Attached files
| file | filename |
|---|---|
| 8-K - 8-K - InspireMD, Inc. | s101800_8k.htm |
| EX-99.2 - EXHIBIT 99.2 - InspireMD, Inc. | s101800_ex99-2.htm |
| EX-10.1 - EXHIBIT 10.1 - InspireMD, Inc. | s101800_ex10-1.htm |
Exhibit 99.1

NYSE MKT: NSPR September 2015

Forward Looking Statements 2 This presentation contains "forward - looking statements." Such statements may be preceded by the words "intends," "may," "will," "plans," "expects," "anticipates," "projects," "predicts," "estimates," "aims," "believes," "hopes," "potential" or similar words. Forward - looking statements are not guarantees of future performance, are based on certain assumptions and are subject to various known and unknown risks and uncertainties, many of which are beyond the Company's control, and cannot be predicted or quantified and consequently, actual results may differ materially from those expressed or implied by such forward - looking statements. Such risks and uncertainties include, without limitation, risks and uncertainties associated with (i) market acceptance of our existing and new products, (ii) negative clinical trial results or lengthy product delays in key markets, (iii) an inability to secure regulatory approvals for the sa le of our products, (iv) intense competition in the medical device industry from much larger, multinational companies, (v) product liability claims, (vi) product malfunctions, (vii) our limited manufacturing capabilities and reliance on subcontractors for assistance, (viii) insufficient or inadequate reimbursement by governmental and other third party payers for our products, (ix) our efforts to successfully obtain and maintain intellectual property protection covering our products, which may not be successful, (x) legislative or regulatory reform of the healthcare system in both the U.S. and foreign jurisdictions, (xi) our reliance on single suppliers for certain product components, (xii) the fact that we will need to raise additional capital to meet our business requirements in the future and that such capital raising may be costly, dilutive or difficult to obtain and (xiii) the fact that we conduct business in multiple foreign jurisdictions, exposing