Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pulmatrix, Inc. | v417390_8k.htm |
Exhibit 99.1

Virtual Investor Conference August 6, 2015 Robert Clarke, Ph.D. President & Chief Executive Officer NASDAQ: PULM Engineering the Future for Inhaled Therapeutics

2 Forward Looking Statements Disclaimers and Forward Looking Statements This presentation contains forward - looking statements . Forward - looking statements may be identified by the use of forward - looking terms such as “anticipates,” “assumes,” “believes,” “can,” “could,” “estimates,” “expects,” “forecasts,” “guides,” “intends,” “is confident that,” “may,” “plans,” “seeks,” “projects,” “targets,” and “would” or the negative of such terms or other variations on such terms or comparable terminology . These statements are not guarantees of future performance, are based on current expectations of future events and are subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements, including risks that we will not have sufficient working capital, that we will have delays in obtaining, or we will be unable to obtain, FDA or other regulatory approvals for our products, that we may be unable to establish collaborations, or that our products will not be commercially viable, among other risks . Additional information about the risk factors that may affect the realization of forward - looking statements is set forth in our joint proxy and consent solicitation statement/prospectus dated May 4 , 2015 , of the Company and Ruthigen, Inc . , which is publicly available at : http : // www . sec . gov/Archives/edgar/data/ 1574235 / 000114420415027327 / v 409291 _ 424 b 3 . htm #TOC . Forward - looking statements contained in this presentation are made as of this date, and the Company undertakes no obligation to publicly update any forward - looking statement, whether as a result of new information, future events, or otherwise .

3 Pulmatrix Overview Clinical - Stage Pipeline PUR1900 could be the 1 st inhaled anti - fungal for CF; clinical data expected 2H 2016 Mylan - partnered Phase II COPD asset PUR0200 back to clinic in Q4 2015 PUR1500 is a novel inhaled treatment for IPF advancing to clinical studies Proprietary iSPERSE Technology Novel inhaled dry powder supports increased drug loading E nables efficient high dose pulmonary drug delivery with reduced side effects Multiple therapeutic classes: small molecules, m acromolecules, biologics, & others Strong Barriers - to - Entry 36 issued patents worldwide with coverage through at least 2030 Single - step manufacturing leveraging Pulmatrix engineering expertise Funded into 2017 $27 million in cash (as of June 16, 2015) Cash projected to fund current pipeline beyond key clinical & corporate milestones Experienced Leadership Management team has accomplished track record in inhaled technologies Strong board has history of success

4 Efficiently Managing Market Opportunities Pursue via partnerships (e.g. COPD with Mylan ) Full clinical development funding De - risked strategy to generate royalties on large market potential Pursue independently or partner post - value infection In - license development stage novel compounds to resolve chemistry/dose issues Capture greater value of market Make iSPERSE particles available through dedicated particle supplier Royalties across broad development Dedicated manufacturing partner without cost/risk of own facility Large Markets Rare/Orphan iSPERSE License

5 iSPERSE Pipeline: Significant Opportunities in Respiratory Disease Product COPD PUR1900 - Anti - fungal Cystic Fibrosis PUR0200 – LAMA PUR1500 - Anti - fibrotic Formulation Feasibility Nonclinical Development Phase 1 Phase 2 IPF

6 iSPERSE Technology Platform Enables New Possibilities for Inhaled Therapies iSPERSE Technology Platform: Small , dense , dispersible , and respirable salt - containing particles Novel IP portfolio and applicability to any drug class from small molecule to biologic

Development Pipeline

8 PUR1900: Anti - Fungal for CF Cystic Fibrosis Rare genetic disorder afflicting 100,000 patients WW Impaired mucus clearance and infection susceptibility resulting in: • Increased lung infection • P ulmonary function decline PUR1900 Drug Profile: Dry powder anti - fungal in iSPERSE matrix High local drug concentrations to treat infection in lung with low systemic exposure

9 0 24 48 72 96 120 144 168 -1 0 1 2 3 4 5 6 Time (h) [ D r u g ] Lung (ng/g) Plasma (ng/mL) PUR1900: Anti - Fungal for CF Lead API PK profile: Anticipated Program Milestones Preclinical data Q3 2015; disclosure of API Non - clinical safety completed Q2 2016 Phase 1b clinical data in CF patients in 2H 2016 High lung levels Low systemic levels

