Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Neos Therapeutics, Inc. | a15-20524_18k.htm |
Exhibit 99.1
|
|
September 2015 CONFIDENTIAL |
|
|
2 This presentation contains forward looking statements including, but not limited to, statements concerning Neos Therapeutics, Inc.’s ability to successfully gain regulatory approvals and commercialize products in the United States and the timing thereof; its ability to successfully advance its pipeline of product candidates; its ability to maintain and protect its intellectual property; the outcome or success of its clinical trials; the rate and degree of market acceptance of its products; and its ability to develop sales and marketing capabilities. In some cases, you can identify forward looking statements by terms such as “may,” ”will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. The forward looking statements of this presentation are only predictions and are subject to a number of risks, uncertainties and assumptions. Moreover, Neos Therapeutics operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for Neos Therapeutics’ management to predict all risks, nor can Neos Therapeutics assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward looking statements it may make. In light of these risks, uncertainties and assumptions, the forward looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward looking statements. You should not rely upon forward looking statements as predictions of future events. Although Neos Therapeutics believes that the expectations reflected in the forward looking statements are reasonable, it cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward looking statements will be achieved or occur. Moreover, except as required by law, neither Neos Therapeutics nor any other person assumes responsibility for the accuracy and completeness of the forward looking statements. Forward looking statements in this presentation represent Neos Therapeutics’ views only as of the date of this presentation. Neos Therapeutics undertakes no obligation to update or review any forward looking statement, whether as a result of new information, future developments or otherwise, except as required by law. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy these securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. |
|
|
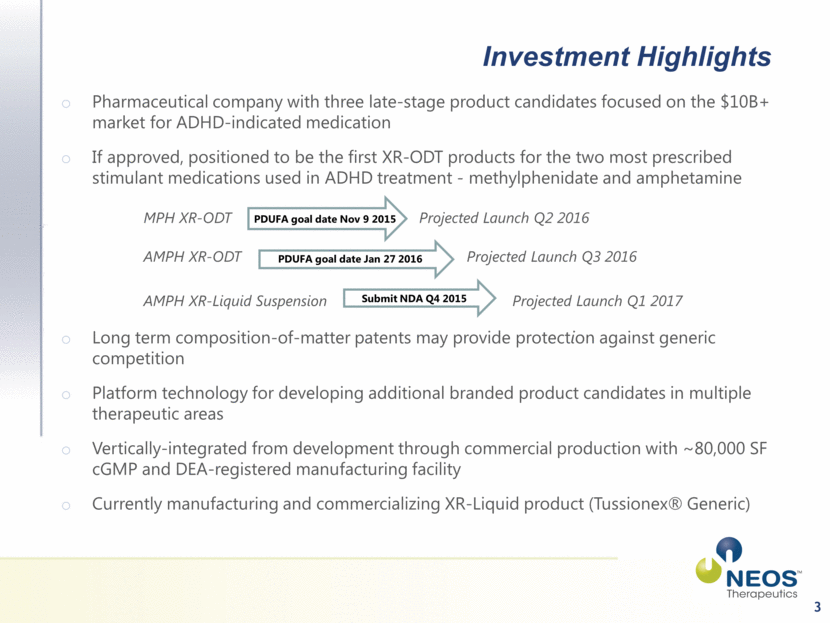
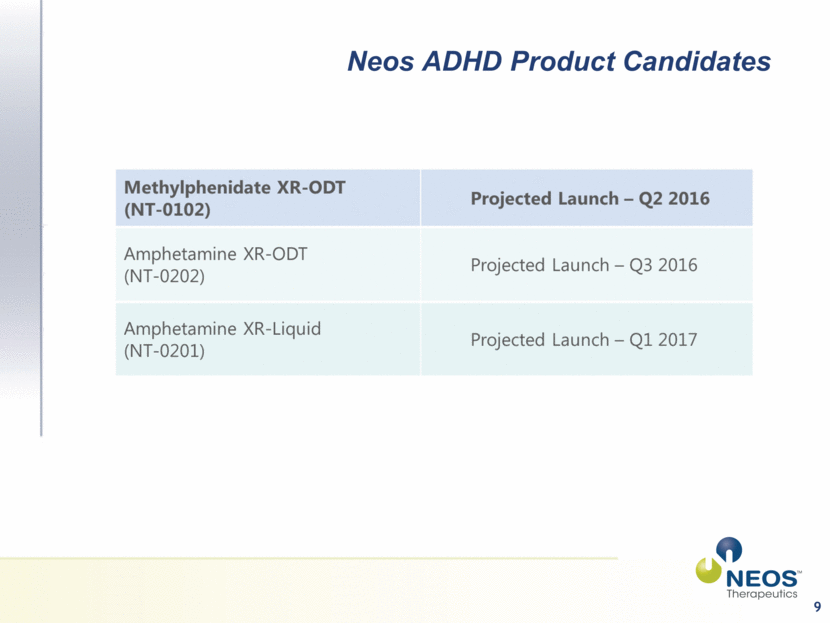
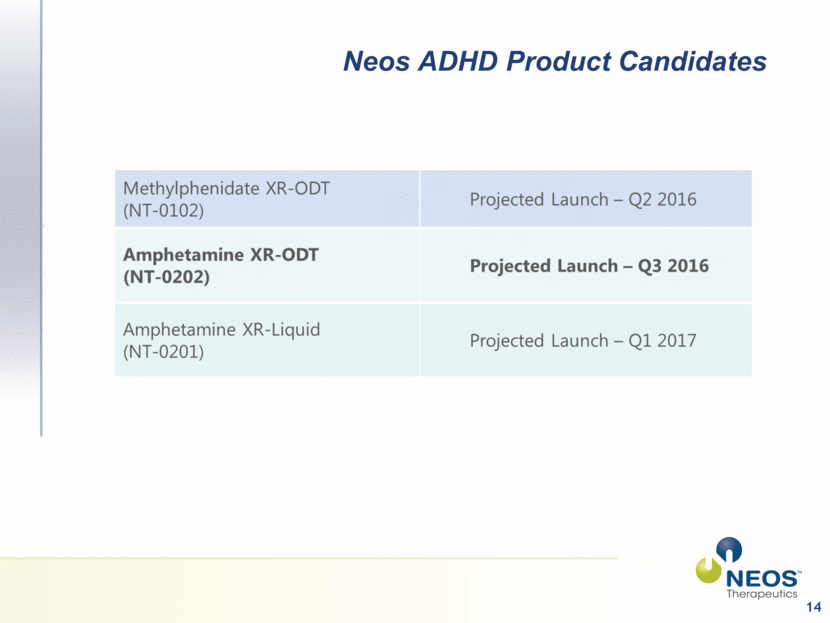
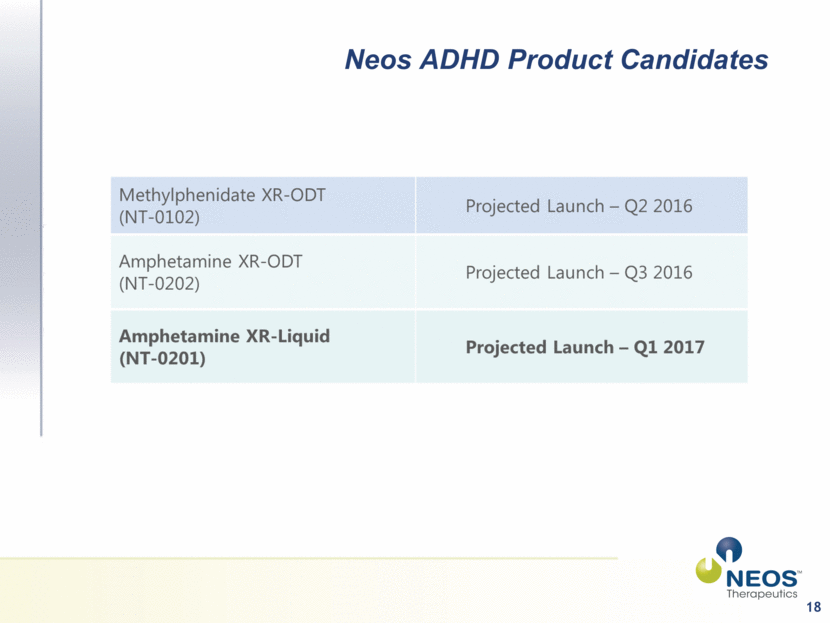
Investment Highlights Pharmaceutical company with three late-stage product candidates focused on the $10B+ market for ADHD-indicated medication If approved, positioned to be the first XR-ODT products for the two most prescribed stimulant medications used in ADHD treatment - methylphenidate and amphetamine MPH XR-ODT Projected Launch Q2 2016 AMPH XR-ODT Projected Launch Q3 2016 AMPH XR-Liquid Suspension Projected Launch Q1 2017 Long term composition-of-matter patents may provide protection against generic competition Platform technology for developing additional branded product candidates in multiple therapeutic areas Vertically-integrated from development through commercial production with ~80,000 SF cGMP and DEA-registered manufacturing facility Currently manufacturing and commercializing XR-Liquid product (Tussionex® Generic) 3 PDUFA goal date Nov 9 2015 PDUFA goal date Jan 27 2016 Submit NDA Q4 2015 |
|
|
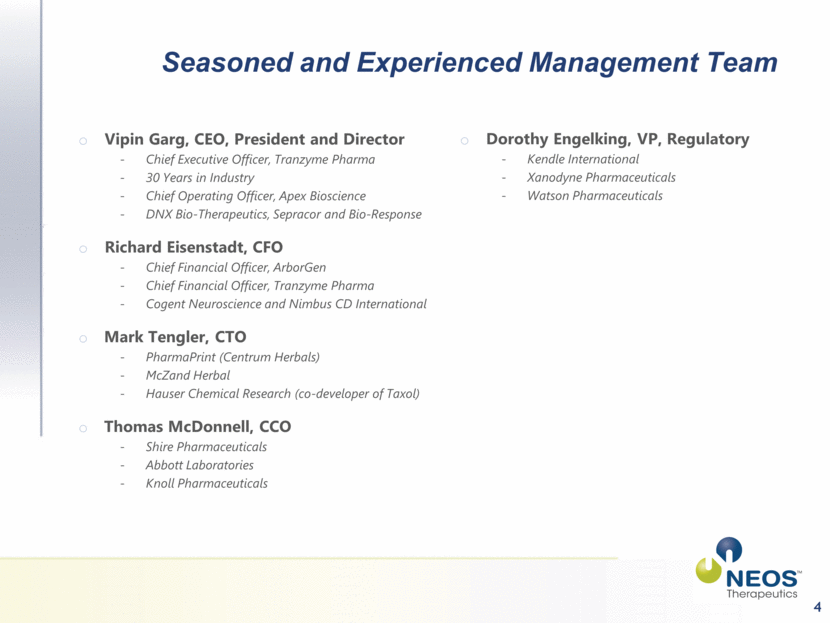
