Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Vyant Bio, Inc. | a8-kcorporatepresentation0.htm |

THE ONCOLOGY DIAGNOSTICS PARTNER FROM BENCH TO BEDSIDE Investor Presentation NASDAQ: CGIX September 2015

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 2 This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development and potential opportunities for Cancer Genetics, Inc. products and services, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management constitute forward-looking statements. Any statements that are not historical fact (including, but not limited to, statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates“, “proformas”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, risks of cancellation of customer contracts or discontinuance of trials, risks that anticipated benefits from acquisitions will not be realized, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, maintenance of intellectual property rights and other risks discussed in the Cancer Genetics, Inc. Forms 10-K for the year ended December 31, 2014 and 10-Q for the quarter ended June 30, 2015 along with other filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof. Cancer Genetics, Inc. disclaims any obligation to update these forward- looking statements. This presentation also contains “forward-looking statements” and Proforma information regarding the Company’s proposed acquisition of Response Genetics, Inc. (“Response Genetics”) and the anticipated benefits from the proposed acquisition. The Company cautions that these statements are subject to certain risks, including, but not limited to, the effects of the bankruptcy proceeding on the business of Response Genetics; risks that the Company will not be the successful bidder or that the proposed acquisition will not close on the proposed terms, or at all; risks that the Company will not realized the anticipated benefits of such transaction; risks that the Proforma financial information included in this presentation may not necessarily reflect the Company’s operating results and financial condition following the proposed acquisition. FORWARD-LOOKING STATEMENT

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 3 Large, Global Market Opportunities $458B GLOBAL ONCOLOGY SPEND BY 2030 Global Footprint Created by Highly Strategic M&A 3 TRANSFORMATIVE ACQUISITIONS IN 2014–2015 Strong & Growing Partnerships with Leading BioPharma 7 of 10 TOP BIOPHARMA +650% INCREASE IN BIOPHARMA REVENUE IN 2012–2015 Innovation Engine & Expertise Driven By Key Collaborations 18 RESEARCH COLLABORATIONS WITH LEADING INSTITUTIONS Unique, Proprietary Portfolio of Genomic Tests & Panels 9 COMMERCIALLY LAUNCHED TESTS 8 ISSUED PATENTS Diversified & High Growth Revenue Streams REVENUE GROWTH: 36% 4-YEAR CAGR / 55% AAGR World-Class Management Team 100+ CUMULATIVE YEARS OF EXPERIENCE INVESTOR HIGHLIGHTS: CANCER GENETICS IS UNIQUELY POSITIONED TO ADDRESS THE TRENDS IN ONCOLOGY FROM BENCH TO BEDSIDE
INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 4 CLINICAL TRIALSRESEARCH PATIENT CARE OUR MISSION IS TO BE THE ONCOLOGY DIAGNOSTICS PARTNER OF CHOICE FROM BENCH TO BEDSIDE PARTNERING WITH LEADING RESEARCH INSTITUTIONS TO DRIVE INNOVATION AND DEVELOP NEW INSIGHTS DELIVERING CRITICAL GENOMIC INSIGHTS TO MEDICAL PROFESSIONALS TO PERSONALIZE TREATMENT & IMPROVE OUTCOMES PROVIDING UNPARALLELED EXPERTISE TO BIOPHARMA COMPANIES FOR IMPROVED THERAPEUTIC DEVELOPMENT

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 5 UNIQUELY POSITIONED TO ADDRESS MACRO-TRENDS IN ONCOLOGY INCREASING GLOBAL NEED FOR IMPROVED DIAGNOSIS AND MANAGEMENT GLOBAL FOOTPRINT DEEP, DISEASE-FOCUSED GENOMIC KNOWLEDGE & INSIGHTS PROVEN DISEASE-KNOWLEDGE CAPABILITIES GROWING AWARENESS AND ADOPTION OF GENOMIC TESTING BY CLINICIANS AND PATIENTS HIGHLY SKILLED SALES FORCE CONTINUOUS NEED TO INCORPORATE NEW TECHNOLOGIES AND IMPROVED ANALYTIC CAPABILITIES COMPREHENSIVE & PLATFORM AGNOSTIC MAJORITY OF ONCOLOGY CLINICAL TRIALS NOW INCORPORATE BIOMARKERS RX, DX, & CDX EXPERTISE FOCUSED ON CREATING DURABLE SHAREHOLDER VALUE

