Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a15-12321_18k.htm |
Exhibit 99.1
|
|
Corporate Overview May 2015 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, cash runway, and the timing and reporting of results from clinical trials evaluating Amicus’ candidate drug products. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2014. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof. |
|
|
Company Mission Amicus Therapeutics is a biopharmaceutical company at the forefront of developing next-generation medicines to treat a range of rare and orphan diseases, with a focus on improved therapies for Lysosomal Storage Disorders |
|
|
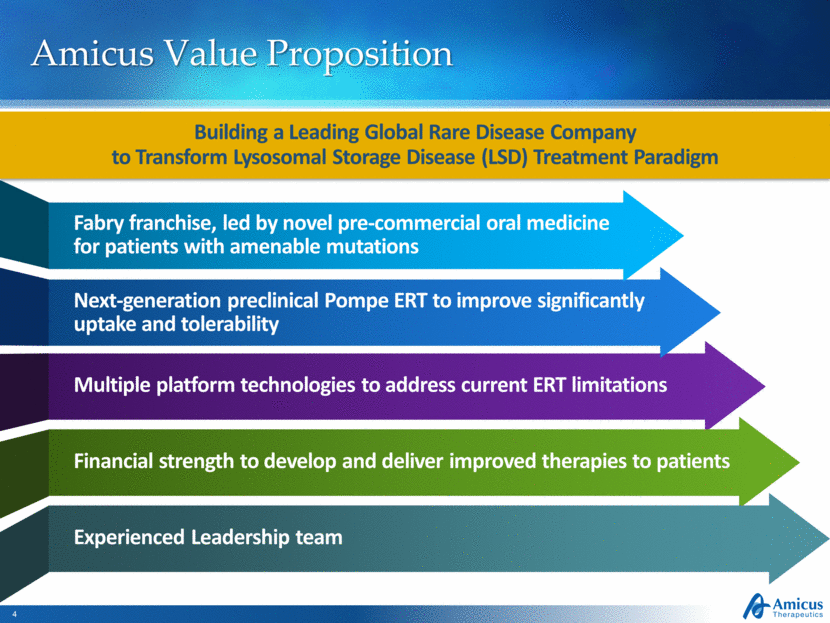
Amicus Value Proposition Building a Leading Global Rare Disease Company to Transform Lysosomal Storage Disease (LSD) Treatment Paradigm Multiple platform technologies to address current ERT limitations Next-generation preclinical Pompe ERT to improve significantly uptake and tolerability Financial strength to develop and deliver improved therapies to patients Experienced Leadership team Fabry franchise, led by novel pre-commercial oral medicine for patients with amenable mutations |
|
|
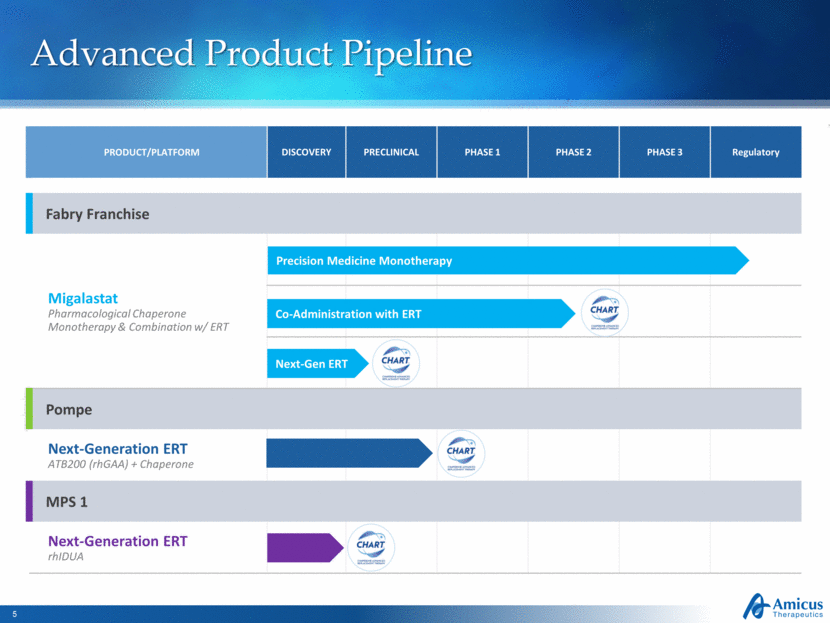
Advanced Product Pipeline PRODUCT/PLATFORM DISCOVERY PRECLINICAL PHASE 1 PHASE 2 PHASE 3 Regulatory Fabry Franchise Migalastat Pharmacological Chaperone Monotherapy & Combination w/ ERT Pompe Next-Generation ERT ATB200 (rhGAA) + Chaperone MPS 1 Next-Generation ERT rhIDUA Precision Medicine Monotherapy Co-Administration with ERT Next-Gen ERT |
|
|
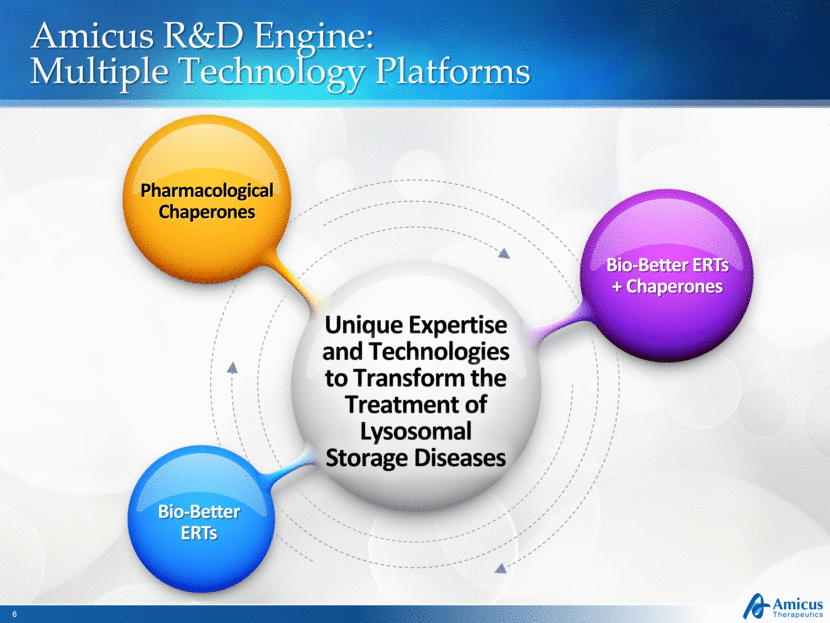
Amicus R&D Engine: Multiple Technology Platforms Unique Expertise and Technologies to Transform the Treatment of Lysosomal Storage Diseases Pharmacological Chaperones Bio-Better ERTs Bio-Better ERTs + Chaperones |
|
|
Fabry Franchise |
|
|
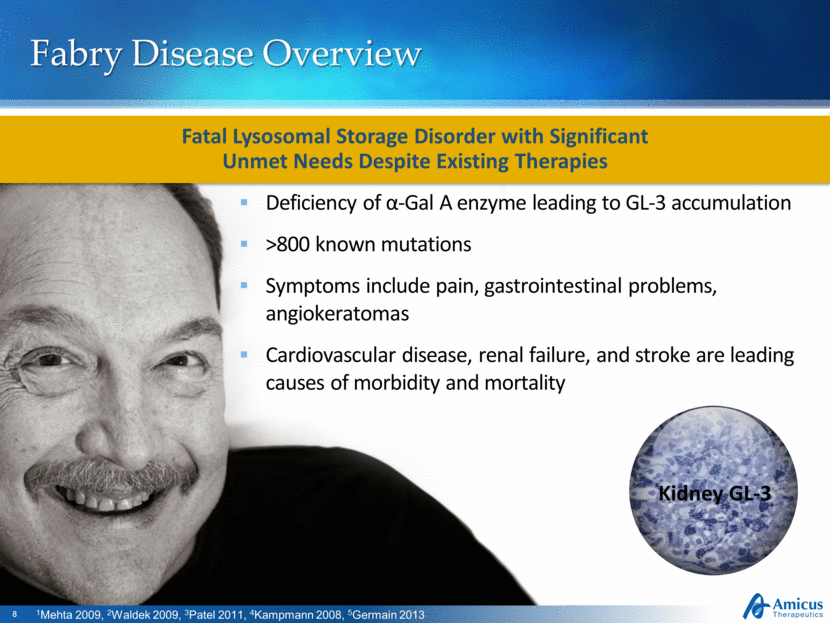
Fabry Disease Overview Deficiency of α-Gal A enzyme leading to GL-3 accumulation >800 known mutations Symptoms include pain, gastrointestinal problems, angiokeratomas Cardiovascular disease, renal failure, and stroke are leading causes of morbidity and mortality Fatal Lysosomal Storage Disorder with Significant Unmet Needs Despite Existing Therapies 1Mehta 2009, 2Waldek 2009, 3Patel 2011, 4Kampmann 2008, 5Germain 2013 Kidney GL-3 |
|
|
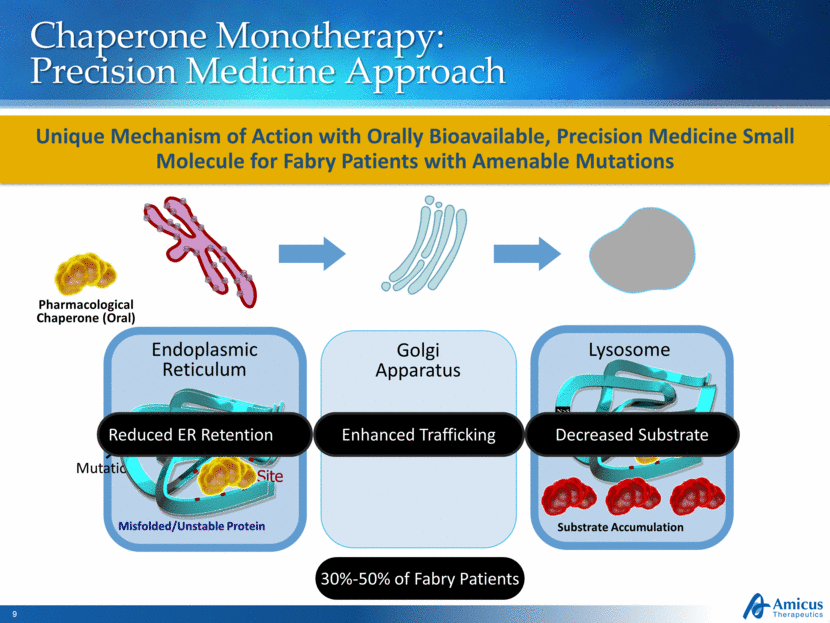
Chaperone Monotherapy: Precision Medicine Approach Substrate Accumulation Endoplasmic Reticulum Golgi Apparatus Lysosome Pharmacological Chaperone (Oral) Misfolded/Unstable Protein Active Site Mutation Enhanced Trafficking Reduced ER Retention Decreased Substrate 30%-50% of Fabry Patients Unique Mechanism of Action with Orally Bioavailable, Precision Medicine Small Molecule for Fabry Patients with Amenable Mutations N > S N > S N > S N > S |
