Attached files
| file | filename |
|---|---|
| EX-5.1 - EX-5.1 - BioSig Technologies, Inc. | ex5-1.htm |
| EX-23.1 - EX-23.1 - BioSig Technologies, Inc. | ex23-1.htm |
| EX-10.44 - EX-10.44 - BioSig Technologies, Inc. | ex10-44.htm |
As filed with the Securities and Exchange Commission on June 10 , 2015
SEC File No. 333- 204335
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
AMENDMENT NO. 1 TO
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
|
BioSig Technologies, Inc.
|
|
(Exact name of registrant as specified in its charter)
|
|
Delaware
|
3845
|
26-4333375
|
||
|
(State or other jurisdiction of
incorporation or organization)
|
(Primary Standard Industrial
Classification Code Number)
|
(I.R.S. Employer Identification No.)
|
|
8441 Wayzata Blvd., Suite 240
Minneapolis, Minnesota 55426
(763) 999-7330
|
|
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
|
|
Kenneth Londoner
Executive Chairman
8441 Wayzata Blvd., Suite 240
Minneapolis, Minnesota 55426
(763) 999-7330
|
|
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
|
|
Copies of all communications, including communications sent to agent for service, should be sent to:
|
Rick A. Werner, Esq.
Haynes and Boone, LLP
30 Rockefeller Plaza, 26th Floor
New York, New York 10112
Tel. (212) 659-7300
Fax (212) 884-8234
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
(Check one):
|
Large accelerated filer o
|
Accelerated filer o
|
|
Non-accelerated filer o
|
Smaller reporting company x
|
|
(Do not check if a smaller reporting company)
|
Calculation of Registration Fee
|
Title of Each Class of Securities to be Registered
|
Amount to be Registered(1)
|
Proposed Maximum Offering Price per Share
|
Proposed Maximum Aggregate Offering Price
|
Amount of Registration Fee
|
||||||||||||
|
Common Stock, $0.001 par value per share
|
2,578,531
|
$
|
2.70
|
(2)
|
$
|
6,962,033.70
|
$
|
808.99
|
||||||||
|
Common Stock issuable upon conversion of Series C Preferred Stock
|
300,000
|
$
|
2.70
|
(2)
|
$
|
810,000.00
|
$
|
94.12
|
||||||||
|
Common Stock underlying Warrants
|
3,678,155
|
$
|
2.70
|
(2)
|
$
|
9,931,018.50
|
$
|
1,153.98
|
||||||||
|
Total
|
6,556,686
|
$
|
2.70
|
(2)
|
$
|
17,703,052.20
|
$
|
2,057.09
|
(3)
|
|||||||
|
(1)
|
Pursuant to Rule 416 under the Securities Act of 1933, as amended (the “Securities Act”), the shares of common stock offered hereby also include an indeterminate number of additional shares of common stock as may from time to time become issuable by reason of stock splits, stock dividends, recapitalizations or other similar transactions.
|
||
|
(2)
|
Estimated solely for purposes of calculating the registration fee pursuant to Rule 457(c) under the Securities Act of 1933, as amended, based upon the average of the high and low sales price of the common stock on June 8, 2015, as reported on the OTCQB.
|
||
|
(3)
|
$1,903.07 of which has been previously paid.
|
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Commission acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JUNE 10, 2015
PRELIMINARY PROSPECTUS

BioSig Technologies, Inc.
2,578,531 Shares of Common Stock
Up to 300,000 Shares of Common Stock Underlying Series C Preferred Stock and up to 3, 678,155 Shares of Common Stock Underlying Warrants
This prospectus relates to the resale of (i) 2,578,531 shares of our common stock to be offered by the selling stockholders, (ii) up to 300,000 shares of our common stock to be offered by the selling stockholders upon the conversion of 450 shares of our Series C Preferred Stock, at a conversion price of $1.50 per share, and (iii) up to 3, 678,155 shares of our common stock to be offered by the selling stockholders upon the exercise of outstanding common stock purchase warrants.
Our common stock trades in the over-the-counter market and is quoted on the OTCQB tier of the OTC Markets Group, Inc. under the symbol “BSGM.” Only a limited public market currently exists for our common stock. On June 9, 2015, the last reported sale price of our shares of common stock on the OTCQB was $2.80 per share.
We will not receive any of the proceeds from the sale of common stock by the selling stockholders. However, we will receive proceeds from the conversion of the Series C Preferred Stock and from the exercise of the warrants if the warrants are exercised for cash. We intend to use those proceeds, if any, for general corporate purposes. All expenses of registration incurred in connection with this offering are being borne by us, but all selling and other expenses incurred by the selling stockholders will be borne by the selling stockholders.
We qualify as an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012, or JOBS Act. Please read the related disclosure beginning on page 15 of this prospectus.
Investing in our common stock is highly speculative and involves a high degree of risk. You should carefully consider the risks and uncertainties in the section entitled “Risk Factors” beginning on page 3 of this prospectus before making a decision to purchase our stock.
We may amend or supplement this prospectus from time to time by filing amendments or supplements as required. You should read the entire prospectus and any amendments or supplements carefully before you make your investment decision.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2015
|
Page
|
|
|
1
|
|
| 3 | |
| 18 | |
| 18 | |
| 18 | |
| 19 | |
| 24 | |
| 37 | |
| 40 | |
| 43 | |
| 43 | |
| 46 | |
| 63 | |
| 64 | |
| 73 | |
| 74 | |
| 74 | |
| 75 | |
|
F-1
|
You should rely only on the information contained in this prospectus. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not making an offer to sell these securities in any jurisdiction where offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
Information contained on our website is not part of this prospectus.
PROSPECTUS SUMMARY
The following summary highlights information contained elsewhere in this prospectus. It may not contain all the information that may be important to you. You should read this entire prospectus carefully, including the sections entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and our historical financial statements and related notes included elsewhere in this prospectus or any accompanying prospectus supplement before making an investment decision. In this prospectus, unless the context requires otherwise, all references to “we,” “our,” “us” and the “Company” refer to BioSig Technologies, Inc.
Overview
We are a development stage medical device company that is developing a proprietary technology platform to minimize noise and artifacts from cardiac recordings during electrophysiology studies, where signals that measure electrical activity of the heart, such as electrocardiograms and electrograms, are measured. These signals are also evaluated during ablation, a procedure that involves delivery of energy through the tip of a catheter that scars or destroys heart tissue in order to correct heart rhythm disturbances. Our product under development, the PURE EP System, is a surface electrocardiogram and intracardiac multichannel recording and analysis system that acquires, processes and displays electrocardiogram and electrograms required during electrophysiology studies and ablation procedures. The PURE EP System is intended to be used in addition to existing electrophysiology recorders. We believe that data provided by the PURE EP System will increase the workload ability and enhance the capabilities of the typical electrophysiology laboratory.
We were formed as BioSig Technologies, Inc., a Nevada corporation, in February 2009 and in April 2011 we merged with our wholly-owned subsidiary, BioSig Technologies Inc., a Delaware corporation, with the Delaware corporation continuing as the surviving entity. We have not generated any revenue to date and consequently our operations are subject to all risks inherent in the establishment of a new business enterprise.
Our principal executive offices are located at 8441 Wayzata Blvd., Suite 240, Minneapolis, Minnesota 55426, telephone number (763) 999-7330. Our website address is www.biosigtech.com. Information accessed through our website is not incorporated into this prospectus and is not a part of this prospectus.
The Offering
|
Common stock offered by the selling stockholders:
|
2,578,531 shares of our common stock to be offered by the selling stockholders and up to 300,000 shares of our common stock to be offered by the selling stockholders upon the conversion of 450 shares of Series C Preferred Stock and up to 3, 678,155 shares of our common stock to be offered by the selling stockholders upon the exercise of outstanding common stock purchase warrants.
|
|||
|
Common stock outstanding prior to the offering:
|
14,203,202
|
|||
|
Common stock outstanding after this offering:
|
18,181,357 (1)
|
|||
|
Use of proceeds:
|
We will not receive any proceeds from the sale of the common stock offered by the selling stockholders. However, we will receive proceeds from the conversion of the Series C Preferred Stock and from the exercise price of the warrants if the warrants are exercised for cash. We intend to use those proceeds, if any, for general corporate purposes.
|
|||
|
The OTCQB trading symbol:
|
“BSGM”
|
|||
|
Risk factors:
|
You should carefully consider the information set forth in this prospectus and, in particular, the specific factors set forth in the “Risk Factors” section beginning on page 3 of this prospectus before deciding whether or not to invest in shares of our common stock.
|
|||
|
(1)
|
The number of shares of common stock outstanding after the offering is based upon 14,203,202 shares outstanding as of June 9, 2015, and assumes the conversion of all shares of Series C Preferred Stock and the exercise of all warrants with respect to those shares being registered for resale pursuant to the registration statement of which this prospectus forms a part.
|
The number of shares of common stock outstanding after this offering excludes:
|
●
|
7,340 ,190 shares of common stock issuable upon the exercise of currently outstanding options at a weighted average exercise price of $2. 34 per share;
|
|
●
|
2,500 ,933 shares of common stock available for future issuance under the BioSig Technologies, Inc. 2012 Equity Incentive Plan; and
|
|
|
●
|
1, 305,348 shares of common stock issuable upon the conversion of 1,958 outstanding shares of our Series C Preferred Stock at a conversion price of $1.50 per share.
|
RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the following factors and other information in this prospectus or any accompanying prospectus supplement before making a decision to invest in our common stock. If any of the risks actually occur, our business, financial conditions and operating results may be materially and adversely affected. In that event, the trading price of our common stock may decline, and you could lose all or part of your investment.
Risks Related to Our Business and Industry
Because our condition as a going concern is in doubt, we will be forced to cease our business operations unless we can raise sufficient funds to satisfy our working capital needs.
As shown in the accompanying financial statements during years ended December 31, 2014 and 2013, we incurred net losses attributable to common stockholders of $8,773,399 and $10,101,846, respectively and used $1,997,072 in cash for operating activities for the year ended December 31, 2014. As of June 9, 2015, we had cash on hand of approximately $1.5 million . These factors, among others, raise substantial doubt that we will be able to continue as a going concern for a reasonable period of time.
Our existence is dependent upon management’s ability to develop profitable operations. We are devoting substantially all of our efforts to developing product candidates and there can be no assurance that our efforts will be successful. There is no assurance that can be given that our actions will result in profitable operations or the resolution of our liquidity problems.
Because we are an early development stage company with no products near commercialization, we expect to incur significant additional operating losses.
We are an early development stage company and we expect to incur substantial additional operating expenses over the next several years as our research, development, pre-clinical testing, regulatory approval and clinical trial activities increase. The amount of our future losses and when, if ever, we will achieve profitability are uncertain. We have no products that have generated any commercial revenue and do not expect to generate revenues from the commercial sale of our products in the near future, if ever. Our ability to generate revenue and achieve profitability will depend on, among other things, the following:
|
●
|
successful completion of the pre-clinical and clinical development of our products;
|
|
|
●
|
obtaining necessary regulatory approvals from the U.S. Food and Drug Administration or other regulatory authorities;
|
|
|
●
|
establishing manufacturing, sales, and marketing arrangements, either alone or with third parties; and
|
|
|
●
|
raising sufficient funds to finance our activities.
|
We might not succeed at all, or at any, of these undertakings. If we are unsuccessful at some or all of these undertakings, our business, prospects, and results of operations may be materially adversely affected.
Our product candidates are at an early stage of development and may not be successfully developed or commercialized.
Our main product candidate, the PURE EP System, is in the early stage of development and will require substantial further capital expenditures, development, testing, and regulatory clearances prior to commercialization, especially given that we have not yet completed pre-clinical testing on this product. The development and regulatory approval process takes several years and it is not likely that the PURE EP System, even if successfully developed and approved by the U.S. Food and Drug Administration, may not be commercially available for a number of years. In addition, due to budgetary constraints, we have not been able to devote the level of resources that we desired to our research and development efforts. The continued development of our product candidates is dependent upon our ability to obtain sufficient financing. However, even if we are able to obtain the requisite financing to fund our development program, we cannot assure you that our product candidates will be successfully developed or commercialized. Our failure to develop, manufacture or receive regulatory approval for or successfully commercialize any of our product candidates could result in the failure of our business and a loss of all of your investment in our company.
We expect to derive our revenue from sales of our PURE EP System and other products we may develop. If we fail to generate revenue from these sources, our results of operations and the value of our business will be materially and adversely affected.
We expect our revenue to be generated from sales of our PURE EP System and other products we may develop. Future sales of these products, if any, will be subject to, among other things, the receipt of regulatory approvals and commercial and market uncertainties that may be outside our control. If we fail to generate our intended revenues from these products, our results of operations and the value of our business and securities would be materially and adversely affected.
We may need to finance our future cash needs through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. Any additional funds that we obtain may not be on terms favorable to us or our stockholders and may require us to relinquish valuable rights.
Until and unless we receive approval from the U.S. Food and Drug Administration and other regulatory authorities for our products, we will not generate revenues from our products. Therefore, for the foreseeable future, we will have to fund all of our operations and capital expenditures from cash on hand, public or private equity offerings, debt financings, bank credit facilities or corporate collaboration and licensing arrangements. We believe that our existing cash on hand will be sufficient to enable us to fund our projected operating requirements for approximately the next six months. However, we may need to raise additional funds more quickly if one or more of our assumptions prove to be incorrect or if we choose to expand our product development efforts more rapidly than we presently anticipate. We also may decide to raise additional funds before we require them if we are presented with favorable terms for raising capital.
If we seek to sell additional equity or debt securities, obtain a bank credit facility or enter into a corporate collaboration or licensing arrangement, we may not obtain favorable terms for us and/or our stockholders or be able to raise any capital at all, all of which could result in a material adverse effect on our business and results of operations. The sale of additional equity or debt securities, if convertible, could result in dilution to our stockholders. The incurrence of indebtedness would result in increased fixed obligations and could also result in covenants that would restrict our operations. Raising additional funds through collaboration or licensing arrangements with third parties may require us to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates, or to grant licenses on terms that may not be favorable to us or our stockholders. In addition, we could be forced to discontinue product development, reduce or forego sales and marketing efforts and forego attractive business opportunities, all of which could have an adverse impact on our business and results of operations.
We may be unable to develop our existing or future technology.
Our product, the PURE EP System, may not deliver the levels of accuracy and reliability needed to make it a successful product in the marketplace, and the development of such accuracy and reliability may be indefinitely delayed or may never be achieved. In addition, we may experience delays in the development of our technology for other reasons, including failure to obtain necessary funding and failure to obtain regulatory approvals. Failure to develop this or other technology could have an adverse material effect on our business, financial condition, results of operations and future prospects.
The results of clinical studies may not support the usefulness of our technology.
Conducting clinical trials is a long, expensive and uncertain process that is subject to delays and failure at any stage. Clinical trials can take months or years. The commencement or completion of any of our clinical trials may be delayed or halted for numerous reasons, including:
|
●
|
the U.S. Food and Drug Administration may not approve a clinical trial protocol or a clinical trial, or may place a clinical trial on hold;
|
|
●
|
subjects may not enroll in clinical trials at the rate we expect or we may not follow up on subjects at the rate we expect;
|
|
●
|
subjects may experience events unrelated to our products;
|
|
●
|
third-party clinical investigators may not perform our clinical trials consistent with our anticipated schedule or the clinical trial protocol and good clinical practices, or other third-party organizations may not perform data collection and analysis in a timely or accurate manner;
|
|
●
|
interim results of any of our clinical trials may be inconclusive or negative;
|
|
●
|
regulatory inspections of our clinical trials may require us to undertake corrective action or suspend or terminate the clinical trials if investigators find us not to be in compliance with regulatory requirements; or
|
|
●
|
governmental regulations or administrative actions may change and impose new requirements, particularly with respect to reimbursement.
|
Results of pre-clinical studies do not necessarily predict future clinical trial results and previous clinical trial results may not be repeated in subsequent medical trials. We may experience delays, cost overruns and project terminations despite achieving promising results in pre-clinical testing or early clinical testing. In addition, the data obtained from clinical trials may be inadequate to support approval or clearance of a submission. The U.S. Food and Drug Administration may disagree with our interpretation of the data from our clinical trials, or may find the clinical trial design, conduct or results inadequate to demonstrate the safety and effectiveness of the product candidate. The U.S. Food and Drug Administration may also require us to conduct additional pre-clinical studies or clinical trials that could further delay approval of our products. If we are unsuccessful in receiving U.S. Food and Drug Administration approval of a product, we would not be able to commercialize the product in the U.S., which could seriously harm our business. Moreover, we face similar risks in other jurisdictions in which we may sell or propose to sell our products.
The medical device industry is subject to stringent regulation and failure to obtain regulatory approval will prevent commercialization of our products.
Medical devices are subject to extensive and rigorous regulation by the U.S. Food and Drug Administration pursuant to the Federal Food, Drug, and Cosmetic Act, by comparable agencies in foreign countries and by other regulatory agencies and governing bodies. Under the Federal Food, Drug, and Cosmetic Act and associated regulations, manufacturers of medical devices must comply with certain regulations that cover the composition, labeling, testing, clinical study, manufacturing, packaging and distribution of medical devices. In addition, medical devices must receive U.S. Food and Drug Administration clearance or approval before they can be commercially marketed in the U.S., and the U.S. Food and Drug Administration may require testing and surveillance programs to monitor the effects of approved products that have been commercialized and can prevent or limit further marketing of a product based on the results of these post-market evaluation programs. The process of obtaining marketing clearance from the U.S. Food and Drug Administration for new products could take a significant period of time, require the expenditure of substantial resources, involve rigorous pre-clinical and clinical testing, require changes to the products and result in limitations on the indicated uses of the product. In addition, if we seek regulatory approval in non-U.S. markets, we will be subject to further regulatory approvals that may require additional costs and resources. There is no assurance that we will obtain necessary regulatory approvals in a timely manner, or at all.
Our product, the PURE EP System, will need to receive 510(k) marketing clearance from the U.S. Food and Drug Administration in order permit us to market this product in the U.S. In addition, if we intend to market our product for additional medical uses or indications, we will need to submit additional 510(k) applications to the U.S. Food and Drug Administration that are supported by satisfactory clinical trial results specifically for the additional indication. The results of our initial clinical trials may not provide sufficient evidence to allow the U.S. Food and Drug Administration to grant us such additional marketing clearances and even additional trials requested by the U.S. Food and Drug Administration may not result in our obtaining 510(k) marketing clearance for our product. The failure to obtain U.S. Food and Drug Administration marketing clearance for the PURE EP System, any additional indications for the PURE EP System or any other of our future products would have a material adverse effect on our business.
Even if regulatory approval is obtained, our products will be subject to extensive post-approval regulation.
Once a product is approved by the relevant regulatory body for our targeted commercialization market, numerous post-approval requirements apply, including but not limited to requirements relating to manufacturing, labeling, packaging, advertising and record keeping. Even if regulatory approval of a product is obtained, the approval may be subject to limitations on the uses for which the product may be marketed, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product. Any such post-approval requirement could reduce our revenues, increase our expenses and render the approved product candidate not commercially viable. If we fail to comply with the regulatory requirements of the applicable regulatory authorities, or if previously unknown problems with any approved commercial products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions or other negative consequences, including:
|
●
|
restrictions on our products, manufacturers or manufacturing processes;
|
|
●
|
warning letters and untitled letters;
|
|
●
|
civil penalties and criminal prosecutions and penalties;
|
|
●
|
fines;
|
|
●
|
injunctions;
|
|
●
|
product seizures or detentions;
|
|
●
|
import or export bans or restrictions;
|
|
●
|
voluntary or mandatory product recalls and related publicity requirements;
|
|
●
|
suspension or withdrawal of regulatory approvals;
|
|
●
|
total or partial suspension of production; and
|
|
●
|
refusal to approve pending applications for marketing approval of new products or of supplements to approved applications.
|
Regulations are constantly changing, and in the future our business may be subject to additional regulations that increase our compliance costs.
We believe that we understand the current laws and regulations to which our products will be subject in the future. However, federal, state and foreign laws and regulations relating to the sale of our products are subject to future changes, as are administrative interpretations of regulatory agencies. If we fail to comply with such federal, state or foreign laws or regulations, we may fail to obtain regulatory approval for our products and, if we have already obtained regulatory approval, we could be subject to enforcement actions, including injunctions preventing us from conducting our business, withdrawal of clearances or approvals and civil and criminal penalties. In the event that federal, state, and foreign laws and regulations change, we may incur additional costs to seek government approvals, in addition to the clearance we intend to seek from the U.S. Food and Drug Administration in order to sell or market our products. If we are slow or unable to adapt to changes in existing regulatory requirements or the promulgation of new regulatory requirements or policies, we or our licensees may, following approval, lose marketing approval for our products which will impact our ability to conduct business in the future.
The market for our technology and revenue generation avenues for our products may be slow to develop, if at all.
The market for our products may be slower to develop or smaller than estimated or it may be more difficult to build the market than anticipated. The medical community may resist our products or be slower to accept them than we anticipate. Revenues from our products may be delayed or costs may be higher than anticipated which may result in our need for additional funding. We anticipate that our principal route to market will be through commercial distribution partners. These arrangements are generally non-exclusive and have no guaranteed sales volumes or commitments. The partners may be slower to sell our products than anticipated. Any financial, operational or regulatory risks that affect our partners could also affect the sales of our products. In the current economic environment, hospitals and clinical purchasing budgets may exercise greater restraint with respect to purchases, which may result in purchasing decisions being delayed or denied. If any of these situations were to occur this could have a material adverse effect on our business, financial condition, results of operations and future prospects.
If we seek to market our products in foreign jurisdictions, we may need to obtain regulatory approval in these jurisdictions.
In order to market our products in the European Union and many other foreign jurisdictions, we may need to obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. Approval procedures vary among countries (except with respect to the countries that are part of the European Economic Area) and can involve additional clinical testing. The time required to obtain approval may differ from that required to obtain U.S. Food and Drug Administration approval. Should we decide to market our products abroad, we may fail to obtain foreign regulatory approvals on a timely basis, if at all. Approval by the U.S. Food and Drug Administration does not ensure approval by regulatory authorities in other countries, and approval by one foreign regulatory authority, including obtaining CE Mark approval, does not ensure approval by regulatory authorities in other foreign countries or by the U.S. Food and Drug Administration. We may be unable to file for, and may not receive, necessary regulatory approvals to commercialize our products in any foreign market, which could adversely affect our business prospects.
If we fail to obtain an adequate level of reimbursement for our system by third-party payors, there may be no commercially viable markets for our system or the markets may be much smaller than expected.
The availability and levels of reimbursement by governmental and other third-party payors significantly affect the market for our system. Reimbursement by third-party payors in the U.S. typically is based on the device’s perceived benefit and whether it is deemed medically reasonable and necessary. Reimbursement levels of third-party payors in the U.S. are also based on established payment formulas that take into account part or all of the cost associated with these devices and the related procedures performed. We cannot assure you the level of reimbursement we might obtain in the U.S., if any, for our system. If we do not obtain adequate levels of reimbursement for our system by third-party payors in the U.S., which we believe is largest potential market for our system, our financial condition, results of operations and prospects would be harmed.
Reimbursement and health care payment systems in international markets vary significantly by country, and include both government-sponsored health care and private insurance. To obtain reimbursement or pricing approval in some countries, we may be required to produce additional clinical data, which may involve one or more additional clinical trials that compares the cost-effectiveness of our system to other available therapies. We may not obtain international reimbursement or pricing approvals in a timely manner, if at all. Our failure to receive international reimbursement or pricing approvals would negatively impact market acceptance of our system in the international markets in which those approvals are sought.
We believe that future reimbursement may be subject to increased restrictions both in the U.S. and in international markets. Future legislation, regulation or reimbursement policies of third-party payors may adversely affect the demand for the PURE EP System or any of our other future products and limit our ability to sell the PURE EP System or any of our other future products on a profitable basis. In addition, third-party payors continually attempt to contain or reduce the costs of health care by challenging the prices charged for health care products and services. If reimbursement for our system is unavailable in any market or limited in scope or amount, or if pricing is set at unsatisfactory levels, market acceptance of our system would be significantly impaired and our future revenues, if any, would be significantly harmed.
The electrophysiology market is highly competitive.
There are a number of groups and organizations, such as healthcare, medical device and software companies in the electrophysiology market that may develop a competitive offering to our products. The largest companies in the electrophysiology market are GE, Johnson & Johnson, Boston Scientific, Siemens and St. Jude Medical. All of these companies have significantly greater resources, experience and name recognition than we possess. There is no assurance that they will not attempt to develop similar or superior products, that they will not be successful in developing such products or that any products they may develop will not have a competitive advantage over our products. If we experience delayed regulatory approvals or disputed clinical claims, we may not have a commercial or clinical advantage over competitors’ products that we believe we currently possess. Should a superior offering come to market, this could have a material adverse effect on our business, financial condition, results of operations and future prospects.
We rely on key officers, consultants and scientific and medical advisors, and their knowledge of our business and technical expertise would be difficult to replace.
We are highly dependent on our officers, consultants and scientific and medical advisors because of their expertise and experience in medical device development. We do not have “key person” life insurance policies for any of our officers. Moreover, if we are unable to obtain additional funding, we will be unable to meet our current and future compensation obligations to such employees and consultants. In light of the foregoing, we are at risk that one or more of our consultants or employees may leave our company for other opportunities where there is no concern about such employers fulfilling their compensation obligations, or for other reasons. The loss of the technical knowledge and management and industry expertise of any of our key personnel could result in delays in product development, loss of customers and sales and diversion of management resources, which could adversely affect our results of operations.
We may fail to attract and retain qualified personnel.
We expect to rapidly expand our operations and grow our sales, research and development and administrative operations. This expansion is expected to place a significant strain on our management and will require hiring a significant number of qualified personnel. Accordingly, recruiting and retaining such personnel in the future will be critical to our success. There is intense competition from other companies, research and academic institutions, government entities and other organizations for qualified personnel in the areas of our activities. Many of these companies, institutions and organizations have greater resources than we do, along with more prestige associated with their names. If we fail to identify, attract, retain and motivate these highly skilled personnel, we may be unable to continue our marketing and development activities, and this could have a material adverse effect on our business, financial condition, results of operations and future prospects.
If we do not effectively manage changes in our business, these changes could place a significant strain on our management and operations.
Our ability to grow successfully requires an effective planning and management process. The expansion and growth of our business could place a significant strain on our management systems, infrastructure and other resources. To manage our growth successfully, we must continue to improve and expand our systems and infrastructure in a timely and efficient manner. Our controls, systems, procedures and resources may not be adequate to support a changing and growing company. If our management fails to respond effectively to changes and growth in our business, including acquisitions, there could be a material adverse effect on our business, financial condition, results of operations and future prospects.
Our strategic business plan may not produce the intended growth in revenue and operating income.
Our strategies ultimately include making significant investments in sales and marketing programs to achieve revenue growth and margin improvement targets. If we do not achieve the expected benefits from these investments or otherwise fail to execute on our strategic initiatives, we may not achieve the growth improvement we are targeting and our results of operations may be adversely affected. We may also fail to secure the capital necessary to make these investments, which will hinder our growth.
In addition, as part of our strategy for growth, we may make acquisitions and enter into strategic alliances such as joint ventures and joint development agreements. However, we may not be able to identify suitable acquisition candidates, complete acquisitions or integrate acquisitions successfully, and our strategic alliances may not prove to be successful. In this regard, acquisitions involve numerous risks, including difficulties in the integration of the operations, technologies, services and products of the acquired companies and the diversion of management’s attention from other business concerns. Although we will endeavor to evaluate the risks inherent in any particular transaction, there can be no assurance that we will properly ascertain all such risks. In addition, acquisitions could result in the incurrence of substantial additional indebtedness and other expenses or in potentially dilutive issuances of equity securities. There can be no assurance that difficulties encountered with acquisitions will not have a material adverse effect on our business, financial condition and results of operations.
We currently have no sales, marketing or distribution operations and will need to expand our expertise in these areas.
We currently have no sales, marketing or distribution operations and, in connection with the expected commercialization of our planned products, will need to expand our expertise in these areas. To increase internal sales, distribution and marketing expertise and be able to conduct these operations, we would have to invest significant amounts of financial and management resources. In developing these functions ourselves, we could face a number of risks, including:
|
●
|
we may not be able to attract and build an effective marketing or sales force;
|
|
●
|
the cost of establishing, training and providing regulatory oversight for a marketing or sales force may be substantial; and
|
|
●
|
there are significant legal and regulatory risks in medical device marketing and sales that we have never faced, and any failure to comply with applicable legal and regulatory requirements for sales, marketing and distribution could result in an enforcement action by the U.S. Food and Drug Administration, European regulators or other authorities that could jeopardize our ability to market our planned products or could subject us to substantial liability.
|
The liability of our directors and officers is limited.
The applicable provisions of the Delaware General Corporation Law and our Amended and Restated Certificate of Incorporation and By-laws limit the liability of our directors to us and our stockholders for monetary damages for breaches of their fiduciary duties, with certain exceptions, and for other specified acts or omissions of such persons. In addition, the applicable provisions of the Delaware General Corporation Law and of our Amended and Restated Certificate of Incorporation and By-laws provide for indemnification of such persons under certain circumstances. In the event we are required to indemnify any of our directors or any other person, our financial strength may be harmed.
Our product development program depends upon third-party researchers who are outside our control and whose negative performance could materially hinder or delay our pre-clinical testing or clinical trials.
We do not have the ability to conduct all aspects of pre-clinical testing or clinical trials ourselves. We depend upon independent investigators and collaborators, such as commercial third-parties, government, universities and medical institutions, to conduct our pre-clinical and clinical trials under agreements with us. These collaborators are not our employees and we cannot control the amount or timing of resources that they devote to our programs. These investigators may not assign as great a priority to our programs or pursue them as diligently as we would if we were undertaking such programs ourselves. The failure of any of these outside collaborators to perform in an acceptable and timely manner in the future, including in accordance with any applicable regulatory requirements, such as good clinical and laboratory practices, or pre-clinical testing or clinical trial protocols, could cause a delay or otherwise adversely affect our pre-clinical testing or clinical trials, our success in obtaining regulatory approvals and, ultimately, the timely advancement of our development programs. In addition, these collaborators may also have relationships with other commercial entities, some of whom may compete with us. If our collaborators assist our competitors at our expense, our competitive position would be harmed.
Negative publicity or unfavorable media coverage could damage our reputation and harm our operations.
In the event that the marketplace perceives our products as not offering the benefits which we believe they offer, we may receive negative publicity. This publicity may result in litigation and increased regulation and governmental review. If we were to receive such negative publicity or unfavorable media attention, whether warranted or unwarranted, our ability to market our products would be adversely affected. We may be required to change our products and services and become subject to increased regulatory burdens, and we may be required to pay large judgments or fines and incur significant legal expenses. Any combination of these factors could further increase our cost of doing business and adversely affect our financial position, results of operations and cash flows.
We may face risks associated with future litigation and claims.
We may, in the future, be involved in one or more lawsuits, claims or other proceedings. These suits could concern issues including contract disputes, employment actions, employee benefits, taxes, environmental, health and safety, personal injury and product liability matters. Due to the uncertainties of litigation, we can give no assurance that we will prevail on any claims made against us in any such lawsuit. Also, we can give no assurance that any other lawsuits or claims brought in the future will not have an adverse effect on our financial condition, liquidity or operating results.
Specifically, we believe we will be subject to product liability claims or product recalls, particularly in the event of false positive or false negative reports, because we plan to develop and manufacture medical diagnostic products. We intend to obtain appropriate insurance coverage once we reach a manufacturing stage. A product recall or a successful product liability claim or claims that exceed our planned insurance coverage could have a material adverse effect on us. In addition, product liability insurance is expensive. In the future we may not be able to obtain coverage on acceptable terms, if at all. Moreover, our insurance coverage may not adequately protect us from liability that we incur in connection with clinical trials or sales of our products. In the event of an award against us during a time when we have no available insurance or insufficient insurance, we may sustain significant losses of our operating capital. In addition, any products liability litigation, regardless of outcome or strength of claims, may divert time and resources away from the day-to-day operation of our business and product development efforts. Any of these outcomes could adversely impact our business and results of operations, as well as impair our reputation in the medical and investment communities.
We may be subject, directly or indirectly, to U.S. federal and state health care fraud and abuse and false claims laws and regulations. Prosecutions under such laws have increased in recent years and we may become subject to such litigation. If we are unable to, or have not fully complied with such laws, we could face substantial penalties.
If we are successful in achieving regulatory approval to market our PURE EP System, our operations will be directly, or indirectly through our customers and health care professionals, subject to various U.S. federal and state fraud and abuse laws, including, without limitation, the federal Anti-Kickback Statute, federal False Claims Act, and federal Foreign Corrupt Practices Act. These laws may impact, among other things, our proposed sales, and marketing and education programs.
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal health care program such as the Medicare and Medicaid programs. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal health care covered business, the statute has been violated. The federal Anti-Kickback Statute is broad and, despite a series of narrow safe harbors, prohibits many arrangements and practices that are lawful in businesses outside of the health care industry. Penalties for violations of the federal Anti-Kickback Statute include criminal penalties and civil and administrative sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal health care programs. An alleged violation of the federal Anti-Kickback Statute may be used as a predicate offense to establish liability pursuant to other federal laws and regulations such as the federal False Claims Act. Many states have also adopted laws similar to the federal Anti-Kickback Statute, some of which apply to the referral of patients for health care items or services reimbursed by any source, not only the Medicare and Medicaid programs.
The federal False Claims Act prohibits persons from knowingly filing, or causing to be filed, a false claim to, or the knowing use of false statements to obtain payment from, the federal government. Suits filed under the federal False Claims Act, known as “qui tam” actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “relators” or “whistleblowers,” may share in any amounts paid by the entity to the government in fines or settlement. The frequency of filing qui tam actions has increased significantly in recent years, causing greater numbers of medical device and health care companies to have to defend a federal False Claim Act action. The federal Patient Protection and Affordable Care Act includes provisions expanding the ability of certain relators to bring actions that would have been previously dismissed under prior law. When an entity is determined to have violated the federal False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. The Deficit Reduction Act of 2005 encouraged states to enact or modify their state false claims act to be at least as effective as the federal False Claims Act by granting states a portion of any federal Medicaid funds recovered through Medicaid-related actions. Most states have enacted state false claims laws, and many of those states included laws including qui tam provisions.
The federal Patient Protection and Affordable Care Act includes provisions known as the Physician Payments Sunshine Act, which requires manufacturers of drugs, biologics, devices and medical supplies covered under Medicare and Medicaid starting in 2012 to record any transfers of value to physicians and teaching hospitals and to report this data beginning in 2013 to the Centers for Medicare and Medicaid Services for subsequent public disclosure. Manufacturers must also disclose investment interests held by physicians and their family members. Failure to submit the required information may result in civil monetary penalties of up to $1 million per year for knowing violations and may result in liability under other federal laws or regulations. Similar reporting requirements have also been enacted on the state level in the U.S., and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with health care professionals. In addition, some states such as Massachusetts and Vermont impose an outright ban on certain gifts to physicians. If we receive U.S. Food and Drug Administration clearance to market our system in the U.S., these laws could affect our promotional activities by limiting the kinds of interactions we could have with hospitals, physicians or other potential purchasers or users of our system. Both the disclosure laws and gift bans will impose administrative, cost and compliance burdens on us.
We are unable to predict whether we could be subject to actions under any of these laws, or the impact of such actions. If we are found to be in violation of any of the laws described above and other applicable state and federal fraud and abuse laws, we may be subject to penalties, including civil and criminal penalties, damages, fines, or an administrative action of suspension or exclusion from government health care reimbursement programs and the curtailment or restructuring of our operations.
In addition, to the extent we commence commercial operations overseas, we will be subject to the federal Foreign Corrupt Practices Act and other countries’ anti-corruption/anti-bribery regimes, such as the U.K. Bribery Act. The federal Foreign Corrupt Practices Act prohibits improper payments or offers of payments to foreign governments and their officials for the purpose of obtaining or retaining business. Safeguards we implement to discourage improper payments or offers of payments by our employees, consultants, sales agents or distributors may be ineffective, and violations of the federal Foreign Corrupt Practices Act and similar laws may result in severe criminal or civil sanctions, or other liabilities or proceedings against us, any of which would likely harm our reputation, business, financial condition and results of operations.
Risks Related to Our Intellectual Property
If we do not obtain protection for our intellectual property rights, our competitors may be able to take advantage of our research and development efforts to develop competing products.
We intend to rely on a combination of patents, trade secrets, and nondisclosure and non-competition agreements to protect our proprietary intellectual property. We have filed a patent application with the U.S. Patent and Trademark Office, and we have filed this patent application under the Patent Cooperation Treaty (PTC) with the U.S. Receiving Office. We plan to file additional patent applications in the U.S. and in other countries as we deem appropriate for our products. Our applications have and will include claims intended to provide market exclusivity for certain commercial aspects of the products, including the methods of production, the methods of usage and the commercial packaging of the products. However, we cannot predict:
|
●
|
the degree and range of protection any patents will afford us against competitors, including whether third parties will find ways to invalidate or otherwise circumvent our patents;
|
|
●
|
if and when such patents will be issued, and, if granted, whether patents will be challenged and held invalid or unenforceable;
|
|
●
|
whether or not others will obtain patents claiming aspects similar to those covered by our patents and patent applications; or
|
|
●
|
whether we will need to initiate litigation or administrative proceedings which may be costly regardless of outcome.
|
Our success also depends upon the skills, knowledge and experience of our scientific and technical personnel, our consultants and advisors as well as our licensors and contractors. To help protect our proprietary know-how and our inventions for which patents may be unobtainable or difficult to obtain, we rely on trade secret protection and confidentiality agreements. To this end, it is our policy to require all of our employees, consultants, advisors and contractors to enter into agreements which prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business. These agreements may not provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure or the lawful development by others of such information. If any of our trade secrets, know-how or other proprietary information is disclosed, the value of our trade secrets, know-how and other proprietary rights would be significantly impaired and our business and competitive position would suffer.
Given the fact that we may pose a competitive threat, competitors, especially large and well-capitalized companies that own or control patents relating to electrophysiology recording systems, may successfully challenge our current and planned patent applications, produce similar products or products that do not infringe our future patents, or produce products in countries where we have not applied for patent protection or that do not respect our patents.
If any of these events occurs, or we otherwise lose protection for our trade secrets or proprietary know-how, the value of our intellectual property may be greatly reduced. Patent protection and other intellectual property protection are important to the success of our business and prospects, and there is a substantial risk that such protections will prove inadequate.
If we infringe upon the rights of third parties, we could be prevented from selling products and forced to pay damages and defend against litigation.
If our products, methods, processes and other technologies infringe the proprietary rights of other parties, we could incur substantial costs and we may be required to:
|
●
|
obtain licenses, which may not be available on commercially reasonable terms, if at all;
|
|
●
|
abandon an infringing product candidate;
|
|
●
|
redesign our product candidates or processes to avoid infringement;
|
|
●
|
cease usage of the subject matter claimed in the patents held by others;
|
|
●
|
pay damages; and/or
|
|
●
|
defend litigation or administrative proceedings which may be costly regardless of outcome, and which could result in a substantial diversion of our financial and management resources.
|
Any of these events could substantially harm our earnings, financial condition and operations.
Risks Related to our Common Stock
The public trading market for our common stock is volatile and may result in higher spreads in stock prices, which may limit the ability of our investors to sell their shares of our common stock at a profit, if at all.
Our common stock trades in the over-the-counter market and is quoted on the OTCQB tier of the OTC Markets Group, Inc. The over-the-counter market for securities has historically experienced extreme price and volume fluctuations during certain periods. These broad market fluctuations may adversely affect the market price of our common stock and result in substantial losses to our investors. In addition, the spreads on stock traded through the over-the-counter market are generally unregulated and higher than on national stock exchanges, which means that the difference between the price at which shares could be purchased by investors in the over-the-counter market compared to the price at which they could be subsequently sold would be greater than on these exchanges. Significant spreads between the bid and asked prices of the stock could continue during any period in which a sufficient volume of trading is unavailable or if the stock is quoted by an insignificant number of market makers. Historically, our trading volume has been insufficient to significantly reduce this spread and we have had a limited number of market makers insufficient to affect this spread. These higher spreads could adversely affect investors who purchase the shares at the higher price at which the shares are sold, but subsequently sell the shares at the lower bid prices quoted by the brokers. Unless the bid price for the stock exceeds the price paid for the shares by the investor, plus brokerage commissions or charges, the investor could lose money on the sale. For higher spreads such as those on over-the-counter stocks, this is likely a much greater percentage of the price of the stock than for exchange listed stocks. There is no assurance that at the time an investor in our common stock wishes to sell the shares, the bid price will have sufficiently increased to create a profit on the sale.
We do not know whether a market for our common stock will be sustained or what the market price of our common stock will be and as a result it may be difficult for you to sell your shares of our common stock.
Although our common stock now trades on the OTCQB, an active trading market for our shares may not be sustained. It may be difficult for our stockholders to sell their shares without depressing the market price for our shares or at all. As a result of these and other factors, our stockholders may not be able to sell their shares. Further, an inactive market may also impair our ability to raise capital by selling shares of our common stock and may impair our ability to enter into strategic partnerships or acquire companies or products by using our shares of common stock as consideration. If an active market for our common stock does not develop or is not sustained, it may be difficult for our stockholders to sell shares of our common stock.
The market price for our common stock may fluctuate significantly, which could result in substantial losses by our investors.
The market price of our common stock may fluctuate significantly in response to numerous factors, some of which are beyond our control, such as:
|
●
|
the outcomes of potential future patent litigation;
|
|
●
|
our ability to monetize our future patents;
|
|
●
|
changes in our industry;
|
|
●
|
announcements of technological innovations, new products or product enhancements by us or others;
|
|
●
|
announcements by us of significant strategic partnerships, out-licensing, in-licensing, joint ventures, acquisitions or capital commitments;
|
|
●
|
changes in earnings estimates or recommendations by security analysts, if our common stock is covered by analysts;
|
|
|
●
|
investors’ general perception of us;
|
|
|
●
|
future issuances of common stock;
|
|
|
●
|
the addition or departure of key personnel;
|
|
●
|
general market conditions, including the volatility of market prices for shares of technology companies, generally, and other factors, including factors unrelated to our operating performance; and
|
|
|
●
|
the other factors described in this “Risk Factors” section.
|
These factors and any corresponding price fluctuations may materially and adversely affect the market price of our common stock and result in substantial losses by our investors.
Further, the stock market in general, and the market for technology companies in particular, has experienced extreme price and volume fluctuations in the past. Continued market fluctuations could result in extreme volatility in the price of our common stock, which could cause a decline in the value of our common stock.
Price volatility of our common stock might be worse if the trading volume of our common stock is low. In the past, following periods of market volatility, stockholders have often instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and attention of management from our business, even if we are successful. Future sales of our common stock could also reduce the market price of such stock.
Moreover, the liquidity of our common stock is limited, not only in terms of the number of shares that can be bought and sold at a given price, but by delays in the timing of transactions and reduction in security analysts’ and the media’s coverage of us, if any. These factors may result in lower prices for our common stock than might otherwise be obtained and could also result in a larger spread between the bid and ask prices for our common stock. In addition, without a large float, our common stock is less liquid than the stock of companies with broader public ownership and, as a result, the trading prices of our common stock may be more volatile. In the absence of an active public trading market, an investor may be unable to liquidate its investment in our common stock. Trading of a relatively small volume of our common stock may have a greater impact on the trading price of our stock than would be the case if our public float were larger. We cannot predict the prices at which our common stock will trade in the future.
Our common stock is a “penny stock,” which makes it more difficult for our investors to sell their shares.
Our common stock is subject to the “penny stock” rules adopted under Section 15(g) of the Securities Exchange Act of 1934, as amended. The penny stock rules generally apply to companies whose common stock is not listed on The NASDAQ Stock Market or other national securities exchange and trades at less than $5.00 per share, other than companies that have had average revenue of at least $6,000,000 for the last three years or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stock to persons other than “established customers” complete certain documentation, make suitability inquiries of investors and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our stockholders sell substantial amounts of our common stock in the public market, it could create a circumstance commonly referred to as an “overhang,” in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
Our stockholders may experience substantial dilution as a result of the conversion of outstanding convertible preferred stock or the exercise of options and warrants to purchase shares of our common stock.
As of June 9, 2015, we have granted options to purchase 7,340 ,190 shares of common stock and have reserved 2,500 ,933 shares of our common stock for issuance upon the exercise of options pursuant to our 2012 Equity Incentive Plan. In addition, as of June 9, 2015, we may be required to issue 1, 605,348 shares of our common stock for issuance upon conversion of outstanding convertible preferred stock and 7,928,379 shares of our common stock for issuance upon exercise of outstanding warrants. Should all of these shares be issued, you would experience dilution in ownership of our common stock and the price of our common stock will decrease unless the value of our company increases by a corresponding amount.
The interests of our controlling stockholders may not coincide with yours and such controlling stockholder may make decisions with which you may disagree.
As of June 9, 2015, two of our stockholders beneficially owned over 56.53 % of our common stock. As a result, our controlling stockholders control substantially all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. In addition, this concentration of ownership may delay or prevent a change in control of our company and make some future transactions more difficult or impossible without the support of our controlling stockholders. The interests of our controlling stockholders may not coincide with our interests or the interests of other stockholders.
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have research coverage by securities and industry analysts and you should not invest in our common stock in anticipation that we will obtain such coverage. If we obtain securities or industry analyst coverage and if one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price and trading volume to decline.
We are subject to financial reporting and other requirements that place significant demands on our resources.
We are subject to reporting and other obligations under the Securities Exchange Act of 1934, as amended, including the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. Section 404 requires us to conduct an annual management assessment of the effectiveness of our internal controls over financial reporting. These reporting and other obligations place significant demands on our management, administrative, operational, internal audit and accounting resources. Any failure to maintain effective internal controls could have a material adverse effect on our business, operating results and stock price. Moreover, effective internal control is necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed.
We are an “emerging growth company” and we cannot be certain that the reduced disclosure requirements applicable to emerging growth companies will not make our common stock less attractive to investors.
