Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Akari Therapeutics Plc | v415534_8k.htm |
Exhibit 99.1

Celsus Therapeutics Plc (to be renamed Akari Therapeutics Plc) Celsus Therapeutics (CLTX) and Volution Immuno Pharmaceuticals SA July 2015

Cautionary Note Regarding Forward - Looking Statements Certain statements in this presentation regarding the proposed business combination transaction with Volution Immuno Pharmaceuticals SA constitute "forward - looking statements" within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are us ual ly identified by the use of words such as "anticipates," "believes," "estimates," "expects," "intends," "may," "plans," "projects," "seeks," "should," "will," and vari ati ons of such words or similar expressions. We intend these forward - looking statements to be covered by the safe harbor provisions for forward - looking statements contained in Section 27A of the Securities Act and Section 21E of the Securities Exchange Act and are making this statement for purposes of complying with those safe harbor provisions. These for ward - looking statements reflect our current views about our plans, intentions, expectations, strategies and prospects, which are based on the information currently avail abl e to us and on assumptions we have made. Although we believe that our plans, intentions, expectations, strategies and prospects as reflected in or suggested by those for ward - looking statements are reasonable, we can give no assurance that the plans, intentions, expectations or strategies will be attained or achieved. Furthermore, actual re sul ts may differ materially from those described in the forward - looking statements and will be affected by a variety of risks and factors that are beyond our control. Risks and uncertainties for Celsus and Volution and of the combined company include, but are not limited to: inability to com ple te the proposed business combination transaction; liquidity and trading market for ADSs prior to and following the consummation of the proposed transaction and an y p roposed financing; costs and potential litigation associated with the proposed transaction; failure or delay in obtaining required approvals by the SEC or any other go vernmental or quasi - governmental entity necessary to consummate the proposed transaction, including our ability to file an effective proxy statement in connection wi th the proposed transaction, which may also result in unexpected additional transaction expenses and operating cash expenditures on the parties; an inability or delay in obtain ing required regulatory approvals for product candidates, which may result in unexpected cost expenditures; risks inherent in drug development in general; uncertainties in ob taining successful clinical results for product candidates and unexpected costs that may result therefrom; failure to realize any value of certain product candidates develop ed and being developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; inability to develop new product candidates and support existing products; the approval by the FDA and EMA and any other similar foreign regulatory authorities of other competing or superior products brought to market; r isk s resulting from unforeseen side effects; risk that the market for the combined company's products may not be as large as expected; inability to obtain, maintain and enforc e p atents and other intellectual property rights or the unexpected costs associated with such enforcement or litigation; inability to obtain and maintain commercial manufacturin g a rrangements with third party manufacturers or establish commercial scale manufacturing capabilities; unexpected cost increases and pricing pressures; failure to obtain the ne cessary shareholder approvals or to satisfy other conditions to the closing of the proposed transaction; uncertainties of cash flows and inability to meet working capital need s; cost reductions that may not result in anticipated level of cost savings or cost reductions prior to or after the consummation of the proposed transaction; and risks associated with the possible failure to realize certain benefits of the proposed transaction, including future financial, tax, accounting treatment, and operating results. Many of these factors th at will determine actual results are beyond Celsus's, Volution's, or the combined company's ability to control or predict. Other risks and uncertainties are more fully des cribed in periodic filings with the Securities and Exchange Commission (the "SEC"), including the factors described in the section entitled "Risk Factors" in our Quarterly Repo rt on Form 10 - Q for the quarter ended March 31, 2015 filed with the SEC, and in other filings that Celsus makes and will make with the SEC in connection with the proposed tr ans actions, including the proxy statement described below under "Important Information and Where to Find It." Existing and prospective investors are cautioned not to place undue re liance on these forward - looking statements, which speak only as of the date hereof. The statements made in this presentation speak only as of the date stated herein, and su bsequent events and developments may cause our expectations and beliefs to change. Unless otherwise required by applicable securities laws, we do not intend, nor do we und ertake any obligation, to update or revise any forward - looking statements contained in this news release to reflect subsequent information, events, results or circumstances or otherwise. While we may elect to update these forward - looking statements publicly at some point in the future, we specifically disclaim any obligation to do so, whether as a result of new information, future events or otherwise, except as required by law. Important Information and Where to Find It This communication does not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitati on of any vote or approval. In connection with the proposed transaction between Celsus and Volution, Celsus will file relevant materials with the Securities and Exchange Commis sio n (the “SEC”), including a definitive proxy statement which will be distributed to Celsus shareholders. INVESTORS AND SECURITY HOLDERS OF CELSUS ARE URGED TO READ THE PR OXY STATEMENT AND OTHER DOCUMENTS THAT WILL BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAI N I MPORTANT INFORMATION. Shareholders may obtain, free of charge, copies of the definitive proxy statement and any other documents filed by Celsus with the SEC in connection with the proposed transaction at the SEC's website (http://www.sec.gov), at Celsus's website or by writing to Dov Elefant, CFO, Celsus Th erapeutics Plc. at 24 West 40th Street, 8th Floor, New York, NY 10018. Additional information regarding the participants in the proxy solicitations and a description of their dir ect and indirect interests, by security holdings or otherwise, will be contained in the proxy statement and other relevant materials to be filed with the SEC when they become av ail able . Copyright 2015 Co nfidential 1

