Attached files
Use these links to rapidly review the document
Table of Contents
Index to Financial Statements
As filed with the Securities and Exchange Commission on July 23, 2015
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 1 TO
FORM S-1
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
ZYNERBA PHARMACEUTICALS, INC.
(Exact Name Of Registrant As Specified In Its Charter)
| Delaware (State or other jurisdiction of incorporation or organization) |
2834 (Primary Standard Industrial Classification Code Number) |
26-0389433 (I.R.S. Employer Identification Number) |
80 W. Lancaster Avenue, Suite 300
Devon, PA 19333
(484) 581-7505
(Address, including zip code and telephone number, including area code, of registrant's principal executive offices)
Armando Anido
Chairman and Chief Executive Officer
80 W. Lancaster Avenue, Suite 300
Devon, PA 19333
(484) 581-7505
(Name, address, including zip code and telephone number, including area code, of agent for service)
| Copies to: | ||
Jeffrey P. Libson, Esq. Steven J. Abrams, Esq. Rachael M. Bushey, Esq. Pepper Hamilton LLP 3000 Two Logan Square 18th and Arch Streets Philadelphia, PA 19103 (215) 981-4241 |
Steven D. Singer, Esq. Lisa Firenze, Esq. Wilmer Cutler Pickering Hale and Dorr LLP 7 World Trade Center 250 Greenwich Street New York, NY 10007 (212) 295-6307 |
|
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended (the "Securities Act"), check the following box. o
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) |
Smaller reporting company o |
CALCULATION OF REGISTRATION FEE
|
||||||||
| Title of Each Class of Securities to be Registered |
Amount to be Registered(1) |
Proposed Maximum Offering Price Per Share(2) |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee(3) |
||||
|---|---|---|---|---|---|---|---|---|
Common Stock, par value $0.001 per share |
3,450,000 | $15.00 | $51,750,000 | $6,013.35 | ||||
|
||||||||
- (1)
- Includes
450,000 shares of common stock that may be sold if the underwriters exercise their option to purchase additional shares.
- (2)
- Estimated
solely for purposes of calculating the registration fee in accordance with Rule 457(a) under the Securities Act of 1933, as amended.
- (3)
- Previously paid.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Commission acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting offers to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JULY 23, 2015
PRELIMINARY PROSPECTUS
3,000,000 Shares

Zynerba Pharmaceuticals, Inc.
Common Stock
We are offering 3,000,000 shares of our common stock. This is our initial public offering, and no public market currently exists for our common stock. We expect the initial public offering price to be between $13.00 and $15.00 per share. We have applied to list our common stock on The NASDAQ Global Market under the symbol "ZYNE." We are an "emerging growth company" as defined by the Jumpstart Our Business Startups Act of 2012 and, as such, we have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock involves a high degree of risk. Please read "Risk Factors" beginning on page 12 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| |
PER SHARE | TOTAL | |||||
|---|---|---|---|---|---|---|---|
Public Offering Price |
$ | $ | |||||
Underwriting Discounts and Commissions(1) |
$ | $ | |||||
Proceeds to Zynerba Pharmaceuticals, Inc. before expenses |
$ | $ | |||||
- (1)
- We refer you to "Underwriting" beginning on page 138 of this prospectus for information regarding expenses reimbursable by us to the underwriters in connection with FINRA filings.
Certain of our existing investors, including Perceptive Advisors, LLC, have indicated an interest in purchasing up to an aggregate of approximately $12 million in our common stock in this offering at the initial public offering price. However, because indications of interest are not binding agreements or commitments to purchase, these entities may determine to not purchase any shares in this offering. It is also possible that these entities could indicate an interest in purchasing more shares of common stock. In addition, the underwriters could determine to sell fewer shares of common stock to any of these entities than such entities indicate an interest in purchasing or to not sell any shares to these entities.
Delivery of the shares of common stock is expected to be made on or about , 2015. We have granted the underwriters an option for a period of 30 days to purchase up to an additional 450,000 shares of common stock. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ .
| Joint Book-Running Managers | ||
| Jefferies | Piper Jaffray | |
| Co-Managers | ||
| Canaccord Genuity | Oppenheimer & Co. | |
Prospectus dated , 2015.
Table of Contents
Neither we nor any of the underwriters has authorized anyone to provide you with information different from, or in addition to, that contained in this prospectus or any free writing prospectus prepared by or on behalf of us or to which we may have referred you in connection with this offering. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. Neither we nor any of the underwriters is making an offer to sell or seeking offers to buy these securities in any jurisdiction where, or to any person to whom, the offer or sale is not permitted. The information in this prospectus is accurate only as of the date on the front cover of this prospectus, regardless of the time of delivery of this prospectus or of any sale of shares of our common stock and the information in any free writing prospectus that we may provide you in connection with this offering is accurate only as of the date of that free writing prospectus. Our business, financial condition, results of operations and future growth prospects may have changed since those dates.
Through and including , 2015 (25 days after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
We obtained the industry, market and competitive position data in this prospectus from our own internal estimates and research as well as from industry and general publications and research surveys and studies conducted by third parties. We believe this data is accurate in all material respects as of the date of this prospectus. In addition, projections, assumptions and estimates of the future performance of the industry in which we operate and our future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in "Risk Factors."
We have applied to register Zynerba™ as a U.S. trademark based on an intent to use in the United States. This prospectus contains references to our trademark and to trademarks belonging to other entities. Solely for convenience, trademarks and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or ™ symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies' trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other companies.
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in shares of our common stock and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus carefully, especially the "Risk Factors" beginning on page 12 and our financial statements and related notes, before deciding to buy shares of our common stock.
Unless the context indicates otherwise, as used in this prospectus, the terms "Zynerba," "Zynerba Pharmaceuticals," "we," "us," "our," "our company" and "our business" refer to Zynerba Pharmaceuticals, Inc.
Company Overview
We are a ten-year-old specialty pharmaceutical company focused on developing and commercializing proprietary next-generation synthetic cannabinoid therapeutics formulated for transdermal delivery. Our management team is highly experienced and has a successful history of development, regulatory approval and commercialization of patch and gel transdermal delivery products. We are evaluating two patent-protected product candidates, ZYN002 and ZYN001, in five indications. We intend to study ZYN002 in patients with refractory epilepsy, Fragile X syndrome, or FXS, and osteoarthritis, or OA. We intend to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain. We expect to initiate Phase 1 clinical trials for ZYN002 in the second half of 2015 and ZYN001 by mid-2016.
Cannabinoids are a class of compounds derived from Cannabis plants. The two primary cannabinoids contained in Cannabis are cannabidiol, or CBD, and D9-tetrahydrocannabinol, or THC. Clinical and preclinical data suggest that CBD has positive effects on treating refractory epilepsy, FXS and arthritis and THC has positive effects on treating pain. Interest in cannabinoid therapeutics has increased significantly over the past several years as preclinical and clinical data has emerged highlighting the potential efficacy and safety benefits of cannabinoid therapeutics. The cannabinoid therapeutics market is expected to grow significantly due to the potential benefits these products may provide over existing therapies. In addition to ZYN002 and ZYN001 potentially offering first-line therapies to patients suffering from FXS, OA, fibromyalgia and peripheral neuropathic pain, we believe ZYN002 may provide a complementary treatment for patients suffering from epilepsy who are refractory to their current treatment regimens.
We believe that we offer an attractive alternative to existing cannabinoid therapies by synthetically manufacturing and transdermally delivering our product candidates. Most cannabinoid therapies have drawbacks and limitations due to their botanical (plant-derived) nature, as well as the fact that they are administered orally. Botanical cannabinoids create significant challenges for drug manufacturers because of the natural resources and security measures required to grow Cannabis, as well as the strict batch controls required by regulatory agencies in pharmaceutical manufacturing. In addition, we believe all currently approved and development-stage cannabinoid therapeutics, except ZYN002 and ZYN001, are designed to be administered orally which can lead to limitations in safety and efficacy including low bioavailability, inconsistent plasma levels, degradation by stomach acids, and significant first-pass liver metabolism. First-pass liver metabolism refers to the process by which the liver breaks down therapeutics ingested directly or indirectly through the gastrointestinal system, such as through oral or oral mucosal delivery methods, allowing only a small amount of drug to be absorbed into the circulatory system. In contrast, transdermal therapeutics are absorbed through the skin directly into the systemic circulation, avoiding first-pass liver metabolism and degradation by stomach acids, and potentially enabling lower dosage levels of active pharmaceutical ingredients and rapid and reliable absorption with high bioavailability, fewer negative psychoactive effects and fewer drug-drug interactions.
1
We have assembled a highly experienced management team, each of whom has over 25 years of pharmaceutical industry experience, including our chief executive officer and president, who have a track record of success for obtaining regulatory approval of and commercializing products using transdermal delivery. Armando Anido, our chairman and chief executive officer, previously served as the chief executive officer of two publicly traded companies, Auxilium Pharmaceuticals Inc., or Auxilium, and NuPathe, Inc., or NuPathe. Terri B. Sebree, our president, previously co-founded NuPathe and served as senior vice president, development at Auxilium, and has successfully developed ten products from Investigational New Drug Application to regulatory approval. Ms. Sebree most recently oversaw the development and regulatory approval of Testim® gel and Zecuity® patch. Richard A. Baron, our chief financial officer, has extensive experience as chief financial officer of public and private pharmaceutical companies, most recently having served as chief financial officer of Globus Medical, Inc. and, prior to that, at Avid Radiopharmaceuticals, Inc.
Our Product Candidates
Our patent-protected synthetic transdermal cannabinoid product candidates, ZYN002 and ZYN001, represent next-generation cannabinoid therapeutics for several indications including refractory epilepsy, FXS, OA, fibromyalgia and peripheral neuropathic pain. Treatments for these indications represent markets with underserved patient populations which we believe can benefit from cannabinoid therapies. With the FXS indication, we have requested orphan drug designation from the Food and Drug Administration, or FDA, in the second half of 2015. We believe our proprietary synthetic transdermal product candidates will effectively address these indications and provide a solution to the limitations of botanically-derived and oral and oral mucosal delivered cannabinoid therapeutics.
ZYN002 is the first and only synthetic CBD formulated as a permeation-enhanced gel for transdermal delivery, and is patent-protected through 2030. In preclinical animal studies, ZYN002's permeation enhancer increased delivery of CBD through the layers of the skin and into the circulatory system. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, an in vitro study performed by us demonstrated that CBD is degraded to THC in an acidic environment such as the stomach. We believe such degradation may lead to increased psychoactive effects, which may be avoided or minimized with the transdermal delivery of ZYN002, which avoids the gastrointestinal tract and potential stomach acid degradation. ZYN002 is being developed as a clear, odorless gel with once- or twice-daily dosing.
We plan to evaluate ZYN002 in patients with refractory epilepsy, FXS and OA. Epilepsy is a disease characterized by an enduring predisposition to generate epileptic seizures (transient symptoms due to abnormal neuronal activity in the brain) and by the neurobiological, cognitive, psychological and social consequences of the condition. FXS is a genetic condition that causes autism-like symptoms including intellectual disability, anxiety disorders, behavioral and learning challenges and various physical characteristics. OA is a degenerative joint disease that leads to wear and tear of the joints and, in some patients, significant inflammation and involves the cartilage, joint lining, ligaments and bone.
2
The table below summarizes our target indications, expected type of therapy and market size with regard to each target indication for ZYN002.
| |
|
2012 U.S. Market Size(1) | ||||
|---|---|---|---|---|---|---|
Target Indication
|
Expected Type Of Therapy | Patient Population | Current Market | |||
Refractory Epilepsy |
Adjunctive, second-line therapy in patients with partial seizures with secondary generalization on a stable dose of an anticonvulsant with a history of failure | 2.2 million | $1.7 billion | |||
Fragile X Syndrome |
Monotherapy, first-line therapy in patients with FXS | 71,000 | There are no FDA-approved therapies | |||
Osteoarthritis |
Monotherapy, first-line therapy in patients with OA | 129.5 million | $670.0 million | |||
- (1)
- Except for FXS data, based on data provided by Decision Resources. Data for epilepsy represents the market size for all types of epilepsy. FXS data based on 2012 U.S. Census data and data provided by the National Fragile X Foundation.
ZYN001 is a pro-drug of THC that enables transdermal delivery via a patch and is patent-protected through 2031. A pro-drug is a drug administered in an inactive or less active form and designed to enable more effective delivery, which is then converted into an active form through a normal metabolic process. In addition, we expect that ZYN001 will be classified by the FDA as a new chemical entity, or NCE. The transdermal patch is a non-invasive, non-oral dosage form that has been proven to be an effective method of delivery in other FDA approved products. In our preclinical animal studies, ZYN001 demonstrated effective skin permeation with sustained delivery and rapid conversion of ZYN001 to THC. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism.
We intend to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain. Fibromyalgia is a chronic pain syndrome that can be considered a neurosensory disorder characterized in part by abnormalities in pain processing by the central nervous system. Patients suffer from widespread pain, stiffness, fatigue, disrupted and unrefreshing sleep, and cognitive difficulties. Patients may also experience symptoms such as headache, anxiety and/or depression and gastrointestinal distress, all of which lead to impairment of daily activities. Fibromyalgia typically presents in middle-aged women, but it can affect patients of either sex and at any age. Neuropathic pain is defined as pain initiated or caused by a primary lesion or dysfunction of the central or peripheral nervous systems. In patients with peripheral neuropathic pain, the pain is a symptom of another disease that has caused nerve damage — such as a herniated disc (lower back pain), diabetes (diabetic neuropathy), cancer (neuropathic cancer pain), or herpes zoster infection (postherpetic neuralgia) — but it is recognized as a clinical condition on its own. Because the damage does not involve the brain or spinal cord, the resulting neuropathic pain is defined as peripheral.
The table below summarizes our target indications, expected type of therapy and market size with regard to each target indication for ZYN001.
| |
|
2012 U.S. Market Size(1) | ||||
|---|---|---|---|---|---|---|
Target Indication
|
Expected Type Of Therapy | Patient Population | Current Market | |||
Fibromyalgia |
Monotherapy, first-line therapy in patients with fibromyalgia | 5.6 million | $1.6 billion | |||
Peripheral Neuropathic Pain |
Monotherapy, first-line therapy in patients with peripheral neuropathic pain | 14.0 million | $4.0 billion | |||
- (1)
- Based on data provided by Decision Resources.
3
Product Development
We plan to evaluate the tolerability and pharmacokinetics, or PK, profile of both ZYN002 and ZYN001 in Phase 1 single rising dose clinical trials in healthy human subjects (and in patients with refractory epilepsy for ZYN002). Subsequent to the single rising dose clinical trials, we intend to conduct Phase 1 multiple rising dose clinical trials to examine the tolerability, PK and pharmacodynamics, or PD, of multiple doses of each compound in healthy human subjects and in patients with refractory epilepsy for ZYN002 and in patients with fibromyalgia for ZYN001. To complete the Phase 1 program for both product candidates, we will conduct bioequivalence clinical trials assessing the PK when applied to various parts of the body (e.g., arm, thigh and back).
We intend to initiate a Phase 2a randomized, double-blind, placebo-controlled clinical trial comparing the efficacy and safety of multiple doses of ZYN002 to placebo in refractory epilepsy and OA. We intend to initiate an open label Phase 2a clinical trial in FXS to evaluate efficacy and safety. We also intend to initiate a Phase 2a randomized, double-blind, placebo-controlled clinical trial comparing the efficacy and safety of multiple doses of ZYN001 to placebo in fibromyalgia and peripheral neuropathic pain.
Depending on the results of the ZYN002 and ZYN001 Phase 2a clinical trials, we may need to further define the dosing in Phase 2b clinical trials or we may proceed directly into Phase 3 clinical trials.
We intend to use the data from the Phase 2 clinical trials outlined above to select doses of ZYN002 and ZYN001 for our Phase 3 program, which will consist of two randomized, double-blind, placebo-controlled clinical trials for each indication and open-label long-term clinical trials.
We plan to conduct our Phase 1, and possibly Phase 2, clinical trials for ZYN002 in Australia (subject to applicable regulatory approval), and do not expect at this time to file an Investigational New Drug Application, or IND, with the FDA prior to the commencement of those clinical trials. We must file an IND with the FDA and receive approval from the U.S. Drug Enforcement Agency, or DEA, prior to commencement of any clinical trial in the United States. We plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. We plan to submit New Drug Applications, or NDAs, for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials.
Our key development programs and expected timelines for the development of ZYN002 and ZYN001 are shown in the table below:
Product Candidate |
Target Indication | Delivery Method | Current Development Status |
Expected Next Steps | ||||
|---|---|---|---|---|---|---|---|---|
| ZYN002 | Refractory Epilepsy | Permeation-enhanced Gel | Preclinical | 2H15: Initiate Phase 1 | ||||
| Fragile X Syndrome | 2H16: Initiate Phase 2a | |||||||
| Osteoarthritis | ||||||||
| ZYN001 | Fibromyalgia | Transdermal Patch | Preclinical | Mid-2016: Initiate Phase 1 | ||||
| Peripheral Neuropathic Pain | 1H17: Initiate Phase 2a | |||||||
| | | | | | | | | |
Our Intellectual Property
Our intellectual property related to ZYN002 and ZYN001 was internally developed. Our ZYN002 patent portfolio currently consists of two issued patents in the United States, five issued patents in France, Germany, Ireland, Switzerland and the United Kingdom and two pending patent applications in Canada and Japan. The issued patents will expire between 2026 and 2029, and any patents that issue from our currently pending patent applications will expire in 2030. Our ZYN001 patent portfolio currently consists of two issued patents in the United States, one issued patent in Japan, one allowed patent in Europe and patent applications pending in the United States, Europe, Canada and Japan. The issued patents will expire
4
between 2028 and 2031, and any patents that issue from our currently pending patent applications will expire in 2028.
Our Strengths
We are the first and only company developing patent-protected synthetic transdermal cannabinoid therapeutics with the following key distinguishing characteristics:
Exceptional and experienced management team with proven track record. We have a sophisticated and experienced management team, each of whom has over 25 years of pharmaceutical industry experience, including our chief executive officer and president, who have a successful history of development, regulatory approval and commercialization of patch and gel transdermal delivery products.
Unique delivery methods. We are the first and only company developing patent-protected synthetic cannabinoid therapeutics for transdermal delivery. Transdermal delivery has a range of potential benefits including the ability to provide sustained and consistent plasma levels, controlled delivery and convenient dosing, as well as the avoidance of the first-pass liver metabolism and stomach acid degradation and an alternative for patients for whom oral formulations are suboptimal.
Synthetically manufactured pure cannabinoid therapeutics. Our product candidates are synthetically manufactured rather than extracted from Cannabis plants. We believe synthetically produced cannabinoids offer several advantages to botanically-derived cannabinoids, including consistent, reproducible pharmaceutical-grade active ingredients with well-defined impurity profiles.
Targeting indications with significant unmet medical need. We believe that our product candidates can provide effective treatment to patients with significant unmet medical needs in large markets, which will increase the probability of commercial success if our product candidates are approved.
Strong intellectual property protection for our product candidates. Our patent portfolio provides a long window for development and commercialization and is not specific to any single indication, which we believe will allow us to develop products for additional patient populations in markets with significant unmet medical need.
Our Business Strategy
Our goal is to become a leader in the cannabinoid pharmaceuticals market by pursuing the following strategies:
- §
- Rapidly advance ZYN002 and ZYN001 through clinical
development to regulatory approval in the United States.
- §
- Explore collaborations to develop and pursue regulatory
approval of ZYN002 and ZYN001 outside the United States.
- §
- Explore additional indications and product candidates for
synthetic CBD and THC.
- §
- Strengthen our competitive position by maintaining
leadership in the transdermal synthetic cannabinoid therapeutics market and broadening our intellectual property rights.
- §
- Commercialize ZYN002 and ZYN001 in the United States independently or with third parties.
5
Risks Associated with Our Business
Our ability to implement our business strategy is subject to numerous risks and uncertainties. As a preclinical-stage specialty pharmaceutical company, we face many risks inherent in our business and our industry generally. You should carefully consider all of the information set forth in this prospectus and, in particular, the information under the heading "Risk Factors," beginning on page 12, prior to making an investment in our common stock. These risks include, among others, the following:
- §
- We have no commercial revenue, may never become profitable and will incur
substantial and increasing net losses for the foreseeable future as we continue development of, and seek regulatory approvals for, ZYN002 and ZYN001;
- §
- We will need to raise additional capital to continue operations, including the
development of ZYN002 and ZYN001;
- §
- We have limited resources which may inhibit our ability to commence clinical
trials of ZYN002 in 2015 and ZYN001 in 2016;
- §
- We are subject to regulatory approval processes that are lengthy, time consuming
and unpredictable, and we may not obtain approval for ZYN002 or ZYN001 from the FDA or foreign regulatory authorities;
- §
- Even if we achieve regulatory approval, our success is dependent on the
effective commercialization of ZYN002 and ZYN001;
- §
- Our product candidates will be subject to controlled substances laws and
regulations, including approval, oversight and scheduling by the DEA;
- §
- It is difficult and costly to protect our intellectual property rights;
- §
- We may be unable to recruit or retain key employees, including our senior
management team;
- §
- We depend on the performance of third parties, including contract research
organizations, or CROs, and third-party manufacturers; and
- §
- Our government grants are conditioned upon audits and could require us to repay funds previously awarded to us.
Our Corporate Information
We were incorporated in Delaware in January 2007 under the name AllTranz, Inc., and in June 2007 we merged with AllTranz LLC, a Kentucky limited liability company that was founded in 2004 by Audra Stinchcomb, a pharmacologist and transdermal expert, with AllTranz, Inc. surviving. In May 2014, we were reorganized and recapitalized pursuant to an agreement and plan of merger whereby BCM Partners IV, Corp., a non-operating entity owned by BCM X1 Holdings, LLC and Audra Stinchcomb, two of our principal stockholders at that time, was merged with and into Alltranz, Inc., with Alltranz, Inc. surviving. In August 2014, AllTranz, Inc. changed its name to Zynerba Pharmaceuticals, Inc. See "Certain Relationships and Related Party Transactions — Agreements with Broadband Capital Management — Agreement and Plan of Merger" in this prospectus.
Our primary executive offices are located at 80 W. Lancaster Avenue, Suite 300, Devon, PA 19333 and our telephone number is (484) 581-7505. Our website address is www.zynerba.com. The information contained in, or that can be accessed through, our website is not part of this prospectus.
We have applied to register Zynerba as a U.S. trademark. All other trademarks, trade names or service marks referred to in this prospectus are the property of their respective owners.
6
Implications of Being an Emerging Growth Company
We are an "emerging growth company," as defined in Section 2(a) of the Securities Act of 1933, as amended, or the Securities Act, as modified by the Jumpstart Our Business Startups Act of 2012, or JOBS Act. As such, we are eligible to take advantage of exemptions from various disclosure and reporting requirements that are applicable to other public companies that are not "emerging growth companies" including, but not limited to:
- §
- our exemption from the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002;
- §
- being permitted to present only two years of audited financial statements and only two years of related Management's Discussion and Analysis of Financial Condition and Results of Operations, in each case, instead of three years;
- §
- being permitted to present the same number of years of selected financial data as the years of audited financial statements presented, instead of five years;
- §
- reduced disclosure obligations regarding executive compensation, including no Compensation Disclosure and Analysis;
- §
- our exemption from any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements; and
- §
- our exemption from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We may rely on these provisions until the last day of our fiscal year following the fifth anniversary of the closing of this offering. However, if certain events occur prior to the end of such period, including if we become a "large accelerated filer," our annual gross revenue exceeds $1.0 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such period.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
The JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. We have irrevocably elected not to avail ourselves of this exemption and, therefore, we will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
7
Common stock offered by us |
3,000,000 shares | |
Common stock to be outstanding after this offering |
8,733,963 shares |
|
Option to purchase additional shares |
We have granted to the underwriters the option, exercisable for 30 days from the date of this prospectus, to purchase up to 450,000 additional shares of common stock. |
|
Use of proceeds |
We estimate that the net proceeds to us from this offering, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us, will be approximately $36.3 million. This assumes a public offering price of $14.00, which is the midpoint of the price range set forth on the cover page of this prospectus. We intend to use the net proceeds from this offering for the following purposes: |
|
|
§ approximately $16.4 million to fund development efforts of ZYN002; |
|
|
§ approximately $14.2 million to fund development efforts of ZYN001; and |
|
|
§ the remainder to fund working capital and research and development and for general corporate purposes. |
|
|
See "Use of Proceeds" for more information. |
|
Directed share program |
The underwriters have reserved for sale, at the initial public offering price, up to approximately 5% of the shares of our common stock being offered. These shares will be offered for sale to our directors and director nominees; officers; existing stockholders and their affiliates and employees of both; and business associates, as well as certain friends and family members of our directors and officers. We will offer these shares to the extent permitted under applicable regulations in the United States. The number of shares available for sale to the general public in this offering will be reduced to the extent these persons purchase reserved shares. Any reserved shares not purchased will be offered by the underwriters to the general public on the same terms as the other shares. |
|
Risk factors |
You should read the "Risk Factors" section beginning on page 12 of this prospectus for a discussion of certain of the factors to consider carefully before deciding to purchase any shares of our common stock. |
|
Listing |
We have applied to list our common stock on The NASDAQ Global Market under the symbol "ZYNE." |
8
Dividend Policy |
We do not anticipate declaring or paying, in the foreseeable future, any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. See "Dividend Policy" for more information. |
|
Lock-Up Agreements |
We, along with our directors, executive officers and substantially all of our other securityholders, have agreed with the underwriters that for a period of 180 days, after the date of this prospectus, subject to specified exceptions, we or they will not offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock. |
The number of shares of our common stock to be outstanding after this offering is based on 2,029,747 shares of common stock outstanding as of March 31, 2015, and assumes:
- §
- the issuance by us of 3,000,000 shares of our common stock in this
offering; and
- §
- the conversion of all of our convertible preferred stock outstanding immediately prior to the closing of this offering into an aggregate of 3,704,216 shares of common stock.
and excludes:
- §
- 606,379 shares of common stock issuable upon the exercise of outstanding
stock options as of July 23, 2015, at a weighted-average exercise price of $3.98 per share; and
- §
- 1,263,739 shares of our common stock reserved for future issuance under our Amended and Restated 2014 Omnibus Incentive Compensation Plan, or the 2014 Equity Plan, as amended effective upon the closing of this offering.
Unless otherwise indicated, all information contained in this prospectus assumes or gives effect to:
- §
- a 1 for 1.88 reverse stock split of our common stock to be effected prior
to the effectiveness of the registration statement of which this prospectus forms a part;
- §
- the filing of our sixth amended and restated certificate of incorporation and
the adoption of our amended and restated bylaws immediately prior to the closing of this offering; and
- §
- no exercise by the underwriters of their option to purchase up to an additional 450,000 shares of our common stock.
9
The following summary financial data should be read together with our financial statements and accompanying notes, "Selected Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing elsewhere in this prospectus. We derived the summary statements of operations data for the years ended December 31, 2013 and 2014 from our audited financial statements and accompanying notes appearing elsewhere in this prospectus. We derived the summary statements of operations data for the three months ended March 31, 2014 and 2015 and the summary balance sheet data as of March 31, 2015 from our unaudited financial statements and accompanying notes appearing elsewhere in this prospectus. The summary financial data in this section are not intended to replace our financial statements and the related notes. Our unaudited financial statements have been prepared on the same basis as the audited financial statements and, in the opinion of management, include adjustments, consisting of normal recurring adjustments and accruals necessary for a fair statement of the information for the interim periods. Our historical results are not necessarily indicative of the results that may be expected in the future and results from our interim period may not necessarily be indicative of the results of the entire year.
Statements of Operation Data:
| |
Years Ended December 31, | Three Months Ended March 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | 2015 | |||||||||
| |
|
|
(unaudited) |
||||||||||
Revenues |
$ | 943,904 | $ | 810,012 | $ | 146,287 | $ | 14,828 | |||||
Operating expenses: |
|||||||||||||
Research and development |
1,134,041 | 2,401,406 | 285,725 | 853,704 | |||||||||
General and administrative |
444,302 | 4,076,339 | 99,704 | 653,773 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
1,578,343 | 6,477,745 | 385,429 | 1,507,477 | |||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(634,439 | ) | (5,667,733 | ) | (239,142 | ) | (1,492,649 | ) | |||||
Other income (expense): |
|||||||||||||
Interest (expense) income, net |
(2,351 | ) | (1,844 | ) | (1,217 | ) | 680 | ||||||
| | | | | | | | | | | | | | |
Net loss |
(636,790 | ) | (5,669,577 | ) | (240,359 | ) | (1,491,969 | ) | |||||
Accretion of redeemable convertible preferred stock |
(161,834 | ) | (87,954 | ) | (87,954 | ) | — | ||||||
| | | | | | | | | | | | | | |
Net loss applicable to common stockholders |
$ | (798,624 | ) | $ | (5,757,531 | ) | $ | (328,313 | ) | $ | (1,491,969 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Per share information: |
|||||||||||||
Net loss per share basic and diluted |
$ | (1.63 | ) | $ | (6.44 | ) | $ | (0.67 | ) | $ | (0.74 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic and diluted weighted average shares outstanding |
490,760 | 894,575 | 490,760 | 2,029,747 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma net loss (unaudited)(1) |
$ |
(5,669,577 |
) |
$ |
(1,491,969 |
) |
|||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma net loss per share basic and diluted (unaudited)(1) |
$ | (2.56 | ) | $ | (0.26 | ) | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma basic and diluted weighted average shares outstanding (unaudited)(1) |
2,215,507 | 5,733,963 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- Refer to note 2(k) of our audited financial statements and note 1(e) to our unaudited financial statements for a description of the method used to calculate net loss per share, basic and diluted, and pro forma net loss per share basic and diluted and the basic and diluted weighted average shares outstanding.
10
| |
As of March 31, 2015 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual | Pro forma(1) | Pro forma as adjusted(2) |
|||||||
| |
|
(unaudited) |
(unaudited) |
|||||||
BALANCE SHEET DATA: |
||||||||||
Cash and cash equivalents |
$ | 7,375,975 | $ | 7,375,975 | $ | 43,635,975 | ||||
Total assets |
10,316,797 | 10,316,797 | 46,576,797 | |||||||
Total liabilities |
3,337,630 | 3,337,630 | 3,337,630 | |||||||
Convertible preferred stock |
16,522,811 | — | — | |||||||
Total stockholders' equity (deficit) |
(9,543,644 | ) | 6,979,167 | 43,239,167 | ||||||
- (1)
- Pro
forma summary balance sheet data includes the effects of the impact of the automatic conversion of all outstanding shares of
Series 1 convertible preferred stock into shares of common stock upon the closing of this offering. The shares of common stock and any related proceeds are excluded from the pro forma
information.
- (2)
- Reflects on a pro forma as adjusted basis the automatic conversion of our Series 1 convertible preferred stock described in (1) and the sale and issuance by us of 3,000,000 shares of common stock in this offering at the assumed initial public offering price of $14.00 per share, the midpoint of the price range set forth on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses. Each $1.00 increase (decrease) in the assumed initial public offering price of $14.00 per share would increase (decrease) each of cash and cash equivalents, total assets and total stockholders' equity (deficit) by approximately $2.8 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. Each increase (decrease) of 100,000 in the number of shares offered by us would increase (decrease) each of cash and cash equivalents, total assets and total stockholders' equity (deficit) by approximately $1.3 million, assuming that the assumed initial public offering price remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will adjust based on the actual initial public offering price and other terms of this offering determined at pricing.
11
Investing in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus, including our financial statements and related notes included elsewhere in this prospectus, before making an investment decision. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that event, the trading price of our common stock could decline and you could lose part or all of your investment.
Risks Related to Our Financial Position and Capital Needs
We have incurred significant losses since our inception and anticipate that we will continue to incur losses in the future.
We are a preclinical stage specialty pharmaceutical company, engaged in developing next-generation transdermal synthetic cannabinoid therapeutics. Since our inception in January 2007, we have devoted substantially all of our resources to the development of our product candidates, ZYN002 and ZYN001. We have generated significant operating losses since our inception. Our net losses for the years ended December 31, 2013 and 2014 and for the three months ended March 31, 2015 were approximately $636,790, $5.7 million and $1.5 million, respectively. As of March 31, 2015, we had an accumulated deficit of $11.5 million. Substantially all of our losses have resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations.
We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate these losses will increase as we continue the research and development of, and clinical trials for, our product candidates. In addition to budgeted expenses, we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. If either of our product candidates fails in clinical trials or does not gain regulatory approval, or even if approved, fails to achieve market acceptance, we may never become profitable. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
Due to our limited operating history and history of losses, any predictions about our future success, performance or viability may not be accurate.
We currently have no commercial revenue and may never become profitable.
To date, the only revenue we have generated has been from the receipt of research grants and payments for research services. Our ability to generate revenue and become profitable depends upon our ability to obtain regulatory approval for, and successfully commercialize, ZYN002, ZYN001 or other product candidates that we may develop, in-license or acquire in the future.
Even if we are able to successfully achieve regulatory approval for these product candidates, we do not know what the reimbursement status of our product candidates will be or when any of these products will generate revenue for us, if at all. We have not generated, and do not expect to generate, any product revenue for the foreseeable future, and we expect to continue to incur significant operating losses for the foreseeable future due to the cost of research and development, preclinical studies and clinical trials and the regulatory approval process for our product candidates. The amount of future losses is uncertain and will depend, in part, on the rate of growth of our expenses.
12
Our ability to generate revenue from our product candidates also depends on a number of additional factors, including our ability to:
- §
- successfully complete development activities, including the remaining
preclinical studies and planned clinical trials for our product candidates;
- §
- complete and submit New Drug Applications, or NDAs, to the U.S. Food and Drug
Administration, or FDA, and Marketing Authorisation Applications, or MAAs, to the European Medicines Agency, or EMA, and obtain regulatory approval for indications for which there is a commercial
market;
- §
- complete and submit applications to, and obtain regulatory approval from, other
foreign regulatory authorities;
- §
- manufacture any approved products in commercial quantities and on commercially
reasonable terms;
- §
- develop a commercial organization, or find suitable partners, to market, sell
and distribute approved products in the markets in which we have retained commercialization rights;
- §
- achieve acceptance among patients, clinicians and advocacy groups for any
products we develop;
- §
- obtain coverage and adequate reimbursement from third parties, including
government payors; and
- §
- set a commercially viable price for any products for which we may receive approval.
We are unable to predict the timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. Even if we are able to complete the processes described above, we anticipate incurring significant costs associated with commercializing our product candidates.
We will require additional capital to fund our operations and if we fail to obtain necessary financing, we will not be able to complete the development and commercialization of ZYN002 or ZYN001.
Our operations have consumed substantial amounts of cash since inception. We expect to continue to spend substantial and increasing amounts to conduct further research and development, preclinical testing and clinical trials of our product candidates, to seek regulatory approvals and reimbursement for our product candidates and to launch and commercialize any product candidates for which we receive regulatory approval. As of March 31, 2015, we had approximately $7.4 million in cash and cash equivalents. We expect that the net proceeds from this offering and our existing cash and cash equivalents will be sufficient to fund our operations and capital requirements for the next 24 months. We believe that these available funds will be sufficient to complete (i) Phase 1 clinical trials for ZYN002 and three Phase 2a clinical trials for this product candidate, one for each target indication of refractory epilepsy, FXS and OA and (ii) Phase 1 clinical trials for ZYN001 and two Phase 2a clinical trials for this product candidate, one for each target indication of fibromyalgia and peripheral neuropathic pain. The progress of ZYN002 and ZYN001 for each target indication is uncertain due to numerous factors, including, without limitation, the rate of progress of clinical trials, the results of preclinical studies and clinical trials for such indication, the costs and timing of seeking and obtaining FDA and other regulatory approvals for clinical trials and FDA guidance regarding clinical trials for such indication. In addition, it is difficult to predict our spending for our product candidates prior to obtaining FDA approval. Moreover, changing circumstances may cause us to expend cash significantly faster than we currently anticipate, and we may need to spend more cash than currently expected because of circumstances beyond our control. For these reasons, we are unable to estimate the actual funds we will require for development and any approved marketing and commercialization activities. Our future funding requirements, both near and long-term, will depend on many factors, including, but not limited to:
- §
- the initiation, progress, timing, costs and results of preclinical studies and
clinical trials for our product candidates;
- §
- the clinical development plans we establish for these product candidates;
- §
- the number and characteristics of product candidates that we develop or may
in-license;
- §
- the terms of any collaboration agreements we may choose to execute;
13
- §
- the outcome, timing and cost of meeting regulatory requirements established by
the U.S. Drug Enforcement Administration, or DEA, the FDA, the EMA or other comparable foreign regulatory authorities;
- §
- the cost of filing, prosecuting, defending and enforcing our patent claims and
other intellectual property rights;
- §
- the cost of defending intellectual property disputes, including patent
infringement actions brought by third parties against us;
- §
- the effect of competing product and market developments;
- §
- costs and timing of the implementation of commercial scale manufacturing
activities; and
- §
- the cost of establishing, or outsourcing, sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own.
We cannot be certain that additional funding will be available on acceptable terms, or at all. If we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of one or more of our product candidates or one or more of our other research and development initiatives.
Our federal and state government grants could subject us to audits and could require us to repay substantial amounts of funds previously awarded to us.
To date, most of our revenue has been from the receipt of state and federal research grants. As of March 31, 2015 we have been granted approximately $7.9 million in federal and state research grants. In connection with these grants, we may be subject to routine audits by government agencies. As part of an audit, these agencies may review our performance, cost structures and compliance with applicable laws, regulations, policies and standards and the terms and conditions of the grant. If any of our expenditures are found to be unallowable or allocated improperly or if we have otherwise violated terms of the grant, the expenditures may not be reimbursed and/or we may be required to repay funds already disbursed. Accordingly, an audit could result in a material adjustment to our results of operations and financial condition.
Raising additional capital may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
We may seek additional capital through a combination of private and public equity offerings, debt financings, strategic partnerships and alliances and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, existing ownership interests will be diluted and the terms of such financings may include liquidation or other preferences that adversely affect the rights of existing stockholders. Debt financings may be coupled with an equity component, such as warrants to purchase shares, which could also result in dilution of our existing stockholders' ownership. The incurrence of indebtedness would result in increased fixed payment obligations and could also result in certain restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire or license intellectual property rights and other operating restrictions that could adversely impact our ability to conduct our business and may result in liens being placed on our assets and intellectual property. If we were to default on such indebtedness, we could lose such assets and intellectual property. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our product candidates, or grant licenses on terms that are not favorable to us.
14
Risks Related to our Business and Industry
We are largely dependant on the success of our product candidates, ZYN002 and ZYN001, which are still in preclinical development and will require significant capital resources and years of clinical development effort.
We currently have no products on the market, and our product candidates, ZYN002 and ZYN001, are still in preclinical development. Our business depends almost entirely on the successful clinical development, regulatory approval and commercialization of ZYN002 and ZYN001, and additional preclinical testing and substantial clinical development and regulatory approval efforts will be required before we are permitted to commence commercialization, if ever. It will be several years before we can commence and complete a pivotal study for ZYN002 or ZYN001, if ever. For ZYN002, we plan to conduct Phase 1, and possibly Phase 2, clinical trials in Australia, subject to applicable regulatory approval. We plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. We plan to submit NDAs for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials. The clinical trials and manufacturing and marketing of ZYN002 and ZYN001 will be subject to extensive and rigorous review and regulation by numerous government authorities in the United States, Australia, the European Union, Canada, and other jurisdictions where we intend to test and, if approved, market our product candidates. Before obtaining regulatory approvals for the commercial sale of any product candidate, we must demonstrate through preclinical testing and clinical trials that the product candidate is safe and effective for use in each target indication, and potentially in specific patient populations. This process can take many years and may include post-marketing studies and surveillance, which would require the expenditure of substantial resources beyond the proceeds we raise in this offering. Of the large number of drugs in development for approval in the United States and the European Union, only a small percentage successfully complete the FDA or EMA regulatory approval processes, as applicable, and are commercialized. Accordingly, even if we are able to obtain the requisite financing to continue to fund our research, development and clinical programs, we cannot assure you that any of our product candidates will be successfully developed or commercialized.
Because the results of preclinical testing are not necessarily predictive of future results, ZYN002 and ZYN001 may not have favorable results in our planned clinical trials.
Any positive results from our preclinical testing of ZYN002 and ZYN001 may not necessarily be predictive of the results from our planned clinical trials in humans. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical trials after achieving positive results in preclinical development, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in clinical trials, including adverse events. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain FDA or EMA approval. If we fail to produce positive results in our clinical trials of ZYN002 and ZYN001, the development timeline and regulatory approval and commercialization prospects for ZYN002 and ZYN001, and, correspondingly, our business and financial prospects, would be materially adversely affected.
We may not be able to commence clinical trials in 2015; even if ZYN002 and ZYN001 advance into clinical trials, we may experience difficulties in managing our growth and expanding our operations.
We have not begun clinical trials for any of our product candidates. While we expect to commence clinical trials in Australia in 2015 for ZYN002, we have limited resources to carry out these objectives. In addition, we do not expect at this time to file an IND with the FDA prior to the commencement of these trials. Our company has no history of conducting clinical trials, which is a time-consuming, expensive and uncertain process. In addition, while we have experienced management and expect to contract out many of the
15
activities related to conducting clinical trials, we are a small company with only seven employees and therefore have limited internal resources both to conduct clinical trials and to monitor third-party providers. As our product candidates enter into and advance through preclinical studies and any clinical trials, we will need to expand our development, regulatory and manufacturing operations, either by expanding our internal capabilities or contracting with other organizations to provide these capabilities for us. In the future, we expect to have to manage additional relationships with collaborators or partners, suppliers and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures.
Failures or delays in the completion of our preclinical studies or the commencement and completion of our planned clinical trials of ZYN002 or ZYN001 could result in increased costs to us and could delay, prevent or limit our ability to generate revenue and continue our business.
To date, we have not commenced any clinical trials for ZYN002 or ZYN001. Successful completion of such clinical trials is a prerequisite to submitting an NDA to the FDA or an MAA to the EMA. Clinical trials are expensive, difficult to design and implement, can take many years to complete and are uncertain as to outcome. A product candidate can unexpectedly fail at any stage of clinical development. The historic failure rate for product candidates is high due to scientific feasibility, safety, efficacy, changing standards of medical care and other variables. We expect to initiate clinical trials for ZYN002 in the second half of 2015. However, we do not know whether our clinical trials will begin or be completed on schedule, if at all, as the commencement and completion of clinical trials can be delayed or prevented for a number of reasons, including, among others:
- §
- delays in reaching or failing to reach agreement on acceptable terms with
prospective clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different clinical trial sites;
- §
- delays or inability in manufacturing or obtaining sufficient quantity or quality
of a product candidate or other materials necessary to conduct clinical trials due to regulatory and manufacturing constraints;
- §
- difficulties obtaining institutional review board, or IRB, DEA or comparable
foreign regulatory authority, or ethics committee approval to conduct a clinical trial at a prospective site or sites;
- §
- challenges in recruiting and enrolling patients to participate in clinical
trials, including the size and nature of the patient population, the proximity of patients to clinical trial sites, eligibility criteria for the clinical trial, the nature of the clinical trial
protocol, the availability of approved effective treatments for the relevant indication and competition from other clinical trial programs for similar indications;
- §
- severe or unexpected toxicities or drug-related side effects experienced by
patients in our clinical trials or by individuals using drugs similar to our product candidates;
- §
- DEA or comparable foreign regulatory authority-related recordkeeping, reporting
or security violations at a clinical trial site, leading the DEA, state authorities or comparable foreign regulatory authorities to suspend or revoke the site's controlled substance license and
causing a delay or termination of planned or ongoing clinical trials;
- §
- regulatory concerns with cannabinoid products generally and the potential for
abuse of those products;
- §
- difficulties retaining patients who have enrolled in a clinical trial who may
withdraw due to lack of efficacy, side effects, personal issues or loss of interest;
- §
- ambiguous or negative interim results; or
- §
- lack of adequate funding to continue the clinical trial.
16
In addition, a clinical trial may be suspended or terminated by us, the FDA, IRBs, ethics committees, data safety monitoring board or other foreign regulatory authorities overseeing the clinical trial at issue or other regulatory authorities due to a number of factors, including, among others:
- §
- failure to conduct the clinical trial in accordance with regulatory requirements
or our clinical trial protocols;
- §
- inspection of the clinical trial operations or clinical trial sites by the FDA,
the DEA, the EMA or other foreign regulatory authorities that reveals deficiencies or violations that require us to undertake corrective action, including the imposition of a clinical hold;
- §
- unforeseen safety issues, including any safety issues that could be identified
in our ongoing toxicology studies;
- §
- adverse side effects or lack of effectiveness; and
- §
- changes in government regulations or administrative actions.
We intend to expend our limited resources to pursue ZYN002 and ZYN001 for certain indications, and may fail to capitalize on other product candidates or other indications for ZYN002 or ZYN001 that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we are focusing on research programs relating to ZYN002 and ZYN001 for certain indications, which concentrates the risk of product failure in the event ZYN002 or ZYN001 proves to be unsafe or ineffective or inadequate for clinical development or commercialization. In particular, we intend to study ZYN002 in patients with refractory epilepsy, Fragile X syndrome, or FXS, and osteoarthritis and we intend to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications for ZYN002 or ZYN001 that could later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on proprietary research and development programs relating to ZYN002 and ZYN001 may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for ZYN002 and ZYN001, we may relinquish valuable rights to ZYN002 or ZYN001 through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to ZYN002 or ZYN001.
The regulatory approval processes of the FDA, the EMA and other comparable foreign regulatory authorities are lengthy, time-consuming and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidates, our business will be substantially harmed.
We are not permitted to market our product candidates in the United States or the European Union until we receive approval of an NDA from the FDA or an MAA from the EMA, respectively, or in any foreign countries until we receive the requisite approval from such countries. Prior to submitting an NDA to the FDA or an MAA to the EMA for approval of our product candidates we will need to complete our ongoing preclinical studies, as well as Phase 1, Phase 2 and Phase 3 clinical trials. We are still conducting preclinical studies and have not yet commenced our clinical program or tested ZYN002 or ZYN001 in humans. For ZYN002, we plan to conduct Phase 1, and possibly Phase 2, clinical trials in Australia, subject to applicable regulatory approval. We plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. We plan to submit NDAs for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials. Successfully initiating and completing our clinical program and obtaining approval of an NDA or MAA is a complex, lengthy, expensive and uncertain process, and the FDA or EMA may delay, limit or deny approval of our product candidates for many reasons, including, among others, because:
- §
- we may not be able to demonstrate that our product candidates are safe and effective in treating patients to the satisfaction of the FDA or EMA;
17
- §
- the results of our clinical trials may not meet the level of statistical or
clinical significance required by the FDA or EMA for marketing approval;
- §
- the FDA or EMA may disagree with the number, design, size, conduct or
implementation of our clinical trials;
- §
- the FDA or EMA may require that we conduct additional clinical trials;
- §
- the FDA or EMA or other applicable foreign regulatory authorities may not
approve the formulation, labeling or specifications of our product candidates;
- §
- the contract research organizations, or CROs, and other contractors that we may
retain to conduct our clinical trials may take actions outside of our control that materially adversely impact our clinical trials;
- §
- the FDA or EMA may find the data from preclinical studies and clinical trials
insufficient to demonstrate that ZYN002's or ZYN001's clinical and other benefits outweigh its safety risks;
- §
- the FDA or EMA may disagree with our interpretation of data from our preclinical
studies and clinical trials;
- §
- the FDA or EMA may not accept data generated at our clinical trial sites or may
disagree with us over whether to accept efficacy results from clinical trial sites outside the United States where the standard of care is potentially different from that in the United States;
- §
- if and when our NDAs or MAAs are submitted to the FDA or EMA, as applicable, the
regulatory agency may have difficulties scheduling the necessary review meetings in a timely manner, may recommend against approval of our application or may recommend or require, as a condition of
approval, additional preclinical studies or clinical trials, limitations on approved labeling or distribution and use restrictions;
- §
- the FDA may require development of a Risk Evaluation and Mitigation Strategy, or
REMS, which would use risk minimization strategies beyond the professional labeling to ensure that the benefits of certain prescription drugs outweigh their risks, as a condition of approval or
post-approval, and the EMA may grant only conditional approval or impose specific obligations as a condition for marketing authorization, or may require us to conduct post-authorization safety
studies;
- §
- the FDA, EMA, DEA or other applicable foreign regulatory agencies may not
approve the manufacturing processes or facilities of third-party manufacturers with which we contract or DEA or other applicable foreign regulatory agency quotas may limit the quantities of controlled
substances available to our manufacturers; or
- §
- the FDA or EMA may change their approval policies or adopt new regulations.
Any of these factors, many of which are beyond our control, could jeopardize our ability to obtain regulatory approval for and successfully market ZYN002 or ZYN001. Moreover, because our business is almost entirely dependent upon these two product candidates, any such setback in our pursuit of regulatory approval would have a material adverse effect on our business and prospects.
We plan to conduct clinical trials for ZYN002 and ZYN001 outside the United States and the FDA may not accept data from such trials.
We plan to conduct clinical trials outside the United States. For ZYN002, we plan to conduct Phase 1, and possibly Phase 2, clinical trials in Australia, subject to applicable regulatory approval. We plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. We plan to submit NDAs for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials. Although the FDA may accept data from clinical trials conducted outside the United States, acceptance of such study data by the FDA is subject to certain conditions. For example, the clinical trial must be conducted in accordance with Good Clinical Practices, or GCP, requirements and the FDA must be able to validate the data from the clinical trial through an onsite inspection if it deems such inspection necessary.
18
Where data from foreign clinical trials are intended to serve as the sole basis for marketing approval in the United States, the FDA will not approve the application on the basis of foreign data alone unless those data are applicable to the U.S. population and U.S. medical practice, the clinical trials were performed by clinical investigators of recognized competence, and the data is considered valid without the need for an on-site inspection by the FDA or, if the FDA considers such an inspection to be necessary, the FDA is able to validate the data through an on-site inspection or other appropriate means. In addition, such clinical trials would be subject to the applicable local laws of the foreign jurisdictions where the clinical trials are conducted. There can be no assurance the FDA will accept data from clinical trials conducted outside of the United States. If the FDA does not accept any such data, it would likely result in the need for additional clinical trials, which would be costly and time-consuming and delay aspects of our development plan.
In addition, the conduct of clinical trials outside the United States could have a significant impact on us. Risks inherent in conducting international clinical trials include:
- §
- foreign regulatory requirements that could burden or limit our ability to
conduct our clinical trials;
- §
- administrative burdens of conducting clinical trials under multiple foreign
regulatory schema;
- §
- foreign exchange fluctuations;
- §
- manufacturing, customs, shipment and storage requirements;
- §
- cultural differences in medical practice and clinical research; and
- §
- diminished protection of intellectual property in some countries.
Even if ZYN002 or ZYN001 receive regulatory approval, they may still face future development and regulatory difficulties.
If we obtain regulatory approval for ZYN002 or ZYN001, such approval would be subject to extensive ongoing requirements by the DEA, FDA, EMA and other foreign regulatory authorities related to the manufacture, quality control, further development, labeling, packaging, storage, distribution, safety surveillance, import, export, advertising, promotion, recordkeeping and reporting of safety and other post-market information. The safety profile of any product will continue to be closely monitored by the FDA, EMA and other comparable foreign regulatory authorities. If the FDA, EMA or any other comparable foreign regulatory authority becomes aware of new safety information after approval of any of our product candidates, these regulatory authorities may require labeling changes or establishment of a REMS, impose significant restrictions on a product's indicated uses or marketing, impose ongoing requirements for potentially costly post-approval studies or post-market surveillance or impose a recall.
In addition, manufacturers of therapeutic products and their facilities are subject to continual review and periodic inspections by the FDA, the EMA and other comparable foreign regulatory authorities for compliance with current good manufacturing practices, or cGMP, regulations. Further, manufacturers of controlled substances must obtain and maintain necessary DEA and state registrations and registrations with applicable foreign regulatory authorities, and must establish and maintain processes to ensure compliance with DEA and state requirements and requirements of applicable foreign regulatory authorities governing, among other things, the storage, handling, security, recordkeeping and reporting for controlled substances. If we or a regulatory agency discover previously unknown problems with a product, such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions on that product, the manufacturing facility or us, including requiring recall or withdrawal of the product from the market or suspension of manufacturing. If we, our product candidates or the manufacturing facilities for our product candidates fail to comply with applicable regulatory requirements, a regulatory agency may, among other things:
- §
- issue untitled letters or warning letters;
19
- §
- mandate modifications to promotional materials or require us to provide
corrective information to healthcare practitioners;
- §
- require us to enter into a consent decree, which can include imposition of
various fines, reimbursements for inspection costs, required due dates for specific actions and penalties for noncompliance;
- §
- seek an injunction or impose civil or criminal penalties or monetary fines;
- §
- suspend or withdraw regulatory approval;
- §
- suspend any ongoing clinical trials;
- §
- refuse to approve pending applications or supplements to applications filed by
us; or
- §
- require us to initiate a product recall.
The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and may otherwise have a material adverse effect on our business, financial condition and results of operations.
ZYN002 and ZYN001 will be subject to controlled substance laws and regulations; failure to receive necessary approvals may delay the launch of our products and failure to comply with these laws and regulations may adversely affect the results of our business operations.
ZYN002 and ZYN001 contain controlled substances as defined in the federal Controlled Substances Act of 1970, or CSA. Controlled substances that are pharmaceutical products are subject to a high degree of regulation under the CSA, which establishes, among other things, certain registration, manufacturing quotas, security, recordkeeping, reporting, import, export and other requirements administered by the DEA. The DEA classifies controlled substances into five schedules: Schedule I, II, III, IV or V substances. Schedule I substances by definition have a high potential for abuse, have no currently "accepted medical use" in the United States, lack accepted safety for use under medical supervision, and may not be prescribed, marketed or sold in the United States. Pharmaceutical products approved for use in the United States may be listed as Schedule II, III, IV or V, with Schedule II substances considered to present the highest potential for abuse or dependence and Schedule V substances the lowest relative risk of abuse among such substances. Schedule I and II drugs are subject to the strictest controls under the CSA, including manufacturing and procurement quotas, security requirements and criteria for importation. In addition, dispensing of Schedule II drugs is further restricted. For example, they may not be refilled without a new prescription.
While Cannabis is a Schedule I controlled substance, products approved for medical use in the United States that contain Cannabis or Cannabis extracts must be placed in Schedules II - V, since approval by the FDA satisfies the "accepted medical use" requirement. If and when ZYN002 or ZYN001 receives FDA approval, the DEA will make a scheduling determination and place it in a schedule other than Schedule I in order for it to be prescribed to patients in the United States. If approved by the FDA, we expect the finished dosage forms of ZYN002 and ZYN001 to be listed by the DEA as a Schedule II or III controlled substance. Consequently, their manufacture, importation, exportation, domestic distribution, storage, sale and legitimate use will be subject to a significant degree of regulation by the DEA. The scheduling process may take one or more years beyond FDA approval, thereby significantly delaying the launch of ZYN002 or ZYN001. Furthermore, if the FDA, DEA or any foreign regulatory authority determines that ZYN002 or ZYN001 may have potential for abuse, it may require us to generate more clinical data than that which is currently anticipated, which could increase the cost and/or delay the launch of ZYN002 or ZYN001.
Because ZYN002 and ZYN001 contain active ingredients of Cannabis, which are Schedule I substances, to conduct preclinical studies and clinical trials with ZYN002 and ZYN001 in the United States prior to approval, each of our research sites must submit a research protocol to the DEA and obtain and maintain a DEA researcher registration that will allow those sites to handle and dispense ZYN002 and ZYN001 and to obtain the product from our manufacturer. If the DEA delays or denies the grant of a research registration to one or more research sites, the preclinical studies or clinical trials could be significantly delayed, and we could lose and be required to replace clinical trial sites, resulting in additional costs.
20
We expect that ZYN002 and ZYN001 will be scheduled as Schedule II or III, as a result of which we will also need to identify wholesale distributors with the appropriate DEA registrations and authority to distribute the products to pharmacies and other healthcare providers, and these distributors would need to obtain Schedule II or III distribution registrations. The failure to obtain, or delay in obtaining, or the loss of any of those registrations could result in increased costs to us. If ZYN002 or ZYN001 is a Schedule II drug, pharmacies would have to maintain enhanced security with alarms and monitoring systems and they must adhere to recordkeeping and inventory requirements. This may discourage some pharmacies from carrying the product. Furthermore, state and federal enforcement actions, regulatory requirements, and legislation intended to reduce prescription drug abuse, such as the requirement that physicians consult a state prescription drug monitoring program, may make physicians less willing to prescribe, and pharmacies to dispense, Schedule II products.
We may manufacture the commercial supply of ZYN002 and ZYN001 outside of the United States. If ZYN002 or ZYN001 is approved by the FDA and classified as a Schedule II or III substance, an importer can import for commercial purposes if it obtains from the DEA an importer registration and files an application with the DEA for an import permit for each import. The DEA provides annual assessments/estimates to the International Narcotics Control Board which guides the DEA in the amounts of controlled substances that the DEA authorizes to be imported. The failure to identify an importer or obtain the necessary import authority, including specific quantities, could affect the availability of ZYN002 or ZYN001 and have a material adverse effect on our business, results of operations and financial condition. In addition, an application for a Schedule II importer registration must be published in the Federal Register, and there is a waiting period for third party comments to be submitted.
Individual states have also established controlled substance laws and regulations. Though state-controlled substance laws often mirror federal law, because the states are separate jurisdictions, they may separately schedule our product candidates as well. While some states automatically schedule a drug based on federal action, other states schedule drugs through rulemaking or a legislative action. State scheduling may delay commercial sale of any product for which we obtain federal regulatory approval and adverse scheduling could have a material adverse effect on the commercial attractiveness of such product. We or our partners must also obtain separate state registrations, permits or licenses in order to be able to obtain, handle, and distribute controlled substances for clinical trials or commercial sale, and failure to meet applicable regulatory requirements could lead to enforcement and sanctions by the states in addition to those from the DEA or otherwise arising under federal law.
We currently manufacture the API for ZYN002 and ZYN001 in the United States and Canada. For ZYN002, we plan to conduct Phase 1, and possibly Phase 2, clinical trials in Australia, subject to applicable regulatory approval. We plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. In addition, we may decide to develop, manufacture or commercialize our product candidates in additional countries. As a result, ZYN002 and ZYN001 will also be subject to controlled substance laws and regulations from the Therapeutic Goods Administration in Australia, Health Canada's Office of Controlled Substances in Canada, and from other regulatory agencies in other countries where we may develop, manufacture or commercialize ZYN002 or ZYN001 in the future. We plan to submit NDAs for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials and will require additional DEA approvals at such time as well.
Product shipment delays could have a material adverse effect on our business, results of operations and financial condition.
The shipment, import and export of ZYN002 and ZYN001 and the API used to manufacture ZYN002 and ZYN001 will require import and export licenses. In the United States, the FDA, U.S. Customs and Border Protection, and the DEA, in Canada, where our API is manufactured, the Canada Border Services Agency and Health Canada, in Australia, where we will commence clinical trials, the Australian Customs and Board Protection Service and the Therapeutic Goods Administration, and in other countries, similar regulatory
21
authorities, regulate the import and export of pharmaceutical products that contain controlled substances. Specifically, the import and export process requires the issuance of import and export licenses by the relevant controlled substance authority in both the importing and exporting country. We may not be granted, or if granted, maintain, such licenses from the authorities in certain countries. Even if we obtain the relevant licenses, shipments of API and our product candidates may be held up in transit, which could cause significant delays and may lead to product batches being stored outside required temperature ranges. Inappropriate storage may damage the product shipment resulting in delays in clinical trials or, upon commercialization, a partial or total loss of revenue from one or more shipments of API or ZYN002 or ZYN001. A delay in a clinical trial or, upon commercialization, a partial or total loss of revenue from one or more shipments of API or ZYN002 or ZYN001 could have a material adverse effect on our business, results of operations and financial condition.
Failure to obtain regulatory approval in jurisdictions outside the United States and the European Union would prevent our product candidates from being marketed in those jurisdictions.
In order to market and sell our products in jurisdictions other than the United States and the European Union, we must obtain separate marketing approvals and comply with numerous and varying regulatory requirements. The regulatory approval process outside the United States and the European Union generally includes all of the risks associated with obtaining FDA and EMA approval, but can involve additional testing. We may need to partner with third parties in order to obtain approvals outside the United States and the European Union. In addition, in many countries worldwide, it is required that the product be approved for reimbursement before the product can be approved for sale in that country. We may not obtain approvals from regulatory authorities outside the United States and the European Union on a timely basis, if at all. Even if we were to receive approval in the United States or the European Union, approval by the FDA or the EMA does not ensure approval by regulatory authorities in other countries or jurisdictions. Similarly, approval by one regulatory authority outside the United States and the European Union would not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA or the EMA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our products in any market. If we are unable to obtain approval of our product candidates by regulatory authorities in other foreign jurisdictions, the commercial prospects of those product candidates may be significantly diminished and our business prospects could decline.
Healthcare legislation, including potentially unfavorable pricing regulations or other healthcare reform initiatives, may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates.
In the United States there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system that could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities or affect our ability to profitably sell any product candidates for which we obtain marketing approval.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or Affordable Care Act, among other things, imposes a significant annual fee on companies that manufacture or import branded prescription drug products. It also contains substantial provisions intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against healthcare fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers, and impose additional health policy reforms, any of which could negatively impact our business. A significant number of provisions are not yet, or have only recently become effective, but the Affordable Care Act is likely to continue the downward pressure on pharmaceutical and medical device pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
22
In addition, other legislative changes have been proposed and adopted since passage of the Affordable Care Act. The Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of an amount greater than $1.2 trillion for the fiscal years 2012 through 2021, triggering the legislation's automatic reduction to several government programs. This included aggregate reductions to Medicare payments to healthcare providers of up to 2.0% per fiscal year, which went into effect in April 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several categories of healthcare providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. If we ever obtain regulatory approval and successfully commercialize ZYN002, ZYN001 or other product candidates that we may develop, these new laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and accordingly, our financial operations.
We expect that the Affordable Care Act, as well as other healthcare reform measures that have been and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product, and could seriously harm our future revenues. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may compromise our ability to generate revenue, attain profitability or commercialize our products.
We have requested orphan drug status for ZYN002 for the treatment of FXS, but we may be unable to obtain such designation or to maintain the benefits associated orphan drug status, including market exclusivity, which may cause our revenue, if any, to be reduced.
Regulatory authorities in some jurisdictions, including the United States and European Union, may designate drugs for relatively small patient populations as orphan drugs. The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals annually in the United States, or, if the disease or condition affects more than 200,000 individuals annually in the United States, if there is no reasonable expectation that the cost of developing and making the drug would be recovered from sales in the United States. In the European Union, the EMA's Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the European Union community. Additionally, designation is granted for products intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the drug.
In the United States, orphan drug designation entitles a party to financial incentives, such as opportunities for grant funding towards clinical trial costs, tax credits for certain research and user fee waivers under certain circumstances. In addition, if a product receives the first FDA approval for the indication for which it has orphan drug designation, the product is entitled to seven years of market exclusivity, which means the FDA may not approve any other application for the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition. In the European Union, orphan drug designation also entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market exclusivity following drug approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable so that market exclusivity is no longer justified.
23
As a result, even if ZYN002 receives orphan drug exclusivity in FXS, the FDA or EMA can still approve other drugs that have a different active ingredient for use in treating the same indication. Furthermore, the FDA can waive orphan drug exclusivity if we are unable to manufacture sufficient supply of ZYN002 or the EMA could reduce the term of exclusivity if ZYN002 is sufficiently profitable.
We have requested orphan drug designation for ZYN002 in FXS with the FDA and may seek orphan drug designation in the future with the EMA, but exclusive marketing rights in the United States may be limited if we seek approval for an indication broader than the orphan designated indication and may be lost if the FDA or EMA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition. In addition, although we have requested orphan drug designation for ZYN002, we may never receive such designation, or there may be a delay in receiving such designation that would impact our expected timeframe for clinical development.
Even if we are able to commercialize ZYN002 or ZYN001, the products may not receive coverage and adequate reimbursement from third-party payors, which could harm our business.
The availability of reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments. Sales of our product candidates, if approved, will depend substantially on the extent to which the costs of these product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by government health administration authorities, private health coverage insurers and other third-party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize ZYN002 or ZYN001. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or Medicare Modernization Act, established the Medicare Part D program and provided authority for limiting the number of drugs that will be covered in any therapeutic class thereunder. The Medicare Modernization Act, including its cost reduction initiatives, could decrease the coverage and reimbursement rate that we receive for any of our approved products. Furthermore, private payors often follow Medicare coverage policies and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, or HHS, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors tend to follow CMS to a substantial degree.
The intended use of a drug product by a physician can also affect pricing. For example, CMS could initiate a National Coverage Determination administrative procedure, by which the agency determines which uses of a therapeutic product would and would not be reimbursable under Medicare. This determination process can be lengthy, thereby creating a long period during which the future reimbursement for a particular product may be uncertain.
Outside the United States, particularly in member states of the European Union, the pricing of prescription drugs is subject to governmental control. In these countries, pricing negotiations or the successful completion of health technology assessment procedures with governmental authorities can take considerable time after receipt of marketing approval for a product. In addition, there can be considerable pressure by governments and other stakeholders on prices and reimbursement levels, including as part of cost
24
containment measures. Certain countries allow companies to fix their own prices for medicines, but monitor and control company profits. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various European Union member states and parallel distribution, or arbitrage between low-priced and high-priced member states, can further reduce prices. In some countries, we or our collaborators may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Publication of discounts by third-party payors or authorities may lead to further pressure on the prices or reimbursement levels within the country of publication and other countries. If reimbursement of any product candidate approved for marketing is unavailable or limited in scope or amount, or if pricing is set at unsatisfactory levels, our business, financial condition, results of operations or prospects could be adversely affected.
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and third-party payors play a primary role in the recommendation and prescription of any product candidates for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. As a pharmaceutical company, even though we do not and will not control referrals of healthcare services or bill directly to Medicare, Medicaid or other third-party payors, certain federal and state healthcare laws and regulations pertaining to fraud and abuse and patients' rights are and will be applicable to our business. Restrictions under applicable federal and state healthcare laws and regulations that may affect our ability to operate include the following:
- §
- the U.S. federal healthcare Anti-Kickback Statute impacts our marketing
practices, educational programs, pricing policies and relationships with healthcare providers or other entities, by prohibiting, among other things, persons from knowingly and willfully soliciting,
offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or
recommendation of, any good or service, for which payment may be made under a federal healthcare program such as Medicare and Medicaid;
- §
- federal civil and criminal false claims laws and civil monetary penalty laws
impose criminal and civil penalties, including through civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal
government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent (including through impermissible promotion of our products for off-label uses) or making a
false statement or record to avoid, decrease or conceal an obligation to pay money to the federal government;
- §
- the U.S. federal Health Insurance Portability and Accountability Act of 1996, or
HIPAA, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also created federal criminal laws that prohibit knowingly and willfully falsifying,
concealing or covering up a material fact or making any materially false statements in connection with the delivery of or payment for healthcare benefits, items or services;
- §
- HIPAA, and the rules and regulations promulgated thereunder, establish federal standards for maintaining the privacy and security of certain patient health information known as Protected Health Information, or PHI. As amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, HIPAA establishes federal standards for administrative, technical and physical safeguards relevant to the electronic transmission of PHI and imposes notification obligations in the event of a breach of the privacy or security of PHI. In addition to adhering to the requirements of HIPAA, entities considered "covered entities" under HIPAA (such as health plans,
25
- §
- the U.S. federal physician payment transparency requirements under the
Affordable Care Act require applicable manufacturers of covered drugs, devices, biologics and medical supplies to report annually to HHS information related to payments and other transfers of value to
physicians, certain other healthcare providers, and teaching hospitals, and ownership and investment interests held by physicians and certain other healthcare providers and their immediate family
members and applicable group purchasing organizations; and
- §
- analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers. Some state laws require pharmaceutical companies to comply with the pharmaceutical industry's voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government and may require drug manufacturers to report information related to payments and other transfers of value to physicians and certain other healthcare providers or marketing expenditures. Additionally, state and foreign laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA/HITECH, thus complicating compliance efforts.
healthcare clearinghouses, and certain healthcare providers) are required to obtain assurances in the form of a written contract from certain business associates to which they transmit PHI (or who create, receive, transmit or maintain PHI on the covered entity's behalf) to ensure that the privacy and security of such information is maintained in accordance with HIPAA requirements. HITECH made changes to HIPAA including extending the reach of HIPAA beyond HIPAA covered entities to business associates, increased the maximum civil monetary penalties for violations of HIPAA, and granted enforcement authority to state attorneys general. Failure to comply with HIPAA/HITECH can result in civil and criminal liability, including civil monetary penalties, fines and imprisonment;
Comparable laws and regulations exist in the countries within the European Economic Area, or EEA. Although such laws are partially based upon European Union law, they may vary from country to country. Healthcare specific, as well as general European Union and national laws, regulations and industry codes constrain, for example, our interactions with government officials and healthcare practitioners, and the handling of healthcare data. Non-compliance with any of these laws or regulations could lead to criminal or civil liability.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, imprisonment, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any physicians or other healthcare providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Also, the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to non-U.S. officials for the purpose of obtaining or retaining business. Our internal control policies and procedures may not protect us from reckless or negligent acts committed by our employees, future distributors, licensees or agents. Violations of these laws, or allegations of such violations, could result in fines, penalties or prosecution and have a negative impact on our business, results of operations and reputation.
26
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could subject us to significant liability and harm our reputation.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with DEA, FDA or EMA regulations or similar regulations of other foreign regulatory authorities or to provide accurate information to the DEA, FDA, EMA or other foreign regulatory authorities. In addition, misconduct by employees could include intentional failures to comply with certain manufacturing standards, to comply with U.S. federal and state healthcare fraud and abuse laws and regulations and similar laws and regulations established and enforced by comparable foreign regulatory authorities, to report financial information or data accurately or to disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and serious harm to our reputation. We plan to adopt, and will implement and enforce, a Code of Business Conduct and Ethics, which will be effective as of the effectiveness of the registration statement of which this prospectus forms a part, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity, such as employee training on enforcement of the Code of Business Conduct and Ethics, may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
If we are unable to develop sales, marketing and distribution capabilities or enter into agreements with third parties to perform these functions on acceptable terms, we may be unable to generate revenue.
We do not currently have any sales, marketing or distribution capabilities. If ZYN002 or ZYN001 is approved, we will need to develop internal sales, marketing and distribution capabilities to commercialize such products, which would be expensive and time-consuming, or enter into collaborations with third parties to perform these services. If we decide to market our products directly, we will need to commit significant financial and managerial resources to develop a marketing and sales force with technical expertise and supporting distribution, administration and compliance capabilities. If we rely on third parties with such capabilities to market our products or decide to co-promote products with collaborators, we will need to establish and maintain marketing and distribution arrangements with third parties, and there can be no assurance that we will be able to enter into such arrangements on acceptable terms or at all. In entering into third-party marketing or distribution arrangements, any revenue we receive will depend upon the efforts of the third parties and there can be no assurance that such third parties will establish adequate sales and distribution capabilities or be successful in gaining market acceptance of any approved product. If we are not successful in commercializing any product approved in the future, either on our own or through third parties, our business, financial condition and results of operations could be materially adversely affected.
Our product candidates, if approved, may be unable to achieve broad market acceptance and, consequently, limit our ability to generate revenue from new products.
Even when product development is successful and regulatory approval has been obtained, our ability to generate significant revenue depends on the acceptance of our products by physicians and patients. The market acceptance of any product depends on a number of factors, including the indication statement and warnings approved by regulatory authorities in the product label, continued demonstration of efficacy and safety in commercial use, physicians' willingness to prescribe the product, reimbursement from third-party
27
payors such as government healthcare systems and insurance companies, the price of the product, the nature of any post-approval risk management plans mandated by regulatory authorities, competition, and marketing and distribution support. Any factors preventing or limiting the market acceptance of our product candidates could have a material adverse effect on our business, results of operations and financial condition.
If we receive regulatory approvals, we intend to market ZYN002 and ZYN001 in multiple jurisdictions where we have limited or no operating experience and may be subject to increased business and economic risks that could affect our financial results.
If we receive regulatory approvals, we plan to market ZYN002 and ZYN001 in jurisdictions where we have limited or no experience in marketing, developing and distributing our products. Certain markets have substantial legal and regulatory complexities that we may not have experience navigating. We are subject to a variety of risks inherent in doing business internationally, including risks related to the legal and regulatory environment in non-U.S. jurisdictions, including with respect to privacy and data security, trade control laws and unexpected changes in laws, regulatory requirements and enforcement, as well as risks related to fluctuations in currency exchange rates and political, social and economic instability in foreign countries. If we are unable to manage our international operations successfully, our financial results could be adversely affected.
In addition, controlled substance legislation may differ in other jurisdictions and could restrict our ability to market our products internationally. Most countries are parties to the Single Convention on Narcotic Drugs 1961, which governs international trade and domestic control of narcotic substances, including Cannabis extracts. Countries may interpret and implement their treaty obligations in a way that creates a legal obstacle to us obtaining marketing approval for ZYN002 or ZYN001 in those countries. These countries may not be willing or able to amend or otherwise modify their laws and regulations to permit ZYN002 or ZYN001 to be marketed, or achieving such amendments to the laws and regulations may take a prolonged period of time. We would be unable to market ZYN002 or ZYN001 in countries with such obstacles in the near future or perhaps at all without modification to laws and regulations.
ZYN002 and ZYN001 contain controlled substances, the use of which may generate public controversy.
Since our product candidates contain controlled substances, their regulatory approval may generate public controversy. Political and social pressures and adverse publicity could lead to delays in approval of, and increased expenses for, our product candidates. These pressures could also limit or restrict the introduction and marketing of our product candidates. Adverse publicity from Cannabis misuse or adverse side effects from Cannabis or other cannabinoid products may adversely affect the commercial success or market penetration achievable by our product candidates. The nature of our business attracts a high level of public and media interest, and in the event of any resultant adverse publicity, our reputation may be harmed.
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of key management and other specialized personnel, including Armando Anido, our chairman and chief executive officer, Terri B. Sebree, our president, Richard A. Baron, our chief financial officer, and Suzanne M. Hanlon, our general counsel and vice president of human resources. The loss of one or more members of our management team or other key employees could delay our research and development programs and materially harm our business, financial condition, results of operations and prospects. The relationships that our team has cultivated within the life sciences industry makes us particularly dependent upon their continued employment with us. Because our management team is not obligated to provide us with continued service, they could terminate their employment or services with us at any time without penalty, subject to providing any required advance notice. We do not maintain key person life insurance policies for any members of our management team.
28
Our future success and growth will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations.
We face substantial competition, which may result in others discovering, developing or commercializing products before or more successfully than we do.
The development and commercialization of drugs is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as products and processes being developed at universities and other research institutions. Our competitors have developed, are developing or will develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community and any new treatments that enter the market. We believe that a significant number of products are currently available, under development, and may become commercially available in the future, for the treatment of indications for which we may try to develop product candidates. If either of our product candidates, ZYN002 or ZYN001, is approved for the indications we are currently pursuing, it will compete with a range of therapeutic treatments that are either in development or currently marketed.
We are aware of multiple companies that are working in the Cannabis therapeutic area, including pharmaceutical companies such as GW Pharmaceuticals PLC, or GW, which markets Sativex, a botanical cannabinoid oral mucosal for the treatment of spasticity due to multiple sclerosis and which is also in development in neuropathic pain in several foreign countries and is seeking FDA approval in the United States, and is developing Epidiolex, a liquid formulation of highly purified CBD extract, as a treatment for Dravet's Syndrome, Lennox Gastaut Syndrome, and various childhood epilepsy syndromes; Insys Therapeutics, Inc., which is seeking FDA approval for an orally-administered liquid formulation of its synthetic CBD compound as a treatment for Dravet's Syndrome, Lennox Gastaut Syndrome, and other childhood epilepsy syndromes; and Nemus Bioscience, Inc. which is focused on the discovery, development and commercialization of Cannabis therapeutics.
More established companies may have a competitive advantage over us due to their greater size, cash flows and institutional experience. Compared to us, many of our competitors may have significantly greater financial, technical and human resources. As a result of these factors, our competitors may have an advantage in marketing their approved products and may obtain regulatory approval of their product candidates before we are able to, which may limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs that are safer, more effective, more widely used and less expensive than ours, and may also be more successful than us in manufacturing and marketing their products. These advantages could materially impact our ability to develop and commercialize ZYN002 or ZYN001 successfully.
Our product candidates may compete with non-synthetic cannabinoid drugs, including therapies such as GW's Sativex. Our product candidates may also compete with medical and recreational marijuana, in markets where the recreational and/or medical use of marijuana is legal. There is support in the United States for further legalization of marijuana. In markets where recreational and/or medical marijuana is not legal, our product candidates may compete with marijuana purchased in the illegal drug market. We cannot assess the extent to which patients may utilize marijuana obtained illegally for the treatment of the indications for which we are developing ZYN002 and ZYN001.
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining
29
qualified scientific, management and commercial personnel, establishing clinical trial sites and subject registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
The market opportunity for refractory epilepsy will be limited to those patients who are not currently receiving adequate relief from current treatment regimens, which may reduce our targeted market.
Pre-existing treatments may be adequate to treat certain patients with refractory epilepsy. Whenever the first-line therapy fails or is unsuccessful, then second-line therapy may be administered. For refractory epilepsy, ZYN002 is particularly targeted to provide an additional treatment option for patients not currently receiving adequate relief from current treatment regimens. If a more successful first-line therapy is developed, it may significantly reduce the patient population to which we can supply, which may affect our ability to successfully commercialize ZYN002 for refractory epilepsy.
Product liability lawsuits against us could cause us to incur substantial liabilities.
Our planned use of ZYN002 and ZYN001 in clinical trials and the sale of ZYN002 and ZYN001, if approved, exposes us to the risk of product liability claims. Product liability claims might be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with ZYN002 or ZYN001. For example, we may be sued if any product we develop allegedly causes injury or is found to be otherwise unsuitable during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, including as a result of interactions with alcohol or other drugs, negligence, strict liability, and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we become subject to product liability claims and cannot successfully defend ourselves against them, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in, among other things:
- §
- withdrawal of patients from our clinical trials;
- §
- substantial monetary awards to patients or other claimants;
- §
- decreased demand for ZYN002 or ZYN001 following marketing approval, if
obtained;
- §
- damage to our reputation and exposure to adverse publicity;
- §
- increased FDA or EMA warnings on product labels;
- §
- litigation costs;
- §
- distraction of management's attention from our primary business;
- §
- loss of revenue; and
- §
- the inability to successfully commercialize ZYN002 or ZYN001, if approved.
We will need to obtain product liability insurance coverage for our clinical trials. We may not be able to obtain such coverage at a reasonable cost or in sufficient amounts to protect us against losses, including if insurance coverage becomes increasingly expensive. Large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. The cost of any product liability litigation or other proceedings, even if resolved in our favor, could be substantial, particularly in light of the size of our business and financial resources. A product liability claim or series of claims brought against us could cause our share price to decline and, if we are unsuccessful in defending such a claim or claims and the resulting judgments exceed our insurance coverage, our financial condition, results of operations, business and prospects could be materially adversely affected.
Our business and operations would suffer in the event of computer system failures.
Despite the implementation of security measures, our information technology and other internal infrastructure systems and those of our CROs and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and
30
telecommunication and electrical failures. A significant disruption in the availability of our information technology and other internal infrastructure systems could cause delays in our research and development work. For instance, the loss of preclinical data or data from any future clinical trial involving our product candidates could result in delays in our development and regulatory filing efforts and significantly increase our costs. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the development of our product candidates could be delayed.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct our preclinical studies and clinical trials. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates.
We rely on CROs, clinical data management organizations and consultants to design, conduct, supervise and monitor preclinical studies of our product candidates and may do the same for our planned clinical trials. We and our CROs are required to comply with various regulations, including GCP, which are enforced by the FDA, and guidelines of the Competent Authorities of Member States of the EEA and comparable foreign regulatory authorities to ensure that the health, safety and rights of patients are protected in clinical development and clinical trials, and that trial data integrity is assured. Regulatory authorities ensure compliance with these requirements through periodic inspections of trial sponsors, principal investigators and trial sites. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. If we or any of our CROs fail to comply with applicable requirements, the clinical data generated in our clinical trials may be deemed unreliable and the FDA, EMA or other comparable foreign regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. We cannot assure you that upon inspection by a given regulatory authority, such regulatory authority will determine that any of our clinical trials comply with such requirements. In addition, our clinical trials must be conducted with products produced under cGMP requirements, which mandate the methods, facilities and controls used in manufacturing, processing and packaging of a drug product to ensure its safety and identity. Failure to comply with these regulations may require us to repeat preclinical and clinical trials, which would delay the regulatory approval process.
Our CROs are not our employees, and except for remedies available to us under our agreements with such CROs, we cannot control whether or not they devote sufficient time and resources to our ongoing clinical and preclinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines or if the quality or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for or successfully commercialize our product candidates. As a result, our operations and the commercial prospects for our product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
Because we have relied on third parties, our internal capacity to perform these functions is limited. Outsourcing these functions involves risk that third parties may not perform to our standards, may not produce results in a timely manner or may fail to perform at all. In addition, the use of third-party service providers requires us to disclose our proprietary information to these parties, which could increase the risk that this information will be misappropriated. We currently have a small number of employees, which limits the internal resources we have available to identify and monitor our third-party providers. To the extent we are unable to identify and successfully manage the performance of third-party service providers in the future, our business may be adversely affected. Though we carefully manage our relationships with our CROs, there can be no assurance that we will not encounter similar challenges or delays in the future or that these delays or challenges will not have a material adverse impact on our business, financial condition and prospects.
31
We rely on third-party manufacturers and suppliers and we intend to rely on third parties to produce preclinical, clinical and commercial supplies of active pharmaceutical ingredients, or APIs, for ZYN002 and ZYN001.
We rely on third parties to supply the materials for, and manufacture, our research and development, preclinical and clinical trial APIs. We do not own manufacturing facilities or supply sources for such components and materials. There can be no assurance that our supply of research and development, preclinical and clinical development drugs and other materials will not be limited, interrupted, restricted in certain geographic regions or of satisfactory quality or continue to be available at acceptable prices. In particular, any replacement of our API manufacturer could require significant effort and expertise because there may be a limited number of qualified manufacturers.
The manufacturing process for our product candidates is subject to FDA, EMA, DEA and other foreign regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards such as cGMP. In addition, our manufacturers must ensure therapeutic consistency among batches, including preclinical, clinical and, if approved, marketing batches. Demonstrating such consistency may require typical manufacturing controls as well as clinical data. Our manufacturers must also ensure that our batches conform to complex release specifications. Further, manufacturers of controlled substances must obtain and maintain necessary DEA and state registrations and registrations with applicable foreign regulatory authorities, and must establish and maintain processes to ensure compliance with DEA and state requirements and requirements of applicable foreign regulatory authorities governing, among other things, the storage, handling, security, recordkeeping and reporting for controlled substances. In the event that any of our suppliers or manufacturers fails to comply with such requirements or to perform its obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to manufacture the materials ourselves, for which we currently do not have the capabilities or resources, or enter into an agreement with another third party, which we may not be able to do on reasonable terms, if at all. In some cases, the technical skills or technology required to manufacture our product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third party and a feasible alternative may not exist. These factors would increase our reliance on such manufacturer or require us to obtain a license from such manufacturer in order to have another third party manufacture our product candidates. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop product candidates in a timely manner or within budget.
We expect to continue to rely on third-party manufacturers if we receive regulatory approval for any product candidate. To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially reasonable terms, we may not be able to develop and commercialize our product candidates successfully. Our or a third party's failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
- §
- an inability to initiate or continue preclinical studies or clinical trials of
product candidates under development;
- §
- delay in submitting regulatory applications, or receiving regulatory approvals,
for product candidates;
- §
- loss of the cooperation of a collaborator;
- §
- subjecting our product candidates to additional inspections by regulatory authorities; and
32
- §
- in the event of approval to market and commercialize a product candidate, an inability to meet commercial demands for our products.
If a collaborative partner terminates or fails to perform its obligations under an agreement with us, the commercialization of ZYN002 or ZYN001, if approved, could be delayed or terminated.
We are not currently party to any collaborative arrangements for the commercialization of ZYN002 or ZYN001, if approved, or similar arrangements, although we may pursue such arrangements before any commercialization of ZYN002 or ZYN001, if approved. If we entered into future collaborative arrangements for the commercialization of any product candidate or similar arrangements and any of our collaborative partners does not devote sufficient time and resources to a collaboration arrangement with us, we may not realize the potential commercial benefits of the arrangement, and our results of operations may be materially adversely affected. In addition, if any such future collaboration partner were to breach or terminate its arrangements with us, the commercialization of any product candidate could be delayed, curtailed or terminated.
Much of the potential revenue from future collaborations may consist of contingent payments, such as payments for achieving regulatory milestones or royalties payable on sales of drugs. The milestone and royalty revenue that we may receive under these collaborations will depend upon our collaborators' ability to successfully develop, introduce, market and sell new products. In addition, collaborators may decide to enter into arrangements with third parties to commercialize products developed under collaborations using our technologies, which could reduce the milestone and royalty revenue that we may receive, if any. Future collaboration partners may fail to develop or effectively commercialize products using our products or technologies, which could have a material adverse effect on our operating results and financial condition.
Business disruptions affecting our third-party suppliers, manufacturers and CROs could harm our future revenues and financial condition and increase our costs and expenses.
We rely on third parties to supply the materials for, and manufacture our APIs for, our preclinical and clinical trials. There are only a limited number of suppliers and manufacturers of our APIs and our ability to obtain these materials could be disrupted if the operations of these manufacturers is affected by earthquakes, power shortages, telecommunications failures, water shortages, floods, hurricanes, typhoons, fires, extreme weather conditions, medical epidemics and other natural or man-made disasters or business interruptions. We also rely on CROs, clinical data management organizations and consultants to design, conduct, supervise and monitor preclinical studies of our product candidates and will do the same for our planned clinical trials. If their facilities are unable to operate because of an accident or incident, even for a short period of time, some or all of our research and development programs may be harmed or delayed and our operations and financial condition could suffer.
Our third-party manufacturers may use hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly.
Our third-party manufacturers may use hazardous materials, including chemicals and compounds that could be dangerous to human health and safety or the environment. The operations of our third-party manufacturers may also produce hazardous waste products. Federal, state and local laws and regulations govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. In the event of contamination or injury, our third-party manufacturers could be held liable for damages or be penalized with fines in an amount exceeding their resources, which could result in our clinical trials or regulatory approvals being delayed or suspended.
33
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property rights or if our intellectual property rights are inadequate for our technology and product candidates, our competitive position could be harmed.
Our commercial success will depend in large part on our ability to obtain and maintain patent and other intellectual property protection in the U.S. and other countries with respect to our proprietary technology and products. We rely on trade secret, patent, copyright and trademark laws, and confidentiality and other agreements with employees and third parties, all of which offer only limited protection. We seek to protect our proprietary position by filing and prosecuting patent applications in the United States and abroad related to our novel technologies and products that are important to our business.
The patent positions of biotechnology and pharmaceutical companies generally are highly uncertain, involve complex legal and factual questions and have in recent years been the subject of much litigation. As a result, the issuance, scope, validity, enforceability and commercial value of our patents are highly uncertain. The steps we have taken to protect our proprietary rights may not be adequate to preclude misappropriation of our proprietary information or infringement of our intellectual property rights, both inside and outside the United States. Our pending applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications. Further, the examination process may require us to narrow the claims for our pending patent applications, which may limit the scope of patent protection that may be obtained if these applications issue. We do not know whether any of the pending patent applications for any of our product candidates will result in the issuance of patents that protect our technology or products, or if any of our issued patents will effectively prevent others from commercializing competitive technologies and products. The rights already granted under any of our currently issued patents and those that may be granted under future issued patents may not provide us with the proprietary protection or competitive advantages we are seeking. If we are unable to obtain and maintain patent protection for our technology and products, or if the scope of the patent protection obtained is not sufficient, our competitors could develop and commercialize technology and products similar or superior to ours, and our ability to successfully commercialize our technology and products may be adversely affected. It is also possible that we will fail to identify patentable aspects of inventions made in the course of our development and commercialization activities before it is too late to obtain patent protection on them.
Because the issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, our issued patents may be challenged in the courts or patent offices in the U.S. and abroad. Such challenges may result in the loss of patent protection, the narrowing of claims in such patents or the invalidity or unenforceability of such patents, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection for our technology and products. Publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing. Therefore we cannot be certain that we were the first to make the inventions claimed in our owned patents or pending patent applications, or that we were the first to file for patent protection of such inventions.
Protecting against the unauthorized use of our patented technology, trademarks and other intellectual property rights is expensive, difficult and may in some cases not be possible. In some cases, it may be difficult or impossible to detect third-party infringement or misappropriation of our intellectual property rights, even in relation to issued patent claims, and proving any such infringement may be even more difficult.
34
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
The United States Patent and Trademark Office, or U.S. PTO, and various foreign national or international patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. Periodic maintenance fees on any issued patent are due to be paid to the U.S. PTO and various foreign national or international patent agencies in several stages over the lifetime of the patent. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Non-compliance events that could result in abandonment or lapse of patent rights include, but are not limited to, failure to timely file national and regional stage patent applications based on our international patent application, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. If we fail to maintain the patents and patent applications covering our product candidates, our competitors might be able to enter the market, which would have a material adverse effect on our business.
We may become subject to claims by third parties asserting that we or our employees have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates, and to use our related proprietary technologies without violating the intellectual property rights of others. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our product candidates, including interference or derivation proceedings before the U.S. PTO. Third parties may assert infringement claims against us based on existing patents or patents that may be granted in the future. If we are found to infringe a third party's intellectual property rights, we could be required to obtain a license from such third party to continue commercializing our product candidates. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Under certain circumstances, we could be forced, including by court order, to cease commercializing the applicable product candidate. In addition, in any such proceeding or litigation, we could be found liable for monetary damages. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Any claims by third parties that we have misappropriated their confidential information or trade secrets could have a similar negative impact on our business.
While our preclinical studies and clinical trials are ongoing, we believe that the use of ZYN002 and ZYN001 in these preclinical studies and clinical trials falls within the scope of the exemptions provided by 35 U.S.C. Section 271(e) in the United States, which exempts from patent infringement liability activities reasonably related to the development and submission of information to the FDA, or the Clinical Development Exemption. As ZYN002 and ZYN001 progress toward commercialization, the possibility of a patent infringement claim against us increases. We attempt to ensure that our product candidates and the methods we employ to manufacture them, as well as the methods for their uses we intend to promote, do not infringe other parties' patents and other proprietary rights. There can be no assurance they do not, however, and competitors or other parties may assert that we infringe their proprietary rights in any event.
35
We are aware of an allowed U.S. patent owned by a third party with claims that are directed to a method of treating partial seizures by administering a medication containing CBD at doses at or above 400mg. This patent could be construed to cover ZYN002 for our refractory epilepsy development program if the therapeutic dose for CBD contained in ZYN002 is determined to be at or above 400mg. According to our current understanding of ZYN002 dosing and gel formulation at 400mg or more, the application of gel quantities required to attain a dosing of 400mg or more would not be practicable. While our preclinical studies are ongoing, we believe that our development during both preclinical and clinical trials will fall within the Clinical Development Exemption. If and when ZYN002 is approved by the FDA for treatment of refractory epilepsy, if it has a label that contains dosing of ZYN002 with CBD at or above 400mg, such third party may then seek to enforce its patent, if issued, by filing a patent infringement lawsuit against us. In such lawsuit, we may incur substantial expenses defending our rights to commercialize ZYN002 for refractory epilepsy, and in connection with such lawsuit and under certain circumstances, it is possible that we could be required to cease or delay the commercialization of ZYN002 for refractory epilepsy and/or be required to pay monetary damages or other amounts, including royalties on the sales of ZYN002 for refractory epilepsy. Moreover, such lawsuit may also consume substantial time and resources of our management team and board of directors. The threat or consequences of such a lawsuit may also result in royalty and other monetary obligations, which may adversely affect our results of operations and financial condition.
We may become involved in lawsuits to protect or enforce our intellectual property, which could be expensive, time consuming and unsuccessful and have a material adverse effect on the success of our business.
Competitors may infringe our patents or misappropriate or otherwise violate our intellectual property rights. To counter infringement or unauthorized use, litigation may be necessary in the future to enforce or defend our intellectual property rights, to protect our trade secrets or to determine the validity and scope of our own intellectual property rights or the proprietary rights of others. Also, third parties may initiate legal proceedings against us to challenge the validity or scope of intellectual property rights we own. These proceedings can be expensive and time consuming. Many of our current and potential competitors have the ability to dedicate substantially greater resources to defend their intellectual property rights than we can. Accordingly, despite our efforts, we may not be able to prevent third parties from infringing upon or misappropriating our intellectual property. Litigation could result in substantial costs and diversion of management resources, which could harm our business and financial results. In addition, in an infringement proceeding, a court may decide that a patent owned by us is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of shares of our common stock.
If we are not able to adequately prevent disclosure of trade secrets and other proprietary information, the value of our technology and products could be significantly diminished.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our current and former employees, consultants, outside scientific collaborators, sponsored researchers, contract manufacturers, vendors and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of
36
confidential information. In addition, we cannot guarantee that we have executed these agreements with each party that may have or have had access to our trade secrets. Any party with whom we or they have executed such an agreement may breach that agreement and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches.
Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they disclose such trade secrets, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor or other third-party, our competitive position would be harmed.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on all of our product candidates throughout the world would be prohibitively expensive. Therefore, we have filed applications and/or obtained patents only in key markets such as the United States, Canada, Japan and Europe. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may be able to export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as that in the United States. These products may compete with our products in jurisdictions where we do not have any issued patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to pharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. For example, an April 2014 report from the Office of the United States Trade Representative identified a number of countries, including India and China, where challenges to the procurement and enforcement of patent rights have been reported. Several countries, including India and China, have been listed in the report every year since 1989. As a result, proceedings to enforce our patent rights in certain foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business and could be unsuccessful.
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. We expect to seek extensions of patent terms in the United States and, if available, in other countries where we are prosecuting patents. In the United States, the Drug Price Competition and Patent Term Restoration Act of 1984 permits a patent term extension of up to five years beyond the normal expiration of the patent, which is limited to the approved indication (or any additional indications approved during the period of extension). However, the applicable authorities, including the FDA and the U.S. PTO, and any equivalent regulatory authorities in other countries, may not agree with our assessment of whether such extensions are available, and may refuse to grant extensions to our patents, or may grant more limited extensions than we request. If this occurs, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data and launch their product earlier than might otherwise be the case.
37
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
- §
- others may be able to make compounds that are the same as or similar to our
product candidates but that are not covered by the claims of the patents that we own;
- §
- we might not have been the first to make the inventions covered by the issued
patents or pending patent applications that we own;
- §
- we might not have been the first to file patent applications covering certain of
our inventions;
- §
- others may independently develop similar or alternative technologies or
duplicate any of our technologies without infringing our intellectual property rights;
- §
- it is possible that our pending patent applications will not lead to issued
patents;
- §
- issued patents that we own may not provide us with any competitive advantages,
or may be held invalid or unenforceable as a result of legal challenges;
- §
- our competitors might conduct research and development activities in the United
States and other countries that provide a safe harbor from patent infringement claims for certain research and development activities, as well as in countries where we do not have patent rights and
then use the information learned from such activities to develop competitive products for sale in our major commercial markets;
- §
- we may not develop additional proprietary technologies that are patentable; and
- §
- the patents of others may have an adverse effect on our business.
Risks Related to This Offering and Ownership of Our Common Stock
We do not know whether an active, liquid and orderly trading market will develop for our common stock or what the market price of our common stock will be and as a result it may be difficult for you to sell your shares of our common stock.
Prior to this offering there has been no market for shares of our common stock. An active trading market for our shares may never develop or be sustained following this offering. The initial public offering price for our common stock in this offering has been determined through negotiations with the underwriters, and the negotiated price may not be indicative of the market price of our common stock after this offering. The market value of our common stock may decrease from the initial public offering price. As a result of these and other factors, you may be unable to resell your shares of our common stock at or above the initial public offering price in this offering. In addition, approximately 66% of our publicly traded common stock will be held by our executive officers, directors and existing investors after the closing of this offering. Certain of our existing investors have indicated an interest in purchasing shares of our common stock in this offering, and if they make such purchases, this percentage could increase. The lack of an active market may impair your ability to sell your shares at the time you wish to sell them or at a price that you consider reasonable. The lack of an active market may also reduce the fair market value of your shares. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into collaborations or acquire companies or products by using our shares of common stock as consideration.
The market price of our stock may be volatile, and you could lose all or part of your investment.
The trading price of our common stock following this offering is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. In addition to the factors discussed in this "Risk Factors" section and elsewhere in this prospectus, these factors include:
- §
- trading volatility of low-priced stock;
38
- §
- the success of competitive products;
- §
- regulatory actions with respect to our product candidates or our competitors'
products and product candidates;
- §
- actual or anticipated changes in our growth rate relative to our competitors;
- §
- announcements by us or our competitors of significant acquisitions, strategic
partnerships, joint ventures, collaborations or capital commitments;
- §
- results of clinical trials of ZYN002, ZYN001 or product candidates of our
competitors;
- §
- regulatory or legal developments in the United States and other countries;
- §
- developments or disputes concerning patent applications, issued patents or
other proprietary rights;
- §
- the recruitment or departure of key personnel;
- §
- the level of expenses related to our preclinical and clinical development
programs;
- §
- the results of our efforts to in-license or acquire additional product
candidates or products;
- §
- actual or anticipated changes in estimates as to financial results, development
timelines or recommendations by securities analysts;
- §
- variations in our financial results or those of companies that are perceived to
be similar to us;
- §
- fluctuations in the valuation of companies perceived by investors to be
comparable to us;
- §
- share price and volume fluctuations attributable to inconsistent trading volume
levels of our common stock;
- §
- announcement or expectation of additional financing efforts;
- §
- sales of our common stock by us, our insiders or our other stockholders;
- §
- changes in the structure of healthcare payment systems;
- §
- market conditions in the pharmaceutical sector; and
- §
- general economic, industry and market conditions.
In addition, the stock market in general, and pharmaceutical companies in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of these companies. Broad market and industry factors may negatively affect the market price of our common stock, regardless of our actual operating performance. Moreover, some institutional investors and mutual funds cannot invest in stocks priced below $5.00 per share. The realization of any of these risks or any of a broad range of other risks, including those described in these "Risk Factors," could have a dramatic and material adverse impact on the market price of our common stock.
We may be subject to securities litigation, which is expensive and could divert our management's attention.
The market price of our common stock may be volatile, and in the past companies that have experienced volatility in the market price of their stock have been subject to securities class action litigation. We may be the target of this type of litigation in the future. Securities litigation against us could result in substantial costs and divert our management's attention from other business concerns, which could seriously harm our business.
Insiders have substantial influence over us and could delay or prevent a change in corporate control.
Prior to this offering, our executive officers, directors, and holders of 5.0% or more of our capital stock collectively beneficially owned approximately 49.78% of our voting stock. After giving effect to this offering, that same group will hold approximately 32.78% of our common stock. Certain of our existing investors have indicated an interest in purchasing shares of our common stock in this offering, and if they make such purchases, this percentage could increase. This concentration of ownership could harm the market price of our common stock by:
- §
- delaying, deferring or preventing a change in control of our company;
39
- §
- impeding a merger, consolidation, takeover or other business combination
involving our company; or
- §
- discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of our company.
The interests of this group of stockholders may not always coincide with your interests or the interests of other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including by seeking a premium value for their common stock, and might negatively affect the prevailing market price for our common stock.
If you purchase shares of our common stock in this offering, you will incur immediate and substantial dilution in the book value of your shares.
The initial public offering price is substantially higher than the net tangible book value per share of our common stock. Investors purchasing shares of common stock in this offering will pay a price per share that substantially exceeds the book value of our tangible assets after subtracting our liabilities. As a result, investors purchasing shares of common stock in this offering will incur immediate dilution of $9.05 per share, which assumes an initial public offering prices of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus).
The exercise of any of our outstanding options would result in additional dilution. These issuances of common stock will result in additional dilution to investors purchasing shares in this offering. As a result of this dilution, investors may receive significantly less than the purchase price paid in this offering, if anything, in the event of our liquidation. Further, because we will need to raise additional capital to fund our clinical development programs, we may in the future sell substantial amounts of common stock or securities convertible into or exchangeable for common stock. These future issuances of equity or equity-linked securities, together with the exercise of outstanding options and any additional shares issued in connection with acquisitions, if any, may result in further dilution to investors.
We are an "emerging growth company" and we intend to take advantage of reduced disclosure and governance requirements applicable to emerging growth companies, which could result in our common stock being less attractive to investors and could make it more difficult for us to raise capital as and when we need it.
We are an "emerging growth company," as defined in the Jumpstart Our Business Startups Act of 2012, or JOBS Act, and we intend to take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile. In addition, we may be less attractive to investors and it may be difficult for us to raise additional capital as and when we need it. Investors may be unable to compare our business with other companies in our industry if they believe that our financial accounting is not as transparent as other companies in our industry. If we are unable to raise additional capital as and when we need it, our financial condition and results of operations may be materially and adversely affected.
We may take advantage of these reporting exemptions until we are no longer an emerging growth company, which in certain circumstances could be for up to five years. See "Summary — Implications of Being an Emerging Growth Company" above.
40
If we fail to maintain an effective system of internal control over financial reporting in the future, we may not be able to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
The Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. Commencing with our annual report on Form 10-K for the year ending December 31, 2016, we will be required, under Section 404 of the Sarbanes-Oxley Act, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. This assessment will need to include disclosure of any material weaknesses identified by our management in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim financial statements will not be prevented or detected on a timely basis. Section 404 of the Sarbanes-Oxley Act also generally requires an attestation from our independent registered public accounting firm on the effectiveness of our internal control over financial reporting. However, for as long as we remain an emerging growth company as defined in the JOBS Act, we intend to take advantage of the exemption permitting us not to comply with the independent registered public accounting firm attestation requirement.
Our compliance with Section 404 will require that we incur substantial accounting expense and expend significant management efforts. We currently do not have an internal audit group, and we will need to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge, and compile the system and process documentation necessary to perform the evaluation needed to comply with Section 404. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective. We cannot assure you that there will not be material weaknesses or significant deficiencies in our internal control over financial reporting in the future. Any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting once that firm begins its Section 404 reviews, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our common stock could decline, and we could be subject to sanctions or investigations by the NASDAQ Stock Market, the Securities and Exchange Commission, or SEC, or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
Upon the closing of this offering, we will become subject to the periodic reporting requirements of the Securities Exchange Act of 1934, as amended, or Exchange Act. Our disclosure controls and procedures are designed to reasonably assure that information required to be disclosed by us in reports we file or submit under the Exchange Act is accumulated and communicated to management, recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the
41
controls. Accordingly, because of the inherent limitations in our control system, misstatements or insufficient disclosures due to error or fraud may occur and not be detected.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company, and these expenses may increase even more after we are no longer an "emerging growth company." We will be subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd-Frank Wall Street Reform and Protection Act, as well as rules adopted, and to be adopted, by the SEC and NASDAQ Stock Market. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, we expect these rules and regulations to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. The increased costs will increase our net loss. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the sufficient coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers.
Because we do not anticipate paying any cash dividends on our capital stock in the foreseeable future, capital appreciation, if any, will be your sole source of gain.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business. In addition, the terms of any future debt agreements may preclude us from paying dividends. As a result, capital appreciation, if any, of our common stock will be your sole source of gain for the foreseeable future.
Sales of a substantial number of shares of our common stock in the public market could cause our stock price to fall.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. Immediately after this offering, we will have 8,733,963 outstanding shares of common stock. This includes the shares that we are selling in this offering, which may be resold in the public market immediately without restriction, unless purchased by our directors, officers or existing securityholders and therefore subject to lock-up agreements. See "Underwriting" in this prospectus. 5,687,367 shares of our common stock will be restricted as a result of securities laws or lock-up agreements but will be able to be sold after the offering as described in the "Shares Eligible for Future Sale" section of this prospectus. Moreover, after this offering, holders of an aggregate of 3,658,895 shares of our common stock will have rights, subject to certain conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We also intend to register all shares of common stock that we may issue under the 2014 Equity Plan. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the "Underwriting" section in this prospectus.
Future sales and issuances of our common stock or rights to purchase common stock pursuant to our equity incentive plan could result in additional dilution of the percentage ownership of our stockholders and could cause our stock price to fall.
Pursuant to our equity incentive plan, our compensation committee is authorized to grant equity-based incentive awards to our directors, executive officers and other employees and service providers. As of July 23, 2015, there are 102,320 shares of our common stock available for future grant under our 2014
42
Equity Plan. Future equity incentive grants and issuances of common stock under the 2014 Equity Plan may result in material dilution to our investors and may have an adverse effect on the market price of our common stock.
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Although we currently intend to use the net proceeds from this offering in the manner described in the "Use of Proceeds" section in this prospectus, our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our business, including our preclinical and clinical development programs, or enhance the value of our common stock. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the market price of our common stock to decline and delay the development of ZYN002 and ZYN001. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or that loses value. If we do not invest the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected clinical development progression, which could cause the price of our common stock to decline.
Some provisions of our charter documents and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management.
Provisions in our certificate of incorporation and bylaws that will become effective in connection with the closing of this offering, as well as provisions of Delaware law, could make it more difficult for a third party to acquire us or increase the cost of acquiring us, even if doing so would benefit our stockholders, or remove our current management. These include provisions that will:
- §
- permit our board of directors to issue up to 10 million shares of
preferred stock, with any rights, preferences and privileges as it may designate;
- §
- provide that all vacancies on our board of directors, including as a result of
newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
- §
- provide that directors can only be removed for cause;
- §
- require that any action to be taken by our stockholders must be effected at a
duly called annual or special meeting of stockholders and not be taken by written consent;
- §
- provide that stockholders seeking to present proposals before a meeting of
stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a
stockholder's notice;
- §
- require that the amendment of certain provisions of our certificate of
incorporation and bylaws relating to anti-takeover measures may only be approved by a vote of 662/3% of our outstanding capital stock;
- §
- not provide for cumulative voting rights, thereby allowing the holders of a
majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election; and
- §
- provide that special meetings of our stockholders may be called only by the board of directors or by such person or persons designated by a majority of the board of directors to call such meetings.
These provisions may frustrate or prevent any attempts by our stockholders to replace or remove our current management by making it more difficult for stockholders to replace members of our board of directors, who are responsible for appointing the members of our management. Because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may discourage, delay or prevent someone from acquiring us or merging with us whether or not it is desired by or beneficial to our stockholders. Under Delaware law, a corporation may not, in general, engage in a
43
business combination with any holder of 15.0% or more of its capital stock unless the holder has held the stock for three years or, among other things, the board of directors has approved the transaction. Any provision of our certificate of incorporation or bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our certificate of incorporation will also provide that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders' ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our certificate of incorporation that will become effective in connection with the closing of this offering provides that the Court of Chancery of the State of Delaware is the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our certificate of incorporation or our bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine. The choice of forum provision may limit a stockholder's ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provision contained in our certificate of incorporation to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could adversely affect our business and financial condition.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on our company. If no securities or industry analysts commence coverage of our company, the trading price for our stock would likely be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our stock or publish inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our stock could decrease, which might cause our stock price and trading volume to decline.
44
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the sections entitled "Prospectus Summary," "Risk Factors," "Management's Discussion and Analysis of Financial Condition and Results of Operations" and "Business," contains forward-looking statements. We may, in some cases, use words such as "anticipate," "believe," "could," "estimate," "expect," "intend," "may," "plan," "potential," "predict," "project," "should," "will," "would" or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes to identify these forward-looking statements. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. Forward-looking statements in this prospectus include, but are not limited to, statements about:
- §
- our estimates regarding expenses, future revenues, capital requirements and needs for additional financing;
- §
- the success and timing of our preclinical studies and clinical trials;
- §
- the potential results of preclinical studies and clinical trials for ZYN002 and ZYN001;
- §
- our dependence on third parties in the conduct of our preclinical studies and clinical trials;
- §
- the difficulties and expenses associated with obtaining and maintaining regulatory approval of ZYN002 and ZYN001;
- §
- our plans and ability to develop and commercialize ZYN002 and ZYN001;
- §
- the successful development of our commercialization capabilities, including sales and marketing capabilities;
- §
- the size and growth of the potential markets for ZYN002 and ZYN001, market acceptance of ZYN002 and ZYN001 and our ability to serve those markets;
- §
- the rate and degree of market acceptance of ZYN002 and ZYN001;
- §
- legal and regulatory developments in the United States and foreign countries;
- §
- our ability to limit our exposure under product liability lawsuits;
- §
- the success of competing therapies and products that are or become available;
- §
- our exposure to additional scrutiny as a public company;
- §
- our use of the proceeds from this offering;
- §
- obtaining and maintaining intellectual property protection for ZYN002 and ZYN001;
- §
- recently enacted and future legislation regarding the healthcare system;
- §
- the performance of third parties upon which we depend, including CROs and third-party manufacturers; and
- §
- our ability to recruit or retain key scientific or management personnel or to retain our executive officers.
ZYN002 and ZYN001 are investigational drugs undergoing preclinical development and have not yet been submitted to the FDA for approval. Neither ZYN002 nor ZYN001 has been, nor may either ever be, approved by any regulatory agency or marketed anywhere in the world. Statements contained in this prospectus should not be deemed to be promotional.
These forward-looking statements reflect our management's beliefs and views with respect to future events and are based on estimates and assumptions as of the date of this prospectus and are subject to risks and uncertainties. We discuss many of these risks in greater detail under "Risk Factors." Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
You should read this prospectus and the documents that we reference in this prospectus and have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this prospectus by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
45
We estimate that we will receive net proceeds of approximately $36.3 million (or approximately $42.1 million if the underwriters' option to purchase additional shares is exercised in full) from the sale of the shares of common stock offered by us in this offering, based on an assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed initial public offering price of $14.00 per share would increase (decrease) the net proceeds to us from this offering by approximately $2.8 million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Similarly, a 100,000 share increase (decrease) in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the net proceeds to us by approximately $1.3 million, based on an assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
The principal purposes of this offering are to obtain additional capital to support our operations, to establish a public market for our common stock and to facilitate our future access to the public equity markets. We intend to use the net proceeds from this offering for the following purposes:
- §
- approximately $16.4 million in the aggregate to fund the development
efforts of ZYN002 for its target indications through Phase 2, of which we expect:
- §
- approximately $9.9 million will fund the general development efforts, including preclinical studies and Phase 1 clinical trials; and
- §
- approximately $6.5 million in the aggregate will fund three Phase 2a clinical trials, one for each target indication of refractory epilepsy, FXS and OA;
- §
- approximately $14.2 million in the aggregate to fund the development
efforts of ZYN001 for its target indications through Phase 2, of which we expect:
- §
- approximately $9.7 million will fund the general development efforts, including preclinical studies and Phase 1 clinical trials; and
- §
- approximately $4.5 million in the aggregate will fund two Phase 2a clinical trials, one for each target indication of fibromyalgia and peripheral neuropathic pain.
The remaining $5.7 million of the net proceeds from this offering will be used to fund working capital, research and development and general corporate purposes.
We may also use a portion of the remaining net proceeds to in-license, acquire, or invest in complementary businesses, intellectual property, products or assets. However, we have no current commitments or obligations to do so.
We expect that the net proceeds from this offering and our existing cash and cash equivalents will be sufficient to fund our operations and capital requirements for the next 24 months. We believe that these available funds will be sufficient to complete (i) Phase 1 clinical trials for ZYN002 and three Phase 2a clinical trials for this product candidate, one for each target indication of refractory epilepsy, FXS and OA and (ii) Phase 1 clinical trials for ZYN001 and two Phase 2a clinical trials for this product candidate, one for each target indication of fibromyalgia and peripheral neuropathic pain. The progress of ZYN002 and ZYN001 for each target indication is uncertain due to numerous factors, including, without limitation, the rate of progress of clinical trials, the results of preclinical studies and clinical trials for such indication, the costs and timing of seeking and obtaining FDA, DEA and other regulatory approvals for clinical trials and FDA guidance regarding clinical trials for such indication. In addition, it is difficult to predict our required
46
spending for our product candidates prior to obtaining FDA approval. Moreover, changing circumstances may cause us to expend cash significantly faster than we currently anticipate, and we may need to spend more cash than currently expected because of circumstances beyond our control.
Our expected use of the net proceeds from this offering represents our current intentions based upon our present plans and business condition. As of the date of this prospectus, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering, or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of the net proceeds will vary depending on numerous factors, including our ability to gain access to additional financing, the relative success and cost of our research, preclinical and clinical development programs and whether we are able to enter into future licensing arrangements. As a result, our management will have broad discretion in the application of the net proceeds, and investors will be relying on our judgment regarding the application of the net proceeds of this offering.
Pending their use, we plan to invest the net proceeds from this offering in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
47
We do not anticipate declaring or paying, in the foreseeable future, any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. Any future determination related to our dividend policy will be made at the discretion of our board of directors and will depend upon, among other factors, our results of operations, financial condition, capital requirements, contractual restrictions, business prospects and other factors our board of directors may deem relevant.
48
The following table sets forth our cash and cash equivalents, and our capitalization as of March 31, 2015:
- §
- on an actual basis;
- §
- on a pro forma basis to give effect to the conversion of all our outstanding convertible preferred stock into an aggregate of 3,704,216 shares of our common stock in connection with the closing of this offering; and
- §
- on a pro forma as adjusted basis, giving effect to the pro forma adjustments set forth above and the sale of 3,000,000 shares of our common stock in this offering at an assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us and to the filing of our sixth amended and restated certificate of incorporation.
The pro forma information below is illustrative only and our capitalization following the closing of this offering will be adjusted based on the actual initial public offering price and other terms of this offering determined at pricing. You should read this table together with "Selected Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and the related notes appearing elsewhere in this prospectus.
| |
As of March 31, 2015 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual | Pro Forma | Pro Forma As Adjusted |
|||||||
Cash and cash equivalents |
$ | 7,375,975 | $ | 7,375,975 | $ | 43,635,975 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Series 1 convertible preferred stock, $0.001 par value per share: 7,807,502 shares authorized, 6,964,053 shares issued and outstanding, actual; 7,807,502 shares authorized, no shares issued or outstanding, pro forma and 10,000,000 shares authorized, and no shares issued or outstanding pro forma as adjusted |
16,522,811 | — | — | |||||||
Stockholders' equity (deficit): |
||||||||||
Common stock, $0.001 par value per share: 50,000,000 shares authorized, 2,029,747 shares issued and outstanding, actual; 50,000,000 shares authorized, 5,733,963 shares issued and outstanding, pro forma; and 200,000,000 shares authorized, 8,733,963 shares issued and outstanding, pro forma as adjusted |
2,030 | 5,734 | 8,734 | |||||||
Additional paid-in capital |
1,975,000 | 18,494,107 | 54,751,107 | |||||||
Accumulated deficit |
(11,520,674 | ) | (11,520,674 | ) | (11,520,674 | ) | ||||
| | | | | | | | | | | |
Total stockholders' equity (deficit) |
(9,543,644 | ) | 6,979,167 | 43,239,167 | ||||||
| | | | | | | | | | | |
Total capitalization |
$ | 6,979,167 | $ | 6,979,167 | $ | 43,239,167 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
The number of shares of our common stock to be outstanding after this offering is based on 2,029,747 shares of common stock outstanding as of March 31, 2015, and assumes:
- §
- the issuance by us of 3,000,000 shares of our common stock in this offering; and
- §
- the conversion of all of our convertible preferred stock outstanding immediately prior to the closing of this offering into an aggregate of 3,704,216 shares of common stock;
and excludes:
- §
- 606,379 shares of common stock issuable upon the exercise of outstanding stock options as of July 23, 2015, at a weighted-average exercise price of $3.98 per share; and
- §
- 1,263,739 shares of our common stock reserved for future issuance under the 2014 Equity Plan, as amended effective upon the closing of this offering.
49
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock after this offering.
Our historical net tangible deficit as of March 31, 2015, was approximately $9.5 million, or $(4.70) per share of our common stock. Our historical net tangible deficit is the amount of our total tangible assets less our liabilities and convertible preferred stock. Historical net tangible deficit per share is our historical net tangible deficit divided by the number of shares of common stock outstanding as of March 31, 2015.
Our pro forma net tangible book value as of March 31, 2015, was $7.0 million, or $1.22 per share of common stock. Pro forma net tangible book value gives effect to the conversion of all shares of our convertible preferred stock outstanding as of March 31, 2015 into an aggregate of 3,704,216 shares of our common stock in connection with the closing of this offering.
Pro forma as adjusted net tangible book value is our pro forma net tangible book value (deficit), plus the effect of the sale of shares of our common stock in this offering at an assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus), and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. This amount represents an immediate increase in pro forma as adjusted net tangible book value of $3.73 per share to our existing stockholders, and an immediate dilution of $9.05 per share to new investors participating in this offering.
The following table illustrates this dilution on a per share basis:
Assumed initial public offering price per share |
$ | 14.00 | |||||
Historical net tangible book value (deficit) per share as of March 31, 2015 |
$ | (4.70 | ) | ||||
Pro forma increase in net tangible book value per share attributable to the conversion of all outstanding shares of our preferred stock into 3,704,216 shares of our common stock immediately prior to the closing of this offering |
5.92 | ||||||
| | | | | | | | |
Pro forma net tangible book value per share as of March 31, 2015 |
1.22 | ||||||
Increase in pro forma net tangible book value per share attributable to investors participating in this offering |
3.73 | ||||||
| | | | | | | | |
Pro forma as adjusted net tangible book value per share after this offering |
4.95 | ||||||
| | | | | | | | |
Dilution per share to investors participating in this offering |
$ | 9.05 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
A $1.00 increase (decrease) in the assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus) would increase (decrease) the pro forma as adjusted net tangible book value per share after this offering by approximately $0.32 per share and decrease (increase) the dilution per share to investors participating in this offering by approximately $0.68 per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Similarly, a 100,000 share increase (decrease) in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the pro forma as adjusted net tangible book value per share after this offering by approximately $0.09 per share and decrease (increase) the dilution per share to investors participating in this offering by approximately $0.09 per share, assuming the assumed initial
50
public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus) remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters exercise in full their option to purchase additional shares of our common stock in this offering, the pro forma as adjusted net tangible book value will increase to $5.35 per share, representing an immediate increase in pro forma as adjusted net tangible book value to existing stockholders of $0.40 per share and an immediate decrease of dilution of $0.40 per share to new investors participating in this offering.
The following table summarizes, on a pro forma as adjusted basis as of March 31, 2015, the number of shares purchased or to be purchased from us, the total consideration paid or to be paid to us, and the average price per share paid or to be paid to us by existing stockholders and investors participating in this offering at an assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus), before deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. As the table below shows, investors participating in this offering will pay an average price per share substantially higher than our existing stockholders paid.
| |
Shares Purchased | Total Consideration | |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Average Price Per Share | |||||||||||||||
| |
Number | Percent | Amount | Percent | ||||||||||||
Existing stockholders before this offering |
5,154,081 | (1) | 63 | % | $ | 18,087,350 | (2) | 30 | % | $ | 3.51 | |||||
Investors participating in this offering |
3,000,000 | 37 | 42,000,000 | 70 | 14.00 | |||||||||||
| | | | | | | | | | | | | | | | | |
Total |
8,154,081 | 100.0 | % | $ | 60,087,350 | 100.0 | % | $ | 7.37 | |||||||
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | |
- (1)
- Shares
exclude 579,882 of restricted stock shares outstanding.
- (2)
- Includes $2,086,196 of noncash consideration.
A $1.00 increase (decrease) in the assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus) would increase (decrease) the total consideration paid by investors participating in this offering, total consideration paid by all stockholders and the average price per share paid by all stockholders by approximately $3.0 million, $3.0 million and $0.37, respectively, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and before deducting the estimated underwriting discount and commissions and estimated offering expenses payable by us.
Similarly, a 100,000 share increase (decrease) in the number of shares offered by us, as set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid by investors participating in this offering, total consideration paid by all stockholders and the average price per share paid by all stockholders by approximately $1.4 million, $1.4 million and $0.08, respectively, assuming the assumed initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus) remains the same, and before deducting the estimated underwriting discount and commissions and estimated offering expenses payable by us.
If the underwriters exercise in full their option to purchase additional shares of our common stock in this offering, the number of shares of common stock held by existing stockholders will be reduced to 60% of the total number of shares of common stock to be outstanding after this offering, and the number of shares of common stock held by investors participating in this offering will be further increased to 3,450,000, or
51
40% of the total number of shares of common stock to be outstanding after this offering (excluding the 579,882 restricted stock shares outstanding).
The number of shares of our common stock to be outstanding after this offering is based on 2,029,747 shares of common stock outstanding as of March 31, 2015, and assumes:
- §
- the issuance by us of 3,000,000 shares of our common stock in this offering; and
- §
- the conversion of all of our convertible preferred stock outstanding immediately prior to the closing of this offering into an aggregate of 3,704,216 shares of common stock;
and excludes:
- §
- 606,379 shares of common stock issuable upon the exercise of outstanding
stock options as of July 23, 2015, at a weighted-average exercise price of $3.98 per share; and
- §
- 1,263,739 shares of our common stock reserved for future issuance under the 2014 Equity Plan, as amended effective upon the closing of this offering.
52
This section should be read together with our financial statements and accompanying notes and "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing elsewhere in this prospectus. We derived the selected statements of operations data for the years ended December 31, 2013 and 2014 and the selected balance sheet data as of December 31, 2013 and 2014 from our audited financial statements and accompanying notes appearing elsewhere in this prospectus. We derived the selected statements of operations data for the three months ended March 31, 2014 and 2015 and the selected balance sheet data as on March 31, 2015 from our unaudited financial statements and accompanying notes appearing elsewhere in this prospectus. The selected financial data in this section are not intended to replace our financial statements and the related notes. Our unaudited financial statements have been prepared on the same basis as the audited financial statements and, in the opinion of management, include adjustments, consisting of normal recurring adjustments and accruals necessary for a fair statement of the information for the interim periods. Our historical results are not necessarily indicative of the results that may be expected in the future and results from our interim period may not necessarily be indicative of the results of the entire year.
Statements of Operation Data:
| |
Years Ended December 31, | Three Months Ended March 31, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | 2014 | 2015 | |||||||||
| |
|
|
(unaudited) |
||||||||||
Revenues |
$ | 943,904 | $ | 810,012 | $ | 146,287 | $ | 14,828 | |||||
Operating expenses: |
|||||||||||||
Research and development |
1,134,041 | 2,401,406 | 285,725 | 853,704 | |||||||||
General and administrative |
444,302 | 4,076,339 | 99,704 | 653,773 | |||||||||
| | | | | | | | | | | | | | |
Total operating expenses |
1,578,343 | 6,477,745 | 385,429 | 1,507,477 | |||||||||
| | | | | | | | | | | | | | |
Loss from operations |
(634,439 | ) | (5,667,733 | ) | (239,142 | ) | (1,492,649 | ) | |||||
Other income (expense): |
|||||||||||||
Interest (expense) income, net |
(2,351 | ) | (1,844 | ) | (1,217 | ) | 680 | ||||||
| | | | | | | | | | | | | | |
Net loss |
(636,790 | ) | (5,669,577 | ) | (240,359 | ) | (1,491,969 | ) | |||||
Accretion of redeemable convertible preferred stock |
(161,834 | ) | (87,954 | ) | (87,954 | ) | — | ||||||
| | | | | | | | | | | | | | |
Net loss applicable to common stockholders |
$ | (798,624 | ) | $ | (5,757,531 | ) | $ | (328,313 | ) | $ | (1,491,969 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Per share information: |
|||||||||||||
Net loss per share basic and diluted |
$ | (1.63 | ) | $ | (6.44 | ) | $ | (0.67 | ) | $ | (0.74 | ) | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Basic and diluted weighted average shares outstanding |
490,760 | 894,575 | 490,760 | 2,029,747 | |||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma net loss (unaudited)(1) |
$ |
(5,669,577 |
) |
$ |
(1,491,969 |
) |
|||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma net loss per share basic and diluted (unaudited)(1) |
$ | (2.56 | ) | $ | (0.26 | ) | |||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Pro forma basic and diluted weighted average shares outstanding (unaudited)(1) |
2,215,507 | 5,733,963 | |||||||||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
- (1)
- Refer to note 2(k) of our audited financial statements and note 1(e) to our unaudited financial statements for a description of the method used to calculate net loss per share, basic and diluted, and pro forma net loss per share basic and diluted and the basic and diluted weighted average shares outstanding.
53
| |
As of December 31, | |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
As of March 31, 2015 |
|||||||||
| |
2013 | 2014 | ||||||||
| |
|
|
(unaudited) |
|||||||
BALANCE SHEET DATA: |
||||||||||
Cash and cash equivalents |
$ | 154,695 | $ | 9,330,681 | $ | 7,375,975 | ||||
Total assets |
1,860,840 | 11,616,671 | 10,316,797 | |||||||
Total liabilities |
3,057,546 | 3,145,535 | 3,337,630 | |||||||
Convertible preferred stock |
3,162,373 | 16,522,811 | 16,522,811 | |||||||
Total stockholders' equity (deficit) |
(4,359,079 | ) | (8,051,675 | ) | (9,543,644 | ) | ||||
54
MANAGEMENT'S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion summarizes the significant factors affecting the operating results, financial condition, liquidity and cash flows of our company as of and for the periods presented below. The following discussion and analysis should be read in conjunction with "Prospectus Summary — Summary Financial Information," "Selected Financial Information" and the financial statements and the related notes thereto included elsewhere in this prospectus. The statements in this discussion regarding industry outlook, our expectations regarding our future performance, liquidity and capital resources and all other non-historical statements in this discussion are forward-looking statements and are based on the beliefs of our management, as well as assumptions made by, and information currently available to, our management. Actual results could differ materially from those discussed in or implied by forward-looking statements as a result of various factors, including those discussed below and elsewhere in this prospectus, particularly in the section entitled "Risk Factors."
Overview
Company Overview
We are a ten-year-old specialty pharmaceutical company focused on developing and commercializing proprietary next-generation synthetic cannabinoid therapeutics formulated for transdermal delivery. Our management team is highly experienced and has a successful history of development, regulatory approval and commercialization of patch and gel transdermal delivery products. We are evaluating two patent-protected product candidates, ZYN002 and ZYN001, in five indications. We intend to study ZYN002 in patients with refractory epilepsy, Fragile X syndrome, or FXS, and osteoarthritis, or OA. We intend to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain. We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently receiving adequate relief from current treatment regimens. We expect to initiate Phase 1 clinical trials for ZYN002 in the second half of 2015 and ZYN001 by mid-2016. We plan to conduct our Phase 1, and possibly Phase 2, clinical trials for ZYN002 in Australia, subject to applicable regulatory approval, and do not expect at this time to file an investigational new drug application, or IND, with the U.S. Food and Drug Administration, or the FDA, prior to the commencement of those clinical trials. We must file an IND with the FDA and receive approval from the U.S. Drug Enforcement Agency, or DEA, prior to commencement of any clinical trials in the United States. We plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. We plan to submit New Drug Applications, or NDAs, for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials.
Cannabinoids are a class of compounds derived from Cannabis plants. The two primary cannabinoids contained in Cannabis are cannabidiol, or CBD, and D9-tetrahydrocannabinol, or THC. Clinical and preclinical data suggest that CBD has positive effects on treating refractory epilepsy, FXS and arthritis and THC has positive effects on treating pain. Interest in cannabinoid therapeutics has increased significantly over the past several years as preclinical and clinical data has emerged highlighting the potential efficacy and safety benefits of cannabinoid therapeutics. The cannabinoid therapeutics market is expected to grow significantly due to the potential benefits these products may provide over existing therapies. In addition to ZYN002 and ZYN001 potentially offering first-line therapies to patients suffering from FXS, OA, fibromyalgia and peripheral neuropathic pain, we believe ZYN002 may provide a complementary treatment for patients suffering from epilepsy who are refractory to their current treatment regimens.
ZYN002 is the first and only synthetic CBD formulated as a permeation-enhanced gel for transdermal delivery, which is patent-protected through 2030. CBD is the primary non-psychoactive component of Cannabis. In preclinical animal studies, ZYN002's permeation enhancer increased delivery of CBD through the layers of the skin and into the circulatory system. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, an in vitro study performed by us demonstrated that CBD is degraded to THC in an acidic environment such as the stomach. We believe such degradation may lead to increased psychoactive effects, which may be
55
avoided or minimized with the transdermal delivery of ZYN002, which avoids the gastrointestinal tract and potential stomach acid degradation. ZYN002 is targeting treatment of refractory epilepsy, FXS and OA, which collectively affect millions of patients using treatments that currently comprise a multi-billion dollar market. FXS may qualify for orphan drug designation in the United States because the number of patients in the United States with FXS is less than 100,000. We have requested orphan drug designation from the FDA in the second half of 2015.
ZYN001 is a pro-drug of THC that enables effective transdermal delivery via a patch and is patent-protected through 2031. In addition, we expect that ZYN001 will be classified by the FDA as a new chemical entity, or NCE. In our preclinical animal studies, ZYN001 demonstrated effective skin permeation with sustained delivery and rapid conversion of ZYN001 to THC. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, preclinical toxicology models conducted to date have not shown any toxicology or genotoxicity findings. ZYN001 is targeting two pain indications (fibromyalgia and peripheral neuropathic pain) which collectively affect millions of patients using treatments that currently comprise a multi-billion dollar market.
Our key development programs and expected timelines for the development of ZYN002 and ZYN001 are shown in the table below:
Product Candidate |
Target Indication | Delivery Method | Current Development Status |
Expected Next Steps | ||||
|---|---|---|---|---|---|---|---|---|
| ZYN002 | Refractory Epilepsy | Permeation-enhanced Gel | Preclinical | 2H15: Initiate Phase 1 | ||||
| Fragile X Syndrome | 2H16: Initiate Phase 2a | |||||||
| Osteoarthritis | ||||||||
| | | | | | | | | |
| ZYN001 | Fibromyalgia | Transdermal Patch | Preclinical | Mid-2016: Initiate Phase 1 | ||||
| Peripheral Neuropathic Pain | 1H17: Initiate Phase 2a |
We have never been profitable and have incurred net losses since inception. Our net losses were $636,790 and $5.7 million for the years ended December 31, 2013 and 2014, respectively, and $1.5 million for the three months ended March 31, 2015. We expect to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates. Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when, or if, we will be able to achieve or maintain profitability.
Financial Operations Overview
The following discussion sets forth certain components of our statements of operations as well as factors that impact those items.
Revenues —
Our revenues consist of state and federal research grants and fees received from research services for third-party product development. These revenues are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable and collectability is reasonably assured.
Research and Development Expenses —
Our research and development expenses consist of expenses incurred in development and preclinical studies relating to our product candidates, including:
- §
- expenses associated with preclinical development;
- §
- personnel-related expenses, such as salaries, benefits, travel and other
related expenses, including stock-based compensation;
- §
- payments to third-party contract research organizations, or CROs, contractor laboratories and independent contractors; and
56
- §
- depreciation, maintenance and other facility-related expenses.
We expense all research and development costs as incurred. Preclinical development expenses for our product candidates are a significant component of our current research and development expenses. Product candidates in later stage clinical development generally have higher research and development expenses than those in earlier stages of development, primarily due to increased size and duration of the clinical trials. We track and record information regarding external research and development expenses for each grant, study or trial that we conduct. From time to time, we use third-party CROs, contractor laboratories and independent contractors in preclinical studies. We recognize the expenses associated with third parties performing these services for us in our preclinical studies based on the percentage of each study completed at the end of each reporting period.
We incurred research and development expenses of $1.1 million and $2.4 million for the years ended December 31, 2013 and 2014, respectively, and $853,704 for the three months ended March 31, 2015.
We expect that our research and development expenses in 2015 and for the next several years will be higher than in 2014 as a result of the work needed for our expected initiation of our Phase 1 clinical trials of ZYN002 in the second half of 2015 and ZYN001 by mid-2016. These expenditures are subject to numerous uncertainties regarding timing and cost to completion. Completion of our preclinical development and clinical trials may take several years or more and the length of time generally varies according to the type, complexity, novelty and intended use of a product candidate. The cost of clinical trials may vary significantly over the life of a project as a result of differences arising during clinical development, including, among others:
- §
- the number of sites included in the clinical trials;
- §
- the length of time required to enroll suitable patients;
- §
- the size of patient populations participating in the clinical trials;
- §
- the duration of patient follow-ups;
- §
- the development stage of the product candidates; and
- §
- the efficacy and safety profile of the product candidates.
Due to the early stages of our research and development, we are unable to determine the duration or completion costs of our development of ZYN002 and ZYN001. As a result of the difficulties of forecasting research and development costs of ZYN002 and ZYN001 as well as the other uncertainties discussed above, we are unable to determine when and to what extent we will generate revenues from the commercialization and sale of an approved product candidate.
General and Administrative Expenses —
General and administrative expenses consist primarily of salaries, benefits and other related costs, including stock-based compensation, for personnel serving in our executive, finance, accounting, legal and human resource functions. Our general and administrative expenses also include facility and related costs not included in research and development expenses, professional fees for legal services, including patent-related expenses, consulting, tax and accounting services, insurance and general corporate expenses. We expect that our general and administrative expenses will increase with the continued development and potential commercialization of our product candidates.
We expect that our general and administrative expenses in 2015 and for the next several years will be higher than in 2014 as we increase our headcount. We also anticipate increased expenses relating to our operations as a public company, including increased costs for the hiring of additional personnel, and for payment to outside consultants, including lawyers and accountants, to comply with additional regulations, corporate governance, internal control and similar requirements applicable to public companies, as well as increased costs for insurance.
Interest Income (Expense), net —
Interest expense consists of interest expense on our note payable that was paid off during 2014. Interest income consists primarily of interest earned on our money market bank account.
57
Income Taxes —
As of December 31, 2014, we had $6.9 million of federal operating loss carryforwards and $161,000 of research tax credit carryforwards available to offset future taxable income. These operating loss and research tax credit carryforwards will begin to expire in 2028 and 2027, respectively. The Tax Reform Act of 1986, or the Act, provides for limitation on the use of net operating loss and research and development tax credit carryforwards following certain ownership changes (as defined by the Act) that could limit our ability to utilize these carryforwards. We may have experienced various ownership changes, as defined by the Act, as a result of past financings. Accordingly, our ability to utilize the aforementioned carryforwards may be limited. Additionally, U.S. tax laws limit the time during which these carryforwards may be applied against future taxes; therefore, we may not be able to take full advantage of these carryforwards for federal income tax purposes.
The closing of this offering, together with private placements and other transactions that have occurred since our inception, may trigger, or may have already triggered, an "ownership change" pursuant to Section 382 of the Internal Revenue Code of 1986. If an ownership change is triggered, it will limit our ability to use some of our net operating loss carryforwards. In addition, since we will need to raise substantial additional funding to finance our operations, we may undergo further ownership changes in the future, which could further limit our ability to use net operating loss carryforwards. As a result, if we generate taxable income, our ability to use some of our net operating loss carryforwards to offset U.S. federal taxable income may be subject to limitations, which could result in increased future tax liability to us.
Critical Accounting Policies and Use of Estimates
We have based our management's discussion and analysis of financial condition and results of operations on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as the reported revenues and expenses during the reporting periods. On an ongoing basis, we evaluate our estimates and judgments, including those related to preclinical development expenses and stock-based compensation. We base our estimates on historical experience and on various other factors that we believe to be appropriate under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully discussed in note 2 to our audited financial statements appearing at the end of this prospectus, we believe that the following accounting policies are critical to the process of making significant judgments and estimates in the preparation of our financial statements.
Research and Development Expenses
We rely on third parties to conduct our preclinical studies and to provide services, including data management, statistical analysis and electronic compilation. Once our clinical trials begin, at the end of each reporting period, we will compare the payments made to each service provider to the estimated progress towards completion of the related project. Factors that we will consider in preparing these estimates include the number of patients enrolled in studies, milestones achieved and other criteria related to the efforts of our vendors. These estimates will be subject to change as additional information becomes available. Depending on the timing of payments to vendors and estimated services provided, we will record net prepaid or accrued expenses related to these costs.
Fair Value of Common Stock and Stock-Based Compensation
We account for grants of stock options and restricted stock to employees based on their grant date fair value and recognize compensation expense over the vesting periods. We estimate the fair value of stock options as of the date of grant using the Black-Scholes option pricing model, and we estimate the fair value of restricted stock based on the fair value of the underlying common stock as determined by our board of directors or the value of the services provided, whichever is more readily determinable. We account for stock
58
options and restricted stock awards to non-employees using the fair value approach. Stock options and restricted stock awards to non-employees are subject to periodic revaluation over their vesting terms.
In the absence of a public trading market for our common stock, on each grant date, we develop an estimate of the fair value of our common stock for the option and restricted stock grants based in part on input from an independent third-party valuation firm. We determined the fair value of our common stock using methodologies, approaches and assumptions consistent with the AICPA Practice Guide, Valuation of Privately-Held Company Equity Securities Issued as Compensation. In addition, our board of directors considered various objective and subjective factors, along with input from management and an independent third-party valuation firm, to estimate the fair value of our common stock, including external market conditions affecting the pharmaceutical industry, trends within the pharmaceutical industry, the prices at which we sold shares of our different series of preferred stock, the superior rights and preferences of each series of preferred stock relative to our common stock at the time of each grant, our results of operations and financial position, the status of our research and development efforts and progress of our preclinical programs, our stage of development and business strategy, the lack of an active public market for our common and our preferred stock, and the likelihood of achieving a liquidity event.
Common stock, restricted stock and stock options issued during and subsequent to the year ended December 31, 2014 —
In connection with and subsequent to our recapitalization in May 2014, through March 31, 2015, we issued 1,172,391 shares of common stock to investors and third-party service providers for services rendered. In addition, our board of directors approved grants of restricted stock and stock options that were issued in October 2014 and January 2015, with the stock options having an exercise price of $3.98 per share. Our common stock fair value was supported by an independent third-party valuation of $1.65 per share of common stock as of August 31, 2014, which management believed was still appropriate as of the dates of these grants in the absence of any substantial progress with our preclinical research and development activities.
In conducting the August 31, 2014 valuation, we utilized the option pricing model backsolve method to calculate our enterprise value utilizing the Series 1 convertible preferred stock financing at $2.12 per share.
We then allocated the aggregate equity value between the common stock and the preferred stock using a Black-Scholes call option pricing method. Under this method, we estimated the fair value of our common stock as the net value of a series of call options, representing the present value of the expected future returns to the common stockholders. We considered the rights of the common stockholders to be equivalent to a call option on our future value in excess of the aggregate liquidation preferences payable on preferred stock, with adjustments to account for the rights retained by the preferred stockholders related to any value in excess of the applicable liquidation preferences. Using this method, we valued the common stock by estimating the value of a share of common stock in each of these call option rights.
We then reduced the value of the common stock using this approach by applying a lack of control discount and an illiquidity discount to account for the heightened level of risk associated with our shares compared to that of comparable, publicly traded companies.
Results of Operations
Comparison of the Three Months Ended March 31, 2014 and March 31, 2015
Revenues
Revenues decreased by $131,459, or 89.9%, to $14,828 for the three months ended March 31, 2015 from $146,287 for the three months ended March 31, 2014. Revenues in each period were entirely related to work performed in connection with grants received. The decrease from 2014 reflected the termination of work on two grants and a temporary slowdown in research activities associated with our remaining grant.
59
Research and Development Expenses
Research and development expenses increased by $567,979, or 198.8%, to $853,704 for the three months ended March 31, 2015 from $285,725 for the three months ended March 31, 2014. The increase was primarily the result of increased consulting and compensation expense of approximately $652,000 related to increased product development activities, which was partly offset by lower spending on university contracted services, repair and maintenance and lab supplies.
Our expenditures associated with ZYN002, ZYN001 and other research and development projects for the three months ended March 31, 2015 were $282,614, $382,097 and $188,993, respectively. Our expenditures associated with ZYN001 and our other projects in the three months ended March 31, 2014 were $111,529 and $174,196, respectively; no expenditures were made for ZYN002 during the three month period.
General and Administrative Expenses
General and administrative expenses increased by $554,069, or 555.7%, to $653,773 for the three months ended March 31, 2015 from $99,704 for the three months ended March 31, 2014. Contributing to the increase were increases of approximately $302,000 in personnel costs and approximately $167,000 in professional service costs. These increases were largely the result of our efforts to establish an infrastructure to support our product development activities and the professional service fees related to the preparation for our planned initial public offering.
Comparison of the Years Ended December 31, 2013 and December 31, 2014
Revenues
Revenues for the years ended December 31, 2013 and December 31, 2014 were comprised of the following:
| |
Years Ended December 31, |
Increase (Decrease) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | $ | % | |||||||||
Grant revenues |
$ | 856,605 | $ | 686,770 | $ | (169,835 | ) | (19.8% | ) | ||||
Research services |
87,299 | 123,242 | 35,943 | 41.2% | |||||||||
| | | | | | | | | | | | | | |
|
$ | 943,904 | $ | 810,012 | $ | (133,892 | ) | (14.2% | ) | ||||
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
| | | | | | | | | | | | | | |
Revenues decreased by $133,892 or 14.2% to $810,012 for the year ended December 31, 2014 from $943,904 for the year ended December 31, 2013. The decrease was due to a $169,835 decrease in grant revenues, which was partly offset by a $35,943 increase in research services related to revenues received from a new contract entered into in November 2013.
Research and Development Expenses
Research and development expenses increased by $1.3 million, or 111.8%, to $2.4 million for the year ended December 31, 2014 from $1.1 million for the year ended December 31, 2013. The increase was primarily the result of increased consulting and compensation expense of $1.1 million for increased development activities, which was partly offset by decreased spending on university contracted services and lab supplies.
Our expenditures associated with ZYN002, ZYN001 and our other research and development projects in 2014 were $578,825, $585,711 and $1,236,870, respectively. Our expenditures associated with ZYN001 and other research and development activities in 2013 were $309,821 and $824,220, respectively; no expenditures were made for ZYN002 during the year ended December 31, 2013.
60
General and Administrative Expenses
General and administrative expenses increased by $3.6 million to $4.1 million for the year ended December 31, 2014 from $444,302 for the year ended December 31, 2013. The increase was primarily the result of $1.9 million of non-cash expense for the issuance of common stock for services rendered by a related party and a third-party service provider in the year ended December 31, 2014 compared to $0 in the prior year. Additionally, during the year ended December 31, 2014, we expensed payments of $250,000 to a related party for strategic advisory and consulting services and $500,000 for the termination of a royalty agreement. The remaining increase is primarily due to legal costs and other administrative costs.
Other Income (Expense)
Other expense, net was $2,351 and $1,844 for the years ended December 31, 2013 and 2014, respectively.
Liquidity and Capital Resources
Since our inception in 2007, we have devoted most of our cash resources to research and development and general and administrative activities. We have financed our operations primarily with the proceeds from the sale of preferred stock and convertible promissory notes, state and federal grants and research services. To date, we have not generated any revenues from the sale of products, and we do not anticipate generating any revenues from the sales of products for the foreseeable future. We have incurred losses and generated negative cash flows from operations since inception. As of March 31, 2015, our principal sources of liquidity were our cash and cash equivalents, which totaled $7.4 million. Our working capital was $7.0 million as of March 31, 2015.
Equity Financings
For the years ended December 31, 2013 and 2014, we received net proceeds of $109,458 and $13.2 million, respectively, from the sale of convertible preferred stock. For the three months ended March 31, 2014, we received net proceeds of $309,411 from the sale of shares of our Series B redeemable convertible preferred stock. There were no proceeds from equity financings for the three months ended March 31, 2015.
Debt
We had no debt outstanding as of December 31, 2014 or March 31, 2015.
In 2009 and 2010, we issued subordinated convertible promissory notes for proceeds of $773,000. In October 2012, the subordinated secured convertible promissory note holders elected to convert their notes into 698,109 shares of Series B redeemable convertible preferred stock.
In October 2009, we converted outstanding fees due to a service provider of $327,791 into a promissory note secured by all of our assets. The note bore interest at a rate of 4.63% per annum and was payable in 36 equal installments of principal and interest. During 2012, the service provider agreed to restructure the remaining note payable balance of $68,389 and $91,952 of additional outstanding fees payable. The agreement terminated all future promissory note payments and all outstanding fees payable in exchange for a payment of $95,000.
In April 2007, we were awarded a grant by the Kentucky Economic Development Finance Authority, or KEDFA, on behalf of the Commonwealth of Kentucky Department of Commercialization and Innovation, or DCI, in the form of a non-interest bearing forgivable loan in the amount of up to $500,000 to be used for the purchase of equipment. The loan was subject to repayment in four annual installments equal to $125,000 and was secured by the assets purchased with the loan funds. The loan contained a provision for the forgiveness of the total loan provided we maintained certain employment positions in existence at the time of the award at the then average annual base salary and created 30 additional high tech employment positions at an average annual base salary of at least $80,000. Under the terms of the initial loan these
61
existing and new employment positions were to be created by December 31, 2012 and retained through December 31, 2015. In December 2012, KEDFA approved an extension of the deadline for creating the new employment positions to December 31, 2014, with repayment beginning in December 2014. Additionally, the loan provided for partial forgiveness had the Company not fully reached the specified employment creation. In January 2014, we granted KEDFA liens on certain of our patents as security for the forgivable loan. In September 2014, we repaid the forgivable loan balance of $499,996 and KEDFA released its security interest.
Future Capital Requirements
We expect that the net proceeds from this offering and our existing cash and cash equivalents will be sufficient to fund our operations and capital requirements through August 2017. We believe that these available funds will be sufficient to complete (i) Phase 1 clinical trials for ZYN002 and three Phase 2a clinical trials for this product candidate, one for each target indication of refractory epilepsy, FXS and OA and (ii) Phase 1 clinical trials for ZYN001 and two Phase 2a clinical trials for this product candidate, one for each target indication of fibromyalgia and peripheral neuropathic pain. However, it is difficult to predict our spending for our product candidates prior to obtaining FDA approval. Moreover, changing circumstances may cause us to expend cash significantly faster than we currently anticipate, and we may need to spend more cash than currently expected because of circumstances beyond our control.
Our expectations regarding future cash requirements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we make in the future. We have no current understandings, agreements or commitments for any material acquisitions or licenses of any products, businesses or technologies. We may need to raise substantial additional capital in order to engage in any of these types of transactions.
We expect to continue to incur substantial additional operating losses for at least the next several years as we continue to develop our product candidates and seek marketing approval and, subject to obtaining such approval, the eventual commercialization of our product candidates. If we obtain marketing approval for either of our product candidates, we will incur significant sales, marketing and outsourced manufacturing expenses. In addition, we expect to incur additional expenses to add operational, financial and information systems and personnel, including personnel to support our planned product commercialization efforts. We also expect to incur significant costs to comply with corporate governance, internal controls and similar requirements applicable to us as a public company following the closing of this offering.
Our future use of operating cash and capital requirements will depend on many forward-looking factors, including the following:
- §
- the initiation, progress, timing, costs and results of preclinical studies and
clinical trials for our product candidates;
- §
- the clinical development plans we establish for these product candidates;
- §
- the number and characteristics of product candidates that we develop or may
in-license;
- §
- the terms of any collaboration agreements we may choose to execute;
- §
- the outcome, timing and cost of meeting regulatory requirements established by
the DEA, the FDA, the EMA or other comparable foreign regulatory authorities;
- §
- the cost of filing, prosecuting, defending and enforcing our patent claims and
other intellectual property rights;
- §
- the cost of defending intellectual property disputes, including patent
infringement actions brought by third parties against us;
- §
- costs and timing of the implementation of commercial scale manufacturing
activities; and
- §
- the cost of establishing, or outsourcing, sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval in regions where we choose to commercialize our products on our own.
62
To the extent that our capital resources are insufficient to meet our future operating and capital requirements, we will need to finance our cash needs through public or private equity offerings, debt financings, collaboration and licensing arrangements or other financing alternatives. We have no committed external sources of funds. Additional equity or debt financing or collaboration and licensing arrangements may not be available on acceptable terms, if at all.
If we raise additional funds by issuing equity securities, our stockholders will experience dilution. Debt financing, if available, would result in increased fixed payment obligations and may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Any debt financing or additional equity that we raise may contain terms, such as liquidation and other preferences that are not favorable to us or our stockholders. If we raise additional funds through collaboration and licensing arrangements with third parties, it may be necessary to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us.
Cash Flows
Three Months Ended March 31, 2014 and March 31, 2015 — The following table summarizes our cash flows from operating, investing and financing activities for the three months ended March 31, 2014 and March 31, 2015.
| |
Three Months Ended March 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2015 | |||||
Statement of Cash Flows Data: |
|||||||
Total net cash provided by (used in): |
|||||||
Operating activities |
$ | (287,018 | ) | $ | (1,915,429 | ) | |
Investing activities |
— | (6,277 | ) | ||||
Financing activities |
309,411 | (33,000 | ) | ||||
| | | | | | | | |
Increase (decrease) in cash and cash equivalents |
$ | 22,393 | $ | (1,954,706 | ) | ||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Operating Activities
For the three months ended March 31, 2014, cash used in operations was $287,018 compared to $1.9 million for the three months ended March 31, 2015. The increase from the comparable 2014 period was primarily the result of increased compensation costs related to an increase in the number of employees hired to support our product development activities as well as higher professional service costs incurred in connection with the preparation for our planned initial public offering.
We expect cash used in operating activities to continue to increase in 2015 as compared to 2014 due to an expected increase in our operating losses associated with ongoing development of our product candidates.
Investing Activities
For the three months ended March 31, 2015, cash used in investing activities was $6,277, representing the cost of computer equipment, furniture and fixtures associated with the establishment of our new corporate headquarters.
Financing Activities
Cash used in financing activities was $33,000 in the three months ended March 31, 2015, representing direct costs related to our planned initial public offering. In the three months ended March 31, 2014, cash provided by financing activities was $309,411, resulting from the issuance of shares of our Series B redeemable convertible preferred stock.
63
Years ended December 31, 2013 and December 31, 2014 — The following table summarizes our cash flows from operating, investing and financing activities for the years ended December 31, 2013 and December 31, 2014.
| |
Years ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
Statement of Cash Flows Data: |
|||||||
Total net cash provided by (used in): |
|||||||
Operating activities |
$ | (117,708 | ) | $ | (3,540,655 | ) | |
Investing activities |
(2,703 | ) | (19,717 | ) | |||
Financing activities |
109,458 | 12,736,358 | |||||
| | | | | | | | |
(Decrease) increase in cash and cash equivalents |
$ | (10,953 | ) | $ | 9,175,986 | ||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Operating Activities
Net cash used in operating activities for the year ended December 31, 2014 was $3.5 million, including a net loss of $5.7 million, partly offset by $1.9 million of net noncash expenses and a $196,526 net change in operating assets and liabilities. The net noncash expenses were predominantly related to the issuance of shares of common stock valued at $1.9 million for services rendered. The change in operating assets and liabilities was primarily due to a $437,691 decrease in prepaid expenses and other assets and an increase in accounts payable and accrued expenses of $365,642 partly offset by the timing of revenue of $641,321 and expense recognition related to our research activities.
Net cash used in operating activities was $117,708 for the year ended December 31, 2013 including a net loss of $636,790, partly offset by depreciation expense of $49,392 and changes in operating assets and liabilities of $469,690 primarily related to the timing of revenue and expense recognition for research activities.
We expect cash used in operating activities to continue to increase in 2015 as compared to 2014 due to an expected increase in our operating losses associated with ongoing development of our product candidates.
Investing Activities
Cash used in investing activities for the purchases of property and equipment was $19,717 for the year ended December 31, 2014.
Cash used in investing activities for the purchase of property and equipment was $2,703 for the year ended December 31, 2013.
Financing Activities
Cash provided by financing activities of $12.7 million for the year ended December 31, 2014 was primarily due to $12.9 million in net proceeds received on the sale of shares of our Series 1 convertible preferred stock and $309,911 in net proceeds received on the sale of 250,000 shares of our Series B redeemable convertible preferred stock offset by $499,996 used to pay the note payable to KEDFA.
Cash provided by financing activities for the year ended December 31, 2013 was $109,458, reflecting $71,958 of proceeds on the issuance of shares of Series B redeemable convertible preferred stock and $37,500 of proceeds on the collection of stock subscription advances.
64
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements, except for operating leases, or relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities.
Recent Accounting Pronouncements
In August 2014, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, No. 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern, which defines management's responsibility to assess an entity's ability to continue as a going concern, and to provide related footnote disclosures if there is substantial doubt about its ability to continue as a going concern. The pronouncement is effective for annual reporting periods ending after December 15, 2016 with early adoption permitted. The adoption of this guidance is not expected to have a material impact on our financial statements.
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to various market risks, which may result in potential losses arising from adverse changes in market rates, such as interest rates and foreign exchange rates. We do not enter into derivatives or other financial instruments for trading or speculative purposes and do not believe we are exposed to material market risk with respect to our cash and cash equivalents.
We currently have no operations outside the United States, but we have contracted with third parties to manufacture our product candidates and conduct clinical trials outside of the United States. At this time, such manufacturing and research costs are paid for in U.S. dollars and, therefore, are not subject to fluctuations in exchange rates. If we conduct additional clinical trials outside of the United States in the future, we may be required or may choose to pay for those clinical trials in a local foreign currency and could incur foreign currency exchange rate risk.
As of March 31, 2015, we had cash and cash equivalents of $7.4 million consisting primarily of cash and money market accounts. We do not engage in any hedging activities against changes in interest rates or foreign currency exchange rates. Because of the short-term maturities of our cash and cash equivalents, we do not believe that an immediate 10% increase in interest rates would have any significant impact on the realized value of our investments.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or JOBS Act, was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an "emerging growth company." As an "emerging growth company," we are electing not to take advantage of the extended transition period afforded by the JOBS Act for the implementation of new or revised accounting standards, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision not to take advantage of the extended transition period is irrevocable.
Subject to certain conditions set forth in the JOBS Act, as an "emerging growth company," we are not required to, among other things, (i) provide an auditor's attestation report on our system of internal controls over financial reporting pursuant to Section 404, (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd-Frank Wall Street Reform and Consumer Protection Act, (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor's report providing additional information about the audit and the financial statements (auditor discussion and analysis), and (iv) disclose certain executive compensation-related items such as the correlation between executive
65
compensation and performance and comparisons of the chief executive officer's compensation to median employee compensation. These exemptions will apply until the fifth anniversary of the completion of our initial public offering or until we no longer meet the requirements for being an "emerging growth company," whichever occurs first.
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
On or about September 30, 2014, we dismissed Mountjoy Chilton Medley LLP, or Mountjoy, as our independent public accounting firm.
The audit report of Mountjoy on our financial statements as of and for the fiscal years ended December 31, 2013 and 2012 did not contain any adverse opinion or disclaimer of opinion, nor was it qualified or modified as to uncertainty, audit scope, or accounting principles, except for modifications for uncertainties related to going concern and a change in accounting principle. Those audits were conducted under United States generally accepted auditing standards and not the standards as prescribed by the Public Company Accounting Oversight Board.
In connection with the audit of our financial statements for the fiscal years ended December 31, 2013 and 2012, and for the subsequent interim period through the date of the dismissal of Mountjoy, (i) there were no disagreements with Mountjoy on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreements, if not resolved to Mountjoy's satisfaction, would have caused Mountjoy to make reference to the subject matter of the disagreement in connection with its report, and (ii) there were no "reportable events," as that term is described in Item 304(a)(1)(v) of Regulation S-K.
On October 20, 2014, we engaged KPMG, to serve as our independent registered public accounting firm and to reaudit the fiscal years ended December 31, 2013 and 2012. The engagement of KPMG has been approved by our board of directors.
During the two most recent fiscal years and in the subsequent interim period through September 30, 2014, neither we, nor anyone acting on our behalf, consulted with KPMG regarding either: (i) the application of accounting principles to a specified transaction, either completed or proposed, or the type of audit opinion that might be rendered on our financial statements, and no written report nor oral advice was provided by KPMG, or (ii) any matter that was either the subject of a disagreement, as that term is defined in Item 304(a)(1)(iv) of Regulation S-K, or a reportable event, as that term is defined in Item 304(a)(1)(v) of Regulation S-K.
We requested that Mountjoy furnish us with a letter addressed to the SEC stating whether it agrees with the above statements. A copy of the letter dated January 12, 2015, is filed as an exhibit to the registration statement of which this prospectus forms a part.
66
Overview
We are a ten-year-old specialty pharmaceutical company focused on developing and commercializing proprietary next-generation synthetic cannabinoid therapeutics formulated for transdermal delivery. Our management team is highly experienced and has a successful history of development, regulatory approval and commercialization of patch and gel transdermal delivery products. We are evaluating two patent-protected product candidates, ZYN002 and ZYN001, in five indications. We intend to study ZYN002 in patients with refractory epilepsy, Fragile X syndrome, or FXS, and osteoarthritis, or OA. We intend to study ZYN001 in patients with fibromyalgia and peripheral neuropathic pain. We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently receiving adequate relief from current treatment regimens. We expect to initiate Phase 1 clinical trials in the second half of 2015 for ZYN002 and by mid-2016 for ZYN001. We plan to conduct our Phase 1, and possibly Phase 2, clinical trials for ZYN002 in Australia (subject to applicable regulatory approval), and do not expect at this time to file an IND with the U.S. Food and Drug Administration, or FDA, prior to the commencement of those clinical trials. We must file an IND with the FDA and receive approval from the U.S. Drug Enforcement Agency, or DEA, prior to commencement of any clinical trial in the United States. We plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. We plan to submit New Drug Applications, or NDAs, for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials.
Cannabinoids are a class of compounds derived from Cannabis plants. The two primary cannabinoids contained in Cannabis are cannabidiol, or CBD, and D9-tetrahydrocannabinol, or THC. Clinical and preclinical data suggest that CBD has positive effects on treating refractory epilepsy, FXS and arthritis and THC has positive effects on treating pain. Interest in cannabinoid therapeutics has increased significantly over the past several years as preclinical and clinical data has emerged highlighting the potential efficacy and safety benefits of cannabinoid therapeutics. The cannabinoid therapeutics market is expected to grow significantly due to the potential benefits these products may provide over existing therapies. In addition to ZYN002 and ZYN001 potentially offering first-line therapies to patients suffering from FXS, OA, fibromyalgia and peripheral neuropathic pain, we believe ZYN002 may provide a complementary treatment for patients suffering from epilepsy who are refractory to their current treatment regimens.
We believe that we offer an attractive alternative to existing cannabinoid therapies by synthetically manufacturing and transdermally delivering our product candidates. Most cannabinoid therapies have drawbacks and limitations due to their botanical (plant-derived) nature, as well as the fact that they are administered orally. Botanical cannabinoids create significant challenges for drug manufacturers because of the natural resources and security measures required to grow Cannabis, as well as the strict batch controls required by regulatory agencies in pharmaceutical manufacturing. In addition, we believe all currently approved and development-stage cannabinoid therapeutics, except ZYN002 and ZYN001, are designed to be administered orally which can lead to limitations in safety and efficacy including low bioavailability, inconsistent plasma levels, degradation by stomach acids, and significant first-pass liver metabolism. First-pass liver metabolism refers to the process by which the liver breaks down therapeutics ingested directly or indirectly through the gastrointestinal system, such as through oral or oral mucosal delivery methods, allowing only a small amount of drug to be absorbed into the circulatory system. In contrast, transdermal therapeutics are absorbed through the skin directly into the systemic circulation, avoiding first-pass liver metabolism and degradation by stomach acids, and potentially enabling lower dosage levels of active pharmaceutical ingredients and rapid and reliable absorption with high bioavailability, fewer negative psychoactive effects and fewer drug-drug interactions.
ZYN002 is the first and only synthetic CBD formulated as a permeation-enhanced gel for transdermal delivery, and is patent-protected through 2030. CBD is the primary non-psychoactive component of Cannabis. In preclinical animal studies, ZYN002's permeation enhancer increased delivery of CBD through
67
the layers of the skin and into the circulatory system. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, an in vitro study performed by us demonstrated that CBD is degraded to THC in an acidic environment such as the stomach. We believe such degradation may lead to increased psychoactive effects, which may be avoided or minimized with the transdermal delivery of ZYN002 which maintains CBD in a neutral pH. ZYN002 is targeting treatment of refractory epilepsy, FXS and OA, which collectively affect millions of patients using treatments that currently comprise a multi-billion dollar market. FXS may qualify for an orphan drug designation in the United States because the number of patients in the United States with FXS is less than 100,000. We have requested orphan drug designation from the FDA in the second half of 2015.
We believe that ZYN002 may provide an effective treatment for refractory epilepsy based on the anticonvulsant effects of CBD shown in multiple third-party in vivo models of epilepsy and the results of a small third-party clinical trial in which CBD-treated epilepsy patients showed improvement. We believe that ZYN002 may provide an effective treatment for FXS based upon the hypothesis that an endocannabinoid deficiency is the underlying cause of abnormal cellular function in FXS, which may enable patients to benefit from therapy with an exogenous cannabinoid. We believe that ZYN002 may provide effective treatment for OA based on animal research that we have conducted, measuring efficacy of ZYN002 for reduction of inflammation and pain in vivo.
ZYN001 is a pro-drug of THC that enables effective transdermal delivery via a patch and is patent-protected through 2031. A pro-drug is a drug administered in an inactive or less active form and designed to enable more effective delivery, which is then converted into an active form through a normal metabolic process. In addition, we expect that ZYN001 will be classified by the FDA as a new chemical entity, or NCE. In our preclinical animal studies, ZYN001 demonstrated effective skin permeation with sustained delivery and rapid conversion of ZYN001 to THC. These preclinical studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism. In addition, preclinical toxicology models conducted to date have not shown any toxicology or genotoxicity findings. ZYN001 is targeting two pain indications (fibromyalgia and peripheral neuropathic pain) which collectively affect millions of patients using treatments that currently comprise a multi-billion dollar market.
We believe ZYN001 may provide an effective treatment for fibromyalgia based on the hypothesis that an endocannabinoid deficiency is the underlying cause of fibromyalgia, which may enable patients to benefit from therapy with an exogenous cannabinoid. We believe ZYN001 may have a role in peripheral neuropathic pain management based on THC's activity as a partial agonist of cannabinoid receptors, as a stimulator of the noradrenergic pathway and as an inducer of antinociception, as well as the results of a third-party study suggesting that Cannabis reduces neuropathic pain.
We have assembled a highly experienced management team, each of whom has over 25 years of pharmaceutical industry experience, including our chief executive officer and president, who have a track record of success for obtaining regulatory approval of and commercializing products using transdermal delivery. Armando Anido, our chairman and chief executive officer, previously served as the chief executive officer of two publicly traded companies, Auxilium Pharmaceuticals Inc., or Auxilium, and NuPathe, Inc., or NuPathe. Terri B. Sebree, our president, previously co-founded NuPathe and served as senior vice president, development at Auxilium, and has successfully developed 10 products from IND to regulatory approval. Ms. Sebree most recently oversaw the development and regulatory approval of Testim® gel and Zecuity® patch. Richard A. Baron, our chief financial officer, has extensive experience as chief financial officer of public and private pharmaceutical companies, most recently having served as chief financial officer of Globus Medical, Inc. and, prior to that, at Avid Radiopharmaceuticals, Inc.
68
Our key development programs and expected timelines for the development of ZYN002 and ZYN001 are shown in the table below:
Product Candidate |
Target Indication | Delivery Method | Current Development Status |
Expected Next Steps | ||||
|---|---|---|---|---|---|---|---|---|
| ZYN002 | Refractory Epilepsy | Permeation-enhanced Gel | Preclinical | 2H15: Initiate Phase 1 | ||||
| Fragile X Syndrome | 2H16: Initiate Phase 2a | |||||||
| Osteoarthritis | ||||||||
| | | | | | | | | |
| ZYN001 | Fibromyalgia | Transdermal Patch | Preclinical | Mid-2016: Initiate Phase 1 | ||||
| Peripheral Neuropathic Pain | 1H17: Initiate Phase 2a |
Our Strengths
We are the first and only company developing patent-protected synthetic transdermal cannabinoid therapeutics with the following key distinguishing characteristics:
Exceptional and experienced management team with proven track record. We have a sophisticated and experienced management team, each of whom has over 25 years of pharmaceutical industry experience, including our chief executive officer and president, who have a successful history of development, regulatory approval and commercialization of patch and gel transdermal delivery products. Our management team has extensive pharmaceutical experience spanning a range of indications and a portion of our management team most recently gained regulatory approval and successfully commercialized Testim® Gel at Auxilium and Zecuity® Patch at NuPathe. Our leadership team is well-positioned to lead us through clinical development, regulatory approval and commercialization for our product candidates.
Unique delivery methods. We are the first and only company developing patent-protected synthetic cannabinoid therapeutics for transdermal delivery. Transdermal delivery is well-established in other FDA approved products, but we believe it is novel to cannabinoid therapeutics. Transdermal delivery has a range of potential benefits including the ability to provide sustained and consistent plasma levels, controlled delivery and convenient dosing, as well as the avoidance of first-pass liver metabolism and stomach acid degradation and an alternative for patients for whom oral formulations are suboptimal.
Synthetically manufactured pure cannabinoid therapeutics. Our product candidates are synthetically manufactured rather than extracted from Cannabis plants. We believe synthetically produced cannabinoids offer several advantages to botanically derived cannabinoids, including consistent, reproducible pharmaceutical-grade active ingredients, or APIs, with well-defined impurity profiles. Through synthetic manufacturing, we are able to isolate THC and CBD, which allows us to develop product candidates that do not contain the impurities and other compounds found in botanical cannabinoid products, which we believe may improve the therapeutic effect and reduce the potential adverse side effects associated with botanical Cannabis compounds. Synthetic manufacturing also allows for a more efficient chemistry, manufacturing and control, or CMC, process. We believe synthetic manufacturing will allow for a more clearly defined and straightforward FDA approval pathway by avoiding the potential problems faced when seeking approval for product candidates containing botanically derived cannabinoids, which include inconsistent API reproduction and additional toxicology studies related to botanical impurities.
Targeting indications with significant unmet medical need. We believe that our product candidates can provide effective treatment to patients with significant unmet medical needs in large markets, which will increase the probability of commercial success if our product candidates are approved. We plan to evaluate ZYN002 as adjunctive, second-line therapy in patients with epilepsy who have poor outcomes with standard therapies, which we believe represents an underserved patient population, and as monotherapy, first-line treatment in patients with FXS and OA. We plan to evaluate ZYN001 as monotherapy, first-line treatment of pain in patients with fibromyalgia and peripheral neuropathic pain, which we believe represent significant
69
markets of unmet need. We believe these patient populations are large enough to provide us with an attractive commercial opportunity.
Strong intellectual property protection for our product candidates. Our patent portfolio provides a long window for development and commercialization. The issued patents in the ZYN002 patent portfolio will expire between 2026 and 2029 and the issued patents in the ZYN001 portfolio will expire between 2028 and 2031. Our patent portfolio is not specific to any single indication, which we believe will allow us to develop products for additional patient populations in markets with significant unmet medical need.
Our Business Strategy
Our goal is to become a leader in the cannabinoid pharmaceuticals market by pursuing the following strategies:
- §
- Rapidly advance ZYN002 and ZYN001 through clinical development to regulatory approval in the United States. We expect to initiate Phase 1 clinical trials in the second half of 2015 for ZYN002 and by mid-2016 for ZYN001. We plan to initiate Phase 2a clinical trials for ZYN002 in the second half of 2016 and for ZYN001 in the first half of 2017. We are collaborating with the FDA and key opinion leaders in refractory epilepsy, FXS, OA, fibromyalgia and peripheral neuropathic pain to develop efficient clinical development plans.
- §
- Explore collaborations to develop and pursue regulatory approval of ZYN002 and ZYN001 outside the United States. We plan to explore out-licensing opportunities for ZYN002 and ZYN001 as appropriate with third parties who have regulatory and commercial expertise outside of the United States.
- §
- Explore additional indications and delivery methods. We plan to explore additional potential indications and product candidates for synthetic THC and CBD. For THC, examples of these additional indications include chronic cancer pain and gastrointestinal disorders. For CBD, examples of these additional indications include autism, autoimmune diseases, generalized anxiety disorder, posttraumatic stress disorder, depression, multiple sclerosis, addictive behaviors such as alcoholism and smoking, attention deficit hyperactivity disorder, and additional orphan disorders such as Neimann Pick Disease. In addition, our patent portfolio for synthetic THC and CBD includes the ability to use different delivery methods.
- §
- Strengthen our competitive position. We plan to continue to strengthen our competitive position by maintaining leadership in the transdermal synthetic cannabinoid therapeutics market and broadening our intellectual property rights including additional patents relating to process technologies, formulations and therapeutic uses of ZYN002 and ZYN001.
- §
- Commercialize ZYN002 and ZYN001 in the United States independently or with third parties. We intend to evaluate our commercialization options in the United States. We may build our own internal sales force; enter into a joint marketing partnership with another pharmaceutical or biotechnology company, whereby we jointly sell and market our product; or out-license our product, whereby another pharmaceutical or biotechnology company sells and markets our product and pays us a royalty on sales.
Cannabinoid Science Overview
Cannabinoids refer to a unique class of compounds derived from the Cannabis plant. Of the over 70 cannabinoid compounds currently identified, THC and CBD are the primary cannabinoids used for pharmaceutical purposes. THC was identified as the major psychoactive cannabinoid and subsequently found to be a partial agonist of the CB1 and CB2 receptors, activation of which stimulates the endogenous noradrenergic pathway, inducing antinociception and suggesting a role for THC in pain management.
CBD, the main non-psychoactive component of Cannabis, has little affinity for the CB1 and CB2 receptors. It does, however, produce multiple effects, including blocking the equilibrative nucleoside transporter, the orphan G-protein-coupled receptor GRP55, and the transient receptor potential of melastatin type 8 channel; enhancing activity of 5-HT1a and glycine receptors and the transient receptor potential of ankyrin type 1 channel; and regulating the intracellular effects of calcium. The influence of CBD on these targets —
70
each of which we believe plays a key role in neuronal excitability — is the scientific basis for its antiepileptic potential.
CBD inhibits fatty acid amide hydroxylase, or FAAH, an enzyme that breaks down anandamide, which is an endogenous cannabinoid ligand, mostly at the CB1 receptor. Inhibition of FAAH is thought to result in increased anandamide availability and greater CB1 activation. In addition, CBD inhibition of FAAH increases 2-arachidonoylglycerol, or 2-AG, availability. 2-AG, an endogenous endocannabinoid, activates both CB1 and CB2 receptors. Therefore, CBD acts as a facilitator of the endogenous endocannabinoid system, which modifies release of other neurotransmitters from presynaptic terminals. This modulation of neurotransmitter release from presynaptic neurons of various classes is the scientific basis for the use of CBD in the treatment of FXS.
CBD exerts a range of anti-inflammatory effects, including attenuation of the endothelial cell activation, chemotaxis of inflammatory cells, suppression of T-cell macrophage reactivity, and induction of apoptosis of T cells, which suggests a possible therapeutic role in the treatment of OA. CBD's agonist effect on TRPV1 receptors leads to antihyperalgesia, which further suggests a possible role in the treatment of OA. Third-party studies suggest that psychotropic effects of Cannabis are caused by THC, not CBD.
Clinical and preclinical data suggest that THC has positive effects on treating pain and CBD has positive effects on treating epilepsy, FXS and arthritis. Clinical data suggest that THC and CBD have a very high therapeutic index. Interest in cannabinoid therapeutics has increased significantly over the past several years as preclinical and clinical data has emerged highlighting the potential efficacy and safety benefits of cannabinoid therapeutics. Dronabinol and nabilone, oral formulations of THC, have been approved by the FDA. In third-party studies, adverse events from oral THC including dronabinol and nabilone were primarily psychotropic and related to peak plasma levels. Many patients have received CBD-enriched Cannabis and Epidiolex®, a liquid formulation of highly concentrated CBD which is currently in development. The cannabinoid therapeutics market is expected to grow significantly due to the potential benefits these products may provide over existing therapies. For example, opioids are often the standard of care for treating various pain diseases but patients frequently experience adverse side effects, including addiction and opioid-induced constipation. In addition to ZYN002 and ZYN001 potentially offering first-line therapies to patients suffering from FXS, OA, fibromyalgia and peripheral neuropathic pain, we believe ZYN002 may provide a complementary treatment for patients suffering from epilepsy who are refractory to their current treatment regimens.
We believe that we offer an attractive alternative to existing cannabinoid therapies by synthetically manufacturing and transdermally delivering our product candidates. Most cannabinoid therapies have drawbacks and limitations due to their botanical (plant-derived) nature, as well as the fact that they are administered orally. Botanical cannabinoids create significant challenges for drug manufacturers because of the natural resources and security measures required to grow Cannabis, as well as the strict batch controls required by regulatory agencies in pharmaceutical manufacturing. In addition, we believe all currently approved and development-stage cannabinoid therapeutics, except ZYN002 and ZYN001, are designed to be administered orally which can lead to limitations in safety and efficacy including low bioavailability, inconsistent plasma levels, degradation by stomach acids and significant first-pass liver metabolism. In contrast, transdermal therapeutics are absorbed through the skin directly into the systemic circulation, avoiding first-pass liver metabolism and degradation by stomach acid and potentially enabling lower dosage levels of active pharmaceutical ingredients and rapid and reliable absorption with high bioavailability, fewer negative psychoactive effects and fewer drug-drug interactions.
Our Product Candidates
Our patent-protected synthetic transdermal cannabinoid product candidates, ZYN002 and ZYN001, represent next-generation cannabinoid therapeutics for several indications including refractory epilepsy, FXS and OA for ZYN002 and fibromyalgia and peripheral neuropathic pain for ZYN001. Treatments for these indications represent markets with underserved patient populations which we believe can benefit from cannabinoid therapies. We believe our proprietary synthetic transdermal product candidates will effectively
71
address these indications and provide a solution to the limitations of botanical and oral and oral mucosal cannabinoid therapeutics.
Our patent-protected synthetic transdermal cannabinoid product candidates, ZYN002 and ZYN001, will be evaluated in five indications beginning with Phase 1 clinical trials commencing in 2015 for ZYN002 and in 2016 for ZYN001. We plan to study ZYN002 in patients with refractory epilepsy, FXS and OA. We plan to evaluate ZYN001 in patients with fibromyalgia and peripheral neuropathic pain. We believe these product candidates will provide new treatment options for patients, as well as additional treatment options for patients not currently receiving adequate relief from current treatment regimens. Additionally, we believe that these indications represent significant unmet medical needs in large markets, which will increase the probability of commercial success if our product candidates are approved.
ZYN002
Overview
ZYN002 is the first and only synthetic CBD formulated as a patent-protected permeation-enhanced gel for transdermal delivery (see Figure 1).
Figure 1 — Chemical structure and delivery of CBD.
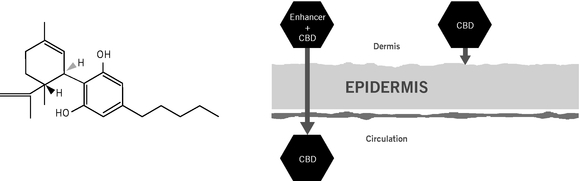
ZYN002 is being developed as a clear, odorless gel that is designed to provide consistent, controlled drug delivery with convenient once- or twice-daily dosing. Because CBD is virtually insoluble in water, we use ethanol and propylene glycol as solubilizing agents and Transcutol® HP as a permeation enhancer. All excipients in the gel have been classified as GRAS and have been used in transdermal products previously approved by the FDA.
The permeation enhancer in ZYN002 increases the delivery of CBD through the layers of the skin and into the circulatory system.
Transdermal delivery allows the CBD in ZYN002 to avoid stomach acid degradation and the first-pass liver metabolism that occurs with oral or oral mucosal delivery methods. Drugs applied transdermally are absorbed across the skin directly into the systemic circulation, enabling the potential to have rapid and reliable absorption with high bioavailability.
Market Overview
We intend to study ZYN002 in the treatment of refractory epilepsy, FXS and OA.
Epilepsy —
Epilepsy is a disease characterized by an enduring predisposition to generate epileptic seizures (transient symptoms due to abnormal neuronal activity in the brain) and by the neurobiological, cognitive, psychological, and social consequences of the condition. Partial seizures usually start in a small area of the temporal lobe or frontal lobe of the brain and quickly involve other areas of the brain that affect alertness and awareness. Partial seizures are the most common type of seizure, representing 35% of all epilepsies.
According to Decision Resources, in 2012 there were approximately 2.2 million epilepsy patients in the United States, and treatments for these patients represented a total U.S. market size of approximately $1.7 billion.
72
FXS —
FXS is a genetic condition that causes intellectual disability, anxiety disorders, behavioral and learning challenges and various physical characteristics. The impairment can range from learning disabilities to more severe cognitive or intellectual disabilities. According to the National Fragile X Foundation, FXS affects 1 in 3,600 to 4,000 males and 1 in 4,000 to 6,000 females of all races and ethnic groups. Approximately 1 in 151 women carry the Fragile X gene and could pass it to their children. Approximately 1 in 468 men carry the Fragile X gene and their daughters will also be carriers. FXS is an autism spectrum disorder, and patients with FXS exhibit autism-like symptoms including cognitive impairment, anxiety and mood swings, attention deficit and heightened stimuli. Approximately 7% of women and 18% of men with FXS have seizures. People with FXS are affected throughout their lives. Currently, there are no known cures or approved therapies for the treatment of FXS. Special education and symptomatic treatments for anxiety and irritability are employed to lessen the burden of illness.
FXS is the most common inherited intellectual disability. Based on the 2012 U.S. Census and FXS prevalence rates according to the National Fragile X Foundation, in 2012 there were approximately 71,000 patients with FXS in the United States.
Osteoarthritis —
OA is a degenerative joint disease that leads to wear and tear of the joints and affects the cartilage, joint lining, ligaments and bone. It is the most common form of joint disease and tends to occur most often in the hand joints, spine, hip, knees and great toes. It is characterized by the breakdown of the joint cartilage, bony changes in the joints and deterioration of the tendons and ligaments leading to pain and inflammation of the joint lining.
According to Decision Resources, which defines OA as radiographically confirmed OA of any joint, in 2008 it was predicted that the total number of prevalent cases of OA would be approximately 129.5 million by 2012. Treatment for patients suffering from OA represented a total U.S. market size of approximately $670.0 million in 2012.
The table below summarizes our target indications, expected type of therapy and 2012 market size with regard to each target indication for ZYN002.
| |
|
2012 U.S. Market Size(1) | ||||
|---|---|---|---|---|---|---|
| Target Indication | Expected Type Of Therapy | Patient Population | Current Market | |||
| Refractory Epilepsy | Adjunctive, second-line therapy in patients with partial seizures with secondary generalization on a stable dose of an anticonvulsant with a history of failure | 2.2 million | $1.7 billion | |||
| Fragile X Syndrome | Monotherapy, first-line therapy in patients with FXS | 71,000 | There are no FDA-approved therapies | |||
| Osteoarthritis | Monotherapy, first-line therapy in patients with OA | 129.5 million | $670.0 million | |||
- (1)
- Except for FXS data, based on data provided by Decision Resources. Data for epilepsy represents the market size for all types of epilepsy. FXS data based on 2012 U.S. Census data and data provided by the National Fragile X Foundation.
CBD Rationale in Epilepsy, FXS and OA
Epilepsy —
We believe that ZYN002 may provide an effective treatment for refractory epilepsy based on the anticonvulsant effects of CBD due to its ability to reduce neuronal hyperexcitability shown in multiple in vivo models of epilepsy conducted by third parties. Epilepsy specialists and patient organizations have
73
shown considerable interest in the potential therapeutic role of CBD in adults with epilepsy and, especially, children with intractable epilepsy. Two companies have active CBD development programs for the treatment of patients with Dravet's syndrome, or DS, or Lennox Gastaut syndrome, or LGS, both of which are rare and severe forms of pediatric epilepsy. Unlike these cannabinoid-based epilepsy development programs, we are conducting development programs for ZYN002 in a much broader subset of the epilepsy population.
No large-scale, placebo-controlled clinical trials of CBD for the treatment of patients with epilepsy (either pediatric or adult) have been published. However, in a small (fifteen patients), third-party Phase 2 clinical trial, patients with a documented history of epilepsy were treated with crystalline CBD or placebo for up to 4.5 months. A total of 88% of the CBD-treated group had a response to treatment, with 50% of patients experiencing considerable improvement and 38% reporting at least partial improvement.
In separate, uncontrolled work, in a third-party survey of parents who have used CBD-enriched Cannabis to treat seizures in their children with Dravet syndrome, Doose syndrome, or LGS, 84% of parents reported a reduction in seizure frequency while their children were taking CBD-enriched Cannabis.
Fragile X Syndrome —
We believe ZYN002 may provide an effective treatment for FXS based on its capacity to interact with the endocannabinoid system, which is compromised in patients with FXS. Specifically, CBD indirectly increases the concentration of the cannabinoids 2-AG and anandamide, which are endogenous ligands at the CB1 and CB2 receptors. Furthermore, the Fragile X mental retardation protein 1, that is diminished in patients with FXS, is required for the production of 2-AG. 2-AG acts in part by interacting with the CBD receptor. Therefore, FXS results in the reduction of endogenous stimulation of endocannabinoid receptors while CBD facilitates the availability of endogenous endocannabinoids, potentially attenuating the pathophysiology of the disease.
Osteoarthritis —
We believe that ZYN002 may provide an effective treatment for OA based on research we have conducted. We examined the efficacy of transdermal ZYN002 for reduction of inflammation and pain in vivo, assessing adverse effects in a rat with complete Freund's adjuvant-induced monoarthritic knee. ZYN002 gel (0.6, 3.1, 6.2, or 62.3 mg/day) was applied for four consecutive days after arthritis induction. The level of inflammation was assessed by knee joint circumference and immune cell invasion in histological sections.
Measurement of CBD plasma concentration revealed linearity with daily doses of 0.6 to 6.2 mg of ZYN002. Compared with baseline, rats treated with ZYN002 gel had significant, dose-dependent reductions in knee joint and synovial membrane swelling, immune cell infiltration, and spontaneous pain rating scores (P£0.05). Paw withdrawal latency, or PWL, recovered to near-baseline levels. Immunohistochemical analysis of spinal cord and dorsal root ganglia revealed dose-dependent reductions of pro-inflammatory biomarkers (CD11b/c, CGRP, TNF). The data suggests that topical ZYN002 has the potential to provide effective relief of pain and inflammation caused by OA.
In vitro and Preclinical Studies
In an in vitro study, we evaluated the effects of exposing CBD to acidic conditions. Gastric fluid has a hydrochloric acid (HCl) concentration of 0.1 M which is highly acidic with a pH of 1.5 to 3.0. We exposed CBD to a 0.1 M HCl solution. Upon high-performance liquid chromatography analysis of this solution, we found D-8 THC and D-9 THC compounds formed from the degradation of CBD in this acidic condition. These results suggest that CBD may degrade and form THC if delivered by oral or oral mucosal routes. Figure 2 illustrates the degradation of CBD in an acidic solution.
74
Figure 2. Degradation of CBD in an Acidic Solution.
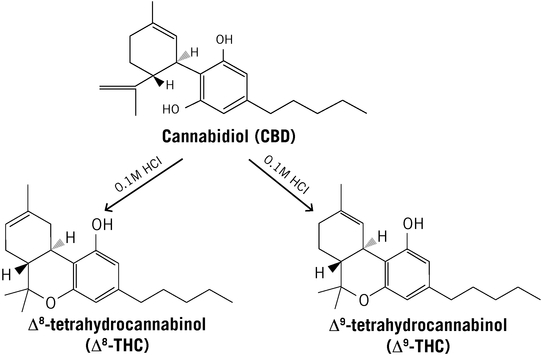
The pharmacokinetics, or PK, of ZYN002 has been studied in three animal species: guinea pigs, Sprague-Dawley, or SD, rats and squirrel monkeys. PK refers to a drug's absorption, distribution, metabolism, and excretion from the body and measures, among other things, the concentration of the drug in the plasma. Based upon preclinical studies, the transdermal route of administration of ZYN002 achieves sustained, consistent CBD plasma levels.
In vivo guinea pig PK —
The PK of CBD in a gel was tested in guinea pigs with and without Transcutol HP, ZYN002's permeation enhancer, in the gel. Plasma concentrations of CBD were measured over the course of 96 hours. CBD with the permeation enhancer demonstrated higher plasma concentrations compared to CBD gel without a permeation enhancer. No skin irritation was shown. Results are reflected in Figure 3.
75
Figure 3. CBD plasma concentrations with and without ZYN002 permeation enhancer in guinea pigs.
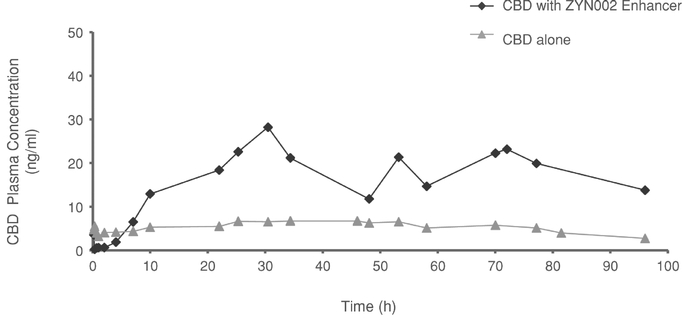
The PK of ZYN002 1% CBD gel was examined in a guinea pig in single and multiple doses. The multiple doses were administered at 24 and 48 hours after the first dose. Blood samples were obtained for 120 hours. The results demonstrated consistent, sustained plasma levels of CBD when ZYN002 was administered transdermally in our patent-protected formulation. No skin irritation was shown. Results are reflected in Figure 4.
Figure 4. CBD plasma concentrations after single and multiple ZYN002 dosing in guinea pig.
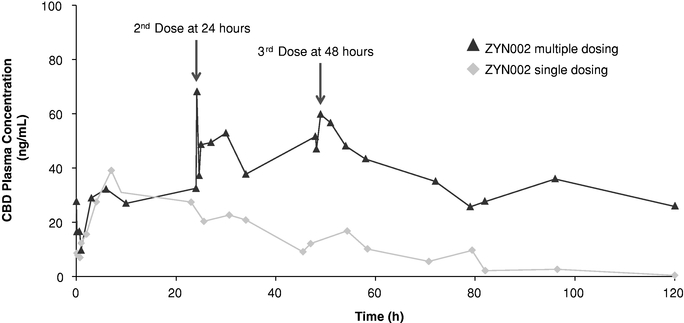
In vivo rat PK —
SD rats were studied to examine the efficacy of ZYN002 for reduction in inflammation and pain in a monoarthritic knee model. As part of this study, plasma concentrations of CBD were determined following topical application of ZYN002 in the following dosages: 0.6, 3.1, 6.2 or 62.3 mg/day of daily for four days. Plasma concentrations of CBD from rats dosed with 0.6, 3.1 and 6.2 mg/day of ZYN002 exhibited a linear
76
dose response. A linear dose response means the drug delivery is directly proportional to the amount of drug formulation transdermally applied. This proportionality will help us predict human doses of ZYN002 and plasma levels of CBD. No skin irritation was shown. The CBD plasma concentrations are shown in Table 1.
Table 1. CBD Plasma Concentrations in Mono-Arthritic Rat Model.
ZYN002 CBD gel dose (mg/day)
|
Plasma concentrations (ng/ml)
|
|
|---|---|---|
| 0.6 | 3.8 ±1.4 | |
| 3.1 | 17.5 ±4.4 | |
| 6.2 | 33.3 ±9.7 | |
| 62.3 | 1629.9 ± 379.0 |
In vivo squirrel monkey PK —
In a study of squirrel monkeys, the PK of ZYN002 2.5% CBD gel was examined in single and multiple doses. As part of this study, plasma concentrations of CBD were determined following topical application of ZYN002 in the following doses: 2.7, 5.4, 10.8 and 16.1 mg/day daily for five days. Blood samples were obtained four hours after daily application of ZYN002 gel. Mean CBD plasma concentrations exhibited a linear dose response (Figure 5).
Figure 5. Mean CBD plasma concentrations of squirrel monkeys after increasing doses of ZYN002 2.5% CBD gel.
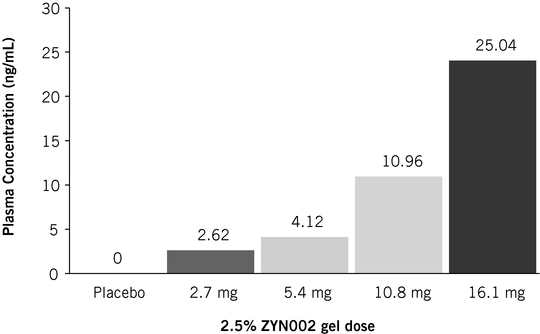
ZYN001
Overview
ZYN001 is a pro-drug of THC that enables effective transdermal delivery via a patch and is patent-protected through 2031. In addition, we expect that ZYN001 will be classified by the FDA as an NCE. The transdermal patch is a non-invasive, non-oral dosage form that has been proven to be an effective method of delivery in other FDA approved products. In our preclinical animal studies, ZYN001 demonstrated effective skin permeation with sustained delivery and rapid conversion of ZYN001 to THC. These preclinical
77
studies suggest increased bioavailability, consistent plasma levels and the avoidance of first-pass liver metabolism.
Due to the structure of the skin, its two main layers, the outer epidermis and inner dermis, effectively inhibit the transdermal delivery of most therapeutics. Drugs classified as hydrophobic, such as THC, add an additional impediment to their transdermal delivery through the skin. THC is naturally hydrophobic and thus is unable to be effectively delivered through human skin.
ZYN001 is a pro-drug, which is a drug administered in an inactive or less active form and designed to enable more effective delivery, and then converted into an active form through a normal metabolic process. Chemically, ZYN001 is the synthetic D-glyceric acid ester of THC. Unlike THC, ZYN001 is able to be efficiently absorbed into the skin through transdermal delivery. After crossing the stratum corneum, ZYN001 is hydrolyzed back to THC and glyceric acid under physiological conditions, mainly due to the action of common enzymes in the skin tissue known as "esterases." See Figure 6 below.
Figure 6. Hydrolysis of ZYN001 into glyceric acid and THC.
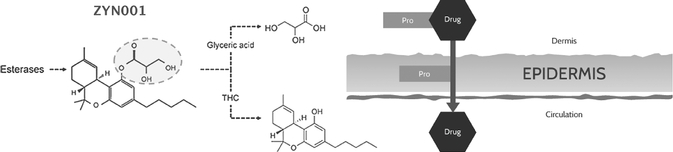
The ZYN001 transdermal patch contains a 4% ZYN001 gel formulation. We intend to test the ZYN001 patch for application to the arm, back and thigh. The drug substance is produced synthetically and is not derived or extracted from botanicals. The excipients in the patch have been classified as Generally Recognized As Safe, or GRAS, and have been used in transdermal products previously approved by the FDA.
As illustrated below in Figure 7, transdermal delivery often provides more consistent plasma levels without the peaks and valleys often associated with an oral dosage form.
Figure 7. Illustration of transdermal delivery.
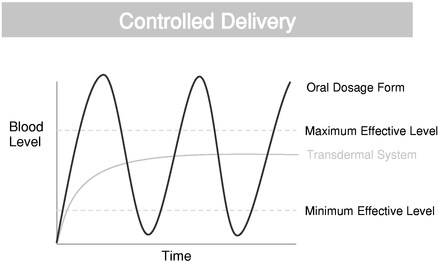
Market Overview
We intend to study ZYN001 for the treatment of fibromyalgia and peripheral neuropathic pain.
78
Fibromyalgia —
Fibromyalgia is a chronic health problem that causes pain throughout the body and other symptoms such as fatigue and cognitive (memory or thought) problems.
According to Decision Resources, in 2012, there were approximately 5.6 million adult fibromyalgia patients in the United States, and pain treatment for these patients represented a total U.S. market size of approximately $1.6 billion.
Peripheral Neuropathic Pain —
Neuropathic pain is defined as pain initiated or caused by a primary lesion or dysfunction of the central or peripheral nervous systems. In patients with peripheral neuropathic pain, the pain is a symptom of another disease that has caused nerve damage — such as a herniated disc (lower back pain), diabetes (diabetic neuropathy), cancer (neuropathic cancer pain), or herpes zoster infection (postherpetic neuralgia) — but it is recognized as a clinical condition on its own. Because the damage does not involve the brain or spinal cord, the resulting neuropathic pain is defined as peripheral.
According to Decision Resources, in 2012 there were approximately 14.0 million peripheral neuropathic pain patients in the United States, and pain treatment for these patients represented a total U.S. market size of approximately $4.0 billion in 2012.
| |
|
2012 U.S. Market Size(1) | ||||
|---|---|---|---|---|---|---|
| Target Indication | Expected Type Of Therapy | Patient Population | Current Market | |||
| Fibromyalgia | § Monotherapy, first-line therapy in patients with fibromyalgia |
§ 5.6 million |
§ $1.6 billion |
|||
Peripheral Neuropathic Pain |
§ Monotherapy, first-line therapy in patients with peripheral neuropathic pain |
§ 14.0 million |
§ $4.0 billion |
|||
- (1)
- Based on data provided by Decision Resources.
THC Rationale in Pain Indications
Fibromyalgia —
We believe that ZYN001 may provide an effective treatment for fibromyalgia based on the hypothesis that an endocannabinoid deficiency is the underlying cause of fibromyalgia, which may enable patients to benefit from therapy with an exogenous cannabinoid. Nabilone, a compound which is chemically similar but not identical to THC, has shown significant reduction in fibromyalgia pain in a third-party randomized clinical trial, but it has also been associated with dose limiting psychoactive adverse effects. We believe ZYN001 has the potential to treat fibromyalgia by delivering sustained, consistent plasma levels of THC to provide a therapeutic benefit with minimal psychoactive adverse effects.
Peripheral Neuropathic Pain —
A strong pharmacologic rationale for the use of ZYN001 in peripheral neuropathic pain provides the experimental basis for our clinical program. Third-party studies in animals have shown that cannabinoid receptors are found in high concentrations in the central nervous system, as well as on peripheral neurons and along the principal nociceptive pathways. The results of a third-party randomized, placebo-controlled clinical trial demonstrated that low-dose vaporized Cannabis may reduce neuropathic pain which we believe suggests a role for THC in peripheral neuropathic pain management.
79
Preclinical Studies
In preclinical studies, the transdermal route of administration of ZYN001 achieved consistent THC plasma levels that we believe will deliver therapeutic benefit with minimal psychoactive side effects thereby distinguishing it from oral and oral mucosal delivery systems.
In vivo guinea pig PK —
The PK of a 4% ZYN001 patch was examined in a hairless guinea pig model by adhering two patches, with a total of 6.25 cm2 active area, to the guinea pig. PK refers to a drug's absorption, distribution, metabolism, and excretion from the body and measures, among other things, the concentration of the drug in the plasma. The patches remained on the guinea pig for 72 hours, and blood samples were obtained for 103 hours. Plasma samples were analyzed by liquid chromatography-tandem mass spectrometry, or LC/MSMS. ZYN001, THC, and metabolites of THC (hydroxy-THC, or THC-OH, and 11-nor-delta-9-THC carboxylic acid, or TCH-COOH), were measured. No skin irritation or erythema was shown. Results of this study are reflected in Figure 8 below.
Figure 8. Plasma concentration vs time in guinea pig after ZYN001 patch application.
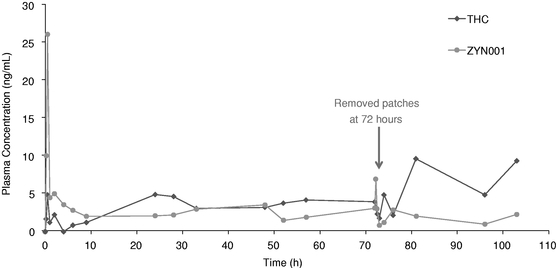
ZYN001 achieved a steady-state plasma concentration of total THC equivalents of 6.90 ± 1.47 ng/mL. These results demonstrate that ZYN001 was rapidly converted to THC and sustained, consistent plasma levels of THC were achieved.
In vivo rat PK —
The PK of ZYN001 was examined in SD rats following a subcutaneous injection of 1 mg/kg of ZYN001 in sesame oil. Plasma samples were obtained and determined by LC/MSMS. Results of this study are shown in Figure 9.
80
Figure 9. Plasma concentration vs time in rats after 1 mg/kg subcutaneous administration of ZYN001.
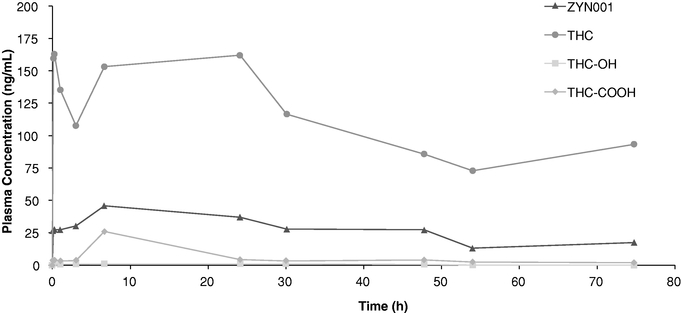
This data demonstrated the rapid conversion of ZYN001 to THC in the rat.
Product Development
ZYN002
We have completed an extensive review of the literature related to preclinical toxicology of CBD and completed a mutagenicity study with ZYN002. This review of the literature related to preclinical toxicology was provided to the FDA for a pre-IND meeting held in the first quarter of 2015 and, the FDA confirmed that no further preclinical work is required prior to the initiation of the Phase 1 clinical program. We expect to initiate Phase 1 clinical trials in Australia beginning in the second half of 2015, and Phase 2 clinical trials in each indication in the second half of 2016. We do not expect at this time to file an IND with the FDA prior to the commencement of these trials. Based upon our method of transdermal delivery of ZYN002, we expect that the ZYN002 dosage administered to patients in our clinical trials will be less than 400 mg of CBD. We intend to initiate a standard toxicology program for ZYN002 concurrently with the clinical program. The toxicology program will consist of standard single-dose toxicology, multiple-dose toxicology, reproductive studies and carcinogenicity studies.
Phase 1 Clinical Trial
We plan to evaluate the tolerability and PK profile of ZYN002 in a Phase 1 single rising dose clinical trial in healthy human subjects and in patients with epilepsy. Subsequent to the single rising dose clinical trial, we intend to conduct a Phase 1 multiple rising dose clinical trial to examine the tolerability, PK and pharmacodynamics, or PD, of multiple doses of ZYN002 in healthy human subjects and in patients with epilepsy. To complete the Phase 1 program, we will conduct a bioequivalence clinical trial assessing the PK of ZYN002 when applied to various parts of the body (e.g., arm, thigh and back).
81
Phase 2a Clinical Trials
We intend to initiate Phase 2a clinical trials for ZYN002 as outlined in the following table:
| |
Phase 2a Clinical Trials | |||||
|---|---|---|---|---|---|---|
Target Indication
|
Patient Population | Expected Type of Therapy | Design | |||
| Refractory Epilepsy | Patients with partial seizures with or without secondary generalization | Adjunctive therapy in patients with partial seizures with secondary generalization on a stable dose of an anticonvulsant with a history of failure | Randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of multiple doses of ZYN002 to placebo | |||
Fragile X syndrome |
Patients diagnosed with FXS |
Monotherapy, first-line therapy in patients with FXS |
Open-label trial to evaluate the efficacy and safety of ZYN002 |
|||
Osteoarthritis |
Patients diagnosed with OA |
Monotherapy, first-line therapy in patients with OA |
Randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of multiple doses of ZYN002 to placebo |
|||
Phase 2b Clinical Trials
Depending on the results of Phase 2a clinical trials, we may need to further define the dosing in Phase 2b trials or we may proceed directly into Phase 3 clinical trials.
Phase 3 Clinical Trials
We intend to use the data from the Phase 2 clinical trials outlined above to select doses of ZYN002 for our Phase 3 program, which will consist of two randomized, double-blind, placebo-controlled clinical trials for each indication and open-label long-term clinical trials.
ZYN001
The standard battery of genotoxicology studies required by the FDA for ZYN001 showed no adverse effects. The safety pharmacology studies required by the FDA demonstrated that ZYN001 has a pharmacology profile consistent with THC. The compound is currently being investigated in preclinical toxicology with completion expected in the first half of 2016. We discussed our planned preclinical studies and clinical trials for ZYN001 with the FDA at a Pre-IND meeting in August 2013. We anticipate initiating Phase 1 clinical trials beginning by mid-2016. Phase 2 clinical trials investigating ZYN001 in each individual indication are planned to begin in the first half of 2017.
Phase 1 Clinical Trial
We plan to evaluate the tolerability and PK profile of ZYN001 in a Phase 1 single rising dose clinical trial in healthy human subjects. Subsequent to the single rising dose clinical trial, we intend to conduct a Phase 1 multiple rising dose clinical trials to examine the tolerability, PK and PD of multiple doses of ZYN001 in healthy human subjects and in patients with fibromyalgia. To complete the Phase 1 program, we will conduct a bioequivalence clinical trial assessing the PK of ZYN001 when applied to various parts of the body (e.g., arm, thigh and back).
82
Phase 2a Clinical Trials
We intend to initiate Phase 2a clinical trials for ZYN001 as outlined in the following table:
| |
|
|||||
|---|---|---|---|---|---|---|
| |
Phase 2a Clinical Trials |
|||||
| Target Indication | Patient Population | Expected Type of Therapy | Design | |||
Fibromyalgia |
Patients who meet the American College of Rheumatology criteria for fibromyalgia syndrome | Monotherapy, first-line therapy in patients with fibromyalgia | Randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of multiple doses of ZYN001 to placebo | |||
Peripheral Neuropathic Pain |
Patients with peripheral neuropathic pain of at least six months' duration |
Monotherapy, first-line therapy in patients with peripheral neuropathic pain |
Randomized, double-blind, placebo-controlled trial comparing the efficacy and safety of multiple doses of ZYN001 to placebo |
|||
Phase 2b Clinical Trials
Depending on the results of Phase 2a clinical trials, we may need to further define the dosing in Phase 2b clinical trials or we may proceed directly into Phase 3 clinical trials.
Phase 3 Clinical Trials
We intend to use the data from the Phase 2 clinical trials outlined above to select doses of ZYN001 for our Phase 3 program, which will consist of two randomized, double-blind, placebo-controlled clinical trials for each indication and open-label long-term clinical trials.
Intellectual Property
The success of our product candidates will depend in large part on our ability to:
- §
- obtain and maintain patent and other legal protections for the proprietary
compounds, technology, inventions and improvements we consider important to our business;
- §
- prosecute our patent applications and defend our issued patents;
- §
- preserve the confidentiality of our trade secrets; and
- §
- operate without infringing the patents and proprietary rights of third parties.
We internally developed our intellectual property related to ZYN002 and ZYN001. We have sought and intend to continue to seek appropriate patent protection for our product candidates, as well as other proprietary technologies and their uses by filing patent applications in the United States and selected other countries.
As of July 23, 2015, we owned a total of seven issued U.S. utility patents and three pending U.S. utility patent applications. These U.S. patents and patent applications will expire between 2029 through 2031. We have already obtained additional patent term for some of the issued patents to compensate us for delays at the U.S. Patent Office, under the patent term adjustment laws. These patents, and any pending
83
applications that ultimately issue as patents, may also be eligible for patent term extension for delay caused by FDA regulatory review, thereby further extending their patent terms.
In addition to our U.S. intellectual property, we own 23 corresponding foreign issued patents and 13 corresponding foreign applications, which will expire between 2026 and 2031. Three of the foreign applications are allowed, and should issue as patents in the next several months.
ZYN002
Our ZYN002 patent portfolio currently consists of two issued patents in the United States, five issued patents in France, Germany, Ireland, Switzerland, and the United Kingdom and two pending patent applications in Canada and Japan. The issued patents claim formulations and methods of use relating to ZYN002. The issued patents cover the permeation-enhanced formulation of ZYN002. The issued patents will expire between 2026 and 2029. Any patents that issue from our currently pending patent applications will expire in 2030.
ZYN001
Our ZYN001 patent portfolio currently consists of two issued patents in the United States, one issued patent in Japan, one allowed patent in Europe and patent applications pending in the United States, Europe, Canada and Japan. The issued patents are composition of matter patents one of which covers the chemical structure of the pro-drug ZYN001, the D-glyceric acid ester of THC, and one of which covers other THC pro-drugs. The issued patents will expire between 2028 and 2031. Any patents that issue from our currently pending patent applications will expire in 2028.
Other
The rest of our patent portfolio relates to patents and applications owned by us and directed to other potential product candidates.
U.S. Government Rights
Under 35 U.S.C §202(c), the U.S. government retains certain rights in inventions funded by U.S. government grants. Although we have received U.S. government grants, some of which were used to fund preclinical research related to ZYN002 and ZYN001, the inventions and patent applications covering ZYN002 and ZYN001 were made prior to our receipt of such grants. Therefore, we believe that the U.S. government does not have any rights in those patents and applications pursuant to 35 U.S.C §202(c).
Trade Secrets and Proprietary Information
We seek to protect our proprietary information, including our trade secrets and proprietary know-how, by requiring our employees, consultants and other advisors to execute confidentiality agreements upon the commencement of their employment or engagement. These agreements generally provide that all confidential information developed or made known during the course of the relationship with us be kept confidential and not be disclosed to third parties except in specific circumstances. In the case of our employees, the agreements also typically provide that all inventions resulting from work performed for us, utilizing our property or relating to our business and conceived or completed during employment shall be our exclusive property to the extent permitted by law. Where appropriate, agreements we obtain with our consultants also typically contain similar assignment of invention obligations. Further, we require confidentiality agreements from entities that receive our confidential data or materials.
Manufacturing
The APIs used in ZYN002 and ZYN001 are synthesized by contract manufacturers. We have inventories that we believe will supply API for the preclinical studies required prior to initiation of clinical trials.
We believe synthetically produced API offers several advantages to botanically derived API because it provides a consistent, reproducible substance with a well-defined, constant impurity profile manufactured in FDA approved facilities.
84
The ZYN002 gel is manufactured and filled into single unit tubes by a contract manufacturer. The ZYN001 transdermal patch is manufactured by contract manufacturers and sub-component fabricators. The patch is manufactured by a contract manufacturer. The drug product formulation is manufactured and filled into the patch by another contract manufacturer.
We selected our contract manufacturers and sub-component fabricators for their specific competencies in manufacturing, product design, and materials. FDA regulations require that products be produced under current Good Manufacturing Practices, or cGMPs. Our key suppliers currently meet cGMPs and have sufficient capacities to meet our projected product requirements through Phase 3 clinical trials.
Commercial Operations
We do not currently have an organization for the sales, marketing and distribution of pharmaceutical products. We may rely on licensing and co-promotion agreements with strategic collaborators for the commercialization of our products in the United States and other territories. If we choose to build a commercial infrastructure to support marketing in the United States, such commercial infrastructure could be expected to include a sales force supported by sales management, internal sales support, an internal marketing group and distribution support. To develop the appropriate commercial infrastructure internally, we would have to invest financial and management resources, some of which would have to be deployed prior to any confirmation that ZYN002 or ZYN001 will be approved.
Competition
The biotechnology and pharmaceutical industries are characterized by rapidly advancing technologies, intense competition, and a strong emphasis on proprietary products. We believe our scientific knowledge, technology, and development capabilities provide us with substantial competitive advantages, but we face potential competition from multiple sources, including major pharmaceutical, specialty pharmaceutical, and biotechnology companies; academic institutions; governmental agencies; and public and private research institutions. Successfully developed and commercialized product candidates must compete not only with existing therapies, but also with agents that may become available in the future.
ZYN002
We intend to study ZYN002, our proprietary synthetic CBD product candidate, as an adjunctive, second-line therapy in refractory epilepsy patients who are not well controlled by their current anti-epileptic drug, or AED. We also intend to study ZYN002 in a clinical development program as monotherapy first-line treatment in patients with FXS and OA.
Cannabinoid Competition
Cannabinoids, such as GW Pharmaceuticals, PLC, or GW's Epidiolex® and CBD more generally, have shown promise clinically and anecdotally as a treatment for epilepsy. No CBD products or combination THC/CBD products have been approved in the United States. GW is currently investigating Epidiolex, a liquid formulation of highly concentrated CBD, in the United States for the treatment of DS, (severe, infantile-onset, genetic drug-resistant epilepsy with no FDA approved treatments) and LGS (a rare disorder with onset typically between three and five years of age featuring multiple seizure types with slow spike wave complexes on EEG) and childhood epilepsy syndromes. Insys Therapeutics Inc., or Insys, is also developing a synthetic CBD compound for the treatment of DS, LGS, childhood epilepsy syndromes and glioblastoma.
In the combination THC/CBD space, GW's Sativex®, a plant extract combination of THC/CBD for treatment of spasticity associated with multiple sclerosis, is approved in 27 countries outside the United States as an alternative to baclofen and tizanidine.
Additional activity in this space includes companies supplying synthetic cannabinoids, Cannabis extracts, and herbal Cannabis to researchers for preclinical and clinical investigation.
85
General Competition and Standard of Care
Within epilepsy, we intend to treat refractory patients who have not responded to previous treatment with AEDs such as depakote, pregabalin, gabapentin, topiramate, and lacosamide. Second and third generation AEDs continue to improve upon first generation therapies, but experts contend that a better understanding of the disorder accompanied by fundamentally innovative treatments will be required to effectively improve treatment outcomes for the high percentage of patients undertreated by AEDs. The majority of AEDs have frequent safety concerns including serious CNS adverse events and drug-drug interactions.
In FXS, our expected type of therapy is monotherapy, first-line therapy in patients with FXS. There are no drugs approved for the treatment of FXS, although various classes of medications are used off-label for the treatment of behavioral and mental health conditions associated with FXS. Some patients with FXS benefit from medications that treat attention deficient disorders and other patients who experience general anxiety, social anxiety and other chronic conditions may benefit from different types of anti-anxiety medications.
We are aware of several drugs in development for the treatment of behavioral and mental health conditions associated with FXS, including a number of generic drugs used for other indications such as donepezil, memantine, sertraline, and minocycline. Companies developing compounds include Roche, Afraxis, Neuren Pharmaceuticals and Marinus Pharmaceuticals.
In OA, our expected type of therapy is monotherapy, first-line therapy in patients with OA. Currently, no disease-modifying treatments for OA have been approved. Consequently, patients try a multitude of prescription and over-the-counter, or OTC, medications to relieve pain including traditional NSAIDs, Cox-2 inhibitors, centrally acting analgesics, topical analgesics, intra-articular corticosteroids, and hyaluronic acid preparations. Though these products offer varying degrees of pain relief, many also have been shown to cause significant adverse effects including potential addiction.
ZYN001
We plan to pursue two different indications for ZYN001, our proprietary synthetic THC product candidate: fibromyalgia and peripheral neuropathic pain.
Cannabinoid Competition
We believe cannabinoids offer a superior treatment paradigm for patients suffering from pain, providing more controlled delivery and therefore treatment effect along with a much more tolerable safety profile. The Cannabis therapeutic area currently includes formulated extracts of the Cannabis sativa plant and synthetic products, all of which use THC, CBD, or a combination of THC/CBD as the active ingredient. In the United States, two oral capsules — Insys' dronabinol (a synthetic THC) and Meda AB's nabilone (a synthetic derivative of THC) — have been approved and distributed for the treatment of nausea and vomiting associated with cancer chemotherapy in patients who have not responded adequately to conventional antiemetic treatments. Dronabinol capsules are also approved for anorexia associated with weight loss in patients with acquired immune deficiency syndrome, or AIDS. Insys is also seeking FDA approval for an orally administered liquid formulation of dronabinol for treatment of anorexia associated with weight loss in patients with AIDS and nausea and vomiting associated with cancer chemotherapy in patients who have failed to respond adequately to conventional antiemetic treatments. Exploratory research into the effects of THC formulations in other indications is also in progress. GW, is also developing Sativex®, a plant extract combination of THC/CBD for treatment of chronic cancer pain and neuropathic pain in an oral mucosal spray.
General Competition and Standard of Care
In fibromyalgia, our expected type of therapy is monotherapy, first-line therapy in patients with fibromyalgia. There are only three drugs approved by the FDA: Pregabalin, duloxetine, and milnacipran. We intend to treat patients whose condition is either newly diagnosed or has not responded to previous treatment with these three currently approved treatments. There is no known cure for the disease and no single therapy is likely to provide significant relief of all symptoms. Low-dose tricyclic antidepressants are prescribed frequently,
86
but are associated with various side-effects. Fibromyalgia is also treated using opioids, but, in addition to debate about their efficacy, these can be addictive for many patients.
In peripheral neuropathic pain, our expected type of therapy is monotherapy, first-line therapy in patients with peripheral neuropathic pain. Peripheral neuropathic pain generally is treated with tricyclic antidepressants, anticonvulsants such as duloxetine, depakote, pregabalin, gabapentin and topiramate, and serotonin/norepinephrine reuptake inhibitors, or SNRIs. Although tricyclic antidepressants, anticonvulsants, and SNRIs often show efficacy in treating neuropathic pain, they also have many drawbacks, including poor tolerability with side effects in most patients.
Government Regulation and Product Approval
As a development stage pharmaceutical company that operates in the United States, we are subject to extensive regulation by the FDA, and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, and its implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for, and marketing of, our products in other countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in Europe are addressed in a centralized way through the EMA, but country-specific regulation remains essential in many respects. The process of obtaining regulatory marketing approvals and the subsequent compliance with appropriate federal, state, local and foreign statutes and regulations require the expenditure of substantial time and financial resources and may not be successful.
U.S. Government Regulation
The FDA is the main regulatory body that controls pharmaceuticals in the United States, and its regulatory authority is based in the FDC Act. Pharmaceutical products are also subject to other federal, state and local statutes. A failure to comply explicitly with any requirements during the product development, approval, or post-approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an institutional review board, or IRB, of a hold on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
The steps required before a new drug may be marketed in the United States generally include:
- §
- completion of preclinical studies, animal studies and formulation studies in
compliance with the FDA's good laboratory protocols, or GLP, regulations;
- §
- submission to the FDA of an IND to support human clinical testing in the United
States;
- §
- approval by an IRB at each clinical site before each trial may be initiated;
- §
- performance of adequate and well-controlled clinical trials in accordance with
federal regulations and with current GCPs to establish the safety and efficacy of the investigational product candidate for each target indication;
- §
- submission of an NDA to the FDA;
- §
- satisfactory completion of an FDA Advisory Committee review, if applicable;
- §
- satisfactory completion of an FDA inspection of the manufacturing facilities at
which the investigational product candidate is produced to assess compliance with cGMP, and to assure that the facilities, methods and controls are adequate; and
- §
- FDA review and approval of the NDA.
87
Clinical Trials
An IND is a request for authorization from the FDA to administer an investigational product candidate to humans. This authorization is required before interstate shipping and administration of any new drug product to humans that is not the subject of an approved NDA. A 30-day waiting period after the submission of each IND is required prior to the commencement of clinical testing in humans. If the FDA has neither commented on nor questioned the IND within this 30-day period, the clinical trial proposed in the IND may begin. Clinical trials involve the administration of the investigational product candidate to patients under the supervision of qualified investigators following GCPs, an international standard meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, administrators and monitors. Clinical trials are conducted under protocols that detail the parameters to be used in monitoring safety, and the efficacy criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND. We plan to conduct our Phase 1, and possibly Phase 2, clinical trials for ZYN002 in Australia, subject to applicable regulatory approval, and do not expect at this time to file an investigational new drug application, or IND, with the FDA prior to the commencement of those clinical trials.
In Australia, the approval process for commencing Phase 1 and 2 clinical trials resides with the Human Research Ethics Committee, or HREC. Prior to commencing a clinical trial, a sponsor must submit to the HREC a study protocol, an investigator brochure and a template informed consent for such clinical trial. The HREC approval process generally takes four to eight weeks.
Once a study is approved by the HREC, a Clinical Trial Notification, or CTN, is submitted to the Australian Government Department of Health, Therapeutic Goods Administration, or TGA. The CTN is a notification that the HREC has approved the safety, efficacy and ethical acceptability of the trial, approved the trial protocol and evaluated the scientific merit of the trial. The TGA sends the clinical trial site a written acknowledgement of the clinical trial, allowing the clinical trial to begin. TGA response time to acknowledge a clinical trial is approximately two weeks from receipt of the CTN from the clinical trial site.
