Attached files
| file | filename |
|---|---|
| EX-10.84 - EXHIBIT 10.84 - Isoray, Inc. | v419853_ex10-84.htm |
| EX-21.1 - EXHIBIT 21.1 - Isoray, Inc. | v419853_ex21-1.htm |
| EX-32 - EXHIBIT 32 - Isoray, Inc. | v419853_ex32.htm |
| EX-10.83 - EXHIBIT 10.83 - Isoray, Inc. | v419853_ex10-83.htm |
| EX-31.2 - EXHIBIT 31.2 - Isoray, Inc. | v419853_ex31-2.htm |
| EX-23.1 - EXHIBIT 23.1 - Isoray, Inc. | v419853_ex23-1.htm |
| EX-31.1 - EXHIBIT 31.1 - Isoray, Inc. | v419853_ex31-1.htm |
United States Securities and Exchange Commission
Washington, d.c. 20549
FORM 10-K
| x | Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the fiscal year ended June 30, 2015
or
| ¨ | Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from __________ to ____________
Commission File No. 001-33407
| IsoRay, Inc |
(Exact name of registrant as specified in its charter)
| Minnesota | 41-1458152 | |
| (State of incorporation) | (I.R.S. Employer Identification No.) | |
| 350 Hills St., Suite 106 | ||
| Richland, Washington | 99354 | |
| (Address of principal executive offices) | (Zip code) |
Registrant's telephone number, including area code: (509) 375-1202
Securities registered pursuant to Section 12(b) of the Exchange Act – Common Stock – $0.001 par value
(NYSE MKT)
Securities registered pursuant to Section 12(g) of the Exchange Act – Series C Preferred Share Purchase Rights
Number of shares outstanding of each of the issuer's classes of common equity:
| Class | Outstanding as of September 11, 2015 | |
| Common stock, $0.001 par value | 55,013,553 |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer x | Non-accelerated filer ¨ | Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act): Yes ¨ No x
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant's most recently completed second fiscal quarter – $79,783,105 as of December 31, 2014.
Documents incorporated by reference – none.
ISORAY, INC.
Table of Contents
Caution Regarding Forward-Looking Information
In addition to historical information, this Form 10-K contains certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA). This statement is included for the express purpose of availing IsoRay, Inc. of the protections of the safe harbor provisions of the PSLRA.
All statements contained in this Form 10-K, other than statements of historical facts, that address future activities, events or developments are forward-looking statements, including, but not limited to, statements containing the words "believe," "expect," "anticipate," "intends," "estimate," "forecast," "project," and similar expressions. All statements other than statements of historical fact are statements that could be deemed forward-looking statements, including any statements of the plans, strategies and objectives of management for future operations; any statements concerning proposed new products, services, developments or industry rankings; any statements regarding future revenue, economic conditions or performance; any statements of belief; and any statements of assumptions underlying any of the foregoing. These statements are based on certain assumptions and analyses made by us in light of our experience and our assessment of historical trends, current conditions and expected future developments as well as other factors we believe are appropriate under the circumstances. However, whether actual results will conform to the expectations and predictions of management is subject to a number of risks and uncertainties described under Item 1A – Risk Factors beginning on page 23 below that may cause actual results to differ materially.
Consequently, all of the forward-looking statements made in this Form 10-K are qualified by these cautionary statements and there can be no assurance that the actual results anticipated by management will be realized or, even if substantially realized, that they will have the expected consequences to or effects on our business operations. Readers are cautioned not to place undue reliance on such forward-looking statements as they speak only of the Company's views as of the date the statement was made. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
PART I
As used in this Form 10-K, unless the context requires otherwise, "we" or "us" or the "Company" means IsoRay, Inc. and its subsidiaries.
General
Century Park Pictures Corporation (Century) was organized under Minnesota law in 1983. Century had no operations since its fiscal year ended September 30, 1999 through June 30, 2005.
On July 28, 2005, IsoRay Medical, Inc. (Medical) became a wholly-owned subsidiary of Century pursuant to a merger. Century changed its name to IsoRay, Inc. (IsoRay or the Company). In the merger, the Medical stockholders received approximately 82% of the then outstanding securities of the Company.
Medical, a Delaware corporation, develops, manufactures and sells isotope-based medical products and devices for the treatment of cancer and other malignant diseases. Medical is headquartered in Richland, Washington.
Medical was formed under Delaware law on June 15, 2004 and merged with IsoRay Products LLC and IsoRay, Inc., each formed under Washington law, on October 1, 2004. The first IsoRay entity was originally organized in 1998 as a Washington limited liability company, IsoRay, LLC, to develop a medical device using the Cesium-131 seed technology and later transferred its operations to IsoRay, Inc. a Washington corporation on May 1, 2002. IsoRay Products LLC was formed in September 2003 to raise capital to fund the operations of IsoRay, Inc. Both IsoRay, Inc. and IsoRay Products LLC merged with Medical on October 1, 2004.
IsoRay International LLC (International), a Washington limited liability company, was formed on November 27, 2007 and is a wholly-owned subsidiary of the Company. International has entered into various international distribution agreements.
| 1 |
Available Information
Our Internet website address is www.IsoRay.com. Information on this website is not a part of this Report. We make our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, Forms 3, 4, and 5 filed on behalf of directors and executive officers, and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, or the Exchange Act, available free of charge on our website as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities and Exchange Commission, or the SEC. You can also read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, NE, Washington, DC 20549. You can obtain additional information about the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet site (www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, including us.
Information regarding our corporate governance, including the charters of our audit committee, our nominations and corporate governance committee and our compensation committee, and our Code of Conduct and Ethics, is available on our Internet site (www.IsoRay.com). We will provide any of the foregoing information without charge upon request to Brien Ragle, 350 Hills Street, Suite 106, Richland, WA, 99354.
Business Operations
Overview
In 2003, IsoRay obtained clearance from the FDA for treatment for all solid tumor applications using Cesium-131. As of the date of this report, such applications include prostate cancer; ocular melanoma; head, neck and lung tumors; breast cancer; liver cancer; brain cancer; colorectal cancer; gynecological cancer; esophageal cancer; and pancreatic cancer. The brachytherapy seed form of Cesium-131 may be used in surface, interstitial and intracavity applications for tumors with known radio sensitivity. Management believes its Cs-131 technology will allow it to become a leader in the brachytherapy market. Management believes that the IsoRay Proxcelan® Cesium-131 brachytherapy seed represents the first major advancement in brachytherapy technology in approximately 30 years with attributes that could make it the long-term "seed of choice" for internal radiation therapy procedures.
Brachytherapy seeds are small devices used in an interstitial radiation procedure. The procedure has become one of the primary treatments for prostate cancer. The brachytherapy procedure places radioactive seeds as close as possible to (in or near) the cancerous tumor (the word "brachytherapy" means close therapy). The seeds deliver therapeutic radiation thereby killing the cancerous tumor cells while minimizing exposure to adjacent healthy tissue. This procedure allows doctors to administer a higher dose of radiation directly to the tumor. Each seed contains a radioisotope sealed within a titanium capsule. When brachytherapy is the only treatment (monotherapy) used in the prostate, approximately 70 to 120 seeds are permanently implanted in the prostate in an outpatient procedure. The number of seeds used varies based on the size of the prostate and the activity level specified by the physician. When brachytherapy is combined with external beam radiation or intensity modulated radiation therapy (dual therapy), then approximately 40 to 80 seeds are used in the procedure. The isotope decays over time and eventually the seeds become inert. The seeds may be used as a primary treatment or as an adjunct therapy with other treatment modalities, such as chemotherapy, or as treatment for residual disease after excision of primary tumors. The number of seeds for other treatment sites commonly vary from as few as 8 seeds to more than 100 seeds depending on the type of cancer, the location of the tumor being treated and the type of therapy being utilized.
IsoRay began production and sales of Proxcelan® Cesium-131 brachytherapy seeds in October 2004 for the treatment of prostate cancer after clearance of its premarket notification (510(k)) by the Food and Drug Administration (FDA). In December 2007, IsoRay began selling its Proxcelan® Cs-131 seeds for the treatment of ocular melanoma, however, the market for the treatment has been limited generating a minimal amount of revenue for the Company. The Company continues to make the treatment available to interested physicians and medical facilities. In June 2009, the Company began selling its Proxcelan® Cs-131 seeds for treatment of head and neck tumors, commencing with treatment of a tumor that could not be accessed by other treatment modalities. The Company obtained clearance in August 2009 from the FDA to permit loading Cesium-131 into bioabsorbable braided sutures which are commonly referred to in the industry as braided strands, facilitating treatment of brain, lung, and head and neck tumors as well as tumors in other organs with Proxcelan® Cs-131. During the fiscal year ended June 30, 2010, the Company expanded the number of areas of the body in which the Proxcelan® Cs-131 seeds were being utilized for treatment by adding lung cancer in August 2009, colorectal cancer in October 2009, and chest wall cancer in December 2009. During the fiscal year ended June 30, 2011, the Company continued the expansion in the number of areas of the body in which the Proxcelan® Cs-131 seeds were being utilized through the addition of the treatment of brain cancer in September 2010 and the treatment of gynecological cancer in December 2010.
| 2 |
In March 2011, the Company received clearance to commercially deliver Proxcelan® Cesium-131 brachytherapy seeds that are preloaded into bioabsorbable braided sutures into Europe. This clearance permits the product to be commercially distributed for treatment of brain, lung, and head and neck tumors as well as tumors in other organs in Europe.
In August 2011, Medical received clearance from the FDA for its premarket notification (510(k)) for the GliaSite® Radiation Therapy System (GliaSite® RTS). The GliaSite® RTS is the only FDA-cleared balloon catheter device used in the treatment of brain cancer.
In May 2012, Medical received a CE mark for the GliaSite® RTS which states that the Company conforms with the product requirements of the European Council Directive 93/42/EEC. The CE mark allows the GliaSite® RTS to be sold in 31 European countries and to be marketed in the European Free Trade Associate member states and the European Union. In June 2012, the first Cesium-131 brachytherapy seed sutured mesh was implanted on a patient suffering from a recurring meningioma tumor.
Management focused in fiscal 2012 and 2013 on obtaining its regulatory clearances and final research and development of its GliaSite® RTS, entering into international distribution agreements to sell the product in Europe and Australia, and marketing its brain and lung products. The GliaSite® RTS is the world’s only system that enables doctors to use liquid radiation in areas where the cancer is most likely to remain after brain surgery and tumor removal. In fiscal 2013, the Company began supporting the use of a system developed at the Barrow Neurological Institute to deliver doses of Cesium-131 to treat malignant meningioma, brain metastases, and primary cancers of the brain. A multi-institutional study was conducted to explore the use of braided sutures containing Proxcelan® Cs-131 seeds placed directly into the cavity following surgical resection of brain metastases.
In August 2013, Medical received an approval for an extension to the scope of the CE mark for the GliaSite® RTS. This approval allows Medical to implement certain product improvements that management believes will enhance GliaSite® RTS’s acceptance by customers in the European market.
In December 2013, the Company received clearance for Cesitrex™ from the US Food and Drug Administration. Cesitrex™ is the liquid form of Cesium-131 and can be used in place of Iotrex®, the liquid form of Iodine-125, in the Company’s GliaSite® RTS. In May 2014, the Company received clearance for Cesitrex™ from the Washington Department of Health. In June 2014, the Company delivered its first order of Cesitrex™ for use in treating a patient.
In October 2014, IsoRay announced early success for a young Peruvian girl utilizing Cesium-131 in the first stereotactic implant for inoperable brain cancer. This 7 year old girl is back in school as of August 2015 with no restrictions and normal activities. In December 2014, Barrow Neurological Institute released its findings that IsoRay’s Cesium-131 therapy stops brain cancers from recurring in treated locations where previous conventional treatments failed.
Also, in December 2014, Cesium-131 was used in the world’s first veterinary implant in a horse with cancer.
In January 2015, the first Cesium-131 prostate cancer treatment was performed in Russia at the Neftyanik Hospital, a center providing cutting edge cancer treatments. In May 2015, the five-year 96% success in local control and 100% survival rates for lung cancer treatment using Cesium-131 were released in a peer-reviewed study.
| 3 |
In June 2015, Cesium-131 was selected by Chicago Prostate Cancer Center for use in the launch of a study of focal treatment of prostate cancer.
While management has not identified additional opportunities to expand treatment to other sites in the body, we continue to investigate potential new opportunities with interested physicians and medical facilities. Management is now focusing primarily on the brain and gynecological markets while the Company continues to research delivery systems other than those historically used by the Company.
Industry Information
Prostate Cancer Treatment
According to the American Cancer Society, approximately one man in seven will be diagnosed with prostate cancer during his lifetime and one man in thirty-eight will die of prostate cancer. It is the most common form of cancer in men after skin cancer, and the second leading cause of cancer deaths in men following lung cancer. The American Cancer Society estimates there will be about 220,800 new cases of prostate cancer diagnosed and an estimated 27,540 deaths associated with the disease in the United States in 2015. (American Cancer Society, 2015)
Prostate cancer treatment remains a key focus of the Company. Most doctors use the American Joint Committee on Cancer (AJCC) TNM system to stage prostate cancer. This system is based on five key pieces of information.
| § | The extent of the main tumor (T category) |
| § | Whether the cancer has spread to nearby lymph nodes (N category) |
| § | Whether the cancer has metastasized (spread) to other parts of the body (M category) |
| § | The PSA level at the time of diagnosis |
| § | The Gleason score, based on the prostate biopsy or surgery |
These factors are combined to determine an overall stage, using Roman numerals I through IV (1-4). The lower the number, the less the cancer has spread. A higher number, such as stage IV (4), means a more advanced cancer.
Once diagnosed, prostate cancer can generally be divided into one of the three “risk groups”: low, intermediate and high risk. As risk increases so does the probability of advanced cancer at diagnosis and the probability of failing treatment with cancer progression or recurrence.
IsoRay’s Proxcelan® Cesium-131 sources are an option in the treatment of prostate cancers of all risk levels, but like most other prostate cancer treatments are most successful in the more prevalent low risk category. The diagnosis of prostate cancer – and especially low risk prostate cancer – has been potentially reduced with the introduction of guidelines dissuading the use of serum PSA screening at the general practitioner level as a means to detect prostate cancer early in men with no symptoms of prostate cancer. Effective July 2012, the U.S Preventative Services Task Force (USPSTF) recommends against the use of the PSA test.
Furthermore, the deferral of cancer-eradicating (definitive) prostate cancer treatments such as surgery and radiation therapy has become more popular as some men with prostate cancer have decided to “watch” the cancer using a variety of diagnostic tools – a trend known as “active surveillance”.
As such, the industry has experienced an overall decrease in the number of low risk cases of prostate cancer diagnosed due to reduced PSA screening, as well as a larger number of men who are deferring treatment altogether at a higher rate than seen historically. Intense competition in the space due to numerous established treatment options along with recently added entrants has further eroded existing market share.
Still, minimally invasive brachytherapy such as that provided by Company’s Proxcelan® Cesium-131 brachytherapy products provides significant advantages over competing treatments including lower cost, equal or better survival data, fewer side effects, faster recovery time and the convenience of a single outpatient implant procedure that generally lasts less than one hour (Grimm, et al., British Journal of Urology International, Vol. 109 (Suppl 1), 2012; Merrick, et al., Techniques in Urology, Vol. 7, 2001; Potters, et al., Journal of Urology, May 2005; Sharkey, et al., Current Urology Reports, 2002).
| 4 |
In addition to permanent, low-dose rate (LDR) brachytherapy, such as Proxcelan®, localized prostate cancer can be treated with prostatectomy surgery (RP for radical prostatectomy), external beam radiation therapy (EBRT), three-dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT), dual or combination therapy, permanent, high dose rate brachytherapy (HDR), cryosurgery, hormone therapy, and watchful waiting. The success of any treatment is measured by the feasibility of the procedure for the patient, morbidities associated with the treatment, overall survival, and cost. When the cancerous tissue is not completely eliminated, the cancer typically returns to the primary site, often with metastases to other areas of the body.
The National Cancer Data Base (NCDB) contains a total of 1,547,941 patients with localized prostate cancer that were identified from 1998 to 2010. Overall, 13.4% of patients were treated with brachytherapy, with an additional 2.6% treated with brachytherapy boost, which is the addition of a brachytherapy implant in addition to external beam radiation therapy, compared with 49.8% treated with surgery, 26.3% with non-brachytherapy radiotherapy, 24.1% who received hormone therapy, and 7.8% who received no treatment. (Martin JM, Handorf EA, Kutikov A, et al. (2014) The rise and fall of prostate brachytherapy: Use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 120:2114–2121)
Prostatectomy Surgery Options. In the radical prostatectomy operation, a surgeon will remove the entire prostate gland plus some of the tissue around it, including the seminal vesicles. New methods such as laparoscopic and robotic prostatectomy surgeries are currently being used more frequently in order to minimize the nerve damage that leads to impotence and incontinence, but these techniques require a high degree of surgical skill. (American Cancer Society, 2015) Surgical resection accounted for approximately 44% of treatments before the introduction of robotic prostatectomy in the early 2000s and then rose to 60% in 2010. (Martin JM, Handorf EA, Kutikov A, et al. (2014) The rise and fall of prostate brachytherapy: Use of brachytherapy for the treatment of localized prostate cancer in the National Cancer Data Base. Cancer 120:2114–2121)
External Radiation Therapy. Primary External Beam Radiation Therapy (EBRT), Three-dimensional Conformal Radiation Therapy (3D-CRT), Stereotactic Radiotherapy (SBRT) and Intensity Modulated Radiation Therapy (IMRT) all involve directing a beam of radiation from outside the body at the prostate gland to destroy cancerous tissue. Treatments are received on an outpatient basis with the patient usually receiving five treatments per week over a period of seven to nine weeks. The use of EBRT as a whole doubled from 11.6% in 2004 to 24% in 2009. The increase in the number of cases being treated with EBRT during 2004 to 2008 were cases that historically would have been treated with brachytherapy. During that period there was a new complete transition to IMRT as the predominant method with IMRT treatment increasing from 0.15% to 95.9% of EBRT treatments from 2000 to 2008. (Mahmood U, Pugh T, Frank S, et al. (2014) Declining use of brachytherapy for the treatment of prostate cancer. Brachytherapy 13:157–162) Side effects of these treatments can include bowel problems, bladder problems, urinary incontinence, impotence, fatigue, lymphedema, and urethral stricture. (American Cancer Society, 2015)
Dual or Combination Therapy. Dual therapy is the combination of IMRT or 3-dimensional conformal external beam radiation and seed brachytherapy to treat extra-prostatic extensions or high risk prostate cancers that have metastasized or grown outside the prostate. Combination therapy treats high risk patients with a full course of IMRT or EBRT over a period of several weeks. When this initial treatment is completed, the patient must then wait for several more weeks to months to have the prostate seed implant. (American Cancer Society, 2015) Management estimates that at least 25% of all U.S. prostate implants are now dual therapy cases.
High Dose Rate Temporary Brachytherapy (HDR). HDR temporary brachytherapy involves placing very tiny plastic catheters into the prostate gland, and then giving a series of radiation treatments through these catheters. The catheters are then removed, and no radioactive material is left in the prostate gland. A computer-controlled machine inserts a single highly radioactive iridium-192 seed into the catheters one by one. This procedure is typically repeated at least three times while the patient is hospitalized for at least 24 hours. (American Cancer Society, 2015)
Additional Treatments. Additional, less frequently used, treatments include cryotherapy, hormone therapy, vaccine treatment and chemotherapy.
| 5 |
Watchful Waiting and Active Surveillance. Because prostate cancer often grows very slowly, some men (especially those who are older or who have other major health problems) may never need treatment for their cancer. Instead, their doctor may suggest watchful waiting or active surveillance.
Some doctors use these terms to mean the same thing. For other doctors the terms mean something slightly different:
| § | Active surveillance is often used to mean watching the cancer closely with PSA blood tests, digital rectal exams (DREs), and ultrasounds at regular intervals to see if the cancer is growing. Prostate biopsies may be done as well to see if the cancer is starting to grow faster. If there is a change in a patient’s test results, the doctor would then talk to the patient about treatment options. |
| § | Watchful waiting (observation) is sometimes used to describe a less intense type of follow-up that may mean fewer tests and relying more on changes in a man’s symptoms to decide if treatment is needed. |
If the cancer seems to be growing or getting worse, the doctor may suggest starting treatment. Some early studies have shown that among men who choose active surveillance, those who elect not to be treated do as well as those who decide to start treatment right away. (American Cancer Society, 2015)
Low Dose Rate Permanent Brachytherapy (LDR). LDR permanent brachytherapy involves placing pellets or seeds of radioactive material directly in the prostate. The pellets/seeds are left in place and emit low dose rate radiation for weeks or months. The pellets/seeds can deliver a large dose of radiation to a small area of the body thereby reducing the damage done to healthy tissue that is close to the prostate. (American Cancer Society, 2015)
Iodine-125 (I-125) and Palladium-103 (Pd-103) are two isotopes, other than Cesium-131, that are currently used for LDR permanent brachytherapy. A number of published studies describing the use of I-125 and Pd-103 brachytherapy in the treatment of early-stage prostate cancer have been very positive when compared to other treatment options. A study of 2,963 prostate cancer patients who underwent brachytherapy as their sole therapeutic modality at 11 institutions across the U.S. concluded that low-risk patients (who make up the majority of localized cases) who underwent adequate implants experienced rates of PSA relapse survival of greater than 90% between eight and ten years (Zelefsky MJ, et al, "Multi-institutional analysis of long-term outcome for stages T1-T2 prostate cancer treated with permanent seed implantation" International Journal of Radiation Oncology Biology Physics, Volume 67, Issue 2, 2007, 327-333).
Other studies have demonstrated similar, durably high rates of control following brachytherapy for localized prostate cancer out to 15 years post-treatment (Sylvester J, et al. "15-year biochemical relapse free survival in clinical stage T1-T3 prostate cancer following combined external beam radiotherapy and brachytherapy; Seattle experience", International Journal of Radiation Oncology Biology Physics, Vol. 67, Issue 1, 2007, 57-64). The cumulative effect of these studies has been the conclusion by leaders in the field that brachytherapy offers a disease control rate as high as surgery, though with a lesser side-effect profile than surgery (Ciezki JP. "Prostate brachytherapy for localized prostate cancer" Current Treatment Options in Oncology, Volume 6, 2005, 389-393).
Long-term survival data is now available for brachytherapy with I-125 and Pd-103, which support the efficacy of brachytherapy in the treatment of clinically localized cancer of the prostate gland. Clinical data indicate that brachytherapy offers success rates for early-stage prostate cancer treatment that are equal to or better than those of RP or EBRT. While historically clinical studies of brachytherapy have focused primarily on results from brachytherapy with I-125 and Pd-103, management believes that these data are also relevant for brachytherapy with Cesium-131. In fact, it appears that Cesium-131 offers improved clinical outcomes over I-125 and Pd-103, perhaps due to its shorter half-life.
Sexual impotence and urinary incontinence are two major concerns men face when choosing among various forms of treatment for prostate cancer. Studies have shown that brachytherapy with existing sources results in lower rates of impotence and incontinence than surgery (Buron C, et al. "Brachytherapy versus prostatectomy in localized prostate cancer: results of a French multicenter prospective medico-economic study". International Journal of Radiation Oncology, Biology, Physics, Volume 67, 2007, 812-822). Combined with the high disease control rates described in many studies, these findings have driven the adoption of brachytherapy as a front-line therapy for localized prostate cancer.
| 6 |
Comparing Cesium-131 to I-125 and Pd-103 Clinical Results
The Company’s Proxcelan® Cesium-131 - based permanent brachytherapy treatment was introduced in 2004, as compared to the other permanent brachytherapy sources - Iodine-125 (introduced 1965) and Palladium-103 (introduced 1986). Thus, it has only been recently that the achievement of significant follow-up in patient studies has occurred for the Company’s Cesium-131 product (introduced 2004).
Management believes that the Proxcelan® Cesium-131 brachytherapy seed has specific clinical advantages for treating cancer over I-125 and Pd-103, the other isotopes currently used in brachytherapy seeds. The table below highlights the key differences of the three seeds. The Company believes that the short half-life, high-energy characteristics of Cesium-131 will increase industry growth and facilitate meaningful penetration into the treatment of other forms of cancer such as lung cancer.
| Isotope Delivery Over Time | ||||
| Isotope | Half-Life | Energy | 90% Dose | Total Dose |
| Cs-131 | 9.7 days | 30.4 KeV | 33 days | 115 Gy |
| Pd-103 | 17 days | 20.8 KeV | 58 days | 125 Gy |
| I-125 | 60 days | 28.5 KeV | 204 days | 145 Gy |
As stated earlier, Company management believes that the long-term results already reported for Iodine-125 and Palladium-103 based prostate brachytherapy confirm the validity of permanent prostate brachytherapy, and at least comparable long-term outcomes are likely with Cesium-131 treatment. A recent clinical report supports this contention (Benoit RM, et al. “Five year prostate-specific antigen outcomes after caesium prostate brachytherapy. Clinical Oncology, Volume 26, 2014, 776-780).
However, management also believes that Cesium-131 will ultimately prove to possess clinical advantages over the two other permanently implantable isotopes. These advantages include better performance in elevated risk cases (especially intermediate risk localized prostate cancers) and a more rapid resolution of side effects. Both advantages are related to the shorter half-life of Cesium-131 as compared to the other two isotopes.
The most recent clinical data was presented at the annual meeting of the American Brachytherapy Society in April 2014. Dr. Brian Moran of the Chicago Prostate Center reported a 92.6% rate of success at five years after treatment for 69 patients with prostate cancer following treatment with Cesium-131 brachytherapy (Moran BJ, Braccioforte MH. PSA Outcomes in a Single Institution, Prospective Randomized 131Cs/125I Prostate Brachytherapy Trial. (Brachytherapy 2014 13(S1)S34). At the same meeting, Dr. Rajagopalan of the University of Pittsburgh Medical Center reported a six year success rate of 95.4% in 243 Cesium-131 treated patients (Six-year biochemical outcome in patients treated with Cs-131 brachytherapy as monotherapy for prostate cancer. Brachytherapy 2014 13(S1)S38).
When taken together with the multi-institutional 5 year outcome presentation by Prestidge and others, where a group of 100 patients from multiple institutions exhibited a PSA disease-free rate of 98% at five years (Prestidge B. et al. Five-year biochemical control following Cesium-131 Permanent Prostate Brachytherapy in a Multi-Institutional Trial. Brachytherapy 2011 10(3S1)S27.), a strong case for an outstanding rate of durable PSA (biochemical) success can be made.
Furthermore, in all three reports a significant proportion of “intermediate risk” patients (who are at greater risk of failure following any treatment compared to most prostate cancer patients) were included in the studies. Despite this added risk – 37% of patients across all three studies were intermediate risk — the three studies together average a 95% rate of success at five years and beyond for a total of 412 patients under study.
| 7 |
Improved side-effect profile.
In addition to the cancer-related outcomes described for prostate brachytherapy, a significant proportion of patients who undergo I-125 or Pd-103 brachytherapy experience acute urinary irritative symptoms following treatment – more so than with surgery or external beam radiation therapy (Frank SJ, et al, "An assessment of quality of life following radical prostatectomy, high dose external beam radiation therapy, and brachytherapy iodine implantation as monotherapies for localized prostate cancer" Journal of Urology, Volume 177, 2007, 2151-2156). These irritative symptoms can range from an increased frequency of urination to significant pain upon urination. Because the portion of the urethra that runs through the prostate takes high doses from the implant, these side effects are fairly common following prostate brachytherapy.
Recent completed studies show that Cesium-131, with the shortest available half-life of the commonly used implantable isotopes, results in a quicker resolution of these irritative symptoms based on the shorter time interval over which normal tissue receives radiation from the implanted sources than for longer lived isotopes such as I-125. (Shah H, et al. A comparison of AUA symptom scores following permanent low-dose-rate prostate brachytherapy with iodine-125 and cesium-131. Brachytherapy 2013:12(SI)S64)).
A Cesium-131 monotherapy trial for the treatment of prostate cancer was fully enrolled in February 2007. The trial was a 100 patient multi-institutional study that sought to (1) document the dosimetric characteristics of Cesium-131, (2) summarize the side effect profile of Cesium-131 treatment, and (3) track biochemical (PSA) results in patients following Cesium-131 therapy. Some of the significant and specific findings were as follows:
1. Patient reported irritative urinary symptoms (IPSS Scores) were mild to moderate with relatively rapid resolution within 4-6 months. (Prestidge BR, Bice WS, "Clinical outcomes of a Phase II, multi-institutional Cesium-131 permanent prostate brachytherapy trial". Brachytherapy, Volume 6, Issue 2, April-June 2007, Page 78).
2. Gland coverage was excellent and the dose delivered to critical structures outside the prostate was well within acceptable limits. (Bice WS, Prestidge BR, "Cesium-131 permanent prostate brachytherapy: The dosimetric analysis of a multi-institutional Phase II trial". Brachytherapy 2007(6); 88-89.).
3. An abstract detailing the outcomes of the 100 patient multi-institutional Cesium-131 study was prepared for the 32nd Annual Meeting of the American Brachytherapy Society (April 2011), Notably, the PSA control rate at 5 years was reported as 98%. No other study of brachytherapy utilizing the competing isotopes Iodine-125 and Palladium-103 has reported five year rates as high as 98%.
The resolution of urinary side effects advantage of Company’s Proxcelan Cesium-131 product as pictured in the graphic below has been observed in a second study, presented at the 2013 Annual Meeting of the American Brachytherapy Society (Shah AB, Shah AA, Fortier GA. A comparison of AUA symptom scores following permanent low dose rate prostate brachytherapy with iodine-125 and cesium-131. Brachytherapy 2013 12(Suppl. 1)S64).
| 8 |
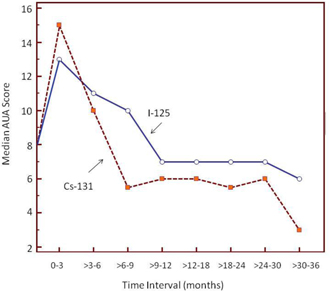
As seen in the plot of these AUA scores, the duration of an elevated side effect (AUA) score profile resolved to pre-treatment levels more quickly with the Cesium-131 group than with the Iodine-125 group. All patients were treated at the same institution by the same physicians, and the difference in the time to resolution was considered significant.
Non-Prostate Product Offerings
Brain Cancer Treatment Options
An estimated 22,850 new cases of malignant primary tumors of the brain or spinal cord are expected in 2015. About 15,320 people are expected to die from brain and spinal cord tumors in 2015 and overall a person has a less than 1% chance of developing a malignant tumor. The chance that a man will develop a malignant tumor of the brain or spinal cord is about 1 in 140 and for a woman is 1 in 180. These numbers would be much higher if benign tumors were also included. (American Cancer Society, 2015).
Most brain and spinal cord tumors are difficult to treat and require several specialists. The most common forms of treatment are resection at surgery (craniotomy); radiation therapy which may include external beam radiation therapy (EBRT), three-dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT), conformal proton beam radiation therapy, stereotactic radiosurgery, and brachytherapy; chemotherapy; targeted therapy; other types of drugs (including corticosteroids and anti-seizure drugs); or a combination of therapies. (American Cancer Society, 2015)
The treatment of brain cancer with Cesium-131 now has several delivery methods, including the use of bioabsorbable mesh to apply the Proxcelan® Cesium-131 brachytherapy seeds which generally dissolves after about 45 days. Cesium-131 delivers 90% of its dose in 33 days and is therefore well-suited to use with bioabsorbable mesh, single seed applications, implantable strands, and by implantable device, including the GliaSite® RTS (which now uses Iotrex®, a form of liquid Iodine 125, and Cesitrex™, a form of liquid Cesium-131), the world’s only liquid radiation balloon catheter device used in the treatment of brain cancer. During the year ended June 30, 2015, there were sixty-six patients treated with Company products for brain cancer.
Lung Cancer Treatment Options
An estimated 221,200 new cases of lung cancer are expected in 2015, accounting for 13% of all cancer diagnoses in the United States. Approximately 27% of all cancer deaths are from lung cancer and it accounts for the most cancer related deaths in both men and women in the United States. An estimated 158,040 deaths will result from lung cancer in 2015. Approximately 2 of 3 people diagnosed with lung cancer will be older than 65 and fewer than 2% will be younger than 45 years old. Overall, the chance of developing lung cancer is 1 in 13 for a woman and 1 in 16 for a man (combined for both smokers and non-smokers). Naturally, the risk for smokers is much higher and for non-smokers the risk is lower. (American Cancer Society 2015)
| 9 |
Lung cancer has historically been treated utilizing surgery, radiofrequency ablation (RFA), radiation therapy, other local treatments, chemotherapy and targeted therapy including LDR brachytherapy. More than one kind of treatment may be used, depending on the stage of the patient's cancer and other factors. (American Cancer Society, 2015)
The Company believes that Cesium-131, with its shorter half-life (faster rate of decay) and relatively high energy, is better suited for treating lung cancer in Stages I and II than I-125. The bioabsorbable mesh used in this procedure to apply the Proxcelan® Cesium-131 brachytherapy seeds generally dissolves after about 45 days. Cesium-131 delivers 90% of its dose in 33 days and is therefore well-suited to use with bioabsorbable mesh. A report was published in November 2011 describing the more technical details applicable to Cesium-131 implants (Parashar B, et al. Cesium-131 Permanent Seed Brachytherapy: Dosimetric Evaluation and Radiation Exposure to Surgeons, Radiation Oncology, and Staff. Brachytherapy 10(6):508-513, 2011).
In April 2012, the Company initiated a 100 patient study of Cesium-131 brachytherapy in the treatment of early stage non-small cell lung cancer (NSCLC). In this study, patients who are poor candidates for large surgical resections undergo a limited (sub-lobar) resection followed by Cesium-131 mesh brachytherapy. This study is based upon strong evidence collected to date suggesting that Iodine-125 mesh implants utilized in a similar way assist the limited surgical resection in achieving high rates of local cancer control. (see Colonias, et al. Mature Follow-up for High Risk Stage I Non-Small Cell Lung Carcinoma Treated with Sub-lobar Resection and Intra-operative Iodine-125 Brachytherapy. International Journal of Radiation Oncology Biology Physics 2011, 79(1), 105.) As of June 30, 2015, eighty-nine patients were enrolled in the study. During the year ended June 30, 2015, there were eighteen patients treated with Company products for lung cancer.
Head and Neck Cancer Treatment Options
An estimated 56,480 new cases of head and neck cancer are expected to be diagnosed in the United States in 2015. (American Cancer Society, 2015)
Surgery is the most common option to treat head and neck cancers. Chemotherapy is often used in conjunction with surgery or radiation therapy depending on the type and stage of the cancer. External beam radiation therapy and brachytherapy have been used together or in combination with surgery or chemotherapy. (American Cancer Society, 2015)
Management believes Proxcelan® Cesium-131 continues to represent an improved approach to brachytherapy treatment of specific head and neck cancers. During the year ended June 30, 2015, there were seven patients that were treated with Company products for head and neck cancers.
Gynecological Cancer Treatment Options (Vaginal and Vulvar Cancer)
An estimated 22,120 new cases of cervical (12,900), vaginal (4,070) and vulvar (5,150) cancers are expected to be diagnosed in the United States in 2015. (American Cancer Society, 2015). In addition to brachytherapy to treat gynecological cancers such as cervical, vaginal and vulvar cancers, other treatment options include surgery, laser surgery, radiation therapy, chemotherapy, and topical treatments. (American Cancer Society, 2015)
During the year ended June 30, 2015, there were eight patients treated with Company products for gynecological cancers.
Colorectal Treatment Options
An estimated 132,700 new cases of colorectal cancer are expected in the United States in 2015 (American Cancer Society, 2015). Colorectal cancer has historically been treated using surgery, radiation therapy, chemotherapy, immunotherapy and other targeted therapies. (American Cancer Society, 2015)
| 10 |
For the treatment of early stage colon and rectal cancers, surgery is often the main treatment. For the treatment of colorectal cancers beyond early stage, other surgery treatments, radiation therapy, chemotherapy, and targeted therapies can be used. (American Cancer Society, 2015)
Low-dose rate (LDR) brachytherapy, including Proxcelan® Cesium-131, is typically utilized in treating individuals with rectal cancer who are not healthy enough to tolerate curative surgery. This is generally a one-time only procedure and does not require ongoing visits as is common with other types of radiation therapy. Management believes that the advantages provided by Cesium-131 shown through the treatment of other cancers will benefit patients utilizing Proxcelan® Cesium-131 brachytherapy seeds in the treatment of their colorectal cancers with low-dose rate brachytherapy. The treatment of colorectal cancer is an additional non-prostate application of the Company’s product which by itself is not a significant portion of the Company’s business. However, when aggregated with the other non-prostate applications, it contributes to the overall growth in the Company’s non-prostate applications.
Ocular Melanoma Treatment Options
The American Cancer Society estimates that 2,580 new cases of cancers of the eye and orbit (primarily melanoma) will be diagnosed in 2015 (American Cancer Society, 2015). In addition to brachytherapy to treat ocular melanoma, other treatment options include surgery, external beam radiation, chemotherapy, and laser therapy.
Brachytherapy has become the most commonly used radiation treatment for most eye melanomas. Studies have shown that in many cases it is as effective as surgery (enucleation). Brachytherapy using Cesium-131, I-125, or Pd-103 is done by placing the seeds in a plaque (shaped like a small cap) that is attached to the eyeball with minute stitches in a procedure that lasts 1 to 2 hours and is usually kept in place for 4 to 7 days. The patient generally stays in the hospital until the plaque is removed from the eye during a procedure that takes less than 1 hour. Brachytherapy cures approximately 9 out of 10 small tumors and can preserve the vision of some patients. (American Cancer Society, 2014) Management believes that while Cesium-131 provides the best treatment alternative, it is at a disadvantage to I-125 or Pd-103 as a result of Cs-131's short half-life, which requires it to be ordered and manufactured for each procedure and unable to be inventoried. Most patients are unwilling to wait for it to be ordered when the other products are often available immediately. The treatment of ocular melanoma was the first opportunity for the Company to utilize the Cs-131 brachytherapy seed in a treatment other than a prostate application but does not comprise a significant portion of the Company’s business.
Financial Information About Segments
The Company has determined that it operates in only one segment, as it only reports profit and loss information on an aggregate basis to its chief operating decision maker.
