |
820 | (643 | ) | (89 | ) | |||||||
| Deferred revenue |
(49 | ) | (130 | ) | (47 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(4,951 | ) | (4,726 | ) | (5,214 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities |
||||||||||||
| Purchases of property and equipment and other assets |
(185 | ) | (319 | ) | (271 | ) | ||||||
| Other intangible assets acquired |
(361 | ) | (162 | ) | (169 | ) | ||||||
| Payment for business acquisition |
— | — | (31 | ) | ||||||||
| Sales of marketable securities |
210 | — | — | |||||||||
| Net proceeds from sale of non-core assets |
— | 851 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash (used) provided by investing activities |
(336 | ) | 370 | (471 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities |
||||||||||||
| Net proceeds from issuance of preferred and common stock and exercise of warrants |
2,118 | 2,855 | 3,964 | |||||||||
| Proceeds from issuance of short-term promissory notes |
2,550 | — | — | |||||||||
| Repayment of short-term promissory notes |
(200 | ) | — | — | ||||||||
| Net (repayments) proceeds from issuance of senior secured notes and warrants |
— | (850 | ) | 3,260 | ||||||||
| Dividends paid |
(18 | ) | (70 | ) | (70 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
4,450 | 1,935 | 7,154 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (decrease) increase in cash and cash equivalents |
(837 | ) | (2,421 | ) | 1,469 | |||||||
| Cash and cash equivalents, beginning of year |
1,084 | 3,505 | 2,036 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 247 | $ | 1,084 | $ | 3,505 | ||||||
|
|
|
|
|
|
|
|||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-6
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements
June 30, 2015, 2014 and 2013
1. Description of Business, Liquidity and Summary of Significant Accounting Policies
Authentidate Holding Corp. (Authentidate or the company) and its subsidiaries provide secure web-based revenue cycle management applications and telehealth products and services that enable healthcare organizations to increase revenues, improve productivity, reduce costs, coordinate care for patients and enhance related administrative and clinical workflows and compliance with regulatory requirements. Our web-based services are delivered as Software as a Service (SaaS) to our customers interfacing seamlessly with billing, information and records management systems. These solutions incorporate multiple features and security technologies such as business-rules based electronic forms, intelligent routing, transaction management, electronic signatures, identity credentialing, content authentication, automated audit trails and remote patient management capabilities. Both web and fax-based communications are integrated into automated, secure and trusted workflow solutions.
Our telehealth solutions provide in-home patient vital signs monitoring systems and services to improve care for patients and reduce the cost of care by delivering results to their healthcare providers via the Internet. Our solutions enable unattended measurements of patients’ vital signs and related health information and are designed to aid wellness and preventative care and deliver better care to specific patient segments that require regular monitoring of medical and behavioral health conditions. Healthcare providers can easily view each specific patient’s vital statistics and make adjustments to the patient’s care plans securely via the Internet. The service provides a combination of care plan schedule reminders and comprehensive disease management education as well as intelligent routing to alert on-duty caregivers whenever a patient’s vital signs are outside of the practitioner’s pre-set ranges. Healthcare providers and health insurers are also expected to benefit by having additional tools to improve patient care and reduce in-person and emergency room patient visits and hospital readmissions. We operate our business in the United States with technology and service offerings that address emerging growth opportunities based on the regulatory and legal requirements specific to each market. Our business is engaged in the development and sale of web-based services largely based on our Inscrybe® platform and related capabilities and our telehealth products and services.
The company has incurred significant recurring losses and negative cash flows from operations and our product development activities have required substantial capital investment to date. These factors, among others, raise substantial doubt about our ability to continue as a going concern. Our consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty. Additionally, on March 6, 2015 we announced that the Department of Veterans Affairs (VA) informed the company that it did not intend to exercise the fourth and final option year under our contract for telehealth products and services. The company’s contract with the VA was originally awarded in April 2011 and consisted of a base year and four one-year option years which were exercisable at the VA’s sole discretion. The current option year under the contract expired on May 15, 2015 and the transition process with the VA was completed by that date. Our VA revenue included both recurring service revenues as well as hardware sales. As a result of the non-renewal of the VA contract we expect to report significantly reduced revenues over the next several quarters and we have taken steps to reduce our operating costs and better align our resources with the growth opportunities we intend to pursue. The VA had been our largest customer, accounting for approximately 45 % and 58% of our total revenue for the years ended June 30, 2015 and 2014, respectively. As a result, we have implemented a number of changes to our business plan with the ultimate goal to increase revenues and generate positive cash flow from operations, including a recalibration of marketing and sales efforts that have already resulted in growth from existing customers and sales to new customers. These changes include cost reductions from reducing our workforce and use of consultants that we made in the third quarter of fiscal 2015 and additional workforce reductions through August 31, 2015. During the third quarter the company recorded charges of approximately $70,000 related to workforce severance during the period and did not incur any additional severance costs for the additional workforce reductions made through June 30, 2015 or the more recent workforce reductions made in August
F-7
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
2015. These reductions are expected to reduce operating expenses on an annualized basis. The company has also taken actions to realign our data center operations and has executed an agreement with its landlord to relocate its offices, which are expected to result in additional annualized savings.
Since February 2015, the company has completed debt financing transactions resulting in total proceeds of approximately $3.5 million, however, the company has an immediate need for additional capital and is exploring additional potential transactions to improve our capital position, ensure we are able to meet our working capital requirements and provide funds to pay these debt obligations which are due in the next twelve months. Based on its business plan, the company expects its existing resources, revenues generated from operations, net proceeds from our debt financing transactions, other transactions we are considering and proceeds received from the exercise of outstanding warrants (of which there can be no assurance) or a restructuring of outstanding debt obligations (of which there can be no assurance) to satisfy its working capital requirements for at least the next twelve months. If necessary, management of the company believes that it can reduce operating expenses and/or raise additional equity or debt financing to satisfy its working capital requirements. However, no assurances can be given that we will be able to support our costs or pay debt obligations through revenues derived from operations or generate sufficient cash flow to satisfy our other obligations. Unless we are able to increase revenues substantially or generate additional capital from other transactions, our current cash resources will only satisfy our working capital needs for a limited period of time and we will need to raise additional funds to meet our obligations in the future. We have an immediate need for additional capital and in addition to our recently announced letter of intent for a business combination transaction, we are continuing to explore additional potential transactions to improve our capital position to ensure we are able to meet our financing and working capital requirements. Currently, the company does not have any definitive agreements with any third parties for such transactions and there can be no assurances that the company will be successful in completing the transaction contemplated by the letter of intent or in raising additional capital or securing financing when needed or on terms satisfactory to the company. Any inability to obtain required financing on sufficiently favorable terms could have a material adverse effect on our business, results of operations and financial condition.
At the company’s adjourned annual meeting of stockholders held on June 30, 2015, our stockholders approved an amendment to the company’s certificate of incorporation to increase the number of authorized shares of the company’s common stock from 100 million shares to 190 million shares of common stock.
Principles of Consolidation
The financial statements include the accounts of Authentidate Holding Corp. and its subsidiaries. Intercompany transactions and balances have been eliminated. Equity investments where we do not exercise significant influence over the investee are accounted for under the cost method.
Cash Equivalents
We consider all highly liquid investments with maturities of three months or less when purchased to be cash equivalents. At June 30, 2015 and 2014 cash equivalents consisted primarily of investments in short term investments such as overnight interest bearing deposits.
Marketable Securities
Our marketable securities as of June 30, 2014 consisted primarily of money market investments. We classify our investments as “available for sale” and they have been recorded at cost which approximates fair
F-8
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
market value due to their variable interest rates. As a result, we have had no cumulative gross unrealized holding gains (losses) or gross realized gains (losses) from such investments. All income generated from these investments was recorded as interest income.
Accounts Receivable
Accounts receivable represent customer obligations due under normal trade terms, net of an allowance for doubtful accounts. The allowance for doubtful accounts reflects our best estimate of probable losses inherent in the accounts receivable balance. We determine the allowance based on known troubled accounts, historical experience and other currently available evidence. The allowance for doubtful accounts is not material for any of the periods presented.
Inventory
Inventory amounts are stated at the lower of cost or market. Cost is determined based on average cost for the related inventory items.
Long-Lived Assets
Long-lived assets, including property and equipment, software development costs, patent costs, trademarks and licenses are reviewed for impairment using an undiscounted cash flow approach whenever events or changes in circumstances such as significant changes in the business climate, changes in product offerings, or other circumstances indicate that the carrying amount may not be recoverable.
Property and Equipment
Property and equipment are stated at cost, net of accumulated depreciation and amortization. Depreciation and amortization are determined using the straight-line method. Estimated useful lives of the assets range from three to seven years.
Repairs and maintenance are charged to expense as incurred. Renewals and betterments are capitalized. When assets are sold, retired or otherwise disposed of, the applicable costs and accumulated depreciation or amortization are removed from the accounts and the resulting gain or loss, if any, is recognized.
Software Development Costs
Software development and modification costs incurred subsequent to establishing technological feasibility are capitalized and amortized based on anticipated revenue for the related product with an annual minimum equal to the straight-line amortization over the remaining economic life of the related product (generally three years). Amortization expense was $0 for the years ended June 30, 2015 and 2014 and $171,000 for the year ended June 30, 2013 which is included in depreciation and amortization expense.
Income Taxes
Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the
F-9
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amounts expected to be realized.
Tax benefits from an uncertain tax position are recognized only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than fifty percent likelihood of being realized upon ultimate settlement. Accrued interest related to unrecognized benefits is recorded as interest expense and penalties are recorded as income tax expense.
Revenue Recognition
Revenue is derived from web-based hosted software services, telehealth products and post contract customer support services. Revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred, the selling price is fixed and collectability is reasonably assured. Multiple-element arrangements are assessed to determine whether they can be separated into more than one unit of accounting. A multiple-element arrangement is separated into more than one unit of accounting if all of the following criteria are met: the delivered item has value to the customer on a standalone basis; there is objective and reliable evidence of the fair value of the undelivered items in the arrangement; if the arrangement includes a general right of return relative to the delivered items, and delivery or performance of the undelivered item is considered probable and substantially in our control. If these criteria are not met, then revenue is deferred until such criteria are met or until the period over which the last undelivered element is delivered, which is typically the life of the contract agreement. If these criteria are met, we allocate total revenue among the elements based on the sales price of each element when sold separately which is referred to as vendor specific objective evidence or VSOE.
Revenue from web-based hosted software and related services and post contract customer support services is recognized when the related service is provided and, when required, accepted by the customer. Revenue from telehealth products is recognized when such products are delivered. Revenue from multiple element arrangements that cannot be allocated to identifiable items is recognized ratably over the contract term which is generally one year.
Warranty Provisions
We provide a limited warranty on the web-based hosted software, telehealth products and services sold. Warranty expense was not significant in any of the periods presented.
Advertising Expenses
We recognize advertising expenses as incurred. Advertising expense was $49,000, $56,000 and $44,000 for the years ended June 30, 2015, 2014 and 2013, respectively.
Product Development Expenses
These costs represent research and development expenses and include salary and benefits, professional and consultant fees and supplies and are expensed as incurred.
F-10
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
Management Estimates
Preparing financial statements requires management to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses. Examples include estimates of loss contingencies and product life cycles, assumptions such as elements comprising a software arrangement, including the distinction between upgrades/enhancements and new products; when technological feasibility is achieved for our products; the potential outcome of future tax consequences; and determining when investment or other impairments exist. We maintain allowances for doubtful accounts for estimated losses resulting from the inability of our customers to make required payments. We make estimates on the future recoverability of capitalized amounts; we record a valuation allowance against deferred tax assets when we believe it is more likely than not that such deferred tax assets will not be realized and we make assumptions in connection with the calculations of share-based compensation expense. Actual results and outcomes may differ from management’s estimates, judgments and assumptions. We have based our estimates on historical experience and on various assumptions that are believed to be reasonable under the circumstances and we evaluate our estimates on a regular basis and make changes accordingly. Historically, our estimates relative to our critical accounting estimates have not differed materially from actual results; however, actual results may differ from these estimates under different conditions. If actual results differ from these estimates and other considerations used in estimating amounts reflected in our consolidated financial statements, the resulting changes could have a material adverse effect on our consolidated statement of operations, and in certain situations, could have a material adverse effect on liquidity and our financial condition.
Share-Based Compensation
Option-based employee compensation expense is determined using the Black-Scholes option pricing model which values options based on the stock price at the grant date, the exercise price of the option, the expected life of the option, the estimated volatility, expected dividend payments and the risk-free interest rate over the expected life of the options. Restricted stock units granted to employees are valued using the closing stock price of the company’s common stock on the grant date.