10 PUR1900 Addresses Multiple Unmet Needs for Patients Aggressive market penetration in CF due to high patient compliance Beyond CF: Multiple other rare/orphan indications served with same inhaled drug product – E.g. Chemotherapy patients, transplant patients in a prophylactic regimen Potential peak annual sales = > $1.1B (all indications & prophylactic dosing) Indication Cystic Fibrosis Class Anti - infective Active Ingredient Undisclosed Dosing Once daily, inhaled Device Dry Powder Inhaler Expected launch date 1H 2021 (WW) Potential est . peak annual sales $335MM (WW)

11 PUR0200: Bronchodilator for COPD Chronic Obstructive Pulmonary Disease Fifth leading cause of death worldwide Chronic mucus overproduction/inflammation reduces airflow and increases morbidity/mortality $25B global market by 2024 Drug Product Profile Branded generic of a marketed $5B WW once daily LAMA bronchodilator in iSPERSE platform Capsule - based passive DPI device

12 PUR0200: Bronchodilator for COPD Product Status Commercial scale capsule based device Low Mid High Matching PD at 1/6 th lactose blend Commercial Assessment EU Potential: $300 - $500MM peak annual sales; Register 2017 US Potential: $450 - $650MM peak annual sales; Register 2020

13 PUR0200: Development Strategy Mylan Partnership Global leader in generics with established commercial infrastructure Announced European partnership June 2015 Pulmatrix to receive royalty on sales European PK Bioequivalence Pathway Venue for expedited regulatory approval Pathway precedence includes Mylan’s Sirdupla ™ , an alternative combo asthma therapy to Seretide ® ( Advair ®) Pulmatrix’s next trial expected to begin Q4 2015; Data expected Q1 2016 US 505(b)2 Pathway R &D Collaboration with major pharma to support a future US partnership

14 PUR1500: Anti - Fibrotic for IPF Idiopathic Pulmonary Fibrosis (IPF) Rare disorder afflicting 130,000 patients in the US; 40,000 deaths per year Fibrotic scarring leads to reduced lung function and mortality within 2 - 5 years Drug Product Profile Dry powder anti - fibrotic in iSPERSE matrix based using approved oncology molecules with a well described mechanism of action applicable to IPF Chronic therapy for preventing or slowing progression of fibrosis • iSPERSE enables consistent delivery of high lung drug loads needed for efficacy • Local targeting overcomes GI or systemic side effects Anticipated Program Milestones Disclose selected API 1H 2016 Pre - clinical pk and pd using established models completed Q4 2015 Precedent pre - clinical and Phase 1 stage deals with large pharma for promising IPF candidates ($444M Galectos deal with BMS in 2014)

15 Anticipated Near Term Milestones PUR0200 Q4 2015 Report Phase 1 data at European Respiratory Society annual meeting Q4 2015 Initiate EU Phase 2 bioequivalence clinical study vs. reference product 1H 2016 Report data from EU Phase 2 bioequivalence clinical study 2H 2016 Initiate Pivotal EU PK Bioequivalence approval study with Mylan 2H 2016 Form U.S. development & commercialization partnership PUR1900 Q4 2015 Report preclinical efficacy at N. American Cystic Fibrosis Conference 2H 2016 Initiate Ph 1/1b trial in fungal infections in cystic fibrosis patients 2H 2016 Finalize development plan for orphan prophylactic indication s PUR1500 1H 2016 Identify active pharmaceutical ingredient for IPS candidate

