Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Theravance Biopharma, Inc. | a15-18164_18k.htm |
Exhibit 99.1
|
|
Investor Relations Presentation August 2015 Theravance Biopharma, Inc. (NASDAQ: TBPH) Rick E Winningham, Chief Executive Officer |
|
|
Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, and the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies). The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products and risks associated with establishing and maintaining sales, marketing and distribution capabilities. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-Q filed with the Securities and Exchange Commission (SEC) on May 13, 2015, under the heading "Risk Factors" contained in the Form S-3 Registration Statement filed by Theravance Biopharma with the SEC on July 9, 2015, and other periodic reports filed with the SEC. Cautionary Statement Regarding Forward-Looking Statements |
|
|
Theravance Biopharma Investment Highlights Value Creation Acute Care U.S. Commercial Strategy Led by VIBATIV® Pipeline of High Value Assets Strong Financial Position Productive Research Engine Team Track Record of Success Financial Assets |
|
|
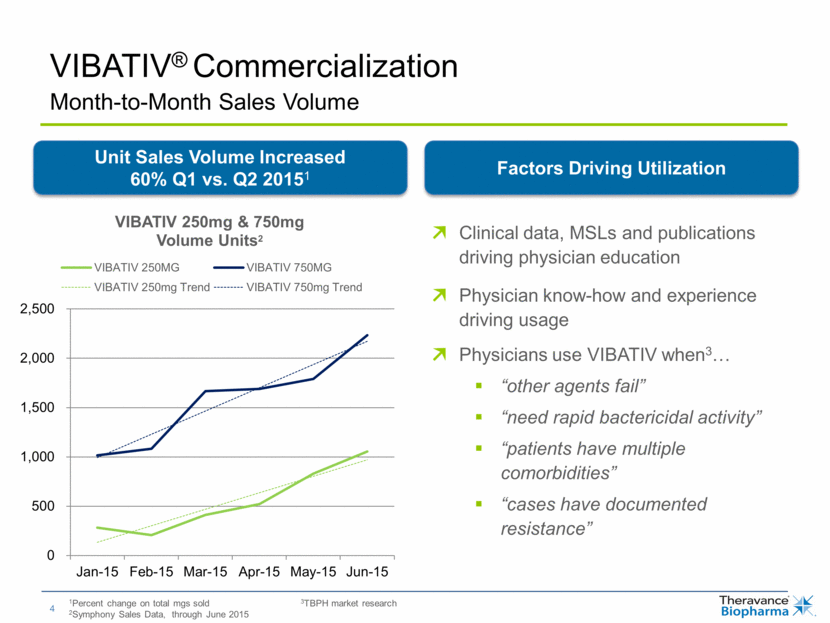
VIBATIV® Commercialization 1Percent change on total mgs sold 2Symphony Sales Data, through June 2015 Clinical data, MSLs and publications driving physician education Physician know-how and experience driving usage Physicians use VIBATIV when3 “other agents fail” “need rapid bactericidal activity” “patients have multiple comorbidities” “cases have documented resistance” Month-to-Month Sales Volume Unit Sales Volume Increased 60% Q1 vs. Q2 20151 Factors Driving Utilization 3TBPH market research 0 500 1,000 1,500 2,000 2,500 Jan-15 Feb-15 Mar-15 Apr-15 May-15 Jun-15 VIBATIV 250mg & 750mg Volume Units 2 VIBATIV 250MG VIBATIV 750MG VIBATIV 250mg Trend VIBATIV 750mg Trend |
