Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Sorrento Therapeutics, Inc. | d31279d8k.htm |
Exhibit 99.1
|
|
Sorrento
Therapeutics
Corporate Presentation
Henry Ji, PhD - President and CEO
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
|
|
Disclaimer
Certain statements contained in this presentation or in other documents of Sorrento Therapeutics, Inc. (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward-looking statements” as de_ned in the Private Securities Litigation Reform Act of 1995. These statements can be identi_ed by the fact that they do not relate strictly to historic or current facts. They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,” “anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or _nancial performance or condition. These statements are based upon the current beliefs and expectations of the Company’s management and are subject to signi_-cant risks and uncertainties. Statements regarding future action, future performance and/or future results including, without limitation, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory _lings related to the same, and receipt by the Company of milestone and royalty payments may di_er from those set forth in the forward-looking statements. Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate.
The Company assumes no obligation to update forward-looking statements as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10-K, 10-Q and 8-K reports.
NASDAQ: SRNE
In presenting this material or responding to inquiries in connection with a presentation, management may refer to results, projections or performance measures that are not prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”) as reported in the Company’s SEC _lings. These results, projections or performance measures are Non-GAAP measures and are not intended to replace or as a substitute for results measured under GAAP, but rather as supplement to the GAAP reported results.
Because actual results are a_ected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may di_er materially from those expressed or implied in any forward-looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identi_es some of these factors in its Securities and Exchange Commission (“SEC”) _lings on Forms 10-K, 10-Q and 8-K, and investors are advised to consult the Company’s _lings for a more complete listing of risk factors, contingencies and uncertainties e_ecting the Company and its business and _nancial performance.
Sorrento™, G-MAB™, CAR.TNK™, TNK Therapeutics™, and the Sorrento logo are trademarks owned by Sorrento Therapeutics, Inc.
All other trademarks and trade names are the property of their respective owners.
Logo - http://photos.prnewswire.com/prnh/20150105/167173LOGO
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
2
|
|
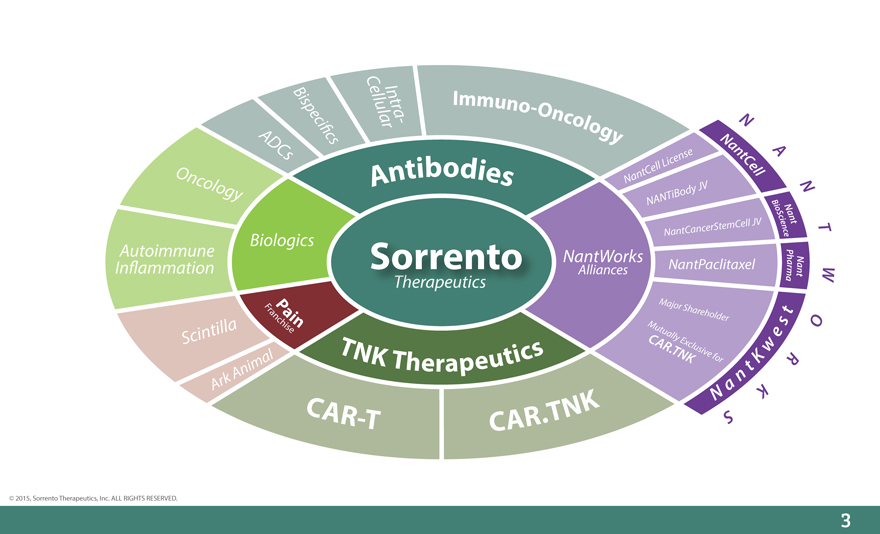
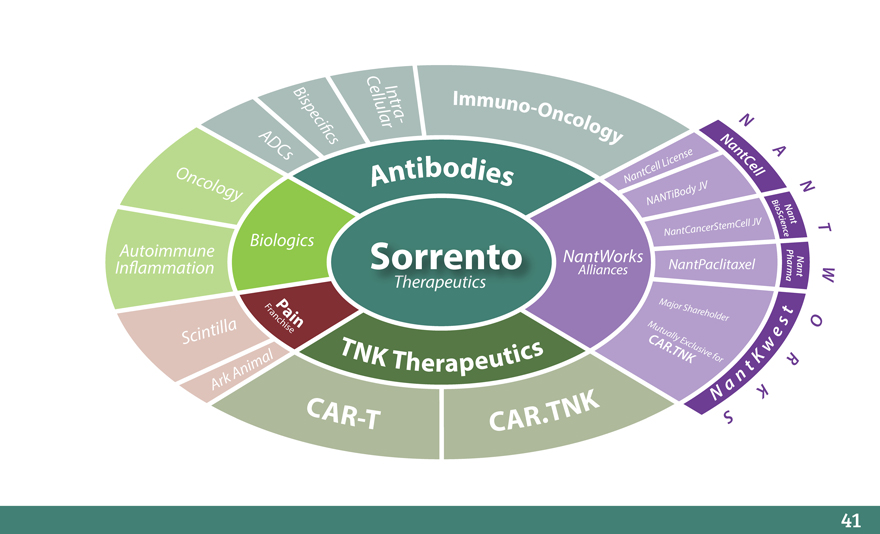
C
Bisp eci_c ellularIntr a - Immuno-Oncology N
ADCs s Nan t A
ellLic ense Cell
Onc Antibodies NantC
olo gy iBody JV N
NANT B
io N
Sc i an
n t
NantC ancerStemCell JV e c T
Biologics e
P
Autoimmune Sorrento NantWorks NantPaclitaxel ha N
In_ammation Alliances r n a
mt W
a
FraP ai Major Shar t
nchise n Mutu eholder s O
Scintilla C ally Ex e
T N K Therapeutics AR.TNKclusive for Kw R
A nimal n t
A rk a
C AR-T CAR.TNK N S K
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
3
|
|
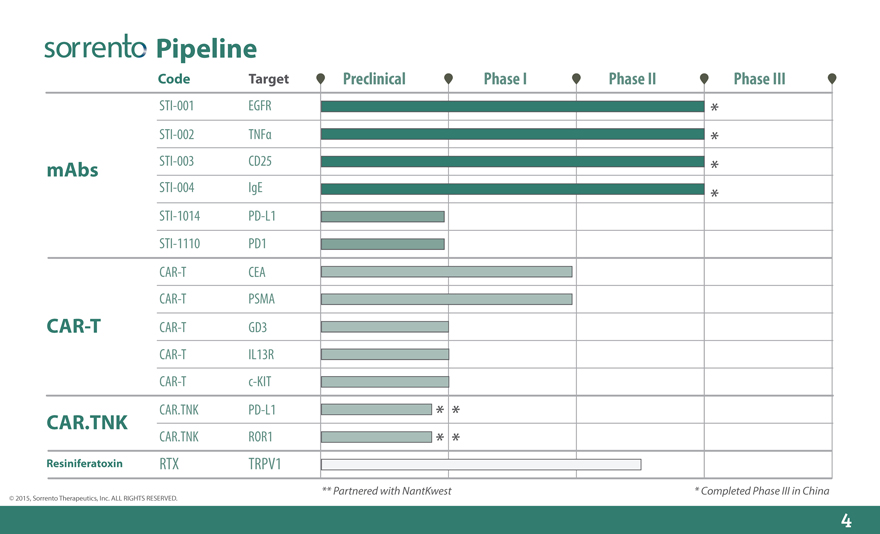
Pipeline
Code Target Preclinical Phase I Phase II Phase III
STI-001 EGFR
STI-002 TNF?