The JOBS Act permits “emerging growth companies” like us to rely on some of the reduced disclosure requirements that are already available to smaller reporting companies. As long as we qualify as an emerging growth company or a smaller reporting company, we would be permitted to omit the auditor’s attestation on internal control over financial reporting that would otherwise be required by the Sarbanes-Oxley Act, as described above, and are also exempt from the requirement to submit “say-on-pay”, “say-on-pay frequency” and “say-on-parachute” votes to our stockholders and may avail ourselves of reduced executive compensation disclosure that is already available to smaller reporting companies.
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the exemption from complying with new or revised accounting standards provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, as long as we are an emerging growth company. An emerging growth company can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We intend to take advantage of the benefits of this until we are no longer an emerging growth company or until we affirmatively and irrevocably opt out of this exemption. Our financial statements may therefore not be comparable to those of companies that comply with such new or revised accounting standards.
We will cease to be an emerging growth company upon the earliest to occur of (i) the last day of the fiscal year during which we had total annual gross revenues of $1 billion (as indexed for inflation); (ii) the last day of the fiscal year following the fifth anniversary of the date of the first sale of common stock under our registration statement on Form S-1 that became effective on June 23, 2014; (iii) the date on which we have, during the previous 3-year period, issued more than $1 billion in non-convertible debt; or (iv) the date on which we are deemed to be a “large accelerated filer,” as defined by the Securities and Exchange Commission, which would generally occur upon our attaining a public float of at least $700 million. Once we lose emerging growth company status, we expect the costs and demands placed upon our management to increase, as we would have to comply with additional disclosure and accounting requirements, particularly if we would also not qualify as a smaller reporting company. In addition, until such time, we cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile and could cause our stock price to decline.
Delaware law and our Amended and Restated Certificate of Incorporation and By-laws contain anti-takeover provisions that could delay or discourage takeover attempts that stockholders may consider favorable.
Our board of directors is authorized to issue shares of preferred stock in one or more series and to fix the voting powers, preferences and other rights and limitations of the preferred stock. Accordingly, we may issue shares of preferred stock with a preference over our common stock with respect to dividends or distributions on liquidation or dissolution, or that may otherwise adversely affect the voting or other rights of the holders of common stock. Issuances of preferred stock, depending upon the rights, preferences and designations of the preferred stock, may have the effect of delaying, deterring or preventing a change of control, even if that change of control might benefit our stockholders. In addition, we are subject to Section 203 of the Delaware General Corporation Law. Section 203 generally prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless (i) prior to the date of the transaction, the board of directors of the corporation approved either the business combination or the transaction which resulted in the stockholder becoming an interested stockholder; (ii) the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding (a) shares owned by persons who are directors and also officers and (b) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or (iii) on or subsequent to the date of the transaction, the business combination is approved by the board and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least 66 2/3% of the outstanding voting stock which is not owned by the interested stockholder.
Section 203 could delay or prohibit mergers or other takeover or change in control attempts with respect to us and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
The terms of our Series C Preferred Stock prohibit us from paying dividends in the future on our common stock. As a result, any return on investment may be limited to the value of our common stock.
The terms of our Series C Preferred Stock prohibit us from paying dividends in the future on our common stock, absent consent from the holders representing a super-majority of the outstanding shares of our Series C Preferred Stock and a certain investor. Because we will likely not pay dividends, our common stock may be less valuable because a return on an investment in our common stock will only occur if our stock price appreciates.
Risks Related to our Series C Preferred Stock
Our Series C Preferred Stock contains covenants that could limit our financing options and liquidity position, which would limit our ability to grow our business.
Covenants in the certificate of designation for our Series C Preferred Stock impose operating and financial restrictions on us. These restrictions prohibit or limit our ability to, among other things:
|
●
|
incur additional indebtedness;
|
|
●
|
permit liens on assets;
|
|
●
|
repay, repurchase or otherwise acquire more than a de minimis number of shares of capital stock;
|
|
●
|
pay cash dividends to our stockholders; and
|
|
●
|
engage in transactions with affiliates.
|
These restrictions may limit our ability to obtain financing, withstand downturns in our business or take advantage of business opportunities. Moreover, debt financing we may seek may contain terms that include more restrictive covenants, may require repayment on an accelerated schedule or may impose other obligations that limit our ability to grow our business, acquire needed assets, or take other actions we might otherwise consider appropriate or desirable.
In addition, the certificate of designation for our Series C Preferred Stock requires us to redeem shares of our Series C Preferred Stock, at each holder’s option and for an amount greater than their stated value, upon the occurrence of certain events, including our being subject to a judgment of greater than $100,000 or our initiation of bankruptcy proceedings.
The holders of our Series C Preferred Stock are entitled to receive a dividend, which may be increased if we do not comply with certain covenants, and are also entitled to receive a make-whole payment if the Series C Preferred Stock is converted into common stock prior to February 12, 2016.
The holders of the Series C Preferred Stock are entitled to a 9% annual dividend on the $1,000 per share stated value of our Series C Preferred Stock, which is payable in cash or, subject to the satisfaction of certain conditions, in pay-in-kind shares. The dividend may be increased to a 18% annual dividend if we fail to comply with certain covenants, including our being subject to a judgment of greater than $100,000 or our initiation of bankruptcy proceedings. In addition, if a holder of the Series C Preferred Stock converts its shares of Series C Preferred Stock into shares of common stock any time prior to February 12, 2016, the holder will be deemed to have earned a make whole amount equal to the amount that would have been due if such shares of Series C Preferred Stock had been outstanding until such date, which may be paid in cash or pay-in-kind shares, depending upon the availability of funds to us to make such payments and the fulfillment of certain conditions relating to our company and our common stock. As a result of the payment of dividends and the make whole amounts related to our Series C Preferred Stock, we may be obligated to pay significant sums of money or issue significantly more shares of common stock than our Series C Preferred Stock would otherwise be convertible into, which could negatively affect our operations or result in the dilution of the holders of our common stock, respectively.
Our Series C Preferred Stock and certain of our warrants contain anti-dilution provisions that may result in the reduction of their conversion prices or exercise prices in the future.
Our Series C Preferred Stock and certain of our warrants contain anti-dilution provisions, which provisions require the lowering of the conversion price or exercise price, as applicable, to the purchase price of future offerings. Furthermore, with respect to such warrants, if we complete an offering below the exercise price of such warrants, the number of shares issuable under such warrants will be proportionately increased such that the aggregate exercise price payable after taking into account the decrease in the exercise price, shall be equal to the aggregate exercise price prior to such adjustment. If in the future we issue securities for less than the conversion or exercise price of our Series C Preferred Stock and such warrants, respectively, we will be required to further reduce the relevant conversion or exercise prices, and the number of shares underlying such warrants will be increased. We may find it more difficult to raise additional equity capital while our Series C Preferred Stock and such warrants are outstanding.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains “forward-looking statements,” which include information relating to future events, future financial performance, strategies, expectations, competitive environment and regulation. Words such as “may,” “should,” “could,” “would,” “predict,” “potential,” “continue,” “expect,” “anticipate,” “future,” “intend,” “plan,” “believe,” “estimate,” and similar expressions, as well as statements in future tense, identify forward-looking statements. Forward-looking statements should not be read as a guarantee of the occurrence or the expected timing of future performance or results. Forward-looking statements are based on information we have when those statements are made or our management’s good faith belief as of that time with respect to future events, and are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in or suggested by the forward-looking statements. Important factors that could cause such differences include, but are not limited to:
|
●
|
inability to manufacture our product candidates on a commercial scale on our own, or in collaboration with third parties;
|
|
●
|
difficulties in obtaining financing on commercially reasonable terms;
|
|
●
|
changes in the size and nature of our competition;
|
|
●
|
loss of one or more key executives or scientists; and
|
|
●
|
difficulties in securing regulatory approval to market our product candidates.
|
You should review carefully the section entitled “Risk Factors” beginning on page 3 of this prospectus for a discussion of these and other risks that relate to our business and investing in shares of our common stock. We undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise.
USE OF PROCEEDS
All shares of our common stock offered by this prospectus are being registered for the accounts of the selling stockholders and we will not receive any proceeds from the sale of these shares. However, we will receive the conversion price of $1.50 per share for the Series C Preferred Stock and the exercise price of the warrants if the warrants are exercised for cash. We intend to use those proceeds, if any, for general corporate purposes.
MARKET FOR COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
Market Information
On October 29, 2014, our common stock commenced trading on OTCQB under the symbol “BSGM.” Prior to October 29, 2014, there was no established trading price for our common stock. The following table sets forth, for the periods indicated, the high and low sales prices per share of our common stock as reported by the OTCQB. The quotations reflect inter-dealer prices, without retail markup, markdown or commissions, and may not represent actual transactions.
|
Fiscal Year 2015
|
||||||||
|
High
|
Low
|
|||||||
|
Second Quarter
|
$
|
4.80
|
|
$
|
2.01
|
|
||
|
First Quarter
|
$
|
2.85
|
$
|
1.31
|
||||
|
Fiscal Year 2014
|
||||||||
|
High
|
Low
|
|||||||
|
Fourth Quarter
|
$
|
3.50
|
$
|
2.56
|
||||
|
Third Quarter
|
$
|
-
|
$
|
-
|
||||
|
Second Quarter
|
$
|
-
|
$
|
-
|
||||
|
First Quarter
|
$
|
-
|
$
|
-
|
||||
|
Fiscal Year 2013
|
||||||||
|
High
|
Low
|
|||||||
|
Fourth Quarter
|
$
|
-
|
$
|
-
|
||||
|
Third Quarter
|
$
|
-
|
$
|
-
|
||||
|
Second Quarter
|
$
|
-
|
$
|
-
|
||||
We have never paid cash dividends on our common stock and do not anticipate paying any cash dividends in the foreseeable future, but intend to retain our capital resources for reinvestment in our business. In addition, the terms of our Series C Preferred Stock prohibit us from paying dividends in the future on our common stock, absent consent from the holders representing a super-majority of the outstanding shares of our Series C Preferred Stock and a certain investor.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our financial statements and the related notes thereto that are included in this prospectus. In addition to historical information, the following discussion and analysis includes forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. Factors that could cause or contribute to these differences include those discussed below and elsewhere in this prospectus, particularly in the section entitled “Risk Factors.” See “Special Note Regarding Forward-Looking Statements.”
Our Business
We are a development stage medical device company that is developing a proprietary technology platform to minimize noise and artifacts from cardiac recordings during electrophysiology studies and ablation. Our product under development, the PURE EP System, is a surface electrocardiogram and intracardiac multichannel recording and analysis system that acquires, processes and displays electrocardiogram and electrograms required during electrophysiology studies and ablation procedures.
We have not generated any revenue to date and consequently our operations are subject to all risks inherent in the establishment of a new business enterprise.
Critical Accounting Policies and Estimates
The following discussion and analysis of our financial condition and results of operations are based upon our financial statements, which have been prepared in accordance with generally accepted accounting principles in the U.S. The preparation of financial statements in accordance with generally accepted accounting principles in the U.S. requires us to make estimates and assumptions that affect the amounts reported in our financial statements. The financial statements include estimates based on currently available information and our judgment as to the outcome of future conditions and circumstances. Significant estimates in these financial statements include allowance for doubtful accounts and accruals for inventory claims. Changes in the status of certain facts or circumstances could result in material changes to the estimates used in the preparation of the financial statements and actual results could differ from the estimates and assumptions.
Among the significant judgments made by management in the preparation of our financial statements are the following:
Research and Development.
We account for research and development costs in accordance with the Accounting Standards Codification subtopic 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and developments costs are expensed when the contracted work has been performed or as milestone results have been achieved. Company-sponsored research and development costs related to both present and future products are expensed in the period incurred.
Stock Based Compensation.
All stock-based payments to employees and to nonemployee directors for their services as directors consisted of grants of restricted stock and stock options, which are measured at fair value on the grant date and recognized in the statements of operations as compensation expense over the relevant vesting period. Restricted stock payments and stock-based payments to nonemployees are recognized as an expense over the period of performance. Such payments are measured at fair value at the earlier of the date a performance commitment is reached or the date performance is completed. In addition, for awards that vest immediately and are non-forfeitable, the measurement date is the date the award is issued.
On October 29, 2014, our common stock commenced trading on OTCQB under the symbol “BSGM.” Fair value is typically determined by the closing price of our common stock on the date of the award.
Income Taxes.
Deferred income tax assets and liabilities are determined based on the estimated future tax effects of net operating loss and credit carryforwards and temporary differences between the tax basis of assets and liabilities and their respective financial reporting amounts measured at the current enacted tax rates. We record an estimated valuation allowance on our deferred income tax assets if it is not more likely than not that these deferred income tax assets will be realized. We recognize a tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position are measured based on the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement.
Results of Operations
We anticipate that our results of operations will fluctuate for the foreseeable future due to several factors, such as the progress of our research and development efforts and the timing and outcome of regulatory submissions. Due to these uncertainties, accurate predictions of future operations are difficult or impossible to make.
Three Months Ended March 31, 2015 Compared to Three Months Ended March 31, 2014
Revenues and Cost of Goods Sold. We had no revenues or cost of goods sold during the three months ended March 31, 2015 and 2014.
Research and Development Expenses. Research and development expenses for the three months ended March 31, 2015 were $302,079, an increase of $179,928, or 147.3%, from $122,151 for the three months ended March 31, 2014. This increase is primarily due to increase in activity level and increased personnel and consulting expenses.
General and Administrative Expenses. General and administrative expenses for the three months ended March 31, 2015 were $2,746,853, an increase of 2,145,288, or 357%, from $601,565 incurred in the three months ended March 31, 2014. This increase is primarily due to stock based compensation issued to employees and consultants in the current period as compared to the same period, last year.
Payroll related expenses increased to $323,617 in the current period from $83,586 for the three months ended March 31, 2014, an increase of $240,031. The increase is due to added personnel and bonus compensation paid. We incurred $2,054,475 in stock based compensation in connection with the vesting of stock and stock options issued to board members, officers, employees and consultants for the three months ended March 31, 2015 as compared to $343,837 stock based compensation for the same period in 2014.
Professional services for the three months ended March 31, 2015 totaled $73,350, a decrease of $36,565, or 33.3%, over the $109,915 recognized for the three months ended March 31, 2014. Of professional services, legal fees totaled $34,850 for the three months ended March 31, 2015, a decrease of $40,435, or 53.7%, from $75,285 incurred for the three months ended March 31, 2014. Accounting fees incurred in the three months ended March 31, 2015 amounted to $38,500, an increase of $3,870, or 11.2%, from $34,630 incurred in same period last year. The net decrease in professional service fees was primarily related to legal and auditing fees incurred associated with our efforts to become a publicly traded entity and our capital raising activities beginning in 2014.
Consulting and investor relations fees for the three months ended March 31, 2015 was $155,790 as compared to none incurred for the three months ended March 31, 2014. During the three months ended March 31, 2015, we incurred services relating to us becoming public company.
Travel, meals and entertainment costs for the three months ended March 31, 2015 were $53,436, an increase of $35,656, or 200.5%, from $17,780 incurred in the three months ended March 31, 2014. Travel, meals and entertainment costs include travel related to business development and financing. Rent for the three months ended March 31, 2015 totaled $22,514, an increase of $4,584 or 25.6%, from $17,930 incurred in the three months ended March 31, 2014, primarily due to changing common area maintenance fees incurred and lease renewal.
Depreciation Expense. Depreciation expense for the three months ended March 31, 2015 totaled $2,860, a decrease of $1,575, or 35.5%, over the expense of $4,435 incurred in the three months ended March 31, 2014, as a result of the aging of office computers and other equipment.
Interest Expense. Interest expense for the three months ended March 31, 2015 totaled $1,114, an increase of $16 from interest expense of $1,098 incurred during the same period last year. In the three months ended March 31, 2015 and 2014, our interest costs were comprised primarily related to credit card financing charges.
Financing Costs. Financing costs for the three months ended March 31, 2015 totaled $0 from $388,285 incurred during the three months ended March 31, 2014. Financing costs were primarily related to the fees paid related to the issuance of our Series A and Series B Preferred Stock in 2011 and 2012 and a beneficial conversion feature in our Series C Preferred Stock. During 2014, the remaining financing costs were amortized to operations.
Preferred Stock Dividend. Our preferred stock dividend for the three months ended March 31, 2015 totaled $79,395, a decrease of $4,629, or 5.5% from $84,024 incurred during the three months ended March 31, 2014. Preferred stock dividends are primarily related to the issuance of our Series A, Series B and Series C Preferred Stock from 2011 through 2013. In the second quarter of 2014, the Series A and Series B Preferred Stock was converted to common.
Net Loss. As a result of the foregoing, net loss for the three months ended March 31, 2015 was $3,090,801, compared to a net loss of $1,201,558 for the three months ended March 31, 2014.
Twelve Months Ended December 31, 2014 Compared to Twelve Months Ended December 31, 2013
Revenues and Cost of Goods Sold. We had no revenues or cost of goods sold during the twelve months ended December 31, 2014 and 2013.
Research and Development Expenses. Research and development expenses for the twelve months ended December 31, 2014 were $547,996, a decrease of $444,211, or 45%, from $992,207 for the twelve months ended December 31, 2013. This decrease is primarily due to a reduction in the costs paid to both personnel and research and development consulting services as we develop our proprietary technology platform. Research and development expenses were comprised of $366,362 of personnel costs and $115,692 consulting services for the twelve months ended December 31, 2014 as compared to $632,881 and $359,326 for the same period the previous year, respectively. This decrease is primarily due to salary and fee reductions in both personnel and research and development consulting services expenses, including accounting for payments to our scientists as personnel costs, as we develop our proprietary technology platform.
General and Administrative Expenses. General and administrative expenses for the twelve months ended December 31, 2014 were $7,304,440, an increase of $2,557,242, or 49%, from $5,229,252 incurred in the twelve months ended December 31, 2013. This increase is primarily due to increases in payroll related expenses and professional services and, to a lesser extent, due to increases in consulting fees and travel, meals and entertainment costs.
Payroll related expenses increased to $5,938,442 in the twelve months ended December 31, 2014 from $3,465,680 for the twelve months ended December 31, 2013, an increase of $2,472,762, or 71%. This increase is due to the value of the stock based compensation increasing to $5,693,425 in 2014, as a result of the vesting of stock and stock options issued to board members, officers and employees, as compared to $3,247,187 of stock based compensation in 2013.
Professional services for the twelve months ended December 31, 2014 totaled $585,472, an increase of $32,991, or 6%, over the $552,481 recognized for the twelve months ended December 31, 2013. Of professional services, legal fees totaled $284,787 for the twelve months ended December 31, 2014, a decrease of $24,406, or 8%, from $309,193 incurred for the twelve months ended December 31, 2013. Accounting fees incurred in the twelve months ended December 31, 2014 amounted to $64,380, a decrease of $29,178, or 32%, from $93,558 incurred for the same period in 2013. The increase in professional fees was primarily related to an increase in legal requirements as we continue to develop our operations, including legal fees associated with our capital raising transactions and the filing of our initial registration statement.
Consulting fees totaled $246,754 for the twelve months ended December 31, 2014, a decrease of $608,902 or 71%, from $855,656 for the twelve months ended December 31, 2013. The consulting fees for the year ended December 31, 2013 included $800,823 in stock based compensation for investment and finance consultants to assist in our fund raising and investor relations efforts to support our increased efforts in market research and potential investor identification.
Travel, meals and entertainment costs for the twelve months ended December 31, 2014 were $125,923, an increase of $35,144, or 39%, from $90,779 incurred during the twelve months ended December 31, 2013. During 2014, more travel was required than in 2013. Rent for the twelve months ended December 31, 2014 totaled $77,063, an increase of $3,058, or 4%, from $74,005 incurred during the same period in 2013.
Depreciation Expense. Depreciation expense for the twelve months ended 2014 totaled $15,809, a decrease of $1,250, or 7%, from the expense of $17,059 incurred during the same period in 2013, as a result of the aging of office computers and other equipment.
Interest Expense. Interest expense for the twelve months ended December 31, 2014 totaled $11,025, a decrease of $59,036 from the expense of $70,061 incurred during the twelve months ended December 31, 2013. For 2014, interest costs were comprised of finance costs and estimated liquidated damages of $6,953. During the twelve months ended December 31, 2013, we accrued estimated liquidated damages of $48,668 relating to our registration rights obligations in connection to the issuance of our Series C Preferred Stock. In addition, other interest costs were comprised primarily of bridge and related party notes issued in late 2012.
Financing Costs. Financing costs for the year ended December 31, 2014 totaled $593,770, a decrease of $2,902,282, or 83%, from $3,496,052 incurred during the year ended December 31, 2013. Financing costs are primarily related to the fees paid related to the issuance of our Series A and Series B Preferred Stock in 2011 and 2012 and a beneficial conversion feature in and the fees paid related to the issuance of our Series C Preferred Stock issued in 2013. The beneficial conversion feature associated with the Series C Preferred Stock is comprised of the allocated fair value of the conversion feature and the allocated fair value of warrants issued in connection with the sale of the Series C Preferred Stock.
Preferred Stock Dividend. Our preferred stock dividend for the twelve months ended December 31, 2014 totaled $300,359, an increase of $3,144, or 3%, from $297,215 incurred during the twelve months ended December 31, 2013. Preferred stock dividends are related to the issuance of our Series A, Series B and C Preferred Stock in 2011, 2012 and 2013.
Net Loss Available to Common Stockholders. Net loss Available to Common Stockholders for the twelve months ended December 31, 2014 was $8,773,399, compared to a net loss of $10,101,846 for the twelve months ended December 31, 2013, a decrease of $1,328,447 or 13%. The primary reasons for the decrease, as described above, is the reduction in financing costs net with the increase in stock based compensation incurred in general and administrative expenses.
Liquidity and Capital Resources
Three Months Ended March 31, 2015 Compared to Three Months Ended March 31, 2014
As of March 31, 2015, we had a working capital deficit of $4,544,839, comprised of cash of $2,059,328 and prepaid expenses of $159,768, which was offset by $320,871 of accounts payable and accrued expenses, stock based payable of $646,066, accrued dividends on preferred stock issuances of $456,964 and an aggregate of $5,340,034 of warrant and derivative liabilities. For the three months ended March 31, 2015, we used $1,219,982 of cash in operating activities and $2,684 of cash in investing activities. Cash provided by financing activities totaled $3,042,213, comprised of proceeds from the sale of our common stock. In the comparable period in 2014, $229,222 was raised through the sale of our common stock, net with repayments of related party advances of $20,281. At March 31, 2015, we had cash of $2,059,328 compared to $5,365 at March 31, 2014. Our cash is held in bank deposit accounts. At March 31, 2015 and December 31, 2014, we had no convertible debentures outstanding.
Cash used in operations for the three months ended March 31, 2015 and 2014 was $1,219,982 and $505,763, respectively, which represent cash outlays for research and development and general and administrative expenses in such periods. Increase in cash outlays principally resulted from increased research and development and general and administrative expenses due to the continued development of our operations, and reduction of our outstanding accounts payable by $274,655.
We used $2,684 cash for investing activities for the three months ended March 31, 2015, compared to $0 for the three months ended March 31, 2014. During the three months ended March 31, 2015, we purchased office furniture and computer equipment.
Twelve Months Ended December 31, 2014 Compared to Twelve Months Ended December 31, 2013
As of December 31, 2014, we had a working capital deficit of $910,082, comprised of cash of $239,781 and prepaid expenses of $75,537, which was offset by $554,026 of accounts payable and accrued expenses, stock based payable of $226,305 and accrued dividends on preferred stock issuances of $445,069. For the twelve months ended December 31, 2014, we used $1,997,072 of cash in operating activities. Cash provided by financing activities totaled $1,938,629, comprised of proceeds from the sale of our common stock of $1,969,410, net with repayments of related party advances of $30,781. In the comparable period in 2013, $1,768,410 was raised from the sale of our Series C Preferred Stock, $299,974 was raised through the sale of our common stock, $13,741 through related party advances, net with $30,000 repayments of related party loans. At December 31, 2014, we had cash of $239,781 compared to $302,187 at December 31, 2013. Our cash is held in bank deposit accounts. At December 31, 2014 and 2013, we had no convertible debentures outstanding as compared to $613,812 at December 31, 2012.
Cash used in operations for the twelve months ended December 31, 2014 and 2013 was $1,997,072 and $1,762,459, respectively, which represent cash outlays for research and development and general and administrative expenses in such periods. Increase in cash outlays principally resulted from increased research and development and general and administrative expenses due to the continued development of our operations.
Cash used in investing activities for the twelve months ended December 31, 2014 was $3,963, compared to $11,716 for the twelve months ended December 31, 2013. During both the twelve months ended December 31, 2014 and the twelve months ended December 31, 2013, we purchased office furniture and computer equipment.
December 2014 Private Placement
On December 19, 2014, we entered into a unit purchase agreement with certain accredited investors, pursuant to which we issued and sold, in multiple closings occurring on each of December 19, 2014, December 30, 2014, January 23, 2015, February 10, 2015, February 27, 2015 and March 31, 2015, an aggregate of 40.09 units, which consisted of, in the aggregate, 1,603,600 shares of our common stock, “A” warrants to purchase 1,603,600 shares of our common stock at an exercise price of $2.50 and “B” warrants to purchase 801,800 shares of our common stock at an exercise price of $3.75 per share, in exchange for aggregate gross proceeds of $4,009,000. As consideration for serving as our placement agent in connection with the private placement, we issued to Laidlaw & Company (UK) Ltd. “B” warrants to purchase an aggregate of 400,900 shares of common stock at an exercise price of $3.75 per share and paid cash fees equal to $481,080.
In their report dated February 20, 2015, our independent registered public accounting firm stated at December 31, 2014, there is substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is an issue raised due to our net losses and negative cash flows from operations since inception and our expectation that these conditions will continue for the foreseeable future. In addition, we will require additional financing to fund future operations. Further, we do not have any commercial products available for sale and have not generated revenues to date, and there is no assurance that, if approval of our products is received, we will be able to generate cash flow to fund operations. In addition, there can be no assurance that our research and development will be successfully completed or that any product will be approved or commercially viable. Our ability to continue as a going concern is subject to our ability to obtain necessary funding from outside sources, including obtaining additional funding from the sale of our securities, obtaining loans from various financial institutions or being awarded grants from government agencies, where possible. Our continued net operating losses increase the difficulty in meeting such goals and there can be no assurances that such methods will prove successful.
Our Series C Preferred Stock contains triggering events which would, among other things, require redemption (i) in cash, at the greater of (a) 120% of the stated value of $1,000 or (b) the product of (I) the variable weighted average price of our common stock on the trading day immediately preceding the date of the triggering event and (II) the stated value divided by the then conversion price or (ii) in shares of our common stock, equal to a number of shares equal to the amount set forth in (i) above divided by 75%. The triggering events include our being subject to a judgment of greater than $100,000 or our initiation of bankruptcy proceedings. If any of the triggering events contained in our Series C Preferred Stock occur, the holders of our Series C Preferred Stock may demand redemption, an obligation we may not have the ability to meet at the time of such demand. We will be required to pay interest on any amounts remaining unpaid after the required redemption of our Series C Preferred Stock at a rate equal to the lesser of 18% per annum or the maximum rate permitted by applicable law.
We expect to incur losses from operations for the near future. We expect to incur increasing research and development expenses, including expenses related to clinical trials. We expect that our general and administrative expenses will increase in the future as we expand our business development, add infrastructure and incur additional costs related to being a public company, including incremental audit fees, investor relations programs and increased professional services.
Our future capital requirements will depend on a number of factors, including the progress of our research and development of product candidates, the timing and outcome of regulatory approvals, the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims and other intellectual property rights, the status of competitive products, the availability of financing and our success in developing markets for our product candidates. We believe our existing cash will not be sufficient to fund our operating expenses and capital equipment requirements. We anticipate we will need approximately $2 million in addition to our current cash on hand to fund our operating expenses and capital equipment requirements for the next 12 months. We will have to raise additional funds to continue our operations and, while we have been successful in doing so in the past, there can be no assurance that we will be able to do so in the future. Our continuation as a going concern is dependent upon our ability to obtain necessary additional funds to continue operations and the attainment of profitable operations.
Future financing may include the issuance of equity or debt securities, obtaining credit facilities, or other financing mechanisms. Even if we are able to raise the funds required, it is possible that we could incur unexpected costs and expenses or experience unexpected cash requirements that would force us to seek alternative financing. Furthermore, if we issue additional equity or debt securities, existing holders of our securities may experience additional dilution or the new equity securities may have rights, preferences or privileges senior to those of existing holders of our securities.
If additional financing is not available or is not available on acceptable terms, we may be required to delay, reduce the scope of or eliminate our research and development programs, reduce our commercialization efforts or obtain funds through arrangements with collaborative partners or others that may require us to relinquish rights to certain product candidates that we might otherwise seek to develop or commercialize independently.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
BUSINESS
History
We were formed as BioSig Technologies, Inc., a Nevada corporation, in February 2009 and in April 2011 we merged with our wholly-owned subsidiary, BioSig Technologies Inc., a Delaware corporation, with the Delaware corporation continuing as the surviving entity. We are principally devoted to improving the quality of cardiac recordings obtained during ablation of atrial fibrillation (AF) and ventricular tachycardia (VT). We have not generated any revenue to date and consequently our operations are subject to all risks inherent in the establishment of a new business enterprise.
Overview
We are a development stage medical device company that is developing a proprietary technology platform to minimize noise and artifacts from cardiac recordings during electrophysiology studies and ablation. We are developing the PURE (Precise Uninterrupted Real-time evaluation of Electrograms) EP System, a surface electrocardiogram and intracardiac multichannel recording and analysis system that acquires, processes and displays electrocardiogram and electrograms required during electrophysiology studies and ablation procedures.
The PURE EP System is designed to assist electrophysiologists in making clinical decisions in real-time by providing information that, we believe, is not always easily obtained, if at all, from any other equipment presently used in electrophysiology labs. The PURE EP System’s ability to acquire high fidelity cardiac signals will potentially increase these signals’ diagnostic value, and therefore offer improved accuracy and efficiency of the EP studies and related procedures. We are developing signal processing tools within the PURE EP System. We believe that these will assist electrophysiologists in further differentiating true signals from noise, and will provide guidance in identifying ablation targets.
Since June 2011, we have collaborated with physicians affiliated with the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin, Texas for initial technology validation. The physicians affiliated with the Texas Cardiac Arrhythmia Institute has provided us with digital recordings obtained with conventional electrophysiology recording systems during different stages of electrophysiology studies. Using our proprietary signal processing tools that are part of the PURE EP System, we analyzed these recordings and successfully removed baseline wander, noise and artifacts from the data thereby providing better diagnostic quality signals.
We are focused on improving the quality of cardiac recordings obtained during ablation of atrial fibrillation, the most common cardiac arrhythmia, and ventricular tachycardia, an arrhythmia evidenced by a fast heart rhythm originating from the lower chambers of the heart, which can be life-threatening. Cardiac ablation is a procedure that corrects conduction of electrical impulses in the heart that cause arrhythmias. During this invasive procedure, a catheter is usually inserted using a venous access into a specific area of the heart. A special radiofrequency generator delivers energy through the catheter to small areas of the heart muscle that cause the abnormal heart rhythm. According to a 2009 article in Circulation: Arrhythmia and Electrophysiology, ablation is superior to pharmacological treatments and is becoming a first line of therapy for certain patients with arrhythmias (“Treatment of Atrial Fibrillation With Antiarrhythmic Drugs or Radiofrequency Ablation,” Circulation: Arrhythmia and Electrophysiology (2009) 2: 349-361).
Our overall goal is to establish our proprietary technology as a new platform that will have the following advantages over the electrophysiology recording systems currently available on the market:
|
●
|
Higher quality cardiac signal acquisition for accurate and more efficient electrophysiology studies;
|
|
●
|
Precise, uninterrupted, real time evaluations of electrograms;
|
|
●
|
Reliable cardiac recordings to better determine precise ablation targets, strategy and end point of procedures; and
|
|
●
|
A portable device that can be fully integrated into existing electrophysiology lab environments.
|
If we are able to develop our product as designed, we believe that the PURE EP System and its signal processing tools will contribute to an increase in the number of procedures performed in each electrophysiology lab and possibly improved patient outcomes.
Our significant scientific achievements to date include:
|
●
|
Initial system concept validation has been performed in collaboration with physicians at the Texas Cardiac Arrhythmia Institute at St. David’s Medical Center in Austin, Texas in June 2011. The Texas Cardiac Arrhythmia Institute provided challenging recordings obtained with electrophysiology recording systems presently in use at the institute during various electrophysiology studies. Our technology team successfully imported the data into the PURE EP System software and using proprietary signal processing, the PURE EP System software was able to reduce baseline wander, noise, and artifacts from the data and therefore provide better diagnostic quality signals.
|
|
●
|
We have established clinical and/or advisory relationships for both technology development and validation studies with physicians and researchers affiliated with the following medical centers: Texas Cardiac Arrhythmia Institute, Austin, TX; Cardiac Arrhythmia Center at the University of California at Los Angeles, Los Angeles, CA; Mount Sinai Medical Center, New York, NY; Beaumont Medical Center, Detroit, MI; University Hospitals Case Medical Center, Cleveland, OH; The Heart Rhythm Institute, University of Oklahoma Health Sciences Center, Oklahoma City, OK; and Mayo Clinic, Rochester, MN.
|
|
●
|
As part of our pre-clinical trials, physicians affiliated with the Texas Cardiac Arrhythmia Institute, University Hospitals Case Medical Center and Mount Sinai Medical Center provide us with recordings from challenging ablation procedures, mainly for ventricular tachycardia and atrial fibrillation, where the attending electrophysiologists face clinical dilemmas with the recordings obtained by their current recording systems. We believe that the recordings that the PURE EP System software has provided them, which show a reduction in baseline wander, noise, and artifacts, are of higher diagnostic value than the original recordings.
|
|
●
|
The Cardiac Arrhythmia Center at the University of California at Los Angeles and Dr. Kalyanam Shivkumar, a former member of our board of directors, have played a significant role in the initial functional testing of our hardware. Dr. Shivkumar and his team have enabled us to learn the connectivity of the lab and its devices that pertain to where our PURE EP System will fit in. In June 2013, we commenced our first proof of concept pre-clinical study with the assistance of Dr. Shivkumar in order to further test the components of the PURE EP System hardware, as further explained below.
|
|
●
|
We are developing signal processing tools within the PURE EP System that will assist electrophysiologists in further differentiating true signals from noise, which may potentially provide guidance in identifying ablation targets. The signal processing tools are expected to be an integral part of the software of the PURE EP System, which we believe will significantly facilitate the locating of ablation targets.
|
|
●
|
In the second and third quarters of 2013, we performed and finalized testing of our proof of concept unit by initially using an electrocardiogram/intracardiac simulator at our lab, and subsequently by obtaining pre-clinical recordings from the lab at the University of California at Los Angeles. As part of the testing, we simultaneously recorded electrocardiogram and intracardiac signals on our proof of concept unit and GE’s CardioLab recording system. An identical signal was applied to the input of both systems and the monitor of our proof of concept unit was positioned next to the monitor of GE’s CardioLab recording system to allow for visual comparison. We believe that our proof of concept unit performed well as compared to GE’s CardioLab recording system, in that the electrocardiogram and intracardiac signals displayed on our proof of concept unit showed less baseline wander, noise and artifacts compared to signals displayed on GE’s CardioLab recording system. However, because this was a proof of concept test, without any clearly established protocols, we cannot present this data for publication and we do not have any independent verification or peer review of these findings.
|
|
●
|
In the third quarter of 2013, we analyzed the results of our proof of concept unit to determine the final design of the PURE EP System prototype, which has since been completed.
|
|
|
●
|
In the fourth quarter of 2014, we appointed Dr. Samuel J. Asirvatham from Mayo Clinic as a member of our Scientific Advisory Board and initiated plans for pre-clinical studies at Mayo Clinic. We expect to perform our initial study there in April 2015.
|
|
|
●
|
In the first quarter of 2015, we appointed Dr. K.L. Venkatachalam from Mayo Clinic as a member of our Scientific Advisory Board. On March 31, 2015, Drs. Asirvatham and Venkatachalam performed our first pre-clinical study at the Mayo Clinic in Rochester, Minnesota.
|
We recently completed testing of the assembled components of the PURE EP System prototype in order to validate the design of the prototype. We conducted our first pre-clinical study on March 31, 2015 at the Mayo Clinic in Rochester, Minnesota with the PURE EP System prototype. We intend to conduct further pre-clinical studies, end-user preference studies, and research studies. The main objective of these studies is to demonstrate the clinical potential of the PURE EP System and show its advantages as compared to electrophysiology recorders currently on the market. We have also begun planning and implementing steps for obtaining 510(k) approval from the U.S. Food and Drug Administration for the PURE EP System.
We believe that by the first half of 2016, we will have obtained 510(k) marketing clearance from the FDA and will be able to commence marketing and commercialization of the PURE EP System. Our ability to achieve the aforementioned milestones will be principally determined by our ability to obtain necessary financing and regulatory approvals, among other factors.
Because we a development stage company, with our initial product under development, we currently do not have any customers. We anticipate that our initial customers will be hospitals and other health care facilities that operate electrophysiology labs.
Our Industry
Electrophysiology is the study of the propagation of electrical impulses throughout the heart. Electrophysiology studies are focused on the diagnosis and treatment of arrhythmias, a medical condition in which conduction of electrical impulses within the heart vary from the normal. Such conditions may be associated with significant health risks to patients. The invasive cardiac electrophysiology study for the evaluation of cardiac conduction disorders has evolved rapidly from a research tool to an established clinical treatment. This technique permits detailed analyses of the mechanism underlying cardiac arrhythmias and determines precise locations of the sites of origin of these arrhythmias, thereby aiding in treatment strategies.
Pharmacological, or medicine-based, therapies have traditionally been used as initial treatments, but they often fail to adequately control the arrhythmia and may have significant side effects. Catheter ablation is now often recommended for an arrhythmia that medicine cannot control. Catheter ablation involves advancing several flexible catheters into the patient’s blood vessels, usually either in the femoral vein, internal jugular vein or subclavian vein. The catheters are then advanced towards the heart. Electrical impulses are then used to induce the arrhythmia and local heating or freezing is used to ablate (destroy) the abnormal tissue that is causing it. Catheter ablation of most arrhythmias has a high success rate and multiple procedures per patient have been found to be more successful.
One study found that arrhythmia-free survival rates after a single catheter ablation procedure were 40%, 37%, and 29% at one, two and five years, respectively, with most recurrences over the first six months (“Catheter Ablation for Atrial Fibrillation - Are Results Maintained at 5 Years of Follow-Up?” J Am Coll Cardiol. (2011) 57(2):160-166). Another study stated that catheter ablation of atrial fibrillation has been shown to be effective in approximately 80% of patients after 1.3 procedures per patient, with approximately 70% of such patients requiring no further antiarrhythmic drugs during intermediate follow-up (Updated Worldwide Survey on the Methods, Efficacy, and Safety of Catheter Ablation for Human Atrial Fibrillation Circulation: Arrhythmia and Electrophysiology (2010) 3: 32-38).
Catheter ablation is usually performed by an electrophysiologist (a specially trained cardiologist) in a catheterization lab or a specialized electrophysiology lab. It is estimated that there are about 2,000 electrophysiology labs in the U.S. and 2,000 electrophysiology labs outside the U.S., each with an electrophysiology recording system costing an average of $250,000. We believe that the current value of the electrophysiology recording device market in the U.S. is approximately $500 million, based upon the number of electrophysiology labs in U.S. and the average cost of the recording system in each lab. With the potential of 12 million atrial fibrillation patients by the year 2050 (according to the Atrial Fibrillation Fact Sheet, February 2010, published by the Centers for Disease Control and Prevention) and improvements in technology for atrial fibrillation ablation therapy, significant growth is predicted for the number of hospitals building electrophysiology labs. A July 2012 report published by the Millennium Research Group predicted rapid growth in the U.S. market for electrophysiology mapping and ablation devices from 2012 to 2016, due to the medical community’s growing focus on treating atrial fibrillation. The report further predicts that even with advances in drug treatments and management devices to treat or manage arrhythmias, the electrophysiology mapping and ablation device market will be sustained by the continued development of advanced technologies that decrease ablation procedure times and improve success rates. According to the report, Electrophysiology Devices Market - Global Industry Analysis, Size, Share, Growth, Trends and Forecast, 2013 – 2019, analysts forecast the global market for EP devices will grow at a 12.1 percent compound annual growth rate, from $2.5 billion in 2012 to $5.5 billion by 2019.
Treatment of Atrial Fibrillation and Ventricular Tachycardia
We believe that the clearer recordings and additional information provided by the PURE EP System may improve outcomes during electrophysiology studies and ablation procedures for a variety of arrhythmias. For patients who are candidates for ablation, an electrophysiology study is necessary to define the targeted sites for the ablation procedure. Two common, yet complex, conditions for which ablation procedures are performed are atrial fibrillation and ventricular tachycardia. We believe that in the near future, the PURE EP System may have a great impact on assisting ablation strategies for these conditions.
Most cardiac arrhythmias are well understood and ablation simply requires destroying a small area of heart tissue possessing electrical abnormality. In contrast, complex arrythmias, such as atrial fibrillation and ventricular tachycardia, have complex pathophysiology and because knowledge of their origins and mechanisms are incomplete, ablation treatments for these arrhythmias are largely empirical. Catheter ablation is now an important option to control recurrent ventricular tachycardias (“EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias,” Europace (2009) 11 (6): 771-817). Catheter ablation of ventricular tachycardia in nonischemic heart diseases can be challenging, and outcomes across different diseases are incompletely defined (“Catheter Ablation of Ventricular Tachycardia in Nonischemic Heart Disease,” Circulation: Arrhythmia and Electrophysiology (2012) 5: 992-1000). In addition, limitations of atrial fibrillation ablation include the use of catheters designed for pinpoint lesions to perform large area ablations in a point-by-point fashion, and the dexterity required to perform the procedure (“New Technologies in Atrial Fibrillation Ablation,” Circulation (2009)). Furthermore, the length of these procedures exposes the physician and staff to extensive radiation, requiring them to wear heavy lead vests. Consequently, ablating atrial fibrillation and ventricular tachycardia have been regarded as being extremely difficult. Therefore, access to these procedures has been limited to being performed by only especially well-trained cardiologists; however, advancements in new technologies and techniques show a strong growth rate for these procedures.
According to the National Institute of Health National Heart Lung and Blood Institute, there are more than 3 million Americans suffering with atrial fibrillation and about 850,000 patients are hospitalized annually. As many as 600,000 new cases of atrial fibrillation are diagnosed each year. Despite the fact that physicians have been performing radiofrequency ablations since the 1990s, catheter-based treatment is offered to less than 3% of the atrial fibrillation patient population in the U.S. and Europe. According to Millenium Research Group (MRG), an increasing proportion of diagnosed atrial fibrillation cases are now being treated via ablation, as both physician confidence and the devices used in these procedures improve. A growing amount of positive clinical data has been demonstrating the efficacy of AF ablation when compared to the traditional first-line treatment of anti-arrhythmic drugs. As a result, AF ablation is becoming the fastest growing procedure type in this market, increasing at an average annual rate of 16 percent from 2012 to 2016. The American College of Cardiology Foundation/American Heart Association Task Force reported that catheter-directed ablation of atrial fibrillation represents a substantial achievement that promises better therapy for a large number of patients presently resistant to pharmacological or electrical conversion to sinus rhythm (“2011 ACCF/AHA/HRS Focused Update on the Management of Patients With Atrial Fibrillation (Updating the 2006 Guideline)”). However, rates of success and complications may vary, sometimes considerably.
According to the Heart Rhythm Society, ventricular tachycardia is the most dangerous arrhythmia since it may result in ventricular fibrillation, a rapid chaotic heartbeat in the lower chambers of the heart. Because the fibrillating muscle cannot contract and pump blood to the brain and vital organs, ventricular fibrillation is the number one cause of sudden cardiac death accounting for more than 350,000 deaths in the U.S. each year. Ventricular tachycardia is typically treated with implantable cardioverter defibrillators, or ICDs, or a combination of ablation along with an ICD. The American College of Cardiology/American Heart Association Task Force on Practice Guidelines/European Society of Cardiology Committee for Practice Guidelines, or ACC/AHA/ESC, 2009 guidelines recommend ablation in patients who either have sustained predominantly monomorphic ventricular tachycardia that is drug resistant, are drug intolerant or do not wish for long-term drug therapy. According to a recent study, catheter ablation has been found to reduce ventricular tachycardia/ventricular fibrillation recurrences and thereby ICD interventions, including ICD shocks, by approximately 75% in patients that have undergone multiple ICD shocks (Kuck, “Should Catheter Ablation be the Preferred Therapy for Reducing ICD Shocks? Ventricular Tachycardia in Patients With an Implantable Defibrillator Warrants Catheter Ablation,” Circulation: Arrhythmia and Electrophysiology (2009) 2: 713-720). More importantly, according to Kuck, catheter ablation is the only treatment that can terminate and eliminate incessant ventricular tachycardia and acutely abolish electrical storm in ICD patients. Typically, patients who receive ICDs are at high risk for recurrent arrhythmia; hence, most patients receive one or more ICD therapies for spontaneous arrhythmias after implantation. Despite the technological evolution of ICD systems, more than 20% of shocks are due to supraventricular arrhythmia and hence are inappropriate. Although the ICD aborts ventricular tachycardia/ventricular fibrillation, many patients continue to have symptoms. These shocks are physically and emotionally painful and lead to poor quality of life and adverse psychological outcomes in patients and their families.
According to Dr. Srijoy Mahapatra, the status of ventricular tachycardia ablation is growing at a 14-17% compound annual growth rate due to the fact that ablation of ventricular tachycardia may help patients feel better and live longer, despite the risks, including the occurrence of stroke, and the modest success rates. The success of ventricular tachycardia ablation varies, depending on the patient’s specific heart condition that caused ventricular tachycardia. The procedure is most effective in patients with otherwise normal hearts, in whom the success rate exceeds 90%. In patients with structural heart disease resulting from scar or cardiomyopathy, success rates range between 50% and 75% at six to 12 months. In cases in which a patient experiences a recurrence, two of three patients will still have less ventricular tachycardia than before the initial ablation (Circulation (2010) 122: e389-e391). Therefore, we believe that ablation will continue to become a preferred treatment for ventricular tachycardia, especially in light of the challenges presented by ICD therapies; this increase in demand for ablation procedures will likely also increase the demand for technological advances in medical devices essential to ablation procedures, including electrophysiology recorders, in order to better support and ablation procedures.