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 6 2014 2018 GLOBAL ONCOLOGY MARKET FORECAST & POTENTIAL GLOBAL SPENDING ($ IN BILLIONS) US EU5 JAPAN PHARMERGING ROW Source: IMS Health MIDAS, Dec. 2014; IMS Health Market Prognosis, March 2015. Oncology includes therapeutic treatments as well as supportive care, radiotherapy and immunotherapies $100 $117–$147 CAGR 2014–2018: 6–8% Community Hospitals Regional Cancer Centers POTENTIAL MARKET 4K+ U.S.HOSPITALS 200+ RESEARCHCENTERS IN THE U.S. 3K+ U.S. CLINICALTRIALS FOR ALL CANCER TYPES Pharmaceutical & Biotechnology Companies Universities & Research Centers 85% ONCOLOGY PATIENTS Treated in Community Setting
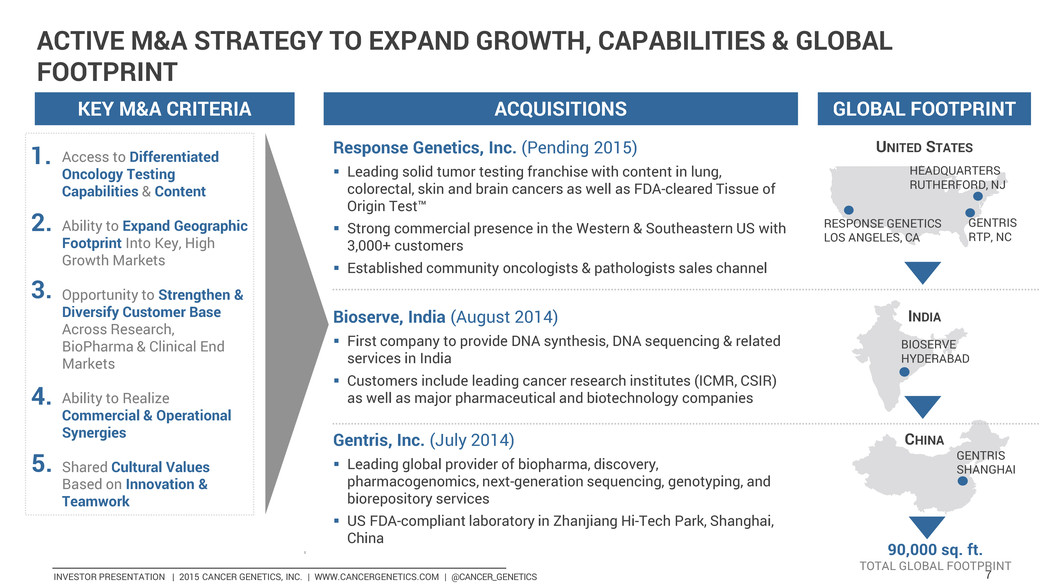
INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 7 KEY M&A CRITERIA ACQUISITIONS GLOBAL FOOTPRINT Response Genetics, Inc. (Pending 2015) Leading solid tumor testing franchise with content in lung, colorectal, skin and brain cancers as well as FDA-cleared Tissue of Origin Test™ Strong commercial presence in the Western & Southeastern US with 3,000+ customers Established community oncologists & pathologists sales channel UNITED STATES Bioserve, India (August 2014) First company to provide DNA synthesis, DNA sequencing & related services in India Customers include leading cancer research institutes (ICMR, CSIR) as well as major pharmaceutical and biotechnology companies INDIA Gentris, Inc. (July 2014) Leading global provider of biopharma, discovery, pharmacogenomics, next-generation sequencing, genotyping, and biorepository services US FDA-compliant laboratory in Zhanjiang Hi-Tech Park, Shanghai, China CHINA RESPONSE GENETICS LOS ANGELES, CA GENTRIS RTP, NC HEADQUARTERS RUTHERFORD, NJ GENTRIS SHANGHAI ACTIVE M&A STRATEGY TO EXPAND GROWTH, CAPABILITIES & GLOBAL FOOTPRINT 90,000 sq. ft. TOTAL GLOBAL FOOTPRINT BIOSERVE HYDERABAD Access to Differentiated Oncology Testing Capabilities & Content Ability to Expand Geographic Footprint Into Key, High Growth Markets Opportunity to Strengthen & Diversify Customer Base Across Research, BioPharma & Clinical End Markets Ability to Realize Commercial & Operational Synergies Shared Cultural Values Based on Innovation & Teamwork 1. 2. 3. 4. 5.

