Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Savara Inc | mstx-8k_20150625.htm |
| EX-99.2 - EX-99.2 - Savara Inc | mstx-ex992_201506256.htm |

NYSE MKT: MSTX Corporate Overview June 25, 2015

Forward-Looking Statements This presentation includes forward-looking statements about our business prospects, financial position, and development of vepoloxamer and AIR001 for therapeutic use in humans. Any statement that is not a statement of historical fact should be considered a forward-looking statement. Because forward-looking statements relate to the future, they are subject to inherent risks, uncertainties and changes in circumstances that are difficult to predict. Actual events or performance may differ materially from our expectations indicated by these forward-looking statements due to a number of factors, including, but not limited to, results of our pending and future clinical studies, the timeline for clinical and manufacturing activities and regulatory approval; our dependency on third parties to conduct our clinical studies and manufacture our clinical trial material; our ability to raise additional capital, as needed; our ability to establish and protect proprietary rights related to our product candidates; and other risks and uncertainties more fully described in our press releases and our filings with the SEC, including our annual report on Form 10-K filed with the SEC on March 24, 2015. We caution you not to place undue reliance on any of these forward-looking statements, which speak only as of the date of this presentation. We do not intend to update any forward-looking statement included in this presentation to reflect events or circumstances arising after the date of the presentation, except as may be required by law.
Publicly-traded biopharmaceutical company based in San Diego Developing vepoloxamer (MST-188) for: Rare (“orphan”) diseases: Sickle Cell Disease Acute Limb Ischemia Large market opportunities: Heart Failure Stroke Developing AIR001 for: Heart failure with preserved ejection fraction Commercially complementary to vepoloxamer Corporate Overview
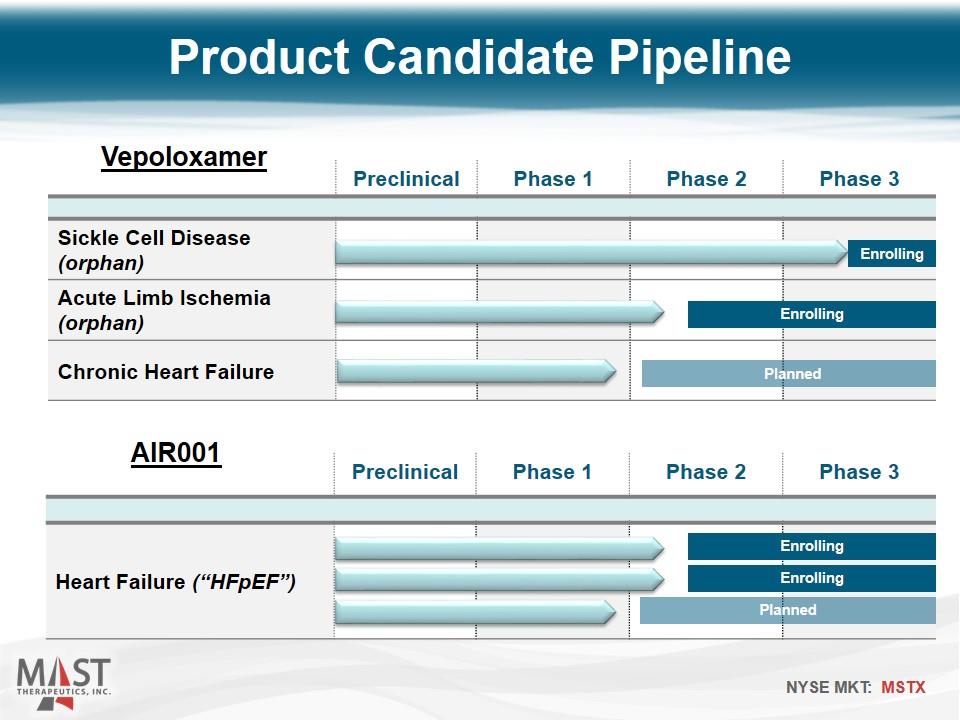
Preclinical Phase 1 Phase 2 Phase 3 Heart Failure (“HFpEF”) Product Candidate Pipeline Preclinical Phase 1 Phase 2 Phase 3 Sickle Cell Disease (orphan) Acute Limb Ischemia (orphan) Chronic Heart Failure Enrolling Planned Enrolling Vepoloxamer AIR001 Enrolling Enrolling Planned

Lead Program Vepoloxamer
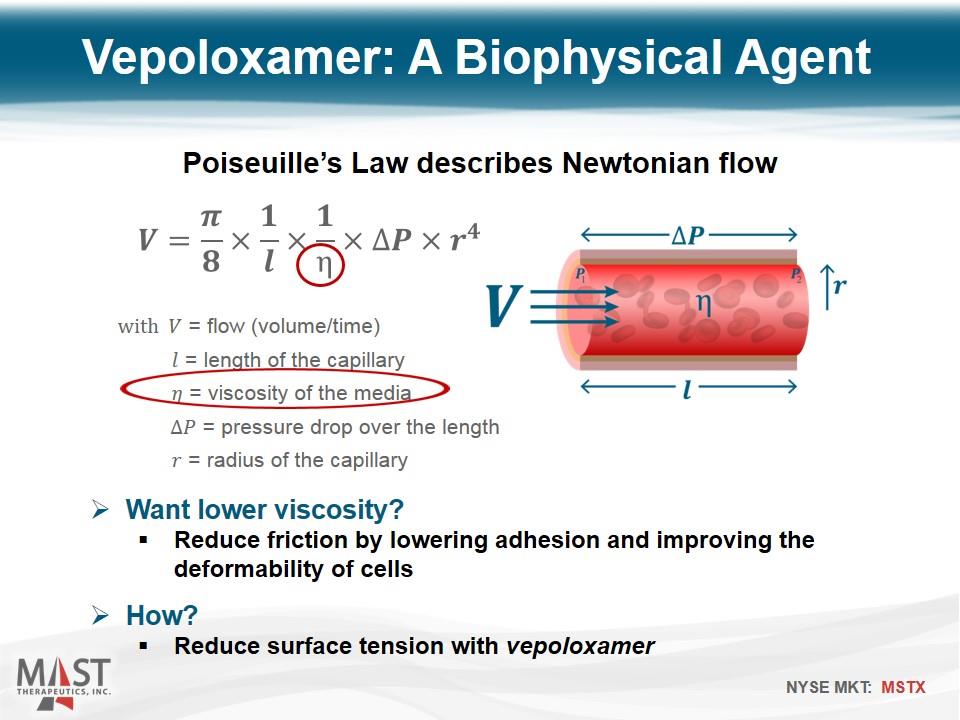
Poiseuille’s Law describes Newtonian flow with = flow (volume/time) = length of the capillary = viscosity of the media = pressure drop over the length = radius of the capillary Vepoloxamer: A Biophysical Agent Want lower viscosity? Reduce friction by lowering adhesion and improving the deformability of cells How? Reduce surface tension with vepoloxamer
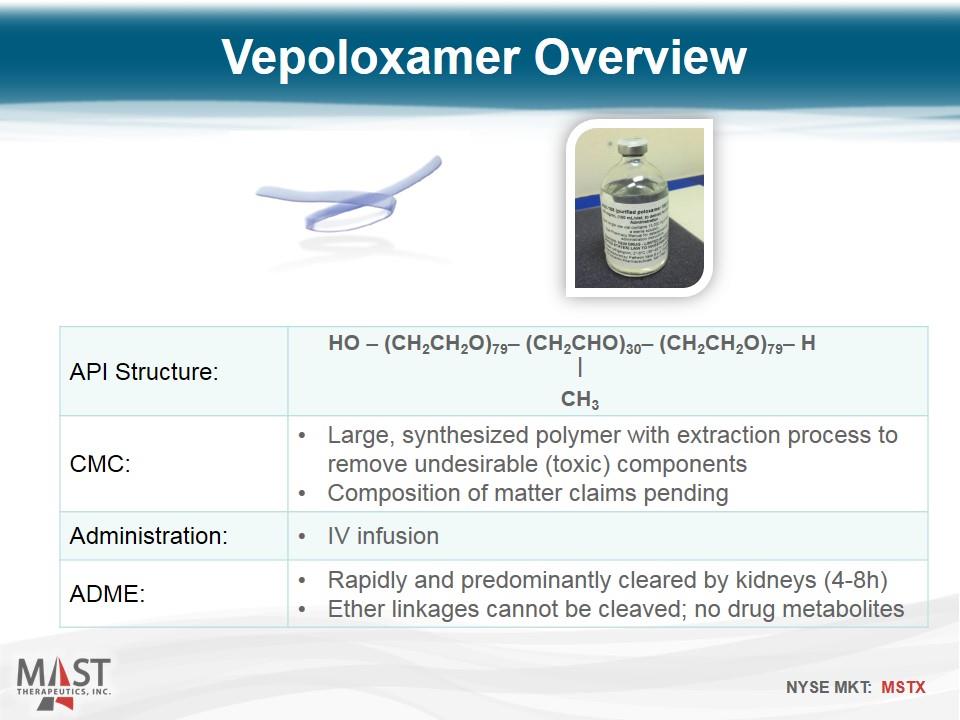
API Structure: CMC: Large, synthesized polymer with extraction process to remove undesirable (toxic) components Composition of matter claims pending Administration: IV infusion ADME: Rapidly and predominantly cleared by kidneys (4-8h) Ether linkages cannot be cleaved; no drug metabolites Vepoloxamer Overview HO – (CH2CH2O)79– (CH2CHO)30– (CH2CH2O)79– H CH3 |
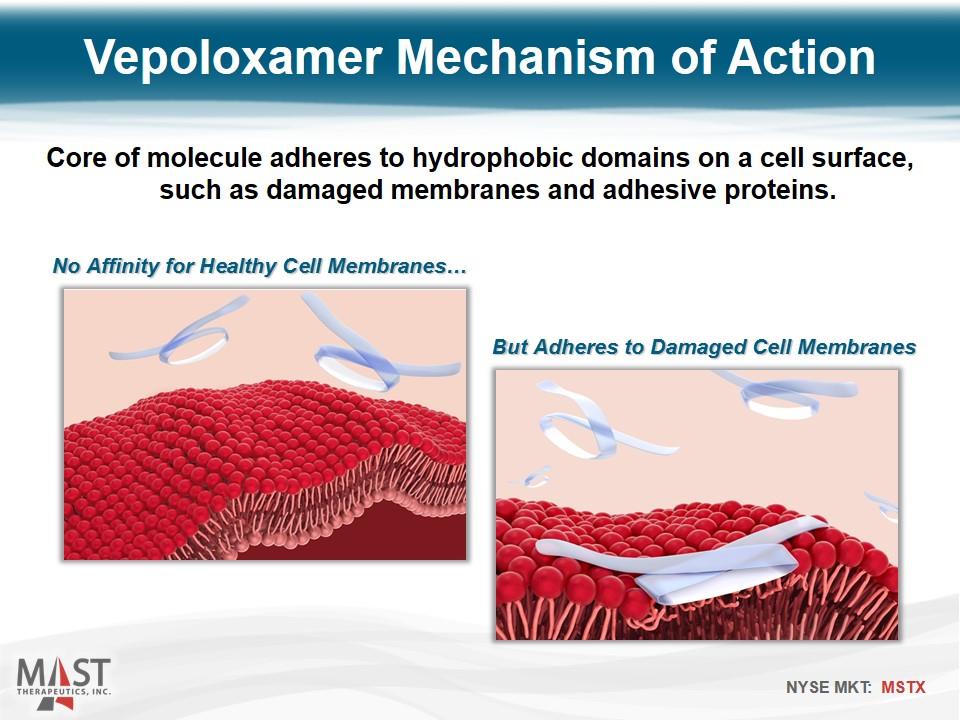
No Affinity for Healthy Cell Membranes… But Adheres to Damaged Cell Membranes Core of molecule adheres to hydrophobic domains on a cell surface, such as damaged membranes and adhesive proteins. Vepoloxamer Mechanism of Action
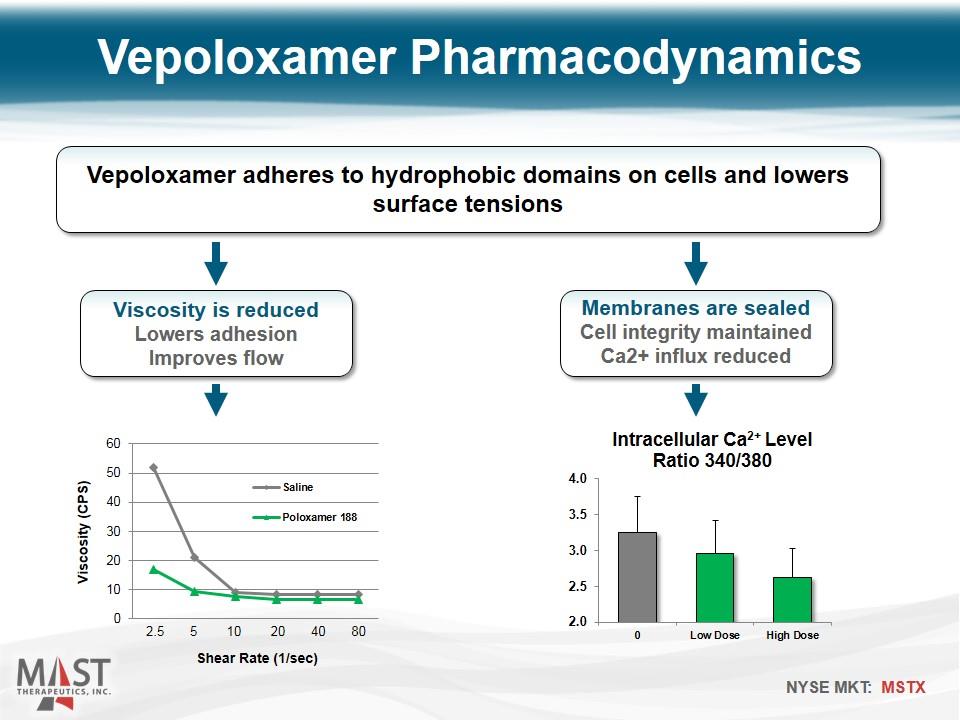
Vepoloxamer Pharmacodynamics Vepoloxamer adheres to hydrophobic domains on cells and lowers surface tensions Viscosity is reduced Lowers adhesion Improves flow Membranes are sealed Cell integrity maintained Ca2+ influx reduced Shear Rate (1/sec) 2.5 5 10 20 40 80