Combined Company Highlights • In line to become 2nd approved and best - in - class complement C5 inhibitor • Powerful inhibitor – 100% complement inhibition in Phase I trial • Evidence of efficacy equivalent to eculizumab in PNH blood model • Evidence of full complement inhibition in patients resistant to eculizumab • Once daily subcutaneous (SQ) injection provides significant patient benefit • Approved C5 inhibitor indications: Paroxysmal Nocturnal Hemoglobinuria (PNH) and atypical Hemolytic - Uremic Syndrome (aHUS) • $2.2 billion in global 2014 revenue, with rapid continuing growth expected • Expanding range of additional C5 - Inhibitor - related target indications • Initiate Phase II PNH trial in 4Q15; full data expected late 2016 • C ompassionate treatment for eculizumab - resistant patients starting late 2015 • Initiate Phase II trials in Guillain - Barré syndrome (GBS) and aHUS in 2016 • S hort duration clinical trials and potential rapid regulatory approvals Coversin Large, Proven Market Upcoming Milestones 2 Copyright 2015

Executive Chairman (Volution) • Lead investor; serial entrepreneur; founder TDL, part of Sonic (SHL) Chief Executive Officer (Celsus) • Celsus; Venrock; Piper Jaffray; Internist Chief Operating Officer (Volution) • LEK Consulting; Head of Research, Investec; Clinisys Ltd. Medical Director (Volution) • European Medical Director, BMS; 25 years development exp. Chief Scientific Officer (Volution) • Coversin inventor; expert on parasite:host interactions Chief Financial Officer (Celsus) • Lev Pharmaceuticals; EpiCept; Synvista; Tetragenix SVP, Corporate Development (Volution) • Aprecia Pharmaceuticals; McKinsey Consulting; Sandoz GmbH Proven Management Team 3 Ray Prudo MD Gur Roshwalb MD, MBA Clive Richardson Dov Elefant Michael King MBA Wynne Weston - Davies, MD Miles Nunn PhD Copyright 2015

PNH Eculizumab Resistance GBS aHUS Phase II Phase III Three Phase II Trials Initiating Within 18 Months 4 2017 2018 2015 2016 Phase II Phase III Compassionate use Phase II Phase III Ph Ib Copyright 2015

Coversin Inhibits C5 – Proven Complement Target C3 C3b Classical Mannose binding lectin Alternative Activation pathways C3 convertase Opsonisation Amplification loop Liver, Macrophages, etc. C5 convertase C3a C5b C6 C7 C9 C8 C5 Inflammation autoimmunity C3b Anaphylatoxins Lysis and cell death C5a C5a and MAC act jointly on granulocytes and many other immune and tissue cell types - potentially causing inflammation and damage Complement system tightly regulated to prevent damage to self; if normal regulation fails or autoantibodies occur, significant tissue damage may result MAC Both Coversin and eculizumab act on C5 to prevent formation of C5a and the MAC 5 LTB4 (Coversin’s secondary binding site) Copyright 2015
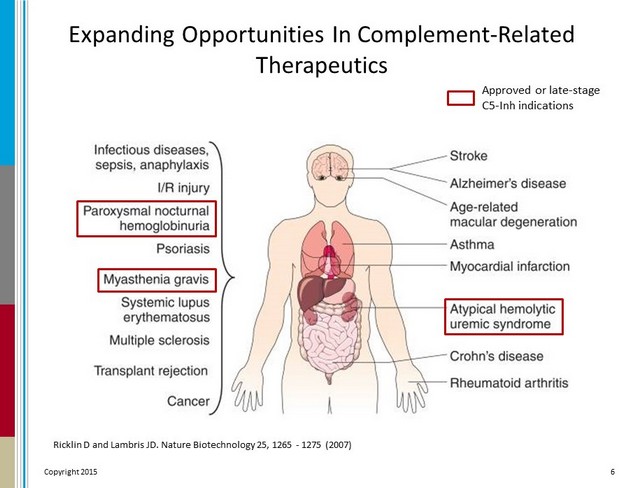
Expanding Opportunities In Complement - Related Therapeutics Ricklin D and Lambris JD. Nature Biotechnology 25, 1265 - 1275 (2007) 6 Approved or late - stage C5 - Inh indications Copyright 2015