mAbs STI-003 CD25
STI-004 IgE
STI-1014 PD-L1
STI-1110 PD1
CAR-T CEA
CAR-T PSMA
CAR-T CAR-T GD3
CAR-T IL13R
CAR-T c-KIT
CAR.TNK CAR.TNK PD-L1
CAR.TNK ROR1
Resiniferatoxin RTX TRPV1
** Partnered with NantKwest* Completed Phase III in China
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
4
|
|
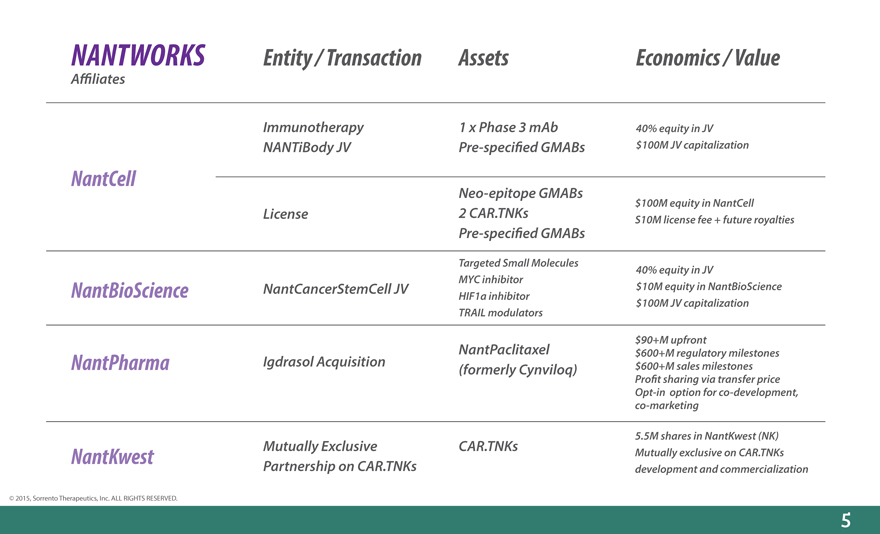
NANTWORKS Entity / Transaction Assets Economics / Value
A_liates
Immunotherapy 1 x Phase 3 mAb 40% equity in JV
NANTiBody JV Pre-speci_ed GMABs $100M JV capitalization
NantCell
Neo-epitope GMABs
$100M equity in NantCell
License 2 CAR.TNKs S10M license fee + future royalties
Pre-speci_ed GMABs
Targeted Small Molecules 40% equity in JV
NantBioScience NantCancerStemCell JV MYC inhibitor $10M equity in NantBioScience
HIF1a inhibitor $100M JV capitalization
TRAIL modulators
$90+M upfront
NantPaclitaxel $600+M regulatory milestones
NantPharma Igdrasol Acquisition(formerly Cynviloq) $600+M sales milestones
Pro_t sharing via transfer price
Opt-in option for co-development,
co-marketing
5.5M shares in NantKwest (NK)
NantKwest Mutually Exclusive CAR.TNKs Mutually exclusive on CAR.TNKs
Partnership on CAR.TNKs development and commercialization
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
5
|
|
Sorrento
Immunotherapy Platform
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
6
|
|
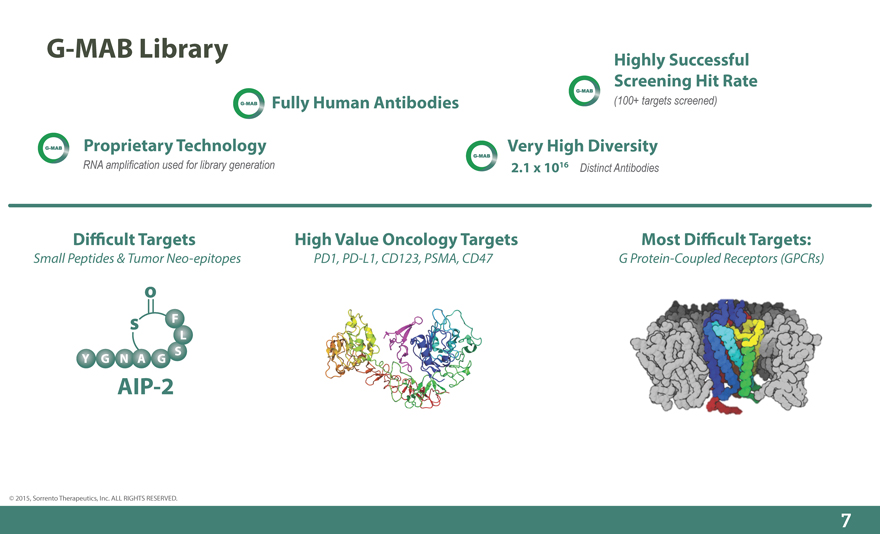
G-MAB Library Highly Successful
Screening Hit Rate
Fully Human Antibodies (100+ targets screened)
Proprietary Technology Very High Diversity
RNA amplification used for library generation 2.1 x 1016 Distinct Antibodies
Di_cult Targets High Value Oncology Targets Most Di_cult Targets:
Small Peptides & Tumor Neo-epitopes PD1, PD-L1, CD123, PSMA, CD47 G Protein-Coupled Receptors (GPCRs)
F
I L
S
Y G N A G
AIP-2
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
7
|
|
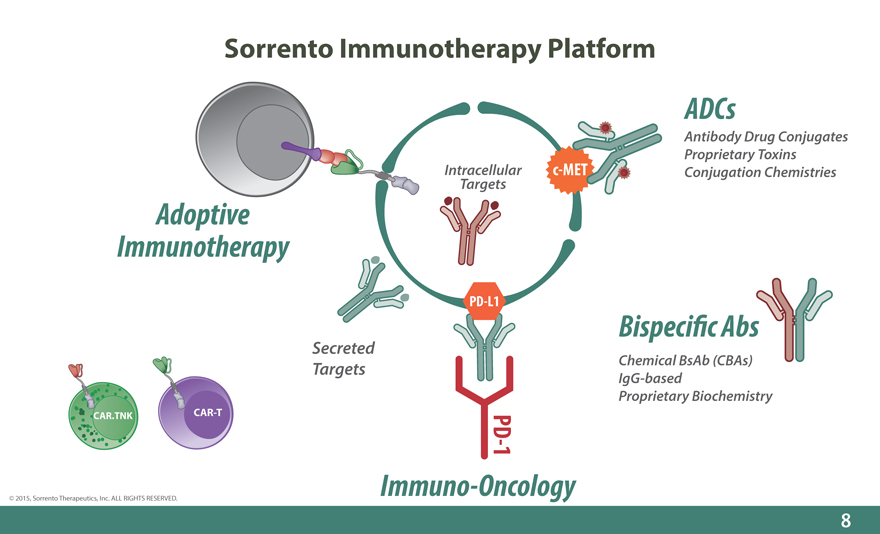
Sorrento Immunotherapy Platform
ADCs
Antibody Drug Conjugates Proprietary Toxins Intracellular c-MET Conjugation Chemistries
Targets
Adoptive Immunotherapy
PD-L1 Bispeci_c Abs
Secreted
Targets Chemical BsAb (CBAs) IgG-based Proprietary Biochemistry
CAR.TNK CAR-T
- PD 1
Immuno-Oncology
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
8
|
|
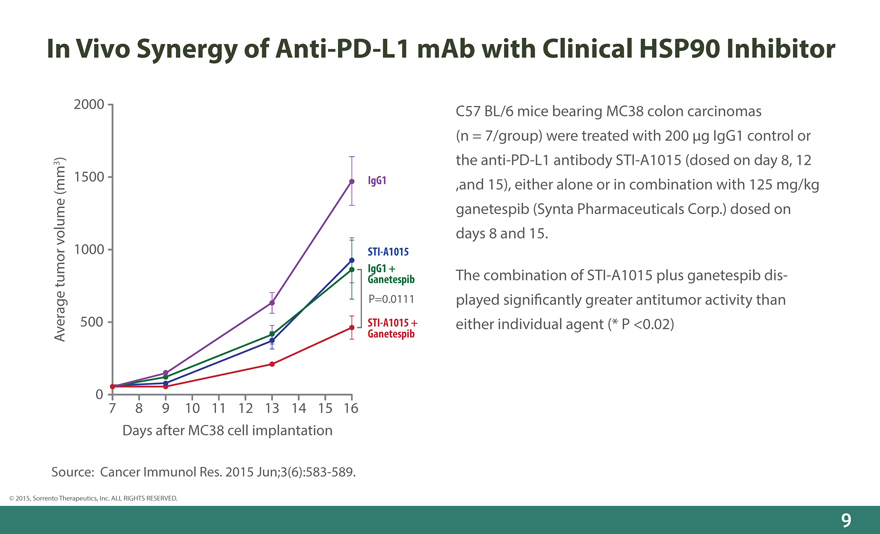
In Vivo Synergy of Anti-PD-L1 mAb with Clinical HSP90 Inhibitor
2000
)
3
(mm 1500 IgG1
volume 1000 STI-A1015
IgG1 +
tumor Ganetespib
P=0.0111
500 STI-A1015 +
Average Ganetespib
0 7 8 9 10 11 12 13 14 15 16
Days after MC38 cell implantation
C57 BL/6 mice bearing MC38 colon carcinomas
(n = 7/group) were treated with 200 _g IgG1 control or the anti-PD-L1 antibody STI-A1015 (dosed on day 8, 12 ,and 15), either alone or in combination with 125 mg/kg ganetespib (Synta Pharmaceuticals Corp.) dosed on days 8 and 15.
