Attached files
| file | filename |
|---|---|
| 8-K - 8-K - bluebird bio, Inc. | blue-8k_20150615.htm |
| EX-99.2 - EX-99.2 - bluebird bio, Inc. | blue-ex992_201506158.htm |
| EX-99.1 - EX-99.1 - bluebird bio, Inc. | blue-ex991_201506157.htm |

Transforming the Lives of Patients with Severe Genetic and Rare Diseases EHA 2015 Clinical Data Update Making Hope a Reality Exhibit 99.3

Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward looking statements. For example, all statements we make regarding the initiation, timing, progress and results of our preclinical and clinical studies and our research and development programs, our ability to advance product candidates into, and successfully complete, clinical studies, and the timing or likelihood of regulatory filings and approvals are forward looking. All forward-looking statements are based on estimates and assumptions by our management that, although we believe to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected. These statements are also subject to a number of material risks and uncertainties that are described in our most recent quarterly report on Form 10-Q, as well as our subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Nasdaq: BLUE
bluebird bio: Why We Do What We Do Our Vision – Make Hope a Reality Seeking to transform the lives of patients with severe genetic and rare diseases through the development of innovative gene therapy products. Ethan Cameron Aidan
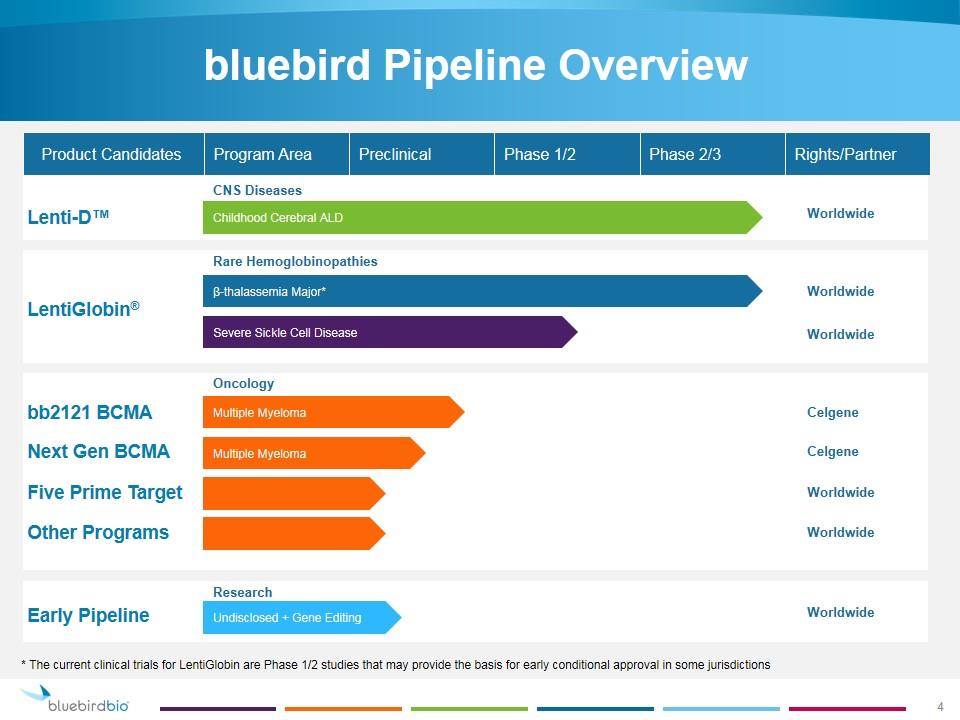
bluebird Pipeline Overview Childhood Cerebral ALD β-thalassemia Major* Multiple Myeloma Lenti-D™ LentiGlobin® bb2121 BCMA Undisclosed + Gene Editing Product Candidates Severe Sickle Cell Disease Early Pipeline CNS Diseases Oncology Rare Hemoglobinopathies Research Multiple Myeloma Next Gen BCMA Five Prime Target Other Programs Program Area Preclinical Phase 1/2 Phase 2/3 Rights/Partner Worldwide Celgene Worldwide Worldwide Worldwide Worldwide Celgene Worldwide * The current clinical trials for LentiGlobin are Phase 1/2 studies that may provide the basis for early conditional approval in some jurisdictions
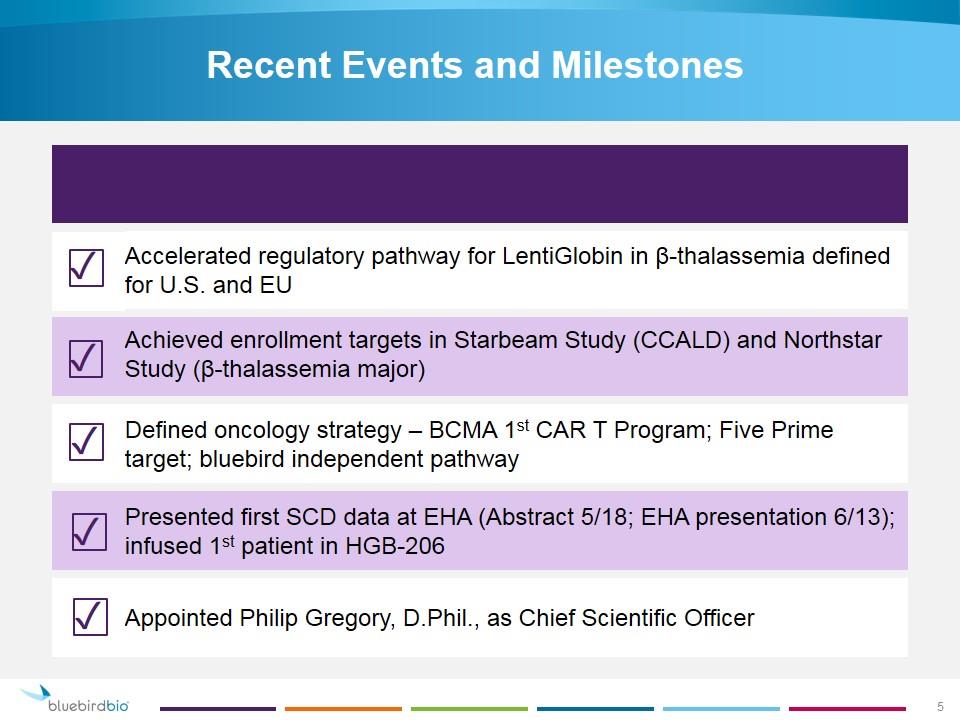
Recent Events and Milestones Accelerated regulatory pathway for LentiGlobin in β-thalassemia defined for U.S. and EU Achieved enrollment targets in Starbeam Study (CCALD) and Northstar Study (β-thalassemia major) Defined oncology strategy – BCMA 1st CAR T Program; Five Prime target; bluebird independent pathway Presented first SCD data at EHA (Abstract 5/18; EHA presentation 6/13); infused 1st patient in HGB-206 Appointed Philip Gregory, D.Phil., as Chief Scientific Officer ✓ ✓ ✓ ✓ ✓ 5

New CSO Appointed 15 years of experience in biopharmaceutical industry Extensive gene therapy and genome editing experience Formerly Chief Scientific Officer and Senior Vice President of Research at Sangamo BioSciences D.Phil. in Biochemistry from Oxford University; B.Sc. in Microbiology from Sheffield University Philip Gregory, D. Phil. Chief Scientific Officer 6

