Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PLURISTEM THERAPEUTICS INC | zk1516846.htm |
| EX-99.1 - EXHIBIT 99.1 - PLURISTEM THERAPEUTICS INC | exhibit_99-1.htm |
Exhibit 99.2

1
Advancing cell therapeutic products for clinical use

Proprietary data of Pluristem Therapeutics Inc.
2
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995
and federal securities laws. For example, we are using forward-looking statements when we discuss moving closer to reaching our objective to bring innovative,
effective treatments to patients, when we discuss the timing of receiving marketing approval for our product candidates, if at all, and the forecasted worldwide
sales of our product candidates, when we discuss the potential and timing of achieving regulatory approval for our product candidates via the EU Adaptive
Pathway or other expedited regulatory pathways, regarding future collaboration with other pharmaceutical companies, when we discuss anticipated milestones
regarding our regulatory approach and the development of current and future product candidates, including the timing and design of future clinical trials, and
whether such trials will be conducted at all. These forward-looking statements and their implications are based on the current expectations of the management
of Pluristem only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the
forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking
statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing our clinical
trials; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be
accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products;
unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than we anticipate; results in the laboratory
may not translate to equally good results in real surgical settings; results of preclinical studies may not correlate with the results of human clinical trials; our
patents may not be sufficient; our products may harm recipients; changes in legislation; inability to timely develop and introduce new technologies, products
and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of Pluristem to
differ materially from those contemplated in such forward-looking statements. In addition, historic results of scientific research do not guarantee that the
conclusions of future research would not suggest different conclusions or that historic results referred to in this presentation would not be interpreted
differently in light of additional research or otherwise. Also, while the company’s program was selected for the European Medicines Agency’s Adaptive
Pathways pilot project, as well as recognized by the PMDA, these agencies are not bound by these communications and accordingly may change their position
in the future due to reasons within or outside the control of Pluristem. Except as otherwise required by law, Pluristem undertakes no obligation to publicly
release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated
events. For a more detailed description of the risks and uncertainties affecting Pluristem, reference is made to Pluristem's reports filed from time to time with
the Securities and Exchange Commission.
and federal securities laws. For example, we are using forward-looking statements when we discuss moving closer to reaching our objective to bring innovative,
effective treatments to patients, when we discuss the timing of receiving marketing approval for our product candidates, if at all, and the forecasted worldwide
sales of our product candidates, when we discuss the potential and timing of achieving regulatory approval for our product candidates via the EU Adaptive
Pathway or other expedited regulatory pathways, regarding future collaboration with other pharmaceutical companies, when we discuss anticipated milestones
regarding our regulatory approach and the development of current and future product candidates, including the timing and design of future clinical trials, and
whether such trials will be conducted at all. These forward-looking statements and their implications are based on the current expectations of the management
of Pluristem only, and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the
forward-looking statements. The following factors, among others, could cause actual results to differ materially from those described in the forward-looking
statements: changes in technology and market requirements; we may encounter delays or obstacles in launching and/or successfully completing our clinical
trials; our products may not be approved by regulatory agencies, our technology may not be validated as we progress further and our methods may not be
accepted by the scientific community; we may be unable to retain or attract key employees whose knowledge is essential to the development of our products;
unforeseen scientific difficulties may develop with our process; our products may wind up being more expensive than we anticipate; results in the laboratory
may not translate to equally good results in real surgical settings; results of preclinical studies may not correlate with the results of human clinical trials; our
patents may not be sufficient; our products may harm recipients; changes in legislation; inability to timely develop and introduce new technologies, products
and applications; loss of market share and pressure on pricing resulting from competition, which could cause the actual results or performance of Pluristem to
differ materially from those contemplated in such forward-looking statements. In addition, historic results of scientific research do not guarantee that the
conclusions of future research would not suggest different conclusions or that historic results referred to in this presentation would not be interpreted
differently in light of additional research or otherwise. Also, while the company’s program was selected for the European Medicines Agency’s Adaptive
Pathways pilot project, as well as recognized by the PMDA, these agencies are not bound by these communications and accordingly may change their position
in the future due to reasons within or outside the control of Pluristem. Except as otherwise required by law, Pluristem undertakes no obligation to publicly
release any revisions to these forward-looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated
events. For a more detailed description of the risks and uncertainties affecting Pluristem, reference is made to Pluristem's reports filed from time to time with
the Securities and Exchange Commission.
Forward Looking Statement

