Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Sorrento Therapeutics, Inc. | d935495d8k.htm |
| Exhibit 99.1
|
Next-Generation Cancer Therapeutics
June 2015
| 1 |
|
|
|
Forward Looking Statements NASDAQ: SRNE
Certain statements contained in this presentation or in other documents of Sorrento Therapeutics, Inc. (the “Company”), along with certain statements that may be made by management of the Company orally in presenting this material, may contain “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995. These statements can be identified by the fact that they do not relate strictly to historic or current facts. They use words such as “estimate,” “expect,” “intend,” “believe,” “plan,”
“anticipate,” “projected” and other words and terms of similar meaning in connection with any discussion of future operating or financial performance or condition. These statements are based upon the current beliefs and expectations of the Company’s management and are subject to significant risks and uncertainties. Statements regarding future action, future performance and/or future results including, without limitation, those relating to the timing for completion, and results of, scheduled or additional clinical trials and the FDA’s or other regulatory review and/or approval and commercial launch and sales results (if any) of the Company’s formulations and products and regulatory filings related to the same, and receipt by the Company of milestone and royalty payments may differ from those set forth in the forward-looking statements. Peak sales and market size estimates have been determined on the basis of market research and comparable product analysis, but no assurances can be given that such sales levels will be achieved, if at all, or that such market size estimates will prove accurate.
The Company assumes no obligation to update forward-looking statements as circumstances change. Investors are advised to consult further disclosures that the Company makes or has made on related subjects in the Company’s Form 10-K, 10-Q and 8-K reports.
In presenting this material or responding to inquiries in connection with a presentation, management may refer to results, projections or performance measures that are not prepared in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”) as reported in the Company’s SEC filings. These results, projections or performance measures are Non-GAAP measures and are not intended to replace or as a substitute for results measured under GAAP, but rather as supplement to the GAAP reported results.
Because actual results are affected by these and other potential risks, contingencies and uncertainties, the Company cautions investors that actual results may differ materially from those expressed or implied in any forward-looking statement. It is not possible to predict or identify all such risks, contingencies and uncertainties. The Company identifies some of these factors in its Securities and Exchange Commission (“SEC”) filings on Forms 10-K, 10-Q and 8-K, and investors are advised to consult the Company’s filings for a more complete listing of risk factors, contingencies and uncertainties effecting the Company and its business and financial performance.
| 2 |
|
|
|
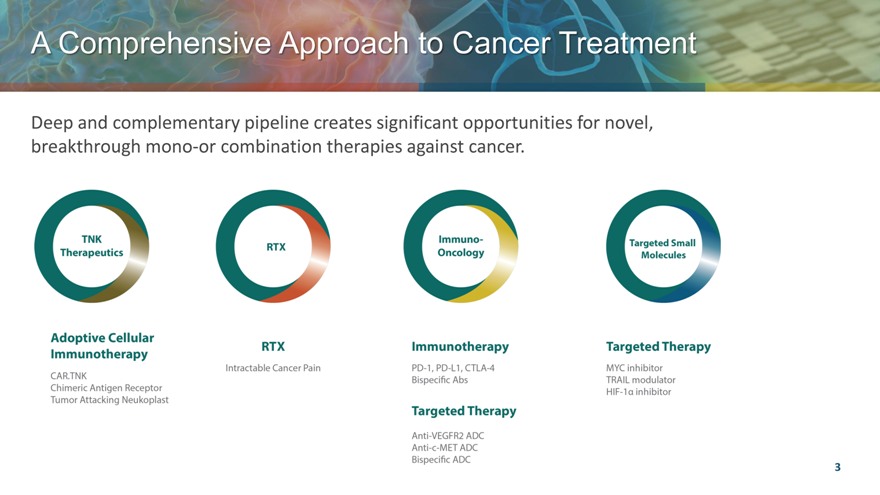
A Comprehensive Approach to Cancer Treatment
Deep and complementary pipeline creates significant opportunities for novel, breakthrough mono-or combination therapies against cancer.