us t o foreign currency exchange rate fluctuations, logistical and communications challenges, burdens and costs of compliance with foreign laws and political and economic instability in each jurisdiction. More detailed information about the Company and the risk factors that may affect the realization of forward looking statements is set forth in the Company's filings with the Securities and Exchange Commission (SEC), including the Company's Annual Report on Form 10 - K and its Quarterly Reports on Form 10 - Q. Investors and security holders are urged to read these documents free of charge on the SEC's web site at http://www.sec.gov. The Company assumes no obligation to publicly update or revise its forward - looking statements as a result of new information, future events or otherwise.
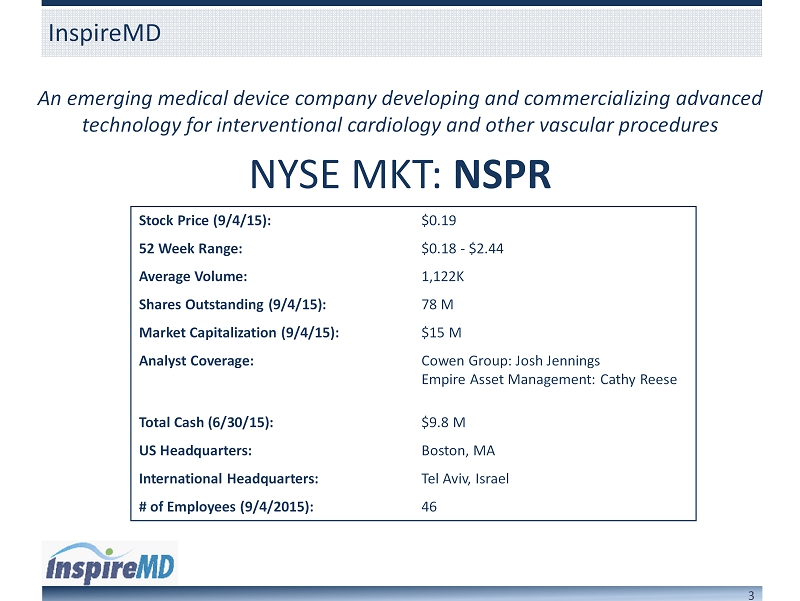
3 An emerging medical device company developing and commercializing advanced technology for interventional cardiology and other vascular procedures InspireMD NYSE MKT: NSPR Stock Price (9/4/15): $0.19 52 Week Range: $0.18 - $2.44 Average Volume: 1,122K Shares Outstanding (9/4/15): 78 M Market Capitalization (9/4/15): $15 M Analyst Coverage: Cowen Group: Josh Jennings Empire Asset Management: Cathy Reese Total Cash (6/30/15): $9.8 M US Headquarters: Boston, MA International Headquarters: Tel Aviv, Israel # of Employees (9/4/2015): 46

Investment Highlights 4 • 2015 return to revenue growth driven by the full launch of carotid platform through strategic distribution partnership with Penumbra, Inc. • Operating and financial realignment inline with development and growth initiatives. • Advancing into highly valued Neuro and Peripheral markets to leverage MicroNet technology into high growth segments. • Expanding collaboration activities on multiple MicroNet technology applications. Effectively Executing a “Neck Up” Interventional Strategy
5

