Attached files
| file | filename |
|---|---|
| EX-4.2 - WARRANT AGREEMENT - INNOVATION ECONOMY Corp | fs12015a3ex4ii_innovation.htm |
| EX-4.4 - FORM OF FACE OF WARRANT CERTIFICATE - INNOVATION ECONOMY Corp | fs12015a3ex4iv_innovation.htm |
| EX-23.1 - INNOVATION ECONOMY Corp | fs12015a3ex23i_innovation.htm |
| EX-4.3 - FORM OF FACE OF WARRANT CERTIFICATE - INNOVATION ECONOMY Corp | fs12015a3ex4iii_innovation.htm |
| EX-1.1 - SELLING AGENCY AGREEMENT - INNOVATION ECONOMY Corp | fs12015a3ex1i_innovation.htm |
As filed with the Securities and Exchange Commission on May 20, 2015
Registration No. 333-203238
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
____________________________________________________________________________________________
Amendment No. 3
to
FORM S-1
REGISTRATION
STATEMENT
UNDER
THE SECURITIES ACT OF
1933
____________________________________________________________________________________________
INNOVATION ECONOMY
CORPORATION
(Exact name of registrant as specified
in its charter)
|
Delaware |
|
7389 |
|
27-3865577 |
|
(State or other jurisdiction of incorporation or organization) |
|
(Primary Standard Industrial Classification Code Number) |
|
(I.R.S. Employer Identification No.) |
1650 Spruce Street,
Suite 500
Riverside, CA 92507
Tel. (951) 824-8669
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Paracorp
Incorporated
2140 S DuPont Hwy
Camden, DE 19934
(302) 730-1320
(Name, address, including zip code, and telephone number, including area code, of agent for service)
with copies to:
Richard
Baumann, Esq. |
|
Louis
Taubman, Esq. |
Approximate date of proposed sale to public: From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
|
¨ |
|
|
|
Accelerated filer |
|
¨ |
|
Non-accelerated filer |
|
¨ |
|
(Do not check if a smaller reporting company) |
|
Smaller reporting company |
|
x |
CALCULATION OF REGISTRATION FEE
|
Title of Each Class of Securities to be Registered |
|
Proposed Maximum Aggregate Offering Price(1) |
|
Amount of Registration Fee(2) |
||||
|
Units, each unit consisting of one share of common stock, par value $0.00001 per share, and one warrant to purchase one-half of one share of common stock |
|
$ |
25,650,000 |
(3) |
|
$ |
2,980.53 |
(3) |
|
Shares of common stock included in the units |
|
|
N/A |
|
|
|
N/A |
(4) |
|
Warrants included in the units |
|
|
N/A |
|
|
|
N/A |
(4) |
|
Shares of common stock underlying the warrants included in the units (at an exercise price of 125% of the price of the shares of common stock included in the units) |
|
$ |
15,775,000 |
(5) |
|
$ |
1,833.06 |
(5) |
|
Shares of common stock issuable upon exercise of the warrants issued to the selling agent in connection with the offering of the units |
|
$ |
1,500,000 |
|
|
$ |
174.30 |
|
|
Shares of common stock issuable upon exercise of the warrants included in the units issued to the placement agent in connection with the 2014 Private Placement |
|
$ |
200,000 |
|
|
$ |
23.24 |
|
|
Total |
|
$ |
43,125,000 |
|
|
$ |
5,011.13 |
(6)(7) |
____________
(1) Estimated solely for purposes of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended.
(2) Calculated pursuant to Rule 457(o) based on the estimated proposed maximum aggregate offering price.
(3) Consists of units issuable (i) in this offering, (ii) upon the forced conversion, immediately after the closing of this offering, of convertible notes (the “Private Placement Convertible Notes”) sold by the registrant in two private placements, one of which had its final closing on October 14, 2014 (the “2014 Private Placement”) and the other of which closed on $2,100,000 of Note purchases as of April 30, 2015 (the “2015 Private Placement” and collectively with the 2014 Private Placement, the “Private Placements”) and (iii) upon the forced conversion of notes whose terms are the same as the Private Placement Convertible Notes, issuable upon the exercise by the placement agent and an additional broker-dealer for the 2014 Private Placement of warrants received as partial compensation for placing Notes in that placement. By their terms, the Private Placement Convertible Notes earn interest at a rate of 8% per annum. The amount of unpaid principal of, and accrued interest on, the Private Placement Convertible Notes, immediately after the closing of this offering shall convert into units at a price equal to $5.10 per unit.
(4) No registration fee required pursuant to Rule 457(g).
(5) Consists of shares of common stock underlying (i) the warrants included in the units issuable in this offering and (ii) the warrants included in the units issuable upon the forced conversion of the Private Placement Convertible Notes, as described in note 3, above. Our registration includes the exercise of the warrants by any purchasers of warrants or units from the selling securityholders identified in this prospectus.
(6) Pursuant to Rule 416 under the Securities Act of 1933, as amended, the number of shares of common stock registered hereby shall also include an indeterminate number of additional shares of common stock issuable as a result of stock splits, stock dividends (including interest payments), recapitalizations, reorganizations or similar transactions.
(7) Previously paid.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date, until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the commission, acting pursuant to said section 8(a), may determine.
EXPLANATORY NOTE
This registration statement contains two forms of prospectus, as set forth below.
• Public Offering Prospectus. A prospectus to be used for the initial public offering by the registrant of up to $20,000,000 of units (the “Public Offering Prospectus”).
• Selling Securityholder Prospectus. A prospectus to be used in connection with the potential resale by certain selling securityholders of up to an aggregate of 994,652 units, each unit consisting of one share of the registrant’s common stock and one warrant to purchase one-half share of the registrant’s common stock, issuable upon the forced conversion, immediately after the closing of the Company’s initial public offering of units, of the Private Placement Convertible Notes sold by the registrant in the Private Placements (the “Selling Securityholder Prospectus”). By their terms, upon the Company’s election to force such conversion of the Private Placement Convertible Notes, the Private Placement Convertible Notes shall convert into units at a price equal to $5.10 per unit. There is no minimum amount of securities that must be sold in the Company’s initial public offering and the conversion of the Private Placement Convertible Notes will occur even if only a few Units are sold. The Selling Securityholder Prospectus will also include 34,824 Units issuable upon the forced conversion of notes issuable upon the exercise of placement agent warrants related to the October 2014 Convertible Notes.
The Public Offering Prospectus and the Selling Securityholder Prospectus will be identical in all respects except for the following principal points:
• they will contain different front covers;
• the Table of Contents in the Selling Securityholder Prospectus (but not in the Public Offering Prospectus) will contain entries for a Shares Registered for Resale section and a Selling Securityholders section;
• they will contain different Use of Proceeds sections;
• a Shares Registered for Resale section will be included in the Selling Securityholder Prospectus (but not in the Public Offering Prospectus);
• a Selling Securityholders section will be included in the Selling Securityholder Prospectus (but not in the Public Offering Prospectus);
• they will contain different Plan of Distribution sections;
• the Legal Matters section in the Selling Securityholder Prospectus will not contain a reference to counsel for the selling agent; and
• they will contain different back covers.
The registrant has included in this registration statement, following the financial statement pages, a set of alternative pages reflecting the foregoing differences between the Public Offering Prospectus and the Selling Securityholder Prospectus.
As of January 16, 2015, we effectuated a 15-1 reverse split of our shares, meaning that 15 of our shares held prior to the reverse split equals one share held after the reverse split. If, as a result of the split, any stockholder would be entitled to receive a fraction of a share, in order to avoid issuing fractional shares we will instead provide that stockholder with an additional whole share. All share and share equivalents numbers presented in this prospectus retroactively reflect the effectuation of the 15-1 reverse split dated January 16, 2015, unless otherwise stated or unless the context otherwise indicates.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where an offer or sale is not permitted.
PRELIMINARY PROSPECTUS |
|
SUBJECT TO COMPLETION, dated MAY 20, 2015 |

Up to
3,125,000 Units
Consisting of One Share of Common Stock and
One Warrant to Purchase One-half Share of Common
Stock
This is the initial public offering of securities of Innovation Economy Corporation, which does business under the name “ieCrowd”. We are offering up to 3,125,000 units (the “Units”), each Unit consisting of one share of our common stock, par value $0.00001 per share, and one warrant to purchase one-half share of our common stock. The Units are being offered at a price of $6.40 per Unit, representing a price of $6.39 for the underlying share of common stock and $0.01 for the underlying warrant. Each warrant will entitle the holder to purchase one-half share of our common stock at an exercise price of 125% of the price of the shares in this offering, or $8.00 per whole share. The warrants will expire 36 months after the date they are issued. The Units will not be issued or certificated. Instead, the shares of common stock and the warrants will be issued separately and may be resold separately, although they will have been purchased together in this offering.
Currently, there is no public market for our Units, common stock or warrants. We have applied to list our common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “MYIE”, and to list our warrants on NASDAQ under the symbol “MYIEW”, on or promptly after the closing date of this offering. We cannot guarantee that our securities will be approved for listing on NASDAQ. If they are not, we may in our sole discretion cancel some or all of this offering and thereafter promptly return any escrow funds you may already have paid. See “Plan of Distribution”.
Investing in our securities involves a high degree of risk. Our company is at an early stage of its development and our securities may only be appropriate for long-term investment. Our independent registered public accounting firm has issued an audit opinion that includes a statement expressing substantial doubt as to our ability to continue as a going concern. You should purchase our securities only if you can afford to lose your entire investment. See “Risk Factors” beginning on page 10.
|
|
Per Unit |
|
Total(3) |
||
Price to the public |
|
$ |
6.40 |
|
$ |
20,000,000 |
Selling agent’s commissions(1) |
|
$ |
0.38 |
|
$ |
1,200,000 |
Proceeds to us (before expenses)(2) |
|
$ |
6.02 |
|
$ |
18,800,000 |
(1) TriPoint Global Equities, LLC is an underwriter of this offering within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended, or the Securities Act, and any commissions received by it and any profit realized on the sale of the securities by it while so acting may be deemed to be underwriting compensation. See “Plan of Distribution”.
(2) We estimate that our total expenses for this offering, excluding selling agent’s commissions, will be approximately $625,000.
(3) Assumes that all of the securities offered are sold.
We plan to market this offering to potential investors through the selling agent and by ourselves, directly, at meetings that we will conduct at selected locations around the United States with prospective investors. The offering will close on June 25, 2015, 45 days after we commence it, unless all the securities are sold before that date, we and the selling agent agree to extend the offering another 45 days or we otherwise decide to close the offering early or cancel it, in our sole discretion. If we and the selling agent extend the offering, we will provide that information in an amendment to this prospectus. If we close the offering early or cancel it, we may do so without notice to you, although if we cancel the offering we will promptly return any escrow funds you may already have paid. See “Plan of Distribution”.
We are an “emerging growth company” under applicable law and rules and we may elect to comply with certain reduced public company reporting requirements following this offering.
Neither the Securities and Exchange Commission nor any state securities commission nor any other regulatory body has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
TriPoint Global Equities, LLC
The date of this prospectus is __________, 2015
TABLE OF CONTENTS
|
|
|
Page |
|
Prospectus Summary |
|
1 |
|
The Company |
|
1 |
|
The Offering |
|
6 |
|
Summary Consolidated Financial Data |
|
9 |
|
Risk Factors |
|
10 |
|
Cautionary Note Regarding Forward-Looking Statements |
|
31 |
|
Use of Proceeds |
|
33 |
|
Dividend Policy |
|
33 |
|
Capitalization |
|
34 |
|
Dilution |
|
36 |
|
Selected Consolidated Financial Data |
|
38 |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
39 |
|
Business |
|
51 |
|
Management |
|
85 |
|
Executive Compensation |
|
91 |
|
Certain Relationships and Related Party Transactions |
|
100 |
|
Principal Stockholders |
|
102 |
|
Description of Securities |
|
104 |
|
Shares Eligible for Future Sale |
|
107 |
|
Certain U.S. Federal Income Tax Considerations |
|
109 |
|
Plan of Distribution |
|
113 |
|
Legal Matters |
|
119 |
|
Experts |
|
119 |
|
Where You Can Find More Information |
|
119 |
|
Index to Financial Statements |
|
F-1 |
We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where such offers and sales are permitted. You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with any information other than the information contained in this prospectus.
The information contained in this prospectus is accurate only as of its date, regardless of the time of its delivery or of any sale or delivery of our securities. Neither the delivery of this prospectus nor any sale or delivery of our securities shall, under any circumstances, imply that there has been no change in our affairs since the date of this prospectus. This prospectus will be updated and made available for delivery to the extent required by the federal securities laws.
This prospectus includes estimates, statistics and other industry data that we obtained from industry publications, research and surveys, from studies conducted by third parties and from publicly available information. All of such data involve a number of assumptions and limitations, and contains projections and estimates of the future performance of the industries in which we operate, all of which is subject to a high degree of uncertainty. We caution you not to give undue weight to any such estimates, statistics, other industry data and projections. Internet addresses for such publicly available information are provided solely for the convenience of the reader. We do not incorporate by reference any information available through such websites, other than to the extent we quote or expressly refer to such information in the body of this prospectus.
As of January 16, 2015, we effectuated a 15-1 reverse split of our shares, meaning that 15 of our shares held prior to the reverse split equals one share held after the reverse split. If, as a result of the split, any stockholder would be entitled to receive a fraction of a share, in order to avoid issuing fractional shares we will instead provide that stockholder with an additional whole share. All references to the number of shares, options, warrants and other common stock equivalents, price per share and weighted-average number of shares of common stock outstanding presented in this prospectus retroactively reflect the effectuation of the 15-1 reverse split dated January 16, 2015, unless otherwise stated or unless the context otherwise indicates.
i
PROSPECTUS SUMMARY
The following summary highlights material information contained in this prospectus. This summary does not contain all of the information you should consider before investing in our securities. Before making an investment decision, you should read the entire prospectus carefully, including our consolidated financial statements and the related notes, and the information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations”.
Unless the context requires otherwise, we use the terms “ieCrowd”, “the Company”, “our Company”, “we”, “us”, and “our” to refer to Innovation Economy Corporation and its consolidated subsidiaries. We do business under the name “ieCrowd”.
The Company
We are an emerging growth company based on a Collaborative Economy model with a mission to bring the world together to unlock the potential of untapped innovations.
We were founded by experienced entrepreneurs who recognized that research institutions can be filled with un-commercialized technology discoveries and breakthrough research (which we refer to throughout this document as “innovations”) which, if successfully commercialized, could solve or help to solve significant global challenges. We believe that the potential of these untapped innovations could be vast. Yet many of these innovations will never see the light of day because of the significant difficulties often experienced in the process of taking laboratory-proven, potentially ground-breaking innovations and transforming them into products, product platforms, services and technologies for distribution to global markets.
Recognizing the gap between the marketplace and un-commercialized but potentially beneficial innovations, the founders of ieCrowd have created a business model that is intended to bridge this gap. ieCrowd’s business goal is to license or acquire innovations with the potential to solve global problems for the purpose of commercializing them into product platforms, products, services and technologies that have substantial value-adding impact over large populations and markets.
When ieCrowd licenses or acquires a new innovation, it places it into a separate, newly formed, majority-owned and ieCrowd-operated subsidiary that benefits from the application of the ieCrowd parent company’s commercialization platform. Now in its fifth year of existence, ieCrowd is in the process of applying its commercialization platform to three innovations, through three separate, majority-owned and ieCrowd-formed and -operated subsidiaries: Olfactor Laboratories, Inc. (“OLI”), Nano Engineered Applications, Inc. (“NEA”) and Smart Oxygen Solutions, Inc. (“SOS”) (formerly known as Breathing Technologies, Inc.). These subsidiaries have reached the following stages of their development:
• OLI, now in its fourth year of operations, is seeking to commercialize a range of patent-pending technologies to control the behavior of mosquitoes and other blood-seeking insects with non-insecticidal agents, with the aim of lowering the spread of diseases such as malaria, Dengue fever, West Nile virus and other diseases that are transmitted by blood-seeking insects. Transmission of disease from insects to humans is a major global health issue, and OLI is working to create a number of product offerings leveraging these technologies for various markets globally. The Company has chosen “Kite” as the brand under which its expected future repellant and attractant product offerings will be marketed. OLI’s initial product offerings must pass through OLI’s final product development stages, including testing, the determination of manufacturing specifications and consumer brand and packaging finalization. OLI must also pursue regulatory approvals for most of its “Kite”-branded product offerings prior to sale to consumers in most countries. In the U.S., OLI must obtain approval for its Kite-branded products from the EPA. We expect to begin seeking regulatory approvals for the first of our Kite-branded products in or about the first half of 2016 (although we can provide no assurance that we will be able to begin seeking approvals within this timeframe, or at all). We would expect to obtain these approvals within approximately 24 to 36 months from the filing of applications for the approvals (although we can provide no assurance that we will be able to obtain the approvals within that timeframe, or at all). We would expect to repeat this process and timing for later-developed Kite-branded products. OLI is also pursuing the development of a range of product offerings for licensing and public health efforts globally. OLI currently expects to begin selling repellant and attractant products, or licensing the distribution of such products to others, or both, as well as beginning to license active ingredients or exclusive rights to active ingredients, in 2016 (although we can provide no assurance that we will be able to fully develop, and sell or license the distribution of, any such products at that time or any time). From April 25 to May 15, 2015,
1
members of ieCrowd’s Kite team have been in Uganda — in the Jinja area and surrounding rural communities — implementing a series of tests relating to Kite’s technologies in development.
• NEA, now in its fourth year of operations, seeks to commercialize patented and patent-pending sensor technologies based on nano materials that can detect airborne gases to the parts-per-billion (PPB) level. (“Nano materials” are materials sized in at least one dimension between 1 and 100 nanometers, with a nanometer being 10−9 of a meter.) NEA’s technologies, if commercialized, could provide a product platform encompassing multiple consumer, commercial, industrial and security-related products with a variety of applications, including air quality monitoring, explosives detection, industrial plant toxic gas detection, gaseous chemical warfare agent detection, food and agricultural safety hazard detection and other applications. Before any products can be made available to the market, laboratory facility and equipment improvements have to be made, product development (including sensor, algorithm, form factor, software and prototype development) must be completed, testing must be conducted, any necessary regulatory and industry safety and industry standard certifications must be acquired and manufacturing and quality assurance facilities and protocols must be established. We expect to begin seeking regulatory approvals for the first of our NEA-branded products in or about the second half of 2016 (although we can provide no assurance that we will be able to begin seeking approvals within this timeframe, or at all). We would expect to obtain these approvals within approximately three to four months after the beginning of the approval process (although we can provide no assurance that we will be able to obtain the approvals within that timeframe, or at all). We expect to repeat this process and timing for later-developed NEA-branded products. We currently expect to complete these steps and begin licensing the distribution of products to others, and possibly selling some initial consumer products ourselves, in 2016 (although we can provide no assurance that we will be able to fully develop, and license the distribution of or sell, any such products at that time or any time).
• SOS, now in its second year of operations, seeks to commercialize supplemental oxygen delivery devices that would incorporate a computer controlled, patient-adaptive dosing system, for people who have been prescribed supplemental oxygen delivery equipment by a doctor. The devices would be configured as add-ons to existing, commercially available supplemental oxygen delivery equipment. If commercialized, the devices would have the potential to be used by people who have been prescribed supplemental oxygen therapy by their doctor in maintaining a more active lifestyle. The current milestone plan for development of the devices extends through a currently ongoing FDA pre-submission process and, thereafter, an expected FDA submission process. We cannot currently foresee when our FDA processes may be completed. Potential revenue-earning options will be considered thereafter.
In addition to the foregoing, effective November 3, 2014, we entered into agreements with the University of California, Los Angeles (UCLA) to secure licenses, subject to the completion of our due diligence efforts, for two additional innovations:
• Head Pain Management. We are exploring potential therapeutic treatments based on technology that stimulates the sensory fibers of a nerve in order to mitigate pain perception. The technology uses mechanical vibration (as opposed to electrical stimulation) to stimulate such fibers and is non-invasive and patient controllable. The target market for the commercialization of this technology would include migraine sufferers, as well as those with trigeminal neuropathy (a debilitating, long-lasting oral pain, often arising from failed or compromised dental procedures) and others.
• Sleep Disorders. We are exploring potential therapeutic treatments based on technology that takes advantage of the fact that signals from the limbs can be interpreted by the brain as movement and can elicit altered breathing rates. The device we would expect to develop would use a nerve stimulation technique to alter the breathing rate without actual limb movement. The target market for the commercialization of this technology would include people who suffer sleep disorders mostly due to irregular breathing, the most common of which is sleep apnea.
More recently, in May 2015, we negotiated options to license up to 20 different families of patents and patent applications. The options run for six months, and can be extended for an additional six months thereafter in the sole discretion of ieCrowd. The patents and applications relate to subject matters in a variety of health-related technology areas. ieCrowd intends to use the time afforded to it under the options to continue its due diligence of the patents and applications and decide which patent families, if any, it wishes to license.
2
ieCrowd intends to pursue commercial success with each of these subsidiaries and innovations, and over time to expand the number of its subsidiaries and innovations. Ultimately, ieCrowd envisions having a group of life and health subsidiaries diversified across various business development stages and product, service and technology markets. ieCrowd expects that achieving such diversification may help it mitigate some of the risks typically associated with investing in early-stage companies and innovative discoveries and technologies.
The Company does business under the name “ieCrowd” in order to emphasize its Collaborative Economy approach to implementing its business model. By “Collaborative Economy”, we mean a system that emphasizes the sharing, repurposing and distribution of ideas, opportunities and other resources among a great number of participants, typically aided by the use of social media and other information technology, in order to increase the value and efficient deployment of those resources not only for the participants but also for larger populations, societies and the world generally. We refer to the individuals and entities we seek to collaborate with as the “Crowd”. The Crowd includes the people and organizations through which we hope to gain access to expertise, innovations, social and business networks, marketing and distribution opportunities and other resources. The Crowd includes, among others, universities, government agencies, municipalities, non-governmental organizations (“NGOs”), stockholders, innovators, investors, employees, vendors, other stakeholders and collaborators and potential marketing and distribution partners and customers. The Crowd plays an integral role in each stage of ieCrowd’s mission to bring commercialization to bear to help solve global problems.
Our Business Strategy
The Company’s strategy for attaining its goals incorporates three principal components:
• Licensing innovations with potential global benefits from universities, federal agencies, innovators, businesses and other sources. In the future, we may also acquire innovations outright or acquire the companies that own the innovations;
• Commercializing these innovations, to seek to turn them into products, product platforms, services and technologies. We seek to do this by assigning each innovation to a commercialization team, made up of, among other people, product development members – scientists and other technical subject matter experts – supported by our “business infrastructure” members –personnel who can provide executive leadership, operations management, sales, marketing and public relations functions and administrative services (human resources, legal, finance, accounting). We also provide office space, laboratory space, specialized equipment, raw materials, supplies and other physical resources; and
• Deploying these innovations into global markets, which we expect to do if and when our first products are fully commercialized, via licensing these products to regional, national and international market partners and distribution channels and, in some cases, through direct-to-consumer sales and marketing campaigns.
Market Opportunity
According to the Scholarly Publishing and Academic Resources Coalition’s January 2014 report (available at http://www.sparc.arl.org/news/omnibus-appropriations-bill-codifies-white-house-directive), over $60 billion of taxpayer funds is spent annually on research projects to “advance science, spur the economy, accelerate innovation, and improve the lives of our citizens”. A large number of these research projects end when their results are published and their government funding is complete. Some of this research is worthy of further development and this is where the ieCrowd process begins. ieCrowd believes that a significant innovation gap exists between laboratory-proven innovations and the global market launch of products and product platforms based on those innovations.
ieCrowd sees significant potential in working with research institutions to search through their research portfolios for possible breakthrough innovations, and then licensing or acquiring the most promising of these innovations for development and commercialization. In regard to these innovations, ieCrowd picks up where government funding left off and seeks to generate value moving the selected innovation through development to commercialization.
Our Key Strengths
There are significant barriers to overcome in taking laboratory-proven technologies and transforming them into products for distribution to global markets. Though simple in concept, the process is difficult in execution. In order to succeed in its business, ieCrowd relies on the following key strengths:
• Past experience: ieCrowd’s management team is comprised of experienced entrepreneurs.
3
• Business model: ieCrowd believes that its business model brings systemization, efficiency and greater predictability to the task of transforming innovations into market-ready products, services and technologies, and that its Collaborative Economy focus helps it find more ideas and resources and reach more collaborators and potential customers and beneficiaries of its efforts.
• Capital efficiency: ieCrowd targets the licensing or acquisition of intellectual property rights that require low initial capital outlays, and then deploys capital efficiently through the shared services and economies of scale available to the ieCrowd operating subsidiaries through the ieCrowd parent company.
• Parent company-level infrastructure: For each operating subsidiary, ieCrowd provides operations, sales, marketing, public relations and administration (human resources, legal, finance, accounting) functions, as well as capital and executive leadership.
• Key relationships: ieCrowd has a demonstrated track record of identifying and establishing partnerships with research institutions and other key stakeholders.
• Flexibility in seeking revenue: Each ieCrowd operating subsidiary can pursue revenue through licensing, distributorships or retail and direct-to-consumer campaigns.
• Diversification: ieCrowd aims eventually to hold a portfolio of innovations across different phases of development, product types and markets.
Going Concern
As of March 31, 2015, the Company had a working capital deficiency of $890,684 and a stockholders’ deficiency of $3,181,300. As of December 31, 2014, the Company had working capital of $723,267 and a stockholders’ deficiency of $1,576,760. The Company has not generated any significant revenues from ongoing operations and has incurred net losses since inception. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements included in this prospectus do not include any adjustments relating to the recoverability and classification of asset amounts or the classification of liabilities that might be necessary should the Company be unable to continue as a going concern. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Going Concern”.
Emerging Growth Company
We are an “emerging growth company” within the meaning of the federal securities laws. For as long as we are an emerging growth company, we will not be required to comply with certain regulatory requirements applicable to other public companies that are not emerging growth companies, including but not limited to: not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and being exempt from the requirements of holding a non-binding advisory vote on executive compensation and securing stockholder approval of golden parachute payments. We intend to take advantage of these reduced regulatory requirements until we are no longer an emerging growth company. For a description of the qualifications and other requirements applicable to emerging growth companies, and certain elections we have made due to our status as an emerging growth company, see “Risk Factors—”As an ‘emerging growth company’ under applicable law, we will be subject to reduced disclosure requirements, which could leave our stockholders without information or rights available to stockholders of more mature companies”.
Risks Associated with Our Business
Our business is subject to numerous significant risks, as more fully described in the section entitled “Risk Factors” immediately following this prospectus summary. You should read and carefully consider the risks summarized immediately below, together with all the risks more fully described in the section entitled “Risk Factors” and all of the other information in this prospectus, including the financial statements and the related notes, before deciding whether to invest in our securities. If any of the risks discussed in this prospectus actually occur, our business, financial condition, operating results, prospects and share price could be materially and adversely affected. Our risks include but are not limited to the following:
• We are an emerging growth company and are subject to the many risks associated with new businesses.
• Our independent registered public accounting firm has issued an audit opinion that includes a statement expressing substantial doubt as to our ability to continue as a going concern.
4
• We have not generated substantial revenue and may not generate revenue in the manner or within the timeframe we anticipate. We do not have positive operating cash flow. We expect to incur losses for the foreseeable future.
• We will need to raise additional investment capital, which may be unavailable to us or, if raised, may cause dilution of our existing investors and place significant restrictions on our ability to operate.
• Our independent registered public accountants have identified material weaknesses in our internal control over financial reporting. In addition, because of our status as an emerging growth company, our independent registered public accountants are not required to provide an attestation report as to our internal control over financial reporting for the foreseeable future.
• Successful testing of our innovations in the laboratory may not be indicative of future results and may not result in commercially viable innovations. Further, our innovations may have to be modified from their original design in order to reach or be successful in the market.
• Our products are still being developed for commercialization, and our business may fail if we are not able to successfully generate significant revenues from these products.
• We focus our efforts on the commercialization of potential products that not only present revenue and profit opportunities, but also promise to do good. However, our goal of doing good may at times undercut our business performance, and we will be our own judge of what it means for a product, service or technology to “do good”.
• If we are unable to establish successful relations with third-party distributors, or these distributors do not focus adequate resources on selling our products or are otherwise unsuccessful in selling them, sales of our products may not develop.
• Our ownership interest in each of our operating subsidiaries is less than 100%, ownership interests in our current and to-be-formed subsidiaries may fluctuate and shares in our subsidiaries may be sold to third parties at the discretion of management.
• Our inability to obtain regulatory approvals, or to comply with ongoing and changing regulatory requirements, could delay or prevent sales of the products we are developing.
• The failure to obtain or maintain patents, licensing agreements and other intellectual property could materially impact our ability to compete effectively.
• We are selling Units in this offering without using the traditional services of an underwriter, and there can be no assurance that all or any of the Units included in this offering will be sold.
• We have applied to list our common stock and warrants on NASDAQ, but we cannot guarantee that our securities will be approved for listing on NASDAQ.
• No public market for our common stock currently exists, and an active trading market may not develop or be sustained following this offering.
• The issuance in the future of a significant number of additional new shares of our common stock may have an adverse effect on our stock price, even if our business is performing well, and will dilute then‑existing shareholders.
Corporate Information
We were formed on October 28, 2010 as a Delaware corporation. Our principal executive offices are located at 1650 Spruce Street, Suite 500, Riverside, CA 92507 and our telephone number is (951) 824-8669.
5
The Offering
|
Issuer: |
|
Innovation Economy Corporation, which does business under the name “ieCrowd” |
|
|
|
|
|
Securities: |
|
Up to 3,125,000 Units, each Unit consisting of: • one share of common stock; and • one warrant to purchase one-half share of common stock |
|
|
|
|
|
Number of shares outstanding before the offering: |
|
9,444,828 shares(1) |
|
|
|
|
|
Number of shares outstanding after the offering: |
|
13,599,304 shares(1) (2) (3) |
|
|
|
|
|
Price per Unit: |
|
$6.40 per Unit, representing a price of $6.39 for the underlying share of common stock and $0.01 for the underlying warrant. |
|
|
|
|
Exercisability of warrant: |
|
Each warrant will entitle the holder to purchase one-half share of our common stock at an exercise price of 125% of the price of the shares in this offering, or $8.00 per whole share. No fractional shares will be issued upon exercise of the warrants. The warrants shall be exercisable from the date of issuance (except for those persons subject to the lock-up agreements discussed under “Shares Eligible for Future Sale”), which is the closing date of this offering, and expire on the 36-month anniversary thereof. If, upon exercise of the warrants, a holder would be entitled to receive a fractional interest in a share, we will, at our election, upon exercise, either pay a cash adjustment in respect of such fraction (in an amount equal to such fraction multiplied by the exercise price) or round the number of shares to be received by the holder up to the next whole number. |
|
|
|
|
|
Redemption of warrants |
|
We may redeem all or a portion of the warrants offered as part of our Units to the extent they remain outstanding and unexercised: • at a price of $0.01 per warrant; • upon a minimum of 30 days’ prior written notice of redemption, which we refer to as the 30-day redemption period; • if, and only if, the last sale price of our common stock equals or exceeds $16.00 per share (as may be adjusted for stock splits and similar transactions) on each of 20 trading days within the 30 trading-day period ending on the third business day prior to the date on which notice of the redemption is given to the warrant holders; and • if, and only if, the average daily trading volume of our common stock exceeds 500,000 shares per day during the redemption period. We will not redeem the warrants unless an effective registration statement under the Securities Act covering the shares of common stock issuable upon exercise of the warrants is effective, except if the warrants may be exercised on a cashless basis and such cashless exercise is exempt from registration under the Securities Act. |
|
|
|
|
|
Separability of shares and warrants:
|
|
The Units will not be issued or certificated. Instead, the shares of common stock and the warrants will be issued separately and may be resold separately, although they will have been purchased together in this offering. |
|
|
|
|
|
Marketing: |
|
TriPoint Global Equities, LLC has agreed to act as our selling agent for this offering. It will not purchase the securities offered by us and it is not required to sell any specific number or dollar amount of securities, but will arrange for the sale of securities to investors on a “best efforts” basis. See “Plan of Distribution”. We plan to market this offering to potential investors through the selling agent and by ourselves, directly, at meetings that we will conduct at selected locations around the United States with prospective investors. |
6
|
Duration of offering: |
|
The offering will close on June 25, 2015, 45 days after we commence it, unless all the securities are sold before that date, we and the selling agent agree to extend the offering another 45 days or we otherwise decide to close the offering early or cancel it, in our sole discretion. If we and the selling agent extend the offering, we will provide that information in an amendment to this prospectus. If we close the offering early or cancel it, including during any extended offering period, we may do so without notice to you, although if we cancel the offering we will promptly return any escrow funds you may already have paid. See “Plan of Distribution”. |
|
|
|
|
Proceeds to us from the offering (after selling agent’s commissions but before expenses): |
|
$18,800,000(3) |
|
|
|
|
Use of proceeds: |
|
If we sell all of the securities being offered, our net proceeds (after the selling agent’s commissions and after our estimated other offering expenses) will be $18,175,000. We will use the net proceeds to pay development and commercialization costs for existing innovations; acquisition, development and commercialization costs for new innovations; operating expenses (including salaries; legal, accounting and consulting fees; subsidiary formation costs, program development, rent; marketing programs; and other general administrative expenses); and working capital. See “Use of Proceeds.” |
|
|
|
|
|
Proposed listings: |
|
Currently, there is no public market for our Units, common stock or warrants. We have applied to list our common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “MYIE”, and to list our warrants on NASDAQ under the symbol “MYIEW”, on or promptly after the closing date of this offering. We cannot guarantee that our securities will be approved for listing on NASDAQ. NASDAQ’s listing standards require, among other things, that we have stockholders’ equity of $5 million. As of March 31, 2015, we had total stockholders’ equity of $2.0 million (on a pro forma basis, taking into account the forced conversion, immediately after the closing of this offering, of our Private Placement Convertible Notes). This means that, to have closed this offering and listed on NASDAQ as of that date, we would have had to raise approximately $3 million. In this offering, if we are unable to raise the amount necessary to meet NASDAQ’s stockholders’ equity requirement, or if we otherwise fail to meet NASDAQ’s listing standards, our securities will not be listed on NASDAQ. In that event, we may in our sole discretion cancel some or all of this offering and thereafter promptly return any escrow funds you may already have paid. |
|
|
|
|
|
CUSIP: |
|
The CUSIP for our common stock is 457696 102 and the CUSIP for our warrants is 457696 110. |
|
|
|
|
|
Emerging growth company: |
|
We are an “emerging growth company” under applicable law and rules and we may elect to comply with certain reduced public company reporting requirements following this offering. |
|
|
|
|
7
|
Risk factors: |
|
Investing in our securities involves a high degree of risk. Our Company is at an early stage of its development and our securities may only be appropriate for long-term investment. Our independent registered public accounting firm has issued an audit opinion that includes a statement expressing substantial doubt as to our ability to continue as a going concern. You should purchase our securities only if you can afford to lose your entire investment. See “Risk Factors”. |
____________
(1) Does not reflect the exercise of (i) outstanding options to purchase an aggregate of 2,645,967 shares of our common stock at a weighted average exercise price of $1.98 per share, issued pursuant to our Amended and Restated Long-Term Incentive Compensation Plan, (ii) outstanding warrants to purchase an aggregate of 240,333 shares of our common stock, at a weighted average exercise price of $2.29 per share, (iii) the warrants that will be issued in this offering as part of the Units, (iv) the warrants that will be issued to our selling agent in connection with this offering, (v) the warrants underlying the Units issuable upon the forced conversion of the Private Placement Convertible Notes immediately after the closing of this offering and (vi) the warrants issued to our placement agent in connection with the 2014 Private Placement (as defined below).
(2) Includes 994,652 shares of common stock underlying the Units issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest immediately after the closing of this offering and units issued to the selling agent, as discussed below. The Private Placement Convertible Notes were issued by us pursuant to two private placements, one of which had its final closing on October 14, 2014 (the “2014 Private Placement”) and the other of which had closed on $2,100,000 of Note purchases as of April 30, 2015 (the “2015 Private Placement” and collectively with the 2014 Private Placement, the “Private Placements”).
(3) Assumes that all of the securities offered are sold. If all of the securities offered are sold, the selling agent’s commissions will be $1,200,000. We estimate that our total expenses for this offering, excluding selling agent’s commissions, will be approximately $625,000.
8
Summary Consolidated Financial Data
These summary consolidated financial data should be read together with our consolidated financial statements and the accompanying notes thereto, and the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations”. The summary consolidated financial data as of and for the years ended December 31, 2014 and 2013 have been derived from our annual consolidated financial statements appearing elsewhere in this prospectus. The summary consolidated financial data as of March 31, 2015 and for the three months periods ended March 31, 2015 and 2014 have been derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus, and include all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for a fair presentation of our financial position as of such date and our results of operations for such periods.
Summary Consolidated Statement of Operations Data
|
|
For the years ended December 31, |
|
For
the three months |
||||||||||||
|
|
2014 |
|
2013 |
|
2015 (Unaudited) |
|
2014 (Unaudited) |
||||||||
Revenue |
|
$ |
— |
|
|
$ |
109,688 |
|
|
$ |
— |
|
|
$ |
— |
|
Gross profit |
|
$ |
— |
|
|
$ |
11,684 |
|
|
$ |
— |
|
|
$ |
— |
|
Total operating expenses |
|
$ |
4,895,129 |
|
|
$ |
3,651,088 |
|
|
$ |
1,590,317 |
|
|
$ |
1,001,889 |
|
Net loss |
|
$ |
(4,939,395 |
) |
|
$ |
(3,411,383 |
) |
|
$ |
(1,715,983 |
) |
|
$ |
(1,000,243 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share |
|
$ |
(0.49 |
) |
|
$ |
(0.37 |
) |
|
$ |
(0.16 |
) |
|
$ |
(0.10 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding-basic and diluted |
|
|
9,261,231 |
|
|
|
8,344,836 |
|
|
|
9,444,828 |
|
|
|
8,881,578 |
|
Summary Consolidated Balance Sheet Data
|
|
As of December31, |
|
As of March 31, |
||||||||
|
|
2014 |
|
2013 |
|
2015 (Unaudited) |
||||||
Cash |
|
$ |
1,881,776 |
|
|
$ |
722,736 |
|
|
$ |
544,699 |
|
Current assets |
|
$ |
1,905,916 |
|
|
$ |
744,026 |
|
|
$ |
574,734 |
|
Total assets |
|
$ |
2,428,504 |
|
|
$ |
938,490 |
|
|
$ |
1,213,472 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
$ |
1,182,649 |
|
|
$ |
1,226,172 |
|
|
$ |
1,465,418 |
|
Total liabilities |
|
$ |
4,005,264 |
|
|
$ |
1,226,172 |
|
|
$ |
4,394,772 |
|
Total Stockholders’ equity (deficiency) |
|
$ |
(1,576,760 |
) |
|
$ |
(287,682 |
) |
|
$ |
(3,181,300 |
) |
Summary Consolidated Cash Flow Statement Data
|
|
For the years ended December 31, |
|
For
the three months |
||||||||||||
|
|
2014 |
|
2013 |
|
2015 (Unaudited) |
|
2014 (Unaudited) |
||||||||
Net cash used in operations |
|
$ |
(3,973,743 |
) |
|
$ |
(1,413,006 |
) |
|
$ |
(1,228,667 |
) |
|
$ |
(715,310 |
) |
Net cash used in investing activities |
|
$ |
(25,613 |
) |
|
$ |
(3,995 |
) |
|
$ |
(30,412 |
) |
|
$ |
(5,591 |
) |
Net cash provided by financing activities |
|
$ |
5,158,396 |
|
|
$ |
1,754,155 |
|
|
$ |
(77,998 |
) |
|
$ |
1,503,250 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash at end of period |
|
$ |
1,881,776 |
|
|
$ |
722,736 |
|
|
$ |
544,699 |
|
|
$ |
1,505,085 |
|
9
RISK FACTORS
Investing in our common stock is highly speculative and involves a significant degree of risk. You should carefully consider the following risk factors and other information in this prospectus before making a decision to invest in our common stock. Additional risks and uncertainties that we are unaware of may become important factors that affect us. If any of the following events occur, our business, financial condition and operating results may be materially and adversely affected. In that event, the trading price of our common stock may decline, and you could lose all or part of your investment.
Risks Related to our Company and our Business
We are an emerging growth company and are subject to the many risks associated with new businesses.
We are an emerging growth company with no history of commercializing our product candidates, and our principal assets consist of cash raised in private placements and the intellectual property licensed to us and developed by us. We have only a short operating history by which you can assess our Company and our prospects. We are, and expect for the foreseeable future to be, subject to all the risks and uncertainties inherent in a new business and the development and sale of new products. As a result, we still must establish many functions necessary to operate a business, including developing marketable products, meeting applicable regulatory requirements, commencing marketing activities, achieving sales revenue and scaling up our operating and financial systems and controls.
Accordingly, you should consider our prospects in light of the costs, uncertainties, delays and difficulties frequently encountered by companies in their pre-revenue-generating stages, particularly those in the life sciences industry. Potential investors should carefully consider the risks and uncertainties that a new company with a limited operating history will face. In particular, potential investors should consider that there is a significant risk that we will not be able to:
• implement a sound business plan;
• raise sufficient funds to effectuate our business plan;
• maintain our management team;
• increase the scale and effectiveness of our management systems and operations;
• determine that the processes we are employing and the products we are developing are commercially viable; and
• attract, enter into contracts with, makes sales to and retain market partners, distributors and customers.
If we cannot execute any one of the foregoing, our business may fail, in which case you could lose the entire amount of your investment in our Company.
Our independent registered public accounting firm has issued an audit opinion that includes a statement expressing substantial doubt as to our ability to continue as a going concern.
We are an early stage company, and the development and commercialization of our products is uncertain and expected to require substantial expenditures. We have not yet generated any recurring revenues from our operations to fund our activities, and are therefore dependent upon external sources for financing our operations. There is a risk that we will be unable to obtain necessary financing to continue our operations on terms acceptable to us or at all. As a result, our independent registered public accounting firm has expressed in its auditors’ report on our financial statements included as part of this prospectus a substantial doubt regarding our ability to continue as a going concern. Our financial statements do not include any adjustments that might result from a negative outcome of the uncertainty regarding our ability to continue as a going concern. If we cannot continue as a going concern, our stockholders may lose their entire investment in our Company.
We have not generated substantial revenue and may not generate revenue in the manner or within the timeframe we anticipate. We do not have positive operating cash flow. We expect to incur losses for the foreseeable future.
We have not generated substantial revenue to date, since our product candidates are not yet commercialized. We have incurred operating losses since our inception in 2010, and we expect to continue to incur operating losses for the foreseeable future. At March 31, 2015, December 31, 2014 and December 31, 2013, we had accumulated deficits of $14,839,743, $13,282,055 and $8,749,750, respectively. For the three months ended March 31, 2015 and the
10
years ended December 31, 2014 and 2013, we had net losses attributable to common shareholders of $1,557,688, $4,532,305 and $3,059,352, respectively. As a result, we will need to generate significant revenues to achieve and maintain profitability. If our revenues grow more slowly than anticipated, or if our operating expenses exceed our expectations, then we may not be able to achieve profitability in the near future or at all.
Because of the various risks and uncertainties associated with developing, obtaining regulatory approvals for and marketing new products, we are unable to predict with any certainty the extent of any future revenues, cash flows, profits or losses or when we will generate positive operating cash flow or become profitable, if at all. We expect to derive future revenues principally from the sale and licensing of our products, but we cannot guarantee the magnitude of any sales or licensing revenues. We expect to continue to require substantial resources to expand our development activities, introduce manufacturing capabilities and sales and marketing activities and take other actions necessary for the future commercialization of our product candidates. We expect that we will continue to incur significant and increasing operating losses for the foreseeable future, and we may never be profitable. Continuing losses will, among other things, have an adverse effect on our stockholders’ equity and working capital. Failure to generate revenue or achieve profitability would materially adversely affect the value of our Company and our ability to grow our business.
We will need to raise additional investment capital, which may be unavailable to us or, if raised, may cause dilution of our existing investors and place significant restrictions on our ability to operate.
As of March 31, 2015, we had cash and cash equivalents of $544,699. According to our management’s estimates, based on these funds and our current budget, and assuming we engage in no other fundraising, we believe that we have sufficient resources to continue our activity at least until August 2015.
Since we expect not to generate sufficient, if any, revenue or cash flow to fund our operations for the foreseeable future, we will likely need to seek additional equity or debt financing to provide the capital required to maintain or expand our operations. We may also need additional funding for developing new product candidates, initiating our sales and marketing capabilities, promoting brand identity and acquiring complementary technologies and assets, as well as for working capital requirements and other operating and general corporate purposes. Moreover, the regulatory compliance arising out of being a publicly registered company following the effectiveness of this offering will dramatically increase our operating costs.
We do not currently have any arrangements or credit facilities in place as a source of funds, and there can be no assurance that we will be able to raise sufficient capital in this offering or additional capital on acceptable terms, if at all. If such financing is not available on satisfactory terms, or is not available, we may be required to delay, scale back or eliminate the development of business opportunities and our operations and financial condition may be materially adversely affected.
If we raise additional capital by issuing equity securities, the percentage ownership of our existing stockholders will be reduced, and these stockholders may experience substantial dilution. We may also issue equity securities that provide for rights, preferences and privileges senior to those of our common stock.
Debt financing, if obtained, may involve agreements that include liens on our assets and covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, would increase our expenses and could require that our assets be provided as security for such debt. Debt financing would also be required to be repaid regardless of our operating results.
If we raise additional funds through collaborations and licensing arrangements, we may be required to relinquish some rights to our technologies or product candidates, or to grant licenses on terms that are not favorable to us.
Funding from any source may be unavailable to us on acceptable terms, if at all. If we do not have sufficient capital to fund our operations and expenses, this could lead to the failure of our business and the loss of your investment.
Our independent registered public accountants have identified material weaknesses in our internal control over financial reporting. In addition, because of our status as an emerging growth company, our independent registered public accountants are not required to provide an attestation report as to our internal control over financial reporting for the foreseeable future.
In connection with the audit of our consolidated financial statements for the year ended December 31, 2014, our independent registered public accountants identified material weaknesses in our internal control over financial
11
reporting. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses relate to our having only one employee assigned to positions that involve processing financial information, resulting in a lack of segregation of duties so that all journal entries and account reconciliations are not reviewed by someone other than the preparer, heightening the risk of error or fraud. In addition, we have identified a material weakness related to accounting for complex transactions. If we are unable to remediate the material weaknesses, or other control deficiencies are identified, we may not be able to report our financial results accurately, prevent fraud or file our periodic reports as a public company in a timely manner.
As a public company, we will be required to maintain internal control over financial reporting and to report any material weaknesses in such internal control. In addition, beginning with our annual report on Form 10-K for the year ending December 31, 2016, we will be required to annually assess the effectiveness of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act. We are in the process of designing, implementing, and documenting the internal control over financial reporting required to comply with this obligation, which process is time consuming, costly and complicated. Because of our limited resources, we may be unable remediate the identified material weaknesses in a timely manner, or additional control deficiencies may be identified. As a result, we may be unable to report our financial results accurately on a timely basis or help prevent fraud, which could cause our reported financial results to be materially misstated, result in the loss of investor confidence and cause the market price of our securities to decline.
Our independent registered public accounting firm has not assessed the effectiveness of our internal control over financial reporting and will not be required to provide an attestation report on the effectiveness of our internal control over financial reporting so long as we qualify as an emerging growth company or until we are no longer a non-accelerated filer, as defined in Rule 12b-2 under the Securities Exchange Act of 1934 (the “Exchange Act”), whichever is later, which may increase the risk that weaknesses or deficiencies in our internal control over financial reporting go undetected. We expect to be an “emerging growth company” for up to five years. Accordingly, you will not be able to depend on any attestation concerning our internal control over financial reporting from our independent registered public accountants for the foreseeable future.
There may be limitations on the effectiveness of our internal controls, and a failure of our control systems to prevent error or fraud may materially harm our Company.
We do not expect that internal control over financial accounting and disclosure, even if timely and well established, will prevent all error and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. Failure of our control systems to prevent error or fraud could materially adversely affect our business.
Successful testing of our innovations in the laboratory may not be indicative of future results and may not result in commercially viable products, services or technologies. Further, our products, services or technologies may have to be modified from their originally conceived versions in order to reach or be successful in the market.
Our business model includes acquiring innovations backed by laboratory-proven results. Positive results from laboratory testing, however, may not be predictive of future successful development, commercialization and sales results and should not be relied upon as evidence that products developed from our innovations will become commercially viable and successful. Further, the products we are developing may have to be significantly modified from their originally conceived versions in order for us to control costs, compete with similar products, receive market acceptance, meet specific development and commercialization timeframes, avoid potential infringement of the proprietary rights of others, or otherwise succeed in developing our business and earning ongoing revenues. What appear to be promising innovations when we acquire them may not lead to viable products, service or technologies or to commercial success.
Our products are still being developed for commercialization, and our business may fail if we are not able to successfully generate significant revenues from these products.
Successful development of our product candidates will require significant additional investment, including costs associated with additional development, completing field trials and obtaining regulatory approval, as well as the
12
ability to manufacture or have others manufacture our products in sufficient quantities at acceptable costs while also preserving product quality. Difficulties often encountered in scaling up production include problems involving production yields, quality control and assurance, shortage of qualified personnel, production costs and process controls. In addition, we are subject to inherent risks associated with new products, services and technologies. These risks include the possibility that any product, service or technology candidate may:
• be found unsafe;
• be ineffective or less effective than anticipated;
• fail to receive necessary regulatory approvals;
• be difficult to competitively price relative to alternative solutions;
• be harmful to consumers or the environment;
• be difficult or impossible to manufacture on an economically viable scale;
• be subject to supply chain constraints for raw materials;
• fail to be developed and accepted by the market prior to the successful marketing of alternative products by competitors;
• be difficult or impossible to market because of infringement on the proprietary rights of third parties; or
• be too expensive for commercial use.
Furthermore, we may be faced with lengthy market partner or distributor evaluation and approval processes. Consequently, we may incur substantial expenses and devote significant management effort in order to customize products for market partner or distributor acceptance. As a result, we cannot accurately predict the volume or timing of any future sales.
We focus our efforts on the commercialization of potential products that not only present revenue and profit opportunities, but also promise to do good. However, our goal of doing good may at times undercut our business performance, and we will be our own judge of what it means for a product, service or technology to “do good”.
Our business focuses on commercializing innovations that provide revenue and profit opportunities, and that also meet our self-imposed mandate that the potential product, product platform, service or technology “do good”, by which we mean “have a beneficial impact on people’s lives, their communities and our planet”. We will be our own judge of how innovations meet or do not meet this self-imposed mandate, and our own judgments as to whether a particular product, product platform, service or technology will or will not do good may not match with, and may even be at odds with, the judgments that others, including our investors, might reach. Potential investors in our securities are cautioned that our business may take longer to generate, or fail to generate, or be less successful in generating, revenues or profits because some or all of our decisions as to which potential products, product platforms, services and technologies to favor will be based on our judgments as to whether they will “do good”, and potential investors are further cautioned that they may not always agree with, and may even be at odds with, some or all of our decisions as to which may indeed “do good”.
We may not be successful in implementing our “Collaborative Economy” business model, which could negatively affect our future achievements or results.
The Company does business under the name “ieCrowd” in order to emphasize its Collaborative Economy approach to implementing its business model. By “Collaborative Economy”, we mean a system that emphasizes the sharing, repurposing and distribution of ideas, opportunities and other resources among a great number of participants, typically aided by the use of social media and other information technology, in order to increase the value and efficient deployment of those resources not only for the participants but also for larger populations, societies and the world generally. We refer to the individuals and entities we seek to collaborate with as the “Crowd”. To the extent we are unable to significantly develop a “Crowd” of such individuals and entities, we may not be able to implement our business model in the way we envision. This could negatively affect our ability to “do good”, and could negatively affect our future operations, results or financial condition.
13
We may not be successful in accomplishing our goal of “doing good” and we may choose not to follow our self-imposed mandate to “do good” in the future.
We have a self-imposed mandate that our potential products, product platforms, services or technologies “do good”, by which we mean “have a beneficial impact on people’s lives, their communities and our planet”. However, we may not be successful in “doing good” or be as successful as we or our investors might wish. Additionally, we may choose not to follow this mandate in the future if we decide not to in order to accomplish competing financial or other objectives. As a result, it is possible that our potential products, product platforms, services or technologies will not do good, or not do as much good as investors or others may hope. Potential investors are cautioned that we may fail to accomplish our goal of “doing good”, and potential investors are further cautioned that they may not always agree with, and may even be at odds with, some or all of our decisions as to whether to continue to pursue the mandate to “do good”.
If we are unable to establish successful relations with third-party market partners or distributors, or these market partners or distributors do not focus adequate resources on selling our products or are otherwise unsuccessful in selling them, sales of our products may not develop.
We anticipate relying on independent market partners and distributors to distribute and assist us with the marketing and sale of our products. Our future revenue generation and growth will depend in large part on our success in establishing and maintaining this sales and distribution channel. If our market partners and distributors are unable to sell our products, or receive negative feedback from end users, they may not continue to purchase or market our products. In addition, there can be no assurance that our market partners and distributors will focus adequate resources on selling our products to end users or will be successful in selling them. Many of our potential market partners and distributors are in the business of distributing and sometimes manufacturing other, possibly competing, products. As a result, these market partners and distributors may perceive our products as a threat to various product lines currently being distributed or manufactured by them. In addition, these market partners and distributors may earn higher margins by selling competing products or combinations of competing products. If we are unable to establish successful relationships with independent market partners and distributors, we will need to further develop our own sales and distribution capabilities, which would be expensive and time-consuming and might not be successful.
Our ownership interest in each of our operating subsidiaries is less than 100%, ownership interests in our current and to-be-formed subsidiaries may fluctuate and shares in our subsidiaries may be sold to third parties at the discretion of management.
We develop discrete innovations through different operating subsidiaries of the Company and will continue to form new subsidiaries to develop additional innovations in the future. In the process of developing particular innovations, we have in the past sought to incentivize employees, pay licensors and otherwise deal with certain third parties by providing them with shares in our subsidiaries. In addition, when developing our first such subsidiary, OLI, we sold shares in that subsidiary to outside investors. It is our intention to reduce our past practice of, and to the extent possible, cease, providing shares in subsidiaries to third parties. Any issuance or transfer of shares of a subsidiary to third parties dilutes our percentage ownership in that subsidiary and our proportionate right to any dividends paid by that subsidiary or any net gain on the occurrence of any liquidation event; in addition, if our percentage ownership drops to too low a level, we make have difficulty controlling the operations of the subsidiary or controlling it in circumstances where law or operative agreements require a super-majority vote of stockholders in order to exercise control. In addition, in circumstances where we control a subsidiary, to the extent there are minority investors in the subsidiary we owe them various duties of fair dealing under applicable state laws, and they may be able to take the subsidiary to court or seek to be bought out of their investments at prices favorable to them if they are sufficiently dissatisfied with our control of the subsidiary. If we are unable to adequately manage the capital structure of our subsidiaries, our operations and results may be substantially affected. Nevertheless, we may continue to issue or transfer shares in our subsidiaries to third parties if and when we determine that the benefits to our Company as a whole from continuing to do so outweigh the considerations against further reducing our ownership in our subsidiaries and permitting third parties to hold ownership stakes in our subsidiaries.
We may experience conflicts of interest with our operating subsidiaries with respect to business opportunities and other matters.
A significant portion of our operations occur through our operating subsidiaries OLI, NEA and SOS. We own 70.4% of the outstanding shares of common stock of OLI, 83.5% of the outstanding shares of common stock of NEA and 95.0% of the outstanding shares of common stock of SOS. Although we control these subsidiaries, they are not wholly owned subsidiaries of ours, and conflicts of interest may arise with respect to transactions involving business dealings between us and these subsidiaries, or other transactions in which our best interests and the best interests of
14
our stockholders may conflict with the best interests of the stockholders of these subsidiaries. Because a portion of each of these subsidiaries is owned by minority stockholders, we cannot treat these entities as we would a wholly owned subsidiary. With respect to our management services agreements and any other agreements with our operating subsidiaries, we cannot assure you that we will negotiate terms that are as favorable to us as if such transactions were with an unaffiliated third party or a wholly owned subsidiary, or that any conflicts of interest will be resolved in our favor. Further, the fiduciary duties of the members of our operating subsidiaries’ boards of directors may result in actions that are not in our stockholders’ best interests. Because of their importance to our business, we may elect to fund our operating subsidiaries’ operations in circumstances in which we undertake substantial risks but which will benefit all of those subsidiaries’ stockholders, and from which we may only receive economic benefits in proportion to our ownership interest in such entities. To the extent we form additional operating subsidiaries in the future and do not retain 100% ownership of them, the risks described above will apply to these subsidiaries and our relationships with them, also.
Customers may not adopt our products quickly, or at all.
Customers in the health- and life science-related sectors are generally cautious in their adoption of new products and technologies. In addition, given the relative novelty of our product candidates, consumers of those products will require education regarding their utility and use, which may delay their adoption. There can be no assurance that customers will adopt our products quickly, or at all.
If our product candidates fail to satisfy customer requirements, we may be required to make significant expenditures to redesign such products, and we may have insufficient resources to do so.
There is a risk that our product candidates will not meet anticipated customer requirements or desires. If we are required to redesign our products to address customer demands or otherwise modify our business model, we may incur significant unanticipated expenses and losses, and we may be left with insufficient resources to engage in such activities. If we are unable to redesign our products, develop new products or modify our business model to meet customer desires or any other customer requirements that may emerge, our operating results would be materially adversely affected and our business might fail.
The high level of competition in the markets for many of our product candidates may result in pricing pressure, reduced margins or the inability of our product candidates to achieve market acceptance.
The markets for many of our product candidates are intensely competitive and rapidly changing. We may be unable to compete successfully, which may result in price reductions, reduced margins and the inability to achieve market acceptance for our products.
Our competitors include major multinational companies and specialized businesses. Many of these organizations have longer operating histories, significantly greater resources, greater brand recognition than we do and large customer bases. As a result, they may be able to devote greater resources to the manufacture, promotion or sale of their products, receive greater resources and support from market partners and independent distributors, initiate or withstand substantial price competition or more readily take advantage of acquisition or other opportunities. Further, many of the large companies have a more diversified product offering than we do, which may give these companies an advantage in meeting customers’ needs by enabling them to offer a broader range of related products.
If our ongoing or future field trials are unsuccessful, we may be unable to obtain regulatory approval of, or commercialize, our product candidates on a timely basis or at all.
The successful completion of multiple field trials in domestic and foreign locations in various settings is likely to be critical to the success of many of our products. If our ongoing or future field trials are unsuccessful or produce inconsistent results or unanticipated adverse side effects, or if we are unable to collect reliable data, regulatory approval of our products could be delayed or we may be unable to commercialize our products. Moreover, the results of our ongoing and future field trials are subject to a number of conditions beyond our control, including weather-related events such as drought or floods, severe heat or frost, hail, tornadoes and hurricanes. Generally, we engage third parties such as consultants, universities or other collaboration partners to conduct field tests on our behalf. Incompatible practices or misapplication of our products by these third parties could impair the success of our field trials.
15
If we are unable to complete required clinical trials, or we experience significant delays in completing such clinical trials, we could experience significant delays in our product launches and impair our viability and business plan.
The completion of any clinical trials that we may be required to undertake could be delayed, suspended or terminated for several reasons, including:
• our failure or inability to conduct the clinical trial in accordance with regulatory requirements;
• sites participating in the trial may drop out of the trial, which may require us to engage new sites for an expansion of the number of sites that are permitted to be involved in the trial;
• patients may not enroll in, remain in or complete, the clinical trial at the rates we expect; and
• clinical investigators may not perform our clinical trial on our anticipated schedule or consistent with the clinical trial protocol and good clinical practices.
If our clinical trials are delayed it will take us longer to ultimately commercialize our products and generate revenues. Moreover, our development costs will increase if we have material delays in our clinical trials or if we need to perform more or larger clinical trials than planned.
If we are unable to identify new product candidates through our Discovery process, we may not achieve or maintain profitability.
Our future success will depend in part on our ability to identify and commercialize additional product candidates. A failure by us to continue identifying viable product candidates could make it difficult to grow our business. In addition, licensing of products requires the identification of new innovations or new applications for existing innovations and the willingness of the licensor. If we are unable to identify or license additional product candidates, our results may not improve over time and our business could suffer.
Our results of operations will be affected by the level of royalty payments that we are required to pay to third parties.
We are a party to license agreements that require us to remit royalty payments related to licensed innovations and meet certain performance milestones. Any failure on our part to pay royalties owed or meet milestones could lead to us losing rights under our licenses and could thereby adversely affect our business. As our product sales increase, we may, from time-to-time, disagree with our third-party collaborators as to the appropriate royalties owed and the resolution of such disputes may be costly and may consume management’s time. Furthermore, we may enter into additional license agreements in the future, which may also include royalty payments.
We will need to expand our sales and marketing infrastructure, including by attracting and retaining additional talented personnel.
We will need to further develop our sales and marketing capabilities in order to successfully commercialize the product candidates we are developing, which may involve substantial costs. There can be no assurance that members of our sales and marketing team will successfully compete against the sales and marketing teams of competitors, many of which may have more established relationships with market partners, distributors and consumers. Our inability to recruit, train and retain sales and marketing personnel or their inability to effectively market and sell the products we are developing could impair our ability to gain market acceptance of our products and cause our sales to suffer.
We will need to expand our management, operational and financial systems and financial and other controls, including by attracting and retaining additional talented personnel. We may have difficulties managing our growth, which could lead to our inability to implement our business plan.
Any growth will require us to expand our management, operational and financial systems and financial and other controls, including by attracting and retaining additional talented personnel. If we are unable to do so successfully, our business and financial condition could be materially harmed. If rapid growth occurs, it may strain our operational, managerial and financial resources.
16
If we fail to maintain and successfully manage our existing strategic collaborations and other relationships, or enter into new strategic collaborations and other relationships, we may not be able to expand commercial development and sales of many of our products.
Our ability to enter into, maintain and manage collaborations and other relationships is fundamental to the success of our business. We currently have entered into various license, distribution and other agreements. We intend to enter into other strategic agreements to produce, market and sell the products we develop. Any failure to enter into new strategic arrangements on favorable terms or to maintain or manage our existing strategic arrangements, in particular with the University of California, could delay or hinder our ability to commercialize our products and could adversely affect our results, financial condition and prospects.
