Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT - Statera Biopharma, Inc. | fs12015a1ex23i_cleveland.htm |
As filed with the Securities and Exchange Commission on May 11, 2015
Registration No. 333- 203365
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1/A
(Amendment No. 1)
REGISTRATION STATEMENT
UNDER THE SECURITIES ACT OF 1933
CLEVELAND BIOLABS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 2834 | 20-0077155 | ||
| (State
or other jurisdiction of incorporation or organization) |
(Primary
Standard Industrial Classification Code Number) |
(I.R.S.
Employer Identification No.) |
73 High Street
Buffalo, NY 14203
(716) 849-6810
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Yakov Kogan, Ph.D., MBA
Chief Executive Officer
Cleveland BioLabs, Inc.
73 High Street
Buffalo, New York 14203
(716) 849-6810
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Gregory Sichenzia, Esq. Marcelle Balcombe, Esq. Jeff Cahlon, Esq. Sichenzia Ross Friedman Ference LLP 61 Broadway, 32nd Floor New York, New York 10006 Tel: (212) 930-9700 Fax: (212) 930-9725 |
Michael Nertney. Esq. Ellenoff Grossman & Schole LLP 1345 Avenue of the Americas New York, New York 10105
Tel: (212) 370-1300 Fax: (212) 370-7889
|
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: þ
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non - accelerated filer ☐ (Do not check if a smaller reporting company) | Smaller reporting company þ |
CALCULATION OF REGISTRATION FEE
| Title
of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price (1) | Amount
of Registration Fee | ||||||
| Class A Units (3) consisting of: | ||||||||
| (i) Common Stock, $0.005 par value | ||||||||
| (ii) Warrants to purchase Common Stock (2) | — | — | ||||||
| Class B Units (3) consisting of: | ||||||||
| (i) Series B Preferred Stock | ||||||||
| (ii) Warrants to purchase Common Stock (2) | — | — | ||||||
| Common Stock issuable upon exercise of Warrants to purchase Common Stock | ||||||||
| Common Stock issuable upon conversion of Series B Preferred Stock (2) | — | — | ||||||
| Total | $ | 11,500,000 | $ | 1,336.30 | * | |||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) | No additional registration fee is payable pursuant to Rule 457 under the Securities Act. |
| (3) | Pursuant to Rule 416 under the Securities Act, the securities being registered hereunder include such indeterminate number of additional shares of common stock as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. |
| * | Previously paid. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED MAY 11, 2015 |

$10,000,000 of
Class A Units consisting of Common Stock and Warrants and
Class B Units consisting of Series B Preferred Stock and Warrants
( shares of Common Stock Underlying the Series B Preferred Stock and Warrants)
Cleveland BioLabs, Inc. is offering $10,000,000 of shares of our common stock and warrants to purchase shares of our common stock (and the shares of common stock that are issuable from time to time upon exercise of the warrants). Each share of common stock is being sold together with a warrant to purchase [ ] shares of our common stock at an exercise price of $ per share. The shares of common stock and warrants are immediately separable and will be issued separately in this offering.
We are also offering to those purchasers, whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 9.9% of our outstanding common stock following the consummation of this offering, the opportunity to purchase, in lieu of the shares of our common stock that would result in ownership in excess of 9.9%, Series B Preferred Stock convertible into shares of our common stock. Each share of Series B Preferred Stock will convert into [ ] share of common stock. Each Series B Preferred share is being sold together with the same warrants described above, with each warrant to purchase [ ] shares of our common stock for each share of common stock issuable upon conversion of the Series B Preferred Stock. The Series B Preferred shares and warrants are immediately separable and will be issued separately in this offering.
Our common stock is listed on The NASDAQ Capital Market under the symbol “CBLI”. On May 8, 2015, the last reported sale price of our common stock on The NASDAQ Capital Market was $2.04 per share.
There is no established public trading market for the Series B Preferred Stock or warrants, and we do not expect a market to develop. In addition, we do not intend to apply for a listing of the Series B Preferred Stock or the warrants on any national securities exchange. The Series B Preferred Stock and the warrants will be issued in book-entry form pursuant to a preferred stock agency agreement between us and Continental Stock Transfer & Trust Company, as preferred stock agent, and a warrant agency agreement between us and Continental Stock Transfer & Trust Company, as warrant agent, respectively.
Our business and an investment in our securities involve significant risks. These risks are described under the caption “Risk Factors” beginning on page 7 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Per Series B Preferred Share | Per Warrant | Total | |||||||||||||
| Public offering price | $ | $ | $ | $ | ||||||||||||
| Underwriting discounts and commissions (1) | $ | $ | $ | $ | ||||||||||||
| Proceeds, before expenses, to us | $ | $ | $ | $ | ||||||||||||
| (1) | The underwriter will receive compensation in addition to the underwriting discount. See “Underwriting” on page 92 of this prospectus for a description of the compensation payable to the underwriter. |
The underwriter has the option, exercisable, in whole or in part, for a period of 45 days from the date of this prospectus, to purchase up to (i) additional shares of common stock, and/or (ii) additional warrants to purchase up to additional shares of common stock solely to cover over-allotments, if any, at the price to the public less the underwriting discounts and commissions. The over-allotment option may be used to purchase shares of common stock, or warrants, or any combination thereof, as determined by the underwriter, but such purchases cannot exceed an aggregate of 15% of the number of shares of common stock (on an as-converted basis with respect to any shares of Series B Preferred Stock sold) and warrants sold in the primary offering.
The underwriter expects to deliver the securities against payment in New York, New York on or about _______________, 2015.
Ladenburg Thalmann
The date of this prospectus is , 2015.
You should rely only on the information contained in this prospectus and any related free writing prospectus that we may provide to you in connection with this offering. We have not, and the underwriter has not, authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriter is not, making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus is accurate only as of the date on the front cover of this prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
This summary highlights information contained in other parts of this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in our securities and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. You should read the entire prospectus especially “Risk Factors” and our consolidated financial statements and the related notes included in this prospectus, before deciding to buy our securities. The “Company,” “CBLI”, “we,” “us” and “our” refer to Cleveland BioLabs, Inc. together with its consolidated subsidiary BioLabs 612, LLC and consolidated joint venture, Panacela Labs, Inc.
The Company
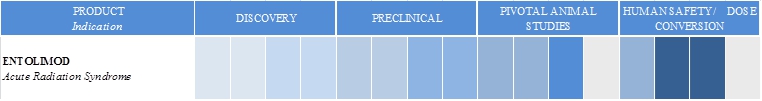
We are an innovative biopharmaceutical company seeking to develop first-in-class pharmaceuticals designed to address diseases with significant unmet medical need. We combine our proven scientific expertise and our depth of knowledge about our products’ mechanisms of action into a passion for developing drugs to save lives. Our programs are focused on the use of novel toll-like receptor agonists to activate the immune system for therapeutic benefit. Our proprietary drug candidates act via unique mechanisms that are designed to kill cancer and protect healthy cells. We conduct business in the United States and the Russian Federation. CBLI and our joint ventures, Panacela Labs, Inc., or Panacela, and Incuron, LLC, or Incuron each have worldwide development and commercialization rights to product candidates in development, subject to certain financial obligations to our current licensors. CBLI’s most advanced product candidate is entolimod, which we are developing as a radiation countermeasure and an immunotherapy for oncology and other indications. Our primary product development programs and their respective development stages are illustrated below:

| (1) | Mobilan is in development by Panacela. |
| (2) | CBL0137 is in development by Incuron. | |
| 1 |
Entolimod is a Toll-like receptor 5, or TLR5, agonist, which we are developing as a radiation countermeasure for prevention of death from Acute Radiation Syndrome, or ARS, and as an oncology drug. We believe that entolimod is the most efficacious radiation countermeasure currently in development. Following is a summary of the clinical development of entolimod to date and regulatory status:
Entolimod is being developed under the U.S. Food & Drug Administration’s, or FDA’s, Animal Efficacy Rule, or the Animal Rule, for the indication of reducing the risk of death following exposure to potentially lethal irradiation occurring as a result of a radiation disaster (see “Government Regulation - Animal Rule”). We have completed two clinical studies designed to evaluate the safety, pharmacokinetics and pharmacodynamics of entolimod in a total of 150 healthy volunteers. We have completed a Good Laboratory Practices, or GLP, randomized, blinded, placebo-controlled, pivotal study designed to evaluate the dose- dependent effect of entolimod on survival and biomarker induction in 179 non-human primates exposed to 7.2 Gy total body irradiation when entolimod or placebo were administered at 25 hours after radiation exposure. We have completed a GLP, randomized, open-label, placebo-controlled, pivotal study designed to evaluate the dose- dependent effect of entolimod on biomarker induction in 160 non-irradiated non-human primates. We met with the FDA in July 2014 to present our human dose-conversion and to discuss our intent to submit a pre-Emergency Use Authorization, or pre-EUA. The FDA confirmed that our existing efficacy and safety data and animal-to- human dose conversion are sufficient to proceed with a pre-EUA submission and agreed to accept a pre-EUA submission for review. We are currently preparing the pre-EUA dossier, which we anticipate filing in the first half of 2015. If the FDA authorizes the application, then Federal agencies are free to procure drug product for stockpiling so that the drug is available to distribute in the event of an emergency, i.e. prior to the drug being formally approved by FDA under a Biologics License Application, or BLA.
In January and April 2015, we announced the receipt of recommendations from the Department of Defense, or DoD, Congressionally Directed Medical Research Programs, or CDMRP in support of DoD funding for two CBLI proposals to support further development of entolimod as a medical radiation countermeasure. These proposals aim to conduct several pivotal animal efficacy studies and a clinical study to support a BLA. The Company’s receipt of these awards is subject to successful negotiations and availability of funds.
Additionally, we completed enrollment in a Phase 1 open-label, dose-escalation trial of entolimod in patients with advanced cancer in the United States and began dosing in a small expansion study in the Russian Federation, which is enrolling additional patients at the highest doses achieved in the US study. Both studies include evaluation of immune cell response to administrations of entolimod. Preliminary evaluations of the completed study in the United States indicate that the tolerability profile in patients with advanced cancer was similar to that observed in the two previously conducted studies in 150 healthy volunteers. Initial assessments of immunological response were consistent with TLR5 activation. Early analyses indicate that stable disease was observed in several patients with heavily pretreated cancers. Complete data for this study will be presented during the Developmental Therapeutics - Immunotherapy poster session at the 2015 annual meeting of the American Society of Clinical Oncology (ASCO) on May 30, 2015 in Chicago, IL.
SA-702 is a new therapeutic approach with entolimod that employs the immunopotentiating properties of the drug together with alum (aluminum salts) as a vaccine adjuvant. In this context, entolimod’s immune activity would be harnessed to enhance the efficacy of vaccines by eliciting a stronger immune response to the vaccine’s particular antigen. Many vaccines require an adjuvant to induce sufficient immune response. It is estimated that about one half of 30 of the most common vaccines approved by FDA contain alum as an adjuvant. Until recently, alum was the only adjuvant approved by FDA, but often alum alone does not allow new vaccines to reach sufficient clinical potency. A shortage of effective and safe adjuvants is a major bottleneck in vaccine development. A newer generation of vaccine boosters combine classic adjuvants mixed with immunomodulators (like entolimod). We have collaborated with academic investigators who have performed preclinical studies that support the adjuvant potential of SA-702 in enhancing vaccine immune and wish to translate these data to clinical studies to document the immunopotentiating effect of the drug.
CBLB612 is a proprietary compound based upon a natural activator of another tissue-specific component of the innate immune system, the TLR2/TLR6 heterodimeric receptor. CBLB612 is a pharmacologically optimized synthetic molecule that structurally mimics naturally occurring lipopeptides of Mycoplasma (a genus of parasitic bacteria) and activates NF-kB pro-survival and immunoregulatory signaling pathways via specific binding to TLR2 on a subset of body tissues and cell types that express this receptor. Preclinical studies have shown that CBLB612 stimulates white blood cell regeneration. More recent research indicates that stimulation of these toll-like receptors may also enhance anti-tumor efficacy. We believe an opportunity may exist for CBLB612 to offer a single-dose alternative to existing hemopoietic growth factors, such as filgrastim (Neupogen™), which comprises a multi-billion-dollar market in support of chemotherapy administration. FIlgrastim modestly shortens the duration of chemotherapy-related neutropenia, but does not improve thrombocytopenia or anemia, and does not provide antitumor efficacy. In October 2014, we initiated a Phase 1, single-center, blind, placebo-controlled, single ascending-dose study in the Russian Federation to evaluate the safety, tolerability, and pharmacodynamic effects of CBLB612 in healthy volunteers. The study was performed under a 139-million-ruble matching funds development contract that we received in July 2012 from The Russian Federation Ministry of Industry and Trade, or MPT. We announced that we had completed dosing in this study in March 2015. A maximum tolerated dose was established and changes in blood counts were observed, including neutrophilia. Induction of a variety of cytokines was also documented. Full results will be reported in 2015. We believe the Phase 1 data support a Phase 2 study in a clinical model of chemotherapy-induced myelosuppression. Plans for this study are already underway and will be supported by the same MPT contract. We licensed CBLB612 to Zhejiang Hisun Pharmaceutical Co., Ltd. for the territories of China, Taiwan, Hong Kong and Macau. We have rest-of-world development and commercialization rights to CBLB612.
| 2 |
Mobilan is the lead product candidate of our consolidated joint venture Panacela. Mobilan is a nanoparticle- formulated recombinant non-replicating adenovirus that directs expression of TLR5 and its agonistic ligand, flagellin. In pre-clinical studies, delivery of Mobilan to tumor cells results in constitutive autocrine TLR5 signaling and strong activation of the innate immune system with subsequent development of adaptive anti-tumor immune responses. An IND was opened in the Russian Federation in March 2015 for a Phase 1 multicenter, randomized, placebo-controlled, single-blinded study evaluating single injections of ascending doses of Mobilan administered directly into the prostate of patients with prostate cancer. This study is being performed under a 149-million-ruble matching funds development contract that Panacela received in October 2013 from MPT. Panacela holds worldwide development and commercialization rights to Mobilan. As of April 30, 2015, we owned 60.47% of Panacela.
CBL0137 is the lead product candidate of our unconsolidated joint venture Incuron. CBL0137 is a small molecule with a multi-targeted mechanism of action that may be broadly useful for the treatment of many different types of cancer. CBL0137 may offer greater efficacy and substantially lower risk for the development of drug resistance than conventional chemotherapeutic agents. CBL0137 inhibits Nuclear Factor kappa-B, or NF- kB, heat shock factor protein-1, or HSF-1, and hypoxia-inducible factor 1-alpha, or HIF1 alpha; these are transcription factors that are important for the viability of many types of tumors. The drug also activates tumor suppressor protein p53 by modulating intracellular localization and activity of chromatin remodeling complex facilitates chromatin transcription, or FACT. CBL0137 has been shown to be efficacious in pre-clinical models of colon, lung, breast, renal, pancreatic, head and neck and prostate cancers; melanoma; glioblastoma; and neuroblastoma. It has also been shown to be efficacious in pre-clinical models of hematological cancers, including lymphoma, leukemia and multiple myeloma.
Incuron is currently enrolling patients with advanced, resistant solid tumors into two Phase 1 studies, one in the Russian Federation evaluating the oral administration of CBL0137 and one in the United States evaluating the intravenous administration of CBL0137. These studies are designed to investigate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of CBL0137. Incuron is conducting these parallel evaluations of oral and intravenous routes of administration and continuous low-dose versus interrupted high-dose schedules to reduce the company’s developmental risk by fully characterizing the clinical pharmacology of CBL0137.
Incuron holds worldwide development and commercialization rights to CBL0137. As of April 30, 2015, we owned 11.74% of Incuron.
Our Partners
In October 2011, we entered into our Panacela joint venture with Open Joint Stock Company “Rusnano” (“Rusnano”) to carry out a complete cycle of development and commercialization in the Russian Federation for the treatment of oncological, infectious or other diseases. We invested $3.0 million in Panacela preferred shares and warrants, and, together with certain third-party owners, assigned and/or provided exclusive licenses, as applicable, to Panacela to provide Panacela with worldwide development and commercialization rights to five preclinical product candidates in exchange for Panacela common shares. Rusnano invested $9.0 million in Panacela preferred shares and warrants. In 2013, Rusnano loaned Panacela $1.5 million through a convertible term loan, or the Panacela Loan, and revised their original investment agreement to remove the predetermined development milestones and timelines for further investment and provide that Rusnano may invest an additional $15.5 million at their option. As of April 30, 2015, we had an ownership stake of 60.47% in Panacela.
In December 2009, we entered into our Incuron joint venture with BioProcess Capital Partners, or BCP, to develop Curaxin compounds for treatment of oncological diseases. According to the terms of the agreement, we transferred rights in the Curaxin molecules to a new joint venture company, Incuron, in which BCP agreed to cause their affiliated fund, BCV, to contribute an aggregate of 549,497,000 Russian rubles (approximately $16.9 million) to support development of the compounds. As of September 30, 2014, Incuron had received all committed funding. On November 25, 2014, we transferred 3.05% of the Company’s participation interest in Incuron to BCV. The transfer of 3.05% of our participation interest was made pursuant to the Participation Agreement dated December 9, 2009, as amended by the First and Third Amendments to Participation Agreement dated April 13, 2010 and June 17, 2014, respectively, that governs the joint ownership of Incuron by the Company and BCV. As described in the Form 8-K filed by the Company on December 2, 2014, as a result of the transfer of 3.05% of our participation interests to BCV, the Company’s participation interest in Incuron decreased to 46.96%, BCV’s participation interest increased to 53.04%. As described in the Form 8-K filed by the Company on May 4, 2015, on April 29, 2015 we entered into and closed an agreement to sell our equity stake in Incuron to Dr. Mikhail Mogutov, Chairman of the Board of Directors of Incuron, LLC and Chairman of the Investment Committee and founder of Bioprocess Capital Ventures. Pursuant to this agreement, we sold 75% of our equity stake in Incuron for approximately $3 million and granted Dr. Mogutov an option to purchase our remaining ownership interest in Incuron for approximately $1 million before the end of 2015. In addition, we have assigned our remaining intellectual property for CBL0137 to Incuron in exchange for a 2% royalty on the future commercialization, licensing or sale of the CBL0137 technology.
| 3 |
Additionally, we leverage close development relationships with Roswell Park Cancer Institute and The Cleveland Clinic. Together, our team of legal entities, financial partners and other collaborators engage in the collective development efforts necessary to advance all of our product candidates towards marketing approval and commercialization.
Corporate Information
We were incorporated in Delaware on June 5, 2003. We conduct operations through several subsidiaries, including our wholly-owned subsidiary, BioLab 612, LLC, our consolidated joint venture Panacela Labs, Inc. and our unconsolidated joint venture, Incuron, LLC.
Our principal executive offices are located at 73 High Street, Buffalo, New York 14203. Our telephone number is (716) 849-6810. Our website address is www.cbiolabs.com. We have included our website address as an inactive textual reference only. The information contained on, or that can be accessed through, our website is not a part of this prospectus.
Our Challenges
Our business and an investment in our securities is subject to numerous risks and uncertainties, including those highlighted in the section entitled “Risk Factors” immediately following this prospectus summary. These risks include, but are not limited to, the following:
| ● | we will require substantial additional financing in order to meet our business objectives. | |
| ● | we have a history of operating losses. We expect to continue to incur losses and may not continue as a going concern. | |
| ● | we may not be able to successfully and timely develop our products. | |
| ● | we may not be able to obtain regulatory approval in a timely manner or at all and the results of future clinical trials and pivotal efficacy studies may not be favorable. | |
| ● | you will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future as we do further financings and transactions. |
| 4 |
The following summary contains basic information about the offering and the securities we are offering and is not intended to be complete. It does not contain all the information that is important to you. For a more complete understanding of the common stock and the warrants, please refer to the sections of this prospectus titled “Description of Capital Stock” and “Description of Securities We Are Offering.”
| Class A Units offered by us | We are offering $10,000,000 of Class A Units and Class B Units (collectively, the “Units”). Each Class A Unit consists of one share of our common stock and a warrant to purchase [ ] shares of our common stock. The Class A Units will not be certificated and the shares of common stock and warrants part of such unit are immediately separable and will be issued separately in this offering. |
| Class B Units offered by us | We are also offering to those purchasers, whose purchase of Class A Units in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 9.9% of our outstanding common stock following the consummation of this offering, the opportunity to purchase, in lieu of Class A Units that would result in ownership in excess of 9.9%, Class B Units. Each Class B Unit consists of [ ] shares of Series B Preferred Stock and [ ] warrants. Each share of Series B Preferred Stock will be convertible into [ ] shares of common stock. The Class B Units and the warrant part of such unit are immediately separable and will be issued separately in this offering. This prospectus also relates to the offering of shares of our common stock issuable upon conversion of the Series B Preferred Stock. |
| Over-allotment option | We have granted the underwriter an option to purchase up to (i) additional shares of common stock, and/or (ii) additional warrants to purchase up to additional shares of common stock. This option is exercisable, in whole or in part, for a period of 45 days from the date of this prospectus. |
| Warrants | Each warrant included in the Class A Units and Class B Units will have an exercise price of $ per share, and will be exercisable upon issuance. The warrants will expire [ ] years from the date of issuance. This prospectus also relates to the offering of shares of common stock issuable upon exercise of the warrants. |
Common stock to be outstanding immediately after this offering |
shares of common stock ( shares of common stock if the underwriter exercises in full the over-allotment option) (on an as-converted basis with respect to any shares of Series B Preferred Stock sold) (1)(2) |
| Use of proceeds | We intend to use the net proceeds from this offering to obtain additional capital to support preparation of a pre-EUA application for entolimod for our defense indication, various other oncology-focused development efforts, as well as for working capital and general corporate purposes. See “Use of Proceeds.” |
| Market for our common stock | Our common stock is listed on the NASDAQ Capital Market under the symbol “CBLI”. |
No market for the Units, Series B Preferred Stock or Warrants |
The Class A Units and Class B Units will not be certificated and the securities part of such units are immediately separable and will be issued separately in this offering. |
| There is no established public trading market for our Series B Preferred Stock or warrants, and we do not intend to apply to list the Series B Preferred Stock or warrants on any securities exchange or automated quotation system |
| (1) | The number of shares of common stock outstanding immediately after the closing of this offering is based on 4,269,176 shares of common stock outstanding as of April 30, 2015 (on an as-converted and exercised basis with respect to the Series B pre-funded warrant and the Series A Convertible Preferred Stock sold in our offering that closed on February 6, 2015), and, as of that date, excludes: |
| ● | 379,307 shares of common stock issuable upon the exercise of outstanding stock options, at a weighted average exercise price of $46.85 per share; | |
| ● | up to 2,281,332 shares of common stock issuable upon the exercise of outstanding warrants, having a weighted average exercise price of $14.49 per share, excluding 40,617 shares of common stock issuable upon the exercise of a warrant held by Rusnano that is only exercisable in the event of a default by Panacela in the repayment of a loan; | |
| ● | 225,000 shares of common stock reserved for future issuance under our 2013 employee stock purchase plan, or the ESPP; | |
| ● | 142,149 shares of common stock reserved for future issuance under our 2006 Equity Incentive Plan, as amended, or the 2006 Plan; and | |
| ● | shares of common stock that may be issued under the warrants to be issued in this offering. |
Unless otherwise stated, outstanding share information throughout this prospectus excludes such outstanding options or warrants to purchase shares of common stock.
| 5 |
The following selected financial data has been derived from our audited financial statements. The information below is not necessarily indicative of the results of future operations and should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and “Risk Factors,” and the financial statements and related notes thereto included in this prospectus, in order to fully understand factors that may affect the comparability of the information presented below:
| Quarter
Ended March 31, | Year Ended December 31, | |||||||||||||||
| (in thousands, except per share data) | 2015 | 2014 | 2013 | 2012 | ||||||||||||
| Consolidated statements of operations data: | ||||||||||||||||
| Government contract or grant revenues | $ | 607 | $ | 3,702 | $ | 8,488 | $ | 3,571 | ||||||||
| Operating expenses (1) | 3,919 | 18,409 | 31,564 | 33,617 | ||||||||||||
| Loss from operations | (3,312 | ) | (14,707 | ) | (23,076 | ) | (30,047 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Change in value of warrant liability | (49 | ) | 2,662 | 2,864 | 7,702 | |||||||||||
| Gain on deconsolidation of Incuron, LLC | - | 14,207 | ||||||||||||||
| Other income (expense) | (338 | ) | (2,126 | ) | 83 | (70 | ) | |||||||||
| Total other income (expense) | (387 | ) | 14,743 | 2,947 | 7,632 | |||||||||||
| Net income (loss) | (3,699 | ) | 35 | (20,129 | ) | (22,415 | ) | |||||||||
| Net loss attributable to noncontrolling interests | 48 | 1,594 | 2,866 | 4,180 | ||||||||||||
| Net income (loss) attributable to Cleveland BioLabs, Inc. | $ | (3,650 | ) | $ | 1,629 | $ | (17,263 | ) | $ | (18,234 | ) | |||||
| Net income (loss) per share, basic and diluted | $ | (1.14 | ) | $ | 0.60 | $ | (7.60 | ) | $ | (9.80 | ) | |||||
| March 31, | December 31, | |||||||||||||||
| (in thousands) | 2015 | 2014 | 2013 | 2012 | ||||||||||||
| Consolidated balance sheet data: | ||||||||||||||||
| Cash and cash equivalents | $ | 4,256 | $ | 3,104 | $ | 10,048 | $ | 25,652 | ||||||||
| Short-term investments | 770 | - | 306 | 2,634 | ||||||||||||
| Total current assets | 5,582 | 3,545 | 11,157 | 29,406 | ||||||||||||
| Total assets | 10,677 | 9,814 | 14,696 | 32,010 | ||||||||||||
| Capital leases (current & noncurrent) | - | 8 | 91 | 169 | ||||||||||||
| Long-term debt (current & noncurrent) | 4,058 | 4,140 | 7,473 | - | ||||||||||||
| Stockholders’ equity (deficit) | (1,407 | ) | 1,786 | 1,581 | 20,486 | |||||||||||
| (1) | Operating expenses in 2015, 2014, 2013 and 2012 included employee stock-based compensation costs of $0.0, $0.5, $1.5 and $2.5 million, net of tax. |
| 6 |
Any investment in our securities involves a high degree of risk. Investors should carefully consider the risks described below and all of the information contained in this prospectus before deciding whether to purchase our common stock. Before you invest you should carefully consider the risks and uncertainties described below and the other information in this prospectus. Our business, operating results and financial condition could be harmed and the value of our securities could go down due to any of these risks, and you could lose all or a part of your investment.
RISKS RELATING TO OUR FINANCIAL POSITION AND NEED FOR ADDITIONAL FINANCING
We will require substantial additional financing in order to meet our business objectives.
Since our inception, most of our resources have been dedicated to the pre-clinical and clinical development of our product candidates. In particular, we are currently conducting multiple clinical trials of our product candidates, each of which will require substantial funds to complete. We believe that we will continue to expend substantial resources for the foreseeable future developing our pre-clinical and clinical product candidates. These expenditures will include costs associated with research and development, conducting pre-clinical and clinical trials, obtaining regulatory approvals and products from third-party manufacturers, as well as marketing and selling any products approved for sale. In addition, other unanticipated costs may arise. Because the outcome of our planned and anticipated clinical trials is highly uncertain, we cannot reasonably estimate the actual amounts of capital necessary to successfully complete the development and commercialization of our product candidates.
As of March 31, 2015, our cash, cash equivalents and short-term investments amounted to $5.0 million. We believe that our existing cash, cash equivalents, and marketable securities (not including proceeds from this offering) will allow us to fund our operating plan into June 2015.
Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amounts of our total capital requirements. Our future capital requirements depend on many factors, including:
| ● | the number and characteristics of the product candidates we pursue; | |
| ● | the scope, progress, results and costs of researching and developing our product candidates, and conducting pre-clinical and clinical trials; | |
| ● | the timing of, and the costs involved in, obtaining regulatory approvals for our product candidates; | |
| ● | the cost of commercialization activities for any of our product candidates that are approved for sale, including marketing, sales and distribution costs; | |
| ● | the cost of manufacturing our product candidates and any products we successfully commercialize; | |
| ● | our ability to establish and maintain strategic partnerships, licensing or other arrangements and the financial terms of such agreements; | |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation; | |
| ● | whether we realize the full amount of any projected cost savings associated with our strategic restructuring; | |
| ● | the occurrence of a breach or event of default under our loan agreement with Hercules or under any other agreements with third parties; | |
| ● | the success of any pre-EUA submission we make with the FDA; and | |
| ● | the timing, receipt and amount of sales of, or royalties on, our future products, if any. |
| 7 |
When our available cash and cash equivalents become insufficient to satisfy our liquidity requirements, or if and when we identify additional opportunities to do so, we will likely seek to sell additional equity or debt securities or obtain additional credit facilities. In addition, the terms of our outstanding share of Series A Preferred Stock restrict our ability to obtain additional credit facilities (see “Description of Capital Stock”). The sale of additional equity or convertible debt securities may result in additional dilution to our stockholders. If we raise additional funds through the issuance of debt securities or preferred stock or through additional credit facilities, these securities and/or the loans under credit facilities could provide for rights senior to those of our common stock and could contain covenants that would restrict our operations. Furthermore, any funds raised through collaboration and licensing arrangements with third parties may require us to relinquish valuable rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us. In any such event, our business prospects, financial condition and results of operations could be materially adversely affected.
We may require additional capital beyond our currently forecasted amounts and additional funds may not be available when we need them, on terms that are acceptable to us, or at all. In particular, the decline in the market price of our common stock could make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem appropriate. In addition, the variable rate clause in our stock purchase agreement from our February 2015 equity transaction prohibits certain types of capital raising activities until April 14, 2016 and pledge of assets in our loan and security agreement with Hercules Technology II, L.P., or Hercules, may inhibit our ability to attract future investors and/or lenders. Additionally, our corporate structure, including the ownership of several of our product candidates in our joint ventures, may deter third parties from entering into collaboration and licensing arrangements with us. If we fail to raise sufficient additional financing, on terms and dates acceptable to us, we may not be able to continue our operations and the development of our product candidates, our patent licenses may be terminated, and we may be required to reduce staff, reduce or eliminate research and development, slow the development of our product candidates, outsource or eliminate several business functions or shut down operations.
The report of our independent registered public accounting firm expresses substantial doubt about the Company’s ability to continue as a going concern.
Our auditors, Meaden & Moore, LLP, have indicated in their report on the Company’s financial statements for the fiscal year ended December 31, 2014, that conditions exist that raise substantial doubt about our ability to continue as a going concern due to our recurring losses and substantial decline in our working capital. This “going concern” opinion could impair our ability to finance our operations through the sale of equity, incurring debt or other financing alternatives. Our ability to continue as a going concern will depend upon the availability and terms of future funding and our ability to limit our expenses. If we are unable to achieve these goals, our business would be jeopardized and the Company may not be able to continue. If we ceased operations, it is likely that all of our investors would lose their investment.
We have a history of operating losses. We expect to continue to incur losses and may not continue as a going concern.
We have incurred significant losses to date. We have incurred net losses of approximately $3.7 million and $138.3 million for the three months ended March 31, 2015 and since inception, respectively. We expect significant losses to continue for the next few years as we spend substantial sums on the continued research and development of our proprietary product candidates, and there is no certainty that we will ever become profitable as a result of these expenditures. As a result of losses that will continue throughout our development stage, we may exhaust our financial resources and be unable to complete the development of our product candidates.
Our ability to become profitable depends primarily on the following factors:
| ● | our ability to obtain adequate sources of continued financing; | |
| ● | our ability to obtain approval for, and if approved, to successfully commercialize our product candidates; | |
| ● | our ability to successfully enter into license, development or other partnership agreements with third-parties for the development and/or commercialization of one or more of our product candidates; |
| 8 |
| ● | our research and development, or R&D, efforts, including the timing and cost of clinical trials; and | |
| ● | our ability to enter into favorable alliances with third-parties who can provide substantial capabilities in clinical development, manufacturing, regulatory affairs, sales, marketing and distribution. |
Even if we successfully develop and market our product candidates, we may not generate sufficient or sustainable revenue to achieve or sustain profitability.
We may be unable to service our existing debt due to lack of cash flow, which could lead to default.
In September 2013, we entered into a loan and security agreement with Hercules under which we borrowed $6.0 million. The current interest rate is 10.45%, with the initial 12 months of the facility requiring interest only payments and the following 30 months requiring interest and principal payments. The loan matures on January 1, 2017. In June 2014, we made a $4.0 million principal pre-payment, and we are currently paying approximately $76,000 per month for interest and principal, with a final principal and interest payment of approximately $305,000 and an end-of-term fee of $550,000 due in January 2017. As of December 31, 2014, the remaining principal and end-of-term obligations owed to Hercules was approximately $2.4 million. We granted Hercules a first priority security interest in substantially all of our assets, with the exception of (i) our intellectual property, where the security interest is limited to proceeds of intellectual property, and (ii) our equity interest in Incuron.
If we do not make the required payments when due, either at maturity, or at applicable installment payment dates, or if we breach the agreement, default under the agreement by having a material adverse event happen to the business of the Company or become insolvent, Hercules could elect to declare all amounts outstanding together with all accrued and unpaid interest and penalties, to be immediately due and payable. In order to continue our planned operations and satisfy our debt obligations with Hercules, we will need to raise additional capital in the future. Additional capital may not be available on terms acceptable to us, or at all. Even if we were able to repay the full amount in cash, any such repayment could leave us with little or no working capital for our business. If we are unable to repay these amounts, Hercules will have a first claim on our assets pledged under the loan agreement. If Hercules should attempt to foreclose on the collateral, there may not be any assets remaining for distribution to shareholders after repayment in full of such secured indebtedness. Any default under the loan agreement and resulting foreclosure would have a material adverse effect on our financial condition and our ability to continue our operations.
Additionally, in September 2013, our majority-owned joint venture Panacela entered into a $1.5 million Convertible Loan Agreement with Rusnano, or the Rusnano Loan, and is required to pay all unpaid principal and interest under the loan in September 2015. The loan may be converted into shares of Panacela stock at any time at Rusnano’s option. In the event Panacela defaults on the loan and such default is not cured, Rusnano shall have the right to exercise a warrant to purchase shares of Cleveland BioLabs common stock equal to 69.2% of the outstanding amount remaining unpaid under the Rusnano Loan at the time of exercise, divided by the exercise price of $33.88 per share. As of March 31, 2015, that would amount to 40,073 shares.
Our ability to use our net operating loss carryforwards may be limited.
As of December 31, 2014, we had federal net operating loss carryforwards, or NOLs, of $120.9 million to offset future taxable income, which begin to expire if not utilized by 2023. Under the provisions of the Internal Revenue Code, substantial changes in our ownership, in certain circumstances, will limit the amount of NOLs that can be utilized annually in the future to offset taxable income. In particular, section 382 of the Internal Revenue Code imposed limitations on a company’s ability to use NOLs if a company experiences a more than 50% ownership change over a three-year period. As we have indicated, we believe that our funds will be sufficient to fund our projected operating requirements into June 2015. As such, we will need to secure additional financing and it is possible that as a result of such additional financing our ability to use our NOLs in future years may be limited. If we are limited in our ability to use our NOLs in future years in which we have taxable income, we will pay more taxes than if we were able to utilize our NOLs fully. A full valuation allowance has been recorded against our deferred tax assets, including the net operating loss carryforwards, as we believe it is more likely than not we will be unable to realize the benefit of these assets.
| 9 |
RISKS RELATED TO PRODUCT DEVELOPMENT
We may not be able to successfully and timely develop our products.
Our product candidates range from ones currently in the research stage to ones currently in the clinical stage of development and all require further testing to determine their technical and commercial viability. Our success will depend on our ability to achieve scientific, clinical and technological advances and to translate such advances into reliable, commercially competitive products in a timely manner. In addition, the success of our subsidiaries and joint ventures will depend on their ability to meet developmental milestones in a timely manner or to fulfill certain other development requirements under contractual agreements, which are pre-requisites to their receipt of additional funding from their non-controlling interest holders or the government agency funding their government contracts. Products that we may develop are not likely to be commercially available for several years. The proposed development schedules for our products may be affected by a variety of factors, including, among others, technological difficulties, proprietary technology of others, the government approval process, the availability of funds, disagreements with the financial partners in our joint ventures, and changes in government regulation, many of which will not be within our control. Any delay in the development, introduction or marketing of our products could result either in such products being marketed at a time when their cost and performance characteristics would not be competitive in the marketplace or in the shortening of their commercial lives. In light of the long-term nature of our projects and the unproven technology involved, we may not be able to complete successfully the development or marketing of any products.
We may fail to develop and commercialize some or all of our products successfully or in a timely manner because:
| ● | pre-clinical or clinical study results may show the product to be less effective than desired (e.g., a study may fail to meet its primary objectives) or to have harmful or problematic side effects; | |
| ● | we fail to receive the necessary regulatory approvals or there may be a delay in receiving such approvals. Among other things, such delays may be caused by slow enrollment in clinical studies, length of time to achieve study endpoints, additional time requirements for data analysis or pre-EUA, NDA or BLA preparation, discussions with the FDA, an FDA request for additional pre-clinical or clinical data or unexpected safety or manufacturing issues; | |
| ● | we fail to receive funding necessary for the development of one or more of our products; | |
| ● | they fail to conform to a changing standard of care for the diseases they seek to treat; | |
| ● | they are less effective or more expensive than current or alternative treatment methods; | |
| ● | of manufacturing costs, pricing or reimbursement issues, or other factors that make the product not economically feasible; | |
| ● | one or more of our financial partners in our joint ventures and us do not agree on the development strategy of our products; | |
| ● | proprietary rights of others and their competing products and technologies may prevent our product from being commercialized; or | |
| ● | our collaborative relationships with third parties could cause us to expend significant resources and incur substantial business risk with no assurance of financial return. |
We anticipate substantial reliance upon strategic collaborations for marketing and commercialization of our product candidates and we may rely even more on strategic collaborations for R&D of our product candidates. Our business depends on our ability to sell drugs to both government agencies and to the general pharmaceutical market. Offering entolimod for its biodefense indication use to government agencies may require us to develop new sales, marketing or distribution capabilities beyond those already existing in the Company and we may not be successful in selling entolimod for its biodefense indication use in the United States or in foreign countries despite our efforts. Selling oncology drugs will require a more significant infrastructure. We plan to sell oncology drugs through strategic partnerships with pharmaceutical companies. If we are unable to establish or manage such strategic collaborations on terms favorable to us in the future, our revenue and drug development may be limited. To date, we have not entered into any strategic collaboration with a third party capable of providing these services and we can make no guarantee that we will be able to enter into a strategic collaboration in the future. In addition, we have not yet marketed or sold any of our product candidates or entered into successful collaborations for these services in order to ultimately commercialize our product candidates. We also rely on third-party collaborations with our manufacturers. Manufacturers producing our product candidates must follow cGMP regulations enforced by the FDA and foreign equivalents.
| 10 |
Establishing strategic collaborations is difficult and time-consuming. Our discussion with potential collaborators may not lead to the establishment of collaborations on favorable terms, if at all. Potential collaborators may reject collaborations based upon their assessment of our financial, regulatory or intellectual property position. Even if we successfully establish new collaborations, these relationships may never result in the successful development or commercialization of our product candidates or the generation of sales revenue. In addition, to the extent that we enter into collaborative arrangements, our drug revenues are likely to be lower than if we directly marketed and sold any drugs that we may develop.
We will not be able to commercialize our product candidates if our pre-clinical development efforts are not successful, our clinical trials do not demonstrate safety or our clinical trials or pivotal animal studies do not demonstrate efficacy.
Before obtaining required regulatory approvals for the commercial sale of any of our product candidates, we must conduct extensive pre-clinical and clinical studies to demonstrate that our product candidates are safe and clinical or pivotal animal trials to demonstrate that our product candidates are efficacious. Pre-clinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. Success in pre-clinical testing and early clinical trials does not ensure that later clinical trials or animal efficacy studies will be successful and interim results of a clinical trial or animal efficacy study do not necessarily predict final results. In addition, we must outsource our clinical trials and our animal studies required to obtain regulatory approval of our products. We are not certain that we will successfully or promptly finalize agreements for the conduct of these studies. Delay in finalizing such agreements would delay the commencement of our pre-clinical and clinical studies, such as animal efficacy studies for entolimod’s biodefense indication and clinical trials of entolimod, CBLB612, Mobilan and CBL0137 for oncology indications. In addition, we are seeking final FDA agreement on the scope and design of our pivotal animal efficacy and human safety program for an entolimod biodefense BLA. Delay in agreement with the FDA on this program will delay conduct of the pivotal animal efficacy and human safety studies.
Agreements with contract research organizations, or CROs, and study investigators, for clinical or animal testing and with other third parties for data management services place substantial responsibilities on these parties, which could result in delays in, or termination of, our clinical trials if these parties fail to perform as expected. For example, if any of our clinical trial sites fail to comply with Good Clinical Practices or our pivotal animal studies fail to comply with Good Laboratory Practices we may be unable to use the data generated at those sites. In these studies, if contracted CROs or other third parties do not carry out their contractual duties or obligations or fail to meet expected deadlines, or if the quality or accuracy of the data they obtain is compromised due to their failure to adhere to our protocols or for other reasons, our clinical or animal studies may be extended, delayed or terminated, and we may be unable to obtain regulatory approval for or successfully commercialize our product candidates.
Our clinical trial operations will be subject to regulatory inspections at any time. If regulatory inspectors conclude that we or our clinical trial sites are not in compliance with applicable regulatory requirements for conducting clinical trials, we or they may receive warning letters or other correspondence detailing deficiencies and we will be required to implement corrective actions. If regulatory agencies deem our responses to be inadequate, or are dissatisfied with the corrective actions that we or our clinical trial sites have implemented, our clinical trials may be temporarily or permanently discontinued, we may be fined, we or our investigators may be the subject of an enforcement action, the government may refuse to approve our marketing applications or allow us to manufacture or market our products or we may be criminally prosecuted.
In addition, a failure of one or more of our clinical trials or animal studies can occur at any stage of testing and such failure could have a material adverse effect on our ability to generate revenue and could require us to reduce the scope of or discontinue our operations. We may experience numerous unforeseen events during, or as a result of, pre-clinical testing and the clinical trial or animal study process that could delay or prevent our ability to receive regulatory approval or commercialize our product candidates, including:
| ● | regulators or IRBs may not authorize us to commence a clinical trial, conduct a clinical trial at a prospective trial site or continue a clinical trial following amendment of a clinical trial protocol or an IACUC may not authorize us to commence an animal study at a prospective study site; |
| 11 |
| ● | we may decide, or regulators may require us, to conduct additional pre-clinical or clinical studies, or we may abandon projects that we expect to be promising, if our pre-clinical tests, clinical trials or animal efficacy studies produce negative or inconclusive results; | |
| ● | we may have to suspend or terminate our clinical trials if the participants are being exposed to unacceptable safety risks; | |
| ● | regulators or IRBs may require that we hold, suspend or terminate clinical development for various reasons, including noncompliance with regulatory requirements or if it is believed that the clinical trials present an unacceptable safety risk to the patients enrolled in our clinical trials; | |
| ● | the cost of our clinical trials or animal studies could escalate and become cost prohibitive; | |
| ● | any regulatory approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the product not commercially viable; | |
| ● | we may not be successful in recruiting a sufficient number of qualifying subjects for our clinical trials or certain animals used in our animal studies or facilities conducting our studies may not be available at the time that we plan to initiate a study; | |
| ● | the effects of our product candidates may not be the desired effects, may include undesirable side effects, or the product candidates may have other unexpected characteristics; and | |
| ● | our collaborators that conduct our clinical or pivotal animal studies could go out of business and not be available for FDA inspection when we submit our product for approval. |
Even if we or our collaborators complete our animal studies and clinical trials and receive regulatory approval, it is possible that a product may be found to be ineffective or unsafe due to conditions or facts that arise after development has been completed and regulatory approvals have been obtained. In this event, we may be required to withdraw such product from the market. To the extent that our success will depend on any regulatory approvals from government authorities outside of the United States that perform roles similar to that of the FDA, uncertainties similar to those stated above will also exist.
Our joint ventures have significant non-controlling interest holders and, as such, are not operated solely for our benefit.
As of the date of this filing, we owned 11.74% of the equity interests in Incuron and 60.47% of the equity interests in Panacela. These entities have significant non-controlling interest holders, including funds regulated by the Russian Federation government. As such, we share ownership and management of these entities with one or more parties who may not have the same goals, strategies, priorities or resources as we do.
In each of these entities, both we and our co-owners have certain rights. Each entity provides the right to each party to designate certain of the board members and certain decisions in respect of these entities may not be made without a supermajority vote of the equity holders or the consent of all of the equity holders. The right to transfer ownership interests in these entities is restricted by provisions such as rights of first refusal and tag along and drag along rights. In addition, the use of funds and other matters are subject to monitoring and oversight by both groups of equity holders. Furthermore, we are required to pay more attention to our relationship with our co-owners as well as with the entitites, and if a co-owner changes, our relationship may be materially adversely affected. These various restrictions may lead to additional organizational formalities as well as time-consuming procedures for sharing information and making decisions. In addition, the benefits from a successful joint venture are shared among the co-owners, so that we would not receive all the benefits from our successful joint ventures.
Panacela is in need of additional financial resources. In addition, as Panacela has not received additional funding since their loan from Rusnano in late 2013 and grant funding under their MPT contract, Panacela has not been able to pay certain of their obligations as they become due and may be unable to continue operations. Management is pursuing sources of additional financing. If Panacela does not receive additional financing and is unable to continue operations, it may cause us to experience a material adverse effect on our business, financial condition and results of operations.
| 12 |
If parties on whom we rely to manufacture our product candidates do not manufacture them in satisfactory quality, in a timely manner, in sufficient quantities or at an acceptable cost, clinical development and commercialization of our product candidates could be delayed.
We do not own or operate manufacturing facilities. Consequently, we rely on third parties as sole suppliers of our product candidates. We do not expect to establish our own manufacturing facilities and we will continue to rely on third-party manufacturers to produce supplies for pre-clinical, clinical and pivotal animal studies and for commercial quantities of any products or product candidates that we market or may supply to our collaborators. We also rely on third parties as sole providers of certain testing of our products. Our dependence on third parties for the manufacture and testing of our product candidates may adversely affect our ability to develop and commercialize any product candidates on a timely and competitive basis.
To date, our product candidates have only been manufactured in quantities sufficient for pre-clinical studies and initial clinical trials. We rely on a single collaborator for production of each of our product candidates. For a variety of reasons, dependence on any single manufacturer may adversely affect our ability to develop and commercialize our product candidates in a timely and competitive basis. In addition, our current contractual arrangements alone may not be sufficient to guarantee that we will be able to procure the needed supplies as we complete clinical development and/or enter commercialization.
Additionally, in connection with our application for commercial approvals and if any product candidate is approved by the FDA or other regulatory agencies for commercial sale, we will need to procure commercial quantities of the product candidate from qualified third-party manufacturers. We may not be able to contract for increased manufacturing capacity for any of our product candidates in a timely or economic manner or at all. A significant scale-up in manufacturing may require additional validation studies and commensurate financial investments by the contract manufacturers. If we are unable to successfully increase the manufacturing capacity for a product candidate, the regulatory approval or commercial launch of that product candidate may be delayed or there may be a shortage of supply, which could limit our sales and could initiate regulatory intervention to minimize the public health risk.
Other risks associated with our reliance on contract manufacturers include the following:
| ● | contract manufacturers may encounter difficulties in achieving volume production, quality control and quality assurance and also may experience shortages in qualified personnel and obtaining active ingredients for our product candidates; | |
| ● | if, for any circumstance, we are required to change manufacturers, we could be faced with significant monetary and lost opportunity costs with switching manufacturers. Furthermore, such change may take a significant amount of time. The FDA and foreign regulatory agencies must approve these manufacturers in advance. This requires prior approval of regulatory submissions as well as successful completion of pre-approval inspections to ensure compliance with FDA and foreign regulations and standards; | |
| ● | contract manufacturers are subject to ongoing periodic, unannounced inspection by the FDA and state and foreign agencies or their designees to ensure strict compliance with cGMP and other governmental regulations and corresponding foreign standards. We do not have control over compliance by our contract manufacturers with these regulations and standards. Our contract manufacturers may not be able to comply with cGMP and other FDA requirements or other regulatory requirements outside the United States. Failure of contract manufacturers to comply with applicable regulations could result in delays, suspensions or withdrawal of approvals, seizures or recalls of product candidates and operating restrictions, any of which could significantly and adversely affect our business; and | |
| ● | contract manufacturers may breach the manufacturing agreements that we have with them because of factors beyond our control or may terminate or fail to renew a manufacturing agreement based on their own business priorities at a time that is costly or inconvenient to us. |
Changes to the manufacturing process during the conduct of clinical trials or after marketing approval also require regulatory submissions and the demonstration to the FDA or other regulatory authorities that the product manufactured under the new conditions complies with cGMP requirements. These requirements especially apply to moving manufacturing functions to another facility. In each phase of investigation, sufficient information about changes in the manufacturing process must be submitted to the regulatory authorities and may require prior approval before implementation with the potential of substantial delay or the inability to implement the requested changes.
| 13 |
RISKS RELATING TO REGULATORY APPROVAL
We may not be able to obtain regulatory approval in a timely manner or at all and the results of future clinical trials and pivotal efficacy studies may not be favorable.
The testing, marketing and manufacturing of any product for use in the United States will require approval from the FDA. We cannot predict with any certainty the amount of time necessary to obtain FDA approval and whether any such approval will ultimately be granted. Obtaining approval for products requires testing in animals and human subjects of substances whose effects on humans are not fully understood or documented. Pre-clinical studies, animal efficacy studies or clinical trials may reveal that one or more products are ineffective or unsafe, in which event, further development of such products could be seriously delayed, terminated or rendered more expensive.
In addition, we expect to rely on an FDA regulation known as the “Animal Rule” to obtain approval for entolimod’s biodefense indication. The Animal Rule permits the use of animal efficacy studies together with human clinical safety trials to support an application for marketing approval of products when human efficacy studies are neither ethical nor feasible. These regulations have limited prior use and we have limited experience in the application of these rules to the product candidates that we are developing. Additionally, we may submit an application with the FDA for pre-EUA, so that entolimod may be used in an emergency situation. If and when we provide the FDA with the data to support a pre-EUA for entolimod’s biodefense indication we cannot guarantee that the FDA will review the data in a timely manner, or that the FDA will accept the data when reviewed. The FDA may decide that our data are insufficient for pre-EUA or BLA approval and require additional pre-clinical, clinical or other studies, refuse to approve our products, or place restrictions on our ability to commercialize those products. If we are not successful in completing the development, licensure and commercialization of entolimod for its biodefense indication, or if we are significantly delayed in doing so, our business will be materially harmed.
The receipt of FDA approval may be delayed for reasons other than the results of pre-clinical studies and clinical trials. For example, in 2011, the IND application for entolimod’s biodefense indication was transferred within the FDA from the Division of Biologic Oncology Products, or DBOP, to the Division of Medical Imaging Products, or DMIP. As a result of this transfer, we requested and participated in nine meetings with DMIP during 2011-2014 to review the product mechanisms of action, safety profile and preliminary estimation of an effective human dose. In 2013, DMIP has agreed on the scope and design of the proposed pivotal animal efficacy program and has acknowledged that specific cytokines do play an important role in entolimod’s mechanism of action and, as such, can be used as biomarkers for animal-to-human dose-conversion. In order to maintain a competitive edge following the March 2015 approval of Neupogen for a related radiation countermeasure indication, we plan to modify the remaining entolimod BLA efficacy program. Therefore, we will return to FDA to reach an agreement on the elements of the design of our remaining clinical studies for entolimod. There can be no guarantee that we will reach a satisfactory agreement in a timely manner, or at all, or that DMIP will not request any additional information related to our pre-clinical or clinical programs.
Delays in obtaining FDA or any other necessary regulatory approvals of any proposed product or the failure to receive such approvals would have an adverse effect on our ability to develop such product, the product’s potential commercial success and/or on our business, prospects, financial condition and results of operations.
Failure to obtain regulatory approval in international jurisdictions could prevent us from marketing our products abroad.
We intend to market our product candidates, including specifically the product candidates being developed by our subsidiaries and joint ventures, in the United States, Russia and other countries and regulatory jurisdictions. In order to market our product candidates in the United States, Russia and other jurisdictions, we must obtain separate regulatory approvals in each of these countries and territories. The procedures and requirements for obtaining marketing approval vary among countries and regulatory jurisdictions and may involve additional clinical trials or other tests. In addition, we do not have in-house experience and expertise regarding the procedures and requirements for filing for and obtaining marketing approval for drugs in countries outside of the United States, Europe and Japan and may need to engage and rely upon expertise of third parties when we file for marketing approval in countries outside of the United States, Europe and Japan. Also, the time required to obtain approval in markets outside of the United States may differ from that required to obtain FDA approval, while still including all of the risks associated with obtaining FDA approval. We may not be able to obtain all of the desirable or necessary regulatory approvals on a timely basis, if at all. Approval by a regulatory authority in a particular country or regulatory jurisdiction, such as the FDA in the United States or the Roszdravnadzor in Russia, does not ensure approval by a regulatory authority in another country.
| 14 |
We may not be able to file for regulatory approvals and may not receive necessary approvals to commercialize our product candidates in any or all of the countries or regulatory jurisdictions in which we desire to market our product candidates. At this time, other countries do not have an equivalent to the Animal Rule and, as a result, such countries do not have established criteria for review and approval for this type of product outside their normal review process. Specifically, because such other countries do not have an equivalent to the Animal Rule, we may not be able to file for or receive regulatory approvals for entolimod’s biodefense indication outside the United States based on our animal efficacy and human safety data.
The Fast Track designation for entolimod may not actually lead to a faster development or regulatory review or approval process.
We have obtained a “Fast Track” designation from the FDA for entolimod’s biodefense indication. However, we may not experience a faster development process, review or approval compared to conventional FDA procedures. The FDA may withdraw our Fast Track designation if the FDA believes that the designation is no longer supported by data from our clinical or pivotal development program. Our Fast Track designation does not guarantee that we will qualify for or be able to take advantage of the FDA’s expedited review procedures or that any application that we may submit to the FDA for regulatory approval will be accepted for filing or ultimately approved.
Any pre-EUA submission we make to the FDA may not be successful and, even if such submission is successful, it may not accelerate BLA approval of entolimod or result in any purchase by the U.S. government for this product.
In July 2014, we met with the FDA regarding human dose-conversion of entolimod and based on the results of that meeting, we plan to submit a pre-EUA dossier in the first half of 2015 in order to inform and expedite the FDA’s issuance of an EUA, should one become necessary in the event of an emergency. The FDA does not have review deadlines with respect to pre-EUA submissions and, therefore, the timing of any approval of a pre-EUA submission is uncertain. If we submit a pre-EUA, the FDA may decide not to accept the data or may decide that our data are insufficient for pre-EUA. The FDA may require additional pre-clinical, clinical or other studies, refuse to approve our products, or place restrictions on our ability to commercialize those products. Additionally, an authorization of our pre-EUA submission will not guarantee, and may not accelerate, BLA approval of entolimod as a radiation countermeasure. Further, even if our pre-EUA submission is authorized, there is no guarantee that such authorization will lead to procurement by the United States or other governments or any additional development funding as it is possible that the United States or other government may not be interested in our product or our proposed terms of sale for any number of reasons including, but not limited to, lack of available funding, potential lack of government co-sponsorship of our pre-EUA, perceptions about the safety and effectiveness of entolimod, the storage requirements for entolimod or one of our competitors receiving pre-EUA authorization for their product. If we are not successful in partnering entolimod or completing the development, licensure and commercialization of entolimod for its biodefense indication use, or if we are significantly delayed in doing so, our business may be materially harmed.
