Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Mallinckrodt plc | d85491d8k.htm |
 Mallinckrodt Strategic
Acquisition Therakos, Inc. August 11, 2015 Exhibit 99.1 |
 1 Forward-Looking Statements Statements in this document that are not strictly historical, including statements regarding, the proposed acquisition of Therakos,
Inc., the expected timetable for completing the transaction, future financial condition
and operating results, economic, business, competitive and/or regulatory
factors affecting Mallinckrodt’s and Therakos’ businesses and any other statements regarding events or developments that we believe or anticipate will or may occur in the future, may be "forward-looking" statements within
the meaning of the Private Securities Litigation Reform Act of 1995, and
involve a number of risks and uncertainties. There are a number of
important factors that could cause actual events to differ materially from those suggested or indicated by such forward-looking statements and you should not place undue reliance on any such forward-looking statements. These factors
include risks and uncertainties related to, among other things:
General economic conditions and conditions affecting the industries in which Mallinckrodt and Therakos operate; The commercial success of Mallinckrodt’s products and of Therakos’ photopheresis platforms; The parties’ ability to satisfy the acquisition agreement conditions (including required regulatory approvals) and complete
the Therakos acquisition on the anticipated timeline or at all; Mallinckrodt’s ability to realize anticipated growth, synergies and cost savings from its recently completed acquisitions and
the Therakos acquisition; Changes in laws and regulations; Mallinckrodt’s ability to identify, acquire or close future acquisitions;
Mallinckrodt’s ability to successfully integrate acquisitions of operations,
technology, products and businesses generally and