| 3 |
|
|
|
Recent Corporate Events
| 4 |
|
|
|
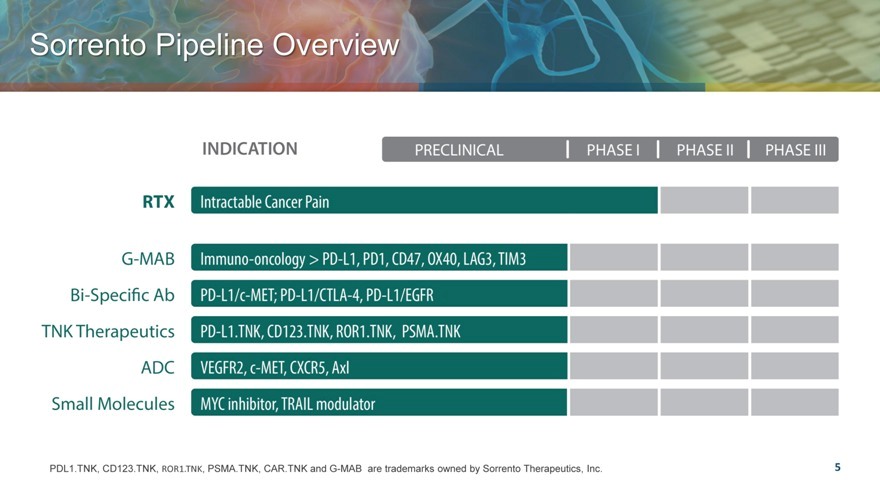
Sorrento Pipeline Overview
PDL1.TNK, CD123.TNK, ROR1.TNK, PSMA.TNK, CAR.TNK and G-MAB are trademarks owned by Sorrento Therapeutics, Inc.
| 5 |
|
|
|
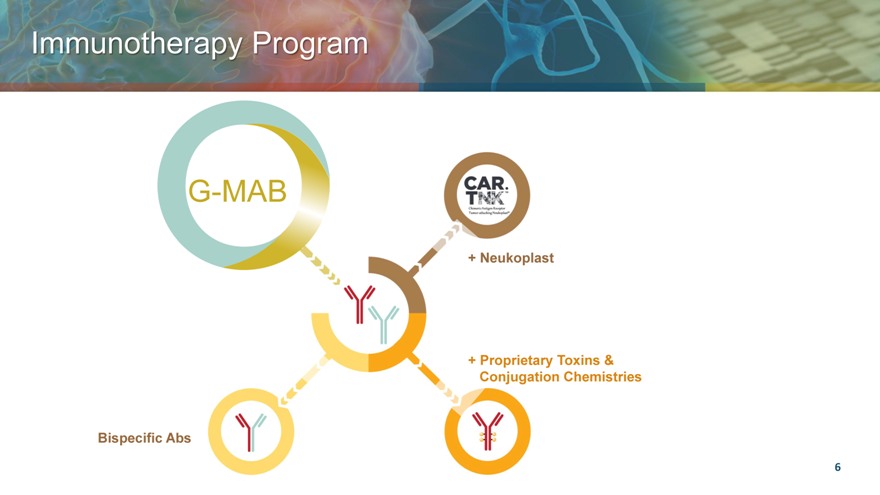
Immunotherapy Program
G-MAB
+ Neukoplast
+ Proprietary Toxins & Conjugation Chemistries
Bispecific Abs
| 6 |
|
|
|
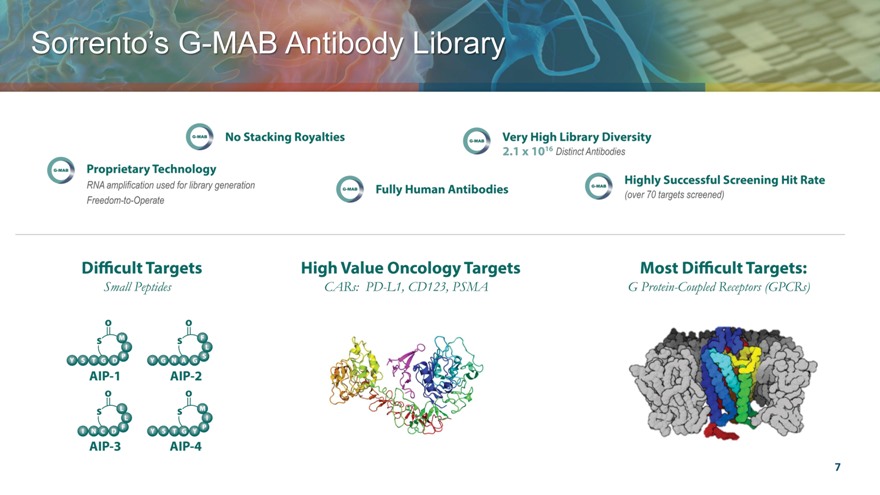
Sorrento’s MAB Antibody Library
| 7 |
|
|
|
Wholly-owned subsidiary of Sorrento Therapeutics
CAR.TNK and TNK Therapeutics are trademarks owned by Sorrento Therapeutics, Inc.