The combination of STI-A1015 plus ganetespib displayed signi_cantly greater antitumor activity than either individual agent (* P <0.02)
Source: Cancer Immunol Res. 2015 Jun;3(6):583-589.
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
9
|
|
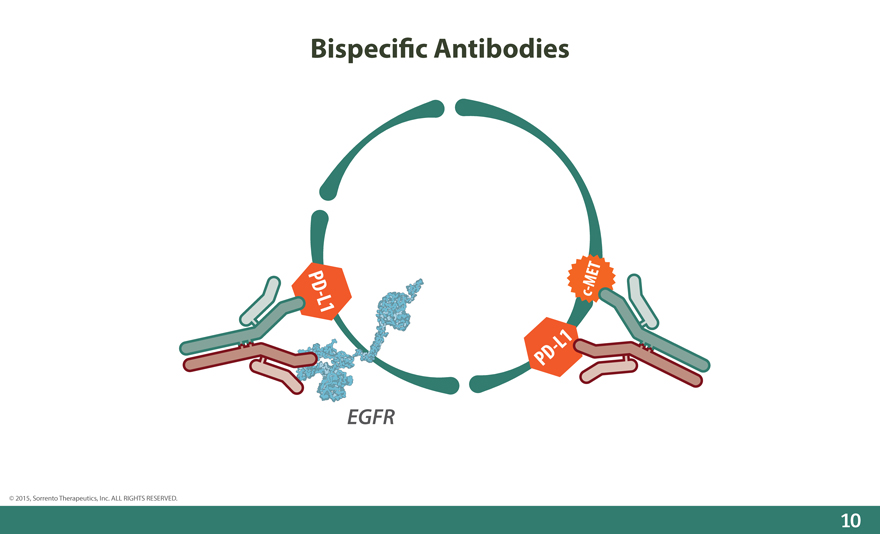
Bispeci_c Antibodies
MET
PD - L1 c -
- L1
PD
EGFR
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
10
|
|
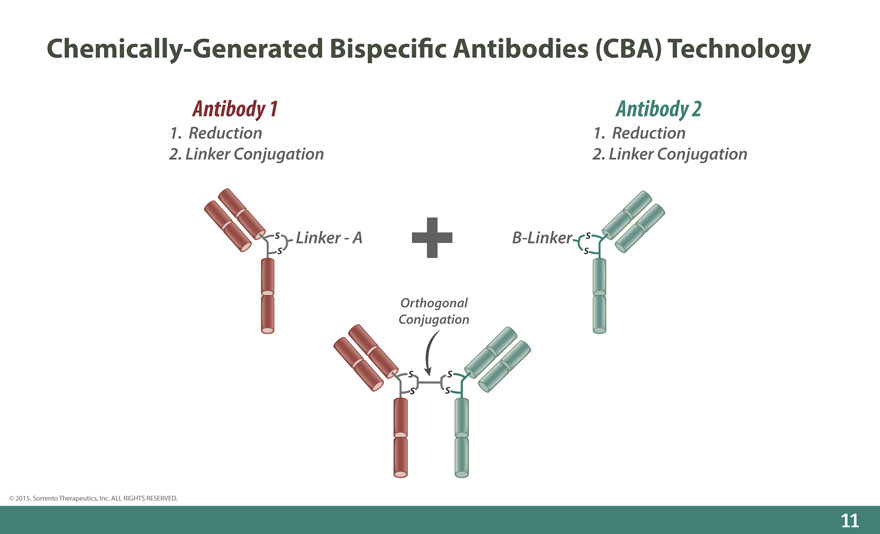
Chemically-Generated Bispeci_c Antibodies (CBA) Technology
Antibody 1 Antibody 2
1. Reduction 1. Reduction
2. Linker Conjugation 2. Linker Conjugation
s Linker - A B-Linker s
s s
Orthogonal Conjugation
s s s s
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
11
|
|
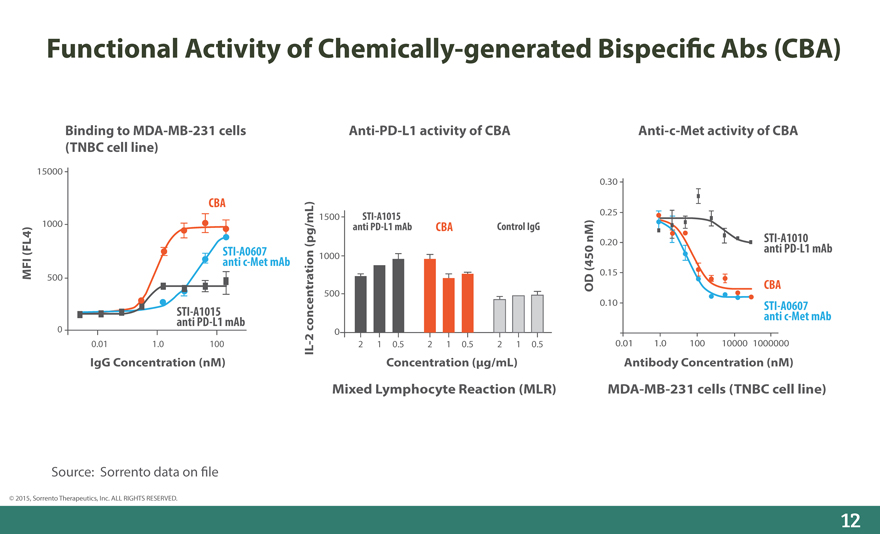
Functional Activity of Chemically-generated Bispeci_c Abs (CBA)
Binding to MDA-MB-231 cells Anti-PD-L1 activity of CBA Anti-c-Met activity of CBA (TNBC cell line)
15000
0.30
CBA
1500 STI-A1015 0.25
1000 anti PD-L1 mAb CBA Control IgG
(pg/mL) 0.20 STI-A1010
(FL4) STI-A0607 1000 nM) anti PD-L1 mAb
anti c-Met mAb(450
MFI 0.15
500 OD CBA
500
0.10 STI-A0607
anti STI-A1015 PD-L1 mAb concentration anti c-Met mAb
0 0
2
0.01 1.0 100 - 2 1 0.5 2 1 0.5 2 1 0.5 0.01 1.0 100 10000 1000000
IL
IgG Concentration (nM) Concentration (_g/mL) Antibody Concentration (nM)
Mixed Lymphocyte Reaction (MLR) MDA-MB-231 cells (TNBC cell line)
Source: Sorrento data on _le
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
12
|
|
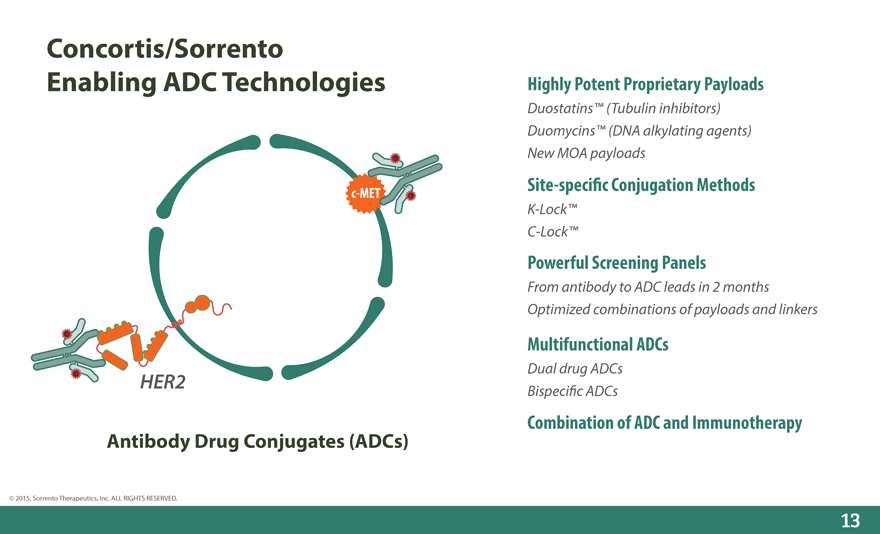
Concortis/Sorrento Enabling ADC Technologies
c-MET
HER2
Antibody Drug Conjugates (ADCs)
Highly Potent Proprietary Payloads
Duostatins™ (Tubulin inhibitors) Duomycins™ (DNA alkylating agents) New MOA payloads
Site-speci_c Conjugation Methods
K-Lock™ C-Lock™
Powerful Screening Panels
From antibody to ADC leads in 2 months Optimized combinations of payloads and linkers
Multifunctional ADCs
Dual drug ADCs Bispeci_c ADCs
Combination of ADC and Immunotherapy
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
13
|
|
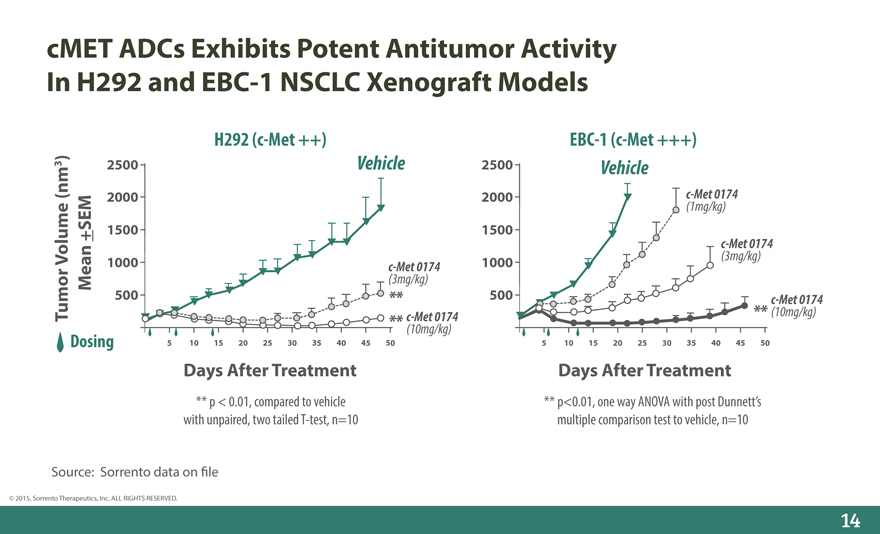
cMET ADCs Exhibits Potent Antitumor Activity In H292 and EBC-1 NSCLC Xenograft Models
H292 (c-Met ++) EBC-1 (c-Met +++)
)
3 2500 Vehicle 2500 Vehicle
(nm 2000 2000 c-Met 0174
(1mg/kg)
1500 1500
c-Met 0174
Volume +SEM 1000 c-Met 0174 1000(3mg/kg)
Mean(3mg/kg)
Tumor 500** c-Met 0174 500** (10mg/kg) c-Met 0174
** (10mg/kg)
Dosing 5 10 15 20 25 30 35 40 45 50 5 10 15 20 25 30 35 40 45 50
Days After Treatment
** p < 0.01, compared to vehicle with unpaired, two tailed T-test, n=10
Days After Treatment
** p<0.01, one way ANOVA with post Dunnett’s multiple comparison test to vehicle, n=10
Source: Sorrento data on _le
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
|
|
BioSimilars
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
15
|
|
STI-001 BioSimilar / BioBetter mAb to Cetuximab
Cetuximab – Eli Lilly/BMS/Merck KGaA
Backgrounds Chimeric monoclonal antibody that binds to the extracellular domain of the epidermal growth factor receptor (EGFR) Binding of Erbitux to EGFR interferes with ligand-mediated activation of the receptor’s intracellular tyrosine kinase activity, thus inhibiting EGFR-mediated signaling.
