Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AERIE PHARMACEUTICALS INC | d945772d8k.htm |
 Aerie
Pharmaceuticals, Inc. Company Overview
June 23, 2015 Building a Major Ophthalmic Pharmaceutical Company Exhibit 99.1 |
 2 Important Information Any discussion of the potential use or expected success of our product candidates is subject to our
product candidates being approved by regulatory authorities. In addition, any
discussion of clinical trial results
for Rhopressa TM relate to the results in its first Phase 3 registration trial, Rocket 1, and for Roclatan TM relate to the results in its Phase 2b clinical trial. The information in this presentation is current only as of its date and may have changed or may change
in the future. We undertake no obligation to update this information in light of new
information, future events or otherwise. We are not making any
representation or warranty that the information in this presentation is
accurate or complete. Certain statements in this presentation are
“forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,”
“expects,” “anticipates,” “plans,”
“intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans
and expectations. Known and unknown risks, uncertainties and other factors could cause
actual results to differ materially from those contemplated by the
statements. In evaluating these statements, you should specifically
consider various factors that may cause our actual results to differ materially from any forward-looking statements. In particular, the preclinical research discussed in this presentation is
preliminary and the outcome of such preclinical studies may not be predictive of the
outcome of later trials. Any future clinical trial results may not
demonstrate safety and efficacy sufficient to obtain regulatory approval
related to the preclinical research findings discussed in this presentation. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC,
particularly in the sections titled “Risk Factors” and
“Management’s Discussion and Analysis of Financial Condition
and Results of Operations.” Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements,
whether because of new information, future events or otherwise, except as otherwise
required by law. |
 3 Current Aerie Products: Once-Daily IOP-Lowering Eye Drops for Glaucoma Pre-Clinical Research Findings • Rhopressa™ shows potential to modify diseased tissue • May block fibrotic response in trabecular meshwork cells • May increase perfusion of the trabecular meshwork • AR-13154 shows potential for the treatment of wet AMD • May inhibit ROCK/JAK/PDGFR- • Lesion size reduction in rats exceeds market-leading product • Triple action Rhopressa™ • Inhibits ROCK and NET, lowers EVP, targets diseased tissue • P3 programs ongoing • Quadruple Action Roclatan™ • Fixed combination of Rhopressa™ and latanoprost • P2b achieved all clinical endpoints, P3 to start mid-2015 • Potentially most efficacious IOP-lowering therapy Aerie – Building a Major Ophthalmic Pharmaceutical Company Full patent protection through at least 2030; Blockbuster Potential These new preclinical discoveries represent potential breakthroughs |