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 8 COMBINED PORTFOLIO COVERS 8 OF THE 10 MOST PREVALENT CANCERS GEOGRAPHIC REACH HIGHLY EXPERIENCED SALES FORCE FOCUSED ON THE COMMUNITY ONCOLOGIST & PATHOLOGIST MARKETS COMMERCIAL PRESENCE ESTIMATED REVENUE CONTRIBUTION(1) $2-3 million BIOPHARMA(2) TISSUE OF ORIGIN™ IS ONLY FDA-CLEARED TEST OF ITS TYPE EXTENSIVE & HIGHLY COMPLEMENTARY SOLID TUMOR TESTING PORTFOLIO STRONG COMMERCIAL PRESENCE IN THE WESTERN & SOUTHEASTERN US $8-9 million CLINICAL (1) Expected over the next 12 months (2) ALCHEMIST, is a group of clinical trials for patients with certain types of early-stage non-small cell lung cancer (NSCLC) that has been treated surgically. (3) Acquisition Pending RESPONSE GENETICS, INC. ACQUISITION HIGHLIGHTS(3) $10-12 million TRANSACTION HIGHLIGHTS GLOBAL FOOTPRINT INCLUDES $7 MILLION IN CASH AND $7 MILLION IN CGI COMMON STOCK ADDS 27,000 SQUARE FEET FOR A TOTAL OF 90,000 SQUARE FEET OF STATE-OF-THE-ART LAB SPACE
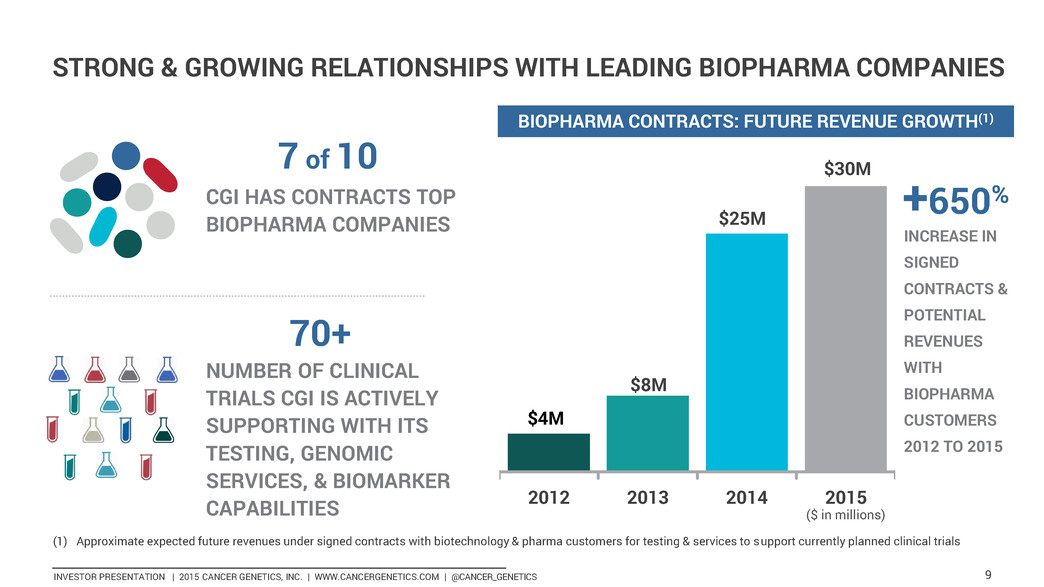
INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 9 2012 2013 2014 2015 $4M $30M +650% INCREASE IN SIGNED CONTRACTS & POTENTIAL REVENUES WITH BIOPHARMA CUSTOMERS 2012 TO 2015 BIOPHARMA CONTRACTS: FUTURE REVENUE GROWTH(1) (1) Approximate expected future revenues under signed contracts with biotechnology & pharma customers for testing & services to support currently planned clinical trials ($ in millions) STRONG & GROWING RELATIONSHIPS WITH LEADING BIOPHARMA COMPANIES CGI HAS CONTRACTS TOP BIOPHARMA COMPANIES 7 of 10 NUMBER OF CLINICAL TRIALS CGI IS ACTIVELY SUPPORTING WITH ITS TESTING, GENOMIC SERVICES, & BIOMARKER CAPABILITIES 70+ $8M $25M

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 10 Global CRO industry leader specializing in Phase I through Phase IV clinical trial testing $1.5B in net annual revenue & 11,000 employees ICON has conducted over 950 oncology studies, assessing over 148,000 patients at nearly 27,500 sites worldwide over the past 5 years ICON has expertise in tumor imaging, laboratory (including PK/PD and Biomarkers) IVRS, central data management, electronic data capture and regulatory oncology issues ICON has ~30% of revenue from oncology Combined capabilities provide unparalleled oncology insights across ICON’s 900+ Phase I - Phase IV clinical trial studies Provide clients access to combined expertise in oncology- focused genomic testing, disease-specific proprietary genomic panels, and world-class bioinformatics. Tests and services offered by Cancer Genetics (CGI) through ICON will be branded as “POWERED BY CGI” Cross training of global sales forces & scientific affairs teams scheduled during Q3 & Q4 of 2015 CGI has already realized cross- and up-selling wins with joint selling efforts Future potential opportunities to partner with other service and technology providers to create enhanced capabilities for BioPharma customers ICON PARTNERSHIP & STRATEGIC ALLIANCE ENABLING UNPARALLELED COMPREHENSIVE ONCOLOGY LABORATORY TESTING AND SOLUTIONS TO THE BIOPHARMA INDUSTRY ICON CGI & ICON PARTNERSHIP

