Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a15-14851_28k.htm |
| EX-2.1 - EX-2.1 - AMAG PHARMACEUTICALS, INC. | a15-14851_2ex2d1.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a15-14851_2ex99d1.htm |
Exhibit 99.2
|
|
AMAG to Acquire Cord Blood Registry® June 29, 2015 |
|
|
Forward-Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including among others, statements regarding expectations as the strategic and commercial fit of Cord Blood Registry (CBR) for AMAG’s maternal health business, its impact on AMAG’s diversification strategy focused on high-value specialty markets and its potential to drive accelerated product growth and acquisitions across AMAG’s product platform; AMAG’s belief regarding favorable market conditions for stem cell collection and storage companies generally and CBR in particular; AMAG’s beliefs regarding CBR being an industry-leading growth business and the #1 stem cell banking brand with healthcare providers and families; AMAG’s beliefs regarding CBR’s marketing capabilities; projections regarding the proposed transaction’s effects on adjusted EBITDA and earnings as well as AMAG’s net leverage ratio; projections regarding pro forma net revenue, anticipated cost synergies and CBR’s projected revenues; the expected timing of the closing of the transaction; expectations regarding CBR’s post-closing organizational structure, current CBR employees joining AMAG following the closing of the transaction and the contributions and responsibilities of those employees to AMAG’s and CBR’s continued operations; AMAG’s expectations for financing the transaction and use of financing proceeds to repay its existing senior secured term loan; expectations regarding CBR’s revenue mix and the