Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Karyopharm Therapeutics Inc. | d69642d8k.htm |
Exhibit 99.1

NASDAQ: KPTI
Corporate Presentation
August 2015
Targeting Disease at the Nuclear Pore

Forward-looking Statements
This presentation contains forward-looking statements within the meaning of the “safe harbor” provisions of The Private Securities Litigation
Reform Act of 1995.
Such forward-looking statements include those regarding the therapeutic potential of and potential clinical development plans and commercialization for Karyopharm’s lead drug candidate, selinexor (KPT-330), including the timing of initiation of certain trials and of the reporting of data from such trials, as well as assumptions of management and financial expectations and projections.
Such statements are subject to numerous important factors, risks and uncertainties that may cause actual events or results to differ materially from the company’s current expectations. For example, there can be no guarantee that Selinexor or any other drug candidate Karyopharm is developing will successfully complete necessary preclinical and clinical development phases or that development of any of Karyopharm’s drug candidates will continue. Further, there can be no guarantee that any positive developments in Karyopharm’s drug candidate portfolio will result in stock price appreciation. In addition, even if Karyopharm receives marketing approval for selinexor or another drug candidate, there can be no assurance that Karyopharm will be able to successfully commercialize that drug candidate. Management’s expectations and, therefore, any forward-looking statements in this presentation could also be affected by risks and uncertainties relating to a number of other factors, many of which are beyond Karyopharm’s control, including the following: Karyopharm’s results of clinical trials and preclinical studies, including subsequent analysis of existing data and new data received from ongoing and future studies; the content and timing of decisions made by the U.S. Food and Drug Administration and other regulatory authorities, investigational review boards at clinical trial sites and publication review bodies; Karyopharm’s ability to obtain and maintain requisite regulatory approvals and to enroll patients in its clinical trials; unplanned cash requirements and expenditures; development of drug candidates by Karyopharm’s competitors for diseases for which Karyopharm is currently developing its drug candidates; and Karyopharm’s ability to obtain, maintain and enforce patent and other intellectual property protection for any drug candidates it is developing.
These and other risks, including those which may impact management’s expectations, are described in greater detail under the heading “Risk
