Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AERIE PHARMACEUTICALS INC | d25675d8k.htm |
 1 Aerie Pharmaceuticals, Inc. Company Overview August 12, 2015 Building a Major Ophthalmic Pharmaceutical Company Exhibit 99.1 |
 2 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our
product candidates being approved by regulatory authorities. In addition, any
discussion of clinical trial results for Rhopressa
TM relate to the results in its first Phase 3 registration trial, Rocket 1, and for
Roclatan TM relate to the results in its Phase 2b clinical trial. The information in this presentation is current only as of its date and may have changed or may change
in the future. We undertake no obligation to update this information in light of new
information, future events or otherwise. We are not making any
representation or warranty that the information in this presentation is
accurate or complete. Certain statements in this presentation are
“forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,”
“expects,” “anticipates,” “plans,”
“intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans
and expectations. Known and unknown risks, uncertainties and other factors could cause
actual results to differ materially from those contemplated by the
statements. In evaluating these statements, you should specifically
consider various factors that may cause our actual results to differ materially from any forward-looking statements. In particular, the preclinical research discussed in this presentation is
preliminary and the outcome of such preclinical studies may not be predictive of the
outcome of later trials. Any future clinical trial results may not
demonstrate safety and efficacy sufficient to obtain regulatory approval
related to the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC,
particularly in the sections titled “Risk Factors” and
“Management’s Discussion and Analysis of Financial Condition
and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements,
whether because of new information, future events or otherwise, except as otherwise
required by law. |
 3 Current Aerie Products: Once-Daily IOP-Lowering Eye Drops for Glaucoma Pre-Clinical Research Findings • Rhopressa™ shows potential to modify diseased tissue • May block fibrotic response in trabecular meshwork cells • May increase perfusion of the trabecular meshwork • AR-13154 shows potential for the treatment of wet AMD • May inhibit ROCK/JAK/PDGFR-ß • Lesion size reduction in rats exceeds market-leading product • Triple action Rhopressa™ • Inhibits ROCK and NET, lowers EVP, targets diseased tissue • P3 programs ongoing • Quadruple Action Roclatan™ • Fixed combination of Rhopressa™ and latanoprost • P2b achieved all clinical endpoints, P3 to start mid-2015 • Potentially most efficacious IOP-lowering therapy Aerie – Building a Major Ophthalmic Pharmaceutical Company These new preclinical discoveries represent potential breakthroughs Full patent protection through at least 2030; Blockbuster Potential |
 4 Ciliary Processes Cornea Uveoscleral Outflow NET RKI NET RKI Trabecular Meshwork Episcleral Veins Schlemm’s Canal Latanoprost Rhopressa ™ Triple-Action Rhopressa TM Quadruple-Action Roclatan™ IOP-Lowering Mechanisms Rhopressa TM : ROCK inhibition relaxes TM, increases outflow NET inhibition reduces fluid production ROCK inhibition lowers Episcleral Venous Pressure (EVP) Roclatan TM also adds latanoprost: PGA receptor activation increases uveoscleral outflow |
