Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Inspyr Therapeutics, Inc. | v413365_8k.htm |
Exhibit 99.01

1 BIO International Presentation June 2015 OTC QB : GNSZ Craig A. Dionne, PhD President & CEO

2 Safe Harbor Statement Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995: Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, the outcome of its clinical trials, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the Company's filings and future filings made with the United States Securities and Exchange Commission. Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera directly. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock. This presentation is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of the presentation and are subject to change without notice.

3 • An innovation - driven biotech company that unlocks conventional thinking • Deep experience in cancer drug discovery and development • A leader in prodrug therapeutics for the treatment of cancer • Robust, global Intellectual Property platform • Highly - differentiated drugs utilizing natural, plant - derived toxin • Global barrier to generic entry via exclusive manufacturing and farming controls of raw goods • World class strategic partners • 2015 is tipping point for value creation GenSpera Profile
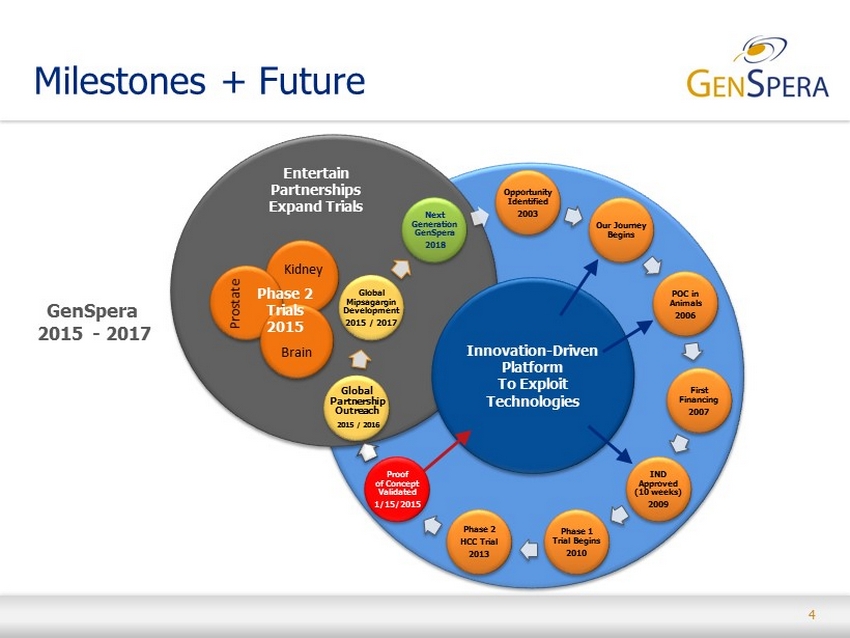
4 Milestones + Future Opportunity Identified 2003 Our Journey Begins POC in Animals 2006 First Financing 2007 IND Approved (10 weeks) 2009 Phase 1 Trial Begins 2010 Phase 2 HCC Trial 2013 Global Partnership Outreach 2015 / 2016 Global Mipsagargin Development 2015 / 2017 Next Generation GenSpera 2018 GenSpera 2015 - 2017 Entertain Partnerships Expand Trials Innovation - Driven Platform To Exploit Technologies Proof of Concept Validated 1/15/2015 Phase 2 Trials 2015 Prostate Brain Kidney

5 Initial Market Focus Tremendous unmet needs in numerous types of cancer Mipsagargin attacks a universal attribute of all solid tumors Liver Cancer • 3rd largest cancer - killer worldwide • $10 billion by 2020 Brain Cancer • 13,000 deaths annually in the U.S. • Avastin expected to generate $460 million in revenue by 2017 Prostate Cancer • 240,000 new cases diagnosed annually in the U.S. • $6.7 billion by 2020 Kidney Cancer • 64,000 new cases diagnosed annually in the U.S. • 14,000 deaths annually in the U.S.