|
|
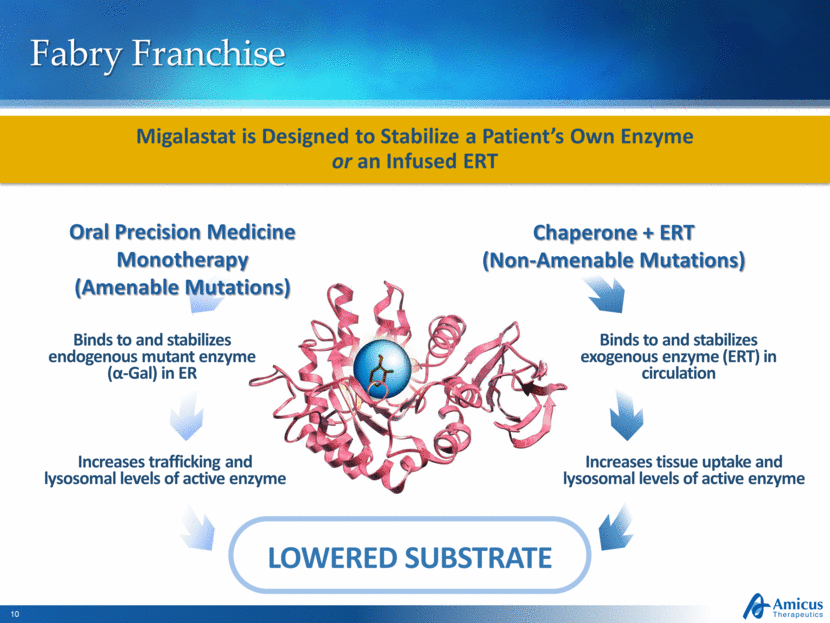
Fabry Franchise Binds to and stabilizes endogenous mutant enzyme (α-Gal) in ER Increases trafficking and lysosomal levels of active enzyme Binds to and stabilizes exogenous enzyme (ERT) in circulation Increases tissue uptake and lysosomal levels of active enzyme LOWERED SUBSTRATE Migalastat is Designed to Stabilize a Patient’s Own Enzyme or an Infused ERT Oral Precision Medicine Monotherapy (Amenable Mutations) Chaperone + ERT (Non-Amenable Mutations) |
|
|
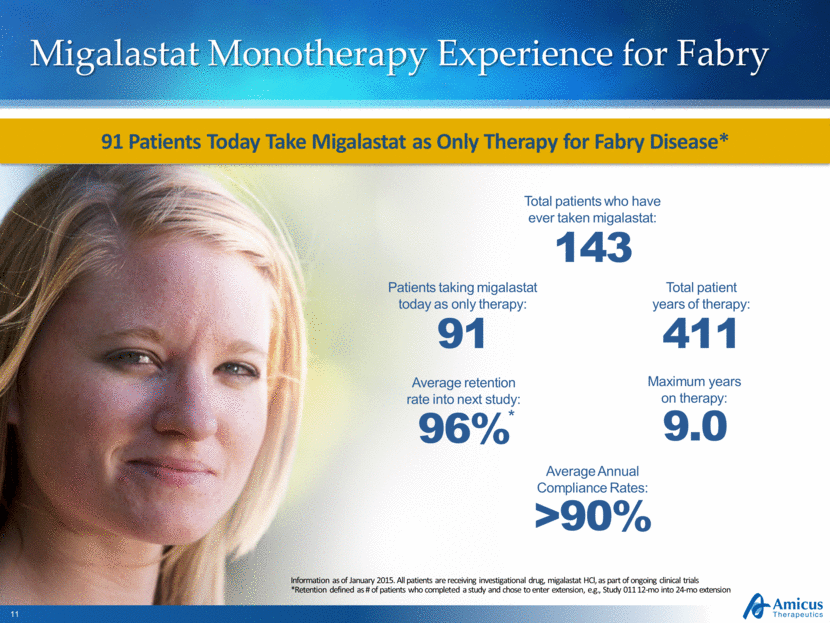
Migalastat Monotherapy Experience for Fabry Information as of January 2015. All patients are receiving investigational drug, migalastat HCl, as part of ongoing clinical trials *Retention defined as # of patients who completed a study and chose to enter extension, e.g., Study 011 12-mo into 24-mo extension Total patients who have ever taken migalastat: 143 Total patient years of therapy: 411 Maximum years on therapy: 9.0 Average retention rate into next study: 96% * Patients taking migalastat today as only therapy: 91 91 Patients Today Take Migalastat as Only Therapy for Fabry Disease* Average Annual Compliance Rates: >90% |
|
|
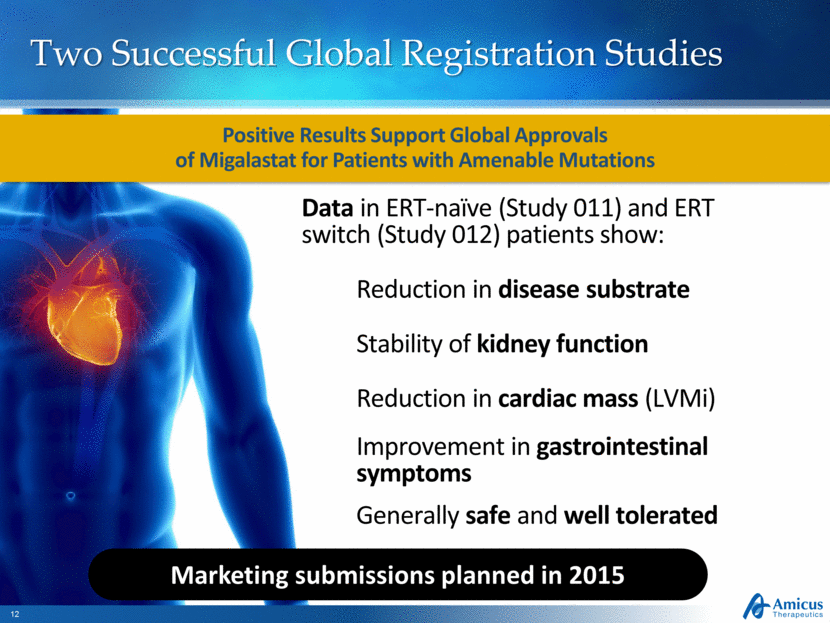
Two Successful Global Registration Studies Positive Results Support Global Approvals of Migalastat for Patients with Amenable Mutations Stability of kidney function Marketing submissions planned in 2015 Data in ERT-naïve (Study 011) and ERT switch (Study 012) patients show: Reduction in disease substrate Reduction in cardiac mass (LVMi) Improvement in gastrointestinal symptoms Generally safe and well tolerated |
|
|
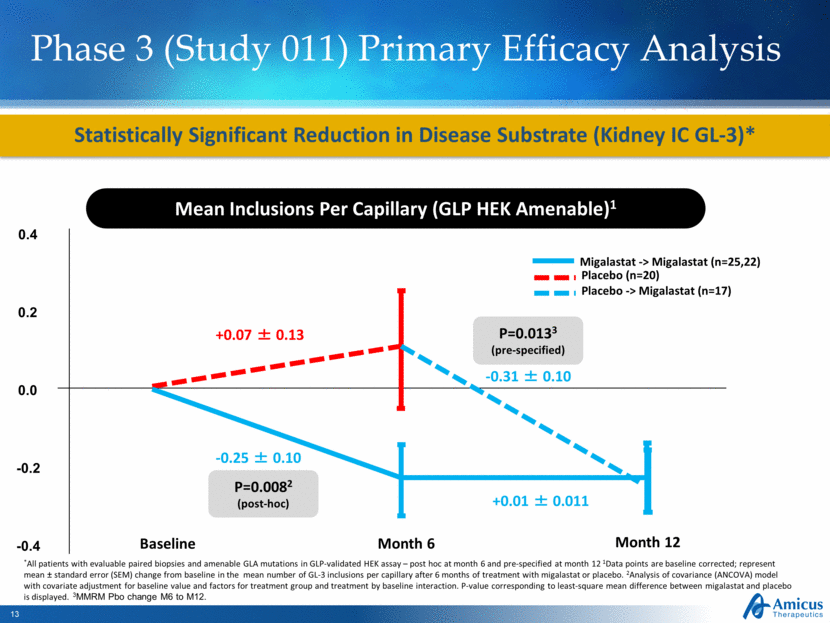
Phase 3 (Study 011) Primary Efficacy Analysis Statistically Significant Reduction in Disease Substrate (Kidney IC GL-3)* Mean Inclusions Per Capillary (GLP HEK Amenable)1 Baseline Month 6 +0.07 ± 0.13 -0.25 ± 0.10 P=0.0082 (post-hoc) *All patients with evaluable paired biopsies and amenable GLA mutations in GLP-validated HEK assay – post hoc at month 6 and pre-specified at month 12 1Data points are baseline corrected; represent mean ± standard error (SEM) change from baseline in the mean number of GL-3 inclusions per capillary after 6 months of treatment with migalastat or placebo. 2Analysis of covariance (ANCOVA) model with covariate adjustment for baseline value and factors for treatment group and treatment by baseline interaction. P-value corresponding to least-square mean difference between migalastat and placebo is displayed. 3MMRM Pbo change M6 to M12. Month 12 -0.31 ± 0.10 +0.01 ± 0.011 P=0.0133 (pre-specified) 0.4 0.0 -0.2 -0.4 0.2 Placebo -> Migalastat (n=17) Migalastat -> Migalastat (n=25,22) Placebo (n=20) |
