Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Inspyr Therapeutics, Inc. | v411260_8k.htm |
| EX-99.02 - EXHIBIT 99.02 - Inspyr Therapeutics, Inc. | v411260_ex99-02.htm |
Exhibit 99.01

1 Mipsagargin Summary May 2015 OTCQB : GNSZ

2 Safe Harbor Statement Safe Harbor Statement Under the Private Securities Litigation Reform Act of 1995: Any statements that are not historical facts are forward - looking statements that involve risks and uncertainties that could cause actual results to differ materially from those in the forward - looking statements, which may include, but are not limited to, factors related to GenSpera's anticipated growth strategies, the outcome of its clinical trials, future business development, ability to develop new products, expand to other related industries or markets in other geographical locations, and other information detailed from time to time in the Company's filings and future filings made with the United States Securities and Exchange Commission. Readers are advised that this information is intended for the use of investment professionals. Anyone interested in obtaining information on GenSpera should contact GenSpera directly. This presentation was developed by GenSpera and is intended solely for informational purposes and is not to be construed as an offer to sell or the solicitation of an offer to buy the Company's stock. This presentation is based upon information available to the public, as well as other information from sources which management believes to be reliable, but is not guaranteed by the Company as being accurate nor does it purport to be complete. Opinions expressed herein are those of management as of the date of the presentation and are subject to change without notice.

3 • An innovation driven biotech company that unlocks conventional thinking • Deep experience in cancer drug discovery and development • A leader in prodrug therapeutics for the treatment of cancer • Robust global IP platform • World class strategic partners • 2015 is tipping point for value creation GenSpera Profile

4 • Technology platform combines a powerful, plant - derived cytotoxin (thapsigargin) with targeted release of drug candidates only within the tumor • Unique mechanism of action • Minimized side effects • Enhanced anti - tumor activity • Target cells unable to acquire resistance • Mipsagargin: Lead drug candidate • Encouraging Phase 2 results: hepatocellular carcinoma (liver cancer) • Phase 2: glioblastoma (brain cancer) underway • Phase 2: prostate cancer soon to enroll Technology Highlights

5 Novel Technology Platform – Creating our Prodrug 12ADT • Thapsigargin — the active ingredient isolated from Thapsia garganica • Well - characterized toxin that kills both slow - and fast - growing cancer cells • Effective potency is 10 - 100x greater than common cytotoxic agents • Potent inhibitor of the intracellular SERCA pump - causes Ca2+ (calcium) to rise significantly and trigger apoptosis (cell death) Extraction and chemical modification • Our toxic “payload” is inactive and soluble in the blood stream. • Different peptides are used to target different tumor sites. • Active drug is released via removal of the targeting peptide only at the tumor site. Masking and targeting peptide attached

6 PSMA is a Pan - tumor Target • Type II transmembrane glycoprotein • Unique proteolytic activity • Highly expressed in normal and malignant prostate epithelial cells • Selective expression in the vascular endothelium of solid tumors – both established and new growth PSMA

7 PSMA is Expressed by Tumor Vasculature Normal Pancreas Normal Skin Normal Lung Normal Bladder Pancreatic Cancer Bladder Cancer Lung Cancer Skin Cancer

8 PSMA Staining in Tumor Vasculature 0.0 20.0 40.0 60.0 80.0 100.0 Positive PSMA Negative PSMA Hepato - Cellular (n=42) Meso - Thelioma (n=36) Ovarian (n=34) Renal (n=46) Breast (n=44) Mela - noma (n=44) Normal Liver (n=9) Normal Kidney (n=9) Normal Breast (n=7) Normal Bladder (n=10) Bladder (n=94)

9 PSMA Positive Vasculature in HCC

10 Mipsagargin: a PSMA - Activated Thapsigargin Prodrug O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O (CH 2 ) 11 His (H) Ser- (S) Ser- (S) Lys- (K) Leu- (L) Gln- (Q) Leu- (L)12ADT - PSA Hydrolysis O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O (CH 2 ) 11 His (H) Ser- (S) Ser- (S) Lys- (K) Leu- (L) Gln- (Q) Leu- (L)12ADT - O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 O O H OH CH 3 CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O H N N H H N N H H N N H H N N H O O NH 2 O O O H 2 N O HO O OH O HN N N O O H N O (CH 2 ) 11 His (H) Ser- (S) Ser- (S) Lys- (K) Leu- (L) Gln- (Q) Leu- (L)12ADT - PSA Hydrolysis O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 (CH 2 ) 11 12ADT- NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O -N H Asp Glu Glu Glu Glu PSMA Hydrolysis O O H OH CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 O O H OH CH 3 CH 3 OH O CH 3 O O O O O CH 3 O H 3 C O H 3 8 1 (CH 2 ) 11 12ADT- NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O NH O O O ONH 2 OH H N N H O HO O H N OH O HO O O HO O -N H Asp Glu Glu Glu Glu PSMA Hydrolysis Thapsigargin Analog (12ADT)