Concentrations of Credit Risk
Financial instruments which subject us to concentrations of credit risk consist of cash and cash equivalents, marketable securities and trade accounts receivable. To reduce credit risk, we place our cash, cash equivalents and investments with several high credit quality financial institutions and typically invest in AA or better rated investments. We monitor our credit customers and establish an allowance for doubtful accounts based upon factors surrounding the credit risk of specific customers, historical trends and other information.
We have no significant off-balance sheet arrangements at June 30, 2015. At June 30, 2015, two customers represented 34% of total accounts receivable. At June 30, 2014, one customer represented 20% of total accounts receivable. For the year ended June 30, 2015, one customer accounted for approximately 45% of consolidated revenues. For the year ended June 30, 2014, one customer accounted for approximately 58% of consolidated revenues. For the year ended June 30, 2013, two customers accounted for 52% of consolidated revenues.
Present Accounting Standards Not Yet Adopted
In May 2014, the FASB issued ASU No. 2014-09, “Revenue from Contracts with Customers” (Topic 606). This ASU is intended to clarify the principles for recognizing revenue by removing inconsistencies and weaknesses in revenue requirements; providing a more robust framework for addressing revenue issues;
F-11
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
improving comparability of revenue recognition practices across entities, industries, jurisdictions and capital markets; and providing more useful information to users of financial statements through improved revenue disclosure requirements. The new standard is required to be applied retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying it recognized at the date of initial application. The provisions of this ASU were effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2016. Early application was not permitted. In August 2015, the FASB issued ASU 2015-14, “Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date”, which defers the effective date to fiscal periods beginning after December 15, 2017. Early adoption is permitted for fiscal periods beginning after December 15, 2016. The standard permits the use of either the retrospective or cumulative effect transition method. We are currently evaluating the impact of this standard on our consolidated financial statements.
In June 2014, the FASB issued ASU No. 2014-12, “Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period.” This ASU requires a reporting entity to treat a performance target that affects vesting and that could be achieved after the requisite service period as a performance condition, and apply existing guidance under the Stock Compensation Topic of the ASC as it relates to awards with performance conditions that affect vesting to account for such awards. The provisions of this ASU are effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2015. We are currently evaluating the impact of this standard on our consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern.” This ASU is intended to define management’s responsibility to evaluate whether there is substantial doubt about an entity’s ability to continue as a going concern and to provide related footnote disclosures. Specifically, ASU 2014-15 provides a definition of the term substantial doubt and requires an assessment for a period of one year after the date that the financial statements are issued (or available to be issued). It also requires certain disclosures when substantial doubt is alleviated as a result of consideration of management’s plans and requires an express statement and other disclosures when substantial doubt is not alleviated. The new standard will be effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2016, with early adoption permitted. We are currently evaluating the impact of this standard on our consolidated financial statements.
In April 2015, the FASB issued ASU 2015-03, “Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs.” This ASU is intended to simplify the presentation of debt issuance costs and conform to the guidance in International Financial Reporting Standards, which requires that transaction costs be deducted from the carrying value of the financial liability and not recorded as separate assets. Additionally, the requirement to recognize debt issuance costs as deferred charges conflicts with the guidance in FASB Concepts Statement No. 6, “Elements of Financial Statements,” which states that debt issuance costs are similar to debt discounts and in effect reduce the proceeds of borrowing, thereby increasing the effective interest rate. FASB Concepts Statement No. 6 further states that debt issuance costs cannot be an asset because they provide no future economic benefit. The amendments in this ASU require that debt issuance costs related to a recognized debt liability be presented in the balance sheet as a direct deduction from the carrying amount of that debt liability, consistent with debt discounts. The recognition and measurement guidance for debt issuance costs are not affected by the amendments in this ASU. For public business entities, the amendments in this ASU are effective for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2015. In August 2015, the FASB issued ASU No. 2015-15, “Interest—Imputation of Interest
F-12
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
(Subtopic 835-30): Presentation and Subsequent Measurement of Debt Issuance Costs Associated with Line-of-Credit Arrangements—Amendments to SEC Paragraphs Pursuant to Staff Announcement at June 18, 2015 EITF Meeting.” This ASU adds SEC paragraphs pursuant to the SEC Staff Announcement at the June 18, 2015, Emerging Issues Task Force meeting about the presentation and subsequent measurement of debt issuance costs associated with line-of-credit arrangements to this topic. Given the absence of authoritative guidance within ASU 2015-03 for debt issuance costs related to line-of-credit arrangements, the SEC staff would not object to an entity deferring and presenting debt issuance costs as an asset and subsequently amortizing the deferred debt issuance costs ratably over the term of the line-of-credit arrangement, regardless of whether there are any outstanding borrowings on the line-of-credit arrangement. We are currently evaluating the impact of this standard on our consolidated financial statements.
In April 2015, the FASB issued ASU No. 2015-05, “Customer’s Accounting for Fees Paid in a Cloud Computing Arrangement”, which provides explicit guidance to help companies evaluate the accounting for fees paid by a customer in a cloud computing arrangement. The new guidance clarifies that if a cloud computing arrangement includes a software license, the customer should account for the license consistent with its accounting for other software licenses. If the arrangement does not include a software license, the customer should account for the arrangement as a service contract. ASU 2015-05 is effective for public business entities for annual reporting periods, including interim periods within those annual periods, beginning after December 15, 2015. An entity can elect to adopt the amendments either prospectively for all arrangements entered into or materially modified after the effective date, or retrospectively. Early adoption is permitted for all entities. We are currently evaluating the impact of this standard on our consolidated financial statements.
2. Share-Based Compensation
The accounting guidance requires all share-based payments, including grants of stock options, to be recognized in the statement of operations as compensation expense based on their fair values. Accordingly, the estimated fair value of options granted under the company’s share based compensation plans are recognized as compensation expense over the option-vesting period.
On May 1, 2014, the stockholders approved an amendment to the Omnibus Equity Incentive Plan (the 2011 Plan) to increase the number of shares available under the plan by 3,400,000 shares. The 2011 Plan, as amended, provides for the issuance of up to 6,750,000 shares of the company’s common stock in connection with stock options, restricted share awards and other stock compensation vehicles. The 2011 Plan is administered by a committee designated by the board of directors comprised entirely of outside directors. The board or the committee, as the case may be, has the discretion to determine eligible participants and the types of awards and the terms of such awards. Vesting of stock options generally occurs one-third per year over three years or subject to performance based vesting conditions and options generally have a life of ten years. The restricted stock units awarded during fiscal 2013, 2014 and 2015 are subject to performance-based vesting conditions. The board or the committee has full authority to interpret the 2011 Plan and to establish and amend rules and regulations relating thereto. Under the 2011 Plan, the exercise price of an option designated as an ISO may not be less than the fair market value of the company’s common stock on the date the option is granted. However, in the event an option designated as an ISO is granted to a ten percent shareholder, the exercise price shall be at least 110% of such fair market value. The aggregate fair market value on the grant date of shares subject to options which are designated as ISOs which become exercisable in any calendar year, shall not exceed $100,000 per optionee.
The board or the committee may in its sole discretion grant bonuses or authorize loans to or guarantee loans obtained by a participant to enable such participant to pay any taxes that may arise in connection with the
F-13
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
exercise or cancellation of an option. As of June 30, 2015, 2014 and 2013, no loans had been granted or guaranteed. Loans may not be granted or guaranteed for directors or executive officers.
Stock option activity under the company’s stock option plans for employees and non-executive directors for the period ended June 30, 2015 is as follows (in thousands, except per share and average life data):
| Employees Information |
Number of Options |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
||||||||||||
| Outstanding, June 30, 2012 |
2,290 | $ | 4.06 | |||||||||||||
| Granted |
233 | 1.41 | ||||||||||||||
| Expired/forfeited |
(252 | ) | 1.87 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2013 |
2,271 | 3.94 | ||||||||||||||
| Granted |
783 | 0.92 | ||||||||||||||
| Expired/forfeited |
(435 | ) | 1.11 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding June 30, 2014 |
2,619 | 3.59 | ||||||||||||||
| Granted |
727 | 0.83 | ||||||||||||||
| Expired/forfeited |
(1,215 | ) | 4.25 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding June 30, 2015 |
2,131 | $ | 2.27 | 6.16 | $ | — | ||||||||||
|
|
|
|||||||||||||||
| Exercisable at June 30, 2015 |
1,209 | $ | 2.89 | 3.87 | $ | — | ||||||||||
|
|
|
|||||||||||||||
| Expected to vest at June 30, 2015 |
723 | $ | 1.42 | 7.42 | $ | — | ||||||||||
|
|
|
|||||||||||||||
| Non-Executive Director Information |
Number of Options |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
||||||||||||
| Outstanding, June 30, 2012 |
243 | $ | 3.86 | |||||||||||||
| Granted |
218 | 1.01 | ||||||||||||||
| Expired |
(89 | ) | 4.76 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2013 |
372 | 1.97 | ||||||||||||||
| Granted |
379 | 0.92 | ||||||||||||||
| Expired |
(9 | ) | 6.08 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2014 |
742 | 1.39 | ||||||||||||||
| Granted |
1,498 | 0.37 | ||||||||||||||
| Expired |
(55 | ) | 3.91 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding June 30, 2015 |
2,185 | $ | 0.62 | 8.20 | $ | — | ||||||||||
|
|
|
|||||||||||||||
Non-executive director options are granted at market price and vest on the grant date.
As of June 30, 2015, there were approximately 431,295 restricted stock units outstanding that were granted to employees on January 15, 2013 and January 28, 2014, in connection with the company’s compensation modification program. These restricted stock units vest when the company achieves cash flow breakeven, as defined.
F-14
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
Under the 2011 Plan, the company’s non-employee directors continued to have the option to elect to receive up to 100% of their cash director compensation, including amounts payable for committee service, service as a committee chair and per meeting fees, in restricted shares of our common stock issued at fair value in accordance with the terms of the 2011 Plan. In December 2012, the board of directors agreed that all non-employee directors would receive all of their cash director compensation, including amounts payable for committee service, service as a committee chair and per meeting fees, in restricted shares of our common stock or stock options issued at fair value in accordance with the terms of the 2011 Plan for periods ending after December 2012. During the year ended June 30, 2015, the company issued 107,776 shares of restricted common stock and 1,363,024 stock options (with a total fair value of approximately $358,000) to certain non-executive directors in connection with this program. In July 2015, the company issued 78,854 shares of restricted common stock (with a fair value of approximately $15,000) to such directors under the program.
Share-based compensation expense by category is as follows (in thousands):
| June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| SG&A |
$ | 415 | $ | 527 | $ | 245 | ||||||
| Product development |
40 | 40 | 40 | |||||||||
| Cost of revenues |
20 | 20 | 20 | |||||||||
|
|
|
|
|
|
|
|||||||
| Share-based compensation expense |
$ | 475 | $ | 587 | $ | 305 | ||||||
|
|
|
|
|
|
|
|||||||
The accounting guidance requires cash flows resulting from the tax benefits from tax deductions in excess of the compensation cost recognized for those options (excess tax benefits) to be classified as financing cash flows. The company did not realize any tax benefits from the exercise of stock options during the years ended June 30, 2015, 2014 and 2013.
We computed the estimated fair values of all option-based compensation using the Black-Scholes option pricing model and the assumptions set forth in the following table. We based our estimate of the life of these options on historical averages over the past five years and estimates of expected future behavior. The expected volatility was based on our historical stock volatility. The assumptions used in our Black-Scholes calculations for fiscal 2015, 2014 and 2013 are as follows:
| Risk Free Interest Rate |
Dividend Yield |
Volatility Factor |
Weighted Average Option Life (Months) |
|||||||||||||
| Fiscal year 2015 |
0.6 | % | 0 | % | 84 | % | 48 | |||||||||
| Fiscal year 2014 |
1.4 | % | 0 | % | 89 | % | 48 | |||||||||
| Fiscal year 2013 |
0.6 | % | 0 | % | 107 | % | 48 | |||||||||
The Black-Scholes option-pricing model requires the input of highly subjective assumptions. Because our stock options have characteristics significantly different from those of traded options, and because changes in the subjective input assumptions can materially affect the fair value estimate, in management’s opinion, the existing models may not provide a reliable single measure of the fair value of share-based compensation for employee and director stock options. Management will continue to assess the assumptions and methodologies used to calculate estimated fair value of share-based compensation as circumstances change and additional data becomes available over time, which may result in changes to these assumptions and methodologies. Such changes could materially impact the company’s fair value determination.
F-15
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
As of June 30, 2015, there was approximately $452,000 of total unrecognized compensation expense related to unvested share-based compensation arrangements which is expected to be recognized over a weighted-average period of 17 months.
