Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Bellerophon Therapeutics, Inc. | a15-19124_18k.htm |
Exhibit 99.1
|
|
Company Presentation September 2015 |
|
|
Forward Looking Statements This presentation contains forward-looking statements that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make due to a number of important factors, including risks and uncertainties relating to: the timing and outcomes of our ongoing and expected clinical trials for our product candidates; our ability to successfully develop, commercialize and market any of our product candidates; our ability to obtain, maintain and enforce intellectual property rights; competition; our reliance on third parties; our ability to obtain necessary financing; and those risk factors discussed in the “Risk Factors” section and elsewhere in our most recent Form 10-K and other periodic filings we make with the SEC. All forward-looking statements contained in this presentation reflect our current views with respect to future events. We assume no obligation, except as required by applicable law, to update any forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. |
|
|
Bellerophon Highlights Focused on developing novel therapies for severe cardiopulmonary and cardiac diseases Product candidates address opportunities at the intersection of drugs and devices with high unmet need INOpulse: Extension of nitric oxide based therapy for Pulmonary Hypertension (PH) in chronic diseases Nitric oxide approved for use in hospital in neonates; over 450,000 patients treated since launch Novel delivery technology allows for use in serious chronic diseases Several potential applications include: PAH: Planning to initiate Phase 3 in 2015 PH-COPD: Completed early Phase 2 testing PH-IPF: Proof of concept work planned Other opportunities include CTEPH, PH-Sarcoidosis, and chronic high altitude sickness BCM: Novel injectable device designed to prevent congestive heart failure following a serious heart attack Compelling animal data and encouraging, early open label study Further exploratory work under consideration following results from recent Phase 2 study but no active development at this point Strong IP protection on core programs |