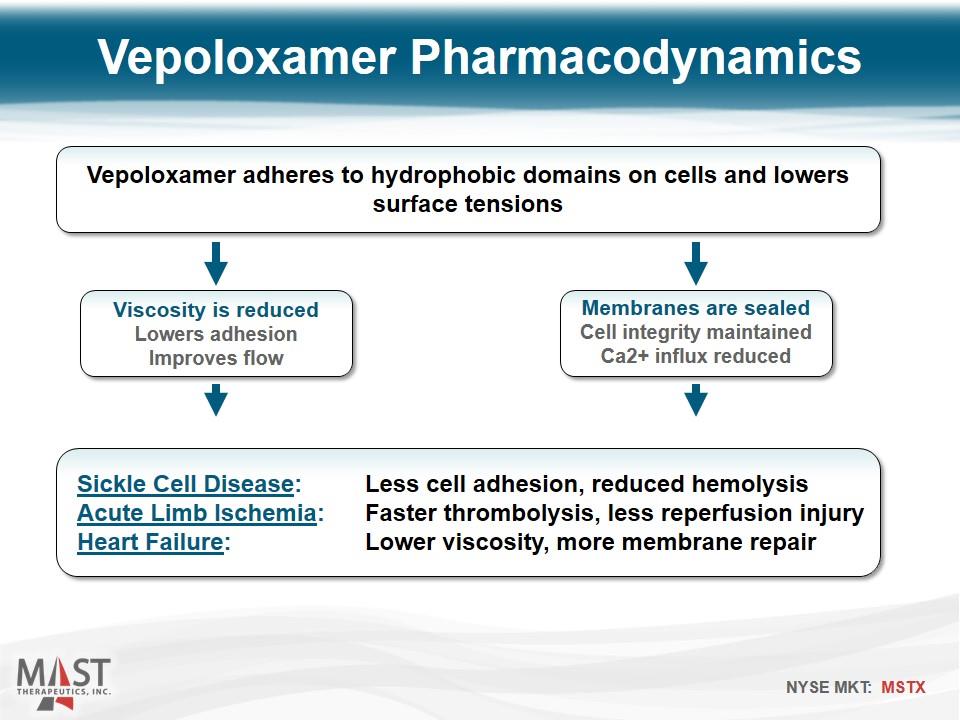
Vepoloxamer Pharmacodynamics Vepoloxamer adheres to hydrophobic domains on cells and lowers surface tensions Viscosity is reduced Lowers adhesion Improves flow Membranes are sealed Cell integrity maintained Ca2+ influx reduced Sickle Cell Disease: Less cell adhesion, reduced hemolysis Acute Limb Ischemia: Faster thrombolysis, less reperfusion injury Heart Failure: Lower viscosity, more membrane repair
Sickle Cell Disease Objective Improve blood flow and shorten the duration of crisis

A chronic, genetic disorder and rare (orphan) disease Affects 90,000 to 100,000 people in the U.S. Characterized by severe deformation (i.e., “sickling”) of red blood cells Hallmark of disease is a “vaso-occlusive crisis” Exceedingly painful condition Leading cause of hospitalization Significant unmet need No approved agents to shorten duration or severity of crisis Standard of care (hydration and analgesics) unchanged for >10 years Vaso-occlusion is associated with early death Obstructed blood flow -> hypoxia -> tissue death -> organ failure Average age at death; 42 years (males), 48 years (females) Overview of Sickle Cell Disease
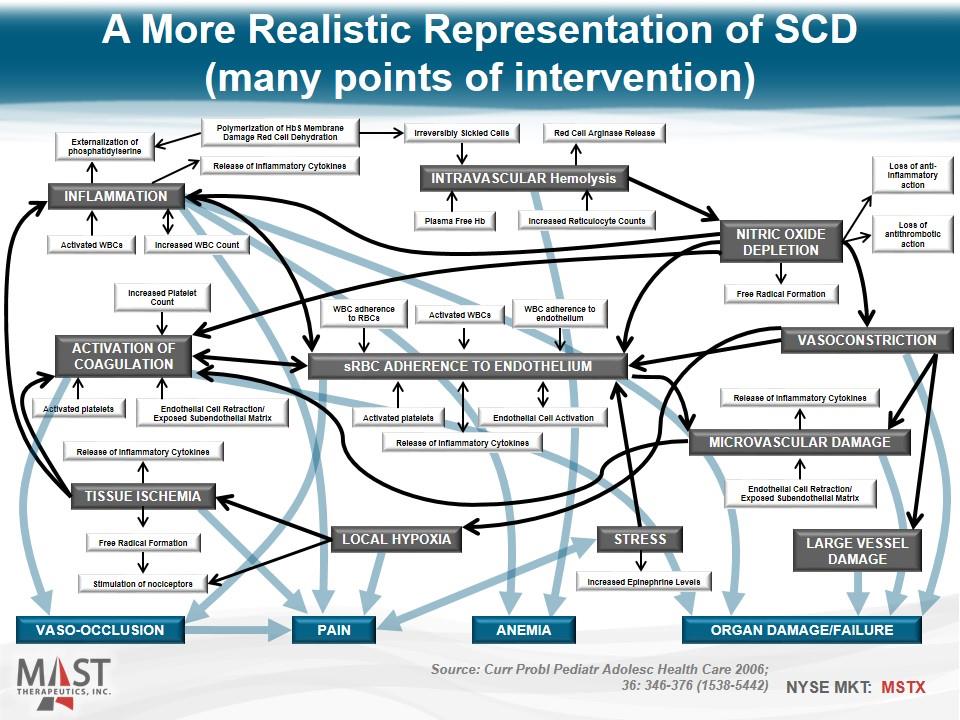
A More Realistic Representation of SCD (many points of intervention) sRBC Adherence to Endothelium VASO-OCCLUSION ORGAN DAMAGE/FAILURE ANEMIA PAIN INTRAVASCULAR Hemolysis NITRIC OXIDE DEPLETION VASOCONSTRICTION INFLAMMATION ACTIVATION OF COAGULATION TISSUE ISCHEMIA LOCAL HYPOXIA STRESS LARGE VESSEL DAMAGE Microvascular DAMAGE WBC adherence to RBCs Activated WBCs WBC adherence to endothelium Activated platelets Release of Inflammatory Cytokines Endothelial Cell Activation Free Radical Formation Loss of antithrombotic action Loss of anti-inflammatory action Release of Inflammatory Cytokines Endothelial Cell Retraction/ Exposed Subendothelial Matrix Release of Inflammatory Cytokines Free Radical Formation Stimulation of nociceptors Increased Epinephrine Levels Activated platelets Endothelial Cell Retraction/ Exposed Subendothelial Matrix Increased Platelet Count Activated WBCs Increased WBC Count Release of Inflammatory Cytokines Externalization of phosphatidylserine Polymerization of HbS Membrane Damage Red Cell Dehydration Increased Reticulocyte Counts Plasma Free Hb Irreversibly Sickled Cells Red Cell Arginase Release Source: Curr Probl Pediatr Adolesc Health Care 2006; 36: 346-376 (1538-5442)
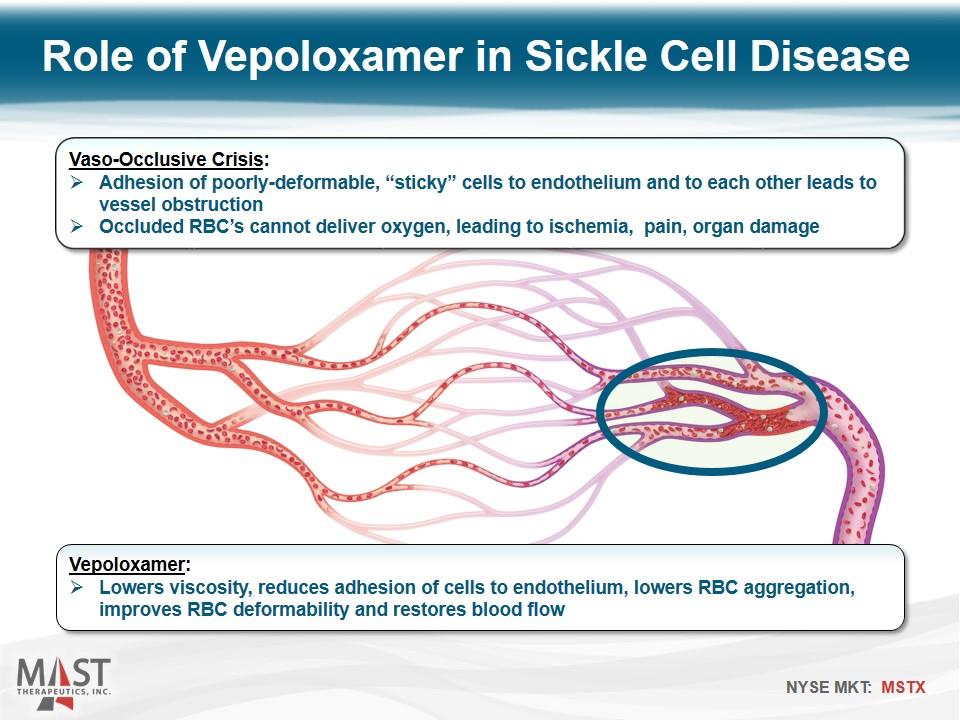
Role of Vepoloxamer in Sickle Cell Disease Vaso-Occlusive Crisis: Adhesion of poorly-deformable, “sticky” cells to endothelium and to each other leads to vessel obstruction Occluded RBC’s cannot deliver oxygen, leading to ischemia, pain, organ damage Vepoloxamer: Lowers viscosity, reduces adhesion of cells to endothelium, lowers RBC aggregation, improves RBC deformability and restores blood flow
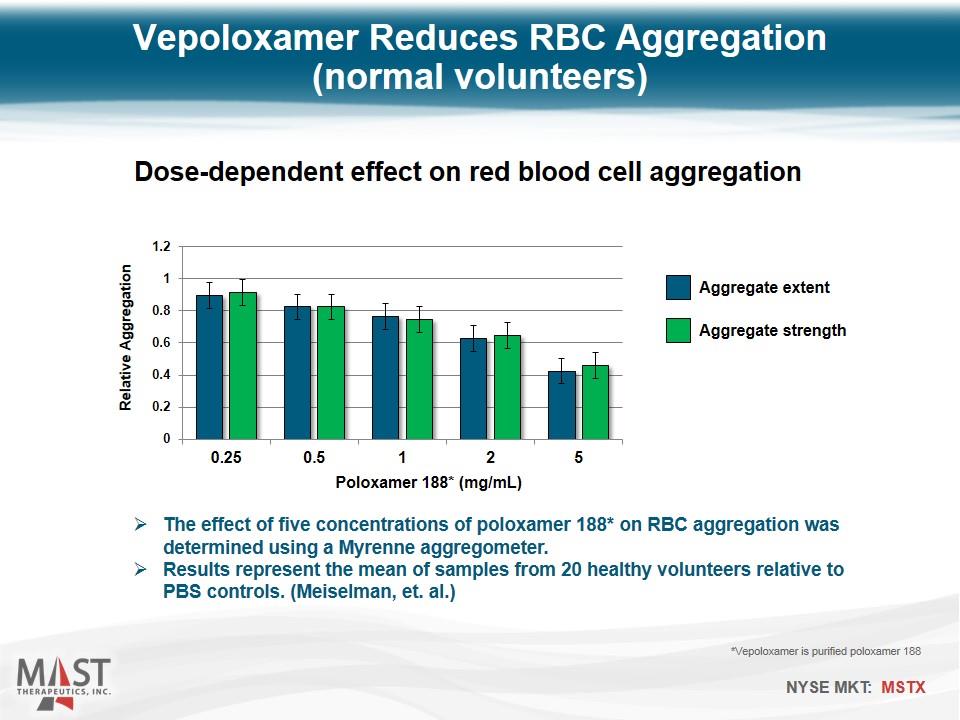
Vepoloxamer Reduces RBC Aggregation (normal volunteers) The effect of five concentrations of poloxamer 188* on RBC aggregation was determined using a Myrenne aggregometer. Results represent the mean of samples from 20 healthy volunteers relative to PBS controls. (Meiselman, et. al.) *Vepoloxamer is purified poloxamer 188 Dose-dependent effect on red blood cell aggregation Aggregate extent Aggregate strength Poloxamer 188* (mg/mL)
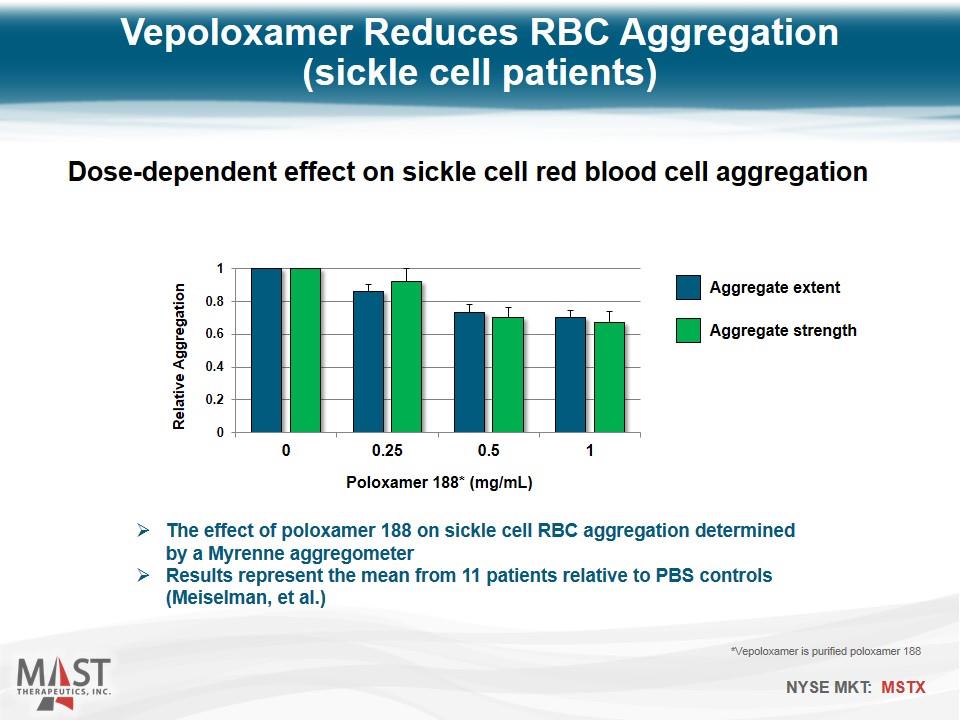
Vepoloxamer Reduces RBC Aggregation (sickle cell patients) Dose-dependent effect on sickle cell red blood cell aggregation Poloxamer 188* (mg/mL) Aggregate extent Aggregate strength *Vepoloxamer is purified poloxamer 188 The effect of poloxamer 188 on sickle cell RBC aggregation determined by a Myrenne aggregometer Results represent the mean from 11 patients relative to PBS controls (Meiselman, et al.)