Factors” in Karyopharm’s Annual Report on Form 10-Q for the quarter ended June 30, 2015, which is on file with the Securities and Exchange Commission, and in subsequent filings filed by Karyopharm with the Securities and Exchange Commission.
Any forward-looking statements contained in this presentation are for informational purposes only and speak only as of the date hereof. Other than as is required by law, Karyopharm expressly disclaims any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
Karyopharm’s website is http://www.karyopharm.com. Karyopharm regularly uses its website to post information regarding its business, drug development programs and governance. Karyopharm encourages investors to use www.karyopharm.com, particularly the information in the section entitled “Investors,” as a source of information about Karyopharm. References to www.karyopharm.com in this presentation are not intended to, nor shall they be deemed to, incorporate information on www.karyopharm.com into this presentation by reference.
Unless otherwise noted, this presentation contains data that are interim and unaudited based on site reports.
| 2 |
|
Karyopharm: At the Nucleus of Cancer Care
Karyopharm’s wholly owned, lead SINE™ compound selinexor offers an entirely new approach to cancer therapy that restores the ability of a cell to detect cancerous changes and commit suicide
Novel mechanism First in class Oral, small molecule Fully Owned
Tumor Suppressor Protein (TSP) activation and oncoprotein reduction
Potentially relevant to any type of cancer
| 4 |
|
ongoing later stage Heme trials |
| 3 |
|
ongoing Ph 2 Solid Tumor trials |
>40 combination studies ongoing or planned
Prepare to commercialize selinexor in NA and Western EU
Seek collaborators for other geographies
Selinexor
New Drug Class in Oncology
Clinical Development Strategy
Commercial Strategy
Initial focus on the regulatory approval and commercialization of oral selinexor as a single-agent, while developing SINE™ compounds as novel approaches to treat a variety of diseases in areas of unmet medical need
| 3 |
|
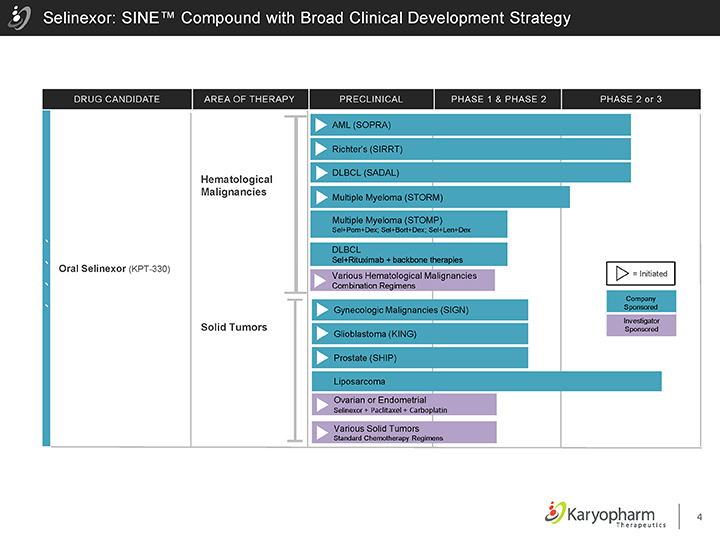
Selinexor: SINE™ Compound with Broad Clinical Development Strategy
DRUG CANDIDATE AREA OF THERAPY PRECLINICAL PHASE 1 & PHASE 2 PHASE 2 or 3
Oral Selinexor (KPT-330)
Hematological Malignancies
Solid Tumors
AML (SOPRA)
Richter’s (SIRRT)
DLBCL (SADAL)
Multiple Myeloma (STORM)
Multiple Myeloma (STOMP)
Sel+Pom+Dex; Sel+Bort+Dex; Sel+Len+Dex
DLBCL
Sel+Rituximab + backbone therapies
Various Hematological Malignancies
Combination Regimens
Gynecologic Malignancies (SIGN) Glioblastoma (KING) Prostate (SHIP)
Liposarcoma
Ovarian or Endometrial
Selinexor + Paclitaxel + Carboplatin
Various Solid Tumors
Standard Chemotherapy Regimens
= Initiated