|
|
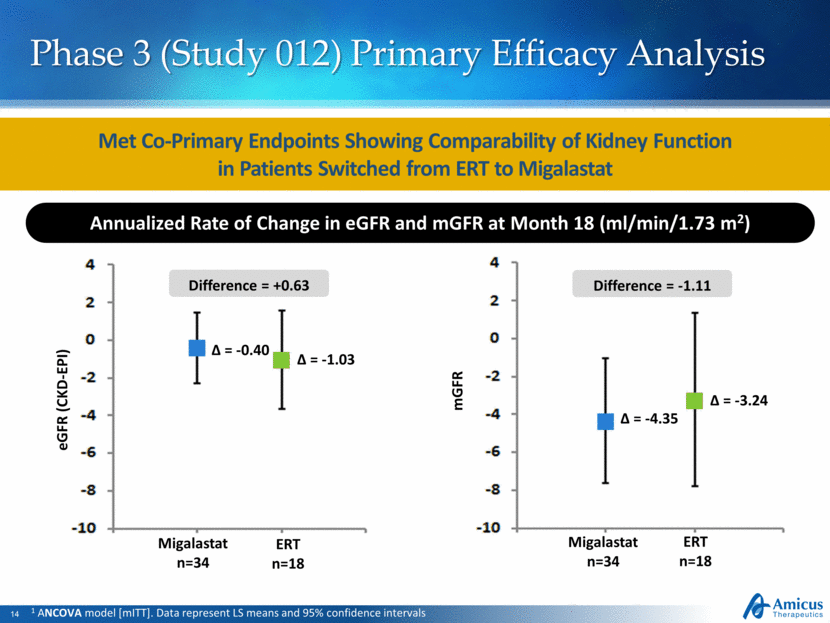
mGFR Migalastat n=34 ERT n=18 ∆ = -4.35 ∆ = -3.24 Migalastat n=34 ERT n=18 ∆ = -0.40 ∆ = -1.03 eGFR (CKD-EPI) Difference = +0.63 Difference = -1.11 Phase 3 (Study 012) Primary Efficacy Analysis 1 ANCOVA model [mITT]. Data represent LS means and 95% confidence intervals Met Co-Primary Endpoints Showing Comparability of Kidney Function in Patients Switched from ERT to Migalastat Annualized Rate of Change in eGFR and mGFR at Month 18 (ml/min/1.73 m2) |
|
|
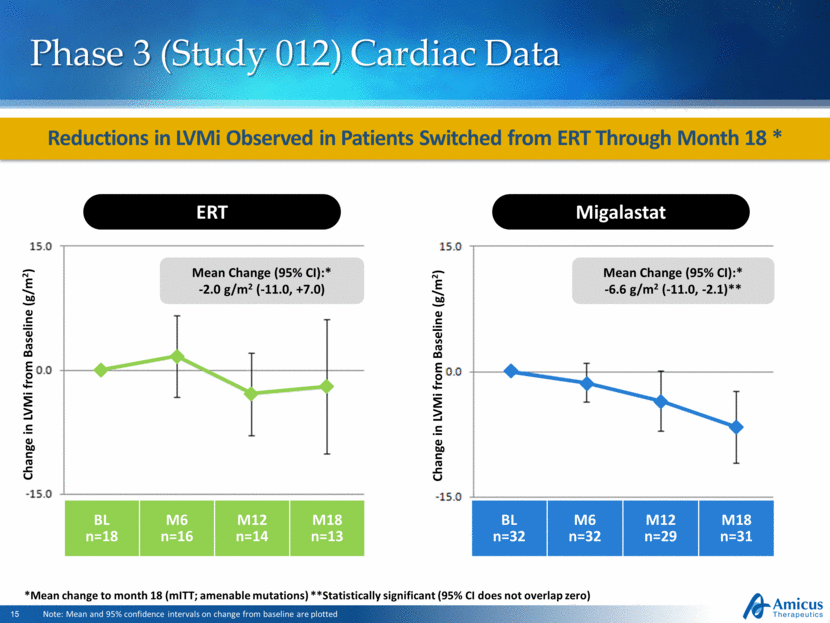
Reductions in LVMi Observed in Patients Switched from ERT Through Month 18 * Phase 3 (Study 012) Cardiac Data Note: Mean and 95% confidence intervals on change from baseline are plotted ERT Change in LVMi from Baseline (g/m2) BL n=18 M6 n=16 M12 n=14 M18 n=13 Mean Change (95% CI):* -2.0 g/m2 (-11.0, +7.0) BL n=32 M6 n=32 M12 n=29 M18 n=31 Migalastat Change in LVMi from Baseline (g/m2) Mean Change (95% CI):* -6.6 g/m2 (-11.0, -2.1)** *Mean change to month 18 (mITT; amenable mutations) **Statistically significant (95% CI does not overlap zero) |
|
|
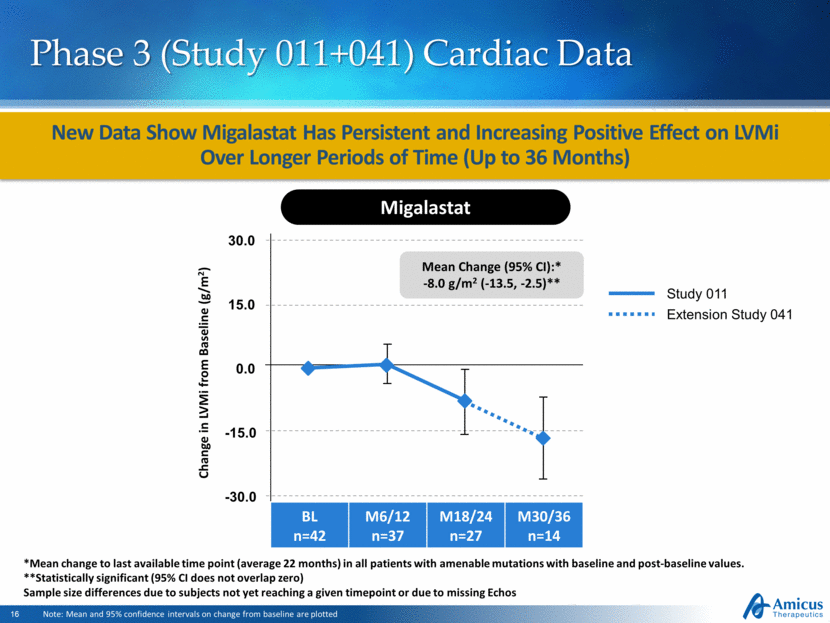
Change in LVMi from Baseline (g/m2) Mean Change (95% CI):* -8.0 g/m2 (-13.5, -2.5)** New Data Show Migalastat Has Persistent and Increasing Positive Effect on LVMi Over Longer Periods of Time (Up to 36 Months) Phase 3 (Study 011+041) Cardiac Data Study 011 Extension Study 041 Migalastat BL n=42 M6/12 n=37 M18/24 n=27 M30/36 n=14 *Mean change to last available time point (average 22 months) in all patients with amenable mutations with baseline and post-baseline values. **Statistically significant (95% CI does not overlap zero) Sample size differences due to subjects not yet reaching a given timepoint or due to missing Echos Note: Mean and 95% confidence intervals on change from baseline are plotted 30.0 0.0 -15.0 -30.0 15.0 |
|
|
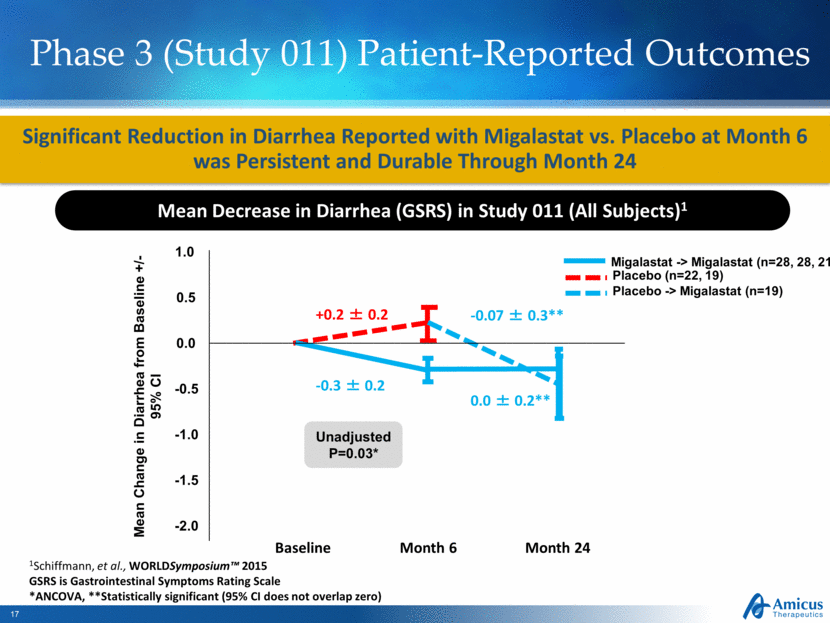
Phase 3 (Study 011) Patient-Reported Outcomes Significant Reduction in Diarrhea Reported with Migalastat vs. Placebo at Month 6 was Persistent and Durable Through Month 24 Mean Decrease in Diarrhea (GSRS) in Study 011 (All Subjects)1 Unadjusted P=0.03* 1Schiffmann, et al., WORLDSymposium™ 2015 GSRS is Gastrointestinal Symptoms Rating Scale *ANCOVA, **Statistically significant (95% CI does not overlap zero) Mean Change in Diarrhea from Baseline +/-95% CI 1.0 0.0 -0.5 -1.0 0.5 -1.5 -2.0 +0.2 ± 0.2 -0.3 ± 0.2 -0.07 ± 0.3** 0.0 ± 0.2** Placebo -> Migalastat (n=19) Migalastat -> Migalastat (n=28, 28, 21) Placebo (n=22, 19) Baseline Month 6 Month 24 |
|
|
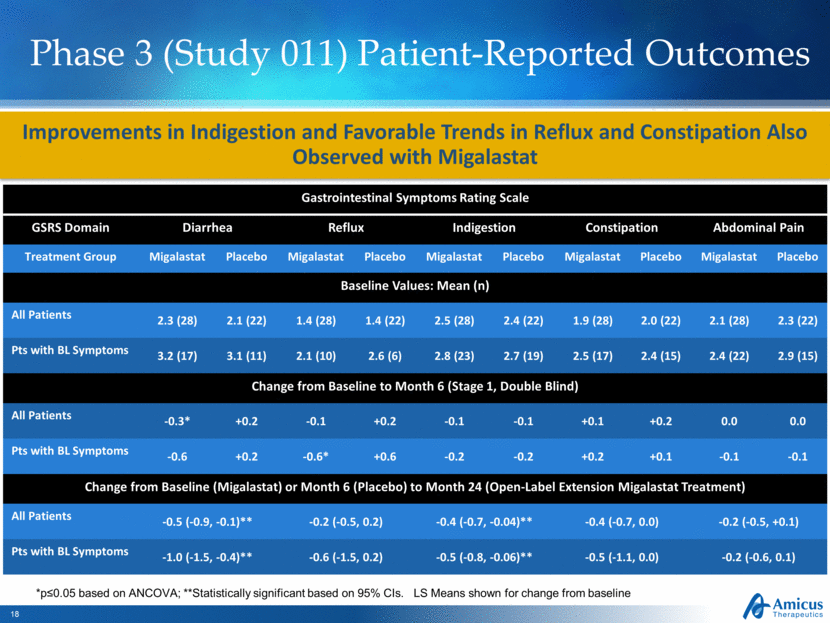