loyalty of its customers; plans to increase clinical and research activities expected to grow the commercial utility of cord blood stem cells; expectations regarding CBR’s target market generally; the role of CBR’s Scientific and Medical Advisory Board; CBR being a high-margin business with a predictable revenue stream; AMAG’s plans to strengthen its maternal health business platform and bolster efforts to reach obstetricians as well as pregnant mothers and their families; beliefs that the CBR brand is poised for continued commercial success with increasing applications for the use of cord blood units in regenerative medicine; and beliefs regarding the importance of cord blood stem cells in general now and in the future are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Statements about AMAG’s or CBR’s past financial results do not, and are not meant to, predict future results. AMAG can provide no assurance that such results and performance will continue. Such risks and uncertainties include, among others, (1) the possibility that the closing conditions set forth in the definitive acquisition agreement, including those conditions related to antitrust clearance, will not be met and that the parties will be unable to consummate the proposed transaction, (2) the chance that, despite having a commitment in place, AMAG will be unable to secure financing, or financing on satisfactory or anticipated terms, in amounts sufficient to consummate the acquisition and the possibility that such financing may have a dilutive effect on AMAG’s current stockholders, (3) the possibility that, if the acquisition is consummated, AMAG may not realize the expected benefits, synergies and opportunities anticipated in connection with