|
|
Major Institutional Stockholders |
|
|
INOpulse Platform |
|
|
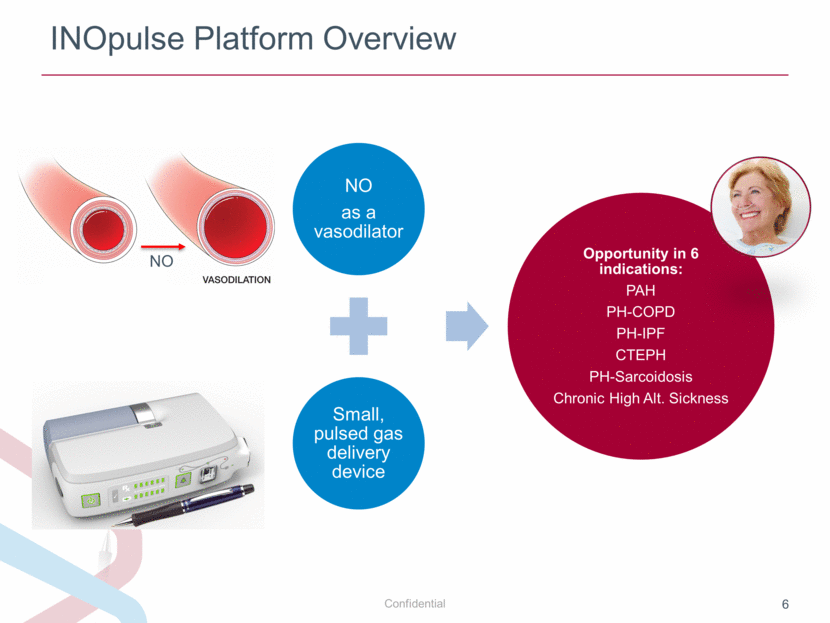
INOpulse Platform Overview NO NO as a vasodilator Small, pulsed gas delivery device Opportunity in 6 indications: PAH PH-COPD PH-IPF CTEPH PH-Sarcoidosis Chronic High Alt. Sickness |
|
|
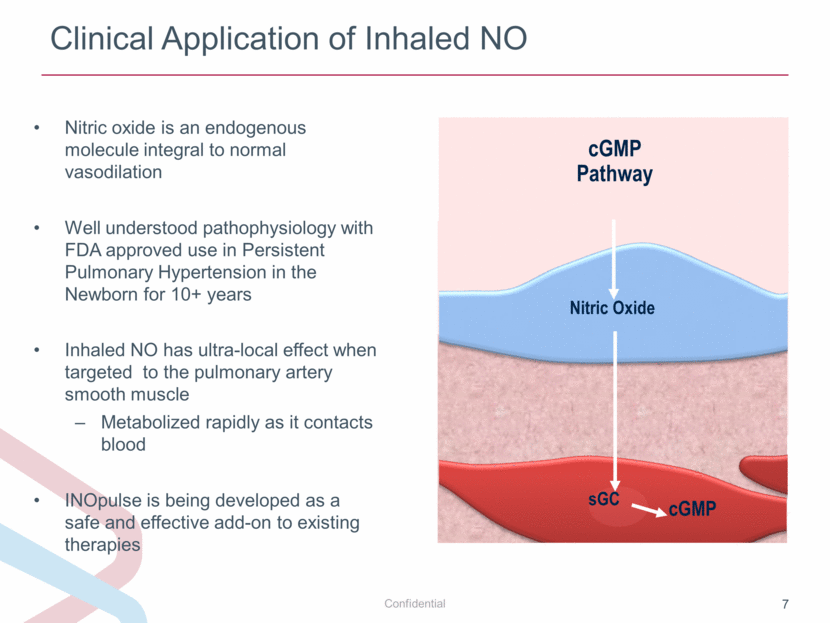
Clinical Application of Inhaled NO Nitric oxide is an endogenous molecule integral to normal vasodilation Well understood pathophysiology with FDA approved use in Persistent Pulmonary Hypertension in the Newborn for 10+ years Inhaled NO has ultra-local effect when targeted to the pulmonary artery smooth muscle Metabolized rapidly as it contacts blood INOpulse is being developed as a safe and effective add-on to existing therapies cGMP Nitric Oxide cGMP Pathway sGC |
|
|
Portable Delivery System Allows Outpatient Chronic Nitric Oxide Therapy Images are not to scale relative to each other Currently well established as a therapy for neonates in hospitals In-hospital device is bulky with large cylinders Continuous dosing is inefficient Pulsed iNO can deliver equivalent dose as continuous delivery with 5% of the volume; Allows for small portable ~2.5 lbs. device Dynamic pulse is designed to deliver the prescribed dose accurately throughout the day Triple-lumen cannula designed to support accurate dosing and for use, when needed, alongside Long Term Oxygen Therapy (LTOT) Pulsing minimizes NO release which is important for chronic at-home use |
|
|
Potential Indications for Chronic Use of Pulsed iNO Pulmonary Arterial Hypertension (PAH) Several approved therapies but median survival still less than 5 years Orphan disease PH associated with Chronic Obstructive Pulmonary Disease (PH-COPD) No current drug therapies PH associated with Idiopathic Pulmonary Fibrosis (PH-IPF) No current drug therapies Orphan disease Chronic Thromboembolic Pulmonary Hypertension (CTEPH) Riociguat recently approved for this condition Orphan disease PH associated with Sarcoidosis No current drug therapies Orphan disease Chronic High Altitude Sickness Currently treated with Oxygen therapy |
