Attached files
| file | filename |
|---|---|
| 8-K - BIOTIME INC 8-K 5-14-2015 - Lineage Cell Therapeutics, Inc. | form8k.htm |
Exhibit 99.1

LEADING THEREGENERATIVE MEDICINE REVOLUTION May 2015NYSE MKT: BTX

The matters discussed in this presentation include forward looking statements which are subject to various risks, uncertainties, and other factors that could cause actual results to differ materially from the results anticipated. Such risks and uncertainties include but are not limited to the success of BioTime in developing new stem cell products and technologies; results of clinical trials of BioTime products; the ability of BioTime and its licensees to obtain additional FDA and foreign regulatory approval to market BioTime products; competition from products manufactured and sold or being developed by other companies; the price of and demand for BioTime products; and the ability of BioTime to raise the capital needed to finance its current and planned operations. Any statements that are not historical fact (including, but not limited to statements that contain words such as "will," "believes," "plans," "anticipates," "expects," "estimates") should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, risks inherent in the development and/or commercialization of potential products, uncertainty in the results of clinical trials or regulatory approvals, need and ability to obtain future capital, and maintenance of intellectual property rights. As actual results may differ materially from the results anticipated in these forward-looking statements they should be evaluated together with the many uncertainties that affect the business of BioTime and its subsidiaries, particularly those mentioned in the cautionary statements found in BioTime's Securities and Exchange Commission filings. BioTime disclaims any intent or obligation to update these forward-looking statements. 2 Safe Harbor Statement

RIGHT TIME to consolidate leadership in regenerative medicine ROBUST PIPELINE addressing large, degenerative diseases UNIQUE CELL DELIVERY PLATFORM: ReneviaTM pivotal trial underway MULTIPLE CLINICAL TRIALS advancing products to commercialization UNLOCKING SUBSIDIARY VALUE (NYSE MKT: AST, $230M1) LEADERSHIP TEAM with clinical development, commercial expertise Investment Higlights BUILDING VALUE AT BIOTIME 3 1 Fair value of AST shares held by BioTime based on 68% ownership on May 1, 2015

Gene cloningtechnology developed Hybridomatechnology developed Isolation of pluripotentstem cells 2015 1970 1980 1990 2000 2010 1974 1975 1998 RECOMBINANT DNA MONOCLONAL ANTIBODIES REGENERATIVE MEDICINE 2010: FIRST-IN-HUMAN TRIAL OF OPC1 $44BNCURRENT GLOBAL MARKET $75BNCURRENT GLOBAL MARKET Regenerative Medicine: The Next Revolution 4

Small MoleculesTechnology in decline Monoclonal AntibodiesTechnology in growth phase Cell TherapyTechnology in clinical trials phase Cell Therapy Has Long Asset Life Potential Product Recombinant ProteinsTechnology in maturity phase hESC MasterCell Bank 5 Lack of regulatory pathway for generics or biosimilars Generics Biosimilars Biosimilars

Pluripotent stem cell platform allows industrial manufacture of all humancell typesSignificant competitive barriers:>600 patents/apps worldwideLarge degenerativedisease markets Multiple near-term clinical milestonesBioTime and subsidiaries have multiple opportunities for value creation Stem Cell Platform Enables Multiple Opportunities 6

HIV-associated lipoatrophy CELL THERAPIES Broad Portfolio Advancing in Clinic CELL DELIVERY MATRIX Renevia DIAGNOSTICS PLATFORM PanC-Dx Pre-Clinical Phase I Phase II Phase III/Pivotal OpRegen OPC1 VAC2 Dry AMD Spinal cord injury rehabilitation NSC lung cancer Screening diagnostics for bladder, lung, and breast cancer Brown Adipocyte Progenitors Metabolic disease 7 Osteochondral Progenitors Bone grafting VAC1 Acute myelogenous leukemia

Retinal Pigment Epithelium (RPE) Choroid Photoreceptors Drusen Age-Related Macular Degeneration (AMD) OpRegen has Proven Mechanism in Dry AMD Photoreceptor function, angiogenesis inhibition depend on RPE cells Loss of RPE cells can cause dry or wet AMD OpRegen integrates into subretinal space to replace missing RPE cells 8 Dry AMD frequently leads to wet AMD, opportunity to prevent dry AMD Data1 show OpRegen’s high purity, safety and efficacy in leading animal models 1 The Association for Research in Vision and Opthalmology, May 2015

hES Cell-Derived RPE Cell Replacement Therapy for Dry AMD Targeting Larger Dry AMD Opportunity 9 800,000 Cases in U.S. Annually 90% Dry AMDNo Approved Therapy 10% Wet AMD>$7B Market Globally