Seasoned and Experienced Management Team 4 Vipin Garg, CEO, President and Director Chief Executive Officer, Tranzyme Pharma 30 Years in Industry Chief Operating Officer, Apex Bioscience DNX Bio-Therapeutics, Sepracor and Bio-Response Richard Eisenstadt, CFO Chief Financial Officer, ArborGen Chief Financial Officer, Tranzyme Pharma Cogent Neuroscience and Nimbus CD International Mark Tengler, CTO PharmaPrint (Centrum Herbals) McZand Herbal Hauser Chemical Research (co-developer of Taxol) Thomas McDonnell, CCO Shire Pharmaceuticals Abbott Laboratories Knoll Pharmaceuticals Dorothy Engelking, VP, Regulatory Kendle International Xanodyne Pharmaceuticals Watson Pharmaceuticals |
|
|
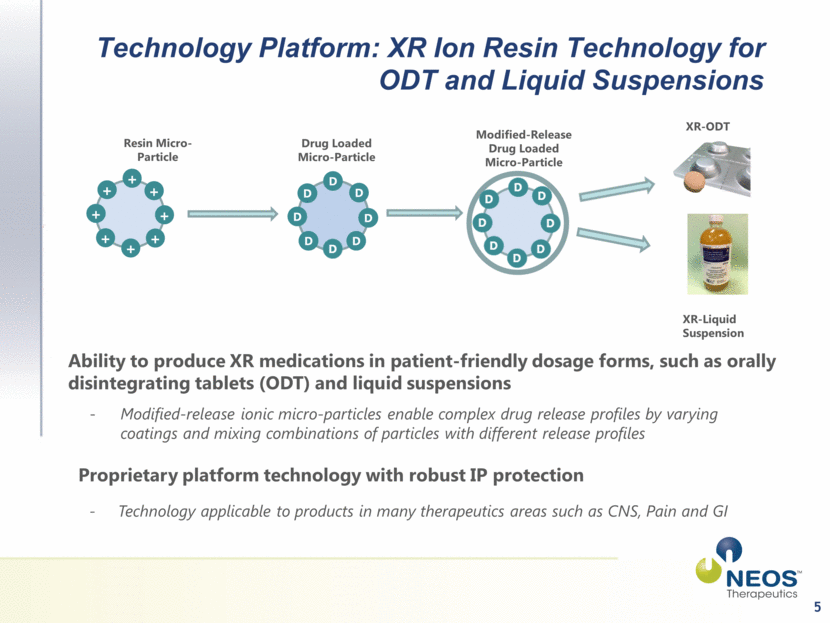
5 Technology Platform: XR Ion Resin Technology for ODT and Liquid Suspensions Resin Micro-Particle Drug Loaded Micro-Particle Modified-Release Drug Loaded Micro-Particle XR-ODT XR-Liquid Suspension Ability to produce XR medications in patient-friendly dosage forms, such as orally disintegrating tablets (ODT) and liquid suspensions Modified-release ionic micro-particles enable complex drug release profiles by varying coatings and mixing combinations of particles with different release profiles Proprietary platform technology with robust IP protection - Technology applicable to products in many therapeutics areas such as CNS, Pain and GI + + + + + + + + D D D D D D D |
|
|
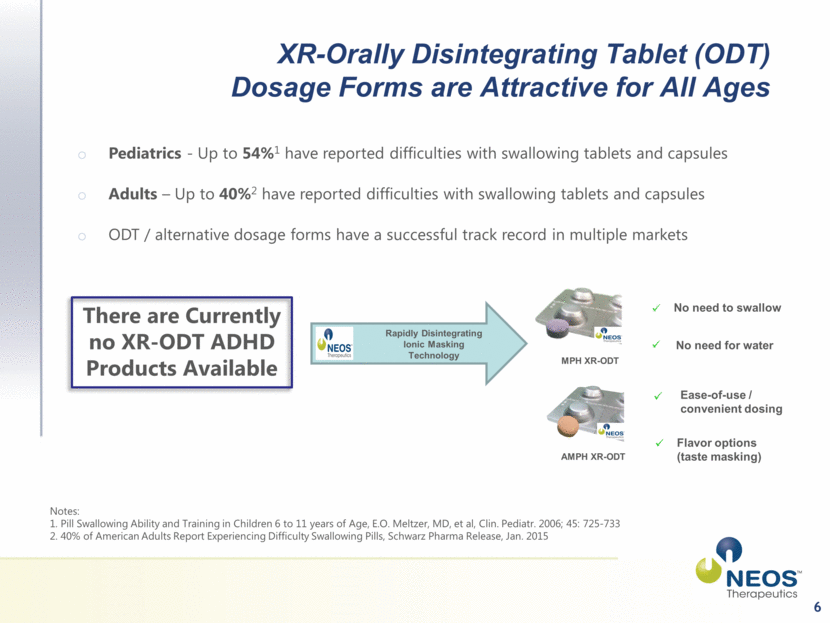