|
|
INOpulse Scientific Advisory Board Senior International Experts in PAH, PH-COPD and PH associated with other lung diseases Greg Elliot, MD, Utah: Professor of Medicine at the University of Utah and Chief of the Pulmonary and Critical Care Medicine Division at the LDS Nazzareno Galie, MD, Italy: Head of the Pulmonary Hypertension Center at the University of Bologna Ardi Ghofrani, MD, Germany: Associate Professor for Internal Medicine at University Hospital, Giessen Fernando Martinez, MD, NYC: Executive Vice Chair of Medicine, Weill Cornell Medical College and New York-Presbyterian Hospital/Weill Cornell Medical Center Vallerie McLaughlin, MD, Detroit: Director of the Pulmonary Hypertension Program at the University of Michigan and Attending Physician at the University of Michigan Health System in Ann Arbor, MI Robert Naeije, MD, PhD, Belgium: Professor and Chairman of the Department of Physiology and Pathophysiology at Erasme University Hospital, Brussels Steve Rennard, MD, Omaha: Larson Professor of Medicine in the Pulmonary and Critical Care Medicine, Department of Internal Medicine at the University of Nebraska Medical Center in Omaha Lewis Rubin, MD, NYC: Emeritus Professor of Medicine at the University of California in San Diego; now consultant out of NY Olivier Sitbon, MD, France: Professor of Respiratory Medicine at the South Paris University |
|
|
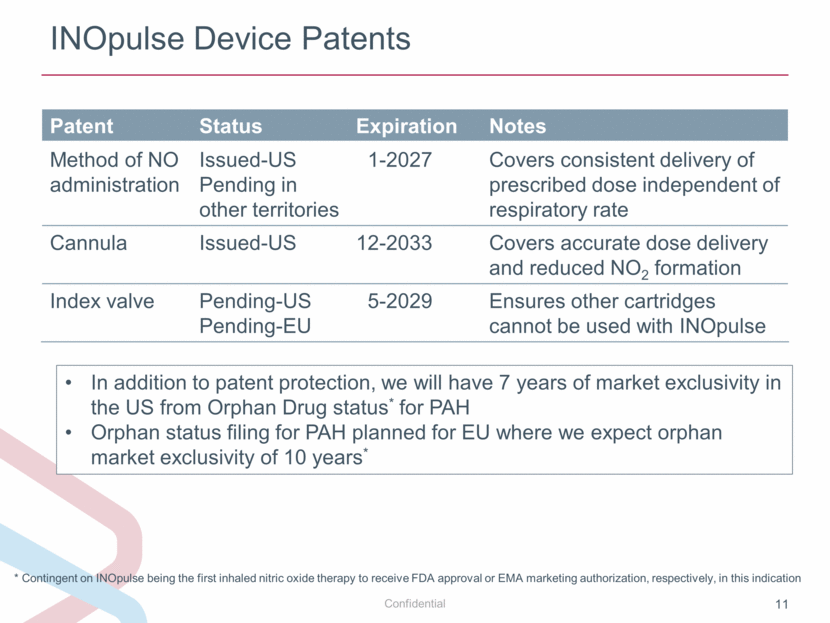
INOpulse Device Patents Patent Status Expiration Notes Method of NO administration Issued-US Pending in other territories 1-2027 Covers consistent delivery of prescribed dose independent of respiratory rate Cannula Issued-US 12-2033 Covers accurate dose delivery and reduced NO2 formation Index valve Pending-US Pending-EU 5-2029 Ensures other cartridges cannot be used