The total intrinsic value of options exercised was $0 for each of the fiscal years ended 2015, 2014 and 2013, respectively. The weighted average grant date fair value of options granted during the fiscal years ended June 30, 2015, 2014 and 2013 was approximately $0.40, $0.64 and $0.86. The values were calculated using the Black-Scholes model.
The total fair value of shares vested was $807,000, $454,000 and $297,000 for the fiscal years ended June 30, 2015, 2014 and 2013, respectively.
3. Loss per Share
The following table sets forth the calculation of basic and diluted loss per share for the periods presented (in thousands, except per share data):
| June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Net loss |
$ | (9,700 | ) | $ | (7,143 | ) | $ | (11,349 | ) | |||
| Preferred stock dividends |
(403 | ) | (402 | ) | (384 | ) | ||||||
| Deemed preferred stock dividends |
— | (2,017 | ) | (906 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Net loss applicable to common shareholders |
$ | (10,103 | ) | $ | (9,562 | ) | $ | (12,639 | ) | |||
|
|
|
|
|
|
|
|||||||
| Weighted average shares |
41,182 | 37,196 | 28,086 | |||||||||
|
|
|
|
|
|
|
|||||||
| Basic and diluted loss per common share |
$ | (0.25 | ) | $ | (0.26 | ) | $ | (0.45 | ) | |||
|
|
|
|
|
|
|
|||||||
The deemed preferred stock dividends included in the loss per share calculation represent the accretion of the fair value of the warrants issued in connection with the extension of the redemption date of the Series C 15% convertible redeemable preferred stock over the period from issuance through the conversion date in April 2013. Beginning in June 2013, the deemed preferred stock dividends also include the accretion of the fair value allocated to a non-cash beneficial conversion feature related to the Series D preferred stock issued in June 2013. The beneficial conversion feature was being amortized over the period from issuance through the earliest permitted conversion date in December 2013. As discussed more fully in Note 11 of Notes to Consolidated Financial Statements, the fair value of the warrants was determined using the Black-Scholes option pricing model. All common stock equivalents were excluded from the loss per share calculation for all periods presented because the impact is antidilutive. At June 30, 2015 options (4,316,000), restricted stock units (888,000), warrants (33,669,000), convertible debt (3,600,000), Series D preferred stock (6,125,000) and Series B preferred stock (250,000) were outstanding. At June 30, 2014 options (3,361,000), restricted stock units (910,000), warrants (26,807,000), Series D preferred stock (6,125,000) and Series B preferred stock (250,000) were outstanding. At June 30, 2013 options (2,643,000), restricted stock units (576,000), warrants (24,966,000), Series D preferred stock (6,125,000) and Series B preferred stock (250,000) were outstanding.
4. Sale of Non-Core Assets
In February 2014, the company completed the sale of all of its shares in Health Fusion, Inc. in connection with a share buy-back program initiated by Health Fusion. Net proceeds from the sale were approximately
F-16
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
$851,000 and the company recorded a gain on the sale of this investment of approximately $101,000 which is included in other income. The company made a $750,000 investment in Health Fusion in fiscal 2005. The investment was accounted for using the cost method and was included in other assets through the date of the sale.
5. Inventory
In connection with our manufacturing and sales plans for our telehealth service, the company has purchased certain components and contract manufacturing services for the production of the monitoring appliance. Inventory consists of the following (in thousands):
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Purchased components, net |
$ | 261 | $ | 2,735 | ||||
| Finished goods |
342 | 202 | ||||||
|
|
|
|
|
|||||
| Total inventory |
$ | 603 | $ | 2,937 | ||||
|
|
|
|
|
|||||
6. Property and Equipment
Property and equipment consists of the following (in thousands):
|
June 30, |
Estimated Useful Life In Years |
|||||||||||
| 2015 | 2014 | |||||||||||
| Machinery and equipment |
$ | 5,256 | $ | 5,071 | 3-6 | |||||||
| Furniture and fixtures |
236 | 236 | 5-7 | |||||||||
| Leasehold improvements |
240 | 240 | 5 | |||||||||
|
|
|
|
|
|||||||||
| 5,732 | 5,547 | |||||||||||
| Less: Accumulated depreciation and amortization |
(5,431 | ) | (5,099 | ) | ||||||||
|
|
|
|
|
|||||||||
| $ | 301 | $ | 448 | |||||||||
|
|
|
|
|
|||||||||
Depreciation and amortization expense on property and equipment for the years ended June 30, 2015, 2014 and 2013 was approximately $332,000, $354,000 and $354,000, respectively. Depreciation and amortization reported in the statement of operations also includes amounts related to other assets for the years ended June 30, 2015, 2014 and 2013 of approximately $242,000, $79,000 and $28,000, respectively. These assets generally have three-year lives and are depreciated or amortized using the straight-line method.
7. Accounts Payable, Accrued Expenses and Other Liabilities
Accounts payable, accrued expenses and other liabilities consist of the following (in thousands):
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Accounts payable |
$ | 1,410 | $ | 2,541 | ||||
| Legal |
365 | — | ||||||
| Accrued vacation |
130 | 155 | ||||||
| Other accrued expenses |
204 | 110 | ||||||
|
|
|
|
|
|||||
| $ | 2,109 | $ | 2,806 | |||||
|
|
|
|
|
|||||
F-17
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
8. Notes Payable
On March 9, 2012 and September 24, 2012, the company entered into securities purchase agreements with certain accredited investors pursuant to which we sold an aggregate principal amount of $7,350,000 of senior secured promissory notes and warrants to purchase a total of 5,580,527 shares of common stock for gross proceeds of $7,350,000. The secured notes were senior secured promissory notes and were not convertible into equity securities. No interest accrued on the secured notes and they contained covenants and events of default customary for similar transactions. The secured notes were collateralized by a first priority lien on all of the company’s assets in accordance with, and subject to, a security agreement. The warrants are exercisable for a period of 54 months commencing on the six month anniversary of the issue date of such warrants at an initial exercise price of $1.34 per share, which is 101% of the consolidated closing bid price reported by the Nasdaq Stock Market on March 9, 2012. The closing of these financings occurred on March 14, 2012 and September 28, 2012 and the net proceeds to us from these transactions, after deducting offering expenses, were approximately $7,260,000. The following investors that participated in these financings are related parties to us. J. David Luce, a member of our board of directors, and his spouse purchased an aggregate principal amount of $2,650,000 of secured notes and 2,010,876 warrants through affiliated entities, John J. Waters, who was a member of our board of directors through May 12, 2013, purchased an aggregate principal amount of $150,000 of secured notes and 119,940 warrants. In addition, our former chief executive officer and a former member of our board of directors, O’Connell Benjamin, and our chief financial officer, William Marshall, each purchased an aggregate principal amount of $100,000 of secured notes and 76,073 warrants. Further, Lazarus Investment Partners LLLP, which was the beneficial owner of approximately 16.2% and 23.8% of our outstanding shares of common stock immediately prior to these offerings, respectively purchased an aggregate principal amount of $2,000,000 of secured notes and 1,521,463 warrants. The manager of the general partner of Lazarus Investment Partners, LLLP is the brother of Dr. Todd A. Borus, a member of our board of directors. The participation by these investors was on the same terms as the other investors in these transactions.
On September 24, 2012, we also entered into a transaction with the holders of the $4,050,000 senior secured promissory notes issued in March 2012, to extend the maturity date of such notes to October 31, 2013 and grant pari passu rights to the new notes issued on September 28, 2012. In connection with and in consideration of this extension, we issued the holders of the notes issued in March 2012 warrants to purchase an aggregate of 2,197,674 shares of common stock with the same terms as the warrants issued to the new note holders. Due to their ownership of secured notes issued in March 2012, extension warrants were issued to the following related parties: entities affiliated with Mr. Luce were issued a total of 813,953 extension warrants; John Waters, a director through May 12, 2013 was issued 81,395 extension warrants, each of the company’s former chief executive officer and chief financial officer were issued 27,131 extension warrants and Lazarus Investment Partners was issued 542,636 extension warrants.
The company allocated the proceeds from the secured note transactions to the secured notes and the warrants based on the relative fair values of such instruments using the present value of the secured notes at a market rate of interest and the fair value of the warrants based on the Black-Scholes option pricing model and the applicable assumptions set forth in Note 2 of Notes to Consolidated Financial Statements. This allocation resulted in an effective interest rate for the secured notes from March 2012 through September 2012 of approximately 54% per annum. Following the extension of the secured notes issued in March 2012 the effective interest rate for the remaining term was approximately 47% per annum. The effective interest rate for the secured notes issued in September 2012 was approximately 41% per annum. However, since the subjective nature of the inputs for the option pricing models can materially affect fair value estimates, in management’s opinion, such models may not provide a reliable single measure of the fair value of the warrants and the resulting effective interest rates. In connection with the secured note transactions the company recorded senior secured promissory notes of $7,350,000, debt discount of $4,726,000, and additional paid-in-capital of $4,676,000 related to the fair value of the warrants.
F-18
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
In connection with the sale of Series D convertible preferred stock discussed in Note 19 of Notes to Consolidated Financial Statements, holders of $6,500,000 of secured notes agreed to cancel their notes in exchange for the Series D shares and warrants issued in the sale. The company completed this transaction on June 20, 2013 and recorded the reduction in secured notes and the related unamortized debt discount and deferred financing costs on such date. The early extinguishment of the secured notes resulted in a non-cash loss on extinguishment of approximately $1,060,000 which is equal to the remaining unamortized debt discount and deferred financing costs related to the cancelled notes as of the extinguishment date. The loss on extinguishment is included in other expense for the year ended June 30, 2013. The aggregate principal amounts of $850,000 of senior notes outstanding following the Series D transaction was repaid on October 31, 2013, the stated maturity date.
On January 29, 2015, the company received a short-term loan of $200,000 from a strategic lender pursuant to which we issued a secured promissory note to the lender in the aggregate principal amount of $200,000. The note was secured by a first priority lien on certain equipment owned by the company with a net present value equivalent to the face value of the note, was due and payable on March 30, 2015 and accrued interest at the rate of 6% per annum. The note and accrued interest was repaid on the maturity date.
On February 17, 2015, the company entered into a securities purchase agreement with an accredited investor pursuant to which we issued a promissory note in the aggregate principal amount of $100,000 and common stock purchase warrants to purchase up to 80,000 shares of common stock for gross proceeds of $100,000. The note was an unsecured obligation of the company, was not convertible into equity securities of the company and accrued interest at a rate of 8% per annum. The note was due and payable on the six month anniversary of the issue date, subject to the right of the investor to extend the maturity date for up to an additional six months. The net proceeds from this transaction were approximately $92,000. The warrants vested in equal monthly installments over twelve months if the notes were outstanding and, subject to vesting requirements, were exercisable for a period of 54 months commencing on the six month anniversary of the issuance date at an initial exercise price of $1.01 per share. Although the securities purchase agreement that the company entered into with this investor contemplated a second closing for $900,000 of additional proceeds, the second closing did not occur and the company did not receive the additional funds. This note and the vested portion of the warrants were exchanged for notes and warrants issued in the June 8, 2015 transaction discussed below and the warrants issued for this transaction were cancelled.
On February 17, 2015, in a separate transaction, the company issued a short-term promissory note in the aggregate principal amount of $950,000 and warrants to purchase 99,500 shares of common stock to an accredited investor for gross proceeds of $950,000. The holder of the short-term note is an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of the board of directors of the company. The short-term note is an unsecured obligation of the company, is not convertible into equity securities of the company and accrues interest at a rate of 5.76% per annum. The short-term note was due and payable on the first to occur of the one month anniversary of the issue date or the date on which the company received at least $950,000 in proceeds from equity or debt financing. The short-term note contains covenants and events of default customary for similar transactions. The net proceeds from this transaction were approximately $940,000. The warrants are exercisable for a period of 54 months commencing on the six month anniversary of the issuance date and have an initial exercise price of $1.01 per share. On April 3, 2015, the company entered into an amendment agreement with the holder of the $950,000 short-term promissory note in order to amend such note to extend its maturity date from March 19, 2015 to July 2, 2015. In addition, pursuant to the amendment agreement, the company also agreed to grant the holder the right to exchange the principal amount of the short-term note (and unpaid interest thereon) into the securities of the company sold in the next financing, as defined in the amendment agreement.
F-19
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
This investor subsequently agreed not to participate in the convertible debt financing for which a closing was held June 8, 2015 and in consideration of such election, the participation right was modified to allow him to exchange such note for comparable securities in an alternative transaction. In consideration of waivers previously granted by the holder of potential events of default under the short-term note and the amendment to extend the maturity date, the company agreed to issue the holder warrants to purchase 3,166,667 shares of common stock of the company. The warrants are exercisable for a period of 54 months commencing six months following the date of issuance and have an exercise price equal to $0.31 per share. Through a series of amendment agreements, this note has been extended through October 16, 2015 and the interest rate has been increased to 12% per annum in connection with such extensions.