to realize anticipated growth, synergies and cost savings (including with respect to the Therakos acquisition); Mallinckrodt’s ability to successfully develop or commercialize new products;
Mallinckrodt’s ability to protect intellectual property rights;
Mallinckrodt’s ability to receive procurement and production quotas granted by
the U.S. Drug Enforcement Administration; Customer concentration;
8/11/15 |
 2 Forward-Looking Statements (continued) Mallinckrodt’s reliance on certain individual products that are material to its financial performance;
Cost containment efforts of customers, purchasing groups, third-party payers and
governmental organizations; The reimbursement practices of a small
number of public or private insurers; Limited
clinical trial data for H.P. Acthar ® Gel; Complex reporting and payment obligations under healthcare rebate programs; Mallinckrodt’s ability to achieve anticipated benefits of price increases;
Mallinckrodt’s ability to achieve expected benefits from restructuring
activities; Complex manufacturing processes;
Competition; Product liability losses and other litigation liability; Ongoing governmental investigations; Material health, safety and environmental liabilities; Retention of key personnel; Conducting business internationally; and The effectiveness of information technology infrastructure. These and other factors are identified and described in more detail in the "Risk Factors" section of Mallinckrodt's Annual Report
on Form 10-K for the fiscal year ended September 26, 2014 and
Quarterly Reports on Form 10-Q for the quarters ended March 27, 2015
and June 26, 2015. The forward-looking statements made herein speak only as of the date hereof and Mallinckrodt does not assume any obligation to update or revise any forward-looking statement, whether as a result of new
information, future events and developments or otherwise, except as required by
law. 8/11/15 |
 3 Mallinckrodt to acquire Therakos, Inc. for $1.325B 1 ECP: Extra Corporeal Photopheresis 2 Therakos ECP system is approved by U.S. Food and Drug Administration (FDA) for cutaneous T-Cell lymphoma (CTCL); broad
