Attached files
| file | filename |
|---|---|
| EX-5.1 - EX-5.1 - NovoCure Ltd | d940664dex51.htm |
| EX-23.2 - EX-23.2 - NovoCure Ltd | d940664dex232.htm |
Table of Contents
As filed with the Securities and Exchange Commission on October 1, 2015
Registration No. 333-206681
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2
to
Form S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
NovoCure Limited
(Exact name of registrant as specified in its charter)
| Jersey (Channel Islands) | 3841 | Not Applicable | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification Number) |
NovoCure Limited
Le Masurier House
La Rue Le Masurier
St. Helier, Jersey JE2 4YE
+44 (0)15 3475 6700
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Wilhelmus Groenhuysen
Chief Financial Officer
NovoCure Limited
c/o Novocure Inc.
20 Valley Stream Pkwy
Suite 300
Malvern, PA 19355
(212) 767-7530
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | ||||
| Julie M. Allen, Esq. Reid Arstark, Esq. Proskauer Rose LLP Eleven Times Square New York, NY 10036 |
Todd Longsworth, Esq. NovoCure Limited c/o Novocure Inc. 20 Valley Stream Pkwy Suite 300 Malvern, PA 19355 |
Richard D. Truesdell, Jr., Esq. Byron Rooney, Esq. Davis Polk & Wardwell LLP 450 Lexington Ave New York, NY 10017 | ||
| (212) 969-3000 | (212) 767-7530 | (212) 450-4000 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ |
Accelerated filer ¨ | Non-accelerated filer x | Smaller reporting company ¨ |
(Do not check if a smaller reporting company)
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of each class of securities to be registered | Amount to be |
Proposed maximum |
Proposed maximum aggregate offering price(2) |
Amount of registration fee(2)(3) | ||||
| Ordinary shares, no par value |
8,625,000 |
$24.00 |
$207,000,000 | $24,053.40 | ||||
|
| ||||||||
|
| ||||||||
| (1) | Includes ordinary shares that may be purchased by the underwriters pursuant to an over-allotment option. |
| (2) | Calculated pursuant to Rule 457(a) under the Securities Act. |
| (3) | Previously paid. |
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
Subject to completion, dated October 1, 2015
Preliminary prospectus
7,500,000 shares

Ordinary shares
We are offering 7,500,000 ordinary shares to be sold in this offering. This is our initial public offering of ordinary shares.
Prior to this offering, there has been no public market for our ordinary shares. The estimated initial public offering price is between $23.00 and $24.00 per share. Our ordinary shares are approved for listing on the NASDAQ Global Select Market under the symbol “NVCR.”
We are an emerging growth company under the federal securities laws and will be subject to reduced public company reporting requirements.
Investing in our ordinary shares involves a high degree of risk. These risks are described under the caption “Risk factors” that begins on page 11 of this prospectus.
| Per share | Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discount(1) |
$ | $ | ||||||
| Proceeds to us, before expenses |
$ | $ | ||||||
| (1) | We have also agreed to reimburse the underwriters for certain FINRA-related expenses. See “Underwriting” for a description of all compensation payable to the underwriters. |
We have granted the underwriters an option for a period of 30 days to purchase up to 1,125,000 additional shares on the same terms and conditions set forth above to cover over-allotments, if any.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the securities that may be offered under this prospectus, nor have any of these organizations determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares to investors on , 2015.
| J.P. Morgan | Deutsche Bank Securities | Evercore | ||
| Wells Fargo Securities | ||||
| JMP Securities |
Wedbush PacGrow | |
Prospectus dated , 2015
Table of Contents

Table of Contents
| Page | ||||
| 1 | ||||
| 11 | ||||
| 49 | ||||
| 50 | ||||
| 51 | ||||
| 52 | ||||
| 53 | ||||
| 56 | ||||
| Management’s discussion and analysis of financial condition and results of operations |
58 | |||
| 73 | ||||
| 110 | ||||
| 118 | ||||
| 150 | ||||
| 152 | ||||
| 156 | ||||
| 168 | ||||
| 171 | ||||
| 178 | ||||
| 191 | ||||
| 191 | ||||
| 191 | ||||
| F-1 | ||||
i
Table of Contents
About this prospectus
Neither we nor the underwriters have authorized anyone to provide you with any information other than information contained in this prospectus or in any free writing prospectus prepared by or on behalf of us or to which we have referred you. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus is an offer to sell only the ordinary shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. You should assume that the information appearing in this prospectus is accurate only as of the date hereof. Our business, prospects, financial condition and results of operations may have changed since that date. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to this offering and the distribution of this prospectus outside the United States.
The terms “NovoCure,” “the Company,” “we,” “us,” “our” and “our company,” as used in this prospectus, refer to NovoCure Limited, a public limited company incorporated under the laws of Jersey, Channel Islands, and its wholly owned subsidiaries.
All ordinary share amounts in this prospectus reflect a 5.913-for-1 stock split effected on September 16, 2015.
This prospectus includes trademarks of NovoCure and other persons. All trademarks or trade names referred to in this prospectus are the property of their respective owners.
References in this prospectus to “regulatory approvals” should be understood to include U.S. Food and Drug Administration, or FDA, approvals, as well as CE Certificates of Conformity issued by notified bodies in the European Union and approvals by the applicable regulatory authorities in Japan and other relevant jurisdictions. References in this prospectus to “regulatory authorities” should be understood to include the FDA, such notified bodies in the European Union and regulatory authorities in Japan and other relevant jurisdictions.
A copy of this document has been delivered to the registrar of companies in Jersey in accordance with Article 5 of the Companies (General Provisions) (Jersey) Order 2002, and it has given, and has not withdrawn, its consent to circulation thereof. The Jersey Financial Services Commission has given, and has not withdrawn, its consent under Article 2 of the Control of Borrowing (Jersey) Order 1958 to the issue of the ordinary shares. It must be distinctly understood that, in giving these consents, neither the registrar of companies in Jersey nor the Jersey Financial Services Commission takes any responsibility for the financial soundness of our company or for the correctness of any statements made, or opinions expressed, with regard to it.
Nothing in this document or anything communicated to holders or potential holders of ordinary shares is intended to constitute or should be construed as advice on the merits of the purchase of or subscription for ordinary shares or the exercise of any rights attached thereto for the purposes of the Financial Services (Jersey) Law, 1998, as amended. If you are in any doubt about the contents of this prospectus, you should consult your stockbroker, bank manager, solicitor, accountant or other financial advisor.
The directors of our company have taken all reasonable care to ensure that the facts stated in this prospectus are true and accurate in all material respects, and that there are no other facts the omission of which would make misleading any statement in this prospectus, whether of facts or of opinion. All the directors accept responsibility accordingly. It should be remembered that the price of securities and the income from them can go down as well as up.
ii
Table of Contents
This summary highlights information contained elsewhere in this prospectus and does not contain all the information that you should consider before investing in our ordinary shares. See “Our business” for more information, including a “Glossary of terms” and “Summary of completed and existing clinical trials and registry data” beginning on page 105. You should carefully read the entire prospectus, including our financial statements and related notes included in this prospectus and the information set forth under the headings “Risk factors” and “Management’s discussion and analysis of financial condition and results of operations,” before making an investment decision. In this prospectus, unless the context otherwise requires, the terms “NovoCure,” “we,” “us,” “our” and “our company” refer to NovoCure Limited, a public limited company incorporated under the laws of Jersey, Channel Islands, and its wholly owned subsidiaries.
Our company
We are a commercial-stage oncology company developing a novel, proprietary therapy called Tumor Treating Fields, or TTFields, for the treatment of solid tumor cancers. TTFields is a low-toxicity anti-mitotic treatment that uses low-intensity, intermediate frequency, alternating electric fields to exert physical forces on key molecules inside cancer cells, disrupting the basic machinery necessary for normal cell division, leading to cancer cell death. Physicians have typically treated patients with solid tumors using one or a combination of three principal treatment modalities—surgery, radiation and pharmacological therapies. Despite meaningful advancements in each of these modalities, a significant unmet need to improve survival and quality of life remains. We believe we will establish TTFields as a new treatment modality for a variety of solid tumors that increases survival without significantly increasing side effects when used in combination with other cancer treatment modalities.
We received FDA approval for Optune, our first TTFields delivery system, in 2011 for use as a monotherapy treatment for adult patients with glioblastoma brain cancer, or GBM, following confirmed recurrence after chemotherapy. We have built a commercial organization and launched Optune in the United States, Germany, Switzerland and Japan, which we refer to as our currently active markets. In November 2014, our Phase 3 pivotal trial of Optune in combination with chemotherapy for patients with newly diagnosed GBM met its endpoints and was halted after a protocol pre-specified interim analysis showed significant improvements in both progression free and overall survival. In April 2015, we filed a premarket approval, or PMA, supplement application with the FDA for the treatment of newly diagnosed GBM based on our Phase 3 data and our application was granted priority review status. Upon FDA approval of Optune for newly diagnosed GBM, we believe TTFields will transform the standard of care for patients with newly diagnosed and recurrent GBM.
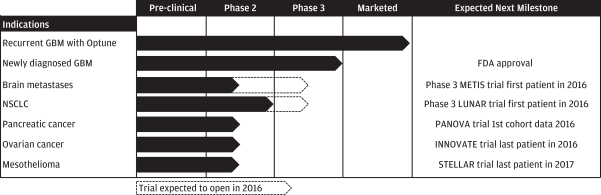
We have researched the biological effects of TTFields extensively. Because TTFields are delivered regionally, act only on mitotic cells and are tuned to target cancer cells of a specific size, there is minimal damage to healthy cells. We believe our pre-clinical and clinical research demonstrates that TTFields’ mechanism of action affects fundamental aspects of cell division and can have broad applicability across a variety of solid tumors. In addition to our clinical and commercial progress in GBM, we are currently planning or conducting clinical trials evaluating the use of TTFields in brain metastases, advanced non-small cell lung cancer, or NSCLC, pancreatic cancer, ovarian cancer and mesothelioma.
We own all commercialization rights to TTFields in oncology,
and have a patent and intellectual property portfolio that, as of June 30, 2015, consists of a total of 52 issued patents, including 36 issued in the United States,
as well as over 30 additional patent applications on file. We believe we will
maintain exclusive rights to market TTFields for all solid tumor indications in our key markets through the life of our patents.
1
Table of Contents
To date, substantially all of our revenues have been derived from patients using Optune in our currently active markets. Our net revenues for the year ended December 31, 2014 were $15.5 million and $11.8 million for the six months ended June 30, 2015. However, we have incurred significant costs in connection with our pre-clinical and clinical trial programs, commercial launch efforts and general and administrative costs. We had net losses of $77.4 million for the year ended December 31, 2013, $80.7 million for the year ended December 31, 2014 and $52.6 million for the six months ended June 30, 2015, and we have an accumulated deficit of $329.1 million as of June 30, 2015. We expect to continue to incur significant expenses and operating losses for at least the next several years.
GBM—our first approved and commercialized indication
GBM is the most common and aggressive form of primary brain cancer. We estimate approximately 27,500 patients are diagnosed with GBM annually in the United States, the top five European Union markets and Japan. GBM has few effective treatment options at present and provides our first opportunity to transform the standard of care for a solid tumor cancer to include TTFields.
We launched Optune in the United States for the treatment of recurrent GBM in 2011 and more recently in our other currently active markets. Since the majority of recurrent GBM patients are treated at large cancer centers, we built a commercial organization to focus primarily on these centers. As of the date of this prospectus, we have trained physicians in over 270 clinical centers. These trained physicians have treated over 1,600 GBM patients using Optune.
We initiated our EF-14 Phase 3 pivotal trial in 2009 to establish TTFields for the treatment of newly diagnosed GBM. The EF-14 trial randomized 700 patients to receive either temozolomide, the established standard of care chemotherapy for newly diagnosed GBM, or TTFields in combination with temozolomide. In November 2014, a protocol pre-specified interim analysis of the first 315 patients demonstrated the trial met its powered endpoints of significant extension of both progression free survival, or PFS, and overall survival, or OS, in patients treated with TTFields in combination with temozolomide versus temozolomide alone. The interim analysis results demonstrated that:
| • | the two-year survival rate among patients treated with TTFields in combination with temozolomide, in the as-treated population, was 48% compared to 32% among patients treated with temozolomide alone (p=0.0058); |
| • | patients treated with TTFields, in combination with temozolomide, in the intent-to-treat population, demonstrated a statistically significant increase in PFS compared to temozolomide alone (median PFS of 7.2 months compared to 4.0 months, hazard ratio=0.62, p=0.001); and |
| • | patients treated with TTFields, in combination with temozolomide, in the as-treated population, demonstrated a statistically significant increase in OS compared to temozolomide alone (median OS of 20.5 months compared to 15.6 months, hazard ratio=0.66, p=0.004). |
The trial’s independent data monitoring committee recommended that patients receiving temozolomide alone be allowed to cross over immediately to receive TTFields. Following FDA approval of this recommendation in December 2014, we allowed patients receiving temozolomide alone to cross over. We submitted a PMA supplement application to the FDA in April 2015 to expand our label for Optune to include the treatment of newly diagnosed GBM. In May 2015, we received priority review status from the FDA. We believe that following FDA approval of Optune for newly diagnosed GBM, Optune in combination with temozolomide will transform the standard of care for the treatment of patients with newly diagnosed GBM.
2
Table of Contents
Our clinical pipeline
We have performed extensive pre-clinical research on TTFields and their effects in multiple solid tumor cancers. We have gained a deep understanding of the underlying mechanism of action and the multiple pathways through which TTFields exert their effects within the dividing cancer cells. Our research shows that TTFields have an anti-mitotic effect in over 15 different solid tumor types in culture and in multiple in vivo tumor models. In vitro and in vivo studies combining TTFields with radiation or chemotherapy, in multiple tumor types, have demonstrated at least additive efficacy, or stronger efficacy than the effect of either treatment alone, and in some cases synergistic efficacy, or stronger efficacy than the sum of the effects of both treatments. An increase in cancer cell sensitivity to chemotherapy when used in combination with TTFields in the range of one to two orders of magnitude suggests additivity, while an increase in the range of three to four orders of magnitude suggests synergism. Certain in vitro experiments using TTFields have suggested both additivity and synergism when used in combination with chemotherapy, as the presence of TTFields was shown to increase cancer cell sensitivity to chemotherapy from approximately 275 times to over 1,250 times depending on the mechanism of action of the particular chemotherapy. The upper end of this range was observed in testing with taxane-based chemotherapies.
We believe our success in delaying disease progression and extending survival in GBM patients, our pre-clinical data and our early clinical data in additional indications validate the potential of TTFields to become a new therapeutic modality for a variety of solid tumors. We have developed a pipeline strategy to advance TTFields through Phase 2 pilot and Phase 3 pivotal clinical trials across multiple solid tumor types, and anticipate expanding our clinical pipeline over time to study the safety and efficacy of TTFields for additional solid tumor indications.
Our competitive advantages
We believe our key competitive advantages are:
| • | Significant market potential addressable via a broadly applicable mechanism of action. Based on our pre-clinical research and clinical experience to date, we believe the anti-mitotic mechanism of action of TTFields is broadly applicable to a variety of solid tumors with an annual incidence of approximately 1.1 million people in the United States alone. Currently, we have ongoing and completed clinical trials for indications with an incidence of approximately 350,000 people annually in the United States. We believe that the global incident population of target solid tumors provides us with significant additional commercial opportunities. |
3
Table of Contents
| • | Immediate commercial opportunity for Optune in GBM. We are currently marketing Optune for the treatment of recurrent GBM in the United States and our other currently active markets. We have applied for FDA approval for the treatment of newly diagnosed GBM based on the results of our successful EF-14 Phase 3 clinical trial. Upon approval, we will begin marketing Optune as a treatment for newly diagnosed GBM, and we expect that Optune will transform the standard of care for the treatment of patients with newly diagnosed GBM. |
| • | Pipeline of Phase 2 trials in five additional indications. In addition to our GBM clinical programs, we have invested in a variety of clinical programs in other solid tumors. We have completed a Phase 2 trial in NSCLC, and are currently enrolling patients in Phase 2 trials for brain metastases, pancreatic cancer, ovarian cancer and mesothelioma. We expect to continue investing in our pipeline over time to broaden our commercial opportunity. |
| • | Established commercial organization and supply chain. We have established our commercial organization and believe we have the experience, expertise and infrastructure to scale our sales and marketing efforts in our key markets. In addition to our commercial organization, we have established a scalable supply chain. |
| • | Significant barriers to entry. We own all commercialization rights to TTFields in oncology and have a patent and intellectual property portfolio that, as of June 30, 2015, consists of a total of 52 issued patents, including 36 issued in the United States, as well as over 30 additional patent applications on file. We have patent protection through 2031 in the United States and through 2026 in other key markets. We believe we will maintain exclusive rights to market TTFields for all solid tumor indications in our key markets for the life of our patents. In addition, even after the expiration of our U.S. patents, potential market entrants applying low-intensity, alternating electric fields to solid tumors in the United States will have to undertake their own clinical trials and PMA submissions to the FDA to demonstrate equivalence to TTFields to market a competing product. |
Our strategies for growth
Our objective is to establish TTFields as a new modality for the treatment of a variety of solid tumors. Our key strategies include the following:
| • | Drive adoption of Optune in GBM. We plan to use the data from our pivotal EF-14 Phase 3 clinical trial and our commercial organization to transform the standard of care for patients with newly diagnosed and recurrent GBM and to drive adoption of Optune by physicians and patients. |
| • | Expand our commercial organization. We plan to expand our direct sales force to call on physicians who treat newly diagnosed GBM patients. We expect to further expand our commercial organization following regulatory approvals for additional indications. |
| • | Advance clinical development of TTFields. We plan to advance our clinical pipeline and evaluate other solid tumor indications that we believe can be targeted with TTFields. |
| • | Evaluate the use of TTFields in combination with other solid tumor therapies. We are supporting independent research into the optimal combinations of TTFields with radiation or pharmacological therapies to expand the population of patients who may benefit from TTFields. For example, we believe that TTFields may be combined with radiation or chemotherapy to allow for dose reductions, leading to reduced toxicity while achieving the same or better treatment outcomes. |
4
Table of Contents
| • | Continue to improve our TTFields delivery systems. We plan to continue to develop and enhance our TTFields delivery systems to improve performance and to provide the optimal patient experience across a variety of approved and potential clinical indications. We intend to seek FDA approval for the second generation of Optune, which is less than half the weight and size of the current version. |
Risk factors
Our business is subject to numerous risks as discussed more fully in the section entitled “Risk factors” immediately following this prospectus summary. Principal risks of our business include:
| • | Our business and prospects depend heavily on Optune, which is currently FDA-approved only for recurrent GBM. If we are unable to increase sales of Optune, obtain regulatory approvals for and commercialize Optune or our other delivery system candidates for the treatment of additional indications or significantly delayed or limited in doing so, our business and prospects will be materially harmed. |
| • | To date, we have incurred substantial operating losses. |
| • | If we do not achieve our projected research and development and commercialization goals, including FDA approval of Optune for newly diagnosed GBM, in the timeframes we announce or expect, our business would be harmed and we may need to raise additional capital to fund our operations. |
| • | We may not be successful in achieving market acceptance of TTFields by healthcare professionals, patients and/or third-party payers in the timeframes we anticipate, or at all, which could have a material adverse effect on our business, prospects, financial condition and results of operations. |
| • | Failure to secure and maintain adequate coverage and reimbursement from third-party payers could adversely affect acceptance of our delivery systems and reduce our revenues. |
| • | We depend on single-source suppliers for some of our components. The loss of these suppliers could prevent or delay shipments of Optune, delay our clinical trials or otherwise adversely affect our business. |
| • | If we encounter difficulties enrolling patients in our clinical trials, our clinical trials could be delayed or otherwise adversely affected. |
| • | We are subject to extensive federal, state and foreign laws and regulations in the conduct of our business. |
| • | If we are unable to protect our proprietary technology, trade secrets or know-how, we may not be able to operate our business profitably. Similarly, if we fail to sustain, further build and enforce our intellectual property rights, competitors may be able to develop competing therapies. |
Implications of being an emerging growth company
We qualify as an emerging growth company as defined in the Jumpstart Our Business Startups Act of 2012, as amended, or the JOBS Act. As an emerging growth company, we may take advantage of reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| • | only two years of audited financial statements in addition to any required unaudited interim financial statements with correspondingly reduced “Management’s discussion and analysis of financial condition and results of operations” disclosure; |
| • | reduced disclosure about our executive compensation arrangements; |
5
Table of Contents
| • | no non-binding advisory votes on executive compensation or golden parachute arrangements; and |
| • | exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company on the date that is the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; and (iv) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, or SEC. We may choose to take advantage of some but not all of these exemptions. We have taken advantage of these reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different from the information you receive from other public companies in which you hold stock. We have irrevocably elected to “opt out” of the exemption for the delayed adoption of certain accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Corporate information
We were incorporated under the laws of Jersey, Channel Islands, in 2000. Our registered office address is located at Le Masurier House, La Rue Le Masurier, St. Helier, Jersey JE2 4YE, and our telephone number is +44 (0)15 3475 6700. Our agent for service of process in the United States is NovoCure Limited, c/o Novocure Inc., located at 20 Valley Stream Pkwy, Suite 300, Malvern, PA 19355 and our telephone number is 212-767-7530. Our register of members is kept at our registered office address at Le Masurier House, La Rue Le Masurier, St. Helier, Jersey JE2 4YE. Our website address is www.novocure.com. We do not incorporate the information on, or accessible through, our website into this prospectus, and you should not consider it part of this prospectus, nor should you rely on any such information in making your decision whether to purchase our ordinary shares.
6
Table of Contents
The offering
| Ordinary shares offered by us |
7,500,000 ordinary shares. |
| Ordinary shares outstanding immediately after this offering |
82,676,810 ordinary shares (or 83,801,810 ordinary shares if the underwriters exercise their over-allotment option in full). |
| NASDAQ symbol |
NVCR. |
| Over-allotment option |
We have granted to the underwriters an option, exercisable within 30 days from the date of this prospectus, to purchase up to 1,125,000 additional ordinary shares to cover over-allotments, if any. |
| Use of proceeds |
We estimate that we will receive net proceeds of approximately $161.0 million from this offering after deducting the underwriting discounts, commissions and estimated offering expenses payable by us and assuming an initial public offering price of $23.50 per share, the mid-point of the range of the initial public offering price shown on the front cover of this prospectus. We plan to use the net proceeds we receive from this offering for working capital and general corporate purposes, including clinical trials and research and development and continued commercialization of Optune and our future delivery systems. In addition, we are required to make a $1.0 million payment to the Technion Research and Development Foundation and the Technion—Israel Institute of Technology, which we refer to collectively as the Technion, with the net proceeds of this offering. See “Use of proceeds” and “Our business—Intellectual property.” |
| Dividend policy |
We do not intend to pay dividends on our ordinary shares. We plan to retain our available cash and any future earnings for use in the operation of our business and to fund future growth. |
Unless we specifically state otherwise, the information in this prospectus assumes:
| • | the conversion of all our outstanding preferred shares in connection with the consummation of this offering after giving effect to a 5.913-for-1 stock split effected on September 16, 2015; |
| • | that we will amend and restate our current memorandum and articles of association, and that such amendment and restatement will be effective upon consummation of this offering; and |
| • | an initial public offering price of $23.50 per share, the mid-point of the price range set forth in the cover of this prospectus. |
7
Table of Contents
Unless we specifically state otherwise, the information in this prospectus does not take into account:
| • | up to an additional 1,125,000 ordinary shares issuable pursuant to the underwriters’ over-allotment option; |
| • | 10,054,321 ordinary shares issuable upon the exercise of outstanding options as of June 30, 2015, at a weighted average exercise price of $5.39 per share (including the option to acquire 1,005,210 ordinary shares held by the Technion Research and Development Foundation); |
| • | 921,488 ordinary shares issuable upon the exercise of options granted conditioned upon the consummation of this offering at an exercise price equal to the price per share to the public in this offering; |
| • | 4,635,317 ordinary shares issuable upon the exercise of outstanding warrants as of June 30, 2015, at a weighted average exercise price of $6.75 per share; or |
| • | 11,978,512 ordinary shares reserved for future issuance under our 2015 Omnibus Incentive Plan. |
8
Table of Contents
Summary consolidated financial and operating data
We present below our summary consolidated financial and operating data for the periods and as of the dates indicated. The following summary consolidated statement of operations data for the years ended December 31, 2013 and 2014 have been derived from our audited consolidated financial statements included elsewhere in this prospectus. The summary consolidated statement of operations data for the six months ended June 30, 2014 and 2015, and the summary consolidated balance sheet data as of June 30, 2015, have been derived from our unaudited consolidated financial statements included elsewhere in this prospectus. The unaudited consolidated financial statements have been prepared on a basis consistent with our audited consolidated financial statements. In the opinion of management, the unaudited financial data reflect all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of the results for those periods. Results for interim periods are not necessarily indicative of results that may be expected for a full fiscal year. Historical results are not necessarily indicative of the results expected in the future.
The summary consolidated financial data should be read in conjunction with our consolidated financial statements and related notes and “Management’s discussion and analysis of financial condition and results of operations” included elsewhere in this prospectus.
| Year ended December 31, |
Six months ended June 30, | |||||||||||||||
| (in thousands except share and per share data) | 2013 | 2014 | 2014 | 2015 | ||||||||||||
| Consolidated statement of operations data |
||||||||||||||||
| Net revenues |
$ | 10,359 | $ | 15,490 | $ | 7,315 | $ | 11,751 | ||||||||
| Cost of revenues |
7,013 | 10,036 | 4,820 | 8,647 | ||||||||||||
|
|
|
|||||||||||||||
| Gross profit |
3,346 | 5,454 | 2,495 | 3,104 | ||||||||||||
|
|
|
|||||||||||||||
| Operating costs and expenses: |
||||||||||||||||
| Research, development and clinical trials |
34,797 | 40,381 | 19,915 | 22,692 | ||||||||||||
| Sales and marketing |
16,406 | 21,177 | 11,055 | 15,221 | ||||||||||||
| General and administrative |
16,602 | 24,052 | 10,767 | 14,343 | ||||||||||||
|
|
|
|||||||||||||||
| Total operating costs and expenses |
67,805 | 85,610 | 41,737 | 52,256 | ||||||||||||
|
|
|
|||||||||||||||
| Operating loss |
(64,459 | ) | (80,156 | ) | (39,242 | ) | (49,152 | ) | ||||||||
| Financial expenses, net |
12,558 | 144 | 38 | 1,467 | ||||||||||||
|
|
|
|||||||||||||||
| Loss before income taxes |
(77,017 | ) | (80,300 | ) | (39,280 | ) | (50,619 | ) | ||||||||
| Income taxes |
353 | 382 | 166 | 2,011 | ||||||||||||
|
|
|
|||||||||||||||
| Net loss |
$ | (77,370 | ) | $ | (80,682 | ) | $ | (39,446 | ) | $ | (52,630 | ) | ||||
|
|
|
|||||||||||||||
| Basic and diluted net loss per ordinary share |
$ | (6.73 | ) | $ | (6.46 | ) | $ | (3.21 | ) | $ | (4.12 | ) | ||||
| Weighted average number of ordinary shares used in computing basic and diluted net loss per share |
11,498,392 | 12,490,017 | 12,269,507 | 12,783,881 | ||||||||||||
|
|
|
|||||||||||||||
| Basic and diluted pro forma net loss per ordinary share(1) |
$ | (1.13 | ) | $ | (0.73 | ) | ||||||||||
| Weighted average number of ordinary shares used in computing basic and diluted pro forma net loss per ordinary share(1) |
71,166,032 | 72,137,939 | ||||||||||||||
|
|
||||||||||||||||
9
Table of Contents
| June 30, 2015
(in thousands) |
Actual |
Pro forma
as adjusted(1)(2) |
||||||
| Consolidated balance sheet data: |
||||||||
| Cash and cash equivalents |
$ | 106,508 | $ | 266,755 | ||||
| Short-term investments |
56,996 | 56,996 | ||||||
| Total assets |
192,770 | 352,223 | ||||||
| Working capital |
158,677 | 320,378 | ||||||
| Total liabilities |
48,463 | 47,009 | ||||||
| Total shareholders’ equity |
$ | 144,307 | $ | 305,214 | ||||
|
|
||||||||
| (1) | Pro forma for the conversion of all our outstanding preferred shares into ordinary shares in connection with the consummation of this offering (see Note 13a to the consolidated financial statements) after giving effect to a 5.913-for-1 stock split effected on September 16, 2015. Does not include any ordinary shares to be sold by us in this offering. |
| (2) | Pro forma as adjusted balances reflect the sale by us of 7,500,000 ordinary shares in this offering and our receipt of the estimated net proceeds from that sale, based on an assumed public offering price of $23.50 per share, which is the midpoint of the range set forth on the cover page of this prospectus, and after deducting underwriting discounts, commissions, estimated offering expenses payable by us and a $1.0 million payment to the Technion. A $1.00 increase (decrease) in the assumed initial public offering price of $23.50 per share would increase (decrease) the pro forma as adjusted amount of each of cash and cash equivalents, total assets, working capital and total stockholders’ equity by approximately $7.0 million, assuming that the number of ordinary shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts, commissions and estimated offering expenses payable by us. |
The following table includes certain commercial patient operating statistics for and as of the end of the periods presented.
| Operating statistics | Year
ended December 31, |
Six months ended June 30, |
||||||||||||||
| 2013 | 2014 | 2014 | 2015 | |||||||||||||
| Prescriptions received in period(1) |
510 | 707 | 298 | 865 | ||||||||||||
| Active patients at period end(2) |
184 | 225 | 172 | 425 | ||||||||||||
|
|
||||||||||||||||
| (1) | A “prescription received” is a commercial order for Optune that is received from a physician certified to treat patients with TTFields therapy for a patient not previously on TTFields therapy. Orders to renew or extend treatment are not included in this total. In the future, we may have regulatory approvals and commercial programs for multiple clinical indications, at which time we will recognize a commercial order as a prescription for the same patient for each clinical indication treated. For example, in the future, a patient may have a prescription for the treatment of lung cancer and a prescription for the treatment of brain metastases from the lung cancer. |
| (2) | An “active patient” is a patient who is on TTFields therapy under a commercial prescription order as of the measurement date, including patients who may be on a temporary break from treatment and who plan to resume treatment in less than 60 days. |
10
Table of Contents
An investment in our ordinary shares involves a high degree of risk. You should carefully consider all of the information in this prospectus, including the risks and uncertainties described below, before you decide to buy our ordinary shares. Any of the following risks could have a material adverse effect on our business, prospects, financial condition and results of operations. In any such case, the trading price of our ordinary shares could decline, and you could lose all or part of your investment. In assessing these risks, you should also refer to the other information contained in this prospectus, including our consolidated financial statements and the related notes.
Risks relating to our business, TTFields and our delivery systems
Our business and prospects depend heavily on Optune, which is currently FDA-approved only for recurrent GBM. If we are unable to increase sales of Optune, obtain regulatory approvals for and commercialize Optune or our other delivery system candidates for the treatment of additional indications or are significantly delayed or limited in doing so, our business and prospects will be materially harmed.
Although we have received the FDA and Japanese Ministry of Health, Labour and Welfare regulatory approvals for Optune for treatment of adult patients with recurrent GBM and have affixed a CE mark to our TTFields delivery systems for certain indications in the European Union, such approvals and the CE mark affixed to our delivery systems do not guarantee future revenues for these indications, and until we receive FDA approval for the use of Optune for newly diagnosed GBM and TTFields delivery systems for other indications, almost all of our revenues in the United States will derive from sales of Optune for recurrent GBM. The commercial success of Optune and any other delivery systems and our ability to generate and maintain revenues from the use of these delivery systems will depend on a number of factors, including:
| • | our ability to develop, obtain regulatory approval for and commercialize Optune and our other TTFields delivery system candidates for additional indications, including newly diagnosed GBM; |
| • | our ability to successfully commercialize Optune and our other delivery system candidates for approved indications in our key markets; |
| • | the acceptance of TTFields by patients and the healthcare community, including physicians and third-party payers (both private and public), as therapeutically effective and safe relative to the cost and safety of alternative therapies; |
| • | the ability to obtain and maintain sufficient coverage or reimbursement by private and public third-party payers; |
| • | the ability of our third-party manufacturers to manufacture Optune and other delivery systems in sufficient quantities with acceptable quality; |
| • | our ability to provide marketing and distribution support for Optune and our other delivery system candidates; |
| • | results of future clinical studies relating to TTFields or our competitors’ products; |
| • | the label and promotional claims allowed by the FDA and by the applicable rules on the promotion of medical devices in other foreign jurisdictions, such as in the EU member states; |
| • | the maintenance of our existing regulatory approvals in the United States, the European Union, Switzerland and Japan; and |
| • | the consequences of any reportable adverse events occurring in the United States, the European Union or other foreign jurisdictions. |
11
Table of Contents
In addition, sales of Optune are limited to approved indications, which vary by geography, and the FDA label for Optune is limited in certain respects (for example, it is not approved for use in the brain stem, and is limited for use by adults ages 22 and older). Optune is also less efficacious in the cerebellum, which may reduce the number of GBM patients to whom it may be prescribed.
Our ability to generate future revenues will depend on achieving regulatory approval of, and eventual commercialization of, our most advanced delivery system candidates. However, obtaining regulatory approval of our delivery systems is not guaranteed. For example, although we expect to receive FDA approval of Optune for newly diagnosed GBM, we cannot assure you that we will receive such approval in a timely manner, or at all. Our near-term prospects are substantially dependent on our ability to obtain regulatory approvals on the timetable we have anticipated, and thereafter to further successfully commercialize our delivery systems. If we are not able to receive such approvals or to further commercialize our delivery systems, or are significantly delayed or limited in doing so, our business and prospects will be materially harmed and we may need to delay our initiatives or even significantly curtail operations.
To date, we have incurred substantial operating losses.
We were founded in 2000, operated as a development stage company through December 31, 2011 and have incurred substantial operating losses to date. In assessing our prospects, you must consider the risks and difficulties frequently encountered by companies in new and rapidly evolving markets, particularly companies engaged in the development and sales of oncology products. These risks include our ability to:
| • | continue to develop and enhance Optune and our delivery system candidates; |
| • | obtain regulatory clearance to commercialize new delivery systems and enhance or modify our existing delivery systems; |
| • | increase our sales, marketing and distribution organization to commercialize our delivery systems; |
| • | perform clinical research and trials on TTFields; |
| • | establish and increase awareness and acceptance of our delivery systems; |
| • | implement and successfully execute our business and marketing strategy; |
| • | respond effectively to competitive pressures and developments; |
| • | maintain, protect and expand our intellectual property portfolio; |
| • | expand our presence and commence operations in our key markets; |
| • | attract, retain and motivate qualified personnel; and |
| • | grow our organization to support our operations as a public company and our clinical pipeline and planned commercialization efforts. |
We anticipate incurring significant costs associated with commercializing our delivery systems for approved indications. Our expenses could increase beyond expectations if we are required by the FDA, or other regulatory agencies, domestic or foreign, to change manufacturing processes for our delivery systems, or to perform clinical, nonclinical or other types of studies in addition to those that we currently anticipate. Our revenues are dependent, in part, upon the size of the markets in the jurisdictions in which we receive regulatory approval, the accepted price for our delivery systems and the ability to obtain reimbursement at such price. If the number of our addressable patients is not as significant as we estimate, the indications approved by regulatory
12
Table of Contents
authorities is narrower than we expect or the population for treatment is narrowed by competition, physician choice or treatment guidelines, we may not generate significant revenues. If we are not able to generate significant revenues, we may never become profitable.
We can also be negatively affected by general economic conditions. We may not have insight into trends that could emerge and negatively affect our business. As a result of these or other risks, our business strategy might not be successful.
If we do not achieve our projected research and development and commercialization goals, including FDA approval of Optune for newly diagnosed GBM, in the timeframes we announce or expect, our business would be harmed and we may need to raise additional capital to fund our operations.
For planning purposes, we estimate the timing of the accomplishment of various scientific, clinical, regulatory and other goals, which we sometimes refer to as milestones. These milestones may include the commencement or completion of scientific studies and clinical trials, the submission of regulatory filings in the United States and other foreign jurisdictions and the receipt of regulatory approvals in such jurisdictions. From time to time, we may publicly announce the expected timing of some of these milestones. All of these milestones are based on a variety of assumptions. The actual timing of the achievement of these milestones can vary dramatically from our estimates, in many cases for reasons beyond our control, depending on numerous factors, including:
| • | the rate of progress, costs and results of our research and development activities and clinical trials; |
| • | our ability to identify and enroll patients who meet clinical trial eligibility criteria; |
| • | the extent of scheduling conflicts with participating clinicians and clinical institutions; |
| • | the occurrence of unanticipated adverse events during clinical trials; |
| • | the receipt of approvals by our competitors and by us of our delivery systems and our competitors’ products; |
| • | our ability to achieve coverage and reimbursement milestones with private and governmental third-party payers; |
| • | our ability to access sufficient, reliable and cost-effective supplies of components used in the manufacture of our delivery systems and delivery system candidates, including the transducer arrays and other materials; |
| • | our ability to develop a sales and marketing organization and/or enter into sales and marketing collaborations for Optune and, if approved, our delivery system candidates; and |
| • | other actions by regulators. |
For example, our key milestones include our expected FDA approval of Optune for newly diagnosed GBM and FDA approval of our second-generation Optune delivery system for GBM, as well as other clinical development milestones for other indications described in this prospectus. We can provide no assurance that we will achieve these milestones on our expected timetable, or at all.
If we do not achieve these milestones in the timeframes we expect, and/or if we are unable to obtain sufficient additional funds through financings, including sales of securities such as those in this offering, the proceeds from long-term loans, strategic collaborations or the license or sale of certain of our assets on a timely basis when necessary, we may be required to reduce expenses by delaying, reducing or curtailing the development of our delivery systems and we may need to raise additional capital to fund our operations, which we may not be able to obtain on favorable terms, if at all. If we fail to commence or complete, or experience delays in or are forced to curtail, our proposed clinical programs or otherwise fail to adhere to our projected development goals
13
Table of Contents
in the timeframes we announce or expect (or within the timeframes expected by analysts or investors), or we fail to raise any required additional capital, any of such events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
We may not be successful in our efforts to create a pipeline of delivery system candidates for future indications for TTFields and successfully commercialize them, or we may expend our resources on indications that do not yield a successful approval and fail to capitalize on other indications that may be more profitable or for which there is a greater likelihood of success.
We are pursuing clinical development of TTFields to treat a variety of solid tumors. For these future indications, we are at an early stage of development and we do not have approvals. Further, we do not intend to pursue indications involving solid tumors of the throat or extremities, and TTFields would not be efficacious for non-solid tumor cancers like lymphoma or other blood cancers.
Even if we are successful in continuing to build our pipeline, obtaining regulatory approvals and commercializing our delivery system candidates for additional indications are prone to risks of failure, including the significant risk that the development of our delivery system candidates for any potential indications will fail to demonstrate adequate efficacy or an acceptable safety profile, gain regulatory approval and become commercially viable. We cannot provide you any assurance that we will be able to advance any of these additional indications through the development and commercialization process. Our research programs may initially show promise in addressing additional indications, yet fail to yield approvals or commercialization for many reasons, including the following:
| • | we may not be able to assemble sufficient resources to pursue clinical trials for additional indications; |
| • | our delivery system candidates may not succeed in pre-clinical or clinical testing; |
| • | our delivery systems may on further study be shown to have harmful side effects for other indications or other characteristics that indicate they are unlikely to be effective or otherwise do not meet applicable regulatory criteria for such indications; |
| • | competitors may develop alternative treatments that render our delivery systems obsolete or less attractive; |
| • | the market for TTFields may change so that the continued development of our pipeline as currently contemplated is no longer appropriate; |
| • | our delivery systems may not be capable of being produced in commercial quantities at an acceptable cost, or at all; and |
| • | our delivery systems may not be accepted as safe, effective, convenient or otherwise desirable by patients, the medical community or third-party payers. |
If any of these events occur, we may be forced to delay or abandon our development efforts for our anticipated pipeline, which would have a material adverse effect on our business and prospects and could potentially cause us to cease operations. Moreover, any such events in respect of any particular indication and/or delivery system candidate may have a negative effect on the approval process for other indications and/or result in losing approval of approved delivery systems for other indications, which may exacerbate the harm to our business and prospects.
We have limited experience in commercializing Optune and, to the extent we do not successfully develop this ability or contract with a third party to assist us, we may not be able to successfully commercialize our delivery systems that may be approved for commercial sale.
We currently have a small sales and marketing organization, and we may not be able to successfully develop adequate sales and marketing capabilities to achieve our growth objectives. The growth of our sales and
14
Table of Contents
marketing organization will require us to commit significant additional management and other resources. We will have to compete with other pharmaceutical and life sciences companies to recruit, hire, train and retain the sales and marketing personnel that we anticipate we will need. If we are unable to establish adequate sales and marketing capabilities, we will need to enter into sales and distribution agreements to market some or all of our delivery systems that may be approved for commercial sale. In addition, because Optune and future delivery systems require physician training and education, our sales and marketing organization must grow substantially as we expand our approved indications and markets. As a consequence, our expenses associated with building up and maintaining our sales force and marketing capabilities may be disproportionate to the revenues we may be able to generate on sales of Optune and other delivery systems.
If we are unable to establish adequate sales and marketing capabilities or successful distribution relationships, we may fail to realize the full sales potential of some or all of our delivery system candidates, and we may not be able to achieve the necessary growth in a cost-effective manner or realize a positive return on our investment. If we establish distribution agreements with other companies, we generally would not have control over the resources or degree of effort that any of these third parties may devote to our delivery systems, and if they fail to devote sufficient time and resources to the marketing of such delivery systems, or if their performance is substandard, it will adversely affect our revenues.
We may not be successful in achieving market acceptance of TTFields by healthcare professionals, patients and/or third-party payers in the timeframes we anticipate, or at all, which could have a material adverse effect on our business, prospects, financial condition and results of operations.
Our business model is predicated on achieving market acceptance of TTFields as a monotherapy or in combination with well established cancer treatment modalities like surgery, radiation and chemotherapy. We may not achieve market acceptance of Optune and other TTFields delivery systems we develop in the amount of time that we have anticipated, or at all, for a number of different reasons. As a general matter, we may not achieve market acceptance of TTFields because of the following factors, among others:
| • | it may be difficult to gain broad acceptance of TTFields because it is a new technology and involves a novel delivery system, and as such physicians may be reluctant to prescribe TTFields delivery systems without prior experience or additional data or training; |
| • | it may be difficult to gain broad acceptance at community hospitals where the number of patients seeking cancer treatment may be more limited than at larger medical centers, and such community hospitals may not be willing to invest in the resources necessary for their physicians to become trained to use TTFields, which could lead to reluctance to prescribe our TTFields delivery systems; |
| • | patients may be reluctant to elect to use our TTFields delivery systems, including Optune, for various reasons, including a perception that the treatment is untested; |
| • | the delivery systems may have some side effects (for example, dermatitis where the transducer arrays are placed) and the delivery system cannot be worn in all circumstances (for example, it cannot get wet and is difficult to wear in high temperatures); and |
| • | the price of the TTFields delivery systems includes a monthly fee for use of the delivery system (including the transducer arrays), so as the duration of the treatment course increases, the price will increase correspondingly, and, when used in combination with other treatments, the overall cost of treatment will be greater than using a single type of treatment. |
15
Table of Contents
In particular, Optune may not achieve market acceptance because of the following additional factors (which may apply to our future delivery systems, to varying degrees):
| • | achieving patient acceptance is difficult because GBM is a devastating disease with a poor prognosis, and not all patients with short lifespans are willing to comply with Optune therapy requirements, such as extended use of Optune, carrying around a battery pack and shaving their heads (which may be of particular concern to women), and other patients may forego Optune treatment for cosmetic or mobility reasons; |
| • | achieving patient compliance is difficult because the recommended average daily use of Optune is at least 18 hours a day, requiring patients to wear the delivery system nearly continuously, which to some extent restricts physical mobility because the battery must be frequently recharged, and the patient or a caregiver must ensure that it remains continuously operable; |
| • | certain patients are not advised to use Optune, including: patients who have an active electronic medical device, which include deep brain stimulators, spinal cord stimulators, vagus nerve stimulators, pacemakers, defibrillators and programmable shunts, because the use of Optune with these devices has not been tested and may lead to malfunctioning of these devices; patients who have a skull defect, a shunt or bullet fragments because the use of Optune with these conditions has not been tested and may lead to tissue damage or render Optune ineffective; and patients who are sensitive to conductive hydrogels because skin contact with the gel used in Optune for patients that are sensitive to conductive hydrogels may commonly cause increased redness and itching, and in rare instances may lead to severe allergic reactions, such as shock or respiratory failure; |
| • | the need to wear Optune nearly continuously in order to achieve efficacy of TTFields may also impact the pool of patients to whom physicians may be willing to prescribe treatment, as physicians may be reluctant to treat patients who are physically frail or lack caregiver support with Optune, and efficacy may also be limited in instances where patients take a break from the delivery system when experiencing skin rashes, while bathing or swimming because Optune cannot get wet, or while traveling because Optune batteries cannot be taken on airplanes, and although we ship batteries to patients, there is inevitably a disruption in continuous use; and |
| • | side effects reported by GBM patients treated with a combination of TTFields and temozolomide, including dermatitis where the transducer arrays are placed, headaches, weakness, falls, fatigue, muscle twitching and skin ulcers (and there may be additional side effects not yet observed). |
In addition, even if we are successful in achieving market acceptance of Optune for GBM, we may be unsuccessful in achieving market acceptance of TTFields as a treatment for other solid tumor cancers, such as brain metastases, NSCLC, pancreatic cancer, ovarian cancer, mesothelioma and other solid tumor cancers, because certain radiation or chemotherapies may remain the preferred standard of care for these indications.
There may be other factors that are presently unknown to us that also may negatively impact our ability to achieve market acceptance of TTFields delivery systems. If we do not achieve market acceptance of our delivery systems in the timeframes we anticipate, or are unable to achieve market acceptance at all, our business, prospects, financial condition and results of operations could be materially adversely affected, and our stock price could decline.
Failure to secure and maintain adequate coverage and reimbursement from third-party payers could adversely affect acceptance of our delivery systems and reduce our revenues.
We expect that the vast majority of our revenues will come from third-party payers either directly to us in markets where we provide our delivery systems to patients or indirectly via payments made to hospitals or other entities providing our delivery systems to patients. Private payers in the United States cover a majority of
16
Table of Contents
the population, with the remainder covered by governmental payers or uninsured. In 2014, United Healthcare and Aetna represented 15% and 12%, respectively, of our net revenues. We anticipate that the majority of the third-party payers outside the United States will be government agencies, government sponsored entities or other payers operating under significant regulatory requirements from national or regional governments.
Medical treatments may not be reimbursed by third-party payers based on a number of factors, such as a determination that it is experimental, not medically necessary or not appropriate for a particular patient. Currently, we are aware that seven private payers in the United States have issued policies that deny coverage for Optune on one or more of these bases. Additionally, private commercial and government payers may be permitted to consider the cost of a treatment in approving coverage or in setting payment for the treatment.
Private and government payers in the United States and around the world are increasingly challenging the prices charged for medical products and services. Additionally, the containment of healthcare costs has become a priority of U.S. federal and state governments and governments around the world. Adoption of additional price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our revenues and operating results. If third-party payers do not consider our delivery system or the combination of our delivery system with additional treatments to be cost-justified under a required cost-testing model, they may not cover our delivery systems for their populations or, if they do, the level of payment may not be sufficient to allow us to sell our delivery systems on a profitable basis.
Reimbursement for the treatment of patients with medical devices in the EU member states, Switzerland and Japan is governed by complex mechanisms established on a national level in each country. In the European Union, these mechanisms vary widely among the EU member states and evolve constantly, reflecting the efforts of these countries to reduce public spending on healthcare. As a result, obtaining reimbursement for the treatment of patients with medical devices has become more challenging. Outside the United States, the European Union and Japan, reimbursement systems vary significantly by country. We cannot, therefore, guarantee that the treatment of patients with Optune or any of our future delivery systems would be reimbursed in any of the EU member states, Switzerland, Japan or any other country.
We provide financial assistance to patients to defray their out-of-pocket costs for Optune, and therefore, absorb any unreimbursed costs of patients who begin treatment and are unable to pay for the costs of their treatment not covered by insurance. Our costs associated with this program could increase if payers increase the cost-sharing burden of patients. We bill $21,000 for each month that a patient uses Optune in the United States. Total cash payments of $12.4 million received during the six months ended June 30, 2015 were recorded as revenues for Optune therapy provided to patients in the current period and prior periods. These cash payments represent an average of approximately $14,700 for a month of use. The difference between billed and paid amounts consists of disputed underpayments, patient financial assistance and discounts. Additionally, during the period ended June 30, 2015, for each month of use, we paid approximately $600 in indirect taxes, primarily the federal medical device excise tax. This metric does not include our experience with patients covered by the Medicare fee-for-service program, as we have not received material payments from that program and the invoices remain open as we appeal the coverage denials. Our average payment amount per month of use in the United States may decrease based on a number of factors, including, but not limited to, agreeing to greater discounts with payers and payers increasing the cost-sharing requirement for patients.
Our failure to secure or maintain adequate coverage or reimbursement for Optune or any of our future delivery systems by third-party payers in the United States or in the other jurisdictions in which we market Optune or any of our future delivery systems, could have a material adverse effect on our business, financial condition and results of operations and cause our stock price to decline.
17
Table of Contents
We may not be successful securing and maintaining reimbursement codes necessary to facilitate accurate and timely billing for Optune, future delivery systems and physician services attendant to TTFields therapy.
Third-party payers, healthcare systems, government agencies or other groups often issue reimbursement codes to facilitate billing for products and physician services used in the delivery of medicine. Within the United States, the billing codes most directly related to Optune and future delivery systems are contained in the Healthcare Common Procedure Coding System, or HCPCS code set. The HCPCS code set contains Level I codes that describe physician services, also known as Common Procedural Terminology codes, or CPT codes, and Level II codes that primarily describe products. The Centers for Medicare and Medicaid Services, or CMS, is responsible for issuing the HCPCS Level II codes. The American Medical Association issues HCPCS Level I codes.
We have secured unique HCPCS Level II codes that describe Optune and we are able to use these codes in the United States to bill third-party payers. Loss of these codes or any alteration in the payment attached to these codes would materially impact our operating results.
No CPT codes exist to describe physician services related to the delivery of TTFields therapy. We may not be able to secure CPT codes for physician services related to Optune based on the relatively low incidence of GBM. Our future revenues and results may be affected by the absence of CPT codes, as physicians may be less likely to adopt the therapy when not adequately reimbursed for the time, effort, skill, practice expense and malpractice costs required to provide the therapy to patients.
We have not secured codes to describe our delivery systems or to document physician services related to the delivery of TTFields therapy in markets outside the United States. Absence of these codes may affect the future growth of our business.
There is no assurance that Medicare or the Medicare Administrative Contractors will provide coverage or adequate payment rates for Optune or our future delivery systems.
Approximately 25% of our patients are beneficiaries under the Medicare fee-for-service program as of June 30, 2015. Failure to secure coverage and adequate payment from Medicare would reduce our revenues and may also affect the coverage and payment decisions of other third-party payers in the United States.
Medicare has the authority to issue national coverage determinations or to defer coverage decisions to its regional Medicare Administrative Contractors, or MACs. Medicare has not issued a national coverage determination for Optune. The four MACs that administer the durable medical equipment benefit for Medicare, or DME MACs, have each issued local coverage determination policies stating that Optune is not reasonable and necessary for the treatment of recurrent GBM. The continuing absence of a positive coverage determination from Medicare or the DME MACs would materially affect our future revenues.
Additionally, Medicare has the authority to publish the price of durable medical equipment products. Medicare may publish prices for Optune or future delivery systems that do not reflect then current prices for Optune or future delivery systems. Medicare price schedules are frequently referenced by private payers in the United States and around the world. Medicare would materially reduce our revenues and operating results by publishing a price for Optune or future delivery systems that is not based on the actual price of Optune or future delivery systems within the private payer market.
We are unable to bill our existing Medicare fee-for-service patients for amounts not paid by Medicare. Therefore, we will absorb the costs of treatment for amounts not paid by Medicare.
We depend on single-source suppliers for some of our components. The loss of these suppliers could prevent or delay shipments of Optune, delay our clinical trials or otherwise adversely affect our business.
We source some of the key components of Optune from only a single vendor. If any one of these single-source suppliers were to fail to continue to provide components to us on a timely basis, or at all, our business and
18
Table of Contents
reputation could be harmed. For example, we currently have a single source for the ceramic discs used in the transducer arrays for Optune, which we source from Exelis Inc., or Exelis (formerly ITT Corporation). We currently do not have alternate suppliers for these ceramic discs, and our existing supplier would be difficult for us to replace because the ceramic discs are manufactured with specialized electrical properties designed specifically for Optune, and as such there are few vendors available to produce these components, and those that can supply them may not be able to do so on terms that are commercially favorable to us. We are in the process of identifying a second source for the ceramic discs, but we can provide no assurance that we will secure an alternate supplier on favorable terms or in time to support our commercialization efforts, or at all. Our current agreement with Exelis continues through July 21, 2017, following which time the agreement will automatically renew for up to three successive two-year periods unless either we provide timely written notice of non-renewal (for any reason) or Exelis provides timely written notice of non-renewal (if we fail to satisfy certain minimum purchase requirements). We currently expect that this agreement will be renewed. In addition to certain other customary termination rights, Exelis can terminate this agreement with 90 days’ written notice if we breach any of our material obligations under the agreement.
Agreements with our other suppliers range from terms of four years to ten years and are terminable by either party, generally between 180 days’ and 12 months’ written notice. Establishing additional or replacement suppliers for any components of our delivery systems, and obtaining any additional regulatory approvals required to add or replace suppliers, will take a substantial amount of time and could result in increased costs and impair our ability to produce Optune, which would have a material adverse effect on our business, prospects, financial condition and results of operations. We may have difficulty obtaining similar components from other suppliers that are acceptable to the FDA or foreign regulatory authorities, or to comply with the Essential Requirements laid down in Annex I to the Directive 93/42/EEC concerning medical devices, commonly known as the Medical Devices Directive, which are the minimum requirements governing design and manufacturing in the European Union. The risks associated with the failure of our suppliers to comply with strictly enforced regulatory requirements as described below are exacerbated by our dependence on single-source suppliers. Furthermore, since some of these suppliers are located outside of the United States, we are subject to foreign export laws and United States import and customs regulations, which complicate and could delay shipments of components to us.
We are currently seeking second-source suppliers, which we expect to have under contract over the next few years, but we can provide no assurance we will achieve this on this timeframe or at all. Various steps must be taken before signing up these suppliers, including qualifying these suppliers in accordance with regulatory requirements.
If we experience any delay or deficiency in the quality of components supplied to us by third-party suppliers, or if we have to switch to replacement suppliers, we may face additional regulatory delays and the manufacture and delivery of Optune would be interrupted for an extended period of time, which could materially adversely affect our business, prospects, financial condition and results of operations. In addition, we may be required to obtain prior regulatory approval if we use different suppliers or components. Such changes could affect our FDA regulatory approvals and the compliance of our delivery systems with the Essential Requirements laid down in Annex I to the Medical Devices Directive and the validity of our current CE Certificates of Conformity. If we are required to obtain prior regulatory approval from the FDA or foreign regulatory authorities or to conduct a new conformity assessment procedure and obtain new CE Certificates of Conformity in the EU to use different suppliers or components for our delivery systems, regulatory approval or the CE Certificates of Conformity for our delivery systems may not be received on a timely basis, or at all, which would have a material adverse effect on our business, prospects, financial condition and results of operations.
19
Table of Contents
Quality control problems with respect to delivery systems and components supplied by third-party vendors could have a material adverse effect on our reputation, our clinical trials or the commercialization of Optune and our future delivery systems and, as a result, a material adverse effect on our business, prospects, financial condition and results of operations.
Our delivery systems, which are manufactured by third parties, are highly technical and are required to meet exacting specifications. Any quality control problems that we experience with respect to the delivery systems and components supplied by third-party vendors could have a material adverse effect on our reputation, our attempts to complete our clinical trials or the commercialization of Optune and our future delivery systems. The failure of our suppliers to comply with strictly enforced regulatory requirements could expose us to regulatory action, including warning letters, product recalls, suspension or termination of distribution, product seizures or civil penalties. If we experience any delay or deficiency in the quality of products supplied to us by third-party suppliers, or if we have to switch to replacement suppliers, we may face additional regulatory delays and the manufacture and delivery of our delivery systems would be interrupted for an extended period of time, which would materially adversely affect our business, prospects, financial condition and results of operations.
If the third parties on which we rely to conduct our clinical trials and to assist us with pre-clinical research and development do not perform as contractually required or expected, we may not be able to obtain regulatory approvals for our future delivery systems or commercialize our future delivery systems.
We do not have the ability to independently conduct our pre-clinical and clinical trials for our delivery systems and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories, to conduct such trials. We and these third parties are required to comply with current good clinical practices, or cGCPs, which are regulations and guidelines enforced by the FDA and comparable foreign regulatory authorities for TTFields in clinical development. Regulatory authorities enforce these cGCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of these third parties fail to comply with applicable cGCP regulations, the clinical data generated in our clinical trials may be deemed unreliable and the FDA or foreign regulatory authorities may require us to perform additional nonclinical or clinical trials before approving our approved applications. We cannot be certain that, upon inspection or review of our files, such regulatory authorities will determine that any of our clinical trials comply with the cGCP regulations.
Any third parties conducting our clinical trials are not and will not be our employees and, except for remedies available to us under our agreements with such third parties, we cannot control whether or not they devote sufficient time and resources to our ongoing pre-clinical, clinical and nonclinical programs. These third parties may also have relationships with other commercial entities, including our competitors, for whom they may also be conducting clinical studies or other cancer treatment development activities, which could affect their performance on our behalf. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our delivery systems or successfully commercialize our delivery systems on a timely basis, if at all, and our business, prospects and results of operations may be adversely affected.
20
Table of Contents
If we encounter difficulties enrolling patients in our clinical trials, our clinical trials could be delayed or otherwise adversely affected.
The timely completion of clinical trials depends, among other things, on our ability to enroll a sufficient number of patients who remain in the trial until its conclusion. We may experience difficulties in patient enrollment in our clinical trials for a variety of reasons, including:
| • | the severity of the disease under investigation; |
| • | the size and nature of the patient population; |
| • | the patient eligibility criteria defined in the protocol; |
| • | the nature of the trial protocol, including the attractiveness of, or the discomforts and risks associated with, the treatments received by enrolled subjects; |
| • | clinicians’ and patients’ perceptions as to the potential advantages and side effects of TTFields in relation to other available therapies, including any new drugs or treatments that may be approved for the indications we are pursuing; |
| • | availability of other clinical trials; |
| • | patient referral practices of physicians; |
| • | the ability to monitor patients adequately during and after treatment; |
| • | the availability of appropriate clinical trial investigators, support staff and proximity of patients to clinical sites; |
| • | our ability to obtain and maintain patient consents; and |
| • | the risk that patients enrolled in clinical trials will choose to withdraw from or otherwise not be able to complete a clinical trial. |
Patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive follow-up to assess the safety and effectiveness of TTFields or if they determine that the treatments received under the trial protocols are not attractive or involve unacceptable risks or discomforts. Patients may also not participate in our clinical trials if they choose to participate in contemporaneous clinical trials of competing products. In addition, the inclusion of critically ill patients in our clinical trials may result in deaths or other adverse medical events for reasons that may not be related to TTFields, or, in those trials where TTFields is being tested in combination with one or more other therapies, for reasons that may be attributable to the other therapies, but which can nevertheless negatively affect clinical trial results. If we have difficulty enrolling a sufficient number or diversity of patients to conduct our clinical trials as planned, we may need to delay or terminate ongoing or planned clinical trials, either of which would have an adverse effect on our business.
Continued testing of Optune or our other delivery system candidates may not yield successful results and could reveal currently unknown safety hazards associated with TTFields.
Our research and development programs are designed to test the safety and efficacy of TTFields through extensive pre-clinical and clinical testing. Even if our ongoing and future clinical trials are completed as planned, we cannot be certain that their results will support our claims or that the FDA and other regulatory authorities will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our delivery system candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a delivery system candidate and may delay development of others. It is also possible that
21
Table of Contents
patients enrolled in clinical trials will experience adverse side effects that have not been previously observed. In addition, our pre-clinical studies and clinical trials for our delivery system candidates involve a relatively small patient population, and as a result, these studies and trials may not be indicative of future results.
We may experience numerous unforeseen events during, or as a result of, the testing process that could delay or prevent further commercialization of Optune and any of our delivery system candidates, including the following:
| • | safety and efficacy results for Optune and any of our delivery system candidates obtained in our pre-clinical and clinical testing may be inconclusive or may not be predictive of results obtained in future clinical trials, following long-term use or in much larger populations; |
| • | unanticipated adverse events may occur during TTFields’ clinical trials; |
| • | the data collected from clinical trials of our delivery system candidates may not reach statistical significance due to limited sample size or otherwise be sufficient to support FDA or other regulatory approval; and |
| • | our delivery system candidates may not produce the desired effects or may result in adverse health effects or other characteristics that are not currently known that preclude additional regulatory approval or limit their commercial use if approved. |
To date, patients treated with Optune in our EF-11 and EF-14 clinical trials have experienced treatment-related side effects, including dermatitis where the transducer arrays are placed, headaches, weakness, falls, fatigue, muscle twitching and skin ulcers. There may be additional side effects observed in future clinical trials and/or through real-world experience with patients using Optune or our other TTFields delivery system candidates. Undesirable side effects caused by our delivery systems could cause us or regulatory authorities to interrupt, delay or terminate clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other regulatory authorities. Results of our clinical trials could reveal a high and unacceptable severity and prevalence of side effects.
If unacceptable side effects arise in the development of our delivery system candidates, we could suspend or terminate our clinical trials or the FDA or other regulatory authorities could order us to cease clinical trials or deny approval of our delivery system candidates for any or all targeted indications, narrow the approved indications for use or otherwise require restrictive product labeling or marketing, or require further clinical trials, which may be time-consuming and expensive and may not produce results supporting FDA or other regulatory approval of our delivery system candidates in a specific indication. Treatment-related side effects could also affect patient recruitment or the ability of enrolled patients to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff. We expect to have to train medical personnel using our delivery system candidates to understand the side effect profiles for our clinical trials and upon any commercialization of any of our delivery system candidates. Inadequate training in recognizing or managing the potential side effects of our delivery system candidates could result in patient injury or death. Any of these occurrences may harm our business, prospects and financial condition significantly.
Any delay or termination of our clinical trials will delay the filing of our delivery systems submissions for regulatory approvals and ultimately our ability to commercialize our delivery systems and generate revenues. Furthermore, we may abandon delivery system candidates that we previously believed to be promising. Any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
22
Table of Contents
We face competition from numerous competitors, most of whom have far greater resources than we have, which may make it more difficult for us to achieve significant market penetration and which may allow them to develop additional oncology treatments to compete with TTFields.
The oncology market is intensely competitive, subject to rapid change and significantly affected by new product introductions and other market activities of industry participants. As a monotherapy, TTFields primarily competes with radiation and pharmacological therapies. We may face additional competition as advancements are made in the field of immuno-oncology and to date, we have not conducted any clinical trials where TTFields is used in combination with an immuno-oncological therapy.
Many of our competitors are large, well capitalized companies with significantly more market share and resources than we have. As a consequence, they are able to spend more aggressively on product development, marketing, sales and other initiatives than we can. Many of these competitors have:
| • | significantly greater name recognition and experience; |
| • | established relations with healthcare professionals, patients and third-party payers; |
| • | established distribution networks; |
| • | additional product lines, and the ability to offer rebates or bundle products to offer higher discounts or more competitive pricing or other incentives to gain a competitive advantage; and/or |
| • | greater financial and human resources for research and development, sales and marketing, patent litigation and/or acquisitions. |
Although we believe TTFields represents a treatment modality that can be used in combination with other cancer treatment modalities, our current competitors or other companies may at any time develop additional drugs and devices for the treatment of GBM and other solid tumors that could reduce the benefits of using our TTFields delivery systems. If an existing or future competitor develops a product that proves to be superior or comparable to Optune or any of our future delivery systems, our revenues may decline. In addition, some of our competitors may compete by changing the price of their cancer treatments. If these competitors’ products were to gain acceptance by healthcare professionals, patients or third-party payers, a downward pressure on prices could result. If prices were to fall, we may not be able to improve our gross margins or sales growth sufficiently to achieve profitability.
As we expand, we may experience difficulties managing our growth.
Our anticipated growth will place a significant strain on our management and on our operational and financial resources and systems. Failure to manage our growth effectively could materially adversely affect us. Additionally, our anticipated growth will increase the demands placed on our suppliers, resulting in an increased need for us to carefully monitor the available supply of components and quality assurance. Any failure by us to manage our growth effectively could have an adverse effect on our ability to achieve our development and commercialization goals.
Because of the specialized nature of our business, the termination of relationships with our key management and scientific personnel may prevent us from developing TTFields, conducting clinical trials and obtaining any necessary financing. Further, the inability to recruit and retain additional personnel may have an adverse effect on our ability to successfully operate our business.
For the majority of our history, Asaf Danziger and Dr. Eilon Kirson have played a significant role in our research efforts. Mr. Danziger is our Chief Executive Officer and a director of our company and Dr. Kirson is our Chief Science Officer and Head of Research and Development. We are highly dependent on these individuals, and they
23
Table of Contents
have played a critical role in our research and development programs, clinical trials and financing. Additionally, we have several scientific personnel with significant expertise in TTFields, some of whom are critical to our research and development efforts. The loss of the services of either of these key members of our company or any of our scientific personnel may prevent us from achieving our business objectives.
The competition for qualified personnel in the oncology field is intense, and we rely heavily on our ability to attract and retain qualified scientific, technical and managerial personnel. Our future success depends upon our ability to attract, retain and motivate highly skilled employees. In order to commercialize TTFields successfully, we will be required to expand our workforce, particularly in the areas of research and development and clinical trials, sales and marketing and supply chain management. These activities will require the addition of new personnel and the development of additional expertise by existing management personnel. We face intense competition for qualified individuals from numerous pharmaceutical, biopharmaceutical and biotechnology companies, as well as academic and other research institutions. We may not be able to attract and retain these individuals on acceptable terms or at all. Failure to do so would materially harm our business.
Changes in tax or other laws, regulations or treaties, changes in our status under U.S. or non-U.S. laws or adverse determinations by taxing or other governmental authorities could increase our tax burden or otherwise affect our financial condition or results of operations, as well as subject our shareholders to additional taxes.
The amount of taxes we pay is subject to a variety of tax laws in the various jurisdictions in which we and our subsidiaries are organized and operate. Our domestic and international tax liabilities are dependent on the location of earnings among these various jurisdictions. Such tax liabilities could be affected by changes in tax or other laws, treaties and regulations as well as the interpretation or enforcement thereof by tax or other governmental entities in any relevant jurisdiction. The amount we pay in tax to any particular jurisdiction depends, in part, on the correct interpretation of the tax laws in such jurisdiction, and we have made a number of determinations as to the effect of such tax laws in our particular circumstances. For example, while our U.S. operations are subject to U.S. federal income tax, we believe that a significant portion of our non-U.S. operations are generally not subject to U.S. tax other than withholding taxes in certain circumstances. In some cases, the determinations we have made as to the effect of the tax laws in a particular jurisdiction depend on the continuing effectiveness of administrative rulings we have received from the tax authorities in that jurisdiction, while in other cases, our determinations are based on the reasoned judgment of our tax advisors. Although we believe that we are in compliance with the administrative rulings we have received, that the assumptions made by our tax advisors in rendering their advice remain correct, and that as a result we are in compliance with applicable tax laws in the jurisdictions where we and our subsidiaries are organized and operate, a taxing authority in any such jurisdiction may challenge our interpretation of those laws and assess us or any of our subsidiaries with additional taxes.
Additionally, from time to time, proposals have been made and legislation has been introduced (for example, the Swiss Corporate Tax Reform III, or CTR III) to change the tax laws, regulations or interpretations thereof (possibly with retroactive effect) of various jurisdictions or limit tax treaty benefits that, if enacted, could materially increase our tax burden, increase our effective tax rate or otherwise have a material adverse impact on our financial condition and results of operations. As an example, recent U.S. legislative proposals would broaden the circumstances under which a foreign corporation like us would be considered a U.S. resident for U.S. federal income tax purposes, in addition to other U.S. legislative proposals that could have a material adverse impact on us by overriding certain tax treaties and limiting the treaty benefits on certain payments, which could increase our tax liability. We cannot predict whether or when any of these potential changes in law might become effective in any jurisdiction.
24
Table of Contents
While we monitor proposals and other developments that would materially impact our tax burden and effective tax rate and investigate our options accordingly, we could still be subject to increased taxation on a going forward and retroactive basis no matter what action we undertake if certain legislative proposals or regulatory changes are enacted, certain tax treaties are amended and/or our interpretation of applicable tax or other laws is challenged and determined to be incorrect. In particular, any alternative interpretations of applicable tax laws asserted by a tax authority or changes in tax laws, regulations or accounting principles that limit our ability to take advantage of tax treaties between jurisdictions, modify or eliminate the deductibility of various currently deductible payments, increase the tax burden of operating or being resident in a particular country, result in transfer pricing adjustments or otherwise require the payment of additional taxes, may have a material adverse effect on our cash flows, financial condition and results of operations.
We believe our ordinary shares should not be treated as stock of a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in the current taxable year or in a future taxable year, but this conclusion is a factual determination that is made annually and thus may be subject to change. If we were to be treated as a PFIC, this could result in adverse U.S. federal income tax consequences to U.S. persons that hold our ordinary shares.
Based on the composition of our assets and the nature of our income, we believe that our shares should not be treated as stock of a PFIC for U.S. federal income tax purposes, but this conclusion is a factual determination that is made annually and thus may be subject to change.
A non-U.S. corporation will be treated as a PFIC for U.S. federal income tax purposes in any taxable year in which a specified percentage of its gross income is “passive income” or a specified percentage of its assets produce or are held for the production of passive income (“passive assets”), including cash. If we are treated as a PFIC, and a U.S. person that holds our ordinary shares, either directly or indirectly, did not make one of the elections described under “Tax considerations—United States taxation—Passive foreign investment company considerations,” such U.S. person would be subject to adverse U.S. federal income tax consequences on distributions with respect to the ordinary shares to the extent the distributions are “excess distributions,” which are generally distributions in excess of a normal rate of distribution as calculated for PFIC purposes. Gain realized on the sale or other disposition of the ordinary shares would generally not be treated as capital gain, but rather would be treated as if such U.S. person had realized such gain and certain “excess distributions” ratably over the holding period for the ordinary shares and would be taxed at the highest tax rate in effect for each such year to which the gain was allocated, together with an interest charge in respect of the tax attributable to each such year. Partial redemptions would also be treated as excess distributions. We will, upon request from any shareholder, prepare and provide information as necessary for “qualified electing fund” elections but we make no representation as to the availability of “mark to market” elections that may mitigate the consequences of our being a PFIC to any U.S. investor. Prospective U.S. investors should consult their own U.S. tax advisors regarding the potential application of the PFIC rules. For more information on the U.S. federal income tax consequences of our ordinary shares being treated as stock of a PFIC, see “Tax considerations—United States taxation—Passive foreign investment company considerations.”
Product liability suits, whether or not meritorious, could be brought against us due to alleged defective delivery systems or for the misuse of our delivery systems. These suits could result in expensive and time-consuming litigation, payment of substantial damages and an increase in our insurance rates.