On April 24, 2015, the company issued a promissory note in the aggregate principal amount of $500,000 to Lazarus Investment Partners LLLP, the beneficial owner of approximately 29.3% of the company’s common stock, in a private transaction. The note is an unsecured obligation of the company and is not convertible into equity securities of the company. The note was originally due and payable on the first to occur of July 2, 2015 or the date on which the company received at least $900,000 in proceeds from equity or debt financing transactions. Interest on the note was originally 5.76% per annum payable at maturity. The note contains terms and events of default customary for similar transactions. In consideration of the loan, the company and Lazarus entered into a warrant amendment agreement pursuant to which the company agreed to amend certain of the terms of the existing 6,233,636 common stock purchase warrants held by Lazarus to reduce the exercise price of such warrants to $0.25, which was $0.01 above the most recently reported closing consolidated bid price of the company’s common stock prior to the execution of the transaction documents, and to extend the expiration date of the warrants to October 25, 2019. The warrants amended were issued in various transactions from 2010 through 2013 at exercise prices ranging between $0.88 and $2.00. The fair value related to the warrant modifications recorded as a debt discount and amortized over the term of the new agreement. Through a series of amendment agreements, this note has been extended through October 16, 2015 and the interest rate has been increased to 12% per annum in connection with such extensions.
On June 8, 2015, the company entered into definitive agreements relating to a private placement of up to $3.0 million in principal amount of senior secured convertible notes and common stock purchase warrants. An initial closing for an aggregate principal amount of $900,000 of notes and warrants to purchase 3,626,667 shares of common stock was held on June 8, 2015. Subject to certain limitations, the warrants are exercisable on or after the six month anniversary of the date of issuance at an initial exercise price of $0.30 per share and will expire 54 months from the initial exercise date. In addition, subject to certain limitations, the warrants provide that, beginning six months from issuance, the company shall have the right to cause the holder to exercise the warrants provided that the following conditions are satisfied: (i) the closing bid price of the company’s common stock is at least $0.45 for the 20 consecutive trading days prior to the date of the mandatory exercise notice and (ii) all of the warrants issued in the transaction are called by the company for a mandatory exercise. A final closing for up to $2.1 million of debentures and warrants to purchase 8,400,000 shares of common stock has not occurred. As a condition of the initial closing, the holder of an aggregate principal amount of $100,000 of previously issued promissory notes was required to exchange such notes and certain warrants held by it for senior secured notes and warrants issued in this transaction. These notes, subject to certain exceptions, rank senior to existing and future indebtedness of the company and are secured to the extent and as provided in the security agreement entered into between the company and the purchasers. Each note matures on the one-year anniversary of the issuance date thereof and, subject to certain limitations, is convertible at any time at the option of the holder into shares of the company’s common stock at an initial conversion price of $0.25 per share. Subject to certain exemptions, if the company issues or sells shares of its common stock, rights to purchase shares of its common stock, or securities convertible into shares of its common stock for a price per share that is less than the conversion price then in effect, the conversion price then in effect will be decreased to equal 85% of such lower
F-20
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
price and the exercise price of the warrants will be decreased to a lower price based on the amount by which the conversion price of the notes was reduced due to such transaction. The foregoing adjustments to the conversion price will not apply to certain exempt issuances, including issuances pursuant to certain employee benefit plans. In addition, the conversion price is subject to adjustment upon stock splits, reverse stock splits, and similar capital changes. The senior secured notes bear interest at 9% per annum with interest payable upon maturity or on any earlier redemption date. At any time after the issuance date, the company will have the right to redeem all or any portion of the outstanding principal balance of the notes, plus all accrued but unpaid interest at a price equal to 110% of such amount and the holders shall have the right to convert any or all of the amount to be redeemed into common stock prior to redemption. The notes are secured by a first priority lien on the company’s assets related to its “Inscrybe Referral and Order Management” and “Inscrybe Hospital Discharge” solutions. Subject to certain exceptions, the notes contain customary covenants against incurring additional indebtedness and granting additional liens and contain customary events of default. Upon the occurrence of an event of default under the notes, a holder may require the company to repay all or a portion of its notes in cash, at a price equal to 110% of the principal and accrued and unpaid interest.
The company also agreed that if it needs to appoint a new president and/or chief executive officer, it would consult with the holders of a majority in interest of the notes on such matter; provided, however, that the company’s board of directors shall retain full discretion and authority over such matters. The company also agreed to file a registration statement covering the resale of the shares of the company’s common stock issuable upon conversion of the notes and exercise of the warrants and to use commercially reasonable efforts to have the registration declared effective in a timely manner. The Company will be subject to certain monetary penalties, as defined in the agreement if the registration statement is not filed, does not become effective on a timely basis, or does not remain available for the resale, as such term is defined in the registration rights agreement.
Except for the June 8, 2015 transaction, the company allocated the proceeds from the note transactions to the notes and the related warrants based on the relative fair values of such instruments using the fair value of the notes at a market rate of interest and the fair value of the warrants based on the Black-Scholes option pricing model and the applicable assumptions set forth in Note 2 of Notes to Consolidated Financial Statements. As a result of the ratchet provision included in the June 8, 2015 transaction, the company recorded a warrant liability and a debt discount based on the fair value of the warrants issued in connection with the new senior secured note. These allocations resulted in effective interest rates for the $100,000 note of approximately 39% per annum, for the $950,000 note of approximately 65% per annum, for the $500,000 note of approximately 313% per annum and for the $800,000 senior secured note of approximately 36% per annum, respectively. However, since the subjective nature of the inputs for the option pricing and other models used to calculate fair values can materially affect fair value estimates, in management’s opinion, such models may not provide a reliable single measure of the fair value of the warrants and the resulting effective interest rates. In connection with the note transactions the company recorded promissory notes of $2,350,000, debt discount of $560,000, warrant liability of $214,000 and additional paid-in-capital of $902,000 related to the fair value of the warrants.
In connection with the modifications of the $950,000 and $100,000 short-term notes discussed above, the company issued additional warrants and agreed to modifications that, for accounting purposes, resulted in the extinguishment of the old notes and the creation of new notes. Accordingly, the company recorded non-cash losses on extinguishment of approximately $557,000 as other expense in the fourth quarter ended June 30, 2015.
The non-cash amortization of the debt discount and deferred financing costs of $386,000, $127,000 and $2,931,000 is included in other expense for the years ended June 30, 2015, 2014 and 2013, respectively.
F-21
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
The following table sets forth the secured notes and unamortized debt discount (in thousands):
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Notes payable |
||||||||
| Secured notes payable |
$ | 900 | $ | — | ||||
| Unsecured notes patable |
1,450 | — | ||||||
| Unamortized debt discount |
(200 | ) | — | |||||
|
|
|
|
|
|||||
| Notes payable |
$ | 2,150 | $ | — | ||||
|
|
|
|
|
|||||
9. Income Taxes
At June 30, 2015, the company had federal and state net operating loss carryforwards for tax purposes of approximately $162,000,000 and $60,000,000, respectively. Approximately $5,300,000 of the federal net operating loss carryforwards are Separate Return Limitation Year (SRLY) and can only be used by the entity that generated these losses in the separate return years. Federal net operating losses expire in various years between fiscal 2019 and fiscal 2035 and state net operating losses expire in various years between fiscal 2016 and fiscal 2035. The company also has a capital loss carryforward for tax purposes of approximately $13,600,000 related to the sale of its German subsidiary which expires in fiscal 2016 and can only be applied towards capital gains.
The provision for income taxes differs from the amount computed by applying the federal statutory rate of 34% as follows (in thousands):
| Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Computed expected tax benefit |
$ | (3,298 | ) | $ | (2,429 | ) | $ | (3,859 | ) | |||
| Benefit attributable to net operating loss and tax credit carryforwards and other deductible temporary differences not recognized |
3,298 | 2,429 | 3,859 | |||||||||
|
|
|
|
|
|
|
|||||||
| Income tax expense |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The components of deferred tax assets and liabilities consist of the following (in thousands):
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Deferred tax asset: |
||||||||
| Intangible assets |
$ | 619 | $ | 690 | ||||
| Deferred compensation |
742 | 775 | ||||||
| Accrued expenses and other |
583 | 262 | ||||||
| Net operating loss and tax credit carryforwards |
64,544 | 61,831 | ||||||
|
|
|
|
|
|||||
| Total gross deferred tax assets |
66,488 | 63,558 | ||||||
| Less: Valuation allowance |
(66,488 | ) | (63,558 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
F-22
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
The company has recorded a full valuation allowance of an amount equal to its deferred tax assets since it believes it is more likely than not that such deferred tax assets will not be realized. The net change in the total valuation allowance for the years ended June 30, 2015, 2014 and 2013 was an increase of approximately $2,930,000 for 2015, a decrease of approximately $1,741,000 for 2014 and an increase of approximately $3,131,000 for 2013 , respectively. As of June 30, 2015, the company has not recorded any unrecognized tax benefits, which remains unchanged from previous years.
10. Lease Commitments
The company is obligated under operating leases for facilities and equipment expiring at various dates through the year 2017. Future minimum payments are as follows (in thousands):
| Operating Leases |
||||||
| Year Ending June 30: |
2016 | $ | 512 | |||
| 2017 | 299 | |||||
| 2018 | — | |||||
| 2019 | — | |||||
| 2020 | — | |||||
| Thereafter | — | |||||
|
|
|
|||||
| $ | 811 | |||||
|
|
|
|||||
Rental expense was approximately $481,000, $481,000 and $477,000 for the years ended June 30, 2015, 2014 and 2013, respectively. At June 30, 2015 and 2014, restricted cash was being held as collateral for a letter of credit securing certain lease payments.
11. Preferred Stock
The board of directors is authorized to issue shares of preferred stock, $.10 par value per share, from time to time in one or more series. The board may issue a series of preferred stock having the right to vote on any matter submitted to shareholders including, without limitation, the right to vote by itself as a series, or as a class together with any other or all series of preferred stock. The board of directors may determine that the holders of preferred stock voting as a class will have the right to elect one or more additional members of the board of directors, or the majority of the members of the board of directors.
At a special meeting of stockholders held on April 5, 2013, our stockholders approved the automatic conversion of outstanding shares of Series C preferred stock into an aggregate of 3,551,541 shares of common stock, including 1,051,541 shares of common stock issued in lieu of accrued but unpaid dividends on the Series C preferred stock. In connection with amendments to the Series C preferred stock in April 2012, the company issued warrants to purchase 825,000 shares of common stock to the holders of the Series C 15% convertible redeemable preferred stock. These warrants are exercisable commencing six months following their issuance for the period of 54 months at an exercise price of $1.60 per share, which is equal to 101% of the closing bid price of the company’s common stock, as reported on the Nasdaq Stock Market, on the trading day immediately before the stockholders’ meeting on April 9, 2012. The warrants are exercisable for cash or by net exercise and the number of shares of common stock issuable upon exercise of these warrants is subject to adjustment in the case of stock splits, stock dividends, combinations and similar recapitalization transactions.
F-23
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
As of June 30, 2015, there are 28,000 shares of Series B preferred stock outstanding. The Series B preferred stock was originally issued in a private financing in October 1999 and the conversion and redemption features were amended in October 2002 to provide for the rights and obligations described in this note. The company has the right to repurchase the outstanding Series B preferred stock at a redemption price equal to $25.00 per share, plus accrued and unpaid dividends. The holder of such shares has the right to convert shares of preferred stock into an aggregate of 250,000 shares of our common stock at a conversion rate of $2.80 per share. In the event the company elects to redeem these securities, the holder will be able to exercise its conversion right subsequent to the date that we issue a notice of redemption but prior to the deemed redemption date as would be set forth in such notice. At June 30, 2015, the company accrued dividends in the amount of $70,000 which have been paid.
As of June 30, 2015, there are 665,000 shares of Series D convertible preferred stock outstanding. The Series D preferred stock was issued in June 2013 and can be converted by the holders into an aggregate of 6,125,024 shares of common stock at an initial conversion rate of $1.08571 per share. The holder of such shares has the right to convert the preferred shares at any time commencing on the six month anniversary of the issue date; however, the shares received upon conversion may not be offered or sold except pursuant to an effective registration statement or an applicable exemption from the registration requirements of the Securities Act and applicable state securities laws. The company has the right to repurchase the outstanding Series D preferred stock at a redemption price equal to $10.00 per share, plus accrued and unpaid dividends, beginning two years after issuance and to require holders to convert their Series D preferred stock beginning three years after issuance. Dividends on the Series D preferred stock accrue at a rate of 5% per annum and are payable semi-annually in cash or stock at the company’s option. The company paid accrued dividends for fiscal 2015 and 2014 of $332,500 and $342,521, respectively, through the issuance of 306,254 and 275,228 shares of restricted common stock, respectively, to holders of outstanding shares of Series D convertible preferred stock in accordance with the applicable terms of the Series D convertible preferred stock. At June 30, 2015, the company has no accrued dividends.