The use of our products may be limited by regulations, and we may be exposed to product liability and remediation claims.
The use of certain health- and life science-related products is regulated by various local, state, federal and foreign environmental and public health agencies. These regulations may include requirements that only certified or professional users may apply the product, that certain products may only be used in certain circumstances or in certain locations, that users must post notices on properties to which products have been or will be applied, that users must notify individuals in the vicinity that products will be applied in the future or that certain substances may not be used at all. Even if we are able to comply with all such regulations and obtain all necessary registrations, we cannot provide assurance that our products will not cause injury to the environment or people under all circumstances. For example, our products may be improperly combined with other chemicals or, even when properly combined, our products may be blamed for damage caused by those other chemicals. The costs of remediation or products liability could materially adversely affect our results, financial condition and operations.
We may be held liable for, or incur costs to settle, liability and remediation claims if any products we develop, or any products that use or incorporate any of our technologies, cause injury or are found unsuitable during product testing, manufacturing, marketing, sale or use. These risks exist even with respect to products that have received, or may in the future receive, regulatory approval, registration or clearance for commercial use. We cannot guarantee that we will be able to avoid product liability exposure.
We maintain product liability insurance at levels we believe are sufficient and consistent with industry standards for companies at our stage of development. However, we cannot guarantee that our product liability insurance will be sufficient to help us avoid product liability-related losses. In the future, it is possible that meaningful insurance coverage may not be available on commercially reasonable terms or at all. In addition, a product liability claim could result in liability to us greater than our assets or insurance coverage. Moreover, even if we have adequate insurance coverage, product liability claims or recalls could result in negative publicity or force us to devote significant time and attention to these matters, which could harm our business.
Our inability to obtain regulatory approvals, or to comply with ongoing and changing regulatory requirements, could delay or prevent sales of the products we are developing.
The field testing, manufacture, sale and use of some of the product candidates we are developing are extensively regulated by the U.S. Environmental Protection Agency, (the “EPA”) and the U.S. Food & Drug Administration (the “FDA”) and other authorities, including at the state, local and foreign governmental levels. These regulations substantially increase the time and cost associated with bringing our product candidates to market. If we do not receive the necessary governmental approvals to test, manufacture and market our products, or if regulatory authorities revoke our approvals, do not grant approvals in a timely manner or grant approvals subject to restrictions on their use, we may be unable to sell our products in the United States or other jurisdictions, which could adversely affect our results and operations. As we introduce new formulations of, and applications for, our products, we will need to seek new regulatory approvals prior to commercial sales.
There can be no assurance that we will be able to obtain regulatory approval for marketing our current product candidates or new product formulations and applications we may develop. Because many of the products that may be sold by us must be registered with one or more government agencies, the registration process can be time consuming and expensive, and there is no guarantee that the product will obtain all needed registrations.
Even if we obtain all necessary regulatory approvals to market and sell our products, they will be subject to continuing review and extensive regulatory requirements, including periodic re-registrations. Regulatory authorities
17
could withdraw a previously approved product from the market upon receipt of newly discovered information, including an inability to comply with regulatory requirements or the occurrence of unanticipated problems with our products, or for other reasons.
The regulatory approval process is expensive, time-consuming and uncertain and may prevent us from obtaining approvals for the commercialization of our future product candidates, if any.
The testing, manufacturing, labeling, approval, selling, marketing and distribution of health- and life science-related products are subject to extensive regulation, which regulations differ from country to country.
In the case of those of our product candidates that qualify as medical devices, we will not be permitted to market them in the United States until we receive a clearance letter under the 510(k) premarket notification process, or approval of a Section 515 premarket approval (or PMA) from the FDA, depending on the nature of the device. We have not submitted an application or premarket notification for or received marketing clearance or approval for any of our product candidates. Obtaining approval of any premarket approval can be a lengthy, expensive and uncertain process. While the FDA normally reviews and clears a premarket notification in three months, there is no guarantee that our products will qualify for this more expeditious regulatory process, which is reserved for Class I and II devices, nor is there any assurance that, even if a device is reviewed under the 510(k) premarket notification process, the FDA will review it expeditiously or determine that the device is substantially equivalent to a lawfully marketed non-premarket approval device. If the FDA fails to make this finding, then we cannot market the device. In lieu of acting on a premarket notification, the FDA may seek additional information or additional data, which would further delay our ability to market the product. In addition, if we modify our FDA approved medical devices, we may need to seek additional clearance or approvals, which, if not granted, would prevent us from selling our modified products. Furthermore, failure to comply with FDA, non-U.S. regulatory authorities or other applicable U.S. and non-U.S. regulatory requirements may, either before or after product clearance or approval, if any, subject us to administrative or judicially imposed sanctions, including:
• restrictions on the products, manufacturers or manufacturing process;
• adverse inspectional observations (Form 483), warning letters or non-warning letters incorporating inspectional observations;
• civil and criminal penalties;
• injunctions;
• suspension or withdrawal of regulatory clearances or approvals;
• product seizures, detentions or import bans;
• voluntary or mandatory product recalls and publicity requirements;
• total or partial suspension of production;
• imposition of restrictions on operations, including costly new manufacturing requirements; and
• refusal to clear or approve pending applications or premarket notifications.
The FDA can delay, limit or deny clearance or approval of a medical device candidate for many reasons, including:
• a medical device candidate may not be deemed safe or effective, in the case of a premarket approval application;
• a medical device candidate may not be deemed to be substantially equivalent to a lawfully marketed non‑premarket approval device in the case of a 510(k) premarket notification;
• FDA officials may not find the data from pre-clinical studies and clinical trials sufficient;
• the FDA might not approve our third-party manufacturer’s processes or facilities; or
• the FDA may change its clearance or approval policies or adopt new regulations.
18
We may need to rely on third parties for the production of our products. If these parties do not produce our products at a satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, our development and commercialization efforts could be delayed or otherwise negatively affected.
We do not currently manufacture products, and we might not manufacture products in the future. As such, we may need to rely on third parties for the manufacture of our products. Our reliance on third parties to manufacture our products may present significant risks to us, including the following:
• reduced control over delivery schedules, yields and product reliability;
• price increases;
• manufacturing deviations from internal and regulatory specifications;
• the failure of a key manufacturer to perform as we require for technical, market or other reasons;
• difficulties in establishing additional manufacturer relationships if we are presented with the need to transfer our manufacturing process technologies to them;
• misappropriation of our intellectual property; and
• other risks in potentially meeting our product commercialization schedule or satisfying the requirements of our market partners, distributors, direct customers and end users.
If we need to enter into agreements for the manufacturing of our products, there can be no assurance we will be able to do so on favorable terms, if at all.
Dependence on others for the manufacture of our products may adversely affect our ability to commercialize products on a timely and competitive basis. If manufacturing capacity is reduced or eliminated at one or more of our manufacturers’ facilities, we could have difficulties fulfilling customer orders, and our results of operations could be adversely affected.
We will need to accurately forecast demand for our products to obtain adequate and cost-effective capacity from third-party manufacturers and to purchase materials used in our products at cost-effective rates. If we inaccurately forecast demand for our products, we may be unable to meet our customers’ delivery requirements, we may cause difficulties for our manufacturers’ in meeting targets and we may accumulate excess inventories of products and materials.
Our future performance will depend on the continued engagement of key members of our management team.
Our future performance depends to a large extent on the continued services of members of our current management and other key personnel, including, among others, Mr. Amro Albanna. In the event that we lose the continued services of such key personnel for any reason, this could have a material adverse effect on our business and prospects.
If we are not able to attract and retain highly skilled managerial, marketing, scientific and technical personnel, we may not be able to implement our business model successfully.
We believe that our management team must be able to act decisively to apply and adapt our business model in the rapidly changing markets in which we will compete. In addition, we will rely upon managerial, marketing, technical and scientific employees or third party contractors to effectively establish, manage and grow our business. Consequently, we believe that our future viability will depend largely on our ability to attract and retain highly skilled managerial, marketing, scientific and technical personnel. In order to do so, we may need to pay higher compensation or fees to our employees or consultants than we currently expect and such higher compensation payments would have a negative effect on our operating results. Competition for experienced, high-quality personnel is intense and we cannot assure that we will be able to recruit and retain such personnel. We may not be able to hire or retain the necessary personnel to implement our business strategy. Our failure to hire and retain such personnel could impair our ability to develop new products and manage our business effectively.
We use hazardous materials in our business and are subject to potential liability under environmental laws. Any claims relating to improper handling, storage or disposal of hazardous materials could be time consuming and costly to resolve.
We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling, disposal and release of hazardous materials and certain waste products. Our development activities and any future manufacturing that we undertake ourselves will involve the controlled use and disposal of such substances. In
19
the event of an accident, or if any hazardous materials are found within our operations or on the premises of our manufacturing facility in violation of the law at any time, we may be liable for cleanup costs, fines, penalties and other amounts. This liability could exceed our resources and, if significant losses arise from hazardous substance contamination, our financial viability could be substantially and adversely affected.
In addition, we may have to incur significant costs to comply with future environmental laws and regulations. We cannot predict the impact of new governmental regulations that might have an adverse effect on the development, production and marketing of our products. We may be required to incur significant costs to comply with future laws or regulations, and our business could be harmed by such costs.
Our collaborators may use hazardous materials in connection with our collaborative efforts. In the event of a lawsuit or investigation, we could be held responsible for any injury caused to persons or property by exposure to, or release of, hazardous materials used by these parties. Further, we may be required to indemnify our collaborators against damages and other liabilities arising out of our development activities or products produced in connection with these collaborations.
Supply problems could harm our business.
We anticipate that in the future we may commit to purchase component parts and substances from suppliers based on sales forecasts of our products. If we cannot change or be released from these purchase commitments, and if orders for our products do not materialize, we could incur significant costs related to the purchase of excess parts and substances. Additionally, a delay in production of the parts or substances or inaccuracies in our sales forecasts could materially adversely affect our results or reputation if we are unable to timely ship ordered products or provide replacements under warranty or maintenance contracts.
Sales of some of our products are expected to be seasonal and subject to weather conditions and other factors beyond our control, which may cause our operating results to fluctuate significantly quarterly and annually.
Sales of some of our products are expected to be seasonal. In addition, weather conditions may affect decisions by our market partners, distributors, direct customers and end users as to how much of our products to purchase and when to use them.
The level of seasonality that may affect our business, once we have products to sell, is difficult to evaluate as a result of our relatively early stage of business. It is possible that our business may be more seasonal, or experience seasonality in different periods, than anticipated. Other factors may also contribute to the unpredictability of our operating results, including the size and timing of significant market partner and distributor transactions, the delay or deferral of use of our products and the fiscal or quarterly budget cycles of our market partners, distributors, direct customers and end users. Customers may purchase large quantities of our products in a particular quarter to store and use over long periods of time or time their purchases to manage their inventories, which may cause significant fluctuations in our operating results for a particular quarter or year, which could result in uncertainty surrounding our level of earnings and possibly a decrease in our stock price.
We expect to derive a portion of our revenues from markets outside the United States, which will subject us to additional business risks.
Our success depends in part on our ability to expand internationally, including by obtaining, directly or indirectly, regulatory approvals to market and sell our products in foreign countries. International expansion of our operations could impose substantial burdens on our resources, divert management’s attention from domestic operations and otherwise harm our business. Furthermore, international operations are subject to several inherent risks, including different regulatory requirements and, depending on the country, reduced protection of intellectual property rights, any of which could adversely affect our ability to compete in international markets and have a negative effect on our operating results. Revenues generated outside the United States could also result in increased difficulty in collecting delinquent or unpaid accounts receivable, adverse tax consequences and losses due to currency fluctuations. It has not been our practice to engage in foreign exchange hedging transactions to manage the risk of fluctuations in foreign exchange rates because of the limited nature of our past international operations. Moreover, it is likely that we will engage market partners in foreign countries to help us secure regulatory approvals in those countries and otherwise navigate the more unfamiliar aspects of operating in those countries. If such market partners fail to provide us with valid regulatory clearances, or otherwise fail to help us manage important local business issues
20
and avoid legal and other pitfalls, our operations in those countries could be damaged or derailed and our business, reputation, results or financial condition could suffer.
In addition, we will be increasingly subject to general geopolitical risks in foreign countries where we operate, such as political and economic instability and changes in diplomatic and trade relationships, which could affect, among other things, customers’ inventory levels and consumer purchasing, which could cause our results to fluctuate and our sales to decline.
We rely on information technology, and if we are unable to protect against service interruptions, data corruption, cyber-based attacks or network security breaches, our operations could be disrupted and our business could be negatively affected.
We rely on information technology networks and systems to process, transmit and store electronic and financial information; to coordinate our business; and to communicate within our Company and with customers, suppliers, partners and other third-parties. These information technology systems may be susceptible to damage, disruptions or shutdowns, hardware or software failures, power outages, computer viruses, cyber-attacks, telecommunication failures, user errors or catastrophic events. If our information technology systems suffer severe damage, disruption or shutdown, and our business continuity plans do not effectively resolve the issues in a timely manner, our operations could be disrupted and our business could be negatively affected. In addition, cyber-attacks could lead to potential unauthorized access and disclosure of confidential information, and data loss and corruption. There is no assurance that we will not experience these service interruptions or cyber-attacks in the future.
Healthcare policy changes, including ongoing efforts to reform the U.S. healthcare system, may have a material adverse effect on us.
Certain health- and life science-related markets have experienced downward pressure on product pricing because U.S. federal and state governments have made various cost containment efforts relating to health care expenditures. Furthermore, the industry faces uncertainty brought by the Patient Protection and Affordable Care Act and other healthcare reform legislation and regulations. The implementation of these laws or the adoption of additional healthcare reform proposals in the future could impose limitations on the prices we will be able to charge for some of our products, or the amounts of reimbursement available for some of our products from governmental agencies or third-party payors. These limitations could have a material adverse effect on our results of operations.
We anticipate that legislation could change access to health care products, increase rebates, reduce prices or the rate of price increases, or cap reimbursement rates for medical devices. We also anticipate that legislation could impose sales or excise tax on the medical device manufacturing sector. Any change on medical device taxation and downward trending reimbursement rates could affect those of our product candidates that are regulated as medical devices, and could affect our results of operations.
We may be subject to federal, state and foreign healthcare fraud and abuse laws and regulations.
We may become subject to certain federal and state regulations, including the federal healthcare programs’ Anti-Kickback Law, the federal Health Insurance Portability and Accountability Act of 1996, and other federal and state false claims laws. The medical device industry has been under heightened scrutiny as the subject of government investigations and enforcement actions involving manufacturers who allegedly offered unlawful inducements to potential or existing customers in an attempt to procure their business, including arrangements with physician consultants. Stringent enforcement of medical device laws could adversely affect our ability to operate our business.
We do not expect to pay dividends in the foreseeable future.
We do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any and all future earnings in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their securities, and holders may be unable to sell their securities on favorable terms or at all. We cannot assure you of a positive return on your investment or that you will not lose the entire amount of your investment.
Our majority stockholders are likely to control our Company for the foreseeable future.
Our executive officers and directors collectively have an approximately 43.8% beneficial ownership of the issued and outstanding shares of our common stock. In addition, two outside investors beneficially own approximately
21
28.2% of the issued and outstanding shares of our common stock. As a result, such individuals will have the ability, acting together, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as a merger or a sale of our Company, a sale of all or substantially all of our assets or amendments to our certificate of incorporation or bylaws. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might be beneficial to our other stockholders but not to our controlling stockholders. Certain of these individuals also have significant control over our business, policies and affairs as officers or directors of our Company.
Upon dissolution of our Company, you may not recoup all or any portion of your investment.
In the event of a liquidation, dissolution or winding-up of our Company, whether voluntary or involuntary, our assets would be used to pay all of our debts and liabilities, and only thereafter would any remaining assets be distributed to our stockholders, on a pro rata basis. There can be no assurance that we will have assets available from which to pay any amounts to our stockholders upon such a liquidation, dissolution or winding-up. In such an event, you would lose all of your investment.
Our continuing compliance with U.S. regulations concerning corporate governance and public disclosure after the completion of this offering will result in additional expenses. Moreover, our ability to comply with all applicable laws, rules and regulations is uncertain given our management’s relative inexperience with operating a U.S. public company.
We will be faced with complicated and evolving disclosure, corporate governance and other compliance laws, regulations and standards relating to corporate governance and public disclosure, including the Sarbanes-Oxley Act of 2002 and the Dodd-Frank Act, enacted in 2010. New or changing laws, regulations and standards are subject to varying interpretations, in many cases due to a lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs due to ongoing revisions to disclosure and governance practices. Our efforts to comply with the evolving laws, regulations and standards governing a U.S. public company are likely to continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities.
Our failure to comply with all the laws, regulations and standards applicable to U.S. public companies could subject us or our management to regulatory scrutiny or sanction, which could harm our reputation and stock price.
As an “emerging growth company” under applicable law, we will be subject to reduced disclosure requirements, which could leave our stockholders without information or rights available to stockholders of more mature companies.
For as long as we remain an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (which we refer to as the JOBS Act), we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies”, including but not limited to:
• not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes‑Oxley Act;
• taking advantage of extensions of time to comply with certain new or revised financial accounting standards;
• being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and
• being exempt from the requirement to hold a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We expect to take advantage of these reporting exemptions until we are no longer an emerging growth company. We will remain an “emerging growth company” for up to five years, although if the market value of our common stock that is held by non-affiliates exceeds $700 million as of any June 30 before that time, we would cease to be an “emerging growth company” as of the following December 31.
22
Because of the lessened regulatory requirements discussed above, our stockholders will be left without information or rights available to stockholders of more mature companies.
Because we have elected to use the extended transition period for complying with new or revised accounting standards for an “emerging growth company”, our financial statements may not be comparable to companies that comply with the usual public company effective dates.
We have elected to use the extended transition period for complying with new or revised accounting standards that is available to us under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. Consequently, our financial statements may not be comparable to companies that comply with the usual public company effective dates. Because our financial statements may not be comparable to companies that comply with such public company effective dates, investors may have difficulty evaluating or comparing our business, performance or prospects in comparison to such public companies, which may have a negative impact on the value and liquidity of our common stock.
Anti-takeover provisions in our charter documents and under Delaware law could discourage, delay or prevent a change in control of our Company and could affect the trading price of our securities.
We are a Delaware corporation and the anti-takeover provisions of the Delaware General Corporation Law may discourage, delay or prevent a change in control by prohibiting us from engaging in a business combination with an interested stockholder for a period of three years after the person becomes an interested stockholder, even if a change in control would be beneficial to our existing stockholders. In addition, our certificate of incorporation and bylaws may discourage, delay or prevent a change in our management or control over us that stockholders may consider favorable. Our certificate of incorporation and bylaws:
• provide that vacancies on our board of directors, including newly created directorships, may be filled only by a majority vote of directors then in office;
• provide that special meetings of stockholders may only be called by the chair of our board of directors, the board of directors or at the request in writing of stockholders of record owning at least 15% of our outstanding common stock; and
• do not provide stockholders with the ability to cumulate their votes.
Risks Related to Our Intellectual Property
The failure to obtain or maintain patents, licensing agreements and other intellectual property could materially impact our ability to compete effectively.
In order for our business to be viable and to compete effectively, we need to develop and maintain, and we will heavily rely on, a proprietary position with respect to our technologies and intellectual property. However, there are significant risks associated with our actual or proposed intellectual property. The risks and uncertainties that we face with respect to our rights principally include the following:
• pending patent applications we have filed or will file may not result in issued patents or may take longer than we expect to result in issued patents;
• we may be subject to interference proceedings;
• we may be subject to reexamination proceedings;
• we may be subject to post grant review proceedings;
• we may be subject to inter partes review proceedings;
• we may be subject to derivation proceedings;
• we may be subject to opposition proceedings in the U.S. or in foreign countries;
• any patents that are issued to us may not provide meaningful protection;
23
• we may not be able to develop additional proprietary technologies that are patentable;
• other companies may challenge patents licensed or issued to us;
• other companies may have independently developed and patented (or may in the future independently develop and patent) similar or alternative technologies, or duplicate our technologies;
• other companies may design around technologies we have licensed or developed;
• enforcement of patents is complex, uncertain and very expensive and we may not be able to secure, enforce and defend our patents; and
• in the event that we were to ever seek to enforce our patents in ligation, there is some risk that they could be deemed invalid, not infringed, or unenforceable.
We cannot be certain that any patents will be issued as a result of any pending or future applications, or that any patents, once issued, will provide us with adequate protection from competing products. For example, issued patents may be circumvented or challenged, declared invalid or unenforceable, or narrowed in scope. In addition, since publication of discoveries in scientific or patent literature often lags behind actual discoveries, we cannot be certain that we or our licensors were the first to invent or to file patent applications covering them.
It is also possible that others may have or may obtain issued patents that could prevent us from commercializing our products or require us to obtain licenses requiring the payment of significant fees or royalties in order to enable us to conduct our business. There is no guarantee that such licenses will be available based on commercially reasonable terms. As to those patents that we have licensed, our rights depend on maintaining our obligations to the licensor under the applicable license agreement, and we may be unable to do so.
In addition to patents and patent applications, we depend upon agreements relating to trade secrets and proprietary know-how to protect our rights in innovations. We require all our consultants, advisors and collaborators to enter into agreements that prohibit the disclosure of our confidential information to other parties. In addition, it is our policy to require our consultants, advisors and collaborators who have access to proprietary and trade secret material to enter into agreements that require them to assign any and all intellectual property rights to us that arise as a result of their work on our behalf. Moreover, we require our employees to enter into an Invention Assignment and Confidentiality Agreement and assign to us their ideas, developments, discoveries and inventions and to prohibit the disclosure of confidential information to other parties. We also require our employees to review and acknowledge our trade secret policies regarding how employees handle trade secrets. These agreements, acknowledgements and policies may not, however, provide adequate protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure in violation of these agreements, and may not be sufficient to secure for us the value in such developments that they are designed to secure.
If we are unable to obtain and maintain patent protection for our products, or if the scope of the patent protection obtained is not sufficiently broad, competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our products could be impaired.
The patent prosecution process is expensive and time-consuming, and we may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost, in a timely manner, or in all jurisdictions. It is also possible that we will fail to identify patentable aspects of our development output before it is too late to obtain patent protection.
The patent position of life science companies generally is highly uncertain, involves complex legal and factual questions and has in recent years been the subject of much litigation. In addition, the laws of foreign countries may not protect our rights to the same extent as the laws of the United States and we may fail to seek or obtain patent protection in all major markets. For example, unlike the U.S., European patent law restricts the patentability of methods of treatment of the human body. Our pending and future patent applications may not result in patents being issued which protect our technology or products, in whole or in part, or which effectively prevent others from commercializing competitive technologies and products. Changes in either the patent laws or interpretation of the patent laws in the United States and other countries may diminish the value of our patents or narrow the scope of our patent protection, even post-grant.
Recent patent reform legislation has increased the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. On September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of
24
significant changes to United States patent law. These include provisions that affect the way patent applications are prosecuted and may also affect patent litigation. The U.S. Patent and Trademark Office, or USPTO, recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business and financial condition.
Moreover, we may be subject to a third-party preissuance submission of prior art to the USPTO, or become involved in opposition, derivation, reexamination, inter partes review, post-grant review or interference proceedings challenging our patent rights (whether licensed or otherwise held) or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate, our patent rights (whether licensed or otherwise held), allow third parties to commercialize our technology or products and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. In addition, if the breadth or strength of protection provided by our patents and patent applications (whether licensed or otherwise held) is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current or future product candidates.
Even if our patent applications (whether licensed or otherwise held) result in the issuance of patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability, and our licensed or owned patents may be challenged in the courts or patent offices in the United States and abroad. Such challenges may result in loss of exclusivity or freedom to operate or in patent claims being narrowed, invalidated or held unenforceable, in whole or in part, which could limit our ability to stop others from using or commercializing similar or identical products, or limit the duration of the patent protection of our products. Given the amount of time required for the development, testing and regulatory review of new life science product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property rights portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
We may become involved in lawsuits to protect or enforce our intellectual property rights, which could be expensive, time-consuming and ultimately unsuccessful.
Competitors may infringe our intellectual property. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. Any claims we assert against perceived infringers could provoke these parties to assert counterclaims against us alleging that we infringe their intellectual property or that our intellectual property is invalid or unenforceable. In addition, in a patent infringement proceeding, a court may decide that a licensed or owned patent of ours is invalid or unenforceable, in whole or in part, construe the patent’s claims narrowly or refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover that technology. Moreover, lawsuits to protect or enforce our intellectual property rights could be expensive, time-consuming and ultimately unsuccessful.
Third parties may initiate legal proceedings alleging that we are infringing their intellectual property rights, the outcome of which would be uncertain.
Our commercial success depends upon our ability to develop, manufacture, market and sell our product candidates without infringing the proprietary rights of third parties. There is considerable intellectual property litigation in the life sciences industry. We cannot guarantee that our product candidates will not infringe third-party patents or other proprietary rights. We may become party to, or threatened with, future adversarial proceedings or litigation regarding intellectual property rights with respect to our products and technology, including inter partes review, interference, or derivation proceedings before the USPTO and similar bodies in other countries. Third parties may assert infringement claims against us based on existing intellectual property rights and intellectual property rights that may be granted in the future.
25
If we are found to infringe a third party’s intellectual property rights, we could be required to obtain a license from such third party to continue developing and marketing our products. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. We could be forced, including by court order, to cease commercializing the infringing technology or product. In addition, we could be found liable for monetary damages, including treble damages and attorneys’ fees if we are found to have willfully infringed a patent. A finding of infringement could prevent us from commercializing our product candidates or force us to cease some of our business operations, which could materially harm our business. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar negative impact on our business.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our own patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees and annuities on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter our markets, which could have a material adverse effect on our business.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
Certain of our employees and contractors were previously employed at universities or other companies, including potential competitors. Although we try to ensure that our employees and contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employer. Litigation may be necessary to defend against these claims, and any such litigation could have an unfavorable outcome.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact develops intellectual property that we regard as our own. Our and their assignment agreements may not be self-executing or may be breached, and we may be forced to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of what we regard as our intellectual property.
If we fail in prosecuting or defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in prosecuting or defending against such claims, litigation could result in substantial costs and adverse results, and be a distraction to management.
A substantial portion of our in-licensed intellectual property will be subject to the provisions of the Bayh-Dole Act, if the underlying inventions were achieved using federal government funding. Such innovations are subject to “march-in” rights and other provisions under that Act.
Should we fail to take “effective steps to achieve practical application of” inventions we have licensed or fail to satisfy “health and safety needs” of consumers, then, under the federal Bayh-Dole Act, a federal government agency that has funded the discovery of the invention may “march in” and, despite our intellectual property rights in the invention, compel the granting of a license, or grant the license itself, to the invention to third-party petitioners who are “reasonable applicants”. There is also nothing prohibiting such government agency in exercising its “march-in” rights by granting such licenses to any of our competitors. Any such “march-in” under this Act could disrupt our operations and business plans with respect to the invention at issue.
26
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, it could have an adverse effect on the price of our common stock. Such litigation or proceedings could increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could compromise our ability to compete in the marketplace.
Risks Related to this Offering and Ownership of Our Common Stock and Warrants
We are selling Units in this offering without using the traditional services of an underwriter, and there can be no assurance that all or any of the Units included in this offering will be sold.
This offering is self-underwritten; that is, we are not going to engage the services of an underwriter to sell the Units. Instead, we have engaged the selling agent to sell Units on a “best efforts” basis and we intend to market and sell our shares to a substantial extent through our officers and directors, who will receive no commissions or other remuneration from any sales made hereunder. Furthermore, our management has no prior experience marketing a best efforts registered offering. There can be no assurance that all or any of the Units included in this offering will be sold. To the extent we fail to sell Units, our proceeds will be reduced. If we are not successful in selling a sufficient number of Units, we may not meet the NASDAQ initial listing requirements and, separately, we may have to seek additional, alternative financing to implement our plans as discussed in “Use of Proceeds”. Furthermore, the conversion of the Private Placement Convertible Notes at a price equal to $5.10 per Unit will occur even if only a few Units are sold, which would cause dilution to existing and new stockholders.
We have applied to list our common stock and warrants on NASDAQ, but we cannot guarantee that our securities will be approved for listing on NASDAQ.
We have applied to list our common stock and warrants on the NASDAQ Capital Market (“NASDAQ”). However, to be approved for listing on NASDAQ, our securities and our Company must meet certain initial listing requirements and thereafter continue to meet ongoing continued listing requirements. Among the initial listing requirements we expect to have to meet are requirements that we have at least one million shares publicly held, with an aggregate market value of at least $15 million and a bid price of at least $4.00 per share, held by at least 300 round-lot holders, and a minimum stockholders’ equity of $5 million. Our ability to meet all of the initial listing requirements applicable to us and thereafter continue to meet ongoing continued listing requirements will depend substantially on the number of shares we are able to sell in this offering and the price at which we are able to sell them. As a result, we cannot guarantee that our securities will be approved for listing on NASDAQ. In particular, as of March 31, 2015, we had total stockholders’ equity of $2.0 million (on a pro forma basis, taking into account the forced conversion, immediately after the closing of this offering, of our Private Placement Convertible Notes). This means that, to have closed this offering and listed on NASDAQ as of that date, we would have had to raise approximately $3 million. In this offering, if we are unable to raise the amount necessary to meet NASDAQ’s stockholders’ equity requirement, or if we otherwise fail to meet NASDAQ’s listing standards, our securities will not be listed on NASDAQ. In that event, we may in our sole discretion cancel some or all of this offering and thereafter promptly return any escrow funds you may already have paid.
In the event we close on some or all of this offering but our securities are not approved for listing on NASDAQ, we expect they will be quoted for trading on one of the “over the counter” markets operated by OTC Markets Group, Inc., such as the OTCQB market. The over-the-counter markets are relatively unorganized, inter-dealer markets that provide significantly less liquidity than NASDAQ. No assurance can be given that our securities, if quoted on one of the over-the-counter markets, will ever trade on an active and liquid basis.
In the event we do not qualify for listing on NASDAQ, if you are in the United States you may purchase securities in this offering only if you reside in one of the states in which we have registered our securities under the applicable state securities laws or in which we have qualified for an exemption from registration, or in which our transactions with you may otherwise be permitted consistent with applicable state securities laws.
We have applied or will apply to register our securities, or qualify for an exemption from registration, under the state securities, or “blue sky”, laws of Colorado, Florida, Georgia, Illinois, Nevada, New York and possibly other states. In the events we do not qualify for listing on NASDAQ, if you are in the United States but are not an “institutional investor,”
27
you will have to be a resident of one of these jurisdictions to purchase our securities in this offering. The definition of an “institutional investor” varies from state to state, but generally includes financial institutions, broker-dealers, banks, insurance companies and other qualified entities. Institutional investors in every state may purchase the securities in this offering pursuant to exemptions provided to such entities under the blue sky laws of such states. Under the National Securities Markets Improvement Act of 1996, or NSMIA, the resale of our securities by investors after this offering will be exempt from state registration requirements to the extent we regularly file periodic and annual reports with the SEC under the Exchange Act. For a more complete discussion of the state securities laws and regulations affecting this offering, please see “Plan of Distribution — State Blue Sky Information”.
In the event we do not qualify our securities for listing on NASDAQ, and must conduct offers and sales in this offering in compliance with all applicable state securities laws, we may fail to comply with all such laws in all instances. In any such instance, we may become subject to fines or other state regulatory actions.
In the event we do not qualify our securities for listing on NASDAQ, this offering will not be exempt from state securities law regulation to the extent available under NSMIA, and as a result we will have to conduct all offers and sales in this offering in compliance with the particular state securities laws that apply to each such offer and sale. The various states can impose fines on us or take other regulatory actions against us if we fail to comply with their state securities laws. Although we are taking steps to help insure that, if our securities do not qualify for listing on NASDAQ, we will conduct all the offers and sales in this offering in compliance with all state securities laws, there can be no assurance that we will be able to achieve such compliance in all instances, or avoid fines or other state regulatory actions if we do not achieve such compliance. See “Plan of Distribution — State Blue Sky Information”.
In the event we close on some or all of this offering but our securities are or in the future become subject to the “penny stock” rules, they will thereby be subject to additional sale and trading regulations that may make it more difficult for you to sell.
An equity security may be considered a “penny stock” if the following conditions are met: (i) it trades at a price less than $5 per share; (ii) it is not traded on a national securities exchange; and (iii) it is issued by a company that has net tangible assets (meaning total assets less intangible assets and liabilities) of $2,000,000 or less if the issuer has been in continuous operation for at least three years, or $5,000,000 or less if the issuer has been in continuous operation for less than three years.
The principal result or effect of being designated a “penny stock” is that broker-dealers participating in sales of such securities will be subject to the “penny stock” regulations set forth in Rules 15g-2 through 15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to: (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives.
If compliance with these requirements is necessary in regard to any of our equity securities, it may be more difficult and time consuming for holders of those securities to resell them to third parties or otherwise dispose of them in the market or otherwise.
The issuance in the future of a significant number of additional new shares of our common stock may have an adverse effect on our stock price, even if our business is performing well.
In connection with this offering, we are registering a substantial number of Units for resale by investors who participated in one or more of the Private Placements, hold Private Placement Convertible Notes and are having their Private Placement Convertible Notes converted into Units immediately after the closing of this offering. The shares and warrants comprising these Units will be eligible for resale by the selling securityholders starting immediately after the closing of this offering. Resales of a large number of our equity securities by one or more selling securityholders, or the perception that such sales could occur, could result in a decrease in the price of our common stock. In addition, TriPoint Global Equities, LLC, as our placement agent in the 2014 Private Placement, received warrants exercisable for the purchase of notes whose terms are identical to the Private Placement Convertible Notes (including their conversion terms), and as our selling agent in this offering will receive warrants exercisable for the purchase of Units. The issuance of additional equity securities of ours upon the conversion of such warrants may substantially increase the number of shares available for sale in the public market, and the sale of such shares, or the perception that such sales could occur, may further depress the price of our common stock. In addition, as shares resellable under Rule 144 under the Securities Act are sold after the closing of this offering, or as restrictions on resale end, the market price of our stock could drop if the holders of restricted shares sell them or are perceived by the market as intending to sell them. For more detailed information, please see “Share Eligible for Future Sale.” In addition to the foregoing, we may issue additional shares and other securities or instruments convertible into shares, to raise additional capital, close transactions or incentivize employees and others. Any such issuances that lead to, or are perceived to anticipate, the addition of shares of our common stock to the market could result in a decrease in the price of our common stock.
28
The issuance in the future of additional new shares of our common stock will dilute the then existing holders of our equity securities, including investors in this offering.
The issuance in the future of additional new shares of our common stock, including without limitation upon the issuance of Units to holders of Private Placement Convertible Notes as a result of the conversion of such Notes into Units immediately after the closing of this offering, the issuance of shares upon the exercise of outstanding or later-issued options or warrants and the issuance of additional shares or other securities or instruments convertible into shares, to raise additional capital, close transactions, incentivize employees and others or otherwise, will all result in the dilution of the percentage ownership of our common stock held by our then existing stockholders, including investors in this offering who are stockholders as of any such time of issuance or additional new shares.
The Company will likely issue additional options, warrants or other equity compensation to its officers, directors, employees and others in the ordinary course of business, and will likely seek to raise, and may succeed in raising, additional equity capital. As a result, investors in our Company are likely to experience dilution of their holdings in the future, and will in fact experience dilution to the extent of any of the potential issuances of securities described above increased the number of our outstanding shares of common stock.
Our stock price may be volatile, and an investment in our Company will likely require a long-term commitment.
There is no established market for our common stock or warrants, and the extent to which an active and liquid market for our securities may develop in the future depends in part on the number of Units we are able to sell in this offering. As we are in the early stages of growing our business, an investment in our Company will likely require a long-term commitment, with no certainty of return. The market prices of our securities are likely to be highly volatile and could fluctuate in response to various factors, many of which will be beyond our control, including the following:
• competition;
• additions or departures of key personnel;
• our ability to execute on our business plan on a timely basis;
• operating results that fall below expectations;
• period-to-period actual or anticipated fluctuations in our operating results;
• the loss of any strategic relationships;
• the extent of our need for additional capital and its availability to us on acceptable terms;
• industry and market developments;
• changes in the economic performance or market valuations of companies similar to ours; and
• economic and other external factors.
In particular, the market prices of health- and life science-related companies such as ours have been highly volatile due to such factors as:
• any delay or failure to conduct a field test or clinical trial for our products or in receiving approval from the EPA, FDA or other regulators;
• developments or disputes concerning our intellectual property rights;
• our or our competitors’ technological innovations;
• changes in market valuations of similar companies;
29
• announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures, capital commitments, new technologies or patents; and
• failure to complete significant transactions or collaborate with vendors in manufacturing our products.
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market prices of our securities.
In addition to negatively affecting the value of your investment, the lack of an active and liquid market for our stock may also impair our ability to raise capital by selling shares and may impair our ability to acquire additional intellectual property assets by using our shares as consideration.
Management may invest or spend the proceeds of this offering in ways with which you may not agree and in ways that may not yield favorable returns.
Our management will exert control over the net proceeds from this offering, and may spend them in ways with which you may not agree and in ways that may not yield favorable returns. Our current intention is to spend our net proceeds as described under “Use of Proceeds”. However, factors beyond our control make render these spending plans inadvisable or impractical prior to the time when all of the net proceeds are spent, in which case management would devise alternative spending plans.
Our board of directors has determined the offering price of the Units and the exercise price of the warrants.
Our board of directors has determined the offering price of the Units and the exercise price of the warrants. They have done so with reference to the value of our Company as reflected in the Private Placements, but not otherwise with reference to our book value or asset values or by the application of any customary, established models for valuing companies or securities. Because such prices have not been so determined, they may not be indicative of any amounts you might receive should you seek to sell your Company securities or should there be a liquidation of the Company. In addition, such prices are not necessarily indicative of any prices at which Company securities may trade, or any value that might be ascribed to the Company after the completion of the offering.
We may redeem all or a portion of the warrants offered as part of our Units prior to their exercise at a time that is disadvantageous to you, thereby making your warrants worthless.
We have the ability to redeem all or a portion of the warrants offered as part of our Units at any time prior to their expiration, at a price of $0.01 per warrant, provided, that the last reported sales price of our common stock equals or exceeds $16.00 per share on each of 20 trading days within the 30 trading-day period ending on the third business day prior to the date on which notice of the redemption is given to the warrant holders and the average daily trading volume of our common stock exceeds 500,000 shares per day during the redemption period. Redemption of the outstanding warrants could force you to exercise your warrants and pay the exercise price therefor at a time when it may be disadvantageous for you to do so, to sell your warrants at the then-current market price when you might otherwise wish to hold your warrants or to accept the nominal redemption price which, at the time the outstanding warrants are called for redemption, is likely to be substantially less than the market value of your warrants.
30
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain of the statements in this prospectus constitute forward-looking statements. All statements other than statements of historical fact contained in this prospectus, including statements regarding our future results of operations and financial condition, business strategy, operations, plans, prospects, projected revenue and costs and objectives of management are forward-looking statements. Forward-looking statements include, without limitation, any statement that may predict, forecast, indicate, or imply future results, performance or achievements, and may contain the words “estimate,” “project,” “intend,” “forecast,” “potential,” “anticipate,” “plan,” “planning,” “expect,” “believe,” “will,” “will likely,” “should,” “could,” “would,” “may” or words or expressions of similar meaning. These risks and uncertainties include, but are not limited to, those factors described under the heading “Risk Factors” in this prospectus. All our forward-looking statements involve significant risks and uncertainties, including, but not limited to, risks and uncertainties relating to:
• our development, marketing and sales programs;
• our ability to obtain FDA, EPA and other applicable regulatory approvals for our product candidates;
• the commercial markets for our product candidates;
• competition;
• the proprietary rights of others;
• the availability of additional financing;
• the effects of existing and future federal, state and foreign regulations;
• joint or licensed development, commercialization, distribution, collaboration, marketing and other arrangements with third parties; and
• the period of time over which the net proceeds of this offering will enable us to fund our operations.
As more fully described in this prospectus under the heading “Risk Factors,” many important factors may affect our ability to achieve our stated objectives and commercialize product candidates, including, among other things, our ability to:
• obtain substantial additional funds;
• obtain and maintain all necessary patents or licenses;
• demonstrate the safety and efficacy of product candidates whenever required to do so;
• meet applicable regulatory standards and receive required regulatory approvals;
• retain key executives and attract, retain and motivate qualified personnel;
• manufacture and distribute products in commercial quantities at reasonable costs;
• compete against others; and
• generate and market our products in a cash-flow-positive and profitable manner.
Prospective investors are cautioned that there can be no assurance that any forward-looking statements included in this prospectus will prove to be accurate. In light of the often significant uncertainties inherent in our forward-looking statements, the inclusion of such statements should not be regarded as a representation or warranty by the Company or any other person that the objectives and plans of the Company will be achieved in any specified time frame, if at all. Except to the extent required by applicable laws or rules, the Company does not undertake any obligation to update any forward-looking statements or to announce revisions to any forward-looking statements.
We caution you that the important risk factors and cautionary statements described in the sections of this prospectus entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results
31
of Operations”, as well as in other portions of this prospectus, may not be all of the factors important to you in determining whether to invest in our securities. We cannot assure you that we will realize the results or developments we expect or anticipate or, even if we realize them substantially, that they will result in the outcomes we expect. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for us to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any one factor, or combination of factors, may cause our actual results to differ materially and adversely from those stated or suggested in our forward-looking statements. The forward-looking statements included in this prospectus are made only as of the date hereof. We undertake no obligation to update or revise any forward-looking statement as a result of new information, future events or otherwise, except to the extent required by law.
32
USE OF PROCEEDS
If we sell all of the Units being offered, our net proceeds (after the selling agent’s commissions of $1,200,000 and after our estimated other offering expenses of $625,000) will be $18,175,000. We will use the net proceeds to pay development and commercialization costs for existing innovations; acquisition, development and commercialization costs for new innovations; operating expenses (including salaries; legal, accounting and consulting fees; subsidiary formation costs, program development, rent; marketing programs; and other general administrative expenses); and working capital. The precise amounts that the Company will devote to each of these items, and the timing of expenditures, will vary depending on numerous factors, including but not limited to the progress of development and commercialization efforts relating to each current and new project. The following table sets forth a breakdown of the estimated use of the net proceeds as we currently expect to use them, assuming the sale of 100%, 75%, 50% and 25% of the Units offered for sale in this offering:
Assumed Percentage of Units Sold |
|
100% |
|
75% |
|
50% |
|
25% |
||||
Price to Public |
|
$ |
20,000,000 |
|
$ |
15,000,000 |
|
$ |
10,000,000 |
|
$ |
5,000,000 |
Selling agent’s commissions |
|
|
1,200,000 |
|
|
900,000 |
|
|
600,000 |
|
|
300,000 |
Other offering expenses |
|
|
625,000 |
|
|
625,000 |
|
|
625,000 |
|
|
625,000 |
Net proceeds |
|
$ |
18,175,000 |
|
$ |
13,475,000 |
|
$ |
8,775,000 |
|
$ |
4,075,000 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Innovation acquisition, development and commercialization |
|
$ |
10,675,000 |
|
$ |
6,475,000 |
|
$ |
2,775,000 |
|
$ |
1,025,000 |
Operating expenses |
|
|
5,000,000 |
|
|
4,500,000 |
|
|
4,000,000 |
|
|
2,000,000 |
Working capital |
|
|
2,500,000 |
|
|
2,500,000 |
|
|
2,000,000 |
|
|
1,050,000 |
Total use of proceeds |
|
$ |
18,175,000 |
|
$ |
13,475,000 |
|
$ |
8,775,000 |
|
$ |
4,075,000 |
As indicated in the table above, if we sell only 75%, or 50%, or 25% of the Units offered for sale in this offering, we would expect to use the resulting net proceeds for the same purposes as we would use the net proceeds from a sale of 100% of the Units, and in approximately the same proportions. However, the lower our net proceeds, the less we would expect to use the funds in the expenditure category “Innovation acquisition, development and commercialization” for the acquisition of new innovations, and the more we would expect to use those funds for the development and commercialization of innovations we have already acquired.
In the event we do not sell all of the Units being offered, we may seek additional financing to support the intended use of proceeds discussed above. If we secure additional equity funding, investors in this offering would be diluted. In all events, there can be no assurance that additional financing would be available when needed and, if available, on terms acceptable to us.
In addition, if we sell all of the Units being offered, and if all of the warrants being offered as part of the Units are exercised, we would receive an additional $12,500,000 in net proceeds. We cannot predict if or when any such warrants will be exercised.
DIVIDEND POLICY
We have not paid any dividends on our common stock since inception, and we currently expect that, in the foreseeable future, all earnings (if any) will be retained for the development of our business and no dividends will be declared or paid. Any future dividends will be subject to the discretion of our board of directors and will depend upon, among other things, our earnings (if any), operating results, financial condition and capital requirements, general business conditions and other pertinent facts.
33
CAPITALIZATION
The following table sets forth our cash and our capitalization as of March 31, 2015:
• on an actual basis;
• on a pro forma basis after the reflection of $2,100,000 of proceeds from the 2015 Private Placement through April 30, 2015 and the conversion of an aggregate of $5,072,723 of the Private Placement Convertible Notes and accrued interest into Units immediately after the closing of this offering, including the reclassification of derivative liabilities aggregating $914,838 to additional paid in capital, and $770,484 of unamortized debt discount charged to expense; and
• on a pro forma basis assuming the sale in this offering of all of the 3,125,000 Units being offered, at the price to the public of $6.40 per unit, resulting in net proceeds to us of $18,175,000, after deducting the selling agent’s commissions and our estimated total expenses for this offering of $625,000.
You should read this table together with our consolidated financial statements as of and for the years ended December 31, 2014 and 2013 and the related notes thereto, the condensed consolidated financial statements as of and for the three months ended March 31, 2015 and 2014 and the related notes thereto, “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” included elsewhere in this prospectus.
|
|
March 31, 2015 |
|||||||||||||||||
|
|
Actual |
|
Pro Forma (unaudited) |
|
Pro Forma as Adjusted (unaudited) |
|||||||||||||
|
|
|
|
Conversion of Notes into Units |
|
Pro Forma before Offering |
|
This Offering |
|
|
|||||||||
Cash and cash equivalents |
|
$ |
544,699 |
|
|
$ |
2,100,000 |
|
|
$ |
2,644,699 |
|
|
$ |
18,175,000 |
|
$ |
20,819,699 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accrued interest on convertible notes |
|
$ |
101,939 |
|
|
$ |
(101,939 |
) |
|
|
— |
|
|
|
|
|
|
— |
|
Convertible notes, net of debt discount |
|
$ |
2,014,516 |
|
|
$ |
(2,014,516 |
) |
|
|
— |
|
|
|
|
|
|
— |
|
Derivative liabilities |
|
$ |
914,838 |
|
|
$ |
(914,838 |
) |
|
|
— |
|
|
|
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock |
|
$ |
94 |
|
|
$ |
10 |
|
|
$ |
104 |
|
|
$ |
31 |
|
$ |
135 |
|
Additional paid-in capital |
|
$ |
13,110,930 |
|
|
$ |
5,987,551 |
|
|
$ |
19,098,481 |
|
|
$ |
18,174,969 |
|
$ |
37,273,450 |
|
Accumulated deficit |
|
$ |
(14,839,743 |
) |
|
$ |
(770,484 |
) |
|
$ |
(15,610,227 |
) |
|
|
|
|
$ |
(15,610,227 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ (deficiency) equity-Innovation Economy |
|
$ |
(1,728,719 |
) |
|
$ |
5,217,077 |
|
|
$ |
3,488,358 |
|
|
$ |
18,175,000 |
|
$ |
21,663,358 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling interest deficiency |
|
$ |
(1,452,581 |
) |
|
|
|
|
|
$ |
(1,452,581 |
) |
|
|
|
|
$ |
(1,452,581 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity (deficiency) |
|
$ |
(3,181,300 |
) |
|
|
|
|
|
$ |
2,035,777 |
|
|
|
|
|
$ |
20,210,777 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total capitalization |
|
$ |
(251,946 |
) |
|
|
|
|
|
$ |
2,035,777 |
|
|
|
|
|
$ |
20,210,777 |
|
34
The table above includes 994,652 shares of common stock included in the Units issuable upon the forced conversion of the Private Placement Convertible Notes and accrued interest immediately after closing of this offering. By the terms of the Private Placement Convertible Notes, this conversion will be effected at a price per unit equal to $5.10. The table above excludes:
• 2,645,967 shares of our common stock issuable upon the exercise of stock options outstanding as of March 31, 2015, at a weighted average exercise price of $1.98 per share;
• 240,333 shares of our common stock issuable upon the exercise of warrants outstanding as of March 31, 2015, at a weighted average exercise price of $2.29 per share;
• 20,700 shares of our common stock available for future issuance under our Amended and Restated Long-Term Incentive Compensation Plan as of the date of this prospectus;
• 1,250,000 shares of our common stock that will be made available for future issuance under our 2015 equity incentive plan upon the closing of this offering;
• 1,562,500 shares of our common stock issuable upon exercise of the warrants included in the Units being offered in this offering, assuming all of the Units being offered are sold;
• 234,375 shares of our common stock issuable upon exercise of the warrants to be issued to the selling agent in connection with this offering;
• 497,326 shares of our common stock issuable upon exercise of the warrants included in the Units issuable upon conversion of the Private Placement Convertible Notes plus accrued interest immediately after the closing of this offering; and
• 34,824 shares of our common stock issuable upon conversion of the notes issuable upon the exercise of placement agent warrants issued in connection with the 2014 Private Placement.
To the extent such stock options or warrants are hereafter exercised, or awards made under such equity compensation plans result in the issuance of additional shares of our common stock, there will be further dilution to our investors.
35
DILUTION
If you purchase Units in this offering, your ownership interest in our common stock will be diluted immediately, to the extent of the difference between the price to the public charged for each unit and the pro forma net tangible book value per share of our common stock after this offering.
Our historical net tangible book value as of March 31, 2015 was a deficit of $(3,181,300), or ($0.34) per then outstanding share of our common stock. Historical net tangible book value per share equals the amount of our total tangible assets less total liabilities, divided by the total number of shares of our common stock outstanding, all as of the date specified.
Our unaudited pro forma net tangible book value as of March 31, 2015 before the offering was $2,035,777, or $0.19 per then outstanding share of our common stock. Unaudited pro forma net tangible book value per share equals the amount of our total tangible assets less our total liabilities, divided by the pro forma total number of shares of our common stock outstanding, as of the date specified, after giving effect to the conversion upon the closing of this offering of the Private Placement Convertible Notes plus accrued interest into 994,652 Units, which include an aggregate of 994,652 shares of our common stock.
The following table illustrates this per share dilution to new investors, assuming the sale of 25%, 50%, 75% and 100% of the Units offered for sale in this offering, after giving effect to the deduction of selling agent’s commissions and our estimated other offering expenses:
|
|
100% |
|
75% |
|
50% |
|
25% |
||||||||
Price to the public charged for each unit in this offering |
|
$ |
6.40 |
|
|
$ |
6.40 |
|
|
$ |
6.40 |
|
|
$ |
6.40 |
|
Historical net tangible book value per share as of March 31, 2015 |
|
$ |
(0.34 |
) |
|
$ |
(0.34 |
) |
|
$ |
(0.34 |
) |
|
$ |
(0.34 |
) |
Increase attributable to the pro forma transactions described in the preceding paragraphs |
|
$ |
0.53 |
|
|
$ |
0.53 |
|
|
$ |
0.53 |
|
|
$ |
0.53 |
|
Pro forma net tangible book value per share as of March 31, 2015 |
|
$ |
0.19 |
|
|
$ |
0.19 |
|
|
$ |
0.19 |
|
|
$ |
0.19 |
|
Increase in net tangible book value per share attributable to new investors |
|
$ |
1.30 |
|
|
$ |
1.02 |
|
|
$ |
0.71 |
|
|
$ |
0.35 |
|
Pro forma net tangible book value per share after this offering |
|
$ |
1.49 |
|
|
$ |
1.21 |
|
|
$ |
0.90 |
|
|
$ |
0.54 |
|
Dilution per share to new investors |
|
$ |
4.91 |
|
|
$ |
5.19 |
|
|
$ |
5.50 |
|
|
$ |
5.86 |
|
These effects on pro forma net tangible book value per share assume the warrants sold in this offering will be accounted for as part of stockholders’ equity. Dilution per share to new investors is determined by subtracting pro forma net tangible book value per share after this offering from the price to the public charged for each Unit in this offering.
The following table sets forth, assuming the sale of 25%, 50%, 75% and 100% of the Units offered for sale in this offering after giving effect to the same factors given effect in the table above, as of December 31, 2014, the total number of shares previously sold to existing stockholders, the total consideration paid or to be paid for each of the foregoing, and the average price paid per share or unit, respectively. As the table shows, new investors purchasing Units may in certain circumstances pay an average price per share substantially higher than the average price per share paid by our existing stockholders.
|
|
|
Shares/Units Purchased |
|
Total Consideration |
|
Average Price |
|||||||
|
Assuming 100% of Units Sold: |
|
Number |
|
Percentage |
|
Amount |
|
Percentage |
|
Per Share/Unit |
|||
|
Existing stockholders |
|
10,474,304 |
|
77.0 |
% |
|
12,645,314 |
|
38.7 |
% |
|
$ |
1.21 |
|
New Investors |
|
3,125,000 |
|
23.0 |
% |
|
20,000,000 |
|
61.3 |
% |
|
$ |
6.40 |
|
Total |
|
13,599,304 |
|
100.0 |
% |
|
32,645,314 |
|
100.0 |
% |
|
$ |
2.40 |
|
|
|
Shares/Units Purchased |
|
Total Consideration |
|
Average Price |
|||||||
|
Assuming 75% of Units Sold: |
|
Number |
|
Percentage |
|
Amount |
|
Percentage |
|
Per Share/Unit |
|||
|
Existing Stockholders |
|
10,474,304 |
|
81.7 |
% |
|
12,645,314 |
|
45.7 |
% |
|
$ |
1.21 |
|
New Investors |
|
2,343,750 |
|
18.3 |
% |
|
15,000,000 |
|
54.3 |
% |
|
$ |
6.40 |
|
Total |
|
12,818,054 |
|
100.0 |
% |
|
27,645,314 |
|
100.0 |
% |
|
$ |
2.16 |
36
|
|
|
Shares/Units Purchased |
|
Total Consideration |
|
Average Price |
|||||||
|
Assuming 50% of Units Sold: |
|
Number |
|
Percentage |
|
Amount |
|
Percentage |
|
Per Share/Unit |
|||
|
Existing Stockholders |
|
10,474,304 |
|
87.0 |
% |
|
12,645,314 |
|
55.8 |
% |
|
$ |
1.21 |
|
New Investors |
|
1,562,500 |
|
13.0 |
% |
|
10,000,000 |
|
44.2 |
% |
|
$ |
6.40 |
|
Total |
|
12,036,804 |
|
100.0 |
% |
|
22,645,314 |
|
100.0 |
% |
|
$ |
1.88 |
|
|
|
Shares/Units Purchased |
|
Total Consideration |
|
Average Price |
|||||||
|
Assuming 25% of Units Sold: |
|
Number |
|
Percentage |
|
Amount |
|
Percentage |
|
Per Share/Unit |
|||
|
Existing Stockholders |
|
10,474,304 |
|
93.1 |
% |
|
12,645,314 |
|
71.7 |
% |
|
$ |
1.21 |
|
New Investors |
|
781,250 |
|
6.9 |
% |
|
5,000,000 |
|
28.3 |
% |
|
$ |
6.40 |
|
Total |
|
11,255,554 |
|
100.0 |
% |
|
17,645,314 |
|
100.0 |
% |
|
$ |
1.57 |
Each of the two tables above in this section includes 994,652, shares of common stock included in the Units issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest immediately after the closing of this offering. By the terms of the Private Placement Convertible Notes, this conversion will be effected at a price per unit equal to $5.10. Each of the two tables above in this section excludes:
• 2,645,967 shares of our common stock issuable upon the exercise of stock options outstanding as of the date of this prospectus, at a weighted average exercise price of $1.98 per share;
• 240,333 shares of our common stock issuable upon the exercise of warrants outstanding as of the date of this prospectus, at a weighted average exercise price of $2.29 per share;
• 20,700 shares of our common stock available for future issuance under our Amended and Restated Long-Term Incentive Compensation Plan as of the date of this prospectus;
• 1,250,000 shares of our common stock that will be made available for future issuance under our 2015 equity incentive plan upon the closing of this offering;
• 1,562,500 shares of our common stock issuable upon exercise of the warrants included in the Units being offered in this offering, assuming all of the Units being offered are sold;
• 234,375 shares of our common stock issuable upon exercise of the warrants to be issued to the selling agent in connection with this offering;
• 497,326 shares of our common stock issuable upon exercise of the warrants included in the Units issuable upon conversion of the Private Placement Convertible Notes plus accrued interest immediately after the closing of this offering; and
• 34,824 shares of our common stock issuable upon conversion of the notes issuable upon the exercise of placement agent warrants issued in connection with the 2014 Private Placement.
To the extent such stock options or warrants are hereafter exercised, or awards made under such equity compensation plans result in the issuance of additional shares of our common stock, there will be further dilution to our investors.
37
SELECTED CONSOLIDATED FINANCIAL DATA
The following selected consolidated financial data should be read together with our financial statements and the accompanying notes thereto, and the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations”, appearing elsewhere in this prospectus. The selected consolidated financial data as of and for the years ended December 31, 2014 and 2013 have been derived from our annual consolidated financial statements appearing elsewhere in this prospectus. The summary consolidated financial data as of March 31, 2015 and for the three months periods ended March 31, 2015 and 2014 have been derived from our unaudited interim condensed consolidated financial statements appearing elsewhere in this prospectus, and include all adjustments, consisting of normal recurring adjustments, which in the opinion of management are necessary for a fair presentation of our financial position as of such date and our results of operations for such periods.
Summary Consolidated Statement of Operations Data
|
|
For the years ended December 31, |
|
For the three months ended March 31, |
||||||||||||
|
|
2014 |
|
2013 |
|
2015 (Unaudited) |
|
2014 (Unaudited) |
||||||||
Revenue |
|
$ |
— |
|
|
$ |
109,688 |
|
|
$ |
— |
|
|
$ |
— |
|
Gross profit |
|
$ |
— |
|
|
$ |
11,684 |
|
|
$ |
— |
|
|
$ |
— |
|
Total operating expenses |
|
$ |
4,895,129 |
|
|
$ |
3,651,088 |
|
|
$ |
1,590,317 |
|
|
$ |
1,001,889 |
|
Net loss |
|
$ |
(4,939,395 |
) |
|
$ |
(3,411,383 |
) |
|
$ |
(1,715,983 |
) |
|
$ |
(1,000,243 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted net loss per share |
|
$ |
(0.49 |
) |
|
$ |
(0.37 |
) |
|
$ |
(0.16 |
) |
|
$ |
(0.10 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding-basic and diluted |
|
|
9,261,231 |
|
|
|
8,344,836 |
|
|
|
9,444,828 |
|
|
|
8,881,578 |
|
Summary Consolidated Balance Sheet Data
|
|
As of December31, |
|
As of March 31, |
||||||||
|
|
2014 |
|
2013 |
|
2015 (Unaudited) |
||||||
Cash |
|
$ |
1,881,776 |
|
|
$ |
722,736 |
|
|
$ |
544,699 |
|
Current assets |
|
$ |
1,905,916 |
|
|
$ |
744,026 |
|
|
$ |
574,734 |
|
Total assets |
|
$ |
2,428,504 |
|
|
$ |
938,490 |
|
|
$ |
1,213,472 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities |
|
$ |
1,182,649 |
|
|
$ |
1,226,172 |
|
|
$ |
1,465,418 |
|
Total liabilities |
|
$ |
4,005,264 |
|
|
$ |
1,226,172 |
|
|
$ |
4,394,772 |
|
Total Stockholders’ equity (deficiency) |
|
$ |
(1,576,760 |
) |
|
$ |
(287,682 |
) |
|
$ |
(3,181,300 |
) |
Summary Consolidated Cash Flow Statement Data
|
|
For the years ended December 31, |
|
For the three months ended March 31, |
||||||||||||
|
|
2014 |
|
2013 |
|
2015 (Unaudited) |
|
2014 (Unaudited) |
||||||||
Net cash used in operations |
|
$ |
(3,973,743 |
) |
|
$ |
(1,413,006 |
) |
|
$ |
(1,228,667 |
) |
|
$ |
(715,310 |
) |
Net cash used in investing activities |
|
$ |
(25,613 |
) |
|
$ |
(3,995 |
) |
|
$ |
(30,412 |
) |
|
$ |
(5,591 |
) |
Net cash provided by financing activities |
|
$ |
5,158,396 |
|
|
$ |
1,754,155 |
|
|
$ |
(77,998 |
) |
|
$ |
1,503,250 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash at end of period |
|
$ |
1,881,776 |
|
|
$ |
722,736 |
|
|
$ |
544,699 |
|
|
$ |
1,505,085 |
|
38
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
This Management’s Discussion & Analysis contains forward-looking statements relating to, among other things, our future economic performance, plans and objectives. These forward-looking statements are often identified by words such as “may”, “will”, “expect”, “intend”, “anticipate”, “believe”, “estimate”, “continue”, “plan”, “potential” and similar expressions. These statements involve estimates, assumptions and uncertainties that could cause actual outcomes to differ materially from those expressed. You should not place undue reliance on these forward-looking statements. You should also consider carefully the statements we make in “Risk Factors” and in other sections of this prospectus, addressing factors that could cause actual outcomes to differ from those expressed in our forward-looking statements and that could materially and adversely affect our business, operating results and financial condition.
This Management’s Discussion and Analysis should be read together with our consolidated financial statements and the notes thereto included elsewhere in this prospectus.
Overview
We are an emerging growth company based on a Collaborative Economy model with a mission to bring the world together to unlock the potential of untapped innovations. We were founded by experienced entrepreneurs who recognized that research institutions can be filled with un-commercialized technology discoveries and breakthrough research (which we refer to throughout this document as “innovations”) which, if successfully commercialized, could solve or help to solve significant global challenges. We believe that the potential of these untapped innovations could be vast. Yet many of these innovations will never see the light of day because of the significant difficulties often experienced in the process of taking laboratory-proven, potentially ground-breaking innovations and transforming them into products, product platforms, services and technologies for distribution to global markets.