Company Sponsored
Investigator Sponsored
| 4 |
|
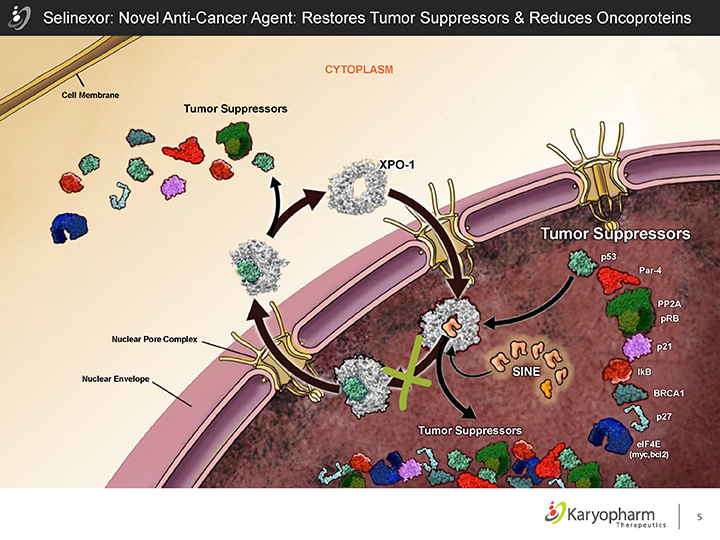
Selinexor: Novel Anti-Cancer Agent: Restores Tumor Suppressors & Reduces Oncoproteins
Cell Membrane
Tumor Suppressors
CYTOPLASM
Nuclear Envelope
Nuclear Pore Complex
XPO-1
Tumor Suppressors
p53
Par-4
PP2A pRB
p21
IkB
BRCA1
p27
elF4E
(myc,bcl2)
Tumor Suppressors
| 5 |
|
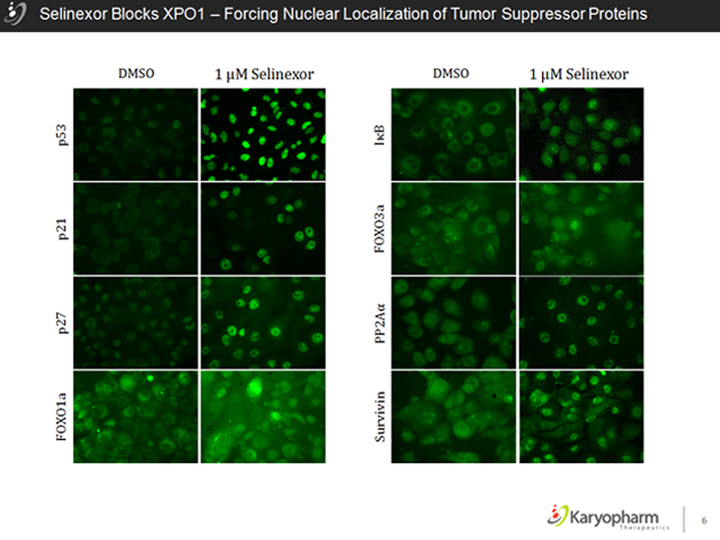
Selinexor Blocks XPO1 – Forcing Nuclear Localization of Tumor Suppressor Proteins
| 6 |
|

Initial Focus for Single-Agent Approval of Selinexor in:
DLBCL
Acute Myeloid Leukemia
Multiple Myeloma
Richter’s Transformation
7 ©2015 – Karyopharm Therapeutics Inc.
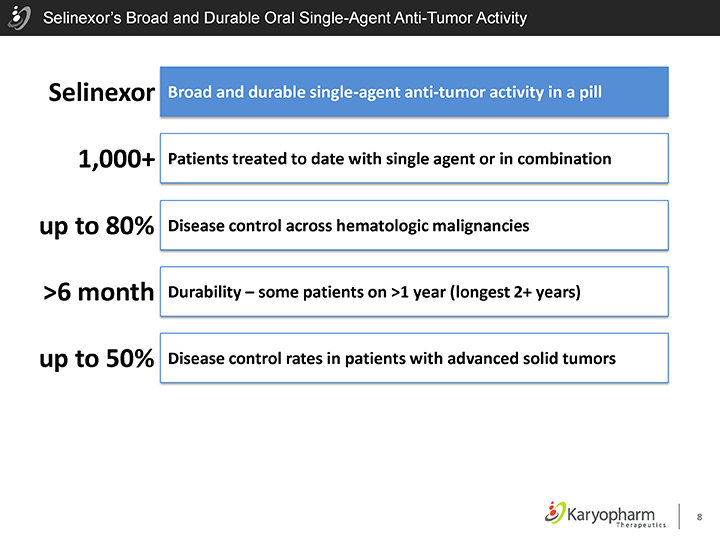
Selinexor’s Broad and Durable Oral Single-Agent Anti-Tumor Activity
Selinexor 1,000+ up to 80% >6 month up to 50%
Broad and durable single-agent anti-tumor activity in a pill Patients treated to date with single agent or in combination Disease control across hematologic malignancies Durability – some patients on >1 year (longest 2+ years)
Disease control rates in patients with advanced solid tumors
| 8 |
|
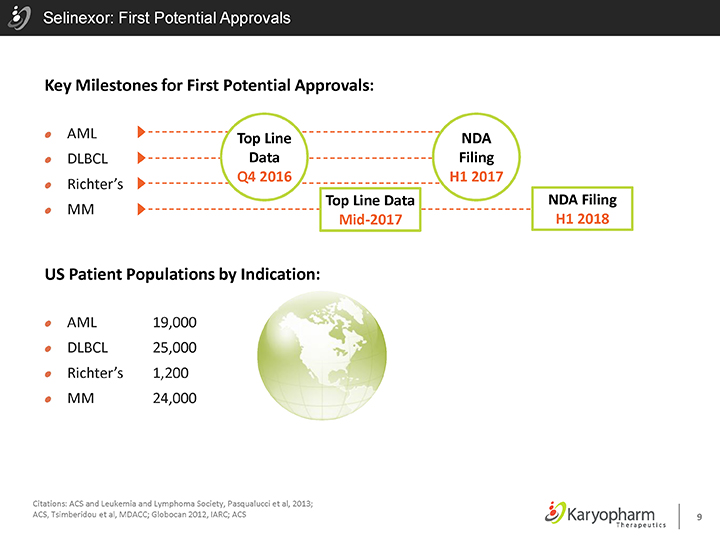
Selinexor: First Potential Approvals
Key Milestones for First Potential Approvals:
AML DLBCL
Richter’s
MM
Top Line Data Q4 2016
NDA Filing H1 2017
Top Line Data Mid-2017
NDA Filing H1 2018
US Patient Populations by Indication:
AML 19,000 DLBCL 25,000
Richter’s 1,200
MM 24,000
Citations: ACS and Leukemia and Lymphoma Society, Pasqualucci et al, 2013;
ACS, Tsimberidou et al, MDACC; Globocan 2012, IARC; ACS 9
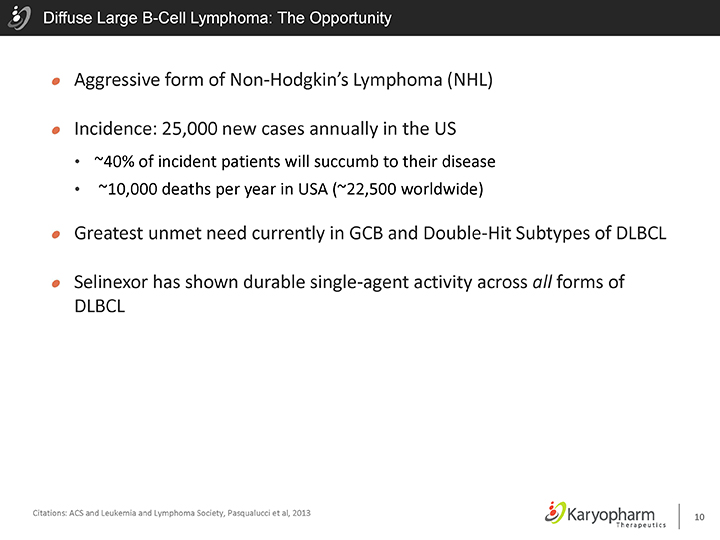
Diffuse Large B-Cell Lymphoma: The Opportunity
Aggressive form of Non-Hodgkin’s Lymphoma (NHL)
Incidence: 25,000 new cases annually in the US
~40% of incident patients will succumb to their disease
~10,000 deaths per year in USA (~22,500 worldwide)
Greatest unmet need currently in GCB and Double-Hit Subtypes of DLBCL
Selinexor has shown durable single-agent activity across all forms of DLBCL
Citations: ACS and Leukemia and Lymphoma Society, Pasqualucci et al, 2013
10
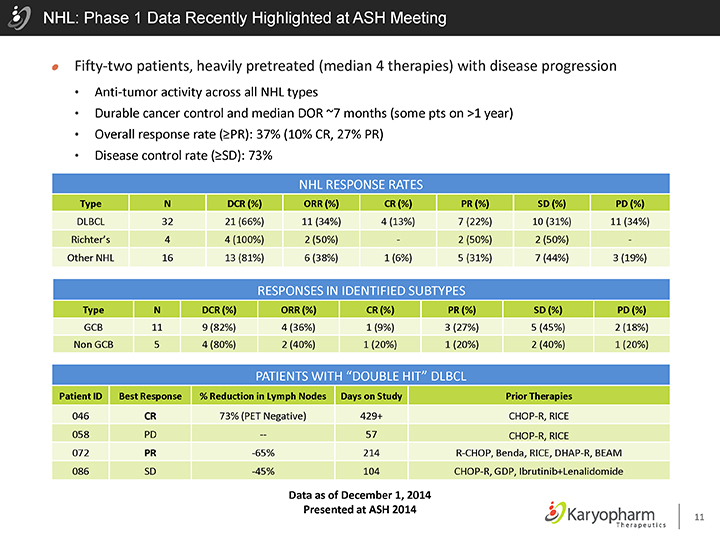
NHL: Phase 1 Data Recently Highlighted at ASH Meeting
Fifty-two patients, heavily pretreated (median 4 therapies) with disease progression
Anti-tumor activity across all NHL types
Durable cancer control and median DOR ~7 months (some pts on >1 year)
Overall response rate (³PR): 37% (10% CR, 27% PR)
Disease control rate (³SD): 73%
NHL RESPONSE RATES
Type N DCR (%) ORR (%) CR (%) PR (%) SD (%) PD (%)
DLBCL 32 21 (66%) 11 (34%) 4 (13%) 7 (22%) 10 (31%) 11 (34%) Richter’s 4 4 (100%) 2 (50%)—2 (50%) 2 (50%)—Other NHL 16 13 (81%) 6 (38%) 1 (6%) 5 (31%) 7 (44%) 3 (19%)
RESPONSES IN IDENTIFIED SUBTYPES
Type N DCR (%) ORR (%) CR (%) PR (%) SD (%) PD (%)
