Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIO REFERENCE LABORATORIES INC | dp57048_8k.htm |
Exhibit 99.1

Investor Presentation June 2015

Important Information for Investors and Shareholders This communication does not constitute an offer to buy or sell or the solicitation of an offer to buy or sell any securities or a solicitation of any vote or approval. This communication relates to a proposed business combination between Bio - Reference Laboratories, Inc. (“Bio - Reference Laboratories”) and OPKO Health, Inc. (“OPKO”). In connection with this proposed business combination, Bio - Reference Laboratories and/or OPKO wi ll file relevant materials with the Securities Exchange Commission (the “SEC”), including an OPKO registration statement on Form S - 4 that will in clude a proxy statement of Bio - Reference Laboratories and constitute a prospectus of OPKO. INVESTORS AND SECURITY HOLDERS OF BIO - REFERENCE LABORATORIES AND OPKO ARE URGED TO READ THE PROXY STATEMENT/PROSPECTUS AND OTHER DOCUMENTS THAT MAY BE FILED WITH THE SEC CAREFULLY AND IN THEIR ENTIRETY IF AND WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION. Any definitive proxy statement (if and when available) will be mailed to shareholders of Bio - Reference La boratories. Investors and security holders will be able to obtain free copies of these documents (if and when available) and other docume nts filed with the SEC by Bio - Reference Laboratories and/or OPKO through the website maintained by the SEC at www.sec.gov. Copies of the documents filed w ith the SEC by Bio - Reference Laboratories will be available free of charge on Bio - Reference Laboratories’ website at http://www.bioreference.co m or by contacting Bio - Reference Laboratories’ Investor Relations Department by email at tmackay@bioreference.com or by phone at (201) 791 - 2600. Copies of the documents filed with the SEC by OPKO will be available free of charge on OPKO’s website at www.opko.com or by contacting OPKO ’s Investor Relations Department by email at contact@opko.com or by phone at (305) 575 - 4100. Participants in Solicitation Bio - Reference Laboratories, OPKO, their respective directors and certain of their respective executive officers may be considered participa nts in the solicitation of proxies in connection with the proposed transaction. Information about the directors and executive officers o f B io - Reference Laboratories is set forth in its Annual Report on Form 10 - K for the year ended October 31, 2014, which was filed with the SEC on January 13, 2015, its Quarterly Report on Form 10 - Q for the quarter ended April 30, 2015 which was filed with the SEC on June 9, 2015 and its Current Reports on Form 8 - K, which were filed with the SEC on March 5, 2015, April 29, 2015 and June 4, 2015. Information about the directors and executive offi cer s of OPKO is set forth in its amended Annual Report on Form 10 - K for the year ended December 31, 2014, which was filed with the SEC on February 27, 201 5 and April 30, 2015, its proxy statement for its 2015 annual meeting of stockholders, which was filed with the SEC on May 7, 2015, its Quart erl y Report on Form 10 - Q for the quarter ended March 31, 2015 which was filed with the SEC on May 11, 2015 and its Current Report on Form 8 - K, which was filed with the SEC on March 19, 2015. These documents can be obtained free of charge from the sources indicated above. Additional information regarding the participants in the proxy solicitations and a description of their direct and indirect interests, by security holdings or otherwise, will be contained in the proxy statement/prospectus and other relevant materials to be filed with the SEC when they become available.