Phase 3 (Study 011) Patient-Reported Outcomes Improvements in Indigestion and Favorable Trends in Reflux and Constipation Also Observed with Migalastat Gastrointestinal Symptoms Rating Scale GSRS Domain Diarrhea Reflux Indigestion Constipation Abdominal Pain Treatment Group Migalastat Placebo Migalastat Placebo Migalastat Placebo Migalastat Placebo Migalastat Placebo Baseline Values: Mean (n) All Patients 2.3 (28) 2.1 (22) 1.4 (28) 1.4 (22) 2.5 (28) 2.4 (22) 1.9 (28) 2.0 (22) 2.1 (28) 2.3 (22) Pts with BL Symptoms 3.2 (17) 3.1 (11) 2.1 (10) 2.6 (6) 2.8 (23) 2.7 (19) 2.5 (17) 2.4 (15) 2.4 (22) 2.9 (15) Change from Baseline to Month 6 (Stage 1, Double Blind) All Patients -0.3* +0.2 -0.1 +0.2 -0.1 -0.1 +0.1 +0.2 0.0 0.0 Pts with BL Symptoms -0.6 +0.2 -0.6* +0.6 -0.2 -0.2 +0.2 +0.1 -0.1 -0.1 Change from Baseline (Migalastat) or Month 6 (Placebo) to Month 24 (Open-Label Extension Migalastat Treatment) All Patients -0.5 (-0.9, -0.1)** -0.2 (-0.5, 0.2) -0.4 (-0.7, -0.04)** -0.4 (-0.7, 0.0) -0.2 (-0.5, +0.1) Pts with BL Symptoms -1.0 (-1.5, -0.4)** -0.6 (-1.5, 0.2) -0.5 (-0.8, -0.06)** -0.5 (-1.1, 0.0) -0.2 (-0.6, 0.1) *p≤0.05 based on ANCOVA; **Statistically significant based on 95% CIs. LS Means shown for change from baseline |
|
|
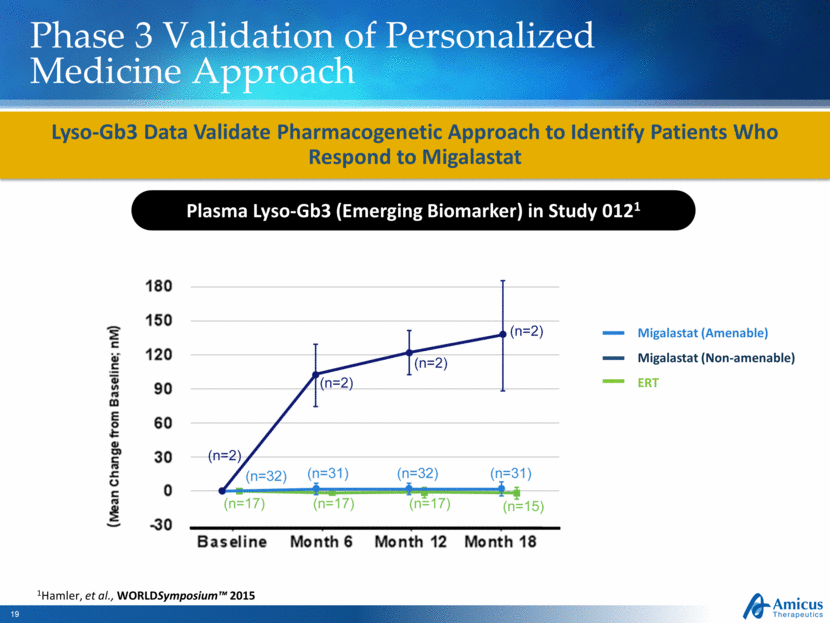
Phase 3 Validation of Personalized Medicine Approach Lyso-Gb3 Data Validate Pharmacogenetic Approach to Identify Patients Who Respond to Migalastat Plasma Lyso-Gb3 (Emerging Biomarker) in Study 0121 Migalastat (Amenable) Migalastat (Non-amenable) ERT 1Hamler, et al., WORLDSymposium™ 2015 |
|
|
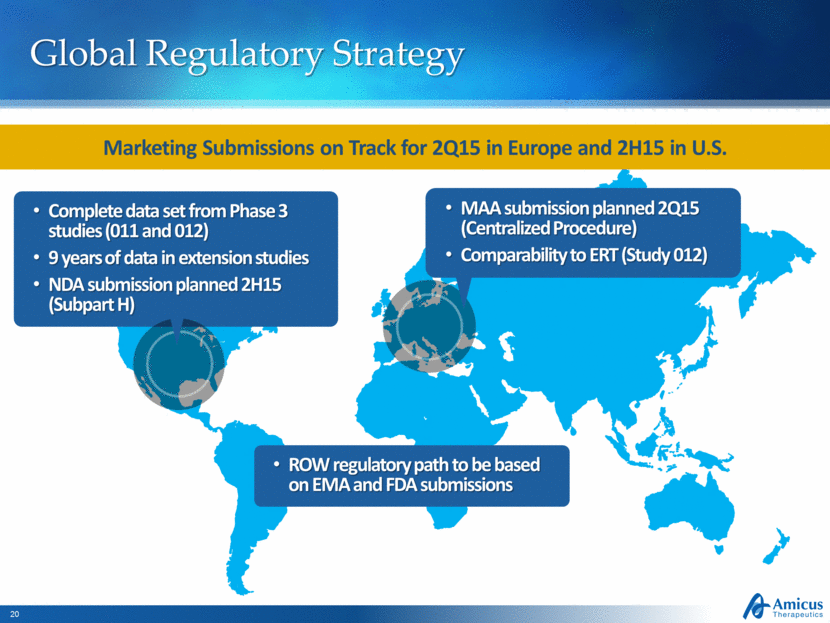
Global Regulatory Strategy Complete data set from Phase 3 studies (011 and 012) 9 years of data in extension studies NDA submission planned 2H15 (Subpart H) MAA submission planned 2Q15 (Centralized Procedure) Comparability to ERT (Study 012) Marketing Submissions on Track for 2Q15 in Europe and 2H15 in U.S. ROW regulatory path to be based on EMA and FDA submissions |
|
|
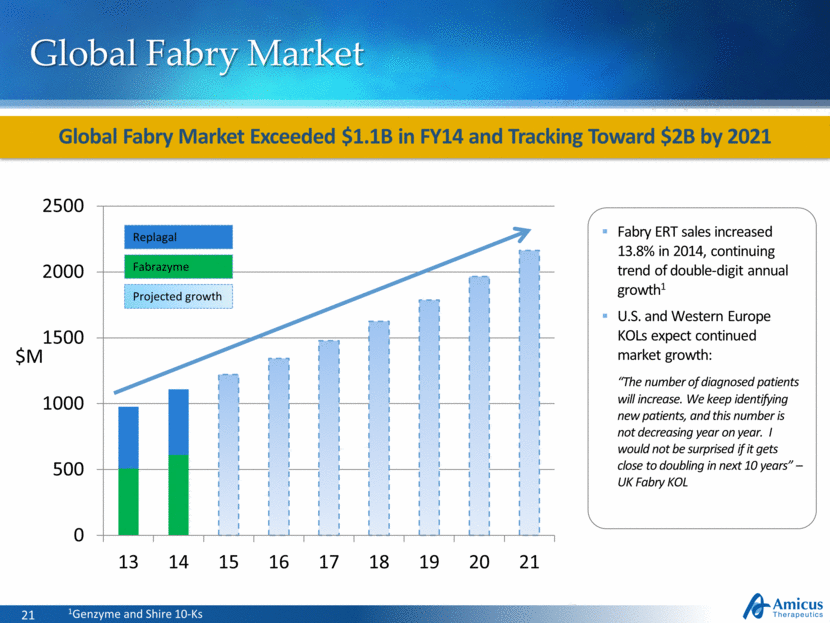
Global Fabry Market Global Fabry Market Exceeded $1.1B in FY14 and Tracking Toward $2B by 2021 Fabrazyme Replagal Projected growth $M Fabry ERT sales increased 13.8% in 2014, continuing trend of double-digit annual growth1 U.S. and Western Europe KOLs expect continued market growth: “The number of diagnosed patients will increase. We keep identifying new patients, and this number is not decreasing year on year. I would not be surprised if it gets close to doubling in next 10 years” – UK Fabry KOL 1Genzyme and Shire 10-Ks |
|
|
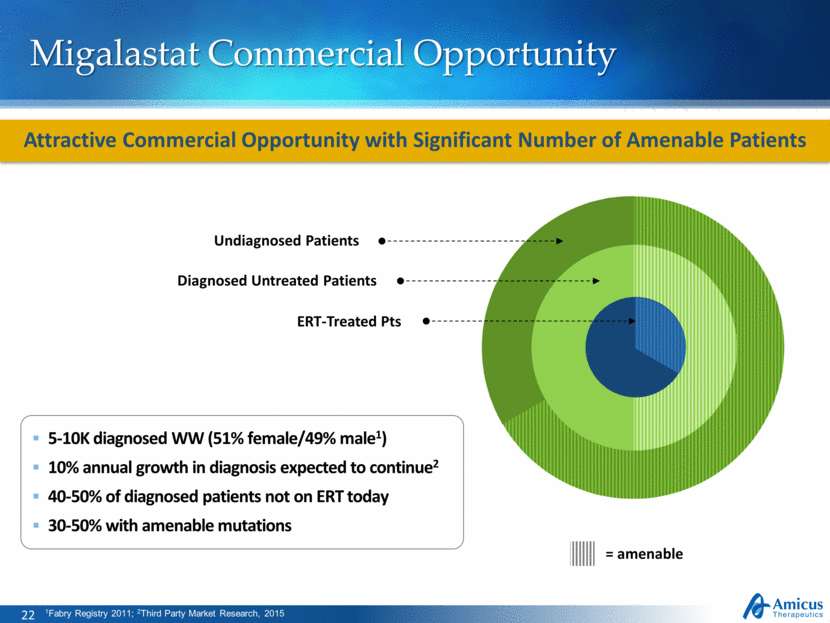