Pulmatrix iSPERSE Technology
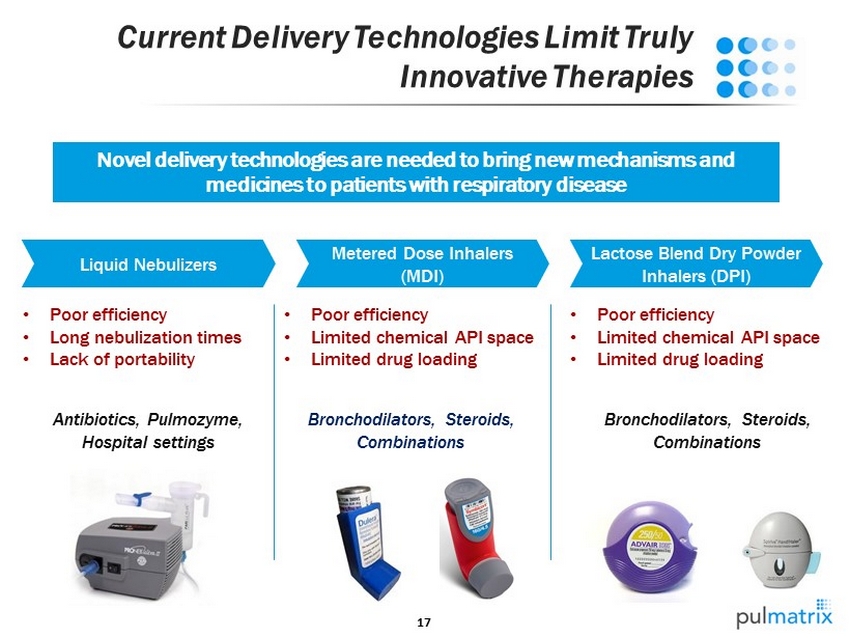
17 Current Delivery Technologies Limit Truly Innovative Therapies Liquid Nebulizers Metered Dose Inhalers (MDI) Lactose Blend Dry Powder Inhalers (DPI) • Poor efficiency • Long nebulization times • Lack of portability Antibiotics, Pulmozyme, Hospital settings • Poor efficiency • Limited chemical API space • Limited drug loading Bronchodilators , Steroids, Combinations • Poor efficiency • Limited chemical API space • Limited drug loading Bronchodilators, Steroids, Combinations Novel delivery technologies are needed to bring new mechanisms and medicines to patients with respiratory disease
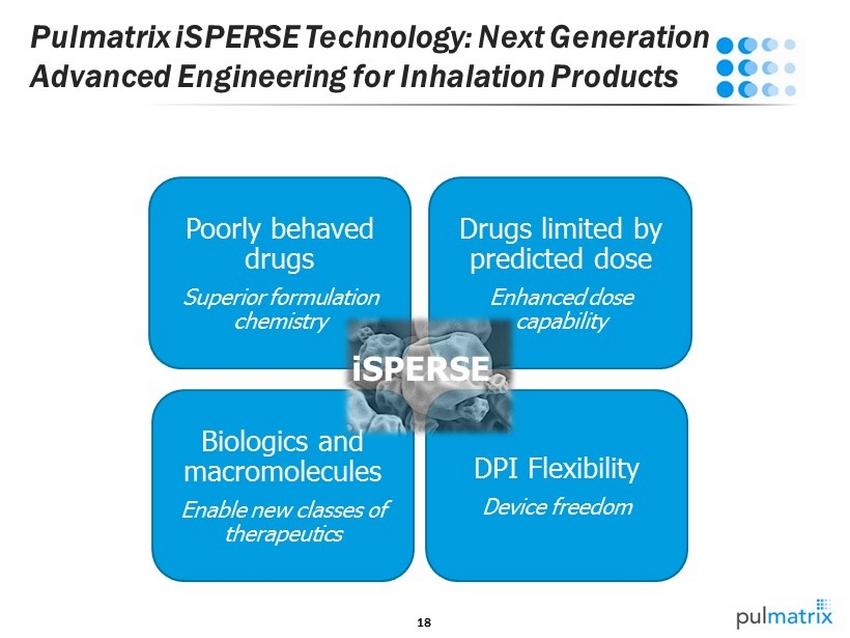
18 Pulmatrix iSPERSE Technology: Next Generation Advanced Engineering for Inhalation Products DPI Flexibility Device freedom Biologics and macromolecules Enable new classes of therapeutics Poorly behaved drugs Superior formulation chemistry Drugs limited by predicted dose Enhanced dose capability iSPERSE
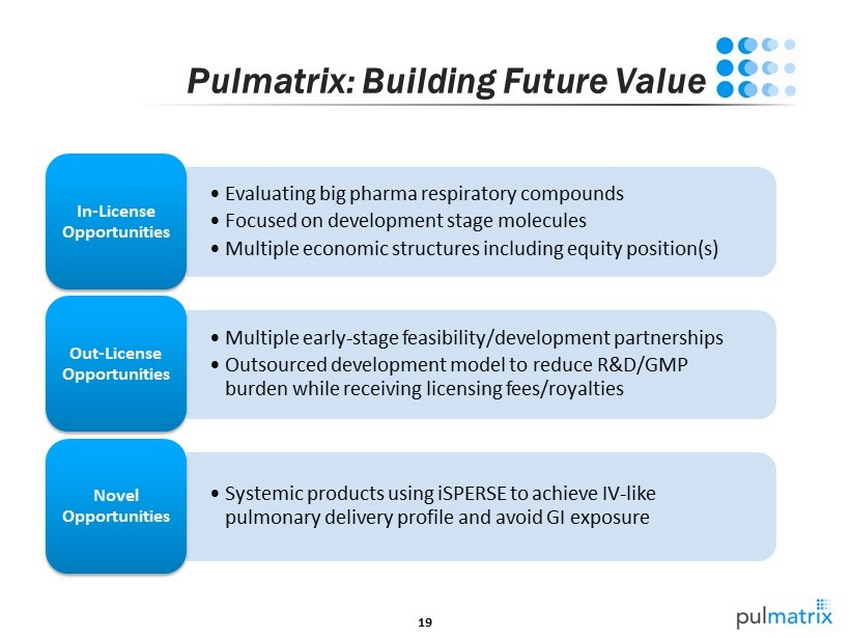
19 Pulmatrix : Building Future Value • Evaluating big pharma r espiratory compounds • Focused on development stage molecules • Multiple economic structures including equity position(s) In - License Opportunities • Multiple early - stage feasibility/development partnerships • Outsourced development model to reduce R&D/GMP burden while receiving licensing fees/royalties Out - License Opportunities • Systemic products using iSPERSE to achieve IV - like pulmonary delivery profile and avoid GI exposure Novel Opportunities