| 8 |
|
|
|
“The Next Generation of the Next Generation”
Neukoplast
G-MAB Library
NK92 cell line (“off-the-shelf”) Vast diversity human antibody library
Broad anti-cancer activity in solid and liquid High successful screening rate (over 70 targets screened) tumors Proprietary technologies with FTO
No clinical DLTs in over 40 patients treated
Ideal for generation of CARs
9
|
|
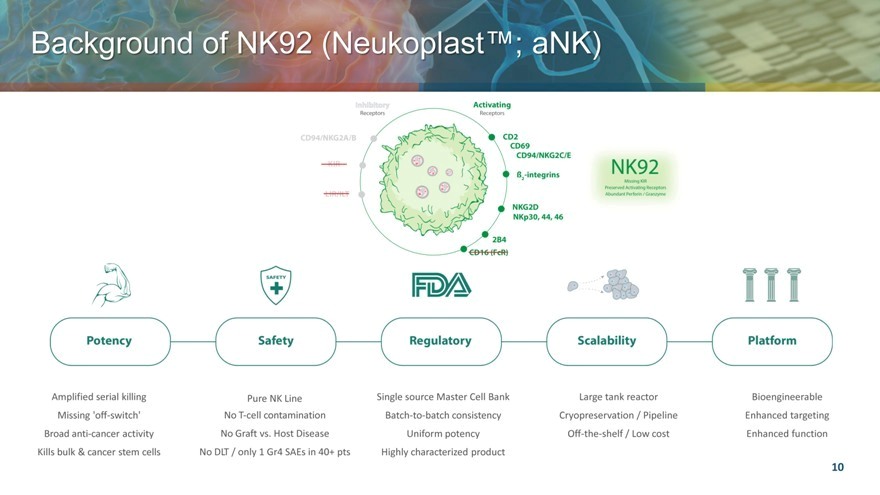
Background of NK92 Neukoplast™; aNK)
Amplified serial killing Pure NK Line Single source Master Cell Bank Large tank reactor Bioengineerable Missing ‘off-switch’ No T-cell contamination Batch-to-batch consistency Cryopreservation / Pipeline Enhanced targeting Broad anti-cancer activity No Graft vs. Host Disease Uniform potency Off-the-shelf / Low cost Enhanced function Kills bulk & cancer stem cells No DLT / only 1 Gr4 SAEs in 40+ pts Highly characterized product
10
|
|
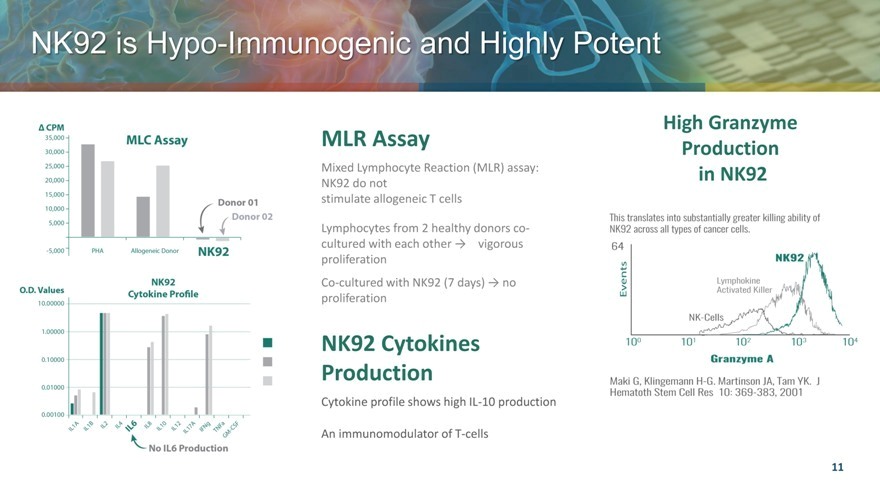
NK92 is Hypo Immunogenic and Highly Potent
MLR Assay
Mixed Lymphocyte Reaction (MLR) assay: NK92 do not stimulate allogeneic T cells
Lymphocytes from 2 healthy donors co-cultured with each other vigorous proliferation
Co-cultured with NK92 (7 days) no proliferation
NK92 Cytokines Production
Cytokine profile shows high IL-10 production
An immunomodulator of T-cells
High Granzyme Production in NK92
11
|
|
Unmodified NK92 Clinically Validated: Phase I
12
|
|
Phase I Studies: Summary of Findings
Excellent Safety Demonstrated
No adverse T-cell induced cytotoxic response