May induce immune system activation through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC)
Approvals / - With irinotecan in advanced colorectal cancer
Indications - With chemotherapy in late-stage metastatic head and neck cancer
- With FOLFIRI for 1st-line treatment of metastatic colorectal cancer
Market Size 2014: approximately $1.9B globally ($680m in US)1 2013: approximately $1.9B globally ($680m in US)1
Future sales forecast – decrease in sales from $1.9B in 2015 to $1B by 20201
Source: DataMonitor Healthcare (www.datamonitorhealthcare.com)
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
16
|
|
STI-002 BioSimilar / BioBetter mAb to In_iximab
Remicade In_iximab – Janssen
INFLIXIMAB
Backgrounds Chimeric monoclonal antibody against tumour necrosis factor alpha (TNF-?)
Approvals / Crohn’s Disease, pediatric Crohn’s Disease, Ulcerative Collitis, pediatric Ulcerative Collitis, Indications Rheumatoid Arthritis, Psoriatic Arthritis, Akylosing Spondylitis, Plaque Psoriasis Market Size Marketing rights divided between Janssen Biotech/Merck/Mitsubishi Tanabe 2014: approximately $9B in sales globally ($5B in US) 2013: approximately $9B in sales globally ($5B in US) Future sales forecast – decrease in sales from $8.9B in 2015 to $7.4B by 2020
Source:
Source: DataMonitor Healthcare (www.datamonitorhealthcare.com)
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
17
|
|
STI-003 BioSimilar / BioBetter mAb to Basiliximab
Basiliximab – Novartis1
basiliximab
Backgrounds Chimeric monoclonal antibody (IgG1k ) that binds to and blocks the binding of IL-2 to IL-2Ra (aka CD25)
on the surface of activated T-lymphocytes.
This speci_c, high a_nity binding to IL-2Ra competitively inhibits IL-2-mediated activation of
lymphocytes, a critical pathway in the cellular immune response involved in allograft rejection.
Approvals / Indicated for the prophylaxis of acute organ rejection in patients receiving renal transplantation when
Indications used as part of an immunosuppressive regimen that includes cyclosporin and corticosteroids.
Market Size 2012: approximately $117M globally
2013: approximately $114M globally
Future sales forecast – decreases in sales as the result of SPC protection loss in EU as of April 20132
1 Source: Simulect® package insert - https://www.pharma.us.novartis.com/product/pi/pdf/simulect.pdf
2 Source: http://www.genericsweb.com/Pipeline%20Watch/Pipeline%20Watch%20April%202013%20-%20Basiliximab.pdf
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
18
|
|
STI-004 BioSimilar mAb to Omalizumab
Omalizumab – Novartis/Genentech
Backgrounds Xolair (omalizumab; Roche/Novartis), a humanized monoclonal antibody product, inactivates
immunoglobulin E (IgE), preventing the in_ammatory events that lead to asthma exacerbations.
Omalizumab inhibits the binding of IgE to the high-a_nity IgE receptor on the surface of mast cells and
basophils.
This is the only FDA approved biologic used in treatment of asthma.