Cautionary Statement Regarding Forward - Looking Statements Certain statements in this communication regarding the proposed acquisition of Bio - Reference Laboratories by OPKO, including any statements regarding the expected timetable for completing the proposed transaction, synergies, benefits and opportunities of the propo sed transaction, future opportunities for the combined company and products, future financial performance and any other statements regarding OPKO’s a nd Bio - Reference Laboratories’ future expectations, beliefs, plans, objectives, financial conditions, assumptions or future events or performa nce that are not historical facts are “forward - looking” statements made within the meaning of Section 27A of the Securities Act of 1933, as amended, and Sec tion 21E of the Securities Exchange Act of 1934, as amended. The words “anticipate,” “believe,” “ensure,” “expect,” “if,” “intend,” “estimat e,” “probable,” “project,” “forecasts,” “predict,” “outlook,” “aim,” “will,” “could,” “should,” “would,” “potential,” “may,” “might,” “anticipate,” “lik ely ” “plan,” “positioned,” “strategy,” and similar expressions, and the negative thereof, are intended to identify forward - looking statements. All forward - looking information are subject to numerous risks and uncertainties, many of which are beyond the control of Bio - Referen ce Laboratories and OPKO, that could cause actual results to differ materially from the results expressed or implied by the statements. These ri sks and uncertainties include, but are not limited to: failure to obtain the required vote of Bio - Reference Laboratories’ shareholders; the timing to consummate the proposed transaction; the risk that a condition to closing of the proposed transaction may not be satisfied or that the closing of the pr oposed transaction might otherwise not occur; the risk that a regulatory approval that may be required for the proposed transaction is not obtained or is obtained subject to conditions that are not anticipated; the diversion of management time on transaction - related issues; ability to successfully int egrate the businesses; risk that the transaction and its announcement could have an adverse effect on Bio - Reference Laboratories’ ability to retain customer s and retain and hire key personnel; the risk that any potential synergies from the transaction may not be fully realized or may take longer to rea liz e than expected; new information arising out of clinical trial results; and the risk that the safety and/or efficacy results of existing