Electrophysiology Lab Environment and Electrophysiology Recording Systems
The electrophysiology lab environment and recording systems create significant amounts of noise and artifacts during electrophysiology procedures. Current surface and intracardiac recording systems typically consist of large workstations interconnected by a complex set of cables that contribute to significant amounts of noise during signal acquisition. Additional noise and artifacts generated from the electrophysiology lab equipment further hamper recordings of small electrophysiological potentials. Preserving spaciotemporal (space and time) characteristics of the signal in a very challenging electrophysiology recording environment is a difficult task. To remove noise and artifacts, recorders that are currently on the market offer a family of low pass, high pass and notch filters, but these filters alter signal information context.
The shape and amplitude of electrocardiograms, unipolar and bipolar electrograms, and, consequently, reconstructed endocardial and epicardial maps, are influenced not only by electrophysiological and structural characteristics of the myocardial tissue involved, but with characteristics of the recording system. Amplitude and morphology of electrocardiogram and intracardiac signals are significantly affected by filters used to remove noise. Because of the number of amplitude and interval measurements made during an electrophysiology study, it is imperative that the recording system faithfully acquires surface electrocardiogram and intracardiac electrograms. We believe that the recording systems that are currently available on the market are ineffective in preserving the optimal amount of original information contained in the cardiac signals.
In addition, the electrophysiology lab consists of sophisticated equipment that requires an electrophysiologist to mentally integrate information from a number of sources during procedures. There are numerous monitors in an electrophysiology lab that provide and display this variety of information. An electrophysiologist needs to evaluate the acquired cardiac signals and the patient’s responses to any induced arrhythmias during the procedure. However, it is difficult for an electrophysiologist to synthesize the disparate information produced by the numerous monitors in the lab and calculate the real-time, three-dimensional orientation of the anatomy and the location of the recording and ablation catheters. As the number of electrophysiology procedures increase, a variety of diagnostic and therapeutic ablation catheters are becoming more widely available and new highly specialized catheters are being developed. In addition, remote robotic and magnetic navigation systems are being developed to address limitations of dexterity in controlling the catheter tip, especially during complex arrhythmia ablation procedures. We believe that, considering the improvements being made with respect to other equipment used in the electrophysiology lab and the continual increase of ablation procedures, the electrophysiology recorders currently available on the market are not sufficiently advanced with respect to the quality of their recordings to deliver adequate results. We believe that the PURE EP System will be able to deliver superior quality of recordings that will allow it to successfully integrate with the other advanced equipment found in the electrophysiology lab.
The requirement for optimal signal integrity is further amplified during ablation treatments of atrial fibrillation and ventricular tachycardia. Presently, one of the main objectives of the atrial fibrillation ablation procedure is to precisely identify, ablate and eliminate pulmonary vein potentials and one of the main objectives of the ventricular tachycardia procedure is to map the arrhythmia substrate and precisely identify, ablate and eliminate small abnormal potentials. The information provided by recorders is essential for an electrophysiologist to determine ablation strategy during termination of both pulmonary vein potentials and ventricular tachycardia. Therefore, it is important that the recording system’s noise removal technique does not alter appearance and fidelity of these potentials. As a result, it is necessary that any new signal processing preserves signal fidelity as much as possible during electrophysiology recordings; otherwise, the signals that are needed to guide the ablation procedures will be difficult to distinguish due to noise interference.
Our Products
We intend to bring to the electrophysiology market the PURE EP System, an electrocardiogram/intracardiac recorder that will be coupled with an array of software tools intended for electrophysiology studies and procedures ranging from simple diagnostic tests to ablation for the most complex cases of arrhythmias. We believe that this system will provide unique recording capabilities because we are developing it to allow precise, uninterrupted, real-time evaluations of electrocardiograms and electrograms, and allow electrophysiologists to obtain data that cannot be acquired from present day recorders.
The PURE EP System uses a combination of analog and digital signal processing to acquire and display cardiac data. Because our technology consists of proprietary hardware, software and algorithms, the original cardiac data is not distorted. In addition, we are developing a library of software tools that are designed to be configured to fit the needs of electrophysiologists in different settings and/or for different arrhythmia treatments. With the software, the PURE EP System can be positioned to provide information that can be used by electrophysiologists to help guide the ablation catheter; shorten procedure times; and can reduce the complexity of maneuvers necessary for identifying ablation targets for various arrhythmias, including atrial fibrillation and ventricular tachycardia. The PURE EP System is intended to be used in addition to existing electrophysiology recorders. We believe that the less distorted cardiac data provided by the PURE EP System will increase the workload ability and enhance the capabilities of the typical electrophysiology laboratory.
Initial Analysis
According to S. J. Asirvatham, MD, et. al. (“Signals and Signal Processing for the Electrophysiologist,” Circ Arrhythm Electrophysiol. (2011) 4:965-973), recording environments in a typical electrophysiology laboratory presents challenging situations. S. J. Asirvatham, MD, et. al., state, “Successful mapping and ablation in the electrophysiology laboratory is critically dependent on acquiring multiple, low-amplitude, intracardiac signals in the presence of numerous sources of electric noise and interference and displaying these signals in an uncomplicated and clinically relevant fashion, with minimal artifacts. This represents a significant engineering challenge and, in real-life electrophysiology laboratory, is not always successful.”
To determine and validate the state of present electrophysiology recording technology in the field, we completed a detailed analysis of the effect of filters used by existing EP recorders to reduce noise on spaciotemporal characteristics of electrocardiograms and intracardiac electrograms. We used a custom built electrocardiogram/intracardiac simulator with a database of various electrocardiogram signals combined with electrophysiology signals, along with waveforms from publicly available databases. The ability to faithfully reproduce database waveforms generated by an electrocardiogram/intracardiac simulator was tested using the PURE EP System and conventional electrophysiology recorders, the GE CardioLab and St. Jude EP-WorkMate.
We evaluated the signal quality (amplitude, morphology and duration) of the different recorders, along with the ability of the recorders to reduce noise level and remove baseline wander, which are the cardiac signals that have shifted from the isoelectric line (the base line of the signal tracing). The electrocardiogram and intracardiac signals subjected to the PURE EP System’s signal processing showed less baseline wander, noise and artifacts compared to the conventional electrophysiology recorders. Further, spaciotemporal characteristics of signals were greatly distorted by the conventional electrophysiology system, particularly when a notch filter was used, as compared to the recording of the same spaciotemporal characteristics by the PURE EP System. A notch filter is used to remove a specific frequency from the signal, especially either 60Hz in the U.S. and 50Hz in Europe, and can be implemented in hardware or software.
To date, we have not conducted any studies of the data produced by our technology that have been subjected to any third-party review, as would be required for the publication of a formal study. If we are able to demonstrate a similar level of success in removing baseline wander and reducing noise level for our planned pre-clinical and clinical studies and trials, we believe that the PURE EP System’s signal processing will become a vital part of electrophysiology labs and will greatly assist in the ablation treatment for complex arrhythmias, including atrial fibrillation and ventricular tachycardia.
Proof of Concept Testing
We developed the PURE EP System’s proof of concept unit, which is the version of the product prior to prototype. The proof of concept unit was designed using separate analog and digital boards to allow for easier debugging and to demonstrate single channel electrocardiogram and intracardiac acquisition capabilities. The proof of concept unit was built to (i) verify that the PURE EP System performs in line with our intended design of the product, (ii) validate a portion of the hardware design that we intend to use in the prototype, and (iii) verify the software used by the PURE EP System. The main objectives of the proof of concept unit were to demonstrate that the system’s hardware and software have the ability to faithfully records small cardiac signals in an electrophysiology laboratory environment and to obtain initial performance results.
In the second and third quarters of 2013, we performed and finalized testing of our proof of concept unit by initially using an electrocardiogram/intracardiac simulator at our lab, and subsequently by obtaining pre-clinical recordings from the lab at the University of California at Los Angeles. As part of the testing, we simultaneously recorded electrocardiogram and intracardiac signals on our proof of concept unit and GE’s CardioLab recording system. An identical signal was applied to the input of both systems and the monitor of our proof of concept unit was positioned next to the monitor of GE’s CardioLab recording system to allow for visual comparison. We believe that our proof of concept unit performed well as compared to GE’s CardioLab recording system, in that the electrocardiogram and intracardiac signals displayed on our proof of concept unit showed less baseline wander, noise and artifacts compared to signals displayed on GE’s CardioLab recording system. However, because this was a proof of concept test, without any clearly established protocols, we cannot present this data for publication and we do not have any independent verification or peer review of these findings.
Subsequently, in the third quarter of 2013, we analyzed the results of our proof of concept unit to determine the final design of the PURE EP System prototype. Because the proof of concept unit was designed to verify the capabilities of the main components of the PURE EP System, we established a list of tasks necessary to complete the prototype (which we intend to use for end-user preference studies, additional pre-clinical studies and research studies), which has since been completed.
Proof of Concept Testing at UCLA’s EP Lab
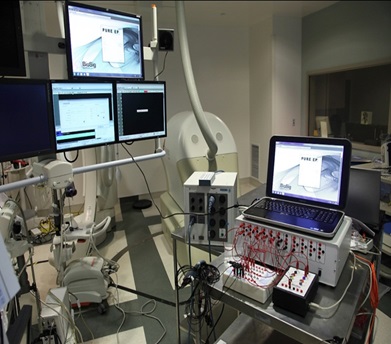
The current Pure EP System prototype
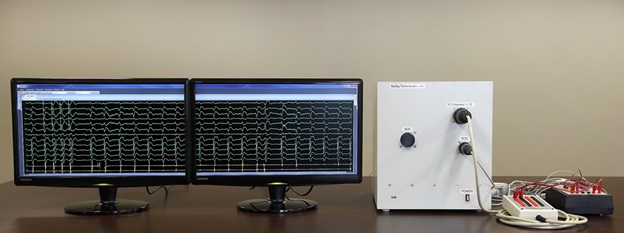
Growth Strategy
Technology and Development Plan
Our technology team consists of six engineers with expertise in digital signal processing, low power analog and digital circuit design, software development, embedded system development, electromechanical design, testing and system integration, and the regulatory requirements for medical devices. We have also entered into collaboration agreements with advisors and medical institutions in the fields of cardiology and electrophysiology, including the Texas Cardiac Arrhythmia Institute (see “–Strategic Alliances”). We currently intend to outsource manufacturing, assembling, and testing.
We recently completed testing of the assembled components of the PURE EP System prototype in order to validate the design of the prototype. We conducted our first pre-clinical study on March 31, 2015 at the Mayo Clinic in Rochester, Minnesota with the PURE EP System prototype. We intend to conduct further pre-clinical studies, end-user preference studies, and research studies. The main objective of these studies is to demonstrate the clinical potential of the PURE EP System and show its advantages as compared to electrophysiology recorders currently on the market. We have also begun planning and implementing steps for obtaining 510(k) approval from the U.S. Food and Drug Administration for the PURE EP System.
We believe that by the first half of 2016, we will have obtained 510(k) marketing clearance from the FDA and will be able to commence marketing and commercialization of the PURE EP System. Our ability to achieve the aforementioned milestones will be principally determined by our ability to obtain necessary financing and regulatory approvals, among other factors.
Competition
The electrophysiology market is characterized by intense competition and rapid technological advances. There are currently four large companies that share the majority of the electrophysiological recording market share. They produce the following electrophysiology recording systems, each with a unit price of approximately $250,000 per unit:
|
●
|
GE’s CardioLab Recording System was developed in the early 1990s by Prucka Engineering and was acquired by GE in 1999.
|
|
|
●
|
Bard’s LabSystem PRO EP Recording System was originally designed in the late 1980s. CR Bard’s electrophysiology business was acquired by Boston Scientific in 2013.
|
|
●
|
Siemens developed the Axiom Sensis XP in 2002.
|
|
|
●
|
St. Jude Medical’s EP-WorkMate Recording System was acquired from EP MedSystems in 2008, which had received approval for the product from the U.S. Food and Drug Administration in 2003.
|
Based upon our analysis of data taken from patent applications filed with the U.S. Patent and Trademark Office and 510(k) approval applications filed with the U.S. Food and Drug Administration, we believe that the above recording systems are built on relatively old technologies and all use the identical approach in applying digital filters to remove noise and artifacts. We are of the opinion that such an approach sacrifices cardiac signal fidelity and, in the case of ablation, the filters have a direct impact on the ablation strategy of an electrophysiologist. The imprecise method to remove noise and artifacts used by the old recorders could be a contributing factor to the multiple (or repeated) ablation procedures that are frequently required in order to completely cure patients from atrial fibrillation and ventricular tachycardia. We are not currently aware of any other companies that are developing new recording technology for electrophysiology recorders.
Suppliers
The PURE EP System contains proprietary hardware and software modules that are assembled into the system. Hardware boards contain components that are available from different distributors. The parts used to manufacture analog and digital boards are readily available from a number of distributors or manufacturers. We obtained components from various suppliers and have assembled our first prototype in-house. We envision outsourcing manufacturing of the complete PURE EP System to a local medical device manufacturer in California.
Research and Development Expenses
Research and development expenses for the fiscal years ended December 31, 2014 and 2013 were $547,996 and $992,207, respectively.
Sales, Marketing and Customer Service
We plan to implement a market development program prior to launch of our PURE EP System. As the product progresses through development and testing, we intend to gather the data produced by the PURE EP System’s processing and presenting electrocardiogram and intracardiac signals and use such data for posters, presentations at cardiology conferences, and, if appropriate, submissions to scientific journals. We believe that as we gather additional data from our existing proof of concept tests and our planned pre-clinical and clinical studies and user preference studies, we will be able to better determine the focus of our marketing efforts. We also plan to leverage our relationships with cardiac research and treatment centers to gain early product evaluation and validation. We believe that through these efforts, we may be able to gain preliminary acceptance of our PURE EP product by experienced professionals and academics in the electrophysiology field.
We also intend to simultaneously develop a branding strategy to introduce and support the PURE EP System. The strategy may include our presence at major relevant cardiology meetings on a national and regional basis to engage and educate physicians concerning the PURE EP System and any of our other products, as well as engaging in a variety of other direct marketing methods. We also intend to develop a small direct sales force together with a distribution network that has existing relationships with hospitals and electrophysiologists. We believe that we may be able to begin commercial sales of the PURE EP System in 2016.
Intellectual Property
Patents
Our success depends in large part on our ability to establish and maintain the proprietary nature of our technology. Our co-founder and former chief technology officer, Budimir S. Drakulic, Ph.D., conceived of the proprietary elements of the PURE EP System in 2009 and 2010. We filed a patent application with the U.S. Patent and Trademark Office in December 2013 directed at systems and methods for the evaluation of electrophysiology systems. In March 2014, the inventors listed on the patent application filed in December 2013 assigned all of their rights to the patent application to us. In December 2014, we filed this patent application under the Patent Cooperation Treaty (PCT) with the U.S. Receiving Office.
We intend to file one or more additional patents in the U.S. in the future. Our patent application filed in December 2013 represents a significant portion of our core proprietary intellectual property. Our patent application filed in December 2013 describes a system that can show comparative output of any two cardiac signal systems—such as the PURE EP System as compared to a competitor system, thus showing the value of the PURE EP System.
This patent application describes signal processing evaluators that assess how well a cardiac signal system reading a cardiac signal (such as the PURE EP System or another system) filters out noise, such as non-cardiac signals or other body-generated artifacts. Such noise is filtered by such systems with varying success, thus, an evaluator such as described in the patent application may be used to provide comparison data for a particular system versus another given the same or similar input. The patent application also describes a simulator that can send a simulated signal to a cardiac signal system (the PURE EP System or another system) in order to challenge such cardiac signal system to filter out typical noise. These are adjunct technologies that can be used to show the value of the PURE EP System as compared to other systems existing in the market. The additional patent applications that we intend to file in the U.S. in the future are expected to represent portions of the hardware and software technology associated with our PURE EP System, which technology includes a cardiac signal system that reads cardiac signals and filters such cardiac signals from noise such as non-cardiac signals or other body-generated artifacts. Upon filing of such patent applications, we believe that the novel aspects of our PURE EP System should be subject to pending patent application; however, we cannot be assured that all of the patents related to our patent applications, if any, will be granted.
Trademarks
Our trademark application to register “PURE EP” in the U.S. is pending.
Government Regulation
Our solutions include software and hardware which will be used for patient diagnosis and, accordingly, are subject to regulation by the U.S. Food and Drug Administration and other regulatory agencies. U.S. Food and Drug Administration regulations govern, among other things, the following activities that we perform and will continue to perform in connection with:
|
●
|
Product design and development;
|
|
|
●
|
Product testing;
|
|
●
|
Product manufacturing;
|
|
|
●
|
Product labeling and packaging;
|
|
●
|
Product handling, storage, and installation;
|
|
|
●
|
Pre-market clearance or approval;
|
|
●
|
Advertising and promotion; and
|
|
|
●
|
Product sales, distribution, and servicing.
|
U.S. Food and Drug Administration’s Pre-market Clearance and Approval Requirements
The U.S. Food and Drug Administration classifies all medical devices into one of three classes. Devices deemed to pose lower risks are placed in either Class I or II, which requires the manufacturer to submit to the U.S. Food and Drug Administration a pre-market notification, known as a PMN, and a 510(k) approval, requesting clearance of the device for commercial distribution in the U.S. Class III devices are devices which must be approved by the pre-market approval process. These tend to be devices that are permanently implanted into a human body or that may be necessary to sustain life. For example, an artificial heart meets both these criteria. Based on analysis of predicate devices, we believe that our products will be classified as Class II. Pursuant to U.S. Food and Drug Administration guidelines, Class II devices include a programmable diagnostic computer, which is a device that can be programmed to compute various physiologic or blood flow parameters based on the output from one or more electrodes, transducers, or measuring devices; this device includes any associated commercially supplied programs. Because the PURE EP System is a surface electrocardiogram and intracardiac multichannel recording and analysis system that acquires, processes and displays electrocardiogram and electrograms, we believe it will be classified as a Class II device. We must, therefore, first receive a 510(k) clearance from the U.S. Food and Drug Administration for our PURE EP System before we can commercially distribute it in the U.S. In the event that our PURE EP System is classified as a Class III device, which we believe is unlikely to occur, the U.S. Food and Drug Administration regulatory approval process and the subsequent commercialization of our product will require significantly greater time and resources than if it is classified as a Class II device, which would require us to reassess our strategic business plan of operations.
510(k) Clearance Process
For our PURE EP System, we must submit a pre-market notification to the U.S. Food and Drug Administration demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device, a device that was in commercial distribution before May 28, 1976 for which the U.S. Food and Drug Administration has not yet called for the submission of pre-market approval applications, or is a device that has been reclassified from Class III to either Class II or I.
The U.S. Food and Drug Administration’s 510(k) clearance process usually takes three to six months from the date the application is submitted and filed with the U.S. Food and Drug Administration, but it can take significantly longer. A device that reaches market through the 510(k) process is not considered to be “approved” by the U.S. Food and Drug Administration. They are generally referred to as “cleared” or “510(k) cleared” devices. Nevertheless, it can be marketed and sold in the U.S.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, will require a new 510(k) clearance or could require a pre-market approval, which requires more data and is generally a significantly longer process than the 510(k) clearance process. The U.S. Food and Drug Administration requires each manufacturer to make this determination initially, but the U.S. Food and Drug Administration can review any such decision and can disagree with a manufacturer’s determination. If the U.S. Food and Drug Administration disagrees with a manufacturer’s determination, the U.S. Food and Drug Administration can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or a pre-market approval is obtained.
Pervasive and continuing U.S. Food and Drug Administration regulation
After a medical device is placed on the market, numerous U.S. Food and Drug Administration regulatory requirements apply, including, but not limited to the following:
|
●
|
Quality System regulation, which requires manufacturers to follow design, testing, control, documentation and other quality assurance procedures during the manufacturing process;
|
|
|
●
|
Establishment Registration, which requires establishments involved in the production and distribution of medical devices intended for commercial distribution in the U.S. to register with the U.S. Food and Drug Administration;
|
|
●
|
Medical Device Listing, which requires manufacturers to list the devices they have in commercial distribution with the U.S. Food and Drug Administration;
|
|
|
●
|
Labeling regulations, which prohibit “misbranded” devices from entering the market, as well as prohibit the promotion of products for unapproved or “off-label” uses and impose other restrictions on labeling; and
|
|
●
|
Medical Device Reporting regulations, which require that manufacturers report to the U.S. Food and Drug Administration if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur.
|
Failure to comply with applicable regulatory requirements can result in enforcement action by the U.S. Food and Drug Administration, which may include one or more of the following sanctions:
|
●
|
Fines, injunctions, and civil penalties;
|
|
|
●
|
Mandatory recall or seizure of our products;
|
|
●
|
Administrative detention or banning of our products;
|
|
|
●
|
Operating restrictions, partial suspension or total shutdown of production;
|
|
●
|
Refusing our request for 510(k) clearance or pre-market approval of new product versions;
|
|
|
●
|
Revocation of 510(k) clearance or pre-market approvals previously granted; and
|
|
●
|
Criminal penalties.
|
International Regulation
International sales of medical devices are subject to foreign government regulations, which vary substantially from country to country. The time required to obtain approval by a foreign country may be longer or shorter than that required for U.S. Food and Drug Administration approval, and the requirements may differ significantly.
The European Union has adopted legislation, in the form of directives to be implemented in each member state, concerning the regulation of medical devices within the European Union. The directives include, among others, the Medical Device Directive that establishes standards for regulating the design, manufacture, clinical trials, labeling, and vigilance reporting for medical devices. Our PURE EP system may be affected by this legislation. Under the European Union Medical Device Directive, medical devices are classified into four classes, I, IIa, IIb, and III, with class I being the lowest risk and class III being the highest risk. Under the Medical Device Directive, a competent authority is nominated by the government of each member state to monitor and ensure compliance with the Medical Device Directive. The competent authority of each member state then designates a notified body to oversee the conformity assessment procedures set forth in the Medical Device Directive, whereby manufacturers demonstrate that their devices comply with the requirements of the Medical Device Directive and are entitled to bear the CE mark. CE is an abbreviation for Conformité Européenne (or European Conformity) and the CE mark, when placed on a product, indicates compliance with the requirements of the applicable directive. Medical devices properly bearing the CE mark may be commercially distributed throughout the European Union. Failure to obtain the CE mark will preclude us from selling the PURE EP System and related products in the European Union.
Employees
As of June 9, 2015, we had eleven full-time employees. Additionally, we use consultants as needed to perform various specialized services. None of our employees are represented under a collective bargaining agreement.
Properties
Our headquarters are located in Minneapolis, Minnesota and our research and development offices are in Los Angeles, California. We lease our office space in both locations. Because we do not have any manufacturing requirements at this time, we believe our current space is sufficient to meet our current needs.
Legal Proceedings
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business. We are currently not aware of any such legal proceedings or claims that we believe will have, individually or in the aggregate, a material adverse effect on our business, financial condition or operating result.
There are no material proceedings in which any of our directors, officers or affiliates or any registered or beneficial shareholder of more than 5% of our common stock is an adverse party or has a material interest adverse to our interest.
EXECUTIVE OFFICERS AND DIRECTORS
The following table sets forth information regarding our executive officers and the members of our board of directors.
|
Name
|
Age
|
Position with the Company
|
||
|
Kenneth L. Londoner
|
47 |
Executive Chairman and Director
|
||
|
Gregory D. Cash
|
58 |
President and Chief Executive Officer, Director
|
||
|
Steve Chaussy
|
61 |
Chief Financial Officer
|
||
|
Asher Holzer, Ph.D.
|
65 |
Chief Scientific Advisor and Director
|
||
|
Roy T. Tanaka
|
67 |
Director
|
||
|
Patrick J. Gallagher
|
50 |
Director
|
||
|
Seth H.Z. Fischer
|
59 |
Director
|
||
|
Jeffrey F. O’Donnell, Sr.
|
55 |
Director
|
||
|
Jerome Zeldis, M.D., Ph.D.
|
64 |
Director
|
||
| David Weild IV | 58 | Director |
Directors are elected at each annual meeting of our stockholders and hold office until their successors are elected and qualified or until their earlier resignation or removal. Officers are appointed by our board of directors and serve at the discretion of the board of directors, absent an employment agreement.
Biographical Information
Kenneth L. Londoner. Mr. Londoner has served as our director since February 2009 and as our executive chairman since November 2013. He previously served as our chairman and chief executive officer from February 2009 to September 2013. Mr. Londoner has served as the managing partner of Endicott Management Partners, LLC, a firm dedicated to assisting emerging growth companies in their corporate development, since February 2010.From April 2007 to October 2009, he served as executive vice president – corporate business development and senior director of business development and, from November 2009 to December 2010, he served as a consultant to NewCardio, Inc., a medical device designer and developer. Mr. Londoner had also served as a director of chatAND Inc. from January 2012 to April 2015. Mr. Londoner is a co-founder and board member of Safe Ports Holdings, Charleston, South Carolina. Mr. Londoner also served as a director of MedClean Technologies, Inc. from November 2008 to September 2010. Mr. Londoner was an investment officer and co-manager of the Seligman Growth Fund, Seligman Capital Fund, and approximately $2 billion of pension assets at J & W Seligman & Co, Inc. in New York from 1991 to 1997. Mr. Londoner graduated from Lafayette College in 1989 with a degree in economics and finance and received his MBA from New York University’s Leonard N. Stern School of Business in 1994.We believe that Mr. Londoner’s extensive experience in financial and venture capital matters, as well as his intimate knowledge of our company as its co-founder make him an asset to our board of directors.
Gregory D. Cash. Mr. Cash has served as our president and chief executive officer and as a director since July 2014. Mr. Cash served as the president, chief executive officer and founder of Argent International LLC, a life sciences consulting firm, from July 2011 until July 2014. Mr. Cash served as a member of the board of directors for Acuity Medical International, Inc. from January 2015 to April 2015. From September 2012 until February 2013, he was also president and chief executive officer of NeuroTherm, Inc., a multinational company in the interventional pain field. Until June 2011, Mr. Cash served as president, chief executive officer and director of HeartSine Technologies, Inc., a start-up company in the automated external defibrillator market. Prior to joining HeartSine Technologies in December 2006, he was President, Vascular Therapy and New Business for Sorin Group based in Milan, Italy and also Senior Vice President, Strategic Alliances based in Denver, Colorado. From 2002 to 2004, Mr. Cash was the president, chief executive officer and a director of Vasomedical, Inc., a NASDAQ traded public company. Prior to 2002, he was corporate vice president at Datascope Corporation and president of its wholly owned subsidiary, InterVascular, Inc., president and chief operating officer of Eminent Technology Partners, Inc. and chief executive officer of its subsidiary, Eminent Research Systems, vice president and general manager of vascular therapies for the U.S. Surgical Corporation and spent five years at Boston Scientific Corporation in numerous roles, including vice president of cardiology sales and marketing in Europe. Mr. Cash began his career at Medtronic, Inc., where he served fourteen years in increasingly senior sales and marketing positions. He currently serves on a number of advisory boards, including the Concordia Language Villages National Board, the University of Minnesota Office for Technology Commercialization as well as the French American Chamber of Commerce of Minneapolis/St. Paul. Mr. Cash holds a B.A. in International Marketing and Business Administration from the College of St. Thomas in St. Paul, Minnesota. We believe that Mr. Cash’s medical business experience, proven leadership skills and cardiac industry technology expertise make him a valuable member of our board.
Steve Chaussy. Mr. Chaussy has served as our chief financial officer on a part time basis since May 2011. Since 2005, Mr. Chaussy has been the sole proprietor of Anna & Co., Inc., a consulting company that offers services to small publicly traded companies. Anna & Co., Inc. provides general financial and accounting services, with a special emphasis towards SEC reporting and compliance, to companies that lack sufficient resources to hire full-time employees to provide such services. From 2001 to 2005, Mr. Chaussy provided services as both a chief financial officer and as a consultant to small publicly traded companies. Prior to 2001, Mr. Chaussy served as chief financial officer for a large private distribution and wholesaling company, where he gained international experience. Mr. Chaussy is a graduate of Virginia Polytechnic Institute and State University and is a licensed certified public accountant in Virginia, California and Florida.
Asher Holzer, Ph.D. Dr. Holzer has served as our chief scientific officer and our director since September 2012. Dr. Holzer served as a director of InspireMD, Inc., an Israeli-based developer of a new stent platform from March 2011 to December 2013, and served as that company’s president from March 2011 until June 2012 and chairman from March 2011 until November 2011. In addition, Dr. Holzer co-founded InspireMD Ltd., the predecessor and later wholly-owned subsidiary of InspireMD, Inc., and served as its president and chairman of the board from April 2007 until June 2012. Previously, Dr. Holzer founded Adar Medical Ltd., an investment firm specializing in medical device startups, and served as its chief executive officer from 2002 through 2004. Dr. Holzer currently serves on the board of directors of Adar Medical Ltd., O.S.H.-IL The Israeli Society of Occupational Safety and Health Ltd., Myocare Inc., Magnetecs Corporation, 2to3D Ltd., and S.P. Market Windows Cyprus. Dr. Holzer earned his Ph.D. in Applied Physics from the Hebrew University. Dr. Holzer is also an inventor and holder of numerous patents. Dr. Holzer brings to the board his more than 25 years of experience in advanced medical devices, as well as expertise covering a wide range of activities, including product development, clinical studies, regulatory affairs, market introduction and the financial aspects of the advance medical device business.
Roy T. Tanaka. Mr. Tanaka has served as our director since July 2012. From 2004 until his retirement in September 2008, Mr. Tanaka served as the worldwide president of Biosense Webster, Inc., a Johnson & Johnson company, a market and technology leader in the field of electrophysiology. He joined Biosense Webster, Inc. as its U.S. president in 1997. Previously he held a variety of senior management positions at Sorin Biomedical, Inc., including president and chief executive officer, and leadership roles at CooperVision Surgical and Shiley, a division of Pfizer, Inc. He currently serves on the boards of directors of Coherex Medical, Inc., Advanced Cardiac Therapeutics Inc., a company using electrophysiology to develop technology to measure the temperature in a lesion during cardiac ablation procedures, and VytronUS Inc. In addition, Mr. Tanaka served as a director of Volcano Corporation until May 2014 and Tomo Therapy until its acquisition in June 2011. Mr. Tanaka brings broad experience in executive leadership in the medical device field. His operational expertise and knowledge of the regulatory environment, both in the U.S. and globally, also bring a valuable perspective.
Patrick J. Gallagher. Mr. Gallagher has served as our director since July 2014. Mr. Gallagher, MBA, CFA, is an accomplished capital markets executive, advisor, and investor with a distinguished record of success in both the public and private markets. He has nearly 20 years of experience on Wall Street and extensive expertise in alternative investments, capital markets, and marketing. Since September 2014, Mr. Gallagher has served as managing director and head of healthcare sales at Laidlaw & Co. (UK) Ltd. Mr. Gallagher serves as a strategic consultant for Kinex Pharmaceuticals, LLC, a biotechnology firm focused on next-generation therapies in oncology and immunology and was the vice president of business development and investor relations from September 2012 to October 2013. In November 2010, he was appointed by broker Concept Capital, a division of Sanders Morris Harris, as a Managing Director and the head of institutional sales. In 2001, Mr. Gallagher co-founded BDR Research Group, LLC, an independent sell-side research firm specializing in healthcare investing, financing and operations, and served as its chief executive officer until November 2010. Prior to 2001, he held various sales positions at investment and research firms Kidder Peabody, PaineWebber and New Vernon Associates. Mr. Gallagher is a CFA charter holder, received his MBA from Pennsylvania State University and holds a B.S. degree in finance from the University of Vermont. We believe that Mr. Gallagher’s experience in capital markets and marketing, with extensive expertise concentrated in the life sciences space, make him a valuable resource on our board.
Seth H. Z. Fischer. Mr. Fischer has served as our director since May 2013. Since September 2013, Mr. Fischer has served as the chief executive officer and director of Vivus, Inc., a biopharmaceutical company focusing on the treatment of obesity, sleep apnea, diabetes and sexual health. Prior to that, Mr. Fischer served in positions of increasing responsibility with Johnson & Johnson from 1983 until his retirement in 2012. Most recently Mr. Fischer served as Company Group Chairman Johnson & Johnson and Worldwide Franchise Chairman Cordis Corporation from 2008 to 2012, which included responsibility for Cordis and Biosense Webster, and as Company Group Chairman North America Pharmaceuticals from 2004 to 2007, which included responsibility for Ortho-McNeil Pharmaceuticals, Janssen and Scios. Since 2013, Mr. Fischer has served as an advisor of MedHab, LLC, a medical device limited liability company. From April 2013 to September 2013, Mr. Fischer served on the board of directors of Trius Therapeutics, Inc., a public pharmaceutical company, until it was acquired by Cubist Pharmaceuticals, now a wholly owned subsidiary of Merck & Co., Inc. We believe that Mr. Fischer’s extensive executive experience in a major health care company and his specific experience in launching and growing new pharmaceutical products make him an ideal member of our board.
Jeffrey F. O’Donnell, Sr. Mr. O’Donnell has served as our director since February 2015; he had previously served as a director from October 2011 until February 2014. Mr. O’Donnell has extensive experience in the healthcare industry, merging a solid, traditional corporate background with emerging growth experience. In July, 2014, Mr. O’Donnell was named chief executive officer of Trice Medical, Inc., where he has been chairman of the board since its founding in December 2011. Trice Medical, Inc. is an early stage medical device company developing and commercializing a camera enabled needle for orthopedic diagnostic procedures. In 2008, Mr. O'Donnell started Embrella Cardiovascular, a medical device startup company which was sold in 2011 to Edwards Lifesciences (EW). Prior to Embrella Cardiovascular, Mr. O'Donnell served as president and chief executive officer of PhotoMedex (PHMD) from 1999 to 2009. He was the president and chief executive officer of Radiance Medical Systems (originally Cardiovascular Dynamics) from 1997 to 1999 after serving as its vice president of sales and marketing from 1995 to 1997. From 1994 to 1995 Mr. O'Donnell held the position of president and chief executive officer of Kensey Nash Corporation (KNSY). Additionally, he has held several senior sales and marketing management positions at Boston Scientific Corporation, Guidant Corporation and Johnson & Johnson's Orthopedic Division. In 2005, Mr. O'Donnell was named life sciences chief executive officer of the year by PriceWaterhouse Coopers. In 2011, Mr. O'Donnell was named the Greater Philadelphia Emerging Entrepreneur Of The Year by Ernst & Young. Mr. O'Donnell is a previous director for Cardiac Science (7 yrs) and Endologix (12 yrs). Mr. O’Donnell is also chairman of the board of Mela Sciences. Mr. O’Donnell brings his experience in the healthcare industry and cardiovascular space, along with his experience with emerging growth companies, which will make him a valuable member of our board of directors.
Jerome Zeldis, M.D., Ph.D. Dr. Zeldis has served as a director since April 2015. Dr. Zeldis is the chief executive officer of Celgene Global Health and the chief medical officer of Celgene Corporation. Dr. Zeldis has been with Celgene since 1997; prior to his current role, he served as senior vice president of clinical research and medical affairs. Prior to Celgene, Dr. Zeldis worked at Sandoz Research Institute and Janssen Research Institute in both clinical research and medical development. He is currently on the board of the Semorex Corporation, Bionor Pharma, Inc., Mali Health and PTC Corporation and serves as the chairman of the board of directors of Alliqua BioMedical, Inc. Dr. Zeldis attended Brown University for a B.A., M.S., followed by Yale University for a M.Phil., M.D., and Ph.D. in molecular biophysics and biochemistry (immunochemistry). He trained in internal medicine at the UCLA Center for the Health Sciences and Gastroenterology at the Massachusetts General Hospital and Harvard Medical School. He was assistant professor of medicine at the Harvard Medical School, associate professor of medicine at University of California, Davis, clinical associate professor of Medicine at Cornell Medical School and professor of clinical medicine at the Robert Wood Johnson Medical School in New Brunswick, New Jersey. Dr. Zeldis has published 122 peer reviewed articles and 24 reviews, book chapters, and editorials. Dr. Zeldis brings his extensive background in the healthcare industry, as well as his experience in emerging growth companies, which will make him a valuable resource on our board of directors.
David Weild IV . Mr. Weild has served as a director since May 2015. Mr. Weild is founder, chairman and CEO of IssuWorks, parent company of the investment banking firm Weild & Co. Holdings. Prior to Weild & Co., Mr. Weild was vice chairman of NASDAQ and head of corporate finance and equity capital markets at Prudential Securities, Inc. Mr. Weild holds an M.B.A. from the Stern School of Business and a B.A. from Wesleyan University. Mr. Weild is currently on the board of PAVmed. From September 2010 to June 2011, Mr. Weild served on the board of Helium.com, until it was acquired by R.R. Donnelly & Sons Co. Since 2003, Mr. Weild has been chairman of the board of the 9-11 charity Tuesday’s Children. Mr. Weild brings extensive financial, economic, stock exchange, capital markets, and small company expertise to the Company gained throughout his career on Wall Street.
Family Relationships
There are no family relationships among any of our officers or executive officers.
Independent Directors
Our board of directors has determined that each of Roy T. Tanaka, David Weild IV , Patrick J. Gallagher , Asher Holzer , Seth H. Z. Fischer, Jerome Zeldis and Jeffrey F. O’Donnell, Sr. is independent within the meaning of Rule 5605(a)( 2) of the NASDAQ Listing Rules and the rules and regulations promulgated by the Securities and Exchange Commission.
Committees of the Board of Directors
Our board of directors has established an audit committee, a nominating and corporate governance committee and a compensation committee , each of which has the composition and responsibilities described below.
Audit Committee
Our audit committee is currently comprised of Messrs. Weild, Gallagher and O’Donnell, each of whom our board has determined to be financially literate and qualifies as an independent director under Section 5605(a)(2) and Section 5605(c)(2) of the rules of the NASDAQ Stock Market. Mr. Weild is the chairman of our audit committee. In addition, Mr. Weild qualifies as a financial expert , as defined in Item 407(d)(5 )(ii ) of Regulation S-K .
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee is currently comprised of Drs. Zeldis and Holzer and Mr. Tanaka, each of whom qualifies as an independent director under Section 5605(a)(2) of the rules of the NASDAQ Stock Market. Dr. Zeldis is the chairman of our nominating and corporate governance committee.
Compensation Committee
Our compensation committee is currently comprised of Messrs. O’Donnell, Tanaka and Fischer, each of whom qualifies as an independent director under Section 5605(a)(2) of the rules of the NASDAQ Stock Market, an “outside director” for purposes of Section 162(m) of the Internal Revenue Code and a “non-employee director” for purposes of Section 16b-3 under the Securities Exchange Act of 1934, as amended, and does not have a relationship to us which is material to his ability to be independent from management in connection with the duties of a compensation committee member, as described in Section 5605(d)(2) of the rules of the NASDAQ Stock Market. Mr. O’Donnell is the chairman of our compensation committee.
Code of Ethics
We have adopted a code of business conduct and ethics that applies to our officers, directors and employees, including our principal executive officer , principal financial officer and principal accounting officer. The full text of our Code of Business Conduct and Ethics is published on the Investors section of our website at www.biosigtech.com. We intend to disclose any future amendments to certain provisions of the Code of Business Conduct and Ethics, or waivers of such provisions granted to executive officers and directors, on this website within four business days following the date of any such amendment or waiver .
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table provides certain summary information concerning compensation awarded to, earned by or paid to table below sets forth, for our last two fiscal years, the compensation earned by our named executive officers: (i) Kenneth L. Londoner, our executive chairman and member of our board, (ii) Gregory Cash, our chief executive officer and (iii) Steven Chaussy, our chief financial officer for fiscal years 2014 and 2013.
|
Name and principal position
|
Year
|
Salary
($)
|
Stock Awards
($)
|
Total
($)
|
|||||||
|
Kenneth L. Londoner, Executive Chairman and Director
|
2014
|
206,913
|
1,000,000
|
(1)
|
1,206,913
|
||||||
|
2013
|
211,500
|
458,400
|
(2)
|
669,900
|
|||||||
|
Gregory D. Cash, President, Chief Executive and Director
|
2014
|
103,126
|
2,383,443
|
(3)
|
2,486,569
|
||||||
|
2013
|
—
|
—
|
—
|
||||||||
|
Steven Chaussy, Chief Financial Officer
|
2014
|
49,500
|
500,000
|
(4)
|
549,500
|
||||||
|
2013
|
40,250
|
58,149
|
(5)
|
98,399
|
(1) Represents a common stock award of 400,000 shares granted on September 1, 2014.
(2) Represents a stock option granted on January 16, 2013 for the purchase of 250,000 shares of common stock, exercisable immediately, at an exercise price of $2.09 per share and a termination date of January 16, 2020.
(3) Represents a stock option granted on July 15, 2014 for the purchase of 1,265,769 shares of common stock, 135,618 of which are exercisable immediately, 406,855 of which are exercisable over two years vesting on a quarterly basis, and the remainder of which shall vest upon the satisfaction of performance goals at $2.21 per share and terminate on July 15, 2024.
(4) Represents a common stock award of 200,000 shares granted on September 1, 2014.
(5) Represents a stock option granted on January 16, 2013 for the purchase of 30,000 shares of common stock, exercisable immediately, at an exercise price of $2.09 per share and a termination date of January 16, 2020.
Agreements with Executive Officers and Change-In-Control Arrangements
Kenneth L. Londoner
We entered into an employment agreement with Kenneth Londoner on March 1, 2013. The employment agreement terminated on March 1, 2015, after which Mr. Londoner’s employment became on an at-will basis. Prior to its termination, Mr. Londoner’s employment agreement required that Mr. Londoner receive an annual base salary of $225,000 and be eligible for annual discretionary bonuses and equity-based incentives, as our board may determine. Mr. Londoner was also subject to non-competition and non-solicitation obligations, whereby, for a period lasting until one year after the termination of his employment with us, Mr. Londoner was not permitted to, directly or indirectly, (i) in any state in the U.S. or country that we conduct business and for which Mr. Londoner had responsibility, work for, invest in, provide financing to or establish a business that competes with our business, other than an exception that permits limited investment in publicly-traded competitors, (ii) solicit business from or do business with any customer, client, manufacturer or vendor with whom we did business or who we solicited within the preceding two years, and (iii) solicit, engage or hire any person employed by or who served as a consultant to us within the preceding twelve months. In September 2013, Mr. Londoner resigned as our chief executive officer, but remained with us in an executive role. In November 2013, Mr. Londoner became our executive chairman. While Mr. Londoner’s employment agreement expired on March 1, 2015, we intend to continue to compensate Mr. Londoner pursuant to the terms of his former employment agreement for his contributions with respect to corporate finance, investor relations, and business development.
Prior to entering into his employment agreement, Mr. Londoner was an at-will employee.
Gregory D. Cash
On July 15, 2014, we entered into an employment agreement with Gregory Cash. The employment agreement has an initial term of three years that expires on July 15, 2017. Under the employment agreement, Mr. Cash is entitled to an annual base salary of $275,000. On March 31, 2015, upon our closing an equity or equity-linked financing with proceeds of at least $3.5 million (a “Qualified Financing”), Mr. Cash’s annual base salary automatically increased to $325,000 and he received (i) a one-time payment equal to the difference between the amount he would have earned if his base salary was $325,000 and the amount he actually earned at his base salary of $275,000 for the time period from the effective date of the agreement until the closing of such Qualified Financing and (ii) a one-time cash bonus of $30,000. Mr. Cash is also eligible to receive an annual bonus equal to at least 50% of the sum of his base salary and one-time payment, based on the achievement of reasonable performance criteria to be determined by the board in consultation with Mr. Cash within 90 days of the effective date.
In accordance with Mr. Cash’s employment agreement, on July 15, 2014, we granted Mr. Cash an incentive stock option to purchase 1,265,769 shares of common stock, made pursuant to an Incentive Stock Option Agreement. The option has an exercise price of $2.21, which was the fair market value of our common stock on the date of grant, and a term that expires ten years from the date of grant. The option will vest as follows (i) 542,473 shares of common stock will vest in eleven equal installments of 45,206 shares of common stock and one final installment of 45,207 shares of common stock on a quarterly basis with the first installment vesting on the effective date of his employment agreement and subsequent installments vesting every three months thereafter; (ii) 180,824 shares of common stock will vest immediately upon completion of a Qualified Financing; (iii) 180,824 shares of common stock will vest upon the listing of our common stock on a recognized U.S. national securities exchange (i.e., NYSE, MKT LLC, The Nasdaq Stock Market LLC or the New York Stock Exchange); (iv) 180,824 shares of common stock will vest upon the 510(k) clearance or any other type of clearance deemed necessary by the U.S. Food and Drug Administration of our PURE EP technology platform; and (v) 180,824 shares of common stock will vest upon our achieving a market capitalization of $150,000,000 and maintaining such market capitalization for at least 90 consecutive calendar days.
Outstanding Equity Awards at Fiscal Year End
The following table sets forth information regarding equity awards that have been previously awarded to each of the named executive officers and which remain outstanding as of December 31, 2014.
|
Name and principal position
|
Number of Securities underlying Unexercised Options (#) Exercisable
|
Number of Securities underlying Unexercised Options (#) Unexercisable
|
Option Exercise Price ($/Sh)
|
Option Expiration Date
|
||||||||||
|
Gregory D. Cash
|
90,412
|
1,175,357
|
$
|
2.21
|
7/15/2024
|
|||||||||
|
Kenneth Londoner
|
250,000
|
—
|
$
|
2.09
|
1/16/2020
|
|||||||||
|
Steven Chaussy
|
30,000
|
—
|
$
|
2.09
|
1/16/2020
|
|||||||||
|
30,000
|
—
|
$
|
2.00
|
6/11/2023
|
||||||||||
BioSig Technologies, Inc. 2012 Equity Incentive Plan
On October 19, 2012, our board of directors adopted the BioSig Technologies, Inc. 2012 Equity Incentive Plan, which provides for the grant of stock options, stock appreciation rights, restricted stock and restricted stock units to employees, directors and consultants, to be granted from time to time as determined by our board of directors or its designees. An aggregate of 11,686,123 shares of common stock are reserved for issuance under the BioSig Technologies, Inc. 2012 Equity Incentive Plan. As of June 9, 2015, the number of options and restricted stock awards granted under the BioSig Technologies, Inc. 2012 Equity Incentive Plan are 8, 935 ,190.