6 Leadership: Significant Track Records of Success EXECUTIVE TEAM BOARD OF DIRECTORS Alan Milinazzo, President, CEO & Director • Medtronic • Boston Scientific Craig Shore, CFO • Pfizer • General Electric Dr. James Barry, COO • Boston Scientific • Howmedica Division of Pfizer Eli Bar, CTO • Nicast Gwen Bame, VP Corporate Development • Boston Scientific • Covidien David Blossom, VP Global Marketing & Strategy • Boston Scientific • Covidien Dr. Sol Barer, Chairman • Former Chairman and CEO, Celgene Alan Milinazzo, President, CEO & Director • Medtronic • Boston Scientific Dr. James Barry • SVP Corporate Technology Development at Boston Scientific • Howmedica Division of Pfizer Michael Berman • Pres. Boston Scientific/Scimed • Founder, Velocimed and Lutonix James Loughlin • KPMG • Celgene Audit Chair Paul Stuka • Founder, Osiris • Fidelity Management and Research Dr. Campbell Rogers • CMO, Heartflow • CSO, Cordis/JNJ • Associate Professor, Harvard School of Medicine

Technology: MicroNet ™ 7 Proprietary MicroNet Mesh for Embolic Prevention and Flow Diversion MicroNet Platform Ultra thin PET enhances clinical benefit of scaffold devices • Provides revascularization benefit • MicroNet acts as safety net by offering greater surface area coverage to prevent large debris flow • Mesh configuration allows perfusion to vessel wall • Made of a single fiber from a biocompatible polymer, widely used in medical implantations
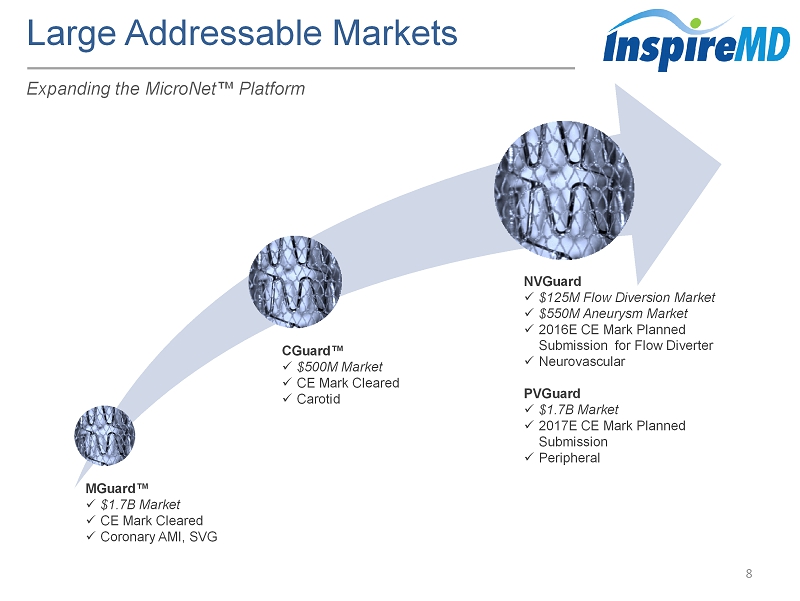
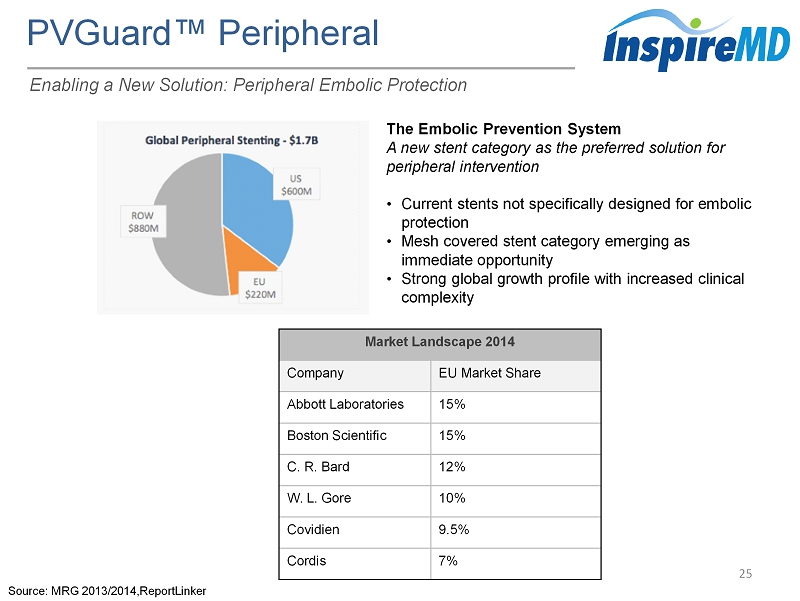
Large Addressable Markets 8 Expanding the MicroNet ™ Platform MGuard ™ x $1.7B Market x CE Mark Cleared x Coronary AMI, SVG CGuard ™ x $500M Market x CE Mark Cleared x Carotid NVGuard x $125M Flow Diversion Market x $550M Aneurysm Market x 2016E CE Mark Planned Submission for Flow Diverter x Neurovascular PVGuard x $1.7B Market x 2017E CE Mark Planned Submission x Peripheral
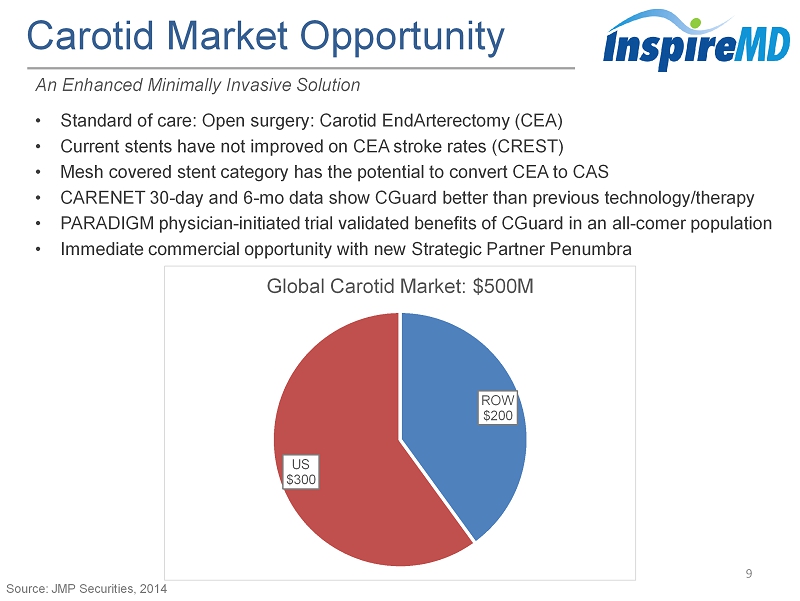
Carotid Market Opportunity 9 An Enhanced Minimally Invasive Solution • Standard of care: Open surgery: Carotid EndArterectomy (CEA) • Current stents have not improved on CEA stroke rates (CREST) • Mesh covered stent category has the potential to convert CEA to CAS • CARENET 30 - day and 6 - mo data show CGuard better than previous technology/therapy • PARADIGM physician - initiated trial validated benefits of CGuard in an all - comer population • Immediate commercial opportunity with new Strategic Partner Penumbra ROW $200 US $300 Global Carotid Market : $ 500M Source: JMP Securities, 2014