clinical tri als will not support continued clinical development, as well as risks inherent in funding, developing and obtaining regulatory approvals of new, commercially - viable and competitive products and treatments. In addition, forward - looking statements may also be adversely affected by general market factors, competitive pr oduct development, product availability, federal and state regulations and legislation, the regulatory process for new products and indications, ma nufacturing issues that may arise, patent positions and litigation, among other factors. The forward - looking statements contained in this communication may become outdated over time. OPKO and Bio - Reference Laboratories do not assume any responsibility for updating any forward - looking statements. Additio nal information concerning these and other factors can be found in Bio - Reference Laboratories’ and OPKO’s respective filings with the SEC and av ailable through the SEC’s Electronic Data Gathering and Analysis Retrieval system at www.sec.gov, including Bio - Reference Laboratories’ and OPKO’s m ost recent Annual Reports on Form 10 - K, Quarterly Reports on Form 10 - Q and Current Reports on Form 8 - K. The foregoing list of important fac tors is not exclusive. Bio - Reference Laboratories and OPKO assume no obligation to update or revise any forward - looking statements as a resu lt of new information, future events or otherwise, except as may be required by law. Readers are cautioned not to place undue reliance on these forward - looking statements that speak only as of the date hereof.
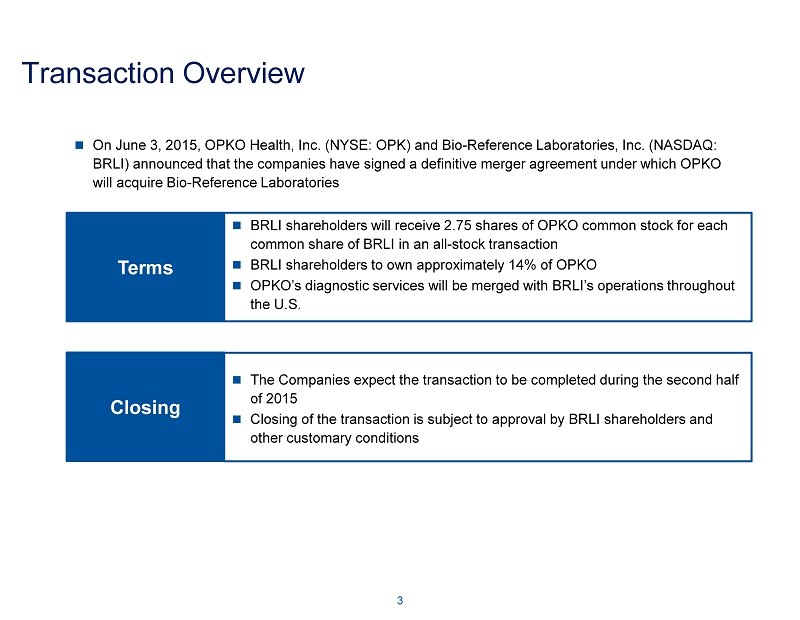
Transaction Overview On June 3, 2015, OPKO Health, Inc. (NYSE: OPK) and Bio - Reference Laboratories, Inc. (NASDAQ: BRLI) announced that the companies have signed a definitive merger agreement under which OPKO will acquire Bio - Reference Laboratories Terms BRLI shareholders will receive 2.75 shares of OPKO common stock for each common share of BRLI in an all - stock transaction BRLI shareholders to own approximately 14% of OPKO OPKO’s diagnostic services will be merged with BRLI’s operations throughout the U.S. 3 Closing The Companies expect the transaction to be completed during the second half of 2015 Closing of the transaction is subject to approval by BRLI shareholders an d other customary conditions

Bio-Reference is a Unique Clinical Lab Asset
0 Demonstrated strong, consistent organic growth - 21 years of -20% compound
annual revenue growth
0 Created and expanded franchises in multiple specialty markets including oncology,