 4 Uveoscleral Outflow NET RKI NET RKI Trabecular Meshwork Episcleral Veins Schlemm’s Canal Latanoprost Rhopressa ™ Triple-Action Rhopressa TM Quadruple-Action Roclatan™ IOP-Lowering Mechanisms Rhopressa TM : ROCK inhibition relaxes TM, increases outflow NET inhibition reduces fluid production ROCK inhibition lowers Episcleral Venous Pressure (EVP) Roclatan TM also adds latanoprost: PGA receptor activation increases uveoscleral outflow Ciliary Processes Cornea |
 5 increase Decreases Fluid Inflow/Production (Ciliary Processes) Increases Fluid Outflow: Secondary Drain (Uveoscleral Pathway) Increases Fluid Outflow: Primary Drain-Trabecular Meshwork
(TM); Lowers EVP -
(Episcleral Venous Pressure) Rhopressa™ Roclatan™ AA, BB, CAI PGAs Aerie Products Cover the IOP-lowering Spectrum |
 6 Baseline IOP* ~80% of U.S. Glaucoma Patients Have IOPs that are 26 mmHg at Time of Diagnosis The Baltimore Eye Survey * Sommer A, Tielsch JM, Katz J et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black
Americans: The Baltimore eye survey. Arch Ophthalmol 1991;109:1090-1095 . 21 mmHg (Normal Tension Glaucoma) >21 - 26 mmHg >26 - <35 mmHg Between 75% and 80% of Patients with IOP 25mmHg 60% 20% 20% |
 7 Latanoprost and Timolol Show Reduced Efficacy at Lower Baseline IOPs Pooled data from three latanoprost registration studies. Hedman and Alm; European Journal Ophthalmology;
2000 Latanoprost and timolol lose efficacy as baseline IOPs decline Timolol at least 1 mmHg less effective than latanoprost across all published baselines Timolol is the standard comparator for glaucoma Phase 3 trials -16 -14 -12 -10 -8 -6 -4 -2 0 16 18 20 22 24 26 28 30 32 34 36 38 Untreated Diurnal IOP (mmHg) Timolol (n=369) Latanoprost (n=460) |
 8 Rhopressa™ Registration Trial Design * PGAs have been shown to be less effective when dosed BID “Rocket 1” 90-Day Efficacy Registration Trial Rhopressa™ 0.02% QD 182 patients Timolol BID 188 patients “Rocket 2” One Year Safety (3 Mo. Interim Efficacy) Registration Trial “Rocket 3” One Year Safety Registration Trial Canada Rhopressa™ 0.02% QD ~230 patients Rhopressa™ 0.02% BID * ~230 patients Timolol BID ~230 patients Rhopressa™ 0.02% QD ~90 patients Rhopressa™ 0.02% BID ~90 patients Timolol BID ~60 patients |
 9 Rocket 1 Study Endpoints Efficacy: • The primary efficacy endpoint is the mean IOP at the following time points: 08:00, 10:00, and 16:00 at the Week 2, Week 6, and Day 90 visits. • IOP range above 20 mmHg and below 27 mmHg • Secondary efficacy endpoints include: • IOP analysis stratified by baseline IOP above and below 24 mmHg • Mean change from baseline IOP • Mean percent change from diurnally adjusted baseline IOP • Mean diurnal and change from baseline diurnal IOP Safety: • Ocular and systemic safety measures |
 10 Rocket 1: Summary Of Rhopressa™ Efficacy Results Baseline IOP (mmHg) Non-inferiority Numerical Superiority <27* Did not meet Met 2 time points <26 Met Met 4 time points <25 Met Met 7 time points <24** Met All (9 time points) <23 Met All (9 time points) <22 Insufficient power All (9 time points) * Primary endpoint ** Pre-specified secondary endpoint |