6 There are Currently no XR-ODT ADHD Products Available Rapidly Disintegrating Ionic Masking Technology MPH XR-ODT AMPH XR-ODT No need to swallow No need for water Ease-of-use / convenient dosing Flavor options (taste masking) Notes: 1. Pill Swallowing Ability and Training in Children 6 to 11 years of Age, E.O. Meltzer, MD, et al, Clin. Pediatr. 2006; 45: 725-733 2. 40% of American Adults Report Experiencing Difficulty Swallowing Pills, Schwarz Pharma Release, Jan. 2015 XR-Orally Disintegrating Tablet (ODT) Dosage Forms are Attractive for All Ages Pediatrics - Up to 54%1 have reported difficulties with swallowing tablets and capsules Adults – Up to 40%2 have reported difficulties with swallowing tablets and capsules ODT / alternative dosage forms have a successful track record in multiple markets |
|
|
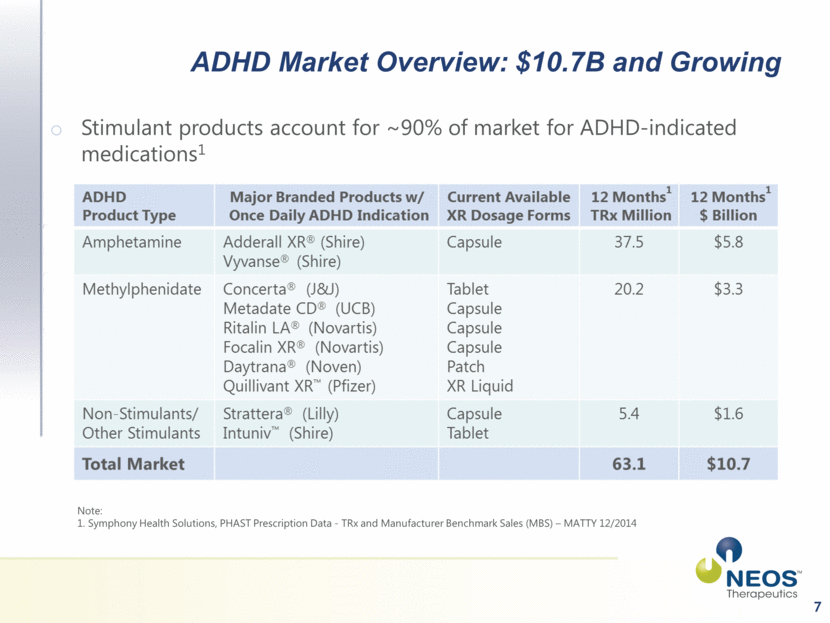
7 ADHD Market Overview: $10.7B and Growing Stimulant products account for ~90% of market for ADHD-indicated medications1 Note: 1. Symphony Health Solutions, PHAST Prescription Data - TRx and Manufacturer Benchmark Sales (MBS) – MATTY 12/2014 |
|
|
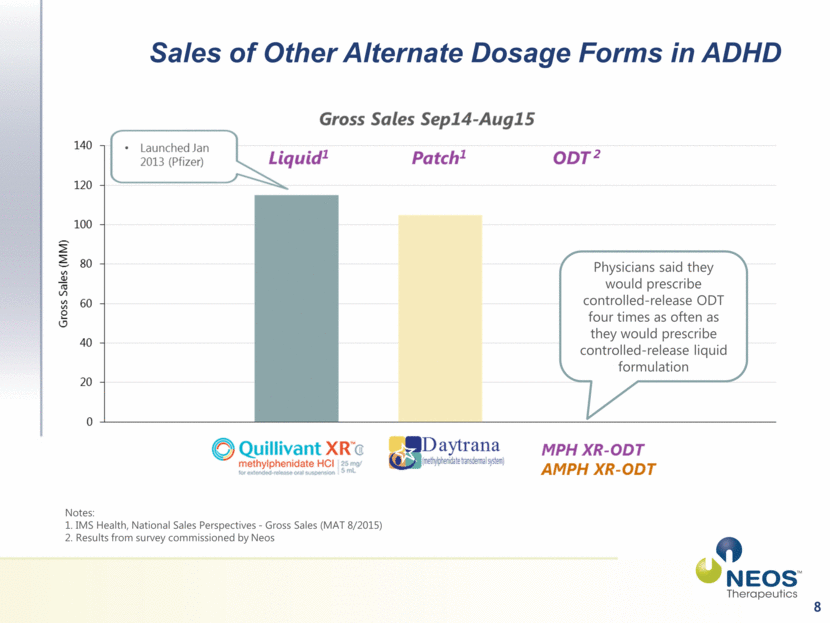
8 Sales of Other Alternate Dosage Forms in ADHD Notes: 1. IMS Health, National Sales Perspectives - Gross Sales (MAT 8/2015) 2. Results from survey commissioned by Neos Physicians said they would prescribe controlled-release ODT four times as often as they would prescribe controlled-release liquid formulation 2 |
|
|
9 Neos ADHD Product Candidates |
|
|
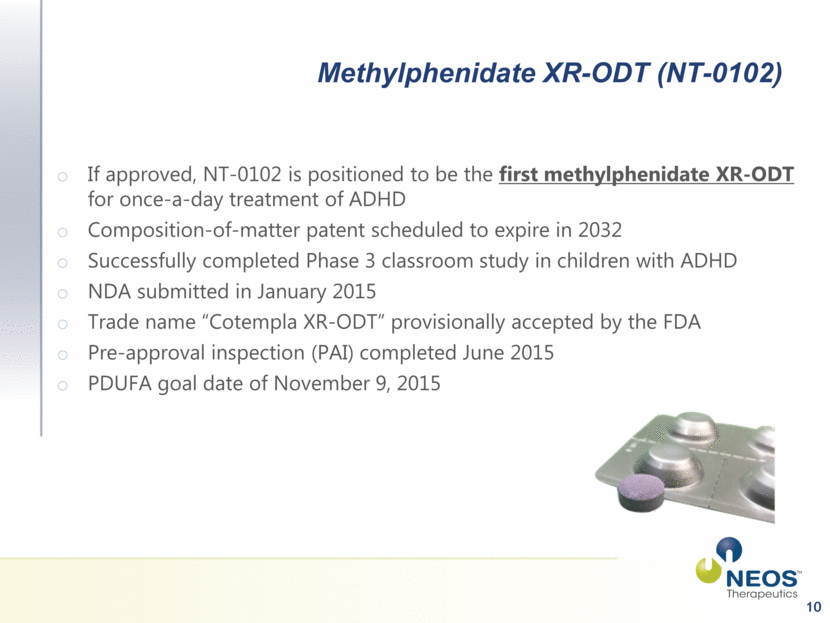
10 Methylphenidate XR-ODT (NT-0102) If approved, NT-0102 is positioned to be the first methylphenidate XR-ODT for once-a-day treatment of ADHD Composition-of-matter patent scheduled to expire in 2032 Successfully completed Phase 3 classroom study in children with ADHD NDA submitted in January 2015 Trade name “Cotempla XR-ODT” provisionally accepted by the FDA Pre-approval inspection (PAI) completed June 2015 PDUFA goal date of November 9, 2015 |