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 11 COLLABORATIONS WITH PREMIER CANCER RESEARCH INSTITUTIONS Disease Target Collaboration Highlights Beth Israel Deaconess Medical Ctr DLBCL (Diffuse Large B-Cell Lymphoma) Biomarker-based outcome prediction Cleveland Clinic Kidney Cancer Genomic marker validation Columbia University AML, MDS, and Myeloid Cancers (Acute Myeloid Leukemia, Myelodysplastic Syndromes) NGS panel development Dana-Farber Cancer Institute CLL (Chronic Lymphocytic Leukemia) CLL outcome scheme validation Georgia Regents University DLBCL Diagnosis, prognosis & management Hackensack University Medical Ctr CLL Further validation of MatBA® -CLL regions Kamineni Hospital, India Cervical Cancer FHACT® evaluation Keck Medicine of USC DLBCL Genomic panel evaluation & optimization Memorial Sloan-Kettering Cancer Ctr CLL, DLBCL, FL, MCL, Kidney (Follicular Lymphoma, Mantle Cell Lymphoma) Multiple collaborations Moffitt Cancer Center CINV and PGx Prediction of Side Effects Associated With Chemo National Cancer Institute Cervical Cancer FHACT® development North Shore LIJ Health System CLL/SLL CLL/SLL validation & BTK inhibitor resistance Stanford University DLBCL Risk stratification University of Alabama Central Nervous System Lymphoma Biomarker investigation University of Iowa Cancer Center Cervical Cancer, DLBCL FHACT® evaluation & MatBA®-DLBCL validation Westchester Medical Center Central Nervous System Lymphoma Genomic biomarker identification using UroGenRA®

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 12 ONCOSPIRE GENOMICS JOINT VENTURE BETWEEN CANCER GENETICS & MAYO CLINIC Joint venture based in Rochester, MN Goal: to develop tests that will become the gold standard in diagnosing and managing patients with selected disease targets Diseases with known clinical dilemmas were selected and informed by clinicians in the Mayo Clinic network OncoSpire has the rights to druggable targets that are discovered SELECTED PROJECTS PIPELINE(1): 200,000 NEW CASESPER YEAR MULTIPLE MYELOMA LUNG CANCER FOLLICULAR LYMPHOMA 1.6M NEW CASESPER YEAR RESEARCH & DISCOVERY CLINICAL DEVELOPMENT COMMERCIAL DEVELOPMENT LAUNCH & MARKET ENTRY Q4 2015 Expected Launch 20,920 NEW CASESPER YEAR (1) Pipeline of projects may change based on business or scientific rationale.

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 13 TISSUE OF ORIGIN™ (2) UROGENRA®-KIDNEY FHACT® CERVICAL FOCUS::CANCER HOTSPOT™ UNIQUE, PROPRIETARY PORTFOLIO OF GENOMIC TESTS & PANELS RESEARCH & DISCOVERY CLINICAL DEVELOPMENT COMMERCIAL DEVELOPMENT FOCUS::MYELOID™ FOCUS::LYMPHOID™ FOCUS::CLL™ MATBA® FOR B-CELL CANCERS TEST IN MARKET 4 TESTS IN MARKET Late 2015 MULTIPLE MYELOMA FOLLICULAR LYMPHOMA LUNG CANCER TBD Late 2015 Late 2015 TEST IN MARKET FOCUS::RENAL™ TEST IN MARKET TEST IN MARKET TEST IN MARKET Early 2016 Late 2015 HEREDITARY PANEL COMPREHENSIVE PHARMACOGENOMICS (PGX) PANEL TBD BLOOD CANCERS ONCOSPIRE GENOMICS(3) SOLID TUMORS HEREDITARY PROVIDE COMPREHENSIVE AND TECHNOLOGY AGNOSTIC METHODOLOGIES DRIVE NOVEL DISCOVERY AND DEVELOPMENT THROUGH PARTNERSHIPS WITH KEY THOUGHT LEADERS TARGET UNMET AND CRITICAL DISEASE STATES MEANINGFULLY IMPACT AND IMPROVE CLINICAL TRIALS AND PATIENT CARE MARKET ENTRY (1) Pipeline of projects may change based on business or scientific rationale. (2) Acquisition Pending (3) Joint Venture with the Mayo Clinic Guiding Principals for Portfolio Development (1) TEST IN MARKET