If our current or future delivery systems are defectively designed or manufactured, contain defective components or are misused, or if someone claims any of the foregoing, whether or not meritorious, we may become subject to substantial and costly litigation. For example, we may be sued if our delivery systems cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. This may occur if Optune is misused or damaged, has a sudden failure or malfunction
25
Table of Contents
(including with respect to safety features) or is otherwise impaired due to wear and tear. Even absent a product liability suit, malfunctions of the device or misuse by the physician or patient would need to be remedied swiftly in order to maintain continuous use and ensure efficacy of TTFields.
Any product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the delivery system, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of Optune and our delivery system candidates. Even successful defense may require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | decreased demand for our TTFields delivery systems; |
| • | injury to our reputation; |
| • | withdrawal of clinical trial participants and inability to continue clinical trials; |
| • | initiation of investigations by regulators; |
| • | costs to defend the related litigation; |
| • | a diversion of management’s time and our resources; |
| • | substantial monetary awards to trial participants or patients; |
| • | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| • | loss of revenues; |
| • | exhaustion of any available insurance and our capital resources; |
| • | the inability to commercialize any delivery system candidate; and |
| • | a decline in our share price. |
Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizable damage awards against us. We may not have sufficient insurance coverage for all claims. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, could harm our reputation in the industry and could reduce revenues. Product liability claims in excess of our insurance coverage would be paid out of cash reserves, if any, which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. Even if our agreements with our manufacturers and suppliers entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Regional instability in Israel may adversely affect business conditions and may disrupt our business and negatively affect our revenues and results of operations.
We have research facilities located in Israel, and one of our key suppliers, which is both a component supplier and finished good manufacturer, manufactures its goods in one physical location in Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its Arab neighbors, as well as incidents of civil unrest. In recent years, these have included hostilities between Israel and Hezbollah in Lebanon and Hamas in the Gaza strip, both of which resulted in rockets being fired into Israel, causing casualties and disruption of economic activities. While we did not sustain damages from the conflicts with
26
Table of Contents
Hezbollah or Hamas, our Israeli operations, which are located in Haifa, in northern Israel, are within range of Hezbollah missiles and we or our immediate surroundings may sustain damages in a missile attack, which could adversely affect our operations. Recent political events, including political uprisings, social unrest and regime change, in various countries in the Middle East and North Africa have weakened the stability of those countries, which could result in extremists coming to power. Any future armed conflicts or political instability in the region could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. Parties with whom we do business have sometimes declined to travel to Israel during periods of heightened unrest or tension, forcing us to make alternative arrangements when necessary. In addition, the political and security situation in Israel may result in parties with whom we have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in the agreements.
Additionally, several countries, principally in the Middle East, restrict doing business with Israeli companies, and additional countries and groups may impose similar restrictions if hostilities in Israel or political instability in the region continue or increase. If recent regime changes and civil wars in neighboring states result in the establishment of fundamentalist Islamic regimes or governments more hostile to Israel, or if Egypt or Jordan abrogates its respective peace treaty with Israel, Israel could be subject to additional political, economic and military confines, which could result in a material adverse effect on our operations.
In addition, our business insurance only covers certain specified events associated with war or terrorism in the Middle East, and may not cover all such events. Additionally, although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, this government coverage may not be maintained, or may be insufficient to cover all losses we incur, even if available. Any losses or damages incurred by us could have a material adverse effect on our business.
If any of our facilities are damaged or our clinical, research and development or other business processes interrupted, our business could be seriously harmed.
We conduct our business in a limited number of facilities in the United States, Switzerland, Israel and Japan. Additionally, one of our key suppliers, which is both a component supplier and finished goods manufacturer, manufactures its goods in one physical location in Israel. Damage or extended periods of interruption to our or our suppliers’ or manufacturers’ corporate, development or research facilities due to fire, natural disaster, power loss, communications failure, unauthorized entry, terrorist attacks or other events could cause us to cease or delay development of some or all of our delivery systems. Our internal computer systems may fail or suffer security breaches, which could result in a material disruption of our business. Our business may be seriously harmed by such delays and interruption.
We have significant debt service obligations and may incur additional indebtedness in the future, which could adversely affect our financial condition and results of operations and our ability to react to changes in our business.
As of June 30, 2015, we had approximately $25.0 million of indebtedness outstanding under our Loan and Security Agreement dated as of January 7, 2015, between us, as borrower, and Biopharma Secured Investments III Holdings Cayman LP, as lender, or the Term Loan Credit Facility, and availability to borrow an additional $75.0 million thereunder. We may incur additional indebtedness in the future, including draws under our Term Loan Credit Facility. We do not intend to use any of the proceeds of this offering to repay indebtedness, and we will continue to have significant debt service obligations following the closing of this offering. Our existing indebtedness and any additional indebtedness we may incur could require us to divert funds identified for other purposes for debt service and impair our liquidity position.
27
Table of Contents
The fact that a substantial portion of our cash flow from operations could be needed to make payments on our indebtedness could have important consequences, including the following:
| • | increasing our vulnerability to general adverse economic and industry conditions or increased interests rates; |
| • | reducing the availability of our cash flow for other purposes; |
| • | limiting our flexibility in planning for or reacting to changes in our business and the markets in which we operate, which would place us at a competitive disadvantage compared to our competitors that may have less debt; |
| • | limiting our ability to borrow additional funds for working capital, capital expenditures and other investments; and |
| • | failing to comply with the covenants in our debt agreements could result in all of our indebtedness becoming immediately due and payable. |
Our ability to obtain necessary funds through borrowing, as well as our ability to service our indebtedness, will depend on our ability to generate cash flow from operations. Our ability to generate cash is subject to general economic, financial, competitive, legislative, regulatory and other factors that are beyond our control. If our business does not generate sufficient cash flow from operations or if future borrowings are not available to us under our Term Loan Credit Facility or otherwise in amounts sufficient to enable us to fund our liquidity needs, our financial condition and results of operations may be adversely affected. Our inability to make scheduled payments on our debt obligations in the future would require us to refinance all or a portion of our indebtedness on or before maturity, sell assets or seek additional equity investment. We may not be able to take any of such actions on a timely basis, on terms satisfactory to us or at all.
Covenants in our debt agreements restrict our operational flexibility.
Our Term Loan Credit Facility contains usual and customary restrictive covenants relating to the operation of our business, including restrictions on our ability:
| • | to incur or guarantee additional indebtedness; |
| • | to incur or permit to exist certain liens; |
| • | to enter into certain sale and lease-back transactions; |
| • | to make certain investments, loans and advances; |
| • | to effect certain mergers, consolidations, asset sales and acquisitions; |
| • | to pay dividends on, or redeem or repurchase, capital stock, enter into transactions with affiliates or materially change our business; and |
| • | to repay or modify certain other agreements with respect to other material indebtedness or modify our organizational documents. |
In addition, our Term Loan Credit Facility has a minimum liquidity covenant, which is tested quarterly. We must also meet certain annual pro forma net sales requirements.
28
Table of Contents
Risks relating to regulation
Our delivery system candidates must undergo rigorous pre-clinical and clinical testing and we must obtain regulatory approvals, which could be costly and time-consuming and subject us to unanticipated delays or prevent us from marketing any delivery systems.
Our research and development activities, as well as the manufacturing and marketing of Optune and our delivery system candidates, are subject to regulation, including regulation for safety, efficacy and quality, by the FDA in the United States and comparable authorities in other countries. FDA regulations and the regulations of comparable foreign regulatory authorities are wide-ranging and govern, among other things:
| • | the conduct of pre-clinical and clinical studies; |
| • | product design, development, manufacturing and testing; |
| • | product labeling; |
| • | product storage and shipping; |
| • | premarket clearance, approval and conformity assessment procedures; |
| • | premarket clearance, approval and conformity assessment procedures for modifications introduced in marketed products; |
| • | post-approval market surveillance and monitoring; |
| • | reporting of adverse events or incidents and implementation of corrective actions, including product recalls; |
| • | pricing and reimbursement; |
| • | interactions with healthcare professionals; |
| • | advertising and promotion; and |
| • | product sales and distribution. |
Clinical testing can be costly and take many years, and the outcome is uncertain and susceptible to varying interpretations. Moreover, success in pre-clinical and early clinical trials does not ensure that large-scale trials will be successful or predict final results. Acceptable results in early trials may not be replicable in later trials. A number of companies in the oncology industry have suffered significant setbacks in advanced clinical trials, even after promising results in earlier trials. Negative or inconclusive results or adverse medical events during a clinical trial could cause it to be redone or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events and could cause a clinical trial to be suspended, redone or terminated. We cannot be certain if or when the FDA, a foreign regulatory agency or our notified body (a private organization designated in an EU member state to conduct conformity assessment procedures under the Medical Devices Directive) might request additional studies, under what conditions such studies might be requested, or what the size or length of any such studies might be. The clinical trials of our delivery system candidates may not be completed on schedule, the FDA, foreign regulatory agencies or our notified body may order us to stop or modify our research, or these agencies or our notified body may not ultimately approve or issue a CE Certificate of Conformity for any of our delivery system candidates for commercial sale. While we have received regulatory approval for Optune for treatment of adult patients with recurrent GBM in the United States, the FDA required us to initiate a post-approval study and we have met this requirement. The data collected from our clinical trials may not be sufficient to support regulatory approval in the United States, Japan and other countries or to obtain CE Certificate of Conformity in the European Union for our various future delivery system candidates. Even if we
29
Table of Contents
believe the data collected from our clinical trials are sufficient, the FDA, equivalent foreign regulatory bodies and notified bodies have substantial discretion in the assessment and approval or conformity assessment processes and may disagree with our interpretation of the data. Our failure to adequately demonstrate the safety and efficacy of any of our delivery system candidates would delay or prevent regulatory approval in the United States, Japan and other countries or the CE marking in the European Union of our delivery system candidates, which could prevent us from achieving profitability.
We currently market Optune in the United States, as well as certain EU member states, Switzerland and Japan. We intend to market our TTFields delivery systems in a number of additional international markets. Although certain of our delivery systems have been approved for commercialization in Australia, Switzerland and Israel and are CE marked in the European Union, in order to market our delivery systems in other foreign jurisdictions and for other indications, we must obtain separate regulatory approvals and CE Certificates of Conformity. The requirements governing the conduct of clinical trials and manufacturing and marketing of our delivery system candidates outside the United States vary widely from country to country. Foreign regulatory approvals and CE Certificates of Conformity may take longer to obtain than FDA approvals and can require, among other things, additional testing and different clinical trial designs. Foreign regulatory approval and CE marking processes include essentially all of the risks associated with the FDA approval processes. Some foreign agencies must also approve prices of the delivery systems. Approval of a product by the FDA does not ensure approval of the same product by the health authorities of other countries or CE marking of Optune in the European Union and vice versa. In addition, changes in regulatory policy in the United States or in foreign countries for the approval or CE marking of a medical device during the period of product development and regulatory agency review or notified body review of each submitted new application may cause delays or rejections. Upcoming changes in the EU rules governing the CE marking of medical devices may also have a potential impact on the CE marking of our delivery systems in the European Union. On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework for medical devices in the European Union. On October 22, 2013, the European Parliament voted on an amended draft of the Regulation. The proposed text is currently being discussed by the Council of the European Union, or the Council. If and when adopted, the proposed new legislation may prevent or delay the CE marking of our delivery systems under development or impact our ability to modify our currently CE marked delivery systems on a timely basis. On June 19, 2015, the Council came to a common position concerning the Council’s proposed amendments to the two draft regulations intended to replace the current Medical Devices Directive, the Active Implantable Medical Devices Directive and the In Vitro Diagnostic Medical Devices Directive. Negotiations among the Council, the European Parliament and the European Commission are now expected to start in the fourth quarter of 2015. Depending on the outcome of the negotiations, the regulation on medical devices and the regulation on in vitro diagnostic medical devices could be definitively adopted in mid-2016.
We may choose to, or may be required to, suspend, repeat or terminate our clinical trials if they are not conducted in accordance with regulatory requirements, the results are negative or inconclusive or the trials are not well designed.
Clinical trials must be conducted in accordance with the FDA’s cGCPs and the equivalent laws and regulations applicable in other jurisdictions in which the clinical trials are conducted. The clinical trials are subject to oversight by the FDA, foreign regulatory agencies, ethics committees and institutional review boards at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with delivery system candidates produced under the FDA’s Good Manufacturing Practices, or GMP, and in accordance with the applicable regulatory requirements in the other jurisdictions in which the clinical trials are conducted. The conduct of clinical trials may require large numbers of test patients. Patient enrollment is a function of many factors, including the size of the patient population for the target indication, the proximity of patients to clinical sites, the eligibility criteria for the trial, the existence of competing clinical trials and the
30
Table of Contents
availability of alternative or new treatments. Clinical trials may be suspended by the FDA or by a foreign regulatory agency at any time if the FDA or the foreign regulatory agency finds deficiencies in the conduct of these trials or it is believed that these trials expose patients to unacceptable health risks.
We, the FDA or foreign regulatory agencies might delay or terminate our clinical trials of a delivery system candidate for various reasons, including:
| • | the delivery system candidate may have unforeseen adverse side effects; |
| • | the time required to determine whether the delivery system candidate is effective may be longer than expected; |
| • | we may not agree with the FDA, a foreign regulatory authority or an ethics committee regarding the protocol for the conduct of a clinical trial; |
| • | fatalities may occur during a clinical trial due to medical problems that may or may not be related to clinical trial treatments; |
| • | the delivery system candidate may not appear to be more effective than current therapies; |
| • | there may be insufficient patient enrollment in the clinical trials; or |
| • | we may not be able to produce sufficient quantities of the delivery system candidate to complete the trials. |
Furthermore, the process of obtaining and maintaining regulatory approvals in the United States and other foreign jurisdictions and CE Certificates of Conformity in the European Union for new therapeutic products is lengthy, expensive and uncertain. It can vary substantially, based on the type, complexity and novelty of the product involved. Accordingly, any of our delivery system candidates could take a significantly longer time than we expect to, or may never, gain regulatory approval or obtain CE Certificates of Conformity in the European Union, which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
Healthcare reform and other legislative and regulatory changes in the United States and in other countries may adversely affect our business and financial results.
In response to perceived increases in healthcare costs in recent years, there have been and continue to be proposals by the U.S. federal government, state governments, regulators and third-party payers to control these costs and, more generally, to reform the United States healthcare system. In the United States, the Patient Protection and Affordable Care Act, or the PPACA, was enacted in 2010 with a goal of reducing the cost of healthcare and substantially changing the way healthcare is financed by both government and private insurers.
In addition, other legislative changes have been proposed and adopted in the United States since the PPACA was enacted. On August 2, 2011, the Budget Control Act of 2011 created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This included aggregate reductions of Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013. On January 2, 2013, the American Taxpayer Relief Act, or the ATRA, was signed into law, which, among other things, further reduced Medicare payments to several providers, including hospitals.
The U.S. Congress could pass additional healthcare laws in the future, including those that affect coverage and reimbursement for healthcare items and services, including our delivery systems. The Centers for Medicare and Medicaid Services, or CMS, also could implement regulatory changes that could affect coverage and
31
Table of Contents
reimbursement for our delivery systems as well. In addition, various healthcare reform proposals have also emerged at the state level. We cannot predict to what extent future healthcare initiatives will be implemented at the federal or state level or the effect any future legislation or regulation will have on us.
We continue to evaluate the impact that PPACA will have on our business. The taxes imposed by the new federal legislation and the expansion in government’s role in the U.S. healthcare industry may result in decreased revenues, lower reimbursements by payers for our delivery systems and reduced medical procedure volumes, all of which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
The competent authorities in the EU member states are increasingly active in their goal of reducing public spending on healthcare. We cannot, therefore, guarantee that the treatment of patients with Optune would be reimbursed in any of the EU member states or, if successfully included on reimbursement lists will remain thereon. If adopted, the recent proposals of the European Commission for new rules governing medical devices in the European Union could impose additional requirements on manufacturers of medical devices placed on the market in the European Union. Failure to comply with these new requirements may affect our ability to market our delivery systems in the European Union.
We are subject to extensive regulation by the FDA and equivalent foreign authorities, which could restrict the sales and marketing of Optune and could cause us to incur significant costs. In addition, we may become subject to additional foreign regulation as we increase our efforts to sell Optune outside of the United States.
We sell Optune, and expect to sell our delivery system candidates, subject to extensive regulation by the FDA and numerous other federal, state and foreign governmental authorities. These regulations are broad and relate to, among other things, the conduct of pre-clinical and clinical studies, product design, development, manufacturing, labeling, testing, product storage and shipping, premarket clearance and approval, conformity assessment procedures, premarket clearance and approval of modifications introduced in marketed products, post-market surveillance and monitoring, reporting of adverse events and incidents, pricing and reimbursement, interactions with healthcare professionals, advertising and promotion and product sales and distribution. Although we have received FDA approval to market Optune in the United States for the treatment of adult patients with recurrent GBM, we will require additional FDA approval to market Optune for newly diagnosed GBM and other indications. We may be required to obtain approval of a new PMA or PMA supplement application for modifications made to Optune. This approval process is costly and uncertain, and it could take one to three years, or longer, from the time the application is filed with the FDA. We may make modifications in the future that we believe do not or will not require additional approvals. If the FDA disagrees, and requires new PMAs or PMA supplements for the modifications, we may be required to recall and to stop marketing the modified versions of Optune.
In addition, before our delivery systems can be marketed in the European Union, they must obtain a CE Certificate of Conformity from a notified body. New therapeutic uses of CE marked medical devices falling outside the scope of the current CE Certificate of Conformity require a completely new conformity assessment before the device can be CE marked and marketed in the European Union for the new intended purpose.
These processes can be expensive and lengthy and entail significant fees. The process preceding CE marking of a medical device in the European Union could also be expensive and lengthy and its outcome would be uncertain. We may make modifications in the future that we believe do not or will not require additional approvals or the notification of our notified body and potentially additional conformity assessment to permit maintenance of current CE Certificate of Conformity. If the competent authorities of the EU member states or our notified body disagree and require the conduct of a new conformity assessment procedure and the modification of the existing CE Certificate of Conformity or the issuance of a new certificate, we may be required to recall or suspend the marketing of the modified versions of Optune.
32
Table of Contents
In the United States and other jurisdictions, we also are subject to numerous post-marketing regulatory requirements, which include quality system regulations related to the manufacturing of our delivery systems, labeling regulations and medical device reporting regulations, which require us to report to the FDA or other foreign regulatory authorities and notified bodies if our delivery systems cause or contribute to a death or serious injury, or malfunction in a way that would likely cause or contribute to a death or serious injury. In addition, these regulatory requirements may change in the future in a way that adversely affects us. If we fail to comply with present or future regulatory requirements that are applicable to us, we may be subject to enforcement action by the FDA or other foreign regulatory authorities and notified bodies, which may include any of the following sanctions:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures to address or defend such actions; |
| • | patient notification, or orders for repair, replacement or refunds; |
| • | voluntary or mandatory recall or seizure of our current or future delivery systems; |
| • | administrative detention by the FDA of medical devices believed to be adulterated or misbranded; |
| • | operating restrictions, suspension or shutdown of production; |
| • | refusal or delay of our requests for PMA approval for new intended uses or modifications to Optune; |
| • | refusal or delay of our requests for PMA approval of new delivery systems; |
| • | refusal or delay in obtaining CE Certificates of Conformity for new intended uses or modifications to Optune; |
| • | suspension, variation or withdrawal of the CE Certificates of Conformity granted by our notified body in the EU member states; |
| • | operating restrictions; |
| • | suspension or withdrawal of PMA approvals that have already been granted; |
| • | refusal to grant export approval for Optune or any delivery system candidates; or |
| • | criminal prosecution. |
The occurrence of any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
Modifications to Optune or any of our future approved delivery systems may require approvals of new PMA or PMA supplement applications, modified or new CE Certificates of Conformity or even require us to cease promoting or to recall the modified versions of Optune until such clearances, approvals or modified or new CE Certificates of Conformity are obtained, and the FDA, foreign regulatory authorities or our notified body may not agree with our conclusions regarding whether new approvals are required.
Any modification to a device approved through the PMA pathway that impacts the safety or effectiveness of the device requires submission to the FDA and FDA approval of a PMA supplement application or even a new PMA application, as the case may be. The FDA requires a company to make the determination as to whether a new PMA or PMA supplement application is to be filed, but the FDA may review the company’s decision. For example, in the past, we have made initial determinations that certain modifications did not require the filing of a new PMA or PMA supplement application and have notified the FDA of these changes in our PMA Annual Report, but after its review of our PMA Annual Report, the FDA requested that we submit these modifications to
33
Table of Contents
the FDA as a PMA supplement application. Currently, we intend to submit a PMA supplement application for our next-generation Optune delivery system, which may require clinical data, and from time to time make other changes to the software and packaging and may submit additional PMA supplement applications for these changes. We can provide no assurance that we will receive FDA approval for these changes on a timely basis, or at all. We also may make additional changes in the future that we may determine do not require the filing of a new PMA or PMA supplement application. The FDA may not agree with our decisions regarding whether the filing of new PMAs or PMA supplement applications are required.
In addition, any substantial change introduced to a medical device CE marked in the European Union or to the quality system review by our notified body requires a new conformity assessment of the device and can lead to changes to the CE Certificates of Conformity or the preparation of a new CE Certificate of Conformity. Substantial changes include, among others, the introduction of a new intended purpose of the device, a change in its design or a change in the company’s suppliers. Responsibility for determination that a modification constitutes a substantial change lies with the manufacturer of the medical device. We must inform the notified body that conducted the conformity assessment of the delivery systems we market or sell in the European Union of any planned substantial changes to our quality system or changes to our devices which could affect compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive or the devices’ intended purpose. The notified body will then assess the changes and verify whether they affect the delivery system’s conformity with the Essential Requirements laid down in Annex I to the Medical Devices Directive or the conditions for the use of the device. If the assessment is favorable, the notified body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive. There is a risk that the competent authorities of the EU member states or our notified body may disagree with our assessment of the changes introduced to our delivery systems. The competent authorities of the EU member states or our notified body also may come to a different conclusion than the FDA on any given product modification.
If the FDA disagrees with us and requires us to submit a new PMA or PMA supplement application for then-existing modifications and/or the competent authorities of the EU member states or our notified body disagree with our assessment of the change introduced in a product, we may be required to cease promoting or to recall the modified product until we obtain approval and/or until a new conformity assessment has been conducted in relation to the product, as applicable. In addition, we could be subject to significant regulatory fines or other penalties. Furthermore, our delivery systems could be subject to recall if the FDA or the competent authorities of the EU member states or our notified body determine, for any reason, that our delivery systems are not safe or effective or that appropriate regulatory submissions were not made. Any recall or requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associated with modification of a product, loss of revenues and potential operating restrictions imposed by the FDA or the competent authorities of the EU member states or our notified body. Delays in receipt or failure to receive approvals, the loss of previously received approvals, the failure to conduct appropriate conformity assessments prior to CE marking of our delivery systems, or the failure to comply with any other existing or future regulatory requirements, could reduce our sales, profitability and future growth prospects.
34
Table of Contents
We will spend considerable time and money complying with federal, state and foreign regulations in addition to FDA regulations, and, if we are unable to fully comply with such regulations, we could face substantial penalties.
We are subject to extensive regulation by the U.S. federal government and the states and foreign countries in which we conduct our business. U.S. federal government healthcare laws apply when we submit a claim on behalf of a U.S. federal healthcare program beneficiary, or when a customer submits a claim for an item or service that is reimbursed under a U.S. federal government-funded healthcare program, such as Medicare or Medicaid. The laws that affect our ability to operate our business in addition to the Federal Food, Drug, and Cosmetic Act and FDA regulations include, but are not limited to, the following:
| • | the federal anti-kickback statute, which prohibits offering or providing remuneration of any kind for the purpose of inducing or rewarding referrals for items or services reimbursable by a federal healthcare program; |
| • | the U.S. federal False Claims Act, or the False Claims Act, which prohibits submitting false claims or causing the submission of false claims to the federal government; |
| • | Medicare laws and regulations that prescribe requirements for coverage and payment, including the conditions of participation for DME suppliers, and laws prohibiting false claims for reimbursement under Medicare and Medicaid; |
| • | healthcare fraud statutes that prohibit false statements and improper claims to any third-party payer; |
| • | the federal physician self-referral prohibition, commonly known as the Stark law, which prohibits physicians from referring Medicare patients to an entity for the provision of certain designated health services (including DME) if the physician (or a member of the physician’s immediate family) has an impermissible financial relationship with that entity; |
| • | similar state anti-kickback, false claims, insurance fraud and self-referral laws, which may not be limited to government-reimbursed items, as well as state laws that require us to maintain permits or licenses to distribute durable medical equipment; |
| • | state accreditation and licensing requirements applicable to DME providers; |
| • | the U.S. Foreign Corrupt Practices Act, which can be used to prosecute companies in the United States for arrangements with physicians or other parties outside the United States if the physician or party is a government official of another country and the arrangement violates the law of that country; |
| • | the Federal Trade Commission Act, the Lanham Act and similar federal and state laws regulating advertising and consumer protection; |
| • | the Physician Payments Sunshine Act, or the Sunshine Act, and similar state and foreign laws, which require reporting of payments and other transfers of value to health care practitioners periodically; and |
| • | the laws and codes of practices applicable in the EU member states, Switzerland and Japan concerning the marketing and promotion of medical devices, interactions with healthcare professionals, consumer protection, comparative advertising and unfair commercial practices, data protection, anti-corruption, bribery and reimbursement of medical devices. |
The laws and codes of practices applicable to us are subject to evolving interpretations. Moreover, certain federal and state laws regarding healthcare fraud and abuse and certain foreign laws regarding interactions with healthcare professionals are broad and we may be required to restrict certain of our practices to be in compliance with these laws. Similar law exists in the European Union, individual EU member states and other
35
Table of Contents
foreign countries. These laws are complemented by EU or national profession codes of practices. Healthcare fraud and abuse laws also are complex and even minor, inadvertent irregularities can potentially give rise to claims that a statute has been violated. If a governmental authority were to conclude that we are not in compliance with applicable laws and regulations, we and our officers and employees could be subject to severe criminal and civil penalties, including, for example, exclusion from participation as a supplier of delivery systems to beneficiaries covered by federal healthcare programs. For example, most states require us to maintain a license as a DME provider. The Medicare program requires that we maintain accreditation with an independent quality body. Loss of this accreditation would result in loss of our billing privileges to Medicare.
Any violation of these laws or equivalent foreign laws and codes of practices regarding interactions with healthcare professionals and bribery could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. Similarly, if there is a change in law, regulation or administrative or judicial interpretations, we may have to change our business practices or our existing business practices could be challenged as unlawful, which likewise could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline. In addition, although we believe that we have the required licenses, permits and accreditation to dispense our delivery systems, a regulator could find that we need to obtain additional licenses or permits. We also may be subject to audits, mandatory reaccreditation and other requirements in order to maintain our billing privileges. Failure to satisfy those requirements or successfully address any issues identified in an audit could cause us to lose our privileges to bill public and private payers. If we are required to obtain permits or licenses that we do not already possess, we also may become subject to substantial additional regulation or incur significant expense. Any penalties, damages, fines, curtailment or restructuring of our operations would adversely affect our ability to operate our business, our prospects and our financial results. In addition, any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation.
If we, our contract manufacturers or our component suppliers fail to comply with the FDA’s quality system regulations or equivalent regulations established in foreign countries, the manufacturing and distribution of our delivery systems could be interrupted, and our delivery system sales and results of operations could suffer.
We, our contract manufacturers and our component suppliers are required to comply with the FDA’s quality system regulations and the equivalent quality system requirements imposed by the laws and regulations in other jurisdictions, which are a complex regulatory framework that covers the procedures and documentation of the design, testing, production, control, quality assurance, labeling, packaging, sterilization, storage and shipping of our delivery systems. All aspects of our supply chain are subject to periodic inspections and audits by the FDA, notified bodies and other regulatory authorities to ensure continuing compliance. We and the two critical finished goods manufacturers listed in our PMA were inspected by the FDA in the first half of 2012 and again in the fall of 2013. No material inspectional observations were identified and no FDA Form 483s were issued following these inspections. We cannot assure you that our facilities or our contract manufacturers’ or component suppliers’ facilities would pass any future quality system inspection. If our or any of our contract manufacturers’ or component suppliers’ facilities fails a quality system inspection, the manufacturing or distribution of our delivery systems could be interrupted and our operations disrupted. Failure to take adequate and timely corrective action in response to an adverse quality system inspection could force a suspension or shutdown of our packaging and labeling operations or the manufacturing operations of our contract manufacturers, and lead to suspension, variation or withdrawal of our regulatory approvals or a recall of our delivery systems. If any of these events occurs, we may not be able to provide our customers with TTFields delivery systems that they require on a timely basis, our reputation could be harmed and we could lose customers, any or all of which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
36
Table of Contents
Our delivery systems may in the future be subject to recalls that could harm our reputation, business and financial results.
The FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our delivery systems in the event of material deficiencies or defects in design or manufacture. Distributors and manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or one of our manufacturers could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. The FDA requires that certain classifications of recalls be reported to the FDA within ten working days after the recall is initiated. Requirements for the reporting of product recalls to the competent authorities are imposed in other jurisdictions in which our delivery systems are or would be marketed in the future. Companies are required to maintain certain records of recalls, even if they are not reportable to the FDA or to the competent authorities of other countries. In the future, we may initiate voluntary recalls involving our delivery systems that we determine do not require notification of the FDA or to other equivalent non-U.S. authorities. If the FDA or the equivalent non-U.S. authorities disagree with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, the FDA and the equivalent non-U.S. authorities could take enforcement action if we fail to report the recalls when they were conducted. Recalls of any of our delivery systems would divert managerial and financial resources and could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
If our delivery systems cause or contribute to a death or a serious injury, or malfunction in certain ways, we will be subject to medical device reporting regulations, which can result in voluntary corrective actions or agency enforcement actions.
Under the FDA medical device reporting regulations and the equivalent regulations applicable in foreign jurisdictions in which our delivery systems are or may be marketed in the future, medical device manufacturers are required to report to the FDA and to the equivalent non-U.S. authorities information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to death or serious injury if the malfunction of the device or one of our similar devices were to recur. If we fail to report these events to the FDA or to the equivalent foreign authorities within the required timeframes, or at all, the FDA or the equivalent foreign authorities could take enforcement action against us. Any such adverse event involving our delivery systems also could result in future voluntary corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business, and may harm our reputation and financial results.
We may be subject to fines, penalties or injunctions if we are determined to be promoting the use of our delivery systems for unapproved or off-label uses.
Medical devices may be marketed only for the indications for which they are approved. Our promotional materials and training materials must comply with FDA regulations and other applicable laws and regulations governing the promotion of our delivery systems in the United States and foreign jurisdictions. Currently, Optune is only approved for treatment of adult patients with recurrent GBM in the United States. In the European Union and Switzerland, we have CE marked the Optune delivery system for the treatment of newly diagnosed GBM (in
37
Table of Contents
combination with temozolomide), recurrent GBM, and advanced NSCLC (in combination with standard-of-care chemotherapy). Optune is also approved in Israel and Japan for the treatment of recurrent GBM and in Australia for the treatment of recurrent GBM and newly diagnosed GBM (in combination with temozolomide).
If the FDA or the competent authorities in other jurisdictions, including the EU member states, determine that our promotional materials or training constitutes promotion of an unapproved use, they could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance of an untitled letter, a warning letter, an injunction, seizure, civil fines and criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our promotional or training materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement. In that event, our reputation could be damaged and commercialization of Optune and future delivery systems would be impaired.
U.S. legislative or FDA regulatory reforms may make it more difficult and costly for us to obtain regulatory approval of our delivery system candidates and to manufacture, market and distribute our delivery systems after approval is obtained.
From time to time, legislation is drafted and introduced in the U.S. Congress that could significantly change the statutory provisions governing the regulatory approval, manufacture and marketing of regulated products or the reimbursement thereof. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our delivery systems. Any new regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of future delivery system candidates. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our delivery systems. It is impossible to predict whether legislative changes will be enacted or FDA regulations, guidance or interpretations changed, and what the impact of such changes, if any, may be. Any change in the laws or regulations that govern the clearance and approval processes relating to our current and future delivery systems could make it more difficult and costly to obtain clearance or approval for new delivery systems, or to produce, market and distribute Optune. Significant delays in receiving clearance or approval, or the failure to receive clearance or approval for our new delivery systems would have an adverse effect on our ability to expand our business in the United States.
As a DME supplier, if we are found to have violated laws protecting the confidentiality of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
There are a number of federal and state laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information, as well as data protection laws applicable in other jurisdictions, such as the EU member states. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under HIPAA. These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information and limiting most use and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose.
The collection and use of personal health data in the European Union is governed by the provisions of Directive 95/46/EC of the European Parliament and of the Council of October 24, 1995, on the protection of individuals with regard to the processing of personal data and on the free movement of such data, commonly known as the Data Protection Directive. The EU member states have adopted national laws and regulations transposing the EU Data Protection Directive into their national laws. The Data Protection Directive and related national laws impose a number of requirements, including an obligation to seek the consent of individuals to whom the personal data relates, the information that must be provided to the individuals, notification of data processing
38
Table of Contents
obligations to the competent national data protection authorities of EU member states and the security and confidentiality of the personal data. The Data Protection Directive also imposes strict rules on the transfer of personal data out of the European Union to the United States. Failure to comply with the requirements of the Data Protection Directive and the related national data protection laws of EU member states may result in fines and other administrative penalties.
We are affected by and subject to environmental laws and regulations that could be costly to comply with or that may result in costly liabilities.
We are affected by federal, state, foreign and local laws and regulations, including those that impose various environmental controls on the manufacturing, transportation, storage, use and disposal of batteries and hazardous chemicals and other materials used in, and hazardous waste produced by, the manufacturing of our delivery systems. We incur and expect to continue to incur costs to comply with these environmental laws and regulations. Additional or modified environmental laws and regulations, including those relating to the manufacture, transportation, storage, use and disposal of materials used to manufacture our delivery systems or restricting disposal or transportation of batteries, may be imposed that may result in higher costs.
In addition, we cannot predict the effect that additional or modified environmental laws and regulations may have on us, our third-party suppliers of equipment, batteries and our delivery systems or our customers. For example, we and our suppliers rely on an exemption from the European Directive 2011/65/EU relating to the restriction of the use of certain hazardous substances in electrical and electronic equipment relating to lead content in our transducer arrays. To the extent this exemption is revoked, it may have a material impact on our business and results of operations.
Regulations on the transportation of lithium ion batteries may affect our business.
The Air Line Pilots Association International has called on the U.S. government to prohibit shipments of lithium-ion batteries on cargo and passenger planes pending new regulations, in light of recent incidents involving a battery pack for an electric bicycle and more recently lithium ion batteries in a shipment of electronic cigarettes that may have been a contributing factor in a fire on a FedEx cargo plane. Rechargeable lithium-ion batteries are not as flammable and can be put out with fire extinguishers, but the National Transportation Safety Board has issued a series of recommendations calling for tighter regulation and testing of the batteries. In March 2014, the U.S. Department of Transportation and the Pipeline and Hazardous Materials Safety Administration issued new standards to strengthen safety conditions for the shipment of lithium-ion batteries and cells. The new rules enhance packaging and hazard communication requirements for lithium-ion batteries transported by air, adopt separate shipping descriptions for lithium-ion batteries, revise provisions for the transport of small and medium lithium-ion batteries packed with, or contained in, equipment, and harmonize the provisions for the transport of low production and prototype lithium cells and batteries with the International Civil Aviation Organization’s Technical Instructions and the International Maritime Dangerous Goods Code. In February 2015, the U.S. Postal Service revised its policies so that shipping carriers are not permitted to ship packages solely containing lithium-ion batteries internationally. Consequently, we use vendors other than the U.S. Postal Service to ship our lithium-ion batteries.
Additionally, lithium ion batteries are classified as Class 9—Miscellaneous Dangerous Goods by the International Air Transport Association, or IATA. Our batteries have passed the UN 3480 test for transport as cargo called out in the IATA guidelines and, as such, when they are properly packaged and labeled (with a class 9 sticker) they can be shipped by air transport as cargo. However, our batteries are not allowed on passenger aircraft according to the IATA standards. Consequently, we offer to ship batteries for patients who are traveling by air. If additional restrictions are put in place that limit our ability to ship our delivery systems by air freight or on water borne cargo, it could have an adverse effect on our supply chain, our inventory management procedures and processes and our ability to fill prescriptions and service patients in a timely manner, which could have a material adverse effect on our business, prospects, financial condition and results of operations.
39
Table of Contents
Risks relating to intellectual property
If we are unable to protect our proprietary technology, trade secrets or know-how, we may not be able to operate our business profitably. Similarly, if we fail to sustain, further build and enforce our intellectual property rights, competitors may be able to develop competing therapies.
Our success depends, in part, on our ability to obtain and maintain protection for our delivery systems and technologies under the patent laws or other intellectual property laws of the United States and other countries. The standards that the U.S. Patent and Trademark Office, or USPTO, and its foreign counterparts use to grant patents are not always applied predictably or uniformly and can change. Consequently, we cannot be certain as to whether pending patent applications will result in issued patents, and we cannot be certain as to the type and extent of patent claims that may be issued to us in the future. Any issued patents may not contain claims that will permit us to stop competitors from using similar technology.
Our existing and future patent portfolio also may be vulnerable to legal challenges. The standards that courts use to interpret patents are not always applied predictably or uniformly and can change, particularly as new technologies develop. On September 16, 2011, President Obama signed into law the Leahy-Smith America Invents Act, or AIA, a significant patent law reform. The AIA implements a first-inventor-to-file standard for patent approval, changes the legal standards for patentability and creates a post-grant review system. As a result of the uncertainties of patent law in general, and surrounding the interpretation of the AIA in particular, we cannot predict with certainty how much protection, if any, will be given to our patents if we attempt to enforce them and they are challenged in court. Any attempt to enforce our intellectual property rights may also be time-consuming and costly, may divert the attention of management from our business, may ultimately be unsuccessful or may result in a remedy that is not commercially valuable. Such attempts may also provoke third parties to assert claims against us or result in our intellectual property being narrowed in scope or declared to be invalid or unenforceable.
In addition, we rely on certain proprietary trade secrets, know-how and other confidential information. We have taken measures to protect our unpatented trade secrets, know-how and other confidential information, including the use of confidentiality and assignment of inventions agreements with our employees, consultants and certain contractors. It is possible, however, that these persons may breach or challenge the agreements, that our trade secrets may otherwise be misappropriated or that competitors may independently develop or otherwise discover our trade secrets. There is therefore no guarantee that we will be able to obtain, maintain and enforce the intellectual property rights that may be necessary to protect and grow our business and to provide us with a meaningful competitive advantage, and our failure to do so could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
The oncology industry is characterized by patent and other intellectual property litigation and disputes, and any litigation, dispute or claim against us may cause us to incur substantial costs, could place a significant strain on our financial resources, divert the attention of management from our business, harm our reputation and require us to remove certain delivery systems from the market.
Whether a product infringes a patent or violates other intellectual property rights involves complex legal and factual issues, the determination of which is often uncertain. Any intellectual property dispute, even a meritless or unsuccessful one, would be time consuming and expensive to defend and could result in the diversion of our management’s attention from our business and result in adverse publicity, the disruption of research and development and marketing efforts, injury to our reputation and loss of revenues. Any of these events could negatively affect our business, prospects, financial condition and results of operations.
40
Table of Contents
Third parties may assert that TTFields, Optune, our other delivery system candidates, the methods employed in the use of our delivery systems or other activities infringe on U.S. or foreign patents. Such claims may be made by competitors seeking to obtain a competitive advantage or by other parties, many of whom have significantly larger intellectual property portfolios than we have. Additionally, in recent years, individuals and groups have begun purchasing intellectual property assets for the purpose of making claims of infringement and attempting to extract settlements from companies like ours. The risk of infringement claims is exacerbated by the fact that there are numerous issued and pending patents relating to the treatment of cancer. Because patent applications can take many years to issue, and in many cases remain unpublished for many months after filing, there may be applications now pending of which we are unaware that may later result in issued patents that our delivery systems may infringe. There could also be existing patents that one or more components of our delivery systems may inadvertently infringe. As the number of competitors in the market for the treatment of cancer grows, the possibility of inadvertent patent infringement by us or a patent infringement claim against us increases. To the extent we gain greater market visibility, our risk of being subject to such claims is also likely to increase.
If a third party’s patent was upheld as valid and enforceable and we were found to be infringing, we could be prevented from making, using, selling, offering to sell or importing Optune or other delivery system candidates, unless we were able to obtain a license under that patent or to redesign our systems to avoid infringement. A license may not be available at all or on terms acceptable to us, and we may not be able to redesign our delivery systems to avoid any infringement. Modification of our delivery systems or development of delivery system candidates to avoid infringement could require us to conduct additional clinical trials and to revise our filings with the FDA and other regulatory bodies, which would be time-consuming and expensive. If we are not successful in obtaining a license or redesigning our delivery systems, we may be unable to make, use, sell, offer to sell or import our delivery systems and our business could suffer. We may also be required to pay substantial damages and undertake remedial activities, which could cause our business to suffer.
We may also be subject to claims alleging that we infringe or violate other intellectual property rights, such as copyrights or trademarks, may have to defend against allegations that we misappropriated trade secrets, and may face claims based on competing claims of ownership of our intellectual property. The confidentiality and assignment of inventions agreements that our employees, consultants and other third parties sign may not in all cases be enforceable or sufficient to protect our intellectual property rights. In addition, we may face claims from third parties based on competing claims to ownership of our intellectual property.
We also employ individuals who were previously employed at other medical device companies, and as such we may be subject to claims that such employees have inadvertently or otherwise used or disclosed the alleged trade secrets or other proprietary information of their former employers.
Any such litigation, dispute or claim could be costly to defend and could subject us to substantial damages, injunctions or other remedies, which could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
As described in “Our business—Intellectual property,” we entered into a settlement agreement in February 2015 with the Technion, whereby we agreed to resolve certain potential disputes among us, the Technion and Professor Yoram Palti arising out of certain intellectual property that Professor Palti developed while affiliated with the Technion and which Professor Palti has assigned to us. As part of the settlement, we have a contingent obligation to pay the Technion $5.5 million upon the earlier of our achieving $250.0 million of cumulative net sales since our inception, as defined in the Settlement Agreement, and any merger, consolidation, reorganization or sale or other disposition of all or substantially all of our assets.
41
Table of Contents
The patent rights on which we rely to protect the intellectual property underlying TTFields delivery systems may not be adequate, which could enable third parties to use our technology or market competing products, which would harm our continued ability to compete in the market.
Our success will depend in part on our continued ability to develop or acquire commercially valuable patent rights and to protect these rights adequately. The scope of some of our patents are limited to certain ranges. For example, some of our patents protect low-intensity (1-3 V/cm), intermediate frequency (100-300 kHz) alternating electric fields, but do not cover intensities and frequencies for electric fields that are outside of these ranges. While intensities and frequencies of electric fields outside of these ranges have not yet proven to be effective treatment modalities, that may not be the case in the future. Our patent position is generally uncertain and involves complex legal and factual questions. The risks and uncertainties that we face with respect to our patents and other related rights include the following:
| • | the pending patent applications we have filed may not result in issued patents or may take longer than we expect to result in issued patents; |
| • | the pending patent applications and patents we own may be subject to interference proceedings or similar disputes over the priority of the inventions claimed; |
| • | the claims of any patents that are issued may not provide meaningful protection; |
| • | we may not be able to develop additional proprietary technologies that are patentable; |
| • | changes in patent laws or their interpretation in the United States and other countries (including the recently enacted AIA) could diminish the value of our patents, narrow the scope of our patent protection or adversely affect our ability to obtain new patents; |
| • | obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements; |
| • | other parties may challenge patents, patent claims or patent applications licensed or issued to us, and such patents, patent claims or patent applications may be narrowed or found to be invalid or unenforceable; and |
| • | other companies may design around technologies we have patented or developed. |
We also may fail to apply for or be unable to obtain patent rights in some foreign countries. In addition, the legal systems of certain countries may not protect our rights to the same extent as the laws of the United States, which could affect our ability to enforce patent rights effectively in such foreign countries. For a variety of reasons, we may decide not to file for patent protection for certain of our intellectual property. Our patent rights underlying TTFields, Optune and our other delivery systems may not be adequate, and our competitors or customers may design around our proprietary technologies or independently develop similar or alternative technologies or products that are equal or superior to ours without infringing on any of our patent rights. In addition, the patents licensed or issued to us may not provide a competitive advantage, and may be insufficient to prevent others from commercializing products similar or identical to ours. The occurrence of any of these events could have a material adverse effect on our business, prospects, financial condition and results of operations and cause our stock price to decline.
We have limited foreign intellectual property rights outside of our key markets and may not be able to protect our intellectual property rights throughout the world.
We have limited intellectual property rights outside of our key markets. In some countries outside the United States, we do not have any intellectual property rights, and our intellectual property rights in other countries outside the United States have a different scope and strength compared to our intellectual property rights in
42
Table of Contents
the United States. Consequently, we will not be able to prevent third parties from practicing our inventions in all countries outside the United States. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but where enforcement rights are not as strong as those in the United States. These products may compete with our delivery systems, and our patents or other intellectual property rights may not be effective or adequate to prevent such competition.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our delivery systems.
As is the case with other medical device companies, our success is heavily dependent on our intellectual property rights, and particularly on our patent rights. Obtaining and enforcing patents in the medical device industry involves both technological and legal complexity, and is therefore costly, time consuming and inherently uncertain. In addition, the United States has recently enacted and is currently implementing wide-ranging patent reform legislation. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents once obtained. Depending on decisions by the U.S. Congress, the federal courts and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could further negatively impact the value of our patents, narrow the scope of available patent protection or weaken the rights of patent owners.
Risks relating to this offering
We are an emerging growth company, and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our ordinary shares less attractive to investors.
We are an emerging growth company, as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including the ability to include only two years of audited financial statements and only two years of related management’s discussion and analysis of financial condition and results of operations disclosure, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act or any Public Company Accounting Oversight Board requirements regarding mandatory audit firm rotation or supplemental disclosures regarding the audit, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our ordinary shares held by non-affiliates exceeds $700 million as of any June 30 before that time, in which case, we would become a large accelerated filer under SEC rules and would no longer be an emerging growth company as of the following December 31. We cannot predict if investors will find our ordinary shares less attractive because we may rely on these exemptions. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares and our share price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
43
Table of Contents
There has been no public market for our ordinary shares prior to this offering, and an active trading market for our ordinary shares may not develop after this offering. As a result, you may not be able to resell your ordinary shares at or above the price you paid, or at all.
Prior to this offering, there has been no public market for our ordinary shares and we cannot predict the extent of investor interest in us. The initial public offering price for our ordinary shares will be determined by negotiations between us and J.P. Morgan Securities LLC and may bear no relationship to the market price for our ordinary shares after the offering. Further, an active trading market for our ordinary shares may not develop or be maintained after this offering and the market price of our ordinary shares may decline below the initial public offering price. Consequently, you may lose part or all of your investment in our ordinary shares.
The market price for our ordinary shares may be volatile, which could result in substantial losses to you.
The market price for our ordinary shares may be volatile and subject to wide fluctuations in response to factors such as publication of clinical studies relating to Optune, our other delivery system candidates or a competitor’s product, actual or anticipated fluctuations in our quarterly results of operations, changes in financial estimates by securities research analysts, negative publicity, studies or reports, changes in the economic performance or market valuations of other companies that operate in our industry, changes in the availability of third-party reimbursement in the United States or other countries, changes in governmental regulations or in the status of our regulatory approvals or applications, announcements by us or our competitors of material acquisitions, strategic partnerships, joint ventures or capital commitments, intellectual property litigation, release of lock-up or other transfer restrictions on our outstanding ordinary shares, and economic or political conditions in the United States, Israel or elsewhere. In addition, the performance, and fluctuation in market prices, of other foreign companies that have listed their securities in the United States may affect the volatility in the price of and trading volumes of our ordinary shares. Volatility in global capital markets, as was experienced during the global financial crisis beginning in 2008 and during the recent European sovereign debt crisis, as well as volatility resulting from the recent economic slowdown in Asia, could also have an adverse effect on the market price of our ordinary shares. Furthermore, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price and liquidity of our ordinary shares.
Our ordinary shares are issued under the laws of Jersey, which may not provide the level of legal certainty and transparency afforded by incorporation in a U.S. state.
We are incorporated under the laws of Jersey, Channel Islands. Jersey legislation regarding companies is largely based on English corporate law principles. A further summary of applicable Jersey company law, including a comparison to Delaware corporate law, is contained in this prospectus under the caption “Description of share capital.” However, there can be no assurance that Jersey law will not change in the future or that it will serve to protect investors in a similar fashion afforded under corporate law principles in the United States, which could adversely affect the rights of investors.
U.S. shareholders may not be able to enforce civil liabilities against us.
We are a Jersey entity with most of our assets located outside of the United States. Although we have appointed an agent for service of process in the United States for purposes of U.S. federal securities laws, a number of our directors and executive officers and a number of directors of each of our subsidiaries are not residents of the United States, and all or a substantial portion of the assets of such persons are located outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them judgments obtained in U.S. courts predicated upon the civil liability provisions of the federal securities laws of the United States.
44
Table of Contents
We have been advised by our Jersey lawyers that the courts of Jersey would recognize any final and conclusive judgment under which a sum of money is payable (not being a sum payable in respect of taxes or other charges of a like nature or in respect of a fine or other penalty) obtained against us in the courts of any other territory in respect of certain enforceable obligations in accordance with the principles of private international law as applied by Jersey law (which are broadly similar to the principles accepted under English common law) and such judgment would be sufficient to form the basis of proceedings in the Jersey courts for a claim for liquidated damages in the amount of such judgment. In such proceedings, the Jersey courts would not re-hear the case on its merits save in accordance with such principles of private international law. Obligations may not necessarily be enforceable in Jersey in all circumstances or in accordance with their terms; and in particular, but without limitation: (a) any agreement purporting to provide for a payment to be made in the event of a breach of such agreement would not be enforceable to the extent that the Jersey courts were to construe such payment to be a penalty that was excessive, in that it unreasonably exceeds the maximum damages that an obligee could have suffered as a result of the breach of an obligation; (b) the Jersey courts may refuse to give effect to any provision in an agreement that would involve the enforcement of any foreign revenue or penal laws; and (c) the Jersey courts may refuse to allow unjust enrichment or to give effect to any provisions of an agreement (including provisions relating to contractual interest on a judgment debt) that it considers usurious.
Our annual and quarterly results may fluctuate due to a number of factors and, as a result, could fall below investor expectations or estimates by securities research analysts, which may cause the trading price of our ordinary shares to decline.
Our revenues and results of operations are difficult to predict, and potentially may vary significantly from period to period. As a result of a number of factors, many of which are beyond our control, it is possible that results of operations for future periods may be below the expectations of public market analysts and investors, which could cause our stock price to decline. Factors that may affect our quarterly results include, but are not limited to:
| • | failure to obtain regulatory approval for our delivery systems; |
| • | failure to effectively commercialize our delivery systems; |
| • | competition; and |
| • | changes in the laws and regulations that affect our operations. |
As a result, investors should not rely on year-to-year or quarter-to-quarter comparisons of results of operations as an indication of future performance.
Substantial future sales of our ordinary shares in the public market, or the perception that such sales may occur, could cause the price of our ordinary shares to decline.
Sales of our ordinary shares in the public market after this offering, or the perception that these sales may occur, could cause the market price of our ordinary shares to decline. All ordinary shares sold in this offering (other than any shares acquired by our affiliates) will be freely transferable without restriction or additional registration under the Securities Act of 1933, as amended, or the Securities Act. Substantially all of the remaining ordinary shares outstanding after this offering will be available for sale upon the expiration of the 180-day lock-up period, subject to volume, notice and manner of sale restrictions as applicable to our affiliates in the United States under Rule 144 and Rule 701 under the Securities Act. See “Shares eligible for future sale” and “Underwriting” for a detailed description of the lock-up and Securities Act restrictions. Any or all of these ordinary shares may be released prior to expiration of the lock-up period at the discretion of J.P. Morgan Securities LLC. To the extent ordinary shares are released before the expiration of the lock-up period and these ordinary shares are sold into the market, the market price of our ordinary shares could decline.
45
Table of Contents
Since the initial public offering price is substantially higher than our net tangible book value per share, you will incur immediate and substantial dilution.
If you purchase our ordinary shares in this offering, you will pay more for your ordinary shares than the average amount paid by our existing shareholders for their ordinary shares on a per share basis. As a result, you will experience immediate and substantial dilution of approximately $19.82 per share (assuming no exercise by the underwriters of the over-allotment option), representing the difference between our net tangible book value per share as of June 30, 2015 after giving effect to this offering at an assumed initial public offering price of $23.50 per share, the midpoint of the estimated initial public offering price range set forth on the cover of this prospectus, and after deducting the underwriting discounts, commissions and estimated offering expenses payable by us. In addition, you may experience further dilution to the extent that our ordinary shares are issued upon exercise of future equity awards under our 2015 Omnibus Incentive Plan or upon the exercise of currently outstanding options or warrants. See “Dilution” for a more complete description of how the value of your investment in our ordinary shares will be diluted upon the consummation of this offering and may be diluted in the future.
Our executive officers, directors and principal shareholders may exert control over us and may be able to exercise influence over matters subject to shareholder approval.
Our executive officers and directors, together with their respective affiliates, beneficially owned approximately 36.7% of our outstanding ordinary shares as of September 21, 2015, and upon consummation of this offering, that same group will beneficially own approximately 33.6% of our outstanding ordinary shares, assuming no exercise of the underwriters’ over-allotment option. Accordingly, these shareholders, if they act together, will be able to exercise substantial influence over all matters requiring shareholder approval, including the election of directors and approval of corporate transactions, such as a merger. This concentration of ownership could have the effect of delaying or preventing a change in our control or otherwise discouraging a potential acquirer from attempting to obtain control of us, which in turn could have a material adverse effect on the market value of our ordinary shares. For information regarding the ownership of our outstanding ordinary shares by our executive officers and directors and their affiliates, please see the section entitled “Principal shareholders.”
Our memorandum and articles of association will contain anti-takeover provisions that could adversely affect the rights of holders of our ordinary shares.
We expect to amend and restate our current memorandum and articles of association effective upon consummation of this offering. Our amended and restated memorandum and articles of association, referred to as the memorandum and articles of association, will contain certain provisions that could limit the ability of third parties to acquire control of our company, including a provision for a classified board of directors and a provision that grants authority to our board of directors to issue from time to time one or more classes of preferred shares without action by our shareholders and to determine, with respect to any class of preferred shares, the terms and rights of that class. See “Description of share capital” for a description of our amended and restated memorandum and articles of association. The provisions could have the effect of depriving our shareholders of the opportunity to sell their ordinary shares at a premium over the prevailing market price by discouraging third parties from seeking to obtain control of our company in a tender offer or similar transactions.
Our management will have broad discretion over the use and investment of the net proceeds we receive in this offering and might not apply the proceeds in ways that increase the value of your investment.
Our management will have broad discretion over the use and investment of the net proceeds we receive from this offering, and you will be relying on, and may not agree with, the judgment of our management regarding the application of these net proceeds. Our management intends to use the net proceeds we receive from this offering for working capital and general corporate purposes, including clinical trials, research and development
46
Table of Contents
and continued commercialization of Optune and future delivery systems. In addition, we are required to make a $1.0 million payment to the Technion with the net proceeds we receive from this offering. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business and cause the price of our ordinary shares to decline. Pending these uses, we may invest the net proceeds we receive from this offering in a manner that does not produce income or loses value. See “Use of proceeds.”
If securities or industry analysts do not publish research or publish unfavorable or inaccurate research about our business, our share price and trading volume could decline.
The trading market for our ordinary shares will continue to depend, in part, on the research and reports that securities or industry analysts publish about us or our business. We may be unable to sustain coverage by well regarded securities and industry analysts. If either none or only a limited number of securities or industry analysts maintain coverage of our company, or if these securities or industry analysts are not widely respected within the general investment community, the trading price for our ordinary shares would be negatively impacted. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who cover us downgrade our ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ordinary shares would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our ordinary shares could decrease, which might cause the price of our ordinary shares and trading volume to decline.
We will incur significant increased costs on an ongoing basis as a result of operating as a company whose ordinary shares are publicly traded in the United States, and our management will be required to devote substantial time to new compliance initiatives, including after we are no longer an emerging growth company.
As a company whose shares are publicly traded in the United States, we will incur significant legal, accounting and other expenses on an ongoing basis that we did not incur prior to this offering. In addition, the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act, and the rules of the SEC and The NASDAQ Stock Market LLC, or NASDAQ, have imposed various requirements on public companies, including requirements for the establishment and maintenance of effective disclosure controls and internal control over financial reporting. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
When the emerging growth company exemptions under the JOBS Act cease to apply, we expect to incur additional expenses and devote increased management effort toward ensuring compliance with reporting requirements. We cannot predict or estimate the amount of additional costs we may incur as a result of losing our emerging growth company status or the timing of such costs.
If we fail to maintain proper and effective internal controls, our ability to produce accurate and timely financial statements could be impaired and investors’ views of us could be harmed.
As a public company, we will be required to maintain internal control over financial reporting and to report any material weaknesses in such internal controls. In addition, beginning with our annual report on Form 10-K for our fiscal year ending December 31, 2016 to be filed in 2017, we will be required to furnish a report by management on the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We are in the process of designing, implementing and testing the internal control over financial reporting required to comply with this obligation, which process is time-consuming, costly and complicated. In addition, our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting beginning with our annual report on Form 10-K following the date on which we are no longer an emerging growth company, which may be up to five full years following the date of this offering. Our
47
Table of Contents
compliance with Section 404 of the Sarbanes-Oxley Act will require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit function, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. If we are not able to comply with the requirements of Section 404 in a timely manner, or if we or our independent registered public accounting firm identify deficiencies in our internal control over financial reporting that are deemed to be material weaknesses, the market price of our ordinary shares could decline and we could be subject to sanctions or investigations by NASDAQ, the SEC or other regulatory authorities, which would require additional financial and management resources.
Our ability to implement our business plan successfully and comply with Section 404 requires us to be able to prepare timely and accurate financial statements. We expect that we will need to continue to improve existing, and implement new, operational and financial systems, procedures and controls to manage our business effectively. Any delay in the implementation of, or disruption in the transition to, new or enhanced systems, procedures or controls, may cause our operations to suffer and we may be unable to conclude that our internal control over financial reporting is effective and to obtain an unqualified report on internal controls from our auditors when required under Section 404 of the Sarbanes-Oxley Act. Moreover, we cannot be certain that these measures would ensure that we implement and maintain adequate controls over our financial processes and reporting in the future. Even if we were to conclude, and, when required, our auditors were to concur, that our internal control over financial reporting provided reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles, because of its inherent limitations, internal control over financial reporting may not prevent or detect fraud or misstatements or omissions.
We have never declared or paid cash dividends on our ordinary shares and we do not anticipate paying dividends in the foreseeable future and, as a result, you must rely on price appreciation of our ordinary shares for a return on your investment.
We have never declared or paid cash dividends on our ordinary shares. We currently intend to retain our funds and any future earnings to support the operation, growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our board of directors and subject to compliance with applicable laws and covenants under our current or any future credit facilities, which may restrict or limit our ability to pay dividends, and the form, frequency and amount of dividends will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that our board of directors may deem relevant. See “Dividend policy” for more information. We do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. As a result, a return on your investment will only occur if our share price appreciates. There is no guarantee that our ordinary shares will appreciate in value after this offering or even maintain the price at which you purchased your ordinary shares. You may not realize a return on your investment in our ordinary shares and you may even lose your entire investment in our ordinary shares.
48
Table of Contents
Cautionary note regarding forward-looking statements
This prospectus contains forward-looking statements that reflect our current views with respect to, among other things, future events and financial performance. You can identify these forward-looking statements by the use of forward-looking words such as “outlook,” “believes,” “expects,” “potential,” “continues,” “may,” “will,” “should,” “seeks,” “approximately,” “predicts,” “intends,” “plans,” “estimates,” “anticipates” or the negative version of those words or other comparable words. The forward-looking statements contained in this prospectus are based on our current plans, expectations, hopes, beliefs, intentions or strategies concerning future developments and their potential effects on us. Forward-looking statements in this prospectus may include, for example, statements about:
| • | our research and development, clinical trial and commercialization activities and projected expenditures; |
| • | the further commercialization of Optune and our delivery system candidates; |
| • | our business strategies and the expansion of our sales and marketing efforts in the United States and in other countries; |
| • | the market acceptance of Optune and our other delivery systems by patients, physicians, third-party payers and others in the healthcare and scientific community; |
| • | our plans to pursue the use of TTFields delivery systems for the treatment of other solid tumor cancers; |
| • | our estimates regarding revenues, expenses, capital requirements and needs for additional financing; |
| • | our ability to obtain regulatory approvals for additional indications and any future delivery systems; |
| • | our ability to acquire the supplies needed to manufacture our delivery systems from third-party suppliers; |
| • | our ability to manufacture adequate supply; |
| • | our ability to secure adequate coverage from third-party payers to reimburse us for our delivery systems; |
| • | our ability to maintain and develop our intellectual property position; |
| • | our use of proceeds from this offering; |
| • | our cash needs; and |
| • | our prospects, financial condition and results of operations. |
These forward-looking statements involve a number of risks and uncertainties (some of which are beyond our control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking statements. Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect, actual results may vary in material respects from those projected in these forward-looking statements. Some of these factors are described in this prospectus under the headings “Summary,” “Risk factors,” “Management’s discussion and analysis of financial condition and results of operations” and “Our business.” There can be no assurance that future developments affecting us will be those that we have anticipated. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable securities laws.
49
Table of Contents
We estimate that we will receive net proceeds from this offering of approximately $161.0 million, or $185.5 million if the underwriters exercise their over-allotment option to purchase additional ordinary shares in full, after deducting the underwriting discounts, commissions and estimated offering expenses payable by us. These estimates are based upon an initial offering price of $23.50 per share, the midpoint of the range of the initial public offering price shown on the cover page of this prospectus. Assuming the number of ordinary shares offered by us set forth on the cover page of this prospectus remains the same, and after deduction of underwriting discounts and estimated offering expenses payable by us, a $1.00 increase (decrease) in the assumed initial public offering price of $23.50 per share would increase (decrease) the net proceeds of this offering by approximately $7.0 million.
We plan to use the net proceeds we receive from this offering for working capital and general corporate purposes, including clinical trials and research and development and continued commercialization of Optune and our future delivery systems. In addition, we are required to make a $1.0 million payment to the Technion with the net proceeds we receive from this offering. See “Our business—Intellectual property.” In particular, we expect to use the net proceeds as follows:
| • | $65.0 million for clinical trials; |
| • | $20.0 million for other research and development; |
| • | $55.0 million for commercialization efforts; |
| • | $1.0 million for our payment to the Technion; and |
| • | $20.0 million for working capital and other general corporate purposes. |
Our management will have significant flexibility and discretion to apply the net proceeds we receive from this offering. If an unforeseen event occurs or business conditions change, we may use our net proceeds differently than as described in this prospectus. See “Risk factors—Risks relating to this offering—Our management will have broad discretion over the use and investment of the net proceeds we receive in this offering and might not apply the proceeds in ways that increase the value of your investment.” We have not identified any alternative use of proceeds other than those set forth above.
Until we use the net proceeds we receive from this offering for the above purposes, we intend to invest the funds in short-term, investment-grade, interest-bearing securities. We cannot predict whether these investments will yield a favorable return.
50
Table of Contents
We have never declared nor paid any cash dividends on our ordinary shares, nor do we have any present plan to pay any dividends on our ordinary shares in the foreseeable future. We currently intend to retain our funds and any future earnings to operate and expand our business.
Our board of directors has complete discretion as to whether we will distribute dividends in the future, subject to restrictions under Jersey law and our current and any future credit facilities. Any payment of dividends would be subject to the Companies (Jersey) Law 1991, as amended, which requires that all dividends be approved by our board of directors. In order to be able to declare any dividends our directors must make a statutory solvency statement to the effect that we will be able to discharge our liabilities as they fall due and that, having regard to our prospects and to the intention of the directors with respect of the management of our business and the amount and character of the financial resources that will in the view of the directors be available to us, we will be able to continue to carry on business; and discharge our liabilities as they fall due for a 12-month period immediately following the date on which the dividend is proposed to be paid (or until we are dissolved on a solvent basis, if earlier). See “Description of share capital.” If our board of directors decides to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial condition, contractual restrictions and other factors that the board of directors may deem relevant. The Term Loan Credit Facility restricts our ability to pay dividends.
51
Table of Contents
The following table sets forth our cash and cash equivalents, including short-term investments, and our capitalization as of June 30, 2015:
| • | on an actual basis; |
| • | on a pro forma basis to give effect to the conversion into ordinary shares of all of our outstanding preferred shares in connection with the consummation of this offering (see Note 13a to the consolidated financial statements) after giving effect to a 5.913-for-1 stock split effected on September 16, 2015; and |
| • | on a pro forma as adjusted basis to reflect the issuance and sale of the 7,500,000 ordinary shares in this offering (assuming no exercise by the underwriters of the over-allotment option), assuming an initial public offering price of $23.50 per share, the midpoint of the estimated range of the initial public offering price, after deducting the underwriting discounts, commissions, estimated offering expenses payable by us as described under “Use of proceeds” and a $1.0 million payment to the Technion. |
You should read this capitalization table together with “Use of proceeds,” “Selected consolidated financial data,” “Management’s discussion and analysis of financial condition and results of operations” and our consolidated financial statements and the related notes appearing elsewhere in this prospectus.
| As of June 30, 2015 | ||||||||||||
| (in thousands, other than share and per share numbers) | Actual | Pro forma | Pro forma as adjusted |
|||||||||
| Cash and cash equivalents, including short-term investments |
$ | 163,504 | $ | 163,504 | $ | 323,751 | ||||||
| Long-term loan, net of discount |
24,539 | 24,539 | 24,539 | |||||||||
| Share capital - |
||||||||||||
| Ordinary shares—unlimited no par value shares authorized; 12,430,419 shares issued and outstanding actual; 75,174,936 shares, issued and outstanding pro forma; 82,674,936 shares issued and outstanding pro forma as adjusted |
— | — | — | |||||||||
| Preferred shares—unlimited no par value shares authorized; 62,744,517 shares issued and outstanding actual; no shares issued and outstanding pro forma; no shares issued and outstanding pro forma as adjusted |
— | — | — | |||||||||
| Additional paid-in capital |
473,437 | 473,437 | 634,390 | |||||||||
| Accumulated deficit |
(329,130 | ) | (329,130 | ) | (329,176 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Total shareholders’ equity |
144,307 | 144,307 | 305,214 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total capitalization |
$ | 329,753 | ||||||||||
|
|
$ | 168,846 | $ | 168,846 |
|
|
||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $23.50 per share would increase (decrease) the as adjusted amount of cash and cash equivalents, including short-term investments, by $7.0 million, total shareholders’ equity by $7.0 million and total capitalization by $7.0 million, assuming the number of ordinary shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts, commissions, estimated expenses payable by us and a $1.0 million payment to the Technion.
52
Table of Contents
If you invest in our ordinary shares, your interest will be diluted immediately for each ordinary share to the extent of the difference between the initial public offering price per share you will pay in this offering and the pro forma as adjusted net tangible book value per share immediately after this offering. Dilution results from the fact that the initial public offering price per share is substantially in excess of the book value per share attributable to the existing shareholders for our presently outstanding ordinary shares.
Our pro forma net tangible book value as of June 30, 2015 was approximately $143.1 million, or $1.90 per share, assuming the conversion of all of our outstanding preferred shares into ordinary shares in connection with the consummation of this offering after giving effect to a 5.913-for-1 stock split effected on September 16, 2015. Pro forma net tangible book value represents the amount of our total consolidated assets, less the amount of our total consolidated liabilities and deferred IPO costs and discount on long-term loan. Dilution is determined by subtracting pro forma net tangible book value per share from the assumed initial public offering price per share, which is based on the midpoint of the estimated initial public offering price range set forth on the cover page of this prospectus, and after deducting the underwriting discounts, commissions and estimated offering expenses payable by us.
Without taking into account any other changes in pro forma net tangible book value after June 30, 2015, other than to give effect to our sale of ordinary shares offered in this offering based on the assumed initial public offering price of $23.50 per share after deducting the underwriting discounts, commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value would have been $304.8 million, or $3.68 per outstanding ordinary share (assuming no exercise by the underwriters of the over-allotment option). This represents an immediate increase in net tangible book value of $1.78 per share to the existing shareholders, and an immediate dilution in net tangible book value of $19.82 per share to investors purchasing ordinary shares in this offering. The following table illustrates such dilution:
| Assumed initial public offering price per share |
$ | 23.50 | ||||||
| Pro forma net tangible book value per share as of June 30, 2015 |
$ | 1.90 | ||||||
| Increase per share attributable to sale of ordinary shares in this offering |
1.78 | |||||||
|
|
|
|||||||
| Pro forma as adjusted net tangible book value per share after giving effect to this offering |
3.68 | |||||||
|
|
|
|||||||
| Amount of dilution in net tangible book value per share to new investors in the offering |
$ | 19.82 | ||||||
|
|
|
|||||||
|
|
||||||||
The foregoing table does not reflect exercise of the underwriters’ over-allotment option to purchase up to an additional 1,125,000 shares. If the underwriters exercise their over-allotment option in full, pro forma as adjusted net tangible book value will decrease to $3.92 per share, representing an increase to existing holders of $2.02 per share, and there will be an immediate dilution of $19.58 per share to new investors.
53
Table of Contents
The following table summarizes, on a pro forma basis as of June 30, 2015, the differences between existing shareholders and the new investors with respect to the number of ordinary shares purchased from us, the total consideration paid and the average price per share before deducting the underwriting discounts, commissions and estimated offering expenses payable by us, assuming an initial public offering price of $23.50 per share, the midpoint of the range of the initial public offering price. The information in the following table is illustrative only and the total consideration paid and the average price per share for new investors is subject to adjustment based on the actual initial public offering price of our ordinary shares and other terms of this offering determined at pricing.
| Ordinary shares purchased |
Total consideration | Average price per ordinary share |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| Existing Shareholders |
75,174,936 | 91% | 456,452,000 | 72% | $ | 6.07 | ||||||||||||||
| New Investors |
7,500,000 | 9% | 176,250,000 | 28% | $ | 23.50 | ||||||||||||||
|
|
|
|||||||||||||||||||
| Total |
82,674,936 | 100% | 632,702,000 | 100% | $ | 7.65 | ||||||||||||||
|
|
||||||||||||||||||||
The foregoing table does not reflect exercise of the underwriters’ over-allotment option to purchase up to an additional 1,125,000 shares.
A $1.00 increase (decrease) in the assumed initial public offering price per share would increase (decrease) our pro forma as adjusted net tangible book value after giving effect to the offering by $7.0 million, the pro forma as adjusted net tangible book value per share after giving effect to this offering by $0.08 per share and the dilution in pro forma as adjusted net tangible book value per share to new investors in this offering by $0.92 per share assuming no change to the number of ordinary shares offered by us as set forth on the cover page of this prospectus, and after deducting the underwriting discounts, commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only. Our net tangible book value following the consummation of this offering is subject to adjustment based on the actual initial public offering price of our ordinary shares and other terms of this offering determined at pricing.