Carotid Solution 10 Emerging Market Opportunity CGuard ™ Embolic Prevention System Combines stent and embolic protection in a single device • CE marked • Self - expanding nitinol stent • Global market valued at $500M* • Strong CARENET FIM data released 9/14 and 1/15 • Impressive all - comer data from PARADIGM presented 5/15 • Full launch planned for Q4 2015 *Source : JMP Securities, 2014
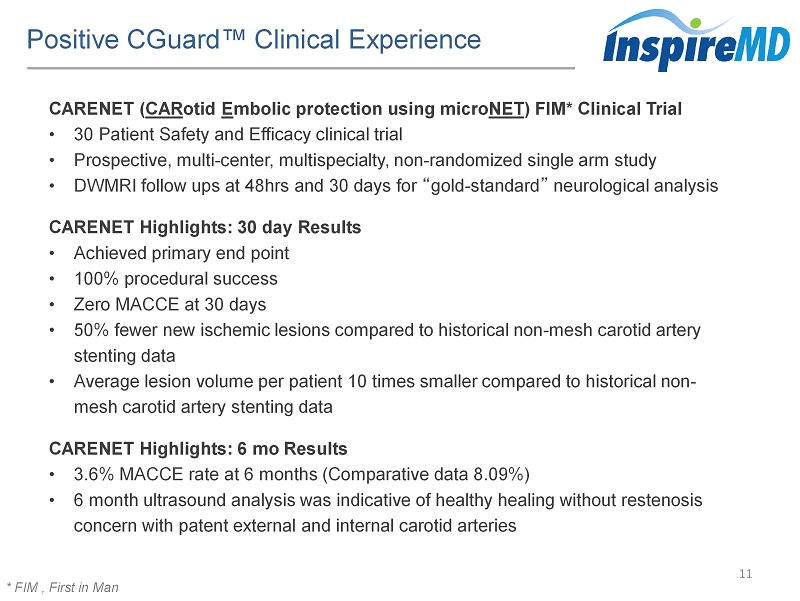
Positive CGuard™ Clinical Experience 11 * FIM , First in Man CARENET ( CAR otid E mbolic protection using micro NET ) FIM* Clinical Trial • 30 Patient Safety and Efficacy clinical trial • Prospective, multi - center, multispecialty, non - randomized single arm study • DWMRI follow ups at 48hrs and 30 days for “ gold - standard ” neurological analysis CARENET Highlights: 30 day Results • Achieved primary end point • 100% procedural success • Zero MACCE at 30 days • 50% fewer new ischemic lesions compared to historical non - mesh carotid artery stenting data • Average lesion volume per patient 10 times smaller compared to historical non - mesh carotid artery stenting data CARENET Highlights: 6 mo Results • 3.6% MACCE rate at 6 months (Comparative data 8.09%) • 6 month ultrasound analysis was indicative of healthy healing without restenosis concern with patent external and internal carotid arteries
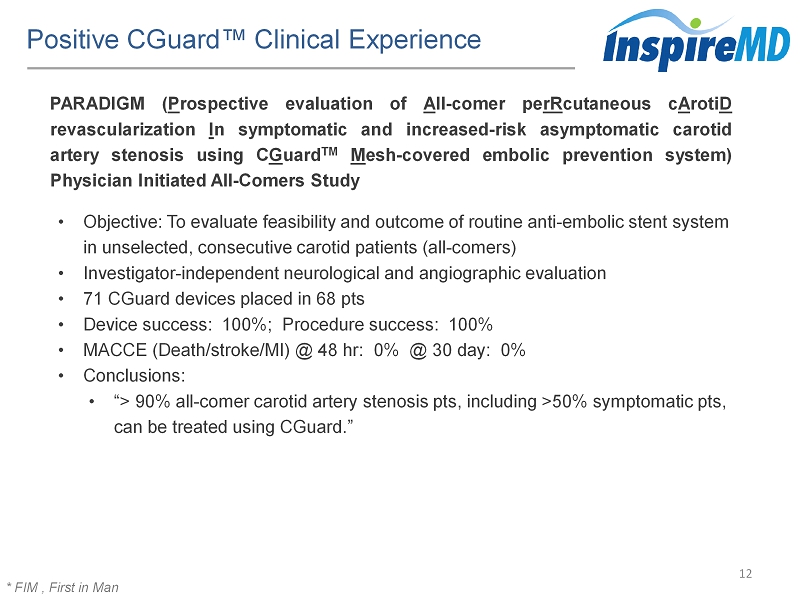
Positive CGuard™ Clinical Experience 12 * FIM , First in Man PARADIGM ( P rospective evaluation of A ll - comer pe rR cutaneous c A roti D revascularization I n symptomatic and increased - risk asymptomatic carotid artery stenosis using C G uard TM M esh - covered embolic prevention system) Physician I nitiated All - Comers Study • Objective: To evaluate feasibility and outcome of routine anti - embolic stent system in unselected, consecutive carotid patients (all - comers) • Investigator - independent neurological and angiographic evaluation • 71 CGuard devices placed in 68 pts • Device success: 100%; Procedure success: 100% • MACCE (Death/stroke/MI) @ 48 hr: 0% @ 30 day: 0% • Conclusions: • “> 90% all - comer carotid artery stenosis pts, including >50% symptomatic pts, can be treated using CGuard .”