EHA Data Update
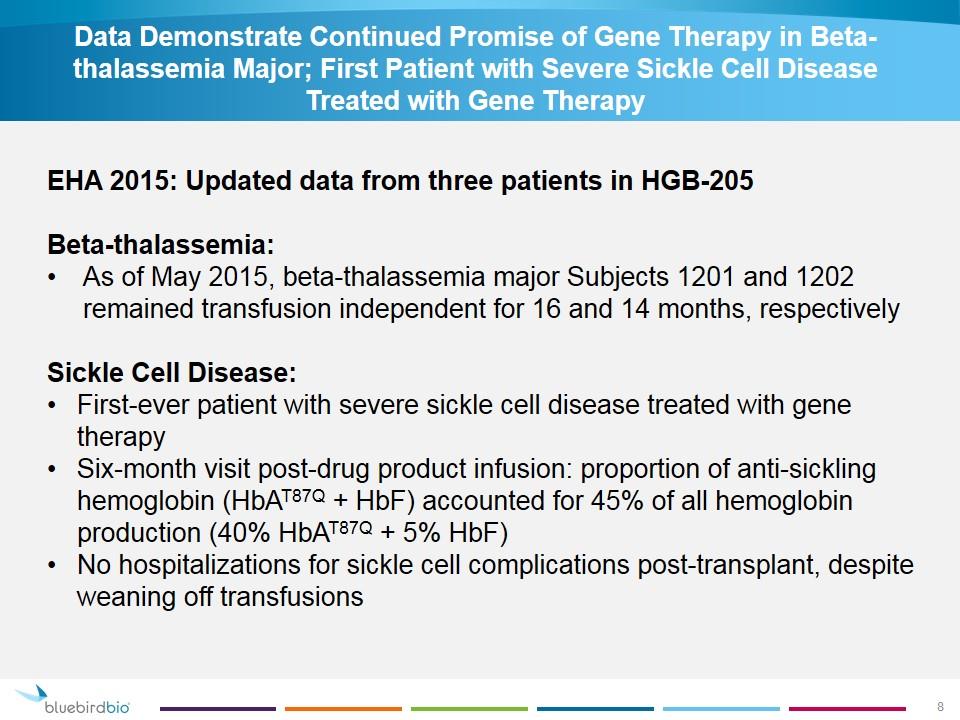
Data Demonstrate Continued Promise of Gene Therapy in Beta-thalassemia Major; First Patient with Severe Sickle Cell Disease Treated with Gene Therapy EHA 2015: Updated data from three patients in HGB-205 Beta-thalassemia: As of May 2015, beta-thalassemia major Subjects 1201 and 1202 remained transfusion independent for 16 and 14 months, respectively Sickle Cell Disease: First-ever patient with severe sickle cell disease treated with gene therapy Six-month visit post-drug product infusion: proportion of anti-sickling hemoglobin (HbAT87Q + HbF) accounted for 45% of all hemoglobin production (40% HbAT87Q + 5% HbF) No hospitalizations for sickle cell complications post-transplant, despite weaning off transfusions
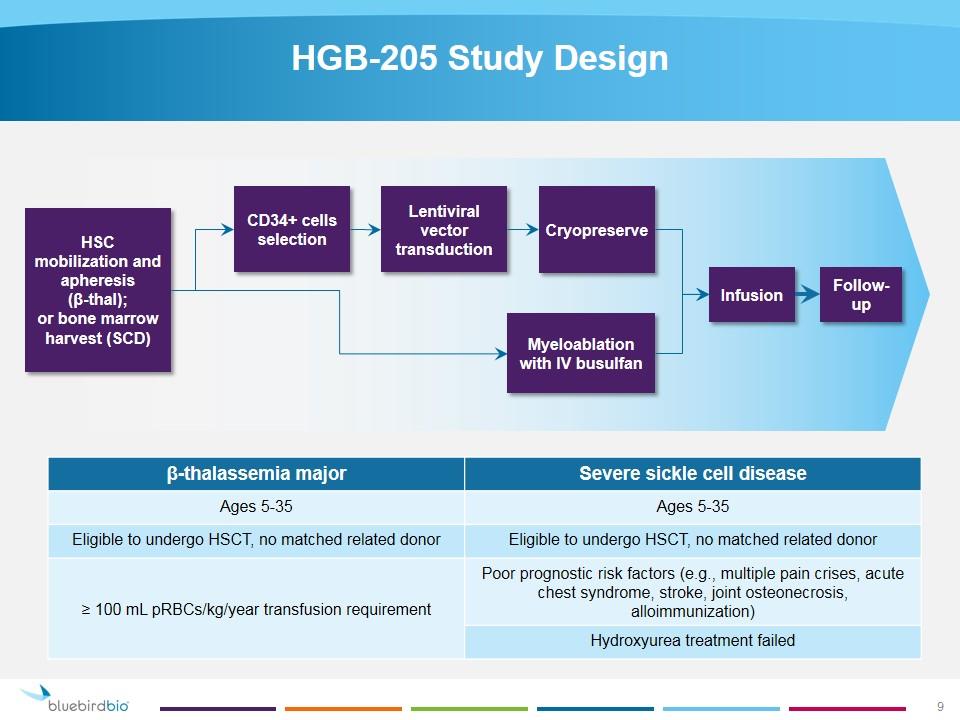
HGB-205 Study Design β-thalassemia major Severe sickle cell disease Ages 5-35 Ages 5-35 Eligible to undergo HSCT, no matched related donor Eligible to undergo HSCT, no matched related donor ≥ 100 mL pRBCs/kg/year transfusion requirement Poor prognostic risk factors (e.g., multiple pain crises, acute chest syndrome, stroke, joint osteonecrosis, alloimmunization) Hydroxyurea treatment failed HSC mobilization and apheresis (β-thal); or bone marrow harvest (SCD) CD34+ cells selection Lentiviral vector transduction Cryopreserve Myeloablation with IV busulfan Infusion Follow-up