12. Common Stock Warrants
As discussed more fully in Notes 8, 11 and 19 of Notes to Consolidated Financial Statements, during fiscal 2012 the company issued warrants to purchase shares of the company’s common stock as follows: 3,022,388 warrants to accredited investors in connection with the issuance of senior secured notes, 825,000 warrants to the holders of Series C preferred stock in connection with the extension of the maturity date for such securities and 1,468,752 warrants to institutional and accredited investors in a registered direct offering. These warrants are fully vested and have exercise prices of $1.34, $1.60 and $2.00, respectively.
As discussed more fully in Notes 8 and 19 of Notes to Consolidated Financial Statements, during fiscal 2013 the company issued warrants to purchase shares of the company’s common stock as follows: 2,558,139 warrants to accredited investors in connection with the issuance of senior secured notes, 2,197,674 warrants to accredited investors in connection with the extension of the maturity date of the senior secured notes issued in fiscal 2012, 6,650,000 warrants to accredited investors in connection with the sale of Series D preferred stock and 4,683,685 warrants to institutional and accredited investors in an underwritten offering. These warrants are fully vested and have exercise prices of $1.34, $1.34, $0.95 and $0.95, respectively. During fiscal 2013, the company also issued warrants to purchase 225,000 shares of the company’s common stock to a consultant for services. These warrants vested on a pro-rata basis through August 31, 2013 and have an exercise price of $1.34 per share. The fair value of these warrants, as determined by the Black Scholes Model was charged to operations over the twelve month term of the contract which expired on November 30, 2013.
F-24
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
As discussed more fully in Note 19 of Notes to Consolidated Financial Statements, during fiscal 2014, the company issued warrants to purchase 774,716 shares of the company’s common stock to accredited investors in connection with a private placement in November 2013. These warrants are fully vested and are exercisable at $1.38 per share. In addition, during fiscal 2014, the company issued warrants to purchase shares of the company’s common stock as follows: (i) 50,000 warrants to a consultant for services with an exercise price of $0.89 per share. These warrants vest monthly in arrears through July 31, 2014, have a five year life and, subject to vesting requirements, are exercisable beginning six months after the grant date, (ii) 500,000 warrants to a consultant for services with an exercise price of $1.34 per share, and (iii) 1,000,000 warrants to a consultant for services with an exercise price of $1.76 per share. These warrants vest in equal installments of 50,000 warrants and 100,000 warrants, respectively, based on performance-based targets related to service revenues recognized by the company from customers covered by such agreements, have a five year life, and, subject to vesting requirements, are exercisable beginning six months after the grant date. The fair value of these warrants, as determined by the Black Scholes Model, is being charged to operations over the service period for the time based warrants and for the performance based warrants costs will be recognized during the periods that performance targets are achieved. During fiscal year 2014, 100,000 warrants vested and the fair value of those warrants of $59,000 was charged to operations.
During fiscal 2015 the company issued warrants to purchase shares of the company’s common stock as follows: 150,000 warrants to a consultant for services with an exercise price of $0.84 per share. These warrants vest monthly in arrears through March 31, 2015, have a two year life and, are exercisable beginning six months after the grant date. The fair value of these warrants, as determined by the Black Scholes Model, is being charged to operations over the service period. Further, as discussed more fully in Notes 8 and 19 of Notes to Consolidated Financial Statements, the company issued warrants to purchase 6,972,834 shares of the company’s common stock, of which 80,000 warrants were cancelled, in connection with the issuance and modification of promissory notes and 1,003,678 shares of the company’s common stock in a registered direct offering in August 2014. These warrants are fully vested and have exercise prices ranging from $0.30 to $1.01 per share.
A schedule of common stock warrant activity is as follows (in thousands, except per share and average life data):
| Number of Shares |
Weighted Average Exercise Price Per Share |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Outstanding, June 30, 2012 |
8,651 | $ | 1.53 | |||||||||||||
| Warrants issued |
16,315 | 1.03 | ||||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2013 |
24,966 | 1.23 | ||||||||||||||
| Warrants issued |
2,324 | 1.52 | ||||||||||||||
| Warrants exercised |
(483 | ) | 0.95 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2014 |
26,807 | 1.26 | ||||||||||||||
| Warrants issued |
8,127 | 0.40 | ||||||||||||||
| Warrants expired |
(1,265 | ) | 1.86 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding, June 30, 2015 |
33,669 | $ | 0.84 | 3.40 | $ | — | ||||||||||
|
|
|
|||||||||||||||
| Exercisable, June 30, 2015 |
26,276 | $ | 0.97 | 3.01 | $ | — | ||||||||||
|
|
|
|||||||||||||||
F-25
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
13. Commitments and Contingencies
On May 22, 2015, the company, together with its subsidiary Express MD Solutions, LLC entered into a license and settlement agreement with Robert Bosch Healthcare Systems, Inc. providing for the resolution and dismissal, with prejudice, of the purported patent infringement lawsuit filed by Bosch against Express MD in January 2012 in the U.S. District Court for the Northern District of California, Case No. 5:12-cv-00068-JW. While the company does not believe that it is infringing any of the asserted patents, in order to mitigate its risk and avoid further costs and distractions of litigation, it entered into the license and settlement agreement. As previously reported by the company, the complaint alleged that the company’s “Electronic House Call” product infringes one or more claims of certain patents allegedly owned by Bosch. The agreement provides for the company to be granted by Bosch a worldwide, non-exclusive, non-assignable, royalty bearing license under the patents and a covenant not to be sued under certain other patents owned by Bosch. In consideration thereof, the company agreed to pay Bosch for the license and related matters and to pay certain royalties to Bosch for a three year period thereafter.
During fiscal 2009, the company purchased certain inventory components under arrangements that required payment only after such components where actually used in production. Based on the company’s current production plans we have determined that these components will not be used and we offset the inventory balance for undelivered items against the related accounts payable balance during the quarter ended March 31, 2015. Additionally, we intend to return approximately $0.2 million of such items that are included in the inventory and accounts payable balances as of June 30, 2015. The vendor that provided these components is currently disputing this arrangement and is requesting payment for these items. The company believes the amount at issue is approximately $1.2 million and intends to vigorously contest this assertion. Management does not believe that the ultimate resolution of this matter will have a material adverse effect on our consolidated financial position, results of operations or cash flows.
We are also subject to claims and litigation arising in the ordinary course of business. Our management considers that any liability from any reasonably foreseeable disposition of such claims and litigation, individually or in the aggregate, would not have a material adverse effect on our consolidated financial position, results of operations or cash flows.
We are not currently engaged in any other litigation which would be anticipated to have a material adverse effect on our financial condition or results of operations.
We have entered into employment agreements with our chief executive officer and chief financial officer that specify the executive’s current compensation, benefits and perquisites, the executive’s entitlements upon termination of employment, and other employment rights and responsibilities.
We have entered into various agreements by which we may be obligated to indemnify the other party with respect to certain matters. Generally, these indemnification provisions are included in contracts arising in the normal course of business under which we customarily agree to hold the indemnified party harmless against losses arising from a breach of representations related to such matters as intellectual property rights. Payments by us under such indemnification clauses are generally conditioned on the other party making a claim. Such claims are generally subject to challenge by us and to dispute resolution procedures specified in the particular contract. Further, our obligations under these arrangements may be limited in terms of time and/or amount and, in some instances, we may have recourse against third parties for certain payments made by us. It is not possible to predict the maximum potential amount of future payments under these indemnification agreements due to the conditional nature of our obligations and the unique facts of each particular agreement. Historically, we have not
F-26
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
made any payments under these agreements that have been material individually or in the aggregate. As of June 30, 2015, we are not aware of any obligations under such indemnification agreements that would require material payments.
14. Supplemental Cash Flow Information
We did not pay any income taxes during the fiscal years ended June 30, 2015, 2014 and 2013. See Notes 8, 11, 18 and 21 of Notes to Consolidated Financial Statements for supplemental cash flow information regarding debt discount, the non-cash loss on extinguishment of certain notes, the non-cash dividends paid in connection with the Series C and Series D preferred stock, the reclassification of the Series B preferred stock to shareholders equity, acquired licenses and interest expense, respectively.
15. Employee Benefit Plan
The company maintains a qualified defined contribution 401(k) profit sharing plan for all eligible employees and may make discretionary contributions in percentages of compensation, or amounts as determined by our board of directors. The company did not make any contributions to the plan for the years ended June 30, 2015, 2014 and 2013.
16. Other Intangible Assets
The following table set forth licenses, net and other intangible assets that are included in other assets as follows (in thousands):
| June 30, 2015 | June 30, 2014 | Useful Life In Years |
||||||||||||||||||||||||||
| Gross Carrying Amount |
Accumulated Amortization |
Net Book Value |
Gross Carrying Amount |
Accumulated Amortization |
Net Book Value |
|||||||||||||||||||||||
| Patents |
$ | 357 | $ | 276 | $ | 81 | $ | 353 | $ | 256 | $ | 97 | 17 | |||||||||||||||
| Trademarks |
214 | 108 | 106 | 205 | 97 | 108 | 20 | |||||||||||||||||||||
| Acquired technologies |
95 | 72 | 23 | 72 | 72 | — | 2 | |||||||||||||||||||||
| Licenses |
4,212 | 2,308 | 1,904 | 3,887 | 1,954 | 1,933 | 3-10 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total |
$ | 4,878 | $ | 2,764 | $ | 2,114 | $ | 4,517 | $ | 2,379 | $ | 2,138 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
As of June 30, 2015 and 2014 goodwill amounted to $50,000 and is included in other assets.
The company amortizes other intangible assets using the straight line method. Amortization expense was approximately $385,000, $333,000 and $291,000 for the years ended June 30, 2015, 2014 and 2013, respectively.
F-27
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
Amortization expense for the next five fiscal years and thereafter is expected to be as follows (in thousands):
| June 30, |
||||
| 2016 |
$ | 518 | ||
| 2017 |
423 | |||
| 2018 |
331 | |||
| 2019 |
303 | |||
| 2020 |
276 | |||
| Thereafter |
263 | |||
|
|
|
|||
| $ | 2,114 | |||
|
|
|
|||
17. Financial Instruments
We use a variety of methods and assumptions to estimate the fair value of each class of financial instruments for which it is practicable to estimate that value, including cash and cash equivalents, marketable securities, accounts receivable, other currents assets, accounts payable and accrued expenses and other current liabilities. To date, the carrying amount of these assets and liabilities approximates fair value because of the short maturity of these instruments. For additional information regarding marketable securities see Note 1 of Notes to Consolidated Financial Statements.
18. Business Acquisition
In June 2008 we formed a joint venture with EncounterCare Solutions, Inc., called ExpressMD™ Solutions LLC to provide in-home patient vital signs monitoring systems and services. The company and EncounterCare Solutions, Inc. each owned fifty percent of the joint venture and neither party had any special rights under the joint venture agreement. ExpressMD Solutions did not have any assets or liabilities and EncounterCare Solutions, Inc. did not have any recourse to our general credit. ExpressMD Solutions was consolidated in our financial statements because the company elected to provide the majority of funding for the joint venture and was deemed to be the primary beneficiary.
On November 21, 2011, the company entered into a definitive joint venture termination agreement with EncounterCare Solutions, Inc. (“ECS”), providing for the assignment and transfer to the company of all of the membership interests held by ECS in ExpressMD Solutions. At the closing on November 21, 2011, the joint venture agreement was terminated, ExpressMD Solutions became a wholly-owned subsidiary of the company and ECS and an affiliated company of ECS entered into an intellectual property license and supply agreement and the company and ECS entered into a registration rights agreement. Pursuant to the license agreement, the company was granted a worldwide, perpetual, irrevocable, royalty-free, non-exclusive license to use the intellectual property of ECS and the affiliated company of ECS to continue to commercialize and develop the remote patient monitoring devices and related software sold by ExpressMD Solutions and to develop improvements and other products based on such intellectual property. Further, pursuant to the license agreement, the company shall supply to ECS, and ECS shall purchase exclusively from the company, such quantities of the ExpressMD Solutions device as is reasonably requested by ECS. Pursuant to the registration rights agreement the company filed a registration statement with the Securities and Exchange Commission to register the resale of the shares of common stock issued at closing which was declared effective on April 5, 2012. The termination agreement also provides that within 90 days immediately prior to the fifth anniversary of the closing date, the company shall assign the trademark “ExpressMD” to ECS. At that time, the company will have a limited period
F-28
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
within which to transition to a new trademark for the ExpressMD products. The company believes that this transaction provides it with greater flexibility to meet emerging market needs in the telehealth space and will enable it to continue to offer innovative and leading-edge products and solutions to its customers. The transaction was accounted for under the purchase method of accounting and, as discussed above, ExpressMD Solutions will continue to be consolidated in the company’s financial statements. The company paid $1,000,000 in cash, forgave a receivable for approximately $58,000 and issued 750,000 shares of company stock valued at $1,215,000, based on the closing market price for the shares at closing, for a total purchase price of approximately $2,273,000. The company also incurred acquisition related costs of approximately $36,000 which were expensed as selling, general and administrative expenses and approximately $15,000 to cover the registration of the common stock issued in connection with the transaction. The company allocated the entire purchase price to the licensed intangible assets referred to above since the joint venture had no assets or liabilities on the closing date. We estimated the fair value of the license using level 3 inputs for future operating results and present value techniques applied to estimated royalty amounts which is a common method used to value licensed intangible assets. There was no bargain purchase gain or goodwill related to the transaction.