Financial Information About Geographic Areas
All of the long-lived assets are located in the United States. Revenue by geographic region is based on the shipping addresses of the Company's customers. The following summarizes revenue by geographic region:
| For the year ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| United States | 99.57 | % | 96.88 | % | 98.20 | % | ||||||
| Europe | 0.13 | % | 3.06 | % | 1.80 | % | ||||||
| Russia | 0.14 | % | 0.00 | % | 0.00 | % | ||||||
| South America | 0.16 | % | 0.07 | % | 0.00 | % | ||||||
| Total | 100.00 | % | 100.00 | % | 100.00 | % | ||||||
Our Strategy
The key elements of IsoRay's strategy for fiscal year 2016 include:
Continue to introduce the Proxcelan® Cesium-131 brachytherapy seed into the U.S. market for prostate cancer. Prostate cancer treatment represents the original and core business for the Company's Proxcelan® Cesium-131 product. With five year data relating to biochemical (PSA) control of prostate cancer now presented to the prostate cancer field, IsoRay intends to continue to seek to increase the number of centers using Proxcelan® through its direct sales force and through its international distributors. Because intermediate- to long-term follow-up data is required to convince clinicians and patients to consider any particular therapy for localized prostate cancer, the availability of five-year data with Proxcelan® in the treatment of prostate cancer represents a significant milestone. IsoRay hopes to capture much of the incremental market growth if and when seed implant brachytherapy recovers market share from other treatments, take market share from existing competitors, and expand the use of Cesium-131 as a dual therapy option where it has experienced success.
| 11 |
Improve distribution of the GliaSite® RTS in the United States and European Union (EU). In June of 2010, the Company acquired exclusive worldwide distribution rights to the GliaSite® RTS, the only FDA-cleared balloon catheter device used in the treatment of brain cancer, from Hologic Inc. The Company received a CE Mark in May 2012 allowing distribution in 31 countries. The Company distributes the product using a German distributor to Germany (the location of the first European sale in July 2012) and other European nations. To date, fifteen cases in Europe and thirteen cases in the U.S. have been treated with GliaSite® RTS sold by the Company directly or through a distributor. In fiscal 2014, the Company entered into an international distribution agreement with an independent distributor in Russia. Additionally in fiscal year 2014, the Company announced that Greek governmental approval was obtained for its entire product line. The Greek distributorship was terminated in 2015 when sales failed to materialize. The Company plans to contact previous users of the product and leverage significant existing clinical data related to the safety and effectiveness of the GliaSite® RTS in order to restore the GliaSite® RTS as a strong treatment option for patients suffering from primary and metastatic brain cancers.
Increase utilization of Cesium-131 in treatment of other solid tumor applications such as lung, brain, head and neck, and colorectal cancers. IsoRay Medical has clearance from the FDA for its premarket notification (510(k)) for Proxcelan® brachytherapy seeds that are preloaded into bioabsorbable braided sutures and bioabsorbable braided sutures attached to bioabsorbable mesh. This FDA clearance allowed commercial distribution for treatment of lung and head and neck tumors as well as tumors in other organs. IsoRay has successfully launched an initiative to market its Proxcelan® source in bioabsorbable carrier material as a lung cancer treatment. It has begun selling its lung cancer treatment product but has not been in the market long enough to determine long-term success of the product. The Company continues to sell product to physicians treating lung cancer while continuing to compile treatment outcomes for publication. IsoRay will continue to explore licenses or joint ventures with other companies to develop the appropriate technologies and therapeutic delivery systems for treatment of other solid tumors.
Early clinical data support management’s initiatives into brain cancers and early stage non-small cell lung cancers. Local control – defined as success in preventing the re-growth of cancer in the immediate vicinity of the treatment area – has been excellent to date.
Support clinical research and sustained product development. The publication and presentation of speculative and real-world data contribute to the acceptability of Cesium-131 in the oncologic marketplace, and discussion in the medico-scientific community of established and novel Cesium-131 applications is considered a prerequisite to expansion into untapped markets. The Company structures and supports clinical studies on the therapeutic benefits of Cesium-131 for the treatment of solid tumors and other patient benefits. We are and will continue to support clinical studies with several leading radiation oncologists to clinically document patient outcomes, provide support for our product claims, and compare the performance of our seeds to competing seeds. IsoRay plans to sustain long-term growth by implementing research and development programs with leading medical institutions in the U.S. and other countries to identify and develop other applications for IsoRay's core radioisotope technology. The Company has deployed a secure, regulatory environment compliant, online information system capable of large usable databases to participating investigators.
Over fiscal year 2015, four presentations were accepted by and presented at the annual meeting of the American Brachytherapy Society describing Cesium-131 treatment of prostate and ocular cancers. Five presentations were accepted by and presented at the annual meeting of the American Society for Radiation Oncology (ASTRO). The Company will continue to seek to increase the number of reports made to society meetings and the peer reviewed literature in order to seek to enhance the standing of its products in the scientific community.
Maintain ISO 13485:2003 certification. In August 2008, the Company obtained its initial ISO 13485:2003 certification. This permitted the Company to register its products in Europe in 2008 and in Canada and Russia during fiscal year 2009. The ISO 13485:2003 certification demonstrates that the Company is in compliance with this internationally recognized quality standard and the initial certification was valid for a three year period. In June 2012, the Company received a recertification to ISO 13485:2003 for an additional three year period, which was affirmed through a surveillance audit in June 2013.
| 12 |
In May 2015, IsoRay completed an annual ISO13485:2003 audit from BSI (British Standards Institution) with no nonconformities. The Company is subject to a recertfication audit every three years, two annual maintenance audits and one additional unannounced audit during each three year period for a total of four audits during each three year period. The successful audit confirms the Company’s success in meeting the standards of manufacturing and quality systems required for the Company to continue to market its products in Canada and Europe.
Products
Proxcelan® Cesium-131
IsoRay markets the Proxcelan® Cesium-131 brachytherapy seed for the treatment of prostate cancer; brain cancer; lung cancer; head and neck cancers; gynecological cancer: pelvic/abdominal cancer; colorectal cancer, and ocular melanoma. The Company intends to market Cesium-131 for the treatment of other malignant diseases as opportunities are identified in the future through the use of existing proven technologies that have received FDA-clearance. The strategy of utilizing existing FDA-cleared technologies reduces the time and cost required to develop new applications of Cesium-131 and deliver them to market.
Cesium-131 Manufacturing Process and Suppliers
Product Overview
Cesium-131 is a radioactive isotope that can be produced by the neutron bombardment of Barium-130 (Ba-130). To produce the Proxcelan® seed, the purified Cesium-131 isotope is adsorbed onto a ceramic core containing a gold X-ray marker. This internal core assembly is subsequently inserted into a titanium capsule that is then welded shut and becomes a sealed radioactive source and a biocompatible medical device.
Isotope Suppliers
The Company has identified key reactor facilities in the U.S. and Russia that are capable of meeting the specific requirements of Cesium-131 production. On June 23, 2014, and again on January 12, 2015, Medical entered into a supply contract (the INM Agreement) with The Open Joint Stock Company, Institute of the Nuclear Materials, a Russian company (JSC INM). With the current INM Agreement, Medical can purchase Cesium-131 from the Institute of Nuclear Materials within the quality standards and within the time periods specified, through January 31, 2016.
The Company also receives irradiated barium from the MURR reactor located in the United States. For the fiscal year ended June 30, 2015, approximately eighty-three percent (83%) of our Cesium-131 was supplied by our Russian supplier and approximately seventeen percent (17%) of Cesium-131 was generated by the irradiated barium from MURR. The Company has demonstrated the capability to expand Cesium-131 manufacturing capability at the MURR reactor in a cost effective manner to meet the current needs of the Company, however, the Company intends to continue to obtain Cesium-131 from its foreign supplier to mitigate the risk of reliance on a single source.
In the past, management believed that failure to obtain deliveries of Cesium-131 from its Russian supplier (JSC INM) would have a material adverse effect on seed production. Management now believes that its existing domestic supplier can meet the Company's isotope requirements for the near future and can mitigate the periodic required shutdowns at the foreign facility. The Company also has a stock of enriched barium that could be utilized to meet isotope requirements.
Quality Controls
In July 2008, IsoRay had its baseline inspection by the FDA at its manufacturing and administrative offices in Richland, WA. This inspection was carried out over a five day period during which the investigator performed a complete inspection following Quality Systems Inspection Techniques (QSIT). At the end of the inspection, no report of deviations from Good Manufacturing Practices or list of observations (FDA Form 483) was issued to IsoRay. An additional inspection of IsoRay was conducted by FDA in April 2013. Again the FDA reported no deviations from Good Manufacturing Practices and did not list any observations (FDA Form 483).
| 13 |
In May 2015, IsoRay completed an annual ISO13485:2003 audit from BSI (British Standards Institution) with no nonconformities. The Company is subject to a recertfication audit every three years, two annual maintenance audits and one additional unannounced audit during each three year period for a total of four audits during each three year period. The successful audit confirms the Company’s success in meeting the standards of manufacturing and quality systems required for the Company to continue to market its products in Canada and Europe.
Order Processing
The Company has implemented a just-in-time production process that is responsive to customer input and orders to ensure that individual customers receive a higher level of customer service than received from our competitors who have the luxury of longer lead times due to longer half-life products. Time from order confirmation to completion of product manufacture is reduced to several working days, including receipt of irradiated barium (from the domestic supplier's reactor) or unpurified Cesium-131 (from the international supplier's reactor), separation and purification of Cesium-131, isotope labeling of the core, loading of cores into pre-welded titanium "cans" for final welding, testing, quality assurance and shipping.
It is up to each physician to determine the dosage necessary for implants and acceptable dosages vary among physicians. Many of the physicians order more seeds than necessary to assure themselves that they have a sufficient quantity. Upon receipt of an order, the Company either delivers the seeds from its facility directly to the physician in either loose or preloaded form or sends the order to an independent preloading service that delivers the seeds preloaded into needles or cartridges just prior to implant. If the implant is postponed or rescheduled, the short half-life of the seeds makes them unsuitable for use and therefore they must be re-ordered.
Due to the lead time for obtaining and processing the Cesium-131 isotope and its short half-life, the Company relies on sales forecasts and historical knowledge to estimate the proper inventory levels of isotope needed to fulfill all customer orders. Consequently, some portion of the isotope is lost through decay and is not used in an end product. Management continues to reduce the variances between ordered isotope and isotope deliveries and is continually improving its ordering process efficiencies. The non-prostate applications have resulted in a greater loss of isotope as cancellations are more frequent due to factors beyond the control of the physicians. These cancellations both increase the costs of the Company for seeds and decrease the revenue as these seeds are not sold.
Pre-loading Services
In addition to providing loose seeds to customers, most brachytherapy manufacturers offer their seed product to the end user packaged in various configurations provided in a sterile or non-sterile package depending on the customer's preference. These include:
| § | Pre-loaded needles (loaded typically with three to five seeds and spacers) |
| § | Pre-loaded Mick™ cartridges (fits the Mick™ applicator) |
| § | Strands of seeds (consists of seeds and spacers in a bioabsorbable rigid "carrier sleeve") |
| § | Preloaded strands (strands of seeds loaded into a needle) |
| § | Pre-loaded braided strands (seeds loaded into a flexible bioabsorbable braided suture) |
| § | Pre-loaded braided strands attached to bioabsorbable mesh (creates planar implants out of braided sutures and bioabsorbable mesh) |
In fiscal year 2015, the Company delivered approximately 53% of its Proxcelan® seeds to customers configured in Mick® cartridges, approximately 29% of the Proxcelan® seeds configured in stranded and pre-loaded in a needle form, 9% of the Proxcelan® seeds configured in a braided strand form, 5% of the Proxcelan® seeds sold in a loose configuration and the remaining 4% are configured in either a pre-loaded in a needle or stranded form.
| 14 |
The role of the pre-loading service is to package, assay and certify the contents of the final product configuration shipped to the customer. A commonly used method of providing this service is through independent radiopharmacies. Manufacturers send loose seeds along with the physician's instructions to the radiopharmacy which, in turn, loads needles and/or strands the seeds according to the doctor's instructions. These radiopharmacies then sterilize the product and certify the final packaging prior to shipping directly to the end user.
As of June 30, 2015, IsoRay had two entities that handled radiopharmacy services at the request of certain individual customers that were able to assay, preload, and sterilize loose seeds. Shipping Cs-131 brachytherapy seeds to independent radiopharmacies requires loading the seeds with additional volume of isotope activity than would be required if the seeds were to be preloaded utilizing our in-house loading facility, which causes the Company to incur additional isotope cost to allow for the additional isotope decay created by the additional processing time. The Company pre-loaded 94% and 97% of the Cs-131 brachytherapy seeds that it sold to customers during the fiscal years ended June 30, 2015 and 2014, respectively. The Company anticipates continuing to load a significant majority of its customer orders during fiscal year 2016 unless there is a specific customer requirement for which the Company does not have the loading capability or capacity.
Independent radiopharmacies traditionally provide the final packaging of the product delivered to the end user thereby eliminating the opportunity for reinforcing the "branding" of our seed product. By providing our own repackaging service, we are able to preserve the product branding opportunity, reduce isotope decay loss, control overall product quality and eliminate any concerns related to the handling of our product by a third party prior to receipt by the end user.
In fiscal year 2012, IsoRay obtained a CE mark which allows shipment of seeds loaded into flexible braided strands and flexible strands attached to bioabsorbable mesh into the European Union.
GliaSite® Radiation Therapy System
IsoRay markets the GliaSite® RTS for the treatment of brain cancer, i.e. primary and recurrent gliomas and metastic brain tumors. Specifically, the intended use of GliaSite® RTS is the management of surgically resectable brain tumors where adjuvant radiation therapy of the post-resection tissue bed is indicated. In August 2013, the Company successfully amended its CE mark on the GliaSite® RTS which incorporated five changes. These changes included a change in the sterilization method of the right angle clip; a change in the packaging of the right angle clip; an extension of the GliaSite® RTS catheter tray expiration date to 3 years; the qualification of a second manufacturer of the Iotrex® solution and the extension of the shelf life of Iotrex® from 19 days to 30 days.
Product Overview
GliaSite® RTS is the only FDA cleared balloon catheter device used in the treatment of brain cancer. The main components included in the GliaSite® RTS are the GliaSite® Catheter Tray, GliaSite® Access Tray, Iotrex® Solidifier and either Iotrex® or Cesitrex™ as the radiotherapy solution. The catheter tray includes a GliaSite® RTS catheter, two non-coring needles, and two right anchoring clips. On one end of the catheter subassembly is a balloon device which is filled with radiotherapy solution and on the other end is an infusion port which is attached to the skull and punctured by a needle to get the solution to the balloon at the end of the catheter.
Manufacturing Process and Key Suppliers
A dual balloon configuration is used to act as a primary and secondary reservoir for the radiotherapy solution within the resection cavity in the brain. The balloon catheter is manufactured by Vesta, Inc. and conforms to the applicable required IsoRay quality standards.
The infusion port consists of a port body, reservoir base, and a self-sealing septum. The infusion port is produced by Smith Medical, a subsidiary of Smiths Group plc., and conforms to the applicable required IsoRay quality standards. It is attached to the catheter subassembly and is bonded in place.
The radiotherapy solution is inserted in the balloon catheter through the infusion port using a needle. Iotrex® is one form of the radiation source used with the GliaSite® RTS catheter to deliver the intracranial radiation therapy. The key suppliers of the Iotrex® radiotherapy solution are Iso-Tex and Anazao. Another relatively new radiation source to deliver the intracranial radiation therapy is liquid Cesium-131 or Cesitrex™. Cesitrex™ was approved for the use in the GliaSite® RTS catheter to deliver the intracranial radiation therapy starting in May 2014 with the first case using Cesitrex™ in June 2014. The dosage of the Cesitrex™ is dependent on the strength at implant and is made to order.
| 15 |
Other accessories sold and packaged with the GliaSite® RTS catheter trays include access trays and solidifier. These accessories assist in the delivery of both Cesitrex™ and Iotrex® and subsequent removal after completion of the radiotherapy treatment. All accessories are obtained from distributors and are sterilized and tested by the Company to ensure compliance with quality standards.
From start to finish, including the creation of the GliaSite® RTS catheter subassemblies, the manufacture of the device takes approximately 4 weeks. The Company maintains on hand a number of subassemblies that reduce the manufacture time to 2 weeks, which includes sterilization of the final product. The subassemblies are maintained in a clean room facility and are not dated until the entire GliaSite® RTS medical device is Gamma sterilized. Management periodically evaluates the appropriate lot sizes in which to manufacture the GliaSite® RTS product to ensure that sterilization capacity is optimized, enough product is on hand to meet customer needs, and to manage the risk of expired product utilizing historical information and sales forecasts.
Order Processing
The Company implements a just-in-time order process for the Iotrex® radiotherapy solution. The Iodine-125 stock is ordered by the Company and drop shipped to Iso-Tex or Anazao, the Company’s contracted manufacturers of Iotrex®. The Iodine-125 is tested by the manufacturer and if accepted, is used to manufacture the Iotrex® radiotherapy solution which has a 30 day shelf life once manufactured. Once manufacture is completed by Iso-Tex or Anazao, testing is performed on the product and the test results are sent to IsoRay along with the batch record for review and acceptance. Facilities performing the implants can choose to receive the isotope in vials or the vials can be preloaded into dose-specific vials.
Due to the lead time for obtaining and processing the Iodine-125 by Iso-Tex, the Company relies on sales forecasts and historical information to estimate the proper inventory levels of catheters as well as Iotrex® given the 1 year and 30 day shelf life, respectively. Consequently, some portions of the product including the Iotrex® or the GliaSite® RTS device itself are lost through decay and are subsequently destroyed.
Since May 2014, another option for the radiotherapy solution is the liquid form of Cesium-131 or Cesitrex™, which is manufactured by the Company. Similar to Iotrex®, the Company implements a just-in-time order process and produces the Cesitrex™ at the time an order is placed and it can take up to a week to manufacture and deliver. Cesitrex™ is manufactured by the Company in its Richland, Washington manufacturing plant, then shipped to Anazao as the radiopharmacy which loads the isotope into a syringe and tests it prior to shipment to the end user. Consequently, some portion of the Cesitrex™ is lost to decay during the process. The Company ensures that the customer receives the dosage specified for the patient treatment by calculating for the decay during shipping and processing at Anazao.
Manufacturing Facility
The Company maintains a production facility located at Applied Process Engineering Laboratory (APEL) in Richland, Washington. The APEL facility became operational in September 2007. The production facility has over 15,000 square feet and includes space for isotope separation, seed production, order dispensing, a clean room for radiopharmacy work, and a dedicated shipping area. A description of the lease terms for the APEL facility is located in the Commitments and Contingencies note included in Item 8 below. Management has exercised the second of three three-year renewal options to extend the APEL facility lease through April 2016.
The Company has negotiated and agreed to a subsequent modification to the lease modification that is awaiting the signatures of both parties that provides modifications to the requirement to return the facility to ground at the time of exit at Company discretion, exercises the additional three year term to April 30, 2019 and modifies the required notice to terminate early from twelve months to six months. This lease modification provides the flexibility required for the Company to plan, design and construct its own production facility which is expected to reduce operational cashflow requirements and provide for long-term security of production capabilities for the Company.
| 16 |
The Company has reached agreement with the owner of a property adjacent to its leased facility with the expectation of planning, designing and constructing a new production facility which will accommodate the facility requirements for production, laboratory, and administrative offices. The new facility is anticipated to be a similar size to the current facility. The property also provides for additional future building as needed or subdivision, if required.
Marketing and Sales
Marketing Strategy
IsoRay has chosen to identify its proprietary Cesium-131 seed with the “Proxcelan®” brand and its liquid Cesium-131 with the brand “Cesitrex™”. Management is using these brands to differentiate Cesium-131 from competing isotopes.
The market for treatments for localized prostate cancer treatment is very competitive and largely hinges upon the demonstration of long term follow-up data that has been presented to the prostate cancer treatment profession. The fact that Proxcelan® Cesium-131 was introduced to the prostate cancer marketplace more than a decade after Iodine-125 and Palladium-103, and the resulting time for mature clinical data to be developed, has proven an obstacle to widespread market acceptance. The time to publish these results is lengthy and includes the time to enroll patients in protocols which may take multiple years depending on the size of the enrollment population; time to aggregate the results at five years from the final patient treatment; time to analyze the data and author the article followed by the time for peer review and publication in a medical journal. The total time for this process may approach a decade in length from start to publication. Management believes that the impressive results achieved for treatment with Cesium-131 at the five-year mark should create further scientific support for Cesium-131 as an attractive treatment for localized prostate cancer, overcoming at least some of the initial resistance predicated on the lack of long-term follow-up reports. The data that was published in fiscal year 2015 is discussed in the section titled Industry Information, Prostate Cancer Treatment, “Comparing Cesium-131 to I-125 and Pd-103 Clinical Results”.
The professional and patient market segments each play a role in the ultimate choice of cancer treatment and the specific isotope chosen for seed brachytherapy treatment. The Company has developed a customized brand message for each audience. The Company's website (www.isoray.com) delivers the message that Cesium-131 is for the treatment of cancers throughout the body. IsoRay also maintains print and visual media (including physician brochures discussing the clinical advantages of Cesium-131, clinical information binders, informational DVDs, single sheet glossies with targeted clinical data, etc.), and advertisements in leading medical journals. In addition, the Company attends national professional meetings, including the following:
| § | American Brachytherapy Society (ABS); |
| § | American Society for Therapeutic Radiation and Oncology (ASTRO); |
| § | Association of American Physicists in Medicine (AAPM); |
| § | Society for Neuro-Oncology (SNO); |
| § | American Association of Neurological Surgeons (AANS); |
| § | American Association for Thoracic Surgery (STS); |
| § | various local chapter meetings. |
The Company also continues to consult with noted contributors from the medical physics community and expects articles for professional journals regarding the benefits of and clinical trials involving Cesium-131 will continue to be submitted.
In addition, the Company continues to promote the clinical findings of the various protocols through presentations by respected thought leaders. The Company will continually review and update all marketing materials as more clinical information is gathered from the protocols and studies.
Apart from clinical studies and papers sponsored by the Company, several physicians across the country have independently published papers and studies on the benefits of Cesium-131.
In today's U.S. health care market, patients are more informed and involved in the management of their health than in the past. Many physicians relate incidents of their patients coming for consultations armed with articles researched on the Internet and other sources describing new treatments and medications. In many cases, these patients are demanding a certain therapy or drug and the physicians are complying when medically appropriate.
| 17 |
Because of this consumer-driven market factor, we also promote our products directly to the general public. We target the prostate cancer patient, his spouse, family and care givers. We emphasize to these segments the specific advantages of the Proxcelan® Cesium-131 brachytherapy seed through our websites (located at www.isoray.com and www.proxcelan.com), patient advocacy efforts, informational patient brochures and DVDs with patient testimonials, patient focused informational website (www.proxcelan.com), and advertisements in specific markets supporting brachytherapy. None of our websites should be considered a part of this Report.
The Company’s marketing plan with regard to non-prostate segments includes identifying and exhibiting at scientific meetings attended by specialty physicians who perform procedures related to Company’s product offerings; direct sales contact with such physicians (for example thoracic surgeons and neuro-surgeons); and the development and dissemination of training videos and other media that outline the Company’s products. The Company also continues to work with its existing radiation oncology physician customers and to educate them as to additional or new Company products. The Company’s sales managers call on existing radiation oncology physicians and other key decision makers within an organization to discuss the results from other organizations in coordination with key Company scientific personnel to engage the customer representatives in discussions on perceptions about Cesium-131 and comparisons to competing treatments.
Sales and Distribution
In the prostate cancer market, we target radiation oncologists and medical physicists as well as urologists as key clinical decision-makers in the type of radiation therapy offered to prostate cancer patients.
With respect to non-prostate applications, the Company targets neurosurgeons and thoracic surgeons in addition to radiation oncologists. After these decision makers determine to use the Company's radiation therapy, the Company then needs approval for the procedure from the medical physicists on staff. The sales cycle for non-prostate applications has proved a longer process than prostate and often takes nine months before the Company is licensed in a new hospital and can make its first sale.
IsoRay has a direct sales organization consisting of five territory sales managers, who are being managed and developed by Dwight Babcock, the Company’s Chairman and Chief Executive Officer. All of the Company's territory sales managers solicit potential specialist physicians in all areas of the body. This approach allows our territory sales managers to call on a single location for all applications of our products, resulting in a more efficient sales approach.
With the assistance of an executive search firm, the Company is currently actively recruiting additional territory sales managers with previous experience in radiation oncology and specifically with brachytherapy sales for sales territories that currently do not have a full-time territory sales manager. The Company is also actively recruiting a new national sales director to lead and mentor the direct sales organization as the former director recently resigned.
The Company expects to continue to expand its customer base outside the U.S. market through use of established distributors in the key markets of other countries. As of September 14, 2015, the Company had independent distributors in Australia and New Zealand, Germany (with a territory covering Germany, Austria, Switzerland, and Luxembourg), Italy, and Russia.
Reimbursement
Reimbursement by third party payers is the primary means of payment for all IsoRay products. The Centers for Medicare and Medicaid Services (CMS) is the primary payer, providing coverage for approximately 65% of all prostate brachytherapy cases. Well established brachytherapy coverage and payment policies are currently in place by CMS and other non-governmental payers. In 2003, CMS established a unique HCPCS code for Cesium-131 brachytherapy seeds that permitted providers to report the use of Cesium-131 directly to payers. In July 2007, CMS established two separate Cesium-131 codes for providers to report loose seeds and stranded seeds due to the cost differential of these two products. Reimbursement for prostate brachytherapy services and sources is well established in the US and most providers (hospitals and physicians) are not faced with reimbursement challenges when providing this treatment option to patients.
| 18 |
Prostate brachytherapy is typically performed in an outpatient setting, and as such, is covered by the CMS Outpatient Prospective Payment System, which since 2010 has provided a fixed reimbursement per seed for stranded and loose seeds. Iodine, palladium and cesium each have their own reimbursement values for stranded and loose seeds. If reported correctly when seeds are submitted for payment to CMS, providers are reimbursed at a flat rate that is equivalent to the cost of the seeds. It is expected that this reimbursement system established in January 2010 will continue as currently scheduled through calendar 2016 but there is no assurance that this will occur. CMS has generally continued its historical trend of declining year over year reimbursement with few exceptions. Private insurance companies have historically followed the CMS reimbursement policies. The Company expects that CMS will continue its annual review of payments provided as reimbursement for our various products and that CMS will continue to provide favorable reimbursement rates for our Cesium-131 brachytherapy seeds. At this time, the costs of our loose seeds (which sometimes is the preferred configuration for the physician) is less than the amount reimbursed by CMS. However, typically physicians order so few loose seeds that it does not appear to be a significant impairment to the sales process.
Unlike prostate brachytherapy implants, lung and brain procedures utilizing either seed brachytherapy or the GliaSite® RTS are performed when the patient has been admitted to the hospital. In-patient procedures, as they are known, are covered by CMS which remits a set amount depending on the kind of surgery being performed and the status of the patient. Under this Diagnostic Related Group or “DRG” system, the hospital pays for all the items involved in the care of the patient excluding physician fees. The brachytherapy seeds or the GliaSite® RTS in these in-patient cases are not paid for separately by CMS, but rather the hospital pays for the seeds out of the DRG payments from CMS. Because the Company's seeds may be more expensive than the cost incurred by a hospital for a competing treatment, this reimbursement method can sometimes result in greater difficulty convincing the hospitals to use the Company's products.
Other Information
Customers
The following top five customers, facilities or physician practices that utilize multiple surgical facilities at which primarily prostate brachytherapy procedures are performed accounted for approximately 49.50% of the total Company product sales for the twelve months ended June 30, 2015:
| Facility | Location | % of revenue | ||||
| El Camino, Los Gatos, & other facilities | Northern CA (1) | 24.16 | % | |||
| Bon Secours DePaul and Maryview Medical Center | MD | 11.72 | % | |||
| University of Pittsburg Medical Center – Mercy | PA | 5.10 | % | |||
| Advanced Radiation Centers of New York | NY | 4.53 | % | |||
| Candler Hospital & other facilities (Savannah, GA) | GA | 4.49 | % | |||
| Total | 49.50 | % | ||||
| (1) | The head of the single largest physician practice also serves as the Company's medical director. As the medical director, this physician is a member of the Medical Advisory Board; advises the Company Board of Directors and management; provides technical advice related to product development and research and development; and provides internal training to the Company sales staff and professional training to our sales staff and to other physicians. Revenue from this practice decreased by $15,887 in the year ended June 30, 2015 when compared to the year ended June 30, 2014. |
The loss of either the single largest physician practice or a combination of the other significant facilities and customers could have a material adverse effect on the Company's revenues, which would continue until the Company located new customers to replace them and there can be no assurance this would occur in a timely manner or at all.
| 19 |
Proprietary Rights
The Company relies on a combination of patent, copyright and trademark laws, trade secrets, software security measures, license agreements and nondisclosure agreements to protect its proprietary rights. Some of the Company's proprietary information may not be patentable. The Company has a registered U.S. trademark for Proxcelan® and a pending application for Cesitrex™.
The Company intends to vigorously defend its proprietary technologies, trademarks, and trade secrets. Members of management, employees, and certain equity holders have previously signed non-disclosure, non-compete agreements, and future employees, consultants, advisors, with whom the Company engages, and who are privy to this information, will be required to do the same. A patent for the cesium separation and purification process was granted on May 23, 2000 by the U.S. Patent and Trademark Office (USPTO) under Patent Number 6,066,302, with an expiration date of April 28, 2019. The process was developed by Lane Bray, our Chief Chemist until his recent passing and a shareholder of the Company, and has been assigned exclusively to IsoRay. IsoRay's predecessor also obtained patent protection in four European countries under the Patent Cooperation Treaty. Those patents have been assigned to IsoRay.
Our management believes that certain aspects of the IsoRay seed design and construction techniques are patentable innovations. These innovations resulted in a patent granted by the USPTO under Patent Number 7,410,458, in August 2008 with an expiration date of December 5, 2025. Certain methodologies regarding isotope production, separation, and seed manufacture are retained as trade secrets and are embodied in IsoRay's procedures and documentation. Five patents have been granted by the USPTO relating to methods of deriving Cesium-131 developed by IsoRay employees: Patent Number 7,479,261 with an expiration date of April 6, 2027; Patent Number 7,517,508 with an expiration date of July 19, 2027; Patent Number 7,531,150 with an expiration date of July 13, 2027; Patent Number 7,316,644 with an expiration date of August 5, 2025; and Patent Number 7,510,691 with an expiration date of July 19, 2027. The Company has two patents allowed in Canada which will become effective at the time of their use in Canada. The Company has patents granted in the Russian Federation which expire at various times in 2024 and 2025. The Company has a single patent granted in the Netherlands and India that each expire on June 22, 2025. The Company has a single patent pending in the EU and Hong Kong. The Company is continuing its efforts on developing and patenting additional methods of deriving Cesium-131 and other isotopes.
There are specific conditions attached to the assignment of the Cesium-131 patent from Lane Bray. In particular, the associated Royalty Agreement provides for 1% of gross profit payment from seed sales to Lane Bray and 1% of gross profit from any use of the Cesium-131 process patent for non-seed products. If IsoRay reassigns the Royalty Agreement to another company, these royalties increase to 2%. The Royalty Agreement has an anti-shelving clause which requires IsoRay to return the patent if IsoRay permanently abandons sales of products using the invention. During fiscal years 2015 and 2014, the Company recorded royalty expense of $14,448 and $10,106, respectively, related to this patent.
The terms of a license agreement with the Lawrence Family Trust (successor to Don Lawrence) for a patent application and related "know-how" require the payment of a royalty based on the Net Factory Sales Price, as defined in the agreement, of licensed product sales. Because the licensor's patent application was ultimately abandoned, only a 1% "know-how" royalty remains applicable. To date, management believes that there have been no product sales incorporating the "know-how;" and therefore believes no royalty is due pursuant to the terms of the agreement. Management believes that ultimately no royalties should be paid under this agreement as there is no intent to use this "know-how" in the future.
The Lawrence Family Trust has disputed management's contention that it is not using this "know-how". On September 25, 2007 and again on October 31, 2007, the Company participated in nonbinding mediation regarding this matter; however, no settlement was reached with the Lawrence Family Trust. After additional settlement discussions, which ended in April 2008, the parties failed to reach a settlement. The parties may demand binding arbitration at any time.
Research and Development
During the three-year period ended June 30, 2015, IsoRay and its subsidiaries incurred approximately $0.62 million in costs related to research and development activities. The Company expects to continue ongoing research and development activities for the foreseeable future.
| 20 |
Government Regulation
The Company's present and future intended activities in the development, manufacture and sale of cancer therapy products are subject to extensive laws, regulations, regulatory approvals and guidelines. Within the United States, the Company's therapeutic radiological devices must comply with the U.S. Federal Food, Drug and Cosmetic Act, which is enforced by the FDA. The Company is also required to adhere to applicable FDA Quality System Regulations, also known as the Good Manufacturing Practices, which include extensive record keeping and periodic inspections of manufacturing facilities. The Company's predecessor obtained FDA 510(k) clearance in March 2003 to market the Proxcelan® Cesium-131 seed for the treatment of localized solid tumors and other malignant disease and IsoRay obtained FDA 510(k) clearance in November 2006 to market preloaded brachytherapy seeds and in August 2009 for preloading flexible braided strands and bioabsorbable mesh.
In the United States, the FDA regulates, among other things, new product clearances and approvals to establish the safety and efficacy of these products. We are also subject to other federal and state laws and regulations, including the Occupational Safety and Health Act and the Environmental Protection Act.
The Federal Food, Drug, and Cosmetic Act and other federal statutes and regulations govern or influence the research, testing, manufacture, safety, labeling, storage, record keeping, approval, distribution, use, reporting, advertising and promotion of such products. Noncompliance with applicable requirements can result in civil penalties, recall, injunction or seizure of products, refusal of the government to approve or clear product approval applications, disqualification from sponsoring or conducting clinical investigations, preventing us from entering into government supply contracts, withdrawal of previously approved applications, and criminal prosecution.
In the United States, medical devices are classified into three different categories over which the FDA applies increasing levels of regulation: Class I, Class II, and Class III. Most Class I devices are exempt from premarket notification (510(k)); most Class II devices require premarket notification (510(k)); and most Class III devices require premarket approval. Our Proxcelan® Cesium-131 seed is a Class II device and received 510(k) clearance in March 2003.
Approval of new Class III medical devices is a lengthy procedure and can take a number of years and require the expenditure of significant resources. There is a shorter FDA review and clearance process for Class II medical devices, the premarket notification or 510(k) process, whereby a company can market certain Class II medical devices that can be shown to be substantially equivalent to other legally marketed devices. Since brachytherapy seeds have been classified by the FDA as a Class II device, we have been able to achieve market clearance for our Cesium-131 seed using the 510(k) process.
In August 2011, IsoRay Medical received clearance from the FDA for its premarket notification (510(k)) for the GliaSite® RTS. The GliaSite® RTS is the only FDA-cleared balloon catheter device used in the treatment of brain cancer.
In May 2014, the Company received clearance from the FDA for its pre-market notification (510k) for the radiotherapy solution Cesitrex™ (liquid Cesium-131) for use with the GliaSite® RTS.
As a registered medical device manufacturer with the FDA, we are subject to inspection to ensure compliance with its current Good Manufacturing Practices, or cGMP. These regulations require that we and any of our contract manufacturers design, manufacture and service products, and maintain documents in a prescribed manner with respect to manufacturing, testing, distribution, storage, design control, and service activities. Modifications or enhancements that could significantly affect the safety or effectiveness of a device or that constitute a major change to the intended use of the device require a new 510(k) premarket notification for any significant product modification.
The Medical Device Reporting regulation requires that we provide information to the FDA on deaths or serious injuries alleged to be associated with the use of our devices, as well as product malfunctions that are likely to cause or contribute to death or serious injury if the malfunction were to recur. Labeling and promotional activities are regulated by the FDA and, in some circumstances, by the Federal Trade Commission.
As a medical device manufacturer, we are also subject to laws and regulations administered by governmental entities at the federal, state and local levels. For example, our facility is licensed as a medical device manufacturing facility in the State of Washington and is subject to periodic state regulatory inspections. Our customers are also subject to a wide variety of laws and regulations that could affect the nature and scope of their relationships with us.
| 21 |
In support of IsoRay's global strategy to expand marketing to Canada, the European Union (EU) and Russia, we initiated the process in fiscal year 2008 to obtain the European CE Mark, Canadian registration, and certification to ISO 13485:2003, an internationally recognized quality system. During the fiscal year ended June 30, 2014, the CE Mark was renewed for an additional five years. European law requires that medical devices sold in any EU Member State comply with the requirements of the European Medical Device Directive (MDD) or the Active Implantable Medical Device Directive (AIMDD). IsoRay's brachytherapy seeds are classified in Europe as an active implantable and are subject to the AIMDD and GliaSite® RTS is an EU Class 3 device subject to the MDD. Compliance with the AIMDD, MDD, and obtaining a CE Mark involves being certified to ISO 13485:2003 and obtaining approval of the product technical file by a notified body that is recognized by competent authorities of a Member State. Compliance with ISO 13485:2003 is also required for registration of a company for sale of its products in Canada. Many of the recognized EU Notified Bodies are also recognized by Health Canada to conduct the ISO 13485:2003 inspections for Canadian registration. During fiscal year 2009, the Company received its certification to ISO 13485:2003 and obtained approval from Health Canada for its Canadian registration. The Company has had no success in selling the product in the Canadian market and through its distributors is currently focusing on the markets in Germany, Austria, Switzerland, Luxembourg, Italy, and the Russian Federation. On June 18, 2014, the Company entered into an agreement with MedikorPharma-Ural LLC as the distributor in the Russian Federation. The agreement provides the distributor with the ability to sell the entire product line with exception of the Cesitrex™ which does not carry the CE mark. The Company has extended its agreement to August 31, 2016 with one modification that removes Italy from the territory with the German distributor whose market includes Germany, Austria, Switzerland and Luxembourg. The Company reached agreement with a distributor for Greece during the fiscal year ended June 30, 2013 and has actively supported this distributor in achieving regulatory clearance in its distribution market. The agreement with the distributor for Greece was effective on May 1, 2013 but has now expired with no sales.
In April 2012, IsoRay Medical received a CE mark for the GliaSite® RTS which states that the Company conforms to the product requirements of the European Council Directive 93/42/EEC. The CE mark allows the GliaSite® RTS to be sold in 31 European countries and to be marketed in the European Free Trade Associate member states and the European Union. In August 2013, the Company successfully amended its CE mark on the GliaSite® RTS which incorporated five changes.
