Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Akebia Therapeutics, Inc. | d75683d8k.htm |
| EX-99.1 - EX-99.1 - Akebia Therapeutics, Inc. | d75683dex991.htm |
 September 8, 2015
Akebia Therapeutics Exhibit 99.2 |
| 2
Forward-Looking Statements
This presentation includes forward-looking statements. Such
forward-looking statements include those about Akebia's
strategy, future plans and prospects, including statements regarding the
potential indications, dosing and benefits of vadadustat, the
development plan for vadadustat, plans for presenting a more detailed analysis of the data from the Phase 2 study, and the initiation of the Phase 3 program. The words "anticipate," "appear," "believe,"
"estimate," "expect," "intend,"
"may," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions are intended to identify forward-looking statements, although not all
forward- looking statements contain these identifying
words. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement,
including the risk that existing preclinical and clinical data may not be
predictive of the results of ongoing or later clinical trials;
the ability of Akebia to successfully complete the clinical development of vadadustat; the funding required to develop Akebia's product candidates and operate the company, and the actual expenses associated therewith; the
cost of our Phase 3 studies and the availability of financing to cover such
cost; the timing and content of decisions made by the FDA and
other regulatory authorities; the acceptance of Akebia's abstract for presentation at a medical meeting; the actual time it takes to prepare for and initiate Phase 3 clinical studies; the success of competitors in developing
product candidates for diseases for which Akebia is currently developing its
product candidates; and Akebia's ability to obtain, maintain and
enforce patent and other intellectual property protection for vadadustat. Other risks and uncertainties include those identified under the heading "Risk Factors" in Akebia's Quarterly Report on Form 10-Q for
the quarter ended June 30, 2015, and other filings that Akebia
may make with the Securities and Exchange Commission in the
future. Akebia does not undertake, and specifically disclaims, any obligation to update any forward-looking statements contained in this presentation. |
 3 Akebia at a Glance Akebia is a biopharmaceutical company focused on delivering innovative therapies to patients with kidney disease through the biology of hypoxia inducible factor (HIF) Experienced Management Team with extensive knowledge in HIF biology combined with renal expertise Lead program: vadadustat (AKB-6548) is a once-daily, oral therapy with
best-in-class potential for the treatment of renal anemia
Completed Phase 2 vadadustat studies in both non-dialysis and dialysis
patients
Additional HIF-PH inhibitor compounds in pipeline
Public (NASDAQ:AKBA), headquartered in Cambridge, MA
|
 Top-Line Results of Phase 2 Vadadustat Study in Dialysis Patients with
Anemia Related to Chronic Kidney Disease
|