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 14 ® SOLUTION MatBA®-CLL/SLL STRATIFIES PATIENTS INTO 3 DISTINCT RISK GROUPS USING 20 BIOMARKERS: 38% OF CASES ARE FAVORABLE FALLING UNDER "WATCH & WAIT" APPROACH 8% OF UNFAVORABLE CASES MISSED BY FISH ARE DETECTED BY MATBA®-CLL/SLL 23% 39% 38% FAVORABLE INTERMEDIATE UNFAVORABLE PROBLEM STANDARD FISH TESTING FOR CLL STRATIFIES PATIENTS INTO 2 RISK GROUPS USING 4 BIOMARKERS FAVORABLE/INTERMEDIATE UNFAVORABLE 85% 15% MatBA® DRIVES IMPROVED INSIGHT AND MANAGEMENT FOR CHRONIC LYMPHOCYTIC LEUKEMIA (CLL) IN PATIENTS

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 15 UroGenRA® - KIDNEY GUIDES MANAGEMENT & APPROPRIATE TREATMENT SELECTION Patients with renal masses often undergo unnecessary nephrectomy for accurate diagnosis & experience delay in treatment Over 15% of needle biopsies of renal masses are rendered non-diagnostic PROBLEM UroGenRA®-Kidney assesses 16 biomarkers in a single assay Provides accurate diagnosis guiding appropriate clinical management and treatment strategies (benign vs. malignant; malignant subtype) Reduction in number of highly invasive procedures & time to treatment initiation SOLUTION PROPRIETARY DIAGNOSTIC ALGORITHM >600 RCC MALIGNANTSUBTYPES in-silico: SNP >100 MALIGNANT & BENIGNRENAL NEOPLASMS in-house: aCGH & FISH RETROSPECTIVE IN-HOUSE FFPE VALIDATION (n>190) 100% DIAGNOSTICYIELD 97% DIAGNOSTICSENSITIVITY to distinguish benign/malignant 93% SENSITIVITYto distinguish malignant RCC subtypes PROSPECTIVE ONGOING PERCUTANEOUS CORE NEEDLE BIOPSY (n>190) DEMONSTRATED ABILITY TO DIAGNOSE PATHOLOGICALLY “UNCLASSIFIABLE” BIOPSIES PRIOR TO SURGICAL INTERVENTION ®

