Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a15-18774_1ex99d1.htm |
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a15-18774_18k.htm |
Exhibit 99.2
|
|
Amicus Acquisition of Scioderm, Inc. Overview August 31, 2015 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to the planned acquisition of Scioderm, the expected financial impact and benefits to Amicus of such acquisition, and anticipated milestones and other expectations regarding Scioderm’s product development activities, clinical trials and commercialization, and business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, cash runway, and the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2014 and our Form 10-Q for the quarter ended June 30, 2015. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. |
|
|
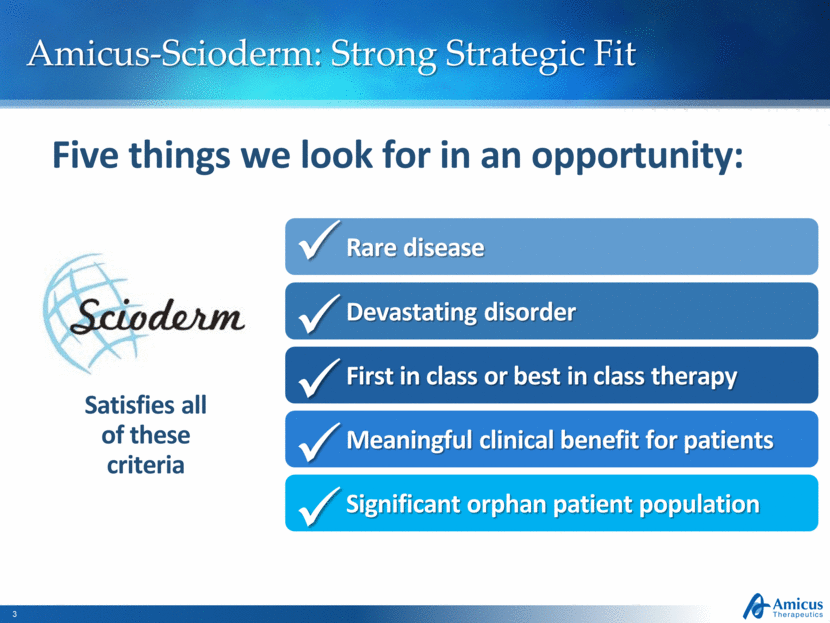
Satisfies all of these criteria Amicus-Scioderm: Strong Strategic Fit Five things we look for in an opportunity: Devastating disorder First in class or best in class therapy Meaningful clinical benefit for patients Significant orphan patient population Rare disease |
|
|
Strategic Acquisition Aligned with Amicus Mission Amicus Therapeutics is a biopharmaceutical company at the forefront of developing advanced therapies to treat a range of devastating rare and orphan diseases |
|
|
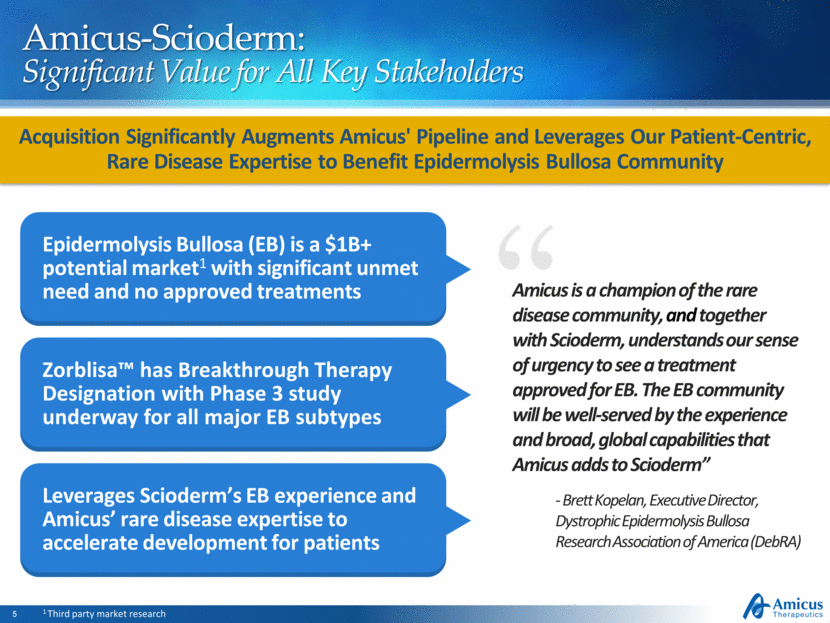
Epidermolysis Bullosa (EB) is a $1B+ potential market1 with significant unmet need and no approved treatments Zorblisa™ has Breakthrough Therapy Designation with Phase 3 study underway for all major EB subtypes Leverages Scioderm’s EB experience and Amicus’ rare disease expertise to accelerate development for patients Amicus-Scioderm: Significant Value for All Key Stakeholders Acquisition Significantly Augments Amicus' Pipeline and Leverages Our Patient-Centric, Rare Disease Expertise to Benefit Epidermolysis Bullosa Community 1 Third party market research - Brett Kopelan, Executive Director, Dystrophic Epidermolysis Bullosa Research Association of America (DebRA) Amicus is a champion of the rare disease community, and together with Scioderm, understands our sense of urgency to see a treatment approved for EB. The EB community will be well-served by the experience and broad, global capabilities that Amicus adds to Scioderm” |
|
|
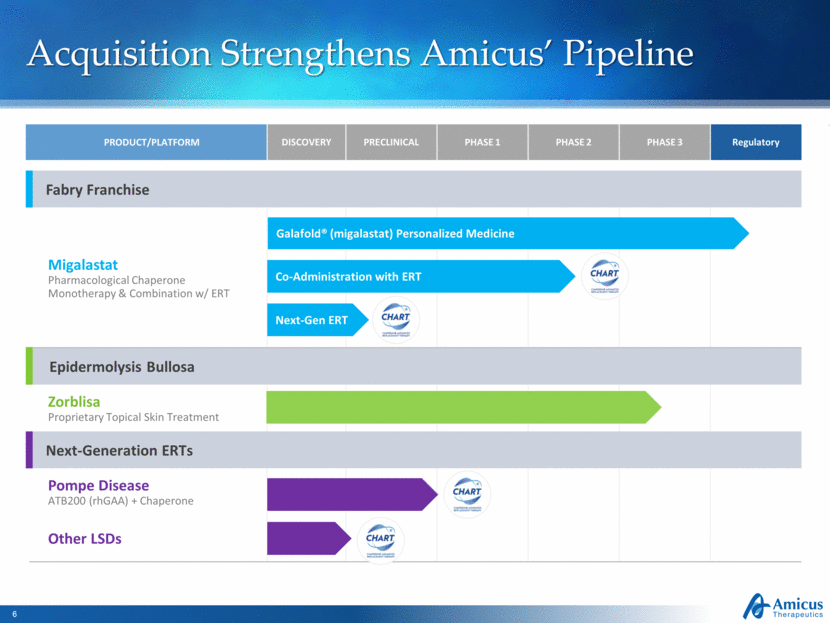
Acquisition Strengthens Amicus’ Pipeline PRODUCT/PLATFORM DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Regulatory Fabry Franchise Migalastat Pharmacological Chaperone Monotherapy & Combination w/ ERT Epidermolysis Bullosa Zorblisa Proprietary Topical Skin Treatment Next-Generation ERTs Pompe Disease ATB200 (rhGAA) + Chaperone Other LSDs Galafold® (migalastat) Personalized Medicine Co-Administration with ERT Next-Gen ERT |
|
|
Epidermolysis Bullosa “EB” |
|
|
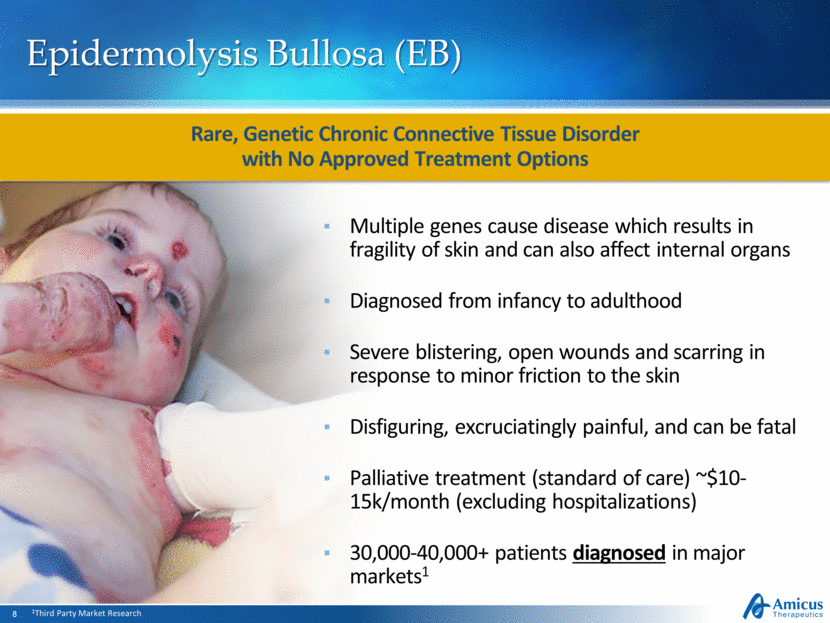
Epidermolysis Bullosa (EB) Multiple genes cause disease which results in fragility of skin and can also affect internal organs Diagnosed from infancy to adulthood Severe blistering, open wounds and scarring in response to minor friction to the skin Disfiguring, excruciatingly painful, and can be fatal Palliative treatment (standard of care) ~$10-15k/month (excluding hospitalizations) 30,000-40,000+ patients diagnosed in major markets1 Rare, Genetic Chronic Connective Tissue Disorder with No Approved Treatment Options 1Third Party Market Research |