the transaction, including that the transaction will further diversify AMAG’s revenue base, be immediately accretive to adjusted EBITDA, and result in expected annual synergies of approximately $15 million annually, (4) the challenges of integrating CBR into the AMAG organization, including CBR’s commercial team, as well as AMAG’s ability to retain key CBR talent and the resulting disruptions to AMAG's and CBR’s operations if it fails to do so, (5) AMAG’s ability to successfully continue the CBR business, including as a result of CBR’s service based business model, the market for stem cell research and the need for strategic pricing skills to optimize the forward looking business, (6) the potential for stem cell science and its recognition adoption and utility among the medical community, (7) the ethical, legal and social implications of stem cell research, and the possibility that negative public opinion about stem cell therapy may damage public perception of AMAG’s overall business and (8) such other risks identified in AMAG's Securities and Exchange Commission (SEC) filings, including AMAG's Annual Report on Form 10-K for the year ended December 31, 2014 and subsequent filings with the SEC. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. |
|
|
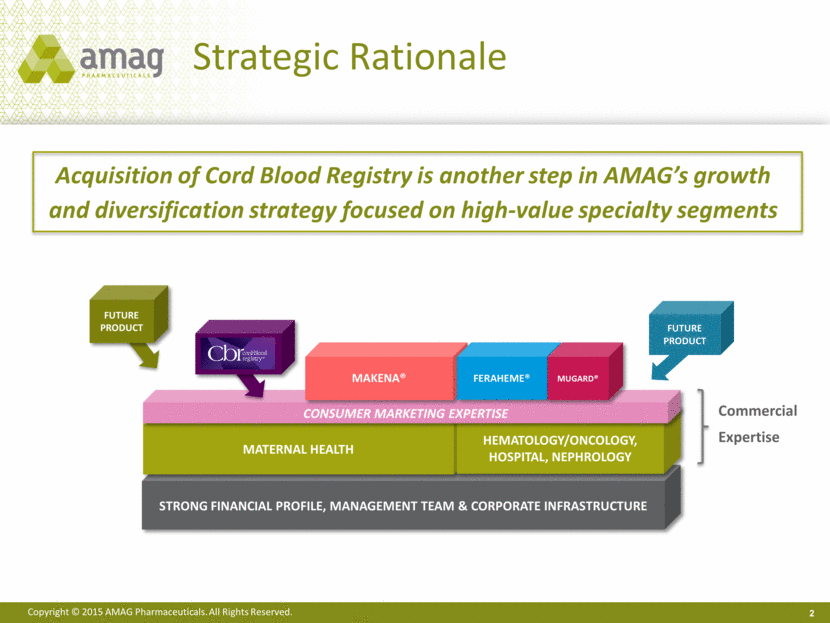
Strong Financial profile, management team & corporate infrastructure Strategic Rationale Acquisition of Cord Blood Registry is another step in AMAG’s growth and diversification strategy focused on high-value specialty segments 2 FUTURE PRODUCT Maternal Health Hematology/Oncology, Hospital, Nephrology Consumer marketing expertise Makena® Feraheme® MuGard® FUTURE PRODUCT CBR Commercial Expertise |
|
|