Strategic Distribution Partnership 13 • Founded in 2005 as a Neurovascular company with a clinically - driven product development strategy • Reputation as the innovation leader in the neurovascular field • Extending success beyond stroke into the periphery and neurosurgical markets • Track record of consistent, profitable growth (S 1 just filed) • Management team with decades of vascular experience • Entering carotid market to complement their stroke portfolio Rationale: Predictable, Sustainable & Profitable Revenue Growth
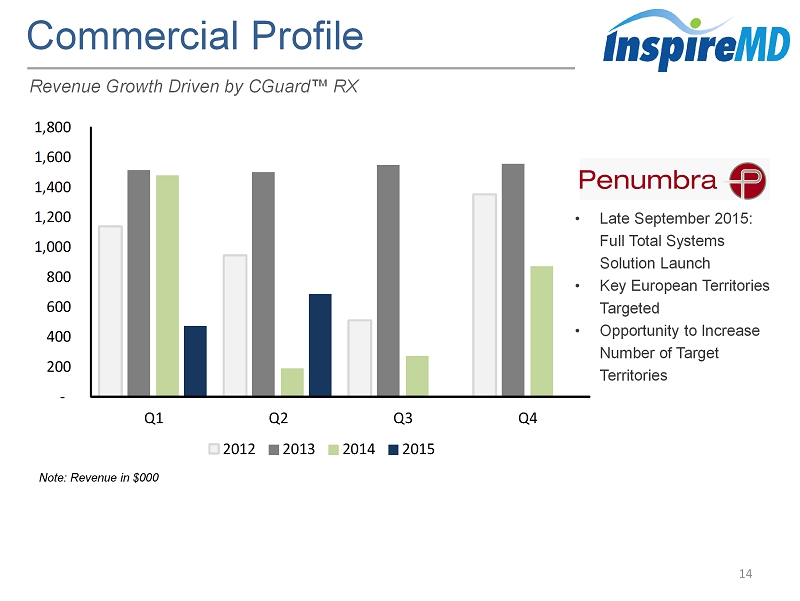
- 200 400 600 800 1,000 1,200 1,400 1,600 1,800 Q1 Q2 Q3 Q4 2012 2013 2014 2015 Commercial Profile 14 Revenue Growth Driven by CGuard ™ RX • Late September 2015: Full Total Systems Solution Launch • Key European Territories Targeted • Opportunity to Increase Number of Target Territories Note: Revenue in $000
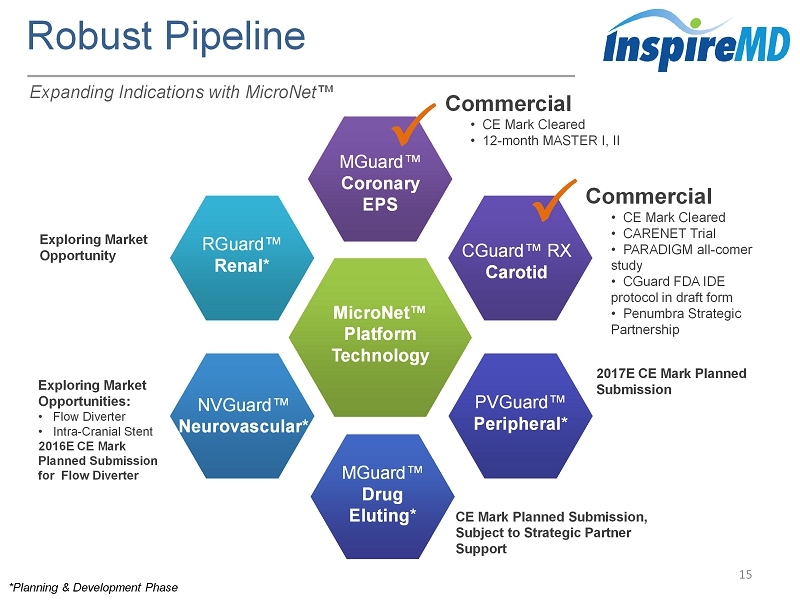
Robust Pipeline 15 Commercial • CE Mark Cleared • 12 - month MASTER I, II MicroNet ™ Platform Technology MGuard ™ Coronary EPS NVGuard ™ Neurovascular* RGuard™ Renal * CGuard ™ RX Carotid PVGuard™ Peripheral * MGuard ™ Drug Eluting * * Planning & Development Phase Commercial • CE Mark Cleared • CARENET Trial • PARADIGM all - comer study • CGuard FDA IDE protocol in draft form • Penumbra Strategic Partnership 2017E CE Mark Planned Submission CE Mark Planned Submission, Subject to Strategic Partner Support Exploring Market Opportunities: • Flow Diverter • Intra - Cranial Stent 2016E CE Mark Planned Submission for Flow Diverter Exploring Market Opportunity Expanding Indications with MicroNet ™