11 Compelling Mipsagargin Preclinical Data Efficacy Pharmacology • Complete tumor regression with monotherapy in mouse tumor models – No need to combine with other drugs – Fewer side effects • Safety margin of 7 - to 10 - fold in mouse • No acquired resistance to mipsagargin Bioanalytical • In blood can only find intact mipsagargin and no released active 12ADT • 12ADT is found at very high levels in tumors, less found in liver and kidneys, not found elsewhere - Targeted Delivery! Toxicology • No effect on liver or cardiovascular system • No effect on bone marrow – no immunosuppression or anemia • Kidney is the affected organ at high doses – dose - dependent, non - cumulative, easily monitored, and 100% rapidly reversible
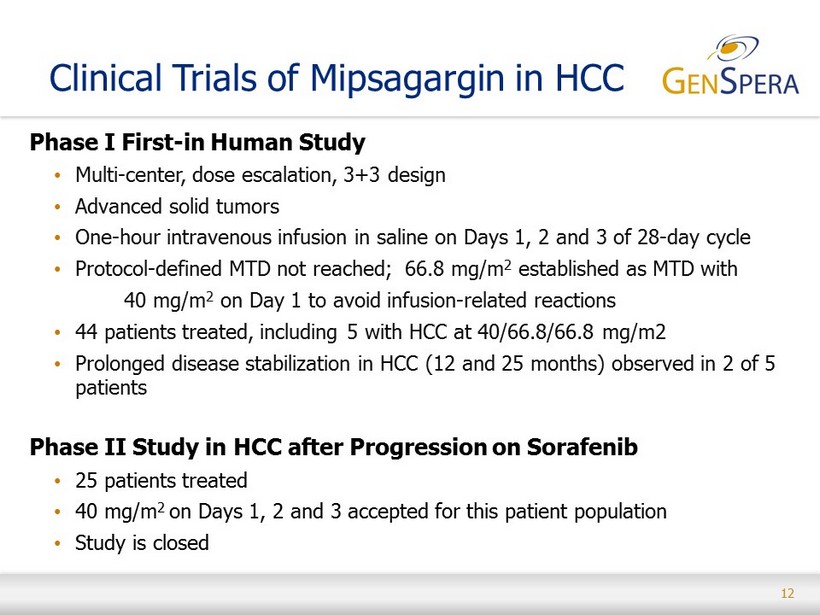
12 Clinical Trials of Mipsagargin in HCC Phase I First - in Human Study • Multi - center, dose escalation, 3+3 design • Advanced solid tumors • One - hour intravenous infusion in saline on Days 1, 2 and 3 of 28 - day cycle • Protocol - defined MTD not reached; 66.8 mg/m 2 established as MTD with 40 mg/m 2 on Day 1 to avoid infusion - related reactions • 44 patients treated, including 5 with HCC at 40/66.8/66.8 mg/m2 • Prolonged disease stabilization in HCC (12 and 25 months) observed in 2 of 5 patients Phase II Study in HCC after Progression on Sorafenib • 25 patients treated • 40 mg/m 2 on Days 1, 2 and 3 accepted for this patient population • Study is closed
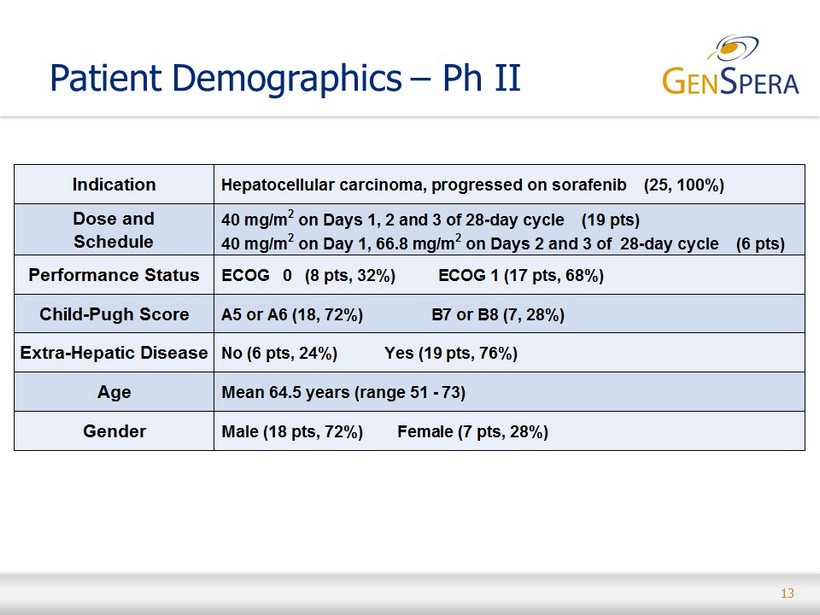
13 Patient Demographics – Ph II Indication Hepatocellular carcinoma, progressed on sorafenib (25, 100%) Dose and Schedule 40 mg/m 2 on Days 1, 2 and 3 of 28-day cycle (19 pts) 40 mg/m 2 on Day 1, 66.8 mg/m 2 on Days 2 and 3 of 28-day cycle (6 pts) Performance Status ECOG 0 (8 pts, 32%) ECOG 1 (17 pts, 68%) Child-Pugh Score A5 or A6 (18, 72%) B7 or B8 (7, 28%) Extra-Hepatic Disease No (6 pts, 24%) Yes (19 pts, 76%) Age Mean 64.5 years (range 51 - 73) Gender Male (18 pts, 72%) Female (7 pts, 28%)