GCB 11 9 (82%) 4 (36%) 1 (9%) 3 (27%) 5 (45%) 2 (18%) Non GCB 5 4 (80%) 2 (40%) 1 (20%) 1 (20%) 2 (40%) 1 (20%)
PATIENTS WITH “DOUBLE HIT” DLBCL
Patient ID Best Response % Reduction in Lymph Nodes Days on Study Prior Therapies
046 CR 73% (PET Negative) 429+ CHOP-R, RICE 058 PD — 57 CHOP-R, RICE
072 PR -65% 214 R-CHOP, Benda, RICE, DHAP-R, BEAM 086 SD -45% 104 CHOP-R, GDP, Ibrutinib+Lenalidomide
Data as of December 1, 2014
Presented at ASH 2014
11
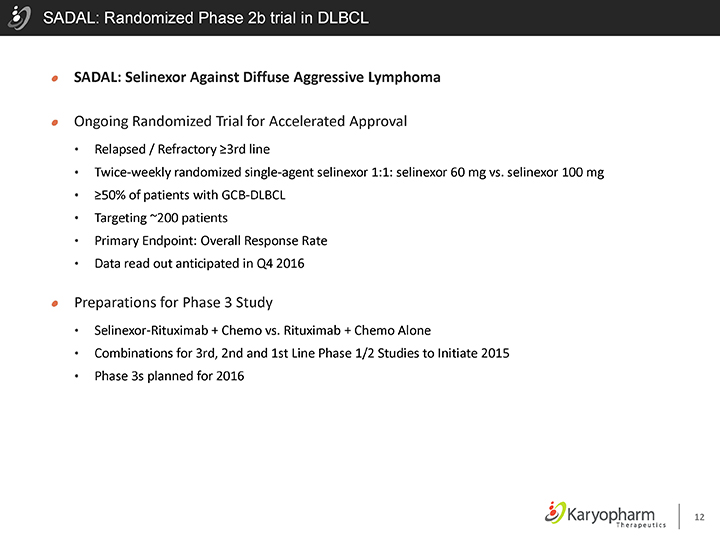
SADAL: Randomized Phase 2b trial in DLBCL
SADAL: Selinexor Against Diffuse Aggressive Lymphoma
Ongoing Randomized Trial for Accelerated Approval
Relapsed / Refractory ³3rd line
Twice-weekly randomized single-agent selinexor 1:1: selinexor 60 mg vs. selinexor 100 mg
³50% of patients with GCB-DLBCL
Targeting ~200 patients
Primary Endpoint: Overall Response Rate
Data read out anticipated in Q4 2016
Preparations for Phase 3 Study
Selinexor-Rituximab + Chemo vs. Rituximab + Chemo Alone
Combinations for 3rd, 2nd and 1st Line Phase 1/2 Studies to Initiate 2015
Phase 3s planned for 2016
12
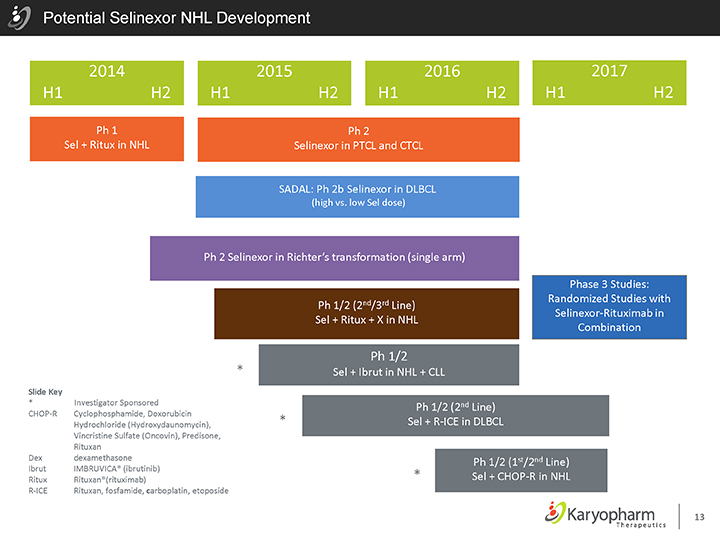
Potential Selinexor NHL Development
2014 2015 2016 2017 H1 H2 H1 H2 H1 H2 H1 H2
Ph 1 Ph 2
Sel + Ritux in NHL Selinexor in PTCL and CTCL
SADAL: Ph 2b Selinexor in DLBCL
(high vs. low Sel dose)
Ph 2 Selinexor in Richter’s transformation (single arm)
Ph 1/2 (2nd/3rd Line) Sel + Ritux + X in NHL
Ph 1/2
| * |
|
Sel + Ibrut in NHL + CLL |
Phase 3 Studies: Randomized Studies with Selinexor-Rituximab in Combination
Slide Key
| * |
|
Investigator Sponsored |
CHOP-R Cyclophosphamide, Doxorubicin Hydrochloride (Hydroxydaunomycin), Vincristine Sulfate (Oncovin), Predisone, Rituxan Dex dexamethasone Ibrut IMBRUVICA® (ibrutinib) Ritux Rituxan®(rituximab) R-ICE Rituxan, fosfamide, carboplatin, etoposide
Ph 1/2 (2nd Line) Sel + R-ICE in DLBCL
Ph 1/2 (1st/2nd Line)
| * |
|
Sel + CHOP-R in NHL |
13

Multiple Myeloma: The Opportunity
Multiple Myeloma represents a significant patient population for selinexor
Second most commonly diagnosed blood cancer, after NHL
114,000 new cases worldwide each year
In the US, a prevalence of approximately 83,000, with 24,050 new cases and 11,090 deaths in 2014
Initial focus on patients with recurrent disease following multiple lines of therapy
Unmet need for patients with recurrent or refractory MM for patients after proteasome inhibitors and immunomodulatory drugs (IMIDs)
Citations: ACS Facts and Figures 2014; GLOBOCAN 2012, IARC, http:///seer.cancer.gov/
14
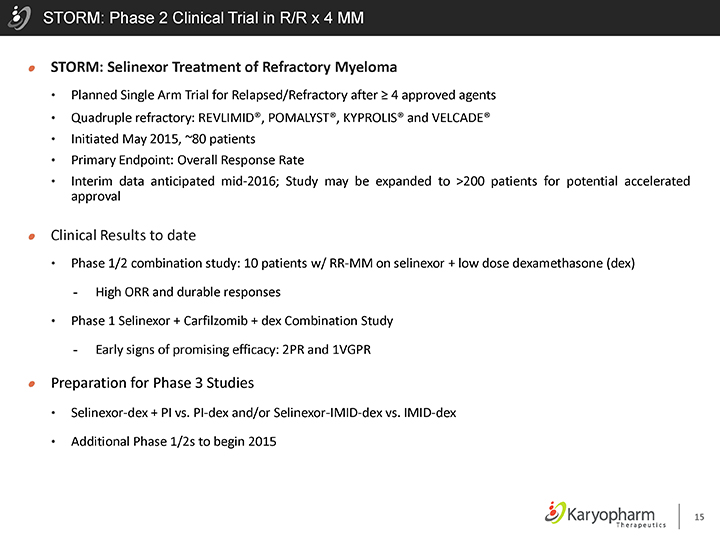
STORM: Phase 2 Clinical Trial in R/R x 4 MM
STORM: Selinexor Treatment of Refractory Myeloma
Planned Single Arm Trial for Relapsed/Refractory after ³ 4 approved agents
Quadruple refractory: REVLIMID®, POMALYST®, KYPROLIS® and VELCADE®
Initiated May 2015, ~80 patients