Approvals / US/EU: Moderate to severe allergy-related asthma that is inadequately controlled with inhaled
corticosteroids alone for patients 12 years or older
Indications chronic idiopathic urticaria/chronic spontaneous urticaria (CIU/CSU)
EU: Children 6-11 years with severe persistent allergic asthma
Market Size 2014: approximately $1.8B globally ($1.1B in US)
2013: approximately $1.5B globally ($850m in US)
Future sales forecast – $2B globally in 2015, $2.2B in 2016, decrease to $1.8B by 2020
Source: DataMonitor Healthcare (www.datamonitorhealthcare.com)
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
19
|
|
Harnessing
T Cell Adaptive and Innate NK92 Immunity
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
20
|
|
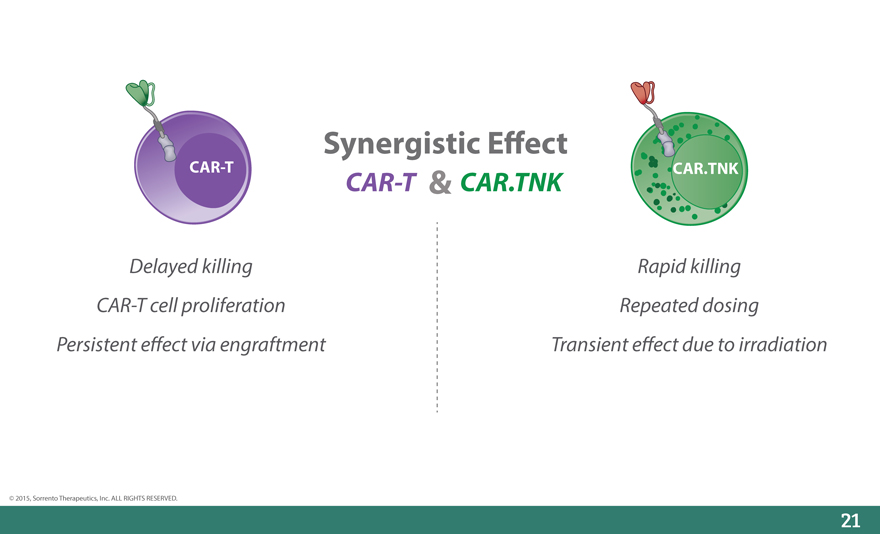
Synergistic E_ect
CAR-T CAR-T & CAR.TNK CAR.TNK
Delayed killing Rapid killing
CAR-T cell proliferation Repeated dosing
Persistent e_ect via engraftment Transient e_ect due to irradiation
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
21
|
|
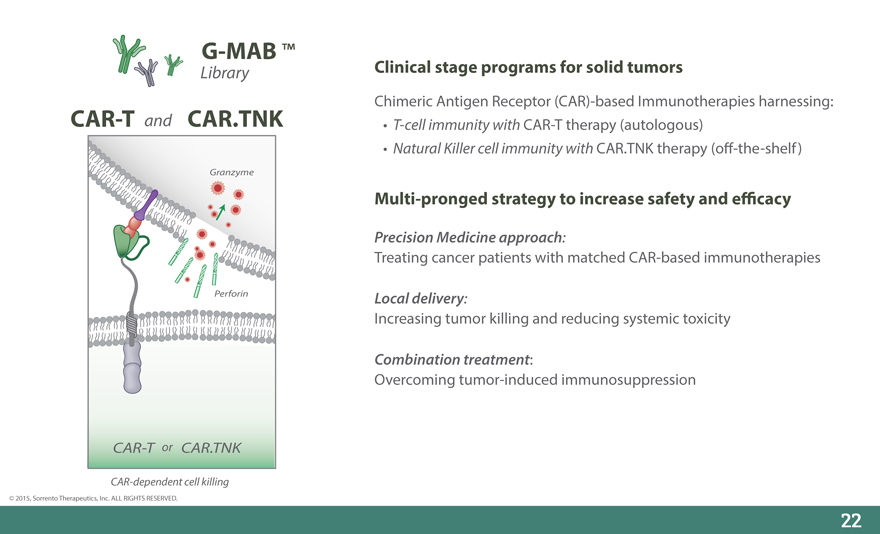
G-MAB
Library
CAR-T and CAR.TNK
Granzyme
Perforin
CAR-T or CAR.TNK
CAR-dependent cell killing
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
Clinical stage programs for solid tumors
Chimeric Antigen Receptor (CAR)-based Immunotherapies harnessing: • T-cell immunity with CAR-T therapy (autologous)
Natural Killer cell immunity with CAR.TNK therapy (o_-the-shelf)
Multi-pronged strategy to increase safety and e_cacy
Precision Medicine approach:
Treating cancer patients with matched CAR-based immunotherapies
Local delivery:
Increasing tumor killing and reducing systemic toxicity
Combination treatment:
Overcoming tumor-induced immunosuppression
22
|
|
Positioning
NK
T-cells Natural Killer cells
CAR-T CAR.TNK
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
23
|
|
Therapeutic Platform
CAR-T Technology Programs Acquisition G-MAB CAR.TNK
CAR-T NK92
Fully human antibody library
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