In the United States, as a manufacturer of medical devices and devices utilizing radioactive byproduct material, we are subject to extensive regulation by not only federal governmental authorities, such as the FDA, but also by state and local governmental authorities, such as the Washington State Department of Health, to ensure such devices are safe and effective. In Washington State, the Department of Health, by agreement with the federal Nuclear Regulatory Commission (NRC), regulates the possession, use, and disposal of radioactive byproduct material as well as the manufacture of radioactive sealed sources to ensure compliance with state and federal laws and regulations. Our Cesium-131 brachytherapy seeds and the GliaSite® RTS constitute both medical devices and radioactive sealed sources and are subject to these regulations. The Company has received sealed source device approval from the State of Washington Department of Health for the GliaSite® RTS, components of which are manufactured at our Richland facility.
Moreover, our use, management, and disposal of certain radioactive substances and wastes are subject to regulation by several federal and state agencies depending on the nature of the substance or waste material. We believe that we are in compliance with all federal and state regulations for this purpose.
Seasonality
The Company believes that some seed implantation procedures are deferred around physician vacations (particularly in the summer months), holidays, and medical conventions and conferences resulting in a seasonal influence on the Company's business. These factors cause a momentary decline in revenue which management believes is ultimately realized later. Because approximately 49.50% of the Company's business is dependent on five customers, physician practices or facilities, simultaneous or extended vacations by the physicians at these facilities or by our single largest physician who accounts for approximately 24% of total revenue could cause significant drops in the Company's productivity during those reporting periods.
| 22 |
Employees
As of September 11, 2015, IsoRay employed thirty-five full-time individuals and one part-time individual. The Company's future success will depend, in part, on its ability to attract, retain, and motivate highly qualified sales, technical and management personnel. From time to time, the Company may employ independent consultants or contractors to support its research and development, marketing, sales, accounting and administrative organizations. None of the Company's employees are represented by any collective bargaining unit. At June 30, 2015, the Company employed seven direct sales people.
Competition
The Company competes in a market characterized by technological innovation, extensive research efforts, and significant competition. In general, the Proxcelan® Cesium-131 brachytherapy seed competes with conventional methods of treating localized cancer, including, but not limited to, all forms of prostatectomy surgery and external beam radiation therapy which includes intensity modulated radiation therapy, as well as competing permanent brachytherapy devices.
The Company's patented Cesium-131 separation process is likely to provide a sustainable competitive advantage. Production of Cesium-131 also requires specialized facilities that represent high cost and long lead time if not readily available. In addition, a competitor would need to develop a method for isotope attachment and seed assembly, would need to conduct testing to meet NRC and FDA requirements, and would need to obtain regulatory clearances before marketing a competing device. Best Medical received FDA 510(k) clearance to market a Cesium-131 seed on June 6, 1993 but to date has not produced any products for sale.
The GliaSite® RTS and the Company’s brachytherapy products used in non-prostate applications typically compete with external beam radiation therapy (EBRT), which can be provided as conventional or intensity modulated radiation therapy, or as stereotactic radiosurgery, a technique that delivers high doses of radiation to a target in a much lower number of sessions than other forms of EBRT.
Manufacturers of EBRT equipment include Varian Medical Systems, Siemens Healthcare, Elekta AB, and Accuray Incorporated, among others.
In the cases of lung and brain tumors (and other solid tumors), a surgeon will remove the tumor if it is medically prudent and this offers the patient some benefit in terms of controlling the growth of the cancer or its symptoms. In many cases, radiation therapy is added following the surgery; this is known as “adjuvant” radiation therapy. The Company believes that its form of adjuvant radiation therapy deployable in such cases offers advantages over external beam methods. However, external beam holds the vast majority of the market for adjuvant radiation therapy.
You should carefully consider the following factors regarding information included in this Annual Report. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations. If any of the following risks actually occur, our business, financial condition and operating results could be materially adversely affected.
Risks Related to Our Industry and Operations
Our Revenues Depend Upon One Product. With the exception of the GliaSite® RTS which the Company began selling in the 2012 fiscal year, our revenues depend solely upon the successful production, marketing, and sales of the Proxcelan® Cesium-131 brachytherapy seed. The rate and level of market acceptance of this product varies depending on the perception by physicians and other members of the healthcare community of its safety and efficacy as compared to that of competing products, if any; the clinical outcomes of the patients treated; the effectiveness of our sales and marketing efforts or those of our distributors in the United States, the European Union (EU), Germany, Australia, New Zealand and the Russian Federation; any unfavorable publicity concerning our product or similar products; our product's price relative to other products or competing treatments; any decrease in current reimbursement rates from the Centers for Medicare and Medicaid Services or third-party payers; regulatory developments related to the manufacture or continued use of the product; availability of sufficient supplies of barium for Cesium-131 seed production; ability to produce sufficient quantities of Cesium-131; the ability of physicians to apply the correct dosage of seeds and avoid excessive levels of radiation to patients; and the ability to use this product to treat multiple types of cancers in various organs. Because of our reliance on this product as the primary source of our revenue, any material adverse developments with respect to the commercialization of this product may cause us to continue to incur losses rather than profits in the future.
| 23 |
Although Cleared To Treat Any Malignant Tissue, Our Principal Product Is Primarily Used To Treat A Single Type Of Cancer. Currently, the Proxcelan® Cesium-131 seed is used almost exclusively for the treatment of prostate cancer (approximately eighty-seven percent (87%) of our sales). We have been treating brain cancer which amounted to approximately 7% of our product sales, lung cancer which amounted to approximately 2% and other cancers including head and neck; colorectal; gynecological and brain that combined constituted approximately 2% of our product sales in fiscal year 2015. The GliaSite® RTS contributed 2% of our product sales in fiscal year 2015. Management believes the Proxcelan® Cesium-131 seed will continue to be used to treat other types of cancers as the Company identifies existing delivery systems that can be utilized or develops new delivery methods for the product, however these delivery systems may not prove as effective as anticipated. Management believes that clinical data gathered by select groups of physicians under treatment protocols specific to other organs will be needed prior to widespread acceptance of our product for treating other cancer sites. If our current and future products do not become accepted in treating cancers of other sites, our sales will depend primarily on treatment of prostate cancer, a market with increasing competition and ongoing loss of market share by all brachytherapy products except for this fiscal year when the Company’s prostate cancer revenue increased by 13% compared to the fiscal year 2014 and revenue overall increased by 9% in fiscal year 2015 compared to fiscal year 2014.
We Rely Heavily On Five Customers. Approximately fifty percent (50%) of the Company’s revenues are dependent on five customers and approximately twenty-four percent (24%) on one customer. The loss of any of these customers would have a material adverse effect on the Company’s revenues which may not be replaced by other customers particularly as these customers are in the prostate sector which is facing substantial competition from other treatments.
We Rely Heavily On A Limited Number Of Suppliers. Some materials used in our products are currently available only from a limited number of suppliers. In fiscal 2015, approximately eighty-three percent (83%) of our Cesium-131 was supplied through JSC INM from a reactor located in Russia. Our current contract with JSC INM terminates on January 31, 2016 and will have to be renegotiated. Management will seek to negotiate favorable pricing but there is no assurance as to the outcome of these negotiations. Management is evaluating other reactors that meet current specifications to yield Cesium-131 of the purity that the Company requires for use in its products but thus far has only confirmed such availability from MURR.
Reliance on any single supplier increases the risks associated with concentrating isotope production at a single reactor facility which can be subject to unanticipated shutdowns and political or civil unrest. Failure to obtain deliveries of Cesium-131 from multiple sources could have a material adverse effect on seed production and there may be a delay before we could locate alternative suppliers beyond the two currently used.
We may not be able to locate additional suppliers outside of Russia, other than MURR, capable of producing the level of output of cesium at the quality standards we require. Additional factors that could cause interruptions or delays in our source of materials include limitations on the availability of raw materials or manufacturing performance experienced by our suppliers and a breakdown in our commercial relations with one or more suppliers. Some of these factors may be completely out of our and our suppliers' control.
Virtually all titanium tubing used in brachytherapy seed manufacture comes from a single source, Accellent Corporation. We currently obtain a key component of our seed core from another single supplier, C5 Medical Werks, LLC. We do not have formal written agreements with Accellent Corporation. We do have a purchase agreement with C5 Medical Werks, LLC which calls for fixed quantity of seed cores to be shipped over a 36 month period at a fixed unit price. Any interruption or delay in the supply of materials required to produce our products could cause harm to our business if we were unable to obtain an alternative supplier or substitute equivalent materials in a cost-effective and timely manner. To mitigate any potential interruptions, the Company continually evaluates its inventory levels and management believes that the Company maintains a sufficient quantity on hand to alleviate any potential disruptions.
| 24 |
Virtually all of the components used in the production of the GliaSite® RTS are from single sources. We do not have formal written agreements with those suppliers. Any interruption or delay in the supply of these components could cause harm to our business as the cost and / or time required to meet the regulatory requirements of the Food and Drug Administration for the United States and our notified body for our CE mark (British Standards Institute) in the European Union may be prohibitive.
While we work closely with suppliers to assure continuity of supply and maintain high quality and reliability, these efforts may not be successful. Manufacturing disruptions experienced by our suppliers may jeopardize our supply of components. The loss or disruption of our relationships with outside vendors could subject us to substantial delays in the delivery of our products to customers. Significant delays in the delivery of our products could result in possible cancellation of orders and the loss of customers.
Due to the stringent regulations and requirements of the FDA and other similar non-U.S. regulatory agencies regarding the manufacture of our products, we may not be able to quickly establish additional or replacement sources for certain components or materials. A change in suppliers could require significant effort or investment in circumstances where the items supplied are integral to product performance or incorporate unique technology. A reduction or interruption in manufacturing, or an inability to secure alternative sources of raw materials or components, could have a material effect on our business, results of operations, financial condition and cash flows.
Any casualty, natural disaster or other significant disruption of any of our sole-source suppliers’ operations, or any unexpected loss of any existing exclusive supply contract could have a material adverse effect on our business.
Although we expect our suppliers to comply with our contract terms, we do not have control over these suppliers. Our inability to provide a product that meets delivery schedules could have a material adverse effect on our reputation in the industry, which could have a material adverse effect on our financial condition and results of operations.
Further, any single source suppliers or contract manufacturers may operate through a single facility. If an event occurred that resulted in material damage to this manufacturing facility or our supplier/manufacturing contractor lacked sufficient labor to fully operate the facility, we may be unable to transfer the manufacture of our product or supply of the component to another facility or location in a cost-effective or timely manner, if at all. This potential inability to transfer production could occur for a number of reasons, including but not limited to a lack of necessary relevant manufacturing capability at another facility, or the regulatory requirements of the FDA or other governmental regulatory bodies. Even if there are many qualified suppliers or contract manufacturers available around the country and our product or its components are relatively easy to manufacture, such an event could have a material adverse effect on our financial condition and results of operations.
Unfavorable Industry Trends in the Prostate Market. Several factors which began in fiscal 2009 have caused our revenues to significantly decline. These factors continued into fiscal year 2014 contributing to our failure to improve sales in the prostate market until this fiscal year when we experienced an increase in sales over fiscal year 2014, but this improvement was not back to the amount of revenues we had in fiscal 2011 or 2012. Beginning in the Fall of 2008, U.S. consumers significantly curtailed all spending (even for life saving medical procedures) which impacted the brachytherapy industry as a whole. In February of 2009 noted urologists announced at a medical conference that prostate specific antigen (PSA) testing was not as necessary as previously believed. Their statements were widely publicized. In May 2012, the U.S. Preventive Services Task Force recommended against routine PSA screenings for healthy men without symptoms. We believe this recommendation has led to a renewed decline in PSA screenings. In addition, we believe there has been an increase in “active surveillance”, a practice where no immediate medical treatment is provided; but the physician and patient closely monitor the patient’s cancer for signs that the cancer is growing. We believe that declines in PSA screenings have led to a decline in the number of men diagnosed with prostate cancer. A decline in the number of PSA screenings would in turn lead to a decline in the number of procedures to treat prostate cancer, including brachytherapy procedures. An increase in the proportion of men diagnosed with prostate cancer but not seeking immediate medical treatment would also lead to a decline in the number of procedures to treat prostate cancer.
As of the end of fiscal 2015, the American Cancer Society has not further revised its advice regarding PSA testing, continuing to advise that the decision to be screened for prostate cancer should be made after getting information about the uncertainties, risks, and potential benefits of prostate cancer screening. This advice has led to an increased number of men electing to forgo PSA testing.
| 25 |
Also the emergence of IMRT as the preferred treatment alternative as a result of a much higher reimbursement rate to physicians compared to brachytherapy treatments has resulted in declining market share for brachytherapy treatment. In fiscal 2015, each of these factors continued to impact the performance of the Company in the prostate market and the industry as a whole and there is no assurance that they will not continue to impact sales of the Company in the prostate market through fiscal 2016.
Doctors And Hospitals May Not Adopt Our Products And Technologies At Levels Sufficient To Sustain Our Business Or To Achieve Our Desired Growth Rate. To date, we have attained very limited penetration of the total potential market for most of our products, particularly our non-prostate applications. Our future growth and success depends upon creating broad awareness and acceptance of our products by doctors, hospitals and freestanding clinics, as well as patients. This will require substantial marketing and educational efforts, which will be costly and may not be successful. The target customers for our products may not adopt these technologies or may adopt them at a rate that is slower than desired. We depend extensively on long term protocol results and publications by independent physicians. Unfavorable protocol results or publications would have an impact on the success of our products. In addition, potential customers who decide to utilize any of our devices may later choose to purchase competitors’ products. Important factors that will affect our ability to attain broad market acceptance of our products include:
| · | doctor and/or patient awareness and acceptance of our products; |
| · | the real or perceived effectiveness and safety of our products; |
| · | the relationship between the cost of our products and the real or perceived medical benefits of our products; |
| · | the relationship between the cost of our products and the financial benefits to our customers using our products, which will be greatly affected by the coverage of, and reimbursement for, our products by governmental and private third-party payors; and |
| · | market perception of our ability to continue to grow our business and develop enhanced products. |
We must promote our products effectively. Factors that could affect our success in marketing our products include:
| · | the adequacy and effectiveness of our sales force and that of any distributor’s sales force; |
| · | the adequacy and effectiveness of our production, distribution and marketing capabilities and those of our distributors; |
| · | the success of competing treatments or products; and |
| · | the availability and extent of reimbursement from third-party payors for our products. |
If any of our products fails to achieve market acceptance, we may not be able to market and sell the products successfully, which would limit our ability to generate revenue and could harm our business.
The Single Russian Supplier For Our Cesium-131. In June 2014 and again in January 2015, the Company entered into an agreement with The Open Joint Stock Company <<Institute of Nuclear Materials>> (JSC INM) to purchase Cesium-131 directly from Institute of Nuclear Materials (INM). As a result, the Company relies on JSC INM to obtain Cesium-131 from its single Russian reactor source. Through the INM Agreement, we have obtained fixed pricing for our Russian Cesium-131 through the termination of the contract on January 31, 2016. There can be no guarantee that JSC INM will always be able to supply us with sufficient Cesium-131 or will renew our existing contract on favorable terms in January 2016, which could be due in part to risks associated with foreign operations and beyond either our or JSC INM's control. If we were unable to obtain supplies of isotopes from Russia in the future, our overall supply of Cesium-131 could be reduced significantly unless we have a source of enriched barium for utilization in domestic reactors beyond the quantity that we already own. The Company has performed a search for enriched barium as part of its annual impairment testing for its existing inventory of enriched barium and has found no other entity that could supply the required quantities of enriched barium. While recent testing of regions within the reactor at MURR has found that Cesium-131 can be produced in economically viable quantities at a viable price, there is no assurance that we can obtain the increased quantity of isotope at the pricing and quantities that the Company requires in the long term. Management estimates that the supply of enriched barium that it currently owns should last from 24 to 36 months which would allow time to expand into other irradiation sites within MURR or at another reactor to supplement its supply of Cs-131.
| 26 |
Increased Prices For, Or Unavailability Of, Raw Materials Used In Our Products Could Adversely Affect Our Revenues. Our revenues are affected by the prices of the raw materials and sub-assemblies used in the manufacture of our products. These prices may fluctuate based on a number of factors beyond our control, including changes in supply and demand, general economic conditions, labor costs, fuel related delivery costs, competition, import duties, tariffs, currency exchange rates, and government regulation. Due to the highly competitive nature of the healthcare industry and the cost containment efforts of our customers and third-party payers, we may be unable to pass along cost increases for key components or raw materials through higher prices to our customers. If the cost of key components or raw materials increases, and we are unable fully to recover these increased costs through price increases or offset these increases through other cost reductions, we could experience lower margins and profitability. Significant increases in the prices of raw materials or sub-assemblies that cannot be recovered through productivity gains, price increases or other methods could adversely affect our results of operations.
We Are Subject To Uncertainties Regarding Reimbursement For Use Of Our Products. Hospitals and freestanding clinics may be less likely to purchase our products if they cannot be assured of receiving favorable reimbursement for treatments using our products from third-party payers, such as Medicare and private health insurance plans. Currently, Medicare reimburses hospitals at fixed rates that cover the cost of stranded and loose seeds. Clinics and physicians performing procedures in a free standing center are reimbursed at the actual cost of the seeds. It is expected that CMS will continue to reimburse providers using this same methodology in 2016 but there is no assurance this will occur.
Brachytherapy seeds have two CMS codes – one code for loose seeds and a second code for stranded seeds. Reimbursement amounts are reviewed and revised annually based upon information submitted to CMS on claims by providers. Changes in reimbursement can positively or negatively affect market demand for our products. We monitor these changes and provide comments, as permitted, when changes are proposed, prior to implementation.
In 2011, IsoRay introduced the GliaSite® RTS, which had an existing reimbursement code. As an in-patient procedure covered by CMS, hospitals are paid based on the type of surgery and the status of the patient. These procedures are done as part of a Diagnostic Related Group or “DRG” system under which the hospital pays for all items involved in the care of the patient exclusive of the physician fees. Hospitals are less receptive to treatments which require out of pocket costs.
Historically, private insurers have followed Medicare guidelines in establishing reimbursement rates. However, third-party payers are increasingly challenging the pricing of certain medical services or devices, and we cannot be sure that they will reimburse our customers at levels sufficient for us to maintain favorable sales and price levels for our products. There is no uniform policy on reimbursement among third-party payers, and we can provide no assurance that our products will continue to qualify for reimbursement from all third-party payers or that reimbursement rates will not be reduced. A reduction in or elimination of third-party reimbursement for treatments using our products would likely have a material adverse effect on our revenues.
Our success in international markets also depends upon the eligibility of our products for coverage and reimbursement through government-sponsored health care payment systems and third-party payors. Reimbursement practices vary significantly by country. Many international markets have government-managed insurance systems that control reimbursement for new products and procedures. Other foreign markets have both private insurance systems and government-managed systems that control reimbursement for new products and procedures. Market acceptance of our products may depend on the availability and level of coverage and reimbursement in any country within a particular time. In addition, health care cost containment efforts similar to those we face in the United States are prevalent in many of the other countries in which we intend to sell our products and these efforts are expected to continue.
Furthermore, any federal and state efforts to reform government and private healthcare insurance programs, such as those passed by the federal government in 2010, could significantly affect the purchase of healthcare services and products in general and demand for our products in particular. Medicare is the payer in approximately 65% of all U.S. prostate brachytherapy cases. We are unable to predict the ultimate impact of the healthcare reform passed in 2010, those reforms that may be enacted in the future both in the United States and in other countries, whether other healthcare legislation or regulations affecting the business may be proposed or enacted in the future or what effect any such legislation or regulations would have on our business, financial condition or results of operations.
| 27 |
Our Operating Results Will Be Subject To Significant Fluctuations. Our quarterly revenues, expenses, and operating results are likely to fluctuate significantly in the future. Fluctuation may result from a variety of factors, which are discussed in detail throughout this "RISK FACTORS" section, including:
| · | demand and pricing for the Company's products; |
| · | effects of aggressive competitors; |
| · | hospital, clinic and physician purchasing decisions; |
| · | research and development and manufacturing expenses; |
| · | patient outcomes from our products; |
| · | physician acceptance of our products; |
| · | government or private healthcare reimbursement policies; |
| · | healthcare reform; |
| · | our manufacturing performance and capacity; |
| · | incidents, if any, that could cause temporary shutdown of our manufacturing facility; |
| · | the amount and timing of sales orders; |
| · | rate and success of future product approvals; |
| · | timing of FDA clearance, if any, of competitive products and the rate of market penetration of competing |
products;
| · | seasonality of purchasing behavior in our market; |
| · | overall economic conditions; |
| · | the 2.3% excise tax on medical devices which began in January 2013; |
| · | the successful introduction or market penetration of alternative therapies; and |
| · | the outcome of the FDA's evaluation of the clearance process for class II devices. |
We Are Subject To The Risk That Certain Third Parties May Mishandle Our Product. We rely on third parties, such as Federal Express, to deliver our Proxcelan® Cesium-131 seed and all components of our GliaSite® RTS including Iotrex® and Cesitrex™, and on other third parties, including various radiopharmacies, to package our products in certain specialized packaging forms requested by customers. We are subject to the risk that these third parties may mishandle our product, which could result in adverse effects, particularly given the radioactive nature of our products.
We May Encounter Manufacturing Problems Or Delays That Could Result In Lost Revenue. Manufacturing our products is a complex process. We (or our critical suppliers) may encounter difficulties in scaling up or maintaining production of our products, including:
| · | problems involving production yields; |
| · | quality control and assurance; |
| · | component supply shortages; |
| · | import or export restrictions on components, materials or technology; |
| · | shortages of qualified personnel; and |
| · | compliance with state, federal and foreign regulations. |
If demand for our products exceeds our manufacturing capacity, we could develop a substantial backlog of customer orders. If we are unable to maintain larger-scale manufacturing capabilities, our ability to generate revenues will be limited and our reputation in the marketplace could be damaged.
| 28 |
Failure Of Any Clinical Studies Or Third-Party Assessments To Demonstrate Desired Outcomes In Proposed Endpoints May Reduce Physician Usage Or Result In Pricing Pressures That Could Have A Negative Impact On Business Performance. We may directly conduct or support third party clinical studies designed to test a variety of endpoints associated with product performance and use across a number of applications. If, as a result of poor design, implementation or otherwise, a clinical study conducted by us or others fails to demonstrate statistically significant results supporting performance or use benefits or comparative or cost effectiveness of our products, physicians may elect not to use our products as a treatment for conditions that may benefit from them. Furthermore, in the event of an adverse clinical study outcome, our products may not achieve “standard-of-care” designations, where they exist, for the conditions in question, which could deter the adoption of our products. Also, if serious device-related adverse events are reported during the conduct of a study it could affect continuation of the study, product approval and product adoption. If we are unable to develop a body of statistically significant evidence from our clinical study program, whether due to adverse results or the inability to complete properly designed studies, domestic and international public and private payers could refuse to cover our products, limit the manner in which they cover our products, or reduce the price they are willing to pay or reimburse for our products. In the case of a pre-approval study or a study required by a regulatory body as a condition of clearance or approval, a regulatory body can revoke, modify or deny clearance or approval of the study and/or the product in question.
It Is Possible That Other Treatments May Be Deemed Superior To Brachytherapy. Our Proxcelan® Cesium-131 seed and GliaSite® RTS face competition not only from companies that sell other radiation therapy products, but also from companies that are developing alternative therapies for the treatment of cancers. It is possible that advances in the pharmaceutical, biomedical, or gene therapy fields could render some or all radiation therapies, whether conventional or brachytherapy, obsolete. If alternative therapies are proven or even perceived to offer treatment options that are superior to brachytherapy, physician adoption of our brachytherapy product could be negatively affected and our revenues from our brachytherapy product could decline.
Our Industry Is Intensely Competitive. The medical device industry is intensely competitive. We compete with both public and private medical device, biotechnology and pharmaceutical companies that have been in existence longer than we have, have a greater number of products on the market, have greater financial and other resources, and have other technological or competitive advantages. As physicians migrate to medical devices such as external beam radiation and robotic surgery that have a much higher capital cost to repay and higher profit margins, this puts increasing pressure on all brachytherapy products to compete regardless of their superior treatment results. The market share for brachytherapy continues to decline as a result of this pressure from increasing usage by oncologists of external beam radiation. In addition, centers that wish to offer the Proxcelan® Cesium-131 seed or the GliaSite® RTS must comply with licensing requirements specific to the state, province, and/or country in which they do business and these licensing requirements may take a considerable amount of time to comply with. Certain centers may choose not to offer our Proxcelan® Cesium-131 seed or the GliaSite® RTS due to the time required to obtain necessary license amendments. We also compete with academic institutions, government agencies, and private research organizations in the development of technologies and processes and in acquiring key personnel. Although we have patents granted and patents applied for to protect our isotope separation processes and Cesium-131 seed manufacturing technology, we cannot be certain that one or more of our competitors will not attempt to obtain patent protection that blocks or adversely affects our product development efforts. The Company’s GliaSite® RTS brachytherapy products typically compete with external beam radiation therapy (EBRT), which can be provided as conventional or intensity modulated radiation therapy, or as stereotactic radiosurgery, a technique that delivers high doses of radiation to a target in a much fewer number of sessions than other forms of EBRT. Manufacturers of EBRT equipment include Varian Medical Systems, Siemens Healthcare, Elekta AB, and Accuray Incorporated, among others. In the case of brain tumors, a surgeon will remove the tumor and radiation therapy is added following the surgery; this is known as “adjuvant” radiation therapy. The Company believes that its form of adjuvant radiation therapy deployable in such cases offers advantages over external beam methods. However, external beam holds the vast majority of the market for adjuvant radiation therapy. Until the fiscal year ended June 30, 2015, when the Company experienced 13% growth in prostate brachytherapy and 9% overall growth in product sales, revenues had declined in each of the prior four fiscal years. Revenues in the fiscal year ended June 30, 2015 rebounded to similar levels as the fiscal year ended June 30, 2013.
Cost-Containment Efforts Of Our Customers, Purchasing Groups, Third-Party Payers And Governmental Organizations Could Adversely Affect Our Sales And Profitability. The continuing efforts of governments, insurance companies and other payors of healthcare costs to contain or reduce these costs, combined with closer scrutiny of such costs, could lead to patients being unable to obtain approval for payment from these third-party payors. The cost containment measures that healthcare providers are instituting both in the U.S. and internationally could harm our business. Some healthcare providers in the U.S. have adopted or are considering a managed care system in which the providers contract to provide comprehensive healthcare for a fixed cost per person. Healthcare providers may attempt to control costs by authorizing fewer elective surgical procedures or by requiring the use of the least expensive devices possible, which could adversely affect the demand for our products or the price at which we can sell our products. Some healthcare providers have sought to consolidate and create new companies with greater market power, including hospitals. As the healthcare industry consolidates, competition to provide products and services has become and will continue to become more intense. This has resulted and likely will continue to result in greater pricing pressures and the exclusion of certain suppliers from important marketing segments.
| 29 |
Outside the United States, we expect to experience pricing pressure from centralized governmental healthcare authorities due to efforts by such authorities to lower healthcare costs. Implementation of healthcare reforms and competitive bidding contract tenders may limit the price or the level at which reimbursement is provided for our products and adversely affect both our pricing flexibility and the demand for our products. Healthcare providers may respond to such cost-containment pressures by substituting lower cost products or other therapies for our products. We may be required to engage in competitive bidding for the sale of our products to governmental purchasing agents and hospital groups. Our failure to offer acceptable prices to these customers could adversely affect our sales and profitability in these markets. Distributors of our products may also negotiate terms of sale more aggressively to increase their profitability. Failure to negotiate distribution arrangements having advantageous pricing and other terms of sale could cause us to lose market share and would adversely affect our business, results of operations, financial condition and cash flows.
If We Fail To Comply With Applicable Healthcare Regulations, We Could Face Substantial Penalties And Our Business, Operations And Financial Condition Could Be Adversely Affected. Certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients' rights may be applicable to our business. We could be subject to healthcare fraud and abuse and patient privacy regulation by both the federal government and the states in which we conduct our business, without limitation. The laws that may affect our ability to operate include, but are not limited to:
| · | the federal Anti-Kickback Statute, which prohibits, among other things, knowingly and willfully soliciting, receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly, in cash or in kind, to induce, or in return for, the referral of an individual for the furnishing or arranging for the furnishing of any item or service, or the purchase, lease, order, arrangement for, or recommendation of the purchase, lease, or order of any good, facility, item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and Medicaid programs; |
| · | the civil federal False Claims Act, which imposes civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for, among other things, knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent; knowingly making, using or causing to be made or used, a false record or statement to get a false or fraudulent claim paid or approved by the government; conspiring to defraud the government by getting a false or fraudulent claim paid or approved by the government; or knowingly making, using or causing to be made or used a false record or statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| · | the criminal federal False Claims Act, which imposes criminal fines or imprisonment against individuals or entities who make or present a claim to the government knowing such claim to be false, fictitious or fraudulent; |
| · | the civil monetary penalties statute, which imposes penalties against any person or entity who, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent; |
| · | the Veterans Health Care Act of 1992 that requires manufacturers of “covered drugs” to offer them for sale to certain federal agencies, including but not limited to, the Department of Veterans Affairs, on the Federal Supply Schedule, which requires compliance with applicable federal procurement laws and regulations and subjects manufacturers to contractual remedies as well as administrative, civil and criminal sanctions; |
| · | the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which created new federal criminal statutes that prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by, or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private), knowingly and willfully embezzling or stealing from a health care benefit program, willfully obstructing a criminal investigation of a health care offense and knowingly and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false statements in connection with the delivery of, or payment for, healthcare benefits, items or services relating to healthcare matters; |
| 30 |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans and healthcare clearinghouses as well as their respective business associates that perform services for them that involve individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization, including mandatory contractual terms as well as directly applicable privacy and security standards and requirements; |
| · | the federal Physician Payment Sunshine Act, created under the Patient Protection and Affordable Care Act (the ACA), and its implementing regulations requires manufacturers of drugs, devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program (with certain exceptions) to report annually to the United States Department of Health and Human Services information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors) and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members, with data collection required reporting to CMS by the 90th day following each calendar year; |
| · | federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; |
| · | the Foreign Corrupt Practices Act, a U.S. law which regulates certain financial relationships with foreign government officials (which could include, for example, certain medical professionals); and |
| · | state law equivalents of each of the above federal laws, such as anti-kickback, false claims, consumer protection and unfair competition laws which may apply to our business practices, including but not limited to, research, distribution, sales and marketing arrangements as well as submitting claims involving healthcare items or services reimbursed by any third-party payors, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances many of which differ from each other in significant ways, with differing effect. |
Additionally, the compliance environment is changing, with more states, such as California and Massachusetts, mandating implementation of compliance programs, compliance with industry ethics codes, and spending limits, and other states, such as Vermont, Maine, and Minnesota, requiring reporting to state governments of gifts, compensation, and other remuneration to physicians. These laws all provide for penalties for non-compliance. The shifting regulatory environment, along with the requirement to comply with multiple jurisdictions with different compliance and/or reporting requirements, increases the possibility that a company may inadvertently run afoul of one or more laws.
If our past or present operations are found to be in violation of any of the laws described above or the other governmental regulations to which we, our distributors or our customers are subject, we may be subject to the applicable penalty associated with the violation, including civil and criminal penalties, damages, fines, exclusion from Medicare, Medicaid and other government programs and the curtailment or restructuring of our operations. If we are required to obtain permits or licensure under these laws that we do not already possess, we may become subject to substantial additional regulation or incur significant expense. Any penalties, damages, fines, curtailment or restructuring of our operations would adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that many of them have not been fully or clearly interpreted by the regulatory authorities or the courts, and their provisions are subject to a variety of interpretations and additional legal or regulatory change. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses, divert our management’s attention from the operation of our business and damage our reputation. Moreover, achieving and sustaining compliance with applicable federal and state privacy, security and fraud laws may prove costly.
Medical Device Tax. Significant reforms to the healthcare system were adopted in the form of the ACA. The ACA includes provisions that, among other things, require the medical device industry to subsidize healthcare reform in the form of a 2.3% excise tax (the Medical Device Tax) on the U.S. sales of most medical devices beginning in 2013. We believe this tax is assessed on 100% of our product sales that are sold in the United States. This tax is subject to change due to, among other things, future IRS guidance and interpretations of the Medical Device Tax regulations, and changes in our product mix. This revenue-based tax will have a material impact on our consolidated results of operations, cash flows, and financial condition.
| 31 |
In the year ended June 30, the Company’s medical device tax expense was:
| Amount | ||||
| 2015 | $ | 99,203 | ||
| 2014 | $ | 96,115 | ||
Healthcare Reform Measures Could Hinder Our Products' Commercial Success. In both the United States and certain foreign jurisdictions there have been, and we anticipate there will continue to be, a number of legislative and regulatory changes to the healthcare system that could impact our ability to sell any of our products profitably. In the United States, the Federal government passed healthcare reform legislation, the ACA. The provisions of the ACA have become or will become effective on various dates. While many of the details regarding the implementation of the ACA are yet to be determined, we believe there will be continuing trends towards expanding coverage to more individuals, containing health care costs and improving quality.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to make and implement healthcare reforms may adversely affect:
| · | our ability to set a price we believe is fair for our products; |
| · | our ability to generate revenues and achieve or maintain profitability; |
| · | the availability of capital; and |
| · | our ability to obtain timely approval of any future product modifications. |
CMS has published final regulations that implement provisions in ACA related to disclosure of payments made by manufacturers to physicians and teaching hospitals, effective April 2013. Because we manufacture devices that are covered by the regulations, all payments that we make to physicians and teaching hospitals would be subject to this reporting requirement even if the payment relates to a device that is not considered a covered device. The tracking and reporting of these payments could have an adverse impact on our business and/or consolidated results of operations and financial condition and on our relationships with customers and potential customers.
We May Be Unable To Adequately Protect Or Enforce Our Intellectual Property Rights Or Secure Rights To Third-Party Patents. Our ability and the abilities of our distributors to obtain and maintain patent and other protection for our products will affect our success. We are assigned, have rights to, or have exclusive licenses to patents and patents pending in the U.S. and numerous foreign countries. The patent positions of medical device companies can be highly uncertain and involve complex legal and factual questions. Our patent rights may not be upheld in a court of law if challenged. Our patent rights may not provide competitive advantages for our products and may be challenged, infringed upon or circumvented by our competitors. We cannot patent our products in all countries or afford to litigate every potential violation worldwide.
Because of the large number of patent filings in the medical device and biotechnology field, our competitors may have filed applications or been issued patents and may obtain additional patents and proprietary rights relating to products or processes competitive with or similar to ours. We cannot be certain that U.S. or foreign patents do not exist or will not be issued that would harm our ability to commercialize our products and product candidates.
Pending And Future Patent Litigation Could Be Costly And Disruptive And May Have An Adverse Effect On Our Financial Condition And Results Of Operations. We operate in an industry characterized by extensive patent litigation. Potential patent claims include challenges to the coverage and validity of the Company’s patents on products or processes as well as allegations that the Company’s products infringe patents held by competitors or other third parties. A loss in any of these types of cases could result in a loss of patent protection or the ability to market products, which could lead to a significant loss of sales, or otherwise materially affect future results of operations.
| 32 |
The Company’s commercial success will depend in part on not infringing the patents or violating the other proprietary rights of third parties. Intellectual property litigation is expensive and complex and outcomes are difficult to predict. Any pending or future patent litigation may result in significant damage awards, including treble damages under certain circumstances, and injunctions that could prevent the manufacture and sale of affected products or force us to make significant royalty payments in order to continue selling the affected products. At any given time, we may be involved as either a plaintiff or a defendant in a number of patent infringement actions, the outcomes of which may not be known for prolonged periods of time. As a healthcare supplier, we can expect to face additional claims of patent infringement in the future. A successful claim of patent or other intellectual property infringement against us could adversely affect our results of operations and financial condition.
The Value Of Our Granted Patents, and Our Patents Pending, Is Uncertain. Although our management strongly believes that our patent on the process for producing Cesium-131, our patents on additional methods for producing Cesium-131 and other isotopes, our patent on the manufacture of the brachytherapy seed, and anticipated future patent applications, which have not yet been filed, have significant value, we cannot be certain that other like-kind processes may not exist or be discovered, that any of these patents is enforceable, or that any of our patent applications will result in issued patents.
Failure To Comply With Government Regulations Could Harm Our Business. As a medical device and medical isotope manufacturer, we are subject to extensive, complex, costly, and evolving governmental rules, regulations and restrictions administered by the FDA, by other federal and state agencies, and by governmental authorities in other countries. Compliance with these laws and regulations is expensive and time-consuming, and changes to or failure to comply with these laws and regulations, or adoption of new laws and regulations, could adversely affect our business.
In the United States, as a manufacturer of medical devices and devices utilizing radioactive by-product material, we are subject to extensive regulation by federal, state, and local governmental authorities, such as the FDA and the Washington State Department of Health, to ensure such devices are safe and effective. Regulations promulgated by the FDA under the U.S. Food, Drug and Cosmetic Act, or the FDC Act, govern the design, development, testing, manufacturing, packaging, labeling, distribution, marketing and sale, post-market surveillance, repairs, replacements, and recalls of medical devices. In Washington State, the Department of Health, by agreement with the federal Nuclear Regulatory Commission (NRC), regulates the possession, use, and disposal of radioactive byproduct material as well as the manufacture of radioactive sealed sources to ensure compliance with state and federal laws and regulations. Our Proxcelan® Cesium-131 brachytherapy seeds and the GliaSite® RTS constitute both medical devices and radioactive sealed sources and are subject to these regulations.
Under the FDC Act, medical devices are classified into three different categories, over which the FDA applies increasing levels of regulation: Class I, Class II, and Class III. Our Proxcelan® Cesium-131 seed has been classified as a Class II device and has received clearance from the FDA through the 510(k) pre-market notification process. Any modifications to the device that would significantly affect safety or effectiveness, or constitute a major change in intended use, would require a new 510(k) submission. As with any submittal to the FDA, there is no assurance that a 510(k) clearance would be granted to the Company.
The FDA has been considering legislative, regulatory and/or administrative changes to the FDA’s 510(k) program. Various committees of the U.S. Congress have also indicated that they may consider investigating the FDA’s 510(k) process. Under the current 510(k) rules, certain types of medical devices can obtain FDA approval without lengthy and expensive clinical trials. Among our product offerings, those products that require FDA approval have received such approval under the 510(k) rules. Our R&D programs and new product programs contemplate obtaining any required FDA approvals under the current 510(k) rules. Any changes to the current 510(k) or related FDA rules that make such rules more stringent or require more clinical data can significantly increase the time and costs associated with bringing new products or product modifications to market. This may have a material adverse effect on our business, financial condition and results of operations.