 5 increase Decreases Fluid Inflow/Production (Ciliary Processes) Increases Fluid Outflow: Secondary Drain (Uveoscleral Pathway) Increases Fluid Outflow: Primary Drain-Trabecular Meshwork
(TM); Lowers EVP -
(Episcleral Venous Pressure) Rhopressa™ Rhopressa™ Roclatan™ Roclatan™ AA, BB, CAI AA, BB, CAI PGAs PGAs Aerie Products Cover the IOP-lowering Spectrum |
 6 Baseline IOP* ~80% of U.S. Glaucoma Patients Have IOPs that are 26 mmHg at Time of Diagnosis The Baltimore Eye Survey * Sommer A, Tielsch JM, Katz J et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black
Americans: The Baltimore eye survey. Arch Ophthalmol 1991;109:1090-1095 . 60% 20% 20% >26 - <35 mmHg Between 75% and 80% of Patients with IOP 25mmHg 21 mmHg (Normal Tension Glaucoma) >21 - 26 mmHg |
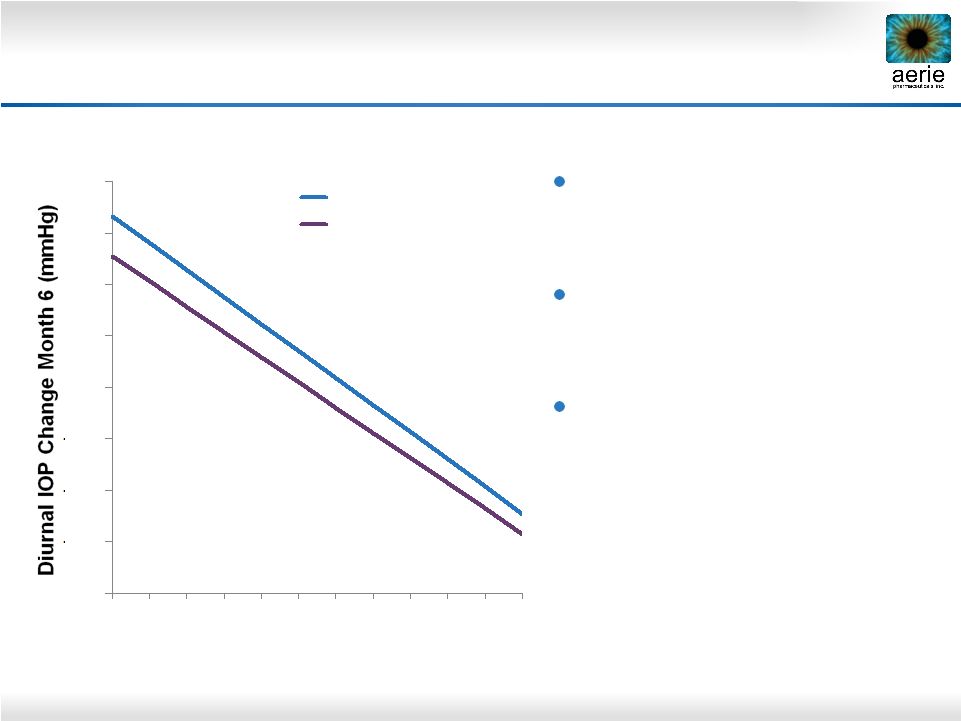 7 Latanoprost and Timolol Show Reduced Efficacy at Lower Baseline IOPs Pooled data from three latanoprost registration studies. Hedman and Alm; European Journal Ophthalmology;
2000 Latanoprost and timolol lose efficacy as baseline IOPs decline Timolol at least 1 mmHg less effective than latanoprost across all published baselines Timolol is the standard comparator for glaucoma Phase 3 trials -16 -14 -12 -10 -8 -6 -4 -2 0 16 18 20 22 24 26 28 30 32 34 36 38 Untreated Diurnal IOP (mmHg) Timolol (n=369) Latanoprost (n=460) |
 8 Rhopressa™ Registration Trial Design * PGAs have been shown to be less effective when dosed BID “Rocket 1” 90-Day Efficacy Registration Trial Rhopressa™ 0.02% QD 182 patients Timolol BID 188 patients “Rocket 2” One Year Safety (3 Mo. Interim Efficacy) Registration Trial “Rocket 3” One Year Safety Registration Trial Canada Rhopressa™ 0.02% QD ~230 patients Rhopressa™ 0.02% BID * ~230 patients Timolol BID ~230 patients Rhopressa™ 0.02% QD ~90 patients Rhopressa™ 0.02% BID ~90 patients Timolol BID ~60 patients |
 9 Rocket 1 Study Endpoints Efficacy: • The primary efficacy endpoint is the mean IOP at the following time points: 08:00, 10:00, and 16:00 at the Week 2, Week 6, and Day 90 visits. • IOP range above 20 mmHg and below 27 mmHg • Secondary efficacy endpoints include: • IOP analysis stratified by baseline IOP above and below 24 mmHg • Mean change from baseline IOP • Mean percent change from diurnally adjusted baseline IOP • Mean diurnal and change from baseline diurnal IOP Safety: • Ocular and systemic safety measures |
 10 Rocket 1: Summary Of Rhopressa™ Efficacy Results Baseline IOP (mmHg) Non-inferiority Numerical Superiority <27* Did not meet Met 2 time points <26 Met Met 4 time points <25 Met Met 7 time points <24** Met All (9 time points) <23 Met All (9 time points) <22 Insufficient power All (9 time points) * Primary endpoint ** Pre-specified secondary endpoint |
 11 Rhopressa TM Update: Rocket 2 • Rocket 2 primary endpoint range changed with FDA agreement - New primary endpoint range is above 20 mmHg to below 25 mmHg - Statistical change allowed; Rocket 2 adequately powered at
below 25 mmHg - No additional patient enrollment necessary • Rocket 2 data base not yet locked; patients still being treated • Efficacy read-out expected September 2015 • If Rocket 2 is successful, NDA filing expected by mid-2016 |
 12 Rocket 1: Baseline IOP < 25 mmHg At All Time Points |
 13 Rocket 1: Rhopressa TM Efficacy in Subjects On PGA Prior To Study (Baseline IOP < 25 mmHg) • Prior PGA use produced enhanced IOP-lowering with Rhopressa TM at weeks 2 and 6 • IOP lowering at month 3 equivalent to IOP lowering in non-PGA subjects • Prior PGA use had no effect on Timolol efficacy |
