Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | a10-7x20158k.htm |
| EX-99.1 - EXHIBIT 99.1 - GENOCEA BIOSCIENCES, INC. | exhibit991pressrelease10-7.htm |

Positive 6 Month Durability Results GEN-003 Immunotherapy for Genital Herpes Phase 2 Dose Optimization Study 7 October 2015 Exhibit 99.2

Safe Harbor Statement This presentation contains “forward-looking” statements that are within the meaning of federal securities laws and are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward- looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. Factors that may materially affect our results of operations include, among other things, those listed in our Annual Report on Form 10-K and other filings with the Securities and Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward- looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings for free by visiting EDGAR on the SEC website at http://www.sec.gov. 2

Highlights 3 • Improved impact on viral activity • Durable clinical efficacy demonstrated across potential Phase 3 endpoints • Potential for GEN-003 to serve as cornerstone treatment for genital herpes reinforced • Clear path to FDA end of Phase 2 meeting in Q4 2016 • Data will be presented at IDWeek 2015TM in San Diego this Friday 9 October

Agenda for Today’s Call • Disease pathology of genital herpes • Current treatment paradigms • Ongoing Phase 2 dose optimization trial – Study goals – Positive 6-month durability data • GEN-003 value proposition • Upcoming GEN-003 milestones • Conclusions • Q&A 4

Viral Shedding Underpins Pathology of Genital Herpes • A serious chronic infection caused by herpes simplex viruses (HSV) • Periodic disease reactivation causes viral shedding at rates specific to individual patients • Shedding necessary for genital lesions, disease transmission 5 Virus moves from nerve cell to skin and mucosa Nerve cell Skin cells Viral Shedding Genital mucosa Virus lays dormant in nerve ganglia

Current Genital Herpes Treatment Paradigms • Approximately two thirds (~4.5m) of treated U.S. patients use oral antivirals episodically – No impact on asymptomatic viral shedding/transmission risk – No impact on frequency of lesion outbreaks – Modest impact on duration of lesion outbreaks • Remaining one third (~2.5m) treat with chronic oral therapy – More durable reduction in viral shedding, visible lesions – Few options if outbreaks persist – Heavy compliance burden 6

Phase 2 Dose Optimization Trial Goals and Objectives • Goal: Select dose for late stage clinical trials • Primary endpoint: Reduction in viral shedding vs. baseline* • Secondary objectives: – Impact on clinical disease • Lesion rates* • Proportion recurrence free at 6* and 12 months • Time to next recurrence* – Safety and tolerability* – Immunogenicity 7 * Data discussed today

-100% -50% 0% Baseline Post dose 3 6 Months Chang e v s. b a se lin e 30/25 µg 30/50 µg 30/75 µg 60/25 µg 60/50 µg 60/75 µg Sustained Reduction in Viral Shedding Rate at 6 Months 8 • Sustained viral shedding reductions across several doses • Stronger response at 6 months than in Ph 1/2 – 58% vs. 40% • Upside potential for durable effect to 12 months Viral Shedding Rate Reduction vs. Baseline *** * Poisson model analysis vs. baseline *** p<0.0001, * p<0.05 Protein dose / adjuvant dose *** *** *** *** *** *** *** ***

-100% -50% 0% Baseline Post dose 3 6 Months Chang e v s. b a se lin e 30/25 µg 30/50 µg 30/75 µg 60/25 µg 60/50 µg 60/75 µg Genital Lesion Rate Reduction Sustained at 6 Months 9 • Sustained reductions at 6 months • Lesion rates results broadly parallel viral shedding rate reductions Genital Lesion Rate Reduction vs. Baseline * Poisson model analysis vs. baseline *** p<0.0001, * p<0.05 Protein dose / adjuvant dose *** *** *** *** *** *** *** *** *** ***

0% 20% 40% 60% 80% % Lesion Free at Six Months 30/25 µg 30/50 µg 30/75 µg 60/25 µg 60/50 µg 60/75 µg Consistent Efficacy on % Lesion Free at 6 Months Post Dosing 10 • Multiple doses similar to suppressive oral anti-viral therapy and superior to placebo from previous trials • Small sample size – ~45 per group vs. 269 for Valtrex % Lesion Free at Six Months* Protein dose / adjuvant dose Placebo** Famvir Valtrex * GEN-003 data displayed as mean result by dose group bounded by 95% confidence intervals ** n=134, Valtrex Phase 3 trial