Recognizing the gap between the marketplace and un-commercialized but potentially beneficial innovations, the founders of ieCrowd have created a business model that is intended to bridge this gap. ieCrowd’s business goal is to license or acquire innovations with the potential to solve global problems for the purpose of commercializing them into product platforms, products, services and technologies that have substantial value-adding impact over large populations and markets.
ieCrowd’s financial condition and results of operations generally reflect the fact that the Company is still in an early stage of its business, and that its three operating subsidiaries, each focused on the development and commercialization of different innovations, are also in early stages of their businesses. As discussed in more detail below, from period to period, we generate little or no revenues and use more cash in our operating activities than we generate from such activities; we have financed and expect to continue to finance our operations substantially through the issuance of equity securities and debt securities convertible into equity; our assets consist mostly of cash and cash equivalents; substantially all of our liabilities are current, rather than long-term, liabilities; and we run a substantial and increasing stockholders’ deficiency. We expect our financial condition and results of operations to continue to be generally loss-making, operating-cash-flow negative and dependent on additional financing, unless and until we are able to begin to successfully commercialize one or more of our acquired innovations, and possibly for a substantial time thereafter.
Going Concern
The accompanying consolidated financial statements of ieCrowd have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As of March 31, 2015, the Company had a working capital deficiency of $890,684 and a stockholders’ deficiency of $3,181,300. As of December 31, 2014, the Company had working capital of $723,267 and a stockholders’ deficiency of $1,576,760. The Company has not generated any significant revenues from ongoing operations and has incurred net losses since inception. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of asset amounts or the classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
The Company’s primary source of operating funds since inception has been equity financings. Subsequent to December 31, 2013, the Company secured additional equity financing through the sale of common stock aggregating
39
$2,840,000 and debt financing in the form of Private Placement Convertible Notes aggregating $2,785,000. Subsequent to December 31, 2014, the Company secured additional debt financing in the form of Private Placement Convertible Notes aggregating $2,100,000. The Company expects that its current cash on hand will fund its operations through August 2015.
The Company needs to raise additional capital in order to be able to accomplish its business objectives, and is continuing its efforts to secure additional funds due to the impending lack of funds. The Company intends to raise additional capital through the initial public offering described in this prospectus and from private debt and equity investors. Management believes that it will be successful in obtaining additional financing based on its history of raising funds; however, no assurance can be provided that the Company will be able to do so. In addition, there can be no assurance that funds raised will be sufficient to enable the Company to attain profitable operations or continue as a going concern. To the extent that the Company is unsuccessful in its fundraising, it may need to curtail or cease its operations and implement a plan to extend payables or reduce overhead until sufficient additional capital is raised to support further operations. There can be no assurance that any such plan would be successful.
Critical Accounting Policies and Estimates
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires management to make estimates and assumptions that affect the reported amounts of our assets, liabilities, revenues and expenses. Certain of these accounting policies are considered to be critical accounting policies, as defined below.
A critical accounting policy is defined as one that is both material to the presentation of our consolidated financial statements and requires management to make difficult, subjective or complex judgments that could have a material effect on our financial condition and results of operations. Critical accounting estimates have the following attributes: (1) they require us to make assumptions about matters that are highly uncertain at the time of the estimate; and (2) different estimates we could reasonably have used, or changes in the estimate we used that are reasonably likely to occur, could have a material effect on our financial condition or results of operations.
Estimates and assumptions about future events and their effects cannot be determined with certainty. We base our estimates on historical experience and on various other assumptions believed to be applicable and reasonable under the circumstances. These estimates may change as new events occur, as additional information is obtained or as our operating environment changes. We believe the following critical accounting policies reflect the more significant estimates and assumptions we have used in the preparation of our consolidated financial statements:
Revenue Recognition — The Company’s revenue recognition policies are in compliance with the Financial Accounting Standards Board’s (“FASB’s”) Accounting Standards Codification (“ASC”) Topic 605, “Revenue Recognition”, which establishes criteria that must be satisfied before revenue is realized or realizable and earned. Revenue is recognized when persuasive evidence of an arrangement exists, delivery of the product or service has occurred, all obligations have been performed pursuant to the terms of the agreement, the sales price is fixed or determinable and collectability is reasonably assured.
The Company has generated revenue from sources other than product sales, namely collaboration and research agreements, prototype development, conference fees and a Kite Patch Internet crowdfunding campaign that offered benefits other than securities. In these cases, revenue is recognized when the contractual milestones are met, prototype delivery is achieved and, in the case of the conference, the event has occurred. For the Kite Patch campaign, revenue was recognized for merchandise delivered, and deferred for amounts received related to the future delivery of still-to-be-commercialized products.
On May 1, 2013 the Company entered into an agreement with a third party to develop an electronic analyzer for a defined market. An initial payment of $30,000 was due and paid on the signing of the agreement. On July 12, 2013, the Company entered into a second agreement to design and deliver a prototype analyzer for the defined market for $5,000. The Company recognized revenue when all milestones relative to the funded amount were completed, the prototype was delivered and collection was reasonably assured.
On May 9, 2013 the Company sponsored the ieCrowd Expo conference event. The Company generated revenues from general-public ticket sales and participation fees from event sponsors for booth and presentation opportunities. Revenue of $74,688 was recognized at the completion of the event.
40
On July 15, 2013, the Company initiated an INDIEGOGO.com campaign for the development and testing of Kite Patch (the “Campaign”). Kite Patch is a mosquito fighting product under development that is based on technology designed to block mosquitos’ ability to track humans. On August 29, 2013 at the close of the Campaign, $516,974 (net of transaction fees) was collected. As part of the Campaign, the Company offered “perks” for various levels of contributions. These items included t-shirts, water bottles, stickers and Kite Patches. As of December 31, 2013, the Company has delivered all the non-Kite Patch perks and recognized $14,482 as Other Income, net of pro rata transaction fees of $4,475 and merchandise product costs of $43,791. On March 31, 2015, December 31, 2014 and 2013, the Company had deferred revenue related to the Campaign of $210,210, net of related transaction fees of $35,000 and estimated Kite Patch production cost of $249,000 (since the Kite Patches will not be shipped until they are ready for commercial distribution).
Research and Development — The Company incurs formulation costs that include salaries, materials and consultant fees. These costs are classified as research and development expenses under the Operating Expenses portion of our consolidated statements of operations. All costs incurred to establish the technological feasibility of a product to be sold, leased or otherwise marketed are research and development costs, and shall be charged to expense when incurred.
Segment Reporting — In accordance with ASC 280 “Segment Reporting”, operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. The Company’s chief decision maker, as defined under the FASB’s guidance, is its Chief Executive Officer. It is determined that the Company operates in one business segment and one geographic segment, the United States of America.
Material Weaknesses
In connection with the contemporaneous audit of our consolidated financial statements for the years ended December 31, 2014 and 2013, our independent registered public accountants identified material weaknesses in our internal control over financial reporting. A “material weakness” is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses relates to having only one employee assigned to positions that involve processing financial information, resulting in a lack of segregation of duties so that all journal entries and account reconciliations are not reviewed by someone other than the preparer. In addition, we have identified a material weakness related to accounting for complex transactions. See “Risk Factors”.
Although we are aware of the risks associated with having a small internal accounting staff, we are also at an early stage in the development of our business. We expect to expand our accounting function and improve its ability to handle complex transactions and other matters as we grow our business and can more readily absorb the costs of such expansion and improvements. In the meantime, management will continue to observe and assess our internal audit function and make necessary improvements whenever they may be required.
Results of Operations
We have been operating since October 2010. Our results of operations for the years ended December 31, 2014 and 2013 reflect, in general, expenses we have incurred in connection with continuing to develop mosquito repellents & attractants, gas sensors and a supplemental oxygen delivery device, and revenue we have received on an intermittent basis, including revenue related to fees charged in connection with our “Innovation Economy Expo” (“ieExpo”), a conference we hosted in May 2013 on high-growth business ventures, revenue received through research collaboration and development contracts, cash receipts from our Kite Patch campaign on Indiegogo.com and government grants from the National Institutes of Health and the City of Riverside, California.
Three Months Ended March 31, 2015 Compared to Three Months Ended March 31, 2014
Revenues. The Company had zero consolidated revenues in the three months ended March 31, 2015 and in the three months ended March 31, 2014.
41
Operating Expenses. The Company’s condensed consolidated operating expenses for the three months ended March 31, 2015 and 2014 consisted of the following:
|
|
Three months ended March 31, |
|
Increase (Decrease) |
|||||||||
|
|
2015 |
|
2014 |
|
$ |
|
% |
|||||
General and administrative expenses |
|
$ |
1,216,108 |
|
$ |
687,025 |
|
$ |
529,083 |
|
|
77.0 |
% |
Marketing and sales |
|
|
130,148 |
|
|
131,891 |
|
|
(1,743 |
) |
|
(1.3 |
)% |
Research and development |
|
|
244,061 |
|
|
182,973 |
|
|
61,088 |
|
|
33.4 |
% |
Total Operating Expenses |
|
$ |
1,590,317 |
|
$ |
1,001,889 |
|
$ |
588,428 |
|
|
58.7 |
% |
The individual components of our consolidated operating expenses are discussed below.
General and Administrative Expenses — General and administrative expenses include payroll, professional and consultant fees, stock-based compensation and stock issued for services. From the three months ended March 31, 2014 to the three months ended March 31, 2015, general and administrative expenses increased $529,083, or 77.0%, from $687,025 to $1.216.108. The increase was due principally to the following:
• Payroll expense increased $155,229, or 68.5%, from $226,502 to $381,731, due to the hiring of additional executive personnel to lead product development efforts and strategic marketing and partnership development efforts, in anticipation of the sale and distribution of products when available.
• Professional and consultant fees increased $303,506, or 229.8%, from $101,238 to $404,744, due to increased legal, audit and consultant fees. Increased legal fees were the result of Company fundraising efforts, intellectual property protection and patent filings. Audit fees increased in connection with the Company’s preparations to begin reporting as a public company. Consultant fees increased due to product development efforts.
• Stock-based compensation cost decreased $28,751, or 20.5%, from $140,194 to $111,443. See “Executive Compensation” for a discussion of our compensation policies.
• The cost of stock and warrants issued for services decreased from $110,950 to $0. During the three months ended March 31, 2014, the Company issued common stock and warrants to vendors and contractors. The Company has from time-to-time used this method of payment to minimize its cash outlays. Issuances are to those willing to accept payment in stock, which are typically communications consulting firms. The Company does not anticipate using this method of payment with vendors and contractors in the future.
Marketing and Sales Expenses — Marketing and sales costs decreased $1,743, or 1.3%, from $131,891 to $130,148.
Research and Development Expenses — Research and development costs increased $61,088, or 33.4%, from $182,973 to $244,061. The increase was due principally to increased research and development payroll costs. All costs incurred to establish the technological feasibility of a product to be sold, leased or otherwise marketed are expensed as research and development costs. This increase occurred despite the absence in the first quarter of 2015 of certain expenses incurred in the first quarter of 2014 for fees paid to the University of California, Riverside.
Loss from Operations — For the reasons discussed above, our consolidated loss from operations increased $588,428, or 58.7%, from $1,001,889 to $1,590,317, from the three months ended March 31, 2014 to the three months ended March 31, 2015.
Other Income (Expense) — Our consolidated other income and expense includes amortization of deferred financing costs and debt discount, change in the value of derivative liability, loss on settlement of accounts payable and other income.
• Amortization of deferred financing costs increased $19,227, from $0 to $19,227, from the three months ended March 31, 2014 to the three months ended March 31, 2015. The increase was due to the amortization of the deferred offering costs on the convertible notes payable issued in October 2014.
• Amortization of debt discount increased $124,944, from $0 to $124,944, from the three months ended March 31, 2014 to the three months ended March 31, 2015. The increase was attributable to amortization of the debt discount associated with the note conversion feature on the convertible notes payable issued in October 2014.
42
• Change in fair value of derivative liability increased $18,205, from $0 to a gain of $18,205, from the three months ended March 31, 2014 to the three months ended March 31, 2015. The gain was associated with a change in value of the note conversion feature on the convertible notes payable issued in October 2014. The gain occurred due to the convertible notes moving three months closer to maturity.
• Loss on settlement on accounts payable decreased $8,354, from $8,354 to $0, from the three months ended March 31, 2014 to the three months ended March 31, 2015. The loss in the prior year was attributable to the difference between the fair value of the warrants issued to settle accounts payable and the value of the accounts payable settled.
• Other income decreased $9,700 or 97.0%, from $10,000 to $300, from the three months ended March 31, 2014 to the three months ended March 31, 2015. The decrease was due to contract revenue earned in the first quarter of 2014.
Net Loss. For the reasons discussed above, our consolidated net loss increased $715,740, or 71.6%, from $1,000,243 to $1,715,983, from the three months ended March 31, 2014 to the three months ended March 31, 2015.
Year Ended December 31, 2014 Compared to Year Ended December 31, 2013
Revenues. The Company’s consolidated revenues were $109,688 in the year ended December 31, 2013, and $0 in the year ended December 31, 2014. The Company’s product candidates are in development and the Company has no products available for sale. The $109,688 in the 2013 period related to fees of $74,688 charged in connection with our ieExpo conference and the revenue earned from an electronic analyzer development contract of $35,000.
The Company’s cost of revenues were $98,004 in the year ended December 31, 2013, and $0 in the year ended December 31, 2014. Cost of revenues in the 2013 period included principally catering, hospitality services and facilities rental for the ieExpo conference.
Operating Expenses. The Company’s consolidated operating expenses for the years ended December 31, 2014 and 2013 consisted of the following:
|
|
|
Years ended December 31, |
|
Increases, 2013 to 2014 |
||||||||
|
|
|
2014 |
|
2013 |
|
$ |
|
% |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
General and administrative expenses |
|
$ |
3,516,043 |
|
$ |
2,762,461 |
|
$ |
753,582 |
|
27.3 |
% |
|
Marketing and sales |
|
|
676,710 |
|
|
317,557 |
|
|
359,153 |
|
113.1 |
% |
|
Research and development |
|
|
702,376 |
|
|
571,070 |
|
|
131,306 |
|
23.0 |
% |
|
Total Operating Expenses |
|
$ |
4,895,129 |
|
$ |
3,651,088 |
|
$ |
1,244,041 |
|
34.1 |
% |
The individual components of our consolidated operating expenses are discussed below.
General and Administrative Expenses — General and administrative expenses include payroll, professional and consultant fees, stock-based compensation and stock issued for services. From year ended December 31, 2013 to year ended December 31, 2014, general and administrative expenses increased $753,582, or 27.3%, from $2,762,461 to $3,516,043. The increase was due principally to the following:
• Payroll expense increased $786,305, or 101.9%, from $771,618 to $1,557,923, due to the hiring of additional executive personnel to lead product development efforts and strategic marketing and partnership development efforts, in anticipation of the sale and distribution of products when available.
• Professional consultant fees increased $72,824, or 16.3%, from $448,126 to $520,950, due to increased legal, audit and consultant fees. Increased legal fees were the result of Company fundraising efforts and patent filings. Audit fees increased in connection with the Company’s engagement of a new auditor to re-audit and preparations to begin reporting as a public company. Consultant fees increased due to product development efforts.
• Stock-based compensation cost decreased $13,838, or 1.9%, from $714,905 to $701,067. See “Executive Compensation” for a discussion of our compensation policies.
43
• The cost of stock issued for services decreased $270,520, or 76.7%, from $352,915 to $82,395. The decrease was due primarily to fewer shares of Company common stock being issued to vendors and contractors. The Company has from time-to-time used this method of payment to minimize its cash outlays. Issuances are to those willing to accept payment in stock, which are typically communications consulting firms. The Company does not anticipate using this method of payment with vendors and contractors in the future.
Marketing and Sales Expenses — Marketing and sales costs increased $359,153, or 113.1%, from $317,557 to $676,710. The increase was due primarily to increased fees for marketing consultants who had been engaged to provide product branding services.
Research and Development Expenses — Research and development costs increased $131,306, or 23.0%, from $571,070 to $702,376. The increase was due principally to increased research and development payroll costs. All costs incurred to establish the technological feasibility of a product to be sold, leased or otherwise marketed are expensed as research and development costs.
Loss from Operations — For the reasons discussed above, our consolidated loss from operations increased $1,255,725, or 34.5%, from $3,639,404 to $4,895,129, from the year ended December 31, 2013 to the year ended December 31, 2014.
Other Income (Expense) — Our consolidated other income and expense includes income from government grants, interest income, research collaboration and development contracts, other income and certain expenses such as amortization of deferred financing costs and debt discount, change in the value of derivative liability and loss on settlement of accounts payable.
• Government grants decreased $271,636, from $271,636 to $0, from the year ended December 31, 2013 to the year ended December 31, 2014. The 2013 grants were $180,636 from the National Institutes of Health to OLI and $91,000 from the City of Riverside, CA to ieCrowd. In 2014, no grants were awarded. The Company and its operating subsidiaries seek to identify and apply for government and private grants from time to time, when grant opportunities relate to their research and development efforts. However, we do not rely on, or budget for, the receipt of grants in order to meet our liquidity or capital needs. As a result, we do not expect to face any cash or financing shortfall at any time in the future due to any failure to receive grants.
• Amortization of deferred financing costs increased $28,297, from $0 to $28,297, from the year ended December 31, 2013 to the year ended December 31, 2014. The increase is due to the amortization of the deferred offering costs on the convertible notes payable issued in October 2014.
• Amortization of debt discount increased $45,996, or 79.1%, from $58,124 to $104,120 from the year ended December 31, 2013 to the year ended December 31, 2014. The increase is attributable to amortization of the debt discount associated with the note conversion feature on the convertible notes payable issued in October 2014.
• Change in fair value of derivative liability increased $66,505, from $0 to a gain of $66,505, from the year ended December 31, 2013 to the year ended December 31, 2014. The gain was associated a change in value of the note conversion feature on the convertible notes payable issued in October 2014. The gain occurred due to the convertible notes moving 2.5 months closer to maturity.
• Loss on settlement on accounts payable increased $8,354, from $0 to $8,354, from the year ended December 31, 2013 to the year ended December 31, 2014. The loss was attributable to the difference between the fair value of the warrants issued to settle accounts payable and the value of the accounts payable settled.
• Other income increased $15,491, or 106.8%, from $14,509 to $30,000, from the year ended December 31, 2013 to the year ended December 31, 2014. The increase was due to consulting fees earned of $20,000.
Net Loss. For the reasons discussed above, our consolidated net loss increased $1,528,012, or 44.8%, from $3,411,383 to $4,939,395, from the year ended December 31, 2013 to the year ended December 31, 2014.
44
Liquidity and Capital Resources
As of March 31, 2015, we had cash and cash equivalents of $544,699 and a working capital deficiency of $890,684. Our operations generally have negative operating cash flows and our working capital and capital investment requirements have been and will continue to be significant. As a result, we depend substantially on financing activities to provide us with the liquidity and capital resources we need to meet our working capital requirements and to make capital investments in connection with ongoing operations and new product commercialization efforts. Since our inception, we have covered these requirements primarily by making private sales of our common stock, issuing debt instruments and through the exercise of warrants.
In addition, from time to time in the past, the Company and its operating subsidiaries have applied for and received government and private grants for research and development efforts. We expect to continue to apply for and receive such grants from time to time in the future. Since the inception of our business, the Company and its operating subsidiaries have received $281,636 from grant sources, including $0 in the three months to March 31, 2015 of $0 in 2014. However, we do not rely on, or budget for, the receipt of grants in order to meet our liquidity or capital needs. As a result, we do not expect to face any cash or financing shortfall at any time in the future due to any failure to receive grants.
There are a number of risks to investors associated with our financial condition. The sale of additional equity securities, or the issuance of debt convertible into equity securities, could result in dilution to our stockholders. We do not have any credit facilities or other access to bank credit. In the event we could raise long-term debt finance, however, its incurrence would result in increased fixed obligations and could result in our being subject to covenants that would restrict our operations. In all events, there can be no assurance that we will be able to raise additional capital to the extent we require it, when we require it, on favorable terms, or at all. See “Risk Factors” for further discussion of the risks inherent in any investment in our securities, given our need for capital, the early stage of our operations and our continuing losses and working capital shortfalls.
Cash Flows and Cash Balances
Three Months Ended March 31, 2015 and 2014
Cash Flows from Operating Activities — During the three months ended March 31, 2015, our operating activities used net cash of $1,228,667, compared to $715,310 of net cash used in our operating activities during the three months ended March 31, 2014. Our principal operating sources and uses of cash during the 2015 period included:
• $161,626 provided by increasing accounts payable, resulting from efforts to manage our outstanding vendor balances.
Cash Flows Used In Investing Activities — During the three months ended March 31, 2015, we used net cash of $30,412 for equipment purchases associated with the expansion of our operations. During the three months ended March 31, 2014, we used $5,591 of net cash for the same purpose.
Cash Flows Provided By Financing Activities — During the three months ended March 31, 2015, we used $77,998 in net cash from financing activities as a result of repayment of a loan and payment of deferred offering costs partially offset by proceeds received from a promissory note. During the three months ended March 31, 2014, we generated $1,503,250 in net cash from financing activities, primarily as a result of receiving the proceeds of the private placement of common stock and partially offset by a repayment of a loan.
Since inception, our principal capital-raising activities have included the following:
• The sale of common stock to private investors, from which we have generated net proceeds of $7,260,314; and
• The exercise of warrants for common stock totaling $500,000;
• The 2014 Private Placement, which we closed in October 2014 and from which we generated net proceeds of $2,631,190; and
• The 2015 Private Placement, from which we have received proceeds of $2,100,000 as of the date hereof.
45
In March 2015, we commenced a “best efforts” private placement offering of up to $6,000,000 of 8% unsecured convertible promissory notes, or the “2015 Convertible Notes,” pursuant to Rule 506(b) of Regulation D of the Securities Act and the JOBS Act. The terms of these Convertible Notes are substantially identical to the terms of the Convertible Notes issued in the 2014 Private Placement. We refer to this offering as the “2015 Private Placement.” As of the date hereof, we have received proceeds of $2,100,000 from the 2015 Private Placement.
On July 14, 2014, we commenced a “best efforts” private placement offering of up to $5,000,000 of 8% unsecured convertible promissory notes, or the “2014 Convertible Notes,” pursuant to Rule 506(c) of Regulation D of the Securities Act and the JOBS Act. In addition, simultaneously on July 14, 2014, we commenced a “best efforts” private placement offering of up to $2,500,000 of 2014 Convertible Notes pursuant to Regulation S of the Securities Act. We refer to these offerings collectively as the “2014 Private Placement.” The final closing of the 2014 Private Placement occurred on October 14, 2014, pursuant to which we issued Convertible Notes in the aggregate amount of $2,785,000.
The 2015 and 2014 Convertible Notes will mature 24 months from their respective issuance dates unless converted earlier, including immediately after the closing of our initial public offering or a transaction pursuant to which we receive at least $7,500,000 in gross proceeds. Immediately after the closing of this offering, the Company will exercise its right to convert all of the Convertible Notes into Units at a conversion price per Unit equal to 80% of the price at which the Units are being offered to the public in this offering. There is no minimum amount of securities that must be sold in the Company’s initial public offering for the conversion of all of the Convertible Notes to occur, and the conversion will occur even if only a few Units are sold.
Cash Balances — As a result principally of the activities discussed above, as of March 31, 2015 we had cash and cash equivalents of $544,699. As of December 31, 2014, we had cash and cash equivalents of $1,881,776.
Cash Flows and Cash Balances
Year Ended December 31, 2014
Cash Flows from Operating Activities — During the year ended December 31, 2014, our operating activities used net cash of $3,973,743, compared to $1,413,006 of net cash used in our operating activities during the year ended December 31, 2013. Our principal operating sources and uses of cash during the 2014 period included:
• $133,430 provided by increasing accounts payable, resulting from efforts to manage our outstanding vendor balances.
• $83,542 used to reduce accrued expenses and other current liabilities, resulting from efforts to reduce certain accrued expenses, including payroll, as well as operating expenses associated with the expansion of our operations.
Cash Flows Used In Investing Activities — During the year ended December 31, 2014, we used net cash of $25,613 for equipment purchases associated with the expansion of our operations. During the year ended December 31, 2013, we used $3,995 of net cash for the same purpose.
Cash Flows Provided By Financing Activities — During the year ended December 31, 2014, we generated $5,158,396 in net cash from financing activities as a result of receiving the proceeds of the private placement of common stock we commenced in December 2013 and completed in 2014, a private placement of convertible notes completed in October 2014 and warrants exercised in July 2014. During the year ended December 31, 2013, we generated $1,754,155 in net cash from financing activities, primarily as a result of receiving the proceeds of the private placement of common stock we commenced in February 2013 and proceeds from notes payable issued.
Cash Balances — As a result principally of the activities discussed above, as of December 31, 2014, we had cash and cash equivalents of $1,881,776. As of December 31, 2013, we had cash and cash equivalents of $722,736.
Year Ended December 31, 2013
Cash Flows from Operating Activities — During 2013, our operating activities used net cash of $1,413,006, compared to $1,718,343 of net cash used in our operating activities during 2012. Our principal operating sources and uses of cash during 2013 included:
46
• $97,691 provided by increasing accounts payable, resulting from efforts to manage our outstanding vendor balances;
• $513,037 from accrued expenses arising from payroll, marketing cost and accrued Kite Patch production cost, as described elsewhere in this document; and
• $210,370 of deferred revenue from the Kite Patch Campaign, which will not be recognized as revenue until the completion of Kite Patch commercialization and the delivery of products.
Cash Flows Used In Investing Activities — During 2013, we used net cash of $3,995 for equipment purchases associated with the expansion of our operations. During 2012, we used $220,527 of net cash for the build-out of the OLI laboratory facilities.
Cash Flows Provided By Financing Activities — During 2013, we generated $1,754,155 in net cash from financing activities, principally as a result of our February 2013 private placement of common stock, which raised $1,627,000, an issuance of promissory notes in December 2013 for advances of ieExpo costs in the principal amount of $123,583 ($122,050 as of December 31, 2013) and a third-party investment of $4,950 at a subsidiary. During 2012, we generated $1,910,504 in net cash from financing activities, as a result of common stock private placements during that period.
Cash Balances — As a result principally of the activities discussed above, as of December 31, 2013, we had cash and cash equivalents of $722,736. As of December 31, 2012, we had cash and cash equivalents of $385,582.
Capital Expenditures
Capital expenditures totaled $30,412 during the three months ended March 31, 2015 for equipment purchases and $5,591 during the three months ended March 31, 2014 for a vehicle.
Capital expenditures totaled $3,995 in 2013 for equipment purchases and $25,613 in 2014 for leasehold improvements, equipment and a vehicle.
Credit Facilities
We do not have any credit facilities or other access to bank credit.
Contractual Obligations, Commitments and Contingencies
We are not subject to any material contingencies.
The Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. Such matters are subject to many uncertainties, and outcomes are not predictable with assurance. Currently, there are no such matters deemed material to the Company.
The Company leases 6,900 square feet of office space in Riverside, CA. The lease agreement runs through October 31, 2015 and imposes rent on an annual escalating basis, currently at $15,144 per month. Payments under this lease are being accounted for on a straight-line basis. The Company also leases 11,846 square feet of laboratory and office space at a nearby location. The lease agreement runs through April 30, 2016 and imposes rent of $7,015 per month. The Company’s total rent expense for the three months ended March 31, 2015 was $61,914 and for the years ended December 31, 2014 and 2013 was $245,505 and $241,285, respectively.
The Company is subject to material contractual obligations and commitments under our various licensing agreements. In general, the continuation of the exclusivity of our rights, and the continuation of non-exclusive rights if exclusivity has been lost or has not been granted, is subject to our payment of various amounts and our achievement of various milestones over time toward the commercialization of the licensed innovations. The material payment obligations applying to our license agreements are summarized below:
• OLI license. As partial consideration for the license, the Company paid a one-time license issue fee of $10,000. The Company must pay an earned royalty of 3% of net sales of licensed products upon becoming cash-flow positive or starting 18 months after the effective date of the license, January 29, 2010, whichever is earlier. The Company must also pay 3% of all non-royalty consideration received
47
from sublicensing. OLI must pay the Board of Regents of the University of California (“the Regents”) a minimum royalty payment of $30,000 annually, irrespective of whether or not OLI has generated any revenue.
• NEA license. As partial consideration for the license, the Company paid a one-time license issue fee of $10,000. The Company must pay an earned royalty of 3% of net sales of licensed products upon becoming cash-flow positive or starting 18 months after the effective date of the license, March 31, 2010, whichever is earlier. The Company must also pay 3% of all non-royalty consideration received from sublicensing. For the year 2017, and for each year thereafter for the duration of the license, The Company must pay the greater of the earned royalty described above or a minimum annual royalty of $5,000 in 2017 and $10,000 in 2018 and each year thereafter.
• SOS license. The Company must pay an earned royalty of 3% of net sales of licensed products. The Company must also pay 50% of all non-royalty consideration received from sublicensing. These fees are reduced, in a tiered fashion, to 15%, upon completion of certain revenue and business performance milestones. In addition, on the anniversary date of entering into the license, October 21, 2013, the Company must pay the following amounts: on the first anniversary, $10,000; on the second anniversary, $15,000; on the third anniversary, $15,000; on the fourth anniversary, $15,000; on the fifth anniversary, $30,000; and on each subsequent anniversary, $30,000. Moreover, after the First Commercial Sale (“FCS”), the Company must pay minimum royalty fees of $25,000 in year one, $37,500 in year two and $50,000 in year three and in each subsequent year.
• Head Pain Management license. As partial consideration for the license, the Company must pay $16,000 within 30 days of entering into the license, November 3, 2014, and another $16,000 within 120 days of entering into the license. The Company must make a milestone payment of $350,000 on the first to occur of: the receipt of a letter of approval or clearance for a 510(k) or PMA application in the U.S. for a licensed product, and the receipt of marketing approval in Europe or any individual European country for a licensed product. In addition, on the anniversary date of entering into the license, the Company must pay $2,500 in each of years one and two, $5,000 in year three and $10,000 in each subsequent year. Moreover, after the First Commercial Sale (“FCS”), the Company must pay minimum royalty fees of $20,000 in year one, $80,000 in year two, $140,000 in each of years three and four and $300,000 in each subsequent year.
• Sleep Disorders license. As partial consideration for the license, the Company must pay $10,000 within 30 days of entering into the license, November 3, 2014, and another $10,000 within 120 days of entering into the license. The Company must make a milestone payment of $140,000 on the first to occur of: the receipt of a letter of approval or clearance for a 510(k) or PMA application in the U.S. for a licensed product, and the receipt of marketing approval in Europe or any individual European country for a licensed product. In addition, on the anniversary date of entering into the license, the Company must pay $2,500 in each of years one and two, $5,000 in year three and $10,000 in each subsequent year. Moreover, after the First Commercial Sale (“FCS”), The Company must pay minimum royalty fees of $20,000 in year one, $80,000 in year two, $140,000 in each of years three and four and $300,000 in each subsequent year.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Quantitative and Qualitative Disclosures about Market Risk
In the ordinary course of our business, we are not exposed to market risks, such as those that may arise from changes in interest rates or changes in foreign currency exchange rates or that may otherwise arise from transactions in derivatives.
Recent Accounting Pronouncements
In April 2015, the Financial Accounting Standards Board issued Accounting Standards Update 2015-03, Interest—Imputation of Interest. To simplify presentation of debt issuance costs, the amendments in this Update would require that debt issuance costs be presented in the balance sheet as a direct deduction from the carrying amount of debt liability, consistent with debt discounts or premiums. The recognition and measurement guidance for
48
debt issuance costs would not be affected by the amendments in this Update. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2014-250—Interest—Imputation of Interest (Subtopic 835-30), which has been deleted. The amendments in this Update are effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company is currently evaluating the effects of ASU 2015-03 on the condensed consolidated financial statements.
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue from Contracts with Customers,” (“ASU 2014-09”). ASU 2014-09 supersedes the revenue recognition requirements in Accounting Standards Codification (“ASC”) 605 - Revenue Recognition, and most industry-specific guidance throughout the ASC. The standard requires that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. ASU 2014-09 is effective on January 1, 2017 and should be applied retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying ASU 2014-09 recognized at the date of initial application. The Company is currently evaluating the impact of the adoption of ASU 2014-09 on its consolidated financial statements.
In June 2014, the FASB issued ASU No. 2014-10, “Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation,” (“ASU 2014-10”). ASU 2014-10 removes the definition of a development stage entity from the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, ASU 2014-10 eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of operations, cash flows, and stockholders’ equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. ASU 2014-10 is effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. Early adoption is permitted. The Company has elected to adopt ASU 2014-10 and, as a result, is not required to present the previously required development stage disclosures.
In June 2014, the FASB issued ASU No. 2014-12, “Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide that a Performance Target Could be Achieved after the Requisite Service Period,” (“ASU 2014-12”). The amendments in ASU 2014-12 require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in ASC Topic No. 718, “Compensation — Stock Compensation” as it relates to awards with performance conditions that affect vesting to account for such awards. The amendments in ASU 2014-12 are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Early adoption is permitted. Entities may apply the amendments in ASU 2014-12 either: (a) prospectively to all awards granted or modified after the effective date; or (b) retrospectively to all awards with performance targets that are outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. The Company does not anticipate that the adoption of ASU 2014-12 will have a material impact on its consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements — Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”). ASU 2014-15, which is effective for annual reporting periods ending after December 15, 2016, extends the responsibility for performing the going-concern assessment to management and contains guidance on how to perform a going-concern assessment and when going-concern disclosures would be required under U.S. GAAP. The Company elected to adopt ASU 2014-15 early. Management’s evaluations regarding the events and conditions that raise substantial doubt regarding the Company’s ability to continue as a going concern have been disclosed in Note 2 to our consolidated financial statements included elsewhere in this prospectus.
In November 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-17, “Business Combinations (Topic 805): Pushdown Accounting” (“ASU 2014-17”). ASU 2014-17 provides an acquired entity with an option to apply pushdown accounting in its separate financial statements upon occurrence of an event in which an acquirer obtains control of the acquired entity. The acquired entity may elect the option to apply pushdown accounting in the reporting period in which the change-in-control event occurs. If pushdown accounting is not applied in the reporting period in which the change-in-control event occurs, an acquired entity will have the option to elect to
49
apply pushdown accounting in a subsequent reporting period as a change in accounting principle in accordance with ASC Topic 250, “Accounting Changes and Error Corrections”. If pushdown accounting is applied to an individual change-in-control event, that election is irrevocable. ASU 2014-17 also requires an acquired entity that elects the option to apply pushdown accounting in its separate financial statements to disclose information in the current reporting period that enables users of financial statements to evaluate the effect of pushdown accounting. The Company has adopted the amendments in ASU 2014-17. The adoption did not have an impact on our consolidated financial statements.
Effects of Inflation
Our results of operations and financial condition are presented based on historical cost. While it is difficult to accurately measure the effects of inflation on our results of operations and financial condition, due to the imprecise nature of the estimates required, we believe such effects, if any, have been immaterial.
50
BUSINESS
Overview
We are an emerging growth company based on a Collaborative Economy model with a mission to bring the world together to unlock the potential of untapped innovations.
We were founded by experienced entrepreneurs who recognized that research institutions can be filled with un-commercialized technology discoveries and breakthrough research (which we refer to throughout this document as “innovations”) which, if successfully commercialized, could solve or help to solve significant global challenges. We believe that the potential of these untapped innovations could be vast. Yet many of these innovations will never see the light of day because of the significant difficulties often experienced in the process of taking laboratory-proven, potentially ground-breaking innovations and transforming them into products, product platforms, services and technologies for distribution to global markets.
These difficulties include the following:
• Innovators are not always entrepreneurs. They may lack the skills, experience or desire to undertake the process of creating products and taking them to market.
• Innovators may not have access to the people and resources needed to move an innovation from the laboratory to the market. In particular, an innovator’s access to investment capital may be limited, because early-stage investors often select investments that have already attracted entrepreneurs and advanced toward commercialization, and avoid investing in innovations alone.
• The process of developing and commercializing innovations, although simple in concept, is difficult in execution. First-time innovators and entrepreneurs may experience high rates of failure.
• There is no efficient system for large, successful, market-driving businesses to learn about innovations with broad or even global market potential. In addition, such businesses are often uninterested in or wary of undertaking the development and commercialization process, and instead prefer to acquire or distribute products already commercialized by others.
As a result of these difficulties, innovations with the potential to improve the lives of people around the globe can often sit languishing in research institutions.
Recognizing the gap between the marketplace and un-commercialized but potentially beneficial innovations, the founders of ieCrowd have created a business model that is intended to bridge this gap. ieCrowd’s business goal is to license or acquire innovations with the potential to solve global problems for the purpose of commercializing them into product platforms, products, services and technologies that have substantial value-adding impact over large populations and markets. The Company’s strategy for attaining this goal incorporates three principal components:
• Licensing innovations with potential global benefits from universities, federal agencies, innovators, businesses and other sources. In the future, we may also acquire innovations outright or acquire the companies that own the innovations;
• Commercializing these innovations, to seek to turn them into products, product platforms, services and technologies. We seek to do this by assigning each innovation to a commercialization team, made up of, among other people, product development members – scientists and other technical subject matter experts – supported by our “business infrastructure” members –personnel who can provide executive leadership, operations management, sales, marketing and public relations functions and administrative services (human resources, legal, finance, accounting). We also provide office space, laboratory space, specialized equipment, raw materials, supplies and other physical resources; and
• Launching these innovations into global markets, which we expect to do if and when our first products are fully commercialized, via licensing these products to regional, national and international market partners and distribution channels, and, in some cases, through direct-to-consumer sales and marketing campaigns.
Now in its fifth year of existence, ieCrowd is in the process of applying its commercialization platform to three innovations, through three separate, majority-owned and ieCrowd-formed and -operated subsidiaries: Olfactor
51
Laboratories, Inc. (“OLI”), Nano Engineered Applications, Inc. (“NEA”) and Smart Oxygen Solutions, Inc. (“SOS”). These subsidiaries have reached the following stages of their development:
• OLI, now in its fourth year of operations, is seeking to commercialize a range of patent-pending technologies to control the behavior of mosquitoes and other blood-seeking insects with non-insecticidal agents, with the aim of lowering the spread of diseases such as malaria, Dengue fever, West Nile virus and other diseases that are transmitted by blood-seeking insects. Transmission of disease from insects to humans is a major global health issue, and OLI is working to create a number of product offerings leveraging these technologies for various markets globally. The Company has chosen “Kite” as the brand under which its expected future repellant and attractant product offerings will be marketed. OLI’s initial product offerings must pass through OLI’s final product development stages, including testing, the determination of manufacturing specifications and consumer brand and packaging finalization. OLI must also pursue regulatory approvals for most of its “Kite”-branded product offerings prior to sale to consumers in most countries. In the U.S., OLI must obtain approval for its Kite-branded products from the EPA. We expect to begin seeking regulatory approvals for the first of our Kite-branded products in or about the first half of 2016 (although we can provide no assurance that we will be able to begin seeking approvals within this timeframe, or at all). We would expect to obtain these approvals within approximately 24 to 36 months from the filing of applications for the approvals (although we can provide no assurance that we will be able to obtain the approvals within that timeframe, or at all). We would expect to repeat this process and timing for later-developed Kite-branded products. OLI is also pursuing the development of a range of product offerings for licensing and public health efforts globally. OLI currently expects to begin selling repellant and attractant products, or licensing the distribution of such products to others, or both, as well as beginning to license active ingredients or exclusive rights to active ingredients, in 2016 (although we can provide no assurance that we will be able to fully develop, and sell or license the distribution of, any such products at that time or any time). From April 25 to May 15, 2015, members of ieCrowd’s Kite team have been in Uganda — in the Jinja area and surrounding rural communities — implementing a series of tests relating to Kite’s technologies in development.
• NEA, now in its fourth year of operations, seeks to commercialize patented and patent-pending sensor technologies based on nano materials that can detect airborne gases to the parts-per-billion (PPB) level. (“Nano materials” are materials sized in at least one dimension between 1 and 100 nanometers, with a nanometer being 10−9 of a meter.) NEA’s technologies, if commercialized, could provide a product platform encompassing multiple consumer, commercial, industrial and security-related products with a variety of applications, including air quality monitoring, explosives detection, industrial plant toxic gas detection, gaseous chemical warfare agent detection, food and agricultural safety hazard detection and other applications. Before any products can be made available to the market, laboratory facility and equipment improvements have to be made, product development (including sensor, algorithm, form factor, software and prototype development) must be completed, testing must be conducted, any necessary regulatory and industry safety and industry standard certifications must be acquired and manufacturing and quality assurance facilities and protocols must be established. We expect to begin seeking regulatory approvals for the first of our NEA-branded products in or about the second half of 2016 (although we can provide no assurance that we will be able to begin seeking approvals within this timeframe, or at all). We would expect to obtain these approvals within approximately three to four months after the beginning of the approval process (although we can provide no assurance that we will be able to obtain the approvals within that timeframe, or at all). We expect to repeat this process and timing for later-developed NEA-branded products. We currently expect to complete these steps and begin licensing the distribution of products to others, and possibly selling some initial consumer products ourselves, in 2016 (although we can provide no assurance that we will be able to fully develop, and license the distribution of or sell, any such products at that time or any time).
• SOS, now in its second year of operations, seeks to commercialize supplemental oxygen delivery devices that would incorporate a computer controlled, patient-adaptive dosing system, for people who have been prescribed supplemental oxygen delivery equipment by a doctor. The devices would be configured as add-ons to existing, commercially available supplemental oxygen delivery equipment. If commercialized, the devices would have the potential to be used by people who have been prescribed supplemental oxygen therapy by their doctor in maintaining a more active lifestyle. The current milestone plan for development of the devices extends through a currently ongoing FDA
52
pre‑submission process and, thereafter, an expected FDA submission process. We cannot currently foresee when our FDA processes may be completed. Potential revenue-earning options will be considered thereafter.
In addition to the foregoing, effective November 3, 2014, we entered into agreements with UCLA to secure licenses, subject to the completion of our due diligence efforts, for two additional innovations:
• Head Pain Management. The other innovation consists of technology designed to take advantage of the fact that stimulating the sensory fibers of a nerve can mask pain perception. The technology uses mechanical vibration (as opposed to electrical stimulation) to stimulate such fibers and is non-invasive and patient controllable. The target market for the commercialization of this technology would include migraine sufferers; those with trigeminal neuropathy, a debilitating, long-lasting oral pain, often arising from failed or compromised dental procedures; and others.
• Sleep Disorders. One innovation consists of technology designed to take advantage of the fact that signals from the limbs interpreted by the brain as movement can elicit enhanced breathing rates. The device we would expect to develop would use a nerve stimulation technique to enhance the breathing rate without actual limb movement. The target market for the commercialization of this technology would include people who suffer sleep disorders mostly due to irregular breathing, the most common of which is sleep apnea.
More recently, in May 2015, we negotiated options to license up to 20 different families of patents and patent applications. The options run for six months, and can be extended for an additional six months thereafter in the sole discretion of ieCrowd. The patents and applications relate to subject matters in a variety of health-related technology areas. The patents and applications are owned or co-owned by the University of New Mexico, Torrey Pines Institute for Molecular Studies, the University of Kansas, New Mexico State University, the University of Michigan, Ball State University and the University of Zurich. ieCrowd intends to use the time afforded to it under the options to continue its due diligence of the patents and applications and decide which patent families, if any, it wishes to license.
ieCrowd intends to pursue commercial success with each of these subsidiaries and innovations, and over time to expand the number of its subsidiaries and innovations. Ultimately, ieCrowd envisions having a group of life and health subsidiaries diversified across various stages of business evolution and various product, service and technology markets. ieCrowd expects that achieving such diversification may help it mitigate some of the risks typically associated with investing in early-stage companies and innovative discoveries and technologies.
The Company does business under the name “ieCrowd” in order to emphasize its Collaborative Economy approach to implementing its business model. By “Collaborative Economy”, we mean a system that emphasizes the sharing, repurposing and distribution of ideas, opportunities and other resources among a great number of participants, typically aided by the use of social media and other information technology, in order to increase the value and efficient deployment of those resources not only for the participants but also for larger populations, societies and the world generally. We refer to the individuals and entities we seek to collaborate with as the “Crowd”. The Crowd includes the people and organizations through which we hope to gain access to expertise, innovations, social and business networks, marketing and distribution opportunities and other resources. The Crowd includes, among others, universities, government agencies, municipalities, non-governmental organizations (“NGOs”), stockholders, innovators, investors, employees, vendors, other stakeholders and collaborators and potential marketing and distribution partners and customers. The Crowd plays an integral role in each stage of ieCrowd’s mission to bring commercialization to bear to help solve global problems.
Innovation Economy Corporation was incorporated in the State of Delaware on October 28, 2010. We are an “emerging growth company” within the meaning of the federal securities laws. For as long as we are an emerging growth company, we will not be required to comply with certain regulatory requirements applicable to other public companies that are not emerging growth companies, including but not limited to: not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; being permitted to comply with reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements; and being exempt from the requirements of holding a non-binding advisory vote on executive compensation and securing stockholder approval of golden parachute payments. We intend to take advantage of these reduced regulatory requirements until we are no longer an emerging growth company. For a description of the qualifications and other requirements applicable to emerging growth companies, and certain elections we have made due to our status as an
53
emerging growth company, see “Risk Factors—We are an ‘Emerging Growth Company’ and we cannot be certain if the reduced disclosure requirements applicable to Emerging Growth Companies will make our common stock less attractive to Investors”.
Market Opportunity
According to the Scholarly Publishing and Academic Resources Coalition’s January 2014 report (available at http://www.sparc.arl.org/news/omnibus-appropriations-bill-codifies-white-house-directive), over $60 billion of taxpayer funds is spent annually on research projects to “advance science, spur the economy, accelerate innovation, and improve the lives of our citizens”. A large number of these research projects end when their results are published and their government funding is complete. Some of this research is worthy of further development, and this is where the ieCrowd process begins. ieCrowd believes that a significant innovation gap exists between laboratory-proven innovations and the global market launch of products and product platforms based on those innovations.
ieCrowd sees significant potential in working with research institutions to search through their research portfolios for possible breakthrough innovations, and then licensing or acquiring the most promising of these innovations for development and commercialization. In regard to these innovations, ieCrowd picks up where government funding left off and seeks to generate value moving the selected innovation through development to commercialization.
Our Key Strengths
There are significant barriers to overcome in taking laboratory-proven technologies and transforming them into products for distribution to global markets. Though simple in concept, the process is difficult in execution. In order to succeed in its business, ieCrowd relies on the following key strengths:
• Past experience: ieCrowd’s management team is comprised of experienced entrepreneurs.
• Business model: ieCrowd believes that its business model brings systemization, efficiency and greater predictability to the task of transforming innovations into market-ready products, services and technologies, and that its Collaborative Economy focus helps it find more ideas and resources and reach more collaborators and potential customers and beneficiaries of its efforts.
• Capital efficiency: ieCrowd targets the licensing or acquisition of intellectual property rights that require low initial capital outlays, and then deploys capital efficiently through the shared services and economies of scale available to the ieCrowd operating subsidiaries through the ieCrowd parent company.
• Parent company-level infrastructure: For each operating subsidiary, ieCrowd provides operations, sales, marketing, public relations and administration (human resources, legal, finance, accounting) functions, as well as capital and executive leadership.
• Key relationships: ieCrowd has a demonstrated track record of identifying and establishing partnerships with research institutions and other key stakeholders.
• Flexibility in seeking revenue: Each ieCrowd operating subsidiary can pursue revenue through licensing, distributorships or retail and direct-to-consumer campaigns.
• Diversification: ieCrowd aims eventually to hold a portfolio of innovations across different phases of development, product types and markets.
54
Our Corporate Organization
ieCrowd currently has three operating subsidiaries, through which it is seeking to commercialize its licensed innovations. Each of these subsidiaries is majority-owned and ieCrowd-formed and -operated: Olfactor Laboratories, Inc. (“OLI”), Nano Engineered Applications, Inc. (“NEA”) and Smart Oxygen Solutions, Inc. (“SOS”) (formerly known as Breathing Technologies, Inc.). The following diagram illustrates the organization of these entities and the ieCrowd parent company in the consolidated ieCrowd group:
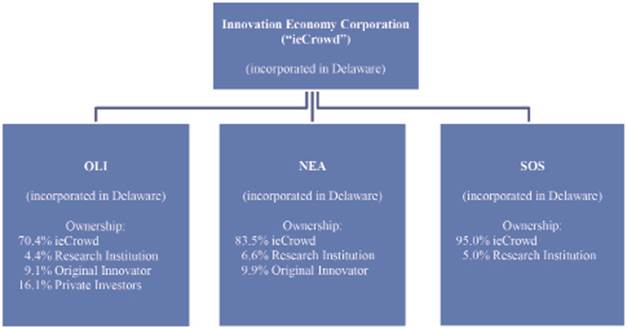
In addition to the foregoing, OLI has issued, to various key OLI employees, options to purchase 675 shares of OLI stock, 592 shares of which have previously vested, 42 shares of which will vest on September 1, 2015 and 41 shares on September 1, 2016. Moreover, in September 2012, the Company granted fully vested options to our President, Dale Hutchins, Chief Discovery Officer, Stephen Abbott, and an OLI employee to purchase a total of 1,028 shares of OLI stock from ieCrowd at an exercise price of $210.00. These options expire in September 2016. If all of the foregoing options were exercised, the key OLI employees would acquire approximately 4.3% of OLI’s capital stock on a fully diluted basis, the employees of ieCrowd would acquire approximately 4.1% of OLI’s capital stock on a fully diluted basis and ieCrowd would have a 63.0% ownership stake in OLI on a fully diluted basis.
NEA and SOS have no options or other potentially dilutive instruments outstanding.
ieCrowd is not the sole owner of its operating subsidiaries, and does not expect to be the sole owner of any future innovation-developing subsidiaries that it may form or acquire, for two reasons. First, in the case of many innovations (including the innovations around which each of our current operating subsidiaries was formed), the university or other research institution where the innovation was initially developed typically makes it a condition of licensing or selling the innovation to us that such institution keep an ownership stake in the innovation’s later development. Second, ieCrowd typically gives the original innovator an ownership stake in the innovation’s development, as part of a policy to encourage maximizing the value of the innovation. In the case of OLI, which was ieCrowd’s first innovation-developing subsidiary, there are also third-party investors with minority ownership stakes. In the future, it is ieCrowd’s intention generally to avoid having such third-party investors in its subsidiaries.
As indicated in the diagram above, it is also a policy of ieCrowd’s to grant, on a case-by-case basis, options, warrants or other rights to acquire subsidiary shares to the original innovator, the originating research institution and employees, again as part of a policy to encourage the maximization of value of the relevant innovations. Options, warrants or other rights, or shares, could also be granted to certain vendors or other partners, if appropriate. Currently, we do not intend to make any grants of options, warrants or other rights, or shares, if as a result of such grants ieCrowd’s fully diluted ownership percentage in any of its subsidiaries would fall below the ownership percentage necessary to retain majority control.
55
ieCrowd — The Parent Company
Business Model
ieCrowd develops its commercialization strategies and methods at the level of its parent company, and then applies them to each of its subsidiaries in order to develop their separate innovations. ieCrowd’s approach is designed to help overcome the difficulties the Company has identified in the current ways individuals, businesses and others try to commercialize laboratory innovations.
ieCrowd’s business model includes the repeated application of the following process:
• DISCOVER — Identify innovations, pre-screen them, subject them to due diligence, license them. ieCrowd’s efforts to locate and secure innovations are overseen by its Chief Discovery Officer. Our discovery process includes the steps described below. As of the date of this prospectus, our innovations pipeline had 9 potential opportunities, from 4 different research institutions, in various stages of review. The opportunities are in various stages of advancement, from initial document review to discussions regarding license or option terms and conditions. The research institutions involved include 3 universities and 1 national laboratory.
• Identification: ieCrowd’s Chief Discovery Officer, along with other Company executives, continually work to develop and maintain ongoing business relationships with key contacts in research institutions, businesses, NGOs and government agencies. Most of these relationships are, or have been, developed through personal introductions, past business and personal relationships and direct “cold call” contacts from ieCrowd personnel. It is principally through these relationships that ieCrowd identifies innovations to evaluate for potential commercialization. On occasion, individual innovators and organizations that have heard of our business approach us.
• Pre-Screening: ieCrowd reviews innovations using a number of selection guidelines, including, as appropriate: whether there is a laboratory-demonstrated proof of concept; whether the innovation has attracted significant prior support through private and public research grants; the potential addressable global market; whether the innovation would improve technologies already in the market; and whether the innovation would provide a platform technology that may lead to multiple market opportunities.
In addition, ieCrowd focuses on commercializing innovations that meet our self-imposed mandate that the potential product, product platform, service or technology “do good”, by which we mean: have a beneficial impact on people’s lives, their communities and our planet. At ieCrowd, we believe that true success for a company should be measured not only by the value it creates for its stockholders, employees and partners, but also by the good it creates in our world.
• Due Diligence: Innovations that have passed pre-screening are subjected to due diligence by ieCrowd’s Discovery Group, which is led by our Chief Discovery Officer and includes, among others, our senior executives, IP attorneys and, as appropriate, other contracted subject matter experts. Our due diligence process is designed to investigate and assess an innovation’s scientific and technical viability; global market viability; legal and financial requirements; and potential social impact. If significant issues are identified during the due diligence period that cannot be resolved to ieCrowd’s satisfaction, ieCrowd will not move forward with the innovation. Under certain circumstances, ieCrowd may negotiate an option to license or otherwise acquire the innovation prior to completing its due diligence, but subject to the completion of due diligence to ieCrowd’s satisfaction.
• Licensing: In the licensing phase, we structure and execute the transactions by which ieCrowd or one of its subsidiaries will take its rights in the innovation. ieCrowd sets up separate subsidiaries for each separate innovation, and the licenses taken reside in the subsidiaries. The specific terms and conditions of each license are negotiated on a case-by-case basis. Generally, however, ieCrowd seeks to license innovations on an exclusive, worldwide basis, in perpetuity, although these terms are on occasion conditioned on our achievement of certain negotiated milestones or minimum performance requirements. The consideration we pay is generally royalty- and license fee-based,
56
although we seek to limit the amounts we may owe in upfront fees. In certain circumstances, we may agree to provide reimbursement for some costs already incurred in respect of the innovation, including for patent protection efforts.
Typically, in the past, ieCrowd has also conveyed to the licensing research institution shares in the relevant ieCrowd subsidiary, subject to ieCrowd retaining majority control over the subsidiary. In the future, ieCrowd expects to seek to license innovations with little or no equity component as consideration. Also, in the past, ieCrowd has on occasion conveyed subsidiary shares or warrants to the individual innovator or innovators. In future, we will evaluate whether to do this on a case-by-case basis. In some cases, ieCrowd may engage the innovator as a technical advisor on a limited consulting basis. However, it is a general goal of ours not to license or otherwise acquire innovations unless they have been developed past the point at which their success is dependent on the continued involvement of any particular individual.
In our first five years of existence, we have established three separate innovation subsidiaries. We have also, in our view, significantly grown and refined our commercialization platform, to the point where we now believe we are ready to substantially increase the pace at which we acquire new innovations and establish new subsidiaries. We expect that, going forward, we may license new innovations and create new subsidiaries at an average rate of approximately 5 per year. Achieving this rate of growth is one of the reasons we are undertaking this IPO. See “Use of Proceeds”.
• DEVELOP — Commercialize innovations through the application of ieCrowd’s commercialization platform. Once ieCrowd has secured a new innovation, it seeks to efficiently and successfully develop the innovation until it is ready to be marketed, by applying what the Company refers to as its “commercialization platform”. Our commercialization platform has the following key components:
• Team: The commercialization team for each project has the following members:
• ieCrowd Leadership Member: ieCrowd’s senior executives assign a member of ieCrowd’s executive team to oversee the commercialization of the innovation. As a commercialization project progresses, ieCrowd may place an individual directly in the relevant subsidiary to lead day-to-day activities, with oversight by the parent company. Currently, as a measure of further control at the subsidiary level, the ieCrowd CEO also holds the position of CEO of each subsidiary.
• Product Development Members: The product development members of the commercialization team are the technical subject matter experts, such as scientists, laboratory technicians, engineers and programmers. Product development team members are placed or hired directly into the relevant subsidiary, to enable those individuals to focus exclusively on the development of that subsidiary’s product platforms, products, services or technologies. The number of product development team members varies from project to project and over time, depending upon the needs of each project.
• Business Infrastructure Members: ieCrowd’s chief-level executives and their support staff constitute the “business infrastructure” members of the commercialization team. Each chief-level executive is responsible for providing each commercialization project with support, resources and the benefit of their expertise, relative to their areas of responsibility. This is an important feature of our model — because these business infrastructure functions are provided by the business infrastructure team members, product development team members can concentrate all of their efforts on technical product development. Our business infrastructure team members also keep ieCrowd’s senior executives apprised of activities across all of our subsidiaries.
Our chief-level executives rely on their own training and experience (as more fully described in the “Management” section of this prospectus) to provide or oversee the provision of the following business infrastructure needs for each operating subsidiary:
• Operations and product development support
57
• Sales, marketing and public relations
• Administration (human resources, legal, finance, accounting)
Business infrastructure support is provided to each operating subsidiary through a management services agreement that the subsidiary enters into with our parent company. This agreement provides for payments to the parent company for the provision of these services. The amounts owed by the subsidiaries under the management services agreements are held on the books of ieCrowd as receivables or notes until such time as the subsidiary has the means to pay the parent company.
• Intellectual Property Member: ieCrowd’s IP legal counsel, Dr. Donna Ward of DT Ward, PC, with offices in Groton, MA and Cambridge, MA, is an active member of each commercialization project.
• Consultant Members: Consultants are engaged and participate as team members when specialized expertise, such as FDA or EPA regulatory experience, is needed for a project. Business infrastructure members are generally responsible for the engagement and management of these consultants.
• Physical and Capital Resources: ieCrowd also provides each operating subsidiary with office space, laboratory space, specialized equipment, raw materials, supplies and other physical resources. ieCrowd’s senior management keeps current on the subsidiary’s cash and capital needs and seeks to address them in the group’s overall capital-raising efforts.
• Efficiencies: ieCrowd’s commercialization approach allows each of its subsidiaries to benefit from the economies of scale presented by the concentration of commercialization personnel and resources in ieCrowd’s parent company. In addition, duplications of effort can be avoided. Finally, the ieCrowd commercialization platform frees scientific and technical experts in our subsidiaries from the need to address business infrastructure issues, allowing them to focus on their areas of technical expertise.
• DEPLOY — Take commercialized innovations to market.
• Once ieCrowd has completed its commercialization of a particular innovation and the resulting products, product platforms, services or technologies are ready to go to market, ieCrowd will commit itself to a market launch on as large a scale globally as is practical. ieCrowd will work to launch each of its future market offerings via three different types of market deployment methods: licensing to market partners; distribution through distribution partners; and through traditional retail and direct-to-consumer marketing and sales campaigns.
While we have yet to advance a technology to the stage of marketing or selling products, or licensing them for distribution by others, we are actively seeking to develop relationships with companies in the Unites States and abroad that could serve as potential licensees, distributors, or marketing or retail partners. In addition, we are working to establish a range of product launch capabilities, including the launching of pre-product campaigns on non-securities crowdfunding websites (e.g., Indiegogo.com); the deployment of national media campaigns; and the development of brands.
Our Deploy efforts will be guided by ieCrowd staff members with experience in sales, marketing, licensing, distribution agreements and retail and direct-to-consumer marketing. The ieCrowd senior management team includes individuals with experience in licensing new innovations and technologies; launching new products to retail markets; developing and executing direct-to‑consumer campaigns; managing multiple retail products and distribution channels; launching e-commerce platforms and offerings; and establishing business-to-business (B2B) and business-to-consumer (B2C) businesses. See “Management” for the biographies of our key executives.
We believe that ieCrowd’s Kite Patch fundraising campaign on Indiegogo.com, in which future OLI products as well as other merchandise was offered in exchange for donations, was
58
a demonstration of our management team’s ability to develop and launch a campaign that can generate global attention and meet (and exceed) financial goals. The Company has chosen “Kite” as the brand under which OLI’s future repellant and attractants product offerings will be marketed, and “Kite Patch” is the name given to one of OLI’s planned future products. In 2013, the Company conducted a six-week crowdfunding campaign on Indiegogo.com to raise funds to support a field test of OLI’s technology in Uganda. The campaign raised $557,254 (or $516,974, net of transaction fees) from 11,254 contributors and as of December 9, 2014 is still listed on Indigogo.com as the most funded campaign in that website’s small business category. The campaign generated significant global media coverage in only six weeks, including coverage by CNN, the BBC, Forbes, Fast Co., Entrepreneur and over 400 more publications. Additionally, the campaign generated over 100,000 social networking mentions, sharing, likes, and commentary. Bill Gates, who otherwise has no involvement with the Company, but whose foundation provided a grant to the University of California, Riverside (“UCR”) to support one of the early stage technologies contributing to the development of the Kite Patch, personally Tweeted to his 12 million followers about a Wired magazine article about Kite. The Kite Patch campaign also generated hundreds of licensing, distribution and direct-to-consumer inquiries relating to the Kite Patch envisioned product, the broader Kite product line that is still in development and the underlying mosquito repellent technology.
Prospective investors should be aware that the Kite Patch Indiegogo.com campaign included drawings, photos and video of the Kite Patch product in a then-envisioned form. However, through the date of this prospectus, the final Kite Patch product does not yet exist in a working, field-tested prototype form. Photos and video from the campaign may still be available on the web, although the Company has taken down, or otherwise posted disclaimers adjacent to, all such photos and video over which it has control.
The Kite Patch campaign led to ieCrowd’s first and, to date, only multi-year distribution partnership. The distribution partner — YuYu Pharma, a Seoul, South Korea-based company — has been granted exclusive rights to distribute any Kite-branded products we may develop in South Korea, Malaysia and Indonesia through March 31, 2023, under an Exclusive License Agreement signed by YuYu Pharma and OLI in March 2014. Under the agreement, no up-front payments were due from YuYu Pharma; in years four through nine of the exclusivity period, YuYu Pharma must make minimum annual payments to OLI, starting at $1 million for the fourth year and increasing each year until reaching $8 million for the last year; and YuYu Pharma agreed to use reasonable efforts to secure regulatory approvals to distribute the Kite-branded products of its choosing in South Korea, Malaysia and Indonesia. However, YuYu Pharma may terminate the agreement at any time without penalty, on notice to OLI. Consequently, there can be no assurance that we will receive any revenue under the agreement, even if we are successful in developing Kite-branded products.