A significant safety concern for CAR-T cell approaches
No pre-conditioning or combination treatment
Notable responses:
Melanoma: 1/1
Lung Cancer: 3/4 (1 MR, 2 PR)
Renal Cell Cancer: 6/11 (1 MR, 5 SD-prolonged survival)
AML (relapsed): 1/1
Lymphoma post transplant: 2/6
MR Mixed PR Partial SD Stable
Response Response Disease
13
|
|
TNK: CAR Modified NK92
Clonal cell lines expressing one or more CARs to establish a range of distinct products
Multiple killing mechanisms—CAR-targeted as well as broad intrinsic anti-cancer activity of NK92 (“off-target / on-tumor”)
Engages the adaptive immune system through cytokine secretion and immune cell recruitment
Titratable: repeat dosing option; controllable dose exposure to manage safety risk
14
|
|
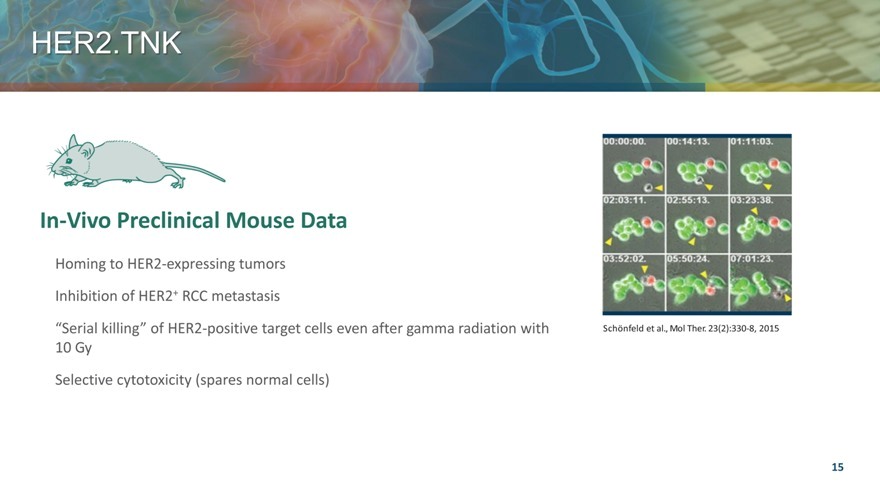
HER2.TNK
In-Vivo Preclinical Mouse Data
Homing to HER2-expressing tumors
Inhibition of HER2+ RCC metastasis
“Serial killing” of HER2-positive target cells even after gamma radiation with
10 Gy
Selective cytotoxicity (spares normal cells)
Schönfeld et al., Mol Ther. 23(2):330-8, 2015
15
|
|
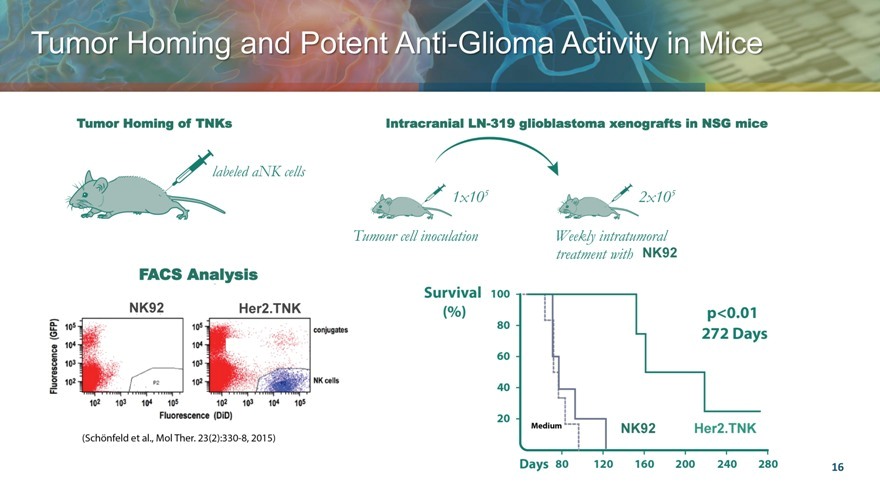
Tumor Homing and Potent Anti Glioma Activity in Mice
Tumor Homing of TNKs
FACS Analysis
NK92 Her2.TNK
Intracranial LN-319 glioblastoma xenografts in NSG mice
NK92
NK92 Her2.TNK
16
|
|
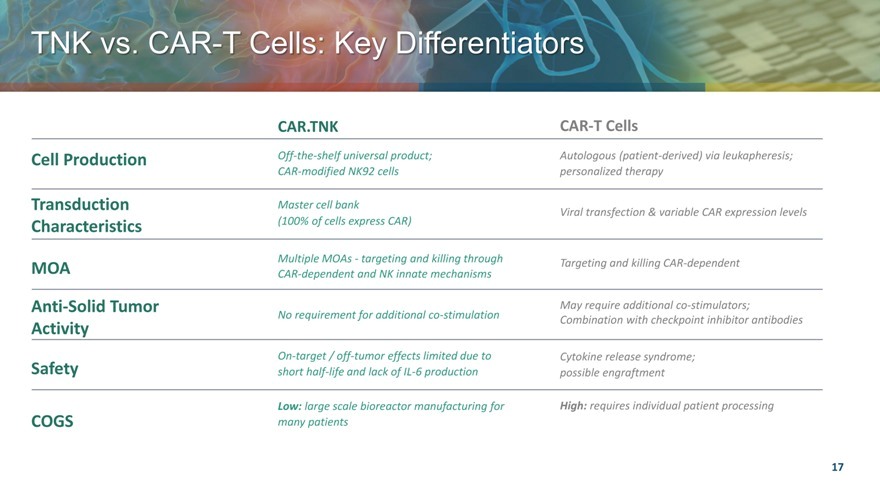
TNK vs. CAR T Cells Key Differentiators
CAR.TNK CAR-T Cells
Cell Production Off-the-shelf universal product; Autologous (patient-derived) via leukapheresis;
CAR-modified NK92 cells personalized therapy