|
|
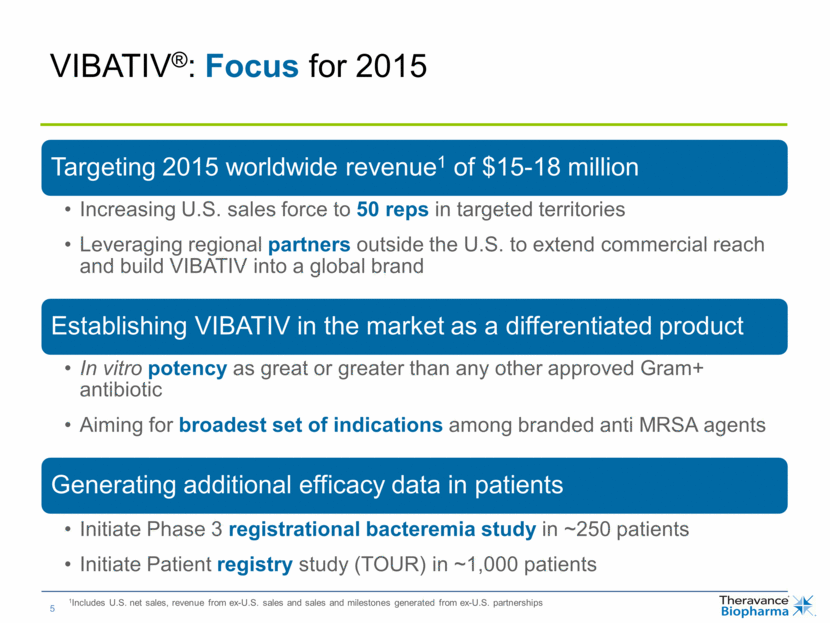
VIBATIV®: Focus for 2015 1Includes U.S. net sales, revenue from ex-U.S. sales and sales and milestones generated from ex-U.S. partnerships Targeting 2015 worldwide revenue1 of $15-18 million Increasing U.S. sales force to 50 reps in targeted territories Leveraging regional partners outside the U.S. to extend commercial reach and build VIBATIV into a global brand Establishing VIBATIV in the market as a differentiated product In vitro potency as great or greater than any other approved Gram+ antibiotic Aiming for broadest set of indications among branded anti MRSA agents Generating additional efficacy data in patients Initiate Phase 3 registrational bacteremia study in ~250 patients Initiate Patient registry study (TOUR) in ~1,000 patients |
|
|
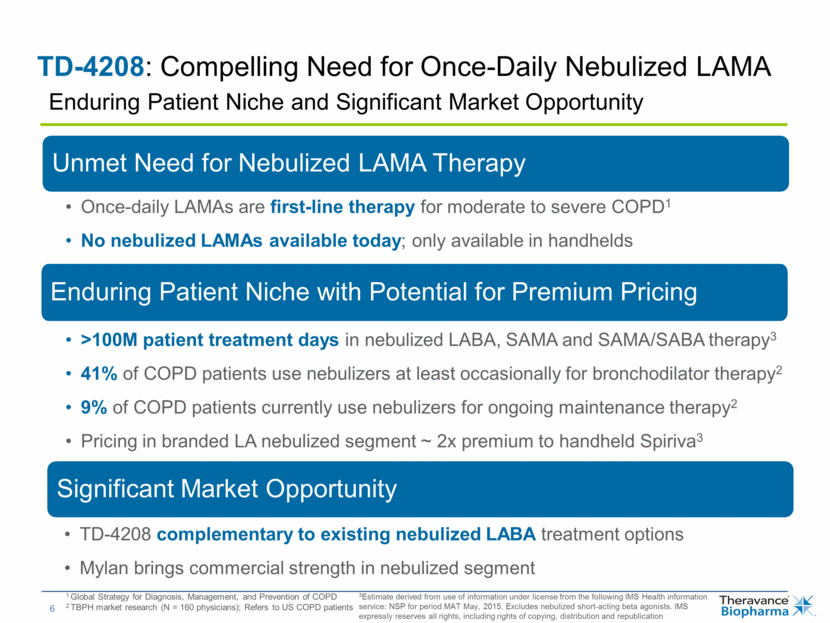
TD-4208: Compelling Need for Once-Daily Nebulized LAMA 1 Global Strategy for Diagnosis, Management, and Prevention of COPD 2 TBPH market research (N = 160 physicians); Refers to US COPD patients 3Estimate derived from use of information under license from the following IMS Health information service: NSP for period MAT May, 2015. Excludes nebulized short-acting beta agonists. IMS expressly reserves all rights, including rights of copying, distribution and republication Enduring Patient Niche and Significant Market Opportunity Unmet Need for Nebulized LAMA