In addition to FDA-required market clearances and approvals for our products, our manufacturing operations are required to comply with the FDA's Quality System Regulation, or QSR, which addresses requirements for a company's quality program such as management responsibility, good manufacturing practices, product and process design controls, and quality controls used in manufacturing. Compliance with applicable regulatory requirements is monitored through periodic inspections by the FDA Office of Regulatory Affairs (ORA). We anticipate both announced and unannounced inspections by the FDA. Such inspections could result in non-compliance reports (Form 483) which, if not adequately responded to, could lead to enforcement actions. The FDA can institute a wide variety of enforcement actions ranging from public warning letters to more severe sanctions such as fines; injunctions; civil penalties; recall of our products; operating restrictions; suspension of production; non-approval or withdrawal of pre-market clearances for new products or existing products and criminal prosecution. There can be no assurance that we will not incur significant costs to comply with these regulations in the future or that the regulations will not have a material adverse effect on our business, financial condition and results of operations.
| 33 |
In addition to the ACA, various healthcare reform proposals have also emerged at the state level. Like the ACA, these proposals could reduce medical procedure volumes and impact the demand for our products or the prices at which we sell our products. The impact of these proposals could have a material adverse effect on our business and/or consolidated results of operations and financial condition.
The automatic spending cuts of nearly $1 trillion over the next 10 years that were included under the Budget Control Act of 2011, including a 2% cut to Medicare providers and suppliers, took effect in 2013. Medicaid is exempt from these cuts. Any cuts to Medicare reimbursement which affect our products could have a material adverse effect on our business and/or our consolidated results of operations and financial condition.
The marketing of our products in foreign countries will, in general, be regulated by foreign governmental agencies similar to the FDA. Foreign regulatory requirements vary from country to country. The time and cost required to obtain regulatory approvals could be longer than that required for FDA clearance in the United States and the requirements for licensing a product in another country may differ significantly from FDA requirements. We will rely, in part, on foreign distributors to assist us in complying with foreign regulatory requirements. We may not be able to obtain these approvals without incurring significant expenses or at all, and the failure to obtain these approvals would prevent us from selling our products in the applicable countries. This could limit our sales and growth.
Quality Problems With Our Products Could Harm Our Reputation For Producing High-Quality Products And Erode Our Competitive Advantage, Sales, And Market Share. Quality is extremely important to us and our customers due to the serious and costly consequences of product failure, which can include patient harm. Our operating results depend in part on our ability to sustain an effective quality control system and effectively train and manage our employee base with respect to our quality system. Our quality system plays an essential role in determining and meeting customer requirements, preventing defects and improving our products and services. While we have a network of quality systems throughout our business lines and facilities, quality and safety issues may occur with respect to any of our products. A quality or safety issue may result in a public warning letter from the FDA, product recalls or seizures, monetary sanctions, injunctions to halt manufacturing and distribution of products, civil or criminal sanctions, refusal of a government to grant clearances or approvals or delays in granting such clearances or approvals, import detentions of any future products made outside the United States, restrictions on operations or withdrawal or suspension of existing approvals. Negative publicity regarding a quality issue could damage our reputation, cause us to lose customers, or decrease demand for our products. Any of the foregoing events could disrupt our business and have an adverse effect on our results of operations and financial condition.
Our Business Exposes Us To Product Liability Claims. Our design, testing, development, manufacture, and marketing of products involve an inherent risk of exposure to product liability claims and related adverse publicity. Our brachytherapy seed products deliver a highly concentrated and confined dose of radiation directly to the organ in which it is implanted from within the patient’s body. Surrounding tissues and organs are typically spared excessive radiation exposure. It is an inherent risk of the industries in which we operate that we might be sued in a situation where one of our products results in, or is alleged to result in, a personal injury to a patient, health care provider, or other user. Although we believe that as of the date of this report, we have adequate insurance to address anticipated potential liabilities associated with product liability, any unforeseen product liability exposure in excess of, or outside the scope of, such insurance coverage could adversely affect our financial condition and operating results. Any such claim brought against us, with or without merit, could result in significant damage to our business. Insurance coverage is expensive and difficult to obtain, and, although we currently have a five million dollar policy, in the future we may be unable to obtain or renew coverage on acceptable terms, if at all. If we are unable to obtain or renew sufficient insurance at an acceptable cost or if a successful product liability claim is made against us, whether fully covered by insurance or not, our business could be harmed. The FDA’s medical device reporting regulations require us to report any incident in which our products may have caused or contributed to a death or serious injury, or in which our products malfunctioned in a way that would be likely to cause or contribute to a death or serious injury if the malfunction reoccurred. Any required filing could result in an investigation of our products and possibly subsequent regulatory action against us if it is found that one of our products caused the death or serious injury of a patient.
| 34 |
Our Business Involves Environmental Risks. Our business involves the controlled use of hazardous materials, chemicals, biologics, and radioactive compounds. Manufacturing is extremely susceptible to product loss due to radioactive, microbial, or viral contamination; material or equipment failure; vendor or operator error; or due to the very nature of the product's short half-life. Although we believe that our safety procedures for handling and disposing of such materials comply with state and federal standards there will always be the risk of accidental contamination or injury. In addition, radioactive, microbial, or viral contamination may cause the closure of the manufacturing facility for an extended period of time. By law, radioactive materials may only be disposed of at state-approved facilities. At our leased facility we use commercial disposal contractors. We are in the planning process of shutting down our leased manufacturing and office facility, planning the construction of a new manufacturing and office facility to be owned by the Company on an adjacent property and moving to the new manufacturing facility. Once it is constructed and licensed, we will incur costs related to the clean-up and disposal of hazardous materials, chemicals and radioactive components of the leased facility. While management believes it has reserved a sufficient amount of funds for this process, the Company may need more than the amount of the asset retirement obligation to meet the lease requirements and to receive clearance from the Washington State Department of Health. We may incur substantial costs related to the disposal of these materials. If we were to become liable for an accident, or if we were to suffer an extended facility shutdown, we could incur significant costs, damages, and penalties that could harm our business.
We Rely Upon Key Personnel. Our success will depend, to a great extent, upon the experience, abilities and continued services of our executive officers, sales staff and key scientific personnel. If we lose the services of several officers, sales personnel, or key scientific personnel, our business could be harmed. Our success also will depend upon our ability to attract and retain other highly qualified scientific, managerial, sales, and manufacturing personnel and their ability to develop and maintain relationships with key individuals in the industry. Competition for these personnel and relationships is intense and we compete with numerous pharmaceutical and biotechnology companies as well as with universities and non-profit research organizations. We are highly dependent on our direct sales organization who promote and support our brachytherapy products. There is intense competition for skilled sales and marketing employees, particularly for people who have experience in the radiation oncology market. Accordingly, we could find it difficult to hire or retain skilled individuals to sell our products. Failure to retain our direct sales force could adversely affect our growth and our ability to meet our revenue goals. There can be no assurance that our direct sales and marketing efforts will be successful. If we are not successful in our direct sales and marketing, our sales revenue and results of operations are likely to be materially adversely affected. We may not be able to continue to attract and retain qualified personnel.
Our Ability To Operate In Foreign Markets Is Uncertain. Our future growth will depend in part on our ability and the ability of our distributors to establish, grow and maintain product sales in foreign markets, particularly in the European Union (EU), and through the German distributor in its territory which includes Germany, Austria, Switzerland, and Luxembourg. However, we have limited experience in marketing and distributing products in other countries. Foreign operations subject us to additional risks and uncertainties, including our customers' ability to obtain reimbursement for procedures using our products in foreign markets; the burden of complying with complex and changing foreign regulatory requirements; time-sensitive delivery requirements due to the short half-life of our product; language barriers and other difficulties in providing long-distance customer service; potentially increased time to collect accounts receivable; significant currency fluctuations, which could cause third-party distributors to reduce the number of products they purchase from us because the cost of our products to them could fluctuate relative to the price they can charge their customers; reduced protection of intellectual property rights in some foreign countries; and the possibility that contractual provisions governed by foreign laws would be interpreted differently than intended in the event of a contract dispute. In addition, the significant appreciation of the U.S. dollar during the past year has made our products much more expensive in overseas markets. Any future foreign sales of our products could also be adversely affected by export license requirements, the imposition of governmental controls, political and economic instability, trade restrictions, changes in tariffs, and difficulties in staffing and managing foreign operations. Many of these factors may also affect our ability to import Cesium-131 from Russia under our contract with JSC INM. Sanctions placed on financial transactions with Russian banking institutions may interfere with the Company’s ability to transact business in Russia on a temporary or other basis resulting in an interruption of the Cs-131 supply which could have a temporary material adverse effect on the Company’s business, operating results and financial condition.
| 35 |
Our Ability To Expand Operations And Manage Growth Is Uncertain. Our efforts to expand our operations will result in new and increased responsibilities for management personnel and will place a strain upon the entire company. To compete effectively and to accommodate growth, if any, we may be required to continue to implement and to improve our management, manufacturing, sales and marketing, operating and financial systems, procedures and controls on a timely basis and to expand, train, motivate and manage our employees. There can be no assurance that our personnel, systems, procedures, and controls will be adequate to support our future operations. If the Proxcelan® Cesium-131 seed were to rapidly become the "seed of choice," it is unlikely that we could immediately meet demand. This could cause customer discontent and invite competition. There can be no assurance that our personnel, systems, procedures, and controls will be adequate to immediately react to that growth.
We Rely On The Performance Of Our Information Technology Systems, The Failure Of Which Could Have An Adverse Effect On Our Business And Performance. Our business requires the continued operation of sophisticated information technology systems and network infrastructure. These systems are vulnerable to interruption by fire, power loss, system malfunction, computer viruses, cyber-attacks and other events, which may be beyond our control. Systems interruptions could reduce our ability to accept customer orders, manufacture our products, or provide service for our customers, and could have an adverse effect on our operations and financial performance. The level of protection and disaster-recovery capability varies from site to site, and there can be no guarantee that any such plans, to the extent they are in place, will be totally effective. In addition, security breaches of our information technology systems could result in the misappropriation or unauthorized disclosure of confidential information belonging to us, our employees, partners, customers, or our suppliers, which may result in significant costs and potential government sanctions. In particular, if we are unable to adequately safeguard individually identifiable health information, we may be subject to additional liability under domestic and international laws respecting the privacy and security of health information.
Fluctuations In Insurance Cost And Availability Could Adversely Affect Our Profitability Or Our Risk Management Profile. We hold a number of insurance policies, including product liability insurance, directors’ and officers’ liability insurance, and workers’ compensation insurance. If the costs of maintaining adequate insurance coverage increase significantly in the future, our operating results could be materially adversely affected. Likewise, if any of our current insurance coverage should become unavailable to us or become economically impractical, we would be required to operate our business without indemnity from commercial insurance providers. If we operate our business without insurance, we could be responsible for paying claims or judgments against us that would have otherwise been covered by insurance, which could adversely affect our results of operations or financial condition.
Risks Of Ongoing Litigation. On May 22, 2015, the first of multiple class action complaints for violation of the federal securities laws were filed in U.S. District Court for the Central District of California and in the Eastern District of Washington against IsoRay, Inc., Dwight Babcock (CEO and Chairman of the Board) and Brien Ragle (CFO). The complaint, purportedly brought on behalf of all purchasers of IsoRay, Inc. common stock from May 20, 2015 through and including May 21, 2015, asserts claims related to a press release on May 20, 2015 regarding a May 19 online publication of the peer-reviewed article in the journal Brachytherapy titled “Analysis of Stereotactic Radiation vs. Wedge Resection vs. Wedge Resection Plus Cesium-131 Brachytherapy in Early-Stage Lung Cancer” by Dr. Bhupesh Parashar, et al. and seeks, among other things, damages and costs and expenses. An order dated August 17, 2015 was filed in the U.S. District Court for the Eastern District of Washington in which the multiple complaints were consolidated into a single action in that district, appointing a group of lead plaintiffs and appointing their choice of lead counsel. The order provided the plaintiffs with the opportunity to amend the complaint. We cannot predict the outcome of such proceedings or provide an estimate of damages, if any. We believe that these claims are without merit and intend to defend them vigorously. While management believes that its insurance will adequately cover costs to defend, settlement and damages, if any, it is too early to determine what those amounts may ultimately be and there is no assurance as to the total costs and outcome of this lawsuit.
We Have Incurred Significant Losses To Date, And There Is No Guarantee That We Will Ever Become Profitable. We incurred net losses of $3,681,050 and $5,959,122 in the fiscal years ended 2015 and 2014, respectively. In addition, we have accumulated deficit from the inception of business through June 30, 2015 of $61,731,506. The costs for research and product development of our products along with marketing and selling expenses and general and administrative expenses have been the principal causes of our losses. We may not ever become profitable and if we do not become profitable your investment could be harmed.
| 36 |
We May Need Additional Capital In The Future For Acquisitions And Expansion Into Other Markets. At June 30, 2015, we had cash and certificates of deposit of $19,696,089. The combination of our current cash and certificates of deposit both current and non-current balance and projected product sales should provide us with sufficient funds to support operations at current levels of expenses and revenues for 5 years. However, we may need to raise capital for strategic acquisitions or expansion into other markets and there is no assurance management will not pursue this additional capital if available.
Risks Related to Our Stock and Reporting Requirements
Our Reporting Obligations As A Public Company Are Costly. Operating a public company involves substantial costs to comply with reporting obligations under federal securities laws that have continued to increase as provisions of the Sarbanes Oxley Act of 2002 have been implemented and may increase again as a result of the Company becoming subject to the accelerated filer requirements of the Securities and Exchange Commission as of the year ended June 30, 2015. The accelerated filing timelines and requirement for the auditor to opine on internal control effectiveness may increase the cost of the quarterly reviews and annual audit and may require additional employees or technology investment to meet these requirements.
Our Stock Price Is Likely To Be Volatile. The market price of our common stock has experienced fluctuations and is likely to fluctuate significantly in the future. For example, during fiscal 2015 the closing price of one share of our common stock reached a high of $3.79 and a low of $1.22. There is generally significant volatility in the market prices and limited liquidity of securities of early stage companies, and particularly of early stage medical product companies. Contributing to this volatility are various events that can affect our stock price in a positive or negative manner. These events include, but are not limited to: governmental approvals of or refusals to approve regulations or actions; market acceptance and sales growth of our products; litigation involving the Company or our industry; developments or disputes concerning our patents or other proprietary rights; changes in the structure of healthcare payment systems; departure of key personnel; future sales of our securities; fluctuations in our financial results or those of companies that are perceived to be similar to us; swings in seasonal demands of purchasers; investors' general perception of us; and general economic, industry and market conditions. In addition, the securities of many medical device companies, including us, have historically been subject to extensive price and volume fluctuations that may affect the market price of their common stock. If any of these events occur, it could cause our stock price to fall.
The Price Of Our Common Stock May Be Adversely Affected By The Future Issuance And Sale Of Shares Of Our Common Stock Or Other Equity Securities. We cannot predict the size of future issuances or sales of our common stock or other equity securities for future acquisitions or capital raising activities, or the effect, if any, that such issuances or sales may have on the market price of our common stock. The issuance and sale of substantial amounts of common stock or other equity securities or announcement that such issuances and sales may occur, could adversely affect the market price of our common stock.
The Issuance Of Shares Upon Exercise Of Derivative Securities May Cause Immediate And Substantial Dilution To Our Existing Shareholders. The issuance of shares upon conversion of the preferred stock and the exercise of common stock warrants and options may result in substantial dilution to the interests of other shareholders since these selling shareholders may ultimately convert or exercise and sell all or a portion of the full amount issuable upon exercise. If all derivative securities outstanding as of September 11, 2015 were converted or exercised into shares of common stock, there would be approximately an additional 2,817,153 shares of common stock outstanding as a result. The issuance of these shares will have the effect of further diluting the proportionate equity interest and voting power of holders of our common stock.
We Do Not Expect To Pay Any Dividends For The Foreseeable Future. We do not anticipate paying any dividends to our shareholders for the foreseeable future except for dividends on the Series B Preferred Stock which we intend to pay on or before December 31, 2015. Shareholders must be prepared to rely on sales of their common stock after price appreciation to earn an investment return, which may never occur. Any determination to pay dividends in the future will be made at the discretion of our Board of Directors and will depend on our results of operations, financial conditions, contractual restrictions, restrictions imposed by applicable laws and other factors that our Board deems relevant.
| 37 |
Certain Provisions of Minnesota Law and Our Charter Documents Have an Anti-Takeover Effect. There exist certain mechanisms under Minnesota law and our charter documents that may delay, defer or prevent a change of control. Anti-takeover provisions of our articles of incorporation, bylaws and Minnesota law could diminish the opportunity for shareholders to participate in acquisition proposals at a price above the then-current market price of our common stock. For example, while we have no present plans to issue any preferred stock, our Board of Directors, without further shareholder approval, may issue shares of undesignated preferred stock and fix the powers, preferences, rights and limitations of such class or series, which could adversely affect the voting power of the common shares. In addition, our bylaws provide for an advance notice procedure for nomination of candidates to our Board of Directors that could have the effect of delaying, deterring or preventing a change in control. Further, as a Minnesota corporation, we are subject to provisions of the Minnesota Business Corporation Act, or MBCA, regarding "business combinations," which can deter attempted takeovers in certain situations. Pursuant to the terms of a shareholder rights plan adopted in February 2007, each outstanding share of common stock has one attached right. The rights will cause substantial dilution of the ownership of a person or group that attempts to acquire the Company on terms not approved by the Board of Directors and may have the effect of deterring hostile takeover attempts. The effect of these anti-takeover provisions may be to deter business combination transactions not approved by our Board of Directors, including acquisitions that may offer a premium over the market price to some or all shareholders. We may, in the future, consider adopting additional anti-takeover measures. The authority of our Board to issue undesignated preferred or other capital stock and the anti-takeover provisions of the MBCA, as well as other current and any future anti-takeover measures adopted by us, may, in certain circumstances, delay, deter or prevent takeover attempts and other changes in control of the Company not approved by our Board of Directors.
ITEM 1B – UNRESOLVED STAFF COMMENTS
We have no unresolved written comments from the SEC staff regarding our filings under the Exchange Act.
The Company's executive offices are located at 350 Hills Street, Suite 106, Richland, WA 99354, (509) 375-1202, where IsoRay currently leases approximately 15,300 square feet of office and laboratory space for approximately $22,850 per month plus janitorial expenses of approximately $400 per month from Energy Northwest, the owner of the Applied Process Engineering Laboratory (the APEL facility). The Company is not affiliated with this lessor. The monthly rent is subject to annual increases based on the Consumer Price Index. The current lease was entered into in May 2013, and expires on April 30, 2016. The lease modification and renewal entered into in May 2013 added one additional three-year renewal option, giving the Company the ability to extend the lease through April 2019.
The Company has negotiated and agreed to a subsequent modification to the lease modification that is awaiting the signatures of both parties that provides modifications to the requirement to return the facility to ground at the time of exit at Company discretion, exercises the additional three year term to April 30, 2019 and modifies the required notice to terminate early from twelve months to six months. This lease modification provides the flexibility required for the Company to plan, design and construct its own production facility which is expected to reduce operational cashflow requirements and provide for long-term security of production capabilities for the Company
The Company has entered into an agreement with the owner of a property adjacent to its leased facility with the expectation of planning, designing and constructing a new production facility which will accommodate the facility requirements for production, laboratory, and administrative offices. The new facility is anticipated to be a similar size to the current facility. The property also provides for additional future building as needed or subdivision, if required. The property is approximately 4.2 acres located within the Technology & Business Campus of the Port of Benton. The agreement provides for a 60 day “Feasibility Period” to determine that the property is acceptable for its intended use and in which the Company at its sole discretion may terminate the agreement. The closing date is to be no later than October 30, 2015.
The Company's management believes that all facilities occupied by the Company are adequate for present requirements, and that the Company's current equipment is in good condition and is suitable for the operations involved.
| 38 |
On May 22, 2015, the first of multiple class action complaints alleging violations of the federal securities laws were filed in U.S. District Court for the Central District of California and in the Eastern District of Washington against IsoRay, Inc., Dwight Babcock (CEO and Chairman of the Board) and Brien Ragle (CFO). The complaints, purportedly brought on behalf of all purchasers of IsoRay, Inc. common stock from May 20, 2015 through and including May 21, 2015, asserts claims related to a press release on May 20, 2015 regarding a May 19 online publication of the peer-reviewed article in the journal Brachytherapy titled “Analysis of Stereotactic Radiation vs. Wedge Resection vs. Wedge Resection Plus Cesium-131 Brachytherapy in Early-Stage Lung Cancer” by Dr. Bhupesh Parashar, et al. and seeks, among other things, damages and costs and expenses. An order dated August 17, 2015 was filed in the U.S. District Court for the Eastern District of Washington in which the multiple complaints were consolidated into a single action in that district, appointing a group of lead plaintiffs and appointing their choice of lead counsel. The order provided the plaintiffs with the opportunity to amend the complaint. We cannot predict the outcome of such proceedings or provide an estimate of damages, if any. We believe that these claims are without merit and intend to defend them vigorously.
ITEM 4 –MINE SAFETY DISCLOSURES
Not applicable
PART II
ITEM 5 – MARKET FOR REGISTRANT'S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
The Company's Articles of Incorporation provide that the Company has the authority to issue 200,000,000 shares of capital stock, which are currently divided into two classes as follows: 192,998,329 shares of common stock, par value of $0.001 per share; and 7,001,671 shares of preferred stock, par value of $0.001 per share. As of September 11, 2015, we had 55,013,553 outstanding shares of Common Stock and 59,065 outstanding shares of Series B Preferred Stock.
On April 19, 2007, our common stock began trading on the American Stock Exchange (now the NYSE MKT) under the symbol "ISR."
| 39 |
The following table sets forth, for the fiscal quarters indicated, the high and low sales prices for our common stock as reported on the NYSE MKT.
| Year ended June 30, 2015 | High | Low | ||||||
| First quarter | $ | 3.24 | $ | 1.35 | ||||
| Second quarter | 2.18 | 1.22 | ||||||
| Third quarter | 1.86 | 1.27 | ||||||
| Fourth quarter | 3.79 | 1.42 | ||||||
| Year ended June 30, 2014 | High | Low | ||||||
| First quarter | $ | 0.82 | $ | 0.52 | ||||
| Second quarter | 0.65 | 0.46 | ||||||
| Third quarter | 3.30 | 0.50 | ||||||
| Fourth quarter | 3.18 | 1.86 | ||||||
The Company has never paid any cash dividends on its Common Stock and does not plan to pay any cash dividends in the foreseeable future. On December 17, 2014, the Board of Directors declared a dividend on the Series B Preferred Stock of all outstanding and cumulative dividends through December 31, 2014. The total Series B accrued dividends of $10,632 were paid as of December 31, 2014. At June 30, 2015, there were 59,065 Series B preferred shares outstanding and cumulative dividends in arrears were $5,316. There are no Series A, Series C or Series D shares of Preferred Stock outstanding as of the date of this Report.
As of September 11, 2015, we had approximately 234 shareholders of record, exclusive of shares held in street name. The closing price of our common stock was $1.46 on September 11, 2015.
Equity Compensation Plans
On May 27, 2005, the Company adopted the 2005 Stock Option Plan (the Option Plan) and the 2005 Employee Stock Option Plan (the 2005 Employee Plan). The Option Plan and the 2005 Employee Plan terminated on May 27, 2015 and no further options may be granted under either Plan. On August 15, 2006, the Company adopted the 2006 Director Stock Option Plan (the Director Plan) pursuant to which it may grant equity awards to eligible persons. Each of the Plans has subsequently been amended. On May 15, 2014, the Company adopted the 2014 Employee Stock Option Plan (the 2014 Employee Plan) pursuant to which it may grant equity awards to eligible persons. The 2014 Employee Plan allows the Board of Directors to grant options to purchase up to 2,000,000 shares of common stock to officers and key employees of the Company. The Director Plan allows the Board of Directors to grant options to purchase up to 1,000,000 shares of common stock to directors of the Company. Options granted under all of the Plans have a ten year maximum term, an exercise price equal to at least the fair market value of the Company's common stock (based on the trading price on the NYSE MKT) on the date of the grant, and with varying vesting periods as determined by the Board.
As of June 30, 2015, the following options had been granted under the option plans.
| Number of | Weighted- | Number of | ||||||||||
| securities to | average | securities | ||||||||||
| be issued on | exercise | remaining | ||||||||||
| exercise of | price of | available for | ||||||||||
| outstanding | outstanding | future | ||||||||||
| options, | options, | issuance | ||||||||||
| warrants, | warrants, | under equity | ||||||||||
| and rights | and rights | compensation | ||||||||||
| Plan Category | # | $ | plans | |||||||||
| Equity compensation plans approved by shareholders | 270,000 | $ | 1.47 | 1,730,000 | ||||||||
| Equity compensation plans not approved by shareholders | 2,148,282 | $ | 1.96 | 133,334 | ||||||||
| Total | 2,418,282 | $ | 1.91 | 1,863,334 | ||||||||
| 40 |
Performance Graph
The graph below matches IsoRay, Inc.'s cumulative 5-year total shareholder return on common stock with the cumulative total returns of the NYSE MKT Composite index and the Russell Microcap® Index. The graph tracks the performance of a $100 investment in our common stock and in each index (with the reinvestment of all dividends) from June 30, 2010 to June 30, 2015.
The stock price performance included in this graph is not necessarily indicative of future stock price performance. The performance graph is furnished solely to accompany this Form 10-K annual report and is not being filed for purposes of the Securities Exchange Act of 1934, as amended, and is not to be incorporated by reference into any filing of the Company, whether made before or after the date hereof, regardless of any general incorporation language in such filing.
Sales of Unregistered Securities
All sales of unregistered securities during the 2015 fiscal year were previously reported.
ITEM 6 – SELECTED FINANCIAL DATA
The following table sets forth our selected consolidated financial data for the periods indicated, derived from consolidated financial statements prepared in accordance with United States generally accepted accounting principles.
| 41 |
Consolidated Statement of Operations Data
| Year Ended June 30, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| Product Sales, net | $ | 4,606,539 | $ | 4,219,158 | $ | 4,525,233 | $ | 5,071,088 | $ | 5,238,973 | ||||||||||
| Cost of product sales | 4,439,146 | 4,415,629 | 4,375,057 | 4,367,884 | 4,081,556 | |||||||||||||||
| Gross income/(loss) | 167,393 | (196,471 | ) | 150,176 | 703,204 | 1,157,417 | ||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Research and development | 614,771 | 668,803 | 627,107 | 780,579 | 981,186 | |||||||||||||||
| Research and development reimbursement | - | - | - | (50,000 | ) | (515,853 | ) | |||||||||||||
| Sales and marketing | 1,488,456 | 1,234,725 | 1,296,149 | 1,215,580 | 1,232,188 | |||||||||||||||
| General and administrative | 2,400,353 | 2,488,219 | 2,294,173 | 2,355,015 | 2,422,884 | |||||||||||||||
| Total operating expenses | 4,503,580 | 4,391,747 | 4,217,429 | 4,301,174 | 4,120,405 | |||||||||||||||
| Operating loss | (4,336,187 | ) | (4,588,218 | ) | (4,067,253 | ) | (3,597,970 | ) | (2,962,988 | ) | ||||||||||
| Non-operating income (select items) | ||||||||||||||||||||
| Interest income | 282,745 | 12,113 | 664 | 747 | 3,381 | |||||||||||||||
| Change in fair value | 374,605 | (1,382,134 | ) | 210,000 | 170,000 | 334,000 | ||||||||||||||
| Net loss applicable to common shareholders | $ | (3,691,683 | ) | $ | (6,696,132 | ) | $ | (3,867,228 | ) | $ | (3,499,537 | ) | $ | (2,852,845 | ) | |||||
| Basic and diluted loss per share | $ | (0.07 | ) | $ | (0.16 | ) | $ | (0.11 | ) | $ | (0.12 | ) | $ | (0.11 | ) | |||||
| Weighted average common Shares used in computing loss per shares | 54,900,828 | 42,675,158 | 34,423,420 | 28,621,831 | 25,131,563 | |||||||||||||||
Consolidated Balance Sheet Data
| As of June 30, | ||||||||||||||||||||
| 2015 | 2014 | 2013 | 2012 | 2011 | ||||||||||||||||
| Cash and cash equivalents | $ | 5,226,740 | $ | 7,680,073 | $ | 2,899,927 | $ | 2,672,711 | $ | 2,112,254 | ||||||||||
| Certificates of deposit | 9,362,574 | 10,002,912 | - | - | - | |||||||||||||||
| Working capital | 15,233,328 | 18,060,973 | 3,650,792 | 3,487,161 | 3,447,795 | |||||||||||||||
| Certificates of deposit, non-current | 5,106,775 | 5,401,398 | - | - | - | |||||||||||||||
| Total assets | 23,003,284 | 26,549,255 | 7,055,356 | 7,505,482 | 7,888,895 | |||||||||||||||
| Long-term liabilities | 1,128,849 | 1,439,560 | 896,242 | 1,038,298 | 662,181 | |||||||||||||||
| Total shareholders’ equity | 20,782,241 | 23,955,768 | 5,366,246 | 5,818,192 | 6,452,516 | |||||||||||||||
ITEM 7 – MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Critical Accounting Policies and Estimates
Management's discussion and analysis of the Company's financial condition and results of operations is based upon its consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosures of contingent liabilities. On an on-going basis, management evaluates past judgments and estimates, including those related to bad debts, inventories, accrued liabilities, and contingencies. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
| 42 |
The Company believes the following critical accounting policies affect its more significant judgments and estimates used in the preparation of its consolidated financial statements.
Accounts Receivable
Accounts receivable are stated at the amount that management of the Company expects to collect from outstanding balances. Management provides for probable uncollectible amounts through an allowance for doubtful accounts. Additions to the allowance for doubtful accounts are based on management's judgment, considering historical write-offs, collections and current credit conditions. Balances which remain outstanding after management has used reasonable collection efforts are written off through a charge to the allowance for doubtful accounts and a credit to the applicable accounts receivable. Payments received subsequent to the time that an account is written off are considered bad debt recoveries.
Inventory
Inventory is reported at the lower of cost or market. Cost of raw materials is determined using the weighted average method. Cost of work in process and finished goods is computed using standard cost, which approximates actual cost, on a first-in, first-out basis.
Licenses
Amortization of licenses is computed using the straight-line method over the estimated economic useful lives of the assets.
Amortization expense of licenses for each fiscal year was:
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Licenses amortization expense | $ | 11,721 | $ | 11,721 | $ | 11,721 | ||||||
Based on the licenses recorded at June 30, 2015, and assuming no subsequent impairment of the underlying assets, the annual amortization expense for each fiscal year ending June 30 is expected to be as follows:
| Fiscal Year | Amount | |||
| 2016 | $ | 15,628 | ||
| All years thereafter | $ | - | ||
Revenue Recognition
The Company applies the provisions of ASC Topic 605, Revenue Recognition. ASC 605 provides guidance on the recognition, presentation and disclosure of revenue in financial statements. ASC 605 outlines the basic criteria that must be met to recognize revenue and provides guidance for the disclosure of revenue recognition policies. The Company recognizes revenue related to product sales when (i) persuasive evidence of an arrangement exists, (ii) shipment has occurred, (iii) the fee is fixed or determinable, and (iv) collectability is reasonably assured.
Revenue for the fiscal years ended June 30, 2015, 2014 and 2013, respectively, was derived primarily from sales of the Proxcelan® Cs-131 brachytherapy seed, which is used in the treatment of cancer. The Company also had sales from the GliaSite® RTS, which is used in the treatment of brain cancer, in the fiscal years ended June 30, 2015, 2014 and 2013, respectively. The Company recognizes revenue once the product has been shipped to the customer. Prepayments, if any, received from customers prior to the time that products are shipped are recorded as deferred revenue. In these cases, when the related products are shipped, the amount recorded as deferred revenue is then recognized as revenue. The Company accrues for sales returns and other allowances at the time of shipment. Although the Company does not have an extensive operating history upon which to develop sales returns estimates, we have used the expertise of our management team, particularly those with extensive industry experience and knowledge, to develop a proper methodology.
| 43 |
Product Returns and Allowances
The Company as part of normal operations allows for customers to receive credit for patient procedures cancelled after shipping to the customer for a variety of criteria. These criteria include but are not limited to a physical symptom on the date of procedure that interferes with the patient's ability to go forward with the procedure, discovery that a patient's condition is beyond treatment during surgery and other criteria as determined acceptable by management.
Stock-Based Compensation
The Company measures and recognizes expense for all share-based payments at fair value. The Company uses the Black-Scholes option valuation model to estimate fair value for all stock options on the date of grant. For stock options that vest over time, the Company recognizes compensation cost on a straight-line basis over the requisite service period for the entire award.
Research and Development Costs
Research and development costs, including salaries, research materials, administrative expenses and contractor fees, are charged to operations as incurred. The cost of equipment used in research and development activities which has alternative uses is capitalized as part of fixed assets and not treated as an expense in the period acquired. Depreciation of capitalized equipment used to perform research and development is classified as research and development expense in the year recognized.
Legal Contingencies
In the ordinary course of business, the Company is involved in legal proceedings involving securities, contractual and employment relationships, product liability claims, patent rights, environmental matters, and a variety of other matters. The Company is also subject to various local, state, and federal environmental regulations and laws due to the isotopes used to produce the Company's product. As part of normal operations, amounts are expended to ensure that the Company is in compliance with these laws and regulations. While there have been no reportable incidents or compliance issues, the Company believes that if it relocates its current production facilities then certain decommissioning expenses will be incurred and has recorded an asset retirement obligation for these expenses.
The Company records contingent liabilities resulting from asserted and unasserted claims against it, when it is probable that a liability has been incurred and the amount of the loss is reasonably estimable. Estimating probable losses requires analysis of multiple factors, in some cases including judgments about the potential actions of third-party claimants and courts. Therefore, actual losses in any future period are inherently uncertain. Currently, the Company does not believe any probable legal proceedings or claims will have a material adverse effect on its financial position or results of operations. However, if actual or estimated probable future losses exceed the Company's recorded liability for such claims, it would record additional charges as other expense during the period in which the actual loss or change in estimate occurred.
Income Taxes
Income taxes are accounted for under the liability method. Under this method, the Company provides deferred income taxes for temporary differences that will result in taxable or deductible amounts in future years based on the reporting of certain costs in different periods for financial statement and income tax purposes. This method also requires the recognition of future tax benefits such as net operating loss carry-forwards, to the extent that realization of such benefits is more likely than not. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment of the change. Management has determined that the Company, its subsidiary Medical, and its subsidiary International are subject to examination of their income tax filings in the United States and state jurisdictions for the 2012 through 2015 tax years. In the event that the Company is assessed penalties and/or interest, penalties will be charged to other operating expense and interest will be charged to interest expense.
| 44 |
Income (Loss) Per Common Share
Basic earnings per share is calculated by dividing net income (loss) available to common shareholders by the weighted average number of common shares outstanding, and does not include the impact of any potentially dilutive common stock equivalents, including preferred stock, common stock warrants or options that are potentially convertible into common stock as those would be anti-dilutive due to the Company's net loss position.
Securities that could be dilutive in the future as of June 30, 2015, 2014 and 2013 were as follows:
| 2015 | 2014 | 2013 | ||||||||||
| Preferred stock | 59,065 | 59,065 | 59,065 | |||||||||
| Common stock warrants | 385,800 | 444,747 | 1,957,033 | |||||||||
| Common stock options | 2,418,282 | 2,314,422 | 2,305,072 | |||||||||
| Total potential dilutive securities | 2,863,147 | 2,818,234 | 4,321,170 | |||||||||
Subsequent Events
Effective April 1, 2009, the Company adopted ASC 855 Subsequent Events. This Statement establishes the accounting for, and disclosure of, material events that occur after the balance sheet date, but before the financial statements are issued. In general, these events will be recognized if the condition existed at the date of the balance sheet, and will not be recognized if the condition did not exist at the balance sheet date. Disclosure is required for non-recognized events if required to keep the financial statements from being misleading. Subsequent events have been evaluated through the date our financial statements were issued—the filing time and date of our 2015 Annual Report on Form 10-K.
Use of Estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the United States of America requires management of the Company to make estimates and assumptions that affect the amounts reported in the financial statements and accompanying notes. Accordingly, actual results could differ from those estimates and affect the amounts reported in the financial statements.
Results of Operations
Financial Presentation
The following sets forth a discussion and analysis of the Company's financial condition and results of operations for the fiscal years ended June 30, 2015, 2014 and 2013. This discussion and analysis should be read in conjunction with our consolidated financial statements appearing elsewhere in this Annual Report on Form 10-K. The following discussion contains forward-looking statements. Our actual results may differ significantly from the results discussed in such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in "Item 1A — Risk Factors," beginning on page 23 of this Annual Report on Form 10-K.
| 45 |
Fiscal 2015 Results Compared to 2014
| Year Ended June 30, | ||||||||||||||||||||
| 2015 | 2014 | 2015-2014 | ||||||||||||||||||
| Amount | % (a) | Amount | % (a) | % Change | ||||||||||||||||
| Product sales, net | 4,606,539 | 100 | % | 4,219,158 | 100 | % | 9 | % | ||||||||||||
| Gross profit / (loss) | 167,393 | 4 | % | (196,471 | ) | -5 | % | 185 | % | |||||||||||
| Research and development | 614,771 | 13 | % | 668,803 | 16 | % | -8 | % | ||||||||||||
| Sales and marketing | 1,488,456 | 32 | % | 1,234,725 | 29 | % | 21 | % | ||||||||||||
| General and administrative | 2,400,353 | 52 | % | 2,488,219 | 59 | % | -4 | % | ||||||||||||
| Non-operating income (Select items) | ||||||||||||||||||||
| Interest income | 282,745 | 6 | % | 12,113 | 0 | % | 2234 | % | ||||||||||||
| Change in fair value of warrant derivative liability | 374,605 | 8 | % | (1,382,134 | ) | -33 | % | 127 | % | |||||||||||
| Net loss | (3,681,051 | ) | -80 | % | (5,959,122 | ) | -141 | % | 38 | % | ||||||||||
| Preferred stock deemed dividends | - | 0 | % | (726,378 | ) | -17 | % | 100 | % | |||||||||||
| Net loss applicable to common shareholders | (3,691,683 | ) | -80 | % | (6,696,132 | ) | -159 | % | 45 | % | ||||||||||
(a) calculated as a percentage of product sales, net
Product sales.
