Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - HEAT BIOLOGICS, INC. | htbx_8k.htm |
| EX-99.1 - PRESS RELEASE - HEAT BIOLOGICS, INC. | htbx_ex99z1.htm |
EXHIBIT 99.2

Combining HS-110 and anti-PD-1 in NSCLC September 1, 2015

Forward Looking Statements This presentation includes statements that are, or may be deemed, ‘‘forward-looking statements.’’ In some cases, these forward-looking statements can be identified by the use of forward-looking terminology, including the terms “believes,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should,” “approximately” or, in each case, their negative or other variations thereon or comparable terminology, although not all forward-looking statements contain these words. They appear in a number of places throughout this presentation and include statements regarding our intentions, beliefs, projections, outlook, analyses or current expectations concerning, among other things, our ongoing and planned discovery and development of drugs targeting cancer stem cells, the strength and breadth of our intellectual property, our ongoing and planned preclinical studies and clinical trials, the timing of and our ability to make regulatory filings and obtain and maintain regulatory approvals for our product candidates, our ability to partner our product development, the degree of clinical utility of our products, particularly in specific patient populations, expectations regarding clinical trial data, our results of operations, financial condition, liquidity, prospects, growth and strategies, the length of time that we will be able to continue to fund our operating expenses and capital expenditures, our expected financing needs and sources of financing, the industry in which we operate and the trends that may affect the industry or us. By their nature, forward-looking statements involve risks and uncertainties because they relate to events, competitive dynamics, and healthcare, regulatory and scientific developments and depend on the economic circumstances that may or may not occur in the future or may occur on longer or shorter timelines than anticipated. Although we believe that we have a reasonable basis for each forward-looking statement contained in this presentation, we caution you that forward-looking statements are not guarantees of future performance and that our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate may differ materially from the forward-looking statements contained in this presentation as a result of, among other factors, the factors referenced in the “Risk Factors” section of our Annual Report on Form 10-K for the year ended December 31, 2014 and our quarterly report on Form 10-Q for the subsequent quarters (collectively, our “SEC Filings”). In addition, even if our results of operations, financial condition and liquidity, and the development of the industry in which we operate are consistent with the forward-looking statements contained in this presentation, they may not be predictive of results or developments in future periods. Any forward-looking statements that we make in this presentation speak only as of the date of such statement, and we undertake no obligation to update such statements to reflect events or circumstances after the date of this presentation, except as required by law.You should read carefully the factors described in the “Risk Factors” sections of our SEC Filings to better understand the risks and uncertainties inherent in our business. *

Checkpoint Combination Rationale Preclinical Synergy withanti-PD-1 Bladder trial informing ImPACT responsive population Immune Response from Phase 2 NSCLC trial Changing landscape in NSCLC Checkpoint Combination Strategy for HS-110 *