with INOpulse In addition to patent protection, we will have 7 years of market exclusivity in the US from Orphan Drug status* for PAH Orphan status filing for PAH planned for EU where we expect orphan market exclusivity of 10 years* * Contingent on INOpulse being the first inhaled nitric oxide therapy to receive FDA approval or EMA marketing authorization, respectively, in this indication |
|
|
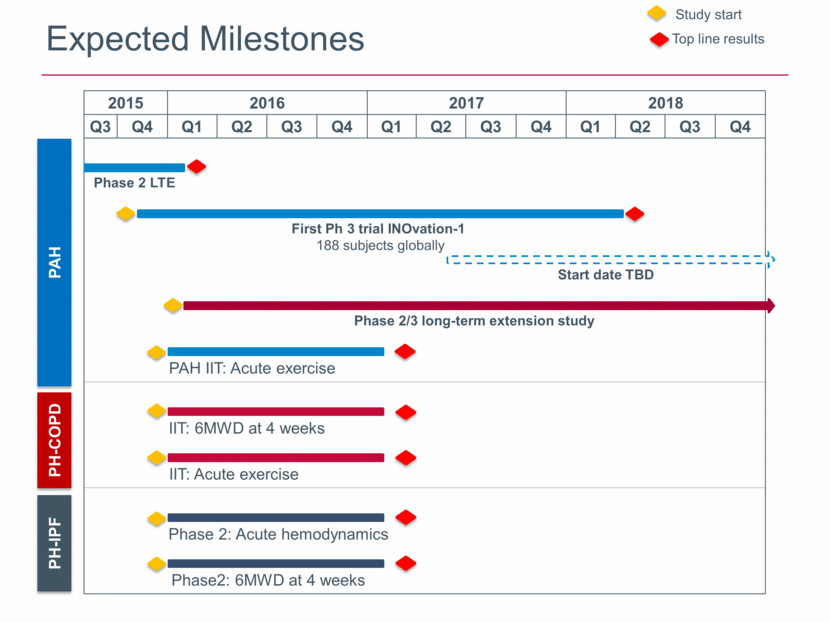
Expected Milestones Top line results Study start PAH PH-COPD PH-IPF 2015 2016 2017 2018 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Phase 2/3 long-term extension study First Ph 3 trial INOvation-1 188 subjects globally Phase 2 LTE PAH IIT: Acute exercise IIT: Acute exercise IIT: 6MWD at 4 weeks Phase2: 6MWD at 4 weeks Start date TBD Phase 2: Acute hemodynamics |
|
|
INOpulse: Primary Indication in Pulmonary Arterial Hypertension (PAH) |
|
|
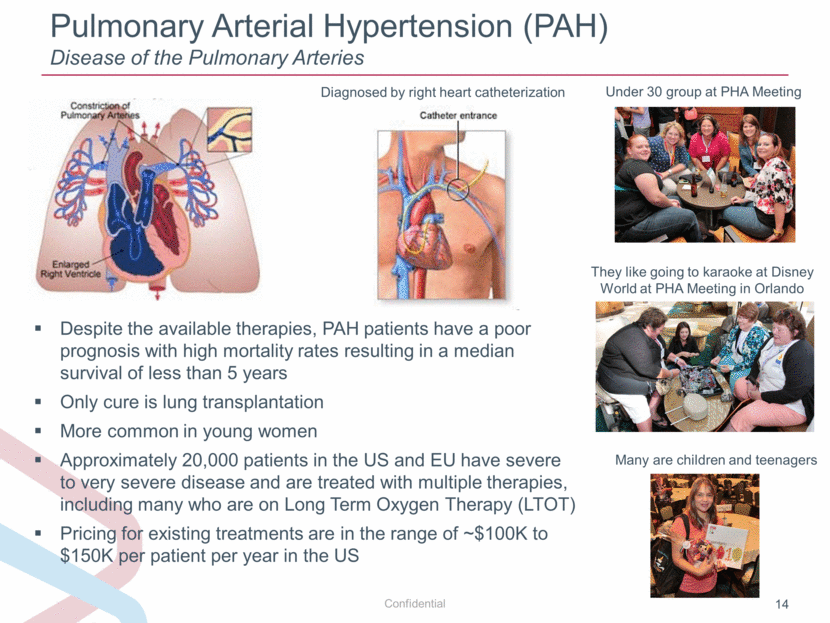
Pulmonary Arterial Hypertension (PAH) Disease of the Pulmonary Arteries Under 30 group at PHA Meeting They like going to karaoke at Disney World at PHA Meeting in Orlando Many are children and teenagers Diagnosed by right heart catheterization Despite the available therapies, PAH patients have a poor prognosis with high mortality rates resulting in a median survival of less than 5 years Only cure is lung transplantation More common in young women Approximately 20,000 patients in the US and EU have severe to very severe disease and are treated with multiple therapies, including many who are on Long Term Oxygen Therapy (LTOT) Pricing for existing treatments are in the range of ~$100K to $150K per patient per year in the US |
|
|
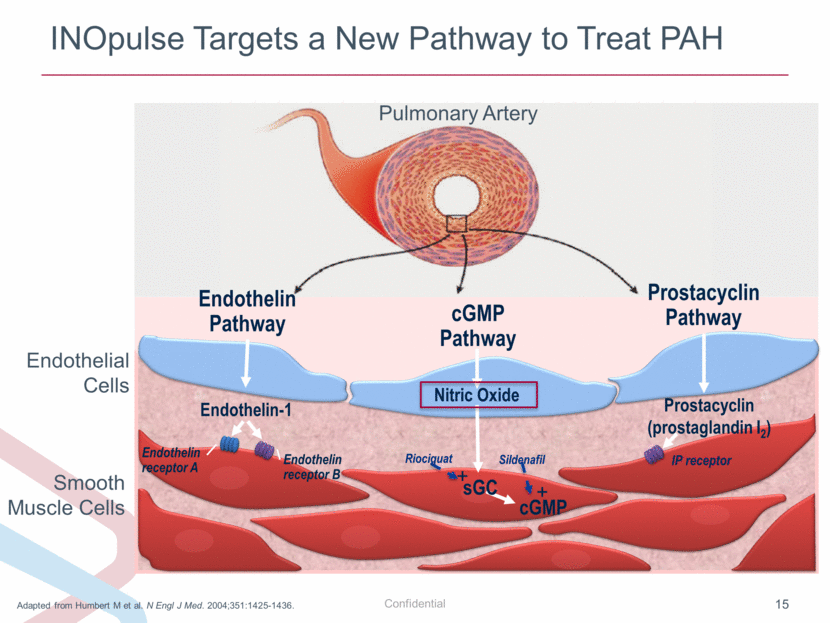
INOpulse Targets a New Pathway to Treat PAH Adapted from Humbert M et al. N Engl J Med. 2004;351:1425-1436. Endothelin receptor A Endothelin receptor B Endothelin-1 Endothelin Pathway Prostacyclin (prostaglandin I2) IP receptor Prostacyclin Pathway + sGC cGMP Sildenafil Nitric Oxide cGMP Pathway Riociguat + Endothelial Cells Smooth Muscle Cells Pulmonary Artery |