Product Sales
Total revenue from product sales increased $0.39 million in fiscal year 2015 when compared to fiscal year 2014. The 9% year over year growth was the result of 13% overall growth in seed brachytherapy treatments which more than offset the 57% decrease in GliaSite® revenues year over year. The growth in revenue of 9% year over year was significantly impacted by the 41% growth in the fourth quarter results year over year. In the fourth quarter, prostate seed brachytherapy treatments increased by 44%, while other seed brachytherapy treatments grew by 49% and combined to more than offset the decrease in revenue from GliaSite® RTS of 24%.
Prostate Brachytherapy.
During the year ended June 30, 2015 prostate brachytherapy increased to 87% of total revenue compared to 84% of total revenue during the year ended June 30, 2014. The growth in revenue was the result of an 8% increase in the number of cases treated and the loading configurations selected by the physicians. Physicians in fiscal year 2015 utilized approximately 38% more seeds configured into stranded and pre-loaded in needles.
Management believes that the declines in the prostate brachytherapy market prior to fiscal year 2015 have now ended and that increased pressure to deliver effective healthcare in both terms of outcome and cost is beginning to drive some treatment decisions. While market trends can shift rapidly and we remain heavily dependent on five or fewer physicians for our prostate revenue, management believes that the knowledge being shared with physicians is reaching a stage of moving beyond the innovating physician and into a broader market acceptance but there is no assurance these trends will continue.
| 46 |
While there is still overall market pressure to use other treatment options with higher reimbursement rates such as Intensity–Modulated Radiation Therapy (IMRT) and robotic-assisted surgery, management believes that the idea of focal therapy in treating prostate cancer using Cesium-131 seeds combined with conversion of physicians in part or in whole from other competing isotopes based on the data published in peer reviewed articles on the performance of Cesium-131 when compared to other isotopes used in seed brachytherapy, is assisting the Company with better prostate revenue. Additionally, the role of brachytherapy in treating low–risk prostate cancer is beginning to be challenged within the physician community in journal publication addressing the overall cost effectiveness of treating low-risk prostate cancer in the Affordable Care Act (ACA) era with low dose rate (LDR) brachytherapy instead of high-dose rate (HDR) brachytherapy, or IMRT. The analysis projects an additional 70,000 cases in the next ten years from the implementation of the ACA of early prostate cancers, providing favorable comparisons for our product. The cost of using HDR will be approximately 175% of LDR and the cost of using IMRT will be approximately 295% of LDR.
Other Brachytherapy.
The strategy implemented by management in diversifying the number of body sites being actively treated with the Proxcelan® Cs-131 brachytherapy seed has continued to provide approximately 11% of total revenue. These treatments continue to be subject to the influence of a small pool of innovative physicians who are the early adopters of the technology who also tend to be faculty at teaching hospitals which provide the next generation of physicians to learn from the innovative physician. This also causes the revenue created by these types of treatment application to be more volatile and vary significantly from quarter to quarter and year to year.
GliaSite® Radiation Therapy System.
All product sales are generated by the brachytherapy seeds and the related methods of application except for the revenue generated by the sales of GliaSite® RTS which come from sale of the liquid isotope, catheter trays and access trays. Product sales from GliaSite ® RTS decreased 57% in the year ended June 30, 2015 when compared to 2014. GliaSite® RTS contributed 2% of total revenue in the year ended June 2015 compared to 5% in the year ended June 30, 2014. The decrease in product sales from GliaSite ® RTS was the direct result of decreased sales to our distributor in Germany and is attributed to the change in the exchange rate between the US dollar and the Euro as the dollar strengthened during fiscal year 2015 which effectively increased the cost to European customers as all the transactions are conducted in the US dollar.
The conversion of prospects to new GliaSite® RTS customers has been a longer process than originally anticipated by the Company. The Company has experienced lengthy timelines in the internal processes of the medical facilities in reviewing and approving the use of the product at the request of their physician(s). These longer than anticipated internal processes are compounded by uncertain timelines and delays in receiving the approval for the requested modification of each facility's nuclear materials license, which is required to begin using GliaSite® RTS and is dependent on external government regulators.
Cost of product sales.
Total cost of product sales overall have remained materially unchanged with an approximate 1% increase during the fiscal year ended June 30, 2015 compared to the fiscal year ended June 30, 2014. Cost of product sales related to seed production increased by 3% during the fiscal year ended June 30, 2015 when compared to the fiscal year ended June 30, 2014. Cost of product sales related to GliaSite® RTS decreased by 67% in the year ended June 30, 2015 compared to the fiscal year ended June 30, 2014 due to the significant decrease in the sales of this product.
During the year ended June 30, 2015 compared to the year ended June 30, 2014, the total cost of product sales related to seed production increased by 3%. This increase was the net result of increases in payroll, taxes, employee benefits and share-based compensation which included cost of living payroll increases granted to employees at the beginning of the fiscal year along with the associated increased payroll taxes, increased cost of accrued paid vacation and the increase in share-based compensation for options granted to production employees. The increase in pre-loading expense was a function of the increased materials cost. Specifically, the increased number of seeds being ordered in a stranded and pre-loaded needle configuration resulted in an increase in the volume of orders and the increased cost of third-party loading for a certain new configuration that require a particular type of sterilization that the Company does not have the capability to perform (the capital investment in that capability is not yet warranted by the current volume). These cost increases were partially offset by the decreased depreciation expense as production equipment has reached the end of depreciable lives. The cost of product sales related to GliaSite® RTS decreased significantly primarily as the result of decreased product sales. This reduction was the result of reduced isotope purchases and lack of an inventory impairment expense which was a non-recurring cost that occurred in the year ended June 30, 2014.
| 47 |
Gross margin.
Gross margin for the fiscal year ended June 30, 2015 increased substantially when compared to the fiscal year ended June 30, 2014 as the direct result of the increased revenue primarily from the increased sales of brachytherapy seeds for the treatment of prostate cancer on similar cost of goods sold. The additional seeds sold were able to be produced using isotope that was purchased and previously would have been expensed as it decayed, thus the similar cost of goods sold year over year.
Research and development expenses.
Research and development costs for fiscal year ended June 30, 2015 compared to the fiscal year ended June 30, 2014 were decreased by the net of an increased legal expense partially offset by decreased other organ research expense and protocol expense. Legal expense increased as the result of legal costs related to the maintenance of US and European patents that have been granted. Other organ research expense decreased as a result of the completion of the Cesitrex™ project with the FDA clearance and management focusing on projects that have the best opportunity for a return with an engaged partner who is focused on developing their own product that utilize our brachytherapy seeds. Protocol expense decreased as the result of the reinstatement of the brain study at Weill Medical College which temporarily increased the cost related to the reinstatement in fiscal year 2014 and did not reoccur in fiscal year 2015.
Sales and marketing expenses.
Sales and marketing expenses increased during the fiscal year ended June 30, 2015 when compared to the fiscal year ended June 30, 2014. Conventions and tradeshow expense increased as there was increased investment in the Company presence at the American Society for Radiation Oncology annual meeting, general attendance at various other tradeshows and conducting a national sales meeting to refine the information being presented to physicians. Payroll, benefits and share-based compensation increased primarily as the result of increased commission compensation based on territory sales managers reaching certain targets in the final four months of the fiscal year ended June 30, 2015, and increased payroll and related taxes and insurance expenses as the result of having an increased number of team members in the year ended June 30, 2015. Travel expense increased as a function of having additional territory sales managers in the field which increased the Company cost of travel, including general ground transportation, including mileage reimbursement, auto rentals, cabs and parking.
General and administrative expenses.
General and administrative expenses decreased by 4% during the fiscal year ended June 30, 2015 when compared to the fiscal year ended June 30, 2014. Costs were reduced related to legal services and public company expense while there were increases to insurance expense and occupancy expense.
The increased cost of insurance expense was the result of a changed insurance market in the directors and officers segment in August of 2014 which resulted in an 84% increase in cost due to the overall directors and officers insurance market adjusting its risk tolerance in the Summer of 2014. The Company renewed its directors and officers insurance for fiscal year 2016 during August 2015 with only a moderate increase in cost and increased coverage. Costs from legal counsel decreased by 13% in the year ended June 30, 2015 as the result of no legal input needed for capital raises which was required in the year ended 2014 and public company expense decreased by 12% as a direct result of a temporary reduction in the number of independent board members from 3 to 2 serving on the board of directors.
Operating loss.
Operating loss for the year ended June 30, 2015 compared to the year ended June 30, 2014 decreased as a result of increased revenue generated from the sales of brachytherapy seeds for the treatment of prostate cancer; increases in product sales from other seed brachytherapy partially reduced by a decrease in the sales of GliaSite® RTS and a minimal increase in cost of product sales.
| 48 |
Change in fair value of warrant derivative liabilities.
During the years ended June 30, 2015 and June 30, 2014, there were changes in the fair value of the warrant derivative liabilities established upon issuance of the warrants during October 2011 and December 2011 to the purchasers and underwriters in the Company’s registered public offering. Per ASC 820, the warrant derivative liability requires periodic evaluation for changes in fair value. As required at June 30, 2015 and June 30, 2014, the Company evaluated the fair value of the warrant derivative liability using the Black-Scholes option pricing model on which the original warrant derivative liability was based and applied updated inputs as of those dates. The resulting change in fair value was recorded as of June 30, 2015 and 2014, respectively.
Fiscal 2014 Results Compared to 2013
| Year Ended June 30, | ||||||||||||||||||||
| 2014 | 2013 | 2014 - 2013 | ||||||||||||||||||
| Amount | % (a) | Amount | % (a) | % Change | ||||||||||||||||
| Product sales, net | 4,219,158 | 100 | % | 4,525,233 | 100 | % | -7 | % | ||||||||||||
| Gross profit / (loss) | (196,471 | ) | -5 | % | 150,176 | 3 | % | -231 | % | |||||||||||
| Research and development | 668,803 | 16 | % | 627,107 | 14 | % | 7 | % | ||||||||||||
| Sales and marketing | 1,234,725 | 29 | % | 1,296,149 | 29 | % | -5 | % | ||||||||||||
| General and administrative | 2,488,219 | 59 | % | 2,294,173 | 51 | % | 8 | % | ||||||||||||
| Non-operating income (Select items) | ||||||||||||||||||||
| Interest income | 12,113 | 0 | % | 664 | 0 | % | 1724 | % | ||||||||||||
| Change in fair value of warrant derivative liability | (1,382,134 | ) | -33 | % | 210,000 | 5 | % | -758 | % | |||||||||||
| Net loss | (5,959,122 | ) | -141 | % | (3,856,596 | ) | -85 | % | -55 | % | ||||||||||
| Preferred stock deemed dividends | (726,378 | ) | -17 | % | - | 0 | % | -100 | % | |||||||||||
| Net loss applicable to common shareholders | (6,696,132 | ) | -159 | % | (3,867,228 | ) | -85 | % | -73 | % | ||||||||||
(a) calculated as a percentage of product sales, net
Product sales.
Prostate Brachytherapy.
Revenue generated from treatment with prostate brachytherapy increased from 82% of total revenue in the fiscal year ended June 30, 2013 to 84% of total revenue in the fiscal year ended June 30, 2014. Prostate brachytherapy revenue decreased at a slower rate than revenue classified as other product sales which resulted in the increase from 82% to 84% of the decreased total revenue during fiscal year 2014 compared to fiscal year 2013. Management believes that the continuing decrease in sales for non-prostate applications has resulted from key physicians being assigned to new roles within their facilities, moving to new facilities that are not licensed for Cesium-131 and the ongoing incentive to recover capital investments in treatment equipment and the required facilities to house the equipment for competing treatment methods by those facilities. Management believes that the overall market for prostate brachytherapy has continued to receive increased pressure from other treatment options with higher reimbursement rates such as Intensity–Modulated Radiation Therapy (IMRT) and robotic-assisted surgery. Although combination treatments incorporating brachytherapy with other modalities in the prostate and treatment of other body sites with brachytherapy have increased, these increases are insufficient to offset the overall decrease in use of prostate brachytherapy.
| 49 |
Other Brachytherapy.
The strategy implemented by management in the prior year in diversifying the number of body sites being actively treated with the Proxcelan® Cs-131 brachytherapy seed has continued to partially mitigate the lost revenue from the prostate brachytherapy segment. The timeline of developing and bringing new products from concept to revenue production in the pharmaceutical/medical device segment is lengthy and is typically measured in years. The probability of any new cancer treatment product reaching the stage at which it produces revenue is very low.
Company management has been investing in development of alternative uses for the Company’s brachytherapy seed that management believes have the ability to generate revenue in the near-term to offset development costs. New treatments such as those being initiated by the Company typically experience a staged entry to market in which primary adopters demonstrate the suitability of a treatment, after which wider adoption is possible. The non-prostate products are very dependent on first adopters as a source of revenue. While there may be a steep growth in revenue, it often plateaus due to capacity constraints until the mainstream adoption occurs, when and if there is favorable publication of the experiences and treatment outcomes of the first adopters. Through June 30, 2014, the Company had only experienced minimal sales to first adopters.
During the fiscal year ended June 30, 2014, there were over seven hundred and eighty cases treated with the Company’s Cs-131 brachytherapy seeds, with approximately 12% of the cases being non-prostate applications. Management’s strategy includes soliciting the use of other applications for the Company's brachytherapy seeds at major medical institutions that are more likely to publish their outcomes and that are training the next generation of decision makers. Company management intends to actively pursue alternative uses for the Company’s brachytherapy seeds in treatments consistent with the FDA clearance granted permitting the Company to utilize other FDA cleared application methods as a means of administering the treatments.
During the year ended June 30, 2014, the revenue from other brachytherapy treatments decreased 13% over the year ended June 30, 2013. While revenue for the GliaSite® RTS increased 33%, gynecological increased 70% and head and neck increased 56%, these areas of growth were offset by a 15% decrease in revenue from the treatment of brain cancer and a 41% decrease in revenue from the treatment of lung cancer. Management believes the decrease in brain and lung treatments were caused by two physicians that were significant early adopters who were on extended leave during the 2014 fiscal year.
GliaSite® Radiation Therapy System.
During the fiscal year ended June 30, 2014, revenue from the GliaSite® RTS increased by approximately 33% or $57,000 compared to the fiscal year ended June 30, 2013. All product sales are generated by the brachytherapy seeds and the related methods of application except for the revenue generated by the sales of GliaSite® RTS which come from sale of the liquid isotope, catheter trays and access trays.
The conversion of prospects to new GliaSite® RTS customers has been a longer process than originally anticipated by the Company. The Company has experienced lengthy timelines in the internal processes of the medical facilities in reviewing and approving the use of the product at the request of their physician(s). These longer than anticipated internal processes are compounded by uncertain timelines and delays in receiving the approval for the requested modification of each facility's nuclear materials license, which is required to begin using GliaSite® RTS and is dependent on external government regulators. On December 17, 2013, the Company received clearance from the US Food and Drug Administration to market Cesitrex™ (Cs-131 in liquid form) with the GliaSite® RTS.
Cost of product sales.
Cost of product sales overall have remained materially unchanged during the fiscal year ended June 30, 2014 compared to the fiscal year ended June 30, 2013 with the exception of two categories of cost, the medical device tax expense which increased by 76% and GliaSite® RTS cost of product sold increased by 31%. The additional medical device tax of approximately $0.04 million during the twelve months ended June 30, 2014 was the result of the medical device tax being applicable to only two quarters during the fiscal year ended June 30, 2013 as compared to all four quarters during the fiscal year ended June 30, 2014. The additional cost of product sales related to the GliaSite® RTS of approximately $0.034 million were from the additional cost of the Iotrex® solution ordered and produced to satisfy the increased volume orders of approximately $0.014 million and a minimum royalty obligation related to the licensing of intellectual property utilized in the GliaSite® RTS system of approximately $0.015 million.
| 50 |
Gross margin.
Gross margin for the fiscal year ended June 30, 2014 decreased 231% when compared to the fiscal year ended June 30, 2013. The change in gross margin was primarily as a result of the previously discussed reduction in sales in the prostate market when combined with the additional cost of the medical device tax, increased cost of isotope to meet the increased number of GliaSite® RTS orders and fixed contractual minimums related to isotope purchases that were lost to decay, partially offset by cost savings in other areas during the fiscal year ended June 30, 2014 when compared to June 30, 2013.
Research and development expenses.
Research and development costs for fiscal year ended June 30, 2014 were increased due to the protocol expense increased by 53% as the Company reinstated an investment in the brain study at Weill Medical College. The Company continued to invest in protocols in support of products that have been developed and sales have begun in support of gaining general acceptance in the market. During the fiscal year ended June 30, 2014, the Company accrued protocol costs in accordance with its agreements with participating facilities.
Sales and marketing expenses.
Sales and marketing expenses decreased during the fiscal year ended June 30, 2014 when compared to the fiscal year ended June 30, 2013 primarily as a result of the decreased hiring costs due to the reduction in the use of outside agencies to hire additional sales staff of 92% and the reduction in costs associated with travel expense of 15% and specifically a reduction in meals expense.
General and administrative expenses.
General and administrative expenses increased by 8% during the fiscal year ended June 30, 2014 when compared to the fiscal year ended June 30, 2013 primarily as the result of increased legal costs of 42% year over year and increased share-based compensation of 367%, which was the result of fully vested options to purchase 100,000 shares of common stock awarded to Dwight Babcock, CEO, valued at $0.116 million at various grant dates.
Operating loss.
Operating loss for the year ended June 30, 2014 compared to the year ended June 30, 2013 increased by 13% as a result of decreased revenue generated from the sales of brachytherapy seeds for the treatment of prostate cancer; which was not offset by a sufficient increase in product sales from other seed brachytherapy and sales of GliaSite® RTS; coupled with cost of product sales which failed to decrease commensurate with the decrease in revenues.
Change in fair value of warrant derivative liabilities.
During the years ended June 30, 2014 and June 30, 2013, there were changes in the fair value of the warrant derivative liabilities established upon issuance of the warrants during October 2011 and December 2011 to the purchasers and underwriters in the Company’s registered public offering. Per ASC 820, the warrant derivative liability requires periodic evaluation for changes in fair value. As required at June 30, 2014 and June 30, 2013, the Company evaluated the fair value of the warrant derivative liability using the Black-Scholes option pricing model on which the original warrant derivative liability was based and applied updated inputs as of those dates. The resulting increase of 758% in fair value was recorded as of June 30, 2014 and 2013, respectively.
| 51 |
Liquidity and capital resources.
At June 30, 2015, we had $5.23 million in cash and cash equivalents and $14.48 million in investments. Cash from operations could be affected by various risks and uncertainties, including, but not limited to, the risks included in Part I, Item 1A titled "Risk Factors." Based on our current business plan and revenue prospects, we believe that we will have sufficient cash resources and anticipated cash flows to fund our operations for at least the next 12 months and, at current levels of revenue and expense, for the next five years.
The Company has historically financed its operations through the sale of common stock and the issuance of related common stock warrants. During fiscal years 2015 and 2014, the Company used existing cash reserves and cash received through sales of common stock of approximately $3.66 million and $3.27 million, respectively, to fund operations and capital expenditures.
Cash flows
| For the years ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Net cash used in operating activities | $ | (3,521,858 | ) | $ | (3,228,221 | ) | $ | (3,056,267 | ) | |||
| Net cash provided by (used in) investing activities | 783,660 | (15,441,156 | ) | (12,816 | ) | |||||||
| Net cash provided by financing activities | 284,865 | 23,449,523 | 3,296,299 | |||||||||
| Net increase (decrease) in cash and cash equivalents | $ | (2,453,333 | ) | $ | 4,780,146 | $ | 227,216 | |||||
Cash flows from operating activities
Net cash used in operating activities was $3.52 million in fiscal 2015 as compared to net cash used in operating activities of $3.23 million in fiscal 2014. Net cash used in operating activities in fiscal 2015 was primarily related to:
| · | Net loss of $3.68 million |
| · | Net loss offset by non-cash items of $0.52 million related to allowance for doubtful accounts; depreciation of fixed assets; amortization of other assets; change in fair value of warrant derivative liability; accretion of asset retirement obligation and share-based compensation. |
| · | Increase in accounts receivable of $0.13 related to the significant increase of product sales in the fourth quarter. |
| · | Increase in inventory of $0.14 million as the result of a purchase of a quantity of inventory items that the Company was able to obtain a volume discount by purchasing a supply that will last beyond the current operating cycle and some of which has been reclassified as inventory, non-current. |
| · | Increase in prepaid expenses and other current assets of $0.06 million, the result of prepayment of certain insurance policies at renewal for fiscal year 2016. |
| · | Decrease in accounts payable of $0.08 million as a result of the timing of payments being issued. |
| · | Increase in accrued protocol expense of $0.04 million as a result of the timing of facilities billing for their expenses incurred. |
| · | Decrease in accrued radioactive waste disposal of $0.01 million as the Company accrued $0.05 million of cost during the year and incurred $0.06 million of actual disposal cost during the fiscal year. |
Net cash used in operating activities was $3.23 million in fiscal 2014 as compared to net cash used in operating activities of $3.06 million in fiscal 2013. Net cash used in operating activities in fiscal 2014 was primarily related to:
| · | Net loss of $5.96 million |
| · | Net loss offset by non-cash items of $2.34 million related to allowance for doubtful accounts; depreciation of fixed assets; amortization of other assets; change in fair value of warrant derivative liability; accretion of asset retirement obligation and share-based compensation. |
| · | Increase in inventory of $0.05 million as the result of the timing of purchases during the fourth quarter. |
| · | Increase in prepaid expenses and other current assets of $0.06 million, the result of prepayment of certain insurance policies at renewal for fiscal year 2016. |
| · | Increase in accounts payable of $0.14 million as a result of the timing of payments being issued. |
| 52 |
| · | Increase in accrued protocol expense of $0.06 million as a result of the timing of facilities billing for their expenses incurred. |
| · | Increase in accrued radioactive waste disposal as the Company accrued $0.05 million of cost during the year and incurred $0.01 million of actual disposal cost during the fiscal year. |
| · | Increase in accrued payroll and related taxes of $0.11 million as the result of federal withholding taxes and FICA taxes payable of $0.91 million related to the exercise of non-qualified employee stock options and timing differences in the timing of the amount of payroll accrual of $0.20 million. |
Cash flows from investing activities
Net cash provided by investing activities was $0.78 million in fiscal 2015, which primarily consisted of maturities of certificates of deposit of $15.87 million, offset by purchases of certificates of deposit of $10.15 million, offset by purchases of certificates of deposit, non-current of $4.8 million, and offset by purchases of fixed assets of $0.13 million.
Net cash used in investing activities was $15.44 million in fiscal 2014, which primarily consisted of purchases of certificates of deposit of $10.0 million and purchases of certificates of deposit, non-current of $5.4 million.
Cash flows from financing activities
Net cash provided by financing activities during fiscal 2015 was $0.29 million, attributable to $0.21 million from proceeds from employee stock option exercises and $0.08 million from proceeds from warrant exercises.
Net cash provided by financing activities during fiscal 2014 was $23.45 million, attributable to $1.48 million from the sale of convertible preferred stock in an underwritten offering, $1.8 million from the sale of common stock in an underwritten offering, $13.82 million from the sale of common stock in a registered public offering, to $0.27 million from proceeds from employee stock option exercises and $6.1 million from proceeds from warrant exercises.
Projected 2016 Liquidity and Capital Resources
Operating Capital and Capital Expenditure Requirements
As of June 30, 2015, the Company had working capital of $15.24 million and certificates of deposit, non-current of $5.11 million that could either be liquidated with an early withdraw penalty or used as security against a debt facility if required.
Our future capital requirements depend on numerous factors. These factors include but are not limited to the following:
| · | Purchase of land and construction of a new manufacturing and office facility on this property. |
| · | Inability to expand international markets due to the strength of the U.S. dollar. |
| · | Facilities, equipment and IT systems required to support current and future operations. |
| · | Our ability to continue to adapt our product within our current regulatory clearances or to obtain regulatory clearances for an adaptation of our product to meet the treatment needs of physicians. |
| · | Stability of costs associated with our manufacturing processes including isotope and other components with a limited number of manufacturers. |
| · | Costs of marketing initiatives. |
| · | Maintaining a quality system that meets the requirements of the FDA and the British Standards Institute, our notified body, to maintain our CE mark. |
| · | Effects of competing products in our markets and changes in the markets as a whole. |
| · | Number and timing of acquisitions. |
Management believes that our current cash and cash equivalents and investments will be sufficient to meet our anticipated cash needs for working capital and capital expenditures for at least 12 months and, at our present levels of revenue and expenses, for the next five years. If these sources of cash, cash equivalents and investments are insufficient to satisfy our liquidity requirements, we may seek to sell additional equity, debt securities or obtain credit facilities. The sale of additional equity or convertible debt securities could result in dilution to our stockholders. If additional funds are raised through the issuance of debt securities, these securities could have rights senior to those associated with our common stock and could contain covenants that would restrict our operations. Additional financing may not be available at all, or in amounts or on terms acceptable to us. If we are unable to obtain this additional financing, we may be required to reduce the scope of our planned product development and marketing efforts.
| 53 |
During fiscal year 2016, the Company intends to continue its existing protocol studies and to begin new protocol studies on lung and inter-cranial cancer treatments using Cesium-131 brachytherapy seeds and the GliaSite® RTS. The Company believes that no more than $250,000 in expense will be incurred during fiscal year 2015 related to protocol expenses relating to lung cancer, inter-cranial cancer and both dual therapy and mono therapy prostate cancer protocols but there is no assurance that these protocols will not cost more than anticipated.
Management plans to attain breakeven and generate additional cash flows by increasing revenues from the Company's existing treatment applications of the Cs-131 brachytherapy seed to both new and existing customers (through our direct sales channels and through our distributors), while expanding into new market applications for Cs-131 and continuing to maintain the Company's focus on cost control.
Additionally, management plans to increase revenue through expanding the sale of the FDA cleared and ISO 13845:2003 certified GliaSite® RTS to current customers, adding new customers in the United States through the Company’s direct sales force, through international sales with the existing distribution agreements which cover Germany, Austria, Switzerland, Italy, Luxembourg, Russia, Australia and New Zealand, and the addition of other distribution channels to European Union countries covered by the ISO certifications. In the past year, the strengthening of the US dollar against other currencies has made expansion of international sales difficult.
Management believes the Company will reach breakeven with revenues of approximately $750,000 per month with cash flow breakeven from operations being reached at approximately $700,000. However, there can be no assurance that the Company will attain profitability or that the Company will be able to attain its revenue targets. Sales in the prostate market have shrunk during three of the past four years, which has not allowed breakeven to be reached during the past four fiscal years. The most recent fiscal year experienced an increase of 13% in prostate brachytherapy revenue compared to the year ended June 30, 2014 but there is no assurance this trend will continue. Sales of other applications and of the GliaSite® RTS have been nominal and historically have not been a substantial contributor to total revenue.
On March 21, 2014, the Company entered into a Securities Purchase Agreement with certain investors providing for the sale of a total of 5,644,300 shares of common stock for an aggregate purchase price of $14,675,180 at a price per share of $2.60 (the Registered Direct Offering). The Company received net proceeds from the offering of approximately $13,814,742 from the Registered Direct Offering which will be used to meet the Company’s working capital needs and general corporate purposes.
On August 29, 2013, the Company entered into an agreement to sell 3,800,985 common units, each consisting of 1 share of the Company’s common stock and a warrant to purchase 0.816 shares of common stock (the Common Units), and 1,670 preferred units, each consisting of 1 share of Series D Convertible Preferred Stock and a warrant to purchase 1,525.23 shares of common stock (the Preferred Units) on a firm commitment underwritten basis. The Common Units were sold at an initial per unit purchase price of $0.535 and the Preferred Units were sold at an initial per unit purchase price of $1,000. The warrants are all exercisable at $0.72 per share and have a twenty-four month term. Each share of the Series D Convertible Preferred Stock is convertible into 1,869.15 shares of common stock at any time at the option of the holder, subject to adjustment, provided that the holder will be prohibited from converting Series D Convertible Preferred Stock into shares of the Company’s common stock if, as a result of such conversion, the holder, together with affiliates, would own more than 9.99% of the total shares of the Company’s common stock then issued and outstanding. The offering yielded approximately $3,279,292 in cash after expenses.
The Company also received over $6 million from warrant and option exercises in fiscal year 2014.
There was no material change in the use of proceeds from our public offerings as described in our final prospectus supplements filed with the SEC pursuant to Rule 424(b) on August 29, 2013 and March 24, 2014. Through June 30, 2014, the Company had used the net proceeds raised through the August 2013 and March 2014 offerings as described in the table below and held the remaining net proceeds in cash and cash equivalents, short-term investments and investments. No offering expenses were paid directly or indirectly to any of our directors or officers (or their associates) or persons owning ten percent or more of any class of our equity securities or to any other affiliates.
| 54 |
| Offering description | Period | Net proceeds | Remaining net proceeds | |||||||||
| Underwritten offering | August 2013 | 3,279,292 | - | |||||||||
| Registered direct offering | March 2014 | 13,814,742 | 13,814,742 | |||||||||
| Total | $ | 17,094,034 | $ | 13,814,742 | ||||||||
| Proceeds used in the year ended June 30, 2015: | ||||
| Indirect payments to directors and officers for database development | $ | 24,000 | ||
| Direct payments for services to directors | 80,867 | |||
| Direct payments of salaries to officers | 569,836 | |||
| Working capital | 3,004,778 | |||
| Total proceeds used in the year ended June 30, 2015 | $ | 3,679,481 |
As a result of these recent capital raises, management does not need to raise financing during fiscal 2016 but may elect to do so in its sole discretion. If financing is obtained, it may be dilutive to shareholders. Of course, funding may not be available to the Company on acceptable terms, or at all.
Other Commitments and Contingencies
In April 2013, Medical exercised the second of two options to renew the original lease that was entered into on May 2, 2007 with Energy Northwest, the owner of the Applied Process Engineering Laboratory (the APEL lease), for an additional 3 years with a new lease expiration date of April 30, 2016. The Company agreed to modification number 14 which became effective on May 1, 2014. The lease modification provided for a contractually permitted rent increase based on a CPI index which was 1.1%. The modification also provided the Company with an additional (third) three year option to extend its tenancy beyond the current expiration date of April 30, 2016. The rent contained in lease modification number 15 beginning on May 1, 2014 is $22,850. The Company is planning to exercise the option to extend the lease to April 30, 2019 and is in the process of exercising that option along with certain modifications that have been negotiated between management and the landlord related to the future construction of a new manufacturing and office facility between now and the expiration of the lease extension on April 30, 2019.
Future minimum lease payments under operating leases, including the one remaining three-year renewal of the APEL lease, are as follows:
| Year ending June 30, | ||||
| 2016 | $ | 278,855 | ||
| 2017 | 278,855 | |||
| 2018 | 278,855 | |||
| 2019 | 232,380 | |||
| $ | 1,068,045 | |||
The Company is subject to various local, state, and federal environmental regulations and laws due to the isotopes used to produce the Company's products. As part of normal operations, amounts are expended to ensure that the Company is in compliance with these laws and regulations. While there have been no reportable incidents or compliance issues, the Company believes that if it relocates its current production facilities then certain decommissioning expenses will be incurred. An asset retirement obligation was established in the first quarter of fiscal year 2008 for the Company's obligations at its new production facility. This asset retirement obligation will be for obligations to remove any residual radioactive materials and to remove all leasehold improvements.
The industry that the Company operates in is subject to product liability litigation. Through its production and quality assurance procedures, the Company works to mitigate the risk of any lawsuits concerning its products. The Company also carries product liability insurance to help protect it from this risk.
| 55 |
The Company received a Qualifying Therapeutic Discovery Project (QTDP) grant in lieu of a QTDP credit for the Company tax years 2010 and 2011. The costs of the Company associated with these grants are subject to examination as are the tax returns of the Company. While there is no indication that the Internal Revenue Service intends to examine these returns or the costs utilized as the underlying basis for the receipt of the grant funds, these grant funds are subject to recapture if the associated costs are determined by the Service to not meet the definition of a "Qualified Investment" during an examination.
Off Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Contractual Obligations and Commitments
The following is a schedule summarizing our obligations to make future payments under contractual obligations as of June 30, 2015:
| Less than | 1 – 3 | 3 – 5 | More than 5 | |||||||||||||||||
| Contractual obligations | Total | 1 year | years | years | years | |||||||||||||||
| Operating lease obligations | $ | 1,068,045 | $ | 278,855 | $ | 790,090 | $ | - | $ | - | ||||||||||
| Seed core purchase obligation | 228,256 | 114,128 | 114,128 | - | - | |||||||||||||||
| Asset retirement obligation | 1,036,764 | - | - | 1,036,764 | - | |||||||||||||||
Our purchase commitments and obligations include all open purchase orders and contractual obligations in the ordinary course of business, including commitments with contract manufacturers and suppliers, for which we have not received the goods or services and acquisition and licensing of intellectual property. A majority of these purchase obligations are due within a year. Although open purchase orders are considered enforceable and legally binding, the terms generally allow us the option to cancel, reschedule, and adjust our requirements based on our business needs prior to the delivery of goods or performance of services, and hence, have not been included in the table above.
Inflation
Management does not believe that the current levels of inflation in the United States have had a significant impact on the operations of the Company. If current levels of inflation hold steady, management does not believe future operations will be negatively impacted.
New Accounting Standards
In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2014-09, "Revenue from Contracts with Customers" (ASU 2014-09), which supersedes the revenue recognition requirements in FASB Accounting Standards Codification (ASC) Topic 605, "Revenue Recognition". The guidance requires that an entity recognize revenue in a way that depicts the transfer of promised goods or services to customers in the amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods and services. The guidance will be effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period and is to be applied retrospectively, with early application not permitted. The Company is currently evaluating the new standard and its impact on the Company's consolidated financial statements.
| 56 |
ITEM 7A – QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We do not utilize derivative financial instruments, derivative commodity instruments or other market risk sensitive instruments, positions or transactions.
Interest Rate Risk
Market risk represents the risk of loss that may impact our consolidated financial position, results of operations or cash flows due to adverse changes in financial and commodity market prices and rates. We are exposed to market risk primarily in the area of changes in United States Treasury interest rates. All investments are in certificates of deposit of varying terms and in FDIC insured amounts.
To minimize market risk, we have in the past and, to the extent possible, will continue in the future, to hold debt securities to maturity at which time the debt security will be redeemed at its stated or face value.
Fair Value Measurements
We account for our common stock warrants pursuant to the authoritative guidance on accounting for derivative financial instruments indexed to, and potentially settled in, a company's own stock, on the understanding that in compliance with applicable securities laws, the registered warrants require the issuance of registered securities upon exercise and do not sufficiently preclude an implied right to net cash settlement. We classify warrants on the consolidated balance sheet as a long-term liability that is revalued at each balance sheet date subsequent to the initial issuance.
Foreign Currency Risk
All of our manufacturing operations are conducted in the United States and all transactions during the year ended June 30, 2015 have been made in United States dollars. Accordingly, we have not had any material exposure to foreign currency rate fluctuations.
ITEM 8 – FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this Item 8 is incorporated by reference to our Consolidated Financial Statements and the Report of Independent Registered Public Accounting Firm beginning at page F-1 of this report.
ITEM 9 – CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
There were no disagreements or reportable events with DeCoria, Maichel & Teague, P.S.
ITEM 9A – CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the design and operation of our disclosure controls and procedures, as such term is defined under Rules 13a-14(c) and 15d-14(c) promulgated under the Securities Exchange Act of 1934, as amended (the "Exchange Act"), as of June 30, 2015. Based on that evaluation, our principal executive officer and our principal financial officer concluded that the design and operation of our disclosure controls and procedures were effective. The design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote. However, management believes that our system of disclosure controls and procedures is designed to provide a reasonable level of assurance that the objectives of the system will be met.
| 57 |
Management's Annual Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Our internal control over financial reporting is designed to provide reasonable assurance concerning both the reliability of our financial reporting and the preparation of our financial statements in accordance with generally accepted accounting principles. This control includes policies and procedures that obligate us to maintain reasonably detailed records that accurately and fairly reflect our transactions and the disposition of our assets, provide assurance that our transactions are properly recorded, ensure that our receipts and expenditures are authorized by management and, where applicable, our board of directors, and prevent or allow us to timely detect material unauthorized acquisitions, uses or dispositions of our assets.
We have evaluated the effectiveness of our internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control Integrated Framework (2013). This evaluation was performed under the supervision and with the participation of our management, including our chief executive officer and our chief financial officer, both of whom concluded that our internal control over financial reporting was effective as of June 30, 2015. Our evaluation of the effectiveness of our internal control over financial reporting in future periods may differ due to changing conditions or non-compliance with the policies and procedures we have established.
Attestation Report of Independent Registered Public Accounting Firm
The independent registered public accounting firm that audited the consolidated financial statements that are included in this Annual Report on Form 10-K has issued an audit report on the effectiveness of our internal control over financial reporting as of June 30, 2015. The report appears below.
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
IsoRay, Inc. and Subsidiaries
Richland, Washington
We have audited IsoRay, Inc. and Subsidiaries’ internal control over financial reporting as of June 30, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). IsoRay, Inc. and Subsidiaries’ management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Item 9A, Management’s Annual Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
| 58 |
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, IsoRay, Inc. and Subsidiaries maintained, in all material respects, effective internal control over financial reporting as of June 30, 2015, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of IsoRay, Inc. and Subsidiaries as of June 30, 2015 and 2014, and the related consolidated statements of operations, changes in shareholders’ equity, and cash flows for each of the three years in the period ended June 30, 2015 and our report dated September 11, 2015 expressed an unqualified opinion thereon.
/s/ DeCoria, Maichel & Teague, P.S.
Spokane, Washington
September 11, 2015
Changes in Internal Control over Financial Reporting
There have not been any changes in our internal control over financial reporting (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) during the most recent fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
There were no items required to be disclosed in a report on Form 8-K during the fourth quarter of the fiscal year ended June 30, 2015 that have not been already disclosed on a Form 8-K filed with the SEC. The following is being reported in lieu of filing a Form 8-K due to the timing of the reportable event occurring just prior to the filing of this report:
Item 1.01 Entry into a Material Definitive Agreement
On September 10, 2015, the Company’s operating subsidiary, IsoRay Medical, Inc. (“IM”) entered into a Real Estate Purchase and Sale Agreement with The Port of Benton, a municipal corporation of the State of Washington. The Agreement is for the sale of undeveloped real property of approximately 4.2 acres located adjacent to the Company’s existing manufacturing facility and corporate offices. The purchase price for the property is One Hundred Sixty-Eight Thousand Dollars ($168,000) which is payable on October 30, 2015, the expected date of closing.