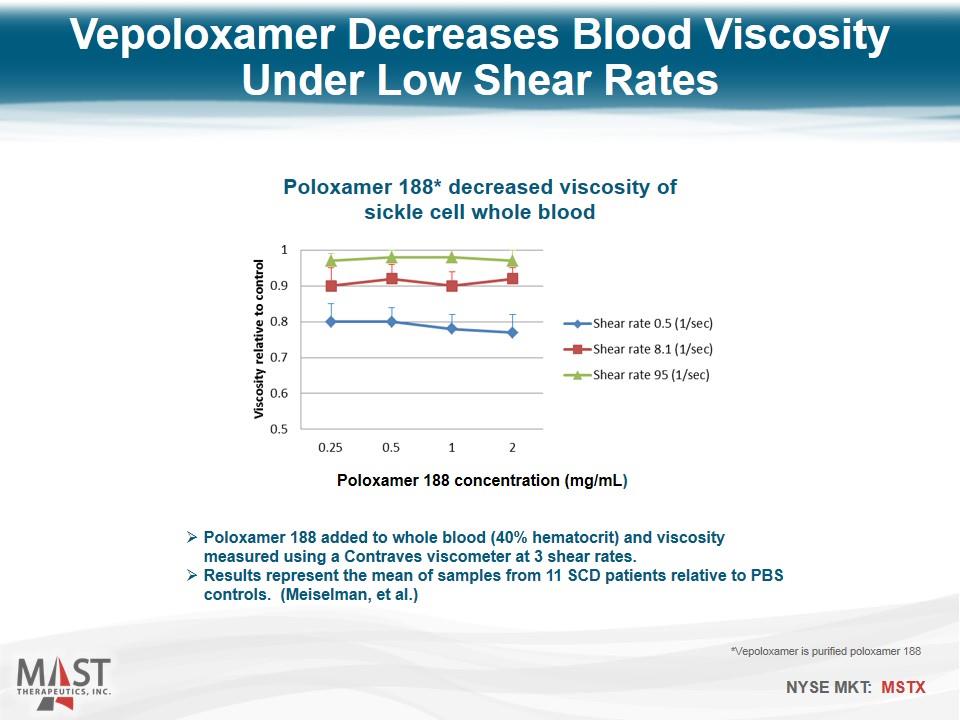
Vepoloxamer Decreases Blood Viscosity Under Low Shear Rates Poloxamer 188 added to whole blood (40% hematocrit) and viscosity measured using a Contraves viscometer at 3 shear rates. Results represent the mean of samples from 11 SCD patients relative to PBS controls. (Meiselman, et al.) *Vepoloxamer is purified poloxamer 188 Poloxamer 188 concentration (mg/mL) Poloxamer 188* decreased viscosity of sickle cell whole blood
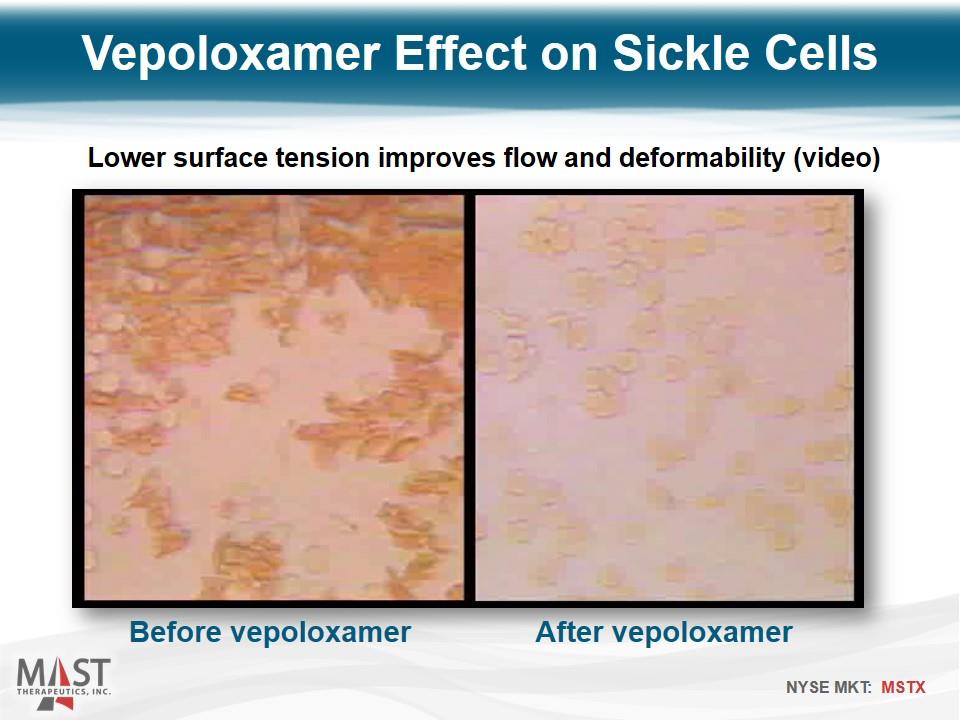
Vepoloxamer Effect on Sickle Cells Before vepoloxamer Lower surface tension improves flow and deformability (video) After vepoloxamer
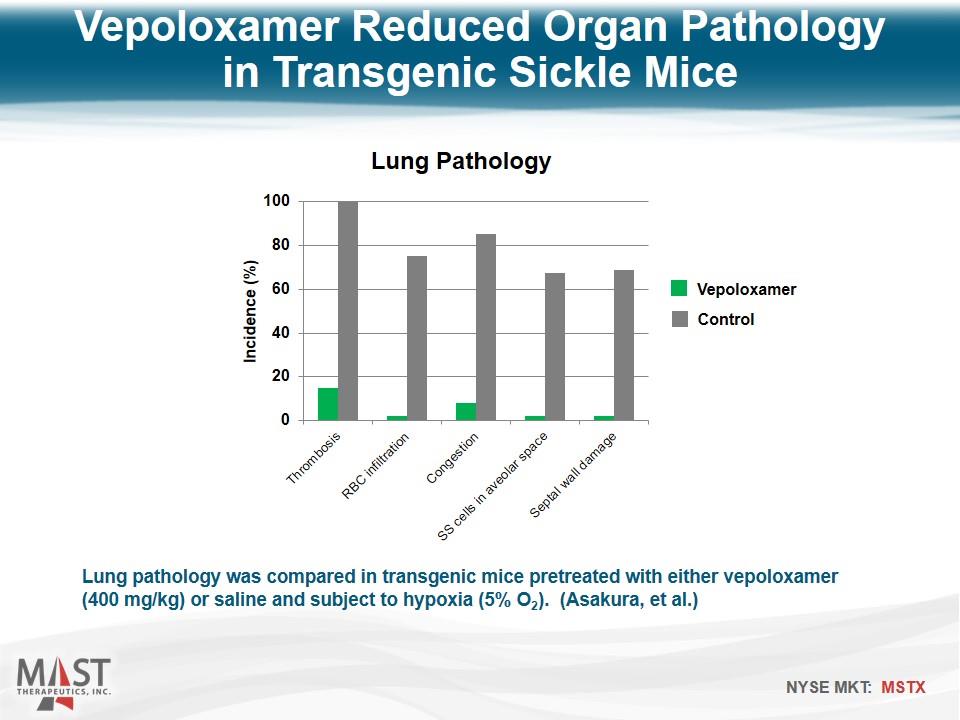
Lung pathology was compared in transgenic mice pretreated with either vepoloxamer (400 mg/kg) or saline and subject to hypoxia (5% O2). (Asakura, et al.) Vepoloxamer Reduced Organ Pathology in Transgenic Sickle Mice Vepoloxamer Control
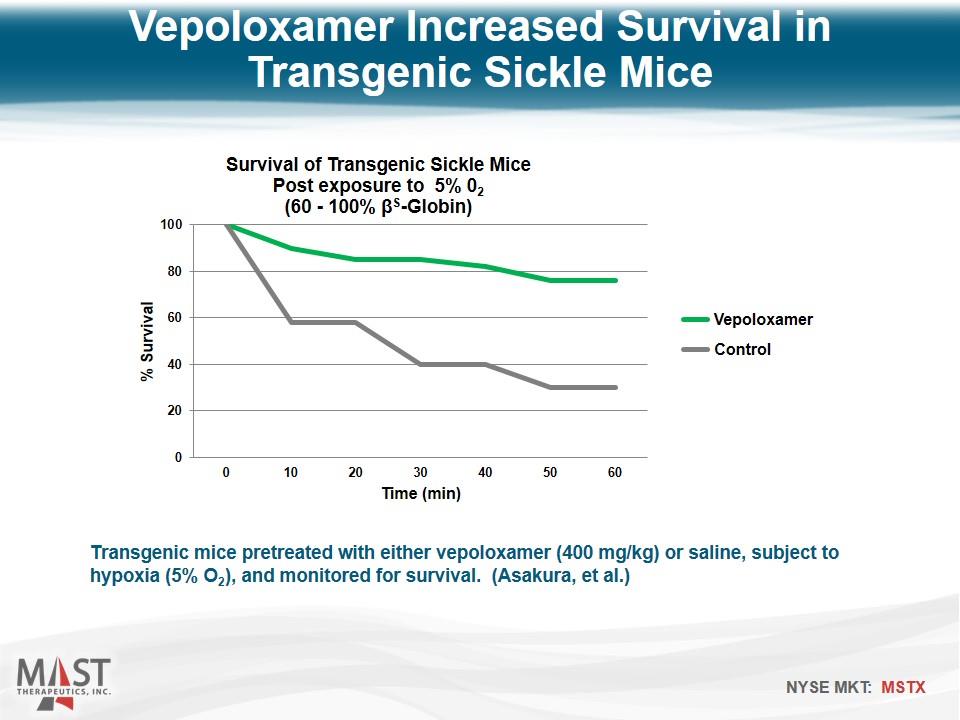
Transgenic mice pretreated with either vepoloxamer (400 mg/kg) or saline, subject to hypoxia (5% O2), and monitored for survival. (Asakura, et al.) Vepoloxamer Increased Survival in Transgenic Sickle Mice Vepoloxamer Control
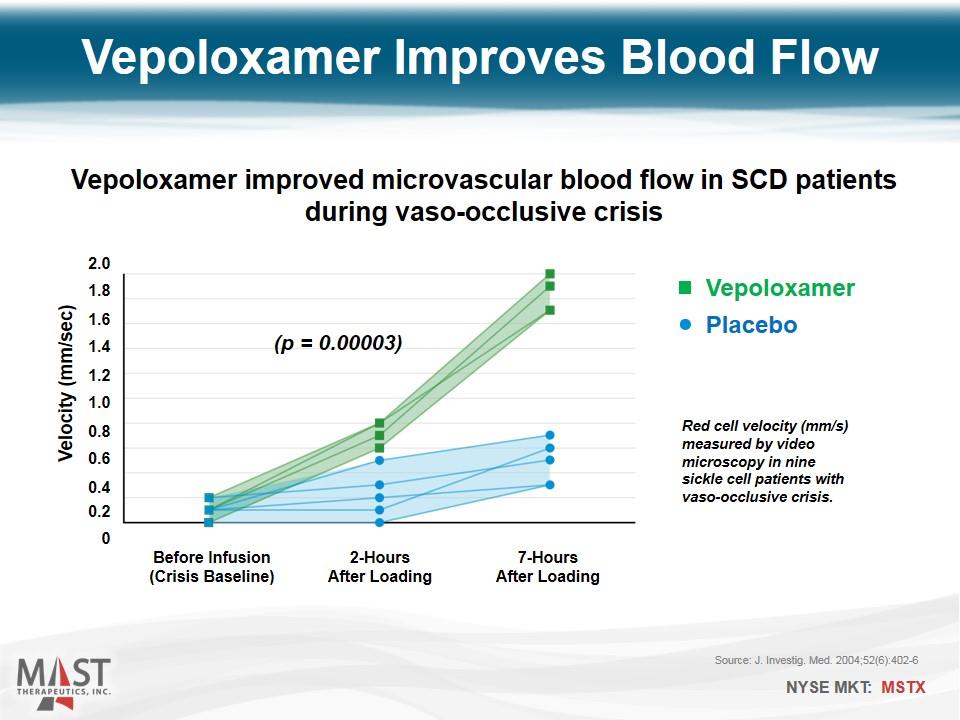
Vepoloxamer Placebo Before Infusion (Crisis Baseline) Velocity (mm/sec) 0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 2-Hours After Loading 7-Hours After Loading Source: J. Investig. Med. 2004;52(6):402-6 (p = 0.00003) Vepoloxamer improved microvascular blood flow in SCD patients during vaso-occlusive crisis Vepoloxamer Improves Blood Flow Red cell velocity (mm/s) measured by video microscopy in nine sickle cell patients with vaso-occlusive crisis.