 14 Rocket 1: Rhopressa TM Efficacy In Subjects Not on PGA Prior To Study (Baseline IOP < 25
mmHg) •
No loss of efficacy seen from week 2 to month 3 for Rhopressa
TM or Timolol |
 15 Rhopressa TM IOP-lowering Effect Enhanced In Subjects On PGA Therapy Prior To Study Entry Prospective (pre-specified) analysis by pre-study medication status showed that prior PGA use enhanced Rhopressa IOP-lowering at week 2 (p=0.003) The PGA effect is lost over time, which we believe creates a false impression that Rhopressa TM loses efficacy over time Retrospective analysis of Phase 2 trial results shows prior PGA use enhanced Rhopressa TM IOP-lowering by 1 mmHg (p=0.007) and 1.2 mmHg (p=0.002) at weeks 2 and 4, respectively, relative to subjects not previously on PGA Daily exposure to both Rhopressa TM and a PGA may result in ongoing synergy – and may also explain positive Roclatan TM P2b results TM |
 16 FY 2014 U.S. Glaucoma Market = $2.2B; 33M TRx Market Share in TRx PGA Market Non-PGA Market Rhopressa TM Synergy with PGAs May Represent a Market Opportunity Once Daily 2-3 Times Daily 10% 9% 33% 14% 15% 10% 8% Bimatoprost Travoprost Latanoprost BB Fixed Combo AA CAI PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor
Source: IMS MIDAS, IMS NPA |
 17 Rhopressa TM Next Steps: Rocket 4 • Planning to commence Rocket 4 in Q3 2015, an additional Rhopressa trial in the U.S. • Baseline IOPs: - Primary <25 mmHg to mirror revised Rocket 2 - Pre-specified secondary <27 mmHg - Considering stratification - May enroll patients up to 30 mmHg • Efficacy evaluated at 3 months (primary) and 6 months (secondary) • Final design to be reviewed with FDA • Read-out expected in approximately one year • Adds over 200 additional Rhopressa patients for 6 month EU safety TM TM |
 18 Roclatan™ Phase 2b Clinical Trial Design Phase 2b Protocol Roclatan™ 0.01% vs. Roclatan™ 0.02% vs. Rhopressa™ 0.02% vs. Latanoprost All Dosed QD PM ~300 Patients 28 Days Primary efficacy endpoint: Mean diurnal IOP on Day 29 Two concentrations of Roclatan™ vs. Rhopressa™ 0.02% and latanoprost Trial design follows FDA requirement for fixed-dose combination Statistically significant difference at measured time points Higher combo efficacy vs. components of at least 1–3 mmHg, as previously accepted by FDA for product approval |
 19 Roclatan™ Phase 2b Clinical Trial Performance Achieved primary efficacy measure Superiority over each of the components on day 29 Achieved statistical superiority over the individual components at all time points More efficacious than latanoprost by 1.6 – 3.2 mmHg More efficacious than Rhopressa™ by 1.7 – 3.4 mmHg Main adverse event was hyperemia (eye redness): Reported in 40 percent of patients Mild for the large majority of patients No systemic drug-related adverse events |
 20 Mean IOP at Each Time Point Primary Efficacy Measure 0.02% Roclatan™ Achieved Statistical Superiority Over Individual Components at All Time Points (p<0.001) Roclatan™ Phase 2b, Intent to Treat |
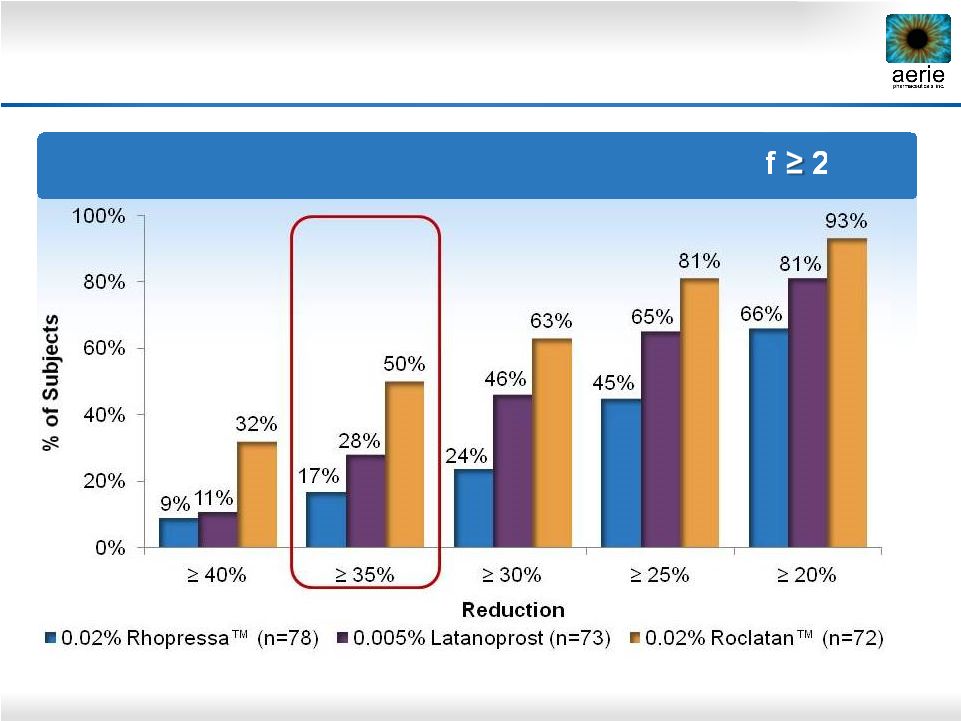 21 Day 29 – % of Patients with IOP Reductions of 20% Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible |
 22 Roclatan™ Phase 2b Responder Analysis: Goal is to Achieve Lowest IOP Possible Day 29 – % of Subjects with IOP Reduced to < 18 mmHg |