Transduction Master cell bank Viral transfection & variable CAR expression levels
Characteristics (100% of cells express CAR)
MOA Multiple MOAs—targeting and killing through Targeting and killing CAR-dependent
CAR-dependent and NK innate mechanisms
Anti-Solid Tumor May require additional co-stimulators;
No requirement for additional co-stimulation Combination with checkpoint inhibitor antibodies
Activity
On-target / off-tumor effects limited due to Cytokine release syndrome;
Safety short half-life and lack of IL-6 production possible engraftment
Low: large scale bioreactor manufacturing for High: requires individual patient processing
COGS many patients
17
|
|
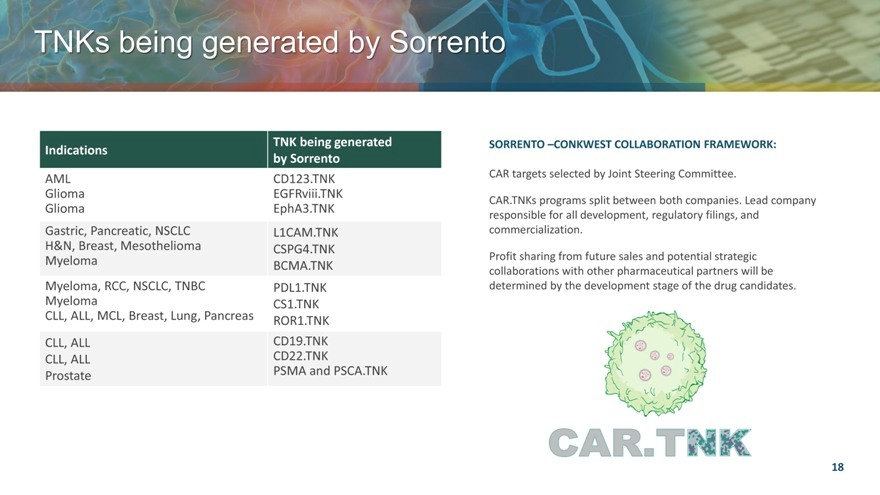
TNKs being generated by Sorrento
TNK being generated
Indications
by Sorrento
AML CD123.TNK
Glioma EGFRviii.TNK
Glioma EphA3.TNK
Gastric, Pancreatic, NSCLC L1CAM.TNK
H&N, Breast, Mesothelioma CSPG4.TNK
Myeloma BCMA.TNK
Myeloma, RCC, NSCLC, TNBC PDL1.TNK
Myeloma CS1.TNK
CLL, ALL, MCL, Breast, Lung, Pancreas ROR1.TNK
CLL, ALL CD19.TNK
CLL, ALL CD22.TNK
Prostate PSMA and PSCA.TNK
SORRENTO –CONKWEST COLLABORATION FRAMEWORK:
CAR targets selected by Joint Steering Committee.
CAR.TNKs programs split between both companies. Lead company responsible for all development, regulatory filings, and commercialization.
Profit sharing from future sales and potential strategic collaborations with other pharmaceutical partners will be determined by the development stage of the drug candidates.