|
|
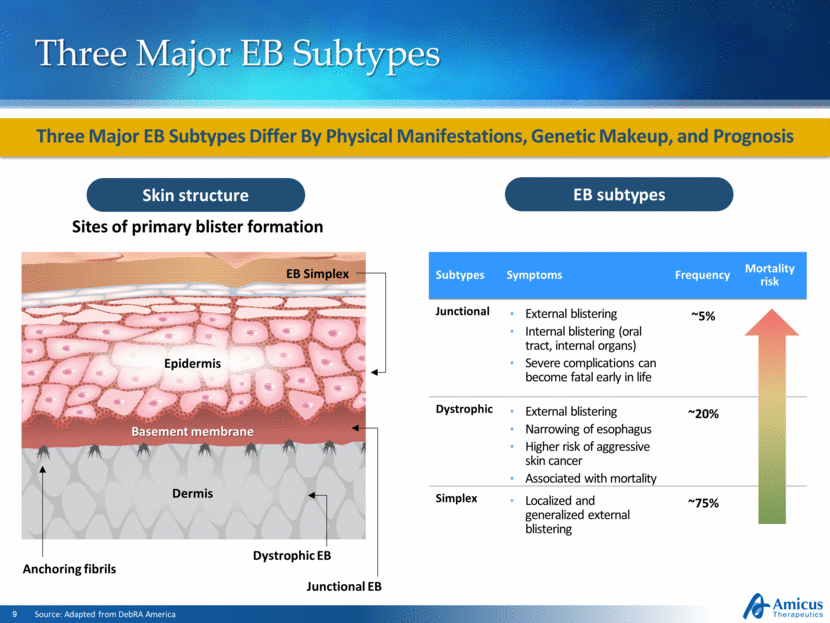
Subtypes Symptoms Frequency Mortality risk Junctional External blistering Internal blistering (oral tract, internal organs) Severe complications can become fatal early in life ~5% Dystrophic External blistering Narrowing of esophagus Higher risk of aggressive skin cancer Associated with mortality ~20% Simplex Localized and generalized external blistering ~75% Three Major EB Subtypes Three Major EB Subtypes Differ By Physical Manifestations, Genetic Makeup, and Prognosis EB subtypes Source: Adapted from DebRA America Skin structure Sites of primary blister formation EB Simplex Junctional EB Dystrophic EB Basement membrane Anchoring fibrils Epidermis Dermis |
|
|
Active ingredient Allantoin RoA Proprietary topical cream containing 6% allantoin, applied to entire body once daily Proposed Indication All major EB subtypes (Simplex, Dystrophic and Junctional) Phase of development Phase 3 ongoing (first to enter Phase 3 for EB) First to show significant benefit in wound closure in EB clinical studies Proposed MOA* Impacts inflammatory response and promotes formation of epithelial and granulation tissue Direct bactericidal action demonstrated in vitro Loosens protein bridges (desmosomes) that hold together hyperkeratinized cells (e.g. in calluses) Formulation Patented high concentration formulation Long-term stability of active ingredient and dose-related penetration of active ingredient in human dermal tissue Zorblisa™ Overview Patented High Concentration Allantoin with Breakthrough Therapy Designation Novel, Proprietary Topical Cream Promotes Healing of Wounds in EB and is Differentiated by Applicability for All Major EB Subtypes *Margraf and Covey 1977; Meixell and Mecca 1966; Settle 1969; Meixell and Mecca 1966; Flesch 1958, Fisher 1981; Cajkovac et al., 1992, Medda 1976 |