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 16 55.0M PAP SMEARS 3.5M UNCLEAR/ ABNORMAL 2.0M BIOPSIES 13K CASES OF CANCER Today, all HPV+ women with abnormal Pap results are referred for colposcopy Several cervical cancer tests are available but the need for less invasive and better informed treatment exists PROBLEM FHACT® helps triage before colposcopy Non-invasive test performed on remnant liquid cytology – no resampling is necessary which means no additional doctor visit Fewer women referred for colposcopy reducing healthcare costs & patient anxiety SOLUTION Today, all these women are referred for colposcopy PROGRESS to a higher grade and increased risk for cancer within 10-30 years of the infection REGRESS within 2 years of the infection Not referred for colposcopy FHACT® Results: Normal Referred for colposcopy FHACT® Results: Abnormal ~90% ~10% HPV+ women with abnormal or unclear liquid-based cytology FHACT® FITS DIRECTLY INTO TODAY’S CERVICAL CANCER SCREENING WORKFLOW RECENT UPDATE Results from two independent cervical cancer-related studies during the 30th International Papillomavirus Conference & Clinical and Public Health Workshops (HPV 2015) “Chromosomal Gains Measured by Fluorescence in situ Hybridization in Cytology Samples” was conducted in collaboration with the National Cancer Institute (NCI) “Evaluation of Gain of Four Chromosomal Loci by Fluorescence in-situ Hybridization on Pap Smears of Women in India” in collaboration with Kamineni Hospital, Hyderabad (India)
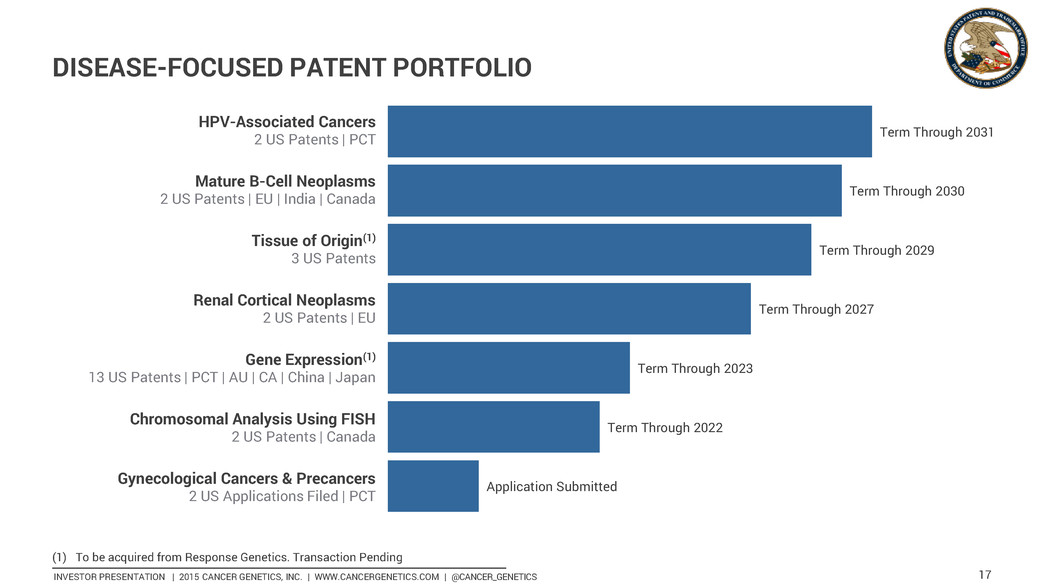
INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 17 Application Submitted Term Through 2022 Term Through 2023 Term Through 2027 Term Through 2029 Term Through 2030 Term Through 2031 2 US Patents | EU | India | Canada Mature B-Cell Neoplasms 3 US Patents Tissue of Origin(1) 2 US Patents | EU Renal Cortical Neoplasms 13 US Patents | PCT | AU | CA | China | Japan Gene Expression(1) 2 US Patents | Canada Chromosomal Analysis Using FISH 2 US Applications Filed | PCT Gynecological Cancers & Precancers 2 US Patents | PCT HPV-Associated Cancers (1) To be acquired from Response Genetics. Transaction Pending DISEASE-FOCUSED PATENT PORTFOLIO

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 18 DIVERSIFIED AND GROWING REVENUE BASE 55% 43% 2% Full Year 2014 $10.2M 70% 25% 5% 1H 2015 $8.6M CGI’s business is not over-dependent on third party reimbursement. In 1H 2015, Medicare represented 6% and other insurers represented 6% of consolidated revenue. • Entered into partnership with ICON to offer access to ICON Laboratory Services combined with Cancer Genetics' Focus::CLL™ chosen for global clinical trial by leading biotechnology company BIOPHARMA GLOBAL BIOTECH AND PHARMACEUTICAL COMPANIES • Launched new NGS panels, including Focus::Myeloid™ • Entered into agreement to acquire Response Genetics (OTCQB:RGDX)** CLINICAL CANCER CENTERS, HOSPITALS, REGIONAL LABS, AND CLINICIANS • New research studies with leading cancer centers and academic institutions • Selected by regenerative medicine company, ReproCELL, Inc., to provide (NGS) services DISCOVERY PRECLINICAL RESEARCH GROUPS AT ACADEMIC,GOVERNMENT & COMMERCIAL ORGANIZATIONS ** Acquisition Pending

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 19 STRONG RECORD OF CONTINUOUS GROWTH REVENUE GROWTH BY YEAR / PERIOD ($ IN MILLIONS) $0.6 $2.7 $5.6 $6.0 $3.2 $3.7 $4.4 $2.1 $0.2 $0.4 $0.6 $0.3 $4.3 $6.6 $10.2 $8.6 2012 2013 2014 1H 2015 BIOPHARMA CLINICAL DISCOVERY OTHER 2013–2014 REVENUE HIGHLIGHTS TOTAL REVENUE GROWTH OF 54% $6.6M TO $10.2M $2.7M TO $5.6M $3.7M TO $4.4M CLINICAL REVENUE GROWTH 0F 21% BIOPHARMA REVENUE GROWTH OF 112%

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 20 $6.6 $19.8 $26.4 $10.2 $16.7 $26.9 $8.6 $7.7 $16.3 FY13 FY14 YTD 15 PROFORMA REVENUES CANCER GENETICS RESPONSE GENETICS PRO FORMA CANCER GENETICS’ AND RESPONSE GENETICS’ PRO FORMA REVENUE ($ IN MILLIONS) * The pro forma revenue numbers above may not be indicative of the consolidated results in the future. Acquisition pending. $5.2M ADDITIONAL POTENTIAL REVENUE FROM BIOPHARMA TRIALS (TOTAL $38M) $32+M PRO FORMA REVENUE RUN RATE*