Even if our drug candidates obtain regulatory approval, we will be subject to on-going government regulation.
Even if our drug candidates obtain regulatory approval, our products will be subject to continuing regulation by the FDA, including record keeping requirements, submitting periodic reports to the FDA, reporting of any adverse experiences with the product and complying with Risk Evaluation and Mitigation Strategies and drug sampling and distribution requirements. In addition, updated safety and efficacy information must be maintained and provided to the FDA. We or our collaborative partners, if any, must comply with requirements concerning advertising and promotional labeling, including the prohibition against promoting non-FDA approved or “off-label” indications or products. Failure to comply with these requirements could result in significant enforcement action by the FDA, including warning letters, orders to pull the promotional materials and substantial fines.
After FDA approval of a product, the discovery of problems with a product or its class, or the failure to comply with requirements may result in restrictions on a product, manufacturer or holder of an approved marketing application. These include withdrawal or recall of the product from the market or other voluntary or FDA-initiated action that could delay or prevent further marketing. Newly discovered or developed safety or effectiveness data, including from other products in a therapeutic class, may require changes to a product’s approved labeling, including the addition of new warnings and contraindications. Also, the FDA requires post-market clinical testing of products approved under the Animal Rule at the time of a declared emergency and may require post-market clinical testing of other products. They may also require surveillance to monitor the product’s safety or efficacy to evaluate long-term effects. It is also possible that rare but serious adverse events not seen in our drug candidates may be identified after marketing approval. This could result in withdrawal of our product from the market.
| 15 |
Compliance with post-marketing regulations may be time-consuming and costly and could delay or prevent us from generating revenue from the commercialization of our drug candidates.
If physicians and patients do not accept and use our drugs, we will not achieve sufficient product revenues and our business will suffer.
Even if we gain marketing approval of our drug candidates, government purchasers, physicians and/or patients may not accept and use them. Acceptance and use of these products may depend on a number of factors including:
| ● | perceptions by members of the government healthcare community, including physicians, about the safety and effectiveness of our drugs; | |
| ● | published studies demonstrating the safety and effectiveness of our drugs; | |
| ● | adequate reimbursement for our products from payors; and | |
| ● | effectiveness of marketing and distribution efforts by us and our licensees and distributors, if any. |
The failure of our drugs, if approved for marketing, to gain acceptance in the market would harm our business and could require us to seek additional financing.
RISKS RELATED TO OUR DEPENDENCE ON U.S. AND FOREIGN GOVERNMENT CONTRACTS AND GRANTS
If we are unable to procure additional government funding, we may not be able to fund future R&D and implement technological improvements, which would materially harm our financial conditions and operating results.
In the three months ended March 31, 2015, we received none of our revenues from government contract and grant development work in connection with grants from the DoD and 58.2% of our revenues from government contract development work in the Russian Federation. In 2014 and 2013, we received 0.6% and 26.8% of our revenues from U.S. government contract and grant development work, and 95.2% and 73.2% of our revenues from Russian government contract development work, respectively.
These revenues have funded some of our personnel and other R&D and general and administrative, or G&A, costs and expenses. It is possible that we may not choose to apply for or, if we do apply, be able to procure new grants and contracts that provide sufficient funding, or any funding at all. If we do submit proposals for new grants or contracts, the review of such proposals may take significant time. In addition, in the event of a positive review of one or more of our proposals, it may take significant time from the time we receive the positive review to the finalization of a new contract or grant. Additionally, a positive review of a proposal in no way indicates that we will ultimately receive a grant or contract award. Contract and grant awards are subject to a significant amount of uncertainty, including, but not limited to, successful negotiation and availability of funds. In addition, in our experience, contracts from Russian government entities require matching funds and posting of performance guarantees. Therefore, we expect that our acceptance of new contracts or grants from Russian government entities will also be subject to our ability to provide matching funds and to post performance guarantees.
As an example of the uncertainty of U.S. government contracting, in January 2014, we announced that the Biomedical Advanced Research and Development Authority, or BARDA, had terminated negotiations related to our proposal for further development of entolimod as a medical radiation countermeasure, noting that all such negotiations are subject to the availability of funds. In addition, we announced in January 2015 that we had received notice that our proposal application to support further development of entolimod as a medical radiation countermeasure has been recommended for funding subject to negotiations by the DoD. Additionally, in April 2015, we announced that we had received notice that another of our proposal applications to support further development of entolimod as a medical radiation countermeasure was recommended for funding subject to negotiations by the DoD, office of CDMRP. That proposal application aims to conduct an additional clinical study to support submission of a BLA. There is no guarantee that either of these recommendations will quickly or ever lead to the funding by DoD of the related proposals. The Company’s receipt of these awards is subject to successful negotiations and availability of funds. Additionally, with regard to our current Russian contracts, in each instance where we have been successful in receiving a contract, the contracts have been subject to matching funds and we have had to post performance guarantees, which have restricted our ability to use funds previously classified as operating funds.
| 16 |
If we are unable to obtain sufficient grants and contracts on a timely basis or if our current grants or contracts are terminated our ability to fund future R&D would be diminished, which would negatively impact our ability to compete in our industry and could materially and adversely affect our business, financial condition and results of operations.
Our future business may be harmed as a result of the foreign and U.S. government contracting process as it involves risks not present in the commercial marketplace.
We expect that a significant portion of the business that we will seek in the near future will be under government contracts or subcontracts, both U.S. and foreign, which may be awarded through competitive bidding. For example, as described above, we are seeking funding from the DoD to support further development of entolimod. Additionally, in the Russian federation we may seek additional funding from the Skolkovo Foundation or MPT. Competitive bidding for government contracts presents a number of risks that are not typically present in the commercial contracting process, which may include:
| ● | the need to devote substantial time and attention of management and key employees to the preparation of bids and proposals for contracts that may not be awarded to us; | |
| ● | the need to accurately estimate the resources and cost structure that will be required to perform any contract that we might be awarded; | |
| ● | the risk that the government will issue a request for proposal to which we would not be eligible to respond; | |
| ● | the risk that third parties may submit protests to our responses to requests for proposal that could result in delays or withdrawals of those requests for proposal; | |
| ● | the expenses that we might incur and the delays that we might suffer if our competitors protest or challenge contract awards made to us pursuant to competitive bidding and the risk that any such protest or challenge could result in the resubmission of bids based on modified specifications, or in termination, reduction or modification of the awarded contract; and | |
| ● | the risk that review of our proposal or award of a contract or an option to an existing contract could be significantly delayed for reasons including, but not limited to, the need for us to resubmit our proposal or limitations on available funds due to government budget cuts. |
The U.S. government may choose to award future contracts for the supply of medical radiation countermeasures to our competitors instead of to us. If we are unable to win particular contracts, or if the government chooses not to fully exercise all options under contracts awarded to us, we may not be able to operate in the market for products that are provided under those contracts for a number of years. If we are unable to consistently win new contract awards, or if we fail to anticipate all of the costs and resources that will be required to secure such contract awards, our growth strategy and our business, financial condition and operating results could be materially adversely affected.
Additionally, government authorities have a high degree of discretion in Russia and have at times exercised their discretion selectively or arbitrarily, without hearing or prior notice, and sometimes in a manner that is perceived to be influenced, or may be influenced, by political or commercial considerations. The government also has the power, in certain circumstances, to interfere with the performance of, nullify or terminate contracts. Selective or arbitrary actions have included withdrawal of licenses, sudden and unexpected tax audits, criminal prosecutions and civil actions. Federal and local government entities have also used common defects in documentation as pretexts for court claims and other demands to invalidate and/or to void transactions, apparently for political purposes. We cannot assure you that regulators, judicial authorities or third parties will not challenge our compliance with applicable laws, decrees and regulations in Russia. Selective or arbitrary government action could have a material adverse effect on our business and on the value of our common stock.
The market for U.S. and other government funding is highly competitive.
We have submitted or plan to submit applications for funding of various research studies of our product candidates to the U.S. and other governments. There is no guarantee that any proposals that we have or plan to submit will be funded even if we receive positive reviews of such proposals as funding by the government is highly competitive and limited to the availability of funds. Failure to receive funding from U.S. and other government sources for the development of our product candidates could impair our ability to fund the development programs for our product candidates and thus could result in delays in development, or even stopping of development, of certain indications for our product candidates.
| 17 |
Notably, our biodefense product candidate, entolimod, faces significant competition for U.S. government funding for both development and procurement of medical countermeasures for biological, chemical and nuclear threats, diagnostic testing systems and other emergency preparedness countermeasures. In addition, we may not be able to compete effectively if entolimod does not satisfy procurement requirements of the U.S. government with respect to biodefense products. Our opportunities to succeed in the biodefense industry could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer side effects, are more convenient or are less expensive than any products that we may develop.
U.S. government agencies have special contracting requirements, which create additional risks.
We have historically entered into contracts with various U.S. government agencies. Due to these contracts with government agencies, we are subject to various federal contract requirements. Future sales to U.S. government agencies will depend, in part, on our ability to meet these requirements, certain of which we may not be able to satisfy.
U.S. government contracts typically contain unfavorable termination provisions and are subject to audit by the government at its sole discretion even after the end of the period of performance under the contract, which subjects us to additional risks. These risks include the ability of the U.S. government to unilaterally:
| ● | suspend or prevent us for a set period of time from receiving new contracts or extending existing contracts based on violations or suspected violations of laws or regulations; | |
| ● | terminate our existing contracts; | |
| ● | reduce the scope and value of our existing contracts; | |
| ● | audit and object to our contract-related costs and fees, including allocated indirect costs; | |
| ● | control and potentially prohibit the export of our products; and | |
| ● | change certain terms and conditions in our contracts. |
Pursuant to our government contracts, we are generally permitted to retain title to any patentable invention or discovery made while performing the contract. However, the U.S. government is generally entitled to receive a non-exclusive, non-transferable, irrevocable, paid-up license to the subject inventions throughout the world. In addition, our government contracts generally provide that the U.S. government retains unlimited rights in the technical data produced under such government contract.
Our business could be adversely affected by a negative audit by the U.S. government.
As a U.S. government contractor, we may become subject to periodic audits and reviews by U.S. government agencies such as the Defense Contract Audit Agency, or the DCAA. These agencies review a contractor’s performance under its contracts, cost structure and compliance with applicable laws, regulations and standards. The DCAA also reviews the adequacy of, and a contractor’s compliance with, its internal control systems and policies, including the contractor’s purchasing, property, estimating, compensation and management information systems. Any costs found to be improperly allocated to a specific contract will not be reimbursed, which such costs already reimbursed must be refunded.
Based on the results of these audits, the U.S. government may adjust our contract-related costs and fees, which have already been paid to us, including allocated indirect costs. In addition, if an audit or review uncovers any improper or illegal activity, we may be subject to civil and criminal penalties and administrative sanctions, including termination of our contracts, forfeiture of profits, suspension of payments, fines and suspension or prohibition from doing business with the U.S. government. We could also suffer serious harm to our reputation if allegations of impropriety were made against us. In addition, under U.S. government purchasing regulations, some of our costs, including most financing costs, amortization of intangible assets, portions of our R&D costs and some marketing expenses, may not be reimbursable or allowed under our contracts. Further, as a U.S. government contractor, we may become subject to an increased risk of investigations, criminal prosecution, civil fraud, whistleblower lawsuits and other legal actions and liabilities to which purely private sector companies are not.
| 18 |
RISKS RELATING TO OUR INTELLECTUAL PROPERTY
We rely upon licensed patents to protect our technology. We may be unable to obtain or protect such intellectual property rights and we may be liable for infringing upon the intellectual property rights of others.
Our ability to compete effectively will depend on our ability to maintain the proprietary nature of our technologies and the proprietary technology of others with which we have entered into licensing agreements. We have entered into five separate exclusive license agreements to license our product candidates that are not owned by us and some product candidates are covered by up to three separate license agreements. Pursuant to these license agreements we maintain patents and patent applications covering our product candidates. We do not know whether any of these patent applications that are still in the approval process will ultimately result in the issuance of a patent with respect to the technology owned by us or licensed to us. The patent position of pharmaceutical or biotechnology companies, including ours, is generally uncertain and involves complex legal and factual considerations. The standards that the United States Patent and Trademark Office use to grant patents are not always applied predictably or uniformly and can change. There is also no uniform, worldwide policy regarding the subject matter and scope of claims granted or allowable in pharmaceutical or biotechnology patents. Accordingly, we do not know the degree of future protection for our proprietary rights or the breadth of claims that will be allowed in any patents issued to us or to others.
Our technology may be found in the future to infringe upon the rights of others or be infringed upon by others. In such a case, others may assert infringement claims against us, and should we be found to infringe upon their patents, or otherwise impermissibly utilize their intellectual property, we might be forced to pay damages, potentially including treble damages, if we are found to have willfully infringed on such parties’ patent rights. Furthermore, parties making claims against us may be able to obtain injunctive or other equitable relief which could effectively block our ability to further develop, commercialize and sell products. In addition to any damages we might have to pay, we may be required to obtain licenses from the holders of this intellectual property, enter into royalty agreements, or redesign our products so as not to utilize this intellectual property, each of which may prove to be uneconomical or otherwise impossible. Conversely, we may not always be able to successfully pursue our claims against others that infringe upon our technology and the technology exclusively licensed by us or developed with our collaborative partners. Thus, the proprietary nature of our technology or technology licensed by us may not provide adequate protection against competitors.
Moreover, the cost to us of any litigation or other proceeding relating to our patents and other intellectual property rights, even if resolved in our favor, could be substantial and the litigation would divert our management’s efforts and our resources. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations.
If we fail to comply with our obligations under our license agreement with third parties, we could lose our ability to develop our product candidates.
The manufacture and sale of any products developed by us may involve the use of processes, products or information, the rights to certain of which are owned by others. Although we have obtained exclusive licenses for our product candidates from The Cleveland Clinic and RPCI with regard to the use of patent applications as described above and certain processes, products and information of others, these licenses could be terminated or expire during critical periods and we may not be able to obtain licenses for other rights that may be important to us, or, if obtained, such licenses may not be obtained on commercially reasonable terms. Furthermore, some of our product candidates require the use of technology licensed from multiple third parties, each of which is necessary for the development of such product candidates. If we are unable to maintain and/or obtain licenses, we may have to develop alternatives to avoid infringing upon the patents of others, potentially causing increased costs and delays in product development and introduction or precluding the development, manufacture, or sale of planned products. Additionally, the patents underlying any licenses may not be valid and enforceable. To the extent any products developed by us are based on licensed technology, royalty payments on the licenses will reduce our gross profit from such product sales and may render the sales of such products uneconomical.
Our current exclusive licenses impose various development, royalty, diligence, record keeping, insurance, solvency and other obligations on us. If we breach any of these obligations and do not cure such breaches within the relevant cure period, the licensor may have the right to terminate the license, which could result in us being unable to develop, manufacture and sell products that are covered by the licensed technology or enable a competitor to gain access to the licensed technology.
| 19 |
In addition, while we cannot currently determine the dollar amount of the royalty and other payments we will be required to make in the future under the license agreements, if any, the amounts may be significant. The dollar amount of our future payment obligations will depend on the technology and intellectual property we use in products that we successfully develop and commercialize, if any.
If we are not able to protect and control our unpatented trade secrets, know-how and other technology, we may suffer competitive harm.
We also rely on a combination of trade secrets, know-how, technology and nondisclosure and other contractual agreements and technical measures to protect our rights in the technology. However, trade secrets are difficult to protect and we rely on third parties to develop our products and thus must share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements will typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication. If any trade secret, know-how or other technology not protected by a patent or intellectual property right were disclosed to, or independently developed by, a competitor, our business, financial condition and results of operations could be materially adversely affected.
RISKS RELATING TO OUR INDUSTRY AND OTHER EXTERNAL FACTORS
The biopharmaceutical market in which we compete is highly competitive.
The biopharmaceutical industry is characterized by rapid and significant technological change. Our success will depend on our ability to develop and apply our technologies in the design and development of our product candidates and to establish and maintain a market for our product candidates. In addition, there are many companies, both public and private, including major pharmaceutical and chemical companies, specialized biotechnology firms, universities and other research institutions engaged in developing pharmaceutical and biotechnology products. Many of these companies have substantially greater financial, technical, research and development resources and human resources than us. Competitors may develop products or other technologies that are more effective than those that are being developed by us or may obtain FDA or other governmental approvals for products more rapidly than us. If we commence commercial sales of products, we still must compete in the manufacturing and marketing of such products, areas in which we have no experience.
Our growth could be limited if we are unable to attract and retain key personnel and consultants.
We have limited experience in filing and prosecuting regulatory applications to obtain marketing approval from the FDA or other regulatory authorities. The loss of services of one or more of our key employees or consultants could have a negative impact on our business or our ability to expand our research, development and clinical programs. We depend on our scientific and clinical collaborators and advisors, all of whom have outside commitments that may limit their availability to us. In addition, to the extent that we are unable to engage certain collaborators or advisors for certain periods of time due to lack of relevant work or lack of available funds, there is a risk that such collaborators or advisors will not be available to provide services in the future at such time when there is available work and/or funds. In addition, we believe that our future success will depend in large part upon our ability to attract and retain highly skilled scientific, managerial and marketing personnel, particularly as we expand our activities in clinical trials, the regulatory approval process, external partner solicitations and sales and manufacturing. We routinely enter into consulting agreements with our scientific and clinical collaborators and advisors, opinion leaders and heads of academic departments in the ordinary course of our business. We also enter into contractual agreements with physicians and institutions who recruit patients into our clinical trials on our behalf in the ordinary course of our business. In addition, as a result of our 2013 corporate restructuring and workforce reductions, we may face additional challenges in retaining our existing senior management and key employees and recruiting new employees to join our company as our business needs change. We face significant competition for this type of personnel and for employees from other companies, research and academic institutions, government entities and other organizations. We cannot predict our success in hiring or retaining the personnel we require for continued growth.
| 20 |
We may be subject to damages resulting from claims that we, our employees or our consultants have wrongfully used or disclosed alleged trade secrets of their former employers.
We engage as employees and consultants individuals who were previously employed at other biotechnology or pharmaceutical companies, including at competitors or potential competitors. Although no claims against us are currently pending, we may become subject to claims that we or our employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and distract management.
We may incur substantial liabilities from any product liability and other claims if our insurance coverage for those claims is inadequate.
We face an inherent risk of product liability exposure related to the testing of our product candidates in human clinical trials and will face an even greater risk if the product candidates are sold commercially. An individual may bring a product liability claim against us if one of the product candidates causes, or merely appears to have caused, an injury. If we cannot successfully defend ourselves against the product liability claim, we will incur substantial liabilities. Regardless of merit or eventual outcome, product liability claims may result in:
| ● | decreased demand for our product candidates; | |
| ● | injury to our reputation; | |
| ● | withdrawal of clinical trial participants; | |
| ● | costs of related litigation; | |
| ● | diversion of our management’s time and attention; | |
| ● | substantial monetary awards to patients or other claimants; | |
| ● | loss of revenues; | |
| ● | the inability to commercialize product candidates; and | |
| ● | increased difficulty in raising required additional funds in the private and public capital markets. |
We currently have product liability insurance and intend to expand such coverage from coverage for clinical trials to include the sale of commercial products if marketing approval is obtained for any of our product candidates. However, insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage that will be adequate to satisfy any liability that may arise.
From time to time, we may also become subject to litigation, such as stockholder derivative claims or securities fraud claims, which could involve our directors and officers as defendants. We currently have director and officer, or D&O, insurance to cover such risk exposure for our directors and officers. Our bylaws require us to indemnify our current and past directors and officers from reasonable expenses related to the defense of any action arising from their service to us. Our certificate of incorporation and by-laws include provisions to indemnify the directors and officers to the fullest extent permitted by the Delaware General Corporation Law, including circumstances under which indemnification is otherwise discretionary. If our D&O insurance is insufficient to cover all such expenses for all directors and officers, we would be obligated to cover any shortfall, which may be substantial. Such expenditure could have a material adverse effect on our results of operation, financial condition and liquidity. Further, if D&O insurance becomes prohibitively expensive to maintain in the future, we may be unable to renew such insurance on economic terms or unable renew such insurance at all. The lack of D&O insurance may make it difficult for us to retain and attract talented and skilled directors and officers to serve our company, which could adversely affect our business.
| 21 |
Our former laboratories used certain chemical and biological agents and compounds that may be deemed hazardous and we are subject to various safety and environmental laws and regulations. Our compliance with these laws and regulations may result in significant costs, which could materially reduce our ability to become profitable.
Until late 2013, we operated laboratories that used hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety or the environment and we currently sublease these laboratories for operation by other companies. As appropriate, we stored these materials and wastes resulting from their use at our laboratory facility pending their ultimate use or disposal and we currently require that our laboratory sub-lessors do the same. We contracted with a third party to properly dispose of these materials and wastes and our laboratory sub-lessors now manage such contracts. We were and continue to be subject to a variety of federal, state and local laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these materials and wastes. We may incur significant costs if we unknowingly failed to comply with environmental laws and regulations.
We rely significantly on information technology and any failure, inadequacy, interruption or security lapse of that technology, including any cybersecurity incidents, could harm our ability to operate our business effectively.
Despite the implementation of security measures, our internal computer systems and those of third parties with which we contract are vulnerable to damage from cyber-attacks, computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. System failures, accidents or security breaches could cause interruptions in our operations, and could result in a material disruption of our product development and clinical activities and business operations, in addition to possibly requiring substantial expenditures of resources to remedy. The loss of product development or clinical trial data could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and our development programs and the development of our product candidates could be delayed.
Political or social factors may delay or impair our ability to market our products.
Entolimod is being developed to treat ARS, which is a disease that may be caused by terrorist acts. The political and social responses to terrorism have been highly charged and unpredictable. Political or social pressures may delay or cause resistance to bringing our products to market or limit pricing of our products, which would harm our business. Changes to favorable laws, such as the Project BioShield Act, could have a material adverse effect on our ability to generate revenue and could require us to reduce the scope of or discontinue our operations.
We hope to receive funding from U.S. or foreign government agencies for the development of entolimod and our products. Changes in government budgets and agendas, however, have previously resulted in termination of our contract negotiations and may, in the future, result in future funding being decreased and de-prioritized. In addition, government contracts contain provisions that permit cancellation in the event that funds are unavailable to the government agency. Furthermore, we cannot be certain of the timing of any future funding and substantial delays or cancellations of funding could result from protests or challenges from third parties. If the U.S. government fails to continue to adequately fund R&D programs, we may be unable to generate sufficient revenues to continue development of entolimod or continue our other operations. Similarly, if our pre-EUA submission for entolimod is authorized by the FDA, but the U.S. government does not place sufficient orders for this product, our future business may be harmed.
Failure to comply with the United States Foreign Corrupt Practices Act and similar foreign laws could subject us to penalties and other adverse consequences.
We are required to comply with the United States Foreign Corrupt Practices Act, or FCPA, which prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some that may compete with us, are not subject to these prohibitions. Furthermore, foreign jurisdictions in which we operate may have laws that are similar to the FCPA to which we are or may become subject. This may place us at a significant competitive disadvantage. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices may occur from time to time in the foreign markets where we conduct business. Although we inform our personnel that such practices are illegal, we can make no assurance that our employees or other agents will not engage in illegal conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
| 22 |
The FCPA also obligates companies whose securities are listed in the United States to comply with certain accounting provisions requiring the company to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries and to devise and maintain an adequate system of internal accounting controls for international operations.
Compliance with the FCPA and similar foreign anti-bribery laws is expensive and difficult, particularly in countries in which corruption is a recognized problem. In addition, such anti-bribery laws present particular challenges in the biotech or pharmaceutical industry, because, in many countries, hospitals are operated by the government and doctors and other hospital employees may be considered foreign officials.
RISKS RELATED TO CONDUCTING BUSINESS IN THE RUSSIAN FEDERATION
Political, economic and governmental instability in Russia could materially adversely affect our operations and financial results.
Political, ethnic, religious, historical and other differences have, on occasion, given rise to tensions within certain regions of Russia. Further, political and economic relations between Russia and the United States, two of the jurisdictions in which we operate, are complex. The current situation in Ukraine and Crimea along with the response of the governments of Russia, the United States, member states of the European Union, the European Union itself and other nations to this situation, have the potential to materially adversely affect our operations in Russia. In connection with the current situation in Ukraine, the United States, the European Union and certain other states have imposed a broad raft of sanctions against Russian and Crimean officials, Russian businesses and certain businessmen, including sectorial sanctions applicable to businesses operating in certain sectors of the economy, including energy and finance. Russia has responded with certain countermeasures, including limiting the import of certain goods from the United States and other countries. While we do not anticipate that the current sanctions will materially affect our business directly, if further sanctions are ordered by the European Union, the United States or other international interests, such sanctions may materially adversely affect our operations in Russia.
In addition to geopolitical events, other factors, including the steady fall in oil prices, the global strengthening of the U.S. dollar and the Russian Central Bank’s reduction of currency rate support, have negatively affected the value of the Russian ruble relative to the U.S. dollar and this has drove increasing inflation. Continuing fluctuations in the rates at which the U.S. dollar are exchanged into Russian rubles may result in both foreign currency transaction and translation losses. We are subject to exchange rate fluctuations as (i) CBLI exchanges U.S. dollar-denominated funds into ruble-denominated funds in order to conduct operations of our Russian-based subsidiary BioLab 612, (ii) Panacela, Incuron (prior to deconsolidation in November of 2014) and BioLab 612 use their ruble-denominated funds to pay for services under U.S. dollar-denominated contracts, including payments to CBLI for services we provide to our subsidiary and joint ventures, and (iii) the U.S. dollar equivalent of ruble denominated assets and liabilities fluctuate from period-to-period causing us to record foreign currency translation adjustments which are reflected as a change in other comprehensive income (loss). As the U.S. dollar strengthens or weakens relative to the ruble, our ruble-denominated revenue and expenses decline or increase respectively, when translated into U.S. dollars for financial reporting purposes. Should exchange rates in effect at the time of this filing as compared to early 2014 and 2013, continue throughout the year, we expect the exchange rates to reduce our revenues and expenses in 2014 compared to 2013, and we would also record other comprehensive losses on our ruble denominated assets and liabilities when translated into the U.S. dollar. Additionally, the purchasing power of U.S. dollar denominated services is reduced, such as those being provided in the U.S. for Incuron’s clinical trial of the intravenous application of CBL0137.
Even before the current events mentioned above, and since the early 1990s, Russia has sought to transform from a one-party state with a centrally planned economy to a democracy with a market economy. As a result of the sweeping nature of various reforms and the failure of some of them, the political system of Russia remains vulnerable to popular dissatisfaction, including demands for autonomy from particular regional and ethnic groups. Current and future changes in the Russian government, major policy shifts or lack of consensus between various branches of the government and powerful economic groups could disrupt or reverse economic and regulatory reforms. Furthermore, the Russian economy is vulnerable to market downturns and economic slowdowns elsewhere in the world, and has experienced periods of considerable instability. Although the Russian economy showed positive trends until 2008, including annual increases in the gross domestic product, a relatively stable currency, strong domestic demand, rising real wages and a reduced rate of inflation, these trends were interrupted by the global financial crisis in late 2008, in which Russia experienced adverse economic and financial effects including a substantial decrease in the growth rate of gross domestic product, depreciation of local currency and a decline in domestic and international demand for its products and services. Economic instability in Russia could materially adversely affect our business, financial condition and results of operations.
| 23 |
Emerging markets, such as Russia, are subject to greater risks than more developed markets and financial turmoil in Russia could disrupt our business.
Investors in emerging markets, such as Russia, should be aware that these markets are subject to greater risks than more developed markets, including significant economic risks. For example, the Russian economy has periodically experienced high rates of inflation and is experiencing increased rates of inflation at present. According to The World Bank, the annual inflation rate in Russia, as measured by the consumer price index, was 5.1% in 2012 6.8% in 2013 and 7.8% in 2014. Periods of higher inflation may slow economic growth. Inflation also is likely to increase some of our costs and expenses including the costs for our subsidiaries and joint ventures to conduct business operations, including any outsourced product testing costs.
Prospective investors in our common stock should note that emerging markets are subject to rapid change and that the information set out in this prospectus about our operations in Russia may become outdated relatively quickly.
Our subsidiary/joint venture research operations are conducted primarily in Russia, making them subject to political uncertainties relating to Russia and U.S.-Russian relations.
The majority of our subsidiary’s and joint ventures’ research activities are in Russia. Given the unprecedented level of hostility between the United States and Russia since the dissolution of the Soviet Union, our operations may be negatively and materially impacted by escalation of measures and counter-measures taken by the United States against Russia and Russia against the United States and their respective citizens and persons organized under their laws, including the adoption of measures that could require us to reduce, suspend or terminate our operations in Russia. For example, the organizations funding our activities in Russia are highly regulated and, in many instances, are controlled by the Russian government so our funding could be delayed, reduced or even terminated under expanded sanction regimes.
The legal system in Russia can create an uncertain environment for business activity, which could materially adversely affect our business and operations in Russia.
The legal framework to support a market economy remains new and in flux in Russia and, as a result, its legal system can be characterized by: inconsistencies between and among laws and governmental, ministerial and local regulations, orders, decisions, resolutions and other acts; gaps in the regulatory structure resulting from the delay in adoption or absence of implementing regulations; selective enforcement of laws or regulations, sometimes in ways that have been perceived as being motivated by political or financial considerations; limited judicial and administrative guidance on interpreting legislation; relatively limited experience of judges and courts in interpreting recent commercial legislation; a perceived lack of judicial and prosecutorial independence from political, social and commercial forces; inadequate court system resources; a high degree of discretion on the part of the judiciary and governmental authorities; and underdeveloped bankruptcy procedures that are subject to abuse.
In addition, as is true of civil law systems generally, judicial precedents generally have no binding effect on subsequent decisions. Not all legislation and court decisions in Russia are readily available to the public or organized in a manner that facilitates understanding. Enforcement of court orders can in practice be very difficult. All of these factors make judicial decisions difficult to predict and effective redress uncertain. Additionally, court claims and governmental prosecutions may be used in furtherance of what some perceive to be political or commercial aims.
Effective August 6, 2014, the Supreme State Commercial (Arbitrazh) Court was merged into the Russian Supreme Court and now exits as a sub-division of the Russian Supreme Court, known as the Judicial Collegium for Economic Disputes of the Supreme Court. A draft law on full merger of the commercial courts and courts of general jurisdiction reportedly is being prepared. As of the date of this Annual Report, the consequences of this merger process on the expeditious resolution of commercial disputes and stability of the prior decisions of the Supreme State Commercial (Arbitrazh) Court is unknown.
The untested nature of much of recent legislation in Russia and the rapid evolution of its legal system may result in ambiguities, inconsistencies and anomalies in the application and interpretation of laws and regulations. Any of these factors may affect our ability to enforce our rights under our contracts or to defend ourselves against claims by others, or result in our being subject to unpredictable requirements. These uncertainties also extend to property rights and the expropriation or nationalization of any of our entities, their assets or portions thereof, potentially without adequate compensation, could materially adversely affect our business, financial condition and results of operations.
| 24 |
Changes in the tax system in Russia or the arbitrary or unforeseen application of existing rules could materially adversely affect our financial condition and results of operations.
There have been significant changes to the taxation system in Russia in recent years as the authorities have gradually replaced legislation regulating the application of major taxes such as corporate income tax, value added tax, corporate property tax and other taxes with new legislation. Effective January 1, 2015, the Russian tax law was amended as part of the government’s “deoffshorization” policy to, among other things, introduce a concept analogous to that of controlled foreign corporations found in other jurisdictions.
Tax authorities in Russia have also been aggressive in their interpretation of tax laws and their many ambiguities, as well as in their enforcement and collection activities. Technical violations of contradictory laws and regulations, many of which are relatively new and have not been subject to extensive application or interpretation, can lead to penalties. High-profile companies, particularly those operating in strategically sensitive sectors, can be perceived to be particularly vulnerable to aggressive application of unclear requirements. Many companies must negotiate their tax bills with tax inspectors who may demand higher taxes than applicable law appears to provide. Our Russian subsidiary’s and joint ventures’ tax liabilities may become greater than the estimated amount that they have expensed to date and paid or accrued on the balance sheets, particularly if the tax benefits currently received in Russia are changed or removed. Any additional tax liability, as well as any unforeseen changes in tax laws, could materially adversely affect our future results of operations, financial condition or cash flows in a particular period.
In October 2006, the Supreme Arbitration Court of Russia issued a ruling that introduced the concept of an “unjustified tax benefit,” which is a benefit that may be disallowed for tax purposes. Specific examples cited by the court include benefits obtained under transactions lacking a business purpose (i.e., when the only purpose of a deal or structure is to derive tax benefits). The tax authorities have actively sought to apply this concept when challenging tax positions taken by taxpayers. Although the intention of the ruling was to combat tax abuse, in practice there is no assurance that the tax authorities will not seek to apply this concept in a broader sense than may have been intended by the court. In addition, the tax authorities and the courts have indicated a willingness to interpret broadly the application of criminal responsibility for tax violations.
The tax system in Russia imposes additional burdens and costs on our operations there and complicate our tax planning and related business decisions. For example, the tax environment in Russia has historically been complicated by contradictions in Russian tax law and ambiguity in areas such as the deductibility of certain expenses. This uncertainty could result in a greater than expected tax burden and potentially exposes us to significant fines and penalties and enforcement measures, despite our best efforts at compliance. These factors raise the risk of a sudden imposition of arbitrary or onerous taxes on our operations in these countries. This could materially adversely affect our financial condition and results of operations.
Selective or arbitrary government action may have an adverse effect on our business and the value of our common stock.
Government authorities have a high degree of discretion in Russia and have at times exercised their discretion selectively or arbitrarily, without hearing or prior notice, and sometimes in a manner that is perceived to be influenced, or may be influenced, by political or commercial considerations. The government also has the power, in certain circumstances, to interfere with the performance of, nullify or terminate contracts. Selective or arbitrary actions have included withdrawal of licenses, sudden and unexpected tax audits, criminal prosecutions and civil actions. Federal and local government entities have also used common defects in documentation as pretexts for court claims and other demands to invalidate and/or to void transactions, apparently for political purposes. We cannot assure you that regulators, judicial authorities or third parties will not challenge our compliance with applicable laws, decrees and regulations in Russia. Selective or arbitrary government action could have a material adverse effect on our business and on the value of our common stock.
| 25 |
Shareholder liability under Russian legislation could cause us to become liable for the obligations of our subsidiaries and joint ventures.
The Russian Civil Code and the Law on Limited Liability Companies generally provide that shareholders in a Russian limited liability company are not liable for the obligations of the company and bear only the risk of loss of their investment. This may not be the case, however, when one person, an effective parent, is capable of determining decisions made by another, an effective subsidiary. The effective parent bears joint and several responsibilities for transactions concluded by the effective subsidiary in carrying out these decisions in certain circumstances.
In addition, a parent is secondarily liable for an effective subsidiary’s debts if an effective subsidiary becomes insolvent or bankrupt as a result of the action or inaction of the parent. This is the case no matter how the parent’s capability to determine decisions of the effective subsidiary arises. For example, this liability could arise through ownership of voting securities or by contract. In these instances, other shareholders of the effective subsidiary may claim compensation for the effective subsidiary’s losses from the parent that caused the effective subsidiary to act or fail to act, knowing that such action or inaction would result in losses. Accordingly, in CBLI’s position as a parent, there is a risk that it could be held liable in certain limited circumstances for the debts of its effective subsidiaries consequently, it is possible that CBLI could face material liability in this regard in the future, which could materially adversely affect our business and our results of operations.
Our Russian subsidiary/joint ventures can be forced into liquidation on the basis of formal noncompliance with certain legal requirements.
Incuron, BioLab 612 and Panacela Labs, LLC, the wholly-owned Russian subsidiary of Panacela, were organized under the laws of the Russian Federation. Certain provisions of Russian law may allow a court to order the liquidation of a locally organized legal entity on the basis of its formal noncompliance with certain requirements during formation, reorganization or during its operations. Additionally, Russian corporate law allows the government to liquidate a company if its net assets fall below a certain threshold. Similarly, there have also been cases in Russia in which formal deficiencies in the establishment process of a legal entity or noncompliance with provisions of law have been used by courts as a basis for liquidation of a legal entity. Weaknesses in the legal systems of Russia create an uncertain legal environment, which makes the decisions of a court or a governmental authority difficult, if not impossible, to predict. If involuntary liquidation of either of the aforementioned entities were to occur, such liquidation could materially adversely affect our financial condition and results of operations.
Crime and corruption could disrupt our ability to conduct our business.
Political and economic changes in Russia in recent years have resulted in significant dislocations of authority. The local and international press has reported the existence of significant organized criminal activity, particularly in large metropolitan centers. In addition, the local and international press has reported high levels of corruption, including the bribing of officials for the purpose of initiating investigations by government agencies. Press reports have also described instances in which state officials have engaged in selective investigations and prosecutions to further the interests of the state and individual officials, as well as private businesses, including competitors and corporate raiders. Corruption in Russia is perceived to be pervasive and, in some cases, is worsening. The government in Russia has recently pursued a campaign against corruption. However, there is no assurance that such laws or other laws enacted elsewhere will be applied with any effectiveness by the local authorities and the continuing effects of corruption, money laundering and other criminal activity could have a negative effect on the Russian economy and could materially adversely affect our business in Russia.
RISKS RELATING TO OUR SECURITIES AND THIS OFFERING
There is uncertainty regarding the application of the federal and state securities laws to our offering of common stock and warrants, and there is a corresponding risk that we could be required to refund the purchase price of securities offered to purchasers who so elect.
We conducted an offering under a registration statement filed with the Securities and Exchange Commission and a concurrent private placement intended to comply with the requirements of Section 4(a)(2) under the Securities Act of 1933, as amended, and Rule 506(b) promulgated thereunder. Shares of common stock and warrants were offered and sold in combination. The shares of common stock and Series B pre-funded warrants were intended to be offered and sold in a transaction registered under the Securities Act, while the other warrants and shares of common stock issuable thereunder were intended to be offered and sold in a private placement exempt from the registration requirements of the Securities Act.
| 26 |
While we are aware of other transactions using a concurrent public/private offering approach, the SEC has not addressed whether concurrent public and private offerings and sales to the same prospective investors would adversely impact the public offering or preclude the private offering from satisfying the requirements of Rule 506(b). If the securities offered in our concurrent private placement do not satisfy the conditions of Rule 506(b), the offering would be a violation of Section 5 of the Securities Act and each purchaser would have the right to rescind its purchase of the securities, meaning that we would be required to refund the purchase price of the securities to each purchaser electing rescission. If that were to occur, we would face severe financial demands and reputational harm that could adversely affect our business and operations. Additionally, if we did not in fact qualify for the exemptions upon which it has relied, we may become subject to significant fines and penalties imposed by SEC. It is also possible that additional remedies may be available to purchasers under applicable state law.
Significant stockholders or potential stockholders may attempt to effect changes to our company, which could adversely affect our corporate governance, results of operations and financial condition.
Stockholders may from time to time attempt to effect changes, engage in proxy solicitations or advance stockholder proposals. Responding to proxy contests and other actions by activist stockholders can generally be costly and time-consuming, disrupting our operations and diverting the attention of our board of directors and senior management from the pursuit of business strategies. Additionally, stockholder campaigns could result in corporate governance changes that could adversely affect our results of operations and financial condition.
The price of our common stock has been and could remain volatile, which may in turn expose us to securities litigation.
The market price of our common stock has historically experienced and may continue to experience significant volatility. From January 2014 through April 2015, the market price of our common stock, which is listed on the NASDAQ Capital Market, fluctuated from a high of $25.40 per share in the first quarter of 2014 to a low of $3.02 in April 2015. The listing of our common stock on the NASDAQ Capital Market does not assure that a meaningful, consistent and liquid trading market will exist, and in recent years, the market has experienced extreme price and volume fluctuations that have particularly affected the market prices of many smaller companies like us. Our common stock is thus subject to this volatility in addition to volatility caused by the occurrence of industry and company specific events. Factors that could cause fluctuations include, but are not limited to, the following:
| ● | our progress in developing and commercializing our products; | |
| ● | price and volume fluctuations in the overall stock market from time to time; |
| 27 |
| ● | fluctuations in stock market prices and trading volumes of similar companies; | |
| ● | actual or anticipated changes in our earnings or fluctuations in our operating results or in the expectations of securities analysts; | |
| ● | general economic conditions and trends; | |
| ● | major catastrophic events; | |
| ● | sales of large blocks of our stock; | |
| ● | departures of key personnel; | |
| ● | changes in the regulatory status of our product candidates, including results of our pre-clinical studies and clinical trials; | |
| ● | status of contract and funding negotiations relating to our product candidates; | |
| ● | events affecting our collaborators; | |
| ● | events affecting our competitors; | |
| ● | announcements of new products or technologies, commercial relationships or other events by us or our competitors; | |
| ● | regulatory developments in the U.S. and other countries; | |
| ● | failure of our common stock to be listed or quoted on the NASDAQ Capital Market, other national market system or any national stock exchange; | |
| ● | changes in accounting principles; and | |
| ● | discussion of us or our stock price by the financial and scientific press and in online investor communities. |
As a result of the volatility of our stock price, we could be subject to securities litigation, which could result in substantial costs and divert management’s attention and company resources from our business.
We may be unable to maintain the listing of our common shares on NASDAQ.
The quantitative listing standards of the NASDAQ Stock Market, or NASDAQ, require, among other things, that listed companies maintain a minimum closing bid price of $1.00 per share and a minimum of $2,500,000 of stockholders equity. We failed to satisfy the bid price threshold for 30 consecutive trading days in 2014 and effected a 1:20 reverse split on January 28, 2015 which allowed us to regain compliance with this NASDAQ listing standard. Additionally, on March 10, 2015, we received a letter from NASDAQ indicating that, as of December 31, 2014 we did not meet the stockholders’ equity threshold and that, as of March 9, 2015, we did not meet the alternatives of market value of listed securities or net income from continuing operations. We filed a plan to regain compliance with NASDAQ and on May 7, 2015 we received a letter indicating that NASDAQ has accepted our plan and granted us until July 15, 2015 to evidence compliance. If we fail to regain compliance, our stock will be subject to delisting by NASDAQ.
The per share market price of the common stock and stockholders’ equity will continue to be affected by our financial and operational results, financial position, including our liquidity and capital resources, product development, industry conditions, the market’s perception of the our business and other factors, which are unrelated to the number of common shares outstanding. There is no assurance we will be able to continually meet such conditions.
Issuance of additional equity may adversely affect the market price of our stock.
We are currently authorized to issue 160,000,000 shares of common stock and 10,000,000 shares of preferred stock. As of this filing, 4,002,264 shares of our common stock were issued and outstanding, we had pre-funded warrants and convertible preferred stock that were convertible into 266,911 shares of our common stock for no additional consideration, outstanding warrants to purchase 2,281,332 shares of our common stock at an average exercise price of $14.49 per share (exclusive of the Rusnano warrant described above as it was not exercisable at the time), and we had outstanding options to purchase 382,220 shares of our common stock at an average exercise price of $47.18 per share. To the extent we issue shares of common stock or our outstanding options and warrants are exercised, holders of our common stock will experience dilution.
| 28 |
In the event of any future issuances of equity securities or securities convertible into or exchangeable for, common stock, holders of our common stock may experience dilution. Furthermore, certain of our outstanding warrants, pre-funded warrants and convertible preferred stock contain provisions that, in certain circumstances, could result in the number of shares of common stock issuable upon the exercise of such securities to increase and/or the exercise price of such warrants to decrease.
Moreover, our board of directors is authorized to issue preferred stock without any action on the part of our stockholders. Our board of directors also has the power, without stockholder approval, to set the terms of any such preferred stock that may be issued, including voting rights, conversion rights, dividend rights, preferences over our common stock with respect to dividends or if we liquidate, dissolve or wind up our business and other terms. For example, on February 6, 2015, we issued 717.4 shares of Series A Convertible Preferred Stock convertible into 239,135 shares of our common stock at a conversion price of $3.00 per share. The Series A Convertible Preferred Stock have a liquidation preference over junior securities, including common stock. Additionally, the Company agreed to comply with negative covenants that limit our ability to incur debt, incur liens, amend our charter documents, repurchase securities, pay dividends or enter into related party transactions, which could adversely impact our operations, until the date that (i) we obtained shareholder approval for the issuance of all of the shares of common stock issuable upon conversion of the Series A convertible preferred stock, exercise of the Series B pre-funded warrants and exercise of the Series A warrants in excess of 19.99% of our issued and outstanding common stock (the “Shareholder Approval”) (which occurred on April 14, 2015), (ii) the Series B Pre-funded Warrants are no longer outstanding, and (iii) there is an effective registration statement registering the resale of all of the shares of common stock underlying the Series A Preferred Stock (which occurred on March 11, 2015. We also may issue shares of Series B Preferred Stock in this offering (see “Description of Securities We Are Offering”). If we issue additional shares of preferred stock in the future that have preference over our common stock with respect to the payment of dividends or upon our liquidation, dissolution or winding up, or if we issue preferred stock with voting rights that dilute the voting power of our common stock, the market price of our common stock could decrease. Additionally, the conversion of the Series A convertible preferred stock, or any preferred stock issued in the future, into our common stock could result in significant dilution to the holders of our common stock.
We also consider from time to time various strategic alternatives that could involve issuances of additional shares of common stock or shares of preferred stock, including but not limited to acquisitions and business combinations.
If securities or industry analysts do not publish research or reports about our business, or publish negative reports about our business, our stock price and trading volume could decline.
The trading market for our common stock depends in part on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these reports and we currently do not have any industry analysts covering us. In the event we do regain analyst coverage, there can be no assurance that analysts will provide favorable coverage. Our stock price may be adversely impacted by our current lack of analyst coverage as we may have less visibility in the financial markets than other companies in our industry, which may cause declined trading volume and stock price.
We have no plans to pay dividends on our common stock and investors may not receive funds without selling their common stock.
We have not declared or paid any cash dividends on our common stock, nor do we expect to pay any cash dividends on our common stock for the foreseeable future. We currently intend to retain any additional future earnings to finance our operations and growth and, therefore, we have no plans to pay cash dividends on our common stock at this time. Any future determination to pay cash dividends on our common stock will be at the discretion of our board of directors and will be dependent on our earnings, financial condition, operating results, capital requirements, any contractual restrictions, regulatory and other restrictions on the payment of dividends by our subsidiaries to us and other factors that our board of directors deems relevant.
Accordingly, investors may have to sell some or all of their common stock in order to generate cash from your investment. Investors may not receive a gain on their investment when they sell our common stock and may lose the entire amount of their investment.
Provisions in our charter documents and Delaware law may inhibit a takeover or impact operational control of our company, which could adversely affect the value of our common stock.
Our certificate of incorporation and bylaws, as well as Delaware corporate law, contain provisions that could delay or prevent a change of control or changes in our management that a stockholder might consider favorable. These provisions include, among others, prohibiting stockholder action by written consent, advance notice for raising business or making nominations at meetings of stockholders and the issuance of preferred stock with rights that may be senior to those of our common stock without stockholder approval. These provisions would apply even if a takeover offer may be considered beneficial by some of our stockholders. If a change of control or change in management is delayed or prevented, the market price of our common stock could decline.
| 29 |
There is no trading market for the Series B Preferred Stock or warrants to be offered hereunder.
There is no trading market for our Series B Preferred Stock or the warrants offered hereunder. We do not intend to apply to have the Series B Preferred Stock or the warrants listed or quoted on any market or exchange. The lack of an active market may impair our stockholders’ ability to sell our Series B Preferred Stock or warrants at the time they wish to sell them or at a price that they consider reasonable. The lack of any market for the Series B Preferred Stock or warrants may also reduce the fair market value of our outstanding Series B Preferred Stock and warrants.
The warrants are speculative in nature.
The warrants do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of common stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the warrants may exercise their right to acquire the common stock and pay an exercise price of $ per share, prior to [ ] years from the date of issuance, after which date any unexercised warrants will expire and have no further value.
Our management will have broad discretion over the use of the net proceeds from this offering and we may use the net proceeds in ways with which you disagree or which do not produce beneficial results.
We currently intend to use the net proceeds from this offering for capital to support preparation of a pre-EUA application for entolimod for our defense indication, various other oncology-focused development efforts, as well as for working capital and general corporate purposes. We have not allocated specific amounts of the net proceeds from this offering for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds of this offering. You will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. It is possible that the net proceeds will be invested in a way that does not yield a favorable, or any, return for us or our stockholders. The failure of our management to use such funds effectively could have a material adverse effect on our business, prospects, financial condition, and results of operation.
You will experience immediate and substantial dilution as a result of this offering and may experience additional dilution in the future as we do further financings and transactions.
You will incur immediate and substantial dilution as a result of this offering. After giving effect to the sale by us of up to _______ shares of common stock in this offering (on an as-converted basis with respect to any shares of Series B Preferred Stock sold) at the assumed public offering price of $______ per Class A Unit, and after deducting the underwriter’s discount and estimated offering expenses payable by us, investors in this offering can expect an immediate dilution of $___ per share. In addition, in the past, we issued options and warrants to acquire shares of common stock and will issue warrants in this offering. To the extent these options or warrants are ultimately exercised, you will sustain further future dilution.
Cautionary Note Regarding Forward-Looking Statements
This prospectus contain forward-looking statements contain forward-looking statements. The forward-looking statements are contained principally in the sections entitled “Prospectus Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business” in this prospectus. We may, in some cases, use words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” or the negative of those terms, and similar expressions that convey uncertainty of future events or outcomes to identify these forward-looking statements. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements.
| 30 |
Forward-looking statements in this prospectus include, but are not limited to, statements about:
| ● | the commercialization of our product candidates, if approved; | |
| ● | our plans to research, develop and commercialize our product candidates; | |
| ● | our ability to attract collaborators with development, regulatory and commercialization expertise; | |
| ● | our plans and expectations with respect to future commercial scale-up activities; | |
| ● | future agreements with third parties in connection with the commercialization of any approved product; | |
| ● | the size and growth potential of the markets for our product candidates, and our ability to serve those markets; | |
| ● | the rate and degree of market acceptance of our product candidates; | |
| ● | regulatory developments in the United States and foreign countries; | |
| ● | the performance of our third-party suppliers and manufacturers; | |
| ● | the success of competing therapies that are or may become available; | |
| ● | our ability to attract and retain key scientific or management personnel; | |
| ● | the accuracy of our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; | |
| ● | our use of the proceeds from this offering; and | |
| ● | our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates. |
These forward-looking statements reflect our management’s beliefs and views with respect to future events and are based on estimates and assumptions as of the date of this prospectus and are subject to risks and uncertainties. We discuss many of these risks in greater detail under “Risk Factors” in this prospectus. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Given these uncertainties, you should not place undue reliance on these forward-looking statements.
You should read this prospectus and the documents we have filed as exhibits to the registration statement, of which this prospectus is a part, completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements by these cautionary statements. Except as required by law, we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
| 31 |
We estimate that the net proceeds from the sale of the Units offered pursuant to this prospectus will be approximately $ million, after deducting the underwriting discount and the estimated offering expenses that are payable by us.
We currently intend to use the net proceeds from this offering to obtain additional capital to support preparation of a pre-EUA application for entolimod for our defense indication, various other oncology-focused development efforts, as well as for working capital and general corporate purposes.
We have not yet determined the amount of net proceeds to be used specifically for any of the foregoing purposes. Accordingly, our management will have significant discretion and flexibility in applying the net proceeds from this offering. Pending any use as described above, we intend to invest the net proceeds in high-quality, short-term, interest-bearing securities.
We have never declared or paid any cash dividends on our common stock, and currently do not plan to declare cash dividends on shares of our common stock or Series B Preferred Stock in the foreseeable future. We expect that we will retain all of our available funds and future earnings, if any, for use in the operation and expansion of our business. Our loan agreement with Hercules Capital, N.A. prohibits us from paying cash dividends on our common stock and the terms of any future loan agreement we enter into or any debt securities we may issue are likely to contain similar restrictions on the payment of dividends. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our board of directors and will depend upon such factors as earnings levels, capital requirements, restrictions imposed by applicable law, our overall financial condition and any other factors deemed relevant by our board of directors.
| 32 |
MARKET PRICE OF OUR COMMON STOCK
The following table sets forth the range of high and low sale prices on The NASDAQ Capital Market, for the periods indicated.
| 2015 | High | Low | ||||||
| First Quarter | $ | 6.20 | $ | 3.11 | ||||
| 2014 | High | Low | ||||||
| First Quarter | $ | 24.80 | $ | 12.80 | ||||
| Second Quarter | 15.60 | 9.20 | ||||||
| Third Quarter | 11.60 | 7.60 | ||||||
| Fourth Quarter | 10.20 | 5.40 | ||||||
| 2013 | High | Low | ||||||
| First Quarter | $ | 45.60 | $ | 26.00 | ||||
| Second Quarter | 45.20 | 28.60 | ||||||
| Third Quarter | 36.80 | 29.20 | ||||||
| Fourth Quarter | 33.60 | 19.40 | ||||||
| 33 |
The following table sets forth our capitalization, as of March 31, 2015:
| ● | on an actual basis; | ||
| ● | on a pro forma basis to give effect to the issuance of common stock from March 31, 2015 through and immediately prior to the date of this prospectus (on an as converted and exercised basis with respect to the Series B pre-funded warrant and the Series A Convertible Preferred Stock sold in our February 6, 2015 offering); and | ||
| ● | on a pro forma, as adjusted basis to give effect to (i) the issuance of shares of common from March 31, 2015 through and immediately prior to the date of this prospectus, (and on an as converted and exercised basis with respect to the Series B pre-funded warrant and the Series A Convertible Preferred Stock sold in our February 6, 2015 offering); and (ii) the sale of the shares in this offering (on an as-converted basis with respect to any shares of Series B Preferred Stock sold) at the assumed public offering price of $ per Class A Unit, after deducting underwriting discounts and commissions and other estimated offering expenses payable by us. | ||
You should consider this table in conjunction with our financial statements and the notes to those financial statements included elsewhere in this prospectus.
| As of March 31, 2015 | ||||||||||||
| Actual | Pro forma | Pro forma, as adjusted | ||||||||||
| Preferred Stock, $.005 par value, 10,000,000 shares authorized, 0 shares issued and outstanding | - | - | ||||||||||
| Common stock; $0.005 par value; 160,000,000 shares authorized, 3,435,354 shares issued and outstanding actual, 4,269,176 shares issued and outstanding Pro forma, _______ shares issued and outstanding Pro forma, as adjusted | 17,173 | 21,346 | ||||||||||
| Additional paid-in capital | 133,235,836 | 134,050,067 | ||||||||||
| Other comprehensive income (loss) | (450,172 | ) | (450,172 | ) | ||||||||
| Accumulated deficit | (138,261,859 | ) | (138,261,859 | ) | ||||||||
| Total Cleveland BioLabs, Inc. stockholders’ equity (deficit) | (5,459,022 | ) | (4,640,618 | ) | ||||||||
| Noncontrolling interest in stockholder’s equity | 4,051,554 | 4,051,554 | ||||||||||
| Total stockholder's equity | (1,407,468 | ) | (589,064 | ) | ||||||||
| Total liabilities and stockholders' equity | $ | 10,677,113 | $ | 10,677,113 | ||||||||
| 34 |
If you invest in our securities, your interest will be immediately and substantially diluted to the extent of the difference between the public offering price per Class A Unit and the pro forma net tangible book value per share of our common stock after giving effect to this offering.
Our pro forma net tangible deficiency in assets as of March 31, 2015 was approximately $(0.6) million or $(0.14) per share of common stock, based upon 4,269,176 shares outstanding after giving effect to issuances of our common stock from March 31, 2015 through and immediately prior to the date of this prospectus (on an as converted and exercised basis with respect to the Series B pre-funded warrant and the Series A Convertible Preferred Stock sold in our February 6, 2015 offering). After giving effect to the sale of the shares in this offering at the assumed public offering price of $ per Class A Unit (on an-converted basis with respect to any shares of Series B Preferred Stock sold) and after deducting underwriting discounts and commissions and other estimated offering expenses payable by us, our pro forma as adjusted net tangible book value at March 31, 2015 would have been approximately $ million or $ per share. This represents an immediate increase in pro forma net tangible book value of approximately $ per share to our existing stockholders, and an immediate dilution of $ per share to investors purchasing shares in the offering.