OpRegen Phase I/IIa: First Patient Dosing 2Q15 Cohort 3 ● 3 Patients BCVA 20/200 or less 500,000 cells TRIAL DESIGNPart 1 Cohort 4 ● 6 Patients BCVA 20/100 or less 500,000 cells Cohort 2 ● 3 Patients BCVA 20/200 or less 200,000 cells Cohort 1 ● 3 Patients BCVA 20/200 or less 50,000 cells Part 2 Phase I/IIa Study Dose escalation safety and efficacy study of OpRegen transplanted subretinally in patients with advanced dry-form of AMD(Geographic Atrophy)Open label, non-randomized, sequential, single center trial Study Site Hadassah University Medical Center, Jerusalem, Israel Dose and Administration Single escalating doses of cells in saline injection into subretinal space 10

Demyelination of neurons impairs rehabilitation from spinal cord injury, plays a role in multiple sclerosis, other diseases Transplantation of oligodendrocyte progenitors can remyelinate damaged nerve axons, improving recovery from spinal cord injury in extensive rat model studies Oligodendrocyte Progenitors OPC1: Promise in Spinal Cord Rehabilitation Nerve Cell Axon Oligodendrocyte 11 Phase I study demonstrated OPC1’s safety and feasibility, with five subjects followed for >4 years after receiving 2 mil OPC1 cells

after 30 Days dose five pts with 20 million AST-OPC1 cells OPC1: Phase I/IIa Trial Underway TRIAL DESIGNSequential Cohort, Dose Escalation Dose three pts with two millionAST-OPC1 cells after 30 Days dose five pts with 10 million AST-OPC1 cells Subject to FDA clearance, expansion of second and third dose cohortsMay result in pathway to registration study Indication: Complete Cervical Spinal Cord Injury 12 Objectives Safety and Preliminary Efficacy Assess effects on upper extremity motor functionInvestigate effects on additional measures of neurological function
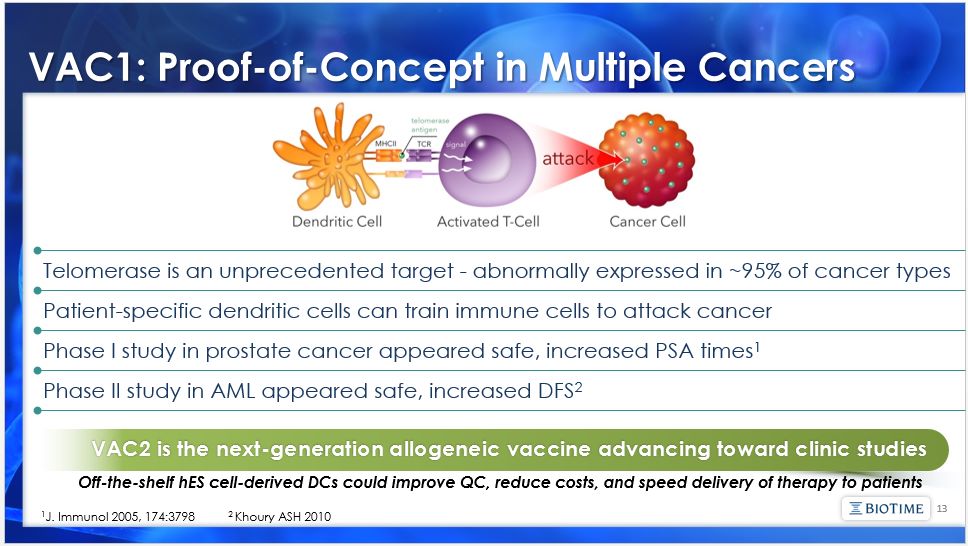
VAC2 is the next-generation allogeneic vaccine advancing toward clinic studies 13 Telomerase is an unprecedented target - abnormally expressed in ~95% of cancer types VAC1: Proof-of-Concept in Multiple Cancers Patient-specific dendritic cells can train immune cells to attack cancer Phase I study in prostate cancer appeared safe, increased PSA times1 Phase II study in AML appeared safe, increased DFS2 Off-the-shelf hES cell-derived DCs could improve QC, reduce costs, and speed delivery of therapy to patients 1J. Immunol 2005, 174:3798 2 Khoury ASH 2010
Most cell therapies without matrix support have reported engraftment of less than 5% Most human cells naturally die if not rapidly attached to matrix Need for Cell Delivery is Significant 14 Current known options not easily adapted to include cells and matrix for delivery to tissues in vivo using a small gauge syringe ReneviaTM