ECP approval in Europe 3
GvHD: Graft v Host Disease
4
DRG: Diagnosis Related Group
Therakos adds value to Mallinckrodt Advances Mallinckrodt’s growth strategy Increases depth in Specialty Brands; further diversifies Hospital portfolio ECP 1,2 immunotherapy treatment used in cancer, transplant, GvHD³, Crohn’s disease, delivered in
hospital-based outpatient clinics
Durable, high-value, integrated drug-device system; commercial model similar to INOMAX ® 800+ devices in 350+ hospitals, major medical centers in 25+ countries Widely reimbursed globally; under DRG 4 in US Mallinckrodt adds value to Therakos Leverages INOMAX model and footprint to potentially expand accounts, broaden patient access Provides clinical support to expand label and seeks FDA approval for ECP applications used globally Leverages Mallinckrodt’s ability to manage complex products and businesses 8/11/15 |
 4 Transaction highlights Acquisition of Therakos for approximately $1.325 billion Expected to be accretive by no less than $0.10 per share to adjusted diluted fiscal 2016 earnings and increasingly accretive thereafter¹ With roughly 60% of revenue in U.S., we expect fiscal 2015 net sales of $185-$195 million, and anticipate high single-digit growth off that base going forward, driven primarily by the U.S. Long term net sales potential >$500 million annually with gross profit as percent of sales above current company average Significant synergies Financing expected to include cash on hand and debt Consideration Therakos Financial Impact Financing Timing Close expected late fiscal 2015, subject to customary conditions 1 Assumes close by end of fiscal 2015 8/11/15 |
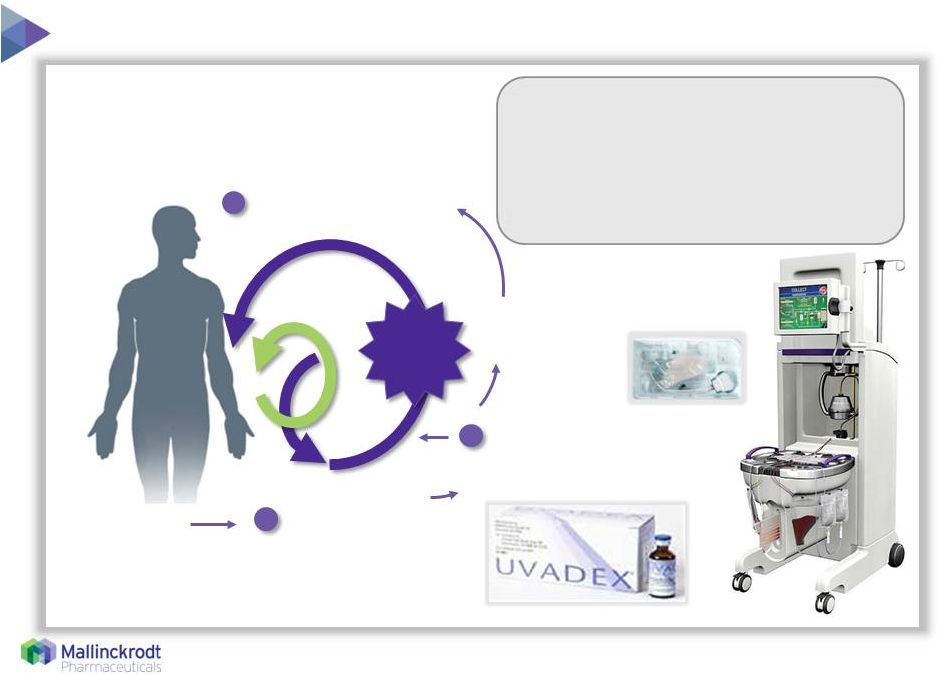 5 Used globally in T-cell mediated diseases – CTCL¹, GvHD, solid organ transplant, Crohn’s disease Exposed to UVA² light UVADEX (methoxsalen) sterile solution used to treat white blood cells Blood separated; red blood cells and plasma returned Instrument draws blood from patient Photoactivated white blood cells returned to the patient 1 2 3 1 Cutaneous T Cell Lymphoma (CTCL) is the only FDA-approved indication; all other U.S. uses are not promoted