Dilution in pro forma net tangible book value per share represents the difference between the amount per share paid by purchasers of our common stock in this offering and the pro forma net tangible book value per share of our common stock immediately after this offering.
The following table illustrates the per share dilution to investors purchasing shares in the offering:
| Assumed public offering price per Class A Unit | $ | |||
| Pro forma net tangible book value per share as of March 31, 2015 | $ | (0.14 | ) | |
| Increase in net tangible book value per share attributable to this offering | $ | |||
| Pro forma as adjusted net tangible book value per share after this offering | $ | |||
| Amount of dilution in net tangible book value per share to new investors in this offering | $ |
| 35 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
OVERVIEW
We are an innovative biopharmaceutical company seeking to develop first-in-class pharmaceuticals designed to address diseases with significant unmet medical need. Our most advanced product candidate is entolimod, which we are developing as a radiation countermeasure and an immunotherapy for oncology and other indications. We conduct business in the United States and in the Russian Federation through several legal entities, one of which is wholly-owned, and two of which are owned in collaboration with financial partners. See Item 1, “Business” for more information on our product candidates and our strategic partnerships. We refer to Cleveland BioLabs, Inc., or CBLI, along with our wholly-owned subsidiary BioLab 612, LLC, or BioLab 612, as CBLI Stand-alone. We refer to CBLI Stand-alone, in combination with, consolidated joint venture Panacela Labs, Inc., or Panacela, as CBLI Consolidated. Our joint venture Incuron, LLC, or Incuron, was deconsolidated on November 25, 2014. As such, the Incuron balance sheet, including cash, cash equivalents and short-term investments and all of its other assets and liabilities are no longer part of our consolidated balance sheet as of December 31, 2014. In addition, Incuron’s detailed results of operations were consolidated through November 25, 2014, after which we recognized only our equitable interest in Incuron’s results of operation as a single line item classified as an operating expense in our Statement of Operations through December 31, 2014, as Incuron’s operations are an extension of our core business.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and judgments that affect our reported amounts of assets, liabilities, revenues and expenses.
On an ongoing basis, we evaluate our estimates and judgments, including those related to accrued expenses, income taxes, stock-based compensation, investments and in-process research and development. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the reported amounts of revenues and expenses that are not readily apparent from other sources. Actual results may differ from these estimates.
We believe the following critical accounting policies affect our more significant judgments and estimates used in the preparation of our financial statements.
Revenue Recognition
Revenue is recognized when persuasive evidence of an arrangement exists, delivery has occurred, the fee is fixed and determinable, collectability is reasonably assured, contractual obligations have been satisfied and title and risk of loss have been transferred to the customer. We generate our revenue from two different types of contractual arrangements: cost-reimbursable grants and contracts and fixed-price grants and contracts. Costs consist primarily of actual internal labor charges, subcontractor and material costs incurred, plus an allocation of fringe benefits, overhead and general and administrative expenses, based on the terms of the contract.
| 36 |
Revenues on cost-reimbursable grants and contracts are recognized in an amount equal to the costs incurred during the period, plus an estimate of the applicable fee earned. The estimate of the applicable fee earned is determined by reference to the contract: if the contract defines the fee in terms of risk-based milestones and specifies the fees to be earned upon the completion of each milestone, then the fee is recognized when the related milestones are earned. Otherwise, we compute fee income earned in a given period by using a proportional performance method based on costs incurred during the period as compared to total estimated project costs and application of the resulting fraction to the total project fee specified in the contract.
Revenues on fixed-price grants and contracts are recognized using a percentage-of-completion method, which uses assumptions and estimates, as appropriate. These assumptions and estimates are developed in coordination with the principal investigator performing the work under the fixed-price grants to determine levels of accomplishments throughout the life of the grant.
Stock-Based Compensation
We expense all share-based awards to employees and consultants, including grants of stock options and shares, based on their estimated fair value at the date of grant. Costs of all share-based payments are recognized over the requisite service period that an employee or consultant must provide to earn the award (i.e., the vesting period) and allocated to the functional operating expense associated with that employee or consultant.
Fair Value of Financial Instruments
The carrying value of cash and cash equivalents, accounts receivable, short-term investments, accounts payable and accrued expenses approximates fair value due to the relatively short maturity of these instruments. Common stock warrants, which are classified as liabilities, are recorded at their fair market value as of each reporting period.
The measurement of fair value requires the use of techniques based on observable and unobservable inputs. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect our market assumptions. The inputs create the following fair value hierarchy:
| ● | Level 1 – Quoted prices for identical instruments in active markets. | |
| ● | Level 2 – Quoted prices for similar instruments in active markets; quoted prices for identical or similar instruments in markets that are not active; and model-derived valuations where inputs are observable or where significant value drivers are observable. | |
| ● | Level 3 – Instruments where significant value drivers are unobservable to third parties. |
We use the Black-Scholes model to determine the fair value of certain common stock warrants on a recurring basis and classify such warrants in Level 3. The Black-Scholes model utilizes inputs consisting of: (i) the closing price of our common stock; (ii) the expected remaining life of the warrants; (iii) the expected volatility using a weighted-average of historical volatilities of CBLI and a group of comparable companies; and (iv) the risk-free market rate.
As of March 31, 2015, we held approximately $4.7 million in accrued expenses classified as Level 3 securities for warrants to purchase common stock and for compensatory stock options not yet issued.
Income Taxes
Determining the consolidated provision for income tax expense, deferred tax assets and liabilities and related valuation allowance, if any, involves judgment. On an on-going basis, we evaluate whether a valuation allowance is needed to reduce our deferred income tax assets to an amount that is more likely than not to be realized. The evaluation process includes assessing historical and current results in addition to future expected results. Upon determining that we would be able to realize our deferred tax assets, an adjustment to the deferred tax valuation allowance would increase income in the period we make such determination.
| 37 |
Revenue
Our revenue originates from grants and contracts from both United States federal government sources and Russian Federation government sources and service contracts with Incuron. U.S. federal grants and contracts are provided to advance research and development for product candidates that are of interest for potential sale to the U.S. Department of Defense, or DoD, or the Biomedical Advanced Research and Development Authority of the U.S. Department of Health and Human Services, or BARDA. State grants are usually designed to stimulate economic activity. Russian government contracts are provided to develop the biotechnology and pharmaceutical industries in Russia. We provide various research, management, business development and clinical advisory and management services to Incuron.
Research and Development Expenses
Research and development, or R&D, costs are expensed as incurred. Advance payments are deferred and expensed as performance occurs. R&D costs include the cost of our personnel consisting of salaries, incentive and stock-based compensation, out-of-pocket pre-clinical and clinical trial costs usually associated with contract research organizations, drug product manufacturing and formulation and a pro-rata share of facilities expense and other overhead items.
General and Administrative Expenses
General and administrative, or G&A, functions include executive management, finance and administration, government affairs and regulations, corporate development, human resources, legal and compliance. The specific costs include the cost of our personnel consisting of salaries, incentive and stock-based compensation, out-of-pocket costs usually associated with attorneys (both corporate and intellectual property), bankers, accountants and other advisors and a pro-rata share of facilities expense and other overhead items.
Other Income and Expenses
Other recurring income and expenses primarily consists of interest income on our investments, changes in the market value of our derivative financial instruments and foreign currency transaction gains or losses.
THREE MONTHS ENDED MARCH 31, 2015 COMPARED TO THREE MONTHS ENDED MARCH 31, 2014
Revenue
Revenue decreased from $1.3 million for the three months ended March 31, 2014 to $0.6 million for the three months ended March 31, 2015, representing a decrease of $0.7 million, or 54%. During these periods, we received revenues associated with our contracts and/or grants from the Department of Defense, or DoD, the Ministry of Industry and Trade of the Russian Federation, or MPT, and the Skolkovo Foundation, or Skolkovo. The revenues related to our contracts and grants are cost-based and vary as a direct function of the underlying contracted work, which varies between periods. DoD and Skolkovo contracts completed in the first half of 2014 and there were differences in the underlying research activities associated with the MPT contracts, which collectively resulted in decreased revenues. Additionally, beginning in December 2014, we recognized service contract revenue from Incuron, LLC, which was deconsolidated in the fourth quarter of 2014. The revenue differences related to our contracts, grants, and service contracts between the periods are set forth in the following table:
| Three Months Ended March 31, | ||||||||||||||
| Funding Source | Program | 2015 | 2014 | Variance | ||||||||||
| DoD | MCS Contract (1) | $ | - | $ | 23,390 | $ | (23,390 | ) | ||||||
| MPT | CBLB612 Pre-clinical (2) | 39,998 | 180,211 | (140,213 | ) | |||||||||
| MPT | Entolimod Colorectal Cancer (2) | 217,070 | 37,186 | 179,884 | ||||||||||
| Incuron | Service Contracts | 253,734 | - | 253,734 | ||||||||||
| 510,802 | 240,787 | 270,015 | ||||||||||||
| Skolkovo | Curaxin research (2) | - | 612,659 | (612,659 | ) | |||||||||
| MPT | Xenomycins Pre-clinical (2) | - | 28,605 | (28,605 | ) | |||||||||
| MPT | Mobilan Pre-clinical (2) | 96,527 | 452,203 | (355,676 | ) | |||||||||
| $ | 607,329 | $ | 1,334,254 | $ | (726,925 | ) | ||||||||
| (1) | The Medical Countermeasure Systems, or MCS, Contract was formerly known as the Chemical Biological Medical Systems-Medical Identification and Treatment Systems, or CBMS-MITS Contract. |
| (2) | The grants received from Russian government entities are denominated in Russian Rubles (RUB). The revenue above was calculated using average exchange rates for the periods presented. |
| 38 |
We anticipate our revenue over the next year will continue to be derived mainly from government grants and contracts. We plan to submit or have submitted proposals for government grants and contracts to various funding sources that have awarded us grants and contracts in the past, but there can be no assurance that we will receive future funding awards. The following table sets forth information regarding our currently active grants and contracts:
| As of March 31, 2015 | ||||||||||||||||||||||
| Funding Source | Program | Total Award Value | FundedAward Value | Cumulative Revenue | Funded Backlog | Unfunded Backlog | ||||||||||||||||
| MPT | CBLB612 Pre-clinical (1) | $ | 3,445,574 | $ | 3,445,574 | $ | 2,916,363 | $ | 529,211 | $ | - | |||||||||||
| MPT | Entolimod Colorectal Cancer (1) | 3,194,560 | 2,513,803 | 2,031,406 | 482,397 | 680,757 | ||||||||||||||||
| 6,640,134 | 5,959,377 | 4,947,768 | 1,011,609 | 680,757 | ||||||||||||||||||
| MPT | Mobilan Pre-clinical (1) | 3,307,134 | 2,626,377 | 2,143,979 | 482,397 | 680,757 | ||||||||||||||||
| $ | 9,947,268 | $ | 8,585,754 | $ | 7,091,748 | $ | 1,494,006 | $ | 1,361,514 | |||||||||||||
| (1) | The grants received from MPT are denominated in Russian Rubles (RUB). Cumulative Revenue includes contract receipts-to-date and outstanding receivables. Backlog amounts are valued at the period end exchange rate. Funded Award Value is the sum of Cumulative Revenue and Funded Backlog. Total Award Value is the sum of Funded Award Value and Unfunded Backlog. |
Research and Development Expenses
R&D expenses decreased from $2.4 million for the three months ended March 31, 2014 to $1.6 million for the three months ended March 31, 2015, representing a decrease of $0.8 million, or 33%. $0.5 million of this decrease was due to the deconsolidation of Incuron, which occurred in the fourth quarter of 2014. The remaining net reduction of $0.3 million was due to variances in the levels of outsourced research as set forth, by drug candidate, as noted in the table below, with the most significant variances being an increase in the cost of CBLB612 development due to an active Phase 1 trial during the three months ended March 31, 2015 that was not active during the first quarter of 2014 and a reduction in Mobilan preclinical development as Panacela was planning for the commencement of a Phase 1 trial during Q1 2015, which was less expensive than the pre-clinical development underway in the first quarter of 2014.
| Three
Months Ended March 31, | ||||||||||||
| 2015 | 2014 | Variance | ||||||||||
| Entolimod for Biodefense Applications | $ | 905,483 | $ | 882,107 | $ | 23,376 | ||||||
| CBLB612 | 250,625 | 135,719 | 114,906 | |||||||||
| Entolimod for Oncology Indications | 224,453 | 197,675 | 26,778 | |||||||||
| 1,380,561 | 1,215,501 | 165,060 | ||||||||||
| Curaxins | 158,277 | 641,209 | (482,932 | ) | ||||||||
| Panacela product candidates | 72,132 | 583,063 | (510,931 | ) | ||||||||
| Total research & development expenses | $ | 1,610,970 | $ | 2,439,773 | $ | (828,803 | ) | |||||
General and Administrative Expenses
G&A expenses decreased from $2.4 million for the three months ended March 31, 2014 to $2.3 million for the three months ended March 31, 2015, representing a decrease of $0.1 million, or 4%. $0.2 million of this decrease was due to the deconsolidation of Incuron, which occurred in the fourth quarter of 2014. In addition, compensation expense decreased by $0.2 million and recurring professional fees decreased by $0.3 million. These reductions were partially offset by a one-time increase of $0.6 million related to costs associated with our equity offering in February 2015, more fully described in Note 5 “Stockholders’ Equity” to the unaudited consolidated financial statements. The majority of the costs of the February equity offering were expensed, and not otherwise charged to equity, as the majority of the net proceeds were considered derivative liabilities.
| 39 |
Other Income and Expenses
Other income decreased from $1.6 million for the three months ended March 31, 2014 to $0.4 million of other expense for the three months ended March 31, 2015, representing a decrease of $2.0 million, or -124%. Variances include an income reduction and a new expense, offset by expense reductions. The income reduction was attributable to a $2.1 million variance related to our warrant liability. The new expense was attributable to $0.2 million related to our equity in the loss of Incuron accounted for as an equity investment during the three months ended March 31, 2015. In combination, these items reduced income by $2.3 million and were offset by expense reductions of $0.2 million of less interest accrued on our lower outstanding loan balance with Hercules and $0.1 million in less foreign exchange losses.
YEAR ENDED DECEMBER 31, 2014 COMPARED TO YEAR ENDED DECEMBER 31, 2013
Revenue
Revenue decreased from $8.5 million for the year ended December 31, 2013 to $3.7 million for the year ended December 31, 2014, representing a decrease of $4.8 million, or 56%. In the year ended December 31, 2014, we received revenues associated with our contracts and/or grants from DoD, Ministry of Industry and Trade of the Russian Federation, or MPT, and Skolkovo Foundation. The revenues related to our contracts and grants are cost-based and vary as a direct function of the underlying contracted work, which varies between periods. For instance, both the DoD and Skolkovo Foundation contracts completed in the first half of 2014, the Xenomycin MPT contract was closed in the third quarter of 2014 and there were differences in the underlying research activities associated with the other MPT contracts, which collectively resulted in decreased revenues. Additionally, we received service contract revenue from Incuron after its deconsolidation. The revenue differences related to our contracts, grants and service contracts between the periods and details regarding the sources of our government grant and contract revenue are set forth in the following table:
| Year Ended December 31, | ||||||||||||||
Funding Source | Program | 2014 | 2013 | Variance | ||||||||||
| DoD | MCS Contract (1) | $ | 23,390 | $ | 1,511,812 | $ | (1,488,422 | ) | ||||||
| MPT | CBLB612 Pre-clinical (2) | 519,302 | 1,065,454 | (546,152 | ) | |||||||||
| MPT | Entolimod Colorectal Cancer (2) | 969,252 | 937,499 | 31,753 | ||||||||||
| DoD | DTRA Contract | — | 765,096 | (765,096 | ) | |||||||||
| Incuron | Service Contracts | 154,687 | — | 154,687 | ||||||||||
| 1,666,631 | 4,279,861 | (2,613,230 | ) | |||||||||||
| Skolkovo | Curaxin research (2) | 1,000,770 | 2,060,080 | (1,059,310 | ) | |||||||||
| MPT | Xenomycins Pre-clinical (2) | 28,605 | 1,210,526 | (1,181,921 | ) | |||||||||
| MPT | Mobilan Pre-clinical (2) | 1,005,893 | 937,499 | 68,394 | ||||||||||
| $ | 3,701,899 | $ | 8,487,966 | $ | (4,786,067 | ) | ||||||||
(1) |
The MCS Contract was formerly known as the CBMS-MITS Contract. The contracts received from Russian government entities are denominated in Russian Rubles (RUR). The revenue above was calculated using average exchange rates for the periods presented. |
Research and Development Expenses
R&D expenses decreased from $19.5 million for the year ended December 31, 2013 to $9.7 million for the year ended December 31, 2014, representing a decrease of $9.8 million, or 50%. $5.3 million of this net decrease related to reduced utilization of third-party vendors including reductions of $3.1 million for entolimod for a biodefense indication, $1.8 million for Panacela compounds, $0.7 million for Curaxin compounds, and $0.7 million for CBLB612, which were partially offset by a $1.0 million increase in entolimod for oncology applications. In addition, compensation expense decreased by $3.7 million primarily attributable to personnel transferred to Buffalo BioLabs, LLC in the fourth quarter of 2013. Of the $3.7 million in reduced compensation expense, $3.6 million relates to cash compensation and $0.1 million relates to non-cash compensation. Reduced facilities and travel costs accounted for $0.8 million of the net decrease. The following table sets forth our total R&D costs by drug candidate:
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Variance | ||||||||||
| Entolimod for Biodefense Indication | $ | 3,926,110 | $ | 9,337,962 | $ | (5,411,852 | ) | |||||
| CBLB612 | 508,247 | 1,149,098 | (640,851 | ) | ||||||||
| Entolimod for Oncology Indications | 1,289,692 | 628,797 | 660,895 | |||||||||
| 5,724,049 | 11,115,857 | (5,391,808 | ) | |||||||||
| Curaxins | 2,708,516 | 4,459,854 | (1,751,338 | ) | ||||||||
| Panacela product candidates | 1,221,579 | 3,950,239 | (2,728,660 | ) | ||||||||
| Total research & development expenses | $ | 9,654,144 | $ | 19,525,950 | $ | (9,871,806 | ) | |||||
| 40 |
General and Administrative Expenses
G&A expenses decreased from $12.0 million for the year ended December 31, 2013 to $8.5 million for the year ended December 31, 2014, representing a decrease of $3.5 million, or 29%. $2.2 million of the reduction was due to a reduction in costs related to our personnel and consultants, of which $1.3 million relates to cash compensation and $0.9 million relates to non-cash compensation. $0.8 million was due to a reduced usage of outside professionals. In addition, $0.3 million was due to charges in 2013 associated with the execution of a bank guarantee at Biolab 612 and a non-cash idle facilities reserve. Travel expenses decreased by $0.2 million.
Other Income and Expenses
Other net income increased from $3.0 million for the year ended December 31, 2013 to $14.5 million for the year ended December 31, 2014, representing a net income increase of $11.5 million, or 38.3%. The net increase was primarily attributable to a $14.2 million gain on the deconsolidation of Incuron described in Note 5 to the Consolidated Financial Statements which was partially offset by $1.1 million of other expenses related to interest associated with our loans, $1.1 million due to foreign exchange losses incurred due the depreciation of the ruble, a $0.2 million loss attributable to changes in the value of our warrant liability, and a $0.3 million loss in our equitable share of Incuron’s operating results for the period post deconsolidation.
YEAR ENDED DECEMBER 31, 2013 COMPARED TO YEAR ENDED DECEMBER 31, 2012
Revenue
Revenue increased from $3.6 million for the year ended December 31, 2012 to $8.5 million for the year ended December 31, 2013, representing an increase of $4.9 million, or 138%. This increase consisted of increases of $1.0 million from U.S. government contracts and $3.9 million from Russian government contracts, primarily due to increased development activities under Russian government contracts, including two new contracts from MPT that were awarded in the fourth quarter of 2013 for development of an oncology application of entolimod and Mobilan.
The following table sets forth details regarding the sources of our government grant and contract revenue in 2012 and 2013:
| Year Ended December 31, | ||||||||||||||
Funding Source | Program | 2013 | 2012 | Variance | ||||||||||
| DoD | MCS Contract (1) | $ | 1,511,812 | $ | 1,113,830 | $ | 397,982 | |||||||
| MPT | CBLB612 Pre-clinical (2) | 1,065,454 | 888,686 | 176,768 | ||||||||||
| MPT | CBLB502 Colorectal Cancer (2) | 937,499 | — | 937,499 | ||||||||||
| DoD | DTRA Contract | 765,096 | 130,149 | 634,947 | ||||||||||
| 4,279,861 | 2,132,665 | 2,147,196 | ||||||||||||
| Skolkovo Foundation | Curaxin research (2) | 2,060,080 | 488,781 | 1,571,299 | ||||||||||
| MPT | Xenomycins Pre-clinical (2) | 1,210,526 | 949,264 | 261,262 | ||||||||||
| MPT | Mobilan Pre-clinical (2) | 937,499 | — | 937,499 | ||||||||||
| $ | 8,487,966 | $ | 3,570,710 | $ | 4,917,256 | |||||||||
| (1) | The MCS Contract was formerly known as the CBMS-MITS Contract. |
| (2) | The contracts received from Russian government entities are denominated in Russian Rubles (RUR). The revenue above was calculated using average exchange rates for the periods presented. |
| 41 |
Research and Development Expenses
R&D expenses decreased from $22.5 million for the year ended December 31, 2012 to $19.5 million for the year ended December 31, 2013, representing a decrease of $3.0 million, or 13%. This net decrease primarily reflected decreases of $2.7 million related to entolimod’s biodefense indication, as the development in 2013 focused on a less expensive, non-irradiated non-human primate study, and $1.6 million related to a narrowed scope of development for the compounds under development by Panacela. These decreases were partially offset by an increase of $1.2 million related to Curaxin development, primarily due to the initiation of a clinical trial in the United States for CBL0137. The following table sets forth our R&D expenses by drug candidate:
| 2013 | 2012 | Variance | ||||||||||
| Entolimod for Biodefense Applications | $ | 9,337,962 | $ | 11,986,020 | $ | (2,648,058 | ) | |||||
| CBLB612 | 1,149,098 | 1,039,832 | 109,266 | |||||||||
| Entolimod for Oncology Indications | 628,797 | 605,365 | 23,432 | |||||||||
| 11,115,857 | 13,631,217 | (2,515,360 | ) | |||||||||
| Curaxins | 4,459,854 | 3,276,866 | 1,182,988 | |||||||||
| Panacela product candidates | 3,950,239 | 5,593,722 | (1,643,483 | ) | ||||||||
| Total research & development expenses | $ | 19,525,950 | $ | 22,501,805 | $ | (2,975,855 | ) | |||||
General and Administrative Expenses
G&A costs increased from $11.1 million for the year ended December 31, 2012 to $12.0 million for the year ended December 31, 2013, representing an increase of $0.9 million, or 8%. This net increase was primarily attributable to increases of $1.0 million related to our Russian-based subsidiary and joint ventures, $0.4 million in corporate legal and intellectual property fees and $0.4 million due to a reduction in incentive tax refunds. These increases were partially offset by decreases of $0.7 million in business development expenses and $0.2 million in non-cash stock-based compensation.
Other Income and Expenses
Other income decreased from $7.6 million for the year ended December 31, 2012 to $2.9 million for the year ended December 31, 2013, representing a decrease of $4.7 million, or 61%. The change in the fair market value of our stock yielded a change in the fair market value of our accrued warrant liability, which was the primary reason for this decrease.
Liquidity and Capital Resources
We have incurred net losses of $138.3 million from our inception through March 31, 2015. Historically, we have not generated, and do not expect to generate in the immediate future, revenue from sales of product candidates. Since our founding in 2003, we have funded our operations through a variety of means:
| ● | Through March 31, 2015, we have raised $121.1 million of net equity capital, including amounts received from the exercise of options and warrants. We have also received $5.8 million in net proceeds from the issuance of long-term debt instruments. | |
| ● | DoD and the Biomedical Advanced Research and Development Authority of the U.S. Department of Health and Human Services, or BARDA, have funded grants and contracts totaling $44.6 million for the development of entolimod for biodefense indication; |
| 42 |
| ● | Entities affiliated with the Russian Federation have awarded us contracts totaling $13.5 million through a series of awards of over $3.2 million each. All awards are valued based on revenue recognized to date, with the remaining backlog valued at the March 31, 2015 exchange rate. These contracts include a requirement for us to contribute matching funds, which we have satisfied or expect to satisfy with both the value of developed intellectual property at the time of award, incurred development expenses and future expenses; | |
| ● | We have been awarded $4.0 million in grant and contracts not described above, all of which has been recognized at March 31, 2015; | |
| ● | Panacela was formed to develop and commercialize preclinical compounds, which were transferred to Panacela through assignment and lease agreements. Open Joint Stock Company “Rusnano” contributed $9.0 million at formation and has options to contribute up to $15.5 million of additional funding. CBLI contributed $3.0 million plus intellectual property at formation and has an option to contribute additional capital based on agreed-upon terms. As of the date of this filing, CBLI owns 60.47% of Panacela; and, | |
| ● | Incuron was formed to develop and commercialize the Curaxins product line, including their lead oncology drug candidate CBL0137. BCV committed to contribute equity capital to Incuron as milestones were achieved, gradually increasing their ownership in Incuron. Since inception, BCV has contributed approximately $17.0 million, growing their ownership percentage to 53.04%, leaving us with an ownership percentage of 46.96%. In April, 2015, we sold 75% of our ownership interest to Dr. Mogutov, BCV’s Chairman, for approximately $3 million and also gave him an option to buy our remaining 25% ownership interest for approximately $1 million through December 31, 2015. Simultaneously we assigned the rest of our Curaxin intellectual property to Incuron for a 2% royalty (see also Note 8, Subsequent Events to our Consolidated Financial Statements). |
At March 31, 2015, we had cash, cash equivalents and short-term investments of $5.0 million. Of that total, $1.4 million was restricted for the use of our consolidated joint venture, Panacela, leaving $3.6 million available for general use. Furthermore, Panacela and Biolab 612 had an additional $0.8 million of restricted cash held for performance bonds in connection with their respective MPT grants, which are classified as a long-term asset.
Variances in cash flows for the three months ended March 31, 2015 compared to the three months ended March 31, 2014
Operating Activities
Net cash used in operations decreased by $0.6 million to $2.2 million for the three months ended March 31, 2015 from $2.8 million for the three months ended March 31, 2014. After adjusting for non-cash items, the net loss decreased by $0.9 million, while changes in working capital used cash and cash equivalents of $0.3 million between the periods.
Investing Activities
Net cash provided by investing activities increased by $0.1 million for the three months ended March 31, 2015 due to the release of $0.8 million of restricted cash which was partially offset by a $0.7 million investment in short-term securities.
Financing Activities
Cash flows provided by financing activities decreased by $3.0 million to $3.3 million for the three months ended March 31, 2015, as compared to $6.3 million for the three months ended March 31, 2014. The decrease is primarily related to the difference in net proceeds received from equity offerings closed in each period.
Variances in cash flows for the year ended December 31, 2015 compared to the year ended December 31, 2014
Operating Activities
Net cash used in operations decreased by $8.6 million to $14.5 million for the year ended December 31, 2014 from $23.1 million for the year ended December 31, 2013. After adjusting for non-cash items, the net loss decreased by $5.7 million, while changes in working capital provided cash and cash equivalents of $2.9 million.
| 43 |
Investing Activities
Net cash (used in)/provided by investing activities changed by $2.3 million to $1.8 million used for the year ended December 31, 2014 from $0.6 million provided for the year ended December 31, 2013. This change was primarily attributable to cash divested upon the deconsolidation of Incuron.
Financing Activities
Cash provided by financing activities increased by $3.3 million to $10.6 million for the year ended December 31, 2014, from $7.3 million for the year ended December 31, 2013. During the year ended December 31, 2014, we raised $9.7 million, net of offering costs, through the sale of equity securities, BCV made an equity investment in Incuron of $5.2 million, we repaid $4.2 million of debt owed to Hercules and we made $0.1 million in capital lease payments.
Other
We have incurred cumulative net losses and expect to incur additional losses related to our research and development activities. We do not have commercial products and have limited capital resources. We will need additional funds to complete the development of our product candidates. Our plans with regard to these matters may include seeking additional capital through a combination of government contracts, collaborative agreements, strategic alliances, research grants and equity and debt financing. There is no assurance that we will be successful in obtaining additional financing on commercially reasonable terms or that we will be able to secure funding from anticipated government contracts and grants. Additionally, our ability to raise funds through equity or debt financing is currently limited by our requirement to receive stockholder approval of certain of the terms of our February 2015 equity transaction.
We believe that our funds as of March 31, 2015, combined with the net proceeds of approximately $3 million from our April 2015 sale of 35.22% of our Incuron equity interests, will be sufficient to fund our projected operating requirements into September 2015. In order to finance the continued development of our product and to otherwise satisfy obligations as they mature, we will likely seek to sell additional equity or debt securities or obtain additional credit facilities. Our success is dependent upon commercializing our research and development programs and our ability to obtain adequate future financing. There can be no assurance that we will be able to obtain future financing or, if obtained, what the terms of such future financing may be, or that any amount that we are able to obtain will be adequate to support our working capital requirements until we achieve profitable operations. If we are unable to raise adequate capital and/or achieve profitable operations, future operations might need to be scaled back or discontinued. The financial statements do not include any adjustments relating to the recoverability of the carrying amount of recorded assets and liabilities that might result from the outcome of these uncertainties.
Our auditors, Meaden & Moore, LLP, have indicated in their report on our financial statements for the fiscal year ended December 31, 2014, that conditions exist that raise substantial doubt about our ability to continue as a going concern due to our recurring losses and substantial decline in our working capital. Our ability to continue as a going concern will depend upon the availability and terms of future funding and our ability to limit our expenses. If we are unable to achieve these goals, our business would be jeopardized and the Company may not be able to continue. If we ceased operations, it is likely that all of our investors would lose their investment.
Off-Balance Sheet Arrangements
The Company did not have any off-balance sheet arrangements at March 31, 2015 or December 31, 2014.
GENERAL OVERVIEW
We are an innovative biopharmaceutical company seeking to develop first-in-class pharmaceuticals designed to address diseases with significant unmet medical need. We combine our proven scientific expertise and our depth of knowledge about our products’ mechanisms of action into a passion for developing drugs to save lives. Our programs are focused on the use of novel toll-like receptor agonists to activate the immune system for therapeutic benefit. Our proprietary drug candidates act via unique mechanisms that are designed to kill cancer and protect healthy cells. We conduct business in the United States and the Russian Federation. CBLI and our joint ventures, Incuron, LLC, or Incuron, and Panacela Labs, Inc., or Panacela, each have worldwide development and commercialization rights to product candidates in development, subject to certain financial obligations to our current licensors. CBLI’s most advanced product candidate is entolimod, which we are developing as a radiation countermeasure and an immunotherapy for oncology and other indications.
Entolimod is a Toll-like receptor 5, or TLR5, agonist, which we are developing as a radiation countermeasure for prevention of death from Acute Radiation Syndrome, or ARS, and as an oncology drug. We believe that entolimod is the most efficacious radiation countermeasure currently in development. Following is a summary of the clinical development of entolimod to date and regulatory status.
| 44 |
Entolimod is being developed under the U.S. Food & Drug Administration’s, or FDA’s, Animal Efficacy Rule, or the Animal Rule, for the indication of reducing the risk of death following exposure to potentially lethal irradiation occurring as a result of a radiation disaster (see “Government Regulation - Animal Rule”). We have completed two clinical studies designed to evaluate the safety, pharmacokinetics and pharmacodynamics of entolimod in a total of 150 healthy volunteers. We have completed a Good Laboratory Practices, or GLP, randomized, blinded, placebo-controlled, pivotal study designed to evaluate the dose-dependent effect of entolimod on survival and biomarker induction in 179 non-human primates exposed to 7.2 Gy total body irradiation when entolimod or placebo were administered at 25 hours after radiation exposure. We have completed a GLP, randomized, open-label, placebo-controlled, pivotal study designed to evaluate the dose-dependent effect of entolimod on biomarker induction in 160 non-irradiated non-human primates. We met with the FDA in July 2014 to present our human dose-conversion and to discuss our intent to submit a pre-Emergency Use Authorization, or pre-EUA. The FDA confirmed that our existing efficacy and safety data and animal-to-human dose conversion are sufficient to proceed with a pre-EUA submission and agreed to accept a pre-EUA submission for review. We are currently preparing the pre-EUA dossier, which we anticipate filing in the first half of 2015. If the FDA authorizes the application, then Federal agencies are free to procure drug product for stockpiling so that the drug is available to distribute in the event of an emergency, i.e. prior to the drug being formally approved by FDA under a Biologics License Application, or BLA.
In January and April 2015, we announced the receipt of recommendations from the Department of Defense, or DoD, Congressionally Directed Medical Research Programs, or CDMRP in support of DoD funding for two independent CBLI proposals to support further development of entolimod as a medical radiation countermeasure. These proposals aim to conduct several pivotal animal efficacy studies and a clinical study to support a BLA. The Company’s receipt of these awards is subject to successful negotiations and availability of funds.
Additionally, we completed enrollment in a Phase 1 open-label, dose-escalation trial of entolimod in patients with advanced cancer in the United States and began dosing in a small expansion study in the Russian Federation, which is enrolling additional patients at the highest doses achieved in the US study. Both studies include evaluation of immune cell response to administrations of entolimod. Preliminary evaluations of the completed study in the United States indicate that the tolerability profile in patients with advanced cancer was similar to that observed in the two previously conducted studies in 150 healthy volunteers. Initial assessments of immunological response were consistent with TLR5 activation. Early analyses indicate that stable disease was observed in several patients with heavily pretreated cancers. Complete data for this study will be presented during the Developmental Therapeutics - Immunotherapy poster session at the 2015 annual meeting of the American Society of Clinical Oncology (ASCO) on May 30, 2015 in Chicago, IL.
SA-702 is a new therapeutic approach with entolimod that employs the immunopotentiating properties of the drug together with alum (aluminum salts) as a vaccine adjuvant. In this context, entolimod’s immune activity would be harnessed to enhance the efficacy of vaccines by eliciting a stronger immune response to the vaccine’s particular antigen. Many vaccines require an adjuvant to induce sufficient immune response. It is estimated that about one half of 30 of the most common vaccines approved by FDA contain alum as an adjuvant. Until recently, alum was the only adjuvant approved by FDA, but often alum alone does not allow new vaccines to reach sufficient clinical potency. A shortage of effective and safe adjuvants is a major bottleneck in vaccine development. A newer generation of vaccine boosters combine classic adjuvants mixed with immunomodulators (like entolimod). We have collaborated with academic investigators who have performed preclinical studies that support the adjuvant potential of SA-702 in enhancing vaccine immune and wish to translate these data to clinical studies to document the immunopotentiating effect of the drug.
CBLB612 is a proprietary compound based upon a natural activator of another tissue-specific component of the innate immune system, the TLR2/TLR6 heterodimeric receptor. CBLB612 is a pharmacologically optimized synthetic molecule that structurally mimics naturally occurring lipopeptides of Mycoplasma (a genus of parasitic bacteria) and activates NF-kB pro-survival and immunoregulatory signaling pathways via specific binding to TLR2 on a subset of body tissues and cell types that express this receptor. Preclinical studies have shown that CBLB612 stimulates white blood cell regeneration. More recent research indicates that stimulation of these toll-like receptors may also enhance anti-tumor efficacy. We believe an opportunity may exist for CBLB612 to offer a single-dose alternative to existing hemopoietic growth factors, such as filgrastim (Neupogen™), which comprises a multi-billion-dollar market in support of chemotherapy administration. Filgrastim modestly shortens the duration of chemotherapy-related neutropenia, but does not improve thrombocytopenia or anemia, and does not provide antitumor efficacy. In October 2014, we initiated a Phase 1, single-center, blind, placebo-controlled, single ascending-dose study in the Russian Federation to evaluate the safety, tolerability, and pharmacodynamic effects of CBLB612 in healthy volunteers. The study was performed under a 139-million-ruble matching funds development contract that we received in July 2012 from MPT. We announced that we had completed dosing in this study in March 2015. A maximum tolerated dose was established and changes in blood counts were observed, including neutrophilia. Induction of a variety of cytokines was also documented. Full results will be reported in 2015. We believe the Phase 1 data support a Phase 2 study in a clinical model of chemotherapy-induced myelosuppression. Plans for this study are already underway and will be supported by the same MPT contract. We licensed CBLB612 to Zhejiang Hisun Pharmaceutical Co., Ltd. for the territories of China, Taiwan, Hong Kong and Macau. We have rest-of-world development and commercialization rights to CBLB612.
CORPORATE INFORMATION
We were incorporated in Delaware in June 2003 as a spin-off company from The Cleveland Clinic. We exclusively license our founding intellectual property from The Cleveland Clinic. In 2007, we relocated our operations to Buffalo, New York and became affiliated with Roswell Park Cancer Institute, or RPCI, through technology licensing and research collaboration relationships. Our common stock is listed on the NASDAQ Capital Market under the symbol “CBLI.”
Our principal executive offices are located at 73 High Street, Buffalo, New York 14203, and our telephone number at that address is (716) 849-6810.
The CBLI logo and CBLI product names are proprietary trade names of CBLI, its subsidiary or joint ventures. We may indicate U.S. trademark registrations and U.S. trademarks with the symbols “®” and “TM”, respectively. Third-party logos and product/trade names are registered trademarks or trade names of their respective owners.
| 45 |
PRODUCT DEVELOPMENT PIPELINE
Our product development programs arise from both internally developed and in-licensed intellectual property from our innovation partners, The Cleveland Clinic and RPCI. In building the Company’s product development pipeline, we intentionally pursued targets with applicability across multiple therapeutic areas and indications. This approach gives us multiple product opportunities and ensures that our success is not dependent on any single product or indication.
Our primary product development programs and their respective development stages are illustrated below:

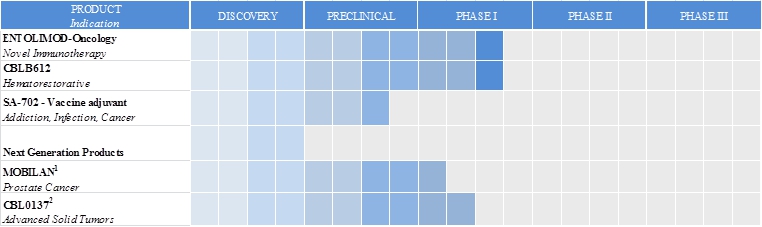
(1) Mobilan is in development by Panacela.
(2) CBL0137 is in development by Incuron.
Entolimod is a Toll-like receptor 5, or TLR5, agonist, which we are developing as a radiation countermeasure for prevention of death from Acute Radiation Syndrome, or ARS, and as an oncology drug. We believe that entolimod is the most efficacious radiation countermeasure currently in development. Following is a summary of the clinical development of entolimod to date and regulatory status:
Our product development efforts were initiated by discoveries related to apoptosis, a tightly regulated form of cell death that can occur in response to internal stresses or external events such as exposure to radiation or toxic chemicals. Apoptosis is a major determinant of the tissue damage that occurs in a variety of medical conditions involving ischemia, or temporary loss of blood flow, such as cerebral stroke, heart attack and acute renal failure. In addition, apoptotic loss of cells of the hematopoietic system and gastrointestinal tract is largely responsible for the acute lethality of high-dose radiation exposure. On the other hand, apoptosis is also an important protective mechanism that allows the body to eliminate defective cells such as those with cancer-forming potential.
We have developed novel strategies to target the molecular mechanisms controlling apoptotic cell death for therapeutic benefit. These strategies take advantage of the fact that tumor and normal cells respond to apoptosis-inducing stresses differently due to tumor-specific defects in cellular signaling pathways such as inactivation of p53 (a pro-apoptosis regulator) and constitutive activation of NF-kB (a pro-survival regulator).
Thus, we designed two oppositely-directed general therapeutic concepts:
| (a) | temporary and reversible suppression of apoptosis in normal cells to protect healthy tissues from stress-induced damage using compounds we categorize as Protectans, which include entolimod and CBLB612; and, |
| (b) | reactivation of apoptosis in tumor cells to eliminate cancer using compounds we categorize as Curaxins, which includes CBL0137. |
| 46 |
Entolimod Biodefense Indication
Our lead Protectan product candidate is entolimod, an engineered derivative of the Salmonella flagellin protein that was designed to retain its specific TLR5-activating capacity while increasing its stability, reducing its immunogenicity and enabling high-yield production. We are developing entolimod for dual indications: (i) as a radiation countermeasure for prevention of death from ARS, which we refer to as a Biodefense Indication; and (ii) as an oncology drug (discussed below).
The market for radiation countermeasures grew dramatically following the September 11, 2001 terrorist attacks and the subsequent use of anthrax in a biological attack in the United States. Terrorist activities worldwide have continued in the intervening years and the possibility of chemical, biological, radiation and nuclear attacks continues to represent a perceived threat for governments world-wide. In addition to the U.S. government, we believe the potential markets for the sale of radiation countermeasures include U.S. and foreign state and local governments, including defense and public health agencies, non-governmental organizations and multinational companies, transportation and security companies, healthcare providers, hospitals and clinics, and nuclear power facilities.
Acute high-dose whole body or significant partial body radiation exposure induces massive apoptosis of cells of the hematopoietic system and gastrointestinal tract, which leads to ARS, a potentially fatal condition for which there is currently only one FDA-approved treatment. The threat of ARS is primarily limited to emergency/defense scenarios and is significant given the possibility of nuclear/radiological accidents, warfare or terrorist incidents. The scale of possible exposure (number of people affected) has been estimated by the U.S. government to be in the range of 500,000 based on a modeled 10-kiloton device detonation in New York City. And we believe the current lack of approved efficacious treatments to deal with such an event makes entolimod a compelling product candidate. It is not feasible or ethical to test the efficacy of entolimod as a radiation countermeasure in humans. Therefore, we are developing entolimod under the FDA’s Animal Rule guidance (see “Government Regulation - Animal Rule”). The Animal Rule authorizes the FDA to rely on data from animal studies to provide evidence of a product’s effectiveness under circumstances where there is a reasonably well-understood mechanism for the activity of the product. Under these requirements, and with the FDA’s prior agreement, medical countermeasures, like entolimod, may be approved for use in humans based on evidence of effectiveness derived from appropriate animal studies, evidence of safety derived from studies in humans and any additional supporting data.
We met with the FDA in July 2014 to discuss our intent to submit an application to obtain pre-EUA status for entolimod. As a result of this meeting, the FDA agreed to accept a pre-EUA submission for review. We plan to submit a pre-EUA dossier in the first half of 2015 using the human dose of entolimod that we determined through our proprietary dose conversion methodology, which utilizes the data from our pivotal non-human primate studies and our clinical studies of entolimod in healthy volunteers. If authorized, pre-EUA status will allow entolimod to be sold into the Strategic National Stockpile and used under a state of emergency. Such authorization is not equivalent to full licensure through approval of a BLA, but precedes full licensure, and, importantly, would position entolimod for potential sales in advance of full licensure in the United States. We also believe pre-EUA status will position us to explore sales opportunities with foreign governments.
Our pivotal efficacy study conducted in 179 non-human primates demonstrated with a high degree of statistical significance that injection of a single dose of entolimod given to rhesus macaques 25 hours after exposure to a 70% lethal dose of total body irradiation improved animal survival by nearly three-fold compared to the control group. Dose-dependence of entolimod’s efficacy was demonstrated with doses above the minimal efficacious dose establishing a plateau at approximately 75% survival at 60 days after irradiation, as compared to 27.5% survival in the placebo-treated group.
Our pivotal study conducted in 160 non-irradiated non-human primates established the dose-dependent effect of entolimod on biomarkers for animal-to-human dose conversion.
Our clinical studies of entolimod in 150 healthy human subjects demonstrated the safety profile of entolimod and established the dose-dependent effect of entolimod on efficacy biomarkers in humans. In these studies, and in a Phase 1 oncology study that concluded enrollment in September 2014, transient decrease in blood pressure and changes in clinical laboratory blood parameters were observed along with transient mild to moderate flu-like syndrome. Such effects are the most common adverse events and they are linked to up-regulation of cytokines that are also biomarkers for efficacy.
| 47 |
The FDA has granted Fast Track status to entolimod (see “Government Regulation – Fast Track Designation”) and Orphan Drug status for prevention of death following a potentially lethal dose of total body irradiation during or after a radiation disaster (see “Government Regulation – Orphan Drug Designation”).
In January and April 2015, we announced the receipt of recommendations from the DoD CDMRP in support of DoD funding for two independent CBLI proposals to support further development of entolimod as a medical radiation countermeasure. These proposals aim to conduct several pivotal animal efficacy studies and a clinical study to support a BLA. The Company’s receipt of these awards is subject to successful negotiations and availability of funds.
Entolimod Oncology Indication
In addition to developing entolimod as a radiation countermeasure for prevention of death from ARS, we are also developing entolimod as an oncology drug. We believe that entolimod has the potential to treat cancer by activating the innate and adaptive immune response in patients. In preclinical studies, entolimod produced tissue-specific activation of innate immune responses via interaction with its receptor, TLR5, and the liver was identified as a primary mediator of entolimod activity. Entolimod has also been shown to have a direct cytotoxic effect on tumors expressing TLR5 in animal models. Evaluations of local administration of entolimod in organs expressing TLR5, such as the bladder, have also been performed in animal models.
We have completed enrollment in a Phase 1, open-label, dose-escalation trial of entolimod in patients with advanced cancer in the United States and have begun dosing in a small expansion study in the Russian Federation enrolling additional patients at the highest doses achieved in the US study. Both studies include evaluation of immune cell response to administrations of entolimod. Preliminary evaluations of the completed study in the United States indicate that the tolerability profile in patients with advanced cancer was similar to that observed in two previously conducted studies in 150 healthy volunteers. Initial assessments of immunological response were consistent with TLR5 activation. Early analyses indicate that stable disease was observed in several patients with heavily pretreated cancers. Complete data for the US study will be presented during the Developmental Therapeutics - Immunotherapy poster session at the 2015 annual meeting of the American Society of Clinical Oncology (ASCO) on May 30, 2015 in Chicago, IL. The study in the Russian Federation is the first of two planned studies under a 149-million ruble matching funds development contract that we received in October 2013 from the Ministry of Industry and Trade of the Russian Federation, or MPT (see Item 7. “Management’s Discussion and Analysis of Financial Condition and Results of Operations”).
We have worldwide development and commercialization rights to entolimod.
SA-702
SA-702 is a new therapeutic approach with entolimod that employs the immunopotentiating properties of the drug together with alum (aluminum salts) as a vaccine adjuvant. In this context, entolimod’s immune activity would be harnessed to enhance the efficacy of vaccines by eliciting a stronger immune response to the vaccine’s particular antigen. Many vaccines require an adjuvant to induce sufficient immune response. It is estimated that about one half of 30 of the most common vaccines approved by FDA contain alum as an adjuvant. Until recently, alum was the only adjuvant approved by FDA, but often alum alone does not allow new vaccines to reach sufficient clinical potency. A shortage of effective and safe adjuvants is a major bottleneck in vaccine development. A newer generation of vaccine boosters combine classic adjuvants mixed with immunomodulators (like entolimod). We have collaborated with academic investigators who have performed preclinical studies that support the adjuvant potential of SA-702 in enhancing vaccine immune and wish to translate these data to clinical studies to document the immunopotentiating effect of the drug.
CBLB612
CBLB612 is a proprietary compound based upon a natural activator of another tissue-specific component of the innate immune system, the TLR2/TLR6 heterodimeric receptor. CBLB612 is a pharmacologically optimized synthetic molecule that structurally mimics naturally occurring lipopeptides of Mycoplasma (a genus of parasitic bacteria) and activates NF-kB pro-survival and immunoregulatory signaling pathways via specific binding to TLR2 on a subset of body tissues and cell types that express this receptor. Preclinical studies have shown that CBLB612 stimulates white blood cell regeneration. More recent research indicates that stimulation of these toll-like receptors may also enhance anti-tumor efficacy. We believe an opportunity may exist for CBLB612 to offer a single-dose alternative to existing hemopoietic growth factors, such as filgrastim (Neupogen™), which comprises a multi-billion-dollar market in support of chemotherapy administration. Filgrastim modestly shortens the duration of chemotherapy-related neutropenia, but does not improve thrombocytopenia or anemia, and does not provide antitumor efficacy. In October 2014, we initiated a Phase 1, single-center, blind, placebo-controlled, single ascending-dose study in the Russian Federation to evaluate the safety, tolerability, and pharmacodynamic effects of CBLB612 in healthy volunteers. The study was performed under a 139-million-ruble matching funds development contract that we received in July 2012 from MPT. We announced that we had completed dosing in this study in March 2015. A maximum tolerated dose was established and changes in blood counts were observed, including neutrophilia. Induction of a variety of cytokines was also documented. Full results will be reported in 2015. We believe the Phase 1 data support a Phase 2 study in a clinical model of chemotherapy-induced myelosuppression. Plans for this study are already underway and will be supported by the same MPT contract. We licensed CBLB612 to Zhejiang Hisun Pharmaceutical Co., Ltd. for the territories of China, Taiwan, Hong Kong and Macau. We have rest-of-world development and commercialization rights to CBLB612.
| 48 |
Mobilan
Mobilan is the lead product candidate of our consolidated joint venture Panacela. Mobilan is a nanoparticle- formulated recombinant non-replicating adenovirus that directs expression of TLR5 and its agonistic ligand, flagellin. In pre-clinical studies, delivery of Mobilan to tumor cells results in constitutive autocrine TLR5 signaling and strong activation of the innate immune system with subsequent development of adaptive anti-tumor immune responses. An IND was opened in the Russian Federation in March 2015 for a Phase 1 multicenter, randomized, placebo-controlled, single-blinded study evaluating single injections of ascending doses of Mobilan administered directly into the prostate of patients with prostate cancer. This study is being performed under a 149-million-ruble matching funds development contract that Panacela received in October 2013 from MPT. Panacela holds worldwide development and commercialization rights to Mobilan. As of April 30, 2014, we owned 60.47% of Panacela.
CBL0137 is the lead product candidate of our unconsolidated joint venture Incuron. CBL0137 is a small molecule with a multi-targeted mechanism of action that may be broadly useful for the treatment of many different types of cancer. CBL0137 may offer greater efficacy and substantially lower risk for the development of drug resistance than conventional chemotherapeutic agents. CBL0137 inhibits Nuclear Factor kappa-B, or NF- kB, heat shock factor protein-1, or HSF-1, and hypoxia-inducible factor 1-alpha, or HIF1 alpha; these are transcription factors that are important for the viability of many types of tumors. The drug also activates tumor suppressor protein p53 by modulating intracellular localization and activity of chromatin remodeling complex facilitates chromatin transcription, or FACT. CBL0137 has been shown to be efficacious in pre-clinical models of colon, lung, breast, renal, pancreatic, head and neck and prostate cancers; melanoma; glioblastoma; and neuroblastoma. It has also been shown to be efficacious in pre-clinical models of hematological cancers, including lymphoma, leukemia and multiple myeloma.
Incuron is currently enrolling patients with advanced, resistant solid tumors into two Phase 1 studies, one in the Russian Federation evaluating the oral administration of CBL0137 and one in the United States evaluating the intravenous administration of CBL0137. These studies are designed to investigate the safety, pharmacokinetics, pharmacodynamics, and antitumor activity of CBL0137. Incuron is conducting these parallel evaluations of oral and intravenous routes of administration and continuous low-dose versus interrupted high-dose schedules to reduce the company’s developmental risk by fully characterizing the clinical pharmacology of CBL0137.
Incuron holds worldwide development and commercialization rights to CBL0137. As of April 30, 2015, we owned 11.74% of Incuron.
| 49 |
STRATEGIC PARTNERSHIPS
Since our inception, strategic alliances and collaborations have been integral to our business. We have leveraged the experience, contacts and knowledge of our founders to engage funding partners in the Russian Federation and to develop and maintain academic-corporate innovation partnerships with The Cleveland Clinic and RPCI. Through these partnerships we have collaborated with world-class scientists to develop our novel technologies and accessed non-traditional funding sources, including federal and foreign government contracts and project-oriented funding to support the development of certain of our technologies. We have received project-oriented funding from Russian Federation based venture funds BCV, and Open Joint Stock Company “Rusnano”, or Rusnano, through the formation of Incuron and Panacela, both of which are co-located in the Russian Federation and the United States. We believe that these companies, as well as our wholly-owned subsidiary BioLab 612, may benefit from programs supporting domestic pharmaceutical industry development in the Russian Federation as well as the relative ease of enrolling patients as compared to western markets. We have negotiated exclusive licenses to rights in each of our technologies from The Cleveland Clinic and RPCI.
Rusnano
In October 2011, we entered into our Panacela joint venture with Rusnano to carry out a complete cycle of development and commercialization in the Russian Federation for the treatment of oncological, infectious or other diseases. We invested $3.0 million in Panacela preferred shares and warrants, and, together with certain third-party owners, assigned and/or provided exclusive licenses, as applicable, to Panacela to provide Panacela with worldwide development and commercialization rights to five preclinical product candidates in exchange for Panacela common shares. Rusnano invested $9.0 million in Panacela preferred shares and warrants. In 2013, Rusnano loaned Panacela $1.5 million through a convertible term loan, or the Panacela Loan, and revised their original investment agreement to remove the predetermined development milestones and timelines for further investment and provide that Rusnano may invest an additional $15.5 million at their option. As of December 31, 2014, we had an ownership stake of 57.78% in Panacela.
BioProcess Capital Partners
In December 2009, we entered into our Incuron joint venture with BioProcess Capital Partners, or BCP, to develop Curaxin compounds for treatment of oncological diseases. According to the terms of the agreement, we transferred rights in the Curaxin molecules to a new joint venture company, Incuron, in which BCP agreed to cause their affiliated fund, BCV, to contribute an aggregate of 549,497,000 Russian rubles (approximately $16.9 million) to support development of the compounds. As of September 30, 2014, Incuron had received all committed funding. On November 25, 2014, we transferred 3.05% of the Company’s participation interest in Incuron to BCV. The transfer of 3.05% of our participation interest was made pursuant to the Participation Agreement dated December 9, 2009, as amended by the First and Third Amendments to Participation Agreement dated April 13, 2010 and June 17, 2014, respectively, that governs the joint ownership of Incuron by the Company and BCV. As described in the Form 8-K filed by the Company on December 2, 2014, as a result of the transfer of 3.05% of our participation interests to BCV, the Company’s participation interest in Incuron decreased to 46.96%, BCV’s participation interest increased to 53.04%. As described in the Form 8-K filed by the Company on May 4, 2015, on April 29, 2015 we entered into an agreement to sell our equity stake in Incuron to Dr. Mikhail Mogutov, Chairman of the Board of Directors of Incuron, LLC and Chairman of the Investment Committee and founder of Bioprocess Capital Ventures. Pursuant to this agreement, on April 29, 2015, we sold 75% of our equity stake in Incuron being sold for approximately $3 million and granted Dr. Mogutov an option to purchase our remaining ownership interest in Incuron for approximately $1 million before the end of 2015. In addition, we have assigned our remaining intellectual property for CBL0137 to Incuron in exchange for a 2% royalty on the future commercialization, licensing or sale of the CBL0137 technology.