In addition to the feasibility studies required on all aspects of the property required by IM to close, IM is also bound to comply with a Development Plan for a ten year period which requirements include but are not limited to the following:
| (1) | Certain specified site configurations and design with a minimum of 12,000 square feet of warehouse and production space and 4,000 square feet of office space; |
| (2) | Completion of all construction in two years; |
| (3) | Use of facility as primary production facility for ten (10) years; and |
| (4) | Provision of jobs for not less than 25 full time employees. |
Failure to comply with these covenants will result in a breach of the Agreement and if not cured, will obligate IM to pay the Port the difference in the sales price and the appraised value of the property at the time of default.
| 59 |
PART III
ITEM 10 – DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Each member of the Board of Directors serves a one-year term and is subject to reelection at the Company's Annual Meeting of Shareholders held each year.
Board Committees
The Board has established an Audit Committee consisting of Thomas LaVoy (Chairman), Michael McCormick and Philip Vitale, MD; a Compensation Committee consisting of Thomas LaVoy, Michael McCormick and Philip Vitale, MD (Chairman); and a Nominating Committee consisting of Thomas LaVoy, Michael McCormick (Chairman), and Philip Vitale, MD. No other committees have been formed.
Audit Committee
The Audit Committee was established on December 8, 2006, the date on which its Charter was adopted. The Audit Committee Charter lists the purposes of the Audit Committee as overseeing the accounting and financial reporting processes of the Company and audits of the financial statements of the Company and providing assistance to the Board of Directors in monitoring (1) the integrity of the Company's financial statements, (2) the Company's compliance with legal and regulatory requirements, (3) the independent auditor's qualifications and independence, and (4) the performance of the Company's internal audit function, if any, and independent auditor.
The Board of Directors has determined that Mr. LaVoy is an "audit committee financial expert" as defined in Item 407(d)(5) of Regulation S-K promulgated by the Securities and Exchange Commission, and each Audit Committee member is independent under applicable NYSE MKT standards. The Board's conclusions regarding the qualifications of Mr. LaVoy as an audit committee financial expert were based on his service as a chief financial officer of a public company, his experience as a certified public accountant and his degree in accounting.
Executive Officers and Directors
The executive officers and directors serving the Company as of June 30, 2015 were as follows:
| Name | Age | Position Held | Term* | |||
| Dwight Babcock | 67 | Chairman, Chief Executive Officer | Annual | |||
| Brien Ragle | 46 | Chief Financial Officer | ||||
| Thomas LaVoy | 55 | Director | Annual | |||
| Michael McCormick | 52 | Director | Annual | |||
| Philip Vitale, MD | 68 | Director | Annual | |||
| William Cavanagh III | 49 | Vice President, Research and Development |
* For directors only
Dwight Babcock – Mr. Babcock was appointed CEO of the Company on February 18, 2009. He was previously appointed Chairman and Interim CEO of the Company on February 26, 2008 and has served as a Director of the Company since 2006. Mr. Babcock has served as Chairman and Chief Executive Officer of Apex Data Systems, Inc., an information technology company, since 1975. Apex Data Systems automates the administration and claims adjudication needs of insurance companies both nationally and internationally. Mr. Babcock was formerly President and CEO of Babcock Insurance Corporation (BIC) from 1974 until 1985. BIC was a nationally recognized third party administrator operating within 35 states. Mr. Babcock has knowledge and experience in the equity arena and has participated in various activities within the venture capital, private and institutional capital markets. Mr. Babcock studied marketing and economics at the University of Arizona where he currently serves on the University of Arizona Astronomy Board. Mr. Babcock brings over 35 years of CEO-level experience to his service on the Company's Board.
Brien Ragle – Mr. Ragle has over 15 years of finance and accounting experience, including SEC reporting, financial reporting, cost, project, and management accounting in addition to performing operational analysis. Mr. Ragle became IsoRay's Chief Financial Officer on October 1, 2013. Mr. Ragle was IsoRay's Controller – Principal Financial and Accounting Officer from October 2009 to September 2013. Mr. Ragle was IsoRay’s Cost Accounting Manager from January 2007 until October 2009. Before joining IsoRay in January 2007 as Cost Accounting Manager, Mr. Ragle was employed by BNG America, LLC, a wholly-owned subsidiary of Energy Solutions, LLC (ES), from 2005 to 2006 as Project Accounting Manager for all projects located in the Western United States and from 2000 to 2004 as a Business Unit Controller by SCM Consultants, Inc, a wholly-owned subsidiary of Tetra Tech, Inc (TTEK). Mr. Ragle holds Bachelor of Arts degrees in Business Administration, with an emphasis in accounting, and in Hospitality Management from Washington State University. Mr. Ragle is a Certified Public Accountant in the State of Washington and designated as a Chartered Global Management Accountant by the American Institute of Certified Public Accountants. Mr. Ragle filed for personal bankruptcy under Chapter 13 of the U.S. Bankruptcy Code on January 26, 2011.
| 60 |
Thomas LaVoy – Mr. LaVoy has been a Director of the Company since 2005. Mr. LaVoy presently serves as Deputy Chief Operations Officer and President of Corporate Services of Veolia Transportation on Demand (VTOD), the parent company of Super Shuttle International Inc. and its subsidiaries, since January 2014. He concurrently serves as Chief Financial Officer of SuperShuttle International, Inc. and its subsidiaries as he has since July 1997 and as Secretary since March 1998. VTOD through SuperShuttle is the largest shuttle transportation company in the US in addition to operating bus and cab services throughout the US. He has also served as a director of Alanco Technologies, Inc. (OTCBB: ALAN) since 1998 and presently serves on its audit committee. From September 1987 to February 1997, Mr. LaVoy served as Chief Financial Officer of NASDAQ-listed Photocomm, Inc. Mr. LaVoy was a Certified Public Accountant with the firm of KPMG Peat Marwick from 1980 to 1983. Mr. LaVoy has a Bachelor of Science degree in Accounting from St. Cloud University, Minnesota, and is a Certified Public Accountant (Inactive) in the State of Minnesota. Mr. LaVoy brings over 25 years of CFO experience for progressively growing companies in multiple industries to his service on the Company's Board.
Michael McCormick – Mr. McCormick has been a Director of the Company since June 2015 and brings over 25 years of senior executive positions in global management, sales, and marketing to the Company. He is currently the CEO of Glukos, one of the fastest growing food energy products in the U.S. He also serves as a founder and partner of GO Intellectual Capital, an advisory firm specializing in medical, aviation, and financial services. GO Intellectual Capital recently provided consulting services to DJO Global, a medical device and services company, to expand its product assortment, add new channels of distribution, and market new category opportunities. Previous to his service with Glukos and GO, Mr. McCormick served as Executive Vice President of Global Sales and Marketing for Columbia Sportswear from 2006-2012, where his team successfully launched several new patented technologies, including Omni-Heat® Reflective and Omni-Freeze® Zero. During Mr. McCormick’s tenure, Columbia built an intellectual property portfolio with over 200 patents. Mr. McCormick started his career with Nike, working in several senior management roles and ultimately becoming the Director of National Sales, US, prior to his departure in 1999. He also served as Chief Marketing Officer of Golf Galaxy from 2003-2006 and Executive Vice President of Global Sales and Marketing of Callaway Golf from 2000-2003. Mr. McCormick brings over 25 years of marketing experience in a diverse group of industries to his service on the Company’s Board.
Philip Vitale, MD – Dr. Vitale has been a Director of the Company since 2014 and is a board certified urologist. He practiced Urology from 1978 to 2005 at Lovelace Health Systems in Albuquerque. He also served on the Board of Governors for 9 years and held various administrative positions including Chief Medical Officer and Senior Vice President at Lovelace. He was a staff urologist at Albuquerque VA Medical Center from 2005 until his retirement in November 2014. He served as Chief of the Urology section from 2008 to November 2013. Dr. Vitale was also an Assistant Professor at the University of New Mexico, Division of Urology. He is a member of the American Urological Association and the South Central Section of the American Urological Association. Prior to his retirement, Dr. Vitale’s clinical trials included: chemotherapy after prostatectomy (cap); a phase III randomized study for high risk prostate carcinoma; RTOG 0415 a phase III randomized study of hypofractionated 3d-crt/IMRT versus conventionally fractionated 3d-crt/IMRT in patients with favorable-risk prostate cancer; RTOG 0815 a phase III prospective randomized trial of dose-escalated radiotherapy with or without short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer; and YP19A1 gene and pharmacogenetics of response to testosterone therapy. Dr. Vitale holds a BA in Biology from LaSalle College and obtained his M.D. from the New Jersey College of Medicine and Dentistry. He received his M.S. in Health Services Administration from the College of St. Francis. Dr. Vitale brings to the Board medical expertise in the industries the Company is targeting.
| 61 |
William Cavanagh III – Mr. Cavanagh joined IsoRay Medical in January 2010 and serves as Vice President, Research and Development. Immediately prior to joining IsoRay Medical, Mr. Cavanagh was engaged in the research and development of dendritic cell therapies for cancer and infectious diseases. He served as Chief Scientific Officer for Sangretech Biomedical, LLC for the six years prior to joining IsoRay Medical. At Sangretech, he oversaw the design and implementation of a novel cancer therapy. Mr. Cavanagh began his extensive career in cancer treatment technologies in the early 1990s, when he helped lead research and development of a therapy involving the insertion of radioactive sources directly into the prostate for the treatment of prostate cancer (prostate brachytherapy). He has designed several cancer treatment-related studies, is listed as an author on 34 peer-reviewed publications, and is the listed inventor on a U.S. patent application detailing a novel treatment for cancer. Mr. Cavanagh has also served as Director of the Haakon Ragde Foundation for Advanced Cancer Studies in Seattle, Washington, where he led the research foundation in the selection of viable research projects directed at treating advanced cancers. Mr. Cavanagh holds a B.S. in Biology from the University of Portland (Oregon) and completed two years of medical school before beginning his career in research management.
The Company's directors, as named above, will serve until the next annual meeting of the Company's shareholders or until their successors are duly elected and have qualified. Directors will be elected for one-year terms at the annual shareholders meeting. There is no arrangement or understanding between any of the directors or officers of the Company and any other person pursuant to which any director or officer was or is to be selected as a director or officer, and there is no arrangement, plan or understanding as to whether non-management shareholders will exercise their voting rights to continue to elect the current directors to the Company's board. There are also no arrangements, agreements or understandings between non-management shareholders that may directly or indirectly participate in or influence the management of the Company's affairs.
There are no agreements or understandings for any officer or director to resign at the request of another person, and none of the officers or directors is acting on behalf of, or will act at the direction of, any other person. There are no family relationships among our executive officers and directors.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934 (the Exchange Act) requires the Company's directors and executive officers, and persons who beneficially own more than ten percent of a registered class of our equity securities, to file with the Securities and Exchange Commission (the Commission) initial reports of beneficial ownership and reports of changes in beneficial ownership of our Common Stock. The rules promulgated by the Commission under Section 16(a) of the Exchange Act require those persons to furnish us with copies of all reports filed with the Commission pursuant to Section 16(a). The information in this section is based solely upon a review of Forms 3, Forms 4, and Forms 5 received by us.
We believe that IsoRay's executive officers, directors and 10% shareholders timely complied with their filing requirements during the year ended June 30, 2015, except as follows – Fredric Swindler (one Form 4) with one transaction; William Cavanagh (two Form 4s) each with one transaction; Brien Ragle (one Form 4) with one transaction and Thomas LaVoy (one Form 4) with one transaction. Each of these Form 4s was filed late.
Code of Ethics
We have adopted a Code of Conduct and Ethics that applies to all of our officers, directors and employees and a separate Code of Ethics for Chief Executive Officer and Senior Financial Officers that supplements our Code of Conduct and Ethics.
The Code of Conduct and Ethics was previously filed as Exhibit 14.1 to our Form 10-KSB for the period ended June 30, 2005, and the Code of Ethics for Chief Executive Officer and Senior Financial Officers was previously filed as Exhibit 14.2 to this same report. The Code of Ethics for Chief Executive Officer and Senior Financial Officers is also available to the public on our website at http://www.isoray.com/corporate_governance. Each of these policies comprises written standards that are reasonably designed to deter wrongdoing and to promote the behavior described in Item 406 of Regulation S-K promulgated by the Securities and Exchange Commission. Any amendments to or waivers of the Codes will be promptly posted on our website at www.IsoRay.com or in a report on Form 8-K, as required by applicable laws.
Nominating Procedures
There have been no material changes to the procedures by which our shareholders may recommend nominees to the Board of Directors during our last fiscal year.
| 62 |
ITEM 11 – EXECUTIVE COMPENSATION
The following summary compensation table sets forth information concerning compensation for services rendered in all capacities during our past three fiscal years awarded to, earned by or paid to each of the following individuals. Salary and other compensation for these officers are set or recommended to the Board by the Compensation Committee. No other executive officer received total compensation of over $100,000 during fiscal year 2015.
Summary Compensation Table
| Nonqualified | ||||||||||||||||||||||||||||||||||||
| Non-equity | deferred | |||||||||||||||||||||||||||||||||||
| Stock | Option | incentive plan | compensation | All other | ||||||||||||||||||||||||||||||||
| Salary | Bonus | awards | awards | compensation | earnings | compensation | Total | |||||||||||||||||||||||||||||
| Name and principal position | Year | ($) | ($) | ($) | ($) (1) | ($) | ($) | ($) | ($) | |||||||||||||||||||||||||||
| Dwight Babcock | 2015 | 291,554 | - | - | 57,095 | - | - | - | 348,650 | |||||||||||||||||||||||||||
| Chairman and CEO | 2014 | 284,712 | 50,000 | 116,095 | - | - | - | 450,807 | ||||||||||||||||||||||||||||
| 2013 | 284,394 | - | - | - | - | - | - | 284,394 | ||||||||||||||||||||||||||||
| Brien Ragle | 2015 | 119,620 | - | - | 20,554 | - | - | - | 140,174 | |||||||||||||||||||||||||||
| CFO | 2014 | 117,834 | - | - | 39,401 | - | - | - | 157,235 | |||||||||||||||||||||||||||
| 2013 | 99,215 | - | - | - | - | - | - | 99,215 | ||||||||||||||||||||||||||||
| William Cavanagh | 2015 | 158,020 | - | - | 20,554 | - | - | - | 178,574 | |||||||||||||||||||||||||||
| VP – R&D | 2014 | 154,500 | - | - | 37,099 | - | - | - | 191,599 | |||||||||||||||||||||||||||
| 2013 | 154,327 | - | - | - | - | - | - | 154,327 | ||||||||||||||||||||||||||||
| 1. | Amounts represent the ASC 718, Compensation – Stock Compensation valuation for the fiscal years ended June 30, 2015, 2014 and 2013, respectively. All such options were awarded under one of the Company's four stock option plans. All options awarded (with the exception of Mr. Babcock's stock option grants that were immediately vested on the grant date) vest in three equal annual installments beginning with the first anniversary from the date of grant and expire ten years after the date of grant. All options were granted at the fair market value of the Company's stock on the date of grant and the Company used a Black-Scholes methodology as discussed in the footnotes to the financial statements to value the options. |
Grants of Plan-Based Awards
The following table sets forth certain information with respect to stock and option awards and other plan-based awards granted to our named executive officers during fiscal 2015.
| All other | Grant | |||||||||||
| option | Exercise | date fair | ||||||||||
| awards: | or base | value of | ||||||||||
| Number of | price of | of stock | ||||||||||
| securities | option | and | ||||||||||
| Grant | underlying | awards | option | |||||||||
| Name | Date | options (#) | ($/Sh) | awards | ||||||||
| Dwight Babcock Chairman/CEO | 617/2015 | 50,000 | $ | 1.47 | $57,095 | |||||||
| Brien Ragle CFO | 617/2015 | 20,000 | 1.47 | 20,554 | ||||||||
| William Cavanagh Vice-President of Research and Development | 617/2015 | 20,000 | 1.47 | 20,554 | ||||||||
| 63 |
Outstanding Equity Awards at Fiscal Year-End
| Option awards | ||||||||||||||||||
| Equity | ||||||||||||||||||
| Incentive | ||||||||||||||||||
| plan | ||||||||||||||||||
| awards: | ||||||||||||||||||
| Number of | Number of | Number of | ||||||||||||||||
| securities | securities | securities | ||||||||||||||||
| underlying | underlying | underlying | ||||||||||||||||
| unexercised | unexercised | unexercised | Option | |||||||||||||||
| Options | options | unearned | exercise | Option | ||||||||||||||
| (#) | (#) | options | price | expiration | ||||||||||||||
| Name | exercisable | unexercisable | (#) | ($) | date | |||||||||||||
| Dwight Babcock, | 50,000 | (1) | - | - | 6.30 | 03/31/2016 | ||||||||||||
| Chairman and CEO | 50,000 | (1) | - | - | 3.80 | 06/23/2016 | ||||||||||||
| 50,000 | (1) | - | - | 3.11 | 08/15/2016 | |||||||||||||
| 100,000 | (1) | - | - | 0.75 | 05/13/2018 | |||||||||||||
| 200,000 | (1) | - | - | 0.26 | 06/01/2019 | |||||||||||||
| 100,000 | (1) | - | - | 1.43 | 06/30/2020 | |||||||||||||
| 100,000 | (1) | - | - | 0.99 | 06/07/2021 | |||||||||||||
| 50,000 | (1) | - | - | 0.98 | 06/27/2022 | |||||||||||||
| 50,000 | (1) | - | - | 0.58 | 09/05/2023 | |||||||||||||
| 50,000 | (1) | - | - | 2.17 | 05/20/2024 | |||||||||||||
| 50,000 | (1) | - | - | 1.47 | 06/17/2025 | |||||||||||||
| Brien Ragle | 5,000 | (2) | - | - | 4.40 | 03/02/2017 | ||||||||||||
| CFO | 2,000 | (3) | - | - | 4.14 | 06/01/2017 | ||||||||||||
| 20,000 | (4) | - | - | 1.43 | 06/30/2020 | |||||||||||||
| 20,000 | (5) | - | - | 0.99 | 06/07/2021 | |||||||||||||
| 1,666 | (7) | 3,334 | - | 0.59 | 09/06/2023 | |||||||||||||
| - | (8) | 20,000 | - | 2.46 | 06/17/2024 | |||||||||||||
| - | (9) | 20,000 | - | 1.47 | 06/17/2025 | |||||||||||||
| William Cavanagh | 6,660 | (6) | - | - | 0.98 | 06/27/2022 | ||||||||||||
| Vice-President, Research | - | (8) | 20,000 | - | 2.46 | 06/17/2024 | ||||||||||||
| And Development | - | (9) | 20,000 | - | 1.47 | 06/17/2025 | ||||||||||||
| 1) | Represents options issued to Mr. Babcock which were all immediately vested and exercisable. The grant dates are 10 years prior to the expiration date in the table above. |
| 2) | Represents the March 2, 2007 grant, all of which were exercisable as of March 2, 2010. |
| 3) | Represents the June 1, 2007 grant, all of which were exercisable as of June 1, 2010. |
| 4) | Represents a June 30, 2010 grant, all of which were exercisable as of June 30, 2013. |
| 5) | Represents a June 7, 2011 grant, all of which were exercisable as of June 30, 2014. |
| 6) | Represents a June 27, 2012 grant, all of which were exercisable as of June 27, 2015. |
| 7) | Represents a September 6, 2013 grant, one-third of which became exercisable on September 6, 2014, one-third of which became exercisable on September 6, 2015, and the final third will become exercisable on September 6, 2016. |
| 8) | Represents a June 17, 2014 grant, one-third of which became exercisable on June 17, 2015, one-third of which will become exercisable on June 17, 2016, and the final third will become exercisable on June 17, 2017. |
| 9) | Represents a June 17, 2015 grant, one-third of which will become exercisable on June 17, 2016, one-third of which will become exercisable on June 17, 2017, and the final third will become exercisable on June 17, 2018. |
Option Exercises and Stock Vested
| Option Awards | Stock Awards | |||||||||||||||
| Number of shares | Value | Number of shares | Value | |||||||||||||
| acquired on exercise | realized on exercise | acquired on vesting | realized on vesting | |||||||||||||
| Name | (#) | ($) | (#) | ($) | ||||||||||||
| Dwight Babcock Chairman/CEO | - | - | - | - | ||||||||||||
| Brien Ragle CFO | - | - | - | - | ||||||||||||
| William Cavanagh Vice-President of Research and Development | 13,340 | 7,337 | - | - | ||||||||||||
The Company has a 401(k) plan that covers all eligible full-time employees of the Company. Contributions to the 401(k) plan are made by participants to their individual accounts through payroll withholding. Additionally, the 401(k) plan provides for the Company to make contributions to the 401(k) plan in amounts at the discretion of management. The Company has not made any contributions to the 401(k) plan and does not maintain any other retirement plans for its executives or employees.
| 64 |
Director Compensation
| Fees earned | Non-equity | Non-qualified | ||||||||||||||||||||||||||
| or paid in | Stock | Option | incentive plan | deferred | All other | |||||||||||||||||||||||
| cash | awards | awards | compensation | compensation | compensation | Total | ||||||||||||||||||||||
| Name | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||||
| Thomas LaVoy (1) | 52,000 | - | - | - | - | - | 52,000 | |||||||||||||||||||||
| Michael McCormick (2) | 433 | - | 28,548 | - | - | - | 28,981 | |||||||||||||||||||||
| Philip Vitale MD | 28,433 | - | - | - | - | - | 28,433 | |||||||||||||||||||||
(1) Mr. LaVoy received an additional $2,000 per month for serving as Audit Committee Chairman.
(2) Mr. McCormick received payment for his service as a non-employee director beginning when his Board service and service on committees of the Board commenced on June 18, 2015.
During fiscal year 2015, each non-employee director received cash compensation of $2,000 per month. In addition, each non-employee director received $1,000 per Board meeting attended in person or $500 per Board meeting attended via telephone and $500 per committee meeting attended.
Each non-employee director had stock options to purchase shares of the Company's common stock outstanding as of June 30, 2015 as follows - Mr. LaVoy had stock options to purchase 150,000 shares of common stock, Mr. McCormick had stock options to purchase 25,000 shares of common stock, and Dr. Vitale had stock options to purchase 25,000 shares of common stock.
Effective June 18, 2015, the Board, on the recommendation of the Compensation Committee, changed its compensation. Since that date, the independent directors receive $3,000 per month for their service, and the Chair of the Audit Committee receives an additional $1,000 per month. The per meeting fees as disclosed above were not changed. Any employee directors do not receive any compensation for their service on the Board.
Compensation Committee Interlocks and Insider Participation
No member of the Compensation Committee is or was during fiscal year 2015 an employee, or is or ever has been an officer of our Company. None of our executive officers has served during fiscal year 2015 as a director or a member of the Compensation Committee of another company.
Compensation Discussion & Analysis
Overview of Our Compensation Process
We design our named executive officer compensation programs to attract, motivate and retain the key executives who drive our success and help us maintain a strong position in our industry. We are committed to industry standards for the region in which we operate for base pay, and equity payable to our named executive officers based on our ability to raise capital and cut costs. In addition, we design our named executive compensation to encourage long-term commitment by our named executive officers to IsoRay.
Please read the "Executive Compensation" section of this annual report, beginning on page 63. That section of the annual report, which includes our named executive officer compensation tables and related narrative discussion, provides historical details on our compensation programs and policies for our named executive officers. The executive officers named in the summary compensation table and deemed to be a "named executive officer" are Dwight Babcock, Brien Ragle, and William Cavanagh.
The compensation paid to the Company's named executive officers is intended to align their interests with the long term interests of the Company's shareholders and is based on a pay-for-performance philosophy. It is straightforward, consisting principally of salary, which must be competitive to retain the skills and experience of excellent employees, and equity compensation to encourage long term commitment and team performance. Not all elements of our compensation package may be provided every year, depending on the performance of the Company and the executive.
| 65 |
At our 2014 annual meeting, our shareholders approved our executive compensation program, and the next advisory vote will be held in 2017.
Highlights of our named executive officer compensation programs and policies are as follows:
| · | We generally do not enter into employment agreements with our named executive officers, which results in a lack of severance pay obligations, lack of change in control payments, and the ability of the Board and the CEO to dismiss named executive officers at will, all of which the Board believes ultimately can save the Company ongoing severance obligations and encourage performance by the named executive officers. |
| · | The discretionary option grants available to our CEO are linked to the success of the Company in overseeing and selecting investment banking firms in raising capital and reducing costs. |
| · | The compensation of our named executive officers is not linked to the performance of the Company, except for discretionary bonuses and option grants, but is instead based on our ability to obtain executives with the experience necessary and willingness to work for a company located in a small community with limited access to a major metropolitan center. |
| · | The compensation of our CFO is less than the industry norm for a CFO, as our CFO does not have past CFO experience. |
| · | We provide named executive officers with long-term incentives in the form of stock options. These equity-based awards, which generally vest over a period of three years (except for grants to our CEO which vest immediately), link compensation with the long-term price performance of our stock, and also provide a substantial retention incentive. |
| · | We do not provide perquisites to our named executive officers. |
Company Background
Historically, our revenue has been difficult to predict and we have not shown a profit for any quarter since the inception of our Company. When the entire prostate cancer brachytherapy industry began to experience annual deceases in demand, our business also suffered declines. These declines were exacerbated by the emergence of alternative radiation therapies which provided greater remuneration to the physician than our brachytherapy solution.
Our CEO, with the assistance of our management team, had the foresight to expand the use of the Company’s products to also include treating brain, gynecologic, lung and other cancers with our brachytherapy products. These non-prostate treatments required significant capital for research and development, protocols and studies. Acceptance by medical professionals unfamiliar with our products is a long term process.
As a consequence of the combination of (i) a decline in the prostate market due to macro-economic factors; and (ii) the need to deploy significant resources in non-prostate applications, our compensation programs have not been structured to award pay increases, bonuses, or stock options based on revenues or profits. Instead, our compensation has rewarded capital raises and cutting costs. Capital is critical to fund new applications. Cost cutting is important as we face a declining prostate market.
This Compensation Discussion and Analysis describes our compensation objectives, our executive compensation process and our policies and actions with respect to each compensation element. We describe the rationale for compensation decisions made in fiscal year 2015 with respect to our President and Chief Executive Officer, our Chief Financial Officer and our Vice-President of Research and Development, whom we refer to as our named executive officers.
| 66 |
Our Executive Compensation Program
Program Objectives
We design our executive compensation program to achieve the following objectives:
| · | Motivate and reward executives whose knowledge, skills and performance are essential to our success; |
| · | Align the performance of our executives and the interests of our shareholders; |
| · | Recruit and retain executive talent; and |
| · | Support the corporate business strategy by rewarding cost control measures and capital raising results. |
Compensation Process
The Compensation Committee of our Board has the primary responsibility for determining compensation of our executives. Our Board has determined that each member of our Compensation Committee is “independent” as that term is defined by applicable NYSE MKT rules, is an “outside director” as defined in Section 162(m) of the Internal Revenue Code, or the Code, and a “non-employee” director as defined under Section 16 of the Exchange Act.
Our Compensation Committee determines all compensation matters for our named executive officers, including base salary, bonuses, and equity compensation. Utilizing input from our Chief Executive Officer, the Compensation Committee makes an independent decision on compensation for each executive other than the CEO. The Compensation Committee also primarily relies on the judgment of the Chief Executive Officer in making compensation determinations of our non-executive staff. The primary goal of our Compensation Committee is to closely align the interests of our named executive officers and staff with those of our shareholders. The Compensation Committee assesses performance on a number of subjective and objective factors.
In making decisions regarding executive compensation, our Compensation Committee considers, among other things:
| · | Past compensation levels of each executive and the executives as a group; |
| · | Consistency of current compensation with previous compensation decisions and benchmarks; |
| · | Existing levels of stock and stock option ownership among our executives, previous stock option grants and vesting schedules to ensure executive retention and alignment with shareholder interests; |
| · | Management recommendations; |
| · | General trends in executive compensation; and |
| · | Meeting ongoing cost control and capital raise objectives. |
The Compensation Committee conducts an annual review of the Chief Executive Officer’s performance and reports its evaluation to the Board. The Board reviews the Compensation Committee’s evaluation and recommendation and also evaluates the Chief Executive Officer’s performance according to the goals and objectives established periodically by the full Board. This review serves as the basis for the recommendation of the Compensation Committee on Chief Executive Officer compensation.
The Compensation Committee did not engage an independent compensation consultant to evaluate executive compensation. It did not survey healthcare industry data or complete a peer group comparison.
Compensation Components
Our executive compensation primarily consists of base salary, bonuses and long-term equity-based compensation.
| 67 |
The factors our Compensation Committee considered for each of our executives in fiscal 2015 included:
| · | Overall corporate performance during fiscal 2015 in achieving certain non-financial milestones; |
| · | The roles and responsibilities of our executives in helping the Company meet these milestones; |
| · | The additional roles and responsibilities of our executives; |
| · | The individual experience and skills of our executives; and |
| · | The location of the Company in a small city and the fact that we are a much smaller company than any of our competitors. |
We have an executive compensation philosophy and goals based on attracting, retaining and rewarding our executive officers. In addition, we believe that executive compensation should be linked to corporate performance and accomplishments that increase shareholder value. As such, our executive compensation policy focuses on aligning the interests of our executive officers with the long-term interests of our shareholders and with our corporate strategies and goals.
Base salaries of executive officers are reviewed and approved annually by our Compensation Committee and adjustments are made based on (i) salary recommendations from our Chief Executive Officer, (ii) individual performance of executive officers for the previous fiscal year, and (iii) historical pay. In addition, in establishing the total compensation package for our Chief Executive Officer, the Compensation Committee pursues the same objectives and policies that apply for our other executive officers.
Base Salary
Base salary reflects job responsibilities, value to us and individual performance, taking into consideration the need to attract and retain our executives. We determine salaries for our named executive officers initially by reference to each executive’s previous year’s salary. The Compensation Committee determines any increase over these salaries based upon recommendations of our Chief Executive Officer, except in the case of the Chief Executive Officer’s own compensation. The Compensation Committee generally reviews base salaries of our executives annually and adjusts salaries from time to time to realign salaries with perceived market increases and individual performance.
Achievement of individual and corporate accomplishments along with the executive officer’s level of responsibility, competitive factors and our internal policies regarding salary increases were considered regarding fiscal 2015 salary increases.
Merit-based salary increases for fiscal 2015 were two and one-half percent (2.5%) for Dwight Babcock, Brien Ragle and William Cavanagh. In June 2015, we set the annual base salary for fiscal 2016 for Dwight Babcock, our President and Chief Executive Officer, at $301,000, for Brien Ragle, our Chief Financial Officer, at $130,000, and for William Cavanagh, our Vice President of Research and Development, at $163,116.
Performance-Based Annual Bonus
We provide for an annual cash incentive that reinforces our pay-for-performance approach. This incentive compensation is a short-term incentive program that rewards achievement. Annual incentive awards are awarded at the sole determination of the Compensation Committee (on behalf of the Board) based on the actual and measurable performance of the Company based on a set of corporate objectives for the previous year.
This past year we did not award any cash incentives and bonuses. Instead, the Compensation Committee requested the CEO to provide some suggested objective quarterly performance goals so that a bonus plan could be structured. The Compensation Committee now believes that the Company has finally reached the stage where it can base bonuses on revenue increases. Effective for the quarter ending September 30, 2015, each named officer will earn a bonus of three percent (3%) of their annual base salary for a fifteen percent (15%) or greater increase in revenue from the prior fiscal year’s comparable quarter. Also, effective for the year ending June 30, 2016, each named officer will earn a bonus of three percent (3%) of their annual base salary for a fifteen percent (15%) or greater increase in revenue over the prior fiscal year. The Compensation Committee will closely monitor the results of this incentive plan this year to determine if it provides a better incentive than the subjective bonuses paid historically from time to time.
| 68 |
Fiscal 2014 and 2015 accomplishments taken into account by the Compensation Committee to determine overall corporate performance included the following:
| 1. | Maintained controls over expenses. |
| 2. | Achieved annual revenue growth. |
| 3. | Received FDA clearance for our liquid Cesium-131. |
| 4. | Performed the first GliaSite case utilizing Cs-131. |
| 5. | Selected for the Russell Microcap Index. |
| 6. | First patient ever was implanted with Cs-131 combined with the C-4 spacer. |
| 7. | First veterinary case performed on a horse. |
| 8. | Several first ever peer reviewed publications were issued. |
| 9. | 2 new focal prostate studies initiated (Moran and UPMC). |
| 10. | Established new Italian distributor. |
| 11. | Received Greek license to ship products. |
| 12. | Introduced Cs-131 in Russia in new cancer medical center grand opening. |
| 13. | CE Mark audit completed with no warnings. |
| 14. | Upgraded manufacturing and enterprise systems with redundancy. |
| 15. | Participated in 6 industry shows. |
Long-Term Equity-Based Incentive Compensation
Our long-term incentive program provides an annual award, with the potential for periodic awards, which is performance based. The objective of the program is to align compensation for named executive officers over a multi-year period directly with the interests of our shareholders by motivating and rewarding creation and preservation of long-term shareholder value. We believe that we can maximize our long-term performance best if we tie the value of the long-term benefits our executives receive to our long-term performance.
The sole form of equity compensation to our executive officers are stock options. Our Compensation Committee receives preliminary recommendations for equity-based awards from our Chief Executive Officer. Our Compensation Committee then reviews the recommendations and recommends equity-based awards for all of our officers, including our Chief Executive Officer and the other named executive officers, to our Board for approval.
Stock option awards provide our executive officers with the right to purchase shares of our common stock at a fixed exercise price typically for a period of up to ten years, subject to continued service with us in accordance with the terms of our equity incentive plans, and generally vest over three years (other than for the CEO whose options vest immediately). We do not grant stock options that have exercise prices below the fair market value of our common stock on the date of grant. We do not reduce the exercise price of stock options if the price of our common stock subsequently declines below the exercise price unless we first obtain shareholder approval. However, we do adjust the exercise price of previously granted stock options to reflect recapitalizations, stock splits, mergers, and similar events as permitted by the applicable stock plans.
We typically grant stock options on an annual basis as part of annual performance reviews of our employees. We grant equity incentive compensation to our executive officers because we believe doing so will motivate our executives by aligning their interest more closely with the interest of our shareholders.
On June 18, 2015, we granted stock options to purchase 50,000 shares, 20,000 shares, and 20,000 shares of our common stock to Dwight Babcock, Brien Ragle, and William Cavanagh, respectively, at an exercise price of $1.47 per share.
| 69 |
Other Aspects of Our Compensation Philosophy
Other Benefits
We provide our named executive officers with the same employee benefits that all of our other employees receive under our broad-based benefit plans. These plans provide for health benefits, life insurance and other welfare benefits.
Perquisites
We do not provide our named executive officers with any retirement or welfare plan benefits that we do not provide to all of our other employees.
Risks Related to Compensation Policies and Practices
The Compensation Committee has considered whether our overall compensation program for employees in 2015 creates incentives for employees to take excessive or unreasonable risks that could materially harm our Company. We believe that several features of our compensation policies for management employees appropriately mitigate such risks, including a mix of long- and short-term compensation incentives that we believe is properly weighted, and the uniformity of compensation practices across our Company, which the Compensation Committee regards as setting an appropriate level of risk taking for us. We also believe our internal legal and financial controls appropriately mitigate the probability and potential impact of an individual employee committing us to a harmful long-term business transaction in exchange for short-term compensation benefits.
Recoupment Policy
In order to align further management’s interests with the interests of our shareholders and to support good corporate governance practices, the Board has adopted a recoupment policy. Subject to rules of the SEC and NYSE MKT, in the event that we are required to prepare an accounting restatement due to the material noncompliance with any financial reporting requirement under the federal securities laws, we will form a committee of the non-management directors to determine whether we will recover from any of our current or former executive officers, as determined in accordance with such rules, who received performance-based compensation (including stock options awarded as compensation) during the period for which we are required to prepare an accounting restatement, based on the erroneous data, in excess of what would have been paid to the executive officer under the accounting restatement. The committee may also take any other actions authorized by our Executive Compensation Clawback Policy.
Compensation Committee Report
The Compensation Committee of the Board of Directors has reviewed and discussed the matters contained under the title Compensation Discussion and Analysis of this Report with our management and, based on such review and discussions we recommended to the Board that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K for the Company’s fiscal year ended June 30, 2015.
Respectfully submitted,
Philip Vitale, MD (Chair)
Thomas LaVoy
Michael McCormick
ITEM 12 – SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following tables set forth certain information regarding the beneficial ownership of the Company's common stock and preferred stock as of September 11, 2015 for (a) each person known by the Company to be a beneficial owner of five percent or more of the outstanding common or preferred stock of the Company, (b) each executive officer, director and nominee for director of the Company, and (c) directors and executive officers of the Company as a group. As of September 11, 2015, the Company had 55,013,553 shares of common stock and 59,065 shares of Series B preferred stock outstanding. Except as otherwise indicated below, the address for each listed beneficial owner is c/o IsoRay, Inc., 350 Hills Street, Suite 106, Richland, Washington 99354.
| 70 |
Common Stock Share Ownership
| Name of Beneficial Owner | Common Shares Owned | Common Stock Options(1) | Percent of Class (2) | |||||||||
| Dwight Babcock(3) | 304,097 | 850,000 | 2.00 | % | ||||||||
| Brien Ragle | - | 56,999 | 0.02 | % | ||||||||
| Thomas LaVoy | 93,523 | 150,000 | 0.44 | % | ||||||||
| Michael McCormick | 25,000 | 0.05 | % | |||||||||
| Philip Vitale M.D. | 10,000 | 25,000 | 0.06 | % | ||||||||
| William Cavanagh III | 13,326 | 0.02 | % | |||||||||
| Directors and Executive Officers as a group | 407,620 | 1,120,325 | 0.02 | % | ||||||||
| 1) | Only includes those common stock options that could be exercised for common stock within 60 days after September 11, 2015. |
| 2) | Percentage ownership is based on 55,013,553 shares of Common Stock outstanding on September 11, 2015. Shares of Common Stock subject to stock options which are currently exercisable or will become exercisable within 60 days after September 11, 2015 are deemed outstanding for computing the percentage ownership of the person or group holding such options but are not deemed outstanding for computing the percentage ownership of any other person or group. |
| 3) | Mr. Babcock's common shares owned include 2,695 shares owned by his spouse. |
Series B Preferred Stock Share Ownership
| Series B | ||||||||
| Preferred | ||||||||
| Shares | Percent of | |||||||
| Name of Beneficial Owner | Owned | Class (1) | ||||||
| Aissata Sidibe (2) | 20,000 | 33.86 | % | |||||
| William and Karen Thompson Trust (3) | 14,218 | 24.07 | % | |||||
| Jamie Granger (4) | 10,529 | 17.83 | % | |||||
| Hostetler Living Trust (5) | 9,479 | 16.05 | % | |||||
| Leslie Fernandez (6) | 3,688 | 6.24 | % | |||||
| (1) | Percentage ownership is based on 59,065 shares of Series B Preferred Stock outstanding on September 11, 2015. |
| (2) | The address of Aissata Sidibe is 99302 E Sidibe PR SE, Kennewick, WA 99338. |
| (3) | The address of the William and Karen Thompson Trust is 285 Dondero Way, San Jose, CA 95119. |
| (4) | The address of Jamie Granger is 53709 South Nine Canyon Road, Kennewick, WA 99337. |
| (5) | The address of the Hostetler Living Trust is 9327 NE 175th Street, Bothell, WA 98011. |
| (6) | The address of Leslie Fernandez is 2615 Scottsdale Place, Richland, WA 99352. |
No officers or directors beneficially own shares of any class of Preferred Stock.
| 71 |
ITEM 13 – CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Certain Relationships and Related Party Transactions
None requiring disclosure under Reg. S-K Item 404.