women's health and genetics in lab testing and healthcare provider communities
0 Commercialized innovations in clin ical testing and informatics: GenPap, PanEthnic
Carrier Screen, OnkoSight, Genome DX, Next-Gen Clinical Testing, Whole Exome
Sequencing, PsiMedica and CareEvolve
0 Positioned itself securely on the cutting edge of Genetic Medicine through GeneDx
0 Built a strong corporate culture with an outstanding industry leading medical and
scientific team with long company tenure and strong commitment to BRLI
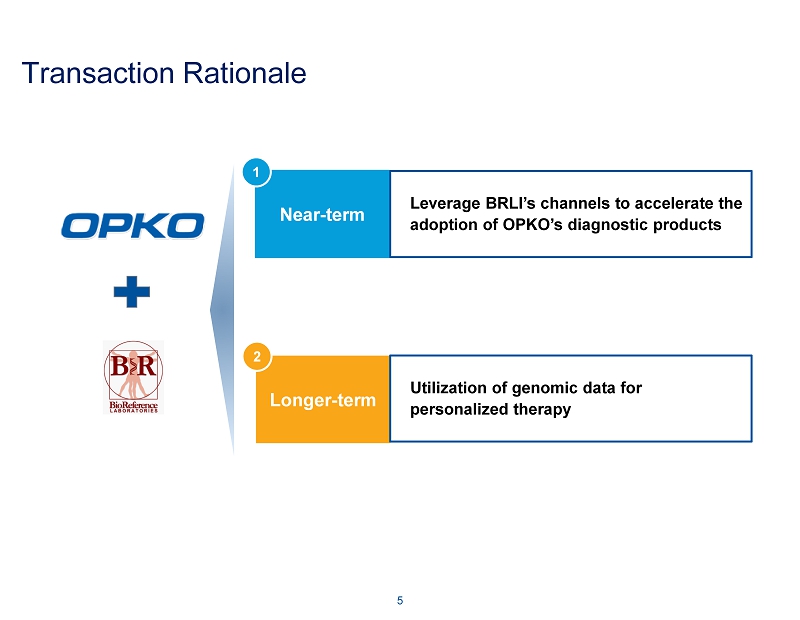
Transaction Rationale 5 Near - term Longer - term Leverage BRLI’s channels to accelerate the adoption of OPKO’s diagnostic products Utilization of genomic data for personalized therapy 1 2

Transaction Rationale: Near - term Extensive phlebotomy draw stations offer synergistic opportunity for efficient commercialization of 4Kscore test for high - grade prostate cancer ~175 BRLI patient service centers located throughout the United States for collection of patient specimens Leverage the national marketing, sales, and distribution resources of BRLI to enhance sales of OPKO’s diagnostic platforms ~420 sales and marketing personnel ~5,000 people working together to support the needs of clients and patients Near - term profitability supports the development of existing pharmaceutical pipeline 6 Leverage BRLI’s channels to accelerate the adoption of OPKO’s diagnostic products 1

Transaction Rationale: Longer - term 7 Utilization of genomic data for personalized therapy 2 BRLI’s vast array of genetics and genomics data should benefit OPKO in its drug discovery and clinical trial programs GeneDx was the first commercial laboratory to offer next generation sequencing for panels Offers 620+ single gene tests along with numerous panel - based tests, including inherited cancers, to over 250 providers in 25 countries, many unique to GeneDx Performs more whole exome testing than any other commercial laboratory in the world OPKO’s research capabilities deepen the insights into the genetic information and further strengthen the GeneDx offering
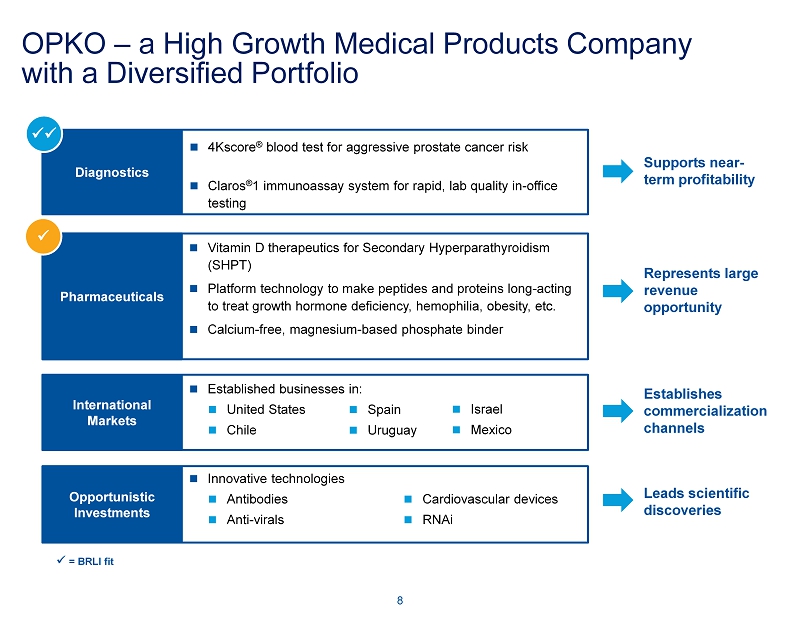
Diagnostics 4Kscore ® blood test for aggressive prostate cancer risk Claros ® 1 immunoassay system for rapid, lab quality in - office testing Pharmaceuticals Vitamin D therapeutics for Secondary Hyperparathyroidism (SHPT) Platform technology to make peptides and proteins long - acting to treat growth hormone deficiency, hemophilia, obesity, etc. Calcium - free, magnesium - based phosphate binder