|
|
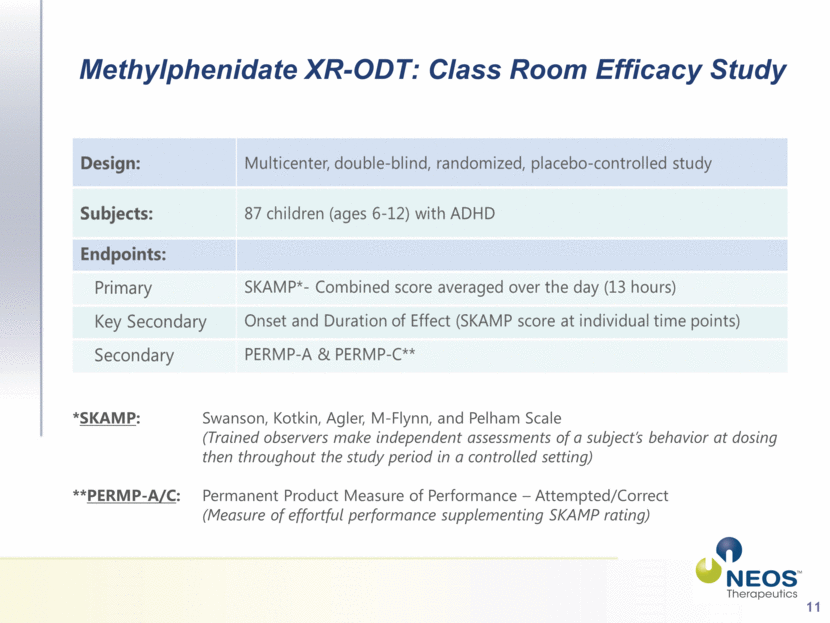
11 Methylphenidate XR-ODT: Class Room Efficacy Study *SKAMP: Swanson, Kotkin, Agler, M-Flynn, and Pelham Scale (Trained observers make independent assessments of a subject’s behavior at dosing then throughout the study period in a controlled setting) **PERMP-A/C: Permanent Product Measure of Performance – Attempted/Correct (Measure of effortful performance supplementing SKAMP rating) |
|
|
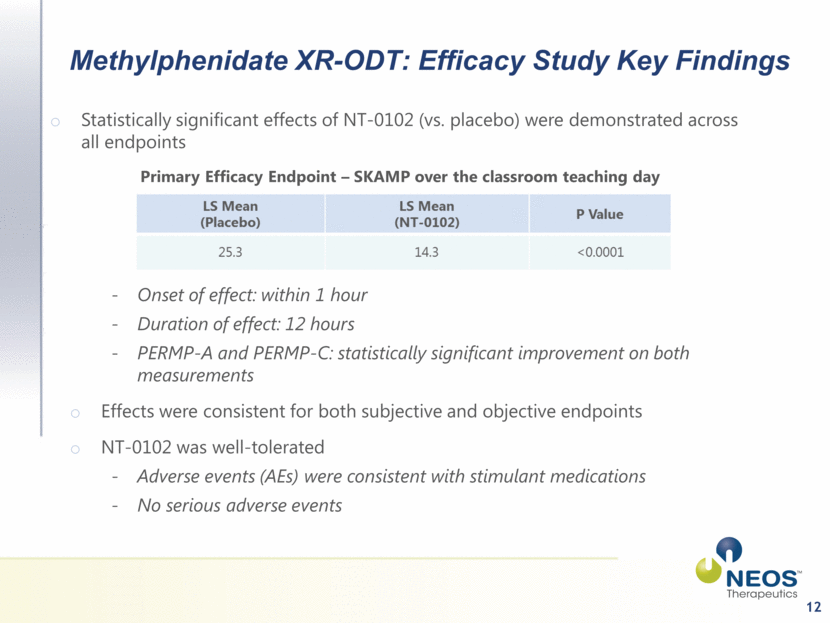
12 Methylphenidate XR-ODT: Efficacy Study Key Findings Statistically significant effects of NT-0102 (vs. placebo) were demonstrated across all endpoints Onset of effect: within 1 hour Duration of effect: 12 hours PERMP-A and PERMP-C: statistically significant improvement on both measurements Effects were consistent for both subjective and objective endpoints NT-0102 was well-tolerated Adverse events (AEs) were consistent with stimulant medications No serious adverse events Primary Efficacy Endpoint – SKAMP over the classroom teaching day |
|
|
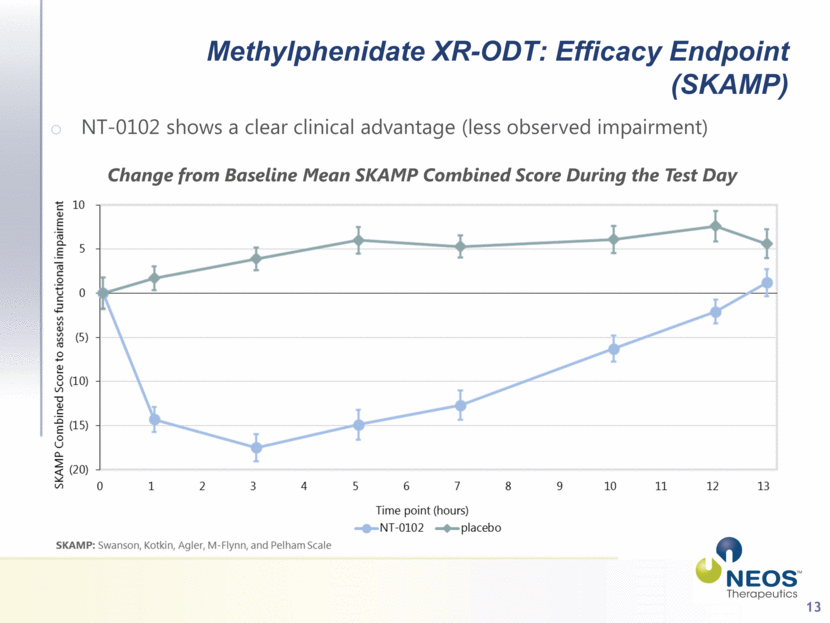
13 Methylphenidate XR-ODT: Efficacy Endpoint (SKAMP) NT-0102 shows a clear clinical advantage (less observed impairment) Change from Baseline Mean SKAMP Combined Score During the Test Day |
|
|
14 Neos ADHD Product Candidates |
|
|
15 If approved, NT-0202 is positioned to be the first amphetamine XR-ODT for once-a-day treatment of ADHD Composition-of-matter patents scheduled to expire in 2032 Completed commercial scale-up and human bioequivalence trial vs. Adderall XR capsules Filed Class 2 re-submission of the NDA in July 2015 PDUFA goal date of January 27, 2016 Amphetamine XR-ODT (NT-0202) |
|
|
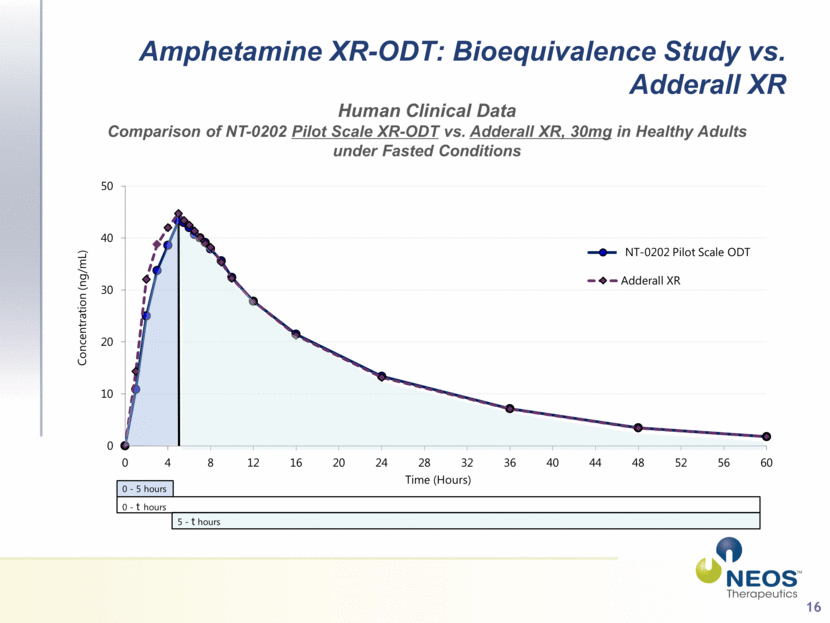