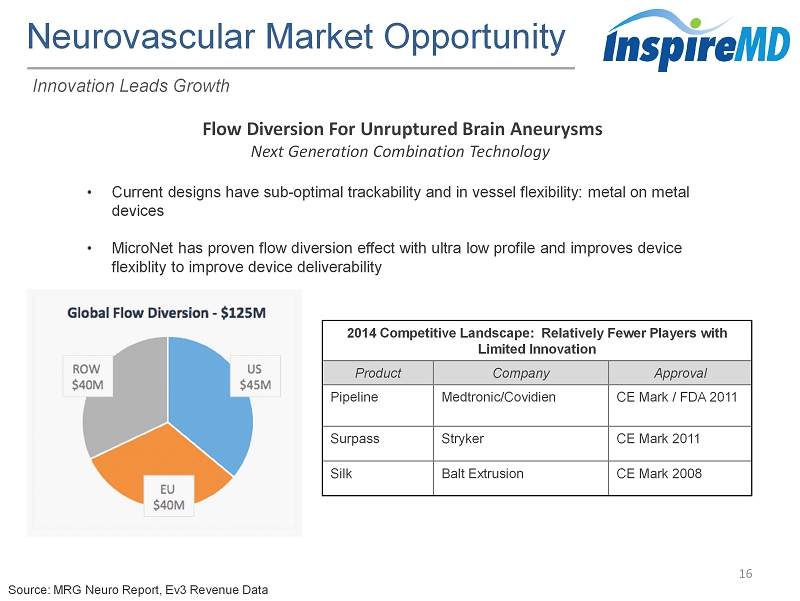
Neurovascular Market Opportunity 16 Innovation Leads Growth • Current designs have sub - optimal trackability and in vessel flexibility: metal on metal devices • MicroNet has proven flow diversion effect with ultra low profile and improves device flexiblity to improve device deliverability Source: MRG Neuro Report, Ev3 Revenue Data 2014 Competitive Landscape: Relatively Fewer Players with Limited Innovation Product Company Approval Pipeline Medtronic/Covidien CE Mark / FDA 2011 Surpass Stryker CE Mark 2011 Silk Balt Extrusion CE Mark 2008 Flow Diversion For Unruptured Brain Aneurysms Next Generation Combination Technology
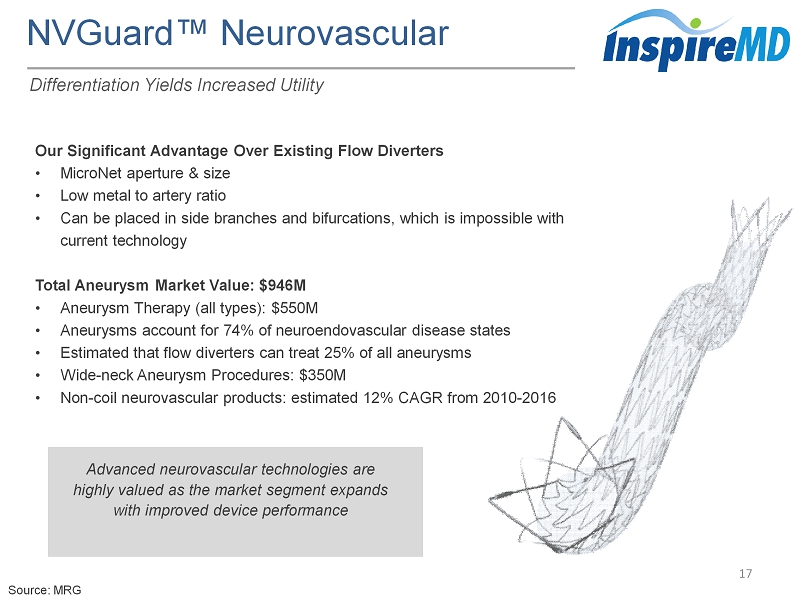
NVGuard ™ Neurovascular 17 Differentiation Yields Increased Utility Our Significant Advantage Over Existing Flow Diverters • MicroNet aperture & size • Low metal to artery ratio • Can be placed in side branches and bifurcations, which is impossible with current technology Total Aneurysm Market Value: $946M • Aneurysm Therapy (all types): $550M • Aneurysms account for 74% of neuroendovascular disease states • Estimated that flow diverters can treat 25% of all aneurysms • Wide - neck Aneurysm Procedures: $350M • Non - coil neurovascular products: estimated 12% CAGR from 2010 - 2016 Advanced neurovascular technologies are highly valued as the market segment expands with improved device performance Source: MRG