18
|
|
TNK Development: Next Steps
H1 2015 Generation of in-house CARs
Generation and evaluation of
H2 2015
stable TNK pools and cell lines
2016 IND-enabling studies, IND submission, and initiation of Phase I
Studies
19
|
|
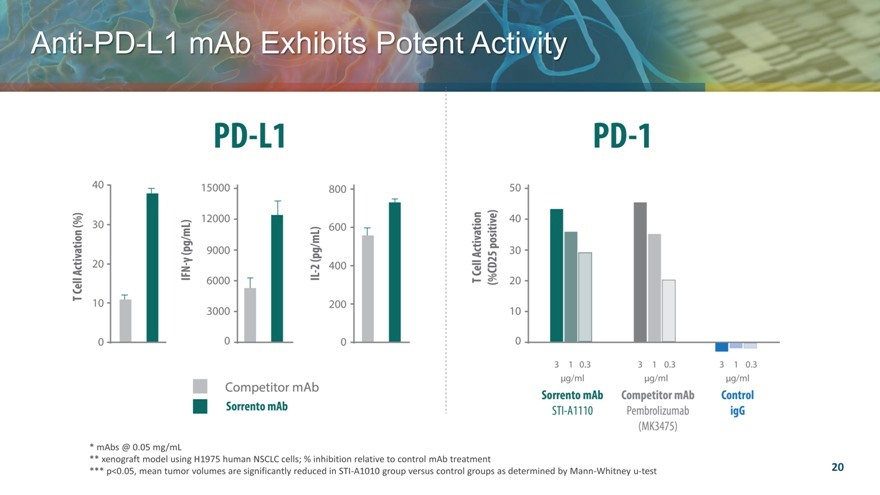
Anti PD L1 mAb Exhibits Potent Activity
mAbs @ 0.05 mg/mL
xenograft model using H1975 human NSCLC cells; % inhibition relative to control mAb treatment
p<0.05, mean tumor volumes are significantly reduced in STI-A1010 group versus control groups as determined by Mann-Whitney u-test
20
|
|
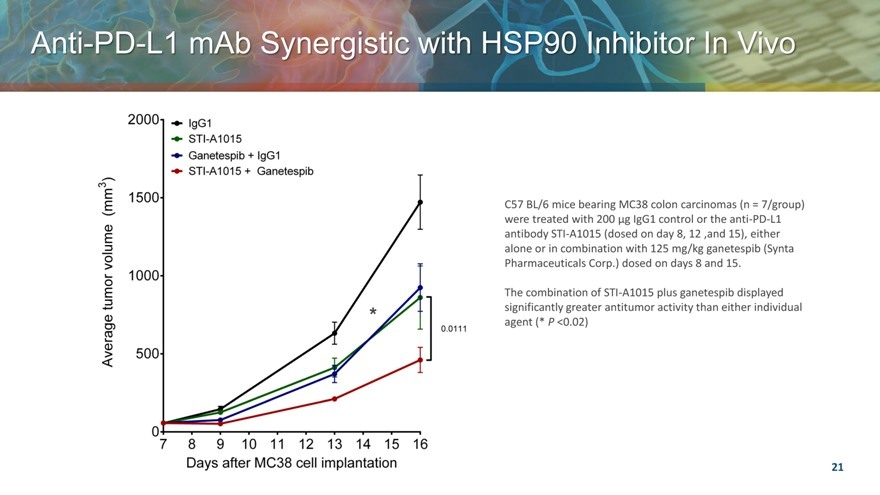
Anti PD L1 mAb Synergistic with HSP90 Inhibitor In Vivo
| * |
|
C57 BL/6 mice bearing MC38 colon carcinomas (n = 7/group) were treated with 200 ìg IgG1 control or the anti-PD-L1 antibody STI-A1015 (dosed on day 8, 12 ,and 15), either alone or in combination with 125 mg/kg ganetespib (Synta Pharmaceuticals Corp.) dosed on days 8 and 15.
The combination of STI-A1015 plus ganetespib displayed significantly greater antitumor activity than either individual agent (* P <0.02)
21
|
|
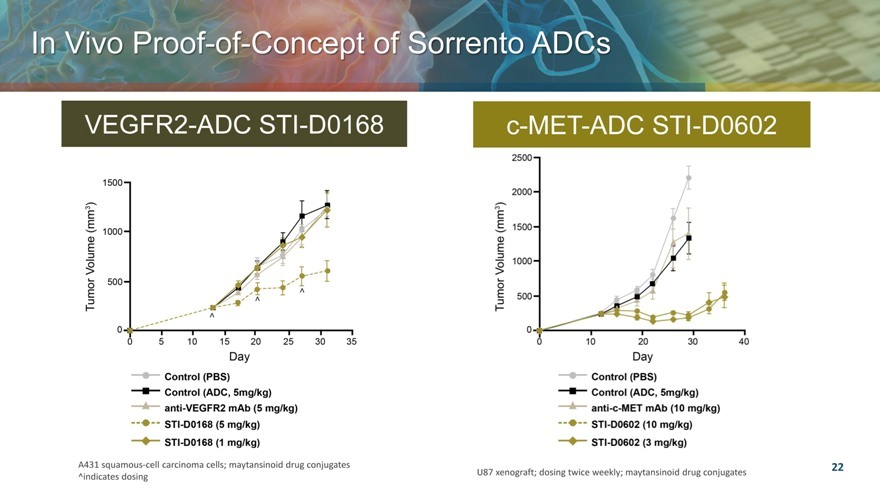
In Vivo Proof Concept of Sorrento ADCs
VEGFR2-ADC STI-D0168
c-MET-ADC STI-D0602
A431 squamous-cell carcinoma cells; maytansinoid drug conjugates U87 xenograft; dosing twice weekly; maytansinoid drug conjugates