 11 Rhopressa TM Update: Rocket 2 • Rocket 2 primary endpoint range being changed with FDA agreement - New primary endpoint range is above 20 mmHg to below 25 mmHg - Statistical change allowed; Rocket 2 adequately powered at below 25 mmHg - No additional patient enrollment necessary • Rocket 2 data base not yet locked; patients still being treated • Efficacy read-out expected end of Q3 2015 • If Rocket 2 is successful, NDA filing expected by mid-2016 |
 12 Rocket 1: Baseline IOP < 25 mmHg At All Time Points |
 13 Rocket 1: Rhopressa TM Efficacy in Subjects On PGA Prior To Study (Baseline IOP < 25 mmHg) • Prior PGA use produced enhanced IOP-lowering with Rhopressa TM at weeks 2 and 6 • IOP lowering at month 3 equivalent to IOP lowering in non-PGA subjects • Prior PGA use had no effect on Timolol efficacy |
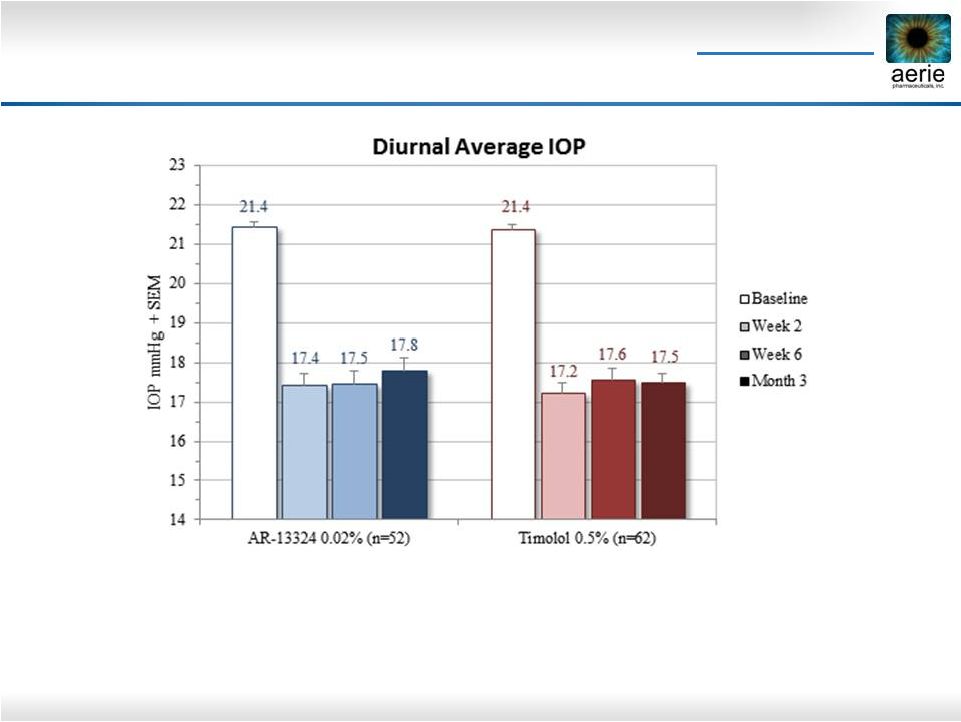 14 Rocket 1: Rhopressa TM Efficacy In Subjects Not on PGA Prior To Study (Baseline IOP < 25
mmHg) •
No loss of efficacy seen from week 2 to month 3 for Rhopressa
TM or Timolol |
 15 Rhopressa TM IOP-lowering Effect Enhanced In Subjects On PGA Therapy Prior To Study Entry Prospective (pre-specified) analysis by pre-study medication status showed that prior PGA use enhanced Rhopressa TM IOP-lowering at week 2 (p=0.003) The PGA effect is lost over time, which we believe creates a false impression that Rhopressa TM loses efficacy over time Retrospective analysis of Phase 2 trial results shows prior PGA use enhanced Rhopressa TM IOP-lowering by 1 mmHg (p=0.007) and 1.2 mmHg (p=0.002) at weeks 2 and 4, respectively, relative to subjects not previously on PGA |
 16 Rhopressa TM /PGA Synergy May Reflect Complementary Actions on TM Extracellular Matrix ROCK inhibition relaxes trabecular meshwork (TM) cells by reducing actin stress fibers and reducing production of ECM 1 PGAs lower IOP by increasing ECM turnover in uveoscleral pathway- but subtle changes in TM pathway also observed with long-term dosing 2 • PGAs have no obvious effect on TM outflow on their own • Subtle PGA-induced changes in ECM may sensitize TM cells to the unique IOP-lowering effects of Rhopressa TM Daily exposure to both Rhopressa TM and a PGA may result in ongoing synergy – and may also explain positive Roclatan TM P2b results References: 1. Pattabiraman P and Rao P (2010) Am J Physiol Cell Physiol 298:C749-63; 2. Richter M et al. (2003) Invest Ophthalmol Vis Sci. 44:4419-26 |
 17 FY 2014 U.S. Glaucoma Market = $2.2B; 33M TRx Market Share in TRx PGA Market Non-PGA Market Rhopressa TM Synergy with PGAs May Represent a Market Opportunity Once Daily 2-3 Times Daily Bimatoprost Travoprost Latanoprost BB Fixed Combo AA CAI PGA: Prostaglandin Analogue; BB: Beta Blocker; AA: Alpha Agonist; CAI: Carbonic Anhydrase Inhibitor
Source: IMS MIDAS, IMS NPA
8% 15% 14% 33% 10% 9% 10% |
 18 Rhopressa TM Next Steps: Rocket 4 • Planning to commence Rocket 4 in Q3 2015, an additional Rhopressa TM trial in the U.S. • Baseline IOPs: - Primary <25 mmHg to mirror revised Rocket 2 - Pre-specified secondary <27 mmHg - Considering stratification - May enroll patients up to 30 mmHg • Efficacy evaluated at 3 months (primary) and 6 months (secondary) • Final design to be reviewed with FDA • Read-out expected in approximately one year • Adds over 200 additional Rhopressa TM patients for 6 month EU safety |