Equity Compensation Plan Information
As of December 31, 2014
|
Plan category
|
Number of securities to be issued upon exercise of outstanding options
(a)
|
Weighted-average exercise price of outstanding options
(b)
|
Securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a))
(c)
|
||||||
|
Equity compensation plans approved by security holders
|
5,990,190
|
2.25
|
2,815,933
|
||||||
|
Equity compensation plans not approved by security holders
|
—
|
—
|
—
|
||||||
|
Total
|
5,990,190
|
2.25
|
2,815,933
|
DIRECTOR COMPENSATION
The following table sets forth summary information concerning the total compensation paid to our non-employee directors during the fiscal year ended December 31, 2014 for services to our company.
|
Name
|
Fees earned or paid in cash ($)
|
Option Awards ($) (1)
|
Total ($)
|
||||||||
|
Asher Holzer, Ph.D.
|
—
|
145,063
|
(1)
|
145,063
|
|||||||
|
Roy T. Tanaka
|
—
|
241,772
|
(2)
|
241,772
|
|||||||
|
Jonathan Steinhouse
|
—
|
338,481
|
(3)
|
338,481
|
|||||||
|
Seth H. Z. Fischer
|
—
|
904,069
|
(4)
|
904,069
|
|||||||
|
Patrick J. Gallagher
|
—
|
307,269
|
(5)
|
307,269
|
|||||||
|
Total:
|
—
|
1,936,654
|
1,936,654
|
||||||||
|
(1)
|
Represents a stock option granted on September 1, 2014 for the purchase of 75,000 shares of common stock, exercisable immediately, at an exercise price of $2.50 per share and a termination date of September 1, 2021.
|
|
(2)
|
Represents a stock option granted on September 1, 2014 for the purchase of 125,000 shares of common stock, exercisable immediately, at an exercise price of $2.50 per share and a termination date of September 1, 2021.
|
|
(3)
|
Represents a stock option granted on September 1, 2014 for the purchase of 175,000 shares of common stock, exercisable immediately, at an exercise price of $2.50 per share and a termination date of September 1, 2021.
|
|
(4)
|
Represents a stock option granted on September 1, 2014 for the purchase of 150,000 shares of common stock of which 50% vested on the date of the grant and 50% vest on the first anniversary, at an exercise price of $2.50 per share and a termination date of September 1, 2021; and a stock option granted on October 14, 2014 for 163,444 shares of common stock, exercisable immediately, at an exercise price of $2.50 per share and a termination date of October 14, 2021.
|
|
(5)
|
Represents a stock option granted on September 1, 2014 for the purchase of 150,000 shares of common stock, vesting over two years with 50% vesting on the first anniversary and the remaining 50% vesting monthly over a one year period, at an exercise price of $2.50 per share and a termination date of September 1, 2021.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
Common Stock
The following table sets forth information with respect to the beneficial ownership of our common stock as of June 9, 2015:
|
●
|
by each person who is known by us to beneficially own more than 5.0% of our common stock;
|
|
●
|
by each of our named executive officers and directors; and
|
|
●
|
by all of our named executive officers and directors as a group.
|
The percentages of common stock beneficially owned are reported on the basis of regulations of the Securities and Exchange Commission governing the determination of beneficial ownership of securities. Under the rules of the Securities and Exchange Commission, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of the security, or investment power, which includes the power to dispose of or to direct the disposition of the security. With respect to the Series C Preferred Stock and warrants held by the beneficial owners listed below, there exist contractual provisions limiting conversion and exercise to the extent such conversion or exercise would cause such beneficial owner, together with its affiliates or members of a “group,” to beneficially own a number of shares of common stock which would exceed from 4.99% to 9.99% of our then outstanding shares of common stock following such conversion or exercise. The shares and percentage ownership of our outstanding shares indicated in the table below do not give effect to these limitations. Except as indicated in the footnotes to this table, to our knowledge and subject to community property laws where applicable, each beneficial owner named in the table below has sole voting and sole investment power with respect to all shares beneficially owned and each person’s address is c/o BioSig Technologies, Inc., 8441 Wayzata Blvd., Suite 240, Minneapolis, Minnesota 55426.
|
Name of Beneficial Owner
|
Number of Shares
Beneficially Owned (1)
|
Percentage of Common
Stock Owned (1)(2)
|
||||||
|
5% Owners
|
||||||||
|
Lora Mikolaitis
|
3,611,224
|
(3)
|
25. 12
|
%
|
||||
|
Alpha Capital Anstalt (4)
|
2, 365,046
|
(5)
|
15.64
|
%
|
||||
|
Laidlaw & Co. (UK) Ltd. (6)
|
876,233
|
(7)
|
5. 81
|
%
|
||||
|
Officers and Directors
|
||||||||
|
Kenneth L. Londoner
|
4,605,314
|
(8)
|
31. 41
|
%
|
||||
|
Asher Holzer, Ph.D.
|
324,083
|
(9)
|
2. 24
|
%
|
||||
|
Gregory D. Cash
|
431,854
|
(10)
|
2.96
|
%
|
||||
|
Roy T. Tanaka
|
633,452
|
(11)
|
4. 28
|
%
|
||||
|
Seth H. Z. Fischer
|
453,027
|
( 12 )
|
3. 10
|
%
|
||||
|
Patrick J. Gallagher
|
27,083
|
( 13 )
|
*
|
|||||
|
Jeffrey F. O’Donnell, Sr.
|
214,550
|
( 14 )
|
1. 50
|
%
|
||||
|
Steve Chaussy
|
368,362
|
( 15 )
|
2. 58
|
%
|
||||
|
Jerome Zeldis, M.D., Ph.D.
|
418,315
|
( 16 )
|
2. 87
|
%
|
||||
|
David Weild IV
|
133,334
|
(17)
|
*
|
|||||
|
All directors and executive officers as a group (10 persons)
|
7, 609,374
|
51.96
|
%
|
|||||
* Less than 1%
|
(1)
|
Shares of common stock beneficially owned and the respective percentages of beneficial ownership of common stock assume the exercise of all options and other securities convertible into common stock beneficially owned by such person or entity currently exercisable or exercisable within 60 days of June 9, 2015, except as otherwise noted. Shares issuable pursuant to the exercise of stock options and other securities convertible into common stock exercisable within 60 days are deemed outstanding and held by the holder of such options or other securities for computing the percentage of outstanding common stock beneficially owned by such person, but are not deemed outstanding for computing the percentage of outstanding common stock beneficially owned by any other person.
|
|
(2)
|
These percentages have been calculated based on 14,203,202 shares of common stock outstanding as of June 9, 2015.
|
|
(3)
|
Comprised of (i) 43,750 shares of common stock, (ii) options to purchase 175,000 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015, and (iii) 3,392,474 shares of common stock held by Miko Consulting Group, Inc. Lora Mikolaitis has sole voting and dispositive power over the securities held for the account of Miko Consulting Group, Inc.
|
|
(4)
|
The address for Alpha Capital Anstalt is Pradafant 7, 9490 Furstentums, Vaduz, Lichtenstein. Konrad Ackermann has sole voting and dispositive power over the securities held for the account of this stockholder.
|
|
(5)
|
Comprised of (i) 824,534 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 516 ,667 shares of common stock, and (iii) warrants to purchase 1,023,845 shares of common stock. With respect to the Series C Preferred Stock and warrants, there exist contractual provisions limiting conversion and exercise to the extent such conversion or exercise would cause Alpha Capital Anstalt, together with its affiliates or members of a “group,” to beneficially own a number of shares of common stock which would exceed from 4.99% to 9.99% of our then outstanding shares of common stock following such conversion or exercise. The shares and percentage ownership of our outstanding shares indicated in the table do not give effect to these limitations.
|
|
(6)
|
Laidlaw & Co. (UK) Ltd. is a registered broker-dealer. Matthew Eitner is the chief executive officer of Laidlaw & Co. (UK) Ltd. and, in such capacity, he may be deemed to have voting and dispositive power over the securities held for the account of this stockholder.
|
|
(7)
|
Comprised of warrants to purchase shares of common stock.
|
|
(8)
|
Comprised of (i) 724,045 shares of common stock directly held by Mr. Londoner, (ii) 3,334,974 shares of common stock held by Endicott Management Partners, LLC, an entity for which Mr. Londoner is deemed the beneficial owner, (iii) warrants to purchase 296,295 shares of common stock, and (v) options to purchase 250,000 shares of common stock that are currently exercisable.
|
|
(9)
|
Comprised of (i) 85,000 shares of common stock, and (ii) options to purchase 239,083 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015.
|
|
(10)
|
Comprised of (i) 25,000 shares of common stock, and (ii) options to purchase 406,854 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015.
|
|
(11)
|
Comprised of (i) 25,000 shares of common stock, and (ii) options to purchase 608,452 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015.
|
|
(12)
|
Consists of (i) 25 ,000 shares of common stock , and (ii) options to purchase 428,027 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015 .
|
|
(13)
|
Consists of (i) 25,000 shares of common stock, and (ii) options to purchase 2,083 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015.
|
|
(14)
|
Consists of (i) 112,500 shares of common stock, and (ii) options to purchase 102,050 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015.
|
|
(15)
|
Consists of (i) 308,362 shares of common stock, and (ii) options to purchase 60,000 shares of common stock that are currently exercisable.
|
|
(16)
|
Consists of (i) 12,245 shares of common stock, (ii) options to purchase 304,167 shares of common stock that are currently exercisable, (iii) shares of Series C Preferred Stock that are convertible into 33,334 shares of common stock, and (iv) warrants to purchase 68,569 shares of common stock.
|
|
(17)
|
Consists of options to purchase 133,334 shares of common stock that are currently exercisable or exercisable within 60 days of June 9, 2015.
|
SELLING STOCKHOLDERS
Up to 6, 556,686 shares of our common stock are currently being offered by the selling stockholders under this prospectus. This reflects the sum of (a) shares of our common stock, (b) the number of shares of common stock into which shares of our Series C Preferred Stock are currently convertible, at a price of $1.50 per share per $1,000 principal amount of Series C Preferred Stock and (c) the number of shares of common stock into which the warrants are exercisable. All of the shares of common stock, Series C Preferred Stock and warrants were purchased by the selling stockholders in (i) two separate closings in December 2013 and January 2014 pursuant to the same securities purchase agreement (the “December 2013 Placement”), (ii) two separate closings in April 2014 pursuant to a separate securities purchase agreement (the “April 2014 Placement”), (iii) two separate closings in August 2014 and September 2014 pursuant to a separate securities purchase agreement (the “August 2014 Placement”), (iv) six separate closings from December 2014 to March 2015 pursuant to a separate securities purchase agreement (the “December 2014 Placement”) and (v) two separate closings on May 11, 2015 pursuant to two separate securities purchase agreements (the “May 2015 Placement”), except for (x) the warrants held by Laidlaw & Co. (UK) Ltd., which were issued as part of the compensation for serving as our placement agent in connection with the private placement of our common stock and the related warrants , and (y) the October 2013 Five-Year Amendment Warrants, which were issued in consideration for amending the terms of the securities purchase agreement to permit the December 2013 Placement.
The selling stockholders that participated in the December 2013 Placement paid a price per unit equivalent to $2.45 for one share of common stock and a five-year warrant to purchase up to 50% of a share of common stock. The selling stockholders that participated in the April 2014 Placement paid a price per unit equivalent to $2.50 for one share of common stock and a five-year warrant to purchase up to 50% of a share of common stock. The selling stockholders that participated in the August 2014 Placement paid a price per unit equivalent to $2.50 for one share of common stock and a five-year warrant to purchase up to 50% of a share of common stock. The selling stockholders that participated in the December 2014 Placement paid a price per unit equivalent to $2.50 for one share of common stock, an “A” warrant expiring July 31, 2015 to purchase up to one share of common stock and a “B” warrant expiring March 31, 2020 to purchase up to 50% of a share of common stock. The selling stockholders that participated in the May 2015 Placement paid a price per unit equivalent to $1,000 for one share of Series C Preferred Stock and a five-year warrant to purchase up to 833.46 shares of common stock. In addition, due to our failure to fulfill our obligations under the registration rights agreement entered into with the participants in the December 2013 Placement, the April 2014 Placement, the August 2014 Placement and the December 2014 Placement, we owe such holders liquidated damages in an amount equal to 1.0% of the aggregate purchase price paid by such holders per month for each monthly period beginning on March 17, 2014, in the case of the December 2013 Placement, June 14, 2014, in the case of the April 2014 Placement, November 14, 2014, in the case of the August 2014 Placement, and May 15, 2015, in the case of the December 2014 Placement, provided that (i) the maximum aggregate liquidated damages due under the registration rights agreements in connection with the December 2013 Placement, the April 2014 Placement and the August 2014 Placement shall be 3% of the aggregate purchase price paid by the purchasers, and (ii) the maximum aggregate liquidated damages due under the registration rights agreement in connection with the December 2014 Placement shall be 6% of the aggregate purchase price paid by the purchasers. Because we have not paid such liquidated damages, we are also obligated to pay interest of 18% per annum, accruing daily, on such unpaid amounts.
The shares of common stock referred to above are being registered to permit public sales of the shares, and the selling stockholders may offer the shares for resale from time to time pursuant to this prospectus. The selling stockholders may also sell, transfer or otherwise dispose of all or a portion of their shares in transactions exempt from the registration requirements of the Securities Act of 1933, as amended, or pursuant to another effective registration statement covering those shares.
The table below sets forth certain information regarding the selling stockholders and the shares of our common stock offered by them in this prospectus. The selling stockholders have not had a material relationship with us within the past three years other than as described in the footnotes to the table below or as a result of their acquisition of our shares or other securities. To our knowledge, subject to community property laws where applicable, each person named in the table has sole voting and investment power with respect to the shares of common stock set forth opposite such person’s name. None of the selling stockholders are broker-dealers or affiliates of broker-dealers, unless otherwise noted.
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission. In computing the percentage of our common stock beneficially owned by each selling stockholder after the offering, we have assumed the exercise by such selling stockholder of all warrants with respect to those shares being offered by such selling stockholder, and therefore the calculation is based on a number of shares of common stock outstanding comprised of (i) 14,203,202 shares of common stock outstanding as of June 9, 2015 plus (ii) the number of shares offered by the selling stockholder in this offering underlying Series C Preferred Stock and warrants held by such selling stockholder. The shares offered by one selling stockholder underlying Series C Preferred Stock and warrants held by such selling stockholder are not deemed outstanding for the purpose of computing the percentage ownership of any other selling stockholder. With respect to the Series C Preferred Stock and warrants held by the selling stockholders, there exist contractual provisions limiting conversion and exercise to the extent such conversion or exercise would cause such selling stockholder, together with its affiliates or members of a “group,” to beneficially own a number of shares of common stock which would exceed from 4.99% to 9.99% of our then outstanding shares of common stock following such conversion or exercise. The shares and percentage ownership of our outstanding shares indicated in the table below do not give effect to these limitations.
|
Ownership Before Offering
|
Ownership After Offering
|
|||||||||||||||
|
Selling Stockholder
|
Number of shares of common stock beneficially owned (1)
|
Number of shares offered
|
Number of shares of common stock
beneficially owned (1)
|
Percentage of common stock beneficially owned (1) (2)
|
||||||||||||
|
Michael C. Bellard
|
62,000
|
(3)
|
62,000
|
(3)
|
0
|
*
|
||||||||||
|
Johnson Revocable Trust DTD Feb 13 2000, Todd Johnson & Luann Johnson TTEES
|
24,000
|
(4)
|
24,000
|
(4)
|
0
|
*
|
||||||||||
|
Herschel Johnson
|
38,565
|
(5)
|
9,539
|
(6)
|
29,026
|
(7)
|
*
|
|||||||||
|
Kenneth R. Klimitchek
|
15,000
|
(8)
|
15,000
|
(8)
|
0
|
*
|
||||||||||
|
James W. Lees
|
71,429
|
(9)
|
21,246
|
(10)
|
50,183
|
(11)
|
*
|
|||||||||
|
Sterne Agee & Leach Inc. C/F David W. Frost IRA
|
48,645
|
(12)
|
39,649
|
(13)
|
8,996
|
(14)
|
*
|
|||||||||
|
David W. Frost
|
537,122
|
(15)
|
281,021
|
(16)
|
256,101
|
(17)
|
1.77 %
|
|||||||||
|
Maree Casatelli
|
12,000
|
(18)
|
12,000
|
(18)
|
0
|
*
|
||||||||||
|
Douglas E. Jasek
|
30,000
|
(19)
|
30,000
|
(19)
|
0
|
*
|
||||||||||
|
Randall L Payne & Kathy S Payne JTWROS
|
92,600
|
(20)
|
92,600
|
(20)
|
0
|
*
|
||||||||||
|
Trevor W. Davies
|
16,000
|
(21)
|
16,000
|
(21)
|
0
|
*
|
||||||||||
|
Bryan J. Hanks & Michelle B. Hanks JTWROS
|
55,000
|
(22)
|
55,000
|
(22)
|
0
|
*
|
||||||||||
|
Koushik & Kamla A. Reddy JTWROS
|
15,000
|
(23)
|
15,000
|
(23)
|
0
|
*
|
||||||||||
|
AAJK Investments, LLC (24)
|
6,000
|
(25)
|
6,000
|
(25)
|
0
|
*
|
||||||||||
|
Bruno J. Casatelli
|
150,612
|
(26)
|
150,612
|
(26)
|
0
|
*
|
||||||||||
|
Jerry Caldwell
|
21,000
|
(27)
|
21,000
|
(27)
|
0
|
*
|
||||||||||
|
Thomas B. D’Agostino
|
30,000
|
(28)
|
30,000
|
(28)
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F Bruce Frost IRA
|
6,000
|
(29)
|
6,000
|
(29)
|
0
|
*
|
||||||||||
|
Alexander Gerould
|
30,000
|
(30)
|
30,000
|
(30)
|
0
|
*
|
||||||||||
|
Benjamin Netick
|
15,000
|
(31)
|
15,000
|
(31)
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F Algis J. Rajeckas IRA
|
15,000
|
(32)
|
15,000
|
(32)
|
0
|
*
|
||||||||||
|
Randolph Swickle
|
6,000
|
(33)
|
6,000
|
(33)
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc C/F Jonathan Steinhouse R/O IRA
|
24,368
|
( 34 )
|
24,368
|
( 34 )
|
0
|
*
|
||||||||||
|
Lee Irish
|
9,000
|
( 35 )
|
9,000
|
( 35 )
|
0
|
*
|
||||||||||
|
Jere D. Peak
|
15,000
|
( 36 )
|
15,000
|
( 36 )
|
0
|
*
|
||||||||||
|
Tim Engels
|
16,000
|
( 37 )
|
16,000
|
( 37 )
|
0
|
*
|
||||||||||
|
Bruce G. Krueger
|
34,000
|
( 38 )
|
34,000
|
( 38 )
|
0
|
*
|
||||||||||
|
Douglas Davies
|
12,000
|
( 39 )
|
12,000
|
( 39 )
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F Benjamin Netick Roth IRA Conversion
|
10,000
|
( 40 )
|
10,000
|
( 40 )
|
0
|
*
|
||||||||||
|
Graham John Nicholson
|
21,000
|
( 41 )
|
21,000
|
( 41 )
|
0
|
*
|
||||||||||
|
Fernando Malvido Olascoaga
|
25,000
|
( 42 )
|
25,000
|
( 42 )
|
0
|
*
|
||||||||||
|
Gustavo Dos Reis Vasques
|
20,000
|
( 43 )
|
20,000
|
( 43 )
|
0
|
*
|
||||||||||
|
Michael Wessner
|
10,000
|
( 44 )
|
10,000
|
( 44 )
|
0
|
*
|
||||||||||
|
Herbert B. Alcorn
|
18,500
|
( 45 )
|
18,500
|
( 45 )
|
0
|
*
|
||||||||||
|
Marvin Dale Martin
|
15,000
|
( 46 )
|
15,000
|
( 46 )
|
0
|
*
|
||||||||||
|
Waleed Suhail Al-Nasrawi
|
25,000
|
( 47 )
|
25,000
|
( 47 )
|
0
|
*
|
||||||||||
|
Kenneth N. Larsen Nancy J. Larsen JTWROS
|
50,000
|
( 48 )
|
50,000
|
( 48 )
|
0
|
*
|
||||||||||
|
Nicholas Osorio & Paulina Veytia JTWROS
|
25,000
|
( 49 )
|
25,000
|
( 49 )
|
0
|
*
|
||||||||||
|
Ecovest Limited ( 50 )
|
20,000
|
( 51 )
|
20,000
|
( 51 )
|
0
|
*
|
||||||||||
|
Jan J. Laskowski & Sofia M. Laskowski JTWROS
|
50,000
|
( 52 )
|
50,000
|
( 52 )
|
0
|
*
|
||||||||||
|
John Lloyd
|
18,000
|
( 53 )
|
18,000
|
( 53 )
|
0
|
*
|
||||||||||
|
Horacio Fajer Cardona
|
9,600
|
( 54 )
|
9,600
|
( 54 )
|
0
|
*
|
||||||||||
|
Ronald P. Geisler
|
10,000
|
( 55 )
|
10,000
|
( 55 )
|
0
|
*
|
||||||||||
|
The William D Woodford & Deborah N Woodford Revoc Living Trust 01/15/13
|
25,000
|
( 56 )
|
25,000
|
( 56 )
|
0
|
*
|
||||||||||
|
Michael D. Watson
|
30,000
|
( 57 )
|
30,000
|
( 57 )
|
0
|
*
|
||||||||||
|
Gonzalo A. Salgueiro
|
72,009
|
( 58 )
|
25,000
|
( 58 )
|
47,009
|
*
|
||||||||||
|
Benjamin Hasty
|
50,000
|
( 59 )
|
50,000
|
( 59 )
|
0
|
*
|
||||||||||
|
Steven A. Hobbs
|
50,000
|
( 60 )
|
50,000
|
( 60 )
|
0
|
*
|
||||||||||
|
Michael L. Turner
|
40,000
|
( 61 )
|
40,000
|
( 61 )
|
0
|
*
|
||||||||||
|
Mariusz J. Klin
|
25,000
|
( 62 )
|
25,000
|
( 62 )
|
0
|
*
|
||||||||||
|
Bernd Albrecht
|
25,000
|
( 63 )
|
25,000
|
( 63 )
|
0
|
*
|
||||||||||
|
Carlo Wolf
|
100,000
|
( 64 )
|
100,000
|
( 64 )
|
0
|
*
|
||||||||||
|
Philip Ireland
|
28,000
|
( 65 )
|
28,000
|
( 65 )
|
0
|
*
|
||||||||||
|
Richard Burgess
|
50,000
|
( 66 )
|
50,000
|
( 66 )
|
0
|
*
|
||||||||||
|
Jorge Enrique Borbolla
|
20,000
|
( 67 )
|
20,000
|
( 67 )
|
0
|
*
|
||||||||||
|
Andreas Wawrla
|
500,000
|
( 68 )
|
500,000
|
( 68 )
|
0
|
*
|
||||||||||
|
Wayne Young
|
16,000
|
( 69 )
|
16,000
|
( 69 )
|
0
|
*
|
||||||||||
|
Charles Morse
|
15,000
|
( 70 )
|
15,000
|
( 70 )
|
0
|
*
|
||||||||||
|
Graeme Farr
|
25,000
|
( 71 )
|
25,000
|
( 71 )
|
0
|
*
|
||||||||||
|
Alois Praxmarer & Sandra Praxmarer JTWROS
|
100,000
|
( 72 )
|
100,000
|
( 72 )
|
0
|
*
|
||||||||||
|
William Wade Brawley
|
25,000
|
( 73 )
|
25,000
|
( 73 )
|
0
|
*
|
||||||||||
|
Patrick S. Thomas
|
25,000
|
( 74 )
|
25,000
|
( 74 )
|
0
|
*
|
||||||||||
|
Robert Dunn & Judy Dunn JTWROS
|
50,000
|
( 75 )
|
50,000
|
( 75 )
|
0
|
*
|
||||||||||
|
Steven K. Nelson
|
50,000
|
( 76 )
|
50,000
|
( 76 )
|
0
|
*
|
||||||||||
|
James M. Wimberly
|
25,000
|
( 77 )
|
25,000
|
( 77 )
|
0
|
*
|
||||||||||
|
William A. Valka & Barbara B. Valka JTWROS
|
100,000
|
( 78 )
|
100,000
|
( 78 )
|
0
|
*
|
||||||||||
|
Bill D. Eischeid
|
25,000
|
( 79 )
|
25,000
|
( 79 )
|
0
|
*
|
||||||||||
|
Douglas Pence
|
100,000
|
( 80 )
|
100,000
|
( 80 )
|
0
|
*
|
||||||||||
|
Ian H. Murray
|
49,076
|
( 81 )
|
34,083
|
( 82 )
|
14,993
|
( 83 )
|
*
|
|||||||||
|
Larry W. Schwartz
|
16,000
|
( 84 )
|
16,000
|
( 84 )
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F Deborah A. Schwartz Roth IRA
|
25,000
|
( 85 )
|
25,000
|
( 85 )
|
0
|
*
|
||||||||||
|
Timothy Williams
|
25,000
|
( 86 )
|
25,000
|
( 86 )
|
0
|
*
|
||||||||||
|
Anders P. Lindholm
|
50,000
|
( 87 )
|
50,000
|
( 87 )
|
0
|
*
|
||||||||||
|
Julian Bavin
|
40,000
|
( 88 )
|
40,000
|
( 88 )
|
0
|
*
|
||||||||||
|
Rudolf Weiss
|
30,000
|
( 89 )
|
30,000
|
( 89 )
|
0
|
*
|
||||||||||
|
Bruce C. Ghrist
|
10,000
|
( 90 )
|
10,000
|
( 90 )
|
0
|
*
|
||||||||||
|
Ronald J. Woodward
|
25,000
|
( 91 )
|
25,000
|
( 91 )
|
0
|
*
|
||||||||||
|
James Ellinwood
|
25,000
|
( 92 )
|
25,000
|
( 92 )
|
0
|
*
|
||||||||||
|
Craig William Bannister
|
30,000
|
( 93 )
|
30,000
|
( 93 )
|
0
|
*
|
||||||||||
|
John Campbell
|
50,000
|
( 94 )
|
50,000
|
( 94 )
|
0
|
*
|
||||||||||
|
Michael F. Tedesco
|
10,000
|
( 95 )
|
10,000
|
( 95 )
|
0
|
*
|
||||||||||
|
Donald Joseph Stroh
|
10,000
|
( 96 )
|
10,000
|
( 96 )
|
0
|
*
|
||||||||||
|
Timothy McCormick & Katheryn E. Murray JTWROS
|
10,000
|
( 97 )
|
10,000
|
( 97 )
|
0
|
*
|
||||||||||
|
Matthew Reid
|
38,910
|
( 98 )
|
25,000
|
( 98 )
|
13,910
|
*
|
||||||||||
|
Gary J. Mabie & Janelle L. Mabie JTWROS
|
50,000
|
( 99 )
|
50,000
|
( 99 )
|
0
|
*
|
||||||||||
|
Tim N. Montgomery
|
50,000
|
( 100 )
|
50,000
|
( 100 )
|
0
|
*
|
||||||||||
|
J.L. Christopher Cheadle
|
100,000
|
( 101 )
|
100,000
|
( 101 )
|
0
|
*
|
||||||||||
|
Kenneth H. Hancock
|
100,000
|
( 102 )
|
100,000
|
( 102 )
|
0
|
*
|
||||||||||
|
Bernard J. Heiles & Gabriele Heiles JTWROS
|
50,000
|
( 103 )
|
50,000
|
( 103 )
|
0
|
*
|
||||||||||
|
Douglas A. Alcott
|
25,000
|
( 104 )
|
25,000
|
( 104 )
|
0
|
*
|
||||||||||
|
James R. Bement & Sheryl Bement JTWROS
|
50,000
|
( 105 )
|
50,000
|
( 105 )
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F David Bowlby IRA
|
50,000
|
( 106 )
|
50,000
|
( 106 )
|
0
|
*
|
||||||||||
|
Jeffery S. Boyer
|
22,500
|
( 107 )
|
10,000
|
( 107 )
|
12,500
|
*
|
||||||||||
|
Donald K. Coffey
|
25,000
|
( 108 )
|
25,000
|
( 108 )
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F John P. Cotis R/O IRA
|
20,000
|
( 109 )
|
20,000
|
( 109 )
|
0
|
*
|
||||||||||
|
James V. Cunningham
|
20,000
|
( 110 )
|
20,000
|
( 110 )
|
0
|
*
|
|
Stephen J. Farley
|
50,000
|
( 111 )
|
50,000
|
( 111 )
|
0
|
*
|
||||||||||
|
David Nahmias
|
9,000
|
( 112 )
|
9,000
|
( 112 )
|
0
|
*
|
||||||||||
|
Randy O. Frost
|
10,000
|
( 113 )
|
10,000
|
( 113 )
|
0
|
*
|
||||||||||
|
Gerard A. Gabriel
|
50,000
|
( 114 )
|
50,000
|
( 114 )
|
0
|
*
|
||||||||||
|
Robert J. Gray
|
83,355
|
( 115 )
|
30,204
|
( 116 )
|
53,151
|
( 117 )
|
*
|
|||||||||
|
Alexander H. Hachiya
|
51,000
|
( 118 )
|
51,000
|
( 118 )
|
0
|
*
|
||||||||||
|
David M. Laurenson
|
50,000
|
( 119 )
|
50,000
|
( 119 )
|
0
|
*
|
||||||||||
|
Bruce Levy
|
16,150
|
( 120 )
|
16,150
|
( 120 )
|
0
|
*
|
||||||||||
|
Lawrence T Juette
|
19,000
|
( 121 )
|
19,000
|
( 121 )
|
0
|
*
|
||||||||||
|
Stuart R. Oliver
|
36,306
|
( 122 )
|
36,306
|
( 122 )
|
0
|
*
|
||||||||||
|
Lennox Jaipersad
|
6,000
|
( 123 )
|
6,000
|
( 123 )
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F Joseph Oppito Bene Owner Patrick Oppito DCSD IRA
|
25,000
|
( 124 )
|
25,000
|
( 124 )
|
0
|
*
|
||||||||||
|
Dhiman Parikh
|
12,500
|
( 125 )
|
12,500
|
( 125 )
|
0
|
*
|
||||||||||
|
Manu Prasad Parikh
|
35,000
|
( 126 )
|
35,000
|
( 126 )
|
0
|
*
|
||||||||||
|
George A. Parmer
|
100,000
|
( 127 )
|
100,000
|
( 127 )
|
0
|
*
|
||||||||||
|
Arthur Pereless
|
20,000
|
( 128 )
|
20,000
|
( 128 )
|
0
|
*
|
||||||||||
|
Gregoy George Pyszczymuka
|
12,500
|
( 129 )
|
12,500
|
( 129 )
|
0
|
*
|
||||||||||
|
Reed Family Trust DTD 06/24/1999 Clayton A Reed & Stephanie S Reed TTEES
|
100,000
|
( 130 )
|
100,000
|
( 130 )
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach Inc. C/F Gary A. Robbins IRA
|
10,000
|
( 131 )
|
10,000
|
( 131 )
|
0
|
*
|
||||||||||
|
Terrence E. Rusin
|
10,900
|
( 132 )
|
10,900
|
( 132 )
|
0
|
*
|
||||||||||
|
Oliver Schulte
|
25,000
|
( 133 )
|
25,000
|
( 133 )
|
0
|
*
|
||||||||||
|
Neha Parikh Shah & Nikesha Shah JTWROS
|
10,000
|
( 134 )
|
10,000
|
( 134 )
|
0
|
*
|
||||||||||
|
Robert T. Stapell
|
25,000
|
( 135 )
|
25,000
|
( 135 )
|
0
|
*
|
||||||||||
|
Jeff L. Stevens
|
25,000
|
( 136 )
|
25,000
|
( 136 )
|
0
|
*
|
||||||||||
|
Daniel P. Wikel
|
50,000
|
( 137 )
|
50,000
|
( 137 )
|
0
|
*
|
||||||||||
|
Kenneth Williamson
|
50,000
|
( 138 )
|
50,000
|
( 138 )
|
0
|
*
|
||||||||||
|
SAL C/F Lance Ziaks Simple IRA
|
10,000
|
( 139 )
|
10,000
|
( 139 )
|
0
|
|||||||||||
|
Scott L. Byer
|
25,000
|
( 140 )
|
25,000
|
( 140 )
|
0
|
*
|
||||||||||
|
Kenneth Londoner ( 141 )
|
4,605,314
|
( 142 )
|
181,220
|
( 143 )
|
4, 424,094
|
( 144 )
|
30. 18 %
|
|||||||||
|
Alpha Capital Anstalt ( 145 )
|
2, 365,046
|
( 146 )
|
745,875
|
( 147 )
|
1, 619,171
|
( 148 )
|
10. 71 %
|
|||||||||
|
Jonathan Steinhouse
|
436,765
|
( 149 )
|
23,572
|
( 150 )
|
413,193
|
( 151 )
|
2. 87 %
|
|||||||||
|
Gary W Chmielewski & Monica R. Chmielewski JTWROS
|
6,123
|
( 152 )
|
6,123
|
( 152 )
|
0
|
*
|
||||||||||
|
Julius E. Talton
|
18,750
|
( 153 )
|
18,750
|
( 153 )
|
0
|
*
|
||||||||||
|
Carlos Javier Jurado & Zulma E. Jurado JTIC
|
7,500
|
( 154 )
|
7,500
|
( 154 )
|
0
|
*
|
||||||||||
|
Stourbridge Investments LLC ( 155 )
|
11,250
|
( 156 )
|
11,250
|
( 156 )
|
0
|
*
|
||||||||||
|
Paul E. Hoffmann
|
9,183
|
( 157 )
|
9,183
|
( 157 )
|
0
|
*
|
||||||||||
|
Jerome B. Zeldis ( 158 )
|
418,315
|
( 159 )
|
28,777
|
( 160 )
|
389,538
|
( 161 )
|
2. 67 %
|
|||||||||
|
Mark S Samuels
|
12,245
|
12,245
|
0
|
*
|
||||||||||||
|
ATA Investments, LLC ( 162 )
|
6,150
|
( 163 )
|
6,150
|
( 163 )
|
0
|
*
|
||||||||||
|
Craig H. Unger
|
7,653
|
( 164 )
|
7,653
|
( 164 )
|
0
|
*
|
||||||||||
|
Reynold Duclas Jr. & Janice Kannikal JTIC
|
6,150
|
( 165 )
|
6,150
|
( 165 )
|
0
|
*
|
||||||||||
|
James Keleher
|
6,000
|
( 166 )
|
6,000
|
( 166 )
|
0
|
*
|
||||||||||
|
Alan Greenhalgh & Angela Greenhalgh JTWROS
|
56,940
|
( 167 )
|
56,940
|
( 167 )
|
0
|
*
|
||||||||||
|
Garfield W. Hardeman T.O.D
|
15,000
|
( 168 )
|
15,000
|
( 168 )
|
0
|
*
|
||||||||||
|
Medardo Villatoro
|
6,780
|
( 169 )
|
6,780
|
( 169 )
|
0
|
*
|
||||||||||
|
Barry G. Pallay
|
6,000
|
( 170 )
|
6,000
|
( 170 )
|
0
|
*
|
||||||||||
|
John Avon
|
21,000
|
( 171 )
|
21,000
|
( 171 )
|
0
|
*
|
||||||||||
|
Jason H Murray as Trustee for The Golden Pond Super Fund
|
30,000
|
( 172 )
|
30,000
|
( 172 )
|
0
|
*
|
||||||||||
|
David C. Metzner
|
12,245
|
( 173 )
|
12,245
|
( 173 )
|
0
|
*
|
||||||||||
|
Lyle Helmick
|
6,150
|
( 174 )
|
6,150
|
( 174 )
|
0
|
*
|
||||||||||
|
Georges Zanellato
|
30,000
|
( 175 )
|
30,000
|
( 175 )
|
0
|
*
|
|
Jan Backvall
|
6,000
|
( 176 )
|
6,000
|
( 176 )
|
0
|
*
|
||||||||||
|
Brad Larson
|
7,080
|
( 177 )
|
7,080
|
( 177 )
|
0
|
*
|
||||||||||
|
Tim Lockner
|
6,000
|
( 178 )
|
6,000
|
( 178 )
|
0
|
*
|
||||||||||
|
Sterne Agee & Leach C/F Robert E Spano IRA
|
9,840
|
( 179 )
|
9,840
|
( 179 )
|
0
|
*
|
||||||||||
|
William Bellinger
|
10,800
|
( 180 )
|
10,800
|
( 180 )
|
0
|
*
|
||||||||||
|
Ted Fiore
|
7,200
|
( 181 )
|
7,200
|
( 181 )
|
0
|
*
|
||||||||||
|
Frank R Deis & Donna R Deis JTWROS
|
6,000
|
( 182 )
|
6,000
|
( 182 )
|
0
|
*
|
||||||||||
|
Joseph A Schuld
|
6,000
|
( 183 )
|
6,000
|
( 183 )
|
0
|
*
|
||||||||||
|
L. Dean Fox
|
74,210
|
( 184 )
|
51,042
|
( 185 )
|
23,168
|
( 186 )
|
*
|
|||||||||
|
Standard Sand & Silica Co., Inc. ( 187 )
|
12,000
|
( 188 )
|
12,000
|
( 188 )
|
0
|
*
|
||||||||||
|
Brio Capital Master Fund Ltd. ( 189 )
|
363,878
|
( 190 )
|
112,440
|
( 191 )
|
251,438
|
( 192 )
|
1. 75 %
|
|||||||||
|
Michael N. Emmerman
|
487,314
|
(193)
|
41,628
|
(194)
|
445,686
|
(195)
|
3.06%
|
|||||||||
|
Lau Family Fund LP (196)
|
105,693
|
(197)
|
10,409
|
(198)
|
95,284
|
(199)
|
*
|
|||||||||
|
R. Ian Chaplin
|
73,204
|
(200)
|
5,204
|
(201)
|
68,000
|
(202)
|
*
|
|||||||||
|
Kenneth Epstein
|
192,084
|
(203)
|
20,814
|
(204)
|
171,270
|
(205)
|
1.19%
|
|||||||||
|
Sterne Agee & Leach Inc. C/F Maree Casatelli SEP IRA
|
47,889
|
(206)
|
5,204
|
(207)
|
42,685
|
(208)
|
*
|
|||||||||
|
Ron D. Craig
|
223,192
|
(209)
|
20,400
|
(210)
|
202,792
|
(211)
|
1.42%
|
|||||||||
|
Michael Engdall & Susan Engdall JTWROS
|
64,965
|
(212)
|
7,287
|
(213)
|
57,678
|
(214)
|
*
|
|||||||||
|
Phillip Todd Herndon
|
123,599
|
(215)
|
10,409
|
(216)
|
113,190
|
(217)
|
*
|
|||||||||
|
Rex A. Jones
|
254,228
|
(218)
|
20,814
|
(219)
|
233,414
|
(220)
|
1.62%
|
|||||||||
|
Nabil M. Yazgi
|
159,128
|
(221)
|
4,164
|
(222)
|
154,964
|
(223)
|
1.09%
|
|||||||||
|
Portofino Ventures LP (224)
|
38,312
|
(225)
|
4,164
|
(226)
|
34,148
|
(227)
|
*
|
|||||||||
|
Thomas G. Hoffman
|
60,736
|
(228)
|
5,204
|
(229)
|
55,532
|
(230)
|
*
|
|||||||||
|
Martin F. Sauer
|
79,228
|
(231)
|
5,204
|
(232)
|
74,024
|
(233)
|
*
|
|||||||||
|
Ray Weber
|
84,118
|
(234)
|
9,368
|
(235)
|
74,750
|
(236)
|
*
|
|||||||||
|
Sterne Agee & Leach Inc. C/F Raymond E. Weber IRA
|
67,042
|
(237)
|
7,285
|
(238)
|
59,757
|
(239)
|
*
|
|||||||||
|
Fourfathom Capital LLC (240)
|
191,549
|
(241)
|
20,814
|
(242)
|
170,735
|
(243)
|
1.19%
|
|||||||||
|
Michael B. Carroll & Sheila J. Carroll JTWROS
|
287,322
|
(244)
|
31,221
|
(245)
|
256,101
|
(246)
|
1.77%
|
|||||||||
|
Scott D. Gamble
|
191,549
|
(247)
|
20,814
|
(248)
|
170,735
|
(249)
|
1.19%
|
|||||||||
|
Brian E. Jones & Peggy A. Jones JTWROS
|
123,599
|
(250)
|
10,409
|
(251)
|
113,190
|
(252)
|
*
|
|||||||||
|
David Patterson
|
38,312
|
(253)
|
4,164
|
(254)
|
34,148
|
(255)
|
*
|
|||||||||
|
George Elefther & Karin Alexa Elefther JTWROS
|
24,210
|
(256)
|
1,042
|
(257)
|
23,168
|
(258)
|
*
|
|||||||||
|
Sterne Agee & Leach Inc. C/F John L. Sommer IRA
|
72,718
|
(259)
|
2,083
|
(260)
|
70,635
|
(261)
|
*
|
|||||||||
|
Allan D. Carlson
|
17,076
|
(262)
|
2,083
|
(263)
|
14,993
|
(264)
|
*
|
|||||||||
|
Sterne Agee & Leach Inc. C/F Randy Payne IRA
|
30,986
|
(265)
|
2,083
|
(266)
|
28,903
|
(267)
|
*
|
|||||||||
|
Dr. Richard Matter & Anita Matter JTWROS
|
34,150
|
(268)
|
4,164
|
(269)
|
29,986
|
(270)
|
*
|
|||||||||
|
Randal E. Margo
|
47,185
|
(271)
|
5,204
|
(272)
|
41,981
|
(273)
|
*
|
|||||||||
|
Eugene E. Eubank
|
94,369
|
(274)
|
10,407
|
(275)
|
83,962
|
(276)
|
*
|
|||||||||
|
Robert W. Baird & Co. Inc. TTEE FBO Brian Mark Miller ROTH IRA
|
170,735
|
(277)
|
20,814
|
(278)
|
149,921
|
(279)
|
1.04%
|
|||||||||
|
Sterne Agee & Leach Inc. C/F Dr. Gary W. Chmielewski IRA
|
17,076
|
(280)
|
2,083
|
(281)
|
14,993
|
(282)
|
*
|
|||||||||
|
Laidlaw & Co (UK) Ltd ( 283 )
|
876,233
|
( 284 )
|
0
|
876,233
|
( 284 )
|
5. 81 %
|
* Less than 1%
|
(1)
|
In computing the percentage of our common stock beneficially owned by each selling stockholder after the offering, we have assumed the exercise by such selling stockholder of all warrants with respect to those shares being offered by such selling stockholder, and therefore the calculation is based on a number of shares of common stock outstanding comprised of (i) 14,203,202 shares of common stock outstanding as of June 9, 2015 plus (ii) the number of shares offered by the selling stockholder in this offering underlying warrants held by such selling stockholder. The shares offered by one selling stockholder underlying warrants held by such selling stockholder are not deemed outstanding for the purpose of computing the percentage ownership of any other selling stockholder.
|
|
(2)
|
In computing the percentage of our common stock beneficially owned by each selling stockholder after the offering, we have assumed that all shares offered by such selling stockholder underlying warrants held by such selling stockholder have been sold, and therefore the calculation is based on a number of shares of common stock outstanding comprised of (i) 14,203,202 shares of common stock outstanding as of June 9, 2015 plus (ii) the number of shares offered by the selling stockholder in this offering underlying warrants held by such selling stockholder. The shares offered by one selling stockholder underlying warrants held by such selling stockholder are not deemed outstanding for the purpose of computing the percentage ownership of any other selling stockholder.
|
|
(3)
|
Includes 34,000 shares of common stock issuable upon the exercise of warrants.
|
|
(4)
|
Includes 8,000 shares of common stock issuable upon the exercise of warrants.
|
|
(5)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 11,334 shares of common stock and (ii) 23,231 shares of common stock issuable upon the exercise of warrants.
|
|
(6)
|
Includes 5,539 shares of common stock issuable upon the exercise of warrants.
|
|
(7)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 11,334 shares of common stock and (ii) 17,692 shares of common stock issuable upon the exercise of warrants.
|
|
(8)
|
Includes 5,000 shares of common stock issuable upon the exercise of warrants.
|
|
(9)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 20,001 shares of common stock and (ii) 41,428 shares of common stock issuable upon the exercise of warrants.
|
|
(10)
|
Includes 11,246 shares of common stock issuable upon the exercise of warrants.
|
|
(11)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 20,001 shares of common stock and (ii) 30,182 shares of common stock issuable upon the exercise of warrants.
|
|
(12)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 4,000 shares of common stock and (ii) 19,045 shares of common stock issuable upon the exercise of warrants.
|
|
(13)
|
Includes 14,049 shares of common stock issuable upon the exercise of warrants.
|
|
(14)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 4,000 shares of common stock and (ii) 4,996 shares of common stock issuable upon the exercise of warrants.
|
|
(15)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 100,000 shares of common stock and (ii) 309,522 shares of common stock issuable upon the exercise of warrants.
|
|
(16)
|
Includes 153,421 shares of common stock issuable upon the exercise of warrants.
|
|
(17)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 100,000 shares of common stock and (ii) 156,101 shares of common stock issuable upon the exercise of warrants.
|
|
(18)
|
Includes 4,000 shares of common stock issuable upon the exercise of warrants.
|
|
(19)
|
Includes 10,000 shares of common stock issuable upon the exercise of warrants.
|
|
(20)
|
Includes 47,400 shares of common stock issuable upon the exercise of warrants.
|
|
(21)
|
Includes 8,000 shares of common stock issuable upon the exercise of warrants.
|
|
(22)
|
Includes 25,000 shares of common stock issuable upon the exercise of warrants.
|
|
(23)
|
Includes 5,000 shares of common stock issuable upon the exercise of warrants.
|
|
(24)
|
Alok K. Agrawal, the Managing Member of AAJK Investments, LLC, has voting and dispositive power over the securities held for the account of this selling stockholder.
|
|
(25)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(26)
|
Includes 50,204 shares of common stock issuable upon the exercise of warrants.