24
|
|
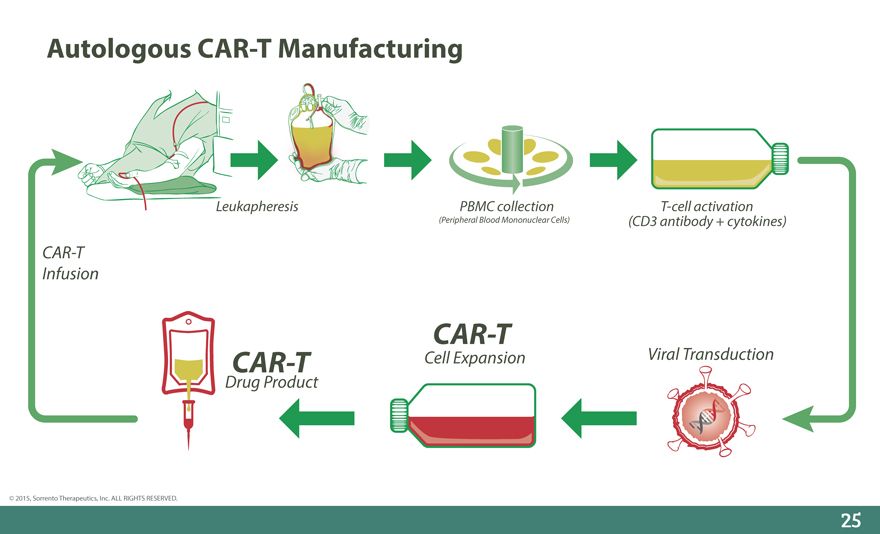
Autologous CAR-T Manufacturing
Leukapheresis PBMC collection T-cell activation
(Peripheral Blood Mononuclear Cells) (CD3 antibody + cytokines)
CAR-T
Infusion
CAR-T
CAR-T Cell Expansion Viral Transduction
Drug Product
ento Therapeutics, Inc. ALL RIGHTS RESERVED.
|
|
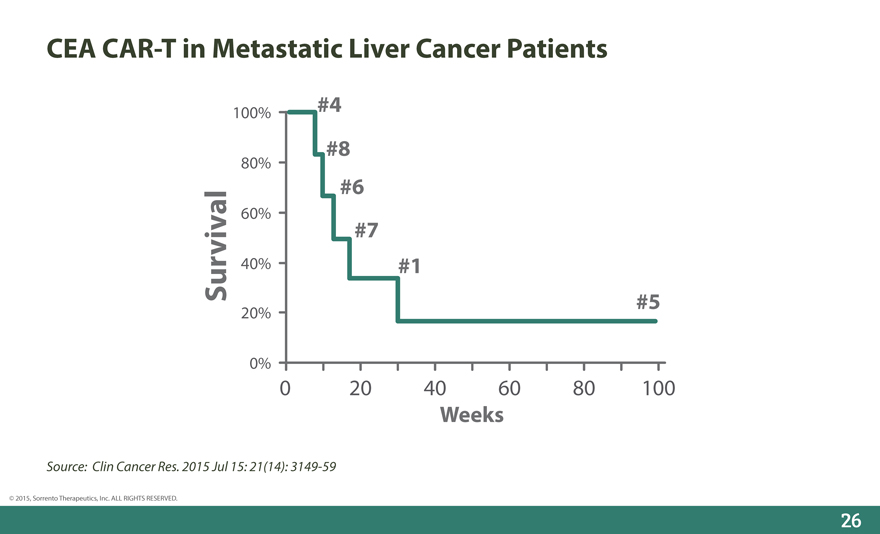
CEA CAR-T in Metastatic Liver Cancer Patients
100% #4
#8
80%
#6
60%
#7
Survival 40% #1
20% #5
0%
0 20 40 60 80 100
Weeks
Source: Clin Cancer Res. 2015 Jul 15: 21(14): 3149-59
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
26
|
|
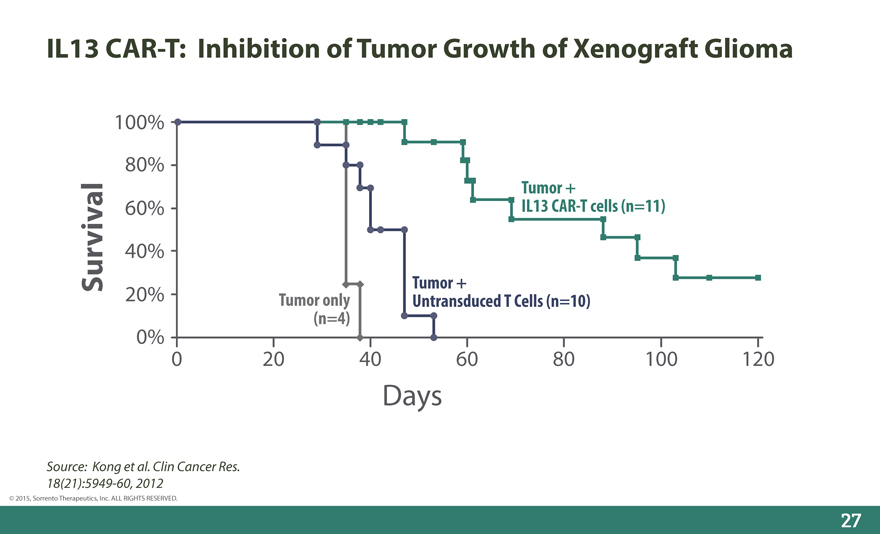
IL13 CAR-T: Inhibition of Tumor Growth of Xenograft Glioma
100%
80%
Tumor +
60% IL13 CAR-T cells (n=11)
40%
Survival Tumor +
20% Tumor only Untransduced T Cells (n=10)
(n=4)
0%
0 20 40 60 80 100 120
Days
Source: Kong et al. Clin Cancer Res._
18(21):5949-60, 2012
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
27
|
|
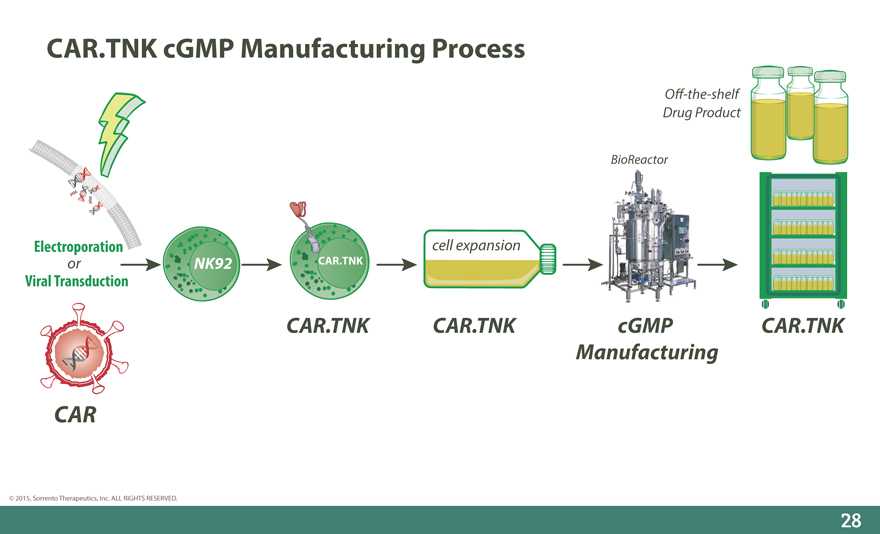
CAR.TNK cGMP Manufacturing Process
O_-the-shelf Drug Product
BioReactor
Electroporation cell expansion
or NK92 CAR.TNK
Viral Transduction
CAR.TNK CAR.TNK cGMP CAR.TNK Manufacturing
CAR
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
28
|
|
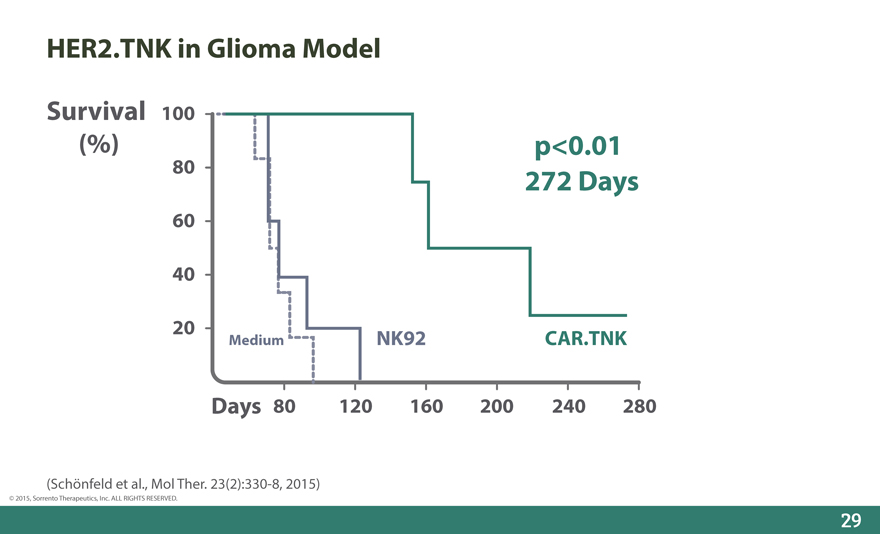
HER2.TNK in Glioma Model
Survival 100
(%) p<0.01
80 272 Days
60
40
20 Medium NK92 CAR.TNK
Days 80 120 160 200 240 280
(Schönfeld et al., Mol Ther. 23(2):330-8, 2015)
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
29
|
|
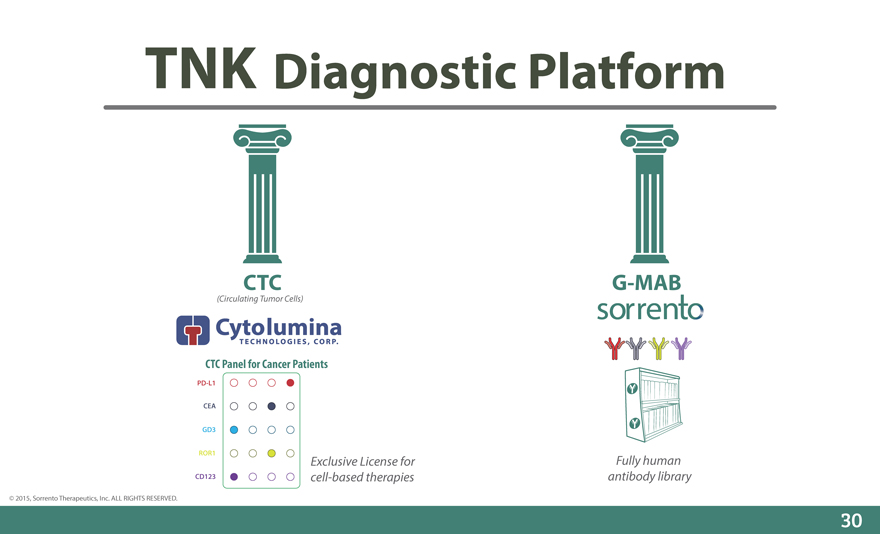
Diagnostic Platform
CTC G-MAB
(Circulating Tumor Cells)
CTC Panel for Cancer Patients
PD-L1
CEA
GD3
ROR1
Exclusive License for Fully human
CD123 cell-based therapies antibody library
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
|
|
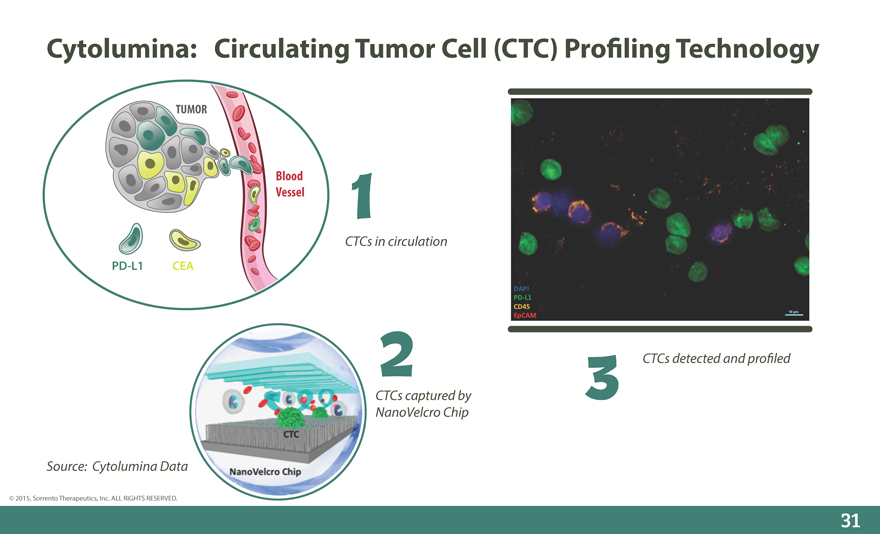
Cytolumina: Circulating Tumor Cell (CTC) Pro_ling Technology
TUMOR
Blood Vessel
CTCs in circulation
PD-L1 CEA
CTCs detected and pro_led