19. Equity Offerings
In February 2004, the company completed a private sale of its common stock to certain accredited investors pursuant to Section 4(2) of the Securities Act of 1933 (the “Securities Act”), as amended and Regulation D, promulgated thereunder. The company sold a total of 2,680,185 common shares at a price of $27.50 per share and realized gross proceeds of $73.7 million. After payment of offering expenses and broker commissions the company realized $69.1 million in net proceeds. The shares of common stock issued are restricted securities and have not been registered under the Securities Act, or any state securities law, and unless so registered, may not be offered or sold in the United States absent a registration or applicable exemption from the registration requirements of the Securities Act and applicable state securities laws.
During fiscal 2000, the company sold 50,000 shares of a newly created class of Series B convertible cumulative preferred stock (the “Series B preferred stock”). The Series B preferred stock was sold at $25.00 per share for an aggregate offering price of $1.2 million. Dividends on the Series B Preferred Stock are payable at the rate of 10% per annum, semi-annually in cash. Each share of Series B preferred stock is convertible into shares of the company’s common stock or is converted into such number of shares of the common stock as shall equal $25.00 divided by the conversion price of $3.75 per share, subject to adjustment under certain circumstances. Commencing three years after the closing, the conversion price shall be the lower of $3.75 per share or the average of the closing bid and asked price of the company’s common stock for the 10 consecutive trading days immediately ending one trading day prior to the notice of the date of conversion; provided, however, that the holders are not entitled to convert more than 20% per month of the Series B preferred shares held by such holder on the third anniversary of the date of issuance. The Series B preferred stock is redeemable at the option of the company at any time commencing one year after issuance or not less than 30 nor more than 60 days after written notice at a redemption price of $25.00 per share plus accrued and unpaid dividends provided; (i) the public sale of the shares of common stock issuable upon conversion of the Series B preferred stock (the “Conversion Shares”) are covered by an effective registration statement or are otherwise exempt from registration; and (ii) during the immediately preceding 20 consecutive trading days ending within 10 days of the date of the notice of redemption, the closing bid price of the company’s common stock is not less than $7.50 per share. The Series B voting rights are limited to any issue which would adversely affect their rights.
On October 30, 2002, the company filed a Certificate of Amendment of the Certificate of Designations, Preferences and Rights and Number of Shares of Series B preferred stock with the Secretary of State of the State
F-29
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
of Delaware. The amendment provides that the conversion rate applicable to the outstanding shares of Series B preferred stock will be fixed at $2.80. Previously, the conversion rate was equal to the lower of $3.75 and the average of the closing bid and asked prices of the company’s common stock for the immediately preceding ten consecutive trading days ending one day prior to the notice of conversion; provided, however, that the conversion rate would not be below $1.75. Accordingly, the outstanding 28,000 shares of Series B preferred stock are presently convertible into an aggregate of 250,000 shares of the company’s common stock. Prior to the amendment, the outstanding shares of Series B preferred stock were convertible into a maximum of 400,000 shares of the company’s common stock. In consideration of obtaining the consent of the holder of the outstanding Series B preferred stock, the company agreed to defer its ability to redeem those shares for a period of two years. The outstanding shares of Series B Preferred Stock are held in the name of Greener Fairways, Inc., an entity affiliated with Douglas B. Luce, who is the brother of J. David Luce, a member of our board of directors.
As of June 30, 2015, 22,000 Series B preferred shares have been converted leaving 28,000 shares outstanding. Commencing October 2004, the Series B preferred stock is redeemable at the option of the company without regard to the closing price of the company’s common stock. During the fiscal years ended June 30, 2015 and 2014, no Series B preferred stock was redeemed.
In December 2009, the company completed a registered direct offering with certain institutional and/or accredited investors pursuant to which the company agreed to sell an aggregate of 1,700,000 shares of its common stock and warrants to purchase a total of 1,700,000 shares of its common stock to the investors for gross proceeds of $3.4 million. The purchase price of a share of common stock and warrant was $2.00 per share. The warrants were exercisable for a period of 90 days following the closing date of the offering at an exercise price of $2.00. Through March 10, 2010, the expiration date of these warrants, an aggregate of 349,493 warrants were exercised and the remainder expired on the stated expiration date. The company received proceeds of $0.7 million from the exercise of such warrants. The net proceeds to the company from the offering and subsequent warrant exercises, after deducting placement agent fees and the company’s offering expenses, were approximately $3.54 million. The company also issued the placement agent a warrant to purchase 85,000 shares of common stock at a per share exercise price of $2.50, which warrants will be exercisable for a period of five years from the effective date of the registration statement. The common stock, warrants to purchase common stock and shares of common stock issuable upon exercise of the warrants were issued pursuant to a prospectus supplement filed with the Securities and Exchange Commission on December 9, 2009 in connection with a takedown from the company’s shelf registration statement on Form S-3 (File No. 333-161220), which was declared effective by the Securities and Exchange Commission on September 30, 2009.
On October 12, 2010, the company entered into a securities purchase agreement with selected institutional and accredited investors to sell and issue $5.0 million of units of its securities in a private placement under Section 4(2) of the Securities Act of 1933, as amended, and Rule 506 of Regulation D promulgated thereunder. In the aggregate, the company agreed to sell 1,250,000 units of securities, at a price of $4.00 per unit, with the units consisting of a total of 3,750,000 shares of common stock, 1,250,000 shares of Series C 15% convertible redeemable preferred stock, and warrants to purchase an additional 3,125,000 shares of common stock. Each individual unit consists of three shares of common stock, one share of preferred stock and two and one half warrants. The transaction closed on October 13, 2010 and the company received net proceeds from the private placement of approximately $4.46 million. The terms of the Series C 15% convertible redeemable preferred stock are discussed in Note 11 of Notes to Consolidated Financial Statements. The warrants issued in this 2010 private placement are exercisable for shares of the company’s common stock at an exercise price of $1.40 per share for a period of 54 months and will be exercisable for cash or by net exercise in the event that there is no effective registration statement covering the resale of the shares of common stock underlying the warrants. The value of these warrants using the Black-Scholes option pricing model was approximately $2.85 million.
F-30
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
In connection with the 2010 private placement, the company entered into a registration rights agreement with the investors. Pursuant to the registration rights agreement, the company agreed to file a registration statement with the Securities and Exchange Commission within 45 days from closing to register the resale of the shares of common stock to be issued at closing and the shares of common stock underlying the preferred stock and the warrants (collectively the “Registrable Securities”). The company also agreed to use its best efforts to have the registration statement declared effective as promptly as possible after the filing thereof, but in any event within 90 days from the filing date. This registration statement was declared effective on December 9, 2010. In the event it ceases to remain continuously effective as required by the registration rights agreement (each such event, a “Registration Default”), then the company has agreed to pay each investor as liquidated damages an amount equal to 1.0% of the purchase price paid by each such investor with respect to any Registrable Securities then held and not registered pursuant to an effective registration statement, per 30-day period or portion thereof during which the Registration Default remains uncured thereafter, subject to a limitation of 6% per Registration Default. In addition, the company agreed to keep the registration statement continuously effective until the earlier to occur of (i) the date after which all of the Registrable Shares registered thereunder shall have been sold and (ii) the date on which 100% of the Registrable Securities covered by such registration statement may be sold without volume restrictions pursuant to Rule 144 under the Securities Act of 1933, as amended.
On October 7, 2011, the company entered into a Placement Agency Agreement (the “Placement Agency Agreement”) with C.K. Cooper & Co., Inc. (“CKCC”) and an Engagement Agreement (the “Engagement Agreement”) with Rodman & Renshaw LLC (“Rodman”) whereby it engaged them as co-placement agents (the “Placement Agents”) relating to a registered direct offering by the Company to select investors. In addition, as of October 7, 2011, the company entered into a securities purchase agreement with certain institutional and/or accredited investors pursuant to which the company agreed to sell an aggregate of 2,937,497 shares of its common stock, par value $0.001 per share, and warrants to purchase a total of 1,468,752 shares of common stock to the investors for gross proceeds of $4.1 million. The purchase price of a share of common stock and warrant was $1.40. The warrants are exercisable for a period of four years commencing on the six month anniversary of the date on which they were issued and will have an initial exercise price of $2.00 per share. The value of these warrants using the Black Scholes option pricing model was approximately $1.83 million. The transaction closed on October 13, 2011 and the net proceeds to the company, after deducting placement agent fees and expenses and the company’s offering expenses, are approximately $3.6 million.
On June 20, 2013, the company completed the sale of 665,000 shares of Series D convertible preferred stock and warrants to purchase 6,650,000 shares of common stock to a number of holders of its senior secured notes and other investors in consideration for the cancellation of $6.5 million of senior secured notes and $0.15 million in additional cash proceeds. After deducting offering expenses, the company received net proceeds of approximately $0.03 million. The terms of the Series D preferred stock are discussed in Note 11 of Notes to Consolidated Financial Statements. The warrants issued with the Series D preferred stock are exercisable commencing six months following their issuance for a period of 54 months at an exercise price of $0.95 per share. The company allocated the proceeds from the transaction to the Series D preferred stock and the warrants based on the relative fair values of such instruments using the fair value of the common stock issuable on conversion of the Series D preferred stock and the fair value of the warrants based on the Black Scholes option pricing model and the applicable assumptions set forth in Note 2 of Notes to Consolidated Financial Statements. This allocation assigned approximately $2.98 million of the proceeds to the fair value of the warrants and resulted in a beneficial conversion feature related to the Series D preferred stock of approximately $2.15 million since the effective conversion rate was below the fair value of the common stock issuable on conversion. As discussed in Note 3 of Notes to Consolidated Financial Statements, the beneficial conversion feature is being treated as a deemed dividend in the company’s loss per share calculation. In connection with the Series D preferred stock transaction, the company also entered into a registration rights agreement with the holders of the
F-31
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
Series D preferred stock and warrants dated June 11, 2013, pursuant to which we granted the holders a right to require us to file a registration statement with the Securities and Exchange Commission covering the resale of the shares of common stock issuable under the Series D preferred stock and exercise of the warrants. We also agreed to grant piggyback registration rights to the purchasers of the Series D preferred stock and warrants.
On June 17, 2013, the company completed an underwritten public offering of 4,683,685 units (including the full exercise by the underwriter of the over-allotment option) of securities at a price of $0.95 per unit. Each unit consists of one share of common stock and one warrant to purchase one share of common stock at an exercise price of $0.95 per share. The warrants are exercisable for a period of five years from the date of issuance. The value of these warrants using the Black-Scholes option pricing model was approximately $3.33 million. J.P. Turner & Company, LLC served as the underwriter for this transaction and the securities were offered by the company pursuant to a shelf registration statement that was previously filed by the company and declared effective by the Securities and Exchange Commission. The company received net proceeds, after deducting underwriting fees and expenses and the company’s offering expenses, of approximately $3.93 million.
On November 12, 2013, the company completed a private placement transaction with certain accredited and/or institutional investors of 2,347,625 shares of common stock and warrants to purchase 774,716 shares of common stock at a unit price of $1.05 per share and warrant. The warrants are exercisable commencing six months following their issuance for a period of 54 months at an exercise price of $1.38 per share. The fair value of these warrants using the Black-Scholes Model was approximately $0.5 million. After deducting offering expenses, the company received net proceeds of approximately $2.4 million. In connection with the offering the company also entered into a registration rights agreement with the investors pursuant to which we granted the investors a right, commencing on the one-year anniversary of the closing, to require us to file a registration statement with the Securities and Exchange Commission covering the resale of the common stock and the shares issuable upon the exercise of the warrants. We also agreed to grant the investors piggyback registration rights related to these securities.