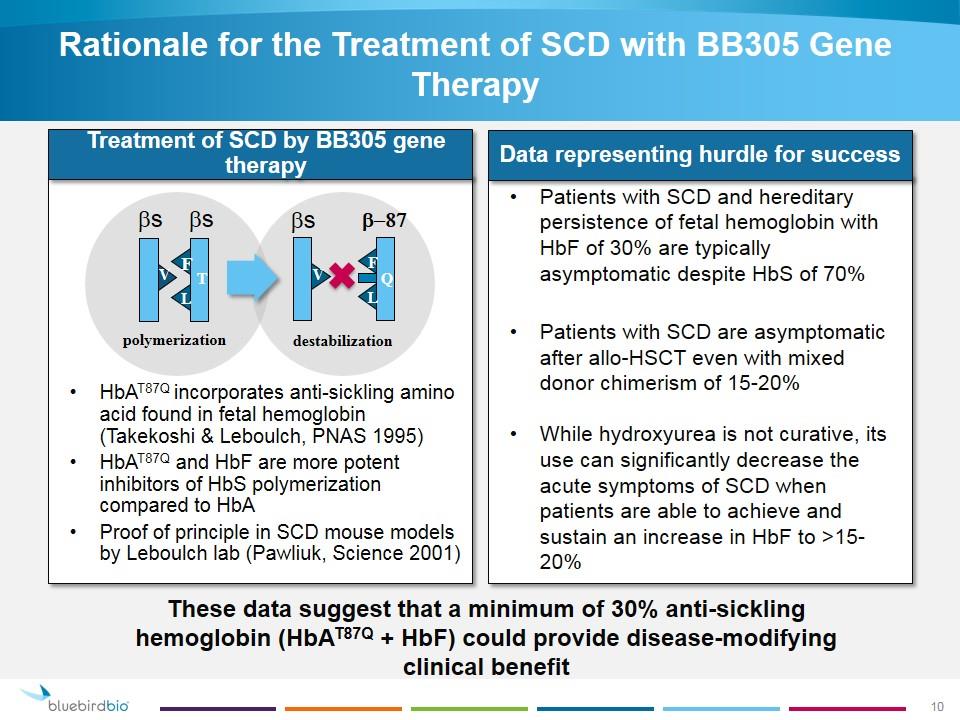
Rationale for the Treatment of SCD with BB305 Gene Therapy Patients with SCD and hereditary persistence of fetal hemoglobin with HbF of 30% are typically asymptomatic despite HbS of 70% Patients with SCD are asymptomatic after allo-HSCT even with mixed donor chimerism of 15-20% While hydroxyurea is not curative, its use can significantly decrease the acute symptoms of SCD when patients are able to achieve and sustain an increase in HbF to >15-20% HbAT87Q incorporates anti-sickling amino acid found in fetal hemoglobin (Takekoshi & Leboulch, PNAS 1995) HbAT87Q and HbF are more potent inhibitors of HbS polymerization compared to HbA Proof of principle in SCD mouse models by Leboulch lab (Pawliuk, Science 2001) polymerization destabilization V F L T bs bs V F L Q bs b-87 Treatment of SCD by BB305 gene therapy Data representing hurdle for success These data suggest that a minimum of 30% anti-sickling hemoglobin (HbAT87Q + HbF) could provide disease-modifying clinical benefit
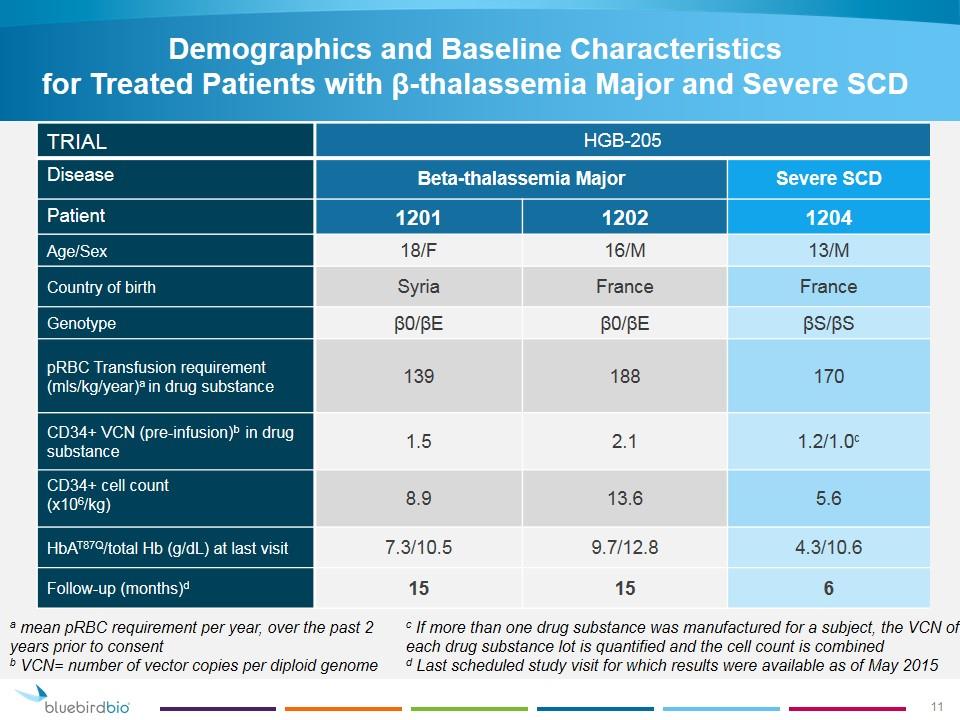