Migalastat Commercial Opportunity Attractive Commercial Opportunity with Significant Number of Amenable Patients 5-10K diagnosed WW (51% female/49% male1) 10% annual growth in diagnosis expected to continue2 40-50% of diagnosed patients not on ERT today 30-50% with amenable mutations 1Fabry Registry 2011; 2Third Party Market Research, 2015 ERT-Treated Pts Diagnosed Untreated Patients Undiagnosed Patients = amenable |
|
|
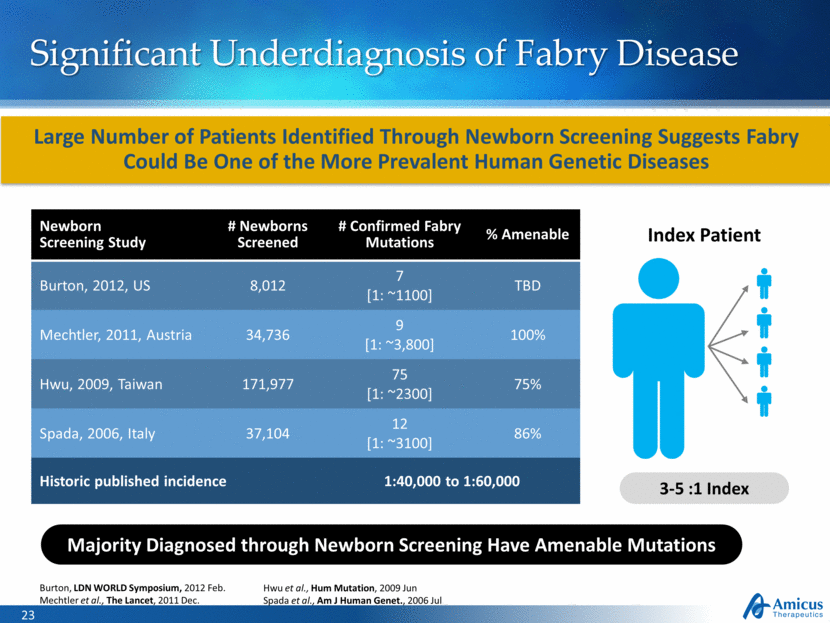
Significant Underdiagnosis of Fabry Disease Index Patient 3-5 :1 Index Burton, LDN WORLD Symposium, 2012 Feb. Mechtler et al., The Lancet, 2011 Dec. Hwu et al., Hum Mutation, 2009 Jun Spada et al., Am J Human Genet., 2006 Jul Large Number of Patients Identified Through Newborn Screening Suggests Fabry Could Be One of the More Prevalent Human Genetic Diseases Newborn Screening Study # Newborns Screened # Confirmed Fabry Mutations % Amenable Burton, 2012, US 8,012 7 [1: ~1100] TBD Mechtler, 2011, Austria 34,736 9 [1: ~3,800] 100% Hwu, 2009, Taiwan 171,977 75 [1: ~2300] 75% Spada, 2006, Italy 37,104 12 [1: ~3100] 86% Historic published incidence 1:40,000 to 1:60,000 Majority Diagnosed through Newborn Screening Have Amenable Mutations |
|
|
Note: *Includes feedback from 33 KOLs in U.S., UK, Germany, Italy, Brazil, Japan and France Based on Target Product Profile, KOLs Would Use Migalastat in Most Naïve and Switch Patients with Amenable Mutations with Signs and Symptoms if Approved Positive KOL Feedback New patients Migalastat preference share Diagnosed, treated 0% 100% 0% 100% 100% 0% Diagnosed, untreated |
|
|
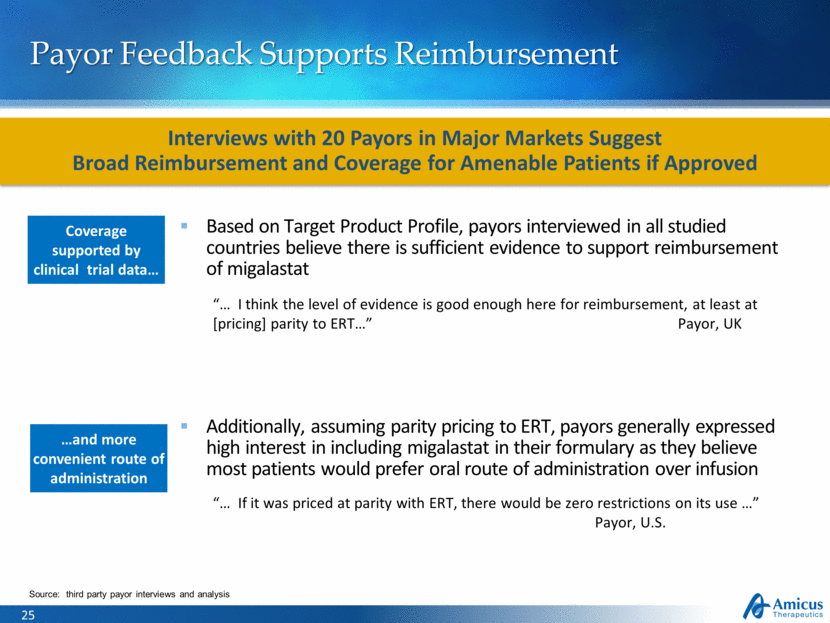
Payor Feedback Supports Reimbursement Coverage supported by clinical trial data Based on Target Product Profile, payors interviewed in all studied countries believe there is sufficient evidence to support reimbursement of migalastat “ I think the level of evidence is good enough here for reimbursement, at least at [pricing] parity to ERT ” Payor, UK Additionally, assuming parity pricing to ERT, payors generally expressed high interest in including migalastat in their formulary as they believe most patients would prefer oral route of administration over infusion “ If it was priced at parity with ERT, there would be zero restrictions on its use ” Payor, U.S. Source: third party payor interviews and analysis and more convenient route of administration Interviews with 20 Payors in Major Markets Suggest Broad Reimbursement and Coverage for Amenable Patients if Approved |
|
|
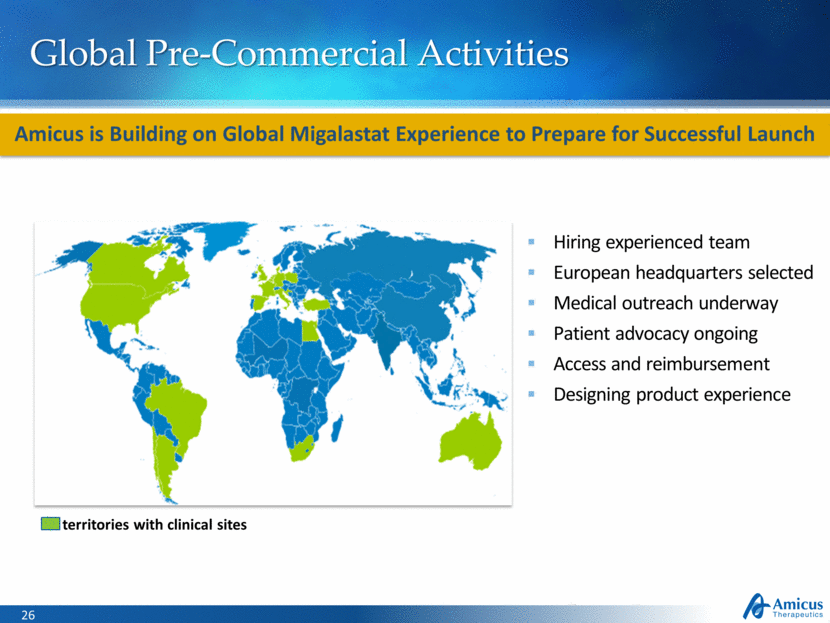
Global Pre-Commercial Activities Hiring experienced team European headquarters selected Medical outreach underway Patient advocacy ongoing Access and reimbursement Designing product experience Amicus is Building on Global Migalastat Experience to Prepare for Successful Launch territories with clinical sites |
|
|
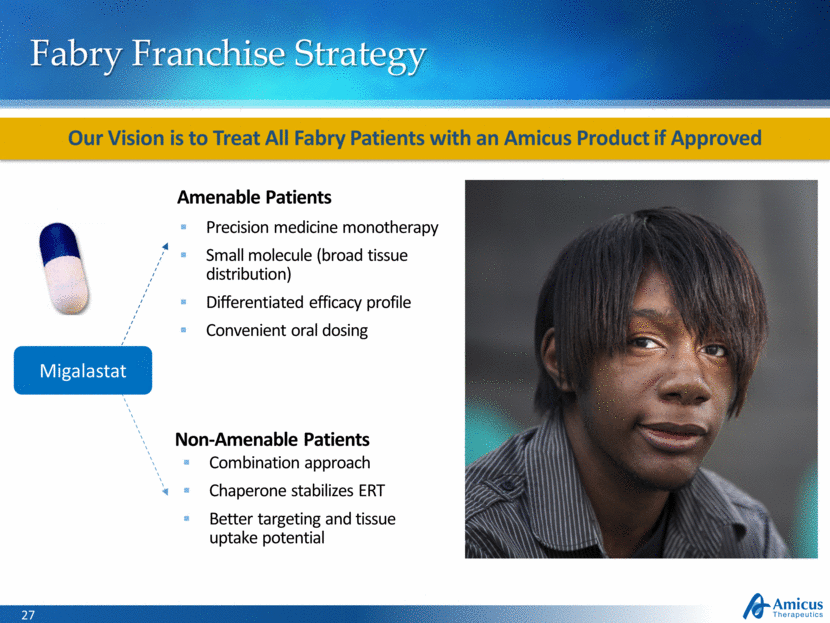