 19 Roclatan™ Phase 2b Clinical Trial Design Phase 2b Protocol Roclatan™ 0.01% vs. Roclatan™ 0.02% vs. Rhopressa™ 0.02% vs. Latanoprost All Dosed QD PM ~300 Patients 28 Days Primary efficacy endpoint: Mean diurnal IOP on Day 29 Two concentrations of Roclatan™ vs. Rhopressa™ 0.02% and latanoprost Trial design follows FDA requirement for fixed-dose combination Statistically significant difference at measured time points Higher combo efficacy vs. components of at least 1–3 mmHg, as previously accepted by FDA for product approval |
 20 Roclatan™ Phase 2b Clinical Trial Performance Achieved primary efficacy measure Superiority over each of the components on day 29 Achieved statistical superiority over the individual components at all time points More efficacious than latanoprost by 1.6 – 3.2 mmHg More efficacious than Rhopressa™ by 1.7 – 3.4 mmHg Main adverse event was hyperemia (eye redness): Reported in 40 percent of patients Mild for the large majority of patients No systemic drug-related adverse events |
 21 0.02% Roclatan™ Achieved Statistical Superiority Over Individual Components at All Time Points (p<0.001) Roclatan™ Phase 2b, Intent to Treat 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Pre- 8AM 8AM 10AM 4PM 8AM 10AM 4PM 8AM 10AM 4PM 8AM 10AM 4PM 8AM Study Qual 1 Baseline Day 8 Day 15 Day 29 Day 30 0.02% Rhopressa™ (n=78) 0.005% Latanoprost (n=73) 0.02% Roclatan™ (n=72) Mean IOP at Each Time Point Primary Efficacy Measure |
 22 Roclatan TM Next Steps • Commencing “Mercury 1” in Q3 2015 in the U.S. - Designed for superiority to individual components, similar to P2b - Baseline IOP range tentatively > 20 mmHg to <36 mmHg, with
stratified enrollment
- Multiple secondary endpoints
- Efficacy trial with one year safety • “Mercury 2” expected to commence in 2016 in the U.S. - Expect same comparators as Mercury 1 - Three month efficacy study • “Mercury 3” expected to commence in 2016 in Europe - Comparing to a leading combo product marketed in EU - Efficacy study, duration TBD |
 23 Aerie Financial Resources • As of March 31, 2015 had $179.3M of cash and investments on balance sheet • Expected to fund Aerie operations for approximately the next 3 years • Proceeding with clinical path outlined for Rhopressa TM and Roclatan TM , and continue to evaluate potential for pre-clinical Aerie molecules and outside opportunities |
 24 Summary • Key Clinical Priorities • Rhopressa TM : Rocket 2 efficacy readout end of Q3
2015
• Roclatan TM : Mercury 1 commencement Q3 2015 • Research Initiatives • Rhopressa TM disease modification and neuro-protection • AR-13154 potential in wet AMD, etc. • Evaluating Aerie’s 3,000+ owned molecules • Potential Business Development Opportunities • Evaluating additions to ophthalmic product pipeline • Exploring drug delivery opportunities – front and back of eye Rocket 4 commencement Q3 2015 Mercury 2 and 3 commencement early 2016 |
 Building a Major Ophthalmic Pharmaceutical Company |