If the underwriters exercise their over-allotment option in full, the following will occur:
| • | the pro forma as adjusted percentage of ordinary shares held by existing shareholders will decrease to approximately 90% of the total number of pro forma as adjusted ordinary shares outstanding after this offering and the total consideration paid for those ordinary shares, as a percentage of the total consideration paid for all ordinary shares outstanding after this offering on a pro forma as adjusted basis, will decrease to 69%; and |
| • | the pro forma as adjusted number of ordinary shares held by new public investors will increase to 8,625,000, or approximately 10% of the total number of pro forma as adjusted ordinary shares outstanding after this offering and the total consideration paid for those ordinary shares, as a percentage of the total consideration paid for all ordinary shares outstanding after this offering on a pro forma as adjusted basis, will increase to 31%. |
The tables and calculations above are based on 82,674,936 ordinary shares issued and outstanding as of June 30, 2015 (after giving effect to this offering) and exclude:
| • | 10,054,321 ordinary shares issuable upon the exercise of outstanding options as of June 30, 2015, at a weighted average exercise price of $5.39 per share (including the option to acquire 1,005,210 ordinary shares held by the Technion Research and Development Foundation); |
54
Table of Contents
| • | 921,488 ordinary shares issuable upon the exercise of options granted conditioned upon the consummation of this offering at an exercise price equal to the price per share to the public in this offering; |
| • | 4,635,317 ordinary shares issuable upon the exercise of outstanding warrants as of June 30, 2015, at a weighted average exercise price of $6.75 per share; and |
| • | 11,978,512 ordinary shares reserved for future issuances under our 2015 Omnibus Incentive Plan. |
To the extent these options or warrants are exercised, or ordinary shares are awarded under our 2015 Omnibus Incentive Plan, there will be further dilution to new investors.
55
Table of Contents
Selected consolidated financial data
We present below our selected consolidated financial data for the periods indicated. The following selected consolidated statement of operations data for the years ended December 31, 2013 and 2014, and the selected consolidated balance sheet data as of December 31, 2013 and 2014, have been derived from our audited consolidated financial statements included elsewhere in this prospectus. The selected consolidated statement of operations data for the six months ended June 30, 2014 and 2015, and the selected consolidated balance sheet data as of June 30, 2015, have been derived from our unaudited consolidated financial statements included elsewhere in this prospectus. The unaudited consolidated financial statements have been prepared on a basis consistent with our audited consolidated financial statements. In the opinion of management, the unaudited financial data reflect all adjustments, consisting only of normal and recurring adjustments, necessary for a fair presentation of the results for those periods. Results for interim periods are not necessarily indicative of results that may be expected for a full fiscal year. Historical results are not necessarily indicative of the results expected in the future.
The selected consolidated financial data should be read in conjunction with our consolidated financial statements and related notes and “Management’s discussion and analysis of financial condition and results of operations” included elsewhere in this prospectus. The consolidated financial statements are prepared and presented in accordance with U.S. GAAP.
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| (in thousands except share and per share data) | 2013 | 2014 | 2014 | 2015 | ||||||||||||
| Consolidated statement of operations data |
||||||||||||||||
| Net revenues |
$ | 10,359 | $ | 15,490 | $ | 7,315 | $ | 11,751 | ||||||||
| Cost of revenues |
7,013 | 10,036 | 4,820 | 8,647 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
3,346 | 5,454 | 2,495 | 3,104 | ||||||||||||
| Operating costs and expenses: |
||||||||||||||||
| Research, development and clinical trials |
34,797 | 40,381 | 19,915 | 22,692 | ||||||||||||
| Sales and marketing |
16,406 | 21,177 | 11,055 | 15,221 | ||||||||||||
| General and administrative |
16,602 | 24,052 | 10,767 | 14,343 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating costs and expenses |
67,805 | 85,610 | 41,737 | 52,256 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(64,459 | ) | (80,156 | ) | (39,242 | ) | (49,152 | ) | ||||||||
| Financial expenses, net |
12,558 | 144 | 38 | 1,467 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before income taxes |
(77,017 | ) | (80,300 | ) | (39,280 | ) | (50,619 | ) | ||||||||
| Income taxes |
353 | 382 | 166 | 2,011 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (77,370 | ) | $ | (80,682 | ) | $ | (39,446 | ) | $ | (52,630 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted net loss per ordinary share |
$ | (6.73 | ) | $ | (6.46 | ) | $ | (3.21) | $ | (4.12) | ||||||
| Weighted average number of ordinary shares used in computing basic and diluted net loss per share |
11,498,392 | 12,490,017 | 12,269,507 | 12,783,881 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Basic and diluted pro forma net loss per ordinary share(1) |
$ | (1.13 | ) | $ | (0.73) | |||||||||||
| Weighted average number of ordinary shares used in computing basic and diluted pro forma net loss per ordinary share(1) |
71,166,032 | 72,137,939 | ||||||||||||||
|
|
||||||||||||||||
56
Table of Contents
| As of December 31, |
As of June 30, | |||||||||||
| (in thousands) | 2013 | 2014 | 2015 | |||||||||
| Consolidated balance sheet data: |
||||||||||||
| Cash and cash equivalents |
$ | 175,894 | $ | 57,613 | $ | 106,508 | ||||||
| Short-term investments |
— | 44,999 | 56,996 | |||||||||
| Total assets |
188,911 | 117,876 | 192,770 | |||||||||
| Working capital |
167,885 | 94,161 | 158,677 | |||||||||
| Total liabilities |
17,132 | 20,001 | 48,463 | |||||||||
| Total shareholders’ equity |
171,779 | 97,875 | 144,307 | |||||||||
|
|
||||||||||||
| (1) | Pro forma for the conversion of all our outstanding preferred shares into ordinary shares in connection with the consummation of this offering (see Note 13a to the consolidated financial statements) after giving effect to a 5.913-for-1 stock split effected on September 16, 2015. Does not include any ordinary shares to be sold by us in this offering. |
57
Table of Contents
Management’s discussion and analysis of financial condition and results of operations
You should read the following discussion of our financial condition and results of operations in conjunction with our selected financial data and our consolidated financial statements and related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. As a result of many factors, such as those set forth under the section entitled “Risk factors” and elsewhere in this prospectus, our actual results may differ materially from those anticipated in these forward-looking statements.
Overview
We are a commercial-stage oncology company developing a novel, proprietary therapy called TTFields for the treatment of solid tumor cancers. TTFields is a low-toxicity anti-mitotic treatment that uses low-intensity, intermediate frequency, alternating electric fields to exert physical forces on key molecules inside cancer cells, disrupting the basic machinery necessary for normal cell division, leading to cancer cell death. Physicians have typically treated patients with solid tumors using one or a combination of three principal treatment modalities—surgery, radiation and pharmacological therapies. Despite meaningful advancements in each of these modalities, a significant unmet need to improve survival and quality of life remains. We believe we will establish TTFields as a new treatment modality for a variety of solid tumors that increases survival without significantly increasing side effects when used in combination with other cancer treatment modalities.
We view our operations and manage our business in one operating segment. We have incurred significant losses and cumulative negative cash flows from operations since our founding in 2000. Our net losses were $77.4 million for the year ended December 31, 2013, $80.7 million for the year ended December 31, 2014 and $52.6 million for the six months ended June 30, 2015. As of June 30, 2015, we had an accumulated deficit of $329.1 million. Our net losses primarily resulted from costs incurred in connection with our pre-clinical and clinical trial programs, costs incurred in our commercial launch efforts, including the U.S. launch of Optune for the treatment of recurrent GBM, and general and administrative costs necessary to operate as a multi-national oncology business. To date, we have financed our operations primarily through the issuance and sale of our convertible preferred shares and the proceeds from long-term loans. As of June 30, 2015, we had received a total of $454.3 million from the sale of our convertible preferred shares, all of which are authorized, issued and outstanding and will convert to our ordinary shares in connection with the consummation of this offering after giving effect to a 5.913-for-1 stock split effected on September 16, 2015.
We expect to continue to incur significant expenses and operating losses for at least the next several years. We expect our research, development and clinical trials expenses to increase in connection with our ongoing activities, particularly as we continue the research, development and clinical trials of TTFields and related delivery system candidates, including multiple simultaneous clinical trials for certain delivery system candidates, some of which are in, or we expect will be entering, late-stage clinical development. In addition, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We may need additional funding to support the continuation of our operating activities subsequent to this offering. Until we can generate substantial revenues (which may not occur), we expect to finance our cash needs through the proceeds of this offering and availability under our Term Loan Credit Facility, and possibly also from collaborations, strategic alliances, licensing arrangements and other marketing and distribution arrangements. We will need to generate significant revenues to achieve profitability, and we may never do so.
58
Table of Contents
Financial overview
Net revenues
Substantially all of our revenues are derived from patients using our TTFields delivery system, marketed as Optune in our currently active markets. We bill patients or their third-party healthcare payer for each month of use. Our potential revenues per patient are determined by the monthly fee we collect and the number of months that the patient remains on therapy. We bill $21,000 for each month that a patient uses Optune in the United States. Total cash payments of $12.4 million received during the six months ended June 30, 2015 were recorded as revenues for Optune therapy provided to patients in the current period and prior periods. These cash payments represent an average of approximately $14,700 for a month of use. The difference between billed and paid amounts consists of disputed underpayments, patient financial assistance and discounts. Additionally, during the period ended June 30, 2015, for each month of use, we paid approximately $600 in indirect taxes, primarily the federal medical device excise tax. This metric does not include our experience with patients covered by the Medicare fee-for-service program, as we have not received material payments from that program and the invoices remain open as we appeal the coverage denials. Patient assistance programs include assistance to reduce cost-share burdens imposed on patients by the payer (for example, co-insurance and co-payments), and the terms and conditions of our assistance program vary by market. Revenues are presented net of indirect taxes, including the U.S. medical device excise and sales tax.
We account for revenues when cash is collected. When we have sufficient history of collections from the third-party payers or third-party payer groups and can demonstrate that we can make a reasonable estimate of amounts that will ultimately be collected, we will recognize revenues ratably over the term of patients’ use of Optune and approved future delivery systems.
Our reported revenues are driven by commercial demand factors, including the number of prescription orders received, the number of active patients on therapy, the actual reimbursement rates and payment practices of private and governmental payers. We depend on third-party payers to provide reimbursement in most instances, and as such we dedicate substantial efforts toward obtaining coverage and pursuing contracts with third-party payers. See “Our business—Billing and reimbursement” for more information regarding our reimbursement and coverage activities.
Cost of revenues
We contract with third-party manufacturers that manufacture our TTFields delivery systems. Our cost of revenues is primarily comprised of the following:
| • | cost of the disposable transducer arrays purchased from third-party manufacturers; |
| • | depreciation expense for the field equipment, including the electric field generator used by patients; and |
| • | personnel, warranty and overhead costs such as facilities, freight and depreciation of property, plant and equipment associated with managing our inventory, warehousing and order fulfillment functions. |
The cost of revenues reported for the years ended December 31, 2013 and 2014 and the six months ended June 30, 2014 and 2015 reflects costs incurred for patients receiving TTFields treatment in the period. Revenue recognized in any period includes collections from amounts billed primarily in prior periods and, to a lesser extent, in the current period. Gross margin as a percentage of revenues is also affected by the timing of revenue recognition based on cash collections, which often result in costs being incurred in one period that relate to revenues recognized in a later period.
59
Table of Contents
Operating expenses
Our operating expenses consist of research, development and clinical trials, sales and marketing and general and administrative expenses. Personnel costs are a significant component for each category of operating expenses and consist of wages, benefits and bonuses. Personnel costs also include share-based compensation. We expect personnel costs to continue to increase as we hire new employees to continue to grow our business.
Research, development and clinical trials
Our research, development and clinical trials activity is focused on advancing TTFields through clinical trials across multiple solid tumor types and improving our delivery systems. Research, development and clinical trials costs, including direct and allocated expenses, are expensed as incurred and consist primarily of the following:
| • | personnel costs (including share-based compensation) for those employees involved in our research, development, clinical trial, regulatory and medical affairs activities; |
| • | costs to conduct research, development and clinical trial activity through agreements with contract research organizations and other third parties; |
| • | facilities, depreciation and other allocated expenses, which include direct and allocated expenses for rent and maintenance of facilities, depreciation of leasehold improvements and equipment and laboratory and other supplies; |
| • | manufacturing expense associated with TTFields delivery systems, including durable components and disposable arrays, utilized in clinical trials and other research; and |
| • | professional fees related to regulatory approvals and conformity assessment procedures. |
We have incurred significant expenditures related to conducting clinical studies to develop TTFields in multiple solid tumor indications. The following table summarizes our principal clinical programs for TTFields for the years ended December 31, 2013 and 2014 and the six months ended June 30, 2014 and 2015:
| Year ended December 31, |
Six months ended June 30, | |||||||||||||||
| (in thousands) | 2013 | 2014 | 2014 | 2015 | ||||||||||||
| Personnel costs |
$ | 12,176 | $ | 13,443 | $ | 6,394 | $ | 7,097 | ||||||||
| General research and development |
8,384 | 12,199 | 4,199 | 6,909 | ||||||||||||
| Materials |
6,224 | 5,397 | 3,640 | 3,129 | ||||||||||||
| Newly diagnosed GBM |
7,677 | 8,415 | 5,137 | 4,899 | ||||||||||||
| Brain metastases |
181 | 157 | 107 | 126 | ||||||||||||
| Pancreatic cancer |
155 | 292 | 183 | 216 | ||||||||||||
| Ovarian cancer |
— | 223 | 129 | 111 | ||||||||||||
| Mesothelioma |
— | 255 | 126 | 205 | ||||||||||||
|
|
|
|||||||||||||||
| Research, development and clinical trials |
$ | 34,797 | $ | 40,381 | $ | 19,915 | $ | 22,692 | ||||||||
|
|
||||||||||||||||
Personnel costs include all pre-clinical, clinical and other research and development company personnel. General research and development costs include costs related to pre-clinical, engineering, regulatory, intellectual property, advisors and subcontractors, travel and other. Materials include the costs of all equipment, arrays and other disposables for use in the clinical trials. Clinical trial costs in these periods include contract research organization services, data managing services, clinic and lab costs, as well as clinical sites costs.
60
Table of Contents
We expect our research and development expenses to increase in absolute dollars as we continue to advance TTFields and develop new delivery systems to address current and possible future indications. We are in different stages in clinical programs evaluating TTFields as a treatment for brain metastases, NSCLC, pancreatic cancer, ovarian cancer and mesothelioma. We also expect to continue or begin a number of significant clinical programs in the future for other solid tumor indications.
Sales and marketing
Sales and marketing expenses consist primarily of personnel costs (including share-based compensation), travel expenses, marketing and promotional activities, and facilities costs. Over the next few years, we expect to continue to make significant expenditures associated with selling and marketing our delivery systems, primarily in connection with the continued commercialization of Optune in the United States, Europe and Japan for the treatment of our approved indications.
General and administrative
General and administrative expenses consist primarily of personnel costs, professional fees and facilities costs. General and administrative personnel costs (including share-based compensation) include our executive, finance, human resources and legal functions. These costs also include our contributions to support industry and patient groups. Our professional fees consist primarily of accounting, legal and other consulting costs. We expect that general and administrative expenses will increase in absolute dollars to support our growth. In addition, following the consummation of this offering, we expect to incur significant additional legal and accounting costs related to compliance with SEC rules and regulations, including the additional costs of achieving and maintaining compliance with Section 404 of the Sarbanes-Oxley Act and compliance with NASDAQ rules, as well as additional insurance, investor relations and other costs associated with being a public company.
Financial expenses, net
Financial expenses, net primarily consists of credit facility interest expense and related debt issuance costs, interest income from cash balances and short-term investments and gains (losses) from foreign currency transactions. Our functional currency is the U.S. dollar. We have historically held substantially all of our cash balances in U.S. dollar denominated accounts to minimize the risk of translational currency exposure.
Income taxes
Our income taxes for the years ended December 31, 2013 and 2014 and the six months ended June 30, 2014 and 2015 are primarily due to statutory tax liabilities incurred by our subsidiaries.
Critical accounting policies and estimates
In accordance with U.S. GAAP, in preparing our financial statements, we must make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of net revenues and expenses during the reporting period. We develop and periodically change these estimates and assumptions based on historical experience and on various other factors that we believe are reasonable under the circumstances. Actual results may differ from these estimates.
The critical accounting policies requiring estimates, assumptions and judgments that we believe have the most significant impact on our consolidated financial statements are described below.
61
Table of Contents
Revenue recognition
The TTFields delivery system, currently marketed for recurrent GBM as Optune, is comprised of two main components: (1) an electric field generator and (2) transducer arrays and related accessories that are disposable supplies to the device, or the transducer arrays. We retain title to the electric field generator, and the patient is provided replacement transducer arrays for the device during the term of treatment. The electric field generator and transducer arrays are always supplied and function together and are not sold on a standalone basis.
Revenues are recognized when persuasive evidence of an arrangement exists, delivery of the electric field generator and transducer arrays has occurred, the price is fixed or determinable and collectability is reasonably assured. The evidence of an arrangement generally consists of a prescription, a patient service agreement and the verification of eligibility and insurance with the patient’s third-party insurance company. We generally bill third-party payers a monthly rental fee for use of Optune by patients. As such, we take assignment of the benefit and risk of collection from the third-party payer. Patients often have out-of-pocket costs for the amount not covered by their third-party payer and we bill the patient directly for the amounts of their co-pays and deductibles subject to our patient assistance programs.
For the reported periods, all revenues were recognized when cash was collected as the price is not fixed or determinable and the collectability cannot be reasonably assured. The price is not fixed or determinable since we do not have sufficient history with third-party payers to reliably estimate their individual payment patterns and as such cannot reliably estimate the amount that would be ultimately collected. Once sufficient history is established and we can demonstrate that we can reliably estimate the amounts that would be ultimately collected per third-party payer or payer group and the above criteria are met, we will recognize revenues ratably over the term of the patient’s use of Optune.
Revenues are presented net of indirect taxes incurred in the reported period, including the U.S. medical device excise tax and sales tax, regardless of whether the revenues associated with those taxes are reported on a cash basis.
Share-based compensation
Under the Financial Accounting Standards Board’s (FASB) Accounting Standards Codification (ASC) 718, Compensation—Stock Compensation, we measure and recognize compensation expense for share options granted to our employees and directors based on the fair value of the awards on the date of grant. The fair value of share options is estimated at the date of grant using the Black-Scholes option pricing model that requires management to apply judgment and make estimates, including:
| • | the fair value of our ordinary shares on the date of grant determined as discussed below; |
| • | the expected term of the stock option award, which we calculate using the simplified method, in accordance with ASC No.718-10-S99-1 (SAB No. 110) as we have insufficient historical information regarding our stock options to provide a basis for an estimate; |
| • | the expected share price volatility of our underlying ordinary shares, which we estimate based on the historical volatility of a representative group of publicly traded biopharmaceutical and medical technology companies with similar characteristics to us; |
| • | the risk-free interest rate, which we base on the yield curve of U.S. Treasury securities with periods commensurate with the expected term of the options being valued; and |
| • | the expected dividend yield, which we estimate to be zero based on the fact that we have never paid cash dividends and have no present intention to pay cash dividends. |
62
Table of Contents
We recognize share-based compensation costs only for those shares expected to vest over the requisite vesting period of the award, which is generally the option vesting term of four years, using the accelerated method. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Our forfeiture rate is based on an analysis of our actual forfeitures since the adoption of our 2003 Share Option Plan.
The table below summarizes the assumptions that were used to estimate the fair value of the options granted to employees during the periods presented:
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| 2013 | 2014 | 2014 | 2015 | |||||||||||||
| Expected term (years) |
6.25 | 6.25 | 6.25 | 6.25 | ||||||||||||
| Expected volatility |
70.4%-75.9% | 73.1%-75.3% | 75.1%-75.3% | 62.5%-65.8% | ||||||||||||
| Risk-free interest rate |
1.4%-2.0% | 1.9%-2.3% | 2.1%-2.3% | 1.8%-1.9% | ||||||||||||
| Dividend yield |
0% | 0% | 0% | 0% | ||||||||||||
|
|
||||||||||||||||
We incurred share-based compensation expense of $5.1 million, $4.6 million, $2.1 million and $4.4 million during the years ended December 31, 2013 and 2014 and the six months ended June 30, 2014 and 2015, respectively. As of June 30, 2015, we have unrecognized compensation expense of $16.7 million, which is expected to be recognized over a weighted average period of approximately 3.40 years. We expect to continue to grant share options in the future, and to the extent that we do, our recognized share-based compensation expense will likely increase.
If any of the assumptions used in the Black-Scholes option pricing model change significantly, share-based compensation for future awards may differ materially from the awards granted previously.
Our management and board of directors determined the fair value of our ordinary shares based on a number of objective and subjective factors consistent with the methodologies, approaches and assumptions consistent with the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the AICPA Guide. These factors included the hiring of key personnel, contemporaneous third-party valuations of our ordinary shares, our financial condition and prospects as of such date, the status of our research and development efforts, the public trading price of comparable companies for the March 5, 2015 grant, the lack of marketability of our ordinary shares as a private company, risk factors relevant to our business, capital markets conditions generally and the prices of our preferred shares sold to investors in arm’s-length transactions, and the rights, preferences and privileges of our preferred shares relative to our ordinary shares.
63
Table of Contents
The per share estimated fair value of ordinary shares in the table below represents the determination of the fair value of our ordinary shares as of the date of grant, taking into consideration the various objective and subjective factors described above. The following table presents the grant dates and related exercise prices of stock options granted to employees and consultants from January 1, 2013 through June 30, 2015:
| Grant date | Options granted | Exercise price | Fair value per ordinary share |
|||||||||
|
|
||||||||||||
| February 20, 2013 |
669,050 | $ | 7.03 | $ | 7.03 | |||||||
| July 24, 2013 |
180,923 | 7.04 | 7.04 | |||||||||
| October 23, 2013 |
193,935 | 7.28 | 7.28 | |||||||||
| February 26, 2014 |
511,460 | 7.48 | 7.48 | |||||||||
| April 23, 2014 |
136,281 | 7.52 | 7.52 | |||||||||
| August 3, 2014 |
73,908 | 7.58 | 7.58 | |||||||||
| October 22, 2014 |
438,148 | 7.73 | 7.73 | |||||||||
| March 5, 2015 |
1,637,887 | 14.37 | 14.37 | |||||||||
| April 22, 2015 |
158,752 | 15.60 | 15.60 | |||||||||
|
|
||||||||||||
Following completion of this offering and so long as our ordinary shares are publicly traded in a liquid market, we will rely on the daily trading price of our ordinary shares when we estimate the fair value of options granted.
Long-lived assets
Property and equipment and field equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful life of the relevant asset. We make estimates of the useful life of our property and equipment and field equipment, based on similar assets purchased in the past and our historical experience with such similar assets, in order to determine the depreciation expense to be recorded for each reporting period.
Our field equipment consists of equipment being utilized under rental agreements accounted for on a monthly basis as an operating lease, as well as service pool equipment. Service pool equipment is equipment owned and maintained by us that is swapped for equipment that needs repair or maintenance by us while being used by a patient. We record a provision for any excess, lost or damaged equipment when warranted based on an assessment of the equipment.
We assess impairment whenever events or changes in circumstances indicate that the carrying amount of the asset is impaired or the estimated useful life is no longer appropriate. Circumstances such as changes in technology or in the way an asset is being used may trigger an impairment review.
Inventories
Inventories are stated at the lower of cost or market. We regularly evaluate the ability to realize the value of inventory. If actual demand for our delivery systems declines or market conditions are less favorable than those projected, inventory write-offs may be required.
Income taxes
As part of the process of preparing our consolidated financial statements, we are required to calculate our income taxes based on taxable income by jurisdiction. We make certain estimates and judgments in determining our income taxes, including valuation of our uncertain tax positions, for financial statement purposes. Significant changes to these estimates may result in an increase or decrease to our tax provision in the subsequent period when such a change in estimate occurs.
64
Table of Contents
Uncertain tax positions are based on estimates and assumptions that have been deemed reasonable by management. Our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes.
Results of operations
The consolidated statement of operations data for the years ended December 31, 2013 and 2014 and the consolidated balance sheet data as of December 31, 2013 and 2014 are derived from our audited consolidated financial statements included elsewhere in this prospectus. The consolidated statement of operations data for the six months ended June 30, 2014 and 2015 and the consolidated balance sheet data as of June 30, 2015 have been derived from our unaudited consolidated financial statements included elsewhere in this prospectus. The unaudited consolidated financial statements include, in the opinion of management, all adjustments, consisting only of normal recurring adjustments, that management considers necessary for a fair presentation of the financial information set forth in those statements. Our historical results are not necessarily indicative of the results that should be expected in the future, and our interim results are not necessarily indicative of results that should be expected for the full year.
| Year ended December 31, |
Six months ended June 30, | |||||||||||||||
| (in thousands) | 2013 | 2014 | 2014 | 2015 | ||||||||||||
| Net revenues |
$ | 10,359 | $ | 15,490 | $ | 7,315 | $ | 11,751 | ||||||||
| Cost of revenues |
7,013 | 10,036 | 4,820 | 8,647 | ||||||||||||
|
|
|
|||||||||||||||
| Gross profit |
3,346 | 5,454 | 2,495 | 3,104 | ||||||||||||
|
|
|
|||||||||||||||
| Operating costs and expenses: |
||||||||||||||||
| Research, development and clinical trials |
34,797 | 40,381 | 19,915 | 22,692 | ||||||||||||
| Sales and marketing |
16,406 | 21,177 | 11,055 | 15,221 | ||||||||||||
| General and administrative |
16,602 | 24,052 | 10,767 | 14,343 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Total operating costs and expenses |
67,805 | 85,610 | 41,737 | 52,256 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(64,459 | ) | (80,156 | ) | (39,242) | (49,152) | ||||||||||
| Financial expenses, net |
12,558 | 144 | 38 | 1,467 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Loss before income taxes |
(77,017 | ) | (80,300 | ) | (39,280) | (50,619) | ||||||||||
| Income taxes |
353 | 382 | 166 | 2,011 | ||||||||||||
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (77,370 | ) | $ | (80,682 | ) | $ | (39,446) | $ | (52,630) | ||||||
|
|
||||||||||||||||
The following table includes certain commercial patient operating statistics for and as of the end of the periods presented.
| Operating statistics | Year ended December 31, | Six months ended June 30, | ||||||||||||||
| 2013 | 2014 | 2014 | 2015 | |||||||||||||
|
|
||||||||||||||||
| Prescriptions received in period(1) |
510 | 707 | 298 | 865 | ||||||||||||
| Active patients at period end(2) |
184 | 225 | 172 | 425 | ||||||||||||
|
|
||||||||||||||||
| (1) | A “prescription received” is a commercial order for Optune that is received from a physician certified to treat patients with TTFields therapy for a patient not previously on TTFields therapy. Orders to renew or extend treatment are not included in this total. In the future, we may have regulatory approvals and commercial programs for multiple clinical indications, at which time we will recognize a commercial order as a prescription for the same patient for each clinical indication treated. For example, in the future, a patient may have a prescription for the treatment of lung cancer and a prescription for the treatment of brain metastases from the lung cancer. |
65
Table of Contents
| (2) | An “active patient” is a patient who is on TTFields therapy under a commercial prescription order as of the measurement date, including patients who may be on a temporary break from treatment and who plan to resume treatment in less than 60 days. |
Six months ended June 30, 2015 compared to six months ended June 30, 2014
| Six months ended June 30, |
||||||||||||||||
| (in thousands, except % change) | 2014 | 2015 | Change | % Change | ||||||||||||
| Net revenues |
$ | 7,315 | $ | 11,751 | $ | 4,436 | 61% | |||||||||
|
|
|
|
|
|
|
|
|
|
||||||||
Net revenues. Net revenues increased by $4.4 million, or 61%, to $11.8 million for the six months ended June 30, 2015 from $7.3 million for the six months ended June 30, 2014. The increase was primarily due to an increase of $3.9 million in U.S. commercial sales of Optune, driven in part by the increased marketing efforts in the United States for recurrent GBM and in part by the announcement of the EF-14 interim results in November 2014, which we believe led to more sales of Optune for recurrent GBM, and to an increase of $0.5 million in commercial sales of Optune in our currently active markets in Europe.
Cost of revenues. Our cost of revenues increased by $3.8 million, or 79%, to $8.6 million for the six months ended June 30, 2015 from $4.8 million for the six months ended June 30, 2014. The increase was due to the increased volume of Optune shipments to commercial patients, comprised of $2.6 million in the cost of transducer arrays shipped to patients mainly in the United States, $1.0 million in personnel, facility and other costs and $0.2 million in field equipment depreciation expense. The personnel, facility and other costs reported for the six months ended June 30, 2015 were driven by our efforts to establish the staff and facility organization necessary to support our commercialization efforts.
| Six months ended June 30, | ||||||||||||||||
| (in thousands, except % change) | 2014 | 2015 | Change | % Change | ||||||||||||
| Research, development and clinical trials |
$ | 19,915 | $ | 22,692 | $ | 2,777 | 14% | |||||||||
| Sales and marketing |
11,055 | 15,221 | 4,166 | 38% | ||||||||||||
| General and administrative |
10,767 | 14,343 | 3,576 | 33% | ||||||||||||
|
|
|
|||||||||||||||
| $ | 41,737 | $ | 52,256 | $ | 10,519 | 25% | ||||||||||
|
|
||||||||||||||||
Research, development and clinical trials expenses. Research, development and clinical trials expenses increased by $2.8 million, or 14%, to $22.7 million in the six months ended June 30, 2015 from $19.9 million for the six months ended June 30, 2014. The change was primarily due to an increase of $0.7 million in personnel costs (including share-based compensation) due to increased headcount related to the expansion of research and development activity for possible future indications, an increase of $1.3 million in facility and other expenses and an increase of $0.6 million in clinical trials and engineering related to the second generation of Optune.
Sales and marketing expenses. Sales and marketing expenses increased by $4.1 million, or 38%, to $15.2 million for the six months ended June 30, 2015 from $11.1 million for the six months ended June 30, 2014. The change was due to an increase of $2.4 million in advertising and other marketing expenses, an increase of $1.3 million for increased headcount to support marketing activities and an increase of $0.4 million related to facility, freight and travel expenses.
General and administrative expenses. General and administrative expenses increased by $3.6 million, or 33%, to $14.3 million for the six months ended June 30, 2015 from $10.7 million for the six months ended June 30, 2014. The change was primarily due to an increase of $2.3 million in personnel costs due to increased
66
Table of Contents
headcount (including $1.1 million of share-based compensation expense) to support the growth and operation of our business, an increase of $0.7 million for costs of professional services relating to the implementation of our new SAP ERP system and legal services, and an increase of $0.6 million in facilities and travel costs.
Financial expenses, net. Financial expenses, net increased by $1.4 million to $1.5 million for the six months ended June 30, 2015, primarily due to the interest expense, including amortization expense of the discount and deferred issuance costs, related to our Term Loan Credit Facility entered into in January 2015.
| Six months ended June 30, |
||||||||||||||||
| (in thousands, except % change) | 2014 |
2015 |
Change | % Change | ||||||||||||
| Income taxes |
$ | 166 | $ | 2,011 | $ | 1,845 | 1,111% | |||||||||
|
|
||||||||||||||||
Income taxes. Income taxes increased by $1.9 million to $2 million for the six months ended June 30, 2015. The change was primarily attributable to an increase in the statutory tax provision related to Switzerland of $1.7 million.
Year ended December 31, 2014 compared to year ended December 31, 2013
| Year ended December 31, | ||||||||||||||||
| (in thousands, except % change) | 2013 | 2014 | Change | % Change | ||||||||||||
| Net revenues |
$ | 10,359 | $ | 15,490 | $ | 5,131 | 50% | |||||||||
|
|
||||||||||||||||
Net revenues. Net revenues increased by $5.1 million, or 50%, to $15.5 million for the year ended December 31, 2014 from $10.4 million for the year ended December 31, 2013. The increase was primarily due to an increase of $4.7 million in U.S. commercial sales of Optune, driven in part by the increased marketing efforts in the United States for recurrent GBM and in part by the announcement of the EF-14 interim results in November 2014, which we believe led to more sales of Optune for recurrent GBM, and to an increase of $0.4 million in commercial sales of Optune in our currently active markets in Europe.
Cost of revenues. Our cost of revenues increased by $3.0 million, or 43%, to $10.0 million for the year ended December 31, 2014 from $7.0 million for the year ended December 31, 2013. The increase was primarily due to an increase of $1.8 million in transducer arrays shipped to U.S. commercial patients, an increase of $0.3 million in transducer arrays shipped to patients in Europe, an increase of $0.4 million for field equipment depreciation and increased personnel costs of $0.5 million associated with new employees.
| Year ended December 31, | ||||||||||||||||
| (in thousands, except % change) | 2013 | 2014 | Change | % Change | ||||||||||||
| Research, development and clinical trials |
$ | 34,797 | $ | 40,381 | $ | 5,584 | 16% | |||||||||
| Sales and marketing |
16,406 | 21,177 | 4,771 | 29% | ||||||||||||
| General and administrative |
16,602 | 24,052 | 7,450 | 45% | ||||||||||||
|
|
|
|||||||||||||||
| $ | 67,805 | $ | 85,610 | $ | 17,805 | 26% | ||||||||||
|
|
||||||||||||||||
Research, development and clinical trials expenses. Research, development and clinical trials expenses increased by $5.6 million, or 16%, to $40.4 million for the year ended December 31, 2014 from $34.8 million for the year ended December 31, 2013. The change is primarily due to an increase in clinical trials expenses of $3.5 million mainly related to EF-14, an increase of $1.3 million of personnel costs (including share-based compensation), and an increase of $0.4 million for regulatory expenses related to the preparation of approval applications to the Japanese Ministry of Health, Labor and Welfare for the treatment of recurrent GBM and the FDA in the United States for the treatment of newly diagnosed GBM. In addition, there was $0.4 million increase in facility expenses.
67
Table of Contents
Sales and marketing expenses. Sales and marketing expenses increased by $4.8 million, or 29%, to $21.2 million for the year ended December 31, 2014 from $16.4 million for the year ended December 31, 2013. The increase was driven by an increase of $3.2 million of personnel costs (including share-based compensation), an increase of $0.7 million in advertising and other marketing expenses and a $0.9 million increase related to facility, freight and travel expenses.
General and administrative expenses. General and administrative expenses increased by $7.5 million, or 45%, to $24.1 million for the year ended December 31, 2014 from $16.6 million for the year ended December 31, 2013. The increase was primarily related to an increase in personnel costs of $3.0 million due to increased headcount to support the growth and operation of our business, an increase of $1.3 million for costs of professional services relating to the implementation of our new SAP ERP system and legal services, and an increase of $1.0 million related to facility and travel costs. In addition, there was an expense related to a provision for settlement with the Technion of $1.9 million.
Financial expenses, net. Financial expenses, net decreased by $12.4 million, or 98%, to $0.1 million for the year ended December 31, 2014 from $12.6 million for the year ended December 31, 2013. The change was primarily due to repayment of an outstanding $52.0 million principal amount loan at the end of 2013, which contributed $12.5 million to the 2013 financial expenses.
| Year ended December 31, | ||||||||||||||||
| (in thousands, except % change) | 2013 | 2014 | Change | % Change | ||||||||||||
| Income taxes |
$ | 353 | $ | 382 | $ | 29 | 8% | |||||||||
|
|
||||||||||||||||
Income taxes. Income taxes did not significantly change from 2014 as compared to 2013. The minor change was primarily attributable to an increase in our provision for current taxes in Switzerland offset by a decrease in provision for unrecognized tax benefits.
Liquidity and capital resources
We have incurred significant losses and cumulative negative cash flows from operations since our founding in 2000. As of June 30, 2015, we had an accumulated deficit of $329.1 million. We expect to continue to incur losses until our delivery systems achieve market acceptance. To date, we have primarily financed our operations through the issuance and sale of our convertible preferred shares and the proceeds from long-term loans. As of June 30, 2015, we had received a total of $454.3 million from the sale of 62,744,517 of our convertible preferred shares, including the sale of 4,068,500 shares of Series J convertible preferred stock in June 2015 for net proceeds of $94.6 million, all of which are authorized, issued and outstanding and will convert to our ordinary shares in connection with the consummation of this offering after giving effect to a 5.913-for-1 stock split effected on September 16, 2015.
As of December 31, 2014 and June 30, 2015, we had $102.6 million and $163.5 million, respectively, of cash and cash equivalents, including short-term investments of $45.0 million and $57.0 million, respectively. In 2013, we raised $194.2 million through the issuance of our Series I convertible preferred shares. In January 2015, we entered into the Loan and Security Agreement dated as of January 7, 2015, between us, as borrower, and Biopharma Secured Investments III Holdings Cayman LP, as lender, or the Term Loan Credit Facility, for up to $100.0 million, of which we drew $25.0 million on entering into the facility. In June 2015, we raised $94.6 million through the issuance of our Series J convertible preferred shares. We believe our cash and cash equivalents as of June 30, 2015, together with the net proceeds of this offering and availability under our Term Loan Credit Facility, are sufficient for our operations for at least the next 12 months based on our existing business plan and our ability to control the timing of significant expense commitments. We expect that our research, development and clinical trials expenses, sales and marketing expenses and general and
68
Table of Contents
administrative expenses will continue to increase over the next several years. As a result, we may need to raise additional capital subsequent to this offering to fund our operations.
The following summary of our cash flows for the periods indicated has been derived from our consolidated financial statements, which are included elsewhere in this prospectus:
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| (in thousands) | 2013 | 2014 | 2014 | 2015 | ||||||||||||
| Net cash used in operating activities |
$ | (52,717 | ) | $ | (74,244 | ) | $ | (38,629 | ) | $ | (51,657 | ) | ||||
| Net cash provided by (used in) investing activities |
10,402 | (46,182 | ) | (69,001 | ) | (16,622 | ) | |||||||||
| Net cash provided by financing activities |
183,318 | 2,145 | 24 | 117,174 | ||||||||||||
|
|
|
|||||||||||||||
| Net increase (decrease) in cash and cash equivalents |
$ | 141,003 | $ | (118,281 | ) | $ | (107,606 | ) | $ | 48,895 | ||||||
|
|
||||||||||||||||
Operating activities
Net cash used in operating activities primarily represents our net loss for the periods presented. Adjustments to net loss for non-cash items include depreciation, share-based compensation and accrued interest. Operating cash flows are also impacted by changes in operating assets and liabilities, principally inventories, prepaid expenses, trade payables and accrued expenses.
Net cash used in operating activities was $51.7 million for the six months ended June 30, 2015, as compared to $38.6 million for the six months ended June 30, 2014, reflecting a net loss of $52.6 million, and a change of $5.5 million in our net operating assets and liabilities offset by non-cash charges of $6.4 million.
The change in our net operating assets and liabilities was primarily the result of an increase in our inventories of $5.1 million necessary to meet anticipated demand, an increase in other receivables of $3.0 million and a decrease of $0.8 million in other long-term liabilities, offset by an increase in trade payables of $3.1 million and other payables of $0.5 million. Non-cash charges included $4.4 million of share-based compensation, $1.1 million of depreciation and $0.7 million of interest related to our Term Loan Credit Facility.
Net cash used in operating activities was $74.2 million in 2014, reflecting a net loss of $80.7 million, offset by non-cash charges of $6.6 million and a change of $0.1 million in our net operating assets and liabilities. The change in our net operating assets and liabilities was the result of an increase in receivables and prepaid expenses of $1.2 million, a decrease of $0.5 million in trade payables and an increase in inventory of $1.6 million primarily due to commercial sales in the United States, offset by an increase in other payables of $2.3 million and an increase in other long-term liabilities of $0.8 million. Non-cash charges included $4.6 million of share-based compensation and $2.0 million of depreciation.
Net cash used in operating activities was $52.7 million in 2013, reflecting a net loss of $77.4 million, offset by non-cash charges of $17.7 million and a change of $7.0 million in our net operating assets and liabilities. The change in our net operating assets and liabilities was primarily the result of an increase in trade payables of $5.7 million due to increased activity, an increase in other payables of $3.0 million, a decrease in inventory of $1.5 million and an increase in other long-term liabilities of $0.3 million, offset by an increase in our receivables and prepaid expenses of $3.6 million. Non-cash charges included $5.1 million of share-based compensation, $1.2 million of depreciation, accrued interest of $6.0 million related to a long-term loan and $5.4 million in related amortization expenses.
Investing activities
Our investing activities consist primarily of capital expenditures to purchase property and equipment and field equipment, as well as investments in and redemptions of our short-term investments.
Net cash provided by investing activities was $16.6 million in the six months ended June 30, 2015 attributable to our receipt of $47.0 million from the maturity of short-term investments, offset by the purchase of new short-
69
Table of Contents
term investments of $59.0 million, purchases of $2.4 million of property and equipment and purchases of $2.2 million of field equipment. Net cash used in investing activities for the same period in 2014 was $69.0 million, attributable to the purchase of $69.0 million of short-term investments. In 2014, purchases of property and equipment of $0.3 million and purchases of field equipment of $0.7 million was offset by a decrease in restricted cash of $1.0 million.
Net cash used in investing activities was $46.2 million in 2014 attributable to our receipt of $93.0 million from the maturity of short-term investments and a decrease of $1.1 million of restricted cash, offset by the purchase of short-term investments of $138.0 million and the purchase of $0.8 million of property and equipment and $1.5 million of field equipment.
Net cash provided by investing activities in 2013 was $10.4 million attributable to proceeds from redemption of bank deposits of $15.0 million, offset by the purchase of property and equipment of $2.2 million, the purchase of field equipment of $1.4 million and an increase in restricted cash of $1.0 million.
Financing activities
To date, our primary financing activities have been the sale of our convertible preferred shares and the proceeds from long-term loans.
Net cash provided by financing activities was $117.2 million for the six months ended June 30, 2015, attributable to the net proceeds from the issuance of Series J preferred shares of $94.6 million and borrowings under our Term Loan Credit Facility of $22.9 million offset by deferred IPO costs of $0.3 million, compared to $0.1 million in the same period of 2014, attributable to a long-term loan.
Net cash provided by financing activities was $2.1 million in 2014, attributable to net proceeds received from the exercise of options. Net cash provided by financing activities was $183.3 million in 2013 attributable mainly to proceeds from the issuance of our Series I preferred shares of $191.7 million and proceeds from a long-term loan of $49.6 million, offset by the repayment of the long-term loan (including interest) of $58.0 million.
Term Loan Credit Facility
Our material outstanding indebtedness consists of our Term Loan Credit Facility, which provides for up to $100.0 million of borrowings in up to four draws, the first of which was made on January 30, 2015 in the amount of $25.0 million. Interest on the outstanding loan is 10% annually, payable quarterly in arrears. As of June 30, 2015, the aggregate principal balance of amounts outstanding under the Term Loan Credit Facility was approximately $25.0 million. The commitments made by the lender to make additional term loans terminate on June 30, 2016. We may prepay the term loans, in whole, at any time, and must prepay in the event of a change of control, in each case, subject to a pay-down fee, prepayment premium and/or make-whole payment. The funding fee payable on the amount drawn on the funding date is 1.5%, the pay-down fee on all principal payments to be paid on the date such payments are made is 0.75% and the pre-payment fee if we prepay outstanding loan amounts prior to the first, second or third year from the initial funding date is 3.0%, 2.0% or 1.0%, respectively.
All obligations under the Term Loan Credit Facility are guaranteed by certain of our current and future domestic direct and indirect subsidiaries. In addition, the obligations under the Term Loan Credit Facility are secured by a first-priority security interest in substantially all of the property and assets of, as well as the equity interests owned by, us and the other guarantors.
The Term Loan Credit Facility has a minimum liquidity covenant, which is tested quarterly. In addition, we must meet certain pro forma net sales requirements. The Term Loan Credit Facility contains other customary covenants.
70
Table of Contents
Contractual obligations and commitments
The following summarizes our significant contractual obligations as of December 31, 2014:
| Payments due by period | ||||||||||||||||||||||||||||
| (in thousands) |
2015 | 2016 | 2017 | 2018 | 2019 | After | Total | |||||||||||||||||||||
| Contractual obligations: |
||||||||||||||||||||||||||||
| Operating leases |
$ | 1,620 | $ | 1,385 | $ | 1,241 | $ | 937 | $ | 801 | $ | 1,395 | $ | 7,379 | ||||||||||||||
| Term Loan Credit Facility(1) |
— | — | — | — | — | 25,188 | 25,188 | |||||||||||||||||||||
| Other long-term loans |
59 | 66 | 69 | 73 | 22 | 90 | 380 | |||||||||||||||||||||
| Settlement agreement |
2,000 | — | — | — | — | — | 2,000 | |||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
| (1) | The Term Loan Credit Facility has a fixed per annum interest rate of 10.0%. Interest due is excluded from the table. |
The total amount of unrecognized tax benefits for uncertain tax positions was $0.3 million at December 31, 2014. Payment of these obligations would result from settlements with taxing authorities. Due to the difficulty in determining the timing of settlements, these obligations are not included in the above table. We do not expect a significant tax payment related to these obligations within the next year.
As described in “Our business—Intellectual property”, we are required to make a $1.0 million payment to the Technion with the net proceeds of this offering. In addition, we have a contingent obligation to pay the Technion an additional $5.5 million upon the earlier of our achieving $250.0 million of cumulative net sales since our inception, as defined in the Settlement Agreement, or any merger, consolidation, reorganization or sale or other disposition of all or substantially all of our assets.
We also have employment agreements with certain employees that require the funding of a specific level of payments if certain events, such as a change in control or termination without cause, occur.
In the course of normal business operations, we also have agreements with third parties to assist in the performance of our research and development (including clinical trials) and manufacturing activities. We could also enter into additional collaborative research, contract research, manufacturing and supplier agreements in the future, which may require up-front payments and even long-term commitments of cash.
Off-balance sheet arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements as defined under SEC rules.
Quantitative and qualitative disclosure about market risk
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily a result of fluctuations in interest rates and foreign currency exchange rates. We do not hold or issue financial instruments for trading purposes.
Interest rate sensitivity
Our exposure to market risk for changes in interest rates relates primarily to our investment portfolio. Our cash, cash equivalents and short-term investment accounts as of December 31, 2014 and June 30, 2015 totaled $102.6 million and $163.5 million, respectively, and consist primarily of cash, cash equivalents and short-term investments with maturities of less than one year from the date of purchase. Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of the interest rates in the
71
Table of Contents
United States. However, because of the short-term nature of the instruments in our portfolio, a 10% change in market interest rates would not be expected to have a material impact on our financial condition or our results of operations.
Foreign currency exchange risk
Our consolidated results of operations and cash flow are subject to fluctuations due to changes in foreign currency exchange rates. All of our revenues are generated in U.S. dollars. Our expenses are generally denominated in the currencies in which our operations are located, which is primarily in the United States and Israel. Our consolidated results of operations and cash flow are, therefore, subject to fluctuations due to changes in foreign currency exchange rates and may be adversely affected in the future due to changes in foreign exchange rates. The effect of a hypothetical 10% change in foreign currency exchange rates applicable to our business would not have a material impact on our historical consolidated financial statements. We do not hedge our foreign currency exchange risk.
72
Table of Contents
Below is a description of our business. Please refer to the “Glossary of terms” and “Summary of completed and existing clinical trials and registry data” beginning on page 105 as you review this section.
Overview
We are a commercial-stage oncology company developing a novel, proprietary therapy called TTFields for the treatment of solid tumor cancers. TTFields is a low-toxicity anti-mitotic treatment that uses low-intensity, intermediate frequency, alternating electric fields to exert physical forces on key molecules inside cancer cells, disrupting the basic machinery necessary for normal cell division, leading to cancer cell death. Physicians have typically treated patients with solid tumors using one or a combination of three principal treatment modalities—surgery, radiation and pharmacological therapies. Despite meaningful advancements in each of these modalities, a significant unmet need to improve survival and quality of life remains. We believe we will establish TTFields as a new treatment modality for a variety of solid tumors that increases survival without significantly increasing side effects when used in combination with other cancer treatment modalities.
We received FDA approval for Optune, our first TTFields delivery system, in 2011 for use as a monotherapy treatment for adult patients with glioblastoma brain cancer, or GBM, following confirmed recurrence after chemotherapy. We have built a commercial organization and launched Optune in the United States, Germany, Switzerland and Japan, which we refer to as our currently active markets. In November 2014, our Phase 3 pivotal trial of Optune in combination with chemotherapy for patients with newly diagnosed GBM met its endpoints and was halted after a protocol pre-specified interim analysis showed significant improvements in both progression free and overall survival. In April 2015, we filed a premarket approval, or PMA, supplement application with the FDA for the treatment of newly diagnosed GBM based on our Phase 3 data and our application was granted priority review status. Upon FDA approval of Optune for newly diagnosed GBM, we believe TTFields will transform the standard of care for patients with newly diagnosed and recurrent GBM.
We have researched the biological effects of TTFields extensively. Because TTFields are delivered regionally, act only on mitotic cells and are tuned to target cancer cells of a specific size, there is minimal damage to healthy cells. We believe our pre-clinical and clinical research demonstrates that TTFields’ mechanism of action affects fundamental aspects of cell division and can have broad applicability across a variety of solid tumors. We have demonstrated in pre-clinical studies that TTFields can offer additive or synergistic benefits in combination with radiation and chemotherapy, which may lead to greater efficacy than either modality alone, without appearing to potentiate the systemic toxicities of either radiation or chemotherapy. In addition to our clinical and commercial progress in GBM, we are currently planning or conducting clinical trials evaluating the use of TTFields in brain metastases, NSCLC, pancreatic cancer, ovarian cancer and mesothelioma.
We own all commercialization rights to TTFields in oncology, and have a patent and intellectual property portfolio that, as of June 30, 2015, consists of a total of 52 issued patents, including 36 issued in the United States, as well as over 30 additional patent applications on file. We believe we will maintain exclusive rights to market TTFields for all solid tumor indications in our key markets through the life of our patents.
GBM—our first approved and commercialized indication
GBM is the most common and aggressive form of primary brain cancer. We estimate approximately 27,500 patients are diagnosed with GBM annually in the United States, the top five European Union markets and Japan. GBM has few effective treatment options at present and provides our first opportunity to transform the standard of care for a solid tumor cancer to include TTFields.
73
Table of Contents
We launched Optune in the United States for the treatment of recurrent GBM in 2011 and more recently in our other currently active markets. Since the majority of recurrent GBM patients are treated at large cancer centers, we built a commercial organization to focus primarily on these centers. As of the date of this prospectus, we have trained physicians in over 270 clinical centers. These trained physicians have treated over 1,600 GBM patients using Optune.
We initiated our EF-14 Phase 3 pivotal trial in 2009 to establish TTFields for the treatment of newly diagnosed GBM. The EF-14 trial randomized 700 patients to receive either temozolomide, the established standard of care chemotherapy for newly diagnosed GBM, or TTFields in combination with temozolomide. In November 2014, a protocol pre-specified interim analysis of the first 315 patients demonstrated the trial met its powered endpoints of significant extension of both progression free survival, or PFS, and overall survival, or OS, in patients treated with TTFields in combination with temozolomide versus temozolomide alone. The interim analysis results demonstrated that:
| • | the two-year survival rate among patients treated with TTFields in combination with temozolomide, in the as-treated population, was 48% compared to 32% among patients treated with temozolomide alone (p=0.0058); |
| • | patients treated with TTFields, in combination with temozolomide, in the intent-to-treat population, demonstrated a statistically significant increase in PFS compared to temozolomide alone (median PFS of 7.2 months compared to 4.0 months, hazard ratio=0.62, p=0.001); and |
| • | patients treated with TTFields, in combination with temozolomide, in the as-treated population, demonstrated a statistically significant increase in OS compared to temozolomide alone (median OS of 20.5 months compared to 15.6 months, hazard ratio=0.66, p=0.004). |
The trial’s independent data monitoring committee recommended that patients receiving temozolomide alone be allowed to cross over immediately to receive TTFields. Following FDA approval of this recommendation in December 2014, we allowed patients receiving temozolomide alone to cross over. We submitted a PMA supplement application to the FDA in April 2015 to expand our label for Optune to include the treatment of newly diagnosed GBM. In May 2015, we received priority review status from the FDA. We believe that following FDA approval of Optune for newly diagnosed GBM, Optune in combination with temozolomide will transform the standard of care for the treatment of patients with newly diagnosed GBM.
Our clinical pipeline
We have performed extensive pre-clinical research on TTFields and their effects in multiple solid tumor cancers. We have gained a deep understanding of the underlying mechanism of action and the multiple pathways through which TTFields exert their effects within the dividing cancer cells. Our research shows that TTFields have an anti-mitotic effect in over 15 different solid tumor types in culture and in multiple in vivo tumor models. In vitro and in vivo studies combining TTFields with radiation or chemotherapy, in multiple tumor types, have demonstrated at least additive efficacy, or stronger efficacy than the effect of either treatment alone, and in some cases synergistic efficacy, or stronger efficacy than the sum of the effects of both treatments. An increase in cancer cell sensitivity to chemotherapy when used in combination with TTFields in the range of one to two orders of magnitude suggests additivity, while an increase in the range of three to four orders of magnitude suggests synergism. Certain in vitro experiments using TTFields have suggested both additivity and synergism when used in combination with chemotherapy, as the presence of TTFields was shown to increase cancer cell sensitivity to chemotherapy from approximately 275 times to over 1,250 times depending on the mechanism of action of the particular chemotherapy. The upper end of this range was observed in testing with taxane-based chemotherapies.
74
Table of Contents
We believe our success in delaying disease progression and extending survival in GBM patients, our pre-clinical data and our early clinical data in additional indications validate the potential of TTFields to become a new therapeutic modality for a variety of solid tumors. We have developed a pipeline strategy to advance TTFields through Phase 2 pilot and Phase 3 pivotal clinical trials across multiple solid tumor types, and anticipate expanding our clinical pipeline over time to study the safety and efficacy of TTFields for additional solid tumor indications.

Our competitive advantages
We believe our key competitive advantages are:
| • | Significant market potential addressable via a broadly applicable mechanism of action. Based on our pre-clinical research and clinical experience to date, we believe the anti-mitotic mechanism of action of TTFields is broadly applicable to a variety of solid tumors with an annual incidence of approximately 1.1 million people in the United States alone. Currently, we have ongoing and completed clinical trials for indications with an incidence of approximately 350,000 people annually in the United States. We believe that the global incident population of target solid tumors provides us with significant additional commercial opportunities. |
| • | Immediate commercial opportunity for optune in GBM. We are currently marketing Optune for the treatment of recurrent GBM in the United States and our other currently active markets. We have applied for FDA approval for the treatment of newly diagnosed GBM based on the results of our successful EF-14 Phase 3 clinical trial. Upon approval, we will begin marketing Optune as a treatment for newly diagnosed GBM, and we expect that Optune will transform the standard of care for the treatment of patients with newly diagnosed GBM. |
| • | Pipeline of Phase 2 trials in five additional indications. In addition to our GBM clinical programs, we have invested in a variety of clinical programs in other solid tumors. We have completed a Phase 2 trial in NSCLC, and are currently enrolling patients in Phase 2 trials for brain metastases, pancreatic cancer, ovarian cancer and mesothelioma. We expect to continue investing in our pipeline over time to broaden our commercial opportunity. |
| • | Established commercial organization and supply chain. We have established our commercial organization and believe we have the experience, expertise and infrastructure to scale our sales and marketing efforts in our key markets. In addition to our commercial organization, we have established a scalable supply chain. |
| • | Significant barriers to entry. We own all commercialization rights to TTFields in oncology and have a patent and intellectual property portfolio that, as of June 30, 2015, includes 52 issued patents, 36 of which are issued in the United States, as well as over 30 additional patent applications on file. We have patent protection through 2031 in the United States and through 2026 in other key markets. We believe we will |
75
Table of Contents
| maintain exclusive rights to market TTFields for all solid tumor indications in our key markets for the life of our patents. In addition, even after the expiration of our U.S. patents, potential market entrants applying low-intensity, alternating electric fields to solid tumors in the United States will have to undertake their own clinical trials and PMA submissions to the FDA to demonstrate equivalence to TTFields to market a competing product. |
Our strategies for growth
Our objective is to establish TTFields as a new modality for the treatment of a variety of solid tumors. Our key strategies include the following:
| • | Drive adoption of optune in GBM. We plan to use the data from our pivotal EF-14 Phase 3 clinical trial and our commercial organization to transform the standard of care for patients with newly diagnosed and recurrent GBM and to drive adoption of Optune by physicians and patients. |
| • | Expand our commercial organization. We plan to expand our direct sales force to call on physicians who treat newly diagnosed GBM patients. We expect to further expand our commercial organization following regulatory approvals for additional indications. |
| • | Advance clinical development of TTFields. We plan to advance our clinical pipeline and evaluate other solid tumor indications that we believe can be targeted with TTFields. |
| • | Evaluate the use of TTFields in combination with other solid tumor therapies. We are supporting independent research into the optimal combinations of TTFields with radiation or pharmacological therapies to expand the population of patients who may benefit from TTFields. For example, we believe that TTFields may be combined with radiation or chemotherapy to allow for dose reductions, leading to reduced toxicity while achieving the same or better treatment outcomes. |
| • | Continue to improve our TTFields delivery systems. We plan to continue to develop and enhance our TTFields delivery systems to improve performance and to provide the optimal patient experience across a variety of approved and potential clinical indications. We intend to seek FDA approval for the second generation of Optune, which is less than half the weight and size of the current version. |
Cancer and solid tumors
Cancer is a disease characterized by unregulated growth of abnormal cells. Normal cells are preprogrammed with genetic information informing them of their function throughout the body. Cells reproduce using a process called mitosis, creating genetic copies of themselves. When normal cells become damaged, the body uses several repair processes to restore function. When normal cells cannot be repaired, they undergo a process of preprogrammed cell death, or apoptosis. Cancer cells avoid the body’s repair and apoptosis pathways and undergo uncontrolled rapid replication, which can lead to the formation of a tumor.
Today, solid tumors are typically treated using one or a combination of three principal treatment modalities—surgery, radiation and pharmacological therapies.
| • | Surgery—For solid tumors, surgical excision of the primary tumor is the most frequently employed form of tumor therapy. If the tumor is detected early enough and confined to a single organ, surgery may serve to remove the entire tumor and be curative. More often, surgery is used to reduce the size of a tumor prior to the initiation of additional treatment modalities, such as radiation and pharmacological therapies. |
| • | Radiation—Radiation is a non-invasive solid tumor therapy that transfers energy to tissues, causing damage to biologically important molecules such as DNA. Radiation kills tumor cells or slows their growth when |
76
Table of Contents
| delivered at high doses. Radiation may be given before, during or after surgery and may also be given before, during or after other tumor treatments to shrink the tumor or to kill tumor cells that might remain. While effective in killing most solid tumors, radiation injures healthy tissues, leading to numerous potential toxic side effects, including bone marrow suppression and inflammation of the esophagus and the mucosal lining of the gastrointestinal tract. These side effects typically result in significant weakening of the patient, discomfort, nausea, vomiting and immune compromise. In the brain, the cumulative dose of radiation is limited due to long-term side effects on normal brain function, including cognitive decline and memory impairment. Advances in radiation have focused largely on limiting the exposure of healthy tissues to toxic radiation. |
| • | Pharmacological therapies—Chemotherapy, one of the earliest pharmacological tumor therapies, kills rapidly proliferating tumor cells by interacting with specific molecular pathways critical to DNA replication or cell reproduction, including mitosis. Chemotherapy acts in an indiscriminate manner, killing all dividing cells, including healthy as well as tumor cells, leading to a variety of side effects. In addition, many solid tumors reside in areas of the body that have poor accessibility to systemically delivered chemotherapies. For example, in GBM, the blood-brain barrier reduces the access of chemotherapies to the tumor. |
Newer agents, such as targeted cancer therapies, are intended to block the growth and spread of cancer by interfering with specific molecules, or molecular targets, in cancer cells. Targeted therapies are designed to kill cancer cells that express the target while attempting to spare normal cells. Targeted therapies have added some benefit to the treatment of solid tumors. However, they have a significant limitation because normal cells often possess variations of the target, leading to damage to healthy tissues. Targeted therapies are often used in combination with one or more traditional chemotherapies to increase efficacy, which can lead to expanded side effects. In addition, tumor cells often develop resistance to both chemotherapies and targeted therapies through mutations, which renders these therapies less effective or ineffective over time.
More recently, immuno-oncology has emerged as a promising therapy for solid tumors. This approach aims to harness a patient’s immune system to fight solid tumors. Similar to the advancements in the development of targeted therapies for cancer, it is widely anticipated that immuno-oncologic approaches to treat solid tumors will be used in combination with existing and newer treatments to increase their overall effectiveness and overcome tumor resistance pathways.
Surgery, radiation and pharmacological therapies have been used as monotherapies or in combination to treat solid tumors for over 100 years. While significant advancements have been made to each of these treatment modalities, they still represent an imperfect solution to cancer care in terms of efficacy and side effects.
Tumor treating fields (TTFields)
TTFields consist of low-intensity, alternating electric fields that operate at intermediate frequencies, changing polarity hundreds of thousands of times a second. TTFields’ anti-mitotic mechanism of action is based on disruption of key electrically charged molecules essential to the mitotic process by which all cells divide. Interference with these key molecules leads to cell death through multiple pathways.
Cell reproduction begins with the replication of the cell’s genetic content. The cell’s DNA is copied to produce two identical copies. Following DNA replication, the cell enters mitosis, a well orchestrated series of events that lead to the formation of two identical progeny of the original reproducing cell, called daughter cells. Each of the newly formed daughter cells has all the necessary molecular and genetic content to reproduce itself.
At the early stages of mitosis, a geometrically organized set of molecular strands or ropes is formed at two opposing ends of the cell, by self-assembly of many thousands of molecules called tubulin dimers. This mitotic spindle acts as a molecular motor to move pairs of exact copies of DNA to the equator of the cell. After the DNA is organized in one plane in the middle of the cell, the mitotic spindle begins to shorten, pulling one copy of the
77
Table of Contents
DNA to each side of the reproducing cell. In parallel to the formation of the mitotic spindle, a circular band, known as a cytokinetic band, forms on the membrane exactly surrounding the DNA plane at the equator of the cell. The location of this band is determined by a group of molecules called septins that are guided by the cell to specific locations on the cell membrane during mitosis. Once the DNA has been pulled to the two opposing ends of the cell by the mitotic spindle, the cytokinetic band begins to contract, physically pinching the cell into an hour glass shape with a narrow bridge between the two forming cells, called the mitotic furrow. At the end of mitosis, the furrow narrows until the membrane between the two forming daughter cells is pinched apart and disconnects in a process called cytokinesis.
There are two well established physical processes that TTFields use to exert their anti-mitotic effect: alignment of large molecules with the direction of the applied field and physical displacement of molecules and organelles.
| • At early stages of mitosis, after the cell has made an exact copy of its genetic content, referred to as metaphase, the mitotic spindle is formed. The mitotic spindle acts as a group of molecular ropes that grab the two copies of the genetic content and pull each copy to an opposite side of the dividing cell. These ropes are formed by self-assembly of many thousands of identical molecules called tubulin dimers. Electrically, tubulin dimers have one highly positive end and one highly negative end. When TTFields are activated, the intracellular environment experiences a uniform electric field and tubulin dimers align with the field instead of with the mitotic spindle. This in turn does not allow the spindle to form properly, and the cells often cannot complete the division process. Cells that cannot complete division and remain arrested in mitosis will ultimately undergo programed cell death, known as apoptosis. In addition, the fact that the mitotic spindle does not form properly leads to improper separation of the two copies of the genetic content into two groups. If these cells do complete mitosis and split into two daughter cells, the genetic content may no longer be evenly divided between the daughter cells. The resulting daughter cells with incomplete genetic content can no longer replicate and will eventually die. |
The application of TTFields to the cell during metaphase aligns the tubulin dimers in the direction of the fields, disrupting the formation of the mitotic spindle, and leads to arrested mitosis and subsequent apoptosis | |
| • In order for the cell to split physically into two cells at the later stages of mitosis, referred to as anaphase, a cytokinetic band forms on the cell surface or membrane. This cytokinetic band must be placed exactly at the equator of the cell, so that when it contracts it will pinch the membrane into two identical daughter cells, each containing one exact copy of the same genetic material. The precise localization of this band depends on septins, which signal its location on the cell surface. Septins, like tubulin dimers, have one negative and one positive end, making them a target for TTFields. By rotating septins and aligning them in the direction of the field, TTFields lead to improper localization of the cytokinetic band, so that when the cell enters cytokinesis and the band receives a signal to contract, the cell is now torn into multiple small bubbles, or blebbing, instead of two equally sized daughter cells, leading to cell death. |
The application of TTFields to the cell during anaphase aligns septins in the direction of the fields, leading to improper formation of the cytokinetic band and subsequent membrane blebbing | |
78
Table of Contents
| • In cells that successfully proceed through the above-described stages of mitosis, the hourglass shape that forms when the cytokinetic band contracts at the cell equator causes the cell to experience a non-uniform electric field. In a non-uniform electric field many macromolecules and intracellular organelles experience electric forces pushing them toward the area of higher field intensity. During cytokinesis, the field intensity is highest at the center of the cell where the membrane is pinching off into two daughter cells, an area called the mitotic furrow. TTFields concentrate macromolecules and organelles toward the mitotic furrow, leading to structural disruption and cell death. |
The application of TTFields to the cell during anaphase and telophase, or cytokinesis, pushes charged and polar subcellular structures towards the mitotic furrow between the dividing cells, leading to cell destruction |
The intensity of the electric field within the cell depends on the frequency of the applied TTFields. Optimal tuning of field frequency to the specific cell type increases electric field intensity and non-uniformity within the cell, maximizing TTFields’ anti-mitotic effect. Pre-clinical data has shown that TTFields’ effects on different tumor cells are specific to the frequency of the electrical field. The optimal frequency for each cell type is dictated by physical properties of the cell, including cell size (which is inversely related to the optimal frequency), membrane thickness, resistance and capacitance. Tumor cells are typically a different size than the normal surrounding cells and may also exhibit other differences in membrane properties. We believe that the ability to frequency tune TTFields is a significant factor in our ability to deliver treatment to the solid tumor site without harming surrounding tissue and normal cell growth.
TTFields delivery
A TTFields delivery system includes a portable electric field generator, transducer arrays, rechargeable batteries and accessories. The electric fields are delivered through the non-invasive, insulated transducer arrays that are placed directly on the skin in the region surrounding the tumor. The therapy is designed to be delivered continuously throughout the day and night.
The portable field generator is designed to allow patients to go about their daily activities while receiving continuous cancer treatment. Transducer arrays are connected to the electric field generator to deliver therapy. Transducer arrays are made of ceramic discs with a very high dielectric constant that are capable of efficient delivery of TTFields into the body and incorporate precision temperature sensors designed to ensure safety. The self-adhesive transducer arrays are placed on the skin after shaving any hair in the treatment area. The sterile, single-use transducer arrays are changed when hair growth or hydrogel dissipation reduces array adhesion to the skin, which is typically two to three times per week for our GBM patients. Each battery provides two to three hours of therapy per charge. The field generator can be run from a standard power outlet for use when the patient is sleeping or stationary. We provide the patient with a specially designed bag to carry the electric field generator and a battery.
We plan to use the same field generator technology across all solid tumor indications for which TTFields are approved. We will specifically target individual solid tumor types by tuning TTFields to the appropriate frequency based on tumor cell size and adjusting the output power to treat the required tissue volume. Our transducer arrays have been developed and are in use, either commercially or clinically, for application on the head, chest and abdomen.
TTFields penetrate the entire volume of tissue between the arrays. Unlike other forms of energy, such as radiation, the strength of the fields does not attenuate over distance. The distribution of the field within a
79
Table of Contents
certain part of the body depends on the exact layout of the transducer arrays and the passive electrical properties, mainly resistance, of the different tissues between them. Physicians or company personnel optimize the placement for each patient using a proprietary software package called NovoTAL, based on morphometric measurements of the patient’s anatomy according to a recent MRI scan and the location of the tumor.
Benefits of TTFields
We believe TTFields offer a number of distinct benefits that will lead to its establishment as a principal solid tumor treatment modality alongside surgery, radiation and pharmacological therapies, including:
| • | Targeted effect on solid tumors. We believe TTFields have a targeted effect on dividing solid tumor cells and limited effect on healthy tissues due to their mechanism of action and regional delivery. |
| • | Acts only on mitotic cells. Based on our research, TTFields do not appear to damage non-mitotic cells since the highly charged tubulin and septin proteins, which the TTFields target, are not assembled when a cell is not in mitosis. In addition, the hourglass shape of cells undergoing cytokinesis is not seen in non-dividing cells. We believe this lack of impact on non-mitotic cells is a significant factor in TTFields’ mild side effect profile as a monotherapy and the limited incremental side effects when used in combination with other cancer treatment modalities. In contrast, radiation and chemotherapy do not differentiate well between healthy cells and rapidly dividing tumor cells, causing damage to healthy tissues. |
| • | Specific to a certain size. TTFields are tuned to target cells of a certain size and with specific membrane properties. Healthy cells in the tissues surrounding or adjacent to a tumor often have different sizes and/or membrane properties than the tumor cells themselves, which leads us to believe that these healthy dividing cells are only minimally affected by TTFields. In contrast, while many chemotherapies also target only dividing cells, they often do not differentiate between dividing cells in healthy surrounding tissues and tumor cells, leading to side effects like hair loss, mucositis and bone marrow suppression. |
| • | Regional delivery. TTFields are regionally delivered to the tumor site rather than systemically delivered throughout the body. As a result, the parts of the body not covered by TTFields are generally not affected, and no systemic toxicities have been observed to date. In contrast, chemotherapy generally is systemic. As it circulates throughout the body, it does not discriminate between healthy tissues and tumors, causing systemic side effects. |
| • | No known resistance or cumulative toxicity. Radiation and pharmacological therapies have well documented toxicity and related side effects, many of which are cumulative. Physicians typically observe maximum dose limits when treating their patients, restricting the total amount of therapy given over time. Radiation in the brain leads to cognitive decline and memory impairment over time when a certain dose is exceeded. No dose-limiting cumulative toxicity has been reported with TTFields and we believe the basic mechanism of action is unlikely to result in a cumulative toxic effect. In our GBM clinical trials, where TTFields has been provided to patients for as long as 24 months, patients have not reported any detrimental effect on cognition or memory. |
| • | Access to sanctuary sites. Certain organs in the body are considered sanctuary sites, since chemotherapy does not enter these organs at sufficiently efficacious doses. For example, chemotherapy doses are limited in the brain due to the blood brain barrier and in the pancreas due to stromal effects. TTFields are not delivered through the bloodstream and can be applied to both the brain and the pancreas, overcoming this known limitation of chemotherapy. |
80
Table of Contents
| • | Complementary to other treatment modalities. We believe TTFields may be combined with existing and future treatments for many solid tumors, offering the potential for more effective, safer treatment. In our pre-clinical and clinical experience to date, TTFields do not appear to potentiate the systemic toxicities of radiation or chemotherapy when administered in combination with either treatment. Also, pre-clinical evidence has shown that the combination of TTFields with radiation or chemotherapy may lead to additive or synergistic efficacy. For example, certain in vitro experiments using TTFields have suggested both additivity and synergism when used in combination with chemotherapy, as the presence of TTFields was shown to increase cancer cell sensitivity to chemotherapy from approximately 275 times to over 1,250 times depending on the mechanism of action of the particular chemotherapy. The upper end of this range was observed in testing with taxane-based chemotherapies. |
GBM—our first approved and commercialized indication
The first indication for TTFields that we pursued was GBM, the most common form of primary brain cancer. GBM is an aggressive disease for which there are few effective treatment options. Median overall survival in newly diagnosed patients is approximately 15 months with standard therapies. We believe that TTFields represents the first therapeutic advance in the treatment of patients with GBM in over 10 years. We received FDA approval for Optune in 2011 for use as a monotherapy treatment for adult patients (ages 22 and older) with recurrent GBM. In November 2014, we presented results from the protocol pre-specified interim analysis of our Phase 3 pivotal trial of TTFields for patients with newly diagnosed GBM. This analysis demonstrated a significant improvement in progression free survival and overall survival. We submitted a PMA supplement application with the FDA in April 2015 to expand our label for Optune to include the treatment of newly diagnosed GBM and our application was granted priority review status. In addition, Optune was recently approved in Japan for recurrent GBM and is a CE marked device approved for sale in the European Union and Switzerland for both recurrent and newly diagnosed GBM for adult patients (ages 18 and older).