The Cleveland Clinic
In July 2004, CBLI entered into an exclusive license agreement with The Cleveland Clinic, or The Cleveland Clinic License, pursuant to which CBLI was granted an exclusive license to The Cleveland Clinic’s research base underlying our therapeutic platform. We entered into an amendment of The Cleveland Clinic License effective as of September 22, 2011, pursuant to which we were granted an exclusive license to The Cleveland Clinic’s research base underlying certain product candidates in development by Panacela, or Panacela Products, including Mobilan and several earlier stage compounds that are not currently material to our business. In consideration for The Cleveland Clinic License, we agreed to issue The Cleveland Clinic common stock and make certain milestone, royalty and sublicense royalty payments as described below.
| 50 |
The Cleveland Clinic License requires milestone payments, which may be credited against future royalties owed to The Cleveland Clinic, as described in the table below. We have also agreed to make milestone payments of up to approximately $6.5 million for each Panacela Product that achieves certain developmental and regulatory milestones, provided that if CBLI or an affiliate of CBLI and The Cleveland Clinic jointly own the Panacela Product, the milestone amounts will be reduced by 50%.
| Milestone Description | For Products Limited to Biodefense Uses | For All
Other Products (Maximum amount)* | ||||||
| For any IND filing for a product | $ | 50,000 | $ | 50,000 | ||||
| For any product entering Phase II clinical trials or similar registration | 100,000 | 250,000 | ||||||
| For any product entering Phase III clinical trials | — | 700,000 | ||||||
| For any product license application, BLA or NDA Filing for a product | 350,000 | 1,500,000 | ||||||
| Upon regulatory approval permitting any product to be sold to the commercial market | 1,000,000 | 4,000,000 | ||||||
| * | Maximum amounts listed for achievement of milestone in United States. If milestones are reached in another country first, milestone payments will be prorated for certain products under the license based on the market size for the product in such country as that market relates to the then current U.S. market. |
The Cleveland Clinic License requires royalty payments of (a) 2% of net sales of any product candidate under a licensed patent solely owned by The Cleveland Clinic; and (b) 1% of net sales of any product candidate under a licensed patent that is jointly owned by The Cleveland Clinic and CBLI or an affiliate of CBLI. Further, if CBLI receives upfront sublicense fees or sublicense royalty payments for sublicenses granted by CBLI to third parties for any licensed patents solely owned by The Cleveland Clinic, CBLI will pay The Cleveland Clinic (i) 35% of such fees if the sublicense is granted prior to filing an IND application, (ii) 20% of such fees if the sublicense is granted after an IND filing but prior to final approval of the Product License Application or NDA, or (iii) 10% of such fees if the sublicense is granted after final approval of the relevant Product License Application or NDA, provided that such sublicense fees shall not be less than 1% of net sales. The above sublicense fees and sublicense royalty payments are reduced by 50% if The Cleveland Clinic and CBLI or an affiliate of CBLI jointly own the licensed patent.
Through December 31, 2014, CBLI had paid The Cleveland Clinic $150,000 for milestone payments on products limited to biodefense uses, and $400,000 for all other products.
As each patent covered by The Cleveland Clinic License expires, the license agreement will terminate as to such patent. The Cleveland Clinic may terminate The Cleveland Clinic License upon a material breach by us, as specified in the agreement. However, we may avoid such termination if we cure the breach within 90 days of receipt of a termination notice. CBLI may terminate The Cleveland Clinic License in its entirety or any specific patent licensed under the agreement by giving at least 90 days written notice of such termination to The Cleveland Clinic. The agreement will, subject to certain exceptions, automatically terminate with respect to a licensed product if The Cleveland Clinic does not receive a royalty payment for more than 1-year after the payment of royalties has begun.
| 51 |
Roswell Park Cancer Institute
We have entered into a number of agreements with RPCI relating to the licensure and development of our product candidates including:
| ● | two exclusive license and option agreements effective December 2007 and September 2011; | |
| ● | various sponsored research agreements entered into between January 2007 to present; and | |
| ● | clinical trial agreements for the conduct of our Phase 1 entolimod oncology study and Incuron’s Phase 1 CBL0137 intravenous administration study. |
In December 2007, CBLI entered into an agreement with RPCI pursuant to which CBLI has an option to exclusively license any technological improvements to our foundational technology developed by RPCI for the term of the agreement. We believe our option to license additional technology under the agreement potentially provides us with access to technology that may supplement our product pipeline in the future. In consideration for this option and exclusive license, we agreed to make certain milestone, royalty and sublicense royalty payments. Additionally, RPCI may terminate the license upon a material breach by us. However, we may avoid such termination if we cure the breach within 90 days of receipt of a termination notice. The license does not have a specified term; however, as each patent covered by this license agreement expires, the royalties to be paid on each product relating to the licensed patent shall cease.
In September 2011, Panacela entered into an agreement with RPCI, or the Panacela-RPCI License, to exclusively license certain rights Panacela Products, including Mobilan and several earlier stage compounds that are not currently material to our business, and to non-exclusively license certain know-how relating to the aforementioned product candidates for the limited purposes of research and development and regulatory, export and other government filings. Additionally, under the Panacela-RPCI Licnese, Panacela has a right to exclusively license (i) any technological improvements to the Panacela Products developed by RPCI before September 2016, and (ii) any technology jointly developed by Panacela and RPCI. In consideration for the Panacela-RPCI License, Panacela agreed to issue RPCI common stock and to make certain milestone, royalty and sublicense royalty payments as described below.
The Panacela-RPCI License requires milestone payments for developmental and regulatory milestones reached in the United States of up to approximately $2.5 million for each Panacela Product that achieves certain developmental and regulatory milestones. Additionally, Panacela will owe additional payments of up to approximately $275,000 for each other country where a licensed Panacela Product achieves similar milestones. Through December 31, 2013, Panacela had not made any milestone payments to RPCI related to the above mentioned license agreement.
The Panacela-RPCI License requires royalty payments on net sales based on percentages in the low single digits. In addition, if Panacela sublicenses any of the licensed Panacela Products, Panacela will owe sublicensing fees ranging from 5% to 15% of fees received from sublicense by Panacela or an affiliate depending upon whether or not an IND has been filed or final approval of the relevant NDA has been obtained for such licensed product.
As each patent covered by the Panacela-RPCI License expires, the license agreement will terminate as to such patent. In addition, the license agreement will terminate with respect of the licensed know-how after 20 years. RPCI may terminate the license upon a material breach by us, as specified in the agreement. However, we may avoid such termination if we cure the breach within 90 days of receipt of a termination notice (or 30 days if notice relates to non-payment of amounts due to RPCI). Panacela may terminate the license agreement in whole or as to any specific patent licensed under the agreement by giving at least 60 days written notice of such termination to RPCI. The agreement will, subject to certain exceptions, automatically terminate with respect to a licensed Panacela Product if Panacela fails to market, promote and otherwise exploit the licensed technology so that RPCI does not receive a royalty payment during any 12-month period after the first commercial sale of such licensed product.
We have also entered into a number of sponsored research agreements with RPCI pursuant to which both parties have sponsored research to be conducted by the other party. Under the sponsored research agreement granted by RPCI to us, title to any inventions under the agreement is determined in a manner substantially similar to U.S. patent law, and we have the option to license, on an exclusive basis, the right to develop any inventions of RPCI (whether solely or jointly developed) under the agreement for commercial purposes. In addition, the sponsored research agreement may be terminated by one party if the other party becomes subject to bankruptcy or insolvency, the other party is debarred by the U.S. government or the other party breaches a material provision of the agreement and fails to cure such breach within 20 days of receiving written notice.
| 52 |
Under the sponsored research agreements granted by us to RPCI, we own any invention that is described in our research plan, co-own any inventions not described in our research plan that are made by Dr. Andrei Gudkov, and RPCI owns any other inventions not described in our research plan. We further have a right to exclusively license RPCI’s ownership in any invention developed under such sponsored research agreements that are owned by RPCI. Such sponsored research agreements with RPCI expire in 2015, although we expect to enter into similar future arrangements.
We entered into an asset transfer and clinical trial agreement with RPCI for the conduct, by RPCI, of our Phase 1 clinical trial to evaluate the safety and pharmacokinetic profile of entolimod in patients with advanced cancers and a clinical trial agreement for RPCI to conduct, as one site in a multi-site trial, our Phase 1 clinical trial to evaluate the safety, pharmacokinetics and pharmacodynamics of intravenous administration of CBL0137 in patients with metastatic or unresectable advanced solid cancers and lymphomas. Either party may terminate these agreements upon 30 days’ notice to the other party.
INTELLECTUAL PROPERTY
Our intellectual property consists of patents, trademarks, trade secrets and know-how. Our ability to compete effectively depends in large part on our ability to obtain patents for our technologies and products, maintain trade secrets, operate without infringing the rights of others and prevent others from infringing our proprietary rights. We will be able to protect our proprietary technologies from unauthorized use by third parties only to the extent that they are covered by valid and enforceable patents, or are effectively maintained as trade secrets. As a result, patents or other proprietary rights are an essential element of our business. Our patent portfolio includes patents and patent applications with claims directed to compositions of matter, pharmaceutical formulations and methods of use. Some of our issued patents, and the patents that may be issued based on our patent applications, may be eligible for patent life extension under the Drug Price Competition and Patent Term Restoration Act of 1984 in the United States, supplementary protection certificates in the European Union or similar mechanisms in other countries or territories. The following are the patent positions relating to our product candidates as of March 31, 2015.
In the U.S., we have 16 issued or allowed patents relating to our clinical-stage programs expiring on various dates between 2024 and 2030, exclusive of regulatory extensions, as well as numerous pending patent applications and foreign counterpart patent filings which relate to our proprietary technologies. These patents and patent applications include claims directed to compositions of matter and methods of use.
We have 11 issued or allowed U.S. patents covering entolimod, which expire between 2024 and 2029. These patents include composition of matter claims, as well as method of use claims relating to our biodefense indication, reducing effects of chemotherapy, and treatment of reperfusion injuries. In addition, we have pending U.S. and international patent applications related to compositions of matter, oncology methods of use, and others biodefense methods, which, if issued, will expire between 2025 and 2035.
We have 2 issued U.S. patents covering CBL0137, which expire in 2030. These patents include composition of matter claims as well as method of use claims relating to apoptosis induction along with inhibition of adaptive heat shock response. In addition, we have two pending U.S. patent applications that include CBL0137 oncology method of use claims, which, if issued, will expire in 2029 and uses in specific cancer claims, which, if issued, will expire in 2033. Further, we have pending international applications that concern various uses of CBL0137 that may give rise to national patents, which, if issued will expire in 2034 or 2035 and pending provisional applications that may give rise to national patents, which, if issued will expire in 2036.
We have 3 issued or allowed U.S. patents covering CBLB612 and related agents, which expire between 2026 and 2027. These patents include composition of matter and methods of use claims. In addition, we have a pending U.S. patent application that includes method of use claims relating to increasing mobility of hematopoietic stem cells, which, if issued will expire in 2028 and another method of use application which, if issued, will expire in 2035.
| 53 |
We have 1 pending international application that concerns uses of CBL0102 that may give rise to national patents, which, if issued will expire in 2034.
In addition, as of March 31, 2015, we had more than a hundred additional patents and patent applications filed worldwide. Any patents that may issue from our pending patent applications would expire between 2024 and 2036, excluding patent term extensions. These patents and patent applications disclose compositions of matter and methods of use.
Our policy is to seek patent protection for the inventions that we consider important to the development of our business. We intend to continue to file patent applications to protect technology and compounds that are commercially important to our business, and to do so in countries where we believe it is commercially reasonable and advantageous to do so. We also rely on trade secrets to protect our technology where patent protection is deemed inappropriate or unobtainable. We protect our proprietary technology and processes, in part, by confidentiality agreements with our employees, consultants, collaborators and contractors.
RESEARCH AND DEVELOPMENT
In 2013, we transferred 26 laboratory and preclinical employee positions to Buffalo BioLabs, LLC, or BBL, an entity then owned in part by our Chief Scientific Officer and director, Dr. Andrei Gudkov, to enable us to better focus our on clinical development activities. In connection with this transaction, we entered into a Master Services Agreement with BBL, pursuant to which BBL agreed to perform laboratory and preclinical research services for us. As of December 31, 2014, our research and development group, including Russian-based personnel, consisted of 14 individuals. Our research and development focuses on management of outsourced preclinical research, clinical trials and manufacturing technologies. We invested $9.7 million, $19.5 million and $22.5 million in research and development in the years ended December 31, 2014, 2013 and 2012, respectively.
SALES AND MARKETING
We currently do not have marketing, sales or distribution capabilities. We do, however, currently have worldwide development and commercialization rights for products arising out of substantially all of our programs. In order to commercialize any of these drugs, if and when they are approved for sale, we will need to enter into partnerships for the commercialization of the approved product(s) or develop the necessary marketing, sales and distribution capabilities.
COMPETITION
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and intense competition. This competition comes from both biotechnology and major pharmaceutical companies. Many of these companies have substantially greater financial, marketing and human resources than we do, including, in some cases, considerably more experience in clinical testing, manufacturing and marketing of pharmaceutical products. There are also academic institutions, governmental agencies and other research organizations that are conducting research in areas in which we are working. They may also develop products that may be competitive with our product candidates, either on their own or through collaborative efforts. We expect to encounter significant competition for any products we develop. Our product candidates’ competitive position among other biotechnology and biopharmaceutical companies will be based on, among other things, time to market, patent position, product efficacy, safety, reliability, availability, patient convenience, delivery devices and price. Additionally, competitive products may have superior safety or efficacy, be manufactured less expensively, or have better concept of operations, or CONOPs, usability for biodefense products. In these cases, we may not be able to commercialize our product candidates or achieve a competitive position in the market. This would adversely affect our business.
| 54 |
Specifically, the competition for entolimod and our other clinical-stage product candidates includes the following:
Entolimod Biodefense Indication
Product candidates for treatment of ARS face significant competition for U.S. government funding for both development and procurement of medical countermeasures and must satisfy government procurement requirements for biodefense products. Currently the only FDA-approved drug for the treatment of ARS is filgrastim (Neupogen™). Filgrastim stimulates neutrophils and may reduce infection related to ARS. Unlike entolimod, it does not improve platelet count or lessen bleeding, or gastrointestinal dysfunction due to ARS. Use of filgrastim requires repeated injections, laboratory monitoring, and intensive supportive care, making filgrastim unsuitable for use in a mass causality situation. By contrast, entolimod is given as a single injection, requires no monitoring, and does not need supplementation with additional medical support. However, we are aware of a number of companies also developing radiation countermeasures to treat the effects of ARS including Aeolus Pharmaceuticals, Araim Pharmaceuticals, Inc., Cellerant Therapeutics, Inc., Humanetics Corporation, Neumedicines, Inc., Onconova Therapeutics, Inc., RxBio, Inc., Soligenix, Inc., and the University of Arkansas Medical Sciences Centers. Although their approaches to treatment of ARS are different, we compete with these companies for U.S. government development funding and may ultimately compete with them for U.S. and foreign government purchase and stockpiling of radiation countermeasures. Additionally, our ability to sell to the government also can be influenced by indirect competition from filgrastim, which was purchased for use as a radiation countermeasure in 2013.
Entolimod Oncology Program
Immunotherapies and targeted therapies are primary drivers of commercial growth in cancer therapy. Examples of marketed drugs in these categories include: Keytruda ® (Merck) for advanced melanoma, Opdivo® (Bristol-Myers Squibb Company) for melanoma and metastatic squamous non-small cell lung cancer, Avastin® (Roche) for a range of solid tumors including colorectal, lung, breast, renal and gastric cancers, Rituximab® (Roche) for CD20-positive, B-cell non-Hodgkin lymphoma and Arzerra® (GlaxoSmithKline) for CD20-positive chronic lymphocytic leukemia; Yervoy® (Bristol-Myers Squibb) for melanoma, Herceptin® (Roche) for human epidermal growth factor receptor-2, or HER-2, positive tumors, Gleevec® (Novartis) for Philadelphia chromosome tumor mutations, Erbitux® (Eli Lilly) and Iressa® (AstraZeneca) for epidermal growth factor receptor, or EGRF, expressing tumors and Zelboraf® (Genentech) for BRAF-mutated tumors. These drugs may also be appropriate combination partners for entolimod in the appropriate treatment settings. However, these drugs may also be competitors for entolimod market share in the treatment of various tumor types.
CBLB612
Mitigation of chemotherapy-induced myelosuppression is a multi-billion dollar commercial category within oncology. Filgrastim, marketed as Neupogen® (Amgen), is the current standard for treatment of this condition. Filgrastim modestly ameliorates chemotherapy-related neutropenia, but does not improve thrombocytopenia, anemia, or antitumor efficacy. CBLB612 may offer improvements in neutrophil, platelet, and red blood cell counts and may also offer the potential for antitumor effects. Thus, CBLB612 may have advantages relative to filgrastim. However, filgrastim is well established as a neutrophil support factor in patients undergoing bone-marrow-toxic chemotherapy for cancer.
MANUFACTURING
Our product candidates are biologics and small molecules that can be readily synthesized by processes that we have developed. We do not own or operate manufacturing facilities for the production of our product candidates for pre-clinical, clinical or commercial quantities. We rely on third-party manufacturers, and in most cases only one third-party, to manufacture critical raw materials, drug substance and final drug product for our research, pre-clinical development and clinical trial activities. Commercial quantities of any drugs we seek to develop will have to be manufactured in facilities and by processes that comply with the FDA and other regulations, and we plan to rely on third parties to manufacture commercial quantities of products we successfully develop.
| 55 |
GOVERNMENT REGULATION
Government authorities in the U.S. and in other countries, regulate the research, development, testing, manufacture, packaging, storage, record-keeping, promotion, advertising, distribution, marketing, quality control, labeling and export and import of pharmaceutical products such as those that we are developing. We cannot provide assurance that any of our product candidates will prove to be safe or effective, will receive regulatory approvals or will be successfully commercialized.
U.S. Drug Development Process
In the U.S., the FDA regulates drugs and drug testing under the Federal Food, Drug, and Cosmetic Act and in the case of biologics, also under the Public Health Service Act. Our product candidates must follow an established process before they may be marketed in the U.S.:
| ● | preclinical laboratory and animal tests performed in compliance with current Good Laboratory Practices, or cGLP; | |
| ● | development of manufacturing processes which conform to current Good Manufacturing Practices, or cGMPs; | |
| ● | submission and acceptance of an IND application which must become effective before human clinical trials may begin; | |
| ● | performance of adequate and well-controlled human clinical trials in compliance with current Good Clinical Practices, or cGCP, to establish the safety and efficacy of the proposed drug for its intended use; provided, however, that for entolimod development under the Animal Rule, we are required to perform pivotal animal studies in compliance with GLP to establish efficacy; and | |
| ● | submission to and review and approval by the FDA of a NDA or BLA prior to any commercial sale or shipment of a product. |
Nonclinical testing. Nonclinical testing includes laboratory evaluation of a product candidate, its chemistry, formulation, safety and stability, as well as animal studies to assess the potential safety and efficacy of the product candidate. The conduct of the nonclinical tests must comply with federal regulations and requirements including GMP and GLP. Prior to the initiation of GLP animal studies, including our pivotal studies for development of entolimod under the Animal Rule, an Institutional Animal Care and Use Committee, or IACUC, at each testing site must review and approve each study protocol and any amendments thereto.
We must submit to the FDA the results of nonclinical studies, which may include laboratory evaluations and animal studies, together with manufacturing information and analytical data, and the proposed clinical protocol for the first clinical trial of the drug as part of an IND. An IND is a request for FDA authorization to administer an investigational drug to humans. Such authorization must be secured prior to the interstate shipment and administration of any new drug that is not the subject of an approved NDA or BLA. Nonclinical tests and studies can take several years to complete, and despite completion of those tests and studies, the FDA may not permit clinical testing to begin.
The IND process. The FDA requires a 30-day waiting period after the submission of each IND application before clinical trials may begin. This waiting period is designed to allow the FDA to review the IND to determine whether human research subjects will be exposed to unreasonable health risks. At any time during this 30-day period or at any time thereafter, the FDA may raise concerns or questions about the conduct of the trials as outlined in the IND and impose a “clinical hold” that may affect one or more specific studies or all studies conducted under the IND. In the case of a clinical hold, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials placed on hold can begin or continue. The IND application process may be extremely costly and could substantially delay development of our products. Moreover, positive results of preclinical animal tests do not necessarily indicate positive results in clinical trials.
| 56 |
Prior to the initiation of clinical studies, each clinical protocol must be submitted to the IND and to an independent Institutional Review Board, or IRB, at each medical site proposing to conduct the clinical trial. The IRB must review and approve each study protocol, and any amendments thereto, and study subjects must sign an informed consent. Protocols include, among other things, the objectives of the study, dosing procedures, subject selection and exclusion criteria and the parameters to be used to monitor patient safety. Progress reports of work performed in support of IND studies must be submitted at least annually to the FDA. Reports of serious and unexpected adverse events must be submitted to the FDA and the investigators in a timely manner.
Clinical trials. Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| ● | Phase 1: The drug is introduced into healthy human subjects or patients (in the case of certain inherently toxic products for severe or life-threatening diseases such as cancer) and tested for safety, dosage tolerance, absorption, distribution, metabolism and excretion; | |
| ● | Phase 2: Involves studies in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage; and | |
| ● | Phase 3: Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide, if appropriate, an adequate basis for product labeling. |
We cannot be certain that we will successfully complete any phase of clinical testing of our product candidates within any specific time period, if at all. Clinical testing must meet requirements of IRB oversight, informed consent and GCP. The FDA, the sponsor, or the IRB at each institution at which a clinical trial is being performed may suspend a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk.
During the development of a new drug, sponsors are given an opportunity to meet with the FDA at certain points. These meetings typically occur prior to submission of an IND, at the end of Phase 2 and before NDA or BLA submission. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and FDA to reach agreement on the next phase of development. Sponsors typically use the end-of-Phase 2 meeting to discuss their Phase 2 clinical results and present their plans for the pivotal Phase 3 clinical trial that they believe will support approval of the new drug.
The NDA or BLA process. If clinical trials are successful, the next step in the drug regulatory approval process is the preparation and submission to the FDA of an NDA or BLA, as applicable. The NDA or BLA, as applicable, is a vehicle through which drug sponsors formally propose that the FDA approve a new pharmaceutical for marketing and sale in the U.S. The NDA or BLA, as applicable, must contain a description of the manufacturing process and quality control methods, as well as results of preclinical tests, toxicology studies, clinical trials and proposed labeling, among other things. A substantial user fee must also be paid with the application, unless an exemption applies. Every newly marketed pharmaceutical must be the subject of an approved NDA or BLA.
| 57 |
Upon submission of an NDA or BLA, the FDA will make a threshold determination of whether the application is sufficiently complete to permit review, and, if not, will issue a refuse-to-file letter. If the application is accepted for filing, the FDA will attempt to review and take action on the application in accordance with performance goal commitments the FDA has made in connection with the prescription drug user fee law in effect at that time. Current timing commitments under the user fee law vary depending on whether an NDA or BLA is for a priority drug or not, and in any event are not a guarantee that an application will be approved or even acted upon by any specific deadline. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA may refer the NDA or BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved, but the FDA is not bound by the recommendation of an advisory committee. The FDA may deny or delay approval of applications that do not meet applicable regulatory criteria or if the FDA determines that the data do not adequately establish the safety and efficacy of the drug. In addition, the FDA may approve a product candidate subject to the completion of post-marketing studies, commonly referred to as Phase 4 trials, to monitor the effect of the approved product. The FDA may also grant approval with restrictive product labeling, or may impose other restrictions on marketing or distribution such as the adoption of a Risk Evaluation and Mitigation Strategies, or REMS. The FDA has broad post-market regulatory and enforcement powers, including the ability to issue warning letters, levy fines and civil penalties, suspend or delay issuance of approvals, seize or recall products, and withdraw approvals.
Manufacturing and post-marketing requirements. If approved, a pharmaceutical may only be marketed in the dosage forms and for the indications approved in the NDA or BLA, as applicable. Special requirements also apply to any samples that are distributed in accordance with the Prescription Drug Marketing Act. The manufacturers of approved products and their manufacturing facilities are subject to continual review and periodic inspections by the FDA and other authorities where applicable, and must comply with ongoing requirements, including the FDA’s cGMP requirements. Once the FDA approves a product, a manufacturer must provide certain updated safety and efficacy information, submit copies of promotional materials to the FDA, and make certain other required reports. Product and labeling changes, as well as certain changes in a manufacturing process or facility or other post-approval changes, may necessitate additional FDA review and approval. Failure to comply with the statutory and regulatory requirements subjects the manufacturer to possible legal or regulatory action, such as untitled letters, warning letters, suspension of manufacturing, seizure of product, voluntary recall of a product, injunctive action or possible criminal or civil penalties. Product approvals may be withdrawn if compliance with regulatory requirements is not maintained or if problems concerning safety or efficacy of the product occur following approval. Because we intend to contract with third parties for manufacturing of our products, our ability to control third party compliance with FDA requirements will be limited to contractual remedies and rights of inspection. Failure of third party manufacturers to comply with cGMP or other FDA requirements applicable to our products may result in, among other things, total or partial suspension of production, failure of the government to grant approval for marketing, and withdrawal, suspension, or revocation of marketing approvals. With respect to post-market product advertising and promotion, the FDA imposes a number of complex regulations on entities that advertise and promote pharmaceuticals, which include, among others, standards for direct-to-consumer advertising, promoting drugs for uses or in patient populations that are not described in the drug’s approved labeling (known as “off-label use”), industry-sponsored scientific and educational activities, and promotional activities involving the internet. Failure to comply with FDA requirements can have negative consequences, including adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not market or promote such off-label uses.
The FDA’s policies may change, and additional government regulations may be enacted which could prevent or delay regulatory approval of our potential products. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or abroad.
Animal Rule
In 2002, the FDA amended its requirements applicable to BLAs/NDAs to permit the approval of certain drugs and biologics that are intended to reduce or prevent serious or life-threatening conditions based on evidence of safety from clinical trial(s) in healthy subjects and effectiveness from appropriate animal studies when human efficacy studies are not ethical or feasible. These regulations, which are known as the “Animal Rule”, authorize the FDA to rely on animal studies to provide evidence of a product’s effectiveness under circumstances where there is a reasonably well-understood mechanism for the activity of the agent. Under these requirements, and with the FDA’s prior agreement, drugs used to reduce or prevent the toxicity of chemical, biological, radiological or nuclear substances may be approved for use in humans based on evidence of effectiveness derived from appropriate animal studies and any additional supporting data. Products evaluated under this rule must demonstrate effectiveness through pivotal animal studies, which are generally equivalent in design and robustness to Phase 3 clinical studies. The animal study endpoint must be clearly related to the desired benefit in humans and the information obtained from animal studies must allow for selection of an effective dose in humans. Safety under this rule is established under preexisting requirements, including safety studies in both animals (toxicology) and humans. Products approved under the Animal Rule are subject to additional requirements including post-marketing study requirements, restrictions imposed on marketing or distribution and requirements to provide information to patients.
| 58 |
We intend to utilize the Animal Rule in seeking marketing approval for entolimod as a radiation countermeasure because we cannot ethically expose humans to lethal doses of radiation. Other countries may not at this time have established criteria for review and approval of these types of products outside their normal review process, i.e. there is no “Animal Rule” equivalent in countries other than the U.S., but some may have similar policy objectives in place for these product candidates. Given the nature of nuclear and radiological threats, we do not believe that the lack of established criteria for review and approval of these types of products in other countries will significantly inhibit us from pursuing sales of entolimod to foreign countries.
All data obtained from the pre-clinical studies and clinical trials of entolimod, in addition to detailed information on the manufacture and composition of the product, would be submitted in a BLA to the FDA for review and approval for the manufacture, marketing and commercial shipment of entolimod.
Emergency Use Authorization
The Commissioner of the FDA, under delegated authority from the Secretary of the U.S. Department of Health and Human Services, or DHHS, may, under certain circumstances, issue an Emergency Use Authorization, or EUA, that would permit the use of an unapproved drug product or unapproved use of an approved drug product. Before an EUA may be issued, the Secretary must declare an emergency based on one of the following grounds:
| ● | a determination by the Secretary of Department of Homeland Security that there is a domestic emergency, or a significant potential for a domestic emergency, involving a heightened risk of attack with a specified biological, chemical, radiological or nuclear agent or agents; | |
| ● | a determination by the Secretary of the DoD that there is a military emergency, or a significant potential for a military emergency, involving a heightened risk to United States military forces of attack with a specified biological, chemical, radiological or nuclear agent or agents; or | |
| ● | a determination by the Secretary of DHHS of a public health emergency that effects, or has the significant potential to effect, national security and that involves a specified biological, chemical, radiological or nuclear agent or agents, or a specified disease or condition that may be attributable to such agent or agent. |
In order to be the subject of an EUA, the FDA Commissioner must conclude that, based on the totality of scientific evidence available, it is reasonable to believe that the product may be effective in diagnosing, treating or preventing a disease attributable to the agents described above, that the product’s potential benefits outweigh its potential risks and that there is no adequate approved alternative to the product.
Although an EUA cannot be issued until after an emergency has been declared by the Secretary of DHHS, the FDA strongly encourages an entity with a possible candidate product, particularly one at an advanced stage of development, to contact the FDA center responsible for the candidate product before a determination of actual or potential emergency. Such an entity may submit a request for consideration that includes data to demonstrate that, based on the totality of scientific evidence available, it is reasonable to believe that the product may be effective in diagnosing, treating, or preventing the serious or life-threatening disease or condition. This is called a pre-EUA submission and its purpose is to allow FDA review considering that during an emergency, the time available for the submission and review of an EUA request may be severely limited. We met with the FDA in July 2014 to present our human dose-conversion and our intent to submit a pre-EUA for entolimod. As a result of this meeting, the FDA confirmed that our existing efficacy and safety data and animal-to-human dose conversion are sufficient to proceed with a pre-EUA submission and agreed to accept a pre-EUA for review. We plan to submit a pre-EUA in the first half of 2015 in order to inform and expedite the FDA’s issuance of an EUA, should one become necessary in the event of an emergency. The FDA does not have review deadlines with respect to pre-EUA submissions. Additionally, if we submit a pre-EUA, there is no guarantee that the FDA will agree that entolimod meets the criteria for EUA, or, if they do agree, that such agreement by the FDA will lead to procurement by the U.S. or other governments or further development funding.
| 59 |
Public Readiness and Emergency Preparedness Act
The Public Readiness and Emergency Preparedness Act, or PREP Act, provides immunity for manufacturers from all claims under state or federal law for “loss” arising out of the administration or use of a “covered countermeasure.” However, injured persons may still bring a suit for “willful misconduct” against the manufacturer under some circumstances. “Covered countermeasures” include security countermeasures and “qualified pandemic or epidemic products”, including products intended to diagnose or treat pandemic or epidemic disease, such as pandemic vaccines, as well as treatments intended to address conditions caused by such products. For these immunities to apply, the Secretary of DHHS must issue a declaration in cases of public health emergency or “credible risk” of a future public health emergency. Since 2007, the Secretary of DHHS has issued 8 declarations and six amendments under the PREP Act to protect countermeasures that are necessary to prepare the nation for potential pandemics or epidemics from liability.
Fast Track Designation
Entolimod has been granted Fast Track designation by the FDA for reducing the risk of death following total body irradiation. The FDA’s Fast Track designation program is designed to facilitate the development and review of new drugs, including biological products that are intended to treat serious or life-threatening conditions and that demonstrate the potential to address unmet medical needs for the conditions. Fast Track designation applies to a combination of the product and the specific indication for which it is being studied. Thus, it is the development program for a specific drug for a specific indication that receives Fast Track designation. The sponsor of a product designated as being in a Fast Track drug development program may engage in early communication with the FDA, including timely meetings and early feedback on clinical trials and may submit portions of an NDA or BLA on a rolling basis rather than waiting to submit a complete application. Products in Fast Track drug development programs also may receive priority review or accelerated approval, under which an application may be reviewed within six months after a complete NDA or BLA is accepted for filing or sponsors may rely on a surrogate endpoint for approval, respectively. The FDA may notify a sponsor that its program is no longer classified as a Fast Track development program if the Fast Track designation is no longer supported by emerging data or the designated drug development program is no longer being pursued. Receipt of Fast Track designation does not guarantee that we will experience a faster development process, review or approval as compared to conventional FDA procedures or that we will qualify or be able to take advantage of the FDA’s expedited review procedures.
Orphan Drug Designation
Entolimod has been granted Orphan Drug designation by the FDA for prevention of death following a potentially lethal dose of total body irradiation. Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition which is defined as one affecting fewer than 200,000 individuals in the United States or more than 200,000 individuals where there is no reasonable expectation that the product development cost will be recovered from product sales in the United States. Orphan Drug designation must be requested before submitting an NDA or BLA and does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
If an Orphan Drug-designated product subsequently receives the first FDA approval for the disease for which it has such designation, the product will be entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances for seven years as compared to five years for a standard new drug approval. As referenced above, we have received Orphan Drug designation for entolimod. We intend to seek Orphan Drug designation for our other products as appropriate, but an Orphan Drug designation may not provide us with a material commercial advantage.
Foreign Regulation
In addition to regulations in the United States, we are and will be subject to a variety of foreign regulations governing clinical trials and will be subject to a variety of foreign regulation governing commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials or marketing of the product in those countries. The approval process varies from country to country and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country. Other countries, at this time, do not have an equivalent to the Animal Rule and, as a result, do not have established criteria for review and approval of these types of products outside their normal review process, but some countries may have similar policy objectives in place for these product candidates.
| 60 |
As in the United States, the European Union may grant orphan drug status for specific indications if the request is made before an application for marketing authorization is made. The European Union considers an orphan medicinal product to be one that affects less than five of every 10,000 people in the European Union. A company whose application for orphan drug designation in the European Union is approved is eligible to receive, among other benefits, regulatory assistance in preparing the marketing application, protocol assistance and reduced application fees. Orphan drugs in the European Union also enjoy economic and marketing benefits, including up to ten years of market exclusivity for the approved indication, unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan designated product.
Our activities in Russia, through our subsidiaries and joint ventures, are regulated by the Ministry of Health and Social Development of the Russian Federation, or Minzdrav. This federal executive authority is responsible for developing state policies as well as normative and legal regulations in the healthcare and pharmaceutical industries, including policies and regulations regarding the quality, efficacy and safety of pharmaceutical products. In addition, the Federal Service on Surveillance in Healthcare and Social Development, or Roszdravnadzor, is the subordinate executive authority to Minzdrav, which, among other things (i) performs control and surveillance of certain activities, including pre-clinical and clinical trials and monitors compliance with state standards for medical products and pharmaceutical activities; (ii) issues licenses for the manufacture of drug products and pharmaceutical activities; (iii) grants allowance for clinical trials, use of new medical technologies and import and export of medical products, including import of products for use in clinical trials; and (iv) reviews and grants or denies registrations of medical products for sale in Russia. The principal statute that governs our activities in Russia is the Federal Law No. 61-FZ “On the Use and Distribution of Medicines” of 12 April 2010 (as amended). This law regulates the research, development, testing, pre-clinical and clinical studies, governmental registration, quality control, manufacture, storage, transporting, export and import, licensing, advertisement, sale, transfer, utilization and destruction of medical products within the Russian Federation, among other things. All medical products must be registered in Russia and comply with stringent safety and quality controls and testing. In addition to Law No. 61-FZ, we are subject to a number of other laws, regulations and orders that regulate our activities in Russia relating to our drug development activities, taxation, corporate existence, labor laws and other areas. In particular, the existence, legal relations and transactions effected by our Russian subsidiaries and joint ventures are governed by the Federal Law No. 14-FZ “On Limited Liability Companies”, which was enacted on February 8, 1998 (as amended). Pursuant to this law, we must comply with equity holder and other approval requirements including those applicable to large transactions and affiliated transactions. Additionally, under the Russian Labor Code, our Russian subsidiaries and joint ventures must enter into employment contracts with each employee, afford them at least 28 paid vacation days, limit the working week to 40 hours per week and follow the code’s specific procedures and safeguards that serve to protect an employee’s rights in the event the employee in Russia is terminated.
EMPLOYEES
As of April 30, 2015, CBLI and its consolidated subsidiaries and joint ventures had 27 employees, 17 of whom are located in the U.S. and 10 of whom are located outside of the U.S. Of these employees, 12 were employed on a full-time basis and 15 were employed on a part-time basis.
ENVIRONMENT
We have made, and will continue to make, expenditures for environmental compliance and protection. Expenditures for compliance with environmental laws and regulations have not had, and are not expected to have, a material effect on our capital expenditures, results of operations or competitive position.
PROPERTIES
Our corporate headquarters is located at 73 High Street, Buffalo, New York 14203. We have approximately 32,000 square feet of laboratory and office space under a twelve-year lease through June of 2019 with successive two-year renewals, of which 8,324 square feet was subleased to various companies. The sublease covering the majority of the subleased space may be terminated by either party upon 90 days written notice to the other party. This space serves as our corporate headquarters and U.S. corporate headquarters for Incuron and Panacela. In addition, we have less than 1,800 square feet under lease outside of the United States expiring at varying times through January 2016. We do not own any real property.
| 61 |
LEGAL PROCEEDINGS
We are not currently a party to any legal proceedings.
AVAILABLE INFORMATION
Our internet website address is http://www.cbiolabs.com/. Through our website, we make available, free of charge, our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, any amendments to those reports, proxy and registration statements, and all of our insider Section 16 reports, as soon as reasonably practicable after such material is electronically filed with, or furnished to, the U.S. Securities and Exchange Commission, or the SEC. These SEC reports can be accessed through the “Investors” section of our website. The information found on our website is not part of this or any other report we file with, or furnish to, the SEC. Paper copies of our SEC reports are available free of charge upon request in writing to Corporate Secretary, Cleveland BioLabs, Inc. 73 High Street, Buffalo NY 14203. The content on any website referred to in this prospectus is not incorporated by reference into this prospectus unless expressly noted.
The Board of Directors
Set forth in the table below are the names of all of the persons currently serving as our directors, their ages, and their offices in the Company.
| Name | Age | Position with the Company | ||
| James J. Antal(1)(2) | 64 | Director | ||
| Andrei Gudkov, Ph.D., D.Sci. | 58 | Director, Chief Scientific Officer | ||
| Yakov Kogan, Ph.D., MBA | 41 | Director, Chief Executive Officer | ||
| Richard S. McGowan, J.D.(1)(2)(3) | 61 | Director, Chairman of the Board | ||
| Anthony J. Principi, J.D.(2)(3) | 70 | Director | ||
| Randy S. Saluck, J.D., MBA(1)(3) | 49 | Director |
| (1) | Member of the Audit Committee |
| (2) | Member of the Compensation Committee |
| (3) | Member of the Nominating and Corporate Governance Committee. |
| 62 |
James J. Antal Mr. Antal has served as one our directors since July 2006. Mr. Antal served as Chief Financial Officer of Experian Group Ltd. from 1996 to 2001 and as Chief Investment Officer from 2001 to 2002. Experian is a leading global provider of consumer and business credit information, direct marketing information services, and integrated customer relationship management processes. From 1997 to 2002, he also served on the board of directors of First American Real Estate Solutions, an Experian joint venture with First American Financial Corp. Starting in 2002, Mr. Antal served as an advisor to the board of directors for Plexus Vaccine, Inc., a biotech company, until it was acquired by SIGA Technologies in 2004. In December 2004, he joined the SIGA board of directors, and also currently serves on its audit and nominating and governance committees. From May 2004 to August 2005, he was engaged as the Chief Financial Advisor to the Black Mountain Gold Coffee Co. From July 2005 to August 2009, he served on a part-time basis as Chief Financial Officer of Pathway Data Inc., a privately-held company engaged in consumer credit notification and identity theft assistance services. Mr. Antal earned a Bachelor of Science degree in Business Administration with an Accounting major from the Ohio State University in 1973. He became a Certified Public Accountant (Ohio) in 1975. Mr. Antal’s experience in accounting and finance, particularly with respect to biotechnology companies and public reporting companies make him an important asset to our board and a qualified Audit Committee Chairman.
Richard S. McGowan, Esq. Mr. McGowan has served as one of our directors since June 2014 and as our chairman since April 2015. Mr. McGowan has been admitted to the practice of law in the states of Connecticut, New York and Massachusetts and numerous Federal Courts for over 30 years, retiring from active practice in 2010. From 1985 to 2009, Mr. McGowan specialized on a national level in the prosecution of mass tort pharmaceutical drug, product liability, and class action cases where he served on several Lead Plaintiff Committees and as Class Counsel, first as a Partner at Rheingold & McGowan, P.C., and then while Of Counsel for Weitz & Luxenberg, P.C. From 2000 to 2008, he was also a partner and President of SFB Holdings, a private investment company that sought to purchase and turn around sub-producing micro-cap companies. Since 2008, he has been involved as a private investor in micro-cap companies. He was an Instructor with the Intensive Trial Advocacy Program at the Cardozo Law School from 1986 to 2001. Mr. McGowan received a Bachelor’s degree from the State University of New York at Stony Brook and a Juris Doctor degree from the University of Boston, School of Law. Mr. McGowan provides our board with stockholder perspective and in-depth legal expertise for both the pharmaceutical industry and micro-cap companies.
Anthony J. Principi, J.D. Mr. Principi has served as one of our directors since April 2013. Mr. Principi serves as principal of The Principi Group, a consulting firm. From March to May 2005 and from 2006 through 2010, he was Senior Vice President of Government Relations of Pfizer, Inc. Prior to joining Pfizer, Inc., Mr. Principi served as Secretary of the U.S. Department of Veterans Affairs from 2001 through 2005. In 2005, he served as the Chairman of the Defense 2005 Base Realignment and Closure Commission. Prior to becoming Secretary of the U.S. Department of Veterans Affairs, Mr. Principi was President of QTC Medical Services Inc. from 1999 through 2001 and Senior Vice President of Lockheed Martin IMS from 1995 through 1996. Prior to joining Lockheed Martin IMS, Mr. Principi was Chief Counsel and Staff Director of the U.S. Senate Armed Services Committee from 1993 through 1994, and was Chief Counsel and Staff Director of the U.S. Senate Committee on Veterans’ Affairs from 1984 through 1988. Mr. Principi serves as a director and member of the corporate governance and compensation and evaluation committees of Mutual of Omaha. He is also a member of the board of directors of Engility Holdings, Inc. and is a member of its compensation and nominating/corporate governance committees. Mr. Principi serves as a director of A. T. Kearney and Onsite Health, Inc. He served as Executive Chairman of QTC Management, and was a director of Perot Systems Corporation. Mr. Principi received a Bachelor of Science from the U.S. Naval Academy and a Juris Doctor from Seton Hall University School of Law. Mr. Principi provides our board with expertise in public health and government affairs.
| 63 |
Randy S. Saluck, J.D., MBA Mr. Saluck has served as one of our directors since May 2013. Mr. Saluck has been the Managing Member of Mortar Rock Capital Management, LLC and the Portfolio Manager of Mortar Rock Capital LP, a value-oriented investment fund, since 2005. From 2002 to 2005, Mr. Saluck was a portfolio manager at the investment fund of Meisenbach Capital, LP and, from 2000 to 2002, Mr. Saluck was a senior analyst at Tyndall Partners, LLC, which invested in value-oriented equities and distressed debt. From 1999 to 2000, Mr. Saluck was an analyst at Highfields Capital Management, LLC, where he was responsible for special situations and risk arbitrage. Prior thereto, Mr. Saluck was an investment banker focused on mergers and acquisitions involving a variety of industries at Salomon Brothers Inc. Before becoming an investment banker, Mr. Saluck was a corporate and securities attorney, working at Cahill Gordon & Reindel LLP and then Tenzer Greenblatt LLP. As an attorney, Mr. Saluck worked with numerous small capitalization companies assisting them in the execution of their financing and strategic plans. He received a Bachelor’s degree from the University of Pennsylvania, a Juris Doctor degree from the University of Virginia and an MBA from the Wharton School of the University of Pennsylvania with a concentration in finance and accounting. Mr. Saluck currently serves on the Board of Directors of the Connecticut Region of the Anti-Defamation League. Mr. Saluck provides our board with stockholder perspective and experience in public finance and investor relationships.
Yakov Kogan, Ph.D., MBA Dr. Kogan has served as one of our directors since our inception in June 2003. Dr. Kogan has served as our Chief Executive Officer since June 2012. Previously, he served as our Chief Operating Officer from February 2008 until June 2012 and as our Interim Chief Executive Officer from January 2012 until June 2012. Dr. Kogan also served as our Executive Vice President of Business Development from our inception until February 2008. From 2002 to 2003, he was Director for Business Development at Integrated Genomics where he was responsible for commercial sales and expansion of the company’s capital base. Prior to his tenure in business development, Dr. Kogan worked as a Group Leader/Senior Scientist at Integrated Genomics and ThermoGen, Inc. and as Research Associate at the University of Chicago. Dr. Kogan holds a Ph.D. degree in Molecular Biology from All-Union Research Institute of Genetics and Selection of Industrial Microorganisms (VNIIGenetika) (Moscow, Russia), as well as an MBA degree from the University of Chicago Graduate School of Business. Dr. Kogan’s day-to-day leadership as Chief Executive Officer provides our board with intimate knowledge of our operations.
Andrei Gudkov, Ph.D., D. Sci. Dr. Gudkov has served as one of our directors and as our Chief Scientific Officer since our inception in June 2003. Since 2007, Dr. Gudkov has served as Senior Vice President of Basic Science and Chairman of the Department of Cell Stress Biology at Roswell Park Cancer Institute. From 2001 to 2007, he was Chairman of the Department of Molecular Biology at the Lerner Research Institute at the Cleveland Clinic and Professor of Biochemistry at Case Western Reserve University. Prior to this, he was a tenured faculty member in the Department of Molecular Genetics at the University of Illinois at Chicago, where his lab concentrated on the development of new functional gene discovery methodologies and the identification of new candidate cancer treatment targets. Before immigrating to the United States in 1990, Dr. Gudkov worked at The National Cancer Research Center in Moscow, where he led a broad research program focused on virology and cancer drug resistance. Dr. Gudkov holds a Ph.D. in Experimental Oncology from the Cancer Research Center (Moscow, Russia). Dr. Gudkov provides our board with invaluable insight into the scientific direction of the Company.
Committees of the Board of Directors
The Board has established an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee, each comprised entirely of directors who are “independent” as that concept is defined in the corporate governance listing requirements of the NASDAQ Marketplace Rules. Each Committee has a written charter that is posted on our website, www.cbiolabs.com, under the link “Investors” and the section therein entitled “Corporate Governance.”
Each of Messrs. Antal, McGowan, Principi and Saluck is independent under The NASDAQ Marketplace Rules and the Securities Exchange Act of 1934 (the “Exchange Act”).
| 64 |
Audit Committee . Our Audit Committee currently has three members, Messrs. Antal (Chair), Saluck and McGowan.
All members of the Audit Committee satisfy the current independence standards promulgated by the Securities and Exchange Commission and by The NASDAQ Stock Market, as such standards apply specifically to members of audit committees. The Board has determined that Mr. Antal is an “audit committee financial expert,” as the Securities and Exchange Commission has defined that term in Item 407 of Regulation S-K.
The Audit Committee generally has direct responsibility and oversight for our accounting policies and internal controls, financial reporting practices, and legal and regulatory compliance. More specifically, the Audit Committee has responsibility to review and discuss the annual audited financial statements and disclosures with management and our independent registered public accounting firm, or our independent auditor; review the financial statements and disclosures provided in our quarterly and periodic reports with management and the independent auditor; and oversee the external audit coverage, including appointment and replacement of the independent auditor and pre-approval of all audit and non-audit services to be performed by the independent auditor.
Compensation Committee. Our Compensation Committee currently has three members, Messrs. McGowan (Chair), Antal and Principi.
The Compensation Committee determines and approves the compensation level of executive officers based on an evaluation of their performance in light of our goals and objectives. The Compensation Committee also considers our performance and relative stockholder return, the level and value of similar incentive awards prevalent in the industry, and awards given to executive officers in past years. The Compensation Committee also has the authority to recommend to the Board compensation for directors and the form of this compensation. The Compensation Committee makes recommendations to the full Board with respect to the adoption, amendment, termination, or replacement of both incentive compensation plans and equity-based plans. The Compensation Committee has the power to retain professionals to assist in the evaluation of director and executive compensation, and has the sole authority to retain and terminate any such professional and to approve the professional’s fees. The Compensation Committee may also establish subcommittees of entirely independent directors to evaluate special or unique matters.
For a discussion concerning the processes and procedures for determining executive and director compensation, see “Compensation Discussion and Analysis” and “Executive Officer and Director Compensation.”
Nominating and Corporate Governance Committee. Our Nominating and Corporate Governance Committee has three members, Messrs. Principi (Chair), McGowan and Saluck.
The Nominating and Corporate Governance Committee generally has responsibility for identifying candidates who are eligible under the qualification standards set forth in our Corporate Governance Guidelines and recommending such eligible individuals to serve as members of the Board. It also makes recommendations to the Board concerning the structure and membership of other Board committees. The Nominating and Corporate Governance Committee is also charged with considering matters of corporate governance generally and reviewing and recommending to the Board, periodically, our corporate governance principles.
In addition, under our current corporate governance policies, the Nominating and Corporate Governance Committee may consider candidates recommended by stockholders as well as from other sources such as other directors or officers, third party search firms or other appropriate sources. For all potential candidates, the Nominating and Corporate Governance Committee may consider all factors it deems relevant, such as a candidate’s personal integrity and sound judgment, business and professional skills and experience, independence, knowledge of the industry in which we operate, possible conflicts of interest, diversity, the extent to which the candidate would fill a present need on the Board, and concern for the long-term interests of the stockholders. In general, persons recommended by stockholders will be considered on the same basis as candidates from other sources.
| 65 |
Corporate Governance Guidelines
The Board has adopted Corporate Governance Guidelines, which it reviews from time to time, to assist the Board in fulfilling its responsibility to exercise its business judgment in what it believes to be the best interests of our stockholders. The Corporate Governance Guidelines are posted on our website, www.cbiolabs.com, under the link “Investors” and the section therein titled “Corporate Governance.”
Code of Ethics for Senior Executives and Financial Officers, Code of Business Conduct and Ethics for Directors and Code of Conduct
The Board has adopted a Code of Ethics for Senior Executives and Financial Officers that is specifically applicable to executive officers and senior financial officers, including our principal executive officer and principal financial officer. Additionally, the Board has adopted the Code of Business Conduct and Ethics for Directors that is specifically applicable to our directors. Both the Code of Ethics for Senior Executives and Financial Officers and the Code of Business Conduct and Ethics for Directors are posted on our website, www.cbiolabs.com, under the link “Investors” and the section therein titled “Corporate Governance.” We have also adopted a Code of Conduct in order to promote honest and ethical conduct and compliance with the laws and governmental rules and regulations to which we are subject. The Code of Conduct is applicable to all of our employees, officers and directors, and is posted on our website, www.cbiolabs.com, under the link “Investors” and the section therein titled “Corporate Governance.”
Board Leadership Structure
Our Corporate Governance Guidelines describe our policies concerning, among other things, the role of the Board and management, proper Board functions, independence, and committee matters. The positions of Chair of the Board and Chief Executive Officer are currently held by different persons, although we do not have a policy requiring that to be the case. Instead, our Board has the authority to choose its Chair in any way it deems best for us at any given point in time. Accordingly, our Board reserves the right to vest the responsibilities of the Chief Executive Officer and Chair in the same person or in two different individuals depending on what it believes is in our best interest. At this time, our Board has determined that separation of these roles most appropriately suits us. Our current Chair, Mr. McGowan is qualified to serve as our Chair given his expertise with both the pharmaceutical industry and micro-cap companies. Further, our Board believes that this division of roles allows our Chief Executive Officer to focus more of his efforts to achieving the goals and objectives of our strategic plan. Our Board believes that there is no single leadership structure that would be most effective in all circumstances and, therefore, retains the authority to modify our Board’s structure to best address our circumstances as and when appropriate.
Role of Our Board in Risk Oversight
The Board, as a whole and at the committee level, has overall responsibility for overseeing our risks, including general oversight of our executive officers’ management of risks relevant to the Company. A fundamental part of risk oversight is not only understanding the material risks a company faces and the steps management is taking to manage those risks, but also understanding what level of risk is appropriate for the Company. The involvement of our Board in reviewing our strategic plan is an integral aspect of the Board’s assessment of management’s tolerance for risk and also its determination of what constitutes an appropriate level of risk for the Company.
At the committee level, the Compensation Committee oversees the management of risks relating to our executive compensation. The Audit Committee oversees our risk policies and processes related to the quality and integrity of our accounting, auditing and financial reporting practices, including our audited and unaudited financial statements and internal controls. The Audit Committee is also responsible for addressing risks arising from related party transactions. The Nominating and Corporate Governance Committee manages risks associated with the independence of the Board and potential conflicts of interest.
Executive Officers
The following table sets forth certain information regarding our executive officers who are not also directors. The Board elects officers annually and such executive officers serve at the discretion of the Board. There are no family relationships among any of our directors or executive officers.
| Name | Age | Position | ||
| C. Neil Lyons, CPA | 58 | Chief Financial Officer | ||
| Langdon L. Miller, MD | 61 | President and Chief Medical Officer |
| 66 |
C. Neil Lyons, CPA Mr. Lyons has been our Chief Financial Officer since September 2012. Mr. Lyons has over 30 years of experience related to operations, finance, SEC compliance, complex financial transactions, strategy, information systems and corporate governance. Prior to joining the Company, from April, 2005 until August, 2011, Mr. Lyons served as Chief Financial Officer and Treasurer of RegeneRx Biopharmaceuticals, Inc., where he led several financial transactions, identified and captured government grant opportunities, directed investor relations activities, developed financial models and implemented investment strategies and employee benefit programs. From 2003 until 2005, Mr. Lyons founded and was the principal of Ironbridge Consulting, a firm that provided financial consulting services, to businesses in the Washington, D.C. metro area. From 1998 until 2003, Mr. Lyons was the Vice President, Finance, for Alcatel’s SkyBridge Limited Partnership, an international satellite broadband start-up, where he secured significant amounts of capital and was an active participant in acquisition and joint venture activities. Prior to that, Mr. Lyons served in various positions at Bell Atlantic (now Verizon), from 1996 to 1998, Honeywell Federal Systems, Inc., a major Department of Defense contractor from 1990 to 1996, and practiced public accounting with Deloitte and Arthur Young from 1979 to 1990. Mr. Lyons is a certified public accountant and received a Bachelor of Science degree in accounting, magna cum laude, from Florida Southern College.
Langdon L. Miller, MD Dr. Miller has served as our President and Chief Medical Officer since May 4, 2015. Prior to accepting his position as President and Chief Medical Officer of the Company, Dr. Miller served as a senior medical advisor to the Company since 2014. Dr. Miller has more than 20 years of experience in the design and conduct of translational and clinical drug development programs in oncology (both in hematological and solid tumors) and orphan diseases (including cystic fibrosis, muscular dystrophy, and hemophilia). From 2013 until he joined the Company, Dr. Miller provided consulting services to a variety of life sciences companies. Before that Dr. Miller held leadership positions in government and in large and small biopharmaceutical companies, including the National Cancer Institute, Pharmacia Corporation, PTC Therapeutics, Calistoga Pharmaceuticals, and Gilead Sciences. He holds a Doctorate of Medicine from Northwestern University and completed his residency in internal medicine at the University of Minnesota and an oncology fellowship at Stanford University.