Fabry Franchise Strategy Our Vision is to Treat All Fabry Patients with an Amicus Product if Approved Amenable Patients Non-Amenable Patients Precision medicine monotherapy Small molecule (broad tissue distribution) Differentiated efficacy profile Convenient oral dosing Combination approach Chaperone stabilizes ERT Better targeting and tissue uptake potential Migalastat |
|
|
Key Milestones – Fabry Franchise Timing Milestone 1Q15 Additional 011 and Phase 2 extension data 1Q15 Scientific Presentations at LDN WORLD 1Q15 US and EU Regulatory Interaction 2Q15 MAA Submission 2H15 NDA Submission 2H15 Phase 2 Co-Administration Study Initiation 2H15 Internal Development of Next-Gen ERT Cell Line |
|
|
Next-Generation ERT for Pompe Disease |
|
|
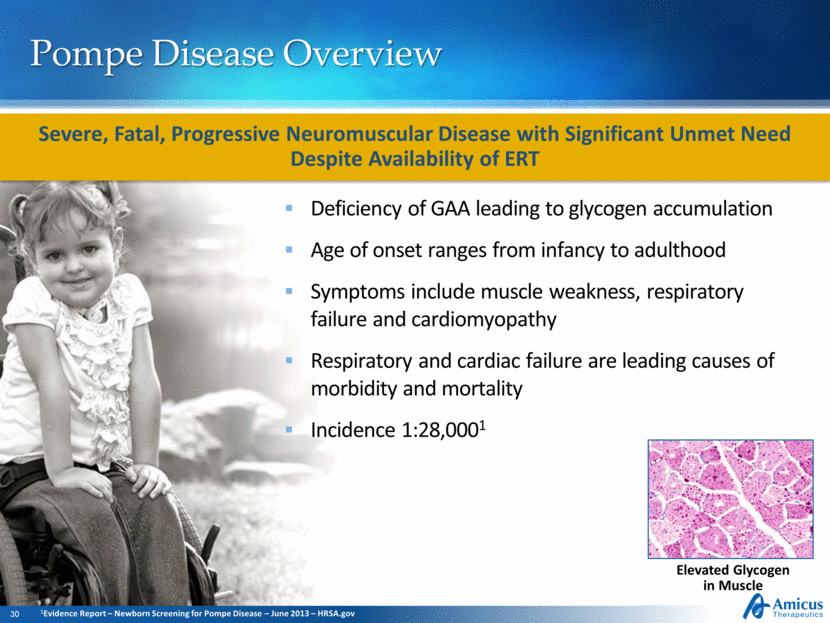
Pompe Disease Overview Deficiency of GAA leading to glycogen accumulation Age of onset ranges from infancy to adulthood Symptoms include muscle weakness, respiratory failure and cardiomyopathy Respiratory and cardiac failure are leading causes of morbidity and mortality Incidence 1:28,0001 Elevated Glycogen in Muscle Severe, Fatal, Progressive Neuromuscular Disease with Significant Unmet Need Despite Availability of ERT 1Evidence Report – Newborn Screening for Pompe Disease – June 2013 – HRSA.gov |
|
|
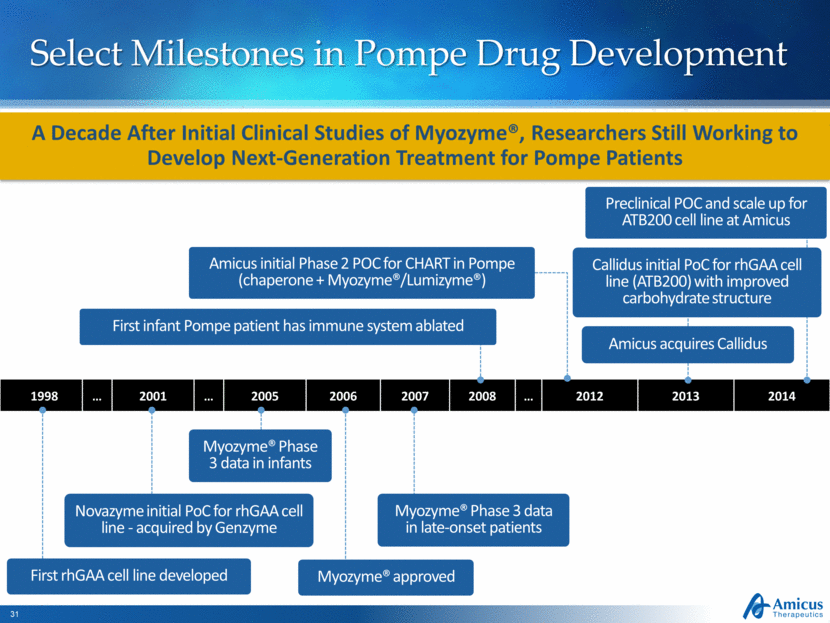
Select Milestones in Pompe Drug Development 1998 2001 2005 2006 2007 2008 2012 2013 2014 A Decade After Initial Clinical Studies of Myozyme®, Researchers Still Working to Develop Next-Generation Treatment for Pompe Patients Myozyme® Phase 3 data in infants Myozyme® approved Novazyme initial PoC for rhGAA cell line - acquired by Genzyme Myozyme® Phase 3 data in late-onset patients First rhGAA cell line developed Callidus initial PoC for rhGAA cell line (ATB200) with improved carbohydrate structure Amicus acquires Callidus Preclinical POC and scale up for ATB200 cell line at Amicus First infant Pompe patient has immune system ablated Amicus initial Phase 2 POC for CHART in Pompe (chaperone + Myozyme®/Lumizyme®) |
|
|
Current Pompe ERT Limitations Significant Unmet Needs Remain Due to Limitations of First-Generation Pompe ERT “ Biologic drugs, including enzyme-replacement therapies, can elicit anti-drug Abs (ADA) that may interfere with drug efficacy and impact patient safety.” (Journal of Immun. 2014) “ recurrent injections of rhGAA during ERT can elicit high titer antibody formation against GAA; this reduces the efficacy of ERT and may prompt infusion associated reactions (IAR) that may be life-threatening.” (Doerfler, et al. WORLD 2014) “All 18 patients who enrolled in the initial [infantile-onset Pompe] study survived significantly longer and with fewer ventilation events However, morbidity and mortality remain substantial, with a 28% mortality rate and a 51% invasive ventilation rate at age 36 months.” (Kishnani, et al. 2009) “ 14% of pts on [Lumizyme] treatment have declining 6-minute walk test and 36% have declining forced vital capacity.” (van der Ploeg, et al. 2010) |