Business

21 Share Statistics Trading Symbol PULM Total Shares Outstanding 14.5 million Financials Cash on Hand At Closing (06/26/15) $27.0 million Runway based on anticipated expenses Mid - 2017 Cap Structure Insider Ownership 57% Key Financial Data
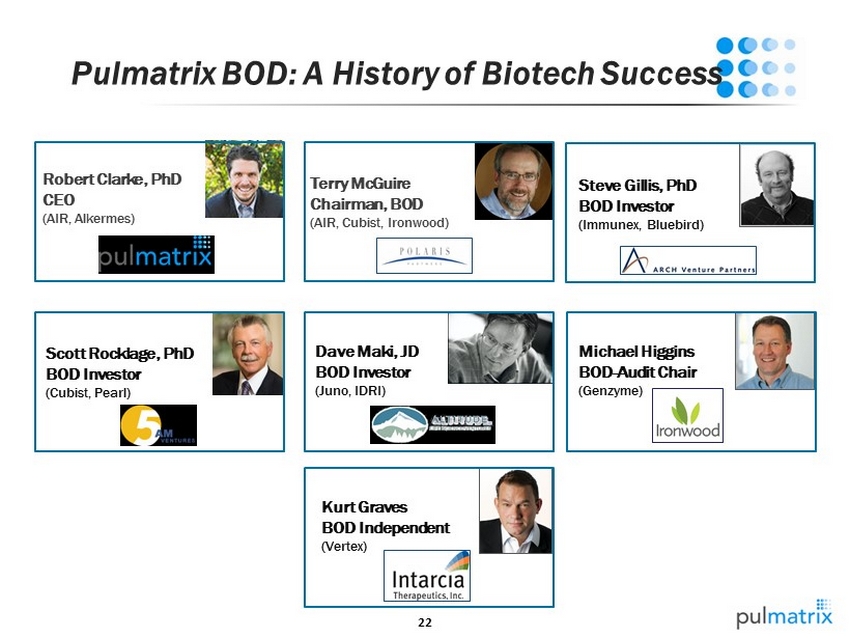
22 Pulmatrix BOD: A History of Biotech Success Terry McGuire Chairman, BOD (AIR, Cubist, Ironwood) Robert Clarke, PhD CEO (AIR, Alkermes ) Scott Rocklage , PhD BOD Investor (Cubist, Pearl) Steve Gillis, PhD BOD Investor ( Immunex , Bluebird) Kurt Graves BOD Independent (Vertex) Michael Higgins BOD - Audit Chair (Genzyme) Dave Maki, JD BOD Investor (Juno, IDRI)

23 Pulmatrix Investment Opportunity Clinical - Stage Pipeline De - risked pipeline with a partnered COPD asset and 2 proprietary rare/orphan programs with multiple inflection points in the next 6 - 18 months Proprietary iSPERSE Technology E nables efficient high dose pulmonary drug delivery with reduced systemic exposure Internal engineering expertise can support development of any therapeutic Funded into 2017 $27 million in cash (as of June 16, 2015) Cash funds current pipeline beyond critical clinical and corporate milestones Experienced Leadership Management team and BOD have a demonstrated history of biotech and technology success Multiple Near Term Milestones Key clinical, corporate, and technology developments over the next 6 - 18 months will drive pipeline value

Virtual Investor Conference August 6, 2015 Robert Clarke, Ph.D. President & Chief Executive Officer NASDAQ: PULM Engineering the Future for Inhaled Therapeutics