Proprietary data of Pluristem Therapeutics Inc.
3
• Cell therapy company (NasdaqCM: PSTI, TASE: PLTR)
• Using off-the-shelf placental expanded cells to achieve both local and
systemic therapeutic effects
systemic therapeutic effects
• First in class 3D cell culturing technology allowing for efficient, controlled
production of different cell products in commercial quantities - “the
process is the product”
production of different cell products in commercial quantities - “the
process is the product”
• Active with regulators in the U.S., EU, Japan, Korea, Australia and Israel
• Demonstrated clinical safety and significant efficacy in 3 clinical
studies (Two Phase I and one Phase I/II study)
studies (Two Phase I and one Phase I/II study)
Corporate Overview
Proprietary data of Pluristem Therapeutics Inc.
4
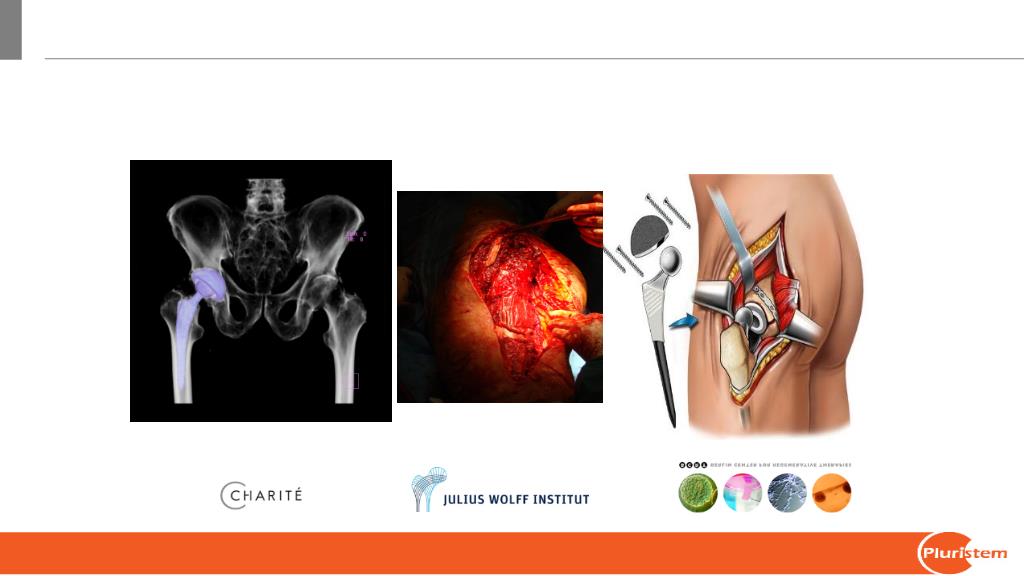
Strong Clinical Data
Muscle Injury following Total Hip Replacement N=20

Proprietary data of Pluristem Therapeutics Inc.
5
Correlation Between Improvement in the Muscle
Force of Injured and Contralateral Leg
Force of Injured and Contralateral Leg
Contralateral (non-operated)
Injured (operated)
Proprietary data of Pluristem Therapeutics Inc.
6
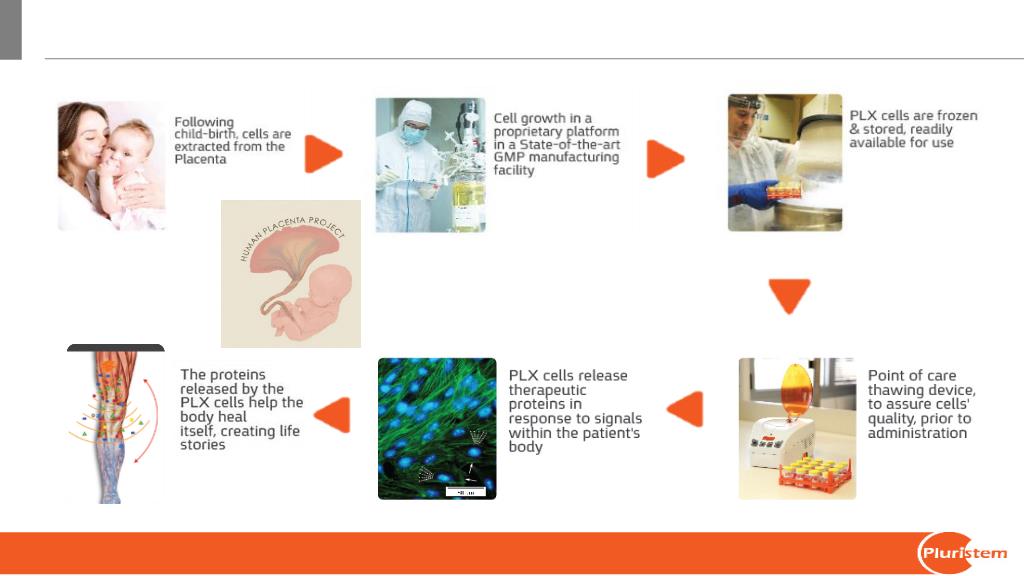
PLX Technology