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 21 CANCER GENETICS STANDALONE SUMMARY STATEMENT OF OPERATIONS SUMMARIZED INCOME STATEMENTS (dollars in thousands) SUMMARIZED BALANCE SHEET (dollars in thousands) 1H, 2014 1H, 2015 REVENUE $2,942 8,555 Gross Profit 149 2,316 Gross Margin (%) 5% 27% Research & Development 1,703 2,533 Sales & Marketing 1,667 2,300 General & Administrative 5,127 6,049 OPERATING PROFIT (Loss) ($8,348) ($8,566) NET INCOME (Loss) ($6,673) ($9,258) NON CASH ADJUSTMENTS $1,637 $2,812 NET INCOME (Loss) EXCLUDING NON CASH ITEMS + ($5,036) ($6,446) Actual 06/30/15 ALL CASH $23,744 STOCKHOLDERS’ EQUITY $26,772 +This is non GAAP measure. Adjustments are depreciation ($228 and $698), equity compensation ($1,294 and $1,453) and other ($115 and $661) respectively.

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 22 COVERED LIVES REPRESENTED BY RECENT AGREEMENTS WITH NATIONAL INTEGRATED NETWORKS America’s Choice Provider Blue Cross Blue Shield of California Blue Cross Blue Shield of Illinois Blue Cross Blue Shield of North Carolina Credentialing Organizations (NCQA, CAQH) Encore Health Network Harvard Pilgrim Health Plan Market-specific Medicaid plans (IL, CA, TX, FL, NY, NJ) Medicare Railroad MultiPlan TRICARE military plan 36% 29% REGIONAL AND NATIONAL PAYERS MEDICARE COMMERCIAL PAYERS CGI PAYMENT HISTORY – 1H 2015 (CLINICAL REVENUES ONLY) 35% DIRECT BILL MORE THAN 50M Cancer Genetics’ business is not over-dependent on third party reimbursement. In 1H 2015, Medicare represented 6% and other insurers represented 6% of consolidated quarterly revenue. HISTORICAL CLINICAL REIMBURSEMENT MIX & PAYER COVERAGE

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 23 MILESTONES EXPECTED IN COMING QUARTERS REIMBURSEMENT & MARKET ADOPTION Increasing covered lives market access through add’l payers & health care organizations Additional international agreements for FHACT® distribution and co-marketing partnerships in key geographies NEXT GENERATION SEQUENCING PANELS Launching multi-marker NGS panel for lymphoid malignancies Multiple Myeloma NGS panel launch, Oncospire Second half of 2015 DATA TO SUPPORT PRODUCTS Lymphoid, Myeloid and CLL Panels - Additional data and results to support clinical usage, patient value and payor coverage FHACT® - Additional data from a study conducted in conjunction with NCI and more published papers focused on FHACT®, including a health economic study Results from two independent cervical cancer-related studies published at HPV 2015 BIOPHARMA REVENUES & MARKET SHARE Additional news on biopharma partners & relationships Major collaborations with US & Asia biopharma companies ADDITIONAL PARTNERSHIPS TO EXPAND BIOPHARMA BUSINESS

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 24 EXPERIENCED LEADERSHIP TEAM Panna Sharma Chief Executive Officer & President, Board Member Edward J. Sitar, CPA Chief Financial Officer & Treasurer Rita Shaknovich, MD, PhD Group Medical Director & VP, Hematopathology Services Mandar Kulkarni, PhD Chief Technology Officer, India Jane Houldsworth, PhD VP, Research & Development Greg Ash VP, Clinical Market Development Randy Goodman, PhD Director, Reimbursement & Managed Care Scott Clark, PhD VP, Global QA & Biopharma Development Rob Fannon, MBA, MPH VP, Biopharma Operations Kamala K Maddali DVM, PhD VP, Biopharma Collaborations and Companion Diagnostics 10+ years operations, client mgmt, and molecular test & panel dev. 5 years in life science and pharmaceutical equity research Roche Molecular Diagnostics, BioServe Biotechnologies, Ltd. 15+ years as advisor to global life science & healthcare companies TSG Partners, iXL Enterprises, Interactive Solutions, Putnam Investment 30+ yrs in finance & deal making in the healthcare industry Healthagen (Aetna), Cadent Holding, MIM Corp, Coopers & Lybrand (PWC) 16+ years at pharma and clinical research companies CAP Biorepository Director and oversees China operations Gentris Corporation 15+ years in both clinical and research capacities Weill Cornell Medial College, Mount Sinai, Columbia Presbyterian Hosp. 10+ years in global P&L scientific and commercial management arena Quest Diagnostics, Quintiles and Merck Schering Plough 25+ years in translational oncology research MSKCC, CALTECH 20+ years in health economics & healthcare policy Healthcare Mgmt Solutions, Scientia Advisors, ImpediMed 5+ years in both clinical and genomic development Sandor Lifesciences, M/Z Diagnostics 16+ years in the healthcare sector Go Path Laboratories, Response Genetics