Acquisition Highlights Complementary business expands AMAG’s maternal health platform World’s largest stem cell collection and storage company #1 stem cell banking brand with healthcare providers and customers Favorable market conditions and large addressable market Strong strategic and commercial fit Complementary commercial capabilities to drive accelerated product growth for Makena and CBR Larger maternal health sales effort CBR’s consumer-driven marketing capabilities Financially compelling Expected to be immediately accretive to adjusted EBITDA and earnings 2014 pro forma revenues of $126MM; Adjusted EBITDA of $45MM before ~$15MM in synergies1 Durable, high-margin business that diversifies AMAG product portfolio Provides foundation for future acquisitions 3 1 See reconciliation tables on slide 14. |
|
|
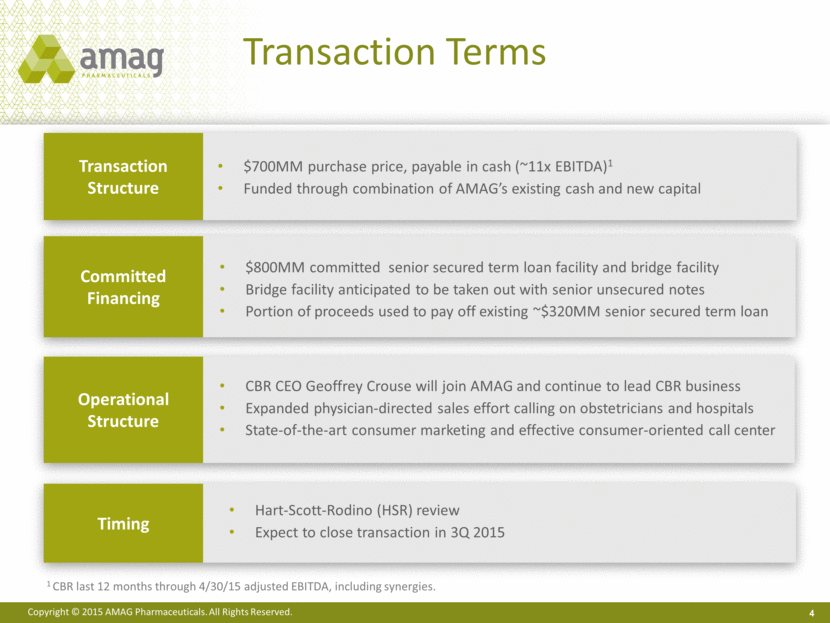
Transaction Terms 4 Transaction Structure Operational Structure Committed Financing Timing $700MM purchase price, payable in cash (~11x EBITDA)1 Funded through combination of AMAG’s existing cash and new capital CBR CEO Geoffrey Crouse will join AMAG and continue to lead CBR business Expanded physician-directed sales effort calling on obstetricians and hospitals State-of-the-art consumer marketing and effective consumer-oriented call center $800MM committed senior secured term loan facility and bridge facility Bridge facility anticipated to be taken out with senior unsecured notes Portion of proceeds used to pay off existing ~$320MM senior secured term loan Hart-Scott-Rodino (HSR) review Expect to close transaction in 3Q 2015 1 CBR last 12 months through 4/30/15 adjusted EBITDA, including synergies. |
|
|
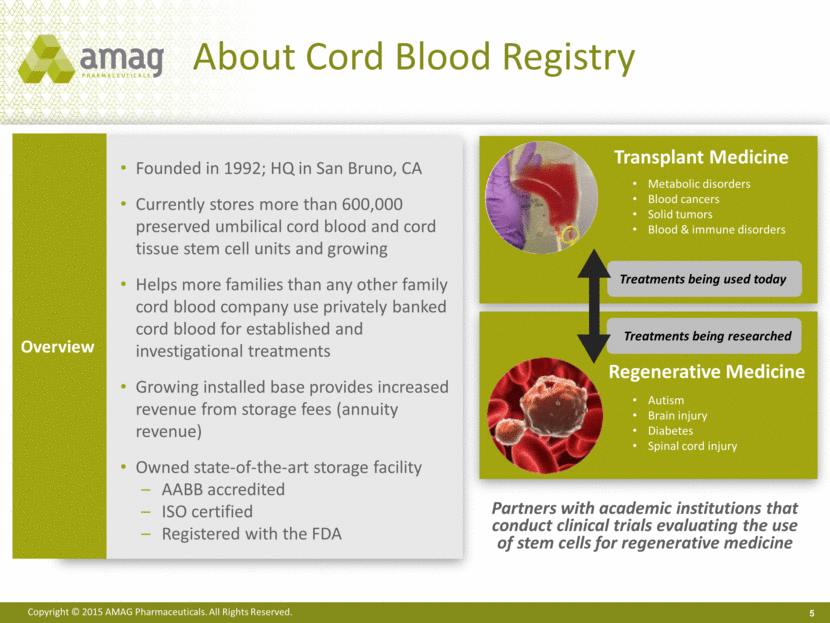