Demographics and Baseline Characteristics for Treated Patients with β-thalassemia Major and Severe SCD TRIAL HGB-205 Disease Beta-thalassemia Major Severe SCD Patient 1201 1202 1204 Age/Sex 18/F 16/M 13/M Country of birth Syria France France Genotype β0/βE β0/βE βS/βS pRBC Transfusion requirement (mls/kg/year)a in drug substance 139 188 170 CD34+ VCN (pre-infusion)b in drug substance 1.5 2.1 1.2/1.0c CD34+ cell count (x106/kg) 8.9 13.6 5.6 HbAT87Q/total Hb (g/dL) at last visit 7.3/10.5 9.7/12.8 4.3/10.6 Follow-up (months)d 15 15 6 a mean pRBC requirement per year, over the past 2 years prior to consent b VCN= number of vector copies per diploid genome c If more than one drug substance was manufactured for a subject, the VCN of each drug substance lot is quantified and the cell count is combined d Last scheduled study visit for which results were available as of May 2015
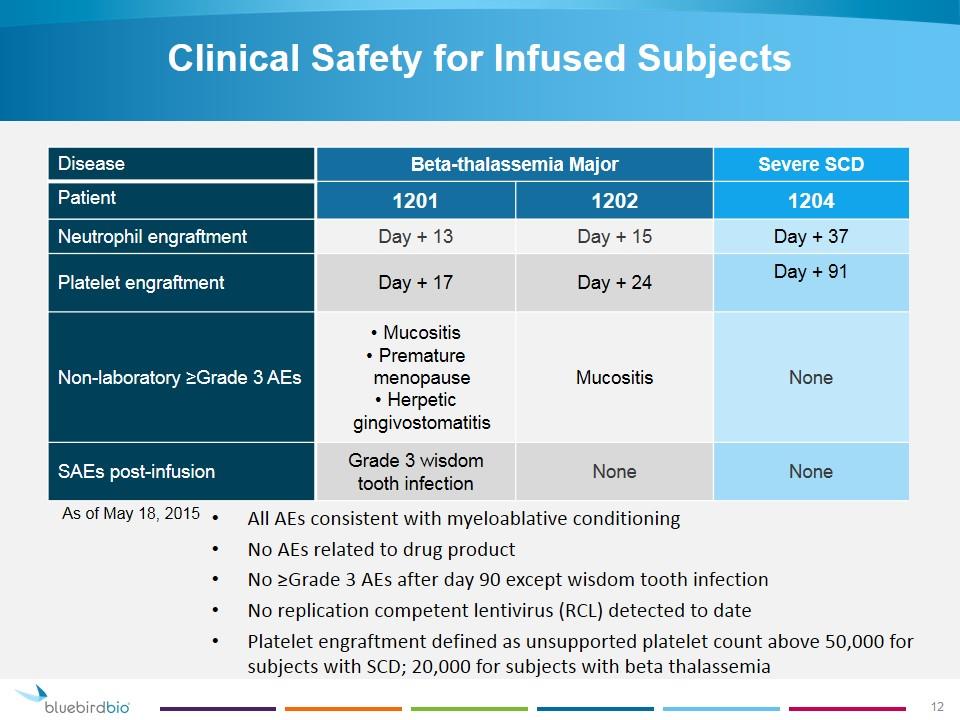
Clinical Safety for Infused Subjects Disease Beta-thalassemia Major Severe SCD Patient 1201 1202 1204 Neutrophil engraftment Day + 13 Day + 15 Day + 37 Platelet engraftment Day + 17 Day + 24 Day + 91 Non-laboratory ≥Grade 3 AEs Mucositis Premature menopause Herpetic gingivostomatitis Mucositis None SAEs post-infusion Grade 3 wisdom tooth infection None None As of May 18, 2015 All AEs consistent with myeloablative conditioning No AEs related to drug product No ≥Grade 3 AEs after day 90 except wisdom tooth infection No replication competent lentivirus (RCL) detected to date Platelet engraftment defined as unsupported platelet count above 50,000 for subjects with SCD; 20,000 for subjects with beta thalassemia