Therapy Once-daily LAMAs are first-line therapy for moderate to severe COPD1 No nebulized LAMAs available today; only available in handhelds Enduring Patient Niche with Potential for Premium Pricing >100M patient treatment days in nebulized LABA, SAMA and SAMA/SABA therapy3 41% of COPD patients use nebulizers at least occasionally for bronchodilator therapy2 9% of COPD patients currently use nebulizers for ongoing maintenance therapy2 Pricing in branded LA nebulized segment ~ 2x premium to handheld Spiriva3 Significant Market Opportunity TD-4208 complementary to existing nebulized LABA treatment options Mylan brings commercial strength in nebulized segment |
|
|
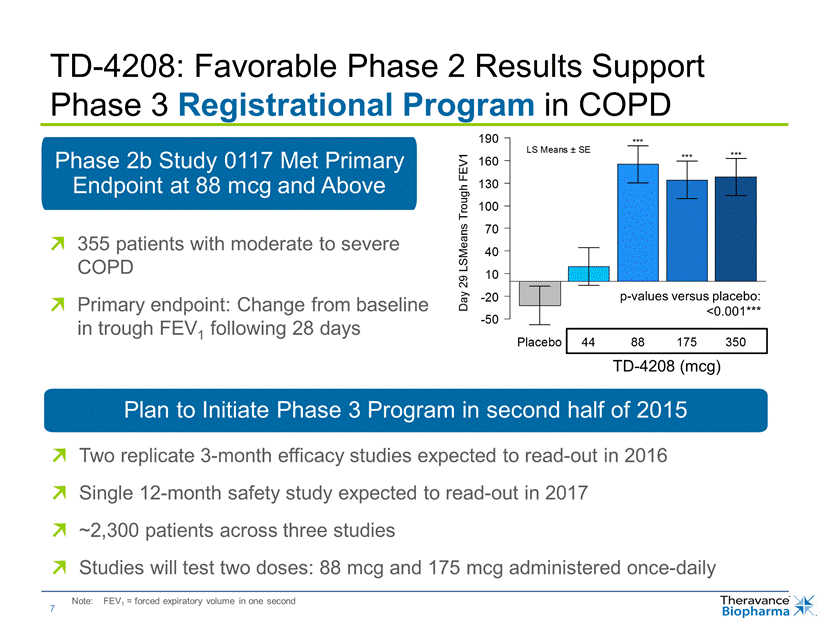
TD-4208: Favorable Phase 2 Results Support Phase 3 Registrational Program in COPD 190 160 130 100 70 40 10 -20 -50 *** *** *** 355 patients with moderate to severe COPD Primary endpoint: Change from baseline in trough FEV1 following 28 days Placebo 44 88 175 350 TD-4208 (mcg) Two replicate 3-month efficacy studies expected to read-out in 2016 Single 12-month safety study expected to read-out in 2017 ~2,300 patients across three studies Studies will test two doses: 88 mcg and 175 mcg administered once-daily Note: FEV1 = forced expiratory volume in one second 7 Day 29 LSMeans Trough FEV1 LS Means ± SE p-values versus placebo: <0.001*** |
|
|
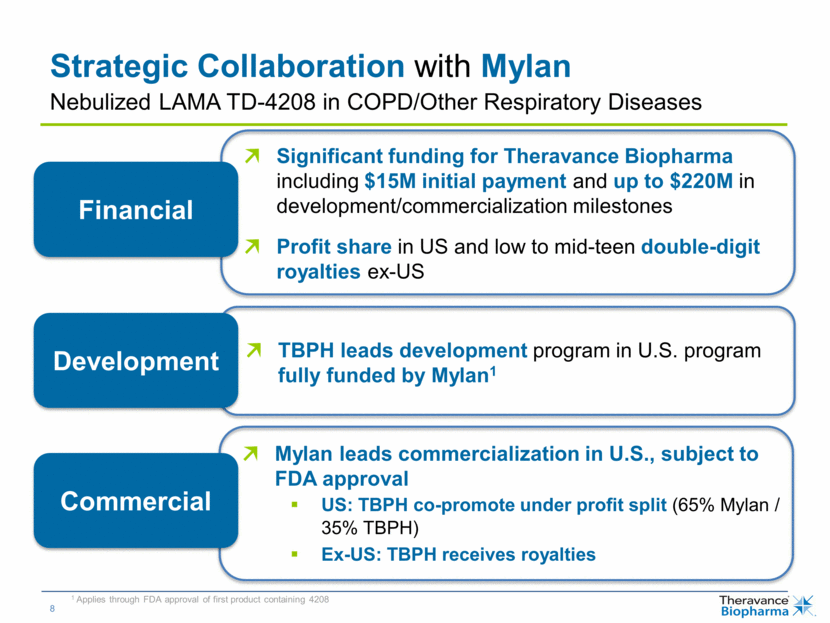