|
|
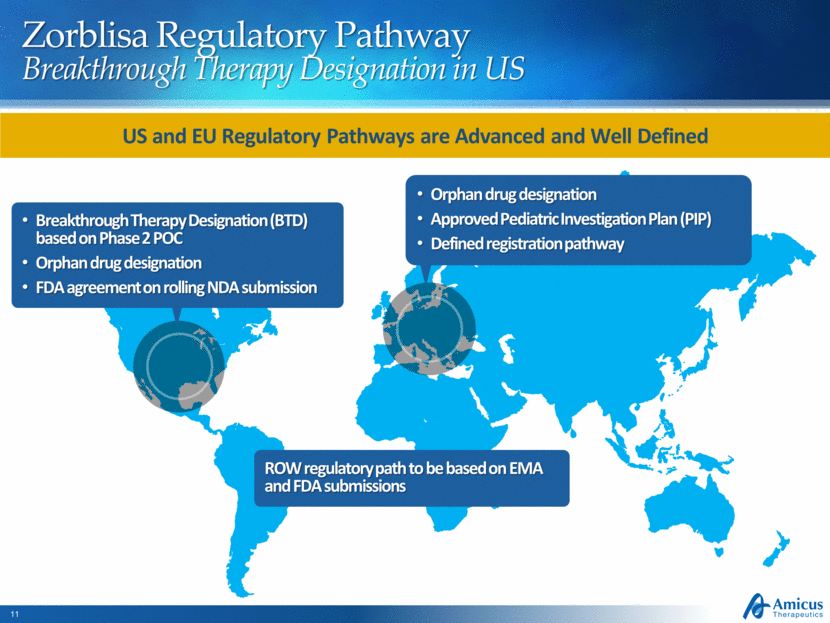
Zorblisa Regulatory Pathway Breakthrough Therapy Designation in US Breakthrough Therapy Designation (BTD) based on Phase 2 POC Orphan drug designation FDA agreement on rolling NDA submission Orphan drug designation Approved Pediatric Investigation Plan (PIP) Defined registration pathway US and EU Regulatory Pathways are Advanced and Well Defined ROW regulatory path to be based on EMA and FDA submissions |
|
|
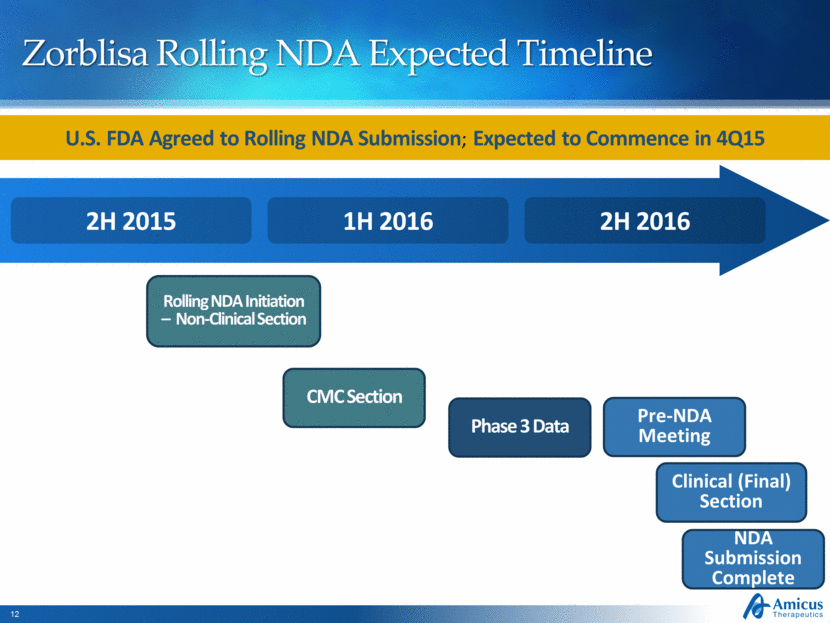
Zorblisa Rolling NDA Expected Timeline Rolling NDA Initiation – Non-Clinical Section 2H 2016 1H 2016 2H 2015 CMC Section Phase 3 Data Pre-NDA Meeting Clinical (Final) Section NDA Submission Complete U.S. FDA Agreed to Rolling NDA Submission; Expected to Commence in 4Q15 |
|
|
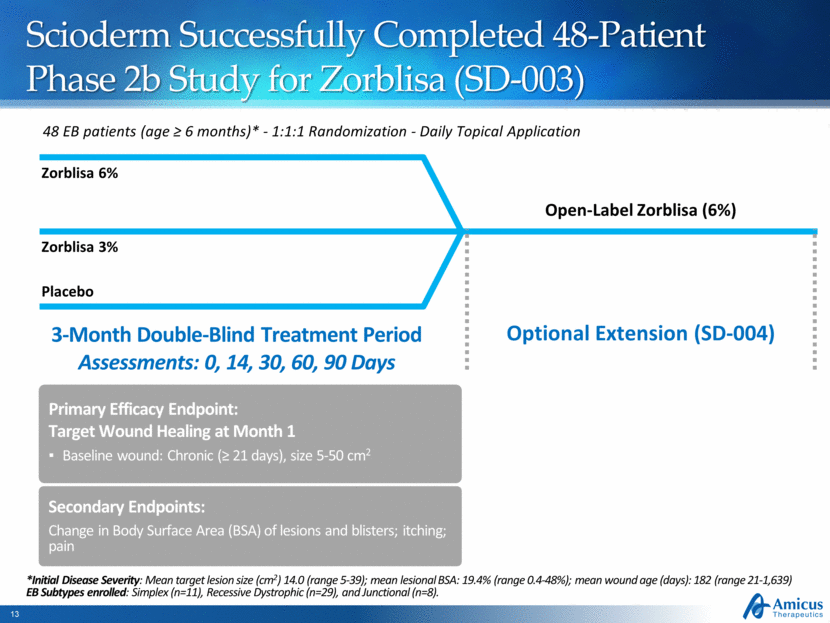
Scioderm Successfully Completed 48-Patient Phase 2b Study for Zorblisa (SD-003) 48 EB patients (age > 6 months)* - 1:1:1 Randomization - Daily Topical Application Placebo Zorblisa 6% Open-Label Zorblisa (6%) 3-Month Double-Blind Treatment Period Assessments: 0, 14, 30, 60, 90 Days Optional Extension (SD-004) Primary Efficacy Endpoint: Target Wound Healing at Month 1 Baseline wound: Chronic (> 21 days), size 5-50 cm2 Zorblisa 3% Secondary Endpoints: Change in Body Surface Area (BSA) of lesions and blisters; itching; pain *Initial Disease Severity: Mean target lesion size (cm2) 14.0 (range 5-39); mean lesional BSA: 19.4% (range 0.4-48%); mean wound age (days): 182 (range 21-1,639) EB Subtypes enrolled: Simplex (n=11), Recessive Dystrophic (n=29), and Junctional (n=8). |