Coversin: Best - in - Class C5 Inhibitor • Coversin : 100% inhibition in 12 hours in Phase I trial ─ Other development - stage systemic C5 inhibitor products appear to inhibit no more than ~90% • Coversin and eculizumab bind to different regions of C5 α domain of C5 ─ Eculizumab inhibits C5 lytic activity by no more than 80% in resistant patients in our studies ─ Coversin inhibits all mammalian species tested ─ Eculizumab only inhibits human C5 • Subcutaneous, once - daily administration ─ Can also be given topically or by inhalation Coversin’s clinical and in - vitro data indicates efficacy for both PNH and eculizumab - resistant subgroups 7 C345C C5a C3b Bb Eculizumab epitope Position of Coversin binding Binding Sites Copyright 2015

Coversin - Nature’s Complement Inhibitor • Coversin, a recombinant compact protein, was successfully synthesized and is now produced in E. coli by leading CMO • Manufacturing optimization and scale - up ongoing • Natural Coversin molecule works by damping down host immune responses, enabling tick to repeatedly feed without damage from inflammatory substances • Coversin derived from saliva of Ornithodoros moubata tick • Ticks have evolved to feed on the same hosts (300 million years of product development) 8 Copyright 2015

Single D ose Phase Ia Clinical Trial Demonstrated 100% Inhibition and SQ Delivery Placebo n=2 Active n=4 • Full complement ablation within 12 hours at 0.57 mg/kg • Inhibition maintained for 24 hours after single dose • Good safety profile: no SAEs or injection site reactions 9 Change from Baseline (Individual) CH50 % activity • 24 normal volunteer subjects (16 active, 8 placebo) • Only highest dosing cohort (n=6) had CH50 testing done through full 96 hour period • Single subcutaneous dose at time 0 Copyright 2015

Coversin Fully Effective in Blood of PNH Patients • Coversin and eculizumab have similar effects on lysis of PNH type III CD59 negative red blood cells • Doses above 10µg/ml Coversin and 50µg/ml eculizumab do not further inhibit lysis of CD59 negative red blood cells Acidified serum + MgCl (lysis) Acidified serum + MgCl + 50µg/ml eculizumab Acidified serum + MgCl + 10µg/ml Coversin Coversin inhibits PNH red blood cell lysis as effectively as eculizumab at a molar equivalent dose 10 PNH cells PNH cells destroyed PNH cell protected by eculizumab PNH cells protected by Coversin Heat inactivated acidified serum + MgCl (no C5, no lysis) Copyright 2015

Coversin Completely Ablated Complement Activity in Patient Unresponsive to E culizumab Serum from European patient with p.Arg885His polymorphism in C5 that confers resistance to eculizumab Resistant serum not fully inhibited by eculizumab Resistant serum and normal human serum both fully inhibited by Coversin (dashed lines). NC (solid red line) fully inhibited by eculizumab Serum with eculizumab (Ecu) S erum with Coversin (Cov) Resistant serum (BJ) Normal control serum (NC) 11 Coversin inhibits complement fully and equally well in normal control AND eculizumab - resistant serum Copyright 2015

Coversin Development Strategy 12 • PNH and aHUS regulatory approvals • Rapid and full ablation • Long term patient safety data • SQ self administration (vs. current IV) • Patients resistant to full eculizumab binding • New indications leverage Coversin’s small physical size Take Advantage of E culizumab’s Proven Success Leverage Coversin’s Differentiation Copyright 2015