Strategic Collaboration with Mylan Significant funding for Theravance Biopharma including $15M initial payment and up to $220M in development/commercialization milestones Profit share in US and low to mid-teen double-digit royalties ex-US TBPH leads development program in U.S. program fully funded by Mylan1 Mylan leads commercialization in U.S., subject to FDA approval US: TBPH co-promote under profit split (65% Mylan / 35% TBPH) Ex-US: TBPH receives royalties Financial Development Commercial 1 Applies through FDA approval of first product containing 4208 Nebulized LAMA TD-4208 in COPD/Other Respiratory Diseases |
|
|
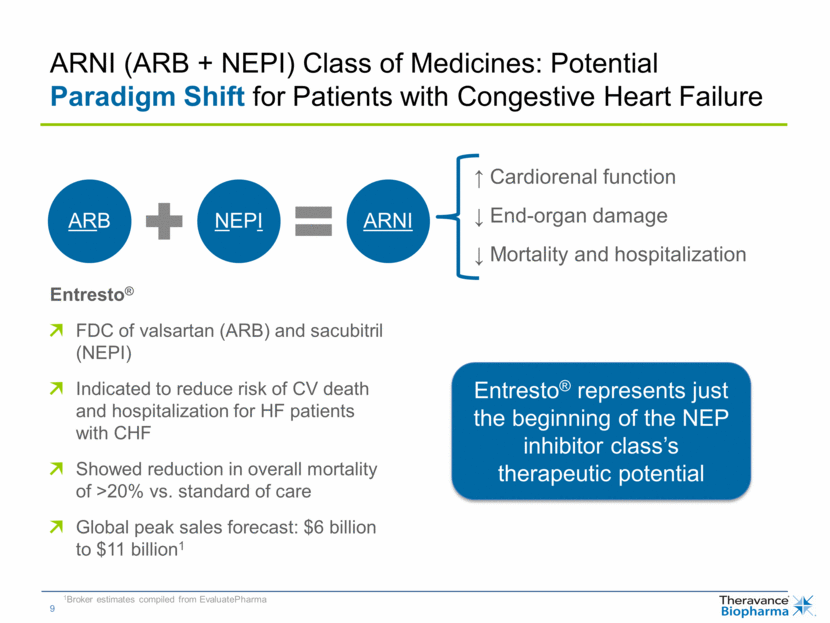
ARNI (ARB + NEPI) Class of Medicines: Potential Paradigm Shift for Patients with Congestive Heart Failure ↑ Cardiorenal function ↓ End-organ damage ↓ Mortality and hospitalization Entresto® FDC of valsartan (ARB) and sacubitril (NEPI) Indicated to reduce risk of CV death and hospitalization for HF patients with CHF Showed reduction in overall mortality of >20% vs. standard of care Global peak sales forecast: $6 billion to $11 billion1 Entresto® represents just the beginning of the NEP inhibitor class’s therapeutic potential 1Broker estimates compiled from EvaluatePharma ARB NEPI ARNI |
|
|
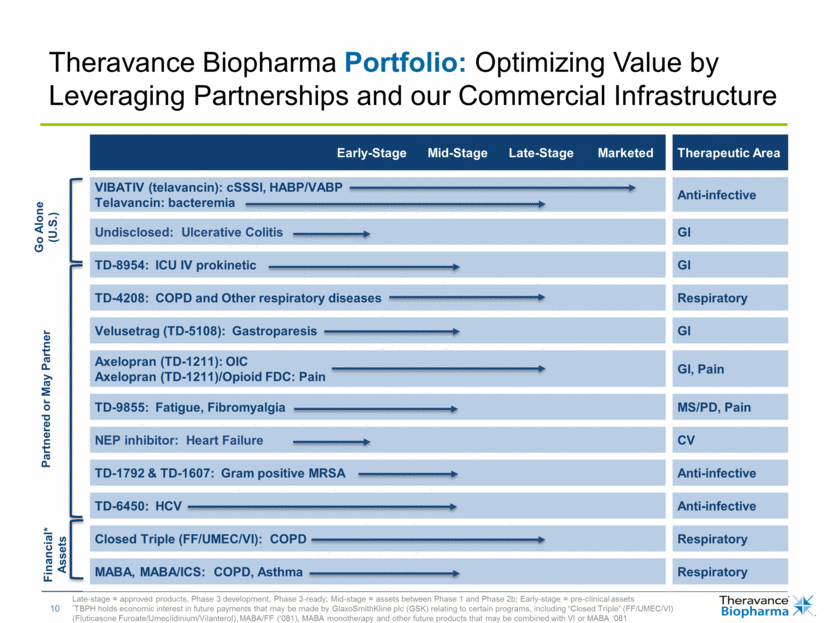
Late-stage = approved products, Phase 3 development, Phase 3-ready; Mid-stage = assets between Phase 1 and Phase 2b; Early-stage = pre-clinical assets *TBPH holds economic interest in future payments that may be made by GlaxoSmithKline plc (GSK) relating to certain programs, including “Closed Triple” (FF/UMEC/VI) (Fluticasone Furoate/Umeclidinium/Vilanterol), MABA/FF (‘081), MABA monotherapy and other future products that may be combined with VI or MABA ‘081 Early-Stage Mid-Stage Late-Stage Marketed Therapeutic Area VIBATIV (telavancin): cSSSI, HABP/VABP Telavancin: bacteremia