Proprietary data of Pluristem Therapeutics Inc.
7
Culture conditions
PLX-R18
Hematological
PLX-PAD
Culture conditions
PLX-CNS
Neuronal
PLX-IMMUNE
Immunological
Human Placenta- A platform for distinct cell products
Four Distinct Products Derived from Placenta

Proprietary data of Pluristem Therapeutics Inc.
8
PLX-PAD CMC approved by 5 Regulatory agencies
Manufacturing facility and PLX-PAD CMC (3D culturing) for Phase II/III and
marketing*
marketing*
approved by FDA, PEI (Germany), EMA, Korean & Israeli regulatory agencies
Batch-to-Batch comparability confirmed,
multiple donors
* Subject to supplier approval

Proprietary data of Pluristem Therapeutics Inc.
9

Proprietary data of Pluristem Therapeutics Inc.
10
Selected for EU
Adaptive Pathway -
potential for
marketing approval in
2018
Adaptive Pathway -
potential for
marketing approval in
2018

Proprietary data of Pluristem Therapeutics Inc.
11
Progression of Atherosclerotic Lesions
From: Peripheral Arterial Disease. Source: Decision Resources
Created for Hillit Mannor Shachar, Pluristem Therapeutics. IP Address: (Unknown)
© 2011 Decision Resources, Inc.

Proprietary data of Pluristem Therapeutics Inc.
12
Therapeutic Angiogenesis by Growth Factors
From: Peripheral Arterial Disease. Source: Decision Resources
Created for Hillit Mannor Shachar, Pluristem Therapeutics. IP Address: (Unknown)
© 2011 Decision Resources, Inc.

Proprietary data of Pluristem Therapeutics Inc.
13
Proangiogenic VEGF, HGF, TGFb, Angiogenin, IL-8,
Angiopoietin, IL-6, MMP-1, TIMP-1, PAI-1, IGFBPs,
Angiopoietin, IL-6, MMP-1, TIMP-1, PAI-1, IGFBPs,
Immunomodulatory HGF, IDO, IL-28,
IL-22, IL-10RA,
Antifibrotic HGF, Decorin
Protrophic- LIF, Angiopoietin, b-NGF
PLX-PAD secretion profile
Proprietary data of Pluristem Therapeutics Inc.
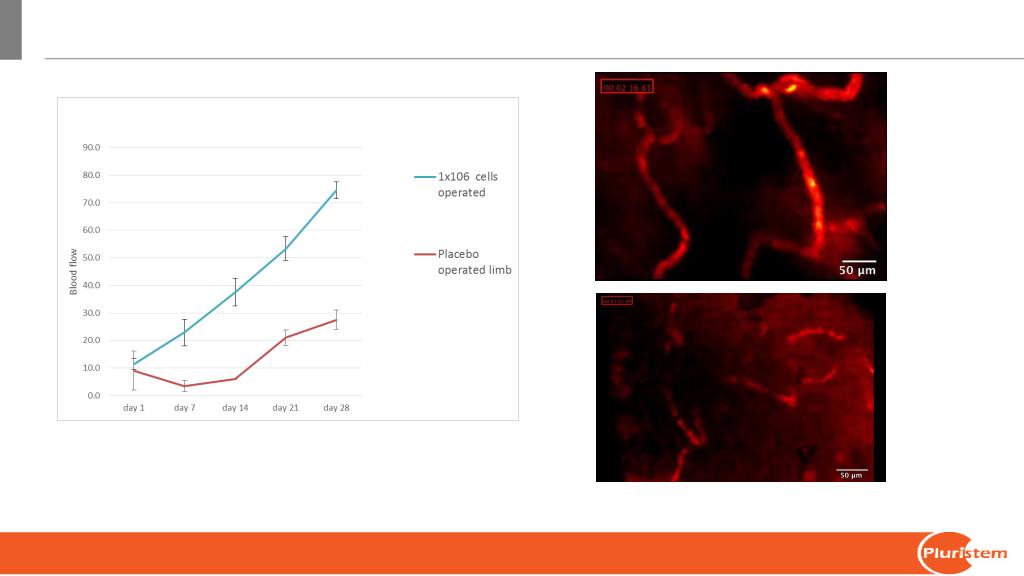
14
Placebo
PLX-PAD treated
PLX-PAD increased blood flow in the tibia
Live Cellvizio imaging following IV
administration of FITC labeled Dextran
administration of FITC labeled Dextran