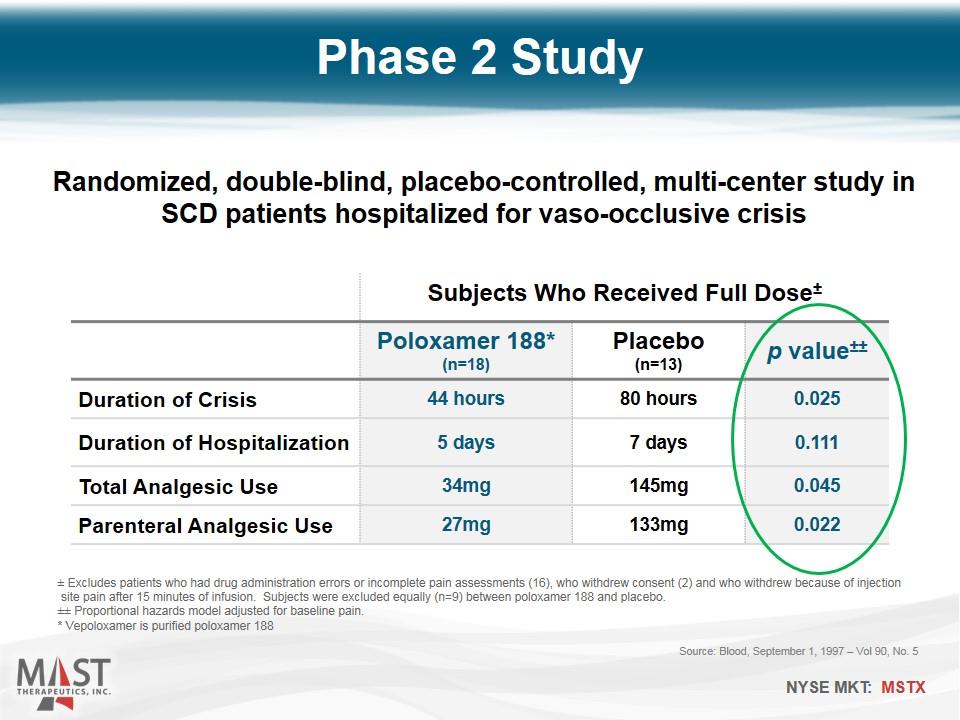
Phase 2 Study Source: Blood, September 1, 1997 – Vol 90, No. 5 Subjects Who Received Full Dose± Poloxamer 188* (n=18) Placebo (n=13) p value±± Duration of Crisis 44 hours 80 hours 0.025 Duration of Hospitalization 5 days 7 days 0.111 Total Analgesic Use 34mg 145mg 0.045 Parenteral Analgesic Use 27mg 133mg 0.022 ± Excludes patients who had drug administration errors or incomplete pain assessments (16), who withdrew consent (2) and who withdrew because of injection site pain after 15 minutes of infusion. Subjects were excluded equally (n=9) between poloxamer 188 and placebo. ±± Proportional hazards model adjusted for baseline pain. * Vepoloxamer is purified poloxamer 188 Randomized, double-blind, placebo-controlled, multi-center study in SCD patients hospitalized for vaso-occlusive crisis
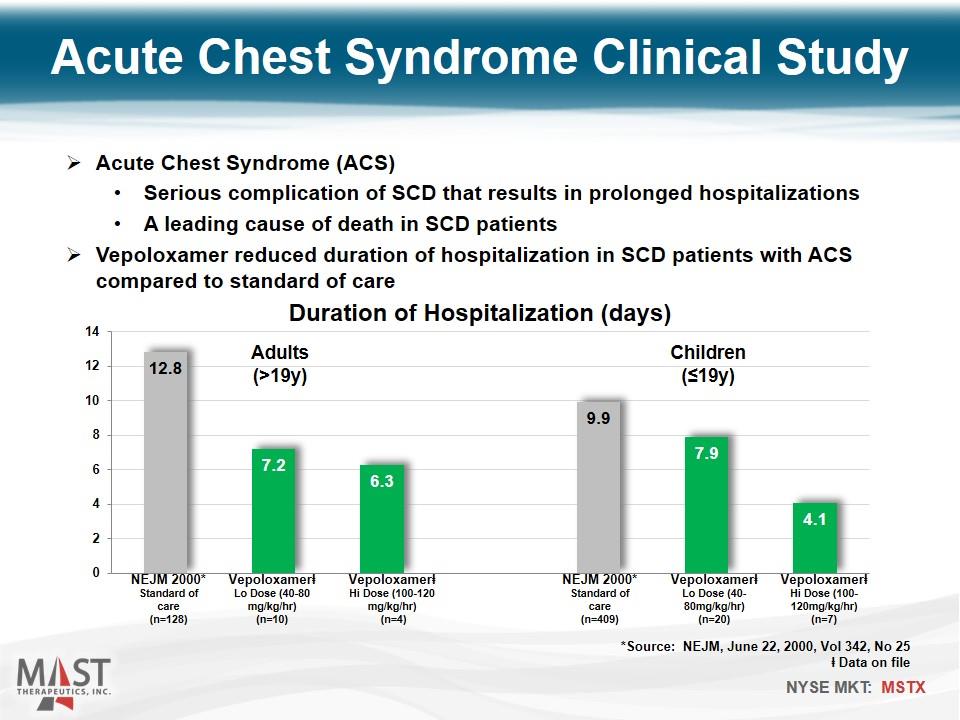
Acute Chest Syndrome Clinical Study Vepoloxamerⱡ Hi Dose (100-120mg/kg/hr) (n=7) Vepoloxamerⱡ Lo Dose (40-80mg/kg/hr) (n=20) NEJM 2000* Standard of care (n=409) Vepoloxamerⱡ Hi Dose (100-120 mg/kg/hr) (n=4) Vepoloxamerⱡ Lo Dose (40-80 mg/kg/hr) (n=10) NEJM 2000* Standard of care (n=128) Children (≤19y) Adults (>19y) Acute Chest Syndrome (ACS) Serious complication of SCD that results in prolonged hospitalizations A leading cause of death in SCD patients Vepoloxamer reduced duration of hospitalization in SCD patients with ACS compared to standard of care *Source: NEJM, June 22, 2000, Vol 342, No 25 ⱡ Data on file
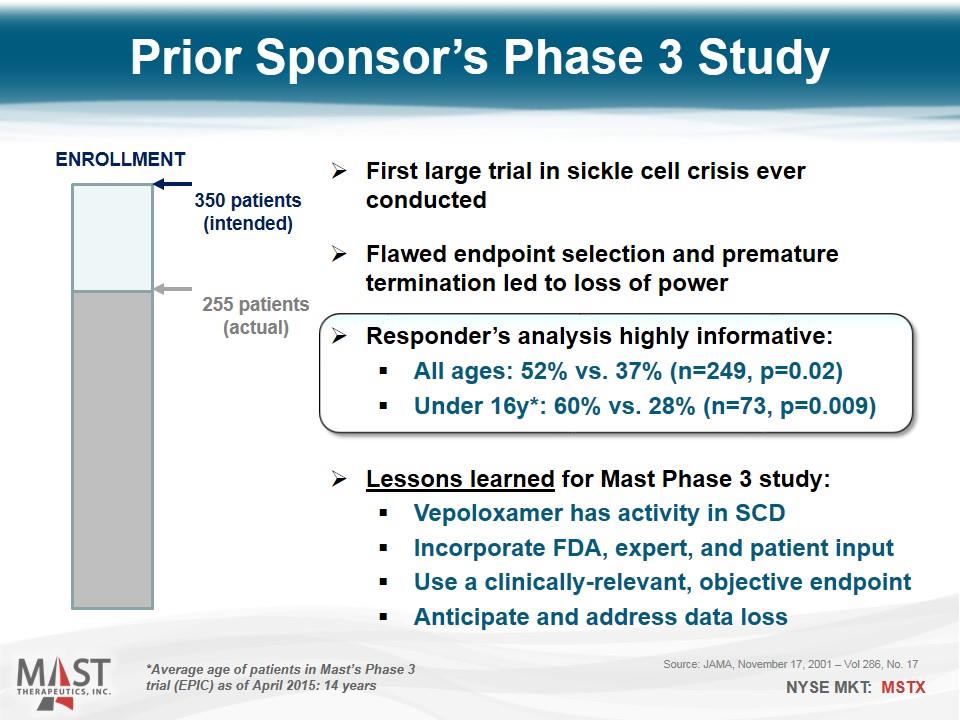
Source: JAMA, November 17, 2001 – Vol 286, No. 17 First large trial in sickle cell crisis ever conducted Flawed endpoint selection and premature termination led to loss of power Responder’s analysis highly informative: All ages: 52% vs. 37% (n=249, p=0.02) Under 16y*: 60% vs. 28% (n=73, p=0.009) Lessons learned for Mast Phase 3 study: Vepoloxamer has activity in SCD Incorporate FDA, expert, and patient input Use a clinically-relevant, objective endpoint Anticipate and address data loss Prior Sponsor’s Phase 3 Study 350 patients (intended) 255 patients (actual) ENROLLMENT *Average age of patients in Mast’s Phase 3 trial (EPIC) as of April 2015: 14 years
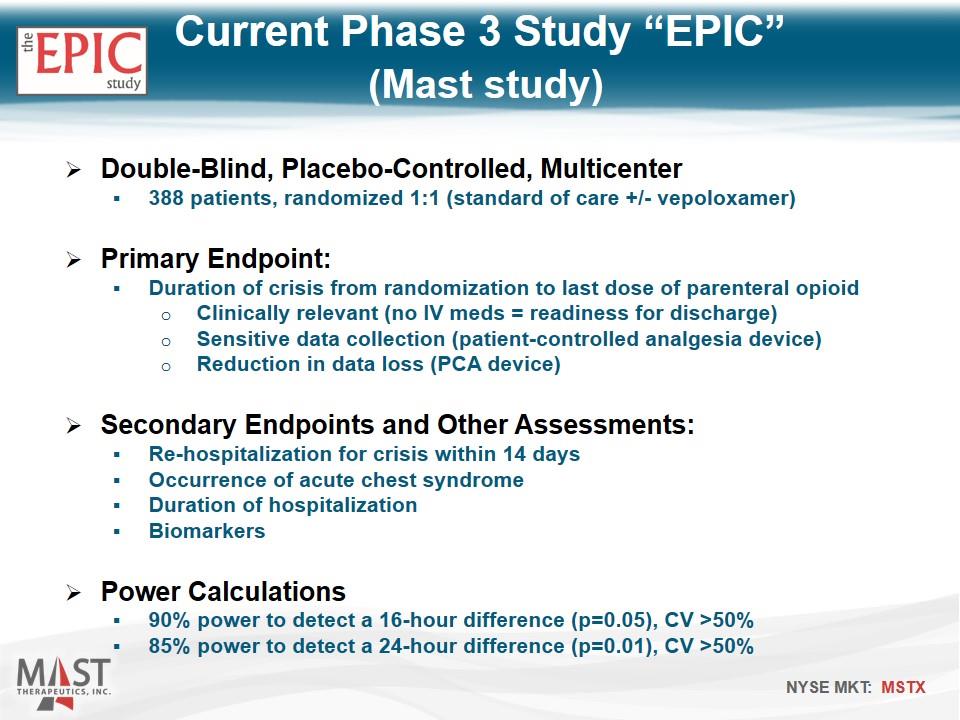
Double-Blind, Placebo-Controlled, Multicenter 388 patients, randomized 1:1 (standard of care +/- vepoloxamer) Primary Endpoint: Duration of crisis from randomization to last dose of parenteral opioid Clinically relevant (no IV meds = readiness for discharge) Sensitive data collection (patient-controlled analgesia device) Reduction in data loss (PCA device) Secondary Endpoints and Other Assessments: Re-hospitalization for crisis within 14 days Occurrence of acute chest syndrome Duration of hospitalization Biomarkers Power Calculations 90% power to detect a 16-hour difference (p=0.05), CV >50% 85% power to detect a 24-hour difference (p=0.01), CV >50% Current Phase 3 Study “EPIC” (Mast study)

Enrollment on-track Enrollment surpassed halfway mark in April 2015 Top-line data anticipated Q1 2016 Most Advanced New Drug in SCD Potential to be 1st drug ever approved to treat on-going vaso-occlusive crisis Substantial head start versus other drugs in development Considerations for Regulatory Decision-Making Significant unmet need – standard of care unchanged for years Increased reliance on disease experts in rare diseases Support among medical / advocacy communities Fast Track designation Orphan Drug designation Healthcare disparity concerns Supportive clinical studies: QT and repeat-administration EPIC Success Factors