|
|
(27)
|
Includes 40,000 shares of common stock issuable upon the exercise of warrants.
|
|
(28)
|
Includes 10,000 shares of common stock issuable upon the exercise of warrants.
|
|
(29)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(30)
|
Includes 14,000 shares of common stock issuable upon the exercise of warrants.
|
|
(31)
|
Includes 5,000 shares of common stock issuable upon the exercise of warrants.
|
|
(32)
|
Includes 5,000 shares of common stock issuable upon the exercise of warrants.
|
|
(33)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(34)
|
Includes 8,123 shares of common stock issuable upon the exercise of warrants.
|
|
(35)
|
Includes 3,000 shares of common stock issuable upon the exercise of warrants.
|
|
(36)
|
Includes 5 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(37)
|
Includes 9,600 shares of common stock issuable upon the exercise of warrants.
|
|
(38)
|
Includes 18,000 shares of common stock issuable upon the exercise of warrants.
|
|
(39)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 40 )
|
Includes 6,000 shares of common stock issuable upon the exercise of warrants.
|
|
(41)
|
Includes 12,600 shares of common stock issuable upon the exercise of warrants.
|
|
(42)
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
(43)
|
Includes 12 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(44)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(45)
|
Includes 9,500 shares of common stock issuable upon the exercise of warrants.
|
|
(46)
|
Includes 5,000 shares of common stock issuable upon the exercise of warrants.
|
|
(47)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(48)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(49)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(50)
|
Gavin Bell, the Founder of Ecovest Limited, has voting and dispositive power over the securities held for the account of this selling stockholder.
|
|
( 51 )
|
Includes 12 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(52)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(53)
|
Includes 10,800 shares of common stock issuable upon the exercise of warrants.
|
|
(54)
|
Includes 5,760 shares of common stock issuable upon the exercise of warrants.
|
|
(55)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(56)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(57)
|
Includes 18 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(58)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(59)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(60)
|
Includes 30,000 shares of common stock issuable upon the exercise of warrants.
|
|
(61)
|
Includes 24 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(62)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(63)
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
(64)
|
Includes 60 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(65)
|
Includes 16,800 shares of common stock issuable upon the exercise of warrants.
|
|
( 66 )
|
Includes 30,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 67 )
|
Includes 12,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 68 )
|
Includes 300,000 shares of common stock issuable upon the exercise of warrants.
|
|
(69)
|
Includes 8,000 shares of common stock issuable upon the exercise of warrants.
|
|
(70)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(71)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(72)
|
Includes 60 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(73)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(74)
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
(75)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(76)
|
Includes 30,000 shares of common stock issuable upon the exercise of warrants.
|
|
(77)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(78)
|
Includes 60 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(79)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(80)
|
Includes 60 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(81)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock and (ii) 29,609 shares of common stock issuable upon the exercise of warrants.
|
|
(82)
|
Includes 21,283 shares of common stock issuable upon the exercise of warrants.
|
|
( 83 )
|
Includes (i) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock and (ii) 8,326 shares of common stock issuable upon the exercise of warrants.
|
|
( 84 )
|
Includes 9,600 shares of common stock issuable upon the exercise of warrants.
|
|
(85)
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
(86)
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
(87)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(88)
|
Includes 24 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(89)
|
Includes 18 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(90)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(91)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 92 )
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 93 )
|
Includes 18,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 94 )
|
Includes 30,000 shares of common stock issuable upon the exercise of warrants.
|
|
(95)
|
Includes 6,000 shares of common stock issuable upon the exercise of warrants.
|
|
(96)
|
Includes 6,000 shares of common stock issuable upon the exercise of warrants.
|
|
(97)
|
Includes 6,000 shares of common stock issuable upon the exercise of warrants.
|
|
(98)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(99)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(100)
|
Includes 30,000 shares of common stock issuable upon the exercise of warrants.
|
|
(101)
|
Includes 60 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(102)
|
Includes 60,000 shares of common stock issuable upon the exercise of warrants.
|
|
(103)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(104)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(105)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(106)
|
Includes 30,000 shares of common stock issuable upon the exercise of warrants.
|
|
(107)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(108)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(109)
|
Includes 12 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(110)
|
Includes 12,000 shares of common stock issuable upon the exercise of warrants.
|
|
(111)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(112)
|
Includes 3 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(113)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(114)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(115)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock and (ii) 41,018 shares of common stock issuable upon the exercise of warrants.
|
|
(116)
|
Includes 20,204 shares of common stock issuable upon the exercise of warrants.
|
|
(117)
|
Includes (i) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock and (ii) 20,814 shares of common stock issuable upon the exercise of warrants.
|
|
(118)
|
Includes 30,600 shares of common stock issuable upon the exercise of warrants.
|
|
(119)
|
Includes 30, 000 shares of common stock issuable upon the exercise of warrants.
|
|
(120)
|
Includes 10,050 shares of common stock issuable upon the exercise of warrants.
|
|
(121)
|
Includes 9,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 122 )
|
Includes 17,702 shares of common stock issuable upon the exercise of warrants.
|
|
(123)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(124)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(125)
|
Includes 7,500 shares of common stock issuable upon the exercise of warrants.
|
|
(126)
|
Includes 21,000 shares of common stock issuable upon the exercise of warrants.
|
|
(127)
|
Includes 60 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(128)
|
Includes 12 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(129)
|
Includes 7,500 shares of common stock issuable upon the exercise of warrants.
|
|
(130)
|
Includes 60,000 shares of common stock issuable upon the exercise of warrants.
|
|
(131)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(132)
|
Includes 6, 540 shares of common stock issuable upon the exercise of warrants.
|
|
(133)
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
(134)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(135)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(136)
|
Includes 15,000 shares of common stock issuable upon the exercise of warrants.
|
|
(137)
|
Includes 30 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(138)
|
Includes 30,000 shares of common stock issuable upon the exercise of warrants.
|
|
(139)
|
Includes 6 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(140)
|
Includes 15 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(141)
|
Kenneth Londoner is our Executive Chairman.
|
|
(142)
|
Comprised of (i) 724,045 shares of common stock directly held by Mr. Londoner, (ii) 3,334,974 shares of common stock held by Endicott Management Partners, LLC, an entity for which Mr. Londoner is deemed the beneficial owner, (iii) warrants to purchase 296,295 shares of common stock, and (iv) options to purchase 250,000 shares of common stock that are currently exercisable.
|
|
(143)
|
Includes 88,159 shares of common stock issuable upon the exercise of warrants.
|
|
(144)
|
Comprised of (i) 630,984 shares of common stock directly held by Mr. Londoner, (ii) 3,334,974 shares of common stock held by Endicott Management Partners, LLC, an entity for which Mr. Londoner is deemed the beneficial owner, (iii) warrants to purchase 208,136 shares of common stock, and (iv) options to purchase 250,000 shares of common stock that are currently exercisable
|
|
(145)
|
Konrad Ackermann has sole voting and dispositive power over the securities held for the account of this stockholder.
|
|
(146)
|
Comprised of (i) 824,534 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 516,667 shares of common stock, and (iii) warrants to purchase 1,023,845 shares of common stock.
|
|
( 147 )
|
Includes 250,000 shares of common stock issuable upon the conversion of shares of our Series C Preferred Stock and 373,426 shares of common stock issuable upon the exercise of warrants.
|
|
( 148 )
|
Comprised of (i) 702,085 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 266 ,667 shares of common stock, and (iii) warrants to purchase 650,419 shares of common stock.
|
|
( 149 )
|
Comprised of (i) 224,420 shares of common stock, (ii) options to purchase 175,000 shares of common stock that are currently exercisable, and (iii) warrants to purchase 37,345 shares of common stock.
|
|
( 150 )
|
Includes 11,327 shares of common stock issuable upon the exercise of warrants.
|
|
( 151 )
|
Comprised of (i) 212,175 shares of common stock, (ii) options to purchase 175,000 shares of common stock that are currently exercisable, and (iii) warrants to purchase 26,018 shares of common stock.
|
|
( 152 )
|
Includes 2,041 shares of common stock issuable upon the exercise of warrants.
|
|
( 153 )
|
Includes 6,250 shares of common stock issuable upon the exercise of warrants.
|
|
( 154 )
|
Includes 2,500 shares of common stock issuable upon the exercise of warrants.
|
|
( 155 )
|
Steve Schnipper, Managing Member of Stourbridge Investments LLC, has sole voting and dispositive power over the securities held for the account of this stockholder.
|
|
( 156 )
|
Includes 3,750 shares of common stock issuable upon the exercise of warrants.
|
|
(157)
|
Includes 3,061 shares of common stock issuable upon the exercise of warrants.
|
|
(158)
|
Jerome B. Zeldis is a member of our board of directors.
|
|
(159)
|
Consists of (i) 12,245 shares of common stock, (ii) options to purchase 304,167 shares of common stock that are currently exercisable, (iii) shares of Series C Preferred Stock that are convertible into 33,334 shares of common stock, and (iv) warrants to purchase 68,569 shares of common stock.
|
|
(160)
|
Includes 16,532 shares of common stock issuable upon the exercise of warrants.
|
|
(161)
|
Consists of (i) options to purchase 304,167 shares of common stock that are currently exercisable, ( ii ) shares of Series C Preferred Stock that are convertible into 33,334 shares of common stock, and ( iii ) warrants to purchase 52,037 shares of common stock.
|
|
(162)
|
Alok K. Agrawal, Managing Member of ATA Investments, LLC, has sole voting and dispositive power over the securities held for the account of this stockholder.
|
|
(163)
|
Includes 2,050 shares of common stock issuable upon the exercise of warrants.
|
|
(164)
|
Includes 2,551 shares of common stock issuable upon the exercise of warrants.
|
|
(165)
|
Includes 2,050 shares of common stock issuable upon the exercise of warrants.
|
|
(166)
|
Includes 2, 000 shares of common stock issuable upon the exercise of warrants.
|
|
(167)
|
Includes 18,980 shares of common stock issuable upon the exercise of warrants.
|
|
(168)
|
Includes 5 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(169)
|
Includes 2,260 shares of common stock issuable upon the exercise of warrants.
|
|
(170)
|
Includes 2 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(171)
|
Includes 11,000 shares of common stock issuable upon the exercise of warrants.
|
|
(172)
|
Includes 10 ,000 shares of common stock issuable upon the exercise of warrants.
|
|
(173)
|
Includes 4,082 shares of common stock issuable upon the exercise of warrants.
|
|
(174)
|
Includes 2,050 shares of common stock issuable upon the exercise of warrants.
|
|
(175)
|
Includes 10,000 shares of common stock issuable upon the exercise of warrants.
|
|
(176)
|
Includes 2, 000 shares of common stock issuable upon the exercise of warrants.
|
|
(177)
|
Includes 2,360 shares of common stock issuable upon the exercise of warrants.
|
|
(178)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(179)
|
Includes 3,280 shares of common stock issuable upon the exercise of warrants.
|
|
(180)
|
Includes 3,600 shares of common stock issuable upon the exercise of warrants.
|
|
( 181 )
|
Includes 2,400 shares of common stock issuable upon the exercise of warrants.
|
|
(182)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(183)
|
Includes 2,000 shares of common stock issuable upon the exercise of warrants.
|
|
(184)
|
Consists of (i) 35,670 shares of common stock , (ii) shares of Series C Preferred Stock that are convertible into 3,334 shares of common stock, and (iii) warrants to purchase 35,206 shares of common stock .
|
|
(185)
|
Includes 31,042 shares of common stock issuable upon the exercise of warrants.
|
|
(186)
|
Consists of (i) 15 ,670 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 3,334 shares of common stock, and (iii) warrants to purchase 4,164 shares of common stock.
|
|
( 187 )
|
Lemuel Baylis Carnes III, CEO of Standard Sand & Silica Co, Inc. has sole voting and dispositive power over the securities held for the account of this stockholder.
|
|
( 188 )
|
Includes 4,000 shares of common stock issuable upon the exercise of warrants.
|
|
( 189 )
|
Shaye Hirsch, director of Brio Capital Master Fund Ltd., has sole voting and dispositive power over the securities held for the account of this selling stockholder.
|
|
( 190 )
|
Comprised of (i) 121,352 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 50,000 shares of common stock, and (iii) warrants to purchase 192,526 shares of common stock.
|
|
( 191 )
|
Includes 50,000 shares of common stock issuable upon the conversion of shares of our Series C Preferred Stock and 62,440 shares of common stock issuable upon the exercise of warrants.
|
|
( 192 )
|
Comprised of (i) 121,352 shares of common stock, and (ii) warrants to purchase 130,086 shares of common stock.
|
|
( 193 )
|
Consists of (i) 104,216 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 133,334 shares of common stock, and (iii) warrants to purchase 249,764 shares of common stock.
|
|
( 194 )
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(195)
|
Consists of (i) 104,216 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 133,334 shares of common stock, and (iii) warrants to purchase 208,136 shares of common stock.
|
|
(196)
|
Steven Lau, manager of S7 Capital, its General Partner of Lau Family Fund LP, has sole voting and dispositive power over the securities held for the account of this selling stockholder.
|
|
(197)
|
Consists of (i) 43,247 shares of common stock, (ii) warrants to purchase 62,446 shares of common stock.
|
|
(198)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(199)
|
Consists of (i) 43,247 shares of common stock, (ii) warrants to purchase 52,037 shares of common stock.
|
|
(200)
|
Comprised of (i) 25,315 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock, and (iii) warrants to purchase 31,222 shares of common stock.
|
|
(201)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(202)
|
Comprised of (i) 25,315 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock, and (iii) warrants to purchase 26,018 shares of common stock.
|
|
(203)
|
Comprised of (i) 535 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (iii) warrants to purchase 124,882 shares of common stock.
|
|
(204)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(205)
|
Comprised of (i) 535 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (iii) warrants to purchase 104,068 shares of common stock.
|
|
(206)
|
Comprised of (i) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock, and (ii) warrants to purchase 31,222 shares of common stock.
|
|
(207)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(208)
|
Comprised of (i) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock, and (ii) warrants to purchase 26,018 shares of common stock.
|
|
(209)
|
Comprised of (i) 110,794 shares of common stock, (ii) warrants to purchase 112,398 shares of common stock.
|
|
(210)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(211)
|
Comprised of (i) 110,794 shares of common stock, (ii) warrants to purchase 91,998 shares of common stock.
|
|
(212)
|
Comprised of (i) shares of Series C Preferred Stock that are convertible into 23,334 shares of common stock, and (ii) warrants to purchase 41,631 shares of common stock.
|
|
(213)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(214)
|
Comprised of (i) shares of Series C Preferred Stock that are convertible into 23,334 shares of common stock, and (ii) warrants to purchase 34,344 shares of common stock.
|
|
(215)
|
Consists of (i) 27,821 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 33,334 shares of common stock, and (iii) warrants to purchase 62,444 shares of common stock.
|
|
(216)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(217)
|
Consists of (i) 27,821 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 33,334 shares of common stock, and (iii) warrants to purchase 52,035 shares of common stock.
|
|
(218)
|
Consists of (i) 62,679 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (iii) warrants to purchase 124,882 shares of common stock.
|
|
(219)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(220)
|
Consists of (i) 62,679 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (iii) warrants to purchase 104,068 shares of common stock.
|
|
(221)
|
Consists of (i) 120,816 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (iii) warrants to purchase 24,978 shares of common stock.
|
|
(222)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(223)
|
Consists of (i) 120,816 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (iii) warrants to purchase 20,814 shares of common stock.
|
|
(224)
|
Michael A. Knudsen, President of Portofino Mgmt Inc., its General Partner of Portofino Ventures LP, has sole voting and dispositive power over the securities held for the account of this selling stockholder.
|
|
(225)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (iii) warrants to purchase 24,978 shares of common stock.
|
|
(226)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(227)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (iii) warrants to purchase 20,814 shares of common stock.
|
|
(228)
|
Consists of (i) 29,514 shares of common stock, (ii) warrants to purchase 31,222 shares of common stock.
|
|
(229)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(230)
|
Consists of (i) 29,514 shares of common stock, (ii) warrants to purchase 26,018 shares of common stock.
|
|
(231)
|
Consists of (i) 31,339 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock, and (iii) warrants to purchase 31,222 shares of common stock.
|
|
(232)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(233)
|
Consists of (i) 31,339 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 16,667 shares of common stock, and (iii) warrants to purchase 26,018 shares of common stock.
|
|
(234)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 30,001 shares of common stock, and (ii) warrants to purchase 54,117 shares of common stock.
|
|
(235)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(236)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 30,001 shares of common stock, and (ii) warrants to purchase 44,749 shares of common stock.
|
|
(237)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 23,334 shares of common stock, and (ii) warrants to purchase 43,708 shares of common stock.
|
|
(238)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(239)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 23,334 shares of common stock, and (ii) warrants to purchase 36,423 shares of common stock.
|
|
(240)
|
Brian Miller, manager of Fourfathom Capital LLC, has sole voting and dispositive power over the securities held for the account of this selling stockholder.
|
|
(241)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (ii) warrants to purchase 124,882 shares of common stock.
|
|
(242)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(243)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (ii) warrants to purchase 104,068 shares of common stock.
|
|
(244)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 100,000 shares of common stock, and (ii) warrants to purchase 187,322 shares of common stock.
|
|
(245)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(246)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 100,000 shares of common stock, and (ii) warrants to purchase 156,101 shares of common stock.
|
|
(247)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (ii) warrants to purchase 124,882 shares of common stock.
|
|
(248)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(249)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (ii) warrants to purchase 104,068 shares of common stock.
|
|
(250)
|
Consists of (i) 27,821 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 33,334 shares of common stock, and (iii) warrants to purchase 62,444 shares of common stock.
|
|
(251)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(252)
|
Consists of (i) 27,821 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 33,334 shares of common stock, and (iii) warrants to purchase 52,035 shares of common stock.
|
|
(253)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (ii) warrants to purchase 24,978 shares of common stock.
|
|
(254)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(255)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (ii) warrants to purchase 20,814 shares of common stock.
|
|
(256)
|
Consists of (i) 15,670 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 3,334 shares of common stock, and (iii) warrants to purchase 5,206 shares of common stock.
|
|
(257)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(258)
|
Consists of (i) 15,670 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 3,334 shares of common stock, and (iii) warrants to purchase 4,164 shares of common stock.
|
|
(259)
|
Consists of (i) 55,642 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (iii) warrants to purchase 10,409 shares of common stock.
|
|
(260)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(261)
|
Consists of (i) 55,642 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (iii) warrants to purchase 8,326 shares of common stock.
|
|
(262)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (ii) warrants to purchase 10,409 shares of common stock.
|
|
(263)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(264)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (ii) warrants to purchase 8,326 shares of common stock.
|
|
(265)
|
Consists of (i) 13,910 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (iii) warrants to purchase 10,409 shares of common stock.
|
|
(266)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(267)
|
Consists of (i) 13,910 shares of common stock, (ii) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (iii) warrants to purchase 8,326 shares of common stock.
|
|
(268)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (ii) warrants to purchase 20,816 shares of common stock.
|
|
(269)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(270)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 13,334 shares of common stock, and (ii) warrants to purchase 16,652 shares of common stock.
|
|
(271)
|
Consists of (i) 21,167 shares of common stock, and (ii) warrants to purchase 26,018 shares of common stock.
|
|
(272)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(273)
|
Consists of (i) 21,167 shares of common stock, and (ii) warrants to purchase 20,814 shares of common stock.
|
|
(274)
|
Consists of (i) 42,334 shares of common stock, and (ii) warrants to purchase 52,035 shares of common stock.
|
|
(275)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(276)
|
Consists of (i) 42,334 shares of common stock, and (ii) warrants to purchase 41,628 shares of common stock.
|
|
(277)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (ii) warrants to purchase 104,068 shares of common stock.
|
|
(278)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(279)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 66,667 shares of common stock, and (ii) warrants to purchase 83,254 shares of common stock.
|
|
(280)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (ii) warrants to purchase 10,409 shares of common stock.
|
|
(281)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
|
(282)
|
Consists of (i) shares of Series C Preferred Stock that are convertible into 6,667 shares of common stock, and (ii) warrants to purchase 8,326 shares of common stock.
|
|
(283)
|
Laidlaw & Co (UK) Ltd is a registered broker-dealer. Matthew Eitner is the chief executive officer of Laidlaw & Co (UK) Ltd and, in such capacity, he may be deemed to have voting and dispositive power over the securities held for the account of this selling stockholder. As consideration for serving as our placement agent in connection with the December 2013 Placement, we issued Laidlaw & Co (UK) Ltd a five-year warrant to purchase up to 40,327 shares of our common stock at an exercise price of $3.67 per share. As consideration for serving as our placement agent in connection with the April 2014 Placement, we issued Laidlaw & Co (UK) Ltd a five-year warrant to purchase up to 22,976 shares of our common stock at an exercise price of $3.75 per share. As consideration for serving as our placement agent in connection with the August 2014 Placement, we issued Laidlaw & Co (UK) Ltd a five-year warrant to purchase up to 38,120 shares of our common stock at an exercise price of $2.75 per share. As consideration for serving as our placement agent in connection with the December 2014 Placement, we issued Laidlaw & Co (UK) Ltd “B” warrants to purchase up to 400,900 shares of our common stock at an exercise price of $3.75 per share.
|
|
(284)
|
Comprised of shares of common stock issuable upon the exercise of warrants.
|
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
On August 1, 2012, we entered into a consulting agreement with Asher Holzer, Ph.D., a member of our board of directors. Pursuant to the consulting agreement, Dr. Holzer was to serve as our chief scientific officer and assist in the development of our technology and our PURE EP System, in exchange for monthly payments of $10,000. We have paid Dr. Holzer an initial payment of $7,500 pursuant to the consulting agreement. In the first quarter of 2014, we agreed to an oral amendment to our consulting agreement with Dr. Holzer, which resulted in Dr. Holzer agreeing to receive (i) a payment of $65,000, which will be paid by us upon our closing of a capital raising transaction that results in proceeds to us of at least $5 million, and (ii) a future option grant to purchase 125,000 shares of our common stock, in exchange for acknowledging that no other payments are due by us to Dr. Holzer pursuant to the consulting agreement.
On September 1, 2014, we entered into a letter agreement and release with Dr. Holzer, pursuant to which Dr. Holzer agreed to cancel, extinguish and terminate all amounts due or owed by us for services performed by Dr. Holzer pursuant to that certain consulting agreement, dated as of August 1, 2012, as amended. In connection with the cancellation of all payment obligations and in exchange for Dr. Holzer waiving and releasing us from all possible claims related to such obligations under the consulting agreement, Dr. Holzer received 26,000 shares of our common stock. Dr. Holzer also agreed to provide us with one additional year of consulting services in exchange for 34,000 shares of common stock.
On November 21, 2012, we issued an unsecured promissory note for $218,000 to Kenneth L. Londoner, our then chairman and chief executive officer, for previously advanced funds with interest payable annually, in arrears, on each anniversary at the short term “Applicable Federal Rate” within the meaning of Section 1274(d) of the Internal Revenue Code of 1986, as amended, which was 0.22% in November 2012, and which will be adjusted each anniversary date. The promissory note matures November 21, 2021 and may be prepaid, without premium or penalty, at any time. In connection with the private placement of our Series C Preferred Stock and warrants, on February 6, 2013, Mr. Londoner agreed not to receive payments (by voluntary prepayment, acceleration, set-off or otherwise) associated with the unsecured promissory note absent the prior written consent of the purchasers holding at least 67% interest of our Series C Preferred Stock outstanding, which purchasers must include Alpha Capital Anstalt so long as Alpha Capital Anstalt holds not less than $100,000 of our Series C Preferred Stock. As of June 30, 2013, aggregate interest of $277.19 had accrued on this unsecured promissory note. The unsecured promissory note was converted into our equity securities, pursuant to a private placement transaction on December 31, 2013, as described below.
On December 6, 2012, we issued an unsecured promissory note for $30,000 to a company under the control of Mr. Londoner for previously advanced funds, interest free and due the earlier of (i) the next financing of not less than $300,000; (ii) February 28, 2013 or (iii) occurrence of an event of default, as defined. The promissory note has been paid in full.
In the fourth quarter of 2012, we sold $600,000 principal amount of certain bridge notes and related warrants in a private placement to selected accredited investors. These bridge notes and related warrants were converted into shares of our Series C Preferred Stock and warrants on February 6, 2013. Kenneth L. Londoner, our then chairman and chief executive officer, purchased $200,000 principal amount of notes, which were converted into 200 shares of Series C Preferred Stock and a warrant to purchase 95,694 shares of our common stock; and Jonathan Steinhouse, a member of our board of directors, purchased $25,000 principal amount of notes, which were converted into 25 shares of Series C Preferred Stock and a warrant to purchase 11,962 shares of our common stock. We also issued to Mr. Londoner and Mr. Steinhouse, in lieu of cash payments on the interest accrued on their respective bridge notes, 2,579 and 383 shares of common stock, respectively. The terms of the Series C Preferred Stock were amended on March 27, 2014 to provide for a decrease of the conversion price of the Series C Preferred Stock from $2.09 per share to $1.50 per share. As a result of the amendment, the full-ratchet anti-dilution protection provision of the related warrants decreased the exercise price of the warrants from $2.61 per share to $1.50 per share and the number of shares issuable under each warrant was increased such that the aggregate exercise price payable under such warrant, after taking into account the decrease in the exercise price, is equal to the aggregate exercise price prior to such adjustment. As such, the number of shares of common stock issuable upon exercise of the warrants increased to 166,508 shares for Mr. Londoner and 20,814 shares for Mr. Steinhouse. In addition, in connection with amendments to the terms of the Series C Preferred Stock, we issued to (i) Mr. Londoner warrants to purchase an aggregate of 83,256 shares of common stock and (ii) Mr. Steinhouse warrants to purchase an aggregate of 10,408 shares of common stock, which such figures reflect the triggering of the full-ratchet anti-dilution protection provision of the warrants.
From 2010 to 2013, Mr. Londoner made four different advances of funds to us in the aggregate amount of $22,000, of which $12,000 has been repaid. In the first quarter of 2013, Mr. Steinhouse made an advance of funds to us in the amount of $20,000, which has been repaid in full. These advances were interest-free and not made on condition of any specific terms. The remaining $10,000 owed to Mr. Londoner was converted into our equity securities, pursuant to a private placement transaction on December 31, 2013, as described below.
On February 12, 2013, as part of a private placement transaction, we issued to Alpha Capital Anstalt 625 shares of Series C Preferred Stock and a warrant to purchase 520,335 shares of our common stock for a purchase price of $625,000. In addition, in connection with amendments to the terms of the Series C Preferred Stock, we issued to Alpha Capital Anstalt warrants to purchase an aggregate of 260,168 shares of common stock. The number of shares of common stock issuable upon exercise of the warrants reflect the triggering of the full-ratchet anti-dilution protection provision of the warrants.
In the fourth quarter of 2013, Steve Chaussy, our chief financial officer, made three different advances of funds to us in the aggregate amount of $21,545, which was repaid in 2014. Also in the fourth quarter of 2013, Mr. Steinhouse made an advance of funds to us in the amount of $6,000, which was repaid in 2014. In addition, in the fourth quarter of 2013, Lora Mikolaitis, who controls Miko Consulting Group, Inc., made an advance of funds to us in the amount of approximately $2,700. These advances were interest-free and not made on condition of any specific terms. Each of these advances was repaid in full in the first quarter of 2014.
On December 31, 2013, as part of a private placement transaction of our common stock and warrants, (i) $228,000 of our outstanding indebtedness that was due to Mr. Londoner was converted into 93,061 shares of common stock and a warrant to purchase 46,531 shares of our common stock; and (ii) we issued to Alpha Capital Anstalt 122,448 shares of our common stock and a warrant to purchase 61,225 shares of our common stock for a purchase price of $300,000.
On January 31, 2014, as part of a private placement transaction of our common stock and warrants, Mr. Steinhouse purchased an aggregate of 24,490 shares of common stock and a warrant to purchase 12,246 shares of common stock for an aggregate purchase price of $60,000.
On March 5, 2014, Mr. Steinhouse made an advance of funds to us in the aggregate amount of $10,000, which was repaid in full on April 3, 2014. The advance was interest-free and not made on condition of any specific terms.
On September 12, 2014, Sterne Agee & Leach Inc. C/F Jonathan Steinhouse R/O IRA, as part of a private placement transaction of our common stock and warrants, purchased an aggregate of 4,000 shares of common stock and a warrant to purchase 2,000 shares of common stock for an aggregate purchase price of $10,000.
On October 22, 2014, the Company issued an aggregate of 21, 167 shares of its common stock to Mr. Steinhouse in exchange for 25 shares of the Company’s Series C Preferred Stock and accrued dividends, at a conversion price of $1.50 per share.
On March 23, 2015, the Company issued an aggregate of 169,334 shares of its common stock to Mr. Londoner in exchange for 200 shares of the Company’s Series C Preferred Stock and accrued dividends, at a conversion price of $1.50 per share.
On May 11, 2015, we entered into a securities purchase agreement with Alpha Capital Anstalt, pursuant to which we issued 375 shares of our Series C Preferred Stock and five-year warrants to purchase 312,201 shares of our common stock for aggregate cash proceeds of $375,000.
On May 21, 2015, the Company issued an aggregate of 190,500 shares of its common stock to Alpha Capital Anstalt in exchange for 225 shares of the Company’s Series C Preferred Stock and accrued dividends, at a conversion price of $1.50 per share.
DESCRIPTION OF SECURITIES
We have authorized 51,000,000 shares of capital stock, par value $0.001 per share, of which 50,000,000 are shares of common stock and 1,000,000 are shares of “blank check” preferred stock, of which 200 are authorized as Series A Preferred Stock, 600 are authorized as Series B Preferred Stock and 4,200 are authorized as Series C Preferred Stock. On June 9, 2015, there were 14,203,202 shares of common stock issued and outstanding, 2, 408 shares of Series C Preferred Stock issued and outstanding and no shares of Series A Preferred Stock or Series B Preferred Stock issued and outstanding.
A portion of the shares of common stock offered by this prospectus are issuable upon the exercise of common stock purchase warrants or the conversion of shares of Series C Preferred Stock. As such, if a selling stockholder converts the Series C Preferred Stock or exercises all or any portion of its warrants on a cash basis, we will receive the aggregate conversion price and exercise price paid by such selling stockholder in connection with any such conversion or warrant exercise. The maximum amount of proceeds we would receive upon the conversion of all the Series C Preferred Stock and the exercise of all the warrants on a cash basis would be approximately $9,160,745. However, certain of the selling stockholders may also exercise their warrants through a cashless exercise. In the event a selling stockholder exercises a warrant through a cashless exercise, we will not receive any proceeds from such exercise. We expect to use the proceeds received from the exercise of the warrants, if any, for general corporate purposes.
Holders of Capital Stock
As of June 9, 2015, we had 245 holders of our common stock, no holders of our Series A Preferred Stock or Series B Preferred Stock, and 34 holders of our Series C Preferred Stock.
Common Stock
The holders of common stock are entitled to one vote per share on all matters to be voted upon by stockholders. Holders of our common stock are entitled to receive ratably dividends as may be declared by the board of directors out of funds legally available for that purpose. In the event of our liquidation, dissolution, or winding up, the holders of common stock are entitled to share ratably in all assets remaining after payment of liabilities. The common stock has no preemptive or conversion rights, other subscription rights, or redemption or sinking fund provisions.
Each share of common stock entitles the holder to one vote, either in person or by proxy, at meetings of stockholders. The holders are not permitted to vote their shares cumulatively. Accordingly, the stockholders of our common stock who hold, in the aggregate, more than fifty percent of the total voting rights can elect all of our directors and, in such event, the holders of the remaining minority shares will not be able to elect any of such directors. The vote of the holders of a majority of the issued and outstanding shares of common stock entitled to vote thereon is sufficient to authorize, affirm, ratify or consent to such act or action, except as otherwise provided by law.
The rights of the holders of our Series C Preferred Stock, as described below, prohibit us from paying cash dividends to our holders of common stock absent the approval of holders representing at least 67% of the outstanding shares of the Series C Preferred Stock, which holders must include Alpha Capital Anstalt, so long as Alpha Capital Anstalt holds not less than $100,000 of the Series C Preferred Stock. We have not paid any dividends since our inception, and, subject to our obligations to pay dividends to the holders of the Series C Preferred Stock as described below, we presently anticipate that all earnings, if any, will be retained for development of our business. Even if we are permitted to pay cash dividends in the future, any future disposition of dividends will be at the discretion of our board of directors and will depend upon, among other things, our future earnings, operating and financial condition, capital requirements, and other factors.
Holders of our common stock have no preemptive rights or other subscription rights, conversion rights, redemption or sinking fund provisions. Subject to the rights of the holders of our preferred stock, upon our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities. There are no provisions in our Amended and Restated Certificate of Incorporation or our By-laws that would prevent or delay a change in our control.
Preferred Stock
On June 23, 2014, upon effectiveness of our registration statement on Form S-1, all outstanding shares of our Series A Preferred Stock and our Series B Preferred Stock, including dividends accrued on the shares of preferred stock, automatically converted into shares of our common stock. As a result, there are presently no issued and outstanding shares of Series A Preferred Stock or Series B Preferred Stock.
Each share of the Series C Preferred Stock is entitled to a nine percent (9%) annual dividend on the $1,000 per share stated value. Unless the Series C Preferred Stock is converted into shares of common stock, the dividends shall accrue and be payable in cash or, subject to the satisfaction of certain conditions, in pay-in-kind shares. Such cumulative dividends are payable quarterly, commencing on September 30, 2013 and on each conversion date; provided, however, that if a holder converts its shares of Series C Preferred Stock into shares of common stock any time prior to February 12, 2016, the holder will be deemed to have earned a make whole amount as if such shares of Series C Preferred Stock had been outstanding until such date. The terms of the Series C Preferred Stock were amended on March 27, 2014 and August 15, 2014. The description herein reflects such amended terms.
In the event that
|
(i)
|
we fail to, or announce our intention not to, deliver common stock share certificates upon conversion of our Series C Preferred Stock prior to the seventh trading day after such shares are required to be delivered,
|
|
|
(ii)
|
we fail for any reason to pay in full the amount of cash due pursuant to our failure to deliver common stock share certificates upon conversion of our Series C Preferred Stock within five calendar days after notice therefor is delivered,
|
|
(iii)
|
we fail to have available a sufficient number of authorized and unreserved shares of common stock to issue upon a conversion of our Series C Preferred Stock,
|
|
|
(iv)
|
we fail to observe or perform any other covenant, agreement or warranty contained in, or otherwise commit any breach of our obligations under, the securities purchase agreement, the registration rights agreement, the certificate of designation or the warrants entered into pursuant to the private placement transaction for our Series C Preferred Stock, which failure or breach could have a material adverse effect, and such failure or breach is not cured within 30 calendar days after written notice was delivered,
|
|
(v)
|
we are party to a change of control transaction,
|
|
|
(vi)
|
we file for bankruptcy or a similar arrangement or are adjudicated insolvent,
|
|
(vii)
|
we are subject to a judgment, including an arbitration award against us, of greater than $100,000, and such judgment remains unvacated, unbonded or unstayed for a period of 45 calendar days,
|
the holders of the Series C Preferred Stock are entitled, among other rights, to redeem their shares of Series C Preferred Stock at any time for greater than their stated value or increase the dividend rate on their shares of Series C Preferred Stock to 18%.
Because we failed to complete a financing or series of related financings by February 12, 2014 that resulted in gross proceeds to us of at least $3 million at a valuation of at least $30 million, and because we failed to maintain the listing of our common stock on a trading market for more than five trading days in any twelve month period at any time after February 12, 2014, the conversion price of the Series C Preferred Stock was reduced to $1.50 per share.
In the event of our liquidation or winding up of affairs, the holders of the Series C Preferred Stock will be entitled to a liquidation preference of the stated value plus any accrued but unpaid dividends or any other fees due the holder. The shares of the Series C Preferred Stock rank senior to the rights of the common stock and all other securities exercisable or convertible into shares of common stock.
Any holder of Series C Preferred Stock is entitled at any time to convert any whole or partial number of shares of Series C Preferred Stock into shares of our common stock at a price of $1.50 per share. The Series C Preferred Stock is subject to full ratchet anti-dilution price protection upon the issuance of equity or equity-linked securities at an effective common stock purchase price of less than $1.50 per share as well as other customary anti-dilution protection.
In the event we issue any equity or equity-linked securities with terms more favorable than those of the Series C Preferred Stock, any holder of the Series C Preferred Stock may request to amend the terms of such holder’s Series C Preferred Stock to be equivalent to the terms of such issued equity or equity-linked securities, subject to certain exempted issuances.
The holders of the Series C Preferred Stock vote together with the holders of our common stock on an as-converted basis, but may not vote the Series C Preferred Stock in excess of the beneficial ownership limitation of the Series C Preferred Stock. The beneficial ownership limitation is 4.99% of our then outstanding shares of common stock following such conversion or exercise, which may be increased to up to 9.99% of our then outstanding shares of common stock following such conversion or exercise upon the request of an individual holder. The beneficial ownership limitation is determined on an individual holder basis, such that the as-converted number of shares of one holder is not included in the shares outstanding when calculating the limitation for a different holder. In addition, absent the approval of holders representing at least 67% of the outstanding shares of the Series C Preferred Stock, which holders must include Alpha Capital Anstalt, so long as Alpha Capital Anstalt holds not less than $100,000 of Series C Preferred Stock, we may not (i) increase the number of authorized shares of preferred stock, (ii) amend our charter documents, including the terms of the Series C Preferred Stock, in any manner adverse to the holders of the Series C Preferred Stock, including authorizing or creating any class of stock ranking senior to, or otherwise pari passu with, the shares of Series C Preferred Stock as to dividends, redemption or distribution of assets upon a liquidation, or (iii) perform certain covenants, including:
|
●
|
incur additional indebtedness;
|
|
●
|
permit liens on assets;
|
|
●
|
repay, repurchase or otherwise acquire more than a de minimis number of shares of capital stock;
|
|
●
|
pay cash dividends to our stockholders; and
|
|
●
|
engage in transactions with affiliates.
|
Pursuant to the securities purchase agreement for the Series C Preferred Stock, each holder of Series C Preferred Stock has a right to participate in any of our financings, subject to certain exceptions, on a pro-rata basis, for a period expiring 12 months after the date our registration statement on Form S-1 became effective on June 23, 2014.
Warrant
Five-Year Warrants
In connection with the private placement of our Series C Preferred Stock, we issued to the holders of our Series C Preferred Stock warrants to purchase up to an aggregate of 1,330,629 shares of common stock at an exercise price of $2.61 per share. The warrants contain full ratchet anti-dilution price protection upon the issuance of equity or equity-linked securities at an effective common stock purchase price of less than $2.61 per share as well as other customary anti-dilution protection. The warrants are exercisable for cash; or if at any time after six months from the issuance date, there is no effective registration statement registering the resale, or no current prospectus available for the resale, of the shares of common stock underlying the warrants, the warrants may be exercised by means of a “cashless exercise”. As a result of an amendment to the conversion price of our Series C Preferred Stock, the full-ratchet anti-dilution protection provision of the warrants decreased the exercise price of the warrants from $2.61 per share to $1.50 per share and increased the aggregate number of shares issuable under the warrants to 2,315,301.
Five-Year Amendment Warrants
As consideration for (i) extending the termination date of the securities purchase agreement and (ii) extending the filing and effectiveness dates for the filing of the registration statement pursuant to the registration rights agreement related to our Series C Preferred Stock, we issued to the holders of our Series C Preferred Stock that purchased shares of our Series C Preferred Stock prior to the July 15, 2013 closing warrants to purchase up to an aggregate of 289,730 shares of common stock. The terms of these warrants are identical to the Five-Year Warrants described above. As a result of an amendment to the conversion price or our Series C Preferred Stock, the full-ratchet anti-dilution protection provision of the warrants decreased the exercise price of the warrants from $2.61 per share to $1.50 per share and increased the aggregate number of shares issuable under the warrants to 504,130.
October 2013 Five-Year Amendment Warrants
As consideration for amending the terms of the securities purchase agreement to permit our private placement of our common stock and warrants in December 2013, we issued to the holders of our Series C Preferred Stock warrants to purchase up to an aggregate of 332,684 shares of common stock. The terms of these warrants are identical to the Five-Year Warrants described above. As a result of an amendment to the conversion price or our Series C Preferred Stock, the full-ratchet anti-dilution protection provision of the warrants decreased the exercise price of the warrants from $2.61 per share to $1.50 per share and increased the aggregate number of shares issuable under the warrants to 578,870.
December 2013 Five-Year Warrants
In connection with the private placement of our common stock in December 2013 and January 2014, we issued to the investors participating in the private placement warrants to purchase up to an aggregate of 177,948 shares of common stock at an exercise price of $3.67 per share. The warrants contain customary anti-dilution protections. The warrants are exercisable for cash; or if at any time after six months from the issuance date, there is no effective registration statement registering the resale, or no current prospectus available for the resale, of the shares of common stock underlying the warrants, the warrants may be exercised by means of a “cashless exercise”.
April 2014 Five-Year Warrants
In connection with the private placement of our common stock in April 2014, we issued to the investors participating in the private placement warrants to purchase up to an aggregate of 114,880 shares of common stock at an exercise price of $3.75 per share. The warrants contain customary anti-dilution protections. The warrants are exercisable for cash; or if at any time after six months from the issuance date, there is no effective registration statement registering the resale, or no current prospectus available for the resale, of the shares of common stock underlying the warrants, the warrants may be exercised by means of a “cashless exercise”.
August 2014 Five-Year Warrants
In connection with the private placement of our common stock in August and September 2014, we issued to the investors participating in the private placement warrants to purchase up to an aggregate of 190,600 shares of common stock at an exercise price of $2.75 per share. The warrants contain customary anti-dilution protections. The warrants are exercisable for cash; or if at any time after six months from the issuance date, there is no effective registration statement registering the resale, or no current prospectus available for the resale, of the shares of common stock underlying the warrants, the warrants may be exercised by means of a “cashless exercise”.
December 2014 “A” and “B” Warrants
In connection with the private placement of our common stock in December 2014 and January, February and March 2015, we issued to the investors participating in the private placement warrants to purchase up to an aggregate of 2,405,400 shares of common stock. Of the warrants issued, 1,603,600 warrants are exercisable at $2.50 per share expiring July 31, 2015 (the “‘A’ Warrants”) and 801,800 warrants are exercisable at $3.75 per share expiring March 31, 2020 (the “‘B’ Warrants”). The warrants contain customary anti-dilution protections. The warrants are exercisable for cash; or if at any time after six months from the issuance date, there is no effective registration statement registering the resale, or no current prospectus available for the resale, of the shares of common stock underlying the warrants, the warrants may be exercised by means of a “cashless exercise”.
Series A Placement Agent Warrant
As consideration for serving as our placement agent in connection with the private placement of Series A Preferred Stock, we issued to Laidlaw & Company (UK) Ltd. a seven-year warrant to purchase up to 35,076 shares of common stock at an exercise price of $1.84 per share. The terms of this warrant are otherwise identical to the Five-Year Warrants described above.
Series B Placement Agent Warrant
As consideration for serving as our placement agent in connection with the private placement of Series B Preferred Stock, we issued to Laidlaw & Company (UK) Ltd. a seven-year warrant to purchase up to 30,755 shares of common stock at an exercise price of $2.02 per share. The terms of this warrant are otherwise identical to the Five-Year Warrants described above.
Series C Placement Agent Warrant
As consideration for serving as our placement agent in connection with the private placement of Series C Preferred Stock, we issued to Laidlaw & Company (UK) Ltd. a warrant to purchase up to 177,057 shares of common stock. The terms of this warrant are identical to the Five-Year Warrants described above. As a result of an amendment to the conversion price or our Series C Preferred Stock, the full-ratchet anti-dilution protection provision of the warrants decreased the exercise price of the warrants from $2.61 per share to $1.50 per share and increased the aggregate number of shares issuable under the warrants to 308,079.
Par Value Warrant
As consideration for providing general financial advisory services, we issued to Jamess Capital Group LLC a seven-year warrant to purchase up to 383,320 shares of common stock at an exercise price of $0.001 per share. The terms of this warrant are otherwise identical to the Five-Year Warrants described above.
Common Stock Placement Agent Warrants
As consideration for serving as our placement agent in connection with a private placement of our common stock, we issued to Laidlaw & Company (UK) Ltd. a warrant to purchase up to 40,327 shares of common stock. The terms of this warrant are identical to the December 2013 Five-Year Warrants described above.
As consideration for serving as our placement agent in connection with a private placement of our common stock, we issued to Laidlaw & Company (UK) Ltd. a warrant to purchase up to 22,976 shares of common stock. The terms of this warrant are identical to the April 2014 Five-Year Warrants described above.
As consideration for serving as our placement agent in connection with a private placement of our common stock, we issued to Laidlaw & Company (UK) Ltd. a warrant to purchase up to 38,120 shares of common stock. The terms of this warrant are identical to the August 2014 Five-Year Warrants described above.
As consideration for serving as our placement agent in connection with a private placement of our common stock, we issued to Laidlaw & Company (UK) Ltd. a warrant to purchase up to 400,900 shares of common stock. The terms of this warrant are identical to the “B” Warrants described above.
Registration Rights
On February 6, 2013, in connection with our private placement of our Series C Preferred Stock and warrants, we entered into a registration rights agreement with the purchasers pursuant to which we agreed to provide certain registration rights with respect to the common stock issuable upon conversion of our Series C Preferred Stock and exercise of the warrants issued to holders of our Series C Preferred Stock. Specifically, we agreed to file a registration statement with the Securities and Exchange Commission covering the resale of the common stock issuable upon conversion of the Series C Preferred Stock and exercise of the warrants on or before July 22, 2013 and to cause such registration statement to be declared effective by the Securities and Exchange Commission, in the event that the registration statement is not reviewed by the Securities and Exchange Commission, within five trading days after we are notified that registration statement is not being reviewed by the Securities and Exchange Commission, and by November 22, 2013 in the event that the registration statement is reviewed by the Securities and Exchange Commission and the Securities and Exchange Commission issues comments.