|
|
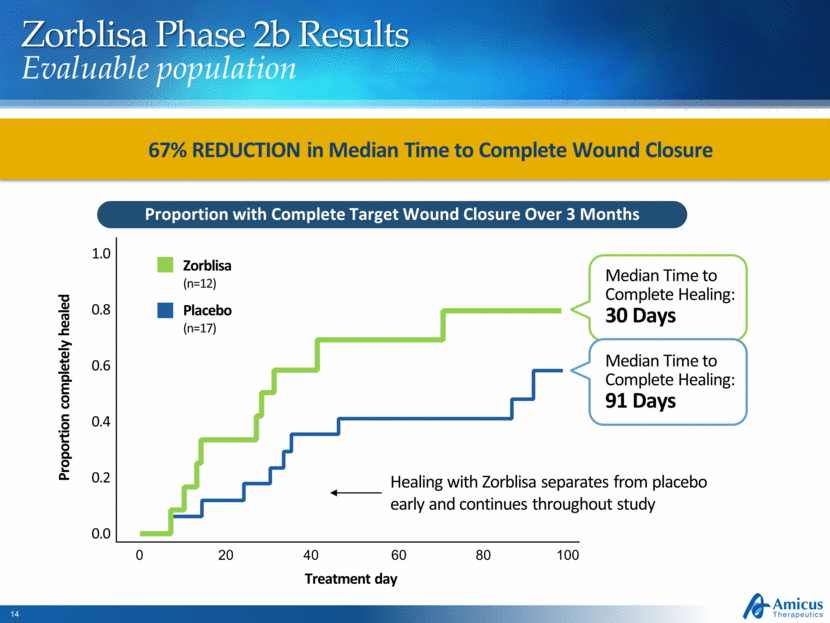
Zorblisa Phase 2b Results Evaluable population Proportion with Complete Target Wound Closure Over 3 Months Treatment day Placebo (n=17) Zorblisa (n=12) Proportion completely healed 0 20 40 60 80 100 1.0 0.8 0.6 0.4 0.2 0.0 Median Time to Complete Healing: 30 Days Median Time to Complete Healing: 91 Days 67% REDUCTION in Median Time to Complete Wound Closure Healing with Zorblisa separates from placebo early and continues throughout study |
|
|
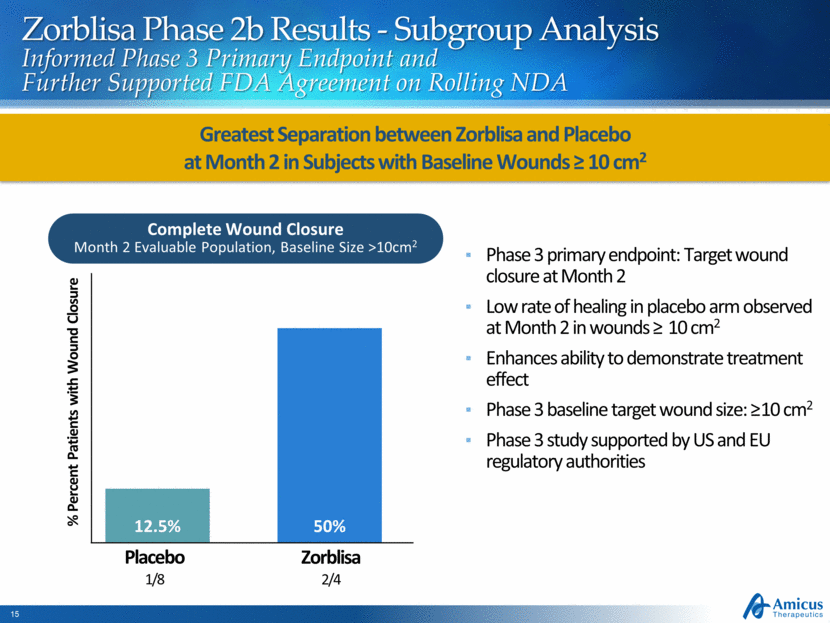
Complete Wound Closure Month 2 Evaluable Population, Baseline Size >10cm2 Zorblisa Phase 2b Results - Subgroup Analysis Informed Phase 3 Primary Endpoint and Further Supported FDA Agreement on Rolling NDA Phase 3 primary endpoint: Target wound closure at Month 2 Low rate of healing in placebo arm observed at Month 2 in wounds > 10 cm2 Enhances ability to demonstrate treatment effect Phase 3 baseline target wound size: >10 cm2 Phase 3 study supported by US and EU regulatory authorities Greatest Separation between Zorblisa and Placebo at Month 2 in Subjects with Baseline Wounds > 10 cm2 Zorblisa 2/4 Placebo 1/8 % Percent Patients with Wound Closure 12.5% 50% |
|
|
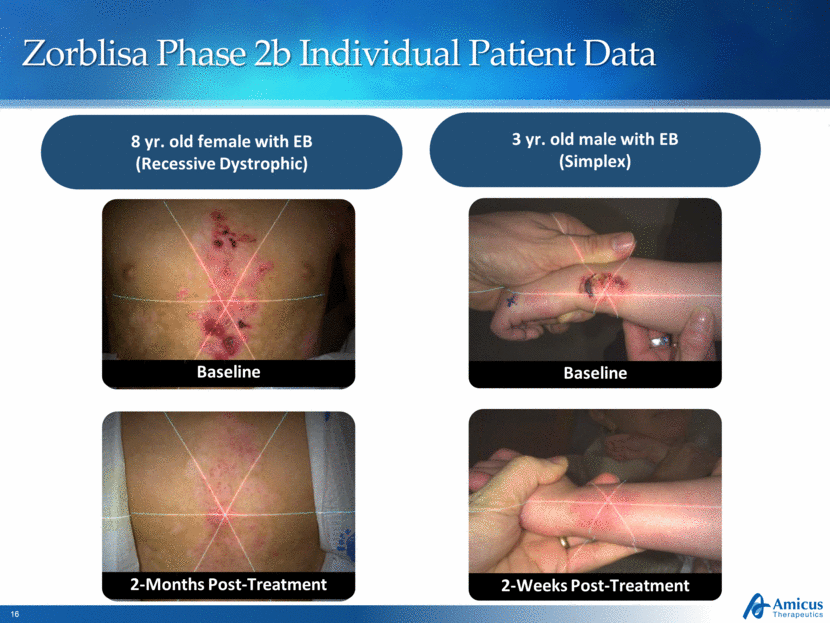
Zorblisa Phase 2b Individual Patient Data Following 2 months of treatment 8 yr. old female with EB (Recessive Dystrophic) 3 yr. old male with EB (Simplex) Baseline 2-Months Post-Treatment Baseline 2-Weeks Post-Treatment |
|
|
Zorblisa Phase 2b Safety Summary Treatment-emergent adverse events (TEAE) similar across treatment groups, including placebo No deaths and no severe TEAEs No serious adverse events reported in Zorblisa 6% group |