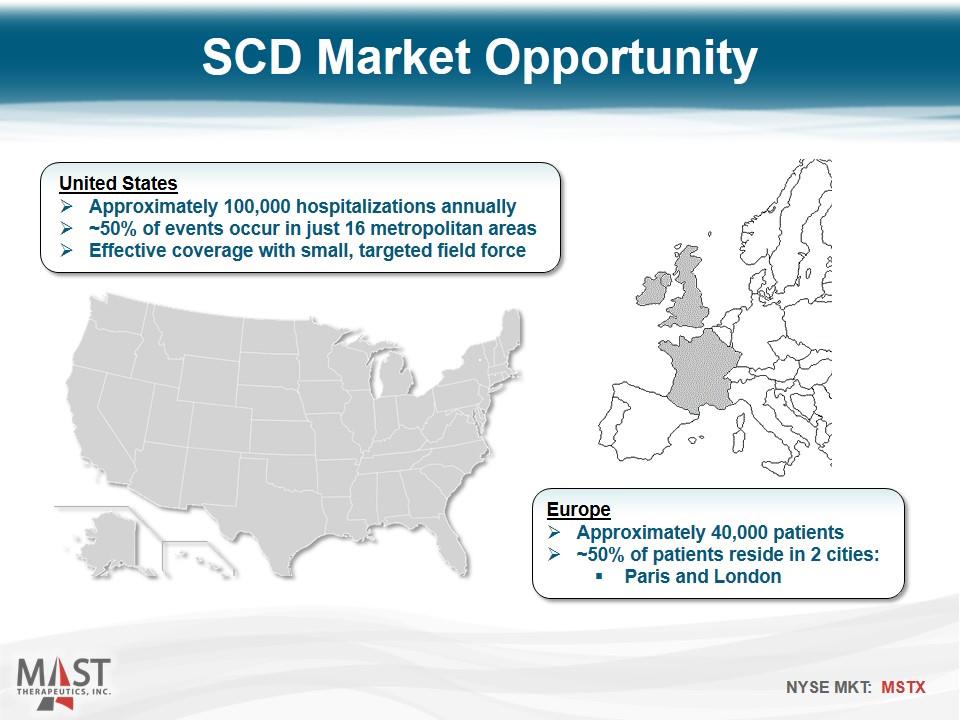
SCD Market Opportunity United States Approximately 100,000 hospitalizations annually ~50% of events occur in just 16 metropolitan areas Effective coverage with small, targeted field force Europe Approximately 40,000 patients ~50% of patients reside in 2 cities: Paris and London
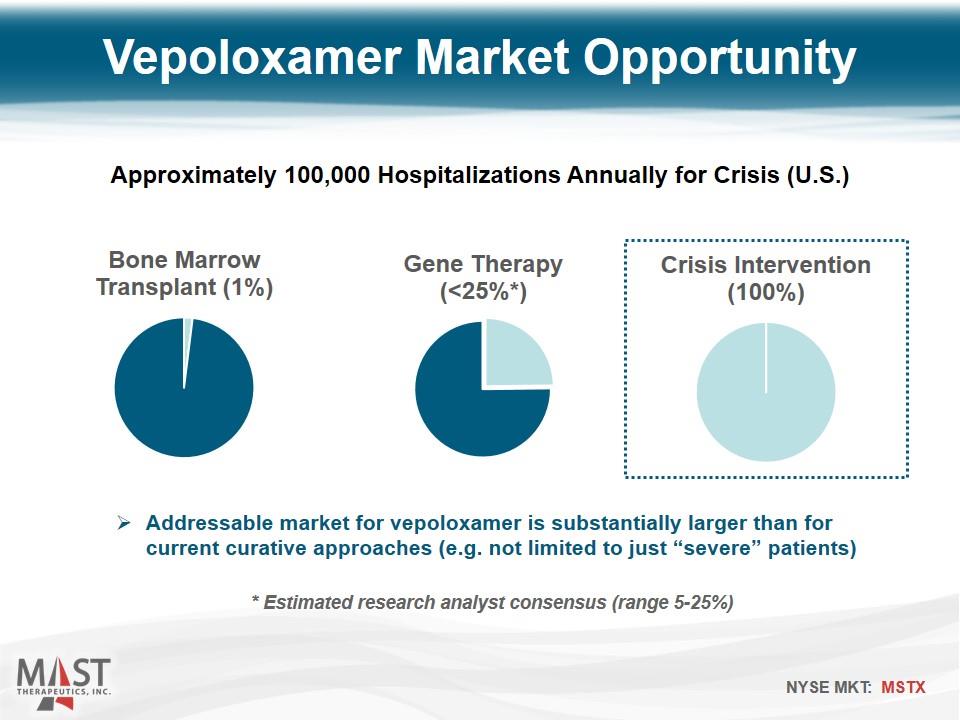
Addressable market for vepoloxamer is substantially larger than for current curative approaches (e.g. not limited to just “severe” patients) Vepoloxamer Market Opportunity Approximately 100,000 Hospitalizations Annually for Crisis (U.S.) * Estimated research analyst consensus (range 5-25%)
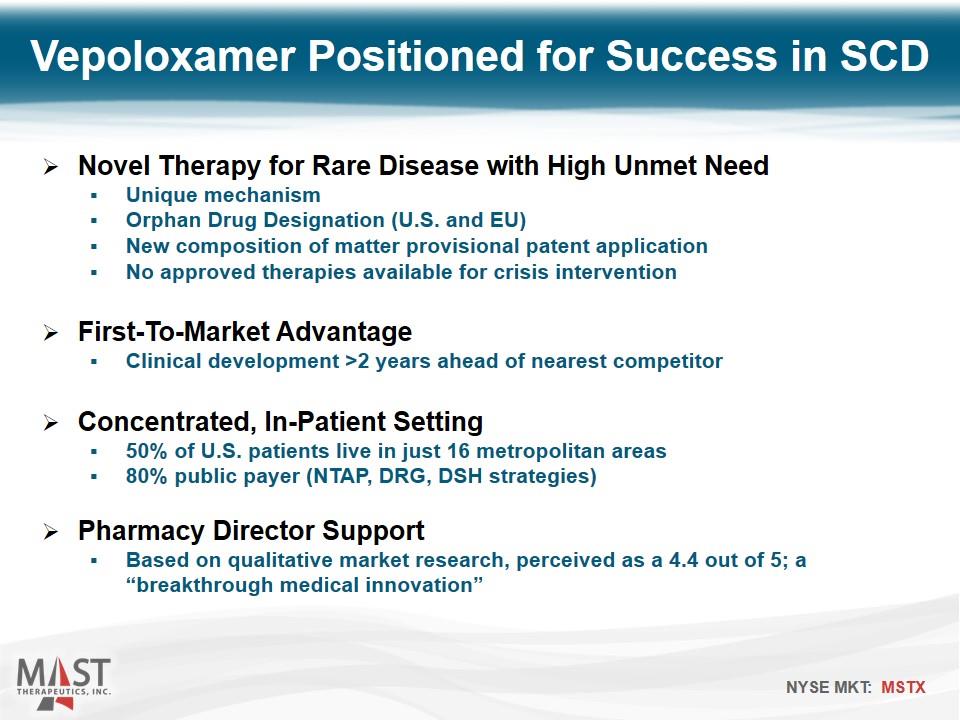
Vepoloxamer Positioned for Success in SCD Novel Therapy for Rare Disease with High Unmet Need Unique mechanism Orphan Drug Designation (U.S. and EU) New composition of matter provisional patent application No approved therapies available for crisis intervention First-To-Market Advantage Clinical development >2 years ahead of nearest competitor Concentrated, In-Patient Setting 50% of U.S. patients live in just 16 metropolitan areas 80% public payer (NTAP, DRG, DSH strategies) Pharmacy Director Support Based on qualitative market research, perceived as a 4.4 out of 5; a “breakthrough medical innovation”
Arterial Disease Vepoloxamer in Combination with Thrombolytics Acute Limb Ischemia Stroke Objective Accelerate time to thrombolysis and restore tissue perfusion
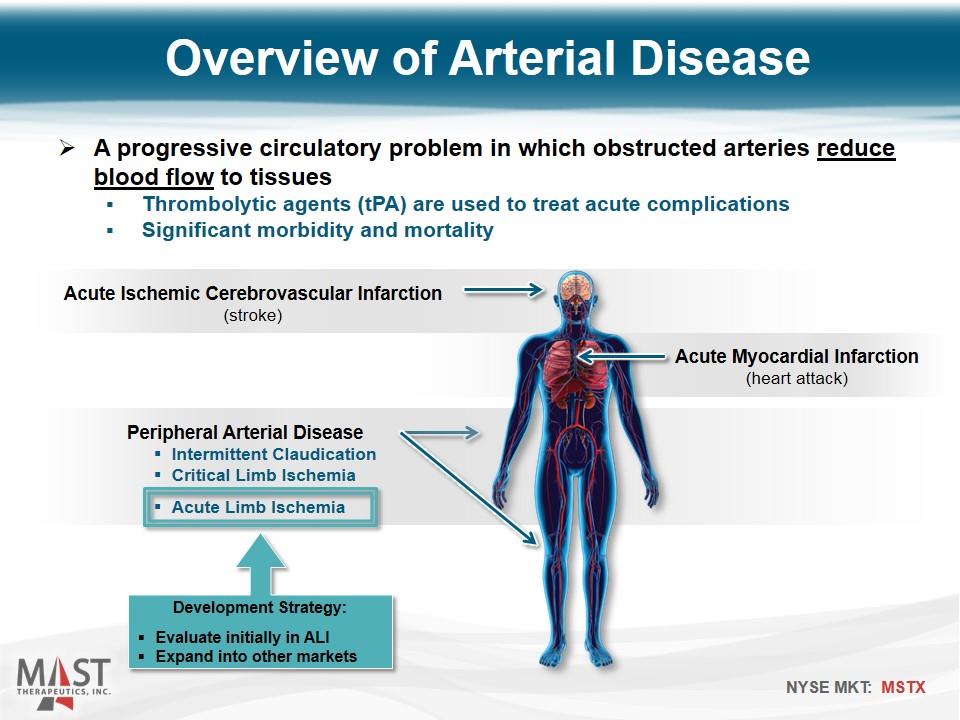
A progressive circulatory problem in which obstructed arteries reduce blood flow to tissues Thrombolytic agents (tPA) are used to treat acute complications Significant morbidity and mortality Acute Ischemic Cerebrovascular Infarction (stroke) Acute Myocardial Infarction (heart attack) Peripheral Arterial Disease Intermittent Claudication Critical Limb Ischemia Acute Limb Ischemia Development Strategy: Evaluate initially in ALI Expand into other markets Overview of Arterial Disease
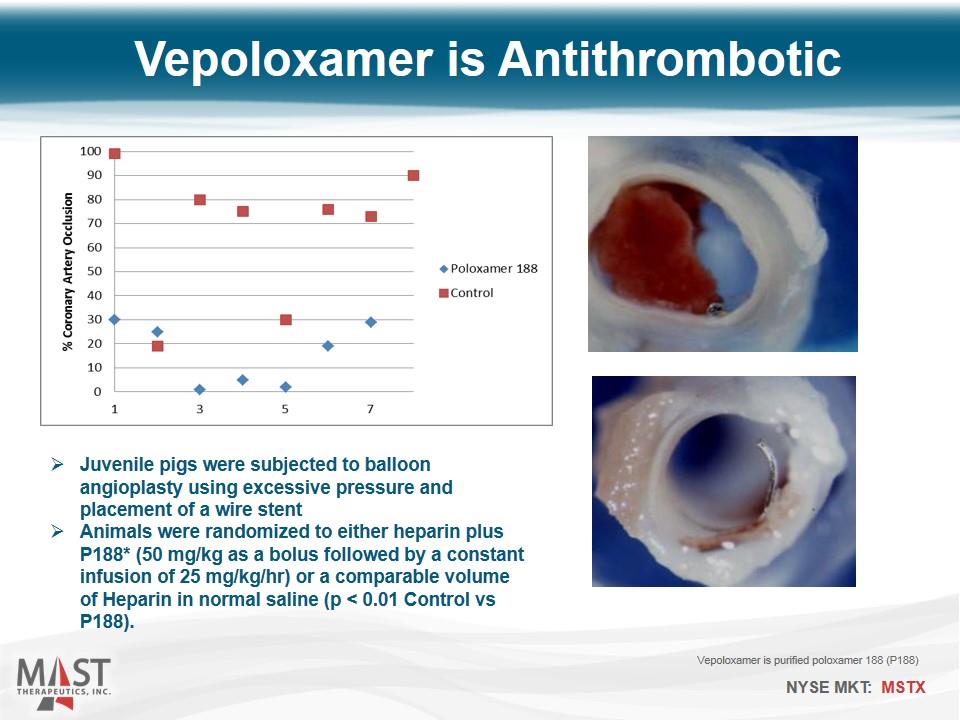
Vepoloxamer is Antithrombotic Juvenile pigs were subjected to balloon angioplasty using excessive pressure and placement of a wire stent Animals were randomized to either heparin plus P188* (50 mg/kg as a bolus followed by a constant infusion of 25 mg/kg/hr) or a comparable volume of Heparin in normal saline (p < 0.01 Control vs P188). Vepoloxamer is purified poloxamer 188 (P188)
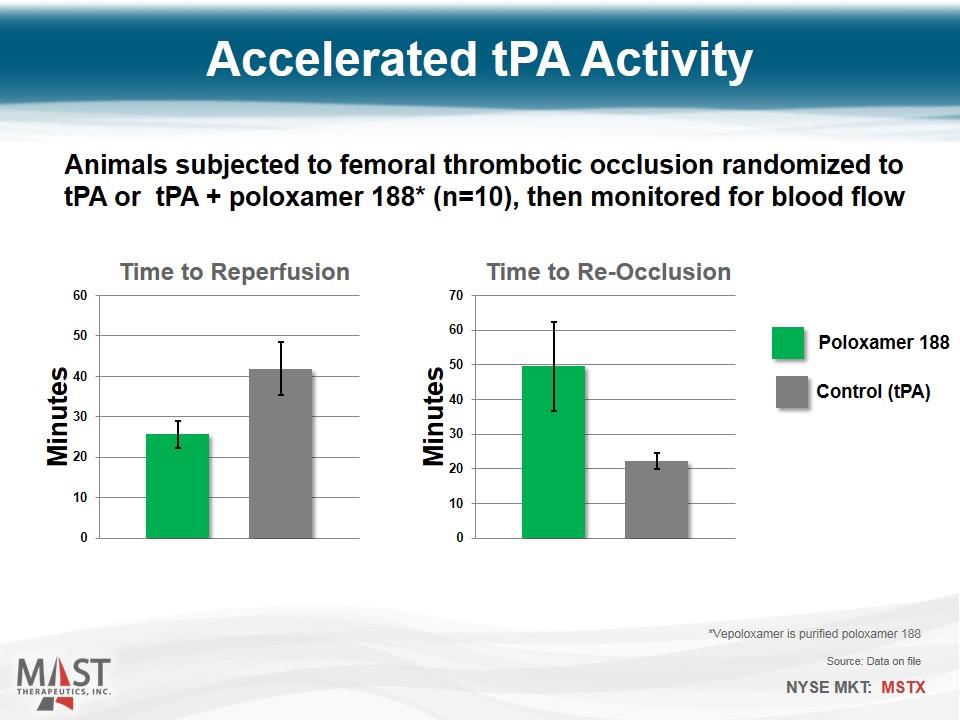
Accelerated tPA Activity Animals subjected to femoral thrombotic occlusion randomized to tPA or tPA + poloxamer 188* (n=10), then monitored for blood flow Control (tPA) Poloxamer 188 *Vepoloxamer is purified poloxamer 188 Source: Data on file
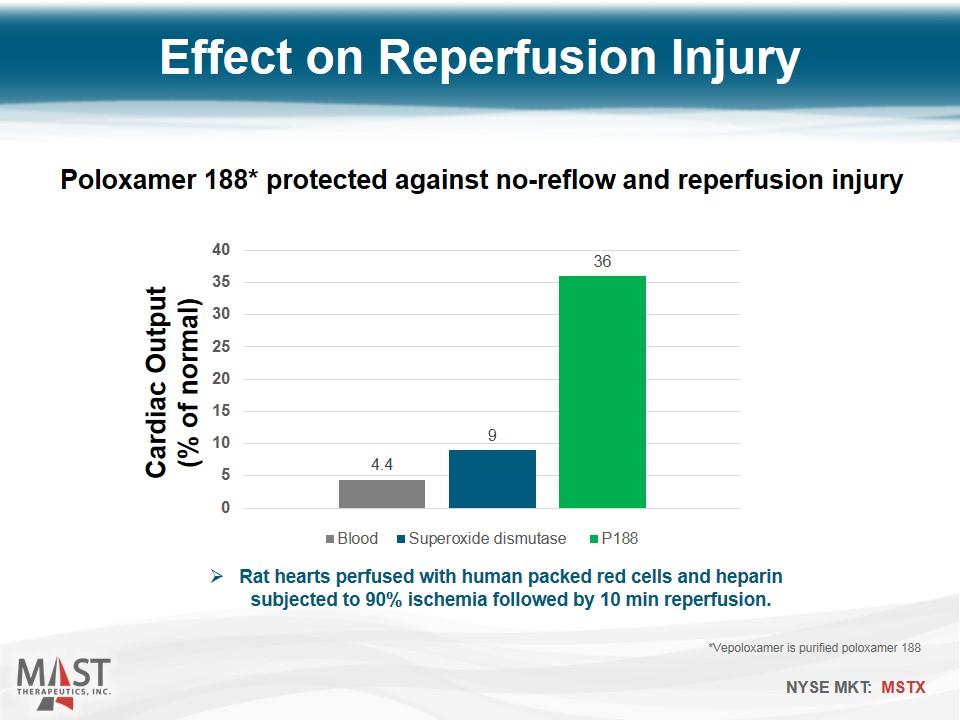
Cardiac Output (% of normal) Effect on Reperfusion Injury Rat hearts perfused with human packed red cells and heparin subjected to 90% ischemia followed by 10 min reperfusion. Poloxamer 188* protected against no-reflow and reperfusion injury *Vepoloxamer is purified poloxamer 188
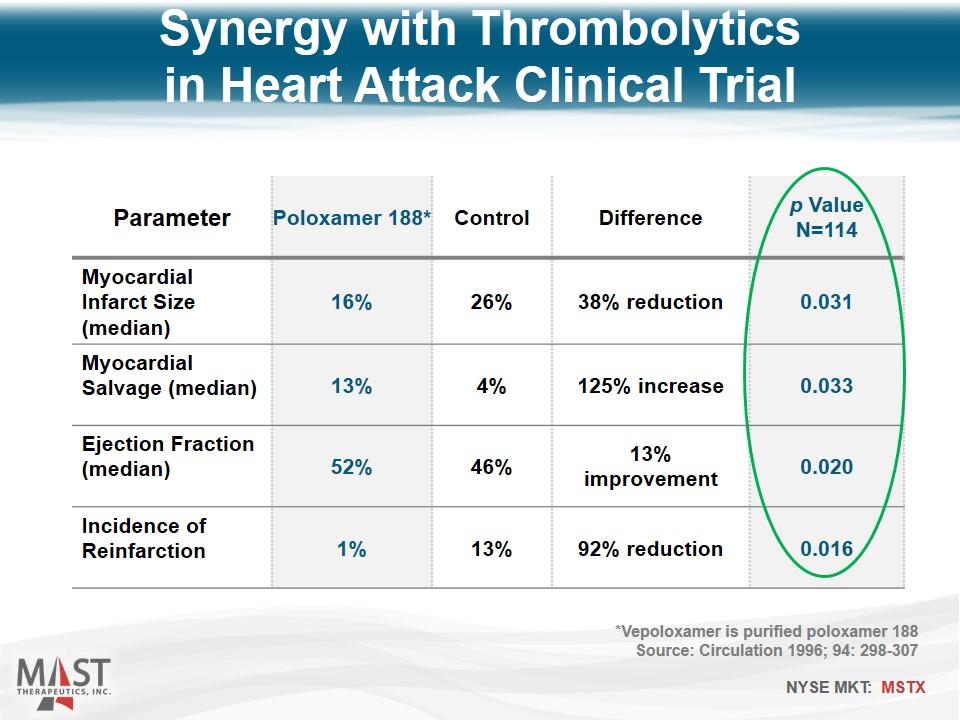
Parameter Poloxamer 188* Control Difference p Value N=114 Myocardial Infarct Size (median) 16% 26% 38% reduction 0.031 Myocardial Salvage (median) 13% 4% 125% increase 0.033 Ejection Fraction (median) 52% 46% 13% improvement 0.020 Incidence of Reinfarction 1% 13% 92% reduction 0.016 Synergy with Thrombolytics in Heart Attack Clinical Trial *Vepoloxamer is purified poloxamer 188 Source: Circulation 1996; 94: 298-307