Neurovascular Market 18 • Medtronic acquires Medina Medical for $150 million • Stryker Acquires Surpass Medical for $135 million • Covidien Acquires Chestnut Medical for $150 million * * Based on milestones achieved as part of structured deal High Strategic interest with attractive valuations
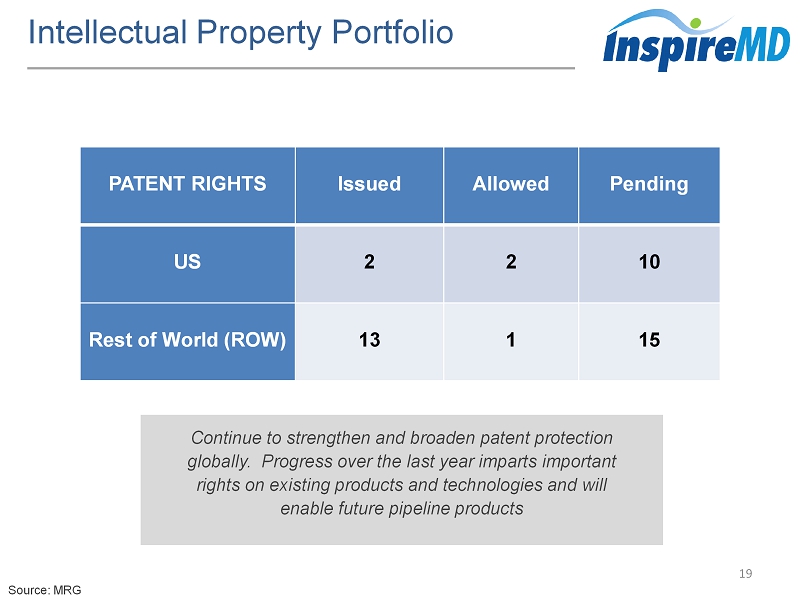
Intellectual Property Portfolio 19 Continue to strengthen and broaden patent protection globally. Progress over the last year imparts important rights on existing products and technologies and will enable future pipeline products Source: MRG PATENT RIGHTS Issued Allowed Pending US 2 2 10 Rest of World (ROW) 13 1 15
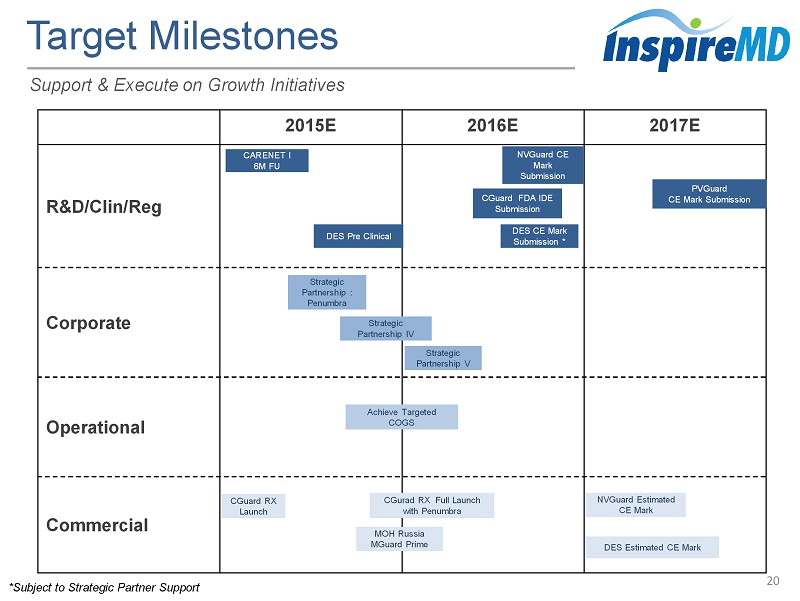
2015E 2016E 2017E R&D/ Clin / Reg Corporate Operational Commercial Target Milestones 20 Support & Execute on Growth Initiatives Strategic Partnership : Penumbra Strategic Partnership V Strategic Partnership IV DES Estimated CE Mark Achieve Targeted COGS NVGuard CE Mark Submission CGuard RX Launch NVGuard Estimated CE Mark CGuard FDA IDE Submission CARENET I 6M FU DES Pre Clinical PVGuard CE Mark Submission DES CE Mark Submission * CGurad RX Full Launch with Penumbra *Subject to Strategic Partner Support MOH Russia MGuard Prime