Proprietary data of Pluristem Therapeutics Inc.
15
• July 2009 - First CLI patient injected in Berlin (N=15)
• October 2010 - last CLI Patient injected in US (N=12)
• April 2012 - EMA declaration - Adaptive Pathways program
• May 2012 - End of 2 years patients safety follow-up in Europe
• April 2012 - EMA initiated the Adaptive Pathways program
• December 2012 - Initiation of IC Phase II clinical study (N=150)
• March 2014 - Pilot project launched by EMA
• May 2014 - Approval of the new facility, ready for Phase III and marketing
• May 2015 - PLX-PAD cells have been selected for the Adaptive Pathways pilot
project
project
PLX-PAD in Peripheral Artery Disease

Proprietary data of Pluristem Therapeutics Inc.
16
COMPANY CONFIDENTIAL
In the base scenario, PLX-PAD has the potential to achieve peak sales of ~$350M in the US
And ~ $290M in Europe
NPV - Base Scenario Peak sales in 2030
Peak worldwide sales: ~$700M
Peak US sales: ~$350M
Peak Europe sales: ~ $290M
NPV for CLI WW sales is: $2,100M
NPV for CLI Sales in Europe : $850M
Without the
EMA approval,
EMA approval,
Peak sales in
2033.
2033.
Reduce the
NPV by $200M
(~25%)
NPV by $200M
(~25%)

Proprietary data of Pluristem Therapeutics Inc.
17
COMPANY CONFIDENTIAL
In the upside scenario, PLX-PAD has the potential to achieve peak sales of ~$1.4B in the US and ~$1B in Europe
NPV Upside Scenario Peak sales in 2030
Peak worldwide sales: ~$2.7B
Peak US sales: ~$1,400M
Peak Europe sales: ~ $1,000M
NPV for CLI WW sales is: $8,300M
NPV for CLI Sales in Europe : $3,100M
Without the
EMA approval,
EMA approval,
Peak sales in
2033.
2033.
Reduce the
NPV by $700M
(~ 25%)
NPV by $700M
(~ 25%)

Proprietary data of Pluristem Therapeutics Inc.
18
PLX-PAD CLI program status in Europe
• 3-4 years earlier in a multi-billion market
• Manufacturing facility approved and inspected by European Qualified Person for
Phase III and marketing
Phase III and marketing
• Granted European patent for the use of PLX in ischemic disease
• Higher probability for a large pharma deal
• EMA new approach to expand the use of PLX-PAD to additional indications
within PAD and other ischemic indications
within PAD and other ischemic indications

Proprietary data of Pluristem Therapeutics Inc.
19

Proprietary data of Pluristem Therapeutics Inc.
20
Zami Aberman
Chairman & CEO
Chairman & CEO
Efrat Livne-Hadass
VP Human Resources
VP Human Resources
Racheli Ofir, Ph.D.
VP Research & Intellectual Property
Sagi Moran
VP Operations
VP Operations
Erez Egozi
VP Finance
Karine Kleinhaus, M.D., MPH
Divisional VP, North America
Divisional VP, North America
Yaky Yanay
President & COO
President & COO
Hillit Mannor Shachar, M.D., M.B.A.
VP Business Development
Ohad Karnieli, Ph.D., M.B.A.
VP Technology & Manufacturing
Esther Lukasiewicz Hagai, M.D., Ph.D.
VP Clinical & Medical Affairs
Orly Amiran
VP Quality Assurance
Management Team