Summary Compensation Table
The following table shows the total compensation paid or accrued during the last two fiscal years ended December 31, 2014 and 2013 to our (1) Chief Executive Officer, (2) Chief Financial Officer and (3) Chief Scientific Officer.
| Name and Principal Position | Year | Salary (1) ($) | Bonus ($) | Stock Awards ($) | Option
Awards (2) ($) | Non-Equity Incentive Plan Compensation (3) ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||||||
| Yakov Kogan | 2014 | 302,304 | — | — | 62,154 | (4) | — | 10,000 | (6) | 374,458 | ||||||||||||||||||||||
| Chief Executive Officer | 2013 | 407,615 | — | — | 121,898 | (5) | — | 10,000 | (6) | 539,513 | ||||||||||||||||||||||
| C. Neil Lyons | 2014 | 283,231 | — | — | 62,154 | (4) | — | 9,995 | (6) | 355,380 | ||||||||||||||||||||||
| Chief Financial Officer | 2013 | 255,479 | — | — | 89,896 | (5) | — | 10,000 | (6) | 355,375 | ||||||||||||||||||||||
| Andrei Gudkov | 2014 | 119,257 | — | — | 62,154 | (4) | — | — | 181,411 | |||||||||||||||||||||||
| Chief Scientific Officer | 2013 | 215,343 | — | — | 97,967 | (5) | — | — | 313,310 | |||||||||||||||||||||||
| (1) | Base salary includes compensation received from our consolidated subsidiary Panacela Labs, Inc. and our deconsolidated joint venture, Incuron, LLC. For 2014, Drs. Kogan and Gudkov earned $0 and $4,166 from these entities, respectively. For 2013, Drs. Kogan and Gudkov earned $60,000 and $76,667 from these entities, respectively. |
| (2) | These amounts represent the aggregate grant date fair value for stock option awards computed in accordance with FASB ASC Topic 718. A discussion of the assumptions used in determining grant date fair value may be found in our Consolidated Financial Statements, included in our respective Annual Reports on Form 10-K. |
| (3) | The Company’s cash bonuses are paid under our executive compensation plans. As such, the bonus amounts are reported in the column “Non-Equity Incentive Plan Compensation.” |
| (4) | Represents (i) options to purchase 7,500 shares of common stock, granted in March 2014 for performance during fiscal 2013, which vested immediately and have an exercise price of $13.60 per share. |
| (5) | Represents options to purchase shares of common stock, granted in May 2013 for performance during fiscal 2012, which vest when the Company’s common stock closes at a price of $100.00 or more for 5 consecutive trading days and have an exercise price of $30.80 per share. The share award amounts for Drs. Kogan, Gudkov and Mr. Lyons were 5,230, 4,203 and 3,857, respectively. |
| (6) | Consists of 401(k) matching contributions. |
| 67 |
Grants of Plan-Based Awards
The following table shows information regarding grants of non-equity incentive plan awards and grants of equity awards that we made during or applicable to the fiscal year ended December 31, 2014 to each of the executive officers named in the Summary Compensation Table. All balances shown in the table below have been adjusted to account for the 1:20 reverse split of the Company’s common stock that was executed on January 28, 2015.
| Name | Grant Date (1) (2) | Compensation Committee Action Date | Estimated Future Payouts Under Non- Equity Incentive Plan Awards | All Other Stock Awards: Number of Shares of Stock or Units (#) | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise or Base Price of Option Awards ($/Sh) | Grant Date Fair Value of Stock and Option Awards | |||||||||||||||||||||||
| Threshold ($) | Target ($) | Maximum ($) | ||||||||||||||||||||||||||||
| Yakov | 2/6/2014 | $ | 41,400 | $ | 82,800 | $ | 165,600 | — | — | — | — | |||||||||||||||||||
| Kogan | 3/13/2014 | 2/6/2014 | — | 7,500 | $ | 13.60 | $ | 62,154 | ||||||||||||||||||||||
| C. Neil | 2/6/2014 | $ | 42,750 | $ | 85,500 | $ | 171,000 | — | — | — | — | |||||||||||||||||||
| Lyons | 3/13/2014 | 2/6/2014 | — | 7,500 | $ | 13.60 | $ | 62,154 | ||||||||||||||||||||||
| Andrei | 2/6/2014 | $ | 33,282 | $ | 66,565 | $ | 133,129 | — | — | — | — | |||||||||||||||||||
| Gudkov | 3/13/2014 | 2/6/2014 | — | 7,500 | $ | 13.60 | $ | 62,154 | ||||||||||||||||||||||
| (1) | All stock option awards granted on March 13, 2014 were for services rendered in fiscal 2013 and were immediately vested upon issuance. |
| (2) | In accordance with the Company’s Equity Incentive Guidelines, grants made under the Equity Plan were made on March 13, 2014, the second day following the end of the blackout period relating to publication of the Company’s periodic financial filings. |
The amounts in the “Estimated Possible Payouts Under Non-Equity Incentive Plan Awards” and “Estimated Possible Payouts Under Equity Incentive Plan Awards” columns represent the minimum, target and maximum amounts that our named executive officers were eligible for pursuant to our executive compensation plan. Actual amounts paid to each of the named executive officers under this plan, if any, are set forth in the Summary Compensation Table above.
As discussed in footnote (2) to the Summary Compensation Table above, the stock awards and stock options in the table above represent awards granted in the year ended December 31, 2014 and the grant date fair value relating thereto computed in accordance with FASB ASC Topic 718. For a discussion of the stock awards and stock options granted in respect of services provided in the year ended December 31, 2014, see the discussion under “Compensation Discussion and Analysis—Our Executive Compensation Plan—Incentive Compensation” and “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table.”
| 68 |
Option Exercises
There were no exercises of stock options by any of our named executive officers during the fiscal year ended December 31, 2014 or the fiscal quarter ended March 31, 2015.
Pension Benefits
We do not have any qualified or non-qualified defined benefit plans.
Nonqualified Deferred Compensation
We do not have any nonqualified defined contribution plans or other deferred compensation plan.
Compensation Discussion and Analysis
Introduction
This section explains our executive compensation program for 2014 as it relates to our “named executive officers,” or “NEOs,” listed below whose compensation information is presented in the tables following this discussion.
Name |
Title |
|
| Dr. Yakov Kogan | Chief Executive Officer | |
| Dr. Andrei Gudkov | Chief Scientific Officer | |
| Mr. C. Neil Lyons | Chief Financial Officer |
Executive Summary
Our Compensation Committee believes that our executive compensation program is appropriately designed to incentivize our NEOs to work for our long-term prosperity through pay-for-performance incentives, is reasonable in comparison with the levels of compensation provided by our peer group companies, discourages our NEOs from assuming excessive risks, and reflects a reasonable cost. We believe our NEOs are critical to the achievement of our corporate goals, through which we can drive stockholder value. We therefore give considerable thought to the design and administration of our NEO compensation program.
Our NEO compensation packages are designed around the following principles:
| ● | align long-term incentive opportunities with stockholder value creation; | |
| ● | attract, motivate and retain qualified individuals to contribute to our growth and success; | |
| ● | provide competitive compensation opportunities consistent with industry practices where we compete for talent; and | |
| ● | maintain a reasonable and responsible cost structure. |
The major aspects of our executive compensation program include the following:
| ● | Voluntary Base Pay Reduction. The Compensation Committee regularly reviews base pay benchmark data to confirm that our NEOs’ base pay is in-line with industry practice and whether to make any adjustments. However, in light of the financial situation of the Company, commencing in May 2014 our Chief Executive Officer and Chief Scientific Officer each agreed to a 20% reduction in their base salaries. |
| 69 |
| ● | Strong Pay-for-Performance Principles. A majority of our NEOs’ total potential compensation is contingent on achieving short-term corporate goals as defined in our annual Executive Compensation Plan, referred to in this discussion as our Annual Plan, and our Long-term Executive Compensation Incentive Plan, referred to in this discussion as our Long-term Plan. Our Annual Plan is intended to focus our NEOs on achieving annual value-driving clinical development goals, pre-commercialization. Our Long-term Plan currently has a performance period that expires on December 31, 2016, and is intended to incentivize our NEOs to attain our commercialization goals, either through out-licensing, marketing approval or direct product sales. Due to the Company’s financial situation and/or stock performance, we did not award cash bonuses to our executives for performance in 2012, 2013 or 2014 under our Annual Plan. In the second half of 2015, we plan to implement new programs and/or amend our current programs and review the entirety of our compensation program in light of the Company’s stock price to better align the interests of our management with those of our stockholders. | |
| ● | Responsible Severance Compensation. Our Severance Plan provides the NEOs with severance benefits only if the NEO’s employment is involuntarily terminated without cause. The cash severance benefits provided are limited to an amount equal to 12-months of base salary. As a condition to provision of any severance benefits, the NEO must provide the Company with a release of claims. We do not provide any tax gross-up payments. | |
| ● | Limited Executive Benefits. We do not offer executive benefits such as car allowances, personal security, financial planning advice, tax preparation services or club memberships. | |
| ● | Stockholder Approval Required to Reprice Options. Our current equity plans do not permit repricing of underwater stock options held by our NEOs or other employees without prior stockholder approval. |
We held our first stockholder advisory vote on executive compensation in 2011. When determining how often to hold an advisory vote on executive compensation, our Board recommended and our stockholders agreed upon, an annual vote. In 2015 and 2014, approximately 79.1% and 62.9%, respectively, of the votes cast approved our executive compensation described in our prior year’s proxy statement. The Compensation Committee considered these votes and has maintained modest base salaries compared to peer companies, has not awarded cash bonuses during the years covered by those proxies and again for the year ended December 31, 2014, but has approved equity incentives in recognition of the progress management has achieved with the United States Food and Drug Administration, or FDA. Of additional note, our founders, Drs. Kogan and Gudkov, each agreed to a 20% reduction in base salary that commenced in May 2014.
Compensation Setting Process
Overview
The overall objectives of our compensation program are to attract and retain the best possible executive talent, to motivate these executives to achieve the goals and objectives within our strategic plan, and to align executive compensation with stockholder interests. To achieve these objectives, we have developed an overall compensation strategy, including specific goals that tie the majority of our NEOs’ compensation to performance.
When creating a NEO’s overall compensation package, the Compensation Committee considers the different components of our compensation elements in light of the role the NEO will play in achieving our near term and longer term goals, as well as the compensation packages provided to similarly situated executives at companies we consider to be our peers. Our NEOs’ compensation components are: Base salary, the Annual Plan and the Long-term Plan, as discussed more thoroughly in this section. We do not predetermine an allocation of the overall compensation to be represented by the various compensation elements. Rather, the Compensation Committee’s intention is that the incentives provided by the Annual Plan and the Long-term Plan provide a majority of the NEOs’ total compensation. As a result, a material amount of our NEOs potential compensation is at risk in any given fiscal year. Our Compensation Committee believes that having a significant portion of our executives’ compensation package at risk has contributed to cultivating a culture in which our NEOs aggressively pursue our corporate performance and strategic goals as they know that their take home pay, to a large extent, depends upon our performance and, to some extent, their contribution to our performance. Additionally, the incorporation of significant equity incentives is designed to mitigate the risk that our NEOs will pursue short-term outcomes at the expense of long-term stockholder value. Performance-based annual cash and stock option compensation awards under our Annual Plan may be made based on the achievement of short-term corporate goals designed to incentivize the executives to create stockholder value and attain short-term performance objectives. Our short-term corporate goals are currently developmental in nature because our product pipeline is pre-commercialization. The corporate goals vary year-to-year, but generally include value-adding achievements such as contract/grant funding, timely completion of research and development objectives, financial performance and cash flow management and stock performance. Performance-based long-term awards under our Long-term Plan are made based on the achievement of corporate commercialization objectives that address out-licensing, drug approval and product sales. The Long-term Plan has a term of three and a half years, was first implemented in June 2012 and expires in December 2016. Any awards granted under the Long-term Plan can be settled in either cash or equity, as determined in the Compensation Committee’s discretion.
| 70 |
We believe that the combined mix of these three pay elements allows us to provide a competitive, cost-effective, total compensation package to our NEOs, largely based on achievement of value-driving milestones. More specifically, the Compensation Committee believes this structure aligns a majority of the NEOs’ potential compensation to performance.
Role of the Chief Executive Officer
The Chief Executive Officer has no role in setting his compensation and is specifically excluded from any discussions related to his compensation. However, the Chief Executive Officer recommends to the Compensation Committee for its approval, proposed corporate performance and strategic goals and their relative weighting for the upcoming fiscal year for the Annual Plan and the Long-term Plan, as well as provides input on the level of attainment of the prior year’s goals, for purposes of determining awards under the Annual Plan and Long-term Plan for all our NEOs, including the Chief Executive Officer. Finally, the Chief Executive Officer regularly provides input to the Compensation Committee during the course of the year regarding the performance and compensation of our other NEOs.
Compensation Committee Decision Making Process
The Compensation Committee approves the compensation packages for all NEOs. When determining the base salary and equity incentive compensation awards, the Compensation Committee considers the ongoing feedback it has received during the prior year from the Chief Executive Officer regarding the performance of each executive, benchmark data, compensation for new executive hires, as well as high-level strategic issues, such as new trends, plans or approaches to compensation. The Compensation Committee also considers the results of our stockholder advisory votes on executive compensation.
In addition, the Compensation Committee approves the goals and performance target levels, if any, relevant to our Annual Plan and Long-term Plan. Generally, the Compensation Committee’s process for determining Annual Plan and Long-term Plan awards involves: (i) the determination of target award levels, (ii) the establishment of performance goals, and (iii) an evaluation of our actual performance in relation to the performance goals. The Compensation Committee has set the cash bonus target levels for all of our NEOs except for Dr. Gudkov at 30% of base compensation, with Dr. Gudkov’s set at 60%. Cash and equity compensation under the Annual Plan and Long-term Plan represents a majority of our NEOs’ total potential compensation, which means that a material amount of our NEOs’ potential compensation is at risk. The Compensation Committee and our full Board typically set the performance goals of the Annual Plan at the beginning of each year and at the beginning of the Long-term Plan’s performance period. The Compensation Committee recognizes that the research and development environment in which management operates is dynamic, requiring changes as new discoveries are made, or opportunities present themselves. As such the Compensation Committee retains discretion to make upward and downward adjustments to final awards based on the Compensation Committee’s assessment of both the Company’s and the executive’s personal performance. When considering the levels of bonus compensation to award, the Compensation Committee also reviews the individual performance of our NEOs and considers the recommendations of our Chief Executive Officer.
| 71 |
Role of Compensation Consultants
The Compensation Committee has the authority under its charter to engage the services of outside advisors, experts and others to assist the Compensation Committee in carrying out its delegated duties. We have not historically hired an outside consulting firm to evaluate our compensation practices or provide recommendations to our Compensation Committee in order to preserve cash to fund our operations. Rather, the Compensation Committee has relied upon significant internally-developed benchmark data to guide its decisions.
Compensation Benchmarking
In any year the Compensation Committee may benchmark the compensation for our NEOs with that of executives with similar positions in our industry, adjusting for known or perceived differences between our NEO’s experience and levels of responsibility with the job descriptions reflected for the generalized survey data. For purposes of setting 2014 compensation the analysis focused on data reported in the 2014 Radford Global Life Sciences Compensation Survey with respect to a comparison of 2013 data for both publicly-traded biotechnology companies with market capitalizations of $250 million or less, and biotechnology companies, both public and private, with less than 50 employees. The Compensation Committee determined that these criteria were appropriate in selecting the peer companies given our market capitalizations at the time the studies were conducted, the complexity of our international operations, and the number of employees that were comparable to our employee base. The Radford survey data as compared to our current NEO compensation indicated that our NEOs were paid at or below the 25th percentile of the survey data, with our Chief Financial Officer falling at approximately the 25th percentile for companies with less than 50 employees and significantly below the 25th percentile for companies with market capitalizations of $250 million or less. As a consequence, a one-time increase to our Chief Financial Officer’s base pay of $30,500 was implemented on January 1, 2014 to better align his base pay with the survey data so that his compensation would be closer to the 25th percentile for companies with market capitalizations of $250 million or less.
Evaluations
The Compensation Committee evaluates the performance of our executive officers in light of performance goals and objectives established for the Annual Plan and Long-term Plan at least once a year. Based upon these evaluations, the Compensation Committee determines the annual compensation for our executive officers, including base cash compensation, cash bonus and equity compensation. In its evaluation of the NEOs, the Compensation Committee considers, among other things, the following:
| ● | overall management of the Company; | |
| ● | progress achieved by our drug candidates; | |
| ● | stockholder return; | |
| ● | the maintenance of successful relationships with our board and stockholders; | |
| ● | our financial performance with respect to the preparation of and compliance with our budget, including capital reserves; | |
| ● | success in securing new government contracts and grants and other third-party funding, and progress under such contracts and other funding arrangements once obtained; and | |
| ● | regulatory compliance (including compliance with NASDAQ rules, the securities laws, FDA regulations, etc.). |
Typically, the Compensation Committee meets at least twice per year to make compensation decisions for our NEOs and with greater frequency if necessary. The Compensation Committee also meets and confers regularly in executive session. The Compensation Committee met nine times during 2014.
The agenda for each meeting is usually developed by the Chair of the Compensation Committee, in consultation with our Corporate Secretary and our other executive officers, as needed. From time-to-time, various members of our management, as well as outside advisors, may make presentations to the Compensation Committee. The Compensation Committee charter grants the Compensation Committee full access to all of our books, records, facilities and personnel, as well as the authority to obtain, at our expense, advice and assistance from external advisors that the Compensation Committee considers appropriate in the performance of its duties. As part of its deliberations, the Compensation Committee may review financial reports, projections, operational data, tax and accounting information. The Compensation Committee also considers historical base salary, bonus and equity information including: (1) equity grant history; (2) vested and unvested potential gain on equity awards using an assumed selected series of stock prices at points in time; and (3) stock option exercise history, in its compensation decisions. In determining 2014 NEO compensation, the Compensation Committee also considered the recommendations of our Chief Executive Officer and each executive’s individual performance.
| 72 |
2014 Executive Compensation Summary
2014 Base Cash Compensation
The purpose of base salary is to provide a level of fixed compensation to our NEOs in order to attract and retain executives with the qualifications desired for the particular position. The Compensation Committee reviews base salaries annually, and usually considers adjusting base salaries to reflect our performance over the preceding year while considering the annual base salary increase trend data reflected by the benchmark data. These guidelines are used throughout our Company in determining appropriate base salary increases for all our employees. For 2014, the Compensation Committee’s aim, in line with CBLI’s general philosophy to set target compensation levels that are competitive while maintaining a reasonable cost structure, was to approve 2014 CBLI base salary increases based upon our 2013 performance levels. Based upon its evaluation of our 2013 performance levels, in January 2014 the Compensation Committee did not generally approve any base cash compensation increases for the NEOs. In taking such action, the Compensation Committee specifically considered our stock price performance during 2013 and the level of attainment of certain of our targeted operating milestones for 2013. However, the Compensation Committee did note that Mr. Lyons’ base compensation was significantly below market and therefore made a one-time increase to his compensation of $30,500 bringing his base pay to $285,000 effective January 1, 2014.
Additionally, in light of the financial situation of the Company, commencing in May 2014 our Chief Executive Officer and Chief Scientific Officer each agreed to a 20% reduction in their base salaries.
Incentive Compensation
The Compensation Committee, in its discretion, may establish incentive plans and otherwise award cash and/or equity bonuses to our executive officers. The amounts of both the cash and equity bonuses are determined based on performance, which is evaluated annually under the Annual Plan, and periodically as goals are achieved under the Long-term Plan. The cash and equity bonuses for each of our executive officers is based on various factors, including, among others, the achievement of various operating milestones based on scientific and business goals, our financial performance, the performance of our stock, and our establishment and compliance with satisfactory corporate governance practices. The operating milestones used in the evaluation of our annual incentive compensation are based on annual proposals made by our executive officers, which are then evaluated and ultimately approved by the Compensation Committee. The purpose of the annual incentive bonuses is to motivate and encourage our executive officers to fulfill the short-term goals required for our long-term strategic plan.
2014 Annual Plan - Cash Bonuses. The target annual cash bonus awarded to each executive officer under the Annual Plan is determined based on a percentage of such executive officer’s base salary. The target cash bonus levels for 2014 were set at 30% of base salary, with a maximum potential bonus of 60% of base salary, except for Dr. Gudkov. Dr. Gudkov’s target cash bonus was set at 60% of his base salary, with a maximum potential bonus of 120% of his base salary. Dr. Gudkov’s incentive compensation percentages in relation to his base salary are doubled to reflect the lesser amount of cash compensation paid to him in his part-time role and that the services services that he provides are critical to the attainment of our performance goals. These target bonus levels for 2014 were approved by our Compensation Committee after taking into account the benchmarking study as well as the financial condition of the Company. The minimum bonus amount is zero, and the maximum is 200% of the target bonus amount. If the Committee determines that bonuses should not be awarded for corporate achievement for any reason, bonuses will not be paid. We believe this fully discretionary bonus structure allows the Committee to be responsive to the uncertainties and lack of predictability associated with being a development-stage biotechnology company. The performance goals established for the annual cash bonus plan for 2014 by the Compensation Committee related to:
| ● | execution on our focused, risk-based development strategy for biodefense and oncology indications; | |
| ● | entering into at least one monetizing partnering event; |
| 73 |
| ● | streamlining corporate structure; and | |
| ● | raising 18 months of capital. |
2014 Annual Plan - Equity Bonuses. The Compensation Committee believes that granting stock options provides executive officers with a strong economic interest in maximizing stock price appreciation over the long term. The Compensation Committee also believes that the practice of granting stock options can be useful in retaining and recruiting the key talent necessary to ensure our continued success. This element of compensation is governed by the Cleveland BioLabs, Inc. Equity Incentive Plan, as amended (the “Equity Plan”). The Equity Plan is administered by our Compensation Committee, which reviews executive management’s recommendations concerning stock option grants, and determines the number of stock options to be granted to each person, and the terms and conditions of any stock options as permitted under the Equity Plan. The exercise price of stock options is based on the value of a share of our common stock on the date of grant. The options, therefore, do not have any value to the executive officer unless the market price of our common stock rises, which aligns the interests of our executive officers with those of our stockholders. Through these option grants, we seek to emphasize the importance of improving the performance of our stock price thereby increasing stockholder value over the long term.
Our target stock option bonus for 2014 under the Annual Plan was set at 6,250 stock options for each NEO, with a maximum of 7,500 stock options. The Compensation Committee determined in its subjective judgment that these target awards levels were appropriate to provide sufficient incentives to the NEOs to attain our 2014 performance goals. If granted, such stock options would be immediately fully vested on the applicable grant date, and would have an exercise price per share equal to the value of our stock on the applicable grant date.
Actual 2014 Annual Plan Awards. In the fourth quarter of 2014, the Compensation Committee determined that in light of our then current cash position, and the lack of returns to our investors as evidenced by our stock price on such date, it would not award any cash bonuses for 2014 performance regardless of the level of attainment of the 2014 performance goals. As such, no 2014 cash bonuses were awarded under our Annual Plan. However, recognizing our executive officers efforts in contributing to the advances achieved with the FDA during 2014, namely the permission to file for pre-Emergency Use Authorization of entolimod as a radiation countermeasure, a 6,250 share option to each executive officer was approved to be granted under the 2014 Annual Plan and the Equity Plan. Pursuant to the terms of the Purchase Agreement described in Proposal 4 in our Proxy Statement for our 2015 annual meeting, filed with the SEC on March 6, 2015, we agreed, among other things, not to grant any stock options or other equity awards until the later of April 22, 2015 or the date that stockholder approval of Proposal 4 is received. Therefore, these share options will be automatically granted to the NEOs on April 22, 2015 if stockholder approval of Proposal 4 is received by that date, or if later, will be automatically granted on the date that stockholder approval of Proposal 4 is received.
2012 Long-Term Executive Compensation Plan
On June 13, 2012, the Compensation Committee approved a 2012 Long-term Plan, which expires on December 31, 2016 and includes three major milestone performance goals for our NEOs. These goals are:
Goal #1 – Approval of a BLA for entolimod (previously known as CBLB502) for treatment as a single agent to reduce the risk of death following total body irradiation during or after radiation disaster (medical radiation countermeasure (“MRC”) application);
Goal #2 – Entolimod MRC - Cumulative Firm Orders (all countries exceed $100 million);
Goal #3 – Cumulative proceeds from upfront and milestone payments from licensing deals for any CBLI compounds exceed $12 million (the licensing deals done for the compounds from our subsidiaries and joint ventures will be adjusted by the percentage of CBLI ownership when the licensing agreement is executed).
| 74 |
These goals were selected for our 2012 Long-term Plan as they were determined by the Compensation Committee to be the best indicators of achieving increased value. The applicable payout levels for attainment of each goal were determined in the Compensation Committee’s subjective judgment to be at levels sufficient to incentivize our NEOs to attain such goals, and that the benefit to the Company of such attainment was greater than the cost. Under the 2012 Long-term Plan, awards would be paid to each NEO upon achievement of each strategic objective, subject to the NEO’s continued services with us through such attainment, as outlined below.
Upon achievement of Goal #1 in the United States, each NEO will be paid a bonus equal to 100% of their CBLI base salary or cash consulting retainer, as applicable, as determined by reference to their respective base salary and cash retainer levels in effect on the applicable Goal #1 achievement date.
Upon the first occurrence of the achievement of Goal #1 in Australia, Brazil, Canada, China, European Union, India, Japan, Mexico or Russian Federation, each NEO will be paid a bonus equal to 33% of their base salary or cash consulting retainer, as applicable, as determined by reference to their respective base salary and cash retainer levels in effect on the applicable Goal #1 achievement date. In addition to the above described bonuses, upon the achievement of Goal #1 in the United States or in another country listed above, an amount equal to 100% of the total of the executive team’s aggregate bonus amount will be placed into an employee bonus pool to be distributed to non-executive employees of CBLI, with such bonus amounts allocated at the sole discretion of the executive team.
The following percentages of cumulative firm order/licensing proceeds will be paid to each executive upon achievement of each strategic goal/milestone:
| ● | Upon achievement of Goal #2 or Goal #3, 4% of any cash that the Company receives from all cumulative orders/licensing payments will be allocated to an executive bonus pool, which will be distributed among the members of the executive team, with the allocation among the executive team members to be determined on a pro-rata basis based on 100% of then current CBLI annual base salaries or cash consulting retainer, as applicable, with respect to each NEO; | |
| ● | An additional 1% of all received cumulative orders/licensing payments will be allocated to an employee bonus pool, which will be distributed among the Company’s non-executive senior employees on a pro rata basis based on salary. |
Based on the Company’s cash position when a goal is achieved, the Compensation Committee will determine whether the incentive payouts will be made in the form of cash or stock, or a combination of both. The 2012 Long-term Plan will expire on December 31, 2016 and no amount will be payable under the Long-term Plan for any goal not achieved by that date.
Severance and Change in Control Agreements
In March 2014, we adopted a Severance Benefit Plan in order to provide for consistent severance benefit terms to each of our NEOs and to conform to the severance benefit market practices of our peer group. Under the terms of the Severance Benefit Plan, each NEO is entitled to certain benefits in the event of an involuntary termination of employment by the Company for a reason other than death, disability, or cause, which is referred to as a Qualifying Termination. In the event of a Qualifying Termination, each NEO is entitled to a cash severance payment in an amount equal to 12-months of salary (and with respect to Drs. Kogan and Gudkov such cash severance benefit will be measured with respect to each officer’s respective base salary as of May 1, 2014 and prior to the voluntary 20% reduction if such amount is greater than the base salary in effect immediately prior to the date of termination). Additionally, the Company will pay the full amount of each officer’s Consolidated Omnibus Budget Reconciliation Act, or COBRA, premiums for a period not to exceed 12-months. In addition, the Company will extend the exercise period of any vested stock option for a period of 1-year from the officer’s last day of employment or until expiration of the stated term (whichever period is shorter), and stock options that would have vested during the 12-month period following the last day of employment shall immediately vest on the last day of employment.
| 75 |
Each of our NEOs became participants in the Severance Benefit Plan during 2014 and as a condition to participation waived their rights with respect to any severance benefits contained in their respective employment agreements or offer letters in the event of a Qualifying Termination. As a condition to provision of any benefits under the Severance Plan, the NEO must provide the Company with a release of claims.
Under the Severance Benefit Plan, cause for termination means any of the following events: (i) the participant’s failure substantially to perform his or her duties and responsibilities to the Company, which is not cured within 30 days of written notice to the participant; (ii) the participant’s commission (including a guilty plea or plea of nolo contendere) of any felony or any other crime involving fraud, dishonesty or moral turpitude; (iii) any intentional or grossly negligent act by the participant that has caused or is reasonably expected to result in material injury to the Company; (iv) the participant’s material breach of any obligation under any written agreement with the Company, including but not limited to the participant’s confidentiality agreement, that is not cured within 30 days of written notice to the participant; (v) the participant’s violation of a Company policy, or commission of any act of fraud, embezzlement, dishonesty or any other willful misconduct, that has caused or is reasonably expected to result in material injury to the Company; or (vi) the material unauthorized use, disclosure or misappropriation by the participant of any proprietary information, trade secret or other asset of the Company or entrusted to the Company by a third party.
Under the terms of the employment agreements with Dr. Kogan and Mr. Lyons, if such executive is terminated due to a permanent disability or death, he would be entitled to receive severance pay equal to the base salary that would have been payable if he had continued his employment for the remaining term under his employment agreement, which period currently may not exceed 12-months as the employment agreements automatically renew for consecutive one year terms. However, if such executive becomes permanently disabled or dies as a result of, or in conduct of, his employment duties under his employment agreement, he would be entitled to severance pay equal to his base salary that would have been payable had he continued his employment for a period of no less than 18 months. In order to comply with Section 409A of the Internal Revenue Code, in certain instances, such severance may be delayed until the earlier of six months and one day after such executive’s separation from service or his death. For purposes of their employment agreements, a “permanent disability” will be deemed to occur if such executive suffers a physical or mental illness, injury or infirmity that prevents him from performing, with or without reasonable accommodations, his essential job functions, for a total period of 120 days in any 360-day period.
Under the terms of the agreement with Dr. Gudkov, if he is terminated without cause during the 12 month period following a change in control he is entitled to payment of the following severance benefits, subject to his timely provision of an effective release of claims against the Company (i) 2.5 times his then current annualized base compensation amount, or if greater, his annualized base compensation amount which was effective as of January 1, 2011, plus (ii) the amount of his target annual cash bonus as in effective for the year that includes his termination. The base compensation severance benefits are payable in installments over the two and half year period following the termination and the target cash bonus severance benefits are payable in a single lump sum at the time the bonus amount would have otherwise been payable absent a termination of service.
Executive Benefits
Our executive benefits are generally limited to the same benefits we offer to all of our employees, except that Dr. Gudkov does not generally participate in any of our employee benefit plans because he is separately employed by the Roswell Park Cancer Institute and participates in their employee benefit plans.
Employee Stock Purchase Plan
At our 2013 Annual Meeting, an Employee Stock Purchase Plan or “ESPP” was approved by our stockholders, and at our 2015 Annual Meeting an amendment thereto was approved by our stockholders. As amended, the ESPP has 225,000 shares available for issuance, which amount will be increase by 100,000 shares annually. The purpose of the ESPP is to provide a means by which our company employees (and any parent or subsidiary of our company designated by the Board to participate in the ESPP) may be given an opportunity to purchase common stock through payroll deductions, to assist us in retaining the services of our employees, to secure and retain the services of new employees, and to provide incentives for such persons to exert maximum efforts for the success of our company and our affiliates.
| 76 |
The rights to purchase common stock granted under the ESPP are intended to qualify as options issued under an “employee stock purchase plan” as that term is defined in Section 423(b) of the Internal Revenue Code of 1986, as amended, or the Code. To date we have not commenced offerings to participate in this plan to our employees, but we plan on implementing the ESPP during the second half of 2015. If and when we do commence offerings to participate in this plan, all of our eligible employees including our named executive officers will be eligible to participate.
Our Compensation Policies
Section 162(m) Policy
Section 162(m) of the Internal Revenue Code limits the amount that a public company may deduct from federal income taxes for remuneration paid to the chief executive officer and the three other most highly paid executive officers (other than the chief financial officer) to $1 million per year per covered executive officer. Section 162(m) provides an exception from this deduction limitation for certain forms of “performance-based compensation,” including the gain recognized by executive officers upon the exercise of certain compensatory stock options and other compensation based on performance criteria that are approved in advance by stockholders. We are mindful of the benefit to the Company and its stockholders of the full deductibility of compensation. However, we believe that there may be times when we need to retain flexibility in compensating our executive officers in a manner that we believe will best promote our corporate objectives even though the compensation may not be fully deductible under Section 162(m). Therefore, we have not adopted a policy that requires that all compensation be deductible.
Accounting Considerations
The accounting impact of our equity compensation program is one of many factors that the Compensation Committee may consider in determining the size and structure of our program.
Common Stock Ownership Requirements
While we have not adopted a formal written policy on common stock ownership requirements, part of our compensation philosophy involves facilitating common stock ownership by our executive officers through the grant of equity awards because we believe that it helps to align their financial interests with those of our stockholders.
Timing of Awards
The Compensation Committee has the authority to grant equity awards under our Equity Plan. The Compensation Committee strives to ensure that any award is made in such a manner as to avoid even the appearance of manipulation because of its award date. It is our policy not to purposely accelerate or delay the public release of material information in consideration of a pending equity grant to allow the grantee to benefit from a more favorable stock price.
Compensation Recovery Policy
We do not have a policy to attempt to recover cash bonus payments paid to our executive officers if the performance objectives that led to the determination of such payments were to be restated, or found not to have been met to the extent the Compensation Committee originally believed. However, as a public company subject to the provisions of Section 304 of the Sarbanes-Oxley Act of 2002, if we are required as a result of misconduct to restate our financial results due to our material noncompliance with any financial reporting requirements under the federal securities laws, our chief executive officer and chief financial officer may be legally required to reimburse us for any bonus or other incentive-based or equity-based compensation they receive. In addition, we will comply with the requirements of the Dodd-Frank Wall Street Reform and Consumer Protection Act and will adopt a compensation recovery policy once the SEC adopts final regulations on the subject.
| 77 |
Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards Table
Yakov Kogan
We entered into an employment agreement dated as of August 1, 2004 with Yakov Kogan, our Chief Executive Officer, which included a three-year initial term and is renewed annually for successive one-year periods, unless earlier terminated in accordance with its terms.
Dr. Kogan was paid a base CBLI salary, exclusive of salaries paid by our subsidiaries, at an annual rate of $345,000 as of December 31, 2014, which Dr. Kogan voluntarily and temporarily reduced to $276,000 effective May 7, 2014. In addition, Dr. Kogan is eligible to earn an annual bonus based on corporate targets set by our board on an annual basis. For fiscal year 2014, in light of our cash position at December 31, 2014, and the lack of returns to our investors as evidenced by our stock price on such date, the Compensation Committee determined that it would not award 2014 cash bonuses to any executives regardless of the level of attainment of the 2014 performance goals. As such, Dr. Kogan did not receive a cash bonus under the 2014 Annual Plan. An equity bonus for Dr. Kogan in the form of a stock option to purchase 6,250 shares of our common stock to be awarded under the 2014 Annual Plan and our Equity Plan was approved by the Compensation Committee in December 2014 and granted on April 22, 2015.
Dr. Kogan’s employment agreement provides that such agreement will automatically be terminated on the date of his death. Furthermore, the employment agreement permits us to terminate Dr. Kogan upon written notice at any time, with or without cause, or due to a permanent disability.
If Dr. Kogan was terminated by us without cause as described in the agreement, he would be entitled to severance pay equal to twelve months of his annual salary, and COBRA health benefits during the severance period. In order to comply with Section 409A of the Internal Revenue Code, in certain instances, such severance may be delayed until the earlier of six months and one day after such executive’s separation from service or his death. The employment agreement also contains confidentiality, assignment of inventions, non-competition and non-solicitation provisions to help protect the value of our intellectual property.
C. Neil Lyons, CPA
We entered into an employment agreement dated as of August 4, 2011 with C. Neil Lyons, our Chief Financial Officer, effective September 1, 2011. The employment agreement provides that Mr. Lyons’ initial employment term extended until August 31, 2012 and, thereafter, his employment term will be renewed pursuant to terms of the employment agreement for successive one-year periods, unless earlier terminated in accordance with its terms.
Mr. Lyons was paid a base salary at an annual rate of $285,000 in fiscal year 2014. As an executive officer of the Company, Mr. Lyons is eligible to earn an annual bonus based on corporate targets set by our board on an annual basis. For fiscal year 2014, in light of our cash position at December 31, 2014, and the lack of returns to our investors as evidenced by our stock price on such date, the Compensation Committee determined that it would not award 2014 cash bonuses to any executives regardless of the level of attainment of the 2014 performance goals. As such, Mr. Lyons did not receive a cash bonus under the 2014 Annual Plan. An equity bonus in the form of a stock option to purchase 6,250 shares of our common stock to be awarded under the 2014 Annual Plan and our Equity Plan was approved by the Compensation Committee in December 2014 and granted on April 22, 2015.
| 78 |
Mr. Lyons’ employment agreement provides that such agreement will automatically be terminated on the date of his death. Furthermore, the employment agreement permits us to terminate Mr. Lyons upon written notice at any time, with or without cause, or due to a permanent disability.
If Mr. Lyons is terminated by us without cause as described in the agreement, he would be entitled to severance pay equal to twelve months of his annual salary, and COBRA health benefits during the severance period. In order to comply with Section 409A of the Internal Revenue Code, in certain instances, such severance may be delayed until the earlier of six months and one day after such executive’s separation from service or his death. The employment agreement also contains confidentiality, assignment of inventions, non-competition and non-solicitation provisions to help protect the value of our intellectual property.
Andrei Gudkov, Ph.D., D.Sci.
During the year ended December 31, 2014, our Chief Scientific Officer, Andrei Gudkov, served in such capacity pursuant to an agreement with us dated as of January 1, 2010, as amended June 10, 2012. Dr. Gudkov’s agreement has an initial term of one year and automatically renews for successive one-year periods, unless earlier terminated in accordance with its terms.
Pursuant to the agreement, Dr. Gudkov received base compensation from CBLI, exclusive of salaries paid by our subsidiaries, at an annual rate of $138,677 in fiscal year 2014, which Dr. Gudkov voluntarily and temporarily reduced to $110,941 effective May 7, 2014. Pursuant to the agreement, Dr. Gudkov is deemed an executive officer of the Company and is eligible to participate in our executive compensation plans. In addition, Dr. Gudkov is eligible to earn an annual bonus based on corporate targets set by our board on an annual basis. For fiscal year 2014, in light of our cash position at December 31, 2014, and the lack of returns to our investors as evidenced by our stock price on such date, the Compensation Committee determined that it would not award 2014 cash bonuses to any executives regardless of the level of attainment of the 2014 performance goals. As such, Dr. Gudkov did not receive a cash bonus under the 2014 Annual Plan. An equity bonus for Dr. Gudkov in the form of a stock option to purchase 6,250 shares of our common stock to be awarded under the 2014 Annual Plan and our Equity Plan was approved by the Compensation Committee in December 2014 and granted on April 22, 2015.
The agreement with Dr. Gudkov permits us to immediately terminate such agreement upon written notice only “for cause.” The agreement also permits either party to terminate such agreement without cause upon 14 days’ written notice to the other party. The agreement also contains confidentiality and assignment of inventions provisions to help protect the value of our intellectual property, and an indemnification provision for the benefit of Dr. Gudkov.
In July of 2014, Dr. Gudkov’s employment status changed from an independent contractor to an employee of CBLI and it was agreed that he would continue to be paid at the same level of base cash compensation and with the same bonus award eligibility as previously provided under his agreement as described above.
| 79 |
Outstanding Equity Awards at Fiscal Year-End
The following table shows grants of stock options outstanding on the last day of the fiscal year ended December 31, 2014, including both awards subject to performance conditions and non-performance-based awards, to each of the executive officers named in the Summary Compensation Table. There were no stock option exercises by any of our named executive officers during the fiscal year ended December 31, 2014. There were no outstanding stock awards to the executive officers named in the Summary Compensation Table on the last day of the fiscal year ended December 31, 2014. All balances shown in the table below have been adjusted to account for the 1:20 reverse split of the Company’s common stock that was executed on January 28, 2015.
| Option Awards | ||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable (#) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | ||||||||||
| Yakov Kogan | 7,500 | 13.60 | 3/13/2024 | |||||||||||
| 5,230 | 30.80 | 5/12/2023 | ||||||||||||
| 5,000 | 35.80 | 6/12/2022 | ||||||||||||
| 2,813 | 67.00 | 1/22/2022 | ||||||||||||
| 7,481 | 143.20 | 3/20/2021 | ||||||||||||
| 5,250 | 68.80 | 5/17/2020 | ||||||||||||
| 6,863 | 80.00 | 2/3/2018 | ||||||||||||
| 1,875 | 167.20 | 4/5/2017 | ||||||||||||
| C. Neil Lyons | 7,500 | 13.60 | 3/13/2024 | |||||||||||
| 3,857 | 30.80 | 5/12/2023 | ||||||||||||
| 938 | 67.00 | 1/22/2022 | ||||||||||||
| 6,250 | 48.20 | 8/31/2021 | ||||||||||||
| Andrei Gudkov | 7,500 | 13.60 | 3/13/2024 | |||||||||||
| 4,203 | 30.80 | 5/12/2023 | ||||||||||||
| 2,813 | 67.00 | 1/22/2022 | ||||||||||||
| 7,481 | 143.20 | 3/20/2021 | ||||||||||||
| 5,250 | 68.80 | 5/17/2020 | ||||||||||||
| 6,863 | 80.00 | 2/3/2018 | ||||||||||||
| 1,875 | 167.20 | 4/5/2017 | ||||||||||||
Potential Payments upon Termination or Change-In-Control
In March 2014, we adopted a Severance Benefit Plan in order to provide for consistent severance benefit terms to each of our NEOs and to conform to the severance benefit market practices of our peer group. Under the terms of the Severance Benefit Plan, each NEO is entitled to certain benefits in the event of an involuntary termination of employment by the Company for a reason other than death, disability, or cause, which is referred to as a Qualifying Termination. In the event of a Qualifying Termination, each NEO is entitled to a cash severance payment in an amount equal to 12-months of salary (and with respect to Drs. Kogan and Gudkov such cash severance benefit will be measured with respect to each officer’s respective base salary as of May 1, 2014 and prior to the voluntary 20% reduction if such amount is greater than the base salary in effect immediately prior to the date of termination). Additionally, the Company will pay the full amount of each officer’s Consolidated Omnibus Budget Reconciliation Act, or COBRA, premiums for a period not to exceed 12-months. In addition, the Company will extend the exercise period of any vested stock option for a period of 1-year from the officer’s last day of employment or until expiration of the stated term (whichever period is shorter), and stock options that would have vested during the 12-month period following the last day of employment shall immediately vest on the last day of employment.
| 80 |
Each of our NEOs became participants in the Severance Benefit Plan during 2014 and as a condition to participation waived their rights with respect to any severance benefits contained in their respective employment agreements or offer letters in the event of a Qualifying Termination. As a condition to provision of any benefits under the Severance Plan, the NEO must provide the Company with a release of claims.
Under the Severance Benefit Plan, cause for termination means any of the following events: (i) the participant’s failure substantially to perform his or her duties and responsibilities to the Company, which is not cured within 30 days of written notice to the participant; (ii) the participant’s commission (including a guilty plea or plea of nolo contendere) of any felony or any other crime involving fraud, dishonesty or moral turpitude; (iii) any intentional or grossly negligent act by the participant that has caused or is reasonably expected to result in material injury to the Company; (iv) the participant’s material breach of any obligation under any written agreement with the Company, including but not limited to the participant’s confidentiality agreement, that is not cured within 30 days of written notice to the participant; (v) the participant’s violation of a Company policy, or commission of any act of fraud, embezzlement, dishonesty or any other willful misconduct, that has caused or is reasonably expected to result in material injury to the Company; or (vi) the material unauthorized use, disclosure or misappropriation by the participant of any proprietary information, trade secret or other asset of the Company or entrusted to the Company by a third party.
Under the terms of the employment agreements with Dr. Kogan and Mr. Lyons, if such executive is terminated due to a permanent disability or death, he would be entitled to receive severance pay equal to the base salary that would have been payable if he had continued his employment for the remaining term under his employment agreement, which period currently may not exceed 12-months as the employment agreements automatically renew for consecutive one year terms. However, if such executive becomes permanently disabled or dies as a result of, or in conduct of, his employment duties under his employment agreement, he would be entitled to severance pay equal to his base salary that would have been payable had he continued his employment for a period of no less than 18 months. In order to comply with Section 409A of the Internal Revenue Code, in certain instances, such severance may be delayed until the earlier of six months and one day after such executive’s separation from service or his death. For purposes of their employment agreements, a “permanent disability” will be deemed to occur if such executive suffers a physical or mental illness, injury or infirmity that prevents him from performing, with or without reasonable accommodations, his essential job functions, for a total period of 120 days in any 360-day period.
Under the terms of the agreement with Dr. Gudkov, if he is terminated without cause during the 12 month period following a change in control he is entitled to payment of the following severance benefits, subject to his timely provision of an effective release of claims against the Company (i) 2.5 times his then current annualized base compensation amount, or if greater, his annualized base compensation amount which was effective as of January 1, 2011, plus (ii) the amount of his target annual cash bonus as in effective for the year that includes his termination. The base compensation severance benefits are payable in installments over the two and half year period following the termination and the target cash bonus severance benefits are payable in a single lump sum at the time the bonus amount would have otherwise been payable absent a termination of service.
The following table summarizes the payments that would have been made to our named executive officers under the employment or consulting agreements, as applicable, upon a termination on December 31, 2014.
| Voluntary Termination/ Termination for Cause | Termination Without Cause | Death or Disability In Performance of Duty | Change-In- Control | |||||||||||||
| Yakov Kogan | $ | — | $ | 345,000 | $ | 414,000 | $ | 345,000 | ||||||||
| Neil Lyons | $ | — | $ | 285,000 | $ | 427,500 | $ | 285,000 | ||||||||
| Andrei Gudkov | $ | — | $ | 138,677 | $ | — | $ | 407,125 | ||||||||
| 81 |
Actual amounts that the named executive officers could receive in the future as a result of a termination of employment could differ materially from the amounts set forth above as a result of, among other things, changes in their base salaries, changes in our stock price and the vesting and grants of additional equity awards.
Director Compensation
The following is a description of the standard cash compensation arrangements under which the members of the Cleveland BioLabs, Inc. Board of Directors are compensated for their service as directors, including as members of the various committees of our board. Each of our directors who also serve as NEOs are not compensated in addition to the compensation they receive as an NEO of the Company as disclosed above.
| Position | Annual Fee | |||
| Board Member | $ | 40,000 | ||
| Board Chair | 20,000 | |||
| Audit Committee Chair | 15,000 | |||
| Audit Committee Members | 10,000 | |||
| Compensation Committee Chair | 7,500 | |||
| Compensation Committee Members | 5,000 | |||
| Nominating and Governance Committee Chair | 2,500 | |||
| Nominating and Governance Committee Members | 2,500 | |||
| (1) | Board Chairman fee commenced in 2014 | |
| (2) | Annual fees were pro-rated as appropriate for the period or each member’s service. | |
In addition to annual cash compensation we also compensate our board with equity in the form of options to purchase shares of our common stock. We grant options to purchase 750 shares of our common stock upon appointment to the board and options to purchase 1,750 shares of common stock upon annual election to continue service. Option grants upon appointment to the board vest in three equal annual installments from the grant date, such that the option is fully vested on the third anniversary of the date of the grant, subject to the director’s continuous service through the applicable vesting date. Annual grants are fully vested when issued. All option grants made to the board are exercisable for ten years. Each of our independent directors is also reimbursed for reasonable out-of-pocket expenses incurred in attending our board or board committee meetings.
The following table shows the total compensation paid or accrued during the fiscal year ended December 31, 2014 to each of our directors.
| Name (a) | Paid In
Cash ($) | Stock Awards (1) ($) | Option Awards (2) ($) | Total ($) | ||||||||||||
| James J. Antal | $ | 61,875 | $ | 0 | $ | 10,159 | $ | 72,034 | ||||||||
| Julia R. Brown (5) | 30,425 | 36,484 | 10,159 | 77,068 | ||||||||||||
| DiCorleto, Paul, M.D (3) | 38,975 | 0 | 0 | 38,975 | ||||||||||||
| Daniel Hoth, M.D. (5) | 24,375 | 0 | 15,159 | 39,534 | ||||||||||||
| Hohn, David, M.D (3) | 37,825 | 15,510 | 0 | 53,335 | ||||||||||||
| Richard S. McGowan, J.D | 24,375 | 0 | 15,159 | 39,534 | ||||||||||||
| Anthony J. Principi, J.D. | 36,475 | 11,526 | 10,159 | 58,160 | ||||||||||||
| Alexander Polinsky, Ph.D. (5) | 28,450 | 0 | 15,159 | 43,609 | ||||||||||||
| Randy S. Saluck, J.D., MBA | 54,600 | 0 | 10,159 | 64,759 | ||||||||||||
| Elena Kasimova, Ph.D. (4) | 0 | 0 | 0 | 0 | ||||||||||||
| (1) | These amounts represent the grant date fair value of stock awards granted to each director in 2014 computed in accordance with FASB ASC Topic 718. A discussion of the assumptions used in determining grant date fair value may be found in our Financial Statements, included in this prospectus. |
| (2) | These amounts represent the grant date fair value of options granted to each director in 2014 computed in accordance with FASB ASC Topic 718. A discussion of the assumptions used in determining grant date fair value may be found in our financial statements, included in this prospectus. |
| (3) | Drs. Hohn and DiCorleto retired from our board at the June 2014 Annual Meeting. |
| (4) | Ms. Kasimova was appointed to the Board in January 2015 and resigned from the board on April 29, 2015. |
| (5) | Ms. Brown and Drs. Hoth and Polisky retired from our board at the April 2015 Annual Meeting. |
| 82 |
EQUITY COMPENSATION PLAN INFORMATION
The following table provides information as of December 31, 2014, regarding shares of common stock that may be issued under the Company’s equity compensation plans, including the Equity Plan. Information is included for both equity compensation plans approved by the Company’s stockholders and not approved by the Company’s stockholders (which date back to before the Company became a reporting company under the Exchange Act). All balances shown in the table below have been adjusted to account for the 1:20 reverse split of the Company’s common stock that was executed on January 28, 2015.
| Plan category | (a) Number of securities to be issued upon exercise of outstanding options, warrant sand rights | (b) Weighted-average exercise price of outstanding options, warrants and rights | (c) Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| Equity compensation plans approved by security holders (1) | 258,476 | $ | 67.64 | 94,389 | ||||||||
| Equity compensation plans not approved by security holders (2) | 2,913 | 90.00 | — | |||||||||
| Total | 261,389 | $ | 67.89 | 94,389 | ||||||||
| (1) | Consists of the Equity Plan. |
| (2) | The number shown consists of shares to be issued upon equity grants made by us prior to our initial public offering when we did not have any defined equity compensation plans approved by our stockholders. |
| 83 |
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
Pursuant to our Related Party Transaction Policy, the Audit Committee must provide written approval in advance for any transaction that could involve an actual, potential or perceived conflict of interest, including transactions where employees or directors have a substantial financial interest in any of our competitors, customers or suppliers, or where gifts or loans of value in excess of $200 are received in a year from our suppliers, customers or competitors. The policy also requires advance written approval for any transaction where an employee or director owns a substantial interest in an entity that has a prospective business relationship with, or is a competitor of, us. The following is a list of related persons with whom all transactions are reviewed and approved by the Audit Committee.
Our Chief Scientific Officer and board member, Dr. Andrei Gudkov, is the Senior Vice President of Basic Science and the Chairman of the Department of Cell Stress Biology at Roswell Park Cancer Institute (“RPCI”). We subcontract Dr. Gudkov’s laboratory at RPCI to perform certain research and development studies for us, and also purchase certain core products and services from RPCI, including mice, the housing and storage of mice, irradiator services, DNA sequencing and blood analysis. RPCI also serves as one of our clinical sites. For the aforementioned services, we paid RPCI approximately $1.1 and $2.7 million in 2014 and 2013, respectively. In addition, we transferred 23 research scientists to Buffalo BioLabs, Inc. (“BBL”) in the later part of 2013, an entity then partially-owned by Dr. Gudkov and of which he is a founder and the Principal Scientific Advisor. We hire BBL on a project basis to perform research work, as needed. For the aforementioned services, we paid BBL approximately $1.2 and $0.3 million in 2014 and 2013, respectively.
On March 1, 2010, we hired Leah Brownlee who serves in the position of Executive Vice President - Compliance and Operations and Corporate Secretary. Ms. Brownlee is the spouse of Dr. Yakov Kogan who is a member of our board and our Chief Executive Officer. During the year ended December 31, 2014, Ms. Brownlee earned a base salary of $225,000 and received options exercisable into 1,200 shares of common stock at a weighted average exercise price of $13.60 per share. During the year ended December 31, 2013, Ms. Brownlee earned a base salary of $200,583 and received options exercisable into 1,250 shares of common stock at a weighted average exercise price of $30.80 per share. On April 14, 2015, we entered into an employment agreement with Ms. Brownlee, pursuant to which Ms. Brownlee will continue to serve as our Executive Vice President, Compliance and Operations. We agreed to pay Ms. Brownlee a base salary of $225,000 per year (subject to review and adjustment from time to time by the Board of Directors), and Ms. Brownlee will also be eligible for participation in the Company’s Annual Executive Bonus Plan and Long Term Incentive Bonus Program. In the event Ms. Brownlee terminates the employment agreement for Good Reason (as defined therein), or the Company terminates the employment agreement without Cause (as defined therein), we will be required to pay Ms. Brownlee’s base salary for a period of 12 months following the termination date.
Dr. Alexander Polinsky, who retired from our board at the 2015 Annual Meeting, is the chief executive officer of Everon Biosciences, Inc. (“Everon”) and Tartis-Aging, Inc., sister companies owned in part with Bioprocess Capital Partners and Dr. Andrei Gudkov, our Chief Scientific Officer and board member. Dr. Gudkov is also a founder of and Chief Scientific Officer of Everon and Tartis Aging, Inc. Bioprocess Capital Partners is our co-shareholder in Incuron, LLC. During 2013, Everon paid CBLI approximately $200,000 for research work during the first nine months of the year, prior to the transfer of our research personnel to BBL.