CTCs captured by NanoVelcro Chip
Source: Cytolumina Data
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED
31
|
|
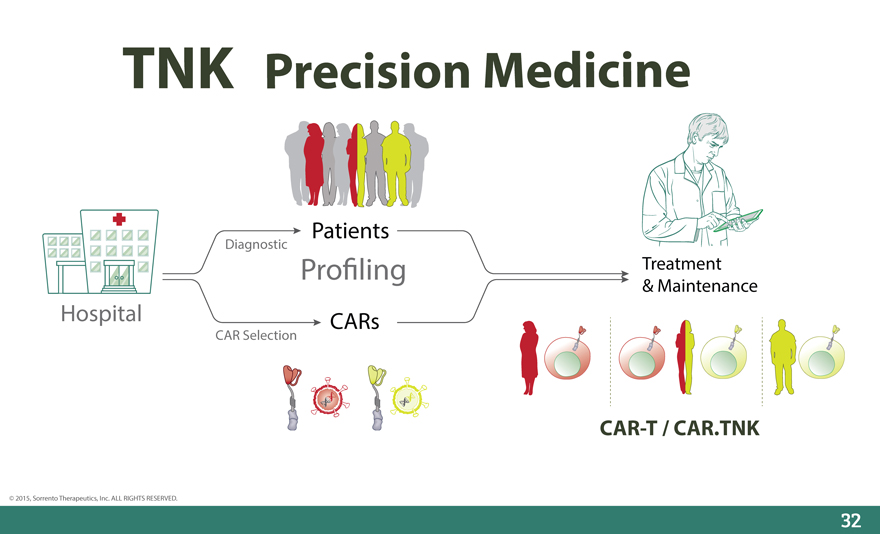
Precision Medicine
Patients
Diagnostic
Pro_ling Treatment
& Maintenance
Hospital CARs
CAR Selection
CAR-T / CAR.TNK
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
32
|
|
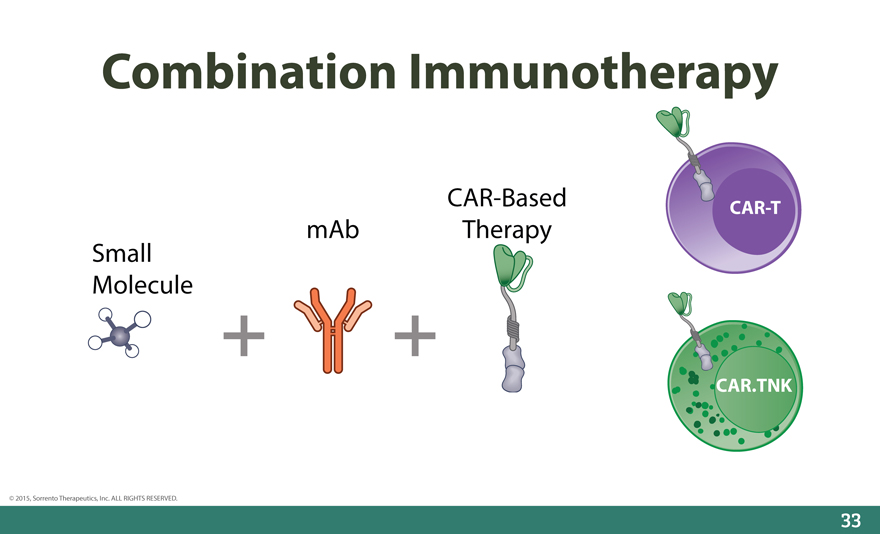
Combination Immunotherapy
CAR-Based CAR-T mAb Therapy Small Molecule
CAR.TNK
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
33
|
|
Pain Franchise
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
34
|
|
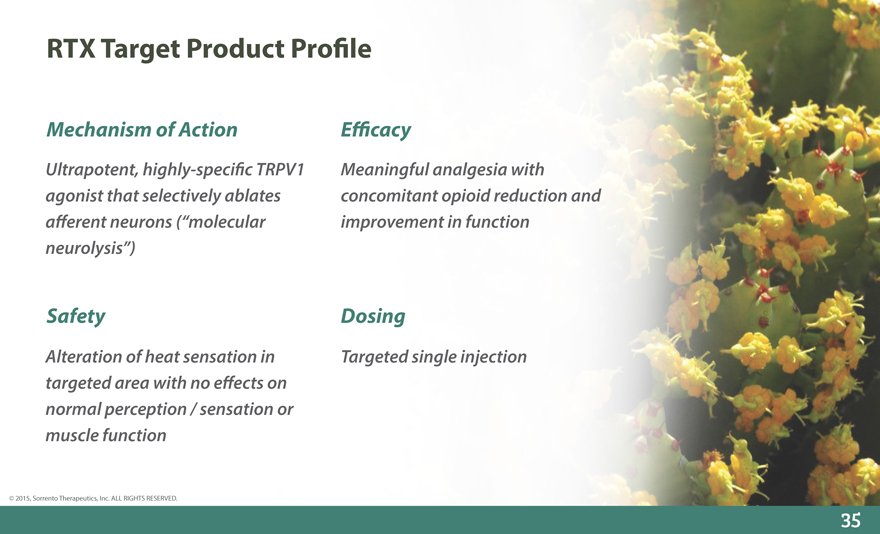
Mechanism of Action E_cacy
Ultrapotent, highly-speci_c TRPV1 Meaningful analgesia with
agonist that selectively ablates concomitant opioid reduction and
a_erent neurons (“molecular improvement in function
neurolysis”)
Safety Dosing
Alteration of heat sensation in Targeted single injection
targeted area with no e_ects on
normal perception / sensation or
muscle function
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
35
|
|
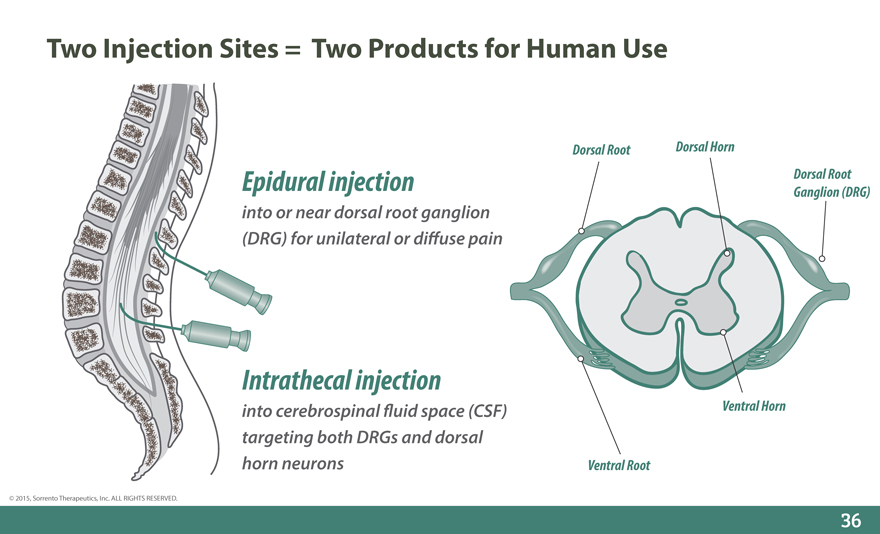
Two Injection Sites = Two Products for Human Use
Dorsal Root Dorsal Horn
Epidural injection Dorsal Root
Ganglion (DRG)
into or near dorsal root ganglion (DRG) for unilateral or di_use pain
Intrathecal injection
into cerebrospinal _uid space (CSF) Ventral Horn targeting both DRGs and dorsal horn neurons Ventral Root
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
36
|
|
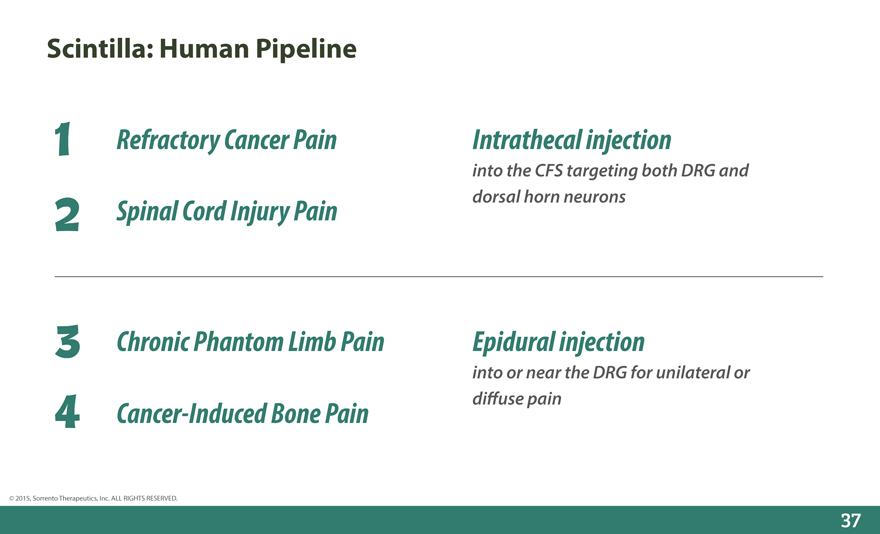
Scintilla: Human Pipeline
1 Refractory Cancer Pain Intrathecal injection
into the CFS targeting both DRG and
2 Spinal Cord Injury Pain dorsal horn neurons
3 Chronic Phantom Limb Pain Epidural injection
into or near the DRG for unilateral or
4 Cancer-Induced Bone Pain di_use pain
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
37
|
|
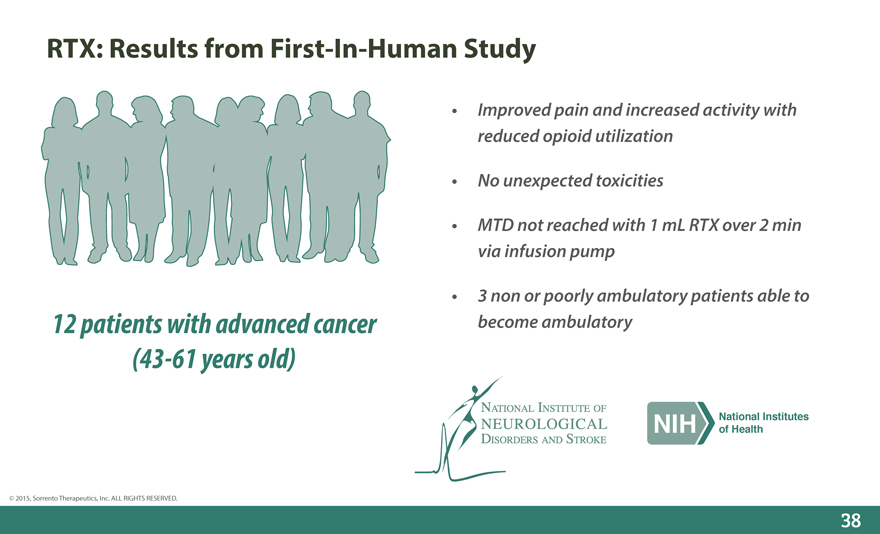
RTX: Results from First-In-Human Study
Improved pain and increased activity with reduced opioid utilization