Primary Endpoint: Overall Response Rate
Interim data anticipated mid-2016; Study may be expanded to >200 patients for potential accelerated approval
Clinical Results to date
Phase 1/2 combination study: 10 patients w/ RR-MM on selinexor + low dose dexamethasone (dex)
- High ORR and durable responses
Phase 1 Selinexor + Carfilzomib + dex Combination Study
- Early signs of promising efficacy: 2PR and 1VGPR
Preparation for Phase 3 Studies
Selinexor-dex + PI vs. PI-dex and/or Selinexor-IMID-dex vs. IMID-dex
Additional Phase 1/2s to begin 2015
15
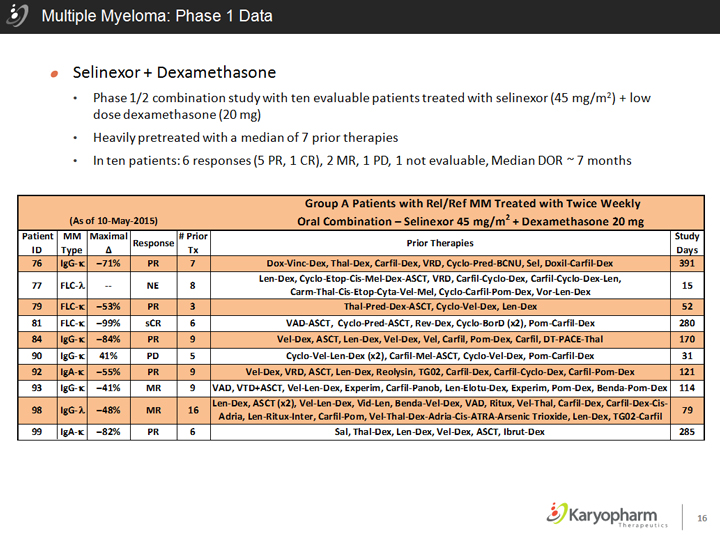
Multiple Myeloma: Phase 1 Data
Selinexor + Dexamethasone
Phase 1/2 combination study with ten evaluable patients treated with selinexor (45 mg/m2) + low dose dexamethasone (20 mg)
Heavily pretreated with a median of 7 prior therapies
In ten patients: 6 responses (5 PR, 1 CR), 2 MR, 1 PD, 1 not evaluable, Median DOR ~ 7 months
Group A Patients with ReI/Ref MM Treated with Twice Weekly
(As of 1D-M ay-2015) 2
Oral Combination—Selinexor 45 mg/m + Dexamethasone 20 mg
Patient MM Maximal Response # Prior Prior Therapies Study ID Type 0. Tx Days
76 IgG-K -71% PR 7 Dox-Vine-Dex, Thal-Dex, Carfil-Dex, VRD, Cyclo-Pred-BCNU, Sel, Doxil-Carfil-Dex 391 FLC-A len-Dex, Cyclo-Etop-Cis-Mel-Dex-ASCT, VRD, CartH-Cycio-Dex, Carfil-Cyclo-Dex-len,
77 — NE 8 Carm-Thal-Cis-Etop-Cyta-Vel-Mel, Cyclo-CartiMultiple Myeloma: Phase 1 Datal-Pom-Dex, Vor-len-Dex 15
79 FLC-K -53% PR 3 Thal-Pred-Dex-ASCT, Cyclo-Vel-Dex, len-Dex 52
81 FLC-K -99% sCR 6 VAD-ASCT, Cyclo-Pred-ASCT, Rev-Dex, Cycio-BorD (xz), Pom-Carfil-Dex 280
84 IgG-K -84% PR 9 Vel-Dex, ASCT, len-Dex, Vel-Dex, Vel, Carfil , Pom-Dex, Carfil, DT-PACE-Thal 170
90 IgG-K 41% PD 5 Cyclo-Vel-len-Dex (x2), Carfil-Mel-ASCT, Cyclo-Vel-Dex, Pom-Carfil-Dex 31
92 IgA-K -55% PR 9 Vel-Dex, VRD, ASCT, len-Dex, Reolysin, TG02, Cart il-Dex, Carfil-Cyclo-Dex, Carfil-Pom-Dex 121
93 IgG-K -41% MR 9 VAD, VTD+ASCT, Vel-len-Dex, Experim, Carfil-Panob, len-Elotu-Dex, Experim, Pom-Dex, Benda-Pom-Dex 114 IgG-A len-Dex, ASCT (x2), Vel-len-Dex, Vid-len, Benda-Vel-Dex, VAD, Ritux , Vel-Thai, Carfil-Dex, Carfil-Dex-Cis-
98 -48% MR 16 Adria, len-Ritux-Inter, Carfil-Pom, Vel-Thal-Dex-Adria-Cis-ATRA-Arsenie Trioxide, len-Dex, TG02-Carfil 79
99 IgA-K -82% PR 6 Sal, Thal-Dex, len-Dex, Vel-Dex, ASCT, Ibrut-Dex 285
16
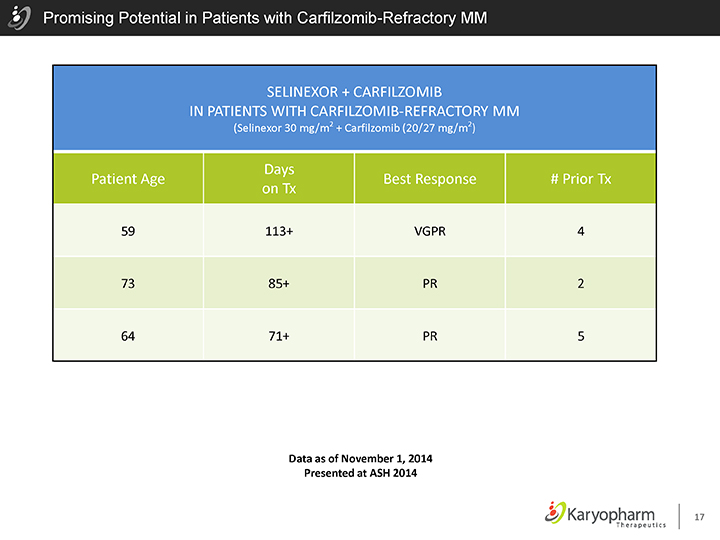
Promising Potential in Patients with Carfilzomib-Refractory MM
SELINEXOR + CARFILZOMIB
IN PATIENTS WITH CARFILZOMIB-REFRACTORY MM
(Selinexor 30 mg/m2 + Carfilzomib (20/27 mg/m2)
Days
Patient Age Best Response # Prior Tx on Tx
59 113+ VGPR 4
73 85+ PR 2
64 71+ PR 5
Data as of November 1, 2014 Presented at ASH 2014
17
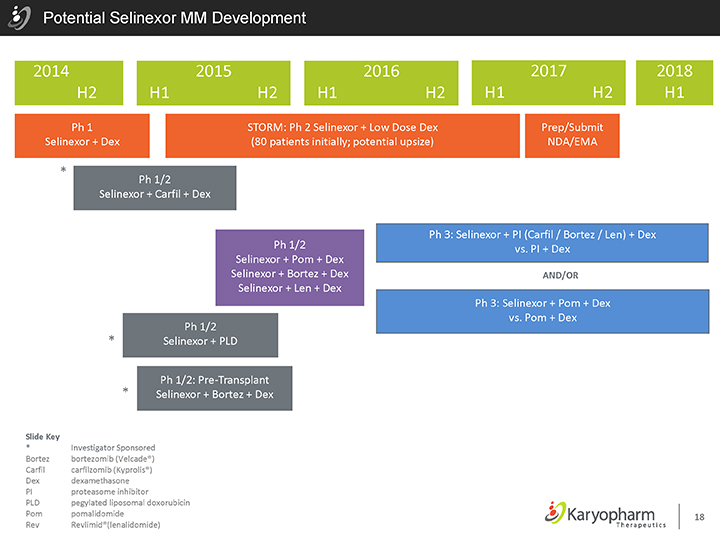
Potential Selinexor MM Development