16 Amphetamine XR-ODT: Bioequivalence Study vs. Adderall XR Human Clinical Data Comparison of NT-0202 Pilot Scale XR-ODT vs. Adderall XR, 30mg in Healthy Adults under Fasted Conditions 0 - 5 hours 0 - t hours 5 - t hours NT-0202 Pilot Scale ODT 0 10 20 30 40 50 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 Concentration (ng/mL) Time (Hours) Pilot Scale ODT Adderall XR |
|
|
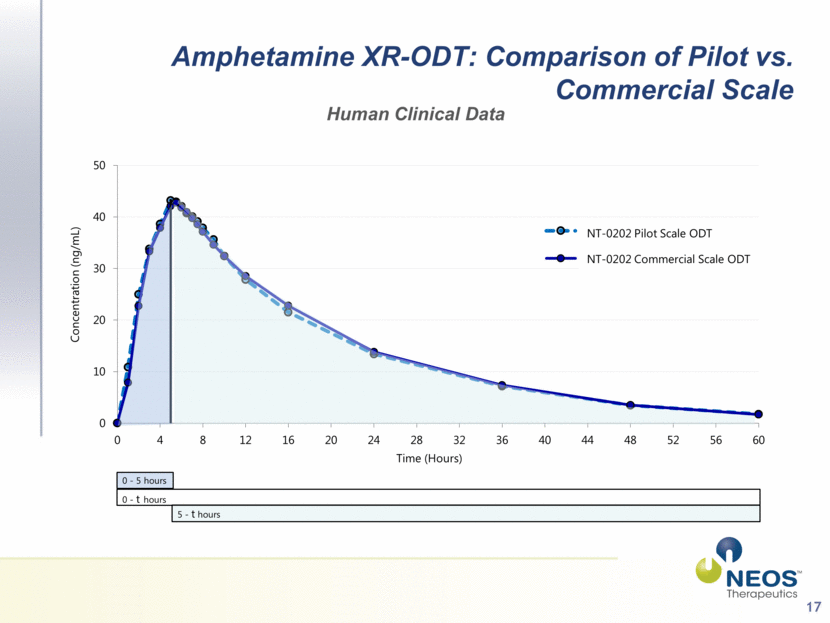
17 Amphetamine XR-ODT: Comparison of Pilot vs. Commercial Scale Human Clinical Data 0 - 5 hours 0 - t hours 5 - t hours NT-0202 Pilot Scale ODT NT-0202 Commercial Scale ODT 0 10 20 30 40 50 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 Concentration (ng/mL) Time (Hours) Pilot Scale ODT Commercial Scale ODT |
|
|
18 Neos ADHD Product Candidates |
|
|
19 Amphetamine XR-Liquid (NT-0201) If approved, NT-0201 is positioned to be an amphetamine XR liquid suspension for once-a-day treatment of ADHD Composition-of-matter patents scheduled to expire in 2032 Completed human bioequivalence trial vs. Adderall XR capsules Expect to submit NDA in Q4 2015 |
|
|
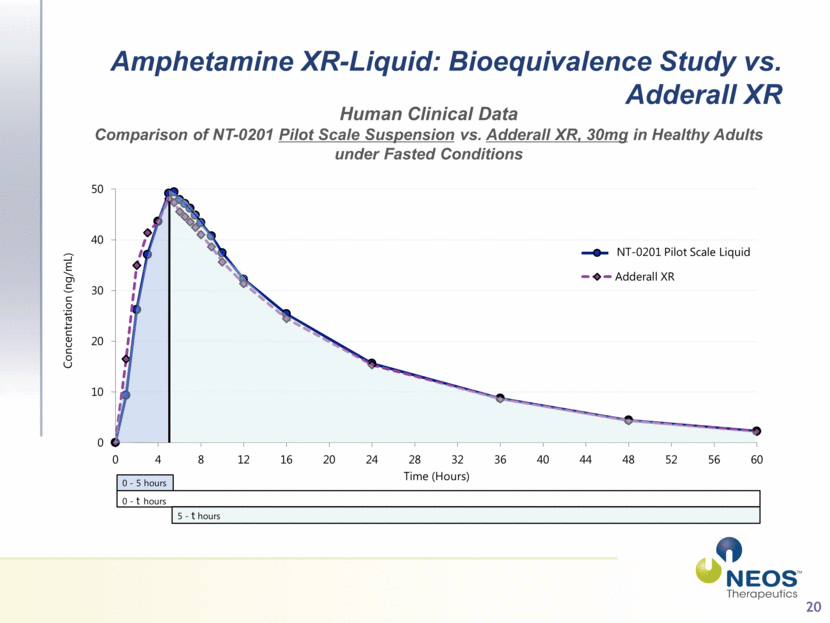
20 d- isomer depicted Amphetamine XR-Liquid: Bioequivalence Study vs. Adderall XR Human Clinical Data Comparison of NT-0201 Pilot Scale Suspension vs. Adderall XR, 30mg in Healthy Adults under Fasted Conditions 0 - 5 hours 0 - t hours 5 - t hours NT-0201 Pilot Scale Liquid 0 10 20 30 40 50 0 4 8 12 16 20 24 28 32 36 40 44 48 52 56 60 Concentration (ng/mL) Time (Hours) Pilot Scale Suspension Adderall XR |
|
|
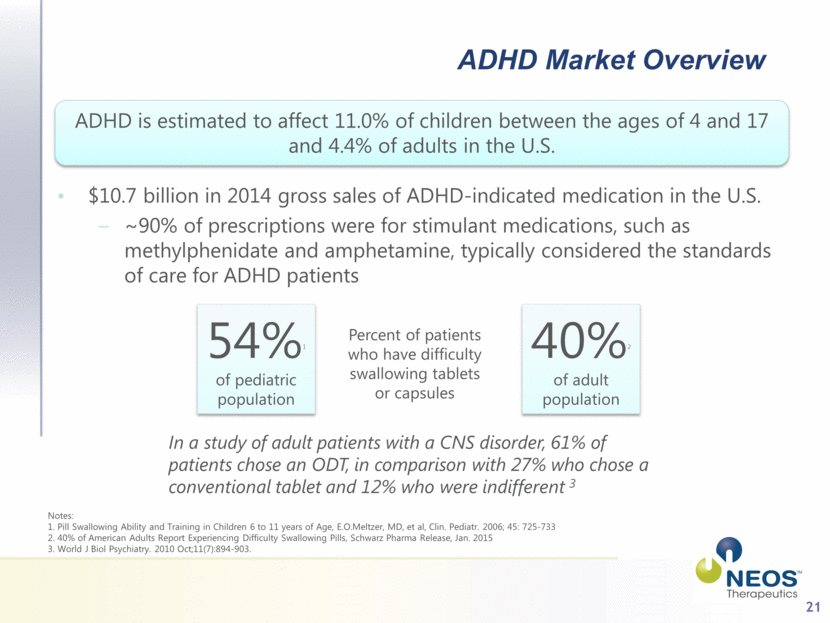