|
|
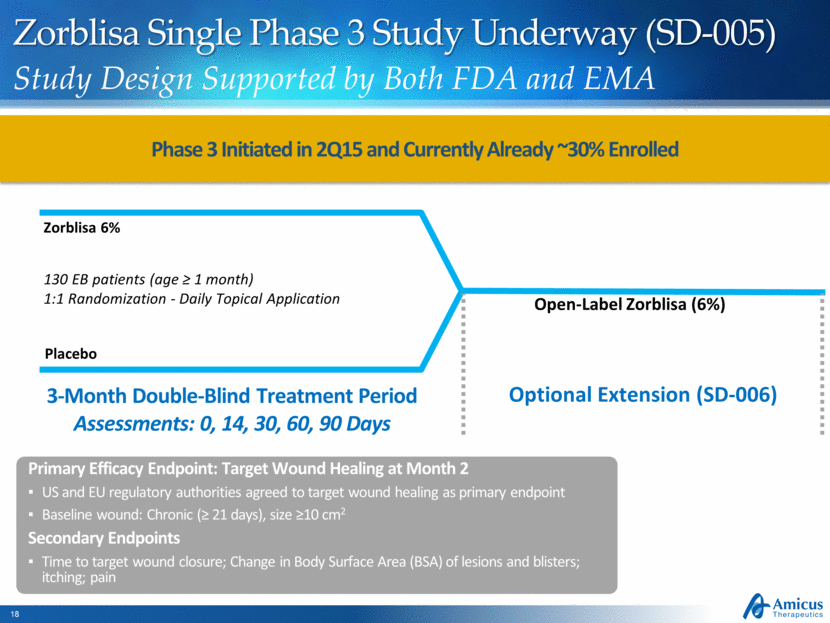
Zorblisa Single Phase 3 Study Underway (SD-005) Study Design Supported by Both FDA and EMA 130 EB patients (age > 1 month) 1:1 Randomization - Daily Topical Application Placebo Zorblisa 6% Open-Label Zorblisa (6%) Slide 27 3-Month Double-Blind Treatment Period Assessments: 0, 14, 30, 60, 90 Days Optional Extension (SD-006) Primary Efficacy Endpoint: Target Wound Healing at Month 2 US and EU regulatory authorities agreed to target wound healing as primary endpoint Baseline wound: Chronic (> 21 days), size >10 cm2 Secondary Endpoints Time to target wound closure; Change in Body Surface Area (BSA) of lesions and blisters; itching; pain Phase 3 Initiated in 2Q15 and Currently Already ~30% Enrolled |
|
|
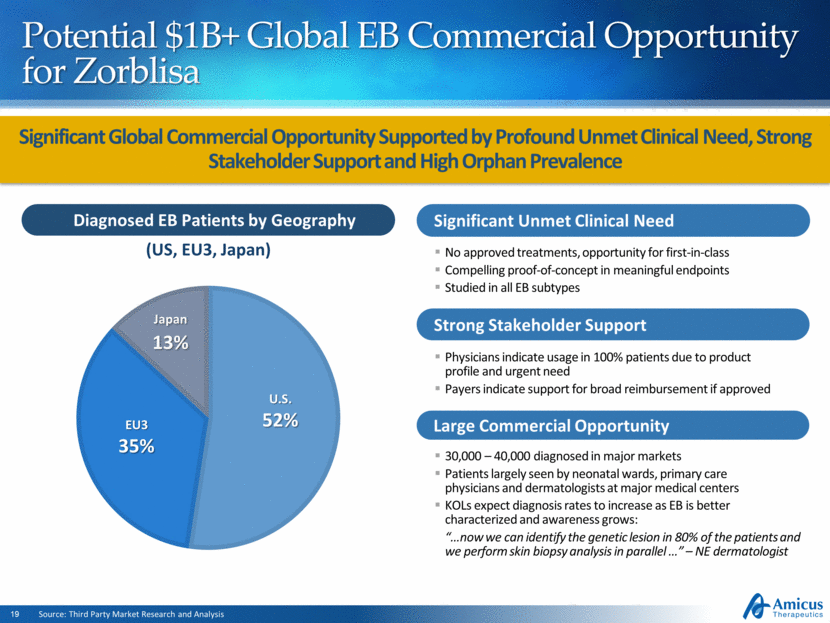
Significant Global Commercial Opportunity Supported by Profound Unmet Clinical Need, Strong Stakeholder Support and High Orphan Prevalence Source: Third Party Market Research and Analysis Diagnosed EB Patients by Geography Significant Unmet Medical Need Significant Unmet Clinical Need No approved treatments, opportunity for first-in-class Compelling proof-of-concept in meaningful endpoints Studied in all EB subtypes Strong Stakeholder Support Physicians indicate usage in 100% patients due to product profile and urgent need Payers indicate support for broad reimbursement if approved Large Commercial Opportunity 30,000 – 40,000 diagnosed in major markets Patients largely seen by neonatal wards, primary care physicians and dermatologists at major medical centers KOLs expect diagnosis rates to increase as EB is better characterized and awareness grows: “ now we can identify the genetic lesion in 80% of the patients and we perform skin biopsy analysis in parallel ” – NE dermatologist Potential $1B+ Global EB Commercial Opportunity for Zorblisa (US, EU3, Japan) U.S. 52% EU3 35% Japan 13% |