Proprietary data of Pluristem Therapeutics Inc.
21
New Regulatory Approaches
for Accelerated Approval
of
PLX-PAD in Critical Limb Ischemia
for Accelerated Approval
of
PLX-PAD in Critical Limb Ischemia
Dr Esther Lukasiewicz-Hagai, MD, PhD
VP Medical and Clinical Affairs

Proprietary data of Pluristem Therapeutics Inc.
22
• Pluristem is highly committed to provide early access of PLX-PAD
to Critical limb ischemia (CLI) patients all over the world
to Critical limb ischemia (CLI) patients all over the world
• Pluristem is taking advantage of each new regulatory opportunity
to achieve this goal:
to achieve this goal:
Ø In Europe: New Adaptive Pathways pilot project of EMA
May 2015: PLX-PAD cells have been selected for the Adaptive Pathways pilot
project
project
Ø In Japan: Accelerated Pathway for Regenerative Medicine
April 2015: PLX-PAD quality and manufacturing processes agreed with PMDA
for use in clinical studies
for use in clinical studies
Introduction

Proprietary data of Pluristem Therapeutics Inc.
23
Completed, ongoing and planned studies in PAOD
• 2 completed Phase I studies in Critical Limb Ischemia (n= 27)
• 1 ongoing Phase 2 study in Intermittent claudication (n=150)
• 2 planned studies in CLI:
ü 1 Phase II/III in Europe via Adaptive Pathways
ü1 Phase I/II in Japan through Expedited Approval for Regenerative
Therapy
Therapy

Proprietary data of Pluristem Therapeutics Inc.
24
Completed Phase I Studies in CLI
• Two open label, dose-escalation, Phase I studies in patients with CLI Rutherford
category 4 (pain at rest) or 5 (minor tissue loss)
category 4 (pain at rest) or 5 (minor tissue loss)
|
PLX-PAD dose
|
U.S. study n=12
( 8 Ruth 4/4 Ruth 5)
|
German study n=15
(9 Ruth 4/6 Ruth 5)
|
|
200x 106
|
-
|
Single course
50 injections (n=3)
|
|
300x 106
|
Single course
30 injections (n=5)
|
Single course
50 injections (n=6)
|
|
600x 106
|
2 courses of 300 106 at 2 weeks
apart (30 injections per course) (n=7)
|
Single course
50 injections
(n=6)
|
• 12 months FU in US study and 24 months FU in German study

Proprietary data of Pluristem Therapeutics Inc.
25
Event Rate
USA data (AFS- 100%)
Germany data (AFS- 73%)
Average (85%)
Published historical data
Strong Clinical Data from 2 Phase I/II Critical Limb Ischemia Trials(N=27)
12 month - 85% Amputation-Free Survival (AFS)

Proprietary data of Pluristem Therapeutics Inc.
26
• Good Safety profile
• Trends in efficacy with improvement from baseline in:
– Transcutaneous Oxygen Pressure (limb perfusion)
– Quality of Life
– Pain score
Main studies results
Pre-Treatment
8 Weeks After Treatment

Proprietary data of Pluristem Therapeutics Inc.
27
EMA Adaptive Pathways

Proprietary data of Pluristem Therapeutics Inc.
28
EMA Adaptive Pathways - Background
Pilot project launched by EMA on March 19th, 2014 :
• European initiative intended to grant earlier access to drugs meant to
treat debilitating and/or life-threatening diseases with unmet
treat debilitating and/or life-threatening diseases with unmet
medical need
• Only for Product at an early stage of clinical development (during or
prior to Phase II)
prior to Phase II)

Proprietary data of Pluristem Therapeutics Inc.
29
EMA Adaptive Pathways - General principles:
• Early approval of a drug for a restricted patient population followed by progressive
adaptations of the marketing authorization to expand access to the drug to broader
patient populations based on data gathered from its use and additional studies
adaptations of the marketing authorization to expand access to the drug to broader
patient populations based on data gathered from its use and additional studies
AND/OR
• Early regulatory approval (e.g. conditional approval) with collection of post-approval
confirmatory data on the drug's use in patients
confirmatory data on the drug's use in patients
• Involves balancing the importance of timely patient access with the need for
adequate, evolving evidence on a drugs’ risks and benefits
adequate, evolving evidence on a drugs’ risks and benefits
• Builds on regulatory processes already in place within the existing European Union
legal framework
legal framework
• Early discussion between a wide range of stakeholders to explore ways of optimizing
development pathways: HTA bodies, payers, patients’ organizations, physicians’
organizations, academic and researchers
development pathways: HTA bodies, payers, patients’ organizations, physicians’
organizations, academic and researchers

