Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Neoleukin Therapeutics, Inc. | d26017d8k.htm |
| EX-99.1 - EX-99.1 - Neoleukin Therapeutics, Inc. | d26017dex991.htm |
 Safety
and Efficacy of AQX-1125 in Interstitial Cystitis/Bladder Pain
Syndrome Results of the Phase 2 LEADERSHIP Trial
Dr. Stephen B. Shrewsbury
MD Chief Medical Officer, Aquinox Pharmaceuticals (Canada) Inc. September 18 , 2015 ESSIC, Rome Exhibit 99.2 th |
 A Novel
First in Class Anti-Inflammatory Therapy AQX-1125, a SHIP1
activator: •
Broad anti-inflammatory potential
• Favourable ADME: • Once daily oral administration • T½ 21hrs • Dose proportional PK, No food effect • High bioavailability; Not metabolized ~60/40 Liver/Renal elimination as unchanged parent • Well tolerated in 5 clinical trials completed • >360 subjects dosed 2 |
 LEADERSHIP
Trial: Endpoints & Selection Criteria Primary
Endpoint:
AQX-1125 vs placebo on average daily pain score utilizing eDiaries Secondary Endpoints: Difference in the change
from baseline in: - Average pain score at
clinic - Maximum daily pain on
eDiary - O’Leary-Sant Interstitial Cystitis Symptom Index and Problem Index (ICSI/PI) - Bladder Pain/Interstitial Cystitis Symptom Score (BPIC-SS) - Voiding frequency over a 24 hour period Safety, Pharmacokinetics Selection Criteria • Key Inclusion: Diagnosed>12m/ 15y; +ve cystoscopy within 36m; BPIC-SS 19 V1/2; ICSI/PI 8 V2; Average pain 5 over 7d; 8 voids in 24h • Key Exclusion: Pelvic floor pain ( 5/10); UTI in 30d; 1+ hematuria; H/o chronic substance abuse, dependency or abuse of opiates; intravesical Tx in 60d ClinicalTrials.Gov: NCT 01882543 3 |
 LEADERSHIP
Trial: Subject Disposition 4
Screened N=137 Randomized to Treatment N=69 Placebo N=32 Discontinued, N=3 (Adverse event, N=2) (Other, N=1) Completed, N=29 AQX-1125 N=37 Discontinued, N=4 (Adverse event, N=2) (Withdrew consent, N=2) Completed, N=33 Screen Failures, N=68 (Pain score, N=19) (Withdrew consent, N=11) (Prohibited meds, N=10) (UTI, N=7) (Cystoscopy, N=4) (Pelvic floor pain, N=3) (IC > 15yrs, N=2) (BMI, N=2) (Substance abuse, N=2) (BPIC-SS score, N=2) (Other, N=6) |