6 Mechanism of Action 1. Blood vessels feed living tumor 2. Drug circulates in bloodstream in benign fashion 3. Enzyme within tumor blood vessels activates drug 4. Activated drug kills blood vessels and tumor cells 5. Death of t umor As a result: • Potential for Complete Tumor Kill • Fewer Side Effects Precision Targeting by Design

7 HCC patient with gastrohepatic metastatic lymph node involvement (yellow arrows). Increased hypoenhancement after mipsagargin treatment (right panel) suggests response. Pharmacodynamic Proof of Principal via DCE - MRI Before After

8 • Unique, naturally derived, cancer killing drug has advantages in potency and significantly reduced side - effects • Highly differentiated from other anti - cancer approaches • Minimal and manageable side effects • No apparent effect on bone marrow – combination with immunotherapy is viable • Prolonged disease stabilization in a significant percentage of HCC patients • Pharmacodynamic proof - of - principle via DCE - MRI imaging Clinical Activity of Mipsagargin

9 GenSpera Clinical Programs – Evaluating Multiple Oncology Indications RESEARCH PRE - CLINICAL PHASE 1 PHASE 2 Liver Cancer (HCC) TARGET SITE DRUG INDICATION Brain Cancer (GBM) Prostate Cancer (PCa) Mipsagargin Blood vessels of most solid tumors DEVELOPMENT STAGE HUMAN CLINICAL TRIALS PHASE 3 25 patients treated in Ph2 study at 5 sites – Study Closed – Results presented 13 patients treated in Ph2 study at UCSD* Ph2 to begin Q3 2015 at UTHSC Houston* * Ph2 costs primarily supported by investigator funds Kidney Cancer (RCC) Ph2 to begin Q4 2015 at UTHSC Houston* 2015 Partner Outreach • Three Phase 2 trials ongoing 2015 • Data with IP = Increased corporate valuation • Provides expanded options for partnering • Global market = partnership opportunities driven by disease type

10 • World Class pedigree - technology developed at Johns Hopkins University and the University of Copenhagen • Current portfolio • Patent coverage in U.S. to 2023 • Orphan Drug designation and patent restoration add significant coverage • Data exclusivity outside of U.S. - up to 10 years after drug approval in EU • Portfolio expansion strategy • New composition of matter PCT - Worldwide exclusivity expected through 2033 • Other applications filed for methods of use, formulations, process improvements • Development of commercial scale thapsigargin production is a barrier to generic intrusion Intellectual Property

11 • Promising business development conversations/negotiations under CDAs with Pharma in US, Asia and EU • Promising glioblastoma clinical trial expanded to 34 patients • National Institute of Health grant support • Prostate clinical trial begins this month • Expanded partnership with Phyton Biotech for manufacture Mid Year Review

12 • Initiate next liver cancer clinical trial 4Q 2015 • Complete glioblastoma and prostate Phase 2 trials by 2 nd quarter 2016 • Expand management and staffing • Complete new financing to support expanded clinical trial operations • Expand corporate partnership discussions/negotiations Near Term Business Goals

13 Proof of Concept/Data create future value • Shareholders • Company options 2015 Tipping Point for growth • Additional Phase 2 studies • Initiate next liver cancer studies • World Class Partnership outreach Undervalued • $ 25mm Market Cap, comparable between $200mm and $600mm • Share price at $ 0.75 vs $3.00 or higher GenSpera in the News • Public Relations • Social Media Channels • 3rd Party Endorsements Summary Recent Events June 15, 2015 – National Institute of Health awards grant supporting GenSpera’s glioblastoma clinical trial June 9, 2015 – KTLA TV’s Health Smart showcases GenSpera’s cancer research June 3, 2015 – GenSpera CEO Craig Dionne interviewed on Sirius Radio May 20, 2015 GenSpera CEO Craig Dionne to present H.C. Wainwright & Co.’s panel discussion during ASCO 2015 May 13, 2015 – GenSpera expands Phase II glioblastoma trial of mipsagargin May 6, 2015 – GenSpera enters national phase with patent application for injectable prodrug compositions April 28, 2015 - GenSpera granted new patent for prodrugs activated by Prostate Specific Antigen

14 GenSpera, Inc. ( OTC QB: GNSZ) 2511 N Loop 1604 W, Suite 204 San Antonio, TX 78258 Craig Dionne, PhD CEO and Chairman (210) 479 - 8112 info@genspera.com Investor Relations Adam Holdsworth PCG Advisory Group (646) 862 - 4607 adamh@pcgadvisory.com Connect with GenSpera https://www.facebook.com/GenSpera https://twitter.com/GenSperaNews https://www.linkedin.com/company/genspera - inc - https://plus.google.com/u/0/b/111021404 725787491742/ 111021404725787491742 / posts https://www.youtube.com/ channel/UCdvATHFsc6Z LVgVMTJtPq3A http://www.thechairmansblog.com/genspera