2 Ultraviolet-A UVA Razor/Razor Blade Model • Average 25-40 treatments/year per patient • One vial of UVADEX ® and one sterile kit per treatment • Over 120,000 treatments globally in 2014 8/11/15 |
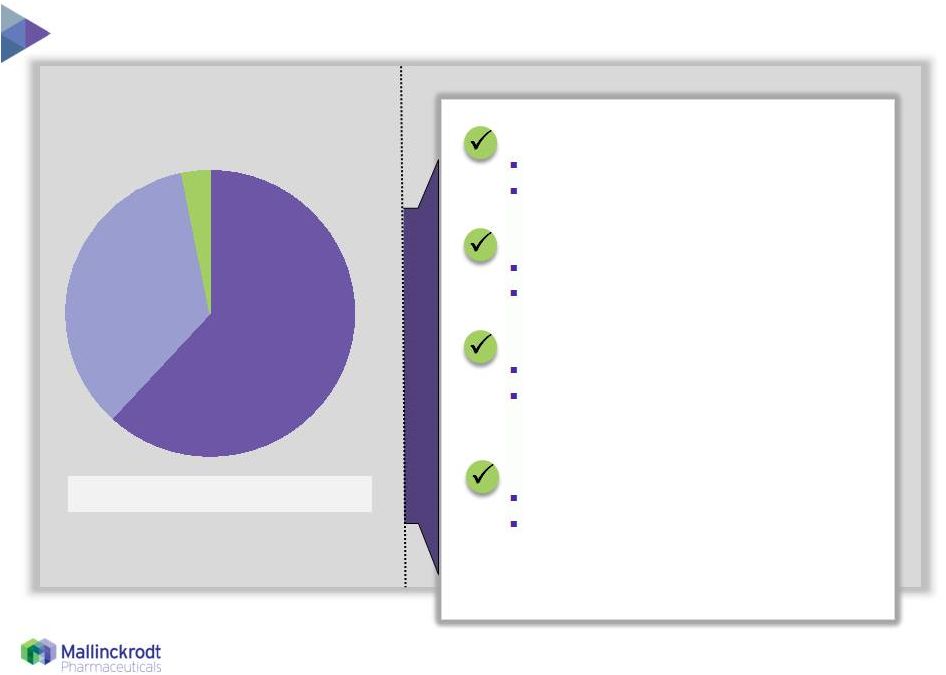 6 Global franchise driven by U.S. growth ROW Europe North America 35% 3% 62% Source: Therakos materials and management estimates Broad global label 10+ years clinical experience U.S. DRG Stable reimbursement ex-U.S. Large installed base 322 active global patents and applications, including 28 in the U.S. Capital efficient Fully outsourced manufacturing and distribution model 2014 Net Sales = $174 million Financially attractive: Strong competitive position: Broadly reimbursed: Widely trusted treatment: 8/11/15 6 |
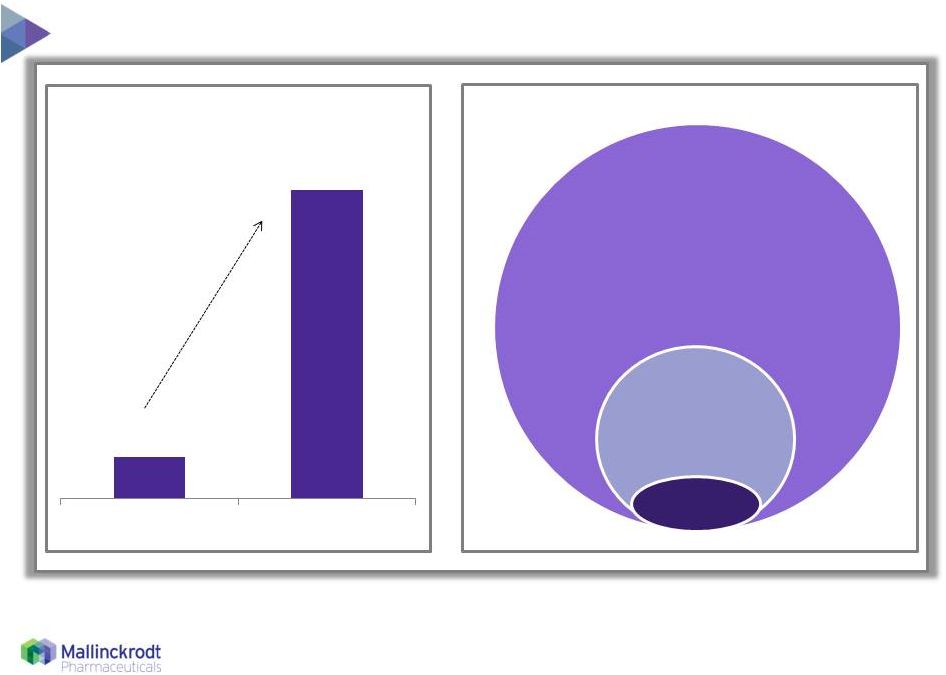 7 Low current penetration, large market potential 1 2 1 Current global treated patients includes promoted and not promoted indications
2 Eligible patients represent patients appropriate for CTCL, GvHD and Solid Organ Transplant
3 Therakos ECP is FDA-approved for the palliative treatment of the skin manifestations of CTCL, that is unresponsive to other
forms of treatment 4
Illustration not to scale
~3,000 CTCL U.S. Patient Population 4 Source: Therakos materials and management estimates 8/11/15 Treated Eligible Total Global Eligible ECP Patient Population ~23,000 CTCL Prevalence (~20,000) Refractory Patients (~2,400) ECP Treated 3 (~400) |
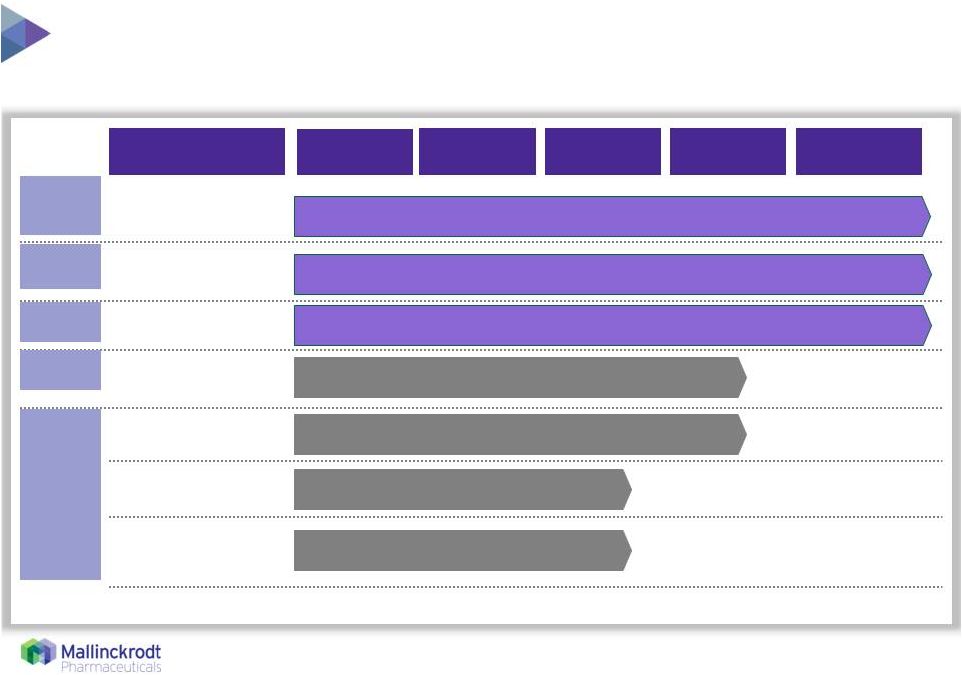 8 Significant label expansion opportunity supported by 10+ years of safety data U.S./ LatAm EU/ AUS U.S. Canada Japan Photopheresis Administration CTCL Scleroderma Chronic GvHD¹ Chronic GvHD Other Indications (e.g., Cystic Fibrosis, Peripheral TCL) Acute GvHD¹ ¹Accelerated regulatory pathways 8/10/15 Pivotal Approved Phase 2 Phase 1 Preclinical Indication(s) |