GBM affects a relatively young population compared to other cancer types. The median age at diagnosis is approximately 55 years. GBM tends to occur more frequently in men than women by a ratio of about 3:2.
We estimate that approximately 12,500 people are diagnosed with GBM or tumors that typically progress to GBM in the United States each year. Of this population, we estimate that approximately 9,300 patients will be medically eligible for treatment with Optune for newly diagnosed GBM once approved. Of that population, we estimate that approximately 5,500 patients will be medically eligible for treatment with Optune for recurrent GBM.
We estimate that approximately 13,500 patients in the top five EU markets (France, Germany, Italy, Spain and the United Kingdom) are diagnosed with GBM or tumors that typically progress to GBM each year. Of this population, we estimate that approximately 10,000 patients will be medically eligible for treatment with Optune for newly diagnosed GBM. Of that population, we estimate that approximately 6,000 patients will be medically eligible for treatment with Optune for recurrent GBM.
We estimate that approximately 1,500 patients in Japan are diagnosed with GBM or tumors that typically progress to GBM each year. Of this population, we estimate that approximately 1,100 patients will be medically eligible for treatment with Optune for newly diagnosed GBM. Of that population, we estimate that approximately 650 patients will be medically eligible for treatment with Optune for recurrent GBM.
The last clinical trial to show a statistically significant survival benefit in GBM was published in 2005 when concomitant and adjuvant temozolomide was added to the prior standard of care of surgical resection, followed by adjuvant radiotherapy. The median overall survival for radiation was 12.1 months versus 14.6 months with radiation plus temozolomide. Since 2005, temozolomide has become the standard of care chemotherapy for newly diagnosed GBM and all subsequent Phase 3 GBM trials have included temozolomide in the control arm.
81
Table of Contents
Standard of care temozolomide in these Phase 3 trials has consistently shown a median overall survival of approximately 15 months and a median two-year survival of less than 30%. No significant advances in GBM patient survival have been made since 2005, and a significant unmet need to improve survival and quality of life remains.
GBM was an optimal initial target for TTFields because the tumor rarely metastasizes outside the brain, allowing us to evaluate the effect of TTFields on the entire scope of the disease. We began to evaluate the use of TTFields for the treatment of GBM in 2004. We initially ran a pilot clinical trial, EF-07, which included 10 recurrent GBM patients who were treated with TTFields alone as salvage therapy and 10 newly diagnosed GBM patients who were treated with a combination of TTFields and maintenance temozolomide after having undergone surgery and radiation with adjuvant temozolomide. Median time to disease progression for the recurrent GBM patients was 26.1 weeks and median overall survival was 62.2 weeks, more than double the reported medians of historical control patients. Median overall survival for the newly diagnosed GBM patients was greater than 39 months in the TTFields in combination with temozolomide arm versus 14.6 months for matched historical control patients who received maintenance temozolomide alone. As of December 1, 2014, four of the 20 patients are alive more than eight years after receiving TTFields in the EF-07 trial. Based on the promising results of the EF-07 trial, we conducted two randomized Phase 3 trials for TTFields in GBM:
| • | EF-11 to evaluate TTFields as a monotherapy for the treatment of patients with recurrent GBM, which we believe established clinical validation and enabled commercial proof of concept; and |
| • | EF-14 to evaluate TTFields in combination with temozolomide for the treatment of patients with newly diagnosed GBM, which we believe will transform the standard of care for patients with newly diagnosed and recurrent GBM. |
Our pivotal trial in recurrent GBM (EF-11)
We received FDA approval in 2011 to market Optune for use as a monotherapy treatment for adult patients with recurrent GBM. The FDA approved Optune based on the EF-11 trial, which was a randomized, active standard of care controlled Phase 3 pivotal clinical trial. While the trial did not achieve its primary endpoint of superiority, the trial results indicate that monotherapy treatment with Optune provides patients with clinically comparable extension of survival compared to chemotherapy and that patients treated with Optune alone had significantly fewer side effects and an overall better quality of life than patients treated with chemotherapy alone.
| • | Overview—The EF-11 trial was a multicenter, randomized (1:1), active controlled clinical trial of 237 adults with recurrent GBM. Participants received either TTFields as a monotherapy or the physician’s choice of chemotherapy. Chemotherapies chosen for the active control arm included mainly bevacizumab, nitrosureas and temozolomide. More than 80% of patients had failed two or more prior lines of chemotherapy and 20% of the patients had failed bevacizumab prior to enrollment. The primary endpoint for the trial was OS. The secondary endpoints included progression free survival at six months, or PFS6, radiological response rate, one-year survival rate, adverse event severity and frequency and quality of life. |
| • | Efficacy—Overall survival times for patients treated with TTFields alone and active chemotherapy were 6.6 months and 6.0 months, respectively (hazard ratio = 0.86; p=0.27). PFS was not significantly different between the groups and PFS6 was numerically higher in the TTFields arm (21.4% vs. 15.2%). The FDA determined that these results represented clinically comparable efficacy outcomes. The overall radiographic response rate was higher for the group treated with TTFields compared with the group treated with chemotherapy (14.0% vs. 9.6%, respectively; p=0.19). Three patients treated with TTFields had complete responses compared to no patients treated with active chemotherapy. A group of principal investigators from the trial published long-term follow-up data on the trial indicating that 8% of the TTFields-treated patients |
82
Table of Contents
| had a long-term survival at 48 months compared to no long-term survivors in the chemotherapy treated group. The EF-11 trial demonstrated that patient compliance is important for successful outcomes. Patients who used TTFields more than 75% of the time had a significant survival advantage compared to those who used it less than 75% of the time (median survival was 7.8 months compared to 4.5 months, respectively; p<0.05). |
| • | Safety and quality of life—Patients treated with TTFields experienced significantly fewer treatment-related adverse events than those treated with active chemotherapy. Specifically, there were significantly fewer hematological, infectious and gastrointestinal adverse events in the TTFields-treated patients than in those treated with chemotherapy. The most commonly reported side effect from the delivery of TTFields was a mild-to-moderate rash on the skin beneath the transducer arrays, which affected 16% of patients. Patients receiving TTFields reported better quality-of-life scores compared to patients treated with active chemotherapy. Importantly, patients reported better quality-of-life outcomes specifically related to cognitive and emotional functioning. |
Our commercial registry (PRiDe)
At the time of our initial commercial launch of Optune for recurrent GBM in 2011, we established a patient registry aimed at capturing information related to the use of TTFields in the real-world commercial setting, which we refer to as PRiDe. We collected Optune treatment data and OS data from all 457 recurrent GBM patients who commenced treatment with Optune in the United States between October 2011 and November 2013. Key findings from this peer-reviewed published data include:
| • | Compelling overall efficacy—Median OS was significantly greater with TTFields in PRiDe than in the EF-11 trial (9.6 months vs. 6.6 months; p=0.0003). OS rates were more than double for TTFields patients in PRiDe than in the EF-11 trial (one-year: 44% vs. 20%; two-year: 30% vs. 9%); |
| • | Efficacy correlated to compliance—Patients for whom compliance data was available (n=287) who used Optune more than 75% of the time (the recommended minimum is 18 hours per day) had a significant survival advantage compared to those who used it less than 75% of the time (median survival was 13.5 months compared to 4.0 months, respectively; p<0.0001); and |
| • | Consistent safety profile—No unexpected adverse events were detected in PRiDe. As in the EF-11 trial, the most frequent side effects were mild to moderate skin reactions associated with application of the transducer arrays. |
Our pivotal trial in newly diagnosed GBM (EF-14)
We began enrolling patients in the EF-14 Phase 3 pivotal trial in 2009 to study the efficacy and safety of TTFields in combination with temozolomide for the treatment of newly diagnosed GBM in comparison with temozolomide alone. The primary endpoint of the trial was PFS and a powered secondary endpoint was OS.
A protocol pre-specified interim analysis of the EF-14 Phase 3 pivotal trial was presented in November 2014. The interim analysis demonstrated that TTFields met both of the trial’s powered endpoints with significant extension of both PFS and OS. The interim analysis was conducted by the trial’s independent data monitoring committee on the first 315 patients, representing approximately 50 percent of the targeted trial population, with a minimum of 18 months follow-up. We submitted a PMA supplement application with the FDA in April 2015 to expand our label for Optune to include the treatment of newly diagnosed GBM and received priority review status. In May 2015, we submitted these results for peer review publication.
83
Table of Contents
Clinical trial design
The EF-14 Phase 3 pivotal clinical trial enrolled newly diagnosed GBM patients following completion of concomitant radiation with temozolomide. Patients were randomized 2:1 to receive either continuous TTFields in combination with monthly maintenance temozolomide or maintenance temozolomide alone. Randomization was stratified by extent of resection (biopsy, partial resection or gross total resection) and O(6)-methylguanine-DNA methyltransferase, or MGMT, methylation status, which are both known prognostic factors in newly diagnosed GBM. In prior clinical studies, positive MGMT status was correlated with better survival outcomes for newly diagnosed GBM patients treated with radiation and temozolomide. Upon disease progression or temozolomide toxicity, patients were allowed to change to a second line chemotherapy. Patients on TTFields continued to receive TTFields until the earlier of the second disease progression or 24 months. Analysis of PFS was performed in the intent-to-treat population. Analysis of OS was analyzed in a pre-specified as-treated population, which excluded 11 patients who crossed over at progression to receive TTFields in the commercial setting against protocol.
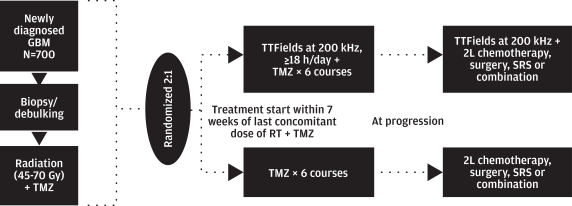
Definitions: TMZ= temozolomide; SRS= stereotactic radiosurgery; 2L= second line; RT= radiation therapy
Baseline characteristics
Baseline characteristics were balanced between the two groups. The median age was 58 years old for patients receiving temozolomide alone and 57 years old for patients treated with TTFields in combination with temozolomide. Sixty-six percent of the participants were male. The median Karnofsky performance score, a standard way of measuring the ability of cancer patients to perform ordinary tasks independently, was 90. The percentage of patients that had either a gross total resection or partial resection was 90% for patients receiving temolozomide alone and 89% for patients treated with TTFields in combination with temolozomide. Tumor tissue for MGMT testing was available for 72% of the patients; the MGMT methylation frequency was 38% and 41% for TTFields in combination with temozolomide and temozolomide alone arm, respectively. Ninety-five percent of the patients were Caucasian, and over 60% of the patients were treated in the United States. Tumor location in the brain was also comparable. The median time from diagnosis to randomization was 3.8 months in both arms. Median time from end of radiation to randomization was 36 and 38 days, respectively. Median time from randomization to initiation of TTFields was five days. Both PFS and OS were measured from the time of randomization, not the time of diagnosis.
84
Table of Contents
Clinical trial results
The interim analysis demonstrated that patients treated with TTFields in combination with temozolomide experienced a significant extension in PFS based on blinded central radiology review and lived significantly longer than patients treated with temozolomide alone:
| • | the two-year survival rate among patients treated with TTFields in combination with temozolomide, in the as-treated population, was 48% compared to 32% among patients treated with temozolomide alone (p=0.0058); |
| • | patients treated with TTFields, in combination with temozolomide, in the intent-to-treat population, demonstrated a statistically significant increase in PFS compared to temozolomide alone (median PFS of 7.2 months compared to 4.0 months, hazard ratio=0.62, p=0.001); and |
| • | patients treated with TTFields, in combination with temozolomide, in the as-treated population, demonstrated a statistically significant increase in OS compared to temozolomide alone (median OS of 20.5 months compared to 15.6 months, hazard ratio=0.66, p=0.004). |
The following graph presents PFS data in the intent-to-treat population from our interim analysis:
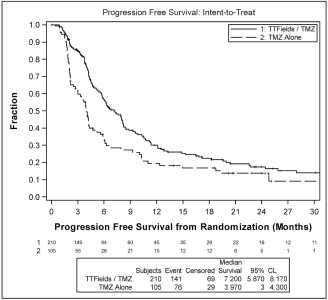
85
Table of Contents
The following graph presents OS data in the as-treated population from our interim analysis:
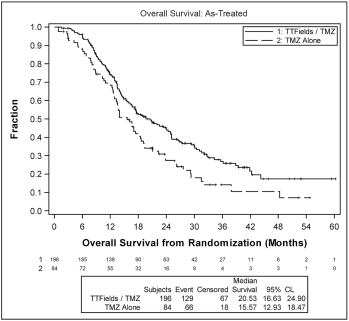
In order to test the effect on OS of crossover of patients from the temozolomide arm to receive TTFields, OS was also analyzed in the intent-to-treat population including all patients randomized according to their original randomization group. This sensitivity analysis showed that even when crossover patients were included, patients treated with TTFields in combination with temozolomide lived significantly longer than patients treated with temozolomide alone (median OS of 19.6 months compared to 16.6 months, hazard ratio=0.74, p=0.034).
The following graph presents OS data in the intent-to-treat population from our interim analysis:
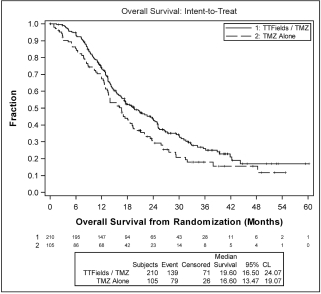
The significant extension of PFS and OS in patients receiving TTFields in the EF-14 trial was seen in all patient subgroups and was not specific to any prognostic subgroup or tumor genetic marker. PFS and OS were
86
Table of Contents
extended in patients with either MGMT methylated or unmethylated tumors. In addition, TTFields had no negative impact on patient quality of life, performance status or cognitive function.
Safety and quality-of-life results
The TTFields in combination with temozolomide arm was not associated with any significant increase in systemic toxicities compared with temozolomide alone. The overall incidence, distribution and severity of adverse events were similar in patients treated with TTFields in combination with temozolomide compared to those treated with temozolomide alone. The only notable exception was, as expected, a higher incidence of localized skin toxicity related to the skin beneath the transducer arrays in patients treated with TTFields. Some mild to moderate skin irritation was observed in 45% of patients, severe skin reaction (grade 3) was observed in 1% of patients. In addition, TTFields had no negative impact on patient quality of life, performance status or cognitive function.
The following graph presents the mean Karnofsky performance score of patients over time, indicating that patients’ ability to perform regular daily activities generally did not decline in both patient populations over time.
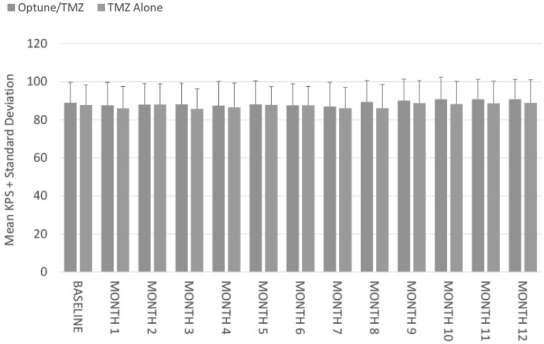
Definitions: TMZ=temozolomide; KPS= Karnofsky performance score.
87
Table of Contents
The following graph presents the mean Mini Mental State Exam (MMSE) of patients over time, indicating generally consistent cognitive function in both patient populations over time:
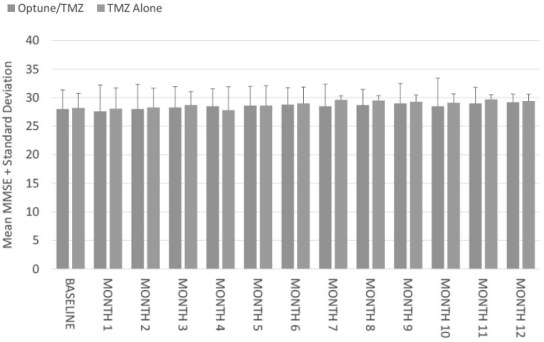
Definitions: TMZ=temozolomide; MMSE= Mini Mental State Exam.
Confirmatory and subgroup analyses
A confirmatory analysis of all 700 patients enrolled in the EF-14 trial was performed after an average follow up of approximately 12 months for all patients. This analysis, which was presented at ASCO in 2015, confirmed the findings of the interim analysis and validated that TTFields in combination with temozolomide extends both PFS and OS significantly compared to temozolomide alone. The significant extension of PFS and OS in patients receiving TTFields in combination with temozolomide in the EF-14 trial was seen in all patient subgroups and was not specific to any prognostic subgroup or tumor genetic marker. PFS and OS were extended in patients with either MGMT methylated or unmethylated tumors. The two-year survival rate among patients treated with TTFields in combination with temozolomide was 43% compared to 29% among patients treated with temozolomide alone.
88
Table of Contents
The following table presents details of the confirmatory and subgroup analyses for OS in the EF-14 trial:
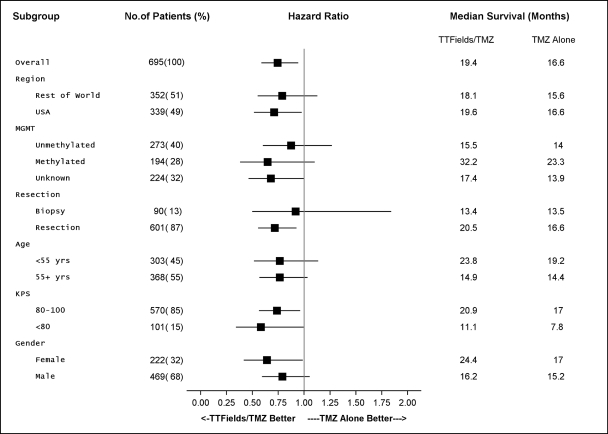
89
Table of Contents
The following table presents details of the confirmatory and subgroup analyses for PFS in the EF-14 trial:
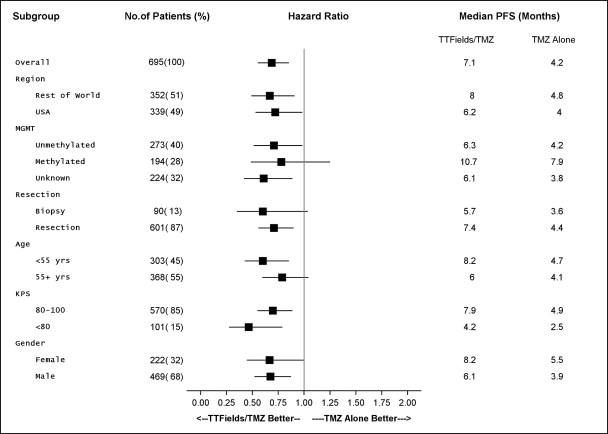
Definitions: TMZ = temozolomide; PFS = progression free survival; MGMT = O-6-methylguanine-DNA methyltransferase; KPS = Karnofsky performance score
We believe based on the reported EF-14 trial data that newly diagnosed patients treated with Optune in combination with temozolomide will have a longer treatment duration than what we have observed in the recurrent GBM population. Median treatment duration was 2.3 months for recurrent GBM patients in the EF-11 trial and 4.1 months in the PRiDe dataset. Median treatment duration increased to 9.0 months in the EF-14 trial.
Pending FDA review
We submitted a PMA supplement application with the FDA in April 2015 to expand our label for Optune to include the treatment of newly diagnosed GBM. The application was accepted for filing and review with a filing date of April 10, 2015. In May 2015, the FDA notified us that it had granted priority review status to the application. See “—Government regulation—Premarket approval pathway (PMA)” below for a discussion of the FDA PMA pathway.
Our commercial capabilities
We established commercial operations in the United States following FDA approval of Optune for the treatment of recurrent GBM in 2011. We believe that the majority of recurrent GBM patients in the United States are treated by physicians in approximately 200 large clinical centers, most of which are major academic teaching hospitals. This has allowed us to focus our commercial efforts and optimize our distribution and support
90
Table of Contents
services to a well defined customer base. As of June 30, 2015, we have trained physicians in the United States at more than 190 centers, including almost every leading cancer center in the United States.
We have built a commercial organization focused on marketing TTFields to physicians treating recurrent GBM. In addition to our commercial functions, we provide health care professionals with educational support and Optune training through a geographically distributed team of clinical science liaisons. We also field a dedicated team of device support specialists who are available locally throughout the United States to assist patients starting Optune and to resolve any technical difficulties patients may encounter. Patients also have access to 24/7 technical support by phone or e-mail as needed. We also work to secure reimbursement for Optune on behalf of patients. We believe our patient-centric approach will enable us to maximize our commercial opportunity by driving patient adoption and therapy compliance.
We plan to expand our commercial team to cover community oncology practices as well as the large cancer centers assuming we receive FDA approval of Optune for newly diagnosed GBM. We believe this will allow us to significantly grow the number of patients treated with Optune and the number of physicians and centers that we target. We believe our existing commercial and supply chain capabilities will allow us to satisfy initial market demand for Optune immediately following anticipated FDA approval. We plan to scale our commercial organization over time to maximize clinical adoption of Optune therapy.
We established commercial operations in Europe in 2014, focusing initially on Germany. We plan to expand to additional European countries in the future. As of June 30, 2015, we have trained physicians at over 20 centers in Germany and plan to expand our commercial efforts after securing reimbursement.
We recently initiated commercial operations in Japan in March 2015. Treatment for recurrent GBM patients in Japan occurs mainly at large academic hospitals. As of June 30, 2015, we have trained physicians at over 20 academic hospitals in Japan. We intend to focus our commercial efforts initially on privately insured patients and are working with the Japanese Ministry of Health, Labour and Welfare to secure Optune reimbursement for patients in Japan.
We believe that we can leverage our commercial capabilities for the GBM market in the United States, Europe and Japan to address additional future indications. We believe that cancer patients are increasingly seeking treatment at large oncology practices, which will allow our sales representatives to market for multiple indications within one practice.
Our clinical pipeline
Based on the results of our pre-clinical research, we have developed a pipeline strategy to advance TTFields through Phase 2 pilot and Phase 3 pivotal clinical trials across multiple solid tumor types, as described in greater detail below. In addition, we anticipate expanding our clinical pipeline over time to apply TTFields to additional solid tumor indications.
Brain metastases
We believe brain metastases will be our next label expansion beyond GBM. We have an ongoing European Phase 2 trial, or the COMET trial, in brain metastases originating from NSCLC, and we plan to open a Phase 3 trial, or the METIS trial, in early 2016 in the United States subject to final protocol approval by the FDA. Metastatic cancer is cancer that has spread from the place where it first started to another place in the body. In metastasis, cancer cells break away from where they first formed (the primary cancer), travel through the blood or lymph system, and form new tumors (the metastatic tumors) in other parts of the body.
The exact incidence of brain metastases is unknown because no national cancer registry documents brain metastases, but it has been estimated that 98,000 to 170,000 new cases are diagnosed in the United States
91
Table of Contents
each year. Approximately 70% of brain metastases are seeded from primary breast cancer, NSCLC or melanoma. Brain metastases cause an estimated 20% of all cancer deaths in the United States annually.
As with GBM, brain metastases are commonly treated with a combination of surgery and radiation. Chemotherapy is often given for the primary tumor; however, many chemotherapy agents do not cross the blood brain barrier and are thus ineffective in the treatment of brain metastases. When brain metastases appear, they are either surgically resected or irradiated using SRS when possible. Whole brain radiation therapy, or WBRT, although effective in delaying progression or recurrence of brain metastases when given either before or after SRS, is associated with neurotoxicity leading to the development of dementia with a significant decline in cognitive and emotional functioning. Thus, WBRT is often delayed until later in the disease course and is often used as a last resort. This practice results in a window of unmet need after localized surgery and SRS are used and before WBRT is administered to delay or prevent additional seeding of brain metastases.
We believe TTFields could be an effective treatment for patients with brain metastases. We have published pre-clinical data showing TTFields can prevent metastatic seeding in vivo. Based on these pre-clinical results and the established safety and efficacy of TTFields in GBM, we commenced the COMET trial examining TTFields as a monotherapy compared to supportive care alone after SRS in patients with brain metastases originating from NSCLC. The primary endpoint of the trial will be local disease control in the brain. We expect the METIS trial to commence in 2016, subject to final protocol approval by the FDA. With an expected 18 months of follow-up, we anticipate Phase 3 data will be available for presentation in 2019.
Non-small cell lung cancer (NSCLC)
We have completed a Phase 2 trial in advanced NSCLC and are planning a randomized Phase 3 trial in NSCLC, or the LUNAR trial. Lung cancer is the leading cause of cancer-related death in the United States. NSCLC accounts for approximately 85% of all lung cancers. The incidence of NSCLC in the United States is approximately 185,000 new cases annually. Approximately 25 to 30% of all lung cancer patients have squamous histology, which is more resistant to chemotherapy. We have received approval to market our NovoTTF-100L system, our TTFields delivery system designed for the treatment of NSCLC (including squamous histology), in combination with standard of care chemotherapy in Europe, based on the efficacy results from our clinical trials completed to date, as described further below.
Physicians use different combinations of surgery, radiation and pharmacological therapies to treat NSCLC, depending on the stage of the disease. Surgery, which may be curative in a subset of patients, is usually used in early stages of the disease. Unfortunately, most patients are likely to relapse sometime after initial surgery. For patients with locally advanced NSCLC, the standard of care is chemotherapy and radiation with or without surgery. Most frequently, however, NSCLC patients are diagnosed at an advanced stage when the cancer has spread outside of the lungs, leaving chemotherapy as the only treatment option. Despite the many advances in chemotherapy made in recent decades, treatment outcomes remain inadequate, especially for patients with the squamous histology, which clinical results suggest may be resistant to or cannot be treated with therapies such as bevacizumab and pemetrexed.
Phase 2 trial design
Based on our pre-clinical findings, we conducted a Phase 2 trial to evaluate the safety and efficacy of TTFields in the treatment of advanced NSCLC (both squamous and adenocarcinoma histologies). Results of this study were published in 2013. To treat NSCLC, we developed the NovoTTF-100L system to deliver TTFields regionally to the lungs, mediastinum and liver. The delivery system output settings differ in frequency and intensity from Optune based on the findings of our pre-clinical studies in NSCLC. The pilot study was a single-arm, open-label,
92
Table of Contents
historically-controlled, multi-center trial. The primary endpoints of the trial were safety and PFS. The secondary endpoints were OS and response rates. Results of the pemetrexed Phase 3 FDA registration trial were used as historical controls in this trial.
A total of 42 patients were recruited to the study at four centers in Switzerland with a minimum follow-up of six months. Before entering the trial, each patient’s disease had progressed after chemotherapy treatment. Patients were 18 years of age or older (median 63 years old) with an Eastern Cooperative Oncology Group, or ECOG, performance of 0-2. ECOG is a standard scoring system of a patient’s general health and well-being running from zero (full health) to five (death). Patients with significant co-morbidities, pregnancy or pacemakers were excluded. All patients were treated with the NovoTTF-100L system in combination with standard of care pemetrexed chemotherapy.
Phase 2 trial results
Efficacy results based on 41 evaluable patients (n=34 non-squamous histology, including 32 adenocarcinoma histology; n=7 squamous histology) showed both PFS and OS for patients receiving TTFields in combination with pemetrexed increased compared to historical control data for pemetrexed alone. Median PFS in the TTFields-treated group was 6.5 months (compared to 2.9 months in pemetrexed historical controls) and median OS was 13.8 months (compared to 8.3 months in historical controls). OS was essentially the same for patients with adenocarcinoma and squamous NSCLC when treated with TTFields and pemetrexed concomitantly (12.8 months and 13.8 months, respectively). As pemetrexed is now known to be inactive in squamous NSCLC, we believe that the OS effect seen in patients in this study with this subtype of lung cancer is due to TTFields alone. Based on these data, the investigators concluded that TTFields in combination with pemetrexed was well tolerated and did not lead to an increase in toxicity. Finally, the data suggests potentially higher efficacy of TTFields in combination with pemetrexed compared to pemetrexed alone.
Adverse events reported in this combination study were comparable to those reported with pemetrexed alone, suggesting minimal added toxicities due to TTFields. TTFields applied to the chest did not cause arrhythmias or other cardiac or pulmonary toxicity for patients in this study. The only delivery system-related toxicities were mild-to-moderate local rash beneath the transducer arrays, which was seen in most of the patients. This condition was managed by topical corticosteroids and transducer array relocation. In persistent cases, either oral corticosteroids were used or transient treatment breaks were made for several days.
Planned Phase 3 trial
Our planned LUNAR trial will examine TTFields in combination with a platinum-based chemotherapy and a taxane versus a platinum-based chemotherapy and a taxane alone as a first-line therapy for the squamous histology of NSCLC. Paclitaxel is a taxane that interferes with microtubule function during mitosis. We expect the LUNAR trial to commence in 2016, subject to final protocol approval by the FDA. With an expected 18 months of follow-up, we anticipate Phase 3 data will be available for presentation in 2019.
Pancreatic cancer
We have an ongoing Phase 2 trial in pancreatic cancer, or the PANOVA trial, with 20 patients currently enrolled, and we plan to open a Phase 3 trial if the results from our PANOVA trial are promising. Pancreatic cancer is one of the most lethal cancers, as most cases are diagnosed once the cancer is at a late stage and/or has metastasized to other parts of the body. It is the fourth most frequent cause of death from cancer in the United States and is responsible for 7% of all U.S. cancer-related deaths. In contrast to the decrease in mortality from other cancers over the past decade, pancreatic cancer death rates have been slowly increasing in the United
93
Table of Contents
States. Pancreatic cancer prognosis remains very poor, with a five-year survival of less than 6%. The National Cancer Institute estimated that, in 2015, there will be approximately 49,000 new cases of pancreatic cancer diagnosed and approximately 40,000 deaths in the United States from pancreatic cancer.
The PANOVA trial commenced in the first quarter of 2014, examining TTFields in combination with the chemotherapy drug gemcitabine for advanced pancreatic adenocarcinoma. The trial is designed to test the feasibility, safety and preliminary efficacy of TTFields in combination with gemcitabine. We completed enrollment of the first patient cohort in the first quarter of 2015. Following the approval of nab-paclitaxel, a taxane-based chemotherapy, for the treatment of advanced pancreatic cancer, we expanded this study to include 20 additional patients that will be treated with TTFields in combination with nab-paclitaxel and gemcitabine. We expect to finish enrollment of the second patient cohort study in 2016. We anticipate Phase 2 data will be available for presentation in 2016 and 2017 for the first and second patient cohorts, respectively.
Ovarian cancer
We have an ongoing Phase 2 trial in ovarian cancer, or the INNOVATE trial, and plan to open a Phase 3 trial if the results from our INNOVATE trial are promising. Ovarian cancer is the fifth most common cause of cancer death in women in the United States. The National Cancer Institute estimated that in 2015, there will be approximately 21,000 new cases of ovarian cancer diagnosed and approximately 14,000 deaths in the United States. Ovarian cancer incidence increases with age, and the median age at time of diagnosis is 63 years old. The five-year survival rate is 44%, and the majority of patients present at advanced stage with 60% having metastatic disease.
In October 2014, we commenced the INNOVATE trial examining TTFields in combination with weekly paclitaxel for recurrent ovarian cancer. The INNOVATE trial is designed to test the feasibility, safety and preliminary efficacy of TTFields in combination with paclitaxel and is expected to enroll 30 patients. We expect to complete enrollment in 2016 and we anticipate Phase 2 data will be available for presentation in 2017.
Mesothelioma
We have an ongoing Phase 2 trial in mesothelioma, or the STELLAR trial. Malignant mesothelioma is a rare thoracic solid tumor cancer that occurs in approximately 3,000 patients in the United States annually. Asbestos exposure has been strongly associated with the development of mesothelioma, which may occur many years following the exposure. The prognosis of mesothelioma patients is very poor, with a median OS of approximately 11 months in most reported studies. Mesothelioma is often limited to the thoracic cavity and progresses regionally making it an attractive target for TTFields.
We commenced the STELLAR trial in mesothelioma in the first quarter of 2015. The STELLAR trial is an open-label trial designed to test the efficacy and safety of TTFields in combination with pemetrexed combined with cisplatin or carboplatin in patients with unresectable, previously untreated malignant mesothelioma compared to a historical, published control. The STELLAR trial is anticipated to enroll 80 patients from Germany, Italy and Poland. We expect to finish enrollment of the trial in 2017. We anticipate Phase 2 data will be available for presentation in 2018.
Our engineering developments
We developed Optune, formerly known as NovoTTF-100A, for GBM, and NovoTTF-100L for NSCLC, and we are developing additional delivery systems for other indications in our pipeline. We have also developed our propriety NovoTAL transducer array layout planning software.
94
Table of Contents
Given the opportunity for TTFields in treating a wide variety of solid tumors, including GBM and other solid tumor cancers we are investigating, we continue to develop and enhance our TTFields delivery systems.
We have recently completed development of improved field generator circuitry, which minimizes power loss and requires a smaller battery. We have incorporated these advancements into a re-designed second generation Optune delivery system, which is less than half of the size and weight of the current system, with improved functionality for physicians and patients. FDA approval to use the second generation Optune device for GBM will require a PMA supplement application, which will include a thorough review of design, verification and validation documentation and complete manufacturing files. In addition, it is possible that the FDA will require a human factors study (user interface). It is also possible that the FDA may require clinical data, although we do not expect that clinical data will be required, as the indications for use, patient contacting element (transducer arrays) and the treatment itself have not changed. In Europe, the second generation Optune device for GBM is CE-marked and we anticipate launching in Europe by the end of 2015.
We also plan to continue to improve the NovoTAL transducer array layout planning software to allow more flexibility and ease of use by prescribing physicians.
Manufacturing
We have established flexible capacity in our principal manufacturing supply chain while driving costs down. We outsource production of all of our system components to qualified partners. Disposable transducer array manufacturing, the dominant activity in our manufacturing supply chain, includes several specialized processes. Production of the durable system components follows standard electronic medical device methodologies.
We have formal supply agreements with our third-party manufacturing partners. We hold safety stocks of single source components to protect our production capacity.
We currently source the ceramic discs used in the transducer arrays for Optune from Exelis, which is our single-source
supplier for these components. We do not currently have alternate suppliers for the ceramic discs, but we are in the process of identifying a second source supplier.
Our current agreement with Exelis continues through July 21, 2017, following
which time the agreement will automatically renew for up to three successive two-year periods unless either we provide timely written notice of non-renewal (for any reason) or Exelis provides timely written notice of non-renewal (if we fail to
satisfy certain minimum purchase requirements). We currently expect that this agreement will be renewed. In addition to certain other customary termination rights, Exelis can terminate this agreement with 90 days’ written notice if we
breach any of our material obligations under the agreement. Agreements with our other suppliers range from terms of four years to ten years and are terminable by either party, generally between 180 days’ and 12 months’ written notice. See
“Risk factors—We depend on single-source suppliers for some of our components. The loss of these suppliers could prevent or delay shipments of Optune, delay our clinical trials or otherwise adversely affect our business.”
Transducer array production represents the main area of focus for the supply chain team. Over the last twelve months, we and our third-party manufacturer have automated the assembly process for the main array components. We utilize standardized printed circuit board continuous flow production techniques including automatic handling and gluing of the ultra-high dielectric constant ceramic transducer elements. Automation has substantially increased capacity while lowering costs. We believe the automated line can be easily duplicated to add capacity as needed.
Billing and reimbursement
We provide Optune directly to patients in the United States and our currently active markets in Europe following receipt of a prescription order. We bear the financial risk of securing payment from patients and
95
Table of Contents
third-party payers. We bill $21,000 for each month that a patient uses Optune in the United States. Total cash payments of $12.4 million received during the six months ended June 30, 2015 were recorded as revenues for Optune therapy provided to patients in the current period and prior periods. These cash payments represent an average of approximately $14,700 for a month of use. The difference between billed and paid amounts consists of disputed underpayments, patient financial assistance and discounts. Additionally, during the period ended June 30, 2015, for each month of use, we paid approximately $600 in indirect taxes, primarily the federal medical device excise tax. This metric does not include our experience with patients covered by the Medicare fee-for-service program, as we have not received material payments from that program and the invoices remain open as we appeal the coverage denials. Physicians may separately bill patients or third-party payers for medical services related to initiating and managing treatment with Optune, including generating personalized transducer array layouts with NovoTAL. We currently employ reimbursement experts devoted to securing coverage for Optune from third-party payers.
For our U.S. patients covered by commercial third-party payers and privately administered government-sponsored plans, specifically Medicare Advantage, Tricare (U.S. military) and federal employee plans, we have been successful in securing coverage for approximately 99% of these patients as of June 30, 2015. Approximately 25% of our active U.S. patients were beneficiaries of the Medicare fee-for-service program as of June 30, 2015. The Medicare fee-for-service program has denied coverage for our claims to date, and we are actively appealing these coverage denials.
We have the ability to contract directly with commercial third-party payers in the United States to set the payment terms for our delivery systems. We will continue to pursue direct contracts with healthcare payers in the United States as part of our overall commercial launch effort after potential FDA approval for newly diagnosed GBM.
The Centers for Medicare and Medicaid Services (CMS) have issued Healthcare Common Procedure Coding System (HCPCS) Level II codes that are specific to our delivery systems. The codes are E0766 “Electrical stimulation device used for cancer treatment, includes all accessories, any type” and A4555 “Electrode/transducer for use with electrical stimulation device used for cancer treatment, replacement only.” We bill payers for a single monthly fee at the start of each month of therapy using the E0766 code and the A4555 code is used as a non-billable tracking code by certain payers.
We started commercial activity in Germany in 2014. We have hired personnel with expertise in reimbursement to support the launch of Optune. We are able to bill healthcare payers in Germany for individual cases currently. We are also pursuing defined coverage and pricing terms with the German healthcare payer industry associations, as well as the relevant regulatory bodies. In Japan we are preparing our application to the MHLW to secure a defined reimbursement rate for Optune. We have initiated a small commercial launch in Japan, and until we secure Japanese Ministry of Health, Labour and Welfare reimbursement approval, our launch efforts will focus on the privately insured patient population.
Intellectual property
As of June 30, 2015, our patent portfolio consists of a total of 52 issued patents, including 36 issued U.S. patents, two issued EU patents, one issued Canadian patent, eight issued Japanese patents and five issued Chinese patents. The U.S. patents have expected expiration dates between 2021 and 2031. The EU, Canadian, Japanese and Chinese patents have expected expiration dates between 2021 and 2026. We have also filed over 30 additional patent applications that, if issued, may protect aspects of our platform beyond 2031 in the United States and beyond 2026 in Europe, Canada, Japan and China.
In addition to our patent portfolio, we further protect our intellectual property by maintaining the confidentiality of our trade secrets, know-how and other confidential information. Given the length of time and
96
Table of Contents
expense associated with bringing delivery systems candidates through development and regulatory approval to the market place, the healthcare industry has traditionally placed considerable importance on obtaining patent protection and maintaining trade secrets, know-how and other confidential information for significant new technologies, products and processes.
Our policy is to require each of our employees, consultants and advisors to execute a confidentiality agreement before beginning their employment, consulting or advisory relationship with us. These agreements generally provide that the individual must keep confidential and not disclose to other parties any confidential information developed or learned by the individual during the course of their relationship with us except in limited circumstances. These agreements also generally provide that we own, or the individual is required to assign to us, all inventions conceived by the individual in the course of rendering services to us.
On February 10, 2015, we entered into a settlement agreement, or the Settlement Agreement, with the Technion, whereby we agreed to resolve certain potential disputes among us, the Technion and Professor Yoram Palti, our Chief Technology Officer and a member of our board of directors, arising out of certain intellectual property that Professor Palti developed while affiliated with the Technion and that Professor Palti has assigned to us. In settlement of these potential disputes, we agreed to pay the Technion an aggregate of $7.5 million, including $1.0 million that was paid on the date of the agreement, an additional $1.0 million that will be payable within five business days of the completion of this offering and an additional $5.5 million that will be payable within five business days (1) if we achieve $250.0 million of cumulative net sales since inception at the end of any given quarter or (2) upon consummation of an M&A transaction, which includes any merger to the extent it involves a change of control, the sale of all or substantially all of our assets or shares, the sale of or exclusive license to our intellectual property or a similar transaction. The $1.0 million payable in connection with the completion of this offering is part of our expected use of proceeds. See “Use of proceeds.”
In addition, pursuant to the terms of the Settlement Agreement, we issued 1,005,210 ordinary shares to the Technion, and further granted the Technion an option to acquire an additional 1,005,210 ordinary shares, which is exercisable at any time until the first to occur of (1) 12 months following this offering and (2) immediately prior to the sale of the company for cash or publicly traded stock. There is no exercise price on this option, and to date this option has not been exercised; however, the Technion will have until 12 months after the completion of this offering to exercise its option. No royalties are owed to the Technion or Professor Palti.
In 2005, we granted an exclusive license to a third party, NovoBiotic LLC, to certain of our key intellectual property for use outside the field of oncology. We are not entitled to any future revenues from this license.
We may be unable to obtain, maintain and protect the intellectual property rights necessary to conduct our business and may be subject to claims that we infringe, misappropriate or otherwise violate the intellectual property rights of others. For more information see “Risk factors—Risks relating to intellectual property.”
Competition
The market for cancer treatments is intensely competitive, subject to rapid change and significantly affected by new product and treatment introductions and other activities of industry participants. The general bases of competition are overall effectiveness, side effect profile, availability of reimbursement and general market acceptance of a product as a suitable cancer treatment.
TTFields is our proprietary method for treating cancer. As such, there is no direct competition from other TTFields delivery systems. We believe our intellectual property rights would provide an obstacle to the introduction of TTFields delivery systems by a competitor, and we intend to protect and enforce our intellectual property. In addition, even after the expiration of our U.S. patents, potential market entrants applying low-
97
Table of Contents
intensity, alternating electric fields to solid tumors in the United States will have to undertake their own clinical trials and regulatory submissions to prove equivalence to TTFields, a necessary step in receiving regulatory approvals for a competing product.
When used as a monotherapy for solid tumor cancers, TTFields competes with a number of existing cancer treatment alternatives, including surgery, radiation and pharmacological therapies. These treatments have been developed, commercialized and established in the market by large, well capitalized companies with significantly more market share and resources than we have. We believe that our direct competition is limited because we intend to use TTFields in combination with cancer treatments offered by other oncology companies.
We also intend to study the use of TTFields in combination with new entrants in the market, including new pharmacological agents such as PD-1 inhibitors and others in the field of immuno-oncology. In this regard, we expect to be less susceptible to competition from such emerging new pharmacological agents.
Government regulation
Our delivery systems and operations are subject to extensive regulation by the FDA and by agencies and notified bodies of the countries or regions in which we develop and market our delivery systems. In addition, our delivery systems must meet the requirements of a large and growing body of international standards that govern the pre-clinical and clinical testing, manufacturing, labeling, certification, storage, recordkeeping, advertising, promotion, export and marketing and distribution, among other things, of TTFields and our delivery systems.
Advertising and promotion of medical devices, in addition to being regulated by the FDA, are also regulated by the Federal Trade Commission and by state regulatory and enforcement authorities. Recently, promotional activities for FDA-regulated products of other companies have been the subject of enforcement action brought under healthcare reimbursement laws and consumer protection statutes. In addition, under the federal Lanham Act and similar state laws, competitors and others can initiate litigation relating to advertising claims. In addition, we are required to meet regulatory requirements in countries outside the United States, which can change rapidly with relatively short notice.
Our research, development and clinical programs, as well as our manufacturing and marketing operations, are subject to extensive regulation in the United States and other countries. Most notably, all of our delivery systems sold in the United States are subject to the Federal Food, Drug, and Cosmetic Act, or the FDCA, as implemented and enforced by the FDA. Marketing our delivery systems sold in the United States requires FDA approval. Foreign countries may require similar or more onerous approvals to manufacture or market these delivery systems.
Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other regulatory authorities, which may result in any number of regulatory enforcement actions.
Food and Drug Administration
The FDA regulates the development, testing, manufacturing, labeling, storage, recordkeeping, promotion, marketing, distribution and service of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. In addition, the FDA regulates the export of medical devices manufactured in the United States to international markets and the importation of medical devices manufactured abroad.
The FDA governs the following activities that we perform or that are performed on our behalf:
| • | product design, development and manufacture; |
98
Table of Contents
| • | product safety, testing, labeling and storage; |
| • | record keeping procedures; |
| • | product marketing, sales and distribution; and |
| • | post-marketing surveillance, complaint handling, medical device reporting, reporting of deaths, serious injuries or device malfunctions and repair or recall of products. |
We have registered three of our facilities with the FDA. The FDA has broad post-market and regulatory enforcement powers. We are subject to announced and unannounced inspections by the FDA to determine our compliance with the Quality System Regulation, or QSR, and other regulations and these inspections include the manufacturing facilities of our suppliers.
FDA’s premarket clearance and approval requirements
Unless an exemption applies, before we can commercially distribute medical devices in the United States, we must obtain, depending on the type of device, either prior 510(k) clearance or PMA from the FDA. The FDA classifies medical devices into one of three classes. Devices deemed to pose lower risks are placed in either class I or II, which typically requires the manufacturer to submit to the FDA a premarket notification requesting permission to commercially distribute the device. This process is generally known as 510(k) clearance. Some low-risk devices are exempted from this requirement. Devices deemed by the FDA to pose the greatest risks, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device, are placed in class III, requiring PMA approval.
Premarket approval (PMA) pathway
Optune, which is the only delivery system we have marketed in the United States, is classified as a Class III device as it is deemed a life-sustaining device. Accordingly, we were required to receive PMA approval for Optune, which the FDA granted in April 2011 for the treatment of recurrent GBM in adult patients. We submitted a PMA supplement application with the FDA in April 2015 to expand our label for Optune to include the treatment of newly diagnosed GBM. We expect that we will be required to receive PMA approval for future indications (and the applicable delivery systems for such indications) using TTFields.
A PMA must be supported by extensive data, including from technical tests, pre-clinical studies and clinical trials, manufacturing information and intended labeling to demonstrate, to the FDA’s satisfaction, the safety and effectiveness of a medical device for its intended use. During the PMA review period, the FDA will typically request additional information or clarification of the information already provided. Also, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the manufacturing facility or facilities to ensure compliance with QSRs. Prior to approval of the Optune PMA for the treatment of recurrent GBM, we and our critical component suppliers were each inspected by the FDA.
New PMAs or PMA supplements are required for modifications that affect the safety or effectiveness of our delivery systems, including, for example, certain types of modifications to a delivery system’s indication for use, manufacturing process, labeling and design. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require any or as extensive clinical data as the original PMA required, or the convening of an advisory panel. The FDA requires a company to make the determination as to whether a new PMA or PMA supplement application is to be filed. If a company determines
99
Table of Contents
that neither a new PMA or PMA supplement application is required for modifications, it must nevertheless notify the FDA of these modifications in its PMA Annual Report. The FDA may review a company’s decision and require the filing of an application.
We have received approval for a number of PMA supplements since approval of the PMA for recurrent GBM, including for modifications to Optune’s electric field generator, transducer arrays, software, manufacturing processes and labeling. Most recently, we submitted a PMA supplement application with the FDA to expand our label for Optune to include the treatment of newly diagnosed GBM. We also intend to file a PMA supplement application for our next-generation delivery system for Optune, as described under “—Our engineering developments.” Future modifications may be considered by us as the need arises, some of which we may deem to require a PMA supplement application and others to require reporting in our PMA Annual Report.
Clinical trials
Clinical trials are generally required to support a PMA. Such trials generally require an investigational device exemption application, or IDE, approved in advance by the FDA for a specified number of patients and study sites, unless the product is deemed a nonsignificant risk device eligible for more abbreviated IDE requirements. Clinical trials are subject to extensive monitoring, recordkeeping and reporting requirements. Clinical trials must be conducted under the oversight of an institutional review board, or IRB, for the relevant clinical trial sites and must comply with FDA regulations, including those relating to good clinical practices. To conduct a clinical trial, we also are required to obtain the patients’ informed consent in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. We, the FDA or the IRB could suspend a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and efficacy of the device or may otherwise not be sufficient to obtain FDA approval to market the product in the United States.
Post-approval studies are also typically required as a condition of PMA approval to demonstrate reasonable assurance of safety and effectiveness. Such studies are conducted in the post-market setting with the approved device, often to address the long-term use of the device or other discrete questions that may have been raised based on the clinical data from the IDE clinical study. The FDA required a post-approval study as a condition of approval for Optune for recurrent GBM. We have obtained approval of the protocol for this study and are currently enrolling patients.
Foreign approvals and CE mark
Sales and marketing of medical devices outside of the United States are subject to foreign regulatory requirements that vary widely from country to country. These include the requirement to affix a CE mark to our medical devices in the European Union. Whether or not we have obtained FDA approval, our delivery systems must be subject to conformity assessment procedure in which a notified body can be involved. Apart from low risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue a declaration of conformity based on a self-assessment of the conformity of its products with the Essential Requirements laid down in the Medical Devices Directive, a conformity assessment procedure requires the intervention of a notified body. The notified body typically audits and examines products’ technical file and the quality system for the manufacture, design and final inspection of our devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant Essential Requirements or the quality system requirements laid down in the relevant Annexes to the Medical Devices Directive. Following the issuance of this CE Certificate of Conformity, we can draw up a declaration of conformity and affix the CE mark to the delivery systems covered by this CE Certificate of Conformity and the declaration of conformity. The time required to CE
100
Table of Contents
mark our delivery systems or to obtain approval from other foreign authorities may be longer or shorter than that required for FDA approval. Pursuant to a mutual recognition agreement, our products bearing a CE mark may be exported to Switzerland. In the European Union, a clinical study must receive a positive opinion from a local ethics committee and approval from the competent authority in the applicable EU member states in which the clinical study is conducted. When a clinical study relates to a CE marked medical device that will be used as part of the study according to its CE mark intended purpose, the approval of the competent authorities is not required. In Japan, we must obtain approvals from the Japanese Ministry of Health, Labour and Welfare to market our delivery systems. The foreign regulatory approval process includes all the risks associated with FDA regulation, as well as country-specific regulations.
Pervasive and continuing regulation
After a device is placed on the market, numerous regulatory requirements apply. These include:
| • | product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action; |
| • | QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process; |
| • | labeling regulations and FDA and equivalent foreign competent authority prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication; |
| • | approval of product modifications that affect the safety or effectiveness of one of our delivery systems that has been approved or is the subject of a CE Certificate of Conformity; |
| • | medical device reporting regulations, which require that manufacturers comply with FDA or equivalent foreign competent authority requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur; |
| • | post-approval restrictions or conditions, including post-approval study commitments; |
| • | post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device; |
| • | the FDA’s and equivalent foreign competent authority’s recall authority, whereby they can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations; |
| • | the Sunshine Act and similar state and foreign laws, which require reporting of payments and other transfers of value to healthcare practitioners periodically; |
| • | regulations pertaining to voluntary recalls; and |
| • | notices of corrections or removals. |
Our delivery systems could be subject to voluntary recall if we, the FDA or an equivalent foreign competent authority determine, for any reason, that our delivery systems pose a risk of injury or are otherwise defective. Moreover, the FDA can order a mandatory recall if there is a reasonable probability that our delivery system would cause serious adverse health consequences or death.
101
Table of Contents
The FDA has broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA to determine our compliance with the QSR and other regulations, and these inspections include the manufacturing facilities of our subcontractors. Failure by us or by our suppliers to comply with applicable regulatory requirements can result in enforcement action by the FDA or other equivalent foreign authorities, which may result in sanctions, including, but not limited to:
| • | untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties; |
| • | unanticipated expenditures to address or defend such actions; |
| • | customer notifications for repair, replacement and/or refunds; |
| • | recall, detention or seizure of our delivery systems; |
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying our requests for approval of delivery system candidates or a modified version of Optune; |
| • | withdrawal of PMA approvals or suspension, variation or withdrawal of CE Certificates of Conformity that have already been granted; |
| • | refusal to grant export approval for our delivery systems; or |
| • | criminal prosecution. |
To date, our facility and those of our critical suppliers have been inspected by the FDA in order to obtain FDA approval of Optune. We and one of our critical component suppliers also were inspected by the FDA in 2012 and 2015. Another one of our suppliers was inspected in the fall of 2013. No inspectional observations were identified and no FDA Form 483s were issued following these inspections.
DME accreditation and licensing
We are subject to accreditation and licensing requirements as a DME supplier in most states. Certain of these states require that DME providers maintain an in-state location. Although we believe we are in compliance with all applicable state regulations regarding licensure requirements, if were found to be noncompliant, we could lose our accreditation or licensure in that state, which could prohibit us from selling our current or future delivery systems to patients in that state.
Healthcare regulatory matters
In addition to FDA restrictions on the marketing of medical devices, in recent years several other types of U.S. federal and state laws have been applied to restrict certain business practices in the healthcare industry and penalize unlawful conduct. These laws include anti-kickback, self-referral and false claims statutes.
The U.S. federal anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between device manufacturers on one hand and prescribers and purchasers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly and practices that involve remuneration intended to induce ordering, purchasing or recommending of a medical device may be subject to scrutiny if they do not qualify for an exemption or safe harbor. In some cases, our practices may not meet all of the criteria for safe harbor protection from anti-kickback liability.
102
Table of Contents
As a DME supplier, we also are subject to a U.S. federal self-referral law, commonly known as the Stark law, which prohibits Medicare payments for DME ordered by physicians who, personally or through an immediate family member, have ownership interests in or compensation arrangements with the furnishing supplier. The Stark law contains a number of specific exceptions that, if met, permit physicians who have certain financial relationships with a DME supplier to make referrals to that entity.
The False Claims Act prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. In recent years, the government has pursued a number of cases under the False Claims Act in connection with the off-label promotion of medical products. In addition, violation of the federal anti-kickback statute and of the Stark law may be actionable under the False Claims Act.
The majority of states also have statutes or regulations similar to the federal anti-kickback, self-referral and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, that apply regardless of the payer.
The Health Insurance Portability and Accountability Act of 1996, or HIPAA, mandated the adoption of standards for the exchange of electronic health information in an effort to encourage overall administrative simplification and enhance the effectiveness and efficiency of the healthcare industry. Ensuring privacy and security of patient information is one of the key factors driving the legislation. We believe we are in substantial compliance with the applicable HIPAA regulation.
HIPAA also included a number of federal criminal provisions, including for healthcare fraud and for false statements relating to healthcare matters. The healthcare fraud provision prohibits knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payers. The false statements provision prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Many states have similar healthcare fraud laws or insurance fraud laws that apply to claims for healthcare reimbursement.
Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines and imprisonment.
Legislation similar to U.S. anti-kickback, self-referral and false claims statutes have been adopted in foreign countries, including a number of EU member states.
The Sunshine Act requires manufacturers of drugs, medical devices, biologicals or medical supplies that participate in U.S. federal health care programs to track and then report certain payments and items of value given to U.S. physicians and U.S. teaching hospitals, which are defined as Covered Recipients. The Sunshine Act requires that manufacturers collect this information on a yearly basis and then report it to Centers for Medicare & Medicaid Services by the 90th day of each subsequent year. We have adopted policies and codes of conduct regarding our interactions with Covered Recipients and believe we are in substantial compliance with the Sunshine Act. However, our failure to adhere to these requirements could materially adversely impact our business and financial results.
In addition, the U.S. Foreign Corrupt Practices Act prohibits corporations and individuals from engaging in certain activities to obtain or retain business outside the United States or to influence a person working in an official capacity in a foreign country. It is illegal to pay, offer to pay or authorize the payment of anything of value to any official of another country, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in that capacity.
103
Table of Contents
Litigation
We are not currently subject to any material pending legal proceedings.
Employees
As of June 30, 2015, we had 258 full-time employees, of which 51 were in sales and marketing, 55 were in research and development, eight were in medical affairs, 41 were device support specialists and 103 were in operations support, finance, legal and administration. As of June 30, 2015, we had 161 employees located in the United States, 54 employees in Israel, 34 employees located in Europe and nine employees located in Japan. We believe relations with our employees are good.
Property
We currently lease space in several countries for our operations, including office, laboratory and/or warehouse space in Portsmouth, New Hampshire, New York, New York, Malvern, Pennsylvania, Haifa, Israel, Lucerne, Switzerland, Tokyo, Japan and Berlin, Germany. We believe that our current facilities are adequate for our present purposes.
104
Table of Contents
Glossary of terms
Adenocarcinoma histology is a cancer of epithelial tissue that has glandular origin, glandular characteristics or both. Adenocarcinoma of the lung is a subtype of lung cancer that responds to certain chemotherapies such as pemetrexed.
Advanced non-small cell lung cancer (NSCLC) is any type of epithelial lung cancer other than small cell lung carcinoma (SCLC). As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small cell lung carcinoma. Advanced NSCLC refers to NSCLC that cannot be treated surgically due to spread of the cancer.
Anaphase is the stage of mitosis when chromosomes are split and the sister chromatids move to opposite poles of the cell.
Anti-mitotic is the description of a substance or treatment that interferes with the replication process of proliferating cells, such as cancer cells.
Apoptosis is the process of programmed cell death characterized by specific biochemical events that lead to characteristic cell changes and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation and global mRNA decay.
Bevacizumab, trade name Avastin (Genentech/Roche), is an angiogenesis inhibitor, a drug that slows the growth of new blood vessels. Bevacizumab is a recombinant humanized monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor A (VEGF-A). VEGF-A is a chemical signal that stimulates angiogenesis in a variety of diseases, especially in cancer.
Blebbing is the formation of bubble-like structures, or blebs, out of the cell membrane. Blebbing is a sign of different types of damage to cells including apoptosis.
Blinded central radiology review is when a group of radiologists assesses whether a cancer tumor has grown or not without knowing which treatment each patient received.
Blood-brain barrier is a highly selective barrier that separates the circulating blood from the brain. The blood brain barrier restricts many types of chemotherapy and other drugs from entering the brain.
Brain metastases are a form of cancer that has metastasized (spread) to the brain from another location in the body, such as the lung.
Cytokinetic band is a ring formed out of contractile molecules (mainly actin and myosin) that is located at the equator between two daughter cells of a replicating parental cell. The contraction of this ring pinches the cell membrane in two, leading to the formation of two independent daughter cells.
Cytokinesis is the process by which a single cell is physically divided to form two daughter cells.
Daughter cells refer to the two identical cells formed during cell division when a dividing cell, known as the mother cell, grows and divides to produce two identical cells.
Dielectric constant is a physical property of a material that determines what part of the electric field magnitude will be reduced within the volume of the material. Materials with higher dielectric constants decrease the field amplitude less.
Karnofsky performance score (KPS) is a quantification of ability to perform activities of daily life independently. The score is used in 10-point intervals from 0 (dead) to 100 (perfect independence in activities of daily life).
Mediastinum is the central compartment of the chest cavity surrounded by loose connective tissue, and is a region that contains a group of structures within the chest. The mediastinum contains mainly the heart and its vessels, the esophagus, trachea, nerves, thymus and lymph nodes of the central chest.
105
Table of Contents
Mesothelioma is a form of cancer that develops from cells of the mesothelium, the protective lining that covers many of the internal organs of the body. Mesothelioma is most commonly caused by exposure to asbestos. The most common anatomical site for mesothelioma is the pleura (the outer lining of the lungs and internal chest wall).
Metaphase is a stage of mitosis in the cell cycle in which chromosomes are at their most condensed and coiled stage. These chromosomes, carrying genetic information, align in the equator of the cell before being separated into each of the two daughter cells.
MGMT is a gene that encodes the enzyme O-6-methylguanine-DNA methyltransferase. When this gene undergoes a chemical reaction called methylation, it is inactivated. Tumor cells that express more of the enzyme MGMT are resistant to certain types of chemotherapy, such as temozolomide. Therefore methylated (inactive) MGMT gene is a marker for tumor cells that are more sensitive to these types of chemotherapy.
Mini Mental State Exam (MMSE) is a quantification of a patient’s cognitive capabilities. The score is used in one point intervals from one to 30, with a higher score indicating better cognitive capabilities.
Mitosis is a part of the cell replication process by which chromosomes in a cell nucleus are separated into two identical sets of chromosomes, each in its own nucleus. In general, mitosis (division of the nucleus) is followed by cytokinesis, which divides the cytoplasm, organelles and cell membrane into two new cells containing roughly equal shares of these cellular components.
Mitotic furrow, or the cleavage furrow, is the indentation of the cell’s surface that begins the progression of cleavage, by which cells undergo cytokinesis, the final splitting of the membrane, in the process of cell replication. The same proteins responsible for muscle contraction, actin and myosin, begin the process of forming the cleavage furrow.
Mitotic spindle, or the spindle apparatus, refers to the subcellular structure of cells that separates chromosomes between daughter cells during cell division. Besides chromosomes, the spindle apparatus is composed of hundreds of proteins. Microtubules comprise the most abundant components of the machinery.
Mucositis is a painful inflammation and ulceration of the mucous membranes lining the digestive tract, usually as an adverse effect of chemotherapy and radiotherapy treatment for cancer.
Nitrosureas compounds are DNA alkylating agents and are often used in chemotherapy. They can cross the blood–brain barrier, making them candidates in the treatment of brain tumors.
Overall survival (OS) is a measure of how long a person lives from a certain starting time point such as diagnosis of a disease or randomization into a clinical trial. Doctors often use median overall survival time to estimate the patient’s prognosis or the effectiveness of a treatment. The FDA considers extension of OS the gold standard in effectiveness of cancer treatments.
Pemetrexed, brand name Alimta, is a chemotherapy drug manufactured and marketed by Eli Lilly and Company. Its indications are the treatment of pleural mesothelioma and non-small cell lung cancer. Pemetrexed is chemically similar to folic acid and is in the class of chemotherapy drugs called folate antimetabolites.
Progression free survival (PFS) is a measure of how long a patient remains alive without a growing cancer tumor from a certain time point such as diagnosis of a disease or randomization into a clinical trial. Doctors often use median PFS to estimate the patient’s prognosis or the effectiveness of a treatment.
PFS6 is the percentage of patients in a group who are alive and did not have tumor growth six months after entering a clinical trial. PFS6 is correlated with OS in recurrent glioblastoma and is sometimes used as a surrogate endpoint for OS in clinical trials.
106
Table of Contents
Platinum-based chemotherapy drugs, informally called platins, are chemotherapeutic agents to treat cancer. The main dose-limiting side effect of cancer treatment with platinum compounds is neurotoxicity, which causes peripheral neuropathies. Platinum-based chemotherapy inhibits DNA repair and/or DNA synthesis in cancer cells.
Salvage therapy is a form of treatment given after a disease does not respond to standard treatment. The term is usually used to describe a final attempt to treat a deadly disease, often with little hope of extending the patient’s life.
Septins are a group of proteins found primarily in cells of animals. Different septins form protein complexes with each other. These complexes can further assemble into filaments. Assembled as such, septins function in cells by localizing other proteins, either by providing a scaffold to which proteins can attach, or by preventing diffusion of molecules from one compartment of the cell to another.
Squamous histology refers to a cancer of a kind of epithelial cell, the squamous cell. These cells are the main part of the epidermis of the skin, and this cancer is one of the major forms of skin cancer. However, squamous cells also occur in the lining of the digestive tract, lungs and other areas of the body. Squamous histology non-small cell lung cancer is a subtype of NSCLC that is usually resistant to many types of chemotherapy, such as pemetrexed.
Stereotactic radiosurgery (SRS) is a distinct neurosurgical discipline that utilizes externally generated ionizing radiation focused to damage very precisely defined targets in the head or spine without the need to make an incision. SRS is often used to treat brain metastases either alone or in combination with standard surgery.
Stromal effects refer to the role of tumor cell microenvironment (stroma) in tumor development and therapy resistance. It is known that the surrounding stroma contributes to pancreatic cancer development and progression, and may contribute to its high resistance to chemotherapy.
Taxane is a type of chemotherapy whose principal mechanism of action is the disruption of microtubule function. Microtubules are essential to cell division, and taxanes stabilize tubulin to tubulin connections in the microtubule, thereby inhibiting the process of cell division, leading to inhibition of mitosis.
Temozolomide, brand names Temodar and Temodal and Temcad, is an oral chemotherapy drug. It is an alkylating agent used as a treatment of some brain cancers, e.g., as a second-line treatment for astrocytoma and a first-line treatment for glioblastoma.
Tubulin dimers are the paired combination of a-tubulin and ß-tubulin, the proteins that make up microtubules in dividing cells. These structural proteins have a very high electric dipole, i.e., there is a large difference between the electric charge on either end of the dimer.
Tumor genetic marker is a gene that is found in a tumor and has either implications regarding prognosis or selection of therapies for a certain patient.
Whole brain radiation therapy (WBRT) is a type of external radiation therapy used to treat patients who have cancer in the brain. It is often used to treat patients whose cancer has spread to the brain, or who have more than one tumor or tumors that cannot be removed by surgery or treated with stereotactic radiotherapy. Radiation is given to the whole brain over a period of many weeks.
107
Table of Contents
Summary of completed and existing clinical trials and registry data
| Indication | Trial/registry name |
Data type | Trial/registry title | Start date | Current status | |||||
| GBM |
EF-07 | Phase 1/2 trial |
A single-arm, pilot trial of the safety and efficacy of TTFields treatment for patients with GBM | 2004 | Completed Results published | |||||
| Recurrent GBM |
EF-11 | Phase 3 trial |
A prospective, multi-center trial of NovoTTF-100A compared to best standard of care in patients with progressive or recurrent GBM (clinicaltrials.gov identifier NCT00379470) | 2006 | Completed Results published | |||||
| NSCLC |
EF-15 | Phase 1/2 trial |
An open label pilot study of NovoTTF-100L in combination with pemetrexed (Alimta®) for advanced non-small cell lung cancer (clinicaltrials.gov identifier NCT00749346) | 2008 | Completed Results published | |||||
| Newly diagnosed GBM |
EF-14 | Phase 3 trial |
A prospective, multi-center trial of NovoTTF-100A together with temozolomide compared to temozolomide alone in patients with newly diagnosed GBM (clinicaltrials.gov identifier NCT00916409) | 2009 | Completed Publication pending | |||||
| Recurrent GBM |
PRiDe | Commercial patient registry |
A registry of 457 recurrent GBM patients who received NovoTTF therapy in the clinical practice setting on the U.S. commercial prescription-use program between October 2011 and November 2013 | 2011 | Completed Results published | |||||
|
| ||||||||||
108
Table of Contents
| Indication | Trial/registry name |
Data type | Trial/registry title | Start date | Current status | |||||
| Brain metastases originating in NSCLC |
COMET | Phase 2 trial |
A phase 2 randomized study of TTFields therapy versus supportive care in non-small cell lung cancer patients with 1-5 brain metastases following optimal standard local treatment (clinicaltrials.gov identifier NCT01755624) | 2013 | Ongoing | |||||
| Pancreatic Cancer |
PANOVA | Phase 1/2 trial |
An open label pilot study of NovoTTF-100L concomitant with gemcitabine for front-line therapy of advanced pancreatic adenocarcinoma (clinicaltrials.gov identifier NCT01971281) | 2013 | Ongoing | |||||
| Ovarian Cancer |
INNOVATE | Phase 1/2 trial |
An open label pilot study of the NovoTTF-100L(O) system (NovoTTF therapy) concomitant with weekly paclitaxel for recurrent ovarian carcinoma (clinicaltrials.gov identifier NCT02244502) | 2014 | Ongoing | |||||
| Mesothelioma |
STELLAR | Phase 2 trial |
Phase 2 trial of pemetrexed and cisplatin or carboplatin in combination with NovoTTF therapy as first-line treatment in malignant pleural mesothelioma (clinicaltrials.gov identifier NCT02397928) | 2015 | Ongoing | |||||
109
Table of Contents
Executive officers and directors
Our executive officers are elected by and serve at the discretion of our board of directors. The following table lists information about our directors and executive officers as of the date of this prospectus:
| Name | Age | Position | ||
| William F. Doyle |
53 | Chairman and Director | ||
| Kinyip Gabriel Leung |
53 | Vice Chairman and Director | ||
| Asaf Danziger |
49 | Chief Executive Officer and Director | ||
| Michael Ambrogi |
52 | Chief Operating Officer | ||
| Wilhelmus Groenhuysen |
58 | Chief Financial Officer | ||
| Eilon Kirson, M.D., Ph.D. |
47 | Chief Science Officer and Head of Research and Development | ||
| Peter Melnyk |
53 | Chief Commercial Officer | ||
| Yoram Palti, M.D., Ph.D. |
78 | Chief Technology Officer and Director | ||
| William Burkoth |
39 | Director | ||
| Timothy Langloss |
39 | Director | ||
| Louis J. Lavigne, Jr. |
67 | Director | ||
| Robert J. Mylod, Jr. |
48 | Director | ||
| Gert Lennart Perlhagen |
72 | Director | ||
| Charles G. Phillips III |
67 | Director | ||
| William A. Vernon |
59 | Director | ||
|
| ||||
William F. Doyle has served as our Chairman since 2009 and has been a director of NovoCure since 2004. Mr. Doyle has also been the Managing Director of WFD Ventures LLC, a venture capital firm he co-founded, since 2002. Prior to 2002, Mr. Doyle was a member of Johnson & Johnson’s Medical Devices and Diagnostics Group Operating Committee and vice president, Licensing and Acquisitions from 1995 to 1999. While at Johnson & Johnson, Mr. Doyle was also chairman of the Medical Devices Research and Development Council, Worldwide president of Biosense-Webster, Inc. and a member of the board of directors of Cordis Corporation and Johnson & Johnson Development Corporation, Johnson & Johnson’s venture capital subsidiary. From 1992 to 1995, Mr. Doyle was a consultant with McKinsey & Company. Mr. Doyle holds a S.B. in materials science and engineering from the Massachusetts Institute of Technology and an M.B.A. from Harvard Business School. We believe Mr. Doyle is qualified to serve on our board of directors due to his business and investment experience and his extensive knowledge of our company and our industry.
Kinyip Gabriel Leung has been our Vice Chairman since 2011 and has been a director of NovoCure since 2011. Mr. Leung serves as our Vice Chairman on a part-time basis. From 2003 to 2010, Mr. Leung worked for OSI Pharmaceuticals, Inc., prior to its acquisition by Astellas Pharma Inc., last serving as executive vice president of
110
Table of Contents
OSI Pharmaceuticals, Inc. and the President of OSI’s Oncology and Diabetes Business. Mr. Leung was responsible for the launch of erlotinib (Tarceva) at OSI Pharmaceuticals, Inc. Prior to his tenure at OSI, from 1999 to 2003, Mr. Leung served as group vice president of the global prescription business at Pharmacia Corporation, leading Pharmacia Corporation’s oncology franchise with business and medical affairs operations in over 80 countries. From 1991 to 1999, Mr. Leung was an executive at Bristol-Myers Squibb Company, where he was responsible for the growth of Taxol and Paraplatin. In addition, Mr. Leung has served as a director for Albany Molecular Research Inc. (AMRI) since 2010 and was also a director of Delcath Systems, Inc. from 2011 to 2014. Mr. Leung is a pharmacist and trained at the University of Texas at Austin, where he earned his B.S. with High Honors. Mr. Leung attended graduate school at the University of Wisconsin-Madison, where he earned his M.S. in Pharmacy, with a concentration in pharmaceutical marketing. We believe that Mr. Leung is qualified to serve on our board of directors due to his service as our Vice Chairman and his extensive knowledge of our company and industry.