Opportunistic Investments Innovative technologies Antibodies Anti - virals Cardiovascular devices RNAi International Markets Established businesses in: United States Chile Spain Uruguay Israel Mexico OPKO – a High Growth Medical Products Company with a Diversified Portfolio 8 Supports near - term profitability Represents large revenue opportunity Establishes commercialization channels Leads scientific discoveries xx x x = BRLI fit
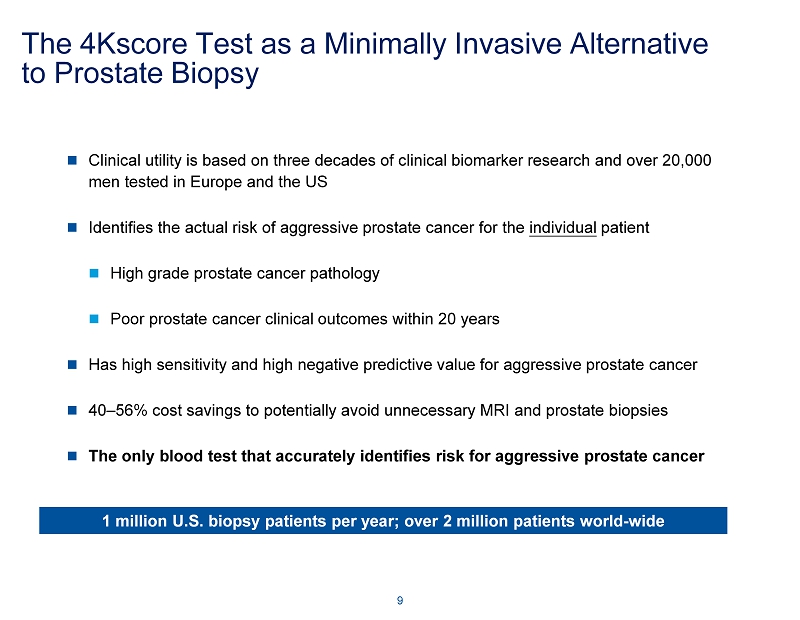
The 4Kscore Test as a Minimally Invasive Alternative to Prostate Biopsy Clinical utility is based on three decades of clinical biomarker research and over 20,000 men tested in Europe and the US Identifies the actual risk of aggressive prostate cancer for the individual patient High grade prostate cancer pathology Poor prostate cancer clinical outcomes within 20 years Has high sensitivity and high negative predictive value for aggressive prostate cancer 40 – 56% cost savings to potentially avoid unnecessary MRI and prostate biopsies The only blood test that accurately identifies risk for aggressive prostate cancer 9 1 million U.S. biopsy patients per year; over 2 million patients world - wide
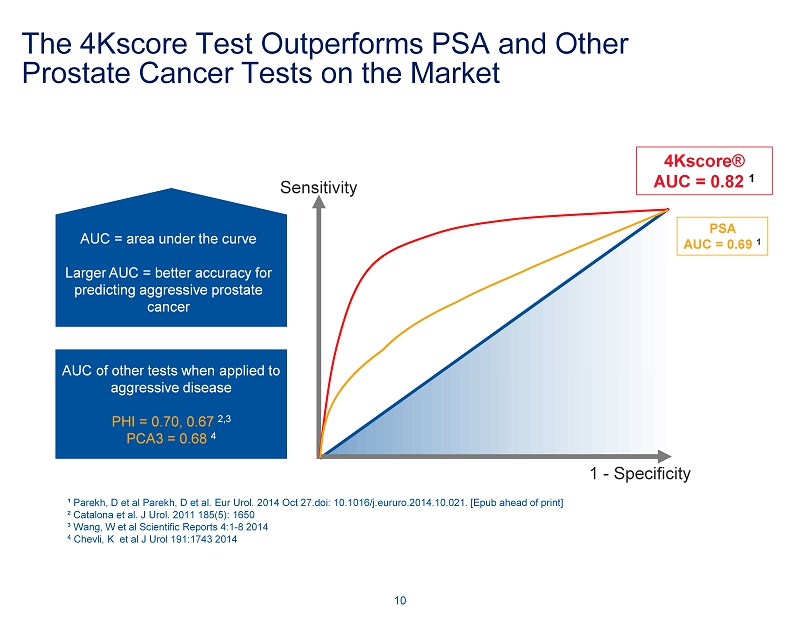
The 4Kscore Test Outperforms PSA and Other Prostate Cancer Tests on the Market 10 Sensitivity 4Kscore® AUC = 0.82 1 AUC = area under the curve Larger AUC = better accuracy for predicting aggressive prostate cancer PSA AUC = 0.69 1 AUC of other tests when applied to aggressive disease PHI = 0.70, 0.67 2,3 PCA3 = 0.68 4 1 - Specificity ¹ Parekh, D et al Parekh, D et al. Eur Urol. 2014 Oct 27.doi: 10.1016/j.eururo.2014.10.021. [Epub ahead of print] ² Catalona et al. J Urol. 2011 185(5): 1650 ³ Wang, W et al Scientific Reports 4:1 - 8 2014 4 Chevli, K et al J Urol 191:1743 2014
11 Leverage BRLI National Reach to Accelerate Diagnostic Products Commercialization
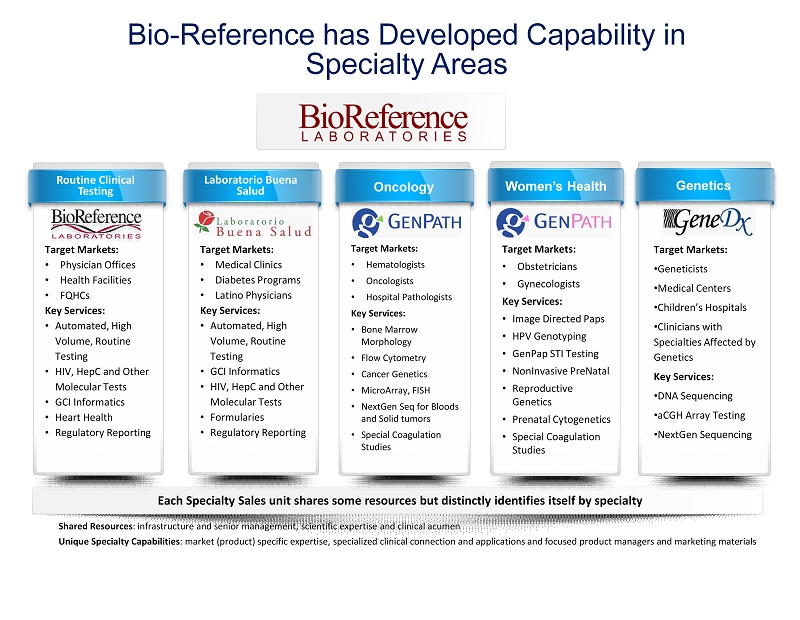
Shared Resources : infrastructure and senior management, scientific expertise and clinical acumen Unique Specialty Capabilities : market (product) specific expertise, specialized clinical connection and applications and focused product managers and mark eti ng materials Each Specialty Sales unit shares some resources but distinctly identifies itself by specialty Target Markets: • Hematologists • Oncologists • Hospital Pathologists Key Services: • Bone Marrow Morphology • Flow Cytometry • Cancer Genetics • MicroArray, FISH • NextGen Seq for Bloods and Solid tumors • Special Coagulation Studies Oncology Routine Clinical Testing Target