|
|
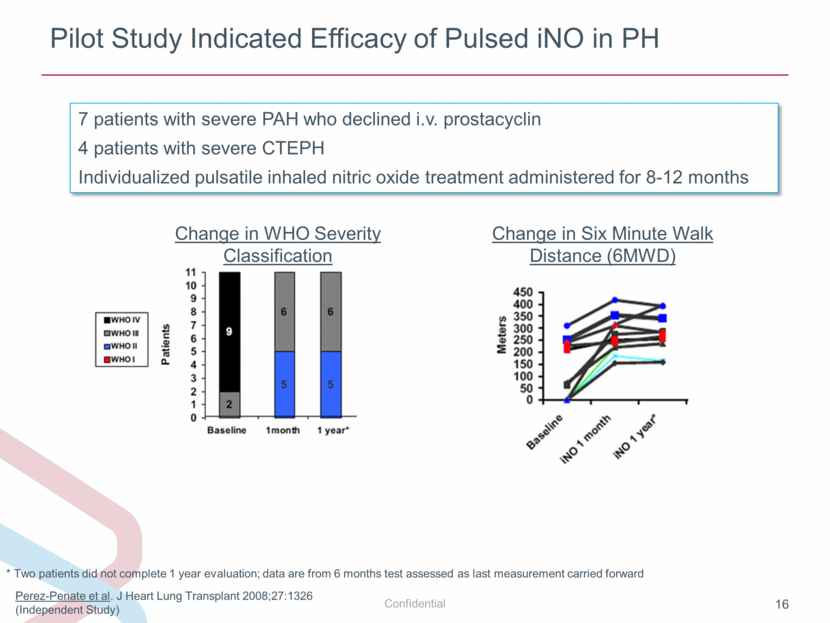
Pilot Study Indicated Efficacy of Pulsed iNO in PH Perez-Penate et al. J Heart Lung Transplant 2008;27:1326 (Independent Study) 7 patients with severe PAH who declined i.v. prostacyclin 4 patients with severe CTEPH Individualized pulsatile inhaled nitric oxide treatment administered for 8-12 months Change in Six Minute Walk Distance (6MWD) * Two patients did not complete 1 year evaluation; data are from 6 months test assessed as last measurement carried forward Change in WHO Severity Classification |
|
|
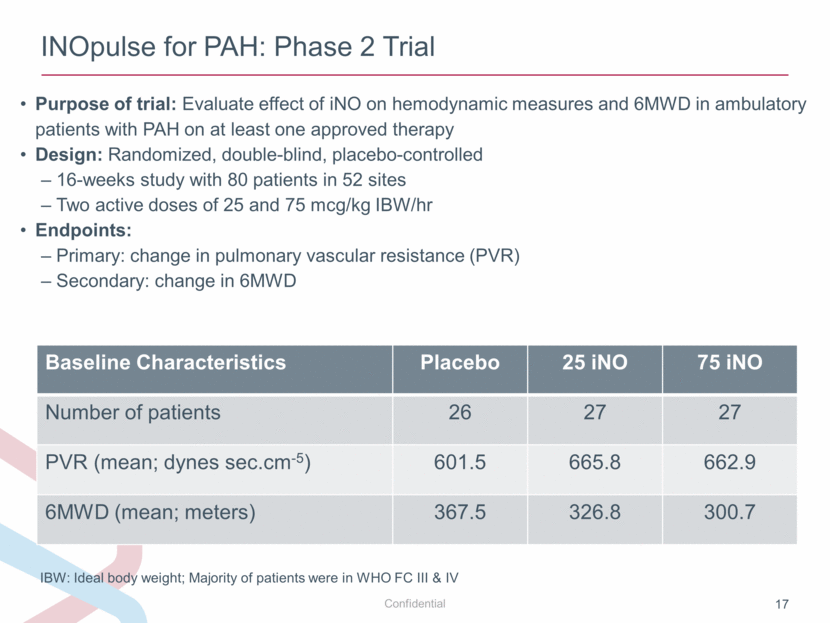
INOpulse for PAH: Phase 2 Trial Purpose of trial: Evaluate effect of iNO on hemodynamic measures and 6MWD in ambulatory patients with PAH on at least one approved therapy Design: Randomized, double-blind, placebo-controlled 16-weeks study with 80 patients in 52 sites Two active doses of 25 and 75 mcg/kg IBW/hr Endpoints: Primary: change in pulmonary vascular resistance (PVR) Secondary: change in 6MWD IBW: Ideal body weight; Majority of patients were in WHO FC III & IV Baseline Characteristics Placebo 25 iNO 75 iNO Number of patients 26 27 27 PVR (mean; dynes sec.cm-5) 601.5 665.8 662.9 6MWD (mean; meters) 367.5 326.8 300.7 |
|
|
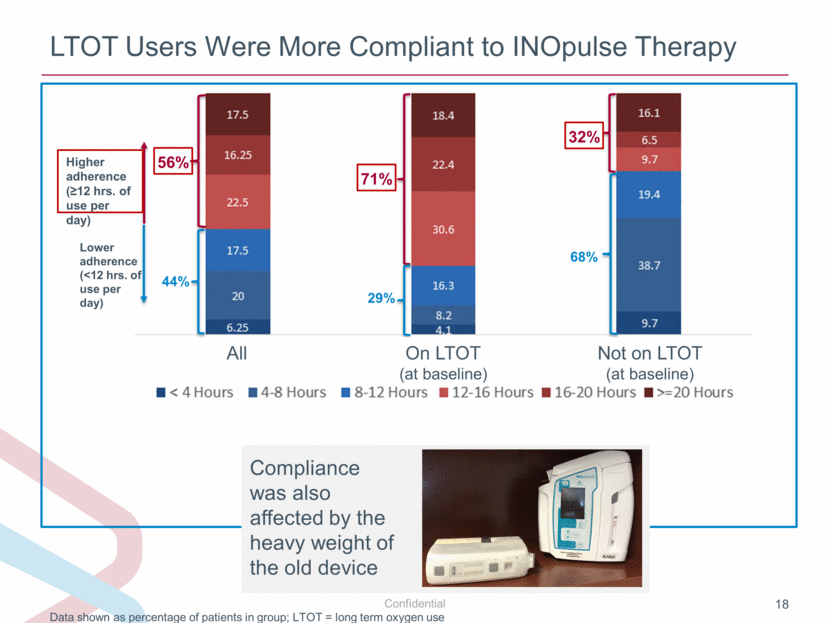
LTOT Users Were More Compliant to INOpulse Therapy Data shown as percentage of patients in group; LTOT = long term oxygen use Higher adherence (≥12 hrs. of use per day) Lower adherence (<12 hrs. of use per day) 71% 29% 56% 44% 32% 68% All On LTOT (at baseline) Not on LTOT (at baseline) Compliance was also affected by the heavy weight of the old device |
|
|
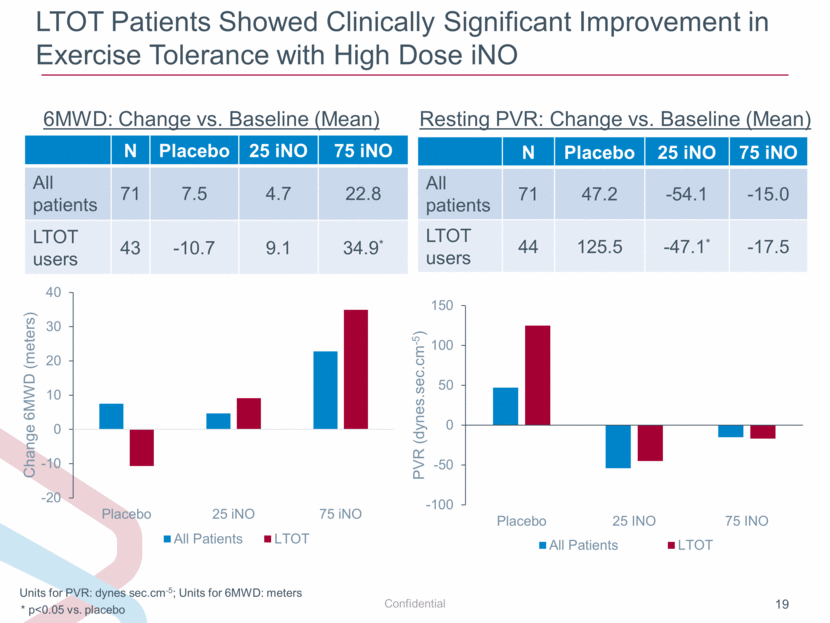
LTOT Patients Showed Clinically Significant Improvement in Exercise Tolerance with High Dose iNO Units for PVR: dynes sec.cm-5; Units for 6MWD: meters N Placebo 25 iNO 75 iNO All patients 71 47.2 -54.1 -15.0 LTOT users 44 125.5 -47.1* -17.5 * p<0.05 vs. placebo Resting PVR: Change vs. Baseline (Mean) N Placebo 25 iNO 75 iNO All patients 71 7.5 4.7 22.8 LTOT users 43 -10.7 9.1 34.9* 6MWD: Change vs. Baseline (Mean) -100 -50 0 50 100 150 Placebo 25 INO 75 INO PVR (dynes.sec.cm - 5 ) All Patients LTOT -20 -10 0 10 20 30 40 Placebo 25 iNO 75 iNO Change 6MWD (meters) All Patients LTOT |