On February 4, 2015, we entered into a Securities Purchase Agreement with certain institutional investors providing for the issuance and sale of 572,205 shares of our common stock, par value $0.005 per share at an offering price of $3.00 per share and Pre-Funded Warrants to purchase an aggregate of 594,688 shares of our common stock. In a concurrent private placement, we sold to the same institutional investors shares of our Series A Convertible Preferred Stock convertible into 239,135 shares of our common stock. In addition, we issued Series A Warrants to purchase one share of our common stock for each share of common stock purchased or prefunded in this offering and each share of Series A Convertible Preferred Stock purchased in the concurrent private placement. Sabby Healthcare Volatility Master Fund, a holder of more than 5% of our capital stock, and its affiliated fund Sabby Volatility Warrant Master Fund participated in the offering and purchased in the aggregate (i) 286,102 shares of common stock, (ii) a Pre-Funded Warrant to purchase 297,344 shares of common stock, (iii) 358.70 shares of Series A Convertible Preferred Stock and (iv) a Series A Warrant to purchase 703,014 shares of common stock.
| 84 |
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of April 30, 2015 for (a) the executive officers named in the Summary Compensation Table in the section titled “Executive Officer and Director Compensation,” (b) each of our directors and director nominees, and (c) all of our current directors and executive officers as a group. As of April 30, 2015, only the stockholders indicated in the table below beneficially owned more than 5% of our common stock. Beneficial ownership is determined in accordance with the rules of the Securities Exchange Commission and includes voting or investment power with respect to the securities. We deem shares of common stock that may be acquired by an individual or group within 60 days of April 30, 2015 pursuant to the exercise of options or warrants to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Except as indicated in footnotes to this table, we believe that the stockholders named in this table have sole voting and investment power with respect to all shares of common stock shown to be beneficially owned by them based on information provided to us by these stockholders. Percentage of ownership is based on 4,002,265 shares of common stock outstanding on April 30, 2015.
| Name and Title | Outstanding Shares Beneficially Owned | Rights to Acquire Beneficial Ownership | Total Shares Beneficially Owned | Percent | |||||||||||||
| Named Directors and Executive Officers | |||||||||||||||||
| James J. Antal | 1,763 | 14,750 | 16,513 | * | |||||||||||||
| Richard S. McGowan, Esq. | - | 16,215 | 16,215 | * | |||||||||||||
| Anthony J. Principi, J.D. | 2,623 | 4,000 | 6,623 | * | |||||||||||||
| Randy S. Saluck, J.D., MBA | (1) | 10,866 | 5,000 | 15,866 | * | ||||||||||||
| Yakov Kogan, Ph.D., MBA | (2) | 35,367 | 55,484 | 90,851 | 2.2 | % | |||||||||||
| Andrei Gudkov, Ph.D., D. Sci. | 75,869 | 38,032 | 113,901 | 2.8 | % | ||||||||||||
| C. Neil Lyons, CPA | 1,013 | 20,938 | 21,951 | * | |||||||||||||
| All current executive officers and directors as a group (7 persons) | 127,501 | 154,419 | 281,920 | 6.8 | % | ||||||||||||
| 5% or greater shareholders | |||||||||||||||||
| Mikhail Mogutov, Ph.D. | (3) | 264,318 | 132,159 | 396,477 | 9.6 | % | |||||||||||
| Sabby Management, LLC | (4) | 416,910 | 155,727 | 572,637 | 13.8 | % | |||||||||||
| Alpha Capital Anstalt | (5) | 150,000 | 266,911 | 416,911 | 9.8 | % | |||||||||||
| * | Represents beneficial ownership of less than 1% of the outstanding shares of our common stock. |
| (1) | Includes 6,641 shares and 3,750 shares of common stock that can be acquired through the exercise of options that are directly owned by Mr. Saluck. Also included are 4,225 shares and 1,250 shares issuable upon the exercise of warrants to purchase common stock, all of which are owned by Mortar Rock LP. Mr. Saluck has voting power and investment power over these shares and investment power over these shares and warrants as he is the Managing Member of Mortar Rock Capital Management, LLC which manages Mortar Rock LP. |
| (2) | Includes 36,782 shares issuable upon the exercise of options to purchase common stock. Also includes 6,202 shares of common stock underlying options to purchase common stock and 2,242 shares of common held by Ms. Leah Brownlee, who is employed by us as Executive Vice President - Compliance and Operations and Corporate Secretary. Dr. Kogan disclaims beneficial ownership over the shares beneficially owned directly by Ms. Brownlee. See “Certain Relationships and Related Person Transactions.” |
| (3) | Mikhail Mogutov, Ph.D. reported sole voting and dispositive power with respect to 264,318 shares of our common stock and 132,159 shares of our common stock issuable upon exercise of warrants in a Schedule 13D filed with the SEC on July 2, 2014. The address of Mikhail Mogutov, Ph.D. is Stoloviy pereulok 6, Moscow, 121069, Russia. |
| (4) | Sabby Management, LLC, Sabby Healthcare Volatility Master Fund, Ltd. and Hal Mintz reported shared voting and dispositive power with respect to 174,583 shares of our common stock as of January 9, 2015 in a Schedule 13G filing with the SEC on January 9, 2015. The address of Sabby Volatile Healthcare Fund is c/o Ogier Fiduciary Services (Cayman) Limited, 89 Nexus Way, Camana Bay, Grand Cayman KY1-9007, Cayman Islands. The address of Sabby Management, LLC and Hal Mintz is 10 Mountainview Road, Suite 205, Upper Saddle River, New Jersey 07458. |
| (5) | Alpha Capital Anstalt reported sole voting and dispositive power with respect to 286,103 shares of our common stock as of February 5, 2015 in a Schedule 13G filing with the SEC on February 5, 2015 . The address of Alpha Capital Anstalt is Pradafant 7, Furstentums 9490, Vaduz, Liechtenstein. |
| 85 |
Our restated certificate of incorporation, as amended, authorizes us to issue up to 160,000,000 shares of common stock, par value $0.005 per share, and 10,000,000 shares of preferred stock, par value $0.005 per share. As of April 30, 2015, we had outstanding
| ● | 4,002,265 shares of common stock; | |
| ● | 358.7 shares of Series A Preferred Stock, which are convertible into 119,567 shares of common stock; | |
| ● | options exercisable for up to 379,307 shares of common stock; and | |
| ● | warrants exercisable for up to 2,428,676 shares of common stock, which number includes 147,344 shares of common stock issuable upon exercise of prefunded warrants and excludes 40,617 shares of common stock issuable upon the exercise of a warrant held by Rusnano that is only exercisable in the event of a default by Panacela in the repayment of a loan. |
As of April 30, 2015, we had approximately 32 holders of record in our common stock. The actual number of stockholders is greater than this number of record holders, and includes stockholders who are beneficial owners, but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities.
The following description of our capital stock is not complete and is subject to and qualified in its entirety by our restated certificate of incorporation, as amended, and by the relevant provisions of the Delaware General Corporation Law.
Common Stock
Voting Rights. The holders of our common stock are entitled to one vote per share with respect to each matter presented to our stockholders on which the holders of common stock are entitled to vote and do not have cumulative voting rights. An election of directors by our stockholders is determined by a plurality of the votes cast by the stockholders entitled to vote on the election.
Dividends. Holders of common stock are entitled to receive ratably any dividends as may be declared by our board of directors, subject to any preferential dividend rights of outstanding preferred stock.
Liquidation and Dissolution. In the event of our liquidation or dissolution, the holders of common stock are entitled to receive ratably all assets available for distribution to stockholders after the payment of all debts and other liabilities and subject to the prior rights of any outstanding preferred stock.
Other Rights. Holders of common stock have no preemptive, subscription, redemption or conversion rights. The rights, preferences and privileges of holders of common stock are subject to and may be adversely affected by the rights of the holders of shares of any series of preferred stock that we may designate and issue in the future.
Listing. Our common stock is listed on The NASDAQ Capital Market under the symbol “CBLI.”
Transfer Agent and Registrar. The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company.
Fully Paid and Nonassessable. All of our outstanding shares of common stock are, and the shares of common stock to be issued in this offering will be, fully paid and nonassessable.
Preferred Stock
Our Board of Directors has the authority, without further action by the stockholders, to issue up to 10,000,000 shares of preferred stock in one or more series, to establish from time to time the number of shares to be included in each such series, to fix the rights, preferences and privileges of the shares of each wholly unissued series and any qualifications, limitations or restrictions thereon and to increase or decrease the number of shares of any such series, but not below the number of shares of such series then outstanding.
| 86 |
Our board of directors may authorize the issuance of preferred stock with voting or conversion rights that could adversely affect the voting power or other rights of the holders of the common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in our control that may otherwise benefit holders of our common stock and may adversely affect the market price of the common stock and the voting and other rights of the holders of common stock. On February 6, 2015, we issued 717.4 shares of Series A convertible preferred stock. As of April 30, 2015, 358.7 shares remain outstanding, which are convertible into 119,567 of our common stock (see “Series A Preferred Stock” below). We have no current plans to issue any additional shares of preferred stock, except as set forth in the section entitled “Description of Securities We Are Offering”.
Series A Preferred Stock
On February 5, 2015, the Company filed a Certificate of Designations of Preferences, Rights and Limitations of Series A Convertible Preferred Stock (the “Series A Certificate of Designation”) with the Secretary of State of the State of Delaware. The number of shares of preferred stock designated as Series A Preferred Stock is 718 and each share of Series A Preferred Stock has a stated value equal to $1,000. Until Shareholder Approval has been obtained, the Series A Preferred Stock cannot be converted into common stock.
Voting Rights. Except as otherwise provided in the Series A Certificate of Designation or as otherwise required by law, the Series A Preferred Stock shall have no voting rights. However, as long as any shares of Series A Preferred Stock are outstanding, the Company shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series A Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Series A Preferred Stock or alter or amend the Series A Certificate of Designation, (b) authorize or create any class of stock ranking as to dividends, redemption or distribution of assets upon a liquidation senior to, or otherwise pari passu with, the Series A Preferred Stock, (c) amend its certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of the Series A Preferred Stock, (d) increase the number of authorized shares of Series A Preferred Stock, or (e) enter into any agreement with respect to any of the foregoing.
Liquidation. Upon any liquidation, dissolution or winding-up of the Company, whether voluntary or involuntary (a “Liquidation”), the holders of Series A Preferred Stock shall be entitled to receive out of the assets, whether capital or surplus, of the Company an amount equal to the Stated Value, plus any other fees, liquidated damages or dividends then due and owing thereon under the Series A Certificate of Designation, for each share of Series A Preferred Stock before any distribution or payment shall be made to the holders of any securities junior to the Series A Preferred Stock, and if the assets of the Company shall be insufficient to pay in full such amounts, then the entire assets to be distributed to the holders of Series A Preferred Stock shall be ratably distributed among the holders in accordance with the respective amounts that would be payable on such shares if all amounts payable thereon were paid in full. A Fundamental Transaction or Change of Control Transaction (as defined in the Series A Certificate of Designation) shall not be deemed a Liquidation. The Company shall mail written notice of any such Liquidation, not less than 45 days prior to the payment date stated therein, to each holder.
Conversion Price. The conversion price for the Series A Preferred Stock shall equal $3.00, subject to certain terms as described therein.
In addition, until the date that (i) Shareholder Approval has been obtained and deemed effective (which occurred on April 14, 2015), (ii) the Pre-funded Warrants are no longer outstanding, and (iii) there is an effective registration statement registering the resale of all of the shares of common stock underlying the Series A Preferred Stock (which occurred on March 11, 2015), we will be required to continue complying with negative covenants that limit our ability to incur debt, incur liens, amend our charter documents, repurchase securities, pay dividends or enter into related party transactions, which could adversely impact our operations.
Stock Options
As of April 30, 2015, there were 379,307 shares of our common stock issuable upon the exercise of outstanding stock options, at a weighted average exercise price of $46.85 per share.
| 87 |
Warrants
As of April 30, 2015, there were 2,281,332 shares of our common stock issuable upon the exercise of outstanding warrants at a weighted average exercise price of $14.49 per share, an additional 147,344 shares of common stock were issuable upon the exercise of prefunded warrants and 40,617 shares of common stock could become issuable upon the exercise of a warrant held by Rusnano, but only in the event of a default by Panacela in the repayment of a loan.
Certain of these warrants provide for cashless exercise at the option of the holder, and all of these warrants contain provisions for the adjustment of the number of shares issuable upon the exercise of the warrant in the event of stock splits, recapitalizations, reclassifications and consolidations. Unless they are exercised, the warrants will expire on various dates between June 2015 and August 2021.
Anti-Takeover Effects of Delaware Law and our Certificate of Incorporation and By-laws
The provisions of Delaware law, our certificate of incorporation and our bylaws, which are discussed below, could discourage or make it more difficult to accomplish a proxy contest or other change in our management or the acquisition of control by a holder of a substantial amount of our voting stock. It is possible that these provisions could make it more difficult to accomplish, or could deter, transactions that stockholders may otherwise consider to be in their best interests or the best interests of the company. These provisions are intended to enhance the likelihood of continuity and stability in the composition of our board of directors and in the policies formulated by the board of directors and to discourage certain types of transactions that may involve an actual or threatened change of control of us. These provisions are also designed to reduce our vulnerability to an unsolicited acquisition proposal and to discourage certain tactics that may be used in proxy fights. Such provisions also may have the effect of preventing changes in our management.
Delaware Law
We are subject to the anti-takeover provisions of Section 203 of the Delaware General Corporation Law, or DGCL. In general, Section 203 prohibits a publicly-held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. For purposes of Section 203, a “business combination” is defined broadly to include a merger, asset sale or other transaction resulting in a financial benefit to the interested stockholder, and, subject to certain exceptions, an “interested stockholder” is a person who, together with his or her affiliates and associates, owns, or within three years prior, did own, 15% or more of the corporation’s voting stock.
Stockholder Action; Special Meeting of Stockholders; Advance Notice Requirements for Stockholder Proposals and Director Nominations
Our certificate of incorporation and bylaws do not permit our stockholders to act by written consent. As a result, any action to be effected by our stockholders must be effected at a duly called annual or special meeting of the stockholders. Our certificate of incorporation and our bylaws also provide that special meetings of the stockholders may be called only by (i) our Chairman of the board of directors and (ii) our board of directors. Our bylaws provide that, for nominations to the board of directors or for other business to be properly brought by a stockholder before a meeting of stockholders, the stockholder must first have given timely notice of the proposal in writing to our Secretary. For an annual meeting, a stockholder’s notice generally must be delivered not less than 90 days nor more than 120 days prior to the anniversary of the date of previous year’s annual meeting; provided, however, that in the event that the annual meeting is called for a date that is not within 30 days before or after such anniversary date, notice by the stockholder in order to be timely must be received not later than the 10th day following the day on which such notice of the date of the annual meeting was mailed or public disclosure was made, whichever occurs first. Detailed requirements as to the form of the notice and information required in the notice are specified in the bylaws. If it is determined that business was not properly brought before a meeting in accordance with our bylaws, such business will not be conducted at the meeting.
| 88 |
Effects of Authorized but Unissued Stock
We have 152,910,049 shares of common stock and 10,000,000 shares preferred stock available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of The NASDAQ Capital Market. We may utilize these additional shares for a variety of corporate purposes including for future public offerings to raise additional capital or facilitate corporate acquisitions or for payment as a dividend on our capital stock. The existence of unissued and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management or to issue preferred stock with terms that could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a controlling interest in our company by means of a merger, tender offer, proxy contest or otherwise. In addition, if we issue preferred stock, the issuance could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation.
Limitation of Liability and Indemnification of Officers and Directors
Our certificate of incorporation contains provisions permitted under the DGCL relating to the liability of directors. The provisions eliminate a director’s liability for monetary damages for a breach of fiduciary duty, except in circumstances involving wrongful acts, such as the breach of a director’s duty of loyalty or acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law. Further, our certificate of incorporation contains provisions to indemnify our directors and officers to the fullest extent permitted by the DGCL. We have also entered into indemnification agreements with certain of our current and former directors and certain of our officers and expect to enter into a similar agreement with any new directors or officers.
| 89 |
DESCRIPTION OF SECURITIES WE ARE OFFERING
We are offering $10,000,000 of Class A Units and Class B Units (collectively, the “Units”). Each Class A Unit consists of one share of our common stock and a warrant to purchase [ ] shares of our common stock. Each Class B Unit consists of [ ] shares of our Series B Preferred Stock and a warrant to purchase [ ] shares of our common stock. The Class A Units and the Class B Units will not be certificated. The shares of common stock, shares of Series B Preferred Stock and warrants part of the units are immediately separable and will be issued separately in this offering.
Common Stock
The material terms of our common stock and our other capital stock are described in the section of this prospectus entitled “Description of Capital Stock”.
Warrants to Purchase Common Stock
The material terms of the warrants to be issued are summarized below. This summary does not purport to be complete in all respects. This description is subject to and qualified entirely by the terms of the form of warrant filed as an exhibit to the registration statement of which this prospectus is a part.
Pursuant to a warrant agency agreement between us and Continental Stock Transfer & Trust Company, as warrant agent, the warrants will be issued in book-entry form and shall initially be represented by one or more book-entry certificates deposited with The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
The warrants will have an initial exercise price of $ per share. Each warrant will be exercisable from their date of issuance and at any time up to the date that is [ ] years after their original date of issuance. A warrant may not be exercised by the holder to the extent that the holder, together with its affiliates, would beneficially own, after such exercise more than 9.99% of the shares of common stock then outstanding (subject to the right of the holder to increase or decrease such beneficial ownership limitation upon not less than 61 days prior notice provided that such limitation cannot exceed 9.99%).
The warrants are exercisable for cash or, solely in the absence of an effective registration statement or prospectus, by cashless exercise.
The exercise price of the warrants is subject to adjustment in the case of stock dividends or other distributions on shares of common stock or any other equity or equity equivalent securities payable in shares of common stock, stock splits, stock combinations, reclassifications or similar events affecting our common stock, and also, subject to limitations, upon any distribution of assets, including cash, stock or other property to our stockholders.
In addition, in the event we consummate a merger or consolidation with or into another person or other reorganization event in which our common shares are converted or exchange for securities, cash or other property, or we sell, lease, license, assign, transfer, convey or otherwise dispose of all or substantially all of our assets or we or another person acquire 50% or more of our outstanding common shares, then following such fundamental transaction, the holders of the warrants will be entitled to receive upon exercise of the warrants the same kind and amount of securities, cash or property which the holders would have received had they exercised the warrants immediately prior to such fundamental transaction. Any successor to us or surviving entity shall assume the obligations under the warrants.
Notwithstanding the foregoing, in the event we consummate a fundamental transaction that is considered a going-private transaction, the holders of the warrants will be entitled to receive, in lieu of our common stock and at the holders’ option, cash in an amount equal to the value of the remaining unexercised portion of the warrant on the date of the transaction determined using Black-Scholes option pricing model with an expected volatility equal to the greater of 100% and the 100-day historical price volatility obtained by Bloomberg L.P. as of the trading day immediately prior to the public announcement of the transaction.
Prior to the exercise of any warrants to purchase common stock, holders of the warrants will not have any of the rights of holders of the common stock purchasable upon exercise, including voting right, however, the holders of the warrants will have certain rights to participate in distributions or dividends paid on our common stock to the extent set forth in the warrants.
| 90 |
We do not plan on applying to list any of the warrants on the NASDAQ Capital Market, any other national securities exchange or any other nationally recognized trading system.
Series B Preferred Stock
The material terms of the Series B Preferred Stock to be issued are summarized below. This summary does not purport to be complete in all respects. This description is subject to and qualified entirely by the terms of the Certificate of Designation filed as an exhibit to the registration statement of which this prospectus is a part.
We are offering to those purchasers, whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 9.9% of our outstanding common stock following the consummation of this offering, the opportunity to purchase, in lieu of the shares of our common stock that would result in ownership in excess of 9.9%, Series B preferred stock convertible into shares of our common stock. Each share of Series B Preferred stock will be convertible at the option of the holder into [ ] shares of common stock. Each Series B Preferred share is being sold together with the same warrants described above. The Series B Preferred shares and warrants are immediately separable and will be issued separately in this offering.
Pursuant to a preferred stock agency agreement between us and Continental Stock Transfer & Trust Company, as preferred stock agent, the Series B Preferred Stock will be issued in book-entry form and shall initially be represented by one or more book-entry certificates deposited with The Depository Trust Company, or DTC, and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
The shares of Series B Preferred Stock will participate in any distributions or dividends on an as-converted basis with the common stock.
The shares of Series B Preferred Stock will not have voting rights except as required by law and except that the Company shall not, without the affirmative vote of the holders of a majority of the then outstanding shares of the Series B Preferred Stock, (a) alter or change adversely the powers, preferences or rights given to the Series B Preferred Stock or alter or amend the Certificate of Designation of Series B Preferred Stock, (b) authorize or create any class of stock ranking as to dividends, redemption or distribution of assets upon a Liquidation (as defined in the Certificate of Designation of Series B Preferred Stock) senior to, or otherwise pari passu with, the Series B Preferred Stock, (c) amend its certificate of incorporation or other charter documents in any manner that adversely affects any rights of the holders of Series B Preferred Stock, (d) increase the number of authorized shares of Series B Preferred Stock, or (e) enter into any agreement with respect to any of the foregoing.
Shares of Series B Preferred Stock may not be converted into common stock to the extent such conversion would result in the holder and its affiliates beneficially owning in excess of 4.99% of our outstanding common stock (subject to the right of the holder to increase or decrease such beneficial ownership limitation upon not less than 61 days prior notice provided that such limitation cannot exceed 9.99%).
| 91 |
We have entered into an underwriting agreement, dated , 2015, with Ladenburg Thalmann & Co. Inc., who we refer to as the “underwriter.” The underwriting agreement provides for the purchase of a specific number of Class A Units and Class B Units. Each Class A Unit consists of one share of our common stock and a warrant to purchase [ ] shares of our common stock. Each Class B Unit consists of [ ] shares of Series B Preferred Stock and a warrant to purchase [ ] shares of our common stock. Subject to the terms and conditions of the underwriting agreement, the underwriter has agreed to purchase the number of our securities set forth opposite its name below
| Underwriter | Class A Units | Class B Units | ||||||
| Ladenburg Thalmann & Co. Inc. | ||||||||
| Total | ||||||||
A copy of the underwriting agreement has been filed as an exhibit to the registration statement of which this prospectus is part.
We have been advised by the underwriter that it proposes to offer the Class A Units and Class B Units, or the Units, directly to the public at the public offering price set forth on the cover page of this prospectus. Any Units sold by the underwriter to securities dealers will be sold at the public offering price less a selling concession not in excess of $ per Unit. The underwriter may allow, and these selected dealers may re-allow, a concession of not more than $ per Unit to other brokers and dealers.
The underwriting agreement provides that the underwriter’s obligation to purchase the securities we are offering is subject to conditions contained in the underwriting agreement. The underwriter is obligated to purchase and pay for all of the securities offered by this prospectus, other than those covered by the over-allotment option described below.
We have granted the underwriter an over-allotment option. This option, which is exercisable for up to 45 days after the date of this prospectus, permits the underwriter to purchase a maximum of (i) additional shares of common stock, and/or (ii) additional warrants to purchase up to additional shares of common stock solely to cover over-allotments, if any, at the price to the public less the underwriting discounts and commissions. The over-allotment option may be used to purchase shares of common stock, or warrants, or any combination thereof, as determined by the underwriter, but such purchases cannot exceed an aggregate of 15% of the number of shares of common stock (on an as-converted basis with respect to any shares of Series B Preferred Stock sold) and warrants sold in the primary offering. If this option is exercised in full, the total price to the public will be $ and the total net proceeds, before expenses, to us will be $ .
No action has been taken by us or the underwriter that would permit a public offering of the Units, or the common stock, Series B Preferred Stock or warrants included in the Units in any jurisdiction where action for that purpose is required. None of our securities included in this offering may be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sales of any of the Units, the common stock, Series B Preferred Stock or warrants be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons who receive this prospectus are advised to inform themselves about and to observe any restrictions relating to this offering of Units, the common stock, Series B Preferred Stock or warrants and the distribution of this prospectus. This prospectus is neither an offer to sell nor a solicitation of any offer to buy the Units, the common stock, the Series B Preferred Stock or the warrants in any jurisdiction where that would not be permitted or legal.
The underwriter has advised us that it does not intend to confirm sales to any accounts over which it exercises discretionary authority.
| 92 |
Underwriting Discount and Expenses
The following table summarizes the underwriting discount and expenses to be paid to the underwriter by us.
| Per Class A Unit | Per Class B Unit | Total Class A Units Without Overallotment | Total Class A Units With Overallotment | Total Class B Units | ||||||||||||||||
| Public offering price | $ | $ | $ | $ | $ | |||||||||||||||
| Underwriting discount to be paid to the underwriter by us | $ | $ | $ | $ | $ | |||||||||||||||
| Proceeds to us (before expenses) (1) | $ | $ | $ | $ | $ | |||||||||||||||
| (1) | We estimate that our total expenses of this offering, excluding the underwriting discount, will be approximately $ . |
The underwriting discount of 8% per unit is subject to a reduction to equal 4% per unit with respect to securities we are offering which are sold to certain existing stockholders of the company, as set forth in the underwriting agreement.
In addition to the underwriting discount to be paid by us, we have agreed to reimburse the underwriter for certain of its out-of-pocket expenses incurred in connection with this offering, including road show costs and expenses incurred in connection with this offering, and the disbursements for the fees and expenses of underwriter’s counsel, subject to a total expense reimbursement cap of $100,000 and subject to compliance with FINRA Rule 5110(f)(2)(D).
The securities we are offering are being offered by the underwriter subject to certain conditions specified in the underwriting agreement.
Other Relationships
Upon completion of this offering and contingent upon this offering raising at least $10 million, we have granted the underwriter a right of first refusal under certain circumstances to act as lead or co-lead underwriter or placement agent in connection with any subsequent public or private offering of equity securities or other capital markets financing by us. This right of first refusal extends for nine months from the closing date of this offering. The terms of any such engagement of the underwriter will be determined by separate agreement.
The underwriter has performed investment banking services for us in the past, for which it has received customary fees and expenses. The underwriter may, from time to time, engage in transactions with or perform services for us in the ordinary course of its business and may continue to receive compensation from us for such services.
Determination of Offering Price
The public offering price of the Units we are offering and the exercise price and other terms of the warrants and Series B Preferred Stock were negotiated between us and the underwriter, based on the trading of our common stock prior to the offering, among other things. Other factors considered in determining the public offering price of the Units we are offering and the exercise price and other terms of the warrants and Series B Preferred Stock include the history and prospects of the Company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Continental Stock Transfer & Trust Company.
| 93 |
Stabilization, Short Positions and Penalty Bids
The underwriter may engage in over-allotment, syndicate covering transactions, stabilizing transactions and penalty bids or purchases for the purpose of pegging, fixing or maintaining the price of our common stock:
| ● | Over-allotment involves sales by the underwriter of securities in excess of the number of securities the underwriter is obligated to purchase, which creates a syndicate short position. The underwriter may close out any short position by purchasing shares in the open market. | |
| ● | Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. |
| ● | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specific maximum. |
| ● | Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These syndicate covering transactions, stabilizing transactions and penalty bids may have the effect of raising or maintaining the market prices of our securities or preventing or retarding a decline in the market prices of our securities. As a result, the price of our common stock may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on the NASDAQ Capital Market, in the over-the-counter market or on any other trading market and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriter also may engage in passive market making transactions in our common stock on the NASDAQ Capital Market in accordance with Regulation M during a period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the prices of our securities. In addition, neither we nor the underwriter make any representation that the underwriter will engage in these transactions or that any transactions, once commenced, will not be discontinued without notice.
Lock-ups
Pursuant to the underwriting agreement, we have agreed to not issue, enter into an agreement to issue or announce the issuance of common stock or securities convertible or exercisable into common stock for a period of 90 days following the closing of the offering, subject to the following exceptions: (a) our issuance of common stock, options to acquire common stock or other equity awards pursuant to our employee benefit plans as such plans now exist, (b) our issuance of common stock pursuant to the valid exercises, vesting or settlements of options, warrants or rights outstanding on the date of this prospectus and (c) our issuance of shares of common stock or securities convertible or exercisable into shares of common stock in connection with any acquisition, strategic partnership, joint venture or collaboration to which we are or may become a party, or the acquisition or license of any products or technology by us, but do not include any such transaction in which we are issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities.
| 94 |
In addition, our directors, executive officers and certain affiliates of the company have agreed to a 90 day lock-up from the date of the pricing of this offering with respect to shares of our common stock (or other securities convertible into or exercisable or exchangeable for our common stock) that they beneficially own, subject to certain exceptions. This means that, for such 90-day period, such persons may not offer, sell, assign, transfer, pledge, contract to sell, or otherwise dispose of these securities, without the prior written consent of the underwriter. The exceptions permit, among other things and subject to restrictions, (1) if the holder is a natural person, certain transfers made as a bona fide gifts to any member of the holder’s immediate family, to a trust the beneficiaries of which are exclusively the holder or members of the holder’s immediate family or to a charity or educational institute, or by will or intestate succession upon the death of the holder, and (2) if the holder is a corporation, partnership, limited liability company or other business entity (a) transfers to any shareholder, partner or member of, or owner of a similar equity interest in, the holder if such transfer is not for value, (b) transfers in connection with the sale of all or substantially all of the holder’s capital stock, partnership interests, membership interests or other similar equity interests, or all or substantially all of the holder’s assets, in any such case not undertaken for the purpose of avoiding the restrictions imposed by the “lock-up” agreement or (c) to another corporation, partnership, limited liability company or other business entity so long as the transferee is an affiliate of the holder and such transfer is not for value.
The applicable restricted period will be automatically extended if (i) during the last 17 days of the restricted period we issue an earnings release or material news or a material event relating to us occurs or (ii) prior to the expiration of the restricted period, we announce that we will release earnings results during the 16-day period beginning on the last day of the restricted period, in either of which case the restrictions described above will continue to apply until the expiration of the 18-day period beginning on the issuance of the earnings release or the occurrence of the material news or material event.
Indemnification
We have agreed to indemnify the underwriter, persons who control the underwriter, and the underwriter’s partners, directors, officers, employees and agents against certain liabilities, including liabilities under the Securities Act of 1933, or to contribute to payments the underwriter may be required to make with respect to any of these liabilities.
The validity of the securities being offered will be passed upon for us by Sichenzia Ross Friedman Ference LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriter by Ellenoff Grossman & Schole, New York, New York.
Our financial statements included in this prospectus as of December 31, 2014 and 2013 and for the years ended December 31, 2014, 2013 and 2012 have been so included in reliance on the report of Meaden & Moore, Ltd., an independent registered public accounting firm, appearing elsewhere herein, given on the authority of said firm as experts in auditing and accounting.
| 95 |
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and special reports, and other information with the Securities and Exchange Commission. Copies of the reports and other information may be read and copied at the SEC’s Public Reference Room at 100 F Street NE, Washington, D.C. 20549. You can request copies of such documents by writing to the SEC and paying a fee for the copying cost. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a web site at http://www.sec.gov that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC.
This prospectus is part of a registration statement on Form S-1 that we filed with the SEC. Certain information in the registration statement has been omitted from this prospectus in accordance with the rules and regulations of the SEC. We have also filed exhibits and schedules with the registration statement that are excluded from this prospectus. For further information you may:
| ● | read a copy of the registration statement, including the exhibits and schedules, without charge at the SEC’s Public Reference Room; or | |
| ● |
obtain a copy from the SEC upon payment of the fees prescribed by the SEC. |
| 96 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
| March 31, 2015 | December 31, 2014 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 4,256,016 | $ | 3,103,969 | ||||
| Short-term investments | 769,700 | — | ||||||
| Accounts receivable | 307,404 | 267,199 | ||||||
| Other current assets | 248,616 | 174,179 | ||||||
| Total current assets | 5,581,736 | 3,545,347 | ||||||
| Equipment, net | 209,641 | 244,537 | ||||||
| Restricted cash | 815,883 | 1,699,759 | ||||||
| Other long-term assets | 48,961 | 56,131 | ||||||
| Investment in Incuron, LLC | 4,020,892 | 4,268,458 | ||||||
| Total assets | $ | 10,677,113 | $ | 9,814,232 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 989,646 | $ | 1,057,743 | ||||
| Accrued expenses | 2,070,984 | 1,804,456 | ||||||
| Deferred revenue | 418,673 | 156,317 | ||||||
| Accrued warrant liability | 4,547,691 | 862,074 | ||||||
| Current portion of notes payable | 2,736,365 | 2,640,968 | ||||||
| Current portion of capital lease obligation | — | 7,522 | ||||||
| Total current liabilities | 10,763,359 | 6,529,080 | ||||||
| Long-term debt | 1,321,218 | 1,499,050 | ||||||
| Commitments and contingencies | — | — | ||||||
| Total liabilities | 12,084,577 | 8,028,130 | ||||||
| Convertible Preferred Stock, $.005 par value, 718 shares designated, 717.4 shares issued and outstanding | 4 | — | ||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, $.005 par value; 10,000,000 shares authorized, 717.4 and 0 shares issued and outstanding as of March 31, 2015 and 2014, respectively | — | — | ||||||
| Common stock, $.005 par value; 160,000,000 shares authorized, 3,435,354 and 2,547,140 shares issued and outstanding as of March 31, 2015 and 2014, respectively | 17,173 | 14,287 | ||||||
| Additional paid-in capital | 133,235,836 | 132,693,988 | ||||||
| Other comprehensive income/(loss) | (450,172 | ) | (380,110 | ) | ||||
| Accumulated deficit | (138,261,859 | ) | (133,935,562 | ) | ||||
| Total Cleveland BioLabs, Inc. stockholders’ equity (deficit) | (5,459,022 | ) | (1,607,397 | ) | ||||
| Noncontrolling interest in stockholders’ equity | 4,051,554 | 3,393,499 | ||||||
| Total stockholders’ equity | (1,407,468 | ) | 1,786,102 | |||||
| Total liabilities and stockholders’ equity | $ | 10,677,113 | $ | 9,814,232 | ||||
See Notes to Consolidated Financial Statements
| F-1 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
| For the Three Months Ended March 31, | ||||||||
| 2015 | 2014 | |||||||
| Revenues: | ||||||||
| Grants and contracts | $ | 607,329 | $ | 1,334,254 | ||||
| Operating expenses: | ||||||||
| Research and development | 1,610,970 | 2,439,773 | ||||||
| General and administrative | 2,307,871 | 2,413,543 | ||||||
| Total operating expenses | 3,918,841 | 4,853,316 | ||||||
| Loss from operations | (3,311,512 | ) | (3,519,062 | ) | ||||
| Other income (expense): | ||||||||
| Interest and other expense | (46,394 | ) | (317,922 | ) | ||||
| Foreign exchange loss | (43,735 | ) | (151,771 | ) | ||||
| Change in value of warrant liability | (49,358 | ) | 2,087,558 | |||||
| Equity in loss of Incuron, LLC | (247,566 | ) | — | |||||
| Total other income | (387,053 | ) | 1,617,865 | |||||
| Net loss | (3,698,565 | ) | (1,901,197 | ) | ||||
| Net loss attributable to noncontrolling interests | 48,243 | 315,825 | ||||||
| Net loss attributable to Cleveland BioLabs, Inc. | $ | (3,650,322 | ) | $ | (1,585,372 | ) | ||
| Net loss available to common stockholders per share of common stock, basic and diluted | $ | (1.14 | ) | $ | (0.63 | ) | ||
| Weighted average number of shares used in calculating net loss per share, basic and diluted | 3,206,249 | 2,498,407 | ||||||
See Notes to Consolidated Financial Statements
| F-2 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
(UNAUDITED)
| For the Three Months Ended March 31, | ||||||||
| 2015 | 2014 | |||||||
| Net loss including noncontrolling interests | $ | (3,698,565 | ) | $ | (1,901,197 | ) | ||
| Other comprehensive loss - | ||||||||
| Foreign currency translation adjustment | (39,739 | ) | (291,466 | ) | ||||
| Comprehensive loss including noncontrolling interests | (3,738,304 | ) | (2,192,663 | ) | ||||
| Comprehensive loss attributable to noncontrolling interests | 63,268 | 417,276 | ||||||
| Comprehensive loss attributable to Cleveland BioLabs, Inc. | $ | (3,675,036 | ) | $ | (1,775,387 | ) | ||
See Notes to Consolidated Financial Statements
| F-3 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
(UNAUDITED)
| Common Stock | Additional Paid-in | Accumulated Other Comprehensive | Accumulated | Noncontrolling | ||||||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Deficit | Interests | Total | ||||||||||||||||||||||
| Balance at December 31, 2014 | 2,858,126 | $ | 14,287 | $ | 132,693,988 | $ | (380,110 | ) | $ | (133,935,562 | ) | $ | 3,393,499 | $ | 1,786,102 | |||||||||||||
| Stock based compensation | 5,023 | 25 | 61,740 | — | — | — | 61,765 | |||||||||||||||||||||
| Issuance of common stock, net of offering costs of $98,846 | 572,205 | 2,861 | 480,108 | — | — | — | 482,969 | |||||||||||||||||||||
| Increased ownership of Panacela, Inc. | — | — | — | (45,348 | ) | (675,975 | ) | 721,323 | — | |||||||||||||||||||
| Net loss | — | — | — | — | (3,650,322 | ) | (48,243 | ) | (3,698,565 | ) | ||||||||||||||||||
| Foreign currency translation | — | — | — | (24,714 | ) | — | (15,025 | ) | (39,739 | ) | ||||||||||||||||||
| Balance at March 31, 2015 | 3,435,354 | $ | 17,173 | $ | 133,235,836 | $ | (450,172 | ) | $ | (138,261,859 | ) | $ | 4,051,554 | $ | (1,407,468 | ) | ||||||||||||
See Notes to Consolidated Financial Statements
| F-4 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| For the Three Months Ended March 31, | ||||||||
| 2015 | 2014 | |||||||
| Cash flows from operating activities: | ||||||||
| Net income (loss) | $ | (3,698,565 | ) | $ | (1,901,197 | ) | ||
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | ||||||||
| Depreciation | 38,620 | 57,811 | ||||||
| Amortization of loan costs | 27,019 | 91,929 | ||||||
| (Gain) loss on equipment disposal | — | 24,685 | ||||||
| Noncash compensation | 33,640 | 48,416 | ||||||
| Warrant issuance costs | 617,776 | 171,116 | ||||||
| Equity in loss of Incuron, LLC | 247,566 | — | ||||||
| Change in value of warrant liability | 49,358 | (2,087,558 | ) | |||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable | (39,959 | ) | 41,901 | |||||
| Other current assets | (73,276 | ) | (54,818 | ) | ||||
| Other long-term assets | 3,986 | 9,847 | ||||||
| Accounts payable | (57,910 | ) | (273,086 | ) | ||||
| Deferred revenue | 254,363 | 108,127 | ||||||
| Accrued expenses | 369,832 | 929,518 | ||||||
| Net cash used in operating activities | (2,227,550 | ) | (2,833,309 | ) | ||||
| Cash flows from investing activities: | ||||||||
| Purchase of short-term investments | (723,661 | ) | — | |||||
| Purchase of equipment | (3,756 | ) | (10,805 | ) | ||||
| Decrease in restricted cash | 770,609 | — | ||||||
| Net cash provided by (used in) investing activities | 43,192 | (10,805 | ) | |||||
| Cash flows from financing activities: | ||||||||
| Issuance of common stock, net of offering costs | 3,501,457 | 6,355,001 | ||||||
| Net proceeds/(repayment) of long-term debt | (182,058 | ) | — | |||||
| Repayment of capital lease obligation | (7,522 | ) | (19,806 | ) | ||||
| Net cash provided by financing activities | 3,311,877 | 6,335,195 | ||||||
| Effect of exchange rate change on cash and equivalents | 24,528 | (147,850 | ) | |||||
| Decrease in cash and cash equivalents | 1,152,047 | 3,343,231 | ||||||
| Cash and cash equivalents at beginning of period | 3,103,969 | 10,048,466 | ||||||
| Cash and cash equivalents at end of period | $ | 4,256,016 | $ | 13,391,697 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Cash paid during the period for interest | $ | 47,782 | $ | 159,780 | ||||
| Supplemental schedule of noncash financing activities: | ||||||||
| Noncash financing costs on common stock offering | — | 50,505 | ||||||
| Noncash warrant issuance costs | — | 15,993 | ||||||
See Notes to Consolidated Financial Statements
| F-5 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(UNAUDITED)
1. Description of Business
Cleveland BioLabs, Inc. is an innovative biopharmaceutical company seeking to develop first-in-class pharmaceuticals designed to address diseases with significant unmet medical need. Our most advanced product candidate is entolimod, which we are developing as a radiation countermeasure and an immunotherapy for oncology and other indications. We conduct business in the United States and in the Russian Federation, or Russia, through several legal entities, one of which is wholly-owned and two of which are owned in collaboration with financial partners. As used throughout these unaudited consolidated financial statements, the terms “Cleveland BioLabs” and “CBLI” refer to Cleveland BioLabs, Inc. and its wholly-owned subsidiary BioLab 612, LLC, but not its consolidated joint venture, Panacela Labs, Inc. or its unconsolidated joint venture, Incuron, LLC. The “Company,” “we,” “us” and “our” refer to Cleveland BioLabs, Inc. together with its consolidated subsidiaries.
CBLI was incorporated in Delaware in June 2003 and is headquartered in Buffalo, New York. As of March 31, 2015, CBLI had one wholly-owned subsidiary, Biolab 612, LLC, or Biolab 612, which began operations in 2012, one consolidated joint venture, Panacela Labs, Inc., or Panacela, which was formed by us and a financial partner in 2011, and one unconsolidated joint venture, Incuron, LLC, or Incuron, which was formed by us and a financial partner in 2010. Additionally, Panacela had a wholly-owned subsidiary, Panacela Labs, LLC, which was formed in 2011.
2. Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The accompanying consolidated financial statements include the accounts of CBLI, BioLab 612, and Panacela. All significant intercompany balances and transactions have been eliminated in consolidation.
The unaudited consolidated financial statements included herein have been prepared in accordance with accounting principles generally accepted in the United States, or GAAP, for interim financial information and in accordance with the instructions to Form 10-Q and Article 10 of Regulation S-X of the Securities and Exchange Commission, or the SEC. Certain information and footnote disclosures normally included in consolidated financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to such rules and regulations. These consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K for the year ended December 31, 2014, as filed with the SEC.
In the opinion of the Company’s management, any adjustments contained in the accompanying unaudited consolidated financial statements are of a normal recurring nature, and are necessary to present fairly the financial position of the Company as of March 31, 2015, along with its results of operations for the three month periods ended March 31, 2015 and 2014 and cash flows for the three month periods ended March 31, 2015 and 2014. Interim results are not necessarily indicative of results that may be expected for any other interim period or for an entire year.
On January 28, 2015, the Company, after receiving authorization from the Company’s shareholders and board of directors, executed a reverse stock split, or Reverse Split, of the Company’s common stock at the ratio of 1:20. All historical share balances and share price-related data have been adjusted based on this ratio.
At March 31, 2015, we had cash, cash equivalents and short-term investments which total $5.0 million. Of that total, $1.4 million ($0.7 million of cash and cash equivalents and $0.7 million of short-term investments) was restricted for the use of our consolidated joint venture, Panacela, leaving $3.6 million available for general use which management believes will be sufficient to support operations into June 2015. To ensure continuing operations beyond that point, management is evaluating all opportunities to secure additional financing, including investments from non-controlling interests, the sale or license of our drug candidates, the issuance of equity and additional revenues from the U.S. or Russian governments. Management believes that sufficient sources of financing will be available to support operations into the future, however there can be no assurances at this time. These matters raise substantial doubt about the Company’s ability to continue as a going concern. These financial statements have been prepared under the assumption that the Company will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty.
| F-6 |
Recent Accounting Pronouncements
In May 2014, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, 2014-09, Revenue from Contracts with Customers, which updates the principles for recognizing revenue. ASU 2014-09 also amends the required disclosures of the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. ASU 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. The Company is evaluating the potential impacts of the new standard on its existing revenue recognition policies and procedures.
In June 2014, the FASB issued ASU 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. ASU 2014-12 requires that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition, and as such, the performance target should not be reflected in estimating the grant-date fair value of the award. ASU 2014-12 is effective for annual reporting periods beginning after December 15, 2015, with early adoption permitted. The Company is evaluating the potential impacts of the new standard on its existing stock-based compensation plans.
In August 2014, the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, ASU 2014-15 requires that an entity’s management evaluate whether there are conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. ASU 2014-15 is effective for annual periods beginning after December 15, 2016 and for interim periods thereafter. The Company is evaluating the potential impacts of this new standard on its quarterly reporting process.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
As of March 31, 2015, $0.7 million of the Company’s cash and cash equivalents was restricted to the use of Panacela, leaving $3.6 million available for general use.
Short-Term Investments
The Company’s short-term investments are classified as held to maturity given the intent and ability to hold the investments to maturity. Accordingly, these investments are carried at amortized cost. Short-term investments classified as held-to-maturity consisted of certificates of deposit with maturity dates beyond three months and less than one year. As of March 31, 2015, all of the Company’s short-term investments were restricted to the use of Panacela.
Significant Customers and Accounts Receivable
Grant and contract revenue from the U.S. government accounted for 0% and 1.8% of total revenue for the three months ended March 31, 2015 and 2014, respectively. Although the Company anticipates ongoing U.S. and Russian government contract and grant revenue, there is no guarantee that these revenue streams will continue in the future.
Grant and contract revenue received by the Company’s subsidiaries from Russian government agencies accounted for 58.2% and 98.2% of total revenues for the three months ended March 31, 2015 and 2014, respectively.
Service contract revenue received by us from Incuron accounted for 41.8% and 0.0% of total revenues for the three months ended March 31, 2015 and 2014, respectively.
Accounts receivable consist of amounts due under reimbursement contracts with certain government and foreign entities. The Company extends unsecured credit to customers under normal trade agreements, which generally require payment within 30 days.
Intellectual Property
Costs related to filing and pursuing patent applications are recognized as general and administrative expenses, or G&A expenses, as incurred, since the recoverability of such expenditures is uncertain. Upon marketing approval by the U.S. Food and Drug Administration, or FDA, or a respective foreign governing body, such costs will be capitalized and depreciated over the expected life of the related patent.
| F-7 |
Accounting for Stock-Based Compensation
The 2006 Equity Incentive Plan, as amended, or the Plan, authorizes CBLI to grant (i) options to purchase common stock, (ii) restricted or unrestricted stock units, and (iii) stock appreciation rights, so long as the exercise or grant price of each are at least equal to the fair market value of the stock on the date of grant. As of March 31, 2015 and taking into consideration the increase in authorized shares under the Plan as approved by our shareholders in April 2015, an aggregate of 650,000 shares of common stock were authorized for issuance under the Plan, of which a total of 262,899 shares of common stock remained available for future awards. A single participant cannot be awarded more than 100,000 shares annually. Awards granted under the Plan have a contractual life of no more than 10 years. The terms and conditions of equity awards (such as price, vesting schedule, term and number of shares) under the Plan are specified in an award document, and approved by the Company’s board of directors, compensation committee or its management delegees.
In June 2013, the Company’s stockholders approved the 2013 Employee Stock Purchase Plan, or ESPP, which provides a means by which eligible employees of the Company and certain designated related corporations may be given an opportunity to purchase shares of Common Stock. As of March 31, 2015, and taking into consideration the increase in authorized shares under the ESPP as approved by our shareholders in April 2015, there are 225,000 shares of Common Stock reserved for purchase under the ESPP. The number of shares reserved for purchase under the ESPP increases on January 1 of each calendar year by the lesser of: (i) 10% of the total number of shares of Common Stock outstanding on December 31st of the preceding year, or (ii) 100,000 shares of Common Stock. The ESPP allows employees to use up to 15% of their compensation to purchase shares of Common Stock at an amount equal to 85% of the fair market value of the Company’s Common Stock on the offering date or the purchase date, whichever is less.
The Company utilizes the Black-Scholes valuation model for estimating the fair value of all stock options granted where the vesting period is based on length of service or performance, while a Monte Carlo simulation model is used for estimating the fair value of stock options with market-based vesting conditions. Set forth below are the assumptions used in valuing the stock options granted and a discussion of the Company’s methodology for developing each of the assumptions used:
| For the three months ended March 31, | ||||||||
| 2015 | 2014 | |||||||
| Risk-free interest rate | 1.43 | % | 1.59 | % | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
| Expected life | 5.5 Years | 5 Years | ||||||
| Expected volatility | 76.66 | % | 74.21 | % | ||||
“Risk-free interest rate” means the range of U.S. Treasury rates with a term that most closely resembles the expected life of the option as of the date the option is granted.
“Expected dividend yield” means the Company does not pay regular dividends on its common stock and does not anticipate paying any dividends in the foreseeable future.
“Expected life” means the period of time that options granted are expected to remain outstanding, based wholly on the use of the simplified (safe harbor) method. The simplified method is used because the Company does not yet have adequate historical exercise information to estimate the expected life the options granted.
“Expected volatility” means a measure of the amount by which a financial variable, such as share price, has fluctuated (historical volatility) or is expected to fluctuate (implied volatility) during a period. Expected volatility is based on the Company’s historical volatility and incorporates the volatility of the common stock of comparable companies when the expected life of the option exceeds the Company’s trading history.
Income Taxes
No income tax expense was recorded for the three months ended March 31, 2015 and 2014, as the Company does not expect to have taxable income for 2015 and did not have taxable income in 2014. A full valuation allowance has been recorded against the Company’s deferred tax asset.
| F-8 |
Earnings (Loss) per Share
Basic net income (loss) per share of common stock excludes dilution for potential common stock issuances and is computed by dividing net income (loss) by the weighted average number of shares outstanding for the period. Diluted net income (loss) per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock. Diluted net loss per share is identical to basic net loss per share as potentially dilutive securities have been excluded from the calculation of diluted net loss per common share because the inclusion of such securities would be antidilutive.
The Company has excluded the following securities from the calculation of diluted net loss per share because all such securities were antidilutive for the periods presented:
| As of March 31, | ||||||||
Common Equivalent Securities | 2015 | 2014 | ||||||
| Convertible Preferred | 239,135 | — | ||||||
| Warrants | 2,876,020 | 822,205 | ||||||
| Options | 261,470 | 306,233 | ||||||
| Total | 3,376,625 | 1,128,438 | ||||||
Contingencies
From time to time, the Company may have certain contingent liabilities that arise in the ordinary course of business. The Company accrues for liabilities when it is probable that future expenditures will be made and such expenditures can be reasonably estimated. For all periods presented, the Company is not a party to any pending material litigation that is estimable and probable of loss.
3. Fair Value of Financial Instruments
The Company measures and records warrant liabilities at fair value in the accompanying financial statements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability, an exit price, in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value, includes:
Level 1 – Observable inputs for identical assets or liabilities such as quoted prices in active markets;
Level 2 – Inputs other than quoted prices in active markets that are either directly or indirectly observable; and
Level 3 – Unobservable inputs in which little or no market data exists, which are therefore developed by the Company using estimates and assumptions that reflect those that a market participant would use.
| F-9 |
The following tables represent the Company’s fair value hierarchy for its financial liabilities measured at fair value on a recurring basis:
| As of March 31, 2015 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Accrued warrant liability | $ | — | $ | — | $ | 4,547,691 | $ | 4,547,691 | ||||||||
| Compensatory stock options not yet issued (1) | — | — | 132,295 | 132,295 | ||||||||||||
| Total liabilities | $ | — | $ | — | $ | 4,679,986 | $ | 4,679,986 | ||||||||
| As of December 31, 2014 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Accrued warrant liability | $ | — | $ | — | $ | 862,074 | $ | 862,074 | ||||||||
| Compensatory stock options not yet issued (1) | — | — | 132,295 | 132,295 | ||||||||||||
| Total liabilities | $ | — | $ | — | $ | 994,369 | $ | 994,369 | ||||||||
| (1) | Included in accrued expenses in the accompanying Consolidated Balance Sheets |
The Company uses the Black-Scholes model to measure the accrued warrant liability and its accrual for compensatory stock options not yet issued. The following are the assumptions used to measure the accrued warrant liability which were determined in a manner consistent with that described for grants of options to purchase common stock as set forth in Note 2:
| March 31, 2015 | December 31, 2014 | |||||||
| Stock Price | $ | 3.56 | $ | 5.60 | ||||
| Exercise Price | $ | 3.00 - 100.00 | $ | 10.10 - 100.00 | ||||
| Term in years | 0.21 - 6.35 | 0.46 - 6.04 | ||||||
| Volatility | 76.18 - 107.53 | % | 70.69 - 100.08 | % | ||||
| Annual rate of quarterly dividends | 0 | % | 0 | % | ||||
| Discount rate- bond equivalent yield | .06 - 1.48 | % | .12 - 1.65 | % | ||||
| F-10 |
The following table sets forth a summary of changes in the fair value of the Company’s Level 3 fair value measurements for the periods indicated:
| Three Months Ended March 31, 2015 | ||||||||
| Accrued
Warrant Liability | Compensatory Stock Options Issued After Year End | |||||||
| Beginning Balance | $ | 862,074 | $ | 132,295 | ||||
| Total (gains) or losses, realized and unrealized, included in earnings (1)(2) | 49,357 | — | ||||||
| Issuances | 3,636,260 | — | ||||||
| Settlements | — | — | ||||||
| Balance, March 31, 2015 | $ | 4,547,691 | $ | 132,295 | ||||
| Three Months Ended March 31, 2014 | ||||||||
| Accrued
Warrant Liability | Compensatory Stock Options Issued After Year End | |||||||
| Beginning Balance | $ | 1,241,311 | $ | 309,450 | ||||
| Issuances | 2,283,092 | — | ||||||
| Total (gains) or losses, realized and unrealized, included in earnings (1)(2) | (2,087,558 | ) | (21,055 | ) | ||||
| Settlements | — | (288,395 | ) | |||||
| Balance, March 31, 2014 | $ | 1,436,845 | $ | — | ||||
| (1) | Unrealized gains or losses related to the accrued warrant liability were included as change in value of accrued warrant liability. There were no realized gains or losses for the three months ended March 31, 2015 and 2014. |
| (2) | Expenses recorded for compensatory stock options not yet issued are included in research & development expense and general and administrative expense. |
As of March 31, 2015 and December 31, 2014, the Company had no assets or liabilities that were measured at fair value on a nonrecurring basis.
| F-11 |
The Company considers the accrued warrant liability and compensatory stock options not yet issued to be Level 3 because some of the inputs into the measurements are neither directly or indirectly observable. Both the accrued warrant liability and compensatory stock options not yet issued use management’s estimate for the expected term. Additionally, the number of compensatory options awarded involves an estimate of management’s performance in relation to the targets set forth in the Company’s Executive Compensation Plan. The following table summarizes the unobservable inputs into the fair value measurement for the accrued warrant liability as of March 31, 2015:
| March 31, 2015 | ||||||||||
Description |
Fair Value | Valuation Technique | Unobservable Input | Range in years | ||||||
| Compensatory stock options not yet issued | $ | 132,295 | Black-scholes pricing model | Expected term | 5 | |||||
| Accrued warrant liability | 4,547,691 | Black-scholes pricing model | Expected term | 0.21 - 6.35 | ||||||
| $ | 4,679,986 | |||||||||
Management believes the value of both the accrued warrant liability and compensatory stock options is more sensitive to a change in the Company’s stock price at the end of the respective reporting period as opposed to a change in one of the unobservable inputs described above.
The carrying amounts of the Company’s short-term financial instruments, which include cash and cash equivalents, accounts receivable and accounts payable, approximate their fair values due to their short maturities.
4. Debt
On September 30, 2013, CBLI and BioLab 612 entered into a Loan and Security Agreement, or the Loan Agreement, with Hercules Technology II, L.P., or Hercules, pursuant to which we issued a $6.0 million note and received net proceeds of $5.9 million. The loan bears interest at the greater of (i) 10.45% per annum or (ii) 10.45% plus the prevailing prime rate minus 4.25%. The loan matures on January 1, 2017, and requires interest-only payments for the initial 12 months and principal and interest payments in 27 monthly installments thereafter. In June 2014, CBLI repaid $4.0 million of the Hercules Loan using net proceeds from a sale of equity and cash.
In connection with the Loan Agreement, CBLI granted a first priority lien in substantially all of CBLI’s assets (exclusive of intellectual property). The Loan Agreement also contains representations and warranties by CBLI and Hercules, indemnification provisions in favor of Hercules, customary covenants (including limitations on other indebtedness, liens, acquisitions, investments and dividends, but excluding any financial covenants), and events of default (including payment defaults, breaches of covenants, material adverse events and events leading to bankruptcy or insolvency). Prepayment of the loan is subject to a penalty rate applied to the balance of the secured obligation and ranges from 1% to 3% based on the date the loan is prepaid. The prepayment penalty was waived in connection with the above referenced prepayment.
Additional loan features, all of which are recorded debt issuance costs and loan discounts in non-current assets, include: $102,000 related to legal fees and a $100,000 facility fee both of which were paid in cash, a $550,000 “end-of-term charge” which is due upon full repayment of the loan or on the maturity date, whichever occurs sooner and is included in long-term liabilities, and a five-year warrant to purchase 7,813 shares of CBLI common stock. The warrant had an initial exercise price of $32.00 per share, which was subsequently lowered to $10.10 per share in accordance with its terms. The Black-Scholes pricing model yielded $117,999 as the fair value of the warrant upon issuance which was recorded as equity.