No unexpected toxicities
MTD not reached with 1 mL RTX over 2 min via infusion pump
3 non or poorly ambulatory patients able to
12 patients with advanced cancer become ambulatory (43-61 years old)
NIH National of Health Institutes
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
38
|
|
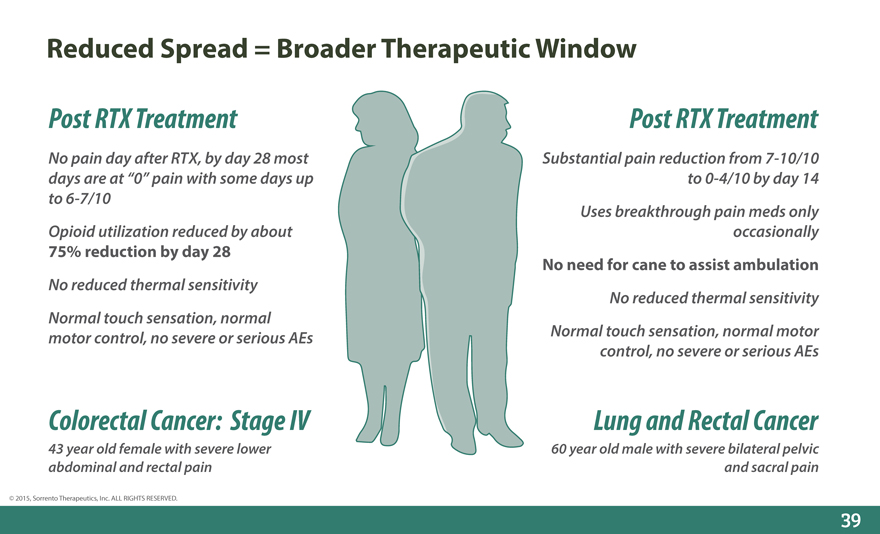
Reduced Spread = Broader Therapeutic Window
Post RTX Treatment Post RTX Treatment
No pain day after RTX, by day 28 most Substantial pain reduction from 7-10/10 days are at “0” pain with some days up to 0-4/10 by day 14 to 6-7/10 Uses breakthrough pain meds only Opioid utilization reduced by about occasionally
75% reduction by day 28
No need for cane to assist ambulation
No reduced thermal sensitivity
No reduced thermal sensitivity Normal touch sensation, normal motor control, no severe or serious AEs Normal touch sensation, normal motor control, no severe or serious AEs
Colorectal Cancer: Stage IV Lung and Rectal Cancer
43 year old female with severe lower 60 year old male with severe bilateral pelvic abdominal and rectal pain and sacral pain
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
|
|
39
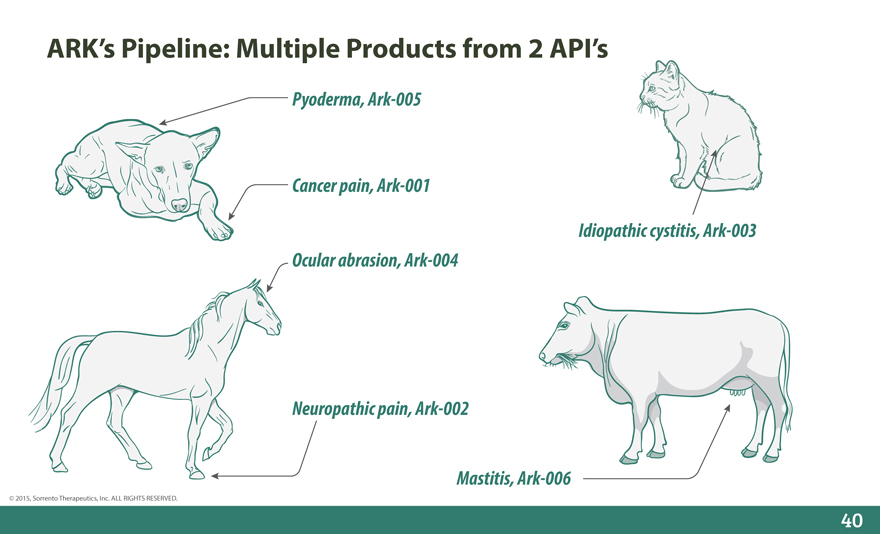
ARK’s Pipeline: Multiple Products from 2 API’s
Pyoderma, Ark-005
Cancer pain, Ark-001
Idiopathic cystitis, Ark-003 Ocular abrasion, Ark-004
Neuropathic pain, Ark-002
Mastitis, Ark-006
© 2015, Sorrento Therapeutics, Inc. ALL RIGHTS RESERVED.
40
|
|
C
Bisp eci_c ellularIntr a - Immuno-Oncology N
ADCs s Nan t A
ellLic ense Cell
Onc Antibodies NantC
olo gy iBody JV N
NANT B
io N
Sc i an
n t
NantC ancerStemCell JV e c T
Biologics e
P
Autoimmune Sorrento NantWorks NantPaclitaxel ha N
In_ammation Alliances r n a
mt W
a
FraP ai Major Shar t
nchise n Mutu eholder s O
Scintilla C ally Ex e
T N K Therapeutics AR.TNKclusive for Kw R
A nimal n t
A rk a
C AR-T CAR.TNK N S K
41
|
|
Sorrento
Therapeutics
Contact
Henry Ji, PhD - President and CEO
hji@sorrentotherapeutics.com (858) 668-6923
42