2014 2015 2016 2017 2018 H2 H1 H2 H1 H2 H1 H2 H1
Ph 1 STORM: Ph 2 Selinexor + Low Dose Dex Prep/Submit Selinexor + Dex (80 patients initially; potential upsize) NDA/EMA
| * |
|
Ph 1/2 Selinexor + Carfil + Dex
Ph 1/2 Selinexor + Pom + Dex Selinexor + Bortez + Dex Selinexor + Len + Dex
Ph 1/2
| * |
|
Selinexor + PLD |
| * |
|
Ph 1/2: Pre-Transplant Selinexor + Bortez + Dex |
Ph 3: Selinexor + PI (Carfil / Bortez / Len) + Dex vs. PI + Dex
AND/OR
Ph 3: Selinexor + Pom + Dex vs. Pom + Dex
Slide Key
| * |
|
Investigator Sponsored
Bortez
bortezomib (Velcade®)
Carfil
carfilzomib (Kyprolis®)
Dex
dexamethasone
PI
proteasome inhibitor
PLD
pegylated liposomal doxorubicin
Pom
pomalidomide
Rev
Revlimid®(lenalidomide)
18

Acute Myeloid Leukemia: The Opportunity
Significant opportunity exists for selinexor in AML as few treatment options exist for patients
Approximately 18,860 new diagnoses of AML and approximately 7,330 deaths per year in the US
The average age of a patient with AML is 66
Older patients with relapsed AML have limited treatment options with poorer outcomes than younger patients due to comorbidities and increased resistance to chemotherapy
Median Survival of older patients unfit for chemotherapy is ~9 months
Approval Plans
SOPRA: Older patients with AML after 1 line of therapy; topline survival data end of 2016
Combination studies ongoing to inform use in first line therapy for older patients
Additional opportunities for younger patients with “high risk” AML in front line
About 20% of patients at first diagnosis
All patients with relapse after initial chemotherapy
Pediatric patients with relapsed disease (>50% of pediatric AML)
Citations: ACS Facts and Figures 2014
19
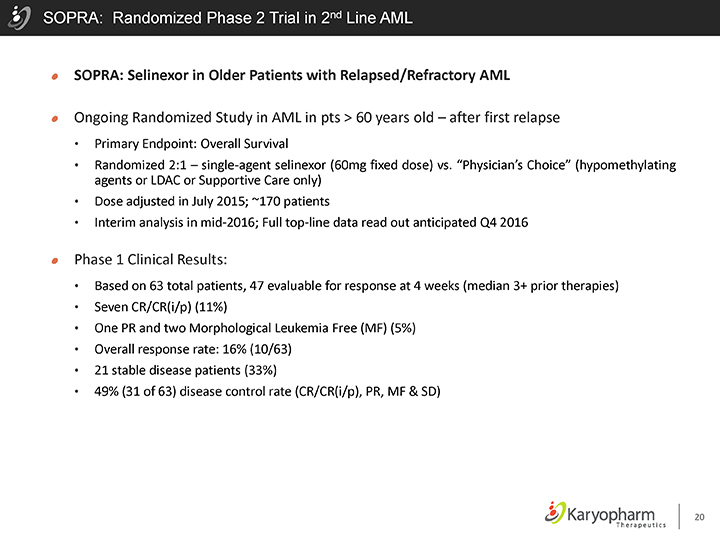
SOPRA: Randomized Phase 2 Trial in 2nd Line AML
SOPRA: Selinexor in Older Patients with Relapsed/Refractory AML
Ongoing Randomized Study in AML in pts > 60 years old – after first relapse
Primary Endpoint: Overall Survival
Randomized 2:1 – single-agent selinexor (60mg fixed dose) vs. “Physician’s Choice” (hypomethylating agents or LDAC or Supportive Care only)
Dose adjusted in July 2015; ~170 patients
Interim analysis in mid-2016; Full top-line data read out anticipated Q4 2016
Phase 1 Clinical Results:
Based on 63 total patients, 47 evaluable for response at 4 weeks (median 3+ prior therapies)
Seven CR/CR(i/p) (11%)
One PR and two Morphological Leukemia Free (MF) (5%)
Overall response rate: 16% (10/63)
21 stable disease patients (33%)
49% (31 of 63) disease control rate (CR/CR(i/p), PR, MF & SD)
20
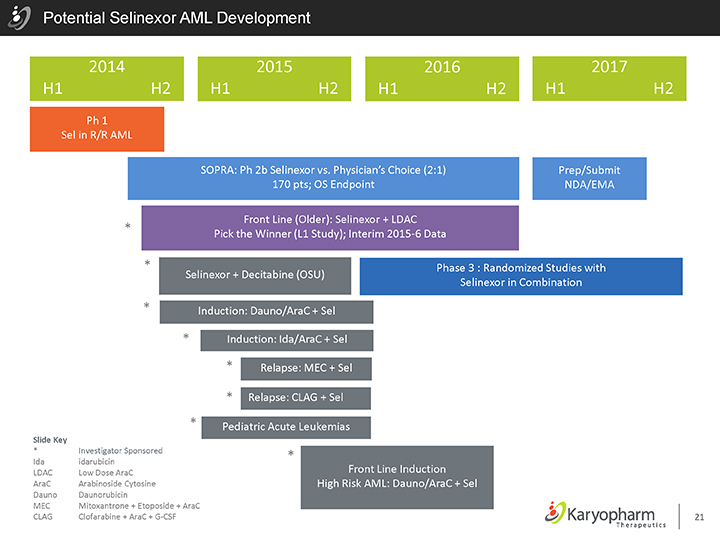
Potential Selinexor AML Development
2014 2015 2016 2017 H1 H2 H1 H2 H1 H2 H1 H2
Ph 1 Sel in R/R AML
SOPRA: Ph 2b Selinexor vs. Physician’s Choice (2:1) Prep/Submit 170 pts; OS Endpoint NDA/EMA
Front Line (Older): Selinexor + LDAC Launch