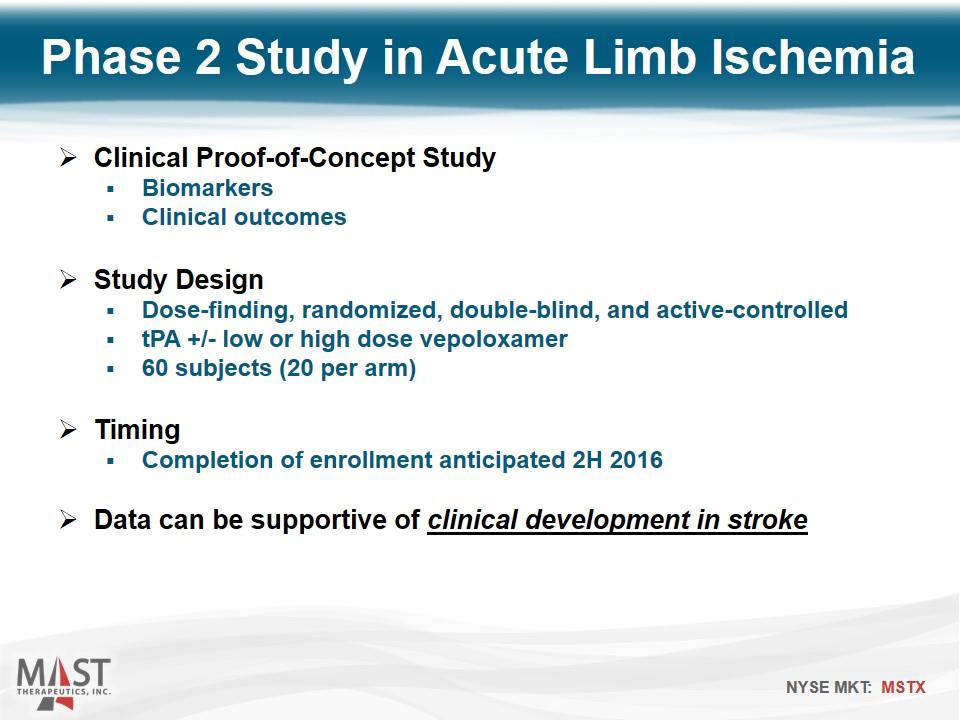
Clinical Proof-of-Concept Study Biomarkers Clinical outcomes Study Design Dose-finding, randomized, double-blind, and active-controlled tPA +/- low or high dose vepoloxamer 60 subjects (20 per arm) Timing Completion of enrollment anticipated 2H 2016 Data can be supportive of clinical development in stroke Phase 2 Study in Acute Limb Ischemia
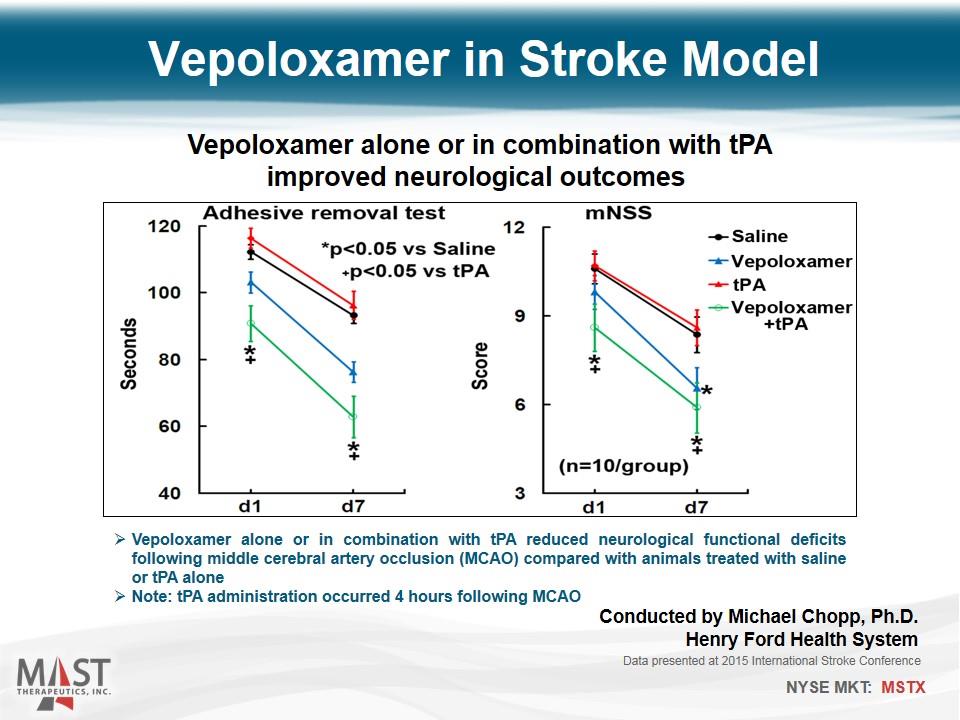
Vepoloxamer in Stroke Model Vepoloxamer alone or in combination with tPA improved neurological outcomes Vepoloxamer alone or in combination with tPA reduced neurological functional deficits following middle cerebral artery occlusion (MCAO) compared with animals treated with saline or tPA alone Note: tPA administration occurred 4 hours following MCAO Data presented at 2015 International Stroke Conference Conducted by Michael Chopp, Ph.D. Henry Ford Health System
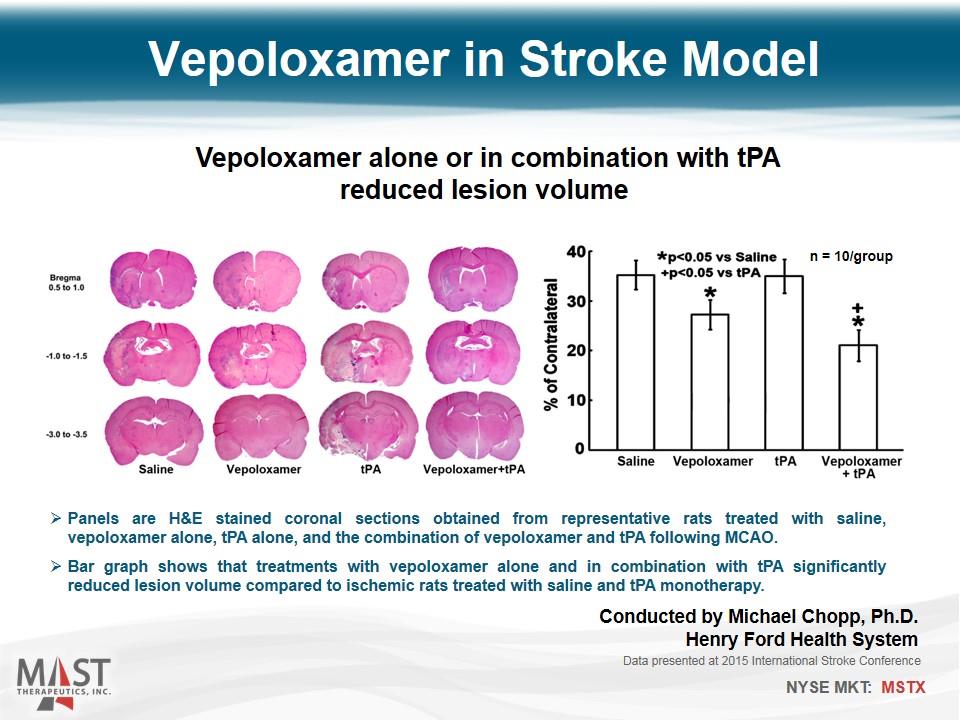
Vepoloxamer in Stroke Model Vepoloxamer alone or in combination with tPA reduced lesion volume Panels are H&E stained coronal sections obtained from representative rats treated with saline, vepoloxamer alone, tPA alone, and the combination of vepoloxamer and tPA following MCAO. Bar graph shows that treatments with vepoloxamer alone and in combination with tPA significantly reduced lesion volume compared to ischemic rats treated with saline and tPA monotherapy. Data presented at 2015 International Stroke Conference n = 10/group Conducted by Michael Chopp, Ph.D. Henry Ford Health System
Heart Failure (Chronic and Acute) Objective Preserve heart cells and improve cardiac function

Overview of Heart Failure Chronic condition characterized by decreasing heart function Heart cannot pump enough blood to meet the body’s needs Primary clinical symptom is difficulty breathing (fluid in lungs – “congestive”) Significant Unmet Medical Need Leading healthcare cost in U.S. and Europe Substantial and Growing Market Opportunity > 5 million individuals with heart failure in the U.S. $21 billion of direct costs for heart failure in the U.S. in 2012 Vepoloxamer Membrane-sealing activity may restore weakened cardiac cell membranes, minimizing calcium overload injury Durable effect may indicate a direct improvement in cardiac function
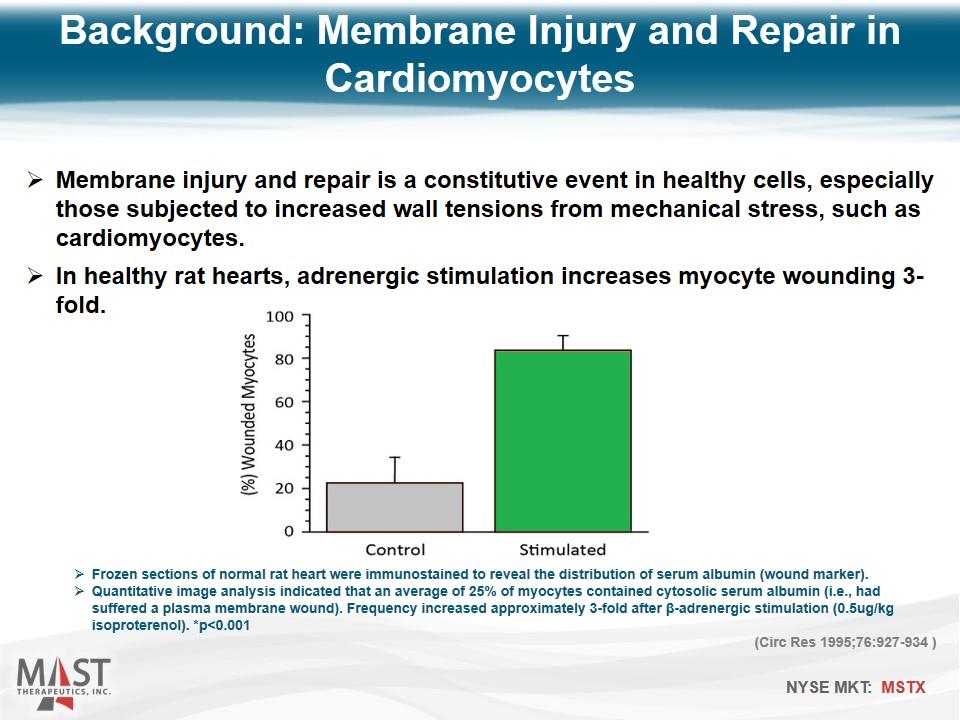
Membrane injury and repair is a constitutive event in healthy cells, especially those subjected to increased wall tensions from mechanical stress, such as cardiomyocytes. In healthy rat hearts, adrenergic stimulation increases myocyte wounding 3-fold. Frozen sections of normal rat heart were immunostained to reveal the distribution of serum albumin (wound marker). Quantitative image analysis indicated that an average of 25% of myocytes contained cytosolic serum albumin (i.e., had suffered a plasma membrane wound). Frequency increased approximately 3-fold after β-adrenergic stimulation (0.5ug/kg isoproterenol). *p<0.001 (Circ Res 1995;76:927-934 ) Background: Membrane Injury and Repair in Cardiomyocytes
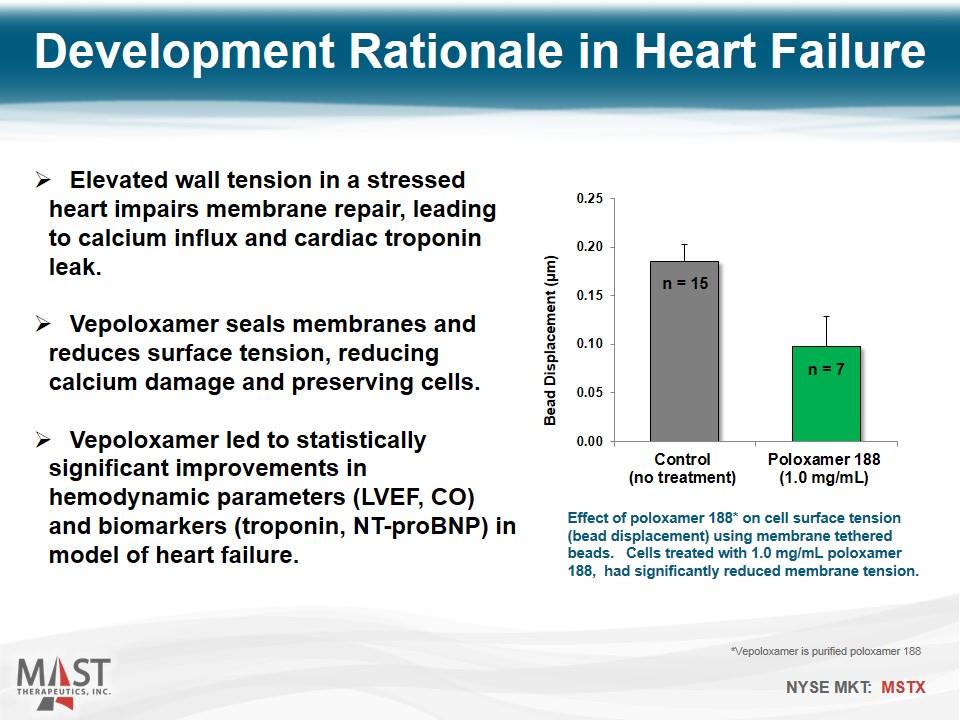