|
|
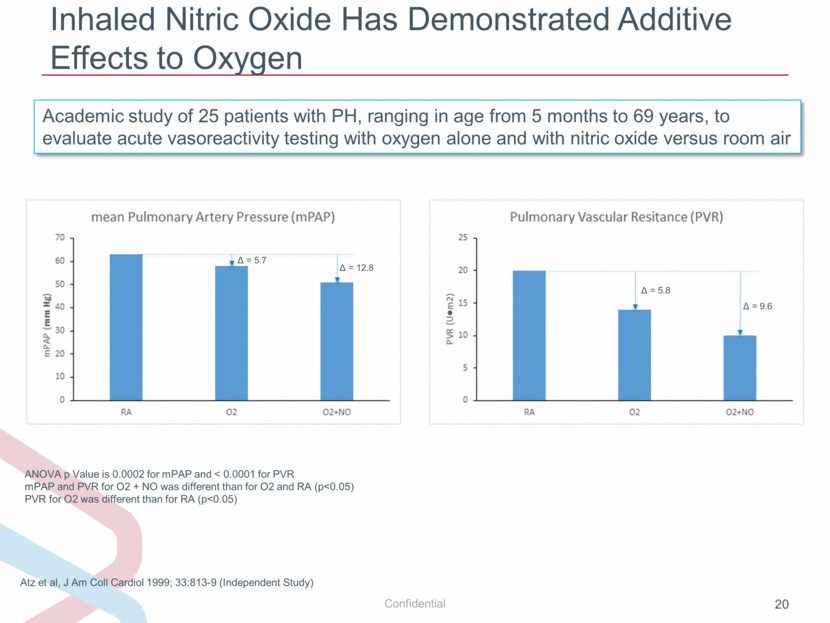
Inhaled Nitric Oxide Has Demonstrated Additive Effects to Oxygen ANOVA p Value is 0.0002 for mPAP and < 0.0001 for PVR mPAP and PVR for O2 + NO was different than for O2 and RA (p<0.05) PVR for O2 was different than for RA (p<0.05) Atz et al, J Am Coll Cardiol 1999; 33:813-9 (Independent Study) Academic study of 25 patients with PH, ranging in age from 5 months to 69 years, to evaluate acute vasoreactivity testing with oxygen alone and with nitric oxide versus room air Δ = 5.7 Δ = 12.8 Δ = 5.8 Δ = 9.6 |
|
|
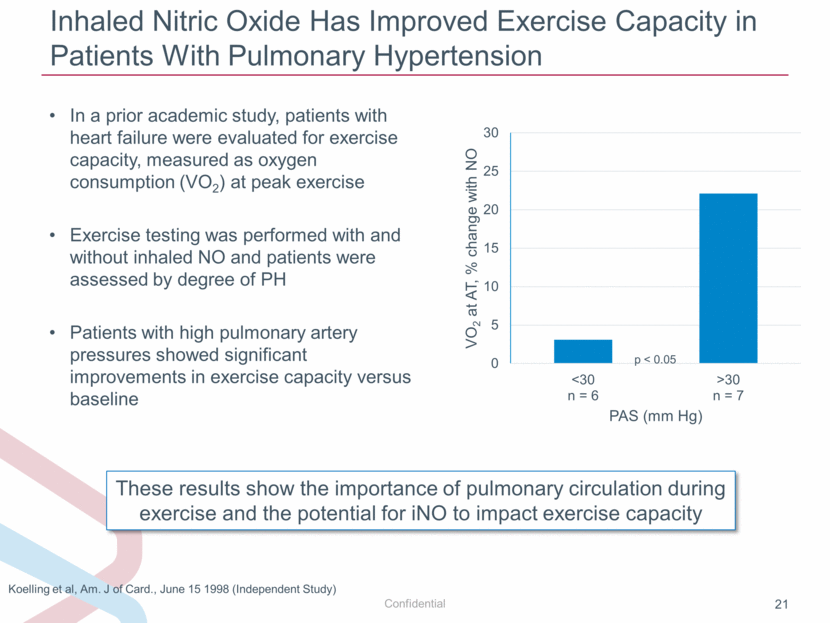
Inhaled Nitric Oxide Has Improved Exercise Capacity in Patients With Pulmonary Hypertension In a prior academic study, patients with heart failure were evaluated for exercise capacity, measured as oxygen consumption (VO2) at peak exercise Exercise testing was performed with and without inhaled NO and patients were assessed by degree of PH Patients with high pulmonary artery pressures showed significant improvements in exercise capacity versus baseline These results show the importance of pulmonary circulation during exercise and the potential for iNO to impact exercise capacity Koelling et al, Am. J of Card., June 15 1998 (Independent Study) p < 0.05 0 5 10 15 20 25 30 <30 n = 6 >30 n = 7 VO 2 at AT, % change with NO PAS (mm Hg) |
|
|
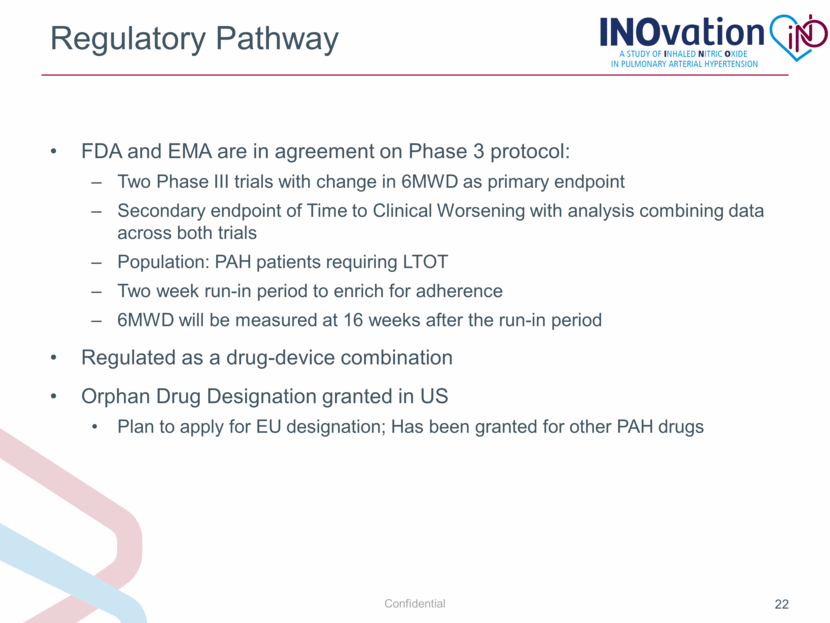
Regulatory Pathway FDA and EMA are in agreement on Phase 3 protocol: Two Phase III trials with change in 6MWD as primary endpoint Secondary endpoint of Time to Clinical Worsening with analysis combining data across both trials Population: PAH patients requiring LTOT Two week run-in period to enrich for adherence 6MWD will be measured at 16 weeks after the run-in period Regulated as a drug-device combination Orphan Drug Designation granted in US Plan to apply for EU designation; Has been granted for other PAH drugs |