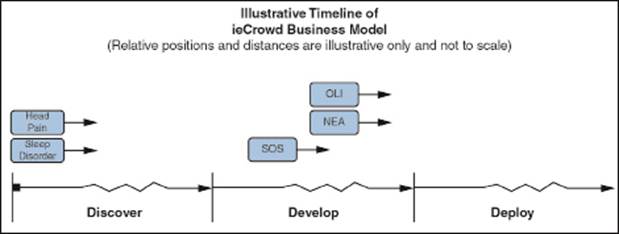
An illustrative timeline of ieCrowd’s Discover-Develop-Deploy business model is shown above.
59
Revenue Model
As an emerging growth company, ieCrowd has not historically earned significant or consistent revenues, and it may not do so in the near future, if at all. See “Management’s Discussion and Analysis of Results of Operations and Financial Condition”. Nevertheless, ieCrowd intends to establish its revenues, and it invests only in innovations that it believes have the potential to achieve substantial revenues, including on a global basis.
Each ieCrowd operating subsidiary will pursue revenue through licensing, sales through distributors and retail and direct-to-consumer marketing and sales campaigns. All of these strategies are contingent on ieCrowd’s operating subsidiaries developing their innovations to the point of market launch. As discussed elsewhere in this prospectus, we do not expect our first products, services or technologies to be ready for market launch until 2016, and there can be no assurance that we will meet that or any market-launch timetable.
Our principal revenue strategies are as follows:
• Licensing Partners: we will target a range of licensing opportunities for our subsidiaries’ product platforms, products, services and technologies, ranging from exclusive licenses with global market partners to specific field-of-use or regional licenses with regional or local market partners. Generally, in the case of licensing arrangements, the market partner would be responsible for product development, manufacturing, distribution and sales, and would compensate ieCrowd in the form of royalties or license fees;
• Distribution Partners: we may distribute our subsidiaries’ products, services and technologies through distribution partners, for penetration into specific markets worldwide. Generally, in the case of distribution arrangements, ieCrowd would be responsible for product development and manufacturing, and the distributor would be responsible for distribution and sales, and would compensate ieCrowd by purchasing products directly from ieCrowd at wholesale prices; and
• Retail and Direct-to-Consumer: ieCrowd subsidiaries, with the support of the parent company’s sales and marketing capabilities, may launch products through traditional retail distribution channels and through direct-to-consumer marketing and sales campaigns, with ieCrowd retaining the responsibility for all product development, manufacturing, distribution and sales.
In addition, as an important additional form of income, in particular while innovations are still being developed, ieCrowd subsidiaries have received, and will routinely continue to seek, Federal, state and private grant money. For example, in 2013 OLI received a grant in the amount of $180,000 from the National Institutes of Health in support of the further development of its insect-repellant product platform.
There can be no assurance that ieCrowd’s revenue model will ever deliver significant or consistent revenues or support profit-making operations. See “Risk Factors”.
Partners
The Company does business under the name “ieCrowd” in order to emphasize its Collaborative Economy model, stakeholders, participants, and potential beneficiaries of its business operations. We refer to these individuals and entities collectively as the “Crowd”. The Crowd includes the people and organizations through which we can gain access to expertise, innovations, social and business networks and resources. When we establish a relationship with a Crowd member who may help us acquire new license rights, secure funding, license or distribute future products or otherwise strengthen and increase the reach of our business, we tend to refer to those Crowd members as our “partners”. Our relationships with our partners can range from informal and temporary associations to structured interactions memorialized in enforceable contracts and licenses.
ieCrowd’s goal is to continuously expand its network of relationships with partners and potential partners, in support of the Company’s business operations. The Company has and will continue to foster relationships with the following types of organizations, among others:
• Research universities
• Federal and state government departments and agencies
60
• Federal and independent research laboratories
• For-profit companies
• Non-profit companies and NGOs
• Foundations
• Communities and city and county governments
• Trade and professional associations
• Crowdfunding websites and other social media platforms
Our focus on developing relationships has helped us form a number of partnerships that we believe have been of tangible value to the Company. For example, from April 25 to May 15, 2015, members of ieCrowd’s Kite team have been in Uganda — in the Jinja area and surrounding rural communities — implementing a series of tests relating to Kite’s technologies in development. They are working with Pilgrim Africa, the Infectious Disease Research Coalition (IDRC) and the Ugandan Ministry of Health. ieCrowd will use the data gathered to help further its Kite product development efforts.
In addition, ieCrowd is in the process of forming a strategic partnership with Children’s Hospital Los Angeles (CHLA), which we expect will provide us with access to certain CHLA-derived innovations that we may wish to consider licensing; access to medical and public health experts who may be able to provide support to our product development efforts; and opportunities to consider entering into collaborations to develop products, services or technologies with a specific focus on pediatric health needs. The partnership is expected to be memorialized in the form of a written, non-binding memorandum of understanding.
The idea for ieCrowd arose initially in connection with the recent world financial crisis and the urgent need for a different approach to transforming innovations into social and economic benefits — a business collaborative approach bringing people and resources together to more effectively commercialize innovations. The City of Riverside became the first regional partner through the Innovation Economy Initiative, a public-private effort to encourage the development of innovation-focused businesses in the Riverside area. ieCrowd secured its corporate headquarters space in Riverside using funds provided by the City of Riverside through the Innovation Economy Initiative. UCR has been a significant partner in ieCrowd’s development since the Company’s early days of operations. ieCrowd licensed its first two innovations from UCR, ieCrowd has sponsored research at the university and a nano-engineering laboratory at UCR has been named the Innovation Economy Corporation Laboratory, in recognition of ieCrowd’s research sponsorship.
ieCrowd Hub
ieCrowd’s success in forming partnerships in the Riverside area has led it to envision creating a network of regional partnership clusters in or around various other U.S. cities. We refer to this potential network as the Regional Innovation Economy Hub program, or “ieCrowd Hub”. ieCrowd’s goal is to work with local partners in each hub to develop an ieCrowd business presence and a network of interconnected relationships. An operating ieCrowd Hub would, we believe, give ieCrowd more access to research institutions, business partners and additional commercialization opportunities in a number of target regions.
Intellectual Property
We consider our license rights, trademarks and other intellectual property to be valuable assets of ours and we seek to both enforce and defend our rights. In regard to the license agreements described below, for a description of the amounts owed in the future by ieCrowd or its subsidiaries to the licensors, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Contractual Obligations, Commitments and Contingencies”.
Licensing Agreements
OLI. On January 29, 2010, OLI and the Regents entered into an exclusive licensing agreement. The agreement was subsequently amended, most recently on September 10, 2012. OLI may not assign the license without the written consent of the Regents, but may sublicense its rights under the terms of the agreement.
61
Pursuant to the agreement, as amended, the Regents granted OLI a license within certain defined fields of use (described below) to make, have made, use, sell, offer for sale and import products and to practice methods covered by specified pending patent applications, and related domestic or foreign patent applications or patents, held by the Regents, in the United States, and in other countries where the Regents may lawfully grant such a license. The fields of use under the license are restricted to use in traps, chemical lures and repellants for certain specified types of insects. Any use outside these fields is excluded.
Should OLI fail to meet certain revenue and business performance milestones set forth in the agreement, the Regents have the right to unilaterally terminate the license or otherwise convert the license to a non-exclusive license at any time. Were the Regents to unilaterally terminate the license or convert it to a non-exclusive license, we do not believe at this time that we would suffer material adverse effects to our business. This is because, subsequent to our licensing of the OLI intellectual property, we discovered chemical compounds that are not covered by the OLI intellectual property and that are, in our view, preferable for us to use in our current product development efforts. Nevertheless, we consider it possible that at some future point in our product development efforts we will wish to use the compounds covered by the OLI intellectual property, and therefore we expect to continue to take the steps necessary to avoid losing our license from the Regents or having it convert to a non-exclusive license.
In exchange for these licensing rights, OLI paid the Regents a cash fee of $10,000 and issued 500 shares of OLI common stock to the Regents on August 9, 2010 (equal to 10% of the then outstanding shares of common stock of OLI). Once OLI is cash flow positive, OLI will be obligated to pay to the Regents a royalty fee equal to 3% or 4% (depending on the nature of the future product) of any future net sales of OLI products developed using the licensed technology covered by the specified patent applications or resulting patents, payable quarterly if and when OLI develops products and generates revenue. Royalty fees for any future sales made below fair market value, as defined in the agreement, must be paid as if such sales were made at fair market value. OLI must pay the Regents a minimum royalty payment of $30,000 annually, irrespective of whether or not OLI has generated any revenue. Late payments bear interest at a rate of 10% per annum.
Until the commencement of sales of products derived from the licensed patent applications or patents, OLI is obligated to submit to the Regents every six months a progress report relating to the commercialization of products derived from the licensed patents. Failure to timely submit a report entitles the Regents to terminate the agreement. We have timely submitted all progress reports to date.
OLI agreed to indemnify the Regents and individuals affiliated with the Regents from all claims arising out of the exercise of the license or any sublicense. OLI is obligated to maintain liability insurance as described in the agreement, until three years after the termination of the agreement.
Unless terminated earlier (by operation of law, upon an event of default under the licensing agreement or upon the filing of a bankruptcy petition or patent challenge by OLI), the license will automatically terminate upon the expiration or abandonment of the licensed patents rights. If granted, patents from the patent portfolio being licensed would have a 20-year expiration period and would expire in 2031 (not including any patent term adjustment).
NEA. On March 31, 2010, NEA and the Regents entered into an exclusive licensing agreement in connection with certain patents held by the Regents. The agreement was subsequently amended, most recently on September 1, 2014. As long as NEA retains exclusive rights under the agreement, NEA retains the right to issue sublicenses in the United States and in other countries where the Regents may lawfully grant such licenses.
Pursuant to the agreement, and as amended, the Regents granted NEA a license with certain defined fields of use (described below) relating to “Bio-receptor Embedded Conductive Polymer Nanowire Sensor Arrays”, “Metal Nanoparticles Decorated Carbon Nanotubes for Gas Sensors”, the “Synthesis of Nanopeapods by Galvanic Displacement of Segmented Nanowires”, “Metal and Metal Oxides Co-Functionalization SWNT’s as High Performance Gas Sensors”, “Ultra-Sensitive Gas Sensors Based on Tellurium-Single Walled Carbon Nanotube Hybrid Nanostructures” and “Selective Nanoscale Asymmetric Gas Sensors”, which we refer to as the NEA Licensed Products. The fields of use under the license are chemical and biochemical detection using carbon nanotube-based sensors and conducting polymer nanowire sensors.
The Regents have the right to terminate the agreement or convert the rights to non-exclusive licenses should NEA fail to achieve certain revenue and business performance milestones set forth in the agreement.
62
Pursuant to the agreement, the Regents granted NEA the exclusive right to make, use and sell the NEA Licensed Products in all territories in which the Regents’ patent rights exist, including the United States. The license is subject to overriding obligations to the U.S. Government, including those set forth in 35 USC Sections 200-212 and applicable governmental implementing regulations and the obligation to report on utilization of the invention set forth in 37 CFR Section 401.14(h). These requirements are commonly referred to as the Bayh-Dole provisions and apply to intellectual property that has been developed using federal government funding. Such intellectual property, if not pursued in a manner to achieve practical application of the inventions, may be subject to the government’s “march-in” rights, by which it can require licenses to be granted in certain circumstances to third parties. In addition, and pursuant to further Bayh-Dole requirements, the agreement requires that NEA Licensed Products be substantially manufactured in the United States, because the inventions relating to the NEA Licensed Products by the Regents were made under funding provided by the U.S. federal government.
NEA paid the Regents a license fee of $10,000, conveyed to the Regents shares of NEA equal to 10% of the then outstanding shares of common stock of NEA and agreed to pay a royalty fee of 3% of all future net sales of NEA Licensed Products. NEA is obligated to pay a minimum royalty of $5,000 in 2017 and $10,000 in 2018 and each year thereafter.
NEA is required to submit to the Regents every six months a progress report summarizing NEA’s activities related to testing and development of NEA Licensed Products. In addition, the progress report must also include milestones achieved or anticipated milestone completion dates, and any activities related to any sublicenses. This reporting requirement will remain in force until the commencement of sale of each NEA Licensed Product. Failure to timely submit a report entitles the Regents to terminate the agreement. We have timely submitted all progress reports to date.
NEA agreed to indemnify the Regents and individuals affiliated with the Regents from all claims arising out of the exercise of the license or any sublicense. NEA is obligated to maintain general liability insurance as described in the agreement, until three years after the termination of the agreement.
Unless terminated earlier (by operation of law, upon an event of default under the licensing agreement or upon the filing of a bankruptcy petition or patent challenge by NEA, or termination for convenience by NEA), the license will automatically terminate upon the expiration or abandonment of the last licensed patents rights. If granted, patents from the patent portfolio being licensed would have a 20-year expiration period and would expire between 2025 and 2033 (not including any patent term adjustment). The termination of the agreement with the Regents will not relieve NEA of its obligations to pay any accrued royalties and other consideration set forth in the agreement.
SOS. On October 21, 2013, SOS and the Regents entered into a licensing agreement in connection with certain patents held by the Regents relating to an Automated Fluid Delivery System, which we refer to as the SOS Licensed Products. Pursuant to the Agreement, the Regents granted SOS the exclusive right to make, use and sell the SOS Licensed Products in all territories in which the Regents’ patent rights exist, including the United States, Europe and China. SOS paid to the Regents a license fee in the form of 5% of the initially issued common stock of SOS, and is obligated to pay a license maintenance fee at one ($10,000), two ($15,000), three ($15,000), four ($15,000), five years ($30,000) and every year thereafter ($30,000). SOS agreed to pay a royalty fee of 3% of all future net sales of SOS Licensed Products and 50% of all non-royalty consideration received from sublicensing, subject to certain adjustments. These fees are reduced, in a tiered fashion, to 15%, upon completion of certain revenue and business performance milestones. In addition, on the anniversary date of entering into the license, we must pay the following amounts: on the first anniversary, $10,000; on the second anniversary, $15,000; on the third anniversary, $15,000; on the fourth anniversary, $15,000; on the fifth anniversary, $30,000; and on each subsequent anniversary, $30,000. Moreover, after the First Commercial Sale (“FCS”), we must pay minimum royalty fees of $25,000 in year one, $37,500 in year two and $50,000 in year three and in each subsequent year.
Head Pain Management. Effective November 3, 2014, ieCrowd and the Regents entered into an agreement that granted to the Company an exclusive license to make, have made, use, sell, offer for sale and import licensed products and to practice licensed methods to treat pain from headaches, migraines, trigeminal neuralgia or neuropathic disorders of the head and mouth. The agreement also includes a non-exclusive license to the copyright over certain user interface software for controlling and monitoring nerve stimulation. The agreement allows for sublicensing with a percentage of income to be paid to the Regents based on sublicense income milestones. Sublicense income due to the Regents includes 25% prior to submission to the FDA of a PMA or 510(k); 15% after submission to the FDA of a PMA or 510(k) but before product approval and 10% after receiving FDA approval.
63
In exchange for these licensing rights, the Company paid the Regents a cash fee of $32,000, with $1,600 attributable to the copyright. License maintenance fees are triggered on the anniversary date of entering into the license and are $2,500 in each of years one and two, $5,000 in year three and $10,000 in each subsequent year, with 5% of the fees attributable to the licensed copyright. Such fees will not be due if the Company is selling a licensed product and paying the earned royalty.
The Company is obligated to pay to the Regents a royalty fee equal to 6% of the net sales of licensed products. There is no royalty due on the copyright. After the First Commercial Sale (“FCS”), we must pay minimum royalty fees of $20,000 in year one, $80,000 in year two, $140,000 in each of years three and four and $300,000 in each subsequent year.
The Company will be obligated to pay the Regents a $350,000 milestone payment on the first to occur of: the receipt of a letter of approval or clearance for a 510(k) or PMA application in the U.S. for a licensed product, and the receipt of marketing approval in Europe or any individual European country for a licensed product.
The Company is obligated to submit to the Regents every six months a progress report relating to the commercialization of products derived from the licenses. Failure to timely submit a report entitles the Regents to terminate the agreement. We have not yet been required to submit a progress report to date.
The Company has agreed to indemnify the Regents and individuals affiliated with the Regents from all claims arising out of the exercise of the license or any sublicense. The Company is obligated to maintain liability insurance as described in the agreement, until three years after the termination of the agreement.
Unless terminated earlier (by operation of law, upon an event of default under the licensing agreement or upon the filing of a bankruptcy petition or patent challenge by the Company), the license will automatically terminate upon the expiration or abandonment of the licensed patents rights. If granted, patents from the patent portfolio being licensed would have a 20-year expiration period and would expire in 2034 (not including any patent term adjustment).
Sleep Disorders. Effective November 3, 2014, ieCrowd and the Regents entered into an agreement that granted to the Company an exclusive license to make, have made, use, sell, offer for sale and import licensed products and to practice licensed methods for treatment of conditions related to sleep-disordered breathing, including sleep apnea, congenital central hypoventilation syndrome and the improvement of sleep quality. The agreement also includes a non-exclusive license to the copyright over certain user interface software for controlling and monitoring nerve stimulation. The agreement allows for sublicensing with a percentage of income paid to the Regents based on sublicense income milestones. Sublicense income due to the Regents includes 25% prior to submission to the FDA of a PMA or 510(k); 15% after submission to the FDA of a PMA or 510(k) but before product approval and 10% after receiving FDA approval.
In exchange for these licensing rights, the Company paid the Regents a cash fee of $20,000 with $1,000 attributable to the copyright. License maintenance fees are triggered on the anniversary date of entering into the license and are $2,500 in each of years one and two, $5,000 in year three and $10,000 in each subsequent year, with 5% of the fees attributable to the licensed copyright. Such fees will not be due if the Company is selling a licensed product and paying the earned royalty.
The Company is obligated to pay to the Regents a royalty fee equal to 4% of the net sales of licensed products. There is no royalty due on the copyright. After the First Commercial Sale (“FCS”), we must pay minimum royalty fees of $20,000 in year one, $80,000 in year two, $140,000 in each of years three and four and $300,000 in each subsequent year.
The Company will be obligated to pay the Regents a $140,000 milestone payment on the first to occur of: the receipt of a letter of approval or clearance for a 510(k) or PMA application in the U.S. for a licensed product, and the receipt of marketing approval in Europe or any individual European country for a licensed product.
The Company is obligated to submit to the Regents every six months a progress report relating to the commercialization of products derived from the licenses. Failure to timely submit a report entitles the Regents to terminate the agreement. We have not yet been required to submit progress report to date.
64
The Company has agreed to indemnify the Regents and individuals affiliated with the Regents from all claims arising out of the exercise of the license or any sublicense. The Company is obligated to maintain liability insurance as described in the agreement, until three years after the termination of the agreement.
Unless terminated earlier (by operation of law, upon an event of default under the licensing agreement or upon the filing of a bankruptcy petition or patent challenge by the Company), the license will automatically terminate upon the expiration or abandonment of the licensed patents rights. If granted, patents from the patent portfolio being licensed would have a 20-year expiration period and would expire in 2034 (not including any patent term adjustment).
Recent Developments
In May 2015, we negotiated options to license up to 20 different families of patents and patent applications. The options run for six months, and can be extended for an additional six months thereafter in the sole discretion of ieCrowd. The patents and applications relate to subject matters in a variety of technology areas, including the treatment of various cancers, ischemic stroke, diabetes and bacterial infections. The patents and applications are owned or co-owned by the University of New Mexico, with certain patent families co-owned by other academic institutions, including the Torrey Pines Institute for Molecular Studies, the University of Kansas, New Mexico State University, the University of Michigan, Ball State University and the University of Zurich.
ieCrowd intends to use the time afforded to it under the options to continue its due diligence of the patents and applications and decide which patent families, if any, it wishes to license.
The opportunity to secure the options arose from ieCrowd’s relationship with one of its principal investors, Sunbelt III Technologies Management, LLC (“Sunbelt”). A company managed by a Sunbelt affiliate had earlier secured options over the patents and applications, and some time thereafter Sunbelt had proposed that the options be conveyed to ieCrowd on arm’s-length terms. Following negotiations, we were able to enter into an agreement with the Sunbelt affiliate that terminated its options, allowing us to negotiate for the options. See “Certain Relationships and Related Party Transactions — Agreement with Company Managed by an Affiliate of Sunbelt Technologies Management”.
Trademarks
We own the registered trademark for “Innovation Economy”. One of our subsidiary companies, OLI, owns a registered trademark for “kite” as a stylized logo. Additionally, we have trademark applications pending for “Democratizing Entrepreneurship”, “Olfactor”, “ieCrowd”, and “Nuuma” in regard to our products and services under development. We have also registered numerous domain names, including, among others: www.iecrowd.com, www.olfactorlabs.com, www.kitepatch.com, www.neaapplications, nuumaair.com, iecorp.co, iecrowdhub.com, iekonnect.com and ieinitiative.com.
Patents
The details of the patents licensed or filed and owned by the ieCrowd subsidiaries are shown in the description of each ieCrowd operating subsidiary. See “— Our Subsidiary, Olfactor Laboratories, Inc.”, “— Our Subsidiary, Nano Engineered Applications, Inc.” and “— Our Subsidiary, Smart Oxygen Solutions, Inc.”, respectively, elsewhere in this Business section.
ieCrowd’s general patent strategy is to license the initial intellectual property for an innovation to be commercialized, followed by ieCrowd subsidiaries filing for additional protections, as appropriate, for advances to the technology made by the subsidiaries.
Competition
ieCrowd believes that its business model represents a new, systematized approach to the commercialization of innovations. Nevertheless, ieCrowd faces competition and potential competition from other entities and enterprises, on a number of fronts:
• Competition for innovations: ieCrowd competes against for-profit and non-profit organizations of various types in seeking to identify and secure commercializable intellectual property from research institutions. Many of these organizations are bigger, better known, better connected and better financed
65
than ieCrowd. In addition, because ieCrowd tries to avoid investing substantial amounts of capital when acquiring its intellectual property rights, it is particularly vulnerable to competition from others who may be willing to pay more for the same rights. Furthermore, in the case of young start-up innovators seeking lucrative exits, ieCrowd’s typical offer to license a technology may be less attractive than other sorts of offers, such as M&A buyout offers.
• Competition for capital: the investor communities from which ieCrowd seeks to raise capital are also targeted by a range of investment funds, crowdfunding companies and venture capital firms. ieCrowd’s value proposition for investors, which is based on an investor’s willingness to invest for a long time period, take high risks and attribute benefit to ieCrowd’s stated goal of investing in opportunities that “do good”, must compete against alternative investment opportunities that may offer higher pecuniary gains, less risk, shorter time horizons for investment and other benefits.
• Competition from non-profit organizations and others: ieCrowd also faces competition from a range of less familiar sources. For example, ieCrowd’s work to commercialize socially beneficial innovations may draw it into competition with the efforts of non-profits, NGOs, foundations and governments to achieve similar goals without the constraint of also pursuing, as ieCrowd does, positive financial returns. Potential markets for ieCrowd products, services and technologies could be disrupted or made less attractive by a foundation, government or other actor spending sizable amounts of money on non-commercial intervention strategies in the same markets.
• Competition facing individual ieCrowd subsidiaries: In addition to the foregoing, each ieCrowd operating subsidiary faces its own competitors and competitive pressures in regard to the specific products, product platforms, services and technologies it is seeking to develop and commercialize. See “— Our Subsidiary, Olfactor Laboratories, Inc.—Competition”, “— Our Subsidiary, Nano Engineered Applications, Inc.—Competition” and “— Our Subsidiary, Smart Oxygen Solutions, Inc.—Competition” elsewhere in this Business section.
Regulation
Given the corporate organization of our various operations, our subsidiaries are more heavily regulated than our parent company, and our regulatory compliance efforts are undertaken principally at the subsidiary level. See “— Our Subsidiary, Olfactor Laboratories, Inc.—Regulation”, “— Our Subsidiary, Nano Engineered Applications, Inc.—Regulation” and “— Our Subsidiary, Smart Oxygen Solutions, Inc.—Regulation” elsewhere in this Business section.
Insurance
We maintain what we believe to be reasonable and customary types and levels of insurance coverage, given the extent of our current operations. We anticipate that, if and when our business expands and matures, we may need to acquire additional types of insurance, such as product liability insurance, and higher levels of insurance coverage.
Employees
ieCrowd and its subsidiaries have in the aggregate 26 full-time employees, 2 part-time employees and 5 independent contractors. They are employed as follows:
• Parent company: 15 full-time employees, 1 part-time employee and 4 independent contractors (1 full‑time and 3 part-time);
• OLI: 8 full-time employees and 1 part-time employee;
• NEA: 3 full-time employees; and
• SOS: 1 part-time independent contractor.
ieCrowd has no unionized employees. Management believes that the relations between the Company and its employees are good.
66
Facilities
Our executive offices are located at 1650 Spruce Street, Suite 500, Riverside, CA 92507. We currently rent this space for approximately $15,000 a month through a lease expiring October 31, 2015. Our 11,846 square-foot OLI laboratory facility is located at 1140 Citrus Street, Riverside, CA 92507. We currently rent this space for $6,872 a month through a lease expiring April 30, 2015. Additionally, we are currently negotiating to rent an additional 6,000 square-foot facility in Riverside, CA to be used as a laboratory for NEA. We do not foresee any significant difficulties in obtaining any required additional space, if needed. We do not own any real property.
Legal Proceedings
From time to time, ieCrowd and its subsidiaries may be subject to lawsuits or other legal proceedings that arise in the ordinary course of business. As of the date of this prospectus, ieCrowd is not aware of any legal proceedings involving it or any of its subsidiaries that may have a material adverse effect on the Company and its consolidated subsidiaries, taken as a whole.
Our Subsidiary, Olfactor Laboratories, Inc.
ieCrowd’s 70.4% owned subsidiary, Olfactor Laboratories, Inc. (“OLI”), is seeking to develop products that will change the behavior of blood-seeking insects, based on chemically modulating those insects’ CO2-detection systems. Mosquitoes, ticks, bed bugs and many other blood-seeking insects use the detection of CO2 as one of the principal mechanisms to find hosts, including humans. The discoveries OLI has licensed arose from research performed at UCR and incorporated into pending patent applications. UCR scientists showed that chemically modulating the mosquito CO2 detection system caused behavioral changes that made mosquitoes less able to find CO2 sources in experimental laboratory tests and semi-field tests.
We believe these discoveries could be developed into three types of new products for managing and controlling of blood-seeking insects:
1) Products that block blood-seeking insects from finding people;
2) Products that lure blood-seeking insects for purposes of surveillance; and
3) Products that lure mosquitoes into traps as an alternative means of mosquito control.
Our hope is that we are able to commercialize new insect-control products that can help people avoid insect bites, and thereby help to substantially reduce the transmission rates of deadly, insect-borne diseases. OLI’s first group of planned future products target mosquitoes, which are both a nuisance and carriers of certain diseases.
The Market
The market for insect control agents is part of the broader market for residential and commercial pest control. Insect pests affect commercial establishments, public health, residences and individuals. Mosquito control products are also used by governmental and public health agencies to control mosquito populations in outdoor areas. Additionally, public health agencies perform indoor residual spraying in some areas to further control mosquito populations and to limit the transmission of diseases such as malaria. The market for mosquito control products is global, because there are over 3,000 species of mosquitoes throughout the world, with different geographic distributions, host preferences, and times of flying and biting activity.
Public health concerns are motivating increased effort in mosquito control around the world. Mosquitoes transmit numerous blood-borne diseases, including malaria, yellow fever, dengue fever, Chikungunya fever, West Nile virus, St. Louis encephalitis and other arboviral encephalitis diseases. Several of these diseases can cause significant morbidity and mortality to those infected. According to the U.S. Centers for Disease Control (CDC) and the World Health Organization (WHO), over half of the world’s population (that is, over 3.4 billion people) live in regions of the world where there is risk of malaria transmission, over 200 million cases of malaria occur each year and these cases result in over 600,000 deaths, the majority of which occur in Africa. (See http://www.cdc.gov/malaria/malaria_worldwide/impact.html.)
67
Product Types
There are a variety of types of insect control products. Some insect pest control products act by killing the insects, while others act by changing their behavior, such as by repelling or attracting them. Furthermore, some insect control products exert their effects on a variety of insects, while others are specialized and act on specific types of insects.
Repellents
Mosquito repellents are used to help people avoid mosquito bites, both for nuisance-avoiding and disease-avoiding purposes. Personal and spatial mosquito repellent products are marketed globally. In developing nations where mosquito-borne diseases pose significant public health problems, WHO, governmental, and non-governmental agencies provide financial support for personal mosquito protection, as well as other forms of mosquito control.
Current mosquito repellent products include a very limited number of active ingredients. Over 500 EPA-registered mosquito repellents include DEET as an active ingredient, making it the most widely used insect repellent on the market. Although widely used, recently published studies from scientists in Europe and the University of Florida have shown that at high levels, DEET can inhibit certain insect and mammalian enzymes that are involved in neuromuscular control. These findings, combined with individual medical case studies in which adverse effects were associated with the use of DEET, have prompted public health organizations such as Health Canada and the American Academy of Pediatrics to issue recommendations that the use of DEET-containing products be limited in children. (See http://www.aap.org/en-us/about-the-aap/aap-press-room/aap-press-room-media-center/Pages/Insect-Repellents.aspx; http://healthycanadians.gc.ca/healthy-living-vie-saine/environment-environnement/pesticides/insect_repellents-insectifuges-eng.php.) Another insect control substance, Permethrin, has been listed by the EPA as a “restricted use” substance due to its high toxicity in aquatic environments, which limits the use of this compound near the vicinity of waterways, the main breeding grounds for mosquitoes. Additionally, the EPA has designated pyrethroids a “likely human carcinogen” at high concentrations.
The U.S. home insect repellent market was valued at $527 million in 2013, according to Spectrum Brands. Spectrum Brands controls 28% of the U.S. market through its subsidiary United Industries Corp., the maker of personal and outdoor repellent products under the brands Cutter and Repel.
Spatial Repellents
Spatial insect repellent products release a repellent in the form of a smoke or vapor into an area. A variety of technologies are used to vaporize the repellent materials by heat (such as coils that are ignited, candles, torches, and heaters with repellent mats), by active dispersion of the repellent with a fan mechanism or by passive vaporization when the repellent is sufficiently volatile to vaporize in ambient conditions.
A research study published in the scientific journal Indoor Air estimated that 45 to 50 billion mosquito coils are used annually by approximately 2 billion people worldwide. Mosquito coils are commonly used in Asia, Africa and South America and for over 100 years have been made from pyrethrum, an extract from the chrysanthemum plant.
Based on our research, the wholesale price for a mosquito coil ranges from $0.01 to $0.25, and the retail price ranges from $0.02 to $0.65. Using the low end of the estimated retail price range of $0.02 per mosquito coil, we estimate total global annual mosquito coil sales to be at least $900 million.
Attractants
There are several mosquito traps currently on the consumer market that aim to control the exposure of people to mosquitoes by luring them away from people and into traps. Most of these traps use CO2 as a mosquito attractant. The CO2 is generated by burning propane, the sublimation of dry ice into CO2 gas or with CO2 gas canisters. These traps are used primarily to control mosquitoes in residential yards, and range from relatively inexpensive ($20-30) to moderately expensive ($200-600) equipment that includes automated controls and timers.
Professional Markets
In the U.S., Europe, Canada, and many other markets, state and local agencies practice integrated pest management (IPM), which aims to limit the amount of insecticide used in the environment to only that amount necessary for
68
the desired level of insect pest population control. Surveillance is a critical part of this strategy, with applications of insecticide made only when pest populations rise to unacceptable levels, or when the prevalence of disease pathogens rises. Typically, surveillance activities are performed by mosquito control and public health agencies at the state or local (county, regional) levels. Budgets for mosquito control agencies vary substantially. In a North Carolina study, available funds in districts throughout the state ranged from nothing to over $50 per resident annually.
Most mosquito control programs use CO2 as an attractant in surveillance traps, which they may supplement with 1-octen-3-ol (“octenol”), other chemical lures sold commercially and light. There are three general methods to generate the CO2 needed for mosquito traps: the burning of propane, the sublimation of dry ice into CO2 gas, and the use of CO2 gas canisters. Although they are commonly used, both gas tanks and dry ice pose challenges in the field, because they are often difficult to obtain, transport and maintain.
We estimate that the annual budget for dry ice used in mosquito surveillance traps in the U.S. is approximately $17.5 million, based on extrapolations from the estimates of the amount of dry ice used by the Coachella Valley Mosquito Control District of California, which has published the acreage they monitor, their number of traps and the number of trap-nights in 2013.
OLI’s Technology
Mosquitoes and many other insects, such as bedbugs, ticks, many flies (such as horse flies, sand flies, deer flies and black flies), midges, fleas and lice have evolved specialized sensory systems that attract them to their blood-hosts. The CO2 in exhaled breath is one of the principal cues used by mosquitoes and bed bugs to track humans and other animals for blood meals. Scientific studies suggest that mosquitoes can detect CO2 from 35 meters away, or more.
The discoveries licensed by OLI identify chemical molecules that change the ability of insects to detect and respond to CO2. Focusing on products that impair the insect’s olfactory system, OLI scientists have identified over 250 single compounds or compositions that alter the normal responses of mosquitoes to CO2. We believe that, if we can commercialize products that use these compounds and compositions, we can make it much more difficult for mosquitoes and other blood-seeking carriers of human disease to find human targets. We intend that these compounds and compositions will serve as active ingredients in a variety of insect repellent and attractant products.
The great majority of the active compositions and many of the active compounds that OLI scientists have discovered are not described in the pending patent applications licensed from the Regents, and are described as novel inventions in pending OLI patent applications.
Currently, OLI scientists are testing the effects of various molecules on the behaviors of mosquito species and bedbugs. These tests provide an initial lab-based assessment of the general or specific nature of the insect behavior modification properties of the compound or composition. Each of our active ingredients and product forms will eventually require field testing in a variety of settings to demonstrate efficacy in preventing mosquito attraction to CO2 sources and human skin odors. Successful field testing and a successful demonstration of efficacy will likely be necessary to secure regulatory reviews, product registrations and acceptance by the market.
Product Development
OLI aims to deliver products that can be used by themselves or with other products to provide superior insect control and environmental safety.
OLI product development requires the selection of appropriate active ingredients and the incorporation of these active ingredients into products that provide the necessary spatial and temporal release of active molecules during the time periods required in order for the product to be effective. OLI intends to develop certain of its products using innovative modes of spatial delivery, which take advantage of the unique chemical, physical and safety properties of the OLI active ingredients. Products derived from OLI active ingredients may also use existing modes of delivery, such as in spray and lotion products that are applied to the skin or clothing, or in mats, coils, aerosols, vaporizers, candles and the like.
69
Each of OLI’s product development projects is managed as a stage-gated project, which must progress through the following stages:
• Discovery (including lab-based experimentation)
• Early Development (progressing to semi-field conditions, and regulatory assessment)
• Advanced Development (field tests, prototyping, interaction with the U.S. Environmental Protection Agency (EPA), including the EPA pre-registration meeting)
• Pre-Market Activities (animal toxicology, environmental testing, market preparation)
• Launch
We consider both our attractant and repellent projects to be in the early stages of Advanced Development. We have performed field tests on attractant prototypes (although we still need to perform additional field studies and prototyping and interact with the EPA). For our repellent prototypes, we are prototyping and planning field tests (though we must perform those field tests, as well as additional prototyping, and interact with the EPA).
We anticipate different formulations of OLI active ingredients will be incorporated into products aimed at different target markets, and may require different characteristics of longevity, mosquito species-specificity, vaporization characteristics, fragrance and possibly many other characteristics desired by particular types of users. For example, for outdoor activities in mosquito-infested areas, we imagine that certain customers will want products that provide protection all day, while other products are desired only for a few hours at night. In addition, we may discover through field testing that products aimed for different geographies may require different active ingredients to achieve appropriate efficacy in repelling particular mosquito species.
We believe that products incorporating OLI’s active ingredients will provide attractive alternatives to current products used for mosquito control purposes in many areas around the world.
OLI’s Intended Products
OLI’s intended mosquito control products have been branded as Kite products. Products may include OLI active ingredients alone, or in combination with other currently marketed insect repellents. Creating such combination products may require business relationships with other companies around the world.
Repellent Products
Most of our Kite repellent active ingredients are approved flavor or fragrance compounds, which are readily available on the market. Costs of these compounds vary, but recent price quotations for compounds of interest were in the $20-500/kg range. OLI has filed U.S. and PCT patent applications seeking intellectual property protection on the active molecules for their uses in insect control.
Kite Patch
OLI’s Kite Patch is intended as an innovative approach to protecting individual humans from mosquitoes. We envision it as a small fabric or plastic patch that would adhere to the user’s clothing, bag, stroller or backpack. The patch would release active ingredients into the air and create a zone of active ingredients around the individual that would block mosquitos’ ability to efficiently find the individual wearing the patch. The patch would work for a period of time, and after its effects have worn off, it could be thrown away. The patch could also be marketed in the form of a pendant or clothing attachment. The design of the Kite Patch is intended to be rugged, simple, and effective. We intend that the Kite Patch will be a superior alternative to current mosquito repellent products.
Kite Yard Repellent
The Kite Yard Repellent is envisioned as a small device that would emit active ingredients to create a zone of protection from mosquito around an outdoor group of people. The Kite Yard Repellent could also be marketed in
70
the form of pellets or granules that would be cast about a yard. OLI scientists have currently selected the candidate active ingredients for the Kite Yard Repellent product, and have a short list of backup candidates. Additional development work is ongoing to identify the optimal placements of the Kite Yard Repellent product, determine the species of mosquitoes that are affected and determine the impact of various environmental variables, such as humidity, temperature, wind and the complex odor environments of mosquito habitats, on the efficacy of the product.
Kite Home Repellent
Many parts of the world lack screens on windows and doors, and in other areas mosquito densities are sufficiently high that mosquitoes enter structures despite the presence of window and door screens. The Kite Home Repellent is envisioned as a small device that emits active ingredients to create a zone of protection from mosquito bites inside of a structure. The Kite Home Repellent must be suitable for use inside of an enclosed space, which diminishes the challenges of environmental influences found in exterior areas, but increases the need for safety and pleasant odors. OLI scientists have currently selected the candidate active ingredients for the Kite Home Repellent product, and have a short list of backup candidates. Additional development work is ongoing to identify the optimal placements of the Kite Home Repellent product, determine the species of mosquitoes that are affected and test for consumer fragrance preferences.
Other Potential Repellent Products
We believe that the active ingredients that we have identified also may be formulated into other more traditional modes of mosquito repellent products, such as sprays or lotions, or traditional area products, such as mats, vaporizers, candles and the like. These products may be developed by ourselves or others, under license from us.
Attractant Products
A variety of mosquito attractant products are planned using OLI active ingredients. Some of these products are intended to serve professionals, such as scientists working with mosquito control agencies, who need more convenient alternatives to dry ice and other CO2 sources for surveillance traps. Other products are intended for users around the world, who use trapping of mosquitoes as an additional form of area mosquito control. These products may include OLI active ingredients alone or in combination with other currently marketed insect attractant products. Creating such combination products may require business relationships with other companies around the world.
Kite Pro
Development of the Kite Pro attractant product will depend on our ability to identify active ingredients that perform as well as CO2 in attracting mosquitoes into a surveillance trap. A short list of active ingredients, and a list of backup candidates, have been created. Recent efforts to create the Kite Pro product have been focused on controlling the release of the active ingredients, and documenting the impact of various control methods on the effectiveness at luring mosquitoes into the surveillance trap. Physical and chemical methods to control release, as well as alternative chemical actives have been explored in OLI’s efforts to meet the characteristics desired in the Kite Pro product. As additional field tests are performed, we may adjust active ingredients for better effectiveness in attracting different species of mosquitoes, for different climatic conditions, or to achieve better temporal or spatial dispersal.
Kite Yard Attractant and Kite Home Attractant Products
Products that use attractants for the purpose of mosquito control (rather than surveillance) either for indoor or outdoor use are regulated in the U.S. by the EPA. We anticipate incorporating OLI active attractant ingredients into existing commercially available, consumer market traps, either through partnerships with the current trap manufacturers or through making our products compatible with existing trap designs. The active ingredients selected for Kite Home Attractant and Kite Yard Attractant products will have many of the same constraints on desirable traits as in the Kite Home Repellent and Kite Yard Repellent products.
71
Other Potential Products
A strategy of great interest today in mosquito and insect control is termed Push-Pull, in which the Push of an insect repellent product located near people is paired with the Pull of an insect attractant product located at a distance from people. OLI believes that novel repellent and attractant products will provide new products that will allow consumers to practice the Push-Pull strategy, providing more powerful solutions for preventing mosquito bites.
OLI has performed laboratory-based screening to identify several molecules with good ability in laboratory tests to attract bed bugs, as well as molecules with the good ability in laboratory tests to repel bed bugs. We may pursue business relationships to couple these active ingredients with others products to produce superior bed bug control products.
Sales and Marketing Strategy
OLI intends to deploy mosquito control products globally. For now, we intend to retain rights for distribution and marketing of OLI products in the U.S. and to use partnerships to expand outside the U.S. OLI products may also be sold directly to consumers with the support of ieCrowd’s sales and marketing expertise. OLI may, in limited cases, launch products through traditional retail and direct to consumer campaigns, with OLI and ieCrowd retaining the responsibility for all product development, manufacturing, distribution and sales.
Partners
OLI will seek licensing and joint development partners who will license OLI’s intended product platforms. In these relationships, we expect that the market partner will be responsible for all aspects of further product development, manufacturing, distribution and sales of products, as well as regulatory approvals that are required to sell the products in the licensed territory.
OLI has used, and will continue to use, paid and unpaid collaboration partners who may help with the discovery, development and performance testing of OLI products. These collaborators include Walter Reed Army Institute of Research, which provided testing of certain OLI products in Peru, Thailand and various U.S. locations; commercial entities; and non-profit organizations that have an interest in OLI products. Furthermore, OLI has collaborations that augment OLI expertise in the area of materials science, in order to help us better achieve the spatial and temporal dispersion of active ingredients that we believe will be necessary in successful products.
Intellectual Property
Patents
OLI has licensed the patent applications described below, which were derived from discoveries in the laboratory of Dr. Anandasankar Ray at UCR. The work at the UCR campus was funded by a variety of public and private agencies, and it is estimated that over $2.6 million of research funding was undertaken by Dr. Ray. The licensed patent applications focus on methods and identification of compounds capable of manipulating CO2 receptors in certain insects, including mosquitoes, bedbugs and other flying blood-seeking insects. Certain of the licensed UCR patent applications have been returned to UCR, primarily because they covered specific applications and markets that we are no longer pursuing (Asian Citrus Psyllid) or because they claim compounds that we do not believe have commercial potential. In addition, OLI has applied for patent protection on discoveries made solely by OLI scientists, and will continue to add to its own portfolio of patents pending and patents.
72
|
Name |
|
Patent Application # |
|
Filing Date |
|
Country |
|
Status |
|
Patent # |
|
Issue |
|
Inventor(s) |
|
Executive License or Original IP |
|
Insect Repellents & Attractants |
|
12/398,164 WO/2010/102049 |
|
3/4/2009 |
|
US/PCT |
|
pending |
|
N/A |
|
N/A |
|
Ray, A Turner, SL |
|
Exclusive License (and terminated by letter March 2014) |
|
Ligands for Olfactory Nuerons |
|
61/325,236 WO/2011/130726 |
|
4/16/2010 |
|
US/PCT |
|
pending |
|
N/A |
|
N/A |
|
Ray, A Boyle, SM |
|
Exclusive License |
|
Odors for Asian Citrus Psyllid, Trapping, Repelling and Control |
|
61/547,559 WO/2013/056176 |
|
10/14/2011 |
|
US/PCT |
|
pending |
|
N/A |
|
N/A |
|
Ray, A Forster, L Gomes, I |
|
Exclusive License (terminated by letter March 2014) |
|
Compositions and Methods for the Attraction and Repulsion of Insects |
|
61/684,242; 61/805,172; 61/858,931; WO/2014/028835 Nationalized US, EP, AE, AP, AU, CA, CN, ID, IL, JP, KR, MX, NG, SA, SG and ZA |
|
8/17/2012 |
|
US/PCT |
|
pending |
|
N/A |
|
N/A |
|
Brown, MA Lomeli, MA Elkashef, S Zion, T Frutos, U |
|
Original IP |
|
Compositions and Methods for Controlling Insect Populations |
|
62,017,963 |
|
6/27/2014
|
|
US/ provisional |
|
pending |
|
N/A |
|
N/A |
|
Brown, MA Lomeli, MA Elkashef, S |
|
Original IP |
|
Compositions and Methods for Capturing, Killing or Relling Bed Bugs |
|
61/932,950 |
|
1/29/2014 |
|
US/ provisional |
|
pending |
|
N/A |
|
N/A |
|
Brown, MA Lomeli, MA Elkashef, S |
|
Original IP |
|
Compositions and Methods for Pest Control |
|
62/058,049 |
|
9/30/2014 |
|
US/ provisional |
|
pending |
|
N/A |
|
N/A |
|
Elkashef, S Lomeli, MA Barkar-McGowan, HL Higa, J Brown, MA Schmid, MB |
|
Original IP |
Government Regulations, Approvals
OLI’s insect repellent and attractant active ingredients are intended for uses that the EPA regulates as pesticides. OLI products may also include devices that will trap or mitigate the effects of insect pests, which the EPA considers pesticide devices. Therefore, most or all of OLI’s intended products will require registration with the EPA. In addition, most U.S. states register or license pesticides for use in those states. Worldwide, most nations have regulations to ensure that products sold in their markets are non-toxic to humans and the environment, including beneficial insects and other wildlife.
EPA registration requires the sponsor company to produce data demonstrating that when the product is used according to its label directions, that there is a reasonable certainty of no harm to human health and that the appropriate use of the product does not pose unreasonable risks to the environment. According to the EPA, the agency generally requires much less data to register a biopesticide than to register a conventional pesticide. Nonetheless, the EPA always conducts rigorous reviews to ensure that pesticides will not have adverse effects on human health or the environment, and therefore requires the submission of a variety of data about the composition, toxicity, degradation and other characteristics of the pesticide.
In the future, we will need to secure legal counsel with expertise in regulatory affairs, so that we can appropriately comply with the regulations of the EPA, US state environmental regulatory agencies and other regulatory agencies around the world. We have not yet secured such legal counsel. Furthermore, regulations can change, and may thereby require revisions to some of our current plans for how we will bring products onto the market.
73
Competition
The development and commercialization of insect repellents and attractants is highly competitive. There are currently approximately 680 skin-applied EPA-registered mosquito repellent products available for sale in the U.S. These products are marketed by over 100 companies. Some of these companies market just one insect repellent, while others, such as S.C. Johnson & Sons, Inc., Avon Products, Inc., ScottsMiracle-Gro Company, United Industries Corporation (a subsidiary of Spectrum Brands Holdings, Inc.), 3M and Sawyer Products market portfolios of insect repellent products.
There are several mosquito traps currently on the market that aim to control the exposure of people to mosquitoes by luring them away from people and into traps. Most of these traps use CO2 as an attractant. These consumer market products include: Mosquito Magnets (American Biophysics), Lentek MK01 (Lentek), Skeeter Vac (BlueRhino), Flowtron Power Trap (Flowtron), Megacatch (Envirosafe), Terminator (Paraclipse), Dragonfly (BioSensory), Mosquito Deleto (Coleman), Sonic Web (Applica, Inc.), Gobblin (MosquitoEater), Black Hole (Advante), Nosquito (Kaz, Inc.) and Bite Shield (Koolatron).
Biological control mechanisms and products are under exploration that involve the purposeful release of genetically modified mosquitoes for the purpose of interbreeding with natural mosquito populations to reduce mosquito population densities. Such approaches are being undertaken by companies such as Oxitech, who are creating sterile male mosquitoes.
We face competition with respect to our intended products, and we expect we will face competition with respect to any additional products we may develop. There can be no assurance that we will be able to complete the development of competitive products and commercialize them on a competitive basis. Our competitors may be able to develop competing or superior technologies and processes, compete more aggressively and sustain their competitive efforts over a longer period of time than we may be able to.
Our Subsidiary, Nano Engineered Applications, Inc.
ieCrowd’s 83.5% owned subsidiary, Nano-Engineered Applications (“NEA”), is seeking to develop nano-materials-based sensors for detecting airborne gases to the parts-per-billion (ppb) level. We are in an early stage of commercializing a core technology platform of gas sensors, which are intended to be easily embedded into existing or new electronic devices, be small in size and use little power, and be put to a variety of uses, including air quality monitoring, food and agricultural safety uses, security-related uses, explosives detection, industrial plant toxic gas detection, gaseous chemical warfare agent detection and other applications.
Examples of existing devices made by others that could incorporate future products from NEA include wearables (such as smart watches and wireless health and fitness monitors), home automation systems, air purifiers, production line systems and professional gas detection equipment. To support the commercial use of our core technology by such product manufacturers and others, we are currently developing certain electronic devices that would serve as components in third-party devices and engaging in application software development. Our future goal is that NEA’s products be adaptable to a wide variety of applications, including portable and stationary gas detection products.
We believe NEA has the capacity to develop gas-sensing products that will represent a substantial improvement over existing technologies. This is because existing technologies are very specific as to which chemical substances (or “analytes”) they can detect and how they detect them. NEA’s technology would include replaceable nano-sensors, useable in either NEA or third-party devices, which could detect a variety of analytes at the same time. In addition, our sensors would be small, easily portable and consume little power. We envision that once our products are fully developed and commercialized, customers would be able to customize their monitoring systems by ordering and installing sensors specifically targeted to one or more analytes of their choosing. Analytes that we expect our sensors to be able to target initially would include carbon monoxide (CO), nitrogen dioxide (NO2), ozone, formaldehyde and other volatile organic compounds (VOCs). Further in the future, we expect we would design sensors to detect a great number of additional analytes, selected in large part based on customer demand.
In both consumer and professional applications, we believe the potential market for the products we hope to develop is extensive, because end users would be able to more accurately, conveniently, efficiently and cheaply inform themselves as to the presence of a large (and expandable) number of specific airborne gases and as to air quality generally. At the consumer level, we believe users with specific health issues such as asthma, respiratory diseases
74
or heart disease would be able to better monitor the air surrounding them. Professional uses could help to detect gas leaks, monitor the exposure of workers and others to airborne analytes, monitor environmental pollution, detect chemicals commonly used in improvised explosive devices and detect food spoilage and contamination when specific gases are emitted. We plan to focus on approximately one area of market potential at a time, depending on such factors as the costs of development and the availability of funding, the amount of estimated additional technology development required, the expected ease or difficulty of reaching the “proof of concept” stage of development, the expected overall development timeline, manufacturability, the quality of available market research, the competitive landscape and access to partners to assist in distribution, marketing and sales. Currently, we are focusing on developing the central product platform from which specific products with specific market applications would be developed.
NEA licenses the underlying technology patents through a license from the Regents in 2010. See “Business — ieCrowd - The Parent Company — Intellectual Property — Licensing Agreements”. More than $2.8M in funding, in the aggregate, from the National Institutes of Health (NIH), Center for Nanoscience Innovation for Defense (CNID), Defense MicroElectronics Activity (DMEA) and other sources was used for research relating to this technology prior to NEA acquiring the license.
The Market
Almost all of us breathe in harmful airborne gases, such as ozone, nitrogen dioxide and sulfur dioxide, every day without knowing it. These gases are difficult to avoid, avert or remove, which makes them all the more problematic for our health. (See EPA, What Are the Six Common Air Pollutants, 2012, available at: www.epa.gov/air/urbanair.) Outdoor air pollution in cities and rural areas is estimated by the World Health Organization (WHO) to have caused 3.7 million premature deaths worldwide in 2012. In addition to outdoor air pollution, indoor smoke is a health risk for an estimated 3 billion people globally who cook and heat their homes with biomass fuels and coal. According to WHO, 4.3 million premature deaths were attributed to indoor air pollution in 2012, with most of the deaths occurring in low- to middle-income countries. (Ambient (outdoor) air quality and health – Fact sheet N°313, WHO, 2014, available at: http://www.who.int/mediacentre/factsheets/fs313/en/.)
By reducing exposure to air pollution levels, we can reduce the burden of disease from stroke, heart disease, lung cancer and chronic and acute respiratory diseases, including asthma. NEA’s technology may potentially be used in a wide variety of applications to test environmental and indoor/outdoor air quality.
NEA’s technology may potentially be used in a wide variety of additional industry sectors, including agriculture, the food and beverage sector, water quality, the military, public safety, manufacturing and the medical sector. The detection of VOCs may be an area of particular opportunity. VOCs are organic chemical compounds whose composition makes it possible for them to evaporate under normal atmospheric conditions of temperature and pressure. They are emitted by a variety of solids and liquids, including common household and workplace items such as paints and lacquers, paint strippers, aerosol sprays, stored fuels, cleaning supplies, pesticides, building materials, furnishings, permanent markers, copiers, printers and craft materials including glues and adhesives. Some VOCs may have short- and long-term adverse health effects. Examples of VOCs include methylene chloride, benzene, perchloroethylene and formaldehyde. (See EPA, Volatile Organic Compounds (VOCs), 2012, available at: www.epa.gov/iaq/voc.html.)
The planned NEA products we envision would fit into a number of market sectors, including gas sensors, wearable electronics (including electronic fitness devices) and indoor air quality monitors. The global gas sensors market was estimated by Grand View Research to be at $1.7 billion in 2012, and is expected to grow at a compound annual growth rate (CAGR) of 5.1% from 2014 to 2020. (Gas Sensors Market Analysis and Segment Forecasts to 2020, March 2014, available at: http://www.grandviewresearch.com/industry-analysis/gas-sensors-market.) As part of its product offerings, NEA expects to include smartphone-enabled wearable products. According to Transparency Market Research, the global wearable technology market stood at $750 million in 2012 and is expected to reach $5.8 billion in 2018, at a CAGR of 40.8% from 2012 to 2018. (Global Wearable Technology Market Research Report 2018, PRWeb, January 14, 2013, available at: http://www.prweb.com/releases/2014/01/prweb11478994.htm.) According to BCC Research, the U.S. market for indoor air quality (IAQ) equipment was valued at over $7.7 billion in 2013 and is projected to grow to $8.1 billion in 2014 and $11.4 billion by 2019, representing a five-year CAGR of 7%. (Report: U.S. Indoor Air Quality Market, September 2014, available at: http://www.bccresearch.com/market-research/environment/indoor-air-quality-market-report-env003e.html.)
75
China Market
China represents an especially large potential market for products we are able to commercialize from our nano-sensor technology. Air pollution is a severe problem in China and has led to concern about the attendant health risks. Only three of the 74 Chinese cities monitored by the central government managed to meet official minimum standards for air quality in 2013, the Ministry of Environmental Protection announced March 2014. (New York Times, Most Chinese Cities Fail Minimum Air Quality Standards, Study Says, March 27, 2014, available at: http://www.nytimes.com/2014/03/28/world/asia/most-chinese-cities-fail-pollution-standard-china-says.html?_r=0.) Chinese concern about air pollution has grown sharply in recent years, according to a 2014 Pew Research Center survey. In that survey, 47% of Chinese citizens said air pollution was a “very big” problem facing the country, up from 31% in 2008. (As China coughs and chokes, public concern about air pollution rises, by Drew Desilver, October 22, 2013, available at: http://www.pewresearch.org/fact-tank/2013/10/22/as-china-coughs-and-chokes-public-concern-about-air-pollution-rises/.)
To combat air pollution, Chinese citizens are increasing their use of purifiers and masks. Total Chinese sales of air purifiers reached $563 million in 2013, according to Daxue Consulting, and Matthieu David-Experton, CEO of Daxue Consulting, has been reported as saying there was an 80-100% growth in sales of air purifiers in China from 2012 to 2013. (theguardian.com, China’s pollution levels spark boom in sale of air purifiers and face masks, by Jennifer Duggan, March 7, 2014, available at: http://www.theguardian.com/environment/2014/mar/07/china‑pollution-smog-air-purifiers-masks.) Chinese state media reported that in February 2014, 217,000 people bought masks in seven days on the online retail platform Tmall.com (a division of Alibaba). One manufacturer, 3M, which sells its products on Tmall, sold out of 26 of its 29 products. (China’s smog suspended, not terminated, February 28, 2014, available at: http://english.peopledaily.com.cn/90882/8549468.html.)
NEA’s Technology
NEA’s core technology platform, which we call “Nuuma”, is a gas sensor in the form of a chip, based on nano materials and capable of detecting airborne gases to the parts-per-billion (ppb) level. (“Nano materials” are materials sized in at least one dimension between 1 and 100 nanometers, with a nanometer being 10−9 of a meter.) The sensor is small and high-density, and built to allow the real-time detection of multiple airborne chemical molecules simultaneously at a low power consumption rate. Using singled-walled carbon nanotubes (SWNT) and solid state electrical sensing, Nuuma is being designed to increase performance while reducing the cost and footprint of the sensing mechanism.
Figure: Sample multiple-analyte sensor
The figure above shows a simulation of a sensor with 14 channels. Each channel would have the ability to detect a specific analyte when exposed to it. Our plan is to design the Nuuma sensor to be miniaturized (as small as 5mm by 5mm) and run using extremely small amounts of power to greatly expand the type, location, reliability, flexibility and accuracy of its operation in diverse settings and for diverse applications. For example, Nuuma chips could be incorporated into small wearable devices (such as activity trackers or smartwatches), allowing consumers to monitor the air quality around them in real time.
76
In addition to Nuuma, we are developing application software that would support multiple NEA product lines. We intend to make such software available for various applications, including smartphones, tablets, personal computers and self-contained stationary devices. In addition, we are developing a cloud-based data repository and reporting software, which would enable real-time gas detection activities to be shared and viewed by a network of voluntary end users.
NEA’s Intended Products
NEA has so far identified four potential product categories:
• NEA Branded Products would carry the NEA brand name “Nuuma” followed by the product name for a specific application. These products would be sold and marketed directly by NEA through sales and marketing strategies yet to be devised.
• NEA White Labeled Products would be available to strategic partners who would rebrand NEA products as their own products.
• NEA Technology Licenses would allow device and product development companies to incorporate NEA’s technology into their product lines.
• NEA Data Licenses would allow companies, organizations and governments to use the gas detection data repository that is expected to be collected and tracked by certain anticipated NEA products; we would expect to market NEA Data Licenses only to the extent that any data privacy issues that might arise in connection with the product were resolved in a commercially acceptable manner.
Before any products can be made available to the market, detailed market research must be carried out, competitive landscape has to be addressed, laboratory facility and equipment improvements or acquisitions have to be made, product development (including sensor, algorithm, form factor, software and prototype development) must be completed, beta-testing must be conducted, any necessary regulatory and industry safety and industry standard certifications must be acquired, manufacturing and quality assurance facilities and protocols must be established and marketing and distribution partner relationships must be established. Beta testing (conducted internally and by third-parties) would involve testing our products in our laboratories as well as field-testing under real-world conditions. None of the products that we are currently developing have reached the completion of their development or the beta-testing stage or are available in any market.
The product candidates that NEA is currently developing are further described below:
NEA Branded Products
Nuuma Personal and Nuuma Air would be smartphone-enabled devices, allowing users to be alerted when specific indoor/outdoor gases known to be harmful to health are detected. We believe the value of these products to consumers would arise principally from the fact that the products would give them sufficiently local, accurate and real-time information about air quality to allow them to take specific actions.
Nuuma Personal. Nuuma Personal would be a wearable activity tracker with a real-time nano sensor designed to detect carbon monoxide and certain VOCs. We believe its customers would include fitness- and health-conscious consumers, niche technology consumers and people with certain health conditions such as heart disease. Users would be able to monitor and track fitness-related metrics in addition to knowing about toxic airborne gas levels around them. The dimensions of the Nuuma Personal device are expected to be approximately 25 mm wide x 55mm long x 12mm high. It would be sold connected to a wristband, bracelet or pendant. We expect that Nuuma Personal will be accompanied by Nuuma Personal software, which would allow users to control the device as well as share information to the Nuuma Personal Cloud, which would act as a repository for the data that multiple Nuuma users might choose to share. Final determination of the exact product look and feel, activity tracking features and analyte detection capabilities would depend on the product deployment strategy, the competitive landscape and prototype field test results.
Nuuma Air. Nuuma Air would be a wearable or stationary device capable of detecting multiple airborne pollutants. We believe its customers would include outdoor and environmental enthusiasts as well as niche technology
77
consumers. Our first version is expected to provide for the detection of carbon monoxide, nitrogen dioxide, ozone and formaldehyde. The dimensions of the Nuuma Air device are expected to be approximately 60mm wide x 60mm long x 20mm high. It would be sold with a holder and different clasps to clip to belts, handbags, backpacks, strollers or bicycles. We expect that Nuuma Air will be accompanied by Nuuma Air software, which would allow users to control the device as well as share information to the Nuuma Personal Cloud, which would act as a repository for the data that multiple Nuuma users might choose to share Final determination of the exact product look and feel and analyte detection capabilities would depend on the product deployment strategy, the competitive landscape and prototype field test results.
Other Branded Products. NEA is currently evaluating other potential branded products, including Nuuma Home and Nuuma Office, to detect analytes specific to the personal, home and office environments. We envision Nuuma Home and Nuuma Office having multiple stationary sensor devices that communicate with a base unit for networked toxic air detection.
NEA Technology Licenses
NEA’s gas sensors could be licensed for future use in a broad spectrum of applications, including:
• Air Quality Monitoring. Gas sensors could help detect air pollution, effluents, toxic spills and hazardous gases that can cause disease, death to humans, damage to other living organisms (including food crops) and damage to the natural environment.
• Agriculture. Air quality issues have become an increasing concern for the agriculture industry. Excessive ammonia emissions, a byproduct of livestock and poultry operations, can be harmful to livestock, humans and the environment as a whole. Personally worn, gas sensors could help alert agriculture workers and their superiors when workers are reaching their permissible exposure limit to ammonia in a given work day (per OSHA standards). Stationary sensors could monitor ammonia levels within animal facilities in order to alert workers to risks to the welfare of the animals.
• Food Packaging & Food Safety. The food packaging industry uses gases such as carbon dioxide in the process of manufacturing and sealing food items for distribution to consumers. Gas sensors could be used to measure and monitor permissible gas levels.
• Homeland Security. Gas sensors could help detect chemical weapons and illegal drugs in various settings, including at ports of entry and in border patrol, governmental and large event locations.
• Industrial. Industrial plants use and produce hazardous and toxic gases that could put their employees and local residents at risk. Gas sensors could help detect the buildup of such gases in order to decrease risks.
• Military. The military has an array of uses for gas sensors, in addition to the obvious use of detecting chemical warfare agents. For example, in the U.S. Navy, sulfide gas detecting systems are used on all surface vessels in spaces containing sanitary waste equipment. Furthermore, in the U.S. Air Force, gas sensors are used in order to detect jet fuel leaks.