Asaf Danziger has served as our Chief Executive Officer since 2002 and has been a director of NovoCure since 2012. From 1998 to 2002, Mr. Danziger was CEO of Cybro Medical, a subsidiary of Imagyn Medical Technologies, Inc. Mr. Danziger holds a B.Sc. in material engineering from Ben Gurion University of the Negev, Israel. We believe that Mr. Danziger is qualified to serve on our board of directors due to his service as our Chief Executive Officer and his extensive knowledge of our company and industry.
Michael Ambrogi has been our Chief Operating Officer since 2010 and previously served as our U.S. General Manager from 2006 to 2010. Mr. Ambrogi has overall responsibility for our ongoing operations, engineering, manufacturing and service and human resources activities worldwide. From 1991 to 2006, Mr. Ambrogi worked for Deka Research and Development Corporation, inventor Dean Kamen’s research and development firm, last serving a general manager. Mr. Ambrogi led Deka’s teams on many products including the Baxter HomeChoice peritoneal dialysis machine, the Davol Hydroflex surgical irrigation device, the Cordis Crowne Stent and the J&J IBOT 3000 mobility system. Earlier in his career, from 1988 to 1990, Mr. Ambrogi was a consultant with McKinsey & Company. Mr. Ambrogi holds a S.B. in mechanical engineering from MIT.
Wilhelmus Groenhuysen has been our Chief Financial Officer since 2012. From 2007 to 2011, Mr. Groenhuysen worked for Cephalon, Inc., last serving as executive vice president and chief financial officer, where he had responsibility for worldwide finance, commercial operations and risk management. From 1987 to 2007, Mr. Groenhuysen worked for Philips Group in various assignments in Europe, Asia and the United States, the latest of which started in 2002 when he was promoted to chief financial officer and senior vice president of Philips Electronics North America Corporation. Mr. Groenhuysen holds a Master’s Degree in Business Economics from VU University Amsterdam and graduated as a Registered Public Controller at VU University Amsterdam.
Eilon Kirson, M.D., Ph.D. has been our Chief Science Officer and Head of Research and Development since 2012 and previously served as our Chief Medical Officer from 2006 to 2012. Dr. Kirson has led the development of TTFields from pre-clinical testing to large, multi-center phase 3 studies and through multiple regulatory approvals. Dr. Kirson previously served as head of electrophysiology at Carmel Biosensors Ltd. Dr. Kirson received his B.Med.Sc., M.D. and Ph.D. in Physiology-Biophysics from the Hebrew University of Jerusalem and served his residency in neurology at Sheba Medical Center, Tel Ha-Shomer Hospital, Israel.
Peter Melnyk has been our Chief Commercial Officer since 2011. Mr. Melnyk has overall responsibility for directing our global marketing and sales efforts and will lead the multi-national commercial launch of Optune. Mr. Melnyk was previously senior vice president for sales and marketing at OSI Pharmaceuticals, Inc. where he led the global commercialization efforts for Tarceva from 2003 to 2011. Prior to OSI, Mr. Melnyk was executive director of oncology at Pfizer Inc. and a director of oncology at Bristol-Myers Squibb Company. Mr. Melnyk also serves on the Cancer Prevention and Early Detection Advisory Workgroup of C-Change. Mr. Melnyk holds a B.Sc in animal science and M.Sc in reproductive endocrinology from McGill University.
111
Table of Contents
Yoram Palti, M.D., Ph.D. founded NovoCure in 2000 and has been our Chief Technology Officer, serving as a consultant, since 2000, and has been a director of NovoCure since 2002. Professor Palti is a professor emeritus of physiology and biophysics at the Technion – Israel Institute of Technology and the inventor of TTFields. Professor Palti has also served as a director of EchoSense Ltd. since 2010, serving as a consultant. From 1982 to 1993, Professor Palti was the head of the Rappaport Family Institute for Research in the Medical Sciences, the research arm of the Technion Medical School. From 1968 to 1970, Professor Palti was an associate professor of physiology at the University of Maryland School of Medicine. Professor Palti also founded and managed Carmel Biosensors Ltd. and CellSense Ltd. from 1992 to 2000. Professor Palti is the author of more than 40 patents and 70 scientific papers. Professor Palti received his M.Sc., Ph.D. and M.D. from The Hebrew Univ. Hadassah Medical School and served his residency at The Hebrew Univ. Hadassah Medical School. We believe that Professor Palti is qualified to serve on our board of directors due to his research qualifications and experience and his extensive knowledge of our technology and our industry.
William Burkoth has been a director of NovoCure since 2009. Mr. Burkoth has worked for Pfizer Inc.’s Venture Investments Team since 2004, currently serving as executive director, where he has responsibility for making direct equity investments in private life-science companies on behalf of Pfizer Inc. Prior to joining Pfizer Inc., from 2002 to 2004, Mr. Burkoth worked in business development at Galileo Pharmaceuticals, Inc. and at IntraBiotics Pharmaceuticals, Inc. From 1999 to 2002, Mr. Burkoth worked as an analyst at Bay City Capital, a life sciences venture capital firm. Mr. Burkoth currently serves as a director of Biodesy, Inc., G-CON Manufacturing Inc., NeuMoDx Molecular, Inc. and RefleXion Medical Inc. Mr. Burkoth received a B.A. in chemistry from Whitman College and an M.B.A. from Columbia Business School. We believe that Mr. Burkoth is qualified to serve on our board of directors due to his business and financial experience as an investor in, and a director of, companies in our industry.
Timothy Langloss has been a director of NovoCure since 2009. Mr. Langloss is a managing director of WFD Ventures LLC, a venture capital firm he co-founded in 2002. As part of his WFD responsibilities, Mr. Langloss served as President of Hand Innovations LLC, a WFD portfolio company acquired in 2006 by DePuy Orthopaedics, a Johnson & Johnson company. From June 1998 to July 2002, Mr. Langloss worked as an investment professional at Insight Venture Partners, a venture capital firm, and at Stonington Partners (formerly Merrill Lynch Capital Partners), a leveraged buyout firm. Mr. Langloss holds an A.B. degree from Harvard University. We believe Mr. Langloss is qualified to serve on our board of directors due to his business and investment experience and his extensive knowledge of our company and our industry.
Louis J. Lavigne, Jr. has been a director of NovoCure since 2012 and has served as the chairperson of our audit committee since 2012. Mr. Lavigne is currently the managing director of Lavrite, LLC, a management consulting firm specializing in corporate finance, accounting, growth strategy and management and the managing member of Spring Development Group, LLC, a specialized investor in growth situations. From 1982 to 2005, Mr. Lavigne worked at Genentech, Inc. He joined Genentech, Inc. in 1982 and was named controller in 1983. In 1986, he was promoted to vice president and assumed the chief financial officer position in 1988. In 1994, Mr. Lavigne became senior vice president and executive vice president in 1997. Mr. Lavigne has served as a director of Accuray, Inc. since 2009, as a director of Depomed, Inc. since 2013, as a director of DocuSign, Inc. since 2013, as a director of Zynga, Inc. since 2015 and as a director of Rodan & Fields, LLC since 2015. Mr. Lavigne previously served as a director of Allergan, Inc. from 2005 to 2014, as a director of BMC Software, Inc. from 2008 to 2013 and as a director of SafeNet, Inc. from 2010 to 2015. Mr. Lavigne is a board member and chairman of the UCSF Benioff Children’s Hospital Oakland, the UCSF Benioff Children’s Hospitals and the UCSF Children’s Hospitals Foundation. Mr. Lavigne also serves as a trustee of Babson College and Babson Global. Mr. Lavigne earned a B.S. in business administration from Babson College and an M.B.A. from Temple University. We believe that Mr. Lavigne is qualified to serve on our board of directors due to his business and accounting experience working as an executive and director of companies in our industry.
112
Table of Contents
Robert J. Mylod, Jr. has been a director of NovoCure since 2012 and has served on our audit committee since 2012. Mr. Mylod has served as managing member of Annox Capital LLC since 2012. From 2009 to 2011, Mr. Mylod served as vice chairman and head of worldwide strategy and planning at priceline.com. From 2000 to 2009, Mr. Mylod was priceline.com’s chief financial officer. Prior to joining priceline.com, Mr. Mylod was a principal at Stonington Partners, a private equity investment firm. Mr. Mylod is on the board of directors of EverBank Financial Corp. Mr. Mylod earned an A.B. in English from the University of Michigan and an M.B.A. from the University of Chicago Graduate School of Business. We believe that Mr. Mylod is qualified to serve on our board of directors due to his business, accounting and corporate finance experience.
Gert Lennart Perlhagen has been a director of NovoCure since 2003 and has served on our compensation committee since 2012. Mr. Perlhagen is an active entrepreneur and investor in emerging healthcare companies and was a founding investor in NovoCure. Mr. Perlhagen founded (as part of its merger with Cross Pharma AB) and, from 1999 to 2006, served as a director of Meda AB, a leading specialty Swedish pharmaceutical company. Prior to founding Meda AB, Mr. Perlhagen was the chief executive officer for Scandinavia and UK for Pharmitalia SpA (later acquired by Pfizer Inc.) where he gained extensive experience in oncology through the launch of the chemotherapy agent doxorubicin (Adriamycin). Outside oncology, Mr. Perlhagen helped lead the commercial launch of omeprazole (Losec/Prilosec) for Astra AB where he served as regional director for South Europe and as a member of the International Marketing Strategy Group. We believe Mr. Perlhagen is qualified to serve on our board of directors due to his business and investment experience and his extensive knowledge of our company and our industry.
Charles G. Phillips III has been a director of NovoCure since 2012 and has served on our audit committee and as the chairperson of our compensation committee since 2012. From 2008 to 2011, Mr. Phillips served as chief operating officer of Prentice Capital Management, LLC, an investment management firm. Prior to joining Prentice Capital Management, LLC, Mr. Phillips was a managing director from 1991 to 2002 and president from 1998 to 2001 of Gleacher & Co., an investment banking and management firm. Prior to joining Gleacher & Co., Mr. Phillips held senior positions at other investment banking firms, including nine years at Morgan Stanley where he served as a managing director within the investment banking division and founded and led that firm’s high-yield finance activities. Mr. Phillips has served on the boards of several public and private companies and private investment funds, including California Pizza Kitchen, Inc. and Fidus Investment Corporation. Mr. Phillips also serves on the governing bodies of a number of educational and non-profit organizations. Mr. Phillips earned an A.B. from Harvard College and an M.B.A from Harvard Business School. We believe Mr. Phillips is qualified to serve on our board of directors due to his business, financial and investment banking experience.
William A. Vernon has been a director of NovoCure since 2006 and has served on our compensation committee since 2012. Mr. Vernon served as the chief executive officer of the Kraft Foods Group from 2011 to 2014 and served as its senior advisor through March 2015. Mr. Vernon has served as a director of Medivation, Inc. since 2006 and as a director of Intersect ENT Inc. since 2015. From 2009 to 2011, Mr. Vernon served as the president of Kraft Foods North America and an executive vice president of Kraft Foods. From 2006 to 2009, Mr. Vernon served as the healthcare industry partner for Ripplewood Holdings, a private equity firm. From 1983 to 2006, Mr. Vernon worked for Johnson & Johnson. He served as company group chairman of DePuy Orthopaedics from 2004 to 2005, president of Centocor from 2001 to 2004 and president of McNeil Consumer Products and Nutritionals, Worldwide and president of The Johnson & Johnson-Merck Joint Venture from 1995 to 2001. Mr. Vernon holds a B.A. in history from Lawrence University and an M.B.A. from Northwestern University’s Kellogg School of Management. We believe Mr. Vernon is qualified to serve on our board of directors due to his business and investment experience as an executive in our industry.
113
Table of Contents
Board of directors
Composition of our board of directors
Our articles of association provide that our board of directors may consist of between two and 13 directors, and our board of directors currently has 11 members, including our Chairman, our Chief Executive Officer and our Chief Technology Officer. Our directors were elected to serve on our board pursuant to an investors rights agreement, which will terminate upon the consummation of this offering.
Upon consummation of this offering, our board of directors will be divided into three classes, class I, class II and class III, with members of each class serving staggered three-year terms. Upon the closing of this offering, the members of the classes will be divided as follows:
| • | the class I directors will be Mr. Burkoth, Mr. Langloss and Mr. Palti; |
| • | the class II directors will be Mr. Leung, Mr. Lavigne, Mr. Perlhagen and Mr. Mylod; and |
| • | the class III directors will be Mr. Vernon, Mr. Danziger, Mr. Doyle and Mr. Phillips. |
Upon the expiration of the term of a class of directors, directors in that class will be eligible to be elected for a new three-year term at the annual meeting of shareholders in the year in which their term expires. To the extent that a vacancy arises on the board of directors during the term of a director’s appointment, the new director appointed as a replacement to fill the vacancy will serve for the remainder of the three-year term of the director he or she has replaced.
Director independence
Our board of directors has determined that each of Messrs. Burkoth, Langloss, Lavigne, Mylod, Perlhagen, Phillips and Vernon has no relationship that, in the opinion of the board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and is an independent director as defined by applicable SEC and NASDAQ rules.
Role of our board of directors in risk oversight
Risk assessment and oversight are an integral part of our governance and management processes. Our board of directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings, and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the board of directors at regular board meetings as part of management presentations that focus on particular business functions, operations or strategies, and presents the steps taken by management to mitigate or eliminate such risks.
Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure. Our audit committee is responsible for overseeing our major financial risk exposures and the steps our management has taken to monitor and control these exposures and our compensation committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage unnecessary risk-taking. In addition, upon completion of this offering, our audit committee will oversee the performance of our internal audit function and consider and approve or disapprove any related-party transactions and our nominating and governance committee will monitor the effectiveness of our corporate governance guidelines.
114
Table of Contents
Indemnification agreements
We will enter into indemnification agreements with each of our directors to indemnify them against certain liabilities and expenses arising from their being a director provided they acted in good faith with a view to our best interests. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Committees of the board of directors
Our board of directors has established an audit committee, a stock option and compensation committee, or compensation committee, and a corporate governance and nominating committee and may from time to time establish other committees. Copies of each committee’s charter will be posted on our website, www.novocure.com, upon our listing on NASDAQ.
Audit committee
Our audit committee oversees our corporate accounting and financial reporting process. Upon completion of this offering, the audit committee will be responsible for, among other things:
| • | appointing our independent registered public accounting firm; |
| • | evaluating the independent registered public accounting firm’s qualifications, independence and performance; |
| • | determining the terms of our engagement of our independent registered public accounting firm; |
| • | reviewing and approving the scope of the annual audit and the audit fee; |
| • | reviewing and discussing the adequacy and effectiveness of our accounting and financial reporting processes and internal controls and the audits of our financial statements; |
| • | reviewing and approving, in advance, all audit and non-audit services to be performed by our independent registered public accounting, taking into consideration whether the independent auditor’s provision of non-audit services to us is compatible with maintaining the independent auditor’s independence; |
| • | monitoring and ensuring the rotation of partners of the independent registered public accounting firm on our engagement team as required by law; |
| • | establishing and overseeing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal controls or auditing matters, including procedures for the confidential, anonymous submission by our employees of complaints regarding questionable accounting or auditing matters and reviewing such complaints; |
| • | reviewing and approving related-party transactions; |
| • | investigating any matter brought to its attention within the scope of its duties and engaging independent counsel and other advisors as the audit committee deems necessary; |
| • | reviewing reports to management prepared by the internal audit function, if any, as well as management’s responses; |
115
Table of Contents
| • | reviewing our financial statements and our management’s discussion and analysis of financial condition and results of operations to be included in our annual quarterly reports to be filed with the SEC; |
| • | reviewing, at least annually, the audit committee charter and the committee’s performance; and |
| • | handling such other matters that are specifically delegated to the audit committee by our board of directors from time to time. |
The audit committee consists of Mr. Lavigne, who currently serves as chairperson of the committee, Mr. Mylod and Mr. Phillips. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and NASDAQ. Mr. Lavigne qualifies and will serve as an audit committee financial expert as defined under the applicable rules and regulations of the SEC. Under the rules and regulations of the SEC and NASDAQ, members of the audit committee must also meet independence standards under Rule 10A-3 of the Exchange Act. All members of our audit committee meet the independence standards.
Compensation committee
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers, directors and employees. See “Executive compensation—Compensation philosophy and objectives” for a description of the responsibilities of our compensation committee.
The compensation committee consists of Mr. Phillips, who currently serves as the chairperson of the committee, Mr. Perlhagen and Mr. Vernon. Each of the members of our compensation committee is a non-employee director as defined in Rule 16b-3 promulgated under the Exchange Act, an outside director as that term is defined in Section 162(m) of the Code and an “independent director” under applicable NASDAQ rules.
Nominating and corporate governance committee
Upon the completion of this offering, the nominating and corporate governance committee will be responsible for, among other things:
| • | identifying and screening candidates for our board of directors and recommending nominees for election as directors, as well as recommending one or more “audit committee financial experts” (as defined under applicable SEC rules) for the audit committee; |
| • | establishing procedures to exercise oversight of the evaluation of our board of directors and management; |
| • | developing and recommending to our board of directors a set of corporate governance guidelines, as well as periodically reviewing these guidelines and recommending any changes to our board of directors; |
| • | reviewing the structure of our board of directors’ committees and recommending to our board of directors for its approval directors to serve as members of each committee, and where appropriate, making recommendations regarding the removal of any member of any committee; |
| • | reviewing and assessing the adequacy of its formal written charter on an annual basis; |
| • | reviewing the relationships that each director has with us for purposes of determining independence; |
| • | generally advising our board of directors on corporate governance and related matters. |
Our nominating and corporate governance committee will consist of Mr. Vernon, who will serve as chairperson of the committee, Mr. Burkoth and Mr. Mylod. Each of the members of our nominating and corporate governance committee is an independent director under the rules of NASDAQ relating to nominating and corporate governance committee independence.
116
Table of Contents
Code of business conduct and ethics
We have adopted a code of business conduct and ethics applicable to all of our employees, officers, directors and consultants, including our principal executive, financial and accounting officers and all persons performing similar functions. A copy of that code will be available on our website prior to completion of this offering. Any amendments to the code, or any waivers of its requirements, will be disclosed on our website or as required by applicable law or NASDAQ listing requirements.
117
Table of Contents
Our named executive officers, or NEOs, for fiscal 2014, which consist of our principal executive officer and our two most highly compensated executive officers other than our principal executive officer, are:
| • | Asaf Danziger, our Chief Executive Officer; |
| • | Wilhelmus Groenhuysen, our Chief Financial Officer; and |
| • | Eilon Kirson, our Chief Science Officer and Head of Research and Development. |
Compensation philosophy and objectives
Historically, our board of directors has primarily been responsible for determining our compensation strategy with respect to our executive officers. In September 2011, our board of directors established the compensation committee. The compensation committee consists of Messrs. Perlhagen, Phillips and Vernon, with Mr. Phillips serving as Chairman.
The compensation committee’s purposes are, among other functions:
| • | to discharge our board of directors’ responsibilities relating to compensation of our directors and executive officers; and |
| • | to oversee the administration of our overall compensation and employee benefits plans, in particular incentive compensation and equity-based plans. |
Pursuant to the compensation committee charter, the compensation committee’s specific authorities and powers will be, among other functions:
| • | to periodically review and approve generally our compensation and benefit strategies and policies; |
| • | to at least annually review and approve the corporate goals and objectives relevant to the compensation of our Chief Executive Officer, to evaluate the Chief Executive Officer’s performance in light of these goals and objectives and set the Chief Executive Officer’s compensation; |
| • | to at least annually review and approve with the input of our Chief Executive Officer, the compensation of our other executive officers and to approve employment, consulting, severance, retirement and/or change in control agreements or provisions with respect to any current or former executive officers; |
| • | to at least annually review and approve succession plans for our Chief Executive Officer and other executive officers; |
| • | to periodically review and make recommendations to the board of directors regarding director compensation; |
| • | to oversee the implementation and administration of our equity compensation plans (including reviewing and approving the adoption of new plans or amendments or modifications to existing plans, subject to shareholder approval, as necessary); |
| • | to approve or review and make recommendations to the board of directors with respect to our share-based compensation plans; |
| • | to retain or obtain the advice of a compensation consultant, independent legal counsel or other adviser (only after taking into consideration certain specified factors identified by the SEC or NASDAQ listing standards), with direct responsibility for the appointment, compensation and oversight of the work of any such compensation consultant, independent legal counsel and other adviser retained by the compensation committee; |
118
Table of Contents
| • | to review from time to time the compensation committee charter and the committee’s performance; and |
| • | to exercise such other authorities and responsibilities as may be delegated to the compensation committee by the board of directors from time to time. |
Compensation decision process
Historically, the design, negotiation and execution of compensatory arrangements in connection with the hiring of our executives has been based in large part on our desire and goal to provide compensation sufficient to enable us to attract and retain talent necessary to further develop our business based on the short- and long-term goals of our board of directors and shareholders, while at the same time most efficiently utilizing available cash and equity in light of the stage of the development of our company. Compensation of our NEOs after the initial period following their hiring was influenced by the amounts of compensation that we initially agreed to pay them in connection with their hiring, as well as by our evaluation of their performance, levels of responsibility, prevailing market conditions and the financial condition and prospects of our company. In addition, we generally have sought a degree of internal equity in the compensation of existing executives relative to the compensation paid to more recently hired executives, using existing pay levels and commitments as a yard-stick in setting and determining our executives’ relative salary, bonus and equity compensation. Finally, certain of the members of our board of directors have had significant experience with private equity-backed companies and the executive compensation practices of such companies and have applied this knowledge and experience to their judgments regarding our past compensation decisions.
Prior to December 2012, our board of directors made compensation decisions for our company. Following the establishment of the compensation committee and adoption of the compensation committee’s charter in December 2012, the compensation committee formalized and refined the compensation approval process. The compensation committee has remained primarily responsible for compensation decisions for our company in accordance with the charter.
In accordance with its charter, our compensation committee determines and approves the annual compensation of our Chief Executive Officer and, with the input of our Chief Executive Officer, our other executive officers. The compensation committee also regularly reports its compensation decisions to our board of directors. Our compensation committee also administers our equity and other incentive compensation plans, including, upon completion of this offering, our 2015 Omnibus Incentive Plan, which is described below. We expect that the specific components of our executive compensation program will continue to be reviewed and adjusted as appropriate to reflect the performance of our company and our executive officers, market and industry practices, consideration of applicable securities and tax regulations as well as our experience as a publicly held company. Accordingly, the compensation paid to our NEOs in fiscal 2014 and paid or payable in fiscal 2015 may not necessarily be indicative of how we may compensate our NEOs following this offering.
119
Table of Contents
The following tables and narratives describe the compensation and benefits provided to our NEOs in fiscal 2014.
2014 Summary compensation table
| Named executive officer and principal position |
Fiscal year |
Salary ($)(1) | Option awards ($)(2) |
Non-equity incentive plan compensation ($)(3) |
All other compensation ($)(4)(5) |
Total ($) | ||||||||||||||||||
| Asaf Danziger Chief Executive Officer |
2014 | 696,174 | 448,161 | 313,568 | 288,528 | 1,746,431 | ||||||||||||||||||
| Wilhelmus Groenhuysen Chief Financial Officer |
2014 | 594,825 | 298,774 | 300,300 | 17,544 | 1,211,443 | ||||||||||||||||||
| Eilon Kirson Chief Science Officer and Head of Research and Development |
2014 | 464,116 | 298,774 | 209,045 | 149,787 | 1,121,722 | ||||||||||||||||||
|
|
||||||||||||||||||||||||
| (1) | Effective January 1, 2014, the compensation committee set Mr. Danziger’s and Mr. Kirson’s annual base salaries, expressed in U.S. dollars, at $624,000 and $416,000, respectively. In accordance with Company practice/policy, such annual salaries are paid in the new Israeli shekel, or NIS. The difference between the salary amounts established by the compensation committee and those reported in the table above are due to currency translations. The salary for Mr. Groenhuysen reflects a blended rate of his base salary at the start of fiscal 2014 of $577,500 and a rate of $600,600 following a performance-based raise in February 2014. |
| (2) | The amounts represent the aggregate grant date fair value of option awards granted by us in 2014, computed in accordance with FASB ASC Topic 718. For further information on how we account for stock-based compensation, see Note 13 to our consolidated financial statements. These amounts reflect our accounting expense for these awards and do not correspond to the actual amounts, if any, that will be recognized by the NEOs. None of our NEOs have been granted stock awards or any equity compensation other than stock options. For Mr. Danziger, the option awards reflect the grant on February 26, 2014 of options to purchase 88,695 ordinary shares under our 2013 Share Option Plan. For Mr. Groenhuysen, the option awards reflect the grant on February 26, 2014 of options to purchase 59,130 ordinary shares under our 2013 Share Option Plan. For Mr. Kirson, the option awards reflect the grant on February 26, 2014 of options to purchase 59,130 ordinary shares under our 2013 Share Option Plan. In each case, 25% of the options become vested on each of the first, second, third and fourth anniversaries of the February 26, 2014 grant date. |
| (3) | Reflects annual cash bonus payments pursuant to the 2014 Cash Bonus Program for achieving certain corporate achievements and personal objectives in respect of 2014 performance, which bonuses were paid in the first quarter of 2015. For additional information, see “Cash bonuses—2014 Cash Bonus Program” below. Mr. Danziger’s and Mr. Kirson’s annual cash bonus payments, expressed in U.S. dollars, are $312,000 and $208,000, respectively; however, like their respective base salaries, in accordance with Company practice/policy, such annual cash bonus payments are paid in NIS. The difference between the salary amounts established by the compensation committee and those reported in the table above are due to currency translations. The amounts reported for Mr. Danziger’s and Mr. Kirson’s annual cash bonus payments pursuant to the 2014 Cash Bonus Program reflect the actual amounts paid in respect of 2014 performance, as expressed in U.S. dollars, based on the actual NIS/US$ exchange rates. |
| (4) | A detailed breakdown of “All other compensation” for Asaf Danziger and Eilon Kirson for fiscal 2014 is provided in the table below, in each case, based on actual cost expressed in U.S. dollars. |
| Name | Company contribution to benefits ($)(a)(b) |
Vacation payout ($)(c)(d) |
Automobile payments ($)(e) |
Total ($) | ||||||||||||
| Asaf Danziger |
173,109 | 69,960 | 45,459 | 288,528 | ||||||||||||
| Eilon Kirson |
95,027 | 32,193 | 22,567 | 149,787 | ||||||||||||
|
|
||||||||||||||||
120
Table of Contents
| (a) | Amount includes $110,592 in severance and pension contributions from us to Mr. Danziger’s Managers Insurance Policy; $52,213 in contributions from us to Mr. Danziger’s advance study fund/professional education fund (keren hishtalmut); $10,304 in payments by us in respect of social security and recuperation pay required by statute in Israel, convalescence pay and holiday gift cards given to Company employees twice per year (Passover and Rosh Hashana) and grossed up for taxes. For additional details with respect to the Managers Insurance Policy and professional education fund, please see “Other employee benefits and compensation” and “Executive employment arrangements—Danziger employment agreement” below. |
| (b) | Amount includes $51,018 in severance and pension contributions from us to Mr. Kirson’s Managers Insurance Policy; $33,642 in contributions from us to Mr. Kirson’s advance study fund/professional education fund (keren hishtalmut); $10,367 in payments by us in respect of social security and recuperation pay required by statute in Israel, convalescence pay and holiday gift cards given to Company employees twice per year (Passover and Rosh Hashana) and grossed up for taxes. For additional details with respect to the Managers Insurance Policy and professional education fund, please see “Other employee benefits and compensation” and “Executive employment arrangements—Kirson employment agreement” below. |
| (c) | Represents payment for 28 days of accrued but unused vacation time paid to Mr. Danziger pursuant to the exercise of his right, in accordance with his employment agreement, to elect, on an annual basis, to receive a cash payment based on Mr. Danziger’s base salary in respect of such accrued but unused vacation time in lieu of using such accrued vacation in the future. For Mr. Danziger, the vacation payment represented 28 days of unused vacation accrued from October 2013 when Mr. Danziger had last made an election to receive his then accrued vacation time in cash. For additional information, see “Executive employment arrangements—Danziger employment agreement” below. |
| (d) | Represents payment for 20 days of accrued but unused vacation time paid to Mr. Kirson pursuant to the exercise of his right, in accordance with Company practice, to elect, on an annual basis, to receive a cash payment based on Mr. Kirson’s base salary in respect of such accrued but unused vacation time in lieu of using such accrued vacation in the future. For Mr. Kirson, the vacation payment represented 20 days of unused vacation accrued from October 2013 when Mr. Kirson had last made an election to receive his then accrued vacation time in cash. For additional information, see “Executive employment arrangements—Kirson employment agreement” below. |
| (e) | In lieu of providing Mr. Danziger with a company car, we currently satisfy our automobile obligations under Mr. Danziger’s employment agreement with us by paying Mr. Danziger cash sufficient to cover the costs of his automobile on an after-tax basis. The total amount paid to Mr. Danziger in 2014 with respect to the automobile payments was $45,459. For additional information, see “Executive employment arrangements—Danziger employment agreement” below. For Mr. Kirson, we pay a third-party leasing company directly for vehicle expenses. |
| (5) | “All other compensation” for Mr. Groenhuysen for fiscal 2014 was comprised of automobile payments totaling $13,200 and insurance premiums paid of $4,344. For additional information, see “Executive employment arrangements—Groenhuysen employment letter agreement.” |
Base salary
Our compensation committee conducts an annual review of each executive officer’s base salary once per year, with input from our Chief Executive Officer (other than with respect to himself), and makes adjustments as it determines appropriate in furtherance of our compensation philosophy and company performance objectives and needs, with revisions generally becoming effective in April of that year.
121
Table of Contents
In 2014, in connection with their respective annual performance reviews, effective January 1, 2014, Mr. Danziger’s base salary was increased to $624,000 and Mr. Kirson’s base salary was increased to $416,000. In addition, in connection with his annual performance review, Mr. Groenhuysen’s base salary was increased to $600,600. These base salary increases were approved by the compensation committee.
The current annual base salaries of our Named Executive Officers and Mr. Groenhuysen are set forth below:
| Named executive officer | Base salary ($) | |||
| Asaf Danziger |
624,000 | |||
| Wilhelmus Groenhuysen |
600,600 | |||
| Eilon Kirson |
416,000 | |||
|
|
||||
The salary actually paid to Mr. Groenhuysen in 2014 and reported in the summary compensation table reflects a blended rate of his base salary at the start of fiscal 2014 of $577,500 and a rate of $600,600 following a performance-based raise in February 2014.
Cash bonuses
2014 Cash Bonus Program
In general, each executive officer’s bonus is determined by the achievement of corporate goals and personal objectives. Under their respective employment agreements, certain executives have pre-established target bonus amounts (expressed as a percentage of base salary) payable at the discretion of the board or compensation committee. For our CEO, the actual amount of his bonus award is determined solely based on our achievement of corporate objectives. For our other executive officers, the actual amount of the bonus award is determined by the executive’s level of achievement against his or her personal objectives (comprising 10% of the bonus), in combination with the achievement of the corporate objectives (comprising 90% of the bonus). The corporate objectives for 2014 included commercial objectives, such as gross billings targets in the United States and launches of certain projects; clinical regulatory and medical affairs objectives, such as opening and enrolling certain trials; finance, health policy and reimbursement and billing operations objectives, including establishing certain payment functions and securing contracts with private payers; and operations, supply chain and engineering objectives.
Our NEOs received the following annual cash bonuses in respect of 2014 performance, in large part in recognition of the continued growth of the business and the successful interim results of the EF-14 clinical trial for newly diagnosed GBM:
| Named executive officer | FY 2014 target bonus (% Base Salary) |
Realization
(%) corporate achievement/personal objectives |
Actual FY 2014 bonus ($) | |||||||||
| Asaf Danziger |
50% | 100%/n/a | 312,000 | |||||||||
| Wilhelmus Groenhuysen |
50% | 100%/100% | 300,300 | |||||||||
| Eilon Kirson |
50% | 100%/100% | 208,000 | |||||||||
|
|
||||||||||||
The target annual cash bonus percentages for Messrs. Groenhuysen and Kirson were as set forth in each of their respective employment agreements (as described below). Mr. Danziger’s target annual cash bonus percentage is established by the compensation committee each year, in its discretion, in accordance with the terms of his employment agreement. The compensation committee determined that for fiscal 2014, Mr. Danziger’s target annual cash bonus would be equal to 50% of his base salary, consistent with the target annual cash bonus percentages for our other named executive officers.
122
Table of Contents
2015 Cash Bonus Program
The target amount of the annual cash bonus for each of our NEOs for fiscal 2015 is described below:
| Named executive officer | FY 2015 target bonus (% Base Salary) |
FY 2015 target bonus ($) | ||||||
| Asaf Danziger |
75% | 468,000 | ||||||
| Wilhelmus Groenhuysen |
50% | 300,300 | ||||||
| Eilon Kirson |
50% | 208,000 | ||||||
|
|
||||||||
For 2015, Messrs. Groenhuysen and Kirson continue to have a target annual cash bonus equal to 50% of their respective base salaries as per their respective employment agreements. The compensation committee determined that for fiscal 2015, Mr. Danziger’s target annual cash bonus would increase to 75% of his base salary to more closely align with market practice.
The corporate objectives for 2015 included business unit objectives (weighted at 30%), such as increasing demand for Optune and launches of certain projects; clinical regulatory and medical affairs objectives (weighted at 30%), such as opening and enrolling certain trials and obtaining approvals for new delivery systems; finance and health policy objectives (weighted at 20%), including securing contracts with private payers; and operations, supply chain, engineering and human resources objectives (weighted at 20%).
Going forward, we expect that we will in the future adopt a formal annual bonus plan or program, which may be a sub-plan under the umbrella of our 2015 Omnibus Incentive Plan (as further described below).
Long-term equity compensation
On February 26, 2014, our board of directors approved stock option awards under our 2013 Share Option Plan to our NEOs as follows:
| Named executive officer | Stock options (#) | Exercise price ($) | ||||||
| Asaf Danziger |
88,695 | 7.48 | ||||||
| Wilhelmus Groenhuysen |
59,130 | 7.48 | ||||||
| Eilon Kirson |
59,130 | 7.48 | ||||||
|
|
||||||||
Set forth below is a summary of the terms of the stock options granted to our NEOs in fiscal 2014.
| • | The options granted to Mr. Danziger and Mr. Kirson were intended to be Section 102(b)(2) options under the Israeli Income Tax Ordinance (as described below). The options granted to Mr. Groenhuysen were intended to be incentive stock options under the Internal Revenue Code. |
| • | The exercise price per share subject to the stock options is equal to the fair market value of an ordinary share on the date of grant. |
| • | The options generally vest in four equal installments of 25% of the shares subject to the option on each of the first four anniversaries of the grant date, subject to the optionee’s continued service through the applicable vesting date. |
| • | The options expire ten years from the date of grant to the extent not theretofore exercised. |
| • | Upon a termination for cause, as defined in our 2013 Share Option Plan, all outstanding options, whether vested or unvested, will immediately be forfeited on the date of termination. |
123
Table of Contents
| • | Upon a termination due to death or disability, unvested options will generally be forfeited on the date of termination, but the optionee will have 12 months to exercise any options that were vested and exercisable at the time of termination. |
| • | Upon a termination by us without cause or a voluntary termination by the optionee, unvested options will generally be forfeited on the date of termination, but the optionee will have 180 days to exercise any options that were vested and exercisable at the time of termination. |
On March 5, 2015, our board of directors approved stock option awards under our 2013 Share Option Plan to our NEOs as follows:
| Named executive officer | Stock options (#) | Exercise price ($) | ||||||
| Asaf Danziger |
354,780 | 14.37 | ||||||
| Wilhelmus Groenhuysen |
266,085 | 14.37 | ||||||
| Eilon Kirson |
266,085 | 14.37 | ||||||
|
|
||||||||
The stock options granted to our NEOs in fiscal 2015 have substantially similar terms as those granted in fiscal 2014 and described above.
Other employee benefits and compensation
We provide limited executive perquisites to some of our NEOs and limited change-in-control benefits as described further below. We generally provide our NEOs in the United States and Israel with the same benefits generally provided to all other employees in the United States and Israel, respectively.
In the United States, we sponsor a tax-qualified 401(k) defined contribution plan. Our 401(k) plan, which is generally available to all employees, allows participants to defer amounts of their annual compensation before taxes, up to the maximum amount specified by the U.S. Internal Revenue Code of 1986, as amended, or the Code, which was $17,500 per person for calendar year 2014 and is $18,000 for calendar year 2015. We do not currently provide , and never have provided, matching contributions or profit-sharing contributions to the plan, although we may do so in the future.
In Israel, we generally provide our executives with severance, pension, disability and education benefits in line with both Israeli law as well as customary compensation practices among technology companies. Israel’s Severance Pay Law generally entitles employees with one or more years of service to a severance benefit equal to one month’s salary (together with any fixed additional payments that are paid unconditionally each month) for each year of service in the event of a termination by the company or, in limited circumstances, a resignation by the employee due to a constructive dismissal. Certain mandatory pension requirements are also required in light of certain expansion orders that apply to all employees in the industrial sector as well as to all employees in Israel, as the case may be. However, by virtue of Section 14 of the Severance Pay Law, the Minister of Economy (formerly the Ministry of Labor and Welfare) promulgated a General Approval Regarding Payments By Employers to a Pension Fund and Insurance Fund in lieu of Severance Pay According to Severance Pay Law, 5723-1963, or the General Approval, which enables a company and an employee to contractually agree to severance benefits that differ from and apply in lieu of the statutory severance obligations. Payments in accordance with Section 14 of the Severance Pay Law release the employer from any future actual severance payments in respect of its employees, as those employees are entitled upon termination of employment only to the sums accrued in their Managers Insurance Policy (bituach menahalim) or pension fund (as the case may be) with respect to severance pay. Such contractual arrangements are common for employees in Israel in the technology sector.
124
Table of Contents
For each of Mr. Danziger and Mr. Kirson, we have contractually agreed to adhere to the provisions of the General Approval and to contribute on a monthly basis to a Managers Insurance Policy (bituach menahalim) on his behalf. Such monthly contributions cover pension and disability benefits as well as severance pay in lieu of our statutory obligation to provide such payment under the Severance Pay Law. Pursuant to their respective employment agreements (as described more fully below), each of Mr. Danziger and Mr. Kirson is the beneficiary of a Managers Insurance Policy pursuant to which we contribute, on a monthly basis, 8.33% of his monthly gross salary in respect of severance, 5% of monthly gross salary in respect of pension benefits and up to 2.5% of monthly gross salary in respect of disability benefits. In addition to these company contributions, each of Mr. Danziger and Mr. Kirson contributes to his Managers Insurance Policy by way of a deduction from his monthly salary, a monthly amount equal to 5% of his monthly gross salary. Earnings on amounts contributed to the Managers Insurance Policy are determined under the terms of the policy that is selected by the executive. Except in extreme cases (as may be adjudicated by a competent Labor Court) or if Mr. Danziger or Mr. Kirson impermissibly draws upon such policy (as described in the General Approval attached as an appendix to the relevant employment agreement), Mr. Danziger or Mr. Kirson, as the case may be, will receive all severance and pension amounts accumulated in his Managers Insurance Policy upon any termination of his employment (including a resignation that otherwise would not result in the payment of statutory severance under the Severance Pay Law). Disability benefits would be paid, if at all, subject to the terms of the specific disability policy acquired by Mr. Danziger or Mr. Kirson (generally only if he is unable to continue working in his profession or to work at all).
In addition, we contribute to an advance study fund/professional education fund (keren hishtalmut) for the benefit of Mr. Danziger and of Mr. Kirson in Israel. These professional education funds are similar to tax-qualified defined contribution savings plans in the United States because contributions up to a certain amount are generally not subject to tax, the funds are managed by insurance companies or investment companies, and their entire activity in this regard is highly regulated by the Ministry of Finance. To qualify for the tax benefits, the employee-beneficiary of the professional education fund is required not to use the funded amounts for a period of at least six years from the establishment of the fund. For each of Mr. Danziger and Mr. Kirson, during each month of his employment, we contribute an amount equal to 7.5% of his monthly gross salary to a professional education fund, but not in excess of the highest amount of such contributions deductible or creditable for Israeli tax purposes. Up to these limits, there is no taxation on the contributions. However, any amount in excess of the highest deductible or creditable amount for such tax purposes is paid to Mr. Danziger or Mr. Kirson concurrently with his monthly salary and taxed like salary at ordinary income rates (but separate from, and not part of, his monthly gross salary as to which the Managers Insurance Policy contributions are required). In addition to contributions that we make to the professional education funds for each of Mr. Danziger and Mr. Kirson, we deduct 2.5% of his monthly gross salary for employee contributions to these funds. Once we make contributions to the funds, Mr. Danziger’s and Mr. Kirson’s rights to the amounts in the funds are generally governed solely by the applicable agreements and policies between the executive and the applicable insurance or investment company.
Except as described above, we do not currently sponsor or contribute to any qualified or non-qualified defined benefit plan or any non-qualified defined contribution plan, and we do not currently maintain (or have any outstanding obligation with respect to) any traditional non-qualified deferred compensation plan or other deferred compensation plans.
125
Table of Contents
Outstanding equity awards at fiscal year end—plan-based awards
The following table sets forth information regarding outstanding option awards under the 2003 Share Option Plan and 2013 Share Option Plan held by our NEOs as of December 31, 2014. This table includes unexercised and unvested option awards. For additional information regarding these option awards, see “Long-term equity compensation” above and “Share option and other equity compensation plans” below.
| Named executive officer | Number
of securities underlying unexercised options (#)—exercisable |
Number of securities underlying unexercised options (#)—unexercisable |
Option exercise price ($ per share) |
Option expiration date |
||||||||||||
| Asaf Danziger |
59,130 | (1) | — | 0.17 | 5/1/2016 | |||||||||||
| 342,954 | (2) | — | 0.17 | 3/28/2017 | ||||||||||||
| 354,780 | (3) | — | 0.38 | 10/12/2020 | ||||||||||||
| 335,147 | (4) | 111,716 | 3.44 | 12/14/2021 | ||||||||||||
| — | (5) | 88,695 | 7.48 | 2/26/2024 | ||||||||||||
| Wilhelmus Groenhuysen |
327,017 | (6) | 196,715 | 3.44 | 12/14/2021 | |||||||||||
| 22,173 | (7) | 66,521 | 7.03 | 2/20/2023 | ||||||||||||
| — | (8) | 59,130 | 7.48 | 2/26/2024 | ||||||||||||
| Eilon Kirson |
29,565 | (9) | — | 0.17 | 5/1/2016 | |||||||||||
| 156,073 | (10) | — | 0.17 | 12/12/2016 | ||||||||||||
| 57,651 | (11) | 19,217 | 3.44 | 9/15/2021 | ||||||||||||
| 53,217 | (12) | 17,739 | 3.44 | 12/14/2021 | ||||||||||||
| 54,695 | (13) | 164,086 | 7.03 | 2/20/2023 | ||||||||||||
| — | (14) | 59,130 | 7.48 | 2/26/2024 | ||||||||||||
|
|
||||||||||||||||
| (1) | These options were granted as Section 102(b)(2) options on May 1, 2006 in respect of 59,130 ordinary shares. One-half of the options became vested on May 1, 2008 and one-fourth of the options became vested on May 1 of each of 2009 and 2010. |
| (2) | These options were granted as Section 102(b)(2) options on March 28, 2007 in respect of 342,954 ordinary shares. One-fourth of the options became vested on March 28, 2008 and one-fourth of the options became vested on March 28 of each of 2009, 2010 and 2011. |
| (3) | These options were granted as Section 102(b)(2) options on October 12, 2010 in respect of 354,780 ordinary shares. One-fourth of the options became vested on October 12 of each of 2011, 2012, 2013 and 2014. |
| (4) | These options were granted as Section 102(b)(2) options on December 14, 2011. One-fourth of the options became vested on December 14 of each of 2012, 2013 and 2014 and one-fourth of the options will become vested on December 14, 2015, subject to Mr. Danziger’s employment through the applicable vesting date. |
| (5) | These options were granted as Section 102(b)(2) options on February 26, 2014. One-fourth of the options became vested on February 26, 2015 and one-fourth of the options would become vested on February 26 of each of 2016, 2017 and 2018, subject to Mr. Danziger’s employment through the applicable vesting date. |
| (6) | These options were granted on December 14, 2011. One-fourth of the options became vested on December 14 of each of 2012, 2013 and 2014 and one fourth of the options will be vested on December 14, 2015, subject to Mr. Groenhuysen’s employment through such date. |
| (7) | These options were granted on February 20, 2013. One-fourth of the options became vested on February 20 of each of 2014 and 2015, and one-fourth of the options will become vested on February 20 of each of 2016 and 2017, subject to Mr. Groenhuysen’s employment through the applicable vesting date. |
126
Table of Contents
| (8) | These options were granted on February 26, 2014. One-fourth of the options became vested on February 26, 2015 and one-fourth of the options will become vested on February 26 of each of 2016, 2017 and 2018, subject to Mr. Groenhuysen’s employment through the applicable vesting date. |
| (9) | These options were granted as Section 102(b)(2) options on May 1, 2006 in respect of 29,565 ordinary shares. One-half of the options became vested on May 1, 2008 and one-fourth of the options became vested on May 1 of each of 2009 and 2010. |
| (10) | These options were granted as Section 102(b)(2) options on December 12, 2006 in respect of 156,073 ordinary shares. One-half of the options became vested on December 12, 2008 and one-fourth of the options became vested on December 12 of each of 2009 and 2010. |
| (11) | These options were granted as Section 102(b)(2) options on September 15, 2011 in respect of 76,869 ordinary shares. One-fourth of the options became vested on September 15 of each of 2012, 2013 and 2014 and one fourth of the options will become vested on September 15, 2015, subject to Mr. Kirson’s employment through the applicable vesting date. |
| (12) | These options were granted as Section 102(b)(2) options on December 14, 2011 in respect of 70,956 ordinary shares. One-fourth of the options became vested on December 14 of each of 2012, 2013 and 2014 and one fourth of the options will become vested on December 14, 2015, subject to Mr. Kirson’s employment through the applicable vesting date. |
| (13) | These options were granted as Section 102(b)(2) options on February 20, 2013 in respect of 218,781 ordinary shares. One-fourth of the options became vested on February 20 of each of 2014 and 2015, and one-fourth of the options will become vested on February 20 of each of 2016 and 2017, subject to Mr. Kirson’s employment through the applicable vesting date. |
| (14) | These options were granted as Section 102(b)(2) options on February 26, 2014 in respect of 59,130 ordinary shares. One-fourth of the options became vested on February 26, 2015 and one-fourth of the options will become vested on February 26 of each of 2016, 2017 and 2018, subject to Mr. Kirson’s employment through the applicable vesting date. |
Executive employment arrangements
In connection with their commencement of employment, we entered into written employment agreements with each of Asaf Danziger, Wilhelmus Groenhuysen and Eilon Kirson, as well as Kinyip Gabriel Leung (whose agreement is described below under “Director compensation”). These employment agreements, as generally described below, were intended to acknowledge and set forth the terms and conditions of each executive’s employment with us, including each executive’s duties and responsibilities, base salary and bonus eligibility, initial sign-on stock option grant, employee benefit entitlement and severance protection. In addition, each of the employment agreements includes certain restrictive covenants, including non-competition, non-solicitation, non-disclosure and/or non-disparagement covenants, which are intended to protect our interests as well as the interests of our shareholders, affiliates, directors, officers and employees.
We do not have any employment or other individual agreements with or in respect of any NEO that provide for an excise tax gross-up payment. To the contrary, the employment agreement with Wilhelmus Groenhuysen provides that in the event that the executive’s receipt of payments or distributions would subject him to the golden parachute excise tax under Section 4999 of the Code, the amount of parachute payments within the meaning of Section 280G of the Code will be reduced to the greatest amount payable that would not result in such tax, but only if it is determined such reduction would cause the executive to be better off, on a net after-tax basis, if such payments were so reduced than without such reduction and payment of the excise tax under Section 4999 of the Code.
127
Table of Contents
Danziger employment agreement
On October 1, 2002, we entered into an employment agreement with Asaf Danziger pursuant to which Mr. Danziger agreed to serve as our Chief Executive Officer. Mr. Danziger’s employment agreement has an indefinite term and may generally be terminated upon six months’ prior written notice to the other party, except in connection with a termination of Mr. Danziger’s employment by us for cause, as defined in his employment agreement, or a termination by Mr. Danziger as a result of our substantial breach of our obligations under the employment agreement. However, regardless of the reason for termination, Mr. Danziger’s employment agreement provides that he will coordinate with his successor in transitioning such successor to be our Chief Executive Officer.
Under Mr. Danziger’s employment agreement, he was initially entitled to a monthly base salary of US $10,800; however, Mr. Danziger’s annual base salary is currently $624,000 per year (and paid in NIS, at a fixed exchange rate of 4 NIS/US $1). The employment agreement also provides that we contribute, on a monthly basis, an amount equal to 8.33% of Mr. Danziger’s monthly gross salary towards a Managers Insurance Policy to cover severance pay benefits (and will deduct 5% of Mr. Danziger’s monthly gross salary towards such Managers Insurance Policy) as well as an amount equal to 5% of his monthly salary to cover pension benefits (tagmulim). Further, we contribute, on a monthly basis, up to 2.5% of Mr. Danziger’s monthly gross salary to cover work disability plan coverage. In addition, the employment agreement requires that we make contributions on a monthly basis on Mr. Danziger’s behalf in an amount equal to 7.5% of Mr. Danziger’s monthly gross salary to cover an advance study fund/professional education fund, but not in excess of the highest amount of such contributions deductible or creditable for Israeli tax purposes (provided that any excess of the 7.5% monthly salary amount and the highest deductible or creditable amount for such tax purposes is paid to Mr. Danziger concurrently with his monthly salary, but not considered part of his monthly gross salary as to which the Managers Insurance Policy and professional education fund contributions relate). In addition to contributions from us, we deduct 2.5% of Mr. Danziger’s monthly gross salary for contribution to such professional education fund. In addition, we are required under the employment agreement to make an automobile available to Mr. Danziger and to bear all fixed and current costs with respect to the automobile, and to pay to Mr. Danziger, on a monthly basis with his salary, an amount sufficient to cover any income tax imposed on his use of the automobile (such amounts specifically excluded from Mr. Danziger’s monthly gross salary to which the Managers Insurance Policy and professional education fund contributions relate). In lieu of providing Mr. Danziger with a company car, we currently satisfy our automobile obligations with Mr. Danziger by paying him cash sufficient to cover the costs of his automobile on an after-tax basis. The total amount paid to Mr. Danziger in 2014 with respect to the automobile payments was $45,049. Mr. Danziger’s employment agreement entitles him to 28 days of paid vacation per year; however, to the extent Mr. Danziger does not take his vacation, his employment agreement permits him to elect, on an annual basis, to either accumulate his accrued but unused vacation time for use in subsequent years or to receive an amount in cash equal to the base salary payable with respect to the number of accrued but unused vacation days.
Upon termination of Mr. Danziger’s employment agreement for any reason, the employment agreement provides that we will assign all of our rights to the Managers Insurance Policy to Mr. Danziger, except that if Mr. Danziger’s right to severance under Israeli Severance Pay Law is deprived under verdict (but only to the extent so deprived) or if Mr. Danziger impermissibly draws upon such policy (as described in the General Approval attached as an appendix to the employment agreement), in either case, Mr. Danziger will not be assigned the rights to such severance benefit policy. In addition, the compensation committee anticipates amending Mr. Danziger’s employment agreement to provide for severance upon certain limited terminations of employment not to exceed 12 months’ base salary and continuation of benefits, or, following a change of control, 24 months’ base salary, two times his bonus and 24 months’ benefit continuation, along with full acceleration of his outstanding equity awards.
128
Table of Contents
Pursuant to his employment agreement, Mr. Danziger is subject to a duty of confidentiality and non-disclosure, and during his employment and for 12 months thereafter, Mr. Danziger agrees that he will not engage in the development, production, sales and/or marketing of tools for medical treatments by means of alternating electric fields anywhere in the world other than for our exclusive benefit or otherwise solicit our business partners with respect to any such activities, and further, that he will not solicit, employ or offer to employ our employees or consultants.
Groenhuysen employment letter agreement
On December 23, 2011, we entered into an employment letter agreement with Wilhelmus Groenhuysen pursuant to which Mr. Groenhuysen serves as our Chief Financial Officer. Pursuant to his employment agreement, Mr. Groenhuysen was to receive an annual base salary of $400,000 per year until January 1 of the year immediately following the completion of an initial public offering, at which time Mr. Groenhuysen’s annual base salary would increase to $550,000 per year; however, Mr. Groenhuysen’s current base salary is $600,600. During his employment, the employment agreement provides that Mr. Groenhuysen is eligible to receive a discretionary annual cash bonus of up to 50% of his annual base salary subject to successful achievement of performance goals set by our board of directors or compensation committee or our Chief Executive Officer in their sole discretion, and further subject to his continued employment through the payment date (which payment date will occur during the first quarter of the following calendar year).
Pursuant to Mr. Groenhuysen’s employment agreement, we granted to Mr. Groenhuysen sign-on options to purchase 786,860 ordinary shares at a price per share of $3.44. These sign-on options vest in four equal installments of 25% of the shares subject to the option on each of the first four anniversaries of his start date, subject to continued service through the applicable vesting date and subject to full acceleration of vesting upon the consummation of a change of control that occurs during his employment or within six months following the termination of his employment. Of these options, Mr. Groenhuysen has exercised his option to purchase 263,128 ordinary shares. Mr. Groenhuysen’s employment agreement provides him with reimbursement for reasonable fees and expenses incurred in negotiating his employment agreement and up to $10,000 per calendar year for financial planning expenses. In addition, Mr. Groenhuysen’s employment agreement entitles him to a monthly automobile allowance of $1,100 per month.
Upon our termination of Mr. Groenhuysen’s employment other than for cause or as a result of his death or disability or a termination of Mr. Groenhuysen’s employment by Mr. Groenhuysen for good reason (as such terms are defined in Mr. Groenhuysen’s employment agreement), then, subject to his execution without revocation of a release of claims, he will receive a lump sum payment equal to 12 months’ base salary and, to the extent he timely elects COBRA continuation coverage and pays the full monthly premiums, a monthly amount equal to the full monthly premium for COBRA continuation coverage for the level of coverage in effect for Mr. Groenhuysen and his eligible dependents as of the date of termination, for up to 12 months following the date of termination. The compensation committee anticipates amending his employment agreement so that if such termination follows a change of control, Mr. Groenhuysen will also receive a severance payment not to exceed the amount of his bonus. In addition, the employment agreement provides that the portion of his sign-on options that would have become vested on the next scheduled vesting date would become vested on the date of his termination and any unvested options would fully vest if a change of control had occurred during his employment or within six months following termination without cause or by him for good reason as of the date of such change of control. In addition, Mr. Groenhuysen’s employment agreement provides that, in the event that Mr. Groenhuysen would be subject to the excise tax under Section 4999 of the Code, his payments will be reduced to the greatest amount payable that would not result in such tax, but only if it is determined such reduction would cause Mr. Groenhuysen to be better off, on a net after-tax basis, than without such reduction and payment of the excise tax under Section 4999 of the Code.
129
Table of Contents
Pursuant to his employment agreement, Mr. Groenhuysen is subject to confidentiality and non-disparagement covenants, as well as covenants of non-competition and non-solicitation of employees, customers and suppliers during his employment and for 12 months thereafter.
Kirson employment agreement
On June 2, 2002, we entered into an employment agreement with Eilon Kirson pursuant to which Mr. Kirson agreed to serve as our Head of Biomedical Engineering. He currently serves as our Chief Science Officer and Head of Research and Development. Mr. Kirson’s employment agreement has an indefinite term and may generally be terminated upon thirty days’ prior written notice to the other party, except in connection with our liquidation, dissolution or winding-up.
During Mr. Kirson’s employment with us, he is entitled to a per year monthly base salary of NIS 25,000, which has been subsequently raised to $416,000 per year (and paid in NIS at a fixed exchange rate of 4 NIS/US $1), and our contributions on his behalf on a monthly basis towards an insurance policy in respect of (i) a professional education fund (7.5% of base salary per month, on top of monthly deductions from Mr. Kirson’s base salary of 2.5% of such monthly salary), (ii) severance pay and pension benefits (13.33% of base salary per month on a monthly basis, on top of monthly deductions from Mr. Kirson’s base salary of 5% of such monthly salary) and (iii) work disability plan coverage (2.5% of base salary per month). In addition, the employment agreement requires us to make available to Mr. Kirson an automobile and to bear all fixed and current costs with respect to the automobile.
Mr. Kirson’s employment agreement provides that he is entitled to participate in our stock option plans.
Upon termination of the employment agreement with Mr. Kirson for any reason, the employment agreement provides that we will assign all of our rights to the severance benefit insurance policy to Mr. Kirson described above, except that if Mr. Kirson’s right to severance under Israeli Severance Pay Law is deprived under verdict (but only to the extent so deprived) or if Mr. Kirson impermissibly draws upon such policy (as described in the General Approval attached as an appendix to the employment agreement), in either case, Mr. Kirson will not be assigned the rights to such severance benefit policy. In addition, the compensation committee anticipates amending Mr. Kirson’s employment agreement to provide for severance in connection with certain limited terminations of employment not to exceed 9 months’ base salary and benefit continuation, or, following a change of control, 12 months’ base salary, one times his bonus and 12 months’ benefit continuation and full acceleration of his outstanding equity awards.
Pursuant to his employment agreement, Mr. Kirson is subject to a duty of confidentiality and non-disclosure. Further, during his employment, Mr. Kirson agreed that he will not engage in the development, production, sales and/or marketing of tools for medical treatments by means of alternating electric fields anywhere in the world other than for the exclusive benefit of us (a “Devoted Activity”) and that during his employment and for 12 months thereafter, he will not solicit our business partners with respect to a Devoted Activity, and further, that he will not solicit or employ our employees or consultants.
Share option and other equity compensation plans
Our equity compensation program prior to 2013 was administered by our board of directors and since 2013 has been administered by our compensation committee. Our equity program is designed to be competitive so that we may attract and retain talented executives. We have historically used stock options as the form of equity award for executives, independent directors and other employees. Our board of directors and our compensation committee believe that stock option awards align the interests of our executive officers with those of our shareholders because they create the incentive to build shareholder value over time. Stock options are granted with an exercise price equal to at least the fair market value of our stock on the applicable date of grant.
130
Table of Contents
We have historically granted stock options to executive officers in connection with their compensation package entered into at the time of their hiring. The size of the initial stock option award is determined based on the executive’s position with us and takes into consideration the executive’s base salary, bonus opportunities and other compensation. The initial stock option awards are intended to provide the executive with an incentive to build value in the organization over an extended period of time, typically vesting in installments on the basis of continued service over a period of four years following the date of grant. We may also grant stock options in connection with a significant change in responsibilities, past performance and anticipated future contributions of the executive officer, as well as achievement of overall company goals, taking into consideration the executive’s overall compensation package and the executive’s existing equity holdings. While these grants may be made based on performance, vesting of the grants has traditionally been based on continued service over time.
The prospect of a change in control can cause significant distraction and uncertainty for executive officers. Accordingly, our compensation committee believes that appropriate change in control provisions in equity award agreements are important tools for aligning executives’ interests in change in control transactions with those of our shareholders by allowing our executive officers to focus on strategic transactions that may be in the best interest of our shareholders without undue concern regarding the effect of such transactions on their continued employment, the value of and rights under their existing stock holdings and equity compensation awards and the tax impact of a transaction on their net compensation. The change of control provisions in each of the 2003 Plan, 2013 Plan and 2015 Plan are described below.
2003 Share Option Plan
We and our shareholders approved and adopted our 2003 Share Option Plan, which we refer to as our 2003 Plan, effective as of March 3, 2003. Since March 2013, when the 2003 Plan expired, we have made grants pursuant to our 2013 Share Option Plan (as described below) and, following completion of this offering, all future equity grants will be made under our 2015 Omnibus Incentive Plan (as described below). However, any awards granted under the 2003 Plan that are outstanding as of the time of this offering will continue to be subject to the terms and conditions of the 2003 Plan and the applicable option award agreement, each as of immediately prior to this offering.
The following description of the 2003 Plan is qualified in its entirety by the full text of the 2003 Plan, which is filed with the SEC as an exhibit to the registration statement of which this prospectus forms a part.
Shares subject to the 2003 Plan
Pursuant to the terms of the 2003 Plan, if outstanding stock options expire or are cancelled without having been fully exercised, the underlying shares would again be available for awards. However, following the adoption of our 2013 Plan, no further awards will be granted under the 2003 Stock Option Plan, and instead, pursuant to the terms of the 2013 Plan, if outstanding stock options under the 2003 Plan expire or are canceled without having been fully exercised, the underlying shares may again be subject to an option under the 2013 Plan (provided that the total number of shares with respect to which options may be issued under the 2013 Plan shall not exceed 14,782,500 shares). However, upon the effectiveness of this offering and the adoption of the 2015 Plan, no further awards will be made under the 2013 Plan.
For companies that become public in connection with an initial public offering, the deduction limit under Section 162(m) of the Code does not apply during a reliance period under the Treasury Regulations under Section 162(m) of the Code, which may be relied upon until the earliest of: (i) the expiration of the 2003 Plan; (ii) the date the 2003 Plan is materially amended for purposes of Treasury Regulation Section 1.162-27(h)(1)(iii);
131
Table of Contents
(iii) the date all ordinary shares available for issuance under this Plan have been allocated; and (iv) the date of the first annual meeting of our shareholders at which directors are to be elected that occurs after the close of the third calendar year following the calendar year in which the initial public offering occurs.
Administration
The 2003 Plan is administered by our board of directors or a committee appointed by the board for such purpose. As described above, the 2003 Plan from inception to 2012 was administered by our board of directors and thereafter has been administered by our compensation committee. Accordingly, except as otherwise described below, references to our compensation committee in the following description of our 2003 Plan should be interpreted as referring to our full board of directors serving in such capacity during the relevant time period. Subject to law and our articles of association, our compensation committee has sole authority to determine to whom, at what time, for what duration, per what vesting schedule and in what amount options may be granted, as well as other terms arising under the 2003 Plan. Our compensation committee has full power and authority to interpret the 2003 Plan’s provisions, to make determinations and adopt rules, regulations or agreements for implementing the 2003 Plan and to determine any other matter necessary, desirable or incidental to the administration of the 2003 Plan. Our compensation committee’s determinations will be final and conclusive.
Eligibility; types of awards
All of our employees (including officers), directors and consultants (including those of any affiliate or subsidiary) were eligible to participate under the 2003 Plan as recipients of awards.
Stock options generally
The 2003 Plan provides for the grant of only stock options. Options granted under the 2003 Plan are designated and structured as either non-qualified stock options or incentive stock options. Certain options granted under the 2003 Plan are intended to provide the recipient with special tax treatment in accordance with the Israeli Income Tax Ordinance, as further described below.
Our compensation committee determines the stock option exercise price, provided that the stock option exercise price is generally at least equal to the fair market value of our ordinary shares on the grant date. However, an incentive stock option will not be granted to any employee who, at the time of grant, holds more than 10% of the total combined voting power of all classes of our stock, unless the option price per share is not less than 110% of the fair market value of our ordinary shares on the date of grant and the option exercise period is not more than five years from the date of grant.
If our ordinary shares are not publicly traded when options are granted (as is the case for all options granted under the 2003 Plan), fair market value will be determined in good faith by our compensation committee.
Our compensation committee determines the option exercise period of each stock option (which may not exceed ten years from the date of grant and will be shorter in certain cases, such as in the case of incentive stock options granted to employees holding more than 10% of our total combined voting power). Stock options become exercisable in accordance with the terms and conditions determined by our compensation committee and specified in the applicable award agreement. If the option holder ceases to be our employee or consultant as a result of termination, unvested and unexercisable stock options generally terminate upon the date of termination. If the option holder ceases to be our employee or consultant as a result of a termination by us for cause (as defined in the 2003 Plan), each stock option generally terminates upon the date of termination, whether vested or unvested. If the option holder ceases to be our employee or consultant as a result of death or
132
Table of Contents
disability, each vested and exercisable stock option generally terminates upon the one-year anniversary of the date of termination, but no later than the expiration of the option exercise period. If the option holder ceases to be our employee or consultant as a result of a termination of employment by us without cause or voluntarily by the employee, each vested stock option generally terminates after 180 days following the date of termination, but no later than the expiration of the option exercise period.
Option holders may exercise their vested stock options by delivering written notice to our compensation committee. Option holders may pay the exercise price in cash, by tendering shares of our ordinary shares or a combination thereof. In order to exercise their stock options, option holders may, pursuant to the award agreement, be required to make certain written representations and acknowledgments about their investment intent. We may also require the option holder to enter into certain other restrictive agreements.
Section 102 Options
Stock options granted under the 2003 Plan to recipients who are residents of the State of Israel, or who are deemed to be residents of the State of Israel for income tax purposes, are generally designated and structured so as to provide the recipient with special tax treatment, to the extent available, in accordance with the Israeli Income Tax Ordinance. In general, stock options granted to Israeli residents are subject to taxation upon exercise at working income tax rates as high as 48% and upon the sale of the underlying shares at capital gains rates (currently 25%). However, the Israeli Tax Authority provides employees, officers and directors who hold less than 10% of the share capital, voting power or profits interests of the issuing corporation with the ability to defer taxation in respect of certain stock options, or Section 102(b)(2) Options, and the shares or other property acquired upon the exercise of such Section 102(b)(2) Options until such time (if any) as the shares acquired upon exercise are transferred from the trustee to the employee or sold by the trustee, and then only at the applicable capital gains tax rate of 25%.
To qualify for this special tax treatment, the option plan must be approved by the corporation’s board of directors and filed with the Israeli Tax Authority for review and approval (which approval is usually deemed to occur thirty days after filing), and the Section 102(b)(2) Options (and any shares or other property issued in respect of such Section 102(b)(2) Options, whether in connection with exercise or pursuant to adjustments made in connection with a corporate transaction) must be deposited with a company-designated trustee within a certain period from actual grant and held in trust until the expiration of a holding period of at least 24 months (unless an exception is granted by the Israeli Tax Authority to sell or transfer the Section 102(b)(2) Options and other restricted property in respect of such Section 102(b)(2) Options). In the event that any of the requirements for Section 102(b)(2) Options are not met, the special tax benefits will not be available and ordinary work income taxes will become due.
The 2003 Plan options granted to Mr. Danziger and Mr. Kirson are intended to qualify as Section 102(b)(2) Options, and as such, are currently held by a trustee in accordance with Section 102(b) so as to qualify for the special tax treatment described above.
By contrast, options granted to Israeli residents that do not satisfy the requirements for 102(b) Options will generally be subject to tax upon the exercise of the option as well as the sale of the underlying shares at working income tax rates of up to 48%. In the case of Professor Palti, the special tax treatment applicable to Section 102(b)(2) Options was not available since Professor Palti, at the time of grant, held more than 10% of our share capital through his company, Bennet Enterprises. As such, his stock options are neither held in trust nor subject to any holding period.
133
Table of Contents
Corporate transactions
Under the 2003 Plan, upon a merger with or into another company, or the sale of all or substantially all of our assets or shares, our board of directors generally will determine whether outstanding options will be assumed or be substituted for options in the company resulting from the change in control or be treated in some other manner deemed equitable and appropriate, and except as otherwise determined by the compensation committee at the time of grant, if the successor company (or its parent) does not agree to assume or substitute the options, the vesting periods will be accelerated and the option will be immediately exercisable in full as of the date ten days prior to the consummation of such change of control transaction and the option holder will be provided with written notice of such acceleration of vesting at least ten days prior to such consummation.
Amendment, expiration and termination
Our board of directors may amend or terminate the 2003 Plan at any time, provided that shareholder approval is generally required to decrease the minimum option exercise price (except in connection with a permitted or required adjustment under the plan), to extend the term of the 2003 Plan or the period during which incentive stock options may be exercised or to modify the 2003 Plan’s eligibility requirements. No amendment to the 2003 Plan may be made in a way that would impair the rights of any holder of an outstanding option except as mutually agreed with the option holder.
Pursuant to its terms, the 2003 Plan expired ten years after its effective date. The 2003 Plan expired in March 2013, but awards granted under the 2003 Plan that were outstanding as of the time of expiration of the 2003 Plan continue to be subject to the terms and conditions of the 2003 Plan and the applicable option award agreement, each as then in effect, and to the extent still outstanding as of the time of this offering shall continue to be subject to such terms and conditions as of immediately prior to this offering.
2013 Share Option Plan
We and our shareholders approved and adopted our 2013 Share Option Plan, which we refer to as our 2013 Plan, effective as of April 24, 2013. The following description of the 2013 Plan is qualified in its entirety by the full text of the 2013 Plan, which is filed with the SEC as an exhibit to the registration statement of which this prospectus forms a part.
Shares subject to the 2013 Plan
Upon the adoption of the 2013 Plan, the total number of our ordinary shares that were initially reserved for future grants under the 2013 Plan was 381,033 shares plus any shares subject to then-outstanding stock options under the 2003 Plan that subsequently expire, terminate or are forfeited without having been fully exercised, which we refer to as Recyclable 2003 Awards, provided that the aggregate number of shares with respect to which options could be issued under the 2013 Plan could not exceed 2,000,000 shares. At the time of adoption of the 2013 Plan, there were 1,234,926 shares then subject to options under the 2003 Plan and, as such, the actual maximum number of shares with respect to which options could be issued under the 2013 Plan (assuming that all 1,234,926 shares then subject to options under the 2003 became Recyclable 2003 Awards) was 1,732,069 shares. Pursuant to the terms of the 2013 Plan, if outstanding stock options granted under the 2013 Plan expire or are canceled without having been fully exercised, the underlying shares may again be subject to an option under the 2013 Plan.
In February and March 2015, our board of directors and shareholders approved an increase in the number of ordinary shares reserved for issuance under the 2013 Plan by 500,000 shares, so that the total number of our
134
Table of Contents
ordinary shares reserved for issuance under the 2013 Plan is 881,033 shares plus any Recyclable 2003 Awards, provided that the aggregate number of shares with respect to which options could be issued under the 2013 Plan cannot exceed 2,500,000 shares.
As of June 30, 2015, there were outstanding awards in respect of 539,725 shares under the 2013 Plan and 990,676 shares under the 2003 Plan, and 413,119 shares remained available for future grants under the 2013 Plan (plus any Recyclable 2003 Awards and shares granted pursuant to the 2013 Plan which expire, terminate or are forfeited without having been fully exercised).
The numbers in the foregoing paragraphs are not adjusted to give effect to the 5.913-for-1 stock split effected on September 16, 2015.
For companies that become public in connection with an initial public offering, the deduction limit under Section 162(m) of the Code does not apply during a reliance period under the Treasury Regulations under Section 162(m) of the Code, as described above under “—2003 Share Option Plan.”