On August 28, 2014, we entered into securities purchase agreements with certain institutional and/or accredited investors, including certain affiliated persons, pursuant to which we sold an aggregate of 3,041,454 shares of common stock and warrants to purchase up to an aggregate of 1,003,678 shares of common stock for gross proceeds of $2,169,040 in a registered direct offering. The purchase price for a unit consisting of one share of common stock and a warrant to purchase 0.33 shares of common stock was $0.71, except that such purchase price per unit was $0.75125 for those investors that are our officers or directors. The warrants are exercisable for a period of 54 months commencing on the six month anniversary of the date on which they are issued and have an exercise price of $0.8875 per share. The net proceeds to us from this transaction, after deducting offering expenses, are approximately $2.18 million. One of the investors in the offering, Lazarus Investment Partners LLLP, was the beneficial owner of approximately 29.6% of our outstanding shares of common stock immediately prior to the offering, agreed to purchase $500,000 worth of shares of common stock and warrants (704,225 shares and 232,394 warrants). The manager of the general partner of Lazarus Investment Partners, LLLP, is the brother of Dr. Todd A. Borus, a member of our board of directors. Dr. Borus agreed to purchase 33,278 shares of common stock and 10,982 warrants (subscription proceeds of $25,000). Further, Sarah Trent Harris, a family member of Charles C. Lucas, the Chairman of our Board, agreed to purchase 211,268 shares of common stock and 69,718 warrants (subscription proceeds of $150,000). In addition, an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of our board of directors, agreed to purchase 352,113 shares of common stock and 116,197 warrants (subscription proceeds of $250,000). O’Connell Benjamin, our former chief executive officer agreed to purchase 133,111 shares of common stock and 43,927 warrants (subscription proceeds of $100,000) and William A. Marshall, our chief financial officer, agreed to purchase 66,556 shares of common stock and 21,963 warrants (subscription proceeds of $50,000).
F-32
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
20. Related Party Transactions
As described in greater detail in Note 8 of Notes to Consolidated Financial Statements, J. David Luce, a member of our board of directors, John J. Waters, who was a member of our board of directors at the time of the transactions, O’Connell Benjamin, our former chief executive officer and a former member of our board of directors, William Marshall, our chief financial officer and Lazarus Investment Partners LLLP, the manager of which is the brother of Dr. Todd A. Borus a member of our board of directors, participated in one or both of the company’s senior secured note transactions completed in March 2012 and September 2012 and the agreement to extend the maturity date of the March 2012 notes.
On September 25, 2012, the company entered into a Board Nomination and Observer Agreement (the “Board Agreement”) with Lazarus Investment Partners, LLLP (“Lazarus Investment”) pursuant to which the company granted Lazarus Investment the right to appoint either an observer to its board of directors or to nominate an individual for election to its board of directors. So long as Lazarus Investment owns 5% or more of the company’s outstanding common stock it may designate an observer to attend all meetings of our board in a non-voting capacity for a period of two years. In addition, in lieu of designating an observer, so long as it owns at least 10% of the company’s outstanding common stock, Lazarus Investment shall have the right to designate one person to be nominated for election to the board. If Lazarus Investment nominates an individual for election to the board, the company shall promptly increase the size of the board, appoint such nominee as a member of the board and, subject to the terms of the Board Agreement, use best efforts to include such nominee in the slate of nominees recommended for election as a director for three years.
On March 22, 2013, the company entered into an agreement with certain purchasers that were a party to the securities purchase agreement dated October 12, 2010 and who held a majority of the currently outstanding securities sold by us in our October 2010 private placement, to amend the terms of the 2010 purchase agreement, in order to provide for a six-month restriction on the transferability of the shares of common stock issuable upon conversion of the Series C preferred stock. We entered into this amendment agreement with the purchasers holding a majority of the outstanding securities which were issued under the 2010 purchase agreement, including Lazarus Investment Partners, LLLP, which as of the record date for our special meeting held on April 5, 2013, owned approximately 23.8% of our common stock and 40% of our Series C preferred stock. In connection with the execution of this amendment agreement, on March 22, 2013, we also amended the Board Agreement discussed above to extend for 90 days the time period within which Lazarus Investment Partners must decide whether to nominate an individual for election to our board of directors.
At the special meeting of stockholders held on April 5, 2013, our stockholders approved the automatic conversion of the outstanding shares of Series C preferred stock into an aggregate of 3,551,541 shares of common stock, including 1,051,541 shares of common stock issued in lieu of accrued but unpaid dividends on the Series C preferred stock. Lazarus Investment Partners was issued 1,420,616 shares of common stock upon conversion of the shares of Series C preferred stock held by it.
As described in greater detail in Note 19 of Notes to Consolidated Financial Statements, in June 2013, we entered into a securities purchase agreement with certain accredited investors pursuant to which we agreed to issue a total of 665,000 shares of Series D preferred stock and warrants to purchase 6,650,000 shares of common stock. The shares of Series D preferred stock and warrants were sold as units, with each unit consisting of one share of Series D preferred stock and ten warrants. Investors that held an aggregate principal amount of $6,500,000 of senior notes agreed to surrender their notes in consideration of the issuance of the shares of Series D preferred stock and warrants and other investors purchased $150,000 of such securities. At closing, which occurred on June 20, 2013, we received an aggregate of $6,650,000 in cancellation of indebtedness and
F-33
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
additional funds. An aggregate principal amount of $4,850,000 of the senior notes surrendered in this transaction were held by certain of our officers, directors and our largest stockholder, as follows: an entity affiliated with J. David Luce, a member of our board of directors, and his spouse, held an aggregate principal amount of $2,650,000 of senior notes, and O’Connell Benjamin, our former chief executive officer and a former member of our board of directors, and our chief financial officer, William Marshall, each held an aggregate principal amount of $100,000 of senior notes. The parties affiliated with Mr. Luce acquired 265,000 shares of Series D preferred stock and 2,650,000 warrants and each of Mr. Benjamin and Mr. Marshall acquired 10,000 shares of Series D preferred stock and 100,000 warrants. Further, Lazarus Investment Partners LLLP, which was the beneficial owner of approximately 24.9% of our outstanding shares of common stock immediately prior to this transaction, held an aggregate principal amount of $2,000,000 of senior notes. We issued to Lazarus Investment Partners a total of 200,000 shares of Series D preferred stock and 2,000,000 warrants. The manager of the general partner of Lazarus Investment Partners, LLLP, is the brother of Dr. Todd A. Borus, a member of our board of directors. In addition, Dr. Todd A. Borus participated in this transaction as an investor and purchased 2,500 shares of Series D preferred stock and 25,000 warrants. As previously reported by the company and as described elsewhere herein, the company has issued the holders of its Series D preferred stock including the related persons noted above, shares of common stock in lieu of the cash payment of dividends on the outstanding shares of Series D preferred stock.
In addition, in connection with the foregoing, we further amended our Board Agreement with Lazarus Investment Partners LLLP. Under this amendment, we again extended by 90 days, to September 19, 2013, the time period within which Lazarus Investment Partners must decide whether to nominate an individual for election to our board of directors. Subsequently, on September 19, 2013, we further amended the Board Agreement to extend by an additional 90 days, to December 18, 2013, the time period within which Lazarus Investment Partners must decide whether to nominate an individual for election to our board of directors. In October 2013, Lazarus Investment Partners exercised its appointment right and nominated Mr. Jeffrey A. Beunier to our board. Our board subsequently increased its size to six members and elected Mr. Beunier to serve as a director on October 31, 2013. He was subsequently elected to serve as a director by the company’s stockholders at our May 2014 annual meeting. Mr. Beunier subsequently resigned from the board on July 1, 2015
In connection with the Series D preferred stock transaction, our former chief executive officer, O’Connell Benjamin and a member of our board of directors, David Luce, agreed to grant a holder of senior notes an option to require them to purchase from him, commencing October 15, 2013, an aggregate of $350,000 of shares of Series D preferred stock and 350,000 warrants pursuant to the terms and conditions of a Put/Call Option Agreement. Under the Put/Call Option Agreement, if the holder declines to exercise its right to require the sale of these securities to Messrs. Luce and Benjamin, then they shall thereafter have a 30-day right to purchase all of such securities from the holder. Following the exercise of the option right by the holder in November 2013, each of Mr. Luce and Mr. Benjamin acquired 50% of these additional securities. Mr. Luce subsequently transferred the securities acquired upon exercise of this right to an entity affiliated with his sibling.
As described more fully in Note19 of Notes to the Consolidated Financial Statements, on August 28, 2014, the company entered into securities purchase agreements with certain institutional and/or accredited investors pursuant to which we sold an aggregate of 3,041,454 shares of common stock and warrants to purchase up to an aggregate of 1,003,678 shares of common stock. One of the investors in the offering, Lazarus Investment Partners LLLP, was the beneficial owner of approximately 29.6% of our outstanding shares of common stock immediately prior to the offering, agreed to purchase $500,000 worth of shares of common stock and warrants (704,225 shares and 232,394 warrants). The manager of the general partner of Lazarus Investment Partners, LLLP, is the brother of Dr. Todd A. Borus, a member of our board of directors. Dr. Borus agreed to purchase
F-34
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
33,278 shares of common stock and 10,982 warrants (subscription proceeds of $25,000). Further, Sarah Trent Harris, a family member of Charles C. Lucas, the Chairman of our Board, agreed to purchase 211,268 shares of common stock and 69,718 warrants (subscription proceeds of $150,000). In addition, an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of our board of directors, agreed to purchase 352,113 shares of common stock and 116,197 warrants (subscription proceeds of $250,000). O’Connell Benjamin, our former chief executive officer agreed to purchase 133,111 shares of common stock and 43,927 warrants (subscription proceeds of $100,000) and William A. Marshall, our chief financial officer, agreed to purchase 66,556 shares of common stock and 21,963 warrants (subscription proceeds of $50,000).
As described more fully in Note 8 of Notes to the Consolidated Financial Statements, on February 17, 2015, the company issued a short-term promissory note in the aggregate principal amount of $950,000 and warrants to purchase 99,500 shares of common stock to an accredited investor for gross proceeds of $950,000. The holder of the short-term note is an entity controlled by Douglas B. Luce, the brother of J. David Luce, a member of the board of directors of the company. On April 3, 2015, the company entered into an amendment agreement with the holder of the $950,000 short-term promissory note in order to amend such note to extend its maturity date from March 19, 2015 to July 2, 2015. In addition, pursuant to the amendment agreement, the company also agreed to grant the holder the right to exchange the principal amount of the short-term note (and unpaid interest thereon) into the securities of the company sold in the next financing, as defined in the amendment agreement. This investor subsequently agreed not to participate in the convertible debt financing for which a closing was held June 8, 2015 and in consideration of such election, the participation right was modified to allow him to exchange such note for comparable securities in an alternative transaction. In consideration of waivers previously granted by the holder of potential events of default under the short-term note and the amendment to extend the maturity date, the company agreed to issue the holder warrants to purchase 3,166,667 shares of common stock of the company. The warrants are exercisable for a period of 54 months commencing six months following the date of issuance and have an exercise price equal to $0.31 per share. As discussed more fully in Note 25 of Notes to the Consolidated Financial Statements, the parties have entered into several amendment agreements to extend the maturity date of this loan.
On April 24, 2015, the company issued a promissory note in the aggregate principal amount of $500,000 to Lazarus Investment Partners LLLP, the beneficial owner of approximately 29.3% of the company’s common stock, in a private transaction. The note was originally due and payable on the first to occur of July 2, 2015 or the date on which the company received at least $900,000 in proceeds from equity or debt financing transactions and has been extended several times. In consideration of the loan, the company and Lazarus entered into a warrant amendment agreement pursuant to which the company agreed to amend certain of the terms of the existing 6,233,636 common stock purchase warrants held by Lazarus to reduce the exercise price of such warrants to $0.25, which was $0.01 above the most recently reported closing consolidated bid price of the company’s common stock prior to the execution of the transaction documents, and to extend the expiration date of the warrants to October 25, 2019. The warrants amended were issued in various transactions from 2010 through 2013 at exercise prices ranging between $0.88 and $2.00. As discussed more fully in Note 25 of Notes to the Consolidated Financial Statements, the parties have entered into several amendment agreements to extend the maturity date of this loan.