• Water Treatment Plants. Water and wastewater treatment often release harmful amounts of chemicals into the air. Gas sensors could help protect employees and the community from toxic and combustible gas hazards such as ammonia, carbon monoxide, chlorine, hydrogen sulfide, nitric oxide, nitrogen dioxide, sulfur dioxide and others.
NEA Data Licenses
To support its technology, NEA is currently developing a cloud-based enterprise application software that will allow end users to track and share content and data relating to gases being detected. If and when NEA devices are used by a substantial number of users, data could be made available through this software to research facilities, news agencies, mapping companies, industries affected by air pollution regulations, environmental organizations and federal, state and local governments. We would expect to market NEA Data Licenses only to the extent that any data privacy issues that might arise in connection with the product were resolved in a commercially acceptable manner.
78
Product Development
Current product development activities for Nuuma Personal and Nuuma Air include team development, lab development, electronics development, materials selection, gas sensing and optimization, pattern recognition algorithm development, mobile application software development, automated and predictive alerts software development, cloud data repository development and regulatory filings. Currently, we have detection capabilities for approximately twenty analytes, and we expect this number will continue to rise. We are currently researching the ability to carry out mass production of sensor chips while ensuring repeatability and consistencies across the manufacturing process. We expect that these activities will be followed by product prototype development, field tests and systems optimization, prior to manufacturing and product launch. Future product development activities may include the development of replaceable chips to detect additional gases for Nuuma Air and other products that NEA may develop. In addition, we will continue to invest in ongoing core technology development activities related to the detection of additional analytes.
Sales and Marketing Strategy
NEA plans to use some or all of the following methods to generate revenues from its intended products:
Retail/Direct to Consumer: NEA may, in limited cases, launch products through traditional retail and direct to consumer campaigns. NEA has not begun direct sales or entered into any sales agreements for any of its intended products.
Master Distributors/OEM: NEA may distribute products via master distributor/OEM partners for penetration into specific markets worldwide. Generally, in the case of master distributor/OEM partnerships, NEA would be responsible for all product development and manufacturing and, once products were sold to a distribution partner, the distribution partner would be responsible for all costs, distribution obligations and sales. On March 19, 2014, NEA entered into an agreement with YuYu Pharma whereby the parties agreed to negotiate an exclusive distribution agreement for South Korea, Malaysia, and Indonesia and NEA agreed not to enter into any agreement regarding the distribution of NEA products in any of those countries with any other parties, during the negotiation period. That period ends on December 31, 2015. NEA has not entered into any other master distributor/OEM agreements or any agreements that might lead to a master distributor/OEM agreement, other than the agreement with YuYu Pharma.
Licensing Partners: NEA may target a range of licensing opportunities for its product platform and products, from exclusive licenses with large, global partners to specific field of use licenses or licenses with regional market partners. NEA has not entered into any such license agreements.
Government Regulations, Approvals and Other Industry Certifications
NEA will comply with all necessary governmental regulations and industry standards to help ensure the quality and safety of its products. NEA believes that the following requirements could apply to its products as currently envisioned:
1. FCC Declaration of Conformity
The FCC Declaration of Conformity, or the FCC label, or the FCC mark, is a certification mark employed on electronic products manufactured or sold in the United States, which certifies that the electromagnetic interference from the device complies with limits approved by the Federal Communications Commission.
2. CSA Mark
CSA International (Canadian Standards Association), a member of the CSA Group, is a provider of product testing and certification services for electrical, mechanical, plumbing, gas and other products. Recognized in the U.S., Canada and around the world, CSA International certification marks indicate that a product, process or service has been tested to a Canadian or U.S. standard and meets the requirements of an applicable CSA standard or other recognized basis for certification. CSA International is accredited by national agencies and organizations, including:
• U.S. Occupational Safety & Health Administration (OSHA)
• American National Standards Institute (ANSI)
• National Voluntary Laboratory Accreditation Program (NVLAP)
79
• National Evaluation Service (NES)
• Standards Council of Canada (SCC)
3. CE Mark
The CE mark, formerly the EC mark, is a mandatory conformity marking for certain products sold within the European Economic Area (EEA). The CE marking is also found on products sold outside the EEA that are manufactured in, or designed to be sold in, the EEA. The CE marking is the manufacturer’s declaration that the product meets the requirements of applicable EC directives.
4. UL Mark
Underwriters Laboratories (UL) is a safety consulting and certification company headquartered in Northbrook, Illinois. It maintains offices in 46 countries. UL is one of several companies approved to perform safety testing by the U.S. Occupational Safety and Health Administration (OSHA).
Intellectual Property
Seven families of patents cover the product platform and products currently being developed by NEA. NEA has licensed the patents below through a license from the Regents in 2010. In addition, NEA has applied for patent protection on discoveries made solely by NEA scientists and engineers and will continue to add to its own portfolio of patents pending and patents. See “Business — ieCrowd - The Parent Company — Intellectual Property — Licensing Agreements”.
|
Name |
|
Patent Application # |
|
Filing Date |
|
Country |
|
Status |
|
Patent # |
|
Issue Date |
|
Inventor(s) |
|
Exclusive License or Original IP |
|
Conducting Polymer Nanowire Sensors |
|
11/259,557 |
|
10/25/2005 |
|
US |
|
Granted |
|
8,034,222 B2 |
|
10/11/2011 (expires 9/3/2029) |
|
Myung, Mulchandani and Chen |
|
Exclusive License |
|
Nanomaterial-Based Gas Sensor |
|
13/672,632 |
|
5/7/2009 |
|
US |
|
Granted |
|
8,683,672 |
|
04/01/2014 (expires 11/10/2027) |
|
Bosze, Deshusses, and Myung |
|
Exclusive License |
|
Metal and Metal Oxide Co-functionalized Single-Walled Carbon Nanotubes for High Performance Gas Sensors |
|
13/111,452 PCT/US2011/000896 |
|
5/19/2010 5/19/2010 |
|
US/PCT |
|
Pending |
|
N/A |
|
N/A |
|
Myung, Mubeen, Mulchandani, and Deshusses |
|
Exclusive License |
|
Synthesis of Nanopeapods by Galvanic Displacement of Segmented Nanowires |
|
13/113,623 PCT/US2011/000918 |
|
5/21/2010 5/23/2011 |
|
US/PCT |
|
Pending |
|
N/A |
|
N/A |
|
Myung and Hangarter |
|
Exclusive License |
|
Ultra-Sensitive Gas Sensors Based on Tellurium-Single Walled Carbon Nanotube Hybrid Nanostructures |
|
61/712,023 14/050,932 |
|
10/12/2012 10/10/2013 |
|
US |
|
Pending |
|
N/A |
|
N/A |
|
Myung and Zhang |
|
Exclusive License |
|
Selective Nanoscale Asymmetric Gas Sensors |
|
61/727,181 14/082,349 |
|
11/15/2012 11/18/2014 |
|
US |
|
Pending |
|
N/A |
|
N/A |
|
Myung and Brooks |
|
Exclusive License |
|
Chemical Sensor |
|
62/131,586 |
|
3/11/2015 |
|
US |
|
Pending |
|
N/A |
|
N/A |
|
Doshi, Su, Chen, and Hutchins |
|
Original IP |
|
Chemical Sensor Device |
|
29524749 |
|
4/23/2015 |
|
US |
|
Pending |
|
N/A |
|
N/A |
|
Frandsen and Smiles |
|
Original IP |
|
Case for a Chemical Sensor Device |
|
29524750 |
|
4/23/2015 |
|
US |
|
Pending |
|
N/A |
|
N/A |
|
Frandsen and Smiles |
|
Original IP |
80
Trademark protection is being sought for “Nuuma” and the trademark application is currently held by NEA’s parent company, ieCrowd.
Competition
The development and commercialization of gas sensors is highly competitive. We face competition with respect to our intended products, and we expect we will face competition with respect to any additional products we may develop, from a variety of entities and enterprises. Some of the products and technologies with which we expect to compete are based on scientific and technological approaches similar to ours. We anticipate facing competition principally from large and small manufacturers of commercial and personal chemical detection systems, including Smiths Detection, Riken Keiki Co. Ltd., Thermo Scientific, RAE Systems, SKC Inc., Honeywell Analytics, Analytical Technology, Inc., Sensigent, Owlstone Inc., Kanomax, USA, Inc., Design West Technologies, Inc., NASA’s Ames Research Center and Topac, Inc. We also anticipate facing competition from wearable product companies such as Fitbit, Nike, Jawbone and Microsoft. General competitive pressures in the sensor market may increase if there is an increase in demand for sensors from manufacturers of smartphones and similar technologically advanced and widely adopted products — in particular if the manufacturers are large, well-established companies, such as Samsung. Potential competitors may also include academic institutions, government agencies and other public and private research organizations and private companies that conduct research into, seek patent protection for and establish collaborative arrangements for the research, development, manufacturing and commercialization of gas sensing technologies and devices.
There can be no assurance that we will be able to complete the development of competitive products and be able to commercialize them on a competitive basis. Our competitors may be able to develop competing or superior technologies and processes, compete more aggressively and sustain their competitive efforts over a longer period of time than we may be able to.
Our Subsidiary, Smart Oxygen Solutions, Inc.
ieCrowd’s 95.0% owned subsidiary, Smart Oxygen Solutions, Inc. (“SOS”) (formerly known as Breathing Technologies, Inc.), is seeking to develop a supplemental oxygen delivery device that would incorporate a computer controlled, patient-adaptive dosing system for use by people who require supplemental oxygen therapy. The underlying technology was originally developed at the University of California, San Diego. SOS licensed the underlying technology from the Regents of the University of California in October 2013. See “Business — ieCrowd - The Parent Company — Intellectual Property — Licensing Agreements”.
The Market
The device being developed by SOS is envisioned to be used by people who have been prescribed supplemental oxygen by their doctor. Doctors prescribe supplemental oxygen for many reasons, such as Chronic Obstructive Pulmonary Disease (“COPD”). According to estimates of the World Health Organization (WHO), in 2012 COPD was among the top four causes of death in the world. (Fact sheet N°310, WHO, 2014, available at: http://www.who.int/mediacentre/factsheets/fs310/en/.) As of 2010, in the United States, COPD afflicted over 14.8 million physician-diagnosed patients and an estimated additional 12 million un-diagnosed individuals. (National Institutes of Health, “Morbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Diseases”, page 5, Feb. 2012, available at: http://www.nhlbi.nih.gov/files/docs/research/2012_ChartBook.pdf.)
In 2010, the American Lung Association in California published a “Strategic Plan to Address COPD in California”, in which it estimated that nearly 1.6 million Californians (4.4% of the State’s population) suffered from COPD. (Strategic Plan to Address COPD in California, page 7, available at: http://www.lung.org/associations/states/california/assets/pdfs/programs/strategic-plan-to-address.pdf.)
In China, which is reportedly the home of approximately one third of all the smokers in the world, smoking-related lung diseases, including COPD, are a “major epidemic”. In 2000, in China, the total cost of COPD and other smoking-related diseases was estimated to be more than U.S. $5 billion, and COPD was the second leading cause of death. (ATS Journals, “Breaking Down the ‘Great Wall’ of COPD Care in China”, 2007, available at: http://www.atsjournals.org/doi/full/10.1164/rccm.200706-927ED.)
81
Supplemental oxygen therapy is a major treatment option for COPD. (“Treatment of Respiratory Failure in COPD”, International Journal of Chronic Obstructive Pulmonary Disease, 2008, available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2650592/.) Supplemental oxygen therapy can help increase survival rates and also help improve exercise tolerance and mobility. In addition, it can help patients increase their activity levels, and in that way improve their health and well-being in a variety of ways.
In addition to COPD, other conditions for which doctors might prescribe supplemental oxygen therapy include:
• Pulmonary fibrosis
• Severe kyphoscoliosis
• Neuromuscular disorders
• Cystic fibrosis
• Chronic asthma
• Chronic heart failure
All of these conditions use supplemental oxygen as a therapy.
The size of the supplemental oxygen therapy market was estimated to approximately $1 billion in 2013, with a projected revenue CAGR of 2.2% from 2013 to 2018. (HIS Technology Abstract, “Oxygen Therapy — World — 2014”, 2014 available at: https://technology.ihs.com/api/binary/510021?attachment=true.)
We are not aware of any currently available supplemental oxygen delivery devices that monitor a person’s depth of breath and blood oxygen levels and automatically adjust the amount of oxygen delivered based on the patient’s needs. The SOS device is intended to address these shortfalls. It is being developed to deliver not only the benefit of an oxygen conserving device, but also the benefit of delivering a dose of oxygen commensurate with demand. It could also incorporate technology that could enable a level of remote monitoring of a patient’s use and lung function by health care professionals.
SOS’s Intended Products
The full function products that SOS plans to develop include stationary and mobile versions of a supplemental oxygen delivery control device that uses, for each breath, blood oxygen readings and inhaled breath depth measurements to deliver an appropriate oxygen flow to satisfy the user’s need. As a user requires a greater volume of oxygen, the SOS device would be designed to automatically respond, breath by breath, to deliver the appropriate oxygen dose. Blood oxygen readings would be taken by an integrated pulse oximeter (a non-invasive monitoring device) attached to the user’s finger or ear. Inhaled breath depth measurements would be taken from the air hose that delivers the oxygen to the user. Unlike continuous-flow supplemental oxygen delivery devices, the planned SOS device would not deliver oxygen flow while the user is exhaling, and thus may serve an oxygen conserving function as well.
SOS envisions that its device would be attached to an oxygen cylinder, between the oxygen source and the user. We anticipate that the initial version of the device will use depth of breath as the algorithm component to determine the size of the oxygen dose to be released to the user and that a subsequent version of the device will monitor the blood oxygen level of the user as well as the depth of breath and deliver its oxygen dose based on an algorithm using both measurements.
Certain iterations of the SOS device are intended to include communications capabilities, whereby the device would be able to deliver information to properly secured health monitoring sites, allowing remote patient care monitoring and responses.
Product Development
The SOS device is planned to be a powered unit (battery or plug-in) that is configured to add to and work with existing oxygen delivery equipment. It would be operable by home and mobile users and clinical and hospital staff and patients.
When ieCrowd secured its license over the SOS technology, the inventors had already designed and built a prototype device based in part on the personal knowledge of one of the inventors, who is a lung function disease sufferer and an engineer. There are currently two versions of the prototype, the original version and the advanced version. The
82
original version does not use pulse oximetry as an input into its dosing calculations, but instead relies entirely on inhalation depth to determine the amount of oxygen to deliver. The advanced version uses both pulse oximetry and inhalation depth, and has been used in a formal human proof-of-concept study, which has been funded by the University of California’s Office of the President and conducted at the Pulmonary Clinic of the University of California, San Diego Medical Center. The study has completed its patient contact phase and is now in the data evaluation phase, during which the information collected during the study is being statistically analyzed to determine if any inferences can be drawn about the value of the device. SOS did not participate directly in the study, but via interaction with the Primary Investigator and the study team we understand that the patients in the study experienced no adverse effects from using the device and reported general subjective satisfaction with it. The completion of the data evaluation phase will allow for more formal declarations to be made on the outcomes of the trial. There is currently no set schedule for the publication of the formal trial results.
SOS has done its review of the results of the trials and in SOS’s opinion the data suggests that the efficacy of the device warrants continued effort toward a commercial product.
SOS now plans to move forward with the following product development plan:
• Device Engineering. SOS will do an engineering review of the advanced version of the prototype to ascertain opportunities for improvements. These improvements could include size changes, electronic signal noise changes, power consumption changes, and parts choice changes for efficiency, stability and cost savings and other changes affecting the device’s ability to perform in a targeted deployment. SOS believes that it may choose to develop a number of different device types based on varying sizes, weights and shapes.
• Software. The control software within the unit will potentially go through an optimization process to create a more refined algorithm for calculating dose response.
• Form Factor. The device will likely be put through an industrial engineering review so as to properly encase the operating electronics to comply with regulations, safety concerns and usability metrics.
• FDA and Other Approvals. SOS intends to assess the particular regulatory requirements for taking the product to market. To the extent additional trials may be needed, SOS will work with established trial clinics to design the appropriate protocols to develop the needed data to satisfy regulatory and permitting requirements. See “—Government Regulation, Approvals”.
Because SOS is in the phase of development in which it needs FDA feedback to develop a full business plan, the size, scope and makeup of the team and operating plan needed to execute the product development plan described above is still to be determined.
Sales and Marketing Strategy
SOS’s device is intended to allow people who require supplemental oxygen to live a more active lifestyle and potentially to provide additional opportunities to users to achieve some level of rehabilitation. Because SOS is in the “Discovery” phase of the ieCrowd business model, it has not yet established a go-to-market strategy. Potential options for revenue generation include field of use or geographic territory licenses, master distributor agreements and retail or direct-to-consumer campaigns. SOS currently expects that its initial revenues would be generated by a third-party license.
The intended users of the SOS device are expected to be home, clinical and hospital users. Given the characteristics of the current prototype device, SOS believes that the device would provide price-performance value in all of these settings.
Government Regulation, Approvals
SOS believes that an FDA medical device approval will be required if the device is to be used in the United States. SOS believes that a submission to the FDA would take the form of a Premarket Notification (“PMN”) under Section 510(k) of the Food, Drug and Cosmetic Act. Under Section 510(k), certain device manufacturers must notify the FDA of their intent to market a medical device by filing a PMN at least 90 days in advance of marketing, to allow the FDA to determine whether the device is equivalent to a device already classified by the FDA. SOS believes that its device has many of the characteristics of an oxygen conserving device, and that existing oxygen conserving devices that have FDA approvals would be used as predicates in a SOS device PMN submission.
83
The regulatory portion of the SOS project does include all facets of governmental and industry approvals. SOS additionally envisions the eventual need for either a CE or UL mark and, if a device is developed that delivers information to health monitors, for adhering to any FCC requirements for devices containing communications capabilities.
Intellectual Property
Patent applications covering the core technology that SOS licenses from the Regents have been filed as follows:
|
Name |
|
Patent Application # |
|
Filling |
|
Country |
|
Status |
|
Patent # |
|
Issue |
|
Inventor(s) |
|
Exclusive License or Original IP |
|
Automated Fluid Delivery System and Method |
|
13/814,934 |
|
2/7/2013 |
|
US/PCT |
|
Pending |
|
N/A |
|
N/A |
|
Lischer and Roberts |
|
Exclusive License |
|
Automated Fluid Delivery System and Method |
|
11816942.2 |
|
2/7/2013 |
|
Europe |
|
Pending |
|
N/A |
|
N/A |
|
Lischer and Roberts |
|
Exclusive License |
|
Automated Fluid Delivery System and Method |
|
201180045992.4 |
|
2/7/2013 |
|
China |
|
Pending |
|
N/A |
|
N/A |
|
Lischer and Roberts |
|
Exclusive License |
|
System and Method for automated control of oxygen |
|
62139285 |
|
3/27/15 |
|
US |
|
Pending |
|
N/A |
|
N/A |
|
Abbott |
|
Original IP |
Competition
Supplemental oxygen delivery devices can be broken into two market segments: continuous flow and intermittent flow. Continuous flow is the traditional form of supplemental oxygen delivery, with a constant and unchanging flow rate provided by the delivery device. This is currently the standard method for administering supplemental oxygen to patients and is typically delivered using an oxygen source, a tank regulator and a nasal cannula. In application, continuous flow delivery can be inefficient and wasteful because gas is delivered both while the patient is breathing in (inhalation) and breathing out (exhalation).
Intermittent flow is a more recently developed mode of delivery, created to conserve oxygen (but not to improve patient outcomes) by delivering a volume of oxygen in response to the start of an inhalation by the user. There are several different types of “oxygen conserving devices” (OCDs) on the market today. OCDs have varying performance characteristics, including those based on the oxygen volume delivered, trigger sensitivity and trigger response time. A user’s exertion can result in shortfalls in the delivery of oxygen by these devices. (American Journal of Respiratory Critical Care Medicine, “Critical Comparisons of the Clinical Performance of Oxygen-conserving Devices”, 2010, available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2874449/.)
The development and commercialization of supplemental oxygen delivery devices is highly competitive. We face competition with respect to our current intended product, and we will face competition with respect to any products that we may develop or commercialize in the future, from a variety of entities and enterprises. We anticipate facing competition principally from large and small manufacturers of supplemental oxygen delivery and general medical devices, including DeVilbiss, Invacare, Southmedic, Inc., Philips Respironics, and CHAD Therapeutics. Potential competitors may also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization of any sort of device or therapy that would compete in and or change the nature of the supplemental oxygen delivery device market.
There can be no assurance that we will be able to complete the development of competitive products and be able to commercialize them on a competitive basis. Factors affecting competition in the supplemental oxygen delivery device industry include the financial, research and development, testing, and marketing strengths of individual competitors, trends in industry consolidation, consumers’ product options, product quality, price and technology, reputation, customer service capabilities and access to market partners and customers. Our competitors may be able to develop competing or superior technologies and processes, compete more aggressively and sustain their competitive efforts over a longer period of time than we may be able to.
84
MANAGEMENT
The following table sets forth the name, age and position of each of our directors, director nominees and executive officers:
|
Name |
|
Age |
|
Position |
|
Amro Albanna |
|
44 |
|
Chief Executive Officer, Director and Chair |
|
Dale Hutchins |
|
51 |
|
President |
|
Vernon Hall |
|
76 |
|
Independent Director Nominee* |
|
George Reyes |
|
61 |
|
Independent Director Nominee* |
|
Albert Cervantes |
|
62 |
|
Chief Financial Officer |
|
Stephen Abbott |
|
60 |
|
Chief Discovery Officer |
|
Rowena Albanna |
|
49 |
|
Chief Operating Officer |
|
Larry Eason |
|
52 |
|
Chief Marketing Officer |
____________
* This individual has indicated his assent to occupy such position immediately after the closing of this offering.
Constitution of Our New Board of Directors
Mr. Albanna currently serves as the sole member of our board of directors. Immediately after the closing of this offering, we intend to expand the size of our board of directors to three members, including the two independent director nominees listed in the table above. In addition, our board of directors intends to establish three standing committees — audit, compensation and nominating and corporate governance — in accordance with NASDAQ rules and as described below.
Backgrounds of Our Directors, Director Nominees and Executive Officers
The following is a brief summary of the background of each of our directors and executive officers. In addition, in regard to our directors, the following includes specific information about each director’s experience, qualifications, attributes or skills that led the board to conclude that the individual was qualified to serve on our board, in light of our business and structure. There are no family relationships among any of the directors and executive officers, except that our Chief Executive Officer, Amro Albanna, and our Chief Operating Officer, Rowena Albanna, are husband and wife.
Directors and Director Nominees
Amro Albanna has served as our Chief Executive Officer and director since our formation in 2010. He also serves as a director of our subsidiaries OLI, NEA, and SOS. Mr. Albanna has a Bachelor of Science degree in Business Administration and Computer Information Systems from California State University, San Bernardino. In 1997, he founded Timely Technology Corporation (TTC), which designed and developed internet-based software systems for organizations and industries seeking to transact online. In 2002, TTC was acquired by Digital Angel Corporation. In 2003, Mr. Albanna founded Qmotions, Inc. (subsequently renamed Deal A Day Group Corp.). He served as its Chief Executive Officer and Chairman until 2011. Qmotions designed and developed a new generation of video game controllers that incorporated body motion. In 2008, Mr. Albanna co-founded the Innovation Economy Initiative, a public-private initiative to encourage the development of innovation-focused businesses in the Riverside, CA area. The initiative led to the creation of Innovation Economy Corporation (ieCrowd). In addition to the foregoing, Mr. Albanna is a co-founder of the Riverside Technology CEO Forum and serves on the board of SmartRiverside, a non-profit organization promoting the local entrepreneurial ecosystem. He has also been involved in establishing the University of California, Riverside Research Park, and has served as a “Practicing Entrepreneur” in the Integrated Technology Transfer Network at California State University, San Bernardino. Awards and honors he has received include the “ComputerWorld Honors Laureate, Economic Development” (ComputerWorld, 2012); “Emerging Entrepreneur of the Year” (Center for Entrepreneurship, California State University, San Bernardino, 2005); “Entrepreneurial Excellence Award” (Bourns College of Engineering, University of California, Riverside, 2004); and “Distinguished Alumni of the Year” (California State University, San Bernardino, 2003).
Vernon Hall is a founding member of the Leonard Financial Group, LLC and has more than 50 years of experience in accounting and financial services. Mr. Hall is a member of the California Society of CPAs (Citrus Belt Chapter), a
85
member of that society’s State PFP (Personal Financial Planner) Committee, and is a past PFP Committee chairman in the Citrus Belt Chapter. He is also a member of the AICPA’s PFP Division, and a member of the Institute of Certified Financial Planners. He was a founding board member of the Riverside Chapter of the International Association for Financial Planning, and a previous adjunct faculty member for the College of Financial Planning in Denver, CO. Mr. Hall has served as Chairman of the Board of Parkview Community Hospital, has served on the board of the local affiliate of the National Lung Association, was on the Joint Powers Commission to build Riverside City Hall and the Raincross Square Convention Center, and has served on gift planning bodies at the University of California, Riverside and the Community Foundation of Riverside. Mr. Hall received his Certified Public Accountant (CPA) license in 1967, earned his Certified Financial PlannerTM certification (CFP®) in 1985 and received his Personal Financial Specialist (PFS) designation in 1995. He earned a Bachelor’s degree in Business Administration from Woodbury College (now Woodbury University) in 1963.
George Reyes has been an attorney at Best & Krieger LLP since 1978 and is a partner in the firm’s Business Services practice group since 1985. His practice includes general counsel work for privately owned businesses and non-profits. He has provided legal representation to various businesses, from start-ups to well-established going concerns. Mr. Reyes has served as the chairman of the board of the Inland Empire Economic Partnership since January 2013, is a director of the Monday Morning Group, the Children’s Fund, the Riverside Community Health Foundation, the La Sierra University Foundation and the California State University San Bernardino Foundation and is a member of the Board of Visitors of California Baptist University. Mr. Reyes is also a former member of the Make-A-Wish Foundation for Orange County and the Inland Empire and a former director of Riverside Community Hospital and the UCR Foundation. He received his B.A. from Harvard University in 1975 and his J.D. from the University of California, Davis in 1978.
Executive Officers
Dale Hutchins has served as our President since our formation in 2010. Additionally, Mr. Hutchins serves as a director of our subsidiaries, OLI, NEA and SOS. Mr. Hutchins is also the co-founder of Innovation Economy Initiative, a public-private initiative to encourage the development of innovation-focused businesses in the Riverside, CA area. Established in 2009, the Initiative lead to the creation of Innovation Economy Corporation. Previously, from 2004 to 2009, Mr. Hutchins served as President, Director, and Corporate Secretary for Qmotions, Inc. and its public successor, a start-up company that developed first-of-its-kind (pre Nintendo Wii), full-motion video game controllers. From 2003 to 2007, Mr. Hutchins was the founder and chief executive of The Canyon Group, a reseller of specialized, component-level, international travel insurance/assistance, as well as a provider of consulting and training services. Prior to this, Mr. Hutchins served as President of Medical Advisory Systems, Inc. and Chief Administrative Officer and Executive Vice President of Digital Angel Corporation, from 2002 to 2004. Digital Angel Corporation, a developer of technologies that enabled the identification, location tracking and condition monitoring of high value assets, animals & people, went public as a result of a merger with Medical Advisory Systems, Inc., in 2002. Prior to this, from 1992 to 2002, Mr. Hutchins served as Chief Operating Officer and Executive Vice President of Medical Advisory Systems, Inc., a publicly-traded company on the American Stock Exchange and provider of 24-hour physician consultations and ancillary medical & pharmaceutical services to ships-at-sea, remote locations and aircraft worldwide, as well as a first-to-market provider of internet-based, one-on-one, confidential medical information chats between physicians and consumers.
Albert “Al” Cervantes has been our Chief Financial Officer since December 2010 and has years of experience in financial, accounting, and strategic planning for large corporations. Prior to joining ieCrowd, from January 2008 to December 2010, Mr. Cervantes served as Chief Financial Officer of Actiga, Inc., a publicly-traded video game software and game controller development company, and its private predecessor. Prior to that, from 2005 to 2008, Mr. Cervantes served as Chief Financial Officer of the Soboba Band of Luiseño Indians where he was responsible for finance, accounting and the strategic planning of future commercial developments. From 2001 to 2005, Mr. Cervantes was Chief Financial Officer of Specialized Direct, Inc. From 1997 to 2001, Mr. Cervantes served as Chief Financial Officer of Locus Direct Marketing Group, Inc. From 1990 to 1997, Mr. Cervantes also served as Vice President of Finance and Administration of KTTV Channel 11, a Fox-owned Los Angeles-based television station, and as Vice President Controller of Fox Inc., a global entertainment corporation. Mr. Cervantes received a Bachelor of Arts in Economics from Stanford University in 1975 and a Master’s in Business Administration from the University of California, Los Angeles in 1978. Mr. Cervantes is a Certified Public Accountant California and a member of the American and California Society of CPAs.
86
Stephen “Steve” Abbott has been ieCrowd’s Chief Discovery Officer since 2012 and has served in other capacities with ieCrowd since 2010. Mr. Abbott is responsible for leading the identification, securing, and integration of new innovative discoveries and intellectual properties. In addition, Mr. Abbott oversees the Company’s initial due diligence process on all pre- and post-acquisition technologies. Mr. Abbott served as a founding executive of Avisio, Inc., a public company focused on transforming underutilized assets into successful companies. Prior to joining Avisio, Inc., Mr. Abbott operated in various capacities, including business unit president and divisional Chief Technology Officer for a multi-billion dollar holding company made up of over 100 operating units. There he was responsible for growing business unit revenue and developing operational strategy. He also served as a member of due diligence teams in connection with acquisitions. Mr. Abbott founded and served as CEO for an independent consulting firm that specialized in mergers and acquisitions transactions for high-value closely held companies, and mentored and coached promising entrepreneurs. Prior to that, Mr. Abbott participated in the formation of Specialty Insurance Services, where he served as Chief Technology Officer, Chief Executive Officer and Partner. Mr. Abbott graduated Magna Cum Laude from Cal Poly San Luis Obispo with a degree in Mathematics in 1976. He earned an accreditation as an Associate of the Society of Actuaries. In 2003, Mr. Abbott earned his EMBA from The Peter Drucker School of Management at the Claremont Graduate University.
Rowena Albanna has been Chief Operating Officer of the Company since August 2013 and prior thereto was Senior Vice President of the Company since its inception. Ms. Albanna also co-founded the Innovation Economy Initiative in 2009. Established in 2009, the initiative lead to the creation of Innovation Economy Corporation. Her responsibilities include serving in both a corporate leadership role and as head of the group that provides operational support to each ieCrowd company. She oversees support services in the areas of manufacturing, regulatory compliance, product development, engineering and IT. Previously, from 2004 to 2009, Ms. Albanna founded and operated two e-commerce and online marketing sites. From 2003 to 2004, Ms. Albanna was Head of Product Development at Qmotions, Inc. where she led the development of the Company’s first golf video game controller system. Prior to this, she served as Vice President of Product Development at Digital Angel Systems in 2002. The company later went public on the American Stock Exchange via a merger. Ms. Albanna has over 20 years of experience managing interdisciplinary teams developing products in a wide variety of industries, including nanotechnology, e-commerce, online marketing, finance, telecommunications, medicine, embedded systems, integrated circuit layout design and defense. She earned her Bachelor of Science Degree in Computer Science with a minor in Mathematics from California State University, San Bernardino in 1988.
Larry Eason has been Chief Marketing Officer for ieCrowd since April 2014 and served as Chief Partnerships Officer from December 2013 to March 2014. Mr. Eason is responsible for leading ieCrowd’s marketing, communications and public relations initiatives. Since September 2013, Mr. Eason has served on the advisory board of big data firm and IBM/IBM Watson strategic partner Findability Sciences. Previously, from 2012 to 2013, Mr. Eason served as Senior Consultant for Business Engagement for Sustain Global Partnership (SGP). SGP is a global collaboration of UPS, TNT, Accenture, Booz Allen Hamilton, World Vision and CARE that is working to bring scale, efficiency and business thinking to the global humanitarian supply chain. Prior to that, in 2008, Mr. Eason founded DotOrgPower, a digital strategy firm focused on communications, technology and social impact, and served as its President until 2014. As President of DotOrgPower, Mr. Eason secured and advised a roster of clients including RAND Corporation, World Vision International (Global Innovation and Collaboration), International Medical Corps, Social Venture Partners International, Charity Navigator, the California Department of Mental Health and more. Prior to that, Mr. Eason co-founded a digital agency in 2001 and served as partner until it was acquired in 2005 by Mindshare Interactive Campaigns (later Virilion), where he served for 3 years as Senior Vice President. From 2009 to 2011, Mr. Eason served as board chair of Healthy Child Healthy World. Mr. Eason is a founding member of Children’s Hospital Los Angeles’ Digital Philanthropy Committee. Mr. Eason graduated with a Bachelor of Arts degree in Art from Occidental College in 1984. In 2005, Mr. Eason won the Institute for Politics, Democracy & the Internet’s Golden Dot award for Best Statewide Internet Campaign for his work developing and directing the online presence for the YesOn63.org campaign, a successful California state ballot measure that supports funding for mental health services in California.
Board Composition
Immediately after the closing of this offering, our board of directors will consist of three members. Our board of directors is authorized to have up to nine members. Each director of the Company serves until the next annual meeting of stockholders and until his successor is elected and duly qualified, or until his earlier death, resignation or
87
removal. Our board is authorized to appoint persons to the offices of Chief Executive Officer, Chief Financial Officer, Secretary and such other offices as may be determined by the board.
We have no formal policy regarding board diversity. In selecting board candidates, we seek individuals who will further the interests of our stockholders through an established record of professional accomplishment, the ability to contribute positively to our collaborative culture, knowledge of our business and understanding of our prospective markets.
Sunbelt III Technologies Management, LLC, or Sunbelt, has a contractual right to designate a member of our board of directors. Sunbelt has informed us that it does not intend to exercise such right until such time as our board of directors is comprised of seven or more members.
Director Independence
Rule 5605 of the NASDAQ Listing Rules requires a majority of a listed company’s board of directors to be comprised of independent directors within one year of listing. In addition, the NASDAQ Listing Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation and nominating and corporate governance committees be independent and that audit committee members also satisfy the independence criteria set forth in Rule 10A-3 under the Exchange Act.
Under Rule 5605(a)(2) of the NASDAQ Listing Rules, a director will only qualify as an “independent director” if, certain specific independence criteria are met and, in the opinion of the board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In order to be considered independent for purposes of Rule 10A-3 of the Exchange Act, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors or any other board committee, accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries or otherwise be an affiliated person of the listed company or any of its subsidiaries.
Our board of directors has reviewed the composition of its committees, to be in effect immediately after the closing of this offering, and the independence of each director and director nominee. Based upon information requested from and provided by each director and director nominee concerning that director’s or director nominee’s background, employment and affiliations, including family relationships, our board has determined that each of our director nominees is an “independent director” as defined under Rule 5605(a)(2) of the NASDAQ Listing Rules. Our board has also determined that Mr. Hall and Mr. Reyes satisfy the independence standards for such committees established by SEC and NASDAQ rules. In making such determinations, our board considered the relationships that each such person has with our Company and all the other facts and circumstances our board deemed relevant in determining independence, including the beneficial ownership of our capital stock by each such person.
Board Committees
Immediately after the closing of this offering, our board will establish three standing committees — audit, compensation and nominating and corporate governance — each of which will operate under a charter that has been approved by our board.
Audit Committee
Immediately after the closing of this offering, the members of our audit committee will be Mr. Hall, Mr. Reyes and Mr. Albanna. Mr. Hall will be the chair of the audit committee. Messrs. Hall and Reyes are independent. We intend to appoint an independent director to the audit committee, in lieu of Mr. Albanna, within one year following the completion of this offering pursuant to the phase-in provisions of the NASDAQ listing standards for initial public offerings. Mr. Albanna owns greater than 10% of our voting equity securities.
Our board of directors has determined that Mr. Hall qualifies as an audit committee financial expert within the meaning of SEC regulations and the NASDAQ Listing Rules. In making this determination, our board has considered the formal education and nature and scope of his previous experience. Our audit committee will assist
88
our board of directors in its oversight of our accounting and financial reporting process and the audits of our financial statements. Our audit committee’s responsibilities will include:
• appointing, approving the compensation of, and assessing the independence of our registered public accounting firm;
• overseeing the work of our registered public accounting firm, including through the receipt and consideration of reports from such firm;
• reviewing and discussing with management and the registered public accounting firm our annual and quarterly financial statements and related disclosures;
• monitoring our internal control over financial reporting, disclosure controls and procedures and code of business conduct and ethics;
• overseeing our internal accounting function;
• discussing our risk management policies;
• establishing policies regarding hiring employees from our registered public accounting firm and procedures for the receipt and retention of accounting-related complaints and concerns;
• meeting independently with our internal accounting staff, registered public accounting firm and management;
• reviewing and approving or ratifying related party transactions; and
• preparing the audit committee reports required by SEC rules.
Compensation Committee
Immediately after the closing of this offering, the members of our compensation committee will be Mr. Hall and Mr. Reyes. Mr. Hall will be the chair of the compensation committee. Our compensation committee will assist our board of directors in the discharge of its responsibilities relating to the compensation of our executive officers. The compensation committee’s responsibilities will include:
• reviewing and approving corporate goals and objectives with respect to Chief Executive Officer compensation;
• making recommendations to our board with respect to the compensation of our Chief Executive Officer and our other executive officers;
• overseeing evaluations of our senior executives;
• overseeing and administering our equity incentive plans;
• reviewing and making recommendations to our board with respect to director compensation;
• reviewing and discussing with management our “Compensation Discussion and Analysis” disclosure; and
• preparing the compensation committee reports required by SEC rules.
Nominating and Corporate Governance Committee
Immediately after the closing of this offering, the members of our nominating and corporate governance committee will be Mr. Hall and Mr. Reyes. Mr. Reyes will be the chair of the nominating and corporate governance committee. The nominating and corporate governance committee’s responsibilities will include:
• identifying individuals qualified to become board members;
• recommending to our board the persons to be nominated for election as directors and to be appointed to each committee of our board of directors;
89
• reviewing and making recommendations to the board with respect to management succession planning;
• developing and recommending corporate governance principles to the board; and
• overseeing periodic evaluations of board members.
Board Leadership Structure, Executive Sessions of Non-Management Directors
Mr. Albanna currently serves as our chief executive officer and chair of our board of directors. Our board has determined not to separate the Company’s chief executive officer and chair positions because the Company is at an early stage in its development, and therefore the additional expense and complexity of maintaining separate chairman and CEO functions and personnel would not be cost-effective for it. As the Company grows, the board plans to re-evaluate this policy from time to time.
Risk Oversight
The board of directors oversees our business and considers the risks associated with our business strategy and decisions. The board currently implements its risk oversight function as a whole. Each of the board committees also provides risk oversight in respect of its areas of concentration and reports material risks to the board for further consideration.
Code of Business Conduct and Ethics
We have adopted a written code of business conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer or controller, or persons performing similar functions. We will post on our website a current copy of the code and all disclosures that are required by law or NASDAQ rules in regard to any amendments to, or waivers from, any provision of the code.
90
EXECUTIVE COMPENSATION
We are providing compensation disclosure that satisfies the requirements applicable to emerging growth companies, as defined in the JOBS Act.
Summary Compensation Table
We are an emerging growth company and have opted to comply with the executive compensation disclosure rules applicable to “smaller reporting companies,” as such term is defined under the Securities Act, which require compensation disclosure for our principal executive officer and the two most highly compensated executive officers other than our principal executive officer. The table below sets forth the annual compensation earned during fiscal 2014 and 2013 by our current principal executive officer and our next two most highly compensated executive officers (collectively, our “Named Executive Officers”).
|
Name and Principal Position |
|
Year End |
|
Salary |
|
All
Other Compensation(1) |
|
Total |
|
Amro Albanna |
|
2014 |
|
154,000 |
|
— |
|
154,000 |
|
Chief Executive Officer |
|
2013 |
|
65,692 |
|
24,538 |
|
90,230 |
|
Dale Hutchins |
|
2014 |
|
143,992 |
|
— |
|
143,992 |
|
President |
|
2013 |
|
65,692 |
|
24,538 |
|
90,230 |
|
Stephen Abbott |
|
2014 |
|
123,977 |
|
— |
|
123,977 |
|
Chief Discovery Officer |
|
2013 |
|
65,692 |
|
24,538 |
|
90,230 |
____________
(1) The amounts reported in the “All Other Compensation” column reflect, for each Named Executive Officer, the sum of the incremental cost to us of all perquisites and other personal benefits. The amounts reported in the “All Other Compensation” column for 2013 reflect deferred compensation that was earned in 2013 but not paid until 2014.
Employment Agreements
Following this offering, we intend to enter into an employment agreement with our Chief Executive Officer, and may enter into employment agreements with other of our executive officers.
Outstanding Equity Awards at December 31, 2014
The following table sets forth information regarding outstanding stock options to acquire shares of ieCrowd common stock held by our Named Executive Officers as of December 31, 2014:
|
Name and Principal Position |
|
Number of securities underlying unexercised options
exercisable |
|
Number of securities underlying unexercised options
unexercisable |
|
Option |
|
Option |
|
Amro Albanna Chief Executive Officer |
|
— |
|
— |
|
— |
|
— |
|
Dale Hutchins President(1) |
|
66,667 |
|
— |
|
2.25 |
|
29-Dec-22 |
|
Stephen Abbott Chief Discovery Officer |
|
866,667 |
|
— |
|
1.50 |
|
31-Dec-21 |
____________
(1) In addition, in September 2012, the Company granted fully vested options to Mr. Hutchins, Mr. Abbott and an OLI employee to purchase a total of 1,028 shares of OLI stock from ieCrowd at an exercise price of $210. These options expire in September 2016. If all of the foregoing options were exercised, these employees would acquire approximately 4.0% of OLI’s capital stock on a fully diluted basis.
Equity and Other Compensation Plans
The equity incentive plans described in this section are our Amended and Restated Long-Term Incentive Plan (the “LTIP”) and our 2015 Equity Incentive Plan (the “2015 Plan”). Under the LTIP, awards can be made in respect of a
91
remaining approximately 20,700 shares of common stock. We may make further awards up to this amount under the LTIP, and we expect shortly to begin making awards under the 2015 Plan.
Amended and Restated Long-Term Incentive Plan
Our board of directors adopted the LTIP on March 1, 2011. The LTIP was most recently amended on July 14, 2011 by our board of directors and our stockholders approved and adopted the amended and restated plan on the same date. The LTIP allows for the grant of incentive stock options and performance bonuses to our employees and for the grant of nonqualified stock options, restricted stock awards, stock appreciation rights and performance units to our employees, directors and consultants.
We set aside 2,666,667 shares of common stock for issuance under the LTIP, and as of March 31, 2015 and December 31, 2014, options in respect of 2,645,967 shares have been awarded. Options awarded under the LTIP have a term of up to 10 years and the exercise price of the outstanding options ranges from $0.03 to $5.25 per share, with a weighted average exercise price of approximately $2.10 per share. No awards will be granted under the LTIP following the completion of this offering. The LTIP will continue to govern outstanding awards previously granted.
Plan Administration. Our board of directors currently administers the LTIP and following this offering our Compensation Committee will administer the LTIP. Subject to the provisions of the LTIP, the administrator has the power to determine the form (including whether awards are granted singly or in combination), conditions (including performance requirements), restrictions and terms (including recipients) of awards, the time and conditions of exercise or vesting, the times at which awards are to be made, the number of shares subject to each award, and the terms of the award agreement for use under the LTIP (which may include the waiver or amendment of prior terms or conditions or acceleration or early vesting or payment of an award). In addition, the administrator has the power to accelerate the vesting, exercise or payment of an award or the performance period of an award, to determine whether and to what extent a performance bonus may be deferred, and to take all other action it deems necessary or advisable for the proper operation or administration of the LTIP. The administrator’s interpretation of the LTIP or any awards thereunder and all decisions and determinations by the administrator with respect to the plan are final, binding and conclusive on all relevant parties.
Grant of Awards. The administrator is prohibited from canceling, reissuing or modifying an award if such action will have the effect of repricing such award. The maximum term of all stock awards is ten years.
Options. Stock options may be granted under the LTIP. The exercise price per share of all options must equal at least the fair market value per share of our common stock on the date of grant. The administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares or certain other property or other consideration acceptable to the administrator.
Restricted Stock. Restricted stock may be granted under the LTIP. Restricted stock awards are grants of shares of our common stock that are subject to various restrictions, including restrictions on transferability. Shares of restricted stock will vest, and the restrictions on such shares will lapse, in accordance with terms and conditions established by the administrator. Restricted stock awards granted to employees must require the holder to remain employed by us or an affiliate of ours for a period of time, as determined by the administrator. Restricted stock awards granted to consultants or directors must require the holder to provide continued services to us for a period of time, as determined by the administrator. In addition to any time vesting conditions determined by the administrator, restricted stock awards may be subject to the achievement by us of specified operational, financial or stock performance criteria established by the administrator from time to time.
Stock Appreciation Rights. The LTIP provides for the grant of stock appreciation rights, or SARs. SARs are governed by the terms provided by the administrator in an award agreement. SARs may be granted in tandem with a stock option or independently. The exercise price of the SAR may not be less than the fair market value of a share of our common stock on the date of grant of the SAR.
Performance Units. The LTIP provides for the grant of performance units. Performance units are governed by the terms provided by the administrator in an award agreement. Each award must state the target, maximum and minimum value of the performance unit upon the achievement of performance goals. The administrator must establish performance targets for each award for a period of no less than one year based upon specified operational, financial or performance criteria. Performance units may be paid in cash or shares of our common stock, as determined in the sole discretion of the administrator.
92
Performance Bonus. The LTIP provides for the grant of performance bonuses to employees. The administrator must determine the amount that may be earned as a performance bonus in any period of one year or more upon the achievement of specified operational, financial or performance criteria. In order for any employee to be entitled to payment of a performance bonus, the applicable targets must first be obtained or exceeded. Generally, payment of a performance bonus must be made within 60 days of the administrator’s determination that performance targets have been achieved and may be paid in cash or shares of our common stock, as determined in the sole discretion of the administrator.
Transferability or Assignability of Awards. The LTIP generally does not allow for the transfer or assignment of nonqualified stock options, other than by gift to an immediate family member.
Certain Adjustments. In the event of certain changes in our capitalization, the exercise prices of and the number of shares subject to outstanding options, and the purchase price of and the numbers of shares subject to outstanding awards will be proportionately adjusted, subject to any required action by our board of directors or stockholders.
Termination of Employment. After an employee’s termination of employment, the employee generally may exercise his or her options or SARs, to the extent vested as of such date of termination, for one year after termination. If termination is due to death, disability or retirement, the options and SARs generally (unless otherwise stated in the award agreement) will remain exercisable, to the extent vested as of such date of termination, until the two-year anniversary of such termination. The administrator may, in its sole discretion, accelerate the vesting of unvested options and SARs in the event of the termination of employment of any employee. The unvested portion of any award is forfeited unless otherwise accelerated pursuant to the terms of the award agreement or by the administrator. However, in no event may an option be exercised later than the expiration of its term. All other benefits that have not been earned, including if performance goals were not met, on the date of termination of employment shall be terminated on the date employment is terminated.
Termination of Service. After a consultant or director ceases to provide services to us, the consultant or director generally may exercise his or her nonqualified stock options or SARs, to the extent vested as of such date of termination, for one year after termination. The unvested portion of any award is forfeited unless otherwise accelerated pursuant to the terms of the award agreement or by the administrator. All other benefits that have not been earned, including if performance goals were not met, on the date of termination of service shall be terminated on the date employment is terminated.
Amendment; Termination. Our board of directors may alter, suspend or terminate the LTIP at any time, provided that, unless approved by our stockholders, such amendment does not allow for the grant of incentive stock options. As noted above, upon completion of this offering, no further awards will be granted under the LTIP.
2015 Equity Incentive Plan
The Company’s Board of Directors has adopted the Company’s 2015 Equity Incentive 2015 Plan (the “2015 Plan”). The purpose of the 2015 Plan is to attract and retain key personnel and to provide a means for directors, officers, employees, consultants and advisors to acquire and maintain an interest in the Company, which interest may be measured by reference to the value of our common stock.
If approved by the Company’s stockholders, the 2015 Plan will be effective on or about May 12, 2015 (the date that the Company’s Board of Directors approved the 2015 Plan). The following description is qualified in its entirety by reference to the 2015 Plan.
Administration. Our Compensation Committee will administer the 2015 Plan. The Committee will have the authority to determine the terms and conditions of any agreements evidencing any Awards granted under the 2015 Plan and to adopt, alter and repeal rules, guidelines and practices relating to the 2015 Plan. Our Compensation Committee will have full discretion to administer and interpret the 2015 Plan and to adopt such rules, regulations and procedures as it deems necessary or advisable and to determine, among other things, the time or times at which the awards may be exercised and whether and under what circumstances an award may be exercised.
Eligibility. Employees, directors, officers, advisors or consultants of the Company or its affiliates are eligible to participate in the 2015 Plan. Our Compensation Committee has the sole and complete authority to determine who will be granted an award under the 2015 Plan, however, it may delegate such authority to one or more officers of the Company under the circumstances set forth in the 2015 Plan.
93
Number of Shares Authorized. The 2015 Plan provides for an aggregate of 1,250,000 shares of common stock to be available for awards. If an award is forfeited or if any option terminates, expires or lapses without being exercised, the common shares subject to such award will again be made available for future grant. Shares that are used to pay the exercise price of an option or that are withheld to satisfy the Participant’s tax withholding obligation will not be available for re-grant under the 2015 Plan.
If there is any change in our corporate capitalization, the Compensation Committee in its sole discretion may make substitutions or adjustments to the number of shares reserved for issuance under our 2015 Plan, the number of shares covered by awards then outstanding under our 2015 Plan, the limitations on awards under our 2015 Plan, the exercise price of outstanding options and such other equitable substitution or adjustments as it may determine appropriate.
The 2015 Plan will have a term of ten years and no further awards may be granted under the 2015 Plan after that date.
Awards Available for Grant. Our Compensation Committee may grant awards of Non-Qualified Stock Options, Incentive (qualified) Stock Options, Stock Appreciation Rights, Restricted Stock Awards, Restricted Stock Units, Stock Bonus Awards, Performance Compensation Awards (including cash bonus awards) or any combination of the foregoing; provided, that our Compensation Committee may not grant funds to any one person in any one calendar year for more than 500,000 common shares in the aggregate.
Options. Our Compensation Committee will be authorized to grant Options to purchase common shares that are either “qualified,” meaning they are intended to satisfy the requirements of Code Section 422 for incentive stock options, or “non-qualified,” meaning they are not intended to satisfy the requirements of Section 422 of the Code. Options granted under the 2015 Plan will be subject to the terms and conditions established by our Compensation Committee. Under the terms of the 2015 Plan, unless our Compensation Committee determines otherwise in the case of an Option substituted for another Option in connection with a corporate transaction, the exercise price of the Options will not be less than the fair market value (as determined under the 2015 Plan) of our common shares at the time of grant. Options granted under the 2015 Plan will be subject to such terms, including the exercise price and the conditions and timing of exercise, as may be determined by our Compensation Committee and specified in the applicable award agreement. The maximum term of an option granted under the 2015 Plan will be ten years from the date of grant (or five years in the case of a qualified option granted to a 10% stockholder). Payment in respect of the exercise of an option may be made in cash or by check, by surrender of unrestricted shares (at their fair market value on the date of exercise) that have been held by the participant for any period deemed necessary by our accountants to avoid an additional compensation charge or have been purchased on the open market, or our Compensation Committee may, in its discretion and to the extent permitted by law, allow such payment to be made through a broker-assisted cashless exercise mechanism, a net exercise method, or by such other method as our Compensation Committee may determine to be appropriate.
Stock Appreciation Rights. Our Compensation Committee will be authorized to award Stock Appreciation Rights (or SARs) under the 2015 Plan. SARs will be subject to the terms and conditions established by our Compensation Committee. An SAR is a contractual right that allows a participant to receive, either in the form of cash, shares or any combination of cash and shares, the appreciation, if any, in the value of a share over a certain period of time. An Option granted under the 2015 Plan may include SARs and SARs may also be awarded to a participant independent of the grant of an Option. SARs granted in connection with an Option shall be subject to terms similar to the Option corresponding to such SARs. SARs shall be subject to terms established by our Compensation Committee and reflected in the award agreement.
Restricted Stock. Our Compensation Committee will be authorized to award Restricted Stock under the 2015 Plan. Unless otherwise provided by our Compensation Committee and specified in an award agreement, restrictions on Restricted Stock will lapse after three years of service with the Company. Our Compensation Committee will determine the terms of such Restricted Stock awards. Restricted Stock consists of common shares that generally are non-transferable and subject to other restrictions determined by our Compensation Committee for a specified period. Unless our Compensation Committee determines otherwise or specifies otherwise in an award agreement, if the participant terminates employment or services during the restricted period, then any unvested restricted stock is forfeited.
Restricted Stock Unit Awards. Our Compensation Committee will be authorized to award Restricted Stock Unit awards. Unless otherwise provided by our Compensation Committee and specified in an award agreement, Restricted Stock Units will vest after three years of service with the Company. Our Compensation Committee will determine
94
the terms of such Restricted Stock Units. Unless our Compensation Committee determines otherwise or specifies otherwise in an award agreement, if the participant terminates employment or services during the period of time over which all or a portion of the units are to be earned, then any unvested units will be forfeited. At the election of our Compensation Committee, the participant will receive a number of common shares equal to the number of units earned or an amount in cash equal to the fair market value of that number of shares at the expiration of the period over which the units are to be earned or at a later date selected by our Compensation Committee.
Stock Bonus Awards. Our Compensation Committee will be authorized to grant awards of unrestricted common shares or other awards denominated in common shares, either alone or in tandem with other awards, under such terms and conditions as our Compensation Committee may determine.
Performance Compensation Awards. Our Compensation Committee will be authorized to grant any award under the 2015 Plan in the form of a Performance Compensation Award by conditioning the vesting of the award on the attainment of specific levels of performance of the Company and/or one or more Affiliates, divisions or operational units, or any combination thereof, as determined by the Committee.
Transferability. Each award may be exercised during the participant’s lifetime only by the participant or, if permissible under applicable law, by the participant’s guardian or legal representative and may not be otherwise transferred or encumbered by a participant other than by will or by the laws of descent and distribution. Our Compensation Committee, however, may permit awards (other than incentive stock options) to be transferred to family members, a trust for the benefit of such family members, a partnership or limited liability company whose partners or stockholders are the participant and his or her family members or anyone else approved by it.
Amendment. The 2015 Plan will have a term of ten years. Our board of directors may amend, suspend or terminate the 2015 Plan at any time; however, shareholder approval to amend the 2015 Plan may be necessary if the law so requires. No amendment, suspension or termination will impair the rights of any participant or recipient of any award without the consent of the participant or recipient.
Change in Control. Except to the extent otherwise provided in an Award agreement, in the event of a Change in Control, all outstanding options and equity awards (other than performance compensation awards) issued under the 2015 Plan will become fully vested and performance compensation awards will vest, as determined by our Compensation Committee, based on the level of attainment of the specified performance goals. In general, our Compensation Committee may, in its discretion, cancel outstanding awards and pay the value of such awards to the participants in connection with a Change in Control. Our Compensation Committee can also provide otherwise in an award agreement under the 2015 Plan.
U.S. Federal Income Tax Consequences. The following is a general summary of the material U.S. federal income tax consequences of the grant and exercise and vesting of awards under the 2015 Plan and the disposition of shares acquired pursuant to the exercise of such awards and is intended to reflect the current provisions of the Code and the regulations thereunder. This summary is not intended to be a complete statement of applicable law, nor does it address foreign, state, local and payroll tax considerations. Moreover, the U.S. federal income tax consequences to any particular participant may differ from those described herein by reason of, among other things, the particular circumstances of such participant.
Options. There are a number of requirements that must be met for a particular option to be treated as a qualified option. One such requirement is that common shares acquired through the exercise of a qualified option cannot be disposed of before the later of (i) two years from the date of grant of the option, or (ii) one year from the date of exercise. Holders of qualified options will generally incur no federal income tax liability at the time of grant or upon exercise of those options. However, the spread at exercise will be an “item of tax preference,” which may give rise to “alternative minimum tax” liability for the taxable year in which the exercise occurs. If the holder does not dispose of the shares before the later of two years following the date of grant and one year following the date of exercise, the difference between the exercise price and the amount realized upon disposition of the shares will constitute long-term capital gain or loss, as the case may be. Assuming both holding periods are satisfied, no deduction will be allowed to the Company for federal income tax purposes in connection with the grant or exercise of the qualified option. If, within two years following the date of grant or within one year following the date of exercise, the holder of shares acquired through the exercise of a qualified option disposes of those shares, the participant will generally realize taxable compensation at the time of such disposition equal to the difference between the exercise price and the lesser of the fair market value of the share on the date of exercise or the amount
95
realized on the subsequent disposition of the shares, and that amount will generally be deductible by the Company for federal income tax purposes, subject to the possible limitations on deductibility under Sections 280G and 162(m) of the Code for compensation paid to executives designated in those Sections. Finally, if an otherwise qualified option becomes first exercisable in any one year for shares having an aggregate value in excess of $100,000 (based on the grant date value), the portion of the qualified option in respect of those excess shares will be treated as a non-qualified stock option for federal income tax purposes.
No income will be realized by a participant upon grant of a non-qualified stock option. Upon the exercise of a non-qualified stock option, the participant will recognize ordinary compensation income in an amount equal to the excess, if any, of the fair market value of the underlying exercised shares over the option exercise price paid at the time of exercise. The company will be able to deduct this same amount for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those Sections.
Restricted Stock. A participant will not be subject to tax upon the grant of an award of restricted stock unless the participant otherwise elects to be taxed at the time of grant pursuant to Section 83(b) of the Code. On the date an award of restricted stock becomes transferable or is no longer subject to a substantial risk of forfeiture, the participant will recognize taxable compensation equal to the difference between the fair market value of the shares on that date over the amount the participant paid for such shares, if any, unless the participant made an election under Section 83(b) of the Code to be taxed at the time of grant. If the participant made an election under Section 83(b), the participant will recognize taxable compensation at the time of grant equal to the difference between the fair market value of the shares on the date of grant over the amount the participant paid for such shares, if any. (Special rules apply to the receipt and disposition of restricted shares received by officers and directors who are subject to Section 16(b) of the Exchange Act). The Company will be able to deduct, at the same time as it is recognized by the participant, the amount of taxable compensation to the participant for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those Sections.
Restricted Stock Units. A participant will not be subject to tax upon the grant of a restricted stock unit award. Rather, upon the delivery of shares or cash pursuant to a restricted stock unit award, the participant will have taxable compensation equal to the fair market value of the number of shares (or the amount of cash) the participant actually receives with respect to the award. The company will be able to deduct the amount of taxable compensation to the participant for U.S. federal income tax purposes, but the deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those Sections.
SARs. No income will be realized by a participant upon grant of an SAR. Upon the exercise of an SAR, the participant will recognize ordinary compensation income in an amount equal to the fair market value of the payment received in respect of the SAR. The company will be able to deduct this same amount for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those Sections.
Stock Bonus Awards. A participant will have taxable compensation equal to the difference between the fair market value of the shares on the date the common shares subject to the award are transferred to the participant over the amount the participant paid for such shares, if any. The company will be able to deduct, at the same time as it is recognized by the participant, the amount of taxable compensation to the participant for U.S. federal income tax purposes, but such deduction may be limited under Sections 280G and 162(m) of the Code for compensation paid to certain executives designated in those Sections.
Section 162(m). In general, Section 162(m) of the Code denies a publicly held corporation a deduction for U.S. federal income tax purposes for compensation in excess of $1,000,000 per year per person to its principal executive officer and the three other officers (other than the principal executive officer and principal financial officer) whose compensation is disclosed in its proxy statement as a result of their total compensation, subject to certain exceptions. The 2015 Plan is intended to satisfy an exception with respect to grants of options to covered employees. In addition, the 2015 Plan is designed to permit certain awards of restricted stock, restricted stock units, cash bonus awards and other awards to be awarded as performance compensation awards intended to qualify under the “performance-based compensation” exception to Section 162(m) of the Code.
96
2015 Plan Benefits. Future grants under the 2015 Plan will be made at the discretion of the Compensation Committee and, accordingly, are not yet determinable. In addition, the value of the awards granted under the 2015 Plan will depend on a number of factors, including the fair market value of our common shares on future dates, the exercise decisions made by the participants and the extent to which any applicable performance goals necessary for vesting or payment are achieved. Consequently, it is not possible to determine the benefits that might be received by participants receiving discretionary grants under, or having their annual bonus paid pursuant to, the 2015 Plan.
Required Vote. Approval of the 2015 Plan will require the affirmative vote of the holders of a majority of the shares of the Company’s common stock represented in person or by proxy and entitled to vote at the meeting called for consideration of the approval of the 2015 Plan.
Interests of Directors of Officers. Our directors may grant awards under the Incentive 2015 Plan to themselves as well as to our officers and others.
Compensation Plans at the Subsidiary Level
Amended and Restated Long-Term Incentive Compensation Plan of OLI
The board of directors and stockholders of our OLI subsidiary adopted the Olfactor Laboratories Inc. Long-Term Incentive Compensation Plan, or OLI LTIP, on July 15, 2010. The OLI LTIP was most recently amended in September 10, 2012 by the OLI board of directors and stockholders. The OLI LTIP allows for the grant of incentive stock options and performance bonuses to OLI employees and for the grant of nonqualified stock options, restricted stock awards, stock appreciation rights and performance units to OLI employees, directors and consultants.
OLI set aside 1,000 shares of its common stock for issuance under the OLI LTIP, and as of March 31, 2015 and December 31, 2014, options in respect of 675 shares have been awarded. Options awarded under the OLI LTIP have a term of up to 10 years and the exercise price is $210.43 per share. No awards will be granted under the OLI LTIP following the completion of this offering. The OLI LTIP will continue to govern outstanding awards previously granted.