Time to First Recurrence Results Consistent Across Dose Groups • Same data as % lesion free, analyzed slightly differently • Consistent time to first recurrence across groups – Range of 152 to >180 days • Efficacy similar to expected performance of orals, superior to placebo 11 Dose Group (protein dose / adjuvant dose) Time to First Recurrence 30 µg / 25 µg 159 30 µg / 50 µg 152 30 µg / 75 µg 160 60 µg / 25 µg >180 60 µg / 50 µg 164 60 µg / 75 µg 161

6 Months Durability Confirms GEN-003 Value Proposition • Convenient, durable efficacy may improve upon dominant treatment paradigm (episodic anti-viral therapy) – Reduce outbreaks – Reduce shedding to reduce transmission risk • Potential benefits vs. chronic suppressive therapy – Durable efficacy via novel mechanism – Orals reserved as rescue therapy during outbreaks – Improved compliance & convenience • GEN-003 profile supports revenue opportunity of >$1bn in US alone 12
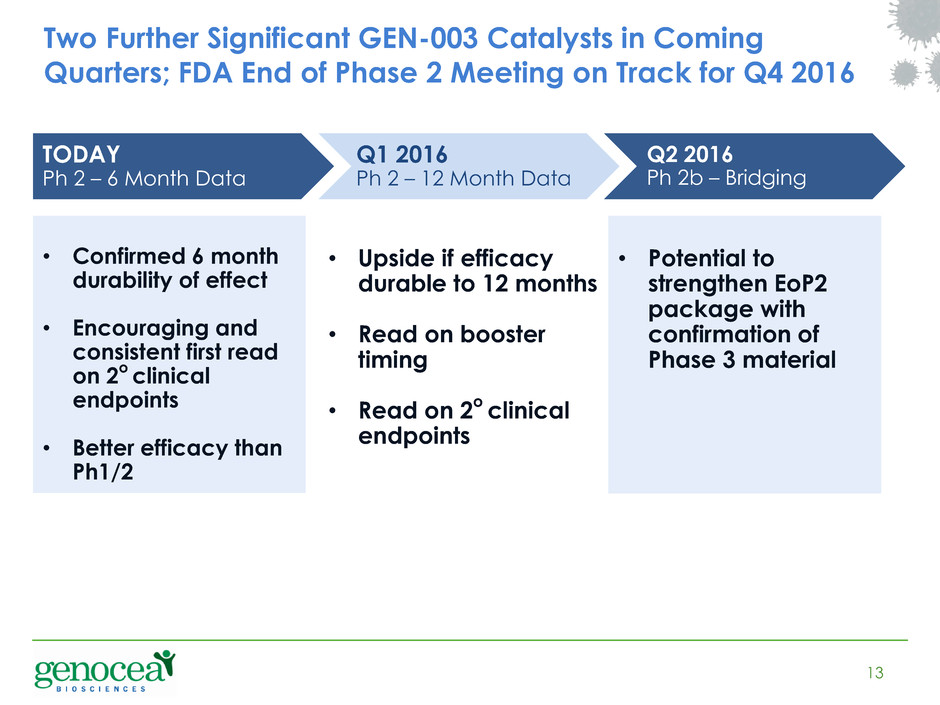
Two Further Significant GEN-003 Catalysts in Coming Quarters; FDA End of Phase 2 Meeting on Track for Q4 2016 13 TODAY Ph 2 – 6 Month Data Q2 2016 Ph 2b – Bridging Q1 2016 Ph 2 – 12 Month Data • Confirmed 6 month durability of effect • Encouraging and consistent first read on 2o clinical endpoints • Better efficacy than Ph1/2 • Upside if efficacy durable to 12 months • Read on booster timing • Read on 2o clinical endpoints • Potential to strengthen EoP2 package with confirmation of Phase 3 material

Conclusions 14 • Improved impact on viral activity • Durable clinical efficacy demonstrated across potential Phase 3 endpoints • Potential for GEN-003 to serve as cornerstone treatment for genital herpes reinforced • Clear path to FDA end of Phase 2 meeting in Q4 2016 • Data will be presented at IDWeek 2015TM in San Diego this Friday 9 October

Questions & Answers 15

Jonathan Poole Chief Financial Officer Phone: +1 617-876-8191 jonathan.poole@genocea.com Megan Lustig Spectrum Science Communications Phone: +1 202-955-6222 mlustig@spectrumscience.com Investor inquiries: Media inquiries:
