Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CYTRX CORP | d93372d8k.htm |
| EX-10.1 - EX-10.1 - CYTRX CORP | d93372dex101.htm |
| EX-10.2 - EX-10.2 - CYTRX CORP | d93372dex102.htm |
| EX-10.3 - EX-10.3 - CYTRX CORP | d93372dex103.htm |
| EX-10.4 - EX-10.4 - CYTRX CORP | d93372dex104.htm |
| EX-99.1 - EX-99.1 - CYTRX CORP | d93372dex991.htm |
| Exhibit 99.2
|
Corporate Overview
July 2015
NASDAQ: CYTR
|
|
CytRx Safe Harbor Statement
THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS THAT INVOLVE CERTAIN RISKS AND UNCERTAINTIES ASSOCIATED WITH A
DEVELOPMENT-STAGE COMPANY. ACTUAL RESULTS COULD DIFFER
MATERIALLY FROM THOSE PROJECTED IN THE FORWARD-LOOKING STATEMENTS AS A RESULT OF THE RISK FACTORS DISCUSSED IN CYTRX
REPORTS ON FILE WITH THE U.S. SECURITIES AND EXCHANGE COMMISSION INCLUDING, BUT NOT LIMITED TO, THE REPORT ON FORM
10-K FOR THE YEAR ENDED DECEMBER 31, 2014 AND FORM 10-Q FOR
THE QUARTER ENDED MARCH 31, 2015.
| 1 |
|
|
|
CytRx Investment Highlights
? Late-stage, technology-validating lead drug candidate
? Pivotal global Ph 3 (SPA) on-going in 2nd-line soft tissue sarcoma (STS)? Potential for first FDA approval in 2017? Follow-on indication: Global 2nd-line small cell lung cancer Ph 2b underway
? Proprietary LADRTM Drug Generation Platform
? Aldoxorubicin is the 1st-Generation drug using LADR (Linker-Activated Drug Release) technology? Next generation linkers can be used with a wide variety of oncology compounds (standard and high potency)? LADR technology concentrates drug release at the tumor, minimizing systemic exposure and allowing for higher doses and additional cycles
? Risk-mitigated strategy
? Creates potential blockbuster oncology therapies from a wide variety of cancer drugs, substantially reducing both development and regulatory risk
2
|
|
Seasoned R&D Team
? Daniel Levitt, MD, PhD – EVP & Chief Medical Officer
? 5 oncology drug approvals
? Formerly at Roche, Sandoz, Protein Design Labs, Affymax, Dynavax, Geron, and Cerimon
? Scott Wieland, PhD – SVP, Drug Development
? 25 years of clinical operations and regulatory experience
? Held clinical, regulatory and product development positions at CytRx, NeoTherapeutics and CoCensys
? Shanta Chawla, MD – VP, Clinical Development
? Board certified in Internal Medicine
? Spent 12 years at Spectrum Pharmaceuticals most recently as VP, Clinical Research? Formerly at Albert Einstein Medical Center and Thomas Jefferson University Hospital
? Felix Kratz, PhD – VP, Drug Discovery
? Inventor of aldoxorubicin
? >25 years of drug discovery and research; heads CytRx’s Freiburg, Germany, lab? Over 260 publications and 23 patents and patent applications
? Andre Warnecke, PhD – Sr. Director, Drug Discovery
? 17 years in chemistry research and over 23 peer-reviewed publications? PhD on albumin-binding prodrugs
3
|
|
Board of Directors
? Steven Kriegsman – Chairman and CEO
? Former CPA with KMPG in New York
? Former investment banker, business manager and healthcare investor
? Joseph Rubinfeld, PhD – Lead Director
? Bristol-Myers International – licensed original anticancer line of products ? Co-founder of Amgen and SuperGen (sold to Otsuka)
? PhD and M.A. in Chemistry from Columbia University; Received the Commonwealth Award for Innovation
? Louis Ignarro, PhD
? Nobel Prize in Medicine recipient in 1998
? PhD in Pharmacology from the University of Minnesota; B.S. in Pharmacy from Columbia University
? Cheryl Cohen
? Led the 2014 US launch of Xtandi® for metastatic castration-resistant prostate cancer generating nearly $400M in the first full year sales; current sales exceed $1B annually? Former Chief Commercial Officer at Medivation, Inc.; VP, Strategic Commercial Group at Johnson & Johnson—Direct responsibility for $1+ billion per year Remicade US rheumatoid arthritis business
? Anita Chawla, PhD
? Economist with >25 years in the health care sector; Managing Principal at Analysis Group
? Formerly Head of Health Economics & Outcomes Research at Genentech responsible for pricing and reimbursement and held positions at the American Medical Association Center for Health Policy Research
? Eric Selter, JD
? Investment advisor and principal at Morton Capital Management, a private wealth management firm? JD from Loyola Law School; B.A. from University of Southern California
4
|
|
Aldoxorubicin: A First-in-Class,, Targeted Anthracycline Using LADRTM Technology
? Superior efficacy to doxorubicin in comparative trial
? Ability to administer 3.5x more doxorubicin equivalents at each cycle? Potential to treat beyond doxorubicin’s black box dose limit
? No clinically significant cardiotoxicity to date
? Patients have received up to 12x more cumulative anthracycline with aldoxorubicin than possible with doxorubicin
? Improved quality of life in clinical trials to date
? Hair loss was less severe and not as frequent as doxorubicin? Very well tolerated at lower doses; few Grade 3/4 adverse events
? Orphan drug status granted
? USA: STS, small cell lung, glioblastoma, pancreatic & ovarian cancers? Europe: STS
5
|
|
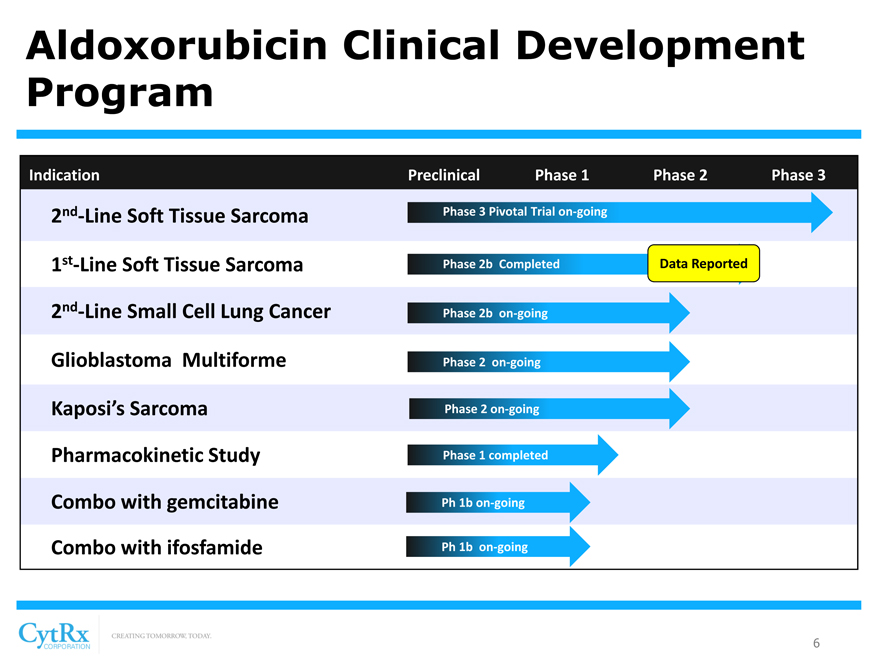
Aldoxorubicin Clinical Development Program
Indication Preclinical Phase 1 Phase 2 Phase 3
2nd-Line Soft Tissue Sarcoma Phase 3 Pivotal Trial on-going
1st-Line Soft Tissue Sarcoma Phase 2b Completed Data Reported
nd
2 -Line Small Cell Lung Cancer Phase 2b on-going Glioblastoma Multiforme Phase 2 on-going Kaposi’s Sarcoma Phase 2 on-going Pharmacokinetic Study Phase 1 completed Combo with gemcitabine Ph 1b on-going Combo with ifosfamide Ph 1b on-going
6
|
|
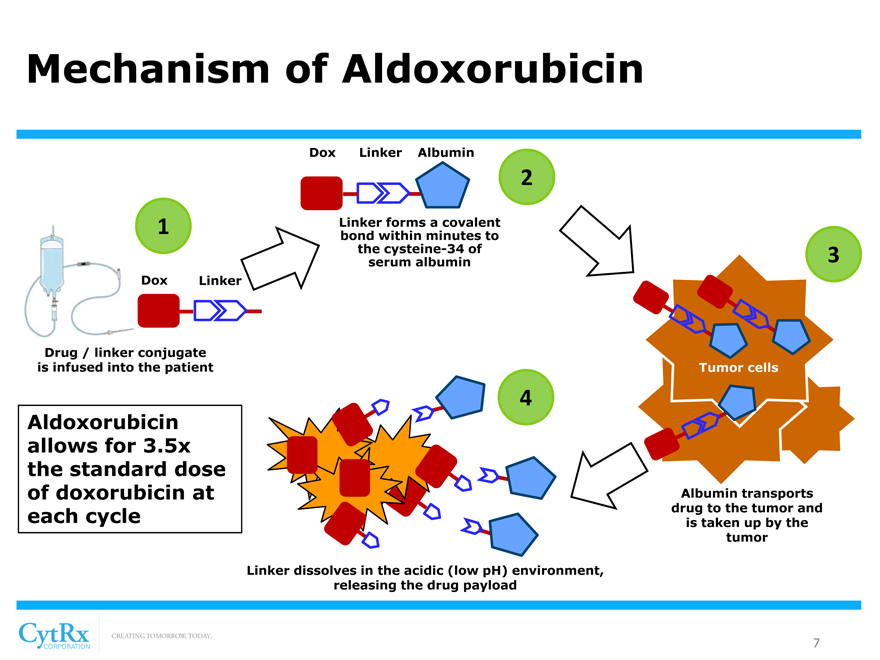
Mechanism of Aldoxorubicin
Dox Linker Albumin
2
1 Linker forms a covalent bond within minutes to the cysteine-34 of 3 serum albumin Dox Linker
Drug / linker conjugate is infused into the patient Tumor cells
4
Aldoxorubicin allows for 3.5x the standard dose of doxorubicin at Albumin transports
each cycle drug to the tumor and is taken up by the tumor
Linker dissolves in the acidic (low pH) environment, releasing the drug payload
7
|
|
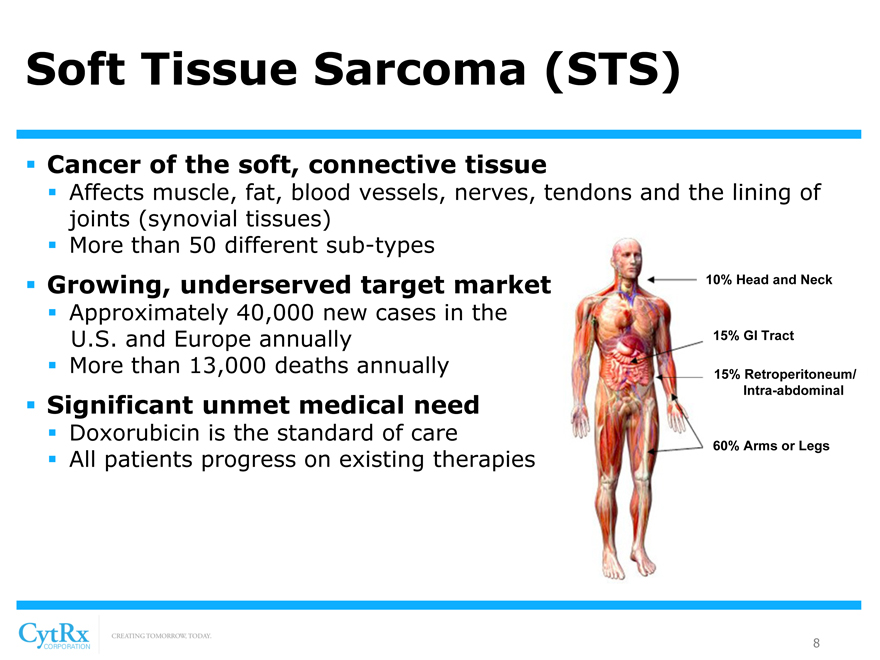
Soft Tissue Sarcoma (STS)
? Cancer of the soft, connective tissue
? Affects muscle, fat, blood vessels, nerves, tendons and the lining of joints (synovial tissues)? More than 50 different sub-types
? Growing, underserved target market 10% Head and Neck
? Approximately 40,000 new cases in the
U.S. and Europe annually 15% GI Tract? More than 13,000 deaths annually 15% Retroperitoneum/
Intra-abdominal
? Significant unmet medical need
? Doxorubicin is the standard of care
60% Arms or Legs
? All patients progress on existing therapies
8
|
|
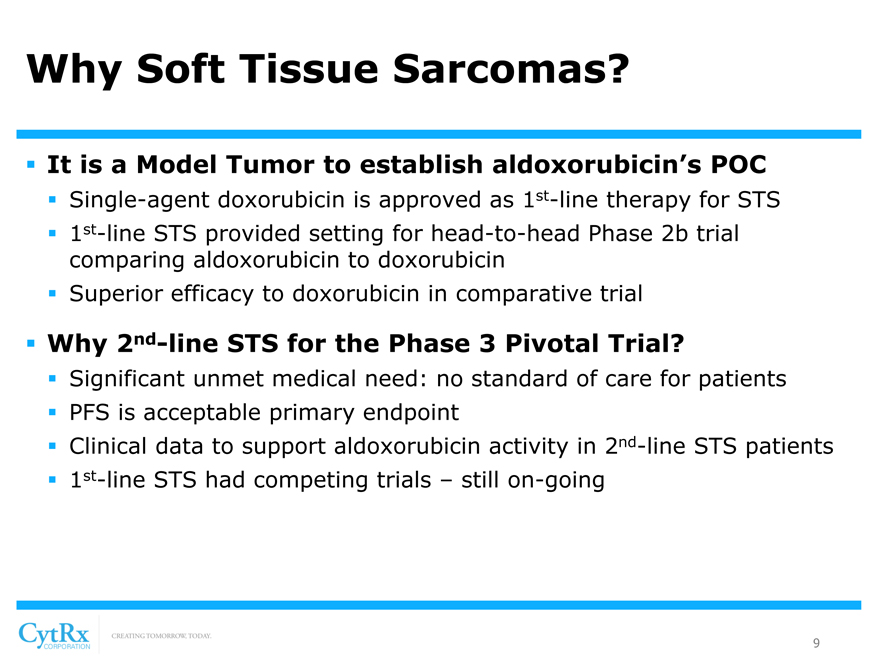
Why Soft Tissue Sarcomas?
? It is a Model Tumor to establish aldoxorubicin’s POC
? Single-agent doxorubicin is approved as 1st-line therapy for STS? 1st-line STS provided setting for head-to-head Phase 2b trial comparing aldoxorubicin to doxorubicin ? Superior efficacy to doxorubicin in comparative trial
? Why 2nd-line STS for the Phase 3 Pivotal Trial?
? Significant unmet medical need: no standard of care for patients? PFS is acceptable primary endpoint? Clinical data to support aldoxorubicin activity in 2nd-line STS patients? 1st-line STS had competing trials – still on-going
9
|
|
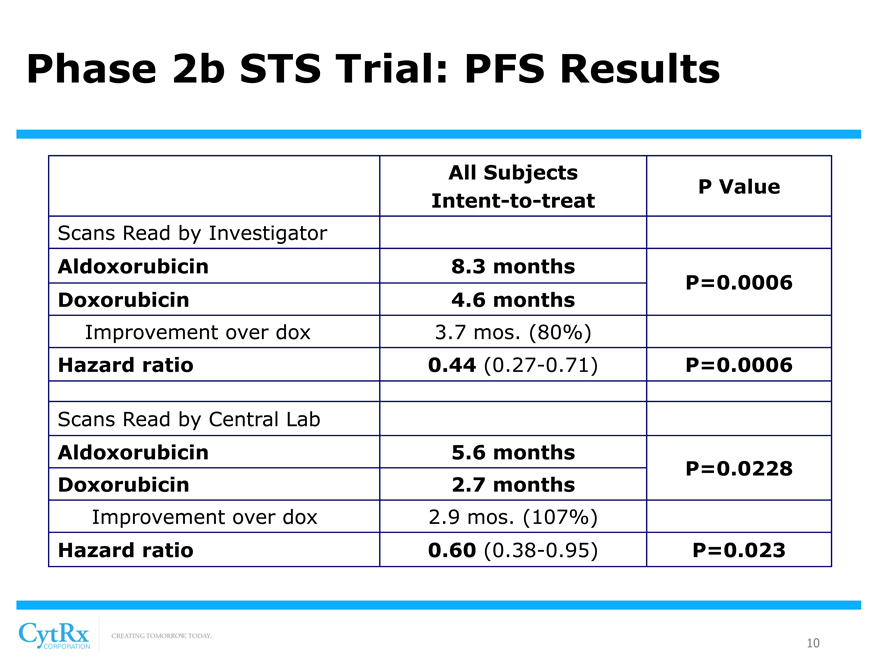
Phase 2b STS Trial: PFS Results
All Subjects
P Value Intent-to-treat
Scans Read by Investigator
Aldoxorubicin 8.3 months
P=0.0006 Doxorubicin 4.6 months
Improvement over dox 3.7 mos. (80%)
Hazard ratio 0.44 (0.27-0.71) P=0.0006
Scans Read by Central Lab
Aldoxorubicin 5.6 months
P=0.0228 Doxorubicin 2.7 months
Improvement over dox 2.9 mos. (107%)
Hazard ratio 0.60 (0.38-0.95) P=0.023
10
|
|
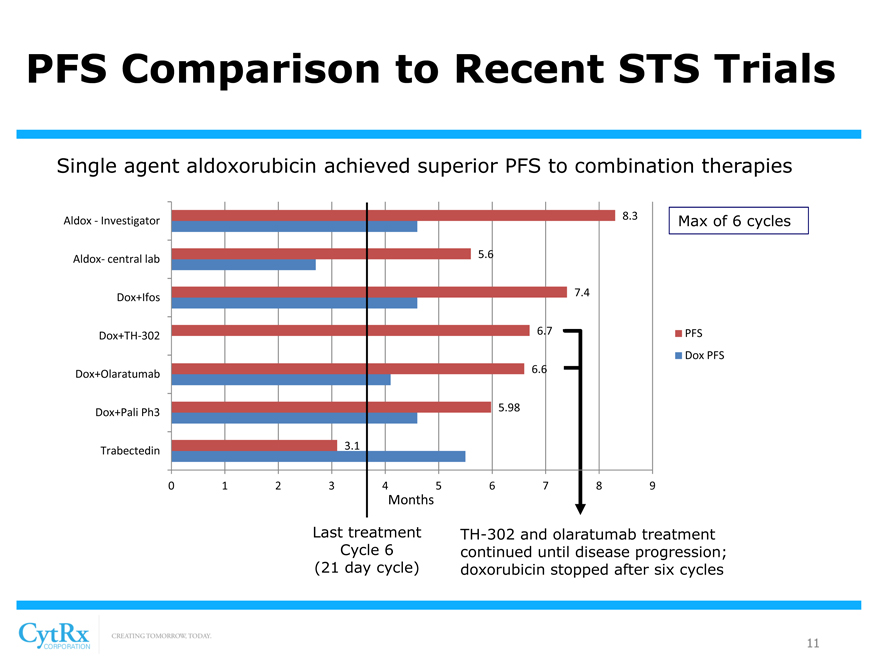
PFS Comparison to Recent STS Trials
Single agent aldoxorubicin achieved superior PFS to combination therapies
8.3
Aldox—Investigator Max of 6 cycles
5.6 Aldox- central lab
7.4 Dox+Ifos
6.7 PFS
Dox+TH-302
Dox PFS
6.6 Dox+Olaratumab
5.98 Dox+Pali Ph3
3.1 Trabectedin
0123 456789
Months
Last treatment TH-302 and olaratumab treatment Cycle 6 continued until disease progression; (21 day cycle) doxorubicin stopped after six cycles
11
|
|
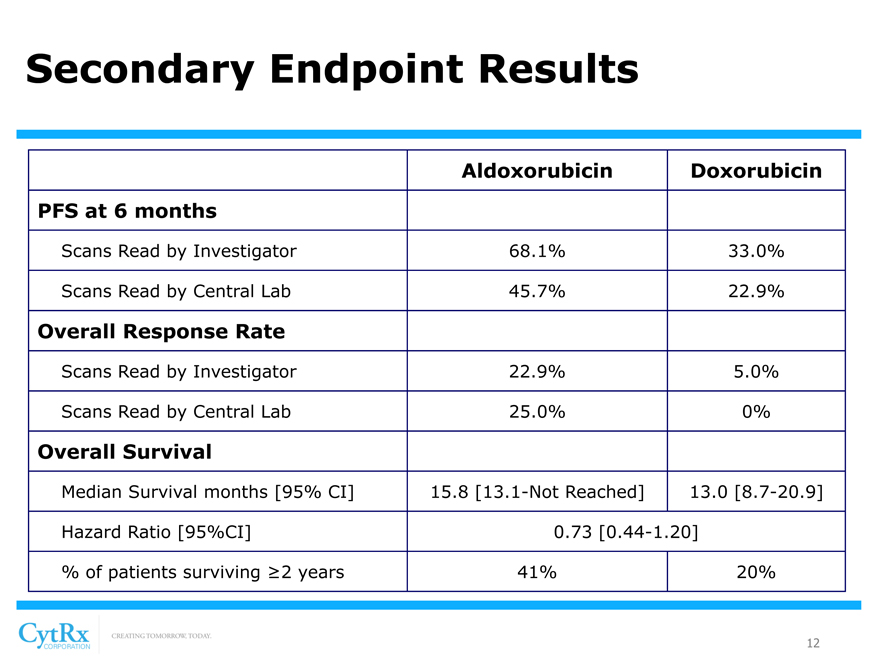
Secondary Endpoint Results
Aldoxorubicin Doxorubicin PFS at 6 months
Scans Read by Investigator 68.1% 33.0%
Scans Read by Central Lab 45.7% 22.9%
Overall Response Rate
Scans Read by Investigator 22.9% 5.0%
Scans Read by Central Lab 25.0% 0%
Overall Survival
Median Survival months [95% CI] 15.8 [13.1-Not Reached] 13.0 [8.7-20.9] Hazard Ratio [95%CI] 0.73 [0.44-1.20] % of patients surviving ?2 years 41% 20%
12
|
|
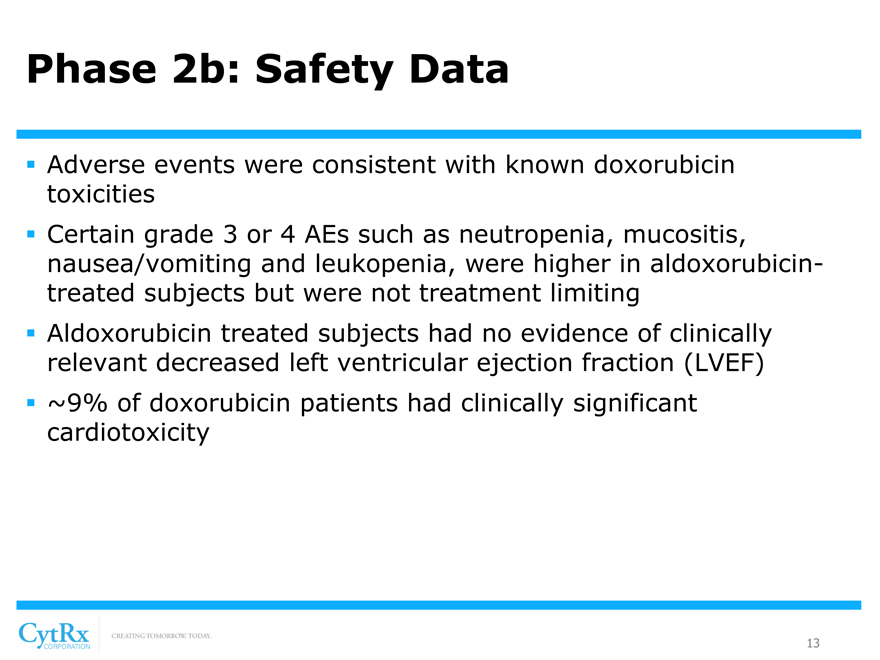
Phase 2b: Safety Data
? Adverse events were consistent with known doxorubicin toxicities? Certain grade 3 or 4 AEs such as neutropenia, mucositis, nausea/vomiting and leukopenia, were higher in aldoxorubicin-treated subjects but were not treatment limiting? Aldoxorubicin treated subjects had no evidence of clinically relevant decreased left ventricular ejection fraction (LVEF)? ~9% of doxorubicin patients had clinically significant cardiotoxicity
13
|
|
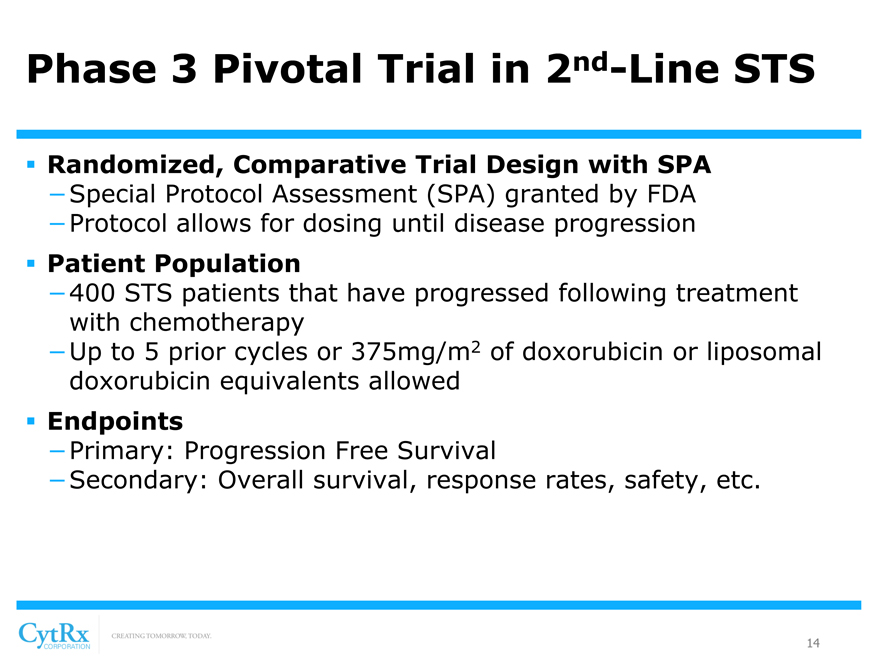
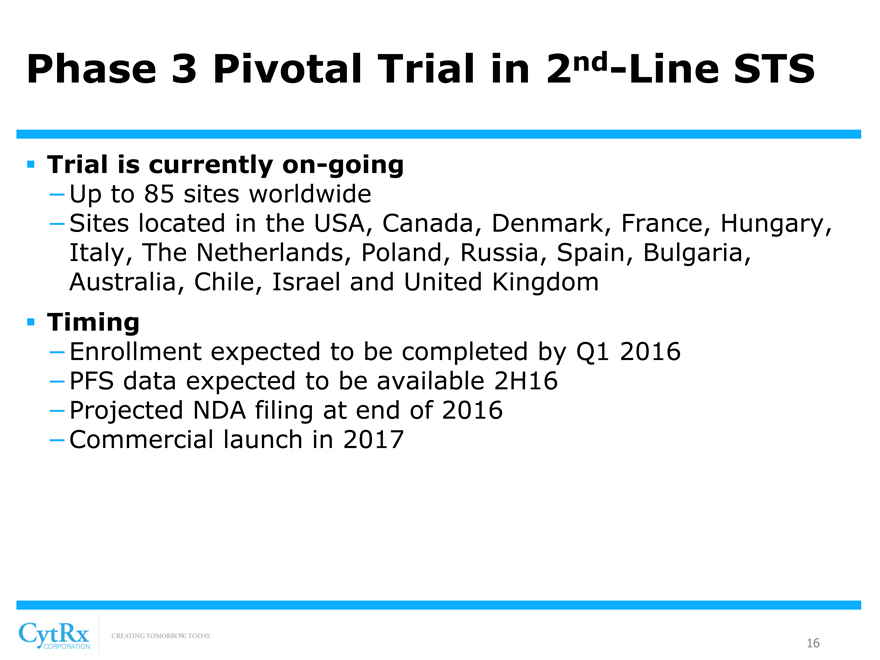
Phase 3 Pivotal Trial in 2nd-Line STS
? Randomized, Comparative Trial Design with SPA
-Special Protocol Assessment (SPA) granted by FDA
-Protocol allows for dosing until disease progression
? Patient Population
-400 STS patients that have progressed following treatment with chemotherapy
-Up to 5 prior cycles or 375mg/m2 of doxorubicin or liposomal doxorubicin equivalents allowed
? Endpoints
-Primary: Progression Free Survival
-Secondary: Overall survival, response rates, safety, etc.
14
|
|
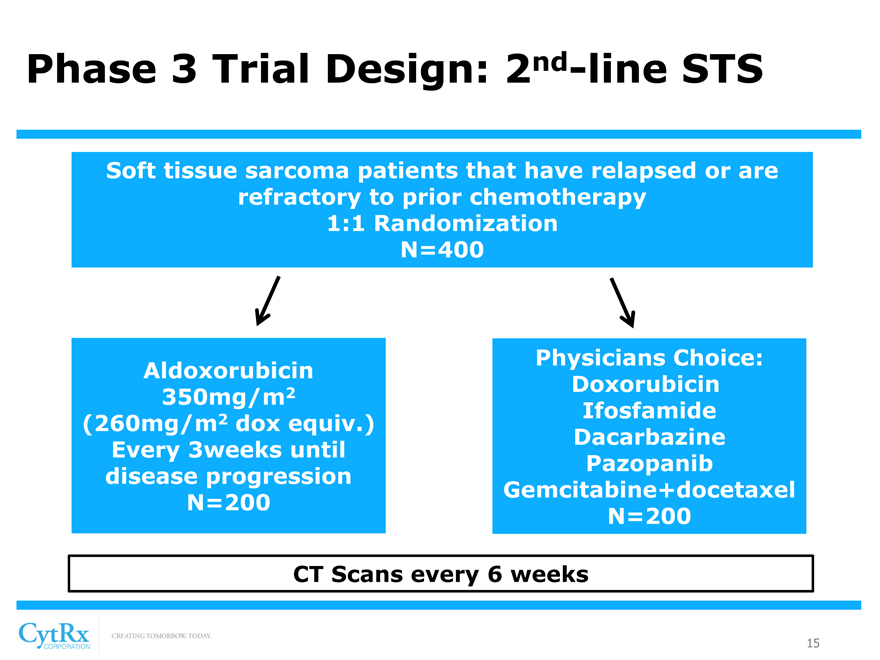
Phase 3 Trial Design: 2nd-line STS
Soft tissue sarcoma patients that have relapsed or are refractory to prior chemotherapy 1:1 Randomization N=400
[Graphic Appears Here]
[Graphic Appears Here]
Physicians Choice: Aldoxorubicin
2 Doxorubicin 350mg/m
2 Ifosfamide (260mg/m dox equiv.) Dacarbazine Every 3weeks until Pazopanib disease progression Gemcitabine+docetaxel N=200 N=200
CT Scans every 6 weeks
15
|
|
Phase 3 Pivotal Trial in 2nd-Line STS
? Trial is currently on-going
-Up to 85 sites worldwide
-Sites located in the USA, Canada, Denmark, France, Hungary, Italy, The Netherlands, Poland, Russia, Spain, Bulgaria, Australia, Chile, Israel and United Kingdom
? Timing
-Enrollment expected to be completed by Q1 2016
-PFS data expected to be available 2H16
-Projected NDA filing at end of 2016
-Commercial launch in 2017
16
|
|
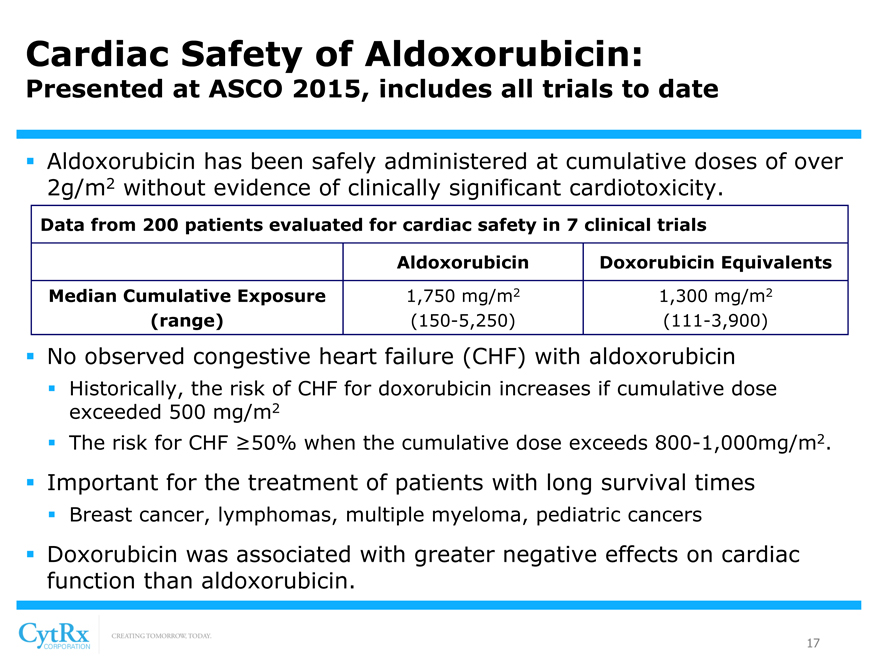
Cardiac Safety of Aldoxorubicin:
Presented at ASCO 2015, includes all trials to date
? Aldoxorubicin has been safely administered at cumulative doses of over 2g/m2 without evidence of clinically significant cardiotoxicity.
Data from 200 patients evaluated for cardiac safety in 7 clinical trials
Aldoxorubicin Doxorubicin Equivalents Median Cumulative Exposure 1,750 mg/m2 1,300 mg/m2
(range) (150-5,250) (111-3,900)
? No observed congestive heart failure (CHF) with aldoxorubicin
? Historically, the risk of CHF for doxorubicin increases if cumulative dose exceeded 500 mg/m2? The risk for CHF ?50% when the cumulative dose exceeds 800-1,000mg/m2.
? Important for the treatment of patients with long survival times
? Breast cancer, lymphomas, multiple myeloma, pediatric cancers
? Doxorubicin was associated with greater negative effects on cardiac function than aldoxorubicin.
17
|
|
Alopecia (hair loss): A hallmark side effect of doxorubicin but not Aldoxorubicin
? 92% of doxorubicin treated patients experience hair loss1
? Typically seen during 1-2 cycles at a standard dose of 75mg/m2
? Aldoxorubicin treated patients reported fewer cases of alopecia with less severity
? Not reported at all or emerges later in treatment beyond cycle 4
? Patient from Phase 1 combo trial with gemcitabine
? No hair loss through cycle 5
? Cumulative exposure to dox equivalents of 625mg/m2
[Graphic Appears Here]
1 Source: doxorubicin package insert
18
|
|
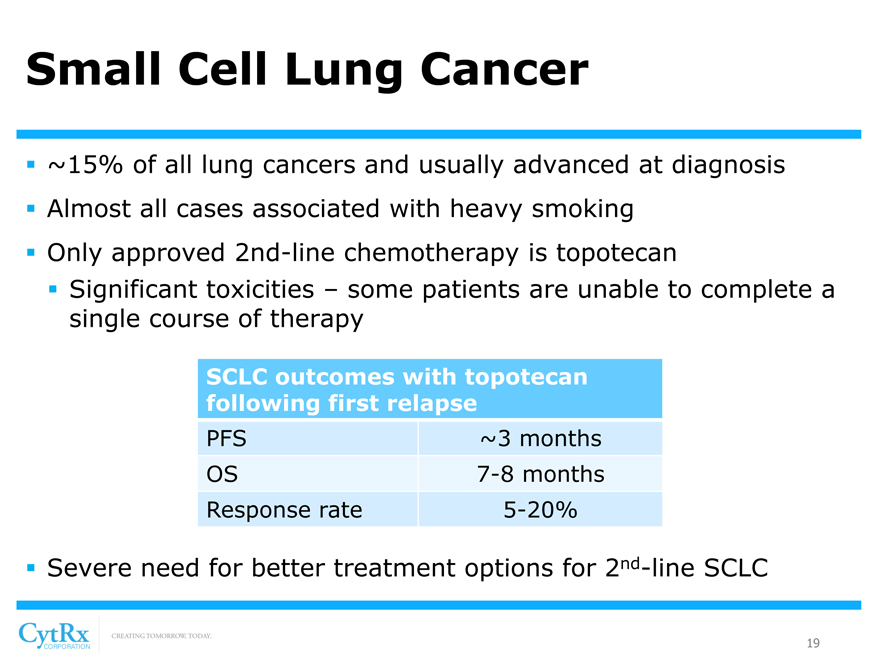
Small Cell Lung Cancer
? ~15% of all lung cancers and usually advanced at diagnosis? Almost all cases associated with heavy smoking? Only approved 2nd-line chemotherapy is topotecan? Significant toxicities – some patients are unable to complete a single course of therapy
SCLC outcomes with topotecan following first relapse
PFS ~3 months OS 7-8 months Response rate 5-20%
? Severe need for better treatment options for 2nd-line SCLC
19
|
|
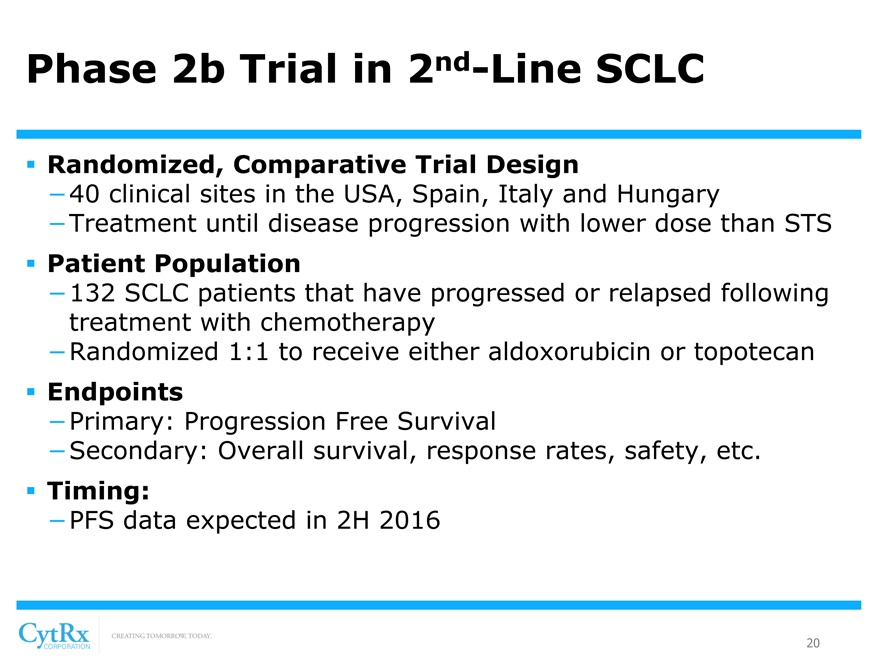
Phase 2b Trial in 2nd-Line SCLC
? Randomized, Comparative Trial Design
-40 clinical sites in the USA, Spain, Italy and Hungary
-Treatment until disease progression with lower dose than STS
? Patient Population
-132 SCLC patients that have progressed or relapsed following treatment with chemotherapy
-Randomized 1:1 to receive either aldoxorubicin or topotecan
? Endpoints
-Primary: Progression Free Survival
-Secondary: Overall survival, response rates, safety, etc.
? Timing:
-PFS data expected in 2H 2016
20
|
|
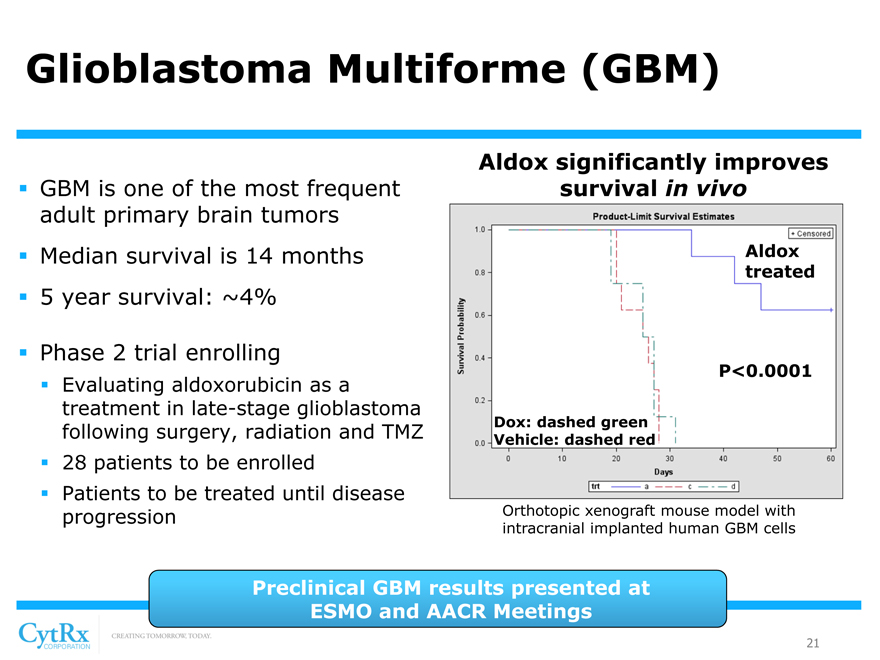
Glioblastoma Multiforme (GBM)
? GBM is one of the most frequent adult primary brain tumors? Median survival is 14 months? 5 year survival: ~4%
? Phase 2 trial enrolling
? Evaluating aldoxorubicin as a treatment in late-stage glioblastoma following surgery, radiation and TMZ? 28 patients to be enrolled? Patients to be treated until disease progression
Aldox significantly improves survival in vivo
Aldox treated
P<0.0001
Dox: dashed green Vehicle: dashed red
Orthotopic xenograft mouse model with intracranial implanted human GBM cells
Preclinical GBM results presented at ESMO and AACR Meetings
21
|
|
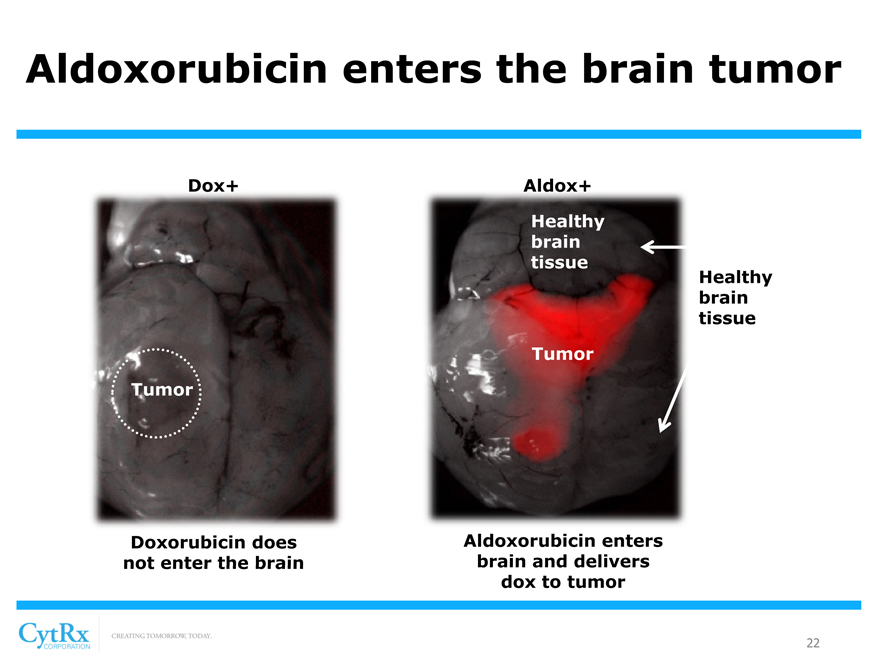
Aldoxorubicin enters the brain tumor
Dox+ Aldox+ Healthy brain tissue
Healthy brain tissue Tumor Tumor
Doxorubicin does Aldoxorubicin enters not enter the brain brain and delivers dox to tumor
22
|
|
Preliminary Ph 2 GBM Results
? Clinical activity including tumor shrinkage and prolonged stable disease seen in the first 18 patients (1-14 cycles received)? Results indicate that aldoxorubicin can cross the blood-brain barrier? Three patients had no microscopic evidence of tumor when the tissue was examined after surgery, representing a complete response
? Case report published in the Journal of Nuclear Medicine & Radiation Therapy in March ‘15
23
|
|
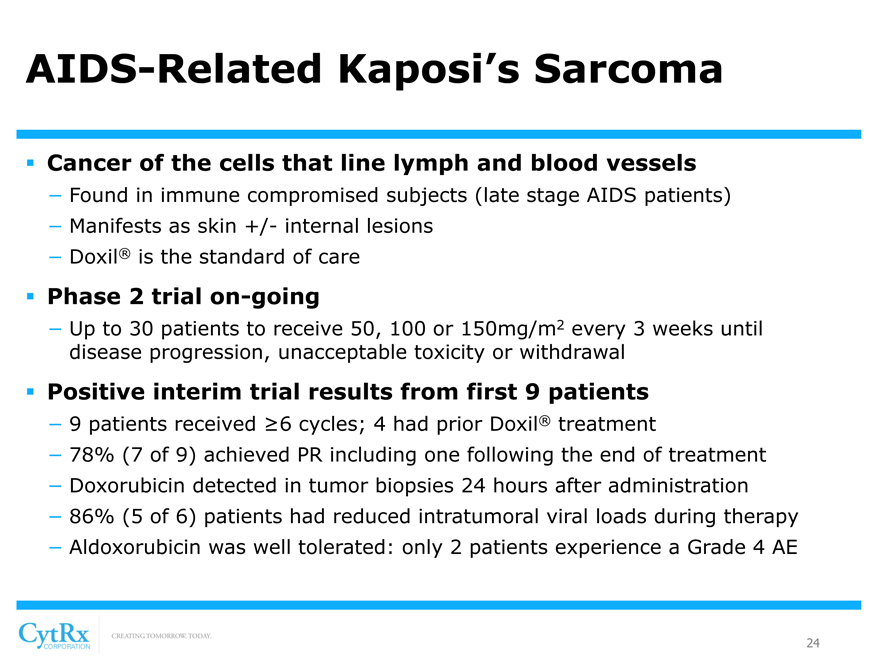
AIDS-Related Kaposi’s Sarcoma
? Cancer of the cells that line lymph and blood vessels
- Found in immune compromised subjects (late stage AIDS patients)
- Manifests as skin +/- internal lesions
- Doxil® is the standard of care
? Phase 2 trial on-going
- Up to 30 patients to receive 50, 100 or 150mg/m2 every 3 weeks until disease progression, unacceptable toxicity or withdrawal
? Positive interim trial results from first 9 patients
- 9 patients received ?6 cycles; 4 had prior Doxil® treatment
- 78% (7 of 9) achieved PR including one following the end of treatment
- Doxorubicin detected in tumor biopsies 24 hours after administration
- 86% (5 of 6) patients had reduced intratumoral viral loads during therapy
- Aldoxorubicin was well tolerated: only 2 patients experience a Grade 4 AE
24
|
|
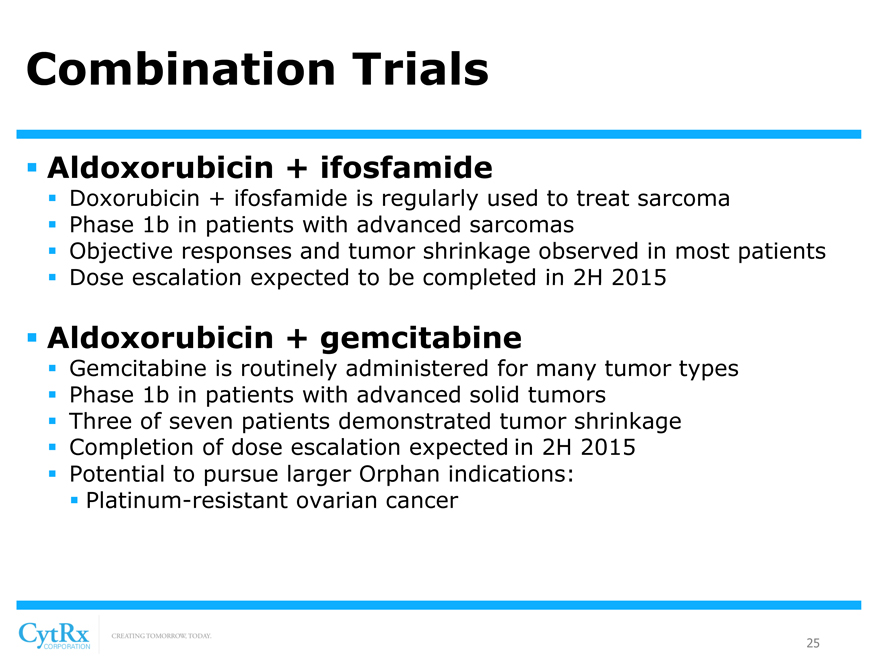
Combination Trials
? Aldoxorubicin + ifosfamide
? Doxorubicin + ifosfamide is regularly used to treat sarcoma? Phase 1b in patients with advanced sarcomas
? Objective responses and tumor shrinkage observed in most patients? Dose escalation expected to be completed in 2H 2015
? Aldoxorubicin + gemcitabine
? Gemcitabine is routinely administered for many tumor types? Phase 1b in patients with advanced solid tumors ? Three of seven patients demonstrated tumor shrinkage? Completion of dose escalation expected in 2H 2015? Potential to pursue larger Orphan indications: ? Platinum-resistant ovarian cancer
25
|
|
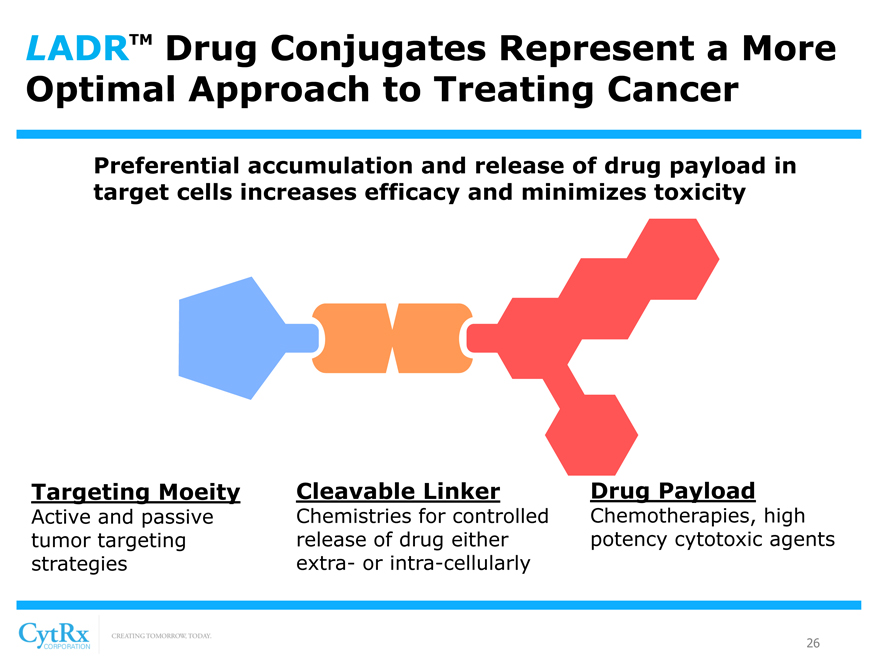
LADRTM Drug Conjugates Represent a More Optimal Approach to Treating Cancer
Preferential accumulation and release of drug payload in target cells increases efficacy and minimizes toxicity
Targeting Moeity Cleavable Linker Drug Payload
Active and passive Chemistries for controlled Chemotherapies, high tumor targeting release of drug either potency cytotoxic agents strategies extra- or intra-cellularly
26
|
|
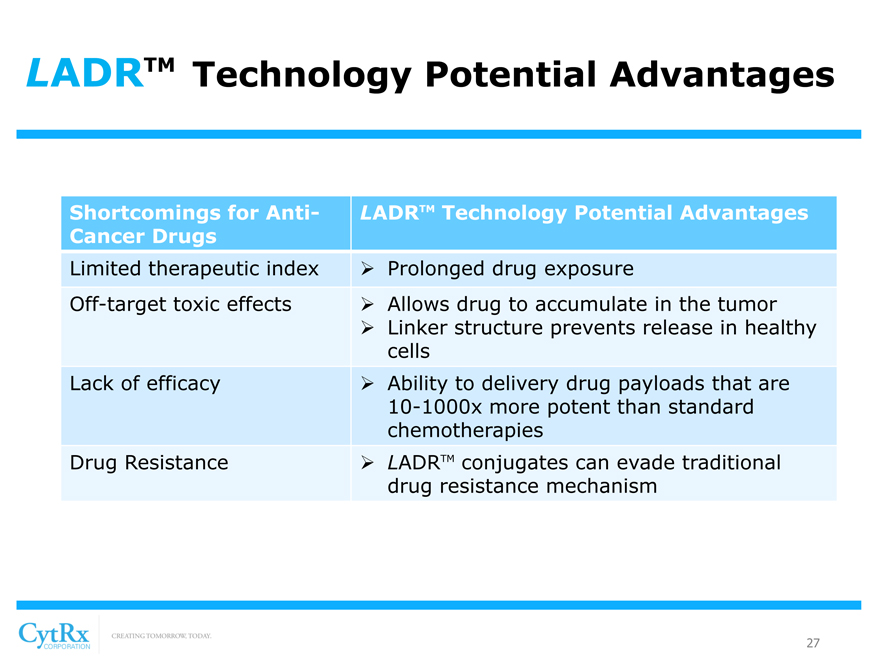
LADRTM Technology Potential Advantages
Shortcomings for Anti-Cancer Drugs
Limited therapeutic index Off-target toxic effects
Lack of efficacy
Drug Resistance
LADRTM Technology Potential Advantages
? Prolonged drug exposure
? Allows drug to accumulate in the tumor? Linker structure prevents release in healthy cells? Ability to delivery drug payloads that are