Elevated wall tension in a stressed heart impairs membrane repair, leading to calcium influx and cardiac troponin leak. Vepoloxamer seals membranes and reduces surface tension, reducing calcium damage and preserving cells. Vepoloxamer led to statistically significant improvements in hemodynamic parameters (LVEF, CO) and biomarkers (troponin, NT-proBNP) in model of heart failure. Effect of poloxamer 188* on cell surface tension (bead displacement) using membrane tethered beads. Cells treated with 1.0 mg/mL poloxamer 188, had significantly reduced membrane tension. Development Rationale in Heart Failure *Vepoloxamer is purified poloxamer 188 n = 15 n = 7
The primary objective of this study was to examine the effects of acute intravenous administration of vepoloxamer on left ventricular (LV) systolic and diastolic function in dogs with advanced heart failure produced by intracoronary microembolizations Chronic Heart Failure Model Study 1: Single-administration Conducted by Hani N. Sabbah, Ph.D., Henry Ford Health System Data presented at American Heart Society Scientific Sessions, November 2014
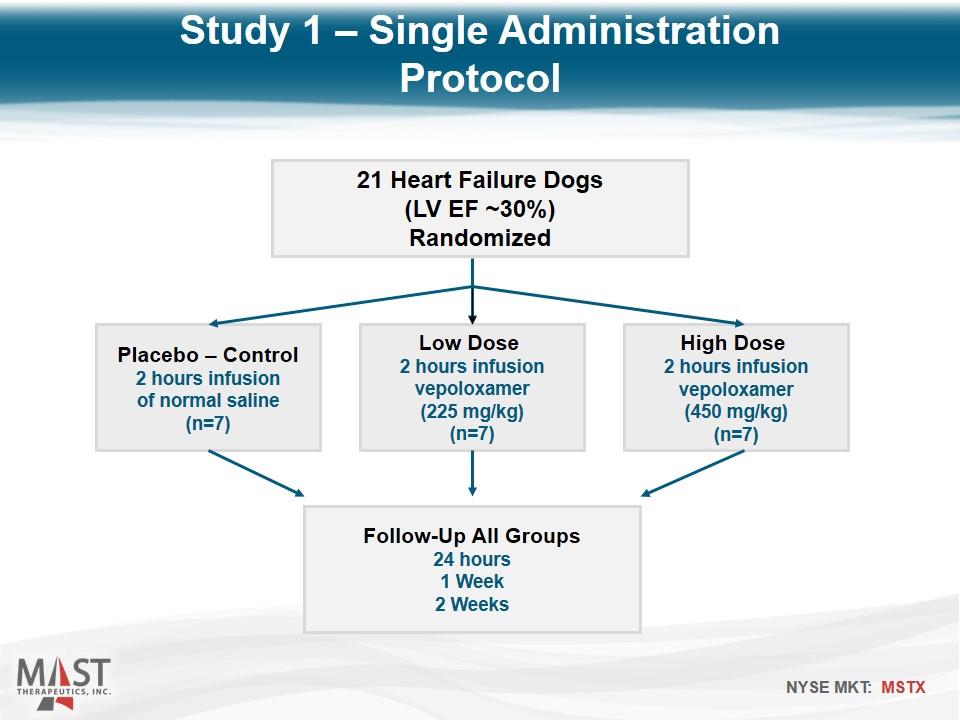
21 Heart Failure Dogs (LV EF ~30%) Randomized Placebo – Control 2 hours infusion of normal saline (n=7) Low Dose 2 hours infusion vepoloxamer (225 mg/kg) (n=7) High Dose 2 hours infusion vepoloxamer (450 mg/kg) (n=7) Follow-Up All Groups 24 hours 1 Week 2 Weeks Study 1 – Single Administration Protocol
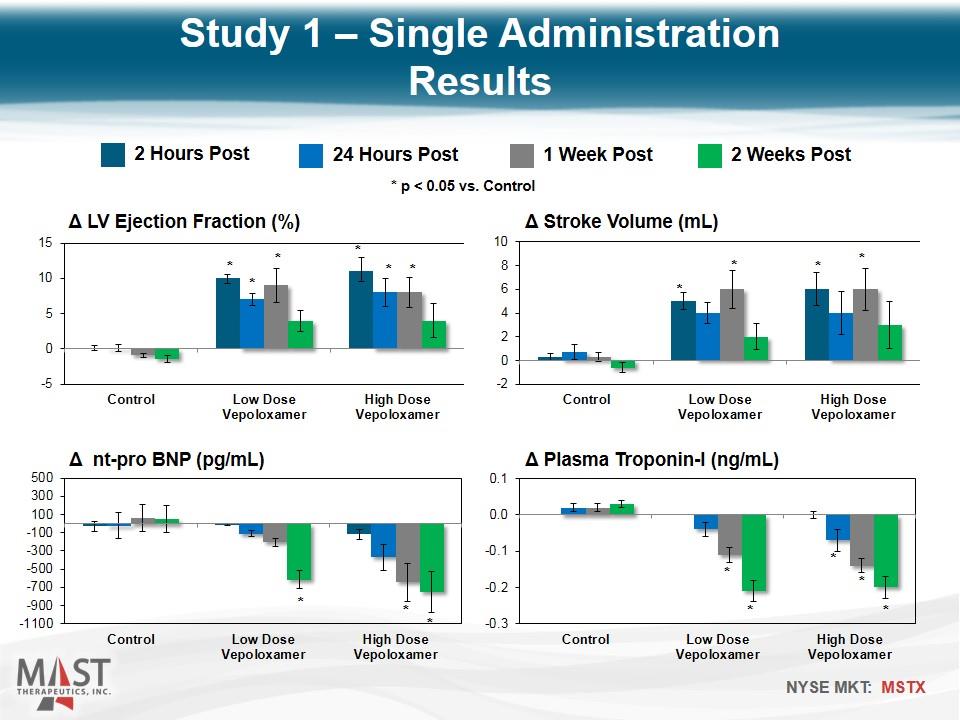
2 Hours Post 24 Hours Post 1 Week Post 2 Weeks Post * p < 0.05 vs. Control * * * * * * * * * * * * * * * * * * Study 1 – Single Administration Results
Intravenous vepoloxamer elicits improvements in LV systolic and diastolic function that last for at least one week after end of drug infusion The functional improvement is supported by significant reductions of NT-proBNP for up to 2 weeks The decline in plasma troponin-I level suggest that vepoloxamer may act to limit ongoing cardiomyocyte loss by limiting unregulated calcium entry into the cell and thus limiting calcium overload Study 1: Single Administration Conclusions
The primary objective of this study was to examine the effects of acute intravenous administration of multiple doses of vepoloxamer on left ventricular (LV) systolic and diastolic function in dogs with advanced heart failure produced by intracoronary microembolizations Chronic Heart Failure Model Study 2: Repeat-administration Conducted by Hani N. Sabbah, Ph.D. Henry Ford Health System
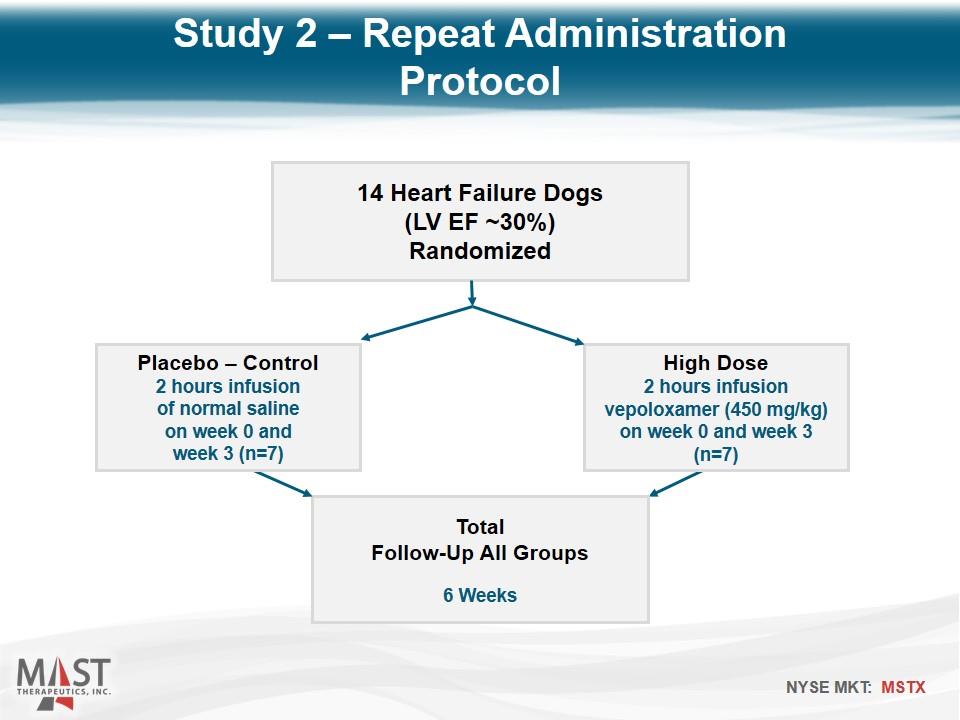
14 Heart Failure Dogs (LV EF ~30%) Randomized High Dose 2 hours infusion vepoloxamer (450 mg/kg) on week 0 and week 3 (n=7) Total Follow-Up All Groups 6 Weeks Placebo – Control 2 hours infusion of normal saline on week 0 and week 3 (n=7) Study 2 – Repeat Administration Protocol
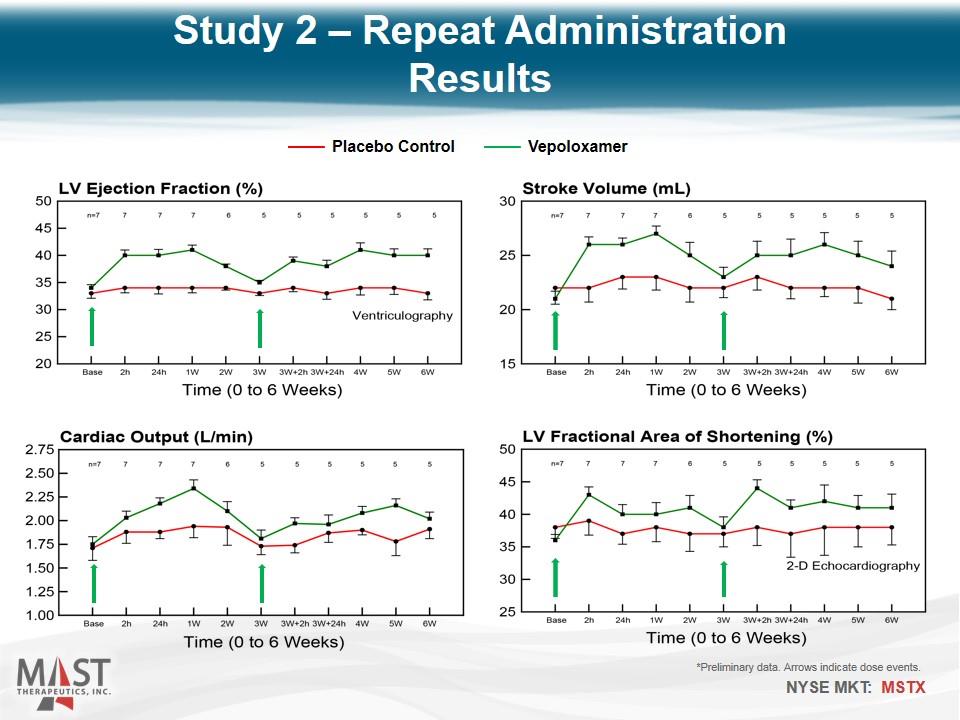
*Preliminary data. Arrows indicate dose events. Placebo Control Vepoloxamer Study 2 – Repeat Administration Results
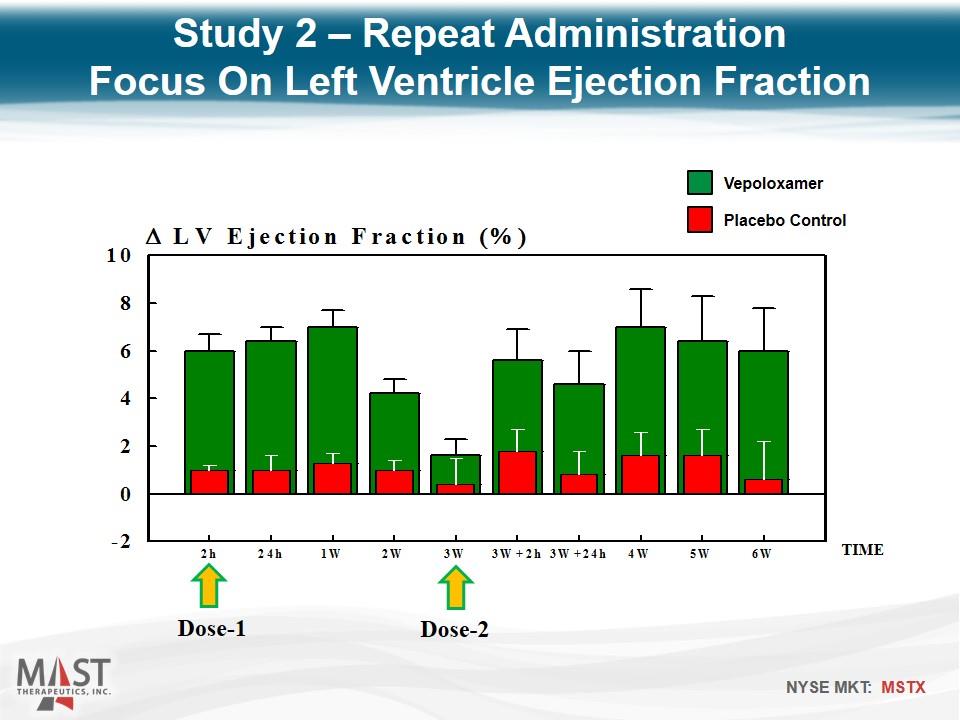
Dose-1 Dose-2 TIME Study 2 – Repeat Administration Focus On Left Ventricle Ejection Fraction Placebo Control Vepoloxamer

Reproduced Study 1 findings In addition, intravenous vepoloxamer pulsed once every 3 weeks elicits improvements in LV systolic and diastolic function that can be sustained for at least 6 weeks. Study 2 – Repeat Administration Conclusions
Protocol: Isolated cardiomyocytes were treated with vepoloxamer at room temperature for 2 hours Cells were then washed and treated with 10 uM Fura-2 AM dye for 1 hour Excess dye was then washed out and cells were resuspended in EDTA (calcium chelator) or 0.5 mM calcium chloride and flourescence intensity readings were obtained after 2 hours at 340/510 and 380/510 Calcium level (based on florescence levels) within the cell was calculated as the ratio of 340/380 Sealing membranes with vepoloxamer Study 2 – Repeat Administration Supplemental Findings Conducted by Hani N. Sabbah, Ph.D. Henry Ford Health System