Proprietary data of Pluristem Therapeutics Inc.
30
Adaptive vs. traditional Pathway to Market
Phase II RCT
CLI (niche population)
Conditional MA
in niche population
2016
2017
2018
2019
2020
2021
Confirmation for the CLI subgroup (Phase III/post-
marketing registries) + Additional studies in broader
populations of CLI patients
marketing registries) + Additional studies in broader
populations of CLI patients
Full MA
Phase II RCT
Two Phase III RCTs
Full MA
Adaptive Pathways
Traditional Pathway

Proprietary data of Pluristem Therapeutics Inc.
31
Adaptive Pathways for PLX-PAD in CLI
• Severely debilitating and life-threatening disease: overall 30% of CLI patients have
amputations and 25% die by 1 year after diagnosis (higher rates in CLI patients that
cannot undergo revascularization)
amputations and 25% die by 1 year after diagnosis (higher rates in CLI patients that
cannot undergo revascularization)
• Unmet medical need: CLI patients who are not eligible for revascularization or have
failed revascularization have no treatment option
failed revascularization have no treatment option
• Phase I data with PLX-PAD in CLI patients with no option for revascularization
showing good safety profile and trends of efficacy
showing good safety profile and trends of efficacy
• Safety data from 150 patients enrolled into IC study to support initial approval in CLI
• Initial Phase II study to get conditional approval in a selected population of CLI
patients with high unmet medical need , then post-marketing extension to more
CLI patients
patients with high unmet medical need , then post-marketing extension to more
CLI patients
• A surrogate endpoint will most probably be accepted for initial approval
(reducing length of the study)

Proprietary data of Pluristem Therapeutics Inc.
32
Clinical development of PLX-PAD
Subgroup of CLI patients at high risk
(Phase II trial /Surrogate endpoint)
Initial approval in selected subgroup of
CLI patients
CLI patients
Expansion to broader CLI population (RCT / RWD)
Expansion to additional indication/s Based on:
-subgroup data/secondary endpoint
- Common clinical endpoints
- Common Mechanism of Action
Final/Additional Approval
Confirmation for selected subgroup of CLI patients
(Phase III/registries of CLI patients treated with PLX-PAD)
Supportive Real-World Data (RWD)
CLI registries

Proprietary data of Pluristem Therapeutics Inc.
33
Expedited approval for
regenerative therapy in Japan
regenerative therapy in Japan

Proprietary data of Pluristem Therapeutics Inc.
34
Background
New Japanese regulations from Nov 2014 to accelerate
development of drugs in the field of regenerative therapy:
development of drugs in the field of regenerative therapy:
• Only for Regenerative therapy
• Seriously-debilitating / Life-threatening indication
• Unmet medical need

Proprietary data of Pluristem Therapeutics Inc.
35
Accelerated vs. traditional approval process
• Conditional time-limited approval can be obtained based on proof of safety and “limited proof of
efficacy” (Phase I/II, surrogate endpoint)
efficacy” (Phase I/II, surrogate endpoint)
• Up to 7 years to confirm efficacy post conditional approval
• Condition to have patients treated post conditional approval signed an ICF and registered in a post
marketing registry for pharmacovigilance
marketing registry for pharmacovigilance

Proprietary data of Pluristem Therapeutics Inc.
36
Clinical development of PLX-PAD for CLI
Phase I/II trial
(limited proof of efficacy)
Time-limited conditional approval
Final/Additional Approval
Registry of patients treated with
PLX-PAD for pharmacovigilance