Plan Administration. The OLI board of directors currently administers the OLI LTIP. Subject to the provisions of the OLI LTIP, the administrator has the power to determine the form (including whether awards are granted singly or in combination), conditions (including performance requirements), restrictions and terms (including recipients) of awards, the time and conditions of exercise or vesting, the times at which awards are to be made, the number of shares subject to each award, and the terms of the award agreement for use under the OLI LTIP (which may include the waiver or amendment of prior terms or conditions or acceleration or early vesting or payment of an award). In addition, the administrator has the power to accelerate the vesting, exercise or payment of an award or the performance period of an award, to determine whether and to what extent a performance bonus may be deferred, and to take all other action it deems necessary or advisable for the proper operation or administration of the OLI LTIP. The administrator’s interpretation of the OLI LTIP or any awards thereunder and all decisions and determinations by the administrator with respect to the plan are final, binding and conclusive on all relevant parties.
Grant of Awards. The administrator is prohibited from canceling, reissuing or modifying an award if such action will have the effect of repricing such award. The maximum term of all stock awards is ten years.
Options. Stock options may be granted under the OLI LTIP. The exercise price per share of all options must equal at least the fair market value per share of OLI common stock on the date of grant. The administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares or certain other property or other consideration acceptable to the administrator.
Restricted Stock. Restricted stock may be granted under the OLI LTIP. Restricted stock awards are grants of shares of OLI common stock that are subject to various restrictions, including restrictions on transferability. Shares of restricted stock will vest, and the restrictions on such shares will lapse, in accordance with terms and conditions established by the administrator. Restricted stock awards granted to employees must require the holder to remain employed by us or an affiliate of OLI’s for a period of time, as determined by the administrator. Restricted stock awards granted to consultants or directors must require the holder to provide continued services to us for a period of time, as determined by the administrator. In addition to any time vesting conditions determined by the administrator, restricted stock awards may be subject to the achievement by us of specified operational, financial or stock performance criteria established by the administrator from time to time.
97
Stock Appreciation Rights. The OLI LTIP provides for the grant of stock appreciation rights, or SARs. SARs are governed by the terms provided by the administrator in an award agreement. SARs may be granted in tandem with a stock option or independently. The exercise price of the SAR may not be less than the fair market value of a share of OLI common stock on the date of grant of the SAR.
Performance Units. The OLI LTIP provides for the grant of performance units. Performance units are governed by the terms provided by the administrator in an award agreement. Each award must state the target, maximum and minimum value of the performance unit upon the achievement of performance goals. The administrator must establish performance targets for each award for a period of no less than one year based upon specified operational, financial or performance criteria. Performance units may be paid in cash or shares of OLI common stock, as determined in the sole discretion of the administrator.
Performance Bonus. The OLI LTIP provides for the grant of performance bonuses to employees. The administrator must determine the amount that may be earned as a performance bonus in any period of one year or more upon the achievement of specified operational, financial or performance criteria. In order for any employee to be entitled to payment of a performance bonus, the applicable targets must first be obtained or exceeded. Generally, payment of a performance bonus must be made within 60 days of the administrator’s determination that performance targets have been achieved and may be paid in cash or shares of OLI common stock, as determined in the sole discretion of the administrator.
Transferability or Assignability of Awards. The OLI LTIP generally does not allow for the transfer or assignment of nonqualified stock options, other than by gift to an immediate family member.
Certain Adjustments. In the event of certain changes in OLI capitalization, the exercise prices of and the number of shares subject to outstanding options, and the purchase price of and the numbers of shares subject to outstanding awards will be proportionately adjusted, subject to any required action by the OLI board of directors or the OLI stockholders.
Termination of Employment. After an employee’s termination of employment, the employee generally may exercise his or her options or SARs, to the extent vested as of such date of termination, for one year after termination. If termination is due to death, disability or retirement, the options and SARs generally (unless otherwise stated in the award agreement) will remain exercisable, to the extent vested as of such date of termination, until the two-year anniversary of such termination. The administrator may, in its sole discretion, accelerate the vesting of unvested options and SARs in the event of the termination of employment of any employee. The unvested portion of any award is forfeited unless otherwise accelerated pursuant to the terms of the award agreement or by the administrator. However, in no event may an option be exercised later than the expiration of its term. All other benefits that have not been earned, including if performance goals were not met, on the date of termination of employment shall be terminated on the date employment is terminated.
Termination of Service. After a consultant or director ceases to provide services to us, the consultant or director generally may exercise his or her nonqualified stock options or SARs, to the extent vested as of such date of termination, for one year after termination. The unvested portion of any award is forfeited unless otherwise accelerated pursuant to the terms of the award agreement or by the administrator. All other benefits that have not been earned, including if performance goals were not met, on the date of termination of service shall be terminated on the date employment is terminated.
Amendment; Termination. The OLI board of directors may alter, suspend or terminate the OLI LTIP at any time, provided that, unless approved by OLI stockholders, such amendment does not allow for the grant of incentive stock options. As noted above, upon completion of this offering, no further awards will be granted under the OLI LTIP.
Pension Benefits
We do not maintain any defined benefit pension plans.
Nonqualified Deferred Compensation
We do not maintain any nonqualified deferred compensation plans.
98
401(k) Retirement Plan
We maintain a 401(k) retirement plan that is intended to be a tax-qualified defined contribution plan under Section 401(k) of the Internal Revenue Code. In general, all of our employees are eligible to participate beginning on the first day of their employment. The 401(k) plan includes a salary deferral arrangement pursuant to which participants may elect to reduce their current compensation by up to the statutorily prescribed limit, equal to $17,500 in 2014, and have the amount of the reduction contributed to the 401(k) plan.
Rule 10b5-1 Sales Plans
Following the closing of this offering, our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from the director or officer. The director or officer may amend or terminate the plan in some circumstances. Our directors and executive officers may also buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
Key-Man Insurance
The Company has a key-man insurance policy in force in respect of Mr. Albanna in the amount of $1.0 million.
Director Compensation
Effective after the closing of this offering, our non-employee directors will be compensated on an annual basis for their services on the board of directors as follows:
• each non-employee director will receive an annual cash retainer of $20,000;
• each non-employee director will receive an annual stock option grant to purchase 2,000 shares of our common stock, generally to be granted in quarterly installments;
• the chair of the Audit Committee will receive an additional annual retainer of $5,000;
• the chair of the Compensation Committee will receive and additional annual retainer of $5,000;
• the chair of the Nominating and Governance Committee will receive an additional annual retainer of $5,000; and
• Non-chair members of the Audit Committee, Compensation Committee and the Nominating and Governance Committee will receive an additional annual retainer of $1,500.
Each member of our board of directors will also be entitled to reimbursement of reasonable travel and other expenses incurred in connection with attending meetings of the board of directors and any committee on which the member serves.
We have not agreed to compensate any member of our board of directors or director nominees until after the closing of this offering.
99
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Transactions with Deal A Day Group Corp. (f/k/a Avisio, Inc.)
Our majority-owned OLI and NEA subsidiaries were previously majority-owned subsidiaries of Deal A Day Group Corp. (formerly known as Avisio, Inc.), or Deal A Day, an entity in which Amro Albanna, our Chairman and CEO, was the Chairman, CEO and holder of a varying aggregate of 34.3% to 24.6% of the issued and outstanding shares of common stock of Deal A Day, from January 1, 2011 to December 31, 2011. We acquired a majority equity stake in each of OLI and NEA from Deal A Day while Mr. Albanna controlled Deal A Day.
As for our shares of OLI common stock, we acquired:
• an aggregate of 4,500 such shares from Deal A Day in a number of private securities purchase transactions from June 2011 to November 2011 in exchange for an aggregate of $31,067 and 533,333 shares of our common stock; and
• 9,400 such shares directly from OLI in exchange for an aggregate of $860,046.
As for our shares of NEA common stock, we acquired:
• an aggregate of 4,500 such shares from Deal A Day in a number of private securities purchase transactions from June 2011 to November 2011 in exchange for an aggregate of $187,024 and 133,333 shares of our common stock; and
• 1,812 such shares directly from NEA in exchange for an aggregate of $90,600.
As result of these transactions, Deal A Day currently owns 6.0% of our issued and outstanding shares of common stock.
Transaction with Innovation Economy Hub, Inc. (f/k/a Innovation Economy Initiative, Inc., Innovation Economy Corporation, Inc., Extreme Entrepreneurship Exchange, Inc.)
On April 1, 2012 we purchased all 100 outstanding shares of Innovation Economy Hub, Inc. (“Hub”), a wholly owned subsidiary of ours that holds the lease of our office and laboratory premises, for a price of $100, from Mr. Albanna. Hub is a California corporation in which Mr. Albanna was the sole shareholder. The assets and liabilities of Hub are consolidated in the financial statements of the Company. As of March 31, 2015 and December 31, 2014, Hub was an inactive company with no assets or liabilities other than the lease.
Management Services Agreement with Olfactor Laboratories, Inc.
On April 1, 2012, our parent company entered into a Management Services Agreement with our majority-owned subsidiary OLI. The agreement calls for our parent company to provide management services, administration, finance, accounting and other corporate functions for a fee of $14,229 per month.
Management Services Agreement with Nano Engineered Applications, Inc.
On January 1, 2013, our parent company entered into a Management Services Agreement with our majority-owned subsidiary NEA. The agreement calls for our parent company to provide management services, administration, finance, accounting and other corporate functions for a fee of $14,229 monthly.
Campaign Management Agreement with Olfactor Laboratories, Inc.
On May 15, 2013, our parent company entered into a Campaign Management Agreement with our majority-owned subsidiary OLI. The agreement called for our parent company to design, develop and conduct a crowdfunding campaign for OLI. The fee for the campaign was 40.0% of the 516,974 net total amount raised in the campaign. See “Business — ieCrowd - The Parent Company — Business Model”.
Advertising Agreement with Sunbelt IV Technologies Management, LLC
On August 21, 2014 we entered into an Advertising Agreement with Sunbelt IV Technologies Management, LLC, or Sunbelt IV. The management of Sunbelt IV controls Sunbelt III Technologies Management, LLC, which is one
100
of our principal stockholders. Under the agreement, Sunbelt IV provided us advertising services related to our 2014 Private Placement, which was conducted under Rule 506(c) of Regulation D under the Securities Act and the JOBS Act. The agreement was amended on August 22, 2014. Sunbelt IV received a total of $16,000 under the agreement.
Gift Agreement with the Regents of the University of California
On March 8, 2012 we entered into a Gift Agreement with the Regents of the University of California (the “Regents”) on behalf of the UCR. The Regents are shareholders in our NEA and OLI subsidiaries under the name of Shellwater & Co. Under the agreement, we gave a gift of $100,000 to UCR in support of research in materials electrochemistry under the direction of Dr. Nosang V. Myung, professor in the UCR Department of Chemical & Environmental Engineering and a shareholder of our NEA subsidiary. In recognition of the gift, UCR named a laboratory in which the research would be conducted the Innovation Economy Corporation Lab.
While we believe we benefitted from all of the above-referenced agreements, due to their related party nature such agreements may not reflect the terms that would have been reached by two unaffiliated parties negotiating at arm’s length. The transactions may have been less favorable to us than would have been the case if they had been negotiated with unaffiliated third parties.
Other than the foregoing, none of our directors or executive officers, nor any person who owned of record or was known to us to own beneficially more than 5% of our outstanding shares of common stock, nor any associate or affiliate of any such persons, has any material interest, direct or indirect, in any transaction that has occurred during the past fiscal year, or in any proposed transaction, which has materially affected or will materially affect us.
In connection with our preparation for this offering, we have established an Audit Committee of the board of directors. Following the completion of this offering, one of the responsibilities of our Audit Committee will be to review and approve or ratify related party transactions.
Agreement with Company Managed by an Affiliate of Sunbelt Technologies Management
On May 1, 2015 ieCrowd executed an agreement with Accelera LLC, a company managed by an affiliate of Sunbelt III Technologies Management, LLC, one of our principal stockholders, pursuant to which Accelera gave up options to license certain patents and patent applications, allowing ieCrowd to negotiate its own options over those patents and applications. As an inducement to Accelera to terminate its options, ieCrowd agreed that if it exercises an option to license any or all of the relevant patent families, ieCrowd shall grant Accelera 125,000 shares of ieCrowd’s common stock (on a restricted basis) and pay Accelera $132,367.77 (as reimbursement for Accelera’s cash expenses related to its option agreements). The agreement between ieCrowd and Accelera was negotiated on an arm’s-length basis.
101
PRINCIPAL STOCKHOLDERS
The following table sets forth the numbers and percentages of our common stock beneficially owned both immediately before and immediately after the closing of this offering by:
• each person known to us to be the beneficial owner of more than 5% of any class of our outstanding voting shares;
• each of our directors and director nominees;
• each of our executive officers; and
• all of our directors, director nominees and executive officers as a group.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock that such person or any member of such group has the right to acquire within 60 days of the date of this prospectus. For purposes of computing the percentage of outstanding shares of our common stock held by each person or group of persons named above, any shares that such person or persons has the right to acquire within 60 days of the date of this prospectus are deemed to be outstanding for such person, but not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares listed as beneficially owned does not constitute an admission of beneficial ownership by any person.
In the table below, the number of shares of common stock outstanding before this offering is deemed to be 9,444,828. Each of this number, the number of shares of common stock shown in the table below to be outstanding after this offering and the percentages shown in the table reflect the inclusion of 994,652 shares of common stock included in the Units issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest that will take place immediately after the closing of this offering. By the terms of the Private Placement Convertible Notes, this conversion will be effected at a price per unit equal to $5.10. The numbers of shares of common stock shown to be outstanding both before and after this offering and the percentages shown exclude:
• 2,645,967 shares of our common stock issuable upon the exercise of stock options outstanding as of the date of this prospectus, at a weighted average exercise price of $1.98 per share;
• 240,333 shares of our common stock issuable upon the exercise of warrants outstanding as of the date of this prospectus, at a weighted average exercise price of $2.29 per share;
• 20,700 shares of our common stock available for future issuance under our Amended and Restated Long-Term Incentive Compensation Plan as of the date of this prospectus;
• 1,250,000 shares of our common stock that will be made available for future issuance under our 2015 equity incentive plan upon the closing of this offering;
• 1,562,500 shares of our common stock issuable upon exercise of the warrants included in the Units being offered in this offering, assuming all of the Units being offered are sold;
• 234,375 shares of our common stock issuable upon exercise of the warrants to be issued to the selling agent in connection with this offering;
• 497,326 shares of our common stock issuable upon exercise of the warrants included in the Units issuable upon conversion of the Private Placement Convertible Notes immediately after the closing of this offering; and
• 34,824 shares of our common stock issuable upon conversion of the notes issuable upon the exercise of placement agent warrants issued in connection with the 2014 Private Placement.
Unless otherwise indicated, the business address of each person listed is c/o Innovation Economy Corporation, 1650 Spruce Street, Suite 500, Riverside, California 92507.
102
|
|
|
Common Stock(1) |
||||||||||
|
|
|
Before the Offering |
|
After the Offering |
||||||||
|
Name and Address of Beneficial Owner |
|
Number of Shares of Common Stock Beneficially Owned |
|
Percentage of Common Stock Beneficially Owned(2) |
|
Number of Shares of Common Stock Beneficially Owned |
|
Percentage of Common Stock Beneficially Owned(3) |
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amro Albanna |
|
3,383,493 |
(4) |
|
32.4 |
% |
|
3,833,493 |
(4) |
|
24.9 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rowena Albanna |
|
750,000 |
(5) |
|
7.2 |
% |
|
750,000 |
(5) |
|
5.5 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dale Hutchins |
|
816,667 |
(6) |
|
7.8 |
% |
|
816,667 |
(6) |
|
6.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stephen Abbott |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vernon Hall |
|
3,334 |
(7) |
|
|
* |
|
3,334 |
(7) |
|
|
* |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
George Reyes |
|
— |
|
|
— |
|
|
— |
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
All directors, director nominees and executive officers as a group (nine individuals) |
|
4,200,160 |
|
|
40.2 |
% |
|
4,200,160 |
|
|
31.0 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sunbelt III Technologies Management, LLC 6040 S Durango Drive, Suite 105, Las Vegas, NV 89113 |
|
2,000,000 |
|
|
19.2 |
% |
|
2,000,000 |
|
|
14.7 |
% |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deal A Day Group Corp. f/k/a Avisio, Inc. 5150 E Pacific Coast Hwy, Suite 200, Long Beach, CA, 90804 |
|
666,667 |
|
|
6.4 |
% |
|
666,667 |
|
|
4.9 |
% |
____________
* Less than 1%
(1) The number and percentage of shares beneficially owned is determined under rules promulgated by the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the individual has sole or shared voting power or investment power and also any shares that the individual has the right to acquire within 60 days through the exercise of any stock option, warrant or other right. The persons named in the table have sole voting and investment power with respect to all shares of common stock shown that are beneficially owned by them, subject to community property laws where applicable and the information contained in the footnotes to this table.
(2) Based on 9,444,828 shares deemed to be issued and outstanding.
(3) Based on 13,599,304 shares deemed to be issued and outstanding, which assumes that all of the Units being offered are sold.
(4) Amro Albanna is Chief Executive Officer and Chair of the Board of Directors of the Company. His beneficial ownership includes: (i) 966,826 shares held by Amro Albanna; and (ii) 1,666,667 shares held by Amro Albanna and Rowena Albanna, as the beneficiaries of the Albanna Family Trust. In addition, Amro Albanna is married to Rowena Albanna. The sum of the shares beneficially owned by either of them is 3,383,493.
(5) Rowena Albanna is Chief Operating Officer of the Company. Her beneficial ownership includes: (i) 750,000 shares held by Rowena Albanna; and (ii) 1,666,667 shares held by Amro Albanna and Rowena Albanna, as the beneficiaries of the Albanna Family Trust. In addition, Rowena Albanna is married to Amro Albanna. The sum of the shares beneficially owned by either of them is 3,383,493.
(6) Dale Hutchins is the President of the Company. His beneficial ownership includes: (i) 483,334 shares held by Dale Hutchins; (ii) 266,667 shares held by Canyon Ridge Development LLC, of which Dale Hutchins is the sole member, with voting and dispositive power over such securities; and (iii) 66,667 shares underlying options that were awarded on December 29, 2012 and are fully vested and exercisable.
(7) Vernon Hall is a director nominee. His beneficial ownership includes 3,334 shares held by the Hall Family Trust.
103
DESCRIPTION OF SECURITIES
The Company’s authorized capital consists of 500,000,000 shares of common stock, par value $0.00001 per share, and no shares of preferred stock.
Units
Each Unit being offering in this offering consists of one share of our common stock and one warrant to purchase one-half share of our common stock. The Units will not be issued or certificated. Instead, the shares of common stock and the warrants will be issued separately and may be resold separately, although they will have been purchased together in this offering.
Common Stock
As of March 31, 2015 and December 31, 2014, there were 9,444,828 shares of common stock outstanding, which were held by approximately 139 stockholders of record. Each outstanding share of common stock entitles the holder thereof to one vote per share on matters submitted to a vote of stockholders. Stockholders do not have preemptive rights to purchase shares in any future issuances of our common stock.
The holders of shares of our common stock are entitled to dividends out of funds legally available when and as declared by our board of directors. Our board of directors has never declared a dividend. See “Dividend Policy”. Each share shall be entitled to the same dividend. In the event of our liquidation, dissolution or winding up, holders of our common stock are entitled to receive, ratably, the net assets available to stockholders after payment of all creditors.
All of the issued and outstanding shares of our common stock are duly authorized, validly issued, fully paid and non-assessable. To the extent additional shares of our common stock are issued, the relative interests of existing stockholders will be diluted.
As of January 16, 2015, we effectuated a 15-1 reverse split of our shares, meaning that 15 of our shares held prior to the reverse split equals one share held after the reverse split. If, as a result of the split, any stockholder would be entitled to receive a fraction of a share, in order to avoid issuing fractional shares we will instead provide that stockholder with an additional whole share. All references to the number of shares, options, warrants and other common stock equivalents, price per share and weighted-average number of shares of common stock outstanding presented in this prospectus retroactively reflect the effectuation of the 15-1 reverse split dated January 16, 2015, unless otherwise stated or unless the context otherwise indicates.
Warrants Included in the Units
Each warrant included in the Units will entitle the holder to purchase one-half share of our common stock at an exercise price of 125% of the price of the shares in this offering. The warrants shall be exercisable from the date of issuance (except for those persons subject to the lock-up agreements discussed under “Shares Eligible for Future Sale”), which is the closing date of this offering, and expire on the 36-month anniversary thereof.
We may redeem all or a portion of these warrants to the extent they remain outstanding and unexercised:
• at a price of $0.01 per warrant;
• upon a minimum of 30 days’ prior written notice of redemption, which we refer to as the 30-day redemption period;
• if, and only if, the last sale price of our common stock equals or exceeds $16.00 per share (as may be adjusted for stock splits and similar transactions) on each of 20 trading days within the 30 trading-day period ending on the third business day prior to the date on which notice of the redemption is given to the warrant holders; and
• if, and only if, the average daily trading volume of our common stock exceeds 500,000 shares per day during the redemption period.
We will not redeem the warrants unless an effective registration statement under the Securities Act covering the shares of common stock issuable upon exercise of the warrants is effective, except if the warrants may be exercised on a cashless basis and such cashless exercise is exempt from registration under the Securities Act.
104
No fractional shares or scrip representing fractional shares shall be issued upon the exercise of the warrants. As to any fraction of a share which the holder would otherwise be entitled to purchase upon such exercise, the Company shall, at its election, either pay a cash adjustment in respect of such fraction (in an amount equal to such fraction multiplied by the exercise price) or round the number of shares to be received by the holder up to the next whole number.
Convertible Notes
Our outstanding Private Placement Convertible Notes are not being offered in this offering, but pursuant to the terms thereof, the Company will elect to convert such notes into Units immediately after the closing of this offering.
Pursuant to the Private Placements, we issued Private Placement Convertible Notes plus accrued interest that are convertible into units of our common stock, including, if the Company so elects, immediately after the closing of this offering, into units identical to the Units being offered in this offering. Up to 994,652 of such units, issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest, and 34,824 additional such units, issuable upon the forced conversion of notes issuable upon the exercise of placement agent warrants, are being offered under a separate prospectus for selling securityholders. As of December 31, 2014, the aggregate amount of outstanding unpaid principal under the Private Placement Convertible Notes was $2,785,000 and the aggregate amount of accrued interest under the Private Placement Convertible Notes was $48,273. Subsequent to December 31, 2014, we have secured additional debt financing in the form of Private Placement Convertible Notes aggregating $2,100,000. Unless converted or repaid in full before maturity, the Private Placement Convertible Notes placed prior to December 31, 2014 mature in October 2016 and the Private Placement Convertible Notes placed subsequent to December 31, 2014 mature in April 2017.
The holders of the Private Placement Convertible Notes (the “Holders”) have certain rights to convert the Private Placement Convertible Notes, in whole or in part, into units of our common stock, at $5.10 per unit, subject to the Company’s right to cause the Holders to convert in the case of a Forced Conversion Event, as described below.
If, during the term of the Private Placement Convertible Notes, the Company completes a Forced Conversion Event, the Company can cause the Holders to convert all or any portion of their outstanding Private Placement Convertible Notes into the securities issued in such event at a price equal to 80% of the price of the common stock issued in such event (the “Discount Conversion Price”). A Forced Conversion Event consists of either an initial public offering of the Company’s common stock or a transaction resulting in gross proceeds of more than $7,500,000 to the Company. Once the Discount Conversion Price is established and used, that shall be the Conversion Price of all Private Placement Convertible Notes, whether or not previously converted in full. Immediately after the closing of this offering, the Company will exercise its right to convert all of the Convertible Notes into Units at a conversion price per Unit equal to $5.10. There is no minimum amount of securities that must be sold in the Company’s initial public offering for the conversion of the Private Placement Convertible Notes to occur, and the conversion will occur even if only a few Units are sold. If the offering does not close and the Company does not otherwise elect to force a conversion when able, the Private Placement Convertible Notes will remain outstanding until the earlier of the time at which the holder converts them in full or maturity.
Notwithstanding any other terms to the contrary, no conversion of the Private Placement Convertible Notes, either by the Holder or by the Company, is permitted if the issuance of shares pursuant to such conversion would cause the Holder’s beneficial ownership of common stock to exceed 9.99% of our then outstanding shares of common stock.
We granted investors in the Private Placement certain “piggyback” registration rights in regard to any registered securities offering by us, on our own behalf or on behalf of selling securityholders, subject to customary exceptions. Contemporaneously with the filing with the SEC of the registration statement of which this prospectus forms a part, a resale registration statement is being filed for the purpose of providing such investors the opportunity to resell the securities they are receiving as a result of the forced conversion of the Private Placement Convertible Notes.
Interest on the unpaid principal balance of the Private Placement Convertible Notes shall accrue at the rate of 8% per annum, which shall increase to 10% upon the occurrence of an event of default, as such term is defined in the Private Placement Convertible Notes. Upon an event of default, as defined in the Private Placement Convertible Notes, the Holders may declare the unpaid principal balance, together with all accrued and unpaid interest thereon, immediately due and payable.
105
Warrants to be Issued to Selling Agent in Connection with this Offering
As part of its compensation for serving as the Company’s selling agent in this offering, TriPoint Global Equities, LLC, will receive warrants exercisable for three years entitling it to purchase for cash such number of shares of common stock as are equal to 5% of the number of Units sold in this offering, at an exercise price equal to 110% of the offering price of the Units sold in this offering, and will also receive warrants exercisable for three years entitling it to purchase for cash such number of shares of common stock as are equal to 5% of the number of shares of common stock underlying the warrants included in the Units sold in this offering, at an exercise price equal to 110% of the exercise price of the warrants in this offering (excluding, in each case, the issuance of securities upon the conversion of the Private Placement Convertible Notes). Such selling agent warrants will be subject to FINRA Rule 5110(g)(1) in that, except as otherwise permitted by FINRA rules, for a period of 180 days following the date of effectiveness or commencement of sales of this offering, such selling agent warrants shall not be (A) sold, transferred, assigned, pledged or hypothecated or (B) the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person. We may redeem all or a portion of these warrants to the extent they remain outstanding and unexercised on terms substantially similar to the terms for the warrants included in the Units, as described under “—Warrants Included in the Units”.
Additional Information
We refer you to our Certificate of Incorporation and Bylaws and the applicable provisions of the Delaware General Corporation Law for a more complete description of the rights and liabilities of holders of our securities.
106
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no market for our common stock or warrants. Future sales of substantial amounts of our common stock, or securities or instruments convertible into our common stock, in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock prevailing from time to time. Furthermore, because there will be limits on the number of shares available for resale shortly after this offering due to contractual and legal restrictions described below, there may be resales of substantial amounts of our common stock in the public market after those restrictions lapse. This could adversely affect the market price of our common stock prevailing at that time.
Upon completion of this offering, assuming that all Units offered in this offering are sold, we will have 13,599,304 shares of common stock outstanding including (i) 3,125,000 shares included in the Units offered in this offering and (ii) 994,652 shares included in the Units issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest immediately after the closing of this offering. In addition, prior to the closing of this offering, we expect the placement agent warrants from the 2014 Private Placement to be exercised for notes which, immediately after the closing of this offering, will be converted into 34,824 units, which would include 34,824 shares. Of these shares, 4,119,652 shares (including (i) 3,125,000 shares included in the Units offered in this offering and (ii) 994,652 shares included in the Units issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest immediately after the closing of this offering) will have been issued in or contemporaneously with the closing of this offering, all of which will be freely transferable without restriction or further registration under the Securities Act, except for any shares acquired by any of our “affiliates,” as that term is defined in Rule 144 under the Securities Act. In addition, the 34,824 shares underlying the units issuable upon the exercise of placement agent warrants from the 2014 Private Placement will be freely tradable without restriction or further registration under the Securities Act. The remaining 9,444,828 shares will be “restricted shares” as defined in Rule 144. Restricted shares may be sold in the public market only if registered under the Securities Act or if they qualify for an exemption from registration, including under Rule 144. In addition, as a result of the lock-up agreements described below, approximately 4,950,159 shares will be subject to the additional contractual lock-up provisions described below.
Rule 144
In general, a person who has beneficially owned restricted shares of our common stock for at least six months would be entitled to sell such securities, provided that (i) such person is not deemed to have been one of our affiliates at the time of, or at any time during the 90 days preceding, the sale and (ii) we are subject to the periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Persons who have beneficially owned restricted shares of our common stock for at least six months but who are affiliates of ours at the time of, or at any time during the 90 days preceding, the sale, would be subject to additional restrictions, by which such person would be entitled to sell within any three-month period only a number of securities that does not exceed the greater of the following:
• 1% of the number of shares of our common stock then outstanding, which will equal approximately 135,993 shares immediately after this offering; or
• the average weekly trading volume of our common stock during the four calendar weeks preceding the filing by such person of a notice on Form 144 with respect to the sale;
provided, in each case, that we are subject to the periodic reporting requirements of the Exchange Act for at least 90 days before the sale. Such sales by non-affiliates and affiliates must also comply with the manner of sale, notice and other provisions of Rule 144, to the extent applicable.
Rule 701
In general, Rule 701 allows a stockholder who purchased shares of our capital stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of ours during the immediately preceding 90 days to sell those shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. All holders of Rule 701 shares, however, are required to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701.
107
Lock-up Agreements
The Company, along with its directors and executive officers and the holders of 5% or more of its shares, have agreed with the selling agent to the following lock-up provisions with regard to future sales of our common stock, warrants and other securities convertible into or exercisable or exchangeable for common stock, for the period beginning on the effective date of this Registration Statement and ending 180 days thereafter, with the following exceptions:
• transfers to spouses, siblings, parents or any natural or adopted children or other descendants or to any personal trust of the Transferor;
• transfers upon death to an estate, executor, administrator or personal representative or to beneficiaries pursuant to a devise or bequest or by laws of descent and distribution;
• gift transfers or other transfer without consideration;
• transfers as a bona fide pledge of shares to a lender; and,
• participation in any transaction in which all holders of the shares of ieCrowd participate or have the opportunity to participate pro rata, including, without limitation, a merger, consolidation or binding share exchange involving ieCrowd, a disposition of the shares in connection with the exercise of any rights, warrants or other securities distributed to ieCrowd’s stockholders, or a tender or exchange offer.
108
CERTAIN U.S. FEDERAL INCOME TAX CONSIDERATIONS
The following are the material U.S. federal income tax consequences to non-U.S. holders, as defined below, of the ownership and disposition of the securities being sold in this offering. The discussion below of the U.S. federal income tax consequences with respect to actual holders of common stock and warrants should also apply to holders of Units (as the deemed owners of the underlying common stock and warrants that comprise the Units). This summary applies to you only if you are a non-U.S. holder of our securities that purchases such securities in this offering and will hold such securities as capital assets within the meaning of section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”) (generally, property held for investment).
For purposes of this discussion, a “non-U.S. holder” means a beneficial owner of our securities that, for U.S. federal income tax purposes, is not any of the following:
• an individual who is a citizen or resident of the United States;
• a corporation (or any other entity treated as a corporation for U.S. federal income tax purposes) created or organized (or deemed to be created or organized) in or under the laws of the United States, any state thereof or the District of Columbia;
• an estate the income of which is subject to U.S. federal income taxation regardless of its source;
• a trust if it (1) is subject to the primary supervision of a court within the United States and one or more U.S. persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable U.S. Treasury Regulations to be treated as a U.S. person for U.S. federal income tax purposes; or
• an entity treated as a partnership or other pass-through entity for U.S. federal income tax purposes.
Additionally, this discussion does not consider the tax treatment of partnerships or other pass-through entities that hold our securities, or persons who hold our securities through such entities. If any entity or arrangement treated as a partnership for U.S. federal income tax purposes holds our securities, the tax treatment of a partner in such partnership generally will depend upon the status of the partner and the activities of the partner and the partnership. If you are a partner or owners of a partnership or other pass-through entity considering an investment in our securities, you should consult your tax advisors.
This summary is based upon provisions of the Code, applicable U.S. Treasury Regulations promulgated thereunder, rulings and other administrative pronouncements, and judicial decisions, all as of the date hereof. These authorities are subject to different interpretations and may be changed, perhaps retroactively, so as to result in U.S. federal income tax consequences different from those summarized below. We cannot assure you that a change in law will not significantly alter the tax considerations described in this summary.
This summary assumes that the common stock and warrants underlying a unit will trade separately and does not address all aspects of U.S. federal income taxation (such as the alternative minimum tax or the 3.8% Medicare tax on certain investment income). This summary also does not address any aspects of other federal taxes (such as gift and estate taxes) or state, local or non-U.S. taxes that may be relevant to non-U.S. holders in light of their particular circumstances. In addition, this summary does not describe the U.S. federal income tax consequences applicable to you if you are subject to special treatment under U.S. federal income tax laws (including if you have income that is effectively connected with a U.S. trade or business or if you are a U.S. expatriate or an entity subject to the U.S. anti-inversion rules, a bank or other financial institution, an insurance company, a tax-exempt organization, a trader, broker or dealer in securities or currencies, a regulated investment company, a real estate investment trust, a “controlled foreign corporation,” a “passive foreign investment company,” an entity treated as a partnership or other pass-through entity for U.S. federal income tax purposes (or an investor in such a partnership or pass-through entity), a person who acquired our securities as compensation or otherwise in connection with the performance of services, a person whose functional currency is other than the United States dollar, or a person who has acquired our securities as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment).
We have not sought and do not expect to seek any rulings from the IRS regarding the matters discussed below. There can be no assurance that the IRS will not take positions concerning the tax consequences of the ownership or disposition of our securities that differ from those discussed below.
109
This discussion is only a summary of the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our securities and is not intended to constitute a complete description of all U.S. federal income tax consequences for non-U.S. holders relating to the acquisition, ownership and disposition of our securities. If you are considering the purchase of our securities, you should consult your tax advisors concerning the particular U.S. federal income tax consequences to you of the acquisition, ownership and disposition of our securities, as well as the consequences to you arising under other U.S. federal tax laws and the laws of any other applicable taxing jurisdiction in light of your particular circumstances.
Allocation of Purchase Price and Characterization of a Unit
There is no authority addressing the treatment, for U.S. federal income tax purposes, of securities with terms substantially the same as the units being offered in this offering, and, therefore, that treatment is not entirely clear. Each unit may be treated for U.S. federal income tax purposes as an investment unit consisting of one shares of our common stock and one warrant to acquire one-half share of our common stock. If this is the case, then for U.S. federal income tax purposes, each holder of a unit may be required to allocate the purchase price of a unit among the shares of common stock and the warrant that comprise the unit based on the relative fair market value of each at the time of issuance. The price allocated to each such share or warrant generally will be the holder’s tax basis in such share, right or warrant, as the case may be.
Neither the foregoing description of the treatment of our common stock and warrants nor a holder’s purchase price allocation is binding on the IRS or the courts. Because there are no authorities that directly address instruments that are similar to the units, no assurance can be given that the IRS or the courts will agree with the characterization described above or the discussion below. Accordingly, each holder is advised to consult its own tax advisor regarding the risks associated with an investment in a unit (including alternative characterizations of a unit) and regarding an allocation of the purchase price among the common stock and the warrant that comprise a unit. The balance of this discussion generally assumes that the characterization of the units described above is respected for U.S. federal income tax purposes.
Dividends
In general, distributions on shares of our common stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent any such distributions exceed both our current and our accumulated earnings and profits, they will first be treated as a return of capital reducing a non-U.S. holder’s tax basis in our common stock (determined on a share by share basis), but not below zero, and thereafter will be treated as gain from the sale of such stock, the treatment of which is discussed below under “Gain on Disposition of Shares of Common Stock.”
As discussed under “Dividend Policy” above, we do not currently expect to pay dividends. In the event that we do pay dividends, dividends paid to a non-U.S. holder generally will be subject to a U.S. federal withholding tax at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty. A non-U.S. holder of shares of our common stock who wishes to claim the benefit of an applicable treaty rate (and avoid backup withholding, as discussed below) for dividends generally will be required (a) to complete IRS Form W-8BEN (or other applicable form) and certify under penalty of perjury that such holder is not a “United States person” as defined under the Code and is eligible for treaty benefits, or (b) if shares of our common stock are held through certain foreign intermediaries (including certain foreign partnerships), satisfy the relevant certification requirements of applicable U.S. Treasury Regulations. This certification must be provided to us or our paying agent prior to the payment to the non-U.S. holder of any dividends, and may be required to be updated periodically.
A non-U.S. holder of shares of our common stock eligible for a reduced rate of United States federal withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
110
Gain on Disposition of our Securities
Subject to the discussions below of backup withholding and the Foreign Account Tax Compliance Act (“FATCA”) legislation, any gain realized by a non-U.S. holder on the sale or other disposition of our securities generally will not be subject to United States federal income tax, unless:
• the non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of such sale or other disposition, and certain other conditions are met; or
• we are or have been a U.S. real property holding corporation (a “USRPHC”) for U.S. federal income tax purposes at any time during the shorter of the five-year period ending on the date of the disposition or the period that the non-U.S. holder held such securities (the “applicable period”).
The determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to the fair market value of other business assets, there can be no assurance that we are not currently or will not become a USRPHC in the future. Even if we are or become a USRPHC, so long as our common stock is regularly traded on an established securities market, a non-U.S. holder will be subject to U.S. federal income tax on any gain not otherwise taxable only if such non-U.S. holder actually or constructively owned more than five percent of our outstanding common stock at some time during the applicable period. You should consult your tax advisor about the consequences that could result if we are, or become, a USRPHC.
Acquisition of Common Stock Pursuant to the Exercise of a Warrant
A non-U.S. holder generally will not recognize gain or loss upon the acquisition of common stock pursuant to the exercise of a warrant for cash. Common stock acquired pursuant to the exercise of a warrant for cash generally will have a tax basis equal to the non-U.S. holder’s tax basis in the warrant, increased by the amount paid to exercise the warrant. The holding period of such common stock generally would begin on the day after the date of receipt of such common stock upon exercise of the warrant and will not include the period during which the non-U.S. holder held the warrant. If a warrant is allowed to lapse unexercised, a non-U.S. holder generally will recognize a capital loss equal to such holder’s tax basis in the warrant.
The tax consequences of a cashless exercise of a warrant are not clear under current tax law. A cashless exercise may be tax-free, either because the exercise is not a gain realization event or because the exercise is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a non-U.S. holder’s basis in the common stock received would equal the holder’s basis in the warrant. If the cashless exercise were treated as not being a gain realization event, a non-U.S. holder’s holding period in the common stock would be treated as commencing on the date following the date of exercise of the warrant. If the cashless exercise were treated as a recapitalization, the holding period of the common stock would include the holding period of the warrant. It is also possible that a cashless exercise could be treated as a taxable exchange in which gain or loss would be recognized. In such event, a non-U.S. holder could be deemed to have surrendered warrants equal to the number of shares of common stock having a value equal to the exercise price for the total number of warrants to be exercised. The non-U.S. holder would recognize capital gain or loss in an amount equal to the difference between the fair market value of the common stock represented by the warrants deemed surrendered and the non-U.S. holder’s tax basis in the warrants deemed surrendered. In this case, a non-U.S. holder’s tax basis in the common stock received would equal the sum of the fair market value of the common stock represented by the warrants deemed surrendered and the non-U.S. holder’s tax basis in the warrants exercised. A non-U.S. holder’s holding period for the common stock would commence on the date following the date of exercise of the warrant. Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise, there can be no assurance which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, non-U.S. holders should consult their tax advisors regarding the tax consequences of a cashless exercise.
If the cashless exercise of a warrant results in taxable gain to a non-U.S. holder, then the consequences to such holder will be as described above under “—Gain on Disposition of our Securities.”
Information Reporting and Backup Withholding
The amount of dividends paid to each non-U.S. holder, and the tax withheld with respect to such dividends generally will be reported annually to the IRS and to each such holder, regardless of whether withholding was reduced or
111
eliminated by an applicable tax treaty. Copies of the information returns reporting such dividends and withholding may also be made available to the tax authorities in the country in which the non-U.S. holder resides or is established under the provisions of an applicable income tax treaty or agreement.
A non-U.S. holder generally will be subject to backup withholding with respect to dividends paid to such holder unless such holder certifies under penalty of perjury (generally on an applicable IRS Form W-8) that it is not a “United States person” as defined under the Code (and the payer does not have actual knowledge or reason to know that such holder is such a United States person), or such holder otherwise establishes an exemption.
Information reporting and, depending on the circumstances, backup withholding will apply to the proceeds of a sale or other disposition by a non-U.S. holder of our securities within the United States or conducted through certain U.S.-related financial intermediaries, unless such non-U.S. holder certifies under penalty of perjury that it is not a “United States person” (as defined under the Code), and the payer does not have actual knowledge or reason to know that the non-U.S. holder is such a United States person, or such non-U.S. holder otherwise establishes an exemption.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s U.S. federal income tax liability provided the required information is timely furnished to the IRS.
Legislation Affecting Taxation of Securities Held by or Through Foreign Entities
Legislation enacted in 2010, known as the “FATCA” legislation, generally will impose a withholding tax of 30% on dividend income from our common stock and on the gross proceeds of a sale or other disposition of our securities paid to certain “foreign financial institutions” (as specifically defined under FATCA), unless such institution enters into an agreement with the U.S. government to, among other things, collect and provide to the U.S. tax authorities substantial information regarding U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are non-U.S. entities with U.S. owners), or another exception applies. The FATCA legislation also generally will impose a withholding tax of 30% on dividend income from our common stock and the gross proceeds of a sale or other disposition of our securities paid to a non-U.S. entity that is not a “foreign financial institution” unless such entity provides the applicable withholding agent with a certification identifying the substantial U.S. owners of the entity (if any), which generally includes any U.S. person who directly or indirectly owns more than 10% of the entity (or more than zero percent in the case of some entities), or another exception applies. Under certain circumstances, a non-U.S. holder of our securities might be eligible for refunds or credits of such withholding taxes, and a non-U.S. holder might be required to file a U.S. federal income tax return to claim such refunds or credits. Under final U.S. Treasury Regulations and subsequent administrative guidance, this legislation only applies to payments of dividends made after June 30, 2014 and payments of gross proceeds made after December 31, 2016. Non-U.S. holders should consult their tax advisors regarding the implications of this legislation on their investment in our securities.
POTENTIAL PURCHASERS OF OUR SECURITIES ARE URGED TO CONSULT THEIR TAX ADVISORS TO DETERMINE THE U.S. FEDERAL, STATE, LOCAL AND NON-U.S. INCOME, ESTATE AND OTHER TAX CONSIDERATIONS OF OWNING AND DISPOSING OF OUR SECURITIES.
112
PLAN OF DISTRIBUTION
We are publicly offering our Units through TriPoint Global Equities, LLC (“TriPoint”) and through their BANQ online brokerage division. TriPoint has agreed to act as our selling agent for this offering. The selling agent is not purchasing any of the securities offered by us and is not required to sell any specific number or dollar amount of securities, but will instead arrange for the sale of securities to investors on a “best efforts” basis, meaning that it need only use its best efforts to sell the securities.
Our board of directors has determined the offering price of the Units and the exercise price of the warrants. They have done so with reference to the value of our Company as reflected in the Private Placements, but not otherwise with reference to our book value or asset values or by the application of any customary, established models for valuing companies or securities. Because such prices have not been so determined, they may not be indicative of any amounts you might receive should you seek to sell your Company securities or should there be a liquidation of the Company. In addition, such prices are not necessarily indicative of any prices at which Company securities may trade, or any value that might be ascribed to the Company after the completion of the offering.
We are offering up to 3,125,000 Units, each Unit consisting of one share of our common stock and one warrant to purchase one-half share of our common stock. Each warrant will entitle the holder to purchase one-half share of our common stock at an exercise price of 125% of the price of the shares in this offering. The warrants will expire 36 months after the date they are issued. The Units will not be issued or certificated. Instead, the shares of common stock and the warrants will be issued separately and may be resold separately, although they will have been purchased together in this offering.
The offering will close on June 25, 2015, 45 days after we commence it, unless all the securities are sold before that date, we and the selling agent agree to extend the offering for another 45 days or we otherwise decide to close the offering early or cancel it, in our sole discretion. If we and the selling agent extend the offering, we will provide that information in an amendment to this prospectus. If we close the offering early or cancel it, including during any extended offering period, we may do so without notice to you, although if we cancel the offering we will promptly return any escrow funds you may already have paid.
We plan to market this offering to potential investors through the selling agent and by ourselves, directly, at meetings that we will conduct at selected locations around the United States and internationally with prospective investors.
In order to participate in this offering, U.S. investors will be required to open an account with BANQ, an online brokerage division of TriPoint, the selling agent. The BANQ website may be found at Banq.co. BANQ is open to qualified U.S. investors and accepts individual, joint, corporate or IRA accounts. The application process takes approximately 10 minutes and there are no account minimums. Deposits to BANQ can be made via wire transfer or ACH deposit or by mailing in a check. Deposits usually post to an account within 3-5 days. BANQ® is a division of TriPoint Global Equities LLC, a member of the Financial Industry Regulatory Authority (FINRA) and the Securities Investor Protection Corporation (SIPC), which protects the securities of its members’ customers up to $500,000 (including $250,000 for claims for cash). Non-U.S. investors may participate in the offering by depositing their funds in an escrow account held at Wilmington Trust, N.A.; any funds Wilmington Trust receives shall be held in escrow until the closing of the offering or such other time as mutually agreed between the Company and the selling agent, and then used to complete securities purchases, or returned if this offering fails to close.
Investors investing through BANQ will be required to open their accounts and deposit funds into their respective BANQ accounts after the effectiveness of the registration statement relating to this offering but prior to the closing of this offering; in all events, no funds may be used to purchase securities issued in this offering until the registration statement relating to this offering and filed by the Company with the SEC has been declared effective by the SEC. After an account is opened but before 48 hours prior to the closing of the offering, the investor will be required to deposit funds into the account sufficient to purchase the amount of securities that the investor intends to purchase in the offering. Such funds will not be held in a separate escrow account or otherwise segregated as part of the offering process. During the marketing period for the offering and after the registration statement has been declared effective, the investor will provide an indication of interest as to the amount of securities the investor intends to purchase. Forty-eight (48) hours prior to the completion of the offering, each investor will be asked by BANQ via e-mail and notification to the secure messages section of the website for the BANQ online brokerage account to confirm and finalize the indication of the amount of securities such investor wishes to purchase. Indications will not be finalized without sufficient funds in the investor’s BANQ online brokerage account. Upon the final closing and completion of the offering, the funds required to purchase that amount of securities will be removed from such investor’s account and transferred to the account of the Company, and the amount of securities purchased will be deposited into such investor’s account. If an investor fails to confirm such investor’s desired investment within the required time, no funds will be withdrawn, no securities will be provided and the investor’s indication will not be confirmed.
113
We will pay the selling agent an aggregate selling agent fee equal to 6.0% of the gross proceeds of the sale of securities in the offering (excluding the issuance of securities upon the conversion of the Private Placement Convertible Notes); provided that the fee shall be equal to 4.0% for sales originating from non-U.S. investors and investors the Company introduces to TriPoint. In addition, subject to compliance with FINRA Rule 5110(f)(2)(D), the Company will reimburse TriPoint’s expenses up to $25,000 (exclusive of the fees of selling agent’s counsel, which the Company will reimburse (but not in excess of $75,000, or $50,000 in the event that less than $10 million of securities are sold in this offering)). Throughout this prospectus, when calculating expected net proceeds of this offering or otherwise calculating or assuming the commissions to be paid to the selling agent in this offering, we have assumed that all sales of Units generate a commission of 6.0% for the selling agent. After deducting the aforementioned fees due to the selling agent and our estimated offering expenses, we expect the net proceeds from this offering to be approximately $18,175,000 if all the securities being offered are sold.
The selling agency agreement between the selling agent and the Company provides that the obligations of the selling agent and the Company under that agreement are subject to certain customary conditions precedent, including the absence of any material adverse changes in our business and the receipt by the selling agent of certain customary legal opinions, letters and certificates prior to the completion of the offering.
TriPoint is an underwriter of this offering within the meaning of Section 2(a)(11) of the Securities Act and any commissions received by it and any profit realized on the sale of the securities by it while so acting may be deemed to be underwriting compensation. TriPoint will be required to comply with the requirements of the Securities Act and the Exchange Act, including, without limitation, Rule 10b-5 and Regulation M under the Exchange Act. These rules and regulations may limit the timing of purchases and sales of Units, shares of common stock and warrants to purchase shares of common stock by TriPoint. Under these rules and regulations, TriPoint may not, among other things, (i) engage in any stabilization activity in connection with our securities or (ii) bid for or purchase any of our securities or attempt to induce any person to purchase any of our securities, other than as permitted under the Exchange Act.
As part of its compensation for serving as selling agent in this offering, TriPoint will receive warrants exercisable for three years entitling it to purchase for cash such number of shares of common stock as are equal to 5% of the Units sold in this offering, at an exercise price equal to 110% of the offering price of the Units sold in this offering, and will also receive warrants exercisable for three years entitling it to purchase for cash such number of shares of common stock as are equal to 5% of the number of shares of common stock underlying the warrants included in the Units sold in this offering, at an exercise price equal to 110% of the exercise price of the warrants in this offering (excluding, in each case, the issuance of securities upon the conversion of the Private Placement Convertible Notes). Such selling agent warrants will be subject to FINRA Rule 5110(g)(1) in that, except as otherwise permitted by FINRA rules, for a period of 180 days following the date of effectiveness or commencement of sales of this offering, such selling agent warrants shall not be (A) sold, transferred, assigned, pledged or hypothecated or (B) the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person.
As part of its compensation for serving as placement agent for the 2014 Private Placement, TriPoint received warrants exercisable for five years entitling it to purchase up to $114,025 of notes with the same terms as those of the Private Placement Convertible Notes. Primary Capital, LLC, which served as a selected broker-dealer in the 2014 Private Placement, received similar warrants entitling it to purchase $4,375 of the same type of notes. Prior to the closing of this offering, TriPoint and Primary Capital, LLC are expected to exercise their warrants privately so as to purchase $114,025 and $4,375, respectively, of such notes. Immediately after the closing of this offering, upon our election to force the conversion of the Private Placement Convertible Notes, the notes held by TriPoint and Primary Capital, LLC will become exercisable into $114,025 and $4,375, respectively, of units, at a price equal to $5.10 per unit, with the same terms as the Units being offered in this offering. Such units would consist of an aggregate of 34,824 shares of common stock and 17,412 warrants with the same terms as the warrants being offered in this offering.
These warrants are considered an additional item of value pursuant to FINRA Rule 5110(c)(2)(C).
TriPoint is subject to the provisions of the lock-up agreement described above.
All of the warrants, and the securities underlying the warrants, issued to TriPoint from this offering and the 2014 Private Placement are subject to FINRA Rule 5110(g).
Pursuant to FINRA Rule 5110(g), the selling agent warrants and any shares issued upon exercise of the selling agent warrants and any securities TriPoint received from the 2014 Private Placement, shall not be sold, transferred, assigned, pledged, or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would
114
result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the date of effectiveness or commencement of sales of this offering, except the transfer of any security:
• by operation of law or by reason of reorganization of our company;
• to any FINRA member firm participating in the offering and the officers or partners thereof, if all securities so transferred remain subject to the lock-up restriction set forth above for the remainder of the time period;
• if the aggregate amount of securities of our company held by the holder of the selling agent warrants or related persons do not exceed 1% of the securities being offered;
• that is beneficially owned on a pro-rata basis by all equity owners of an investment fund, provided that no participating member manages or otherwise directs investments by the fund, and participating members in the aggregate do not own more than 10% of the equity in the fund; or
• the exercise or conversion of any security, if all securities received remain subject to the lock-up restriction set forth above for the remainder of the time period.
The selling agent does not have any present intention or arrangement to release any shares of our common stock subject to lock-up agreements prior to the expiration of the 180-day lock-up period.
The Company, along with its directors and executive officers and the holders of 5% or more of its shares, have agreed with the selling agent to certain lock-up provisions as described under “Share Eligible for Future Sale - Lock-up Agreements”.
We have agreed to indemnify the selling agent against certain liabilities, including liabilities under the Securities Act. We have also agreed to contribute to payments the selling agent may be required to make in respect of such liabilities.
We have applied to list our common stock and warrants on the NASDAQ Capital Market (“NASDAQ”) under the symbols “MYIE” and “MYIEW”, respectively, on or promptly after the date this offering is completed. We cannot guarantee that our securities will be approved for listing on NASDAQ. NASDAQ’s listing standards require, among other things, that we have stockholders’ equity of $5 million. As of March 31, 2015, we had total stockholders’ equity of $2.0 million (on a pro forma basis, taking into account the forced conversion, immediately after the closing of this offering, of our Private Placement Convertible Notes). This means that, to have closed this offering and listed on NASDAQ as of that date, we would have had to raise approximately $3 million. In this offering, if we are unable to raise the amount necessary to meet NASDAQ’s stockholders’ equity requirement, or if we otherwise fail to meet NASDAQ’s listing standards, our securities will not be listed on NASDAQ. In that event, we may in our sole discretion cancel some or all of this offering and thereafter promptly return any escrow funds you may already have paid. In the event we close on some or all of this offering but our securities are not listed on NASDAQ, we expect they will be quoted for trading on one of the “over the counter” markets operated by OTC Markets Group, Inc., such as the OTCQB market. The over-the-counter markets are relatively unorganized, inter-dealer markets that provide significantly less liquidity than NASDAQ. No assurance can be given that our securities, if quoted on one of the over-the-counter markets, will ever trade on an active and liquid basis.
The CUSIP for our common stock is 457696 102 and the CUSIP for our warrants is 457696 110.
The transfer agent for our shares is V-Stock Transfer & Trust Company.
State Blue Sky Information
In the event we do not qualify for listing on NASDAQ, we intend to offer and sell the Units to retail customers in the United States only in Colorado, Florida, Georgia, Illinois, Nevada and New York, where we have applied to have the Units registered for sale. We will not sell the Units to retail customers in these states until registration is effective in each such state (including in Colorado, pursuant to 11-51-302(6) of the Colorado Revised Statutes). Notwithstanding the foregoing, we may choose to register, or otherwise qualify, the Units for sale in additional states.
In the event we do not qualify for listing on NASDAQ and you are in the United States but not an “institutional investor”, you may purchase our securities in this offering only in the states in which we have registered or otherwise qualified them for sale. Institutional investors in every state may purchase the Units in this offering pursuant to exemptions provided to such entities under the blue sky laws of such states. The definition of an “institutional investor” varies from state to state but generally includes financial institutions, broker-dealers, banks, insurance companies and other qualified entities.
115
The National Securities Markets Improvement Act of 1996 (“NSMIA”), which is a federal statute, prevents or preempts the states from regulating transactions in certain securities, which are referred to as “covered securities”. NSMIA nevertheless allows the states to investigate if there is a suspicion of fraud or deceit, or unlawful conduct by a broker or dealer, in connection with the sale of securities. If there is a finding of fraudulent activity, the states can regulate or bar the sale of covered securities in a particular case.
State securities laws require that a company’s securities be registered for sale or that the securities themselves or the transaction under which they are issued, be exempt from registration. When a state law provides an exemption from
registration, it is excusing an issuer from the general requirement to register securities before they may be sold in that state. States may, by rule or regulation, place conditions on the use of exemptions, so that certain companies may not be allowed to rely on such exemptions for the sale of their securities. If an exemption is not available and the securities the company wishes to sell are not covered securities under NSMIA, then the company must register its securities for sale in the state in question.
We will file periodic and current reports under the Exchange Act. Therefore, under NSMIA, the states and territories of the United States are preempted from regulating the resale by stockholders of the Units and the common stock and warrants comprising the Units once they become separately transferable. However, NSMIA does allow states and territories to require notice filings and collect fees with regard to resale transactions, and a state may suspend the offer and resale of securities within such state if any such required filing is not made or fee is not paid. As of the date of this prospectus, the following states and territories do not require any resale notice filings or fee payments and stockholders may resell the Units, and the common stock and warrants comprising the Units once they become separately transferable: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Illinois, Indiana, Iowa, Kansas, Kentucky, Louisiana, Maine, Massachusetts, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Jersey, New Mexico, New York, North Carolina, Ohio, Oklahoma, Pennsylvania, Rhode Island, South Carolina, South Dakota, Utah, Virginia, Virgin Islands, Washington, West Virginia, Wisconsin and Wyoming.
As of the date of this prospectus, in the following states and territories, stockholders may resell the Units, and the common stock and warrants comprising the Units once they become separately transferable, if the proper notice filings have been made and fees paid: the District of Columbia, Maryland, Montana, New Hampshire, North Dakota, Oregon, Puerto Rico, Tennessee, Texas and Vermont. As of the date of this prospectus, we have not determined in which of these states and territories, if any, we will submit the required filings or pay the required fees. Additionally, if any of the states that have not yet adopted a statute, rule or regulation requiring a filing or fee or if any state amends its existing statutes, rules or regulations with respect to its requirements, we would need to comply with those new requirements in order for the securities to continue to be eligible for resale by stockholders in those jurisdictions.
In addition, aside from the exemption from registration provided by NSMIA, we believe that the Units, and the common stock and warrants comprising the Units once they become separately transferable, may be eligible for resale in various states without any notice filings or fee payments, based upon the availability of applicable exemptions from such states’ registration requirements, in certain instances subject to waiting periods, notice filings or fee payments.
The various states can impose fines on us or take other regulatory actions against us if we fail to comply with their state securities laws. Although we are taking steps to help insure that, if our securities do not qualify for listing on NASDAQ, we will conduct all the offers and sales in this offering in compliance with all state securities laws (which steps include, among other things, registering our securities in certain states; restricting our marketing efforts to avoid making offers or sales in states in which we are not registered; relying on institutional investor exemptions from registration as available from state to state; and restricting access to this offering on the BANQ website to residents of states in which our securities are registered), there can be no assurance that we will be able to achieve such compliance in all instances, or avoid fines or other state regulatory actions if we do not achieve such compliance.
Other Selling Restrictions
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a ‘‘Relevant Member State’’), from and including the date on which the European Union Prospectus Directive (the ‘‘EU Prospectus Directive’’) was implemented in that Relevant Member State (the ‘‘Relevant Implementation Date’’) an offer of securities described in this prospectus may not be made to the public in that Relevant Member State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant Member State or, where appropriate, approved in another Relevant Member State and
116
notified to the competent authority in that Relevant Member State, all in accordance with the EU Prospectus Directive, except that, with effect from and including the Relevant Implementation Date, an offer of securities described in this prospectus may be made to the public in that Relevant Member State at any time:
• to any legal entity which is a qualified investor as defined under the EU Prospectus Directive;
• to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150 natural or legal persons (other than qualified investors as defined in the EU Prospectus Directive); or
• in any other circumstances falling within Article 3(2) of the EU Prospectus Directive, provided that no such offer of securities described in this prospectus shall result in a requirement for the publication by us of a prospectus pursuant to Article 3 of the EU Prospectus Directive.
For the purposes of this provision, the expression an ‘‘offer of securities to the public’’ in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and the securities to be offered so as to enable an investor to decide to purchase or subscribe for the securities, as the same may be varied in that Member State by any measure implementing the EU Prospectus Directive in that Member State. The expression “EU Prospectus Directive” means Directive 2003/71/EC (and any amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State) and includes any relevant implementing measure in each Relevant Member State, and the expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Hong Kong
The shares may not be offered or sold by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap.571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap.32, Laws of Hong Kong), and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”), (ii) to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares are subscribed or purchased under Section 275 by a relevant person which is: (a) a corporation (which is not an accredited investor) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or (b) a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary is an accredited investor, shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest in that trust shall not be transferable for 6 months after that corporation or that trust has acquired the shares under Section 275 except: (1) to an institutional investor under Section 274 of the SFA or to a relevant person, or any person pursuant to Section 275(1A), and in accordance with the conditions, specified in Section 275 of the SFA; (2) where no consideration is given for the transfer; or (3) by operation of law.
117
Japan
The securities have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (the Financial Instruments and Exchange Law) and each underwriter has agreed that it will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to a resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the Financial Instruments and Exchange Law and any other applicable laws, regulations and ministerial guidelines of Japan.
118
LEGAL MATTERS
Ellenoff Grossman & Schole LLP, New York, New York, is acting as counsel in connection with the registration of our securities under the Securities Act, and as such will pass upon the validity of the securities offered in this prospectus. The selling agent is being represented by Hunter Taubman Weiss LLP, New York, New York.
EXPERTS
The consolidated financial statements of the Company as of and for the years ended December 31, 2014 and 2013 appearing in this prospectus have been audited by Marcum LLP, independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph relating to substantial doubt about the Company’s ability to continue as a going concern, as discussed in Note 2 to the consolidated financial statements), appearing elsewhere in this prospectus, and are included in reliance on such report given on the authority of such firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus constitutes a part of the registration statement that we have filed with the SEC in connection with this offering. It does not contain all of the information in the registration statement and in the exhibits thereto. Statements contained in this prospectus as to the contents of any contract or other document that we file as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such exhibit. The registration statement and the exhibits thereto may be inspected and copied at the principal office of the SEC, 100 F Street, NE, Washington, D.C. 20549, and copies of all or any part thereof may also be obtained at prescribed rates from the SEC’s Public Reference Section, at that address. In addition, the registration statement and the exhibits thereto may be viewed on and printed out from the SEC’s website at www.sec.gov. We will also make available, free of charge, copies of the registration statement and the exhibits thereto, upon written request to Mr. Amro Albanna, our Chief Executive Officer, c/o the Company at its principal executive offices.