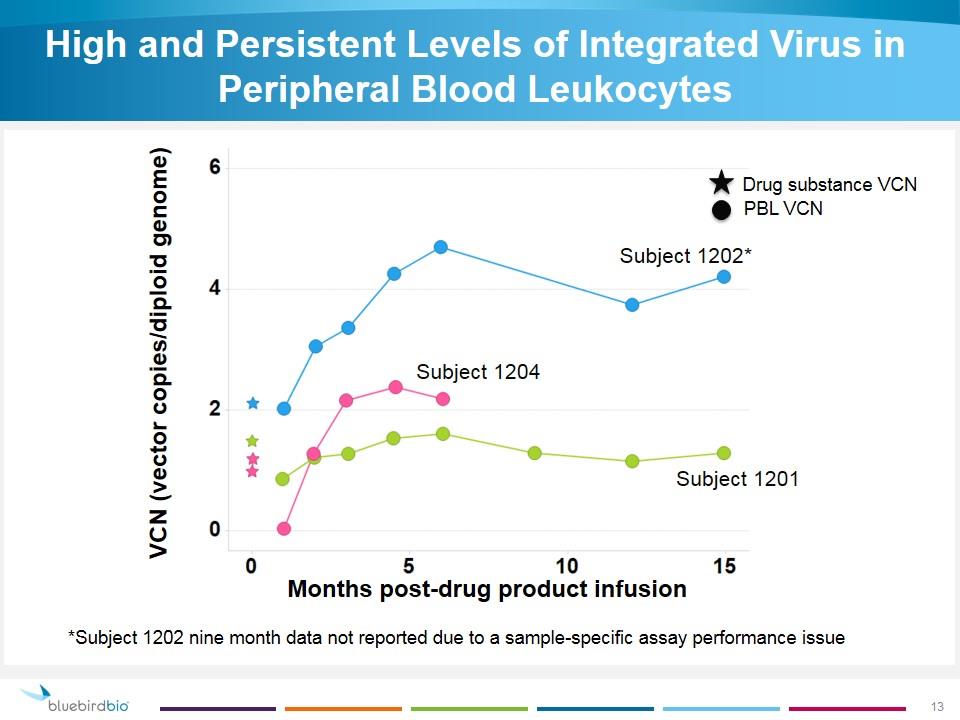
High and Persistent Levels of Integrated Virus in Peripheral Blood Leukocytes VCN (vector copies/diploid genome) Months post-drug product infusion *Subject 1202 nine month data not reported due to a sample-specific assay performance issue Drug substance VCN PBL VCN Subject 1202* Subject 1201 Subject 1204
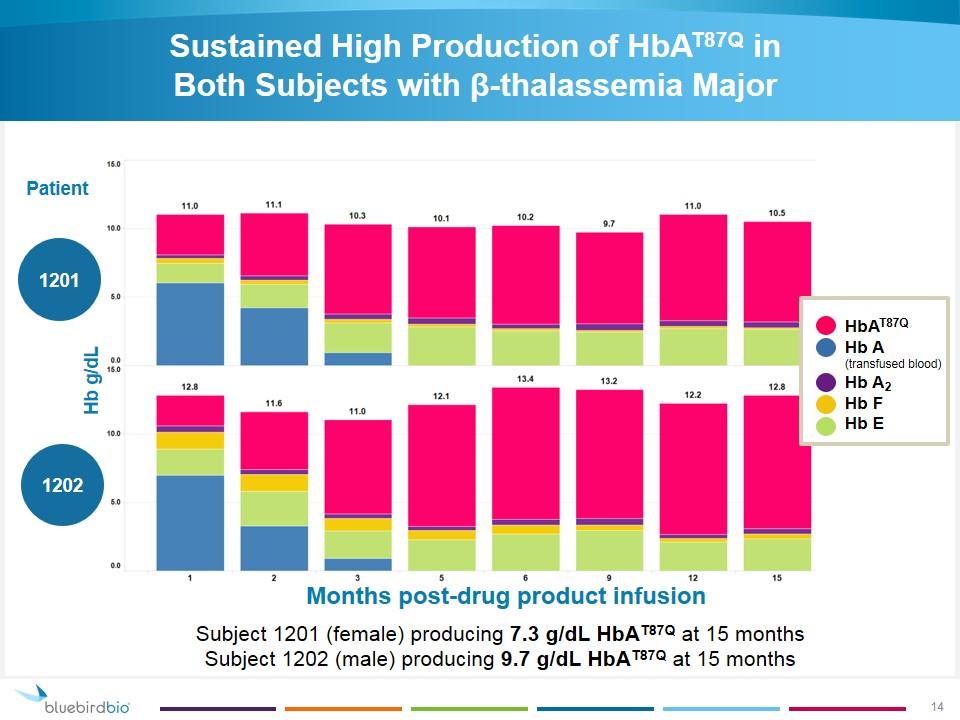
Sustained High Production of HbAT87Q in Both Subjects with β-thalassemia Major Hb g/dL Months post-drug product infusion Patient 1201 1202 Subject 1201 (female) producing 7.3 g/dL HbAT87Q at 15 months Subject 1202 (male) producing 9.7 g/dL HbAT87Q at 15 months HbAT87Q Hb A (transfused blood) Hb A2 Hb F Hb E
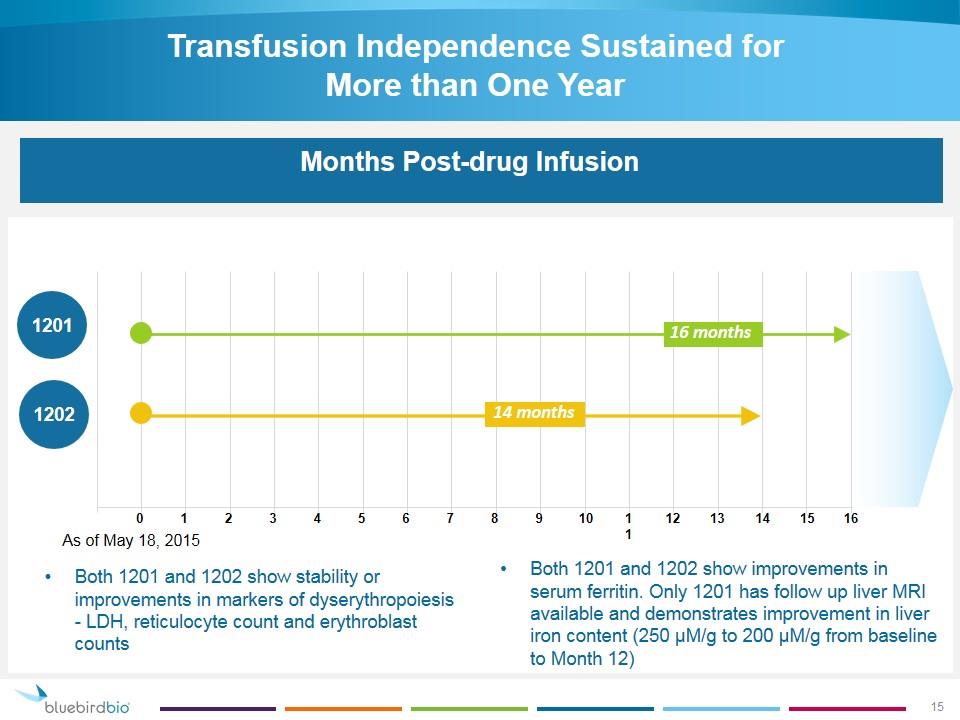
Transfusion Independence Sustained for More than One Year Days Transfusion-Free Months Post-drug Infusion Both 1201 and 1202 show stability or improvements in markers of dyserythropoiesis - LDH, reticulocyte count and erythroblast counts 1201 1202 0 1 2 3 4 5 6 7 8 9 10 16 11 12 13 14 15 14 months 16 months Both 1201 and 1202 show improvements in serum ferritin. Only 1201 has follow up liver MRI available and demonstrates improvement in liver iron content (250 μM/g to 200 μM/g from baseline to Month 12) As of May 18, 2015
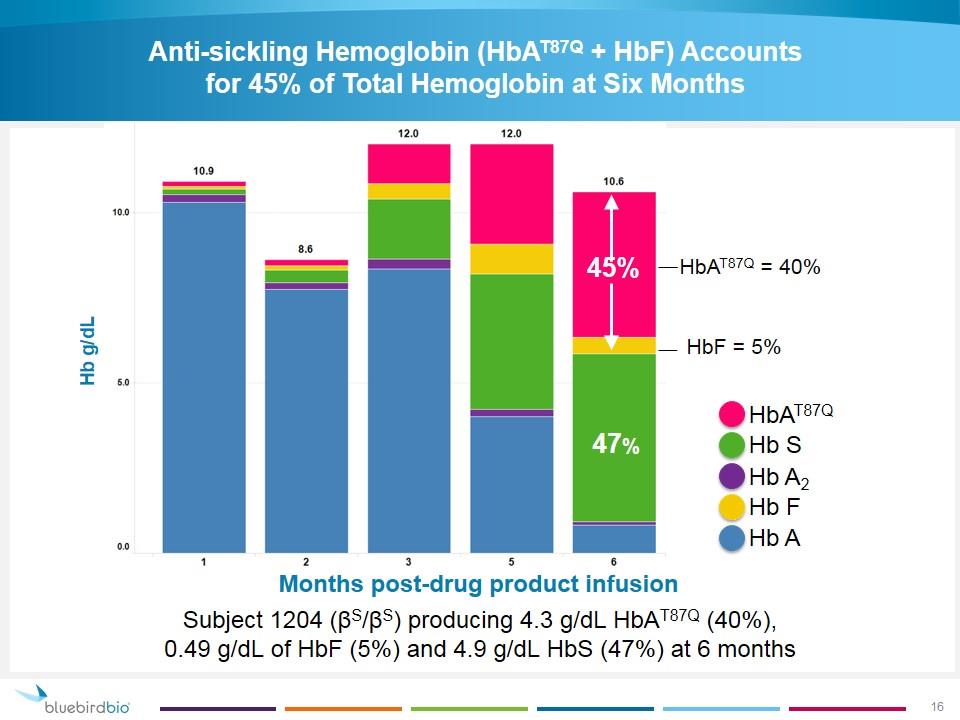