|
|
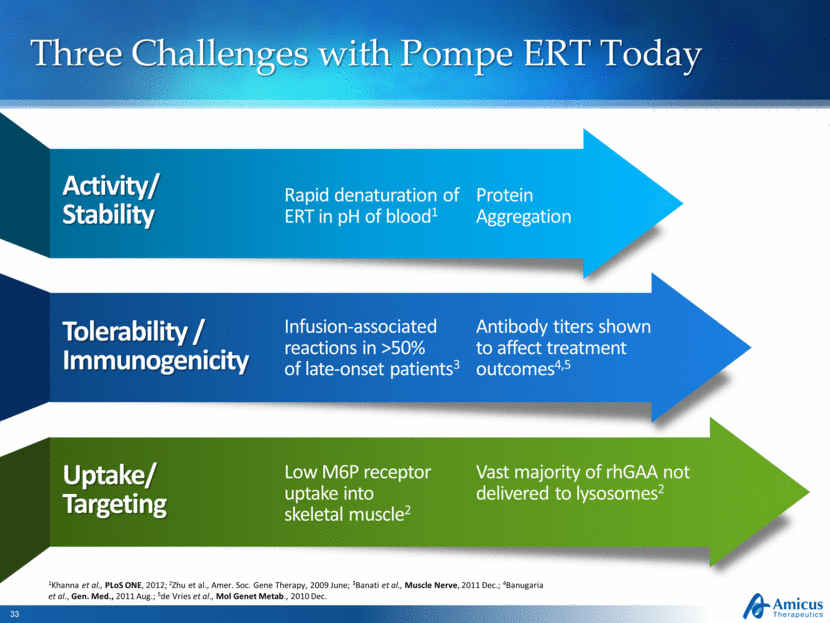
Three Challenges with Pompe ERT Today Activity/ Stability Rapid denaturation of ERT in pH of blood1 Tolerability / Immunogenicity Infusion-associated reactions in >50% of late-onset patients3 Uptake/ Targeting Low M6P receptor uptake into skeletal muscle2 Antibody titers shown to affect treatment outcomes4,5 Vast majority of rhGAA not delivered to lysosomes2 1Khanna et al., PLoS ONE, 2012; 2Zhu et al., Amer. Soc. Gene Therapy, 2009 June; 3Banati et al., Muscle Nerve, 2011 Dec.; 4Banugaria et al., Gen. Med., 2011 Aug.; 5de Vries et al., Mol Genet Metab., 2010 Dec. Protein Aggregation |
|
|
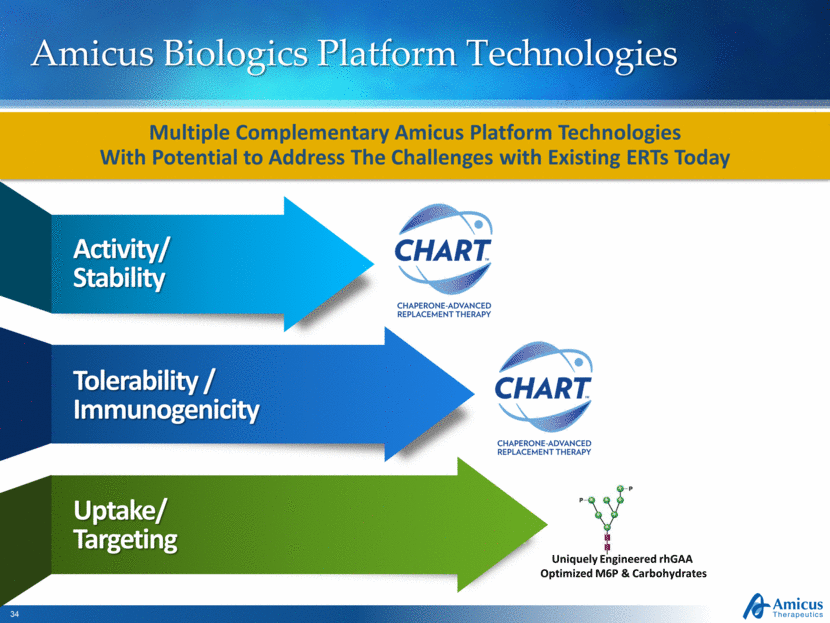
Amicus Biologics Platform Technologies Activity/ Stability Tolerability / Immunogenicity Uptake/ Targeting Multiple Complementary Amicus Platform Technologies With Potential to Address The Challenges with Existing ERTs Today Uniquely Engineered rhGAA Optimized M6P & Carbohydrates |
|
|
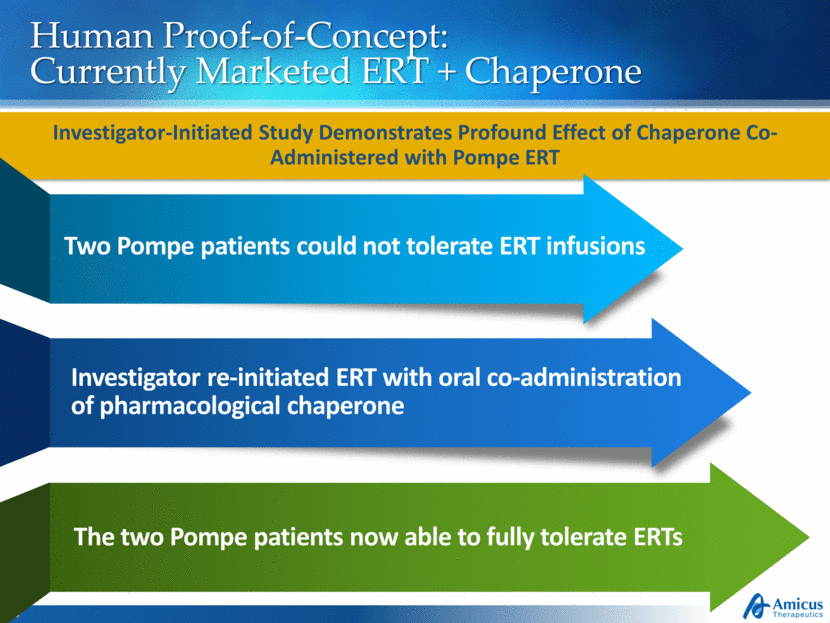
Human Proof-of-Concept: Currently Marketed ERT + Chaperone Investigator-Initiated Study Demonstrates Profound Effect of Chaperone Co-Administered with Pompe ERT Two Pompe patients could not tolerate ERT infusions Investigator re-initiated ERT with oral co-administration of pharmacological chaperone The two Pompe patients now able to fully tolerate ERTs |
|
|
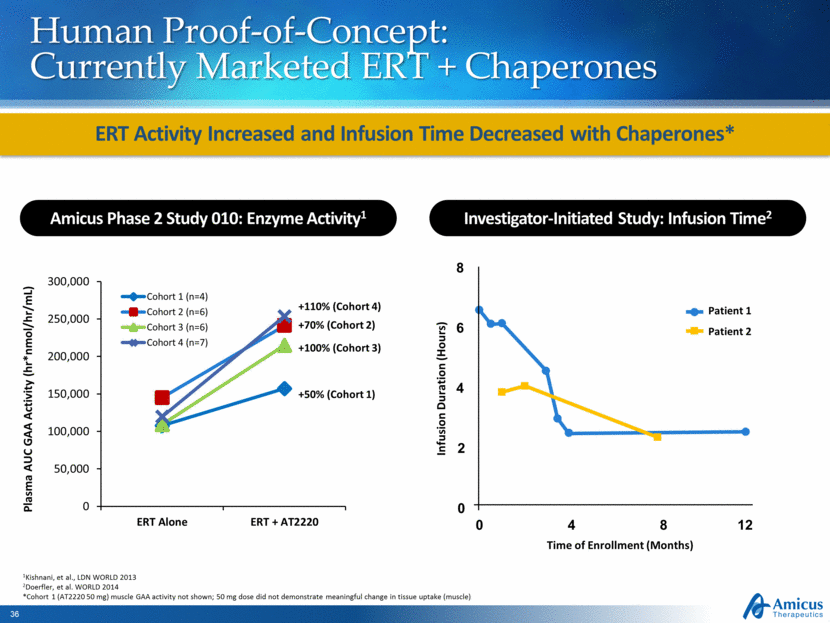
Human Proof-of-Concept: Currently Marketed ERT + Chaperones ERT Activity Increased and Infusion Time Decreased with Chaperones* 1Kishnani, et al., LDN WORLD 2013 2Doerfler, et al. WORLD 2014 *Cohort 1 (AT2220 50 mg) muscle GAA activity not shown; 50 mg dose did not demonstrate meaningful change in tissue uptake (muscle) Amicus Phase 2 Study 010: Enzyme Activity1 Plasma AUC GAA Activity (hr*nmol/hr/mL) Investigator-Initiated Study: Infusion Time2 Time of Enrollment (Months) Infusion Duration (Hours) Patient 1 Patient 2 8 4 0 12 8 4 2 0 6 0 50,000 100,000 150,000 200,000 250,000 300,000 ERT Alone ERT + AT2220 Cohort 1 (n=4) Cohort 2 (n=6) Cohort 3 (n=6) Cohort 4 (n=7) +110% (Cohort 4) +70% (Cohort 2) +100% (Cohort 3) +50% (Cohort 1) |
|
|
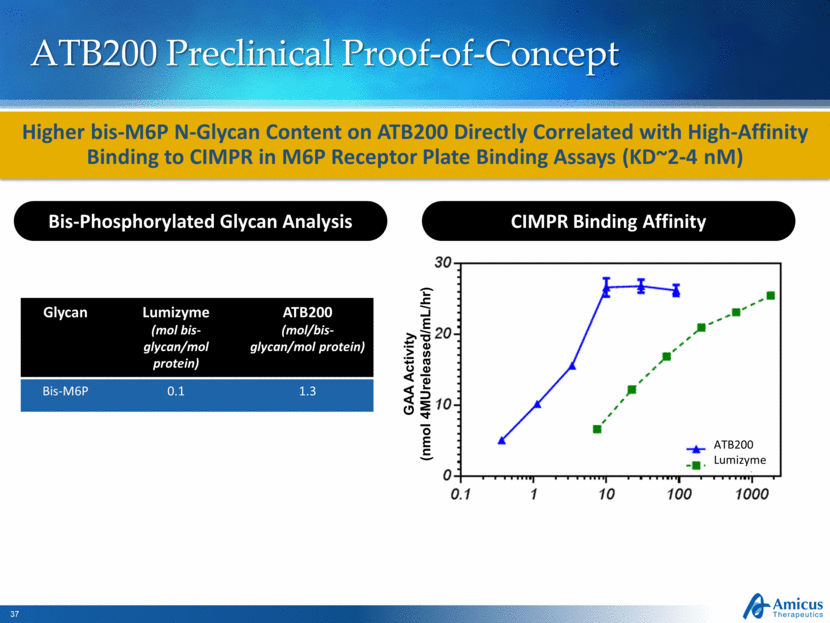
ATB200 Preclinical Proof-of-Concept CIMPR Binding Affinity GAA Activity (nmol 4MUreleased/mL/hr) ATB200 Lumizyme Bis-Phosphorylated Glycan Analysis Glycan Lumizyme (mol bis-glycan/mol protein) ATB200 (mol/bis-glycan/mol protein) Bis-M6P 0.1 1.3 Higher bis-M6P N-Glycan Content on ATB200 Directly Correlated with High-Affinity Binding to CIMPR in M6P Receptor Plate Binding Assays (KD~2-4 nM) |