|
|
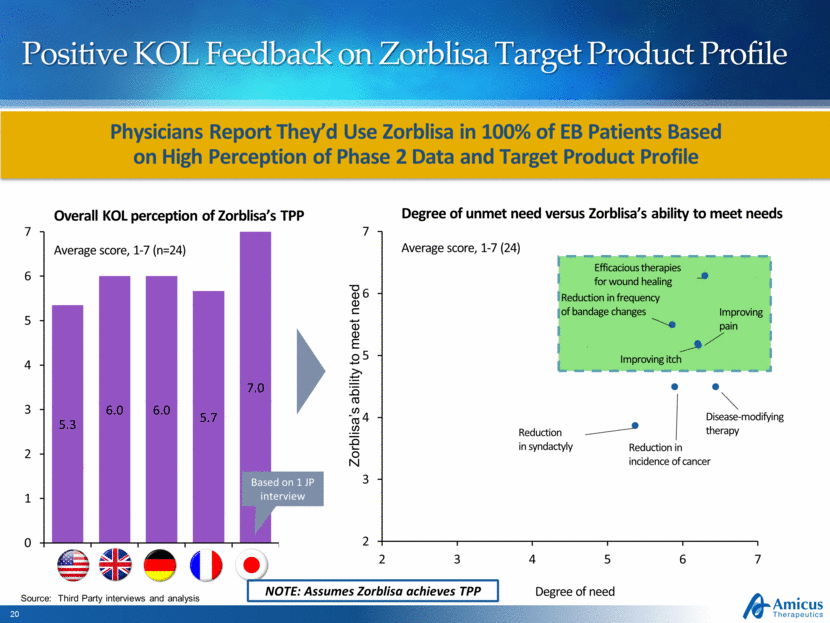
Positive KOL Feedback on Zorblisa Target Product Profile Source: Third Party interviews and analysis JP FR DE Overall KOL perception of Zorblisa’s TPP Average score, 1-7 (n=24) UK US Degree of unmet need versus Zorblisa’s ability to meet needs Average score, 1-7 (24) Degree of need Reduction in frequency of bandage changes Disease-modifying therapy Efficacious therapies for wound healing Reduction in incidence of cancer Reduction in syndactyly Improving itch Improving pain Zorblisa’s ability to meet need Based on 1 JP interview NOTE: Assumes Zorblisa achieves TPP “ We are using Scioderm’s compound in trials with good results, we have seen fast improvements in wounds healing and it is well tolerated ” Dermatologist, Necker Hospital, Paris Physicians Report They’d Use Zorblisa in 100% of EB Patients Based on High Perception of Phase 2 Data and Target Product Profile 5.3 6.0 6.0 5.7 7.0 0 1 2 3 4 5 6 7 2 3 4 5 6 7 2 3 4 5 6 7 |
|
|
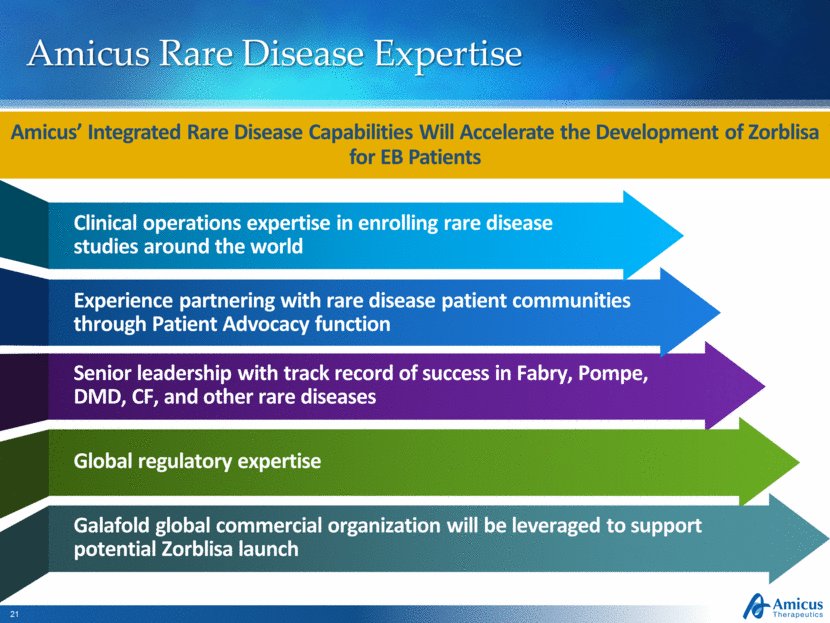
Amicus Rare Disease Expertise Amicus’ Integrated Rare Disease Capabilities Will Accelerate the Development of Zorblisa for EB Patients Senior leadership with track record of success in Fabry, Pompe, DMD, CF, and other rare diseases Experience partnering with rare disease patient communities through Patient Advocacy function Global regulatory expertise Galafold global commercial organization will be leveraged to support potential Zorblisa launch Clinical operations expertise in enrolling rare disease studies around the world |