We submitted a protocol, an investigator brochure and a template informed consent for our Phase 1 single rising dose clinical trial for ZYN002 to the HREC in June 2015. We believe the approval process will be completed in time to allow us to commence our Phase 1 single rising dose clinical trial for ZYN002 in the second half of 2015.
We must file an IND with the FDA and receive approval from the DEA prior to commencement of any clinical trials in the United States. Subject to the submission of an IND, we plan to conduct our Phase 1 clinical trials for ZYN001 in the United States, subject to applicable regulatory approval. We plan to submit NDAs for ZYN002 and ZYN001 to the FDA upon completion of all requisite clinical trials. The informed written consent of each participating subject is required. The clinical investigation of an investigational product candidate is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of an investigation are as follows:
- §
- Phase 1. Phase 1 includes the initial introduction of an investigation product candidate into
humans. Phase 1 clinical trials may be conducted in patients with the target disease or condition or healthy volunteers. These studies are designed to evaluate the safety, metabolism, PKs and
pharmacologic actions of the investigational product candidate in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During
Phase 1 clinical trials, sufficient information about the investigational product candidate's PKs and pharmacological effects may be obtained to permit the design of Phase 2 clinical
trials. The total number of participants included in Phase 1 clinical trials varies, but is generally in the range of 20 to 80.
- §
- Phase 2. Phase 2 includes the controlled clinical trials conducted to evaluate the effectiveness of the investigational product candidate for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the product candidate. Phase 2 clinical trials are
88
- §
- Phase 3. Phase 3 clinical trials are controlled clinical trials conducted in an expanded subject population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the investigational product candidate has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the product candidate, and to provide an adequate basis for drug approval. Phase 3 clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase 3 clinical trials to demonstrate the efficacy of the drug.
typically well-controlled, closely monitored, and conducted in a limited subject population, usually involving no more than several hundred participants.
The decision to terminate development of an investigational product candidate may be made by either a health authority body, such as the FDA or IRB/ethics committees, or by a company for various reasons. The FDA may order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. In some cases, clinical trials are overseen by an independent group of qualified experts organized by the trial sponsor, or the clinical monitoring board. This group provides authorization for whether or not a trial may move forward at designated check points. These decisions are based on the limited access to data from the ongoing trial. The suspension or termination of development can occur during any phase of clinical trials if it is determined that the participants or patients are being exposed to an unacceptable health risk. In addition, there are requirements for the registration of ongoing clinical trials of product candidates on public registries and the disclosure of certain information pertaining to the trials as well as clinical trial results after completion.
A sponsor may be able to request a special protocol assessment, or SPA, the purpose of which is to reach agreement with the FDA on the Phase 3 clinical trial protocol design and analysis that will form the primary basis of an efficacy claim. A sponsor meeting the regulatory criteria may make a specific request for an SPA and provide information regarding the design and size of the proposed clinical trial. An SPA request must be made before the proposed trial begins, and all open issues must be resolved before the trial begins. If a written agreement is reached, it will be documented and made part of the record. The agreement will be binding on the FDA and may not be changed by the sponsor or the FDA after the trial begins except with the written agreement of the sponsor and the FDA or if the FDA determines that a substantial scientific issue essential to determining the safety or efficacy of the product candidate was identified after the testing began. An SPA is not binding if new circumstances arise, and there is no guarantee that a study will ultimately be adequate to support an approval even if the study is subject to an SPA. We expect to test ZYN002 AND ZYN001 in several advanced stage clinical trials, including Phase 3 clinical trial for which we may request an SPA. Having an SPA does not guarantee that a product will receive FDA approval.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational product candidate information is submitted to the FDA in the form of an NDA to request regulatory approval for the product in specified indications.
New Drug Applications
In order to obtain approval to market a drug in the United States, a marketing application must be submitted to the FDA that provides data establishing the safety and effectiveness of the product candidate for the proposed indication. The application includes all relevant data available from pertinent preclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product's chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of a product, or from a number of alternative sources, including studies initiated by investigators. To support marketing approval, the data submitted must be sufficient in quality and quantity
89
to establish the safety and effectiveness of the investigational product candidate to the satisfaction of the FDA.
In most cases, the NDA must be accompanied by a substantial user fee; there may be some instances in which the user fee is waived. The FDA will initially review the NDA for completeness before it accepts the NDA for filing. The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency's threshold determination that it is sufficiently complete to permit substantive review. After the NDA submission is accepted for filing, the FDA begins an in-depth review. The FDA has agreed to certain performance goals in the review of NDAs. Most such applications for standard review product candidates are reviewed within ten to twelve months. The FDA can extend this review by three months to consider certain late-submitted information or information intended to clarify information already provided in the submission. The FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP. The FDA may refer applications for novel product candidates which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving an NDA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing or information in order for the FDA to reconsider the application. If, or when, those deficiencies have been addressed to the FDA's satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications. As a condition of NDA approval, the FDA may require risk evaluation and mitigation strategies, or REMS, to help ensure that the benefits of the drug outweigh the potential risks. REMS can include medication guides, communication plans for healthcare professionals, and elements to assure safe use, or ETASU. ETASU can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. The requirement for a REMS can materially affect the potential market and profitability of the drug. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug's safety or efficacy. Once granted, product approvals may be withdrawn if compliance with regulatory standards is not maintained or problems are identified following initial marketing.
Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing NDA supplements as it does in reviewing NDAs.
90
Disclosure of Clinical Trial Information
Sponsors of clinical trials of certain FDA-regulated products, including prescription drugs, are required to register and disclose certain clinical trial information on a public website maintained by the U.S. National Institutes of Health, or NIH. Information related to the product, patient population, phase of investigation, study sites and investigator, and other aspects of the clinical trial is made public as part of the registration. Sponsors are also obligated to disclose the results of these trials after completion. Disclosure of the results of these trials can be delayed until the product or new indication being studied has been approved. Competitors may use this publicly-available information to gain knowledge regarding the design and progress of our development programs.
Advertising and Promotion
The FDA and other federal regulatory agencies closely regulate the marketing and promotion of drugs through, among other things, standards and regulations for direct-to-consumer advertising, communications regarding unapproved uses, industry-sponsored scientific and educational activities, and promotional activities involving the Internet. A product cannot be commercially promoted before it is approved. After approval, product promotion can include only those claims relating to safety and effectiveness that are consistent with the labeling approved by the FDA. Healthcare providers are permitted to prescribe drugs for "off-label" uses — that is, uses not approved by the FDA and therefore not described in the drug's labeling — because the FDA does not regulate the practice of medicine. However, FDA regulations impose stringent restrictions on manufacturers' communications regarding off-label uses. Broadly speaking, a manufacturer may not promote a drug for off-label use, but may engage in non-promotional, balanced communication regarding off-label use under specified conditions. Failure to comply with applicable FDA requirements and restrictions in this area may subject a company to adverse publicity and enforcement action by the FDA, the DOJ, or the Office of the Inspector General of HHS, as well as state authorities. This could subject a company to a range of penalties that could have a significant commercial impact, including civil and criminal fines and agreements that materially restrict the manner in which a company promotes or distributes drug products.
Post Approval Regulations
After regulatory approval of a drug is obtained, a company is required to comply with a number of post-approval requirements. For example, as a condition of approval of an NDA, the FDA may require post-marketing testing, including Phase 4 clinical trials, and surveillance to further assess and monitor the product's safety and effectiveness after commercialization. In addition, as a holder of an approved NDA, a company would be required to report adverse reactions and production problems to the FDA, to provide updated safety and efficacy information, and to comply with requirements concerning advertising and promotional labeling for any of its products. Also, quality control and manufacturing procedures must continue to conform to cGMP after approval to assure and preserve the long term stability of the drug or biological product. The FDA periodically inspects manufacturing facilities to assess compliance with cGMP, which imposes extensive procedural and substantive record keeping requirements. In addition, changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP and impose reporting and documentation requirements upon a company and any third-party manufacturers that a company may decide to use. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMP and other aspects of regulatory compliance.
Controlled Substances
The federal Controlled Substances Act of 1970, or CSA, and its implementing regulations establish a "closed system" of regulations for controlled substances. The CSA imposes registration, security, recordkeeping and reporting, storage, manufacturing, distribution, importation and other requirements under the oversight of the DEA. The DEA is the federal agency responsible for regulating controlled substances, and requires those individuals or entities that manufacture, import, export, distribute, research, or dispense
91
controlled substances to comply with the regulatory requirements in order to prevent the diversion of controlled substances to illicit channels of commerce.
Facilities that research, manufacture, distribute, import or export any controlled substance must register annually with the DEA. The DEA registration is specific to the particular location, activity(ies) and controlled substance schedule(s). For example, separate registrations are required for importation and manufacturing activities, and each registration authorizes which schedules of controlled substances the registrant may handle. However, certain coincident activities are permitted without obtaining a separate DEA registration, such as distribution of controlled substances by the manufacturer that produces them.
The DEA categorizes controlled substances into one of five schedules — Schedule I, II, III, IV, or V — with varying qualifications for listing in each schedule. Schedule I substances by definition have a high potential for abuse, have no currently "accepted medical use" in treatment in the United States and lack accepted safety for use under medical supervision. They may be used only in federally-approved research programs and may not be marketed or sold for dispensing to patients in the United States. Pharmaceutical products having a currently accepted medical use that are otherwise approved for marketing may be listed as Schedule II, III, IV or V substances, with Schedule II substances presenting the highest potential for abuse and physical or psychological dependence, and Schedule V substances presenting the lowest relative potential for abuse and dependence. The regulatory requirements are more restrictive for Schedule II substances than Schedule III substances. For example, all Schedule II drug prescriptions must be signed by a physician, physically presented to a pharmacist in most situations, and cannot be refilled. While Cannabis is a Schedule I controlled substance, products approved for medical use in the United States that contain Cannabis or Cannabis extracts must be placed in Schedules II-V, since approval by the FDA satisfies the "acceptable medical use" requirement.
The DEA inspects all manufacturing facilities to review security, record keeping, reporting and handling prior to issuing a controlled substance registration. The specific security requirements vary by the type of business activity and the schedule and quantity of controlled substances handled. The most stringent requirements apply to manufacturers of Schedule I and Schedule II substances. Required security measures commonly include background checks on employees and physical control of controlled substances through storage in approved vaults, safes and cages, and through use of alarm systems and surveillance cameras. An application for a manufacturing registration as a bulk manufacturer (not a dosage form manufacturer or a repacker/relabeler) for a Schedule I or II substance must be published in the Federal Register, and is open for 30 days to permit interested persons to submit comments, objections, or requests for a hearing. A copy of the notice of the Federal Register publication is forwarded by the DEA to all those registered, or applicants for registration, as bulk manufacturers of that substance. Once registered, manufacturing facilities must maintain records documenting the manufacture, receipt and distribution of all controlled substances. Manufacturers must submit periodic reports to the DEA of the distribution of Schedule I and II controlled substances, Schedule III narcotic substances, and other designated substances. Registrants must also report any controlled substance thefts or significant losses, and must obtain authorization to destroy or dispose of controlled substances. As with applications for registration as a bulk manufacturer, an application for an importer registration for a Schedule I or II substance must also be published in the Federal Register, which remains open for 30 days for comments. Imports of Schedule I and II controlled substances for commercial purposes are generally restricted to substances not already available from a domestic supplier or where there is not adequate competition among domestic suppliers. In addition to an importer or exporter registration, importers and exporters must obtain a permit for every import or export of a Schedule I and II substance or Schedule III, IV and V narcotic, and submit import or export declarations for Schedule III, IV and V non-narcotics. In some cases, Schedule III non-narcotic substances may be subject to the import/export permit requirement, if necessary to ensure that the United States complies with its obligations under international drug control treaties.
92
For drugs manufactured in the United States, the DEA establishes annually an aggregate quota for the amount of substances within Schedules I and II that may be manufactured or produced in the United States based on the DEA's estimate of the quantity needed to meet legitimate medical, scientific, research and industrial needs. This limited aggregate amount of Cannabis that the DEA allows to be produced in the United States each year is allocated among individual companies, which, in turn, must annually apply to the DEA for individual manufacturing and procurement quotas. The quotas apply equally to the manufacturing of the active pharmaceutical ingredient and production of dosage forms. The DEA may adjust aggregate production quotas a few times per year, and individual manufacturing or procurement quotas from time to time during the year, although the DEA has substantial discretion in whether or not to make such adjustments for individual companies.
The states also maintain separate controlled substance laws and regulations, including licensing, recordkeeping, security, distribution, and dispensing requirements. State Authorities, including Boards of Pharmacy, regulate use of controlled substances in each state. Failure to maintain compliance with applicable requirements, particularly as manifested in the loss or diversion of controlled substances, can result in enforcement action that could have a material adverse effect on our business, operations and financial condition. The DEA may seek civil penalties, refuse to renew necessary registrations, or initiate proceedings to revoke those registrations. In certain circumstances, violations could lead to criminal prosecution.
We currently manufacture the API for ZYN002 and ZYN001 in the United States and Canada and plan to conduct Phase 1 and Phase 2 clinical trials in the United States and Australia, and we may decide to develop, manufacture or commercialize our product candidates in additional countries. As a result, we will also be subject to controlled substance laws and regulations from the Therapeutic Goods Administration in Australia, Health Canada's Office of Controlled Substances in Canada, and from other regulatory agencies in other countries where we develop, manufacture or commercialize ZYN002 or ZYN001 in the future.
The Hatch-Waxman Amendments to the FDC Act
Orange Book Listing
In seeking approval for a drug through an NDA, applicants are required to list with the FDA each patent whose claims cover the applicant's product or a method of using the product. Upon approval of a drug, each of the patents listed in the application for the drug is then published in the FDA's Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book. Drugs listed in the Orange Book can, in turn, be cited by potential generic competitors in support of approval of an abbreviated new drug application, or ANDA, or 505(b)(2) application. An ANDA provides for marketing of a drug product that has the same active ingredients, same strengths and dosage form, as the listed drug and has been shown through PK testing to be bioequivalent to the listed drug. Other than the requirement for bioequivalence testing, ANDA applicants are generally not required to conduct, or submit results of, preclinical studies or clinical tests to prove the safety or effectiveness of their drug product. Drugs approved in this way are commonly referred to as "generic equivalents" to the listed drug, and can often be substituted by pharmacists under prescriptions written for the original listed drug. 505(b)(2) applications provide for marketing of a drug product that may have the same active ingredients as the listed drug and contains full safety and effectiveness data as an NDA, but at least some of this information comes from studies not conducted by or for the applicant. The ANDA or 505(b)(2) applicant is required to certify to the FDA concerning any patents listed for the approved product in the FDA's Orange Book. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired, but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The ANDA or 505(b)(2) applicant may also elect to submit a statement certifying that its proposed ANDA label does not contain (or carves out) any language regarding a patented method of use rather than certify to such listed method of use patent. If the applicant does not challenge the listed patents by filing a certification that the
93
listed patent is invalid or will not be infringed by the new product, the ANDA or 505(b)(2) application will not be approved until all the listed patents claiming the referenced product have expired.
A certification that the new product will not infringe the already approved product's listed patents, or that such patents are invalid, is called a Paragraph IV certification. If the ANDA or 505(b)(2) applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the NDA and patent holders once the ANDA or 505(b)(2) application has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement lawsuit in response to the notice of the Paragraph IV certification. The filing of a patent infringement lawsuit within 45 days of the receipt of a Paragraph IV certification automatically prevents the FDA from approving the ANDA or 505(b)(2) application until the earliest of 30 months, expiration of the patent, settlement of the lawsuit, and a decision in the infringement case that is favorable to the ANDA or 505(b)(2) applicant.
The ANDA or 505(b)(2) application also will not be approved until any applicable non-patent exclusivity listed in the Orange Book for the referenced product has expired.
Marketing Exclusivity
Upon NDA approval of a new chemical entity, which is a drug that contains no active moiety that has been approved by the FDA in any other NDA, that drug receives five years of marketing exclusivity during which the FDA cannot approve any ANDA seeking approval of a generic version of that drug. Certain changes to a drug, such as the addition of a new indication to the package insert, are associated with a three-year period of exclusivity during which the FDA cannot approve an ANDA for a generic drug that includes the change. A Section 505(b)(2) NDA may be eligible for three-year marketing exclusivity, assuming the NDA includes reports of new clinical studies (other than bioequivalence studies) essential to the approval of the NDA.
An ANDA may be submitted one year before marketing exclusivity expires if a Paragraph IV certification is filed. In this case, the 30 months stay, if applicable, runs from the end of the five year marketing exclusivity period. If there is no listed patent in the Orange Book, there may not be a Paragraph IV certification, and, thus, no ANDA may be filed before the expiration of the exclusivity period.
Additionally, six months of marketing exclusivity in the United States is available under Section 505A of the FDCA if, in response to a written request from the FDA, a sponsor submits and the agency accepts requested information relating to the use of the approved drug in the pediatric population. This six month pediatric exclusivity period is not a standalone exclusivity period, but rather is added to any existing patent or non-patent exclusivity period for which the drug product is eligible.
Patent Term Extension
The term of a patent that covers an FDA approved drug may be eligible for patent term extension, which provides patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other foreign jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when our pharmaceutical products receive FDA approval, we expect to apply for patent term extensions on patents covering those products.
The Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring such companies to maintain books and records that accurately and fairly reflect all transactions of the corporation, including
94
international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
European and Other International Government Regulation
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Some countries outside of the United States have a similar process that requires the submission of a clinical trial application, or CTA, much like the IND prior to the commencement of human clinical trials. In Europe, for example, a CTA must be submitted to each country's national health authority and an independent ethics committee, much like the FDA and IRB, respectively. Once the CTA is approved in accordance with a country's requirements, clinical trial development may proceed.
To obtain regulatory approval to commercialize a new drug under European Union regulatory systems, we must submit a marketing authorization application, or MAA. The MAA is similar to the NDA, with the exception of, among other things, country-specific document requirements.
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. Internationally, clinical trials are generally required to be conducted in accordance with GCP, applicable regulatory requirements of each jurisdiction and the medical ethics principles that have their origin in the Declaration of Helsinki.
Compliance
During all phases of development (pre- and post-marketing), failure to comply with applicable regulatory requirements may result in administrative or judicial sanctions. These sanctions could include the FDA's imposition of a clinical hold on trials, refusal to approve pending applications, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, product detention or refusal to permit the import or export of products, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
Other Special Regulatory Procedures
Orphan Drug Designation
The FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition that affects fewer than 200,000 individuals in the United States, or, if the disease or condition affects more than 200,000 individuals in the United States, if there is no reasonable expectation that the cost of developing and making the drug would be recovered from sales in the United States. In the European Union, the EMA's Committee for Orphan Medicinal Products grants orphan drug designation to promote the development of products that are intended for the diagnosis, prevention or treatment of life-threatening or chronically debilitating conditions affecting not more than five in 10,000 persons in the European Union community. Additionally, orphan drug designation is granted for products intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and when, without incentives, it is unlikely that sales of the drug in the European Union would be sufficient to justify the necessary investment in developing the drug.
In the United States, orphan drug designation entitles a party to financial incentives, such as opportunities for grant funding towards clinical trial costs, tax credits for certain research and user fee waivers under certain circumstances. In addition, if a product receives the first FDA approval for the indication for which it has orphan drug designation, the product is entitled to seven years of market exclusivity, which means the FDA may not approve any other application for the same drug for the same indication for a period of seven years, except in limited circumstances, such as a showing of clinical superiority over the product with
95
orphan drug exclusivity. Orphan drug exclusivity does not prevent the FDA from approving a different drug for the same disease or condition, or the same drug for a different disease or condition.
In the European Union, orphan drug designation also entitles a party to financial incentives such as reduction of fees or fee waivers and ten years of market exclusivity following drug approval. This period may be reduced to six years if the orphan drug designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity.
Orphan drug designation must be requested before submission of an application for marketing approval. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Priority Review (United States) and Accelerated Review (European Union)
Based on results of the Phase 3 clinical trial(s) submitted in an NDA, upon the request of an applicant, a priority review designation may be granted to a product by the FDA, which sets the target date for FDA action on the application at six months from the FDA's decision on priority review application, or eight months from the NDA filing. Priority review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a safe and effective therapy where no satisfactory alternative therapy exists, or a significant improvement compared to marketed products is possible. If criteria are not met for priority review, the standard FDA review period is ten months from the FDA's decision on priority review application, or 12 months from the NDA filing. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
Under the Centralized Procedure in the European Union, the maximum timeframe for the evaluation of a MAA is 210 days (excluding "clock stops," when additional written or oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human Use, or CHMP). Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest, which takes into consideration: the seriousness of the disease (e.g., heavy disabling or life-threatening diseases) to be treated; the absence or insufficiency of an appropriate alternative therapeutic approach; and anticipation of high therapeutic benefit. In this circumstance, EMA ensures that the opinion of the CHMP is given within 150 days.
Healthcare Reform
In March 2010, President Obama signed into law the Patient Protection and Affordable Care Act and the associated reconciliation bill, which we refer to collectively as the Affordable Care Act. The Affordable Care Act substantially changes the way healthcare will be financed by both governmental and private insurers, and significantly impacts the pharmaceutical industry. The Affordable Care Act is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Among the Affordable Care Act's provisions of importance to the pharmaceutical industry are the following:
- §
- an annual, nondeductible fee on any covered entity engaged in manufacturing or
importing certain branded prescription drugs and biological products, apportioned among such entities in accordance with their respective market share in certain government healthcare programs;
- §
- an increase in the statutory minimum rebates a manufacturer must pay under the
Medicaid Drug Rebate Program, retroactive to January 1, 2010, to 23.1% and 13.0% of the average manufacturer price, or AMP, for most branded and generic drugs, respectively;
- §
- expansion of healthcare fraud and abuse laws, including the False Claims Act and the Anti-Kickback Statute, new government investigative powers, and enhanced penalties for noncompliance;
96
- §
- a new partial prescription drug benefit for Medicare recipients, or Medicare
Part D, coverage gap discount program, in which manufacturers must agree to offer 50.0% point-of-sale discounts off negotiated prices of applicable brand drugs to eligible beneficiaries during
their coverage gap period, as a condition for the manufacturers' outpatient drugs to be covered under Medicare Part D;
- §
- extension of manufacturers' Medicaid rebate liability to covered drugs
dispensed to individuals who are enrolled in Medicaid managed care organizations;
- §
- expansion of eligibility criteria for Medicaid programs by, among other things,
allowing states to offer Medicaid coverage to additional individuals and by adding new mandatory eligibility categories for individuals with income at or below 133.0% of the Federal Poverty Level,
thereby potentially increasing manufacturers' Medicaid rebate liability;
- §
- expansion of the entities eligible for discounts under the Public Health
Service pharmaceutical pricing program;
- §
- new requirements to report annually specified financial arrangements with
physicians and teaching hospitals, as defined in the Affordable Care Act and its implementing regulations, including reporting any "payments or transfers of value" made or distributed to prescribers,
teaching hospitals, and other healthcare providers and reporting any ownership and investment interests held by physicians and other healthcare providers and their immediate family members and
applicable group purchasing organizations during the preceding calendar year, with data collection required beginning August 1, 2013 and reporting to the Centers for Medicare and Medicaid
Services required beginning March 31, 2014 and by the 90th day of each subsequent calendar year;
- §
- a new requirement to annually report drug samples that manufacturers and
distributors provide to physicians;
- §
- a new Patient-Centered Outcomes Research Institute to oversee, identify
priorities in, and conduct comparative clinical effectiveness research, along with funding for such research; and
- §
- a mandatory nondeductible payment for employers with 50 or more full-time employees (or equivalents) who fail to provide certain minimum health insurance coverage for such employees and their dependents, beginning in 2015 (pursuant to relief enacted by the Treasury Department).
The Affordable Care Act also established an Independent Payment Advisory Board, or IPAB, to reduce the per capita rate of growth in Medicare spending. Beginning in 2014, the IPAB was mandated to propose changes in Medicare payments if it determines that the rate of growth of Medicare expenditures exceeds target growth rates. The IPAB has broad discretion to propose policies to reduce expenditures, which may have a negative impact on payment rates for pharmaceutical products. A proposal made by the IPAB is required to be implemented by the U.S. federal government's Centers for Medicare & Medicaid Services unless Congress adopts a proposal with savings greater than those proposed by the IPAB. The IPAB has not yet been called upon to act as the annual determinations by the CMS Office of the Actuary have not identified a savings target for implementation years 2015 or 2016.
In addition, other legislative changes have been proposed and adopted since passage of the Affordable Care Act. The Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee did not achieve its targeted deficit reduction of an amount greater than $1.2 trillion for the fiscal years 2012 through 2021, triggering the legislation's automatic reductions to several government programs. These reductions included aggregate reductions to Medicare payments to healthcare providers of up to 2.0% per fiscal year, which went into effect in April 2013. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several categories of healthcare providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These laws may result in additional reductions in Medicare and other healthcare funding, which could have a material adverse effect on our customers and accordingly, our financial operations.
97
Coverage and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we receive regulatory approval for commercial sale will depend in part on the availability of reimbursement from third-party payors. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA approved drugs for a particular indication. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain FDA approvals. Our product candidates, if approved, may not be considered medically necessary or cost-effective. A payor's decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
The Medicare Modernization Act, or MMA, enacted by the U.S. Congress in 2003, changed the way Medicare covers and pays for pharmaceutical products, including creating the Medicare Part D prescription drug benefit, which became effective at the beginning of 2006. Government payment for some of the costs of prescription drugs may increase demand for any products for which we receive marketing approval. However, to obtain payments under this program, we would be required to sell products to Medicare recipients through prescription drug plans operating pursuant to this legislation. These plans will likely negotiate discounted prices for our products.
Existing federal law requires pharmaceutical manufacturers to pay rebates to state governments, based on a statutory formula, on covered outpatient drugs reimbursed by the Medicaid program as a condition of having their drugs paid for by Medicaid. Rebate amounts for a product are determined by a statutory formula that is based on prices defined in the statute: AMP, which must be calculated for all products that are covered outpatient drugs under the Medicaid program, and best price, which must be calculated only for those covered outpatient drugs that are a single source drug or innovator multiple source drug, such as biologic products. Manufacturers are required to report AMP and best price for each of their covered outpatient drugs to the government on a regular basis. Additionally, some state Medicaid programs have imposed a requirement for supplemental rebates over and above the formula set forth in federal law, as a condition for coverage. In addition to the Medicaid rebate program, federal law also requires that if a pharmaceutical manufacturer wishes to have its outpatient drugs covered under Medicaid as well as under Medicare Part B, it must sign a "Master Agreement" obligating it to provide a formulaic discount of approximately 24% known as the federal ceiling price for drugs sold to the U.S. Departments of Defense (including the TRICARE retail pharmacy program), Veterans Affairs, the Public Health Service and the Coast Guard, and also provide discounts through a drug pricing agreement meeting the requirements of Section 340B of the Public Health Service Act, for outpatient drugs sold to certain specified eligible healthcare organizations. The formula for determining the discounted purchase price under the 340B drug pricing program is defined by statute and is based on the AMP and rebate amount for a particular product as calculated under the Medicaid drug rebate program, discussed above.
Different pricing and reimbursement schemes exist in other countries. In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national healthcare systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed upon. To obtain reimbursement or pricing approval, some of these
98
countries may require the completion of clinical trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. Other member states allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on healthcare costs in general, particularly prescription drugs, has become more intense. As a result, increasingly high barriers are being erected to the entry of new products. The European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. We may face competition for our product candidates from lower-priced products in foreign countries that have placed price controls on pharmaceutical products. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, an increasing emphasis on managed care in the United States has increased and will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time.
Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other Healthcare Laws and Compliance Requirements
In the United States, our activities are potentially subject to additional regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services, other divisions of HHS (for example, the Office of Inspector General), the DOJ and individual U.S. Attorney offices within the DOJ, and state and local governments.
The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce or in return for purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting some business arrangements from prosecution, the exemptions and safe harbors are drawn narrowly and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from federal Anti-Kickback Statute liability. The reach of the Anti-Kickback Statute was broadened by the Affordable Care Act, which, among other things, amends the intent requirement of the federal Anti-Kickback Statute. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act (discussed below) or the civil monetary penalties statute, which imposes penalties against any person who is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
The federal False Claims Act prohibits any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or knowingly making, using, or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. As a result of a modification made by the Fraud Enforcement and Recovery Act of 2009, a claim includes "any
99
request or demand" for money or property presented to the U.S. government. Pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies' marketing of the product for unapproved, and thus non-reimbursable, uses. HIPAA created new federal criminal statutes that prohibit knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Also, many states have similar fraud and abuse statutes or regulations that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payor.
In addition, we may be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. HIPAA, as amended by HITECH, and its implementing regulations, imposes requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA's privacy and security standards directly applicable to "business associates" — independent contractors or agents of covered entities that receive or obtain protected health information in connection with providing a service on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney's fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in specified circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our business activities could be subject to challenge under one or more of such laws. If our operations are found to be in violation of any of the federal and state laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including criminal and significant civil monetary penalties, damages, fines, imprisonment, exclusion from participation in government programs, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, private "qui tam" actions brought by individual whistleblowers in the name of the government or refusal to allow us to enter into supply contracts, including government contracts, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations. To the extent that any of our products are sold in a foreign country, we may be subject to similar foreign laws and regulations, which may include, for instance, applicable post-marketing requirements, including safety surveillance, anti-fraud and abuse laws, and implementation of corporate compliance programs and reporting of payments or transfers of value to healthcare professionals.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in a state, including, in some states, manufacturers and distributors who ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical companies to, among other things, establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing specified physician prescribing data to pharmaceutical companies for use in sales and
100
marketing, and to prohibit other specified sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
Government Contracts and Grants
To date we have been awarded approximately $7.9 million in state and federal grants which have provided us with funding and resources to continue the development of our product candidates and contributed to research and development efforts outside of our primary therapeutic focus. Approximately $6.0 million of our grant funds have been received from the NIH, and some of these funds support research related to ZYN001.
Scientific Advisors
We have established a clinical advisory board and we regularly seek advice and input from these experienced clinical leaders on matters related to our research and development programs. The members of our clinical advisory board consist of experts across a range of key disciplines relevant to our programs. We intend to continue to leverage the broad expertise of our advisors by seeking their counsel on important topics relating to our product development and clinical development programs. Our scientific advisors are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, our clinical advisors may have arrangements with other companies to assist those companies in developing products or technologies that may compete with ours. All of our clinical advisors are affiliated with other entities and devote only a small portion of their time to us. Our current clinical advisors are set forth in the table below:
Name
|
Title | |
|---|---|---|
Jacqueline French, MD |
Professor of Neurology, NYU Langone Medical Center | |
John Messenheimer, MD |
Consultant, Neurologist/Epileptologist, John Messenheimer PLLC |
|
Rodney Radtke, MD |
Professor of Neurology, Duke University Medical Center |
|
Steven J. Siegel, MD, PhD. |
Professor of Psychiatry, University of Pennsylvania, Perelman School of Medicine; Director, Translational Neuroscience Program; Director, Clinical Neuroscience Track |
|
Daniel Clauw, MD |
Professor of Anesthesiology, Medicine (Rheumatology) and Psychiatry, University of Michigan |
|
Philip Mease, MD |
Clinical Professor, University of Washington, Seattle; Director of Rheumatology Research, Swedish Medical Center |
|
Lesley Arnold, MD |
Professor of Psychiatry and Behavioral Neuroscience, University of Cincinnati |
|
Donald Abrams, MD |
Professor of Clinical Medicine, University of California San Francisco School of Medicine; Chief of Hematology/Oncology, San Francisco General Hospital |
|
Miroslav Backonja, MD |
Clinical Professor, University of Wisconsin School of Medicine and Public Health; Medical Director, CRILifetree |
|
Mark Wallace, MD |
Professor of Clinical Anesthesia, University of California San Diego |
101
Corporate History and Information
We were incorporated in Delaware in January 2007 under the name AllTranz Inc., and in June 2007 we merged with AllTranz LLC, a Kentucky limited liability company that was founded in 2004 by Audra Stinchcomb, a pharmacologist and transdermal expert, with Alltranz, Inc. surviving. In May 2014, we were reorganized and recapitalized pursuant to an agreement and plan of merger whereby BCM Partners IV, Corp. a non-operating entity owned by BCM X1 Holdings, LLC and Audra Stinchcomb, two of our principal stockholders at that time, was merged with and into Alltranz, Inc., with Alltranz, Inc. surviving. In August 2014, AllTranz, Inc. changed its name to Zynerba Pharmaceuticals, Inc. See "Certain Relationships and Related Party Transactions — Agreements with Broadband Capital Management — Agreement and Plan of Merger" in this Prospectus.
Transitional Agreements
Following our recapitalization and reorganization in May 2014 and as part of our transition to narrow our focus on ZYN002 and ZYN001, we completed the following material transactions related to obligations to third parties and intellectual property:
Albany College of Pharmacy
In August 2014 we entered into a patent assignment consideration agreement, or ACP Agreement, with Albany College of Pharmacy, or ACP, pursuant to which we paid ACP a lump sum of $500,000 in exchange for the termination of a royalty agreement executed in December 2004. In December 2004 ACP assigned to us all of the rights, title and interest to certain patent applications directed to transdermal delivery of cannabinoids and to any divisionals, reissues, continuations, continuations in-part (including patents related to ZYN002), renewals, extensions, revisions and foreign counterparts thereof resulting from research performed at ACP, or the Assigned Rights. In connection with the assignment, we entered into a royalty agreement with ACP under which we would have been obligated to pay ACP a royalty on net income from licenses or sales related to the Assigned Rights. While the assigned patent applications are no longer pending and did not result in any issued patents, we entered into the ACP Agreement to eliminate any obligation to pay royalties to ACP in connection with the Assigned Rights. Under the ACP Agreement ACP also acknowledged and confirmed the validity and binding effect of the original assignment by ACP of the Assigned Rights, which are exclusively held by the Company.
Buzzz Pharmaceuticals Ltd.
In October 2014 we entered into a termination and release agreement, or Termination Agreement, with Buzzz Pharmaceuticals Ltd., or Buzzz, pursuant to which the parties mutually terminated a development services agreement, an option agreement and a license agreement, all of which related to certain research studies for the development of an abuse deterrent transdermal system for delivery of oxymorphone and other opioids. In accordance with the Termination Agreement, we assigned to Buzzz, for no monetary consideration, all of our right, title and interest in and to one patent in the United States, which had been exclusively licensed to Buzzz under the license agreement, one patent application in the United States, which arose out of work performed in connection with the development services agreement and their respective foreign equivalents. The transferred patent and patent application are directed to abuse deterrent transdermal formulations containing opioids. In addition, we transferred to Buzzz, documents, data and other know-how directly related to the assigned patent and patent application and the work performed by us under the development services agreement with Buzzz. By entering into the Termination Agreement, we eliminated our obligations to perform extensive research and development outside our primary therapeutic focus area and to engage in an ongoing licensing relationship with Buzzz. We believe that the transfer of the patent, patent application and know-how to Buzzz under the Termination Agreement will not impact development of ZYN002 or ZYN001.
Under the Termination Agreement, the parties also agreed to a mutual general release of claims arising prior to the effective date of the Termination Agreement, excluding claims related to a breach of, or inaccurate representation or warranty made under, the termination and release agreement. Buzzz also agreed not to
102
directly or indirectly compete with us in the market relating to transdermal or topical products containing THC or CBD, including any pro-drugs thereof, for a period of two years following the effective date of the termination and we agreed not to directly or indirectly compete with Buzzz in the market relating to oxymorphone products, including oxymorphone abuse deterrent products, for the same time period. We also covenanted not to sue Buzzz or any of our former employees specifically related to past, present or future work performed by such former employees for Buzzz in connection with an abuse deterrent patch to deliver specified opioid compounds. However, neither this covenant nor any other provision in the Termination Agreement prevents us from filing suit against Buzzz or any of our former employees in connection with any breach by Buzzz or any former employee of any surviving, existing or ongoing confidentially obligations they have to us.
Kentucky Economic Development Finance Authority
In September 2014, we repaid in full an outstanding $500,000 loan from the Kentucky Economic Development Finance Authority, or KEDFA, issued under a forgivable loan agreement dated April 2007, which was amended in December 2012. Under the forgivable loan agreement, our intellectual property, as specified in the agreement, was subject to a conditional assignment to KEDFA as security for the loan. Upon our repayment of the loan in full, KEDFA executed and delivered to us a full release and cancellation of the conditional assignment and security interest in and liens on our intellectual property.
Legal Proceedings
We are not currently a party to any legal proceedings.
Employees
As of July 23, 2015, we had seven full-time equivalent employees. In addition to our full-time employees, we contract with third-parties for the conduct of certain preclinical, manufacturing, accounting and administrative activities. We have no collective bargaining agreements with our employees and none are represented by labor unions.
Property
Our company headquarters are located in Devon, Pennsylvania where we occupy approximately 3,800 square feet of office space pursuant to a five-year lease which expires on May 31, 2020. In addition, one of our employees works in Covington, Kentucky where we lease office space and a shared conference room and business facilities pursuant to a lease agreement that expires in September 2015.
103
Executive Officers, Directors and Director Nominees
The following table sets forth information regarding our current executive officers and the directors and director nominees:
Name
|
Age | Position(s) | |||
|---|---|---|---|---|---|
Executive Officers |
|||||
Armando Anido |
57 | Chairman and Chief Executive Officer | |||
Terri B. Sebree |
57 | President | |||
Richard A. Baron |
59 | Chief Financial Officer and Treasurer | |||
Suzanne M. Hanlon |
58 | Secretary, General Counsel and Vice President, Human Resources | |||
Directors and Director Nominees |
|||||
Philip R. Wagenheim |
44 | Director | |||
Steven Gailar |
68 | Director | |||
Warren D. Cooper, MB, BS, BSc, MPFM |
62 | Director Nominee | |||
William J. Federici |
56 | Director Nominee | |||
Thomas L. Harrison, LH.D |
67 | Director Nominee | |||
Daniel L. Kisner, MD |
68 | Director Nominee | |||
Kenneth I. Moch |
60 | Director Nominee | |||
Cynthia A. Rask, MD |
61 | Director Nominee | |||
Executive Officers
Armando Anido has served as chairman of our board of directors and as our chief executive officer since October 2014. Prior to joining our company, Mr. Anido served as our business consultant from May 2014 to October 2014. Mr. Anido has more than 30 years of executive, operational and commercial leadership experience in the pharmaceutical industry. Mr. Anido served as chief executive officer and as a director of NuPathe, Inc., or NuPathe, a publicly-traded specialty pharmaceutical company, from July 2012 through March 2014, during which time he led the company through FDA approval of its lead product, Zecuity, a transdermal patch for migraines. Prior to joining NuPathe, Mr. Anido served as chief executive officer and president and as a director of Auxilium Pharmaceuticals, Inc., or Auxilium, a specialty pharmaceutical company, from July 2006 through December 2011, where he led the company through FDA approval and commercialization of its lead product, Testim, a testosterone gel. Mr. Anido served as a director of Respira Therapeutics, Inc., a pharmaceutical company, from May 2012 through September 2014 and as a director of Adolor Corporation, a pharmaceutical company, from September 2003 through December 2011. Mr. Anido was retired from January 2012 through June 2012. Mr. Anido holds a B.S. in Pharmacy and an MBA, both from West Virginia University. With more than 35 years of experience in the pharmaceutical industry, Mr. Anido brings valuable executive, operational and commercial expertise to our board of directors.
Terri B. Sebree has served as our president since October 2014, and served as our treasurer from October 2014 to December 2014. Prior to joining our company, Ms. Sebree served as our business consultant from May 2014 to October 2014. Ms. Sebree has more than 30 years of executive, development and operational experience in the pharmaceutical industry, particularly in central nervous system product development including epilepsy and pain. Ms. Sebree founded and served as president of NuPathe, a specialty pharmaceutical company, from February 2005 until April 2014, where she led the effort to develop, achieve regulatory approval for and complete manufacturing of the company's lead product, Zecuity, a transdermal patch for migraines. Prior to founding NuPathe, Ms. Sebree served as senior vice president, development of
104
Auxilium, a specialty pharmaceutical company, where she led the development and approval program of Testim, a testosterone gel. Prior to joining Auxilium, Ms. Sebree served as executive vice president, U.S. Operations at IBAH, Inc., a contract research organization. Prior to that, Ms. Sebree served in a variety of management roles with Abbott Laboratories Inc., a global healthcare company, for over nine years. Ms. Sebree currently serves on the board of directors of Serodus ASA, a publicly traded company on the Oslo Stock Exchange. Ms. Sebree holds a B.S. from Texas A&M University.
Richard A. Baron has served as our chief financial officer and treasurer since January 2015. Prior to joining our company, Mr. Baron served as senior vice president and chief financial officer of Globus Medical Inc., a publicly traded musculoskeletal implant manufacturer, from January 2012 to December 2014. Prior to joining Globus Medical Inc., Mr. Baron served as an independent consultant to various early stage biotech and technology companies from April 2011 to January 2012. From May 2008 through April 2011, Mr. Baron served as vice president, finance and chief financial officer of Avid Radiopharmaceuticals, Inc., a biotech company that developed an imaging agent for Alzheimer's, which was sold to Eli Lilly and Company in November 2011. Mr. Baron served as chief financial officer for eResearch Technology, Inc. from February 2007 to June 2008, and for Animas Corporation from May 2000 through its sale to Johnson & Johnson in February 2006. Prior to that time, Mr. Baron served as chief financial officer for Genex Services, LLC, a managed care provider for workers compensation and disability, and Marsam Pharmaceuticals Inc., a generic manufacturer of injectable anti-infectives. Mr. Baron also sits on the board of directors of Apire Bariatric, Inc. and EIC Solutions, Inc., both privately held companies. Mr. Baron holds a B.S. in Economics, with a concentration in Accounting, from the Wharton School of the University of Pennsylvania.
Suzanne M. Hanlon has served as our secretary, general counsel and vice president, human resources since October 2014. Prior to joining our company, Ms. Hanlon served as our legal consultant from May 2014 to October 2014. Ms. Hanlon has more than 25 years of legal experience in the pharmaceutical industry. Ms. Hanlon served as vice president, associate general counsel of NuPathe from July 2005 to April 2014, where she worked with Mr. Anido and Ms. Sebree on the regulatory approval of Zecuity, a transdermal patch for migraines. Prior to joining NuPathe, Ms. Hanlon served as chief development counsel of Auxilium, a specialty pharmaceutical company. Prior to joining Auxilium, Ms. Hanlon served as vice president of global contracts and general counsel at IBAH, Inc. Prior to joining IBAH, Inc., Ms. Hanlon was a partner at Montgomery McCracken Walker & Rhoads LLP. Ms. Hanlon holds a B.A. from Pennsylvania State University and a J.D. from Villanova University School of Law.
Non-Employee Directors and Director Nominees
Philip R. Wagenheim has served as one of our directors since May 2014 and served as our president from May 2014 until October 2014. Mr. Wagenheim intends to resign from our board of directors upon the closing of this offering. Mr. Wagenheim co-founded and has served as vice chairman of Broadband Capital Management, or BCM, a broker-dealer, since January 2000 and has served as secretary and as a director of BCM since May 2011, and as president of Committed Capital Acquisition Corporation II, a blank check company that was formed for the purpose of acquiring or merging with an operating business, since February 2012. Mr. Wagenheim served as secretary and as a director of Committed Capital Acquisition Corporation, a blank check company that consummated a business combination with The One Group, LLC in October 2013, from March 2006 through October 2013. Mr. Wagenheim holds a B.A. in Business Administration from the University of Miami. Mr. Wagenheim's experience as a co-founder and vice-chairman of BCM, as well as his business experience and education make him a valuable member of our board of directors.
Steven Gailar has served as one of our directors since November 2013. Mr. Gailar intends to resign from our board of directors upon the closing of this offering. Mr. Gailar has served as Managing Partner of Kentucky Seed Capital Fund I, L.P., a company that invests in early-stage Kentucky or Louisville metropolitan-based businesses that specialize in biomedical and healthcare services, medical device development and healthcare information technology, since 2005. Mr. Gailar previously served as president
105
and chief executive officer and as a director of MetaCyte Business Lab, LLC, a company that co-founds and provides start-up services to early-stage companies, from December 2004 through December 2012. Prior to MetaCyte Business Lab, LLC, Mr. Gailar held various management positions in finance, sales and marketing at Eli Lilly and Company, a global pharmaceutical company, from July 1973 to June 1987, served as president and chief executive officer of Marlstone Corporation, a rational drug design firm, from September 1988 through June 1990 and served as managing director of Senmed Medical Ventures, a private evergreen venture capital firm making investments in university spin-outs and mid- to late-stage private life sciences companies, from July 1992 through April 2003. Mr. Gailar has served as a director of numerous early stage life sciences companies from July 1992 through the present. Mr. Gailar holds a B.A. from Indiana University and a M.S. in Industrial Administration from Purdue University. Mr. Gailar has more than 35 years of experience in managing and investing in life sciences companies, which makes him a valuable member of our board of directors.
Warren D. Cooper, MB, BS, BSc, MPFM will serve on the board of directors upon the closing of this offering. Dr. Cooper is a U.K.-trained physician with more than 35 years of experience in the global pharmaceutical industry. Dr. Cooper currently serves as the president of Coalescence Inc., a healthcare and pharmaceutical development consultancy, where he has held various positions since 1999. Dr. Cooper was the chief executive officer of Prism Pharmaceuticals, Inc., a venture-backed, specialty pharmaceutical company that he led from inception in September 2004 until the sale of the Company to Baxter International in May 2011. His career in the pharmaceutical industry began with Merck, Sharp and Dohme and spanned 12 years, initially as a clinical research physician in the United Kingdom, then as head of European and, subsequently, Worldwide Clinical Research Operations for Merck Research Laboratories across all therapeutic areas. Moving to AstraMerck (now AstraZeneca PLC) in a broad clinical development role, he eventually led that company's cardiovascular business division, a role with full business lifecycle leadership from in-licensing through development, to P&L responsibility for sales and marketing. Dr. Cooper is a member of the Faculty of Pharmaceutical Medicine of the Royal Colleges of Physicians of the United Kingdom and since March 2015 he has been a member of the board of directors of Cardiorentis AG, a privately held Swiss pharmaceutical company. He has previously served on the boards of directors of Nutrition 21, Inc., Nuron Biotech Inc. and the World Affairs Council of Philadelphia. Dr. Cooper studied at the London Hospital where he earned degrees in Physiology (1974) and Medicine and Surgery (1977) granted by the University of London.
William J. Federici will serve on the board of directors upon the closing of this offering. Mr. Federici has served as vice president and chief financial officer of West Pharmaceutical Services, Inc., a publicly traded global pharmaceutical technology company, since August 2003. He served as a member of the board of directors at NuPathe and chairman of the Audit committee from January 2011 until February 2014. From June 2002 until August 2003, he was national industry director for Pharmaceuticals of KPMG LLP, and prior thereto, he was an audit partner with Arthur Andersen, LLP. Mr. Federici holds a B.A. in Economics and an M.B.A. in Professional Accounting from Rutgers University and is a Certified Public Accountant.
Thomas L. Harrison, LH.D will serve on the board of directors upon the closing of this offering. Dr. Harrison is a noted author and speaker and since April 2013, has served as chairman emeritus of Diversified Agency Services, the world's largest group of marketing services companies, and a division of Omnicom Group, Inc., or Omnicom. In 1987 he founded Harrison & Star Business Group, a healthcare marketing agency that was acquired by Omnicom in 1992. From 1992 until 1997, he served as chairman of the Harrison & Star Group and chairman of diversified healthcare communications for Omnicom. In 1997, Dr. Harrison was appointed president of diversified agency services, and in 1998 was named chairman and chief executive, serving until 2011. In 1980 he began his agency career at Rolf Werner Rosenthal, a mid-sized healthcare advertising agency. From 1974 until 1980 he served in sales and marketing roles at Pfizer, Inc. Dr. Harrison is a member of the Executive Committee of the Montefiore Hospital, a fellow of the New York Academy of Medicine, and governor of the New York Academy of Sciences where he sits on the Sackler Global Nutrition Committee. In 2013, Dr. Harrison became a partner and board member of Dipexium
106
Pharmaceuticals, Inc. and a board member of rVue Inc., a digital out-of-home media company. In 2014, he was appointed to the boards of Social Growth Technologies, Inc. and of Fifth Street Asset Management Inc. where he serves as chairman of the Audit committee. He previously served as a board member of the New York Chapter of the Arthritis Foundation; a board member for ePocrates, Inc., a publicly traded healthcare information company, until its March 2013 acquisition by athenahealth, Inc.; and The Morgans Hotel Group (2006-2013). Dr. Harrison holds an advanced degree in Cell Biology and Physiology and received an honorary doctorate from West Virginia University in 2007.
Daniel L. Kisner, MD will serve on the board of directors upon the closing of this offering. Dr. Kisner has served as an independent consultant to the pharmaceutical/biotech industry since 2011. From 2003 until 2011 he served as a venture partner/partner at Aberdare Ventures, a venture firm with a focus on investing in healthcare technology companies. Prior to that he was president and chief executive officer of Caliper Technologies Corp., or Caliper, from 1999 until 2003, and served as chairman of Caliper until 2008. He led Caliper from a startup dealing with microfluidic lab-on-a-chip technology to a publically traded commercial company. From 1991 until 1999 He served as chief operating officer and president of Isis Pharmaceuticals, Inc., a biomedical pharmaceutical company. Previously, Dr. Kisner was division vice president of Pharmaceutical Development at Abbott Laboratories and vice president of Clinical Research at SmithKline Beckman Laboratories. In addition he previously held a tenured position at the University of Texas School of Medicine at San Antonio and is certified by the American Board of Internal Medicine in Internal Medicine and Medical Oncology. Dr. Kisner served on the board of directors of Tekmira Pharmaceuticals from 2010 until March 2015, and he currently serves on the boards of directors of Dynavax Technologies Corporation, Lpath, Inc. and Conatus Pharmaceuticals Inc. He holds a B.A. degree from Rutgers University and an M.D. from Georgetown University.
Kenneth I. Moch will serve on the board of directors upon the closing of this offering. Mr. Moch has more than 25 years of experience in managing and financing biomedical technologies, and has played a key role in building five life science companies. He is currently the president of Euclidean Life Science Advisors LLC, where he provides strategic and tactical counsel to the biotechnology and pharmaceutical industries. Prior to that, he served as president and chief executive officer, and as a director, of Chimerix, Inc. from April 2010 to April 2014, having joined the company as chief operating officer in June 2009. Previously, he was president and chief executive officer of three life science companies—BioMedical Enterprises, Inc., Alteon, Inc., and Biocyte Corporation—and was a co-founder and vice president of The Liposome Company, Inc. He also served as managing director of Healthcare Investment Banking at ThinkEquity Partners and as a management consultant with McKinsey & Company. In the public policy arena, Mr. Moch has served as chairman of BioNJ and as a member of the board of the Biotechnology Industry Organization, and is a member of the National Advisory Board of the Johns Hopkins Berman Institute of Bioethics. Mr. Moch received an A.B. in Biochemistry from Princeton University and an M.B.A. with emphasis in Finance and Marketing from the Stanford Graduate School of Business.
Cynthia A. Rask, MD will serve on the board of directors upon the closing of this offering. Dr. Rask has more than 20 years of experience working in the pharmaceutical/biotech industry, seven years of FDA experience, and several years of experience as a clinical neurologist and clinical investigator. Dr. Rask trained in Internal Medicine and Neurology at the University of Rochester and is board-certified in Neurology. Following her residency, she did a fellowship in Epilepsy at the Minnesota Comprehensive Epilepsy Program (MINCEP), and is board certified in Clinical Neurophysiology. She was an assistant professor of Neurology at the University of Minnesota and at Dartmouth College. In 1991, she began her industry career at Abbott Laboratories working on development of a new antiepileptic drug, then joined Genentech Inc. where she led efforts on developing neurotrophic factors for treatment of various types of neuropathic pain. In January of 1999 Dr. Rask began working at the FDA, first as a medical reviewer on INDs and BLAs; in 2002 she was appointed as the division director for the Division of Clinical Evaluation and Pharmacology/Toxicology in the Office of Cellular, Tissue and Gene Therapies (OCTGT). Beginning in 2004, she also served as the acting deputy office director in OCTGT, helping to develop FDA policies and
107
guidance documents and manage the office budget until leaving FDA in June of 2005 to return to the San Francisco Bay area to join a non-profit pharmaceutical company as vice president of Development, an organization funded primarily by grants from the Gates Foundation. In 2006, Dr. Rask began her own consulting company and has worked for a variety of clients including biotech and pharmaceutical companies, ranging in size from small startups to medium and large pharma, academic groups, including Harvard University and the Huntington Study Group, and non-profit organizations, including The Michael J. Fox Foundation. She is a graduate of Cornell University and the University of Minnesota Medical School.
Board Composition
Our business and affairs are managed under the direction of our board of directors, which currently consists of three members, two of whom intend to resign upon the closing of this offering. We expect that upon the closing of this offering, our board of directors will consist of seven members. Upon the closing of this offering, we intend to appoint Warren D. Cooper, MB, BS, BSc, MPFM, William J. Federici, Thomas L. Harrison, LH.D, Daniel L. Kisner, MD, Kenneth I. Moch and Cynthia A. Rask, MD to our board of directors and they have consented to serve. Philip R. Wagenheim and Steven Gailar intend to resign from our board of directors upon the closing of this offering.
Upon the closing of this offering, our sixth amended and restated certificate of incorporation and amended bylaws will provide that our board of directors will consist of a number of directors to be fixed exclusively by resolution of the board of directors. Each of our current directors and, once appointed, our director nominees shall serve a term expiring at the annual meeting of stockholders to be held in 2016 after which directors will be elected to annual terms.