21 ADHD Market Overview ADHD is estimated to affect 11.0% of children between the ages of 4 and 17 and 4.4% of adults in the U.S. $10.7 billion in 2014 gross sales of ADHD-indicated medication in the U.S. ~90% of prescriptions were for stimulant medications, such as methylphenidate and amphetamine, typically considered the standards of care for ADHD patients 54%1 of pediatric population Percent of patients who have difficulty swallowing tablets or capsules 40%2 of adult population In a study of adult patients with a CNS disorder, 61% of patients chose an ODT, in comparison with 27% who chose a conventional tablet and 12% who were indifferent 3 Notes: 1. Pill Swallowing Ability and Training in Children 6 to 11 years of Age, E.O.Meltzer, MD, et al, Clin. Pediatr. 2006; 45: 725-733 2. 40% of American Adults Report Experiencing Difficulty Swallowing Pills, Schwarz Pharma Release, Jan. 2015 3. World J Biol Psychiatry. 2010 Oct;11(7):894-903. |
|
|
22 Building an Efficient and Targeted Commercial Model Healthcare Professional Promotion Caregiver and Patient Promotion Market Access and Reimbursement |
|
|
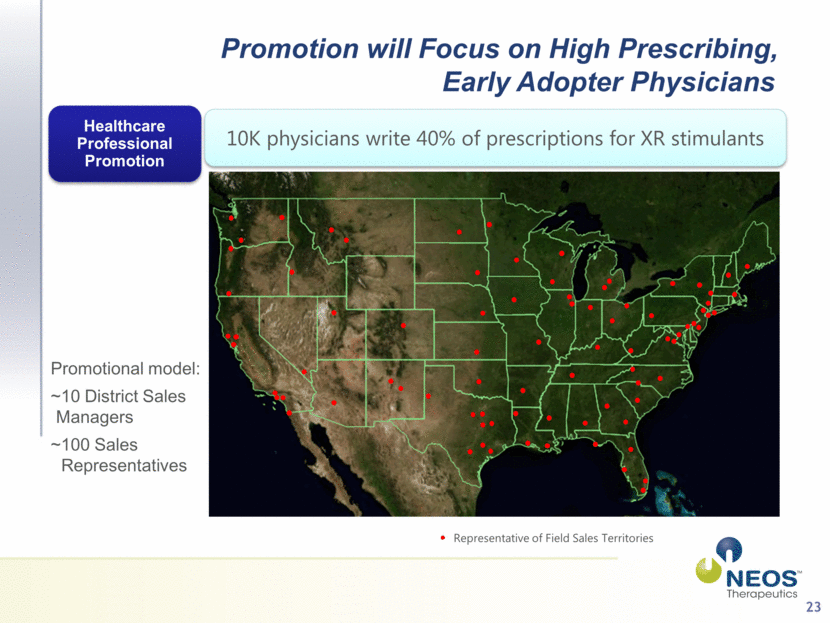
23 Promotion will Focus on High Prescribing, Early Adopter Physicians 10K physicians write 40% of prescriptions for XR stimulants Healthcare Professional Promotion Representative of Field Sales Territories Promotional model: ~10 District Sales Managers ~100 Sales Representatives |
|
|
24 Consumer Promotion will Focus on Active Online Information Seekers Caregiver and Patient Promotion Extremely active and vocal advocacy groups, patients and caregivers Targeted Direct-to-Patient Campaign Social media In-office Pharmacy |
|
|
25 Strategy to Focus On Achieving Profitable Access Across the Channels Access and Reimbursement Payor Pharmacy |
|
|