|
|
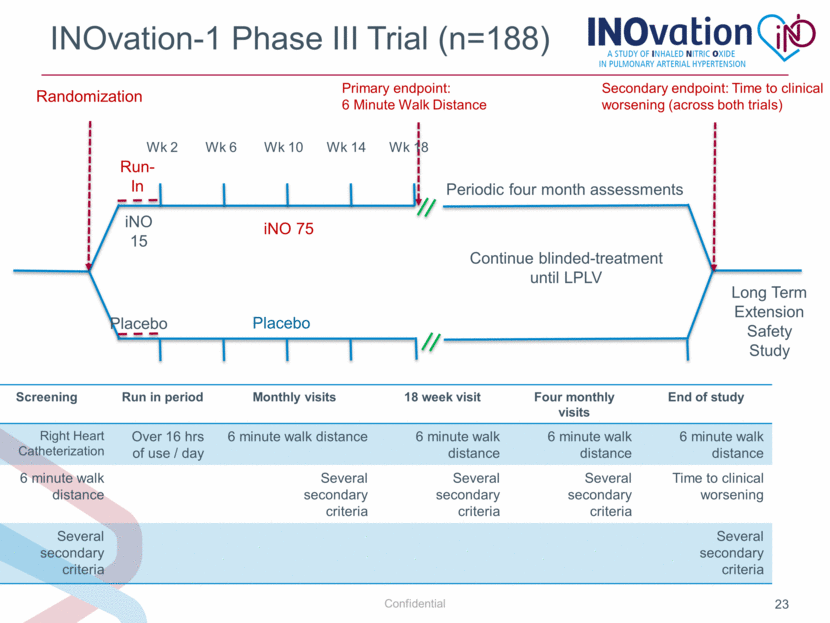
INOvation-1 Phase III Trial (n=188) Wk 2 Wk 6 Wk 10 Wk 14 Wk 18 iNO 75 Placebo iNO 15 Placebo Run- In Long Term Extension Safety Study Continue blinded-treatment until LPLV Secondary endpoint: Time to clinical worsening (across both trials) Periodic four month assessments Screening Run in period Monthly visits 18 week visit Four monthly visits End of study Right Heart Catheterization Over 16 hrs of use / day 6 minute walk distance 6 minute walk distance 6 minute walk distance 6 minute walk distance 6 minute walk distance Several secondary criteria Several secondary criteria Several secondary criteria Time to clinical worsening Several secondary criteria Several secondary criteria Primary endpoint: 6 Minute Walk Distance Randomization |
|
|
INOpulse: PH-COPD |
|
|
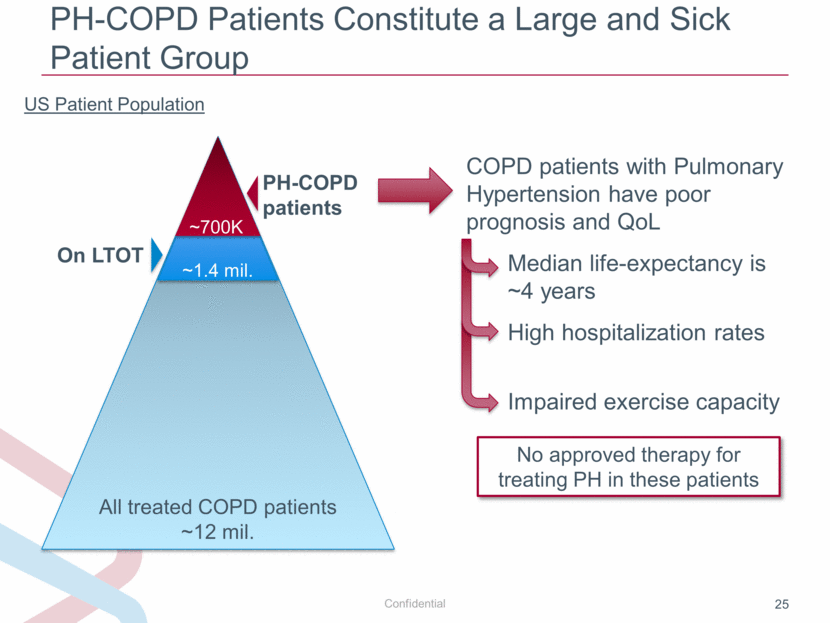
PH-COPD Patients Constitute a Large and Sick Patient Group US Patient Population All treated COPD patients ~12 mil. ~1.4 mil. PH-COPD patients COPD patients with Pulmonary Hypertension have poor prognosis and QoL Median life-expectancy is ~4 years High hospitalization rates Impaired exercise capacity No approved therapy for treating PH in these patients ~700K On LTOT |
|
|
INOpulse May Offer Unique Benefits in Treating COPD Patients with PH Existing PAH therapy lowers pulmonary pressures but negatively influences oxygenation in PH-COPD Pulsed iNO can be targeted to the alveoli Short pulse early in inspiration dilates vessels in best ventilated alveoli only, reduces pulmonary pressures, and prevents admixture of less oxygenated blood Local and fast metabolism prevents delivery to non-targeted alveoli Bianco 2010, Lederer 2012, Stolz 2008 |
|
|
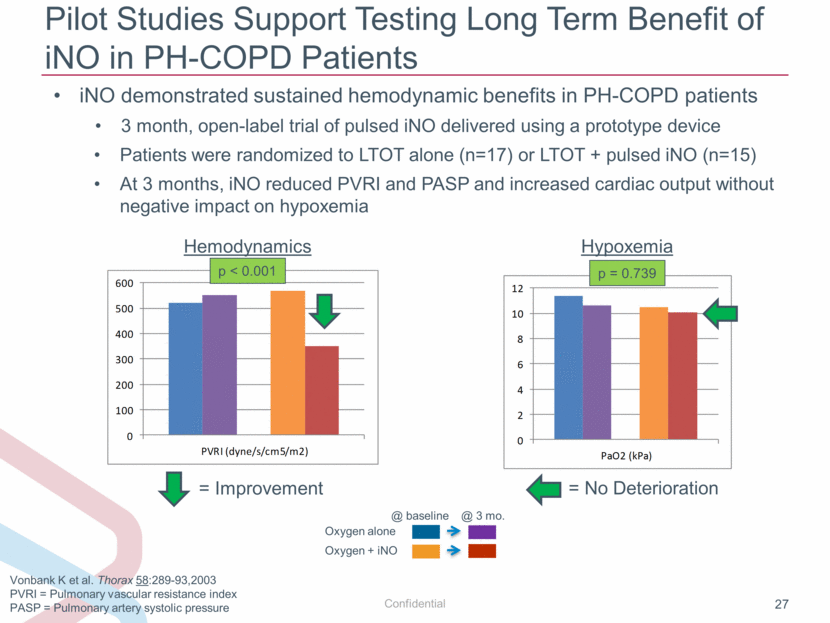