Review and Approval of Related Party Transactions
The Company's Code of Ethics emphasizes the importance of avoiding situations or transactions in which personal interests may interfere with the best interests of the Company or its shareholders. In addition, the Company's general corporate governance practice includes Board-level discussion and assessment of procedures for discussing and assessing relationships, including business, financial, familial and nonprofit, among the Company and its officers and directors or their immediate family members, to the extent that they may arise. The Board and either the Audit Committee or the Nominations and Corporate Governance Committee review any transaction with an officer or director or their immediate family members to determine, on a case-by-case basis, whether a conflict of interest exists. The Board ensures that all directors voting on such a matter have no interest in the matter and discusses the transaction with counsel as the Board deems necessary. The Board will generally delegate the task of discussing, reviewing and approving transactions between the Company and any related persons to either the Audit Committee or the Nominations and Corporate Governance Committee.
As required under SEC rules, transactions that are determined to be directly or indirectly material to the Company or a related party would be disclosed in our Annual Report; however, during our fiscal year ended June 30, 2015, we did not have any related party transactions requiring disclosure under Reg. S-K Item 404.
Director Independence
Using the standards of the NYSE MKT, the Company's Board has determined that Mr. LaVoy, Mr. McCormick and Dr. Vitale each qualify under such standards as an independent director. Mr. LaVoy, Mr. McCormick and Dr. Vitale each meet the NYSE MKT listing standards for independence both as a director and as a member of both the Audit Committee and the Compensation Committee. No other directors are independent under these standards. The Company did not consider any relationship or transaction between itself and these independent directors not already disclosed in this report in making this determination.
ITEM 14 – PRINCIPAL ACCOUNTANT FEES AND SERVICES
The Company paid or accrued the following fees in each of the prior three fiscal years to its principal accountant, DeCoria, Maichel & Teague, P.S.:
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| 1. Audit fees | $ | 76,566 | $ | 63,471 | $ | 60,372 | ||||||
| 2. Audit-related fees | - | - | 2,733 | |||||||||
| 3. Tax fees | 11,988 | 9,000 | 8,250 | |||||||||
| 4. All other fees | - | - | 3,750 | |||||||||
| Totals | $ | 88,554 | $ | 72,471 | $ | 75,105 | ||||||
Audit fees include fees for the audit of our annual financial statements, reviews of our quarterly financial statements, and related consents for documents filed with the SEC, as well as, in fiscal 2015, the fees for the audit of our internal control over financial reporting. Audit-related fees include cost of attendance at the annual shareholder meeting. Tax fees include fees for the preparation of our federal and state income tax returns. All other fees are related to consulting costs related to the review of documents related to equity offerings.
| 72 |
As part of its responsibility for oversight of the independent registered public accountants, the Audit Committee has established a pre-approval policy for engaging audit and permitted non-audit services provided by our independent registered public accountants, DeCoria, Maichel & Teague, P.S. In accordance with this policy, each type of audit, audit-related, tax and other permitted service to be provided by the independent auditors is specifically described and each such service, together with a fee level or budgeted amount for such service, is pre-approved by the Audit Committee. The Audit Committee has delegated authority to its Chairman to pre-approve additional non-audit services (provided such services are not prohibited by applicable law) up to a pre-established aggregate dollar limit. All services pre-approved by the Chairman of the Audit Committee must be presented at the next Audit Committee meeting for review and ratification. All of the services provided by DeCoria, Maichel & Teague, P.S. described above were approved by our Audit Committee.
The Company's principal accountant, DeCoria, Maichel & Teague P.S., did not engage any other persons or firms other than the principal accountant's full-time, permanent employees.
ITEM 15 – EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
EXHIBIT INDEX
(Except as otherwise indicated, all exhibits were previously filed and all omitted exhibits are intentionally omitted)
| Exhibit # | Description | |
| 3.2 | Certificate of Designation of Rights, Preferences and Privileges of Series A and B Convertible Preferred Stock, filed with the Minnesota Secretary of State on June 29, 2005 incorporated by reference to the Form 8-K filed on August 3, 2005. | |
| 3.3 | Restated and Amended Articles of Incorporation incorporated by reference to the Form 10-KSB filed on October 11, 2005. | |
| 3.4 | Certificate of Designation of Rights, Preferences and Privileges of Series C Junior Participating Preferred Stock, incorporated by reference to the Company's Registration Statement on Form 8-A filed February 7, 2007. | |
| 3.5 | Amended and Restated By-Laws of the Company dated as of January 8, 2008, incorporated by reference to the Form 8-K filed on January 14, 2008. | |
| 3.6 | Certificate of Designation and Preferences, Rights and Limitations of Series D Convertible Preferred Stock dated August 29, 2013 of IsoRay, Inc., incorporated by reference to the Form 8-K filed on August 29, 2013. | |
| 4.16 | Amended and Restated 2006 Director Stock Option Plan, incorporated by reference to the Form S-8/A1 filed on December 18, 2006. | |
| 4.19 | Rights Agreement, dated as of February 1, 2007, between the Computershare Trust Company N.A., as Rights Agent, incorporated by reference to the Company's Registration Statement on Form 8-A filed on February 7, 2007. | |
| 4.20 | Certificate of Designation of Rights, Preferences and Privileges of Series C Junior Participating Preferred Stock, incorporated by reference to Exhibit 1 to the Company's Registration Statement on Form 8-A filed February 7, 2007. | |
| 4.26 | Form of Common Stock Purchase Warrant, incorporated by reference to the Form 8-K filed on October 13, 2011. | |
| 4.32 | 2014 Employee Stock Option Plan incorporated by reference to the Form 10-Q filed on March 15, 2014. | |
| 4.33 | Form of Stock Option Agreement of IsoRay, Inc., incorporated by reference to the Form 10-Q filed on March 15, 2014. | |
| 10.2 | Universal License Agreement, dated November 26, 1997 between Donald C. Lawrence and William J. Stokes of Pacific Management Associates Corporation, incorporated by reference to the Form SB-2 filed on November 10, 2005. | |
| 10.3 | Royalty Agreement of Invention and Patent Application, dated July 12, 1999 between Lane A. Bray and IsoRay LLC, incorporated by reference to the Form SB-2 filed on November 10, 2005. | |
| 10.5 | Section 510(k) Clearance from the Food and Drug Administration to market Lawrence CSERION Model CS-1, dated March 28, 2003, incorporated by reference to the Form SB-2 filed on November 10, 2005. | |
| 10.10 | Registry of Radioactive Sealed Sources and Devices Safety Evaluation of Sealed Source, dated September 17, 2004, incorporated by reference to the Form SB-2/A2 filed on April 27, 2006. | |
| 10.18 | State of Washington Radioactive Materials License dated October 6, 2005, incorporated by reference to the Form SB-2 filed on November 10, 2005. | |
| 10.22 | Agreement dated August 9, 2005 between the Curators of the University of Missouri and IsoRay Medical, Inc., incorporated by reference to the Form SB-2/A2 filed on April 27, 2006 (confidential treatment granted for redacted portions). | |
| 10.35 | Form of Officer and Director Indemnification Agreement, incorporated by reference to the Form SB-2 Post Effective Amendment No. 2 filed on October 13, 2006. | |
| 10.59 | License Agreement, dated effective June 14, 2010, by and between IsoRay Medical, Inc. and Hologic Inc., incorporated by reference to the Form 8-K filed on June 23, 2010 (confidential treatment granted for redacted portions). | |
| 10.66 | License Agreement dated as of June 1, 2011, by and between Dr. Reddy's Laboratories (EU) Ltd. and IsoRay Medical, Inc., incorporated by reference to the Form 8-K/A filed on August 19, 2011 (confidential treatment granted for redacted portions). |
| 73 |
| 10.68 | International Distribution Agreement, dated October 31, 2011, by and between IsoRay Medical, Inc. and Karlheinz Goehl-Medizintechnik Göhl, incorporated by reference to the Form 8-K filed on November 3, 2011 (confidential treatment granted for redacted portions). | |
| 10.74 | Letter Agreement, dated August 27, 2013, between IsoRay Medical, Inc. and Karlheinz Goehl-Medizintechnik Göhl, incorporated by reference to the Form 10-K filed on September 30, 2013. | |
| 10.76 | Securities Purchase Agreement dated as of March 1, 2014, between IsoRay, Inc., and each Purchaser identified on the signature pages thereto, incorporated by reference to the Form 8-K filed on March 21, 2014. | |
| 10.77 | Letter Agreement dated March 21, 2014, between Maxim Group LLC and IsoRay, Inc., incorporated by reference to the Form 8-K filed on March 21, 2014. | |
| 10.78 | Contract dated June 23, 2014, by and between IsoRay Medical, Inc. and The Open Joint Stock Company ‹‹ Institute of the Nuclear Materials ›› (confidential treatment granted for redacted portions), incorporated herein by reference to the Form 8-K filed June 25, 2014. | |
| 10.79 | Addendum No. 1 to Contract No. 840/08624332/1609-14 dated July 31, 2014, by and between Open Joint Stock Company ‹‹ Institute of the Nuclear Materials ›› (JSC ‹‹INM››) and IsoRay Medical, Inc., incorporated by reference to the Form 8-K filed on August 7, 2014. | |
| 10.81 | Letter Agreement dated August 28, 2014, by and between IsoRay Medical, Inc. and Karlheinz Goehl-Medizintechnik Göhl (confidential treatment granted for redacted portions), incorporated herein by reference to the Form 10-K filed September 29, 2014. | |
| 10.82 | Contract, dated January 12, 2015, by and between IsoRay Medical, Inc. and Joint Stock Company “Institute of Nuclear Materials” (confidential treatment granted for redacted portions); incorporated herein by reference to the Form 10-Q filed May 15, 2015. | |
| 10.83* | Letter Agreement dated August 31, 2015, by and between IsoRay Medical, Inc. and Karlheinz Goehl - Medizintechnik Göhl. | |
10.84* |
Real Estate Purchase and Sale Agreement, dated September 10, 2015, by and between IsoRay Medical, Inc. and The Port of Benton. | |
| 14.1 | Code of Conduct and Ethics, incorporated by reference to the Form 10-KSB filed on October 11, 2005. | |
| 14.2 | Code of Ethics for Chief Executive Officer & Senior Financial Officers, incorporated by reference to the Form 10-KSB filed on October 11, 2005. | |
| 21.1* | Subsidiaries of the Company. | |
| 23.1* | Consent of DeCoria, Maichel & Teague, P.S. | |
| 31.1* | Rule 13a-14(a)/15d-14(a) Certification - Chief Executive Officer. | |
| 31.2* | Rule 13a-14(a)/15d-14(a) Certification – Principal Financial Officer. | |
| 32.1** | Section 1350 Certifications. | |
| 101.INS* | XBRL Instance Document. | |
| 101.SCH* | XBRL Taxonomy Extension Schema Document. | |
| 101.CAL* | XBRL Taxonomy Extension Calculation Linkbase Document. | |
| 101.DEF* | XBRL Taxonomy Extension Definition Linkbase Document. | |
| 101.LAB* | XBRL Taxonomy Extension Label Linkbase Document. | |
| 101.PRE* | XBRL Taxonomy Extension Presentation Linkbase Document. |
| * | Filed herewith. | ||
| ** | Furnished herewith. |
Reports on Form 8-K
On May 18, 2015, the Company filed a Current Report on Form 8-K announcing its financial results for the quarter and nine months ended March 31, 2015.
On June 22, 2015, the Company filed a Current Report on Form 8-K announcing the appointment of Michael (Mick) McCormick to the Board; and a change to the Board’s compensation.
On September 2, 2015, the Company filed a Current Report on Form 8-K announcing the extension and amendment of IsoRay Medical, Inc.’s International Distribution Agreement with Karlheinz Goehl-Medizintechnik Göhl.
| 74 |
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders
IsoRay, Inc. and Subsidiaries
Richland, Washington
We have audited the accompanying consolidated balance sheets of IsoRay, Inc. and Subsidiaries as of June 30, 2015 and 2014 and the related consolidated statements of operations, changes in shareholders’ equity, and cash flows for each of the three years in the period ended June 30, 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of IsoRay, Inc. and Subsidiaries at June 30, 2015 and 2014, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2015, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), IsoRay, Inc. and Subsidiaries’ internal control over financial reporting as of June 30, 2015, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) and our report dated September 11, 2015 expressed an unqualified opinion thereon.
/s/ DeCoria, Maichel & Teague, P.S.
Spokane, Washington
September 11, 2015
| F-1 |
IsoRay, Inc. and Subsidiaries
Consolidated Balance Sheets
| June 30, 2015 | June 30, 2014 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 5,226,740 | $ | 7,680,073 | ||||
| Certificates of deposit (Note 3) | 9,362,574 | 10,002,912 | ||||||
| Accounts receivable, net of allowance for doubtful accounts of $30,000 and $38,607, respectively | 1,049,041 | 913,049 | ||||||
| Inventory | 403,955 | 359,737 | ||||||
| Other receivables | 19,615 | 53,082 | ||||||
| Prepaid expenses and other current assets | 263,597 | 206,047 | ||||||
| Total current assets | 16,325,522 | 19,214,900 | ||||||
| Fixed assets, net of accumulated depreciation | 574,840 | 1,017,915 | ||||||
| Certificates of deposit, non-current (Note 3) | 5,106,775 | 5,401,398 | ||||||
| Restricted cash | 181,262 | 181,208 | ||||||
| Inventory, non-current | 569,854 | 469,758 | ||||||
| Other assets, net of accumulated amortization | 245,031 | 264,076 | ||||||
| Total assets | $ | 23,003,284 | $ | 26,549,255 | ||||
| LIABILITIES AND SHAREHOLDERS' EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable and accrued expenses | $ | 498,253 | $ | 574,855 | ||||
| Accrued protocol expense | 124,131 | 80,433 | ||||||
| Accrued radioactive waste disposal | 129,500 | 141,592 | ||||||
| Accrued payroll and related taxes | 212,795 | 236,282 | ||||||
| Accrued vacation | 127,515 | 120,765 | ||||||
| Total current liabilities | 1,092,194 | 1,153,927 | ||||||
| Long-term liabilities: | ||||||||
| Warrant derivative liability | 181,000 | 573,000 | ||||||
| Asset retirement obligation | 947,849 | 866,560 | ||||||
| Total liabilities | 2,221,043 | 2,593,487 | ||||||
| Commitments and contingencies (Note 16) | ||||||||
| Shareholders' equity: | ||||||||
| Preferred stock, $.001 par value; 7,001,671 shares authorized: | ||||||||
| Series A: 1,000,000 shares allocated; no shares issued and outstanding | - | - | ||||||
| Series B: 5,000,000 shares allocated; 59,065 shares issued and outstanding | 59 | 59 | ||||||
| Series C: 1,000,000 shares allocated; no shares issued and outstanding | - | - | ||||||
| Series D: 1,671 and 0 shares allocated; no shares issued and outstanding | - | - | ||||||
| Common stock, $.001 par value; 192,998,329 shares authorized; 54,967,559 and 54,701,708 shares issued and outstanding | 54,968 | 54,702 | ||||||
| Treasury stock, at cost, 13,200 shares | (8,390 | ) | (8,390 | ) | ||||
| Additional paid-in capital | 82,467,111 | 81,959,853 | ||||||
| Accumulated deficit | (61,731,507 | ) | (58,050,456 | ) | ||||
| Total shareholders' equity | 20,782,241 | 23,955,768 | ||||||
| Total liabilities and shareholders' equity | $ | 23,003,284 | $ | 26,549,255 | ||||
The accompanying notes are an integral part of these financial statements.
| F-2 |
IsoRay, Inc. and Subsidiaries
Consolidated Statements of Operations
| Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Product sales, net | $ | 4,606,539 | $ | 4,219,158 | $ | 4,525,233 | ||||||
| Cost of product sales | 4,439,146 | 4,415,629 | 4,375,057 | |||||||||
| Gross profit / (loss) | 167,393 | (196,471 | ) | 150,176 | ||||||||
| Operating expenses: | ||||||||||||
| Research and development | 614,771 | 668,803 | 627,107 | |||||||||
| Sales and marketing | 1,488,456 | 1,234,725 | 1,296,149 | |||||||||
| General and administrative | 2,400,353 | 2,488,219 | 2,294,173 | |||||||||
| Total operating expenses | 4,503,580 | 4,391,747 | 4,217,429 | |||||||||
| Operating loss | (4,336,187 | ) | (4,588,218 | ) | (4,067,253 | ) | ||||||
| Non-operating income (expense): | ||||||||||||
| Interest income | 282,745 | 12,113 | 664 | |||||||||
| Change in fair value of warrant derivative liability | 374,605 | (1,382,134 | ) | 210,000 | ||||||||
| Financing and interest expense | (2,214 | ) | (883 | ) | (7 | ) | ||||||
| Non-operating income (expense) , net | 655,136 | (1,370,904 | ) | 210,657 | ||||||||
| Net loss | (3,681,051 | ) | (5,959,122 | ) | (3,856,596 | ) | ||||||
| Preferred stock deemed dividends (Note 11) | - | (726,378 | ) | - | ||||||||
| Preferred stock dividends | (10,632 | ) | (10,632 | ) | (10,632 | ) | ||||||
| Net loss applicable to common shareholders | $ | (3,691,683 | ) | $ | (6,696,132 | ) | $ | (3,867,228 | ) | |||
| Basic and diluted loss per share | $ | (0.07 | ) | $ | (0.16 | ) | $ | (0.11 | ) | |||
| Weighted average shares used in computing net loss per share: | ||||||||||||
| Basic and diluted | 54,882,350 | 42,675,158 | 34,423,420 | |||||||||
The accompanying notes are an integral part of these financial statements.
| F-3 |
IsoRay, Inc. and Subsidiaries
Consolidated Statement of Changes in Shareholders' Equity
| Series B Preferred Stock | Series D Preferred Stock | Common Stock | Treasury Stock | Additional Paid- | Accumulated | |||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | in Capital | Deficit | Total | ||||||||||||||||||||||||||||||||||
| Balances at June 30, 2012 | 59,065 | $ | 59 | - | $ | - | 30,950,108 | $ | 30,950 | 13,200 | $ | (8,390 | ) | $ | 54,030,311 | $ | (48,234,738 | ) | $ | 5,818,192 | ||||||||||||||||||||||||
| Issuance of common stock pursuant to registered public offering, net | 3,626,943 | 3,627 | 3,288,350 | 3,291,977 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock pursuant to exercise of warrants, net | 2,766 | 3 | 1,822 | 1,825 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock pursuant to exercise of options | 38,700 | 38 | 13,091 | 13,129 | ||||||||||||||||||||||||||||||||||||||||
| Payment of dividend to preferred shareholders | (10,632 | ) | (10,632 | ) | ||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | 108,351 | 108,351 | ||||||||||||||||||||||||||||||||||||||||||
| Net loss | (3,856,596 | ) | (3,856,596 | ) | ||||||||||||||||||||||||||||||||||||||||
| Balances at June 30, 2013 | 59,065 | $ | 59 | - | $ | - | 34,618,517 | $ | 34,618 | 13,200 | $ | (8,390 | ) | $ | 57,431,293 | $ | (52,091,334 | ) | $ | 5,366,246 | ||||||||||||||||||||||||
| Issuance of preferred stock pursuant to underwritten public offering, net | 1,670 | 2 | 1,478,701 | 1,478,703 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock pursuant to underwritten public offering, net | 3,800,985 | 3,801 | 1,796,788 | 1,800,589 | ||||||||||||||||||||||||||||||||||||||||
| Conversion of Series D preferred stock to common stock | (1,670 | ) | (2 | ) | 3,121,480 | 3,121 | (3,119 | ) | - | |||||||||||||||||||||||||||||||||||
| Issuance of common stock pursuant to exercise of warrants, net | 7,165,443 | 7,166 | 7,005,775 | 7,012,941 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock pursuant to exercise of options | 350,983 | 351 | 265,963 | 266,314 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock pursuant to registered public offering, net | 5,644,300 | 5,645 | 13,809,097 | 13,814,742 | ||||||||||||||||||||||||||||||||||||||||
| Payment of dividend to preferred shareholders | (10,632 | ) | (10,632 | ) | ||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | 185,987 | 185,987 | ||||||||||||||||||||||||||||||||||||||||||
| Net loss | (5,959,122 | ) | (5,959,122 | ) | ||||||||||||||||||||||||||||||||||||||||
| Balances at June 30, 2014 | 59,065 | $ | 59 | - | $ | - | 54,701,708 | $ | 54,702 | 13,200 | $ | (8,390 | ) | $ | 81,959,853 | $ | (58,050,456 | ) | $ | 23,955,768 | ||||||||||||||||||||||||
| Issuance of common stock pursuant to exercise of warrants, net | 58,947 | 59 | 99,585 | 99,644 | ||||||||||||||||||||||||||||||||||||||||
| Issuance of common stock pursuant to exercise of options | 206,904 | 207 | 213,041 | 213,248 | ||||||||||||||||||||||||||||||||||||||||
| Payment of dividend to preferred shareholders | (10,632 | ) | (10,632 | ) | ||||||||||||||||||||||||||||||||||||||||
| Share-based compensation | 205,264 | 205,264 | ||||||||||||||||||||||||||||||||||||||||||
| Net loss | (3,681,051 | ) | (3,681,051 | ) | ||||||||||||||||||||||||||||||||||||||||
| Balances at June 30, 2015 | 59,065 | $ | 59 | - | $ | - | 54,967,559 | $ | 54,968 | 13,200 | $ | (8,390 | ) | $ | 82,467,111 | $ | (61,731,507 | ) | $ | 20,782,241 | ||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
| F-4 |
IsoRay, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
| Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | (3,681,051 | ) | $ | (5,959,122 | ) | $ | (3,856,596 | ) | |||
| Adjustments to reconcile net loss to net cash used by operating activities: | ||||||||||||
| Allowance for doubtful accounts | (8,607 | ) | (13,992 | ) | (5,006 | ) | ||||||
| Depreciation of fixed assets | 576,380 | 685,396 | 739,147 | |||||||||
| Amortization of other assets | 36,987 | 30,189 | 31,302 | |||||||||
| Change in fair value of derivative warrant liabilities | (374,605 | ) | 1,382,134 | (210,000 | ) | |||||||
| Accretion of asset retirement obligation | 81,289 | 74,318 | 67,944 | |||||||||
| Share-based compensation | 205,264 | 185,987 | 108,351 | |||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | (127,385 | ) | 24,723 | (53,718 | ) | |||||||
| Inventory | (144,314 | ) | 45,834 | 38,774 | ||||||||
| Other receivables | 33,467 | (41,580 | ) | (1,577 | ) | |||||||
| Prepaid expenses and other current assets | (57,550 | ) | (3,167 | ) | (58,764 | ) | ||||||
| Accounts payable and accrued expenses | (76,602 | ) | 142,289 | 43,461 | ||||||||
| Accrued protocol expense | 43,698 | 55,128 | 25,305 | |||||||||
| Accrued radioactive waste disposal | (12,092 | ) | 41,592 | 48,000 | ||||||||
| Accrued payroll and related taxes | (23,487 | ) | 108,863 | 7,538 | ||||||||
| Accrued vacation | 6,750 | 13,187 | 19,572 | |||||||||
| Net cash used by operating activities | (3,521,858 | ) | (3,228,221 | ) | (3,056,267 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||||||
| Purchases of fixed assets | (133,305 | ) | (19,029 | ) | (6,576 | ) | ||||||
| Additions to licenses and other assets | (17,942 | ) | (17,758 | ) | (6,118 | ) | ||||||
| Proceeds from the maturity of certificates of deposit | 15,873,376 | - | - | |||||||||
| Purchases of certificates of deposit | (10,143,741 | ) | (10,002,912 | ) | - | |||||||
| Purchases of certificates of deposit - non-current | (4,794,674 | ) | (5,401,398 | ) | - | |||||||
| Change in restricted cash | (54 | ) | (59 | ) | (122 | ) | ||||||
| Net cash provided by (used in) investing activities | 783,660 | (15,441,156 | ) | (12,816 | ) | |||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Preferred dividends paid | (10,632 | ) | (10,632 | ) | (10,632 | ) | ||||||
| Proceeds from sales of preferred stock, pursuant to underwritten offering, net | - | 1,478,703 | - | |||||||||
| Proceeds from sales of common stock, pursuant to underwritten offering, net | - | 1,800,589 | - | |||||||||
| Proceeds from sales of common stock, pursuant to registered public offering, net | - | 13,814,742 | 3,291,977 | |||||||||
| Proceeds from sales of common stock, pursuant to exercise of warrants | 82,249 | 6,099,807 | 1,825 | |||||||||
| Proceeds from sales of common stock, pursuant to exercise of options | 213,248 | 266,314 | 13,129 | |||||||||
| Net cash provided by financing activities | 284,865 | 23,449,523 | 3,296,299 | |||||||||
| Net increase (decrease) in cash and cash equivalents | (2,453,333 | ) | 4,780,146 | 227,216 | ||||||||
| Cash and cash equivalents, beginning of year | 7,680,073 | 2,899,927 | 2,672,711 | |||||||||
| CASH AND CASH EQUIVALENTS, END OF YEAR | $ | 5,226,740 | $ | 7,680,073 | $ | 2,899,927 | ||||||
| Supplemental disclosures of cash flow information: | ||||||||||||
| Cash paid for interest | $ | 2,214 | $ | 748 | $ | 7 | ||||||
| Non-cash investing and financing activities: | ||||||||||||
| Preferred stock deemed dividends (Note 11) | $ | - | $ | (726,378 | ) | $ | - | |||||
| Reclassification of derivative warrant liability to equity upon exercise | (17,395 | ) | (913,134 | ) | - | |||||||
| Reclassification of convertible preferred stock to common stock upon conversion | - | (1,478,703 | ) | - | ||||||||
The accompanying notes are an integral part of these financial statements.
| F-5 |
IsoRay, Inc.
Notes to Consolidated Financial Statements
For the years ended June 30, 2015, 2014 and 2013
| 1. | Organization |
Century Park Pictures Corporation (Century) was organized under Minnesota law in 1983. Century had no operations during the period from September 30, 1999 through June 30, 2005.
On July 28, 2005, IsoRay Medical, Inc. (Medical) became a wholly-owned subsidiary of Century pursuant to a merger. Century changed its name to IsoRay, Inc. (IsoRay or the Company). In the merger, the Medical stockholders received approximately 82% of the then outstanding securities of the Company.
Medical, a Delaware corporation, was incorporated effective June 15, 2004 to develop, manufacture and sell isotope-based medical products and devices for the treatment of cancer and other malignant diseases.
IsoRay International LLC, a Washington limited liability company, which is wholly-owned by IsoRay Inc. was formed on November 27, 2007 to serve as an owner in a Russian LLC that planned to distribute the Company’s products to the Russian market and also license the Company’s technology for use in manufacturing Cs-131 brachytherapy seeds in Russia. During fiscal year 2009, the Company divested its ownership in the Russian LLC. IsoRay International, LLC is now used to conduct business with the international distributors of Medical’s products.
IsoRay, Inc., together with its subsidiaries, (“IsoRay” or the “Company”), are headquartered in Richland, Washington.
| 2. | Summary of Significant Accounting Policies |
Basis of Presentation and Principles of Consolidation
The accompanying consolidated financial statements have been prepared in accordance with US GAAP, pursuant to the rules and regulations of the Securities and Exchange Commission, or SEC. The consolidated financial statements include the accounts of the Company and its wholly-owned subsidiaries (collectively the Company). All significant inter-company transactions and balances have been eliminated in consolidation.
Cash Equivalents
The Company considers currency on hand, demand deposits, time deposits, and all highly liquid investments with an original maturity of three months or less at the date of purchase to be cash and cash equivalents. Cash and cash equivalents are held in various financial institutions in the United States.
Certificates of Deposit
Certificates of deposit with original maturities greater than three months and remaining maturities less than one year are classified as "Certificates of deposit" and included in current assets. Certificates of deposit with remaining maturities greater than one year are classified as ”Certificates of deposit, non-current” and are included in noncurrent assets.
Accounts Receivable
Accounts receivable are stated at the amount that management of the Company expects to collect from outstanding balances. Management provides for probable uncollectible amounts through an allowance for doubtful accounts. Additions to the allowance for doubtful accounts are based on management's judgment, considering historical experience with write-offs, collections and current credit conditions. Balances which remain outstanding after management has used reasonable collection efforts are written off through a charge to the allowance for doubtful accounts and a credit to the applicable accounts receivable. Payments received subsequent to the time that an account is written off are treated as bad debt recoveries.
| F-6 |
Inventory
Inventory is reported at the lower of cost or market. Cost of raw materials is determined using the weighted average method. Cost of work in process and finished goods is computed using standard cost, which approximates actual cost, on a first-in, first-out basis.
The cost of materials and production costs contained in inventory that are not useable due to the passage of time, and resulting loss of bio-effectiveness, are written off to cost of product sales at the time it is determined that the product is no longer useable.
Fixed Assets
Fixed assets are capitalized and carried at the lower of cost or net realizable value. Normal maintenance and repairs are charged to expense as incurred. When any assets are sold or otherwise disposed of, the cost and accumulated depreciation are reversed with any resulting gain or loss being recognized in the non-operating income / (expense) section of the consolidated statement of operations.
Depreciation is computed using the straight-line method over the following estimated useful lives:
| Production equipment | 3 to 7 years | |
| Office equipment | 2 to 5 years | |
| Furniture and fixtures | 2 to 5 years |
Leasehold improvements and capital lease assets are amortized over the shorter of the lease term or the estimated useful life of the asset.
Management of the Company periodically reviews the net carrying value of all of its long-lived assets on an asset by asset basis. These reviews consider the net realizable value of each asset to determine whether there is impairment in the value of an asset which has occurred, and there is a need for any asset impairment write-down.
Although management has made its best estimate of the factors that affect the carrying value based on current conditions, it is reasonably possible that changes could occur which could adversely affect management's estimate of net cash flows expected to be generated from its assets that could result in an impairment adjustment.
Prepaid Expenses and Other Assets
Prepaid expenses and other assets, which include deferred charges, patents and licenses, are stated at cost, less accumulated amortization. Amortization of patents is computed using the straight-line method over the estimated economic useful lives of the assets. Licenses include costs related to licenses pertaining to the use of technology or operational licenses. These licenses are recorded at stated cost, less accumulated amortization. Amortization of licenses is computed using the straight-line method over the estimated economic useful lives of the assets. The Company periodically reviews the carrying values of licenses and evaluates the recorded basis for any impairment. Any impairment is recognized when the expected future operating cash flows to be derived from the licenses are less than their carrying value. The Company periodically reviews the carrying values of patents and any related impairments are recognized when the expected future operating cash flows to be derived from such assets are less than their carrying value.
| F-7 |
Asset Retirement Obligation
The estimated fair value of the future retirement costs of the Company's leased assets and the costs for the decontamination and reclamation of equipment located within the footprint leased asset are recorded as a liability on a discounted basis when a contractual obligation exists; an equivalent amount is capitalized to fixed assets. The initial recorded obligation is discounted using the Company's credit-adjusted risk-free rate and is reviewed periodically for changes in the estimated future costs underlying the obligation. The Company amortizes the initial amount capitalized to property and equipment and recognizes accretion expense in connection with the discounted liability over the estimated remaining useful life of the leased assets.
Financial Instruments
At June 30, 2015 and 2014, the carrying value of financial instruments such as certificates of deposit approximated fair value based on the short-term maturities of these instruments. The Company discloses the fair value of financial instruments, both assets and liabilities, recognized and not recognized in the balance sheet, for which it is practicable to estimate the fair value. The fair value of a financial instrument is the amount at which the instrument could be exchanged in a current transaction between willing parties, other than a forced liquidation sale.
Fair Value Measurement
ASC Topic 820, Fair Value Measurements, establishes a fair value hierarchy for those assets and liabilities measured at fair value which distinguishes between assumptions based on market data (observable inputs). The hierarchy consists of: Level 1 – quoted market prices in active markets for identical instruments; Level 2 – inputs other than Level 1 inputs that are observable; and Level 3 – unobservable inputs developed using estimates and assumptions determined by the Company.
At June 30, 2015 and 2014, there were no assets or liabilities measured at fair-value on a recurring basis which were measured using Level 3 inputs. The Company had a single liability, the derivative warrant liability, that was measured at fair value on a recurring basis using Level 2 inputs during the years ended June 30, 2015 and 2014. Certain assets and liabilities are measured at fair value on a non-recurring basis; that is, the instruments are not measured at fair value on an ongoing basis, but are subject to fair value adjustments only in certain circumstances (for example, when there is evidence of impairment). The Company had no assets or liabilities measured at fair value on a nonrecurring basis during the years ended June 30, 2015 and 2014.
The following table sets forth the Company’s financial assets and liabilities measured at fair value on a recurring basis by level within the fair value hierarchy. Assets and liabilities are classified in their entirety base on the lowest level of input that is significant to the fair value measurement.
Fair value at June 30, 2015
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Cash and cash equivalents | $ | 5,226,740 | $ | 5,226,740 | $ | - | $ | - | ||||||||
| Warrant derivative liability | 181,000 | - | 181,000 | - | ||||||||||||
| F-8 |
Fair value at June 30, 2014
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Cash and cash equivalents | $ | 7,680,073 | $ | 7,680,073 | $ | - | $ | - | ||||||||
| Warrant derivative liability | 573,000 | - | 573,000 | - | ||||||||||||
The Company’s cash and cash equivalent instruments are classified within Level 1 of the fair value hierarchy because they are valued using quoted market prices.
The Company’s warrant derivative liability is valued using the Black-Scholes option pricing model which requires a variety of inputs as described in Note 11. Such instruments are typically included in Level 2.
Warrant Derivative Liabilities
For the warrant derivative liabilities which are measured at fair value on a recurring basis, the Company uses the Black-Scholes valuation model as described in Note 11.
Revenue Recognition
The Company recognizes revenue related to product sales when (i) persuasive evidence of an arrangement exists, (ii) shipment has occurred, (iii) the fee is fixed or determinable, and (iv) collectability is reasonably assured.
The Company recognizes revenue once the product has been shipped to the customer. Prepayments, if any, received from customers prior to the time that products are shipped are recorded as deferred revenue. In these cases, when the related products are shipped, the amount recorded as deferred revenue is then recognized as revenue. The Company accrues for sales returns and other allowances at the time of shipment.
Shipping and Handling Costs
Shipping and handling costs include charges associated with delivery of goods from the Company's facilities to its customers and are reflected in cost of product sales. Shipping and handling costs paid to the Company by its customers are classified as product sales.
Share-Based Compensation
The Company measures and recognizes expense for all share-based payments at fair value. The Company uses the Black-Scholes option valuation model to estimate fair value for all stock options on the date of grant. For stock options that vest over time, the Company recognizes compensation cost on a straight-line basis over the requisite service period for the entire award.
| F-9 |
Research and Development Costs
Research and development costs, including salaries, research materials, administrative expenses and contractor fees, are charged to operations as incurred. The cost of equipment used in research and development activities which has alternative uses is capitalized as part of fixed assets and not treated as an expense in the period acquired. Depreciation of capitalized equipment used to perform research and development is classified as research and development expense in the year recognized.
Advertising and Marketing Costs
Advertising costs are expensed as incurred except for the cost of tradeshows and related marketing materials which are deferred until the tradeshow occurs.
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Advertising and marketing costs expensed (including tradeshows) | $ | 151,197 | $ | 114,313 | $ | 86,705 | ||||||
| At June 30, | ||||||||
| 2015 | 2014 | |||||||
| Prepaid marketing expenses deferred until event occurs | $ | 9,600 | $ | 14,124 | ||||
Legal Contingencies
The Company records contingent liabilities resulting from asserted and unasserted claims against it, when it is probable that a liability has been incurred and the amount of the loss is reasonably estimable. Estimating probable losses requires analysis of multiple factors, in some cases including judgments about the potential actions of third-party claimants and courts. Therefore, actual losses in any future period are inherently uncertain. Currently, the Company does not believe any probable legal proceedings or claims will have a material adverse effect on its financial position or results of operations. However, if actual or estimated probable future losses exceed the Company's recorded liability for such claims, it would record additional charges as other expense during the period in which the actual loss or change in estimate occurred.
Income Taxes
Income taxes are accounted for under the liability method. Under this method, the Company provides deferred income taxes for temporary differences that will result in taxable or deductible amounts in future years based on the reporting of certain costs in different periods for financial statement and income tax purposes. This method also requires the recognition of future tax benefits such as net operating loss carry-forwards, to the extent that realization of such benefits is more likely than not. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in operations in the period that includes the enactment of the change. Management has determined that the Company and its subsidiaries Medical and International are subject to examination of their income tax filings in the United States and state jurisdictions for the 2012 through 2015 tax years. In the event that the Company is assessed penalties and or interest, penalties will be charged to other operating expense and interest will be charged to interest expense in the period that they are assessed.
| F-10 |
Income (Loss) Per Common Share
Basic earnings per share is calculated by dividing net income (loss) available to common shareholders by the weighted average number of common shares outstanding, and does not include the impact of any potentially dilutive common stock equivalents, including preferred stock, common stock warrants or options that are potentially convertible into common stock, as those would be antidilutive due to the Company's net loss position.