CBLI will amortize the loan discounts and debt issuance costs to interest expense over the term of the loan using the effective interest rate method, which approximates 16.6%.
| F-12 |
The following schedule shows the remaining payments for principal and the end of term charge on the Hercules loan by calendar year:
| March 31, | ||||
| 2016 | $ | 768,179 | ||
| 2017 | 1,484,212 | |||
| Total | $ | 2,252,391 | ||
On September 3, 2013, Panacela entered into a Master Agreement, or the Panacela Loan, with Rusnano and CBLI pursuant to which Panacela issued a $1,530,000 note to Rusnano. The Panacela Loan bears interest at a rate of 16.3% per annum and matures on September 10, 2015, at which time Panacela must repay all unpaid principal and accrued interest. As of March 31, 2015, Panacela had $431,274 of interest accrued associated with the Panacela Loan. Rusnano has the option to convert the unpaid principal plus interest into shares of Panacela preferred stock at a conversion price of $1,057 per share, or if Panacela has a qualified financing event, at a discounted price of 0.75 times the purchase price per share.
5. Stockholders’ Equity
On February 4, 2015, CBLI entered into a Securities Purchase Agreement with certain institutional investors providing for the issuance and sale of 572,205 registered shares (the “Shares”) of the Company’s common stock, at an offering price of $3.00 per share (the “Share Offering”) and Series B pre-funded warrants (the “Pre-Funded Warrants,”) to purchase an aggregate of 594,688 registered shares of its common stock (the “Pre-Funded Warrants Offering”).
| F-13 |
In a concurrent private placement (the “Private Placement Transaction” and, together with the Share Offering and the Pre-Funded Warrants Offering, the “Offerings”), CBLI sold to the purchasers of our Shares and Pre-Funded Warrants, 717.4 shares of our Series A Convertible Preferred Stock, stated value of $1000 per share (the “Preferred Stock”), which are convertible into 239,134 shares of our common stock. Gross proceeds from the Offerings amounted to approximately $4.2 million before deducting placement agent fees and expenses. In addition, a Series A warrant (the “Series A Warrants” and, together with the Shares, the Pre-Funded Warrants and the Preferred Stock, the “Securities”) will be issued to purchase one share of our common stock for each share of common stock purchased or prefunded in this offering and each share of Series A Convertible Preferred Stock purchased in the concurrent private placement. The Series A Warrants cover, in the aggregate, 1,406,028 shares of common stock and become exercisable six months following the date of issuance at an exercise price of $3.64 and expire six years from the date they become exercisable.
The Series A Warrants and Series B Warrants contain provisions that could require CBLI to settle the warrants in cash, and also provide for price or share issuance adjustments in the event of a subsequent qualified issuance of common stock at a price below $3.64 for the Series A Warrants or $3.00 for the Pre-Funded Warrants, and accordingly have been classified as a liability. The fair value of the Series A Warrants and Series B Warrants amounted to $3,636,260 and was determined based on the following assumptions using the Black-Scholes valuation model, as of February 6, 2015, the closing date:
| Series A | Series B | |||||||
| Stock Price | $ | 3.16 | $ | 3.16 | ||||
| Exercise Price | $ | 3.64 | $ | 3.00 | ||||
| Term in years | 6.50 | 1.00 | ||||||
| Volatility | 0.83 | % | 0.88 | % | ||||
| Annual rate of quarterly dividends | 0 | % | 0 | % | ||||
| Discount rate- bond equivalent yield | 0.01 | % | 0.00 | % | ||||
The preferred stock certificate of designation contains provisions for settlement in cash in certain circumstances and a share conversion adjustment in the event a subsequent, qualifying equity issuance for less than $3.00 occurs. As such, the preferred stock is classified outside of equity.
As of May 5, 2015, 4,002,264 shares of our common stock were outstanding, which includes 447,344 shares of our common stock issued pursuant to the exercise of Pre-Funded Warrants, and 119,567 shares of our common stock issued pursuant to the conversion of 358.7 shares of Preferred Stock. After such issuances, 147,344 shares of our common stock are reserved for the exercise of the remaining Pre-Funded Warrants, and 119,567 shares of our common stock are reserved for the conversion of the remaining shares of Preferred Stock.
| F-14 |
The Company has granted options to purchase shares of common stock. The following is a summary of option award activity during the three months ended March 31, 2015:
| Total
Stock Options Outstanding | Weighted Average Exercise Price per Share | Nonvested Stock Options | Weighted Average Grant Date Fair Value per Share | |||||||||||||
| December 31, 2014 | 261,389 | $ | 67.89 | 21,287 | $ | 22.31 | ||||||||||
| Granted | 750 | 4.80 | 750 | 3.10 | ||||||||||||
| Vested | — | — | (250 | ) | 3.10 | |||||||||||
| Exercised | — | — | — | — | ||||||||||||
| Forfeited, Canceled | (669 | ) | 75.93 | — | — | |||||||||||
| March 31, 2015 | 261,470 | $ | 67.69 | 21,787 | $ | 21.87 | ||||||||||
The following is a summary of outstanding stock options as of March 31, 2015:
| Stock Options Outstanding | Vested Stock Options | |||||||
| Quantity | 261,470 | 239,683 | ||||||
| Weighted-average exercise price | $ | 67.69 | $ | 28.45 | ||||
| Weighted Average Remaining Contractual Term (in Years) | 6.24 | 6.05 | ||||||
| Intrinsic value | $ | — | $ | — | ||||
For the three months ended March 31, 2015 and 2014, the Company granted 750 and 34,800 stock options, respectively, with a weighted-average grant date fair value of $3.10 and $8.29 respectively. For the three months ended March 31, 2015 and 2014, the total fair value of options vested was $2,324 and $288,395 respectively.
As of March 31, 2015, total compensation cost not yet recognized related to unvested stock options was $33,678. The Company expects to recognize this cost over a weighted average period of approximately 0.77 years.
6. Warrants
In connection with sales of the Company’s common stock and the issuance of debt instruments, warrants were issued which presently have exercise prices ranging from $3.00 to $100.00. The warrants expire between one and seven years from the date of grant, and are subject to the terms applicable in each agreement. As of March 31, 2015, exclusive of the 594,688 Pre-Funded Warrants described in Note 5, the Company had warrants outstanding that are exercisable for up to 2,281,332 shares of common stock, with a weighted average exercise price of $14.49 per share. In addition, the Company has issued a warrant to Rusnano, our financial partner in Panacela, the “Rusnano Warrant,” that can only be exercised in the event Panacela defaults on a loan from Rusnano, the “Rusnano Loan.” The maximum number of shares issuable under the Rusnano Warrant as of March 31, 2015 is 40,073. At March 31, 2015, Panacela was not in default of the Rusnano Loan.
7. Significant Alliances and Related Parties
Roswell Park Cancer Institute
The Company has entered into several agreements with Roswell Park Cancer Institute, or RPCI, including: various sponsored research agreements, an exclusive license agreement and clinical trial agreements for the conduct of the Phase 1 entolimod oncology study and the Phase 1 CBL0137 intravenous administration study. Additionally, the Company’s Chief Scientific Officer, or CSO, Dr. Andrei Gudkov, is the Senior Vice President of Basic Research at RPCI.
| F-15 |
The Company incurred $229,634 and $103,547 in expense to RPCI related to research grants and agreements for the quarters ended March 31, 2015 and 2014, respectively. The Company had $318,314 and $208,092 included in accounts payable owed to RPCI at March 31, 2015 and 2014, respectively. In addition, the Company had $266,484 and $571,557 in accrued expenses payable to RPCI at March 31, 2015 and 2014, respectively.
The Cleveland Clinic
CBLI entered into an exclusive license agreement, or the License, with The Cleveland Clinic pursuant to which CBLI was granted an exclusive license to The Cleveland Clinic’s research base underlying our therapeutic platform and certain product candidates licensed to Panacela. CBLI has the primary responsibility to fund all newly developed patents; however, The Cleveland Clinic retains ownership of those patents covered by the agreement. CBLI also agreed to use commercially diligent efforts to bring one or more products to market as soon as practical, consistent with sound and reasonable business practices and judgments. There were no milestone or royalty payments to CCF in the three months ending 2015 or 2014
The Company did not have any liabilities to The Cleveland Clinic at March 31, 2015 or 2014.
Buffalo BioLabs, et. al.
Our CSO, Dr. Andrei Gudkov has business relationships with several entities with which we transact business, the most significant of which is Buffalo BioLabs, Inc., or BBL, where Dr. Gudkov was a founder and currently serves as their Principal Scientific Advisor. Pursuant to a Master Services Agreement, more thoroughly discussed Note 8, “Restructuring” of our consolidated financial statements contained in our Annual Report on Form 10-K for the year ended December 31, 2014, as filed with the SEC, the Company recognized $171,386 and $245,423 as research and development expense for the quarters ended March 31, 2015, and 2014, respectively, and included $48,287 and $0 in accounts payable to BBL at March 31, 2015 and 2014. We also recognized $58,519 and $93,745 from BBL for sublease and other income for the quarters ended March 31, 2015 and 2014, respectively. Pursuant to our real estate sublease and equipment lease with BBL, we had accounts receivable of $256,644 and $98,285 at March 31, 2015 and 2014, respectively.
8. Subsequent Events
On April 29, 2015 we entered into an agreement to sell our equity stake in Incuron to Dr. Mikhail Mogutov, Chairman of Incuron’s Board of Directors, and Chairman of the Investment Committee and founder of Bioprocess Capital Ventures and/or his designee. The transaction has been split into two tranches, with 75% of the Company’s equity stake in Incuron being sold for approximately $3 million on April 29, 2015, and an option being given to Dr. Mogutov to purchase CBLI’s remaining ownership interest in Incuron for approximately $1 million before the end of 2015. In addition, Cleveland BioLabs has assigned its remaining intellectual property relating to Curaxin CBL0137 to Incuron in exchange for a 2% royalty on the future commercialization, licensing or sale of the Curaxin CBL0137 technology. For additional information regarding these transactions, see our Current Report on Form 8-K, filed with the SEC on May 4, 2015, and the documents incorporated by reference thereto.
| F-16 |

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and
Stockholders of Cleveland BioLabs, Inc. and Subsidiaries
We have audited the accompanying consolidated balance sheets of Cleveland BioLabs, Inc. and Subsidiaries as of December 31, 2014 and 2013, and the related consolidated statements of operations, comprehensive income (loss), stockholders’ equity (deficit), and cash flows for each of the years in the three-year period ended December 31, 2014. These financial statements are the responsibility of the entity’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Cleveland BioLabs, Inc. and Subsidiaries as of December 31, 2014 and 2013, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2014, in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the entity will continue as a going concern. As discussed in Note 2 to the financial statements, the entity has suffered recurring losses from operations and has a net capital deficiency that raises substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Meaden & Moore, Ltd.
MEADEN & MOORE, LTD.
Certified Public Accountants
Cleveland, Ohio
February 27, 2015
| F-17 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| 2014 | 2013 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 3,103,969 | $ | 10,048,466 | ||||
| Short-term investments | — | 305,538 | ||||||
| Accounts receivable | 267,199 | 458,391 | ||||||
| Other current assets | 174,179 | 344,386 | ||||||
| Total current assets | 3,545,347 | 11,156,781 | ||||||
| Equipment, net | 244,537 | 457,912 | ||||||
| Restricted cash | 1,699,759 | 2,921,724 | ||||||
| Other long-term assets | 56,131 | 159,224 | ||||||
| Investment in Incuron, LLC | 4,268,458 | — | ||||||
| Total assets | $ | 9,814,232 | $ | 14,695,641 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 1,057,743 | $ | 794,397 | ||||
| Accrued expenses | 1,804,456 | 2,445,446 | ||||||
| Deferred revenue | 156,317 | 1,069,438 | ||||||
| Accrued warrant liability | 862,074 | 1,241,311 | ||||||
| Current portion of notes payable | 2,640,968 | 351,527 | ||||||
| Current portion of capital lease obligation | 7,522 | 83,634 | ||||||
| Total current liabilities | 6,529,080 | 5,985,753 | ||||||
| Noncurrent portion of capital lease obligation | — | 7,522 | ||||||
| Long-term debt | 1,499,050 | 7,121,388 | ||||||
| Commitments and contingencies | — | — | ||||||
| Total liabilities | 8,028,130 | 13,114,663 | ||||||
| Stockholders’ equity: | ||||||||
| Preferred stock, $.005 par value; 10,000,000 shares authorized, 0 shares issued and outstanding as of December 31, 2014 and 2013, respectively | — | — | ||||||
| Common stock, $.005 par value; 160,000,000 shares authorized, 2,858,126 and 2,259,818 shares issued and outstanding as of December 31, 2014 and 2013, respectively | 14,287 | 11,299 | ||||||
| Additional paid-in capital | 132,693,988 | 125,723,083 | ||||||
| Other comprehensive income/(loss) | (380,110 | ) | 307,339 | |||||
| Accumulated deficit | (133,935,562 | ) | (135,564,666 | ) | ||||
| Total Cleveland BioLabs, Inc. stockholders’ equity (deficit) | (1,607,397 | ) | (9,522,945 | ) | ||||
| Noncontrolling interest in stockholders’ equity | 3,393,499 | 11,103,923 | ||||||
| Total stockholders’ equity | 1,786,102 | 1,580,978 | ||||||
| Total liabilities and stockholders’ equity | $ | 9,814,232 | $ | 14,695,641 | ||||
See Notes to Consolidated Financial Statements
| F-18 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Revenues: | ||||||||||||
| Grants and contracts | $ | 3,701,899 | $ | 8,487,966 | $ | 3,570,710 | ||||||
| Operating expenses: | ||||||||||||
| Research and development | 9,654,144 | 19,525,950 | 22,501,805 | |||||||||
| General and administrative | 8,469,690 | 12,038,775 | 11,115,511 | |||||||||
| Total operating expenses | 18,123,834 | 31,564,725 | 33,617,316 | |||||||||
| Loss from operations | (14,421,935 | ) | (23,076,759 | ) | (30,046,606 | ) | ||||||
| Other income (expense): | ||||||||||||
| Interest and other income (expense) | (1,089,582 | ) | 44,925 | 295,123 | ||||||||
| Foreign exchange gain (loss) | (1,036,459 | ) | 38,202 | (365,138 | ) | |||||||
| Gain on deconsolidation of Incuron, LLC | 14,206,555 | — | — | |||||||||
| Change in value of warrant liability | 2,662,329 | 2,864,348 | 7,701,981 | |||||||||
| Equity in loss of Incuron, LLC | (285,542 | ) | — | — | ||||||||
| Total other income | 14,457,301 | 2,947,475 | 7,631,966 | |||||||||
| Net income (loss) | 35,366 | (20,129,284 | ) | (22,414,640 | ) | |||||||
| Net loss attributable to noncontrolling interests | 1,593,738 | 2,866,407 | 4,180,498 | |||||||||
| Net income (loss) attributable to Cleveland BioLabs, Inc. | $ | 1,629,104 | $ | (17,262,877 | ) | $ | (18,234,142 | ) | ||||
| Net income (loss) available to common stockholders per share of common stock, basic and diluted | $ | 0.60 | $ | (7.67 | ) | $ | (9.75 | ) | ||||
| Weighted average number of shares used in calculating net income (loss) per share, basic and diluted | 2,702,884 | 2,250,142 | 1,869,443 | |||||||||
See Notes to Consolidated Financial Statements
| F-19 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
| For the Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Net income (loss) including noncontrolling interests | $ | 35,366 | $ | (20,129,284 | ) | $ | (22,414,640 | ) | ||||
| Other comprehensive loss | ||||||||||||
| Foreign currency translation adjustment | (1,816,840 | ) | (421,345 | ) | 797,558 | |||||||
| Comprehensive income (loss) including noncontrolling interests | (1,781,474 | ) | (20,550,629 | ) | (21,617,082 | ) | ||||||
| Comprehensive loss attributable to noncontrolling interests | 2,477,469 | 3,048,618 | 3,844,800 | |||||||||
| Comprehensive income (loss) attributable to Cleveland BioLabs, Inc. | $ | 695,995 | $ | (17,502,011 | ) | $ | (17,772,282 | ) | ||||
See Notes to Consolidated Financial Statements
| F-20 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Cash flows from operating activities: | ||||||||||||
| Net income (loss) | $ | 35,366 | $ | (20,129,284 | ) | $ | (22,414,640 | ) | ||||
| Adjustments to reconcile net income (loss) to net cash used in operating activities: | ||||||||||||
| Depreciation | 200,792 | 376,213 | 479,595 | |||||||||
| Amortization of loan costs | 640,248 | 94,995 | — | |||||||||
| Unrealized currency loss on short-term investments | — | 52,726 | ||||||||||
| (Gain) loss on equipment disposal | 24,685 | (3,222 | ) | 18,997 | ||||||||
| Impairment loss on property and equipment | 293,162 | — | ||||||||||
| Noncash compensation | 496,470 | 1,473,449 | 2,535,217 | |||||||||
| Warrant issuance costs | 171,116 | — | 244,857 | |||||||||
| Equity in loss of Incuron, LLC | 285,542 | — | — | |||||||||
| Change in value of warrant liability | (2,662,329 | ) | (2,864,348 | ) | (7,701,981 | ) | ||||||
| Gain on deconsolidation of Incuron, LLC | (14,206,555 | ) | — | — | ||||||||
| Changes in operating assets and liabilities: | ||||||||||||
| Accounts receivable | 629,397 | (416,495 | ) | 1,736,199 | ||||||||
| Other current assets | (1,242 | ) | 697,966 | (182,428 | ) | |||||||
| Other long-term assets | 15,690 | (29,732 | ) | (6,414 | ) | |||||||
| Accounts payable | 459,337 | (713,502 | ) | 609,522 | ||||||||
| Deferred revenue | (756,808 | ) | (2,062,332 | ) | 3,238,124 | |||||||
| Accrued expenses | 213,104 | 180,483 | 740,723 | |||||||||
| Net cash used in operating activities | (14,455,187 | ) | (23,102,647 | ) | (20,649,503 | ) | ||||||
| Cash flows from investing activities: | ||||||||||||
| Purchase of short-term investments | (1,408,169 | ) | — | (5,220,781 | ) | |||||||
| Sale of short-term investments | 1,689,670 | 2,197,940 | 8,312,120 | |||||||||
| Purchase of equipment | (20,222 | ) | (139,491 | ) | (178,271 | ) | ||||||
| Cash divested upon deconsolidation of Incuron, LLC | (2,048,023 | ) | — | — | ||||||||
| Increase in restricted cash | — | (1,497,740 | ) | (1,541,366 | ) | |||||||
| Net cash provided by (used in) investing activities | (1,786,744 | ) | 560,709 | 1,371,702 | ||||||||
| Cash flows from financing activities: | ||||||||||||
| Issuance of common stock, net of offering costs | 9,697,501 | — | 15,675,727 | |||||||||
| Net proceeds/(repayment) of long-term debt | (4,176,275 | ) | 7,327,675 | — | ||||||||
| Noncontrolling interest capital contribution to Incuron, LLC | 5,152,438 | — | 5,893,557 | |||||||||
| Exercise of options | — | 12,392 | 2,375 | |||||||||
| Repayment of capital lease obligation | (83,634 | ) | (78,125 | ) | (52,410 | ) | ||||||
| Net cash provided by financing activities | 10,590,030 | 7,261,942 | 21,519,249 | |||||||||
| Effect of exchange rate change on cash and equivalents | (1,292,595 | ) | (323,621 | ) | 538,046 | |||||||
| Decrease in cash and cash equivalents | (6,944,497 | ) | (15,603,617 | ) | 2,779,494 | |||||||
| Cash and cash equivalents at beginning of period | 10,048,466 | 25,652,083 | 22,872,589 | |||||||||
| Cash and cash equivalents at end of period | $ | 3,103,969 | $ | 10,048,466 | $ | 25,652,083 | ||||||
| Supplemental disclosure of cash flow information: | ||||||||||||
| Cash paid during the period for interest | $ | 451,327 | $ | 17,105 | $ | 23,708 | ||||||
| Supplemental schedule of noncash financing activities: | ||||||||||||
| Allocation of equity proceeds to fair value of warrants | $ | 2,216,593 | $ | — | $ | — | ||||||
| Noncash warrant issuance costs | $ | 15,993 | $ | 117,999 | $ | — | ||||||
| Equipment acquired through lease financing | $ | — | $ | — | $ | 221,690 | ||||||
See Notes to Consolidated Financial Statements
| F-21 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
| Common Stock | Additional Paid-in | Accumulated Other Comprehensive | Accumulated | Noncontrolling | ||||||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Deficit | Interests | Total | ||||||||||||||||||||||
| Balance at December 31, 2011 | 1,781,319 | 8,907 | $ | 109,034,800 | $ | 84,613 | $ | (100,067,647 | ) | $ | 13,184,824 | $ | 22,245,496 | |||||||||||||||
| — | ||||||||||||||||||||||||||||
| Issuance of common stock net of offering costs of $1,129,916 | 426,250 | 2,131 | 15,918,453 | — | — | — | 15,920,584 | |||||||||||||||||||||
| Allocation of financing proceeds to fair value of warrants | — | — | (4,521,681 | ) | — | — | — | (4,521,681 | ) | |||||||||||||||||||
| Noncontrolling interest capital contribution to Incuron, LLC | — | — | 1,081,040 | — | — | 4,812,517 | 5,893,557 | |||||||||||||||||||||
| Stock based compensation | 29,601 | 148 | 2,562,311 | — | — | — | 2,562,459 | |||||||||||||||||||||
| Exercise of options | 63 | 0 | 2,375 | — | — | — | 2,375 | |||||||||||||||||||||
| Net loss | — | — | — | — | (18,234,142 | ) | (4,180,498 | ) | (22,414,640 | ) | ||||||||||||||||||
| Foreign currency translation | — | — | — | 461,860 | — | 335,698 | 797,558 | |||||||||||||||||||||
| Balance at December 31, 2012 | 2,237,233 | 11,186 | 124,077,297 | 546,473 | (118,301,789 | ) | 14,152,541 | 20,485,708 | ||||||||||||||||||||
| Stock based compensation | 22,100 | 111 | 1,515,399 | — | — | — | 1,515,509 | |||||||||||||||||||||
| Exercise of options | 485 | 2 | 12,389 | — | — | — | 12,391 | |||||||||||||||||||||
| Allocation of debt proceeds to fair value of warrants | — | — | 117,999 | — | — | — | 117,999 | |||||||||||||||||||||
| Net loss | — | — | — | — | (17,262,877 | ) | (2,866,407 | ) | (20,129,284 | ) | ||||||||||||||||||
| Foreign currency translation | — | — | — | (239,134 | ) | — | (182,211 | ) | (421,345 | ) | ||||||||||||||||||
| Balance at December 31, 2013 | 2,259,818 | 11,299 | 125,723,083 | 307,339 | (135,564,666 | ) | 11,103,923 | 1,580,978 | ||||||||||||||||||||
| Stock based compensation | 3,052 | 12 | 645,488 | — | — | — | 645,500 | |||||||||||||||||||||
| Issuance of common stock, net of offering costs of $697,882 | 595,256 | 2,976 | 9,799,142 | — | — | — | 9,802,118 | |||||||||||||||||||||
| Allocation of equity proceeds to fair value of warrants | — | — | (2,216,593 | ) | — | — | — | (2,216,593 | ) | |||||||||||||||||||
| Noncontrolling interest capital contribution | — | — | 1,176,982 | — | — | 3,975,301 | 5,152,283 | |||||||||||||||||||||
| Deconsolidation of Incuron, LLC | — | — | (2,434,114 | ) | 245,660 | — | (9,208,256 | ) | (11,396,710 | ) | ||||||||||||||||||
| Net income (loss) | — | — | — | — | 1,629,104 | (1,593,738 | ) | 35,366 | ||||||||||||||||||||
| Foreign currency translation | — | — | — | (933,109 | ) | — | (883,731 | ) | (1,816,840 | ) | ||||||||||||||||||
| Balance at December 31, 2014 | 2,858,126 | $ | 14,287 | $ | 132,693,988 | $ | (380,110 | ) | $ | (133,935,562 | ) | $ | 3,393,499 | $ | 1,786,102 | |||||||||||||
See Notes to Consolidated Financial Statements
| F-22 |
CLEVELAND BIOLABS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Description of Business
Cleveland BioLabs, Inc. is an innovative biopharmaceutical company seeking to develop first-in-class pharmaceuticals designed to address diseases with significant unmet medical need. Our most advanced product candidate is entolimod, which we are developing as a radiation countermeasure and an immunotherapy for oncology and other indications. We conduct business in the United States and in the Russian Federation, or Russia, through several legal entities, one of which is wholly-owned and two of which are owned in collaboration with financial partners. As used throughout these consolidated financial statements, the terms “Cleveland BioLabs” and “CBLI” refer to Cleveland BioLabs, Inc. and its wholly-owned subsidiary BioLab 612, LLC, but not its consolidated joint venture, Panacela Labs, Inc. or its unconsolidated joint venture, Incuron, LLC. The “Company,” “we,” “us” and “our” refer to Cleveland BioLabs, Inc. together with its wholly-owned subsidiary BioLab 612, LLC, its consolidated joint venture, Panacela Labs, Inc., and its unconsolidated joint venture, Incuron, LLC.
CBLI was incorporated in Delaware in June 2003 and is headquartered in Buffalo, New York. As of December 31, 2014, CBLI had one wholly-owned subsidiary, Biolab 612, LLC, or Biolab 612, which began operations in 2012, one consolidated joint venture, Panacela Labs, Inc., or Panacela, which was formed by us and a financial partner in 2011, and one unconsolidated joint venture, Incuron, LLC, or Incuron, which was formed by us and a financial partner in 2010. Additionally, Panacela had a wholly-owned subsidiary, Panacela Labs, LLC, which was formed in 2011.
2. Summary of Significant Accounting Policies
Basis of Presentation and Consolidation
The accompanying consolidated financial statements include the accounts of CBLI and the subsidiary and joint venture in which CBLI held a controlling interest in as of December 31, 2014, BioLab 612 and Panacela. The accounts of Incuron are also included through November 25, 2014, the date at which CBLI no longer maintained a controlling interest in Incuron. For the period from November 25, 2014 through December 31, 2014, the Company’s interest in Incuron has been presented under the equity method of accounting. All significant intercompany balances and transactions have been eliminated in consolidation. These financial statements have been prepared on the accrual basis in accordance with accounting principles generally accepted in the United States, or GAAP.
On January 28, 2015, the Company, after receiving authorization from the Company’s shareholders and board of directors, executed a reverse stock split, or Reverse Split, of the Company’s common stock at the ratio of 1:20. All historical share balances and share price-related data have been adjusted based on this ratio.
At December 31, 2014, we had cash, cash equivalents and short-term investments of $3.1 million, $6.8 million after giving effect to our equity raise on February 6, 2015 described in Note 14 to the Consolidated Financial Statements. Of that total, $0.5 million was restricted for the use of our consolidated joint venture, Panacela, leaving $2.6 million available for general use, or $6.3 million giving effect to the February 2015 raise, which management believes will be sufficient to support operations into June 2015. To ensure continuing operations beyond that point, management is evaluating all opportunities to secure additional financing, including investments from non-controlling interests, the sale or license of our drug candidates, the issuance of equity and additional revenues from the U.S. or Russian governments. Management believes that sufficient sources of financing will be available to support operations into the future, however there can be no assurances at this time. These matters raise substantial doubt about the Company’s ability to continue as a going concern. These financial statements have been prepared under the assumption that the Company will continue as a going concern and do not include any adjustments that might result from the outcome of this uncertainty.
| F-23 |
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates.
Cash and Cash Equivalents
Of the $3.1 million and $10.0 million of cash and cash equivalents at December 31, 2014 and December 31, 2013, respectively, $0 million and $3.5 million, respectively, consisted of highly liquid investments with maturities of 90 days or less when purchased. These investments consist of commercial paper, short-term debt securities, time deposits and investments in money market funds with commercial banks and financial institutions. As of December 31, 2014, $0.5 million of the Company’s cash and cash equivalents were restricted to the use of our consolidated joint venture, Panacela, leaving $2.6 million available for general use.
Short-Term Investments
The Company’s short-term investments are classified as held to maturity given the intent and ability to hold the investments to maturity. Accordingly, these investments are carried at amortized cost. Short-term investments classified as held-to-maturity consisted of certificates of deposit with maturity dates beyond three months and less than one year. The Company’s short-term investments were $0.0 million and $0.3 million as of December 31, 2014 and 2013, respectively and were restricted for use by its consolidated joint ventures.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to a significant concentration of credit risk primarily consist of cash and cash equivalents and short-term investments. The Company maintains cash balances with financial institutions in excess of insured limits. The Company does not believe it is exposed to significant credit risk due to the financial position of the depository institutions in which those deposits are held.
As of December 31, 2014, the Company held 43% of its cash and cash equivalents in accounts located outside of the United States.
As of February 12, 2015, the Russian Ruble: Dollar exchange rate increased from 56.2584 to 66.0585, resulting in a decrease of $0.2 million to the Company’s cash and cash equivalents as compared to December 31, 2014.
Significant Customers and Accounts Receivable
Grant and contract revenue from the United States government accounted for 0.6%, 26.8% and 34.8% of total revenue for the years ended December 31, 2014, 2013 and 2012, respectively. Although the Company anticipates ongoing U.S. and Russian government contract and grant revenue, there is no guarantee that these revenue streams will continue in the future.
Grant and contract revenue received by our subsidiary/joint ventures from Russian government agencies accounted for 95.2%, 73.2% and 65.2% of total revenues for the years ended December 31, 2014, 2013 and 2012, respectively.
Service contract revenue received by us from Incuron accounted for 4.2%, 0.0% and 0.0% of total revenues for the years ended December 31, 2014, 2013 and 2012, respectively.
Accounts receivable consist of amounts due under contracts with certain government and foreign entities. The Company extends unsecured credit to its government customers under normal trade agreements and contracted terms, which generally require payment within 30 days.
| F-24 |
Management estimates an allowance for doubtful accounts that is based upon management’s review of delinquent accounts and an assessment of the Company’s historical evidence of collections. There were allowances for doubtful accounts of $0.1 and $0.0 million at December 31, 2014 and December 31, 2013, respectively, pertaining to accounts receivable from our subleases.
Equipment
Equipment is stated at cost, net of accumulated depreciation. Upon retirement or sale, the cost of assets disposed of and the related accumulated depreciation are removed from the accounts and any resulting gain or loss is credited or charged to operations. Repair and maintenance costs are expensed as incurred.
Equipment is depreciated using the straight-line method over the estimated useful lives of the respective assets as follows:
| Asset Category | Estimated Useful Life (in Years) | |||
| Laboratory equipment | 5 | |||
| Furniture and fixtures | 5 | |||
| Computer equipment | 3 | |||
Impairment of Long-Lived Assets
Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amounts of the assets or related asset group may not be recoverable. Determination of recoverability is based on an estimate of discounted future cash flows resulting from the use of the asset. In the event that such cash flows are not expected to be sufficient to recover the carrying amount of the asset or asset group, the carrying amount of the asset is written down to its estimated net realizable value.
In connection with the restructuring discussed in Note 8, “Restructuring,” the Company entered into an equipment lease agreement with Buffalo BioLabs, LLC, or BBL, effective January 1, 2014 for the equipment used by the employees transitioned to BBL. The estimated fair value of those assets using a discounted cash flow approach was $243,000. Comparing this fair value to the carrying amount of the equipment as of December 31, 2013 of $536,000 resulted in a $293,000 write-down of the assets, which was included in research and development expenses for the year ended December 31, 2013.
Restricted Cash
Restricted cash at December 31, 2014 includes certificates of deposit denominated in Russian Rubles and posted by Panacela and BioLab 612 as collateral for performance guarantees for their contracts with the Ministry of Industry and Trade of the Russian Federation. The guarantees require Panacela and BioLab 612 to satisfactorily perform their statements of work under the contracts. Both Panacela and BioLab 612 anticipate receiving the full proceeds of their deposits at the completion of the contracts, which for each contract is more than a year away. As a consequence, all of the Company’s restricted cash is classified as a noncurrent asset.
Equity Method Investment
The Company’s equity method investment includes its minority holdings in Incuron. The opening equity investment amount as of November 25, 2014, the date at which we no longer maintained a controlling interest, was determined to be $4,554,000. This determination was made by an independent valuation expert based on the commercial potential of Incuron’s drug candidates and market values assigned to other early-stage oncology drug candidates.
Under the equity method, the carrying amount of the investment is adjusted for the Company’s share of earnings and losses, as well as any capital contributions to and distributions from Incuron. The Company classifies income and losses related to its equity method investments as a component of operating income or loss as Incuron is an extension of the Company’s core business. The Company evaluates equity investments for impairment whenever events or changes in circumstances indicate that the carrying amount of the investment may have experienced an “other-than-temporary” decline in value. If such conditions exist, the Company compares the estimated fair value of the investment to its carrying value to determine if an impairment is indicated and whether the impairment is “other-than-temporary” based on an assessment of all relevant factors, including consideration of the intent and ability to retain the investment.
| F-25 |
Intellectual Property
Costs related to filing and pursuing patent applications are recognized as general and administrative expenses as incurred, since the recoverability of such expenditures is uncertain. Upon marketability approval by the U.S. Food and Drug Administration, or FDA, or a respective foreign governing body, such costs will be capitalized and depreciated over the expected life of the related patent.
Deferred Revenue
Deferred revenue represents cash received under grants and contracts in excess of the revenue recognizable through the end of the respective financial reporting period. The revenue associated with these advances will be recognized in future periods as the applicable costs are incurred.
Accrued Warrant Liability
Certain warrants are accounted for as derivative instruments in accordance with the Financial Accounting Standards Board Accounting Standards Codification, or the Codification, on derivatives and hedging as the warrant holders, under certain change of control situations, could require settlement in cash. As such, the warrants were initially recorded as liabilities based on their fair values on the date of issuance. Subsequent changes in the value of the warrants are recorded in the statements of operations as “Change in value of warrant liability.”
The Company’s remaining outstanding warrants were treated as equity upon issuance and continue to be treated as equity since they did not contain any mandatory redemption features or other provisions that would require a different classification of these warrant instruments outside of permanent equity.
Foreign Currency Translation
The Russian ruble is the functional currency of our foreign subsidiary and joint ventures, which are all located in the Russian Federation. Assets and liabilities of these companies are translated into U.S. dollars at the period-end exchange rate. Income and expense items are translated at the average exchange rates during the period. The net effect of this translation is recorded in the consolidated financial statements as accumulated other comprehensive income (loss).
Revenue Recognition
The Company generates grant and contract revenue from two different types of contractual arrangements: cost reimbursable grants and contracts and fixed-price grants and contracts. Costs consist primarily of internal labor charges, subcontractors and materials, as well as an allocation of fringe benefits, overhead and general and administrative expenses, based on the terms of the contract. Under cost reimbursable grants and contracts, revenue is recognized during the period that the associated research and development costs are incurred. Under fixed-price grants and contracts, revenue is recognized using the percentage-of-completion method. The assumptions and estimates used in determination of the percentage-of-completion are developed in coordination with the principal investigator performing the work.
| F-26 |
Research and Development
Research and development costs are expensed as incurred. Research and development costs primarily consist of salaries, fringe benefits, materials and related expenses for personnel and facility expenses. Other research and development expenses include fees paid to consultants and outside service providers, the costs of materials used in clinical trials and research and development and stock-based compensation.
Accounting for Stock-Based Compensation
The 2006 Equity Incentive Plan, as amended, or the “Plan”, authorizes CBLI to grant (i) options to purchase common stock, (ii) restricted or unrestricted stock units, and (iii) stock appreciation rights, so long as the exercise or grant price of each are at least equal to the fair market value of the stock on the date of grant. At the 2012 annual meeting of stockholders, an amendment to increase the maximum number of shares of common stock reserved for issuance under the Plan was approved, and as of December 31, 2014, an aggregate of 500,000 shares of common stock were authorized for issuance under the Plan, of which a total of approximately 100,000 shares of common stock remained available for future awards. A single participant cannot be awarded more than 20,000 shares annually. Awards granted under the Plan have a contractual life of no more than 10 years. The terms and conditions of equity awards (such as price, vesting schedule, term and number of shares) under the Plan are specified in an award document, and approved by compensation committee of the CBLI board of directors.
The Company utilizes the Black-Scholes valuation model for estimating the fair value of all stock options granted. Set forth below are the assumptions used in valuing the stock options granted and a discussion of the Company’s methodology for developing each of the assumptions used:
| For the year ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Risk-free interest rate | 1.59 - 1.98 | % | .02 - 1.92 | % | .65 - 1.49 | % | ||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||
| Expected life | 5 - 6 Years | 5 - 7.3 Years | 5 - 6 Years | |||||||||
| Expected volatility | 71.24 - 78.02 | % | 78.62 - 89.66 | % | 86.58 - 92.60 | % | ||||||
“Risk-free interest rate” means the range of U.S. Treasury rates with a term that most closely resembles the expected life of the option as of the date the option is granted.
“Expected dividend yield” means the Company does not pay regular dividends on its common stock and does not anticipate paying any dividends in the foreseeable future.
“Expected life” means the period of time that options granted are expected to remain outstanding, based wholly on the use of the simplified (safe harbor) method. The simplified method is used because the Company does not yet have adequate historical exercise information to estimate the expected life the options granted.
“Expected volatility” means a measure of the amount by which a financial variable, such as share price, has fluctuated (historical volatility) or is expected to fluctuate (implied volatility) during a period. Expected volatility is based on the Company’s historical volatility and incorporates the volatility of the common stock of comparable companies when the expected life of the option exceeds the Company’s trading history.
In June 2013, CBLI’s stockholders approved the 2013 Employee Stock Purchase Plan, or ESPP, which provides a means by which eligible employees of CBLI, and certain designated related corporations may be given an opportunity to purchase shares of CBLI common stock. As of December 31, 2014, there were 115,000 shares of common stock reserved for purchase under the ESPP. The number of shares reserved for purchase under the ESPP increases on January 1 of each calendar year by the lesser of (i) 10% of the total number of shares of common stock outstanding on December 31 of the preceding year, or (ii) 10,000 shares of common stock. The ESPP, when implemented, will allow employees to use up to 15% of their compensation, up to $25,000 per year, to purchase shares of common stock at an amount equal to 85% of the fair market value of the our common stock on the offering date or the purchase date, whichever is less.
| F-27 |
Income taxes
No income tax expense was recorded for the years ended December 31, 2014, 2013 and 2012, as the Company did not have taxable income for any of the years presented. A full valuation allowance has been recorded against the Company’s net deferred tax asset.
Earnings/(loss) per share
Basic net income (loss) per share of common stock excludes dilution for potential common stock issuances and is computed by dividing net income (loss) by the weighted average number of shares outstanding for the period. Diluted net income (loss) per share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock. Diluted net loss per share is identical to basic net loss per share as potentially dilutive securities have been excluded from the calculation of diluted net loss per common share because the inclusion of such securities would be antidilutive.
The Company has excluded the following outstanding warrants and options from the calculation of diluted net loss per share because all such securities were antidilutive for the periods presented:
| As of December 31, | ||||||||||||
| Common Equivalent Securities | 2014 | 2013 | 2012 | |||||||||
| Warrants | 875,304 | 526,752 | 518,939 | |||||||||
| Options | 261,389 | 278,242 | 250,849 | |||||||||
| Total | 1,136,693 | 804,994 | 769,788 | |||||||||
Comprehensive Income (Loss)
The Company applies the Codification on comprehensive income (loss) that requires disclosure of all components of comprehensive income (loss) on an annual and interim basis. Comprehensive income (loss) is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources.
Recently Issued Accounting Pronouncements
From time to time, new accounting pronouncements are issued by the Financial Accounting Standards Board or other standard setting bodies that are adopted by us as of the specified effective date. Unless otherwise discussed, we believe that the impact of recently issued standards that are not yet effective will not have a material impact on our financial position or results of operations upon adoption.
In May 2014, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update, or ASU, 2014-09, Revenue from Contracts with Customers, which updates the principles for recognizing revenue. ASU 2014-09 also amends the required disclosures of the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. ASU 2014-09 is effective for annual reporting periods beginning after December 15, 2016, including interim periods within that reporting period. The Company is evaluating the potential impacts of the new standard on its existing revenue recognition policies and procedures.
In June 2014, the FASB issued ASU 2014-12, Accounting for Share-Based Payments When the Terms of an Award Provide That a Performance Target Could Be Achieved after the Requisite Service Period. ASU 2014-12 requires that a performance target that affects vesting and that could be achieved after the requisite service period be treated as a performance condition, and as such, the performance target should not be reflected in estimating the grant-date fair value of the award. ASU 2014-12 is effective for annual reporting periods beginning after December 15, 2015, with early adoption permitted. The Company is evaluating the potential impacts of the new standard on its existing stock-based compensation plans.
In August 2014, the FASB issued ASU 2014-15, Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern, ASU 2014-15 requires that an entity’s management evaluate whether there are conditions or events that raise substantial doubt about the entity’s ability to continue as a going concern within one year after the date that the financial statements are issued. ASU 2014-15 is effective for annual periods beginning after December 15, 2016 and for interim periods thereafter. The Company is evaluating the potential impacts of this new standard on its quarterly reporting process.
| F-28 |
3. Fair Value Measurements
The Company measures and records cash equivalents and warrant liabilities at fair value in the accompanying financial statements. Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability, an exit price, in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value include:
| ● | Level 1 – Observable inputs for identical assets or liabilities such as quoted prices in active markets; | |
| ● | Level 2 – Inputs other than quoted prices in active markets that are either directly or indirectly observable; and | |
| ● | Level 3 – Unobservable inputs in which little or no market data exists, which are therefore developed by the Company using estimates and assumptions that reflect those that a market participant would use. |
The following tables represent the Company’s fair value hierarchy for its financial assets and liabilities measured at fair value on a recurring basis as of December 31, 2014 and December 31, 2013:
| As of December 31, 2014 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Accrued warrant liability | $ | — | $ | — | $ | 862,074 | $ | 862,074 | ||||||||
| Compensatory stock options not yet issued (1) | — | — | 132,295 | 132,295 | ||||||||||||
| Total liabilities | $ | — | $ | — | $ | 994,369 | $ | 994,369 | ||||||||
| As of December 31, 2013 | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| Liabilities: | ||||||||||||||||
| Accrued warrant liability | $ | — | $ | — | $ | 1,241,311 | $ | 1,241,311 | ||||||||
| Compensatory stock options not yet issued (1) | — | — | 309,450 | 309,450 | ||||||||||||
| Total liabilities | $ | — | $ | — | $ | 1,550,761 | $ | 1,550,761 | ||||||||
| (1) | Included in accrued expenses in the accompanying consolidated balance sheets. |
The Company has certain warrants that could require settlement in cash if a fundamental transaction occurs, as defined in the respective agreements. These agreements specify the amount due to warrant holders is based on the Black-Scholes pricing model. The following are the assumptions used to measure the accrued warrant liability at December 31, 2014 and 2013, which were determined in a manner consistent with that described for grants of options to purchase common stock as set forth in Note 2, “Summary of Significant Accounting Policies”:
| December 31, 2014 | December 31, 2013 | |||||||
| Stock Price | $ | 5.60 | $ | 23.40 | ||||
| Exercise Price | $ | 10.10 - 100.00 | $ | 32.00 - 100.00 | ||||
| Term in years | 0.46 - 6.04 | 0.59 - 1.91 | ||||||
| Volatility | 70.69 - 100.08 | % | 42.52 - 76.03 | % | ||||
| Annual rate of quarterly dividends | 0 | % | 0 | % | ||||
| Discount rate- bond equivalent yield | .12 - 1.65 | % | .08 - .36 | % | ||||
| F-29 |
The following are the assumptions used to measure the compensatory stock options not yet issued at December 31, 2014:
| December 31, 2014 | ||||
| Stock price | $ | 5.60 | ||
| Term in years | 5 | |||
| Volatility | 73.99 | % | ||
| Annual rate of quarterly dividends | 0 | % | ||
| Discount rate – bond equivalent yield | 1.45 | % | ||
The following table sets forth a summary of changes in the fair value of the Company’s Level 3 fair value measurements for the year ended December 31, 2014 and 2013:
| Year Ended December 31, 2014 | ||||||||
| Accrued Warrant Liability | Compensatory Stock Options Issued After Year End | |||||||
| Beginning Balance | $ | 1,241,311 | $ | 309,450 | ||||
| Total (gains) or losses, realized and unrealized, included in earnings (1)(2) | (2,572,035 | ) | (21,055 | ) | ||||
| Issuances | 2,283,092 | 132,295 | ||||||
| Settlements | (90,294 | ) | (288,395 | ) | ||||
| Balance, December 31, 2014 | $ | 862,074 | $ | 132,295 | ||||
| Year Ended December 31, 2013 | ||||||||
| Accrued Warrant Liability | Compensatory Stock Options Issued After Year End | |||||||
| Beginning Balance | $ | 4,105,659 | $ | — | ||||
| Total (gains) or losses, realized and unrealized, included in earnings (1)(2) | (2,864,348 | ) | — | |||||
| Estimates and other changes in fair value | — | 309,450 | ||||||
| Balance, December 31, 2013 | $ | 1,241,311 | $ | 309,450 | ||||
| (1) | Unrealized gains or losses related to the accrued warrant liability were included as change in value of accrued warrant liability. For the year ended December 31, 2014 we realized a gain of $90,294 in connection with the elimination of the Series B warrants issued to an investor in the January 2014 equity investment transaction. For the year ended December 31, 2013 there were no realized gains or losses. |
| (2) | Expenses recorded for compensatory stock options not yet issued are included in research and development expense and general and administrative expense. |
Separate disclosure is required for assets and liabilities measured at fair value on a recurring basis, as documented above, from those measured at fair value on a nonrecurring basis. As of December 31, 2014 and 2013, the Company had no assets or liabilities that were measured at fair value on a nonrecurring basis.
| F-30 |
The Company considers the accrued warrant liability and compensatory stock options not yet issued to be Level 3 because some of the inputs into the measurements are neither directly or indirectly observable. The compensatory stock options not yet issued use management’s estimate for the expected term, which is based on the safe harbor method as historical exercise information over the term of each security is not readily available. The following table summarizes the unobservable inputs into the fair value measurements:
| December 31, 2014 | ||||||||||||
| Description | Fair Value | Valuation Technique | Unobservable Input | Range in years | ||||||||
| Compensatory stock options not yet issued | $ | 132,295 | Black-scholes pricing model | Expected term | 5 | |||||||
| Accrued warrant liability | 862,074 | Black-scholes pricing model | Expected term | 0.46 - 6.04 | ||||||||
| $ | 994,369 | |||||||||||
Management believes the value of the accrued warrant liability and compensatory stock options are more sensitive to changes in the Company’s stock price at the end of the respective reporting period as opposed to changes in the expected term. At December 31, 2014, a 10% increase in the expected term of the Company’s warrants measured using the Black-Scholes pricing model would increase the warrant liability by approximately 13%, while a 10% decrease in the expected term would decrease the warrant liability by approximately 14%. A 10% increase in the Company’s stock price would result in an increase in the accrued warrant liability of approximately 17%, while a 10% decrease in the stock price would decrease the warrant liability by approximately 16%. At December 31, 2014, a 10% increase or decrease in the expected term of the Company’s compensatory stock options not yet issued would increase or decrease the amount accrued by approximately 4%, while a 10% increase or decrease in the stock price would increase or decrease the amount accrued by approximately 10%.
The carrying amounts of the Company’s remaining financial instruments, which include cash, short-term investments, accounts receivable and accounts payable, approximate their fair values due to their short maturities.
4. Equipment
The following table summarizes the Company’s gross equipment costs for the years ended December 31, 2014 and 2013:
| As of December 31, | ||||||||
| 2014 | 2013 | |||||||
| Lab equipment | $ | 1,062,679 | $ | 1,083,463 | ||||
| Computer equipment | 293,663 | 332,386 | ||||||
| Furniture | 528,807 | 528,807 | ||||||
| 1,885,149 | 1,944,656 | |||||||
| Less accumulated depreciation | (1,640,612 | ) | (1,486,744 | ) | ||||
| Equipment, net | $ | 244,537 | $ | 457,912 | ||||
As part of the sublease income received from Buffalo BioLabs, Inc. mentioned in Note 9, Significant Alliances and Related Parties, the Company leases lab equipment to Buffalo BioLabs, Inc. The original cost and net book value of that equipment as of December 31, 2014 was $1,054,166 and $137,553, respectively. The monthly income we receive from Buffalo BioLabs, Inc. for this equipment is $4,500 and is cancellable upon 90 days notice.
| F-31 |
5. Noncontrolling Interests
On May 31, 2012, Bioprocess Capital Ventures, or BCV, our financial partner in Incuron, contributed approximately 194.0 million Russian rubles (approximately $5.9 million) to Incuron, which increased its ownership percentage to 40.78% and decreased CBLI’s ownership percentage to 59.22%, which is the ownership percentage at December 31, 2013. On June 20, 2014, BCV contributed 100.0 million Russian rubles (approximately $2.9 million) to Incuron, which increased its ownership interest from 40.78% to 46.06% and decreased CBLI’s ownership percentage from 59.22% to 53.94%. The effect of this change in CBLI’s ownership interest in Incuron on CBLI’s equity is shown on the consolidated statement of stockholders’ equity. On August 5, 2014, BCV contributed an additional 79.9 million Russian rubles (approximately $2.3 million) to Incuron which increased its ownership interest from 46.06% to 49.98%. On November 25, 2014, BCV exercised their rights under Amendment 1 to the Participation Agreement and purchased 3.05% of Incuron from us for a nominal amount. As a result, effective November 25, 2014 CBLI no longer maintained control of Incuron, owning 46.96%, and deconsolidated Incuron. The Company’s consolidated income statement includes revenue, research and development, and general and administrative expenses recognized by Incuron through November 25, 2014 in the amounts of $1,000,770, $1,664,094 and $907,643, respectively.
Beginning on November 25, 2014, CBLI accounted for its ownership interest in Incuron using the equity method of accounting, and recognized its share of equity method losses in the amount of $285,542. The gain on deconsolidation of Incuron amounted to $14,206,555, and was determined based on comparison of the fair value of CBLI’s retained investment in Incuron of $4,554,000 and the carrying amount of the non-controlling interest on the deconsolidation date of $9,797,083, less the carrying amount of the net assets of Incuron on the deconsolidation date of $144,528.
The fair value of Incuron at the date of deconsolidation was determined by an independent valuation. This fair value is categorized within Level 3 of the fair value hierarchy. The value was determined by weighting a market approach and an income approach (Risk adjusted debt-free discounted cash flow) using equal weights of 50% and adjusted for uncertainties associated with the Russian capital markets. The unobservable input of the market approach was a 0.5 multiple of market value to invested capital. The unobservable input to the income approach was a discount rate based upon a weighted average cost of capital of 22%.
We anticipate that CBLI will continue to provide services to Incuron in a related party capacity following the date of deconsolidation to assist in furthering the development of CBL0137. Since the date of the deconsolidation, the Company recognized $154,687 of revenues associated with executed services contracts. As of the year ended December 31, 2014, the Company has $156,464 in accounts receivable with Incuron. A summary of Incuron’s financial position and results of operations follows:
| December 31, 2014 | ||||||||||
| ASSETS | LIABILITIES AND EQUITY | |||||||||
| Cash and cash equivalents | $ | 1,103,384 | Accounts payable | $ | 33,303 | |||||
| Short-term investments | 248,852 | Accounts payable to CBLI | 156,464 | |||||||
| Other current assets | 47,846 | Accrued expenses | 253,915 | |||||||
| Total current assets | 1,400,082 | Total current liabilities | 443,681 | |||||||
| Long-term assets | 6,900 | Net equity | 963,300 | |||||||
| Total assets | $ | 1,406,982 | Total liabilities and equity | $ | ,406,982 | |||||
| STATEMENT OF OPERATIONS | ||||||||||
| For the period from November 26, 2014 to December 31, 2014 | ||||||||||
| REVENUES | ||||||||||
| Grant and contract revenue | $ | — | ||||||||
| OPERATING EXPENSES | ||||||||||
| Research and development | 215,392 | |||||||||
| CBLI development costs | 169,511 | |||||||||
| General and administrative | 106,277 | |||||||||
| Total operating expenses | 491,180 | |||||||||
| LOSS FROM OPERATIONS | (491,180 | ) | ||||||||
| OTHER EXPENSES | ||||||||||
| Foreign exchange gain (loss) | (116,874 | ) | ||||||||
| NET INCOME/(LOSS) | $ | (608,054 | ) | |||||||
| F-32 |
In 2011, CBLI and Open Joint Stock Company “Rusnano”, or Rusnano, and certain other third-party technology providers formed Panacela to develop and commercialize a early-stage drug candidates for the treatment of oncological, infectious or other diseases. CBLI invested $3.0 million, certain third-party owners, assigned and/or provided exclusive licenses, and Rusnano invested $9.0 million, with an additional $17.0 million available for investment. $1.5 million of the $17.0 million was invested in the form of a convertible loan, discussed in Note 6, “Debt,” leaving $15.5 million currently available to be invested at Rusnano’s option. At December 31, 2014, CBLI had an ownership stake of 57.78% in Panacela.
6. Debt
On September 30, 2013, CBLI and BioLab 612 entered into a Loan and Security Agreement, or the Loan Agreement, with Hercules Technology II, L.P., or Hercules, pursuant to which we issued a $6.0 million note and received net proceeds of $5.9 million. The loan bears interest at the greater of (i) 10.45% per annum or (ii) 10.45% plus the prevailing prime rate minus 4.25%. The loan matures on January 1, 2017, and requires interest-only payments for the initial 12 months and principal and interest payments in 27 monthly installments thereafter. In June 2014, CBLI repaid $4.0 million of the Hercules Loan using net proceeds from a sale of equity and cash.
In connection with the Loan Agreement, CBLI granted a first priority lien in substantially all of CBLI’s assets (exclusive of intellectual property). The Loan Agreement also contains representations and warranties by CBLI and Hercules, indemnification provisions in favor of Hercules, customary covenants (including limitations on other indebtedness, liens, acquisitions, investments and dividends, but excluding any financial covenants), and events of default (including payment defaults, breaches of covenants, material adverse events and events leading to bankruptcy or insolvency). Prepayment of the loan is subject to a penalty rate applied to the balance of the secured obligation and ranges from 1% to 3% based on the date the loan is prepaid. The prepayment penalty was waived on connection with the above referenced June 2014 prepayment.
Additional loan features, all of which are recorded debt issuance costs and loan discounts in non-current assets, include: $102,000 related to legal fees and a $100,000 facility fee both of which were paid in cash. A $550,000 “end-of-term charge” which is due upon full repayment of the loan or on the maturity date, whichever occurs sooner and is included in long-term liabilities. And a five-year warrant to purchase 7,813 shares of CBLI common stock. The warrant had an initial exercise price of $32.00 per share, which was subsequently lowered to $10.10 per share in accordance with its terms. The Black-Scholes pricing model yielded $117,999 as the fair value of the warrant upon issuance which was recorded as equity.
CBLI will amortize the loan discounts and debt issuance costs to interest expense over the term of the loan using the effective interest rate method, which approximates 16.6%.
The following schedule shows the payments for principal and the end of term charge on the Hercules loan by calendar year:
| 2015 | $ | 748,738 | ||
| 2016 | 831,721 | |||
| 2017 | 852,365 | |||
| Total | $ | 2,432,824 |
On September 3, 2013, Panacela entered into a Master Agreement, or the Panacela Loan, with Rusnano and CBLI pursuant to which Panacela issued a $1,530,000 note to Rusnano. The Panacela Loan bears interest at a rate of 16.3% per annum and matures on September 10, 2015, at which time Panacela must repay all unpaid principal and accrued interest. As of the year ended December 31, 2014, Panacela had $355,493 of interest accrued associated with the Panacela Loan. Prior to March 10, 2015, the loan is mandatorily convertible into shares of Panacela preferred stock at a conversion price of $1,057 per share if Panacela completes a qualified financing in accordance with the terms of the Panacela Loan. Subsequent to March 10, 2015, Rusnano has the option to convert the unpaid principal plus interest into shares of Panacela preferred stock at a conversion price of $1,057 per share, or if Panacela has a qualified financing event, at a discounted price of 0.75 times the purchase price per share.
| F-33 |
In connection with the Panacela Loan, CBLI issued Rusnano a warrant that has an exercise period that begins upon an event of default on the Panacela Loan and expires on December 31, 2016. Upon an event of default, Rusnano has the option to assign 69.2% of the unpaid principal and interest under the Panacela Loan to CBLI in exchange for shares of CBLI common stock at a price of $33.88 per share.