About Cord Blood Registry 5 Overview Founded in 1992; HQ in San Bruno, CA Currently stores more than 600,000 preserved umbilical cord blood and cord tissue stem cell units and growing Helps more families than any other family cord blood company use privately banked cord blood for established and investigational treatments Growing installed base provides increased revenue from storage fees (annuity revenue) Owned state-of-the-art storage facility AABB accredited ISO certified Registered with the FDA Partners with academic institutions that conduct clinical trials evaluating the use of stem cells for regenerative medicine Transplant Medicine Treatments being researched Treatments being used today Metabolic disorders Blood cancers Solid tumors Blood & immune disorders Regenerative Medicine Autism Brain injury Diabetes Spinal cord injury |
|
|
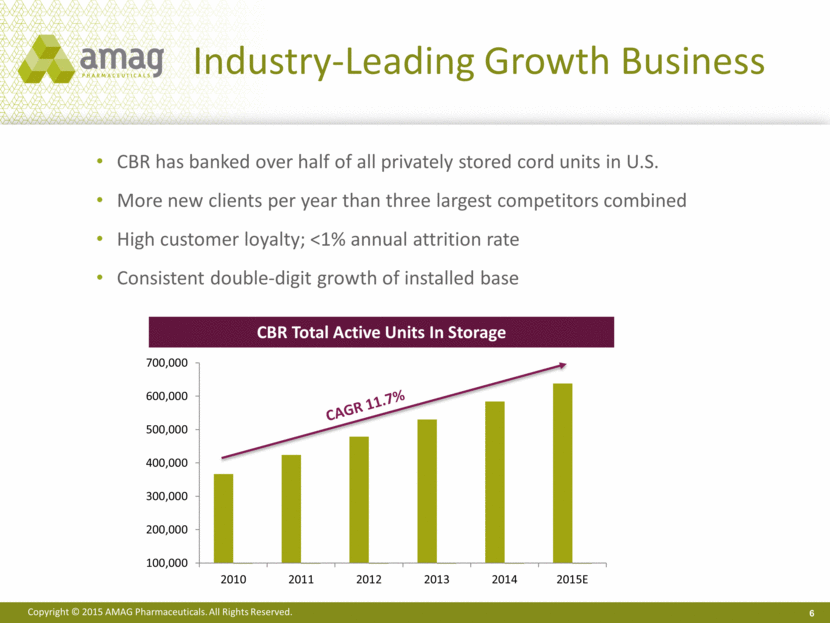
CBR has banked over half of all privately stored cord units in U.S. More new clients per year than three largest competitors combined High customer loyalty; <1% annual attrition rate Consistent double-digit growth of installed base Industry-Leading Growth Business 6 6 CBR Total Active Units In Storage 100,000 200,000 300,000 400,000 500,000 600,000 700,000 2010 2011 2012 2013 2014 2015E |
|
|
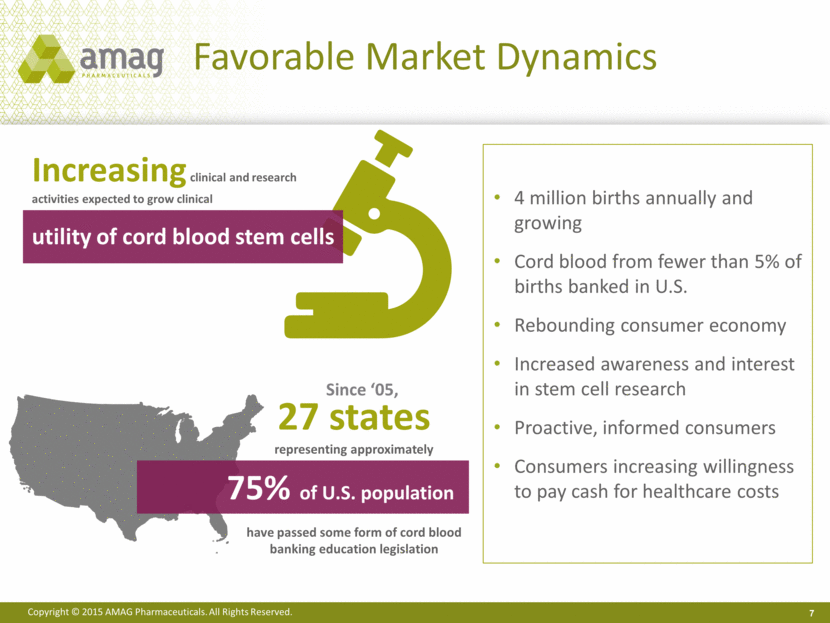
Favorable Market Dynamics 7 4 million births annually and growing Cord blood from fewer than 5% of births banked in U.S. Rebounding consumer economy Increased awareness and interest in stem cell research Proactive, informed consumers Consumers increasing willingness to pay cash for healthcare costs Increasing clinical and research activities expected to grow clinical utility of cord blood stem cells 27 states representing approximately have passed some form of cord blood banking education legislation 75% of U.S. population Since ‘05, |