Securities that could be dilutive in the future are as follows:
| June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Preferred stock | 59,065 | 59,065 | 59,065 | |||||||||
| Common stock warrants | 385,800 | 444,747 | 1,957,033 | |||||||||
| Common stock options | 2,418,282 | 2,314,422 | 2,305,072 | |||||||||
| Total potential dilutive securities | 2,863,147 | 2,818,234 | 4,321,170 | |||||||||
Use of Estimates
The preparation of consolidated financial statements in accordance with generally accepted accounting principles in the United States of America requires management of the Company to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes of the Company including the allowance for doubtful accounts receivable; net realizable value of the enriched barium inventory; the estimated useful lives used in calculating depreciation and amortization on the Company's fixed assets, patents, trademarks and other assets; estimated amount and fair value of the asset retirement obligation related to the Company's production facilities; inputs used in the calculation of expense related to share-based compensation including volatility, estimated lives and forfeiture rates of options granted; and the inputs to the Black-Scholes calculation to estimate the fair value of the derivative warrant liability and the related gain or loss. Accordingly, actual results could differ from those estimates and affect the amounts reported in the financial statements.
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2014-09, "Revenue from Contracts with Customers" (ASU 2014-09), which supersedes the revenue recognition requirements in FASB Accounting Standards Codification (ASC) Topic 605, "Revenue Recognition". The guidance requires that an entity recognize revenue in a way that depicts the transfer of promised goods or services to customers in the amount that reflects the consideration to which the entity expects to be entitled to in exchange for those goods and services. The guidance will be effective for annual reporting periods beginning after December 15, 2017, including interim periods within that reporting period and is to be applied retrospectively, with early application not permitted. The Company is currently evaluating the new standard and its impact on the Company's consolidated financial statements.
3. Certificates of deposit
| As of June 30, 2015 | ||||||||||||||||
| Under 90 | 91 days to | Six months to | Greater | |||||||||||||
| Days | six months | 1 year | than 1 year | |||||||||||||
| CDARS | $ | 3,523,167 | $ | 500,064 | $ | 5,339,343 | $ | 5,106,775 | ||||||||
| As of June 30, 2014 | ||||||||||||||||
| Under 90 | 91 days to | Six months to | Greater | |||||||||||||
| Days | six months | 1 year | than 1 year | |||||||||||||
| CDARS | $ | - | $ | 5,000,902 | $ | 5,002,010 | $ | 5,401,398 | ||||||||
| F-11 |
Certificate of Deposit Account Registry Service (CDARS) is a system that allows the Company to invest in certificates of deposit through a single financial institution that exceed the $250,000 limit to be fully insured by the Federal Deposit Insurance Corporation (FDIC). That institution utilizes the CDARS system to purchase certificates of deposit at other financial institutions while keeping the investment at each institution fully insured by the Federal Deposit Insurance Corporation (FDIC).
| 4. | Inventory |
Inventory consisted of the following:
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Raw materials | $ | 143,669 | $ | 173,417 | ||||
| Work in process | 204,760 | 151,321 | ||||||
| Finished goods | 55,526 | 34,999 | ||||||
| Total inventory | $ | 403,955 | $ | 359,737 | ||||
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Enriched barium, non-current | $ | 469,758 | $ | 469,758 | ||||
| Raw materials, non-current | 100,096 | - | ||||||
| Total inventory, non-current | $ | 569,854 | $ | 469,758 | ||||
Inventory, non-current is raw materials that were ordered in quantities to obtain volume cost discounts which based on current and anticipated sales volumes will not be consumed within an operating cycle and the enriched barium which is classified as non-current is only expected to be utilized if required to obtain volumes of isotope that is not able to be purchased from an existing source in the short or long-term. Management does not anticipate the need to utilize the enriched barium within the current operating cycle.
| 5. | Prepaid Expenses and Other Current Assets |
Prepaid expenses and other current assets consisted of the following:
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Prepaid insurance | $ | 30,578 | $ | 24,763 | ||||
| Prepaid rent | - | 22,701 | ||||||
| Other prepaid expenses | 206,326 | 131,890 | ||||||
| Other current assets | 26,693 | 26,693 | ||||||
| $ | 263,597 | $ | 206,047 | |||||
| 6. | Fixed Assets, net |
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Production equipment | $ | 3,180,933 | $ | 3,120,782 | ||||
| Office equipment | 224,576 | 167,706 | ||||||
| Furniture and fixtures | 148,265 | 148,265 | ||||||
| Leasehold improvements | 4,129,977 | 4,129,977 | ||||||
| Capitalized assets, not in service1 | 5,925 | - | ||||||
| 7,689,676 | 7,566,730 | |||||||
| Less accumulated depreciation | (7,114,836 | ) | (6,548,815 | ) | ||||
| Fixed assets, net | $ | 574,840 | $ | 1,017,915 | ||||
| F-12 |
1 – Capitalized assets, not placed in service are items that meet the capitalization threshold or which management believes will meet the threshold at the time of completion and which have yet to be placed into service as of the date of the balance sheet.
| Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Depreciation expense | $ | 576,380 | $ | 685,396 | $ | 739,147 | ||||||
During the fiscal year ended June 30, 2015, the Company disposed of certain production equipment that were fully depreciated in the amount of $10,359 which were beyond their useful lives. During the fiscal year ended June 30, 2014, the Company disposed of certain information technology assets that were fully depreciated in the amount of $59,723 that were beyond their depreciable lives. No gain or loss was recognized on these assets as they were fully depreciated and had no salvage value. In the year ended June 30, 2013, no disposal of assets occurred.
| 7. | Restricted Cash |
The Washington Department of Health requires the Company to provide collateral for the decommissioning of its facility. To satisfy this requirement, the Company funded two certificates of deposits (CDs) totaling $172,500 in separate banks. The CDs both have original maturities of three months but are termed restricted cash and classified as a long-term asset as the Company does not anticipate decommissioning the facility until the end of the current lease plus the one remaining three-year lease option period. The end date of the current lease including the one remaining three-year renewal option is April 2019. Interest earned on the CDs is rolled-over at the maturity of each CD and becomes part of the restricted cash balance. These funds will be used to settle a portion of the Company's remaining asset retirement obligations (See Note 9).
| 8. | Other Assets, net |
Other assets, net of accumulated amortization consisted of the following:
| June 30, | ||||||||
| 2015 | 2014 | |||||||
| Deferred charges | $ | 46,541 | $ | 40,320 | ||||
| Patents and trademarks, net of accumulated amortization of $134,559 and $109,292 | 198,490 | 223,756 | ||||||
| $ | 245,031 | $ | 264,076 | |||||
| Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Amortization expense on patents and trademarks | $ | 25,226 | $ | 18,468 | $ | 19,581 | ||||||
| F-13 |
Future amortization is expected to be as follows:
| FY2016 | $ | 17,160 | ||
| FY2017 | 17,133 | |||
| FY2018 | 17,005 | |||
| FY2019 | 15,919 | |||
| FY2020 | 15,390 | |||
| Thereafter | 115,883 | |||
| $ | 198,490 |
| 9. | Asset Retirement Obligation |
The asset retirement obligations changed as follows:
| Year Ended June 30, | ||||||||
| 2015 | 2014 | |||||||
| Beginning balance | $ | 866,560 | $ | 792,242 | ||||
| Accretion of discount | 81,289 | 74,318 | ||||||
| Ending balance | $ | 947,849 | $ | 866,560 | ||||
| 10. | Share-Based Compensation |
The Company currently provides share-based compensation under four equity incentive plans approved by the Board of Directors: the Amended and Restated 2005 Stock Option Plan (the Option Plan), the Amended and Restated 2005 Employee Stock Option Plan (the 2005 Employee Plan), the Amended and Restated 2006 Director Stock Option Plan (the Director Plan) and 2014 Employee Stock Option Plan (the 2014 Employee Plan). The Option Plan allowed the Board of Directors to grant options to purchase up to 1,800,000 shares of common stock to directors, officers, key employees and service providers of the Company until it expired in May 2015. The 2005 Employee Plan and 2014 Employee Plan each allow the Board of Directors to grant options to purchase up to 2,000,000 shares of common stock to officers and key employees of the Company. The 2005 Employee Plan expired in May 2015. The Director Plan allows the Board of Directors to grant options to purchase up to 1,000,000 shares of common stock to directors of the Company. Options granted under all of the plans have a ten year maximum term, an exercise price equal to at least the fair market value of the Company's common stock on the date of the grant, and varying vesting periods as determined by the Board. For stock options with graded vesting terms, the Company recognizes compensation cost on a straight-line basis over the requisite service period for the entire award.
The Black-Scholes option valuation model was developed for use in estimating the fair value of traded options which have no vesting restrictions and are fully transferable. In addition, option valuation models require the input of highly subjective assumptions, including the expected stock price volatility. The Company uses the Black-Scholes option valuation model because management believes the model is appropriate for the Company. However, management understands that because changes in the subjective input assumptions can materially affect the fair value estimate, this valuation model does not necessarily provide a reliable single measure of the fair value of its stock options. The risk-free interest rate is based on the U.S. treasury security rate with an equivalent term in effect as of the date of grant. The expected option lives, volatility, and forfeiture assumptions are based on historical data of the Company.
The weighted average fair value of stock option awards granted and the key assumptions used in the Black-Scholes valuation model to calculate the fair value are as follows:
| F-14 |
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Weighted average fair value of options granted | $ | 1.11 | $ | 1.28 | $ | 0.80 | ||||||
| Key assumptions used in determining fair value | ||||||||||||
| Weighted average risk-free interest rate | 1.56 | % | 1.66 | % | 0.71 | % | ||||||
| Weighted average life of the option (in years) | 4.63 | 4.79 | 4.77 | |||||||||
| Weighted average historical stock price volatility | 106.75 | % | 120.89 | % | 132.47 | % | ||||||
| Expected dividend yield | 0.00 | % | 0.00 | % | 0.00 | % | ||||||
The following table presents the share-based compensation expense:
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Cost of product sales | $ | 44,798 | $ | 17,818 | $ | 38,729 | ||||||
| Research and development expense | 17,107 | 13,486 | 30,350 | |||||||||
| Sales and marketing expense | 11,608 | 3,588 | 6,943 | |||||||||
| General and administrative expense | 131,751 | 151,095 | 32,329 | |||||||||
| Total share-based compensation | $ | 205,264 | $ | 185,987 | $ | 108,351 | ||||||
The total value of the stock options awards is expensed ratably over the vesting period of the employees receiving the awards. As of June 30, 2015, total unrecognized compensation cost related to stock-based options and awards was $408,424 and the weighted-average period over which it is expected to be recognized is approximately 1.35 years.
A summary of stock option information within the Company's share-based compensation plans during the fiscal years is presented below:
| Options | ||||||||||||||||
| Outstanding | Price (a) | Life (b) | Value (c) | |||||||||||||
| Balance at June 30, 2012 | 2,381,306 | $ | 1.82 | 5.64 | $ | 473,951 | ||||||||||
| Expired/Forfeited | (37,534 | ) | 2.58 | |||||||||||||
| Exercised | (38,700 | ) | 0.34 | |||||||||||||
| Balance at June 30, 2013 | 2,305,072 | $ | 1.83 | 4.93 | $ | 115,302 | ||||||||||
| Granted (d) | 430,000 | 1.81 | ||||||||||||||
| Expired/Forfeited | (19,667 | ) | 0.61 | |||||||||||||
| Exercised | (350,983 | ) | 0.76 | |||||||||||||
| Balance at June 30, 2014 | 2,314,422 | $ | 2.00 | 4.69 | $ | 3,186,916 | ||||||||||
| Granted (d) | 395,000 | 1.41 | ||||||||||||||
| Expired/Forfeited | (84,236 | ) | 4.15 | |||||||||||||
| Exercised | (206,904 | ) | 1.03 | |||||||||||||
| Balance at June 30, 2015 | 2,418,282 | $ | 1.91 | 4.71 | $ | 691,789 | ||||||||||
| Vested and expected to vest at June 30, 2015 | 2,318,741 | $ | 1.93 | 4.58 | $ | 666,081 | ||||||||||
| Exercisable at June 30, 2015 | 2,015,926 | $ | 1.94 | 3.73 | $ | 680,238 | ||||||||||
| (a) | Weighted average exercise price per share. |
| (b) | Weighted average remaining contractual life. |
| (c) | Aggregate intrinsic value. |
| F-15 |
| (d) | All options granted had exercise prices equal to or greater than the ending closing market price of the Company's common stock on the grant date. The options were granted to employees and management by the Board of Directors and had vesting periods from immediate to three years. |
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Aggregate intrinsic value of options exercised | $ | 306,620 | $ | 548,928 | $ | 16,246 | ||||||
The Company's current policy is to issue new shares to satisfy option exercises.
| 11. | Shareholders’ Equity |
The authorized capital structure of the Company consists of $.001 par value preferred stock and $.001 par value common stock.
Preferred Stock
The Company's Articles of Incorporation authorize 7,001,671 shares of $0.001 par value preferred stock available for issuance with such rights and preferences, including liquidation, dividend, conversion, and voting rights, as described below.
Series A
Series A preferred shares are entitled to a 10% dividend annually on the stated par value per share. These shares are convertible into shares of common stock at the rate of one share of common stock for each share of Series A preferred stock, and are subject to automatic conversion into common stock upon the closing of an underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933 covering the offer and sale of common stock in which the gross proceeds to the Company are at least $4 million. Series A preferred shareholders have voting rights equal to the voting rights of common stock, except that the vote or written consent of a majority of the outstanding preferred shares is required for any changes to the Company's Articles of Incorporation, Bylaws or Certificate of Designation, or for any bankruptcy, insolvency, dissolution or liquidation of the Company. Upon liquidation of the Company, the Company's assets are first distributed ratably to the Series A preferred shareholders, second, to the Series B preferred shareholders, third, to the Series C preferred shareholders, and fourth, to the Series D Convertible preferred shareholders on an “as converted” basis on parity with the holders of the Common Stock. At June 30, 2015 and 2014, there were no shares of Series A preferred stock outstanding.
Series B
Series B preferred shares are entitled to a cumulative 15% dividend annually on the stated par value per share. These shares are convertible into shares of common stock at the rate of one share of common stock for each share of Series B preferred stock, and are subject to automatic conversion into common stock upon the closing of an underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933 covering the offer and sale of common stock in which the gross proceeds to the Company are at least $4 million. Series B preferred shareholders have voting rights equal to the voting rights of common stock, except that the vote or written consent of a majority of the outstanding preferred shares is required for any changes to the Company's Articles of Incorporation, Bylaws or Certificate of Designation, or for any bankruptcy, insolvency, dissolution or liquidation of the Company. Upon liquidation of the Company, the Company's assets are first distributed ratably to the Series A preferred shareholders, second, to the Series B preferred shareholders, third, to the Series C preferred shareholders, and fourth, to the Series D Convertible preferred shareholders on an “as converted” basis on parity with the holders of the Common Stock.
| F-16 |
On December 17, 2014, the Board of Directors declared a dividend on the Series B Preferred Stock of all outstanding and cumulative dividends through December 31, 2014. The total dividends of $10,632 were paid as of December 31, 2014. At June 30, 2015, there were 59,065 Series B preferred shares outstanding and cumulative dividends in arrears were $5,316 and upon any liquidation, dissolution, or winding up of the Company, whether voluntary or involuntary, the assets of the Company legally available for distribution, if any, shall be distributed ratably first, to the holders of the Series A Preferred Stock, second, to the holders of the Series B Preferred Stock, third, to the Series C preferred shareholders, and fourth, to the Series D preferred shareholders on an “as converted” basis on parity with the holders of the Common Stock.
Series C
Series C preferred shares are entitled to a quarterly dividend equal, per share, equal to the greater of $1.00 or 100 times the dividends declared on the common stock in such quarter. Each share of Series C preferred stock has voting rights equal to the voting rights of 100 shares of common stock. The Series C preferred stock was created upon adoption of the Company's share rights plan in 2007. Upon liquidation of the Company, the Company's assets are first, distributed to the holders of the Series A Preferred Stock, second, to the holders of the Series B Preferred Stock and third, to the Series C preferred shareholders and fourth, to the Series D preferred shareholders on an “as converted” basis on parity with the holders of the Common Stock. At June 30, 2015 and 2014, there were no shares of Series C preferred stock outstanding.
Series D
Established in August 2013, Series D preferred shares are entitled to dividends in the same form as dividends actually paid on shares of common stock. Additionally, the Company shall not pay any dividends on shares of common stock (other than dividends in the form of common stock) unless the holders of Series D Convertible Preferred Stock shall first receive dividends on shares of Series D Convertible Preferred Stock held by them (on an as-if-converted-to-common-stock-basis) in an amount equal to and in the same form as any such dividends (other than dividends in the form of common stock) to be paid on shares of common stock. Except as required by law, shares of Series D Convertible Preferred Stock shall not have the right to vote on any matter other than those set forth in the Certificate of Designation with the potential to specifically adversely affect the Series D Convertible Preferred Stock. Series D Convertible Preferred shares are convertible into shares of common stock at the rate of 1,869.15 shares of common stock for each share of Series D Convertible Preferred Stock at any time at the option of the holder, provided that the holder will be prohibited from converting shares of Series D Convertible Preferred Stock into shares of our common stock if, as a result of the conversion, the holder, together with its affiliates, would beneficially own more than 9.99% of the total number of shares of our common stock then issued and outstanding, which is referred to herein as the “Beneficial Ownership Limitation”. At June 30, 2015 and 2014, respectively, there were no shares of Series D Convertible Preferred Stock outstanding. In addition to the previously outstanding shares of common stock and Series B preferred stock, the Company had the following transactions that affected shareholders' equity during the years ended June 30, 2015 and 2014.
Common and Preferred Stock Transactions
Series D Preferred
On August 29, 2013, the Company entered into an agreement to sell 3,800,985 common units, each consisting of 1 share of common stock and a warrant to purchase 0.816 shares of common stock (the Common Units), and 1,670 preferred units, each consisting of 1 share of Series D Convertible Preferred Stock and a warrant to purchase 1,525.23 shares of common stock (the Preferred Units) on a firm commitment underwritten basis. The Common Units were sold at an initial per unit purchase price of $0.535 and the Preferred Units were sold at an initial per unit purchase price of $1,000. The warrants were all exercisable at $0.72 per share and had a twenty-four month term. Each share of the Series D Convertible Preferred Stock was convertible into 1,869.15 shares of common stock at any time at the option of the holder, subject to adjustment and certain ownership percentage restrictions. The preferred shares which were convertible into shares of common stock contained a beneficial conversion feature of $726,378 which was recognized as a deemed dividend to the Series D preferred shareholders on the date of issuance. This public offering resulted in gross proceeds of $3.7 million. The offering yielded approximately $3,279,292 in cash after expenses.
| F-17 |
During January 2014, the holder of the 1,670 shares of Series D convertible preferred stock fully exercised its right to convert the 1,670 shares of Series D convertible preferred stock into 3,121,480 shares of common stock which at the time of conversion resulted in an increase in shares of common stock outstanding from 38,419,502 to 41,540,982. Subsequent to the conversion, no shares of Series D convertible preferred stock remain outstanding.
Common Stock
On March 21, 2014, the Company entered into a Securities Purchase Agreement with certain investors providing for the sale of a total of 5,644,300 shares of common stock for an aggregate purchase price of $14,675,180 at a price per share of $2.60. The Company received net proceeds from the offering of approximately $13,814,742 which will be used to meet the Company’s working capital needs and general corporate purposes.
| F-18 |
Warrant derivative liability
Based on the guidance contained in ASC 815 “Derivatives and Hedging”, management has concluded that the warrants issued in the 2011 offering should be classified as a derivative liability and has recorded a liability at fair value.
Change in fair value of the warrant derivative liability is as follows.
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Change in fair value of the warrant derivative liability | $ | 374,605 | $ | (1,382,134 | ) | $ | 210,000 | |||||
A summary of the change in fair value of derivative warrant liability is as follows for the fiscal years presented.
| Quantity1 | Amount | |||||||
| Balance at June 30, 2012 | 713,601 | $ | 314,000 | |||||
| Change in fair value | (210,000 | ) | ||||||
| Balance at June 30, 2013 | 713,601 | $ | 104,000 | |||||
| Change in fair value | 1,382,000 | |||||||
| Warrants corrected | 10,869 | - | ||||||
| Warrants redeemed in cashless exercise | (22,072 | ) | - | |||||
| Warrants exercised | (463,702 | ) | (913,000 | ) | ||||
| Balance at June 30, 2014 | 238,696 | $ | 573,000 | |||||
| Change in fair value | (374,605 | ) | ||||||
| Warrants exercised | (13,209 | ) | (17,395 | ) | ||||
| Balance at June 30, 2015 | 225,087 | $ | 181,000 | |||||
1 Quantity of derivative warrants issued and/or outstanding as of the date of valuation.
Warrants
The following table summarizes the activity of all stock and weighted average exercise prices for each category. No warrants expired during any of the periods.
| Warrants | Price (a) | |||||||
| Balance at June 30, 2012 | 1,959,799 | $ | 1.38 | |||||
| Warrants exercised | (2,766 | ) | 0.66 | |||||
| Balance at June 30, 2013 | 1,957,033 | $ | 1.38 | |||||
| Corrections | 26,939 | 1.31 | ||||||
| Warrants redeemed in cashless exercise | (22,520 | ) | 0.96 | |||||
| Warrants exercised | (7,165,443 | ) | 0.86 | |||||
| Warrants granted | 5,648,738 | 0.72 | ||||||
| Balance at June 30, 2014 | 444,747 | $ | 1.43 | |||||
| Warrants exercised | (58,947 | ) | 1.38 | |||||
| Balance at June 30, 2015 | 385,800 | $ | 1.22 | |||||
(a) Weighted average exercise price per share.
| F-19 |
The following table summarizes additional information about the Company’s common warrants outstanding as of June 30, 2015:
| Number of | Range of | Expiration | ||||||
| Warrants | Exercise Prices1 | Date | ||||||
| 25,000 | $ | 2.00 | July 2015 | |||||
| 130,713 | 1.56 | November 2015 | ||||||
| 199,437 | 0.94 | October 2016 | ||||||
| 25,650 | 0.94 | December 2016 | ||||||
| 5,000 | 0.98 | June 2017 | ||||||
| 385,800 | ||||||||
1 – Exercise prices have been rounded to the nearest whole cent.
12. Income Taxes
Due to net losses, the Company did not record an income tax provision or benefit for the years ending June 30, 2015, 2014 and 2013.
The significant deferred tax components using a 35% federal income tax rate for the years ended June 30, 2015 and 2014 are as follows:
| As of June 30, | ||||||||
| 2015 | 2014 | |||||||
| Fixed assets | $ | 649,350 | $ | 473,859 | ||||
| Share-based compensation | 443,200 | 371,358 | ||||||
| Reserves | 10,500 | 13,512 | ||||||
| Other accruals | 85,784 | 67,446 | ||||||
| Asset retirement obligation | 331,747 | 303,296 | ||||||
| Net operating loss carryforwards | 17,405,312 | 16,305,768 | ||||||
| Total deferred tax assets | 18,925,893 | 17,535,239 | ||||||
| Valuation allowance | (18,925,893 | ) | (17,535,239 | ) | ||||
| Total | $ | - | $ | - | ||||
As management of the Company cannot determine that it is more likely than not that the Company will realize the benefit of the net deferred tax asset, a valuation allowance equal to 100% of the net deferred tax asset has been recorded at both June 30, 2015 and 2014.
The Company has federal net operating loss carryforwards of approximately $49.7 million at June 30, 2015 that can be used to offset future regular taxable income. These net operating loss carryforwards expire at various times through the years 2026 to 2034.
| F-20 |
The Company's statutory rate reconciliation is as follows:
| For the year ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Expected income tax benefit base on statutory rate of 35% | $ | (1,288,368 | ) | $ | (2,085,693 | ) | $ | (1,349,809 | ) | |||
| Meals and entertainment | 10,129 | 10,130 | 17,966 | |||||||||
| Non-deductible penalties | 18,697 | 9,122 | 849 | |||||||||
| Warrant derivative liability | (131,112 | ) | 483,747 | (73,500 | ) | |||||||
| Valuation allowance | 1,390,654 | 1,582,694 | 1,257,494 | |||||||||
| Income tax expense (benefit) | $ | - | $ | - | $ | - | ||||||
The Company has reviewed the tax positions taken and concluded that it does not have to book a liability for uncertain tax positions.
Management has determined that the Company and its subsidiaries Medical and International are subject to examination of their income tax filings in the United States and state jurisdictions for the 2012 through 2015 tax years. In the event that the Company is assessed penalties and/or interest, penalties will be charged to other operating expense and interest will be charged to interest expense.
13. 401(k) and Profit Sharing Plan
The Company has a 401(k) plan, which commenced in fiscal year 2007, covering all eligible full-time employees of the Company. Contributions to the 401(k) plan are made by the participants to their individual accounts through payroll withholding. The 401(k) plan also allows the Company to make contributions at the discretion of management. To date, the Company has not made any contributions to the 401(k) plan.
14. Foreign Isotope Supply
In January 2015, the Company completed negotiations on a contract to purchase Cs-131 from The Open Joint Stock Company ‹‹Institute of Nuclear Materials››, located in Russia. Under the contract, the Company will purchase Cs-131 from The Open Joint Stock Company ‹‹Institute of Nuclear Materials››. The contract provides for the supply of Cs-131 from a single reactor at the Institute of Nuclear Materials. The agreement expires on January 31, 2016.
15. Distribution Agreements
In June 2014, the Company entered into an exclusive distribution agreement through the wholly-owned subsidiary IsoRay International, LLC with MedikorPharma-Ural LLC for the rights to distribute all of the Company’s products in Russia. The agreement terminates on December 31, 2015 unless terminated earlier as provided in the agreement. The agreement is renewable from year to year beyond the initial term upon mutual written consent of the parties. The distributor is responsible for obtaining regulatory clearance in Russia.
16. Commitments and Contingencies
Royalty Agreement for Invention and Patent Application
A shareholder of the Company previously assigned his rights, title and interest in an invention to IsoRay Products LLC (a predecessor company) in exchange for a royalty equal to 1% of the Gross Profit, as defined, from the sale of "seeds" incorporating the technology. The patent and associated royalty obligations were transferred to the Company in connection with the merger transaction.
| F-21 |
The Company must also pay a royalty of 2% of Gross Sales, as defined, for any sub-assignments of the aforesaid patented process to any third parties. The royalty agreement will remain in force until the expiration of the patents on the assigned technology, unless earlier terminated in accordance with the terms of the underlying agreement.
During fiscal years 2015, 2014 and 2013, the Company recorded royalty expenses of $14,448, $10,106 and 14,168, respectively.
Patent and Know-How Royalty License Agreement
The Company is the holder of an exclusive license to use certain "know-how" developed by one of the founders of a predecessor to the Company and licensed to the Company by the Lawrence Family Trust, a Company shareholder. The terms of this license agreement require the payment of a royalty based on the Net Factory Sales Price, as defined in the agreement, of licensed product sales. Because the licensor's patent application was ultimately abandoned, only a 1% "knowhow" royalty based on Net Factory Sales Price, as defined in the agreement, remains applicable. To date, management believes that there have been no product sales incorporating the "know-how" and therefore no royalty is due pursuant to the terms of the agreement. Management believes that the possibility of a negative outcome in this matter is remote.
The licensor of the "know-how" has disputed management's contention that it is not using this "know-how". On September 25, 2007 and again on October 31, 2007, the Company participated in nonbinding mediation regarding this matter; however, no settlement was reached with the Lawrence Family Trust. After additional settlement discussions, which ended in April 2008, the parties failed to reach a settlement. The parties may demand binding arbitration at any time.
Operating Lease Agreements
The Company leases office and laboratory space and production and office equipment under non-cancelable operating leases. The lease agreements require monthly lease payments and expire on various dates through April 2019 (including renewal dates). In May 2015, the Company agreed to a modification which was retroactively effective on January 1, 2015. The lease modification included a contractually permitted rent increase which is based on a CPI index which was 1.1%. The Company, at its sole option, may exercise a remaining three year option to extend its tenancy beyond the current expiration date of April 30, 2016, to April 30, 2019. The Company's significant lease is described below.
Future minimum lease payments including the one three year option to extend remaining under operating leases are as follows:
| Year ending June 30 | Amount | |||
| 2016 | $ | 278,855 | ||
| 2017 | 278,855 | |||
| 2018 | 278,855 | |||
| 2019 | 232,380 | |||
| $ | 1,068,945 | |||
| F-22 |
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Rental expense | $ | 280,007 | $ | 276,395 | $ | 284,097 | ||||||
Royalty Agreements for Licensed Intellectual Property related to the GliaSite® RTS
The Company is required to pay a royalty to Dr. Reddy’s Laboratory Ltd for the exclusive use of its intellectual property in the field of treating brain cancer related to the production of Iotrex®, which is a component of the GliaSite® RTS. The term of the royalty agreement is from the date of first sale until the expiration of the last patent. The agreement provides for certain minimum payments, as presented below based on calendar year periods and a rate of 2.75% of net sales as defined in the agreement. The initial royalty year began on January 1, 2012.
| Royalty Period | Amount | |||
| Calendar Year 2015 | $ | 25,000 | ||
| Calendar Year 2016 and beyond | $ | 30,000 | ||
The Company recorded royalty expenses related to the licensed intellectual property utilized in the manufacture and sale of the Iotrex®.
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Royalty expense | $ | 20,138 | $ | 20,366 | $ | 5,440 | ||||||
The amount recorded for fiscal year 2015 includes an accrual for the first six months of calendar year 2015 contractual minimum royalty amount of $25,000.
The Company is required to pay a royalty to Hologic, Inc. for the exclusive worldwide use of intellectual property associated with the GliaSite® RTS in the field of intracavity radiation therapy of the brain exclusive of the radioisotope. The term of the royalty agreement is from the effective date of the agreement (January 1, 2012) and continues thereafter unless terminated earlier as defined in the agreement. The agreement provides for a royalty payment based on a rate of 5% of net sales as defined in the agreement.
The Company recorded royalty expenses related to the licensed intellectual property utilized in the manufacture and sale of the GliaSite® RTS.
| For the Year Ended June 30, | ||||||||||||
| 2015 | 2014 | 2013 | ||||||||||
| Royalty expense | $ | 898 | $ | 2,214 | $ | 2,394 | ||||||
Class Action Lawsuit Related to Press Release
On May 22, 2015, a class action complaint for violation of the federal securities laws were filed in U.S. District Court against IsoRay, Inc. and certain of our officers, one of whom is also a director. The complaint related to a press release issued by the Company on May 20, 2015 and is purportedly brought on behalf of all purchasers of IsoRay, Inc common stock from May 20, 2015 through and including May 21, 2015. The complaint asserts that the purchasers were misled by the press release, and seeks, among other things, damages and costs and expenses. We cannot predict the outcome of such proceedings or an estimate of damages, if any. We believe that these claims are without merit and intend to defend them vigorously.
| F-23 |
17. Concentrations of Credit and Other Risks
The Company's financial instruments that were exposed to concentrations of credit risk consist primarily of cash and cash equivalents, certificates of deposit, accounts receivable and certificates of deposit, non-current.
The Company's certificates of deposit and certificates of deposit, non-current are maintained in the Certificate of Deposit Account Registry Service (CDARS®) through Alliance Bank of Arizona and at Columbia State Bank at June 30, 2015. The CDARS system provides the Company access to Federal Deposit Insurance Corporation (FDIC) guarantees on multi-million dollar CD deposits through a single financial institution.
The Company's cash and cash equivalents were maintained with high-quality financial institutions at June 30, 2015 and 2014, respectively. The accounts are guaranteed by the (FDIC) up to $250,000. At June 30, 2015 and 2014, respectively, all cash balances were guaranteed by the FDIC.
Two groups of customers, facilities or physician practices have revenues that aggregate to greater than 10% of total Company product sales:
| Fiscal Year 2015 | Fiscal Year 2014 | Fiscal Year 2013 | ||||||||||
| Facility | % of total revenue | % of total revenue | % of total revenue | |||||||||
| El Camino, Los Gatos, & other facilities1 | 24.16 | % | 26.75 | % | 25.31 | % | ||||||
| Bon Secours DePaul and Maryview Medical Center2 | 11.72 | % | 6.51 | % | 0.00 | % | ||||||
1 – This group of facilities individually do not aggregate to more than 10% of total Company product sales. They are serviced by the same physician group, one of whom is our Medical Director.
2 – These two facilities are part of the same network and currently share one physician who performs procedures in both facilities. Individually, these facilities would not meet the 10% criteria, however, in aggregate, they do.
The Company routinely assesses the financial strength of its customers and provides an allowance for doubtful accounts as necessary.
Inventories
Most components used in the Company's product are purchased from outside sources. Certain components are purchased from single suppliers. The failure of any such supplier to meet its commitment on schedule could have a material adverse effect on the Company's business, operating results and financial condition. If a sole-source supplier, a supplier of Cs-131 or a supplier of irradiated barium were to go out of business or otherwise become unable to meet its supply commitments, the process of locating and qualifying alternate sources could require up to several months, during which time the Company's production could be delayed. Such delays could have a material adverse effect on the Company's business, operating results and financial condition. Sanctions placed on financial transactions with Russian banking institutions may interfere with the Company’s ability to transact business in Russia on a temporary or other basis resulting in an interruption of the Cs-131 supply which could have a temporary material adverse effect on the Company’s business, operating results and financial condition.
Virtually all of the components used in the production of the GliaSite® RTS are from single sources. We do not have formal written agreements with those suppliers. Any interruption or delay in the supply of these components could harm our business as the cost and/or time required meet the regulatory requirements of the Food and Drug Administration for the United States and our notified body for our CE mark (the British Standards Institute) in the European Union may be prohibitive.
18. Related Party Transaction
During the fiscal years ended June 30, 2015, 2014 and 2013, the Company engaged the services of APEX Data Systems, Inc., owned by Dwight Babcock, Chairman and Chief Executive Officer, to build and maintain a web interfaced data collection application to aggregate patient data in a controlled environment. For the fiscal year 2015, the Company incurred costs for website modifications and maintenance of $12,000 (2014 - $12,000 and 2013 - $13,000); and maintenance support for a CRM system of $12,000 (2014 - $12,000 and 2013 - $1,000); and maintenance costs related to the registries of $0 (2014 - $3,720 and 2013 - $1,960). The amount accrued for payment to APEX Data Systems, Inc. was $2,000 at June 30, 2015 and 2014, respectively.
| F-24 |
19. Quarterly Financial Data (unaudited)
The following table provides the selected quarterly financial data for fiscal years 2015 and 2014:
| Quarters ended | ||||||||||||||||
| September 30, | December 31, | March 31, | June 30, | |||||||||||||
| 2014 | 2014 | 2015 | 2015 | |||||||||||||
| Net revenue | $ | 1,042,101 | $ | 1,065,585 | $ | 1,158,109 | $ | 1,340,744 | ||||||||
| Gross profit/(loss) | $ | (54,802 | ) | $ | (37,964 | ) | $ | 55,197 | $ | 204,962 | ||||||
| Net loss | $ | (785,862 | ) | $ | (906,954 | ) | $ | (953,553 | ) | $ | (1,034,682 | ) | ||||
| Net loss per share – basic and diluted | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.02 | ) | $ | (0.02 | ) | ||||
| Shares used in basic and diluted per share calculation | 54,868,053 | 54,883,445 | 54,883,551 | 54,900,828 | ||||||||||||
| Quarters ended | ||||||||||||||||
| September 30, | December 31, | March 31, | June 30, | |||||||||||||
| 2013 | 2013 | 2014 | 2014 | |||||||||||||
| Net revenue | $ | 1,049,915 | $ | 1,085,408 | $ | 1,134,319 | $ | 949,516 | ||||||||
| Gross profit/(loss) | $ | (77,308 | ) | $ | (33,906 | ) | $ | 50,906 | $ | (136,163 | ) | |||||
| Net loss | $ | (1,270,906 | ) | $ | (926,972 | ) | $ | (2,083,439 | ) | $ | (1,677,805 | ) | ||||
| Net loss per share – basic and diluted | $ | (0.06 | ) | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.04 | ) | ||||
| Shares used in basic and diluted per share calculation | 35,921,712 | 38,419,502 | 42,506,077 | 54,435,706 | ||||||||||||
20. Subsequent Events
On September 10, 2015, the Company’s operating subsidiary, Medical, entered into a Real Estate Purchase and Sale Agreement with The Port of Benton, a municipal corporation of the State of Washington. The Agreement is for the sale of undeveloped real property of approximately 4.2 acres located adjacent to the Company’s existing manufacturing facility and corporate offices. The purchase price for the property is One Hundred Sixty-Eight Thousand Dollars ($168,000) which is payable on October 30, 2015, the expected date of closing.
In addition to the feasibility studies required on all aspects of the property required by Medical to close, Medical is also bound to comply with a Development Plan for a ten year period which requirements include but are not limited to the following:
| (1) | Certain specified site configurations and design with a minimum of 12,000 square feet of warehouse and production space and 4,000 square feet of office space; |
| (2) | Completion of all construction in two years; |
| (3) | Use of facility as primary production facility for ten (10) years; and |
| (4) | Provision of jobs for not less than 25 full time employees. |
Failure to comply with these covenants will result in a breach of the Agreement and if not cured, will obligate Medical to pay the Port the difference in the sales price and the appraised value of the property at the time of default.
| F-25 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: September 14, 2015
| ISORAY, INC., a Minnesota corporation | |||
| By | /s/ Dwight Babcock | ||
| Dwight Babcock, Chief Executive Officer and Chairman | |||
| By | /s/ Brien L. Ragle | ||
| Brien L. Ragle, Chief Financial Officer, | |||
| Principal Financial and Accounting Officer | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
Dated: September 14, 2015
| /s/ Dwight Babcock | ||
| Dwight Babcock, Chief Executive Officer and Chairman | ||
| /s/ Brien L. Ragle | ||
| Brien L. Ragle, Chief Financial Officer, | ||
| Principal Financial and Accounting Officer | ||
| /s/ Thomas LaVoy | ||
| Thomas LaVoy, Director | ||
| /s/ Michael McCormick | ||
| Michael McCormick, Director | ||
| /s/ Philip Vitale | ||
| Philip Vitale, Director | ||
75