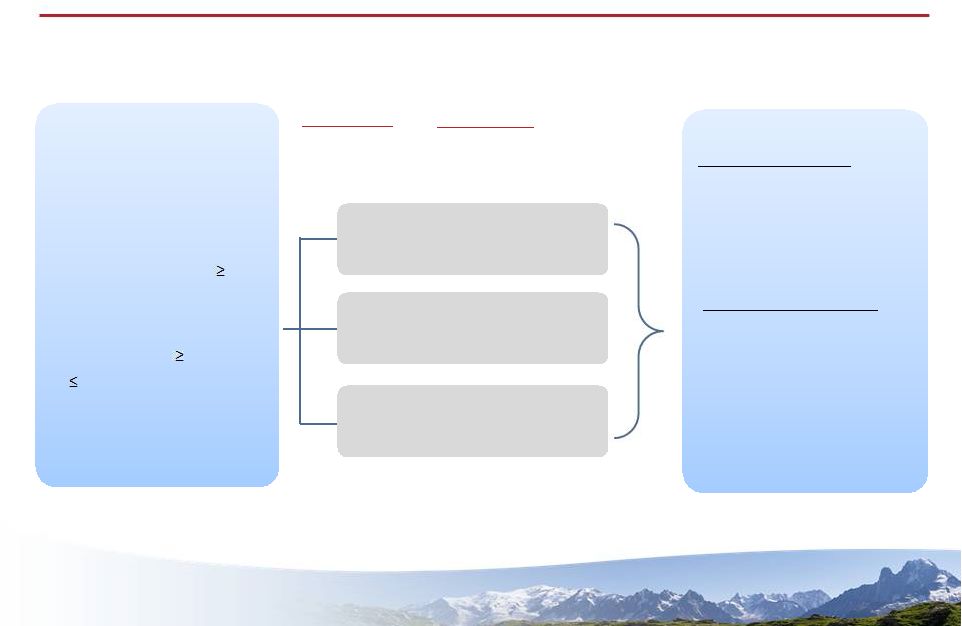 5 5 1st analysis: change in HGB at Week 8 Vadadustat (AKB-6548) dialysis efficacy study Patient Criteria Open Label 16 Weeks of Treatment Endpoints 2nd analysis: subsequent change in HGB with dose adjustment starting at Week 8 Vadadustat 300 mg QD Vadadustat 450 mg QD Vadadustat 450 mg TIW Primary endpoint 1. Compare the change in HGB from baseline for three different doses of Vadadustat Secondary endpoint 1. Safety of Vadadustat in ESRD subjects on dialysis 2. Effect of dialysis on the PK of Vadadustat Primary endpoint 1. Compare the change in HGB from baseline for three different doses of Vadadustat Secondary endpoint 1. Safety of Vadadustat in ESRD subjects on dialysis 2. Effect of dialysis on the PK of Vadadustat (1) First eight weeks include Weeks 1 – 8. (2) Second eight weeks include Weeks 9 – 16. • n= 90 • Age: 18 – 79 years old • CKD Stage 5 undergoing chronic hemodialysis for 3 months and dialysis 3x per week • Mean HGB: 9.0 and 12.0 g/dL • Receives epoetin alfa regularly • n= 90 • Age: 18 – 79 years old • CKD Stage 5 undergoing chronic hemodialysis for 3 months and dialysis 3x per week • Mean HGB: 9.0 and 12.0 g/dL • Receives epoetin alfa regularly |
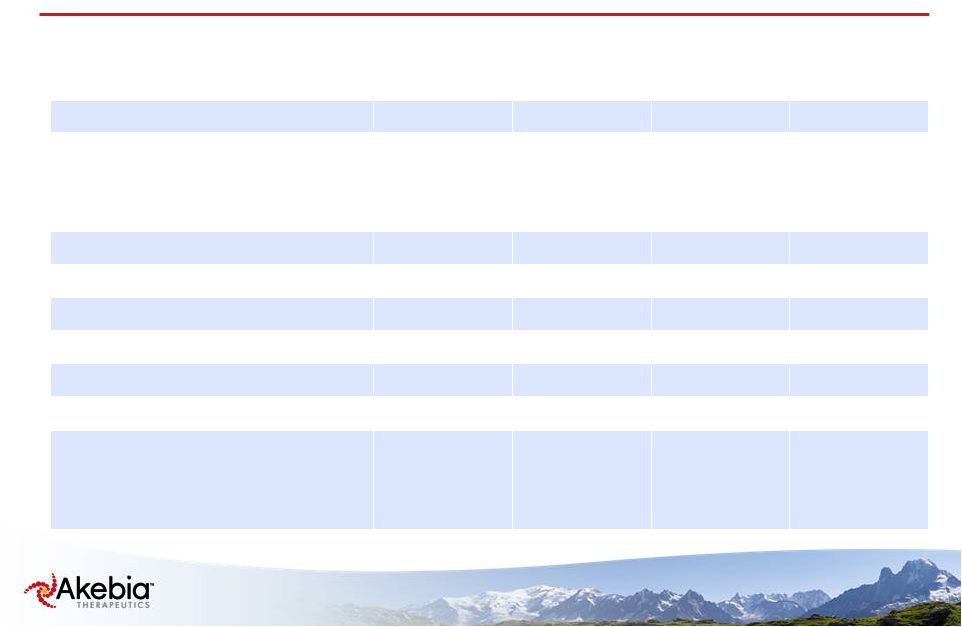 6 Demographics 300 mg QD N=30 450 mg QD N=33 450 mg TIW N=31 All Subjects N=94 Sex-female 13 (43.3%) 15 (45.5%) 12 (38.7%) 40 (42.6%) Race White/Caucasian Black or African American Other 24 (80.0%) 6 (20.0%) 0 (0.0%) 21 (63.6%) 9 (27.3%) 3 (9.1%) 19 (61.3%) 9 (29.0%) 3 (9.7%) 64 (68.1%) 24 (25.5%) 6 (6.4%) Mean Age (years) 55.5 59.4 57.8 57.6 Mean Weight (kg) 82.7 82.1 84.2 83.0 Mean BMI (kg/m²) 29.8 29.6 29.2 29.5 Mean time on dialysis (years) 4.92 4.93 3.87 4.58 Mean Hemoglobin (g/dL) 10.4 10.6 10.5 10.5 Mean Ferritin (ng/mL) 763 782 808 784 Etiology of CKD Diabetes Hypertension & Large Vessel Disease Other 16 (53.3%) 15 (50.0%) 7 (23.3%) 23 (69.7%) 17 (51.5%) 4 (12.1%) 21 (67.7%) 21 (67.7%) 1 (3.2%) 60 (63.8%) 53 (56.4%) 12 (12.8%) |
 7 7 Mean HGB Average Results at Study Stages MITT Population Mean Hemoglobin levels (g/dL) Baseline Week 7/8 Week 15/16 300mg Daily Dose 10.4 10.4 10.3 450mg Daily Dose 10.6 10.3 10.5 450mg Three Times-per-Week Dose 10.5 10.2 10.4 PP Population Mean Hemoglobin levels (g/dL) Baseline Week 7/8 Week 15/16 300mg Daily Dose 10.3 10.4 10.3 450mg Daily Dose 10.6 10.4 10.6 450mg Three Times-per-Week Dose 10.5 10.5 10.5 |
 Summary of adverse events (AEs) and serious AEs (SAEs)
ITT Population
300 mg QD N=30 450 mg QD N=33 450 mg TIW N=31 All Subjects N=94 Number of AEs 110 95 89 294 Subjects with 1 AE 26 (86.7%) 26 (78.8%) 26 (83.9%) 78 (83.0%) Subjects with 1 SAE 2 (6.7%) 6 (18.2%) 5 (16.1%) 13 (13.8%) Subjects with SAE Reported as Related 0 0 0 0 Deaths 0 0 0 0 8 |
 Dialysis Phase 2 Study Top Line Summary
Vadadustat maintained stable HGB levels across the 3 dosing
cohorts following conversion from rESA
• Daily and three times-a-week regimens are viable dosing options in dialysis
subjects • Hemoglobin level was maintained within the desired range while minimizing
excursions 13.0 g/dL • Improvements in iron mobilization consistent with previous studies Vadadustat was generally well-tolerated across the 3 dose cohorts • No apparent differences in the type or frequency of AEs across the 3 dosing
groups • Observed SAEs were consistent with events described in dialysis population
• There were no SAEs reported as related to study drug, no strokes, and no deaths
9 |
 Akebia Therapeutics
September 8, 2015 |