|
|
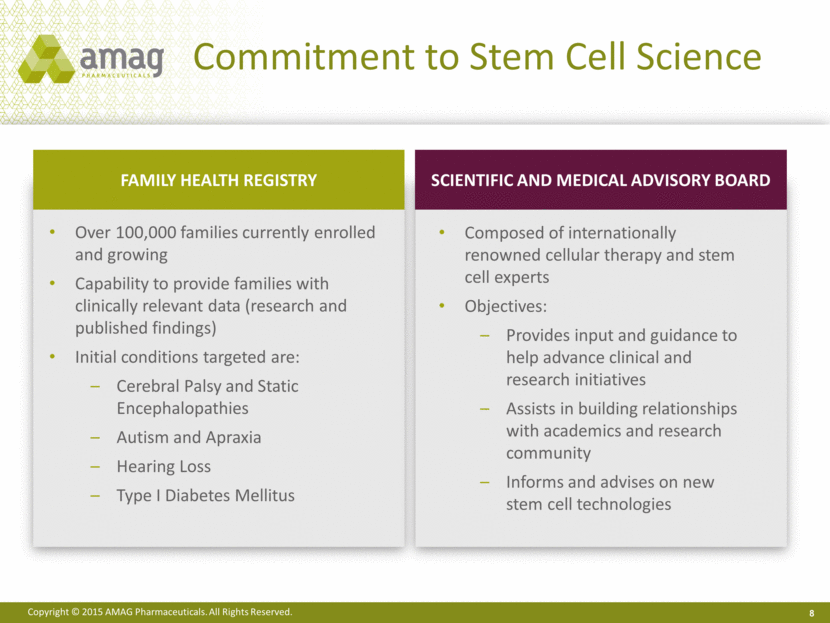
Commitment to Stem Cell Science 8 Over 100,000 families currently enrolled and growing Capability to provide families with clinically relevant data (research and published findings) Initial conditions targeted are: Cerebral Palsy and Static Encephalopathies Autism and Apraxia Hearing Loss Type I Diabetes Mellitus Composed of internationally renowned cellular therapy and stem cell experts Objectives: Provides input and guidance to help advance clinical and research initiatives Assists in building relationships with academics and research community Informs and advises on new stem cell technologies FAMILY HEALTH REGISTRY SCIENTIFIC AND MEDICAL ADVISORY BOARD |
|
|
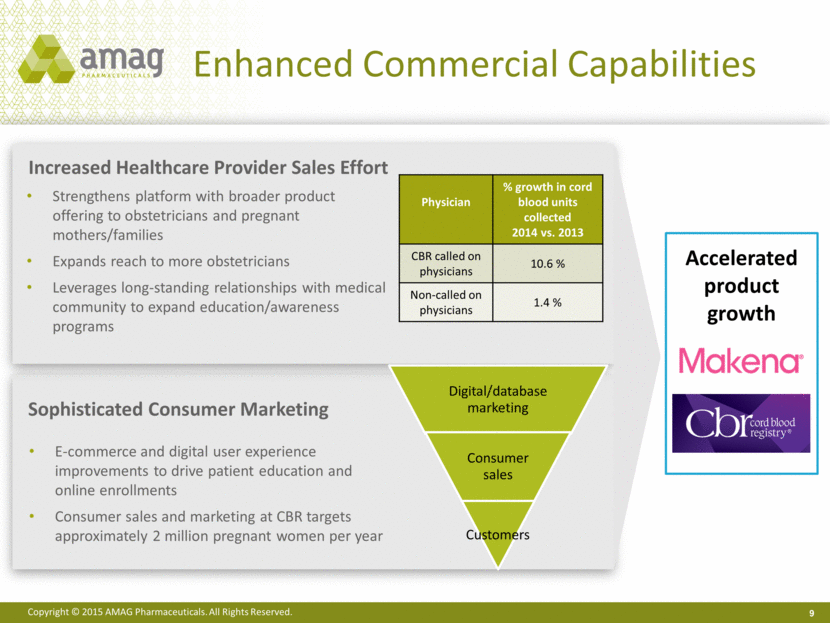
Enhanced Commercial Capabilities 9 Increased Healthcare Provider Sales Effort Sophisticated Consumer Marketing Strengthens platform with broader product offering to obstetricians and pregnant mothers/families Expands reach to more obstetricians Leverages long-standing relationships with medical community to expand education/awareness programs E-commerce and digital user experience improvements to drive patient education and online enrollments Consumer sales and marketing at CBR targets approximately 2 million pregnant women per year Physician % growth in cord blood units collected 2014 vs. 2013 CBR called on physicians 10.6 % Non-called on physicians 1.4 % Accelerated product growth Digital/database marketing Consumer sales Customers |
|
|
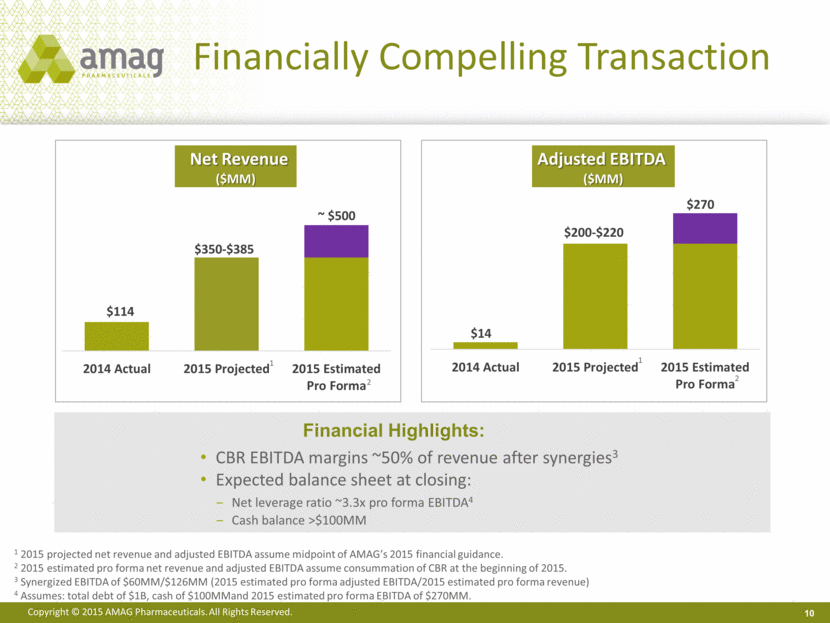