|
|
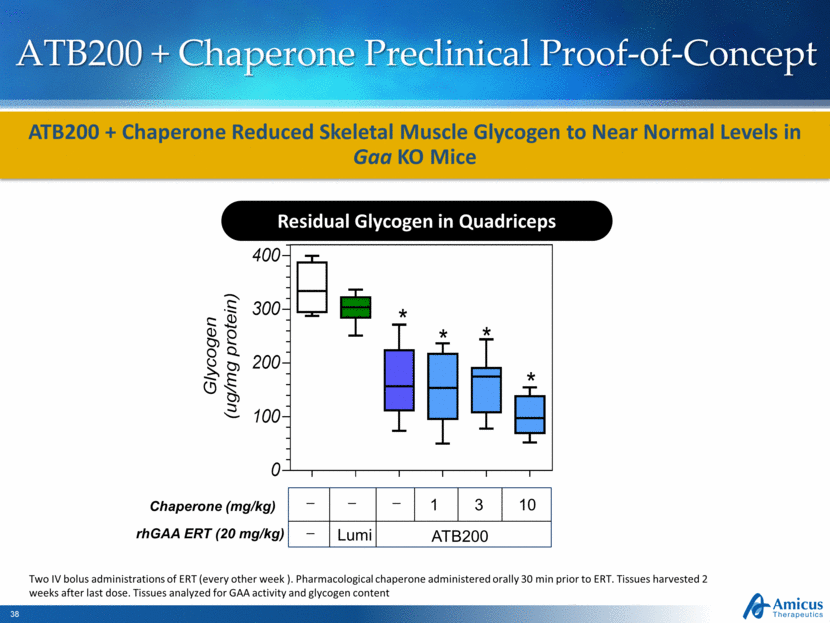
ATB200 + Chaperone Preclinical Proof-of-Concept Chaperone (mg/kg) 1 10 3 _ rhGAA ERT (20 mg/kg) Lumi ATB200 _ _ _ ATB200 + Chaperone Reduced Skeletal Muscle Glycogen to Near Normal Levels in Gaa KO Mice Two IV bolus administrations of ERT (every other week ). Pharmacological chaperone administered orally 30 min prior to ERT. Tissues harvested 2 weeks after last dose. Tissues analyzed for GAA activity and glycogen content Residual Glycogen in Quadriceps Residual Glycogen- Quadriceps G l y c o g e n ( u g / m g p r o t e i n ) 0 100 200 300 400 * * * * |
|
|
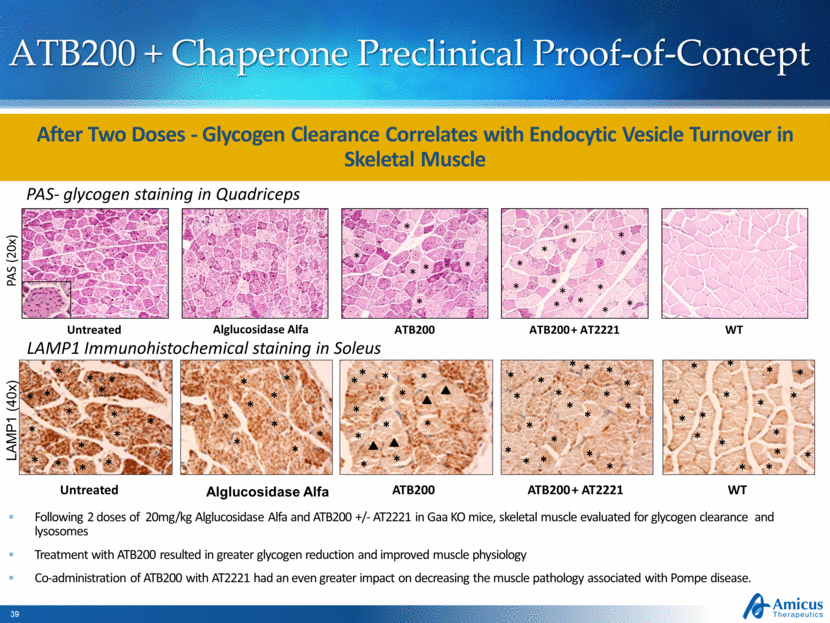
ATB200 + Chaperone Preclinical Proof-of-Concept PAS- glycogen staining in Quadriceps LAMP1 Immunohistochemical staining in Soleus Following 2 doses of 20mg/kg Alglucosidase Alfa and ATB200 +/- AT2221 in Gaa KO mice, skeletal muscle evaluated for glycogen clearance and lysosomes Treatment with ATB200 resulted in greater glycogen reduction and improved muscle physiology Co-administration of ATB200 with AT2221 had an even greater impact on decreasing the muscle pathology associated with Pompe disease. Untreated Alglucosidase Alfa ATB200 ATB200 + AT2221 WT * * * * * * * * * * * * * * * * * * * * Untreated Alglucosidase Alfa ATB200 ATB200 + AT2221 WT LAMP1 (40x) PAS (20x) After Two Doses - Glycogen Clearance Correlates with Endocytic Vesicle Turnover in Skeletal Muscle * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * * |
|
|
Amicus Biologics Capabilities ATB200 Successfully Manufactured at Clinical Scale While Maintaining Optimized Carbohydrate Structure Cell line scaled to 250 L 2 engineering batches completed in 2014 IND-enabling tox underway |
|
|
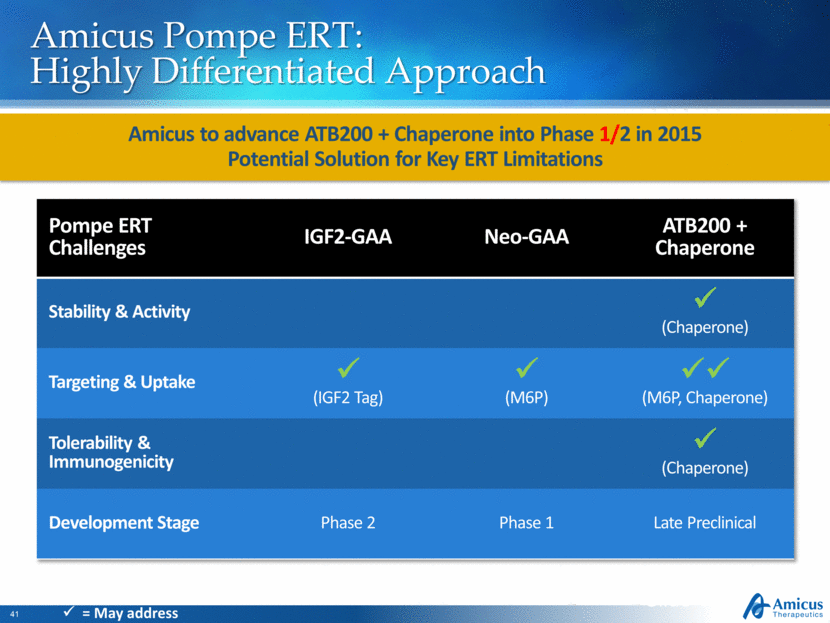
Amicus Pompe ERT: Highly Differentiated Approach Amicus to advance ATB200 + Chaperone into Phase 1/2 in 2015 Potential Solution for Key ERT Limitations Slide 41 Pompe ERT Challenges IGF2-GAA Neo-GAA ATB200 + Chaperone Stability & Activity (Chaperone) Targeting & Uptake (IGF2 Tag) (M6P) (M6P, Chaperone) Tolerability & Immunogenicity (Chaperone) Development Stage Phase 2 Phase 1 Late Preclinical = May address |
|
|
Pompe: Multiple Milestones to Clinic Timing Milestone 1Q15 Initiate GMP Batch 3Q15 Tox Studies Mid-2015 Pre-IND Meeting 2H15 Phase 1/2 study initiation |
|
|
Financial Summary |
|
|
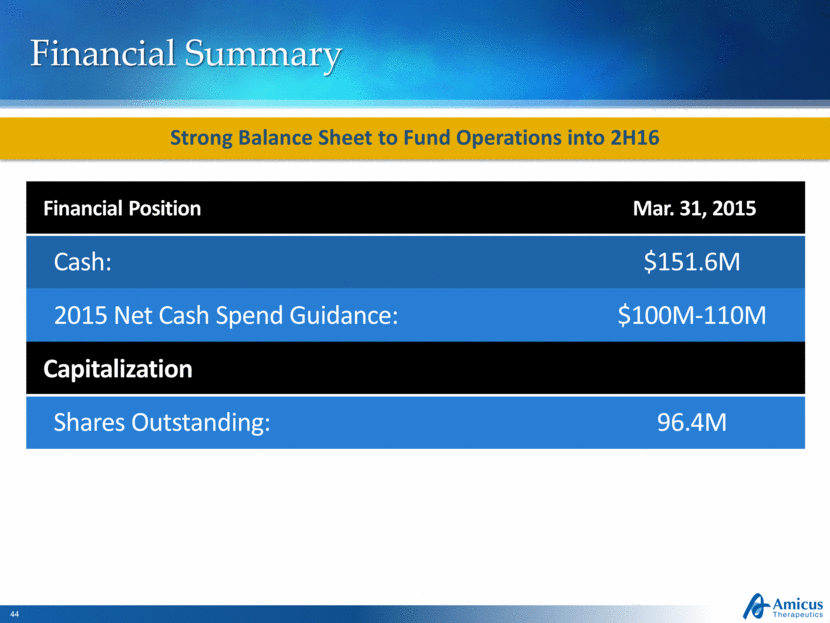
Financial Summary Financial Position Mar. 31, 2015 Cash: $151.6M 2015 Net Cash Spend Guidance: $100M-110M Capitalization Shares Outstanding: 96.4M Strong Balance Sheet to Fund Operations into 2H16 |