Pilot Studies Support Testing Long Term Benefit of iNO in PH-COPD Patients Vonbank K et al. Thorax 58:289-93,2003 PVRI = Pulmonary vascular resistance index PASP = Pulmonary artery systolic pressure iNO demonstrated sustained hemodynamic benefits in PH-COPD patients 3 month, open-label trial of pulsed iNO delivered using a prototype device Patients were randomized to LTOT alone (n=17) or LTOT + pulsed iNO (n=15) At 3 months, iNO reduced PVRI and PASP and increased cardiac output without negative impact on hypoxemia p < 0.001 = Improvement Oxygen alone Oxygen + iNO @ baseline @ 3 mo. p = 0.739 = No Deterioration Hemodynamics Hypoxemia 0 2 4 6 8 10 12 PaO2 (kPa) 0 100 200 300 400 500 600 PVRI (dyne/s/cm5/m2) |
|
|
INOpulse Testing Confirmed Impact on Pulmonary Hemodynamics Without Hypoxemia in PH-COPD PASP = Pulmonary artery systolic pressure *PASP data consistently favor iNO at doses 10 – 75 mcg/kg IBW/hr, but statistical significance compared to placebo was not reached FDA asked us to establish a dose range with the INOpulse device and to confirm the efficacy and safety results seen in the open label study We have completed randomized placebo controlled acute test with 159 patients using the INOpulse device with doses ranging from 3 to 75 mcg/kg IBW/hr Oxygenation levels were similar to placebo across all doses tested PASP change vs. baseline was very similar to the open label study for doses ≥10 mcg/kg IBW/hr |
|
|
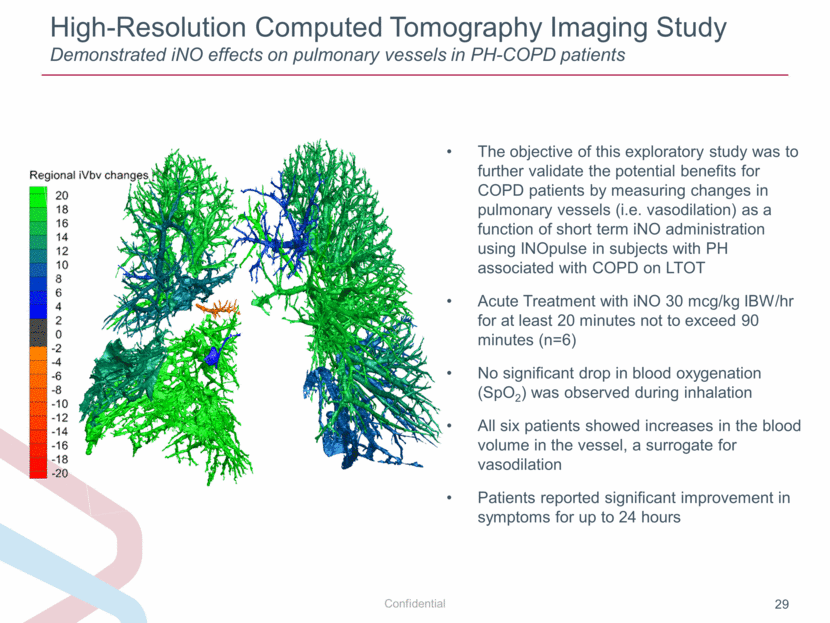
High-Resolution Computed Tomography Imaging Study Demonstrated iNO effects on pulmonary vessels in PH-COPD patients The objective of this exploratory study was to further validate the potential benefits for COPD patients by measuring changes in pulmonary vessels (i.e. vasodilation) as a function of short term iNO administration using INOpulse in subjects with PH associated with COPD on LTOT Acute Treatment with iNO 30 mcg/kg IBW/hr for at least 20 minutes not to exceed 90 minutes (n=6) No significant drop in blood oxygenation (SpO2) was observed during inhalation All six patients showed increases in the blood volume in the vessel, a surrogate for vasodilation Patients reported significant improvement in symptoms for up to 24 hours |