119
INNOVATION ECONOMY CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
Condensed Consolidated Financial Statements (Unaudited) for the Three Months Ended March 31, 2015 and 2014 |
|
|
Condensed Consolidated Balance Sheets as of March 31, 2015 (unaudited) and December 31, 2014 |
|
F - 2 |
Condensed Consolidated Statements of Operations for the three months ended March 31, 2015 and 2014 (unaudited) |
|
F - 3 |
Condensed Consolidated Statements of Cash Flows for the three months ended March 31, 2015 and 2014 (unaudited) |
|
F - 4 |
Notes to Condensed Consolidated Financial Statements |
|
F - 5 – F - 9 |
|
|
|
Consolidated Financial Statements for Years Ended December 31, 2014 and 2013 |
|
|
Report of Independent Registered Public Accounting Firm |
|
F - 10 |
Consolidated Balance Sheets as of December 31, 2014 and 2013 |
|
F - 11 |
Consolidated Statements of Operations for the years ended December 31, 2014 and 2013 |
|
F - 12 |
Consolidated Statements of Stockholders’ Deficiency for the years ended December 31, 2014 and 2013 |
|
F - 13 |
Consolidated Statements of Cash Flows for the years ended December 31, 2014 and 2013 |
|
F - 14 |
Notes to Consolidated Financial Statements |
|
F - 15 – F - 32 |
F-1
INNOVATION ECONOMY CORPORATION
CONDENSED CONSOLIDATED BALANCE SHEETS
|
|
March
31, |
|
December 31, 2014 |
||||
|
|
(Unaudited) |
|
|
||||
ASSETS |
|
|
|
|
|
|
|
|
Current Assets |
|
|
|
|
|
|
|
|
|
$ |
544,699 |
|
|
$ |
1,881,776 |
|
|
|
|
30,035 |
|
|
|
24,140 |
|
|
Total Current Assets |
|
|
574,734 |
|
|
|
1,905,916 |
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
173,160 |
|
|
|
159,031 |
|
Deferred financing costs, net |
|
|
106,286 |
|
|
|
125,513 |
|
Deferred offering costs |
|
|
359,292 |
|
|
|
238,044 |
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS |
|
$ |
1,213,472 |
|
|
$ |
2,428,504 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIENCY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
Current Liabilities |
|
|
|
|
|
|
|
|
|
$ |
573,849 |
|
|
$ |
412,223 |
|
|
|
|
590,654 |
|
|
|
512,761 |
|
|
|
|
90,705 |
|
|
|
47,455 |
|
|
|
|
210,210 |
|
|
|
210,210 |
|
|
|
|
|
|
|
|
|
|
|
Total Current Liabilities |
|
|
1,465,418 |
|
|
|
1,182,649 |
|
|
|
|
|
|
|
|
|
|
Non-Current Liabilities |
|
|
|
|
|
|
|
|
|
|
2,014,516 |
|
|
|
1,889,572 |
|
|
|
|
914,838 |
|
|
|
933,043 |
|
|
|
|
|
|
|
|
|
|
|
Total Non-Current Liabilities |
|
|
2,929,354 |
|
|
|
2,822,615 |
|
|
|
|
|
|
|
|
|
|
Total Liabilities |
|
|
4,394,772 |
|
|
|
4,005,264 |
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficiency |
|
|
|
|
|
|
|
|
|
|
94 |
|
|
|
94 |
|
|
|
|
13,110,930 |
|
|
|
12,999,487 |
|
|
|
|
(14,839,743 |
) |
|
|
(13,282,055 |
) |
|
|
|
|
(1,728,719 |
) |
|
|
(282,474 |
) |
|
|
|
|
|
|
|
|
|
Non-controlling interest |
|
|
(1,452,581 |
) |
|
|
(1,294,286 |
) |
|
|
|
|
|
|
|
|
|
Total Stockholders’ Deficiency |
|
|
(3,181,300 |
) |
|
|
(1,576,760 |
) |
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIENCY |
|
$ |
1,213,472 |
|
|
$ |
2,428,504 |
|
See accompanying notes to condensed consolidated financial statements.
F-2
INNOVATION ECONOMY CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(unaudited)
|
|
Three Months Ended March 31, |
||||||
|
|
2015 |
|
2014 |
||||
Revenues |
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
|
1,216,108 |
|
|
|
687,025 |
|
|
|
|
130,148 |
|
|
|
131,891 |
|
|
|
|
244,061 |
|
|
|
182,973 |
|
|
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
|
1,590,317 |
|
|
|
1,001,889 |
|
|
|
|
|
|
|
|
|
|
Loss From Operations |
|
|
(1,590,317 |
) |
|
|
(1,001,889 |
) |
|
|
|
|
|
|
|
|
|
Other Income (Expense) |
|
|
|
|
|
|
|
|
|
|
(19,227 |
) |
|
|
— |
|
|
|
|
(124,944 |
) |
|
|
— |
|
|
|
|
18,205 |
|
|
|
— |
|
|
|
|
— |
|
|
|
(8,354 |
) |
|
|
|
300 |
|
|
|
10,000 |
|
|
|
|
|
|
|
|
|
|
|
Total Other Income (Expense) |
|
|
(125,666 |
) |
|
|
1,646 |
|
|
|
|
|
|
|
|
|
|
Net Loss |
|
|
(1,715,983 |
) |
|
|
(1,000,243 |
) |
|
|
|
|
|
|
|
|
|
Net loss attributable to non-controlling interests |
|
|
(158,295 |
) |
|
|
(115,497 |
) |
|
|
|
|
|
|
|
|
|
Net Loss attributed to stockholders of Innovation Economy Corporation |
|
$ |
(1,557,688 |
) |
|
$ |
(884,746 |
) |
|
|
|
|
|
|
|
|
|
Net Loss Per Share: Basic and Diluted |
|
$ |
(0.16 |
) |
|
$ |
(0.10 |
) |
|
|
|
|
|
|
|
|
|
Weighted Average Common Shares Outstanding: Basic and Diluted |
|
|
9,444,828 |
|
|
|
8,881,578 |
|
See accompanying notes to condensed consolidated financial statements.
F-3
INNOVATION ECONOMY CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(unaudited)
|
|
Three Months Ended March 31, |
||||||
|
|
2015 |
|
2014 |
||||
CASH FLOWS FROM OPERATING ACTIVITIES |
|
|
|
|
|
|
|
|
|
$ |
(1,715,983 |
) |
|
$ |
(1,000,243 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
111,443 |
|
|
|
251,144 |
|
|
|
|
16,283 |
|
|
|
14,706 |
|
|
|
|
(18,205 |
) |
|
|
— |
|
|
|
|
— |
|
|
|
8,354 |
|
|
|
|
19,227 |
|
|
|
— |
|
|
|
|
124,944 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
(5,895 |
) |
|
|
3,000 |
|
|
|
|
161,626 |
|
|
|
48,358 |
|
|
|
|
77,893 |
|
|
|
(40,629 |
) |
|
|
|
|
|
|
|
|
|
|
NET CASH USED IN OPERATING ACTIVITIES |
|
|
(1,228,667 |
) |
|
|
(715,310 |
) |
|
|
|
|
|
|
|
|
|
CASH FLOWS USED IN INVESTING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
(30,412 |
) |
|
|
(5,591 |
) |
|
|
|
|
|
|
|
|
|
|
CASH FLOWS PROVIDED BY FINANCING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
(6,750 |
) |
|
|
(56,750 |
) |
|
|
|
50,000 |
|
|
|
— |
|
|
|
|
(121,248 |
) |
|
|
— |
|
|
|
|
— |
|
|
|
1,560,000 |
|
|
|
|
|
|
|
|
|
|
|
NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES |
|
|
(77,998 |
) |
|
|
1,503,250 |
|
|
|
|
|
|
|
|
|
|
NET CHANGE IN CASH AND CASH EQUIVALENTS |
|
|
(1,337,077 |
) |
|
|
782,349 |
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of period |
|
|
1,881,776 |
|
|
|
722,736 |
|
Cash and cash equivalents, end of period |
|
$ |
544,699 |
|
|
$ |
1,505,085 |
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
|
$ |
— |
|
|
$ |
— |
|
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL NON-CASH INVESTING AND FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
Warrants issued to settle accounts payable |
|
$ |
— |
|
|
$ |
26,855 |
|
See accompanying notes to condensed consolidated financial statements.
F-4
INNOVATION ECONOMY CORPORATION
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE MONTHS ENDED MARCH 31, 2015 AND 2014
(unaudited)
NOTE 1 — NATURE OF BUSINESS
Innovation Economy Corporation (“IEC” or the “Company”) was incorporated in Delaware on October 28, 2010. The Company intends to position itself as a commercialization and global distribution company that supports the innovation sector in the Inland Empire Southern California region. The Company’s business model is to create a diversified portfolio of subsidiary operating companies by harvesting high-quality innovation-based assets and resources from the Inland Empire Southern California region; developing and commercializing the assets and resources; forming a line of business and corporate structure around each asset or resource; securing funding as needed; and marketing and selling the resulting products and services through national and global distribution channels.
The accompanying unaudited condensed consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) for interim financial information. Accordingly, they do not include all of the information and disclosures required by U.S. GAAP for annual financial statements. In the opinion of management, such statements include all adjustments (consisting only of normal recurring items) that are considered necessary for a fair presentation of the condensed consolidated financial statements of the Company as of March 31, 2015 and for the three months ended March 31, 2015 and 2014. The results of operations for the three months ended March 31, 2015 are not necessarily indicative of the operating results for the full year ending December 31, 2015 or any other period. These unaudited condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and related disclosures of the Company as of December 31, 2014 and for the year then ended, which are included elsewhere in this document. The Company’s primary activities since inception, have been the design and development of its products, negotiating strategic alliances and other agreements, and raising capital. The Company has not commenced its principal operations, nor has it generated any significant revenues from its ongoing operations through March 31, 2015.
On January 16, 2015, the Company effected a 1-for-15 reverse stock split of its issued and outstanding shares of common stock. All references in these condensed consolidated financial statements to the number of shares, options, warrants and other common stock equivalents, price per share and weighted-average number of shares outstanding of common stock have been adjusted to retroactively reflect the effect of the reverse stock split.
NOTE 2 — GOING CONCERN AND MANAGEMENT’S PLANS
The accompanying condensed consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As of March 31, 2015, the Company had a working capital deficiency of $890,684 and stockholders’ deficiency of $3,181,300. The Company has not generated any significant revenues from operations and has incurred net losses since inception. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of asset amounts or the classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Subsequent to March 31, 2015, the Company filed a Registration Statement on Form S-1 with the Securities & Exchange Commission in connection with a best efforts primary offering of units consisting of shares of the Company, common stock and warrants to purchase common stock. In addition, the Company received proceeds of $2,100,000 from unsecured convertible promissory notes (see Note 7). The Company needs to raise additional capital in order to be able to accomplish its business plan objectives. The Company is continuing its efforts to secure additional funds through debt or equity instruments due to the impending lack of funds. Management believes that it will be successful in obtaining additional financing based on its limited history of raising funds; however, no assurance can be provided that the Company will be able to do so. There is no assurance that any funds it raises will be sufficient to enable the Company to attain profitable operations or continue as a going concern. To the extent that the Company is unsuccessful, the Company may need to curtail or cease its operations and implement a plan to extend payables or reduce overhead until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
F-5
INNOVATION ECONOMY CORPORATION
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE MONTHS ENDED MARCH 31, 2015 AND 2014
(unaudited)
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The accompanying condensed consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
At March 31, 2015, the Company owns 100% of Innovation Economy HUB, Inc. and Innovation Economy Konnect, Inc., 95.0% of Smart Oxygen Solutions, Inc. (formerly Breathing Technologies, Inc.) (“SOS”), 70.4% of OlFactor Laboratories, Inc. (“OLI”) and 83.5% of Nano Engineered Applications, Inc. and all are included in the condensed consolidated financial statements of the Company. The non-controlling interest represents the remaining ownership of the majority-owned subsidiaries.
Use of Estimates
The condensed consolidated financial statements and accompanying notes are prepared in accordance with generally accepted accounting principles in the United States of America. The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results may differ from those estimates. The Company’s significant estimates include reserves related to the recoverability and useful lives of long-lived assets, the valuation allowance related to deferred tax assets, and the valuation of equity instruments, derivatives and debt discounts.
Fair Value of Financial Assets and Liabilities Measured on a Recurring Basis
Level 3 Financial Liabilities – Derivative conversion features and warrant liabilities
Financial assets and liabilities measured at fair value on a recurring basis are summarized below and disclosed on the condensed consolidated balance sheet as of March 31, 2015:
|
|
Carrying |
|
Fair Value Measurement Using |
|||||||||||
|
|
Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|||||
Conversion option liabilities |
|
$ |
914,838 |
|
$ |
— |
|
$ |
— |
|
$ |
914,838 |
|
$ |
914,838 |
The table below provides a summary of the changes in fair value, including net transfers in and/or out, of all financial assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) during the three months ended March 31, 2015:
|
|
Fair
Value Measurement |
||
Balance, January 1, 2015 |
|
$ |
933,043 |
|
Change in fair value of derivative liabilities |
|
|
(18,205 |
) |
Balance, March 31, 2015 |
|
$ |
914,838 |
|
Net Loss Per Share of Common Stock
Basic loss per common share is computed by dividing net loss by the weighted average number of vested common shares outstanding during the period. Diluted earnings per common share is computed by dividing net income by the weighted average number of vested common shares outstanding, plus the impact of common shares, if dilutive, resulting from the exercise of outstanding stock options and warrants, plus the conversion of convertible notes.
F-6
INNOVATION ECONOMY CORPORATION
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE MONTHS ENDED MARCH 31, 2015 AND 2014
(unaudited)
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
The following securities are excluded from the calculation of weighted average dilutive common shares outstanding because their inclusion would have been anti-dilutive:
|
|
March 31, |
||
|
|
2015 |
|
2014 |
Options |
|
2,645,967 |
|
2,439,834 |
Warrants |
|
267,637 |
|
219,333 |
Convertible Promissory Notes |
|
546,078 |
|
— |
Total potentially dilutive shares |
|
3,459,682 |
|
2,659,167 |
Recently Issued Accounting Pronouncements
In April 2015, the Financial Accounting Standards Board issued Accounting Standards Update 2015-03, Interest—Imputation of Interest. To simplify presentation of debt issuance costs, the amendments in this Update would require that debt issuance costs be presented in the balance sheet as a direct deduction from the carrying amount of debt liability, consistent with debt discounts or premiums. The recognition and measurement guidance for debt issuance costs would not be affected by the amendments in this Update. This Accounting Standards Update is the final version of Proposed Accounting Standards Update 2014-250—Interest—Imputation of Interest (Subtopic 835-30), which has been deleted. The amendments in this Update are effective for financial statements issued for fiscal years beginning after December 15, 2015, and interim periods within those fiscal years. The Company is currently evaluating the effects of ASU 2015-03 on the condensed consolidated financial statements.
Subsequent Events
Management has evaluated subsequent events or transactions occurring through the date on which the financial statements were issued. Based upon the evaluation, the Company did not identify any recognized or non-recognized subsequent events that would have required adjustment or disclosure in the condensed consolidated financial statements, except as disclosed.
NOTE 4 — CONVERTIBLE NOTES PAYABLE
During the three months ended March 31, 2015, the Company recognized $124,944 and $19,227 in amortization of the debt discount and deferred financing costs, respectively, relating to the convertible notes payable.
The fair value of the conversion feature of the convertible notes payable on March 31, 2015 was calculated using a binomial option model valued with the following weighted average assumptions:
Dividend Yield |
|
|
0 |
% |
Volatility |
|
|
67.55 |
% |
Risk-free interest rate |
|
|
0.56 |
% |
Contractual term |
|
|
1.542 years |
|
Weighted average unit fair value |
|
$ |
1.675 |
|
Common shares assumed issued |
|
|
546,078 |
|
Aggregate fair value |
|
$ |
914,838 |
|
During the three months ended March 31, 2015, the Company marked the conversion feature of the convertible notes payable to fair value and recorded a gain of $18,205 relating to the change in fair value.
In March 2015, the Company received proceeds of $50,000 in an intended purchase of an unsecured convertible promissory note. However, as of March 31, 2015, the investor did not complete the necessary documentation for the Company to issue the note. As a result, as of March 31, 2015, $50,000 was accounted for as a non-interest bearing short term promissory note.
F-7
INNOVATION ECONOMY CORPORATION
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE MONTHS ENDED MARCH 31, 2015 AND 2014
(unaudited)
NOTE 5 — STOCKHOLDERS’ DEFICIENCY
Stock-Based Compensation
The Company uses the Black-Scholes option pricing model to determine the fair value of the options and warrants granted. No options were granted during the three months ended March 31, 2015 or 2014.
In applying the Black-Scholes option pricing model to warrants granted, the Company used the following weighted average assumptions:
|
|
For The Three Months Ended March 31, |
|||
|
|
2015 |
|
2014 |
|
Risk free interest rate |
|
— |
|
1.76 |
% |
Dividend yield |
|
— |
|
0.00 |
% |
Expected volatility |
|
— |
|
154 |
% |
Expected life in years |
|
— |
|
4 |
|
Forfeiture Rate |
|
— |
|
0.00 |
% |
The weighted average fair value of the warrants granted during the three months ended March 31, 2014 was $4.18 per warrant.
Stock-based compensation for stock options and warrants has been recorded in the condensed consolidated statements of operations and totaled $111,443 and $200,575 for the three months ended March 31, 2015 and 2014, respectively. As of March 31, 2015, the remaining balance of unamortized expense is $385,596 and is expected to be amortized over a period of 2.25 years.
NOTE 6 — LITIGATIONS, CLAIMS AND ASSESSMENTS
In the normal course of business, the Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. Such matters are subject to many uncertainties, and outcomes are not predictable with assurance. There are no such matters that are deemed material to the condensed consolidated financial statements as of March 31, 2015.
NOTE 7 — SUBSEQUENT EVENTS
In April 2015, the Company through private placements of unsecured convertible promissory notes (the “2015 Convertible Notes”) raised an aggregate principal amount of $2,100,000. The notes bear interest at 8% per annum and mature in two years. Any unpaid principal and accrued interest due under the notes will automatically convert into shares of common stock at the maturity date.
The principal of the notes are convertible into 411,765 shares of common stock at a conversion price of $5.10 per share. The notes contain a provision to lower the conversion price to any subsequent common stock issuance at a lower price, if any convertible debt subsequently issued has a lower conversion price or if any options or warrants have lower exercise prices. If the Company completes a registered initial public offering (“IPO”) prior to the maturity date, the Company shall have the right to force conversion of any unpaid principal and accrued interest owed under the notes, in whole, or in part, into the securities issued pursuant to the IPO at a 20% discounted conversion price to the price of the securities sold in the IPO. If the Company completes a qualifying transaction that results in the Company receiving gross proceeds of more than $7,500,000 prior to the maturity date, the Company shall also have the right to force conversion of any unpaid principal and accrued interest owing under the previous convertible notes, in whole, or in part, into the securities issued pursuant to the IPO at a 20% discounted conversion price to the price of the qualifying transaction. The Company determined that the conversion feature of the notes did not contain fixed settlement provisions because the conversion price can be adjusted based on new issuances, and
F-8
INNOVATION ECONOMY CORPORATION
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE MONTHS ENDED MARCH 31, 2015 AND 2014
(unaudited)
NOTE 7 — SUBSEQUENT EVENTS (cont.)
accordingly, the Company will record the note conversion feature as a liability and mark to market the derivative to fair value each reporting period.
In May 2015, the Company negotiated options to license certain patents and patent applications from various third parties (the “IP Portfolio”). The options run for six months, and can be extended for an additional six months thereafter in the sole discretion of the Company. The patents and applications relate to subject matters in a variety of technology areas, including the treatment of various cancers, ischemic stroke, diabetes and bacterial infections. The opportunity to secure the options arose from the Company relationship with one of its principal investors, Sunbelt III Technologies Management, LLC (“Sunbelt”). A company managed by a Sunbelt affiliate had earlier secured options over the patents and applications, and some time thereafter Sunbelt had proposed that the options be conveyed to the Company on arm’s-length terms. Following negotiations, we were able to enter into an agreement with the Sunbelt affiliate that terminated its options (the “Termination Agreement”), allowing us to negotiate for the options. As per the terms of the Termination Agreement should the Company exercise its right to license the IP Porfolio then the Company shall grant and issue to the Sunbelt affiliate 125,000 shares of the Company’s restricted common stock and a cash payment of approximately $132,000.
In May 2015, the Company extended its office lease for an additional 12 months through on April 30, 2016. The Company is obligated to pay monthly base rental payments of $5,641.
F-9
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
of Innovation Economy Corporation
We have audited the accompanying consolidated balance sheets of Innovation Economy Corporation (the “Company”) as of December 31, 2014 and 2013, and the related consolidated statements of operations, stockholders’ deficiency and cash flows for the years then ended. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Innovation Economy Corporation as of December 31, 2014 and 2013, and the consolidated results of its operations and its cash flows for the years then ended in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 2, the Company has not generated any significant revenues from operations and has incurred losses since inception. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Marcum LLP
Marcum LLP
New York, NY
April 3, 2015, except for Note 8a and Note 14, as to which the date is May 12, 2015
F-10
INNOVATION ECONOMY CORPORATION
CONSOLIDATED BALANCE SHEETS
|
|
|
December 31, |
||||||
|
|
|
2014 (revised) |
|
2013 |
||||
|
ASSETS |
|
|
|
|
|
|
|
|
|
Current Assets |
|
|
|
|
|
|
|
|
|
|
$ |
1,881,776 |
|
|
$ |
722,736 |
|
|
|
|
|
24,140 |
|
|
|
21,290 |
|
|
|
Total Current Assets |
|
|
1,905,916 |
|
|
|
744,026 |
|
|
|
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
159,031 |
|
|
|
194,464 |
|
|
Deferred financing costs, net |
|
|
125,513 |
|
|
|
— |
|
|
Deferred offering costs |
|
|
238,044 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL ASSETS |
|
$ |
2,428,504 |
|
|
$ |
938,490 |
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIENCY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities |
|
|
|
|
|
|
|
|
|
Current Liabilities |
|
|
|
|
|
|
|
|
|
|
$ |
412,223 |
|
|
$ |
297,294 |
|
|
|
|
|
512,761 |
|
|
|
596,303 |
|
|
|
|
|
47,455 |
|
|
|
122,205 |
|
|
|
|
|
210,210 |
|
|
|
210,370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Current Liabilities |
|
|
1,182,649 |
|
|
|
1,226,172 |
|
|
|
|
|
|
|
|
|
|
|
|
Non-Current Liabilities |
|
|
|
|
|
|
|
|
|
|
|
1,889,572 |
|
|
|
— |
|
|
|
|
|
933,043 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Non-Current Liabilities |
|
|
2,822,615 |
|
|
|
— |
|
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities |
|
|
4,005,264 |
|
|
|
1,226,172 |
|
|
|
|
|
|
|
|
|
|
|
|
Commitments and contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Deficiency |
|
|
|
|
|
|
|
|
|
|
|
94 |
|
|
|
88 |
|
|
|
|
|
12,999,487 |
|
|
|
9,349,176 |
|
|
|
|
|
(13,282,055 |
) |
|
|
(8,749,750 |
) |
|
|
Total Stockholders’ (Deficiency) Equity-Innovation Economy Corporation |
|
|
(282,474 |
) |
|
|
599,514 |
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling interest |
|
|
(1,294,286 |
) |
|
|
(887,196 |
) |
|
|
|
|
|
|
|
|
|
|
|
Total Stockholders’ Deficiency |
|
|
(1,576,760 |
) |
|
|
(287,682 |
) |
|
|
|
|
|
|
|
|
|
|
|
TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIENCY |
|
$ |
2,428,504 |
|
|
$ |
938,490 |
|
See accompanying notes to consolidated financial statements.
F-11
INNOVATION ECONOMY CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS
|
|
|
For the Year Ended December 31, |
||||||
|
|
|
2014 |
|
2013 |
||||
|
Revenues |
|
|
|
|
|
|
|
|
|
|
$ |
— |
|
|
$ |
109,688 |
|
|
|
|
|
— |
|
|
|
(98,004 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Gross Profit |
|
|
— |
|
|
|
11,684 |
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
|
|
3,516,043 |
|
|
|
2,762,461 |
|
|
|
|
|
676,710 |
|
|
|
317,557 |
|
|
|
|
|
702,376 |
|
|
|
571,070 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
|
4,895,129 |
|
|
|
3,651,088 |
|
|
|
|
|
|
|
|
|
|
|
|
Loss From Operations |
|
|
(4,895,129 |
) |
|
|
(3,639,404 |
) |
|
|
|
|
|
|
|
|
|
|
|
Other Income (Expense) |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
271,636 |
|
|
|
|
|
(28,297 |
) |
|
|
— |
|
|
|
|
|
(104,120 |
) |
|
|
(58,124 |
) |
|
|
|
|
66,505 |
|
|
|
— |
|
|
|
|
|
(8,354 |
) |
|
|
— |
|
|
|
|
|
30,000 |
|
|
|
14,509 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Other Income (Expense) |
|
|
(44,266 |
) |
|
|
228,021 |
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss |
|
|
(4,939,395 |
) |
|
|
(3,411,383 |
) |
|
|
|
|
|
|
|
|
|
|
|
Net loss attributable to non-controlling interests |
|
|
(407,090 |
) |
|
|
(352,031 |
) |
|
|
|
|
|
|
|
|
|
|
|
Net Loss attributed to stockholders of Innovation Economy Corporation |
|
$ |
(4,532,305 |
) |
|
$ |
(3,059,352 |
) |
|
|
|
|
|
|
|
|
|
|
|
Net Loss per Share: Basic and Diluted |
|
$ |
(0.49 |
) |
|
$ |
(0.37 |
) |
|
|
|
|
|
|
|
|
|
|
|
Weighted Average Common Shares Outstanding: Basic and Diluted |
|
|
9,261,231 |
|
|
|
8,344,836 |
|
See accompanying notes to consolidated financial statements.
F-12
INNOVATION ECONOMY CORPORATION
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ DEFICIENCY
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
|
|
|
Common Stock |
|
Additional |
|
Non-Controlling |
|
Accumulated |
|
|
||||||||||
|
|
|
Shares |
|
Amount |
|
Capital |
|
Subsidiaries |
|
Deficit |
|
Total |
||||||||
|
Balance – January 1, 2013 |
|
8,133,622 |
|
$ |
81 |
|
$ |
6,380,163 |
|
$ |
(535,165 |
) |
|
$ |
(5,690,398 |
) |
|
$ |
154,681 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued
for |
|
434,950 |
|
|
4 |
|
|
1,626,996 |
|
|
— |
|
|
|
— |
|
|
|
1,627,000 |
|
|
Stock issued for services |
|
85,040 |
|
|
1 |
|
|
344,164 |
|
|
— |
|
|
|
— |
|
|
|
344,165 |
|
|
Stock in subsidiary issued for services |
|
— |
|
|
— |
|
|
8,750 |
|
|
— |
|
|
|
— |
|
|
|
8,750 |
|
|
Stock-based compensation |
|
— |
|
|
— |
|
|
714,905 |
|
|
— |
|
|
|
— |
|
|
|
714,905 |
|
|
Stock issued for conversion of note payable |
|
179,500 |
|
|
2 |
|
|
269,248 |
|
|
— |
|
|
|
— |
|
|
|
269,250 |
|
|
Stock issued in subsidiary for cash |
|
— |
|
|
— |
|
|
4,950 |
|
|
— |
|
|
|
— |
|
|
|
4,950 |
|
|
Net loss for the year ended December 31, 2013 |
|
— |
|
|
— |
|
|
— |
|
|
(352,031 |
) |
|
|
(3,059,352 |
) |
|
|
(3,411,383 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance – December 31, |
|
8,833,112 |
|
$ |
88 |
|
$ |
9,349,176 |
|
$ |
(887,196 |
) |
|
$ |
(8,749,750 |
) |
|
$ |
(287,682 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued
for |
|
490,417 |
|
|
5 |
|
|
2,339,995 |
|
|
— |
|
|
|
— |
|
|
|
2,340,000 |
|
|
Stock issued for exercise of warrants |
|
104,167 |
|
|
1 |
|
|
499,999 |
|
|
— |
|
|
|
— |
|
|
|
500,000 |
|
|
Stock issued for services |
|
17,132 |
|
|
|
|
|
82,395 |
|
|
|
|
|
|
|
|
|
|
82,395 |
|
|
Stock-based compensation |
|
|
|
|
|
|
|
701,067 |
|
|
|
|
|
|
|
|
|
|
701,067 |
|
|
Warrants issued to settle accounts payable |
|
|
|
|
|
|
|
26,855 |
|
|
|
|
|
|
|
|
|
|
26,855 |
|
|
Net loss for the year ended December 31, 2014 |
|
|
|
|
|
|
|
|
|
|
(407,090 |
) |
|
|
(4,532,305 |
) |
|
|
(4,939,395 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance – December 31, |
|
9,444,828 |
|
$ |
94 |
|
$ |
12,999,487 |
|
$ |
(1,294,286 |
) |
|
$ |
(13,282,055 |
) |
|
$ |
(1,576,760 |
) |
See accompanying notes to consolidated financial statements
F-13
INNOVATION ECONOMY CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
|
|
For the Year Ended December 31, |
||||||
|
|
|
2014 (revised) |
|
2013 |
||||
|
CASH FLOWS FROM OPERATING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
$ |
(4,939,395 |
) |
|
$ |
(3,411,383 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
701,067 |
|
|
|
714,905 |
|
|
|
|
|
82,395 |
|
|
|
352,915 |
|
|
|
|
|
61,046 |
|
|
|
58,210 |
|
|
|
|
|
(66,505 |
) |
|
|
— |
|
|
|
|
|
8,354 |
|
|
|
— |
|
|
|
|
|
28,297 |
|
|
|
— |
|
|
|
|
|
104,120 |
|
|
|
58,124 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,850 |
) |
|
|
(6,875 |
) |
|
|
|
|
133,430 |
|
|
|
97,691 |
|
|
|
|
|
(83,542 |
) |
|
|
513,037 |
|
|
|
|
|
(160 |
) |
|
|
210,370 |
|
|
|
|
|
|
|
|
|
|
|
|
|
NET CASH USED IN OPERATING ACTIVITIES |
|
|
(3,973,743 |
) |
|
|
(1,413,006 |
) |
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS USED IN INVESTING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
(25,613 |
) |
|
|
(3,995 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
CASH FLOWS PROVIDED BY FINANCING ACTIVITIES |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
123,583 |
|
|
|
|
|
(74,750 |
) |
|
|
(1,378 |
) |
|
|
|
|
2,785,000 |
|
|
|
— |
|
|
|
|
|
(153,810 |
) |
|
|
— |
|
|
|
|
|
(238,044 |
) |
|
|
— |
|
|
|
|
|
2,340,000 |
|
|
|
1,627,000 |
|
|
|
|
|
500,000 |
|
|
|
— |
|
|
|
|
|
— |
|
|
|
4,950 |
|
|
|
|
|
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY FINANCING ACTIVITIES |
|
|
5,158,396 |
|
|
|
1,754,155 |
|
|
|
|
|
|
|
|
|
|
|
|
INCREASE IN CASH AND CASH EQUIVALENTS |
|
|
1,159,040 |
|
|
|
337,154 |
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents, beginning of year |
|
|
722,736 |
|
|
|
385,582 |
|
|
Cash and cash equivalents, end of year |
|
$ |
1,881,776 |
|
|
$ |
722,736 |
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL NON-CASH INVESTING AND FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
Note payable and accrued interest converted to common stock |
|
$ |
— |
|
|
$ |
269,250 |
|
|
Warrants issued to settle accounts payable |
|
$ |
26,855 |
|
|
$ |
— |
|
|
Debt discount in conjunction with the recording of the original value of the derivative liability |
|
$ |
999,548 |
|
|
$ |
— |
|
See accompanying notes to consolidated financial statements.
F-14
INNOVATION ECONOMY CORPORATION
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 1 — NATURE OF BUSINESS
Innovation Economy Corporation (“IEC” or the “Company”) was incorporated in Delaware on October 28, 2010. The Company intends to position itself as a commercialization and global distribution company that supports the innovation sector in the Inland Empire Southern California region. The Company’s business model is to create a diversified portfolio of subsidiary operating companies by harvesting high-quality innovation-based assets and resources from the Inland Empire Southern California region; developing and commercializing the assets and resources; forming a line of business and corporate structure around each asset or resource; securing funding as needed; and marketing and selling the resulting products and services through national and global distribution channels.
The Company’s primary activities since inception, have been the design and development of its products, negotiating strategic alliances and other agreements, and raising capital. The Company had not commenced its principal operations, nor had it generated any significant revenues from its ongoing operations through December 31, 2014.
On January 16, 2015, the Company effected a 1-for-15 reverse stock split of its issued and outstanding shares of common stock. All references in these consolidated financial statements to the number of shares, options, warrants and other common stock equivalents, price per share and weighted-average number of shares outstanding of common stock have been adjusted to retroactively reflect the effect of the stock split.
NOTE 2 — GOING CONCERN AND MANAGEMENT’S PLANS
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. As of December 31, 2014, the Company had a stockholders’ deficiency of $1,458,932. The Company has not generated any significant revenues from operations and has incurred net losses since inception. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of asset amounts or the classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Subsequent to December 31, 2014, the Company filed a Registration Statement on Form S-1 with the Securities & Exchange Commission in connection with a best efforts primary offering of units consisting of shares of the Company, common stock and warrants to purchase common stock. The Company needs to raise additional capital in order to be able to accomplish its business plan objectives. The Company is continuing its efforts to secure additional funds through debt or equity instruments due to the impending lack of funds. Management believes that it will be successful in obtaining additional financing based on its limited history of raising funds; however, no assurance can be provided that the Company will be able to do so. There is no assurance that any funds it raises will be sufficient to enable the Company to attain profitable operations or continue as a going concern. To the extent that the Company is unsuccessful, the Company may need to curtail or cease its operations and implement a plan to extend payables or reduce overhead until sufficient additional capital is raised to support further operations. There can be no assurance that such a plan will be successful.
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its subsidiaries. All significant intercompany accounts and transactions have been eliminated in consolidation.
At December 31, 2014 and 2013, the Company owns 100% of Innovation Economy HUB, Inc. and Innovation Economy Konnect, Inc., 95.0% of Smart Oxygen Solutions, Inc. (formerly Breathing Technologies, Inc.) (“SOS”), 70.4% of Olfactor Laboratories, Inc. (“OLI”) and 83.5% of Nano Engineered Applications, Inc. and all are included in the consolidated financial statements of the Company. The non-controlling interest represents the remaining ownership of the majority-owned subsidiaries.
F-15
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Use of Estimates
The consolidated financial statements and accompanying notes are prepared in accordance with generally accepted accounting principles in the United States of America. The preparation of financial statements in conformity with United States generally accepted accounting principles (“U.S. GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results may differ from those estimates. The Company’s significant estimates include reserves related to the recoverability and useful lives of long-lived assets, the valuation allowance related to deferred tax assets, and the valuation of equity instruments, derivatives and debt discounts.
Segment Reporting
In accordance with ASC 280 “Segment Reporting”, operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. The Company’s chief decision maker, as defined under the FASB’s guidance, is its Chief Executive Officer. It is determined that the Company operates in one business segment and one geographic segment, the United States of America.
Concentrations of Credit Risk
The Company maintains its cash accounts at financial institutions which are insured by the Federal Deposit Insurance Corporation (“FDIC”). At times, the Company has deposits in excess of federally insured limits.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less when purchased to be cash equivalents. As of December 31, 2014 and 2013, cash equivalents included money market accounts.
Property and Equipment
Property and equipment are stated at cost, less accumulated depreciation. Depreciation is provided over the estimated useful life of each class of assets using the straight-line method. Expenditures for maintenance and repairs are charged to expense as incurred. Additions and betterments that substantially extend the useful life of the asset are capitalized. Upon the sale, retirement, or other disposition of property and equipment, the cost and related accumulated depreciation are removed from the balance sheet, and any gain or loss on the transaction is included in the statement of operations.
Deferred Financing Costs
The Company classifies costs related to the issuance of Notes Payable as Deferred Financing Costs. Deferred Financing Costs are amortized using the straight-line method over the term of the notes.
Deferred Offering Costs
The Company classifies amounts related to a potential future equity financing not closed as of the balance sheet date as Deferred Offering Costs. As of December 31, 2014, the Company capitalized costs in the amount of $238,044 that relates to a potential future financing as Deferred Offering Costs in the accompanying balance sheet.
F-16
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Impairment of Long-Lived Assets
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be held and used is measured by a comparison of the carrying amount of an asset to estimated undiscounted future cash flows expected to be generated by the asset.
If the carrying amount of an asset exceeds its undiscounted estimated future cash flows, an impairment review is performed. An impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of would be separately presented in the balance sheet and reported at the lower of the carrying amount or fair value less costs to sell, and are no longer depreciated. The assets and liabilities of a disposed group classified as held for sale would be presented separately in the appropriate asset and liability sections of the balance sheet.
The Company determined that there was no indication of impairment of long-lived assets during the years ended December 31, 2014 and 2013.
Convertible Instruments
The Company bifurcates conversion options from their host instruments and accounts for them as free standing derivative financial instruments according to certain criteria. The criteria includes circumstances in which (a) the economic characteristics and risks of the embedded derivative instrument are not clearly and closely related to the economic characteristics and risks of the host contract, (b) the hybrid instrument that embodies both the embedded derivative instrument and the host contract is not re-measured at fair value under otherwise applicable generally accepted accounting principles with changes in fair value reported in earnings as they occur and (c) a separate instrument with the same terms as the embedded derivative instrument would be considered a derivative instrument. An exception to this rule is when the host instrument is deemed to be conventional as that term is described under applicable U.S. GAAP.
For instruments in which the Company has determined that the embedded conversion options should not be bifurcated from their host instruments, the Company records, when necessary, discounts to convertible notes for the intrinsic value of conversion options embedded in debt instruments based upon the differences between the fair value of the underlying common stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are amortized over the term of the related debt to their stated date of redemption.
Fair Value of Financial Instruments
The Company’s financial instruments consist of cash and cash equivalents, other current assets, accounts payable, accrued expenses, other current liabilities and notes payable. The carrying amount of these financial instruments approximates fair value due either to length of maturity or interest rates that approximate prevailing market rates unless otherwise disclosed in these financials statements.
Fair value is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. These fair value measurements apply to all financial instruments that are measured and reported on a fair value basis.
F-17
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
Based on the observability of the inputs used in the valuation techniques, financial instruments are categorized according to the fair value hierarchy, which ranks the quality and reliability of the information used to determine fair values. Financial assets and liabilities carried at fair value are classified and disclosed in one of the following three categories:
Level 1 — Observable inputs such as quoted prices in active markets.
Level 2 — Inputs, other than the quoted prices in active markets, that are observable either directly or indirectly.
Level 3 — Unobservable inputs in which there is little or no market data, which require the reporting entity to develop its own assumptions.
Financial assets are considered Level 3 when their fair values are determined using pricing models, discounted cash flow methodologies or similar techniques and at least one significant model assumption or input is unobservable.
In certain cases, the inputs used to measure fair value may fall into different levels of the fair value hierarchy. In such cases, the assignment of an asset or liability within the fair value hierarchy is based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment, and considers factors specific to the asset or liability.
The Company uses Level 3 of the fair value hierarchy to measure the fair value of the derivative liabilities and revalues its derivative liabilities at every reporting period and recognizes gains or losses in the consolidated statements of operations that are attributable to the change in the fair value of the derivative liabilities.
Fair Value of Financial Assets and Liabilities Measured on a Recurring Basis
Level 3 Financial Liabilities – Derivative conversion features and warrant liabilities
Financial assets and liabilities measured at fair value on a recurring basis are summarized below and disclosed on the consolidated balance sheet as of December 31, 2014:
|
|
|
Carrying |
|
Fair Value Measurement Using |
|||||||||||
|
|
|
Value |
|
Level 1 |
|
Level 2 |
|
Level 3 |
|
Total |
|||||
|
Conversion option liabilities |
|
$ |
933,043 |
|
$ |
— |
|
$ |
— |
|
$ |
933,043 |
|
$ |
933,043 |
The table below provides a summary of the changes in fair value, including net transfers in and/or out, of all financial assets and liabilities measured at fair value on a recurring basis using significant unobservable inputs (Level 3) during the year ended December 31, 2014:
|
|
|
Fair
Value Measurement |
||
|
|
|
Total |
||
|
Balance, December 31, 2013 |
|
$ |
— |
|
|
Aggregate fair value of derivatives issued |
|
|
999,548 |
|
|
Change in fair value of derivative liabilities |
|
|
(66,505 |
) |
|
Balance, December 31, 2014 |
|
$ |
933,043 |
|
Changes in the unobservable input values could potentially cause material changes in the fair value of the Company’s Level 3 financial instruments. The significant unobservable inputs used in the fair value measurements are the expected volatility assumption and fair value of the Company’s common stock price. A significant increase
F-18
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
(decrease) in the expected volatility and/or fair value of the Company’s common stock price assumptions could potentially result in a higher (lower) fair value measurement.
Level 3 liabilities are valued using unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the warrant liabilities. For fair value measurements categorized within Level 3 of the fair value hierarchy, the Company’s Chief Financial Officer, who reports to the Chief Executive Officer, determines its valuation policies and procedures.
The development and determination of the unobservable inputs for Level 3 fair value measurements and fair value calculations are the responsibility of the Company’s Chief Financial Officer and are approved by the Chief Executive Officer.
Level 3 financial liabilities consist of the warrant liabilities for which there is no current market for these securities such that the determination of fair value requires significant judgment or estimation. Changes in fair value measurements categorized within Level 3 of the fair value hierarchy are analyzed each period based on changes in estimates or assumptions and recorded as appropriate.
Income Taxes
Income taxes are computed using the asset and liability method. Under the asset and liability method, deferred income tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities and are measured using the currently enacted tax rates and laws. A valuation allowance is provided for the amount of deferred tax assets that, based on available evidence, are not expected to be realized.
U.S. GAAP prescribes a recognition threshold and measurement process for financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return.
Management has evaluated and concluded that there were no material uncertain tax positions requiring recognition in the Company’s financial statements as of December 31, 2014 and 2013. The Company does not expect any significant changes in the unrecognized tax benefits within twelve months of the reporting date.
The Company classifies interest expense and any related penalties related to income tax uncertainties as a component of income tax expense. No interest or penalties have been recognized during the years ended December 31, 2014 and 2013.
Debt Discounts
The Company records, as a discount to notes and convertible notes, the relative fair value of warrants issued in connection with the issuances and the intrinsic value of any conversion options based upon the differences between the fair value of the underlying Common Stock at the commitment date of the note transaction and the effective conversion price embedded in the note. Debt discounts under these arrangements are amortized to interest expense using the interest method over the earlier of the term of the related debt or their earliest date of redemption.
Net Loss per Share of Common Stock
Basic loss per common share is computed by dividing net loss by the weighted average number of vested common shares outstanding during the period. Diluted earnings per common share is computed by dividing net income by the weighted average number of vested common shares outstanding, plus the impact of common shares, if dilutive, resulting from the exercise of outstanding stock options and warrants, plus the conversion of convertible notes.
F-19
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
The following securities are excluded from the calculation of weighted average dilutive common shares outstanding because their inclusion would have been anti-dilutive:
|
|
|
December 31, |
||
|
|
|
2014 |
|
2013 |
|
Options |
|
2,645,967 |
|
2,439,834 |
|
Warrants |
|
267,637 |
|
219,333 |
|
Convertible Promissory Notes |
|
546,078 |
|
— |
|
Total potentially dilutive shares |
|
3,459,682 |
|
2,659,167 |
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, delivery of the product or service has occurred, all obligations have been performed pursuant to the terms of the agreement, the sales price is fixed or determinable, and collectability is reasonably assured.
The Company has generated revenue from sources other than product sales, namely collaboration and research agreements, development of prototypes, conference fees and from a Kite Patch campaign.
Revenue is recognized when the contractual milestones are met, proto-type delivery and in the case of the conference the event has occurred. For the Kite Patch campaign revenue was recognized for merchandise delivered and deferred for contributions related to the delivery of the product. The Company had minimal revenue during the year ended December 31, 2013, and did not generate any revenue during the year ended December 31, 2014.
On May 1, 2013, the Company entered into an agreement with third party to develop an electronic analyzer for a defined market. An initial payment of $30,000 was due and paid on signing of the agreement. On July 12, 2013, the Company entered into a second agreement to design and deliver a proto-type analyzer for the defined market for $5,000. The Company recognized revenue when all milestones relative to the funded amount were completed and the proto-type delivered and collection reasonably assured.
On May 9, 2013, the Company sponsored the ieCrowd Expo conference event. The Company generated revenues from general public ticket sales and participation fees from event sponsors for booth and presentation opportunities. Revenue of $74,688 was recognized at the completion of the event.
On July 15, 2013, the Company initiated an INDIEGOGO.com campaign for the development and testing of Kite Patch (the “Campaign”). Kite Patch is a mosquito fighting technology designed to block mosquitos’ ability to track humans and spread disease. On August 29, 2013 at the close of the Campaign, $516,974 (net of transaction fees) was collected. As part of the Campaign, the Company offered “perks” for various levels of contributions. These items included t-shirts, water bottles, stickers and Kite Patches. As of December 31, 2013, the Company has delivered all the non-kite perks and recognized $14,482 as Other Income “Income from sale of non-operating merchandise”, net of pro-rata transaction fees of $4,475 and merchandise product cost of $43,791. As of December 31, 2014 and 2013, the Company has Deferred Revenue related to the Campaign of approximately $210,000, net of related transaction fees of $35,000 and estimated Kite Patch production cost of approximately $249,000 since the underlying products have not been shipped.
Stock-Based Compensation
On March 1, 2011, the Company adopted its long-term incentive compensation plan (the “Plan”). A total of 1,000,000 options were allocated to the Plan. On July 14, 2011, the Company amended and restated the Plan to a total of 2,666,667 options allocated. Under the Plan, 2,650,300 options have been issued as of December 31, 2014.
F-20
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
The Company measures the cost of services received in exchange for an award of equity instruments based on the fair value of the award. For employees, the fair value of the award is measured on the grant date and for non-employees, the fair value of the award is generally re-measured on vesting dates and interim financial reporting dates until the service period is complete. The fair value amount is then recognized over the period during which services are required to be provided in exchange for the award, usually the vesting period. Awards granted to directors are treated on the same basis as awards granted to employees.
Common Stock Warrants and Other Derivative Financial Instruments
The Company classifies as equity any contracts that (i) require physical settlement or net-share settlement or (ii) provide the Company with a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement) providing that such contracts are indexed to the Company’s own stock. The Company classifies as assets or liabilities any contracts that (i) require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside the Company’s control) or (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement).
The Company assesses classification of its Common Stock warrants and other free standing derivatives at each reporting date to determine whether a change in classification between assets and liabilities is required. The Company evaluated its free standing warrants to purchase Common Stock to assess their proper classification in the balance sheets as of December 31, 2014 and 2013 using the applicable classification criteria enumerated under GAAP and determined that the Common Stock purchase warrants contain fixed settlement provisions.
Advertising
Advertising costs are charged to operations as incurred. For the years ended December 31, 2014 and 2013, the Company incurred advertising costs of $3,586 and $135, respectively.
Research and Development
Research and development expenses are charged to operations as incurred. For the years ended December 31, 2014 and 2013, the Company incurred research and development costs of $702,736 and $571,070, respectively.
Recently Issued Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2014-09, “Revenue from Contracts with Customers,” (“ASU 2014-09”). ASU 2014-09 supersedes the revenue recognition requirements in Accounting Standards Codification (“ASC”) 605 - Revenue Recognition and most industry-specific guidance throughout the ASC. The standard requires that an entity recognizes revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the Company expects to be entitled in exchange for those goods or services. ASU 2014-09 is effective on January 1, 2017 and should be applied retrospectively to each prior reporting period presented or retrospectively with the cumulative effect of initially applying ASU 2014-09 recognized at the date of initial application. The Company is currently evaluating the impact of the adoption of ASU 2014-09 on its consolidated financial statements.
In June 2014, the FASB issued ASU No. 2014-10, “Development Stage Entities (Topic 915): Elimination of Certain Financial Reporting Requirements, Including an Amendment to Variable Interest Entities Guidance in Topic 810, Consolidation,” (“ASU 2014-10”). ASU 2014-10 removes the definition of a development stage entity from the ASC, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, ASU 2014-10 eliminates the requirements for development stage entities to (1) present inception-to-date information in the statements of operations, cash flows, and stockholders’ equity, (2) label the financial statements as those of a development stage entity, (3) disclose a description of the development stage activities in which the entity is engaged, and (4) disclose in the first year in which the entity is no
F-21
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 3 — SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (cont.)
longer a development stage entity that in prior years it had been in the development stage. ASU 2014-10 is effective for annual reporting periods beginning after December 15, 2014, and interim periods therein. Early adoption is permitted. The Company has elected to adopt ASU 2014-10 and as a result, is not required to present the previously required development stage disclosures. The Company’s planned principal operations are to develop its business model. The Company’s activities are subject to significant risks and uncertainties, which are detailed in Note 2 — Going Concern and Management’s Plans.
In June 2014, the FASB issued ASU No. 2014-12, “Compensation - Stock Compensation (Topic 718): Accounting for Share-Based Payments When the Terms of an Award Provide that a Performance Target Could be Achieved after the Requisite Service Period,” (“ASU 2014-12”). The amendments in ASU 2014-12 require that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition. A reporting entity should apply existing guidance in ASC Topic No. 718, “Compensation — Stock Compensation” as it relates to awards with performance conditions that affect vesting to account for such awards. The amendments in ASU 2014-12 are effective for annual periods and interim periods within those annual periods beginning after December 15, 2015. Early adoption is permitted. Entities may apply the amendments in ASU 2014-12 either: (a) prospectively to all awards granted or modified after the effective date; or (b) retrospectively to all awards with performance targets that are outstanding as of the beginning of the earliest annual period presented in the financial statements and to all new or modified awards thereafter. The Company does not anticipate that the adoption of ASU 2014-12 will have a material impact on its consolidated financial statements.
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements — Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” (“ASU 2014-15”). ASU 2014-15, which is effective for annual reporting periods ending after December 15, 2016, extends the responsibility for performing the going-concern assessment to management and contains guidance on how to perform a going-concern assessment and when going-concern disclosures would be required under U.S. GAAP. The Company elected to early adopt ASU 2014-15. Management’s evaluations regarding the events and conditions that raise substantial doubt regarding the Company’s ability to continue as a going concern have been disclosed in Note 2.
In November 2014, the FASB issued Accounting Standards Update (“ASU”) No. 2014-17, “Business Combinations (Topic 805): Pushdown Accounting” (“ASU 2014-17”). ASU 2014-17 provides an acquired entity with an option to apply pushdown accounting in its separate financial statements upon occurrence of an event in which an acquirer obtains control of the acquired entity. The acquired entity may elect the option to apply pushdown accounting in the reporting period in which the change-in-control event occurs. If pushdown accounting is not applied in the reporting period in which the change-in-control event occurs, an acquired entity will have the option to elect to apply pushdown accounting in a subsequent reporting period as a change in accounting principle in accordance with ASC Topic 250, “Accounting Changes and Error Corrections”. If pushdown accounting is applied to an individual change-in-control event, that election is irrevocable. ASU 2014-17 also requires an acquired entity that elects the option to apply pushdown accounting in its separate financial statements to disclose information in the current reporting period that enables users of financial statements to evaluate the effect of pushdown accounting. The Company has adopted the amendments in ASU 2014-17. The adoption did not have an impact on our consolidated financial statements.
NOTE 4 — INVESTMENTS IN SUBSIDIARIES
On April 17, 2013, as part of an OLI equity call to existing shareholders, the Company sold 99 shares of common stock of OLI for $4,950 to third parties.
F-22
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 5 — PROPERTY AND EQUIPMENT
Property and equipment consists of the following at December 31, 2014 and 2013:
|
|
|
2014 |
|
2013 |
||||
|
Lab equipment |
|
$ |
82,834 |
|
|
$ |
70,611 |
|
|
Vehicles |
|
|
5,591 |
|
|
|
— |
|
|
Leasehold improvements |
|
|
228,326 |
|
|
|
220,527 |
|
|
Total fixed assets |
|
|
316,751 |
|
|
|
291,138 |
|
|
Less: Accumulated depreciation |
|
|
(157,720 |
) |
|
|
(96,674 |
) |
|
Property and equipment, net |
|
$ |
159,031 |
|
|
$ |
194,464 |
|
Depreciation and amortization expense was $61,046 and $58,210 for the years ended December 31, 2014 and 2013, respectively.
NOTE 6 — ACCRUED EXPENSES AND OTHER CURRENT LIABILITIES
Accrued expenses and other current liabilities consists of the following at December 31, 2014 and 2013:
|
|
|
2014 |
|
2013 |
||
|
Accrued payroll and payroll taxes |
|
$ |
30,470 |
|
$ |
107,885 |
|
Accrued professional fees |
|
|
— |
|
|
8,300 |
|
Other current liabilities |
|
|
5,714 |
|
|
50,543 |
|
Accrued interest |
|
|
47,002 |
|
|
— |
|
Accrued marketing cost |
|
|
181,244 |
|
|
181,244 |
|
Accrued Kite production cost |
|
|
248,331 |
|
|
248,331 |
|
Total accrued expenses |
|
$ |
512,761 |
|
$ |
596,303 |
NOTE 7 — NOTES PAYABLE
On December 12, 2013, the Company executed two promissory notes for a total of $123,583 for advances made on behalf of the Company to fund the Company’s conference event. The notes bear interest at 7.75% per annum. The notes matured on December 31, 2014 and the maturity dates were subsequently extended to December 31, 2015. At December 31, 2014, the amount outstanding under these notes is $47,455.
NOTE 8 — CONVERTIBLE NOTES PAYABLE
On November 3, 2011, the Company entered into a two year unsecured convertible promissory note for the principal sum of $250,000 plus simple interest at a rate of 4.0% per annum. At the option of the holder, the note was convertible into common shares of the Company at a share price of $1.50. Interest payments were due and payable quarterly in arrears on the date that is thirty days after the end of each calendar quarter. Payments of principal and any accrued but unpaid interest were to be made on or before the maturity date. The convertible promissory note was recorded net of a beneficial conversion feature of $148,540, based on the relative fair value of the conversion feature. The beneficial conversion feature is being amortized over the term of the debenture. During the year ended December 31, 2013, the Company recorded amortization of the beneficial conversion feature related to this convertible promissory note of $58,124. On October 4, 2013, the convertible promissory note plus interest of $19,250 was converted into 179,500 common shares of the Company.
On October 15, 2014, the Company closed private placements of Unsecured Convertible Promissory Notes (the “October 2014 Notes”) in the aggregate principal amount of $2,785,000. The October 2014 Notes bear interest at 8% per annum and mature on October 15, 2016. Any unpaid principal and accrued interest due under the October 2014 notes will automatically convert into shares of common stock at the maturity date. If the Company completes a registered initial public offering (“IPO”) prior to the Maturity Date, the Company shall have the right to force
F-23
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 8 — CONVERTIBLE NOTES PAYABLE (cont.)
conversion of any unpaid principal and accrued interest owed under the October 2014 Notes, in whole, or in part, into the securities issued pursuant to the IPO at a 20% discounted conversion price to the price of the securities sold in the IPO. If the Company completes a qualifying transaction that results in the Company receiving gross proceeds of more than $7,500,000 prior to the Maturity Date, the Company shall also have the right to force conversion of any unpaid principal and accrued interest owing under the October 2014 Notes, in whole, or in part, into the securities issued pursuant to the IPO at a 20% discounted conversion price to the price of the qualifying transaction.
The principal of the October 2014 Notes is convertible into 546,078 shares of common stock at a conversion price of $5.10 per share. The October 2014 Notes contain a provision to lower the conversion price to any subsequent common stock issuance at a lower price, if any convertible debt subsequently issued has a lower conversion price or if any options or warrants have lower exercise prices. The Company determined that the conversion feature of the October 2014 Notes did not contain fixed settlement provisions because the conversion price can be adjusted based on new issuances, and accordingly, the Company recorded the note conversion feature as a liability and mark to market the derivative to fair value each reporting period.
The aggregate fair value of the conversion feature of $999,548 was applied to the principal amount of the October 2014 Notes to determine the debt discount. Accordingly, the Company allocated $999,548 of the offering proceeds to the fair value of the note conversion feature on the date of issuance and recorded it as a derivative liability in the accompanying consolidated balance sheet. Such amount was being amortized over the terms of the October 2014 notes using the effective interest method. During the year ended December 31, 2014, the Company recognized $104,120 in amortization of the debt discount relating to the October 2014 Notes.
The fair value of the conversion feature of the October 2014 Notes on the issuance date was calculated using a binomial option model valued with the following weighted average assumptions:
|
Dividend Yield |
|
|
0 |
% |
|
Volatility |
|
|
65.55 |
% |
|
Risk-free interest rate |
|
|
0.34 |
% |
|
Contractual term |
|
|
2 years |
|
|
Weighted average unit fair value |
|
$ |
1.336 |
|
|
Common shares assumed issued |
|
|
546,078 |
|
|
Aggregate fair value |
|
$ |
999,548 |
|
During the year ended December 31, 2014, the Company marked the conversion feature of the October 2014 Notes to fair value and recorded a gain of $66,505 relating to the change in fair value.
Note 8a — Warrants to Placement Agent (Revised — See Note 14)
In connection with the October 2014 Notes, the Company granted warrants to the placement agent exercisable for the purchase of convertible notes, whose terms are identical to the October 2014 Notes, aggregating approximately $115,000.
In addition, the Company also paid financing fees totaling $153,810. Which recorded as Deferred Financing Costs and are being amortized on a straight line basis over the stated term of the October 2014 Notes. During the year ended December 31, 2014, amortization of deferred financing costs relating to the October 2014 Notes totaled $28,297.
F-24
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 9 — STOCKHOLDERS’ EQUITY (DEFICIENCY)
Authorized Capital
The Company is authorized to issue up to 500,000,000 shares of common stock, $0.00001 par value. The holders of the Company’s common stock are entitled to one vote per share.
Common Stock
For the year ended December 31, 2013, 434,950 shares of common stock were sold resulting in proceeds of $1,627,000. In addition, the Company issued 85,040 shares of common stock to vendors for services provided of $344,165.
For the year ended December 31, 2014, 490,417 shares of common stock were sold resulting in proceeds of $2,340,000. Warrants were exercised into 104,167 shares of common stock for $500,000. In addition, the Company issued 17,132 shares of common stock to vendors for services provided with a value of $82,395.
Conversion of Accounts Payable into Warrants
In February 2014, the Company reached a settlement agreement with a vendor on $18,500 of outstanding accounts payable by issuing five year warrants to purchase 6,667 shares common stock at an exercise price of $3.75 per share. In applying the Black-Scholes option pricing model to the warrants granted, the Company used the following assumptions:
|
Risk free interest rate |
|
0.70% |
|
|
Dividend yield |
|
0.00% |
|
|
Expected Volatility |
|
153.0% |
|
|
Expected life in years |
|
3.01 |
|
|
Forfeiture Rate |
|
0.00% |
|
A loss on settlement of accounts payable of $8,354 was recorded in the consolidated statement of operations, representing the difference between the fair value of the warrants and the outstanding accounts payable.
Stock-Based Compensation
The Company uses the Black-Scholes option pricing model to determine the fair value of the options and warrants granted. In applying the Black-Scholes option pricing model to stock options granted, the Company used the following weighted average assumptions:
|
|
|
For
The Years Ended |
||||
|
|
|
2014 |
|
2013 |
||
|
Risk free interest rate |
|
1.66% to 2.77% |
|
|
1.66% to 2.78% |
|
|
Dividend yield |
|
0.00% |
|
|
0.00% |
|
|
Expected volatility |
|
125.0% to 134.0% |
|
|
130.0% to 134.0% |
|
|
Expected life in years |
|
5.0 to 6.5 |
|
|
5.0 to 7.0 |
|
|
Forfeiture Rate |
|
0.00% |
|
|
0.00% |
|
The weighted average fair value of the stock options granted during the years ended December 31, 2014 and 2013 was $4.81 and $3.15 per option, respectively.
F-25
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 9 — STOCKHOLDERS’ EQUITY (DEFICIENCY) (cont.)
In applying the Black-Scholes option pricing model to warrants granted, the Company used the following weighted average assumptions:
|
|
|
For
The Years Ended |
||
|
|
|
2014 |
|
2013 |
|
Risk free interest rate |
|
1.37% to 1.76% |
|
0.85% to 0.87% |
|
Dividend yield |
|
0.00% |
|
0.00% |
|
Expected volatility |
|
138.20% to 154.0% |
|
159.0% to 160.0% |
|
Expected life in years |
|
4.00 to 5.00 |
|
4.00 to 4.99 |
|
Forfeiture Rate |
|
0.00% |
|
0.00% |
The weighted average fair value of the warrants granted during the years ended December 31, 2014 and 2013 was $4.81 and $3.00 per warrant, respectively.
A summary of the stock option activity during the years ended December 31, 2014 and 2013 is presented below:
|
|
Number of Options |
|
Weighted Average Exercise Price |
|
Weighted Average Remaining Life In Years |
|
Aggregate Intrinsic Value |
|||
Outstanding, January 1, 2013 |
|
2,160,500 |
|
|
$ |
1.50 |
|
7.98 |
|
$ |
7,068,650 |
Granted |
|
279,333 |
|
|
|
3.30 |
|
9.33 |
|
|
|
Exercised |
|
— |
|
|
|
|
|
|
|
|
|
Forfeited |
|
— |
|
|
|
|
|
|
|
|
|
Outstanding, December 31, 2013 |
|
2,439,833 |
|
|
$ |
1.65 |
|
8.14 |
|
$ |
7,452,400 |
Granted |
|
210,467 |
|
|
|
4.81 |
|
8.87 |
|
|
|
Exercised |
|
— |
|
|
|
|
|
|
|
|
|
Forfeited |
|
(4,333 |
) |
|
|
|
|
|
|
|
|
Outstanding, December 31, 2014 |
|
2,645,967 |
|
|
$ |
1.99 |
|
7.28 |
|
$ |
8,254,840 |
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, December 31, 2014 |
|
2,423,500 |
|
|
$ |
1.67 |
|
7.14 |
|
$ |
8,096,100 |
During the year ended December 31, 2014, the Company granted options to purchase an aggregate of 210,467 shares of Common Stock to certain employees. These options vest over a three year period, have a term of 10 years, and contain an exercise price between $4.80 and $5.10 per share. The options were granted under a previously approved plan and had an aggregate grant date fair value of $950,270.
During the year ended December 31, 2013, the Company granted options to purchase an aggregate of 279,333 shares of Common Stock to certain employees. These options vest over a one year to three year period, have a term of 10 years, and contain an exercise price between $2.25 and $5.25 per share. The options were granted under a previously approved plan and had an aggregate grant date fair value of $1,147,656.
F-26
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 9 — STOCKHOLDERS’ EQUITY (DEFICIENCY) (cont.)
A summary of the stock warrant activity during the years ended December 31, 2014 and 2013 is presented below:
|
|
|
Number of Warrants |
|
Weighted Average Exercise Price |
|
Weighted Average Remaining Life In Years |
|
Aggregate Intrinsic Value |
|||
|
Outstanding, January 1, 2013 |
|
205,667 |
|
|
$ |
2.10 |
|
1.17 |
|
$ |
566,200 |
|
Granted |
|
14,000 |
|
|
|
3.00 |
|
3.16 |
|
|
|
|
Exercised |
|
— |
|
|
|
— |
|
|
|
|
|
|
Forfeited |
|
— |
|
|
|
— |
|
|
|
|
|
|
Outstanding, December 31, 2013 |
|
219,667 |
|
|
$ |
2.10 |
|
1.29 |
|
$ |
590,050 |
|
Granted |
|
152,137 |
|
|
|
4.85 |
|
0.96 |
|
|
|
|
Exercised |
|
(104,167 |
) |
|
|
4.80 |
|
|
|
|
|
|
Forfeited |
|
— |
|
|
|
— |
|
|
|
|
|
|
Outstanding, December 31, 2014 |
|
267,637 |
|
|
$ |
2.77 |
|
2.14 |
|
$ |
650,550 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable, December 31, 2014 |
|
267,637 |
|
|
$ |
2.77 |
|
2.14 |
|
$ |
650,550 |
During the year ended December 31, 2014, the Company granted warrants to purchase an aggregate of 13,999 shares of Common Stock to certain contract vendors. The warrants vest immediately, have a term of three to four years and contain exercise prices between $3.75 and $4.80 per share. The warrants had an aggregate grant date fair value of $87,235.