Markets: • Physician Offices • Health Facilities • FQHCs Key Services: • Automated, High Volume, Routine Testing • HIV, HepC and Other Molecular Tests • GCI Informatics • Heart Health • Regulatory Reporting Women’s Health Target Markets: • Obstetricians • Gynecologists Key Services: • Image Directed Paps • HPV Genotyping • GenPap STI Testing • NonInvasive PreNatal • Reproductive Genetics • Prenatal Cytogenetics • Special Coagulation Studies Genetics Target Markets: • Geneticists • Medical Centers • Children’s Hospitals • Clinicians with Specialties Affected by Genetics Key Services: • DNA Sequencing • aCGH Array Testing • NextGen Sequencing Laboratorio Buena Salud Target Markets: • Medical Clinics • Diabetes Programs • Latino Physicians Key Services: • Automated, High Volume, Routine Testing • GCI Informatics • HIV, HepC and Other Molecular Tests • Formularies • Regulatory Reporting Bio - Reference has Developed Capability in Specialty Areas
GeneDx Accumulates Deep Insights in Genetic Information 13 Establish a genomics partnership that will focus on all sequencing based genetic testing, including but not limited to the following key disease states: Prenatal (Non - Invasive pre - natal testing, Next - generation carrier screening) Pediatric and Postnatal (Arrays, Single Gene Assays for Rare Disorders, Exomes ) Cancer (Next Generation Tumor Sequencing, Arrays, other Pathology and Molecular testing)* Inherited Cancers (Including BRCA, Breast, Ovarian, Lynch etc.) Cardiac Disorders (Next Generation Panels including HCM, LQT, Brugada , Noonan) Neurological Disorders (Neuro - muscular, Epilepsy Next Generation Panels, Exomes) * Includes pathology and molecular tests necessary to offer a comprehensive cancer diagnostic solution Comprehensive Testing Capabilities
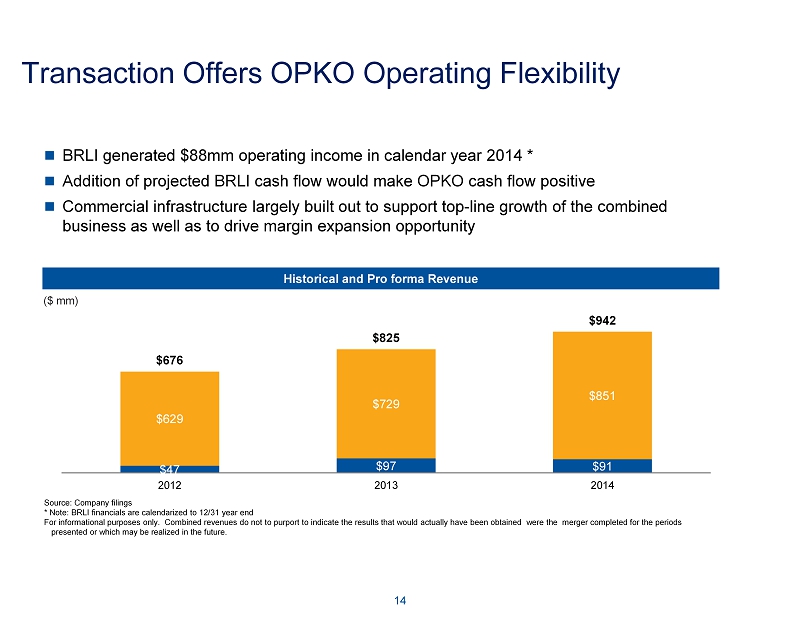
Transaction Offers OPKO Operating Flexibility 14 $47 $97 $91 $629 $729 $851 $676 $825 $942 2012 2013 2014 Historical and Pro forma Revenue ($ mm) BRLI generated $88mm operating income in calendar year 2014 * Addition of projected BRLI cash flow would make OPKO cash flow positive Commercial infrastructure largely built out to support top - line growth of the combined business as well as to drive margin expansion opportunity Source: Company filings * Note : BRLI financials are calendarized to 12/31 year end For informational purposes only. Combined revenues do not to purport to indicate the results that would actually have been o bta ined were the merger completed for the periods presented or which may be realized in the future .