|
|
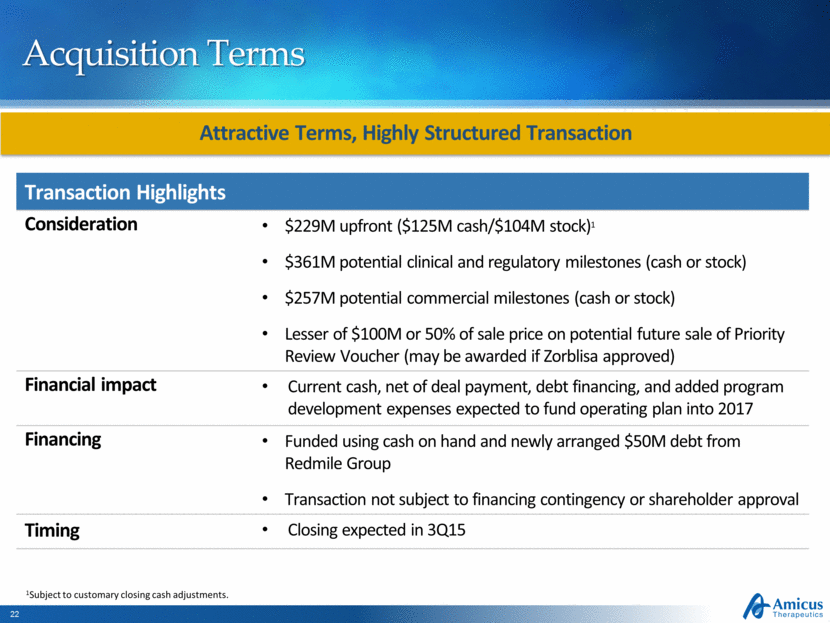
Acquisition Terms Attractive Terms, Highly Structured Transaction Transaction Highlights Consideration $229M upfront ($125M cash/$104M stock)1 $361M potential clinical and regulatory milestones (cash or stock) $257M potential commercial milestones (cash or stock) Lesser of $100M or 50% of sale price on potential future sale of Priority Review Voucher (may be awarded if Zorblisa approved) Financial impact Current cash, net of deal payment, debt financing, and added program development expenses expected to fund operating plan into 2017 Financing Funded using cash on hand and newly arranged $50M debt from Redmile Group Transaction not subject to financing contingency or shareholder approval Timing Closing expected in 3Q15 1Subject to customary closing cash adjustments. |
|
|
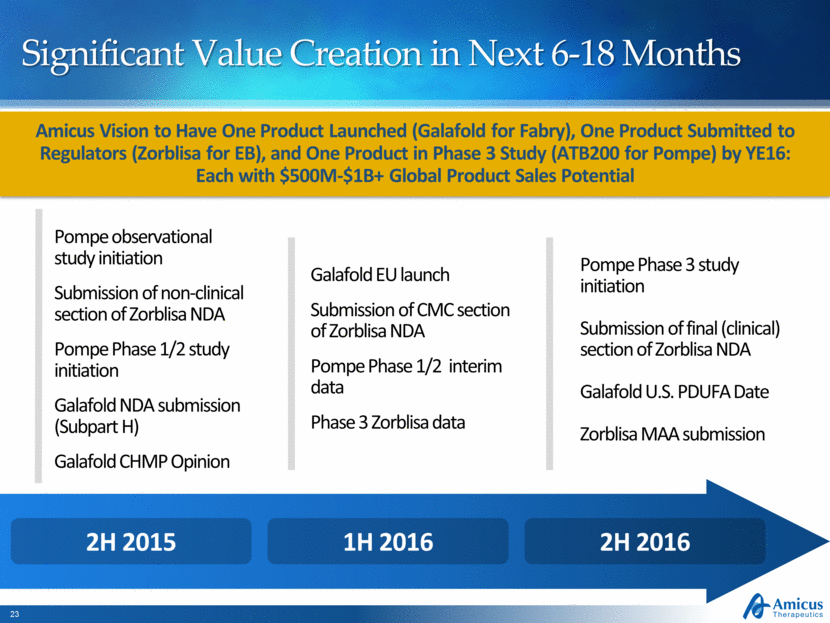
Significant Value Creation in Next 6-18 Months Amicus Vision to Have One Product Launched (Galafold for Fabry), One Product Submitted to Regulators (Zorblisa for EB), and One Product in Phase 3 Study (ATB200 for Pompe) by YE16: Each with $500M-$1B+ Global Product Sales Potential Pompe Phase 3 study initiation Submission of final (clinical) section of Zorblisa NDA Galafold U.S. PDUFA Date Zorblisa MAA submission Galafold EU launch Submission of CMC section of Zorblisa NDA Pompe Phase 1/2 interim data Phase 3 Zorblisa data Pompe observational study initiation Submission of non-clinical section of Zorblisa NDA Pompe Phase 1/2 study initiation Galafold NDA submission (Subpart H) Galafold CHMP Opinion 2H 2016 1H 2016 2H 2015 |
|
|
Amicus Acquisition of Scioderm, Inc. Overview August 31, 2015 |