* 1 2 3 4 Copyright Heat Biologics 2015

Scientific Support for HS-110 & Nivolumab Combination * Pre-clinical studies demonstrate synergy between ImPACT vaccines and anti-PD-1Phase 2 clinical data demonstrate that HS-110 treated patients upregulate PD-1 on CD8+ T cellsPhase 2 tumor biopsies show strong PD-L1 staining Cohen, R.B. et al, ASCO 2015 Schreiber T.H., et al. Keystone 2014 Phase 2 Tumor Biopsy from Patient Treated with HS-110
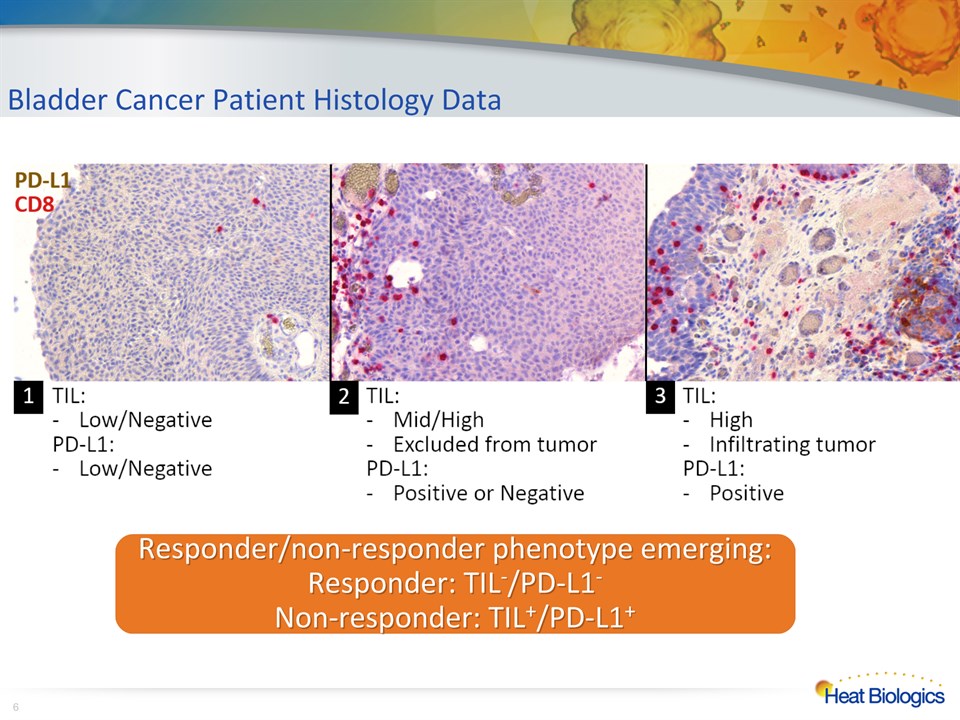
Bladder Cancer Patient Histology Data * Responder/non-responder phenotype emerging:Responder: TIL-/PD-L1-Non-responder: TIL+/PD-L1+

* Design Intended to Broaden Responses to Nivolumab

Design Intended to Broaden Responses to Nivolumab * Estimated 45% NSCLC patients being underserved by single-agent anti-PD-(L)1 and may benefit from vaccine combination Teng et al., 2015 Can ResGettinger et al., 2015 JCO Approx. 50% PD-1 ORR Approx. 10% PD-1 ORR

Checkpoint Combination Rationale Preclinical Synergy withanti-PD-1 Bladder trial informing ImPACT responsive population Immune Response from Phase 2 NSCLC trial Changing landscape in NSCLC Checkpoint Combination Strategy for HS-110 *

Response to Checkpoint Inhibitors by PD-L1 Status * Population HS-110 combination intends to address Source: Velcheti et al. Lab Inv 2014; Gettinger et al., 2015 JCO

DURGA Multi-arm Combination Trial * Provides operational platform to:React quickly to changing landscapeLeverage potential synergy with PD-1 mAbEvaluate TILs as a potential biomarker to improve response rate (Yale Cancer Center’s Translational Immuno-Oncology Laboratory collaboration) Expand cohorts based on efficacy signal Primary Endpoint: SafetySecondary Endpoints: ORR, 6-mo PFSExploratory Endpoints: Ag-specific immune response in PBMCs and tissue

Clinical Development Milestones Milestone Target Date First dose combination with nivo in NSCLC (Durga) 31-Aug-2015 Durga 18 pts enrolled Q2 2016 Durga immune response Q2 2016 Durga top-line readout for nivo (18 pts) Q4 2016 Ph 2 combo with cyclophosphamide readout (~65 pts) Q4 2016 Registration-directed trial initiation in NSCLC Q4 2016 Ph 2 Bladder Trial enrollment complete (75 pts) Q3/Q4 2015 Ph 1 Bladder Readout (10 pts) Q4 2015 Ph 2 Bladder Readout Q4 2016 Registration-directed trial initiation in NMIBC Q4 2016 Announce new ComPACT indication Q1 2016 * 1 2 3 4 5 6 7 LUNG(NSCLC) BLADDER(NMIBC) 8 = NEW 9 10 11 2H 2015 1H2016 2H2016 7 8 9 5 6

Summary * First combination vaccine + nivolumab in NSCLCStrong preclinical/clinical rationale for combinationTargeting patients unresponsive to checkpoints Data for nivo combo expected by end 2016 Registration-directed trial initiation remains on schedule (end 2016/early 2017)Bladder program on track (readout Q4 2016) Cost savings from closing Ph 2 combination with cyclophosphamide to fund DURGA arms with nivolumab