Proprietary data of Pluristem Therapeutics Inc.
37
|
|
2015
|
2016
|
2017
|
2018
|
2019
|
2020
|
2021
|
|||||||
|
EMA initiation
|
PIP
Waiver |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ATMP
Class. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SA on
CMC |
SA with
EMA/HTA |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Phase II/III
(EU)
|
|
CTA Subm.
|
Phase II
|
Phase III/Long term follow up
Registry of patients treated with PLX-PAD
|
|
|
|
|||||||
|
MA
(EMA)
|
|
|
|
|
|
|
CMA
|
|
|
|
Presub.
meeting |
MAA
subm. |
MA
Approval |
|
|
PMDA initiation
|
R&D
meeting |
Pre Phase I/II
meeting |
|
|
|
|
|
|
|
|
|
|
|
|
|
Phase I/II
(Japan)
|
|
CTN
submission
|
Phase I/II
|
Safety
follow up |
Confirmation of efficacy
Registry of Patients treated with PLX-PAD
|
|
|
|
||||||
|
Conditional Time-
Limited Authorization Application (PMDA) |
|
|
|
|
Pre-NDA
meetings |
CAA
subm. |
CAA
Approval |
|
|
|
|
|
|
|
|
Marketing
Authorization Application (PMDA)
|
|
|
|
|
|
|
|
|
|
|
Pre-NDA
Meeting |
NDA
Subm. |
NDA
Approv. |
|

Proprietary data of Pluristem Therapeutics Inc.
38
Conclusion
In the framework of the 2 new regulatory approaches that exist
in Europe and in Japan , Pluristem plan to initiate 2 clinical
studies in CLI in 2016 with the aim of obtaining initial approval
already in 2018
in Europe and in Japan , Pluristem plan to initiate 2 clinical
studies in CLI in 2016 with the aim of obtaining initial approval
already in 2018

Proprietary data of Pluristem Therapeutics Inc.
39
• Time to Market (TTM)- we will be focus on adaptive pathways
Ø Europe
Ø Japan
Ø Our goal is to file early/conditional approval by the end of
2017/early 2018
2017/early 2018
• Launching PLX-R18 studies for hematology and ARS
• Building platform for new products- serum free based
• Entering into Life changing indication
• Entering into licensing deal
So, what’s next?

Proprietary data of Pluristem Therapeutics Inc.
40

Proprietary data of Pluristem Therapeutics Inc.
41
|
Partner
|
Indication
|
Deal structure
|
|
|
Upfront payment of $7M, additional
$48M in milestones, cells supply (cost +) and royalties in gross margin |
|
|
|
IC, CLI
South Korea only
|
JV following marketing authorization
of the Korean authorities |
|
|
Acute Radiation Syndrome
|
U.S. National Institutes of Health to
support development of Pluristem's PLX-R18 |
Pluristem keeps IP and manufacturing rights in all collaborations
Collaborations

Proprietary data of Pluristem Therapeutics Inc.
42
|
IC
|
|||||||||
|
CLI (Adaptive EU)
|
|||||||||
|
CLI (Japan)
|
|
|
|
|
|||||
|
Orthopedic indication
|
|
|
|
|
|
|
|
|
|
|
Hematology
|
|
|
|
||||||
|
ARS
|
|
|
|
|
|
||||
|
PAH
|
|
|
|
|
|
|
|
|
|
|
Preeclampsia
|
|
|
|
|
|
|
|
|
|
End of
enrollment
enrollment
Proprietary data of Pluristem Therapeutics Inc.
Company milestones
indication
End of
enrollment
enrollment
Data Readout
Licensing deal
Study
initiation
initiation
Adaptive
Pathway
approval
Pathway
approval
Initial
approval
approval
Data
Readout
Readout
End of
enrollment
enrollment
Data
Readout
Study initiation
Conditional
approval
approval
We will seek to include the indication under the Adaptive licensing
pathway- based on discussion with EMA
pathway- based on discussion with EMA
IND
Study
initiation
End of
enrollment
enrollment
Data
Readout
Adaptive or
phase II/III
phase II/III
Pivotal study
Data
Readout
Readout
BLA application
UT will pursue IND upon completion of the phase I
We will seek to include preeclampsia under the Adaptive licensing
pathway- based on discussion with EMA
pathway- based on discussion with EMA
Proprietary data of Pluristem Therapeutics Inc.
43
Proprietary data of Pluristem Therapeutics Inc.
43
indication
|
Technologies
|
|
|
|
|
|||||
|
New products
(Tox ready)
|
|
|
|
|
Thawing
device
Plurispheare
MP4
NewPro1
PLX-R18
MP5
NewPro2
NewPro3
Company milestones