Anti-sickling Hemoglobin (HbAT87Q + HbF) Accounts for 45% of Total Hemoglobin at Six Months Hb g/dL Months post-drug product infusion HbAT87Q Hb S Hb A2 Hb F Hb A Subject 1204 (βS/βS) producing 4.3 g/dL HbAT87Q (40%), 0.49 g/dL of HbF (5%) and 4.9 g/dL HbS (47%) at 6 months HbAT87Q + HbF = 45% 47% 45% 47% HbAT87Q = 40% HbF = 5%
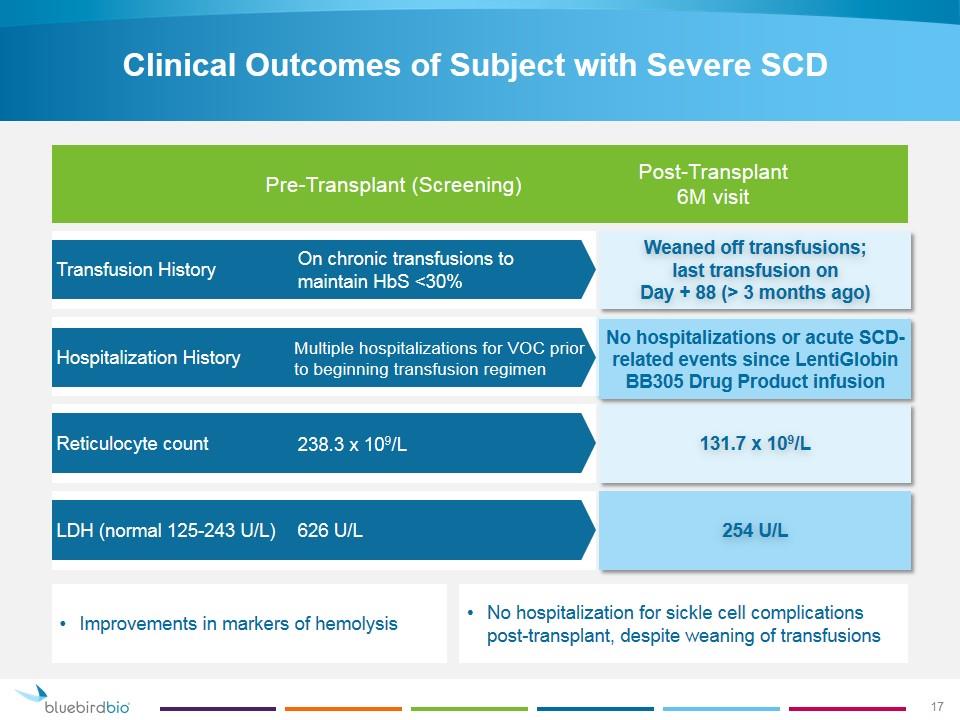
Clinical Outcomes of Subject with Severe SCD Transfusion History Hospitalization History Reticulocyte count LDH (normal 125-243 U/L) Weaned off transfusions; last transfusion on Day + 88 (> 3 months ago) 131.7 x 109/L No hospitalizations or acute SCD-related events since LentiGlobin BB305 Drug Product infusion 254 U/L Pre-Transplant (Screening) Post-Transplant 6M visit On chronic transfusions to maintain HbS <30% Multiple hospitalizations for VOC prior to beginning transfusion regimen 238.3 x 109/L 626 U/L No hospitalization for sickle cell complications post-transplant, despite weaning of transfusions Improvements in markers of hemolysis 17
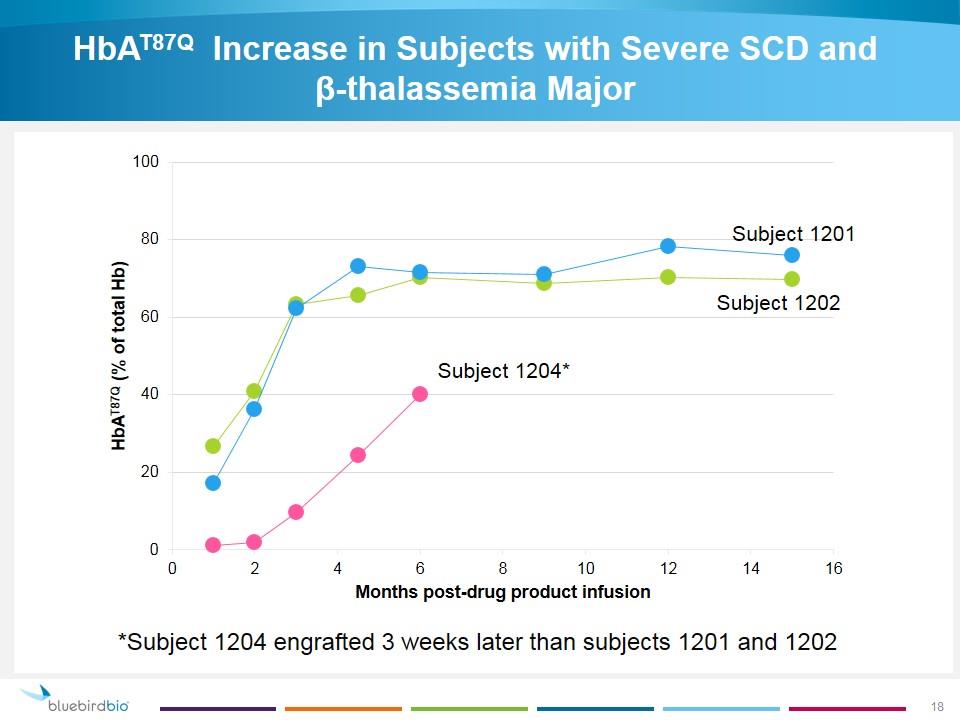
HbAT87Q Increase in Subjects with Severe SCD and β‑thalassemia Major *Subject 1204 engrafted 3 weeks later than subjects 1201 and 1202 Subject 1201 Subject 1202 Subject 1204*
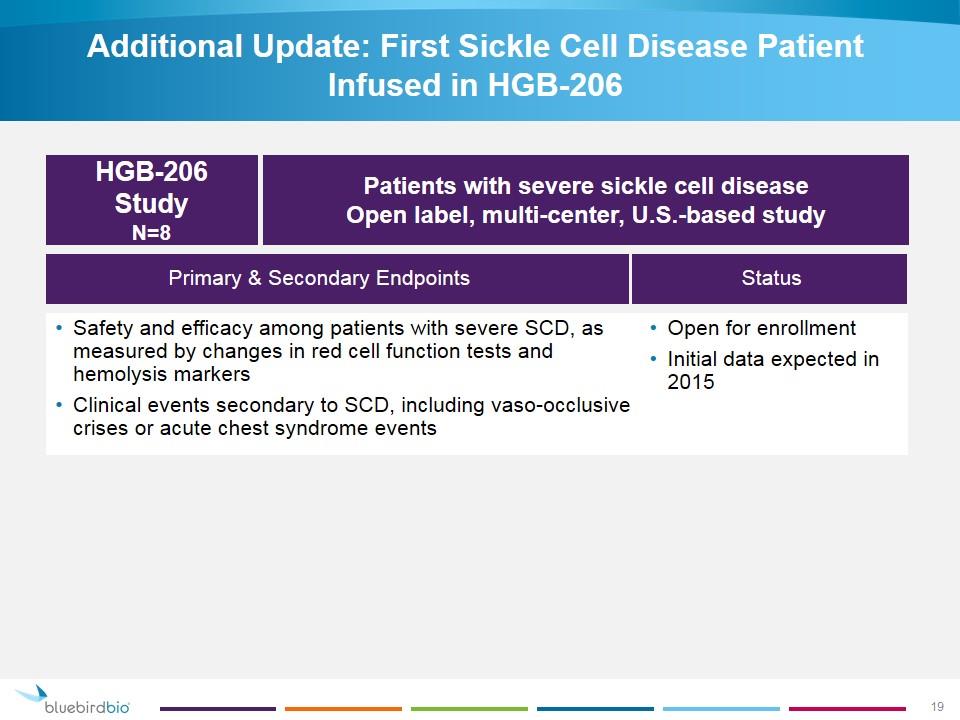