If (i) the registration statement is not filed by July 22, 2013, (ii) the registration statement is not declared effective by the Securities and Exchange Commission within five trading days after we are notified that registration statement is not being reviewed by the Securities and Exchange Commission, in the case of a no review, (iii) the registration statement is not declared effective by the Securities and Exchange Commission by November 22, 2013 in the case of a review by the Securities and Exchange Commission pursuant to which the Securities and Exchange Commission issues comments or (iv) the registration statement ceases to remain continuously effective for more than 20 consecutive calendar days or more than an aggregate of 45 calendar days during any 12-month period after its first effective date, then we are subject to liquidated damage payments to the holders of the shares sold in the private placement in an amount equal to 0.25% of the aggregate purchase price paid by such purchasers per month of delinquency. Notwithstanding the foregoing, (i) the maximum aggregate liquidated damages due under the registration rights agreement shall be 3% of the aggregate purchase price paid by the purchasers, and (ii) if any partial amount of liquidated damages remains unpaid for more than seven days, we shall pay interest of 18% per annum, accruing daily, on such unpaid amount.
Pursuant to the registration rights agreement, we must maintain the effectiveness of the registration statement from the effective date until the date on which all securities registered under the registration statement have been sold, or are otherwise able to be sold pursuant to Rule 144 without volume or manner-of-sale restrictions, subject to the our right to suspend or defer the use of the registration statement in certain events.
On each of December 31, 2013, April 4, 2014, August 15, 2014, and December 19, 2014, in connection with concurrent private placements of our common stock and warrants, we entered into registration rights agreements with the purchasers in such private placements pursuant to which we agreed to provide certain registration rights with respect to the common stock issued to the investors participating in such private placements and the common stock issuable upon exercise of the related warrants issued such investors. Specifically, we agreed to file a registration statement with the Securities and Exchange Commission covering the resale of the shares of common stock issued pursuant to the private placement and issuable upon the exercise of the warrants within 45 days of the termination date of such private placement and to cause such registration statement to be declared effective by the Securities and Exchange Commission, in the event that the registration statement is not reviewed by the Securities and Exchange Commission, within 30 calendar days after we are notified that registration statement is not being reviewed by the Securities and Exchange Commission, and within 180 calendar days of the initial filing date of the registration statement in the event that the registration statement is reviewed by the Securities and Exchange Commission and the Securities and Exchange Commission issues comments.
If (i) the registration statement is not filed within 45 days of the applicable termination date, (ii) the registration statement is not declared effective by the Securities and Exchange Commission within 30 calendar days after we are notified that registration statement is not being reviewed by the Securities and Exchange Commission, in the case of a no review, (iii) the registration statement is not declared effective by the Securities and Exchange Commission within 180 calendar days of the initial filing date of the registration statement in the case of a review by the Securities and Exchange Commission pursuant to which the Securities and Exchange Commission issues comments or (iv) the registration statement ceases to remain continuously effective for more than 10 consecutive calendar days or more than an aggregate of 15 calendar days during any 12-month period after its first effective date, then we are subject to liquidated damage payments to the holders of the shares sold in the private placement in an amount equal to 1.0% of the aggregate purchase price paid by such purchasers per month of delinquency, provided, however, that we will not be required to make any payments any of the foregoing events occurred at such time that all securities registered or to be registered in the registration statement are eligible for resale pursuant to Rule 144 (without volume restrictions or current public information requirements) promulgated by the Securities and Exchange Commission pursuant to the Securities Act of 1933, as amended and provided, further, that we will not be required to make any liquidated damage payments with respect to any securities registered or to be registered in the registration statement that we are unable to register due to limits imposed by the Securities and Exchange Commission’s interpretation of Rule 415 under the Securities Act of 1933, as amended. Notwithstanding the foregoing, (i) the maximum aggregate liquidated damages due under the registration rights agreements dated December 31, 2013, April 4, 2014 and August 15, 2014 shall be 3% of the aggregate purchase price paid by the purchasers, (ii) the maximum aggregate liquidated damages due under the registration rights agreement dated December 19, 2014 shall be 6% of the aggregate purchase price paid by the purchasers and (iii) if any partial amount of liquidated damages remains unpaid for more than seven days, we shall pay interest of 18% per annum, accruing daily, on such unpaid amount.
Pursuant to the registration rights agreement, we must maintain the effectiveness of the registration statement from the effective date until the date on which all securities registered under the registration statement have been sold, or are otherwise able to be sold pursuant to Rule 144 without volume or manner-of-sale restrictions, subject to the our right to suspend or defer the use of the registration statement in certain events.
Delaware Anti-Takeover Law and Provisions of our Amended and Restated Certificate of Incorporation and By-laws
Section 203 of the Delaware General Corporation Law, in general, prohibits a business combination between a corporation and an interested stockholder within three years of the time such stockholder became an interested stockholder, unless:
|
●
|
prior to such time the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder;
|
|
●
|
upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, exclusive of shares owned by directors who are also officers and by certain employee stock plans; or
|
|
●
|
at or subsequent to such time, the business combination is approved by the board of directors and authorized by the affirmative vote at a stockholders’ meeting of at least 66 2/3% of the outstanding voting stock that is not owned by the interested stockholder.
|
The term “business combination” is defined to include, among other transactions between an interested stockholder and a corporation or any direct or indirect majority owned subsidiary thereof: a merger or consolidation; a sale, lease, exchange, mortgage, pledge, transfer or other disposition (including as part of a dissolution) of assets having an aggregate market value equal to 10% or more of either the aggregate market value of all assets of the corporation on a consolidated basis or the aggregate market value of all the outstanding stock of the corporation; certain transactions that would result in the issuance or transfer by the corporation of any of its stock to the interested stockholder; certain transactions that would increase the interested stockholder’s proportionate share ownership of the stock of any class or series of the corporation or such subsidiary; and any receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation or any such subsidiary.
In general, Section 203 defines an “interested stockholder” as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with, or controlling, or controlled by, the entity or person. The term “owner” is broadly defined to include any person that individually, with or through that person’s affiliates or associates, among other things, beneficially owns the stock, or has the right to acquire the stock, whether or not the right is immediately exercisable, under any agreement or understanding or upon the exercise of warrants or options or otherwise or has the right to vote the stock under any agreement or understanding, or has an agreement or understanding with the beneficial owner of the stock for the purpose of acquiring, holding, voting or disposing of the stock.
The restrictions in Section 203 do not apply to corporations that have elected, in the manner provided in Section 203, not to be subject to Section 203 of the Delaware General Corporation Law or, with certain exceptions, which do not have a class of voting stock that is listed on a national securities exchange or held of record by more than 2,000 stockholders. Our Amended and Restated Certificate of Incorporation and By-laws do not opt out of Section 203.
Section 203 could delay or prohibit mergers or other takeover or change in control attempts with respect to us and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Provisions of our Amended and Restated Certificate of Incorporation and By-laws may delay or discourage transactions involving an actual or potential change in our control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, our Amended and Restated Certificate of Incorporation and By-laws:
|
●
|
do not provide for cumulative voting rights (therefore allowing the holders of a majority of the shares of common stock entitled to vote in any election of directors to elect all of the directors standing for election, if they should so choose);
|
|
●
|
provide that special meetings of our stockholders may be called only by our board of directors, chairman, chief executive officer, president or secretary; and
|
|
●
|
provide advance notice provisions with which a stockholder who wishes to nominate a director or propose other business to be considered at a stockholder meeting must comply.
|
Indemnification of Directors and Officers
Pursuant to Section 145 of the Delaware General Corporation Law, a corporation has the power to indemnify its directors and officers against expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with a third-party action, other than a derivative action, and against expenses actually and reasonably incurred in the defense or settlement of a derivative action, provided that there is a determination that the individual acted in good faith and in a manner reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe the individual’s conduct was unlawful. Such determination will be made, in the case of an individual who is a director or officer at the time of such determination:
|
●
|
by a majority of the disinterested directors, even though less than a quorum;
|
|
●
|
by a committee of such directors designated by a majority vote of such directors, even though less than a quorum;
|
|
●
|
if there are no disinterested directors, or if such directors so direct, by independent legal counsel; or
|
|
●
|
by a majority vote of the stockholders, at a meeting at which a quorum is present.
|
Without court approval, however, no indemnification may be made in respect of any derivative action in which such individual is adjudged liable to the corporation.
The Delaware General Corporation Law requires indemnification of directors and officers for expenses relating to a successful defense on the merits or otherwise of a derivative or third-party action.
The Delaware General Corporation Law permits a corporation to advance expenses relating to the defense of any proceeding to directors and officers contingent upon such individuals’ commitment to repay any advances unless it is determined ultimately that such individuals are entitled to be indemnified.
Under the Delaware General Corporation Law, the rights to indemnification and advancement of expenses provided in the law are non-exclusive, in that, subject to public policy issues, indemnification and advancement of expenses beyond that provided by statute may be provided by by law, agreement, vote of stockholders, disinterested directors or otherwise.
Limitation of Personal Liability of Directors
The Delaware General Corporation Law provides that a corporation’s certificate of incorporation may include a provision limiting the personal liability of a director to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director. However, no such provision can eliminate or limit the liability of a director for:
Our Amended and Restated Certificate of Incorporation provides that our directors will not be personally liable to us or any of our stockholders for monetary damages for breach of fiduciary duty as a director to the fullest extent permitted by the Delaware General Corporation Law.
Disclosure of Commission Position on Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to our directors, officers and persons controlling us, we have been advised that it is the Securities and Exchange Commission’s opinion that such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable.
PLAN OF DISTRIBUTION
Each Selling Stockholder (the “Selling Stockholders”) of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on the OTCQB or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A Selling Stockholder may use any one or more of the following methods when selling securities:
|
●
|
ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
|
●
|
block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
|
●
|
purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
|
●
|
an exchange distribution in accordance with the rules of the applicable exchange;
|
|
|
●
|
privately negotiated transactions;
|
|
|
●
|
settlement of short sales entered into after the effective date of the registration statement of which this prospectus is a part;
|
|
|
●
|
in transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security;
|
|
|
●
|
through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
|
|
|
●
|
a combination of any such methods of sale; or
|
|
|
●
|
any other method permitted pursuant to applicable law.
|
The Selling Stockholders may also sell securities under Rule 144 under the Securities Act of 1933, as amended, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
In connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Stockholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker- dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Stockholder has informed us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities. In no event shall any broker- dealer receive fees, commissions and markups which, in the aggregate, would exceed eight percent (8%).
We are required to pay certain fees and expenses incurred by us incident to the registration of the securities. We have agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
Because Selling Stockholders may be deemed to be “underwriters” within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements of the Securities Act including Rule 172 thereunder. In addition, any securities covered by this prospectus which qualify for sale pursuant to Rule 144 under the Securities Act may be sold under Rule 144 rather than under this prospectus. The Selling Stockholders have advised us that there is no underwriter or coordinating broker acting in connection with the proposed sale of the resale securities by the Selling Stockholders.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for us to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of securities of the common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
LEGAL MATTERS
Haynes and Boone, LLP, New York, New York, will pass upon the validity of the shares of our common stock offered by the selling stockholders under this prospectus.
EXPERTS
Our financial statements as of December 31, 2014 and 2013 and for the years then ended included in this prospectus have been audited by Liggett, Vogt & Webb, P.A., an independent registered public accounting firm, as stated in its report appearing in the registration statement, and are included in reliance upon the report of such firm given upon its authority as experts in accounting and auditing.
Change in Our Public Accounting Firm
On May 28, 2013, we advised Rosenberg Rich Baker Berman & Company that it was dismissed as our independent registered public accounting firm. On June 11, 2013, we engaged Liggett, Vogt & Webb P.A., as our independent registered public accounting firm. The decision to dismiss Rosenberg Rich Baker Berman & Company as our independent registered public accounting firm was approved by our board of directors.
The report of Rosenberg Rich Baker Berman & Company on our financial statements for the fiscal years ended December 31, 2011 and December 31, 2012 did not contain an adverse opinion or disclaimer of opinion, nor was it qualified or modified as to uncertainty, audit scope or accounting principles, except that the report raised substantial doubt as to our ability to continue as a going concern.
From our inception through May 28, 2013, there was a disagreement with Rosenberg Rich Baker Berman & Company with regard to the application of accounting principles to certain anti-dilution provisions embedded within our Series C Preferred Stock and related warrants issued during the three months ended March 31, 2013. This disagreement was not discussed by our board of directors. We authorized Rosenberg Rich Baker Berman & Company to respond fully to the inquiries of Liggett, Vogt & Webb P.A. concerning the application of accounting principles with certain anti-dilution provisions embedded within our Series C Preferred Stock and related warrants issued during the three months ended March 31, 2013.
From our inception through date of engagement (June 11, 2013), we did not consult Liggett, Vogt & Webb P.A. regarding either: (i) the application of accounting principles to a specific completed or contemplated transaction, or the type of audit opinion that might be rendered on our financial statements; or (ii) any matter that was the subject of a disagreement as defined in Item 304(a)(1)(iv) of Regulation S-K.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the Securities and Exchange Commission a registration statement on Form S-1, together with any amendments and related exhibits, under the Securities Act of 1933, as amended, with respect to our shares of common stock offered by this prospectus. The registration statement contains additional information about us and our shares of common stock that the selling stockholders are offering in this prospectus.
We are required to file annual, quarterly and current reports and other information with the Securities and Exchange Commission under the Securities Exchange Act of 1934, as amended. Our Securities and Exchange Commission filings are available to the public over the Internet at the Securities and Exchange Commission’s website at http://www.sec.gov. You may also read and copy any document we file at the Securities and Exchange Commission’s public reference room located at 100 F Street, N.E., Washington, D.C. 20549. Please call the Securities and Exchange Commission at 1-800-SEC-0330 for further information on the public reference rooms and their copy charges. Access to those electronic filings is available as soon as practicable after filing with the Securities and Exchange Commission. You may also request a copy of those filings, excluding exhibits, from us at no cost. Any such request should be addressed to us at: 8441 Wayzata Blvd., Suite 240, Minneapolis, Minnesota 55426, Attention: Kenneth L. Londoner, Executive Chairman.
BIOSIG TECHNOLOGIES, INC.
|
Report of Independent Registered Public Accounting Firm
|
F-2
|
|
Balance Sheets as of December 31, 2014 and 2013
|
F-3
|
|
Statements of Operations for the Years Ended December 31, 2014 and 2013
|
F-4
|
|
Statements of Changes in Stockholders’ Deficit for the Years Ended December 31, 2014 and 2013
|
F-5
|
|
Statements of Cash Flows for the Years Ended December 31, 2014 and 2013
|
F-7
|
|
Notes to Financial Statements
|
F-8
|
|
|
|
|
Condensed Balance Sheets as of March 31, 2015 (unaudited) and December 31, 2014
|
F-26
|
|
Condensed Statements of Operations for the Three Months Ended March 31, 2015 and 2014 (unaudited)
|
F-27
|
|
Condensed Statement of Stockholders’ Deficit for the Three Months Ended March 31, 2015 (unaudited)
|
F-28
|
|
Condensed Statements of Cash Flows for the Three Months Ended March 31, 2015 and 2014 (unaudited)
|
F-29
|
|
Notes to Condensed Financial Statements (unaudited)
|
F-30
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
BioSig Technologies, Inc.
We have audited the accompanying balance sheet of BioSig Technologies. Inc. (“the Company”) as of December 31, 2014 and 2013, and the related statements of operations, stockholders’ deficit, and cash flows for the years then ended. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of BioSig Technologies, Inc. as of December 31, 2014 and 2013, and the results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred losses from operations since its inception and has a net stockholders’ deficiency. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
|
/s/ Liggett, Vogt & Webb, P.A.
|
|
|
Liggett, Vogt & Webb, P.A.
|
February 20, 2015
New York, New York
BIOSIG TECHNOLOGIES, INC.
BALANCE SHEETS
DECEMBER 31, 2014 AND 2013
|
2014
|
2013
|
|||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash
|
$
|
239,781
|
$
|
302,187
|
||||
|
Prepaid expenses
|
75,537
|
-
|
||||||
|
Total current assets
|
315,318
|
302,187
|
||||||
|
Property and equipment, net
|
13,020
|
24,866
|
||||||
|
Other assets:
|
||||||||
|
Deposits
|
25,000
|
25,000
|
||||||
|
Total assets
|
$
|
353,338
|
$
|
352,053
|
||||
|
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued expenses, including $40,293 and $92,308 to related parties as of December 31, 2014 and 2013, respectively
|
$
|
554,026
|
$
|
819,330
|
||||
|
Stock based payable
|
226,305
|
-
|
||||||
|
Advances, related party
|
-
|
30,781
|
||||||
|
Liability to placement agent
|
-
|
52,800
|
||||||
|
Redeemable Series A Preferred Stock, 184.4 shares issued and outstanding, liquidation preference of $922,000, net of debt discount of $37,399 as of December 31, 2013
|
-
|
884,601
|
||||||
|
Redeemable Series B Preferred Stock, 177.5 shares issued and outstanding, liquidation preference of $887,500, net of debt discount of $72,478 as of December 31, 2013
|
-
|
815,022
|
||||||
|
Dividends payable
|
445,069
|
414,967
|
||||||
|
Total current liabilities
|
1,225,400
|
3,017,501
|
||||||
|
Series C 9% Convertible Preferred stock, 2,711 and 2,781 shares issued and outstanding liquidation preference of $2,711,000 and $2,781,000, net of debt discount of $-0- and $483,893, respectively
|
2,711,000
|
2,297,107
|
||||||
|
Stockholders' deficit
|
||||||||
|
Preferred stock, $0.001 par value, authorized 1,000,000 shares, designated 200 shares of Series A, 600 shares of Series B and 4,200 shares of Series C Preferred Stock
|
||||||||
|
Common stock, $0.001 par value, authorized 50,000,000 shares, 11,179,266 and 8,412,101 issued and outstanding as of December 31, 2014 and 2013, respectively
|
11,179
|
8,412
|
||||||
|
Additional paid in capital
|
19,186,163
|
9,036,038
|
||||||
|
Accumulated deficit
|
(22,780,404
|
)
|
(14,007,005
|
)
|
||||
|
Total stockholders' deficit
|
(3,583,062
|
)
|
(4,962,555
|
)
|
||||
|
Total liabilities and stockholders' deficit
|
$
|
353,338
|
$
|
352,053
|
||||
BIOSIG TECHNOLOGIES, INC.
STATEMENTS OF OPERATIONS
|
Year ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
Operating expenses:
|
||||||||
|
Research and development
|
$ | 547,996 | $ | 992,207 | ||||
|
General and administrative
|
7,304,440 | 5,229,252 | ||||||
|
Depreciation
|
15,809 | 17,059 | ||||||
|
Total operating expenses
|
7,868,245 | 6,238,518 | ||||||
|
Loss from operations
|
(7,868,245 | ) | (6,238,518 | ) | ||||
|
Other income (expense):
|
||||||||
|
Interest income (expense)
|
(11,025 | ) | (70,061 | ) | ||||
|
Financing costs
|
(593,770 | ) | (3,496,052 | ) | ||||
|
Loss before income taxes
|
(8,473,040 | ) | (9,804,631 | ) | ||||
|
Income taxes (benefit)
|
- | - | ||||||
|
Net loss
|
(8,473,040 | ) | (9,804,631 | ) | ||||
|
Preferred stock dividend
|
(300,359 | ) | (297,215 | ) | ||||
|
NET LOSS AVAILABLE TO COMMON STOCKHOLDERS
|
$ | (8,773,399 | ) | $ | (10,101,846 | ) | ||
|
Net loss per common share, basic and diluted
|
$ | (0.91 | ) | $ | (1.23 | ) | ||
|
Weighted average number of common shares outstanding, basic and diluted
|
9,650,275 | 8,187,648 | ||||||
BIOSIG TECHNOLOGIES, INC.
STATEMENT OF STOCKHOLDERS' DEFICIT
TWO YEARS ENDED DECEMBER 31, 2014
|
Additional
|
||||||||||||||||||||
|
Common stock
|
Paid in
|
Accumulated
|
||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
Balance, December 31, 2012
|
8,166,238 | $ | 8,166 | $ | 833,647 | $ | (3,905,159 | ) | $ | (3,063,346 | ) | |||||||||
|
Common stock issued for services rendered
|
21,412 | 22 | 44,729 | - | 44,751 | |||||||||||||||
|
Common stock issued as payment for accrued interest to note holders at $2.09 per share
|
8,941 | 9 | 18,668 | - | 18,677 | |||||||||||||||
|
Beneficial conversion feature in connection with note payable
|
- | - | 20,000 | - | 20,000 | |||||||||||||||
|
Beneficial conversion feature and warrants issued in connection with the Series C Preferred Stock
|
- | - | 2,404,830 | - | 2,404,830 | |||||||||||||||
|
Fair value of warrants issued to Series C investors for certificate of designation amendment
|
- | - | 1,074,833 | - | 1,074,833 | |||||||||||||||
|
Fair value of warrants issued for services
|
- | - | 916,677 | - | 916,677 | |||||||||||||||
|
Common stock issued in settlement of related party note and advances payable
|
93,061 | 93 | 228,415 | - | 228,508 | |||||||||||||||
|
Sale of common stock
|
122,449 | 122 | 247,052 | 247,174 | ||||||||||||||||
|
Fair value of vested options
|
- | - | 3,247,187 | - | 3,247,187 | |||||||||||||||
|
Preferred stock dividend
|
- | - | - | (297,215 | ) | (297,215 | ) | |||||||||||||
|
Net loss
|
- | - | - | (9,804,631 | ) | (9,804,631 | ) | |||||||||||||
|
Balance, December 31, 2013
|
8,412,101 | $ | 8,412 | $ | 9,036,038 | $ | (14,007,005 | ) | $ | (4,962,555 | ) | |||||||||
BIOSIG TECHNOLOGIES, INC.
STATEMENT OF STOCKHOLDERS' DEFICIT
TWO YEARS ENDED DECEMBER 31, 2014
|
Additional
|
||||||||||||||||||||
|
Common stock
|
Paid in
|
Accumulated
|
||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
Balance, December 31, 2013
|
8,412,101 | $ | 8,412 | $ | 9,036,038 | $ | (14,007,005 | ) | $ | (4,962,555 | ) | |||||||||
|
Sale of common stock
|
956,179 | 956 | 1,968,454 | - | 1,969,410 | |||||||||||||||
|
Common stock issued for services
|
654,000 | 654 | 1,634,346 | - | 1,635,000 | |||||||||||||||
|
Common stock issued in settlement of related party debt
|
26,000 | 26 | 64,974 | - | 65,000 | |||||||||||||||
|
Common stock issued upon conversion of Series A preferred stock and accrued dividends at $1.84 per share
|
577,901 | 578 | 1,062,753 | - | 1,063,331 | |||||||||||||||
|
Common stock issued upon conversion of Series B preferred stock and accrued dividends at $2.02 per share
|
493,818 | 494 | 997,032 | - | 997,526 | |||||||||||||||
|
Common stock issued upon conversion of Series C preferred stock and accrued dividends at $1.50 per share
|
59,267 | 59 | 88,841 | - | 88,900 | |||||||||||||||
|
Donated capital
|
- | - | 87,500 | - | 87,500 | |||||||||||||||
|
Equity warrants issued to placement agent for sale of common stock
|
- | - | 52,800 | - | 52,800 | |||||||||||||||
|
Fair value of vested options
|
- | - | 4,193,425 | - | 4,193,425 | |||||||||||||||
|
Preferred stock dividend
|
- | - | - | (300,359 | ) | (300,359 | ) | |||||||||||||
|
Net loss
|
- | - | - | (8,473,040 | ) | (8,473,040 | ) | |||||||||||||
|
Balance, December 31, 2014
|
11,179,266 | $ | 11,179 | $ | 19,186,163 | $ | (22,780,404 | ) | $ | (3,583,062 | ) | |||||||||
See the accompanying notes to the financial statements
BIOSIG TECHNOLOGIES, INC.
STATEMENTS OF CASH FLOWS
|
Year ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net loss
|
$
|
(8,473,040
|
)
|
$
|
(9,804,631
|
)
|
||
|
Adjustments to reconcile net loss to cash used in operating activities:
|
||||||||
|
Depreciation
|
15,809
|
17,059
|
||||||
|
Amortization of debt discount
|
593,770
|
2,441,220
|
||||||
|
Stock based compensation
|
5,743,425
|
3,305,063
|
||||||
|
Fair value of warrants issued in connection with Series C preferred stock modifications
|
-
|
1,074,833
|
||||||
|
Fair value of warrants issued for services
|
-
|
837,243
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Prepaid expenses
|
8,715
|
20,000
|
||||||
|
Accounts payable
|
(110,844
|
)
|
349,809
|
|||||
|
Stock based payable
|
226,305
|
-
|
||||||
|
Deferred rent payable
|
(1,212
|
)
|
(3,055
|
)
|
||||
|
Net cash used in operating activities
|
(1,997,072
|
)
|
(1,762,459
|
)
|
||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Purchase of property and equipment
|
(3,963
|
)
|
(11,716
|
)
|
||||
|
Net cash used in investing activity
|
(3,963
|
)
|
(11,716
|
)
|
||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Net proceeds from the sale of Series C preferred stock and warrants
|
-
|
1,768,410
|
||||||
|
Proceeds from sale of common stock
|
1,969,410
|
299,974
|
||||||
|
Payments of related party notes
|
-
|
(30,000
|
)
|
|||||
|
Net repayments of related party advances
|
(30,781
|
)
|
13,741
|
|||||
|
Net cash provided by financing activities
|
1,938,629
|
2,052,125
|
||||||
|
Net (decrease) increase in cash and cash equivalents
|
(62,406
|
)
|
277,950
|
|||||
|
Cash and cash equivalents, beginning of the period
|
302,187
|
24,237
|
||||||
|
Cash and cash equivalents, end of the period
|
$
|
239,781
|
$
|
302,187
|
||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Cash paid during the period for interest
|
$
|
-
|
$
|
-
|
||||
|
Cash paid during the period for income taxes
|
$
|
-
|
$
|
-
|
||||
|
Non cash investing and financing activities:
|
||||||||
|
Common stock issued upon conversion of Series A preferred stock and accrued dividends
|
$
|
1,063,331
|
$
|
-
|
||||
|
Common stock issued upon conversion of Series B preferred stock and accrued dividends
|
$
|
997,526
|
$
|
-
|
||||
|
Common stock issued upon conversion of Series C preferred stock and accrued dividends
|
$
|
88,900
|
$
|
-
|
||||
|
Common stock options for future services, related party
|
$
|
85,000
|
$
|
-
|
||||
|
Common stock options in settlement of accounts payable, related party
|
$
|
65,000
|
$
|
-
|
||||
|
Related party donated capital
|
$
|
87,500
|
$
|
-
|
||||
|
Common stock issued in settlement of related party note and advances payable
|
$
|
-
|
$
|
228,508
|
||||
|
Common stock issued in settlement of accrued interest
|
$
|
-
|
$
|
18,677
|
||||
|
Convertible bridge notes payable exchanged for preferred shares
|
$
|
-
|
$
|
600,000
|
||||
See the accompanying notes to the financial statements
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 1 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A summary of the significant accounting policies applied in the preparation of the accompanying financial statements follows.
Business and organization
BioSig Technologies Inc. (the “Company”) was initially incorporated on February 24, 2009 under the laws of the State of Nevada and subsequently re-incorporated in the state of Delaware in 2011. The Company and its efforts are principally devoted to improving the quality of cardiac recordings obtained during ablation of atrial fibrillation (AF) and ventricular tachycardia (VT). The Company has not generated any revenue to date and consequently its operations are subject to all risks inherent in the establishment of a new business enterprise.
Revenue Recognition
The Company recognizes revenue in accordance with Accounting Standards Codification subtopic 605-10, Revenue Recognition (“ASC 605-10”) which requires that four basic criteria must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) the selling price is fixed and determinable; and (4) collectability is reasonably assured. Determination of criteria (3) and (4) are based on management's judgments regarding the fixed nature of the selling prices of the products delivered and the collectability of those amounts. Provisions for discounts and rebates to customers, estimated returns and allowances, and other adjustments are provided for in the same period the related sales are recorded.
Use of estimates
The preparation of financial statements in accordance with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and expenses and disclosures of contingent assets and liabilities at the date of the financial statements. Actual results could differ from those estimates. Significant estimates include the useful life of fixed assets and assumptions used in the fair value of stock-based compensation.
Concentrations of Credit Risk
Financial instruments and related items, which potentially subject the Company to concentrations of credit risk, consist primarily of cash and cash equivalents. The Company places its cash and temporary cash investments with credit quality institutions. At times, such amounts may be in excess of the FDIC insurance limit.
Prepaid Expenses
From time to time, the Company issues shares of its common stock for services to be performed. The fair value of the common stock is determined at the date of the contract for services and is amortized ratably over the term of the contract. As of December 31, 2014 and 2013, prepaid expenses relating to stock based payments were $56,667 and $-0-, respectively.
Property and Equipment
Property and equipment are stated at cost and depreciated using the straight-line method over their estimated useful lives of 3 to 5 years. When retired or otherwise disposed, the related carrying value and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition, is reflected in earnings.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
Long-Lived Assets
The Company follows Accounting Standards Codification 360-10-15-3, “Impairment or Disposal of Long-lived Assets,” which established a “primary asset” approach to determine the cash flow estimation period for a group of assets and liabilities that represents the unit of accounting for a long-lived asset to be held and used. Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
Fair Value of Financial Instruments
The Company’s short-term financial instruments, including cash, prepaid expenses and other assets, accounts payable and accrued expenses and other liabilities, consist primarily of instruments without extended maturities, the fair value of which, based on management’s estimates, reasonably approximate their book value. The fair value of the Company’s convertible securities is based on management estimates and reasonably approximates their book value.
Research and development costs
The Company accounts for research and development costs in accordance with the Accounting Standards Codification subtopic 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and developments costs are expensed when the contracted work has been performed or as milestone results have been achieved. Company-sponsored research and development costs related to both present and future products are expensed in the period incurred. The Company incurred research and development expenses of $547,996 and $992,207 for the years ended December 31, 2014 and 2013, respectively.
Net Income (loss) Per Common Share
The Company computes earnings (loss) per share under Accounting Standards Codification subtopic 260-10, Earnings Per Share (“ASC 260-10”). Net loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share, if presented, would include the dilution that would occur upon the exercise or conversion of all potentially dilutive securities into common stock using the “treasury stock” and/or “if converted” methods as applicable.
The computation of basic and diluted loss per share as of December 31, 2014 and 2013 excludes potentially dilutive securities when their inclusion would be anti-dilutive, or if their exercise prices were greater than the average market price of the common stock during the period.
Potentially dilutive securities excluded from the computation of basic and diluted net income (loss) per share are as follows:
|
2014
|
2013
|
|||||||
|
Series A convertible preferred stock
|
-
|
501,089
|
||||||
|
Series B convertible preferred stock
|
-
|
451,726
|
||||||
|
Series C convertible preferred stock
|
1,807,333
|
1,330,627
|
||||||
|
Options to purchase common stock
|
5,990,190
|
2,990,977
|
||||||
|
Warrants to purchase common stock
|
5,113,990
|
2,717,258
|
||||||
|
Totals
|
12,911,513
|
7,991,667
|
||||||
The Company follows Accounting Standards Codification subtopic 740-10, Income Taxes (“ASC 740-10”) for recording the provision for income taxes. Deferred tax assets and liabilities are computed based upon the difference between the financial statement and income tax basis of assets and liabilities using the enacted marginal tax rate applicable when the related asset or liability is expected to be realized or settled. Deferred income tax expenses or benefits are based on the changes in the asset or liability during each period. If available evidence suggests that it is more likely than not that some portion or all of the deferred tax assets will not be realized, a valuation allowance is required to reduce the deferred tax assets to the amount that is more likely than not to be realized. Future changes in such valuation allowance are included in the provision for deferred income taxes in the period of change. Deferred income taxes may arise from temporary differences resulting from income and expense items reported for financial accounting and tax purposes in different periods. Deferred taxes are classified as current or non-current, depending on the classification of assets and liabilities to which they relate. Deferred taxes arising from temporary differences that are not related to an asset or liability are classified as current or non-current depending on the periods in which the temporary differences are expected to reverse.
Stock Based Compensation
The Company measures the cost of services received in exchange for an award of equity instruments based on the fair value of the award. For employees and directors, the fair value of the award is measured on the grant date and for non-employees, the fair value of the award is generally re-measured on vesting dates and interim financial reporting dates until the service period is complete. The fair value amount is then recognized over the period during which services are required to be provided in exchange for the award, usually the vesting period.
As of December 31, 2014, the Company had 5,990,190 options outstanding to purchase shares of common stock, of which 3,799,559 were vested.
As of December 31, 2013, the Company had 2,990,977 options outstanding to purchase shares of common stock, of which 1,675,658 were vested.
Registration Rights
The Company accounts for registration rights agreements in accordance with the Accounting Standards Codification subtopic 825-20, Registration Payment Arraignments (“ASC 825-20”). Under ASC 825-20, the Company is required to disclose the nature and terms of the arraignment, the maximum potential amount and to assess each reporting period the probable liability under these arraignments and, if exists, to record or adjust the liability to current period operations. On June 23, 2014, the Company filed Form S-1/A became effective with the Securities and Exchange Commission. As such, the Company determined that payments were due under its registration rights agreement and therefore accrued $55,620 as interest expense for the liability under the registration rights agreements.
Recent Accounting Pronouncements
There are various updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to a have a material impact on the Company's financial position, results of operations or cash flows.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 2 – GOING CONCERN MATTERS
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As shown in the accompanying financial statements during the years ended December 31, 2014 and 2013, the Company incurred net losses attributable to common stockholders of $8,773,399 and $10,101,846, respectively and used $1,997,072 in cash for operating activities for the year ended December 31, 2014. These factors among others raise substantial doubt about the Company’s ability to continue as a going concern for a reasonable period of time.
The Company's existence is dependent upon management's ability to develop profitable operations. The Company completed financing subsequent to the date of these financial statements (See Note 16). However additional capital will be needed to continue developing its products and services and there can be no assurance that the Company's efforts will be successful. There is no assurance that can be given that management's actions will result in profitable operations or the resolution of its liquidity problems. The accompanying statements do not include any adjustments that might result should the Company be unable to continue as a going concern.
NOTE 3 – RELATED PARTY TRANSACTIONS
The Company’s President and shareholders have advanced funds to the Company for working capital purposes since the Company’s inception in February 2009. No formal repayment terms or arrangements exist and the Company is not accruing interest on these advances. The net amount outstanding at December 31, 2014 and 2013 was $-0- and $30,781, respectively.
On December 31, 2013, as part of a private placement transaction of our common stock and warrants, (i) $228,000 of our outstanding indebtedness that was due to a related party was converted into 93,061 shares of common stock and a warrant to purchase 46,531 shares of our common stock; and (ii) we issued to a related party 122,448 shares of our common stock and a warrant to purchase 61,225 shares of our common stock for a purchase price of $300,000
Accrued interest and expenses due related parties as of December 31, 2014 and 2013 was $40,293 and $123,089, respectively.
During 2014, one of the Company’s board of directors forgave an outstanding obligation of $87,500 for services. Accordingly, the Company reclassified the liability to equity as donated capital.
During 2014, the Company issued 34,000 shares of its common stock for future services to a board member totaling $85,000 ($2.50 per share), unrelated to his services as a board member. The fair value of the services is amortized over the service period. As of December 31, 2014, the unamortized portion of $56,667 is included in prepaid expenses in the accompanying balance sheet.
During 2014, the Company issued 26,000 shares of its common stock in settlement of $65,000 debt to a board of directors’ member ($2.50 per share).
During 2013, in connection with the amendments of the Series C 9% Convertible Preferred stock, the Company issued to Company’s president and a Director of the Company (Series C holders) an aggregate of 53,830 warrants to purchase the Company’s common stock at $2.61 per share for five years. See Note 9 below.
The Company has informal compensation and consulting agreements with employees and outside contractors, certain of whom are also Company stockholders. The Agreements are generally month to month. As of December 31, 2014 and 2013, total due under these agreements and related expenses were $11,250 and $-0-, respectively.
On January 31, 2014, as part of a private placement transaction of our common stock and warrants, a related party purchased an aggregate of 24,490 shares of common stock and a warrant to purchase 12,246 shares of common stock for an aggregate purchase price of $60,000.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 4 – PROPERTY AND EQUIPMENT
Property and equipment as of December 31, 2014 and 2013 is summarized as follows:
|
|
|
2014
|
|
|
2013
|
|
||
|
Computer equipment
|
|
$
|
54,900
|
|
|
$
|
50,937
|
|
|
Furniture and fixtures
|
|
|
7,803
|
|
|
|
7,803
|
|
|
Subtotal
|
|
|
62,703
|
|
|
|
58,740
|
|
|
Less accumulated depreciation
|
|
|
(49,683
|
)
|
|
|
(33,874
|
)
|
|
Property and equipment, net
|
|
$
|
13,020
|
|
|
$
|
24,866
|
|
Property and equipment are stated at cost and depreciated using the straight-line method over their estimated useful lives of 3 to 5 years. When retired or otherwise disposed, the related carrying value and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition, is reflected in earnings.
Depreciation expense was $15,809 and $17,059 at December 31, 2014 and 2013, respectively.
NOTE 5 – ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses at December 31, 2014 and 2013 consist of the following:
|
|
|
2014
|
|
|
2013
|
|
||
|
Accrued accounting and legal
|
|
$
|
190,767
|
|
|
$
|
300,893
|
|
|
Accrued reimbursements
|
|
|
26,792
|
|
|
|
17,797
|
|
|
Accrued consulting
|
|
|
16,334
|
|
|
|
214,481
|
|
|
Accrued research and development expenses
|
|
|
93,407
|
|
|
|
64,670
|
|
|
Accrued credit card obligations
|
|
|
13,278
|
|
|
|
20,425
|
|
|
Accrued payroll
|
|
|
62,068
|
|
|
|
35,896
|
|
|
Accrued liquidated damages
|
|
|
55,620
|
|
|
|
48,668
|
|
|
Accrued office and other
|
|
|
29,093
|
|
|
|
16,500
|
|
|
Accrued settlement related to arbitration
|
|
|
66,667
|
|
|
|
100,000
|
|
|
|
|
$
|
554,026
|
|
|
$
|
819,330
|
|
NOTE 6 – NOTES PAYABLE, RELATED PARTY
On November 21, 2012, the Company issued an unsecured promissory note for $218,000 to the Company’s President for previously advanced funds with interest payable annually, in arrears, on each anniversary at the short term “Applicable Federal Rate” within the meaning of Section 1274(d) of the Internal Revenue Code of 1986, as amended adjusted each anniversary date. The promissory note matures November 21, 2021 and may be prepaid, without premium or penalty, at any time.
In connection with the issuance of the unsecured promissory note, the Company’s President agreed not to receive payments (by voluntary prepayment, acceleration, set-off or otherwise) associated with the unsecured promissory note absent the prior written consent of the purchasers holding at least 67% interest of the preferred stock outstanding, which purchasers must include Alpha Capital Anstalt so long as Alpha Capital Anstalt holds not less than $100,000 of preferred stock. On December 31, 2013, the Company converted the promissory note and accrued interest to 93,061 shares of the Company’s common stock and 46,531 warrants to purchase the Company’s common stock at $3.67 per share for five years.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
On December 6, 2012, the Company issued an unsecured promissory note for $30,000 to a company under the control of the Company’s President for previously advanced funds, interest free and due the earlier of (i) the next financing of not less than $300,000; (ii) February 28, 2013 or (iii) occurrence of an event of default, as defined.
During year ended December 31, 2013, the Company paid off the promissory note in full.
NOTE 7 – CONVERTIBLE BRIDGE NOTES
In 2012, the Company issued an aggregate of $600,000 unsecured Senior Convertible Promissory Notes ($225,000 related party) with interest due at maturity at 8% per annum and may be paid, at the Company’s discretion, in cash or the Company’s common stock. The Notes, together with unpaid accrued interest, if any, is due upon written notice by the majority in interest of the holders on or after February 15, 2014 or (ii) upon the occurrence of an event of default, as defined. The Notes may be prepaid in whole or in part prior to the maturity date at the Company’s discretion.
The Convertible Bridge Notes and any accrued and unpaid interest automatically converts at the earlier of (i) (A) a completion of a transaction whereby the Company merges or consolidates with another company that has its common stock approved for quotation on any domestic national stock exchange and (B) the new entity thereafter issues and sells shares for no less than $3.0 million aggregate gross proceeds or (ii) a qualified IPO. The Convertible Bridge Notes shall convert into the new securities issued at 95% of the purchase price of the Conversion Securities offered to investors.
In connection with the issuance of the Senior Convertible Promissory Notes, the Company issued the right to purchase at any time, on or after the Public Financing Closing Date,(as defined above) hereof until the fifth anniversary of the Public Financing Closing date, the number of fully paid and nonassessable shares (the “Warrant Shares”) of the Company’s common stock equal to the quotient of (a) the Warrant Coverage Amount (as defined below), divided by (b) the applicable Conversion Price of the Notes, at the per share exercise price (the “Exercise Price”), which shall initially be, as of the Public Financing Closing Date, equal to the Initial Exercise Price (as defined below), subject to further adjustments, as defined.
Initial Exercise Price” means one hundred twenty-five percent (125%) of the Conversion Price.
Warrant Coverage Amount” shall be the amount obtained by multiplying (x) the Warrant Coverage Percentage by (y) the principal amount outstanding (and not including any accrued and unpaid interest) of the Note, in connection with which this Warrant is concurrently issued.
“Warrant Coverage Percentage” shall be equal to fifty percent (50%) as defined in the Bridge Loan Agreement.
On February 6, 2013, the Convertible Bridge Notes and the above described contingent warrants previously issued as described above were converted into 600 shares of Series C Convertible Preferred Stock and an aggregate of 287,082 warrants to purchase the Company’s common stock at an exercise price of $2.09 per share for 5 years. On August 7, 2013, the Company issued an aggregate of 8,941 shares of its common stock in settlement of accrued interest of $18,677.
NOTE 8 – REDEEMABLE PREFERRED STOCK
Series A Preferred Stock
In May 2011, the Board of Directors authorized the issuance of up to 200 shares of Series A Preferred Stock (the “Series A preferred stock”).
The Series A preferred stock is entitled to preference over holders of junior stock upon liquidation in the amount of $5,000 plus any accrued and unpaid dividends; entitled to dividends as a preference to holders of junior stock at a rate of 5% per annum of the Stated Value of $5,000 per share, payable quarterly beginning on August 31, 2011 and are cumulative. The holders of Series A preferred stock have no voting rights, however without the affirmative vote of all the holders of then outstanding shares of the Series A preferred stock, the Company cannot, (a) alter or change adversely the powers, preferences or rights given to the Series A preferred stock or alter or amend the Certificate of Designation.
The Series A preferred stock is mandatorily redeemable on December 31, 2014 (as modified) at a price equal to the Stated Value ($5,000) plus an amount equal to all accumulated and unpaid dividends. If the Company fails to redeem at redemption, the unpaid redemption price will accrue at 14% per annum until paid.
The Series A preferred stock is convertible (as amended), automatically, inclusive of any accrued and unpaid dividends, immediately into the Company’s common stock upon the Company becoming subject to the reporting requirements under Section 13 or 15(d) of the Securities and Exchange Act of 1934, as amended at conversion price of $1.84 per share.
On February 6, 2013, in connection with the amendment to the Series A preferred stock defining the conversion feature, the Company reclassified the associated financing costs as a debt discount against the carrying value of the preferred stock.
As of December 31, 2013, 184.4 shares of Series A preferred stock were issued and outstanding with accrued dividends of $119,355 payable on the Series A preferred stock.
On June 23, 2014, upon the effectiveness of the Company’s registration statement, the Company issued an aggregate of 577,901 shares of its common stock in exchange for all the outstanding Series A preferred stock and accrued dividends of $141,331.
Series B Preferred Stock
On November 28, 2011, the Board of Directors authorized the issuance of up to 600 shares of Series B Preferred Stock (the “Series B preferred stock”).
The Series B preferred stock is entitled to preference over holders of junior stock upon liquidation in the amount of $5,000 plus any accrued and unpaid dividends; entitled to dividends as a preference to holders of junior stock at a rate of 5% per annum of the Stated Value of $5,000 per share, payable quarterly beginning on December 31, 2011 and are cumulative. The holders of Series B preferred stock have no voting rights, however without the affirmative vote of all the holders of then outstanding shares of the Series B preferred stock, the Company cannot (a) alter or change adversely the powers, preferences or rights given to the Series A preferred stock or alter or amend the Certificate of Designation.
The Series B preferred stock is mandatorily redeemable on December 31, 2014 at a price equal to the Stated Value ($5,000) plus an amount equal to all accumulated and unpaid dividends. If the Company fails to redeem at redemption, the unpaid redemption price will accrue at 14% per annum until paid.
The Series B preferred stock is convertible (as amended), automatically, inclusive of any accrued and unpaid dividends, immediately into the Company’s common stock upon the Company becoming subject to the reporting requirements under Section 13 or 15(d) of the Securities and Exchange Act of 1934, as amended at conversion price of $2.02 per share.
On February 6, 2013, in connection with the amendment to the Series B preferred stock defining the conversion feature, the Company reclassified the associated financing costs as a debt discount against the carrying value of the preferred stock.
As of December 31, 2013, 177.5 shares of Series B preferred stock were issued and outstanding. With accrued dividends of $88,872 payable on the Series B preferred stock.
On June 23, 2014, upon the effectiveness of the Company’s registration statement, the Company issued an aggregate of 493,818 shares of its common stock in exchange for all the outstanding Series B preferred stock and accrued dividends of $110,026.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 9 – SERIES C 9% CONVERTIBLE PREFERRED STOCK
On January 9, 2013, the Board of Directors authorized the issuance of up to 4,200 shares of Series C Convertible Preferred Stock (the “Series C Convertible Preferred Stock”).
The Series C convertible preferred stock is entitled to preference over holders of junior stock upon liquidation in the amount of $1,000 plus any accrued and unpaid dividends; entitled to dividends as a preference to holders of junior stock at a rate of 9% per annum of the Stated Value of $1,000 per share, payable quarterly beginning on September 30, 2013 and are cumulative. The holders of Series C preferred stock have no voting rights, however without the affirmative vote of all the holders of then outstanding shares of the Series C preferred stock, the Company cannot (a) alter or change adversely the powers, preferences or rights given to the Series C preferred stock or alter or amend the Certificate of Designation.