26 Modern cGMP / DEA-Registered Facility cGMP Drug Manufacturing Facility DEA schedule II - V Dosage forms include XR-liquid suspension, XR-ODTs, XR-chewable, capsules and tablets Robust IP portfolio and extensive know-how Composition of matter, method-of-use and method-of manufacture patents Orange Book patents provide protection on Methylphenidate XR-ODT, Amphetamine XR-ODT and XR-Liquid Suspension until 2032 Generic Tussionex approved and shipping Proof of technology and regulatory pathway Proven commercial scale and capacity |
|
|
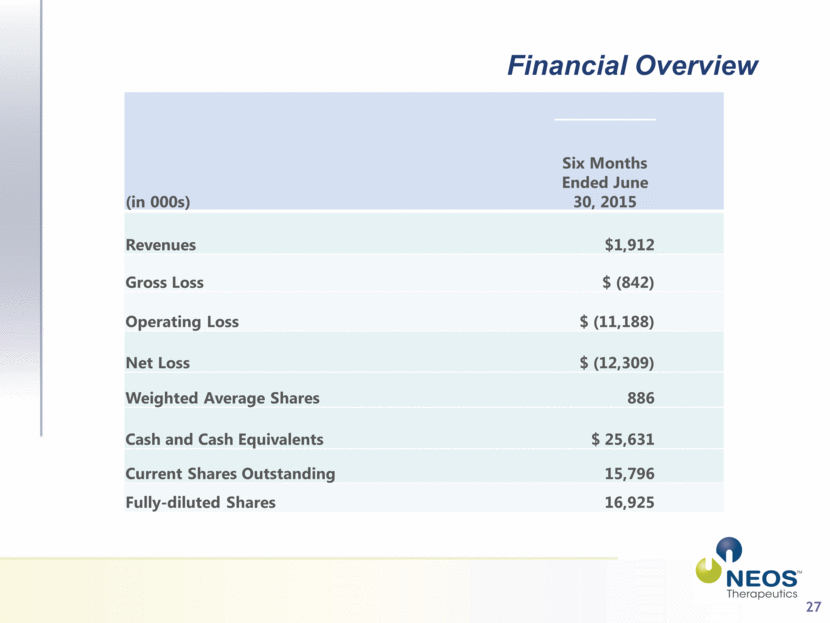
27 Financial Overview (in 000s) Six Months Ended June 30, 2015 Revenues $1,912 Gross Loss $ (842) Operating Loss $ (11,188) Net Loss $ (12,309) Weighted Average Shares 886 Cash and Cash Equivalents $ 25,631 Current Shares Outstanding 15,796 Fully-diluted Shares 16,925 |
|
|
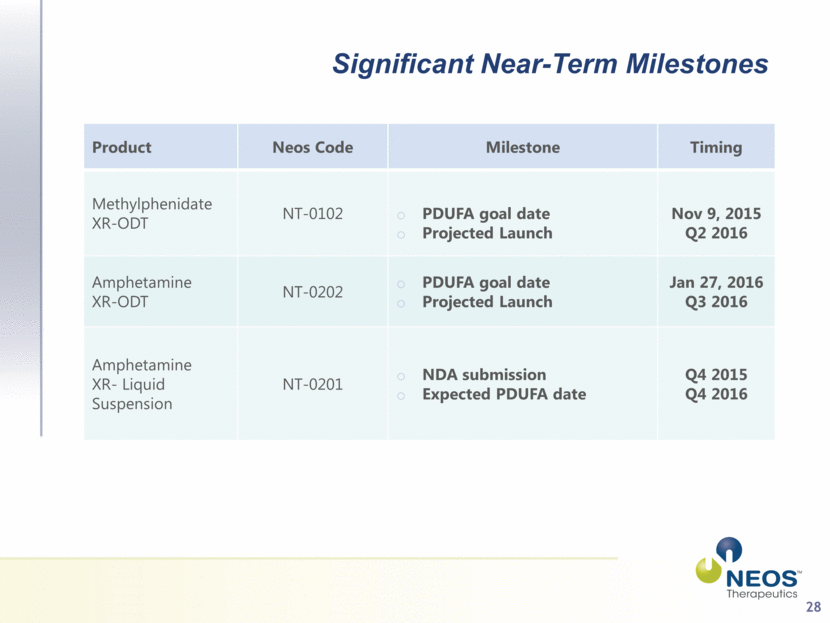
28 Significant Near-Term Milestones Product Neos Code Milestone Timing Methylphenidate XR-ODT NT-0102 PDUFA goal date Projected Launch Nov 9, 2015 Q2 2016 Amphetamine XR-ODT NT-0202 PDUFA goal date Projected Launch Jan 27, 2016 Q3 2016 Amphetamine XR- Liquid Suspension NT-0201 NDA submission Expected PDUFA date Q4 2015 Q4 2016 |
|
|
29 Summary Three late-stage product candidates focused on the $10B+ market for ADHD-indicated medication If approved, our product candidates are positioned to be first XR-ODTs for once a day treatment of ADHD Two PDUFA goal dates in the near future (Nov 9, 2015 and Jan 27, 2016) Anticipate two launches in 2016 and one launch in 2017 Significant protection against generic competition through long term composition-of-matter patents Proprietary platform technology for additional branded product candidates in multiple therapeutic areas |