14 Safety Observations – Ph II Most Frequently - Occurring Mipsagargin - Related AEs Grade ≥ 2 * Number (%) of Patients Creatinine increased 8 (32%) Fatigue 7 (28%) ALT increased 6 (24%) AST increased 6 (24%) Bilirubin increased 6 (24%) LDH increased 5 (20%) Bood urea increased 5 (20%) Thrombocytopenia 4 (16%) Hyperkalemia 3 (12%) * Observed in at least 3 patients

15 Safety Observations – Ph II Mipsagargin - Related SAEs All Mipsagargin - Related Serious Adverse Events Number (%) of Patients Mipsagargin Dose (Day 1 / Day 2 / Day 3) Acute renal failure/acute renal injury* 3 (12%) 40/66.8/66.8 Congestive cardiac failure* 1 (4%) 40/40/40 Chest pain* 1 (4%) 40/40/40 * Grade 3

16 Clinical Observations – Ph II Number of Cycles (n=25 patients) 75 total (average 3 per pt; range 1 - 9) 63 cycles at 40/40/40 12 cycles at 40/66.8/66.8 Evaluable for Response (EE Population)19 patients (76%) Best Response (EE Population) CR + PR 0 pts SD 12 pts (63%) SD ≥ 5 cycles 6 pts (24%) TTP (EE Population) 134 days* (4.5 months; 95% Cl: 58.0, NE)** OS (EE Population) 205 days (6.8 months; 95% Cl: 138, NE)*** PFS (EE Population) 129 days (4.3 months; 95% Cl: 58.0, 205.0)**** * p < 0.001 vs. historic control of 2.1 months ** 8 patients censored *** 2 patients censored **** 8 patients censored

17 Best Change in Tumor Size – Ph II C h a ng e f r o m B ase lin e ( % ) 100 80 60 40 20 0 -20 -30% -40 -60 -80 -100 03 20 16 12 05 19 14 01 09 22 07 15 18 08 02 21 11 23 *10 Patient Number Note: *Patient 010 exhibited new metastatic disease and was removed from study; post-treatment target lesion measurement not performed.

18 DCE - MRI – Decreased Vascular Enhancement DCE - MRI on 28 Apr 2014; measured K trans = 0.72 - 0.76 min - 1 DCE - MRI on 02 Jun 2014; measured K trans = 0.14 - 0.16 min - 1 Red: aorta; blue: kidney; green: tumor. Range reflects use of larger and smaller regions of interest (ROIs) within the lesion.

19 DCE - MRI – Decreased Vascular Enhancement Patient with gastrohepatic metastatic lymph node involvement (green arrows). Increased hypoenhancement after treatment (right panel) suggests response. Before After

20 Distinctive Mipsagargin Characteristics Compared to other chemotherapeutic agents being tested in liver cancer patients: • Unique molecular mechanism of action. • Prolonged disease stabilization in a significant percentage of patients – 24% with stable disease > 5 months. • Side - effect profile – Minimal and manageable (fatigue, nausea, rash, reversible creatinine increase). No apparent effect on bone marrow. • Proof of Concept – Imaging studies demonstrate dramatic effects on tumor blood flow.