Each share of Series C preferred stock is convertible, at the holder’s option, inclusive of any accrued and unpaid dividends, at conversion price of $1.50 (as reset).
If, at any time while the Series C preferred stock is outstanding, the Company sells or grants any option to purchase or sells or grants any right to re-price, or otherwise disposes of or issues any common stock or common stock equivalents entitling any Person to acquire shares of Common Stock at an effective price per share that is lower than the then conversion price (“Base Conversion Price”), then the conversion price shall be reduced to equal the Base Conversion Price. Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. During the year ended December 31, 2014, the resets provisions as described above resulted in the conversion price reset to $1.50.
The Series C preferred stock contains triggering events which would require redemption at (i) the greater of 120% of the stated value of $1,000 or the product of the variable weighted average price of the Company’s common stock on the trading day immediately preceding the date of the triggering event and the stated value divided by the then conversion price or (ii) either (a) redeem each Series C preferred share for a redemption price, in shares of the Company’s common stock, equal to a number of shares equal to the (i) above divided by 75%. The Company determined that certain of the defined triggering events were outside the Company’s control and therefore classified the Series C preferred stock outside of equity.
In connection with the sale of the Series C preferred stock, the Company issued an aggregate of 1,330,627 warrants to purchase the Company’s common stock at $2.61 per share expiring five years from the initial exercise date. The warrant provides if, at any time while the warrant is outstanding, the Company sells or grants any option to purchase or sells or grants any right to re-price, or otherwise disposes of or issues any common stock or common stock equivalents entitling any person to acquire shares of common stock at an effective price per share that is lower than the then conversion price (“base conversion price”), then the warrants outstanding will be subject anti-dilution provisions.
Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. In addition, the warrants provides for at any time after the six month anniversary of the initial exercise date, there is no effective registration statement registering, or no current prospectus available for the resale of the warrant shares by the holder, then the warrant may only be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the holder shall be entitled to receive a number of Warrant Shares equal to defined formula. During the year ended December 31, 2014, the resets provisions as described above resulted in an additional 984,674 warrants issued with an exercise price reset to $1.50 all Series C warrants..
In accordance with ASC 470-20, the Company recognized an embedded beneficial conversion feature present in the Series C preferred stock when it was issued. The Company allocated the net proceeds between the intrinsic value of the conversion option ($1,303,671) and the warrants ($1,064,739) to additional paid-in capital. The aggregate debt discount, comprised of the relative intrinsic value the conversion option ($1,303,671), relative fair value of the warrants ($1,064,739), and the issuance costs ($412,590); total of $2,781,000, is amortized over one year as interest expense, the date a possible redemption feature, outside of the Company’s control, would be available to the Series C stockholders.
The Company valued the warrants in accordance with ASC 470-20 using the Black-Scholes pricing model and the following assumptions: contractual terms of 5 years, an average risk free interest rate of 0.39% to 1.40%, a dividend yield of 0%, and volatility of 123.41% to 125.33%.
During the month of February 2013, the holders of the Convertible Bridge Notes (See Note 7) converted into 600 shares of the Company’s Series C 9% Convertible Preferred Stock.
During the months of February, March, May, and July 2013, the Company sold an aggregate of 2,181 shares of the Company’s Series C 9% Convertible Preferred Stock for net proceeds of $1,814,910.
During October 2014, the Company issued an aggregate of 59,267 shares of its commons stock in exchange for 70 shares of the Company’s Series C 9% Convertible Preferred Stock and accrued dividends.
The Company determined that the anti-dilutive provisions embedded in the Series C 9% Convertible Preferred Stock and related issued warrants did not meet the defined criteria of a derivative in such that the net settlement requirement of delivery of common shares does not meet the “readily convertible to cash” as described in Accounting Standards Codification 815 and therefore bifurcation is not required. As of December 31, 2014, the Company’ s common stock was thinly traded and there was no active trading market.
Series C preferred stock outstanding totaled 2,711 and 2,781 as of December 31, 2014 and 2013. As of December 31, 2014 and 2013, the Company has accrued $445,069 and $206,740 dividends payable on the Series C preferred stock.
Registration Rights Agreement
The Company entered into a Registration Rights Agreement in connection with the sale and issuance of the Series C preferred stock. The Company is required to file a registration statement registering for resale the (a) common stock issuable upon conversion in full of the Preferred Stock (assuming on such date the shares of Preferred Stock are converted in full without regard to any conversion limitations therein), (b) all shares of Common Stock issuable as dividends and “Make-Whole Payments” (as defined in the Certificate of Designation) on the Preferred Stock assuming all dividend and Make-Whole Payments are made in shares of Common Stock and the Preferred Stock is held for at least 3 years, (c) all warrant shares then issuable upon exercise of the Warrants (assuming on such date the warrants are exercised in full without regard to any exercise limitations therein), (d) any additional shares of Common Stock issuable in connection with any anti-dilution provisions in the Preferred Stock or the Warrants (in each case, without giving effect to any limitations on conversion set forth in the Certificate of Designation or limitations on exercise set forth in the Warrants) and (e) any securities issued or then issuable upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing. The Company is required to file a registration statement and must be declared effective no later than 210 days from the date of termination of the sale the Series C preferred stock.
The Company is required to maintain the effectiveness of the registration statement from its effective date unless all securities registered under the registration statement have been sold or are otherwise able to be sold. If the Company fails to comply with the registration statement effective date requirements, the Company is required to pay the investors a fee equal to 0.25% of the Purchaser’s investment, for each 30-day period of delay, subject to a maximum payment of 3% to each Purchaser.
On June 23 2014, the Company became effective and met its required filing requirement. The Company did not meet the effectiveness obligation by November 22, 2013. As a result, the Company accrued $55,620 as interest expense for liquidating damages due under the registration rights agreement.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 10 – STOCKHOLDER EQUITY
There is not a viable market for the Company’s common stock to determine its fair value; therefore, management is required to estimate the fair value to be utilized in the determining stock based compensation costs. In estimating the fair value, management considers recent sales of its common stock to independent qualified investors, placement agents’ assessments of the underlying common shares relating to our sale of preferred stock and validation by independent fair value experts. Considerable management judgment is necessary to estimate the fair value. Accordingly, actual results could vary significantly from management’s estimates.
Preferred stock
The Company is authorized to issue 1,000,000 shares of $0.001 par value preferred stock. As of December 31, 2014 and 2013, the Company has designated and issued 200 and 184.4 shares of Series A preferred stock, respectively, designated and issued 600 and 177.5 shares of Series B preferred stock, respectively. See Note 8.
As of December 31, 2014 and 2013, the Company designated and issued 4,200 and 2,781 shares of Series C 9% convertible preferred stock, respectively. See Note 9.
On June 23, 2014, the Company issued an aggregate of 577,901 and 493,818 shares of its common stock in exchange of all the issued and outstanding Series A and Series B preferred stock.
During December 2014, the Company issued an aggregate of 59,267 shares of its commons stock in exchange for 70 shares of the Company’s Series C 9% Convertible Preferred Stock and accrued dividends.
Common stock
The Company is authorized to issue 50,000,000 shares of $0.001 par value common stock. As of December 31, 2014 and 2013, the Company has 11,179,266 and 8,412,101 shares issued and outstanding, respectively.
During the year ended December 31, 2013, the Company issued an aggregate of 21,412 shares of common stock for services rendered totaling $44,751 ($2.09 per share).
During the year ended December 31, 2013, the Company issued an aggregate of 122,449 shares of common stock for cash rendered totaling $247,174 ($2.45 per share).
During the year ended December 31, 2014, the Company issued 654,000 shares of its common stock (net of shares exchanged) under the terms of its 2012 Equity Plan for services rendered totaling $1,635,000 ($2.50 per share).
During the year ended December 31, 2014, the Company issued 26,000 shares of its common stock in settlement of $65,000 related party debt ($2.50 per share).
During the year ended December 31, 2014, the Company entered into a securities purchase agreement with investors pursuant to which the Company issued 956,179 shares of common stock and five-year warrants for aggregate net proceeds of $1,969,410.
Stock based payable
The Company is obligated to issue an aggregate of 417,500 shares of its common stock to consultants for past and future services. The estimated liability as of December 31, 2014 of $226,305 ($2.50 per share) was determined based on services rendered in 2014.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 11 – OPTIONS AND WARRANTS
There is not a viable market for the Company’s common stock to determine its fair value, therefore management is required to estimate the fair value to be utilized in the determining stock based compensation costs. In estimating the fair value, management considers recent sales of its common stock to independent qualified investors, placement agents’ assessments of the underlying common shares relating to our sale of preferred stock and validation by independent fair value experts. Considerable management judgment is necessary to estimate the fair value. Accordingly, actual results could vary significantly from management’s estimates
On October 19, 2012, the Company’s Board of Directors approved the 2012 Equity Incentive Plan (“the “2012 Plan) and terminated the Long-Term Incentive Plan (the “2011 Plan”). The Plan provides for the issuance of options to purchase up to 8,806,123,( as amended) shares of the Company’s common stock to officers, directors, employees and consultants of the Company (as amended). Under the terms of the Plan the Company may issue Incentive Stock Options as defined by the Internal Revenue Code to employees of the Company only and nonstatutory options. The Board of Directors of the Company determines the exercise price, vesting and expiration period of the grants under the Plan. However, the exercise price of an Incentive Stock Option should not be less than 110% of fair value of the common stock at the date of the grant for a 10% or more stockholder and 100% of fair value for a grantee who is not 10% stockholder. The fair value of the common stock is determined based on quoted market price or in absence of such quoted market price, by the Board of Directors in good faith.
Additionally, the vesting period of the grants under the Plan will be determined by the Committee, in its sole discretion, and expiration period not more than ten years. The Company reserved 1,250,000 shares of its common stock for future issuance under the terms of the Plan.
During the year ended December 31, 2014, the Company granted an aggregate of 3,478,498 options and 654,000 stock grants (net of shares exchanged) to officers, directors and key consultants.
A summary of the stock option activity and related information for the 2012 Plan for the year ended December 31, 2014 and 2013 is as follows:
|
Weighted-Average
|
||||||||||||||||
|
Weighted-Average
|
Remaining
|
Aggregate
|
||||||||||||||
|
Shares
|
Exercise Price
|
Contractual Term
|
Intrinsic Value
|
|||||||||||||
|
Outstanding at January 1, 2013
|
1,298,927
|
$
|
2.04
|
6.85
|
-
|
|||||||||||
|
Grants
|
1,692,050
|
2.09
|
7.00
|
-
|
||||||||||||
|
Exercised
|
||||||||||||||||
|
Canceled
|
||||||||||||||||
|
Outstanding at December 31, 2013
|
2,990,977
|
$
|
2.05
|
6.02
|
$
|
-
|
||||||||||
|
Grants
|
3,478,498
|
$
|
2.39
|
8.10
|
$
|
-
|
||||||||||
|
Exercised
|
-
|
|||||||||||||||
|
Canceled
|
(479,285
|
)
|
(2.00
|
)
|
||||||||||||
|
Outstanding at December 31,2014
|
5,990,190
|
$
|
2.25
|
6.65
|
$
|
3,267,692
|
||||||||||
|
Vested and expected to vest at December 31, 2014
|
5,990,190
|
$
|
2.25
|
6.65
|
$
|
3,267,692
|
||||||||||
|
Exercisable at December 31, 2014
|
3,799,559
|
$
|
2.24
|
5.91
|
$
|
2,111,368
|
||||||||||
The aggregate intrinsic value in the preceding tables represents the total pretax intrinsic value, based on options with an exercise price less than the Company’s estimated market stock price of $2.80 as of December 31, 2014, which would have been received by the option holders had those option holders exercised their options as of that date.
Option valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards was estimated using the Black-Scholes option model with a volatility figure derived from an index of historical stock prices of comparable entities until sufficient data exists to estimate the volatility using the Company’s own historical stock prices. Management determined this assumption to be a more accurate indicator of value. The Company accounts for the expected life of options based on the contractual life of options for non-employees. For employees, the Company accounts for the expected life of options in accordance with the “simplified” method, which is used for “plain-vanilla” options, as defined in the accounting standards codification. The risk-free interest rate was determined from the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the options. The fair value of stock-based payment awards during the years ended December 31, 2014 and 2014 was estimated using the Black-Scholes pricing model.
In addition, the Company is required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. In estimating the Company’s forfeiture rate, the Company analyzed its historical forfeiture rate, the remaining lives of unvested options, and the number of vested options as a percentage of total options outstanding.
If the Company’s actual forfeiture rate is materially different from its estimate, or if the Company reevaluates the forfeiture rate in the future, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
The Company estimated forfeitures related to option grants at a weighted average annual rate of 0% per year, as the Company does not yet have adequate historical data, for options granted during the years ended December 31, 2014 and 2013.
During the year ended December 31, 2013, the Company granted an aggregate of 1,692,050 options to purchase the Company stock in connection with the services rendered at the exercise price of $2.09 per share for a term of seven to ten years with 1,095,000 vesting immediately, 145,833 vesting over three months, 30,000 vesting over nine months, 283,300 options vesting at ratably over one year and 137,917 vesting over two years.
The fair value of the granted options for year ended December 31, 2013 was determined using the Black Scholes option pricing model with the following assumptions:
|
Dividend yield:
|
|
-0-
|
%
|
|
Volatility
|
|
110.70% to 115.03
|
%
|
|
Risk free rate:
|
|
1.07% to 3.04
|
%
|
|
Expected life:
|
|
7 to 10 years
|
|
|
Estimated fair value of the Company’s common stock
|
|
$2.09
|
|
The following assumptions were used in determining the fair value of options during the year ended December 31, 2014:
|
Dividend yield:
|
-0-
|
%
|
|
|
Volatility
|
119.43% to 129.88
|
%
|
|
|
Risk free rate:
|
0.48% to 2.53
|
%
|
|
|
Expected life:
|
7 to 10 years
|
||
|
Estimated fair value of the Company’s common stock
|
$2.21 to $2.50
|
In July 2014, the Company awarded 1,265,769 of stock options to Company’s Chief Executive Officer. The stock options have exercise price of $2.21 per share, with 45,206 options vesting immediately and 497,267 options vesting quarterly over a two year period with the remainder contingent on performance, and have an approximate fair value of $2,383,443 using the Black Scholes model.
In September 2014, the Company awarded an aggregate of 880,000 of stock options to certain employees and key consultants. The stock options have exercise price of $2.50 per share, with 605,000 vested immediately, 125,000 in one year and 150,000 over a two year period, and have an approximate fair value of $1,753,616 using the Black Scholes model.
In September 2014, the Company canceled an aggregate of 479,285 previously issued, unvested (contingent) options issued in July 2012 at an exercise price of $2.00 per share to a board member in exchange for issuance of 479,285 options at an exercise price of $2.50, vesting quarterly over two years and expiring 7 years from the date of issuance. The greater of the approximate fair value of the options exchanged of $981,798 was determined using the Black Scholes option model.
In October 2014, the Company awarded an aggregate of 853,444 stock options to certain employees and a key consultant. The stock options have an exercise price of $2.50 per share with 841,777 vested immediately and a remainder of 11,667 based on future performance conditions, and have an approximate fair value of $1,339,151.
In October 2014, one of the Company’s board of directors exchanged 125,000 common shares issued in September 2014 for services and debt repayment for 163,444 stock options. The stock options have an exercise price of $2.50, vesting immediately. The approximate fair value of the exchange was determined to be the same.
The following table presents information related to stock options at December 31, 2014:
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||
|
Weighted
|
||||||||||||||
|
Average
|
Exercisable
|
|||||||||||||
|
Exercise
|
Number of
|
Remaining Life
|
Number of
|
|||||||||||
|
Price
|
Options
|
In Years
|
Options
|
|||||||||||
|
$
|
1.01-2.00
|
819,642
|
4.7
|
526,642
|
||||||||||
|
2.01-2.50
|
5,170,548
|
7.0
|
3,272,917
|
|||||||||||
|
5,990,190
|
6.7
|
3,799,559
|
||||||||||||
The fair value of all options vesting during the year ended December 31, 2014 and 2013 of $4,193,425 and $3,247,187, respectively, was charged to current period operations. Unrecognized compensation expense of $3,778,589 and $862,066 at December 31, 2014 and 2013, respectively, will be expensed in future periods.
Warrants
The following table summarizes information with respect to outstanding warrants to purchase common stock of the Company, all of which were exercisable, at December 31, 2014:
|
Exercise
|
Number
|
Expiration
|
|||
|
Price
|
Outstanding
|
Date
|
|||
|
$
|
0.001
|
383,320
|
January 2020 | ||
|
$
|
1.50
|
3,721,518
|
February 2018 to September 2018 | ||
|
$
|
1.84
|
35,076
|
January 2020 | ||
|
$
|
2.02
|
30,755
|
January 2020 | ||
|
$
|
2.50
|
204,840
|
July 2015 | ||
|
$
|
2.75
|
228,720
|
August 2019 to September 2019 | ||
|
$
|
3.67
|
218,275
|
December 2018 to January 2019 | ||
|
$
|
3.75
|
291,486
|
April 2019 to March 2020 | ||
|
5,113,990
|
|||||
On January 13, 2013, the Company issued an aggregate of 65,831 warrants to purchase the Company stock in connection with the placement services at the exercise prices of $1.84 (35,076 warrants) and $2.02 (30,775 warrants) per share for a term of five years exercisable immediately.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
The fair value of the issued warrants were determined using the Black Scholes option pricing model with the following assumptions:
|
Dividend yield:
|
|
|
-0-
|
%
|
|
Volatility
|
|
|
123.30
|
%
|
|
Risk free rate:
|
|
|
0.72
|
%
|
|
Expected life:
|
|
5 years
|
|
|
|
Estimated fair value of the Company’s common stock
|
|
$
|
2.09
|
|
The fair value of $115,854 was charged to operations ratably as financing costs through December 31, 2014.
During the year ended December 31, 2013, the Company issued an aggregate of 1,516,386 warrants to purchase the Company stock in connection with the sale of the Series C 9% Convertible Preferred Stock at the exercise price of $2.61 per share for a term of five years exercisable immediately.
During the months of July and September, 2013, the Company issued an aggregate of 622,414 warrants to purchase the Company’s stock to holders of Series C preferred stock as an inducement to amend and waive certain defined provisions of the Series C preferred stock.
The fair value of the issued warrants were determined using the Black Scholes option pricing model with the following assumptions:
|
Dividend yield:
|
|
|
-0-
|
%
|
|
Volatility
|
|
|
125.33
|
%
|
|
Risk free rate:
|
|
|
1.40
|
%
|
|
Expected life:
|
|
5 years
|
|
|
|
Estimated fair value of the Company’s common stock
|
|
$
|
2.09
|
|
During the year ended December 31, 2013, the fair value of $1,074,833 was charged to current period operations
On December 31, 2013, the Company issued an aggregate of 129,307 warrants to purchase the Company’s common stock at $3.67 per share for five years in connection with the sale of the Company’s common stock.
On January 31, 2014, the Company issued an aggregate of 64,626 warrants to purchase the Company’s common stock at $3.67 per share for five years in connection with the sale of the Company’s common stock.
In February 2014, as described in the terms of the warrants issued in connection with the sale of the Series C preferred stock, the Company reset 2,138,800 previously issued warrants from a exercise price of $2.61 per share to $1.50. In addition, the Company was required to increase the number of issued warrants to an aggregate total of 3,721,518 warrants.
In April 2014, the Company issued an aggregate of 137,856 warrants to purchase the Company’s common stock at $3.75 per share for five years in connection with the sale of the Company’s common stock.
In August 2014, the Company issued an aggregate of 135,120 warrants to purchase the Company’s common stock at $2.75 per share for five years in connection with the sale of the Company’s common stock.
In September 2014, the Company issued an aggregate of 93,600 warrants to purchase the Company’s common stock at $2.75 per share for five years in connection with the sale of the Company’s common stock.
In December 2014, the Company issued an aggregate of 358,470 warrants to purchase the Company’s common stock in connection with the sale of the Company’s common stock. Of the aggregate issued, 204,840 warrants are exercisable at $2.50 expiring six months from the date of issuance and 153,630 warrants exercisable at $3.75 per share expiring March 31, 2020.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
A summary of the warrant activity for the years ended December 31, 2014 and 2013 is as follows:
|
Weighted-Average
|
||||||||||||||||
|
Weighted-Average
|
Remaining
|
Aggregate
|
||||||||||||||
|
Shares
|
Exercise Price
|
Contractual Term
|
Intrinsic Value
|
|||||||||||||
|
Outstanding at January 1, 2013
|
-
|
$
|
-
|
-
|
-
|
|||||||||||
|
Grants
|
2,717,258
|
2.28
|
7.00
|
-
|
||||||||||||
|
Exercised
|
||||||||||||||||
|
Canceled
|
||||||||||||||||
|
Outstanding at December 31, 2013
|
2,717,258
|
$
|
2.28
|
6.02
|
$
|
-
|
||||||||||
|
Grants
|
2,396,732
|
$
|
4.64
|
2.05
|
$
|
-
|
||||||||||
|
Exercised
|
-
|
|||||||||||||||
|
Canceled
|
-
|
-
|
||||||||||||||
|
Outstanding at December 31,2014
|
5,113,990
|
$
|
1.71
|
3.6
|
$
|
6,041,436
|
||||||||||
|
Vested and expected to vest at December 31, 2014
|
5,113,990
|
$
|
1.71
|
3.6
|
$
|
6,041,436
|
||||||||||
|
Exercisable at December 31, 2014
|
5,113,990
|
$
|
1.71
|
3.6
|
$
|
6,041,436
|
||||||||||
The aggregate intrinsic value in the preceding tables represents the total pretax intrinsic value, based on options with an exercise price less than the Company’s estimated market stock price of $2.80 as of December 31, 2014, which would have been received by the option holders had those option holders exercised their options as of that date.
NOTE 12 – FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company follows the provisions of ASC 825-10. For financial assets and liabilities included within the scope of ASC 825-10, the Company was required to adopt the provisions of ASC 825-10 prospectively as of the beginning of Fiscal 2009. The adoption of ASC 825-10 did not have a material impact on our consolidated financial position or results of operations.
The Company determined that the anti-dilutive provisions embedded in the Series C 9% Convertible Preferred Stock and related issued warrants did not meet the defined criteria of a derivative in such that the net settlement requirement of delivery of common shares does not meet the “readily convertible to cash” as described in Accounting Standards Codification 815 and therefore bifurcation is not required. As of December 31, 2014, the Company’ s common stock was thinly traded and there was no active trading market.
The Company determined that there were no items required to be measured at fair value on a recurring basis in the consolidated financial statements as of December 31, 2014 and 2013.
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 13 – COMMITMENTS AND CONTINGENCIES
Operating leases
On May 31, 2014, the Company extended its expiring three-year lease for office space in Los Angeles, California for one additional year, with monthly payments of $6,530 beginning September 1, 2014.
Employment agreements
On July 14, 2014, the Company’s Board Of Directors (the “Board”) increased the size of the Board to eight members and appointed Gregory D. Cash and Patrick J. Gallagher as members of the Board, effective as of July 15, 2014, to serve for a term expiring at the Company’s 2015 annual meeting of stockholders. In addition, the Board appointed Mr. Cash to serve as the Company’s president and chief executive officer.
In connection with the appointment of Mr. Cash, on July 15, 2014 (the “Effective Date”), the Company entered into an employment agreement with Mr. Cash (the “Employment Agreement”). The Employment Agreement has an initial term of three years that expires on July 15, 2017. Under the Employment Agreement, Mr. Cash is entitled to an annual base salary of $275,000. Upon the Company closing an equity or equity-linked financing with proceeds to the Company of at least $3.5 million (a “Qualified Financing”), Mr. Cash’s annual base salary will automatically increase to $325,000 and he will receive (i) a one-time payment equal to the difference between the amount he would have earned if his base salary was $325,000 and the amount he actually earned at his base salary of $275,000 for the time period from the Effective Date until the closing of such Qualified Financing and (ii) a one-time cash bonus of $30,000. If the Company does not complete a Qualified Financing within six months after the Effective Date, Mr. Cash’s annual base salary will nonetheless increase to $325,000 and he will receive the same one-time payment unless the Company reasonably determines that the failure to complete such Qualified Financing was within the reasonable control of Mr. Cash. Mr. Cash is also eligible to receive an annual bonus equal to at least 50% of the sum of his base salary and one-time payment, based on the achievement of reasonable performance criteria to be determined by the Board in consultation with Mr. Cash within 90 days of the Effective Date.
In accordance with the Employment Agreement, on July 15, 2014, the Company granted Mr. Cash an incentive stock option to purchase 1,265,769 shares of the Company’s common stock, made pursuant to an Incentive Stock Option Agreement. The option has an exercise price of $2.21, which was the fair market value of the Company’s common stock on the date of grant, and a term that expires ten years from the date of grant. The option will vest as follows (i) 542,473 shares of common stock will vest in eleven equal installments of 45,206 shares of common stock and one final installment of 45,207 shares of common stock on a quarterly basis with the first installment vesting on the Effective Date and subsequent installments vesting every three months thereafter; (ii) 180,824 shares of common stock will vest immediately upon completion of a Qualified Financing; (iii) 180,824 shares of common stock will vest upon the listing of the Company’s common stock on a recognized U.S. national securities exchange (i.e., NYSE, MKT LLC, The Nasdaq Stock Market LLC or the New York Stock Exchange); (iv) 180,824 shares of common stock will vest upon the 510(k) clearance or any other type of clearance deemed necessary by the U.S. Food and Drug Administration of the Company’s PURE (Precise Uninterrupted Real-time evaluations of Electrograms) EP technology platform; and (v) 180,824 shares of common stock will vest upon the Company achieving a market capitalization of $150,000,000 and maintaining such market capitalization for at least 90 consecutive calendar days.
Litigation
On January 7, 2014, David Drachman, the Company’s former chief executive officer and president, filed a statement of claim against the Company with the American Arbitration Association with respect to his resignation from his positions with us in November 2013. Mr. Drachman alleges, among other things, that (i) the Company misled him with respect to the status of our technology and required him to perform capital raising duties that had not been previously agreed upon, (ii) he resigned from his positions with us for good reason, as such term was defined in his employment agreement with the Company, and (iii) he, in his individual capacity, has full rights to the ownership and control of a patent application describing a combined ablation and recording unit directed at the use of electrocardiography sensing for control of radiofrequency renal denervation that we filed with the U.S. Patent and Trademark Office during the time Mr. Drachman served in his positions with the Company.
During the year ended December 31, 2014, the Company settled the above claim for $100,000 with payments over six months. As of December 31, 2014, $66,667 was outstanding.
The Company is subject at times to other legal proceedings and claims, which arise in the ordinary course of its business. Although occasional adverse decisions or settlements may occur, the Company believes that the final disposition of such matters should not have a material adverse effect on its financial position, results of operations or liquidity. There was no outstanding litigation as of December 31, 2014.
NOTE 14 – INCOME TAXES
At December 31, 2014, the Company has available for federal income tax purposes a net operating loss carry forward of approximately $6,800,000, expiring in the year 2034, that may be used to offset future taxable income. The Company has provided a valuation reserve against the full amount of the net operating loss benefit, since in the opinion of management based upon the earnings history of the Company; it is more likely than not that the benefits will not be realized. Due to possible significant changes in the Company's ownership, the future use of its existing net operating losses may be limited. All or portion of the remaining valuation allowance may be reduced in future years based on an assessment of earnings sufficient to fully utilize these potential tax benefits. During the year ended December 31, 2014, the Company has increased the valuation allowance from $1,400,000 to $2,300,000.
We have adopted the provisions of ASC 740-10-25, which provides recognition criteria and a related measurement model for uncertain tax positions taken or expected to be taken in income tax returns. ASC 740-10-25 requires that a position taken or expected to be taken in a tax return be recognized in the financial statements when it is more likely than not that the position would be sustained upon examination by tax authorities. Tax position that meet the more likely than not threshold are then measured using a probability weighted approach recognizing the largest amount of tax benefit that is greater than 50% likely of being realized upon ultimate settlement. The Company had no tax positions relating to open income tax returns that were considered to be uncertain.
The Company is required to file income tax returns in the U.S. Federal jurisdiction and in California. The Company is no longer subject to income tax examinations by tax authorities for tax years ending before December 31, 2010.
The effective rate differs from the statutory rate of 34% for due to the following:
|
2014
|
2013
|
|||||||
|
Statutory rate on pre-tax book loss
|
(34.00 | )% | (34.00 | )% | ||||
|
Stock based compensation
|
23.0 | % | 11.70 | % | ||||
|
Financing costs
|
2.4 | % | 2.40 | % | ||||
|
Valuation allowance
|
8.6 | % | 19.90 | % | ||||
| 0.00 | % | 0.00 | % | |||||
The Company’s deferred taxes as of December 31, 2014 and 2013 consist of the following:
|
2014
|
2013
|
|||||||
|
Non-Current deferred tax asset:
|
||||||||
|
Net operating loss carry-forwards
|
$ | 2,300,000 | $ | 1,400,000 | ||||
|
Valuation allowance
|
(2,300,000 | ) | (1,400,000 | ) | ||||
|
Net non-current deferred tax asset
|
$ | - | $ | - | ||||
BIOSIG TECHNOLOGIES INC.
NOTES TO THE FINANCIAL STATEMENTS
DECEMBER 31, 2014
NOTE 15 – SUBSEQUENT EVENTS
Common stock:
On January 13, 2015, the Company issued 42,334 shares of its Common stock upon conversion of 50 shares of Series C Preferred stock and accrued dividends.
In January, 2015, the Company issued an aggregate of 302,500 shares of its common stock to consultants for services . See Note 10.
January 2015 Private Placement
On January 23, 2015, the Company entered into a securities purchase agreement with investors, pursuant to which we issued 365,200 shares of the Company’s our common stock, and "A" warrants expiring July 31, 2015 to purchase 365,200 shares of our common stock for aggregate cash proceeds of $913,000, and "B" warrants expiring March 21, 2020 to purchase 182,600 shares of our common stock for aggregate net cash proceeds of $917,480. In connection with the private placement, the Company issued 91,300 warrants to purchase our stock in connection with the placement services at the exercise price of $3.75 per share expiring March 21, 2020 for investment banking services.
February 2015 Private Placement
On February 10, 2015, the Company entered into a securities purchase agreement with investors, pursuant to which we issued 337,000 shares of the Company’s common stock, and "A" warrants expiring July 31, 2015 to purchase 337,000 shares of our common stock for aggregate cash proceeds of $842,500, and "B" warrants expiring March 21, 2020 to purchase 168,500 shares of our common stock for aggregate net cash proceeds of $731,400. In connection with the private placement, the Company issued 84,250 warrants to purchase our stock in connection with the placement services at the exercise price of $3.75 per share expiring March 21, 2020 for investment banking services.
|
BIOSIG TECHNOLOGIES, INC.
|
||||||||
|
CONDENSED BALANCE SHEETS
|
||||||||
|
March 31,
|
December 31,
|
|||||||
|
2015
|
2014
|
|||||||
|
(unaudited)
|
||||||||
|
ASSETS
|
||||||||
|
Current assets:
|
||||||||
|
Cash
|
$ | 2,059,328 | $ | 239,781 | ||||
|
Prepaid expenses
|
159,768 | 75,537 | ||||||
|
Total current assets
|
2,219,096 | 315,318 | ||||||
|
Property and equipment, net
|
12,844 | 13,020 | ||||||
|
Other assets:
|
||||||||
|
Deposits
|
25,000 | 25,000 | ||||||
|
Total assets
|
$ | 2,256,940 | $ | 353,338 | ||||
|
LIABILITIES AND STOCKHOLDERS' DEFICIT
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable and accrued expenses, including $14,605 and $40,293 to related parties as of March 31, 2015 and December 31, 2014 respectively
|
$ | 320,871 | $ | 554,026 | ||||
|
Stock based payable
|
646,066 | 226,305 | ||||||
|
Dividends payable
|
456,964 | 445,069 | ||||||
|
Warrant liability
|
4,097,444 | - | ||||||
|
Derivative liability
|
1,242,590 | - | ||||||
|
Total current liabilities
|
6,763,935 | 1,225,400 | ||||||
|
Series C 9% Convertible Preferred stock, 2,461 and 2,711 shares issued and outstanding, liquidation preference of $2,461,000 and $2,711,000, respectively
|
2,461,000 | 2,711,000 | ||||||
|
Stockholders' deficit
|
||||||||
|
Preferred stock, $0.001 par value, authorized 1,000,000 shares, designated 200 shares of Series A, 600 shares of Series B and 4,200 shares of Series C Preferred Stock
|
||||||||
|
Common stock, $0.001 par value, authorized 50,000,000 shares, 13,159,694 and 11,179,266 issued and outstanding as of March 31, 2015 and December 31, 2014, respectively
|
13,160 | 11,179 | ||||||
|
Additional paid in capital
|
18,852,156 | 19,186,163 | ||||||
|
Accumulated deficit
|
(25,833,311 | ) | (22,780,404 | ) | ||||
|
Total stockholders' deficit
|
(6,967,995 | ) | (3,583,062 | ) | ||||
|
Total liabilities and stockholders' deficit
|
$ | 2,256,940 | $ | 353,338 | ||||
See the accompanying notes to the unaudited condensed financial statements
|
BIOSIG TECHNOLOGIES, INC.
|
||||||||
|
CONDENSED STATEMENTS OF OPERATIONS
|
||||||||
|
(unaudited)
|
||||||||
|
Three Months Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
Operating expenses:
|
||||||||
|
Research and development
|
$ | 302,079 | $ | 122,151 | ||||
|
General and administrative
|
2,746,853 | 601,565 | ||||||
|
Depreciation
|
2,860 | 4,435 | ||||||
|
Total operating expenses
|
3,051,792 | 728,151 | ||||||
|
Loss from operations
|
(3,051,792 | ) | (728,151 | ) | ||||
|
Other income (expense):
|
||||||||
|
Interest income (expense)
|
(1,114 | ) | (1,098 | ) | ||||
|
Financing costs
|
- | (388,285 | ) | |||||
|
Loss before income taxes
|
(3,052,906 | ) | (1,117,534 | ) | ||||
|
Income taxes (benefit)
|
- | - | ||||||
|
Net loss
|
(3,052,906 | ) | (1,117,534 | ) | ||||
|
Preferred stock dividend
|
(79,395 | ) | (84,024 | ) | ||||
|
NET LOSS AVAILABLE TO COMMON STOCKHOLDERS
|
$ | (3,132,301 | ) | $ | (1,201,558 | ) | ||
|
Net loss per common share, basic and diluted
|
$ | (0.26 | ) | $ | (0.14 | ) | ||
|
Weighted average number of common shares outstanding, basic and diluted
|
12,256,418 | 8,482,710 | ||||||
See the accompanying notes to the unaudited condensed financial statements
|
BIOSIG TECHNOLOGIES, INC.
|
||||||||||||||||||||
|
CONDENSED STATEMENT OF STOCKHOLDERS' DEFICIT
|
||||||||||||||||||||
|
THREE MONTHS ENDED MARCH 31, 2015
|
||||||||||||||||||||
|
(unaudited)
|
||||||||||||||||||||
|
Additional
|
||||||||||||||||||||
|
Common stock
|
Paid in
|
Accumulated
|
||||||||||||||||||
|
Shares
|
Amount
|
Capital
|
Deficit
|
Total
|
||||||||||||||||
|
Balance, January 1, 2015
|
11,179,266 | $ | 11,179 | $ | 19,186,163 | $ | (22,780,404 | ) | $ | (3,583,062 | ) | |||||||||
|
Sale of common stock
|
1,398,760 | 1,399 | 3,040,814 | - | 3,042,213 | |||||||||||||||
|
Common stock issued upon conversion of Series C preferred stock and accrued dividends at $1.50 per share
|
211,668 | 212 | 317,288 | - | 317,500 | |||||||||||||||
|
Common stock issued for services
|
370,000 | 370 | 928,530 | - | 928,900 | |||||||||||||||
|
Reclassify fair value of warrant liability from equity
|
- | - | (4,097,444 | ) | - | (4,097,444 | ) | |||||||||||||
|
Reclassify fair value of derivative liability from equity
|
- | - | (1,242,590 | ) | - | (1,242,590 | ) | |||||||||||||
|
Fair value of vested options
|
- | - | 798,789 | - | 798,789 | |||||||||||||||
|
Preferred stock dividend
|
- | - | (79,395 | ) | - | (79,395 | ) | |||||||||||||
|
Net loss
|
- | - | - | (3,052,906 | ) | (3,052,906 | ) | |||||||||||||
|
Balance, March 31, 2015
|
13,159,694 | $ | 13,160 | $ | 18,852,156 | $ | (25,833,311 | ) | $ | (6,967,995 | ) | |||||||||
See the accompanying notes to the unaudited condensed financial statements
|
BIOSIG TECHNOLOGIES, INC.
|
||||||||
|
CONDENSED STATEMENTS OF CASH FLOWS
|
||||||||
|
(unaudited)
|
||||||||
|
Three Months Ended March 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES:
|
||||||||
|
Net loss
|
$ | (3,052,906 | ) | $ | (1,117,534 | ) | ||
|
Adjustments to reconcile net loss to cash used in operating activities:
|
||||||||
|
Depreciation
|
2,860 | 4,435 | ||||||
|
Amortization of debt discount
|
- | 388,285 | ||||||
|
Equity based compensation
|
1,634,714 | 343,837 | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Prepaid expenses
|
8,744 | (3,924 | ) | |||||
|
Accounts payable
|
(233,155 | ) | (119,856 | ) | ||||
|
Stock based payable
|
419,761 | - | ||||||
|
Deferred rent payable
|
- | (1,006 | ) | |||||
|
Net cash used in operating activities
|
(1,219,982 | ) | (505,763 | ) | ||||
|
CASH FLOWS FROM INVESTING ACTIVITIES:
|
||||||||
|
Purchase of property and equipment
|
(2,684 | ) | - | |||||
|
Net cash used in investing activity
|
(2,684 | ) | - | |||||
|
CASH FLOWS FROM FINANCING ACTIVITIES:
|
||||||||
|
Proceeds from sale of common stock
|
3,042,213 | 229,222 | ||||||
|
Net repayments of related party advances
|
- | (20,281 | ) | |||||
|
Net cash provided by financing activities
|
3,042,213 | 208,941 | ||||||
|
Net (decrease) increase in cash and cash equivalents
|
1,819,547 | (296,822 | ) | |||||
|
Cash and cash equivalents, beginning of the period
|
239,781 | 302,187 | ||||||
|
Cash and cash equivalents, end of the period
|
$ | 2,059,328 | $ | 5,365 | ||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Cash paid during the period for interest
|
$ | 1,115 | $ | 1,098 | ||||
|
Cash paid during the period for income taxes
|
$ | - | $ | - | ||||
|
Non-cash investing and financing activities:
|
||||||||
|
Common stock issued upon conversion of Series C preferred stock and accrued dividends
|
$ | 317,500 | $ | - | ||||
|
Reclassify fair value of derivative and warrant liability from equity
|
$ | 5,440,034 | $ | - | ||||
See the accompanying notes to the unaudited condensed financial statements
NOTE 1 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A summary of the significant accounting policies applied in the preparation of the accompanying financial statements follows.
Business and organization
BioSig Technologies Inc. (the “Company”) was initially incorporated on February 24, 2009 under the laws of the State of Nevada and subsequently re-incorporated in the state of Delaware in 2011. The Company is principally devoted to improving the quality of cardiac recordings obtained during EP studies and catheter ablation procedures. The Company has not generated any revenue to date and consequently its operations are subject to all risks inherent in the establishment of a new business enterprise.
Interim Financial Statements
The unaudited condensed interim financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) for interim financial information and the instructions to Form 10-Q and Rule 8-03 of Regulation S-X. Accordingly, they do not include all of the information and footnotes required by GAAP for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included.
The condensed balance sheet as of December 31, 2014 has been derived from audited financial statements.
Operating results for the three months ended March 31, 2015 are not necessarily indicative of results that may be expected for the year ending December 31, 2015. These condensed financial statements should be read in conjunction with the audited financial statements for the year ended December 31, 2014 filed with the Company’s Form 10-K with the Securities and Exchange Commission on February 20, 2015.
Basis of presentation
The Company's primary efforts are devoted to conducting research and development principally devoted to improving the quality of cardiac recordings obtained during EP studies and catheter ablation procedures. The Company has experienced net losses and negative cash flows from operations since inception and expects these conditions to continue for the foreseeable future. In addition, the Company has stockholders' deficiencies at March 31, 2015 and requires additional financing to fund future operations. Further, the Company does not have any commercial products available for sale and there is no assurance that if approval of their products is received that the Company will be able to generate cash flow to fund operations. In addition, there can be no assurance that the Company's research and development will be successfully completed or that any product will be approved or commercially viable.
The above factors raise substantial doubt as to the Company's ability to continue as a going concern. The accompanying condensed financial statements have been prepared assuming that the Company will continue as a going concern and do not include any adjustments that may result from the outcome of this uncertainty.
Use of estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include the recoverability and useful lives of long-lived assets, the fair value of the Company’s stock, stock-based compensation, fair values relating to warrant and other derivative liabilities and the valuation allowance related to deferred tax assets. Actual results may differ from these estimates.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
Fair Value of Financial Instruments
The Company’s short-term financial instruments, including cash, prepaid expenses and other assets, accounts payable and accrued expenses and other liabilities, consist primarily of instruments without extended maturities, the fair value of which, based on management’s estimates, reasonably approximate their book value. The fair value of the Company’s convertible securities is based on management estimates and reasonably approximates their book value.
Derivative Instrument Liability
The Company accounts for derivative instruments in accordance with ASC 815, which establishes accounting and reporting standards for derivative instruments and hedging activities, including certain derivative instruments embedded in other financial instruments or contracts and requires recognition of all derivatives on the balance sheet at fair value, regardless of hedging relationship designation. Accounting for changes in fair value of the derivative instruments depends on whether the derivatives qualify as hedge relationships and the types of relationships designated are based on the exposures hedged. At March 31, 2015 and December 31, 2014, the Company did not have any derivative instruments that were designated as hedges.
Research and development costs
The Company accounts for research and development costs in accordance with the Accounting Standards Codification subtopic 730-10, Research and Development (“ASC 730-10”). Under ASC 730-10, all research and development costs must be charged to expense as incurred. Accordingly, internal research and development costs are expensed as incurred. Third-party research and developments costs are expensed when the contracted work has been performed or as milestone results have been achieved. Company-sponsored research and development costs related to both present and future products are expensed in the period incurred. The Company incurred research and development expenses of $302,079 and $122,151 for the three months ended March 31, 2015 and 2014, respectively.
Income Taxes
The Company follows Accounting Standards Codification subtopic 740-10, Income Taxes (“ASC 740-10”) for recording the provision for income taxes. Deferred tax assets and liabilities are computed based upon the difference between the financial statement and income tax basis of assets and liabilities using the enacted marginal tax rate applicable when the related asset or liability is expected to be realized or settled. Deferred income tax expenses or benefits are based on the changes in the asset or liability during each period. If available evidence suggests that it is more likely than not that some portion or all of the deferred tax assets will not be realized, a valuation allowance is required to reduce the deferred tax assets to the amount that is more likely than not to be realized. Future changes in such valuation allowance are included in the provision for deferred income taxes in the period of change. Deferred income taxes may arise from temporary differences resulting from income and expense items reported for financial accounting and tax purposes in different periods. Deferred taxes are classified as current or non-current, depending on the classification of assets and liabilities to which they relate. Deferred taxes arising from temporary differences that are not related to an asset or liability are classified as current or non-current depending on the periods in which the temporary differences are expected to reverse and are considered immaterial.
Net Income (loss) Per Common Share
The Company computes earnings (loss) per share under Accounting Standards Codification subtopic 260-10, Earnings Per Share (“ASC 260-10”). Net loss per common share is computed by dividing net loss by the weighted average number of shares of common stock outstanding during the year. Diluted earnings per share, if presented, would include the dilution that would occur upon the exercise or conversion of all potentially dilutive securities into common stock using the “treasury stock” and/or “if converted” methods as applicable.
The computation of basic and diluted loss per share as of March 31, 2015 and 2014 excludes potentially dilutive securities when their inclusion would be anti-dilutive, or if their exercise prices were greater than the average market price of the common stock during the period.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
Potentially dilutive securities excluded from the computation of basic and diluted net income (loss) per share are as follows:
|
March 31,
2015
|
March 31,
2014
|
|||||||
|
Series A convertible preferred stock
|
-
|
501,089
|
||||||
|
Series B convertible preferred stock
|
-
|
451,726
|
||||||
|
Series C convertible preferred stock
|
1,640,667
|
1,854,019
|
||||||
|
Options to purchase common stock
|
6,205,190
|
2,990,977
|
||||||
|
Warrants to purchase common stock
|
7,561,820
|
4,353,831
|
||||||
|
Totals
|
15,407,677
|
10,151,642
|
||||||
Stock Based Compensation
The Company measures the cost of services received in exchange for an award of equity instruments based on the fair value of the award. For employees and directors, the fair value of the award is measured on the grant date and for non-employees, the fair value of the award is generally re-measured on vesting dates and interim financial reporting dates until the service period is complete. The fair value amount is then recognized over the period during which services are required to be provided in exchange for the award, usually the vesting period.
As of March 31, 2015, the Company had 6,205,190 options outstanding to purchase shares of common stock, of which 4,130,240 were vested.
As of December 31, 2014, the Company had 5,990,190 options outstanding to purchase shares of common stock, of which 3,799,559 were vested.
Registration Rights
The Company accounts for registration rights agreements in accordance with the Accounting Standards Codification subtopic 825-20, Registration Payment Arraignments (“ASC 825-20”). Under ASC 825-20, the Company is required to disclose the nature and terms of the arraignment, the maximum potential amount and to assess each reporting period the probable liability under these arraignments and, if exists, to record or adjust the liability to current period operations. On June 23, 2014, the Company filed Form S-1/A became effective with the Securities and Exchange Commission. As such, the Company determined that payments were due under its registration rights agreement and therefore accrued $55,620 as interest expense during the year ended December 31, 2014 for the liability under the registration rights agreements.
Recent Accounting Pronouncements
There are various updates recently issued, most of which represented technical corrections to the accounting literature or application to specific industries and are not expected to a have a material impact on the Company's financial position, results of operations or cash flows.