Role of the Board in Risk Oversight
One of the key functions of our board of directors is informed oversight of our risk management process. Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through the board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure and our audit committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including adopting guidelines and policies to govern the process by which risk assessment and management is undertaken. While our board of directors maintains the ultimate oversight responsibility for the risk management process, its committees oversee risk in certain specified areas. Our audit committee oversees management of enterprise risks and financial risks, as well as potential conflicts of interests. Additionally, our compensation committee is responsible for overseeing management of risks relating to our executive compensation plans and arrangements, and the incentives created by the compensation awards it administers. Our nominating and corporate governance committee is responsible for overseeing management of risks associated with the independence of our board of directors. Pursuant to our board of directors' instruction, our management regularly reports on applicable risks to the relevant committee or the board of directors, as appropriate, with additional review or reporting on risks conducted as needed or as requested by our board of directors and its committees.
Board Committees
Our board of directors has established an audit committee, a compensation committee and a nominating and corporate governance committee. Each committee will operate under a charter that was approved by our board of directors and will be available on our website, www.zynerba.com, under the "Investor Relations" section, upon the effective date of the registration statement of which this prospectus is a part. The information contained in, or that can be accessed through, our website is not part of this prospectus.
108
Audit Committee
Upon the closing of this offering, our audit committee will consist of Messrs. Federici and Moch and Dr. Cooper, and will be chaired by Mr. Federici. The primary purpose of our audit committee is to assist the board of directors in the oversight of our accounting and financial reporting processes, the audit and integrity of our financial statements, and the qualifications and independence of our independent auditor, and to prepare any reports required of the audit committee under the rules of the SEC. The audit committee has the following responsibilities, among other things, as set forth in the audit committee charter that will be effective upon the closing of this offering:
- §
- hiring our independent registered public accounting firm and pre-approving the
audit and permitted non-audit and tax services to be performed by our independent registered public accounting firm;
- §
- reviewing and approving the planned scope of the annual audit and reviewing the
results of the annual audit;
- §
- reviewing the significant accounting and reporting principles to understand
their impact on our financial statements;
- §
- reviewing quarterly with management its assessment of the effectiveness and
adequacy of our internal control structure and procedures for financial reporting, and reviewing annually with our independent registered public accounting firm the attestation to and report on the
assessment made by management;
- §
- reviewing with management, our independent registered public accounting firm
and legal counsel, as appropriate, our financial reports, earnings announcements and our compliance with legal and regulatory requirements;
- §
- establishing procedures for the treatment of complaints received by us
regarding accounting, internal accounting controls or auditing matters and confidential submissions by our employees of concerns regarding questionable accounting or auditing matters;
- §
- preparing for adoption by our board of directors a Code of Business Conduct and
Ethics, and periodically reviewing and recommending appropriate changes thereto;
- §
- reviewing and approving related-party transactions; and
- §
- reviewing and evaluating, at least annually, our audit committee's charter.
Our audit committee will review related-party transactions for potential conflicts of interests in accordance with our related party transactions policy. See "Certain Relationships and Related Party Transactions—Policies and Procedures for Related Party Transactions."
The financial literacy requirements of the SEC require that each member of our audit committee be able to read and understand fundamental financial statements. In addition, our board of directors has determined that each of Messrs. Federici and Moch and Dr. Cooper qualifies as an audit committee financial expert, as defined in Item 407(d)(5) of Regulation S-K promulgated under the Securities Act of 1933, as amended, or the Securities Act, and has financial sophistication in accordance with the NASDAQ Stock Market Rules.
Both our independent registered public accounting firm and management periodically will meet privately with our audit committee.
Compensation Committee
Upon the closing of this offering, our compensation committee will consist of Drs. Kisner, Harrison and Rask, and will be chaired by Dr. Kisner. The primary purpose of our compensation committee is to review the performance and development of our management in achieving corporate goals and objectives and to assure that our executive officers are compensated effectively in a manner consistent with the strategy of our company, competitive practice, sound corporate governance principles and stockholder interests. In carrying out these responsibilities, this committee oversees, reviews and administers all of our
109
compensation, equity and employee benefit plans and programs. The functions of our compensation committee include, among other things:
- §
- reviewing and approving the corporate goals and objectives relevant to
executive compensation, evaluating performance in light of those goals and objectives and setting the compensation for our executive officers;
- §
- reviewing and recommending the terms of employment agreements and other
employment-related arrangements with our executive officers;
- §
- reviewing and approving our compensation strategy for our employees;
- §
- reviewing and recommending to our board of directors the compensation of our
directors;
- §
- administering our equity compensation plan and benefits plans and approving the
grant of equity awards to our employees and directors under these plans;
- §
- overseeing and periodically reviewing the operation of all of our employee
benefit plans;
- §
- when required, reviewing and discussing with management our Compensation
Discussion and Analysis and recommending to the full board its inclusion in our periodic reports and proxy statement to be filed with the SEC;
- §
- when required, preparing the report of the compensation committee to be included
in our annual proxy statement;
- §
- engaging compensation consultants or other advisors it deems appropriate to
assist with its duties; and
- §
- reviewing and evaluating, at least annually, our compensation committee's charter.
Nominating and Corporate Governance Committee
Upon the closing of this offering, our nominating and corporate governance committee will consist of Drs. Harrison, Cooper and Rask, and will be chaired by Dr. Harrison. The primary purpose of our nominating and corporate governance committee is to assist our board of directors by identifying individuals qualified to become members of our board of directors, recommending a slate of nominees to be proposed by our board of directors to stockholders for election to our board of directors, developing and recommending corporate governance principles and guidelines of our company and monitoring compliance therewith, and recommending directors to serve on the committees of our board of directors. The functions of our nominating and corporate governance committee include, among other things:
- §
- assisting our board of directors in identifying prospective director nominees
and recommending nominees for each annual meeting of stockholders to our board of directors;
- §
- reviewing developments in corporate governance practices and developing and
recommending governance principles applicable to our board of directors;
- §
- reviewing independence of the board of directors;
- §
- evaluating and making recommendations as to the size and composition of the
board of directors;
- §
- recommending members for each board committee of our board of directors;
- §
- determining qualifications for service on our board;
- §
- developing, as appropriate, a set of corporate governance principles and
guidelines, and reviewing and recommending to our board any changes to such guidelines;
- §
- reviewing the adequacy of our certificate of incorporation and bylaws and
recommending to our board of directors, as conditions dictate, amendments for consideration by our stockholders; and
- §
- reviewing and evaluating, at least annually, our nominating and corporate governance committee's charter.
Code of Business Conduct and Ethics
Upon the closing of this offering, our board of directors intends to adopt a Code of Business Conduct and Ethics applicable to all of our employees, executive officers and directors. The Code of Business Conduct and Ethics will be available on our website at www.zynerba.com upon the listing of our common stock on
110
The NASDAQ Global Market. Our board of directors will be responsible for overseeing the Code of Business Conduct and Ethics, and our board of directors or an appropriate committee thereof must approve any waivers of the Code of Business Conduct and Ethics for employees, executive officers or directors. Disclosure regarding any amendments to the Code of Business Conduct and Ethics, or any waivers of its requirements, will be disclosed on our website. The information contained in, or that can be accessed through, our website is not part of this prospectus.
Compensation Committee Interlocks and Insider Participation
No member of our compensation committee has ever been an executive officer or employee of ours. None of our officers currently serves, or has served during the last completed year, on the board of directors, compensation committee or other committee serving an equivalent function, of any other entity that has one or more officers serving as a member of our board of directors or compensation committee.
Director Independence
The NASDAQ Stock Market Rules require that each committee of our board of directors has at least one independent director on the listing date of our common stock, has a majority of independent directors no later than 90 days after such date and be fully independent within one year after such date. The composition of our audit, compensation and nominating and corporate governance committees will satisfy these independence requirements in accordance with the phase-in schedule allowed by the NASDAQ Stock Market Rules.
Upon the closing of this offering, our board of directors will observe all applicable criteria for independence established by the NASDAQ Stock Market Rules and other governing laws and applicable regulations. No director will be deemed to be independent unless our board of directors determines that the director has no relationship which would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our board of directors has determined that Drs. Cooper, Harrison, Kisner and Rask and Messrs. Federici and Moch are independent as defined under the corporate governance rules of the NASDAQ Stock Market Rules. Of these six independent directors, our board has determined that: (i) Messrs. Federici and Moch and Dr. Cooper, who will comprise our audit committee; (ii) Drs. Kisner, Harrison and Rask, who will comprise our compensation committee; and (iii) Drs. Harrison, Cooper and Rask, who will comprise our nominating and corporate governance committee, each satisfy the independence standards for those committees established by the applicable rules and regulations of the SEC and the NASDAQ Stock Market Rules.
Limitation on Liability and Indemnification of Directors and Officers
Our sixth amended and restated certificate of incorporation, which will be effective immediately prior to the closing of this offering, limits our directors' liability to the fullest extent permitted under Delaware corporate law. Delaware corporate law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability:
- §
- for any transaction from which the director derives an improper personal
benefit;
- §
- for any act or omission not in good faith or that involves intentional
misconduct or a knowing violation of law;
- §
- under Section 174 of the Delaware General Corporation Law (unlawful
payment of dividends or redemption of shares); or
- §
- for any breach of a director's duty of loyalty to the corporation or its stockholders.
If the Delaware General Corporation Law is amended to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of our directors will be eliminated or limited to the fullest extent permitted by the Delaware General Corporation Law, as so amended.
111
Delaware law and our amended and restated bylaws provide that we will, in certain situations, indemnify our directors, officers, employees and other agents, to the fullest extent permitted by law. Any indemnified person is also entitled, subject to certain limitations, to payment or reimbursement of reasonable expenses (including attorneys' fees and disbursements) in advance of the final disposition of the proceeding.
In addition, we intend to enter into separate indemnification agreements with our directors and executive officers. These agreements, among other things, require us to indemnify our directors and executive officers for certain expenses, including attorneys' fees, judgments, fines and settlement amounts incurred by a director or executive officer in any action or proceeding arising out of their services as one of our directors or officers or any other company or enterprise to which the person provides services at our request.
We maintain a directors' and officers' insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers. We believe that these provisions in our sixth amended and restated certificate of incorporation and amended bylaws and these indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or control persons, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
112
EXECUTIVE AND DIRECTOR COMPENSATION
Summary Compensation Table
The following table provides information regarding the total compensation for services rendered in all capacities that was earned by our executive officers during 2014 and two additional individuals who served as our principal executive officer during 2014. We refer to these executives as our named executive officers.
Name and Principal Position
|
Year | Salary ($) |
Bonus ($) |
Stock Awards ($)(1) |
All Other Compensation ($)(2) |
Total ($) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Armando Anido, Chief Executive Officer |
2014 | 128,750 | 0 | 759,066 | 102,702 | 990,518 | |||||||||||||
Terri B. Sebree, President |
2014 |
98,461 |
0 |
400,198 |
117,264 |
615,923 |
|||||||||||||
Suzanne M. Hanlon, General Counsel |
2014 |
61,538 |
0 |
96,394 |
87,107 |
245,039 |
|||||||||||||
Philip R. Wagenheim, Former President (principal executive officer, May 2014–October 2014) |
2014 |
0 |
0 |
0 |
0 |
0 |
|||||||||||||
Audra Stinchcomb, Former Chief Scientific Officer (principal executive officer, January 2014–April 2014) |
2014 |
21,668 |
0 |
0 |
143,600 |
165,268 |
|||||||||||||
- (1)
- This
amount reflects the aggregate grant date fair value of stock options and restricted stock awards computed in accordance with FASB
accounting standards codification, or ASC, 718.
- (2)
- This amount reflects (i) payments to Mr. Anido, Ms. Sebree and Ms. Hanlon for consulting services performed prior to their employment with us, in the amounts of $100,400, $115,980 and $84,375, respectively; (ii) payment for 90% of the premiums for medical and dental insurance for each of Mr. Anido, Ms. Sebree and Ms. Hanlon in the amounts of $1,888, $870 and $2,318, respectively; (iii) payments for life and long term disability insurance for each of Mr. Anido, Ms. Sebree and Ms. Hanlon in the amounts of $414 each; and (iv) payment in the amount of $60,000 and transfer of certain office and laboratory equipment with a then-fair market value of $83,600, each to Ms. Stinchcomb in respect of the Release Agreement, as further described below.
Employment Agreements
We have entered into employment agreements with each of our currently employed named executive officers. We did not enter into employment agreements with Mr. Wagenheim or Ms. Stinchcomb. In addition, on January 2, 2015, we entered into an employment agreement with Mr. Baron, our chief financial officer and treasurer.
The employment agreements entered into with each of our currently employed named executive officers provide for a base salary, an annual performance bonus and stock options (subject to vesting requirements) and, other than with respect to Mr. Baron, restricted stock. Mr. Anido's base salary is $515,000, Ms. Sebree's is $400,000, Mr. Baron's is $300,000 and Ms. Hanlon's is $250,000.
Each of our currently employed named executive officers is eligible for an annual performance bonus which is set as a percentage of base salary based upon the achievement of certain individual and/or corporate performance goals. The target bonus for each of Mr. Anido and Ms. Sebree is 60% of base salary, and for Mr. Baron and Ms. Hanlon is 40% and 25% of base salary, respectively.
Each of Mr. Anido, Ms. Sebree and Ms. Hanlon received stock options and restricted stock grants as described below under the heading "Outstanding Equity Awards at Fiscal Year-End." Mr. Baron was granted options to purchase 63,829 shares of our common stock at an exercise price of $3.98 per share in January 2015. Mr. Baron's stock options have the same vesting schedule as the stock options granted to our currently employed named executive officers.
113
Upon the listing of our common stock on the NASDAQ Global Market, each of our currently employed named executive officers is entitled to additional stock options to purchase an aggregate number of shares of our common stock such that, should they exercise such additional stock options plus any previously granted stock options, Mr. Anido, Ms. Sebree, Mr. Baron and Ms. Hanlon will hold shares of our common stock including shares of restricted stock equal to 9.5%, 4.9%, 1.3% and 1.2%, respectively, of our issued and outstanding capital stock on a fully diluted basis immediately following the closing of this offering. These stock option grants will have a per share exercise price equal to the initial public offering price and will vest in quarterly increments over a four year period.
Our currently employed named executive officers are entitled to participate in all of our retirement and group welfare plans available to our senior level executives as a group or our employees generally, subject to the terms and conditions applicable to such plans. Further, each such person's employment agreement contains standard confidentiality and assignment of inventions provisions and post-employment non-compete provisions for, in the case of Mr. Anido 18 months, in the case of Ms. Sebree and Mr. Baron 12 months, and in the case of Ms. Hanlon nine months.
Separation Agreement and Patent Assignment with Ms. Stinchcomb
In August 2014, Ms. Stinchcomb, our former chief scientific officer and director, resigned all positions with us. In connection with Ms. Stinchcomb's separation, in September 2014 we and Ms. Stinchcomb entered into a severance agreement and release of claims, or the Release Agreement, effective as of August 31, 2014, and a patent assignment, or the Patent Assignment, dated October 2, 2014. The Release Agreement provides for (i) a release of all employment-related claims by Ms. Stinchcomb in favor of us and our affiliates and (ii) mutual non-disparagement obligations. For a period of two years following the effective date of the Release Agreement, Ms. Stinchcomb is also prohibited from (i) acquiring an interest in or becoming engaged in or employed by, either directly or indirectly, any business or activity which develops, markets or sells transdermal or topical drugs containing tetrahydrocannabinol or cannabidiol, including any pro-drugs thereof, anywhere in the world; and (ii) willfully or intentionally interfering with or damaging any relationship between us and any of our clients, customers, suppliers or consultants or enticing, inducing or soliciting, directly or indirectly, any of our then current employees to leave us to work with Ms. Stinchcomb or any entity with which Ms. Stinchcomb becomes affiliated. Pursuant to the Release Agreement, Ms. Stinchcomb was paid $60,000, subject to withholding taxes and deductions, and we transferred certain office and laboratory equipment with a then-fair market value of $83,600 to Ms. Stinchcomb.
Pursuant to the Patent Assignment, we transferred our rights in any inventions, discoveries and applications disclosed in the U.S. Patent application entitled "Methods and Compositions for Enhancing the Viability of Microneedle Pores," filed with the U.S. Patent and Trademark Office on December 1, 2008 and assigned Serial No. 12/325,919, or the 2008 Patent Application. The Patent defined and described in the 2008 Patent Application had a fair market value of $3,000.
Potential Payments upon Termination or Change of Control
If any of our currently employed named executive officers' or Mr. Baron's employment by us is terminated without "cause" or such person resigns for "good reason," as such terms are defined in the respective employment agreements, and provided, other than in the case of Ms. Hanlon, such termination or resignation occurs following the first anniversary of the employment agreement effective date, the consummation of an initial public offering or the consummation of a private placement resulting in at least $15 million in gross proceeds, then, subject to his or her execution and delivery of a general release of claims and compliance with all the terms and provisions of his or her employment agreement that survive the executive's termination of employment, such person shall be entitled to: (i) receive continuation of his or her base salary for a period of, in the case of Mr. Anido 18 months, in the case of Ms. Sebree and Mr. Baron 12 months, and in the case of Ms. Hanlon nine months; (ii) continued medical and dental benefits for, in the case of Mr. Anido 18 months, in the case of Ms. Sebree and Mr. Baron 12 months, and in the case of Ms. Hanlon nine months at the same premium rates charged to active employees; and
114
(iii) pro rata vesting of all outstanding stock option and other equity-based awards that would have vested had such executive remained employed by us for an additional 12 month period.
If any of our currently employed named executive officers' or Mr. Baron's employment by us is terminated without "cause" or such person resigns for "good reason" within the 90 day period preceding a "change of control," as such term is defined in the respective employment agreements, or on or within 12 months following a change of control or if such person resigns his or her employment for any reason within 30 days following a change of control, then, subject to his or her execution and delivery of a general release of claims and compliance with all the terms and provisions of his or her employment agreement that survive the executive's termination of employment, such person shall be entitled to the severance benefits described in the preceding paragraph, provided that (i) all outstanding stock options and other equity-based awards shall become fully vested and exercisable (to the extent applicable) as of the date of such termination of employment, (ii) such person's outstanding vested stock options and other equity-based awards (after giving effect to the vesting acceleration described in the preceding clause) shall remain exercisable for three years following such termination of employment or, if earlier, until the stated expiration of the stock option or other equity-based award, and (iii) if such change in control results in net proceeds per share of capital stock to investors in excess of two times the price per share of our Series 1 convertible preferred stock, such person shall receive a payment equal to such person's target annual bonus.
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth certain information concerning unexercised stock options and stock options that have not vested and stock awards that have not vested for each of the named executive officers as of December 31, 2014:
| |
Option Awards | Stock Awards | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name
|
Number of Securities Underlying Unexercised Options (#) (Exercisable) |
Number of Securities Underlying Unexercised Options (#) (Unexercisable)(1) |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Units of Stock That Have Not Vested (#)(2) |
Market Value of Shares or Units of Stock That Have Not Vested ($) |
|||||||||||||
Armando Anido |
— | 292,553 | 3.98 | 10/02/2024 | 332,512 | 550,108 | |||||||||||||
Terri B. Sebree |
— | 146,276 | 3.98 | 10/02/2024 | 178,746 | 295,719 | |||||||||||||
Suzanne M. Hanlon |
— | 42,553 | 3.98 | 10/02/2024 | 39,893 | 66,000 | |||||||||||||
- (1)
- This
option shall become vested with respect to 25% of the shares subject to the option upon the closing of this offering, with remainder of
the option vesting in 12 equal quarterly installments thereafter.
- (2)
- 25% of the restricted shares shall become vested upon the closing of this offering, with remainder of the shares vesting in 12 equal quarterly installments thereafter.
Equity Benefit Plans
Amended and Restated 2014 Omnibus Incentive Compensation Plan, as Amended
Our board of directors has adopted the 2014 Equity Plan and has approved an amendment to the 2014 Equity Plan which will become effective prior to the closing of this offering. The 2014 Equity Plan provides for grants of stock options, stock appreciation rights, or SARS, restricted stock, restricted stock units, or RSUs, performance units, other stock-based awards and bonus awards. Our directors, employees and, generally, our consultants and advisors will be eligible for grants under the 2014 Equity Plan. This summary may not include all of the provisions of the 2014 Equity Plan. For further information about the 2014 Equity Plan, we refer you to the complete copy of the 2014 Equity Plan and the first amendment thereto, each filed as an exhibit to the registration statement of which this prospectus forms a part.
115
Administration. The 2014 Equity Plan will be administered by a committee designated by our board of directors. The committee has full authority to administer and interpret the 2014 Equity Plan, to grant awards under the 2014 Equity Plan, to determine the persons to whom awards will be granted, to determine the types of awards to be granted, to determine the terms and conditions of each award, to determine the number of shares of common stock to be covered by each award, to make all other determinations in connection with the 2014 Equity Plan and the awards thereunder as the committee deems necessary or desirable in its sole discretion.
Available shares. Initially, the aggregate number of shares of our common stock that may be issued pursuant to awards under the 2014 Equity Plan, as amended effective prior to the closing of this offering, is 2,450,000. In addition, as of the first trading day of January during the term of the 2014 Equity Plan (excluding any extensions), beginning with the calendar year following the calendar year of this offering, an additional positive number of shares of our common stock shall be added to the number of shares of our common stock authorized to be issued or transferred under the 2014 Equity Plan and the number of shares authorized to be issued or transferred pursuant to incentive stock options, equal to 10% of the total number of shares of our common stock outstanding on the last trading day in December of the immediately preceding calendar year, or 1.5 million shares, whichever is less.
If there is any change in the number or kind of shares of common stock outstanding by reason of a stock dividend, spinoff, recapitalization, stock split, or combination or exchange of shares, a merger, reorganization or consolidation, a reclassification or change in par value, or any other extraordinary or unusual event affecting our common stock, or if the value of our common stock is substantially reduced as a result of a spinoff or the payment of an extraordinary dividend or distribution, the maximum number of shares available for issuance under the 2014 Equity Plan, the maximum number of shares for which any individual may receive awards in any year, the kind and number of shares covered by outstanding awards, the kind and number of shares issued and to be issued under the 2014 Equity Plan, and the price per share or the applicable market value of such awards shall be equitably adjusted to reflect any increase or decrease in the number of, or change in the kind or value of, the issued shares to preclude the enlargement or dilution of rights and benefits under the 2014 Equity Plan and such outstanding awards.
Individual limits. For awards measured in shares of our common stock, the maximum number of shares of our common stock for which such awards may be made to any one person in any calendar year shall not exceed 1.5 million shares in the aggregate. For awards measured in cash dollars, the maximum dollar amount for which such awards may be made to any one person in any calendar year shall not exceed $3.0 million dollars in the aggregate. Such limitations are designed to help assure that any deductions to which we would otherwise be entitled with respect to such awards will not be subject to the $1.0 million dollar limitation on the income tax deductibility by us of compensation paid to any covered executive officer imposed by Section 162(m) of the Internal Revenue Code of 1986, or the Code.
Eligibility for participation. All of our employees, directors, consultants and advisors (other than consultants and advisors whose services are in connection with the offer and sale of securities in a capital-raising transaction or who directly or indirectly promote or maintain a market for our securities) shall be eligible to receive awards under the 2014 Equity Plan.
Award agreements. Awards granted under the 2014 Equity Plan will be evidenced by award agreements, which need not be identical, and that provide additional terms, conditions, restrictions or limitations covering the grant of the award, including, without limitation, additional terms providing for the acceleration of exercisability or vesting of awards in the event of a change in control or conditions regarding the participant's employment, as determined by the committee.
Stock options. The committee may grant nonqualified stock options to any individuals eligible to participate in the 2014 Equity Plan but may grant incentive stock options only to eligible employees. The committee will determine: (i) the number of shares of our common stock subject to each option; (ii) the term of each option, which may not exceed ten years, or five years in the case of an incentive stock option granted to a greater than 10% stockholder; (iii) the exercise price, which may not be less than the fair
116
market value of our common stock as of the date of grant or less than 110% of the fair market value of our common stock as of the date of grant in the case of an incentive stock option granted to a greater than 10% stockholder; (iv) the vesting schedule, if any, and (v) the other material terms of each option. Options will be exercisable at such time or times and subject to such terms and conditions as determined by the committee at the time of grant and the exercisability of any or all of such options may be accelerated by the committee at any time for any reason. Unless otherwise determined by the committee, following the termination of the option holder's service, vested options will expire (i) as of the date of such termination if such termination results from a termination by us for cause, (ii) one year following the date of such termination if such termination results from the option holder's death or disability (or, if the holder's death occurs within the 90 day period described in (iii)), and (iii) 90 days following such termination if such termination is for any other reason.
Stock awards/Restricted stock. The committee may award shares of our common stock for consideration or for no consideration, and subject to restrictions or no restrictions. Except as otherwise provided by the committee upon the award of restricted stock, the recipient generally will have the rights of a stockholder with respect to the shares, including the right to vote the shares of restricted stock and the right to receive dividends or other distributions paid on the shares, subject to the conditions and restrictions generally applicable to restricted stock or specifically set forth in the recipient's restricted stock agreement. Recipients of restricted stock will be required to enter into a restricted stock agreement with us that states the restrictions to which the shares are subject, which may include satisfaction of pre-established performance goals, and the criteria or date or dates on which such restrictions will lapse. If the grant of restricted stock or the lapse of the relevant restrictions is based on the attainment of performance goals, the committee will establish for each recipient the applicable performance goals, formulae or standards and the applicable vesting percentages with reference to the attainment of such goals or satisfaction of such formulae or standards in accordance with section 162(m) of the Code and in all events while the outcome of the performance goals are substantially uncertain. Section 162(m) of the Code requires that performance awards be based upon objective performance measures. The performance goals for performance-based restricted stock will be based on one or more of the objective criteria described under the heading "Performance goals" below. Except as the committee deems appropriate, if a recipient of restricted stock ceases to be employed by, or provide service to, us during the period while his or her shares are still subject to restriction, the restricted stock award will terminate as to all shares covered by the award as to which restrictions have not lapsed.
Stock units, or RSUs. RSUs are granted in reference to a specified number of shares of common stock and entitle the holder to receive, on achievement of specific performance goals and/or after a period of continued service as set forth in the applicable award agreement, one share of common stock for each such share of common stock covered by the RSU or an amount of cash based on the value of a share of common stock. If the grant of RSUs or the lapse of the relevant restrictions is based on the attainment of performance goals, the committee will establish for each recipient the applicable performance goals, formulae or standards and the applicable vesting percentages with reference to the attainment of such goals or satisfaction of such formulae or standards in accordance with section 162(m) of the Code and in all events where the outcome of the performance goals are substantially uncertain. Section 162(m) of the Code requires that performance awards be based upon objective performance measures. The performance goals for performance-based RSUs will be based on one or more of the objective criteria described under the heading "Performance goals" below. Except as the committee deems appropriate, if a recipient of RSUs ceases to be employed by, or provide service to, us prior to the vesting of the RSUs, or if other conditions established by the committee are not met, the recipient's RSUs shall be forfeited.
Stock appreciation rights, or SARs. The committee may grant SARs representing the right to receive a payment in shares of our common stock, cash or a combination thereof, as determined by the committee, equal in value to the excess of the fair market value of one share of our common stock on the date of exercise over the SAR's base price, which is the fair market value of one share of our common stock on the date the SAR is granted. SARs may be granted separately or in tandem with a stock option. SARs will be
117
exercisable at such time or times and subject to such terms and conditions as determined by the committee at the time of grant and the exercisability of any or all of such SARs may be accelerated by the committee at any time for any reason. Unless otherwise determined by the committee, following the termination of the SAR holder's service, vested SARs will expire (i) as of the date of such termination if such termination results from a termination by us for cause, (ii) one year following the date of such termination if such termination results from the option holder's death or disability (or, if the holder's death occurs within the 90 day period described in (iii)), and (iii) 90 days following such termination if such termination is for any other reason.
Performance units. The committee may grant performance units to eligible individuals payable upon the attainment of specific performance goals. A performance unit award shall represent a participating interest in a special bonus pool tied to the attainment of pre-established corporate performance objectives based on one or more performance goals or the right to receive a targeted dollar amount tied to the attainment of pre-established corporate performance objectives based on one or more performance goals. The performance goals for performance units will be based on one or more of the objective criteria described under the heading "Performance goals" below. Unless otherwise determined by the committee, if a holder of a performance unit ceases to provide service to us prior to the vesting of performance units, or if other conditions established by the committee are not met, the awarded performance units shall be forfeited. Payments with respect to performance units shall be made in cash, our common stock or any combination thereof, as determined by the committee.
Other stock-based awards. The committee may grant "other stock-based awards," which are awards (other than options, stock awards, RSUs, SARs and performance units) that are based on or measured by our common stock, on such terms and conditions as the committee shall determine. Other stock-based awards may be awarded subject to the achievement of performance goals or other conditions and may be payable in cash, our common stock or any combination thereof, as determined by the committee.
Dividend equivalents. The committee may grant dividend equivalents in connection with RSUs or other stock-based awards. A dividend equivalent is an amount determined by multiplying the number of shares of our common stock subject to an award by the per-share cash dividend paid by us on our outstanding common stock, or the per-share fair market value of any dividend paid on our outstanding common stock in consideration other than cash. Dividend equivalents may be paid currently or accrued and may be payable in cash or shares of common stock, and upon such terms as the committee may establish, including the achievement of specific performance goals.
Bonus awards. The committee may grant bonus awards that shall be considered "qualified performance-based compensation" under section 162(m) of the Code to employees who are executive officers, upon such terms and conditions as the committee deems appropriate. The committee shall select the executive employees who will be eligible for bonus awards, specify the performance period and establish target bonus awards and performance goals for the performance period in accordance with section 162(m) of the Code. The performance period shall be our fiscal year or such other period (of not more than 12 months) as the committee determines. A target bonus award shall be based on the executive's responsibility level, position or such other criteria as the committee determines and may provide for differing amounts to be paid based on differing thresholds of performance. The performance goals for bonus awards will be based on one or more of the objective criteria described under the heading "Performance goals" below but need not be uniform among recipients. Unless otherwise determined by the committee, no person shall have any right to receive payment of a bonus award for a performance period unless that person remains in our employ through the last day of the applicable performance period. The committee may also grant executive employees bonus awards that do not constitute "qualified performance-based compensation" under section 162(m) of the Code, which may be based on individual performance, our performance or such other criteria as the committee determines.
Performance goals. The committee may grant stock awards, RSUs, performance units, other stock-based awards and dividend equivalents that are intended to qualify as performance-based compensation for
118
purposes of Section 162(m) of the Code. These awards may be granted, vest and be paid based on attainment of specified performance goals established by the committee. These performance goals may be based upon one or more of the following measures selected by the committee: cash flow; earnings (including gross margin, earnings before interest and taxes, earnings before taxes, earnings before interest, taxes, depreciation, amortization and charges for stock-based compensation, earnings before interest, taxes, depreciation and amortization, and net earnings); earnings per share; growth in earnings or earnings per share; stock price; return on equity or average stockholder equity; total stockholder return or growth in total stockholder return either directly or in relation to a comparative group; return on capital; return on assets or net assets; revenue, growth in revenue or return on sales; income or net income; operating income, net operating income or net operating income after tax; operating profit or net operating profit; operating margin; return on operating revenue or return on operating profit; regulatory filings; regulatory approvals, litigation and regulatory resolution goals; other operational, regulatory or departmental objectives; budget comparisons; growth in stockholder value relative to established indexes, or another peer group or peer group index; development and implementation of strategic plans and/or organizational restructuring goals; development and implementation of risk and crisis management programs; improvement in workforce diversity; compliance requirements and compliance relief; safety goals; productivity goals; workforce management and succession planning goals; economic value added (including typical adjustments consistently applied from generally accepted accounting principles required to determine economic value added performance measures); measures of customer satisfaction, employee satisfaction or staff development; development or marketing collaborations, formations of joint ventures or partnerships or the completion of other similar transactions intended to enhance our revenue or profitability or enhance our customer base; merger and acquisitions; and other similar criteria consistent with the foregoing.
Change of control. Unless the committee determines otherwise, effective upon the date of the change of control, (i) all outstanding options and SARs shall become fully vested and exercisable, (ii) the restrictions and conditions on all outstanding stock awards shall immediately lapse, and (iii) all RSUs, performance units, other stock based awards and dividend equivalents shall become fully vested and shall be paid at their target values, or in such greater amounts as the committee may determine. Notwithstanding the foregoing, in the event of a change of control, the committee may take one or more of the following actions with respect to any or all outstanding awards: (i) require the surrender by holders of their outstanding options and SARs in exchange for one or more payments by us, in cash or common stock as determined by the committee, in an amount equal to the amount by which the then fair market value of the common stock subject to the unexercised options and SARs exceeds the exercise price of the options or the base amount of the SARs, as applicable; (ii) after giving holders an opportunity to exercise their outstanding options and SARs, terminate any or all unexercised options and SARs; or (iii) determine that outstanding and unexercised options and SARs shall be assumed by, or replaced with comparable options or rights by, the surviving corporation, (or a parent or subsidiary of the surviving corporation), and other outstanding awards that remain in effect after the change of control shall be converted to similar grants of the surviving corporation (or a parent or subsidiary of the surviving corporation).
Under the 2014 Equity Plan, unless otherwise set forth in an award agreement, a change of control shall be deemed to have occurred if: (i) any person becomes a beneficial owner, directly or indirectly, of our securities representing more than 50% of the voting power of our then outstanding securities (other than a transaction in which we become a subsidiary of another corporation and in which our stockholders, immediately prior to the transaction, beneficially own, immediately after the transaction, shares entitling such stockholders to more than 50% of all votes to which all stockholders of the parent corporation would be entitled in the election of directors); (ii) the consummation of a merger or consolidation of us with another corporation where our stockholders, immediately prior to the merger or consolidation, will not beneficially own in substantially the same proportion as ownership immediately prior to the merger or consolidation, immediately after the merger or consolidation, shares entitling such stockholders to more than 50% of all votes to which all stockholders of the surviving corporation would be entitled in the election of directors, or where the members of our board of directors, immediately prior to the merger or consolidation, would not, immediately after the merger or consolidation, constitute a majority of the board
119
of directors of the surviving corporation; (iii) a sale or other disposition of all or substantially all of our assets, or a liquidation or dissolution of us; or (iv) a change in the composition of our board of directors over a period of 12 consecutive months or less such that a majority of the members of our board of directors ceases, by reason of one or more contested elections for board membership, to be comprised of individuals who either (A) have been members of our board of directors continuously since the beginning of such period or (B) have been elected or nominated for election as members of our board of directors during such period by at least a majority of the members described in clause (A) who were still in office at the time our board of directors approved such election or nomination.
Amendment and termination. Our board of directors may at any time amend any or all of the provisions of the 2014 Equity Plan, or suspend or terminate it entirely provided that our board of directors may not amend the 2014 Equity Plan without stockholder approval if such approval is required in order to comply with the Code or other applicable law, or to comply with applicable stock exchange requirements. The 2014 Equity Plan will terminate automatically on the day immediately preceding the 10th anniversary of the plan's effective date, unless extended by our board of directors with the approval of our stockholders.
Transferability. Awards granted under the 2014 Equity Plan generally will be nontransferable, other than by will or the laws of descent and distribution and, for awards other than incentive stock options, pursuant to a domestic relations order, except that the committee may provide for the transferability of nonqualified stock options at the time of grant or thereafter to certain family members.
Repricing. The committee may at any time and from time to time, without obtaining prior approval of our stockholders but with the consent of the affected holders: (i) implement a cancellation/re-grant program pursuant to which outstanding options and/or SARs under the 2014 Equity Plan are cancelled in exchange for (A) new options and/or SARs for the same or a different number of shares of common stock and with an exercise price or base price not less than the per share fair market value of our common stock as of the date of grant of the new option or SAR, or (B) cash or shares of our common stock (vested or unvested) equal in value to the cancelled options or SARs; or (ii) reduce the exercise price or base price of an option or SAR to the then current per share fair market value of our common stock.
Clawback rights. The committee may provide in an applicable award agreement that, if an award recipient breaches any restrictive covenant agreement with us or otherwise engages in activities that constitute "cause," all awards held by that person shall terminate, and we may rescind any exercise of an option or SAR and the vesting of any other award and delivery of shares upon such exercise or vesting, as applicable on such terms as the committee shall determine.
Effective date. The 2014 Equity Plan became effective on October 2, 2014, and was amended and restated on January 9, 2015. On July 22, 2015, our board of directors approved an amendment to the 2014 Equity Plan, to be effective prior to the closing of this offering.
Director Compensation
During 2014, we did not pay any cash compensation to our directors. Our board of directors has not adopted a formal non-employee director compensation policy. All of our directors are eligible to receive awards under the 2014 Equity Plan, provided that non-employee directors may not receive incentive stock options. After this offering, each of our non-employee directors will be paid:
- §
- an annual cash retainer of $35,000;
- §
- an additional retainer of $12,500 for the audit committee chair, $10,000 for the
compensation committee chair and $5,000 for the nominating and corporate governance committee chair;
- §
- an additional retainer of $7,500 for members of the audit committee, $5,000 for
members of the compensation committee and $3,500 for members of the nominating and corporate governance committee; and
- §
- an initial equity grant of 35,000 options upon election and an annual equity grant of 15,000 options.
120
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following includes a summary of transactions since January 1, 2012 to which we have been a party, in which the amount involved in the transaction exceeded $120,000, and in which any of our directors, executive officers or, to our knowledge, beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest, other than equity and other compensation, termination, change of control and other arrangements, which are described under "Executive and Director Compensation."
Employment Arrangements
We currently have written employment agreements with our chairman and chief executive officer, Armando Anido, our president, Terri B. Sebree, our chief financial officer and treasurer, Richard A. Baron, and our secretary, general counsel and vice president, human resources, Suzanne M. Hanlon. For more information, refer to the section entitled "Executive and Director Compensation — Employment Agreements."
Agreements with Broadband Capital Management
Engagement Letter
On March 7, 2014, we entered into an engagement letter with BCM, which was co-founded by Philip R. Wagenheim, one of our directors, and of which Mr. Wagenheim serves as vice chairman. BCM is an affiliate of BCM X1 Holdings, LLC or BCM Holdings, which was one of our principal stockholders until February 2015, of which Mr. Wagenheim is a managing member, and Michael D. Rapoport, another one of our principal stockholders, is chairman of BCM. Pursuant to the engagement letter, we engaged BCM as our exclusive agent in connection with a private placement of equity securities. The engagement letter entitled BCM to an issuance of 10% of our common stock upon closing of the private placement and also provided that, for a period of two years following the closing of the private placement, BCM would be entitled to 10% of the gross proceeds raised from any financing arranged with any investors who were contacted by BCM during the term of the engagement. Pursuant to the engagement letter, we reimbursed expenses of BCM in the amount of $250,000. BCM has not received any other compensation under the engagement letter.
On July 16, 2014, we entered into a letter agreement, or waiver letter, with BCM pursuant to which BCM agreed to waive certain of its rights under the engagement letter with regard to future equity issuances by us upon our request and the payment of $500,000. The engagement letter and waiver letter will be terminated prior to the closing of this offering pursuant to the termination agreement described below.
Agreement and Plan of Merger
On May 6, 2014, we entered into an agreement and plan of merger by and among us, BCM Holdings, BCM Partners IV, Corp., a subsidiary of BCM Holdings, or Merger Corp, Audra Stinchcomb, our former principal executive officer, and Steven Gailar, one of our directors, as stockholder representative. Pursuant to the merger agreement, Merger Corp was merged with and into us, with us surviving, which we refer to as the Merger. By virtue of the Merger, each outstanding share of our common stock was cancelled in exchange for the right to receive 0.57434 shares of our Series 1 convertible preferred stock, each issued and outstanding share of common stock of Merger Corp was converted into the right to receive one share of our common stock, and each issued and outstanding share of Series 1 convertible preferred stock of Merger Corp was converted into the right to receive one share of our Series 1 convertible preferred stock. Immediately following the Merger, BCM Holdings and Ms. Stinchcomb beneficially owned 60.0% and 40.0% of our common stock, respectively.
Prior to the Merger, Mr. Rapoport purchased 94,500 shares of Series 1 convertible preferred stock in Merger Corp for an investment of $200,000, which shares were converted into 94,500 shares of our Series 1 convertible preferred stock in the Merger.
121
Advisory Services Agreement
We are party to an advisory services agreement with BCM dated July 16, 2014, as amended on September 3, 2014 and September 18, 2014, pursuant to which BCM provided us with certain advisory services, as mutually agreed between us and BCM. Pursuant to the terms of the advisory services agreement, we have paid BCM $250,000 and issued 579,614 shares of our common stock to BCM Holdings, of which BCM Holdings subsequently forfeited 41,667 shares. The advisory services agreement will be terminated prior to the closing of this offering pursuant to the termination agreement described below.
Termination Agreement
On January 7, 2015, we entered into a termination agreement with BCM pursuant to which we and BCM agreed that, effective upon our payment to BCM of $500,000, the engagement letter, waiver letter and advisory services agreement shall be terminated and all rights thereunder shall be relinquished, other than customary indemnification and confidentiality rights. We intend to make such payment to BCM prior to the closing of this offering.
Stock Transfer Agreement
We are party to a stock transfer agreement, dated September 26, 2014, by and among us, Mr. Rapoport and Ms. Stinchcomb, pursuant to which Mr. Rapoport agreed to purchase from Ms. Stinchcomb 319,148 shares of our common stock for an aggregate purchase price of approximately $1.2 million. Pursuant to the stock transfer agreement, the shares were purchased in two closings, the first for 31,914 shares, which took place on October 7, 2014, and the second for 287,234 shares, which took place on July 15, 2015.
Stockholders' Agreement
On May 6, 2014, we entered into a third amended and restated stockholders' agreement with certain of our stockholders, or the stockholders' agreement. The stockholders' agreement contains provisions with respect to the election of our board of directors, restrictions on transfer of shares, preemptive rights, drag-along rights, rights of first refusal and registration rights. For a description of the registration rights, see "Description of Capital Stock — Registration Rights." Certain of our current directors were elected pursuant to the terms of the stockholders' agreement or an antecedent version thereof. The provisions of the stockholders' agreement, as amended, relating to the election of our board of directors, restrictions on transfer of shares, preemptive rights, drag-along rights and rights of first refusal shall terminate upon the closing of this offering.
Severance Agreement and Patent Assignment with Ms. Stinchcomb
In September and October 2014, we entered into a Severance and Release of Claims and a Patent Assignment, respectively, with our former chief scientific officer, Audra Stinchcomb. For more information, refer to the section entitled "Executive and Director Compensation — Separation Agreement and Patent Assignment with Ms. Stinchcomb."
122
Preferred Stock Issuances
In 2014, we issued and sold an aggregate of 6,149,551 shares of our Series 1 convertible preferred stock at a purchase price per share of $2.12, for an aggregate purchase price of approximately $13.0 million. The table below sets forth the purchases of our Series 1 convertible preferred stock by directors and persons who hold more than 5.0% our outstanding capital stock:
Stockholder
|
Shares of Series 1 Convertible Preferred Stock Purchased |
Aggregate Investment | |||||
|---|---|---|---|---|---|---|---|
Michael D. Rapoport(1) |
1,066,911 | $ | 2,258,012 | ||||
Jaime Hartman and Ethan Benovitz(2) |
945,001 | $ | 2,000,002 | ||||
- (1)
- Includes
94,500 shares of Series 1 convertible preferred stock in Merger Corp that Mr. Rapoport purchased prior to the Merger
for an investment of $200,000, which shares were converted into 94,500 shares of our Series 1 convertible preferred stock in the Merger.
- (2)
- Consists of: (a) 555,188 shares of Series 1 convertible preferred stock held by G-Ten Partners LLC, (b) 342,563 shares of Series 1 convertible preferred stock held by Genesis Capital Advisors LLC and (c) 47,250 shares of Series 1 convertible preferred stock held by Genesis Asset Opportunity Fund L.P. Ethan Benovitz and Jaime Hartman are managing members of both G-Ten Partners LLC and Genesis Capital Advisors LLC, and as a result they may be deemed to beneficially own the shares of our common stock held by G-Ten Partners LLC and Genesis Capital Advisors LLC. Mr. Benovitz and Jaime Hartman are managing members of Genesis Capital GP LLC, which is the general partner of Genesis Asset Opportunity Fund L.P., and as a result they may be deemed to beneficially own the shares of our common stock held by Genesis Asset Opportunity Fund L.P. Mr. Benovitz and Mr. Hartman disclaim beneficial ownership of these shares, except to the extent of their pecuniary interest therein.
In October 2012, we issued and sold an aggregate of 257,144 shares of our Series B participating convertible preferred stock at a purchase price per share of $1.75, for an aggregate purchase price of approximately $450,000. In October 2012, we issued an aggregate of 698,109 shares of our Series B participating convertible preferred stock upon the conversion of our convertible notes. In March 2013, we issued and sold an aggregate of 42,856 shares of our Series B participating convertible preferred stock at a purchase price per share of $1.75, for an aggregate purchase price of approximately $75,000. In January 2014, we issued and sold an aggregate of 200,002 shares of Series B participating convertible preferred stock at a purchase price of $1.75, for an aggregate purchase price of $350,000. The shares were sold with warrants to purchase 49,998 shares of Series B participating convertible preferred stock which were exercised in April 2014 for an aggregate of $500.
Stockholder
|
Shares of Series B Participating Preferred Stock Purchased(1) |
Aggregate Investment | |||||
|---|---|---|---|---|---|---|---|
Kentucky Seed Capital Fund I, L.P. |
440,138 | $ | 550,109 | ||||
Commonwealth Seed Capital, LLC |
260,417 | $ | 350,143 | ||||
Kentucky Science & Technology Corporation |
146,760 | $ | 160,000 | ||||
Russell S. King |
142,858 | $ | 225,144 | ||||
Judd Berlin |
110,715 | $ | 175,109 | ||||
Bluegrass Angel Venture Fund, LLC |
69,671 | $ | 75,000 | ||||
- (1)
- Numbers include warrants that were exercised.
123
Research and Development Grants
We are party to several research and development grants from the Kentucky Science & Technology Corporation, or KSTC, which, prior to the Merger, was one of our principal stockholders. Pursuant to the grant agreements, we received funds of $0, $110,000 and $149,000 from KSTC during the years ended December 31, 2014, 2013 and 2012, respectively and $0 during the three months ended March 31, 2015. The grant agreements required us, for a minimum period of at least 60 months following the dates of final disbursements under the grants, to maintain our status as a "Kentucky-Based Company," which, among other things, required us to maintain our principal office of operation in Kentucky and no less that 51% of each of our property and payroll in Kentucky. We are no longer headquartered in Kentucky, and, as a result, we were required to repay the amounts previously granted to us under these grants, which we did in February 2015.
Policies and Procedures for Related Party Transactions
Upon the closing of this offering, our board of directors will adopt a related party transactions policy for us. Pursuant to the related party transactions policy, we will review all transactions with a dollar value in excess of $120,000 involving us in which any of our directors, director nominees, significant stockholders and executive officers and their immediate family members will be participants, to determine whether such person has a direct or indirect material interest in the transaction. This policy was not in effect when we entered into the transactions described above. All directors, director nominees and executive officers will be required to promptly notify our chief financial officer of any proposed transaction involving us in which such person has a direct or indirect material interest. Such proposed transaction will then be reviewed by the audit committee to determine whether the proposed transaction is a related party transaction under our policy. In reviewing any related party transaction, the audit committee will determine whether or not to approve or ratify the transaction based on all relevant facts and circumstances, including the following:
- §
- the materiality and character of the related person's interest in the
transaction;
- §
- the commercial reasonableness of the terms of the transaction;
- §
- the benefit and perceived benefit, or lack thereof, to us;
- §
- the opportunity costs of alternate transactions;
and
- §
- the actual or apparent conflict of interest of the related person.
In the event that any member of the audit committee is not a disinterested member with respect to the related person transaction under review, that member will be excluded from the review and approval or rejection of such related party transaction and another director may be designated to join the committee for purposes of such review. Whenever practicable, the reporting, review and approval will occur prior to entering into the transaction. If management becomes aware of a related party transaction that has not been previously approved, it will notify the audit committee of such transaction. The audit committee will review the transaction and, based on its review, will: (i) if the transaction is ongoing, (a) ratify the transaction; (b) direct that we terminate the transaction; or (c) ratify the transaction subject to any changes or modifications that it deems appropriate (taking into consideration our contractual obligations); or (ii) if the proposed transaction has been completed, (a) ratify the transaction; (b) direct that we rescind the transaction (taking into consideration our contractual obligations); and/or (c) direct that we take any other action which it deems appropriate in the circumstances. After any such review, the audit committee will approve or ratify the transaction only if it determines that the transaction is in, or not inconsistent with, the best interests of us and our stockholders. Our related party transaction policy will be available on our website, www.zynerba.com, under the "Investor Relations" section, upon the effective date of this offering. The information contained in, or that can be accessed through, our website is not part of this prospectus.
124
The following table sets forth information regarding beneficial ownership of our capital stock by:
- §
- each person, or group of affiliated persons, known by us to beneficially own
more than 5% of our common stock;
- §
- each of our directors;
- §
- each of our named executive officers; and
- §
- all of our current executive officers and directors as a group.
The percentage ownership information under the column entitled "Before Offering" is based on 5,733,963 shares of common stock outstanding as of July 23, 2015, assuming the conversion of all outstanding shares of our convertible preferred stock into 3,704,216 shares of common stock. The percentage ownership information under the column entitled "After Offering" is based on the sale of shares of common stock in this offering, and assumes (1) no exercise of the underwriters' option to purchase additional shares and (2) no exercise of outstanding options, in each case assuming an initial public offering price of $14.00 per share (the mid-point of the price range set forth on the cover page of this prospectus).
Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common stock. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of our common stock issuable pursuant to the exercise of stock options or warrants that are either immediately exercisable or exercisable within 60 days of July 23, 2015. As noted in the applicable footnotes to the table, some of the options are not vested but are exercisable at any time. These shares are deemed to be outstanding and beneficially owned by the person holding those options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Except as otherwise noted below, the address for each person or entity listed in the table is c/o Zynerba Pharmaceuticals, Inc., 80 W. Lancaster Avenue, Suite 300, Devon, PA 19333.
125
| |
|
Percentage of shares beneficially owned(1) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of shares beneficially owned |
Before offering | After offering | |||||||
| Name and Address of Beneficial Owner 5% or greater stockholders: |
||||||||||
|
1,344,115 |
23.44 |
% |
15.39 |
% |
|||||
|
||||||||||
|
502,658 |
8.77 |
% |
5.76 |
% |
|||||
|
||||||||||
|
502,658 |
8.77 |
% |
5.76 |
% |
|||||
|
||||||||||
Other Directors, Director Nominees and Named Executive Officers: |
||||||||||
|
405,650 |
6.99 |
% |
4.61 |
% |
|||||
|
331,796 |
5.79 |
% |
3.80 |
% |
|||||
|
||||||||||
|
215,315 |
3.73 |
% |
2.45 |
% |
|||||
|
94,744 |
1.65 |
% |
1.08 |
% |
|||||
|
||||||||||
|
50,531 |
* |
* |
|||||||
|
15,957 |
* |
* |
|||||||
|
1,113,993 |
18.98 |
% |
12.56 |
% |
|||||
- *
- Represents
beneficial ownership of less than 1%.
- (1)
- Beneficial
ownership is determined in accordance with the rules of the SEC, and includes voting and investment power with respect to shares.
Unless otherwise indicated below, to our knowledge, all persons listed in the table have sole voting and dispositive power with respect to their shares of common stock, except to the extent authority
is shared by spouses under applicable law. Pursuant to the rules of the SEC, the number of shares of common stock deemed outstanding includes shares issuable upon vesting of shares of restricted stock
held by the respective person or group that will vest within 60 days of July 23, 2015 and pursuant to options held by the respective person or group that are currently exercisable or may
be exercised within 60 days of July 23, 2015, which we refer to as presently exercisable stock options.
- (2)
- Consists of: (a) 696,143 shares of common stock, (b) 567,505 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held by Michael D. Rapoport and (c) 80,467 shares of common stock held by BCM. Mr. Rapoport is the chairman of BCM, and as a result he may be deemed to beneficially own the shares of our common stock held by BCM. Mr. Rapoport disclaims beneficial ownership of these shares, except to the extent of his pecuniary interest therein.
126
- (3)
- Consists
of: (a) 295,312 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held
by G-Ten Partners LLC, (b) 182,214 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held by Genesis Capital Advisors LLC
and (c) 25,132 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held by Genesis Asset Opportunity Fund L.P. Ethan Benovitz is a
managing member of both G-Ten Partners LLC and Genesis Capital Advisors LLC, and as a result he may be deemed to beneficially own the shares of our common stock held by G-Ten
Partners LLC and Genesis Capital Advisors LLC. Mr. Benovitz is a managing member of Genesis Capital GP LLC, which is the general partner of Genesis Asset Opportunity
Fund L.P., and as a result he may be deemed to beneficially own the shares of our common stock held by Genesis Asset Opportunity Fund L.P. Mr. Benovitz disclaims beneficial
ownership of these shares, except to the extent of his pecuniary interest therein.
- (4)
- Consists
of: (a) 295,312 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held
by G-Ten Partners LLC, (b) 182,214 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held by Genesis Capital Advisors LLC
and (c) 25,132 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held by Genesis Asset Opportunity Fund L.P. Jaime Hartman is a
managing member of both G-Ten Partners LLC and Genesis Capital Advisors LLC, and as a result he may be deemed to beneficially own the shares of our common stock held by G-Ten
Partners LLC and Genesis Capital Advisors LLC. Mr. Hartman is a managing member of Genesis Capital GP LLC, which is the general partner of Genesis Asset Opportunity
Fund L.P., and as a result he may be deemed to beneficially own the shares of our common stock held by Genesis Asset Opportunity Fund L.P. Mr. Hartman disclaims beneficial
ownership of these shares, except to the extent of his pecuniary interest therein.
- (5)
- Includes
(a) 73,138 shares of common stock issuable upon the exercise of options to purchase common stock that will vest upon the closing of
the offering (and excludes 219,415 shares of common stock issuable upon the exercise of options to purchase common stock that will not be vested within 60 days of July 23, 2015) and
(b) 332,512 shares of restricted stock, all of which have voting rights and 25% of which will vest upon the closing of this offering.
- (6)
- Consists
of: (a) 251,329 shares of common stock held by Philip R. Wagenheim and (b) 80,467 shares of common stock held by
BCM. Mr. Wagenheim is the vice chairman of BCM, and as a result he may be deemed to beneficially own the shares of our common stock held by BCM. Mr. Wagenheim disclaims beneficial
ownership of these shares, except to the extent of his pecuniary interest therein.