21 Mipsagargin vs Sorafenib Mipsagargin Phase IIa Study (25 pts) Sorafenib SHARP Study (299 pts) Population Sorafenib Failures Chemo Naïve ECOG 0/ 1 or 2 (%) 32/68 54/46 Child - Pugh A/B (%) 72/28 95/5 Extra hepatic disease (%) 76 53 DCR (Disease Control Rate) (%) 63* 43** Increase in TTP symptomatic (months) 2.4*** 0.8**** Side effects Minimal, easily managed Significant * At 56 days ** At 28 days *** p < 0.001 vs historical control of 2.1 months **** Not significant vs placebo Source: Llovet , JM, et.al., “Sorafenib in Advanced Hepatocellular Carcinoma,” The New England Journal of Medicine 359:4 (2008): 378 - 90.
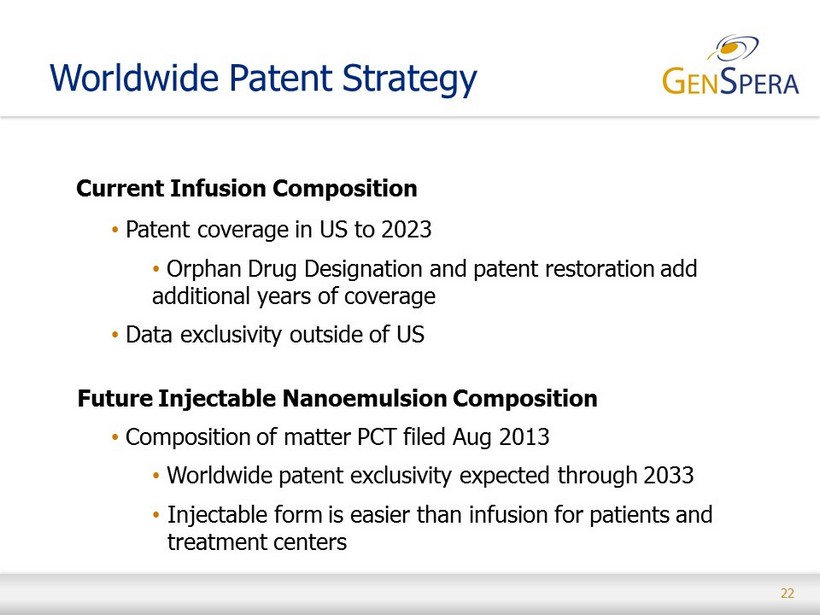
22 Worldwide Patent Strategy Current Infusion Composition • Patent coverage in US to 2023 • Orphan Drug Designation and patent restoration add additional years of coverage • Data exclusivity outside of US Future Injectable Nanoemulsion Composition • Composition of matter PCT filed Aug 2013 • Worldwide patent exclusivity expected through 2033 • Injectable form is easier than infusion for patients and treatment centers

23 GenSpera Clinical Programs – Evaluating Multiple Oncology Indications RESEARCH PRE - CLINICAL PHASE 1 PHASE 2 Liver Cancer (HCC) TARGET SITE DRUG INDICATION Brain Cancer (GBM) Prostate Cancer (PCa) Mipsagargin Blood vessels of most solid tumors DEVELOPMENT STAGE HUMAN CLINICAL TRIALS PHASE 3 25 patients treated in Ph2 study at 5 sites – Study Closed – Results presented 12 patients treated in Ph2 study at UCSD* Ph2 to begin Q2 2015 at UTHSC Houston* * Ph2 costs primarily supported by investigator funds Kidney Cancer (RCC) Ph2 to begin Q3 2015 at UTHSC Houston* 2015 Partner Outreach • 3 additional Phase II trials ongoing 2015 • Provides expanded options for partnering • Evaluating multiple oncology indications for next generation drugs

24 GenSpera Profile • GenSpera is a leader in developing prodrug therapeutics for the treatment of cancer • Unique, naturally derived, cancer killing drug has advantages in potency and significantly reduced side - effects • Experienced scientific and development team • Robust global IP position • Mipsagargin, GenSpera’s lead candidate, is showing excellent clinical results • Highly differentiated from other anti - cancer approaches • Outstanding clinical safety profile • Data strongly suggestive of clinical activity in HCC • Will be evaluated in 4 different cancer indications by Q3 2015 • Multiple development opportunities based on our prodrug platform • Four drug candidates in the pipeline

25 Contact GenSpera, Inc. (OTC QB: GNSZ) 2511 N Loop 1604 W, Suite 204 San Antonio, TX 78258 Craig Dionne, PhD CEO and Chairman +1 210 479 8112 info@genspera.com