Indicates dosing
22
|
|
Resiniferatoxi (RTX): A Novel, Non opiate Analgesic
Intractable RTX Cancer Pain
23
|
|
RTX Target Product Profile
MOA:
Ultrapotent, highly specific TRPV1 agonist selectively targeting afferent neurons
Efficacy:
Meaningful analgesia with concomitant opioid reduction and improvement in function
Safety:
Alteration of heat sensation in the targeted area with effect on normal perception / sensation or muscle function
Dosing:
Targeted single injection
24
|
|
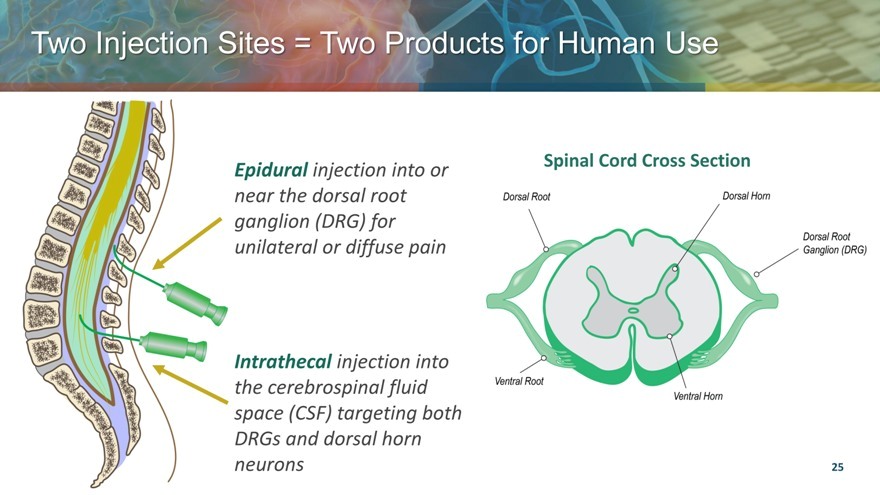
Two Injection Sites = Two Products for Human Use
Epidural injection into or near the dorsal root ganglion (DRG) for unilateral or diffuse pain
Intrathecal injection into the cerebrospinal fluid space (CSF) targeting both DRGs and dorsal horn neurons
Spinal Cord Cross Section
25
|
|
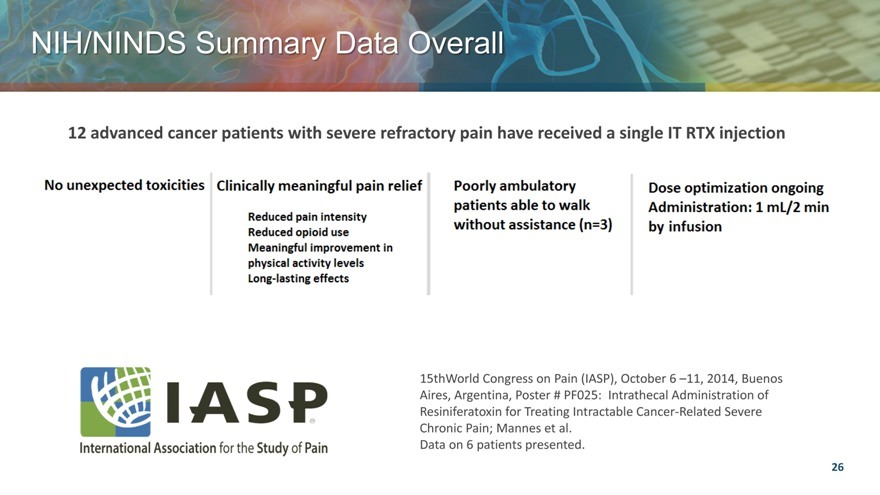
NIH/NINDS Summary Data Overall
12 advanced cancer patients with severe refractory pain have received a single IT RTX injection
15thWorld Congress on Pain (IASP), October 6 –11, 2014, Buenos Aires, Argentina, Poster # PF025: Intrathecal Administration of Resiniferatoxin for Treating Intractable Cancer-Related Severe Chronic Pain; Mannes et al.
Data on 6 patients presented.