During the year ended December 31, 2014, the Company granted warrants to purchase an aggregate of 104,167 shares to an investor for a ninety day period. The warrants were issued to the investor in conjunction with common stock purchased by the investor. The warrants vest immediately and have an exercise price of $4.80 per share. The investor exercised the warrant for $500,000.
During the year ended December 31, 2013, the Company granted warrants to purchase an aggregate of 14,000 shares of Common Stock to certain contract vendors. These warrants vest over a one year or three year period, have a term of 4 years, and contain an exercise price between $3.00 and $4.80 per share. The warrants had an aggregate grant date fair value of $36,327.
Stock-Based Compensation: Olfactor Laboratories Inc.
A subsidiary of the Company has granted options to purchase its common stock to certain employees of the Company and the subsidiary. The Company uses the Black-Scholes option pricing model to determine the fair value of the options granted. In applying the Black-Scholes option pricing model to stock options granted, the Company used the following weighted average assumptions:
|
|
|
For
The Years Ended |
||
|
|
|
2014 |
|
2013 |
|
Risk free interest rate |
|
— |
|
1.56% to 2.03% |
|
Dividend yield |
|
— |
|
0.00% |
|
Expected volatility |
|
— |
|
128.0% to 136.0% |
|
Expected life in years |
|
— |
|
2.0 to 6.5 |
|
Forfeiture Rate |
|
— |
|
0.00% |
The weighted average fair value of the stock options granted during the year ended December 31, 2013 was $210.43 per option.
F-27
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 9 — STOCKHOLDERS’ EQUITY (DEFICIENCY) (cont.)
A summary of the stock option activity of the subsidiary during the years ended December 31, 2014 and 2013 is presented below:
|
|
Number of Options |
|
Weighted Average Exercise Price |
|
Weighted Average Remaining Life In Years |
|
Aggregate Intrinsic Value |
||
Outstanding, January 1, 2013 |
|
1,578 |
|
$ |
210.15 |
|
3.22 |
|
$ |
— |
Granted |
|
125 |
|
|
210.43 |
|
5.55 |
|
|
|
Exercised |
|
— |
|
|
— |
|
|
|
|
|
Forfeited |
|
— |
|
|
— |
|
|
|
|
|
Outstanding, December 31, 2013 |
|
1,703 |
|
$ |
210.17 |
|
4.85 |
|
$ |
— |
Granted |
|
— |
|
|
— |
|
|
|
|
|
Exercised |
|
— |
|
|
— |
|
|
|
|
|
Forfeited |
|
— |
|
|
— |
|
|
|
|
|
Outstanding, December 31, 2014 |
|
1,703 |
|
$ |
210.17 |
|
3.85 |
|
$ |
— |
|
|
|
|
|
|
|
|
|
|
|
Exercisable, December 31, 2014 |
|
1,620 |
|
$ |
210.16 |
|
3.60 |
|
$ |
— |
During the year ended December 31, 2013, the Company granted options to purchase an aggregate of 125 shares of common stock in one of its subsidiaries to certain employees. These options vest over a one year to three year period, have a term of 10 years, and contain an exercise price of $210.43 per share. The options had an aggregate grant date fair value of $23,361.
Stock-based compensation for stock options and warrants has been recorded in the consolidated statements of operations and totaled $701,067 and $714,905 for the years ended December 31, 2014 and 2013, respectively. As of December 31, 2014, the remaining balance of unamortized expense is $497,039, which is expected to be amortized over a period of 2.5 years.
NOTE 10 — RELATED PARTY TRANSACTIONS
On August 21, 2014, the Company entered into an Advertising Agreement with Sunbelt IV Technologies Management, LLC, or Sunbelt IV, an entity under common control. The management of Sunbelt IV controls Sunbelt III Technologies Management, LLC, which is one of the Company’s principal stockholders. Under the agreement, Sunbelt IV provided the Company advertising services related to an upcoming private placement of convertible notes. The services were completed at the close of the private placement on the October 2014 notes. Sunbelt IV received a total of $16,000 under the agreement, and this was included as a part of deferred financing costs (see Note 8).
NOTE 11 — COMMITMENTS AND CONTINGENCIES
Operating Leases
The Company leases 6,900 square feet of office space. The lease agreement calls for payment on an annual escalating basis currently at $15,144 and is through October 31, 2015. Such payments are being accounted for on a straight-line basis. The Company leases laboratory and office space of 11,846 square feet. The lease payment is $7,015 per month through October 31, 2015. Rent expense for the year ended December 31, 2014 and 2013 was $245,505 and $241,285 respectively. Deferred rent as of December 31, 2014 and 2013 was immaterial to the consolidated financial statements.
F-28
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 11 — COMMITMENTS AND CONTINGENCIES (cont.)
Financial Advisory and Investment Banking Agreement
The Company entered into an exclusive Financial Advisory and Investment Banking Agreement with TriPoint Global Securities, LLC (“TriPoint”) effective June 18, 2014 (the “TriPoint Advisory Agreement”). Pursuant to the TriPoint Advisory Agreement, TriPoint will act as the Company’s exclusive financial advisor and placement agent to assist the Company in connection with a best efforts registered primary offering (the “Offering”) of up to $20 million of the Company’s common stock. The Company upon closing of the Offering shall pay consideration to TriPoint a fee in an amount equal to 6.5% of the aggregate gross proceeds raised in the Offering. The Company shall grant and deliver to TriPoint at the closing of the Offering three year warrants covering a number of securities equal to 5% of the total number of securities sold in the Offering. The warrants shall be exercisable at any time after the first six months of the offering at a price equal to 110% of the offering price in connection with the Offering. Along with the above fees, the Company paid a $20,000 engagement fee upon execution of the agreement. In addition, the Company will pay (i) all expenses related to the Offering and (ii) all legal fees incurred by TriPoint up to a maximum of $75,000. As of December 31, 2014 and through the date of this report, the Offering had not been completed. The Company has capitalized $238,044 in Deferred Offering Costs as of December 31, 2014.
Licenses
The Company is subject to material contractual obligations and commitments under the Company’s various licensing agreements. In general, the continuation of the exclusivity of the Company’s rights, and the continuation of non-exclusive rights if exclusivity has been lost or has not been granted, is subject to the Company payment of various amounts and the Company’s achievement of various milestones over time toward the commercialization of the licensed innovations. The material payment obligations applying to the Company’s license agreements are summarized below:
OLI license
OLI is a party to a license agreement relating to patent-pending technologies to control the behavior of mosquitoes and other blood-seeking insects with non-insecticidal agents. As partial consideration for the license, the Company paid a one-time license issue fee of $10,000. The Company must pay an earned royalty of 3% of net sales of licensed products upon becoming cash-flow positive or starting 18 months after the effective date of the license, January 29, 2010, whichever is earlier. The Company must also pay 3% of all non-royalty consideration received from sublicensing. OLI must pay the Board of Regents of the University of California a minimum royalty payment of $30,000 annually, irrespective of whether or not OLI has generated any revenue. In addition, the Company is responsible for patent maintenance costs. The Company has expensed $95,287 and $30,000 for the years ended December 31, 2014 and 2013, respectively, related to such agreement. The agreement is cancellable at any time by OLI.
NEA license
NEA is a party to a license agreement relating to patented and patent-pending sensor technologies based on nano materials that can detect airborne gases to the parts-per-billion (PPB) level. As partial consideration for the license, the Company paid a one-time license issue fee of $10,000. The Company must pay an earned royalty of 3% of net sales of licensed products upon becoming cash-flow positive or starting 18 months after the effective date of the license, March 31, 2010, whichever is earlier. The Company must also pay 3% of all non-royalty consideration received from sublicensing for the year 2017, and for each year thereafter for the duration of the license. The Company must pay the greater of the earned royalty described above or a minimum annual royalty of $5,000 in 2017 and $10,000 in 2018 and each year thereafter. In addition, the Company is responsible for patent maintenance costs. The Company has expensed $13,520 and $117,341 for the years ended December 31, 2014 and 2013, respectively, related to such agreement. The agreement is cancellable at any time by NEA.
F-29
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 11 — COMMITMENTS AND CONTINGENCIES (cont.)
SOS license
SOS is a party to a license agreement relating to oxygen delivery devices that would incorporate a computer controlled, patient-adaptive dosing system, for people who use supplemental oxygen therapy. As partial consideration for the license, the Company paid a one-time license issue fee of $10,000. The Company must pay an earned royalty of 3% of net sales of licensed products upon becoming cash-flow positive or starting 18 months after the effective date of the license, October 21, 2013, whichever is earlier. The Company must also pay 3% of all non-royalty consideration received from sublicensing for the year 2017, and for each year thereafter for the duration of the license. The Company must pay the greater of the earned royalty described above or a minimum annual royalty of $5,000 in 2017 and $10,000 in 2018 and each year thereafter. In addition, the Company is responsible for patent maintenance costs The Company has expensed $16,630 and $23,392 for the years ended December 31, 2014 and 2013, respectively, related to such agreement. The agreement is cancellable at any time by SOS.
On November 3, 2014, the Company executed the following license agreements with the Regents of the University of California:
Head Pain Management license
The Company is a party to a license agreement, dated November 3, 2014, relating to potential therapeutic treatments based on technology that stimulates the sensory fibers of a nerve in order to mitigate pain perception. As partial consideration for the license, the Company must pay $16,000 within 30 days of entering into the license and another $16,000 within 120 days of entering into the license. The Company must make a milestone payment of $350,000 on the first to occur of: the receipt of a letter of approval or clearance for the premarket notifications process required by the FDA under section 510(k) of the Food, Drug, and Cosmetic Act (“501(k)”) or premature approval under section 515 of the Food, Drug, and Cosmetic Act (“PMA”) application in the U.S. for a licensed product, and the receipt of marketing approval in Europe or any individual European country for a licensed product. In addition, on the anniversary date of entering into the license, the Company must pay $2,500 in each of years one and two, $5,000 in year three and $10,000 in each subsequent year. Moreover, after the First Commercial Sale (“FCS”), the Company must pay minimum royalty fees of $20,000 in year one, $80,000 in year two, $140,000 in each of years three and four and $300,000 in each subsequent year. The Company has expensed $16,000 for the year ended December 31, 2014 related to such agreement. The agreement is cancellable at any time by the Company.
Sleep Disorders license
The Company is a party to a license agreement, dated November 3, 2014, relating to potential therapeutic treatments based on technology that takes advantage of the fact that signals from the limbs can be interpreted by the brain as movement and can elicit altered breathing rates. As partial consideration for the license, the Company must pay $10,000 within 30 days of entering into the license and another $10,000 within 120 days of entering into the license. The Company must make a milestone payment of $140,000 on the first to occur of: the receipt of a letter of approval or clearance for a 510(k) or PMA application in the U.S. for a licensed product, and the receipt of marketing approval in Europe or any individual European country for a licensed product. In addition, on the anniversary date of entering into the license, the Company must pay $2,500 in each of years one and two, $5,000 in year three and $10,000 in each subsequent year. Moreover, after the First Commercial Sale (“FCS”), the Company must pay minimum royalty fees of $20,000 in year one, $80,000 in year two, $140,000 in each of years three and four and $300,000 in each subsequent year. The Company has expensed $10,000 for the year ended December 31, 2014 related to such agreement. The agreement is cancellable at any time by the Company.
Litigations, Claims and Assessments
In the normal course of business, the Company may be involved in legal proceedings, claims and assessments arising in the ordinary course of business. Such matters are subject to many uncertainties, and outcomes are not predictable with assurance. There are no such matters that are deemed material to the consolidated financial statements as of December 31, 2014 and 2013.
F-30
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 12 — INCOME TAXES
As of December 31, 2014 and 2013, the Company had $12,440,374 and $8,256,254, respectively, of federal and state net operating loss carryforwards (“NOL’s”) that may be available to offset future taxable income. The net operating loss carryforwards, if not utilized, will expire from 2031 to 2034. In accordance with Section 382 of the Internal Revenue Code, the usage of the Company’s net operating loss carryforwards could be limited in the event of a change in ownership.
The Company files income tax returns in the U.S. federal jurisdiction and various state and local jurisdictions and is subject to examination by the various taxing authorities. The Company’s federal, state and local income tax returns beginning in 2011 remain subject to examination.
The income tax provision (benefit) for the years ended December 31, 2014 and 2013 was as follows:
|
|
|
For
The Years Ended |
||||||
|
|
|
2014 |
|
2013 |
||||
|
Federal: |
|
|
|
|
|
|
|
|
|
|
$ |
— |
|
|
$ |
— |
|
|
|
|
|
(1,660,926 |
) |
|
|
(909,148 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
State and local: |
|
|
|
|
|
|
|
|
|
|
|
— |
|
|
|
— |
|
|
|
|
|
(284,995 |
) |
|
|
(155,090 |
) |
|
|
|
|
|
(1,945,921 |
) |
|
|
(1,064,238 |
) |
|
Valuation allowance |
|
|
1,945,921 |
|
|
|
1,064,238 |
|
|
Income tax provision (benefit) |
|
$ |
— |
|
|
$ |
— |
|
The effects of temporary differences that give rise to significant portions of the deferred tax assets and liabilities for the years ended December 31, 2014 and 2013 are as follows:
|
|
|
December 31, |
||||||
|
|
|
2014 |
|
2013 |
||||
|
Deferred tax assets: |
|
|
|
|
|
|
|
|
|
|
$ |
4,955,498 |
|
|
$ |
3,288,829 |
|
|
|
|
|
41,475 |
|
|
|
— |
|
|
|
|
|
(41,475 |
) |
|
|
— |
|
|
|
|
|
1,371,204 |
|
|
|
1,091,952 |
|
|
|
Total deferred tax assets |
|
|
6,326,702 |
|
|
|
4,380,781 |
|
|
|
|
(6,326,702 |
) |
|
|
(4,380,781 |
) |
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred tax assets |
|
$ |
— |
|
|
$ |
— |
|
The Company assesses the likelihood that deferred tax assets will be realized. To the extent that realization is not likely, a valuation allowance is established. Management believes that it is more likely than not that future benefits of deferred tax assets will not be realized. The valuation allowance increased by $1,945,921 and $1,064,238 at December 31, 2014 and 2013, respectively.
F-31
INNOVATION ECONOMY CORPORATION
NOTES TO THE
CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2014 AND 2013
NOTE 12 — INCOME TAXES (cont.)
For the years ended December 31, 2014 and 2013, the expected tax expense (benefit) based on the statutory rate is reconciled with the actual tax expense (benefit) as follows:
|
|
|
For
The Years Ended |
||||
|
|
|
2014 |
|
2013 |
||
|
US federal statutory rate |
|
(34.0 |
)% |
|
(34.0 |
)% |
|
State tax rate, net of federal benefit |
|
(5.8 |
)% |
|
(5.8 |
)% |
|
Permanent differences: |
|
|
|
|
|
|
|
|
— |
% |
|
8.4 |
% |
|
|
|
0.4 |
% |
|
0.1 |
% |
|
|
Valuation allowance |
|
39.4 |
% |
|
31.3 |
% |
|
|
|
|
|
|
|
|
|
Income tax provision (benefit) |
|
0.0 |
% |
|
0.0 |
% |
NOTE 13 — SUBSEQUENT EVENTS
Management has evaluated subsequent events or transactions occurring through the date on which the financial statements were issued. Based upon the evaluation, the Company did not identify any recognized or non-recognized subsequent events that would have required adjustment or disclosure in the consolidated financial statements, except as disclosed.
NOTE 14 — REVISED CONSOLIDATED BALANCE SHEET AND STATEMENT OF STOCKHOLDERS’ DEFICIENCY
During the preparation of the Company’s Form S-1 (Amendment #2), the Company identified an error related to the overstatement of deferred financing costs. The error has a de minimus effect on the Company’s consolidated statement of operations for the year ended December 31, 2014. On the consolidated balance sheet, deferred financing costs and additional paid-in capital were overstated by $117,828 at December 31, 2014.
In accordance with SEC Staff Accounting Bulletin Nos. 99 and 108 (“SAB 99” and “SAB 108”), the Company has evaluated this error, based on an analysis of quantitative and qualitative factors, as to whether it was material to the consolidated balance sheet as of December 31, 2014 and if amendments of previously filed registration statements with the SEC are required. The Company has determined that, although quantitatively material to the consolidated balance sheet as of December 31, 2014, qualitatively the Company believes the overstatement of deferred financing costs would not have influenced an investor’s decision making process. In accordance with SAB 108, the Company will include this revised financial information in subsequent filings.
A summary of the effects of the correction on the consolidated balance sheet as of December 31, 2014 are presented in the table below:
|
|
|
As Previously Reported |
|
As Corrected |
||
|
Deferred financing costs |
|
$ |
243,341 |
|
$ |
125,513 |
|
Additional paid in capital |
|
$ |
13,117,315 |
|
$ |
12,999,487 |
Note 8a to the consolidated financial statements for the year ended December 31, 2014 relating to warrants issued to a placement agent has also been added.
F-32
Up to
3,125,000 Units
Consisting of One Share
of Common Stock and
One Warrant to Purchase One-half Share of Common
Stock

________________
PROSPECTUS
________________
TriPoint Global Equities, LLC
The date of this prospectus is __________, 2015
Through and including the 25th day after the date of this prospectus, all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any jurisdiction where an offer or sale is not permitted.
[Alternative page for Selling Securityholder Prospectus]
Preliminary Prospectus |
|
|
|
Subject to completion, dated MAY 20, 2015 |

Units
Consisting of One Share of Common Stock and
One Warrant to Purchase One-half Share of Common
Stock
This prospectus relates to the offer for sale of units (“Units”), each Unit consisting of one share of common stock, par value $0.00001 per share, and one warrant to purchase one-half share of common stock, of Innovation Economy Corporation, which does business under the name “ieCrowd” (the “Company”), by the holders of the Units named in this prospectus, referred to throughout this prospectus as the “selling securityholders”. Each warrant will entitle the holder to purchase one-half share of ieCrowd’s common stock at an exercise price of 125% of the price of the shares in the Company’s initial public offering. The warrants will expire 36 months after the date they are issued. The Units will not be issued or certificated. Instead, the shares of common stock and the warrants will be issued separately and may be resold separately. This prospectus also relates to the exercise of warrants by any purchaser of warrants from the selling securityholders.
The Units being offered hereunder are issuable upon the forced conversion, immediately after the closing of the Company’s initial public offering of Units, of the Private Placement Convertible Notes sold by the registrant in the Private Placements. Upon the Company’s election to force their conversion, the Notes shall convert into Units at a price equal to $5.10 per Unit. That offering is expected to close on June 25, 2015.
Currently, there is no public market for ieCrowd’s Units, common stock or warrants. ieCrowd has applied to list its common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “MYIE”, and to list its warrants on NASDAQ under the symbol “MYIEW”, on or promptly after the closing of ieCrowd’s initial public offering of Units. ieCrowd cannot guarantee that its securities will be approved for listing on NASDAQ or that, if so approved, a liquid market for the securities will develop.
If and when the Company’s securities are listed on NASDAQ, the sale of securities offered hereby may be effected on NASDAQ, including through ordinary brokers’ transactions. Sales may also be effected in over-the-counter markets, including through dealers who would resell such securities as principals, or through privately negotiated transactions. Depending in part on the type of transaction effected, the securities may be sold at market prices prevailing at the time of sale, at prices related to such prevailing market prices or at negotiated prices. Until there is a market for the securities, securityholders will sell their securities at the fixed price of $5.10 per unit. Parties to the transactions may be required to pay usual and customary, or specifically negotiated, brokerage or other fees or commissions.
The selling securityholders and any intermediaries by or through whom any of the securities are offered and sold may be deemed “underwriters” within the meaning of the Securities Act of 1933, as amended, or the Securities Act, and any profits realized or commissions received may be deemed underwriting compensation. Any such “underwriters” within the meaning of the Securities Act will be subject to the prospectus delivery requirements of the Securities Act. See “Plan of Distribution”.
In the 2014 Private Placement, TriPoint Global Equities, LLC, which served as the Company’s placement agent in the 2014 Private Placement, together with Primary Capital, LLC, received five-year warrants entitling the holders thereof to purchase up to $114,025 and $4,375, respectively, of notes with the same terms as those of the Private Placement Convertible Notes. Prior to the closing of the Company’s initial public offering, TriPoint and Primary Capital, LLC exercised their warrants privately so as to purchase $114,025 and $4,375, respectively, of such notes. Immediately after the closing of the Company’s initial public offering, upon the Company’s election to force the conversion of the Private Placement Convertible Notes, the notes held by TriPoint and Primary Capital, LLC were converted into $114,025 and $4,375, respectively, of units, at a price equal to $5.10 per unit, with the same terms as the Units being offered in the Company’s initial public offering. Securities offered by TriPoint and Primary Capital, LLC pursuant to this prospectus are securities converted from notes received from the exercise of warrants to purchase Private Placement Convertible Notes, issued to TriPoint and Primary Capital, LLC in connection with the 2014 Private Placement.
In connection with the 2014 Private Placement, ieCrowd paid TriPoint a cash commission of up to eight percent (8%) of the gross proceeds of the Convertible Notes, which resulted in total cash placement agent fees of $118,400 to TriPoint and certain other selected broker-dealers. Additionally, ieCrowd paid a $20,000 due diligence fee to TriPoint in connection with the 2014 Private Placement.
Investing in ieCrowd’s securities involves a high degree of risk. ieCrowd is at an early stage of its development and its securities may only be appropriate for long-term investment. Its independent registered public accounting firm has issued an audit opinion that includes a statement expressing substantial doubt as to its ability to continue as a going concern. You should purchase ieCrowd securities only if you can afford to lose your entire investment. See “Risk Factors” beginning on page 10.
The Company is an “emerging growth company” under applicable law and rules and it may elect to comply with certain reduced public company reporting requirements following its initial public offering.
Neither the Securities and Exchange Commission nor any state securities commission nor any other regulatory body has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is __________, 2015
[Alternative page for Selling Securityholder Prospectus]
TABLE OF CONTENTS
|
|
|
Page |
|
Prospectus Summary |
|
1 |
|
|
1 |
|
|
|
6 |
|
|
|
8 |
|
|
Risk Factors |
|
9 |
|
Cautionary Note Regarding Forward-Looking Statements |
|
30 |
|
Use of Proceeds |
|
32 |
|
Dividend Policy |
|
33 |
|
Capitalization |
|
34 |
|
Dilution |
|
36 |
|
Selected Consolidated Financial Data |
|
38 |
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
|
39 |
|
Business |
|
51 |
|
Management |
|
85 |
|
Executive Compensation |
|
91 |
|
Certain Relationships and Related party transactions |
|
100 |
|
Principal Stockholders |
|
102 |
|
Description of Securities |
|
104 |
|
Shares Eligible for Future Sale |
|
107 |
|
Certain U.S. Federal Income Tax Considerations |
|
109 |
|
Shares Registered for Resale |
|
|
|
Selling Securityholders |
|
|
|
Plan of Distribution |
|
|
|
Legal Matters |
|
|
|
Experts |
|
|
|
Where You Can Find More Information |
|
|
|
Index to Financial Statements |
|
|
We are offering to sell, and seeking offers to buy, the Company’s securities only in jurisdictions where such offers and sales are permitted. You should rely only on the information contained in this prospectus. We have not authorized anyone to provide you with any information other than the information contained in this prospectus.
The information contained in this prospectus is accurate only as of its date, regardless of the time of its delivery or of any sale or delivery of the Company’s securities. Neither the delivery of this prospectus nor any sale or delivery of the Company’s securities shall, under any circumstances, imply that there has been no change in the Company’s affairs since the date of this prospectus. This prospectus will be updated and made available for delivery to the extent required by the federal securities laws.
This prospectus includes estimates, statistics and other industry data obtained from industry publications, research and surveys, from studies conducted by third parties and from publicly available information. All of such data involve a number of assumptions and limitations, and contains projections and estimates of the future performance of the industries in which the Company operates, all of which is subject to a high degree of uncertainty. We caution you not to give undue weight to any such estimates, statistics, other industry data and projections.
As of January 16, 2015, the Company effectuated a 15-1 reverse split of the Company’s shares, meaning that 15 of the Company’s shares held prior to the reverse split equals one share held after the reverse split. If, as a result of the split, any stockholder would be entitled to receive a fraction of a share, in order to avoid issuing fractional shares the Company will instead provide that stockholder with an additional whole share. All references to the number of shares, options, warrants and other common stock equivalents, price per share and weighted-average number of shares of common stock outstanding presented in this prospectus retroactively reflect the effectuation of the 15-1 reverse split dated January 16, 2015, unless otherwise stated or unless the context otherwise indicates.
[Alternative page for Selling Securityholder Prospectus]
USE OF PROCEEDS
The Company will not receive any of the proceeds from the sale of the common stock by the selling securityholders named in this prospectus. All proceeds from the sale of the common stock will be paid directly to the selling securityholders.
[Alternative page for Selling Securityholder Prospectus]
SECURITIES REGISTERED FOR RESALE
2014 Private Placement of Convertible Notes
On July 14, 2014, the Company commenced a “best efforts” private placement offering of up to $5,000,000 of 8% unsecured convertible promissory notes, or the “Private Placement Convertible Notes,” pursuant to Rule 506(c) of Regulation D of the Securities Act and the JOBS Act. In addition, simultaneously on July 14, 2014, the Company commenced a “best efforts” private placement offering of up to $2,500,000 of Private Placement Convertible Notes pursuant to Regulation S of the Securities Act. Although these offerings were conducted separately and were separate and distinct offerings, these offerings are referred to, collectively, as the “2014 Private Placement” in this Prospectus for convenience purposes. The final closing of the 2014 Private Placement occurred on October 14, 2014, pursuant to which the Company issued Private Placement Convertible Notes in the aggregate amount of $2,785,000 ($2,760,000 pursuant to the Regulation D offering and $25,000 pursuant to the Regulation S offering).
Under the terms of the Private Placement Convertible Notes, the Company may elect to force the holders of the Private Placement Convertible Notes to convert the Private Placement Convertible Notes into the securities offered in the Company’s initial public offering, immediately after the closing of such offering, at a conversion price equal to $5.10 per Unit. Immediately after the closing of the Company’s initial public offering, the Company intends to force such a conversion. There is no minimum amount of securities that must be sold in the Company’s initial public offering and the conversion of the Private Placement Convertible Notes will occur even if only a few Units are sold. If the offering does not close and the Company does not otherwise elect to force a conversion upon the occurrence of a qualified transaction, the Private Placement Convertible Notes will remain outstanding until the earlier of such time as the holder chooses to convert them in full or maturity.
In connection with this prospectus, the Company is registering for resale 576,479 Units, issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest issued in the 2014 Private Placement upon the closing of the Company’s initial public offering.
The Company granted investors in the 2014 Private Placement certain “piggyback” registration rights in regard to any registered securities offering by the Company, on the Company’s own behalf or on behalf of selling securityholders, subject to customary exceptions. The registration statement of which this prospectus forms a part is a resale registration statement being filed with the SEC for the purpose of providing such investors the opportunity to resell the securities they are receiving as a result of the forced conversion of the Private Placement Convertible Notes.
2015 Private Placement of Convertible Notes
In March 2015, the Company commenced a “best efforts” private placement offering of up to $6,000,000 of Convertible Notes, pursuant to Rule 506(b) of Regulation D of the Securities Act and the JOBS Act. The terms of these Convertible Notes are substantially identical to the terms of the Convertible Notes issued in the 2014 Private Placement. This offering is referred to as the “2015 Private Placement” in this prospectus for convenience purposes. As of the date hereof, the Company has issued Private Placement Convertible Notes in the aggregate amount of $2,100,000 in the 2015 Private Placement.
In connection with this prospectus, the Company is registering for resale 418,173 Units, issuable upon the forced conversion of the Private Placement Convertible Notes plus accrued interest issued in the 2015 Private Placement upon the closing of the Company’s initial public offering.
The Company granted investors in the 2015 Private Placement certain “piggyback” registration rights in regard to any registered securities offering by the Company, on the Company’s own behalf or on behalf of selling securityholders, subject to customary exceptions. The registration statement of which this prospectus forms a part is a resale registration statement being filed with the SEC for the purpose of providing such investors the opportunity to resell the securities they are receiving as a result of the forced conversion of the Private Placement Convertible Notes.
[Alternative page for Selling Securityholder Prospectus]
SELLING SECURITYHOLDERS
An aggregate of up to 994,652 shares of common stock and 497,326 warrants may be sold from time to time by the selling securityholders (other than the placement agents for the 2014 Private Placement) set forth in the table below. In addition, an aggregate of 34,824 shares of common stock and 17,412 warrants may be sold from time to time by the placement agents for the 2014 Private Placement set forth in the table below, following the conversion of notes issuable upon the exercise of placement agent warrants.
The shares of common stock being offered by the selling securityholders are those issuable to:
• holders of Private Placement Convertible Notes issued pursuant to the terms of the Private Placements, including shares of common stock issuable upon exercise of the warrants such holders will receive upon forced conversion of the Private Placement Convertible Notes into Units, and
• the placement agents, such shares of common stock underlying warrants to purchase Private Placement Convertible Notes in connection with the 2014 Private Placement.
For additional information regarding the issuance of those Private Placement Convertible Notes, see “Securities Registered for Resale—2014 Private Placement of Convertible Notes” and “Securities Registered for Resale—2015 Private Placement of Convertible Notes” above. The Company is registering the shares of common stock and warrants in order to permit the selling securityholders to offer the securities for resale from time to time. The following table sets forth certain information with respect to each selling securityholder for whom the Company is registering securities for resale. Except for the ownership of Private Placement Convertible Notes, warrants and shares of the Company’s common stock, no selling securityholder has had any material relationship with the Company within the past three years.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock that such person or any member of such group has the right to acquire within 60 days of the date of this prospectus. For purposes of computing the percentage of outstanding shares of the Company’s common stock held by each person or group of persons named below, any shares that such person or persons has the right to acquire within 60 days of the date of this prospectus are deemed to be outstanding for such person, but not deemed to be outstanding for the purpose of computing the percentage ownership of any other person.
In the table below, the number of shares of common stock outstanding before this offering is deemed to be 9,444,828.By the terms of the Private Placement Convertible Notes, this conversion will be effected at a price per unit equal to $5.10. The numbers of shares of common stock shown to be outstanding both before and after this offering and the percentages shown exclude:
• 2,645,967 shares of the Company’s common stock issuable upon the exercise of stock options outstanding as of March 31, 2015, at a weighted average exercise price of $1.98 per share;
• 240,333 shares of the Company’s common stock issuable upon the exercise of warrants outstanding as of March 31, 2015, at a weighted average exercise price of $2.29 per share;
• 20,700 shares of the Company’s common stock available for future issuance under the Company’s Amended and Restated Long-Term Incentive Compensation Plan as of the date of this prospectus;
• 1,250,000 shares of the Company’s common stock that will be made available for future issuance under the Company’s 2015 equity incentive plan upon the closing of this offering;
• 1,562,500 shares of the Company’s common stock issuable upon exercise of the warrants included in the Units being offered in this offering, assuming all of the Units being offered are sold;
• 234,375 shares of the Company’s common stock issuable upon exercise of the warrants to be issued to the selling agent in connection with this offering;
• 497,326 shares of the Company’s common stock issuable upon exercise of the warrants included in the Units issuable upon conversion of the Private Placement Convertible Notes plus accrued interest immediately after the closing of this offering; and
• 34,824 shares of the Company’s common stock issuable upon conversion of the notes issuable upon the exercise of private placement warrants issued in connection with the 2014 Private Placement.
The table below lists the selling securityholders and other information regarding the beneficial ownership of the shares of common stock by each of the selling securityholders. Column II lists the number of shares of common stock
beneficially owned by each selling securityholder prior to the offering. The number of shares being offered included in Column III includes the shares underlying the Units issuable to the holders of the Private Placement Convertible Notes, issuable upon the forced conversion of such Notes into Units immediately after the closing of the Company’s initial public offering. The entire outstanding balance, including all accrued interest, of the Private Placement Convertible Notes, will be converted into Units once the Company elects to force such conversion. The Private Placement Convertible Notes earn interest at 8% per annum and convert into Units at a price equal to $5.10 per Unit. The number of warrants being offered included in Column IV includes the number of warrants underlying the Units issuable upon conversion of the Private Placement Convertible Notes. Column V assumes the sale of all of the shares offered by the selling securityholders pursuant to this prospectus, to the extent permitted under the Ownership Cap, as defined below.
Because the number of shares of common stock issuable may increase as a result of an increase in the accrued interest due on the Private Placement Convertible Notes, and a resultant increase in the number of Units issued, the number of shares that will actually be issued may be more than the number of shares being offered. In the event that additional Units are issued, the Company will be obligated to register, up to a certain point, those Units as well as any additional common shares and warrants underlying the Units that may be owed to the investors as a result of interest on the Notes.
Under the terms of the Private Placement Convertible Notes, a selling securityholder may not convert the Notes to the extent such conversion would cause such selling securityholder, together with its affiliates, to beneficially own a number of shares of common stock that would exceed 9.99% of the Company’s then outstanding shares of common stock following such conversion (the “Ownership Cap”), excluding for purposes of such determination shares of common stock not yet issuable upon conversion of Notes which have not been converted. The number of shares in Columns III and IV do reflect this limitation. The selling securityholders may sell all, some or none of their securities in this offering. See “Plan of Distribution.”
The following table sets forth certain information with respect to each selling securityholder for whom the Company is registering securities for resale to the public. Except for the ownership of Private Placement Convertible Notes, warrants and shares of the Company’s common stock, no material relationships exist between any of the selling securityholders and the Company, nor have any such material relationships existed within the past three years.
|
|
|
Number of Shares of |
|
|
|
|
|
Shares Beneficially Owned After Offering |
||||
|
Selling Securityholder |
|
Common Stock Beneficially Owned(1) |
|
Shares Being Offered(2)(3) |
|
Warrants Being Offered |
|
Number of Shares |
|
Percent of Shares^^ |
||
|
Alan C Schwartz and Kerry G Schwartz JTWROS(4) |
|
|
|
20,700 |
|
10,350 |
|
|
|
|
* |
% |
|
Alsava Corporation(5) |
|
|
|
413,989 |
|
206,995 |
|
|
|
|
* |
% |
|
Bridglal Ramkissoon(6) |
|
44,167 |
|
5,175 |
|
2,588 |
|
44,167 |
|
|
* |
% |
|
Bruce A. Shear(7) |
|
|
|
5,175 |
|
2,588 |
|
|
|
|
* |
% |
|
J. Randall White(8) |
|
6,667 |
|
5,175 |
|
2,588 |
|
6,667 |
|
|
* |
% |
|
Jayanth Bolaram(9) |
|
10,667 |
|
7,245 |
|
3,623 |
|
10,667 |
|
|
* |
% |
|
John Altieri(10) |
|
30,417 |
|
5,175 |
|
2,588 |
|
30,417 |
|
|
* |
% |
|
JW Opportunities Fund, LLC(11) |
|
|
|
4,140 |
|
2,070 |
|
|
|
|
* |
% |
|
JW Partners, LP(12) |
|
|
|
16,550 |
|
8,280 |
|
|
|
|
* |
% |
|
Mal Warwick Trust Dated March 4, 1995(13) |
|
13,333 |
|
10,350 |
|
5,175 |
|
13,333 |
|
|
* |
% |
|
Margileth Living Trust(14) |
|
|
|
20,700 |
|
10,350 |
|
|
|
|
* |
% |
|
Philip W. Wong(15) |
|
18,750 |
|
5,175 |
|
2,588 |
|
18,750 |
|
|
* |
% |
|
Ramon Torres(16) |
|
29,875 |
|
5,175 |
|
2,588 |
|
29,875 |
|
|
* |
% |
|
Roger Arumugam(17) |
|
2,083 |
|
5,175 |
|
2,588 |
|
2,083 |
|
|
* |
% |
|
Ukeme Umana & Christina Umana JTWROS(18) |
|
|
|
5,175 |
|
2,588 |
|
|
|
|
* |
% |
|
William R. Jordan III(19) |
|
47,500 |
|
31,050 |
|
15,525 |
|
47,500 |
|
|
* |
% |
|
Rick E. Briggs(20) |
|
|
|
5,175 |
|
2,588 |
|
|
|
|
* |
% |
|
Helmsbridge Holdings Limited(21) |
|
|
|
5,175 |
|
2,588 |
|
|
|
|
* |
% |
|
TriPoint Global Equities, LLC ^(22) |
|
|
|
22,358 |
|
11,179 |
|
|
|
|
* |
% |
|
Primary Capital, LLC^(23) |
|
|
|
858 |
|
429 |
|
|
|
|
* |
% |
|
Roger D. Gurganus(24) |
|
120,000 |
|
9,957 |
|
4,979 |
|
120,000 |
|
1.1 |
% |
|
|
YuYu Pharma Inc.(25) |
|
208,334 |
|
398,260 |
|
199,130 |
|
208,334 |
|
2.0 |
% |
|
|
Jobikan Nigeria Limited(26) |
|
|
|
9,957 |
|
4,979 |
|
|
|
|
* |
% |
____________
* Less than 1%
+ Except as indicated by +, no selling securityholder is an officer, director, affiliate or 5% securityholder.
^ Indicates such selling securityholder is a broker-dealer or an affiliate of a broker-dealer, and therefore an underwriter.
^^ Based on 13,599,304 shares deemed to be issued and outstanding. Assumes the sale of all of the shares offered by the selling securityholders pursuant to this prospectus.
(1) The number of shares of common stock beneficially owned before this offering consists of shares of common stock such selling securityholder owned prior to the 2014 Private Placement.
(2) The number of shares of common stock being offered hereby includes 994,652 Units composed of 994,652 shares of common stock and 497,326 shares of common stock underlying warrants acquired by the Company’s existing Private Placement Convertible Note holders (based upon an initial offering price of $5.10 per Unit) and 34,824 shares of common stock and 17,412 shares of common stock underlying warrants issuable to the placement agents for the 2014 Private Placement upon the conversion of notes issuable upon the exercise of placement agent warrants.
(3) The number of shares of common stock beneficially owned by the placement agents includes 34,824 shares of the Company’s common stock issuable upon conversion of the notes issuable upon the exercise of the private placement warrants issued in connection with the 2014 Private Placement (based upon an exercise price of $5.10 per unit)
(4) Indicates 20,700 Units. Alan C. Schwartz and Kerry G. Schwartz are the natural persons with voting and investment control over such securities.
(5) Indicates 413,989 Units. Mauricio Pontones Simon is the natural person with voting and investment control over such securities held by Alsava Corporation.
(6) Indicates 5,175 Units held by Bridglal Ramkissoon.
(7) Indicates 5,175 Units held by Bruce A. Shear
(8) Indicates 5,175 Units held by. J. Randall White.
(9) Indicates 7,245 Units held by Jayanth Bolaram.
(10) Indicates 5,175 Units held by. John Altieri.
(11) Indicates 4,140 Units. Jason Wild is the natural person with voting and investment control over such securities held by JW Opportunities Fund, LLC.
(12) Indicates 16,560 Units. Jason Wild is the natural person with voting and investment control over such securities held by JW Partners, LP.
(13) Indicates 10,350 Units. Malvin Warwick is the natural person with voting and investment control over such securities held by Mal Warwick Trust Dated March 4, 1995.
(14) Indicates 20,700 Units. David Andrew Margileth is the natural person with voting and investment control over such securities held by Margileth Living Trust.
(15) Indicates 5,175 Units held by Philip W. Wong.
(16) Indicates 5,175 Units held by Ramon Torres.
(17) Indicates 5,175 Units held by Roger Arumugam.
(18) Indicates 5,175 Units Ukeme Umana & Christina Umana are the natural persons with voting and investment control over such securities.
(19) Indicates 31,050 Units held by William R. Jordan III.
(20) Indicates 5,175 Units held by Rick E. Briggs.
(21) Indicates 5,175 Units. Anthony Heller is the natural person with voting and investment control over such securities held by Helmsbridge Holdings Limited.
(22) Includes 33,537 shares of the Company’s common stock issuable upon conversion of the notes issuable upon the exercise of the private placement warrants issued in connection with the 2014 Private Placement (based upon an exercise price of $5.10 per unit). Mark Elenowitz is the natural person with voting and investment control over such securities held by TriPoint Global Equities, LLC.
(23) Includes 1,287 shares of the Company’s common stock issuable upon conversion of the notes issuable upon the exercise of the private placement warrants issued in connection with the 2014 Private Placement (based upon an exercise price of $5.10 per unit). John Leo is the natural persons with voting and investment control over such securities held by Primary Capital, LLC.
(24) Indicates 9,957 Units held by Roger D. Gurganus.
(25) Indicates 398,260 Units held by YuYu Pharma Inc. Inseok Choi is the natural person with voting and investment control over such securities held by YuYu Pharma Inc.
(26) Indicates 9,957 Units held by Jobikan Nigeria Limited. Tim Kim is the natural person with voting and investment control over such securities held by Jobikan Nigeria Limited.
Each of the selling securityholders that is an affiliate of a broker-dealer, which is indicated in the table above, has represented to the Company that it purchased the securities offered by this prospectus in the ordinary course of business and, at the time of purchase of those securities, did not have any agreements, understandings or other plans, directly or indirectly, with any person to distribute those securities. To the extent that the Company becomes aware that such selling securityholder did not acquire its securities in the ordinary course of business or did have such an agreement or understanding, the Company will file a post-effective amendment to the registration statement to designate such affiliate as an “underwriter” within the meaning of the Securities Act.
[Alternative page for Selling Securityholder Prospectus]
PLAN OF DISTRIBUTION
The selling securityholders, which term, as used herein, includes donees, pledgees, transferees or other successors-in-interest selling securities received after the date of this prospectus from a selling securityholder as a gift, pledge, partnership distribution or other similar transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of their securities on any stock exchange, market or trading facility on which the securities are traded or in private transactions. These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale or at negotiated prices.
Currently, there is no public market for ieCrowd’s Units, common stock or warrants. ieCrowd has applied to list its common stock on the NASDAQ Capital Market (“NASDAQ”) under the symbol “MYIE”, and to list its warrants on NASDAQ under the symbol “MYIEW”, on or promptly after the closing date of ieCrowd’s initial public offering of Units; however, there can be no assurance that ieCrowd’s securities will be approved for listing on NASDAQ. No public exchange sales of the securities covered by this prospectus shall occur until the shares of common stock and warrants included in the Units begin separate trading on NASDAQ, if ever. Following the date on which the registration statement of which this prospectus forms a part is declared effective and until such time as the shares of common stock and warrants included in the Units begin trading on NASDAQ, if ever, there may be no public market in which any selling securityholder will be able to sell its securities covered by this prospectus. In addition, there can be no assurance that a listing on NASDAQ will provide an active public market for the securities.
If and when the Company’s securities are listed on NASDAQ, the sale of securities offered hereby may be effected on NASDAQ, including through ordinary brokers’ transactions. Sales may also be effected in over-the-counter markets, including through dealers who would resell such securities as principals, or through privately negotiated transactions. Depending in part on the type of transaction effected, the securities may be sold at market prices prevailing at the time of sale, at prices related to such prevailing market prices or at negotiated prices. Until there is a market for the securities, securityholders will sell their securities at the fixed price of $5.10 per unit. Parties to the transactions may be required to pay usual and customary, or specifically negotiated, brokerage or other fees or commissions.
The selling securityholders may use any one or more of the following methods when disposing of securities:
• ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
• block trades in which the broker-dealer will attempt to sell the securities as agent, but may position and resell a portion of the block as principal to facilitate the transaction;
• purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
• an exchange transaction in accordance with the rules of the applicable exchange;
• privately negotiated transactions;
• short sales;
• through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise;
• broker-dealers selling on behalf of a selling securityholder a specified number of securities at a stipulated price per security;
• a combination of any such methods of sale; and
• any other method permitted pursuant to applicable law.
The selling securityholders may, from time to time, pledge or grant a security interest in some or all of the securities owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the securities, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling securityholders to include the pledgee, transferee or other successors in interest as selling securityholders under this prospectus. The selling securityholders also may transfer the securities in other circumstances, in which case the transferees,
pledgees or other successors in interest will be the selling beneficial owners for purposes of this prospectus; provided, however, that prior to any such transfer the following information (or such other information as may be required by the federal securities laws from time to time) with respect to each such selling beneficial owner must be added to the prospectus by way of a prospectus supplement or post-effective amendment, as appropriate: (1) the name of the selling beneficial owner; (2) any material relationship the selling beneficial owner has had within the past three years with the Company or any of the Company’s predecessors or affiliates; (3) the amount of securities of the class owned by such security beneficial owner before the offering; (4) the amount to be offered for the security beneficial owner’s account; and (5) the amount and (if one percent or more) the percentage of the class to be owned by such security beneficial owner after the offering is complete.
In connection with the sale of the Company’s securities, the selling securityholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The selling securityholders may also sell the Company’s securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The selling securityholders may also enter into option or other transactions with broker-dealers or other financial institutions or participate in the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to the selling securityholders from the sale of the securities offered by them will be the purchase price of the securities less discounts or commissions, if any. Each of the selling securityholders reserves the right to accept and, together with their agents from time to time, to reject, in whole or in part, any proposed purchase of securities to be made directly or through agents. The Company will not receive any of the proceeds from selling securityholder transaction. Upon any exercise of warrants by payment of cash, however, the Company will receive the exercise price of the warrants.
The selling securityholders may also resell all or a portion of the securities in open market transactions in reliance upon Rule 144 under the Securities Act, provided that they meet the criteria and conform to the requirements of that rule.
The selling securityholders and any underwriters, broker-dealers or agents that participate in the sale of the securities may be deemed “underwriters” within the meaning of the Securities Act. Any discounts, commissions, concessions or profits they receive may be deemed underwriting compensation. Any such “underwriters” within the meaning of the Securities Act will be subject to the prospectus delivery requirements of the Securities Act. The selling securityholders may indemnify any broker-dealer that participates in transactions involving the sale of the securities against certain liabilities, including liabilities arising under the Securities Act.
The maximum amount of compensation to be received by any FINRA member or independent broker-dealer for the sale of any securities registered under this prospectus will not be greater than 8.0% of the gross proceeds from the sale of such securities.
In order to comply with the securities laws of some states, if applicable, the securities may be sold in those jurisdictions only through brokers or dealers registered or licensed in those jurisdictions. In addition, in some states the securities may not be sold unless it has been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
The Company has advised the selling securityholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of securities in the market and to the activities of the selling securityholders and their affiliates.
The Company will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling securityholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act.
To the Company’s knowledge, except for TriPoint Global Equities, LLC and Primary Capital, LLC, no selling securityholder is a broker-dealer or an affiliate of a broker-dealer.
[Alternative page for Selling Securityholder Prospectus]
LEGAL MATTERS
Ellenoff Grossman & Schole LLP, New York, New York, is acting as counsel in connection with the registration of the Company’s securities under the Securities Act, and as such will pass upon the validity of the securities offered in this prospectus.
EXPERTS
The consolidated financial statements of the Company as of and for the years ended December 31, 2014 and 2013 appearing in this prospectus have been audited by Marcum LLP, independent registered public accounting firm, as set forth in their report thereon (which contains an explanatory paragraph relating to substantial doubt about the Company’s ability to continue as a going concern, as discussed in Note 2 to the consolidated financial statements), appearing elsewhere in this prospectus, and are included in reliance on such report given on the authority of such firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus constitutes a part of the registration statement that the Company has filed with the SEC in connection with this offering. It does not contain all of the information in the registration statement and in the exhibits thereto. Statements contained in this prospectus as to the contents of any contract or other document that the Company files as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such exhibit. The registration statement and the exhibits thereto may be inspected and copied at the principal office of the SEC, 100 F Street, NE, Washington, D.C. 20549, and copies of all or any part thereof may also be obtained at prescribed rates from the SEC’s Public Reference Section, at that address. In addition, the registration statement and the exhibits thereto may be viewed on and printed out from the SEC’s website at www.sec.gov. The Company will also make available, free of charge, copies of the registration statement and the exhibits thereto, upon written request to Mr. Amro Albanna, the Company’s Chief Executive Officer, c/o the Company at its principal executive offices.
[Alternative page for Selling Securityholder Prospectus]
Units
Consisting of One Share of Common Stock and
One Warrant to Purchase One-half Share of Common
Stock

________________
PROSPECTUS
________________
The date of this prospectus is __________, 2015
PART II – INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The following table sets forth all expenses to be paid by us, other than selling agent’s commissions, in connection with the sale and distribution of the securities being registered. All amounts shown are estimates except the SEC registration fee and the FINRA filing fee.
|
SEC registration fee |
|
$ |
4,636 |
|
FINRA filing fee |
|
$ |
6,485 |
|
Listing Fee |
|
$ |
50,000 |
|
Accounting fees and expenses |
|
$ |
15,000 |
|
Legal fees and expenses |
|
$ |
275,000 |
|
Printing and marketing expenses |
|
$ |
250,000 |
|
Transfer agent and registrar fees |
|
$ |
10,000 |
|
Miscellaneous expenses |
|
$ |
13,879 |
|
Total |
|
$ |
625,000 |
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Section 145 of the Delaware General Corporation Law provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of such person being or having been a director, officer, employee or agent to the registrant. The Delaware General Corporation Law provides that Section 145 is not exclusive of other rights to which those seeking indemnification may be entitled under any bylaw, agreement, vote of stockholders or disinterested directors or otherwise. Section 102(b)(7) of the Delaware General Corporation Law permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (1) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) for unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions, or (4) for any transaction from which the director derived an improper personal benefit. The registrant’s amended and restated certificate of incorporation will provide for such limitation of liability.
Our amended and restated certificate of incorporation to be in effect upon the completion of this offering shall provide for indemnification of our directors and officers to the maximum extent permitted by the Delaware General Corporation Law, and our amended and restated bylaws to be in effect upon the completion of this offering shall provide for indemnification of our directors, officers, Advisory Board members (if any) and employees to the maximum extent permitted by the Delaware General Corporation Law.
Further, prior to the completion of this offering, we expect to enter into indemnification agreements with each of our directors and executive officers that may be broader than the specific indemnification provisions contained in the Delaware General Corporation Law. We expect these indemnification agreements will require us, among other things, to indemnify our directors and executive officers against liabilities that may arise by reason of their status or service. We expect these indemnification agreements will also require us to advance all expenses incurred by the directors and executive officers in investigating or defending any such action, suit, or proceeding. We believe that these agreements will be necessary to attract and retain qualified individuals to serve as our directors and executive officers.
We have obtained insurance policies under which, subject to the limitations of the policies, coverage is provided to our directors and officers against loss arising from claims made by reason of breach of fiduciary duty or other wrongful acts as a director or officer, including claims relating to public securities matters, and to us with respect to payments that may be made by us to these directors and officers pursuant to our indemnification obligations or otherwise as a matter of law.
II-1
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
As of January 16, 2015, we effectuated a 15-1 reverse split of our shares, meaning that 15 of our shares held prior to the reverse split equals one share held after the reverse split. If, as a result of the split, any stockholder would be entitled to receive a fraction of a share, in order to avoid issuing fractional shares we will instead provide that stockholder with an additional whole share. All references to the number of shares, options, warrants and other common stock equivalents, price per share and weighted-average number of shares of common stock outstanding presented in this prospectus retroactively reflect the effectuation of the 15-1 reverse split dated January 16, 2015, including as set forth in this section below, unless otherwise stated or unless the context otherwise indicates.
During the period from January 1, 2012 to the filing of this Registration Statement, the registrant has made the following sales of unregistered securities:
During April 2012, the Company issued 103,467 shares of its common stock to three U.S. accredited investors and one U.S. non-accredited investor, at a per share price of $1.88, pursuant to a private placement subscription offering, for total cash proceeds of $194,000.
From July 2012 to October 2012, the Company issued 79,556 shares of its common stock to three U.S. accredited investors and five U.S. non-accredited investors, at a per share price of $2.25, pursuant to a private placement subscription offering, for total cash proceeds of $179,000.
From October 2012 to November 2012, the Company issued 240,000 shares of its common stock to nine U.S. accredited investors and four U.S. non-accredited investors, at a per share price of $1.50, pursuant to a private placement subscription offering, for total cash proceeds of $360,000.
During December 2012, the Company issued 66,669 shares of its Common Stock to three U.S. accredited investors at a per share price of $2.25, pursuant to a private placement subscription offering, for total cash proceeds of $150,005.
From December 2012 to January 2013, the Company issued 83,333 shares of its common stock to nine U.S. accredited investors and six U.S. non-accredited investors, at a per share price of $3.00, pursuant to a private placement subscription offering, for total cash proceeds of $250,000.
From December 2012 to January 2013, the Company issued 11,458 shares of its common stock to three U.S. accredited investors and five U.S. non-accredited investors, at a per share price of $4.80, pursuant to a private placement subscription offering, for total cash proceeds of $55,000.
From May 2013 to January 2014, the Company issued 437,867 shares of its common stock to 25 U.S. accredited investors, five U.S. non-accredited investors, and two non-U.S. non-accredited investors at a per share price of $3.75, pursuant to a private placement subscription offering, for total cash proceeds of $1,642,000.
On October 4, 2013 at the option of the holder, a two-year unsecured convertible promissory note dated November 3, 2011 was converted into 179,500 common shares of the Company. The note was for a principal amount of $250,000 plus simple interest at a rate of 4.0%. The note, in the amount of its principal face amount plus interest of $19,250, was converted into common shares of the Company at a share price of $1.50.
From February 2014 to April 2014, the Company issued 164,583 shares of its common stock to 17 U.S. accredited investors, at a per share price of $4.80, pursuant to a private placement subscription offering, for total cash proceeds of $790,000.
In March 2014, the Company issued 208,333 shares of its common stock to one investor at a per share price of $4.80, pursuant to a private placement offering, for total cash proceeds of $1,000,000.
In April 2014, the Company issued 104,167 shares of its common stock to one investor, at a per share price of $4.80, pursuant to a private placement offering, for total cash proceeds of $500,000.
On October 14, 2014, the Company issued $2,760,000 convertible promissory notes to 17 accredited investors; the notes are initially convertible into shares of the Company’s common stock at a conversion price of $5.10 per share; provided however, that all notes not previously converted shall convert immediately after the closing of this offering into the securities issued in this offering at a price equal to $5.10 per unit.
II-2
On October 14, 2014, the Company issued $25,000 convertible promissory notes to one non-U.S. Investor; the notes are convertible at the same price as those issued to the U.S. Investors on October 14, 2014 and contain the same conversion terms.
In April 2015, the Company issued $2,100,000 convertible promissory notes to three accredited investors; the notes are initially convertible into shares of the Company’s common stock at a conversion price of $5.10 per share; provided however, that all notes not previously converted shall convert immediately after the closing of this offering into the securities issued in this offering at a price equal to $5.10 per unit.
The sales of the securities described above were exempt from registration under the Securities Act in reliance on one or more of Section 3(a)(9) of the Securities Act, Regulation D under the Securities Act and Regulation S under the Securities Act. All securities sold contained a restrictive legend on the share certificate stating that the securities have not been registered under the Securities Act and setting forth or referring to the applicable restrictions on transferability and sale.
ITEM 16. EXHIBITS.
|
Exhibit Number |
|
|
|
Description |
|
1.1 |
|
|
|
Form of Selling Agent Agreement |
|
3.1 |
|
* |
|
Amended and Restated Certificate of Incorporation filed with the Delaware Secretary of State on July 25, 2011 |
|
3.2 |
|
* |
|
Certificate of Correction filed with the Delaware Secretary of State on January 14, 2015 |
|
3.3 |
|
* |
|
Amendment to Certificate of Incorporation filed with the Delaware Secretary of State on January 16, 2015 |
|
3.4 |
|
* |
|
Bylaws |
|
4.1 |
|
* |
|
Specimen Private Placement Convertible Note |
|
4.2 |
|
|
|
Form of Warrant Agreement |
|
4.3 |
|
|
|
Specimen Warrant |
4.4 |
|
|
|
Specimen Selling Agent Warrant |
5.1 |
|
* |
|
Opinion of Ellenoff Grossman & Schole LLP |
|
10.1 |
|
* |
|
License Agreement between The Regents of the University of California and Olfactor Laboratories, Inc., dated as of January 29, 2010 |
|
10.2 |
|
* |
|
First Amendment to License Agreement between The Regents of the University of California and Olfactor Laboratories, Inc. dated December 16, 2010 |
|
10.3 |
|
* |
|
Second Amendment to License Agreement between The Regents of the University of California and Olfactor Laboratories, Inc. dated February 16, 2012 |
|
10.4 |
|
* |
|
Third Amendment to License Agreement between The Regents of the University of California and Olfactor Laboratories, Inc. dated September 10, 2012 |
|
10.5 |
|
* |
|
Fourth Amendment to License Agreement between The Regents of the University of California and Olfactor Laboratories, Inc. dated March 17, 2015 |
|
10.6 |
|
* |
|
License Agreement between The Regents of the University of California and Nano Engineered Applications, Inc. dated as of March 31, 2010 |
|
10.7 |
|
* |
|
First Amendment to License Agreement between The Regents of the University of California and Nano Engineered Applications, Inc. dated December 14, 2010 |
|
10.8 |
|
* |
|
Second Amendment to License Agreement between The Regents of the University of California and Nano Engineered Applications, Inc. dated April 21, 2011 |
|
10.9 |
|
* |
|
Third Amendment to License Agreement between The Regents of the University of California and Nano Engineered Applications, Inc. dated February 29, 2012 |
|
10.10 |
|
* |
|
Third Amendment to License Agreement between The Regents of the University of California and Nano Engineered Applications, Inc. dated June 3, 2013 |
|
10.11 |
|
* |
|
Fifth Amendment to License Agreement between The Regents of the University of California and Nano Engineered Applications, Inc. dated September 1, 2014 |
|
10.12 |
|
* |
|
License Agreement between The Regents of the University of California and Breathing Technologies, Inc. dated as of October 21, 2013 |
|
10.13 |
|
* |
|
License Agreement between The Regents of the University of California and Breathing Technologies, Inc. dated as of May 19, 2014 |
|
10.14 |
|
* |
|
First Amendment to License Agreement between The Regents of the University of California and Breathing Technologies, Inc. dated as of August 8, 2014 |
II-3
|
Exhibit Number |
|
|
|
Description |
|
10.15 |
|
* |
|
License Agreement between The Regents of the University of California and Innovation Economy Corporation dated as of November 3, 2014 (UCLA 2014-299 “Head Pain Management”) |
|
10.16 |
|
* |
|
License Agreement between The Regents of the University of California and Innovation Economy Corporation dated as of November 3, 2014 (UCLA 2014-781 “Sleep Disorders”) |
|
10.17 |
|
* |
|
Management Services Agreement between the Company and Olfactor Laboratories, Inc. dated April 1, 2012 |
|
10.18 |
|
* |
|
Management Services Agreement between the Company and Nano Engineered Applications, Inc. dated January 1, 2013 |
|
10.19 |
|
* |
|
Amended and Restated Long-Term Incentive Compensation Plan |
|
10.20 |
|
* |
|
2015 Equity Incentive Plan |
|
10.21 |
|
* |
|
Exclusive License Agreement between YuYu Pharma, Inc. and Olfactor Laboratories, Inc. dated as of March 19, 2014 |
|
10.22 |
|
* |
|
Form of Indemnity Agreement |
|
10.23 |
|
* |
|
Olfactor Laboratories Inc. Long-Term Incentive Compensation Plan |
10.24 |
|
* |
|
Form of Closing Escrow Agreement by and among the Company, the Selling Agent and Wilmington Trust, N.A. |
10.25 |
|
* |
|
Termination of Option and Compensation Agreement dated May 1, 2015. |
|
14 |
|
* |
|
Code of Ethics |
|
21 |
|
* |
|
List of Subsidiaries |
|
23.1 |
|
|
|
Consent of Marcum LLP, independent registered public accounting firm |
23.2 |
|
* |
|
Consent of Ellenoff Grossman & Schole LLP (included in Exhibit 5.1) |
|
99.1 |
|
* |
|
Audit Committee Charter |
|
99.2 |
|
* |
|
Compensation Committee Charter |
|
99.3 |
|
* |
|
Nominating and Corporate Governance Committee Charter |
99.4 |
|
* |
|
Consent of Vernon Hall |
99.5 |
|
* |
|
Consent of George Reyes |
____________
* Previously filed
Filed herewith
ITEM 17. UNDERTAKINGS.
(a) The undersigned Registrant hereby undertakes:
1. To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
2. That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
3. To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
II-4
4. That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
i. each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
5. That, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
i. Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
ii. Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
iii. The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
iv. Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
(b) The undersigned registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser.
(c) The undersigned registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(d) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Riverside, State of California, on May 20, 2015.
|
|
|
INNOVATION ECONOMY CORPORATION |
|
|
|
|
|
|
|
/s/ Amro Albanna |
|
|
|
By: Amro Albanna |
|
|
|
Title: Chair of the Board of Directors and |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Amro Albanna such person’s true and lawful attorney-in-fact, with full power of substitution and re-substitution for such person and in such person’s name, place and stead, in any and all capacities, to sign any and all amendments, including post-effective amendments, to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the SEC, hereby ratifying and confirming all that said attorney-in-fact or his substitute, each acting alone, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/ Amro Albanna |
|
Chair of the Board of Directors, Sole Director and |
|
May 20, 2015 |
By: Amro Albanna |
|
Chief Executive Officer (Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Albert Cervantes |
|
Chief Financial Officer (Principal Financial Officer |
|
May 20, 2015 |
By: Albert Cervantes |
|
and Principal Accounting Officer) |
|
|
II-6
EXHIBIT INDEX
Exhibit Number |
|
Description |
1.1 |
|
Form of Selling Agent Agreement |
4.2 |
|
Form of Warrant Agreement |
4.3 |
|
Specimen Warrant |
4.4 |
|
Specimen Selling Agent Warrant |
23.1 |
|
Consent of Marcum LLP, independent registered public accounting firm |
II-7