| * |
|
Pick the Winner (L1 Study); Interim 2015-6 Data
| * |
|
Phase 3 : Randomized Studies with Selinexor + Decitabine (OSU) Selinexor in Combination |
| * |
|
Induction: Dauno/AraC + Sel |
| * |
|
Induction: Ida/AraC + Sel |
| * |
|
Relapse: MEC + Sel |
| * |
|
Relapse: CLAG + Sel |
Slide Key
| * |
|
Investigator Sponsored Ida idarubicin LDAC Low Dose AraC AraC Arabinoside Cytosine Dauno Daunorubicin |
MEC Mitoxantrone + Etoposide + AraC CLAG Clofarabine + AraC + G-CSF
Pediatric Acute Leukemias
| * |
|
Front Line Induction
High Risk AML: Dauno/AraC + Sel
21

Selinexor in Solid Tumors & Combination Studies
22 ©2015 – Karyopharm Therapeutics Inc.
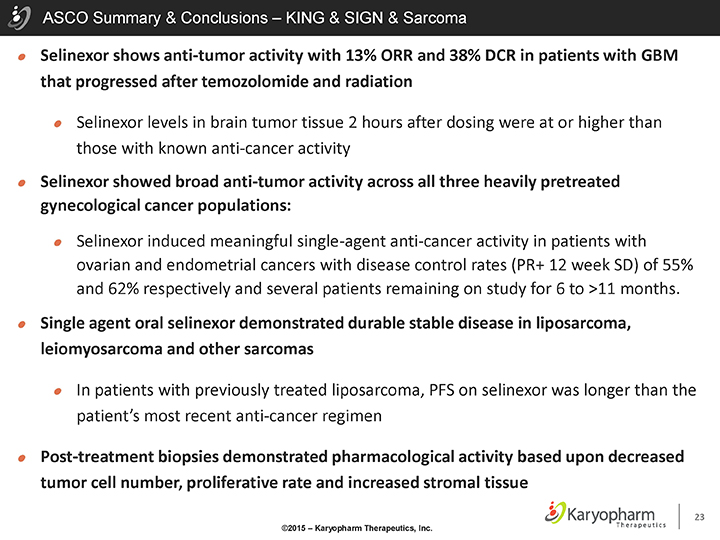
ASCO Summary & Conclusions – KING & SIGN & Sarcoma
Selinexor shows anti-tumor activity with 13% ORR and 38% DCR in patients with GBM that progressed after temozolomide and radiation
Selinexor levels in brain tumor tissue 2 hours after dosing were at or higher than those with known anti-cancer activity
Selinexor showed broad anti-tumor activity across all three heavily pretreated gynecological cancer populations:
Selinexor induced meaningful single-agent anti-cancer activity in patients with ovarian and endometrial cancers with disease control rates (PR+ 12 week SD) of 55% and 62% respectively and several patients remaining on study for 6 to >11 months.
Single agent oral selinexor demonstrated durable stable disease in liposarcoma, leiomyosarcoma and other sarcomas
In patients with previously treated liposarcoma, PFS on selinexor was longer than the patient’s most recent anti-cancer regimen
Post-treatment biopsies demonstrated pharmacological activity based upon decreased tumor cell number, proliferative rate and increased stromal tissue
23
©2015 – Karyopharm Therapeutics, Inc.

ISTs Using Selinexor: Planned or Ongoing in Combination With Other Therapies
Hematological Malignancies
Selinexor + Carfilzomib + Dexamethasone in patients with R/R MM
Selinexor + Fludarabine + Cytarabine in pediatric patients with relapsed or refractory leukemia or myelodysplastic syndrome
Selinexor + Bortezomib + Dexamethasone in patients with progressive of refractory MM
Selinexor + Ibrutinib in patients with R/R CLL and NHL
Selinexor + Decitabine in patients with AML
Solid Tumors
Selinexor + Paclitaxel + Carboplatin in patients with advanced ovarian or endometrial malignancies
Selinexor + Gemcitabine + Abraxane in patients with advanced pancreatic cancer
Selinexor + FOLFOX in patients with metastatic colorectal cancer
Selinexor + Irinotecan in patients with adenocarcinoma of the stomach and distal esophagus
Selinexor + Docetaxel in patients with relapsed squamous cell lung cancer
24

Financial and Commercial Overview
25 ©2015 – Karyopharm Therapeutics Inc.