Notwithstanding the foregoing, as of the effectiveness of this offering, no further awards will be granted under the 2013 Plan and instead, future equity grants will be made under our 2015 Omnibus Incentive Plan (as described below). However, any awards granted under the 2013 Stock Option Plan that are outstanding as of the time of this offering shall continue to be subject to the terms and conditions of the 2013 Stock Option Plan as of immediately prior to this offering.
Administration
The 2013 Plan is administered by our board of directors or a committee appointed by the board for such purpose, and our board has appointed our compensation committee to administer the 2013 Plan. Subject to law and our articles of association, our compensation committee has sole authority to determine to whom, at what time, for what duration, per what vesting schedule and in what amount options may be granted, as well as other terms of awards under the 2013 Plan. Our compensation committee has full power and authority to interpret the 2013 Plan’s provisions, to make determinations and adopt rules, regulations or agreements for implementing the 2013 Plan and to determine any other matter necessary, desirable or incidental to the administration of the 2013 Plan. Our compensation committee’s determinations will be final and conclusive.
Eligibility; types of awards
All of our employees (including officers), directors and consultants (including those of any affiliate or subsidiary) are eligible for participation under the 2013 Plan as recipients of awards.
Stock options generally
The 2013 Plan provides for the grant of only stock options. Options granted under the 2013 Plan are designated and structured as either non-qualified stock options or incentive stock options. Certain options granted under the 2013 Plan are intended to provide the recipient with special tax treatment in accordance with the Israeli Income Tax Ordinance, as described above.
Our compensation committee will determine the stock option exercise price, provided that the stock option exercise price is generally at least equal to the fair market value of our ordinary shares on the grant date. However, an incentive stock option will not be granted to any employee who, at the time of grant, holds more than 10% of the total combined voting power of all classes of our stock, unless the option price per share is not less than 110% of the fair market value of our ordinary shares on the date of grant and the option exercise period is not more than five years from the date of grant.
135
Table of Contents
If our ordinary shares are not publicly traded when options are granted (as is the case for all options granted under our 2013 Plan), fair market value will be determined in good faith by our compensation committee.
Our compensation committee determines the option exercise period of each stock option (which may not exceed ten years from the date of grant and will be shorter in certain cases, such as in the case of incentive stock options granted to employees holding more than 10% of our total combined voting power). Stock options become exercisable in accordance with the terms and conditions determined by our compensation committee and specified in the applicable award agreement. If the option holder ceases to be our employee or consultant as a result of termination, unvested and unexercisable stock options generally terminate upon the date of termination. If the option holder ceases to be our employee or consultant as a result of a termination by us for cause (as defined in the 2013 Plan), each stock option generally terminates upon the date of termination, whether vested or unvested. If the option holder ceases to be our employee or consultant as a result of death or disability, each vested and exercisable stock option generally terminates upon the one-year anniversary of the date of termination, but no later than the expiration of the option exercise period. If the option holder ceases to be our employee or consultant as a result of a termination of employment by us without cause or voluntarily by the employee, each vested stock option generally terminates after 180 days following the date of termination, but no later than the expiration of the option exercise period.
Option holders may exercise their vested stock options by delivering written notice to our compensation committee. Option holders may pay the exercise price in cash, by tendering shares of our ordinary shares or a combination thereof. In order to exercise their stock options, option holders may, pursuant to the award agreement, be required to make certain written representations and acknowledgments about their investment intent. We may also require the option holder to enter into certain other restrictive agreements.
Section 102 options
The 2013 Plan options granted to Mr. Danziger and Mr. Kirson are intended to qualify as Section 102(b)(2) Options, and as such, are currently held by a trustee in accordance with Section 102(b) so as to qualify for the special tax treatment described above.
In the case of Professor Palti, the special tax treatment applicable to Section 102(b)(2) Options was not available since Professor Palti, at the time of grant, held more than 10% of our share capital through his company, Bennet Enterprises. As such, his stock options are neither held in trust nor subject to any holding period.
Corporate transactions
Under the 2013 Plan, upon a merger with or into another company, or the sale of all or substantially all of our assets or shares, our board of directors generally will determine whether outstanding options will be assumed or be substituted for options in the company resulting from the change in control or be treated in some other manner deemed equitable and appropriate, and except as otherwise determined by the compensation committee at the time of grant, if the successor company (or its parent) does not agree to assume or substitute the options, the vesting periods will be accelerated and the option will be immediately exercisable in full as of the date ten days prior to the consummation of such change of control transaction and the option holder will be provided with written notice of such acceleration of vesting at least ten days prior to such consummation.
Amendment, expiration and termination
Our board of directors may amend or terminate the 2013 Plan at any time, provided that shareholder approval is generally required to decrease the minimum option exercise price (except in connection with a permitted or
136
Table of Contents
required adjustment under the plan), to extend the term of the 2013 Plan or the period during which incentive stock options may be exercised or to modify the 2013 Plan’s eligibility requirements. No amendment to the 2013 Plan may be made in a way that would impair the rights of any holder of an outstanding option except as mutually agreed with the option holder.
Pursuant to its terms, the 2013 Plan will expire ten years after its effective date unless earlier terminated by our board of directors, but any then-outstanding awards will continue subject to the terms of the 2013 Plan. However, as noted above, as of the effectiveness of this offering, no further awards will be granted under the 2013 Plan and instead, future equity grants will be made under our 2015 Omnibus Incentive Plan (as described below), but awards granted under the 2013 Plan that are outstanding as of the time of this offering shall continue to be subject to the terms and conditions of the 2013 Plan as of immediately prior to this offering.
2015 omnibus incentive plan
On August 31, 2015, in anticipation of this offering, our board of directors adopted and established our 2015 omnibus incentive plan, or the 2015 Plan. Our shareholders approved the 2015 Plan on September 16, 2015 (which is the effective date of the 2015 Plan). We believe that a new omnibus incentive plan is appropriate in connection with a public offering of our ordinary shares not only to continue to enable us to grant awards to senior management to reward and incentivize their performance and retention, but also to have a long-term equity plan that is appropriate for us as a publicly held company. By contrast to our 2003 and 2013 Plans, which permitted the issuance of only stock options, the 2015 Plan will give us the flexibility and authority to issue various types of equity compensation awards such as restricted shares, performance shares, restricted stock units, performance units, long-term cash award and other share-based awards, as well as to provide us with the ability to structure tax-efficient equity and incentive award programs.
The material terms of the 2015 Plan are summarized below. The following summary is qualified in its entirety by reference to the complete text of the 2015 Plan, a copy of which is filed as an exhibit to the registration statement of which this prospectus forms a part.
Administration of the plan
The board of directors has appointed the compensation committee to administer the 2015 Plan. The compensation committee (or the board of directors, with respect to non-employee directors) are authorized to grant awards to eligible employees, consultants and non-employee directors. All members of the compensation committee are non-employee directors within the meaning of Rule 16b-3 under the Exchange Act, outside directors within the meaning of Section 162(m) of the Code and independent directors under applicable NASDAQ rules. Under the 2015 Plan, the full board of directors has the authority to grant awards to non-employee directors.
Number of authorized shares and award limits
The aggregate number of our ordinary shares that may be issued or used for reference purposes under the 2015 Plan may not exceed 11,980,000 shares, which amount shall automatically increase on December 31 of each year during the term of the 2015 Plan in an amount equal to 4% of the total number of ordinary shares outstanding on December 30 of such year unless otherwise determined by the board of directors. As of the date of this offering, 11,978,512 ordinary shares were available for grant under the 2015 Plan after taking into account the grants made in September 2015 conditioned upon the consummation of this offering.
Our ordinary shares that are subject to awards will be counted against the overall limit as one share for every share granted. If any award is cancelled, expires or terminates unexercised for any reason, the shares covered
137
Table of Contents
by such award will again be available for the grant of awards under the 2015 Plan, except that any shares that are not issued as the result of a net settlement or that are used to pay any exercise price or tax withholding obligation will not be available for the grant of awards.
The maximum number of our ordinary shares that may be subject to any award of share options or restricted shares or any other share-based award denominated in shares that is subject to the attainment of specified performance goals that may be granted under the 2015 Plan during any fiscal year to each employee or consultant is 2,900,000 shares per type of award, provided that the maximum number of our ordinary shares for all types of awards during any fiscal year does not exceed 4,400,000 shares.
In addition, the maximum aggregate grant date value of any other stock-based awards with respect to any fiscal year of the company that are denominated in cash that may be granted under the 2015 Plan to an employee or consultant with respect to any fiscal year is $15,000,000; and the maximum payment under any performance-based cash award granted under the 2015 Plan with respect to any calendar year to an employee or consultant is $15,000,000. However, the foregoing limits will not apply (i) to options, restricted shares or other stock-based awards that constitute “restricted property” under Code Section 83 to the extent granted during the Reliance Period (as defined below) or (ii) to performance-based cash awards or other types of other stock-based awards to the extent paid or otherwise settled during the Reliance Period.
For companies that become public in connection with an initial public offering, the deduction limit under Section 162(m) of the Code does not apply during a reliance period under the Treasury Regulations under Section 162(m) of the Code as described above under “—2003 Share Option Plan.”
The compensation committee will, in accordance with the terms of the 2015 Plan, make appropriate adjustments to the above aggregate and individual limits, to the number and/or kind of ordinary shares or other property (including cash) underlying awards and to the purchase price of ordinary shares underlying awards, in each case, to reflect any change in our capital structure or business by reason of any share split, reverse share split, share dividend, combination or reclassification of shares, any recapitalization, merger, consolidation, spin off, reorganization or any partial or complete liquidation, issuance of rights or warrants to purchase any ordinary shares or securities convertible into ordinary shares, any sale or transfer of all or part of our assets or business, or any other corporate transaction or event that would be considered an equity restructuring within the meaning of FASB ASC Topic 718.
Eligibility and participation
All of our and our affiliates’ employees and consultants and our and our affiliates’ prospective employees and consultants, as well as our non-employee directors, are eligible to be granted non-qualified share options, restricted share awards, performance-based cash awards and other share-based awards under the 2015 Plan. Only our and our subsidiaries’ employees are eligible to be granted incentive stock options, or ISOs, under the 2015 Plan. Eligibility for awards under the 2015 Plan is determined by the compensation committee in its sole discretion.
Types of awards
Stock options. The 2015 Plan authorizes the compensation committee to grant ISOs to eligible employees and non-qualified share options to purchase shares to employees, consultants, prospective employees, prospective consultants and non-employee directors. The compensation committee will determine the number of ordinary shares subject to each option, the term of each option, the exercise price (which may not be less than the fair market value of the ordinary shares at the time of grant, or 110% of fair market value in the case of ISOs granted to 10% shareholders), the vesting schedule and the other terms and conditions of each option. Options will be exercisable at such times and subject to such terms as are determined by the compensation committee at grant. The maximum term of options under the 2015 Plan is ten years (or five years in the case of ISOs
138
Table of Contents
granted to 10% shareholders). Upon the exercise of an option, the participant must make payment of the full exercise price, either in cash or by check, bank draft or money order; solely to the extent permitted by law, through the delivery of irrevocable instructions to a broker, reasonably acceptable to us, to promptly deliver to us an amount equal to the aggregate exercise price; or on such other terms and conditions as may be acceptable to the compensation committee (including, without limitation, the relinquishment of options or by payment in full or in part in the form of ordinary shares). Share options granted under the 2015 Plan to Israeli residents are expected to be designated and structured so as to qualify as Section 102(b)(2) Options, to the extent available, in accordance with the Israeli Income Tax Ordinance, as described above.
Restricted shares. The 2015 Plan authorizes the compensation committee to grant restricted share awards. Recipients of restricted share awards enter into an agreement with us subjecting the restricted share awards to transfer and other restrictions and providing the criteria or dates on which such awards vest and such restrictions lapse. The restrictions on restricted share awards may lapse and the awards may vest over time, based on performance criteria or other factors (including, without limitation, performance goals that are intended to comply with the performance-based compensation exception under Section 162(m) of the Code, as discussed below), as determined by the compensation committee at grant. Except as otherwise determined by the compensation committee, a holder of a restricted share award has all of the attendant rights of a shareholder, including the right to receive dividends, if any, subject to and conditioned upon vesting and restrictions lapsing on the underlying restricted stock award, the right to vote shares and, subject to and conditioned upon the vesting and restrictions lapsing for the underlying shares, the right to tender such shares. However, the compensation committee may in its discretion provide at grant that the right to receive dividends on a restricted share award will not be subject to the vesting or lapsing of the restrictions on the restricted share award.
Other share-based awards. The 2015 Plan authorizes the compensation committee to grant awards of ordinary shares and other awards that are valued in whole or in part by reference to, or are payable in or otherwise based on, ordinary shares, including, but not limited to, ordinary shares awarded purely as a bonus in lieu of cash and not subject to any restrictions or conditions; ordinary shares in payment of the amounts due under an incentive or performance plan sponsored or maintained by us or an affiliate; share appreciation rights; share equivalent units; restricted share units; performance awards entitling participants to receive a number of ordinary shares (or cash in an equivalent value) or a fixed dollar amount, payable in cash, shares or a combination of both, with respect to a designated performance period; or awards valued by reference to book value of our ordinary shares. In general, other share-based awards that are denominated in ordinary shares will include the right to receive dividends, if any, subject to and conditioned upon vesting and restrictions lapsing on the underlying award, but the compensation committee may in its discretion provide at grant that the right to receive dividends on a share-denominated award will not be subject to the vesting or lapsing of the restrictions on the other share-based award.
Certain performance-based awards
As noted above, following the Reliance Period, performance-based awards granted under the 2015 Plan that are intended to satisfy the performance-based compensation exception under Section 162(m) of the Code will vest based on attainment of specified performance goals established by the compensation committee. These performance goals will be based on the attainment of a certain target level of, or a specified increase (or decrease where noted) in, one or more of the following criteria selected by the compensation committee:
| • | enterprise value or value creation targets; |
| • | income or net income; operating income; net operating income or net operating income after tax; operating profit or net operating profit; |
139
Table of Contents
| • | cash flow, including, but not limited to, from operations or free cash flow; |
| • | specified objectives with regard to limiting the level of increase in all or a portion of our bank debt or other long-term or short-term public or private debt or other similar financial obligations, or other capital structure improvements, which may be calculated net of cash balances and/or other offsets and adjustments as may be established by the compensation committee; |
| • | net revenues, net income or earnings before income tax or other exclusions; |
| • | operating margin, return on operating revenue or return on operating profit; |
| • | return measures (after tax or pre-tax), including return on capital employed, return on invested capital, return on equity, return on assets or return on net assets; |
| • | market capitalization, earnings per share, fair market value of our ordinary shares, franchise value (net of debt) or economic value added; |
| • | total shareholder return or growth in total shareholder return (with or without dividend reinvestment); |
| • | proprietary investment results; |
| • | estimated market share; |
| • | expense management, control or reduction (including, without limitation, compensation and benefits expense); |
| • | customer satisfaction measures; |
| • | technological improvements and implementation, new product innovation and delivery system improvements; |
| • | research and development, pre-clinical and clinical trial results and FDA or other regulatory approvals; |
| • | collections and reimbursement recoveries; |
| • | property or asset purchases; |
| • | litigation and regulatory resolution or implementation goals; |
| • | leases, contracts or financings (including renewals, overhead, savings, G&A and other expense control goals); |
| • | risk management/implementation; |
| • | development and implementation of strategic plans and/or organizational restructuring goals; |
| • | development and implementation of risk and crisis management programs, compliance requirements and compliance relief, productivity goals, workforce management and succession planning goals; |
| • | employee satisfaction or staff development; |
| • | formation of joint ventures or partnerships or the completion of other similar transactions intended to enhance our revenues or profitability or to enhance our prescriber base; or |
| • | completion of a merger, acquisition or any transaction that results in the sale of all or substantially all of our stock or assets. |
Such performance goals may be based upon the attainment of specified levels of company, subsidiary, division or other operational unit performance under one or more of the measures described above relative to the
140
Table of Contents
performance of other companies. The compensation committee may designate additional business criteria on which the performance goals may be based or adjust, modify or amend those criteria, to the extent permitted by Section 162(m) of the Code. Unless the compensation committee determines otherwise, to the extent permitted by Section 162(m) of the Code, the compensation committee will disregard and exclude the impact of special, unusual or non-recurring items, events, occurrences or circumstances; discontinued operations or the disposal of a business; the operations of any business that we acquire during the fiscal year or other applicable performance period; or a change in accounting standards required by generally accepted accounting principles.
Effect of certain transactions; change in control
In the event of a change in control, except as otherwise provided by the compensation committee in an award agreement, unvested awards will not vest. Instead, the compensation committee may, in its sole discretion, provide that outstanding awards will be assumed and continued; purchased based on the price per share paid in the change in control transaction subject to adjustment as determined by the compensation committee in its sole discretion, but in no event less than the fair market value (less, in the case of options and other stock-based awards with an exercise price, the exercise price); and/or in the case of stock options or other stock-based appreciation awards where the change in control price is less than the applicable exercise price, cancelled. However, the compensation committee may in its sole discretion provide for the acceleration of vesting and lapse of restrictions of an award at any time.
For the purposes of the foregoing, a change in control generally means the occurrence of one of the following events:
| • | a person acquires at least 50% of our voting power; |
| • | a change in the majority of the board of directors, other than individuals approved by a vote of at least two-thirds of the directors then in office who either were directors as of the effective date of the 2015 Plan or whose election or nomination for election was previously so approved; |
| • | a merger or consolidation with another company in which new shareholders hold 50% or more of our voting securities; or |
| • | approval by the shareholders of a complete liquidation or a sale of substantially all of our assets. |
Deferral arrangements
The compensation committee may permit or require the deferral of any payment of a restricted stock unit or performance award pursuant to a deferred compensation arrangement in a manner intended to comply with, or be exempt from, Sections 409A and 457A of the Code.
Non-transferability of awards
Except as the compensation committee may permit, at the time of grant or thereafter, awards granted under the 2015 Plan are generally not transferable by a participant other than by will or the laws of descent and distribution. Ordinary shares acquired by a permissible transferee will continue to be subject to the terms of the 2015 Plan and the applicable award agreement.
Term
Awards under the 2015 Plan may not be made after more than ten years after the effective date of the 2015 Plan, but awards granted prior to such date may extend beyond that date. Awards (other than stock options
141
Table of Contents
and stock appreciation rights) that are intended to be performance-based under Section 162(m) of the Code will not be made unless the performance goals in the 2015 Plan are reapproved, or new goals approved, on or before the first shareholders’ meeting in the fifth year following the year of the last shareholder approval of the performance goals in the 2015 Plan.
Amendment and termination
Subject to the rules referred to in the balance of this paragraph, our board of directors may at any time amend, in whole or in part, any or all of the provisions of the 2015 Plan, or suspend or terminate it entirely, retroactively or otherwise. Except as required to comply with applicable law, no such amendment may reduce the rights of a participant with respect to awards previously granted without the consent of such participant. In addition, without the approval of shareholders, no amendment may be made that would increase the aggregate number of ordinary shares that may be issued under the 2015 Plan, increase the maximum individual participant share limitations for a fiscal year or year of a performance period, change the classification of individuals eligible to receive awards under the 2015 Plan, extend the maximum option term, decrease the minimum exercise price of (i.e., reprice) any award, reduce the exercise price of any option or other share-based award with an exercise price or cancel any outstanding in-the-money option or other share-based award with an exercise price in exchange for cash, substitute any option or other share-based award with an exercise price in exchange for an option or other stock-based award (or similar other award) with a lower exercise price, alter the performance goals or require shareholder approval in order for the 2015 Plan to continue to comply with Section 162(m) of the Code or Section 422 of the Code.
Equity awards under the 2015 Omnibus Incentive Plan
In September 2015, the compensation committee granted options to purchase 921,488 ordinary shares to certain of our eligible employees pursuant to the 2015 Plan, conditioned upon the consummation of this offering. The actual exercise price will be equal to the price per share at which the shares are sold to the public in this offering. These awards are subject to continued employment and generally vest over four years. Of these options granted in September 2015, 413,910 were granted to Mr. Danziger. Messrs. Groenhuysen and Kirson did not receive grants at that time.
The compensation committee anticipates entering into an employment agreement with Mr. Doyle following the consummation of this offering that would provide, among other things, for an equity award (or combination of equity awards) under the 2015 Plan that has an economic value no greater than the value of 3,250,000 options to purchase ordinary shares had such options been granted at the time of the offering with an exercise price equal to the price at which the ordinary shares are sold to the public in this offering. The compensation committee may adjust such equity award as necessary to account for then-current market conditions.
Registration on Form S-8
Following consummation of this offering, we intend to file a registration statement on Form S-8 under the Securities Act to register the full number of ordinary shares that will be available for issuance under the 2003 Plan, 2013 Plan and 2015 Plan.
Other compensation practices and policies
Tax considerations
Section 162(m) of the Code generally disallows a federal income tax deduction to public companies for compensation greater than $1.0 million paid for any fiscal year to a company’s chief executive officer and to
142
Table of Contents
certain other highly compensated executive officers. Our board of directors has not previously taken the deductibility limit imposed by Section 162(m) into consideration in setting compensation because we were not previously a public company. We expect that our compensation committee will adopt a policy at some point in the future that, where reasonably practicable and advisable, as determined by the compensation committee in the best interests of our company, will seek to qualify the variable compensation paid to our executive officers for an exemption from the deductibility limitations of Section 162(m). Until such policy is implemented and thereafter as may be permitted thereunder, our compensation committee may, in its judgment, authorize compensation payments that do not consider the deductibility limit imposed by Section 162(m) when it believes that such payments are appropriate to attract, incentivize and retain executive talent. Under a transition rule in the regulations issued under Section 162(m) of the Code, for the Reliance Period (as described above), the deduction limits do not apply to any compensation paid pursuant to a compensation plan or agreement that existed when the company was not publicly held.
Policy regarding the timing of equity awards
There has been no market for our ordinary shares prior to the consummation of this offering. Accordingly, in fiscal 2014, we had no program, plan or practice pertaining to the timing of stock option grants to executive officers coinciding with the release of material non-public information. We do not, as of yet, have any plans to implement such a program, plan or practice after becoming a public company. However, we intend to implement policies to ensure that equity awards are granted at fair market value on the date of grant as well as any policies required by applicable securities law requirements.
Employee Share Purchase Plan
On August 31, 2015, in anticipation of this offering, our board of directors adopted our employee share purchase plan, or ESPP, subject to shareholder approval, which was obtained on September 16, 2015. We adopted the ESPP to encourage and enable our eligible employees to acquire ownership of our ordinary shares purchased through accumulated payroll deductions on an after-tax basis. The ESPP is intended to be an “employee stock purchase plan” within the meaning of Section 423 of the Code and the provisions of the ESPP will be construed in a manner consistent with the requirements of such section. We intend to begin offerings under the ESPP at a future date determined by the compensation committee, in its sole discretion.
Description of the ESPP
Under the ESPP, initially an aggregate of 830,000 ordinary shares as determined following the stock split (subject to certain adjustments to reflect changes in our capitalization) may be purchased by eligible employees who become participants in the ESPP; which amount shall be automatically increased on December 31 of each year during term of the ESPP to an amount equal to 1% of the total number of ordinary shares outstanding on December 30 of such year unless otherwise determined by the board of directors. Our employee, or an employee of one of our designated subsidiaries who has at least 90 days of continuous service, customarily works more than 20 hours per week and more than five months per year and does not possess five percent or more of the total combined voting power or value of all classes of our ordinary shares or the common shares or a subsidiary or parent, if any, is eligible to participate in the ESPP commencing on the first day of any offering under the ESPP. However, to the extent allowable under Section 423 of the Code, the compensation committee may determine in the future that an offering will not be extended to highly compensated employees with compensation above a certain level or who are officers or subject to the disclosure requirements of Section 16(a) of the Exchange Act.
The ESPP is administered by the compensation committee. The compensation committee may delegate its duties and responsibilities under the ESPP, as determined by the compensation committee in its sole discretion.
143
Table of Contents
The term “designated subsidiary” means each existing subsidiary and future subsidiaries and parents (if any) that are specifically designated for participation by the compensation committee. A foreign subsidiary or parent will not be a designated subsidiary unless specifically designated by the compensation committee. The compensation committee may exclude the employees of any specified designated subsidiary from any offering under the ESPP.
No person will be eligible to participate in the ESPP if such person, immediately after the grant, would own ordinary shares and/or hold options to purchase ordinary shares, possessing five percent or more of the total combined voting power or value of all classes of our common shares or a subsidiary or parent, or which permits his or her rights to purchase common shares under all of our employee stock purchase plans to accrue at a rate which exceeds $25,000 in fair market value of the ordinary shares (determined at the time such option is granted) for each calendar year in which such option is outstanding.
Once we begin offerings under the ESPP, on January 1 and July 1 of each calendar year while the ESPP is effective, we will commence an offer by granting each eligible employee an option to purchase ordinary shares on the purchase date at the end of each six-month period during a calendar year (i.e., June 30 and December 31) or any other period of time designated by the compensation committee. The purchase price per ordinary share subject to an offering will be determined by the compensation committee, but in no event will the price be less than the lesser of 85% of the fair market value of an ordinary share on (i) the purchase date or (ii) the offering date (the first date of the applicable six-month offering period).
Once we begin offerings under the ESPP, an eligible employee may become a participant in the ESPP by enrolling in accordance with the procedures developed by the compensation committee (or designee), indicating, where required, the amount of the deductions to be taken from his or her pay. An eligible employee may purchase ordinary shares through payroll deductions (on an after-tax basis) from the employee’s compensation received each payroll period, up to a limit specified by the compensation committee, but in no event will such deductions be less than 1% or exceed 10% of an employee’s compensation during an offering period. A participant generally may cancel his or her election at any time with respect to any purchase period and receive in cash the cash balance (without interest) then credited to his or her account.
On each purchase date, the maximum number of ordinary shares, including fractional ordinary shares, will be purchased for such participant at the applicable purchase price with the accumulated payroll deductions in the participant’s account. If all or a portion of the ordinary shares cannot reasonably be purchased on such date because of unavailability or any other reason, such purchase will be made as soon as thereafter feasible. A participant is entitled to all rights as a shareholder as soon as the ordinary shares are credited to his or her account.
If a participant’s continuous service terminates for any reason, or if a participant ceases to be an eligible employee, the entire payroll deduction amount of such employee on the effective date of any such occurrence will be returned to the participant. Other rules apply if a participant is granted a leave of absence or is laid off.
The board of directors (or a duly authorized committee thereof) may at any time and for any reason terminate, freeze or amend the ESPP. No options shall be granted under the ESPP, and the ESPP will automatically terminate, immediately prior to the ten year anniversary of the effective date. Except as otherwise described in the ESPP, no termination may adversely affect any purchase right previously granted and no amendment may change any purchase right theretofore granted which adversely affects the rights of any participant. No amendment will be effective unless approved by our shareholders if shareholder approval of such amendment is required to comply with Section 423 of the Code or to comply with any other applicable law, regulation or stock exchange rule.
144
Table of Contents
Neither payroll deductions credited to a participant’s account nor any rights with regard to the purchase of or right to receive ordinary shares under the ESPP may be assigned, transferred, pledged or otherwise disposed of in any way (other than by will, the laws of descent and distribution or as otherwise provided in the ESPP) by the participant.
Because future rights to purchase ordinary shares under the ESPP will be based upon prospective factors, including the amount of payroll deductions elected by a participant, actual rights to purchase ordinary under the ESPP cannot be determined at this time.
United States Federal Income Tax Consequences
The following is a summary of the principal effect of U.S. federal income taxation consequences to us and participants subject to U.S. taxation with respect to the ESPP, in effect as of the date of this proxy statement. This summary is not intended to be exhaustive and does not discuss the tax consequences arising in the context of the participant’s death or the income tax laws of any other jurisdiction in which the participant’s income or gain may be taxable or the gift, estate or any other tax law other than U.S. federal income tax law.
The ESPP is not, nor is it intended to be, qualified under Section 401(a) of the Code.
The ESPP is intended to qualify as an “employee stock purchase plan” under Section 423 of the Code. Neither the grant of a right to purchase ordinary shares under the ESPP nor the purchase of such ordinary will have any immediate tax consequence for a participating employee. If the participating employee does not dispose of the ordinary shares within two years from the date the right to purchase was granted to him or her or within one year from the date the ordinary was purchased, any gain or loss realized upon the disposition of shares will be treated as long term capital gain or loss to the participant, as the case may be, provided that where the purchase price is less than 100% (but not less than 85%) of fair market value the participant will realize ordinary income equal to the lesser of: (i) the amount, if any, by which the purchase price was exceeded by the fair market value of the ordinary shares at the time the right to purchase was granted or (ii) the amount, if any, by which the purchase price was exceeded by the fair market value of the ordinary shares on the date of the disposition or the participant’s death. No income tax deduction will be allowed to us with respect to ordinary shares purchased under the ESPP by a participant provided such ordinary shares are held for the required periods.
In the event of an earlier disposition of the ordinary shares (i.e., a “disqualifying disposition”), the excess of the fair market value of the ordinary shares at the time of purchase over the purchase price will be treated as income to the participant and taxed at ordinary income tax rates in the year in which the disposition occurred (regardless of the price at which the ordinary shares are sold). The balance of any gain will be treated as capital gain. Also, we will generally be entitled to a corresponding deduction.
Stock ownership policies
We have not established stock ownership or similar guidelines with regard to our executive officers. All of our executive officers currently have a direct or indirect equity interest in us through their stock and/or stock option holdings, and we believe that they regard the potential returns from these interests as a significant element of their potential compensation for services to us.
Recoupment policy
We currently do not have a recoupment policy to adjust or recover bonuses or incentive compensation paid to executive officers where such bonuses or payments were based on financial statements that were subsequently restated or otherwise amended in a manner that would have reduced the size of such bonuses or payments. Upon
145
Table of Contents
the consummation of this offering, we will become subject to the recoupment requirements under the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Consumer Protection Act and other applicable laws. However, at the time of this offering, the final rules relating to such recoupment requirements under the Dodd-Frank Wall Street Reform and Consumer Protection Act have not been issued, and as a result, we expect to implement a recoupment policy once final guidance has been issued so that our recoupment policy may satisfy all then-applicable requirements and regulations (including disclosure obligations with respect to such policy).
Risk considerations in our compensation program
We believe that the mix and design of the elements of our employee compensation policies and practices do not motivate imprudent risk-taking. Consequently, we are satisfied that any potential risks arising from our employee compensation policies and practices are not reasonably likely to have a material adverse effect on us.
Director compensation
Prior to this offering, members of our board of directors have not received or been entitled to receive cash compensation for their services as directors, except for the reimbursement of reasonable and documented costs and expenses incurred by directors in connection with attending any board of director or committee meetings. Nonetheless, certain of our directors did receive compensation from us in their capacity as an employee of or consultant to us. However, our directors who are also one of our employees, such as Mr. Danziger and Mr. Leung, or who are one of our consultants, such as Professor Palti, do not now, and are not expected in the future to, receive any compensation for their services as a director. In the case of Mr. Danziger, who is an NEO, his compensation for fiscal 2014 is described above and reported in the summary compensation tables above. In the case of Mr. Leung and Professor Palti, neither of whom are NEOs, compensation is generally payable pursuant to and in accordance with an employment agreement or consulting agreement, respectively, with us, each as described in more detail below.
The following table shows the total compensation paid to our directors (other than Mr. Danziger, whose compensation is reported in the summary compensation table above) for the year ended December 31, 2014:
| Name | Fees earned or paid in cash ($)(1) |
Option awards ($)(2) |
All other compensation ($)(3) |
Total ($) |
||||||||||||
| William F. Doyle |
— | — | 300,000 | 300,000 | ||||||||||||
| Kinyip Gabriel Leung |
— | 149,387 | 203,311 | 352,698 | ||||||||||||
| Yoram Palti, M.D., Ph.D. |
— | — | 282,634 | 282,634 | ||||||||||||
| William Burkoth |
— | — | — | — | ||||||||||||
| Timothy Langloss |
— | — | — | — | ||||||||||||
| Louis J. Lavigne, Jr. |
— | — | — | — | ||||||||||||
| Robert J. Mylod, Jr. |
— | — | — | — | ||||||||||||
| Gert Lennart Perlhagen |
— | — | — | — | ||||||||||||
| Charles G. Phillips III |
— | — | — | — | ||||||||||||
| William A. Vernon |
— | — | — | — | ||||||||||||
|
|
||||||||||||||||
| (1) | Prior to this offering, members of our board of directors have not received or earned, nor been entitled to receive or earn, fees or other cash compensation for their services as directors, except for the reimbursement of reasonable and documented costs and expenses incurred by directors in connection with attending any board of director or committee meetings. |
| (2) | The amounts represent the aggregate grant date fair value of stock and option awards granted by us in 2014, computed in accordance with FASB ASC Topic 718. For further information on how we account for |
146
Table of Contents
| stock-based compensation, see Note 13 to our consolidated financial statements. These amounts reflect our accounting expense for these awards and do not correspond to the actual amounts, if any, that will be recognized by the directors. None of our directors have been granted stock awards or any other equity compensation other than stock options. |
| On February 26, 2014, in connection with annual grants to our employees, we approved the grant of options to Mr. Leung under our 2013 Plan to purchase 29,565 ordinary shares at an exercise price per share equal to $7.48 (as evidenced by a stock option agreement entered into on February 26, 2014). Pursuant to their terms, these options vest in installments over a period of four years, with 25% vesting on each of the first four anniversaries of the date of grant, subject to Mr. Leung’s continued employment through the applicable vesting date. |
| For each director listed in the director compensation table above, the aggregate number of stock option awards outstanding at fiscal year end for the year ended December 31, 2014 is set forth below; other than these option awards, no shares of unvested restricted stock, stock units or other stock-based awards were held by our directors as of December 31, 2014: |
| Aggregate number of option awards | ||||||||
| Director | Number of securities underlying unexercised options (#)—exercisable |
Number of securities underlying unexercised options (#)—unexercisable |
||||||
| William F. Doyle |
— | — | ||||||
| Kinyip Gabriel Leung |
236,198 | 196,805 | ||||||
| Yoram Palti, M.D., Ph.D. |
147,825 | — | ||||||
| William Burkoth |
— | — | ||||||
| Timothy Langloss |
— | — | ||||||
| Louis J. Lavigne, Jr. |
81,303 | 81,304 | ||||||
| Robert J. Mylod, Jr. |
81,303 | 81,304 | ||||||
| Gert Lennart Perlhagen |
— | — | ||||||
| Charles G. Phillips III |
81,304 | 81,304 | ||||||
| William A. Vernon |
249,085 | — | ||||||
|
|
||||||||
| (3) | For Mr. Leung, the amount includes base salary paid in 2014 pursuant to his employment agreement (as described below); Mr. Leung did not receive an annual bonus or any other compensation in 2014. For Professor Palti, the amount includes $240,000 in consulting fees and $42,634 for automobile payments made in 2014 to Palti Consultants Ltd. pursuant to his consulting agreement with us (as described below). For Mr. Doyle, the amount includes $300,000 paid as consulting fees pursuant to his consulting agreement with us (as described below). |
Leung employment agreement
On August 24, 2011, we entered into an employment agreement with Kinyip Gabriel Leung pursuant to which Mr. Leung agreed to serve as the Vice Chairman of our board of directors for an initial term of three years commencing August 24, 2011, working for us on a part-time basis, with automatic renewal for successive one-year extensions unless either party gives notice not to renew and extend at least 90 days prior to the end of the initial term or any renewal term. Mr. Leung’s employment agreement contemplates part-time employment with us for at least 90 working days per year, and Mr. Leung agreed to devote not less than 40% of his working time and attention to us. Subsequently, we and Mr. Leung have agreed orally that Mr. Leung will devote not less than 25% of his working time and attention to us.
147
Table of Contents
Pursuant to his employment agreement, Mr. Leung is entitled to receive an annual base salary of $324,000 per year and is eligible to receive an annual discretionary incentive payment under our annual bonus plan, if any, in the sole discretion of our board of directors. Subsequent to entry into his employment agreement, Mr. Leung’s base salary was changed by the compensation committee to $162,000 effective March 2014 to reflect an adjustment to his time commitment to us.
Pursuant to Mr. Leung’s employment agreement, in 2011, we granted Mr. Leung an option to purchase 314,743 ordinary shares at a price per share of $3.44. These options vest in six installments, whereby 20% of the shares subject to the option vested on the date of grant and 16% of the shares subject to the option will become vested on each of the first five anniversaries of the date of grant, subject to continued service through the applicable vesting date, provided that the options will fully vest upon the consummation of a change of control that occurs during his employment. In 2013, we granted Mr. Leung options to purchase 88,695 ordinary shares at a price per share of $7.03 and in 2014, we granted Mr. Leung options to purchase 29,565 ordinary shares at a price of $7.48. The options granted in 2013 and 2014 vest in four equal installments on each of the first four anniversaries of the grant date, subject to continued service through the applicable vesting date. As of the end of fiscal 2014, no portion of these options has been exercised.
If we terminate Mr. Leung’s employment other than for cause or if Mr. Leung terminates his employment for good reason (as such terms are defined in Mr. Leung’s employment agreement) or as a result of our non-extension of the term of Mr. Leung’s employment, then, subject to his execution without revocation of a release of claims, he will receive continued payment of his base salary at the time of termination for a period of six months following the date of termination and an additional portion of his sign-on options will become vested equal to the portion that would have become vested on the next scheduled vesting date.
Pursuant to his employment agreement, Mr. Leung is subject to certain confidentiality and non-disparagement covenants, as well as covenants of non-solicitation of employees during his employment and for two years thereafter and non-solicitation of business partners during his employment and for one year thereafter. Mr. Leung is not subject to a non-competition agreement, but his employment agreement requires that his other business activities not unreasonably interfere with his duties under the employment agreement.
Palti consulting arrangement
We are party to a consulting agreement with Palti Consultants Ltd., a private Israeli company, pursuant to which Professor Palti provides research and development services to us relating to Optune and other of our projects. Under the consulting agreement, Palti Consultants Ltd. receives an aggregate of $20,000 per month for Professor Palti’s services. We also pay for the use of a company automobile and reimbursement for actual business expenses arising out of travel, lodging, meals and entertainment in connection with his services to us. In lieu of providing Professor Palti with a company car, we currently satisfy our automobile obligations under the consulting agreement by paying Palti Consultants Ltd. cash sufficient to cover the costs of his automobile on an after-tax basis. The total amount paid to Palti Consultants Ltd. in 2014 with respect to the automobile payments was $42,634.
The consulting agreement generally continues for an indefinite term that is terminable by either party by prior written notice of at least six months, by mutual consent or by us in the event of a breach by Palti Consultants Ltd. that is uncurable or uncured after 14 days’ notice. The consulting agreement includes certain non-competition and non-disclosure covenants with respect to Optune and other of our projects, technologies and inventions other than in the performance of Palti Consultants Ltd.’s services to us.
Doyle consulting arrangement
On June 24, 2014, we entered into a consulting agreement with Mr. Doyle that expired on December 31, 2014. The consulting agreement provided for four quarterly installments of $75,000 each to be paid each quarter for
148
Table of Contents
a total consulting fee of $300,000. The consulting agreement with Mr. Doyle was extended through December 31, 2015 on the same terms and conditions and at the same consulting fee.
The compensation committee anticipates entering into an employment agreement with Mr. Doyle following the consummation of this offering that would provide, among other things, for an equity award (or combination of equity awards) under the 2015 Plan that has an economic value no greater than the value of 3,250,000 options to purchase ordinary shares had such options been granted at the time of the offering with an exercise price equal to the price at which the ordinary shares are sold to the public in this offering. The compensation committee may adjust such equity award as necessary to account for then-current market conditions.
Non-employee director option awards
In November 2012, our board of directors approved grants of options to purchase 162,607 ordinary shares at an exercise price of $6.72 per share to each of the following directors: Louis J. Lavigne, Jr., Robert J. Mylod, Jr. and Charles G. Phillips III. The grant date of each of these options is September 29, 2012. These options vest in installments on the basis of continued service over a period of four years, with 25% vesting on each of the first four anniversaries of the date of grant.
Post-offering
After the consummation of this offering, our executive officers who are members of our board of directors, as well as our directors who are our consultants or otherwise not considered independent under the corporate governance rules of the SEC and NASDAQ will not receive compensation from us for their service on our board of directors. Accordingly, Messrs. Danziger, Doyle, Leung and Palti will not receive compensation from us for their service on our board of directors. Only those directors who are considered independent directors under the corporate governance rules of NASDAQ and the SEC will be eligible to receive compensation from us for their service on our board of directors. Our independent directors are expected to receive the following amounts:
| • | a base annual retainer of $45,000 in cash; |
| • | an additional annual retainer of $25,000 in cash to the chairman of the audit committee; |
| • | an additional annual retainer of $15,000 in cash to the chairman of the compensation committee; |
| • | an additional annual retainer of $10,000 in cash to the chairman of the nominating and corporate governance committee; |
| • | an additional annual retainer of $15,000 in cash to each member of the audit committee; |
| • | an additional annual retainer of $7,500 in cash to each member of the compensation committee; and |
| • | an additional annual retainer of $5,000 in cash to each member of the nominating and corporate governance committee. |
In addition, we expect that each independent director will be granted awards under our 2015 Plan; however, the form and size and other details, terms and conditions of any such awards have not yet been determined.
We also reimburse, and expect that we will continue to reimburse, all of our directors for reasonable and necessary expenses incurred to attend board of director or committee meetings.
149
Table of Contents
Certain relationships and related-party transactions
Issuances of preferred shares
In February 2011, we sold an aggregate of 5,106,269 Series G convertible preferred shares at a purchase price of $8.64 per share. In 2012, we sold an aggregate of 4,073,020 Series H convertible preferred shares at a purchase price of $13.50 per share. In 2013, we sold 13,360,252 Series I convertible preferred shares at a purchase price of $14.53. Lastly, in June 2015, prior to the initial submission to the SEC of the registration statement of which this prospectus forms a part, we sold 4,068,500 Series J convertible preferred shares at a purchase price of $23.33 per share. Certain of our shareholders that beneficially own at least 5% of our voting securities, as well as certain of our directors and executive officers and their affiliates participated in these equity financings. See “Principal shareholders.”
Investors Rights Agreement
On June 1, 2015, we entered into an Eleventh Amended and Restated Investors Rights Agreement, or the Investors Rights Agreement, with the holders of our preferred shares and certain other holders of our securities. The Investors Rights Agreement sets forth the size of our board of directors, provides the procedures through which directors can be elected and removed and through which our securities may be transferred, and also enumerates the corporate actions that require the consent of certain holders of our securities (in addition to that of our board of directors), among other things. The Investors Rights Agreement will terminate upon the completion of this offering.
Registration Rights Agreement
See “Description of share capital—Registration rights of certain holders.”
Technion—letter of agreement
In connection with our entry into the Technion settlement agreement as described in “Our business—Intellectual property” and a previous indemnification provided by Professor Palti to us in 2009, on February 10, 2015, we entered into a letter agreement (the “Letter Agreement”) with Professor Palti and an entity controlled by Professor Palti (together, the “Palti Parties”), pursuant to which the Palti Parties agreed to redeem and cancel 2,010,420 ordinary shares at an aggregate price of £3,400, to enable us to satisfy the obligations to issue shares to the Technion. In addition, to enable us to satisfy (in part) our obligations to pay the Technion cash payments, the Palti Parties agreed to pay up to $2 million solely from net proceeds of the sale of the Palti Parties’ ordinary shares. Following the completion of this offering, we will have the right to require the Palti Parties to sell their shares in market transactions to satisfy any remaining portion of the $2 million amount not yet paid to us, so long as the market price of our ordinary shares is not less than 80% of the price of the ordinary shares being sold in this offering (as set forth on the cover of this prospectus).
Indemnification agreements
In connection with this offering, we will enter into indemnification agreements with our directors and officers. See “Management—Board of Directors—Indemnification agreements.” The terms of our indemnification agreements with our officers are substantially the same as those with our directors.
Related-party transaction policy
Our board of directors expects to adopt a written related-party transaction policy, to be effective and publicly available upon the completion of this offering, setting forth the policies and procedures for the review and
150
Table of Contents
approval or ratification of transactions involving us and related persons. For the purposes of this policy, related persons will include our executive officers, directors and director nominees or their immediate family members, or stockholders owning 5% or more of our outstanding ordinary shares and their immediate affiliates.
The policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person.
Our executive officers and directors will be discouraged from entering into any transaction that may cause a conflict of interest for us. If such a transaction shall arise, they must report any potential conflict of interest, including related-party transactions, to our General Counsel, who will then review and summarize the proposed transaction for our audit committee. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s-length transaction with an unrelated party and the extent of the related person’s interest in the transaction. All related-party transactions may only be consummated if our audit committee has approved or ratified such transaction in accordance with the guidelines set forth in the policy. Any member of the audit committee who is a related person with respect to a transaction under review will not be permitted to participate in the deliberations or vote respecting approval or ratification of the transaction. However, such director may be counted in determining the presence of a quorum at a meeting of the audit committee that considers the transaction.
All of the transactions described in this section occurred prior to the adoption of this policy.
151
Table of Contents
The following table lists information regarding the beneficial ownership of our ordinary shares as of September 21, 2015 by (i) each person whom we know to beneficially own more than 5% of our outstanding ordinary shares (a “greater-than-5% shareholder”), (ii) each director, (iii) each named executive officer and (iv) all directors and executive officers as a group. Unless otherwise indicated, the address of each officer and director is that of our registered office, c/o NovoCure Limited, Le Masurier House, La Rue le Masurier, St. Helier, Jersey JE2 4YE.
The number of ordinary shares beneficially owned by each shareholder is determined under rules issued by the SEC regarding the beneficial ownership of securities. This information is not necessarily indicative of beneficial ownership for any other purpose. Under these rules, beneficial ownership of ordinary shares includes (1) any shares as to which the person or entity has sole or shared voting power or investment power and (2) any ordinary shares of which the person or entity has the right to acquire beneficial ownership within 60 days after September 21, 2015, including any ordinary shares that could be purchased by the exercise of outstanding options or warrants. Each holder’s percentage ownership before this offering is based on 75,176,810 ordinary shares outstanding as of September 21, 2015 (as adjusted to reflect the conversion of all our outstanding preferred shares into ordinary shares in connection with the consummation of this offering after giving effect to a 5.913 for 1 stock split effected on September 16, 2015) plus the number of ordinary shares that may be acquired by such shareholder upon exercise of options or warrants that are exercisable at or within 60 days after September 21, 2015. Each holder’s percentage ownership after this offering is based on 82,676,810 ordinary shares to be outstanding immediately after the consummation of this offering plus the number of ordinary shares that may be acquired by such holder upon exercise of options that are exercisable at or within 60 days after September 21, 2015 (each, as adjusted to reflect the conversion of all our outstanding preferred shares into ordinary shares in connection with the consummation of this offering after giving effect to a 5.913for-1 stock split effected on September 16, 2015).
We have granted the underwriters an option to purchase up to 1,125,000 additional ordinary shares to cover over-allotments, if any, and the table below assumes no exercise of that option.
152
Table of Contents
Unless otherwise indicated below, to our knowledge, all persons named in the table have sole voting and investment power with respect to their ordinary shares.
| Number
of ordinary shares beneficially owned |
Number of ordinary shares offered |
Percentage of ordinary shares beneficially owned |
||||||||||||||
| Name of beneficial owner | Before offering |
After offering |
||||||||||||||
| Directors and Named Executive Officers |
||||||||||||||||
| William F. Doyle(1) |
22,929,589 | — | 29.5% | 26.9% | ||||||||||||
| Kinyip Gabriel Leung(2) |
316,122 | — | * | * | ||||||||||||
| Asaf Danziger(3) |
2,037,741 | — | 2.7% | 2.4% | ||||||||||||
| Wilhelmus Groenhuysen(4) |
649,274 | — | * | * | ||||||||||||
| Eilon Kirson(5) |
588,341 | — | * | * | ||||||||||||
| Yoram Palti, M.D., Ph.D.(6) |
1,818,879 | — | 2.4% | 2.2% | ||||||||||||
| William Burkoth(7) |
— | — | — | — | ||||||||||||
| Timothy Langloss |
39,876 | — | * | * | ||||||||||||
| Louis J. Lavigne, Jr.(8) |
129,358 | — | * | * | ||||||||||||
| Robert J. Mylod, Jr.(9) |
270,069 | — | * | * | ||||||||||||
| Gert Lennart Perlhagen(10) |
— | — | — | — | ||||||||||||
| Charles G. Phillips III(11) |
190,360 | — | * | * | ||||||||||||
| William A. Vernon(12) |
249,085 | — | * | * | ||||||||||||
| All directors and executive officers as a group (15 persons) |
29,884,589 | — | 36.7% | 33.6% | ||||||||||||
| Greater-than 5% holders |
||||||||||||||||
| WFD Ventures Fund II(13) |
20,589,468 | — | 26.5% | 24.2% | ||||||||||||
| Hansjoerg Wyss(14) |
9,954,748 | — | 13.2% | 12.0% | ||||||||||||
| Volati Limited(15) |
8,599,282 | — | 11.3% | 10.3% | ||||||||||||
|
|
||||||||||||||||
| * | Represents less than 1% of our ordinary shares outstanding as of September 21, 2015. |
| (1) | Mr. William F. Doyle is a managing director of WFD Ventures LLC, which is the managing member of WFD Ventures Fund A. WFD Ventures LLC is also the sole member of WFD-GP II, LLC, which is the general partner of WFD Ventures Fund II. As such, Mr. Doyle’s ownership includes the beneficial ownership of the following: (i) 981,215 ordinary shares, 453,621 ordinary shares issuable upon conversion of Series A convertible preferred shares, 4,590,439 ordinary shares issuable upon conversion of Series B convertible preferred shares, 1,598,626 ordinary shares issuable upon conversion of Series C convertible preferred shares, 3,380,361 ordinary shares issuable upon conversion of Series D convertible preferred shares, 4,849,836 ordinary shares issuable upon conversion of Series E convertible preferred shares, 1,121,329 ordinary shares issuable upon conversion of Series F convertible preferred shares, and 1,156,837 ordinary shares issuable upon conversion of Series G convertible preferred shares, all of which are held by WFD Ventures Fund II, and 2,457,204 ordinary shares underlying warrants exercisable by WFD Ventures Fund II within 60 days of September 21, 2015; and (ii) 2,242,664 ordinary shares issuable upon conversion of Series F convertible preferred shares held by WFD Ventures Fund A. Mr. Doyle possesses sole voting and investment power over our shares owned by WFD Ventures Fund II (subject to certain rights of the advisory board of WFD Ventures Fund II to approve sales of any of the shares owned by WFD Ventures Fund II) and WFD Ventures Fund A. Mr. Doyle disclaims beneficial ownership in such shares to the extent that he does not have a pecuniary interest. |
| (2) | Represents 316,122 ordinary shares underlying share options exercisable by Mr. Leung within 60 days of September 21, 2015. |
| (3) | Represents 923,557 ordinary shares and 1,114,184 ordinary shares underlying share options exercisable by Mr. Danziger within 60 days of September 21, 2015. |
153
Table of Contents
| (4) | Represents 263,128 ordinary shares and 386,146 ordinary shares underlying share options exercisable by Mr. Groenhuysen within 60 days of September 21, 2015. |
| (5) | Represents 148,445 ordinary shares and 439,896 ordinary shares underlying share options exercisable by Mr. Kirson within 60 days of September 21, 2015. |
| (6) | Professor Palti shares voting and investment power for the shares held by Bennet Limited. As such, Professor Palti’s beneficial ownership includes 1,540,927 ordinary shares and 130,127 ordinary shares issuable upon conversion of Series A convertible preferred shares held by Bennet Limited and 147,825 ordinary shares underlying share options exercisable by Palti within 60 days of September 21, 2015. Following completion of this offering, we have the right to require Professor Palti and Bennet Limited to sell up to $2.0 million of their ordinary shares in market transactions, so long as the market price of our ordinary shares is not less than 80% of the price of the ordinary shares being sold in this offering (as set forth on the cover of this prospectus). Bennet Limited is a company incorporated in the British Virgin Islands with a registered address at Road Town, Tortola, P.O. Box 3820, British Virgin Islands. |
| (7) | Mr. William Burkoth is an executive director of Pfizer Inc., a subsidiary of which, C.P. Pharmaceuticals International C.V., owns 2,242,664 ordinary shares issuable upon conversion of Series F convertible preferred shares, 266,664 ordinary shares issuable upon conversion of Series G convertible preferred shares and 116,018 ordinary shares issuable upon conversion of Series H convertible preferred shares. Mr. Burkoth currently possesses no voting or investment power over ordinary shares held by C.P. Pharmaceuticals International C.V. and disclaims ownership of such shares. C.P. Pharmaceuticals International C.V. is a Dutch partnership (commanditaire vennootschap) with a registered address at 235 East 42nd Street, New York, New York 10017. |
| (8) | Represents 121,955 ordinary shares underlying share options exercisable by Mr. Lavigne, within 60 days of September 21, 2015. Includes 7,403 ordinary shares issuable upon conversion of Series H convertible preferred shares held by Spring Development Group, LLC, of which Mr. Lavigne is a managing member. Mr. Lavigne shares investment power with respect to these shares. Mr. Lavigne disclaims beneficial ownership in such shares to the extent that he does not have a pecuniary interest. |
| (9) | Represents 148,114 ordinary shares issuable upon conversion of Series H convertible preferred shares and 121,955 ordinary shares underlying share options exercisable by Mr. Mylod within 60 days of September 21, 2015. |
| (10) | Mr. Perlhagen is the settlor of, and a beneficiary of shares held by, the Oden Trust, the beneficial owner of Volati Limited. See footnote 15 below. Mr. Perlhagen possesses no voting or investment power over shares held in the Oden Trust. |
| (11) | Represents 121,955 ordinary shares underlying share options exercisable by Mr. Phillips, within 60 days of September 21, 2015. Includes 44,856 ordinary shares issuable upon conversion of Series F convertible preferred shares, 5,333 ordinary shares issuable upon conversion of Series G convertible preferred shares, 3,701 ordinary shares issuable upon conversion of Series H convertible preferred shares, 10,672 ordinary shares issuable upon conversion of Series I convertible preferred shares and 3,843 ordinary shares issuable upon conversion of Series J convertible preferred shares held by the wife of Mr. Phillips. |
| (12) | Represents 249,085 ordinary shares underlying share options exercisable by Mr. Vernon within 60 days of September 21, 2015. |
| (13) | Represents 981,215 ordinary shares, 453,621 ordinary shares issuable upon conversion of Series A convertible preferred shares, 4,590,439 ordinary shares issuable upon conversion of Series B convertible preferred shares, 1,598,626 ordinary shares issuable upon conversion of Series C convertible preferred shares, 3,380,361 ordinary shares issuable upon conversion of Series D convertible preferred shares, 4,849,836 ordinary shares issuable upon conversion of Series E convertible preferred shares, 1,121,329 |
154
Table of Contents
| ordinary shares issuable upon conversion of Series F convertible preferred shares, and 1,156,837 ordinary shares issuable upon conversion of Series G convertible preferred shares, all of which are held by WFD Ventures Fund II, and 2,457,204 ordinary shares underlying warrants exercisable by WFD Ventures Fund II within 60 days of September 21, 2015. WFD Ventures LLC is the sole member of WFD-GP II, LLC, which is the general partner of WFD Ventures Fund II. Mr. Doyle is the Managing Director of WFD Ventures LLC. Mr. Doyle has sole voting and dispositive power over the shares owned by WFD Ventures Fund II (subject to certain rights of the advisory board of WFD Ventures Fund II to approve sales of any of the shares owned by WFD Ventures Fund II). Each of WFD Ventures LLC and WFD-GP II, LLC is a limited liability company incorporated and existing under the laws of the State of Delaware, United States, having its registered office at 2711 Centreville Road, Suite 400, Wilmington, New Castle County, Delaware 19808, United States. WFD Ventures Fund II is an investment fund, incorporated and existing under the laws of Cayman Islands, having its registered office at c/o Maples Corporate Services Limited, PO Box 309, Ugland House, Grand Cayman, KY1-1104. |
| (14) | Includes the beneficial ownership of 9,954,748 ordinary shares, issuable upon conversion of Series I convertible preferred shares. Mr. Wyss currently possesses no voting or investment power over 825,649 ordinary shares, issuable upon conversion of Series I convertible preferred shares held by The Hansjoerg Wyss 2004 Descendants Trust, and Mr. Wyss disclaims ownership of such shares. The address for Mr. Wyss is c/o Loreda, 138 Mt. Auburn St, Cambridge, MA 02138. |
| (15) | Represents the 1,775,673 ordinary shares, 131,380 ordinary shares issuable upon conversion of Series A convertible preferred shares, 618,411 ordinary shares issuable upon conversion of Series C convertible preferred shares, 829,546 ordinary shares issuable upon conversion of Series D convertible preferred shares, 2,823,670 ordinary shares issuable upon conversion of Series E convertible preferred shares, 1,121,329 ordinary shares issuable upon conversion of Series F convertible preferred shares, and 231,369 ordinary shares issuable upon conversion of Series G convertible preferred shares, all of which are held by Volati Limited, and 1,067,904 ordinary shares underlying warrants exercisable by Volati Limited within 60 days of September 21, 2015. Volati Limited is a company incorporated under the laws of Jersey (Channel Islands) with a registered address at Charter Place, 23-27 Seaton Place, St. Helier, Jersey JE115Y and is beneficially owned by the Oden Trust. The trustee of the Oden Trust is Church Street Trustees Limited, whose directors are Richard M. Kearsey, Brian H. Morris, Juan L. Medina, Siobhan M. McGrath, Elizabeth A. Nursey, Stuart E. McInnes and Marilyn M. Conolly. The Oden Trust was settled by Mr. Perlhagen and the beneficiaries include Mr. Perlhagen. Mr. Perlhagen currently possesses no voting or investment power over the shares owned by Volati Limited, and Mr. Perlhagen disclaims ownership of such shares. |
155
Table of Contents
General
NovoCure Limited is a public limited company, which was incorporated under the laws of Jersey, Channel Islands on February 11, 2000.
Immediately following the consummation of this offering, our authorized share capital will consist of an unlimited number of no par value shares, comprising (i) an unlimited number of ordinary shares, of which 82,676,810 ordinary shares will be issued and outstanding, including 7,500,000 ordinary shares issued in this offering (assuming no exercise by the underwriters of the over-allotment option), and (ii) an unlimited number of preferred shares, of which no preferred shares will be issued and outstanding as a result of the conversion of all our outstanding preferred shares into ordinary shares in connection with the consummation of this offering, in each case after giving effect to a 5.913-for-1 stock split effected on September 16, 2015.
Ordinary shares
Upon the consummation of this offering and the conversion of all our outstanding preferred shares into ordinary shares, there will be 82,676,810 ordinary shares outstanding (assuming no exercise by the underwriters of the over-allotment option). In addition, as of June 30, 2015, 11,978,512 ordinary shares were reserved for future issuances under the 2015 Omnibus Incentive Plan, 10,054,321 ordinary shares were issuable by us upon the exercise of outstanding options (including the option to acquire 1,005,210 ordinary shares held by the Technion Research Development Foundation), 921,488 ordinary shares were issuable by us upon the exercise of options granted conditioned upon the consummation of this offering and 4,635,317 ordinary shares issuable upon the exercise of outstanding warrants. Holders of our ordinary shares will be entitled to one vote per share on matters to be voted on by shareholders.
Preferred shares
Upon the consummation of this offering, all previously outstanding preferred shares will be converted into our ordinary shares and no preferred shares will be outstanding. Our memorandum and articles of association will provide that preferred shares may be issued from time to time in one or more classes. Our board of directors will be authorized to fix the voting rights, if any, designations, powers, preferences, the relative participating, optional or other special rights and any qualifications, limitations and restrictions thereof, applicable to the shares of each class. Our board of directors will be able, without shareholder approval, to issue any authorized but unissued preferred shares with voting and other rights that could adversely affect the voting power and other rights of the holders of the ordinary shares. We have no present plans to issue any preferred shares after the consummation of this offering. The ability of our board of directors to issue preferred shares without shareholder approval could have the effect of delaying, deferring or preventing a change of control of us or the removal of existing management.
Memorandum and articles of association
Public limited companies incorporated under the laws of Jersey are governed in general by two organizational documents, a memorandum of association and articles of association. The memorandum of association sets forth the basic constitutional details of the company and its authorized share capital. Our authorized share capital is described above under “—General.” The articles of association set forth other general corporate matters, including the rights of shareholders and provisions concerning shareholder and director meetings and directors’ terms and fees.
156
Table of Contents
The following description of both our memorandum of association and articles of association does not purport to be complete and describes certain provisions of our memorandum and articles of association, as amended and restated immediately upon the consummation of this offering.
The full text of both our memorandum of association and articles of association are filed as exhibits to the registration statement of which this prospectus forms a part and are also available on our website, www.novocure.com. We do not incorporate the information on, or accessible through, our website into this prospectus, and you should not consider it part of this prospectus, and you should not rely on any such information in making a decision whether to purchase such ordinary shares.
Corporate power
Under the Jersey Companies Law, the capacity of a Jersey company is not limited by anything contained in its memorandum or articles of association. Accordingly, we are able to operate in any markets and to provide any services that are legally permissible and that the directors deem appropriate.
Voting rights
Subject to the rights attaching to the relevant shares in the memorandum and articles of association, each holder of shares is entitled to one vote per share. There is no cumulative voting of shares.
Dividends and other distributions
Holders of our ordinary shares are entitled to receive such dividends, if any, as may be approved by our board of directors in its discretion. In order to be able to declare any dividends, our directors must make a statutory solvency statement to the effect that we will be able to discharge our liabilities as they fall due and that, having regard to our prospects as to the intention of the directors with respect to the management of our business, and with the amount and character of the financial resources that will in the view of the directors be available to us, we will be able to continue to carry on business and discharge our liabilities as they fall due for a 12-month period immediately following the date on which the dividend is proposed to be paid (or until we are dissolved on a solvent basis, if earlier). Dividends must be apportioned and paid pro rata according to the amounts paid on shares, unless otherwise specified in the rights attached to a specific class or classes of shares. Dividends do not accrue interest and may, if unclaimed, be invested by our board of directors on our behalf until claimed. Any dividend unclaimed after a period of seven years from the date of declaration of such dividend or the date on which such dividend became due for payment is forfeited and becomes our property.
Our articles of association provide that our board of directors may offer our shareholders the right to receive in lieu of any cash dividend (or part thereof) that we declare on our ordinary shares, such number of our ordinary shares that are (or nearly as possible) equivalent in value to the cash dividend, based on the market price of such shares determined in accordance with our articles of association.
We do not intend to pay dividends on our ordinary shares. We plan to retain our available cash and any future earnings for use in the operation of our business and to fund future growth. See “Dividend policy.”
Winding up
If we are wound up (whether the liquidation is voluntary, under supervision or by the courts of Jersey), the liquidator (or the board, where no liquidator is appointed) may, with the authority of a special resolution of our shareholders, divide among our shareholders part or all of our assets, or transfer any part of our assets to a trustee for the benefit of our shareholders.
157
Table of Contents
Changes in capital and allotment of securities
We may, by special resolution of our shareholders, alter our memorandum of association to change the amount of our share capital, consolidate all or any of our shares (whether issued or not) into fewer shares or divide all or any of our shares (whether issued or not) into more shares, cancel any unissued shares or alter our share capital in any other way permitted by the Jersey Companies Law. Subject to the provisions of the Jersey Companies Law, our board has the discretion to issue authorized but unissued shares.
Variation of class rights
The rights attaching to any class of shares may only be altered by approval of holders of not less than two-thirds in number of the issued shares of that class, or by special resolution of the relevant class passed at a class shareholder meeting by the holders of not less than two-thirds in number of the issued shares of that class, in each case, being voted in person or by proxy at such meeting. In addition, unless otherwise expressly provided by the conditions of issue of, or statement of rights relating to, any shares or class of shares, the rights conferred upon the holders of any shares or class of shares (regardless of whether they are issued with preferred, deferred or other special rights) will not be deemed to be varied or abrogated by the creation or issue of further shares or classes of shares (including additional shares of such class), the conversion and redemption of shares in accordance with our articles of association or any applicable statement of rights, or the purchase or redemption by us of our own shares.
General meetings
An annual general meeting and any other shareholders’ meeting (whether convened for the passing of an ordinary or a special resolution) shall be called by at least 14 days’ notice (or more where a longer period of notice is specified in the articles of association) given to all of the members, directors and auditors.
Special meetings
Under the Jersey Companies Law, only our board of directors or shareholders holding at least 10% of the total voting rights of our share capital can requisition a shareholders’ meeting. A meeting requisitioned by shareholders must be held within two months of receipt by us of the written request, but such shareholders may call the meeting if our board of directors does not call the meeting within 21 days of the date of deposit of the written request at our registered office, in which event such meeting must be held within three months of the date of deposit of the written request of our registered office. Our articles of association specifies the information that a shareholder requisitioning a shareholders’ meeting is required to provide with its written request for the requisition of a shareholders’ meeting.
Actions by written consent
Our articles of association provide that shareholder actions by written consent are prohibited.
Directors
Our board of directors may vary the minimum or maximum number of directors (subject to a minimum of two and a maximum of 13 directors), and may appoint directors to fill any vacancies. We currently have 11 members on our board of directors.
Shareholders are only able to appoint a person as a director at a shareholder meeting if (i) the relevant person has been recommended by our board or is a serving director who is retiring at that shareholder meeting; or
158
Table of Contents
(ii) if a shareholder (other than the person proposed as a director) who is entitled to attend and vote at that shareholder meeting has submitted written notice to us of their intention to nominate the relevant person no less than 90 and no more than 120 full days prior to the date of that shareholder meeting, along with a notice from the relevant person confirming their willingness to be appointed.
Directors are required to disclose any conflicts of interest with respect to any contract or proposed contract or any other arrangement or proposed arrangement with us.
Other Jersey law considerations
Share repurchases
In most United States jurisdictions, the Board of Directors is permitted to authorize share repurchases without shareholder consent. Jersey law does not permit share repurchases without the sanction of a special resolution of our shareholders. However, our articles of association will permit our Board of Directors to convert any of our shares that we wish to purchase into redeemable shares, provided that we continue to have issued shares that are not redeemable, which will effectively allow our Board of Directors to authorize share repurchases (which shall be effected by way of redemption) without the need to obtain such shareholder consent, consistent with the practice in most United States jurisdictions.
As with declaring a dividend (that would have the effect of reducing our net assets), we may not buy back or redeem our shares unless our directors have made a statutory solvency statement that, immediately following the date on which the buy back or redemption is proposed, we will be able to discharge our liabilities as they fall due and, having regard to our prospects and the intentions of the directors with respect to the management of our business and the amount and character of the financial recourses that will in the view of the directors be available to us, we will be able to continue to carry on business and discharge our liabilities as they fall due for a 12-month period immediately following the date which the buy back or redemption is proposed to be paid (or we are dissolved on a solvent basis, if earlier).
We may fund a redemption or purchase of our own shares from any source, and a payment for a redemption or purchase may be made in cash or otherwise than in cash (or partly in cash and partly otherwise than in cash). We cannot purchase our ordinary shares if, as a result of such purchase, only redeemable shares would remain in issue.
If authorized by a resolution of our shareholders, any shares that we redeem or purchase may be held by us as treasury shares. Any shares held by us as treasury shares may be cancelled, sold, transferred for the purposes of or under an employee share plan or held without cancelling, selling or transferring them. Ordinary shares redeemed or purchased by us are cancelled where we have not been authorized to hold these as treasury shares.
Mandatory purchases and acquisitions
The Jersey Companies Law provides that where a person, or an offeror, has made an offer to acquire all of our outstanding shares not already held by the offeror and has as a result of such offer acquired or contractually agreed to acquire not less than 90% in number of the shares of any class to which the offer relates, that the offeror is then entitled to give notice to the holders of any shares of that class that it has not acquired or contractually agreed to acquire, to acquire the remaining shares. Similarly, in such circumstances, a holder of any such remaining shares may, by written communication addressed to the offeror, require him to acquire its shares. In either case, the holder of such remaining shares may also apply to the Jersey court for an order that the offeror not be entitled to purchase the holder’s shares or that the offeror purchase the holder’s shares on terms different than those under which the offeror made such offer.
159
Table of Contents
Other than as described above, we are not subject to any regulations under which a shareholder that acquires a certain level of share ownership is then required to offer to purchase all of our remaining shares on the same terms as such shareholder’s prior purchase.
Compromises and arrangements
Where we and our creditors or shareholders or a class of either of them propose a compromise or arrangement between us and our creditors or our shareholders or a class of either of them (as applicable) the Jersey court may order a meeting of the creditors or class of creditors or of our shareholders or class of shareholders (as applicable) to be called in such a manner as the court directs. Any compromise or arrangement approved by a majority in number representing 75% or more in value of the creditors or 75% or more of the voting rights of shareholders or class of either of them (as applicable) if sanctioned by the court, is binding upon us and all the creditors, shareholders or members of the specific class of either of them (as applicable).
No pre-emptive rights
The Jersey Companies Law does not confer any pre-emptive rights to purchase our shares on our shareholders.
Rights of minority shareholders
Under Article 141 of the Jersey Companies Law, a shareholder may apply to court for relief on the ground that the conduct of our affairs, including a proposed or actual act or omission by us, is unfairly prejudicial to the interests of our shareholders generally or of some part of our shareholders, including at least the shareholder making the application. What amounts to unfair prejudice is not defined in the Jersey Companies Law. There may also be common law personal actions available to our shareholders.
Under Article 143 of the Jersey Companies Law (which sets out the types of relief a court may grant in relation to an action brought under Article 141 of the Jersey Companies Law), the court may make an order regulating our affairs, requiring us to refrain from doing or continuing to do an act complained of, authorizing civil proceedings and providing for the purchase of shares by us or by any of our other shareholders.
Comparison of Jersey law and Delaware law
Set forth below is a comparison of certain shareholder rights and corporate governance matters under Delaware law and Jersey law:
| Corporate law issue | Delaware law | Jersey law | ||
| Special meetings of shareholders | Shareholders generally do not have the right to call meetings of shareholders unless that right is granted in the certificate of incorporation or by-laws. However, if a corporation fails to hold its annual meeting within a period of 30 days after the date | Shareholders holding 10% or more of the company’s voting rights and entitled to vote at the relevant meeting may legally require our directors to call a meeting of shareholders.
| ||
| designated for the annual meeting, or if no date has been designated for a period of 13 months after its last annual meeting, the Delaware Court of | The Jersey Financial Services Commission, or JFSC, may, at the request of any officer, secretary or shareholder, call or direct the calling of an annual | |||
|
| ||||
160
Table of Contents
| Corporate law issue | Delaware law | Jersey law | ||
| Chancery may order a meeting to be held upon the application of a shareholder. | general meeting. Failure to call an annual general meeting in accordance with the requirements of the Jersey Companies Law is a criminal offense on the part of a Jersey company and its directors and secretary. | |||
| Interested director transactions | Interested director transactions are permissible and may not be legally voided if:
• either a majority of disinterested directors, or a majority in interest of holders of shares of the corporation’s capital stock entitled to vote upon the matter, approves the transaction in good faith upon disclosure of all material facts; or
• the transaction is determined to have been fair to the corporation as of the time it is authorized, approved or ratified by the board of directors, a committee thereof or the shareholders. |
An interested director must disclose to the company the nature and extent of any interest in a transaction with the company or one of its subsidiaries. Failure to disclose an interest entitles the company or a shareholder to apply to the court for an order setting aside the transaction concerned and directing that the director account to the company for any profit, and the court may so order or make such other order as it deems fit.
A transaction is not voidable and a director is not accountable notwithstanding a failure to disclose an interest if the transaction is confirmed by special resolution and the nature and extent of the director’s interest in the transaction are disclosed in reasonable detail in the notice calling the meeting at which the resolution is passed.