Additional Update: First Sickle Cell Disease Patient Infused in HGB-206 Status Primary & Secondary Endpoints Open for enrollment Initial data expected in 2015 HGB-206 Study N=8 Patients with severe sickle cell disease Open label, multi-center, U.S.-based study Safety and efficacy among patients with severe SCD, as measured by changes in red cell function tests and hemolysis markers Clinical events secondary to SCD, including vaso-occlusive crises or acute chest syndrome events
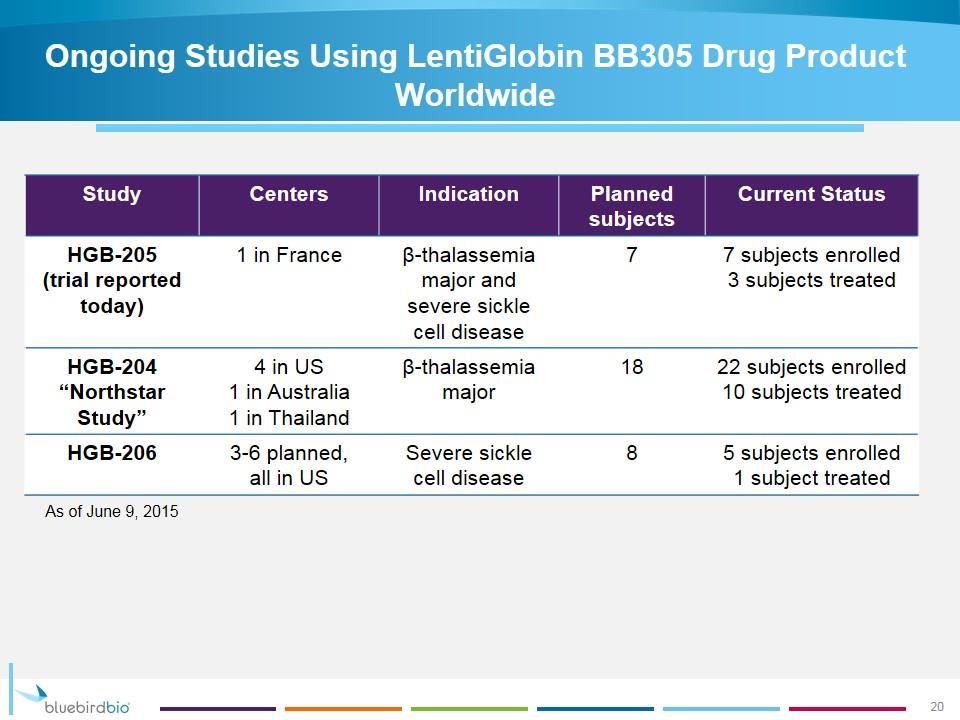
Ongoing Studies Using LentiGlobin BB305 Drug Product Worldwide Study Centers Indication Planned subjects Current Status HGB-205 (trial reported today) 1 in France β-thalassemia major and severe sickle cell disease 7 7 subjects enrolled 3 subjects treated HGB-204 “Northstar Study” 4 in US 1 in Australia 1 in Thailand β-thalassemia major 18 22 subjects enrolled 10 subjects treated HGB-206 3-6 planned, all in US Severe sickle cell disease 8 5 subjects enrolled 1 subject treated As of June 9, 2015 20
Recent Events and Milestones Accelerated regulatory pathway for LentiGlobin in β-thalassemia defined for U.S. and EU Achieved enrollment targets in Starbeam Study (CCALD) and Northstar Study (β-thalassemia major) Defined oncology strategy – BCMA 1st CAR T Program; Five Prime target; bluebird independent pathway Presented first SCD data at EHA (Abstract 5/18; EHA presentation 6/13); infused 1st patient in HGB-206 Appointed Philip Gregory, D.Phil., as Chief Scientific Officer ✓ ✓ ✓ ✓ ✓ 21

Transforming the Lives of Patients with Severe Genetic and Rare Diseases Making Hope a Reality