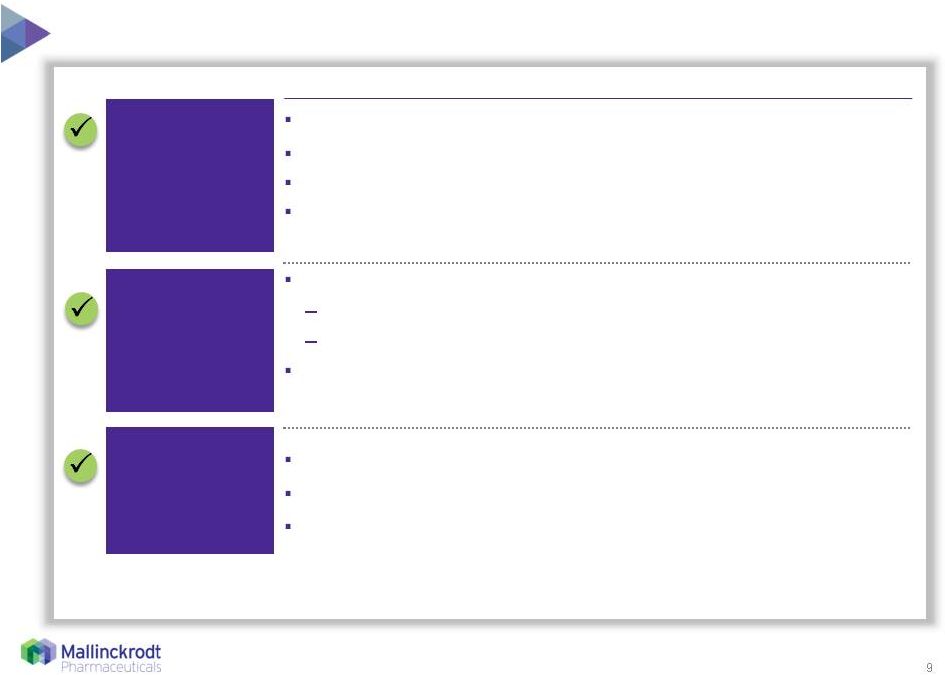 9 Mallinckrodt is the best owner of Therakos 8/11/15 Significant opportunity to maximize synergy Accretive, profitable business Growing revenue and cash flow Multiple levers for financial value creation Mallinckrodt is experienced in managing complex businesses Integrated drug-device with complex account management Leverages INOMAX customer service center of excellence Opportunity to leverage hospital channel to grow installed base, targeting rare diseases and conditions Strong strategic fit Only FDA-approved closed ECP system Highly durable asset with opportunity for near-term expansion Large, established base, 800+ devices, 350+ hospitals, 25+ countries Broad global reimbursement Differentiated product expands existing franchises 1 FDA-approved for the palliative treatment of the skin manifestations of CTCL that is unresponsive to other forms of
treatment. 1 |
 10 Therakos continues Mallinckrodt’s development of a diversified, durable, high-value portfolio • Expand system placement, kit/drug volume • Label expansion • Clinical, HEOR data generation/dissemination • Select ANDA and formulation technology development • High single-digit revenue growth • >$500 million net sales annually over time • Maximize cash generation Key Value Drivers Performance Objective • Label expansion • Enhance patient penetration • Contracting, 24/7 customer intimacy • Mid-single digit revenue growth • Clinical, HEOR data generation/dissemination • Patient penetration in on-label indications • Payer engagement at policy level • Mid-single to low-double digit revenue growth • Expanded formulary access • Increased procedure penetration • Clinical, HEOR data generation/dissemination • >$500mm peak annual revenue 8/11/15 ¹HEOR: Healthcare Economic Outcomes Research 2 ANDA: Abbreviated New Drug Application + Value creating business development 2 1 |