- (7)
- Includes
(a) 36,569 shares of common stock issuable upon the exercise of options to purchase common stock that will vest upon the closing of
the offering (and excludes 109,707 shares of common stock issuable upon the exercise of options to purchase common stock that will not be vested within 60 days of July 23, 2015) and
(b) 178,746 shares of restricted stock, all of which have voting rights and 25% of which will vest upon the closing of this offering.
- (8)
- Consists
of 94,744 shares of common stock issuable upon conversion of shares of Series 1 convertible preferred stock held by Kentucky
Seed Capital Fund I, L.P. Mr. Gailar is managing partner of Kentucky Seed Capital Fund I, L.P., and as a result he may be deemed to beneficially own the shares of our common stock
held by Kentucky Seed Capital Fund I, L.P. Mr. Gailar disclaims beneficial ownership of these shares, except to the extent of his pecuniary interest therein.
- (9)
- Includes
(a) 10,638 shares of common stock issuable upon the exercise of options to purchase common stock that will vest upon the closing of
the offering (and excludes 31,915 shares of common stock issuable upon the exercise of options to purchase common stock that will not be vested within 60 days of July 23, 2015) and
(b) 39,893 shares of restricted stock, all of which have voting rights and 25% of which will vest upon the closing of this offering.
- (10)
- Includes 15,957 shares of common stock issuable upon the exercise of options to purchase common stock that will vest upon the closing of this offering (and excludes 47,872 shares of common stock issuable upon the exercise of options to purchase common stock that will not be vested within 60 days of July 23, 2015).
127
The following is a summary of the rights of our common and preferred stock and some of the provisions of our amended and restated certificate of incorporation and amended and restated bylaws, which will become effective upon the closing of this offering, and of the Delaware General Corporation Law. This summary is not complete. For more detailed information, please see our amended and restated certificate of incorporation and amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus is a part, as well as the relevant provisions of the Delaware General Corporation Law.
General
Upon closing of this offering and the filing of our sixth amended and restated certificate of incorporation immediately prior to the closing of this offering, our authorized capital stock will consist of 210,000,000 shares, 200,000,000 of which will be designated as common stock with a par value of $0.001 per share and 10,000,000 of which will be designated as preferred stock with a par value of $0.001 per share.
Common Stock
Outstanding Shares
As of March 31, 2015, there would have been 5,733,963 shares of common stock outstanding, held by 129 stockholders of record, after giving effect to the conversion of all preferred stock outstanding as of March 31, 2015.
Voting Rights
Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, other than election of directors, which shall be determined by a plurality of the votes cast by the stockholders entitled to vote on the election of such director. In addition, the affirmative vote of the holders of at least 662/3% of the voting power of all of the then outstanding voting stock will be required to take certain actions, including amending certain provisions of our amended and restated certificate of incorporation, such as the provisions relating to director liability, amending our bylaws, removing directors without cause or changing the Court of Chancery of the State of Delaware from being the sole and exclusive forum for certain actions brought by our stockholders against us or our directors, officers or employees.
Dividends
Subject to the preferences that may be applicable to any outstanding preferred stock, holders of our common stock shall be entitled to receive ratably any dividends that may be declared by the board of directors out of funds legally available for that purpose.
Liquidation
In the event of our liquidation, dissolution or winding up, holders of our common stock shall be entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preference of any outstanding preferred stock.
No Preemptive or Similar Rights
Our common stock shall not be entitled to preemptive rights and is not subject to conversion, redemption or sinking fund provisions.
Convertible Preferred Stock
Immediately prior to the closing of this offering, all outstanding shares of our preferred stock will be converted into an aggregate of 3,704,216 shares of common stock. Under our certificate of incorporation that will be in effect following the closing of this offering, our board of directors has the authority, subject to limitations prescribed by Delaware law, to issue preferred stock in one or more series, to establish from time to time the number of shares to be included in each series and to fix the designation, powers,
128
preferences and rights of the shares of each series and any of its qualifications, limitations and restrictions. Our board of directors also can increase or decrease the number of shares of any series, but not below the number of shares of that series then outstanding.
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of our company and may adversely affect the market price of our common stock and the voting and other rights of the holders of common stock. We have no current plan to issue any shares of preferred stock.
Registration Rights
We are party to a stockholders' agreement with certain holders of shares of our common and preferred stock. Under the stockholders' agreement, certain holders of our preferred stock will have registration rights with respect to the shares of common stock issuable upon conversion as further described below. After registration of these shares of common stock pursuant to these rights, these shares will become freely tradable without restriction under the Securities Act. These holders may also be able to sell shares without registration pursuant to Rule 144 as described in this prospectus. See "Shares Eligible for Future Sale — Rule 144." If not otherwise exercised, the rights described below will expire five years after the closing of this offering.
Demand Registration Rights
Following six months after this offering and subject to specified limitations set forth in the stockholders' agreement, the holders of greater than 50.0% of the then outstanding registrable shares may demand in writing that we register all or a portion of the registrable shares under the Securities Act by filing a registration statement on Form S-1 (or any successor form), in an underwritten offering at the registrable share holders' option, if the total amount of registrable shares registered have an aggregate offering price of at least $5.0 million (based on the then current market price or fair value). We are not obligated to file a registration statement pursuant to this provision with respect to an offering in which the number of registrable shares included in the offering was at least 80% of the registrable shares requested by the registrable shareholders to be so included on more than two occasions (excluding any registrations pursuant to which securities are sold by us), provided that, if a registration is withdrawn at the request of the registrable shareholders requesting such registration and such registrable shareholders elect not to have the registration counted under this provision, the fees and expenses relating to the registration will be borne pro rata by the selling stockholders and the registration will not count toward the demand registration limit.
In addition, subject to specified limitations set forth in the stockholders' agreement, at any time after we become eligible to file a registration statement on Form S-3, holders of greater than 50.0% of the registrable shares then outstanding may request that we register their registrable securities on Form S-3 for purposes of a public offering if the total amount of registrable shares registered have an aggregate offering price of at least $1.0 million (based on the then current market price or fair value). We are not obligated to file a registration statement pursuant to this provision on more than two occasions in any 12-month period, provided that, if a registration is withdrawn at the request of the registrable shareholders requesting such registration and such registrable shareholders elect not to have the registration counted under this provision, the fees and expenses relating to the registration will be borne pro rata by the selling stockholders and the registration will not count toward the demand registration limit.
Piggyback Registration Rights
If, at any time, excluding pursuant to this offering, we propose to file a registration statement to register any of our securities under the Securities Act, either for our own account or for the account of any of our stockholders, other than pursuant to the demand registration rights described above, the holders of our registrable securities are entitled to notice of registration and, subject to specified exceptions, we will be
129
required upon the holder's request to use our reasonable best efforts to register their then-held registrable securities.
Other Provisions
The stockholders' agreement provides that, in connection with this offering and upon the managing underwriters' request, holders of registrable securities will be subject to a "lock-up" provision prohibiting the sale or other disposition of our securities for up to 180 days.
We will pay all registration expenses, other than the underwriting discount, selling commissions, and the fees and expenses of the selling stockholders' own counsel (other than the counsel selected to represent all of the selling stockholders), related to any demand registration. The stockholders' agreement contains customary cross-indemnification provisions, pursuant to which we are obligated to indemnify the selling stockholders in the event of material misstatements or omissions in the registration statement attributable to us, and they are obligated to indemnify us for material misstatements or omissions in the registration statement attributable to them.
Delaware Anti-Takeover Law and Provisions of Our Certificate of Incorporation and Bylaws
Delaware Anti-Takeover Law
We are subject to Section 203 of the Delaware General Corporation Law, or Section 203. Section 203 generally prohibits a public Delaware corporation from engaging in a "business combination" with an "interested stockholder" for a period of three years after the date of the transaction in which the person became an interested stockholder, unless:
- §
- prior to the date of the transaction, the board of directors of the corporation
approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder;
- §
- the interested stockholder owned at least 85% of the voting stock of the
corporation outstanding upon consummation of the transaction, excluding for purposes of determining the number of shares outstanding (1) shares owned by persons who are directors and also
officers and (2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered
in a tender or exchange offer; or
- §
- on or subsequent to the consummation of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 662/3% of the outstanding voting stock which is not owned by the interested stockholder.
Section 203 defines a business combination to include:
- §
- any merger or consolidation involving the corporation and the interested
stockholder;
- §
- any sale, transfer, pledge or other disposition involving the interested
stockholder of 10% or more of the assets of the corporation;
- §
- subject to exceptions, any transaction involving the corporation that has the
effect of increasing the proportionate share of the stock of any class or series of the corporation beneficially owned by the interested stockholder;
- §
- subject to exceptions, any transaction that results in the issuance or transfer
by the corporation of any stock of the corporation to the interested stockholder; and
- §
- the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation.
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by the entity or person.
130
Certificate of Incorporation and Bylaws
Provisions of our certificate of incorporation and bylaws that will be in effect upon the closing of this offering may delay or discourage transactions involving an actual or potential change of control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our certificate of incorporation and bylaws will:
- §
- permit our board of directors to issue up to 10 million shares of
preferred stock, with any rights, preferences and privileges as it may designate;
- §
- provide that all vacancies on our board of directors, including as a result of
newly created directorships, may, except as otherwise required by law, be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum;
- §
- require that any action to be taken by our stockholders must be effected at a
duly called annual or special meeting of stockholders and not be taken by written consent;
- §
- provide that stockholders seeking to present proposals before a meeting of
stockholders or to nominate candidates for election as directors at a meeting of stockholders must provide advance notice in writing, and also specify requirements as to the form and content of a
stockholder's notice;
- §
- provide that a director may be removed only for cause and only by the
affirmative vote of the holders of 662/3% or more of the outstanding shares of capital stock then entitled to vote at an election of directors;
- §
- provide that the authorized number of directors may be changed only by the
resolution of our board of directors;
- §
- provide that special meetings of our stockholders may be called only by the
board of directors or by such person or persons requested by a majority of the board of directors to call such meetings;
- §
- provide that the Court of Chancery of the State of Delaware is the exclusive
forum in which we and our directors may be sued by our stockholders; and
- §
- provide that our certificate of incorporation and bylaws can only be amended to remove or revise the anti-takeover measures discussed above upon consent of 662/3% of the outstanding capital stock.
Listing on the NASDAQ Global Market
We have applied to list our common stock on the NASDAQ Global Market under the symbol "ZYNE."
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is American Stock Transfer and Trust Company, LLC.
131
SHARES ELIGIBLE FOR FUTURE SALE
Immediately prior to this offering, there has been no public market for our common stock. Future sales of substantial amounts of our common stock in the public market could adversely affect prevailing market prices. Furthermore, since only a limited number of shares will be available for sale shortly after this offering because of contractual and legal restrictions on resale described below, sales of substantial amounts of common stock in the public market after the restrictions lapse could adversely affect the prevailing market price for our common stock as well as our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as of March 31, 2015, upon the closing of this offering, 8,733,963 shares of common stock will be outstanding, assuming (1) the conversion of all outstanding shares of our preferred stock into 3,704,216 shares of common stock immediately prior to the closing of this offering and (2) the issuance by us of 3,000,000 shares of common stock in this offering. All of the shares sold in this offering will be freely tradable unless purchased by our "affiliates" as that term is defined in Rule 144 under the Securities Act or purchased by existing stockholders and their affiliated entities who are subject to lock-up agreements. The remaining 5,733,963 shares of common stock outstanding after this offering will be restricted as a result of securities laws or lock-up agreements. These remaining 5,733,963 shares will generally become available for sale in the public market as follows:
- §
- 29,209 restricted shares will be eligible for immediate sale upon the
closing of this offering; and
- §
- 5,704,754 restricted shares will be eligible for sale upon expiration of lock-up agreements 180 days after the date of this offering, subject in certain circumstances to the volume, manner of sale and other limitations under Rule 144 and Rule 701.
Rule 144
In general, pursuant to Rule 144 under the Securities Act, as currently in effect, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, any person who is not an affiliate of ours at any time during the three months preceding a sale and has held their shares for at least six months, including the holding period of any prior owner other than one of our affiliates, may sell shares without restriction, provided current public information about us is available. In addition, under Rule 144, any person who is not an affiliate of ours at any time during the three months preceding a sale and has held their shares for at least one year, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell an unlimited number of shares immediately upon the closing of this offering without regard to whether current public information about us is available.
Beginning 90 days after the effective date of the registration statement of which this prospectus is a part, a person who is an affiliate of ours and who has beneficially owned restricted securities for at least six months, including the holding period of any prior owner other than one of our affiliates, is entitled to sell a number of restricted shares within any three-month period that does not exceed the greater of:
- §
- 1% of the number of shares of our common stock then outstanding, which will
equal approximately 87,340 shares immediately after this offering; or
- §
- the average weekly trading volume of our common stock on the NASDAQ Global Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to the sale.
Sales of restricted shares under Rule 144 held by our affiliates are also subject to requirements regarding the manner of sale, notice and the availability of current public information about us. Rule 144 also provides that affiliates relying on Rule 144 to sell shares of our common stock that are not restricted shares must nonetheless comply with the same restrictions applicable to restricted shares, other than the holding period requirement.
132
Notwithstanding the availability of Rule 144, the holders of our restricted shares have entered into lock-up agreements as described below and their restricted shares will become eligible for sale at the expiration of the restrictions set forth in those agreements.
Rule 701
Pursuant to Rule 701 under the Securities Act, shares of our common stock acquired upon the exercise of currently outstanding options or pursuant to other rights granted under our stock plans may be resold by:
- §
- persons other than affiliates, beginning 90 days after the effective date
of the registration statement of which this prospectus is a part, subject only to the manner-of-sale provisions of Rule 144; and
- §
- our affiliates, beginning 90 days after the effective date of the registration statement of which this prospectus is a part, subject to the manner-of-sale and volume limitations, current public information and filing requirements of Rule 144, in each case, without compliance with the six-month holding period requirement of Rule 144.
As of July 23, 2015, options to purchase a total of 606,379 shares of common stock were outstanding, of which 151,592 vest upon the closing of this offering. Of the total number of shares of our common stock issuable under these options, substantially all are subject to contractual lock-up agreements with us or the underwriters described below under "Underwriting" and will become eligible for sale in accordance with Rule 701 at the expiration of those agreements.
Lock-up Agreements
We, along with our directors, executive officers and substantially all of our other securityholders, have agreed with the underwriters that for a period of 180 days, or the restricted period, after the date of this prospectus, subject to specified exceptions, we or they will not sell, offer to sell, contract to sell or lend, effect any short sale or establish or increase any put equivalent position or liquidate or decrease any call equivalent position, pledge, hypothecate, grant any security interest in or in any other way transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or exchangeable for shares of common stock, or enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock. Upon expiration of the restricted period, certain of our stockholders will have the right to require us to register their shares under the Securities Act. See " — Registration Rights" below and "Description of Capital Stock — Registration Rights."
After this offering, certain of our employees, including our executive officers and/or directors, may enter into written trading plans that are intended to comply with Rule 10b5-1 under the Exchange Act. Sales under these trading plans would not be permitted until the expiration of the lock-up agreements described above.
Registration Rights
Upon the closing of this offering, the holders of 3,658,895 shares of our common stock will be entitled to rights with respect to the registration of their shares under the Securities Act, subject to the lock-up agreements described above. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates. Any sales of securities by these stockholders could have a material adverse effect on the trading price of our common stock. See "Description of Capital Stock — Registration Rights" in this prospectus.
Equity Incentive Plans
We intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the shares of common stock reserved for issuance under our 2014 Equity Plan. The registration statement is expected to be filed and become effective as soon as practicable after the closing of this offering. Accordingly, shares registered under such registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations for affiliates and the lock-up agreements described above, if applicable.
133
MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS
FOR NON-U.S. HOLDERS OF OUR COMMON STOCK
The following is a general discussion of material U.S. federal income and estate tax considerations relating to the ownership and disposition of our common stock by a non-U.S. holder. For purposes of this discussion, the term "non-U.S. holder" means a beneficial owner of our common stock that is not, for U.S. federal income tax purposes:
- §
- an individual who is a citizen or resident of the United States;
- §
- a corporation, or other entity treated as a corporation for U.S. federal income
tax purposes, created or organized in or under the laws of the United States or of any political subdivision of the United States;
- §
- an estate the income of which is subject to U.S. federal income taxation
regardless of its source; or
- §
- a trust, if (1) a U.S. court is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust or (2) if the trust has a valid election to be treated as a U.S. person under applicable U.S. Treasury Regulations.
An individual may be treated as a resident instead of a nonresident of the United States in any calendar year for U.S. federal income tax purposes if the individual was present in the United States for at least 31 days in that calendar year and for an aggregate of at least 183 days during the three-year period ending with that calendar year. For purposes of this calculation, all of the days present in the tested year, one-third of the days present in the immediately preceding year and one-sixth of the days present in the second preceding year are counted. Residents are taxed for U.S. federal income tax purposes as if they were U.S. citizens.
This discussion is based on current provisions of the Internal Revenue Code of 1986, as amended, or the Code, existing and proposed U.S. Treasury Regulations promulgated thereunder, current administrative rulings and judicial decisions, all as in effect as of the date of this prospectus and all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any change could alter the tax consequences to non-U.S. holders described in this prospectus. In addition, the Internal Revenue Service, or the IRS, could challenge one or more of the tax consequences described in this discussion.
We assume in this discussion that each non-U.S. holder holds shares of our common stock as a capital asset within the meaning of the Code (generally, property held for investment). This discussion does not address all aspects of U.S. federal income and estate taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder's individual circumstances nor does it address any aspects of U.S. state, local or non-U.S. taxes. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder, including the alternative minimum tax and the Medicare contribution tax on investment income, and does not address the special tax rules applicable to particular non-U.S. holders, such as:
- §
- insurance companies;
- §
- tax-exempt organizations or tax-qualified retirement plans;
- §
- financial institutions;
- §
- brokers or dealers in securities;
- §
- regulated investment companies;
- §
- pension plans;
- §
- controlled foreign corporations;
- §
- passive foreign investment companies;
- §
- owners that hold our common stock as part of a straddle, hedge, conversion
transaction, synthetic security or other integrated investment; and
- §
- certain U.S. expatriates.
134
In addition, this discussion does not address the tax treatment of partnerships or persons who hold their common stock through partnerships or other entities that are pass-through entities for U.S. federal income tax purposes.
Dividends
If we pay distributions on our common stock, those distributions generally will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder's investment, up to such non-U.S. holder's tax basis in the common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described in this prospectus under the heading "— Gain on Disposition of Common Stock." Any such distribution will also be subject to the discussion in this prospectus under the heading "— Withholding and Information Reporting Requirements — FATCA."
Dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30.0% rate or such lower rate as may be specified by an applicable income tax treaty between the U.S. and such holder's country of residence. If we determine, at a time reasonably close to the date of payment of a distribution on our common stock, that the distribution will not constitute a dividend because we do not anticipate having current or accumulated earnings and profits, we may elect not to withhold U.S. federal income tax from such distribution as permitted by U.S. Treasury Regulations.
A non-U.S. holder of our common stock who claims the benefit of an applicable income tax treaty between the U.S. and such holder's country of residence generally will be required to provide a properly executed IRS Form W-8BEN (or successor form) and satisfy applicable certification and other requirements.
A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing an appropriate claim with the IRS.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the U.S., and, if an applicable income tax treaty so provides, that are attributable to a permanent establishment or a fixed base maintained by the non-U.S. holder within the United States, are generally exempt from the 30.0% withholding tax if the non-U.S. holder provides a properly executed IRS Form W-8ECI (or successor form). However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same graduated U.S. federal income tax rates applicable to "United States persons" (within the meaning of the Code). Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional "branch profits tax" at a 30.0% rate or such lower rate as may be specified by an applicable income tax treaty between the U.S. and such non-U.S. holder's country of residence.
Gain on Disposition of Common Stock
Subject to the discussion below regarding backup withholding and the Foreign Account Tax Compliance Act, or FATCA, a non-U.S. holder generally will not be subject to U.S. federal income tax on gain recognized on a disposition of our common stock unless:
- §
- the gain is effectively connected with the non-U.S. holder's conduct of a trade or business in the United States, and, if an applicable income tax treaty so provides, the gain is attributable to a permanent establishment or fixed base maintained by the non-U.S. holder in the U.S.; in these cases, the non-U.S. holder will be taxed on a net income basis at the regular graduated rates and in the manner applicable to U.S. persons, and, if the non-U.S. holder is a foreign corporation, an additional branch profits tax at a rate of 30.0%, or a lower rate as may be specified by an applicable income tax treaty, may also apply;
135
- §
- the non-U.S. holder is an individual present in the United States for
183 days or more in the taxable year of the disposition and certain other requirements are met (in which case the non-U.S. holder will be subject to a 30.0% tax, or such lower rate as may be
specified by an applicable income tax treaty, on the net gain derived from the disposition, which may be offset by U.S.-source capital losses of the non-U.S. holder, if any); or
- §
- we are or have been, at any time during the five-year period preceding such disposition (or the non-U.S. holder's holding period, if shorter) a "United States real property holding corporation" within the meaning of the Code, unless during such applicable period our common stock is regularly traded on an established securities market and the non-U.S. holder held no more than 5.0% of our outstanding common stock, directly or indirectly. Generally, a corporation is a "United States real property holding corporation" if the fair market value of its "United States real property interests" equals or exceeds 50.0% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we believe that we are not currently, and we do not anticipate becoming, a "United States real property holding corporation" for U.S. federal income tax purposes. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rule described above.
Information Reporting and Backup Withholding
The gross amount of the distributions on our common stock paid to each non-U.S. holder and the tax withheld, if any, with respect to such distributions must be reported annually to the IRS. Non-U.S. holders may have to comply with specific certification procedures to establish that they are not "United States persons" (within the meaning of the Code) in order to avoid backup withholding at the applicable rate, currently 28.0%, with respect to dividends on our common stock. Generally, a non-U.S. holder will comply with such procedures if it provides a properly executed IRS Form W-8BEN (or other applicable IRS Form W-8) or otherwise meets documentary evidence requirements for establishing that it is a non-U.S. holder, or otherwise establishes an exemption. Dividends paid to non-U.S. holders subject to withholding of U.S. federal income tax, as described above under the heading "Dividends," will generally be exempt from backup withholding.
Information reporting and backup withholding generally will apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through a U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder (generally an IRS Form W-8BEN) and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker.
Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder can be refunded or credited against the non-U.S. holder's U.S. federal income tax liability, if any, provided that an appropriate claim is timely filed with the IRS.
Withholding and Information Reporting Requirements — FATCA
Legislation known as the Foreign Account Tax Compliance Act, or FATCA, imposes U.S. federal withholding tax of 30.0% on payments of dividends on, and (to the extent described below) on gross proceeds from the sale or disposition of, our common stock if paid to a foreign entity unless (i) in the case of a foreign entity
136
that is a "foreign financial institution" (within the meaning of the Code), the foreign entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) in the case of a foreign entity that is not a foreign financial institution, the foreign entity identifies certain of its U.S. investors or (iii) the foreign entity is otherwise exempt under FATCA. Withholding under FATCA will only apply (1) to payments of dividends on our common stock and (2) to payments of gross proceeds from a sale or other disposition of our common stock made after December 31, 2016. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of such taxes.
Federal Estate Tax
Common stock owned or treated as owned by an individual (including by reason of holding interests in certain entities) who is not a citizen or resident of the United States (as specially determined for U.S. federal estate tax purposes) at the time of death will be included in the individual's gross estate for U.S. federal estate tax purposes and, therefore, may be subject to U.S. federal estate tax, unless an applicable estate tax or other treaty provides otherwise.
THE PRECEDING DISCUSSION OF MATERIAL U.S. FEDERAL INCOME AND ESTATE TAX CONSIDERATIONS IS FOR GENERAL INFORMATION ONLY. IT IS NOT TAX ADVICE. PROSPECTIVE INVESTORS SHOULD CONSULT THEIR OWN TAX ADVISORS REGARDING THE PARTICULAR U.S. FEDERAL, STATE, LOCAL AND NON-U.S. TAX CONSEQUENCES OF PURCHASING, HOLDING AND DISPOSING OF OUR COMMON STOCK (DIRECTLY OR THROUGH ENTITIES), INCLUDING THE CONSEQUENCES OF ANY PROPOSED CHANGES IN APPLICABLE LAWS.
137
Subject to the terms and conditions set forth in the underwriting agreement, dated , 2015, among us, Jefferies LLC and Piper Jaffray & Co., as the representatives of the underwriters named below and the joint book-running managers of this offering, we have agreed to sell to the underwriters, and each of the underwriters has agreed, severally and not jointly, to purchase from us, the respective number of shares of common stock shown opposite its name below:
Underwriter
|
Number of Shares |
|||
|---|---|---|---|---|
Jefferies LLC |
||||
Piper Jaffray & Co. |
||||
Canaccord Genuity Inc. |
||||
Oppenheimer & Co. Inc. |
||||
| | | | | |
Total |
3,000,000 | |||
| | | | | |
| | | | | |
| | | | | |
The underwriting agreement provides that the obligations of the several underwriters are subject to certain conditions precedent such as the receipt by the underwriters of officers' certificates and legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the underwriters will purchase all of the shares of common stock if any of them are purchased. If an underwriter defaults, the underwriting agreement provides that the purchase commitments of the nondefaulting underwriters may be increased or the underwriting agreement may be terminated. We have agreed to indemnify the underwriters and certain of their controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriters may be required to make in respect of those liabilities.
The underwriters have advised us that, following the completion of this offering, they currently intend to make a market in the common stock as permitted by applicable laws and regulations. However, the underwriters are not obligated to do so, and the underwriters may discontinue any market-making activities at any time without notice in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for the common stock, that you will be able to sell any of the common stock held by you at a particular time or that the prices that you receive when you sell will be favorable.
The underwriters are offering the shares of common stock subject to their acceptance of the shares of common stock from us and subject to prior sale. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part. In addition, the underwriters have advised us that they do not intend to confirm sales to any account over which they exercise discretionary authority except sales to accounts over which they have discretionary authority to exceed % of the common stock being offered.
Commission and Expenses
The underwriters have advised us that they propose to offer the shares of common stock to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers, which may include the underwriters, at that price less a concession not in excess of $ per share of common stock. The underwriters may allow, and certain dealers may reallow, a discount from the concession not in excess of $ per share of common stock to certain brokers and dealers. After the offering, the initial public offering price, concession and reallowance to dealers may be reduced by the representatives. No
138
such reduction will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus.
The following table shows the public offering price, the underwriting discounts and commissions that we are to pay the underwriters and the proceeds, before expenses, to us in connection with this offering. Such amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase additional shares.
| |
Per Share | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Without Option to Purchase Additional Shares |
With Option to Purchase Additional Shares |
Without Option to Purchase Additional Shares |
With Option to Purchase Additional Shares |
|||||||||
Public offering price |
$ | $ | $ | $ | |||||||||
Underwriting discounts and commissions paid by us |
$ | $ | $ | $ | |||||||||
Proceeds to us, before expenses |
$ | $ | $ | $ | |||||||||
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $2.8 million. We have also agreed to pay the filing fees incident to, and the fees and disbursements of counsel for the underwriters in connection with, the required review by the Financial Industry Regulatory Authority, Inc., or FINRA, in connection with this offering in an amount not to exceed $30,000.
Determination of Offering Price
Prior to this offering, there has not been a public market for our common stock. Consequently, the initial public offering price for our common stock will be determined by negotiations between us and the representatives. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
We offer no assurances that the initial public offering price will correspond to the price at which the common stock will trade in the public market subsequent to the offering or that an active trading market for the common stock will develop and continue after the offering.
Listing
We have applied to list our common stock on the NASDAQ Global Market under the symbol "ZYNE."
Stamp Taxes
If you purchase shares of common stock offered in this prospectus, you may be required to pay stamp taxes and other charges under the laws and practices of the country of purchase, in addition to the offering price listed on the cover page of this prospectus.
Option to Purchase Additional Shares
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase, from time to time, in whole or in part, up to an aggregate of 450,000 shares from us at the public offering price set forth on the cover page of this prospectus, less underwriting discounts and commissions. If the underwriters exercise this option, each underwriter will be obligated, subject to
139
specified conditions, to purchase a number of additional shares proportionate to that underwriter's initial purchase commitment as indicated in the table above.
No Sales of Similar Securities
We, our officers, directors and holders of all or substantially all our outstanding capital stock and other securities have agreed, subject to specified exceptions, not to directly or indirectly:
- §
- sell, offer, contract or grant any option to sell (including any short sale),
pledge, transfer, establish an open "put equivalent position" within the meaning of Rule 16a-l(h) under the Exchange Act, as amended, or
- §
- otherwise dispose of any shares of common stock, options or warrants to acquire
shares of common stock, or securities exchangeable or exercisable for or convertible into shares of common stock currently or hereafter owned either of record or beneficially, or
- §
- publicly announce an intention to do any of the foregoing for a period of 180 days after the date of this prospectus without the prior written consent of Jefferies LLC and Piper Jaffray & Co.
This restriction terminates after the close of trading of the common stock on and including the 180th day after the date of this prospectus.
Jefferies LLC and Piper Jaffray & Co. may, in their sole discretion and at any time or from time to time before the termination of the 180-day period, release all or any portion of the securities subject to lock-up agreements. There are no existing agreements between the underwriters and any of our stockholders who will execute a lock-up agreement, providing consent to the sale of shares prior to the expiration of the lock-up period.
Stabilization
The underwriters have advised us that, pursuant to Regulation M under the Exchange Act, certain persons participating in this offering may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of the common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either "covered" short sales or "naked" short sales.
"Covered" short sales are sales made in an amount not greater than the underwriters' option to purchase additional shares of our common stock in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares of our common stock or purchasing shares of our common stock in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
"Naked" short sales are sales in excess of the option to purchase additional shares of our common stock. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares of our common stock in the open market after pricing that could adversely affect investors who purchase in this offering.
A stabilizing bid is a bid for the purchase of shares of common stock on behalf of the underwriters for the purpose of fixing or maintaining the price of the common stock. A syndicate covering transaction is the bid for or the purchase of shares of common stock on behalf of the underwriters to reduce a short position incurred by the underwriters in connection with the offering. Similar to other purchase transactions, the underwriters' purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common
140
stock. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriters to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the common stock originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we nor any of the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. The underwriters are not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
Electronic Distribution
A prospectus in electronic format may be made available by e-mail or on the web sites or through online services maintained by one or more of the underwriters or their affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The underwriters may agree with us to allocate a specific number of shares of common stock for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriters on the same basis as other allocations. Other than the prospectus in electronic format, the information on the underwriters' web sites and any information contained in any other web site maintained by any of the underwriters is not part of this prospectus, has not been approved and/or endorsed by us or the underwriters and should not be relied upon by investors.
Other Activities and Relationships
The underwriters and certain of their affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriters and certain of their affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and certain of their affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the underwriters or their respective affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The underwriters and their respective affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the common stock offered hereby. Any such short positions could adversely affect future trading prices of the common stock offered hereby. The underwriters and certain of their respective affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
At our request, the underwriters have also reserved for sale at the initial public offering price up to approximately 5% of the shares of our common stock being offered for our directors and director nominees; officers; existing stockholders and their affiliates and employees of both; and business associates, as well as certain friends and family members of our directors and stockholders, who have expressed an interest in purchasing shares in the offering. The number of shares of common stock available for sale to the general public in the offering will be reduced to the extent these persons purchase the directed shares in the
141
program. Any directed shares not so purchased will be offered by the underwriters to the general public on the same terms as the other shares. Shares purchased in the directed share program will be subject to the 180-day lock-up restriction in the lock-up agreements described above. Jefferies LLC and Piper Jaffray & Co., in their sole discretion, may release any of the securities subject to these lock-up agreements at any time. We have agreed to indemnify the underwriters against certain liabilities and expenses, including liabilities under the Securities Act, in connection with sales of the directed shares.
Disclaimers About Non-U.S. Jurisdictions
European Economic Area
In relation to each member state of the European Economic Area which has implemented the Prospectus Directive, each referred to herein as a Relevant Member State, an offer to the public of any common stock which are the subject of this offering may not be made in that Relevant Member State except that an offer to the public in that Relevant Member State of any common stock may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
- §
- to any legal entity which is a "qualified investor" as defined in the Prospectus
Directive;
- §
- to fewer than 100 or, if the Relevant Member State has implemented the relevant
provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject
to obtaining the prior consent of the underwriters or the underwriters nominated by us for any such offer; or
- §
- in any other circumstances falling within Article 3(2) of the Prospectus Directive,
provided that no such offer of common stock shall require us or any of the underwriters to publish a prospectus pursuant to Article 3 of the Prospectus Directive or supplement a prospectus pursuant to Article 16 of the Prospectus Directive.
For the purposes of this provision, the expression an "offer of common shares to the public" in relation to the common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the common stock to be offered so as to enable an investor to decide to purchase or subscribe to the common stock, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression "Prospectus Directive" means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
United Kingdom
This prospectus is only being distributed to, and is only directed at, persons in the United Kingdom that are qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive that are also (i) investment professionals falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, referred to herein as the Order, and/or (ii) high net worth entities falling within Article 49(2)(a) to (d) of the Order and other persons to whom it may lawfully be communicated. Each such person is referred to herein as a Relevant Person.
This prospectus and its contents are confidential and should not be distributed, published or reproduced (in whole or in part) or disclosed by recipients to any other persons in the United Kingdom. Any person in the United Kingdom that is not a Relevant Person should not act or rely on this document or any of its contents.
142
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Pepper Hamilton LLP. Wilmer Cutler Pickering Hale and Dorr LLP, New York, New York is counsel for the underwriters in connection with this offering.
The financial statements of Zynerba Pharmaceuticals, Inc. as of December 31, 2013 and 2014 and for the years then ended, have been included herein in reliance upon the report of KPMG LLP, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1, including exhibits and schedules, under the Securities Act, with respect to the shares of common stock being offered by this prospectus. This prospectus, which constitutes part of the registration statement, does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, we refer you to the registration statement and its exhibits. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
You can read our SEC filings, including the registration statement, over the Internet at the SEC's website at www.sec.gov. You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street, NE, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, NE, Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing us at Zynerba Pharmaceuticals, Inc., 80 W. Lancaster Avenue, Suite 300, Devon, PA 19333, or by calling (484) 581-7505.
Upon the closing of this offering, we will be subject to the information reporting requirements of the Exchange Act and we will file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available for inspection and copying at the public reference room and web site of the SEC referred to above. We also maintain a website at www.zynerba.com, at which, following the closing of this offering, you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
143
ZYNERBA PHARMACEUTICALS, INC.
Index to Financial Statements
F-1
When the recapitalization referred to in Note 2(l) of the Notes to Financial Statements has been consummated, we will
be in a position to render the following report:
/s/ KPMG LLP
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Stockholders
Zynerba Pharmaceuticals, Inc.:
We have audited the accompanying balance sheets of Zynerba Pharmaceuticals, Inc. (formerly AllTranz, Inc.) as of December 31, 2013 and 2014, and the related statements of operations, redeemable convertible preferred stock, convertible preferred stock, and stockholders' equity (deficit), and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Zynerba Pharmaceuticals, Inc. as of December 31, 2013 and 2014, and the results of its operations and its cash flows for the years then ended, in conformity with U.S. generally accepted accounting principles.
Philadelphia, Pennsylvania
February 20, 2015, except as to
Note 2(l), which is as of , 2015
F-2
ZYNERBA PHARMACEUTICALS, INC.
BALANCE SHEETS
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
Assets |
|||||||
Current assets: |
|||||||
Cash and cash equivalents |
$ | 154,695 | $ | 9,330,681 | |||
Grant receivables |
34,514 | — | |||||
Deferred offering costs |
— | 1,080,199 | |||||
Prepaid expenses |
500,065 | 1,183,949 | |||||
| | | | | | | | |
Total current assets |
689,274 | 11,594,829 | |||||
Property and equipment, net |
47,791 | 19,642 | |||||
Other assets |
1,123,775 | 2,200 | |||||
| | | | | | | | |
Total assets |
$ | 1,860,840 | $ | 11,616,671 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Liabilities, Convertible Preferred Stock and Stockholders' Equity (Deficit) |
|||||||
Current Liabilities: |
|||||||
Accounts payable |
$ | 308,282 | $ | 313,937 | |||
Accrued expenses |
50,322 | 1,711,473 | |||||
Deferred grant revenue |
1,041,321 | 1,120,125 | |||||
Stock subscription advances |
37,500 | — | |||||
Note payable, current portion |
125,000 | — | |||||
| | | | | | | | |
Total current liabilities |
1,562,425 | 3,145,535 | |||||
Note payable |
374,996 | — | |||||
Deferred grant revenue |
1,120,125 | — | |||||
| | | | | | | | |
Total liabilities |
3,057,546 | 3,145,535 | |||||
| | | | | | | | |
Commitments and contingencies (note 10) |
|||||||
Redeemable convertible preferred stock: |
|||||||
Series A redeemable convertible preferred stock; $0.0001 par value; 720,002 shares authorized; 720,002 shares issued and outstanding at December 31, 2013 |
1,646,179 | — | |||||
Series B redeemable convertible preferred stock; $0.0001 par value; 1,544,673 shares authorized; 998,109 shares issued and outstanding at December 31, 2013 |
1,516,194 | — | |||||
Series 1 convertible preferred stock; $0.001 par value; 7,807,502 shares authorized; 6,964,053 shares issued and outstanding at December 31, 2014, (liquidation preference of $14,763,792 at December 31, 2014) |
— | 16,522,811 | |||||
| | | | | | | | |
Stockholders' equity (deficit): |
|||||||
Common stock; $0.001 par value; 50,000,000 shares authorized; 490,760 and 2,029,747 shares issued and outstanding at December 31, 2013 and 2014, respectively |
49 | 2,030 | |||||
Additional paid-in capital |
— | 1,975,000 | |||||
Accumulated deficit |
(4,359,128 | ) | (10,028,705 | ) | |||
| | | | | | | | |
Total stockholders' equity (deficit) |
(4,359,079 | ) | (8,051,675 | ) | |||
| | | | | | | | |
Total liabilities, redeemable convertible preferred stock and stockholders' equity (deficit) |
$ | 1,860,840 | $ | 11,616,671 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See accompanying notes to financial statements.
F-3
ZYNERBA PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
| |
Years ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
Revenues |
$ | 943,904 | $ | 810,012 | |||
Operating expenses: |
|||||||
Research and development |
1,134,041 | 2,401,406 | |||||
General and administrative |
444,302 | 4,076,339 | |||||
| | | | | | | | |
Total operating expenses |
1,578,343 | 6,477,745 | |||||
| | | | | | | | |
Loss from operations |
(634,439 | ) | (5,667,733 | ) | |||
Other income (expense): |
|||||||
Interest expense, net |
(2,351 | ) | (1,844 | ) | |||
| | | | | | | | |
Net loss |
(636,790 | ) | (5,669,577 | ) | |||
Accretion of redeemable convertible preferred stock |
(161,834 | ) | (87,954 | ) | |||
| | | | | | | | |
Net loss applicable to common stockholders |
$ | (798,624 | ) | $ | (5,757,531 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Per share information: |
|||||||
Net loss per share basic and diluted |
$ | (1.63 | ) | $ | (6.44 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Basic and diluted weighted average shares outstanding |
490,760 | 894,575 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Pro forma net loss (unaudited) |
$ | (5,669,577 | ) | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Pro forma net loss per share basic and diluted (unaudited) |
$ | (2.56 | ) | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Pro forma basic and diluted weighted average shares outstanding (unaudited) |
2,215,507 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See accompanying notes to financial statements.
F-4
ZYNERBA PHARMACEUTICALS, INC.
STATEMENTS OF REDEEMABLE CONVERTIBLE PREFERRED STOCK, CONVERTIBLE PREFERRED STOCK, AND STOCKHOLDERS' EQUITY (DEFICIT)
YEARS ENDED DECEMBER 31, 2013 AND 2014
| |
Redeemable convertible preferred stock | Convertible preferred stock |
Stockholders' equity (deficit) | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Series A | Series B | Series 1 | Common stock | |
|
|
|||||||||||||||||||||||||||
| |
Additional paid-in capital |
Accumulated deficit |
Total stockholders' equity (deficit) |
|||||||||||||||||||||||||||||||
| |
Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | ||||||||||||||||||||||||||
Balance at January 1, 2013 |
720,002 | $ | 1,569,764 | 955,253 | $ | 1,358,817 | — | $ | — | 490,760 | $ | 49 | $ | 108,432 | $ | (3,668,936 | ) | $ | (3,560,455 | ) | ||||||||||||||
Issuance of Series B redeemable convertible preferred stock, net of stock issuance costs of $3,040 |
— | — | 42,856 | 71,958 | — | — | — | — | — | — | — | |||||||||||||||||||||||
Accretion of redeemable convertible preferred stock to redemption value |
— | 76,415 | — | 85,419 | — | — | — | — | (108,432 | ) | (53,402 | ) | (161,834 | ) | ||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | — | (636,790 | ) | (636,790 | ) | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2013 |
720,002 | 1,646,179 | 998,109 | 1,516,194 | — | — | 490,760 | 49 | — | (4,359,128 | ) | (4,359,079 | ) | |||||||||||||||||||||
Issuance of Series B redeemable convertible preferred stock, net of stock issuance costs of $3,089 |
— | — | 250,000 | 347,411 | — | — | — | — | — | — | — | |||||||||||||||||||||||
Accretion of redeemable convertible preferred stock to redemption value |
— | 25,443 | — | 62,511 | — | — | — | — | (87,954 | ) | — | (87,954 | ) | |||||||||||||||||||||
Issuance of common stock (pre recapitalization) |
— | — | — | — | — | — | 74,923 | 7 | 125,360 | — | 125,367 | |||||||||||||||||||||||
Forfeiture of common stock (pre recapitalization) |
— | — | — | — | — | — | (359,042 | ) | (36 | ) | 36 | — | — | |||||||||||||||||||||
Recapitalization transactions (note 7) |
(720,002 | ) | (1,671,622 | ) | (1,248,109 | ) | (1,926,116 | ) | 720,002 | 3,597,777 | 591,230 | 778 | 791,183 | — | 791,961 | |||||||||||||||||||
Issuance of Series 1 convertible preferred stock, net of offering costs of $289,878 |
— | — | — | — | 6,244,051 | 12,925,034 | — | — | — | — | — | |||||||||||||||||||||||
Issuance of common stock (post recapitalization) |
— | — | — | — | — | — | 693,661 | 694 | 1,146,913 | — | 1,147,607 | |||||||||||||||||||||||
Forfeiture of common stock (post recapitalization) |
— | — | — | — | — | — | (41,667 | ) | (42 | ) | 42 | — | — | |||||||||||||||||||||
Issuance of restricted stock |
— | — | — | — | — | — | 579,882 | 580 | (580 | ) | — | — | ||||||||||||||||||||||
Net loss |
— | — | — | — | — | — | — | — | — | (5,669,577 | ) | (5,669,577 | ) | |||||||||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Balance at December 31, 2014 |
— | $ | — | — | $ | — | 6,964,053 | $ | 16,522,811 | 2,029,747 | $ | 2,030 | $ | 1,975,000 | $ | (10,028,705 | ) | $ | (8,051,675 | ) | ||||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to financial statements.
F-5
ZYNERBA PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
| |
Years ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
Cash flows from operating activities: |
|||||||
Net loss |
$ | (636,790 | ) | $ | (5,669,577 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: |
|||||||
Depreciation |
49,392 | 27,063 | |||||
Forgiveness of accounts payable |
— | (180,782 | ) | ||||
Loss on disposal of equipment |
— | 22,550 | |||||
Common stock issued for services |
— | 2,063,565 | |||||
Changes in operating assets and liabilities: |
|||||||
Grant receivables |
102,120 | 34,514 | |||||
Prepaid expenses and other assets |
(1,599,580 | ) | 437,691 | ||||
Deferred grant revenue |
1,838,334 | (641,321 | ) | ||||
Accounts payable |
86,233 | 140,105 | |||||
Accrued expenses |
42,583 | 225,537 | |||||
| | | | | | | | |
Net cash used in operating activities |
(117,708 | ) | (3,540,655 | ) | |||
| | | | | | | | |
Cash flows from investing activities: |
|||||||
Purchases of property and equipment |
(2,703 | ) | (19,717 | ) | |||
| | | | | | | | |
Net cash used in investing activities |
(2,703 | ) | (19,717 | ) | |||
| | | | | | | | |
Cash flows from financing activities: |
|||||||
Proceeds from issuance of Series 1 convertible preferred stock, net |
— | 12,925,034 | |||||
Proceeds from issuance of Series B redeemable convertible preferred stock, net |
71,958 | 309,911 | |||||
Proceeds from stock subscription advances |
37,500 | — | |||||
Proceeds from issuance of common stock |
— | 1,409 | |||||
Payments on note payable |
— | (499,996 | ) | ||||
| | | | | | | | |
Net cash provided by financing activities |
109,458 | 12,736,358 | |||||
| | | | | | | | |
Net (decrease)increase in cash and cash equivalents |
(10,953 | ) | 9,175,986 | ||||
Cash and cash equivalents at beginning of year |
165,648 | 154,695 | |||||
| | | | | | | | |
Cash and cash equivalents at end of year |
$ | 154,695 | $ | 9,330,681 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Supplemental disclosures of cash flow information: |
|||||||
Accrued dividends on redeemable convertible preferred stock |
$ | 88,681 | $ | 48,078 | |||
Accretion of redeemable convertible preferred stock |
73,153 | 39,876 | |||||
Deferred offering costs included in accounts payable and accrued expenses |
— | 1,080,199 | |||||
Cash paid for interest |
2,378 | 1,920 | |||||
See accompanying notes to financial statements.
F-6
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS
DECEMBER 31, 2013 AND 2014
(1) Nature of Business and Liquidity
Zynerba Pharmaceuticals, Inc. (the Company) is a specialty pharmaceutical company focused on developing and commercializing proprietary next-generation synthetic cannabinoid therapeutics formulated for transdermal delivery. The Company was incorporated on January 31, 2007 under the laws of the State of Delaware as AllTranz, Inc. and changed its name to Zynerba Pharmaceuticals, Inc. in August 2014. The Company operated in Lexington, Kentucky until October 2014 when it moved its operations to Radnor, Pennsylvania.
The Company has incurred losses and negative cash flows from operations since inception and had an accumulated deficit of $10.0 million as of December 31, 2014. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant revenues from its product candidates currently in development. The Company's primary source of liquidity to date has been the issuance of convertible promissory notes and equity securities. Management believes that the cash and cash equivalents as of December 31, 2014 are sufficient to fund operations through the third quarter of 2016. Substantial additional financing will be needed by the Company to fund its operations and to commercially develop its product candidates. There is no assurance that such financing will be available when needed or on acceptable terms.
Management is currently evaluating different strategies to obtain the required funding of future operations. These strategies may include, but are not limited to: additional funding from current or new investors, borrowings of debt, and/or an initial public offering (IPO) of the Company's common stock. There can be no assurance that these future funding efforts will be successful.
The Company is subject to those risks associated with any specialty pharmaceutical company that has substantial expenditures for research and development. There can be no assurance that the Company's research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition the Company operates in an environment of rapid technological change, and is largely dependent on the services of its employees and consultants.
(2) Summary of Significant Accounting Policies
- (a)
- Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
- (b)
- Fair Value of Financial Instruments
The carrying amounts of the Company's financial instruments, including cash equivalents, grant receivables, accounts payable, accrued expenses and notes payable approximate fair value given their short-term nature.
- (c)
- Cash and Cash Equivalents
The Company considers all highly liquid investments that have maturities of three months or less when acquired to be cash equivalents. As of December 31, 2013 and 2014, the Company invested a portion of its cash balances in money market funds, which has been included as cash equivalents on the balance sheets.
F-7
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(2) Summary of Significant Accounting Policies (Continued)
- (d)
- Prepaid Expenses and Other Assets
Prepaid expenses and other assets primarily consist of prepaid preclinical trial expenses of $491,453 and $1,120,125 respectively, as of December 31, 2013. Prepaid expenses primarily consist of prepaid preclinical trial expenses of $1,120,125 as of December 31, 2014.
- (e)
- Property and Equipment
Property and equipment are recorded at cost and are depreciated on a straight-line basis over their estimated useful lives. The Company estimates a life of three years for computer equipment and five years for furniture and fixtures and lab equipment. When property and equipment are sold or otherwise disposed of, the cost and related accumulated depreciation are removed from the accounts and the resulting gain or loss is included in operating expenses. Repairs and maintenance costs are expensed as incurred.
- (f)
- Impairment of Long-Lived Assets
The Company assesses the recoverability of its long-lived assets, which include property and equipment, whenever significant events or changes in circumstances indicate impairment may have occurred. If indicators of impairment exist, projected future undiscounted cash flows associated with the asset are compared to its carrying amount to determine whether the asset's value is recoverable. Any resulting impairment is recorded as a reduction in the carrying value of the related asset in excess of fair value and a charge to operating results. For the years ended December 31, 2013 and 2014, the Company determined that there was no impairment of its long-lived assets.
- (g)
- Research and Development
Research and development costs are expensed as incurred and are primarily comprised of external research and development expenses incurred under arrangements with third parties, such as contract research organizations (CROs), consultants and employee related expenses including salaries and benefits.
- (h)
- Stock-Based Compensation
The Company measures employee and nonemployee stock-based awards at grant-date fair value and records compensation expense on a straight-line basis over the vesting period of the award. Stock-based awards issued to non-employees are revalued until the award vests.
Estimating the fair value of stock-based payment awards requires the input of subjective assumptions, including the fair value of the Company's common stock, and, for stock options, the expected life of the options and stock price volatility. The Company uses the Black-Scholes option pricing model to value its stock option awards. The assumptions used in calculating the fair value of stock-based payment awards represent management's estimates and involve inherent uncertainties and the application of management's judgment. As a result, if factors change and management uses different assumptions, stock-based compensation expense could be materially different for future awards.
- (i)
- Revenue Recognition
Revenues related to research grants and research services for third party product development are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed and determinable, and collectability is reasonably assured. Research services revenues of $87,299 and $123,242 in 2013 and 2014, respectively, represent fees for research and
F-8
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(2) Summary of Significant Accounting Policies (Continued)
development activities. The remaining revenues represent grant revenues. Grant revenues received are deferred until the related expenditures are incurred.
- (j)
- Income Taxes
The Company recognizes deferred tax assets and liabilities for temporary differences between the financial reporting basis and the tax basis of the Company's assets and liabilities and the expected benefits of net operating loss carryforwards. The impact of changes in tax rates and laws on deferred taxes, if any, applied during the years in which temporary differences are expected to be settled, is reflected in the financial statements in the period of enactment. The measurement of deferred tax assets is reduced, if necessary, if, based on weight of the evidence, it is more likely than not that some, or all, of the deferred tax assets will not be realized. As of December 31, 2013 and 2014, the Company has concluded that a full valuation allowance is necessary for their net deferred tax assets. The Company had no material amounts recorded for uncertain tax positions, interest or penalties in the accompanying financial statements.
- (k)
- Net Loss Per Share
Basic loss per share is computed by dividing net loss applicable to common stockholders by the weighted average number of shares of common stock outstanding during each period. Diluted loss per share includes the effect, if any, from the potential exercise or conversion of securities, such as redeemable convertible preferred stock, convertible preferred stock, restricted stock, and stock options, which would result in the issuance of incremental shares of common stock. In computing the basic and diluted net loss per share applicable to common stockholders, the weighted average number of shares remains the same for both calculations due to the fact that when a net loss exists, dilutive shares are not included in the calculation as the impact is anti-dilutive.
The following potentially dilutive securities outstanding as of December 31, 2013 and 2014 have been excluded from the computation of diluted weighted average shares outstanding, as they would be anti-dilutive:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
Redeemable convertible preferred stock |
913,873 | — | |||||
Convertible preferred stock |
— | 3,704,216 | |||||
Stock options |
196,726 | 542,550 | |||||
Unvested restricted stock |
— | 579,882 | |||||
| | | | | | | | |
|
1,110,599 | 4,826,648 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Amounts in the table reflect the common stock equivalents of the noted instruments.
The unaudited pro forma net loss per share is computed using the weighted average number of shares of common stock outstanding after giving effect to the conversion of all issued and outstanding shares of Series 1 convertible preferred stock into shares of common stock upon the closing of the Company's IPO, as if they had occurred at the beginning of the period, or the date of original issuance, if later.
F-9
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(2) Summary of Significant Accounting Policies (Continued)
The following table summarizes the calculation of unaudited pro forma basic and diluted net loss per share of common stock for the year ended December 31, 2014:
Numerator: |
||||
Net loss applicable to common stockholders |
$ | (5,757,531 | ) | |
Effect of pro forma adjustments: |
||||
Accretion of convertible preferred stock |
87,954 | |||
| | | | | |
Unaudited proforma net loss applicable to common stockholders |
$ | (5,669,577 | ) | |
| | | | | |
| | | | | |
| | | | | |
Denominator: |
||||
Weighted average shares of common stock outstanding |
894,575 | |||
Effect of pro forma adjustments: |
||||
Conversion of convertible preferred stock |
1,320,932 | |||
| | | | | |
Shares used in computing unaudited pro forma weighted average basic diluted shares of common stock outstanding |
2,215,507 | |||
| | | | | |
| | | | | |
| | | | | |
Unaudited pro forma basic and diluted net loss per share of common stock |
$ | (2.56 | ) | |
| | | | | |
| | | | | |
| | | | | |
- (l)
- Recapitalization
On July 22, 2015, the Board of Directors approved a reverse stock split of the Company's common stock at a ratio of one share for every 1.88 shares previously held. All common stock share and per share data included in the financial statements reflect the reverse stock split.
- (m)
- Segment Information
Operating segments are defined as components of an enterprise about which separate discrete information is available for evaluation by the chief operating decision maker, or decision-making group, in deciding how to allocate resources and in assessing performance. The Company views its operations and manages its business in one segment.
- (n)
- Recent Accounting Pronouncements
In August 2014, the FASB issued ASU No. 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern, which defines management's responsibility to assess an entity's ability to continue as a going concern, and to provide related footnote disclosures if there is substantial doubt about its ability to continue as a going concern. The pronouncement is effective for annual reporting periods ending after December 15, 2016 with early adoption permitted. The adoption of this guidance is not expected to have a material impact on the Company's financial statements.