 LEADERSHIP
Trial: Baseline Demographics 5
Placebo (N=32) AQX-1125 (N=37) Age (years; mean ± SD) 53.1 (12.9) 52.1 (14.9) Duration of Diagnosis (months; mean ± SD) 76.3 (57.3) 63.5 (54.4) Number on at Least One Concomitant Medication for Pain 1 (%) 22 (68.8) 22 (59.5) - Number on Pentosan Polysulphate Sodium (%) 9 (28.1) 12 (32.4) - Number on Amitriptyline
(%) 9 (28.1) 9 (24.3) - Others 14 (43.8) 13 (35.1) Average Daily Pain (mean ± SD) 6.7 (1.01) 6.4 (0.88) Maximum Pain (mean ± SD) 7.9 (1.05) 7.6 (1.04) O’Leary-Sant ICSI/PI (mean ± SD) 30.2 (4.40) 27.3 (5.04) - ICSI (mean ± SD) 16.1 (2.87) 14.4 (2.94) - ICPI (mean ± SD) 14.1 (1.92) 12.9 (2.36) BPIC-SS (mean ± SD) 31.6 (3.28) 29.6 (3.62) Voiding Frequency, 24h (mean ± SD) 20.3 (11.97) 17.0 (7.97) 1 Includes PPS, Amitriptyline and Others. Some patients on more than one medication. |
 6 LEADERSHIP Trial: Average Pain (eDiary) - Reduced Average Daily Pain (11-Point NRS, eDiary) |
 BPIC-SS Voiding Frequency (24 hrs) Average Daily Pain (eDiary) Average Pain (Clinic) Maximum Daily Pain (eDiary) O’Leary-Sant ICSI LEADERSHIP Trial: Pain and Symptoms at 6 Weeks - Reduced 7 O’Leary Sant ICPI |
 LEADERSHIP Trial: Responder Rates - Improved Endpoint (at Week 6) Reduction AQX-1125 (N=37) % (n) Placebo (N=32) % (n) Proportion of responders AQX-1125:Placebo 1) Avg NRS Pain (e-Diary) 30% 51.4% (19) 28.1%
(9) 1.83 50% 29.7% (11) 15.6% (5) 1.90 2) Max NRS Pain (e-Diary) 30% 46.0%
(17) 25.0%
(8) 1.84 50% 35.1%
(13) 12.5%
(4) 2.81 8 |
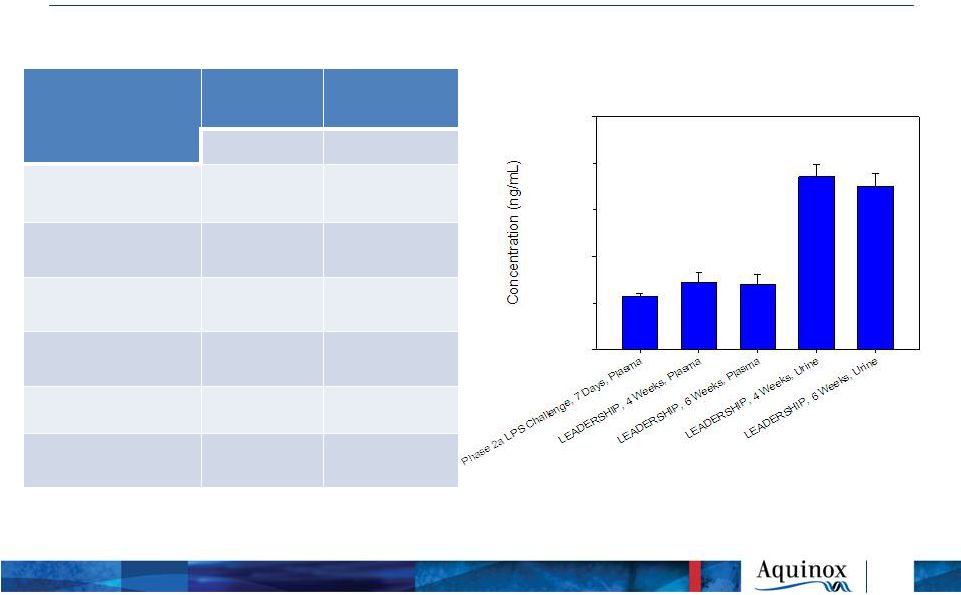 LEADERSHIP
Trial: Safety & PK Summary AEs
Placebo N=32 AQX-1125 N=37 N (%) N (%) Total AEs 25 (78.1) 19 (51.4) GI Disorders 11 (34.4) 12 (32.4) Eye Disorders 3 (9.4) 2 (5.4) SAEs 0 (0.0) 0 (0.0) Deaths 0 (0.0) 0 (0.0) AEs Leading to Discontinuation 2 (6.3) 2 (5.4) *AEs by subject. Subjects counted once per SOC, PT and Treatment group 9 Treatment Emergent Adverse
Events* Pharmacokinetics (Plasma & Urine)
10 100 1000 10000 100000 1000000 |