10-1000x more potent than standard chemotherapies? LADRTM conjugates can evade traditional drug resistance mechanism
27
|
|
LADRTM Technology Platform
Linker-Activated Drug Release
? Discovery engine for generating novel drug conjugates targeting albumin and antibodies (ADCs)? Leverages deep knowledge of drug-linker conjugation chemistry expertise ? Developed to minimize toxicity, overcome drug resistance and maximize anti-cancer efficacy? Drug payloads designed to be up to 1,000x more powerful than traditional cytotoxics? 2nd and 3rd generation LADR derived drug candidates expected to enter clinical development in 2016
[Graphic Appears Here]
28
|
|
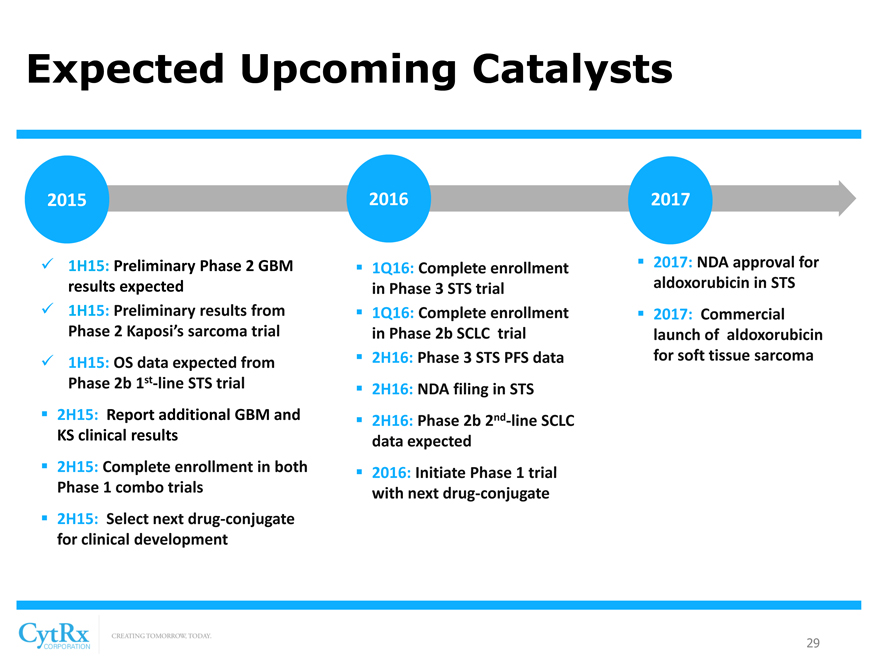
Expected Upcoming Catalysts
2015
? 1H15: Preliminary Phase 2 GBM results expected? 1H15: Preliminary results from Phase 2 Kaposi’s sarcoma trial
? 1H15: OS data expected from Phase 2b 1st-line STS trial
? 2H15: Report additional GBM and KS clinical results
? 2H15: Complete enrollment in both Phase 1 combo trials
? 2H15: Select next drug-conjugate for clinical development
2016
? 1Q16: Complete enrollment in Phase 3 STS trial? 1Q16: Complete enrollment in Phase 2b SCLC trial? 2H16: Phase 3 STS PFS data? 2H16: NDA filing in STS? 2H16: Phase 2b 2nd-line SCLC data expected
? 2016: Initiate Phase 1 trial with next drug-conjugate
2017
? 2017: NDA approval for aldoxorubicin in STS
? 2017: Commercial launch of aldoxorubicin for soft tissue sarcoma
29
|
|
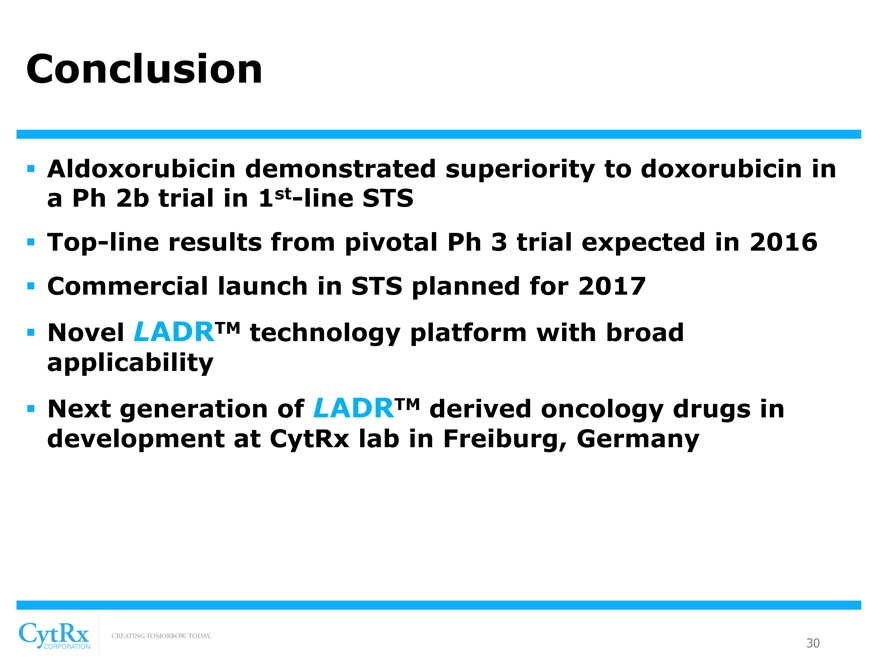
Conclusion
? Aldoxorubicin demonstrated superiority to doxorubicin in a Ph 2b trial in 1st-line STS
? Top-line results from pivotal Ph 3 trial expected in 2016 ? Commercial launch in STS planned for 2017? Novel LADRTM technology platform with broad applicability? Next generation of LADRTM derived oncology drugs in development at CytRx lab in Freiburg, Germany
30