F-35
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
21. Other Expense, Net
Other income (expense) consists of the following (in thousands):
| June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Amortization of debt discount |
$ | (361 | ) | $ | (126 | ) | $ | (2,904 | ) | |||
| Loss on extinguishment of notes |
(557 | ) | — | (1,060 | ) | |||||||
| Amortization of deferred financing costs |
(25 | ) | (1 | ) | (27 | ) | ||||||
| Interest expense |
(35 | ) | — | — | ||||||||
| Net gain from sale of non-core assets |
— | 101 | — | |||||||||
| Miscellaneous income |
(16 | ) | — | 13 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total other (expense) income, net |
$ | (994 | ) | $ | (26 | ) | $ | (3,978 | ) | |||
|
|
|
|
|
|
|
|||||||
22. Fair Value Measurements
The company measures fair value for financial assets and liabilities in accordance with the provisions of the accounting guidance regarding fair value measurements. The guidance utilizes a fair value hierarchy that prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. A brief description of those three levels is as follows:
| • | Level 1: Observable inputs such as quoted prices in active markets for identical assets or liabilities. |
| • | Level 2: Inputs other than quoted prices for identical assets or liabilities that are observable for the asset or liability, either directly or indirectly. |
| • | Level 3: Significant unobservable inputs. |
The company’s assets subject to fair value measurements as of June 30, 2015 and 2014 are as follows (in thousands):
| Fair Value Measurements Using Fair Value Hierarchy |
||||||||||||||||
| Fair value | Level 1 | Level 2 | Level 3 | |||||||||||||
| June 30, 2015 |
||||||||||||||||
| Current marketable securities—available for sale |
$ | — | $ | — | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | — | $ | — | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| June 30, 2014 |
||||||||||||||||
| Current marketable securities—available for sale |
$ | 210 | $ | 210 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 210 | $ | 210 | $ | — | $ | — | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
For the years ended June 30, 2015 and 2014, no gains or losses resulting from the fair value measurement of financial assets were included in the company’s earnings.
The accounting guidance regarding fair value measurements permits entities to choose to measure many financial instruments and certain other items at fair value that are not currently required to be measured at fair value and establishes presentation and disclosure requirements designed to facilitate comparisons between entities that choose different measurement attributes for similar assets and liabilities. The company has elected not to measure any eligible items at fair value.
F-36
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
23. Accounting Standards Adopted in Fiscal 2015
The company adopted the following FASB Accounting Standards Update (ASU) during fiscal 2015:
| • | ASU No. 2013-11, Income Taxes (Topic 740)—Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward or Tax Credit Carryforward Exists (a consensus of FASB Emerging Issues Task Force), which finalizes Proposed ASU No. EITF-13C, and provides explicit guidance regarding the presentation in the statement of financial position of an unrecognized tax benefit when a net operating loss carryforward or a tax credit carryforward exists. In particular, ASU No. 2013-11 provides that an entity’s unrecognized tax benefit, or a portion of its unrecognized tax benefit, should be presented in its financial statements as a reduction to a deferred tax asset for a net operating loss carryforward, a similar tax loss, or a tax credit carryforward, with one exception. That exception states that, to the extent a net operating loss carryforward, a similar tax loss, or a tax credit carryforward is not available at the reporting date under the tax law of the applicable jurisdiction to settle any additional income taxes that would result from the disallowance of a tax position, or the tax law of the applicable jurisdiction does not require the entity to use, and the entity does not intend to use, the deferred tax asset for such purpose, the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. As to the foregoing exception, ASU No. 2013-11 explains that the determination of whether a deferred tax asset is available is based on the unrecognized tax benefit and deferred tax asset that exist at the reporting date and should be made presuming disallowance of the tax position at the reporting date. For example, an entity should not evaluate whether the deferred tax asset expires before the statute of limitations on the tax position or whether the deferred tax asset may be used prior to the unrecognized tax benefit being settled. ASU No. 2013-11 applies prospectively to all entities that have unrecognized tax benefits when a net operating loss carryforward, a similar tax loss or a tax credit carryforward exists at the reporting date and is effective for fiscal years, and interim periods within those years, beginning after December 15, 2013. |
The adoption of the ASU described above had no impact on the company’s results of operations or financial position.
F-37
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
24. Quarterly Financial Data (Unaudited)
| (in thousands) | ||||||||||||||||
| Fiscal Year Ended June 30, |
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
||||||||||||
| 2015 |
||||||||||||||||
| Revenues |
$ | 1,052 | $ | 1,347 | $ | 769 | $ | 521 | ||||||||
| Operating expenses |
3,163 | 3,435 | 3,148 | 2,649 | ||||||||||||
| Net loss |
(2,111 | ) | (2,088 | ) | (2,449 | ) | (3,052 | ) | ||||||||
| Loss per share |
$ | (0.06 | ) | $ | (0.05 | ) | $ | (0.06 | ) | $ | (0.08 | ) | ||||
| 2014 |
||||||||||||||||
| Revenues |
$ | 1,805 | $ | 1,461 | $ | 1,221 | $ | 1,070 | ||||||||
| Operating expenses |
3,584 | 3,006 | 2,903 | 3,181 | ||||||||||||
| Net loss |
(1,874 | ) | (1,577 | ) | (1,581 | ) | (2,111 | ) | ||||||||
| Loss per share |
$ | (0.09 | ) | $ | (0.07 | ) | $ | (0.04 | ) | $ | (0.06 | ) | ||||
| 2013 |
||||||||||||||||
| Revenues |
$ | 921 | $ | 1,051 | $ | 1,410 | $ | 1,445 | ||||||||
| Operating expenses |
2,888 | 2,960 | 3,268 | 3,082 | ||||||||||||
| Net loss |
(2,515 | ) | (2,733 | ) | (2,670 | ) | (3,431 | ) | ||||||||
| Loss per share |
$ | (0.11 | ) | $ | (0.11 | ) | $ | (0.11 | ) | $ | (0.12 | ) | ||||
25. Subsequent Events
On August 7, 2015, the company issued a senior secured promissory note in the aggregate principal amount of $320,000 to an accredited investor in a private transaction dated August 7, 2015. The note is due and payable on December 31, 2015 and interest shall accrue on the note at the rate of 10.0% per annum. The note is not convertible into equity securities of the company and it contains terms and events of default customary for similar transactions. The note is secured by a first priority lien on certain of our assets, as described in a security agreement entered into between the company and the purchaser dated as of August 7, 2015. In consideration of the loan, the company and the purchaser entered into a warrant amendment agreement pursuant to which the company agreed to amend certain of the terms of the existing 5,474,829 common stock purchase warrants currently held by the purchaser and an affiliate to reduce the exercise price of such warrants to $0.17 and to extend the expiration date of the warrants to December 13, 2019. The warrants amended were issued in various transactions from 2012 through 2013 at exercise prices ranging between $0.95 and $1.34. The purchaser is an entity affiliated with J. David Luce, a member of our board of directors. The company is using the net proceeds from the transaction for general business and working capital purposes.
On August 24, 2015 the company entered into an employment agreement with Mr. Henry which sets forth the terms of Mr. Henry’s employment as interim chief strategy officer. The start date of Mr. Henry’s employment as an interim officer was July 23, 2015 and the employment letter is retroactive to that date and provides that Mr. Henry shall serve as our interim chief strategy officer on an at-will basis on the following terms and conditions: Mr. Henry will be entitled to receive a base salary payable at the rate of $250,000 per year, which will be payable upon the expiration of the term of the employment letter. In addition, Mr. Henry will be entitled to a bonus of $200,000 in the event the company completes a transaction resulting in a “change in control” during the term of the employment letter or within 150 days thereafter. Mr. Henry was granted an initial equity award under the company’s 2011 Omnibus Equity Incentive Plan of 475,000 stock options that vest and are exercisable immediately upon the execution of the employment letter. These options are exercisable for a period
F-38
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
of ten years, subject to the terms of the plan and the stock option agreement, at an exercise price of $0.25 per share. On August 24, 2015, the closing bid price of the company’s common stock was $0.12 per share. Mr. Henry will also be eligible to receive grants of additional stock options under the plan based on the duration of the term of the employment letter. Under this arrangement, commencing on August 24, 2015, Mr. Henry shall be granted one or more additional awards of 125,000 stock options under the plan for each thirty (30) day period thereafter while the employment letter remains in effect. Awards of additional options shall be granted on (i) August 24, 2015 and (ii) each monthly anniversary thereafter during the term of the employment letter. Each grant of additional options shall vest on the thirty (30) day anniversary date following the date on which such award was granted. Each grant of additional options shall be subject to the terms and conditions of the plan and each stock option agreement issued to evidence such award. Further, each award of additional options is exercisable immediately upon the approval of the company’s shareholders of an amendment to the plan to increase the number of shares of common stock available for awards to be issued thereunder. The exercise price of each grant of additional options shall be equal to the greater of $0.25 per share or fair market value as determined under the plan, and, to the extent exercisable, such additional options shall be exercisable for a term of ten years. The company will reimburse Mr. Henry for reasonable business-related expenditures. Mr. Henry has also entered into the company’s standard form of employee invention assignment and confidentiality agreement. If Mr. Henry’s employment with the company is terminated for any reason, the company shall pay him all accrued compensation due and owing to him and he is not entitled to any severance or other benefits following any termination of his employment with the company except that if his employment is terminated for any reason other than cause (as defined in the employment letter), any unvested option awards granted under the employment letter shall automatically vest and the exercise period of the options granted under the employment letter shall be extended to the duration of their original term. Further, Mr. Henry, who also serves as a member of the company’s board of directors, will not receive remuneration for serving as a director while he is also serving as an executive officer.
On August 25, 2015 the company announced that it had entered into a non-binding letter of intent with Peachstate Health Management, LLC d/b/a AEON Clinical Laboratories, an expanding clinical laboratory based in Gainesville, GA (“AEON”) for the acquisition of all of the outstanding membership interests of AEON in exchange for shares of a newly created class of Series E preferred stock of Authentidate (the “Series E Shares”). The letter of intent contemplates the AEON members will be issued Series E Shares convertible into 19.9% of the outstanding shares of the company’s common stock on the date of the closing of the merger transaction, and an additional number of Series E Shares convertible into 5% of the outstanding shares of the company’s common stock upon approval of the merger transaction by the shareholders of the company. Additional Series E Shares will be issued to AEON members in 2016 and 2020 if AEON achieves certain financial results. The additional 2016 Series E Shares will be convertible into 24% of the outstanding shares of the company’s common stock on the date of the closing and will be issued provided AEON achieves $16 million of EBITDA in calendar year 2015. The AEON members will be issued another tranche of Series E Shares in 2020 which, including the previously issued Series E Shares, will be convertible into 85% of the outstanding shares of the company’s common stock (on a partially diluted basis as defined) provided AEON achieves $65.9 million in EBITDA, in the aggregate, in calendar years 2017 and 2018, or $99 million in EBITDA, in the aggregate, for calendar years 2016, 2017 and 2018. The letter of intent also provides for the issuance of Series E Shares as bonus shares for the achievement of $117 million in net income for the four fiscal years ending December 31, 2019, convertible into 5% of the outstanding shares of the company’s common stock (on a partially diluted basis as defined ). The holders of the Series E Shares will have certain preferential rights, including the right to vote separately as a class to nominate and elect one director for each 10% of the outstanding shares of the company’s common stock into which the outstanding Series E Shares shall be convertible.
F-39
Table of Contents
Authentidate Holding Corp. and Subsidiaries
Notes to Consolidated Financial Statements—(Continued)
June 30, 2015, 2014 and 2013
On August 26, 2015, the company issued promissory notes in the aggregate principal amount of $400,000 to Lazarus Investment Partners LLLP, the beneficial owner of approximately 29.3% of the company’s common stock, and an entity affiliated with J. David Luce, a member of our board of directors, in a private transaction. The notes are unsecured obligations of the company and are not convertible into equity securities of the company. The notes bear interest at 20% per annum, payable in arrears, and are due upon the earlier of (i) August 26, 2016, or (ii) within 30 days of the closing of the contemplated acquisition, merger or similar transaction with Peachstate Health Management, LLC (d/b/a AEON Clinical Laboratories) as described above, or a similar alternative acquisition, merger or similar transaction with an unaffiliated third party, or (iii) the closing of a sale of equity or debt securities of the company, or series of closings, as part of the same transaction, of equity or debt securities within a period of 90 days, in the gross amount of at least $5,000,000 in cash proceeds. The holders have the right, at their option, to convert interest and principal due on the note into any alternative financing that may be undertaken by the company while the notes are outstanding.
In September 2015, the company issued promissory notes in the aggregate principal amount of $525,000 to accredited investors in a private transaction. The notes are unsecured and are not convertible into equity securities of the company. The notes bear interest at 20% per annum, payable in arrears, and are due upon the earlier of (i) September 18, 2016, or (ii) within 30 days of the closing of a sale of equity or debt securities of the company, or series of closings, as part of the same transaction, of equity or debt securities within a period of 90 days, in the gross amount of at least $5,000,000 in cash proceeds. The company also issued the investors warrants to purchase an aggregate of 1,050,000 shares of common stock. The warrants are exercisable commencing twelve months following their issuance for a period of 54 months at an exercise price of $0.30 per share. These closings are part of an offering of up to $1 million of notes and 2 million warrants.
F-40