For the years ended December 31, 2014 and 2013, we recognized interest expense of $1,340,639 and $338,112 for these loans, respectively. Included in the $1,340,639 of interest expense recognized for the year ended December 31, 2014 was a $401,803 non-cash write-off of debt issuance costs associated with the $4.0 million early loan repayment in June 2014.
7. Stockholders’ Equity
On January 27, 2015, the Company’s stockholders approved the Reverse Split of one share for each twenty shares outstanding (1:20). The Reverse Split became effective as of the open of trading on the NASDAQ Capital Market on January 28, 2015. All shares of Common Stock, warrants, options, per share data and exercise prices included in these financial statements and notes for all periods presented have been retroactively adjusted to reflect the Reverse Split with respect to the Company’s shares of Common Stock.
On June 20, 2014, the Company completed a sale of units that were immediately separable into an aggregate of 308,370 shares of the Company’s common stock and warrants to purchase up to 154,186 additional shares of the Company’s common stock issuable upon the exercise of a warrant. Each unit was sold for $11.35, which qualified as an “at market” transaction as determined by NASDAQ, resulting in net proceeds of approximately $3.3 million after deducting for placement agent fees and offering expenses. In connection with the sale, the Company issued Series J warrants for 154,186 shares of common stock to the purchasers, and 1,324 Series J warrants to the placement agent. Each Series J warrant has an exercise price of $11.20 per share, and will expire five years from the date of issuance. The sale also triggered a reduction in the exercise price of 228,891 of the Company’s warrants to $10.10.
On January 16, 2014, the Company completed a public offering of 286,886 shares of the Company’s common stock at a price of $24.40 per share, resulting in net proceeds of approximately $6.4 million after deducting for placement agent fees and offering expenses. In connection with the offering, the Company issued Series A warrants for 143,445 shares of common stock and Series B warrants for 143,445 shares of common stock to the purchasers. Each Series A warrant has an exercise price of $24.40 per share, and will become exercisable nine months following the date of issuance and expire five years from the date of issuance. Each Series B warrant has an exercise price of $24.40 per share, and became exercisable nine months following the date of issuance and will expire 18 months from the date of issuance. In addition to the warrants issued to the purchasers, the Company also issued Series A warrants for an aggregate of 4,306 shares of common stock and Series B warrants for an aggregate of 4,306 shares of common stock to the placement agent as compensation for completing the offering. The warrants to the placement agent have the same terms, including exercise price, as the warrants issued to investors. The offering also triggered a reduction in the exercise price of 221,078 of the Company’s warrants to $24.40.
The January 2014 Series A and B warrants contain provisions that could require the Company to settle the warrants in cash, and accordingly, have been classified as a liability. The fair value of the January Series A and B warrants amounted to $2,283,092 and was determined based on the following assumptions using the Black-Scholes valuation model:
| January 16, 2014 | ||||
| Stock price | $ | 24.60 | ||
| Exercise price | $ | 24.40 | ||
| Term in years | .75 - 2.50 | |||
| Expected volatility | 43.06 - 79.86 | % | ||
| Expected dividend yield | 0 | % | ||
| Risk-free interest rate | .09 - .58 | % | ||
| F-34 |
On September 4, 2014, the Company and certain investors in the January 16, 2014 public offering discussed above amended the Securities Purchase Agreement to remove restrictions on the Company’s ability to issue securities involving a variable rate transaction, as therein defined. In addition, the same investors returned January 2014 Series B warrants to purchase, in the aggregate, 102,460 shares of the Company’s common stock for cancellation. In exchange for these concessions, the Company agreed to extend the expiration dates from January 16, 2019 to January 16, 2021 for Series A warrants to purchase 102,460 shares of the Company’s common stock, and reduced the exercise price from $24.40 to $20.40.
On September 29, 2014, the Company and certain investors amended a Securities Purchase Agreement, dated as of February 13, 2009, to remove restrictions on the Company’s ability to issue securities involving a variable rate transaction, as therein defined. In exchange, the Company agreed to extend the expiration dates from February 13, 2016 to March 30, 2018 on Series D warrants to purchase 174,307 shares of the Company’s common stock, and revised the anti-dilution provision in the Series D warrants to include variable rate transactions.
On January 9, 2015, the Company and certain investors amended a Securities Purchase Agreement, dated as of February 25, 2010, to remove restrictions on the Company’s ability to issue securities involving a variable rate transaction, as therein defined. In exchange, the Company agreed to extend the expiration dates from March 2, 2015 to March 2, 2017 on warrants to purchase 46,771 shares of the Company’s common stock, and revised the anti-dilution provision to include variable rate transactions.
In October 2012, CBLI completed a public offering of 187,500 units at a price of $40.00 per unit, with each unit consisting of one share of CBLI common stock, and one warrant to purchase 0.5 of a share of CBLI common stock at an exercise price of $60.00 per whole share, or the 2012 Offering. The shares of CBLI common stock and the warrants issued in the 2012 Offering were issued separately. Under the terms of the related underwriting agreement, CBLI granted the Underwriters an option, exercisable for 30 days, to purchase up to an additional 56,250 shares of CBLI common stock (with an over-allotment price of $39.90 per share) and/or additional warrants to purchase up to 28,125 shares of CBLI common stock (with an over-allotment price of $0.19 for each warrant to purchase a whole share) to cover over-allotments, if any. The underwriter exercised their option in part by purchasing 51,250 shares and 28,125 warrants of the over-allotment option within 30 days of the closing.
Certain warrants issued during the 2012 offering contain provisions that could require the Company to settle the warrants in cash and, accordingly, were originally recorded as a liability in the amount of $4,521,681 determined by the Black-Scholes valuation model with the following assumptions:
| Stock price | $ | 49.60 | ||
| Exercise price | $ | 60.00 | ||
| Term in years | 2.51 | |||
| Expected volatility | 79.70 | % | ||
| Expected dividend yield | 0 | % | ||
| Risk-free interest rate | 0.35 | % |
The 2012 Offering triggered a reduction in the exercise price of 46,771 of the Company’s warrants to $40.00.
| F-35 |
The following table sets forth the changes in the number of warrants outstanding for the periods presented, exclusive of the warrants issued to Rusnano in connection with their loan to Panacela as those warrants are not exercisable until the occurrence of an event of default, which has not occurred:
| Number of Warrants | Weighted Average Exercise Price | |||||||
| Outstanding at December 31, 2012 | 518,939 | $ | 58.80 | |||||
| Granted | 7,813 | 32.00 | ||||||
| Forfeited, Canceled | — | — | ||||||
| Outstanding at December 31, 2013 | 526,752 | 54.00 | ||||||
| Granted | 451,012 | 19.85 | ||||||
| Forfeited, Canceled | (102,460 | ) | 24.40 | |||||
| Outstanding at December 31, 2014 | 875,304 | 33.72 | ||||||
Equity Incentive Plan
The following is a summary of option award activity under the Plan for the year ended December 31, 2014:
| Year Ended December 31, 2014 | ||||||||||||||||
| Total Stock Options Outstanding | Weighted Average Exercise Price per Share | Nonvested Stock Options | Weighted Average Grant Date Fair Value per Share | |||||||||||||
| December 31, 2013 | 278,390 | $ | 82.90 | 29,724 | $ | 29.97 | ||||||||||
| Granted | 49,550 | 12.48 | 2,405 | 6.73 | ||||||||||||
| Vested | — | — | (5,155 | ) | 51.85 | |||||||||||
| Exercised | — | — | — | — | ||||||||||||
| Forfeited, Canceled | (66,551 | ) | 89.45 | (5,687 | ) | 28.97 | ||||||||||
| December 31, 2014 | 261,389 | 67.89 | 21,287 | 22.31 | ||||||||||||
The following is a summary of outstanding stock options under the Plan as of December 31, 2014:
| Stock Options Outstanding | Non-Vested Stock Options | |||||||
| Quantity | 261,389 | 21,287 | ||||||
| Weighted-average exercise price | $ | 67.89 | $ | 28.97 | ||||
| Weighted Average Remaining Contractual Term (in Years) | 6.48 | 6.30 | ||||||
| Intrinsic value | $ | — | $ | — | ||||
For the years ended December 31, 2014, 2013 and 2012, the Company granted 49,550, 45,191 and 56,646 stock options, respectively, with a weighted-average grant date fair value of $7.60, $24.15 and $26.33, respectively. For the years ended December 31, 2014, 2013 and 2012, the total fair value of options vested was $267,382, $887,603 and $1,549,888, respectively. The total intrinsic value of options exercised for the years ended December 31, 2014, 2013 and 2012 was $0, $5,736 and $1,485, respectively.
As of December 31, 2014, total compensation cost not yet recognized related to non-vested stock options was $63,013. The Company expects to recognize this cost over a weighted average period of 0.57 years.
8. Restructuring
On September 30, 2013, we transferred 26 laboratory and pre-clinical employee positions to BBL, an entity then owned in part by our Chief Scientific Officer, or CSO, and director, Dr. Andrei Gudkov, to enable us to better focus on clinical development activities. In connection with this transition, we entered into a Master Services Agreement, or MSA, with BBL, pursuant to which BBL agreed to perform laboratory and pre-clinical research services for us. We plan to engage BBL for pre-clinical research services in the future. Such services are discussed further in Note 9, “Significant Alliances and Related Parties.”
| F-36 |
As a result of the above described restructuring, we recorded $101,000 for one-time employee transition costs, $112,000 for an idle facilities reserve and $293,000 for an impairment loss on research equipment. All of these costs were recorded as R&D expense during the year ended December 31, 2013. During the year ended December 31, 2014, a subsequent measurement adjustment of $33,126 was recorded against our idle facilities reserve.
9. Significant Alliances and Related Parties
Roswell Park Cancer Institute
The Company has entered into several agreements with Roswell Park Cancer Institute, or RPCI, including: various sponsored research agreements, an exclusive license agreement and clinical trial agreements for the conduct of the Phase 1 entolimod oncology study and the Phase 1 CBL0137 intravenous administration study. Additionally, the Company’s CSO is the Senior Vice President of Basic Research at RPCI.
The Company incurred $1,042,859, $2,323,078 and $3,876,073 in expense to RPCI related to research grants and agreements for the years ended December 31, 2014, 2013 and 2012, respectively. The Company had $0 and $172,295 included in accounts payable owed to RPCI at December 31, 2014 and 2013, respectively. In addition, the Company had $324,194 and $491,397 in accrued expenses payable to RPCI at December 31, 2014 and 2013, respectively.
The Cleveland Clinic
CBLI entered into an exclusive license agreement, or the License, with The Cleveland Clinic pursuant to which CBLI was granted an exclusive license to The Cleveland Clinic’s research base underlying our therapeutic platform and certain product candidates in development by Panacela. CBLI has the primary responsibility to fund all newly developed patents; however, The Cleveland Clinic retains ownership of those patents covered by the agreement. CBLI also agreed to use commercially diligent efforts to bring one or more products to market as soon as practical, consistent with sound and reasonable business practices and judgments. In consideration for the License, CBLI agreed to issue The Cleveland Clinic common stock and make certain milestone, royalty and sublicense royalty payments. Milestone payments, which may be credited against future royalties, amounted to $0, $0 and $100,000 for the years ended December 31, 2014, 2013 and 2012, respectively. No royalty or sublicense royalty payments were made to The Cleveland Clinic during the three-year period ended December 31, 2014.
The Company did not have any liabilities to The Cleveland Clinic at December 31, 2014 or 2013.
Buffalo BioLabs, et. al.
Our CSO, Dr. Andrei Gudkov has business relationships with several entities with which we transact business, the most significant of which is BBL where Dr. Gudkov was a founder and currently serves as their Principal Scientific Advisor. Pursuant to the MSA that was executed with BBL (see Note 8, “Restructuring”), the Company recognized $1,159,963, $419,284 and $0 as research and development expense for the years ended December 31, 2014, 2013 and 2012, respectively, and included $34,800 and $111,356 in accounts payable at December 31, 2014 and 2013. We also recognized $230,398, $64,362 and $6,500 from BBL for sublease and other income for the years ended December 31, 2014, 2013 and 2012, respectively. Pursuant to our real estate sublease and equipment lease with BBL, we had accounts receivable of $198,124 and $843 at December 31, 2014 and 2013, respectively. As far as other entities with which Dr. Gudkov is affiliated, the Company recognized other income of $0 and $217,347, and $0; and, recognized research and development expenses of $0, $29,607 and $0 for the years ended December 31, 2014, 2013 and 2012, respectively. As of December 31, 2014 and 2013, there were no amounts owed to, or receivable from these entities.
10. Income Taxes
The Company accounts for income taxes using the asset and liability method. Deferred taxes are determined by calculating the future tax consequences attributable to differences between the financial accounting and tax bases of existing assets and liabilities. A valuation allowance is recorded against deferred tax assets when, in the opinion of management, it is more likely than not that the Company will not be able to realize the benefit from its deferred tax assets.
| F-37 |
The Company files income tax returns, as prescribed by the national, state and local jurisdictions in which it operates. The Company’s uncertain tax positions are related to tax years that remain subject to examination and are recognized in the financial statements when the recognition threshold and measurement attributes are met. Interest and penalties related to tax deficiencies and uncertain tax positions are recorded as income tax expense.
Income (loss) from continuing operations consists of the following:
| For the Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| US operations | $ | 6,234,092 | $ | (13,505,266 | ) | $ | (14,317,608 | ) | ||||
| Foreign operations | (6,198,726 | ) | (6,624,018 | ) | (8,097,032 | ) | ||||||
| $ | 35,366 | $ | (20,129,284 | ) | $ | (22,414,640 | ) | |||||
The provision for income taxes charged to continuing operations is $0 for all periods presented.
Deferred tax assets (liabilities) were comprised of the following as of the periods presented below:
| As of December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Deferred tax assets: | ||||||||||||
| Operating loss carryforwards | $ | 48,643,000 | $ | 44,120,000 | $ | 37,642,000 | ||||||
| Accrued expenses | 8,916,000 | 8,861,000 | 8,576,000 | |||||||||
| Tax credit carryforwards | 3,449,000 | 3,186,000 | 2,921,000 | |||||||||
| Intellectual property | 3,214,000 | 4,846,000 | 3,377,000 | |||||||||
| Outside tax basis difference in affiliate | 3,948,000 | 2,825,000 | 1,616,000 | |||||||||
| Equipment | 365,000 | 388,000 | 237,000 | |||||||||
| Other | — | — | 4,000 | |||||||||
| Total deferred tax assets | 68,535,000 | 64,226,000 | 54,373,000 | |||||||||
| Deferred tax liabilities: | — | — | — | |||||||||
| Net deferred tax asset | 68,535,000 | 64,226,000 | 54,373,000 | |||||||||
| Valuation allowance | (68,535,000 | ) | (64,226,000 | ) | (54,373,000 | ) | ||||||
| $ | — | $ | — | $ | — | |||||||
The provision for income taxes differs from the amount of income tax determined by applying the applicable U.S. statutory federal income tax rate to the pretax loss from continuing operations as a result of the following differences:
| For the Year Ended December 31, | ||||||||||||
| 2014 | 2013 | 2012 | ||||||||||
| Tax at the U.S. statutory rate | $ | 12,000 | $ | (6,810,000 | ) | $ | (7,621,000 | ) | ||||
| Change in value of warrant liability | (905,000 | ) | (974,000 | ) | (2,619,000 | ) | ||||||
| Stock option expenses | — | — | — | |||||||||
| Valuation allowance | 5,717,000 | 7,723,000 | 10,204,000 | |||||||||
| Deconsolidation of Incuron | (4,830,000 | ) | ||||||||||
| Other | 6,000 | 61,000 | 36,000 | |||||||||
| $ | — | $ | — | $ | — | |||||||
| F-38 |
At December 31, 2014, the Company had U.S. federal net operating loss carryforwards of approximately $120,934,000, which begin to expire if not utilized by 2023, and approximately $3,565,000 of tax credit carryforwards which begin to expire if not utilized by 2024. The Company also has U.S. state net operating loss carryforwards of approximately $110,957,000, which begin to expire if not utilized by 2027 and state tax credit carryforwards of approximately $337,000, which begin to expire if not utilized by 2022.
The Company files U.S. federal tax returns, along with various state and foreign income tax returns. All federal, state and foreign tax returns for the years ended December 31, 2013, 2012 and 2011 are still open for examination.
The following presents a roll-forward of the unrecognized tax benefits and the associated interest and penalties:
| Unrecognized Tax Benefits | Interest and Penalties | |||||||
| Balance at January 1, 2013 | $ | 437,000 | $ | — | ||||
| Prior year tax position | — | — | ||||||
| Current year tax position | — | — | ||||||
| Deferred tax position | 8,000 | — | ||||||
| Settlements with tax authorities | — | — | ||||||
| Expiration of the statute of limitations | — | — | ||||||
| Balance at December 31, 2013 | 445,000 | — | ||||||
| Prior year tax position | — | — | ||||||
| Current year tax position | — | — | ||||||
| Deferred tax position | 8,000 | — | ||||||
| Settlements with tax authorities | — | — | ||||||
| Expiration of the statute of limitations | — | — | ||||||
| Balance at December 31, 2014 | $ | 453,000 | $ | — | ||||
CBLI received New York State incentive tax credit refunds of $0, $62,000 and $532,000 during 2014, 2013 and 2012, respectively. These refundable tax credits were based on the Company’s research and development activities, real estate tax payments, employment levels and equipment purchases. Since there is no state tax liability or refund of prior year tax payments, these refundable tax credits were recorded against operating expenses in the year of receipt, instead of being recorded as an income tax benefit.
11. Employee Benefit Plan
CBLI maintains an active defined contribution retirement plan for its employees, referred to herein as the Benefit Plan. All employees satisfying certain service requirements are eligible to participate in the Benefit Plan. The Company makes matching cash contributions each payroll period, up to 4% of employees’ salaries. The Company’s expense relating to the Benefit Plan was $79,737, $196,257 and $201,510 for the years ended December 31, 2014, 2013 and 2012, respectively.
12. Commitments and Contingencies
The Company has entered into various agreements with third parties and certain related parties in connection with the research and development activities of its existing product candidates as well as discovery efforts on potential new product candidates. These agreements include fixed obligations to sponsor research and development activities and minimum royalty payments for licensed patents. These amounts do not include any additional amounts that the Company may be required to pay under its license agreements upon the achievement of scientific, regulatory and commercial milestones, including milestones such as the submission of an IND to the FDA and the first commercial sale of the Company’s products in various countries. As of December 31, 2014 the Company is uncertain as to whether any of these contingent events will become realized. The Company is also party to five agreements that require it to make milestone payments, pay royalties on net sales of the Company’s products, and make payments on sublicense income received by the Company relating to certain products. There were no milestone payments or royalties on net sales accrued for any of these agreements as of December 31, 2014 and 2013 as none were due.
| F-39 |
From time-to-time, the Company may have certain contingent liabilities that arise in the ordinary course of business. The Company accrues for liabilities when it is probable that future expenditures will be made and such expenditures can be reasonably estimated. For all periods presented, the Company was not a party to any pending material litigation or other material legal proceedings.
The Company has entered into agreements with substantially all of our employees who, if terminated by the Company without cause as described in these agreements, would be entitled to severance pay.
As of December 31, 2014, the Company had unconditional purchase obligations totaling $861,257 for goods and services, substantially all of which the Company anticipates to incur during 2015.
Capital Lease
In December 2011, the Company entered into a capital lease for scientific equipment in the amount of $304,673. The terms of the lease required an upfront payment of $82,983 and monthly payments of $7,616 for 36 months once the lease term began in March 2012. Principal payments under the capital lease obligation were $83,634, $78,125 and $52,410; and, interest payments were $7,707, $20,827 and $23,708 for the years ended December 31, 2014, 2013 and 2012, respectively. During the year ended December 31, 2013, the Company recognized an impairment loss of $105,491 related to this leased asset as the anticipated discounted future cash flows resulting from the lease with BBL exceeded the carrying value of the asset. As of December 31, 2014, future minimum lease payments amount to $4,500. As of December 31, 2014, accumulated depreciation for the leased equipment was $27,622.
Operating Leases
The Company leases laboratory facilities and office facilities at various locations with expiration dates ranging from 2015 to 2019. The Company recognizes rent expense on a straight-line basis over the term of the related operating leases. For the years ended December 31, 2014, 2013 and 2012, total rent expense related to the Company’s operating leases was $466,770, $511,029 and $459,150, respectively. In addition, the Company has subleased some of its facilities.
As of December 31, 2014, future minimum payments under operating leases are as follows:
| 2015 | $ | 377,623 | ||
| 2016 | 354,918 | |||
| 2017 | 365,565 | |||
| 2018 | 376,532 | |||
| 2019 | 191,048 | |||
| Total minimum lease payments | $ | 1,665,686 |
| F-40 |
13. Quarterly Financial Data (Unaudited)
The following is a summary of the quarterly consolidated results of operations for the years ended December 31, 2014 and December 31, 2013:
| For the Quarter Ended | ||||||||||||||||
| March 31, 2014 | June 30, 2014 | September 30, 2014 | December 31, 2014 | |||||||||||||
| Revenues | $ | 1,334,254 | $ | 562,087 | $ | 415,126 | $ | 1,390,432 | ||||||||
| Loss from Operations | (3,519,062 | ) | (4,012,504 | ) | (3,438,739 | ) | (3,451,630 | ) | ||||||||
| Net Income (Loss) | (1,901,197 | ) | (4,131,571 | ) | (4,615,663 | ) | 10,683,797 | |||||||||
| Net Income (Loss) Attributable to Cleveland BioLabs, Inc. | (1,585,372 | ) | (3,961,161 | ) | (4,096,134 | ) | 11,271,771 | |||||||||
| Basic Earnings (Loss) Per Share Available for Common Shareholders | $ | (0.63 | ) | $ | (1.53 | ) | $ | (1.43 | ) | $ | 3.95 | |||||
| Fully Diluted Earnings (Loss) Per Share Available for Common Shareholders | $ | (0.63 | ) | $ | (1.53 | ) | $ | (1.43 | ) | $ | 3.95 | |||||
| Weighted Average Shares Outstanding, Basic | 2,498,407 | 2,594,582 | 2,855,510 | 2,856,461 | ||||||||||||
| Weighted Average Shares Outstanding, Diluted | 2,498,407 | 2,594,582 | 2,855,510 | 2,856,461 | ||||||||||||
| For the Quarter Ended | ||||||||||||||||
| March 31, 2013 | June 30, 2013 | September 30, 2013 | December 31, 2013 | |||||||||||||
| Revenues | $ | 1,367,472 | $ | 1,613,262 | $ | 1,635,600 | $ | 3,871,632 | ||||||||
| Loss from Operations | (7,447,515 | ) | (6,776,618 | ) | (5,856,468 | ) | (2,996,158 | ) | ||||||||
| Net Loss | (10,787,148 | ) | (3,886,421 | ) | (4,610,961 | ) | (844,754 | ) | ||||||||
| Net Loss Attributable to Cleveland BioLabs, Inc. | (9,764,323 | ) | (3,042,111 | ) | (4,091,196 | ) | (365,247 | ) | ||||||||
| Basic Loss Per Share Available for Common Shareholders | $ | (4.36 | ) | $ | (1.35 | ) | $ | (1.82 | ) | $ | (0.16 | ) | ||||
| Fully Diluted Loss Per Share Available for Common Shareholders | $ | (4.36 | ) | $ | (1.35 | ) | $ | (1.82 | ) | $ | (0.16 | ) | ||||
| Weighted Average Shares Outstanding, Basic | 2,241,329 | 2,247,430 | 2,253,064 | 2,258,507 | ||||||||||||
| Weighted Average Shares Outstanding, Diluted | 2,241,329 | 2,247,430 | 2,253,064 | 2,258,507 | ||||||||||||
| F-41 |
14. Subsequent Events
On February 6, 2015, we sold 572,205 shares of common stock, plus an additional 594,688 shares of common stock subject to Series B pre-funded warrants all at a price of $3.00 per share. An additional $717,400 was invested in Series A preferred stock convertible into common stock at $3.00 per share for 239,135 shares and issued in a private placement. The total investment amounted to $4.2 million and the total common shares issuable under these securities are 1,406,028 after full exercise and conversion, subject to shareholder approval. In addition, a total of 1,406,028 Series A warrants to purchase shares of common stock at an exercise price of $3.64 per share were issued in a private placement, which shall be initially exercisable six months following issuance and expire six years from the date they become exercisable.
After giving effect to the forgoing transaction and 5,023 shares issued in January 2015 to certain Board members in lieu of cash compensation, CBLI’s capitalization structure as of February 6, 2015 follows:
Series A convertible preferred stock, $1,000 stated value: 717.4 shares outstanding, convertible into 239,135 common shares. Common shares outstanding: 3,435,354, along with 594,688 shares of common stock issuable under Series B pre-funded warrants. Upon the full conversion of all Series A convertible preferred shares and issuance of stock under all Series B pre-funded warrants, the Company would have 4,269,177 shares of common stock outstanding. All of the common shares issuable under the Series A convertible preferred stock and Series B pre-funded warrants are currently convertible/issuable at $3.00 per share, subject to adjustment if the Company sells stock at a lesser price.
Shares issuable under outstanding options increased since December 31, 2014 and, as of February 6, 2014, there were 261,470 shares issuable at a weighted average price of $67.89 per share.
The following table sets forth the details of our outstanding warrants as of February 6, 2015, giving effect exercise price adjustments triggered by the equity offering discussed above.
| Warrant Series | Expiration Date | Current Exercise Price | Outstanding Warrants | |||||||||||
| Jun 2011 Series F agent | 6/17/2015 | $ | 100.00 | 8,809 | ||||||||||
| Jan 2014 Series B | 7/16/2015 | 24.40 | 45,291 | |||||||||||
| Jun 2011 Series F | 6/22/2016 | 100.00 | 73,414 | |||||||||||
| Mar 2010 | * | ** | 3/2/2017 | 3.00 | 46,771 | |||||||||
| Oct 2012 Series G | *** | 10/24/2017 | 60.00 | 215,638 | ||||||||||
| 2009 Series D | ** | 3/30/2018 | 3.00 | 174,307 | ||||||||||
| Hercules | * | 9/29/2018 | 10.10 | 7,813 | ||||||||||
| Jan 2014 Series A | 1/16/2019 | 24.40 | 45,291 | |||||||||||
| Jun 2014 Series J | * | 6/20/2019 | 11.20 | 155,510 | ||||||||||
| Jan 2014 Series A Modified | 1/16/2021 | 20.40 | 102,460 | |||||||||||
| Feb 2015 Series A | ** | 8/4/2021 | 3.64 | 1,406,028 | ||||||||||
| 2,281,332 | ||||||||||||||
| Weighted average exercise price | $ | 14.49 | ||||||||||||
| * | can be exercised cashlessly |
| ** | have price protection only, no increase in shares |
| *** | trade under own cusip |
In addition, a warrant for 38,504 shares could be exercised at $33.88 per share in the event of a default on a loan between Rusnano and Panacela. That loan is currently not in default and therefore this warrant is excluded from the above.
| F-42 |

$10,000,000 of
Class A Units consisting of Common Stock and Warrants and
Class B Units consisting of Series B Preferred Stock and Warrants
( shares of Common Stock Underlying the Series B Preferred Stock and Warrants)
PROSPECTUS
Ladenburg Thalmann
, 2015
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table provides information regarding the various actual and anticipated expenses (other than underwriting discounts) payable by us in connection with the issuance and distribution of the securities being registered hereby. All amounts shown are estimates except the Securities and Exchange Commission registration fee, the FINRA filing fee and the NASDAQ initial listing fee.
| Nature of Expense | Amount | |||
| SEC registration fee | $ | 1,336 | ||
| FINRA filing fee | * | |||
| NASDAQ initial listing fee | * | |||
| Accounting fees and expenses | * | |||
| Legal fees and expenses | * | |||
| Transfer agent’s fees and expenses | * | |||
| Printing and related fees | * | |||
| Miscellaneous | * | |||
| Total | ||||
* To be completed by amendment
Item 14. Indemnification of Directors and Officers.
Section 145 of the Delaware General Corporation Law authorizes a court to award, or a corporation’s board of directors to grant, indemnity to directors and officers in terms sufficiently broad to permit such indemnification under certain circumstances for liabilities, including reimbursement for expenses incurred, arising under the Securities Act.
Our second amended and restated bylaws provide for indemnification of our directors and executive officers to the maximum extent permitted by the Delaware General Corporation Law.
In addition, we have entered into indemnification agreements with certain of our current directors and executive officers. These agreements will require us to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to us and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified.
In any underwriting agreement we enter into in connection with the sale of common stock being registered hereby, the underwriter will agree to indemnify, under certain conditions, us, our directors, our officers and persons who control us, within the meaning of the Securities Act, against certain liabilities.
Item 15. Recent Sales of Unregistered Securities.
During the last three years, we made sales of the following unregistered securities:
| (1) | During 2012, as consideration for consulting services provided, we issued an aggregate of 75,000 shares of our common stock to various consultants. |
| (2) | During 2013, as consideration for consulting services provided, we issued an aggregate of 175,000 shares of our common stock to various consultants. |
| II-1 |
| On September 30, 2013, pursuant to the Loan and Security Agreement between us, our wholly owned subsidiary, Biolab 612, LLC, and Hercules Technology II, L.P., or Hercules, we issued to Hercules a warrant to purchase 156,250 shares of our common stock at an exercise price of $1.60 per share. The exercise price of the warrant may be adjusted downward if during the one-year period following the closing date, we sell and issue shares of common stock or convertible stock in a transaction not registered under the Securities Act at a price per share less than the exercise price. The warrant will expire five years from the date of the grant. |
| (3) | In January 2014, in connection with our public offering, we issued to the placement agent and its affiliates warrants to purchase an aggregate of 172,132 shares of our common stock with an exercise price of $1.22 per share. |
| (4) | On June 20, 2014, we completed a sale of units that are immediately separable into an aggregate of 6,167,400 shares of our common stock and warrants to purchase up to 3,083,700 additional shares of the Company’s common stock. Each unit was sold for $0.5675, which qualified as an “at market” transaction as determined by NASDAQ, resulting in net proceeds of approximately $3.3 million after deducting for placement agent fees and offering expenses. In connection with the sale, we issued Series J warrants for 3,083,700 shares of common stock to the purchasers, and 26,432 Series J warrants to the placement agent. Each Series J warrant has an exercise price of $0.56 per share, and will expire five years from the date of issuance. |
| (5) | From January 1, 2011 to November 21, 2014, we granted stock options under our 2006 Equity Incentive Plan to purchase up to an aggregate of 5,799,914 shares of its common stock to its employees, directors and consultants, at a weighted-average exercise price of $3.52 per share. |
| (6) | On February 4, 2015, Cleveland BioLabs, Inc. (the “Company”) entered into a Securities Purchase Agreement (the “Purchase Agreement”) with certain institutional investors providing for the issuance and sale by the Company of 572,205 shares (the “Shares”) of the Company’s common stock, par value $0.005 per share at an offering price of $3.00 per share (the “Share Offering”) and Series B pre-funded warrants to purchase an aggregate of 594,688 shares of its Common Stock (the “Pre-Funded Warrants,”) (the “Pre-Funded Warrants Offering”). The Shares and the Pre-Funded Warrants were offered by the Company pursuant to an effective shelf registration statement on Form S-3, which was initially filed with the Securities and Exchange Commission on December 10, 2013 and declared effective on January 10, 2014 (File No. 333-192755) (the “Registration Statement”). |
In a concurrent private placement (the “Private Placement Transaction” and, together with the Share Offering and the Pre-Funded Warrants Offering, the “Offerings”), we sold to the purchasers of our Shares and Pre-Funded Warrants in this offering shares of our Series A Convertible Preferred Stock (the “Preferred Stock”) convertible into 239,135 of our common stock. Gross proceeds from the offerings amounted to approximately $4.2 million before deducting placement agent fees and expenses. In addition, a Series A warrant (the “Series A Warrants” and, together with the Shares, the Pre-Funded Warrants and the Preferred Stock, the “Securities”) were issued to purchase one share of our common stock for each share of common stock purchased or pre-funded in this offering and each share of Series A Convertible Preferred Stock purchased in the concurrent private placement. The Series A Warrants cover, in the aggregate, 1,406,028 shares of common stock and become exercisable six months following the date of issuance at an exercise price of $3.64 and expire six years from the date they become exercisable.
Pursuant to the terms of the Placement Agency Agreement between the Company and Ladenburg Thalmann & Co. Inc. (“Ladenburg Thalmann”) dated February 4, 2015, Ladenburg Thalmann has no obligation to buy any of the Securities or to arrange for the purchase or sale of any specific number or dollar amount of Securities. The Company has agreed to pay Ladenburg Thalmann a fee equal to 8% on aggregate gross proceeds in this offering, excluding the proceeds, if any, from the exercise of the Series A Warrants. The offering closed on February 6, 2015 (the “Closing”).
| II-2 |
On February 6, 2015, the Company amended the terms of the Securities as described below.
Pursuant to the terms of the Purchase Agreement, the Company has agreed that during the 75-day period following execution of the Purchase Agreement, the Company will not issue (or enter into any agreement to issue) any shares of common stock or common stock equivalents, subject to certain exceptions including securities issuable pursuant to the Purchase Agreement or pursuant to exercises, exchanges or conversions of the Company’s outstanding securities and issuances pursuant to acquisitions or strategic transactions. In addition, pursuant to the Purchase Agreement, the purchasers in the Offerings have the right, until one year after shareholder approval for the Offerings is obtained, to participate in subsequent financings by the Company in an amount up to 50% of the financing in the aggregate subject to certain exceptions as specified in the Purchase Agreement. Under the terms of the Securities, until shareholder approval has been obtained, the Company cannot issue any Shares and the investors in the Offerings cannot exercise the Pre-Funded Warrants into Common Stock, nor convert the Preferred Stock into Common Stock. On February 6, 2015, the Company and investors amended the terms of the Securities to also include the Series A Warrants from being exercised until shareholder approval has been obtained.
The offers, sales and issuances of the securities described in paragraphs (1) through (6) above were deemed to be exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act and Rule 506 promulgated under Regulation D as transactions by an issuer not involving a public offering. The recipients of securities in each of these transactions acquired the securities for investment only and not with a view to or for sale in connection with any distribution thereof and appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions was an accredited investor within the meaning of Rule 501 of Regulation D under the Securities Act and had adequate access, through employment, business or other relationships, to information about us. No underwriters were involved in these transactions.
The offers, sales and issuances of the securities described in paragraph (7) above were deemed to be exempt from registration under the Securities Act in reliance on Rule 701 in that the transactions were under compensatory benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of such securities were our employees, directors or bona fide consultants and received the securities under the Registrant’s 2006 Equity Incentive Plan. Appropriate legends were affixed to the securities issued in these transactions. Each of the recipients of securities in these transactions had adequate access, through employment, business or other relationships, to information about us.
All certificates representing the securities issued in the transactions described in this Item 15 included appropriate legends setting forth that the securities had not been offered or sold pursuant to a registration statement and describing the applicable restrictions on transfer of the securities. There were no underwriters employed in connection with any of the transactions set forth in this Item 15.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
A list of exhibits filed with this registration statement on Form S-1 is set forth on the Exhibit Index and is incorporated herein by reference.
(b) Financial Statement Schedule.
All schedules have been omitted because either they are not required, are not applicable or the information is otherwise set forth in the financial statements and related notes thereto.
Item 17. Undertakings
(a) The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933, as amended (the “Securities Act”);
| II-3 |
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent posteffective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purposes of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of the securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) For the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
| II-4 |
(b) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities, other than the payment by the registrant of expenses incurred and paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding, is asserted by such director, officer or controlling person in connection with the securities being registered hereby, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
(c) The undersigned Registrant hereby undertakes that it will:
(1) for determining any liability under the Securities Act, treat the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant under Rule 424(b)(1), or (4) or 497(h) under the Securities Act as part of this registration statement as of the time the Commission declared it effective.
(2) for determining any liability under
the Securities Act, treat each post-effective amendment that contains a form of prospectus as a new registration statement for
the securities offered in the registration statement, and that offering of the securities at that time as the initial bona fide
offering of those securities.
| II-5 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of Buffalo, New York, on the 11th day of May, 2015.
| CLEVELAND BIOLABS, INC. | ||
| By: | /s/ Yakov Kogan | |
|
Yakov Kogan, Ph.D., MBA Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ YAKOV KOGAN | Chief Executive Officer and Director | |||
| Yakov Kogan | (Principal Executive Officer) | May 11, 2015 | ||
| /s/ C. NEIL LYONS | Chief Financial Officer | |||
| C. Neil Lyons | (Principal Financial and Accounting Officer) | May 11, 2015 | ||
| /s/ ANDREI GUDKOV | ||||
| Andrei Gudkov | Director | May 11, 2015 | ||
| /s/ JAMES J. ANTAL | ||||
| James J. Antal | Director | May 11, 2015 | ||
| /s/ ANTHONY PRINCIPI | ||||
| Anthony Principi | Director | May 11, 2015 | ||
| /s/ RANDY S. SALUCK | ||||
| Randy S. Saluck | Director | May 11, 2015 | ||
| /s/ RICHARD MCGOWAN | ||||
| Richard McGowan | Director | May 11, 2015 |
| II-6 |
EXHIBIT INDEX
| Exhibit No. | Identification of Exhibit | |
| 1.1 # | Form of Underwriting Agreement | |
| 2.1 | Master Purchase Agreement, dated April 29, 2015, between Cleveland BioLabs, Inc., Mikhail Mogutov, and Incuron LLC (Incorporated by reference to Form 8-K filed on May 4, 2015). | |
| 2.2 | Option Agreement, dated April 29, 2015, between Cleveland BioLabs, Inc. and Mikhail Mugutov (Incorporated by reference to Form 8-K filed on May 4, 2015). | |
| 3.1 | Restated Certificate of Incorporation filed with the Secretary of State of Delaware on March 18, 2010 (Incorporated by reference to Form 10-K for the year ended December 31, 2009, filed on March 22, 2010). | |
| 3.2 | Certificate of Amendment to the Restated Certificate of Incorporation, filed with the Secretary of State of Delaware on June 20, 2013 (Incorporated by reference to Form 10-Q for the period ended June 30, 2013, filed on August 9, 2013). | |
| 3.3 | Certificate of Designation of Series A Convertible Preferred Stock (Incorporated by reference to Form 8-K filed on February 6, 2015). | |
| 3.4 # | Certificate of Designation of Series B Preferred Stock | |
| 3.5 | Second Amended and Restated By-Laws (Incorporated by reference to Form 8-K filed on December 5, 2007). | |
| 4.1 | Form of Warrants issued to underwriters (Incorporated by reference to Amendment No. 3 to Registration Statement on Form SB-2 filed on July 10, 2006 (File No. 333-131918)). | |
| 4.2 | Form of Common Stock Purchase Warrant (Series D Transaction) (Incorporated by reference to Form 8-K filed on March 30, 2009). | |
| 4.3 | Form of Common Stock Purchase Warrant (Private Placement closed on March 2, 2010) (Incorporated by reference to Form 8-K/A filed on February 26, 2010). | |
| 4.4 | Form of Series E and F Warrants (Incorporated by reference to Form 8-K filed on June 21, 2011). | |
| 4.5 | Form of Warrant Agreement by and between Cleveland BioLabs, Inc. and Continental Stock Transfer & Trust Company (Incorporated by reference to Form 8-K filed on October 22, 2012). | |
| 4.6 | Warrant to Purchase Common Stock issued to Open Joint Stock Company “Rusnano” (Incorporated by reference to Form 8-K filed on September 5, 2013). | |
| 4.7 | Warrant Agreement, dated September 30, 2013, between Cleveland BioLabs, Inc. and Hercules Technology II, L.P. (Incorporated by reference to Form 10-Q for the period ended September 30, 2013, filed on November 8, 2013). | |
| 4.8 | Form of Series A/B Warrant to Purchase Common Stock (Incorporated by reference to Form 8-K filed on January 15, 2014). | |
| 4.9 | Form of Series J Warrant (Incorporated by reference to Form 8-K filed on June 20, 2014). | |
| 4.10 | Amendment to Series A Common Stock Purchase Warrant, dated September 4, 2014, by and between Cleveland BioLabs, Inc. and Sabby Healthcare Volatility Master Fund, Ltd. (Incorporated by reference to Form 8-K filed on September 8, 2014). | |
| 4.11 | Amendment to Series A Common Stock Purchase Warrant, dated September 4, 2014, by and between Cleveland BioLabs, Inc. and Sabby Volatility Warrant Master Fund, Ltd. (Incorporated by reference to Form 8-K filed on September 8, 2014). | |
| 4.12 # | Form of Warrant for this offering | |
| 5.1 # | Opinion of Sichenzia Ross Friedman Ference LLP | |
| 10.1 | Library Access Agreement by and between ChemBridge Corporation and Cleveland BioLabs, Inc., effective as of April 27, 2004 (Incorporated by reference to Amendment No. 1 to Registration Statement on Form SB-2 filed on April 25, 2006 (File No. 333-131918)). | |
| 10.2 | Restricted Stock and Investor Rights Agreement between Cleveland BioLabs, Inc. and ChemBridge Corporation, dated as of April 27, 2004 (Incorporated by reference to Amendment No. 1 to Registration Statement on Form SB-2 filed on April 25, 2006 (File No. 333-131918)). |
| II-7 |
| 10.3.1 | Exclusive License Agreement by and between The Cleveland Clinic Foundation and Cleveland BioLabs, Inc., effective as of July 1, 2004 (Incorporated by reference to Amendment No. 1 to Registration Statement on Form SB-2 filed on April 25, 2006 (File No. 333-131918)). | |
| 10.3.2 | Second Amendment to Exclusive License Agreement, dated September 22, 2011, by and between The Cleveland Clinic Foundation and the registrant (Incorporated by reference to Form 10-Q for the period ended September 30, 2011, filed on November 9, 2011).† | |
| 10.4.1 | Employment Agreement by and between Cleveland BioLabs, Inc. and Dr. Michael Fonstein, dated August 1, 2004 (Incorporated by reference to Amendment No. 1 to Registration Statement on Form SB-2 filed on April 25, 2006 (File No. 333-131918)). | |
| 10.4.2 | Amendment to Employment Agreement by and between Cleveland BioLabs, Inc. and Dr. Michael Fonstein, dated as of December 31, 2008 (Incorporated by reference to Form 10-K for the year ended December 31, 2008, filed on March 30, 2009). | |
| 10.5.1 | Employment Agreement by and between Cleveland BioLabs, Inc. and Dr. Yakov Kogan, dated August 1, 2004 (Incorporated by reference to Amendment No. 1 to Registration Statement on Form SB-2 filed on April 25, 2006 (File No. 333-131918)). | |
| 10.5.2 | Amendment to Employment Agreement by and between Cleveland BioLabs, Inc. and Dr. Yakov Kogan, dated as of December 31, 2008 (Incorporated by reference to Form 10-K for the year ended December 31, 2008, filed on March 30, 2009). | |
| 10.6 | Employment Agreement made as of April 4, 2013 and effective as of April 1, 2013 by and between Cleveland BioLabs, Inc. and Jean Viallet (Incorporated by reference to Form 8-K filed on April 9, 2013). | |
| 10.7 | Cooperative Research and Development Agreement by and between the Uniformed Services University of the Health Sciences, the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., the Cleveland Clinic Foundation, and Cleveland BioLabs, Inc., dated as of August 1, 2004 (Incorporated by reference to Form 10-Q for the period ended September 30, 2010, filed on November 15, 2010). | |
| 10.8.1 | Cleveland BioLabs, Inc. Equity Incentive Plan (Incorporated by reference to Proxy Statement on Schedule 14A filed on April 1, 2008). | |
| 10.8.2 | First Amendment to Cleveland BioLabs, Inc. Equity Incentive Plan (Incorporated by reference to Form 8-K filed on June 9, 2010). | |
| 10.8.3 | Second Amendment to Cleveland BioLabs, Inc. Equity Incentive Plan (Incorporated by reference to Form 8-K filed on June 15, 2012). | |
| 10.8.4 | Form of Stock Award Grant Agreement (Incorporated by reference to Form 8-K filed on June 15, 2012). | |
| 10.8.5 | Form of Non-Qualified Stock Option Agreement (Incorporated by reference to Form 8-K filed on June 15, 2012). | |
| 10.9 | Cleveland Biolabs, Inc. 2013 Employee Stock Purchase Plan (Incorporated by reference to Form 8-K filed on June 20, 2013). | |
| 10.10.1 | Contract (W9113M-10-C-0088), effective as of September 15, 2010, between Cleveland BioLabs, Inc. and the U.S. Army Space and Missile Defense Command/Army Forces Strategic Command (the “2010 DoD Contract”) (Incorporated by reference to Form 10-Q for the period ended September 30, 2010, filed on November 15, 2010). | |
| 10.10.2 | Amendment of Solicitation/Modification of Contract No. 1, effective as of September 17, 2010, to the 2010 DoD Contract (Incorporated by reference to Form 10-Q for the period ended September 30, 2010, filed on November 15, 2010). | |
| 10.10.3 | Amendment of Solicitation/Modification of Contract No. 2, effective as of June 23, 2011, to the 2010 DoD Contract (Incorporated by reference to Form 8-K filed on June 29, 2011). |
| II-8 |
| 10.11 | Process Development and Manufacturing Agreement between Cleveland BioLabs, Inc. and SynCo Bio Partners B.V., effective as of August 31, 2006 (Incorporated by reference to Form 8-K filed on October 25, 2006). | |
| 10.12 | Sponsored Research Agreement between Cleveland BioLabs, Inc. and Roswell Park Cancer Institute Corporation, effective as of January 12, 2007 (Incorporated by reference to Form 8-K filed on January 12, 2007). | |
| 10.13 | Form of Securities Purchase Agreement (Incorporated by reference to Form 8-K filed on March 30, 2009). | |
| 10.14 | Form of Registration Rights Agreement (Incorporated by reference to Form 8-K filed on March 30, 2009). | |
| 10.15 | Amendment and Waiver Agreement, dated March 20, 2009 (Incorporated by reference to Form 8-K filed on March 30, 2009). | |
| 10.16 | Form of Amendment and Reaffirmation Agreement (Incorporated by reference to Form 8-K filed on March 30, 2009). | |
| 10.17 | License Agreement between Cleveland BioLabs, Inc. and Zhejiang Hisun Pharmaceutical Co., Ltd., dated September 3, 2009 (Incorporated by reference to Form 8-K filed on September 9, 2009). | |
| 10.18.1 | Participation Agreement, dated December 30, 2009, by and between Cleveland BioLabs, Inc. and Bioprocess Capital Partners, LLC (Incorporated by reference to Form 8-K filed on January 5, 2010). | |
| 10.18.2 | First Amendment to Participation Agreement, dated April 13, 2010, by and between Cleveland BioLabs, Inc. and Bioprocess Capital Partners, LLC (Incorporated by reference to Form 10-Q for the period ended June 30, 2010, filed on August 16, 2010). | |
| 10.18.3 | Third Amendment to Participation Agreement, dated June 17, 2014, by and among Cleveland BioLabs, Inc. and Bioprocess Capital Partners, LLC (Incorporated by reference to Form 8-K filed on August 6, 2014). | |
| 10.19.1 | Securities Purchase Agreement dated February 25, 2010 (Incorporated by reference to Form 8-K filed on February 26, 2010). | |
| 10.19.2 | Form of Amendment to Securities Purchase Agreement, dated December 23, 2010, among the Company and the amending purchasers identified on the signature pages thereto (Incorporated by reference to Form 8-K filed on December 29, 2010). | |
| 10.20.1 | Consulting Agreement, dated January 1, 2010, between Cleveland BioLabs, Inc. and Andrei Gudkov (Incorporated by reference to Form 8-K filed on June 13, 2011). | |
| 10.20.2 | First Amendment to Consulting Agreement, dated June 10, 2011, between Cleveland BioLabs, Inc. and Andrei Gudkov (Incorporated by reference to Form 8-K filed on June 13, 2011). | |
| 10.20.3 | Amendment to Consulting Agreement, dated June 10, 2011, between Cleveland BioLabs, Inc. and Andrei Gudkov. Gudkov (Incorporated by reference to Form 10-K filed on February 27, 2015). | |
| 10.21 | Engagement letter, dated as of June 16, 2011, by and between Cleveland BioLabs, Inc. and Rodman & Renshaw, LLC (Incorporated by reference to Form 8-K filed on June 21, 2011). | |
| 10.22 | Form of Securities Purchase Agreement, dated June 17, 2011, by and between Cleveland BioLabs, Inc. and the investors in the Offering (Incorporated by reference to Form 8-K filed on June 21, 2011). | |
| 10.23 | Employment Agreement, dated August 4, 2011, between the Company and C. Neil Lyons (Incorporated by reference to Form 8-K filed on August 4, 2011). | |
| 10.24 | Investment Agreement, dated September 19, 2011, by and among Panacela Labs, Inc., the Registrant and Open Joint Stock Company Rusnano (Incorporated by reference to Form 10-Q for the period ended September 30, 2011, filed on November 9, 2011). | |
| 10.25 | Exclusive License and Option Agreement, dated September 23, 2011, by and between Children’s Cancer Institute Australia for Medical Research and Panacela Labs, Inc (Incorporated by reference to Form 10-Q for the period ended September 30, 2011, filed on November 9, 2011).† |
| II-9 |
| 10.26 | Exclusive License and Option Agreement, dated September 23, 2011, by and between Health Research, Inc., Roswell Park Institute Division, Roswell Park Cancer Institute Corporation, and Panacela Labs, Inc (Incorporated by reference to Form 10-Q for the period ended September 30, 2011, filed on November 9, 2011).† | |
| 10.27 | Amended and Restated Exclusive Sublicense Agreement, dated September 23, 2011, by and between the registrant and Panacela Labs, Inc. (Incorporated by reference to Form 10-Q for the period ended September 30, 2011, filed on November 9, 2011). | |
| 10.28 | Assignment Agreement, dated September 23, 2011, by and between Panacela Labs, Inc. and the registrant (Incorporated by reference to Form 10-Q for the period ended September 30, 2011, filed on November 9, 2011). | |
| 10.29 | Master Agreement dated September 3, 2013 by and among Panacela Labs, Inc., Cleveland Biolabs, Inc. and Open Joint Stock Company “Rusnano” (Incorporated by reference to Form 8-K filed on September 5, 2013). | |
| 10.30 | Convertible Loan Agreement dated September 3, 2013 between Open Joint Stock Company “Rusnano” and Panacela Labs, Inc. (Incorporated by reference to Form 8-K filed on September 5, 2013). | |
| 10.31 | Master Services Agreement, dated October 14, 2014, between Buffalo BioLabs, LLC and Cleveland BioLabs, Inc. (Incorporated by reference to Form 8-K filed on October 18, 2013). | |
| 10.32.1 | Loan and Security Agreement, dated September 30, 2013, by and among Cleveland BioLabs, Inc., Biolab 612, LLC and Hercules Technology II, L.P. (Incorporated by reference to Form 10-Q for the period ended September 30, 2013, filed on November 8, 2013). | |
| 10.32.2 | First Amendment to Loan and Security Agreement, dated June 17, 2014, by and among (a) (i) Cleveland BioLabs, Inc., and (ii) Biolab 612, LLC, and (b) Hercules Technology II, L.P., (Incorporated by reference to Form 8-K filed on June 20, 2014). | |
| 10.33 | Letter Agreement, dated January 9, 2014, by and among Cleveland BioLabs, Inc. and H.C. Wainwright & Co., LLC (Incorporated by reference to Form 8-K filed on January 15, 2014). | |
| 10.34 | Securities Purchase Agreement, dated January 14, 2014, by and among Cleveland BioLabs, Inc. and the Purchasers set forth therein (Incorporated by reference to Form 8-K filed on January 15, 2014). | |
| 10.35 | 2012 Executive Compensation Plan (Incorporated by reference to Form 8-K/A filed on April 30, 2012). | |
| 10.36 | 2012 Long-term Executive Compensation Plan (Incorporated by reference to Form 8-K filed on June 15, 2012). | |
| 10.37 | Severance Benefit Plan (Incorporated by reference to Form 8-K filed on May 13, 2014). | |
| 10.38 | Securities Purchase Agreement, dated June 17, 2014, by and among Cleveland BioLabs, Inc., and the purchasers on Exhibit A thereto (Incorporated by reference to Form 8-K filed on June 20, 2014). | |
| 10.39 | Registration Rights Agreement, dated June 17, 2014, by and among Cleveland BioLabs, Inc., and the purchasers on Exhibit A thereto (Incorporated by reference to Form 8-K filed on June 20, 2014). | |
| 10.40 | Rights Agreement, dated June 17, 2014, by and among Cleveland BioLabs, Inc., and Mikhail Mogutov (Incorporated by reference to Form 8-K filed on June 20, 2014). | |
| 10.41 | Amendment No. 1 to Securities Purchase Agreement, dated September 4, 2014, by and among Cleveland BioLabs, Inc. and the parties named on the signature pages thereto (Incorporated by reference to Form 8-K filed on September 8, 2014). | |
| 10.42 | Amendment No. 1 to Securities Purchase Agreement and Series D Warrants, dated September 29, 2014, by and among Cleveland BioLabs, Inc. and the parties on the signature pages thereto (Incorporated by reference to Form 8-K filed on October 1, 2014). | |
| 10.43 | Warrant Agency Agreement # | |
| 10.44 | Preferred Stock Agency Agreement # |
| 10.45 | Third Amendment to Cleveland BioLabs, Inc. Equity Incentive Plan (Incorporated by reference to Form 8-K filed on April 17, 2015). | |
| 10.46 | First Amendment to 2013 Employee Stock Purchase Plan (Incorporated by reference to Form 8-K filed on April 17, 2015). | |
| 10.47 | Royalty Agreement, dated April 29, 2015, between Cleveland BioLabs, Inc. and Incuron LLC (Incorporated by reference to Form 8-K filed May 4, 2015). |
| II-10 |
| 10.48 | Fourth Amendment to Participation Agreement, dated April 29, 2015, between Cleveland BioLabs, Inc. and Limited Liability Company Bioprocess Capital Partners (Incorporated by reference to Form 8-K filed May 4, 2015). | |
| 10.49 | Employment Agreement, dated May 4, 2015, between Cleveland BioLabs, Inc. and Langdon L. Miller, MD (Incorporated by reference to Form 8-K filed May 11, 2015). |
| 23.1 * | Consent of Meaden & Moore, Ltd. | |
| 23.2 | Consent of Sichenzia Ross Friedman Ference LLP (included in Exhibit 5.1) # | |
| 24.1 | Power of Attorney (previously filed) | |
|
101.1 |
The following financial statements and supplementary data are filed as a part of this registration statement: (i) Consolidated Balance Sheets at December 31, 2014 and 2013; (ii) Consolidated Statements of Operations for years ended December 31, 2014, 2013, and 2012; (iii) Consolidated Statements of Stockholders’ Equity for period from December 31, 2011 to December 31, 2014; (iv) Consolidated Statements of Cash Flows for years ended December 31, 2014, 2013, and 2012; and (v) Notes to Consolidated Financial Statements as blocks of text. # |
| # | To be filed by amendment |
| * | Filed herewith |
| † | Confidential treatment has been requested from the Securities and Exchange Commission as to certain portions of this document. |
II-11