(3) Fair Value Measurements
The Company utilizes a valuation hierarchy that prioritizes fair value measurements based on the types of inputs used for the various valuation techniques related to its financial assets and financial liabilities. The three levels of inputs used to measure fair value are described as follows:
Level 1 — Unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs and quoted prices in active markets for similar assets and liabilities.
F-10
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(3) Fair Value Measurements (Continued)
Level 3 — Unobservable inputs and models that are supported by little or no market activity.
In accordance with the fair value hierarchy described above, the following table sets forth the Company's cash equivalents measured at fair value on a recurring basis:
| |
|
Fair value measurement as of December 31, 2013 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying Value as of December 31, 2013 |
||||||||||||
| |
Level 1 | Level 2 | Level 3 | ||||||||||
Cash equivalents |
$ | 1,716 | $ | 1,716 | — | — | |||||||
| |
|
Fair value measurement as of December 31, 2014 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying Value as of December 31, 2014 |
||||||||||||
| |
Level 1 | Level 2 | Level 3 | ||||||||||
Cash equivalents |
$ | 9,004,991 | $ | 9,004,991 | — | — | |||||||
In addition, the Company considered its warrant liability (note 7) as a Level 3 financial instrument. The valuation of the liability required inputs that reflect the Company's own assumptions that are significant to the fair value measurement and unobservable. The Company utilized the Black-Scholes model to calculate the fair value of the warrant liability.
The reconciliation of the warrant liability measured at fair value on a recurring basis using significant unobservable inputs (Level 3) is as follows:
Balance at January 1, 2014 |
$ | — | ||
Additions |
87,161 | |||
Settlements |
(87,161 | ) | ||
| | | | | |
Balance at December 31, 2014 |
$ | — | ||
| | | | | |
| | | | | |
| | | | | |
(4) Property and Equipment
Property and equipment consisted of the following:
| |
|
December 31, | |||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Estimated useful life (in years) |
||||||||
| |
2013 | 2014 | |||||||
Lab Equipment |
5 | $ | 682,949 | $ | 4,325 | ||||
Computer equipment |
3 | 7,368 | 17,139 | ||||||
Furniture and Fixtures |
5 | 1,781 | 1,781 | ||||||
| | | | | | | | | | |
Total Cost |
692,098 | 23,245 | |||||||
Less accumulated depreciation |
(644,307 |
) |
(3,603 |
) |
|||||
| | | | | | | | | | |
Property and equipment, net |
$ | 47,791 | $ | 19,642 | |||||
| | | | | | | | | | |
| | | | | | | | | | |
| | | | | | | | | | |
Depreciation expense was $49,392 and $27,063 for the years ended December 31, 2013 and 2014, respectively. In September 2014, equipment with a cost of $682,949 and accumulated depreciation of $660,399 was transferred to a founder upon termination from the Company.
F-11
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(5) Accrued Expenses
Accrued expenses consisted of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
Deferred offering costs |
$ | — | $ | 1,080,199 | |||
Grants payable (note 10) |
— | 400,000 | |||||
Other |
50,322 | 231,274 | |||||
| | | | | | | | |
Total accrued expenses |
$ | 50,322 | $ | 1,711,473 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
(6) Debt
In April 2007, the Company was awarded a grant by the Kentucky Economic Development Finance Authority (KEDFA) on behalf of the Commonwealth of Kentucky Department of Commercialization and Innovation (DCI) in the form of a non interest bearing forgivable loan in the amount of up to $500,000 to be used for the purchase of equipment. The grant was subject to repayment in four annual installments equal to $125,000 and was secured by the assets purchased with the grant funds. The grant contained a provision for the forgiveness of the total loan provided the Company maintained certain employment positions in existence at the time of the award at the then average annual base salary and created 30 additional high tech employment positions at an average annual base salary of at least $80,000. Under the terms of the initial grant, these existing and new employment positions were to be created by December 31, 2012 and retained through December 31, 2015. In December 2012, KEDFA approved an extension of the deadline for creating the new employment positions to December 31, 2014, with repayment beginning in December 2014. Additionally, the grant provided for partial forgiveness had the Company not fully reached the specified employment creation. In January 2014, the Company granted KEDFA liens on certain of its patents as security for the forgivable loan.
In September 2014, the Company repaid the forgivable loan balance of $499,996 as management determined they would not meet the performance criteria and KEDFA released its security interest.
(7) Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit)
Series A and Series B Redeemable Convertible Preferred Stock
As of December 31, 2013, the Company was authorized to issue 720,002 shares of $0.0001 par value Series A Participating Preferred Stock (Series A) and 1,544,673 shares of $0.0001 par value Series B Participating Preferred Stock (Series B).
In connection with the Company's recapitalization in 2014, all Series A and Series B shares were converted to Series 1 convertible preferred stock (see below).
The Series B ranked senior to the Series A and the common stock in liquidation. The Series A and B were convertible into common stock at the option of the holder, automatically converted into common stock upon a public offering of stock with a public offering price of not less than $25,000,000, or the consent of a majority of the holders and had a liquidation value of $1.75 per share plus unpaid dividends. Dividends were cumulative, accrued beginning in June 2008 and October 2013 for the Series A and Series B, respectively, at a rate of 6% per year and became payable upon declaration by the board of directors of the Company, redemption or liquidation.
F-12
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(7) Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit) (Continued)
At the earlier of a Company Default (as defined) or August 30, 2017, the Series A and B Preferred Stock were redeemable at the option of a majority of the holders at the greater of a price per share of $1.75 plus unpaid dividends, if any, or the then fair market value. As of December 31, 2013, cumulative dividends for Series A and B of $400,585 and $16,196, respectively, were accrued (within accretion of Redeemable Convertible Preferred Stock), although the dividends were not declared as payable by the board of directors.
Total accretion of the Series A unamortized issuance cost towards redemption value was $3,930 and $1,309 for the years ended December 31, 2013 and 2014, respectively. Total accretion of the Series A dividends towards the redemption value was $72,485 and $24,134 for the years ended December 31, 2013 and 2014, respectively.
Total accretion of the Series B unamortized issuance cost and beneficial conversion feature towards the redemption value was $69,223 and $38,568 for the years ended December 31, 2013 and 2014, respectively. Total accretion of the Series B dividends towards the redemption value was $16,196 and $23,943 for the years ended December 31, 2013 and 2014, respectively.
In March 2013, the Company issued 42,856 shares of Series B for total proceeds of $74,998 less stock issuance costs of $3,040 for net proceeds to the Company of $71,958.
In December 2013, the Company received $37,500 from an investor to purchase Series B shares that were issued in January 2014.
In January 2014, the Company issued 200,002 shares of Series B and warrants to purchase 49,998 shares of Series B for total proceeds of $350,000 less stock issuance costs of $3,089 for net proceeds to the Company of $346,911. In April 2014, the warrants to purchase 49,998 shares of Series B were exercised for total proceeds to the Company of $500. Since the Series B underlying the warrants could have been redeemed for cash upon an event that is not within the Company's control, these warrants were classified as a derivative liability with changes to fair value, if any, recorded through earnings at each reporting period through the exercise date.
Recapitalization Transactions
In May 2014, a series of transactions, referred to as the Recapitalization Transactions, were executed resulting in the issuance of 720,002 shares of Series 1 convertible preferred stock (Series 1) and 797,871 shares of new common stock, as follows:
- §
- A shareholder who was an original founder of the Company elected to forfeit
359,042 shares of the original common stock. The forfeited shares were canceled and retired by the Company.
- §
- All outstanding options that were previously issued were cancelled.
- §
- The Company issued 74,923 shares of common stock to certain existing investors
and employees for total proceeds of $1,409. As a result of the issuance, the Company recognized $123,958 of noncash general and administrative expense during the year ended December 31, 2014.
- §
- Prior to the recapitalization, all outstanding shares of the Series A and the Series B were converted into shares of common stock. The Company converted 720,002 shares of Series A and 1,248,109 shares of Series B into 1,046,847 shares of common stock.
F-13
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(7) Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit) (Continued)
- §
- On May 6, 2014, the Company recapitalized. In connection with the
recapitalization, each share of common stock was exchanged into shares of Series 1 by multiplying each share of common stock issued and outstanding immediately prior to the recapitalization by
0.57434. As a result, 720,002 shares of Series 1 were issued pursuant to such exchange.
- §
- In connection with the recapitalization, 478,723 shares of new common stock were issued to a new investor (New Investor). The Company recognized $792,000 of noncash general and administrative expense during the year ended December 31, 2014 as a result of the issuance. In addition, the Company issued 319,148 shares of new common stock to the same shareholder that forfeited 359,042 shares of common stock prior to the recapitalization.
Series 1 Convertible Preferred Stock
From May 2014 through October 2014, the Company issued an aggregate of 6,244,051 shares of Series 1 for total proceeds of $13,214,912 less stock issuance costs of $289,878 for net proceeds of $12,925,034.
Authorized
Pursuant to the Amended and Restated Certificate of Incorporation of the Company filed in September 2014, the Company had 20,000,000 authorized shares of Preferred Stock, of which 7,807,502 were designated for Series 1.
Voting
Holder of the Series 1, voting as a class, shall be entitled to elect four members of the board of directors. Holders of the common stock, voting as a single class, shall be entitled to elect one member of the board of directors.
Dividends
Holders of Series 1 are entitled to receive a dividend on each outstanding share of Series 1, if and when declared by the board of directors, issuable upon the conversion of a share of Series 1 on the record date in the case of a dividend on common stock or any class or series convertible into common stock.
Liquidation
In the event of a liquidation, dissolution, or winding-up, or in the event the Company is merged with, or is acquired by another entity, the holders of each share of Series 1 shall be entitled to receive an amount equal to the greater of the $3.98 per share plus any dividends declared but unpaid thereon or such amount per share as would have been payable had all shares of Series 1 been converted to common stock immediately prior to such liquidation. With respect to the liquidation preference, after payment has been made to the holders of Series 1, the remaining assets available for distribution will be distributed to the holders of common stock.
Redemption
The Series 1 is subject to redemption under certain "deemed liquidation events" and as such, the Series 1 is considered contingently redeemable for financial accounting purposes. The Company has concluded that none of these events are probable at December 31, 2014.
Common Stock Transactions
In September 2014, the Company issued 579,614 shares of common stock to the New Investor for providing certain advisory service, including management services. The Company recognized $958,914 of noncash general and administrative expense for these issuances during the year ended December 31, 2014.
F-14
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(7) Redeemable Convertible Preferred Stock and Stockholders' Equity (Deficit) (Continued)
An additional 114,047 shares of common stock were issued to third parties for providing consulting services. As a result of the issuances, the Company recognized $188,693 of noncash general and administrative expense during the year ended December 31, 2014.
In October 2014, the New Investor forfeited 41,667 shares of common stock.
(8) Stock Option Plans
During May 2014, all outstanding stock options under the 2007 stock option plan (the 2007 Plan) were cancelled in connection with the recapitalization (note 7).
The following table summarizes the stock option activity under the 2007 Plan:
| |
Options | Weighted average exercise price per share |
Aggregate Intrinsic Value |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Balance as of January 1, 2013 |
35,722 | $ | 0.47 | |||||||
Granted |
176,073 | 0.47 | ||||||||
Forfeited |
(15,069 | ) | 0.47 | |||||||
| | | | | | | | | | | |
Balance as of December 31, 2013 |
196,726 | 0.47 | ||||||||
Cancelled |
(196,726 | ) | 0.47 | |||||||
| | | | | | | | | | | |
Balance as of December 31, 2014 |
— | $ | — | $ | — | |||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Options exercisable, December 31, 2014 |
— | $ | — | $ | — | |||||
The Company determined that the options granted during 2013 had no value as the fair value of the common stock was de minimis, and accordingly, no compensation expense was recorded related to the options granted during 2013.
In September 2014, the Company established the 2014 Omnibus Incentive Compensation Plan (the 2014 Plan), which allows for the granting of incentive stock options, nonqualified stock options, stock appreciation rights, stock awards, stock units, performance units and other stock-based awards to purchase an aggregate of 1,288,581 shares of the Company's common stock to employees, officers, directors, consultants, and advisors. In addition, the 2014 Plan provides selected executive employees with the opportunity to receive bonus awards that are considered qualified performance-based compensation.
Options issued under the 2014 Plan have a contractual life of 10 years and may be exercisable in cash or as otherwise determined by the board of directors. The Company has granted options to employees and non-employees.
In October and December 2014, the Company entered into employment contracts and agreements in connection with the hiring of its key executives and certain consultants and issued stock options to purchase 542,550 shares of common stock with an exercise price of $3.98 per share and 579,882 shares of restricted common stock that have certain performance-based and time-based vesting criteria. The stock options and restricted stock awards vest 25% upon the closing of the Company's IPO and then ratably over three years following the closing of the Company's IPO. No expense has been recorded for the 2014 stock
F-15
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(8) Stock Option Plans (Continued)
option and restricted stock grants as the vesting of the awards is contingent upon the closing of the Company's IPO.
The grant date fair value of the restricted stock was $959,361. The weighted average fair value of options granted in 2014 was estimated at $0.73 per share using the Black-Scholes option pricing model with the following assumptions: expected volatility of 76%, risk free interest rate of 2.0%, expected term of 6 years and 0% expected dividend yield.
(9) Income Taxes
As of December 31, 2013 and 2014, the Company has $2,258,972 of start-up expenses capitalized for income tax purposes. Additionally, the Company has approximately $1,380,000 and $6,913,000 of federal net operating loss carryforwards and $72,000 and $161,000 of research tax credit carry-forwards as of December 31, 2013 and 2014, respectively. The net operating loss carry-forwards and research tax credit carry-forwards begin to expire in 2028 and 2027, respectively.
The Tax Reform Act of 1986 (the Act) provides for limitation on the use of net operating loss and research and development tax credit carryforwards following certain ownership changes (as defined by the Act) that could limit the Company's ability to utilize these carryforwards. The Company may have experienced various ownership changes, as defined by the Act, as a result of past financings. Accordingly, the Company's ability to utilize the aforementioned carryforwards may be limited. Additionally, U.S. tax laws limit the time during which these carryforwards may be applied against future taxes; therefore, the Company has determined it is more likely than not that the these net operating losses will not be realized.
The components of the net deferred income tax asset as of December 31, 2013 and 2014 are as follows:
| |
2013 | 2014 | |||||
|---|---|---|---|---|---|---|---|
Deferred tax assets: |
|||||||
Net operating loss carry-forwards |
$ | 599,368 | $ | 2,411,209 | |||
Startup costs |
909,688 | 916,994 | |||||
Research and development credit carry-forwards |
72,133 | 161,174 | |||||
Deferred revenue |
— | 454,697 | |||||
| | | | | | | | |
Gross deferred tax assets |
1,581,189 | 3,944,074 | |||||
| | | | | | | | |
Deferred tax liabilities: |
|||||||
Accumulated depreciation |
(7,368 | ) | (4,262 | ) | |||
Stock-based compensation |
— | (389,438 | ) | ||||
| | | | | | | | |
Gross deferred tax liabilities |
(7,368 | ) | (393,700 | ) | |||
Less valuation allowance |
(1,573,821 | ) | (3,550,374 | ) | |||
| | | | | | | | |
Net deferred tax asset |
$ | — | $ | — | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
In assessing the realizability of deferred tax assets, the Company considers whether it is more-likely-than-not that some portion or all of the deferred tax assets will not be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which the temporary differences representing net future deductible amounts become deductible. After consideration of all the evidence, both positive and negative, the Company has recorded a full
F-16
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(9) Income Taxes (Continued)
valuation allowance against its net deferred tax assets as of December 31, 2013 and 2014, respectively, because the Company has determined that is it more likely than not that these assets will not be fully realized due to historic net operating losses incurred. The valuation allowance increased by $301,983 and $1,976,553 during the years ended December 31, 2013 and 2014, respectively, due primarily to the generation of net operating loss carryforwards during 2013 and 2014.
The Company does not have unrecognized tax benefits as of December 31, 2013 and 2014, respectively. The Company recognizes interest and penalties accrued on any unrecognized tax benefits as a component of income tax expense.
A reconciliation of income tax expense (benefit) at the statutory federal income tax rate and income taxes as reflected in the financial statements is as follows:
| |
Year ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2013 | 2014 | |||||
Federal income tax benefit at statutory rate |
34.0 | % | 34.0 | % | |||
State income tax, net of federal benefit |
6.3 | (0.3 | ) | ||||
Permanent differences |
— | (0.7 | ) | ||||
Research and development credit benefit |
7.1 | 1.6 | |||||
Change in valuation allowance |
(47.4 | ) | (34.6 | ) | |||
| | | | | | | | |
Effective income tax rate |
— | % | — | % | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The Company files income tax returns in the U.S. federal jurisdiction and various state jurisdictions. The Company's 2011 to 2014 tax years remain open and subject to examination.
(10) Commitments and Contingencies
- (a)
- Federal Grants
One U.S. Federal agency provided 91% and 85% of grant revenues during the years ended December 31, 2013 and 2014, respectively.
As of December 31, 2013 and 2014, there was $1,596,512 and $1,120,125, respectively, included in deferred revenue and $1,566,848 and $1,120,125, respectively, included in prepaid expenses and other assets for prepaid research and development contracts related to the federal grant "ARRA — Transdermal Cannabinoid Prodrug Treatment for Cannabis Withdrawal and Dependence."
During 2007 through 2013, the Company received an aggregate amount of $400,000 related to grants received from the Kentucky Science and Technology Corporation (KSTC), a shareholder of the Company. As part of the grant agreement, the Company was required to have its principal office of operations and 51% of its property and payroll located in the state of Kentucky for at least 60 months after the final disbursement of grant funds, which occurred in October 2013. Upon transfer of the Company's office of operations to another state, a default would occur and all funds received prior to the date of default would be required to be repaid to KSTC. As a result of this contingency, the Company recorded the $400,000 in grant funds as deferred revenue at December 31, 2013. Due to the Company's relocation to Pennsylvania in 2014, this
F-17
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(10) Commitments and Contingencies (Continued)
amount is reflected in accrued expense at December 31, 2014. In February 2015, the Company repaid the KSTC grants in full.
- (b)
- Research and Development Agreement
In August 2014, the Company entered into a patent assignment consideration agreement with Albany College of Pharmacy (ACP) pursuant to which the Company paid $500,000 in exchange for the termination of a royalty agreement that was executed in December 2004. This payment was expensed and included in general and administrative expenses for the year ended December 31, 2014.
- (c)
- Employee Retirement Plan
The Company has established a retirement plan authorized by section 401(k) of the Internal Revenue Code. In accordance with the plan, all employees are eligible to participate in the plan after one year of full-time employment and attainment of age 21 or older. Each employee can contribute a percentage of compensation up to a maximum of the statutory limits per year. The Company has the option to make discretionary matching contributions and profit sharing contributions. The Company made no contributions during 2013 and 2014.
- (d)
- Leases
The Company has entered into lease agreements for office and laboratory space. The Company's current lease for $3,500 per month expires on May 31, 2015. The Company executed a new five-year lease in February 2015 with minimum lease commitments of $56,294 for the remainder of 2015, $97,631 for 2016, $99,563 for 2017, $101,488 for 2018 and $146,845 for 2019 and thereafter.
Total lease expense during the years ended December 31, 2013 and 2014 was $52,665 and $63,880, respectively.
- (e)
- Employment Agreements
The Company has entered into employment contracts with its officers and certain employees that provide for severance and continuation of benefits in the event of termination of employment either by the Company without cause or by the employee for good reason, both as defined in the agreements. In addition, in the event of termination of employment following a change in control, as defined in the employment contracts, either by the Company without cause or by the employee for good reason, any unvested portion of the employee's stock options become immediately vested.
- (f)
- Litigation
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, penalties, and other sources are recorded when it is probable that a liability has been incurred and the amount can be reasonably estimated. The Company believes no matters will have a material impact to the Company's financial position or results of operations.
(11) Related Party Transactions
The Company paid affiliates of the New Investor $250,000 for the reimbursement of stock issuance costs during the year ended December 31, 2014. Additionally, the Company paid $250,000 for various services,
F-18
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO FINANCIAL STATEMENTS (Continued)
DECEMBER 31, 2013 AND 2014
(11) Related Party Transactions (Continued)
including management services, rendered in 2014. Furthermore, the Company paid legal expenses on the behalf of the New Investor totaling $200,000, of which, $37,366 related to stock issuance costs. In January 2015, the Company entered into a termination agreement with the New Investor pursuant to which certain agreements will be terminated upon payment of $500,000. The Company plans to pay the New Investor $500,000 prior to the effectiveness of an IPO.
(12) Subsequent Events
The Company has evaluated subsequent events from the balance sheet date through the date of the filing, the date at which the financial statements were available to be issued, and determined there are no other items requiring disclosure.
F-19
ZYNERBA PHARMACEUTICALS, INC.
BALANCE SHEETS
(UNAUDITED)
| |
December 31, 2014 |
March 31, 2015 | Pro forma March 31, 2015 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
(see Note 1) |
|||||||
Assets |
||||||||||
Current assets: |
||||||||||
Cash and cash equivalents |
$ | 9,330,681 | $ | 7,375,975 | $ | 7,375,975 | ||||
Deferred offering costs |
1,080,199 | 1,424,756 | 1,424,756 | |||||||
Prepaid expenses |
1,183,949 | 1,491,142 | 1,491,142 | |||||||
| | | | | | | | | | | |
Total current assets |
11,594,829 | 10,291,873 | 10,291,873 | |||||||
Property and equipment, net |
19,642 | 22,724 | 22,724 | |||||||
Other assets |
2,200 | 2,200 | 2,200 | |||||||
| | | | | | | | | | | |
Total assets |
$ | 11,616,671 | $ | 10,316,797 | $ | 10,316,797 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Liabilities, Convertible Preferred Stock and Stockholders' Equity (Deficit) |
||||||||||
Current liabilities: |
||||||||||
Accounts payable |
$ | 313,937 | $ | 1,518,839 | $ | 1,518,839 | ||||
Accrued expenses |
1,711,473 | 713,494 | 713,494 | |||||||
Deferred grant revenue |
1,120,125 | 1,105,297 | 1,105,297 | |||||||
| | | | | | | | | | | |
Total current liabilities |
3,145,535 | 3,337,630 | 3,337,630 | |||||||
Commitments and contingencies |
||||||||||
Series 1 convertible preferred stock; $0.001 par value; 7,807,502 shares authorized; 6,964,053 shares issued and outstanding at December 31, 2014 and March 31, 2015 (liquidation preference of $14,763,792 at March 31, 2015) |
16,522,811 | 16,522,811 | — | |||||||
| | | | | | | | | | | |
Stockholders' equity (deficit): |
||||||||||
Common stock; $0.001 par value; 50,000,000 shares authorized; 2,029,747, 2,029,747 and 5,733,963 shares issued and outstanding at December 31, 2014, March 31, 2015 and March 31, 2015 pro forma, respectively |
2,030 | 2,030 | 5,734 | |||||||
Additional paid-in capital |
1,975,000 | 1,975,000 | 18,494,107 | |||||||
Accumulated deficit |
(10,028,705 | ) | (11,520,674 | ) | (11,520,674 | ) | ||||
| | | | | | | | | | | |
Total stockholders' equity (deficit) |
(8,051,675 | ) | (9,543,644 | ) | 6,979,167 | |||||
| | | | | | | | | | | |
Total liabilities, convertible preferred stock and stockholders' equity (deficit) |
$ | 11,616,671 | $ | 10,316,797 | $ | 10,316,797 | ||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
See accompanying notes to unaudited financial statements.
F-20
ZYNERBA PHARMACEUTICALS, INC.
STATEMENTS OF OPERATIONS
(UNAUDITED)
| |
Three Months Ended March 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2015 | |||||
Revenues |
$ | 146,287 | $ | 14,828 | |||
Operating expenses: |
|||||||
Research and development |
285,725 | 853,704 | |||||
General and administrative |
99,704 | 653,773 | |||||
| | | | | | | | |
Total operating expenses |
385,429 | 1,507,477 | |||||
| | | | | | | | |
Loss from operations |
(239,142 | ) | (1,492,649 | ) | |||
Other income (expense): |
|||||||
Interest (expense) income, net |
(1,217 | ) | 680 | ||||
| | | | | | | | |
Net loss |
(240,359 | ) | (1,491,969 | ) | |||
Accretion of redeemable convertible preferred stock |
(87,954 | ) | — | ||||
| | | | | | | | |
Net loss applicable to common stockholders |
$ | (328,313 | ) | $ | (1,491,969 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Per share information: |
|||||||
Net loss per share basic and diluted |
$ | (0.67 | ) | $ | (0.74 | ) | |
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Basic and diluted weighted average shares outstanding |
490,760 | 2,029,747 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Pro forma net loss (unaudited) |
$ | (1,491,969 | ) | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Pro forma net loss per share basic and diluted (unaudited) |
$ | (0.26 | ) | ||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Pro forma basic and diluted weighted average shares outstanding (unaudited) |
5,733,963 | ||||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
See accompanying notes to unaudited financial statements.
F-21
ZYNERBA PHARMACEUTICALS, INC.
STATEMENT OF CONVERTIBLE PREFERRED STOCK, AND STOCKHOLDERS' EQUITY (DEFICIT)
THREE MONTHS ENDED MARCH 31, 2015
(UNAUDITED)
| |
|
|
Stockholders' Equity (Deficit) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Convertible Preferred Stock Series 1 |
|||||||||||||||||||||
| |
Common stock | |
|
|
||||||||||||||||||
| |
Additional paid-in capital |
Accumulated deficit |
Total stockholders' equity (deficit) |
|||||||||||||||||||
| |
Shares | Amount | Shares | Amount | ||||||||||||||||||
Balance at January 1, 2015 |
6,964,053 | $ | 16,522,811 | 2,029,747 | $ | 2,030 | $ | 1,975,000 | $ | (10,028,705 | ) | $ | (8,051,675 | ) | ||||||||
Net loss |
— | — | — | — | — | (1,491,969 | ) | (1,491,969 | ) | |||||||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
Balance at March 31, 2015 |
6,964,053 | $ | 16,522,811 | 2,029,747 | $ | 2,030 | $ | 1,975,000 | $ | (11,520,674 | ) | $ | (9,543,644 | ) | ||||||||
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | |
See accompanying notes to unaudited financial statements
F-22
ZYNERBA PHARMACEUTICALS, INC.
STATEMENTS OF CASH FLOWS
(UNAUDITED)
| |
Three Months Ended March 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2015 | |||||
Cash flows from operating activities: |
|||||||
Net loss |
$ | (240,359 | ) | $ | (1,491,969 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: |
|||||||
Depreciation |
9,635 | 1,447 | |||||
Changes in operating assets and liabilities: |
|||||||
Grant receivables |
(13,953 | ) | — | ||||
Prepaid expenses and other assets |
80 | (307,193 | ) | ||||
Deferred grant revenue |
3,036 | (14,828 | ) | ||||
Accounts payable |
(7,246 | ) | 995,093 | ||||
Accrued expenses |
(38,211 | ) | (1,097,979 | ) | |||
| | | | | | | | |
Net cash used in operating activities |
(287,018 | ) | (1,915,429 | ) | |||
| | | | | | | | |
Cash flows from investing activities: |
|||||||
Purchases of property and equipment |
— | (6,277 | ) | ||||
| | | | | | | | |
Net cash used in investing activities |
— | (6,277 | ) | ||||
| | | | | | | | |
Cash flows from financing activities: |
|||||||
Proceeds from issuance of Series B Redeemable Convertible preferred stock, net |
309,411 | — | |||||
Offering costs |
— | (33,000 | ) | ||||
| | | | | | | | |
Net cash provided by (used in) financing activities |
309,411 | (33,000 | ) | ||||
| | | | | | | | |
Net increase (decrease) in cash and cash equivalents |
22,393 | (1,954,706 | ) | ||||
Cash and cash equivalents at beginning of period |
154,695 | 9,330,681 | |||||
| | | | | | | | |
Cash and cash equivalents at end of period |
$ | 177,088 | $ | 7,375,975 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
Supplemental disclosures of cash flow information: |
|||||||
Accrued dividends on redeemable convertible preferred stock |
$ | 48,078 | $ | — | |||
Accretion of redeemable convertible preferred stock |
39,876 | — | |||||
Deferred offering costs included in accounts payable and accrued expenses |
— | 1,391,756 | |||||
Cash paid for interest |
1,257 | — | |||||
See accompanying notes to unaudited financial statements.
F-23
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO INTERIM FINANCIAL STATEMENTS
(UNAUDITED)
(1) Summary of Significant Accounting Policies
- a.
- Basis of Presentation
The accompanying unaudited interim financial statements of Zynerba Pharmaceuticals, Inc. (the Company) have been prepared in accordance with U.S. generally accepted accounting principles (U.S. GAAP) for interim financial information. In the opinion of management, the accompanying financial statements of the Company, include all normal and recurring adjustments (which consist primarily of accruals, estimates and assumptions that impact the financial statements) considered necessary to present fairly the Company's financial position as of March 31, 2015 and its results of operations and cash flows for the three months ended March 31, 2014 and March 31, 2015. Operating results for the three months ended March 31, 2015 are not necessarily indicative of the results that may be expected for the year ending December 31, 2015. The interim financial statements, presented herein, do not contain the required disclosures under U.S. GAAP for annual financial statements. The accompanying unaudited interim financial statements should be read in conjunction with the annual audited financial statements and related notes as of and for the year ended December 31, 2014.
- b.
- Liquidity
The Company has incurred losses and negative cash flows from operations since inception and has an accumulated deficit of $10.0 million and $11.5 million as of December 31, 2014 and March 31, 2015, respectively. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant revenues from its product candidates currently in development. The Company's primary source of liquidity has been the issuance of convertible promissory notes and equity securities. Management believes that the cash and cash equivalents as of March 31, 2015 are sufficient to fund operations through the third quarter of 2016. Substantial additional financings will be needed by the Company to fund its operations and to commercially develop its product candidates. There is no assurance that such financing will be available when needed or on acceptable terms.
Management is currently evaluating different strategies to obtain the required funding of future operations. These strategies may include, but are not limited to: additional funding from current or new investors, borrowings of debt, and/or an initial public offering (IPO) of the Company's common stock. There can be no assurance that these future funding efforts will be successful.
The Company is subject to those risks associated with any specialty pharmaceutical company that has substantial expenditures for research and development. There can be no assurance that the Company's research and development projects will be successful, that products developed will obtain necessary regulatory approval, or that any approved product will be commercially viable. In addition, the Company operates in an environment of rapid technological change and is largely dependent on the services of its employees and consultants.
- c.
- Unaudited Pro Forma Balance Sheet Presentation
The unaudited pro forma balance sheet as of March 31, 2015 reflects the automatic conversion of all outstanding shares of Series 1 convertible preferred stock into 3,704,216 shares of common stock upon the closing of the Company's IPO. The shares of common stock and any related estimated proceeds from the IPO are excluded from the pro forma information.
F-24
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO INTERIM FINANCIAL STATEMENTS (Continued)
(UNAUDITED)
(1) Summary of Significant Accounting Policies (Continued)
- d.
- Use of Estimates
The preparation of financial statements in conformity with U.S. generally accepted accounting principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and reported amounts of revenues and expenses during the reporting period. Actual results could differ from such estimates.
- e.
- Net Loss per Share
Basic loss per share is computed by dividing net loss applicable to common stockholders by the weighted average number of shares of common stock outstanding during each period. Diluted loss per share includes the effect, if any, from the potential exercise or conversion of securities, such as redeemable convertible preferred stock, convertible preferred stock, restricted stock, and stock options, which would result in the issuance of incremental shares of common stock. In computing the basic and diluted net loss per share applicable to common stockholders, the weighted average number of shares remains the same for both calculations due to the fact that when a net loss exists, dilutive shares are not included in the calculation as the impact is anti-dilutive.
The following potentially dilutive securities outstanding as of March 31, 2014 and 2015 have been excluded from the computation of diluted weighted average shares outstanding, as they would be anti-dilutive:
| |
March 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2014 | 2015 | |||||
Redeemable convertible preferred stock |
1,046,847 | — | |||||
Convertible preferred stock |
— | 3,704,216 | |||||
Stock options |
196,726 | 606,379 | |||||
Unvested restricted stock |
— | 579,882 | |||||
| | | | | | | | |
|
1,243,573 | 4,890,477 | |||||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
The unaudited pro forma net loss per share is computed using the weighted average number of shares of common stock outstanding after giving effect to the conversion of all issued and outstanding shares of Series 1 convertible preferred stock into shares of common stock upon the closing of the Company's IPO, as if it had occurred at the beginning of the period, or the date of original issuance, if later.
- f.
- Recapitalization
On July 22, 2015, the Board of Directors approved a reverse stock split of the Company's common stock at a ratio of one share for every 1.88 shares previously held. All common stock share and per share data included in the financial statements reflect the reverse stock split.
F-25
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO INTERIM FINANCIAL STATEMENTS (Continued)
(UNAUDITED)
(1) Summary of Significant Accounting Policies (Continued)
The following table summarizes the calculation of unaudited pro forma basic and diluted net loss per share of common stock for the year ended March 31, 2015:
Numerator: |
||||
Net loss applicable to common stockholders |
$ | (1,491,969 | ) | |
Denominator: |
||||
Weighted average shares of common stock outstanding |
2,029,747 | |||
Effect of pro forma adjustments: |
||||
Conversion of convertible preferred stock |
3,704,216 | |||
| | | | | |
Shares used in computing unaudited pro forma weighted average basic diluted shares of common stock outstanding |
5,733,963 | |||
| | | | | |
Unaudited pro forma basic and diluted net loss per share of common stock |
$ | (0.26 | ) | |
| | | | | |
| | | | | |
| | | | | |
(2) Fair Value Measurements
The Company utilizes a valuation hierarchy that prioritizes fair value measurements based on the types of inputs used for the various valuation techniques related to its financial assets and financial liabilities. The three levels of inputs used to measure fair value are described as follows:
Level 1 — Unadjusted quoted prices in active markets for identical assets or liabilities.
Level 2 — Observable inputs and quoted prices in active markets for similar assets and liabilities.
Level 3 — Unobservable inputs and models that are supported by little or no market activity
In accordance with the fair value hierarchy described above, the following table sets forth the Company's cash equivalents measured at fair value on a recurring basis:
| |
|
Fair Value Measurement as of December 31, 2014 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying value as of December 31, 2014 |
||||||||||||
| |
Level 1 | Level 2 | Level 3 | ||||||||||
Cash equivalents |
$ | 9,004,991 | $ | 9,004,991 | — | — | |||||||
| |
|
Fair Value Measurement as of March 31, 2015 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Carrying value as of March 31, 2015 |
||||||||||||
| |
Level 1 | Level 2 | Level 3 | ||||||||||
Cash equivalents |
$ | 7,200,680 | $ | 7,200,680 | — | — | |||||||
F-26
ZYNERBA PHARMACEUTICALS, INC.
NOTES TO INTERIM FINANCIAL STATEMENTS (Continued)
(UNAUDITED)
(3) Property and Equipment
Property and equipment consisted of the following:
| |
Estimated useful life (in years) |
December 31, 2014 |
March 31, 2015 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Lab Equipment |
5 | $ | 4,325 | $ | 4,325 | |||||
Computer equipment |
3 | 17,139 | 18,918 | |||||||
Furniture and Fixtures |
5 | 1,781 | 4,531 | |||||||
| | | | | | | | | | | |
Total cost |
23,245 | 27,774 | ||||||||
Less accumulated depreciation |
(3,603 | ) | (5,050 | ) | ||||||
| | | | | | | | | | | |
Property and equipment, net |
$ | 19,642 | $ | 22,724 | ||||||
| | | | | | | | | | | |
| | | | | | | | | | | |
| | | | | | | | | | | |
Depreciation expense was $9,635 and $1,447 for the three months ended March 31, 2014 and 2015, respectively.
(4) Accrued Expenses
Accrued expenses consisted of the following:
| |
December 31, 2014 |
March 31, 2015 |
|||||
|---|---|---|---|---|---|---|---|
Deferred offering costs |
$ | 1,080,199 | $ | 245,060 | |||
Grants payable |
400,000 | — | |||||
Accrued research and development |
— | 315,144 | |||||
Other |
231,274 | 153,290 | |||||
| | | | | | | | |
Total accrued expenses |
$ | 1,711,473 | $ | 713,494 | |||
| | | | | | | | |
| | | | | | | | |
| | | | | | | | |
(5) Stock-Based Compensation
Options issued under the 2014 Plan have a contractual life of 10 years and may be exercisable in cash or as otherwise determined by the board of directors. The Company has granted options to employees and non-employees.
In January 2015, the Company entered into an employment contract in connection with the hiring of an executive and issued stock options to purchase 63,829 shares of common stock with an exercise price of $3.98 per share that have certain performance-based and time-based vesting criteria. The stock options vest 25% upon the closing of the Company's IPO and then ratably over three years following the closing of the Company's IPO. No expense has been recorded for the January 2015 stock option grants as the vesting of the awards is contingent upon the closing of the Company's IPO.
The weighted average fair value of options granted in the three months ended March 31, 2015 was estimated at $0.73 per share using the Black-Scholes option pricing model with the following assumptions: expected volatility of 76%, risk free interest rate of 2.0%, expected term of 6 years and 0% expected dividend yield.
As of March 31, 2015, there are options to purchase 606,379 shares of common stock outstanding and 579,882 shares of restricted stock outstanding. None of the options or restricted stock have vested.
F-27
3,000,000 Shares

Zynerba Pharmaceuticals, Inc.
Common Stock
PRELIMINARY PROSPECTUS
Joint Book-Running Managers
Jefferies
Piper Jaffray
Co-Managers
Canaccord Genuity
Oppenheimer & Co.
, 2015
Through and including , 2015 (25 days after the date of this prospectus), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This delivery requirement is in addition to a dealer's obligation to deliver a prospectus when acting as an underwriter and with respect to their unsold allotments or subscriptions.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth all costs and expenses, other than the underwriting discounts and commissions, payable by Zynerba Pharmaceuticals, Inc. (the "Registrant") in connection with the sale of the common stock being registered. All amounts shown are estimates except for the Securities and Exchange Commission, or SEC, registration fee, the Financial Industry Regulatory Authority, Inc., or FINRA, filing fee and The NASDAQ Global Market listing fee.
| |
Amount | |||
|---|---|---|---|---|
SEC registration fee |
$ | 6,013 | ||
FINRA filing fee |
9,125 | |||
NASDAQ Global Market filing fee |
125,000 | |||
Printing expenses |
275,000 | |||
Legal fees and expenses |
1,600,000 | |||
Accounting fees and expenses |
770,000 | |||
Transfer agent and registrar fees and expenses |
7,500 | |||
Miscellaneous expenses |
7,362 | |||
| | | | | |
Total |
$ | 2,800,000 | ||
| | | | | |
| | | | | |
| | | | | |
Item 14. Indemnification of Directors and Officers.
We are incorporated under the laws of the State of Delaware. Section 145 of the Delaware General Corporation Law provides that a Delaware corporation may indemnify any persons who are, or are threatened to be made, parties to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation, or is or was serving at the request of such person as an officer, director, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys' fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation's best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was illegal. A Delaware corporation may indemnify any persons who are, or are threatened to be made, a party to any threatened, pending or completed action or suit by or in the right of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of such corporation, or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys' fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit provided that such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation's best interests except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against the expenses which such officer or director has actually and reasonably incurred. Our certificate of incorporation and bylaws, each of which will become effective upon the closing of this offering, provide for the indemnification of our directors and officers to the fullest extent permitted under the Delaware General Corporation Law.
II-1
Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duties as a director, except for liability for any:
- §
- transaction from which the director derives an improper personal benefit;
- §
- act or omission not in good faith or that involves intentional misconduct or a
knowing violation of law;
- §
- unlawful payment of dividends or redemption of shares;
or
- §
- breach of a director's duty of loyalty to the corporation or its stockholders.
Our sixth amended and restated certificate of incorporation which will become effective upon the closing of this offering includes such a provision. Expenses incurred by any officer or director in defending any such action, suit or proceeding in advance of its final disposition shall be paid by us upon delivery to us of an undertaking, by or on behalf of such director or officer, to repay all amounts so advanced if it shall ultimately be determined that such director or officer is not entitled to be indemnified by us.
As permitted by the Delaware General Corporation Law, we intend to enter into indemnification agreements with our directors and executive officers. These agreements, among other things, will require us to indemnify each director and officer to the fullest extent permitted by law and advance expenses to each indemnitee in connection with any proceeding in which indemnification is available.
At present, there is no pending litigation or proceeding involving any of our directors or executive officers as to which indemnification is required or permitted, and we are not aware of any threatened litigation or proceeding that may result in a claim for indemnification.
We have an insurance policy covering our officers and directors with respect to certain liabilities, including liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, or otherwise.
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding shares of common and preferred stock, options and warrants granted by us within the past three years that were not registered under the Securities Act. Also included is the consideration, if any, received by us for such shares, notes and options and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
(a) Issuances of Capital Stock:
(1) In October 2012, we issued 257,144 shares of our Series B Participating Preferred Stock at a purchase price of $1.75 per share, for an aggregate purchase price of approximately $450,000, less stock issue costs of approximately $40,000, for aggregate net proceeds of approximately $410,000. In addition, we issued 698,109 shares of our Series B Participating Preferred Stock in a conversion of previously outstanding convertible notes.
(2) In March 2013, we issued and sold 42,856 shares of our Series B Participating Preferred Stock at a purchase price of $1.75 per share, for an aggregate purchase price of approximately $75,000, less stock issue costs of approximately $3,000, for aggregate net proceeds of approximately $72,000.
(3) In January 2014, we issued and sold 200,002 shares of our Series B Participating Preferred Stock and warrants to purchase 49,998 shares of our Series B at a purchase price of $1.75 per share, for an aggregate purchase price of approximately $350,000, or the January 2014 Series B Issuance.
(4) In April 2014, we issued 49,998 shares of our Series B Participating Preferred Stock as a result of the exercise of warrants to purchase such preferred stock at a purchase price of $0.01 per share, for aggregate proceeds of approximately $500.
II-2
(5) In April 2014, we issued 1,046,847 shares of our common stock as a result of the conversion of all Series A and Series B Participating Preferred Stock.
(6) In May 2014, in connection with the Merger (described above in "Certain Relationships and Related Party Transactions"), we issued an aggregate of 814,502 shares of our Series 1 convertible preferred stock upon conversion of all of the outstanding shares of preferred stock held in Merger Corp and all of the outstanding shares of our common stock. Of the 814,502 shares of our Series 1 convertible preferred stock issued in connection with the Merger, 94,500 of such shares were issued to one stockholder upon conversion of shares of preferred stock in Merger Corp that was purchased by such stockholder immediately prior to the Merger for an aggregate purchase price of $200,000. Also in May 2014, in connection with the Merger, we issued 478,723 shares of our common stock to BCM Holdings and 319,148 shares of our common stock to Audra Stinchcomb.
(7) In June 2014, we issued and sold 94,500 shares of our Series 1 convertible preferred stock at a purchase price of $2.12 per share, for an aggregate purchase price of approximately $200,000.
(8) In July 2014, we issued and sold 756,000 shares of our Series 1 convertible preferred stock to a stockholder at a purchase price of $2.12 per share, for an aggregate purchase price of approximately $1.6 million.
(9) In September 2014, we issued an aggregate of 693,668 shares of common stock to three stockholders in consideration for services provided to us valued at approximately $1,148,000.
(10) In September and October 2014, we issued and sold an aggregate of 5,299,051 shares of our Series 1 convertible preferred stock at a purchase price of $2.12 per share, for an aggregate purchase price of approximately $11 million.
(b) Issuances of Warrants:
(1) In January 2014, in connection with the January 2014 Series B Issuance, we issued warrants to purchase 49,998 shares of our Series B Participating Preferred Stock with an exercise price of $0.01 per share. All of the warrants were exercised in April 2014 for $500.
(2) In May 2014, prior to the Merger, we issued warrants to purchase 74,926 shares of our common stock with an exercise price of $0.0188 per share. All of the warrants were exercised immediately following the issuance and prior to the Merger for aggregate proceeds of approximately $1,409.
(c) Restricted Stock Grants:
(1) On October 2, 2014, we issued a total of 572,427 shares of restricted common stock to four employees pursuant to our Amended and Restated 2014 Omnibus Incentive Compensation Plan, or 2014 Equity Plan.
(2) On December 15, 2014, we issued a total of 7,455 shares of restricted common stock to two employees pursuant to the 2014 Equity Plan.
(d) Stock Option Grants:
(1) On January 1, 2013, we granted stock options to purchase a total of 163,781 shares of common stock at an exercise price of $0.47 per share to nine employees, board members, service providers and grant partners pursuant to our 2009 Amended Stock Plan.
(2) On July 31, 2013, we granted stock options to purchase a total of 15,069 shares of common stock at an exercise price of $0.47 per share to four employees pursuant to our 2009 Amended Stock Plan.
II-3
(3) On October 31, 2013, we granted stock options to purchase a total of 1,861 shares of common stock at an exercise price of $0.47 per share to one employee pursuant to our 2009 Amended Stock Plan. All options outstanding prior to the Merger were terminated in connection with the Merger.
(4) On October 2, 2014, we granted stock options to purchase a total of 518,615 shares of common stock at an exercise price of $3.98 per share to four employees and a consultant pursuant to the 2014 Equity Plan.
(5) On October 9, 2014, we granted stock options to purchase a total of 7,978 shares of common stock at an exercise price of $3.98 per share to one employee pursuant to the 2014 Equity Plan.
(6) On December 15, 2014, we granted stock options to purchase a total of 15,957 shares of common stock at an exercise price of $3.98 per share to one consultant pursuant to the 2014 Equity Plan.
(7) On January 2, 2015, we granted stock options to purchase a total of 63,829 shares of common stock at an exercise price of $3.98 per share to one employee pursuant to the 2014 Equity Plan.
The offers, sales and issuances of the securities described in paragraphs (a) and (b) above, other than those described in paragraph (a)(5) were exempt from registration under the Securities Act in reliance on Regulation D of the Securities Act, relative to transactions by an issuer not involving a public offering.
The issuances of the securities described in paragraph (a)(5) above were exempt from registration under Section 3(a)(9) of the Securities Act.
The issuances of the restricted securities described in paragraph (c) above and the grants of stock options described in paragraph (d) above were exempt from registration under the Securities Act in reliance on Rule 701 as offers and sales of securities under written compensatory benefit plans and contracts relating to compensation in compliance with Rule 701. Each of the recipients of securities in any transaction exempt from registration either received or had adequate access, through employment, business or other relationships, to information about us.
All purchasers of securities in transactions exempt from registration pursuant to Regulation D described above represented to us in connection with their purchase that they were "accredited investors" and were acquiring the securities for investment purposes only and not with a view to, or for sale in connection with, any distribution thereof and that they could bear the risks of the investment and could hold the securities for an indefinite period of time. The purchasers received written disclosures that the securities had not been registered under the Securities Act and that any resale must be made pursuant to a registration statement or an available exemption from the registration requirements of the Securities Act.
All of the foregoing securities are deemed restricted securities for purposes of the Securities Act. The certificates representing the issued securities described in this Item 15 included appropriate legends setting forth that the applicable securities have not been registered and reciting the applicable restrictions on transfer. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
The list of exhibits is set forth under "Exhibit Index" at the end of this registration statement and is incorporated herein by reference herein.
(b) Financial Statement Schedules.
No financial statement schedules are provided because the information called for is not required or is shown either in the financial statements or the notes thereto.
II-4
The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-5
Pursuant to the requirements of the Securities Act, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Devon, Commonwealth of Pennsylvania, on the 23rd day of July, 2015.
| ZYNERBA PHARMACEUTICALS, INC. | ||||
| By: | /s/ ARMANDO ANIDO Armando Anido Chief Executive Officer |
|||
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ ARMANDO ANIDO Armando Anido |
Chief Executive Officer and Chairman of the Board of Directors (Principal Executive Officer) |
July 23, 2015 | ||
* Richard A. Baron |
Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) |
July 23, 2015 |
||
* Terri B. Sebree |
President |
July 23, 2015 |
||
* Philip R. Wagenheim |
Director |
July 23, 2015 |
||
* Steven Gailar |
Director |
July 23, 2015 |
||
/s/ ARMANDO ANIDO Name: Armando Anido Title: Attorney-in-Fact |
II-6
| Exhibit Number | Description of Document | |
|---|---|---|
| 1.1 | Form of Underwriting Agreement | |
| 3.1* | Amended and Restated Certificate of Incorporation, as currently in effect | |
| 3.2* | By-laws, as currently in effect | |
| 3.3 | Form of Amendment to the Amended and Restated Certificate of Incorporation | |
| 3.4 | Form of Sixth Amended and Restated Certificate of Incorporation, to be in effect upon completion of the offering | |
| 3.5 | Form of Amended and Restated By-laws, to be in effect upon completion of the offering | |
| 4.1† | Form of Common Stock Certificate | |
| 4.2* | Third Amended and Restated Stockholders Agreement, dated May 6, 2014, by and among the registrant and certain stockholders | |
| 5.1 | Opinion of Pepper Hamilton LLP | |
| 10.1* | Lock-Up Agreement, dated May 2014, by and between the registrant and Audra Stinchcomb | |
| 10.2(A)+* | Employment Agreement, dated September 4, 2014, by and between the registrant and Armando Anido | |
| 10.2(B)+* | Amendment to the Employment Agreement, dated October 2, 2014, by and between the registrant and Armando Anido | |
| 10.3+* | Employment Agreement, dated October 2, 2014, by and between the registrant and Terri B. Sebree | |
| 10.4+* | Employment Agreement, dated October 2, 2014, by and between the registrant and Suzanne M. Hanlon | |
| 10.5+* | Employment Agreement, dated January 2, 2015, by and between the registrant and Richard A. Baron | |
| 10.6* | Severance Agreement and Release of Claims, dated September 26, 2014, by and between the registrant and Audra Stinchcomb | |
| 10.7* | Patent Assignment, dated October 2, 2014, by and between the registrant and Audra Stinchcomb | |
| 10.8* | Engagement Letter, dated March 7, 2014, by and between Broadband Capital Management LLC and the registrant | |
| 10.9* | Letter agreement, dated July 16, 2014, by and between Broadband Capital Management LLC and the registrant | |
| 10.10* | Agreement and Plan of Merger, dated May 6, 2014, by and among BCM X1 Holdings, LLC, BCM Partners IV, Corp., the registrant, Audra Stinchcomb and Steven Gailar | |
| 10.11(A)* | Advisory Services Agreement, dated July 16, 2014, by and between Broadband Capital Management LLC and the registrant | |
| 10.11(B)* | Amendment No. 1 to the Advisory Services Agreement, dated September 3, 2014, by and between Broadband Capital Management LLC and the registrant | |
| 10.11(C)* | Amendment No. 2 to the Advisory Services Agreement, dated September 18, 2014, by and between Broadband Capital Management LLC and the registrant |
II-7
| Exhibit Number | Description of Document | |
|---|---|---|
| 10.12* | Stock Transfer Agreement, dated September 26, 2014, by and between Michael Rapoport, Audra Stinchcomb and the registrant | |
| 10.13* | Grant no. 5RC2DA028984-02 dated September 17, 2010, from the National Institutes of Health to the registrant | |
| 10.14* | Grant no. 1R43DA032161-01 dated July 14, 2011, from the National Institutes of Health to the registrant | |
| 10.15* | Grant no. 1RC2DA028984-01 dated September 30, 2009, from the National Institutes of Health to the registrant | |
| 10.16* | Grant no. 1RC2DA028984-01, revised award letter dated June 9, 2011, from the National Institutes of Health to the registrant | |
| 10.17* | Patent Assignment Consideration Agreement, dated August 21, 2014, by and between Albany College of Pharmacy and the registrant | |
| 10.18* | Termination and Release Agreement, dated October 1, 2014, by and between Buzzz Pharmaceuticals Ltd. and the registrant | |
| 10.19(A)+* | Amended and Restated 2014 Omnibus Incentive Compensation Plan | |
| 10.19(B)+ | Form of Amendment to Amended and Restated 2014 Omnibus Incentive Compensation Plan | |
| 10.19(C)+* | Form of Incentive Stock Option Grant under Amended and Restated 2014 Omnibus Incentive Compensation Plan | |
| 10.19(D)+* | Form of Nonqualified Stock Option Grant under Amended and Restated 2014 Omnibus Incentive Compensation Plan | |
| 10.19(E)+* | Form of Restricted Stock Grant Agreement under Amended and Restated 2014 Omnibus Incentive Compensation Plan | |
| 10.20+ | Form of Indemnification Agreement for directors and officers | |
| 10.21* | Grant Agreement No. KSTC-184-512-12-140 dated October 1, 2012 by and between Kentucky Science and Technology Corporation and the registrant | |
| 10.22* | Grant Agreement No. KSTC-184-512-11-114 dated September 29, 2011 by and between Kentucky Science and Technology Corporation and the registrant | |
| 10.23* | Grant Agreement No. KSTC-184-184-512-07-029 dated November 21, 2007 by and between Kentucky Science and Technology Corporation and the registrant | |
| 10.24* | Grant no. 5RC2DA028984-02, revised award letter dated May 9, 2012, from the National Institutes of Health to the registrant | |
| 10.25* | Termination of Letter Agreements, dated January 7, 2015, by and between Broadband Capital Management LLC and the registrant | |
| 10.26* | Lease Agreement dated February 12, 2015 by and between Provco Devon, L.L.C. and the registrant | |
| 16.1* | Letter of Mountjoy Chilton Medley LLP, as to change in accountant, dated as of January 9, 2015 | |
| 21.1 | List of subsidiaries of the registrant | |
| 23.1 | Consent of independent registered public accounting firm | |
| 23.2 | Consent of Pepper Hamilton LLP (included in Exhibit 5.1) | |
| 24.1* | Power of Attorney (included in signature page) |
II-8
| Exhibit Number | Description of Document | |
|---|---|---|
| 99.1 | Consent of Warren D. Cooper, MB, BS, BSc, MPFM | |
| 99.2 | Consent of William J. Federici | |
| 99.3 | Consent of Thomas L. Harrison, LH.D | |
| 99.4 | Consent of Daniel L. Kisner, MD | |
| 99.5 | Consent of Kenneth I. Moch | |
| 99.6 | Consent of Cynthia A. Rask, MD |
- *
- Previously
filed.
- †
- To
be filed by amendment.
- +
- Indicates management contract or compensatory plan.
II-9