Anti-infective Undisclosed: Ulcerative Colitis GI TD-8954: ICU IV prokinetic GI TD-4208: COPD and Other respiratory diseases Respiratory Velusetrag (TD-5108): Gastroparesis GI Axelopran (TD-1211): OIC Axelopran (TD-1211)/Opioid FDC: Pain GI, Pain TD-9855: Fatigue, Fibromyalgia MS/PD, Pain NEP inhibitor: Heart Failure CV TD-1792 & TD-1607: Gram positive MRSA Anti-infective TD-6450: HCV Anti-infective Closed Triple (FF/UMEC/VI): COPD Respiratory MABA, MABA/ICS: COPD, Asthma Respiratory Theravance Biopharma Portfolio: Optimizing Value by Leveraging Partnerships and our Commercial Infrastructure Go Alone (U.S.) Partnered or May Partner Financial* Assets |
|
|
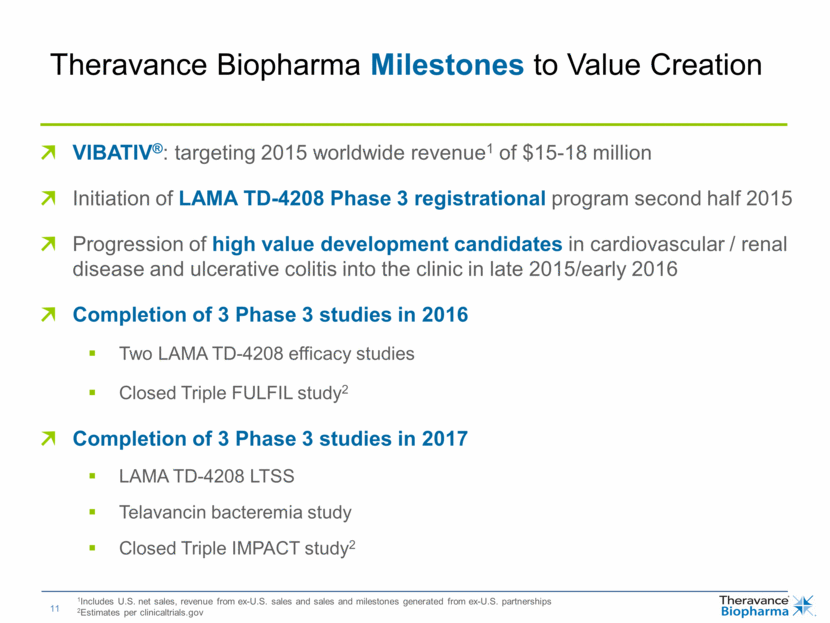
VIBATIV®: targeting 2015 worldwide revenue1 of $15-18 million Initiation of LAMA TD-4208 Phase 3 registrational program second half 2015 Progression of high value development candidates in cardiovascular / renal disease and ulcerative colitis into the clinic in late 2015/early 2016 Completion of 3 Phase 3 studies in 2016 Two LAMA TD-4208 efficacy studies Closed Triple FULFIL study2 Completion of 3 Phase 3 studies in 2017 LAMA TD-4208 LTSS Telavancin bacteremia study Closed Triple IMPACT study2 1Includes U.S. net sales, revenue from ex-U.S. sales and sales and milestones generated from ex-U.S. partnerships 2Estimates per clinicaltrials.gov Theravance Biopharma Milestones to Value Creation |
|
|
Theravance Biopharma Investment Highlights Value Creation Acute U.S. Care Commercial Strategy Led by VIBATIV® Pipeline of High Value Assets Strong Financial Position Productive Research Engine Team Track Record of Success Financial Assets |