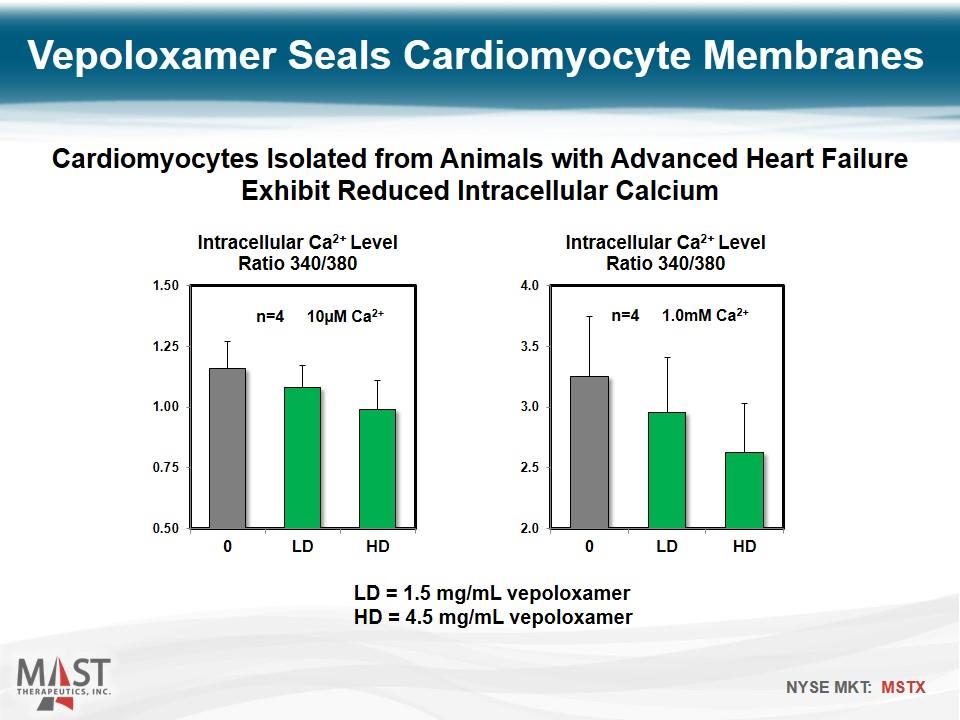
LD = 1.5 mg/mL vepoloxamer HD = 4.5 mg/mL vepoloxamer Vepoloxamer Seals Cardiomyocyte Membranes Cardiomyocytes Isolated from Animals with Advanced Heart Failure Exhibit Reduced Intracellular Calcium n=4 10µM Ca2+ n=4 1.0mM Ca2+
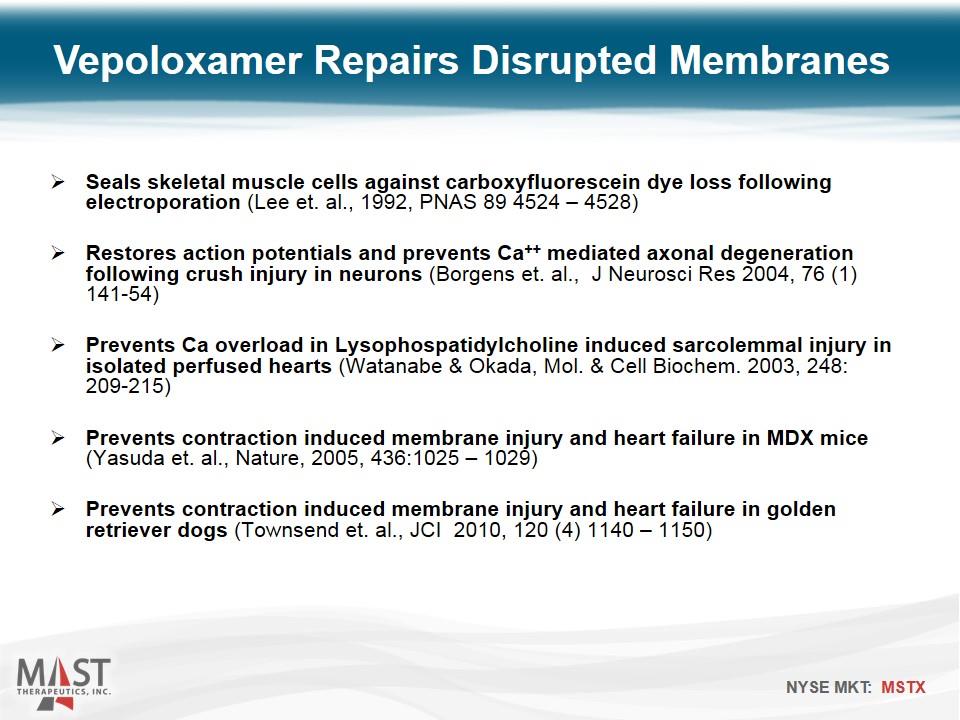
Vepoloxamer Repairs Disrupted Membranes Seals skeletal muscle cells against carboxyfluorescein dye loss following electroporation (Lee et. al., 1992, PNAS 89 4524 – 4528) Restores action potentials and prevents Ca++ mediated axonal degeneration following crush injury in neurons (Borgens et. al., J Neurosci Res 2004, 76 (1) 141-54) Prevents Ca overload in Lysophospatidylcholine induced sarcolemmal injury in isolated perfused hearts (Watanabe & Okada, Mol. & Cell Biochem. 2003, 248: 209-215) Prevents contraction induced membrane injury and heart failure in MDX mice (Yasuda et. al., Nature, 2005, 436:1025 – 1029) Prevents contraction induced membrane injury and heart failure in golden retriever dogs (Townsend et. al., JCI 2010, 120 (4) 1140 – 1150)
AIR001 (sodium nitrite) inhalation solution Objective Improve hemodynamics and exercise tolerance of patients with heart failure
Nitrite for intermittent inhalation (via nebulizer) Different molecule and activity than organonitrates or nitric oxide Beneficial effects include dilation of blood vessels and reduced inflammation Hemodynamic benefits include reductions in pulmonary vascular resistance pulmonary capillary wedge pressure right atrial pressure Safety data available in 124 subjects (well-tolerated) including exposures beyond 52 weeks AIR001 Overview
Three Phase 1 studies: Established MTD and safe dose level Acute improvements in hypoxia-induced pulmonary hypertension No drug-drug interaction with sildenafil One Phase 2 study: Well-tolerated; no treatment-related serious adverse events Showed improvement in median pulmonary vascular resistance (PVR) & median distances in 6-minute walk test Methemoglobin levels remained normal (< 1.5%) AIR001 Clinical Data
AIR001 for Heart Failure with Preserved Ejection Fraction (HFpEF) Responsible for ~50% of heart failure hospitalizations 80% develop pulmonary hypertension No approved medications Supporting three institutional-sponsored Phase 2a studies to: Evaluate the acute hemodynamic effects Evaluate the acute effects versus placebo on submaximal oxygen consumption and exercise hemodynamics Evaluate inhaled versus intravenous administration of nitrite and safety of multiple doses Preliminary data announcements 2H 2015 AIR001 Clinical Development Plan

Cash/investments at 3/31/15: $49.9 million Market capitalization: ~$83 million* Shares outstanding: ~163 million* Average daily volume (3 mo): ~1.1 million* No debt * As of June 19, 2015 MSTX Financial Overview

A Leader in Areas of Significant Unmet Need Sickle Cell Disease: Most advanced new drug in development Acute Limb Ischemia: Phase 2 ongoing; gateway to stroke Heart Failure: two distinct programs with new mechanisms Mast Therapeutics is committed to showing the clinical benefit of improving blood flow and sealing cell membranes in dysfunctional circulatory diseases Mast Therapeutics Summary