3-Dimensional Cell Delivery Matrix Renevia™: Novel Injectable Cell Delivery Matrix 15 Renevia™: Novel Injectable Cell Delivery Matrix
Applications in Multiple Types of Facial Atrophy ReneviaTM designed to safely produce 3-D tissue in vivoCells remain where placed by surgeon~350,000 in EU have HIV-related lipoatrophy$5,000-7,000 spent per patient annually for currently available treatment optionsTrauma or age-related lipoatrophy even more prevalentMany other potential applications incombination with adult and ESC therapies Age-Related Lipoatrophy 16

Renevia™: Pivotal Trial Initiated 1Q15 Secondary Endpoint- Mid-face volume deficit score - - Global aesthetic improvement scale - TRIAL DESIGNMulticenter, randomized, controlled, single-blind trial Treated vs. delayed treatment control25 completers in each group with treatment effect measured at 1, 3, and 6 months Primary EndpointIncrease in skin thickness as measured by ultrasound at 6 months Pivotal trial to support CE mark for use in HIV-associated lipoatrophy in combination with autologous lipotransfer HIV-Related Lipoatrophy 17 Enrollment completion expected within a year

8 Million Patients Screened Annually in U.S. LUNG CANCER 18 PanC-Dx™: Platform with Broad Oncology Potential Noninvasive Cancer Screening Diagnostics Less invasive, easily implemented, novel blood and urine-based diagnostic Potential for expanded use in numerous solid tumor types 3.5 Million Diagnostic Mammograms Annually in U.S. BREAST CANCER 1.5 Million Tests Annually in U.S. BLADDER CANCER Final clinical validation data expected in 4Q15 (bladder), in 1Q16 (breast) Interim data presented on breast and bladder cancer in April 2015; lung data expected in 2H15

Financial Strength for Growth ASSETS $283M Liquid Assets $26M Cash1 $27M Registered BTX shares2 held by subsidiaries $230M AST shares2(BTX owns 68%) No Debt ~$452M BioTime Market cap3 ~45% Shares owned bylong-term stockholders 1 At March 31, 2015, 2 Fair value of AST shares and BTX shares based on May 1, 2015 data, 3 As of May 1, 2015 19

$20-30 million $14.3 million Grant Financial De-Risking AST-OPC1 AST-VAC2 OpRegen $42-52 million of Total Non-Dilutive Funding Includes funding for: Execution ofPhase 1/2a studyAssay developmentFacilities and indirect costs CRUK provides fundingfor personnel, cGMP manufacturing, regulatory filing, Phase 1/2a trialAsterias has first option to reacquire program on preset, reasonable termsor majority revenue share Funding of pre-clinical studies leading toIND filingNondilutive grantwith potential forfollow-on funding ~$8 million 20

Significant Stakes in Key Subsidiaries 68% % BTX Ownership as of March 31, 2015 63%2 75% 1 Fair value of AST shares held by BioTime based on May 1, 2015 2 Includes shares owned by BioTime, Asterias, and ESI 21 $230M 1

Leadership TeamScientific, Clinical Development, Commercial Expertise LEADER ROLE EXPERIENCE Michael D. West, Ph.D. President andChief Executive Officer Ocata Therapeutics, Geron (Founder) Adi Mohanty Chief Operating Officer Shire, Baxter EXPERIENCED BOARD OF DIRECTORS + 3 RECENT ADDITIONS WITH COMMERCIAL EXPERIENCE+ Stephen L. Cartt (Questcor Pharmaceuticals, Inc.)+ Michael H. Mulroy (Mallinckrodt and Questcor)+ Angus C. Russell (Shire, Former Chief Executive Officer) 22 Robert W. Peabody Senior Vice President,Chief Financial Officer Ocata Therapeutics, Ecolab
Near-Term Value Creation Results from multiple clinical studies Commercializing cancer screening diagnostics Completing Renevia pivotal clinical trial enrollment Implementing clinical and financial de-risking strategies Unlocking subsidiary company value for BioTime shareholders Building management & Board for commercial phase POSITIONED FOR SUCCESS 23

LEADING THEREGENERATIVE MEDICINE REVOLUTION NYSE MKT: BTX