PNH Phase II Data By YE 2016 • Ascending dose trial • 60 mg loading dose – then daily fixed doses for 5 days • Continuation under single CTA direct into Phase II • O pen label trial • Primary efficacy endpoint: LDH at 28 days • Secondary efficacy endpoints: Hgb , transfusions, hemoglobinuria and QOL • Projected first patient dosing by YE 2015 • Randomised comparative trial with eculizumab • Open label design; 1:1 randomisation; patients treated for six months and then enter long - term extension study • Two groups: treatment naïve and switching from eculizumab 13 2015 Phase 1b 2016 Phase II 2017 Phase III Copyright 2015

Coversin Compassionate Use Program in E culizumab - Resistant Patients • Eculizumab resistance recently identified – First identified mutation*: 3.5% of Japanese population – Ongoing efforts to determine prevalence of this & other mutations • Preclinical in - vitro validation activities – Identified and successfully tested non - Japanese cases • Clinical treatment – Demand from clinicians to treat patients on compassionate use – Treatment to begin on EU patients in 2015 – Reimbursement possible in many major markets 14 * Nishimura et al . , New England Journal of Medicine 2014 ; 370 : 632 - 9 Copyright 2015

Coversin Targeting First GBS Approval • Guillain Barré syndrome ( GBS): acute immune - mediated polyneuropathy leading to destruction of myelin sheath − Standard of care is treatment with IVIG or PE ─ Short term: 20 – 30% require me chanical ventilation ─ Long term: 2 – 12% die; 10 – 35 % have permanent impairment • Coversin demonstrated robust preclinical efficacy • Over 6,000 patients treated in US per year • Attractive clinical trial duration: 28 days + 6 month follow up ─ Primary endpoint: 1 point improvement in GBS Disability Rating Scale relative to Coversin vs. placebo added to standard of care ─ 1 point = difference between wheelchair - bound or ambulatory 15 Copyright 2015

Coversin Targeting Approval for aHUS • Complement - mediated hemolytic uremic syndrome (aHUS ): chronic and life - threatening genetic disease characterized by microangio - pathic hemolytic anemia, thrombocytopenia, and kidney injury • Estimated 10,000 patients across North America and Europe • Eculizumab received accelerated FDA approval in 2011 – Data from prospective and retrospective trials in 67 patients • Coversin open label Phase II aHUS clinical trial planned for 2H 2016 – Endpoints include normalization of hematologic parameters, kidney function and discontinuation of plasma therapy – Approximately 10 patients 16 Copyright 2015

IP Summary • 2 US and 18 foreign issued patents, expiring 2024 - 2031* • Current patent portfolio covers US, major European countries, Japan, Australia, Brazil, Canada, China , and New Zealand • Ongoing activities to further extend IP barriers to market entry in specific indications • Coversin will have orphan drug and BLA market exclusivity barriers in both US and EU for each approved indication 17 * Excluding patent term extensions Copyright 2015

1H 2017 2H 2016 1H 2016 Near - Term Milestones 2H 2015 18 • Phase Ib trial • Initiate PNH Phase II trial • Initiate treatment of eculizumab - resistant patients • Long - term toxicity studies • CMC optimization • Initial data from compassionate use program • US IND - including Orphan and Fast Track status • Initiate GBS Phase II trial • CMC scale - up • Data from PNH Phase II trial • Initiate aHUS Phase II trial • Second CMO • Initiate PNH Phase III trial • Data from GBS Phase II trial Copyright 2015

Coversin - Best - In - Class C5 Inhibitor • Coversin - Nature’s C omplement C5 Inhibitor – In line to become 2nd approved and best - in - class complement C5 inhibitor – Powerful inhibitor – 100% complement inhibition in Phase I trial – Evidence of efficacy equivalent to eculizumab in PNH blood model – Evidence of full complement inhibition in patients resistant to eculizumab – Once daily subcutaneous (SQ) injection provides significant patient benefit • Multiple near - term clinical readouts within 18 months – Initiate Phase II PNH trial in 4Q15; full data expected late 2016 – Compassionate treatment for eculizumab - resistant patients starting late 2015 – Initiate Phase II trials in Guillain - Barré syndrome (GBS) and aHUS in 2016 – Short duration clinical trials and rapid regulatory approvals 19 Copyright 2015

Celsus Therapeutics Plc (to be renamed Akari Therapeutics Plc) Celsus Therapeutics (CLTX) and Volution Immuno Pharmaceuticals SA July 2015