 23 Roclatan TM Next Steps • Commencing “Mercury 1” in Q3 2015 in the U.S. - Designed for superiority to individual components, similar to P2b - Baseline IOP range tentatively > 20 mmHg to <36 mmHg, with
stratified enrollment
- Multiple secondary endpoints
- Efficacy trial with one year safety • “Mercury 2” expected to commence in 2016 in the U.S. - Expect same comparators as Mercury 1 - Three month efficacy study • “Mercury 3” expected to commence in 2016 in Europe - Comparing to a leading combo product marketed in EU - Efficacy study, duration TBD |
 24 AR-13154 – Preclinical Product for AMD • Aerie-owned small molecule inhibits AMD targets ROCK, JAK2 and PDGFRß • AR-13154 demonstrated significant lesion size reduction in preclinical laser-induced choroidal neovascularization (CNV) study in rats - Additional research underway • Since AR-13154 is a small molecule, a delivery mechanism is necessary for sustained release to back of the eye |
 25 Laser-induced choroidal neovascularization (CNV) in rats Compounds delivered by intravitreal injection AR-13154 vs. Eylea in Preclinical AMD Model ROCK/JAK2/PDGFR Inhibitor AR-13154 Numerically More Effective than Eylea ® in Rat Model of AMD 20000 30000 40000 50000 60000 70000 80000 90000 100000 110000 Saline n=49 0.06 ug/mL AR-13154 n=28 0.6 ug/mL AR-13154 n=25 6 ug/mL AR-13154 n=25 800 ug/mL Eylea n=20 Total CNV Lesion Area (Day 21) ** * * p<0.05 vs. Saline ** p<0.001 ® |
 26 Collaboration with GrayBug • GrayBug • Spin-out from Johns Hopkins University • Founders include Justin Hanes, Peter McDonnell and Peter Campochiaro • Sustained release injectable technology with capability of delivering compounds to the front and back of eye • Versatility for particle and implant formulations with potential for multi-month
delivery • Collaboration overview • GrayBug will dedicate efforts to developing candidates for multiple Aerie compounds • One-year research term with possibility for extension • Aerie has exclusive option to license multiple products from collaboration • Aerie funds GrayBug’s R&D activities and will make additional payments to GrayBug upon certain milestone events |
 27 Injectable High Capacity Reservoir and Localization of Microparticles Biodegradability Biocompatibility Bioabsorbability Polymer Modified to Reduce Inflammation ENCAPSULATED DRUG INTRAVITREAL INJECTION WITH SMALL GAUGE NEEDLE Microparticle Localization Designed to Aggregate and Remain Outside Visual Axis FUNDUS PHOTOS, DAY 7 |
 28 Aerie Financial Resources • As of June 30, 2015 had $163M of cash and investments on balance sheet • Expected to fund Aerie operations through approximately 2017 • Proceeding with clinical path outlined for Rhopressa TM and Roclatan TM , and continue to evaluate potential for pre-clinical Aerie molecules and outside opportunities - e.g.; GrayBug collaboration |
 29 Summary • Key Clinical Priorities • Rhopressa TM : Rocket 2 efficacy readout September
2015
Rocket 4 commencement Q3 2015
• Roclatan TM : Mercury 1 commencement Q3 2015 Mercury 2 and 3 commencement early 2016 • Research Initiatives • Rhopressa TM disease modification and neuro-protection • AR-13154 potential in wet AMD, etc. • Evaluating Aerie’s 3,000+ owned molecules • Business Development Opportunities • Evaluating additions to ophthalmic product pipeline • Drug delivery opportunities – front and back of the eye - GrayBug collaboration |
 Building a Major Ophthalmic Pharmaceutical Company |