Investment Highlights 21 • 2015 return to revenue growth driven by the full launch of carotid platform through strategic distribution partnership with Penumbra, Inc. • Operating and financial realignment inline with development and growth initiatives. • Advancing into highly valued Neuro and Peripheral markets to leverage MicroNet technology into high growth segments. • Expanding collaboration activities on multiple MicroNet technology applications. Effectively Executing a “Neck Up” Interventional Strategy

Alan Milinazzo , CEO (888) 776 - 6804 alanm@ inspiremd.com Craig Shore, CFO (888) 776 - 6804 craigs@inspiremd.com
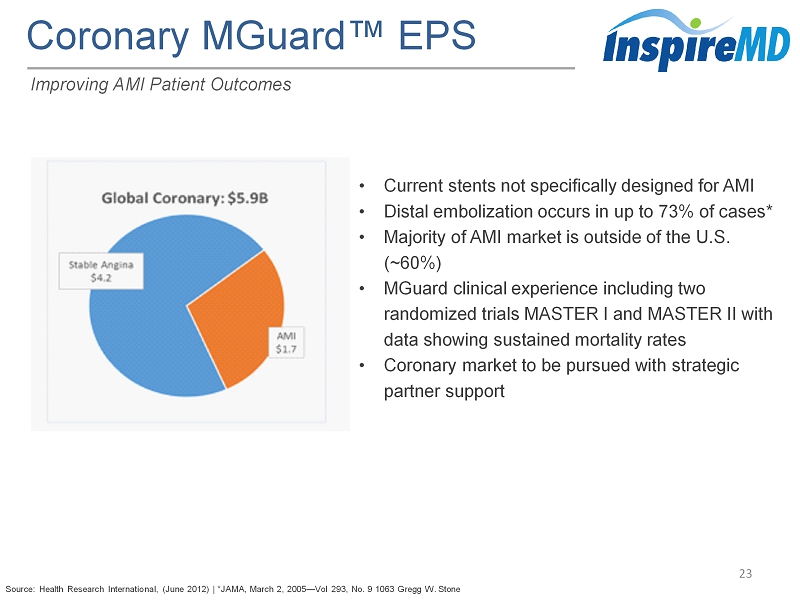
Coronary MGuard ™ EPS 23 Improving AMI Patient Outcomes • Current stents not specifically designed for AMI • Distal embolization occurs in up to 73% of cases* • Majority of AMI market is outside of the U.S . (~ 60%) • MGuard clinical experience including two randomized trials MASTER I and MASTER II with data showing sustained mortality rates • Coronary market to be pursued with strategic partner support Source: Health Research International, (June 2012 ) | *JAMA , March 2, 2005 — Vol 293, No. 9 1063 Gregg W. Stone
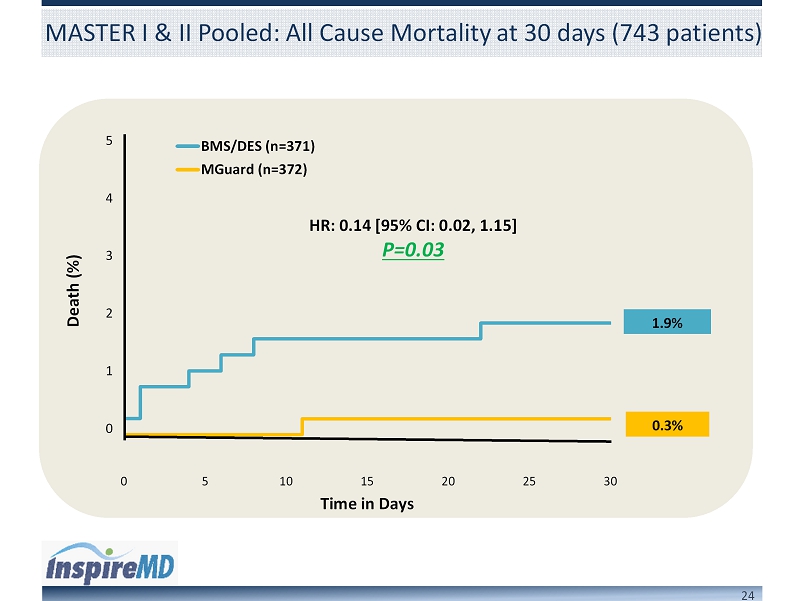
24 MASTER I & II Pooled: All Cause Mortality at 30 days (743 patients) 0 5 10 15 20 25 30 Time in Days BMS/DES (n=371) MGuard (n=372) 5 4 3 2 1 0 Death (%) HR: 0.14 [95% CI: 0.02, 1.15] P=0.03 1.9% 0.3%