Although it may still order that a director account for any profit, a court shall not set aside a transaction unless it is satisfied that the interests of third parties who have acted in good faith would not thereby be | ||
| unfairly prejudiced and the transaction was not reasonable and fair in the interests of the company at the time it was entered into. | ||||
|
| ||||
161
Table of Contents
| Corporate law issue | Delaware law | Jersey law | ||
| Cumulative voting | The certificate of incorporation of a Delaware corporation may provide that shareholders of any class or classes or of any series may vote cumulatively either at all elections or at elections under specified circumstances. | There are no provisions in the Jersey Companies Law relating to cumulative voting. | ||
| Shareholder approval of corporate matters by written consent | Unless otherwise specified in a corporation’s certificate of incorporation, shareholders may take action permitted to be taken at an annual or special meeting, without a meeting, notice or a vote, if consents, in writing, setting forth the action are signed by shareholders with not less than the minimum number of votes that would be necessary to authorize the action at a meeting. All consents must be dated and are only effective if the requisite signatures are collected within 60 days of the earliest dated consent delivered. | A unanimous written consent by each shareholder entitled to vote on the matter may effect any matter that otherwise may be brought before a shareholders’ meeting, except for the removal of our auditors. Such consent shall be deemed effective when the instrument, or the last of several instruments, is last signed or on such later date as is specified in the resolution. However, subject to the Companies Law, it is possible to either specifically include in a company’s memorandum and articles of association for a written resolution to be passed by a specified majority (as with meetings) or for shareholder resolutions in writing to be prohibited.
Our articles of association provide that shareholder resolutions in writing are prohibited. | ||
| Business combinations | With certain exceptions, a merger, consolidation or sale of all or substantially all of the assets of a Delaware corporation must be approved by the board of directors and a majority of the outstanding shares entitled to vote thereon. | Jersey Companies Law requires, in the case of a merger, the approval of a special resolution passed by at least two-thirds of the shares being voted in person or by proxy at a meeting of the shareholders of the company and, where there is more than one class of members, a special resolution of a separate meeting of each class. | ||
|
| ||||
162
Table of Contents
| Corporate law issue | Delaware law | Jersey law | ||
| Jersey Companies Law does not have such a requirement for the sale of all or substantially all of a company’s assets. | ||||
| Limitations on directors liability and indemnification of directors and officers |
A Delaware corporation may include in its certificate of incorporation provisions limiting the personal liability of its directors to the corporation or its shareholders for monetary damages for many types of breach of fiduciary duty. However, these provisions may not limit liability for any breach of the duty of loyalty, acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, the authorization of unlawful dividends, stock repurchases or shares barring redemptions, or any transaction from which a director derived an improper personal benefit. Moreover, these provisions would not be likely to bar claims arising under U.S. federal securities laws. | The Jersey Companies Law does not contain any provision permitting Jersey companies to limit the liabilities of directors for breach of fiduciary duty.
However, a Jersey company may exempt from liability, and indemnify directors and officers for, liabilities:
(1) incurred in defending any civil or criminal legal proceedings where:
the person is either acquitted or receives a judgment in their favor;
where the proceedings are discontinued other than by reason of such person (or someone on their behalf) giving some benefit or suffering some detriment; or
where the proceedings are settled on terms that such person (or someone on their behalf) gives some benefit or suffers some detriment but in the opinion of a majority of the disinterested directors, the person was substantially successful on the merits in | ||
| the person’s resistance to the proceedings.
(2) incurred to anyone other than to the company if the person acted in good faith with a view to the best interests of the company; | ||||
|
| ||||
163
Table of Contents
| Corporate law issue | Delaware law | Jersey law | ||
| (3) incurred in connection with an application made to the court for relief from liability for negligence, | ||||
| default, breach of duty or breach of trust under Article 212 of the Jersey Companies Law in which relief is granted to the person by the court; or
(4) incurred in a case in which the company normally maintains insurance for persons other than directors. | ||||
| Appraisal rights | A shareholder of a Delaware corporation participating in certain major corporate transactions may, under certain circumstances, be entitled to appraisal rights under which the shareholder may receive cash in the amount of the fair value of the shares held by that shareholder (as determined by a court) in lieu of the consideration the shareholder would otherwise receive in the transaction. | A shareholder of a Jersey company does not have statutory appraisal rights. | ||
| Shareholder suits |
Class actions and derivative actions generally are available to the shareholders of a Delaware corporation for, among other things, breach of fiduciary duty, corporate waste and actions not taken in accordance with applicable law. In such actions, the court has discretion to permit the winning party to recover | Under Article 141 of the Jersey Companies Law, a shareholder may apply to court for relief on the ground that the conduct of a company’s affairs, including a proposed or actual act or omission by us, is unfairly prejudicial to the interests of our shareholders generally or of some part of our shareholders, | ||
| attorneys’ fees incurred in connection with such action. | including at least the shareholder making the application.
Under Article 143 of the Jersey Companies Law (which sets out the types of relief a court may grant in relation to an action brought under Article 141 of the Jersey Companies Law), the court | |||
|
| ||||
164
Table of Contents
| Corporate law issue | Delaware law | Jersey law | ||
| may make an order regulating the affairs of a company, requiring a company to refrain from doing or continuing to do an | ||||
| act complained of, authorizing civil proceedings and providing for the purchase of shares by a company or by any of its other shareholders.
There may also be common law personal actions available to shareholders. | ||||
| Inspection of books and records | All shareholders of a Delaware corporation have the right, upon written demand, to inspect or obtain copies of the corporation’s shares ledger and its other books and records for any purpose reasonably related to such person’s interest as a shareholder. | The register of shareholders and books containing the minutes of general meetings or of meetings of any class of shareholders of a Jersey company must during business hours (Jersey time) be open to the inspection of a shareholder of the company without charge. The register of directors and secretaries must during business hours (subject to such reasonable restrictions as the company may by its articles or in general meeting impose, but so that not less than two hours in each business day be allowed for inspection) be open to the inspection of a shareholder or director of the company without charge. | ||
| Amendments to organizational documents | Amendments to the certificate of incorporation of a Delaware corporation require the affirmative vote of the holders of | The memorandum of association and articles of association of a Jersey company each may only be amended by | ||
| a majority of the outstanding shares entitled to vote thereon or such greater vote as is provided for in the certificate of incorporation. A provision in the certificate of incorporation requiring the vote of a greater number or proportion of the directors or of the holders of any | special resolution approved by holders of at least two-thirds of the shares being voted in person, by phone or by proxy at a shareholder meeting. | |||
|
| ||||
165
Table of Contents
| Corporate law issue | Delaware law | Jersey law | ||
| class of shares than is required by Delaware corporate law may not be amended, altered or repealed except by such greater vote. | ||||
| If provided for in a Delaware corporation’s certificate of incorporation, the board of directors of a Delaware corporation may amend its bylaws without stockholder consent. | ||||
|
| ||||
Registration rights of certain holders
In connection with our Series J financing, we entered into the Tenth Amended and Restated Registration Rights Agreement dated June 1, 2015, or the Registration Rights Agreement, pursuant to which we have granted certain registration rights to holders of our registrable securities, which include ordinary shares issuable upon conversion of our preferred shares or exercise of warrants, in each case that are outstanding prior to this offering. Set forth below is a description of the registration rights granted under the agreement. The Registration Rights Agreement has been filed as an exhibit to the registration statement of which this prospectus forms a part.
Demand registration rights
At any time after the effective date of this offering (subject to applicable lock-up restrictions), certain holders, or the Initiating Holders, have the right to demand that we file a registration statement covering the offer and sale of their registrable securities, so long as the aggregate offering price of securities to be sold under the registration statement is no less than $5.0 million. Upon such demand we are required to use our best efforts to file a registration statement; however, we are not obligated to effect a demand registration (1) if we have already effected one demand registration for the Initiating Holders that were either holders of Series B or Series F convertible preferred shares prior to this offering, one demand registration for the Initiating Holders that were holders of Series G convertible preferred shares prior to this offering, one demand registration for the Initiating Holders that were holders of Series H convertible preferred shares prior to this offering, one demand registration for the Initiating Holders that were holders of Series I convertible preferred shares prior to this offering and one demand registration for the Initiating Holders that were holders of Series J convertible preferred shares prior to this offering, subject to certain exceptions, (2) during a period of 180 days after the effective date of this offering or any other registration pursuant to the Registration Rights Agreement, (3) during a period beginning on the 60th day prior to our good faith estimate of the effective date of, and ending on the 120th day after the effective date of, a firm commitment public offering of our securities initiated by us other than pursuant to the demand registration rights described herein, or (4) if the securities to be registered can be registered on Form S-3. We have the right to defer filing of a registration statement for up to 90 days if we provide the Initiating Holders a certificate signed by our Chief Executive Officer stating that in the good faith judgment of our board of directors such registration will be materially detrimental to us and our shareholders, but we cannot exercise this deferral right more than once in any 12-month period.
166
Table of Contents
Piggyback registration rights
At any time after this offering (subject to applicable lock-up restrictions), if we propose to register any of our share capital or other securities in connection with the public offering of such securities solely for cash (other than a registration on Form S-8 or similar successor form relating solely to the sale of securities to participants in a share option plan or other compensatory arrangement to the extent includable on Form S-8 or similar successor form), then we must offer each holder of the registrable securities the opportunity to include their securities in the registration statement or otherwise register such registrable securities. However, we are not obligated to make any offering of our securities or to complete an offering of our securities that we propose to make.
Form S-3 registration rights
Upon our company becoming eligible to use Form S-3, if certain holders of registrable securities request that we file a registration statement on Form S-3, we (i) shall promptly give at least 30 days’ written notice of the proposed registration to all other holders and (ii) shall use our reasonable best efforts to effect such registration and include therein all or such portion of the registrable securities as are specified in such request, together with all or such portion of the registrable securities of any other holder electing to join in such registration by furnishing to us a written request within 20 days after receipt of the written notice specified in clause (i) above.
However, we are not obligated to file a registration statement on Form S-3 if (i) the aggregate offering price of securities to be sold under the registration statement does not exceed $2.0 million, net of any underwriters’ discounts or commissions, (ii) we provide the requesting holders of the registrable securities a certificate signed by our Chief Executive Officer stating that in the good faith judgment of our board of directors the filing of a registration statement on Form S-3 will be materially detrimental to us and our shareholders or (iii) we have already effected one registration on Form S-3 in the 12-month period preceding the date of such request. Such requests for registrations are not counted as demand registrations.
Transfer agent and registrar
The transfer agent and registrar for our ordinary shares in the United States is Computershare Inc. and its address is 250 Royall Street, Canton, Massachusetts 02021. Computershare Investor Services (Jersey) Limited acts as our Jersey registrar and its address is Queensway House, Hilgrove Street, St. Helier, Jersey JE1 1ES.
Company secretary
Our company secretary, whose duties include keeping board and shareholder minutes, maintaining a register of directors and ensuring that Jersey statutory requirements are met, including the filing of the annual return and accounts with the Jersey company registry, is Citco Jersey Limited, First Floor, Le Masurier House, La Rue Le Masurier, St. Helier, Jersey JE2 4YE.
167
Table of Contents
Shares eligible for future sale
Before this offering, there has not been a public market for our ordinary shares, and while our ordinary shares are approved for listing on the NASDAQ Global Select Market, we cannot assure you that a significant public market for the ordinary shares will develop or be sustained after this offering. Substantial future sales of our ordinary shares in the public markets after this offering, or the perception that such sales may occur, could adversely affect market prices prevailing from time to time. As described below, only a limited number of our ordinary shares currently outstanding will be available for sale immediately after this offering due to contractual and legal restrictions on resale. Nevertheless, after these restrictions lapse, future sales of substantial amounts of our ordinary shares, including ordinary shares issued upon exercise of outstanding options, in the public market in the United States, or the possibility of such sales, could negatively affect the market price in the United States of our ordinary shares and our ability to raise equity capital in the future.
Upon the consummation of this offering, we will have 82,676,810 ordinary shares outstanding (assuming no exercise by the underwriters of the over-allotment option). Of that amount, 7,500,000 ordinary shares will be publicly held by investors participating in this offering, and 75,176,810 ordinary shares will be held by our existing shareholders, who may be our affiliates as that term is defined in Rule 144 under the Securities Act. As defined in Rule 144, an affiliate of an issuer is a person that directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, the issuer.
All of the ordinary shares sold in the offering will be freely transferable by persons (other than our affiliates) without restriction or further registration under the Securities Act. Ordinary shares purchased by one of our affiliates may not be resold, except pursuant to an effective registration statement or an exemption from registration, including an exemption under Rule 144 of the Securities Act described below.
The ordinary shares held by existing shareholders are, and those ordinary shares issuable upon conversion of our convertible preferred shares or exercise of options or warrants outstanding following the consummation of this offering, will be restricted securities, as that term is defined in Rule 144 under the Securities Act. These restricted securities may be sold in the United States only if they are registered or if they qualify for an exemption from registration under Rule 144 or Rule 701 under the Securities Act. These rules are described below. In addition, substantially all of our existing shareholders are subject to a 180-day lock-up period following this offering, as described further below.
Lock-up agreements
We and substantially all of our existing shareholders have agreed that we and such shareholders will not, during the period ending 180 days after the date of this prospectus:
(a) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, any ordinary shares or any other securities convertible into or exercisable or exchangeable for ordinary shares; or
(b) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the ordinary shares,
whether any such transaction described above is to be settled by delivery of ordinary shares or such other securities, in cash or otherwise, subject to certain exceptions. See “Underwriting.”
The foregoing lock-up period is subject to waiver or release by J.P. Morgan Securities LLC at its sole discretion.
168
Table of Contents
After the waiver, release or expiration of the lock-up agreements, the ordinary shares subject to the lock-up agreements will be freely eligible for sale in the public market, subject to restrictions under the Securities Act as described below.
Rule 144
In general, under Rule 144 as currently in effect, once we have been a reporting company under the Exchange Act for a period of 90 days after the date of this prospectus, a person who is not deemed to have been our affiliate at any time during the three months preceding a sale and who has beneficially owned our restricted ordinary shares for at least six months would be entitled to sell an unlimited number of those shares, subject only to the availability of current public information about us. A non-affiliate who has beneficially owned our restricted ordinary shares for at least one year from the later of the date these shares were acquired from us or from one of our affiliates would be entitled to freely sell those shares.
A person who is deemed to be an affiliate of ours and who has beneficially owned our restricted ordinary shares for at least six months would be entitled to sell, within any three-month period, a number of shares that is not more than the greater of:
| (a) | 1.0% of the number of our ordinary shares then outstanding, which will equal approximately 826,768 ordinary shares immediately after this offering (assuming no exercise by the underwriters of the over-allotment option); or |
| (b) | the average weekly reported trading volume of our ordinary shares on NASDAQ during the four calendar weeks preceding the date on which a notice of the sale on Form 144 is filed with the SEC by such person. |
Sales under Rule 144 by persons who are deemed to be our affiliates are also subject to manner-of-sale provisions, notice requirements and the availability of current public information about us.
In addition, in each case, these shares would remain subject to lock-up arrangements described in this prospectus and would only become eligible for sale when the lock-up period expires.
Rule 701
Beginning 90 days after the date of this prospectus, persons other than affiliates who purchased ordinary shares under a written compensatory plan or contract may be entitled to sell such shares in the United States in reliance on Rule 701. Rule 701 permits affiliates to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. Rule 701 further provides that non-affiliates may sell these shares in reliance on Rule 144 subject only to its manner-of-sale requirements. However, the Rule 701 shares would remain subject to lock-up arrangements described in this prospectus and would only become eligible for sale when the lock-up period expires.
Share options
Following the consummation of this offering, we intend to file a registration statement on Form S-8 with the SEC covering ordinary shares reserved for issuance under our 2003 Share Option Plan, 2013 Share Option Plan and 2015 Omnibus Incentive Plan. This registration statement is expected to become effective upon filing. Shares covered by this registration statement will then be eligible for sale in public markets, subject to any applicable lock-up agreements described in this prospectus and Rule 144 limitations applicable to affiliates.
169
Table of Contents
Registration rights
As described in the section entitled “Description of share capital—Registration rights of certain holders,” upon the consummation of this offering, holders of 66,609,559 ordinary shares, including shares issued upon conversion of our convertible preferred shares, and the holders of options and warrants to purchase 5,542,711 ordinary shares, or 78% of our ordinary shares on a fully diluted basis after this offering, will have rights, subject to various conditions and limitations, to require us to file registration statements covering their shares or to include their shares in registration statements that we file for ourselves or other shareholders, subject to the 180-day lock-up period described above. By exercising their registration rights and causing a large number of shares to be registered and sold in the public market, these holders could cause the price of our ordinary shares to fall. In addition, any demand to include such shares in our registration statements could have a material adverse effect on our ability to raise any needed capital.
170
Table of Contents
United States taxation
This section describes the material U.S. federal income tax consequences of acquiring, holding and disposing of our ordinary shares. It only applies to a U.S. Holder that acquires its ordinary shares in this offering at the initial public offering price, and holds such ordinary shares as capital assets for U.S. federal income tax purposes. This section does not address certain particular considerations that might apply to any holder that holds any of our share capital acquired other than in this offering. This section does not address any holder that is a member of a special class of holders subject to special rules, including:
| • | a dealer in securities, |
| • | a trader in securities that elects to use a mark-to-market method of accounting for securities holdings, |
| • | a tax-exempt organization, |
| • | a life insurance company, |
| • | a regulated investment company, real estate investment trust, S corporation or other entity taxed as a financial conduit for U.S. federal income tax purposes, |
| • | a bank or other financial institution, |
| • | an entity classified as a partnership for U.S. federal income tax purposes, |
| • | a person liable for the U.S. alternative minimum tax, |
| • | a person that actually or constructively owns, as of the date hereof or at any other time, 10% or more of our voting stock, |
| • | a person that holds shares as part of a straddle or a hedging or conversion transaction, or |
| • | a U.S. Holder (as defined below) whose functional currency is not the United States dollar. |
This section is based on the Internal Revenue Code of 1986, as amended (the “Code”), its legislative history, final, temporary and proposed regulations (together, the “Regulations”), and published rulings and court decisions, all of which are subject to change, possibly on a retroactive basis. There is currently no comprehensive income tax treaty between the United States and Jersey.
A holder is a “U.S. Holder” if such holder is a beneficial owner of ordinary shares and such holder is:
| • | a citizen or resident of the United States, |
| • | a corporation (or other entity taxable as a corporation) created or organized in or under the laws of the United States, any state thereof, or the District of Columbia, |
| • | an estate whose income is subject to U.S. federal income tax regardless of its source, or |
| • | a trust, if a United States court can exercise primary supervision over the trust’s administration and one or more United States persons are authorized to control all substantial decisions of the trust, or if the trust has a valid election in effect under applicable Regulations to be treated as a United States person. |
This disclosure does not address any holder that is not a U.S. Holder.
The U.S. federal income tax treatment of a partnership (including any entity treated as a partnership for U.S. federal income tax purposes) that is a beneficial owner of ordinary shares generally will depend upon the status
171
Table of Contents
of the partner and the activities of the partnership. A beneficial owner of ordinary shares that is a partnership (including the partners in such partnership) should consult its own tax advisors regarding the U.S. federal income tax consequences of owning and disposing of the ordinary shares.
| This discussion addresses only U.S. federal income taxation. You should consult your own tax advisor as to the potential application of U.S. state and local tax laws, as well as any other U.S. tax laws (such as the estate tax) or other U.S. laws, as well as the laws of Jersey and other non-U.S. laws. |
Taxation of dividends
Subject to the passive foreign investment company (“PFIC”) rules discussed below, the gross amount of any dividend we pay out of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes) will be subject to U.S. federal income taxation for U.S. Holders.
Distributions in excess of current and accumulated earnings and profits, as determined for U.S. federal income tax purposes, generally will be treated as a non-taxable return of capital to the extent of the U.S. Holder’s basis in the ordinary shares and thereafter as capital gain; however, since we do not intend to maintain books and records in accordance with U.S. tax principles, it is expected that all amounts we distribute will be reported to U.S. Holders as dividends for U.S. federal income tax purposes. Dividends paid to a noncorporate U.S. Holder that constitute “qualified dividend income” will be taxable to such noncorporate U.S. Holder at preferential tax rates, provided the ordinary shares are held for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and provided that such noncorporate U.S. Holder meets other holding period requirements, unless such noncorporate U.S. Holder takes the dividend income into account as investment income.
Dividends that we pay with respect to the ordinary shares, if any, generally will be qualified dividend income provided that, in the year that a U.S. Holder receives the dividend, the ordinary shares are readily tradable on an established securities market in the United States. Our ordinary shares are approved for listing on the NASDAQ Global Select Market and we expect such ordinary shares to be considered readily tradable on an established securities market. However, even if the ordinary shares are readily tradable on an established securities market in the United States, any dividends paid with respect to the ordinary shares will not be treated as qualified dividend income if we are treated as a PFIC for the taxable year in which we pay a dividend, or if it was treated as a PFIC for the preceding taxable year.
Dividends are taxable to a U.S. Holder when such dividend is received, actually or constructively. Such dividends will not be eligible for the dividends-received deduction generally allowed to United States corporations in respect of dividends received from other United States corporations.
Dividends generally will be income from sources outside the United States, and dividends paid will, depending on a U.S. Holder’s circumstances, be “passive” or “general” income which, in either case, is treated separately from other types of income for purposes of computing the allowable foreign tax credit. A U.S. Holder may make an election to treat all foreign taxes paid as deductible expenses in computing taxable income, rather than as a credit against tax, subject to generally applicable limitations. Such an election, once made, applies to all foreign taxes paid for the taxable year subject to the election. The rules governing foreign tax credits are complex and, therefore, U.S. Holders are strongly encouraged to consult their own tax advisors to determine whether they are subject to any special rules that may limit their ability to use foreign tax credits and whether or not an election to treat foreign taxes paid as deductions rather than credits would be appropriate based on their particular circumstances.
172
Table of Contents
Taxation of capital gains
Subject to the PFIC rules discussed below, if a U.S. Holder sells or otherwise disposes of its ordinary shares, it should recognize capital gain or loss for U.S. federal income tax purposes equal to the difference between the U.S. dollar value of the amount that it realizes and its tax basis in its ordinary shares. Capital gain of a noncorporate U.S. Holder is generally taxed at preferential tax rates, where such U.S. Holder has held its ordinary shares for more than one year. Such gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes. The deductibility of capital losses is subject to certain limitations.
Passive foreign investment company considerations
We believe that our ordinary shares should not be treated as stock of a PFIC for U.S. federal income tax purposes, but this conclusion is a factual determination that is made annually and thus may be subject to change.
In general, for U.S. Holders, we will be a PFIC with respect to a U.S. Holder if for any taxable year in which such U.S. Holder holds ordinary shares:
| • | at least 75% of our gross income for the taxable year is passive income within the meaning of the PFIC rules; or |
| • | at least 50% of the value, determined on the basis of a quarterly average, of our assets is attributable to assets that produce or are held for the production of passive income within the meaning of the PFIC rules. |
For purposes of the PFIC rules, passive income generally includes dividends, interest, royalties, rents (other than certain rents and royalties derived in the active conduct of a trade or business), annuities and gains from assets that produce passive income. Cash is generally treated as an asset that produces passive income, for purposes of the PFIC rules. If a foreign corporation owns at least 25% by value of the stock of another corporation, the foreign corporation is treated for purposes of the PFIC tests as owning its proportionate share of the assets of the other corporation, and as receiving directly its proportionate share of the other corporation’s income. Our ordinary shares will be treated as stock of a PFIC with respect to a U.S. Holder if we are a PFIC at any time during the period in which such U.S. Holder holds the ordinary shares, even if we cease to be treated as a PFIC, unless special elections (described below) are made.
If we are treated as a PFIC, and a U.S. Holder does not make one of the elections described below, the U.S. Holder will be subject to special PFIC tax rules with respect to:
| • | any gain realized on the sale or other disposition of the ordinary shares; and |
| • | any excess distribution that we make to the U.S. Holder (generally, any distributions during a single taxable year that are greater than 125% of the average annual distributions received by a U.S. Holder in respect of the ordinary shares during the three preceding taxable years or, if shorter, the U.S. Holder’s holding period for the ordinary shares). |
Under these rules:
| • | the gain or excess distribution will be allocated ratably over the U.S. Holder’s holding period for the ordinary shares; |
| • | the amount allocated to the taxable year in which the U.S. Holder realizes the gain or excess distribution and to any years before we became a PFIC will be taxed as ordinary income; and |
173
Table of Contents
| • | the amount allocated to any other year, with certain exceptions, will be taxed at the highest tax rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed in respect of the amount allocated to each such year. |
Special rules apply for calculating the amount of foreign tax credits available with respect to excess distributions by a PFIC.
The special PFIC tax rules described above will not apply to a U.S. Holder that makes a qualified electing fund or “QEF” election, and we provide certain required information to such electing U.S. Holder. We intend to provide U.S. Holders with such information as may be required to make a QEF election effective.
If a U.S. Holder makes a QEF election, such U.S. Holder will be currently subject to tax on its pro rata share of our ordinary earnings and net capital gain, at ordinary income and capital gain rates, respectively, for each of our taxable years that we are classified as a PFIC, regardless of whether or not such U.S. Holder receives distributions. Such U.S. Holder’s basis in the ordinary shares will be increased to reflect taxed but undistributed income. Distributions of income that had been taxed previously will result in a corresponding reduction of basis in the shares and will not be taxed again as a distribution to such U.S. Holder. U.S. Holders should consult their own tax advisors regarding making QEF elections in their particular circumstances.
If a U.S. Holder owns ordinary shares in a PFIC that are treated as “marketable stock,” as such term is defined under the PFIC rules, such U.S. Holder may also make a mark-to-market election. If a U.S. Holder makes this election, it will not be subject to the PFIC rules described above. Instead, in general, such U.S. Holder will include as ordinary income each year the excess, if any, of the fair market value of its ordinary shares at the end of the taxable year over its adjusted basis in its ordinary shares. These amounts of ordinary income will not be eligible for the favorable tax rates applicable to qualified dividend income or long-term capital gains. A U.S. Holder will also be allowed to take an ordinary loss in respect of the excess, if any, of the adjusted basis of its ordinary shares over the fair market value at the end of the taxable year (but only to the extent of the net amount of previously included income as a result of the mark-to-market election). A U.S. Holder’s basis in the ordinary shares will be adjusted to reflect any such amounts of income or loss. Gains from an actual sale or other disposition of the ordinary shares in a year when we are a PFIC will be treated as ordinary income, and any losses incurred on a sale or other disposition of the ordinary shares will be treated as an ordinary loss to the extent of any net mark-to-market gains previously included. Any remaining loss on the sale of such ordinary shares will be treated as a capital loss. Once made, the election cannot be revoked without the consent of the IRS unless the ordinary shares cease to be marketable. For purposes of this rule, if a U.S. Holder makes a mark-to-market election with respect to its ordinary shares, it will be treated as having a new holding period in its ordinary shares beginning on the first day of the first taxable year beginning after the last taxable year for which the mark-to-market election applies.
Notwithstanding any election made with regard to the ordinary shares, dividends received from us will not constitute qualified dividend income if we are treated as a PFIC either in the taxable year of the distribution or the preceding taxable year. Dividends received that do not constitute qualified dividend income are not eligible for taxation at the preferential tax rates applicable to qualified dividend income. Instead, a U.S. Holder must include the gross amount of any such dividend paid by us out of our accumulated earnings and profits (as determined for U.S federal income tax purposes) in its gross income, and it will be subject to tax at rates applicable to ordinary income.
If we were to be treated as a PFIC for any taxable year, a U.S. Holder would be required to file an annual report for that taxable year on IRS Form 8621 “Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund.” U.S. Holders should consult their own tax advisors concerning our PFIC status and the potential application of the PFIC rules.
174
Table of Contents
Net investment income tax
An additional 3.8% tax is imposed on the “net investment income” of noncorporate U.S. Holders, and on the undistributed “net investment income” of certain estates and trusts. Among other items, “net investment income” generally includes dividends paid on our ordinary shares and certain net gain from the sale or other taxable disposition of such ordinary shares, less certain deductions. This tax applies whether or not we are a PFIC. U.S. Holders should consult their own tax advisors concerning the potential effect, if any, of this tax on holding ordinary shares in such U.S. Holder’s particular circumstances.
Backup withholding and information reporting
For noncorporate U.S. Holders, information reporting requirements, on IRS Form 1099, generally will apply to:
| • | dividend payments or other taxable distributions made to the noncorporate U.S. Holder within the United States or by a United States payor; and |
| • | the payment of proceeds to the noncorporate U.S. Holder from the sale of ordinary shares effected at a United States office of a broker or through certain U.S.-related financial intermediaries. |
Additionally, backup withholding may apply to such payments if the noncorporate U.S. Holder:
| • | fails to provide an accurate taxpayer identification number; |
| • | is notified by the IRS that it has failed to report all interest and dividends required to be shown on its U.S. federal income tax returns; or |
| • | in certain circumstances, fails to comply with applicable certification requirements. |
Backup withholding is not an additional tax. Noncorporate U.S. Holders generally may obtain a refund of any amounts withheld under the backup withholding rules that exceed their income tax liability by filing a timely refund claim with the IRS.
Disclosure of information with respect to foreign financial assets
Certain U.S. Holders who hold any interest in “specified foreign financial assets,” including our ordinary shares, during such U.S. Holder’s taxable year must attach to their U.S. federal income tax return for such year certain information with respect to each such asset if the aggregate value of all of such assets exceeds $50,000 on the last day of the tax year or more than $75,000 at any time during the tax year (or a higher dollar amount prescribed by the IRS), unless such shares are held in an account maintained by a U.S. payor, such as a U.S. financial institution or the U.S. branch of certain foreign banks or insurers. For this purpose, a “specified foreign financial asset” includes any depositary, custodial or other financial account maintained by a foreign financial institution (other than a U.S. branch of certain foreign financial institutions), and certain assets that are not held in an account maintained by a financial institution, including any stock or security issued by a person other than a United States person. A taxpayer subject to these rules who fails to furnish the required information may be subject to a penalty of $10,000, and an additional penalty may apply if the failure continues for more than 90 days after the taxpayer is notified of such failure by the IRS, unless the taxpayer demonstrates a reasonable cause for such failure to comply. An accuracy-related penalty of 40% is imposed for an underpayment of tax that is attributable to an “undisclosed foreign financial asset understatement,” which for this purpose is the portion of the understatement of gross income for any taxable year that is attributable to any transaction involving an “undisclosed foreign financial asset,” including any asset that is subject to these information reporting requirements, which would include our ordinary shares if the dollar threshold described above were satisfied.
175
Table of Contents
The applicable statute of limitations for assessment of U.S. federal income taxes is extended to six years if a taxpayer omits from gross income more than $5,000 and such omission is attributable to a foreign financial asset as to which reporting is required under the rules described in the preceding paragraph or would be so required if such rules were applied without regard to the dollar threshold and any other exceptions specified by the IRS. In addition, the statute of limitations will be suspended if a taxpayer fails to provide in a timely manner either information with respect to specified foreign financial assets required to be reported or the annual information reports required for holders of PFIC stock. U.S. Holders should consult their own tax advisors concerning any obligation that they may have to furnish information to the IRS as a result of holding our ordinary shares.
Jersey taxation
This section describes the material Jersey tax consequences of owning shares. It applies to you only if you acquire your shares in this offering and you hold your shares as capital assets for tax purposes. This section is based on the tax laws of Jersey and all regulations thereunder as currently in effect. These laws are subject to change, possibly on a retroactive basis.
Taxation of the company
We believe that we are a Jersey resident for tax purposes. The Income Tax (Jersey) Law 1961, as amended, provides that the general basic rate of income tax on the profits of a company, other than a “financial services company” or utility company (of which we are neither), will be 0%.
Taxation of dividends
We will be entitled to pay dividends to holders of our ordinary shares without any withholding or deduction for or on account of Jersey tax. Holders of our ordinary shares (other than residents of Jersey) will not be subject to any tax in Jersey with respect to the acquisition, ownership or other disposition of our ordinary shares.
Taxation of capital gains
Under current Jersey law, there is no capital gains tax.
Jersey stamp duty, estate and gift taxation
Under current Jersey law, there are no death or estate duties or gift, wealth, inheritance or capital transfer taxes. No stamp duty or other transfer tax is levied in Jersey on the issue or transfer of our ordinary shares. In the event of the death of an individual who is a shareholder (whether or not a resident in Jersey), stamp duty at rates of up to 0.75% of the value of the estate held may be payable on the registration of a Jersey probate or letters of administration that may be required in order to transfer or otherwise dispose of our ordinary shares held by the deceased individual’s estate.
Jersey goods and services taxation
We are an “international services entity” for the purposes of the Goods and Services Tax (Jersey) Law 2007, or the GST Law. Consequently, we are not required to:
| • | register as a taxable person pursuant to the GST Law; |
| • | charge goods and services tax in Jersey with respect to any supply made by us; or |
176
Table of Contents
| • | subject to limited exceptions that are not expected to apply to us, pay goods and services tax in Jersey with respect to any supply received by us. |
Our directors intend to conduct our business such that no goods and services tax in Jersey will be incurred by us.
Agreements regarding the EU savings directive
Jersey is not part of the European Union and is not subject to the EU directive on the taxation of savings income in the form of interest payments, or the EU Savings Tax Directive, or other EU fiscal legislation. However, in keeping with Jersey’s policy of constructive international engagement (and in line with steps taken by other relevant countries) Jersey has entered into various agreements regarding the EU Savings Tax Directive.
Jersey introduced a system that permits the disclosure of information concerning details of payments of interest (or other similar payments) and the identity of an individual beneficial owner of the interest to the tax authority of the EU jurisdiction where the owner of the interest payment is resident. In relation to payments of interest made by a Jersey paying agent on and from January 1, 2015, Jersey requires mandatory reporting of such information.
Prior to January 1, 2015, the system permitted either (1) the disclosure described above or (2) the imposition of a retention or withholding tax in respect of payments of interest (or other similar income) made to an individual beneficial owner resident in an EU member state by a paying agent situated in Jersey or an EU member state. However, since January 1, 2015 the latter alternative has ceased to be available.
The terms “beneficial owner” and “paying agent” are defined in the bilateral agreements entered into between Jersey and each of the EU member states relating to the treatment of savings income.
The requirement in respect of information disclosure should not apply to companies, partnerships or to most types of trusts, nor should they apply to individuals who are resident outside the European Union. However, to the extent that any such requirement does apply, shareholders may be required to provide information regarding their tax status, identity and residency in order to satisfy the exchange of information requirements referred to above and will be deemed by their subscription for ordinary shares to have agreed to provide to the Company any information requested by it and to have authorised the automatic disclosure by us to the relevant tax authorities of any information held or provided to us.
177
Table of Contents
We are offering the ordinary shares described in this prospectus through a number of underwriters. J.P. Morgan Securities LLC, Deutsche Bank Securities Inc. and Evercore Group L.L.C. are acting as joint book-running managers of this offering and J.P. Morgan Securities LLC is acting as representative of the underwriters. We have entered into an underwriting agreement with the underwriters. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriters, and each underwriter has severally agreed to purchase, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of ordinary shares listed next to its name in the following table:
| Name | Number of shares | |||
| J.P. Morgan Securities LLC |
||||
| Deutsche Bank Securities Inc. |
||||
| Evercore Group L.L.C. Wells Fargo Securities, LLC JMP Securities LLC Wedbush Securities Inc. |
||||
|
|
|
|||
| Total |
7,500,000 | |||
|
|
||||
The underwriters are committed to purchase all the ordinary shares offered by us if they purchase any ordinary shares. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may also be increased or this offering may be terminated.
The underwriters propose to offer the ordinary shares directly to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. Any such dealers may resell ordinary shares to certain other brokers or dealers at a discount of up to $ per share from the initial public offering price. After the initial public offering of the ordinary shares, the offering price and other selling terms may be changed by the underwriters. Sales of ordinary shares made outside of the United States may be made by affiliates of the underwriters. The offering of the ordinary shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
The underwriters have an option to buy up to 1,125,000 additional ordinary shares from us to cover sales of ordinary shares by the underwriters that exceed the number of ordinary shares specified in the table above. The underwriters have 30 days from the date of this prospectus to exercise this over-allotment option. If any ordinary shares are purchased with this over-allotment option, the underwriters will purchase ordinary shares in approximately the same proportion as shown in the table above. If any additional ordinary shares are purchased, the underwriters will offer the additional ordinary shares on the same terms as those on which the ordinary shares are being offered.
The underwriting fee is equal to the public offering price per share less the amount paid by the underwriters to us per share. The underwriting fee is $ per share. The following table shows the per share and total underwriting discounts and commissions to be paid to the underwriters assuming both no exercise and full exercise of the underwriters’ option to purchase additional ordinary shares.
| Without
over- allotmentexercise |
With full over- allotment exercise |
|||||||
| Per share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
178
Table of Contents
The expenses of this offering, not including the underwriting discounts and commissions, are estimated at approximately $3.0 million and are payable by us. We have agreed with the underwriters to pay all expenses and application fees (including the legal fees of counsel for the underwriters) incurred and invoiced in connection with any filing with, and clearance of this offering by, the Financial Industry Regulatory Authority, Inc., or FINRA, in an aggregate amount not to exceed $100,000. The underwriters have agreed to reimburse us for certain out-of-pocket expenses incurred in connection with this offering.
A prospectus in electronic format may be made available on the web sites maintained by one or more underwriters, or selling group members, if any, participating in this offering. The underwriters may agree to allocate a number of ordinary shares to underwriters and selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to underwriters and selling group members that may make Internet distributions on the same basis as other allocations.
We have agreed that we will not (i) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, or file with the Securities and Exchange Commission a registration statement under the Securities Act relating to, any ordinary shares or securities convertible into or exchangeable or exercisable for any ordinary shares, or publicly disclose the intention to make any such offer, sale, pledge, disposition or filing, or (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the ordinary shares or any such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of ordinary shares or such other securities, in cash or otherwise, without the prior written consent of J.P. Morgan Securities LLC, other than (A) the ordinary shares to be sold pursuant to this prospectus, (B) any of our ordinary shares issued upon the conversion of our outstanding convertible preferred shares into ordinary shares in connection with this offering or the exercise of options granted under company share plans or warrants described as outstanding in this prospectus, (C) any options and other awards granted under company share plans as described in this prospectus, provided that we will cause each recipient of any such grant to execute and deliver to J.P. Morgan Securities LLC a lock-up agreement substantially in the form described in the next paragraph prior to such grants if such recipient has not already delivered one, (D) our filing of any registration statement on Form S-8 or a successor form thereto and (E) any of our ordinary shares or any securities convertible into or exercisable or exchangeable for our ordinary shares issued in connection with a joint venture, marketing or distribution arrangement, collaboration agreement, strategic alliance, partnership, equipment leasing arrangement or intellectual property license agreement or any acquisition of assets or not less than a majority or controlling portion of the equity of another entity, provided that (x) the aggregate number of our ordinary shares or securities convertible into or exercisable for our ordinary shares issued pursuant to this clause (E) may not exceed 5.0% of the total number of our ordinary shares issued and outstanding immediately following the closing of this offering and (y) the recipient of any such ordinary shares and securities issued pursuant to this clause (E) during the 180-day restricted period described above must execute and deliver to J.P. Morgan Securities LLC a lock-up agreement substantially in the form described in the next paragraph prior to such issuance.
Our directors and executive officers and substantially all of our existing shareholders and optionholders have entered into lock-up agreements with the underwriters prior to the commencement of this offering pursuant to which each of these persons or entities, with limited exceptions, for a period of 180 days after the date of this prospectus, may not, without the prior written consent of J.P. Morgan Securities LLC, (1) offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, or otherwise transfer or dispose of, directly or indirectly, any ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares (including, without limitation, ordinary shares or such other securities which may be deemed to be beneficially owned by such directors,
179
Table of Contents
executive officers, shareholders, managers and members in accordance with the rules and regulations of the SEC and securities which may be issued upon exercise of an option or warrant), or publicly disclose the intention to make any such offer, sale, pledge or disposition or (2) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the ordinary shares or such other securities, whether any such transaction described in clause (1) or (2) above is to be settled by delivery of ordinary shares or such other securities, in cash or otherwise, or (3) make any demand for or exercise any right with respect to the registration of any ordinary shares or any security convertible into or exercisable or exchangeable for our ordinary shares, in each case, other than:
(a) transactions relating to ordinary shares or other securities acquired in open market transactions after the completion of this offering, provided that no filing under Section 16(a) of the Exchange Act shall be required or shall be voluntarily made in connection with subsequent sales of ordinary shares or other securities acquired in such open market transactions (other than a filing on a Form 5 made after the expiration of the 180-day period referred to above);
(b) the offering shares to be sold, if any, by the locked-up person or entity pursuant to the underwriting agreement;
(c) the transfer of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares to any immediate family member, or to a trust formed for the benefit of an immediate family member, in a transaction not involving a disposition for value;
(d) the transfer of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares to a trust or other entity formed for estate planning purposes in which the locked-up person or entity is a beneficiary;
(e) the transfer of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares as a bona fide gift;
(f) if the locked-up person or entity is a corporation, partnership or other business entity, the transfer of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares to another corporation, partnership or other business entity that is a direct or indirect affiliate (as defined in Rule 405 under the Securities Act) of such locked-up entity, or that otherwise controls, is controlled by or is under common control with such locked-up entity;
(g) if the locked-up person or entity is a corporation, partnership or other business entity, the distribution of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares to members, limited partners, stockholders or other equity holders of such locked-up entity in a transaction not involving a disposition for value;
(h) the transfer of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares that occurs by operation of law, such as pursuant to a qualified domestic order or divorce decree;
(i) the transfer of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares that occurs by will or intestacy;
(j) (x) any vesting event of our securities or any exercise of options or warrants to purchase our ordinary shares, provided that the underlying ordinary shares remain subject to a lock-up agreement or (y) any transfer of our securities to cover tax withholding obligations in connection with the vesting or exercise of any options, warrants, restricted stock or similar rights, provided that any filing made pursuant to Section 16(a) of the Exchange Act shall include a footnote describing the purpose of the transaction;
180
Table of Contents
(k) the conversion of our outstanding convertible preferred shares into ordinary shares in connection with this offering, provided that such ordinary shares remain subject to the terms of a lock-up agreement;
(l) the transfer of ordinary shares or any securities convertible into or exercisable or exchangeable for ordinary shares to us in exercise of our right to repurchase or reacquire securities from the locked-up person or entity pursuant to agreements entered into with us that permit us to repurchase or reacquire such securities upon the termination of such individual’s employment with us, and in the case of Professor Yoram Palti, pursuant to the Letter Agreement described under “Certain relationships and related-party transactions”;
(m) in response to a bona fide third-party tender offer, merger, amalgamation or consolidation made to all holders of ordinary shares or any other acquisition transaction whereby all of the ordinary shares are acquired by a third party, provided that if such tender offer, merger, amalgamation, consolidation or other acquisition transaction is not completed, any ordinary shares or any security convertible into or exercisable or exchangeable for ordinary shares subject to the lock-up agreement shall remain subject to the restrictions contained in the lock-up agreement;
(n) the establishment of a trading plan pursuant to Rule 10b5-1 under the Exchange Act for the transfer of ordinary shares, provided that such plan does not provide for the transfer of ordinary shares during the 180-day period referred to above and no public announcement or filing under the Exchange Act regarding the establishment of such plan shall be required or voluntarily made; or
(o) in the case of certain shareholders, the transfer of ordinary shares purchased in connection with this offering;
provided that in the case of any transfer or distribution pursuant to clauses (c) through (i) and (m) above, each transferee, donee, trustee or distributee shall sign and deliver to J.P. Morgan Securities LLC a lock-up agreement and in the case of any transfer or distribution pursuant to clauses (c) through (h) and (j) above, no filing under Section 16(a) of the Exchange Act reporting a reduction in beneficial ownership of ordinary shares, shall be required or shall be voluntarily made during the restricted period referred to in the foregoing sentence (other than a filing on a Form 5 made after the expiration of the 180-day period referred to above). For purposes of the lock-up agreements, “immediate family” means the spouse, domestic partner, any lineal descendent, father, mother, brother or sister of the locked-up individual.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act of 1933.
Our ordinary shares are approved for listing on the NASDAQ Global Select Market under the symbol “NVCR.”
In connection with this offering, the underwriters may engage in stabilizing transactions, which involves making bids for, purchasing and selling ordinary shares in the open market for the purpose of preventing or retarding a decline in the market price of the ordinary shares while this offering is in progress. These stabilizing transactions may include making short sales of the ordinary shares, which involves the sale by the underwriters of a greater number of ordinary shares than they are required to purchase in this offering, and purchasing ordinary shares on the open market to cover positions created by short sales. Short sales may be “covered” shorts, which are short positions in an amount not greater than the underwriters’ over-allotment option referred to above, or may be “naked” shorts, which are short positions in excess of that amount. The underwriters may close out any covered short position either by exercising their over-allotment option, in whole or in part, or by purchasing ordinary shares in the open market. In making this determination, the underwriters will consider, among other things, the price of ordinary shares available for purchase in the open market compared to the price at which the underwriters may purchase ordinary shares through the over-allotment option. A naked short position is more likely to be created if the underwriters are concerned that
181
Table of Contents
there may be downward pressure on the price of the ordinary shares in the open market that could adversely affect investors who purchase in this offering. To the extent that the underwriters create a naked short position, they will purchase ordinary shares in the open market to cover the position.
The underwriters have advised us that, pursuant to Regulation M of the Securities Act of 1933, they may also engage in other activities that stabilize, maintain or otherwise affect the price of the ordinary shares, including the imposition of penalty bids. This means that if J.P. Morgan Securities LLC purchases ordinary shares in the open market in stabilizing transactions or to cover short sales, the representative can require the underwriters that sold those ordinary shares as part of this offering to repay the underwriting discount received by them.
These activities may have the effect of raising or maintaining the market price of the ordinary shares or preventing or retarding a decline in the market price of the ordinary shares, and, as a result, the price of the ordinary shares may be higher than the price that otherwise might exist in the open market. If the underwriters commence these activities, they may discontinue them at any time. The underwriters may carry out these transactions on NASDAQ, in the over-the-counter market or otherwise.
Prior to this offering, there has been no public market for our ordinary shares. The initial public offering price will be determined by negotiations between us and J.P. Morgan Securities LLC. In determining the initial public offering price, we and J.P. Morgan Securities LLC expect to consider a number of factors, including:
| • | the information set forth in this prospectus and otherwise available to J.P. Morgan Securities LLC; |
| • | our prospects and the history and prospects for the industry in which we compete; |
| • | an assessment of our management; |
| • | our prospects for future earnings; |
| • | the general condition of the securities markets at the time of this offering; |
| • | the recent market prices of, and demand for, publicly traded ordinary shares of generally comparable companies; and |
| • | other factors deemed relevant by the underwriters and us. |
Neither we nor the underwriters can assure investors that an active trading market will develop for our ordinary shares, or that the ordinary shares will trade in the public market at or above the initial public offering price.
The underwriters and their respective affiliates are full-service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their affiliates have provided in the past to us and our affiliates and may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services in the ordinary course of their business, for which they have received and may continue to receive customary fees and commissions. In addition, from time to time, certain of the underwriters and their respective affiliates may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account or the account of customers, and hold on behalf of themselves or their customers, and such investment and trading activities may involve or relate to our assets, securities and/or instruments (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with us, and may do so in the future. The underwriters and their respective affiliates may also communicate independent investment
182
Table of Contents
recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
Perella Weinberg Partners, or Perella Weinberg, a FINRA member, is acting as our independent financial advisor in connection with the offering. We have agreed with Perella Weinberg to pay certain fees upon the successful completion of this offering, to reimburse certain expenses incurred in connection with the engagement and, in our sole discretion, to pay an additional incentive fee in connection with this offering. Perella Weinberg is not acting as an underwriter and will not sell or offer to sell any securities and will not identify, solicit or engage directly with potential investors. In addition, Perella Weinberg will not underwrite or purchase any of the offered securities or otherwise participate in any such undertaking.
J. P. Morgan Securities LLC’s address is 383 Madison Avenue, New York, New York 10179. Deutsche Bank Securities Inc.’s address is 60 Wall Street, New York, New York 10005. Evercore Group L.L.C.’s address is 55 East 52nd Street, New York, New York 10055.
Selling restrictions
General
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
United Kingdom
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in the Prospectus Directive) (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended (the “Order”) and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”).
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons. Any person in the United Kingdom that is not a relevant person should not act or rely on this document or any of its contents.
European Economic Area
In relation to each Member State of the European Economic Area (each, a “Relevant Member State”), no offer of ordinary shares may be made to the public in that Relevant Member State other than:
| • | to any legal entity which is a qualified investor as defined in the Prospectus Directive; |
183
Table of Contents
| • | to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of the representative; or |
| • | in any other circumstances falling within Article 3(2) of the Prospectus Directive, |
provided that no such offer of ordinary shares shall require the Company or the representative to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
Each person in a Relevant Member State who initially acquires any ordinary shares or to whom any offer is made will be deemed to have represented, acknowledged and agreed that it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive. In the case of any ordinary shares being offered to a financial intermediary as that term is used in Article 3(2) of the Prospectus Directive, each such financial intermediary will be deemed to have represented, acknowledged and agreed that the ordinary shares acquired by it in the offer have not been acquired on a non-discretionary basis on behalf of, nor have they been acquired with a view to their offer or resale to, persons in circumstances which may give rise to an offer of any ordinary shares to the public other than their offer or resale in a Relevant Member State to qualified investors as so defined or in circumstances in which the prior consent of the representative has been obtained to each such proposed offer or resale.
The Company, the representative and their affiliates will rely upon the truth and accuracy of the foregoing representations, acknowledgements and agreements.
This prospectus has been prepared on the basis that any offer of ordinary shares in any Relevant Member State will be made pursuant to an exemption under the Prospectus Directive from the requirement to publish a prospectus for offers of ordinary shares. Accordingly any person making or intending to make an offer in that Relevant Member State of ordinary shares which are the subject of the offering contemplated in this prospectus may only do so in circumstances in which no obligation arises for the Company or any of the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive in relation to such offer. Neither the Company nor the underwriters have authorized, nor do they authorize, the making of any offer of ordinary shares in circumstances in which an obligation arises for the Company or the underwriters to publish a prospectus for such offer.
For the purpose of the above provisions, the expression “an offer to the public” in relation to any ordinary shares in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the ordinary shares to be offered so as to enable an investor to decide to purchase or subscribe the ordinary shares, as the same may be varied in the Relevant Member State by any measure implementing the Prospectus Directive in the Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC (including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member States) and includes any relevant implementing measure in the Relevant Member State and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Australia
This prospectus:
| • | does not constitute a disclosure document under Chapter 6D.2 of the Corporations Act 2001 (Cth) (the “Corporations Act”); |
184
Table of Contents
| • | has not been, and will not be, lodged with the Australian Securities and Investments Commission (“ASIC”), as a disclosure document for the purposes of the Corporations Act and does not purport to include the information required of a disclosure document under Chapter 6D.2 of the Corporations Act; and |
| • | may only be provided in Australia to select investors who are able to demonstrate that they fall within one or more of the categories of investors, or Exempt Investors, available under section 708 of the Corporations Act. |
The ordinary shares may not be directly or indirectly offered for subscription or purchased or sold, and no invitations to subscribe for or buy the ordinary shares may be issued, and no draft or definitive offering memorandum, advertisement or other offering material relating to any ordinary shares may be distributed in Australia, except where disclosure to investors is not required under Chapter 6D of the Corporations Act or is otherwise in compliance with all applicable Australian laws and regulations. By submitting an application for the ordinary shares, you represent and warrant to us that you are an Exempt Investor.
As any offer of ordinary shares under this document will be made without disclosure in Australia under Chapter 6D.2 of the Corporations Act, the offer of those securities for resale in Australia within 12 months may, under section 707 of the Corporations Act, require disclosure to investors under Chapter 6D.2 if none of the exemptions in section 708 applies to that resale. By applying for the ordinary shares you undertake to us that you will not, for a period of 12 months from the date of issue of the ordinary shares, offer, transfer, assign or otherwise alienate those securities to investors in Australia except in circumstances where disclosure to investors is not required under Chapter 6D.2 of the Corporations Act or where a compliant disclosure document is prepared and lodged with ASIC.
Bermuda
Ordinary shares may be offered or sold in Bermuda only in compliance with the provisions of the Investment Business Act of 2003 of Bermuda which regulates the sale of securities in Bermuda. Additionally, non-Bermudian persons (including companies) may not carry on or engage in any trade or business in Bermuda unless such persons are permitted to do so under applicable Bermuda legislation.
British Virgin Islands
The ordinary shares are not being, and may not be offered to the public or to any person in the British Virgin Islands for purchase or subscription by or on behalf of the Company. The ordinary shares may be offered to companies incorporated under the BVI Business Companies Act, 2004 (British Virgin Islands),“BVI Companies”), but only where the offer will be made to, and received by, the relevant BVI Company entirely outside of the British Virgin Islands.
This prospectus has not been, and will not be, registered with the Financial Services Commission of the British Virgin Islands. No registered prospectus has been or will be prepared in respect of the ordinary shares for the purposes of the Securities and Investment Business Act, 2010 (“SIBA”) or the Public Issuers Code of the British Virgin Islands.
The ordinary shares may be offered to persons located in the British Virgin Islands who are “qualified investors” for the purposes of SIBA. Qualified investors include (i) certain entities which are regulated by the Financial Services Commission in the British Virgin Islands, including banks, insurance companies, licensees under SIBA and public, professional and private mutual funds; (ii) a company, any securities of which are listed on a recognised exchange; and (iii) persons defined as “professional investors” under SIBA, which is any person (a) whose ordinary business involves, whether for that person’s own account or the account of others, the acquisition or disposal of property of the same kind as the property, or a substantial part of the property of the
185
Table of Contents
Company; or (b) who has signed a declaration that he, whether individually or jointly with his spouse, has net worth in excess of US$1,000,000 and that he consents to being treated as a professional investor.
Canada
The ordinary shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the ordinary shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
China
This prospectus does not constitute a public offer of ordinary shares, whether by sale or subscription, in the People’s Republic of China (the “PRC”). The ordinary shares are not being offered or sold directly or indirectly in the PRC to or for the benefit of, legal or natural persons of the PRC.
Further, no legal or natural persons of the PRC may directly or indirectly purchase any of the ordinary shares or any beneficial interest therein without obtaining all prior PRC’s governmental approvals that are required, whether statutorily or otherwise. Persons who come into possession of this document are required by the issuer and its representatives to observe these restrictions.
Dubai International Financial Centre (“DIFC”)
This document relates to an Exempt Offer in accordance with the Markets Rules 2012 of the Dubai Financial Services Authority (“DFSA”). This document is intended for distribution only to persons of a type specified in the Markets Rules 2012 of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt Offers. The DFSA has not approved this document nor taken steps to verify the information set forth herein and has no responsibility for this document. The securities to which this document relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the securities offered should conduct their own due diligence on the securities. If you do not understand the contents of this document you should consult an authorized financial advisor.
In relation to its use in the DIFC, this document is strictly private and confidential and is being distributed to a limited number of investors and must not be provided to any person other than the original recipient, and may not be reproduced or used for any other purpose. The interests in the securities may not be offered or sold directly or indirectly to the public in the DIFC.
186
Table of Contents
Hong Kong
The ordinary shares have not been offered or sold and will not be offered or sold in Hong Kong, by means of any document, other than (a) to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong and any rules made under that Ordinance; or (b) in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong or which do not constitute an offer to the public within the meaning of that Ordinance. No advertisement, invitation or document relating to the ordinary shares has been or may be issued or has been or may be in the possession of any person for the purposes of issue, whether in Hong Kong or elsewhere, which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted to do so under the securities laws of Hong Kong) other than with respect to ordinary shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the Securities and Futures Ordinance and any rules made under that Ordinance.
This document is being distributed only to, directed only at, and in relation to products that are only to be sold to people and/or entities meeting the “professional investor” requirements under the SFO.
Japan
The ordinary shares have not been and will not be registered under the Financial Instruments and Exchange Act. Accordingly, the ordinary shares may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to or for the benefit of a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Act and any other applicable laws, regulations and ministerial guidelines of Japan.
Korea
The ordinary shares have not been and will not be registered under the Financial Investments Services and Capital Markets Act of Korea and the decrees and regulations thereunder (the “FSCMA”), and the ordinary shares have been and will be offered in Korea as a private placement under the FSCMA. None of the ordinary shares may be offered, sold or delivered directly or indirectly, or offered or sold to any person for re-offering or resale, directly or indirectly, in Korea or to any resident of Korea except pursuant to the applicable laws and regulations of Korea, including the FSCMA and the Foreign Exchange Transaction Law of Korea and the decrees and regulations thereunder (the “FETL”). The ordinary shares have not been listed on any of securities exchanges in the world including, without limitation, the Korea Exchange in Korea. Furthermore, the purchaser of the ordinary shares shall comply with all applicable regulatory requirements (including but not limited to requirements under the FETL) in connection with the purchase of the ordinary shares. By the purchase of the ordinary shares, the relevant holder thereof will be deemed to represent and warrant that if it is in Korea or is a resident of Korea, it purchased the ordinary shares pursuant to the applicable laws and regulations of Korea.
Malaysia
No prospectus or other offering material or document in connection with the offer and sale of the ordinary shares has been or will be registered with the Securities Commission of Malaysia (“Commission”) for the Commission’s approval pursuant to the Capital Markets and Services Act 2007. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the ordinary shares may not be circulated or distributed, nor may the ordinary shares be offered or sold, or be made
187
Table of Contents
the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Malaysia other than (i) a closed end fund approved by the Commission; (ii) a holder of a Capital Markets Services Licence; (iii) a person who acquires the ordinary shares, as principal, if the offer is on terms that the ordinary shares may only be acquired at a consideration of not less than RM250,000 (or its equivalent in foreign currencies) for each transaction; (iv) an individual whose total net personal assets or total net joint assets with his or her spouse exceeds RM3 million (or its equivalent in foreign currencies), excluding the value of the primary residence of the individual; (v) an individual who has a gross annual income exceeding RM300,000 (or its equivalent in foreign currencies) per annum in the preceding twelve months; (vi) an individual who, jointly with his or her spouse, has a gross annual income of RM400,000 (or its equivalent in foreign currencies), per annum in the preceding twelve months; (vii) a corporation with total net assets exceeding RM10 million (or its equivalent in a foreign currencies) based on the last audited accounts; (viii) a partnership with total net assets exceeding RM10 million (or its equivalent in foreign currencies); (ix) a bank licensee or insurance licensee as defined in the Labuan Financial Services and Securities Act 2010; (x) an Islamic bank licensee or takaful licensee as defined in the Labuan Financial Services and Securities Act 2010; and (xi) any other person as may be specified by the Commission; provided that, in the each of the preceding categories (i) to (xi), the distribution of the ordinary shares is made by a holder of a Capital Markets Services Licence who carries on the business of dealing in securities. The distribution in Malaysia of this prospectus is subject to Malaysian laws. This prospectus does not constitute and may not be used for the purpose of public offering or an issue, offer for subscription or purchase, invitation to subscribe for or purchase any securities requiring the registration of a prospectus with the Commission under the Capital Markets and Services Act 2007.
Saudi Arabia
This document may not be distributed in the Kingdom of Saudi Arabia except to such persons as are permitted under the Offers of Securities Regulations as issued by the board of the Saudi Arabian Capital Market Authority (“CMA”) pursuant to resolution number 2-11-2004 dated 4 October 2004 as amended by resolution number 1-28-2008, as amended (the “CMA Regulations”). The CMA does not make any representation as to the accuracy or completeness of this document and expressly disclaims any liability whatsoever for any loss arising from, or incurred in reliance upon, any part of this document. Prospective purchasers of the securities offered hereby should conduct their own due diligence on the accuracy of the information relating to the securities. If you do not understand the contents of this document, you should consult an authorised financial adviser.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of ordinary shares may not be circulated or distributed, nor may the ordinary shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the ordinary shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
(a) a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
188
Table of Contents
(b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor, securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the ordinary shares pursuant to an offer made under Section 275 of the SFA except:
(a) to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA;
(b) where no consideration is or will be given for the transfer;
(c) where the transfer is by operation of law;
(d) as specified in Section 276(7) of the SFA; or
(e) as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
South Africa
Due to restrictions under the securities laws of South Africa, the ordinary shares are not offered, and this offering shall not be transferred, sold, renounced or delivered, in South Africa or to a person with an address in South Africa, unless one or other of the following exemptions applies:
(i) the offer, transfer, sale, renunciation or delivery is to duly registered banks, mutual banks, financial services provider, financial institution, the Public Investment Corporation (in each case registered as such in South Africa), a person who deals with securities in their ordinary course of business, or a wholly owned subsidiary of a bank, mutual bank, authorised services provider or financial institution, acting as agent in the capacity of an authorised portfolio manager for a pension fund (duly registered in South Africa), or as manager for a collective investment scheme(registered in South Africa); or
(ii) the contemplated acquisition cost of the securities, for any single addressee acting as principal is equal to or greater than R1,000,000.
This document does not, nor is it intended to, constitute an “offer to the public” (as that term is defined in the South African Companies Act, 2008 (the “SA Companies Act”) and does not, nor is it intended to, constitute a prospectus prepared and registered under the SA Companies Act. This document is not an “offer to the public” and must not be acted on or relied on by persons who do not fall within Section 96(1)(a) of the SA Companies Act (such persons being referred to as “relevant persons”). Any investment or investment activity to which this document relates is available only to relevant persons and will be engaged in only with relevant persons.
A South African resident person or company or any non-South African company which is a subsidiary of a South African company is not permitted to acquire the ordinary shares unless such person has obtained exchange control approval to do so.
Switzerland
The ordinary shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document does not constitute a prospectus within the meaning of, and has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the
189
Table of Contents
disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the ordinary shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, the ordinary shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of ordinary shares will not be supervised by, the Swiss Financial Market Supervisory Authority (“FINMA”), and the offer of ordinary shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of ordinary shares.
Taiwan
The ordinary shares have not been and will not be registered with the Financial Supervisory Commission of Taiwan pursuant to relevant securities laws and regulations and may not be sold, issued or offered within Taiwan through a public offering or in circumstances which constitutes an offer within the meaning of the Securities and Exchange Act of Taiwan that requires a registration or approval of the Financial Supervisory Commission of Taiwan. No person or entity in Taiwan has been authorised to offer, sell, give advice regarding or otherwise intermediate the offering and sale of the ordinary shares in Taiwan.
United Arab Emirates
The ordinary shares have not been, and are not being, publicly offered, sold, promoted or advertised in the United Arab Emirates (including the Dubai International Financial Centre) other than in compliance with the laws of the United Arab Emirates (and the Dubai International Financial Centre) governing the issue, offering and sale of securities. Further, this prospectus does not constitute a public offer of securities in the United Arab Emirates (including the Dubai International Financial Centre) and is not intended to be a public offer. This prospectus has not been approved by or filed with the Central Bank of the United Arab Emirates, the Securities and Commodities Authority or the Dubai Financial Services Authority.
190
Table of Contents
We are being represented by Proskauer Rose LLP with respect to certain legal matters as to U.S. federal securities and New York State law. The underwriters are being represented by Davis Polk & Wardwell LLP with respect to certain legal matters as to U.S. federal securities and New York State law. The validity of the ordinary shares in this offering and certain other legal matters as to Jersey law will be passed upon for us by Ogier, with offices located at 44 The Esplanade, St. Helier, Jersey JE4 9WG.
Our consolidated financial statements as of December 31, 2013 and 2014 and for each of the years in the two-year period ended December 31, 2014 appearing in this prospectus and registration statement have been audited by Kost Forer Gabbay & Kasierer, a member of Ernst & Young Global, an independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, with offices located at 3 Aminadav Street, Tel-Aviv 6706703, Israel, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
Where you can get more information
We have filed with the SEC a registration statement on Form S-1 (File No. 333-206681), including relevant exhibits and schedules under the Securities Act with respect to the ordinary shares offered hereby. This prospectus, which constitutes a part of the registration statement, does not contain all of the information contained in the registration statement. For further information about us and our ordinary shares, we refer you to the registration statement and the exhibits and schedules filed thereto.
On the consummation of this offering, we will be subject to the information requirements of the Securities Exchange Act of 1934 and will file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facility at 100 F Street, N.E., Washington, D.C. 20549.
You may also obtain copies of the documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities.
191
Table of Contents
NovoCure Limited
Index to consolidated financial statements
| Page | ||||
| F-2 | ||||
| Consolidated Balance Sheets as of December 31, 2013 and 2014 and June 30, 2015 (unaudited) |
F-3 | |||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-9—F-31 | ||||
F-1
Table of Contents
Report of independent registered public accounting firm
To the board of directors and shareholders of
NovoCure Limited
We have audited the accompanying consolidated balance sheets of NovoCure Limited and subsidiaries (the “Company”) as of December 31, 2013 and 2014, and the related consolidated statements of operations, changes in shareholders’ equity and cash flows for each of the two years in the period ended December 31, 2014. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of the Company and subsidiaries at December 31, 2013 and 2014 and the consolidated results of their operations and their cash flows for each of the two years in the period ended December 31, 2014, in conformity with U.S. generally accepted accounting principles.
| Tel-Aviv, Israel |
/s/ KOST FORER GABBAY & KASIERER | |
| June 24, 2015 |
A Member of Ernst & Young Global | |
| except for Notes 9, 13, 15, 18 as to which the date is September 21, 2015 |
F-2
Table of Contents
NovoCure Limited and subsidiaries
| December 31, | June 30, | |||||||||||
| U.S. dollars in thousands | 2013 | 2014 | 2015 (unaudited) | |||||||||
| Assets |
||||||||||||
| Current Assets: |
||||||||||||
| Cash and cash equivalents |
$ | 175,894 | $ | 57,613 | $ | 106,508 | ||||||
| Short-term investments |
— | 44,999 | 56,996 | |||||||||
| Restricted cash |
1,178 | 61 | 94 | |||||||||
| Receivables and prepaid expenses |
4,519 | 5,711 | 8,737 | |||||||||
| Inventories |
1,892 | 3,446 | 8,593 | |||||||||
|
|
|
|||||||||||
| Total current assets |
183,483 | 111,830 | 180,928 | |||||||||
|
|
|
|||||||||||
| Long-term Assets: |
||||||||||||
| Property and equipment, net |
3,539 | 3,732 | 5,417 | |||||||||
| Field equipment, net |
1,637 | 2,017 | 3,535 | |||||||||
| Severance pay fund |
69 | 70 | 71 | |||||||||
| Deferred IPO costs |
— | — | 794 | |||||||||
| Other long-term assets |
183 | 227 | 2,025 | |||||||||
|
|
|
|||||||||||
| Total long-term assets |
5,428 | 6,046 | 11,842 | |||||||||
|
|
|
|||||||||||
| Total Assets |
$ | 188,911 | $ | 117,876 | $ | 192,770 | ||||||
|
|
||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-3
Table of Contents
NovoCure Limited and subsidiaries
Consolidated balance sheets
| December 31, | June 30, | Pro forma shareholders’ equity as of June 30, |
||||||||||||||
| U.S. dollars in thousands, except share and per share data | 2013 | 2014 | 2015 (unaudited) | 2015 (unaudited) | ||||||||||||
| Liabilities And Shareholders’ Equity |
||||||||||||||||
| Current Liabilities: |
||||||||||||||||
| Trade payables |
$ | 10,286 | $ | 10,033 | $ | 13,379 | ||||||||||
| Other payables and accrued expenses |
5,312 | 7,636 | 8,872 | |||||||||||||
|
|
|
|||||||||||||||
| Total current liabilities |
15,598 | 17,669 | 22,251 | |||||||||||||
|
|
|
|||||||||||||||
| Long-term Liabilities: |
||||||||||||||||
| Long-term loan, net of discount |
— | — | 24,539 | |||||||||||||
| Accrued severance pay |
203 | 246 | 254 | |||||||||||||
| Other long-term liabilities |
1,331 | 2,086 | 1,419 | |||||||||||||
| Total long-term liabilities |
1,534 | 2,332 | 26,212 | |||||||||||||
|
|
|
|||||||||||||||
| Total Liabilities |
17,132 | 20,001 | 48,463 | |||||||||||||
|
|
|
|||||||||||||||
| Commitments and Contingencies |
||||||||||||||||
| Shareholders’ Equity: |
||||||||||||||||
| Share capital— |
||||||||||||||||
| Ordinary shares—unlimited no par value shares authorized; Issued and outstanding: 11,891,421 shares, 13,431,414 shares and 12,430,419 shares at December 31, 2013, December 31, 2014, and June 30, 2015 (unaudited), respectively; 75,174,936 shares issued and outstanding pro forma (unaudited) |
— | — | — | — | ||||||||||||
| Preferred shares—unlimited no par value shares authorized; Issued and outstanding: 58,676,017 shares, 58,676,017 shares and 62,744,517 shares at December 31, 2013, December 31, 2014 and June 30, 2015 (unaudited), respectively; Aggregate liquidation preference of $454,277 at June 30, 2015 (unaudited); no shares issued and outstanding pro forma (unaudited) |
— | — | — | — | ||||||||||||
| Additional paid-in capital |
367,597 | 374,375 | 473,437 | 473,437 | ||||||||||||
| Accumulated deficit |
(195,818 | ) | (276,500 | ) | (329,130 | ) | (329,130 | ) | ||||||||
|
|
|
|||||||||||||||
| Total shareholders’ equity |
171,779 | 97,875 | 144,307 | 144,307 | ||||||||||||
|
|
|
|||||||||||||||
| Total Liabilities and Shareholders’ Equity |
$ | 188,911 | $ | 117,876 | $ | 192,770 | ||||||||||
|
|
||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-4
Table of Contents
NovoCure Limited and subsidiaries
Consolidated statements of operations
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| U.S. dollars in thousands, except share and per share data |
2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | ||||||||||||
| Net revenues |
$ | 10,359 | $ | 15,490 | $ | 7,315 | $ | 11,751 | ||||||||
| Cost of revenues |
7,013 | 10,036 | 4,820 | 8,647 | ||||||||||||
|
|
|
|||||||||||||||
| Gross profit |
3,346 | 5,454 | 2,495 | 3,104 | ||||||||||||
|
|
|
|||||||||||||||
| Operating costs and expenses: |
||||||||||||||||
| Research, development and clinical trials |
34,797 | 40,381 | 19,915 | 22,692 | ||||||||||||
| Sales and marketing |
16,406 | 21,177 | 11,055 | 15,221 | ||||||||||||
| General and administrative |
16,602 | 24,052 | 10,767 | 14,343 | ||||||||||||
|
|
|
|||||||||||||||
| Total operating costs and expenses |
67,805 | 85,610 | 41,737 | 52,256 | ||||||||||||
|
|
|
|||||||||||||||
| Operating loss |
(64,459 | ) | (80,156 | ) | (39,242) | (49,152) | ||||||||||
| Financial expenses, net |
12,558 | 144 | 38 | 1,467 | ||||||||||||
|
|
|
|||||||||||||||
| Loss before income taxes |
(77,017 | ) | (80,300 | ) | (39,280) | (50,619) | ||||||||||
| Income taxes |
353 | 382 | 166 | 2,011 | ||||||||||||
|
|
|
|||||||||||||||
| Net loss |
$ | (77,370 | ) | $ | (80,682 | ) | $ | (39,446) | $ | (52,630) | ||||||
|
|
|
|||||||||||||||
| Basic and diluted net loss per ordinary share |
$ | (6.73 | ) | $ | (6.46 | ) | $ | (3.21) | $ | (4.12) | ||||||
| Weighted average number of ordinary shares used in computing basic and diluted net loss per share |
11,498,392 | 12,490,017 | 12,269,507 | 12,783,881 | ||||||||||||
|
|
|
|||||||||||||||
| Basic and diluted pro forma net loss per ordinary share |
$ | (1.13 | ) | $ | (0.73) | |||||||||||
| Weighted average number of ordinary shares used in computing basic and diluted pro forma net loss per ordinary share (unaudited) |
71,166,032 | 72,137,939 | ||||||||||||||
|
|
||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
Table of Contents
NovoCure Limited and subsidiaries
Statements of changes in shareholders’ equity
| Ordinary shares |
Preferred shares |
Additional paid-in capital |
Accumulated deficit |
Total shareholders’ equity |
||||||||||||||||
| U.S. dollars in thousands, except share data | Shares | Shares | ||||||||||||||||||
| Balance as of January 1, 2013 |
11,419,786 | 45,315,765 | $ | 167,873 | $ | (118,448 | ) | $ | 49,425 | |||||||||||
| Share-based compensation to employees |
— | — | 5,120 | — | 5,120 | |||||||||||||||
| Issuance of Series I Preferred shares, net (a) |
— | 13,360,252 | 191,738 | — | 191,738 | |||||||||||||||
| Issuance of warrants, net (b) |
— | — | 2,864 | — | 2,864 | |||||||||||||||
| Exercise of options |
471,635 | — | 2 | — | 2 | |||||||||||||||
| Net loss |
— | — | — | (77,370 | ) | (77,370 | ) | |||||||||||||
|
|
|
|||||||||||||||||||
| Balance as of December 31, 2013 |
11,891,421 | 58,676,017 | 367,597 | (195,818 | ) | 171,779 | ||||||||||||||
| Share-based compensation to employees |
— | — | 4,624 | — | 4,624 | |||||||||||||||
| Exercise of options and warrants |
1,539,993 | — | 2,154 | — | 2,154 | |||||||||||||||
| Net loss |
— | — | — | (80,682 | ) | (80,682 | ) | |||||||||||||
|
|
|
|||||||||||||||||||
| Balance as of December 31, 2014 |
13,431,414 | 58,676,017 | 374,375 | (276,500 | ) | 97,875 | ||||||||||||||
| Share-based compensation to employees |
— | — | 4,444 | — | 4,444 | |||||||||||||||
| Exercise of options and warrants |
4,215 | — | 19 | — | 19 | |||||||||||||||
| Issuance of Series J preferred shares, net (c) |
— | 4,068,500 | 94,599 | — | 94,599 | |||||||||||||||
| Issuance of shares and options in respect of settlement, net of fair value of shares provided as indemnification (Note 13c) |
(1,005,210 | ) | — | — | — | — | ||||||||||||||
| Net loss |
— | — | — | (52,630 | ) | (52,630 | ) | |||||||||||||
|
|
|
|||||||||||||||||||
| Balance as of June 30, 2015 (unaudited) |
12,430,419 | 62,744,517 | $ | 473,437 | $ | (329,130 | ) | $ | 144,307 | |||||||||||
|
|
||||||||||||||||||||
| (a) | Net of issuance expenses of $2,440 |
| (b) | Net of issuance expenses of $115 |
| (c) | Net of issuance expenses of $319 |
The accompanying notes are an integral part of the consolidated financial statements.