26
|
|
| 2 |
|
Patients from New Cohort (2015) |
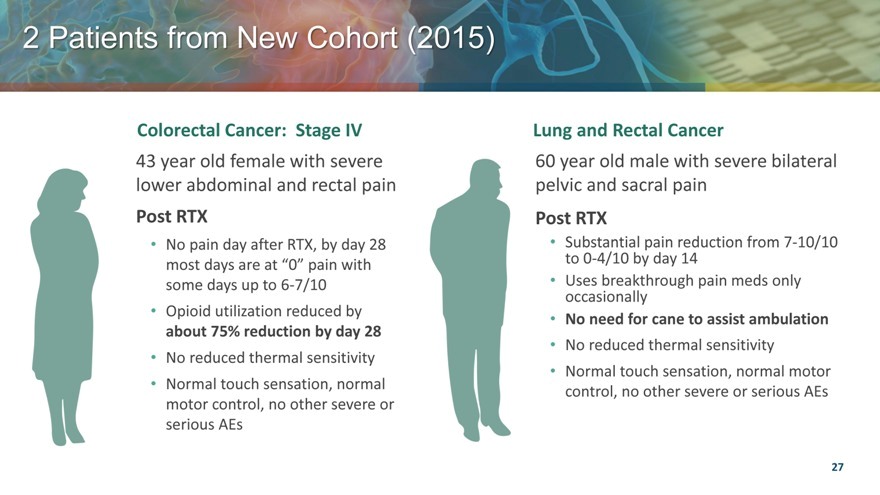
Colorectal Cancer: Stage IV
43 year old female with severe lower abdominal and rectal pain
Post RTX
| • |
|
No pain day after RTX, by day 28 most days are at “0” pain with some days up to 6-7/10 |
| • |
|
Opioid utilization reduced by about 75% reduction by day 28 |
| • |
|
No reduced thermal sensitivity |
| • |
|
Normal touch sensation, normal motor control, no other severe or serious AEs |
Lung and Rectal Cancer
60 year old male with severe bilateral pelvic and sacral pain
Post RTX
| • |
|
Substantial pain reduction from 7-10/10 to 0-4/10 by day 14 |
| • |
|
Uses breakthrough pain meds only occasionally |
| • |
|
No need for cane to assist ambulation |
| • |
|
No reduced thermal sensitivity |
| • |
|
Normal touch sensation, normal motor control, no other severe or serious AEs |
27
|
|
Next Steps for RTX Development
2015
Complete NIH dose ranging IT safety study (cancer pain) Start NIH POC EpiPG study (cancer-induced bone pain [CIBP]) Complete tox package to support corporate IND for IT RTX Begin tox package to support corporate IND for EpiPG RTX (upper thoracic and lumbar approaches)
2016
Begin corporate P2 IT POC/pivotal trial in cancer pain Begin corporate P1b EpiPG safety trial in CIBP
Begin corporate P1b IT safety trial in spinal cord injury w/refractory pain Begin corporate P1b EpiPG trial in refractory phantom pain
28
|
|
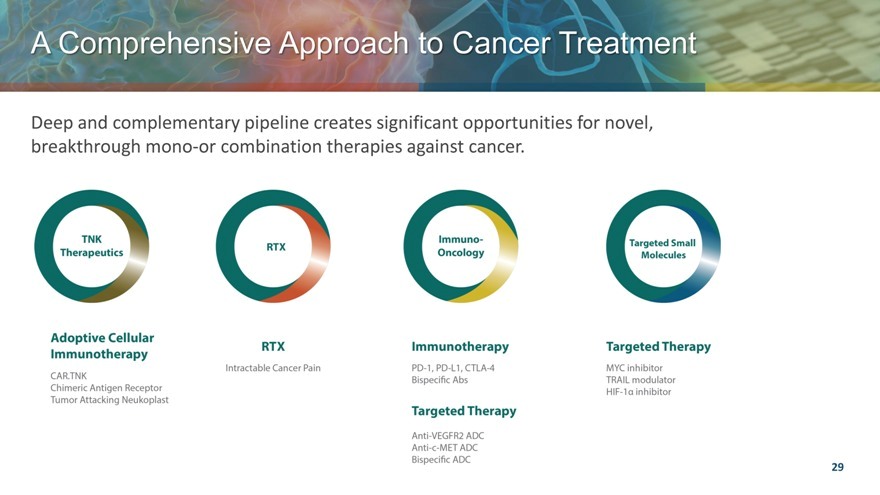
A Comprehensive Approach to Cancer Treatment
Deep and complementary pipeline creates significant opportunities for novel, breakthrough mono-or combination therapies against cancer.
29
|
|
Next-Generation Cancer Therapeutics
CONTACT:
George Uy Henry Ji, Ph.D. Executive Vice President and CCO President and CEO guy@sorrentotherapeutics.com hji@sorrentotherapeutics.com (661) 607-4057 (858) 668-6923
30