Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Flexion Therapeutics Inc | d50263d8k.htm |
 FX006 Pivotal Ph 2b Data
September , 2015
Exhibit 99.1 |
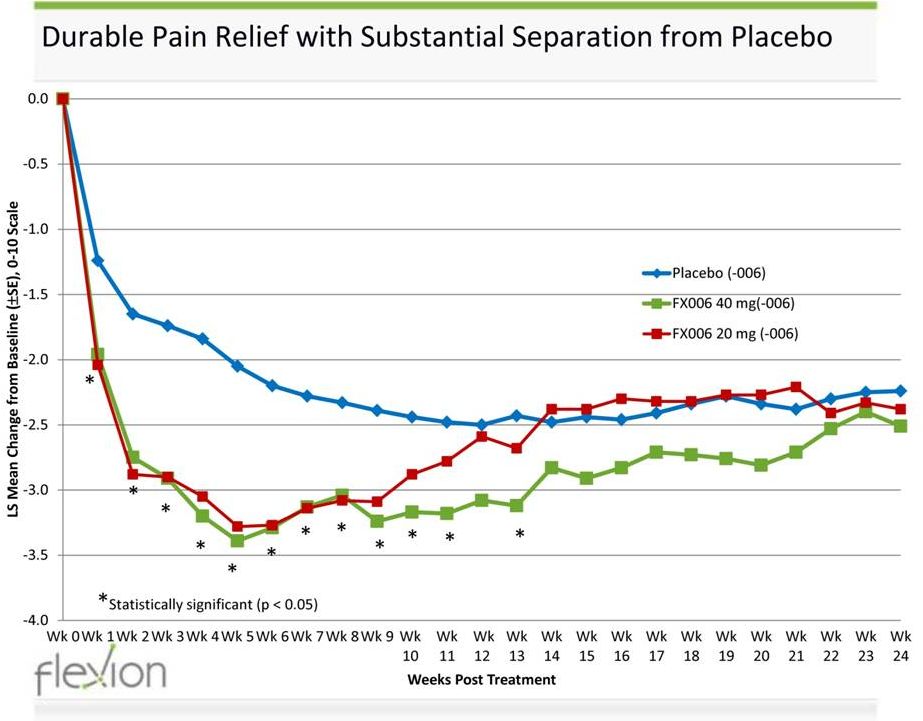
 Pivotal Phase 2b Study Design
-100 OA patients on FX006 (40mg)
-100 OA patients on FX006 (20mg)
-100 OA patients on Placebo (saline)
Study Objective • Identify a safe and well tolerated dose of FX006 which demonstrates superiority to placebo in magnitude of pain relief at 12 weeks Ph 2b Pivotal Study (n=300) 5ml IA single-dose knee injection Location: US and Canada - 45 Centers 2 FX006 20mg (100) FX006 40mg (100) Placebo Saline (100) Pain measured on 0 – 10 Numeric Rating Scale • 0 = no pain; 10 = pain as bad as you can imagine • Baseline index knee pain between 5 and 9 • Primary outcome measure - weekly mean of average daily pain intensity score • Primary endpoint: 12 week landmark analysis vs. placebo • Patients were evaluated for a total of 24 weeks Secondary outcome measures • Pain by WOMAC A (pain), B (stiffness), C (function) • Time to onset of pain relief • Responder status • Patient and clinical global impression of change • Rescue medication consumption Completed enrollment February 2015 |
 |