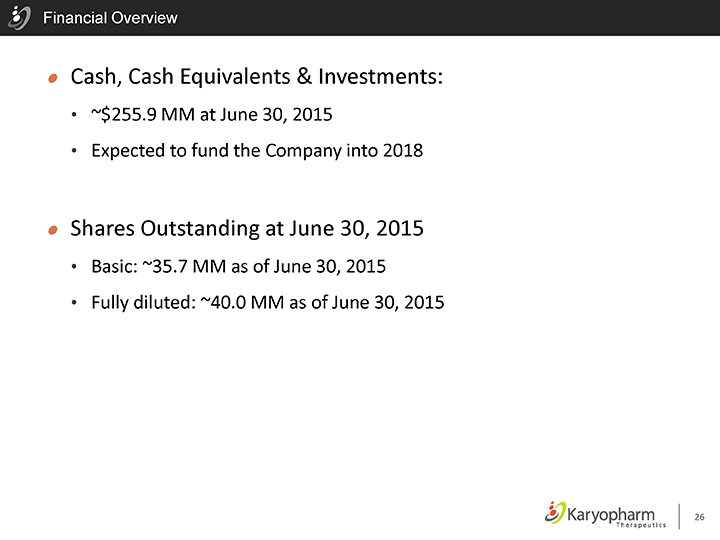
Financial Overview
Cash, Cash Equivalents & Investments:
~$255.9 MM at June 30, 2015
Expected to fund the Company into 2018
Shares Outstanding at June 30, 2015
Basic: ~35.7 MM as of June 30, 2015
Fully diluted: ~40.0 MM as of June 30, 2015
26
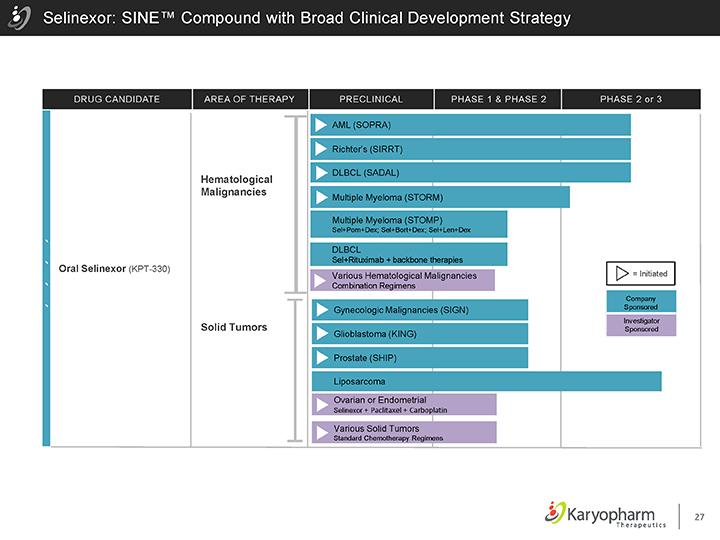
Selinexor: SINE™ Compound with Broad Clinical Development Strategy
DRUG CANDIDATE AREA OF THERAPY PRECLINICAL PHASE 1 & PHASE 2 PHASE 2 or 3
Oral Selinexor (KPT-330)
Hematological Malignancies
Solid Tumors
AML (SOPRA)
Richter’s (SIRRT)
DLBCL (SADAL)
Multiple Myeloma (STORM)
Multiple Myeloma (STOMP)
Sel+Pom+Dex; Sel+Bort+Dex; Sel+Len+Dex
DLBCL
Sel+Rituximab + backbone therapies
Various Hematological Malignancies
Combination Regimens
Gynecologic Malignancies (SIGN) Glioblastoma (KING) Prostate (SHIP)
Liposarcoma
Ovarian or Endometrial
Selinexor + Paclitaxel + Carboplatin
Various Solid Tumors
Standard Chemotherapy Regimens
= Initiated
Company Sponsored
Investigator Sponsored
27
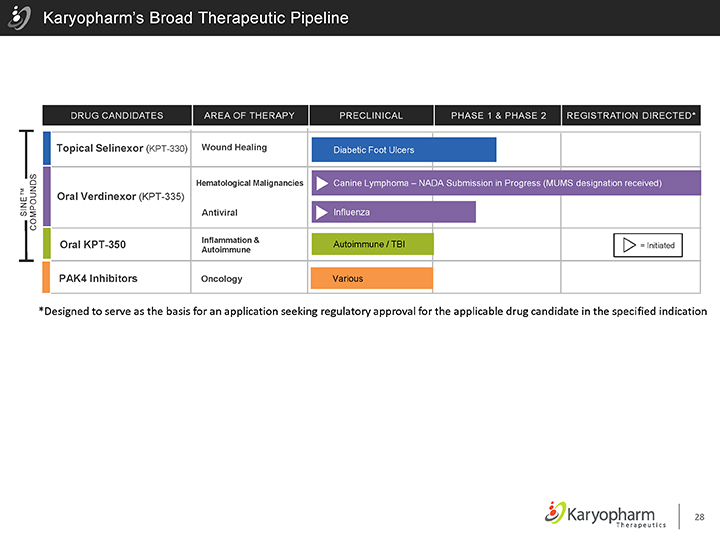
Karyopharm’s Broad Therapeutic Pipeline
SINE™
COMPOUNDS
DRUG CANDIDATES AREA OF THERAPY PRECLINICAL PHASE 1 & PHASE 2 REGISTRATION DIRECTED*
Topical Selinexor (KPT-330) Wound Healing Diabetic Foot Ulcers
Hematological Malignancies Canine Lymphoma – NADA Submission in Progress (MUMS designation received)
Oral Verdinexor (KPT-335)
Antiviral Influenza
Inflammation & Autoimmune / TBI
Oral KPT-350 = Initiated
Autoimmune
PAK4 Inhibitors Oncology Various
*Designed to serve as the basis for an application seeking regulatory approval for the applicable drug candidate in the specified indication
28
Karyopharm: At the Nucleus of Cancer Care
Selinexor is a novel, oral selective inhibitor of XPO1-mediated nuclear export with broad single-agent anti-cancer activity
Karyopharm wholly owns the worldwide rights to selinexor with patent protection through at least 2032
Three ongoing later phase studies (SOPRA, SADAL and SIRRT) are expected to have topline data by the end of 2016
Two ongoing later phase studies (SOPRA and STORM) are expected to have interim analyses in mid-2016
Multiple combination studies are ongoing or planned to incorporate selinexor into treatment regimens across many cancer types
29