F-6
Table of Contents
NovoCure Limited and subsidiaries
Consolidated statements of cash flows
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| U.S. dollars in thousands | 2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | ||||||||||||
| Cash flows from operating activities: |
||||||||||||||||
| Net loss |
$ | (77,370 | ) | $ | (80,682 | ) | $ | (39,446) | $ | (52,630) | ||||||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||||||
| Depreciation |
1,216 | 1,962 | 954 | 1,112 | ||||||||||||
| Asset write-downs and impairment |
19 | 23 | 18 | 42 | ||||||||||||
| Accrued interest expense |
6,070 | — | 2 | 719 | ||||||||||||
| Share-based compensation to employees |
5,120 | 4,624 | 2,149 | 4,444 | ||||||||||||
| Amortization of discount (premium) |
5,432 | (19 | ) | (10) | 143 | |||||||||||
| Decrease (increase) in receivables and prepaid expenses |
(3,611 | ) | (1,192 | ) | 494 | (3,026) | ||||||||||
| Decrease (increase) in inventories |
1,479 | (1,554 | ) | (1,005) | (5,147) | |||||||||||
| Decrease (increase) in other long-term assets |
(44 | ) | (44 | ) | 3 | (171) | ||||||||||
| Increase (decrease) in trade payables |
5,695 | (492 | ) | (833) | 3,085 | |||||||||||
| Increase (decrease) in other payables and accrued expenses |
2,955 | 2,324 | (1,040) | 518 | ||||||||||||
| Increase in severance pay, net |
20 | 42 | 63 | 7 | ||||||||||||
| Increase (decrease) in other long-term liabilities |
302 | 764 | 22 | (753) | ||||||||||||
|
|
|
|||||||||||||||
| Net cash used in operating activities |
$ | (52,717 | ) | $ | (74,244 | ) | $ | (38,629) | $ | (51,657) | ||||||
|
|
|
|||||||||||||||
| Cash flows from investing activities: |
||||||||||||||||
| Purchase of property and equipment |
(2,201 | ) | (849 | ) | (285) | (2,417) | ||||||||||
| Purchase of field equipment |
(1,426 | ) | (1,470 | ) | (766) | (2,180) | ||||||||||
| Decrease (increase) in restricted cash |
(1,019 | ) | 1,117 | 1,034 | (33) | |||||||||||
| Proceeds from maturity of short-term investments |
15,048 | 93,000 | — | 47,000 | ||||||||||||
| Purchase of short-term investments |
— | (137,980 | ) | (68,984) | (58,992) | |||||||||||
|
|
|
|||||||||||||||
| Net cash provided by (used in) investing activities |
$ | 10,402 | $ | (46,182 | ) | $ | (69,001) | $ | (16,662) | |||||||
|
|
||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-7
Table of Contents
NovoCure Limited and subsidiaries
Consolidated statements of operations
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| U.S. dollars in thousands, except share and per share data |
2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | ||||||||||||
| Cash flows from financing activities: |
||||||||||||||||
| Proceeds from issuance of Preferred shares, net |
$ | 191,738 | $ | — | $ | — | $ | 94,599 | ||||||||
| Proceeds from long-term loan, net |
49,432 | — | — | 22,886 | ||||||||||||
| Repayment of long-term loan |
(58,047 | ) | — | — | — | |||||||||||
| Proceeds from issuance of other long-term loans |
193 | 54 | 54 | — | ||||||||||||
| Deferred IPO costs |
— | — | — | (294 | ) | |||||||||||
| Repayment of other long-term loan |
— | (63 | ) | (31 | ) | (31 | ) | |||||||||
| Exercise of options and warrants |
2 | 2,154 | 1 | 19 | ||||||||||||
| Purchase of shares |
— | — | — | (5 | ) | |||||||||||
|
|
|
|||||||||||||||
| Net cash provided by financing activities |
$ | 183,318 | $ | 2,145 | $ | 24 | $ | 117,174 | ||||||||
|
|
|
|||||||||||||||
| Increase (decrease) in cash and cash equivalents |
141,003 | (118,281 | ) | (107,606 | ) | 48,895 | ||||||||||
| Cash and cash equivalents at the beginning of the period |
34,891 | 175,894 | 175,894 | 57,613 | ||||||||||||
|
|
|
|||||||||||||||
| Cash and cash equivalents at the end of the period |
$ | 175,894 | $ | 57,613 | $ | 68,288 | $ | 106,508 | ||||||||
|
|
|
|||||||||||||||
| Supplemental cash flow activities: |
||||||||||||||||
| Cash paid during the period for: |
||||||||||||||||
| Income taxes |
$ | 689 | $ | 282 | $ | 107 | $ | 266 | ||||||||
|
|
|
|||||||||||||||
| Interest |
$ | 7,131 | $ | 25 | $ | 13 | $ | 428 | ||||||||
|
|
|
|||||||||||||||
| Non-cash investing and financing activities: |
||||||||||||||||
| Purchase of property and equipment |
$ | 585 | $ | 239 | $ | — | $ | — | ||||||||
|
|
|
|||||||||||||||
| Deferred IPO costs |
$ | — | $ | — | $ | — | $ | 500 | ||||||||
|
|
||||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-8
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 1: General
a. NovoCure Limited (including its consolidated subsidiaries, “the Company”) was incorporated in Jersey (Channel Islands) and is principally engaged in the development, manufacture and commercialization of tumor treating fields (“TTFields”) for the treatment of solid tumors. Since inception, the Company has devoted substantially all of its efforts to developing a family of products to deliver TTFields for a variety of solid tumor indications, raising capital and recruiting personnel. The Company commenced selling and marketing activities in the United States at the end of 2011, and began commercial launch in Europe during 2014.
b. NovoCure Limited wholly owns the following subsidiaries: Novocure Luxembourg Sarl (“Novocure Luxembourg”), Novocure Inc. (“Inc.”) (the U.S. subsidiary of NovoCure Limited) and Novocure (Israel) Ltd. (“Ltd.”). Inc. wholly owns Novocure (USA) LLC. Novocure Luxembourg wholly owns Novocure GmbH, NovoCure Limited’s Swiss subsidiary (“Novocure Switzerland”) and Novocure GmbH, the German subsidiary of NovoCure Limited (“Novocure Germany”). Novocure Switzerland wholly owns Novocure KK, the Japanese subsidiary.
The Company’s research and development activity is conducted in Ltd. and clinical trials are conducted on behalf of the Company mainly by Ltd., Novocure Switzerland and Inc. Novocure KK will conduct clinical trials and market TTFields in Japan. Novocure Switzerland manages the global supply chain operations for the Company, manages clinical trials conducted outside the United States and overseas marketing of TTFields in Europe. Novocure Germany supports and markets TTFields in Germany.
c. In September 2015, the Company’s shareholders approved the restructuring of the Company’s share capital by converting the Company’s Ordinary and Preferred shares to no par value shares and by effecting a sub division of the issued and outstanding share capital of the Company based on a proportion of 1 :5.913 (“Share Split Ratio”), such that each Ordinary and Preferred share nominal value of £0.01 of the Company, shall be divided into 5.913 shares of such applicable class of shares of the Company each with no par value. It was also resolved to apply the Split Ratio to the Company’s outstanding options and warrants, in accordance with their terms. All share information included in these consolidated financial statements has been retroactively adjusted to reflect the conversion and the Share Split Ratio.
Note 2: Significant accounting policies
The consolidated financial statements are prepared according to United States generally accepted accounting principles (“U.S. GAAP”).
a. Use of estimates:
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. The Company evaluates on an ongoing basis its assumptions, including those related to contingencies, deferred taxes, tax liabilities, useful-life of field equipment and share-based compensation costs. The Company’s management believes that the estimates, judgment and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities at the dates of the consolidated financial statements, and the reported amounts of net revenue and expenses during the reporting period. Actual results could differ from those estimates.
F-9
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 2: Significant accounting policies (cont.)
b. Financial statements in U.S. dollars:
The accompanying financial statements have been prepared in U.S. dollars in thousands.
The Company finances its operations in U.S. dollars and a substantial portion of its costs and revenues from its primary markets is incurred in U.S. dollars. As such, the Company’s management believes that the dollar is the currency of the primary economic environment in which the Company operates. Thus, the functional and reporting currency of the Company is the U.S. dollar.
Transactions and balances denominated in U.S. dollars are presented at their original amounts. Monetary accounts maintained in currencies other than the dollar are re-measured into dollars in accordance with Accounting Standards Codification No. 830-10, “Foreign Currency Matters.” All transaction gains and losses of the re-measurement of monetary balance sheet items are reflected in the consolidated statements of operations as financial income or expenses, as appropriate.
c. Principles of consolidation:
The consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. Intercompany transactions and balances, including profits from intercompany sales not yet realized outside the Company, have been eliminated upon consolidation.
d. Unaudited interim financial information:
The accompanying consolidated balance sheet as of June 30, 2015, the consolidated statements of operations and cash flows for the six months ended June 30, 2014 and 2015, and the statement of changes in shareholders’ equity for the six months ended June 30, 2015 are unaudited. The unaudited interim financial information has been prepared by the Company on the same basis as the audited annual consolidated financial statements and, in management’s opinion, reflects all adjustments (consisting of only normal recurring adjustments) necessary to present fairly the Company’s financial position as of June 30, 2015 and results of operations and cash flows for the six months ended June 30, 2014 and 2015. The financial data and the other information disclosed in these notes to the consolidated financial statements related to the six-month period ended June 30, 2015 are not necessarily indicative of the results to be expected for the fiscal year ending December 31, 2015 or any other interim period or for any other future year.
e. Unaudited pro forma shareholders’ equity:
The Company’s board of directors has authorized the filing of a Registration Statement with the U.S. Securities and Exchange Commission to register certain of the Company’s ordinary shares in connection with the Company’s planned initial public offering (“IPO”). Upon the closing of the IPO, all of the authorized, issued and outstanding preferred shares will be automatically converted into ordinary shares in accordance with the Company’s articles of association in effect prior to the IPO. See Note 13a below. Unaudited pro forma shareholders’ equity as of June 30, 2015 gives effect to the assumed conversion of the preferred shares as described above.
f. Cash equivalents:
Cash equivalents are short-term, highly liquid investments that are readily convertible into cash with an original maturity of three months or less at the date acquired.
F-10
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 2: Significant accounting policies (cont.)
g. Short-term investments and restricted cash:
1. Short-term investments:
The Company accounts for investments in debt securities in accordance with ASC 320, “Investments-Debt and Equity Securities.” Management determines the appropriate classification of its investments in marketable debt securities at the time of purchase and reevaluates such determinations at each balance sheet date. For the year ended December 31, 2014, all securities are classified as held-to-maturity since the Company has the intent and ability to hold the securities to maturity and, accordingly, debt securities are stated at amortized cost.
The amortized cost of held-to-maturity securities is adjusted for amortization of premiums and accretion of discounts to maturity and any other than temporary impairment losses. Such amortization and interest are included in the consolidated statement of operations as financial income or expenses, as appropriate.
For the year ended December 31, 2014 and the six-month period ended June 30, 2015 (unaudited), no other than temporary impairment losses have been identified.
2. The Company has restricted cash as of December 31, 2013 of $1,178 related to share capital increase requirements in Novocure Switzerland in accordance with Swiss law, and as of December 31, 2014 of $61 used as security to cover bank guarantees in respect of use of Company credit cards in the Swiss operations.
h. Inventories:
Inventories are stated at the lower of cost or market. Cost is determined using the weighted average method. The Company regularly evaluates the ability to realize the value of inventory. If actual demand for the Company’s delivery systems deteriorates, or market conditions are less favorable than those projected, inventory write-offs may be required.
There were no inventory write-offs for the years ended December 31, 2013 and 2014 and for the six-month periods ended June 30, 2014 and 2015 (unaudited).
i. Property and equipment:
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets at the following rates:
| % | ||
| Computers and laboratory equipment |
15 - 33 | |
| Office furniture |
6 - 33 | |
| Leasehold improvements |
Over the shorter of the term of the lease or its useful life | |
|
| ||
j. Field equipment under operating leases:
Field equipment is stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful life of the field equipment which was determined to be two years. Field equipment consists of equipment being utilized under rental agreements accounted for in accordance with ASC
F-11
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 2: Significant accounting policies (cont.)
840 on a monthly basis as an operating lease, as well as “service pool” equipment. Service pool equipment is equipment owned and maintained by the Company that is swapped for equipment that needs repairs or maintenance by the Company while being rented by a patient. The Company records a provision for any excess, lost or damaged equipment when warranted based on an assessment of the equipment. Write-downs for equipment are included in cost of revenues. During the years ended December 31, 2013 and 2014, $19 and $12, respectively and the six-month periods ended June 30, 2014 and 2015 (unaudited) $9 and $32, respectively, write-downs had been identified.
k. Impairment of long-lived assets:
The Company’s long-lived assets are reviewed for impairment in accordance with ASC 360-10, “Property, Plant and Equipment”, whenever events or changes in circumstances indicate that the carrying amount of the asset may not be recoverable. Recoverability of an asset to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the asset exceeds its fair value. During the years ended December 31, 2013 and 2014 and the six-month periods ended June 30, 2014 and 2015 (unaudited), no impairment losses have been identified.
l. Long-term lease deposits:
Long-term lease deposits in respect of office rent and vehicles under operating leases are presented in other long-term assets.
m. Revenue recognition:
The TTFields delivery system (“System”) for GBM, Optune, is comprised of two main components: (1) an Electric Field Generator (the “device”) and (2) Transducer Arrays and related accessories that are disposable supplies to the device (“disposables”). Title is retained by the Company for the device and the patient is provided replacement disposables for the device during the rental period. The device and disposables are always supplied and functioning together and are not sold on a standalone basis.
Revenues are recognized when persuasive evidence of an arrangement exists, delivery of the system has occurred, the price is fixed or determinable and collectability is reasonably assured. The evidence of an arrangement generally consists of a prescription, a patient service agreement and the verification of eligibility and insurance with the patient’s third-party insurance company (“payer”). The Company generally bills third-party payers a monthly fee for use of the System by patients. As such, the Company takes assignment of benefits and risk of collection from the third-party payer. Patients have out-of-pocket costs for the amount not covered by their payer and the Company bills the patient directly for the amounts of their co-pays and deductible, subject to the Company’s patient assistance programs.
For the reported periods, all revenues are recognized when cash is collected assuming that all other revenue recognition criteria have been met, as the price is not fixed or determinable and the collectability cannot be reasonably assured. The price is not fixed or determinable since the Company does not have sufficient history with payers to reliably estimate their individual payment patterns and as such cannot reliably estimate the
F-12
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 2: Significant accounting policies (cont.)
amount that would be ultimately collected. Once sufficient history is established and the Company can reliably estimate the amounts that would be ultimately collected per payer/payer group and the above criteria are met, the Company will recognize revenues from the use of the System on an accrual basis ratably over the lease term.
Revenues are presented net of indirect taxes, which include excise tax of $584 and $1,010 for the years ended December 31, 2013 and 2014, respectively and $507 and $998 for the six months ended June 30, 2014 and 2015 (unaudited), respectively and sales tax of $300 and $257 for the years ended December 31, 2013 and 2014, and $69 and $257 for the six months ended June 30, 2014 and 2015 (unaudited), respectively.
n. Research, development and clinical trials:
Research, development and clinical trials, including direct and allocated expenses are expensed as incurred.
o. Shipping and handling costs:
The Company does not bill its customers for shipping and handling costs associated with shipping its delivery systems to its customers. These shipping and handling costs of $431 and $553 for the years ended December 31, 2013 and 2014, respectively, and $258 and $532 for the six-month period ended June 30, 2014 and 2015 (unaudited), respectively, are included in selling and marketing costs.
p. Accounting for share-based payments:
The Company accounts for share-based compensation in accordance with ASC 718, “Compensation—Stock Compensation.” ASC 718 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in the company’s consolidated statements of operations.
The Company recognizes compensation costs net of a forfeiture rate only for those shares expected to vest using the accelerated method over the requisite service period of the award, which is generally the option vesting term of four years. ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
The Company selected the Black-Scholes option-pricing model as the most appropriate fair value method for its option awards. The option-pricing model requires a number of assumptions, of which the most significant are the share price expected, expected volatility and the expected option term.
The fair value of ordinary shares underlying the options has historically been determined by management and the board of directors. Because there has been no public market for the Company’s ordinary shares, the board of directors has determined fair value of an ordinary share at the time of grant of the option by considering a number of objective and subjective factors including operating and financial performance, the lack of liquidity of share capital, general and industry specific economic outlook and valuations performed amongst other factors. The fair value of the underlying ordinary shares will be determined by the board of directors until such time as the Company’s ordinary shares are listed on an established share exchange or national market system. The Company’s board of directors determined the fair value of ordinary shares for the reported periods, among
F-13
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 2: Significant accounting policies (cont.)
other factors, based on valuations performed using the hybrid method, which is the hybrid between the probability weighted expected return method (PWERM) and the option pricing method, as the Company began to consider initial public offering (IPO) activities commencing in January 2012.
The computation of expected volatility is based on actual historical share price volatility of comparable companies. Expected term of options granted is calculated using the average between the vesting period and the contractual term to the expected term of the options in effect at the time of grant. The Company has historically not paid dividends and has no foreseeable plans to pay dividends and, therefore, uses an expected dividend yield of zero in the option pricing model. The risk-free interest rate is based on the yield of U.S. treasury bonds with equivalent terms.
q. Fair value of financial instruments:
The carrying amounts of cash and cash equivalents, short-term investments, restricted cash, receivables and prepaid expenses, trade payables and other accounts payable and accrued expenses approximate their fair value due to the short-term maturity of such instruments. Based upon the borrowing terms and conditions currently available to the Company, the carrying values of the long-term loans approximate fair value.
r. Basic and diluted net loss per share:
The Company applies the two class method as required by ASC 260-10, “Earnings Per Share.” ASC 260-10 requires the income or loss per share for each class of shares (ordinary and preferred shares) to be calculated assuming 100% of the Company’s earnings are distributed as dividends to each class of shares based on their contractual rights. No dividends were declared or paid during the reported periods.
According to the provisions of ASC 260-10, the Company’s preferred shares are not participating securities in losses and, therefore, are not included in the computation of net loss per share.
Basic and diluted net loss per share is computed based on the weighted average number of ordinary shares outstanding during each year. Diluted loss per share is computed based on the weighted average number of ordinary shares outstanding during the period, plus dilutive potential shares considered outstanding during the period, in accordance with ASC 260-10. Basic and diluted net loss per ordinary share was the same for each period presented as the inclusion of all potential ordinary shares (all preferred shares, options and warrants) outstanding was anti-dilutive.
Basic and diluted pro forma net loss per share (unaudited), as presented in the statements of operations has been calculated as described above and also gives effect to the automatic conversion of all series of preferred shares that will occur upon closing of the IPO.
s. Income taxes:
The Company accounts for income taxes in accordance with ASC 740-10, “Income Taxes.” ASC 740-10 prescribes the use of the liability method whereby deferred tax asset and liability account balances are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to
F-14
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 2: Significant accounting policies (cont.)
reverse. The Company provides a valuation allowance, to reduce deferred tax assets to their estimated realizable value, if needed.
ASC 740 clarifies the accounting for uncertainty in income taxes by prescribing a minimum recognition threshold for a tax position taken or expected to be taken in a tax return that is required to be met before being recognized in the financial statements. ASC 740 also provides guidance on measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition.
t. Concentration of risks:
Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash and cash equivalents, restricted cash and short-term investments.
Cash and cash equivalents and restricted cash are invested in major banks or financial institutions in Jersey (Channel Islands), the United States, Israel, Luxemburg, Switzerland, Japan and Germany. Such investments may be in excess of insured limits and are not insured in other jurisdictions. Generally, these investments may be redeemed upon demand and, therefore, bear minimal risk.
The Company has no off-balance sheet concentrations of credit risk such as foreign exchange contracts, option contracts or other foreign hedging arrangements.
In 2014, two payers represented $2,372 and $2,014 or 15% and 12% of net revenues, respectively. In 2013, the same two payers represented $2,056 and $1,160 or 18% and 10% of net revenues, respectively.
u. Retirement plans and severance pay:
The Company has a 401(k) retirement savings plan for its U.S. employees. Each eligible employee may elect to contribute a portion of the employee’s compensation to the plan. The Company does not make any matching contributions to the plan.
The Company has an occupational benefit plan with a private pension fund for its Swiss employees, whereby the employee and the Company contribute to the pension fund.
The pension expense for the years ended December 31, 2013 and 2014 was $70 and $205, respectively.
The majority of the Company’s employees in Israel have subscribed to Section 14 of Israel’s Severance Pay Law, 5723-1963 (“Section 14”). Pursuant to Section 14, Ltd.’s employees covered by this section are entitled to monthly deposits at a rate of 8.33% of their monthly salary, made on their behalf by the Company. Payments in accordance with Section 14 release the Company from any future severance liabilities in respect of those employees. Neither severance pay liability nor severance pay fund under Section 14 for such employees is recorded on the Company’s consolidated balance sheet.
With regard to employees in Israel that are not subject to Section 14, the Company’s liability for severance pay is calculated pursuant to Israeli Severance Pay Law, based on the most recent salary of the relevant employees multiplied by the number of years of employment as of the balance sheet date. These employees are entitled to one month’s salary for each year of employment or a portion thereof. The Company’s liability for these employees is fully provided for through monthly deposits to the employees’ pension and management
F-15
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 2: Significant accounting policies (cont.)
insurance policies and an accrual. The value of these deposits is recorded as an asset on the Company’s consolidated balance sheet.
The carrying value of the deposited funds is based on the cash surrender value and includes profits accumulated up to the balance sheet date. The deposited funds may be withdrawn only upon the fulfillment of the obligation pursuant to the Israeli Severance Pay Law. Severance pay expense for the years ended December 31, 2013 and 2014 amounted to $288 and $307, respectively.
v. Contingent liabilities:
The Company accounts for its contingent liabilities in accordance with ASC 450, “Contingencies”. A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated.
With respect to legal matters, provisions are reviewed and adjusted to reflect the impact of negotiations, estimated settlements, legal rulings, advice of legal counsel and other information and events pertaining to a particular matter. As of December 31, 2013 and 2014, the Company was not a party to any ligation that could have a material adverse effect on the Company’s business, financial position, results of operations or cash flows.
Note 3: Short-term investments
The Company invests in marketable US Treasury Bills (“T-bills”) that are classified as held-to-maturity securities. The amortized cost and recorded basis of the T-bills are presented as short-term investments and their estimated fair value as of December 31, 2014 was $44,999. As of June 30, 2015 (unaudited), the amortized cost of the T-bills was $56,996 while the estimated fair value was $56,999.
Note 4: Receivables and prepaid expenses
| December 31, | June 30, | |||||||||||
| 2013 |
2014 |
2015 (unaudited) |
||||||||||
| Government authorities |
$ | 732 | $ | 501 | $ | 1,123 | ||||||
| Prepaid expenses |
638 | 853 | 886 | |||||||||
| Advances and receivables from suppliers |
2,941 | 4,262 | 6,565 | |||||||||
| Others |
208 | 95 | 163 | |||||||||
|
|
|
|
|
|||||||||
| $ | 4,519 | $ | 5,711 | $ | 8,737 | |||||||
|
|
|
|
||||||||||
Note 5: Inventories
| December 31, | June 30, | |||||||||||
| 2013 |
2014 |
2015 (unaudited) | ||||||||||
| Raw materials |
$ | 237 | $ | 526 | $ | 835 | ||||||
| Work in process |
513 | 1,280 | 3,043 | |||||||||
| Finished goods |
1,142 | 1,640 | 4,715 | |||||||||
|
|
|
|||||||||||
| $ | 1,892 | $ | 3,446 | $ | 8,593 | |||||||
|
|
||||||||||||
F-16
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 6: Property and equipment, net
| December 31, | ||||||||
| 2013 |
2014 |
|||||||
| Cost: |
||||||||
| Computers and laboratory equipment |
$ | 3,166 | $ | 3,745 | ||||
| Office furniture |
889 | 995 | ||||||
| Leasehold improvements |
1,004 | 1,343 | ||||||
|
|
|
|||||||
| 5,059 | 6,083 | |||||||
|
|
|
|||||||
| Accumulated depreciation |
(1,520 | ) | (2,351 | ) | ||||
|
|
|
|
|
|||||
| Depreciated cost |
$ | 3,539 | $ | 3,732 | ||||
|
|
||||||||
Depreciation expense was $520 and $886 for the years ended December 31, 2013 and 2014, respectively.
Note 7: Field equipment, net
| December 31, | June 30, | |||||||||||
| 2013 |
2014 |
2015 (unaudited) | ||||||||||
| Field equipment |
$ | 2,531 | $ | 3,942 | $ 5,912 | |||||||
| Less: accumulated depreciation |
(894 | ) | (1,925 | ) | (2,377) | |||||||
|
|
|
|||||||||||
| Field equipment, net |
$ | 1,637 | $ | 2,017 | $ 3,535 | |||||||
|
|
||||||||||||
Depreciation expense was $696 and $1,076 for the years ended December 31, 2013 and 2014, respectively. Depreciation expense for the six months ended June 30, 2014 and 2015 (unaudited) was $514 and $655, respectively.
Note 8: Other payables and accrued expenses
| December 31, | June 30, | |||||||||||
| 2013 |
2014 |
2015 (unaudited) |
||||||||||
| Employees and payroll accruals |
$ | 4,867 | $ | 5,846 | $ | 4,521 | ||||||
| Taxes payable and others |
384 | 693 | 2,657 | |||||||||
| Provision for settlement |
— | 1,000 | 954 | |||||||||
| Other |
61 | 97 | 740 | |||||||||
|
|
|
|
|
|||||||||
| $ | 5,312 | $ | 7,636 | $ | 8,872 | |||||||
|
|
|
|
||||||||||
Note 9: Long-term loan, net of discount
In January 2013, the Company entered into a Credit Agreement (the “Credit Agreement”) with the lenders named therein (the “Lenders”) for a three-year term (the “Credit Facility”). Upon the closing of the Credit Agreement in January 2013, the Company withdrew the full $52,000 and issued the Lenders 975,644 warrants to purchase preferred H shares at an exercise price of $18.77 per share. The Credit Facility was secured by a
F-17
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 9: Long-term loan, net of discount (cont.)
first lien security over all assets of the Company. The Credit Facility accrued an 11% per year payment-in-kind interest charge, capitalized as additional principal quarterly, and a quarterly cash coupon of 3-month US$ LIBOR, subject to a LIBOR floor of 0.5% plus 1.5% per annum. The Company recorded the relative fair value of the warrants of $2,864 as shareholder’s equity and as a discount to the related loan outstanding. The total discount of $4,927 (including an original issue discount of $2,000) and additional deferred issuance costs of $500 in respect of the loan are amortized to interest expense over the three-year term using the effective interest method.
In December 2013, the Company repaid the entire outstanding Credit Facility of $58,000 of principal and accrued interest. Upon repayment of the Credit Facility, the discount and the deferred issuance costs were recorded immediately as interest expense. Financial expenses related to the Credit Facility for the year ended December 31, 2013 were $12,577.
In January 2015, the Company entered into a five-year term loan agreement (the “Loan Agreement”) with a lender to draw up to $100,000. In January 2015, the Company drew $25,000 from the lender. The Company may draw the remaining $75,000 at its option at any time through June 30, 2016. Interest on the outstanding loan is 10% annually, payable quarterly in arrears. In addition, there is a 1.5% funding fee payable on the amount drawn on the funding date, a 0.75% pay-down fee on all principal amount repayments to be paid on the date such payments of principal are made and a pre-payment fee of 3.0%, 2.0% or 1.0% if the Company prepays outstanding loan amounts prior to the first, second or third year, respectively, from the initial funding date. The entire outstanding principal loan is due on January 2020. The loan is secured by a first priority security interest in substantially all assets of the Company. The Loan Agreement sets forth certain affirmative and negative covenants with which the Company must comply on a quarterly basis commencing March 31, 2015 through the term of loan. As of June 30, 2015, the Company complies with its debt covenants.
The total discount of $491 presented net of the loan and additional deferred issuance costs of approximately $1,739 in respect of the loan (presented in other long-term assets in the balance sheet) are amortized to interest expense over the five year term of the loan using the effective interest method.
Note 10: Other long-term liabilities
| December 31, | ||||||||
| 2013 |
2014 |
|||||||
| Deferred rent liability |
$ | 436 | $ | 590 | ||||
| Long-term loans (see a and b below) |
346 | 321 | ||||||
| Unrecognized tax benefits (Note 12e) |
549 | 308 | ||||||
| Provision for settlement (Note 13c) |
— | 867 | ||||||
|
|
|
|||||||
| $ | 1,331 | $ | 2,086 | |||||
|
|
||||||||
Long-term loans:
a. In July 2013, the Company entered into a loan agreement with the landlord of its facility in Switzerland whereby the landlord will offer a loan of up to CHF 400 for the purpose of financing leasehold improvements in the facility. As of December 31, 2013 and 2014, the Company received CHF 185 ($193) and CHF 220 ($232) of this loan. The loan and interest is due in monthly payments from January 1, 2014 through December 31, 2018 and bears an annual interest of 5%.
F-18
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 10: Other long-term liabilities (cont.)
b. In May 2013 and January 2014, the Company entered into a loan agreement with the landlord of its facility in the U.S. and a leasing company of $226 for the purpose of financing leasehold improvements in the facility and a lease of machinery. The loan and interest is due in monthly payments from June 1, 2013 through May 1, 2023 and bears an annual interest of 7%.
The above principal long-term loans repayments as of December 31, 2014 are as follows:
| 2015 |
$ | 59 | ||
| 2016 |
66 | |||
| 2017 |
69 | |||
| 2018 |
73 | |||
| 2019 |
22 | |||
| Thereafter |
90 | |||
|
|
|
|||
| $ | 380 | |||
| Less: current portion of long-term loans |
(59 | ) | ||
|
|
|
|||
| Long-term loans, net of current portion |
$ | 321 | ||
|
|
|
|
Note 11: Commitments and contingent liabilities
a. The facilities of the Company are leased under various operating lease agreements for periods ending no later than 2023. The Company also leases motor vehicles under various operating leases, which expire on various dates, the latest of which is in 2017.
Future minimum lease payments under non-cancelable operating leases as of December 31, 2014, are as follows:
| 2015 |
$ | 1,620 | ||
| 2016 |
1,385 | |||
| 2017 |
1,241 | |||
| 2018 |
937 | |||
| 2019 |
801 | |||
| Thereafter |
1,395 | |||
|
|
|
|||
| $ | 7,379 | |||
|
|
||||
Lease and rental expense for the years ended December 31, 2013 and 2014 was $1,356 and $1,794, respectively.
As of December 31, 2014 and June 30, 2015 (unaudited), the Company pledged bank deposits of $130 and $133 respectively to cover bank guarantees in respect of its leases of operating facilities and obtained guarantees by the bank for the fulfillment of the Company’s lease commitments of $281 and $287, respectively.
b. For an additional commitment, see note 13c.
F-19
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 12: Income taxes
The Company is, or may be, subject to statutory tax rates in the jurisdictions in which it operates.
a. Parent taxation:
NovoCure Limited is subject to a statutory corporate tax rate of 0% in Jersey (Channel Islands).
Subsidiaries taxation:
1. Tax rates applicable to the income of the Israeli subsidiary:
The statutory corporate tax rate in Israel for general activities in the years 2013 and 2014 is 25% and 26.5%, respectively. The Company’s Israeli subsidiary is qualified to receive tax benefits for its research and development activity under the Law for the Encouragement of Capital Investments (the “Investment Law”) and the Law for Economic Policy (“Amendment No. 68”).
For 2013 and 2014, under Amendment No. 68, the Israeli subsidiary qualified for a 12.5% and 16% corporate tax rate, respectively, for its research and development activity.
2. Tax rates applicable to the income of the Swiss subsidiary:
The Swiss federal corporate income tax is 8.5%. In addition, the Company received a ruling in respect of the cantonal/communal tax rate in Lucerne, whereby the Swiss subsidiary is taxed on 10% to 30% of its foreign-sourced income at the cantonal ordinary tax rate (currently approximately 7%) and all Swiss-generated income is taxed at the cantonal ordinary tax rate.
3. The Company’s other subsidiaries are separately taxed under the federal, state and local tax laws of the state/country of incorporation of each entity.
b. Net operating losses carryforwards:
As of December 31, 2014, the Company’s U.S. and Luxemburg subsidiaries have accumulated net operating losses of $3,178. The U.S. federal net operating loss carryforward of $1,745 expires between 2032 and 2034. The U.S. state net operating loss carryforward of $715 expires between 2017 and 2034.
Management currently believes that since the Company has a history of losses, and uncertainty with respect to future taxable income, it is more likely than not that the deferred tax assets regarding the loss carryforwards will not be utilized in the foreseeable future. Thus, a valuation allowance was provided to reduce deferred tax assets to their realizable value.
F-20
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 12: Income taxes (cont.)
c. Deferred income taxes:
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial purposes and the amounts used for income tax purposes. Significant components of the Company’s deferred tax assets and liabilities are as follows:
| 2013 | 2014 | |||||||
| Deferred tax assets: |
||||||||
| Allowance for doubtful accounts |
$ | 1,822 | $ | 4,001 | ||||
| Revenue recognition (timing differences) |
3,930 | 9,042 | ||||||
| Net operating loss carryforwards |
1,141 | 850 | ||||||
| Other temporary differences |
396 | 482 | ||||||
|
|
|
|||||||
| Total deferred tax assets |
7,289 | 14,375 | ||||||
| Less—valuation allowance |
(6,699 | ) | (13,926 | ) | ||||
|
|
|
|||||||
| Net deferred tax assets |
$ | 590 | 449 | |||||
|
|
|
|||||||
| Deferred tax liabilities: |
||||||||
| Fixed assets |
$ | 590 | 442 | |||||
|
|
|
|||||||
| Total deferred tax liabilities |
590 | 442 | ||||||
|
|
|
|||||||
| Net deferred tax assets |
$ | — | $ | 7 | ||||
|
|
||||||||
d. Income taxes are comprised as follows:
| Year ended December 31, |
||||||||
| 2013 | 2014 | |||||||
| Current taxes and reserves (1) |
$ | 290 | $ | 389 | ||||
| Deferred taxes (1) |
63 | (7 | ) | |||||
|
|
|
|||||||
| $ | 353 | $ | 382 | |||||
|
|
||||||||
| (1) | Foreign taxes. |
F-21
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 12: Income taxes (cont.)
e. Accounting for uncertainty in income taxes (“ASC 740”):
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Year ended December 31, |
||||||||
| 2013 | 2014 | |||||||
| Balance at the beginning of the year |
$ | 343 | $549 | |||||
| Additions based on tax positions taken during the current year |
243 | 79 | ||||||
| Reduction related to a lapse of applicable statute of limitations |
(37 | ) | (320 | ) | ||||
|
|
|
|||||||
| Balance at the end of the year |
$ | 549 | $308 | |||||
|
|
||||||||
The Company recognizes interest and penalties related to unrecognized tax benefits in tax expense. During the years ended December 31, 2013 and 2014, the Company recorded and accrued $20 and $2, respectively, for interest and penalties expenses related to uncertain tax positions.
No audits are currently in process by any federal or state taxing authority, except for an audit of the Company’s Israeli subsidiary for the tax years 2011 through 2013.
f. A reconciliation of the Company’s theoretical income tax expenses to actual income tax expenses as follows:
| 2013 | 2014 | |||||||
| Loss before income taxes |
$ | (77,017 | ) | $ | (80,682 | ) | ||
|
|
|
|||||||
| Statutory tax rate |
0% | 0% | ||||||
|
|
|
|||||||
| Theoretical income tax expenses |
$ | — | $ | — | ||||
|
|
|
|||||||
| Non-deductible expenses |
$ | 1,411 | $ | 1,242 | ||||
| Foreign tax rate differential |
(5,991 | ) | (7,844 | ) | ||||
| Valuation allowance |
4,727 | 7,226 | ||||||
| Unrecognized tax expense (benefit) |
206 | (242 | ) | |||||
|
|
|
|||||||
| Income tax expenses |
$ | 353 | $ | 382 | ||||
|
|
||||||||
F-22
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Share capital
Share capital is composed as follows:
| December 31, 2013 | December 31, 2014 | June 30, 2015 (unaudited) | ||||||||||
| Issued and outstanding |
Issued and outstanding |
Issued and outstanding |
||||||||||
| Number of shares | ||||||||||||
| Ordinary shares no par value |
11,891,421 | 13,431,414 | 12,430,419 | |||||||||
|
|
|
|||||||||||
| Series A Convertible Preferred shares no par value |
4,177,235 | 4,177,235 | 4,177,235 | |||||||||
| Series B Convertible Preferred shares no par value |
4,590,439 | 4,590,439 | 4,590,439 | |||||||||
| Series C Convertible Preferred shares no par value |
2,261,666 | 2,261,666 | 2,261,666 | |||||||||
| Series D Convertible Preferred shares no par value |
4,451,913 | 4,451,913 | 4,451,913 | |||||||||
| Series E Convertible Preferred shares no par value |
7,790,861 | 7,790,861 | 7,790,861 | |||||||||
| Series F Convertible Preferred shares no par value |
12,864,362 | 12,864,362 | 12,864,362 | |||||||||
| Series G Convertible Preferred shares no par value |
5,106,269 | 5,106,269 | 5,106,269 | |||||||||
| Series H Convertible Preferred shares no par value |
4,073,020 | 4,073,020 | 4,073,020 | |||||||||
| Series I Convertible Preferred shares no par value |
13,360,252 | 13,360,252 | 13,360,252 | |||||||||
| Series J Convertible Preferred shares no par value |
— | — | 4,068,500 | |||||||||
|
|
|
|||||||||||
| Total |
58,676,017 | 58,676,017 | 62,744,517 | |||||||||
|
|
||||||||||||
a. The rights, preferences and restrictions of series A through J preferred and ordinary shares are as follows:
Conversion—each preferred share is convertible into ordinary shares, at the holder’s option, or automatically upon a qualified initial public offering, which is defined in the Company’s articles of association in effect prior to the IPO as an offering whereby the aggregate gross proceeds received by the Company are (1) at least $50,000 if the equity securities of the Company that are sold in such offering are listed or traded on the New York Stock Exchange (NYSE) or the National Association of Securities Dealers Automated Quotations (NASDAQ) or (2) at least $100,000 if the equity securities of the Company that are sold in such offering are listed or traded on any other public stock exchange. The Company expects that the IPO will constitute such a qualified initial public offering and therefore will trigger such conversion.
At the current conversion prices, each share of series A, B, C, D, E, F, G, H, I and J will convert to ordinary shares on a 1-for-1 ratio. The current conversion price per preferred share will be adjusted for certain recapitalizations, splits, ordinary share dividends and similar standard anti-dilution events.
Dividend rights—the holders of series A-J preferred shares shall be entitled to receive on a pari passu basis, prior and in preference to the declaration or payment of any dividend or distribution to the holders of ordinary shares on an as-
F-23
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Share capital (cont.)
converted basis when, as and if any dividend or distribution is declared by the Company’s board of directors in respect of ordinary shares, up to an amount equal to the applicable original issue price for such preferred shares.
In addition, any additional dividends or distributions shall be distributed among the holders of the ordinary shares and preferred shares in proportion to the number of ordinary shares that would be held by each holder if all preferred shares were converted to ordinary shares at the then effective conversion rate.
Liquidation preference—the holders of series A, B, C, D, E, F, G, H and I preferred shares have a liquidation preference equal to $1.52, $1.09, $1.77, $1.80, $1.80, $2.23, $8.64, $13.50, $14.53 and $23.33 per share, respectively, plus any declared but unpaid dividends.
Preferred shareholders will be entitled to their liquidation preferences based on their seniority. Series I preferred shareholders shall be the first to receive their liquidation preferences and they will be followed by the holders of series H, G, F, E, D, C, B and A preferred shares in turn.
Voting rights—each holder of an ordinary share and each holder of a series A, B, C, D, E, F, G, H and I preferred shares is entitled to one vote.
b. Investment rounds:
1) Series I preferred shares investment round:
In 2013, the Company entered into a series I preferred shares subscription agreement and an expansion to the agreement (the “agreement”) with certain investors. Pursuant to the agreement, the Company issued to the investors 13,360,252 series I preferred shares at $14.53 per share, for a total consideration of $191,738, net of issuance expenses. The series I preferred shares are senior to the other series of preferred shares on payment of the liquidation preference ($14.53 per share), but otherwise have similar participating preferred rights, dividend rights and voting rights to the other series of preferred shares.
2) Series J Preferred shares investment round:
In June 2015, the Company issued 4,068,500 Series J convertible preferred shares at $23.33 per share to certain investors for a total consideration of $94,599 (net of issuance expenses of $319). The Series J preferred shares are senior to the other series of preferred shares on payment of the liquidation preference (equal to $23.33 per share), but otherwise have similar participating preferred rights, dividend rights and voting rights of the other series of preferred shares.
c. Settlement agreement
In February 2015, the Company entered into a settlement agreement (the “Agreement”) with a third party with respect to a resolution of certain potential disputes regarding intellectual property developed by the Company’s founder and historically assigned to the Company. In exchange for a release of potential disputes from the third party, the Company paid $1,000 on execution of the Agreement and will pay an additional $1,000 (“Additional Payment”) at the earliest of (i) 18 months after signing of the Agreement, (ii) an IPO and (iii) the earlier of consummation of a merger/acquisition (“M&A”) or achievement of a Cumulative Net Sales milestone of $250,000 (as defined pursuant to the Agreement). The Company will pay an additional $5,500 on the earlier of
F-24
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Share capital (cont.)
(i) achievement of the Cumulative Net Sales milestone per above and (ii) consummation of an M&A. In addition, the Company agreed to issue 1,005,210 ordinary shares (the “issued shares”) to the third party and grant options to the third party to purchase 1,005,210 ordinary shares (the “granted options”) that are fully vested and at no cost. The options terminate at the earlier of (i) 12 months subsequent to an IPO and (ii) immediately prior to an M&A.
In February 2015, the Company contemporaneously entered into a Letter of Agreement (“Letter of Agreement”) with the founder mentioned above and a related party (together, the “Founder”). Pursuant to the Letter of Agreement, in furtherance to a previous indemnification provided by the Founder in 2009, in connection with the intellectual property he assigned to the Company and the Agreement signed above, the Founder agreed to indemnify the Company for the compensation incurred to the third party by providing 2,010,420 ordinary shares which were redeemed and cancelled (the “Redeemed Shares”) in March 2015 to the Company at $5, and may be obligated to pay an additional $2,000 in cash to the Company upon its request out of the net proceeds from the sale of any ordinary shares by the Founder in a private transaction or following the consummation of a qualified initial public offering (as defined above in Note 13a—Conversion) in an open market transaction if the closing price of the ordinary shares is at least 80% of the price per share for which the ordinary shares were sold in the initial public offering (after deducting underwriting discounts and commissions and offering expenses). In March 2015, the Company provided the Issued Shares and Granted Options to the third party.
Accordingly, for the year ended December 31, 2014, the Company recorded a provision for a net settlement expense of $1,867 in general and administrative expense, in accordance with ASC 450 reflecting the present value of the cash obligation of $2,000 and the fair value of the Issued Shares and Granted Options to the third party, net of the fair value of the consideration provided by the Founder (Redeemed Shares), which amounted to nil as presented in the statement of shareholder’s equity, in connection with the indemnification provided and the Letter of Agreement.
d. Warrants:
As part of the Series B, C, D and E preferred share investment agreements, the investors received warrants to purchase ordinary shares.
The Company accounted for these warrants as equity instruments based on the guidance of ASC 815, “Derivatives and Hedging”, ASC 480-10, “Distinguishing Liabilities from Equity”, its related FASB staff positions, ASC 815-40 “Contracts in Entity’s Own Stock” and the AICPA Technical Practice Aid for accounting for preferred shares and warrants, including the roadmap for accounting for freestanding financial instruments indexed to, and potentially settled in, a company’s own stock.
F-25
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Share capital (cont.)
Significant terms investors’ warrants to purchase ordinary shares are as follows as of December 31, 2013 and 2014:
| Expiration date | Warrants for ordinary shares | Exercise price per share | ||||||||||
| 2013 | 2014 | |||||||||||
| April 26, 2014 (1) |
573,803 | — | $ | 2.18 | ||||||||
| November 30, 2014 (2) |
573,803 | — | 2.18 | |||||||||
| October 11, 2015 |
565,411 | 565,411 | 3.54 | |||||||||
| May 8, 2016 |
1,112,983 | 1,112,983 | 3.59 | |||||||||
| July 31, 2017 |
556,678 | 556,678 | 3.59 | |||||||||
| January 22, 2018 |
556,678 | 556,678 | 3.59 | |||||||||
| July 21, 2018 |
834,355 | 834,355 | 3.59 | |||||||||
|
|
|
|||||||||||
| 4,773,711 | 3,626,105 | |||||||||||
|
|
||||||||||||
| (1) | The warrants were cashless exercised in 2014 to 407,411 ordinary shares. |
| (2) | The warrants were exercised in 2014 to 573,803 ordinary shares. |
Pursuant to the Credit Agreement (see Note 9), the Company issued the Lenders 975,644 warrants to purchase series H preferred shares at an exercise price of $18.77 per share. The warrants are exercisable until January 2016.
e. Share option plans:
The Company authorized through its 2013 Equity Incentive Share Option Plan approved by the Company’s board of directors and its shareholders, the grant of options to officers, directors, advisors, management and other key employees. The options granted generally have a four-year vesting period and expire ten years after the date of grant. Options granted under the Company’s option plan that are cancelled or forfeited before expiration become available for future grant.
In February and March 2015, the Company’s board of directors and its shareholders approved an increase in the number of ordinary shares reserved for grant of options pursuant to the 2013 Plan by 2,956,500 ordinary shares to 13,198,224 ordinary shares.
As of June 30, 2015 (unaudited), 2,442,936 options are available for future grants.
The fair value of share-based awards was estimated using the Black-Scholes option-pricing model for all grants, with the following underlying assumptions:
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| 2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | |||||||||||||
| Expected term (years) |
6.25 | 6.25 | 6.25 | 6.25 | ||||||||||||
| Expected volatility |
70.4%-75.9% | 73.1%-75.3% | 75.1%-75.3% | 62.5%-65.8% | ||||||||||||
| Risk-free interest rate |
1.4%-2.0% | 1.9%-2.3% | 2.1%-2.3% | 1.8%-1.9% | ||||||||||||
| Dividend yield |
0% | 0% | 0% | 0% | ||||||||||||
|
|
||||||||||||||||
F-26
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Share capital (cont.)
A summary of the status of the Company’s option plan as of December 31, 2014 and June 30, 2015 (unaudited) and changes during the relevant period ended on that date is presented below:
| Six months ended June 30, | ||||||||||||||||||||||||
| Year ended December 31, 2014 | 2015 (unaudited) | |||||||||||||||||||||||
| Number of options |
Weighted average exercise price |
Aggregate intrinsic value |
Number of options |
Weighted average exercise price |
Aggregate intrinsic value |
|||||||||||||||||||
| Outstanding at beginning of period |
7,063,717 | $ | 3.26 | 7,426,159 | $ | 3.98 | ||||||||||||||||||
| Granted |
1,159,797 | 7.58 | 1,796,639 | 14.47 | ||||||||||||||||||||
| Exercised |
(558,778 | ) | 1.62 | (1,034) | 7.04 | |||||||||||||||||||
| Forfeited and cancelled |
(238,577 | ) | 5.69 | (172,653) | 7.86 | |||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Outstanding at end of period |
7,426,159 | $ | 3.98 | $ | 27,810 | 9,049,111 | $ | 5.99 | $ | 86,948 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Exercisable options |
4,489,873 | $ | 2.30 | $ | 24,347 | 4,837,614 | $ | 2.66 | $ | 62,699 | ||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Vested and expected to vest |
7,361,585 | $ | 3.95 | $ | 27,784 | 8,983,813 | $ | 5.97 | $ | 86,520 | ||||||||||||||
|
|
||||||||||||||||||||||||
The total equity-based compensation expense related to all of the Company’s equity-based awards recognized for the years ended December 31, 2013 and 2014 and for the six months ended June 30, 2014 and 2015 (unaudited), was comprised as follows:
| Year ended December 31, |
Six months ended June 30, | |||||||||||||||
| 2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | |||||||||||||
| Cost of revenues |
$ | 52 | $ | 32 | $ | 17 | $ | 19 | ||||||||
| Research, development and clinical trials |
1,137 | 820 | 289 | 1,049 | ||||||||||||
| Sales and marketing |
791 | 1,104 | 545 | 979 | ||||||||||||
| General and administrative |
3,140 | 2,668 | 1,298 | 2,397 | ||||||||||||
|
|
|
|||||||||||||||
| Total share-based compensation expense |
$ | 5,120 | $ | 4,624 | $ | 2,149 | $ | 4,444 | ||||||||
|
|
||||||||||||||||
As of December 31, 2014 and June 30, 2015 (unaudited), there were unrecognized compensation costs of $6,119 and $16,743, respectively, which are expected to be recognized over a weighted average period of approximately 2.93 and 3.40 years, respectively.
The weighted average grant date fair values of the Company’s options granted during the years ended December 31, 2013 and 2014 and for the six months ended June 30, 2015 (unaudited) were $4.59, $5.08 and $8.86 per share, respectively. The weighted average grant date fair values of the Company’s unvested options for the years ended December 31, 2013 and 2014 and for the six months ended June 30, 2015 (unaudited) were $3.42, $4.26 and $6.15 per share, respectively and the unvested options for the respective periods amounted to 3,125,543, 2,934,974 and 4,204,865 options.
The weighted average grant date fair values of the Company’s vested options during the year ended December 31, 2013 and 2014 and for the six months ended June 30, 2015 (unaudited) were $2.26, $2.89 and
F-27
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Share capital (cont.)
$4.72 per share, respectively, and the vested options for the respective periods amounted to 1,030,933, 1,166,974 and 364,471 options. The weighted average grant date fair values of the Company’s options forfeited and cancelled during the years ended December 31, 2013 and 2014 and for the six months ended June 30, 2015 (unaudited) were $1.84, $3.59 and $5.11 per share, respectively.
The aggregate intrinsic values for the options exercised during the years ended December 31, 2013 and 2014 and for the six months period ended June 30, 2015 (unaudited) were $3,431, $3,339 and $8 respectively. The aggregate intrinsic value is calculated as the difference between the per-share exercise price and the deemed fair value of the Company’s ordinary shares for each share subject to an option multiplied by the number of shares subject to options at the date of exercise. The Company’s board of directors deemed the fair value of the Company’s ordinary shares to be $7.28 and $7.73 per share as of December 31, 2013 and 2014, respectively.
The options outstanding as of December 31, 2014 have been separated into exercise prices, as follows:
| Exercise price |
Options as
of |
Weighted average remaining contractual term |
Options exercisable as of December 31, 2014 |
Weighted average remaining contractual term |
||||||||||||
| $ | (years) | (years) | ||||||||||||||
| 0.01 |
98,995 | 1.20 | 98,995 | 1.20 | ||||||||||||
| 0.17 |
1,403,857 | 1.71 | 1,403,857 | 1.71 | ||||||||||||
| 0.23 |
440,531 | 4.42 | 440,531 | 4.42 | ||||||||||||
| 0.38 |
488,331 | 5.78 | 488,331 | 5.78 | ||||||||||||
| 3.44 |
1,779,072 | 6.88 | 1,246,473 | 6.88 | ||||||||||||
| 6.72 |
1,034,587 | 7.68 | 524,065 | 7.68 | ||||||||||||
| 6.83 |
94,001 | 7.94 | 46,997 | 7.94 | ||||||||||||
| 7.03 |
614,356 | 8.14 | 154,319 | 8.14 | ||||||||||||
| 7.04 |
141,754 | 8.46 | 36,206 | 8.46 | ||||||||||||
| 7.28 |
179,746 | 8.66 | 44,928 | 8.66 | ||||||||||||
| 7.48 |
504,365 | 9.14 | 4,432 | 8.78 | ||||||||||||
| 7.52 |
134,508 | 9.24 | 739 | 8.92 | ||||||||||||
| 7.58 |
73,908 | 9.48 | — | — | ||||||||||||
| 7.73 |
438,148 | 9.77 | — | — | ||||||||||||
|
|
|
|||||||||||||||
| 7,426,159 | 6.30 | 4,489,873 | 4.95 | |||||||||||||
|
|
||||||||||||||||
F-28
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 13: Share capital (cont.)
The options outstanding as of June 30, 2015 (unaudited) have been separated into exercise prices, as follows:
| Exercise price | Number of options outstanding as of June 30, 2015 |
Weighted average remaining contractual term |
Number of options exercisable as of June 30, 2015 |
Weighted average remaining contractual term |
||||||||||||
| $ | (years) | (years) | ||||||||||||||
| 0.01 |
98,995 | 0.71 | 98,995 | 0.71 | ||||||||||||
| 0.17 |
1,403,857 | 1.22 | 1,403,857 | 1.22 | ||||||||||||
| 0.23 |
440,531 | 3.93 | 440,531 | 3.93 | ||||||||||||
| 0.38 |
488,331 | 5.29 | 488,331 | 5.29 | ||||||||||||
| 3.44 |
1,779,072 | 6.39 | 1,246,482 | 6.39 | ||||||||||||
| 6.72 |
940,424 | 7.18 | 555,405 | 7.15 | ||||||||||||
| 6.83 |
92,819 | 7.45 | 46,406 | 7.45 | ||||||||||||
| 7.03 |
613,765 | 7.64 | 307,172 | 7.64 | ||||||||||||
| 7.04 |
137,616 | 7.97 | 50,693 | 7.93 | ||||||||||||
| 7.28 |
169,103 | 8.16 | 43,155 | 8.16 | ||||||||||||
| 7.48 |
504,365 | 8.64 | 126,077 | 8.64 | ||||||||||||
| 7.52 |
116,769 | 8.74 | 23,417 | 8.74 | ||||||||||||
| 7.58 |
53,510 | 8.99 | 7,093 | 8.89 | ||||||||||||
| 7.73 |
433,418 | 9.27 | — | — | ||||||||||||
| 14.37 |
1,617,784 | 9.66 | — | — | ||||||||||||
| 15.60 |
158,752 | 9.82 | — | — | ||||||||||||
|
|
|
|||||||||||||||
| 9,049,111 | 6.54 | 4,837,614 | 4.72 | |||||||||||||
|
|
||||||||||||||||
Note 14: Financial expenses, net
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| 2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | |||||||||||||
| Financial expenses: |
||||||||||||||||
| Interest expense |
$ | (7,158 | ) | $ | (41 | ) | $ | (15) | $ | (1,152) | ||||||
| Amortization of credit facility costs |
(5,432 | ) | — | — | (147) | |||||||||||
| Foreign currency transaction losses |
(157 | ) | (104 | ) | (62) | (145) | ||||||||||
| Others |
(78 | ) | (142 | ) | (48) | (78) | ||||||||||
|
|
|
|||||||||||||||
| (12,825 | ) | (287 | ) | (125) | (1,522) | |||||||||||
|
|
|
|||||||||||||||
| Financial income: |
||||||||||||||||
| Interest income |
267 | 143 | 87 | 55 | ||||||||||||
|
|
|
|||||||||||||||
| Total financial expenses, net |
$ | 12,558 | $ | 144 | $ | 38 | $ | 1,467 | ||||||||
|
|
||||||||||||||||
F-29
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 15: Basic and diluted net loss per share
The following table sets forth the computation of the Company’s basic and diluted net loss per ordinary share:
| Year ended December 31, | Six months ended June 30, | |||||||||||||||
| 2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | |||||||||||||
| Net loss attributable to ordinary shares as reported |
$ | (77,370 | ) | $ | (80,682 | ) | $ | (39,446) | $ | (52,630) | ||||||
|
|
|
|||||||||||||||
| Shares used in computing net loss per ordinary share, basic and diluted |
11,498,392 | 12,490,017 | 12,269,507 | 12,783,881 | ||||||||||||
|
|
|
|||||||||||||||
| Net loss per ordinary share, basic and diluted |
$ | (6.73 | ) | $ | (6.46 | ) | $ | (3.21) | $ | (4.12) | ||||||
|
|
||||||||||||||||
For the years ended December 31, 2013 and 2014, and for the six months ended June 30, 2014 and 2015 (unaudited), all outstanding preferred shares, options and warrants have been excluded from the calculation of the diluted net loss per share since their effect was anti-dilutive.
Note 16: Subcontractor
The Company is dependent upon sole source suppliers for certain key components used in its delivery systems. The Company’s management believes that in most cases other suppliers could provide similar components at comparable terms. A change of suppliers which requires FDA or other regulatory approval, however, could cause a material delay in manufacturing and a possible loss of sales, which could adversely affect the Company’s operating results and financial position.
Note 17: Supplemental information
The following table presents long-lived assets by location:
| December 31, | June 30, | |||||||||||
| 2013 | 2014 | 2015 (unaudited) | ||||||||||
| Europe, Middle East and Asia (“EMEA”) |
||||||||||||
| Israel |
$920 | $1,009 | $1,121 | |||||||||
| Switzerland |
522 | 1,259 | 1,945 | |||||||||
| Other EMEA countries |
6 | 13 | 388 | |||||||||
|
|
|
|||||||||||
| Total EMEA |
1,448 | 2,281 | 3,454 | |||||||||
| United States |
3,728 | 3,468 | 5,498 | |||||||||
|
|
|
|||||||||||
| $5,176 | $5,749 | $8,952 | ||||||||||
|
|
||||||||||||
F-30
Table of Contents
NovoCure Limited and subsidiaries
Notes to consolidated financial statements
U.S. dollars in thousands (except share and per share data)
Note 17: Supplemental information (cont.)
The Company’s net revenues by geographic region, based on the patient’s location are summarized as follows:
| Year ended December 31, |
Six months ended June 30, | |||||||||||||||
| 2013 | 2014 | 2014 (unaudited) | 2015 (unaudited) | |||||||||||||
| EMEA |
$ | 29 | $ | 539 | $28 | $587 | ||||||||||
| United States |
10,330 | 14,951 | 7,287 | 11,164 | ||||||||||||
|
|
|
|||||||||||||||
| $ | 10,359 | $ | 15,490 | $7,315 | $11,751 | |||||||||||
|
|
||||||||||||||||
Note 18: Subsequent events
The Company evaluates events or transactions that occur after the balance sheet date but prior to the issuance of financial statements to provide additional evidence relative to certain estimates or to identify matters that require additional disclosure. For its consolidated financial statements as of December 31, 2014, the Company evaluated subsequent events through June 24, 2015, which is the date the financial statements were issued. For its interim financial statements as of June 30, 2015 (unaudited) and for the six-month period then ended (unaudited), the Company evaluated subsequent events through October 1, 2015.
F-31
Table of Contents
7,500,000 shares

Ordinary shares
Prospectus
| J.P. Morgan | Deutsche Bank Securities | Evercore | ||
| Wells Fargo Securities | ||||
| JMP Securities | Wedbush PacGrow | |
, 2015
Until , 2015, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
Table of Contents
Part II
Information not required in prospectus
Item 13. Other expenses of issuance and distribution
The following table sets forth the various expenses and costs incurred or to be incurred by us in connection with the sale of the ordinary shares offered hereby. All the amounts shown are estimated except the SEC filing fee, the FINRA filing fee and the NASDAQ Global Select Market listing fee.
| Expense | Dollar Amount | |||
| SEC filing fee |
$ | 24,053.40 | ||
| FINRA filing fee |
63,031.25 | |||
| NASDAQ Global Select Market listing fee |
200,000 | |||
| Legal fees and expenses |
1,500,000 | |||
| Accounting fees and expenses |
550,000 | |||
| Printing expenses |
380,000 | |||
| Transfer agent and registrar fees and expenses |
6,000 | |||
| Miscellaneous |
237,000 | |||
|
|
|
|||
| Total |
$ | 2,960,084.65 | ||
|
|
||||
Item 14. Indemnification of directors and officers
We will enter into indemnification agreements with each of our directors to indemnify them against certain liabilities and expenses arising from their being a director to the maximum extent permitted by Jersey law. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Subject to the Jersey Companies Law, our Articles permit us to indemnify any director against any liability, to purchase and maintain insurance against any liability for any director and to provide any director with funds (whether by loan or otherwise) to meet expenditures incurred or to be incurred by him in defending any criminal, regulatory or civil proceedings or in connection with an application for relief (or to enable any such director to avoid incurring such expenditure).
However, Article 77 of the Jersey Companies Law limits the ability of a Jersey company to exempt or indemnify a director from any liability arising from acting as a director. It provides that neither a company (or any of its subsidiaries) nor any other person for some benefit conferred or detriment suffered directly or indirectly by the company may exempt or indemnify any director from, or against, any liability incurred by him as a result of being a director of the company except where the company exempts or indemnifies him against:
| (a) | any liabilities incurred in defending any proceedings (whether civil or criminal): |
| (i) | in which judgment is given in his or her favor or he or she is acquitted; |
| (ii) | which are discontinued otherwise than for some benefit conferred by him or her or on his or her behalf or some detriment suffered by him or her; or |
II-1
Table of Contents
| (iii) | which are settled on terms which include such benefit or detriment and, in the opinion of a majority of the directors of the company (excluding any director who conferred such benefit or on whose behalf such benefit was conferred or who suffered such detriment), he or she was substantially successful on the merits in his or her resistance to the proceedings; or |
| (b) | any liability incurred otherwise than to the company if he or she acted in good faith with a view to the best interests of the company; |
| (c) | any liability incurred in connection with an application made under Article 212 of the Jersey Companies Law in which relief is granted to him or her by the court; or |
| (d) | any liability against which the company normally maintains insurance for persons other than directors. |
Article 77 of the Jersey Companies Law permits a company to purchase and maintain directors’ and officers’ insurance and we maintain a directors’ and officers’ liability insurance policy for the benefit of our directors and officers.
Item 15. Recent sales of unregistered securities
During the past three years, we have issued the following securities, which include convertible preferred shares and options to acquire our ordinary shares. We believe that each of the following issuances was exempt from registration under the Securities Act in reliance on Regulation S under the Securities Act, under Section 4(2) of the Securities Act regarding transactions not involving a public offering and under Rule 701 promulgated under the Securities Act.
| Purchaser | Date of sale or issuance |
Number of securities |
Consideration in United States dollars |
|||||||
| Warrants to Purchase Series H Preferred Shares(1) |
January 2013 | 975,644 | $ | — | ||||||
| Series I Preferred Share Purchasers |
May 2013, September 2013 and December 2013 | 13,360,252 | $ | 194,179,109.62 | ||||||
| Ordinary Shares(2) |
March 2015 | 1,005,210 | — | |||||||
| Series J Preferred Share Purchasers |
June 2015 | 4,068,500 | $ | 94,918,290.85 | ||||||
| Options to Purchase Ordinary Shares |
September 2012—September 2015 | 7,148,003 | $ | — | ||||||
|
|
||||||||||
| (1) | Issued to previous lenders under a credit agreement. |
| (2) | See “Our business—Intellectual property” in the accompanying prospectus for a description of the settlement agreement with the Technion whereby the Registrant issued shares to the Technion. |
Item 16. Exhibits and financial statement schedules
Exhibits. See the Exhibit index beginning on page II-6 of this registration statement.
Financial statement schedules. Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in our consolidated financial statements or the notes thereto.
Item 17. Undertakings
The undersigned Registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
II-2
Table of Contents
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned Registrant hereby undertakes that:
| (1) | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (2) | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-3
Table of Contents
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Malvern, Commonwealth of Pennsylvania, on October 1, 2015.
| NovoCure Limited | ||
| By: |
/s/ Asaf Danziger | |
| Asaf Danziger | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| Date: | Signature | Title | ||
| October 1, 2015 | /s/ Asaf Danziger Asaf Danziger |
Chief Executive Officer and Director (Principal Executive Officer) | ||
| October 1, 2015 | /s/ Wilhelmus Groenhuysen Wilhelmus Groenhuysen |
Chief Financial Officer (Principal Financial and Accounting Officer) and Authorized Representative in the United States | ||
| October 1, 2015 | /s/ William F. Doyle William F. Doyle |
Chairman and Director | ||
| October 1, 2015 | * Kinyip Gabriel Leung |
Vice Chairman and Director | ||
| October 1, 2015 | * Yoram Palti, M.D., Ph.D. |
Director | ||
| October 1, 2015 | * William Burkoth |
Director | ||
| October 1, 2015 | * Timothy Langloss |
Director | ||
| October 1, 2015 | * Louis J. Lavigne, Jr. |
Director | ||
| October 1, 2015 | * Robert J. Mylod, Jr. |
Director | ||
| October 1, 2015 | * Gert Lennart Perlhagen |
Director | ||
|
| ||||
II-4
Table of Contents
| Date: | Signature | Title | ||
| October 1, 2015 | * Charles G. Phillips III |
Director | ||
| October 1, 2015 | * William A. Vernon |
Director | ||
|
| ||||
| By: | /s/ Wilhelmus Groenhuysen | |
| Wilhelmus Groenhuysen | ||
| Attorney-in-Fact (pursuant to previously filed power of attorney) |
II-5
Table of Contents
Exhibit index
| Exhibit number |
Description | |||
| 1.1 | Form of Underwriting Agreement** | |||
| 3.1 | Amended and Restated Memorandum of Association, effective June 1, 2015** | |||
| 3.2 | Amended and Restated Articles of Association, effective June 1, 2015** | |||
| 3.3 | Amended and Restated Memorandum of Association (to effect stock split)** | |||
| 3.4 | Amended and Restated Articles of Association (to effect stock split)** | |||
| 3.5 | Memorandum and Articles of Association (to be effective in connection with initial public offering)** | |||
| 4.1 | Form of Ordinary Shares Certificate** | |||
| 4.2 | Eleventh Amended and Restated Investors Rights Agreement, dated June 1, 2015** | |||
| 4.3 | Tenth Amended and Restated Registration Rights Agreement, dated June 1, 2015** | |||
| 5.1 | Opinion of Ogier to the legality of the securities* | |||
| 10.1 | Loan and Security Agreement between the Company and Biopharma Secured Investments III Holdings Cayman LP, dated January 7, 2015** | |||
| 10.2 | Strategic Supplier Agreement between the Company and ITT Corporation (assigned to Exelis Inc.), dated July 21, 2011, as amended on April 29, 2014†** | |||
| 10.3 | 2003 Share Option Plan** | |||
| 10.4 | 2013 Share Option Plan** | |||
| 10.5 | 2015 Omnibus Incentive Plan** | |||
| 10.6 | Employment Agreement with Asaf Danziger and the Company, dated October 1, 2002** | |||
| 10.7 | Employment Agreement with Wilhelmus C.M. Groenhuysen and the Company, dated December 23, 2011†** | |||
| 10.8 | Employment Agreement with Kinyip Gabriel Leung and the Company, dated August 24, 2011†** | |||
| 10.9 | Employment Agreement with Eilon Kirson and the Company, dated June 2, 2002†** | |||
| 10.10 | Consulting Agreement with Palti Consultants Ltd. and the Company, dated May 1, 2002†** | |||
| 10.11 | Consulting Agreement with William F. Doyle, dated June 24, 2014†** | |||
| 10.12 | Form of Indemnification Agreement** | |||
| 10.13 | Settlement Agreement with the Technion, dated February 10, 2015** | |||
| 10.14 | Director Compensation Plan** | |||
| 10.15 | Employee Share Purchase Plan** | |||
| 10.16 | Amendment to Consulting Agreement with William F. Doyle, dated September 16, 2015** | |||
| 10.17 | Form of Non-Qualified Stock Option Agreement pursuant to the 2015 Omnibus Incentive Plan (U.S. individuals)** | |||
| 10.18 | Form of Incentive Stock Option Agreement pursuant to the 2015 Omnibus Incentive Plan (U.S. individuals)** | |||
| 21.1 | List of Subsidiaries** | |||
| 23.1 | Consent of Ogier (included in Exhibit 5.1)* | |||
| 23.2 | Consent of Kost Forer Gabbay & Kasierer, a Member of Ernst & Young Global, Independent Registered Public Accounting Firm* | |||
| 24.1 | Power of Attorney (included in signature page to Registration Statement Filed on August 31, 2015)** | |||
|
|
| |||
| * | Filed herewith. |
| ** | Previously filed. |
| † | Confidential treatment requested. |
II-6