Financially Compelling Transaction 10 Net Revenue ($MM) Adjusted EBITDA ($MM) 1 2015 projected net revenue and adjusted EBITDA assume midpoint of AMAG’s 2015 financial guidance. 2 2015 estimated pro forma net revenue and adjusted EBITDA assume consummation of CBR at the beginning of 2015. 3 Synergized EBITDA of $60MM/$126MM (2015 estimated pro forma adjusted EBITDA/2015 estimated pro forma revenue) 4 Assumes: total debt of $1B, cash of $100MMand 2015 estimated pro forma EBITDA of $270MM. 1 1 2 2 CBR EBITDA margins ~50% of revenue after synergies3 Expected balance sheet at closing: Net leverage ratio ~3.3x pro forma EBITDA4 Cash balance >$100MM Financial Highlights: $200-$220 |
|
|
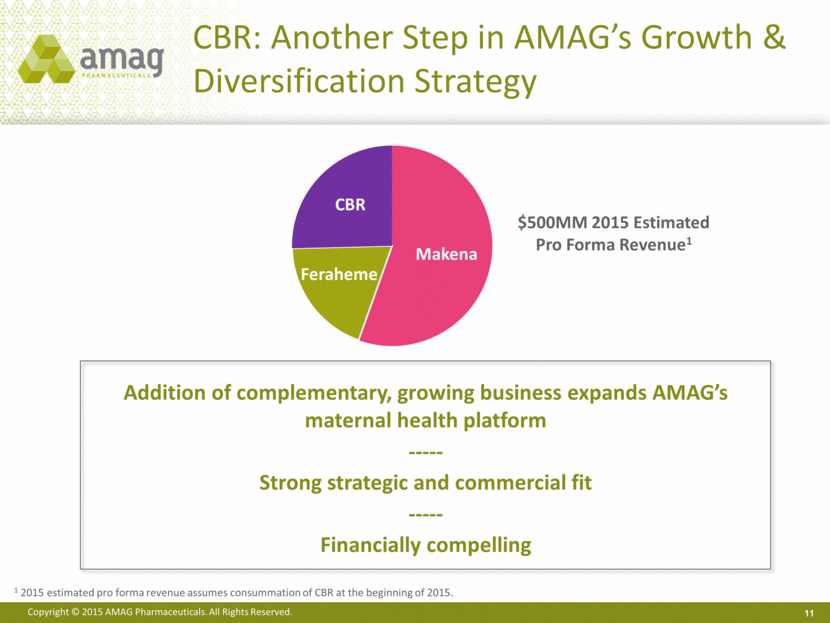
11 CBR: Another Step in AMAG’s Growth & Diversification Strategy $500MM 2015 Estimated Pro Forma Revenue1 Addition of complementary, growing business expands AMAG’s maternal health platform ----- Strong strategic and commercial fit ----- Financially compelling 1 2015 estimated pro forma revenue assumes consummation of CBR at the beginning of 2015. Makena Feraheme CBR |
|
|
AMAG to Acquire Cord Blood Registry® June 29, 2015 |
|
|
Appendix |
|
|
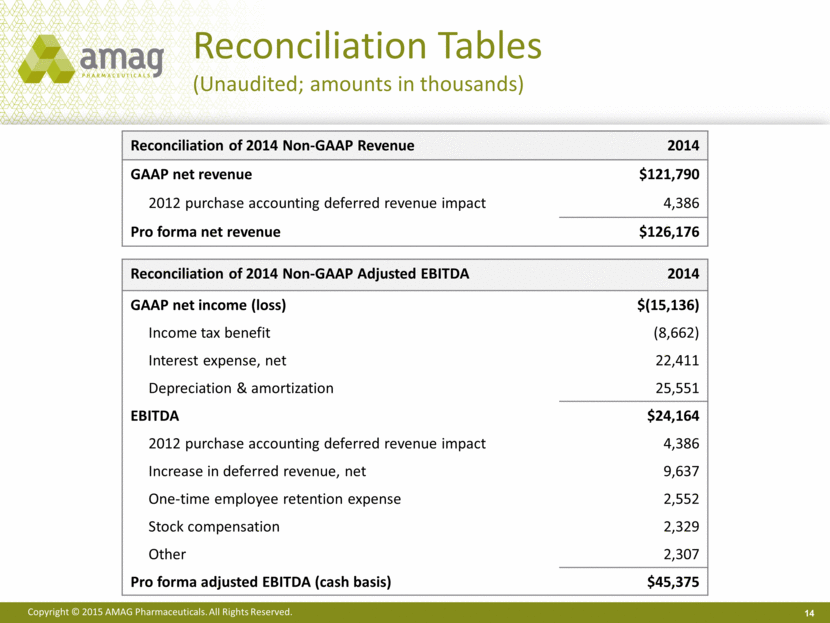
Reconciliation Tables (Unaudited; amounts in thousands) Reconciliation of 2014 Non-GAAP Revenue 2014 GAAP net revenue $121,790 2012 purchase accounting deferred revenue impact 4,386 Pro forma net revenue $126,176 14 Reconciliation of 2014 Non-GAAP Adjusted EBITDA 2014 GAAP net income (loss) $(15,136) Income tax benefit (8,662) Interest expense, net 22,411 Depreciation & amortization 25,551 EBITDA $24,164 2012 purchase accounting deferred revenue impact 4,386 Increase in deferred revenue, net 9,637 One-time employee retention expense 2,552 Stock compensation 2,329 Other 2,307 Pro forma adjusted EBITDA (cash basis) $45,375 |