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 25 BOARD OF DIRECTORS John Pappajohn Non-Executive Chairman Raju S.K. Chaganti, PhD Howard McLeod, PharmD g Panna Sharma Michael J. Welsh, MD c, g Geoffrey Harris, CFA a Ted Cannon a,c Franklyn Prendergast, MD, PhD a,c, g Founder & President of Clinical Research Center of Cape Cod Previously at Franey Medical Labs; Pharmacia Diagnostics; Alletess Involved in 100+ start-up companies Served as director of 40+ public companies Currently on boards of: American CareSource Holdings; ConMed Healthcare Mgmt; CNS Response Founded CGI & served as Chairman until 2014 Internat’ly recognized leader in molecular genetics Co-discovered lymphoma & kidney cancer patents Incumbent of the William Snee E. Chair at MSKCC 30+ years experience as healthcare analyst & portfolio manager for biotech/life sci companies Portfolio manager/managing partner at c7 Advisors Previously: Cantor Fitzgerald; Gleacher & Company Personalized Medicine Medical Director at Moffitt Founding Director of the Univ. of NC Institute for Pharmacogenomics (PGx) & Individualized Therapy 475+ peer-reviewed papers (PGx, applied therapeutics) Director of Mayo Clinic for Individualized Medicine (Retired) Currently on boards of: Translational Genomics Research Inst.; Infectious Disease Research Inst.; DemeRx, Inc.; Ativa; Eli Lilly & Co. CEO & president of Cancer Genetics General Manager of OncoSpire Genomics Previously managing partner/founder of TSG Partners 70+ buy & sell-side transactions (healthcare companies) Investigator at the Howard Hughes Medical Inst. Roy J. Carver Biomed Research Chair in Internal Medicine & Molecular Physiology & Biophysics Director of Univ. of Iowa Inst. for Biomed Discovery a: Audit Committee c: Compensation Committee g: Governance and Nominating Committee

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 26 Large, Global Market Opportunities $458B GLOBAL ONCOLOGY SPEND BY 2030 Global Footprint Created by Highly Strategic M&A 3 TRANSFORMATIVE ACQUISITIONS IN 2014–2015 Strong & Growing Partnerships with Leading BioPharma 7 of 10 TOP BIOPHARMA +650% INCREASE IN BIOPHARMA REVENUE IN 2012–2015 Innovation Engine & Expertise Driven By Key Collaborations 18 RESEARCH COLLABORATIONS WITH LEADING INSTITUTIONS Unique, Proprietary Portfolio of Genomic Tests & Panels 9 COMMERCIALLY LAUNCHED TESTS 8 ISSUED PATENTS Diversified & High Growth Revenue Streams REVENUE GROWTH: 36% 4-YEAR CAGR / 55% AAGR World-Class Management Team 100+ CUMULATIVE YEARS OF EXPERIENCE INVESTOR HIGHLIGHTS: CANCER GENETICS IS UNIQUELY POSITIONED TO ADDRESS THE TRENDS IN ONCOLOGY FROM BENCH TO BEDSIDE

INVESTOR PRESENTATION | 2015 CANCER GENETICS, INC. | WWW.CANCERGENETICS.COM | @CANCER_GENETICS 27 THE ONCOLOGY DIAGNOSTICS PARTNER FROM BENCH TO BEDSIDE CGI Headquarters 201 Route 17 North, 2nd Fl. Rutherford, NJ 07070 Phone: +1 201-528-9200 Fax: +1 201-528-9235 RUTHERFORD, NJ Research Triangle Park 133 Southcenter Court, S.400 Morrisville, NC 27569 Phone: +1 919-465-0100 Fax: +1 919-465-0554 RALEIGH, NC #3-1-135/1A CNR Complex Mallapur Main Road, R.R. Dst. Hyderabad – 500 076, Telangana Toll-free: +91 040-2717-8178 Fax: +91 040-2717-8176 HYDERABAD, INDIA 781 Cai Lun Road, Room 803 Shanghai 201203 P.R. China Toll-free: +91 040-2717-8178 Fax: +91 040-2717-8176 SHANGHAI, CHINA WWW.CGIX.COM WWW.CANCERGENETICS.COM