NOTE 2 – PROPERTY AND EQUIPMENT
Property and equipment as of March 31, 2015 and December 31, 2014 is summarized as follows:
|
March 31,
2015
|
December 31,
2014
|
|||||||
|
Computer equipment
|
$
|
57,584
|
$
|
54,900
|
||||
|
Furniture and fixtures
|
7,803
|
7,803
|
||||||
|
Subtotal
|
65,387
|
62,703
|
||||||
|
Less accumulated depreciation
|
(52,543
|
)
|
(49,683
|
)
|
||||
|
Property and equipment, net
|
$
|
12,844
|
$
|
13,020
|
||||
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
Property and equipment are stated at cost and depreciated using the straight-line method over their estimated useful lives of 3 to 5 years. When retired or otherwise disposed, the related carrying value and accumulated depreciation are removed from the respective accounts and the net difference less any amount realized from disposition, is reflected in earnings.
Depreciation expense was $2,860 and $4,435 for the three months ended March 31, 2015 and 2014, respectively.
NOTE 3 – ACCOUNTS PAYABLE AND ACCRUED EXPENSES
Accounts payable and accrued expenses at March 31, 2015 and December 31, 2014 consist of the following:
|
March 31,
2015
|
December 31,
2014
|
|||||||
|
Accrued accounting and legal
|
$
|
71,952
|
$
|
190,767
|
||||
|
Accrued reimbursements
|
17,305
|
26,792
|
||||||
|
Accrued consulting
|
82,935
|
16,334
|
||||||
|
Accrued research and development expenses
|
42,409
|
93,407
|
||||||
|
Accrued credit card obligations
|
4,735
|
13,278
|
||||||
|
Accrued payroll
|
-
|
62,068
|
||||||
|
Accrued liquidated damages
|
55,620
|
55,620
|
||||||
|
Accrued office and other
|
5,915
|
29,093
|
||||||
|
Accrued settlement related to arbitration
|
40,000
|
66,667
|
||||||
|
$
|
320,871
|
$
|
554,026
|
|||||
NOTE 4 – SERIES C 9% CONVERTIBLE PREFERRED STOCK
On January 9, 2013, the Board of Directors authorized the issuance of up to 4,200 shares of Series C Convertible Preferred Stock (the “Series C Convertible Preferred Stock”).
The Series C convertible preferred stock is entitled to preference over holders of junior stock upon liquidation in the amount of $1,000 plus any accrued and unpaid dividends ; entitled to dividends as a preference to holders of junior stock at a rate of 9% per annum of the Stated Value of $1,000 per share, payable quarterly beginning on September 30, 2013 and are cumulative. The holders of Series C preferred stock have no voting rights, however without the affirmative vote of all the holders of then outstanding shares of the Series C preferred stock, the Company cannot (a) alter or change adversely the powers, preferences or rights given to the Series C preferred stock or alter or amend the Certificate of Designation.
Each share of Series C preferred stock is convertible, at the holder’s option, inclusive of any accrued and unpaid dividends, at conversion price of $1.50 (as reset).
If, at any time while the Series C preferred stock is outstanding, the Company sells or grants any option to purchase or sells or grants any right to re-price, or otherwise disposes of or issues any common stock or common stock equivalents entitling any Person to acquire shares of Common Stock at an effective price per share that is lower than the then conversion price (“Base Conversion Price”), then the conversion price shall be reduced to equal the Base Conversion Price. Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. During 2014, the resets provisions as described above resulted in the conversion price reset to $1.50.
The Series C preferred stock contains triggering events which would require redemption at (i) the greater of 120% of the stated value of $1,000 or the product of the variable weighted average price of the Company’s common stock on the trading day immediately preceding the date of the triggering event and the stated value divided by the then conversion price or (ii) either (a) redeem each Series C preferred share for a redemption price, in shares of the Company’s common stock, equal to a number of shares equal to the (i) above divided by 75%. The Company determined that certain of the defined triggering events were outside the Company’s control and therefore classified the Series C preferred stock outside of equity.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
In connection with the sale of the Series C preferred stock, the Company issued an aggregate of 1,330,627 warrants to purchase the Company’s common stock at $2.61 per share expiring five years from the initial exercise date. The warrant provides if, at any time while the warrant is outstanding, the Company sells or grants any option to purchase or sells or grants any right to re-price, or otherwise disposes of or issues any common stock or common stock equivalents entitling any person to acquire shares of common stock at an effective price per share that is lower than the then conversion price (“base conversion price”), then the conversion price shall be reduced to equal the Base Conversion Price.
Such adjustment shall be made whenever such Common Stock or Common Stock Equivalents are issued. In addition, the warrants provides for at any time after the six month anniversary of the initial exercise date, there is no effective registration statement registering, or no current prospectus available for the resale of the warrant shares by the holder, then the warrant may only be exercised, in whole or in part, at such time by means of a “cashless exercise” in which the holder shall be entitled to receive a number of Warrant Shares equal to defined formula. During 2014, the resets provisions as described above resulted in an additional 984,674 warrants issued with an exercise price reset to $1.50 all Series C warrants.
In accordance with ASC 470-20, the Company recognized an embedded beneficial conversion feature present in the Series C preferred stock when it was issued. The Company allocated the net proceeds between the intrinsic value of the conversion option ($1,303,671) and the warrants ($1,064,739) to additional paid-in capital. The aggregate debt discount, comprised of the relative intrinsic value the conversion option ($1,303,671), relative fair value of the warrants ($1,064,739), and the issuance costs ($412,590); total of $2,781,000, is amortized over one year as interest expense, the date a possible redemption feature, outside of the Company’s control, would be available to the Series C stockholders
During the month of February 2013, the holders of previously issued convertible bridge notes converted into 600 shares of the Company’s Series C 9% Convertible Preferred Stock.
During the months of February, March, May, and July 2013, the Company sold an aggregate of 2,181 shares of the Company’s Series C 9% Convertible Preferred Stock for net proceeds of $1,814,910.
At the time of issuance and till March 31, 2015, the Company determined that the anti-dilutive provisions embedded in the Series C 9% Convertible Preferred Stock and related issued warrants did not meet the defined criteria of a derivative in such that the net settlement requirement of delivery of common shares does not meet the “readily convertible to cash” as described in Accounting Standards Codification 815 and therefore bifurcation is not required. There was no established market for the Company’s common stock. As described in Note 6, March 31, 2015, the Company determined a market has been established for the Company’s common stock and accordingly, reclassified the fair value of the embedded reset provisions of the Series C Preferred Stock and warrants of $1,242,590 and $4,097,444, respectively, from equity to liabilities.
As of March 31, 2015, the Company valued the reset provisions of the Series C Preferred Stock and warrants in accordance with ASC 470-20 using the Multinomial Lattice pricing model and the following assumptions: contractual terms of 2.78 to 3.50 years, a risk free interest rate of 0.56% to 0.89%, a dividend yield of 0%, and volatility of 141.00%.
During January 2015, the Company issued an aggregate of 42,334 shares of its commons stock in exchange for 50 shares of the Company’s Series C 9% Convertible Preferred Stock and accrued dividends.
During March 2015, the Company issued an aggregate of 169,334 shares of its commons stock in exchange for 200 shares of the Company’s Series C 9% Convertible Preferred Stock and accrued dividends.
Series C preferred stock issued and outstanding totaled 2,461 and 2,711 as of March 31, 2015 and December 31, 2014, respectively. As of March 31, 2015 and December 31, 2014, the Company has accrued $456,964 and $445,069 dividends payable on the Series C preferred stock.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
Registration Rights Agreement
The Company entered into a Registration Rights Agreement in connection with the sale and issuance of the Series C preferred stock. The Company is required to file a registration statement registering for resale the (a) common stock issuable upon conversion in full of the Preferred Stock (assuming on such date the shares of Preferred Stock are converted in full without regard to any conversion limitations therein), (b) all shares of Common Stock issuable as dividends and “Make-Whole Payments” (as defined in the Certificate of Designation) on the Preferred Stock assuming all dividend and Make-Whole Payments are made in shares of Common Stock and the Preferred Stock is held for at least 3 years, (c) all warrant shares then issuable upon exercise of the Warrants (assuming on such date the warrants are exercised in full without regard to any exercise limitations therein), (d) any additional shares of Common Stock issuable in connection with any anti-dilution provisions in the Preferred Stock or the Warrants (in each case, without giving effect to any limitations on conversion set forth in the Certificate of Designation or limitations on exercise set forth in the Warrants) and (e) any securities issued or then issuable upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing. The Company is required to file a registration statement and must be declared effective no later than 210 days from the date of termination of the sale the Series C preferred stock.
The Company was required to maintain the effectiveness of the registration statement from its effective date unless all securities registered under the registration statement have been sold or are otherwise able to be sold. If the Company failed to comply with the registration statement effective date requirements, the Company is required to pay the investors a fee equal to 0.25% of the Purchaser’s investment, for each 30-day period of delay, subject to a maximum payment of 3% to each Purchaser.
On June 23 2014, the Company became effective and met its required filing requirement. The Company did not meet the effectiveness obligation by November 22, 2013. As a result, the Company accrued $55,620 as interest expense for liquidating damages due under the registration rights agreement.
NOTE 5 – WARRANT AND DERIVATIVE LIABILITIES
At the time of issuance and till March 31, 2015, the Company determined that the anti-dilutive provisions embedded in the Series C 9% Convertible Preferred Stock and related warrants (see Note 5) did not meet the defined criteria of a derivative in such that the net settlement requirement of delivery of common shares does not meet the “readily convertible to cash” as described in Accounting Standards Codification 815 and therefore bifurcation was not required. There was no established market for the Company’s common stock. As of March 31, 2015, the Company determined a market had been established for the Company’s common stock and accordingly, reclassified from equity to liability treatment the fair value of the embedded reset provisions of the Series C Preferred Stock and warrants of $1,242,590 and $4,097,444, respectively. .
The Company valued the reset provisions of the Series C Preferred Stock in accordance with ASC 470-20 using the Multinomial Lattice pricing model and the following assumptions: estimated contractual terms, a risk free interest rate of 0.56% to 0.89, a dividend yield of 0%, and volatility of 141.00%.
NOTE 6 – STOCKHOLDER EQUITY
Preferred stock
The Company is authorized to issue 1,000,000 shares of $0.001 par value preferred stock. As of March 31, 2015 and December 31, 2014, the Company has designated 200 shares of Series A preferred stock, 600 shares of Series B preferred stock and 4,200 , shares of Series C preferred stock. As of March 31, 2015 and December 31, 2014, there were no outstanding shares of Series A and Series B preferred stock.
During January 2015, the Company issued an aggregate of 42,334 shares of its commons stock in exchange for 50 shares of the Company’s Series C 9% Convertible preferred stock and accrued dividends.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
During March 2015, the Company issued an aggregate of 169,334 shares of its commons stock in exchange for 200 shares of the Company’s Series C 9% Convertible preferred stock and accrued dividends.
As of March 31, 2015 and December 31, 2014, the Company has 2,461 and 2,711 Series C 9% Convertible Preferred Stock issued and outstanding.
Common stock
The Company is authorized to issue 50,000,000 shares of $0.001 par value common stock. As of March 31, 2015 and December 31, 2014, the Company has 13,159,694 and 11,179,266 shares issued and outstanding, respectively.
During the three months ended March 31, 2015, the Company issued an aggregate of 370,000 shares of common stock under the terms of its 2012 Equity Plan for services rendered totaling $928,900 ($2.51 average per share).
During the three months ended March 31, 2015, the Company entered into securities purchase agreements with investors pursuant to which the Company issued 1,398,760 shares of common stock and warrants for aggregate net proceeds of $3,042,213.
In connection with the securities purchase agreements described above, the Company entered into a registration rights agreement whereas the Company is required to file a registration statement registering for resale the a) all of the purchase agreement Shares, (b) all investor warrant Shares then issuable upon exercise of the investor warrants, (c) the shares of common stock underlying the investment banker warrants; (d) any additional shares of Common Stock issuable in connection with any anti-dilution provisions in the investor warrants and the investment banker warrants (e) any securities issued or then issuable upon any stock split, dividend or other distribution, recapitalization or similar event with respect to the foregoing. The Company is required to file a registration statement the 45 days following the final closing date and must be declared effective no later than 180 days from the filing date.
The Company was required to maintain the effectiveness of the registration statement from its effective date unless all securities registered under the registration statement have been sold or are otherwise able to be sold. If the Company failed to comply with the registration statement filing and effective date requirements, the Company is required to pay the investors a fee equal to 1% of the Purchaser’s investment, for each 30-day period of delay, subject to a maximum payment of 6% to each Purchaser.
Stock based payable
The Company is obligated to issue shares of its common stock to board members and consultants for past and future services. The estimated liability as of March 31, 2015 and December 31, 2014 of $646,066 and $226,305 was determined based on services rendered for past services as of March 31, 2015 and December 31, 2014, respectively.
As of March 31, 2015, the Company is obligated to issue an aggregate of 352,500 shares of its common stock. These shares were considered issued as of the date of obligation in determining the weighted average number of outstanding shares used in the basic loss per common share calculation.
NOTE 7 – OPTIONS AND WARRANTS
On October 19, 2012, the Company’s Board of Directors approved the 2012 Equity Incentive Plan (“the “2012 Plan) and terminated the Long-Term Incentive Plan (the “2011 Plan”). The Plan provides for the issuance of options to purchase up to 8,806,123,( as amended) shares of the Company’s common stock to officers, directors, employees and consultants of the Company (as amended). Under the terms of the Plan the Company may issue Incentive Stock Options as defined by the Internal Revenue Code to employees of the Company only and nonstatutory options. The Board of Directors of the Company determines the exercise price, vesting and expiration period of the grants under the Plan. However, the exercise price of an Incentive Stock Option should not be less than 110% of fair value of the common stock at the date of the grant for a 10% or more stockholder and 100% of fair value for a grantee who is not 10% stockholder. The fair value of the common stock is determined based on quoted market price or in absence of such quoted market price, by the Board of Directors in good faith.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
Additionally, the vesting period of the grants under the Plan will be determined by the Committee, in its sole discretion, and expiration period not more than ten years. The Company reserved 1,250,000 shares of its common stock for future issuance under the terms of the Plan.
During the three months ended March 31, 2015, the Company granted an aggregate of 200,000 options and 370,000 stock grants to officers, directors and key consultants.
The following table presents information related to stock options at March 31, 2015:
|
Options Outstanding
|
Options Exercisable
|
|||||||||||||
|
Weighted
|
||||||||||||||
|
Average
|
Exercisable
|
|||||||||||||
|
Exercise
|
Number of
|
Remaining Life
|
Number of
|
|||||||||||
|
Price
|
Options
|
In Years
|
Options
|
|||||||||||
|
$
|
1.01-2.00
|
834,642
|
4.5
|
541,642
|
||||||||||
|
2.01-2.50
|
5,370,548
|
6.7
|
3,588,598
|
|||||||||||
|
6,205,190
|
6.4
|
4,130,240
|
||||||||||||
A summary of the stock option activity and related information for the 2012 Plan for the three months ended March 31, 2015 is as follows:
|
Weighted-Average
|
||||||||||||||||
|
Weighted-Average
|
Remaining
|
Aggregate
|
||||||||||||||
|
Shares
|
Exercise Price
|
Contractual Term
|
Intrinsic Value
|
|||||||||||||
|
Outstanding at January 1, 2015
|
5,990,190
|
$
|
2.25
|
6.7
|
3,267,692
|
|||||||||||
|
Grants
|
215,000
|
2.47
|
7.0
|
-
|
||||||||||||
|
Exercised
|
||||||||||||||||
|
Canceled
|
||||||||||||||||
|
Outstanding at March 31, 2015
|
6,205,190
|
$
|
2.26
|
6.4
|
$
|
1,098,888
|
||||||||||
|
Exercisable at March 31, 2015
|
4,130,240
|
$
|
2.25
|
5.9
|
$
|
797,753
|
||||||||||
The aggregate intrinsic value in the preceding tables represents the total pretax intrinsic value, based on options with an exercise price less than the Company’s estimated market stock price of $2.40 as of March 31, 2015, which would have been received by the option holders had those option holders exercised their options as of that date.
Option valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards was estimated using the Black-Scholes option model with a volatility figure derived from an index of historical stock prices of comparable entities until sufficient data exists to estimate the volatility using the Company’s own historical stock prices. Management determined this assumption to be a more accurate indicator of value. The Company accounts for the expected life of options based on the contractual life of options for non-employees. For employees, the Company accounts for the expected life of options in accordance with the “simplified” method, which is used for “plain-vanilla” options, as defined in the accounting standards codification. The risk-free interest rate was determined from the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the options. The fair value of stock-based payment awards during the three months ended March 31, 2015 was estimated using the Black-Scholes pricing model.
In addition, the Company is required to estimate the expected forfeiture rate and only recognize expense for those shares expected to vest. In estimating the Company’s forfeiture rate, the Company analyzed its historical forfeiture rate, the remaining lives of unvested options, and the number of vested options as a percentage of total options outstanding.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
If the Company’s actual forfeiture rate is materially different from its estimate, or if the Company reevaluates the forfeiture rate in the future, the stock-based compensation expense could be significantly different from what the Company has recorded in the current period.
The Company estimated forfeitures related to option grants at a weighted average annual rate of 0% per year, as the Company does not yet have adequate historical data, for options granted during the three months ended March 31, 2015.
During the months ended March 31, 2015, the Company granted an aggregate of 215,000 options to purchase the Company stock in connection with the services rendered at the exercise prices from $2.00 to $2.50 per share for a term of seven years with 15,000 vesting immediately and 200,000 over two years at 50% for the first and second anniversary.
The fair value of the granted options for three months ended March 31, 2015 was determined using the Black Scholes option pricing model with the following assumptions:
|
Dividend yield:
|
-0- | % | ||
|
Volatility
|
129.54% to 130.30 | % | ||
|
Risk free rate:
|
1.19% to 1.79 | % | ||
|
Expected life:
|
7 years
|
|||
|
Estimated fair value of the Company’s common stock
|
$ | 1.99 to $2.24 | ||
|
Estimated forfeiture rate
|
0 | % | ||
The fair value of all options vesting during the three months ended March 31, 2015 and 2014 of $798,789 and $343,837, respectively, was charged to current period operations. Unrecognized compensation expense of $1,970,428 at March 31, 2015 will be expensed in future periods.
Warrants
The following table summarizes information with respect to outstanding warrants to purchase common stock of the Company, all of which were exercisable, at December 31, 2014:
|
Exercise
|
Number
|
Expiration
|
|||||
|
Price
|
Outstanding
|
Date
|
|||||
| $ | 0.001 | 383,320 |
January 2020
|
||||
| $ | 1.50 | 3,721,518 |
February 2018 to September 2018
|
||||
| $ | 1.84 | 35,076 |
January 2020
|
||||
| $ | 2.02 | 30,755 |
January 2020
|
||||
| $ | 2.50 | 1,603,600 |
July 2015
|
||||
| $ | 2.75 | 228,720 |
August 2019 to September 2019
|
||||
| $ | 3.67 | 218,275 |
December 2018 to January 2019
|
||||
| $ | 3.75 | 1,340,556 |
April 2019 to March 2020
|
||||
| 7,561,820 | |||||||
On January 23, 2015, the Company issued an aggregate of 428,400 and 321,300 warrants to purchase the Company’s common stock at $2.50 and $3.75 per share, respectively, expiring on July 31, 2015 and March 31, 2020, respectively, in connection with the sale of the Company’s common stock.
On February 10, 2015, the Company issued an aggregate of 337,000 and 252,750 warrants to purchase the Company’s common stock at $2.50 and $3.75 per share, respectively, expiring on July 31, 2015 and March 31, 2020, respectively, in connection with the sale of the Company’s common stock.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
On February 27, 2015, the Company issued an aggregate of 223,000 and 167,250 warrants to purchase the Company’s common stock at $2.50 and $3.75 per share, respectively, expiring on July 31, 2015 and March 31, 2020, respectively, in connection with the sale of the Company’s common stock.
On March 31, 2015, the Company issued an aggregate of 410,360 and 307,770 warrants to purchase the Company’s common stock at $2.50 and $3.75 per share, respectively, expiring on July 31, 2015 and March 31, 2020, respectively, in connection with the sale of the Company’s common stock.
A summary of the warrant activity for the three months ended March 31, 2015 is as follows:
|
Weighted-Average
|
||||||||||||||||
|
Weighted-Average
|
Remaining
|
Aggregate
|
||||||||||||||
|
Shares
|
Exercise Price
|
Contractual Term
|
Intrinsic Value
|
|||||||||||||
|
Outstanding at January 1, 2015
|
5,113,990
|
$
|
1.71
|
3.6
|
6,041,436
|
|||||||||||
|
Grants
|
2,447,830
|
3.04
|
2.4
|
-
|
||||||||||||
|
Exercised
|
||||||||||||||||
|
Canceled
|
||||||||||||||||
|
Outstanding at March 31, 2015
|
7,561,820
|
$
|
2.14
|
3.0
|
$
|
4,300,280
|
||||||||||
|
Vested and expected to vest at March 31, 2015
|
7,561,820
|
$
|
2.14
|
3.0
|
$
|
4,300,280
|
||||||||||
|
Exercisable at March 31, 2015
|
7,561,820
|
$
|
2.14
|
3.0
|
$
|
4,300,280
|
||||||||||
The aggregate intrinsic value in the preceding tables represents the total pretax intrinsic value, based on options with an exercise price less than the Company’s estimated market stock price of $2.40 as of March 31, 2015, which would have been received by the option holders had those option holders exercised their options as of that date.
NOTE 8 – FAIR VALUE MEASUREMENT
The Company adopted the provisions of Accounting Standards Codification subtopic 825-10, Financial Instruments (“ASC 825-10”). ASC 825-10 defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required or permitted to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact and considers assumptions that market participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance. ASC 825-10 establishes a fair value hierarchy that requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 825-10 establishes three levels of inputs that may be used to measure fair value:
Level 1 – Quoted prices in active markets for identical assets or liabilities.
Level 2 – Observable inputs other than Level 1 prices such as quoted prices for similar assets or liabilities; quoted prices in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which all significant inputs are observable or can be derived principally from or corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – Unobservable inputs to the valuation methodology that are significant to the measurement of fair value of assets or liabilities.
All items required to be recorded or measured on a recurring basis are based upon level 3 inputs.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
To the extent that valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, for disclosure purposes, the level in the fair value hierarchy within which the fair value measurement is disclosed and is determined based on the lowest level input that is significant to the fair value measurement.
The carrying value of the Company’s cash and cash equivalents, accounts payable and other current assets and liabilities approximate fair value because of their short-term maturity.
As of March 31, 2015 or December 31, 2014, the Company did not have any items that would be classified as level 1 or 2 disclosures.
The Company recognizes its derivative and warrant liabilities as level 3 and values its derivatives using the methods discussed in Note 6. While the Company believes that its valuation methods are appropriate and consistent with other market participants, it recognizes that the use of different methodologies or assumptions to determine the fair value of certain financial instruments could result in a different estimate of fair value at the reporting date. The primary assumptions that would significantly affect the fair values using the methods discussed in Note 6 are that of volatility and market price of the underlying common stock of the Company.
As of March 31, 2015 and December 31, 2014, the Company did not have any derivative instruments that were designated as hedges.
The derivative and warrant liability as of March 31, 2015, in the amount of $1,242,590 and $4,097,444 has a level 3 classification, respectively.
The following table provides a summary of changes in fair value of the Company’s Level 3 financial liabilities as of March 31, 2015:
| Warrant
Liability
|
Derivative |
|
||||||
|
Balance, December 31, 2014
|
$
|
-
|
$
|
-
|
||||
|
Total (gains) losses
|
||||||||
|
Initial fair value of derivative at March 31, 2015, reclassified from equity
|
—
|
1,242,590
|
||||||
|
Initial fair value of warrant liability at March 31, 2015, reclassified from equity
|
4,097,444
|
—
|
||||||
|
Balance, March 31, 2015
|
$
|
4,097,444
|
$
|
1,242,590
|
||||
Fluctuations in the Company’s stock price are a primary driver for the changes in the derivative valuations during each reporting period. As the stock price increases for each of the related derivative instruments, the value to the holder of the instrument generally increases, therefore increasing the liability on the Company’s balance sheet. Additionally, stock price volatility is one of the significant unobservable inputs used in the fair value measurement of each of the Company’s derivative instruments.
BIOSIG TECHNOLOGIES, INC.
NOTES TO THE CONDENSED FINANCIAL STATEMENTS
MARCH 31, 2015
(unaudited)
NOTE 9 – SUBSEQUENT EVENTS
On April 9, 2015, the Company granted options to acquire 300,000 shares of its common stock at an exercise price of $3.99 for seven years, vesting immediately upon appointment of a new board of directors member.
In April 2015, the Company issued an aggregate of 131,234 shares of its common stock in exchange for 155 shares of Series C 9% Convertible preferred stock and accrued dividends.
In April 2015, the Company issued 84,033 shares of its common stock in exchange for the exercise of 130,084 warrants exercisable at $1.50, on a cashless basis.
In April 2015, the Company issued an aggregate of 200,000 shares of its common stock to board of directors for services rendered.
In April 2015, the Company issued 50,000 shares of its common stock to a consultant for services rendered.
In April 2015, the Company issued an aggregate of 10,000 shares of its common stock for the exercise of previously issued options at $2.09 per share.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
We are paying all of the selling stockholders’ expenses related to this offering, except that the selling stockholders will pay any applicable underwriting discounts and commissions. The fees and expenses payable by us in connection with this Registration Statement are estimated as follows:
|
Securities and Exchange Commission Registration Fee
|
$
|
1,903.08
|
||
|
Accounting Fees and Expenses
|
$
|
4,000
|
||
|
Legal Fees and Expenses
|
$
|
30,000
|
||
|
Printing Expenses
|
$
|
3,000
|
||
|
Miscellaneous Fees and Expenses
|
$
|
500
|
||
|
Total
|
$
|
39,403.08
|
Item 14. Indemnification of Directors and Officers.
Section 145 of the General Corporation Law of the State of Delaware provides, in general, that a corporation incorporated under the laws of the State of Delaware, as we are, may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding (other than a derivative action by or in the right of the corporation) by reason of the fact that such person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another enterprise, against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe such person’s conduct was unlawful. In the case of a derivative action, a Delaware corporation may indemnify any such person against expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit if such person acted in good faith and in a manner such person reasonably believed to be in or not opposed to the best interests of the corporation, except that no indemnification will be made in respect of any claim, issue or matter as to which such person will have been adjudged to be liable to the corporation unless and only to the extent that the Court of Chancery of the State of Delaware or any other court in which such action was brought determines such person is fairly and reasonably entitled to indemnity for such expenses.
We are also permitted to apply for, and currently maintain, insurance on behalf of any director, officer, employee or other agent for liability arising out of his actions, whether or not the General Corporation Law of the State of Delaware would permit indemnification.
Item 15. Recent Sales of Unregistered Securities.
In August and December 2012, we issued an aggregate of 30,000 shares of our common stock as payment for services provided by a total of two service providers. The shares were valued at $2.00 per share. The securities issued were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended.
On January 18, 2013, in connection with the previous private placement of our Series A Preferred Stock, we issued a seven-year warrant to purchase up to 35,076 shares of common stock at an exercise price of $1.84 per share, to Laidlaw & Company (UK) Ltd., our placement agent in the private placement. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
On April 30, 2012, we entered into a securities purchase agreement with 24 accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued 177.5 shares of our Series B Preferred Stock for aggregate cash proceeds of $877,500. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On January 18, 2013, in connection the above described private placement, we issued a seven-year warrant to purchase up to 30,755 shares of common stock at an exercise price of $2.02 per share, to Laidlaw & Company (UK) Ltd., our placement agent in the private placement. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
From July to December 2012, we entered into a securities purchase agreement with 6 accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued bridge notes in the aggregate amount of $600,000 and related warrants. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) of the Securities Act of 1933, as amended.
On January 7, 2013, as consideration for providing general financial advisory services, we issued to Jamess Capital Group LLC a seven-year warrant to purchase up to 383,320 shares of common stock at an exercise price of $0.001 per share. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
On February 6, 2013, we entered into a securities purchase agreement with 9 accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued 1,400 shares of our Series C Preferred Stock and five-year warrants to purchase 669,857 shares of our common stock for aggregate cash proceeds of $800,000 and the conversion of $600,000 of our outstanding bridge notes. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On February 7, 2013, as consideration for providing placement support services, we issued to Ellis International Ltd. 15,500 shares of common stock, with a value of $.001 per share. The shares of common stock were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and issued in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
On February 12, 2013, as consideration for providing placement support services, we issued to Ellis International Ltd. a five-year warrant to purchase up to 8,700 shares of common stock at an exercise price of $2.61 per share. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and issued in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
On February 12, 2013, as consideration for consulting services, we granted 283,750 non-employee options to a consultant exercisable at a price equal to $2.09 per share. The options vest at 48,611 shares on each of the first, second and third month anniversaries, with the remainder of the shares vesting in equal amounts each monthly anniversary for the 24 months thereafter. The options and the underlying shares of common stock were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and issued in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
From February to July 2013, over four separate closings, we entered into a securities purchase agreement with 32 accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued 1,381 shares of our Series C Preferred Stock and five-year warrants to purchase 1,330,629 shares of our common stock for aggregate cash proceeds of $1,381,000. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On May 2, 2013, as consideration for providing advisory services, we issued to Mr. Chaussy, our chief financial officer, 5,862 shares of common stock, with a value of $2.09 per share. The shares of common stock were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and issued in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
On July 15, 2013, we issued five-year warrants to purchase 289,730 shares of our common stock to certain holders of our Series C Preferred Stock in consideration for amending certain provisions of the securities purchase agreement and registration rights agreement related to our Series C Preferred Stock. The warrants issued were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were issued in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On July 15, 2013, in connection with the above described private placement, we issued a five-year warrant to purchase up to 177,057 shares of common stock at an exercise price of $2.61 per share, to Laidlaw & Company (UK) Ltd., our placement agent in the private placement. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering. Laidlaw & Company (UK) Ltd. was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the private placement.
On August 7, 2013, we issued to the holders of our bridge notes in lieu of cash for payment of the interest accrued on the bridge notes that were exchanged for shares of our Series C Preferred Stock and the related warrants on February 6, 2013 an aggregate of 8,941 shares of common stock, with a value of $2.09 per share. The shares of common stock were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and issued in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering.
On October 14, 2013, we issued five-year warrants to purchase 332,684 shares of our common stock to certain holders of our Series C Preferred Stock in consideration for amending certain provisions of the securities purchase agreement related to our Series C Preferred Stock. The warrants issued were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were issued in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On December 31, 2013 and January 31, 2014, in two separate closings, we entered into a securities purchase agreement with Mr. Londoner, Mr. Steinhouse and thirteen other accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued 323,218 shares of our common stock and five-year warrants to purchase 161,611 shares of our common stock for aggregate cash proceeds of $791,885, including a conversion of debt of $228,000. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On December 31, 2013 and January 31, 2014, in connection with the above described private placement, we issued five-year warrants to purchase up to 40,327 shares of common stock upon the same terms as the warrants in the above described private placement to Laidlaw & Company (UK) Ltd., our placement agent in the private placement. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering. Laidlaw & Company (UK) Ltd. was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the private placement.
On April 4, 2014 and April 30, 2014, in two separate closings, we entered into a securities purchase agreement with twenty eight accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued 229,760 shares of our common stock and five-year warrants to purchase 114,880 shares of our common stock for aggregate cash proceeds of $574,400. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On April 30, 2014, in connection with the above described private placement, we issued a five-year warrant to purchase up to 22,976 shares of common stock upon the same terms as the warrants in the above described private placement to Laidlaw & Company (UK) Ltd., our placement agent in the private placement. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering. Laidlaw & Company (UK) Ltd. was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the private placement.
On August 15, 2014 and September 12, 2014, in two separate closing, we entered into a securities purchase agreement with 23 accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued 381,200 shares of our common stock and five-year warrants to purchase 190,600 shares of our common stock for aggregate cash proceeds of $953,000. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On August 15, 2014 and September 12, 2014, in connection with the above described private placement, we issued a warrant to purchase up to an aggregate of 38,120 shares of common stock upon the same terms as the warrants in the above described private placement to Laidlaw & Company (UK) Ltd., our placement agent in the private placement. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering. Laidlaw & Company (UK) Ltd. was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the private placement.
On December 19, 2014, December 30, 2014, January 23, 2015, February 10, 2015, February 27, 2015 and March 31, 2015, in six separate closing, we entered into a securities purchase agreement with 102 accredited investors (as defined by Rule 501 under the Securities Act of 1933, as amended), pursuant to which we issued 1,603,600 shares of our common stock, “A” warrants expiring July 31, 2015 to purchase 1,603,600 shares of our common stock and “B” warrants expiring March 21, 2020 to purchase 801,800 shares of our common stock for aggregate cash proceeds of $4,009,000. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
On March 31, 2015, in connection with the above described private placement, we issued a warrant to purchase up to 400,900 shares of common stock upon the same terms as the “B” warrants in the above described private placement to Laidlaw & Company (UK) Ltd., our placement agent in the private placement. The warrant was not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and was offered and sold in reliance on the exemption from registration afforded by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended, and corresponding provisions of state securities laws, which exempt transactions by an issuer not involving a public offering. Laidlaw & Company (UK) Ltd. was an accredited investor (as defined by Rule 501 under the Securities Act of 1933, as amended) at the time of the private placement.
On May 11, 2015, we entered into a securities purchase agreement with Alpha Capital Anstalt and a securities purchase agreement with Brio Capital Master Fund Ltd., pursuant to which we issued 450 shares of our Series C Preferred Stock and five-year warrants to purchase 374,641 shares of our common stock for aggregate cash proceeds of $450,000. The securities sold in this offering were not registered under the Securities Act of 1933, as amended, or the securities laws of any state, and were offered and sold in reliance on the exemption from registration under the Securities Act of 1933, as amended, provided by Section 4(2) and Regulation D (Rule 506) under the Securities Act of 1933, as amended.
Item 16. Exhibits and Financial Statement Schedules.
|
Exhibit No.
|
Description
|
|
|
3.1
|
Amended and Restated Certificate of Incorporation of BioSig Technologies, Inc. (incorporated by reference to Exhibit 3.1 to the Form S-1 filed on July 22, 2013)
|
|
|
3.2
|
Certificate of Amendment of the Amended and Restated Certificate of Incorporation of BioSig Technologies, Inc. (incorporated by reference to Exhibit 3.2 to the Form S-1 filed on July 22, 2013)
|
|
|
3.3
|
Certificate of Second Amendment to the Amended and Restated Certificate of Incorporation of BioSig Technologies, Inc. (incorporated by reference to Exhibit 3.3 to the Form S-1 filed on July 22, 2013)
|
|
|
3.4
|
Certificate of Third Amendment to the Amended and Restated Certificate of Incorporation of BioSig Technologies, Inc. (incorporated by reference to Exhibit 3.5 to the Form S-1/A filed on January 21, 2014)
|
|
|
3.5
|
Certificate of Fourth Amendment to the Amended and Restated Certificate of Incorporation of BioSig Technologies, Inc. (incorporated by reference to Exhibit 3.6 to the Form S-1/A filed on March 28, 2014)
|
|
|
3.6
|
Certificate of Fifth Amendment to the Amended and Restated Certificate of Incorporation of BioSig Technologies, Inc. (incorporated by reference to Exhibit 3.1 to the Form 8-K filed on August 21, 2014)
|
|
|
3.7
|
Bylaws of BioSig Technologies, Inc. (incorporated by reference to Exhibit 3.4 to the Form S-1 filed on July 22, 2013)
|
|
|
5.1
|
||
|
10.1
|
BioSig Technologies, Inc. 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.1 to the Form S-1 filed on July 22, 2013)
|
|
|
10.2
|
Form of Stock Option Agreement under the 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 to the Form S-1 filed on July 22, 2013)
|
|
|
10.3
|
Securities Purchase Agreement, dated September 19, 2011, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.3 to the Form S-1 filed on July 22, 2013)
|
|
|
10.4
|
Securities Purchase Agreement, dated December 27, 2011, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.4 to the Form S-1 filed on July 22, 2013)
|
|
|
10.5
|
Securities Purchase Agreement, dated February 6, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.5 to the Form S-1 filed on July 22, 2013)
|
|
|
10.6
|
Registration Rights Agreement, dated February 6, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.6 to the Form S-1 filed on July 22, 2013)
|
|
|
10.7
|
Form of Warrant used in connection with February 6, 2013 private placement (incorporated by reference to Exhibit 10.7 to the Form S-1 filed on July 22, 2013)
|
|
10.8
|
Amendment Agreement No. 1 to Securities Purchase Agreement and Registration Rights Agreement, dated February 25, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.8 to the Form S-1 filed on July 22, 2013)
|
|
|
10.9
|
Amendment Agreement No. 2 to Securities Purchase Agreement, dated April 12, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.9 to the Form S-1 filed on July 22, 2013)
|
|
|
10.10
|
Amendment Agreement No. 3 to Securities Purchase Agreement and Registration Rights Agreement, dated June 25, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.10 to the Form S-1 filed on July 22, 2013)
|
|
|
10.11
|
Office Lease Agreement, dated August 9, 2011, by and between BioSig Technologies, Inc. and Douglas Emmett 1993, LLC (incorporated by reference to Exhibit 10.11 to the Form S-1 filed on July 22, 2013)
|
|
|
10.12
|
Employment Agreement, dated March 1, 2013, by and between BioSig Technologies, Inc. and Kenneth Londoner (incorporated by reference to Exhibit 10.12 to the Form S-1 filed on July 22, 2013)
|
|
|
10.13
|
Indemnity Agreement, dated May 2, 2013 by and between BioSig Technologies, Inc. and Seth H. Z. Fischer (incorporated by reference to Exhibit 10.14 to the Form S-1 filed on July 22, 2013)
|
|
|
10.14
|
Consulting Agreement, dated August 1, 2012, by and between BioSig Technologies, Inc. and Asher Holzer (incorporated by reference to Exhibit 10.15 to the Form S-1 filed on July 22, 2013)
|
|
|
10.15
|
Unsecured Promissory Note made by BioSig Technologies, Inc. in favor of Kenneth Londoner, dated November 21, 2012 (incorporated by reference to Exhibit 10.19 to the Form S-1/A filed on September 11, 2013)
|
|
|
10.16
|
Form of 8% Senior Convertible Promissory Note issued pursuant to Bridge Loan Agreement, dated July 20, 2012 (incorporated by reference to Exhibit 10.20 to the Form S-1/A filed on September 11, 2013)
|
|
|
10.17
|
Promissory Note made by BioSig Technologies, Inc. in favor of Kenneth Londoner, dated December 6, 2012 (incorporated by reference to Exhibit 10.21 to the Form S-1/A filed on September 11, 2013)
|
|
|
10.18
|
Amendment Agreement No. 4 to Securities Purchase Agreement, dated October 14, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.23 to the Form S-1/A filed on January 21, 2014)
|
|
10.19
|
Securities Purchase Agreement, dated December 31, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.24 to the Form S-1/A filed on January 21, 2014)
|
|
|
10.20
|
Registration Rights Agreement, dated December 31, 2013, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.25 to the Form S-1/A filed on January 21, 2014)
|
|
|
10.21
|
Form of Warrant used in connection with December 31, 2013 private placement (incorporated by reference to Exhibit 10.26 to the Form S-1/A filed on January 21, 2014)
|
|
|
10.22
|
Amendment No. 1 to the BioSig Technologies, Inc. 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.27 to the Form S-1/A filed on March 28, 2014)
|
|
|
10.23
|
Amendment Agreement No. 5 to Securities Purchase Agreement, dated March 24, 2014, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.28 to the Form S-1/A filed on March 28, 2014)
|
|
|
10.24
|
Patent Assignment, dated March 17, 2014, by and among Budimir Drakulic, Thomas Foxall, Sina Fakhar and Branislav Vlajinic and BioSig Technologies, Inc. (incorporated by reference to Exhibit 10.29 to the Form S-1/A filed on May 1, 2014)
|
|
|
10.25
|
Securities Purchase Agreement, dated April 4, 2014, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.30 to the Form S-1/A filed on May 1, 2014)
|
|
|
10.26
|
Registration Rights Agreement, dated April 4, 2014, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.31 to the Form S-1/A filed on May 1, 2014)
|
|
|
10.27
|
Form of Warrant used in connection with April 4, 2014 private placement (incorporated by reference to Exhibit 10.32 to the Form S-1/A filed on May 1, 2014)
|
|
|
10.28
|
Consulting Agreement, dated December 10, 2010, by and between BioSig Technologies, Inc. and Jonathan Steinhouse (incorporated by reference to Exhibit 10.33 to the Form S-1/A filed on May 22, 2014)
|
|
|
10.29
|
Executive Employment Agreement, dated July 15, 2014, by and between BioSig Technologies, Inc. and Gregory Cash (incorporated by reference to Exhibit 10.1 to the Form 8-K filed on July 21, 2014)
|
|
|
10.30
|
Incentive Stock Option Agreement, dated July 15, 2014, by and between BioSig Technologies, Inc. and Gregory Cash (incorporated by reference to Exhibit 10.2 to the Form 8-K filed on July 21, 2014)
|
|
|
10.31
|
Securities Purchase Agreement, dated as of August 15, 2014, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.2 to the Form 8-K filed on August 21, 2014)
|
|
|
10.32
|
Registration Rights Agreement, dated as of August 15, 2014, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.3 to the Form 8-K filed on August 21, 2014)
|
|
|
10.33
|
Form of Warrant used in connection with August 15, 2014 private placement (incorporated by reference to Exhibit 10.2 to the Form 8-K filed on August 21, 2014)
|
|
|
10.34
|
Letter Agreement and Release, dated as of September 1, 2014, by and between BioSig Technologies, Inc. and Asher Holzer, Ph.D (incorporated by reference to Exhibit 10.1 to the Form 8-K filed on September 5, 2014)
|
|
|
10.35
|
Form of Restricted Stock Award Agreement under the 2012 Equity Incentive Plan (incorporated by reference to Exhibit 10.2 to the Form 8-K filed on September 5, 2014)
|
|
|
10.36
|
Settlement and Mutual Release Agreement, dated November 3, 2014, by and between BioSig Technologies, Inc. and David Drachman (incorporated by reference to Exhibit 10.1 to the Form 8-K filed on November 5, 2014)
|
|
|
10.37
|
Composite of Unit Purchase Agreement, dated December 19, 2014, as amended by Supplement No. 1, dated December 17, 2014, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.37 to the Form 10-K filed on February 20, 2015)
|
|
|
10.38
|
Registration Rights Agreement, dated December 19, 2014, by and between BioSig Technologies, Inc. and certain purchasers set forth therein (incorporated by reference to Exhibit 10.38 to the Form 10-K filed on February 20, 2015)
|
|
|
10.39
|
Form of “A” Warrant used in connection with December 19, 2014 private placement (incorporated by reference to Exhibit 10.37 to the Form 10-K filed on February 20, 2015)
|
|
|
Form of “B” Warrant used in connection with December 19, 2014 private placement (incorporated by reference to Exhibit 10.37 to the Form 10-K filed on February 20, 2015)
|
||
|
10.40
|
Amendment No. 2 to the BioSig Technologies, Inc. 2012 Equity Incentive Plan (incorporated by reference to Exhibit 99.3 to the Form S-8 filed on April 17, 2015)
|
|
|
10.41 *
|
Amendment No. 3 to the BioSig Technologies, Inc. 2012 Equity Incentive Plan
|
|
|
10.42
|
Securities Purchase Agreement, dated as of May 11, 2015, by and between BioSig Technologies, Inc. and Alpha Capital Anstalt (incorporated by reference to Exhibit 10.1 to the Form 8-K filed on May 15, 2015)
|
|
|
10.43
|
Securities Purchase Agreement, dated as of May 11, 2015, by and between BioSig Technologies, Inc. and Brio Capital Master Fund Ltd. (incorporated by reference to Exhibit 10.2 to the Form 8-K filed on May 15, 2015
|
|
| 10.44 | ||
| 10.45 | Amendment No. 4 to the BioSig Technologies, Inc. 2012 Equity Incentive Plan (incorporated by reference to Exhibit 99.1 to the Form 8-K filed on May 29, 2015) | |
|
16.1 *
|
Letter of Rosenberg Rich Baker Berman & Company, dated May 20, 2015
|
|
|
23.1
|
||
|
23.2
|
Consent of Haynes and Boone, LLP (included in Exhibit 5.1)
|
|
|
24.1 *
|
Power of Attorney (included on signature page)
|
* Previously filed.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act of 1033, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of the securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered that remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) That, for the purpose of determining liability of the undersigned registrant under the Securities Act of 1033 to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Minneapolis, State of Minnesota on June 10, 2015.
|
BIOSIG TECHNOLOGIES, INC.
|
||
|
By:
|
/s/ Kenneth L. Londoner
|
|
|
Name: Kenneth L. Londoner
|
||
|
Title: Executive Chairman
|
||
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature
|
Title
|
Date
|
||
|
/s/ Kenneth L. Londoner
|
Executive Chairman and Director
|
June 10 , 2015
|
||
|
Kenneth L. Londoner
|
||||
|
*
|
President and Chief Executive Officer, Director
|
June 10 , 2015
|
||
|
Gregory D. Cash
|
||||
|
*
|
Chief Financial Officer
|
June 10 , 2015
|
||
|
Steve Chaussy
|
||||
|
*
|
Chief Scientific Advisor and Director
|
June 10 , 2015
|
||
|
Asher Holzer
|
||||
|
*
|
Director
|
June 10 , 2015
|
||
|
Roy T. Tanaka
|
||||
|
*
|
Director
|
June 10 , 2015
|
||
|
Patrick J. Gallagher
|
||||
|
*
|
Director
|
June 10 , 2015
|
||
|
Seth H. Z. Fischer
|
||||
|
*
|
Director
|
June 10 , 2015
|
||
|
Jeffrey F. O’Donnell, Sr.
|
||||
|
*
|
Director
|
June 10 , 2015
|
||
|
Jerome Zeldis
|
|
/s/ David Weild IV
|
Director
|
June 10, 2015
|
||
|
David Weild IV
|
|
* By:
|
/s/ Kenneth L. Londoner | |
| Kenneth L. Londoner | ||
| Attorney-in-fact | ||
II-10
